Note: Descriptions are shown in the official language in which they were submitted.
COLORED SUTURE CONSTRUCTION
[0001] This is a division of Canadian patent application no. 2,701,546
filed April 26,
2010.
FIELD OF THE INVENTION
[0002] The present invention generally relates to a suture. More
particularly, the
present invention pertains to a colored construction for sutures.
BACKGROUND OF THE INVENTION
[0003] Sutures are commonly used to hold together tissue that has been
severed by
injury, incision, or surgery to aid in the healing process or to apply
pressure to blood
vessels to stop bleeding. Sutures can be absorbable or non-absorbable. Non-
absorbable sutures are made from inherently colorless materials such as silk,
polypropylene, polyester, nylon, or ultra high molecular weight polyethylene
(UHMWPE).
Sutures are commonly in either braided multifilament or monofilament
construction, but
are sometimes also known in a twisted construction. The form of a suture is
usually
dictated by the stiffness or fiber modulus of its constituent fiber. Sutures
require good
pliability for their handling and knotting properties. Thus, fibers that are
too stiff cannot
be used in a monofilament form for larger suture sizes but rather in bundles
of small
denier filaments. These strands of multifilament are then braided together to
form a
suture. As the suture diameter gets larger, a core is usually added inside the
braided
sheath. Fibers with a modulus below about 600,000 psi can be used in
monofilament
form, although lower modulii are preferred. Thus, polyester and UHMWPE sutures
are
used in multifilament braids, while polypropylene is used as a monofilament
suture.
Some materials like nylon 66 with borderline properties are made in both
multifilament
and monofilament constructions. Natural fibers like silk that are fine denier
can only be
used in multifilament constructions.
[0004] Synthetic and most natural materials used to make sutures are
without color.
With the two basic constructions of braided multifilament and monofilament
many types
of sutures would be indistinguishable from other sutures even though their
properties
might be quite different. Thus, the value of coloring in sutures has long been
1
CA 2988667 2017-12-12
recognized. Not only does color provide distinction between different types of
sutures
but is also known to aid the surgeon to keep track of sutures in the blood
field during
surgery.
[0005] Sutures are implantable devices and only specific colorants listed
in the Code
of Federal Regulations (CFR) for a particular suture material can be used. The
regulations also put a limit on the maximum weight percent for each colorant
for each
suture material. Thus, there are limitations in colors available for all
suture materials.
There are also certain inherent limitations to how color can be added to some
suture
materials based on their polymer properties and how they are processed into
sutures.
[0006] The advent of endoscopic surgery procedures has put additional
pressure on
surgeons to correctly identify sutures and their respective ends for proper
tying of knots
in confined spaces. During suturing it may be necessary for a physician to
distinguish
between the ends of similar sutures, which becomes more difficult when both
ends of the
sutures have an identical appearance of either no color or the same color.
[0007] One way to aid a surgeon in distinguishing an incoming end from an
outgoing
end of a suture, is to use a "half and half" suture, in which one half or one
end is colored
and the other half or end remains white. For example, Teleflex Medical of 1295
Main
Street, Coventry, CT 06238, has made a polyester braided tape since 1998 in
which one
half of it has been dyed with D&C Green No. 6. So the tape has one end that is
green
and one end that is undyed white. However, a suture made from white UHMWPE
yarn
is not dyeable owing in part to the high crystallinity of the fiber and
therefore cannot be
dip dyed to distinguish one side from the other. To add color to a colorless
braid of
UHMWPE, it is therefore necessary to incorporate either a monofilament or a
multifilament of a dyeable fiber material into the braided suture
construction. This
construction can then be immersed half way into a dye bath that will only
color the
dyeable fiber and not the UHMWPE fiber. Although it is highly unusual to co-
braid a
monofilament with multifilament yarn in a suture construction, monofilaments
can be
used for this application as they can bring a brighter color to the overall
braid.
2
CA 2988667 2017-12-12
[0008] Although the half and half does allow the surgeon to distinguish
between the
incoming and outgoing ends of one individual suture strand, it offers no help
when two or
more of the same type of suture is being used. In these cases, there is no
differentiation
between the various incoming and outgoing ends of suture from each other.
[0009] Additionally, while the ends are distinguishable in an individual
half and half
suture, one half of the suture remains white and there can still be
considerable white
glare observed under bright lights, such as those used in endoscopic surgery.
It is
therefore also necessary to reduce the glare on the colorless portions of
these sutures.
[0010] Accordingly, it is desirable to provide a UHMWPE suture which has
distinguishable ends, a distinct junction, and has a reduced glare in the
surgical field.
SUMMARY OF THE INVENTION
[0011] The foregoing needs are met, to a great extent, by the present
invention,
wherein in some embodiments a suture that is capable of overcoming the
disadvantages
described herein at least to some extent is provided.
[0012] In accordance with an embodiment of the present invention, a suture
includes an elongate woven braid of filaments. The elongate woven braid of
filaments
is made up of one or more first ends of ultra-high molecular weight
polyethylene
(UHMWPE). At least one second end is made from a colorable material. The at
least
one second end is a single continuous color. At least one third end is made
from a
dyeable material. The at least one third end is dyed a continuous color for a
portion of
its length for any one colorant.
[0013] In accordance with another embodiment of the present invention, a
suture
includes an elongate woven braid of filaments. The elongate woven braid of
filaments
is made up of at least a first end including ultra-high molecular weight
polyethylene
(UHMWPE) multi-filament fiber and at least a second made from a dyeable fiber
material. The second end is a single continuous first color. At least a third
end is made
from a dyeable fiber material. At least a first portion of the third end is
dyed a
continuous second color. The second color can be different from the first
color. A
second portion of the third end can also be dyed a continuous third color,
different from
3
CA 2988667 2017-12-12
,
the second color. The third color can also be different from the first color.
