Note: Descriptions are shown in the official language in which they were submitted.
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
FLUORESCENCE HISTO-TOMOGRAPHY (FHT) SYSTEMS AND
METHODS
RELATED APPLICATIONS
This application claims priority to U.S. Provisional Application No.
62/211,930, filed on
August 31, 2015, entitled "CRYOFLUORESCENCE TOMOGRPAHY (FHT) SYSTEMS AND
METHODS," by Hoppin, et al., and to U.S. Non-Provisional Application No.
15/158,928, filed
on May 19, 2016, entitled "MULTI-SPECTRAL THREE DIMENSIONAL IMAGING
SYSTEM AND METHOD," which claims priority to U.S. Provisional Application No.
62/164,800, filed on May 21, 2015, entitled "MULTI-SPECTRAL THREE DIMENSIONAL
IMAGING SYSTEM AND METHOD," the contents all of which are herein incorporated
by
reference.
TECHNICAL FIELD
The present disclosure relates generally to imaging and, more specifically, to
fluorescence histo-tomography (FHT) systems and methods.
BACKGROUND
Multispectral fluorescence tissue slice imaging can be used to measure drug
distribution
ex-vivo in standalone or retrofitted slice imagers. Such specificity in a
single imaging system can
result in a high cost per scan. For high throughput and low cost, it would be
valuable to
construct a fluorescence histological (histo-) imager with a corresponding
software package that
can work in tandem with slicing instruments. To that end, the methods outlined
here
demonstrate a workflow of fluorescence imaging techniques for a versatile,
transportable add-on
to existing histological slicing instruments.
1
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
SUMMARY
According to one or more embodiments of the disclosure as described in greater
detail
below, a fluorescence histo-tomography (FHT) system is disclosed. The FHT
system includes a
housing, a fluorescence camera located within the housing, a white light
camera located within
the housing, and a fluorescence light source located within the housing. The
FHT system further
includes a support mount configured to support the housing within a chamber of
a slicing
apparatus such that the cameras and fluorescence light source are aimed
towards a block face of
a tissue specimen retained within the chamber.
In further embodiments, a method for performing FHT is disclosed. The method
includes
capturing, by an imaging device mounted within a chamber of a slicing
apparatus, a white light
image of a block face of a tissue specimen retained within the chamber. The
method also
includes capturing, by the imaging device, a fluorescence image of the block
face under white
light and fluorescence illumination. The method further includes co-
registering, by the imaging
device, the white light and fluorescence images to form a combined image. The
method
additionally includes providing, by the imaging device, the combined image to
an electronic
display.
In additional embodiments, a FHT system is disclosed. The FHT system includes
means
for imaging a block face of a tissue specimen retained within a chamber of a
slicing apparatus.
The FHT system also includes means for supporting the imaging means within the
chamber of
the slicing apparatus.
2
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
BRIEF DESCRIPTION OF THE DRAWINGS
The patent. or application file contains at least one drawing executed in
color. Copies of
this patent or patent application publication with color drawing(s) will be
provided by the Office
upon request and payment of the necessary fee.
The foregoing and other objects, features, aspects and advantages of the
embodiments
disclosed herein will become more apparent from the following detailed
description when taken
in conjunction with the following accompanying drawings.
FIGS. 1A-1B illustrate an example cryostat/cryomicrotome, according to various
embodiments.
FIGS. 2-4 illustrate an example fluorescence histo-tomography (FHT) imaging
device for
use in a microtome or cryomicrotome, according to various embodiments.
FIG. 5 illustrates an example computing device of a FHT system, according to
various
embodiments.
FIG. 6 illustrates an example simplified procedure for preparing a tissue
specimen for
FHT imaging, according to various embodiments.
FIG. 7 illustrates an example simplified procedure for imaging a block face of
a tissue
specimen using FHT imaging, according to various embodiments.
FIG. 8 illustrates an example registration scheme for images captured by a FHT
system,
according to various embodiments.
FIGS. 9A-9I illustrate test results of FHT imaging of a fluorophore-infused
piece of
twine, according to various embodiments.
3
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
FIGS. 10A-10B illustrate test results of FHT imaging of brain tissue,
according to various
embodiments.
FIGS. 11A-11F illustrate further test results of FHT imaging of brain tissue,
according to
various embodiments.
FIGS. 12A-12I illustrate additional examples of FHT imaging of brain tissue,
according
to various embodiments.
FIG. 13A-13E illustrate examples of the imaging of intrathecally-administered
anti-sense
oligonucleotides, according to various embodiments.
FIGS. 14A-14C illustrate examples of a mechanism to remove stuck tissues
slices from
an FHT system.
FIGS. 15A-15B illustrate an example apparatus for precisely placing fiducial
markers in
a tissue block.
FIG. 16 illustrates an example of multi-colored fiducial markers.
It should be understood that the above-referenced drawings are not necessarily
to scale,
presenting a somewhat simplified representation of various preferred features
illustrative of the
basic principles of the disclosure. The specific design features of the
present disclosure,
including, for example, specific dimensions, orientations, locations, and
shapes, will be
determined in part by the particular intended application and use environment.
4
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
DETAILED DESCRIPTION
Various challenges persist to find otherwise invisible, small objects in a
body. Labeling
such objects with fluorophores is a possible technique to introduce contrast,
but visible
fluorophores are problematic because excitation light and emission light are
both absorbed and
scattered. Near-infrared (NIR) fluorescent light has the ability to provide
high contrast in the
context of normal bodily tissues and fluids.
Histological analysis of sections from previously living tissue is time-
consuming and
laborious because each section needs to be mounted, processed, and scanned
(e.g., using a
microscope). Block face imaging is the opposite of conventional analysis
because the tissue
slice is thrown away and only the exposed block face of the tissue specimen is
imaged. When
using NIR fluorescent light to image the block face, extremely high signal to
noise is achieved,
although one also has to attempt to compensate for the "extra depth" that NIR
can see. In some
implementations, video information (e.g., color, grayscale, etc.) as well as
assumptions about
light propagation in the tissue can be used to generate at least an
approximation of the actual
fluorescence.
In some aspects, the techniques described herein utilize simultaneous
color/NIR
acquisition to acquire data sets and mathematical methods applied to these
data sets to
reconstruct in ultra-high resolution, at least an approximation of the actual
NIR fluorescence
present in the tissue block and therefore the original living tissue. NIR also
provides high
sensitivity and less interference from endogenous chromophores that would
otherwise preclude
visible fluorescence imaging.
