Note: Descriptions are shown in the official language in which they were submitted.
- 1 -
RESPIRATORY UNIT WITH MULTIPLE MEDICATION CHAMBERS
FIELD OF THE INVENTION
[001] The present invention is in the field of medical devices.
Specifically,
the present invention is in the field of respiratory aid units for use at
hospitals, home or
other non-hospital settings.
BACKGROUND OF THE DISCLOSURE
[002] Acute and chronic respiratory mortality and morbidity are
significant,
common and prevalent health problems that affect patients' quality of life,
and are a big
burden on the public health system. Recently, the incidents of respiratory
diseases have
been increasing. In some countries, the prevalence of bronchial asthma alone
can reach up
to 21%.
[003] A variety of respiratory units are used for the treatment of
obstructive
airway diseases, such as bronchial asthma, chronic obstructive pulmonary
disease (COPD),
cystic fibrosis, viral and bacterial pneumonia, bronchiolitis, emphysema,
hyperactive
airway disease, wheezing attacks (e.g., as associated with seasonal flu or
allergies) and
others.
[004] Inhaled medications, such as salbutamol, corticosteroids and
ipratropium bromide, are very effective in the treatment of a wide spectrum of
acute and
chronic respiratory conditions. In addition, in some cases such as croup
(common viral
upper respiratory infection), inhalation of water steam alone is the main core
of treatment.
In some cases, a patient greatly benefits from receiving a cocktail of
medications as
opposed to just a single one. Current respiratory units do not allow for
multiple
medications to be administered by inhalation simultaneously.
[005] It is not uncommon for a patient suffering from a respiratory disease
to
require rapid adjustment to medications due to an acute onset of severe
symptoms. Given
the current state of the art, it is nearly impossible for patients to reach
their doctor in time.
Instead, these patients end up at emergency rooms, causing significant
economic damage
due to their own loss of productivity and the additional burden on the
emergency rooms.
CA 2985939 2017-11-17
- 2 -
[006] Therefore, a need exists in the art for a respiratory unit that can
administer multiple medications simultaneously, and can be in communication
with the
patient's health care provider.
SUMMARY OF THE INVENTION
[007] Disclosed are respiratory devices comprising at least four components
selected from a control unit 102, a filling unit 104, a generation and output
unit 106, and
an input unit 108, wherein the respiratory device comprises a nebulizer mode
and a steam
mode of operation. Also disclosed are plungers 402, comprising a shaft 404; a
handle 406;
and a plunger joint 414 at the plunger distal end 412, wherein the plunger
joint 414 connects
securely with a seal joint 410 of a moveable seal 408 of an administrable
material chamber
302,304. The plunger is used to fill administrable material chambers 302,304.
BRIEF DESCRIPTION OF THE DRAWINGS
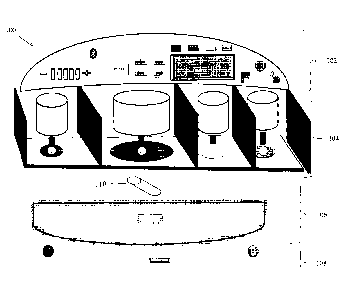
[008] FIG. 1 illustrates an embodiment of the disclosed respiratory
devices.
[009] FIG.2 illustrates an embodiment of the disclosed control unit of the
disclosed respiratory devices.
[0010] Fig. 3 illustrates an embodiment of the disclosed filling
unit of the
disclosed respiratory devices.
[0011] FIG. 4 illustrates an embodiment of the disclosed filling
mechanism.
[0012] FIG. 5 illustrates an embodiment of the disclosed water tank.
[0013] FIG. 6 illustrates an embodiment of the disclosed generator
unit.
[0014] FIG. 7 illustrates an embodiment of the disclosed internal
design.
[0015] FIG. 8 illustrates an embodiment of the disclosed output
stick.
CA 2985939 2017-11-17
- 3 -
DETAILED DESCRIPTION OF THE EMBODIMENTS
PARTS LIST
10016] The following list of parts refers to the accompanying
drawings:
= 100: An embodiment of the disclosed device.
= 102: Control unit.
= 104: Filling unit.
= 106: Generation and output unit.
= 108: Input unit.
= 110: Output stick.
= 202: Power button.
= 204: Display monitor.
