Note: Descriptions are shown in the official language in which they were submitted.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
1
COMPOSITIONS COMPRISING BACTERIAL STRAINS
TECHNICAL FIELD
This invention is in the field of compositions comprising bacterial strains
isolated from the mammalian
digestive tract and the use of such compositions in the treatment of disease.
BACKGROUND TO THE INVENTION
The human intestine is thought to be sterile in utero, but it is exposed to a
large variety of maternal and
environmental microbes immediately after birth. Thereafter, a dynamic period
of microbial
colonization and succession occurs, which is influenced by factors such as
delivery mode,
environment, diet and host genotype, all of which impact upon the composition
of the gut microbiota,
particularly during early life. Subsequently, the microbiota stabilizes and
becomes adult-like [1]. The
human gut microbiota contains more than 500-1000 different phylotypes
belonging essentially to two
major bacterial divisions, the Bacteroidetes and the Firmicutes [2]. The
successful symbiotic
relationships arising from bacterial colonization of the human gut have
yielded a wide variety of
metabolic, structural, protective and other beneficial functions. The enhanced
metabolic activities of
the colonized gut ensure that otherwise indigestible dietary components are
degraded with release of
by-products providing an important nutrient source for the host. Similarly,
the immunological
importance of the gut microbiota is well-recognized and is exemplified in
germfree animals which
have an impaired immune system that is functionally reconstituted following
the introduction of
commensal bacteria [3-5].
Dramatic changes in microbiota composition have been documented in
gastrointestinal disorders such
as inflammatory bowel disease (IBD). For example, the levels of Clostridium
cluster XIVa bacteria
are reduced in IBD patients whilst numbers of E. coli are increased,
suggesting a shift in the balance
of symbionts and pathobionts within the gut [6-9]. Interestingly, this
microbial dysbiosis is also
associated with imbalances in T effector cell populations.
In recognition of the potential positive effect that certain bacterial strains
may have on the animal gut,
various strains have been proposed for use in the treatment of various
diseases (see, for example, [10-
13]). Also, certain strains, including mostly Lactobacillus and
Bifidobacterium strains, have been
proposed for use in treating various inflammatory and autoimmune diseases that
are not directly linked
to the intestines (see [14] and [15] for reviews). However, the relationship
between different diseases
and different bacterial strains, and the precise effects of particular
bacterial strains on the gut and at a
systemic level and on any particular types of diseases, are poorly
characterised.
There is a requirement in the art for new methods of treating inflammatory and
autoimmune diseases.
There is also a requirement for the potential effects of gut bacteria to be
characterised so that new
therapies using gut bacteria can be developed.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
2
SUMMARY OF THE INVENTION
The inventors have developed new therapies for treating and preventing
inflammatory and autoimmune
diseases. In particular, the inventors have developed new therapies for
treating and preventing diseases
and conditions mediated by IL-17 or the Th17 pathway. In particular, the
inventors have identified that
bacterial strains from the genus Roseburia can be effective for reducing the
Th17 inflammatory
response. As described in the examples, oral administration of compositions
comprising Roseburia
hominis may reduce the severity of the inflammatory response, including the
Th17 inflammatory
response, in mouse models of asthma, rheumatoid arthritis and multiple
sclerosis.
Therefore, in a first embodiment, the invention provides a composition
comprising a bacterial strain of
the genus Roseburia, for use in a method of treating or preventing a disease
or condition mediated by
IL-17 or the Th17 pathway. The inventors have identified that treatment with
bacterial strains from
this genus can reduce levels of cytokines that are part of the Th17 pathway,
including IL-17, can
alleviate the Th17 inflammatory response and can provide clinical benefits in
mouse models of
inflammatory and autoimmune diseases mediated by IL-17 and the Th17 pathway.
In particular embodiments, the invention provides a composition comprising a
bacterial strain of the
genus Roseburia, for use in a method of treating or preventing a disease or
condition selected from the
group consisting of: multiple sclerosis; arthritis, such as rheumatoid
arthritis, osteoarthritis, psoriatic
arthritis, or juvenile idiopathic arthritis; neuromyelitis optica (Devic's
disease); ankylosing spondylitis;
spondyloarthritis; psoriasis; systemic lupus erythematosus; inflammatory bowel
disease, such as
Crohn's disease or ulcerative colitis; celiac disease; asthma, such as
allergic asthma or neutrophilic
asthma; chronic obstructive pulmonary disease (COPD); cancer, such as breast
cancer, colon cancer,
lung cancer or ovarian cancer; uveitis; scleritis; vasculitis; Behcet's
disease; atherosclerosis; atopic
dermatitis; emphysema; periodontitis; allergic rhinitis; and allograft
rejection. The effect shown for the
bacterial strains from the genus Roseburia on the Th17 inflammatory response
may provide therapeutic
benefits for diseases and conditions mediated by IL-17 and the Th17 pathway,
such as those listed
above.
In preferred embodiments, the invention provides a composition comprising a
bacterial strain of the
genus Roseburia, for use in a method of treating or preventing asthma, such as
neutrophilic asthma or
allergic asthma. The inventors have identified that treatment with Roseburia
strains can reduce
recruitment of neutrophils and eosinophils into the lungs, which can help
treat or prevent asthma.
Furthermore, the inventors have tested and demonstrated the efficacy of
Roseburia strains in mouse
models of asthma. In certain embodiments, the composition is for use in a
method of treating or
preventing neutrophilic asthma or eosinophilic asthma. The effect shown for
the compositions of the
invention on neutrophils and eosinophils mean that they may be particularly
effective for treating or
preventing neutrophilic asthma and eosinophilic asthma. Indeed, in certain
embodiments, the
composition is for use in a method of reducing a neutrophilic inflammatory
response in the treatment
or prevention of asthma, or the composition is for use in a method of reducing
an eosinophilic
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
3
inflammatory response in the treatment or prevention of asthma. In preferred
embodiments, the
invention provides a composition comprising a bacterial strain of the species
Roseburia hominis for
use in the treatment of asthma, and in particular eosinophilic or allergic
asthma. Roseburia hominis is
shown to have a particularly pronounced effect on eosinophils in asthma models
and treatment with
Roseburia hominis may be particularly effective for treating eosinophilic or
allergic asthma.
In further preferred embodiments, the invention provides a composition
comprising a bacterial strain
of the genus Roseburia, for use in a method of treating or preventing
rheumatoid arthritis. The inventors
have identified that treatment with Roseburia strains can provide clinical
benefits in a mouse model of
rheumatoid arthritis and can reduce joint swelling. In preferred embodiments,
the invention provides a
composition comprising a bacterial strain of the species Roseburia hominis,
for use in the treatment of
rheumatoid arthritis. Compositions using Roseburia hominis may be particularly
effective for treating
rheumatoid arthritis.
In further preferred embodiments, the invention provides a composition
comprising a bacterial strain
of the genus Roseburia, for use in a method of treating or preventing multiple
sclerosis. The inventors
have identified that treatment with Roseburia strains can reduce disease
incidence and disease severity
in a mouse model of multiple sclerosis. In preferred embodiments, the
invention provides a
composition comprising a bacterial strain of the species Roseburia hominis,
for use in the treatment of
multiple sclerosis. Compositions using Roseburia hominis may be particularly
effective for treating
multiple sclerosis.
In certain embodiments, the compositions of the invention are for use in a
method of reducing IL-17
production or reducing Th17 cell differentiation in the treatment or
prevention of a disease or condition
mediated by IL-17 or the Th17 pathway. In particular, the compositions of the
invention may be used
in reducing IL-17 production or reducing Th17 cell differentiation in the
treatment or prevention of
asthma, rheumatoid arthritis or multiple sclerosis. Preferably, the invention
provides compositions
comprising a bacterial strain of the species Roseburia hominis, for use in
reducing IL-17 production or
reducing Th17 cell differentiation in the treatment or prevention of asthma,
rheumatoid arthritis or
multiple sclerosis.
In certain embodiments, the composition is for use in a patient with elevated
IL-17 levels or Th17 cells.
The effect on the Th17 inflammatory response shown for Roseburia strains may
be particularly
beneficial for such patients.
In preferred embodiments of the invention, the bacterial strain in the
composition is of Roseburia
hominis. Closely related strains may also be used, such as bacterial strains
that have a 16s rRNA
sequence that is at least 95%, 96%, 97%, 98%, 99%, 99.5% or 99.9% identical to
the 16s rRNA
sequence of a bacterial strain of Roseburia hominis. Preferably, the bacterial
strain has a 16s rRNA
sequence that is at least 95%, 96%, 97%, 98%, 99%, 99.5% or 99.9% identical to
SEQ ID NO:1, 2 or
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
4
3. Preferably, the sequence identity is to SEQ ID NO:3. Preferably, the
bacterial strain for use in the
invention has the 16s rRNA sequence represented by SEQ ID NO:3.
In certain embodiments, the composition of the invention is for oral
administration. Oral administration
of the strains of the invention can be effective for treating IL-17- or Th17
pathway-mediated diseases
and conditions. Also, oral administration is convenient for patients and
practitioners and allows
delivery to and / or partial or total colonisation of the intestine.
In certain embodiments, the composition of the invention comprises one or more
pharmaceutically
acceptable excipients or carriers.
In certain embodiments, the composition of the invention comprises a bacterial
strain that has been
lyophilised. Lyophilisation is an effective and convenient technique for
preparing stable compositions
that allow delivery of bacteria.
In certain embodiments, the invention provides a food product comprising the
composition as
described above.
In certain embodiments, the invention provides a vaccine composition
comprising the composition as
described above.
Additionally, the invention provides a method of treating or preventing a
disease or condition mediated
by IL-17 or the Th17 pathway, comprising administering a composition
comprising a bacterial strain
of the genus Roseburia.
In developing the above invention, the inventors have identified and
characterised a bacterial strain
that is particularly useful for therapy. The Roseburia hominis strain of the
invention is shown to be
effective for treating the diseases described herein, such as arthritis,
asthma and multiple sclerosis.
Therefore, in another aspect, the invention provides a cell of the Roseburia
hominis strain deposited
under accession number NCIMB 42383, or a derivative thereof The invention also
provides
compositions comprising such cells, or biologically pure cultures of such
cells. The invention also
provides a cell of the Roseburia hominis strain deposited under accession
number NCIMB 42383, or
a derivative thereof, for use in therapy, in particular for the diseases
described herein.