5
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
Referring now to FIG. 1A, a cryostat/cryomicrotome 100 is shown, according to
various
embodiments. In general, a microtome is a device typically used to prepare
histologic samples
of a tissue block for use in microscopy. For example, a microtome may shave a
thin layer of a
tissue specimen from a tissue block that can then be mounted to a slide for
observation using a
microscope. A cryomicrotome is a specialized form of microtome operable to
keep the tissue
specimen at a reduced temperature during slicing. While the FHT system
disclosed herein is
described with respect to a cryomicrotome, the teachings herein are not
limited as such. In
particular, the FHT system can be implemented using any number of different
slicing devices
(e.g., other microtomes, histological sample preparation devices, macrotomes,
etc.).
As shown, cryomicrotome 100 may include a housing 102 that encompasses a
sealed
chamber 106 in which the cutting operation is performed. Cryomicrotome 100 may
also include
a door 104 or other access mechanism (e.g., lid, etc.) that afford the user of
cryomicrotome 100
access to chamber 106. During use, cryomicrotome 100 may regulate the internal
temperature of
chamber 106 to prevent a frozen tissue specimen being sliced from thawing back
to room
temperature.
FIG. 1B illustrates an example chamber 106 of cryomicrotome 100 in greater
detail. As
shown, cryomicrotome 100 may include a specimen retainer 110 that holds a
frozen specimen
112 in place. In addition, cryomicrotome 100 may also include one or more
cutting blades 108
that, when actuated, moves across the block face of specimen 112 to remove a
thin layer of
specimen 112. In many cases, specimen 112 may be any form of biological
material extricated
from a subject and may be frozen in an optimal cutting temperature (OCT)
material, prior to
placement in cryomicrotome 100.
6
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
The alignment of cutting blade 108 and specimen retainer 110 may be
configurable in
some cryomicrotomes to adjust, e.g., the thickness of the resulting tissue
slice and/or to ensure
proper contact of cutting blade 108 with tissue specimen 112. For example, a
user of
cryomicrotome 100 may first ensure the correct positions of tissue specimen
112 before initiating
slicing of the tissue specimen 112. In some embodiments, actuation of cutting
blade 108 may be
performed manually by the user. In other embodiments, actuation of cutting
blade 108 and/or
movement of specimen retainer 110 may be performed automatically by
cryomicrotome 100
under computerized control.
An exemplary cryomicrotome that can be used to implement the techniques herein
is the
Leica 3050 cryomicrotome available from Leica Camera AG, Wetzlar, Germany.
However, as
would be appreciated, the techniques herein can be applied to any number of
different
cryomicrotomes and are not limited to a particular make, model, or type of
cryomicrotome.
As noted above, NIR fluorescence imaging has emerged in recent years and
demonstrates
the ability to produce images with high contrast between fluorophore infused
tissue and regular
tissue. Table 1 below provides a listing of exemplary fluorophore agents with
their peak
excitation wavelengths (Ex) and peak emission wavelengths (Em).
Type Agent Ex (lam) Em (nm)
Reactive and
Hydroxycoumarin 325 386
conjugated probes
Aminocoumarin 350 455
Methoxycoumarin 360 410
Cascade Blue 375;400 421
Lucifer Yellow 425 528
NBI ) 466 539
R-Phycoerythrin (PE) 480; 565 578
PE-Cy5 conjugates 480; 565; 650 670
7
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
PE-Cy7 conjugates 480; 565; 743 767
APC-Cy7 conjugates 650; 755 767
Red 613 480;565 613
Fluorescein 495 519
FluorX 494 520
BODIPY-FL 503 512
TRITC 547 574
X-Rhodamine 570 576
Lissamine Rhodamine
570 590
B
PerCP 490 675
Texas Red 589 615
Allophycocyanin
650 660
(APC)
TruRed 490, 675 695
Alexa Huor 350 346 445
Alexa Fluor 430 430 545
Alexa Fluor 488 494 517
Alexa Fluor 532 530 555
Alexa Fluor 546 556 573
Alexa Huor 555 556 573
Alexa Fluor 568 578 603
Alexa Fluor 594 590 617
Alexa Fluor 633 621 639
Alexa Fluor 647 650 688
Alexa Fluor 660 663 690
Alexa Fluor 680 679 702
Alexa Fluor 700 696 719
Alexa Fluor 750 752 779
Cy2 489 506
Cy3 (512); 550 570; (615)
Cy3, 5 581 596; (640)
Cy5 (625); 650 670
Cy5, 5 675 694
Cy7 743 767
ZW800-1 760 790
ZW700-1 Forte 675 700
Lipo800-Forte 760 790
8
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
Lipo700-Forte 675 700
Nucleic acid probes Hoeschst 33342 343 483
DAP1 345 455
Hoechst 33258 345 478
SYTOX BIIIC 431 ,480
Chromomycin A3 445 575
Mithramycin 445 575
YOY0-1 491 509
SYTOX Green 504 523
SYTOX Orarwe 547 570
Ethidium Borrnide 493 620
7-AAD 546 647
Acridine Orange 503 530/640
Torro-1, TO-PRO-1 509 533
Thiazole Orange 510 530
Propidium Iodide (PI) 536 617
TOTO-3, TO-PRO-3 642 661
LDS 751 543;59() 712;6O7
Fluorescent Proteins Y66F 360 508
5[16611 360 442
EBFP 380 440
Wild-type 396, 475 50, 503
GFPuv 385 508
ECEP 434 477
'Y'66W 436 485
S65 A 471 504
S65C 479 507
S65L 484 510
S65T 488 511
EGEP 489 508
E'YFP 514 527
DsRed 558 583
Other probes Monochlorobimane 380 461
Calcein 496 517
Table 1
9
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
In many cases, the fluorophores that can be used for purposes of fluorescence
imaging
may exhibit emission spectra in the range of approximately 200 nm to 1000 nm.
Further, a
plurality of fluorophores can be used on a single specimen if each of the used
fluorophores has
different emission spectra. Other fluorophores not listed in Table 1 can also
be used without
deviating from the teachings herein.
According to various embodiments, NIR fluorescence imaging can be leveraged
with a
cryomicrotome to perform fluorescence histo-tomography (FHT) of a tissue
specimen. As noted,
cryomicrotomes are typically used to prepare tissue slices for further
analysis (e.g., by mounting
a slice to a microscope slide, etc.). In contrast, the FHT techniques herein
propose the exact
opposite, i.e., by imaging the exposed block face of the tissue specimen
itself while in the
cryomicrotome without any regard to the resultant tissue slices, which may be
discarded or
retained as desired by the user.
