Note: Descriptions are shown in the official language in which they were submitted.
CA 02976009 2017-08-04
WO 2016/137805
PCT/US2016/018392
ANTIMICROBIAL TREATMENT SOLUTIONS
TECHNOLOGY
[0001] The present disclosure is directed to antimicrobial treatments of food
products and,
more particularly, to antimicrobial treatments and treatment solutions
comprising quaternary
ammonium compounds and associated methods of reducing quaternary ammonium
compound residues following such treatments.
BACKGROUND
[0002] Food products are often treated with antimicrobial solutions to kill or
control
microbial populations that may otherwise deleteriously affect the food
products. For
example, food products may be covered in microbial populations that impact the
safety or the
quality of the food products. While such antimicrobial solutions play an
essential role in food
safety by reducing or eliminating microbial populations, antimicrobial
solutions may also
include various levels of toxicity if excessive levels are consumed. Thus,
controlling
microbial populations on food products with antimicrobial solutions may
require achieving a
delicate balance between the desired control of the microbial populations and
the residual
presence of the antimicrobial components used to control the microbial levels.
SUMMARY
[0003] In one aspect, an aqueous antimicrobial solution for treatment of food
products
comprises a quaternary ammonium compound and an acidifying agent. The
acidifying agent
may be present in an amount effective to provide the solution with a pH value
between about
2.0 and about 1.0, between about 1.5 to about 1.2, or between about 1.5 to
about 1.3. The
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
acidifying agent may comprise a wide range of acids, including but not limited
to acids that
are generally recognized as safe (GRAS). In one embodiment, the acidifying
agent may
comprise one or more acids selected from generally GRAS acids. In one
embodiment, the
acidifying agent may comprise at least one of citric acid and hydrochloric
acid. The
quaternary ammonium compound may be present in a wide range of concentrations.
In one
embodiment, the quaternary ammonium compound may be present in an amount of
about
0.2% to about 0.5% by weight, about 0.3% to about 0.4% by weight, or about
0.3% to about
0.35% by weight. The quaternary ammonium compound may be an alkylpyridinium
salt. In
one embodiment, the alkylpyridinium salt may be cetylpyridinium chloride.
[0004] The solution may further comprise a solubility agent. In one
embodiment, the
solubility agent is selected from propylene glycol and glycerin. The solution
may be formed
from dilution of a concentrated antimicrobial solution comprising the
quaternary ammonium
compound and the solubility agent. The concentrated antimicrobial solution may
have a pH
value less than 7Ø
[0005] In one embodiment, the quaternary ammonium compound may be present in a
concentration between one of 0.2% and 0.5% by weight, 0.3% and 0.4% by weight,
and 0.3%
and 0.35 A) by weight and the acidifying agent may be present in an amount
effective to
provide the solution with a pH value such that treatment of a food product
with the solution
results in an average residue of the quaternary ammonium compound below about
35 ppm.
In a further embodiment, the concentrations of the quaternary ammonium
compound and
acidifying agent are selected such that treatment of the food product with the
solution results
in an average residue of the quaternary ammonium compound below about 31.5
ppm. In yet
a further embodiment, the concentration of the quaternary ammonium compound
and
acidifying agent are selected such that treatment of the food product with the
solution results
2
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
in an average residue of the quaternary ammonium compound below about 7 ppm.
The
treatment may comprise spraying the solution onto the food product or
submerging the food
product in the solution for between about 15 seconds and about 3 seconds,
between about 12
seconds and between about 4 seconds, or about 10 seconds and about 5 seconds.
[0006] In another aspect, a concentrated antimicrobial solution comprises a
quaternary
ammonium compound and a solubility agent, wherein the concentrated
antimicrobial solution
has a pH value below 7Ø In some such embodiments, the pH value of the
concentrated
antimicrobial solution may be below about 6.5, below about 6.0, or below about
5.5.
[0007] The quaternary ammonium compound may be present in an amount about 40%
by
weight. The quaternary ammonium compound may be an alkylpyridinium salt. In
one
embodiment, the alkylpyridinium salt is cetylpyridinium chloride.
[0008] The solubility agent may be selected from one of an alcohol and
glycerin. In one
embodiment, the solubility agent is propylene glycol. The propylene glycol may
be present
in a wide range of values. In one embodiment, the propylene glycol may be
present in an
amount between about 55% and about 60% by weight of the concentrated
antimicrobial
solution.
[0009] In yet another aspect, a method of preparing an antimicrobial treatment
solution
comprises adding an acidifying agent to a solution comprising a quaternary
ammonium
compound. The acidifying agent may be added in an amount effective to provide
the solution
with a pH value between one of about 2.0 and about 1.0, about 1.5 and about
1.2, and about
1.5 and about 1.3. In one embodiment, the acidifying agent comprises one or
more acids
generally recognized as safe (GRAS) acids. The acidifying agent may comprise
at least one
of citric acid and hydrochloric acid.
3
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
[0010] The quaternary ammonium compound may be present in the solution in an
amount
between about 0.2% and about 0.5% by weight, between about 0.3% and about 0.4%
by
weight, or between about 0.3% to about 0.35% by weight. In one embodiment, the
quaternary ammonium compound may be an alkylpyridinium salt. In a further
embodiment,
the alkylpyridinium salt may be cetylpyridinium chloride. The concentrated
quaternary
ammonium compound may be provided in a concentrated antimicrobial solution
comprising
the quaternary ammonium compound and a solubility agent. The solubility agent
may be
selected from an alcohol, such as propylene glycol, and glycerin.
[0011] In one embodiment, the concentration of the quaternary ammonium
compound in the
solution is between one of about 0.2% and about 0.5% by weight, about 0.3% and
about 0.4%
by weight, and about 0.3% and about 0.35% by weight, and the method further
comprises
adding the acidifying agent in an amount effective to provide the solution
with a pH value
such that treatment of a food product with the solution results in an average
residue of the
quaternary ammonium compound below about 35 ppm. The treatment may comprise
spraying the solution onto the food product or submerging the food product in
the solution for
between about 15 seconds and about 3 seconds, between about 12 seconds and
about 4
seconds, or between about 10 seconds and about 5 seconds. In a further
embodiment, the
concentrations of the quaternary ammonium compound and acidifying agent are
selected
such that treatment of a food product with the solution results in an average
residue of the
quaternary ammonium compound below about 31.5 ppm. In yet a further
embodiment, the
concentrations of the quaternary ammonium compound and the acidifying agent
are selected
such that treatment of a food product with the solution results in an average
residue of the
quaternary ammonium compound below about 7 ppm.
4
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
[0012] In still yet another aspect, a method of making a concentrated
antimicrobial solution
comprises formulating a concentrated antimicrobial solution comprising a
quaternary
ammonium compound, a solubility agent, and an acidifying agent in a solvent,
wherein the
acidifying agent may be added to the concentrated solution in an amount
sufficient to provide
a pH value of the concentrated antimicrobial solution below 7Ø In some
embodiments, the
pH value of the concentrated antimicrobial solution may be below about 6.5,
below about 6.0,
or below about 5.5.
[0013] In one embodiment, the quaternary ammonium compound may be an
alkylpyridinium salt. In a further
embodiment, the alkylpyridinium salt may be
cetylpyridinium chloride. The cetylpyridinium chloride may be present in an
amount of
about 40% by weight. The solubility agent may be selected from an alcohol and
glycerin. In
one embodiment, the solubility agent is propylene glycol. The propylene glycol
may be
present in a wide range of values. In one embodiment, the propylene glycol may
be present
in an amount between about 55% and about 60% by weight of the concentrated
antimicrobial
solution. In one embodiment, the solvent is water.