BRIEF DESCRIPTION OF DRAWINGS
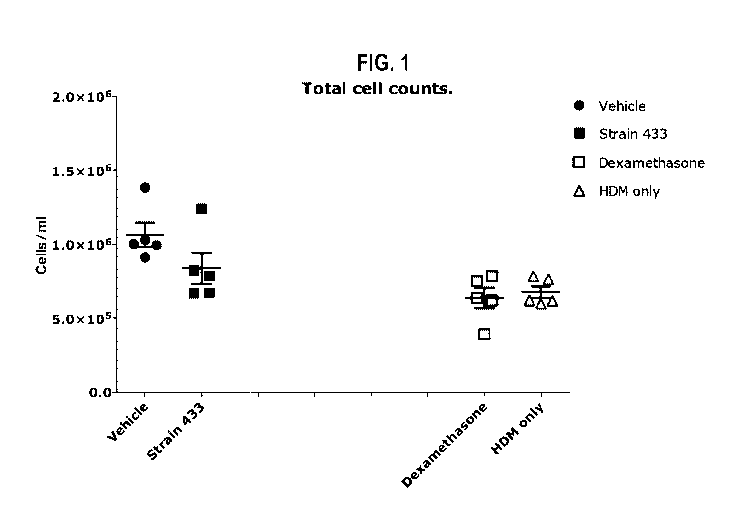
Figure 1: Mouse model of house dust mite-induced asthma - Total BAL fluid cell
counts.
Figure 2: Mouse model of house dust mite-induced asthma - Total eosinophil
count in BALF.
Figure 3: Mouse model of house dust mite-induced asthma - Proportion of
eosinophils in BALF.
Figure 4: Mouse model of house dust mite-induced asthma - Total macrophage
count in BALF.
Figure 5: Mouse model of house dust mite-induced asthma - Proportion of
macrophages in BALF.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
Figure 6: Mouse model of house dust mite-induced asthma - Total neutrophil
count in BALF.
Figure 7: Mouse model of house dust mite-induced asthma - Proportion of
neutrophils in BALF.
Figure 8: Mouse model of house dust mite-induced asthma - Total lymphocyte
count in BALF.
Figure 9: Mouse model of house dust mite-induced asthma - Proportion of
lymphocytes in BALF.
5 Figure 10: Mouse model of severe neutrophilic asthma - Total BAL fluid
cell counts.
Figure 11: Mouse model of severe neutrophilic asthma - Total eosinophil count
in BALF.
Figure 12: Mouse model of severe neutrophilic asthma - Proportion of
eosinophils in BALF.
Figure 13: Mouse model of severe neutrophilic asthma - Total macrophage count
in BALF.
Figure 14: Mouse model of severe neutrophilic asthma - Proportion of
macrophages in BALF.
Figure 15: Mouse model of severe neutrophilic asthma - Total neutrophil count
in BALF.
Figure 16: Mouse model of severe neutrophilic asthma - Proportion of
neutrophils in BALF.
Figure 17: Mouse model of severe neutrophilic asthma - Total lymphocyte count
in BALF.
Figure 18: Mouse model of severe neutrophilic asthma - Proportion of
lymphocytes in BALF.
Figure 19: Mouse model of rheumatoid arthritis - Bodyweights, days -14 to O.
Data are presented as
Mean + SEM percentages of the initial (Day -14) bodyweights.
Figure 20: Mouse model of rheumatoid arthritis - Bodyweights, days 0 to 42.
Data are presented as
Mean SEM percentages of the initial (Day 0) bodyweights.
Figure 21: Mouse model of rheumatoid arthritis - Clinical Scores. Data are
presented as Mean SEM.
**** p < 0.0001 when compared to Day 21 in the vehicle-treated group.
Figure 22: Mouse model of rheumatoid arthritis - Splenocyte proliferative
response to Collagen II.
Media background subtracted [CII-stimulated - media background] counts per
minute based on 3H-
TdR incorporation. All data are presented as Mean SEM. *** p < 0.001
compared to Vehicle group.
Figure 23: Mouse model of rheumatoid arthritis - Levels of IFNy in tissue
culture supernatants. Lines
represent group median values.
Figure 24: Mouse model of rheumatoid arthritis - Levels of IL-17A in tissue
culture supernatants.
Lines represent group median values.
Figure 25: Mouse model of rheumatoid arthritis - Levels of IL-10 in tissue
culture supernatants. Lines
represent group median values.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
6
Figure 26: Mouse model of rheumatoid arthritis - Levels of IL-6 in tissue
culture supernatants. Lines
represent group median values.
Figure 27: Mouse model of house dust mite-induced asthma ¨ Total IgE in Serum
Figure 28: Mouse model of house dust mite-induced asthma ¨ HDM specific IgG1
in Serum
Figure 29: Mouse model of house dust mite-induced asthma ¨ Total IgE in BALF
Figure 30: Mouse model of house dust mite-induced asthma ¨ HDM specific IgG1
in BALF
Figure 31: Mouse model of house dust mite-induced asthma ¨ Histological
Analysis ¨ Mean
Peribronchiolar Infiltration Score
Figure 32: Mouse model of house dust mite-induced asthma ¨ Histological
Analysis ¨ Mean
Perivascular Infiltration Score
Figure 33: Mouse model of house dust mite-induced asthma ¨ Histological
Analysis ¨ Mean
Inflammatory Score (Average of both Peribronchiolar and Perivascular
Infiltration Score)
Figure 34: Mouse model of house dust mite-induced asthma ¨ Histological
Analysis ¨ Mucus Score
Figure 35: Mouse model of house dust mite-induced asthma ¨ IL-9 level in lung
tissue
Figure 36: Mouse model of house dust mite-induced asthma ¨ IL-la level in lung
tissue
Figure 37: Mouse model of house dust mite-induced asthma ¨ IFNT level in lung
tissue
Figure 38: Mouse model of house dust mite-induced asthma ¨ IL-17A level in
lung tissue
Figure 39: Mouse model of house dust mite-induced asthma ¨ IL-4 level in lung
tissue
Figure 40: Mouse model of house dust mite-induced asthma ¨ IL-5 level in lung
tissue
Figure 41: Mouse model of house dust mite-induced asthma - IL-lb level in lung
tissue
Figure 42: Mouse model of house dust mite-induced asthma - RANTES level in
lung tissue
Figure 43: Mouse model of house dust mite-induced asthma - 1VIIP-la level in
lung tissue
Figure 44: Mouse model of house dust mite-induced asthma - KC level in lung
tissue
Figure 45: Mouse model of house dust mite-induced asthma - 1VIIP-2 level in
lung tissue
Figure 46: Mouse model of severe neutrophilic asthma ¨ HDM specific IgG1 in
Serum
Figure 47: Mouse model of severe neutrophilic asthma ¨ HDM specific IgG2a in
Serum
Figure 48: Mouse model of severe neutrophilic asthma ¨ HDM specific IgG1 in
BALF
Figure 49: Mouse model of severe neutrophilic asthma ¨ HDM specific IgG2a in
BALF
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
7
Figure 50: Mouse model of severe neutrophilic asthma ¨ Histological Analysis ¨
Mean
Peribronchiolar Infiltration Score
Figure 51: Mouse model of severe neutrophilic asthma ¨ Histological Analysis ¨
Mean Perivascular
Infiltration Score
Figure 52: Mouse model of severe neutrophilic asthma¨ Histological Analysis ¨
Mean Inflammatory
Score (Average of both Peribronchiolar and Perivascular Infiltration Score)
Figure 53: Mouse model of severe neutrophilic asthma ¨ TNFa level in lung
tissue
Figure 54: Mouse model of severe neutrophilic asthma ¨ IL-la level in lung
tissue
Figure 55: Mouse model of severe neutrophilic asthma ¨ IFNy level in lung
tissue
Figure 56: Mouse model of severe neutrophilic asthma ¨ IL-17F level in lung
tissue
Figure 57: Mouse model of severe neutrophilic asthma ¨ IL-lb level in lung
tissue
Figure 58: Mouse model of severe neutrophilic asthma - RANTES level in lung
tissue
Figure 59: Mouse model of severe neutrophilic asthma - MIP-2 level in lung
tissue
Figure 60: Mouse model of severe neutrophilic asthma - KC level in lung tissue
Figure 61: Mouse model of severe neutrophilic asthma ¨ IL-17A level in lung
tissue
Figure 62: Mouse model of severe neutrophilic asthma ¨MIP-la level in lung
tissue
Figure 63: Mouse model of severe neutrophilic asthma ¨ IL-33 level in lung
tissue
Figure 64: Mouse model of rheumatoid arthritis - Visual Template for
Histopathology Scoring.
Representative images showing composite scores from mouse tarsal joints in a
collagen-induced
arthritis study.
Figure 65: Mouse model of rheumatoid arthritis - Histopathology: Inflammation
Scores. Data are
presented as Mean SEM.
Figure 66: Mouse model of rheumatoid arthritis - Histopathology: Cartilage
Scores. Data are presented
as Mean I SEM.
Figure 67: Mouse model of rheumatoid arthritis - Histopathology: Bone Scores.
Data are presented as
Mean I SEM.
Figure 68: Mouse model of rheumatoid arthritis - Histopathology: Total Scores.
Data are presented as
Mean + SEM.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
8
Figure 69: Mouse model of rheumatoid arthritis - Histopathology:
Representative Pictures. Animal ID
(#n.n) and limb (R for right, L for left) are indicated between brackets. Left
image (vehicle): extensive
joint and bone destruction with inflammation and fibrosis extending to the
peri-articular soft tissues.
Figure 70: Mouse model of multiple sclerosis - clinical score.
Figure 71: Mouse model of multiple sclerosis - disease incidence.
DISCLOSURE OF THE INVENTION
Bacterial strains
The compositions of the invention comprise a bacterial strain of the genus
Roseburia. The examples
demonstrate that bacteria of this genus are useful for treating or preventing
diseases and conditions
mediated by IL-17 or the Th17 pathway. The preferred bacterial strains are of
the species Roseburia
hominis.
Examples ofRoseburia species for use in the invention include Roseburia
hominis, Roseburia cecicola,
Roseburia faecis, Roseburia intestinalis, and Roseburia inulinivorans.
Roseburia bacteria are slightly
curved rod-shaped cells that are strictly anaerobic and indigenous to the
mammalian intestine. They
are of the phylogenetic cluster XIVa within the Firmicutes phylum. The
bacteria are butyrate-
producing and are actively motile through multiple flagella present along the
concave side and in a
cluster at one end [16]. Roseburia hominis and Roseburia intestinalis are
recently described examples.