A junction
line between the first portion of the at least one third end and the second
portion of the
third end is distinct due to an application of an anti-wicking compound during
a step in
formation of the suture.
[0014] In accordance with another embodiment of the present invention,
the at least
one second end of the suture can be made from a fiber than can be dyed like
one of
nylon or polyester. The at least one third end can be made from one of
polypropylene
monofilament, nylon, polyethylene, or polyester. Additionally, the suture can
include a
core. One half of a length of the elongate woven braid can be dipped into a
dye bath to
color the at least one third end. Alternately, the elongate woven braid can be
dipped
into a dye bath to color the third end and to etch the first ends comprising
the ultra-high
molecular weight polyethylene. A dye used to color the at least one second end
can be
a mordant dye and the dye used to color the at least one third end is one of
an acid, vat
or solvent dye. In addition, the braid can include a first color pattern on a
first portion of
the braid and a second color pattern different from the first color pattern on
a second
portion of the braid.
[0015] In accordance with another aspect of the present invention, a
method of
manufacture of a suture includes braiding an elongate suture from at least a
first end of
ultra-high molecular weight polyethylene, at least a second end made from a
dyeable
material, and at least a third end made from a dyeable material. The method
also
includes dyeing the second end a single continuous first color and dyeing at
least a
portion of the third end a continuous second color. The method can also
include dyeing
the second and third ends before braiding or alternately dyeing the first
portion of the
third end after the third end is braided into the suture. A second portion of
the third end
of the suture can be dyed a third continuous color. Additionally, the method
can include
dipping at least a half of the elongate suture into a dye bath.
[0016] There has thus been outlined, rather broadly, certain embodiments
of the
invention in order that the detailed description thereof, herein may be better
understood,
and in order that the present contribution to the art may be better
appreciated. There
4
CA 2988667 2017-12-12
are, of course, additional embodiments of the invention that will be described
below and
which will form the subject matter of the claims appended hereto.
[0017] In this respect, before explaining at least one embodiment of the
invention in
detail, it is to be understood that the invention is not limited in its
application to the details
of construction and to the arrangements of the components set forth in the
following
description or illustrated in the drawings. The invention is capable of
embodiments in
addition to those described and of being practiced and carried out in various
ways. Also,
it is to be understood that the phraseology and terminology employed herein,
as well as
the abstract, are for the purpose of description and should not be regarded as
limiting.
[0017a] Another embodiment of the invention relates to a suture comprising:
an elongate braid of woven fibers comprising:
at least a first end comprising ultra-high molecular weight polyethylene;
at least a second end wherein the second end is dyed a single continuous
first color along the entire length of the second end; and
at least a third end wherein only a first portion of the third end is dyed a
continuous second color; wherein a junction line between the dyed first
portion of the at
least one third end and an undyed second portion of the third end is distinct
due to an
application of an anti-wicking compound during a step in formation of the
suture and the
dyed first portion is distinct in color from the undyed second portion.
[0017b] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the second color is different from the first color.
[0017c] Another embodiment of the invention relates to the suture defined
hereinabove, wherein a second portion of the third end is dyed a continuous
third color,
different from the second color.
CA 2988667 2017-12-12
[0017d] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the braid includes a first color pattern on a first
portion of the
braid and a second color pattern different from the first color pattern on a
second
portion of the braid.
[0017e] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the second end comprises one of polypropylene
monofilament,
nylon, polyethylene, or polyester.
[0017f] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the third end comprises one of nylon or polyester.
[0017g] Another embodiment of the invention relates to the suture defined
hereinabove, wherein one half of a length the elongate woven braid is dipped
into a dye
bath to color the third end.
[0017h] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the elongate woven braid is dipped into a dye bath to
color the
third end and to etch or shadow the first ends comprising the ultra-high
molecular
weight polyethylene.
[0017i] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the dye used to color the second end is a mordant dye.
[0017j] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the dye used to color the third end is an acid, vat, or
solvent dye.
[0017k] Another embodiment of the invention relates to a comprising:
an elongate woven braid of fibers comprising:
one or more first ends comprising ultra-high molecular weight
polyethylene;
at least one second end wherein the second end is dyed a single first
continuous color; and
at least one third end wherein only a portion of the third end is dyed a
second continuous color and the first color is different than the second
color.
6
CA 2988667 2017-12-12
[00171] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the at least one second end comprises one of
polypropylene
monofilament, nylon, polyethylene, or polyester.
[0017m] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the at least one third end comprises one of nylon or
polyester.
[0017n] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the braid includes a first color pattern on a first
portion of the
braid and a second color pattern different from the first color pattern on a
second
portion of the braid.
[00170] Another embodiment of the invention relates to the suture defined
hereinabove, wherein one half of a length of the elongate woven braid is
dipped into a
dye bath to color the at least one third end.
[0017p] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the elongate woven braid is dipped into a dye bath to
color the
third end and to etch or shadow the first ends comprising the ultra-high
molecular
weight polyethylene.
[0017q] Another embodiment of the invention relates to the suture defined
hereinabove, wherein a dye used to color the at least one second end is a
mordant dye.
[0017r] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the dye used to color the at least one third end is one
of an acid,
vat, or a solvent dye.
[0017s] Another embodiment of the invention relates to the suture defined
hereinabove, wherein a junction line between the first portion of the at least
one third
end and a second portion of the third end is distinct due to an application of
an anti-
wicking compound during a step in formation of the suture.
[0017t] Another embodiment of the invention relates to a method of
manufacture of
a suture comprising:
7
CA 2988667 2017-12-12
braiding an elongate suture from at least a first end of ultra-high molecular
weight polyethylene, at least a second end and at least a third end;
dyeing the second end a single continuous first color along the entire length
of the second end;
dyeing only a first portion of the third end a continuous second color
different
from the first color.
