Note: Descriptions are shown in the official language in which they were submitted.
CA 02982686 2017-10-13
WO 2016/174120 1
PCT/EP2016/059461
REMOVAL OF AROMATIC HYDROCARBONS FROM LEAN ACID GAS FEED FOR
SULFUR RECOVERY
The present invention relates to a process for the removal of aromatic
hydrocarbons,
such as benzene, toluene, ethyl benzene and xylene (BTX) and aliphatic
hydrocarbons
having 4 carbon atoms or more (C4+) from a lean acid gas containing CO2 and
less than 20
mol.% of H2S prior to sulfur recovery.
BACKGROUND OF THE INVENTION
Natural gas, as it is captured from naturally occurring deposits, is composed
primarily
of light aliphatic hydrocarbons such as methane, propane, butane, pentane, and
their
isomers. Certain contaminants are naturally present in the gas, and must be
removed prior to
delivery of the purified gas for private use or commercial conditioning. These
contaminants
include aliphatic hydrocarbons having 4 carbon atoms or more (C4+) and
aromatic
hydrocarbons such as benzene, toluene, ethyl benzene and xylenes collectively
referred to
as "BTX", but more importantly acid components such as hydrogen sulfide (H2S)
and carbon
dioxide (002).
The presence of hydrogen sulfide in industrial gases causes significant
environmental
problems and is detrimental to the plant structure, requiring constant
maintenance. Strict
requirements are therefore in place to remove H25 from gas streams, in
particular in natural
gas plants.
Removal and disposal of H25 from natural gas is customarily accomplished by
contacting the natural gas containing the H25 with a liquid amine solvent at
the pressure of
the natural gas, which is usually from 40 to 100 bar (considered "high
pressure"), thus having
the H25 adsorbed by the amine solvent. Carbon dioxide (002), aromatic
hydrocarbons and
C4+ aliphatic hydrocarbons are simultaneously adsorbed by the amine solvent
due to the high
pressure maintained during the absorption of H25. A "sweet" or purified
natural gas meeting
environmental standards is thus obtained and an amine containing most of the
contaminants
(002, H25, aromatic hydrocarbons and C4+ aliphatic hydrocarbons) is recovered.
The
contaminated amine solvent is then carried to a regeneration zone where it is
recovered
under elevated temperature (generally about 130 C) and low pressure conditions
(generally
about 2 to 3 barA). An acid gas containing CO2, H25, aromatic hydrocarbons and
C4+
aliphatic hydrocarbons is also obtained.
The presence of H25 in the acid gas obtained after purification of natural gas
remains
problematic and sulfur recovery units (SRU) are thus installed to convert
poisonous sulfur
compounds, as H25, into harmless elemental sulfur.
CA 02982686 2017-10-13
WO 2016/174120 2
PCT/EP2016/059461
A widespread method for desulfurization of H2S-containing gas streams is the
Claus
process which operates in two major process steps. The first process step is
carried out in a
furnace where hydrogen sulfide is converted to elemental sulfur and sulfur
dioxide at
temperatures of approximately 1100 to 1400 C by the combustion of about one
third of the
hydrogen sulfide in the gas stream. The so obtained sulfur dioxide reacts with
hydrogen
sulfide in the furnace to elemental sulfur by Claus reaction. Thus, in this
first step of the
Claus process, about 60 to 70% of the H2S in the feed gas are converted and
most of the
aromatic and C4+ aliphatic hydrocarbons are eliminated.
To achieve higher sulfur recovery rates, at least one catalytic step follows
where the
Claus reaction according to Eq. 1:
2 H2S + SO2 <---> 3/x Sx + 2 H20
Eq. 1
continues.
The Claus process is very well adapted to acid gas feeds containing more than
55
mol.% of H2S where the first combustion step operated at a temperature higher
than 1200 C
can be fully conducted thus converting 60 to 70% of H2S and simultaneously
destroying the
aromatic hydrocarbons and C4+ aliphatic hydrocarbons. However, recovering
sulfur from an
acid gas feed containing less than 55 mol.% of H2S applying the Claus process
happens to
be more complicated: the first combustion step cannot be conducted at
sufficiently elevated
temperatures or cannot be conducted at all due to the presence of significant
amounts of
CO2 in the feed that cools down the combustion reaction below 1100 C or even
inhibits the
combustion reaction when the content of CO2 exceeds 85%. This allows the
aromatic
hydrocarbons and C4+ aliphatic hydrocarbons to avoid combustion in the first
thermal step of
the Claus process and to pass unreacted in the catalytic step. These aromatic
hydrocarbons
and C4+ aliphatic hydrocarbons are however harmful to the installed unit
because they
deactivate the catalysts operated in the catalytic step of the Claus process.
This results in
poor sulfur recovery and frequent catalyst replacement.
Several methods have been investigated in order to remove aromatic
hydrocarbons
and C4+ aliphatic hydrocarbons from lean acid gas feeds containing less than
55 mol.% of
H25 to make them suitable for sulfur plants. For example, acid gas enrichment
(AGE)
processes where the lean acid gas (obtained from the regeneration zone
generally operated
at about 130 C and 2 to 3barA), is treated in an absorber at its pressure of 3
barA
(considered "low pressure") using a selective solvent can be performed. Due to
the "low
pressure" operated in the AGE, the solvent preferentially absorbs H25 over
CO2, and much
lower levels of aromatic hydrocarbons and C4+ aliphatic hydrocarbons. After
regeneration of
the solvent, an acid gas enriched in H25 and depleted CO2, aromatic
hydrocarbons and C4+
aliphatic hydrocarbons is obtained. AGE processes is usually selected when it
can increase
the H25 content in the acid gas over 55 vol.% allowing the obtained acid gas
to be treated
CA 02982686 2017-10-13
WO 2016/174120 3
PCT/EP2016/059461
conventionally by Claus, with treatment in a Claus furnace at temperatures
higher than
1100 C, thus eliminating the aromatic hydrocarbons and C4+ aliphatic
hydrocarbons prior to
the Claus catalytic step. Application EP2402068 for example discloses the
treatment of acid
gases with two absorption steps. In this process, the solvent enriched in H2S
obtained from
the first absorption zone is sent to a desorption zone where heat is supplied
to desorb H2S
and promote formation of H2S-enriched gas. A portion of this of H2S-enriched
gas is then
sent to another H2S absorption zone for further enrichment. AGE however can be
found
unsatisfactory when the initial concentration of H2S in the acid gas is too
low (usually less
than 20 mol.%) to reach a concentration higher than 55 mol.% of H2S after
enrichment.
Another proposed solution for aromatic and C4+ aliphatic hydrocarbons removal
is the
gas stripping process, conventionally fuel gas stripping, of a rich amine
solvent obtained from
"high pressure" sour natural gas absorber before its regeneration. The fuel
gas stream will
strip off the aromatic and C4+ aliphatic hydrocarbons of the lean acid gas and
an amine
solvent depleted in aromatic and C4+ aliphatic hydrocarbons will thus be
obtained. The fuel
gas containing the aromatic and C4+ aliphatic hydrocarbons will be used as a
combustible for
an incinerator or a utility boiler where the pollutants will be destructed.
However, in such gas
stripping processes, the removal of the aromatic and C4+ aliphatic
hydrocarbons from the rich
amine is a function of stripping fuel gas flow rate: the higher the fuel gas
flowrate, the more
removal can be achieved. The fuel gas, however, is used in the unit as a feed
for an
incinerator and/or utility boilers and, its flowrate thus remains limited by
the demand of the
incinerator or utility boilers. In order to properly remove the aromatic and
C4+ aliphatic
hydrocarbons, it could be necessary to use important amounts of fuel gases,
much higher
than what would be necessary to run the incinerator and/or utility boilers.
