Note: Descriptions are shown in the official language in which they were submitted.
CA 02972864 2017-06-30
- 1
REDUCED IRON POWDER AND METHOD FOR PREPARING SAME
AND BEARING
TECHNICAL FIELD
[0001] The disclosure relates to reduced iron powder, a method for preparing
the same, and a bearing produced from the reduced iron powder.
BACKGROUND
[0002] Two main types of iron powder based on its preparation method are
typically known: reduced iron powder; and atomized iron powder. The
apparent density of iron powder currently known is 2.3 Mg/m3 or more in
reduced iron powder, and 2.5 Mg/m3 or more in atomized iron powder. The
specific surface area of iron powder is 0.10 m2/g or less in reduced iron
powder, and 0.07 m2/g or less in atomized iron powder.
[0003] Iron powder having such characteristics has many uses, and
particularly its uses in chemical reaction material, sintered machine parts,
etc.
make up a high proportion. In chemical reaction material, large specific
surface area is required for efficient reaction. In sintered machine parts,
high porosity is required as oilless bearings (which is also referred to as
"oil
retaining bearings").
[0004] The specific surface area is larger when the apparent density is lower.
Iron powder with low apparent density is needed to produce sintered machine
parts with high porosity.
[0005] As an example of sintered machine parts, an oilless bearing is
described below. It is important that the oilless bearing maintains
appropriate oil content. If the oil content is low, adequate lubricity and
durability cannot be obtained. To maintain appropriate oil content, the
sintered body needs to be increased in porosity. JP 2001-132755 A (PTL 1)
describes a relevant technique.
[0006] With reduction in size of' machine parts, oilless bearings of
approximately 2 mm in outer diameter and 0.6 mm in inner diameter have
been produced in recent years. However, the use of conventional reduced
iron powder for smaller parts causes poor formability and poor yield rate
because conventional reduced iron powder has coarse pores and iron portions,
P0154754-PCT-ZZ (1/15)
CA 02972864 2017-06-30
- 2 -
..
making production difficult. This has increased demand for iron powder that
is finer in microstructure, is more porous, and has fewer inclusions than
conventional iron powder.
[0007] If a part requires contacting with another part as in the case of a
bearing, the presence of inclusions in the part damages the other part and
shortens the life of the product. Besides, in the case where the inclusions do
not sinter with the surrounding iron powder, the inclusions cause structural
defects. This significantly decreases the yield rate or the
strength,
particularly when producing small machine parts.
[0008] The "inclusions" mentioned here has the following meaning.
Reduced iron powder is produced from iron ore or mill scale. The purity of
the reduced iron powder as the product is determined by the purity of the iron
oxide as the raw material. The most common impurity is oxygen. Oxygen
mostly appears as a thin film of surface oxide. Basic impurities include
carbon, magnesium, aluminum, silicon, phosphorus, sulfur, chromium,
manganese, nickel, and copper. Many of these impurities are present as
oxides, and are called inclusions.
[0009] For use in chemical reaction material, iron powder with large specific
surface area, i.e. low apparent density, is known to be useful as described in
JP 4667835 B2 (PTL 2) and JP 4667937 B2 (PTL 3), given that larger specific
surface area of powder contributes to more efficient reaction.
CITATION LIST
Patent Literatures
[0 0 1 Oj PTL 1: JP 2001-132755 A
PTL 2: JP 4667835 82
PTL 3: JP 4667937 B2
SUMMARY
(Technical Problem)
[0011] In the case of using conventional reduced iron powder to produce a
bearing, the shaft is damaged or the bearing develops structural defects
because the reduced iron powder contains inclusions exceeding 200 tun.
