Note: Descriptions are shown in the official language in which they were submitted.
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
1
METHOD FOR INDIVIDUALIZED DRUG THERAPY
BACKGROUND OF THE INVENTION
[0001] The metabolism of a particular drug, and as a result the blood
concentration and
the duration of action achieved by that drug, can vary significantly in a
general population.
(See Chun-Yu et al.: Pharmacogenomics of adverse drug reactions: Implementing
personalized medicine, Human Molecular Genetics, 2012, 21, Review Issue 1, B58-
B65). In
the care of a patient suffering from a particular disease, a drug's efficacy
against disases is a
fundamental issue. However, side effects or "adverse drug reactions" ("ADRs")
caused by a
drug can profoundly impact the patient, thus requiring alterations in the
treatment plan. (See
Lazarou et al.: Incidence of adverse drug reactions in hospitalized patients:
a meta-analysis of
prospective studies. JAMA 1998, 279(15):1200-5). ADRs account for ¨7% of all
hospitalizations and consistently rank as one of the most common causes of
inpatient death in
western countries. (See Pirmohamed & Park: Adverse drug reactions: back to the
future. Br.
J. Clin. Pharmacol., 2003, 55, 486-492; Wester et al.: Incidence of fatal
adverse drug
reactions: a population based study, Br. J. Clin. Pharmacol. 2007, 65:4, 573-
579).
[0002] To guard against ADRs, administering the lowest dose of a drug to a
patient that
achieves the greatest efficacy of the drug is of paramount importance because
75%-80% of
all ADRs are dose-related (i.e., the patient experiences a side effect because
they are taking
too high of a dose of a particular medication). (See Routlege et al.: Adverse
drug reactions in
elderly patients, Br J Clin Pharmacol 2004, 57:2 121-126; Lazarou et al.:
Incidence of
adverse drug reactions in hospitalized patients: a meta-analysis of
prospective studies. JAMA
1998,279 (15):1200-5.; Melmon, KL, Morrelli, HF, Hoffman, BB, Nierenberg, DW.
Melmon
and Morrelli's Clinical Pharmacology: Basic Principles in Therapeutics. (3rd
Edition), New
York: McGraw-Hill, Inc., 1993). Despite the risks from ADRs, finding the
lowest effective
dose is often not addressed by conventional prescribing regimens.
Additionally, the
variability in individual responses to a drug significantly complicates
finding the the lowest
effective dose. The experiences ofprior patients may not be relevant to
aparticular individual
patient's regimen. Prescribers may be detered by a complexity of finding the
lowest effective
dose, and as a result, because many patients are maintained on an effective
dose rather than
the lowest effective dose, inadequate patient responses to the drug and/or
responses with
significant ADRs may occur.
[0003] Although differences in age, gender, and size contribute to the
heterogeneity in
drug metabolism, patients that are the same age, gender, and size can
experience markedly
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
2
different responses to the same drug dosage. Other factors which can influence
the likelihood
of ADRs include, without limitation, the administration of multiple drugs,
disease state, past
history of ADRs, allergic reactions, and genetic factors effecting the
absorption, distribution,
chemical alteration, and excretion of the drug. The ADRs from the class of
drugs known as
"non-steroidal anti-inflammatory drugs" ("NSAIDS") are well documented. (See,
e.g.,
Dieppe et al.: Balancing benefits and harms: the example of non-steroidal anti-
inflammatory
drugs, BMJ 2004, 329, 31-34; McGettigan & Henry: Cardiovascular Risk with Non-
Steroidal
Anti-Inflammatory Drugs: Systematic Review of Population-Based Controlled
Observational
Studies, PLoS Med 2011, 8(9): e1001098. doi:10.1371/joumal.pmed.1001098;
Aagaard &
Hansen: Information about ADRs explored by pharmacovigilance approaches: a
qualitative
review of studies on antibiotics, SSRIs and NSAIDs, BMC Clinical Pharmacology
2009, 9:4;
Siileyman: Anti-inflammatory and side effects of cyclooxygenase inhibitors,
Pharm. Reports
2007, 59, 247-58). Further, although newer NSAIDS have somewhat reduced the
risk for
gastrointestinal bleeding, ulceration, and perforation, they still present
risks to patients such
as kidney failure, hepatic dysfunction, and cardiovascular events (e.g.,
stroke, hypertension,
congestive heart failure) (See Celebrex package insert; see also Bing, et
al.:
Cyclooxygenase-2 inhibitors: is there an association with coronary or renal
events?, Current
Atherosclerosis Reports 2003;5:114-7.)