[0017u] Another embodiment of the invention relates to the method defined
hereinabove, wherein the second and third ends are dyed before braiding.
[0017v] Another embodiment of the invention relates to the method defined
hereinabove, wherein the first portion of the third end is dyed after the
third end is
braided into the suture.
[0017w] Another embodiment of the invention relates to the method defined
hereinabove, further comprising dyeing a second portion of the third end a
third
continuous color.
[00174 Another embodiment of the invention relates to the method defined
hereinabove, further comprising dipping at least a half of the elongate suture
into a dye
bath.
[0017y] Another embodiment of the invention relates to a suture comprising:
a woven braid of fibers comprising:
at least one first fiber;
at least one second fiber comprising a colorable material wherein
the at least one second fiber has been colored a single color along an
entire length of the at least one second fiber; and
at least one third fiber comprising a colorable material wherein
only a portion of the at least one third fiber is colored a continuous
color.
8
CA 2988667 2020-02-07
. ,
[00174 Another embodiment of the invention relates to the suture defined
hereinabove, wherein the at least one first fiber is ultra-high molecular
weight
polyethylene and is at least one of either: white and clear.
[0017aa] Another embodiment of the invention relates to the suture defined
hereinabove, wherein either the at least one second fiber or the at least one
third fiber
includes at least one material of the following materials: polypropylene
monofilament,
nylon, polyethylene, and polyester.
[0017a13] Another embodiment of the invention relates to the suture defined
hereinabove, wherein each of the at least one first fiber, the at least one
second fiber
and the at least one third fiber has a distinct color, wherein the at least
one first fiber is
at least one of: white and clear; and wherein the at least one second fiber
and at least
one third fiber are colored different colors from each other and the at least
one first
fiber.
[0017ac] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the at least one of the at least one first fiber, the at
least one
second fiber and the at least one third fiber is at least partly blue and at
least one of the
at least one first fiber, the at least one second fiber and the at least one
third fiber is at
least partly green.
[0017ad] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the at least one third fiber is colored only about
halfway along its
length.
[0017ae] Another embodiment of the invention relates to the suture defined
hereinabove, wherein one or both of the at least one second fiber and the at
least one
third fiber are co-plied with the at least one first fiber.
[0017af] Another embodiment of the invention relates to the suture defined
hereinabove, wherein the at least one first fiber, the at least second fiber,
and the
colored portion of the at least one third fiber are all different colors from
each other.
9
CA 2988667 2020-02-07
[0017ag] Another embodiment of the invention relates to a method of
manufacturing
a suture comprising:
braiding an elongate suture from at least one first fiber, at least one
second fiber comprising a colorable material, and at least one third fiber
comprising a colorable material;
coloring the at least one second fiber a single first color along an entire
length of the at least one second fiber; and
coloring only a first portion of the at least one third fiber a second color.
[0017ah] Another embodiment of the invention relates to the method defined
hereinabove, wherein the at least one second fiber and the first portion of
the at least
one third fiber are colored before braiding.
[0017ai] Another embodiment of the invention relates to the method defined
hereinabove, wherein the first portion of the at least one third fiber is
colored after the at
least one third fiber is braided into the suture.
[0017aj] Another embodiment of the invention relates to the method defined
hereinabove, further comprising coloring a second portion of the at least one
third fiber
a third color.
[0017ak] Another embodiment of the invention relates to the method defined
hereinabove, further comprising dipping at least a half of the elongate suture
into a dye
bath.
[0017a1] Another embodiment of the invention relates to the method defined
hereinabove, wherein the at least one first fiber is ultra-high molecular
weight
polyethylene.
[0017am] Another embodiment of the invention relates to the method defined
hereinabove, further comprising co-plying one or both of the at least one
second fiber
and the at least one third fiber with the at least one first fiber.
CA 2988667 2020-02-07
. ,
[0017an] Another embodiment of the invention relates to the method defined
hereinabove, further comprising co-plying one or both of the at least one
second fiber
and the at least one third fiber on a braider bobbin of the at least one first
fiber.
[0017a0] Another embodiment of the invention relates to the method defined
hereinabove, wherein the color of the at least one first fiber, the color of
the at least one
second fiber and the color of the at least one third fiber are all different
from each other.
[0017ap] Another embodiment of the invention relates to a suture comprising:
a woven braid of fibers having:
a first material component;
a second material component wherein the second material component
is a colorable material colored a single color along an entire length of the
second
material component; and
a third material component wherein the third material component is
colored a continuous color only along a portion of an entire length of the
third
material component.
[0017aq] Another embodiment of the invention relates to the suture defined
hereinabove, wherein one or both of the second and third material components
are
monofilaments co-plied with the first material component.
[0017ar] Another embodiment of the invention relates to the method defined
hereinabove, wherein one or both of the second and third material components
are
multifilaments co-plied with the first material component.
[0017as] Another embodiment of the invention relates to the method defined
hereinabove, wherein the first material component is ultra-high molecular
weight
polyethylene and one or both of the second and third material components
include at
least one of the following materials: polypropylene monofilament, nylon,
polyethylene,
or polyester.
[0018] As such, those skilled in the art will appreciate that the
conception upon
which this disclosure is based may readily be utilized as a basis for the
designing of
11
CA 2988667 2020-02-07
. .
other structures, methods and systems for carrying out the several purposes of
the
present invention.
BRIEF DESCRIPTION OF THE DRAWINGS
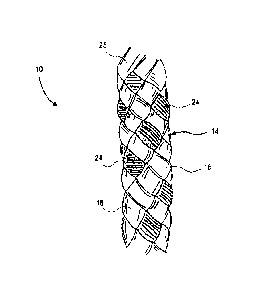
[0019] FIG. 1 illustrates a colored suture in accordance with an
embodiment of the
invention.