[0012] Besides, in the production of bearings, there is a possibility that the
P0154754-PCT-ZZ (2/15)
CA 02972864 2017-06-30
- 3
circulation performance of lubricating oil cannot be obtained because, with
reduction in size of bearings, pores or iron microstructure becomes large
relative to a bearing as mentioned above. In other
words, although
conventional reduced iron powder has fine pores, its many inclusions cause
the product to fail. Bearings with inner diameter of 0.6 mm and outer
diameter of 2.0 mm can be produced at a relatively high yield rate even when
conventional reduced iron powder is used. In the case of producing smaller
bearings, for example, with inner diameter of 0.4 mm and outer diameter of
1.4 mm using conventional reduced iron powder, however, formability is
insufficient and the yield rate drops significantly, making mass production
difficult.
[00131 Atomized iron powder is not suitable for use in the aforementioned
small bearings, as its smooth surface causes insufficient bonding power
between iron powder particles during forming and leads to a significantly
lower rattler value. Moreover, in the production of oilless bearings,
atomized iron powder has a major drawback of having few pores and
hindering sufficient circulation of oil. Atomized
iron powder is also
problematic in that it has few fine pores, although its inclusions are few.
[0014] From the perspective of using reduced iron powder as chemical
reaction material, the powder is required to have large specific surface area
so
that the powder has excellent reactivity per unit mass and even the particle
inside can be effectively used as reaction material.
[00151 As described above, reduced iron powder whose apparent density is
much lower than 2.0 Mg/m3 and whose specific surface area is 0.2 m3/g or
.. more, which is much higher than 0.1 m3/g, is needed in order to produce
bearings with inner diameter of less than 0.6 mm and outer diameter of less
than 2.0 mm at a high yield rate. Such reduced iron powder, however, cannot
be prepared by conventional production methods.
[0016] It could therefore be helpful to provide reduced iron powder that has
fewer coarse inclusions, has excellent formability, has high porosity after
sintering, has excellent reactivity per unit mass, and can be effectively used
as
reaction material even to the particle inside, a method for preparing the
same,
and a bearing produced from the reduced iron powder.
P0154754-PCT-ZZ (3/15)
CA2972864
-4-
(Solution to Problem)
10017) We thus provide:
I . Reduced iron powder haying an apparent density of 1.00 Mg/m3 to
1.40 Mg/m3.
100181 2. The reduced iron powder according to 1., having an amount of
oxygen of 0.38 mass% or less.
100191 1 The reduced iron powder according to 1. or 2., having a specific
surface area or 0.20 m2/g or more.
100201 4. A method for preparing reduced iron powder, for use in preparing
.. the reduced iron powder according to any one of 1. to 3., comprising:
agglomerating precursor iron oxide powder whose mean particle size
measured by a laser diffraction method is 3.0 pun or less to obtain iron oxide
powder; and thereafter reducing the iron oxide powder at 800 C to 1000 C
with hydrogen to obtain the reduced iron powder.
100211 5. The method for preparing reduced iron powder according to 4.,
comprising classifying and selecting the iron oxide powder so that its mean
particle size measured by the laser diffraction method is 50 jam to 200 p.m,
before the reduction of the iron oxide powder.
100221 6. The method for preparing reduced iron powder according to 4. or 5.,
wherein the iron oxide powder has an iron content of 68.8 mass% or more. ,
100231 7. A bearing produced from the reduced iron powder according to any
one of 1. to 3. as a raw material.
[0023A.1 The present specification discloses and claims a method for preparing
a
reduced iron powder comprising: agglomerating a precursor iron oxide powder
having a mean particle size as measured by a laser diffraction method is 3.0
1..tin or
less to obtain an agglomerated iron oxide powder; selecting the agglomerated
iron
oxide powder having the mean particle size as measured by the laser
diffraction
method is 50 pm to 200 pm; and thereafter reducing the selected iron oxide
powder
in a single reduction step at a temperature of 800 C to 1000 C for 120 min or
more
with a hydrogen gas to obtain a reduced iron powder having an apparent density
of
1.00 Mg/m3to 1.40 Mg/m3.
[002313] The present specification also discloses and claims a use of the
reduced iron
CA 2972864 2018-12-14
CA2972864
- 4a -
powder prepared by such a method as a raw material in the production of a
bearing.