This would thus
result in high waste of fuel gas, particularly when the content of aromatics
and aliphatic
hydrocarbons is high in rich amine solvent obtained from "high pressure" sour
natural gas
absorber. In this context, the fuel gas stripping would not provide a
satisfactory solution to
the problem of aromatic and C4+ aliphatic hydrocarbons removal from lean acid
gas prior to
sulfur recovery.
A further option considered in industry is the adsorption of the aromatic
hydrocarbons
and C4+ aliphatic hydrocarbons from the acid gas in regenerable activated
carbon beds or
molecular sieves. Although technically feasible, these processes however
remain costly due
to the necessary regeneration cycles of the carbon beds and the difficulties
to valorize the
products issued from these regenerations due to the presence of pollutants
such as H2S.
Thus, there remains a need for a process that efficiently removes aromatic
hydrocarbons, such as benzene, toluene, ethyl benzene and xylene (BTX) and
aliphatic
hydrocarbons having 4 carbon atoms or more (C4+) from a lean acid gas
containing less than
CA 02982686 2017-10-13
WO 2016/174120 4
PCT/EP2016/059461
20 mol.% of H2S prior to sulfur recovery, when AGE cannot achieve sufficient
H2S
enrichment.
The object of the present invention a process for the removal of aromatic
hydrocarbons, such as benzene, toluene, ethyl benzene and xylene (BTX) and
aliphatic
hydrocarbons having 4 carbon atoms or more (04k) from a lean acid gas
containing CO2 and
less than 20 mol.% of H2S, which process comprises:
a) contacting the lean acid gas stream (1) with a H2S-selective liquid
absorbent
solution (29) in a first absorption zone (2) to produce a gas stream depleted
in H2S (3) and
containing 002, aromatic hydrocarbons and C4+ aliphatic hydrocarbons, and an
absorbent
solution enriched in H2S (4), also containing co-absorbed C4+ aliphatic
hydrocarbons,
aromatic hydrocarbons and 002,
b) introducing the absorbent solution enriched in H2S (4) into a non-thermic
stripping
zone (8) where it is contacted with a stripping gas stream (7), preferably
fuel gas, to obtain
an absorbent solution depleted in C4+ aliphatic hydrocarbons and aromatic
hydrocarbons (9)
and containing H2S and CO2 and a stripping gas stream enriched in aromatic
hydrocarbons
and C4+ aliphatic hydrocarbons (10), also containing H2S and 002,
c) contacting the stripping gas stream enriched in aromatic hydrocarbons and
C4+
aliphatic hydrocarbons (10), also containing H2S and CO2 obtained in step b)
with a H2S-
selective liquid absorbent solution (28) in a second absorption zone (12) to
obtain a stripping
gas stream depleted in H2S and containing aromatic hydrocarbons, C4+ aliphatic
hydrocarbons and CO2 (13), and an absorbent solution enriched in H2S (14) also
containing
co-absorbed aromatic hydrocarbons, C4+ aliphatic hydrocarbons and 002, said
H2S-selective
liquid absorbent solution being preferably identical to that used in step a),
d) introducing the absorbent solution depleted in C4+ aliphatic hydrocarbons
and
aromatic hydrocarbons (9) obtained in step b) into a desorption zone (16)
wherein the H2S-
selective liquid absorbent solution (17) is recovered and a lean acid gas
containing H2S and
002, depleted in 04+ aliphatic hydrocarbons and aromatic hydrocarbons (21) is
produced.
The invention is also directed to a process for sulfur recovery from a lean
acid gas
containing 002 and less than 20 mol.% of H2S , which process comprises:
i) pretreating the lean acid gas stream (1) for the removal of aromatic
hydrocarbons
and 04+ aliphatic hydrocarbons according to the above described process to
obtain a lean
acid gas depleted in 04+ aliphatic hydrocarbons and aromatic hydrocarbons (21)
or (26),
ii) mixing at least part of the pretreated lean acid gas depleted in 04+
aliphatic
hydrocarbons and aromatic hydrocarbons (21) or (26) with an oxygen containing
gas, for
example air, to obtain a gas stream containing both H2S and oxygen,
CA 02982686 2017-10-13
WO 2016/174120 5
PCT/EP2016/059461
iii) optionally introducing part of the obtained lean acid gas depleted in
aromatic
hydrocarbons (21) or (26) and oxygen into a furnace to recover elemental
sulfur,
iv) passing the lean acid gas depleted in C4+ aliphatic hydrocarbons and
aromatic
hydrocarbons recovered from step ii) and optionally step iii), after having
optionally being
preheated, into a catalytic reactor containing a catalyst system which
catalyzes the direct
oxidation of H2S with oxygen and/or the Claus reaction of H2S with sulfur
dioxide (SO2) so as
to recover a lean acid gas stream depleted in H2S and elemental sulfur.
Brief description of the drawings
In the following the present invention will be explained in more detail with
reference to
the figures.
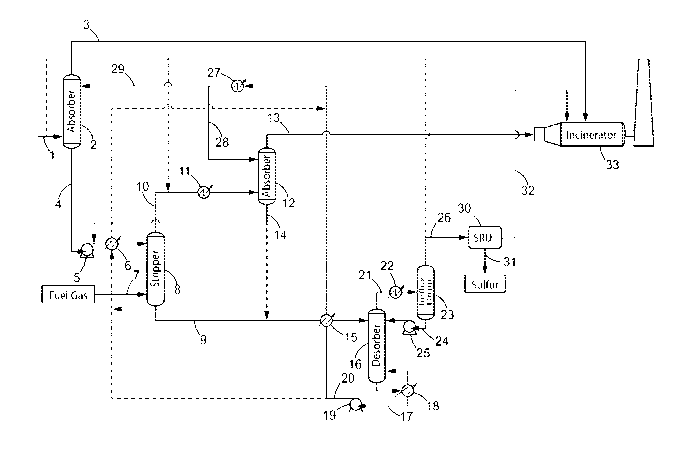
FIG. 1 schematically shows a preferred process of the present invention. The
dotted
lines represent optional embodiments of the invention.
FIG. 2 shows a specific embodiment of the process of the present invention,
operated
in the illustrative examples.
Step a
The process for the removal of aromatic hydrocarbons (BTX) from a lean acid
gas
according to the present invention comprises a first step a) of contacting the
lean acid gas
stream (1) with a H25-selective liquid absorbent solution (29) in a first
absorption zone (2) to
produce a gas stream depleted in H25 (3) and containing CO2, aromatic
hydrocarbons and
C4+ aliphatic hydrocarbons, and an absorbent solution enriched in H25 (4),
also containing
co-absorbed C4+ aliphatic hydrocarbons, aromatic hydrocarbons and CO2.
The aim of step a) is to decrease as much as possible the H25 content in the
gas feed
in order to obtain a gas stream depleted in H25 (3) suitable for combustion in
the incinerator
(33) and emission to atmosphere. The gas (3) exiting the first absorption zone
(2) is depleted
in H25 and contains CO2, aromatic hydrocarbons and C4+ aliphatic hydrocarbons.
The decrease of H25 content in the lean acid gas is obtained by adsorption of
the H25
by the H25-selective liquid absorbent solution (29). Therefore, at the bottom
of the first
absorption zone (2), a liquid absorbent solution enriched in H25 is obtained.
However, even
though the first absorption step a) is operated at quite low pressure (1 to 8
barA), parts of the
aromatic hydrocarbons and C4+ aliphatic hydrocarbons contained in the lean
acid gas (1) will
be simultaneously co-absorbed by the liquid absorbent solution (29) and will
need to be
further treated.