[0020] FIG. 2 illustrates a braided colored suture in accordance
with an embodiment
of the invention.
[0021] FIG. 3 schematically illustrates an in-line pattern for
the colored suture in
accordance with an embodiment of the invention.
[0022] FIG. 4 schematically illustrates a side by side pattern
for the colored suture in
accordance with an embodiment of the invention.
11a
CA 2988667 2020-02-07
[0023] FIG. 5 schematically illustrates a cross pattern for the colored
suture in
accordance with an embodiment of the invention.
[0024] FIG. 6 illustrates a sectional view of a braided, colored suture in
accordance
with an embodiment of the invention.
[0025] FIG 7 illustrates a partially sectional view in accordance with an
embodiment
of the invention.
DETAILED DESCRIPTION
[0026] The present invention provides, in some embodiments, a braided,
colored
suture construction. The colored suture is an elongate woven braid of
filaments
including at least a first end made of an ultra high molecular weight
polyethylene
(UHMWPE). The suture preferably includes several ends of UHMWPE braided
together.
The ends of UHMWPE are a multifilament fiber as is well known in the art. The
braided,
colored suture also includes an end made of a material which contains a
colorant which
can be either a dye or a pigment in a single continuous color, and another end
made of a
dyeable material of which at least a portion of its length is dyed a different
color.
Preferably such dyed portion of the different colored end constitutes only
half the length
of the suture. This invention provides surgeons with improved recognition of
suture ends
in surgery by construction of a bi-colored co-braid with at least two ends of
different
colors braided into a UHMWPE construction. One of the colored ends runs
continuously
from one end of the suture to the other end. The other colored end is colored
only on
one half of the end. This provides a suture with two distinguishable ends,
while still
maintaining a continuous line of color along the length of the suture.
[0027] The invention will now be described with reference to the drawing
figures, in
which like reference numerals refer to like parts throughout. FIG. 1
illustrates an end of
a braided, colored suture 10 in accordance with the present invention. The
braided,
colored suture 10 has a first half 12 above axis A and a second half 14 below
axis A, as
shown in FIG. 1. The point where the first half 12 and the second half 14 meet
is a
junction 16 of the braided, colored suture 10. In one embodiment, the braided,
colored
suture 10 can be made from UHMWPE, a material that contains a continuous
length of
12
CA 2988667 2017-12-12
colorant, like for example, polypropylene monofilament with the pigment
[phthalocyaninato(2-)] copper and another material that is dyeable, such as
nylon or
polyester. UHMWPE is generally colorless and is shown in FIG. 1 as colorless
regions
18. Additionally, the first half 12 includes colored regions 20 and 22, and
the second half
14 includes colored regions 24, which match the color of region 20. These
colored
regions 20, 22, and 24 are created by including a monofilament or
multifilament end
materials containing colorants approved for use by the United States Food and
Drug
Administration (FDA).
[0028] FIG. 2 illustrates a close-up view of the second half 14 of the
braided, colored
suture 10, shown in FIG. 1. The braided, colored suture 10 takes the form of
an
elongate woven braid of ends and has a woven appearance. The ends of the
braided,
colored suture 10 can be monofilaments or multifilament ends. The ends of the
continuous colored material, which is present in both half lengths 12 and 14,
are
interwoven with the UHMWPE to form colored regions labeled 24. The braided,
colored
suture 10 can be braided into different patterns depending on the placement of
the
bobbins holding the ends on the braiding machine. The possible patterns will
be
discussed further below.
[0029] The braided, colored suture 10 illustrated in FIGS. 1 and 2 can be
made in
one example for a USP size 2 suture by using a sixteen carrier braider to co-
braid
fourteen ends of a non-colored multifilament UHMWPE yarn 18 with one end of a
colored polypropylene monofilament, such as a blue polypropylene monofilament
and
one end of a clear monofilament nylon. The braided, colored suture 10 is then
wound on
a frame and the first half 12 is dipped into an FDA approved color bath to
color the nylon
in the first half 12 to a color such as a dark green. Neither the UHMWPE nor
the colored
polypropylene will absorb any of the dark green dye. This results in the first
half 12 of
the braided, colored suture 10 having colorless, blue, and dark green regions,
18, 20,
and 22 respectively. The second half 14 of the braided, colored suture 10 has
colorless
regions 18 and 25 and blue regions 24.
[0030] Alternately, as an example of another embodiment, the braided,
colored
suture 10 illustrated in FIGS. 1 and 2 can be made by using a sixteen carrier
braider to
13
CA 2988667 2017-12-12
co-braid fourteen ends of UHMWPE with one end of a black nylon monofilament
that is
entirely dyed with logwood extract, an FDA approved colorant, and one end of
clear
monofilament nylon. A wound frame of the braided, colored suture 10 is then
dipped
halfway into a dye bath of an FDA approved colorant to color half of the end
of the clear
nylon. The color is not absorbed by the UHMWPE or the continuous end of black
nylon
that was already entirely dyed and only half of the colorless nylon is dyed.
It appears
that since the logwood extract is a mordant dye, it is not displaced by the
dye.
[0031] Since it has been found that a dye will not affect the black color
of nylon dyed
with logwood extract, this discovery can be used to prepare two tone half and
half
sutures of the invention. For example, if the first ends are colorless UHMWPE
yarn, the
second end a continuous blue polypropylene monofilament and the third end half
dyed
black monofilament nylon, then a two tone half and half suture is created when
this braid
is processed in a dye bath. The only portion of the braid that will absorb
color is the
colorless half of the nylon to give a two tone half and half suture.
[0032] As illustrated in FIG. 1, the braided, colored suture 10 made in the
manner
described above, or made in another similar manner, will have a first half 12
which has a
color pattern that is distinguishable from a color pattern on the second half
14.