[0014] In still yet another aspect, a method of treating a food product
comprises applying an
antimicrobial solution to a food product. The antimicrobial solution may
comprise a
quaternary ammonium compound and an acidifying agent. The quaternary ammonium
compound may be present in an amount between one of about 0.2% and about 0.5%
by
weight, about 0.3% and about 0.4% by weight, and about 0.3% and about 0.35% by
weight.
The solution may have a pH value between one of about 2.0 and about 1.0, about
1.5 and
about 1.2, and about 1.5 and 1.3. Applying the antimicrobial solution may
comprise spraying
the solution onto the food product or submerging the food product in the
solution.
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
[0015] In one embodiment, the solution may be applied to the food product for
an
application time between about 15 seconds and about 3 seconds. In one
embodiment, the
food product comprises a poultry food product selected from one of a carcass,
breast, tender,
thigh, giblet, and wing.
[0016] In still yet another aspect, a method of formulating an antimicrobial
treatment
solution comprises selecting a solution concentration of a quaternary ammonium
compound.
The quaternary ammonium compound may be cetylpyridinium chloride present in an
amount
of about 0.2% to about 0.5% by weight, about 0.3% to about 0.4% by weight, and
about 0.3%
to about 0.35% by weight. The method may further comprise selecting a solution
pH value
between one of about 2.0 and about 1.0, about 1.5 and about 1.2, and about 1.5
and about 1.3.
The treatment may comprise application of the solution to the food product by
one of
spraying the solution onto the food product and submerging the food product in
the solution
for an application time between one of about 15 seconds to about 3 seconds,
about 12
seconds to about 4 seconds, and about 10 seconds to about 5 seconds.
[0017] The method may further comprise selecting an acidifying agent to adjust
the pH of
the solution to obtain the selected solution pH value. The selected
quaternary, ammonium
compound may be combined with a solubility agent prior to adjusting the pH
with the
selected acidifying agent to obtain the selected solution pH value. Any number
of different
acidifying agents may be used, but the acidifying agent may preferably be
selected from one
or more acids comprising generally recognized as safe (GRAS) acids. In one
embodiment,
the selected acidifying agent comprises at least one of citric acid and
hydrochloric acid. The
method may further comprise selecting a solubility agent selected from an
alcohol and
glycerin. In one embodiment, the method includes selecting a solubility agent
comprising
6
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
propylene glycol. The selected quaternary ammonium compound may be provided in
a
concentrated antimicrobial solution comprising the selected solubility agent.
BRIEF DESCRIPTION OF THE DRAWINGS
[0018] FIG. 1 schematically illustrates an overview of the experimental
protocol of
Example 1;
[0019] FIG. 2 graphically illustrates residue data obtained in Example 1;
[0020] FIG. 3 schematically illustrates an overview of the experimental
protocol of
Example 2; and
[0021] FIG. 4 graphically illustrates residue data obtained in Example 2.
[0022] FIG. 5 schematically illustrates an overview of the experimental
protocol of
Example 3; and
[0023] FIG. 6 graphically illustrates microbial data obtained in Example 3.
DESCRIPTION
[0024] Various embodiments of antimicrobial treatment solutions comprising
quaternary
ammonium compounds are described herein. The particular quaternary ammonium
compound is preferably suitable for use in preventing the growth of a broad
spectrum of
microorganisms on and in food products by application of the quaternary
ammonium
compound or treatment solution comprising the quaternary ammonium compound to
such
food products. In various embodiments, the food products treated may include
poultry, beef,
7
pork, lamb, venison, and other edible meat products, such as seafood, fish and
shellfish, as
well as fruits, vegetables, and dairy products.
[0025] Treatment Solutions
[0026] In various embodiments, an aqueous antimicrobial solution for treatment
of a food
product comprises a quaternary ammonium compound and an acidifying agent. The
quaternary ammonium compound may be present in the solution in an amount
preferably
about 0.2% to about 0.5% by weight, more preferably about 0.3% to about 0.4%
by weight,
and even more preferably about 0.3% to about 0.35% by weight. The quaternary
ammonium
compound may be present in a concentrated solution formulated for dilution to
prepare the
antimicrobial treatment solution. Accordingly, in some embodiments,
concentrated solutions
of antimicrobial compositions comprising a concentrated quaternary ammonium
compound
are also described herein.
[0027] The quaternary ammonium compound may be any quaternary ammonium
compound
known in the art for use in antimicrobial treatment of food products. For
example, various
suitable quaternary ammonium compounds are described in U.S. Patent
Application No.
6,039,992, issued March 21, 2000, to Compadre et al. for METHOD FOR THE BROAD
SPECTRUM PREVENTION AND REMOVAL OF MICROBIAL CONTAMINATION OF
FOOD PRODUCTS BY QUATERNARY AMMONIUM COMPOUNDS. In various
embodiments, the quaternary ammonium compound may be selected from the group
consisting of alkylpyridinium, tetra-alkylammonium and alkylalicyclic ammonium
salts. The
quaternary ammonium compound is preferably an alkylpyridinium salt. In various
embodiments, the alkylpyridinium salt is cetylpyridinium chloride. The
quaternary
ammonium compound may be selected from the group consisting of
alkylpyridinium, tetra-
alkylammonium and
8
CA 2976009 2019-11-26
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
alkylalicyclic ammonium salts. The quaternary ammonium compound concentrations
are
described by concentrations as either parts per million (ppm) or % by weight,
where 100,000
ppm is equal to 10% by weight. The examples utilize cetylpyridinium chloride
and use both
ppm or % to designate concentration.
[0028] Any wide range of different acidifying agents may be used, but the
acidifying agent
preferably comprises one or more acids generally recognized as safe (GRAS) for
food uses
related to human consumption as defined or recognized by the United States
Food and Drug
Administration or the United States Department of Agriculture, including but
not limited to
those identified in 21 CFR Parts 182, 184, and 186. For example, the
acidifying agent may
comprise one or more of acidic acid, citric acid, and hydrochloric acid. The
acidifying agent
is preferably present in an amount to provide the antimicrobial treatment
solution with an
acidic pH resulting in reduced quaternary ammonium compound residue on the
food product
treated with the solution compared to treatment of the food product with the
antimicrobial
treatment solution having a neutral pH value. The acidifying agent may be
present in an
amount effective to provide the solution with a pH value preferably between
about 2.0 and
about 1.0, more preferably between about 1.5 to about 1.2, or even more
preferably between
about 1.5 to about 1.3.
[0029] In one embodiment, the antimicrobial treatment solution further
comprises at least
one solubility agent to maintain the quaternary ammonium compound in solution.
The
solubility agent may be any compatible solubility agent that solubilizes
quaternary
ammonium compounds in solution at concentrations of greater than preferably
about 0.2% to
about 0.5% by weight, more preferably about 0.3% to about 0.4% by weight, and
even more
preferably about 0.3% to about 0.35% by weight of the solution. However, the
solubility
agent may also include a solubility agent from a concentrated solution
suitable to solubilized
9
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
quaternary.' ammonium compound or maintain the quaternary ammonium compound in
solvent at about 10% by weight is contemplated. The solvent may generally
comprise water
and the antimicrobial treatment solutions described herein may further
generally be aqueous
solutions. The solubility agent may comprise alcohols, which may include
polyglycols such
as polyethylene glycol. A suitable alcohol may be selected from the group
consisting of a
monohydric alcohol, a dihydric alcohol, a trihydric alcohol, and a combination
thereof In
various embodiments, any one of these types of alcohols may be used alone or
in
combination with one or more of the other types of alcohols to obtain the
desired % by
weight of the solubility agent. The choice of the alcohol may depend upon the
food product
treated and may be selected to be compatible with treatment steps prior to or
after the
quaternary ammonium compound treatment with the food product.