Within the meaning of the present invention, the lean acid gas preferably
contains:
- 75 to 99.925 mol.% of CO2,
CA 02982686 2017-10-13
WO 2016/174120 6 PCT/EP2016/059461
- 250 mol. ppm to 20 mol.% of H2S, preferably 500 mol. ppm to 15 mol.% of
H2S, more preferably 500 mol. ppm to 10 mol.% of H2S and even more
preferably 500 mol. ppm to 5 mol.% H2S,
- 500 mol. ppm to 5 mol.% of C4+ aliphatic hydrocarbons and aromatic
hydrocarbons,
the percentages being expressed on a dry basis, in moles, relative to the
total mole of
the lean acid gas. Indeed, the lean acid gas is generally saturated with
water.
In a preferred embodiment, the lean acid gas containing CO2 and less than 20
mol.%
of H2S entering the process of the invention is obtained from a natural gas
comprising
methane (CH4) and ethane (02H6), 002, H2S and C4+ aliphatic hydrocarbons and
aromatic
hydrocarbons.
Indeed, such natural gas is intended to be used as a combustible and therefore
should not contain any pollutant such as acid gas (002, H2S). Specifications
relative to H25
concentration in natural gas are very strict and its maximal concentration
should remain
below 4 ppm mol. The concentration in CO2 on the other hand should preferably
remain
below 2%, depending on the later use of the natural gas, and on the
legislation. The natural
gas thus needs to be treated to remove the acid gases (002, H25) contained
therein. A
purified natural gas meeting the standards for transport, storage and also
private or
commercial use is then obtained, but a lean acid gas containing 002, H25 and
also aromatic
hydrocarbons and 04+ aliphatic hydrocarbons is simultaneously produced. This
lean acid gas
needs to be treated prior to sulfur recovery.
Thus, in a preferred embodiment, the lean acid gas containing CO2 and less
than 20
mol.% of H25 is obtained according to a process comprising:
a) contacting natural gas comprising methane (CH4) and ethane (02H6), 002, H25
and C4+ aliphatic hydrocarbons and aromatic hydrocarbons, with a liquid
absorbent solution
in an absorption zone to produce a natural gas stream depleted in H25 and CO2
and
comprising methane (CH4) and ethane (02H6), and an absorbent solution enriched
in H25
and 002, and also containing co-absorbed C4+ aliphatic hydrocarbons and
aromatic
hydrocarbons and,
b) introducing the absorbent solution enriched in H25 and 002, and also
containing
co-absorbed C4+ aliphatic hydrocarbons and aromatic hydrocarbons into a
desorption zone
wherein the liquid absorbent solution is recovered and a lean acid gas
containing CO2 and
less than 20 mol.% of H25 is produced.
The natural gas used to obtain a lean acid gas for the purpose of the
invention
contains C4+ aliphatic hydrocarbons and aromatic hydrocarbons, and few H25
compared to
CA 02982686 2017-10-13
WO 2016/174120 7 PCT/EP2016/059461
CO2 amount (for example, the amount of CO2 being at least 4 times higher than
the amount
of H2S.
Step a) can preferably be operated:
- at a temperature ranging from 50 and 200 C, preferably from 110 and 145 C
and,
- at a pressure ranging from 1 barA to 8 barA and preferably from1.5 to 3
barA,
The H25-selective liquid absorbent solution can be any of the known absorbents
conventionally used by the skilled person, such as chemical solvents, physical
solvents and
mixtures thereof. When a chemical solvent is used as liquid absorbent
solution, it may be
associated with a physical solvent to enhance the absorption of the
contaminants commonly
found in the lean acid gas streams.
Chemical solvents can for example include alkali metal carbonate and
phosphate, or
alkanolamines, preferably in the form of aqueous solutions.
Alkanolamines are preferably chosen from tertiary alkanolamines and sterically
hindered alkanolamines. The sterically hindered alkanolamine can be selected
from the
group consisting of 2-amino-2-methylpropanol, 2-amino-2-methyl-1,3-
propanediol, 2-amino-
2-hydroxymethy1-1,3-propanediol, 2-amino-2-ethyl-1,3-propanediol,
2-hydroxymethyl
piperidine, 2-(2-hydroxyethyl) piperidine, 3-amino-3-methyl-1-butanol and
mixtures thereof.
Suitable alkanolamines include methyldiethanolamine (MDEA), triethanolamine,
or
one or more dipropanolamines, such as di-n-propanolamine or
diisopropanolamine.
Physical solvents can for example include a substituted or unsubstituted
tetramethylene sulfone or thioglycols,
In a preferred embodiment, the H25-selective liquid absorbent solution
contains an
amine, preferably an alkanolamine, more preferably a tertiary alkanolamine or
sterically
hindered alkanolamine, and even more preferably methyldiethanolamine (MDEA).
Aqueous
methyldiethanolamine (MDEA) solutions are preferred liquid absorbent solution
according to
the invention.
In another preferred embodiment, the H25-selective liquid absorbent solution
can be a
mixture of alkanolamines and thioglycols.
Additives components capable of enhancing the selectivity of H25 adsorption
towards
CO2 such as acidic components like phosphoric acid (H3PO4) can also be
introduced in the
liquid absorbent solution.
Concentrations of aqueous alkanolamine solutions may vary widely, and those
skilled
in the art can adjust solution concentrations to achieve suitable absorption
levels. In general,
the concentration of alkanolamine in aqueous solutions will be from 5 to 60%
by weight, and
preferably between 25 and 50% by weight. If a physical solvent is employed as
a component
CA 02982686 2017-10-13
WO 2016/174120 8
PCT/EP2016/059461
of the absorbent liquid, it can be present in an amount from 2 to 50% by
weight, preferably
from 5 to 45% by weight.
The absorption step a) is preferably conducted:
- at a temperature ranging from 10 to 100 C, preferably 30 to 70 C and more
preferably 40 to 60 C, and
- at a pressure ranging from 1 to 8 barA, preferably 1,5 to 4 barA.
The gas stream depleted in H2S (3) exiting the first absorption zone (2)
preferably
contains 002, aromatic hydrocarbons and C4+ aliphatic hydrocarbons, and in
particular:
-60 to 99 mol.% of the CO2 contained in the lean acid gas (1) and more
preferably 80
to 98 mol.%,
- 60 to 99% of aromatic hydrocarbons and C4+ aliphatic hydrocarbons
contained in the
lean acid gas (1) and more preferably 80 to 98 mol.%,
- 0.001 to 20 mol. % of H2S contained in the lean acid gas (1) and more
preferably
0.002 to 5 mol.%.
The gas stream depleted in H2S (3) exiting the first absorption zone (2) can
then be
transferred to an incinerator (33) where it will be combusted to destruct
remaining H2S as
well as the aromatic hydrocarbons and C4+ aliphatic hydrocarbons contained
therein, thus
reaching the standards requirements for air emission. Alternatively gas stream
depleted in
H2S (3) can be compressed, injected and disposed in an underground storage
reservoir
rather than being incinerated and released to the atmosphere.
The absorbent solution enriched in H2S (4) exiting the first absorption zone
(2) also
contains co-absorbed C4+ aliphatic hydrocarbons, aromatic hydrocarbons and
002. In a
preferred embodiment, the absorbent solution enriched in H2S (4) exiting the
first absorption
zone (2) contains:
- 80 to 99.999 mol.% of the H2S contained in the lean acid gas (1), and
more
preferably 95 to 99,99 mol.%,
- 0.5 to 40 mol.% of the CO2 contained in the lean acid gas (1), more
preferably
1 to 15 mol.%,
- 0.5 to 40 mol.% of the C4+ aliphatic hydrocarbons and aromatic
hydrocarbons
contained in the lean acid gas (1), more preferably 1 to 10 mol.%.