[00041 The danger of NSAID ADRs is particularly important in the elderly
(i.e., age >65)
where drug metabolism is quite heterogeneous. (See Singh et al:
Gastrointestinal Drug
Interactions Affecting the Elderly, Clin. Geriatr. Med 2014, 30:1-15). As
individuals age,
they begin to experience diminished organ function, suffer from various
diseases, and often
take drugs that can interact resulting in an increased susceptibility to
environmental and
physical stressors (e.g., medications). As a result, seniors are generally
more susceptible to
the harmful side effects of NSAIDs, and yet generally receive the same dosing
regimens as
larger, younger individuals. (See McMillan & Hubbard: Frailty in older
inpatients: what
physicians need to know, Q. J. Med. 2012; 105:1059-1065.; Smucker & Kontak:
Adverse
drug reactions causing hospital admission in an elderly population: experience
with a
decision algorithm, Journal of the American Board of Family Practice 1990,
3(2):105-9;
Montamat et al.: Management of drug therapy in the elderly, New England
Journal of
Medicine 1989, 321(5):303-9; and Recchia & Shear: Organization And Function Of
An
Adverse Drug Reaction Clinic. Journal Of Clinical Psychiatry 1994, 34:68-79).
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
3
[0005] Celecoxib (sold under the brand name "Celebrex6") is a NSAID that
has been
approved for the treatment of arthritis for over 15 years. In vitro assays
demonstrate that
celecoxib is a potent inhibitor of prostaglandin synthesis with most of its
activity resulting
from its inhibition of COX-2. (See Saleyman et al.: Anti-inflammatory and side
effects of
cyclooxygenase inhibitors, Phann. Reports 2007, 59, 247-58). Like other
NSAIDs, celecoxib
puts patients, in particular elderly patients, at risk for a number of serious
ADRs. For
example, the 1999 Celebrex0 package insert warns that "the incidence of
adverse
experiences tended to be higher in elderly patients" (Celebrex0 Package
Insert. Searle &
Co., 1999). Similarly, the 2003 Celebrex0 package insert states that "there
have been more
spontaneous post-marketing reports of fatal gastrointestinal events and acute
renal failure"
regarding its use of in the elderly. The 2013 package insert indicates that
"Celebrex0 should
be used with caution in [elderly] patients." The Celebrex0 package insert
advises that for
osteoarthritis and rheumatoid arthritis, the lowest effective dose of
Celebrexe should be
sought for each patient.
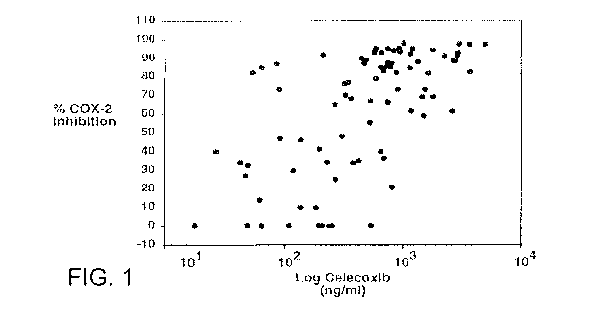
[0006] Even the cyclooxgenase inhibitary activity of celecoxib appears to
be variable
(See McAdam et al.: Systemic biosynthesis of prostacyclin by cyclooxygenase
(COX)-2: the
human pharmacology of a selective inhibitor of COX-2. PNAS. 1999; 96:272-7.)
FIG. 1 is a
scatterplot graph from McAdams et al. displaying the relationship between LPS-
stimulated
plasma PGE2 ex vivo, an index of COX-2 activity, and log plasma concentrations
of
celecoxib at 2, 4, 6, and 24 hours after dosing. PGE2 is expressed as a
percentage of
predosing values. A variable dose-response is evident. (P , 0.01 vs. placebo).
[0007] A study by Bensen, et al. indicates that higher doses of celecoxib
(100 mg and 200
mg twice a day) are similarly efficacious, while patients receiving the 100 mg
dose of
celecoxib also reported fewer side effects than those taking a 200 mg dose.
(Table 1).
