Note: Descriptions are shown in the official language in which they were submitted.
CA 02972416 2017-06-27
WO 2016/122752 PCT/US2015/060893
PULSATING ELECTROMAGNETIC AND ULTRASOUND THERAPY FOR STIMULATING
TARGETED HEAT SHOCK PROTEINS AND FACILITATING PROTEIN REPAIR
BACKGROUND OF THE INVENTION
[Para 1] The present invention is generally directed to the activation of heat
shock proteins and the facilitation of protein repair. More particularly, the
present invention is directed to a system and method for selectively
stimulating
targeted heat shock protein activation or production and facilitating protein
repair utilizing a pulsating electromagnetic or ultrasound energy source.
[Para 2] Heat shock proteins (HSPs) are a family of proteins that are produced
by cells in response to exposure to stressful conditions. Production of high
levels of heat shock proteins can be triggered by exposure to different kinds
of
environmental stress conditions, such as infection, inflammation, exercise,
exposure of the cell to toxins, starvation, hypoxia, or water deprivation.
[Para 3] It is known that heat shock proteins play a role in responding to a
large number of abnormal conditions in body tissues, including viral
infection,
inflammation, malignant transformations, exposure to oxidizing agents,
cytotoxins, and anoxia. Several heat shock proteins function as intra-cellular
chaperones for other proteins and members of the HSP family are expressed or
activated at low to moderate levels because of their essential role in protein
maintenance and simply monitoring the cell's proteins even under non-
CA 02972416 2017-06-27
WO 2016/122752 PCMJS2015/060893
stressful conditions. These activities are part of a cell's own repair system,
called the cellular stress response or the heat-shock response.
[Para 4] Heat shock proteins are typically named according to their molecular
weight. For example, Hsp60, Hsp70 and Hsp80 refer to the families of heat
shock proteins on the order of 60, 70 and 80 kilodaltons in size,
respectively.
They act in a number of different ways. For example, Hsp70 has peptide-
binding and ATPase domains that stabilize protein structures in unfolded and
assembly-competent states. Mitochondria! Hsp60s form ring-shaped
structures facilitating the assembly of proteins into native states. Hsp90
plays a
suppressor regulatory role by associating with cellular tyrosine kinases,
transcription factors, and glucocorticoid receptors. Hsp27 suppresses protein
aggregation.
[Para 5] Hsp70 heat shock proteins are a member of extracellular and
membrane bound heat-shock proteins which are involved in binding antigens
and presenting them to the immune system. Hsp70 has been found to inhibit
the activity of influenza A virus ribonucleoprotein and to block the
replication
of the virus. Heat shock proteins derived from tumors elicit specific
protective
immunity. Experimental and clinical observations have shown that heat shock
proteins are involved in the regulation of autoimmune arthritis, type 1
diabetes,
mellitus, arterial sclerosis, multiple sclerosis, and other autoimmune
reactions.
[Para 6] Accordingly, it is believed that it is advantageous to be able to
selectively induce the heat shock response in order to increase the number or
activity of heat shock proteins in body tissue in response to infection or
other
2
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
abnormalities. However, this must be done in a controlled manner in order not
to damage or destroy the tissue or the area of the body being treated. The
present invention fulfills these needs, and provides other related advantages.
SUMMARY OF THE INVENTION
[Para 7] The present invention is directed to a method for stimulating heat
shock protein activation in tissue without damaging the target tissue. The
method comprises the steps of providing a source of pulsed ultrasound or
electromagnetic energy. The electromagnetic energy may comprise ultraviolet
waves, microwaves, radio frequency waves or laser light at a predetermined
wavelength. The laser light may have a wavelength between 530nm to 1300
nm, a duty cycle of less than 10% and a pulse length of 500 milliseconds or
less.
[Para 8] The pulsed ultrasound or electromagnetic radiation energy is applied
to the target tissue to create a thermal time-course that stimulates cells of
the
target tissue to activate heat shock proteins without damaging the target
tissue. This includes raising the target tissue temperature to at least 10 C
transiently, while only 1 C or less over several minutes.
[Para 9] In one embodiment, a plurality of laser light spots are
simultaneously applied to the target tissue. In another embodiment, a
plurality
of ultrasound beams are focused on the target tissue.
3
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
[Para 10] A device may be inserted into a cavity of the body in order to
apply
the pulsed ultrasound or electromagnetic radiation energy to the tissue. The
device may comprise an endoscope.
[Para 11] The pulsed ultrasound or electromagnetic radiation energy may be
applied to an exterior area of a body which is adjacent to the target tissue,
or
has a blood supply close to a surface of the exterior area of the body. For
example, the body area may comprise an earlobe and the pulse electromagnetic
radiation energy is applied to the blood flowing through the earlobe.
[Para 12] Other features and advantages of the present invention will
become
apparent from the following more detailed description, taken in conjunction
with the accompanying drawings, which illustrate, by way of example, the
principles of the invention.
BRIEF DESCRIPTION OF THE DRAWINGS
[Para 1 3] The accompanying drawings illustrate the invention. In such
drawings:
[Para 14] FIGURE 1 is a diagrammatic view of a light generating unit that
produces timed series of pulses, having a light pipe extending therefrom, in
accordance with the present invention;
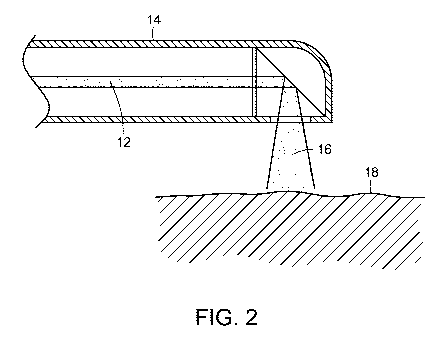
[Para 1 5] FIGURE 2 is a cross-sectional view of a photostimulation
delivery
device delivering electromagnetic energy to target tissue, in accordance with
the present invention;
4
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
[Para 16] FIGURE 3 is a diagrammatic view illustrating a system used to
generate a laser light beam, in accordance with the present invention;
[Para 17] FIGURE 4 is a diagrammatic view of optics used to generate a
laser
light geometric pattern, in accordance with the present invention;
[Para 18] FIGURE 5 is a diagrammatic view illustrating an alternate
embodiment of the system used to generate laser light beams for treating
tissue, in accordance with the present invention;
[Para 19] FIGURE 6 is a diagrammatic view illustrating yet another
embodiment of a system used to generate laser light beams to treat tissue in
accordance with the present invention;
[Para 20] FIGURE 7 is a cross-sectional and diagrammatic view of an end of
an endoscope inserted into the nasal cavity and treating tissue therein, in
accordance with the present invention;
[Para 21] FIGURE 8 is a diagrammatic and partially cross-sectioned view of
a
bronchoscope extending through the trachea and into the bronchus of a lung
and providing treatment thereto, in accordance with the present invention;
[Para 22] FIGURE 9 is a diagrammatic view of a colonoscope providing
photostimulation to an intestinal or colon area of the body, in accordance
with
the present invention;
[Para 23] FIGURE 10 is a diagrammatic view of an endoscope inserted into a
stomach and providing treatment thereto, in accordance with the present
invention;
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
[Para 24] FIGURE 11 is a partially sectioned perspective view of a capsule
endoscope, used in accordance with the present invention;
[Para 25] FIGURE 12 is a diagrammatic view of a pulsed high intensity
focused ultrasound for treating tissue internal the body, in accordance with
the
present invention;
[Para 26] FIGURE 13 is a diagrammatic view for delivering therapy to the
bloodstream of a patient, through an earlobe, in accordance with the present
invention; and
[Para 27] FIGURE 14 is a cross-sectional view of a stimulating therapy
device
of the present invention used in delivering photostimulation to the blood, via
an
earlobe, in accordance with the present invention.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
[Para 28] As shown in the accompanying drawings, and as more fully
described herein, the present invention is directed to a system and method for
delivering an energy source, such as laser, ultrasound, ultraviolet
radiofrequency, microwave radiofrequency and the like, to cause a pulsing
thermal time-course in tissue that stimulates heat shock protein activation or
production and facilitates protein repair without causing any damage.