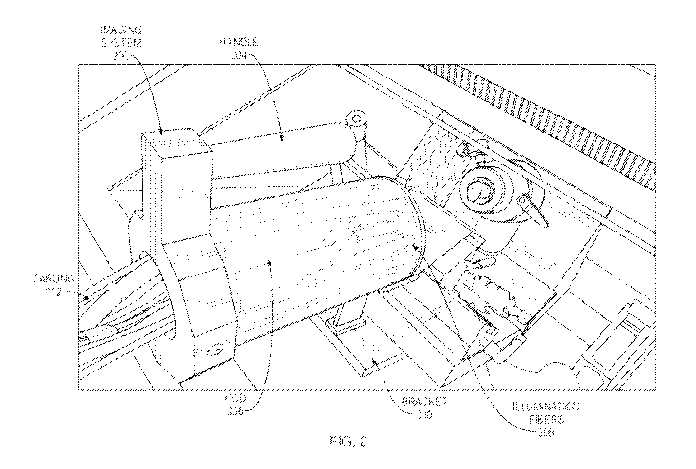
Referring now to FIGS. 2-4, a fluorescence histo-tomography (FHT) system is
shown, in
various embodiments. As shown in FIG. 2, the FHT system may include an imaging
device/system 200 that is operable to image the exposed block face of tissue
specimen 112
within chamber 106 of cryomicrotome 100. As would be appreciated, imaging
system 200 may
be transportable, allowing imaging system 200 to be used with any number of
different types of
cryomicrotomes. Advantageously, this allows a user to adapt a cryomicrotome to
perform FHT
imaging without having to make significant modifications to the cryomicrotome.
Imaging system 200 may include a housing 202 that houses the various imaging
components of system 200. In some embodiments, housing 202 may include a
handle 204 that
allows the user to position housing 202 within chamber 106 of cryomicrotome
100 and/or
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
remove imaging system 200 therefrom, as desired. Housing 202 may be formed of
any suitable
materials such as plastics, ceramics, or sheet metal and may protect the
imaging components of
imaging system 200 from the internal climate of chamber 106 of cryomicrotome
100 (e.g., to
protect a camera from exposure to the colder temperatures, etc.). At least a
portion of housing
202 may also be at least semi-transparent such that light in the white and NIR
spectrums may
pass through housing 202. Further, while housing 202 is shown with a primarily
cylindrical
shape, other implementations of housing 202 may take on other geometric
shapes, e.g., to fit
within the chambers of certain types or models of cryomicrotomes.
In various embodiments, the FHT system may also include one or more support
brackets
or other retaining members, to position the imaging components in housing 202
at a suitable
distance within chamber 106 of cryomicrotome 100 relative to tissue specimen
112. For
example, as shown, support mount/bracket 210 may contact the floor of chamber
106 and
support housing 202 at a distance therefrom. While housing 202 may be
positioned at any
desired distance from the block face of tissue specimen 112, testing has shown
that a distance of
approximately ten inches yields suitable FHT imaging results of the sample
while not impinging
the motion of cutting blade 108, as shown in greater detail in FIG. 3.
In some embodiments, bracket 210 may be coupled or otherwise fastened to the
floor of
chamber 106 (e.g., via one or more screws, bolts, etc.). In other embodiments,
bracket 210 may
be shaped to engage one or more components of cryomicrotome 100 (e.g., to
slide under the
structure associated with cutting blade 108, etc.). In addition, bracket 210
may be a separate
component from that of housing 202 (e.g., housing 202 rests on bracket 210),
may be fastened or
11
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
coupled thereto, or may be directly formed as part of housing 202, according
to various
embodiments.
The imaging components of imaging system 200 may include one or more cameras,
such
as one or more charge-coupled device (CCD) camera(s) 206 and one or more
illumination light
sources/fibers 208. For example, camera(s) 206 may include a white light
camera configured to
capture images within the visible spectrum and/or a fluorescence camera
configured to capture
images in the NIR or IR spectrum. Similarly, illumination light sources/fibers
208 may include
one or more fibers to shine fluorescent and/or white light onto the block face
of tissue specimen
112 during imaging. Extending out of the back of housing 202 may be cabling
212 that connect
camera(s) 206 and illumination sources/fibers 208 to a computing device 300,
as shown in FIG.
4.
In some cases, ambient illumination by room lighting may be sufficiently
diffuse such
that imaging system 200 does not require a dedicated white light illumination
source. However,
in other embodiments, imaging system 200 may further include one or more white
light sources
as part of imaging system 200, such as part of illumination fibers 208 or
lights located on the end
of housing 202, or external to housing 202 (e.g., a surgical lamp, a camera
flash, etc.).
Camera(s) 206 may be of any suitable type operable to capture images in the
white light
and NIR spectrums. For example, one prototype of the FHT system herein uses a
high resolution
Canon EOS 700 white light camera available from Canon, Melville, NY, although
any other
suitable white light camera can be used in other implementations. For the
fluorescence imaging,
suitable systems include the K-FLARE , and Lab-FLARE models R1TM, R1vTM,
RP1TM,
RP2TM, RC2TM, FLARE (FLuorescence-Assisted Resection and Exploration) imaging
systems
12
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
available from Curadel LLC, Marlboro, MA. Other suitable system components may
be used, as
desired, without deviating from the teachings herein.
To reduce specular reflections, imaging system 200 may include polarizers with
camera(s) 206 and/or the illumination sources (e.g., illumination fibers 208).
For example,
imaging system 200 may use concentric linear polarizers with excitation and
emission rotated at
90 degrees, which will reduce specular reflections from the illuminated tissue
specimen 112.
FIG. 5 illustrates an example schematic block diagram of computing device 300,
according to various embodiments. As shown, computing device 300 may comprise
one or more
interfaces 310 (e.g., wired, wireless, etc.), at least one processor 320, and
a memory 340
interconnected by a system bus 350 and powered by a power supply 360.
Interface(s) 310 contain the mechanical, electrical, and signaling circuitry
for
communicating data with other computing devices in the FHT system. For
example, interfaces
310 may be communicatively coupled to camera(s) 206 and illumination fibers
208 of imaging
system 200 via cabling 212 either directly or via any number of intermediate
components. For
example, interface(s) 310 may be in communication with one or more light
sources for
illumination fibers 208, to provide control over when the light sources are
activated (e.g., to
shine fluorescent light on tissue specimen 112). Further, interface(s) 310 may
receive captured
image data from the white light and fluorescence cameras of imaging system 200
for further
image processing.