(Bensen, et al. :Treatment of Osteoarthritis With Celecoxib, a Cyclooxygenase-
2 Inhibitor: A
Randomized Controlled Trial, Mayo Clinic Proceedings 1999, 74(11):1095-1105)
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
4
Table 1. Effect of Treatment on Signs and Symptoms of Osteoarthritis at 12
Weeks
Assessment Placebo 50 mg BID 100 mg BID 200 mg BID Naproxen
(n=198)
(n=203) (n=203) (n=197) (n=202)
Patient global 24 271: 351: 361: 291:
assessment
condition improved
(%)t
Patient global 9 <1 1 <1 2
assessment
condition
worsened (%)t
Physician's global 21 301: 361: 32* 331:
assessment
condition improved
(%)t
Physician's global 4 2 2 <1 1
assessment
condition
worsened (%)t
Mean +/- SEM -6.1 +/- -9.5 +/- 1.111: -13.3 +/-
1.171:/I -12.0 +/- -11.9 +/- 1.291:
WOMAC
1.09 1.221:
Osteoarthritis Index
composite score
Mean +/- SEM -2.0 +/- -3.3 +/- 0.321: -3.8 +/- 0.2944t -3.4
+/- 0.271: -3.1 +/- 0.304
Osteoarthritis
0.29
Severity Index
Withdrawal due to 39 30 201:// 241: 261:
treatment failure
(%)
*BID= twice a day; WOMAC = Western Ontario and McMaster Universities
Scale ranged from 1 (very good) to 5 (very poor). Patient improvement was
defined as a
reduction of at least 2 grades from baseline for grades 3 through 5 or a
change in grade from
2 ti. Patient worsening was defined as an increase of at least 2 grades from
baseline for
grades 1 through 3, or a change in grade from 4 to 5. KEY: t = P.05 vs
placebo; = Scale
ranged from 0 to 96 with negative change indicating improvement // = 13 .05 vs
celecoxib, 50
mg BID; 11= Scale ranged from 0 to 24 with negative change indicating
improvement; # =
P_..05 vs naproxen
10008] Despite these acknowledgements, the Celebrex0 dosage prescribed for
the
treatment of osteoarthritis, is standardized (e.g., 50, 100, 200 or 400 mg
dosage forms)
regardless of the gender and age of the patient receiving celecoxib.
Furthermore, studies
have shown that, on average, elderly patients receiving 100 mg celecoxib doses
over a six
week period reported almost identical reductions in pain as compared to
patients receiving
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
200 mg celecoxib doses over a six week period. The subjects receiving the 100
mg dose of
celecoxib also reported fewer side effects for the duration of the study. The
results indicate
that patients may experience the same celecoxib efficacy at lower, safer
doses. Accordingly,
there is a need for improved methods of using NSAIDs in the therapy of
arthritic pain.
[0009] The present invention addresses the the wide variability in the
responses
individuals have to the same dosage of a drug by setting forth methods for
individualizing
drug therapy. The methods of the present invention individualize dosages a
patient takes,
thus maximizing the efficacy of one or more drugs at the lowest doses possible
and at the
point of care. The present invention can increase drug compliance, reduce
costs, and most
importantly improve the quality and safety of patient lives.
BRIEF SUMMARY OF THE INVENTION
[0010] The invention provides methods for individualized therapy of
arthritic pain using
a non-steroidal anti-inflammatory drug (NSAID). The high patient to patient
variability in
response to a dose of any NSAID makes the mere clinical monitoring of patients
an
inadequate way to treat patients with this class of drugs. Even the
measurement of "blood
levels' (i.e., the ocasional measurement of the drug's concentration in the
blood) is unlikely
to lead to effective nontoxic regimens. Given the complexity of NSAIDs' dose
response
relationships, a more compressive set of metrics must employed in each
patient. The
methods claimed herein take advantage of a pharmacokinetic ("PK") analysis for
each
patient. As such, the claimed methods go beyond the measurement of a single
blood level at
a single time point. Instead, the claimed methods make use of data from
several time points
and take advantage of the full scope of PK parameters. There is no known
method of
predicting individual PK for celecoxib due to the complexity of human
pharmacokinetics. As
such there is no natural law known that can explain human pharmacokinetics; or
if there are,
the multitude of potential determining factors make defining such law
impractical.
Therefore, the claimed method seeks to determined the individual PK directly.
