Note: Descriptions are shown in the official language in which they were submitted.
1
USE OF ISOTHIOCYANATE DERIVATIVES AS MODULATORS OF
PERIPHERAL AND NEUROPATHIC PAIN
Field of the invention
The present invention relates to the field of modulators of peripheral pain,
with specific reference to that linked with diabetic peripheral neuropathy and
chemotherapy-induced neuropathy.
State of the art
Diabetic peripheral neuropathy and chemotherapy-induced neuropathy are
two conditions directly linked with generation of peripheral pain (Galuppo M.,
et al.
Molecules 19, 2877-2895, 2014; Argyriou A.A. et al. Cancer Management and
Research 6, 135-147, 2014). Pain is one of the first manifestations of
inflammation,
but other possible causes such as traumatic events, burns, post-operative or
disease-related pain can be at its origin. Pain can be categorized as:
nociceptive pain,
a protective sensation associated as a reaction to a potentially harmful
noxious
stimuli; inflammatory pain, linked to tissue damage and infiltration of immune
cells;
pathological pain, as a damage to the peripheral or central nervous system
(neuropathic) or as an altered alerting/response leading to an uncontrolled
sensation
of pain (dysfunctional). Chronic pain (longer than 6 months), and in
particular
neuropathic pain, are difficult to treat due to their severity and resistance
to simple
analgesics and are common features in many pathologies, such as diabetes,
multiple
sclerosis, Guillain-Barre syndrome and Parkinson's disease characterized by
peripheral nerve fibers that mainly due to demyelinization transmit abnormal
painful
sensations including hyperalgesia and allodynia. A peripheral neuropathy
leading to
pain is also associated with the clinical use of cancer chemotherapy.
Chemotherapy
drugs currently used to treat cancer (taxanes, cisplatin, oxaliplatin,
epothilones,
bortezomid, vincristine and others), in fact, can be neurotoxic by either
exerting a
direct noxious effect on the brain or the peripheral nerves. Depending on its
severity,
Date Recue/Date Received 2021-03-30
2
chemotherapy-induced neuropathy mainly linked to pain generation can be
dose-limiting and may also markedly compromise quality of life of patients.
Symptoms usually improve or resolve within 3 months after discontinuation of
treatment, whereas severe symptoms may persist for a long period.
Therapeutic agents usually employed for chronic peripheral and neuropathic
pain include off-label utilization of amitriptyline, glutamine, low-dose oral
prednisone. More recently, gabapentin, duloxetine alone or in combination with
pregabalin have provided additional measures in reducing pain, myalgia and
arthralgia. In addition, several neuroprotective agents including amifostine,
acetyl-L-carnitine and vitamin E have demonstrated some promising results but
their
routine use is not recommended in current clinical practice.
Despite of this list of products, a real unique treatment designed to relieve
and counteract chronic peripheral and neuropathic pain has not been found to
date
and pharmacological researchers are still looking for active products even
considering natural and botanical derivatives. In this light, some plants
containing
phenolic compounds, cannabinoids, alkaloids and vanilloids have already been
investigated with some success, leading to the development of oral and topical
formulations such as Sativex (containing a standardized extract of Cannabis
sativa)
and Qutenza (patches containg capsaicin).
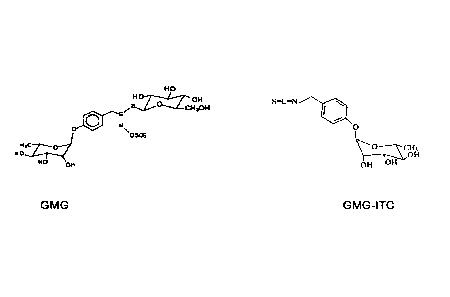
Glucomoringin (4-(a-L-rhamnosyloxy)-benzyl glucosinolate; GMG) is an
uncommon member of the glucosinolates (GLs) family and presents a unique
characteristic consisting in a second saccharidic residue in its side chain.
Its structure
is depicted in Figure along with that of the corresponding des-thioglucoside
isothiocyanate (4-(a-L-rhamnosyloxy)-benzyl isothiocyanate; glucomoringin-des-
thioglucoside; GMG-ITC) which is formed by bioactivation of GMG with the
enzyme myrosinase.
GMG is a typical secondary metabolite present in vegetables belonging to the
genus Moringaceae that consists of 14 species, among which Moringa oleifera is
the
Date Recue/Date Received 2021-03-30
3
most widely distributed. Moringa oleifera L. (horseradish tree) is a pan-
tropical
species also known with the following different names in relationship to the
geographical area: benzolive, drumstick tree, kelor, marango, mionge,
mulangay,
saijhan and saijna (Fahey J.W. et al. Trees of Life Journal 2005 1:5; Mahmood
K.T.