In some cases, interface(s) 310 may also be in communication with one or more
user
interface devices. Generally, a user interface device provides sensory
information to a user
and/or receives input from the user via one or more sensors. For example, user
interface devices
13
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
may include, but are not limited to, electronic displays (e.g., to display the
resulting images of
the tissue block face to the user), pointing devices (e.g., track pads, touch
screens, etc.), audio
equipment (e.g., speakers, microphones, etc.), and the like. Additionally,
interface(s) may also
communicatively couple computing device 300 to other computing devices via a
hardwired or
wireless network (e.g., to convey image data to another device, to receive
instructions from
another device, etc.).
The memory 340 comprises a plurality of storage locations that are addressable
by the
processor 320 and interface(s) 310 for storing software programs and data
structures associated
with the embodiments described herein. The processor 320 may comprise hardware
elements or
hardware logic adapted to execute the software programs and manipulate the
data structures 345,
which may include received sensor data (e.g., captured image data, etc.),
operating parameters or
settings, and the like. An operating system 342, portions of which are
typically resident in
memory 340 and executed by processor 320, functionally organizes device 300
by, inter alia,
invoking operations in support of software processes and/or services executing
on device 300.
These software processes and/or services may comprise, in various embodiments,
an
illumination controller process 347 and/or an imaging process 248, as
described herein.
It will be apparent to those skilled in the art that other processor and
memory types,
including various computer-readable media, may be used to store and execute
program
instructions pertaining to the techniques described herein. Also, while the
description illustrates
various processes, it is expressly contemplated that various processes may be
embodied as
modules configured to operate in accordance with the techniques herein (e.g.,
according to the
functionality of a similar process). Further, while the processes have been
shown separately,
14
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
those skilled in the art will appreciate that processes may be routines or
modules within other
processes.
In general, illumination controller 347 may be configured to control when the
fluorescent
light source, and possibly the white light source, is activated. As would be
appreciated, the light
source itself, such as a light emitting diode (LED), laser, etc., may be in
communication with
computing device 300 and may be optically coupled to illumination fibers 208,
to emit the
corresponding light onto the block face of tissue specimen 112. For example,
illumination
controller 347 may control when imaging system 200 illuminates tissue specimen
112 with NIR
or IR wavelengths, to provoke an excitation response from the fluorophore(s)
present within the
tissue.
Imaging process 248 may be operable to acquire and/or process images captured
by
imaging system 200. For example, imaging process 248 may send control signals
to camera(s)
206 to capture white light and/or fluorescence images of the block face of
tissue specimen 112.
In turn, imaging process 248 may receive the captured image data from
camera(s) 206 and
perform image processing, as described below, to generate a finalized image
for output to an
electronic display. In some embodiments, imaging process 348 may be further
configured to
control one or more automated functions of cryomicrotome 100, such as
automated actuation of
cutting blade 108, movement of specimen retainer 110, etc.
Referring now to FIG. 6, an example simplified procedure 600 is shown for
preparing a
tissue specimen for FHT image acquisition, according to various embodiments.
As shown,
procedure 600 may start at step 605 and continue on to step 610 at which the
subject
sample/specimen is prepared. In various embodiments, this step may entail
selecting a suitable
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
fluorophore for the tissue specimen and injecting the selected fluorophore(s)
into the specimen.
For example, as shown previously in Table 1, different fluorophores may have
different spectral
properties and applications. After a suitable accumulation time subsequent to
the fluorophore
injection, the soft tissue for analysis may be excised.
At step 615, after preparation of the subject specimen to undergo FHT imaging,
the
subject may be embedded in a block of OCT compound. Any suitable OCT compound
may be
selected for this step. The OCT-encased subject tissue block may then be
installed into position
within the cryomicrotome (e.g., as shown in FIG. 1B).
Procedure 600 may also include a step 620 in which the FHT imaging system
components described above are positioned and pointed at the block face of the
subject block.
For example, imaging system 200 may be positioned in front of the specimen
block face of tissue
specimen 112 within chamber 106 of cryomicrotome 100 for imaging of the block
face, as
described above. Notably, the fluorescence components may be positioned such
that as much of
the subject specimen as possible is in focus for the camera and the subject
subtends the largest
possible field of view without occlusion.
Procedure 600 may also include a step 625 at which a fluorescence channel is
selected for
the imaging system. Notably, different NIR channels may be selected, based on
the fluorophore
used on the tissue specimen in step 610. If multiple fluorophores are used on
the specimen, the
corresponding NIR channels may be selected to overlap the spectral properties
of the
fluorophores.
Similar to step 620, procedure 600 may include a step 630 at which a high
resolution
white light camera is pointed at the tissue specimen. If, for example, the
white light camera and
16
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
fluorescence camera are both located within the same housing (e.g., housing
202), steps 620 and
630 may be performed at the same time by positioning the housing within the
chamber of the
cryomicrotome relative to the tissue specimen.
At step 635 of procedure 600, the user may adjust the positions of the
fluorescence and
white light cameras, as needed. For example, based on test images acquired by
the cameras, the
positions of the cameras may be further adjusted to ensure that the desired
area of the block face
of the tissue is captured, the cameras are in focus, or for any other reason.
In step 640 of procedure 600, once the white light and fluorescence imaging
systems are
positioned at a desirable location relative to the block face of the OCT
block, the components
may be locked into position. For example, if the imaging components are housed
within a single
housing, the position of the housing within the chamber of the cryomicrotome
may be solidified,
once the desired position is achieved.
At step 645 of procedure 600, after preparing and mounting the tissue
specimen/sample,
the blade(s) of the cryomicrotome may be actuated to shave the OCT block until
the tissue is
nearly exposed. In other words, on completion of step 645, the exposed block
face may
comprise only a very fine layer of OCT compound in front of the encased tissue
for imaging.
Procedure 600 then ends at step 650.
Referring now to FIG. 7, an example simplified procedure 700 for performing
FHT on a
tissue specimen is shown, according to various embodiments. In some
embodiments, procedure
700 may be performed in whole, or in part, by operating a FHT system having a
computing
device (e.g., device 300) in communication with an imaging system (e.g.,
imaging system 200).
Procedure 700 may start at step 705 and continue on to step 710 where, as
described in greater
17
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
detail above, the room lights may be activated in the room in which the FHT
system is located.
Depending on the capabilities of the imaging system, ambient light from the
room lights may
provide sufficient white light for purposes of imaging.
At step 715 of procedure 700, the FHT system may acquire an image of the block
face of
the tissue specimen using its white light camera. In particular, the computing
device of the FHT
system may signal the white light camera to capture a high definition, white
light image of the
block face of the tissue specimen within the chamber of the cryomicrotome.
At step 720 of procedure 700, the FHT system may also capture one or more
images
using its fluorescence imaging components simultaneously with the step 715 or
within a short
time before or thereafter. In some embodiments, the fluorescence imaging
components may
capture both white light and fluorescence/NIR images of the block face of the
tissue specimen.