= 206: Chamber lights.
= 208,210: Navigation buttons.
= 212: Nebulization rate indicator.
= 214: Bluetooth0 light indicator.
= 216: Nebulization mode display light.
= 218: Steam mode display light.
= 220: Speaker.
= 302: Medication chambers.
= 304: Carrier chamber.
= 306: Chamber housing walls.
= 308: Chamber outlet tube.
= 310: Opening from the chamber to the conduit.
= 312: Housing for medication or carrier chamber.
= 402: Plunger.
= 404: Plunger shaft.
= 406: Plunger handle.
= 408: Chamber moveable seal.
= 410: Seal joint.
= 412: Plunger distal end.
CA 2985939 2017-11-17
- 4 -
= 414: Plunger joint.
= 416: Upper end of the chamber.
= 418: Chamber fill lines.
= 502: Water tank.
= 504: Water tank filling cap.
= 506: Conduit connecting the water tank to the device.
= 508: Conduit valve.
= 510: Connector to secure the water tank to the device.
= 512: Lower end of the device.
= 514: Upper end of the device.
= 602: Ultrasound generator.
= 604: Water sink.
= 606: Medication collector.
= 702: Air pressure pump.
= 704: Air pressure line.
= 706: Air inlet for medication or carrier chamber.
= 708: Conduit connecting medication or carrier chamber
to the medication
collector.
= 710: Air pressure valve.
= 802: Proximal section of output stick.
= 804: Distal section of output stick.
= 806: Connector.
[0017] Disclosed herein are ultrasonic nebulizer and steam generator
respiratory devices having a plurality of chambers for medication. The
disclosed
respiratory devices send real time physiological data of the user to a health
care
professional (HCP) via the internet. Based on the data, the HCP can then
submit an order
to manipulate the dosage of the various medications stored in the respiratory
device. The
respiratory device will then implement the order, e.g., change the medication
dosage, once
the device obtains the user's approval, or will issue various alerts.
CA 2985939 2017-11-17
- 5 -
[0018] The respiratory devices disclosed herein generally comprise
at least four
components that work in concert to achieve the end result. These components
include a
control unit, a filling unit, a generator and output unit, and an input or
accessories unit.
[0019] The control unit comprises the electronic panel and switches
that allow
for the function of the device, such as, without limitation, mode selector
(nebulizer or steam
generator), dose selectors, timers, and any message or alert displays. The
filling unit
comprises the plurality of chambers or reservoirs for the various medications,
water, or any
other compound that is to be administered nasally (e.g., by inhalation) to the
patient. The
generator and output unit nebulizes the medications or generates steam for
their
administration, as is necessary. Finally, the input or accessories unit
provides the means
for a water tank connection, wired or wireless electronic communications with
between the
disclosed respiratory devices and other.
[0020] In some embodiments, the disclosed respiratory devices are
configured
to communicate with one or more devices that obtain physiological data on the
user of the
device. The data includes, but is not limited to, respiratory rate, peak
expiratory respiratory
volume, pulse rate, blood oxygen saturation limits, blood pressure, body
temperature, and
the like. In some embodiments, the disclosed respiratory devices comprise
sensors for
measuring the physiological data. In other embodiments, the data is obtained
by separate
instruments and are relayed to the disclosed respiratory devices either by
wired or wireless
communication.
[0021] In certain embodiments, the disclosed respiratory devices
communicate
the data to an HCP via the internet. In some embodiments, the data is relayed
continuously
while in other embodiments, the data is relayed at certain time intervals. In
certain
embodiments, the HCP sets up certain threshold maxima and minima for the data.
Once
the measured physiological function passes a threshold, the device sends and
urgent alert
to the HCP to review the data immediately. In some of these embodiments, when
the
threshold value is passed, the disclosed respiratory devices emit and audible
signal to alert
someone in the user's vicinity that immediate action is to be taken.
[0022] In other embodiments, the HCP reviews the data and determines
if any
changes to the prescription need to be made. In some embodiments, the data is
reviewed
on a health care professional interface app on a smart device, such as a
computer, a smart
CA 2985939 2017-11-17
- 6 -
phone, or a smart tablet. In certain embodiments, the HCP then sends the
instructions for
the change either to the disclosed respiratory devices, or to the user smart
device, or both.