[0011] The inventive methods disclosed herein comprise: administering to a
first patient
suffering from arthritic pain a first NSAID formulation; determining the
NSAID's
concentration in the first patient's blood at a plurality of time points after
the first NSAID
formulation was administered to the first patient; transforming the first
patient's NSAID
concentration/time data points in to one or more PK parameters; comparing the
first patient's
values for said PK parameters to a predetermined ranges of values for each PK
parameter and
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
6
if one or more of the first patient's PK parameters fall outside of a
predetermined range,
designing a new NSAID formulation, wherein the dose of said NSAID can be
different from
that of the first NSAID formulation. As such, the types and amounts of
excipients can also
differ from those of the first NSAID formulation, or both. The new NSAID
formulation is
then administered to the first patient and the steps used to determine the PK
parameters are
repeated until all the PK parameters used are within said predetermined
ranges, and if pain
control is adequate and toxicity is tolerable, maintaining the first patient
on the NSAID
formulation at frequency of administration that satisfied the comparison
within predetermined
ranges.
[0012] Related methods for individualizing therapy of arthritic pain are
disclosed herein.
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] FIG. 1 is a scatterplot graph displaying the relationship between
LPS-stimulated
plasma PGE2 ex vivo, an index of COX-2 activity, and log plasma concentrations
of
celecoxib 2, 4, 6, and 24 hours after dosing. PGE2 is expressed as a
percentage of predosing
values. A steep but variable dose-response is evident. (P , 0.01 vs. placebo)
(from McAdam
et al. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: the
human
pharmacology of a selective inhibitor of COX-2. PNAS. 1999; 96:272-7.)
[0014] FIG. 2 depicts the pharmacokinetic parameters produced by different
doses of
celecoxib.
[0015] FIG. 3 displays the result of a meta-analysis of the one dose AUC
from patients in
different age groups.
DETAILED DESCRIPTION OF THE INVENTION
[0016] As used herein the phrase "individualized therapy" refers to a
specific treatment
regimen for a patient comprising the administration of one or more drugs,
which is the result
of analyzing pharmacokinetic and/or pharmacodynamic parameters of the subject
to
maximize drug efficacy at about the lowest dosage of the drug(s) possible.
[0017] As used herein "Arthritic pain" refers to any pain arising
anatomically from the
joints and their adjacent bones and non-osseous tissues. Any arthritic pain
can be treated by
the invention including, without limitation, any pain resulting from an auto-
immune,
infectious, inflammatory, proliferative, regenerative or degenerative process
so involving the
joints of an animal or human patient. As such, suitable pain treatable with
the current
invention includes pain from rheumatoid or osteo arthritis.
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
7
100181 As used herein, "formulation" or "a formulation" refers to a
combination of active
ingredients and excipients wherein each is present in a dosage form at fixed
ratios to one
another (i.e., fixed percentages of each ingrediant in the dosage form.)
[0019] As used herein, "first NSAID formulation" is the NSAID formulation
that is
administered to the patient to begin the process by which PK parameters are
determined and
"new NSAID fotinulation" is the formulation designed based on the PK
parameters produced
by the first NSAID formulation. To evaluate the PK parameters produced by the
new NSAID
formulation becomes the first NSAID formulation and the process is repeated.
The new
NSAID formulation may be a second formulation.
[0020] As used herein, the phrases "second formulation" and "second
formulation under
a second drug regimen" refer to the dosage of one or more drugs an individual
receives after
performing certain steps of the claimed invention (e.g., after comparing an
patient's
pharmacokinetic parameters to a predetermined range of values). The second
formulation
under a second regimen, can be, e.g., the same dosage of a drug administered
in the first
regimen, a lower dose, or a higher dose. In addition, there can be changes in
any non-NSAID
components of any combination therapy.
[0021] As used herein the phrases "first formulation under a first regimen"
and "first
formulation" refer to the dosage of one or more drugs an individual initially
receives prior to
performing one or more steps of the claimed invention. The first formulation
under a first
regimen, can be, e.g., the standard 100 mg or 200 mg dosages of celecoxib
prescribed to
patients over 60 kg twice daily for osteoarthritis.
[0022] As used herein, the phrase "NSAID concentration/time data points"
refers to the
amount of NSAID in a unit of volume (e.g., 1 ml of blood from a subject) at a
given point in
time before or after administration of the NSAID.
[0023] As used herein, the phrase "transforming the patient's NSAID
concentration/time
data points" refers to the application of mathematical operations, formulas,
theories, and/or
principles (e.g., a formula for calculating half life or a formula for
calculating AUC), to the
NSAID concentrations/time data points of an individual to derive PK
parameters.