[0030] Concentrated Solutions
[0031] In various embodiments, the antimicrobial treatment solution may be
formed from
dilution of a concentrated antimicrobial solution. In various embodiments, the
concentrated
antimicrobial solution may be formulated to have an acidic pH value below
about 6.5, about
6.0, or about 5.5 or otherwise suitable for dilution and acidification with an
acidifying agent
to form a antimicrobial treatment solution having a pH value preferably
between 2.0 and 1.0,
more preferable between 1.5 and 1.2, even more preferably between 1.5 and 1.3.
When using
a composition in an industrial process, for example, it may be preferable to
work with only
small volumes of liquid concentrates rather than large volumes of liquid
solutions. Suitable
formulations of concentrated solutions of quaternary- ammonium compound, for
example,
may include concentrations up to 1000-fold greater than those made in water
alone. In one
embodiment, the concentrated antimicrobial solution comprises at least one
solubility agent
to provide a soluble concentrate for easy dilution to the final concentration
for use in large
scale industrial processing. The solubility agent functions to maintain the
solubility of the
quaternary ammonium compound so that it does not precipitate out of solution.
[0032] The concentrated amount of quaternary ammonium compound may be in
combination with at least one solubility agent or solvent that is suitable for
use in methods of
preventing the growth of a broad range of microorganisms on and in food
products, as well as
on surfaces that come in contact with food products in the home or in an
industrial
environment. The concentrated antimicrobial solution may comprise a
concentrated amount
of the antimicrobial quaternary ammonium compound that utilizes GRAS
(generally
recognized as safe) components to form a true solution, not an emulsion, of
the quaternary
ammonium compound. The concentrated antimicrobial solution may be formulated
to have
an acidic pH value below about 6.5, about 6.0, or about 5.5 or otherwise
suitable for dilution
and acidified by an acidifying agent to an acidic pH value preferably between
2.0 and 1.0,
more preferable between 1.5 and 1.2, even more preferably between 1.5 and 1.3.
The
formulations may contain solubility components which allow more concentrated
compositions of quaternary ammonium compounds to be prepared. The concentrated
antimicrobial solution may be a concentrated quaternary ammonium compound
solution as
described in U.S. Patent 6,864,269, issued March 8, 2005, to Compadre et al.
for
CONCENTRATED, NON-FOAMING SOLUTION OF QUATERNARY AMMONIUM
COMPOUNDS AND METHODS OF USE. Such concentrated antimicrobial solutions may
be acidified or diluted and acidified to formulate antimicrobial solutions for
treatment of food
products as described above. For example, in various embodiments, the dilute
antimicrobial
treatment solution is formed from a dilution of such concentrated
antimicrobial solutions
comprising the quaternary ammonium compound and the solubility agent, wherein
the
concentrated antimicrobial solution has been modified to have a pH value less
than 7Ø The
pH value of
11
CA 2976009 2019-11-26
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
the concentrated antimicrobial solution for example may be below about 6.5,
below about
6.0, or below about 5.5. Acidification of the concentrated solution may be by
the same or
different acidifying agents used to further acidify the dilute antimicrobial
treatment solution
to a pH value preferably between 2.0 and 1.0, more preferable between 1.5 and
1.2, even
more preferably between 1.5 and 1.3.
[0033] One or more solubility agents may be used. Solubility agents may
include alcohols
such as polygylcols including polyethylene glycol for example. In one
embodiment, the
alcohol is selected from the group consisting of a monohydric alcohol, a
dihydric alcohol, a
trihydric alcohol, and a combination thereof Any one of these types of
alcohols can be used
alone or in combination with one or more of the other types of alcohols to
obtain the desired
% by weight of the solubility agent. If a monohydric alcohol is utilized, then
this type of
alcohol is preferably an aliphatic alcohol, and more preferably is ethyl
alcohol. If a dihydric
alcohol is utilized, then a glycol or a derivative thereof, is preferred. Of
the glycols,
propylene glycol is most preferred and is available from any number of
suppliers. Propylene
glycol provides advantages over other alcohols, as a solubility agent of high
concentrations of
quaternary ammonium compounds, such as cetylpyridinium chloride. Trihydric
alcohols,
such as glycerol or derivatives thereof, are also useful as a solubility agent
in the present
concentrated cetylpyridinium chloride solution. The choice of the alcohol may
depend upon
the food product that is contacted and is selected to be compatible with
treatment steps prior
to or after the quaternary ammonium compound contact with the food product. If
a
polyglycol is used as the solubility agent, for example, then polyethylene
glycol may be
preferred, and particularly the lower molecular weight species with an average
molecular
weight of less than or equal to 600, which are well known and possess
properties similar to
propylene glycol. If ethyl alcohol is used as the solubility agent, it may be
present at a
concentration up to about 49% by weight, for example. Ranges of ethyl alcohol
about 0.5%
12
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
weight to about 49% by weight, from about 10% by weight to about 40% by
weight, from
about 15% by weight to about 30% by weight, and within the range at about 20%
may be
used.
[0034] In some embodiments, any compatible solubility agent may be used,
however, ethyl
alcohol or glycerin may be preferred. For example, in one embodiment, the
antimicrobial
solution, either concentrated or a dilute treatment solution, contains ethyl
alcohol, glycerin or
both. In a concentrated solution for example, the solution may contain about
100,000 ppm to
about 300,000 ppm quaternary ammonium compound, about 0% to about 49% ethyl
alcohol
and about 0 to about 20% glycerin in water. A preferred formulation may
contain about
150,000 ppm to about 250,000 ppm quaternary ammonium compound, about 10% to
about
40% ethyl alcohol and about 0.5 to about 10% glycerin in water. More
preferably, the ethyl
alcohol concentration may range from about 15% to about 30% and the glycerin
concentration can range from about 0.5 to about 5%. Preferably, this
formulation contains
about 200,000 ppm quaternary ammonium compound, about 20% ethyl alcohol, and
about
1% glycerin. This formulation may be particularly useful as a concentrate to
be added to the
storage tanks for use in immersion treatment of food products with quaternary
ammonium
compound but may also be useful in a spraying method at a final concentration
of about
5,000 ppm quaternary ammonium compound. In one embodiment, the dilute
antimicrobial
treatment solution is formed from a dilution of the concentrated antimicrobial
solution
comprising the quaternary ammonium compound and the solubility agent, wherein
the
concentrated antimicrobial solution has a pH value less than 7Ø The pH value
of the
concentrated antimicrobial solution for example may be below about 6.5, below
about 6.0, or
below about 5.5. Acidification of the concentrated solution may be by the same
or different
acidifying agents used to further acidify the dilute antimicrobial treatment
solution to a pH
13
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
value preferably between 2.0 and 1.0, more preferable between 1.5 and 1.2,
even more
preferably between 1.5 and 1.3.