Step b
The absorbent solution enriched in H25 (4) exiting the first absorption zone
(2) is then
sent to a non-thermic stripping zone (8) where it is contacted with a
stripping gas stream (7),
preferably fuel gas, to obtain an absorbent solution depleted in C4+ aliphatic
hydrocarbons
CA 02982686 2017-10-13
WO 2016/174120 9
PCT/EP2016/059461
and aromatic hydrocarbons (9) and containing H2S and CO2 and a stripping gas
stream
enriched in aromatic hydrocarbons and C4+ aliphatic hydrocarbons (10), also
containing H2S
and 002.
Indeed, further to the first absorption step a), the absorbent solution
enriched in H2S
(4) also contains co-absorbed C4+ aliphatic hydrocarbons and aromatic
hydrocarbons.
The aim of step b) is thus to remove as much C4+ aliphatic hydrocarbons and
aromatic hydrocarbons as possible from the absorbent solution, with as few H2S
as possible,
so that when the lean acid gas is recovered, it does not contain high
concentration of
impurities capable of poisoning the sulfur recovery unit catalysts. This is
done by contacting
the absorbent solution enriched in H2S (4) with a countercurrent of stripping
gas, such as fuel
gas stream (7), in a non-thermic stripping step.
Prior art stripping steps are conventionally operated either simply by heating
the
absorbent solution enriched in H2S to produce steam as stripping stream, or by
injecting
directly stream in the stripping zone. The provision of heat to the stripping
zone increases the
chemical desorption of acid gas, in particular H2S, from the absorbent
solution and favors its
removal with the stripping stream. An absorbent solution substantially
depleted in H2S is
therefore obtained with the conventional stripping steps of the prior art. To
the contrary, the
stripping step of the claimed process is athermic in the sense that no
significant heat or
energy is provided to the process at this stage. By operating the stripping
step with no
significant heating, it was possible to more selectively strip the C4+
aliphatic hydrocarbons
and aromatic hydrocarbons over H2S in order to obtain an absorbent solution
depleted in C4+
aliphatic hydrocarbons and aromatic hydrocarbons (9) and containing H2S and
CO2 and a
stripping gas stream enriched in aromatic hydrocarbons and C4+ aliphatic
hydrocarbons (10),
also containing a small quantity of H2S but significantly less than what would
be obtained
with a thermal stripping step.
The obtained absorbent solution (9) is depleted in C4+ aliphatic hydrocarbons
and
aromatic hydrocarbons. The stripping gas stream (7) will preferentially strip
off the C4+
aliphatic hydrocarbons and aromatic hydrocarbons over H2S from the absorbent
solution
enriched in H2S (4), but will however also drag away part of the H2S and CO2
contained in
the absorbent solution (4). The stripping gas stream exiting the stripping
zone (8) is thus
enriched in aromatic hydrocarbons and C4+ aliphatic hydrocarbons (10) but also
contains H2S
and 002.
The stripping gas used in the stripping zone of the process of the invention
can
preferably be a fuel gas stream, but may also be any combustible gas meeting
standards
requirements for a combustible, for example natural gas, hydrogen, and/or
synthetic gas
containing mostly H2 and CO, or any other inert gas containing mainly nitrogen
or helium for
CA 02982686 2017-10-13
WO 2016/174120 10
PCT/EP2016/059461
example. The fuel gas, or any combustible gas, used in the stripping zone can
thus be used
as a feed/combustible in the incinerator (33) and/or in the utility boilers.
In a preferred embodiment, the stripping gas is a fuel gas and, preferably,
the fuel gas
(7) used in the stripping zone (8) is the combustible gas used to run the
incinerator (33)
and/or utility boilers in the unit. Indeed, the plant where the process of the
invention is
conducted generally comprises incinerators and/or utility boilers for various
purposes. Said
incinerators and boilers have to be fed with fuel gas. One advantage of the
present invention
is that the fuel gas that is needed to feed the incinerators and/or utility
boilers of the plant is
used first as a stripping gas, and then recovery and rerouted to its original
path to feed the
incinerators and/or utility boilers. The utility boilers (not shown in the
figure) produce steam
which could feed boilers of the plant where the claimed process is conducted,
as for example
the boiler (18) in figure 1. The fuel gas flow rate in the stripping zone is
limited by the
combustible gas flow rate necessary to run the incinerator (33) and/or the
utility boilers. In
this embodiment, the fuel gas is used consecutively as a stripping gas in the
stripping zone
(8) and as a combustible gas to run the incinerator and/or the utility
boilers, which is
economically advantageous.
In this stripping step, the stripping gas stream (7) is preferably introduced
at the
bottom of the stripping zone in order to be contacted with a countercurrent of
absorbent
solution enriched in H25 (4).
The stripping in step b) is preferably conducted:
- at a temperature ranging from 50 to 150 C, preferably 60 to 130 C and
more
preferably 70 to 110 C, and
- at a pressure ranging from 1 to 8 barA, preferably 1,5 to 4 barA.
In order for the absorbent solution enriched in H25 (4) to meet pressure
conditions
required in the stripping zone, it may be necessary to pass it though a pump
(5) or
alternatively though a valve before it enters the stripping zone.
In some cases where the installation is restricted, for example in terms of
flow rate of
the stripping gas stream, it may be interesting that the absorbent solution
enriched in H25 (4)
also passes through a heater (6) to increase its temperature before entering
the stripping
zone (8) to efficiently remove the aromatic hydrocarbons and C4+ aliphatic
hydrocarbons. In
this embodiment, the heat provided to the stripping step should be controlled
to increase the
removal of aromatic hydrocarbons and C4+ aliphatic hydrocarbons while ensuring
that only a
minimal amount of H25 is desorbed from the absorbent solution to avoid
obtaining a stripping
gas stream (7) that would be enriched with H25. Indeed, in such case where the
stripping gas
stream (7) is enriched with H25, an increase of the size of the second
absorption zone will be
needed to ensure complete removal of H25. In a preferred embodiment, the
temperature
increase in the heater (6) may be obtained by recirculating in the heater (6),
at least a part of
CA 02982686 2017-10-13
WO 2016/174120 11
PCT/EP2016/059461
the H2S-selective liquid absorbent solution (17) recovered from the desorption
zone (16),
and/or exiting the heat exchanger (heater 15). The H2S-selective liquid
absorbent solution
thus acts as a heating source for the heater (6).
As mentioned above, the stripping gas stream (10) exiting the stripping zone
(8) is
enriched in aromatic hydrocarbons and 04+ aliphatic hydrocarbons but also
contains H2S and
002. It preferably contains:
- 50 to 99 mol.%, preferably 85 a 99 mol. % of the aromatic hydrocarbons
and
C4+ aliphatic hydrocarbons contained in the absorbent solution (4) entering
the
stripping zone (8),
- 5 to 40 mol.%, preferably 5 a 20 mol. % of the CO2 contained in the
absorbent
solution (4) entering the stripping zone (8), and
- 1 to 20 mol.%, preferably 1 to 10 mol.% of the H2S contained in the
absorbent
solution (4) entering the stripping zone (8).
The absorbent solution (9) exiting the stripping zone (8) is depleted in C4+
aliphatic
hydrocarbons and aromatic hydrocarbons, and preferably contains:
- 80 to 99 mol.%, preferably 90 to 99 mol.% of the H2S contained in the
absorbent solution (4) entering the stripping zone (8)
- 1 to 50 mol.%, preferably 1 a 15 mol. % of the aromatic hydrocarbons and
C4+
aliphatic hydrocarbons contained in the absorbent solution (4) entering the
stripping zone (8), and
- 60 to 95 mol.%, preferably 70 to 90 mol. % of the CO2 contained in the
absorbent solution (4) entering the stripping zone (8).
In a preferred embodiment, the absorbent solution (9) exiting the stripping
zone (8)
contains 0.01 to 10 mol.% of the aromatic hydrocarbons and C4+ aliphatic
hydrocarbons
contained in the lean acid gas (1), more preferably 0.1 to 5 mol.%.