An example of Roseburia hominis is the strain deposited under the terms of the
Budapest Treaty at
National Collections of Industrial, Food and Marine Bacteria (NCIMB) at NCIMB
Ltd, Ferguson
Building, Craibstone Estate, Bucksburn, Aberdeen, UK, AB21 9YA, on 21 October
2004 by the
Rowett Research Institute under the accession number NCIMB 14029T Roseburia
hominis
A2-183T(DSM = 16839T). Other exemplary Roseburia hominis strains are described
in [17].
GenBank/EMBL/DDBJ accession numbers for the 16S rRNA gene sequence of strains
of Roseburia
hominis are AY804148 and AJ270482 (disclosed herein as SEQ ID NO:1 and SEQ ID
NO:2).
An example ofRoseburia intestinalis is the strain deposited under the
accession number NCIMB 13810
Roseburia intestinalis L1-82T(DSM = 14610T). Another example is the Roseburia
intestinalis strain as
described in [17]. Reference [17] also describes exemplary Roseburia faecis
and Roseburia
inulinivorans strains.
The Roseburia hominis bacterium deposited under accession number NCIMB 42383
was tested in the
Examples and is also referred to herein as strain 433. A 16S rRNA sequence for
the 433 strain that was
tested is provided in SEQ ID NO:3. Strain 433 was deposited with the
international depositary authority
NCIMB, Ltd. (Ferguson Building, Aberdeen, AB21 9YA, Scotland) by GT Biologics
Ltd. (Life
Sciences Innovation Building, Aberdeen, AB25 2ZS, Scotland) on 12th March 2015
as "Roseburia
hominis 433" and was assigned accession number NCIMB 42383. GT Biologics Ltd.
subsequently
changed its name to 4D Pharma Research Limited.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
9
A genome sequence for strain 433 is provided in SEQ ID NO:4. This sequence was
generated using
the PacBio RS II platform.
Bacterial strains closely related to the strain tested in the examples are
also expected to be effective for
treating or preventing diseases and conditions mediated by IL-17 or the Th17
pathway. In certain
embodiments, the bacterial strain for use in the invention has a 16s rRNA
sequence that is at least 95%,
96%, 97%, 98%, 99%, 99.5% or 99.9% identical to the 16s rRNA sequence of a
bacterial strain of
Roseburia intestinalis . Preferably, the bacterial strain for use in the
invention has a 16s rRNA sequence
that is at least 95%, 96%, 97%, 98%, 99%, 99.5% or 99.9% identical to SEQ ID
NO:1, 2 or 3.
Preferably, the sequence identity is to SEQ ID NO: 3. Preferably, the
bacterial strain for use in the
invention has the 16s rRNA sequence represented by SEQ ID NO:3.
Bacterial strains that are biotypes of the bacterium deposited under accession
number 42383 are also
expected to be effective for treating or preventing diseases and conditions
mediated by IL-17 or the
Th17 pathway. A biotype is a closely related strain that has the same or very
similar physiological and
biochemical characteristics.
Strains that are biotypes of the bacterium deposited under accession number
NCIMB 42383 and that
are suitable for use in the invention may be identified by sequencing other
nucleotide sequences for
the bacterium deposited under accession number NCIMB 42383. For example,
substantially the whole
genome may be sequenced and a biotype strain for use in the invention may have
at least 95%, 96%,
97%, 98%, 99%, 99.5% or 99.9% sequence identity across at least 80% of its
whole genome (e.g.
across at least 85%, 90%, 95% or 99%, or across its whole genome). Other
suitable sequences for use
in identifying biotype strains may include hsp60 or repetitive sequences such
as BOX, ERIC, (GTG)5,
or REP or [18]. Biotype strains may have sequences with at least 95%, 96%,
97%, 98%, 99%, 99.5%
or 99.9% sequence identity to the corresponding sequence of the bacterium
deposited under accession
number NCIMB 42383.
In certain embodiments, the bacterial strain for use in the invention has a
genome with sequence
identity to SEQ ID NO:4. In preferred embodiments, the bacterial strain for
use in the invention has a
genome with at least 90% sequence identity (e.g. at least 92%, 94%, 95%, 96%,
97%, 98%, 99% or
100% sequence identity) to SEQ ID NO:4 across at least 60% (e.g. at least 65%,
70%, 75%, 80%, 85%,
95%, 96%, 97%, 98%, 99% or 100%) of SEQ ID NO:4. For example, the bacterial
strain for use in the
invention may have a genome with at least 90% sequence identity to SEQ ID NO:4
across 70% of SEQ
ID NO:4, or at least 90% sequence identity to SEQ ID NO:4 across 80% of SEQ ID
NO:4, or at least
90% sequence identity to SEQ ID NO:4 across 90% of SEQ ID NO:4, or at least
90% sequence identity
to SEQ ID NO:4 across 100% of SEQ ID NO:4, or at least 95% sequence identity
to SEQ ID NO:4
across 70% of SEQ ID NO:4, or at least 95% sequence identity to SEQ ID NO:4
across 80% of SEQ
ID NO:4, or at least 95% sequence identity to SEQ ID NO:4 across 90% of SEQ ID
NO:4, or at least
95% sequence identity to SEQ ID NO:4 across 100% of SEQ ID NO:4, or at least
98% sequence
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
identity to SEQ ID NO:4 across 70% of SEQ ID NO:4, or at least 98% sequence
identity to SEQ ID
NO:4 across 80% of SEQ ID NO:4, or at least 98% sequence identity to SEQ ID
NO:4 across 90% of
SEQ ID NO:4, or at least 98% sequence identity to SEQ ID NO:4 across 100% of
SEQ ID NO:4.
Alternatively, strains that are biotypes of the bacterium deposited under
accession number NCIMB
5 42383 and that are suitable for use in the invention may be identified by
using the accession number
NCIMB 42383 deposit and restriction fragment analysis and/or PCR analysis, for
example by using
fluorescent amplified fragment length polymorphism (FAFLP) and repetitive DNA
element (rep)-PCR
fingerprinting, or protein profiling, or partial 16S or 23s rDNA sequencing.
In preferred embodiments,
such techniques may be used to identify other Roseburia hominis strains.
10 In certain embodiments, strains that are biotypes of the bacterium
deposited under accession number
NCIIVIB 42383 and that are suitable for use in the invention are strains that
provide the same pattern
as the bacterium deposited under accession number NCIMB 42383 when analysed by
amplified
ribosomal DNA restriction analysis (ARDRA), for example when using Sau3AI
restriction enzyme
(for exemplary methods and guidance see, for example,[19]). Alternatively,
biotype strains are
identified as strains that have the same carbohydrate fermentation patterns as
the bacterium deposited
under accession number NCIMB 42383.
Other Roseburia strains that are useful in the compositions and methods of the
invention, such as
biotypes of the bacteria deposited under accession number NCIMB 42383, may be
identified using any
appropriate method or strategy, including the assays described in the
examples. For instance, strains
for use in the invention may be identified by culturing in anaerobic YCFA
and/or administering the
bacteria to the type II collagen-induced arthritis mouse model and then
assessing cytokine levels. In
particular, bacterial strains that have similar growth patterns, metabolic
type and/or surface antigens to
the bacterium deposited under accession number NCIMB 42383 may be useful in
the invention. A
useful strain will have comparable immune modulatory activity to the NCIMB
42383 strain. In
particular, a biotype strain will elicit comparable effects on the asthma,
arthritis and multiple sclerosis
disease models and comparable effects on cytokine levels to the effects shown
in the Examples, which
may be identified by using the culturing and administration protocols
described in the Examples.
A particularly preferred strain of the invention is the Roseburia hominis
strain deposited under
accession number NCIMB 42383. This is the exemplary 433 strain tested in the
examples and shown
to be effective for treating disease. Therefore, the invention provides a
cell, such as an isolated cell, of
the Roseburia hominis strain deposited under accession number NCIMB 42383, or
a derivative thereof
The invention also provides a composition comprising a cell of the Roseburia
hominis strain deposited
under accession number NCIMB 42383, or a derivative thereof. The invention
also provides a
biologically pure culture of the Roseburia hominis strain deposited under
accession number NCIMB
42383. The invention also provides a cell of the Roseburia hominis strain
deposited under accession
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
11
number NCIMB 42383, or a derivative thereof, for use in therapy, in particular
for the diseases
described herein.
A derivative of the strain deposited under accession number NCIMB 42383 may be
a daughter strain
(progeny) or a strain cultured (subcloned) from the original. A derivative of
a strain of the invention
may be modified, for example at the genetic level, without ablating the
biological activity. In particular,
a derivative strain of the invention is therapeutically active. A derivative
strain will have comparable
immune modulatory activity to the original NCIMB 42383 strain. In particular,
a derivative strain will
elicit comparable effects on the asthma, arthritis and multiple sclerosis
disease models and comparable
effects on cytokine levels to the effects shown in the Examples, which may be
identified by using the
culturing and administration protocols described in the Examples. A derivative
of the NCIMB 42383
strain will generally be a biotype of the NCIMB 42383 strain.
References to cells of the Roseburia hominis strain deposited under accession
number NCIMB 42383
encompass any cells that have the same safety and therapeutic efficacy
characteristics as the strains
deposited under accession number NCIMB 42383, and such cells are encompassed
by the invention.
In preferred embodiments, the bacterial strains in the compositions of the
invention are viable and
capable of partially or totally colonising the intestine.
Therapeutic uses
As demonstrated in the examples, the bacterial compositions of the invention
are effective for reducing
the Th17 inflammatory response. In particular, treatment with compositions of
the invention achieves
a reduction in IL-17A levels and other Th17 pathway cytokines, and clinical
improvements in animal
models of conditions mediated by IL-17 and the Th17 pathway. Therefore, the
compositions of the
invention may be useful for treating or preventing inflammatory and autoimmune
diseases, and in
particular diseases or conditions mediated by IL-17. In particular, the
compositions of the invention
may be useful for reducing or preventing elevation of the IL-17 inflammatory
response.
Th17 cells are a subset of T helper cells that produce, for example, IL-17A,
1L17-F, IL-21 and IL-22.