[0024] As used herein, the phrase "predetermined range of values" refers to
a known
range of values (e.g., pharmacokinetic parameters) associated with desirable
drug efficacy at
lower risk doses of a drug (e.g., the Cmax range corresponding to patients
experiencing high
drug efficacy at a low dose). The predetermined range of values may be derived
from a
statistical analysis of a population receiving variable and/or identical doses
of one or more
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
8
drugs. The predetermined range of values can readily be compared to a
patient's values (e.g.,
pharmacokinetic values), associated with one or more drugs. In particular, the
predetermined
ranges are determined (i.e. derived) from other patients for whom there was
improved pain
levels without significant side effects.
[0025] As used herein, "Significant adverse drug reactions" refer to ADRs
that the patient
finds intolerable, impair physiologic functions, and/or put the patient at
risk for immobility
and/or death or combinations thereof
[0026] As used herein "Significant side effects" refer to side effects that
the patient finds
intolerable, impair physiologic functions, and/or put the patient at risk for
immobility and/or
death or combinations thereof
[0027] As used herein, "designing" refers to changes in the active agent's
dose,
formulation and/or regiment based on the patient data, using logic and the
experience of one
of ordinary skill in the art.
[0028] As used herein, "determining the level of efficacy" refers to the
use of objective
(e.g., pharmacokinetic) and subjective tests (e.g., pharmacodynamic), signs
and symptoms to
characterize, quantify or evaluate how well symptoms (e.g., pain) are
controlled by the
administration of the active ingredient (e.g., celecoxib).
[0029] As used herein, "determining the level of toxicity" refers to the
use of objective
and subjective tests, signs and symptoms to characterize, quantify or evaluate
the significance
of any side effects produced by the administration of the active ingredient.
[0030] As used herein, "pain control is adequate" refers to a level of pain
the patient is
willing to live with and does not significantly impair the patient's
functioning in society or
the patient's physiologic functions.
[0031] As used herein, "toxicity is acceptable" refers to the absence of
significant side
effects and a level of toxicity that the patient is willing to live with and
does not significantly
impair the patient's functioning in society or the patient's physiologic
functions.
[0032] As used herein, a "COX-1 inhibitor" refers to a non-steroidal anti-
inflammatory
drug that is capable of directly targeting the COX-1 enzyme in a subject and
inhibits at least
some COX-1 activity, e.g., aspirin.
[0033] As used herein, a "COX-2 inhibitor" refers to a non-steroidal anti-
inflammatory
drug that is capable of directly targeting the COX-2 enzyme in a subject and
inhibits at least
some COX-1 activity, e.g., celecoxib. As used herein, a "mixed COX-1 and COX-2
inhibitor" refers to a non-steroidal anti-inflammatory drug that is capable of
directly targeting
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
9
both the COX-1 and COX-2 enzymes in a subject and inhibits at least some COX-1
and
COX-2 activity, e.g., ibuprofen.
100341 As used here a mixed COX-2 inhibitor can also include a COX-2
inhibitor such as
celecoxib combined with one or more other therapeutic drugs for chronic
diseases such as
arthritis, diabetes, hypertension, hypercholesterolemia, and dementia.
Suitable therapeutic
drugs may include, but are not limited to: aspirin, diclofenac (Cataflam,
Voltaren), diflusnisal
(Dolobid), etodolac (Lodine), fenoprofen (Nalfon), flubiprofen (Ansaid),
ibuprofen (Motrin,
Advil, Nuprin), indomethacin (Indocin), ketoprofen (Orudis), ketorolac
(Toradol),
meclofenamate, nabumetone (Relafen), naproxen (Naprosyn), oxaprozin (Daypro),
phenylbutazone, piroxicam (Feldene), salicylate, sulindac (Clinoril), tolmetin
(Tolectin),
pregbalin, neurontin, beclomethasone, betamethasone, cortisone, dexamethasone,
hydrocortisone, methylprednisolone, prednisolone, prednisone, triamcinolone,
sulfonylureas,
acetohexamide (Dymelor), chlorpropamide (Diabinese), glimepiride (Amaryl),
glipizide
(Glucotrol), glyburide (Micronase, DiaBeta), tolazamide (Tolinase),
tolbutamide (Orinase),
Biguanides, metformin (Glucophage), Alpha-glucosidase Inhibitors, acarbose
(Precose),
miglitol (Glyset), Thiazolidinedione Derivatives, pioglitazone (Actos),
rosiglitazone
(Avandia), troglitazone (Rezulin), enalapril, lisinopril perindopril,
losartan, valsartan,
diltiazem, nifedipine, amlodipine, diuretics, amiloride, frusemide,
indapamide, atenolol,
metoprolol, propanolol, alpha-blockers, doxazosin, prazosin, methyldopa,
vasodilators, and
hydralazine.