The disclosed respiratory devices then alert the patient of the new
instructions. Once the
patient approves the instructions, the disclosed respiratory devices change
the medication
cocktail accordingly.
[0023] The instructions from the HCP may include increase or
decrease of the
medications' doses, changes to the combination of medications, increase or
decrease the
frequency of treatment with nebulized drug or hot steam, increase or decrease
the session
duration, contact the HCP immediately, go to an urgent care facility, or alert
the emergency
medical services. In some embodiments, the HCP instructs the disclosed
respiratory
devices to automatedly alert EMS either by a phone call with a recorded
message, or by a
text or other type of electronic message. In these embodiments, if the user
has become
incapacitated, then the HCP can summon immediate help by the push of the
button, without
wasting time to obtain the user's location to relay to the EMS.
[0024] An advantage of the presently disclosed devices 100 is that
they are
always ready to be used. Even when they are not being operated they are in
stand-by mode
where the device can be made operative in a matter of a few short seconds.
Therefore, the
present devices 100 are particularly suited to be used in emergency
situations, where by
just a single press on the start button the appropriate medication cocktail is
delivered to the
user.
[0025] The various aspects of the disclosed respiratory devices are
now
discussed with a view to the accompanying drawings, in which like numbers
refer to like
parts.
[0026] FIG. 1 illustrates an embodiment of the disclosed respiratory
devices.
The respiratory device 100 comprises four components: a control unit 102, a
filling unit
104, a generation and output unit 106, and an input unit 108. In some
embodiments, the
device 100 comprises additional units. In other embodiments, some of the units
are
combined together. An output stick 110 is provided that delivers the output of
the
generation and output unit 106 to the user. Thus, the aforementioned units are
not to be
construed as physically separate components, but rather as one or more
components that
allow the function of the unit as described herein.
CA 2985939 2017-11-17
-7-
100271 FIG.2 illustrates an embodiment of the disclosed control unit
102. A
power button 202 is provided. Activating the power button 202 turns the device
100 on,
while deactivating the power button 202 turns the device 100 off. In some
embodiments,
the power button 202 also acts as a start button. The start button initiates
the nebulization
of the medication or steam generation, depending on the selected function. In
other
embodiments, the start button is a separate button.
[0028] Throughout the present disclosure, the word "button" is
interchangeable
with "switch" and denotes any device that can turn the flow of electricity on
or off.
[0029] In some embodiments, the power button 202 is a toggle switch
that
alternates through a series of options. The following scenario is offered as
an example of
how various functions can be chosen. One press of the power button 202 powers
the device
100 on and enables it to generate nebulized particles from the medications
chambers 302
(see below) (nebulization mode). Two presses, either immediately consecutively
or about
2-3 seconds apart switches the function of the device 100 to generate steam
from a water
tank, described below (steam mode). By long pressing, e.g., 2-3 seconds, on
the power
button 202 will allow you to the settings panel (see below) is accessed. In
some
embodiments, the user can choose only the nebulization mode or the steam mode,
but not
both. In certain embodiments, when it is clinically beneficial to have the
nebulized
medication delivered along with steam, the user can choose both nebulization
and steam
modes simultaneously.
[0030] In some embodiments, the control unit 102 further comprises a
display
monitor 204. The monitor 204 displays various numbers, messages, alerts, etc.
In some
embodiments, the monitor 204 is a liquid crystal display (LCD) monitor. Other
types of
displays, such as a dot matrix display, cathode ray tube, and the like, or
display types
developed in the future, are contemplated. In some embodiments, the display is
well-
illuminated, with clear and high resolutions figures and letters displayed in
large font. It is
contemplated that a significant number of the users of the device 100 will be
very ill and/or
elderly patients who may have difficulty reading digital displays. The monitor
204 is especially
configured with such limitations in mind.
[0031] In some embodiments, the control unit 102 further comprises a
set of
chamber lights 206. Each chamber light 206 corresponds to one of the chambers
302,304
CA 2985939 2017-11-17
- 8 -
(discussed below). When the light 206 for a particular chamber 302,304 is on,
then that
particular chamber 302,304 is on active mode. Any value that is displayed on
the monitor
204 relates to the particular chamber 302,304. Thus, for example, if the
dosage is adjusted,
then the dosage for the medication in the chamber 302 whose light 206 is
illuminated is
being adjusted. By way of example only, in some embodiments, each particular
chamber
302,304 is color-coded. For example, a red chamber holds Drug A, while a
yellow chamber
holds Drug B. During the operation, a lit red light 206 indicates that the red
chamber 302
settings are being adjusted, while a lit yellow light 206 is lit indicates
that the red chamber
302 settings are being adjusted.