Th17 cell differentiation and IL-17 expression may be driven by IL-23. These
cytokines and others
form important parts of the Th17 pathway, which is a well-established
inflammatory signalling
pathway that contributes to and underlies a number of inflammatory and
autoimmune diseases (as
described in, for example, [20-25]). Diseases wherein the Th17 pathway is
activated are Th17 pathway-
mediated diseases. Th17 pathway-mediated diseases can be ameliorated or
alleviated by repressing the
Th17 pathway, which may be through a reduction in the differentiation of Th17
cells or a reduction in
their activity or a reduction in the level of Th17 pathway cytokines. Diseases
mediated by the Th17
pathway may be characterised by increased levels of cytokines produced by Th17
cells, such as IL-
17A, IL-17F, IL-21, IL-22, IL-26, IL-9 (reviewed in [26]). Diseases mediated
by the Th17 pathway
may be characterised by increased expression of Th-17-related genes, such as
Stat3 or IL-23R.
Diseases mediated by the Th17 pathway may be associated with increased levels
of Th17 cells.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
12
IL-17 is a pro-inflammatory cytokine that contributes to the pathogenesis of
several inflammatory and
autoimmune diseases and conditions. IL-17 as used herein may refer to any
member of the IL-17
family, including IL-17A, IL-17B, IL-17C, IL-17D, IL-1 tE, and IL-17F. IL-17-
mediated diseases and
conditions are characterised by high expression of IL-17 and/or the
accumulation or presence of IL-
17-positive cells in a tissue affected by the disease or condition. Similarly,
IL-17-mediated diseases
and conditions are diseases and conditions that are exacerbated by high IL-17
levels or an increase in
IL-17 levels, and that are alleviated by low IL-17 levels or a reduction in IL-
17 levels. The IL-17
inflammatory response may be local or systemic.
Examples of diseases and conditions that may be mediated by IL-17 or the Th17
pathway include
multiple sclerosis; arthritis, such as rheumatoid arthritis, osteoarthritis,
psoriatic arthritis, or juvenile
idiopathic arthritis; neuromyelitis optica (Devic's disease); ankylosing
spondylitis; spondyloarthritis;
psoriasis; systemic lupus erythematosus; inflammatory bowel disease, such as
Crohn's disease or
ulcerative colitis; celiac disease; asthma, such as allergic asthma or
neutrophilic asthma; chronic
obstructive pulmonary disease (COPD); cancer, such as breast cancer, colon
cancer, lung cancer or
ovarian cancer; uveitis; scleritis; vasculitis; Behcet's disease;
atherosclerosis; atopic dermatitis;
emphysema; periodontitis; allergic rhinitis; and allograft rejection. In
preferred embodiments, the
compositions of the invention are used for treating or preventing one or more
of these conditions or
diseases. In further preferred embodiments, these conditions or diseases are
mediated by 1L-17 or the
Th17 pathway.
In certain embodiments, the compositions of the invention are for use in a
method of reducing IL-17
production or reducing Th17 cell differentiation in the treatment or
prevention of a disease or condition
mediated by 1L-17 or the Th17 pathway. In certain embodiments, the
compositions of the invention
are for use in treating or preventing an inflammatory or autoimmune disease,
wherein said treatment
or prevention is achieved by reducing or preventing elevation of the Th17
inflammatory response. In
certain embodiments, the compositions of the invention are for use in treating
a patient with an
inflammatory or autoimmune disease, wherein the patient has elevated IL-17
levels or elevated Th17
cells or is exhibiting a Th17 inflammatory response. In certain embodiments,
the patient may have
been diagnosed with a chronic inflammatory or autoimmune disease or condition,
or the composition
of the invention may be for use in preventing an inflammatory or autoimmune
disease or condition
developing into a chronic inflammatory or autoimmune disease or condition. In
certain embodiments,
the disease or condition may not be responsive to treatment with TNF-a
inhibitors. These uses of the
invention may be applied to any of the specific disease or conditions listed
in the preceding paragraph.
IL-17 and the Th17 pathway are often associated with chronic inflammatory and
autoimmune diseases,
so the compositions of the invention may be particularly useful for treating
or preventing chronic
diseases or conditions as listed above. In certain embodiments, the
compositions are for use in patients
with chronic disease. In certain embodiments, the compositions are for use in
preventing the
development of chronic disease.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
13
The compositions of the invention may be useful for treating diseases and
conditions mediated by IL-
17 or the Th17 pathway and for addressing the Th17 inflammatory response, so
the compositions of
the invention may be particularly useful for treating or preventing chronic
disease, treating or
preventing disease in patients that have not responded to other therapies
(such as treatment with TNF-
a inhibitors), and/or treating or preventing the tissue damage and symptoms
associated with IL-17 and
Th17 cells. For example, IL-17 is known to activate matrix destruction in
cartilage and bone tissue and
IL-17 has an inhibitory effect on matrix production in chondrocytes and
osteoblasts, so the
compositions of the invention may be useful for treating or preventing bone
erosion or cartilage
damage.
In certain embodiments, treatment with compositions of the invention provides
a reduction or prevents
an elevation in IL-17 levels, in particular IL-17A levels. In certain
embodiments, treatment with
compositions of the invention provides a reduction or prevents an elevation in
IFN-y or IL-6 levels.
Such reduction or prevention of elevated levels of these cytokines may be
useful for treating or
preventing inflammatory and autoimmune diseases and conditions, in particular
those mediated by IL-
17 or the Th17 pathway.
Asthma
In preferred embodiments, the compositions of the invention are for use in
treating or preventing
asthma. The examples demonstrate that the compositions of the invention
achieve a reduction in the
recruitment of neutrophils and/or eosinophils into the airways following
sensitisation and challenge
with house dust mite extract and so they may be useful in the treatment or
prevention of asthma.
Asthma is a chronic disease characterised by inflammation and restriction of
the airways. The
inflammation in asthma may be mediated by IL-17 and/or Th17 cells, and so the
compositions of the
invention may be particularly effective for preventing or treating asthma. The
inflammation in asthma
may be mediated by eosinophils and/or neutrophils.
In certain embodiments, the asthma is eosinophilic or allergic asthma.
Eosinophilic and allergic asthma
are characterised by increased numbers of eosinophils in peripheral blood and
in airway secretions and
is associated pathologically with thickening of the basement membrane zone and
pharmacologically
by corticosteroid responsiveness [27]. Compositions that reduce or inhibit
eosinophil recruitment or
activation may be useful for treating or preventing eosinophilic and allergic
asthma.
In additional embodiments, the compositions of the invention are for use in
treating or preventing
neutrophilic asthma (or non-eosinophilic asthma). High neutrophil numbers are
associated with severe
asthma that may be insensitive to corticosteroid treatment. Compositions that
reduce or inhibit
neutrophil recruitment or activation may be useful for treating or preventing
neutrophilic asthma.
Eosinophilic and neutrophilic asthma are not mutually exclusive conditions and
treatments that help
address either the eosinophil and neutrophil responses may be useful for
treating asthma in general.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
14
Increased IL-17 levels and activation of the Th17 pathway are associated with
severe asthma, so the
compositions of the invention may be useful for preventing the development of
severe asthma or for
treating severe asthma.
In certain embodiments, the compositions of the invention are for use in
methods reducing an
eosinophilic inflammatory response in the treatment or prevention of asthma,
or for use in methods of
reducing a neutrophilic inflammatory response in the treatment or prevention
of asthma. As noted
above, high levels of eosinophils in asthma is associated pathologically with
thickening of the
basement membrane zone, so reducing eosinophilic inflammatory response in the
treatment or
prevention of asthma may be able to specifically address this feature of the
disease. Also, elevated
neutrophils, either in combination with elevated eosinophils or in their
absence, is associated with
severe asthma and chronic airway narrowing. Therefore, reducing the
neutrophilic inflammatory
response may be particularly useful for addressing severe asthma.
In certain embodiments, the compositions reduce peribronchiolar infiltration
in allergic asthma, or are
for use in reducing peribronchiolar infiltration in the treatment of allergic
asthma. In certain
embodiments, the compositions reduce peribronchiolar and/or perivascular
infiltration in neutrophilic
asthma, or are for use in reducing peribronchiolar and/or perivascular
infiltration in the treatment of
allergic neutrophilic asthma.
In certain embodiments, treatment with compositions of the invention provides
a reduction or prevents
an elevation in IFI\Ty levels.
In certain embodiments, the compositions of the invention are for use in a
method of treating asthma
that results in a reduction of the eosinophilic and/or neutrophilic
inflammatory response. In certain
embodiments, the patient to be treated has, or has previously been identified
as having, elevated
neutrophil or eosinophil levels, for example as identified through blood
sampling or sputum analysis.
The compositions of the invention may be useful for preventing the development
of asthma in a new-
born when administered to the new-born, or to a pregnant woman. The
compositions may be useful for
preventing the development of asthma in children. The compositions of the
invention may be useful
for treating or preventing adult-onset asthma. The compositions of the
invention may be useful for
managing or alleviating asthma. The compositions of the invention may be
particularly useful for
reducing symptoms associated with asthma that is aggravated by allergens, such
as house dust mites.
Treatment or prevention of asthma may refer to, for example, an alleviation of
the severity of symptoms
or a reduction in the frequency of exacerbations or the range of triggers that
are a problem for the
patient.
Arthritis
In preferred embodiments, the compositions of the invention are for use in
treating or preventing
rheumatoid arthritis (RA). The examples demonstrate that the compositions of
the invention achieve a
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
reduction in the clinical signs of RA in a mouse model, reduce cartilage and
bone damage, and reduce
the IL-17 inflammatory response, and so they may be useful in the treatment or
prevention of RA. RA
is a systemic inflammatory disorder that primarily affects joints. RA is
associated with an inflammatory
response that results in swelling of joints, synovial hyperplasia, and
destruction of cartilage and bone.
5 IL-17 and Th17 cells may have a key role in RA, for example because IL-17
inhibits matrix production
in chondrocytes and osteoblasts and activates the production and function of
matrix metalloproteinases
and because RA disease activity is correlated to IL-17 levels and Th-17 cell
numbers [28,29], so the
compositions of the invention may be particularly effective for preventing or
treating RA.
In certain embodiments, the compositions of the invention are for use in
lowering IL-17 levels or
10 preventing elevation of IL-17 levels in the treatment or prevention of
RA. In certain embodiments,
treatment with compositions of the invention provides a reduction or prevents
an elevation in IL-17
levels, in particular IL-17A levels. In certain embodiments, treatment with
compositions of the
invention provides a reduction or prevents an elevation in IFN-y or IL-6
levels.
In certain embodiments, treatment with the compositions of the invention
results in a reduction in the
15 swelling of joints. In certain embodiments, the compositions of the
invention are for use in patients
with swollen joints or patients identified as at risk of having swollen
joints. In certain embodiments,
the compositions of the invention are for use in a method of reducing joint
swelling in RA.