[Para 29] The inventors of the present invention have discovered that
applying electromagnetic radiation, in the form of various wavelengths of
laser
light, to retinal tissue in a manner that does not destroy or damage the
retinal
tissue has achieved beneficial effects on eye diseases. It is believed that
this
6
may be due, at least in part, to the stimulation and activation of heat shock
proteins and the facilitation of protein repair in the retinal tissue. This is
- disclosed in United States patent application serial numbers 14/607,959
filed
January 28, 2015, 13/798,523 filed March 13, 2013, and 13/481,124 filed May
25, 2012.
[Para 301 The
inventors have found that a laser light beam can be generated
that is therapeutic, yet sublethal to retinal tissue cells and thus avoids
damaging photocoagulation in the retinal tissue which provides preventative
and protective treatment of the retinal tissue of the eye. The inventors have
discovered that generating a subthreshold, sublethal micropulse laser light
beam which has a wavelength greater than 532nm and a duty cycle of less than
10% at a predetermined intensity or power and a predetermined pulse length or
exposure time creates desirable retinal photostimulation without any visible
burn areas or tissue destruction. More particularly, a laser light beam having
a
wavelength of between 550nm-1300nm, and in a particularly preferred
embodiment 810nm, having a duty cycle of approximately 5% or less and a
predetermined intensity or power (such as between 100-590 watts per square
centimeter at the retina or approximately 1 watt per laser spot for each
treatment spot at the retina) and a predetermined pulse length or exposure
time (such as 500 milliseconds or less) creates a sublethal, "true
subthreshold"
retinal photostimulation in which all areas of the retinal pigment epithelium
exposed to the laser irradiation are preserved and available to contribute
7
CA 2972416 2018-10-01
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
therapeutically. In other words, the inventors have found that raising the
retinal tissue at least up to a therapeutic level but below a cellular or
tissue
lethal level recreates the benefit of the halo effect of the prior art methods
without destroying, burning or otherwise damaging the retinal tissue. This is
referred to herein as subthreshold diode micropulse laser treatment (SDM).
[Para 31] As SDM does not produce laser-induced retinal damage
(photocoagulation), and has no known adverse treatment effect, and has been
reported to be an effective treatment in a number of retinal disorders
(including
diabetic macular edema (DME) proliferative diabetic retinopathy (PDR), macular
edema due to branch retinal vein occlusion (BRVO), central serous
chorioretinopathy (CSR), reversal of drug tolerance, and prophylactic
treatment
of progressive degenerative retinopathies such as dry age-related macular
degeneration, Stargardts' disease, cone dystrophies, and retinitis pigmentosa.
The safety of SDM is such that it may be used transfoveally in eyes with 20/20
visual acuity to reduce the risk of visual loss due to early fovea-involving
DME.
[Para 32] A mechanism through which SDM might work is the generation or
activation of heat shock proteins (HSPs). Despite a near infinite variety of
possible cellular abnormalities, cells of all types share a common and highly
conserved mechanism of repair: heat shock proteins (HSPs). HSPs are elicited
almost immediately, in seconds to minutes, by almost any type of cell stress
or
injury. In the absence of lethal cell injury, HSPs are extremely effective at
repairing and returning the viable cell toward a more normal functional state.
Although HSPs are transient, generally peaking in hours and persisting for a
few
8
CA 02972416 2017-06-27
WO 2016/122752 PCMJS2015/060893
days, their effects may be long lasting. HSPs reduce inflammation, a common
factor in many disorders.
[Para 33] Laser treatment can induce HSP production or activation and alter
cytokine expression. The more sudden and severe the non-lethal cellular stress
(such as laser irradiation), the more rapid and robust HSP activation. Thus, a
burst of repetitive low temperature thermal spikes at a very steep rate of
change (¨ 7 C elevation with each 100ps micropulse, or 70,000 C/sec)
produced by each SDM exposure is especially effective in stimulating
activation
of HSPs, particularly compared to non-lethal exposure to subthreshold
treatment with continuous wave lasers, which can duplicate only the low
average tissue temperature rise.
[Para 34] Laser wavelengths below 550nm produce increasingly cytotoxic
photochemical effects. At 810nm, SDM produces photothermal, rather than
photochemical, cellular stress. Thus, SDM is able to affect the tissue without
damaging it. The clinical benefits of SDM are thus primarily produced by sub-
morbid photothermal cellular HSP activation. In dysfunctional cells, HSP
stimulation by SDM results in normalized cytokine expression, and
consequently improved structure and function. The therapeutic effects of this
"low-intensity" laser/tissue interaction are then amplified by "high-density"
laser application, recruiting all the dysfunctional cells in the targeted
tissue
area by densely / confluently treating a large tissue area, including all
areas of
pathology, thereby maximizing the treatment effect. These principles define
the
treatment strategy of SDM described herein.