(Advantageous Effect)
100241 It is thus possible to obtain reduced iron powder that has fewer coarse
inclusions, has excellent formability, has high porosity after sintering, has
excellent reactivity per unit mass, and can be effectively used as reaction
material even to the particle inside,
BRIEF DESCRIPTION OF THE DRAWINGS
P1125) in the accompanying drawings:
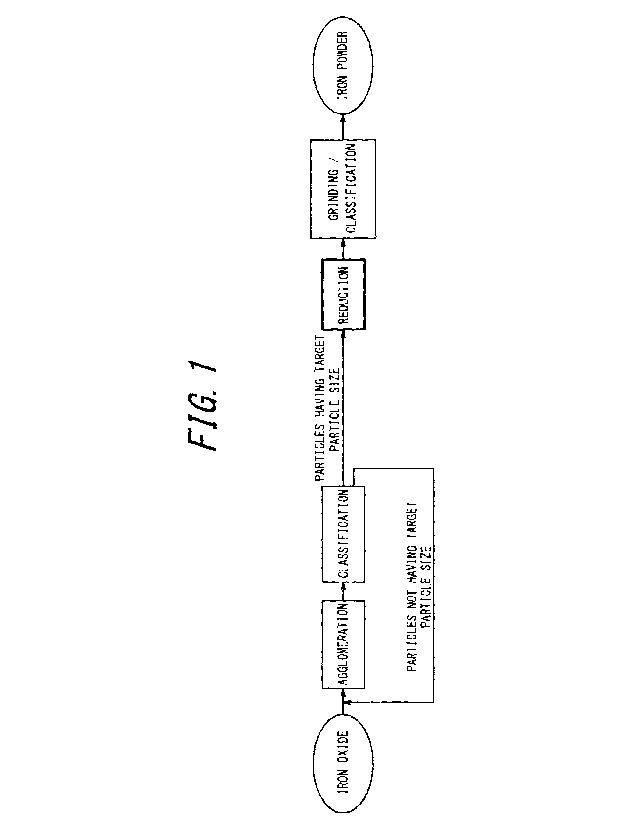
FIG. 1 is a flow diagram illustrating a process of preparing reduced
iron powder according to one of the disclosed embodiments; and
110. 2 is a diagram illustrating an appearance image and
CA 2972864 2018-12-14
CA 02972864 2017-06-30
- 5 -
cross-sectional image of each of a conventional example and Examples 1 and
2.
DETAILED DESCRIPTION
[0026] We succeeded in producing new reduced iron powder having an
apparent density of 1.00 Mg/m3 to 1.40 Mg/m3 and a specific surface area of
0.20 m 2/g or more by a new preparation method. The reduced iron powder
according to the disclosure has sufficiently low apparent density, and
therefore has excellent formability, has excellent reactivity per unit mass,
and
can be effectively used as reaction material even to the particle inside. The
reduced iron powder according to the disclosure also has fine iron
microstructure (see the white portions in the cross-sectional images in FIG.
2),
as a result of which inclusions are finely dispersed. Hence, the reduced iron
powder can be used as raw material to produce high-strength bearings at a
.. high yield rate. For example, bearings with inner diameter of 0.4 mm and
outer diameter of 1.4 mm can be mass-produced at a high yield rate.
100271 The following describes a method for preparing reduced iron powder
according to one of the disclosed embodiments, with reference to FIG. I.
First, iron oxide powder (precursor iron oxide powder) having a
.. predetermined mean particle size is agglomerated to obtain iron oxide
powder.
The obtained iron oxide powder is then classified and selected so that its
mean
particle size is set to a predetermined range. After this, the iron oxide
powder is reduced with hydrogen gas (Fe2O3 + 3H2 = 2Fe + 3H20) and crushed
as appropriate to obtain reduced iron powder (porous iron powder).