J. Pharm. Sci &Res. 2, 775, 2010). Moringa oleifera is the most widely
cultivated
species of a monogenetic family, the Moringaceae that is native of the
sub-Himalayan tracts of India, Pakistan, Bangladesh and Afghanistan. The plant
was
already utilized by the ancient Romans, Greeks and Egyptians; all parts of the
tree
are edible and in more modern era have long been consumed by humans. Moringa
trees have been used to fight malnutrition, in particular among infants. More
recently, attention has also focused on a modern confirmation of traditionally
described potential health benefit deriving from utilization of both oral and
topical
preparation based on Moringa oleifera derivatives. The medical value of the
seeds
and other part of the plant have long been recognized in folk medicine (Longo
PL
(2001). Plasma cell disorders. In Braunwald E, Kasper D, Faucci A. eds
Harrison's
principles of internal medicine, 15th edn, vol. 1:727- 33.). A long list of
traditional
medicine references on the plant are available which include effects such as:
antibiotic, antitrypanosomal, hypotensive,
antispasmodic, antiulcer,
hypocholesterolemic, hypoglycemic. However, most of these effects in humans
are
not supported by controlled studies and are generally observed through the
usage of
non-standardized derivatives of the plant.
GMG-ITC has been shown to exhibit a broad biological activity and it was
also shown to exert an effective antitumor promoting activity (Guevara AP et
al,
Mutation Research, 440, 181-188, 1999) and in counteracting the inflammatory
response (Galuppo M et al, Fitoterapia, 95, 160-174, 2014).
The use of GMG and of GMG-ITC for the treatment of myeloma is described
in W02009/089889 Al.
Date Recue/Date Received 2021-03-30
4
Summary
Certain exemplary embodiments provide glucomoringin, or glucomoringin
des-thioglucoside, or an extract of Moringa oleifera seeds containing
glucomoringin
and/or glucomoringin des-thioglucoside, for use to treat peripheral
neuropathic pain
or chemotherapy-induced neuropathy.
Other exemplary embodiments provide glucomoringin, or glucomoringin
des-thioglucoside, or an extract of Moringa oleifera seeds containing
glucomoringin
and/or glucomoringin des-thioglucoside, for use in the manufacture of a
medicament
for treating peripheral neuropathic pain or chemotherapy-induced neuropathy.
Yet other exemplary embodiments provide use of glucomoringin, or
glucomoringin des-thioglucoside, or an extract of Moringa oleifera seeds
containing
glucomoringin and/or glucomoringin des-thioglucoside, to treat peripheral
neuropathic pain or chemotherapy-induced neuropathy.
Brief description of the figure
Figure: Structure of glucomoringin (GMG) and of glucomoringin
des-thioglucoside (GMG-ITC).
Description of the invention
It has now surprisingly been found that GMG, its des-thio-glucoside
GMG-ITC, or an extract of Moringa oleifera seeds containing GMG and/or
GMG-ITC are endowed with a remarkable effect in modulating peripheral
neuropathic pain in in vivo experimental models of diabetic animals and
chemotherapy-induced neuropathy.
The object of the present invention is therefore GMG, GMG-ITC, or an
extract of Moringa oleifera seeds containing GMG and/or GMG-ITC, for the
treatment of peripheral neuropathic pain and chemotherapy-induced neuropathy.
In a preferred embodiment, the invention relates to isolated GMG or to an
extract of Moringa oleifera seeds containing GMG, for the treatment of
peripheral
neuropathic pain and chemotherapy-induced neuropathy.
Date Recue/Date Received 2021-03-30
5
An extract of Moringa oleifera seeds containing GMT and GMT-ITC can be
obtained treating the de-fatted flour of the pealed seeds with a 70% ethanol
aqueous
solution at about 75-80 C or with water at 80-90 C. The water extract is then
freeze-dried and sealed in vials under vacuum. If aqueous ethanol is used in
the
extraction, ethanol is removed by distillation and the concentrated extract,
after
proper dilution with water, is freeze-dried. The content of GMG in the
obtained solid
is from 30% to 50% by weight, preferably 40% by weight.
GMG and GMG-ITC can be obtained in purified form by means of the
procedure described in WO 2009/089889 Al.
According to said process, GMG is purified in two sequential steps by anion
exchange and size exclusion chromatography and characterized by Ili and 13C
NMR
spectrometry. The purity is assayed by HPLC analysis of the desulfo-derivative
yielding about 99% based on peak area value.