9
CA 02972416 2017-06-27
WO 2016/122752 PCMJS2015/060893
[Para 35] Because normally functioning cells are not in need of repair, HSP
stimulation in normal cells would tend to have no notable clinical effect. The
"patho-selectivity" of near infrared laser effects, such as SDM, affecting
sick
cells but not affecting normal ones, on various cell types is consistent with
clinical observations of SDM. SDM has been reported to have a clinically broad
therapeutic range, unique among retinal laser modalities, consistent with
American National Standards Institute "Maximum Permissible Exposure"
predictions. While SDM may cause direct photothermal effects such as entropic
protein unfolding and disaggregation, SDM appears optimized for clinically
safe
and effective stimulation of HSP-mediated repair.
[Para 36] As noted above, while SDM stimulation of HSPs is non-specific
with
regard to the disease process, the result of HSP mediated repair is by its
nature
specific to the state of the dysfunction. HSPs tend to fix what is wrong,
whatever that might be. Thus, the observed effectiveness of SDM in retinal
conditions as widely disparate as BRVO, DME, PDR, CSR, age-related and
genetic retinopathies, and drug-tolerant NAMD. Conceptually, this facility can
be considered a sort of "Reset to Default" mode of SDM action. For the wide
range of disorders in which cellular function is critical, SDM normalizes
cellular
function by triggering a "reset" (to the "factory default settings") via HSP-
mediated cellular repair.
[Para 37] The inventors have found that SDM treatment of patients suffering
from age-related macular degeneration (AMD) can slow the progress or even
stop the progression of AMD. Most of the patients have seen significant
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
improvement in dynamic functional logMAR mesoptic visual acuity and
mesoptic contrast visual acuity after the SDM treatment. It is believed that
SDM
works by targeting, preserving, and "normalizing" (moving toward normal)
function of the retinal pigment epithelium (RPE).
[Para 38] SDM has also been shown to stop or reverse the manifestations of
the diabetic retinopathy disease state without treatment-associated damage or
adverse effects, despite the persistence of systemic diabetes mellitus. On
this
basis it is hypothesized that SDM might work by inducing a return to more
normal cell function and cytokine expression in diabetes-affected RPE cells,
analogous to hitting the "reset" button of an electronic device to restore the
factory default settings. Based on the above information and studies, SDM
treatment may directly affect cytokine expression via heat shock protein (HSP)
activation in the targeted tissue.
[Para 39] As heat shock proteins play a role in responding to a large
number
of abnormal conditions in body tissue other than eye tissue, it is believed
that
similar systems and methodologies can be advantageously used in treating
such abnormal conditions, infections, etc. As such, the present invention is
directed to the controlled application of ultrasound or electromagnetic
radiation
to treat abnormal conditions including inflammations, autoimmune conditions,
and cancers that are accessible by means of fiber optics of endoscopes or
surface probes as well as focused electromagnetic/sound waves. For example,
cancers on the surface of the prostate that have the largest threat of
metastasizing can be accessed by means of fiber optics in a proctoscope.
11
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
Colon tumors can be accessed by an optical fiber system, like those used in
colonoscopy.
[Para 40] As indicated above, subthreshold diode micropulse laser (SDM)
photostimulation has been effective in stimulating direct repair of slightly
misfolded proteins in eye tissue. Besides HSP activation, another way this may
occur is because the spikes in temperature caused by the micropulses in the
form of a thermal time-course allows diffusion of water inside proteins, and
this allows breakage of the peptide-peptide hydrogen bonds that prevent the
protein from returning to its native state. The diffusion of water into
proteins
results in an increase in the number of restraining hydrogen bonds by a factor
on the order of a thousand. Thus, it is believed that this process could be
applied to other diseases advantageously as well.
[Para 41] Photostimulation, in accordance with the present invention, can
be
effectively transmitted to an internal surface area or tissue of the body
utilizing
an endoscope, such as a bronchoscope, proctoscope, colonoscope or the like.
Each of these consist essentially of a flexible tube that itself contains one
or
more internal tubes. Typically, one of the internal tubes comprises a light
pipe
or multi-mode optical fiber which conducts light down the scope to illuminate
the region of interest and enable the doctor to see what is at the illuminated
end. Another internal tube could consist of wires that carry an electrical
current
to enable the doctor to cauterize the illuminated tissue. Yet another internal
tube might consist of a biopsy tool that would enable the doctor to snip off
and
hold on to any of the illuminated tissue.
12
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
[Para 42] In the present invention, one of these internal tubes is used as
an
electromagnetic radiation pipe, such as a multi-mode optical fiber, to
transmit
the SDM or other electromagnetic radiation pulses that are fed into the scope
at
the end that the doctor holds. With reference now to FIG. 1, a light
generating
unit 10, such as a laser having a desired wavelength and/or frequency is used
to generate electromagnetic radiation, such as laser light, in a controlled,
pulsed manner to be delivered through a light tube or pipe 12 to a distal end
of
the scope 14, illustrated in FIG. 2, which is inserted into the body and the
laser
light or other radiation 16 delivered to the target tissue 18 to be treated.
[Para 43] With reference now to FIG. 3, a schematic diagram is shown of a
system for generating electromagnetic energy radiation, such as laser light,
including SDM. The system, generally referred to by the reference number 20,
includes a laser console 22, such as for example the 810nm near infrared
micropulsed diode laser in the preferred embodiment. The laser generates a
laser light beam which is passed through optics, such as an optical lens or
mask, or a plurality of optical lenses and/or masks 24 as needed. The laser
projector optics 24 pass the shaped light beam to a delivery device 26, such
as
an endoscope, for projecting the laser beam light onto the target tissue of
the
patient. It will be understood that the box labeled 26 can represent both the
laser beam projector or delivery device as well as a viewing system/camera,
such as an endoscope, or comprise two different components in use. The
viewing system/camera 26 provides feedback to a display monitor 28, which
may also include the necessary computerized hardware, data input and
13
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
controls, etc. for manipulating the laser 22, the optics 24, and/or the
projection/viewing components 26.
[Para 44] With reference now to FIG. 4, in one embodiment, the laser light
beam 30 may be passed through a collimator lens 32 and then through a mask
34. In a particularly preferred embodiment, the mask 34 comprises a
diffraction grating. The mask/diffraction grating 34 produces a geometric
object, or more typically a geometric pattern of simultaneously produced
multiple laser spots or other geometric objects. This is represented by the
multiple laser light beams labeled with reference number 36. Alternatively,
the
multiple laser spots may be generated by a plurality of fiber optic
waveguides.
Either method of generating laser spots allows for the creation of a very
large
number of laser spots simultaneously over a very wide treatment field. In
fact,
a very high number of laser spots, perhaps numbering in the hundreds even
thousands or more could be simultaneously generated to cover a given area of
the target tissue, or possibly even the entirety of the target tissue. A wide
array
of simultaneously applied small separated laser spot applications may be
desirable as such avoids certain disadvantages and treatment risks known to be
associated with large laser spot applications.