In certain embodiments, treatment with the compositions of the invention
results in a reduction in
cartilage damage or bone damage. In certain embodiments, the compositions of
the invention are for
use in reducing or preventing cartilage or bone damage in the treatment of RA.
In certain embodiments,
the compositions are for use in treating patient with severe RA that are at
risk of cartilage or bone
damage.
Increased IL-17 levels and Th17 cell numbers are associated with cartilage and
bone destruction in RA
[28,29]. IL-17 is known to activate matrix destruction in cartilage and bone
tissue and IL-17 has an
inhibitory effect on matrix production in chondrocytes and osteoblasts.
Therefore, in certain
embodiments, the compositions of the invention are for use in preventing bone
erosion or cartilage
damage in the treatment of RA. In certain embodiments, the compositions are
for use in treating
patients that exhibit bone erosion or cartilage damage or patients identified
as at risk of bone erosion
or cartilage damage.
TNF-a is also associated with RA, but TNF-a is not involved in the
pathogenesis of the later stages of
the disease. In contrast, IL-17 has a role throughout all stages of chronic
disease [30]. Therefore, in
certain embodiments the compositions of the invention are for use in treating
chronic RA or late-stage
RA, such as disease that includes joint destruction and loss of cartilage. In
certain embodiments, the
compositions of the invention are for treating patients that have previously
received anti-TNF-a
therapy. In certain embodiments, the patients to be treated do not respond or
no longer respond to anti-
TNF-a therapy.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
16
The compositions of the invention may be useful for modulating a patient's
immune system, so in
certain embodiments the compositions of the invention are for use in
preventing RA in a patient that
has been identified as at risk of RA, or that has been diagnosed with early-
stage RA. The compositions
of the invention may be useful for preventing the development of RA.
The compositions of the invention may be useful for managing or alleviating
RA. The compositions
of the invention may be particularly useful for reducing symptoms associated
with joint swelling or
bone destruction. Treatment or prevention of RA may refer to, for example, an
alleviation of the
severity of symptoms or a reduction in the frequency of exacerbations or the
range of triggers that are
a problem for the patient.
Multiple sclerosis
In preferred embodiments, the compositions of the invention are for use in
treating or preventing
multiple sclerosis. The examples demonstrate that the compositions of the
invention achieve a
reduction in the disease incidence and disease severity in a mouse model of
multiple sclerosis (the EAE
model), and so they may be useful in the treatment or prevention of multiple
sclerosis. Multiple
sclerosis is an inflammatory disorder associated with damage to the myelin
sheaths of neurons,
particularly in the brain and spinal column. Multiple sclerosis is a chronic
disease, which is
progressively incapacitating and which evolves in episodes. IL-17 and Th17
cells may have a key role
in multiple sclerosis, for example because IL-17 levels may correlate with
multiple sclerosis lesions,
IL-17 can disrupt blood brain barrier endothelial cell tight junctions, and
Th17 cells can migrate into
the central nervous system and cause neuronal loss [31,32]. Therefore, the
compositions of the
invention may be particularly effective for preventing or treating multiple
sclerosis.
In certain embodiments, treatment with the compositions of the invention
results in a reduction in
disease incidence or disease severity. In certain embodiments, the
compositions of the invention are
for use in reducing disease incidence or disease severity. In certain
embodiments, treatment with the
compositions of the invention prevents a decline in motor function or results
in improved motor
function. In certain embodiments, the compositions of the invention are for
use in preventing a decline
in motor function or for use in improving motor function. In certain
embodiments, treatment with the
compositions of the invention prevents the development of paralysis. In
certain embodiments, the
compositions of the invention are for use in preventing paralysis in the
treatment of multiple sclerosis.
The compositions of the invention may be useful for modulating a patient's
immune system, so in
certain embodiments the compositions of the invention are for use in
preventing multiple sclerosis in
a patient that has been identified as at risk of multiple sclerosis, or that
has been diagnosed with early-
stage multiple sclerosis or "relapsing-remitting" multiple sclerosis. The
compositions of the invention
may be useful for preventing the development of sclerosis. Indeed, the
examples show that
administration of compositions of the invention prevented the development of
disease in many mice.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
17
The compositions of the invention may be useful for managing or alleviating
multiple sclerosis. The
compositions of the invention may be particularly useful for reducing symptoms
associated with
multiple sclerosis. Treatment or prevention of multiple sclerosis may refer
to, for example, an
alleviation of the severity of symptoms or a reduction in the frequency of
exacerbations or the range
of triggers that are a problem for the patient.
Modes of administration
Preferably, the compositions of the invention are to be administered to the
gastrointestinal tract in order
to enable delivery to and / or partial or total colonisation of the intestine
with the bacterial strain of the
invention. Generally, the compositions of the invention are administered
orally, but they may be
administered rectally, intranasally, or via buccal or sublingual routes.
In certain embodiments, the compositions of the invention may be administered
as a foam, as a spray
or a gel.
In certain embodiments, the compositions of the invention may be administered
as a suppository, such
as a rectal suppository, for example in the form of a theobroma oil (cocoa
butter), synthetic hard fat
(e.g. suppocire, witepsol), glycero-gelatin, polyethylene glycol, or soap
glycerin composition.
In certain embodiments, the composition of the invention is administered to
the gastrointestinal tract
via a tube, such as a nasogastric tube, orogastric tube, gastric tube,
jejunostomy tube (J tube),
percutaneous endoscopic gastrostomy (PEG), or a port, such as a chest wall
port that provides access
to the stomach, jejunum and other suitable access ports.
The compositions of the invention may be administered once, or they may be
administered sequentially
as part of a treatment regimen. In certain embodiments, the compositions of
the invention are to be
administered daily.
In certain embodiments of the invention, treatment according to the invention
is accompanied by
assessment of the patient's gut microbiota. Treatment may be repeated if
delivery of and / or partial or
total colonisation with the strain of the invention is not achieved such that
efficacy is not observed, or
treatment may be ceased if delivery and / or partial or total colonisation is
successful and efficacy is
observed.
In certain embodiments, the composition of the invention may be administered
to a pregnant animal,
for example a mammal such as a human in order to prevent an inflammatory or
autoimmune disease
developing in her child in utero and / or after it is born.
The compositions of the invention may be administered to a patient that has
been diagnosed with a
disease or condition mediated by IL-17 or the Th17 pathway, or that has been
identified as being at
risk of a disease or condition mediated by IL-17 or the Th17 pathway. The
compositions may also be
administered as a prophylactic measure to prevent the development of diseases
or conditions mediated
by IL-17 or the Th17 pathway in a healthy patient.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
18
The compositions of the invention may be administered to a patient that has
been identified as having
an abnormal gut microbiota. For example, the patient may have reduced or
absent colonisation by
Roseburia, and in particular Roseburia hominis.
The compositions of the invention may be administered as a food product, such
as a nutritional
supplement.
Generally, the compositions of the invention are for the treatment of humans,
although they may be
used to treat animals including monogastric mammals such as poultry, pigs,
cats, dogs, horses or
rabbits. The compositions of the invention may be useful for enhancing the
growth and performance
of animals. If administered to animals, oral gavage may be used.
Compositions
Generally, the composition of the invention comprises bacteria. In preferred
embodiments of the
invention, the composition is formulated in freeze-dried form. For example,
the composition of the
invention may comprise granules or gelatin capsules, for example hard gelatin
capsules, comprising a
bacterial strain of the invention.
Preferably, the composition of the invention comprises lyophilised bacteria.
Lyophilisation of bacteria
is a well-established procedure and relevant guidance is available in, for
example, references [33-35].
Alternatively, the composition of the invention may comprise a live, active
bacterial culture.
In preferred embodiments, the composition of the invention is encapsulated to
enable delivery of the
bacterial strain to the intestine. Encapsulation protects the composition from
degradation until delivery
at the target location through, for example, rupturing with chemical or
physical stimuli such as
pressure, enzymatic activity, or physical disintegration, which may be
triggered by changes in pH. Any
appropriate encapsulation method may be used. Exemplary encapsulation
techniques include
entrapment within a porous matrix, attachment or adsorption on solid carrier
surfaces, self-aggregation
by flocculation or with cross-linking agents, and mechanical containment
behind a microporous
membrane or a microcapsule. Guidance on encapsulation that may be useful for
preparing
compositions of the invention is available in, for example, references [36]
and [37].
The composition may be administered orally and may be in the form of a tablet,
capsule or powder.
Encapsulated products are preferred because Roseburia are anaerobes. Other
ingredients (such as
vitamin C, for example), may be included as oxygen scavengers and prebiotic
substrates to improve
the delivery and / or partial or total colonisation and survival in vivo.
Alternatively, the probiotic
composition of the invention may be administered orally as a food or
nutritional product, such as milk
or whey based fermented dairy product, or as a pharmaceutical product.
The composition may be formulated as a probiotic.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
19
A composition of the invention includes a therapeutically effective amount of
a bacterial strain of the
invention. A therapeutically effective amount of a bacterial strain is
sufficient to exert a beneficial
effect upon a patient. A therapeutically effective amount of a bacterial
strain may be sufficient to result
in delivery to and / or partial or total colonisation of the patient's
intestine.
A suitable daily dose of the bacteria, for example for an adult human, may be
from about 1 x 103 to
about 1 x 10" colony forming units (CFU); for example, from about 1 x 107 to
about 1 x 1010 CFU; in
another example from about 1 x 106 to about 1 x 1016 CFU.
In certain embodiments, the composition contains the bacterial strain in an
amount of from about 1 x
106to about 1 x 10" CFU/g, respect to the weight of the composition; for
example, from about 1 x 108
to about 1 x 1016 CFU/g. The dose may be, for example, 1 g, 3g, 5g, and 10g.
Typically, a probiotic, such as the composition of the invention, is
optionally combined with at least
one suitable prebiotic compound. A prebiotic compound is usually a non-
digestible carbohydrate such
as an oligo- or polysaccharide, or a sugar alcohol, which is not degraded or
absorbed in the upper
digestive tract. Known prebiotics include commercial products such as inulin
and transgalacto-
oligosaccharides.