For example, the fluorescence camera may capture images of the block face with
the room lights
activated, with and without fluorescence illumination, as well.
At step 725 of procedure 700, the room lights may be disabled to remove the
white light
source from the tissue specimen. Alternatively, if a dedicated white light
source is used, steps
710 and 725 may entail turning the white light source on and off, as needed.
At step 730 of procedure 700, the FHT system may also capture an image of the
block
face under fluorescence illumination with the room lights deactivated. Thus,
as a result of steps
715, 720, and 730, the FHT system may have any or all of the following
distinct images of the
block face: 1.) a white light image captured by the white light camera while
the block face was
illuminated with white light (e.g., with the room lights on), 2.) a
fluorescence image captured by
the fluorescence imaging system while the block face was illuminated solely
with white light, 3.)
18
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
a fluorescence image captured by the fluorescence imaging system while the
room lights and
fluorescent light sources were both on, 4.) a fluorescence image captured by
the fluorescence
imaging system while all light sources were off, and 5.) a fluorescence image
captured by the
fluorescence imaging system while the white light source was off and the
fluorescent lighting
source was turned on.
At step 735, the room lights may be reactivated, after completing the imaging
of the
block face. In turn, at step 740, the blade(s) of the cryomicrotome may be
activated to cut the
OCT block, thereby exposing another portion of the sample for imaging. At or
around this time,
any breaks in the protocol defined by the steps above may be noted at a step
745. For example,
if a superfluous image was taken, a note may be may and associated with any of
the images
captured of the exposed block face, thereby allowing these images to be
discarded or otherwise
ignored.
At step 750 of procedure 700, a decision may be made as to whether the removed
layer of
tissue from the OCT block is the last layer of interest. If not, procedure 700
may return to step
715, thereby repeating steps 715-745 for the newly exposed layer. However, if
the final layer of
tissue has been imaged, procedure 700 may continue on to step 755 where
procedure 700 then
ends.
It should be noted that while certain steps within procedures 600-700 may be
optional as
described above, the steps shown in FIGS. 6-7 are merely examples for
illustration, and certain
other steps may be included or excluded as desired. Further, while a
particular order of the steps
is shown, this ordering is merely illustrative, and any suitable arrangement
of the steps may be
utilized without departing from the scope of the embodiments herein. Moreover,
while
19
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
procedures 600-700 are described separately, certain steps from each procedure
may be
incorporated into each other procedure, and the procedures are not meant to be
mutually
exclusive.
Referring now to FIG. 8, an illustration 800 is shown of the registration of
images within
a FHT system, according to various embodiments. In particular, the FHT
computing device
(e.g., device 300 executing imaging process 248) may co-register the various
captured images
(e.g., images captured via procedure 700 shown in FIG. 7), to generate the
finalized image(s).
The finalized images may then be provided to an electronic display or another
user interface
device, for review by a human user.
For purposes of illustration, assume the following labels are assigned to
their
corresponding images:
WL FLARE ON: the image 805 captured in the white light channel of the
fluorescence
imaging system of the block face illuminated with white light.
WL FLARE OFF: the image captured in the white light channel of the
fluorescence
imaging system of the block face with all white lights off but fluorescence
excitation light on.
FL FLARE ON: the image 815 captured in the fluorescence channel of the
fluorescence
imaging system with the block face illuminated with both fluorescence
excitation and white
light.
FL FLARE OFF: the image 820 captured in the fluorescence channel of the
fluorescence imaging system with the block face illuminated solely with
fluorescent excitation
light.
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
WL HIGH ON: the image(s) 810 captured by the high resolution white light
camera
with the block face illuminated with white light.
In various embodiments, the computing device may perform image processing by
performing any or all of the following, as shown in FIG. 8:
1. Co-register WL HIGH ON images to themselves, creating an aligned image
stack.
2. Co-register WL FLARE ON to WL HIGH ON.
a. FL FLARE ON is natively co-registered to WL FLARE ON.
3. Co-register WL FLARE OFF to FL FLARE OFF.
4. Perform next-image fluorescence processing on the aligned FL FLARE OFF.
Notably, the computing device may perform co-registration on a per-image
basis, with the
exception of the WL HIGH ON images.
As would be appreciated, the above procedures are exemplary only and are not
intended
to limit the teachings herein. Notably, the above procedures may be of
particular use in cases
where the output of the fluorescent light source is comparable to the white
light source (e.g.,
stray room lighting, etc.). However, if the fluorescent light signal is
sufficiently high, the above
techniques may be modified to allow for single-slice white light and
fluorescence images to be
captured simultaneously with the room lights on and natively co-registered to
each other. In
particular, optical filters may be used on the fluorescence image in such
cases, thereby
simplifying the image captures under different lighting conditions. In some
embodiments, the
imaging components may instead comprise the LAB-FLARE imaging system from
Curadel,
LLC or a similar system. Such systems may allow for the simultaneous
acquisition of different
21
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
images (e.g., color and NIR, etc.), thereby eliminating the need to switch off
and on the white
light source (e.g., the room lights).
Example ¨ Twine Imaging
FIGS. 9A-9I illustrate test results of FHT imaging of a fluorophore-infused
piece of
twine, according to various embodiments. In particular, imaging of a piece of
twine comprising
a plurality of individual strands was performed as a proof-of-concept using a
prototype FHT
system employing the techniques herein. During testing, the following steps
were performed:
= Kitchen twine was soaked in fluorophore (AlexaFluor 647, 100nM
concentration) for 10
minutes.
= The twine was dried and wrapped around a OCT pillow.
= The wrapped pillow was embedded in a larger OCT block and frozen for
slicing by the
FHT system.
= Image data was acquired at 50 um thick sections through the OCT block.
= The camera sub-system was positioned approximately 15 cm above the block,
resulting
in a field of view (FOV) of ¨5 x 5 cm and a transverse pixel size of ¨0.085
mm.
FIGS. 9A-9B illustrate the original captured images 900-910 of the twine using
the FHT
system. As shown, images 900-910 demonstrate a contrast between the portions
of the twine
that have high concentrations of the fluorophore and the portions of the twine
that do not.
According to various embodiments, the FHT system may further employ
subtraction-
based deblurring, to produce an image for display. FIGS. 9C-9D illustrate
images 900-910,
respectively, after performing subtraction-based deblurring. According to some
embodiments,
22
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
the deblurring may also involve running a Monte Carlo simulation, using a
point spread function
method, or performing a deconvolution method. The deconvolution method may
include one of
a measured point spread function kernel, a simulated point spread function
kernel, a Richardson-
Lucy method, a Weiner filter deconvolution, a Van Cittert deconvolution, a
blind deconvolution,
or a regularized deconvolution method.