[0032] The embodiment shown in FIG. 2 shows four chamber lights 206.
The
ordinary artisan recognizes that there could be any number of chamber lights
206. In
practice, the quantity of lights 206 should correspond to the quantity of
chambers 302,304.
However, in some embodiments, the device 100 is configured to accommodate 5,
7, 8, 10,
or more chambers 302,304, but not all of which are placed in the device 100
because the
user only requires two or three medications. In that case, the control unit
102 exhibits more
chamber lights 206 than there are chambers 302,304.
[0033] In some embodiments, the chamber lights 206 are color coded,
such that
each light has a different color. In certain of these embodiments, the
chambers 302,304
are also color coded and the color of the chamber 302,304 matches the color of
the
respective chamber light 206.
[0034] In some embodiments, the control unit 102 further comprises
at least
one set of navigation buttons 208. In some embodiments, there are at least two
buttons per
each set of navigation buttons 208. One button increases a value while another
button
decreases a value. In other functions, one button moves the cursor to the
right, while
another button moves the cursor to the left. In still other functions, button
moves the cursor
up, while another button moves the cursor down. In these embodiments, another
button
(not shown) allows the user to toggle through the various options that require
adjustment.
[0035] In other embodiments, the control unit 102 comprises a
plurality of sets
of navigation buttons. For example, in the embodiment shown in FIG. 2, the
control unit
102 comprises a set of navigation buttons 208 and a second set of navigation
buttons 210.
In these embodiments, the functions of the navigation buttons are distributed
between the
CA 2985939 2017-11-17
- 9 -
plurality of the sets of navigation buttons. In the example of FIG. 2, one set
of navigation
buttons 208 is used to select the drug whose dose is to be adjusted, while the
second set of
navigation buttons 210 is used to increase or decrease the dose. The ordinary
artisan
recognizes other functions, such as settings, functions, etc., can be adjusted
using the
illustrated navigation buttons 208,210 or any other set of, or individual,
navigation buttons.
[0036] The following example is provided for illustration only and
is not to be
considered as limiting the scope of the accompanying claims in any way. The
navigation
buttons 208 are used to move between, or select, prompts and functions that
are displayed
on the monitor 204. The buttons 208 can also be used to move between the
chambers 302,304
to adjust the dose of the drug whose chamber 302 is selected or the amount of
distilled water
used from chamber 304 to mix with the drug (i.e., change the concentration of
the drug that is
introduced into the nebulizer. Once a chamber 302,304 is selected, the chamber
light 206
corresponding with that chamber 302,304 is illuminated. The user now knows
that all further
modifications will be made to the chamber 302,304 whose light is illuminated.
[0037] The user then adjusts the selected variable using the
navigation buttons
210. For example, Chamber 1 contains salbutamol. To adjust its dose from 0.1
mL to 0.5 mL,
Chamber 1 is selected using the buttons 208. Dose setting for Chamber 1 is
also selected by
using the buttons 208. The dose is then adjusted upwards using the buttons
210. In some
embodiments, the dose changes in 0.1 mL increments, while in other
embodiments, the
increments are smaller, such as 0.01 or 0.05 mL, and in still other
embodiments the increments
are greater, for example 0.5 or 1.0 mL. In some embodiments, the HCP can
determine what
the dose adjustment increment for a particular chamber 302,304 should be. This
is determined
by the characteristics of the particular drug and its approved dosing regimen.
[0038] In some embodiments, the control unit 102 further comprises a
nebulization rate indicator 212. In these embodiments, the indicator 212 is
used to specify
the length of the nebulization period. Based on the physiological data that
the HCP has
received, the HCP can determine whether the user requires the medication to be
administered as rapidly as possible, or whether the medication should be
administered over
several minutes. Adjusting the nebulization rate allows for the adjusting of
the
administration time. lithe nebulization rate is increased, the time needed to
complete the
administration of the dose becomes shorter, and vice versa.