In certain embodiments, the probiotic composition of the present invention
includes a prebiotic
compound in an amount of from about 1 to about 30% by weight, respect to the
total weight
composition, (e.g. from 5 to 20% by weight). Carbohydrates may be selected
from the group consisting
of fructo- oligosaccharides (or FOS), short-chain fructo-oligosaccharides,
inulin, isomalt-
oligosaccharides, pectins, xylo-oligosaccharides (or XOS), chitosan-
oligosaccharides (or COS), beta-
glucans, arable gum modified and resistant starches, polydextrose, D-tagatose,
acacia fibers, carob,
oats, and citrus fibers. In one aspect, the prebiotics are the short-chain
fructo-oligosaccharides (for
simplicity shown herein below as FOSs-c.c); said FOSs-c.c. are not digestible
carbohydrates, generally
obtained by the conversion of the beet sugar and including a saccharose
molecule to which three
glucose molecules are bonded.
The compositions of the invention may comprise pharmaceutically acceptable
excipients or carriers.
Examples of such suitable excipients may be found in the reference [38].
Acceptable carriers or
diluents for therapeutic use are well known in the pharmaceutical art and are
described, for example,
in reference [39]. Examples of suitable carriers include lactose, starch,
glucose, methyl cellulose,
magnesium stearate, mannitol, sorbitol and the like. Examples of suitable
diluents include ethanol,
glycerol and water. The choice of pharmaceutical carrier, excipient or diluent
can be selected with
regard to the intended route of administration and standard pharmaceutical
practice. The
pharmaceutical compositions may comprise as, or in addition to, the carrier,
excipient or diluent any
suitable binder(s), lubricant(s), suspending agent(s), coating agent(s),
solubilising agent(s). Examples
of suitable binders include starch, gelatin, natural sugars such as glucose,
anhydrous lactose, free-flow
lactose, beta-lactose, corn sweeteners, natural and synthetic gums, such as
acacia, tragacanth or sodium
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
alginate, carboxymethyl cellulose and polyethylene glycol. Examples of
suitable lubricants include
sodium oleate, sodium stearate, magnesium stearate, sodium benzoate, sodium
acetate, sodium
chloride and the like. Preservatives, stabilizers, dyes and even flavouring
agents may be provided in
the pharmaceutical composition. Examples of preservatives include sodium
benzoate, sorbic acid and
5 esters of p-hydroxybenzoic acid. Antioxidants and suspending agents may
be also used.
The compositions of the invention may be formulated as a food product. For
example, a food product
may provide nutritional benefit in addition to the therapeutic effect of the
invention, such as in a
nutritional supplement. Similarly, a food product may be formulated to enhance
the taste of the
composition of the invention or to make the composition more attractive to
consume by being more
10 similar to a common food item, rather than to a pharmaceutical
composition. In certain embodiments,
the composition of the invention is formulated as a milk-based product. The
term "milk-based product"
means any liquid or semi-solid milk- or whey- based product having a varying
fat content. The milk-
based product can be, e.g., cow's milk, goat's milk, sheep's milk, skimmed
milk, whole milk, milk
recombined from powdered milk and whey without any processing, or a processed
product, such as
15 yoghurt, curdled milk, curd, sour milk, sour whole milk, butter milk and
other sour milk products.
Another important group includes milk beverages, such as whey beverages,
fermented milks,
condensed milks, infant or baby milks; flavoured milks, ice cream; milk-
containing food such as
sweets.
In certain embodiments, the compositions of the invention contain a single
bacterial strain or species
20 and do not contain any other bacterial strains or species. Such
compositions may comprise only de
minimis or biologically irrelevant amounts of other bacterial strains or
species. Such compositions may
be a culture that is substantially free from other species of organism.
The compositions for use in accordance with the invention may or may not
require marketing approval.
In some cases, the lyophilised bacterial strain is reconstituted prior to
administration. In some cases,
the reconstitution is by use of a diluent described herein.
The compositions of the invention can comprise pharmaceutically acceptable
excipients, diluents or
carriers.
In certain embodiments, the invention provides a pharmaceutical composition
comprising: a bacterial
strain of the invention; and a pharmaceutically acceptable excipient, carrier
or diluent; wherein the
bacterial strain is in an amount sufficient to treat a disorder when
administered to a subject in need
thereof; and wherein the disorder is selected from the group consisting of
asthma, allergic asthma,
neutrophilic asthma, osteoarthritis, psoriatic arthritis, juvenile idiopathic
arthritis, neuromyelitis optica
(Devic's disease), ankylosing spondylitis, spondyloarthritis, systemic lupus
erythematosus, celiac
disease, chronic obstructive pulmonary disease (COPD), cancer, breast cancer,
colon cancer, lung
cancer, ovarian cancer, uveitis, scleritis, vasculitis, Behcet's disease,
atherosclerosis, atopic dermatitis,
emphysema, periodontitis, allergic rhinitis, and allograft rejection.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
21
In certain embodiments, the invention provides pharmaceutical composition
comprising: a bacterial
strain of the invention; and a pharmaceutically acceptable excipient, carrier
or diluent; wherein the
bacterial strain is in an amount sufficient to treat or prevent a disease or
condition mediated by IL-17
or the Th17 pathway. In preferred embodiments, said disease or condition is
selected from the group
consisting of rheumatoid arthritis, multiple sclerosis, psoriasis,
inflammatory bowel disease, Crohn's
disease, ulcerative colitis, celiac disease, asthma, allergic asthma,
neutrophilic asthma, osteoarthritis,
psoriatic arthritis, juvenile idiopathic arthritis, neuromyelitis optica
(Devic's disease), ankylosing
spondylitis, spondyloarthritis, systemic lupus erythematosus, chronic
obstructive pulmonary disease
(COPD), cancer, breast cancer, colon cancer, lung cancer, ovarian cancer,
uveitis, scleritis, vasculitis,
Behcet's disease, atherosclerosis, atopic dermatitis, emphysema,
periodontitis, allergic rhinitis, and
allograft rejection.
In certain embodiments, the invention provides the above pharmaceutical
composition, wherein the
amount of the bacterial strain is from about 1 x 103 to about 1 x 1011 colony
forming units per gram
with respect to a weight of the composition.
In certain embodiments, the invention provides the above pharmaceutical
composition, wherein the
composition is administered at a dose of 1 g, 3 g, 5 g or 10 g.
In certain embodiments, the invention provides the above pharmaceutical
composition, wherein the
composition is administered by a method selected from the group consisting of
oral, rectal,
subcutaneous, nasal, buccal, and sublingual.
In certain embodiments, the invention provides the above pharmaceutical
composition, comprising a
carrier selected from the group consisting of lactose, starch, glucose, methyl
cellulose, magnesium
stearate, mannitol and sorbitol.
In certain embodiments, the invention provides the above pharmaceutical
composition, comprising a
diluent selected from the group consisting of ethanol, glycerol and water.
In certain embodiments, the invention provides the above pharmaceutical
composition, comprising an
excipient selected from the group consisting of starch, gelatin, glucose,
anhydrous lactose, free-flow
lactose, beta-lactose, corn sweetener, acacia, tragacanth, sodium alginate,
carboxymethyl cellulose,
polyethylene glycol, sodium oleate, sodium stearate, magnesium stearate,
sodium benzoate, sodium
acetate and sodium chloride.
In certain embodiments, the invention provides the above pharmaceutical
composition, further
comprising at least one of a preservative, an antioxidant and a stabilizer.
In certain embodiments, the invention provides the above pharmaceutical
composition, comprising a
preservative selected from the group consisting of sodium benzoate, sorbic
acid and esters of p-
hydroxybenzoic acid.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
22
In certain embodiments, the invention provides the above pharmaceutical
composition, wherein said
bacterial strain is lyophilised.
In certain embodiments, the invention provides the above pharmaceutical
composition, wherein when
the composition is stored in a sealed container at about 4 C or about 25 C and
the container is placed
in an atmosphere having 50% relative humidity, at least 80% of the bacterial
strain as measured in
colony forming units, remains after a period of at least about: 1 month, 3
months, 6 months, 1 year, 1.5
years, 2 years, 2.5 years or 3 years.
Culturing methods
The bacterial strains for use in the present invention can be cultured using
standard microbiology
techniques as detailed in, for example, references [40-42]
The solid or liquid medium used for culture may be YCFA agar or YCFA medium.
YCFA medium
may include (per 100m1, approximate values): Casitone (1.0 g), yeast extract
(0.25 g), NaHCO3 (0.4
g), cysteine (0.1 g), K2HPO4 (0.045 g), KH2PO4 (0.045 g), NaC1 (0.09 g),
(NH4)2SO4 (0.09 g), MgSO4
= 7H20 (0.009 g), CaC12 (0.009 g), resazurin (0.1 mg), hemin (1 mg), biotin
(1 ig), cobalamin (1 ig),
p-aminobenzoic acid (3 lag), folic acid (5 lag), and pyridoxamine (15 mg).
Bacterial strains for use in vaccine compositions
The inventors have identified that the bacterial strains of the invention are
useful for treating or
preventing diseases or conditions mediated by IL-17 or the Th17 pathway. This
is likely to be a result
of the effect that the bacterial strains of the invention have on the host
immune system. Therefore, the
compositions of the invention may also be useful for preventing diseases or
conditions mediated by
IL-17 or the Th17 pathway, when administered as vaccine compositions. In
certain such embodiments,
the bacterial strains of the invention may be killed, inactivated or
attenuated. In certain such
embodiments, the compositions may comprise a vaccine adjuvant. In certain
embodiments, the
compositions are for administration via injection, such as via subcutaneous
injection.
General
The practice of the present invention will employ, unless otherwise indicated,
conventional methods
of chemistry, biochemistry, molecular biology, immunology and pharmacology,
within the skill of the
art. Such techniques are explained fully in the literature. See, e.g.,
references [43] and [44-50], etc.
The term "comprising" encompasses "including" as well as "consisting" e.g. a
composition
"comprising" X may consist exclusively of X or may include something
additional e.g. X + Y.
The term "about" in relation to a numerical value x is optional and means, for
example, x+10%.
The word "substantially" does not exclude "completely" e.g. a composition
which is "substantially
free" from Y may be completely free from Y. Where necessary, the word
"substantially" may be
omitted from the definition of the invention.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
23
References to a percentage sequence identity between two nucleotide sequences
means that, when
aligned, that percentage of nucleotides are the same in comparing the two
sequences. This alignment
and the percent homology or sequence identity can be determined using software
programs known in
the art, for example those described in section 7.7.18 of ref. [51]. A
preferred alignment is determined
by the Smith-Waterman homology search algorithm using an affine gap search
with a gap open penalty
of 12 and a gap extension penalty of 2, BLOSU1VI matrix of 62. The Smith-
Waterman homology search
algorithm is disclosed in ref. [52].