FIGS. 9E-9F illustrate further images 930-940 of the twine in both original
and deblurred
forms, respectively. FIGS. 9G-9I also depict images 950-970 of area 942 of
image 940, to
illustrate the application of different image processing techniques to the
images. In particular,
image 950 illustrates area 942 in its original form from the fluorescence
imaging system. Image
960 in FIG. 9H then illustrates image 950 after performing subtraction-based
deblurring.
Finally, in some embodiments, the FHT system may further apply edge-preserving
smoothing to
image 960, resulting in image 970 shown in FIG. 91.
Example ¨ Brain Tissue Imaging
Ex vivo imaging methodologies such as immunohistochemistry, fluorescence
imaging,
and autoradiography have been used to study anatomy, physiology and drug or
tracer distribution
in either whole bodies or excised organs. These methodologies can follow and
accompany in
vivo imaging studies or serve as stand-alone studies themselves. Because ex
vivo processing is
relatively expensive and time consuming, often sections and/or images are
taken at large
intervals (0.1-1 mm) throughout the entire specimen. Information is lost in
these gaps where
sections are not collected or imaged. Further, if a three dimensional model of
the specimen is
required, interpolation of largely spaced sections is required and the model
may suffer.
23
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
To address some of these potential drawbacks, as described above, high
resolution white
light and multispectral (700 and 800 nm) fluorescence images may be captured
off the block
after every pass of the micro- or macrotome blade using an intraoperative
fluorescence imaging
system, thus vastly improving the amount of information captured throughout
the specimen and
decreasing total acquisition time. Generally, the process of fluorescent and
high resolution white
light data collection and subsequent co-registration and/or three-dimensional
reconstruction, is
referred to herein as cryofluorescence tomography or FHT.
Using the FHT techniques herein, organs or small animal whole bodies may be
sectioned
(e.g., at 25 microns, etc.) and all images may be captured and acquired in
less than 2 hours,
improving some shortcomings of various ex vivo techniques, while enabling the
creation of high
resolution 3D models.
To investigate the ability of FHT to study the physiology of the brain,
XenoLight
RediJect 2-Deoxy-D-glucose (2-DG, PerkinElmer, 750 nm excitation), a
fluorescent glucose
metabolism tracer, was injected into the intrathecal space of a rat. After 1.5
hours of
distribution, the animal was sacrificed, the brain tissue excised, and the
sample was
cryopresevered in OCT. With each pass of the microtome blade (25 micron
spacing), a high
resolution white light and fluorescent image was acquired.
Further, to study the anatomy of ventricles and subarachnoid space, a
fluorescent
zwitterionic compound ZW800-1 (Curadel, LLC) was injected into the rat
intrathecal space.
This compound is not expected to cross into the brain parenchyma due to its
chemical properties.
As shown, ZW800-1 signal is constrained in the ventricles and subarachnoid
space, while it is
24
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
noticeably absent in brain parenchyma. One can begin to build a high
resolution, three-
dimensional map of the ventricles and subarachnoid space.
Various images captured during testing are shown in FIGS. 10A-121. Notably,
FIG.
10A-10B depict images 1000 and 1010 of the brain tissue with the white light
captures colored
gray and the fluorescence images colored orange/purple. FIG. 11A illustrates
FHT images 1100
using maximum intensity projection (MIP). FIGS. 111B-111D illustrate sagittal,
coronal, and
transverse dual camera static images 1110-1130, respectively (e.g., images
1110-1130 are
combined images of the white light and fluorescence captures). FIG. 11E-11F
illustrate dual
camera, M1P images 1140-1150, respectively.
FIGS. 12A-12C illustrate white light images 1200-1220 of the coronal, axial,
and saggital
planes, respectively. FIGS. 12D-12F illustrate fluorescent images 1230-1250 of
the 2-DG taken
up in the brain parenchyma along the coronal, axial, and saggital planes
respectively. Finally,
images 1260-1280 depict the fluorophore, ZW800-1 constrained in the ventricles
and
subarachnoid space.
Thus, as would be appreciated, FHT serves as a multispectral, high resolution,
and time
efficient ex vivo tool to study anatomy, physiology, and drug/tracer
distribution, either in concert
with in vivo studies or as a stand-alone study.
Example - Pharmacokinetic and Pharmacodynamic Imaging of Intrathecally
Administered Anti-Sense Oligonucleotides
Antisense oligonucleotides (AS0s) are promising drugs for treating central
nervous
system (CNS) disorders due to their specific targeting and extended
pharmacological effect. The
development of therapeutics for CNS disorders has been impeded by the
inability of most drug
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
molecules to cross the blood brain barrier (BBB) and engage their targets. The
intrathecal (IT)
dosing route offers a solution for bypassing the BBB and delivering drugs
directly to the CNS.
However, determining the pharmacokinetics (PK) and pharmacodynamics (PD)
presents unique
challenges imposed by anatomical and functional properties of the IT space and
reliance upon ex
vivo histological molecular techniques.
In some aspects, imaging approaches are disclosed herein using radio and
fluorophore-
labeled ASOs tracking PK. Further aspects of the techniques herein employ
neuroreceptor
targeting ASOs to enable tracking of PD using receptor-targeting radiotracers.
During testing,
these PK/PD principles were evaluated using two ASOs which target the MALAT1
house
keeping gene and the GABA-A receptor subunit GABRA1. Dynamic SPECT/CT imaging
with
the 125I-MALAT1 ASO showed widespread time and dose dependent exposure of the
neuroaxis
tissues following lumbar IT injections, with increased exposure in cortical
structures versus basal
ganglia. A dosing study using either unlabeled GABRA1 or MALAT1 ASO (n=4 per
cohort)
demonstrated progressive decline in 18F-flumazenil uptake specific to the
GABRA1 ASO, with
the effect being much greater in cortical structures as compared to basal
ganglia. We confirmed
that the reduction of 18F-flumazenil uptake corresponded to GABRA1 mRNA and
protein
reduction produced by the ASO.
According to various embodiments, a 3D-FHT imaging technique was developed to
demonstrate the correlation between the distributions of the IT administered
Cy7-labeled
GABRA1 ASO with the regional receptor knockdown demonstrated in the 18F-
flumazenil. This
3D cryofluorescence imaging technique offers a bridge between in vivo
molecular imaging and
ex vivo histology enabling the 3D visualization of the PK/PD relationship for
ASO therapy.