[Para 45] Using optical features with a feature size on par with the
wavelength of the laser employed, for example using a diffraction grating, it
is
possible to take advantage of quantum mechanical effects which permits
simultaneous application of a very large number of laser spots for a very
large
target area. The individual spots produced by such diffraction gratings are
all
14
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
of a similar optical geometry to the input beam, with minimal power variation
for each spot. The result is a plurality of laser spots with adequate
irradiance to
produce harmless yet effective treatment application, simultaneously over a
large target area. The present invention also contemplates the use of other
geometric objects and patterns generated by other diffractive optical
elements.
[Para 46] The laser light passing through the mask 34 diffracts, producing
a
periodic pattern a distance away from the mask 34, shown by the laser beams
labeled 36 in FIG. 4. The single laser beam 30 has thus been formed into
hundreds or even thousands of individual laser beams 36 so as to create the
desired pattern of spots or other geometric objects. These laser beams 36 may
be passed through additional lenses, collimators, etc. 38 and 40 in order to
convey the laser beams and form the desired pattern. Such additional lenses,
collimators, etc. 38 and 40 can further transform and redirect the laser beams
36 as needed.
[Para 47] Arbitrary patterns can be constructed by controlling the shape,
spacing and pattern of the optical mask 34. The pattern and exposure spots
can be created and modified arbitrarily as desired according to application
requirements by experts in the field of optical engineering. Photolithographic
techniques, especially those developed in the field of semiconductor
manufacturing, can be used to create the simultaneous geometric pattern of
spots or other objects.
[Para 48] FIG. 5 illustrates diagrammatically a system which couples
multiple
light sources into the pattern-generating optical subassembly described above.
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
Specifically, this system 20' is similar to the system 20 described in FIG. 3
above. The primary differences between the alternate system 20' and the
earlier described system 20 is the inclusion of a plurality of laser consoles,
the
outputs of which are each fed into a fiber coupler 42. The fiber coupler
produces a single output that is passed into the laser projector optics 24 as
described in the earlier system. The coupling of the plurality of laser
consoles
22 into a single optical fiber is achieved with a fiber coupler 42 as is known
in
the art. Other known mechanisms for combining multiple light sources are
available and may be used to replace the fiber coupler described herein.
[Para 49] In this system 20' the multiple light sources 22 follow a similar
path as described in the earlier system 20, i.e., collimated, diffracted,
recollimated, and directed to the projector device and/or tissue. In this
alternate system 20' the diffractive element must function differently than
described earlier depending upon the wavelength of light passing through,
which results in a slightly varying pattern. The variation is linear with the
wavelength of the light source being diffracted. In general, the difference in
the
diffraction angles is small enough that the different, overlapping patterns
may
be directed along the same optical path through the projector device 26 to the
tissue for treatment.
[Para 50] Since the resulting pattern will vary slightly for each
wavelength, a
sequential offsetting to achieve complete coverage will be different for each
wavelength. This sequential offsetting can be accomplished in two modes. In
the first mode, all wavelengths of light are applied simultaneously without
16
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
identical coverage. An offsetting steering pattern to achieve complete
coverage
for one of the multiple wavelengths is used. Thus, while the light of the
selected wavelength achieves complete coverage of the tissue, the application
of the other wavelengths achieves either incomplete or overlapping coverage of
the tissue. The second mode sequentially applies each light source of a
varying
wavelength with the proper steering pattern to achieve complete coverage of
the tissue for that particular wavelength. This mode excludes the possibility
of
simultaneous treatment using multiple wavelengths, but allows the optical
method to achieve identical coverage for each wavelength. This avoids either
incomplete or overlapping coverage for any of the optical wavelengths.
[Para 51] These modes may also be mixed and matched. For example, two
wavelengths may be applied simultaneously with one wavelength achieving
complete coverage and the other achieving incomplete or overlapping coverage,
followed by a third wavelength applied sequentially and achieving complete
coverage.
[Para 52] FIG. 6 illustrates diagrammatically yet another alternate
embodiment of the inventive system 20". This system 20" is configured
generally the same as the system 20 depicted in FIG. 3. The main difference
resides in the inclusion of multiple pattern-generating subassembly channels
tuned to a specific wavelength of the light source. Multiple laser consoles 22
are arranged in parallel with each one leading directly into its own laser
projector optics 24. The laser projector optics of each channel 44a, 44b, 44c
comprise a collimator 32, mask or diffraction grating 34 and recollimators 38,
17
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
40 as described in connection with FIG. 4 above - the entire set of optics
tuned
for the specific wavelength generated by the corresponding laser console 22.
The output from each set of optics 24 is then directed to a beam splitter 46
for
combination with the other wavelengths. It is known by those skilled in the
art
that a beam splitter used in reverse can be used to combine multiple beams of
light into a single output.
[Para 53] The combined channel output from the final beam splitter 46c is
then directed through the projector device 26.
[Para 54] In this system 20" the optical elements for each channel are
tuned
to produce the exact specified pattern for that channel's wavelength.
Consequently, when all channels are combined and properly aligned a single
steering pattern may be used to achieve complete coverage of the tissue for
all
wavelengths.
[Para 55] The system 20" may use as many channels 44a, 44b, 44c, etc. and
beam splitters 46a, 46b, 46c, etc. as there are wavelengths of light being
used
in the treatment.
[Para 56] Implementation of the system 20" may take advantage of different
symmetries to reduce the number of alignment constraints. For example, the
proposed grid patterns are periodic in two dimensions and steered in two
dimensions to achieve complete coverage. As a result, if the patterns for each
channel are identical as specified, the actual pattern of each channel would
not
need to be aligned for the same steering pattern to achieve complete coverage
18
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
for all wavelengths. Each channel would only need to be aligned optically to
achieve an efficient combination.
[Para 57] In system 20", each channel begins with a light source 22, which
could be from an optical fiber as in other embodiments of the pattern-
generating subassembly. This light source 22 is directed to the optical
assembly 24 for collimation, diffraction, recollimation and directed into the
beam splitter which combines the channel with the main output.