[0035] The concentration of the solubility agent may vary depending on the %
weight of the
quaternary ammonium compound, which is to be dissolved in solution, as well as
the
particular intended use of the concentrated quaternary ammonium compound
solution and
dilutions thereof The concentrated antimicrobial solution may comprise
quaternary
ammonium compound in solution at concentrations ranging from greater than
about 10% or
greater than about 15% by weight to about 60% by weight. Although a greater
than about
60% by weight concentration of quaternary ammonium compound can be used in the
concentrated quaternary ammonium compound solution, the upper limit that is
useful is
governed by the interaction between the % (or weight) of quaternary ammonium
compound
and the solubility agent(s) used to prepare the concentrated antimicrobial
solution. Specific
solubility agents or combinations of these agents may result in higher than
60% quaternary
ammonium compound concentrated formulations. In one embodiment, the
concentrated
antimicrobial solution may contain at least one solubility agent, such as an
alcohol at a
concentration of up to about 70% by weight. In one preferred embodiment,
solubility agent
comprises an alcohol present at a concentration of up to about 60% by weight,
and may range
from about 10% by weight to about 60% by weight.
[0036] In one embodiment, the concentration of the quaternary ammonium
compound in the
concentrated antimicrobial solution may be about 40% by weight and at least
one alcohol at a
concentration of up to about 50% by weight, and preferably about 50% by
weight. In this
concentrated aqueous solution, the solubility agent may be a combination of
alcohols, such as
ethyl alcohol and propylene glycol. But glycerol also is useful as the
solubility agent, alone
or in combination with other alcohols or polyglycols. Glycerol is useful for
this purpose at a
14
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
concentration of up to and including about 20% by weight, and also is useful
at
concentrations ranging from about 0.5% to about 10% by weight, and within this
range at
about 1%. Glycerol is useful in methods where propylene glycol is not the
alcohol of choice
for solubilizing the quaternary ammonium compound. A further useful
concentrated
quaternary ammonium compound solution comprises a quaternary ammonium compound
at a
concentration of about 20% by weight and at least one alcohol at a
concentration of about
50% by weight, such as a combination of ethyl alcohol and propylene glycol,
and preferably
where each alcohol is present at about 25% by weight.
[0037] In further such embodiments, the concentrated antimicrobial solution
also includes
an acidifying agent and is formulated to have an acidic pH, as described
above. For example,
the acidic pH value, such as less than about 6.5, less than about 6.0, or less
than about 5.5
may be used to reduce the pH of an antimicrobial treatment solution formulated
by dilution of
the concentrated antimicrobial solution or the amount of a second acidifying
agent needed to
reach a desired acidic pH range, e.g., preferably about 2.0 and about 1.0,
more preferable
between about 1.5 and about 1.2, and even more preferably between about 1.5
and about 1.3,
upon dilution prior to treatment of a food product. In various embodiments,
the concentrated
antimicrobial solution comprises an acidic pH value formulated with a low pH
version of
propylene glycol. The solubility agent may be selected from an alcohol,
propylene glycol,
and glycerin. The quaternary ammonium compound may comprise an alkylpyridinium
salt
such as cetylpyridinium chloride. The acidifying agent is preferably a GRAS
acid and is
more preferably a dilute solution of citric acid and hydrochloric acid. The
acidifying agent
may be present in the concentrated antimicrobial solution in an amount
sufficient to achieve
an acidic pH value below 7.0, preferably below about 6.5, and more preferably
between about
6.0 and about 5.5. In one embodiment, the solubility agent is preferably
propylene glycol. In
one such embodiment, the concentrated antimicrobial solution comprises about
40%
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
quaternary ammonium compound, about 55% to about 60% propylene glycol, and up
to
about 5% water by weight.
[0038] In various embodiments, the quaternary ammonium compound is present at
a
concentration from greater than about 10% or greater than about 15% by weight
to about
50% by weight, and more preferably at a concentration from greater than about
10% or about
15% by weight to about 40% by weight. The concentration of the quaternary
ammonium
compound in the range of greater than about 10% to about 30% by weight or
between about
15% to about 25% by weight and within this range about 20% by weight is also
useful in the
present concentrate solution. In one embodiment, a concentrated antimicrobial
solution
comprises a quaternary ammonium compound with a concentration from greater
than about
10% by weight and at least one solubility agent. The solubility agent or a
combination of
these agents, and water if necessary, to make up the remaining weight of the
solution, is
added to reach 100% by weight. The solubility agent is any compatible
solubility agent that
solubilizes quaternary ammonium compounds at concentrations of greater than
about 10% by
weight is contemplated, but alcohols are the preferred solubility agent. The
solubility agent
may be any water-miscible organic solvent that enhances the solubility of the
quaternary
ammonium compound in an aqueous solution so that it forms a solution at
concentrations of
greater than 10% by weight. A 10% by weight solution is made by weighing 10
grams of
quaternary ammonium compound and dissolving it in 90 grams of liquid that
comprises at
least one solubility agent and water, if water is necessary to bring the
weight to 90 grams of
liquid. The concentrated quaternary ammonium compound solution may comprise
quaternary
ammonium compound in solution at concentrations of greater than about 10% by
weight, and
more preferably at concentrations of greater than about 15% by weight.
16
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
[0039] In one embodiment, the concentrated antimicrobial solution comprises
the quaternary
ammonium compound in solution at a concentration of about 40% by weight and at
least one
alcohol at a concentration ranging from between about 500/s by weight to about
60% by
weight with water making up the remaining % weight. The preferred alcohol in
this solution
may be propylene glycol. More preferably, the concentrated quaternary ammonium
compound solution comprises a quaternary ammonium compound at a concentration
of about
40% by weight and at least one alcohol at a concentration ranging between
about 55% by
weight to about 60% by weight and water present at about 5% by weight. The
more preferred
concentrated quaternary ammonium compound solution comprises a quaternary
ammonium
compound at a concentration of about 40% by weight, an alcohol at a
concentration of about
57% by weight and water present at about 3% by weight. In one embodiment, the
dilute
antimicrobial treatment solution is formed from a dilution of one of the above
concentrated
antimicrobial solutions comprising the quaternary ammonium compound and the
solubility
agent, wherein the concentrated antimicrobial solution has a pH value less
than 7Ø The pH
value of the concentrated antimicrobial solution for example may be below
about 6.5, below
about 6.0, or below about 5.5. Acidification of the concentrated solution may
be by the same
or different acidifying agents used to further acidify the dilute
antimicrobial treatment
solution to a pH value preferably between about 2.0 and about 1.0, more
preferable between
about 1.5 and about 1.2, even more preferably between about 1.5 and about 1.3.
[0040] Residue Reduction Formulations
[0041] In one example, meat products such as poultry parts treated with an
antimicrobial
treatment solution of a quaternary ammonium compound such as cetylpyridinium
chloride
may result in different average levels of cetylpyridinium chloride residue for
different types
of parts. For instance, applying cetylpyridinium chloride of a given
concentration to whole
17
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
carcasses, breasts, thighs, and chicken tenders may result in lower average
residues in poultry
carcasses and breasts and in higher average residues in thighs and chicken
tenders. As
described herein addition of an acidifying agent in an amount sufficient to
provide the
solution with a low acidic pH value may beneficially lower average quaternary
ammonium
residue, such as cetylpyridinium chloride residue, on parts treated with the
solution compared
to treatment of the parts under the same conditions with the same solution but
having a
neutral pH value. Furthermore, treatment with low acidic pH solution may
maintain the
desired efficacy of the antimicrobial treatment solution and provide the
desired microbial
reductions, but with relatively reduced residue compared to the same
conditions and solution
but having a neutral pH value.
[0042] The herein described antimicrobial treatment solutions of a quaternary
ammonium
compound such as cetylpyridinium chloride having a pH value within a range of
from 1.2 to
2.0, for example, may be used to significantly reduce the average residue on
treated product
without adversely affecting the organoleptic properties of the product. In one
currently
preferred embodiment, antimicrobial treatment solutions of a quaternary
ammonium
compound such as cetylpyridinium chloride having a low acidic pH value within
a range of
from about 1.5 to 1.3 may be used to provide a preferred balance of reducing
residue and
providing antimicrobial performance without adversely affecting the
organoleptic properties
of the treated product.