Step c
The stripping gas stream enriched in aromatic hydrocarbons and C4+ aliphatic
hydrocarbons (10), also containing H25 and CO2 obtained in step b) is
contacted with a H25-
selective liquid absorbent solution (28) in a second absorption zone (12) to
obtain a stripping
gas stream depleted in H25 and containing aromatic hydrocarbons, C4+ aliphatic
hydrocarbons and CO2 (13), and an absorbent solution enriched in H25 (14) also
containing
co-absorbed aromatic hydrocarbons, C4+ aliphatic hydrocarbons and 002, said
H25-selective
liquid absorbent solution preferably being identical to that used in step a).
In particular, more than 80% of the stripping gas stream enriched in aromatic
hydrocarbons and 04+ aliphatic hydrocarbons (10), preferably more than 90% and
more
CA 02982686 2017-10-13
WO 2016/174120 12
PCT/EP2016/059461
preferably all the stripping gas stream (10) obtained in step b) is sent to
the second
absorption zone (12).
Indeed, further to the stripping step b), the stripping gas stream is enriched
in
aromatic hydrocarbons and 04+ aliphatic hydrocarbons (10) but also contains
H2S and 002.
The aim of step c) is thus to remove as much H2S as possible from the
stripping gas
stream (10) so as to recover a stripping gas stream suitable for further use
as a combustible,
such as fuel gas for the incinerator (33) and/or the utility boilers.
Alternatively, the stripping
gas stream depleted in H2S can be compressed, injected and disposed in an
underground
storage reservoir rather than being incinerated and released to the
atmosphere. This is done
by contacting the stripping gas stream enriched in aromatic hydrocarbons and
C4+ aliphatic
hydrocarbons (10) with a countercurrent of H2S-selective liquid absorbent
solution (28), said
H2S-selective liquid absorbent solution being preferably the one used in step
a).
The H2S-selective liquid absorbent solution (28) may for example be obtained
by
derivation of the main solvent stream (29) entering the first absorption zone.
The conditions of temperature and pressure operated in the second absorption
zone
(12) are preferably the same as those previously disclosed for the first
absorption zone in
step a).
Optionally, in order for the stripping gas stream enriched in aromatic
hydrocarbons
and C4+ aliphatic hydrocarbons (10) but also containing H2S and 002, to meet
temperature
conditions required in the second absorption zone (12), it may be necessary to
pass it though
a cooler (11) and, optionally, a separator to recover condensed water, before
it enters the
second absorption zone (12).
The stripping gas stream (13) exiting the second absorption zone (12)
preferably
contains aromatic hydrocarbons, C4+ aliphatic hydrocarbons and 002, and in
particular:
- 60 to 99 mol.% of the CO2 contained in the stripping gas stream (10) exiting
the stripping zone (8), more preferably 80 to 98 mol.%
- 60 to 99 mol.% of the aromatic hydrocarbons (BTX) and C4+ aliphatic
hydrocarbons contained in the stripping gas stream (10) exiting the stripping
zone (8), more preferably 80 to 98 mol.% and
- 0.01 to 20 mol.% of H2S contained in the stripping gas stream (10) exiting
the
stripping zone (8), more preferably 0.02 to 5 mol.%.
The stripping gas stream depleted in H2S and containing aromatic hydrocarbons,
04+
aliphatic hydrocarbons and CO2 (13) exiting the second absorption zone (12)
meets
standards requirements as a combustible, such as fuel gas, and can thus be
used as a feed
in the incinerator (33) and/or in the utility boilers where associated
aromatic hydrocarbons
(BTX) and 04+ aliphatic hydrocarbons, as well as remaining sulfur species,
will be destructed.
Alternatively, the stripping gas stream depleted in H2S (13) can be
compressed, injected and
CA 02982686 2017-10-13
WO 2016/174120 13
PCT/EP2016/059461
disposed in an underground storage reservoir rather than being incinerated and
released to
the atmosphere.
The absorbent solution enriched in H2S (14) exiting the second absorption zone
(12)
also contains co-absorbed aromatic hydrocarbons (BTX), C4+ aliphatic
hydrocarbons and
002.
In a preferred embodiment, the absorbent solution enriched in H2S (14) exiting
the
second absorption zone (12) contains:
- 1 to 40 mol.% of the CO2 contained in the stripping gas stream (10)
exiting the
stripping zone (8), more preferably 2 to 20 mol.%
- 1 to 40 mol.% of the aromatic hydrocarbons (BTX) and C4+ aliphatic
hydrocarbons contained in the stripping gas stream (10) exiting the stripping
zone (8), more preferably 2 to 20 mol.% and
- 80 to 99.99 mol.% of H2S contained in the stripping gas stream (10)
exiting the
stripping zone (8), more preferably 95 to 99.98 mol.%.
Depending on the amount of aromatic hydrocarbons (BTX), C4+ aliphatic
hydrocarbons co-absorbed therein, the absorbent solution enriched in H2S (14)
exiting the
second absorption zone (12) can be recycled back to the stripping zone (8) to
supplement
the absorbent solution enriched in H2S (4) and/or can be directly introduced
into the
desorption zone (16) to supplement the absorbent solution depleted in aromatic
hydrocarbons (9) obtained in step b).
In a preferred embodiment, the absorbent solution enriched in H2S (14) exiting
the
second absorption zone (12) is entirely recycled to the stripping zone to
supplement the
absorbent solution enriched in H2S (4) in order to decrease aromatic (BTX) and
C4+ aliphatic
hydrocarbons content in the absorbent solution enriched in H2S (9) sent to the
desorption
zone (16).
The stripping zone (8) and the second absorption zone (12) are designed to
significantly reduce the amount of aromatic hydrocarbons (BTX) and C4+
aliphatic
hydrocarbons in the absorbent solution depleted in H2S entering the desorption
zone (16) in
comparison to their amount in the absorbent solution enriched in H2S (4)
exiting the first
absorption zone and even more in comparison with their initial amount in the
lean acid gas
stream (1).
Step d
The absorbent solution depleted in C4+ aliphatic hydrocarbons and aromatic
hydrocarbons (9) exiting the stripping zone (8), optionally supplemented with
the absorbent
solution enriched in H25 (14) exiting the second absorption zone (12), is
further introduced
into a desorption zone (16) wherein the H25-selective liquid absorbent
solution (17) is
CA 02982686 2017-10-13
WO 2016/174120 14
PCT/EP2016/059461
recovered and a lean acid gas containing H2S and 002, depleted in C4+
aliphatic
hydrocarbons and aromatic hydrocarbons (21) is produced.
Indeed, the absorbent solution exiting the stripping step b) and the second
absorption
step c) are depleted in C4+ aliphatic hydrocarbons and aromatic hydrocarbons
(9) but still
contain H2S and 002.
The aim of step d) is thus to desorb as much H2S and CO2 as possible from the
absorbent solution (9) so as to recover a purified absorbent solution that can
be recycled
back to the first and/or second absorption zones. This is done by heating the
absorbent
solution (9) in a desorption zone (16).
The desorption step d) is preferably conducted:
- at a temperature ranging from 50 to 200 C, preferably 70 to 180 C and
more
preferably 110 to 145 C, and
- at a pressure ranging from 1 to 4 barA, preferably 1,5 to 3 barA.
In a preferred embodiment, the absorbent solution depleted in C4+ aliphatic
hydrocarbons and aromatic hydrocarbons (9) recovered from the stripping zone
(8) may also
pass through a heater (15) to increase its temperature before entering the
desorption zone in
order to reduce the energy consumption for steam circulation in the desorption
zone. The
temperature increase in the heater (15) can preferably be obtained by
recirculating in the
heater (15), at least a part of the regenerated liquid absorbent solution (17)
recovered from
the desorption zone (16). The regenerated liquid absorbent solution (17) thus
acts as a
heating medium for the heater (15).