[Para 58] It will be understood that the laser light generating systems
illustrated in FIGS. 3-6 are exemplary. Other devices and systems can be
utilized to generate a source of SDM laser light which can be operably passed
through to a projector device, typically in the form of an endoscope having a
light pipe or the like. Other forms of electromagnetic radiation may also be
generated and used, including ultraviolet waves, microwaves, other
radiofrequency waves, and laser light at predetermined wavelengths. Moreover,
ultrasound waves may also be generated and used to create a thermal time-
course temperature spike in the target tissue sufficient to activate or
produce
heat shock proteins in the cells of the target tissue without damaging the
target
tissue itself. In order to do so, typically, a pulsed source of ultrasound or
electromagnetic radiation energy is provided and applied to the target tissue
in
a manner which raises the target tissue temperature, such as at least 10 C,
transiently while only 1 C or less for the long term, such as over several
minutes, such as two or more minutes.
19
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
[Para 59] For deep tissue that is not near an internal orifice, a light
pipe is
not an effective means of delivering the pulsed energy. In that case, pulsed
low
frequency electromagnetic energy or preferably pulsed ultrasound can be used
to cause a series of temperature spikes in the target tissue.
[Para 60] Thus, in accordance with the present invention, a source of
pulsed
ultrasound or electromagnetic radiation is applied to the target tissue in
order
to stimulate HSP production or activation and to facilitate protein repair in
the
living animal tissue. In general, Electromagnetic radiation may be ultraviolet
waves, microwaves, other radiofrequency waves, laser light at predetermined
wavelengths, etc. On the other hand, if electromagnetic energy is to be used
for deep tissue targets away from natural orifices, absorption lengths
restrict
the wavelengths to those of microwaves or radiofrequency waves, depending on
the depth of the target tissue. However, as explained later, ultrasound is to
be
preferred to long wavelength electromagnetic radiation for deep tissue targets
away from natural orifices.
[Para 61] The ultrasound or electromagnetic radiation is pulsed so as to
create a thermal time-course in the tissue that stimulates HSP production or
activation and facilitates protein repair without causing damage to the cells
and
tissue being treated. The area and/or volume of the treated tissue is also
controlled and minimized so that the temperature spikes are on the order of
several degrees, e.g. approximately 10 C, while maintaining the long-term rise
in temperature to be less than the FDA mandated limit of 1 C. It has been
found that if too large of an area or volume of tissue is treated, the
increased
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
temperature of the tissue cannot be diffused sufficiently quickly enough to
meet the FDA requirements. However, limiting the area and/or volume of the
treated tissue as well as creating a pulsed source of energy accomplishes the
goals of the present invention of stimulating HSP activation or production by
heating or otherwise stressing the cells and tissue, while allowing the
treated
cells and tissues to dissipate any excess heat generated to within acceptable
limits.
[Para 62] It is believed that stimulating HSP production in accordance with
the present invention can be effectively utilized in treating a wide array of
tissue abnormalities, ailments, and even infections. For example, the viruses
that cause colds primarily affect a small port of the respiratory epithelium
in the
nasal passages and nasopharynx. Similar to the retina, the respiratory
epithelium is a thin and clear tissue. With reference to FIG. 7, a cross-
sectional
view of a human head 48 is shown with an endoscope 14 inserted into the nasal
cavity 50 and energy 16, such as laser light or the like, being directed to
tissue
18 to be treated within the nasal cavity 50. The tissue 18 to be treated could
be within the nasal cavity 50, including the nasal passages, and nasopharynx.
[Para 63] To assure absorption of the laser energy, or other energy source,
the wavelength can be adjusted to an infrared (IR) absorption peak of water,
or
an adjuvant dye can be used to serve as a photosensitizer. In such a case,
treatment would then consist of drinking, or topically applying, the adjuvant,
waiting a few minutes for the adjuvant to permeate the surface tissue, and
then
administering the laser light or other energy source 16 to the target tissue
18
21
CA 02972416 2017-06-27
WO 2016/122752 PCMJS2015/060893
for a few seconds, such as via optical fibers in an endoscope 14, as
illustrated
in FIG. 7. To provide comfort of the patient, the endoscope 14 could be
inserted after application of a topical anesthetic. If necessary, the
procedure
could be repeated periodically, such as in a day or so.
[Para 64] As discussed above, the treatment would stimulate the activation
or
production of heat shock proteins and facilitate protein repair without
damaging the cells and tissues being treated. As discussed above, certain heat
shock proteins have been found to play an important role in the immune
response as well as the well-being of the targeted cells and tissue. The
source
of energy could be monochromatic laser light, such as 810nm wavelength laser
light, administered in a manner similar to that described in the above-
referenced patent applications, but administered through an endoscope or the
like, as illustrated in FIG. 7. The adjuvant dye would be selected so as to
increase the laser light absorption. While this comprises a particularly
preferred method and embodiment of performing the invention, it will be
appreciated that other types of energy and delivery means could be used to
achieve the same objectives in accordance with the present invention.
[Para 65] With reference now to FIG. 8, a similar situation exists for the
flu
virus, where the primary target is the epithelium of the upper respiratory
tree,
in segments that have diameters greater than about 3.3mm, namely, the upper
six generations of the upper respiratory tree. A thin layer of mucous
separates
the targeted epithelial cells from the airway lumen, and it is in this layer
that
the antigen-antibody interactions occur that result in inactivation of the
virus.
22
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
[Para 66] With continuing reference to FIG. 8, the flexible light tube 12
of a
bronchoscope 14 is inserted through the individual's mouth 52 through the
throat and trachea 54 and into a bronchus 56 of the respiratory tree. There
the
laser light or other energy source 16 is administered and delivered to the
tissue
in this area of the uppermost segments to treat the tissue and area in the
same
manner described above with respect to FIG. 7. It is contemplated that a
wavelength of laser or other energy would be selected so as to match an IR
absorption peak of the water resident in the mucous to heat the tissue and
stimulate HSP activation or production and facilitate protein repair, with its
attendant benefits.
[Para 67] With reference now to FIG. 9, a colonoscope 14 could have
flexible
optical tube 12 thereof inserted into the anus and rectum 58 and into either
the
large intestine 60 or small intestine 62 so as to deliver the selected laser
light
or other energy source 16 to the area and tissue to be treated, as
illustrated.
This could be used to assist in treating colon cancer as well as other
gastrointestinal issues.
[Para 68] Typically, the procedure could be performed similar to a
colonoscopy in that the bowel would be cleared of all stool, and the patient
would lie on his/her side and the physician would insert the long, thin light
tube portion 12 of the colonoscope 14 into the rectum and move it into the
area of the colon, large intestine 60 or small intestine 64 to the area to be
treated. The physician could view through a monitor the pathway of the
inserted flexible member 12 and even view the tissue at the tip of the
23
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
colonoscope 14 within the intestine, so as to view the area to be treated.
Using
one of the other fiber optic or light tubes, the tip 64 of the scope would be
directed to the tissue to be treated and the source of laser light or other
radiation 16 would be delivered through one of the light tubes of the
colonoscope 14 to treat the area of tissue to be treated, as described above,
in
order to stimulate HSP activation or production in that tissue 18.