[0035] As used here the combination can also include a "fixed dose
combination" (FDC)
or simply dosing with multiple pills each of a single agent to achieve a
desired effect.
[0036] The invention provides methods for individualized therapy of
arthritic pain using
a non-steroidal anti-inflammatory drug (NSAID), preferably celecoxib. In
addition, the
invention provides methods for predicting the outcome of the therapy arthritic
pain with a
composition comprising NSAID, preferably celecoxib. Further, the invention
provides
methods of using a NSAID, including aspirin, preferably celecoxib, in the
manufacture of
medicament for the treatment of arthritic pain.
[0037] The invention provides endpoints (i.e., an individualized drug
therapy) based on
the achievement of predetermined PK results, as well as the clinical condition
of the patient.
[0038] This disclosure provides processes in accordance with invention
comprising:
administering a first formulation under a first regimen comprising a NSAID,
preferably
celecoxib, at a first dose to a patient suffering from arthritic pain; at a
pre-specified time or
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
times after administration of the first dose of the NSAID, measuring the
NSAID' s
concentration in the patient's blood (e.g., by a point of care device, or by
other suitable
techniques known in the art) at a plurality of time points after administering
the first
formulation resulting in a set of NSAID concentration/time data points;
transforming the
patient's NSAID concentration/time data points in to one or more
pharmacokinetic (PK)
parameters; comparing the patient's PK parameters to a predetermined ranges of
values for
such PK parameters and if one or more of the PK parameters fall outside of the
predetermined range, providing a second formulation under a second regimen
wherein the
second formulation has a different dose of NSAID, preferably celecoxib, from
the first
formulation, or different regimen. These steps will be repeated until the PK
parameters are
within a second predetermined range.
[0039] The invention further provides methods for individualized drug
therapy of arthritic
pain with a NSAID, preferably celecoxib, comprising: administering a NSAID at
a first dose
under a first regimen to a patient suffering from arthritic pain; determining
the concentration
of the NSAID at a plurality of pre-specified time points after administration
of the first dose
of the NSAID; using the concentration/time data, deriving one or more
pharmacokinetic (PK)
parameters exhibited by the NSAID when administered to said patient;
determining the level
of efficacy and toxicity produced by the first dose and regiment; using the
level of efficacy
and toxicity produced by said first dose to determine a new dose of the NSAID;
administering the new dose of the NSAID to the patient; and repeating the
forgoing steps
until pain control is adequate, toxicity is acceptable, and PK parameters are
stable. The
patient will thereafter be maintained at the final dose (i.e., an
individualized drug therapy)
that produced the results. However, if the patient has recurrent pain or
toxicity again, one
measures the PK parameters and adjust dose to provide the PK results
determined by the
original trials of the NSAID.
[0040] Any suitable NSAID can be used in accordance with the invention,
including
without limitation, a COX- 1-specific inhibitor, a COX-2-specific inhibitor, a
mixed COX-1
and 2 inhibitor or a combination thereof As such, the NSAID can be a
salicylate, propionic
acid derivative, acetic acid derivative, enolic acid (oxicam) derivative,
anthranilic acid
derivative (fenamatea ) or combinations thereof Accordingly, the NSAID can be,
aspirin
(acetylsalicylic acid), ibuprofen, naproxen, indomethacin, sulindac,
piroxicam, clonixin,
preferably celecoxib or a combination thereof In addition, the invention can
be used with
combinations of NSAIDs and other analgesic drugs such as lidocaine, opiates,
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
11
acetaminophen, tricylic antidepressants, anticonvulsants, carbamazepine,
gabapentin, and
pregabalin; other anti-inflammatory drugs such as steroids and
immunosuppressants. Further,
the invention can be used with combinations of NSAIDs and other therapies for
arthritis,
including but not limited to, methotrexate and gold-salts.
[0041] The NSAID can be administered in accordance with the invention via
any suitable
route including, without limiting, orally, rectally, by inhalation, trans-
cutaneously, by
injection, intra-venously or intra-arterially. The non-NSAID component of any
combination
therapy can be administered in accordance with the invention by any suitable
route including,
without limiting, orally, rectally, by inhalation, trans-cutaneously, by
injection, intra-
venously or intra-arterially. Any suitable regimen can be used in accordance
with the
invention to administer two or more drug components, including without
limitation,
simultaneously (within minutes of one another), substantially simultaneously
(within an hour
of one another) or at different times.