[0028] It is important to refine the precursor iron oxide powder as starting
material so that its mean particle size (D50) measured by a laser diffraction
method is 3.0 1..tm or less, in order to set the apparent density of the
reduced
iron powder to 1.40 Mg/m3 or less to thus make the inclusions in the reduced
iron powder finer. The refinement makes pores smaller, which contributes to
.. finer inclusions. The mean particle size of the precursor iron oxide powder
is preferably 2.0 pm or less. No lower limit is placed on the mean particle
size of the precursor iron oxide powder, yet in industrial terms the lower
limit
is approximately 0.5 [tm.
[0029] An example of the method for preparing the precursor iron oxide
P0154754-PCT-ZZ (5/15)
CA2972864
- 6 -
powder is a method of neutralizing and extracting waste acid after pickling
steel sheets in a steelworks. For example, a method using a spray roasting
furnace by the Ruthneriprocess and a fluidized roasting method by the LurgiTm
process are available.
100301 It is essential to agglomerate the precursor iron oxide powder to
obtain
iron. oxide powder formed by the coagulation of the precursor iron oxide
powder. Effective methods of agglomerating the precursor iron oxide
powder include a method of mixing a binder and water into the precursor iron
TM
oxide powder using a Henschel mixer and drying the mixture, and a method of
1.0 dissolving the precursor iron oxide powder in water together with a
binder to
form slurry and then drying the droplets with hot air (spray dryer). In both
methods, the binder may be PVA, starch, or the like.
(00311 When a vessel or a reducing furnace is charged with the iron oxide
powder to reduce the iron oxide powder, voids formed between coagulated
particles ensure appropriate air permeability, thus facilitating the
reduction.
To achieve this, the mean particle size of the iron oxide powder after the
agglomeration is important. Moreover, the particle site of the iron oxide
powder to be reduced correlates with the particle size of the reduced iron
powder. It is therefore preferable to classify and select the iron
oxide
powder after the agglomeration to control its mean particle size, before
reducing the iron oxide powder.
[00321 The mean particle size of the iron oxide powder after the
agglomeration is important, as mentioned above. However, not all particles
necessarily maintain their shape. For example, a plurality of particles may
bond with each other, or one particle may be broken. Accordingly, we made
careful examination, and discovered that the mean particle size of the reduced
iron powder effective in practical terms is 50 pin to 100 lam and, to achieve
this, the mean particle size of the iron oxide powder is preferably 50 )tm to
200 pm. Thus, it is preferable to appropriately classify and select the iron
oxide powder after the agglomeration so that its mean particle size is set to
50
pm to 200 pm.
100331 It is also preferable that the iron content in the iron oxide powder is
68.8 mass% or more. This sufficiently reduces the amount of oxygen in the
reduced iron powder, and further enhances the effect of improving chemical
CA 2972864 2018-12-14
CA 02972864 2017-06-30
- 7 -
reactivity and the effect of producing high-strength bearings at a high yield
rate. No upper limit is placed on the iron content in the iron oxide powder,
yet the upper limit is approximately 77 mass%.
[0034] The iron oxide powder after the agglomeration is reduced to obtain
reduced iron powder (also simply referred to as iron powder). We discovered
the conditions for preparing iron powder that has low apparent density, i.e.
approximately half that of conventional reduced iron powder or atomized iron
powder, and in which inclusions are finely dispersed, by appropriately
managing the reduction temperature in this reduction step which is hydrogen
reduction of iron oxide. It is important to set the reduction temperature
during the reduction to 800 C or more and 1000 C or less. If the reduction
temperature is less than 800 C, it is difficult to remove oxygen in the
reduced
iron powder by reduction reaction. As a result, a large amount of oxygen
remains in the iron powder. This causes insufficient chemical reactivity and
decreases formability, and leads to a lower yield rate in the production of
bearings. If the reduction temperature is more than 1000 C, the sintering of
the iron powder progresses and the apparent density exceeds 1.40 Mg/m3.
This causes insufficient chemical reactivity, and leads to a lower yield rate
in
the production of bearings.
10035] The reduction time is preferably 120 min or more, to sufficiently
reduce iron oxide powder yielded from fine precursor iron oxide powder of
3.0 um or less in mean particle size to obtain reduced iron powder of 1.00
Mg/m3 to 1.40 Mg/m3 in apparent density. No upper limit is placed on the
reduction time, yet the upper limit may be approximately 240 min in terms of
process efficiency.