[Para 69] With reference now to FIG. 10, another example in which the
present invention can be advantageously used is what is frequently referred to
as "leaky gut" syndrome, a condition of the gastrointestinal (GI) tract marked
by
inflammation and other metabolic dysfunction. Since the GI tract is
susceptible
to metabolic dysfunction similar to the retina, it is anticipated that it will
respond well to the treatment of the present invention. This could be done by
means of subthreshold, diode micropulse laser (SDM) treatment, as discussed
above, or by other energy sources and means as discussed herein and known in
the art.
[Para 70] With continuing reference to FIG. 10, the flexible light tube 12
of
an endoscope or the like is inserted through the patient's mouth 52 through
the throat and trachea area 54 and into the stomach 66, where the tip or end
64 thereof is directed towards the tissue 18 to be treated, and the laser
light or
other energy source 16 is directed to the tissue 18. It will be appreciated by
those skilled in the art that a colonoscope could also be used and inserted
through the rectum 58 and into the stomach 66 or any tissue between the
stomach and the rectum.
24
CA 02972416 2017-06-27
WO 2016/122752 PCMJS2015/060893
[Para 71] If necessary, a chromophore pigment could be delivered to the GI
tissue orally to enable absorption of the radiation. If, for instance,
unfocused
810nm radiation from a laser diode or LED were to be used, the pigment would
have an absorption peak at or near 810nm. Alternatively, the wavelength of the
energy source could be adjusted to a slightly longer wavelength at an
absorption peak of water, so that no externally applied chromophore would be
required.
[Para 72] It is also contemplated by the present invention that a capsule
endoscope 68, such as that illustrated in FIG. 11, could be used to administer
the radiation and energy source in accordance with the present invention. Such
capsules are relatively small in size, such as approximately one inch in
length,
so as to be swallowed by the patient. As the capsule or pill 68 is swallowed
and
enters into the stomach and passes through the GI tract, when at the
appropriate location, the capsule or pill 68 could receive power and signals,
such as via antenna 70, so as to activate the source of energy 72, such as a
laser diode and related circuitry, with an appropriate lens 74 focusing the
generated laser light or radiation through a radiation-transparent cover 76
and
onto the tissue to be treated. It will be understood that the location of the
capsule endoscope 68 could be determined by a variety of means such as
external imaging, signal tracking, or even by means of a miniature camera with
lights through which the doctor would view images of the GI tract through
which the pill or capsule 68 was passing through at the time. The capsule or
pill 68 could be supplied with its own power source, such as by virtue of a
CA 02972416 2017-06-27
WO 2016/122752 PCMJS2015/060893
battery, or could be powered externally via an antenna, such that the laser
diode 72 or other energy generating source create the desired wavelength and
pulsed energy source to treat the tissue and area to be treated.
[Para 73] As in the treatment of the retina in previous applications, the
radiation would be pulsed to take advantage of the micropulse temperature
spikes and associated safety, and the power could be adjusted so that the
treatment would be completely harmless to the tissue. This could involve
adjusting the peak power, pulse times, and repetition rate to give spike
temperature rises on the order of 10 C, while maintaining the long term rise
in
temperature to be less than the FDA mandated limit of 1 C. If the pill form 68
of delivery is used, the device could be powered by a small rechargeable
battery
or over wireless inductive excitation or the like. The heated/stressed tissue
would stimulate activation or production of HSP and facilitate protein repair,
and the attendant benefits thereof.
[Para 74] From the foregoing examples, the technique of the present
invention is limited to the treatment of conditions at near body surfaces or
at
internal surfaces easily accessible by means of fiber optics or other optical
delivery means. The reason that the application of SDM to activate HSP
activity
is limited to near surface or optically accessibly regions of the body is that
the
absorption length of IR or visible radiation in the body is very short.
However,
there are conditions deeper within tissue or the body which could benefit from
the present invention. Thus, the present invention contemplates the use of
ultrasound and/or radio frequency (RE) and even shorter wavelength
26
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
electromagnetic (EM) radiation which have relatively long absorption lengths
in
body tissue. As will be more fully described below, the use of pulsed
ultrasound is preferable to RE electromagnetic radiation to activate remedial
HSP activity in abnormal tissue that is inaccessible to surface SDM or the
like.
Pulsed ultrasound sources can also be used for abnormalities at or near
surfaces as well.
[Para 75] With reference now to FIG. 12, with ultrasound, a specific region
deep in the body can be specifically targeted by using one or more beams that
are each focused on the target site. The pulsating heating will then be
largely
only in the targeted region where the beams are focused and overlap.
[Para 76] As illustrated in FIG. 12, an ultrasound transducer 78 or the
like
generates a plurality of ultrasound beams 80 which are coupled to the skin via
an acoustic-impedance-matching gel, and penetrate through the skin 82 and
through undamaged tissue in front of the focus of the beams 80 to a target
organ 84, such as the illustrated liver, and specifically to a target tissue
86 to
be treated where the ultrasound beams 80 are focused. As mentioned above,
the pulsating heating will then only be at the targeted, focused region 86
where
the focused beams 80 overlap. The tissue in front of and behind the focused
region 86 will not be heated or affected appreciably.
[Para 77] Examples of parameters giving a desired HSP activation Arrhenius
integral greater than 1 and damage Arrhenius integral less than 1 is a total
ultrasound power between 5.8-17 watts, a pulse duration of 0.5 seconds, an
interval between pulses of 5 seconds, with total number of pulses 10 within
the
27
CA 02972416 2017-06-27
WO 2016/122752
PCT/1JS2015/060893
total pulse stream time of 50 seconds. The target treatment volume would be
approximately lmm on a side. Larger treatment volumes could be treatable by
an ultrasound system similar to the laser diffracted optical system (described
in
paragraph 45), by applying ultrasound in multiple simultaneously applied
adjacent but separated and spaced columns. As mentioned above, the multiple
focused ultrasound beams converge on a very small treatment target within the
body, the convergence allowing for a minimal heating except at the overlapping
beams at the target. This area would be heated and stimulate the activation of
HSPs and facilitate protein repair by transient high temperature spikes.
However, given the pulsating aspect of the invention as well as the relatively
small area being treated at any given time, the treatment is in compliance
with
FDA/FCC requirements for long term (minutes) average temperature rise <1K.
An important distinction of the invention from existing therapeutic heating
treatments for pain and muscle strain is that there are no high T spikes in
existing techniques, and these are required for efficiently activating HSPs
and
facilitating protein repair to provide healing at the cellular level.