[0042] Other treatments for chronic diseases can be included such as
treatments for
diabetes, cardiovascular diseases, dementia, cholesterol, and hypertension.
For example, a
method of individualizing an NSAID drug therapy (e.g., a celecoxib therapy),
may be
practiced in conjunction with the administration of a prescribed cholesterol
regulator, such as
atorvastatin.
[0043] Any suitable PK parameter or parameters can be used in accordance
with the
invention, including without limiting concentration, concentration time
course, peak
concentration, and time after administration to peak concentration, terminal
half-life, AUC,
bioavailability, absorption, distribution, metabolism, excretion,
biotransformation, or a
combination thereof
[0044] Any suitable pharmacodynamic parameter or parameters can be used in
accordance with the invention, including without limiting the physiological
changes of cells,
tissues and ligaments of a patient, patient or physician reported pain level,
the frequency of
side effects, or a combination thereof
[0045] Any suitable method for the assessment of pain known to those of
ordinary skill in
the art can be used in accordnce with the invention, including, but not
limited to, one-
dimensional pain intensity scales, Wisconsin Brief Pain Questionnaire, Brief
Pain Inventory,
The McGill Pain Questionnaire and the short-form, McGill Pain Questionnaire
(See Breivik
et al.: Assessment of pain, British Journal of Anaesthesia 2008, 101 (1): 17-
24).
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
12
[0046] The following examples further illustrate the invention but, of
course, should not
be construed as in any way limiting its scope.
EXAMPLE 1
[0047] The approved prescribing information for Celebrex0 as listed on its
package
insert for US/EU/ROW instructs that a physician should use lowest effective
dose for the
shortest duration consistent with treatment goals for the individual patient.
For four of the six
approved indications the package insert includes a 100 mg BID regimen:
[0048] 1) Osteoarthritis (OA): 200 mg QD or 100 mg BID
[0049] 2) Rheumatoid Arthritis (RA): 100 mg BID or 200 mg BID
[0050] 3) Juvenile Rheumatoid Arthritis (JRA): 50 mg BID in patients 10-25
kg. 100 mg
BID in patients more than 25 kg
[0051] 4) Ankylosing Spondylitis (AS): 200 mg once daily single dose or 100
mg BID.
[0052] 5) Acute Pain (AP) and 5) Primary Dysmenorrhea (PD). 400 mg
initially,
followed by 200 mg dose if needed on first day. On subsequent days, 200 mg BID
as needed
[0053] Unexpectedly, however, the inventor's analysis of the actual
prescribing behavior
using Evaluate Pharma/IMS database deteremined that the 200 mg is the
predominant dose
being prescribed by physicians by more than 10 to 1. These data are consistent
with data
from a MEPS survey (Table 2) and Medicaid survey (Table 3). In view of
predominance of
the 200 mg dosage form sales and the evidence that the 100 mg and 200 mg doses
produce
overlapping PK and pharmacodynamic results, it is questionable that the
package insert's
admonition that "the lowest dose of Celebrex0 should be sought for each
patient" is
followed. Instead, the data indicates that it is likely that there are
numerous patients a risk for
ADRs because their celecoxib dose is higher than it needs to be (i.e., e.g.,
200 mg BID rather
than 100 mg BID).
Table 2. MEPS Survey Data
Proprietary Package description Strength USA sales
USA sales
Name (mg) 2008 ($m)
2011 ($m)
10ELEBREX 100 capsule in bottle (0025-1525- 200 1,553 1,650
31)
2CELEBREX 500 capsule in 1 bottle (0025- 200 323 132
1525-51)
3CELEBREX 100 capsule in 1 bottle (0025- 100 65 142
1520-31)
CA 02973569 2017-07-11
WO 2016/114761
PCT/US2015/011148
13
5CELEBREX 500 capsule in 1 bottle (0025- 100 17
1520-51)
lOCELEBREX 100 blister pack in 1 carton (0025- 100 7
1520-34) > 1 capsule in 1 blister
pack
CELEBREX 120 capsule in 1 bottle (63629-3021- 200 - 2
5)
CELEBREX 30 capsule in 1 bottle, plastic (67544- 200 32
204-30)
TOTAL 1,989 1,982
Table 3. Prescribing information derived from Medicaid
Name Dose Number Number Number Number Number Number Number
(mg) of RXs of RXs of RXs of RXs of RXs of RXs of RXs
2008 2009 2010 2011 2012 2013 2014
CELEBREX 50 293 485 856 1169 1167 1396 726
CELEBREX 100 33,436 36,023 43,755 47,524 35,399 34,178 16,628
CELEBREX 200 320,628 330,521 380,546 384,404 285,764 250,985 114,532
CELEBREX 400 1,383 1,636 3,116 3,476 2,565 2,240
1,093
TOTAL 355,740 368,665 428,273 436,573 324,895 288,799 132,979
EXAMPLE 2
[0054] The
combined plots of published pharmacokinetic data including those from the
Summary basis for approval are shown in FIG. 2. The variability of Celebrex0
pharmacokinetics were unexpectedly high. The PK results for the 200 mg dose
shows a
substantial overlap with that of the 100 mg dose. Accordingly, the dose
proportionality may
not be as is described by the package insert for Celebrex0. As a result of the
failure to
determine and pursue target PK ranges, in some instances patients receiving
100 mg patients
may not get enough of the drug and the 200 mg patients may receive too much of
the drug.