Therefore, a surgeon can distinguish a first end 26 from a second end 28 of
the braided,
colored suture 10. This prevents confusion and allows for ease and speed of
suturing
and an overall faster procedure. Having a color pattern on both the first half
12 and the
second half 14 of the braided, colored suture 10 also decreases the "white
glare" from
intense lights used in endoscopic and other surgeries. Additionally, having a
color
pattern on both the first half 12 and the second half 14 of the braided,
colored suture 10
allows a surgeon to easily distinguish the junction 16 of the first half 12 of
the braided,
colored suture 10 and the second half 14 of the braided colored suture 10. The
junction
16 appears more distinct.
[0033] FIGS. 3, 4, and 5 illustrate example patterns such as in-line, side
by side, and
cross patterns, which are a few of the many patterns that can be made by
setting up a
braiding machine in different ways. FIG. 3 illustrates an in-line pattern in
accordance
with an embodiment of the invention. A first half 112 of a braided colored
suture 110 has
14
CA 2988667 2017-12-12
a pattern with two colors and each color is repeated in-line. The field 118 of
the suture is
made from a braid of UHMWPE ends which are colorless. A first color is
represented by
areas labeled 120 and a second color is represented by areas labeled 122. The
first
color is incorporated by using a first co-braided end of a colored material
such as nylon
or polypropylene. The second color is incorporated by using a second co-
braided end of
a colorless dyeable material such as nylon which is subsequently dipped for a
portion of
the length of the suture in a dye or colorant to provide the second color. In
this example,
a first half 112 of the length of the suture is dipped. The second half 114 of
the braided,
colored suture 110 has a single colored pattern represented by areas labeled
124, which
is the result of the incorporation of the first co-braided end of colored
material and
matches the color of areas 120. Because the second half 114 of the braided,
colored
suture 110 is not dipped into a colorant, the other half of the second co-
braided end, of
colorless dyeable material, remains colorless.
[0034] FIG. 4 illustrates a side by side pattern in accordance with an
embodiment of
the invention. A first half 212 of a braided colored suture 210 has a pattern
with two
colors side by side. The field 218 of the suture is made from a braid of
UHMWPE ends
which are colorless. A first color is represented by areas labeled 220 and a
second color
is represented by areas labeled 222. The first color is above the second color
and the
two colored regions are generally parallel. The first color is incorporated by
using a first
co-braided end of a colored material such as nylon or polypropylene. The
second color
is incorporated by using a second co-braided end of a colorless dyeable
material such
as nylon which is subsequently dipped for a portion of the length of the
suture in a dye or
colorant to provide the second color. In this example, one half 212 of the
length of the
suture is dipped. The second half 214 of the of the braided, colored suture
210 has a
single colored pattern represented by areas labeled 224, which is the result
of the
incorporation of the first co-braided end of colored material and matches the
color of one
of the colored areas 220 or 222. Because the second half 214 of the braided,
colored
suture 210 is not dipped into a colorant, the other half of the second co-
braided end, of
colorless dyeable material, remains colorless.
[0035] FIG. 5 illustrates a cross pattern in accordance with an embodiment
of the
invention. A first half 312 of a braided colored suture 310 has a pattern with
two colors
CA 2988667 2017-12-12
side by side. The field 318 of the suture is made from a braid of UHMWPE ends
which
is colorless. A first color is represented by areas labeled 320 and a second
color is
represented by areas labeled 322. The first color areas are at an angle to the
second
color areas, forming a v-shaped cross pattern. The first color is incorporated
by using a
first co-braided end of a colored material such as nylon or polypropylene. The
second
color is incorporated by using a second co-braided end of a colorless dyeable
material
such as nylon which is subsequently dipped for a portion of the length of the
suture in a
dye or colorant to provide the second color. In this example, one half 312 of
the length
of the suture is dipped. The second half 314 of the of the braided, colored
suture 310
has a single colored pattern represented by areas labeled 324, which is the
result of the
incorporation of the first co-braided end of colored material such as a nylon.
Because
the second half 314 of the braided, colored suture 310 is not dipped into a
colorant, the
other half of the second co-braided end of colorless dyeable material remains
colorless.
[0036] FIGS. 6 and 7 illustrate a braided, colored suture 410 in accordance
with the
present invention. The suture 410 includes monofilament or multifilament ends
made
from materials such as UHMWPE, nylon, or polyethylene. Ends 440 are made from
UHMWPE and are generally colorless. End 442 is made from a monofilament or
multifilament end of continuously colored nylon or polyethylene. End 444 is
made from a
monofilament or multifilament of colorless nylon. The braided, colored suture
410 can
then be wound on a frame and half dipped into an FDA approved color bath to
color half
of a length of the nylon end 444 to a color such as a dark green.
Additionally, the suture
410 can optionally include a core 446, which can take the form of a
multifilament yarn, a
monofilament yarn, or a braid of monofilaments or multifilament ends 447. As
illustrated
in FIG. 7, the end 442 made from the monofilament or multifilament end of
continuously
colored nylon or polyethylene creates a color pattern 448 on the colored
braided suture
410.
[0037] In addition to, the color combinations already mentioned other
combinations
might be envisioned with other approved colorants listed by the FDA for
existing suture
materials. Thus, the dyes and colored filaments are not limited to those
discussed so far.
Any suitable FDA approved colorant or colored material can be used. For
example, a
high density polyethylene monofilament colored with a blue colorant, chromium-
cobalt-
16
CA 2988667 2017-12-12
aluminum oxide, can also be used as the second component containing continuous
color. For
the third component, polyester (polyethylene terephthalate (PET) or
polybutylene terephthalate (PBT)) could also be incorporated into the braided,
colored
suture and half-dyed.