[Para 78] The
pulse train mode of energy delivery has a distinct advantage
over a single pulse or gradual mode of energy delivery, as far as the
activation
of remedial HSPs and the facilitation of protein repair is concerned. There
are
two considerations that enter into this advantage:
[Para 79] First, a
big advantage for HSP activation and protein repair in an
SDM energy delivery mode comes from producing a spike temperature of the
order of 10 C. This large rise in temperature has a big impact on the
Arrhenius
28
CA 02972416 2017-06-27
WO 2016/122752 PCMJS2015/060893
integrals that describe quantitatively the number of HSPs that are activated
and
the rate of water diffusion into the proteins that facilitates protein repair.
This
is because the temperature enters into an exponential that has a big
amplification effect.
[Para 80] It is important that the temperature rise not remain at the high
value (10+ degrees) for long, because then it would violate the FDA and FCC
requirements that over periods of minutes the average temperature rise must
be less than 1 C.
[Para 81] An SDM mode of energy delivery uniquely satisfies both of these
foregoing considerations by judicious choice of the power, pulse time, pulse
interval, and the volume of the target region to be treated. The volume of the
treatment region enters because the temperature must decay from its high
value of the order of 10 C fairly rapidly in order for the long term average
temperature rise not to exceed the long term FDA/FCC limit of 1 C.
[Para 82] For a region of linear dimension L, the time that it takes the
peak
temperature to e-fold in tissue is roughly L2/1 6D, where D = 0.00143 cm2/sec
is the typical heat diffusion coefficient. For example, if L = 1 mm, the decay
time is roughly 0.4 sec. Accordingly, for a region 1 mm on a side, a train
consisting of 10 pulses each of duration 0.5 seconds, with an interval between
pulses of 5 second can achieve the desired momentary high rise in temperature
while still not exceeding an average long term temperature rise of 1 C. This
is
demonstrated further below.
29
CA 02972416 2017-06-27
WO 2016/122752 PCMJS2015/060893
[Para 83] The limitation of heated volume is the reason why RE
electromagnetic radiation is not as good of a choice for SDM-type treatment of
regions deep with the body. The long skin depths (penetration distances) and
Ohmic heating all along the skin depth results in a large heated volume whose
thermal inertia does not allow both the attainment of a high spike temperature
that activates HSPs and facilitates protein repair, and the rapid temperature
decay that satisfies the long term FDA and FCC limit on average temperature
rise.
[Para 84] Ultrasound has already been used to therapeutically heat regions
of
the body to ease pain and muscle strain. However, the heating has not
followed the SDM-type protocol and does not have the temperature spikes that
are responsible for the excitation of HSPs.
[Para 85] Consider, then, a group of focused ultrasound beams that are
directed at a target region deep within the body. To simplify the mathematics,
suppose that the beams are replaced by a single source with a spherical
surface
shape that is focused on the center of the sphere. The absorption lengths of
ultrasound can be fairly long. Table 1 below shows typical absorption
coefficients for ultrasound at 1 MHz. The absorption coefficients are roughly
proportional to the frequency.
[Para 86] Table 1. Typical absorption coefficients for 1 MHz ultrasound in
body tissue:
Body Tissue Attenuation Coefficient at 1 MHz (cm-1)
Water 0.00046
CA 02972416 2017-06-27
WO 2016/122752
PCMJS2015/060893
Blood 0.0415
Fat 0.145
Liver 0.115-0.217
Kidney 0.23
Muscle 0.3-0.76
Bone 1.15
[Para 87] Assuming
that the geometric variation of the incoming radiation
due to the focusing dominates any variation due to attenuation, the intensity
of
the incoming ultrasound at a distance r from the focus can be written
approximately as:
= P/(47r2) [1]
where P denotes the total ultrasound power.
The temperature rise at the end of a short pulse of duration tp at r is then
dT(tp) = Pot p / (47Cvr2) [2]
where a is the absorption coefficient and Cv is the specific volume heat
capacity
This will be the case until the r is reached at which the heat diffusion
length at
tp becomes comparable to r, or the diffraction limit of the focused beam is
reached. For smaller r, the temperature rise is essentially independent of r.
As
an example, suppose the diffraction limit is reached at a radial distance that
is
smaller than that determined by heat diffusion. Then
rdif = (4Dtp)112 [3]
where D is the heat diffusion coefficient, and for r<rdif, the temperature
rise at
tp is
31
CA 02972416 2017-06-27
WO 2016/122752
PCMJS2015/060893
dT(rdif, tp) = 31)(x/(8-r[CD) when r< rdif [4]
Thus, at the end of the pulse, we can write for the temperature rise:
dTp(r) = {Patp/(4TrCv11(6/rchf2)U{rdif-r) +(1 /r2)U(r-rdif)] [5]
On applying the Green's function for the heat diffusion equation,
G(r,t) = (40Dt)-3/2 exp[-r2/(4Dt)] [6]
to this initial temperature distribution, we find that the temperature dT(t)
at the
focal point r=0 at a time t is
dT(t) = [dT0/{(1 /2)+(u1 /2/6)}1R1 /2)(tp/t)3/2 + (71/2/6)(tp/t)] [7]
with
dT0 = 313a/(87CD) [8]
[Para 88] A good approximation to eq. [7] is provided by:
dT(t) dT0 (tp/t)3/2 [9]
as can be seen in Graph 1 below.
dT(t)/dT0
1.0
0.8
0.6
0.4
0.2
........ ,
0.4 0.6 0.8 1.0
tp/t
Graph 1. Comparison of eqs. {7} and [9] for dT(t)/ dT0 at the target treatment
zone. The bottom curve is the approximate expression of eq [9].
32
CA 02972416 2017-06-27
WO 2016/122752 PCMJS2015/060893
The Arrhenius integral for a train of N pulses can now be evaluated with the
temperature rise given by eq. [9]. In this expression,
dTN(t) = dT(t-nti) [11]
where dT(t-nti) is the expression of eq. [9] with t replaced by t-ntrand with
ti
designating the interval between pulses.
[Para 89] The Arrhenius integral can be evaluated approximately by dividing
the integration interval into the portion where the temperature spikes occur
and
the portion where the temperature spike is absent. The summation over the
temperature spike contribution can be simplified by applying Laplace's end
point formula to the integral over the temperature spike. In addition, the
integral over the portion when the spikes are absent can be simplified by
noting
that the non-spike temperature rise very rapidly reaches an asymptotic value,
so that a good approximation is obtained by replacing the varying time rise by
its asymptotic value. When these approximations are made, eq. [10] becomes:
= AN[ftp(2kBT02/(3EdTo)}exp[-(E/kB)1/(T0 + dT0+ dTN(Nti))]
+exp[-(E/kB)1/(T0 + dTN(Nti))]] [12]
where
dTN(Nti) 2.5 dTo (to/ti)3/2 [13]
(The 2.5 in eq. [13] arises from the summation over n of (N-n)-3/2 and is the
magnitude of the harmonic number (N,3/2) for typical N of interest.)