CA 2985939 2017-11-17
- 10 -
[0039] In some embodiments, the device 100 is configured to connect
wirelessly with other devices using Bluetooth technology. In some of these
embodiments, a light indicator 214 is provided to show when a connection is
successfully
made. In some embodiments, the light indicator 214 is also a button that can
be depressed
to activate the Bluetooth pairing function of the device 100.
[0040] In some embodiments, the control unit 102 comprises
indicators that
show the user whether the nebulization or the steam mode is selected. In some
embodiments, a notice on the monitor 204 (e.g., the words "Nebulization" and
"Steam")
appears to indicate the status. In other embodiments, a separate display shows
which mode
is selected. In the embodiment shown in FIG. 2, a display light 216 is
illuminated when
the nebulization mode is selected, and a different display light 218 is
illuminated when the
steam mode is selected.
[0041] In some embodiments, the control unit 102 comprises a speaker
220. In
some embodiments, the speaker 220 provides audible alerts for the user or a
caregiver. In
other embodiments, when the user has difficulty reading the messages displayed
on the
monitor 204, the messages are transmitted in spoken word through the speaker
220. In
some embodiments, the user chooses whether some or all the information on the
monitor
204 is to be vocalized. If a subset of the messages is to be vocalized, then
the user can
choose the members of the subset. In some embodiments, the selection is made
through
the use of the navigation buttons 208,210 and followed on the monitor 204.
100421 Fig. 3 illustrates an embodiment of the filling unit 104. The
filling unit
104 comprises a plurality of medication chambers 302 and at least one
distilled water
chamber 304. In some embodiments, each chamber 302,304 is placed in its own
separate
housing 312, or chamber room, that is defined by walls 306 that separate the
housing 312
from the adjoining housings 312, and by walls (not shown) in the front and
back of the
housing 312. In some embodiments, the entire filling unit 104 is pulled out of
the device
100 to fill the chambers 302,304 with the appropriate medications or water and
then the
filling unit 104 is replaced in the device 100. In other embodiments, each
chamber 302,304
is removed individually from its respective housing 312 in the filling unit
104 of the device
100 to be filled with medication or water. Each chamber is then replaced in
its
corresponding housing 312. In some of the embodiments where the chambers
302,304 are
CA 2985939 2017-11-17
- 11 -
color-coded, the housings 312 are also color-coded and the color of the
housing 312
corresponds to the color of the chambers 302,304.
[0043] The embodiment shown in FIG. 3 comprises three medication
chambers
302 and one distilled water chamber 304. Consequently, the illustrated
embodiment
depicts a device that is useful for delivering at most three medications at a
time to the
patient. The ordinary artisan recognizes that other embodiments are directed
to those
having more, or less, than three medication chambers 302.
[0044] In some embodiments chamber 304 contains distilled water. The
water
is used to dilute the medication(s) before their use in the nebulizer. In some
embodiments
the patient is administered a combination of several drugs. The water in the
chamber 304
is used to make a solution out of the one or a mixture of medications, thereby
diluting the
medication. A dilute solution of a medication results in minimizing the
adverse events
associated with the medication. Because the amount of medication being
administered is
not changed, the dilute solution results in better pharmacokinetic profile,
i.e., a lower Cmax
but the same AUG as compared to the administration of the concentrated drug.
In some
embodiments, the dilution step is employed while the device 100 is used in the
nebulization
mode.
[0045] While the above discussion was presented in terms of
distilled water
being the carrier, other carriers, i.e., physiologically acceptable solvents
that can be
volatilized, can also be used in lieu of water if the HCP prescribes the use
of such carriers.
[0046] In some embodiments, each chamber 302,304 is connected by an
outlet
tube 308 to an opening 310. The administered material from chamber 302,304 is
dispensed
from the chamber 302,304 and into the opening 310 from where it is delivered
through a
conduit (not shown) to a medication collector (606, FIG. 6) in the generation
and output
unit 106 to form the prescribed medication cocktail. The cocktail is then
nebulized and
delivered via output stick 110 to the user.