[0036] The conditions other than the reduced iron powder preparation
conditions described above may be well-known reduced iron powder
preparation conditions. An example of the reduction method is a method of
heating iron powder at atmospheric pressure using a belt furnace or the like
in
a reducing atmosphere such as hydrogen.
[0037] The following describes reduced iron powder according to one of the
disclosed embodiments. The reduced iron powder has an apparent density of
1.00 Mg/m3 to 1.40 Mg/m3, and can be prepared by the preparation method
described above for the first time. If the apparent density of the reduced
iron
P0154754-PCT-ZZ (7/15)
CA 02972864 2017-06-30
8 -
powder is less than 1.00 Mg/m3, the specific surface area is excessively
large,
which increases the risk of a dust explosion, i.e. rapidly progressing
reaction
with oxygen in the air. If the apparent density of the reduced iron powder is
more than 1.40 Mg/m3, chemical reactivity is insufficient. Besides, the
strength of the green compact decreases. This facilitates failures in
subsequent steps, and leads to a lower yield rate in the production of
bearings.
[0038] When the apparent density of the reduced iron powder is in the range
of 1.00 Mg/m3 to 1.40 Mg/m3, the green strength increases, and bearings can
be produced at a high yield rate. Moreover, by limiting the apparent density
to this range, coarse inclusions are effectively reduced, and the strength
after
sintering is improved, thus contributing to higher bearing quality. Further,
the reduced iron powder has excellent reactivity per unit mass, and can be
effectively used as reaction material even to the particle inside. The
apparent density is measured according to ES-Z-2504.
[0039] The amount of oxygen in the reduced iron powder is preferably 0.38
mass% or less. This further enhances the effect of improving chemical
reactivity and the effect of producing high-strength bearings at a high yield
rate. No lower limit is placed on the amount of oxygen in the reduced iron
powder, yet the lower limit is approximately 0.10 mass%.
[0040] If the specific surface area of the reduced iron powder is less than
0.20
m2/g, iron powder particles characteristic of the disclosure are not formed
sufficiently, leading to insufficient chemical reactivity. The specific
surface
area of the reduced iron powder is therefore preferably 0.20 m2/g or more.
No upper limit is placed on the specific surface area of the iron powder, yet
the upper limit is preferably approximately 0.4 m2/g in terms of handling and
the like. The specific surface area is measured by a BET method using
nitrogen gas.
[0041] A bearing can be produced from the reduced iron powder as raw
material. The bearing has an excellent yield rate in bearing production and
excellent strength and porosity, and has high chemical reactivity, as
described
in the following examples. The method for producing the bearing from the
reduced iron powder as raw material may be a conventional method except
that the reduced iron powder is used as raw material.
P0154754-PCT-ZZ (8/15)
CA 02972864 2017-06-30
- 9 -
EXAMPLES
[0042] Table 1 compares conventional reduced iron powder (reduced iron
powder obtained through two reduction steps), conventional atomized iron
powder, and reduced iron powders (Comparative Examples 1 to 5, Examples 1
to 4) obtained through the preparation process illustrated in FIG. 1. In
Comparative Examples 1 to 5 and Examples 1 to 4, hydrogen was used as
reducing gas. The conventional reduced iron powder was prepared as
follows: Using iron ore or mill scale as raw material, coke powder was added
and primary reduction using a tunnel furnace was performed without an
agglomeration step and a classification step in FIG. 1, and then reduction in
the thick-line box was performed.
[0043] The iron powder evaluation items listed in Table 1 were evaluated as
to llows.
The mean particle size of precursor iron oxide powder was measured
by a volume-based laser diffraction method.
The iron content in iron oxide powder was measured according to
JIS-M-8212.
The mean particle size of iron oxide powder after agglomeration was
measured by a laser diffraction method, and set as 50% particle size.