Unless specifically stated, a process or method comprising numerous steps may
comprise additional
steps at the beginning or end of the method, or may comprise additional
intervening steps. Also, steps
may be combined, omitted or performed in an alternative order, if appropriate.
Various embodiments of the invention are described herein. It will be
appreciated that the features
specified in each embodiment may be combined with other specified features, to
provide further
embodiments. In particular, embodiments highlighted herein as being suitable, -
typical or preferred may
be combined with each other (except when they are mutually exclusive).
MODES FOR CARRYING OUT THE INVENTION
Example 1 ¨ Efficacy of bacterial inocula in a mouse model of house dust mite-
induced asthma
Summary
Mice were administered with compositions comprising bacterial strains
according to the invention and
were subsequently challenged with house dust mite (HDM) extract to elicit an
allergic inflammatory
response. The inflammatory response to HDM includes eosinophilic and
neutrophilic components, is
mediated by IL-17 and the Th17 pathway, and is a model for asthma. The
magnitude and characteristics
of the inflammatory response exhibited by mice treated with compositions of
the invention were
compared to control groups. The compositions of the invention were found to
alleviate the
inflammatory response, and to reduce recruitment of eosinophils and
neutrophils, indicating that they
may be useful for treating IL-17- and Thl 7-mediated conditions such as
eosinophilia, neutrophilia and
asthma.
Strain
433: Roseburia hominis
Study design
Groups:
1. Negative control group. Treatment with vehicle control (per oral).
2. Treatment with therapeutic bacteria inoculum strain 433 (per oral).
7. Positive control group. Treatment with Dexamethasone (i.p.).
8. Untreated Control Group.
Number of mice per group = 5
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
24
Day -14 to day 13: Daily administration of vehicle control per oral (Group 1).
Day -14 to day 13: Daily administration of therapeutic bacteria inoculum per
oral (Group 2-6).
Day 0, 2, 4, 7, 9, 11 Administration of 15ug HDM (house dust mite extract ¨
Catalogue number:
XPB70D3A25, Lot number: 231897, Greer Laboratories, Lenoir, NC, USA) in a
volume of 30u1 PBS
per nasal (Group 1-8).
Day 0, 2, 4, 7, 9, 11 Administration of Dexamethasone (i.p., 3mg/kg, Sigma-
Aldrich, Catalogue
number D1159) (Group 7).
Day 14 Sacrifice of all animals for analysis.
Total number of mice = 40.
Endpoints and analysis
On day 14 animals were sacrificed by lethal intraperitoneal injection with
pentabarbitol (Streuli
Pharma AG, Uznach, Cat: 1170139A) immediately followed by a bronchoalveolar
lavage (BAL).
Cells were isolated from the BAL (bronchoalveolar lavage) fluid and
differential cell counts performed
(200 cell counts/ samples).
Material and Methods
Mice. Female 7 week old BALB/c mice were purchased from Charles River
Laboratories and
randomly allocated to cages totally 5 mice per cage (Ventilated cages sourced
from Indulab AG, Gams,
Switzerland Cage type: "The SealsafeTM ¨ IVC cage. Product number 1248L).
Cages were labeled
with study number, group number and experimental starting date. Mice were
monitored weekly and
acclimatized to facility for 7 days prior to initiation of study (Study Day -
14). Animals were 8 weeks
old on Study Day -14. Potable water and food were available ad libitum. Cage
enrichment was present.
Daily care of the animals was performed according to local authorization
license number 2283.1
(issued and approved by: Service de la consommation et des affaires
veterinaires du Canton de Vaud).
Potable water and food were available ad libitum and refreshed once daily.
Cage enrichment was
present. Animal welfare regulations were observed as given by official
authorities of Switzerland under
ordinance 455.163 of the FVO (Federal Veterinary Office) on laboratory animal
husbandry, production
of genetically modified animals, and methods of animal experimentation.
Culturing of bacteria inoculum. Within a sterile workstation, a cryo-vial of
bacteria was thawed by
warming in gloved hand and ¨0.7 ml of contents injected into a Hungate tube
(Cat Number, 1020471,
Glasgeratebau Ochs, Boyenden-Lenglern, Germany), containing 8 ml of anaerobic
YCFA. Two tubes
per strain were usually prepared. The Hungate tubes were then incubated
(static) at 37 C for up to 24-
26 hours (for strain 433).
Culturing of vehicle control. A Hungate tube containing 8 ml of anaerobic YCFA
was incubated
(static) at 37 C for 16h.
Administration of bacteria inoculum or vehicle control. 400u1 of cultured
bacteria inoculum or
vehicle control were administered per day per oral gavage.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
Intranasal sensitization. Mice were anesthetized by i.p. injection with 9.75
mg xylasol and 48.75 mg
ketasol per kg (Dr. E. Graeub AG, Bern, Switzerland) and administered with
15ug of HDM (Catalogue
number: XPB70D3A25, Lot number: 231897, Greer Laboratories, Lenoir, NC, USA)
in a volume of
30u1 PBS per nasal.
5 Preparation and administration of positive control compound
Dexamethasone. Dexamethasone
21-phosphate disodium salt (Sigma-Aldrich, Catalogue number D1159, Lot N
SLBD.1030V) was
solved in H20 and administered to the animals in a dose of 3mg/kg in a volume
of 200u1 per oral at
days indicated in study protocol above.
Terminal procedure. On day 14 animals were sacrificed by lethal i.p. injection
with pentabarbitol
10 (Streuli Pharma AG, Uznach, Cat: 1170139A) immediately followed by
bronchoalveolar lavage (BAL)
in 500 ul of saline.
Measurement of cellular infiltrates into BAL. Cells were isolated from the BAL
fluid and
differential cell counts were performed based upon standard morphological and
cytochemical criteria.
Graphs and statistical analysis. All graphs were generated with Graphpad Prism
Version 6 and a
15 one-way ANOVA was applied. Results from the statistical analysis were
provided with the individual
data tables. Error bars represent Standard Error of the Mean (SEM).
Results and analysis
The results of the experiments are shown in Figures 1-9.
No morbidity or mortality was noted in the mice treated with the bacteria or
the vehicle. The two
20 controls, vehicle treatment (negative control) and the dexamethasone
treatment (positive control)
behaved as expected, with impaired eosinophilia and neutrophilia noted
following dexamethasone
treatment.
Strain 433 was efficacious in alleviating the magnitude of the allergic
inflammatory response. As
shown in Figures 2 and 3, administration of strain 433 reduced total
eosinophils and the proportion of
25 eosinophils in BAL, which indicates reduced eosinophilia. In addition,
administration of strain 433
resulted in a statistically significant reduction in total neutrophils and the
proportion of neutrophils in
BAL relative to the vehicle-only control, as shown in Figures 6 and 7.
Example 2 ¨ Efficacy of bacterial inocula in a mouse model of severe
neutrophilic asthma
Summary
Mice were administered with compositions comprising bacterial strains
according to the invention and
were subsequently sensitised with subcutaneous administrations of house dust
mite (HDM) extract and
challenged with an intranasal administration of HDM in order to model the
inflammatory response of
severe neutrophilic asthma. The magnitude and characteristics of the
inflammatory response exhibited
by mice treated with compositions of the invention were compared to control
groups. The compositions
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
26
of the invention were found to alleviate the inflammatory response, and in
particular to reduce
recruitment of neutrophils, in a manner comparable to the positive control
comprising administrations
of anti-IL-17 antibodies. The data therefore indicate that the compositions of
the invention may be
useful for treating IL-17- and Th17-mediated conditions such as neutrophilia
and asthma.
Strain
433: Roseburia hominis
Study design
Groups:
1. Negative control group. Treatment with vehicle control (per oral).
2. Treatment with therapeutic bacteria inoculum strain 433 (per oral).
7. Positive control group. Treatment anti-IL-17 (i.p.).
8. Untreated Control Group.
9: Healthy mice (baseline).
Number of mice per group (Group 1-8) = 5
Day -14 to day 17: Daily administration of vehicle control per oral (Group 1).
Day -14 to day 17: Daily administration of therapeutic bacteria inoculum per
oral (Group 2-6).
Day 0: Sensitization with HDM in CFA (s.c.) (Group 1-8).
Day 7: Sensitization with HDM in CFA (s.c.) (Group 1-8).
Day 13, 15, 17: Administration of anti IL-17 neutralizing antibody per i.p.
(Group 7).
Day 14, 15, 16, 17: Challenge with HDM in 30u1 PBS per nasal (Group 1-8).
Day 18: Sacrifice of all animals for analysis.
Endpoints and analysis:
On day 14 animals were sacrificed by lethal intraperitoneal injection with
pentabarbitol (Streuli
Pharma AG, Umach, Cat: 1170139A) immediately followed by a bronchoalveolar
lavage (BAL). Cells
were isolated from the BAL fluid and differential cell counts performed (200
cell counts/ samples).
Material and Methods.
Mice. Female 7 week old C57BL/6 mice were purchased from Charles River
Laboratories and
randomly allocated to cages totally 5 mice per cage (Ventilated cages sourced
from Indulab AG, Gams,
Switzerland Cage type: "The SealsafeTM ¨ IVC cage. Product number 1248L).
Cages were labelled
with study number, group number and experimental starting date. Mice were
monitored weekly and
acclimatized to facility for 7 days prior to initiation of study (Study Day -
14). Animals were 8 weeks
old on Study Day -14. Potable water and food were available ad libitum. Cage
enrichment was present.
Daily care of the animals was performed according to local authorization
license number 2283.1
(issued and approved by: Service de la consommation et des affaires
veterinaires du Canton de Vaud).
Potable water and food were available ad libitum and refreshed once daily.
Cage enrichment was
present. Animal welfare regulations were observed as given by official
authorities of Switzerland under
ordinance 455.163 of the FVO (Federal Veterinary Office) on laboratory animal
husbandry, production
of genetically modified animals, and methods of animal experimentation.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
27
Culturing of bacteria inoculum. Within a sterile workstation, a cryo-vial of
bacteria was thawed by
warming in gloved hand and ¨0.7 ml of contents injected into a Hungate tube
(Cat Number, 1020471,
Glasgeratebau Ochs, Bovenden-Lenglern, Germany), containing 8 ml of anaerobic
YCFA. Two tubes
per strain were usually prepared. The Hungate tubes were then incubated
(static) at 37 C for up to 24-
26 hours (for strain 433).