[0038] It is
also possible that existing colorants and dyes already approved in colors
for one material will eventually be approved for other materials. For example,
for the
second component, continuous monofilaments of polypropylene monofilament might
be
colored with D&C Green #6 or D&C Violet #2. For the third component, a
multifilament
poly-L-lactide (PLLA) absorbable yarn could be incorporated into the braided,
colored
suture and half-dyed with colorants such as D&C Green # 6 or D&C Violet #2.
Although
neither of these colorants is listed for polypropylene or PLLA suture
materials, their safe
use in absorbable sutures like PGA where all the contained dye is released is
a
consideration for their use in other suture materials. Thus, the invention
described
herein cannot be limited to the current inventory of approved suture materials
and listed
color colorants, but can be put to practice with newly listed colorants, and
approved
suture materials.
[0039] In
addition to substituting one end of a continuous colored fiber and one end
of a colorless, but dyeable end into a standard braided construction, this
invention can
also be put into practice by first co-plying small monofilaments or low denier
multifilament of both the second and third component materials into the UHMWPE
multifilament braiding bobbins. This optional method is particularly suited
for suture
sizes that use small carrier braiding machines where the substitution of a
lower strength
colored material for a high strength material might affect the overall
performance
properties of the braided suture. For example, a USP size 5-0 high strength
suture is
braided on a three carrier braider. If one end of the braid is substituted
with an end with
continuous color and another end with a colorless, but dyeable fiber, then
about 67% of
the high strength fiber will be lost. This situation is remedied by co-plying
the second and
third components of the invention into the braider bobbins of the UHMWPE yarn.
When
this construction is half dyed, it will contain the benefits of this
invention. Although this
option is directed at braids that contain a small number of ends, it can
certainly be used
in all sized suture constructions. However, the amount of tensile strength
reduction
17
CA 2988667 2017-12-12
observed when replacing a high strength UHMWPE end with a lower strength end
is
always less than expected as the replacement ends appear to cushion or
insulate the
UHMWPE ends from each other which leads to higher retained tensile strength
from the
remaining UHMWPE ends.
[0040] Since
the invention is directed at improvements in the cover or outer sheath
of the suture, this invention is applicable to both coreless sutures and
sutures with cores.
The cores can be of any construction including twisted or non-twisted
multifilament,
braided cores or monofilament cores. However, for most sizes of UHMWPE braided
suture a coreless construction is preferred. This is because a coreless
construction
gives better knotting properties for sutures made from high modulus UHMWPE
yarn.
For other suture materials a core is normally needed to prevent the sheath
from
collapsing in suture sizes greater than about USP size 5-0 to a flat profile
which is not
optimum for suturing. The extremely high fiber modulus of UHMWPE of over 75
GPa is
probably the main factor that keeps it from flattening during its handling, as
opposed to
the fiber modulus of about 3 ¨ 10 GPa in other synthetic braided fibers. The
coreless
UHMWPE braid will flatten when it compressed by the forces knotting. This
phenomenon contributes to its lower knot profile and improved knotting
properties.
[0041] In the
course of the experiments for this invention is has also been
recognized that certain dyeing conditions of dye concentration, added acid
concentrations, temperature and time of dyeing can sometimes yield UHMWPE
fiber
which contains some degree of shadowing or surface modification. In some
cases, it
appears the normal bright finish of the UHMWPE has been diminished.
Accordingly it
may be possible to also reduce the "glare" of the non-colored UHMWPE sutures
by a
surface modification technique. It is well known that the surface of
polyethylene can be
modified by chemical treatment, photochemical treatment, surface grafting,
surface
oxidation, halogenation, plasma, halogenation or corona treatment. The
addition of
shadowing to the UHMWPE surface appears to enhance the differentiation
provided by
the half dyed end of the suture of this invention. Thus, shadowing of fibers
used in
suture constructions may be an important property for distinguishing sutures.
18
CA 2988667 2017-12-12
[0042] The half dyeing process can be carried out by a number of different
process
methods. One of the preferred methods is to wind the suture on a frame and
then dip
half of it into a dye bath. Of course, the frames can be of a variety of sizes
and
configurations including flat, square, rectangular or round. For some sutures
that dyeing
could also take place on other devices or even in a skein form. The half
dyeing process
can also be carried out on pre-cut pieces of suture that are then half dyed in
bundles of
the sutures. Certainly other aids to enhance the dyeing process can be
envisioned. For
example, it has also been discovered that the junction between the half dyed
end and
the other end can also be improved by applying anti-wicking compounds like
silicone
fluids to the junction line before the dye dipping process.
[0043] The colored sutures of this invention can also be made by performing
the
half dyeing before the braiding process. In this method the colorless, but
dyeable fiber
is half dyed on a skein or a frame, and then transferred to a braider bobbin.
The suture
of this invention can then be made by braiding all three end types together on
the
braider. No additional dyeing step is necessary in this method. The procedures
for
mixing and using dye baths for the half and half process will generally follow
those
known to practitioners of the art. For example, in the green half dyeing an
aqueous
solution is prepared of D&C Green No. 5 at a concentration of about 0.02 to
0.2 % by
weight with an added concentration of about 2 to 10 % of acetic acid. The dye
bath is
heated and half of the suture is dipped into the dye bath for a time period of
about 5 to
120 minutes. The suture is rinsed of the excess dye, washed and dried for
further
processing.
[0044] It is also important to note that the palate of colors available to
color sutures
is strictly controlled, because sutures are an implantable device. Use of
any
unauthorized colorant is considered adulteration by the Color Division of the
FDA.
Colors for particular polymers must be listed in the Code of Federal
Regulations (CFR).
Therefore, colors or color combinations in sutures are extremely limited by
FDA
regulations and not by technology available to color polymers or fibers that
are used to
construct suture products.
19
CA 2988667 2017-12-12
[0045] Colorants available to provide color to polymers, fibers or braided
yarns
must be listed by the FDA and can be classified as either dyes or pigments.