[0043] In one embodiment, the quaternary ammonium compound is present in a
concentration selected such that treatment of a food product with the solution
results in an
average residue of the quaternary ammonium compound below about 35 ppm
following
treatment comprising spraying the solution onto the food product or submerging
the food
product in the solution for preferably between about 15 seconds and about 3
seconds, more
18
CA 02976009 2017-08-04
WO 2016/137805
PCT/1JS2016/018392
preferably between about 12 seconds and about 4 seconds, and even more
preferably between
about 10 seconds and about 5 seconds.
[0044] In another embodiment, the concentration of the quaternary ammonium
compound
may be selected such that treatment of the food product with the solution
results in an average
residue of the quaternary ammonium compound below about 31.5 ppm following
treatment
comprising spraying the solution onto the food product or submerging the food
product in the
solution for preferably between about 15 seconds and about 3 seconds, more
preferably
between about 12 seconds and about 4 seconds, and even more preferably between
about 10
seconds and about 5 seconds.
[0045] In yet another embodiment, the concentration of the quaternary ammonium
compound is selected such that treatment of the food product with the solution
results in an
average residue of the quaternary ammonium compound below about 7 ppm where
the
treatment comprises spraying the solution onto the food product or submerging
the food
product in the solution for preferably between about 15 seconds and about 3
seconds, more
preferably between about 12 seconds and about 4 seconds, and even more
preferably between
about 10 seconds and about 5 seconds.
[0046] Enhanced Contact Formulations
[0047] Various embodiments of the treatment solution may be formulated to
enhance
contact, such as duration, quality, quantity of contact, of the quaternary
ammonium
compound with treated food products. For example, a treatment solution may be
formulated
for enhanced access within crevasses or to penetrate materials obstructing or
obscuring
microbial on a surface of a food product.
19
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
[0048] These or other formulations of the solution may include formulations
designed to
increase the contact time of a quaternary ammonium compound solution on food
products
during processing, particularly when delivered by a spraying method. Such a
formulation
may potentially allow a longer contact time of the quaternary ammonium
compound with the
food product without additional steps which would increase the processing
time. The
treatment solution, by virtue of its properties, may thus be formulated to
provide potential
increases the antimicrobial effectiveness of the process.
[0049] In various embodiments, a concentrated treatment solution formulated
for enhanced
contact with a food product, such as enhanced duration of contact particularly
when delivered
by spraying food products, may preferably contain about 50 ppm to about 20,000
ppm
quaternary ammonium compound, and at least one of a solubility agent selected
from about 0
to about 10% ethyl alcohol, and about 0 to about 20% glycerin or both. More
preferably this
formulation may contain about 50 ppm to about 5,000 ppm, about 0 to about 10%
ethyl
alcohol, and about 1.0% to about 100/0 glycerin in water. Even more preferably
this
formulation may contain about 500 ppm to about 5,000 ppm quaternary ammonium
compound, 0 to 10% ethyl alcohol, and about 1.0 to about 5% glycerin in water
and more
preferably about 1.0 to about 3% glycerin. In one embodiment, the dilute
antimicrobial
treatment solution may be formed from a dilution of the above concentrated
antimicrobial
solution comprising the quaternary ammonium compound and the solubility agent,
wherein
the concentrated antimicrobial solution has a pH value less than 7Ø The pH
value of the
concentrated solution may be provided by an acidifying agent. The pH value of
the
concentrated antimicrobial solution for example may be below about 6.5, below
about 6.0, or
below about 5.5. The acidifying agent providing the acidific pH value of the
concentrated
solution may comprise the same or different acidifying agents than those that
may be
subsequently used in dilution or acidification of the dilute antimicrobial
treatment solution to
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
a pH value preferably between 2.0 and 1.0, more preferable between 1.5 and
1.2, even more
preferably between 1.5 and 1.3.
[0050] Methods of Formulating Solutions
[0051] The present disclosure also describes methods of formulating an aqueous
antimicrobial treatment solution for treatment of a food product. The method
may comprise
formulating any of the antimicrobial solutions described above, including
formulation of the
antimicrobial treatment solutions from aqueous dilution of concentrated
solutions of
quaternary ammonium compound, which may further include addition of an
acidifying agent
comprising one or more acids. It is to be appreciated that as described above
the solution
may include the listed components at the identified percentages in an aqueous
solution. The
components may be added within or to various intermediate aqueous solutions to
combine the
components. Water may be added to obtain the remaining weight of the solution.
The
quaternary ammonium compound may be a quaternary ammonium compound selected
from
the group consisting of alkylpyridinium, tetra-alkylammonium and
alkylalicyclic ammonium
salts. In one
preferred embodiment, the quaternary ammonium compound is an
alkylpyridinium salt comprising cetylpyridinium chloride; however, other
quaternary
ammonium compounds may be used. The quaternary ammonium compound may be
present
in or added in an amount preferably between about 0.2% and about 0.5% by
weight, more
preferably between about 0.3% and about 0.4% by weight, and even more
preferably between
about 0.3% to about 0.35% by weight of the resulting solution. The method may
include
adding the acidifying agent in an amount effective to provide the solution
with a pH value
preferably between about 2.0 and about 1Ø more preferably between about 1.5
and about
1.2, and even more preferably between about 1.5 and about 1.3. In one
embodiment, the
quaternary ammonium compound is combined with a solvent prior to adjusting the
pH with
21
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
the selected acidifying agent to the selected pH value. The acidifying agent
is preferably one
or more acids comprising generally recognized as safe (GRAS) acids. In one
embodiment,
the acidifying agent is at least one of citric acid and hydrochloric acid;
however, other acids
may be used.
[0052] In one embodiment, the method of formulating the antimicrobial
treatment solution
comprises dilution of a concentrated antimicrobial solution of the quaternary
ammonium
compound and a solubility agent with an aqueous solvent and addition of the
acidifying agent
to obtain the desired pH value and quaternary ammonium compound concentration.
In one
such example, the solubility agent is selected from an alcohol, propylene
glycol, and glycerin.
In one embodiment, the quaternary ammonium compound is combined with a solvent
prior to
adjusting the pH with the selected acidifying agent to the selected pH value.
[0053] In one embodiment, a method of formulating a concentrated antimicrobial
solution of
quaternary ammonium compound comprises combining a quaternary ammonium
compound
and a solubility agent in a solvent, wherein the concentrated antimicrobial
solution has a pH
value preferably below 7.0, more preferably below about 6.5, even more
preferably below
about 6.0, and most preferably below about 5.5. The quaternary ammonium
compound may
be a quaternary ammonium compound selected from the group consisting of
alkylpyridinium,
tetra-alkylammonium and alkylalicyclic ammonium salts. In one preferred
embodiment, the
quaternary ammonium compound is an alkylpyridinium salt comprising
cetylpyridinium
chloride; however, other quaternary ammonium compounds may be used. The
cetylpyridinium chloride, for example, may be present or added in an amount
about 40% by
weight of the concentrated antimicrobial solution. In another
embodiment, the
cetylpyridinium chloride may be present or added in an amount about between
about 10%
and about 65%, between about 20% and 50%, or between about 35% and about 45%
by
22
weight of the concentrated antimicrobial solution. The method may further
comprise adding
a solubility agent. The solubility agent may be selected from an alcohol,
propylene glycol,
and glycerin. The solubility agent may preferably be propylene glycol.