[0047] In some embodiments, the outlet tube 308 is a part of the
chamber
302,304 while in other embodiments, the outlet tube 308 is a separate piece
from the
chamber 302,304. As described in detail below, each chamber 302,304 is
connected by a
conduit 708 (FIG. 7) to the generation and output unit 106, where a medication
or a
medication cocktail is ultrasonically nebulized to be delivered to the user.
CA 2985939 2017-11-17
- 12 -
[0048] In some embodiments, each chamber 302 has sufficient volume
to hold
enough medication to be used for a period of five or more days. In some
embodiments,
each chamber 302 has a volume of between about 1 to about 100 mL, or between
about
5-75 mL, or between about 10-50 mL, or between about 20-40 mL. In some
embodiments,
each chamber 302 has a volume of about 30 mL. Thus, for example, if the
medication in
a chamber 302 is a salbutamol, budesonide, or ipratropium bromide (AtroventS)
solution,
the chamber volume of 30 mL will provide a dose of 1 mL of the drug solution
up to six times
a day for a minimum 5 days.
[0049] In some embodiment, chamber 304 has sufficient volume to hold
enough distilled water to be used for medication dilution for a period of five
or more days.
In some embodiments, chamber 304 has a volume of about 150 mL.
[0050] Throughout the present disclosure the term "about" a certain
value
means that a range of value 25%, preferably about 10%, and more preferably a
range of
value 5%, is contemplated. Thus, for example, having a volume of about 10 mL
includes
volume being between 7.5 mL and 12.5 mL, and preferably between 9 mL and 11
mL, and
more preferably between 9.5 mL and 10.5 mL. Furthermore, when about a range is
given,
it is understood that the word "about" qualifies both termini of the range.
Thus, for example
"about 7-10" means "about 7 to about 10."
[0051] In the discussion herein reference is made to an
"administrable material"
in a chamber 302,304. In the present context, "administrable material" refers
to a medication
in a chamber 302, or water in a chamber 304.
[0052] In some embodiments, each chamber 302,304 comprises a sensor
that
obtains information regarding the level of administrable material (medication
or water) in the
chamber 302,304 and causes the level to be displayed on the monitor 204. In
certain
embodiments, if the administrable material levels fall below a certain pre-
determined threshold
level, then the sensor causes and audible sound to be emitted to alert the
user.
[0053] In some embodiments, the device 100 is used on a permanent
basis by the
user. In other embodiments, an HCP provides the user with a device 100 in some
acute or
chronic settings for the user to use for a period of days or a few weeks.
After the prescribed
period of use is over, the user returns the device 100 to the HCP, who will in
turn provide it to
another user in need thereof after the device 100 is thoroughly cleaned and
disinfected. In
either case, there is a need to refill the chambers 302,304 with the proper
medication frequently.
CA 2985939 2017-11-17
- 13 -
The refilling is done either by the user, or a caregiver, when the user is a
permanent user, or by
the HCP or their staff when the HCP provides the device 100 to the user.
[0054] Accordingly, there is provided a mechanism to fill each
chamber 302,304
with the corresponding medication. FIG. 4 illustrates an embodiment of the
disclosed filling
mechanism. The chamber 302 is separated from the filling unit 104 of the
device 100. Each
chamber 302 comprises a movable seal 408, which in turn comprises a joint 410.
A plunger
402 is provided, which comprises a shaft 404 and a handle 406 at its proximal
end. The
distal end 412 of shaft 404 terminates in a plunger joint 414.
[0055] To fill a chamber 302,304, the distal end 412 of the plunger
402 is
inserted through an opening in the upper end 416 of the chamber 302,304. The
plunger
joint 414 is then connected with the chamber joint 410. The connection of the
two joints
can be through any known connection means. For example, the two joints can be
connected
by threading the shaft joint 414 into the chamber joint 410, or connecting
them through a
friction lock mechanism. The plunger 402 is then pressed all the way down by
pressing on
the handle 406. When the seal 408 has reached the bottom, the outlet tube 308
is connected
to a medication source. When the plunger 402 is pulled back by pulling on the
handle 406,
the seal 408 moves up, which causes a vacuum to be generated within the
chamber 302,304,
which in turn causes the medication to be sucked into the chamber 302,304.
Fill lines 418
show the volume of medication within the chamber 302,304. Once sufficient
administrable
material has been added to the chamber administrable material, the plunger 402
movement
is stopped, the plunger 402 is separated from the seal 408 and the chamber
administrable
material are returned to the filling unit 104, where the outlet 308 once again
is connected
with the opening 310.