A steam is generated in the desorption zone (16) thus providing the energy
necessary
to remove H2S, 002, hydrocarbons, and aromatics such as BTX from the absorbent
solution.
The steam may be produced by heat exchange with the liquid absorbent solution
present in
the bottom of the desorption zone (16) through any heating means (steam, hot
oil, furnace,
burner, boiler).
The desorption zone (16) can thus preferably comprise a boiler (18) at its
bottom in
which steam circulates in order to permit the regeneration of the absorbent
solution enriched
in H2S.
The regenerated liquid absorbent solution (17) leaving the bottom of the
desorption
zone (16) may then be sent back to the first adsorption zone (2) as the H2S-
selective liquid
absorbent solution (29) and/or to the second adsorption zone (12) as the H2S-
selective liquid
absorbent solution (28).
In order for the regenerated liquid absorbent solution (17) to meet
temperature and
pressure conditions required in the first and second absorption zones (2) and
(12), it may be
CA 02982686 2017-10-13
WO 2016/174120 15
PCT/EP2016/059461
necessary to pass it though heat exchanger (27) and pump (19) or alternatively
though a
valve before it enters the absorption zones.
The lean gas (21) exiting the desorption zone (16) further contains steam and
vaporized absorbent solution. The water issued from the steam and the
vaporized absorbent
solution carried with the lean gas (21) exiting the desorption zone (16) can
be partially
separated from the lean acid gas depleted in aromatic hydrocarbons (21) in the
condenser
(22) and further trapped in the reflux drum (23) which acts as an accumulator.
The water and
the absorbent solution can then be recycled to the desorption zone (16) though
a pump (25)
in order to limit water and absorbent solution loss. A lean acid gas depleted
in aromatic
hydrocarbons (26) is recovered. The condenser is preferably operated at a
temperature
ranging from more preferably from 20 to 70 C and even more preferably from 40
to 60 C.
The lean acid gas (21) or (26) is depleted in aromatic hydrocarbons and C4+
aliphatic
hydrocarbons and preferably contains 0.01 to 10 mol.% of the aromatic
hydrocarbons (BTX)
and C4+ aliphatic hydrocarbons contained in the lean acid gas entering the
process, more
preferably 0.1 to 5 mol.%.
In addition, the lean acid gas depleted in aromatic hydrocarbons (21) or (26)
recovered at the end of the process of the invention has preferably a H2S/CO2
ratio higher
than the H2S/CO2 ratio of the lean acid gas (1) entering the process.
In a preferred embodiment, the lean acid gas depleted in aromatic hydrocarbons
(21)
or (26) recovered after the desorption zone (16) may be partially recycled to
supplement the
lean acid gas stream (1) entering the process, and/or to supplement the
stripping gas stream
enriched in aromatic hydrocarbons and C4+ aliphatic hydrocarbons (10) but also
containing
H2S and 002.
It is of the merit of the inventors to have discovered that the specific
succession of
absorption and stripping steps according to the present invention made it
possible to remove
high amounts of aromatic hydrocarbons such as BTX and C4+ aliphatic
hydrocarbons from
lean acid gases containing less than 20 mol.% of H2S although none of these
steps
conducted separately was considered sufficient to fulfil this goal. Contrary
to the skilled
person's expectations, the lean acid gas depleted in aromatic hydrocarbons and
C4+ aliphatic
hydrocarbons produced is suitable for a subsequent sulfur recovery treatment
because even
if it is not enriched in H2S to reach a proportion of more than 55 mol.%,
which would be
necessary to be properly operated in a Claus furnace (first step of sulfur
recovery), it
contains a sufficiently low amount of aromatic hydrocarbons such as BTX and
C4+ aliphatic
hydrocarbons to allow its use in a sulfur recovery unit operating with partial
by-pass of the
furnace or even no thermal step at all. In a preferred embodiment, the
stripping gas stream
CA 02982686 2017-10-13
WO 2016/174120 16
PCT/EP2016/059461
(7) is a combustible and its flow rate is adapted to the incinerator (33) and
/ or utility boilers
needs.
In a preferred embodiment, the content of aromatic hydrocarbons, such as
benzene,
toluene, ethylbenzene and xylene (BTX) and C4+ aliphatic hydrocarbons in the
lean acid gas
(21) or (26) recovered at the end of the process of the invention should be as
low as possible
and no higher than 500 mol.ppm, preferably between 1 and 500 mol. ppm, in
order to
prevent Claus catalyst deactivation in a further sulfur recovery unit.
Process for sulfur recovery from a lean acid gas containing less than 20 mol.%
of HS
The obtained lean acid gas depleted in aromatic hydrocarbons (21) or (26) is
suitable
for use as a feed in a sulfur recovery unit (30).
Therefore another object of the invention is a process for sulfur recovery
from a lean
acid gas containing CO2 and less than 20 mol.% of H2S , which process
comprises :
i) pretreating the lean acid gas stream (1) for the removal of aromatic
hydrocarbons
and C4+ aliphatic hydrocarbons according to the process previously described
to obtain a
lean acid gas depleted in C4+ aliphatic hydrocarbons and aromatic hydrocarbons
(21) or (26),
ii) mixing at least part of the pretreated lean acid gas depleted in C4+
aliphatic
hydrocarbons and aromatic hydrocarbons (21) or (26) with an oxygen containing
gas, for
example air, to obtain a gas stream containing both H2S and oxygen,
iii) optionally introducing part of the obtained lean acid gas depleted in
aromatic
hydrocarbons (21) or (26) and oxygen into a furnace to recover elemental
sulfur,
iv) passing the lean acid gas depleted in C4+ aliphatic hydrocarbons and
aromatic
hydrocarbons recovered from step ii) and optionally step iii), after having
optionally being
preheated, into a catalytic reactor containing a catalyst system which
catalyzes the direct
oxidation of H2S with oxygen and/or the Claus reaction of H2S with sulfur
dioxide (SO2) so as
to recover a lean acid gas stream depleted in H2S (32) and elemental sulfur.
Usually, the elemental sulfur is recovered in a condenser.
Step iv) may preferably be repeated several times, more preferably at least
twice.
The process for sulfur recovery will be easily adapted by the skilled person
depending
on the content of H25 in the lean acid gas depleted in C4+ aliphatic
hydrocarbons and
aromatic hydrocarbons (21) or (26) obtained after the pretreating step i).
When the content of H25 in the lean acid gas depleted in C4+ aliphatic
hydrocarbons
and aromatic hydrocarbons (21) or (26) is below 15 mol.%, the sulfur recovery
process can
preferably be only a catalytic direct oxidation process (without thermal step
iii)). In this case,
CA 02982686 2017-10-13
WO 2016/174120 17
PCT/EP2016/059461
the lean acid gas depleted in C4+ aliphatic hydrocarbons and aromatic
hydrocarbons (21) or
(26) may be preheated before entering the catalytic reactor.
When the content of H2S in the lean acid gas depleted in C4+ aliphatic
hydrocarbons
and aromatic hydrocarbons (21) or (26) is ranging between 15 and 55 mol.%, a
conventional
Claus process coupling thermal step iii) and catalytic Claus step iv) can be
operated. In such
case, generally only a part of the lean acid gas depleted in C4+ aliphatic
hydrocarbons and
aromatic hydrocarbons (21) or (26) is sent to the furnace and the rest of the
lean acid gas by-
passes the burner of the furnace to directly undergo oxidation in the furnace.
This partial by-
pass of the burner of the thermal step iii) is needed to maintain a stable
flame in the burner
considering the high content of inert gas such as CO2 and/or N2, in the lean
acid gas.