Propylene glycol
may be present in or added in an amount to between about 55% and about 60% by
weight of
the concentrated antimicrobial solution. The solvent may preferably be water.
[0054] Methods of Treating Food Products
[0055] A method of treating food products may comprise applying the
antimicrobial
treatment solution according to any of the above embodiments to a food
product. The food
product may comprise, for example, poultry, beef, pork, lamb, venison, and
other edible meat
products, which may include seafood, fish and shellfish. In one embodiment,
the food
product comprises a poultry food product selected from poultry carcass,
breast, tender, thigh,
giblet, and wing. The food product may also include fruits, vegetables, or
dairy products.
[0056] Application of the antimicrobial treatment solutions to treat the food
product may
include covering a treatment surface of the food product with the
antimicrobial treatment
solution comprising the quaternary ammonium compound in solution. For example,
the food
product may treated by submerging, e.g., dipping, the food product in the
antimicrobial
treatment solution comprising the quaternary ammonium compound or the solution
may be
sprayed onto a surface to be treated with a saturating spray as are known in
the art. One
example of a dip system suitable for treatment of food products employing a
treatment
solution as described herein is described in U.S. Patent Application No.
14/510,439, filed
October 9, 2014, titled CLOSED LOOP RECYCLING SYSTEM AND DIP TANK FOR
ANTIMICROBIAL COMPOUNDS. Additional examples of antimicrobial treatment
systems, including spray application, that may employ a treatment solution as
described
herein are described in U.S. Patent No. 6,742,720, issued June 1, 2004, to
Noland for SPRAY
23
CA 2976009 2019-11-26
APPLICATION SYSTEM; U.S. Patent Application No. 14/471,846, filed August 28,
2014,
titled APPLICATION SYSTEM AND RECYCLE AND RELATED USE OF
ANTIMICROBIAL QUATERNARY AMMONIUM COMPOUND; and U.S. Patent
Application No. 14/510,385, filed October 9, 2014, titled ANTIMICROBIAL
APPLICATION SYSTEM WITH RECYCLE AND CAPTURE.
[0057] The method may include positioning or transmitting a food product
within or through
a treatment zone. The length of the treatment zone or period of time the food
product is
within or transmitted through the treatment zone for application of the
solution, e.g., in a dip,
spray, or drench zone, may be designed so that a treated product will be
present in the
treatment zone for a wide range of desired times. In one embodiment, the
treated product
will be present in the treatment zone for a time that is preferably within a
range of from about
15 seconds to about 3 seconds, more preferably in a range of from about 12
seconds to about
4 seconds, and more preferably within a range of from about 10 seconds to
about 5 seconds.
[0058] Hereinafter, non-limiting experimental examples are described
illustrating various
example applications and reduced residues and methods of formulating various
antimicrobial
treatment solutions.
[0059] EXAMPLE 1
[0060] Comparative quaternary ammonium compound residue levels on breast
tenders
following treatment with quaternary ammonium compound treatment solutions
having
neutral and acidic pH values.
24
CA 2976009 2019-11-26
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
[0061] Materials and Solution Preparation
[0062] Treatment solutions identified in TABLE 1 were prepared in containers
capable of
holding 2500 mL of solution with extra room for dipping and agitating samples.
The
containers were initially weighed and labeled A (Citrilowlm pH 1.2), B
(Propionic 1000 parts
per million (ppm)), and C (Cecure ). About 2200 mL of tap water was then added
to each
container. Container A was pH adjusted to 1.2 with a stock CitrilowTM solution
(CitrilowTM
is a blend of citric acid and hydrochloric acid (HCl) sold by Safe Foods
Corporation, North
Little Rock, Arkansas). Propionic acid (99%) was added to container B to
achieve a
concentration of 1000 ppm (2.2 mL). Containers and solution were weight
adjusted to
achieve a 2000 mL volume. 22 grams of Cecure 43.) solution was added to each
container to
target 0.4%. Cecure is an antimicrobial quaternary ammonium compound
solution sold by
Safe Foods Corporation, North Little Rock, Arkansas, that includes
concentrated
cetylpyridinium chloride, propylene glycol, and water. The concentrated Cecure
solution
has neutral pH and includes approximately 40% cetylpyridinium chloride by
weight.
Containers with solutions were mixed, sealed, and refrigerated overnight.
[0063] Pretreatment and rinse solutions listed in TABLE 1 were prepared as
follows:
containers capable of holding 2500 mL of solution with extra room for dipping
and agitating
samples in the solution labeled D (CitrilowTM pH 1.2), E (Propionic 1000 ppm),
F (Tween
20), and (Water). About 2200 mL of tap water was added to each container.
Container D
was pH adjusted to 1.2 using CitrilowTM solution. Propionic acid (99%) was
added to
container E to achieve a concentration of 1000 ppm (2.2 mL to 2200 mL water).
Tween 20
(100%) was added to container F to achieve a concentration of 20%.
[0064] Treatment Protocol
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
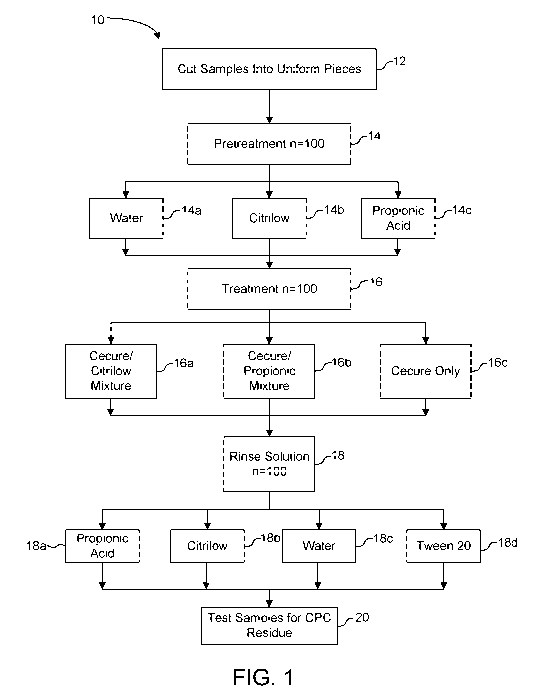
[0065] FIG. 1 provides an overview of the experimental treatment protocol 10.
Breast
tenders where purchased from a local grocery store (control tests (not shown)
confirmed no
initial cetylpyridinium chloride residue). Uniform sized tenders were selected
and/or cut 12.
The selected tenders were grouped for treatment as listed in TABLE 1. Samples
subjected to
pretreatment 14 were placed in one of the pretreatment solutions water 14a,
CitrilowTM 14b,
or propionic acid 14c and were immediately removed following apretreatment
duration of
about 3 seconds. All samples were then treated 16 with treatment solution by
placement in
respective treatment containers containing Cecure (0.4%)/CitrilowTM (pH 1.2)
16a, Cecure
(0.49/0)/Propionic (1000 ppm) 16b, or Cecure (0.4%) 16c with agitation for 3
seconds.
Sample numbers 91-100 were treated with Cecure CC (0.4%) 16c by spray (18 mL,
6 sec) at 3
sec per side. Samples were then removed from the treatment containers and
allowed to drip.
Samples were then rinsed 18 by placement in a rinse solution Propionic (1000
ppm) 18a,
CitrilowTM (pH 1.2) 18b, water 18c, or Tween 20 with agitation for 6 seconds.
Samples were
then allowed to drain for 20 seconds. Sample bags (plastic) were labeled with
sample
numbers as shown in TABLE 1. Each sample was then individually bagged
(plastic) and
refrigerated overnight.