[0056] In some embodiments, both the plunger 402 and the chamber
302,304
are made of materials that are easily cleaned and do not lend themselves to
the
accumulation of bacteria, mold, or other noxious organisms. In some
embodiments, one
or more of the chambers 302 are opaque to protect any light sensitive
medications
contained therein.
[0057] The ordinary artisan recognizes that the above is just one
example of the
mechanism by which chambers 302,304 can be filled. Other mechanisms are known
to the
skilled artisan. For example, in some embodiments the plunger movement is
automated
CA 2985939 2017-11-17
- 14 -
and is controlled by an electrical motor. In some of these embodiments, the
plunger
permanently stays attached to the seal 408. Conventional means of filling the
chambers
302,304 are also contemplated. For example, a syringe can be used to obtain
the proper
amount of a material and then the syringe is emptied into the chamber 302,304.
[0058] In some embodiments, the device 100 further comprises a water
tank.
FIG. 5 illustrates an embodiment of the water tank 502. The water tank 502 is
connected
to the device 100 when it is going to be used to produce steam. In some
embodiments, the
water tank 502 is removable. In certain of these embodiments, the water tank
502 is
attached to the back of the device 100. In some of these embodiments, the
water tank 502
is connected to the input unit 108. In some embodiments, the tank capacity is
under 10 L,
or under 8 L. In certain embodiments, the tank capacity is 5 L. In other
embodiments, the
tank capacity is less than 5 L, for example 4 L. In some embodiments, the tank
comprises
a sensor that alerts the user, and/or shuts off the steam generator, if the
water level has
fallen below a threshold level.
[0059] In some embodiments, the water tank 502 comprises a filling
cap 504
through which the water tank 502 is filled with water, such as sterilized,
deionized, steam-
condensed, or filtered water.
[0060] In some embodiments, the water tank 502 is connected to the
back of
the device 100 by a conduit 506. In certain embodiments, the conduit 506 is
located at the
bottom of the water tank 502, whereas in other embodiments, the conduit 506 is
located at
the side of the water tank 502. In some embodiments, the conduit 506 has a one-
way valve
508 that allows the water to pass from the water tank 502 to the unit 100
only, but not in
the reverse direction. Accordingly, once the water tank 502 is connected to
the device 100,
water will pass from the water tank 502 to a heat generator (not shown) in the
generation
and output unit 106, where the heat generator heats up the water to boiling to
generate
steam. The steam is then delivered to the user via the output stick 110. The
duration of hot
steam inhalation session is determined through the control unit 102.
[0061] Thus, as can be seen, in some embodiments the output stick
110 is
configured to deliver nebulized product, while in other embodiments, the
output stick 110
is configured to deliver steam. In still other embodiments, such as the one
contemplated
CA 2985939 2017-11-17
- 15 -
by the illustrated embodiments, the output stick 110 delivers the nebulized
product while
operating in nebulization mode, and delivers steam while operating in steam
mode.
[0062] In some embodiments, the water tank 502 comprises one or more
connector(s) 510 that secure the water tank 502 to the device 100. In some
embodiments,
the water tank 502 comprises four connectors 510: two connectors 510 connects
the water
tank 502 to the lower end 512 of the device 100; and another two connectors
510 connects
the water tank 502 to the upper end 514 of the device 100.
[0063] In some embodiments, the generation unit 106 comprises an
ultrasound
generator 602, as shown in FIG. 6. Adjacent to the ultrasound generator 602 is
a water
sink 604. The water sink 604 is in vibrational communication with the
ultrasound generator
602. In other words, when the ultrasound generator generates an ultrasonic
wave, the wave
causes motional disturbances in the water of the water sink 604. In some
embodiments,
the water sink 604 is located above the ultrasonic generator 602. In some
embodiments,
the water sink 604 is sealed so that no water spills once the vibrations due
to the ultrasound
waves begin.
[0064] In some embodiments, the water sink 604 contains enough water
to
partially submerge the medication collector 606 within the water of the water
sink 604. In
some embodiments, between about one-fifth to about four-fifths of the
medication collector
606 is submerged in the water. In some embodiments, between about one-fourth
to about
three-fourths of the medication collector 606 is submerged in the water. In
some
embodiments, between about one-third to about two-thirds of the medication
collector 606
is submerged in the water. In some embodiments, about one-half of the
medication
collector 606 is submerged in the water.