Pigments
are not soluble in any solvents and must be compounded into the base polymer
resin
before it is extruded into either multifilament or monofilament yarn. Pigments
can also
be suspended in gel-like solutions to color gel spun fibers. Dyes can also be
compounded into polymer resins or added to a molten polymer at the end of a
polymerization. However, because dyes have solubility in solvent, they can be
used to
"solvent dye" fibers or braided yarns used to make sutures. Of course, this
method is
limited by the ability of the fiber to accept a particular dye. Higher
crystalline polymer
fibers like those of polypropylene or UHMWPE cannot be solvent dyed by normal
methods. The FDA names certifiable dyes as either "FD&C" for solubility in
water or
"D&C" for solubility in organic solvents. Some D&C dyes, like D&C Green # 5,
do have
limited amounts of solubility in water and can be used to dye fibers in
aqueous
solutions.
[0046] Table 1, below, lists examples of colorants for use in coloring the
sutures.
Table 1
SUTURE COLORANT Solvent MAX. WT%
MATERIAL COLORANT TYPE Dye PERMITTED
Polyethylene Cr-Co-Al Oxide pigment NO 2.0
Polypropylene [Phthalocyaninato(2-)] pigment NO 0.5
copper
Polyester (PET) D&C Green #6 dye YES 0.75
Polyester (PET) D&C Blue #6 dye YES 0.2
Nylon 66, Nylon 6 D&C Green #5 dye YES 0.6
Nylon 66, Nylon 6 Logwood Extract dye YES 1.0
Nylon 66, Nylon 6 FD&C Blue #2 dye YES 1.0
Silk Logwood Extract dye YES 1.0
PGA D&C Green #6 dye NO 0.1
PGA D&C Violet #2 dye NO 0.2
PGA-TMC D&C Green #6 dye NO 0.21
PGA-PLA (90:10) D&C Violet #2 dye NO 0.2
PLLA None NA YES NA
PDO D&C Violet #2 dye NO 0.3
PDO D&C Blue #6 dye NO 0.5
CA 2988667 2017-12-12
[0047] Table 2, below, includes examples of various embodiments, for a USP
size 2
suture having fourteen ends of 110 dtex UHMWPE yarn, of possible color
combinations
for non-absorbable and absorbable co-braids. However, some colorants included
in
this chart are not yet approved by the FDA for use in the polymers listed.
Table 2
Continuous 6-0 Dyeable
Example # Fiber Type Fiber Type Half Dye Color
1 BLUE PP 1E-MONO CLR NYL 1E-MONO GRN NYL
2 BLK NYL 1E-MONO CLR NYL 1E-MONO GRN NYL
3 GRN NYL 1E-MONO CLR NYL 1E-MONO GRN NYL
4 BLUE UPE 1E-MULTI CLR NYL 1E-MONO GRN NYL
BLUE PE 1E-MONO CLR NYL 1E-MONO GRN NYL
6 BLK NYL 1E-MONO CLR NYL 1E-MONO BLUE NYL
7 GRN PGA 1E-MULTI UND PLA 1E-MULTI VIOL
PLA
BLUE
8 PDO 1E-MONO UND PLA 1E-MULTI VIOL
PLA
9 VIOL PDO 1E-MONO UND PLA 1E-MULTI GRN PLA
GRN PGT 1E-MONO UND PLA 1E-MULTI VIOL PLA
11 VIOL PGA 1E-MULTI UND PLA 1E-MULTI GRN PLA
12 GRN PGA 1E-MULTI UND PLA 1E-MULTI VIOL PLA
[0048] Additionally, Table 3, below, details examples of braided, colored
sutures in
accordance with various embodiments of the present invention. The
configurations in
Table 3 are a subset of possible combinations described more fully in Table 5.
21
CA 2988667 2017-12-12
,
Table 3
Material configuration (UHMWPE w/CLR NYL & BLU PP)
UHMWPE Yarn CLR NYL BLU PP
Lot # Size dtex # of ends size # of ends size # of ends Pattern Example
R-452 5 440 6 5-0 1 5-0 1 Crossed X X X X
X X X
R-453 5 440 6 5-0 1 5-0 1 In-line
//////////
R-454 3/4 220 10 6-0 1 6-0 1 Crossed X X X X
X X X
R-455 3/4 220 10 6-0 1 6-0 1 In-line
//////////
R-456 2 110 14 6-0 1 6-0 ' 1 Standard //
// // 11 11
R-457 2 110 14 - 6-0 1 6-0 1 Crossed
X X X X X X X
R-458 2 110 14 6-0 1 6-0 1 In-line
//////////
R-459 1 110 10 6-0 1 6-0 1 Crossed X X X X
X X X
R-460 1 110 10 6-0 1 6-0 1 In-line
//////////
_ ___________________________________________________________________________
R-461 0 110 6 6-0 1 6-0 1 Crossed X X X X
X X X
R-462 0 110 6 6-0 1 6-0 1 In-line
//////////
R-463 2-0 55 10 7-0 1 7-0 1 Crossed X X X X
X X X
R-464 2-0 55 10 7-0 1 7-0 1 In-line
//////////
R-465 3-0 55 6 7-0 1 7-0 1 Crossed X X X X
X X X
R-466 3-0 55 6 - 7-0 1 7-0 1 In-
line //////////
[0049] Table 4, below, includes a key to the abbreviations used in
Tables 1, 2, and
3. Given the number of suture materials and colorants available there are many
other
combinations possible.
22
CA 2988667 2017-12-12
Table 4
BLUE PP: Polypropylene monofilament with [phthalocyaninato(2-)] copper
colorant.
VIOL PP: Polypropylene monofilament with D&C Violet #2 colorant.
GRN PP: Polypropylene monofilament with D&C Green #6 colorant.
CLR NYL: Clear nylon monofilament.