GMG-ITC is obtained by enzymatic conversion of GMG by using
myrosinase isolated from seeds of Sinapis alba L.. The total conversion of GMG
into GMG-ITC is confirmed by HPLC analysis of the desulfo-derivative, which
allows monitoring the reduction until complete disappearance of GMG in the
reaction mixture. GMG-ITC is then purified (peak purity > 99%) by reverse-
phase
chromatography, and analytically characterized by HPLC-DAD. Identification is
then confirmed by means of and 13C NMR.
GMG, GMG-ITC and a Moringa oleifera seeds extract containing GMG
and/or GMG-ITC are able to modulate the peripheral neuropathic pain due to
diabetes or induced by chemotherapy, in particular the peripheral neuropathic
pain
induced by chemotherapy with taxanes, platinum complexes such as cisplatin and
oxaliplatin, epothilones, boronic acids such as bortezomid, vinca alkaloids
such as
vincristine.
According to the present invention, the Moringa oleifera seeds extract, GMG
and GMG-ITC will be administered orally or topically, either alone or in
Date Recue/Date Received 2021-03-30
6
combination with other substances with useful or complementary activity,
formulated as tablets, capsules, granules, powders, syrups, ointment, gel and
the like.
The pharmaceutical formulations can be prepared with conventional procedures,
using ingredients known in the technique, such as excipients, ligands,
disintegrants,
lubricants, stabilizing agents, and the like. Dosage may vary, according to
the
symptoms, weight of patients, severity of the disease and the like. A skilled
practitioner will easily determine the most effective dosage regimen according
to
established methods. It is believed that the effective therapeutic doses in
humans
will range between 1 mg/Kg/die to 30 mg/Kg/die, even though higher dosages
cannot be ruled out also in view of the limited toxicity of both GMG and GMG-
ITC.
The invention is now further illustrated by the following example.
Biological results
The acute oral effect of Moringa oleifera L. seeds extract and purified GMG
and GMG-ITC on oxaliplatin induced hyperalgesia in mice (Cold plate test) were
evaluated.
The method utilized is a modification of that described by Cavalletti et al.
(Cavalletti G. et al., Eur. J. Cancer 37(18), 2457, 2001). Oxaliplatin (2.4
mg/kg) was
dissolved in 5% glucose solution and administered i.p. for 5 consecutive days
every
week for 14 days. On day 14, the test products were suspended in 1% CMC and
administered orally. Pregabalin was used, in the same experimental conditions,
as a
reference active compound at the dose of 15 mg/kg s.c. dissolved in saline.
Pain-related behavior (i.e. lifting and licking of the hind paw) were observed
and the time (seconds) of the first sign was recorded.
The results are shown in Table.
Date Recue/Date Received 2021-03-30
Table - Acute oral effect of Moringa oleifera seed extract, GMG and GMG-ITC on
oxaliplatin induced hyperalgesia in the mouse:
Cold plate test
Licking latency (s)
Pre-test
m
Treatment Dose_1 (48 hrs after last After
Treatment with Moringa oleifera seed extract
g kg
oxaliplatin adm.)
15 min 1 30
min 1 45 min 60 min
vehicle + vehicle 19.9 0.6
21.3 0.5 1 21.9 1.1 1 20.8 0.6 21.4 0.6
oxaliplatin + vehicle 10.5 0.4** 11.1 0.6** i
11.7 1.0** i 10.2 0.7** 11.5 0.9** --I
oxaliplatin + Moringa oleifera seeds
100 11.0 0.8**
21.4 1.2A 1 17.8 0.9" i 16.1 0.3A 10.9 0.9
extract
oxaliplatin + GMG 10 10.3 0.5**
21.1 1.9A i 16.2 0.7" i 15.1 1.3" 13.5 2.3
oxaliplatin + GMG-ITC 10 10.2 0.4**
20.9 1.8A i 16.0 0.6" i 15.0 1.2" 13.3 2.1
Oxaliplatin + pregabalin (s.c.) 15 11.0 0.7**
, 19.9 1.9" 1 16.1 0.6" i 14.9 1.3" 13.1 2.0
1
1
. . 1
. .
**P<0.01 vs vehicle + vehicle treated animals; AP<0.05 and AAP<0.01 vs
oxaliplatin + vehicle treated animals. Each value represents the mean
of 10 mice.
Date Recue/Date Received 2021-03-30
8
The results showed a significant activity of Moringa oleifera seeds extract
and its purified major constituent on hyperalgesia induced by oxaliplatin in
mouse.
It is important to underline that this activity is comparable to that of
pregabalin
considered as the standard for this test.
Date Recue/Date Received 2021-03-30