[0065] As described above, medications that are dispensed from
chambers
302,304 ultimately collect in the medication collector 606 to be nebulized.
Once the
medication or the cocktail of medications is completely collected, the
ultrasound generator
602 generates an ultrasound wave that causes the medication or the cocktail of
medications
within the medication collector 606 to become nebulized. In certain of these
embodiments,
the ultrasonic generator generates a sound wave having a frequency of about
100 KHz.
This frequency produces nebulized particles of size 1-5-micron, which is the
proper particle
size for a successful delivery to medium and small airways as well as the
alveoli.
CA 2985939 2017-11-17
- 16 -
Delivering the medication down to the alveoli makes the nebulization treatment
more
effective and more efficient compared to delivering the nebulized particles to
the small and
medium size airways only.
[0066] Accordingly, the generator and output unit 106 comprises the
ultrasonic
generator 602 for nebulization production, the heat generator for steam
production, the
sealed water sink 604, the medication collector 606, and the output stick 110
through which
the nebulized product and/or steam are delivered to the user.
[0067] The input unit 108 comprises a plurality of USB ports that
allow for
other devices or flash memory drives to be connected to the device 100 for
data transfer,
either from the device 100 to the accessory or vice versa. In certain
embodiments, other
accessories, such as a pulse oximeter, an expiratory peak flowmeter, other
physiological
function sensors, and the water tank, communicate with the device 100 through
the input
unit 108.
[0068] FIG. 7 is a schematic showing the mechanism of the
dispensation of
administrable materials in the medication collector 606. In some embodiments,
according
to the HCP prescription the dose for each administrable material in each
chamber 302,304
is set by using buttons in the control unit 102. When a dose is to be
administered to the
user, a pump 702 (or actuator) generates pressurized air that is sent through
air pressure
lines 704 to an air inlet 706. (In FIG. 7 for purposes of clarity the air
inlet 706 of chamber
304 is not shown.) Each air inlet 706 is in pneumatic communication with the
seal 408 of
its respective chamber 302,304. Therefore, when the pressurized air hits the
air inlet 706,
the air pressure is transferred to the seal 408. The air pressure pushes the
seal 408 down to
eject an exact dose in volume of the administrable material from the outlet
308 of the
chamber 302,304. Each outlet 308 is connected by a conduit 708 to the
medication collector
606. In the absence of air pressure, or when the air pressure is not what it
is set to be, a
pressure valve 710 prevents any administrable material from inadvertently
entering the
conduit 708. In some embodiments, the conduit 708 is vibrated to ensure all
the
administrable material is delivered from the conduit 708 to the medication
collector 606.
[0069] In some embodiments, the device 100 comprises a single
conduit 708
that connects to each chamber 302,304 individually to deliver its
administrable material to
CA 2985939 2017-11-17
- 17 -
the medication collector 606. In other embodiments, each chamber 302,304 has
its own
conduit 708 which is separate and distinct from the conduits 708 of other
chambers 302.
[0070] As stated above, in some embodiments, the device 100
comprises an
output stick. FIG. 8 illustrates an embodiment of the disclosed output stick
110. The output
stick 110 comprises a proximal section 804 and a distal section 806. The
proximal section
804 connects to the device 100, whereas the distal section 806 connects to the
user's
respiration line. In some embodiments, each of the sections 804,806 is tubular
having a
generally circular or oval cross section. The two sections 804,806 are
connected by a
connector 808. In some embodiments, the connector 808 has rotating components
that
allow for the angle between the sections 804,806 to vary when needed. In some
embodiments, the angle varies between 0 , i.e., when the two sections 804 and
806 are
adjacent to each other, and 180 , i.e., when the two sections 804 and 806 form
a straight
line. In other embodiments, the connector 808 is a swivel which allows section
804 to
swivel with respect to section 806, and vice versa.
[0071] In some embodiments, the output stick 110 is made of
materials, such
as plastics, that are easily cleanable. The output stick 110 can be cleaned
with usual
disinfecting and cleaning agents used for medical devices or by autoclave.
CA 2985939 2017-11-17