[0044] The apparent density of reduced iron powder was measured according
to JIS-Z-2504.
The mean particle size of reduced iron powder was measured by a
volume-based laser diffraction method, and set as 50% particle size.
The specific surface area of reduced iron powder was measured by a
BET method using nitrogen gas.
The amount of oxygen in reduced iron powder was measured by an
inert gas fusion infrared absorption method (GFA).
[0045] The yield rate in bearing production was evaluated as pass when the
failure rate from the green compacting in the shape of a cylinder with an
inner
diameter of 0.4 mm, an outer diameter of l .4 mm, and a height of 2 mm to 2.5
mm to the completion of sintering was 5% or less (a yield rate of 95% or
more). The strength was evaluated as pass when the strength upon
compressing the cylinder in a lying state was 17 N/mm2 or more, and fail
when the strength was less than 17 N/mm2.
P0154754-PCT-ZZ (9/15)
CA 02972864 2017-06-30
- 10 -
[0046] The porosity is a factor determining the performance of an oilless
bearing, and its appropriate value is 18% to 22%.
The porosity was measured by mercury porosimetry.
[0047] The reaction rate in chemical reaction was evaluated based on the
reaction in which sulfur content in soil adsorbed to iron (Fe + S = FeS).
Adsorptivity by this reaction is required to be a predetermined level or more
in practical terms. Accordingly, in Table 1, chemical reactivity is set as an
index represented by a ratio to 1 as the minimum required level.
[0048] FIG. 2 illustrates an appearance image and cross-sectional image of
the reduced iron powder of each of Examples 1 and 2, in comparison with the
conventional reduced iron powder. The appearance image was taken using a
scanning electron microscope (S EM), and the cross-sectional image was taken
using an optical microscope. Many pores were contained inside particles in
Examples 1 and 2, as compared with the conventional reduced iron powder.
P0154754-PCT-ZZ (10/15)
Table 1
c,
CD
416
Y:P
Preparation conditions for reduced iron powder Characteristics of
reduced iron powder Characteristics in bearing production
Chemical
Mean particle size Iron content Mean particle Mean
Specific reactivity Remarks
Reduction Reduction Apparent Amount of
of precursor iron in iron oxide size of iron oxide , particle surface
oxygen Yield rate
Strength Pomsity (-)
time temperature density
oxide powder powder powder (A))
(1V/) 0/(9
(min) ( C) (Km- =') size area (mass%)
(m) (mass%) (Pn) (tint) (m /g)
2.2 to 55 to 0.07 to Conventional
- - - - <0.40 84 15 18
0.7
2.7 105 0.10 reduced iron powder
2.5 to 50 to 0.04 to Conventional
- - - - - <0.30 62 9 14
0.5
3.1 90 0.08 atomized
iron powder g
2.8 68.8 120 240 1050 1.48 100 0.20 0.2/ 92
18 , 20 0.8 Comparative Example 1 0
s,
'
0
2.8 68.8 120 240 780 0.98 80 0.31 0.40 92
19 11 0.9 Comparative Example 2 ..,
s,
0
3.2 68.8 150 240 850 0.95 90 0.28 0.45 92
19 20 , 0.9 Comparative Example 3
2.8 68.8 45 240 850 1.49 60 0.22 0.21 88
16 16 0.8 Comparative Example 4
.
....3
1
0
2.8 68.8 220 240 850 0.95 150 0.33 0.55 92
19 26 0.8 Comparative Example 5 0
,
,..
2.8 68.8 50 240 1000 1.38 80 0.22 0.25 96
21 22 1.4 Example I 0
2.8 68.8 120 240 , 1000 1.32 80 0.25 0.27
98 25 21 1.3 Example 2
c-F 2.8 68.8 120 240 800 1.03 60 0.28 0,36 98
24 20 1.3 Example 3
z 2.8 68.2 110 240 850 1.12 80 0.25 0.43 95
17 19 1.2 Example 4
P
-ci 0.7 69.0 90 240 850 1.05 75 0.30 0.37 97
23 22 1.3 Example 5
0
-
tn
4=,
---.]