Alternatively and preferably, catalytic direct oxidation process can be
performed
isothermally or pseudo-isothermally with the help of internal cooler, such as
thermoplates like
the SmartSulfTM technology. Such technology is advantageous for catalytic
reactor following
a Claus thermal step (iii)), and even more advantageous in the case of direct
oxidation
without Claus thermal step (iii)), as it could be considered in the case of a
content of H25
below 15 mol.% in the lean acid gas (21) or (26).
The SmartSulfTM technology is disclosed in details in document U52013/0129589,
the
entire content of which is hereby incorporated by reference.
According to a preferred embodiment, step iv) of the process for sulfur
recovery from
a lean acid gas containing CO2 and less than 20 mol.% of H25, includes and/or
is followed
by:
iv.1 transferring the lean acid gas stream containing both H25 and oxygen into
a
first section of a first reactor, after having optionally being preheated,
which
first section contains a non-cooled adiabatic bed containing a first catalyst
which catalyzes the oxidation of H25 with oxygen and the oxidation of H25 with
sulfur dioxide, wherein the maximum temperature of the adiabatic bed is Ti,
iv.2transferring the lean acid gas stream from the first section of the first
reactor to
a second section of the first reactor, which second section contains a second
catalyst which can be different from the first catalyst and which second
section
is kept at a temperature T2 wherein T2 T1 and T2 is higher than the dew
point temperature of elemental sulfur, whereby a gas stream depleted in H25
is obtained,
iv.3 transferring the gas stream depleted in H25 to a sulfur condenser to
obtain a
gas stream depleted in sulfur,
iv.4 optionally preheating the gas stream depleted in sulfur
CA 02982686 2017-10-13
WO 2016/174120 18
PCT/EP2016/059461
iv.5transferring the gas stream depleted in sulfur into the first section of a
second
reactor, which first section contains a non-cooled adiabatic bed containing
the
same catalyst as the first section of the first reactor, wherein the first
section of
the second reactor is operated at a temperature that is above the dew point of
the elemental sulfur so that in the first section of the second reactor no
elemental sulfur deposits as liquid or solid on the catalyst,
iv.6transferring the gas stream from the first section of the second reactor
to the
second section of the second reactor which contains the same catalyst as the
second section of first reactor and which second section is kept at a
temperature that is at or below the dew point of elemental sulfur so that in
the
second section of the second reactor elemental sulfur deposits as liquid or
solid on the catalyst, and desulfurized gas stream meeting standard
requirements for air emissions is obtained,
iv.7 after a defined time switching the operation conditions of the first
reactor and
the second reactor and switching the gas flow simultaneously so that the
previous second reactor becomes the new first reactor and the previous first
reactor becomes the new second reactor.
In this preferred embodiment, in steps iv.2) and iv.6), the second section of
the
reactors can be kept at a temperature that is at or below the dew point of
elemental sulfur
with the help of an internal cooler such as thermoplates.
In this process, steps iv.1 to iv.7 correspond to the SmartSulfTM technology.
Step iv.7 of switching the operation conditions of the first reactor and the
second
reactor and switching the gas flow simultaneously makes it possible to desorb
the elemental
sulfur condensed on the catalyst operated in the second reactor. Indeed, when
operated in
the first place (at higher temperature), the second reactor is run at higher
temperatures thus
desorbing the sulfur that condensed on the catalyst when the reactor was
previously
operated in a second place (at lower temperature).
The lean acid gas stream depleted in H25 (32) exiting the sulfur recovery unit
in step
iv) can then be transferred to an incinerator (33) where it will be combusted
to destruct
remaining H25 as well as the aromatic hydrocarbons and C4+ aliphatic
hydrocarbons
contained therein, thus reaching the standards requirements for air emission.
Alternatively
gas stream depleted in H25 (3) can be compressed, injected and disposed in an
underground storage reservoir rather than being incinerated and released to
the atmosphere.
The present invention will be further illustrated in the following non-
limiting examples.
CA 02982686 2017-10-13
WO 2016/174120 19
PCT/EP2016/059461
EXAMPLE 1
A process for sulfur recovery from a lean acid gas (1) as illustrated in Fig.
2 was
operated using an acid gas containing:
o 10.0 mol.% of H2S,
O 82.1 mol.% of 002, and
O 2500 mol. ppm of BTX
O 180 mol. ppm of C4+
O 7.3 mol % of water, the rest being other hydrocarbons such as methane,
ethane and
propane as well as sulfur species such as mercaptans.
This lean acid gas was sent to a first absorption zone (2) at a flow rate of
2800 kmol/h
at a pressure of 1.7 bar. In this first absorption zone (2), the lean acid gas
contacted a 45
wt% (11 mol.%) methyldiethanolamine (MDEA) aqueous solution (29), introduced
at a flow
rate of 480 m3/h, at a temperature of 45 C and at a pressure of 1.55 barA.
The gas stream (3) exiting the first absorption zone (2) at a flow rate of
2145 kmol/h
contained:
O 92.8 mol% of CO2
O <100 ppm mol of H2S, and
O 3000 ppm mol of BTX (representing 92% of the initial amount of BTX in the
lean acid
gas).
O 230 ppm C4+ (representing the entire initial amount of C4+ in the lean
acid gas).
O 6.4 mol % of water, the rest being other hydrocarbons such as methane,
ethane and
propane as well as sulfur species such as mercaptans.
The MDEA solution (4) exiting the first absorption zone (2) absorbs almost the
entire
amount of H2S of the lean acid gas and co-absorbs about 8% of the BTX
initially present in
the lean acid gas. The solvent reaches a temperature of about 62 C.
The MDEA solution (4) exiting the first absorption zone (2) then passed
through a
pump (5) and through a heater (6) to increase its temperature and pressure in
order to enter
the stripping zone (8) at a temperature of 92.0 C and at a pressure of 5 barA.
As can be seen from Fig.2, the temperature increase in the heater (6) was
obtained
by recirculating in the heater (6) the MDEA solution (20) recovered from the
desorption zone
(16).
The heated MDEA solution entered the stripping zone (8) where it was contacted
with
a countercurrent of a natural gas stream (7) introduced at the bottom of the
stripping zone.
The natural gas stream (7) had the following specifications:
O 95.0 mol.% of methane
O 5.0 mol.% of ethane
CA 02982686 2017-10-13
WO 2016/174120 20
PCT/EP2016/059461
It entered the stripping zone in the following conditions:
O Flow rate : 200 kmol/h
O Temperature: 15 C
O Pressure: 7.0 barA.
The stripper was operated at a pressure of 5.0 barA.
The fuel gas stream (10) exiting the stripping zone (8) had the following
specifications:
O Flow rate: 318 kmol/h
O Temperature: 90 C
0 18.5 mol.% of CO2
O 6.4 mol.% of H2S, and
O 1275 mol. ppm of BTX (65% of the BTX entering the stripping zone).
This fuel gas stream (10) exiting the stripping zone (8) was then passed
through a
heat exchanger and entered a second adsorption zone (12) at a temperature of
45 C.
In the second adsorption zone (12) the fuel gas stream (10) exiting the
stripping zone (8)
contacted a methyldiethanolamine (MDEA) solution (28) introduced at a flow
rate of 30 m3/h,
at a temperature of 45 C and a pressure of 4.0 barA.
The methyldiethanolamine (MDEA) solution is the same as the one used in the
first
absorption zone (2).
A fuel gas stream (13) exited the second absorption zone (12) at a flow rate
of 255
kmol/h with the following composition:
O 19.6 mol.% of CO2
O <100 mol. ppm of H2S, and
O 1 350 mol. ppm of BTX (85% of the BTX entering the second absorption zone
in stream 16 and about 5% of the BTX entering the process in stream 1).