26
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
Specifically, during testing, two groups of four rats were treated with a
single dose of
anti-sense oligonucleotide (ASO) targeting MALAT1 or GABRA A. The rats
underwent 1 hour
dynamic 18F-flumazenil PET scans at baseline (day prior to ASO treatment),
then at 1, 2, 3, 4
weeks post treatment. Phamacodynamics of treatment is followed by 18F-
flumazenil PET,
pharmacokinetics and distribution demonstrated by Cy7-labeled GABRA1 ASO.
Image 1300 in FIG. 13A illustrates averaged MIP, sagittal, coronal and
transverse 18F-
flumazenil PET area under the curve (AUC) images for MALAT1 and GABRA A ASO-
treated
groups at 4 weeks post single-dose treatment. Images were co-registered to a
common atlas
space and scaled to units of decay-corrected uCi-min. Note the decreased 18F-
flumazenil uptake
post GABRA A versus MALAT1 ASO treatment.
FIG. 13B shows an image 1310 illustrating the MIP, sagittal, coronal and
transverse 18F-
flumazenil PET (AUC) difference images between MALAT1 and GABRA A ASO treated
groups at 4 weeks post single-dose treatment for N=4 rats per group. Note most
voxels show
positive or no change between groups.
FIG. 13C illustrates a graph 1320 of the 18F-flumazenil PET (AUC axis 1324)
for volume
of interest on cerebral cortex at baseline and at 1, 2, 3 and 4 weeks post
single dose ASO
treatment (time axis 1322). The cortex uptake showed significant reduction in
GABRA A versus
MALAT1 targeted treatment a 2, 3, and 4 weeks.
FIG. 13D depicts an image 1330 of the MIP sagittal, coronal and transverse 3D-
FHT
images of IT administered Cy7-labeled GABRA1 ASO at 1 hour post
administration.
FIG. 13E shows an image 1340 of the MIP sagittal, coronal and transverse 3D-
FHT
images of IT administered Cy7-labeled GABRA1 ASO at 4 days post
administration.
27
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
Clearing Tissue Slices
As noted during implementation of the techniques herein, the cut tissue slice
may "stick"
to the remaining tissue block after slicing, either due to electrostatic,
hydrophobic, or other
interactions between the slice and the block. Accordingly, in some
embodiments, the FHT
system may further include a mechanism that blows a puff of gas, such as air,
or a non-humidity-
containing gas such as nitrogen, onto the block face at the end of a slicing
cycle to remove any
tissue that might otherwise stick to the block and obscure imaging.
Referring now to FIGS. 14A-14C, examples are shown of a mechanism to remove
stuck
tissues slices from an FHT system, according to various embodiments. As shown,
the
mechanism may generally include a gas cylinder 1402 that stores a gas such as
air, nitrogen, or
argon. As would be appreciated, any form of gas may be selected as desired,
depending on the
type of tissue specimen, environmental conditions, etc. For example, in the
case of cryo-
applications (e.g., in a cryomicrotome), it is important that the gas does not
have water vapor
(i.e., humidity), as water vapor can freeze and block the flow the gas. Thus,
for such cryo-
applications, gasses such as nitrogen and argon may be preferable.
Generally, gas cylinder 1402 may be pneumatically coupled to the FHT system
via a
tubing system 1404 and a control mechanism 1406, as shown in FIG. 14A. In
particular, control
mechanism 1406 may be configured to regulate the flow of gas from gas cylinder
1402 through
tubing system 1404 and onto the face of the tissue block, to dislodge stuck
tissue slices. In other
words, control mechanism may adjust the force of the gas puff so that the gas
will reliably
dislodge the tissue slice completely from the block. Similarly, tubing system
1404 may convey
the gas from gas cylinder 1402 onto the tissue block face via control
mechanism 1406.
28
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
As shown in FIG. 14B, control mechanism 1406 may include a pressure regulator
1408, a
solenoid valve 1410, and control electronics 1412, in various embodiments.
Generally, pressure
regulator 1408 controls the amount of gas pressure in tubing system 1404,
allowing the user to
adjust the pressure such that any stuck tissue slices are dislodged by the gas
exiting the nozzle of
tubing system 1404. Solenoid valve 1410 may also be coupled to tubing system
1404 and
control the flow of gas through tubing system 1404. For example, when
actuated, solenoid valve
1410 may block or unblock the flow of gas through tubing system 1404 so as to
provide a puff of
gas onto the block face of the tissue specimen. In some embodiments, as shown,
control
electronics 1412 may provide electronic or computerized control over solenoid
valve 1410
and/or pressure regulator 1408, to control when the system supplies a puff of
gas and/or the
pressure of the supplied gas. Such electronics 1412 may either fully automate
the actuation of
the system or may allow a user to manually trigger the puff of gas.
FIG. 14C shows tubing system 1404 in greater detail, according to various
embodiments.
As shown, tubing system 1404 may include tubing 1414 that couples a nozzle
1416 to gas
cylinder 1402 via control mechanism 1406. Generally, nozzle 1416 may be
located at a suitable
distance from the block face of the tissue specimen and may, in some
embodiments, be coupled
to the slicing apparatus (e.g., a microtome, etc.), to ensure that nozzle 1416
remains pointed
towards the tissue specimen after each puff of gas. For example, in the case
of a cryomicrotome,
tubing 1414 and nozzle 1416 may be located within the temperature-controlled
chamber of the
cryostat, thereby cooling the gas to the temperature of the frozen ice block.
This prevents
melting of the tissue block that would occur if the gas were at room
temperature.
Fiducial Placement
29
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
Precise positioning of fiducial markers in a tissue block can be a difficult
and time-
consuming procedure. Fiducial markers are critical for FHT applications
because they permit
orientation of each slice relative to the original block, and permit
correction for geometric and
color aberrations during slicing and imaging. In some embodiments, they also
permit calculation
of the point-spread function, which is required to correct for the attenuation
of light due to
absorption and scattering, and thus reconstruction of the proper fluorescence
image.
Referring now to FIGS. 15A-15B, an example apparatus 1500 for precisely
placing
fiducial markers in a tissue block is shown, according to various embodiments.
As shown,
apparatus 1500 may be configured to engage a tissue chamber 1504 in which a
tissue specimen is
retained. Extending through apparatus 1500 may be any number of apertures
through which
fiducials 1502 may be placed, thereby inserting fiducials 1502 into the tissue
specimen.
Apparatus 1500 may be formed of any suitable material such as ABS plastic or
the like.
Preventing Damage to the Cutting Blade
Damage to the cutting blade of the histological slicing instrument may also be
problematic in an FHT system, especially with automated and semi-automated
systems. This
occurs when the specimen retainer 110, typically made of metal, moves into the
path of the
cutting blade.