C.11
'CI
n
'7
N
N
--,
_
-
--,
-.
.,...,
CA 02972864 2017-06-30
- 12 -
[0050] Comparative Example 1 is iron powder obtained by reducing iron
oxide powder at 1050 C. Its apparent density was 1.48 Mg/m3, which is
outside the range according to the disclosure. While the degree of reduction
was relatively favorable, the yield rate in bearing production was evaluated
as
fail. The chemical reactivity was also evaluated as fail.
[0051] Comparative Example 2 is iron powder obtained by reducing iron
oxide powder at 780 C. Its apparent density was 0.98 Mg/m3, which is
outside the range according to the disclosure. The yield rate in bearing
production and the chemical reactivity were evaluated as fail.
10052] Comparative Example 3 is iron powder obtained using precursor iron
oxide powder of 3.2 um in mean particle size and by reducing iron oxide
powder after agglomeration at 850 C. Its apparent density was 0.95 Mg/m3,
which is outside the range according to the disclosure. The degree of
reduction was relatively low. The yield rate in bearing production was poor,
and the chemical reactivity was evaluated as fail.
[0053] Comparative Example 4 is reduced iron powder obtained using iron
oxide powder after agglomeration of 45 um in mean particle size. Its
apparent density was 1.49 Mg/m3, which is outside the range according to the
disclosure. The degree of reduction was high, and the strength of the bearing
was evaluated as pass. Meanwhile, the yield rate in bearing production was
evaluated as fail. The chemical reactivity was also evaluated as fail.
[0054] Comparative Example 5 is reduced iron powder obtained using iron
oxide powder after agglomeration of 220 i.tm in mean particle size. Its
apparent density was 0.95 Mg/m3, which is outside the range according to the
disclosure. The strength of the bearing was evaluated as pass, but the yield
rate in bearing production was evaluated as fail. The porosity was excessive,
and the chemical reactivity was evaluated as fail.
[0055] Example 1 is iron powder obtained using iron oxide powder after
agglomeration of 50 pm in mean particle size and by reducing the iron oxide
powder after agglomeration at 1000 C. Its apparent density was 1.38 Mg/m3.
The degree of reduction was high, and the yield rate in bearing production and
the strength and porosity of the bearing were all evaluated as pass. The
chemical reactivity was also favorable.
[0056[ Example 2 is iron powder obtained using iron oxide powder after
Ref. No. P0154754-PCT-ZZ (12/15)
CA 02972864 2017-06-30
- 13 -
agglomeration of 120 [tm in mean particle size and by reducing the iron oxide
powder after agglomeration at 1000 C. Its apparent density was 1.32 Mg/m3.
The degree of reduction was favorable, and the yield rate in bearing
production and the strength and porosity of the bearing were all evaluated as
pass. The chemical reactivity was also favorable.
[00571 Example 3 is iron powder obtained using iron oxide powder after
agglomeration of 120 urn in mean particle size and by reducing the iron oxide
powder after agglomeration at 800 C. Its apparent density was 1.03 Mg/m3.
The degree of reduction was favorable, and the yield rate in bearing
production and the strength and porosity of the bearing were all evaluated as
pass. The chemical reactivity was also favorable.
[0058] Example 4 is iron powder obtained using iron oxide powder after
agglomeration having an iron content of 68.2 mass%. Its apparent density
was 1.12 Mg/m3, while the amount of oxygen in the reduced iron powder was
0.43 mass%. The chemical reactivity was favorable, and the yield rate in
bearing production and the strength and porosity of the bearing were all
evaluated as pass.
100591 Example 5 is iron powder obtained using precursor iron oxide powder
of 0.7 um in mean particle size, with the mean particle size of iron oxide
powder being 90 gm. Its apparent density was 1.05 Mg/m3. The chemical
reactivity was favorable, and the yield rate in bearing production and the
strength and porosity of the bearing were all evaluated as pass.
Ref. No. P0154754-PCT-ZZ (13/15)