The fuel gas stream (13) meets standards requirements for a combustible and
can
thus be used as a feed in the incinerator (33) and/or in the utility boilers
where associated
aromatic hydrocarbons (BTX) and C4+ aliphatic hydrocarbons, as well as
remaining sulfur
species, will be destructed.
The MDEA solution enriched in H2S (14) exiting the second absorption zone (12)
was
recycled to the stripping zone to supplement the MDEA solution enriched in H2S
(4).
An MDEA solution depleted in BTX (9) exited the stripping zone (8) at a
temperature
of 87 C and contained 35% of the BTX entering the stripping zone (a flow
corresponding to
about 3% of the flow of BTX entering the process).
This MDEA solution depleted in BTX (9) was then introduced into a desorption
zone
(16) equipped with a boiler (18) operating at a temperature of 130 C and a
pressure of 2.4
barA.
CA 02982686 2017-10-13
WO 2016/174120 21
PCT/EP2016/059461
The regenerated MDEA solution (17) leaving the bottom of the desorption zone
(16)
was sent back to the first adsorption zone (2) and to the second adsorption
zone (12).
The lean acid gas depleted in BTX (21) exiting the desorption zone (16) was
passed
through a condenser (22) and a reflux drum (23).
The lean acid gas depleted in BTX (26) recovered at the end of the process of
the
invention had a temperature of 45 C and a flow rate of 570kmol/h. It had the
following
composition:
O 45.5 mol.% of CO2
O 49.2 mol.% of H2S,
0 390 mol. ppm of BTX (3% of the BTX entering the process), and
O 4.9 mol.% of H20.
The process of the present invention made it possible to decrease the BTX
content of
the lean acid gas treated of 97%. The treated lean acid gas was then suitable
for a
subsequent treatment in a sulfur recovery unit even with an H2S content lower
than 55mol%.
The obtained acid gas was then treated in a Claus process with 10% of the flow
of
acid gas by-passing the thermal step (furnace), and 2 reactors (SmartSulfTM
technology)
being used to operate the catalytic step. A sulfur recovery rate of 99.3% was
obtained. 107
tons per day of bright yellow solid sulfur, reaching standards for sulfur
recovery were
recovered without further treatment.
EXAMPLE 2
A process for sulfur recovery from a lean acid gas as illustrated in Fig. 2
was
operated using an acid gas containing:
O 0.2 mol.% of H2S,
0 92.0 mol.% of 002, and
O 1500 mol. ppm of BTX.
O 180 mol. ppm C4+
O 7.3 mol % of water, the rest being other hydrocarbons such as methane,
ethane and
propane as well as sulfur species such as mercaptans
This lean acid gas was sent to a first absorption zone (2) at a flow rate of 2
800
kmol/h. In this a first absorption zone (2), the lean acid gas contacted a 45
wt% (11 mol.%)
methyldiethanolamine (MDEA) aqueous solution (29) introduced at a flow rate of
335 m3/h, at
a temperature of 45 C and at a pressure ranging of 1.55 barA.
The gas stream (3) exiting the first absorption zone (2) at a flow rated of
2580 kmol/h
contained:
O 92.8 mol.% of CO2
O <100 mol. ppm of H2S, and
CA 02982686 2017-10-13
WO 2016/174120 22
PCT/EP2016/059461
o 1510 mol. ppm of BTX (92%).
O 195 mol. ppm of C4+ (representing the entire initial amount of C4+ in the
lean acid
gas).
O 6.6 mol. % of water, the rest being other hydrocarbons such as methane,
ethane and
propane as well as sulfur species such as mercaptans
The MDEA solution (4) exiting the first absorption zone (2) absorbs almost the
entire
amount of H2S of the lean acid gas and co-absorbs about 8% of the BTX and
reached a
temperature of about 55 C.
The MDEA solution (4) exiting the first absorption zone (2) then passed
through a
pump (5) and through a heater (6) to increase its temperature and pressure in
order to enter
the stripping zone (8) at a temperature of 93.0 C and at a pressure of 5 barA.
As can be seen from Fig.2, the temperature increase in the heater (6) was
obtained
by recirculating in the heater (6) the MDEA solution (20) recovered from the
desorption zone
(16).
The heated MDEA solution entered the stripping zone (8) where it was contacted
with
a countercurrent of a natural gas stream (7) introduced at the bottom of the
stripping zone.
The natural gas stream (7) had the following specifications:
O 95.0 mol.% of methane
O 5.0 mol.% of ethane
It entered the stripping zone in the following conditions:
O Flow rate: 360 kmol/h
O Temperature: 15 C
O Pressure: 7.0 barA.
The stripper was operated at a pressure of 5.0 barA.
The fuel gas stream exiting the stripping zone (8) had the following
specifications:
O Flow rate : 465 kmol/h
O Temperature: 93 C
O 8.4 mol.% of CO2
O 0.1 mol.% of H2S, and
o 625 mol. ppm of BTX (89% of the BTX entering the stripping zone).
This fuel gas stream exiting the stripping zone (8) was then passed through a
heat
exchanger and entered a second adsorption zone (12) at a temperature of 45 C.
In the second adsorption zone (12) the fuel gas stream exiting the stripping
zone (8)
contacted a methyldiethanolamine (MDEA) solution (28) introduced at a flow
rate of 12 m3/h,
at a temperature of 45 C and a pressure of 4.0 barA.
The methyldiethanolamine (MDEA) solution is the same as the one used in the
first
absorption zone (2).
CA 02982686 2017-10-13
WO 2016/174120 23
PCT/EP2016/059461
A fuel gas stream (13) exited the second absorption zone (12) at a flow rate
of 406
kmol/h with the following composition:
O 9.1 mol.% of CO2
O <100 mol. ppm of H2S, and
0 680 mol.
ppm of BTX (94% of the BTX entering the second absorption zone in
stream 16 and about 7% of the BTX entering the process in stream 1).
The fuel gas stream (13) meets standards requirements for a combustible and
can
thus be used as a feed in the incinerator (33) and/or in the utility boilers
where associated
aromatic hydrocarbons (BTX) and C4+ aliphatic hydrocarbons, as well as
remaining sulfur
species, will be destructed.
The MDEA solution enriched in H2S (14) exiting the second absorption zone (12)
was
recycled to the stripping zone to supplement the MDEA solution enriched in H2S
(4).
An MDEA solution depleted in BTX (9) exited the stripping zone (8) at a
temperature
of 89 C and contained 11% of the BTX entering the stripping zone (a flow
corresponding to
about 1% of the BTX entering the process).
This MDEA solution depleted in BTX (9) was then introduced into a desorption
zone
(16) equipped with a boiler (18) operating at a temperature of 130 C and a
pressure of 2.4
barA.
The regenerated MDEA solution (17) leaving the bottom of the desorption zone
(16)
was sent back to the first adsorption zone (2) and to the second adsorption
zone (12).
The lean acid gas depleted in BTX (21) exiting the desorption zone (16) was
passed
through a condenser (22) and a reflux drum (23).
The lean acid gas depleted in BTX (26) recovered at the end of the process of
the
invention had a temperature of 45 C and a flow rate of 160 kmol/h. It had the
following
composition:
O 90.8 mol.% of CO2
O 3.4 mol.% of H2S, and
O 220 mol. ppm of BTX (1% of the BTX entering the process).
O 5.8 mol.% of H20.
The process of the present invention made it possible to decrease the BTX
content of
the lean acid gas treated of 99 mol.%. The treated lean acid gas was then
suitable for a
subsequent treatment in a sulfur recovery unit even with an H2S content much
lower than
55 mol%.
The obtained acid gas was then treated by direct oxidation in 2 reactors
(SmartSulfTM
technology). This resulted in a sulfur recovery rate of 98%. 2 tons per day of
bright yellow
solid sulfur, reaching standards for sulfur recovery were recovered without
further treatment.