EXAMPLE 3
[0055]
Applicant's meta analysis of the reported PK parameters in different
populations
demonstrates that the elderly show a higher variability than younger patients.
For Example,
when the applicant's meta analysis is presented in age-based subgroups, the
elderly and
younger patients demonstrate highly significant differences in the variability
of AUC (FIG.
3). In other words, the most efficacious celecoxib dosage is highly
individualized among the
CA 02973569 2017-07-11
WO 2016/114761
PCT/US2015/011148
14
elderly. Unexpectedly, elderly here is defined as patients greater than >40 or
>50, not the
usually definition of elderly (age greater >65). Previously, there has been
reported impaired
PK with elderly and the package insert issued warning on impaired PK in
elderly but did not
suggest dose reduction. Our finding suggests that the issue is more
substantial and more
widespread and includes middle aged groups also.
[0056] That the variability of PK results within groups and the Cm ax and
AUC overlap
between the 100 mg and 200 mg groups indicates that correctly dosing elderly
patients to
maximize celecoxib efficacy at the lowest doses possible depends on many
individualized,
unpredictable variables. Baased on the wide range of AUC values, some
instances patients
receiving 100 mg patients may not get enough of the drug and the 200 mg
patients may
receive too much of the drug (FIG. 2).
[0057] All references, including publications, patent applications, and
patents, cited
herein are hereby incorporated by reference to the same extent as if each
reference were
individually and specifically indicated to be incorporated by reference and
were set forth in
its entirety herein.
[0058] The use of the terms "a" and "an" and "the" and "at least one" and
similar
referents in the context of describing the invention (especially in the
context of the
following claims) are to be construed to cover both the singular and the
plural, unless
otherwise indicated herein or clearly contradicted by context. The use of the
term "at least
one" followed by a list of one or more items (for example, "at least one of A
and B") is to
be construed to mean one item selected from the listed items (A or B) or any
combination
of two or more of the listed items (A and B), unless otherwise indicated
herein or clearly
contradicted by context. The terms "comprising," "having," "including," and
"containing"
are to be construed as open-ended terms (i.e., meaning "including, but not
limited to,")
unless otherwise noted. Recitation of ranges of values herein are merely
intended to serve
as a shorthand method of referring individually to each separate value falling
within the
range, unless otherwise indicated herein, and each separate value is
incorporated into the
specification as if it were individually recited herein. All methods described
herein can be
performed in any suitable order unless otherwise indicated herein or otherwise
clearly
contradicted by context. The use of any and all examples, or exemplary
language (e.g.,
"such as") provided herein, is intended merely to better illuminate the
invention and does
CA 02973569 2017-07-11
WO 2016/114761 PCT/US2015/011148
not pose a limitation on the scope of the invention unless otherwise claimed.
No language
in the specification should be construed as indicating any non-claimed element
as essential
to the practice of the invention.
[0059] Preferred embodiments of this invention are described herein,
including the best
mode known to the inventors for carrying out the invention. Variations of
those preferred
embodiments may become apparent to those of ordinary skill in the art upon
reading the
foregoing description. The inventors expect skilled artisans to employ such
variations as
appropriate, and the inventors intend for the invention to be practiced
otherwise than as
specifically described herein. Accordingly, this invention includes all
modifications and
equivalents of the subject matter recited in the claims appended hereto as
permitted by
applicable law. Moreover, any combination of the above-described elements in
all possible
variations thereof is encompassed by the invention unless otherwise indicated
herein or
otherwise clearly contradicted by context.