[0066] Residue Analysis
[0067] Residue analysis was performed by high pressure liquid chromatograph
(HPLC) for
cetylpyridinium chloride residue analysis. Et0H was added to target weight
about 2 mL per
gram of sample. Samples were stomached for 1 minute. Samples were then
extracted for 30
minutes in the extraction fluid on a rotating platform. Extract samples were
taken from the
extraction fluid (approx. 10 mL aliquot) and allowed to sediment for 10
minutes to allow for
gravity separation of large particles. The extract samples were then run on
HPLC for
cetylpyridinium chloride residue analysis. Parts per million (ppm) of
cetylpyridinium
26
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
chloride in each treated part were calculated using HPLC results. A 30%
extraction
efficiency was added to the final ppm concentration.
[0068] TABLE I. Sample Number and Treatment Descriptions
Pretreatment Treatment Rinse
Conditions (All
Sample Time treatments will be Time
Time
Numbers Conditions (seconds) cold) (seconds)
Conditions (seconds)
Cecure
(0.4%)/CitrilowTM
1-10 None None (pH 1.2) 3 Water 6
Cecure 0
(0.4%)/Propionic
11-20 None None (1000 ppm) 3 Water 6
CitrilowTM
21-30 , (pH 1.2) , 3 Cecure 0 (0.4%) , 3 , Water 6
Propionic
31-40 (1000 ppm) 3 Cecure Ck) (0.4%) 3 Water 6
CitrilowTM
41-50 None None Cecure 0 (0.4%) 3 (pH 1.2) 6
51-60 None None Cecure CD (0.4%) 3 Tween 20 6
Propionic
61-70 None None Cecure (0.4%) 3 (1000 ppm) 6
71-80 Water 3 Cecure 0 (0.4%) 3 Water 6
81-90 None None Cecure (0.4%) 3 Water 6
Cecure 0 (0.4%)
91-100 None None Spray 3 Water 6
[0069] A summary of the data collected in this example is shown in FIG. 2 with
the
corresponding treatment types described and summarized in TABLE 2. Average
cetylpyridinium chloride (CPC) concentration of the 10 samples (Sample
Treatment 1-10
labeled along the x-axis in FIG. 2) within each treatment group is shown.
These data show
that treatment of breast tenders with acidified quaternary ammonium compound
solution
(Cecure 0. (0.4%)), at low pH reduces quaternary ammonium compound residues as
reflected
in measured CPC concentration. In particular, CPC concentration of sample
treatment group
number 1, treated with low pH CitrilowTM (pH 1.2) and Citrilow, was
significantly reduced
compared to the other treatments. Sample treatment group 2 included quaternary
ammonium
27
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
treatment with propionic acid, which provided a solution having a greater pH
than the
treatment of group 1, and provided reduced residue compared to groups such as
8 and 9 that
were not treated with acidic solutions. Pretreatment with acid prior to
treatment with
quaternary ammonium showed reduced residue when low pH CitrilowTM (pH 1.2)
(group 3)
but not with the higher pH propionic acid. Rinsing with the low pH CitrilowTM
(pH 1.2)
(group 5) or Tween 20 (group 6) showed reduced residue while rinsing with
propionic acid
(group 7) or water (group 8) did not.
[0070] TABLE 2. Summary of Residue Data with Sample Treatment Description
Sample
Treatment CPC
Group Pretreatment Treatment Rinse Concentration
Number (3 sec) (cold, 3 sec) (6 sec) (PP11)
Cecure k (0.4%) / CitrilowTM
1 None (pH 1.2) Water 5.66
Cecure k (0.4%) / Propionic
2 None (1000ppm) Water 15.75
CitrilowTM
3 (pH 1.2) Cecure k (0.4%) Water 15.01
Propionic
4 (1000ppm) Cecure k (0.4%) Water 29.74
CitrilowTM (pH
None Cecure k (0.4%) 1.2) 13.61
Tween 20
6 None Cecure 44) (0.4%) (20%) 15.28
Propionic
7 None Cecure k (0.4%) (1000ppm) 22.38
8 Water Cecure k (0.4%) Water 27.86
9 None Cecure k (0.4%) Water 36.98
Cecure k (0.4%) / Spray
None (18mL, 6 sec) Water 17.56
28
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
[0071] EXAMPLE 2
[0072] Quaternary ammonium compound residue levels on breast tenders following
treatment with quaternary ammonium compound treatment solutions having acidic
and
basic pH values.
[0073] Materials and Solution Preparation
[0074] Containers capable of holding 2500 mL of solution with extra room for
dipping and
agitating samples in treatment solution were weighed and labeled A (CitrilowTM
pH 1.2), B
(HC1 pH 1.2), and C (NaOH pH 9.0) as identified in TABLE 3. Treatment
solutions
identified in TABLE 3 were prepared in the containers by addition of about
2200 mL of tap
water. The solutions were pH adjusted to the identified pH with the identified
pH agent as
listed in TABLE 3. CitrilowIm was taken from a stock Citrilowrm solution
(citric acid /HC1).
Containers and solution were weight adjusted to achieve a 2000 mL volume. 22
grams of
Cecure 0 solution was added to each container to target 0.4%. Containers with
solutions
were mixed, sealed, and refrigerated overnight. Rinse solutions identified in
TABLE 3 were
prepared from tap water, Tiveen 20 (100%) diluted with tap water to 20%, and a
stock 1.5 pH
CitnlowTM solution, all held at room temperature.
[0075] Treatment Protocol
[0076] FIG. 3 provides an overview of the experimental treatment protocol 30.
Treatment
32 of non-Cecure treated in a treatment solution Cecure Cg.)
(0.4%)/CitrilowTM (pH 1.19)
32a, Cecure (0.4%);HC1
(pH 1.21) 32b, or Cecure 0 (0.4%)/Na0H(pH 11.1) 32c, as
identified in TABLE 3 (samples 1-90), for 3 seconds with agitation. The
samples were then
removed from the treatment container and allowed to drip for less than 10
seconds. The
samples were next rinsed with rinse solution 34 by placement in a rinse
solution water 34a,
29
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
Tween 20 34b, or CitrilowTM (pH 1.5) 34c, as identified in TABLE 3, for 6
seconds with
agitation. The samples were then allowed to drain for 20 seconds. The rinsed
samples were
bagged individually and refrigerated overnight.
[0077] Residue Analysis
[0078] Residue analysis was performed by high pressure liquid chromatograph
(HPLC) for
cetylpyridinium chloride residue analysis. Et0H was added to target weight
about 2 mL per
gram of sample. Samples where stomached for 1 minute. Samples were then
extracted for
30 minutes in the extraction fluid on a rotating platform. Extract samples
were taken from
the extraction fluid (approx. 10 mL aliquot) and allowed to sediment for 10
minutes to allow
for gravity separation of large particles. The extract samples were then run
on HPLC for
cetylpyridinium chloride residue analysis. Parts per million (ppm) of
cetylpyridinium
chloride in each treated part were calculated using HPLC results. A 30%
extraction
efficiency was added to the final ppm concentration.