BLK NYL: Nylon monofilament with logwood extract colorant.
GRN NYL: Nylon monofilament with D&C Green #5 colorant.
BLUE NYL: Nylon monofilament with FD&C Blue #2 colorant.
BLUE UPE: Blue UHMWPE multifilament with chromium-cobalt-aluminum oxide
colorant.
Blue high density polyethylene (HDPE) monofilament with chromium-cobalt-
aluminum
BLUE PE oxide colorant.
UND PLA: Undyed poly-L-lactic acid (PLLA) multifilament.
VIOL PLA: Poly-L-lactic acid (PLLA) multifilament with D&C Violet #2
colorant.
GRN PLA Poly-L-lactic acid (PLLA) multifilament with D&C Green #6
colorant.
BLUE PDO: Polydioxanone monofilament with D&C Blue #6 colorant.
VIOL PDO: Polydioxanone monofilament with D&C Violet #2 colorant.
Polyglycolic acid-trimethylene carbonate copolymer monofilament with D&C Green
#6
GRN PGT: colorant.
GRN PGA: Polyglycolic acid multifilament with D&C Green #6 colorant.
VIOL PGA: Polyglycolic acid multifilament with D&C Violet #2 colorant.
[0050] Table 5, below provides additional details regarding another type of
braid
construction for sutures in accordance with various embodiments of the
invention.
Table 5 does not list the possible arrangements of the different fibers on
bobbins to be
set up on a braider machine that will give corresponding different
arrangements of non-
colored and colored fiber in the produced suture products, some of which by
example
were presented in Table 3. Additionally, there are numerous additional
arrangements of
non-colored and colored fibers that are possible, and the examples contained
in Table 5
are not meant to limit the invention in any way. In Table 5, the "CLR Nylon
size/dtex"
refers to the at least one third end" of a dyeable fiber. The "colored
size/dtex" refers to
the "at least one second end" of a continuous colored fiber. The "size" listed
for use in
the dyeable and continuous fibers is an estimate of the size of the ends based
upon
USP diameter ranges needed to braid the suture of the invention. However,
dependent
on other factors, such as handling properties or surgeon's preferences, either
larger or
smaller ends can be used in braiding.
23
CA 2988667 2017-12-12
Table 5
UHMWPE
Total Max # Ends Max # Ends Yarn CLR Nylon size//dtex
colored size/dtex
UHMWPE clear/colored
US P - # of # of # of
Size # Ends = N N - 2 N/2 dtex ends size dtex ends size dtex
ends
8 6 4 440 6 5-0 110-220 1 5-0 110-220 1 -
5 8 6 4 440 5 5-0 110-220 2 5-0 110-220 1
5 8 6 4 440 4 5-0 110-220 3 5-0 110-220 1
3/4 12 10 6 220 10 6-0 55-110 1 6-0 55-110 1
3/4 12 10 6 220 9 6-0 55-110 2 6-0 55-110 1
3/4 12 10 6 220 8 6-0 55-110 3 6-0 55-110 1
3/4 12 10 6 220 7 6-0 55-110 4 6-0 55-110 1
3/4 12 10 6 220 6 6-0 55-110 5 6-0 55-110 1 -
2 16 14 8 110 14 6-0 55-110 1 6-0 55-110 1
2 16 14 8 110 13 6-0 55-110 2 6-0 55-110 1
2 16 14 8 110 12 6-0 55-110 3 6-0 55-110 1
2 16 14 8 110 11 6-0 55-110 4 6-0 55-110 1
2 16 14 8 110 10 6-0 55-110 5 6-0 55-110 1
2 16 14 8 110 9 6-0 55-110 6 6-0 55-110 1
2 16 14 8 110 8 6-0 55-110 7 6-0 55-110 1
1 12 10 6 110 10 6-0 55-110 1 6-0 55-110 1 '
1 12 10 6 110 9 6-0 55-110 2 6-0 55-110 1
1 12 10 6 110 8 6-0 55-110 3 6-0 55-110 1
1 12 10 6 110 7 6-0 55-110 4 6-0 55-110 1
1 12 10 6 110 6 6-0 55-110 5 6-0 55-110 1
0 8 6 4 110 6 6-0 55-110 1 6-0 55-110 1
0 8 6 4 110 5 6-0 55-110 2 6-0 55-110 1
0 8 6 4 110 4 6-0 55-110 3 6-0 55-110 1
2-0 12 10 6 55 10 7-0 20-55 1 7-0 20-55 1
2-0 12 10 6 55 9 7-0 20-55 2 7-0 20-55 1
2-0 12 10 6 55 8 7-0 20-55 3 7-0 20-55 1
2-0 12 10 6 55 7 7-0 20-55 4 7-0 20-55 1
2-0 12 10 6 55 6 7-0 20-55 5 7-0 20-55 1
3-0 8 6 4 55 6 7-0 20-55 1 7-0 20-55 1 '
3-0 8 6 4 55 5 7-0 20-55 2 7-0 20-55 1
3-0 8 6 4 55 4 7-0 20-55 3 7-0 20-55 1
24
CA 2 98 8 6 67 2 0 1 7 -1 2 -1 2
,
[0051] In the braid construction detailed in Table 5, the total
number of ends is
represented by "N." The maximum number of ends made from ultra-high molecular
weight polyethylene is "N-2," and the maximum number of ends that are clear is
N/2.
[0052] The many features and advantages of the invention are
apparent from the
detailed specification, and thus, it is intended by the appended claims to
cover all such
features and advantages of the invention which fall within the true spirit and
scope of
the invention. Further, because numerous modifications and variations will
readily
occur to those skilled in the art, it is not desired to limit the invention to
the exact
construction and operation illustrated and described, and accordingly, all
suitable
modifications and equivalents may be resorted to falling within the scope of
the
invention.
CA 2988667 2017-12-12