[Para 90] It is interesting to compare this expression with that for SDM
applied to the retina. The first term is very similar to that from the spike
contribution in the retina case, except that the effective spike interval is
33
CA 02972416 2017-06-27
WO 2016/122752 PCMJS2015/060893
reduced by a factor of 3 for this 3D converging beam case. The second term,
involving dTN(Nti) is much smaller than in the retina case. There the
background temperature rise was comparable in magnitude to the spike
temperature rise. But here in the converging beam case, the background
temperature rise is much smaller by the ratio (tp/ti)3/2. This points up the
importance of the spike contribution to the activation or production of HSP's
and the facilitation of protein repair, as the background temperature rise
which
is similar to the rise in a continuous ultrasound heating case is
insignificant
compared to the spike contribution. At the end of the pulse train, even this
low
background temperature rise rapidly disappears by heat diffusion.
[Para 91] Graph 2 below shows the magnitude of the logarithm of the
Arrhenius integrals for damage and for HSP activation or production as a
function of dT0 for a pulse duration tp = 0.5 sec, pulse interval ti = 10 sec,
and
total number of pulses N = 10.
Log [Chamage]
0.06
0.04
0.02 1
6 8 10 12 14
0.04
0.06
Graph 2a. Damage integral
34
CA 02972416 2017-06-27
WO 2016/122752 PCMJS2015/060893
Log [nhSp]
218
217
216
215
214
213
212
6 8 10 12 14 dTo
Graph 2b. HSP activation integral
Graph 2. Logarithm of Arrhenius integrals [eq. 12] for damage and for HSP
activation as a function of the temperature rise in degrees Kelvin from a
single
pulse dTo, for a pulse duration tp = 0.5 sec., pulse interval ti = 10 sec.,
and a
total number of ultrasound pulses N = 10. Graph 2a shows the logarithm of the
damage integral with the Arrhenius constants A = 8.71x1033 sec-1 and E =
3.55x10-12 ergs. Graph 2b shows the logarithm of the HSP activation integral
with the Arrhenius constants A = 1.24x1027 5ec-1 and E = 2.66x10-12 ergs. The
graphs show that n ¨damage does not exceed 1 until dToexceeds 11.3 K, whereas
lisp is greater than 1 over the whole interval shown, the desired condition
for
cellular repair without damage.
[Para 92] Equation [8] shows that when cc = 0.1 cm-1, a dT0 of 11.5 K can
be
achieved with a total ultrasound power of 5.8 watts. This is easily
achievable.
If a is increased by a factor of 2 or 3, the resulting power is still easily
achievable. The volume of the region where the temperature rise is constant
(i.e. the volume corresponding to r=rd = (4Dtp)1/2 ) is 0.00064 cc. This
corresponds to a cube that is 0.86 mm on a side.
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
[Para 93] This simple example demonstrates that focused ultrasound should
be usable to stimulate reparative HSP's deep in the body with easily
attainable
equipment:
Total ultrasound power: 5.8 watts - 17 watts
Pulse time 0.5 sec
Pulse interval 5 sec
Total train duration (N=10) 50 sec
To expedite the treatment of larger internal volumes, a SAPRA system can be
used.
[Para 94] The present invention contemplates not only the treatment of
surface or near surface tissue, such as using the laser light or the like,
deep
tissue using, for example, focused ultrasound beams or the like, but also
treatment of blood diseases, such as sepsis. As indicated above, focused
ultrasound treatment could be used both at surface as well as deep body
tissue,
and could also be applied in this case in treating blood. However, it is also
contemplated that the SDM and similar treatment options which are typically
limited to surface or near surface treatment of epithelial cells and the like
be
used in treating blood diseases at areas where the blood is accessible through
a
relatively thin layer of tissue, such as the earlobe.
[Para 95] With reference now to FIGS. 13 and 14, treatment of blood
disorders simply requires the transmission of SDM or other electromagnetic
radiation or ultrasound pulses to the earlobe 88, where the SDM or other
radiation source of energy could pass through the earlobe tissue and into the
36
CA 02972416 2017-06-27
WO 2016/122752 PCT/1JS2015/060893
blood which passes through the earlobe. It would be appreciated that this
approach could also take place at other areas of the body where the blood flow
is relatively high and/or near the tissue surface, such as fingertips, inside
of the
mouth or throat, etc.
[Para 96] With reference now to FIGS. 13 and 14, an earlobe 88 is shown
adjacent to a clamp device 90 configured to transmit SDM radiation or the
like.
This could be, for example, by means of one or more laser diodes 92 which
would transmit the desired frequency at the desired pulse and pulse train to
the
earlobe 88. Power could be provided, for example, by means of a lamp drive
94. Alternatively, the lamp drive 94 could be the actual source of laser
light,
which would be transmitted through the appropriate optics and electronics to
the earlobe 88. The clamp device 90 would merely be used to clamp onto the
patient's earlobe and cause that the radiation be constrained to the patient's
earlobe 88. This may be by means of mirrors, reflectors, diffusers, etc. This
could be controlled by a control computer 96, which would be operated by a
keyboard 98 or the like. The system may also include a display and speakers
100, if needed, for example if the procedure were to be performed by an
operator at a distance from the patient.
[Para 97] The proposed treatment with a train of electromagnetic or
ultrasound pulses has two major advantages over earlier treatments that
incorporate a single short or sustained (long) pulse. First, the short
(preferably
subsecond) individual pulses in the train activate cellular reset mechanisms
like
HSP activation with larger reaction rate constants than those operating at
longer
37
CA 02972416 2017-06-27
WO 2016/122752
PCT/1JS2015/060893
(minute or hour) time scales. Secondly, the repeated pulses in the treatment
provide large thermal spikes (on the order of 10,000) that allow the cell's
repair
system to more rapidly surmount the activation energy barrier that separates a
dysfunctional cellular state from the desired functional state. The net result
is a
"lowered therapeutic threshold" in the sense that a lower applied average
power
and total applied energy can be used to achieve the desired treatment goal.
[Para 98] Although
several embodiments have been described in detail for
purposes of illustration, various modifications may be made without departing
from the scope and spirit of the invention. Accordingly, the invention is not
to
be limited, except as by the appended claims.
38