Referring now to FIG. 16, an example of multi-colored fiducial markers 1502 is
shown.
To prevent damage to the blade of the slicing instrument, the fiducial markers
1502, such as
pieces of dry Angel hair pasta, may be dipped in a solution of chemical or
paint to mark one end
with a color different from the original color. For example, if fiducial 1502a
is gray in color, the
distal 1-2 mm might be colored black. Or, if fiducial 1502b is black, such is
the case with squid
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
ink-infused pasta, the distal 1-2 mm might be painted or bleached to a gray or
white color.
Then, the fiducial 1502 is placed onto the specimen retainer 110 such that the
end closest to the
block support has the alternate color of a specified length ("the unsafe
zone").
After the tissue block is placed in the slicer, and slicing begins, custom
imaging software
may continuously identify both the position and color of the fiducial 1502.
When the color
changes from the primary color to the alternate color, the software will stop
all slicing, thus
preventing damage to the blade. In additional embodiments, the colors
throughout the vertical
height of the fiducial could be selected so that the exact depth of each slice
could be estimated.
For example, if a 5 mm fiducial changed color every mm from red, to orange, to
blue, to green,
to yellow, the depth of cutting could be estimated by imaging the transition
from one color to
another and interpolating based on a known tissue slice depth.
The techniques herein, therefore, provide for the mounting of a FHT imaging
system
within the chamber of a cryomicrotome, to perform FHT imaging on the block
face of a tissue
specimen within the chamber. In some aspects, the imaging components of the
system may be
located within a transportable housing, thereby protecting the components from
the conditions
within the chamber and allowing a user to install, position, and remove the
imaging components
from the cryomicrotome as desired. Thus, the FHT system herein can be easily
adapted for use
with any number of existing cryomicrotomes without significant modification.
The methods according to the inventive concepts may be embodied as a non-
transitory
computer program product. Any combination of one or more computer readable
storage
device(s) or computer readable media may be utilized. The computer readable
medium may be a
computer readable storage medium. A computer readable storage device may be,
for example,
31
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
but not limited to, an electronic, magnetic, optical, electromagnetic, or
semiconductor system,
apparatus, or device, or any suitable combination of the foregoing. More
specific examples (a
non-exhaustive list) of the computer readable storage device would include the
following: a
portable computer diskette, a hard disk, a random access memory (RAM), a read-
only memory
(ROM), an erasable programmable read-only memory (EPROM or Flash memory), an
optical
fiber, a portable compact disc read-only memory (CD-ROM), an optical storage
device, a
magnetic storage device, or any suitable combination of the foregoing. In the
context of this
document, a computer readable storage device may be any tangible device or
medium that can
store a program for use by or in connection with an instruction execution
system, apparatus, or
device. The term "computer readable storage device," or variations thereof,
does not encompass
a signal propagation media such as a copper cable, optical fiber or wireless
transmission media.
Program code embodied on a computer readable storage device or computer
readable
medium may be transmitted using any appropriate medium, including but not
limited to wireless,
wireline, optical fiber cable, RF, etc., or any suitable combination of the
foregoing.
Computer program code for carrying out operations for aspects of the present
invention
may be written in any combination of one or more programming languages,
including an object
oriented programming language such as Java, Smalltalk, C++ or the like and
conventional
procedural programming languages, such as the "C" programming language or
similar
programming languages. The program code may execute entirely on the user's
computer, partly
on the user's computer, as a stand-alone software package, partly on the
user's computer and
partly on a remote computer or entirely on the remote computer or server. In
the latter scenario,
the remote computer may be connected to the user's computer through any type
of network,
32
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
including a local area network (LAN) or a wide area network (WAN), or the
connection may be
made to an external computer (for example, through the Internet using an
Internet Service
Provider).
Aspects of the present invention are described herein with reference to
flowchart
illustrations and/or block diagrams of methods, apparatus (systems) and
computer program
products according to embodiments of the invention. It will be understood that
each block of the
flowchart illustrations and/or block diagrams, and combinations of blocks in
the flowchart
illustrations and/or block diagrams, can be implemented by computer program
instructions.
These computer program instructions may be provided to one or more processors
of one or more
general purpose computers, special purpose computers, or other programmable
data processing
apparatuses to produce a machine, such that the instructions, which execute
via the one or more
processors of the computers or other programmable data processing apparatuses,
create means
for implementing the functions/acts specified in the flowchart and/or block
diagram block or
blocks.
These computer program instructions may also be stored in one or more computer
readable storage devices or computer readable media that can direct one or
more computers, one
or more other programmable data processing apparatuses, or one or more other
devices to
function in a particular manner, such that the instructions stored in the one
or more computer
readable storage devices or computer readable medium produce an article of
manufacture
including instructions which implement the function/act specified in the
flowchart and/or block
diagram block or blocks.
33
CA 02987919 2017-11-29
WO 2017/040671
PCT/US2016/049708
The computer program instructions may also be loaded onto one or more
computers, one
or more other programmable data processing apparatuses, or one or more other
devices to cause
a series of operational steps to be performed on the one or more computers,
one or more other
programmable data processing apparatuses, or one or more other devices to
produce a computer
implemented process such that the instructions which execute on the one or
more computers, one
or more other programmable data processing apparatuses, or one or more other
devices provide
processes for implementing the functions/acts specified in the flowchart
and/or block diagram
block or blocks.
The terminology used herein is for the purpose of describing particular
embodiments only
and is not intended to be limiting of the invention. As used herein, the
singular forms "a," "an"
and "the" are intended to include the plural forms as well, unless the context
clearly indicates
otherwise. It will be further understood that the terms "comprises" and/or
"comprising," when
used in this specification, specify the presence of stated features, integers,
steps, operations,
elements, and/or components, but do not preclude the presence or addition of
one or more other
features, integers, steps, operations, elements, components, and/or groups
thereof.
The corresponding structures, materials, acts, and equivalents of all means or
step plus
function elements in the claims below are intended to include any structure,
material, or act for
performing the function in combination with other claimed elements as
specifically claimed.
The description of the present invention has been presented for purposes of
illustration and
description, but is not intended to be exhaustive or limited to the invention
in the form disclosed.
Many modifications and variations will be apparent to those of ordinary skill
in the art without
departing from the scope and spirit of the invention. The embodiments were
chosen and
34
CA 02987919 2017-11-29
WO 2017/040671 PCT/US2016/049708
described in order to best explain the principles of the invention and the
practical application,
and to enable others of ordinary skill in the art to understand the invention
for various
embodiments with various modifications as are suited to the particular use
contemplated.