[0079] TABLE 3. Sample Number and Treatment Descriptions
Treatment Rinse
Conditions Conditions
Sample Time Time
(All treatments will be (Room
Numbers (seconds) (seconds)
cold) Temperature)
1-10 3 Water 6
Cecure CgD
11-20 3 Tween 20% 6
(0.4%)/CitrilowTM
21-30 (pH 1.19) 3 CitrilowTM (pH
6
1.5)
31-40 3 Water 6
41-50 Cecure 6g) (0.4%)/HC1 3 Tween 20% 6
51 60 (pH 1.21) CitrilowTM (pH 6
3
- 1.5)
61-70 3 Water 6
71-80 Cecure 3 Tween 20% 6
(0.4%)/Na0H(pH 11.1) CitrilowTM (pH
81-90 3 6
1.5)
91-91 None None None None
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
[0080] A summary of the residue data collected in this example is shown in
FIG. 4. Bars
represent average cetylpyridinium chloride (CPC) residue of the 10 samples
(identified along
the x-axis) in each treatment subgroup of the 3 treatment groups. These data
show that
treatment with acidified quaternary ammonium compound solution at low pH
reduces
quaternary ammonium compound residues. Quaternary ammonium compound treatment
with quaternary ammonium compound solution at alkaline pH, pH 11.1-samples 61-
90, does
not appear to reduce quaternary ammonium compound residues to levels
corresponding to the
solution at acidic pH values, pH 1.19-samples 1-30 and pH 1.21-samples 31-60.
The identity
of the acidifying agents does not appear to have a significant effect on the
reduction of
quaternary ammonium compound residue.
[0081] EXAMPLE 3
[0082] Treatment efficacy following antimicrobial treatment of thighs with
acidic pH
quaternary ammonium compound solution using commercial application equipment.
[0083] Materials and Solution Preparation
[0084] Treatment solutions and conditions tested are identified in TABLE 4.
Thigh samples
were treated using a commercial part dip system. The system was initially
filled with ice and
water. For acidified treatments, CitrilowTM was then added to obtain a pH of
1.2. The pH
was verified using a pH meter. Cecure (g) was added to the concentration
listed in TABLE 4
(verified by titration using Cecure lk titration kit, sold by Safe Foods
Corporation, North
Little Rock, Arkansas).
[0085] Treatment Protocol
[0086] Treatment solutions and conditions tested are identified in TABLE 4.
FIG. 5
provides an overview of the experimental treatment protocol 50 used in this
example. Whole
31
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
post-chill untreated poultry carcasses were obtained from a local processing
plant 51 and
transported to lab on ice. Thighs (n=200) were cut from the carcasses 52.
Treatments with
CitrilowTM (pH of 1.2)/ Cecure (0.4%) agitation on 54a, CitrilowTM (pH of
1.2)/ Cecure
(0.4%) agitation off 54b, Cecure Eg) neutral solution (0.4%), or agitation on
54c were
performed by placing 50 parts onto the dip system belt and treating the parts
for 3 seconds in
the identified treatment solution with flow system pumps were turned on. The
commercial
belt system was operated at the application parameters. The parts were caught
in a wire rack
cart, placed into a large water rinse bucket, and rinsed for 6 seconds with
agitation 56. The
application system was drained and refilled for each treatment solution. The
parts were then
removed from the rinse water and placed on the wire rack and allowed to drain
for 5 minutes
58. The parts used for the control samples were not treated 54d. All parts
were then
individually bagged in labeled sterile sample bags and refrigerated overnight
60, 62.
[0087] APC Plate Count Assays
[0088] Microbial samples were removed from refrigeration. Butterfield
phosphate diluent
(BPD) was added at 100 mL per 454 grams of sample. Samples were then rinsed
for 1
minute. Dilutions 1-3 were made. Dilutions 0-3 on Aerobic Plate Count (APC)
petrifilmTM.
bag will be zero dilution.
[0089] TABLE 4. Sample Numbers and Treatment Descriptions
Application
Sample Number Solution pH Cecure Concentration
Parameter
1-25 Flow Agitation On 1.2 0.40%
Flow Agitation
26-50 1.2 0.40%
OFF
76-100 Flow Agitation On Neutral 0.40%
101-125 N/A None None
126-147 Flow Agitation On Neutral 0.60%
32
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
[0090] A summary of the APC microbial data obtained in this example is shown
in FIG. 6.
Bars represent average counts (colony forming unites per mL) of the 25 samples
tested in
each treatment group with the exception of the control, which represents
average counts from
the 23 samples tested in the control treatment group. These data show that
increased
concentration of quaternary ammonium compound (cf. samples 76-100 and samples
126-147)
does not increase efficacy of the treatments and acidity of the treatment
solution does not
impair efficacy of the treatments.
[0091] Other Matters
[0092] All numerical quantities stated herein are approximate unless stated
otherwise,
meaning that the term "about" or "approximately" may be inferred when not
expressly stated.
The numerical quantities disclosed herein may be nominal numerical quantities
and are to be
understood as not being strictly limited to the exact numerical values
recited. Instead, unless
stated otherwise, each numerical value is intended to mean both the recited
value and a
functionally, for example pharmaceutically, equivalent range surrounding that
value. All
numerical ranges stated herein include all sub-ranges subsumed therein. For
example, a
range of approximately or about 1 to 10 is intended to include all sub-ranges
between and
including the recited minimum value of 1 and the recited maximum value of 10.
Any
maximum numerical limitation recited herein is intended to include all lower
numerical
limitations. Any minimum numerical limitation recited herein is intended to
include all
higher numerical limitations. Additionally, in some illustrative embodiments,
quantities or
ranges may be given. It is to be understood that any such quantity or range is
provided as an
illustrative example or instance of an embodiment and is not intended to limit
that or other
embodiments.
33
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
[0093] This disclosure describes various elements, features, aspects, and
advantages of
various embodiments antimicrobial solutions, concentrated solutions, methods
of making and
formulating such solutions, and methods of treating food products with such
solutions. It is
to be understood that certain descriptions of the various embodiments have
been simplified to
illustrate only those elements, features and aspects that are relevant to a
more clear
understanding of the disclosed embodiments, while eliminating, for purposes of
brevity or
clarity, other elements, features and aspects. Any references to "various
embodiments,"
"certain embodiments,- "some embodiments,- "one embodiment,- or "an
embodiment"
generally means that a particular element, feature and/or aspect described in
the embodiment
is included in at least one embodiment. The phrases "in various embodiments,"
"in certain
embodiments,- "in some embodiments,- "in one embodiment,- or "in an embodiment-
may
not refer to the same embodiment." Furthermore, the phrases "in one such
embodiment", "in
a further embodiment", or "in certain such embodiments," while generally
referring to and
elaborating upon a preceding embodiment, is not intended to suggest that the
elements,
features, and aspects of the embodiment introduced by the phrase are limited
to the preceding
embodiment; rather, the phrase is provided to assist the reader in
understanding the various
elements, features, and aspects disclosed herein and it is to be understood
that those having
ordinary skill in the art will recognize that such elements, features, and
aspects presented in
the introduced embodiment may be applied in combination with other various
combinations
and sub-combinations of the elements, features, and aspects presented in the
disclosed
embodiments. The present disclosure is not intended to be limited by the
percent
composition of the examples unless claimed otherwise. Percent compositions are
to be
understood as being by weight unless specified otherwise.
[0094] "Consisting of' refers to the inclusion of exactly one element of a
number or list of
elements. The grammatical articles "one", "a", "an", and "the", as used in
this specification,
34
CA 02976009 2017-08-04
WO 2016/137805
PCMJS2016/018392
are intended to include "at least one" or "one or more", unless otherwise
indicated. Thus, the
articles are used in this specification to refer to one or more than one
(i.e., to "at least one") of
the grammatical objects of the article. By way of example, "a component" means
one or
more components, and thus, possibly, more than one component is contemplated
and may be
employed or used in an implementation of the described embodiments. Further,
the use of a
singular noun includes the plural, and the use of a plural noun includes the
singular, unless
the context of the usage requires otherwise.