Culturing of vehicle control. A Hungate tube containing 8 ml of anaerobic YCFA
was incubated
(static) at 37 C for 16h.
Administration of bacteria inoculum or vehicle control. 400u1 of cultured
bacteria inoculum or
vehicle control were administered per day per oral gavage.
HDM sensitization. 50 [ig of HDM (Catalogue number: XPB70D3A25, Lot number:
231897, Greer
Laboratories, Lenoir, NC, USA) in PBS was emulsified in equal volume of
complete Freund's adjuvant
(CFA Chondrex Inc. Washington, USA) and administered subcutaneously in a
volume of 200 0, twice
over two weeks on opposite flanks. A week after the second immunization, mice
were anesthetized by
i.p. injection with 9.75 mg xylasol and 48.75 mg ketasol per kg (Dr. E. Graeub
AG, Bern, Switzerland)
and then given intranasal challenges of 15 lig of HDM in a volume of 30u1 PBS
on 4 consecutive days.
Analysis was performed one day after the final challenge.
Preparation and administration of positive control compound anti mouse IL-17
antibody.
Anti-IL-17 neutralizing antibody was sourced from Bio X Cell and was stored at
4 C (Clone 17F3,
Cat. Number BE0173, Bio X Cell) and administered per i.p. at a dose of 12.5
mg/kg at days indicated
in study protocol above.
Terminal procedure. On day 18 animals were sacrificed by lethal i.p. injection
with pentabarbitol
(Streuli Pharma AG, Uznach, Cat: 1170139A) immediately followed by
bronchoalveolar lavage (BAL)
in 500 ul of saline.
Measurement of cellular infiltrates into BAL. Cells were isolated from the BAL
fluid and
differential cell counts were performed based upon standard morphological and
cytochemical criteria.
Graphs and statistical analysis. All graphs were generated with Graphpad Prism
Version 6 and a
one-way ANOVA was applied. Results from the statistical analysis are provided
with the individual
data tables. Error bars represent Standard Error of the Mean (SEM).
Results and analysis
The results of the experiment are shown in Figures 10-18.
No morbidity or mortality was noted in the mice treated with the bacteria or
the vehicle. As shown in
Figures 11, 12, 15 and 16, certain mice treated with strain 433 exhibited
reduced eosinophilia and
neutrophilia.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
28
Example 3 ¨ Efficacy of bacterial inocula to treat arthritis in a type II
collagen-induced arthritis
mouse model
Materials and methods
Strain
433: Roseburia hominis
Bacterial cultures
Bacterial cultures were grown up for administration in an anaerobic
workstation (Don Whitley
Scientific).
Bacterial strain #433 was grown using glycerol stocks. The glycerol stocks
were stored at -80 C. Three
times per week, glycerol stocks were thawed at room temperature and streaked
on YCFA plates. A
new glycerol aliquot was used on each occasion. Bacteria were allowed to grow
on a given plate for
up to 72 hours.
Solutions to be administered to the animals were prepared twice daily with an
eight hour interval for
morning (AM) and afternoon (PM) treatments. A bacterial colony was picked from
the streaked plate
and transferred into a tube containing YCFA media. Bacterial strain #433 was
allowed to grow for 24
hours before AM administrations. Bacteria were sub-cultured at 1% into YCFA
media for PM
administrations. OD values were recorded for each strain after morning and
afternoon treatment
preparations.
Type II collagen-induced arthritis mouse model
Adult male DBA/1 mice were randomly allocated to experimental groups and
allowed to acclimatise
for two weeks. On Day 0, animals were administered by subcutaneous injection
with 100 microliters
of an emulsion containing 100 micrograms of type II collagen (CII) in
incomplete's Freund's adjuvant
supplemented with 4 mg/ml Mycobacterium tuberculosis H37Ra. On Day 21, animals
were
administered by subcutaneous injection with a booster emulsion containing 100
jig of type II collagen
in incomplete Freund's adjuvant.
Treatments were given according to the administration schedule below. From Day
-14 until the end of
the experiment on Day 45, animals were weighed three times per week. From Day
21 until the end of
the experiment, animals were scored three times per week for clinical signs of
arthritis to include
swelling of the hind- and front paws, radio-carpal (wrist) joints and tibio-
tarsal (ankle) joints.
On Day 45 mice were culled and terminal blood samples were taken for cytokine
analysis.
On Day -14, Day 0 and Day 45, faecal samples were collected for
microbiological analysis,
immediately snap-frozen and stored at -80 C.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
29
The collagen-induced arthritis (CIA) mouse model is a well-established mouse
model for rheumatoid
arthritis [53]. Immunisation with CII causes a pathogenesis that includes
several important pathological
features of rheumatoid arthritis, including synovial hyperplasia, mononuclear
cell infiltration and
cartilage degradation. Significantly, the development of CIA is mediated by
Th17 cells through
secretion of IL-17A [54]. The immune response underlying the arthritis model
is enhanced by the use
of Freund's adjuvant supplemented with Mycobacterium tuberculosis.
On Day 21, spleens were collected from three satellite animals in each group.
Cells were cultured for
72 hours in the presence or absence of type II collagen. Cytokines, including
TNF-a, IL-6, IFN-y, IL-
4, IL-10 and IL-17, were quantified in the culture supernatants and in
terminal serum by Luminex. Cell
proliferation was quantified using a tritiated thymidine incorporation method.
Treatment Groups and Dosages
All Groups were n=15 (n=12 for the main study group and n=3 for satellite
groups)
The vehicle used for the biotherapeutics was Yeast extract-Casitone-Fatty
Acids (YCFA) medium.
Administration Disease
Group Dose Induction
Route Regimen
5m1/kg Day 0:
1 Vehicle Collagen/CFA,
BID: once, SC
5 ml/kg P0 Day-14-End Day 21:
6 Biotherapeutic #433 Collagen/IFA,
once, SC
PO: oral gavage, SC: subcutaneous injection, BID: twice a day, CFA: complete
Freund's adjuvant.
Bodyweights
From Day -14 until the end of the experiment, animals were weighed three times
per week. Data were
graphed (Mean SEM).
Non-specific clinical observations
From Day -14 until the end of the experiment, animals were checked daily for
non-specific clinical
signs to include abnormal posture (hunched), abnormal coat condition
(piloerection) and abnormal
activity levels (reduced or increased activity).
Clinical Observations
From Day 21 until the end of the experiment on Day 45, animals were scored
three times per week for
clinical signs of arthritis to include swelling of the hind- and front paws,
radio-carpal (wrist) joints and
tibio-tarsal (ankle) joints. Each limb was scored using the following scale:
(0) normal, (1) slight
swelling, (2) mild swelling, (3) moderate swelling and (4) severe swelling. A
clinical score was
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
calculated by adding each limb score. The maximum possible clinical score for
an animal was (16).
Animals with a score equal to (12) on two consecutive occasions and animals
with a score greater than
(12) on any one occasion were culled. Data were graphed (Mean SEM).
Cell proliferation analysis
5 On Day 21, three satellite animals per group were culled and spleens were
dissected out. Spleen cells
were cultured for 72 hours in presence or absence of type II Collagen. After
72 hours, cells were pulsed
overnight in the presence of tritiated thymidine. Cell proliferation was
quantified by measuring
thymidine incorporation. Data were graphed (Mean SEM). Supernatants were
taken and tested for
the presence of key cytokines.
10 Cytokine analysis
Terminal supernatants from the spleen cell cultures were tested in order to
quantitate TNF-c, IL-6,
IFN-y, IL-4, IL-10 and IL-17 by Luminex. Data were graphed (Mean SEM).
Microbiological analysis
On Day -14, Day 0 and Day 45, faecal samples were collected from each animal,
immediately snap-
15 frozen, and stored at -80 C. Caeca (including content) were immediately
snap-frozen and stored
at -80 C. A bacterial identification test was performed daily by plating the
bacteria.
Histopathology
At the end of the experiment, hind paws were stored in tissue fixative.
Samples were transferred into
decalcification solution. Tissue samples were processed, sectioned and stained
with Haematoxylin &
20 Eosin. Sections were scored by a qualified histopathologist, blind to
the experimental design, for signs
of arthritis to include inflammation, articular cartilage damage and damage to
the underlying
metaphyseal bone. A detailed scoring system was used (see below). Data were
graphed (Mean SEM).
Raw and analysed data were provided as well as representative pictures.
Table 1: Histopathology Scoring System
Grade Description
Inflammation
0 Normal joint
1 Mild synovial hyperplasia with inflammation dominated by
neutrophils. Low
numbers of neutrophils and macrophages in joint space.
2 Synovial hyperplasia with moderate to marked inflammation
involving both
neutrophils and macrophages. Neutrophils and macrophages in joint space;
may be some necrotic tissue debris.
3 Synovial hyperplasia with marked inflammation involving both
neutrophils
and macrophages. Loss of synoviocyte lining. Inflammation may extend from
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
31
synovium to surrounding tissue including muscle. Numerous neutrophils and
macrophages in joint space, together with significant necrotic tissue debris.
Articular cartilage damage
0 Normal joint
1 Articular cartilage shows only mild degenerative change. Early
pannus
formation may be present peripherally.
2 Articular cartilage shows moderate degenerative change and
focal loss. Pannus
formation is present focally.
3 Significant disruption and loss of articular cartilage with
extensive pannus
formation.
Damage to the underlying metaphyseal bone
0 Normal joint
1 No change to underlying metaphyseal bone.
2 May be focal necrosis or fibrosis of metaphyseal bone.
3 Disruption or collapse of metaphyseal bone. Extensive
inflammation, necrosis
or fibrosis extending to medullary space of the metaphysis.
Results and analysis
Survival and Non-specific Clinical Observations
Some animals were culled prior to the scheduled end of the study due to the
severity of the clinical
signs of arthritis or due to the severity of the non-specific clinical
observations.
Two animals were culled or found dead during the pre-treatment period (Day -14
to Day 0): one animal
in Group 1 (vehicle-treated, animal arrived from supplier with broken leg and
was culled) and one
animal in Group 6 (biotherapeutic 4433-treated).
Ten animals were culled due to the severity of the clinical signs of
arthritis: five animals in Group 1
(vehicle-treated) and five animals in Group 6 (biotherapeutic 4433-treated).
Four animals were culled due to the severity of the non-specific clinical
signs including abnormal
posture (hunched), abnormal coat condition (piloerection), abnormal activity
levels (reduced activity):
three animals in Group 1 (vehicle-treated) and one animal in Group 6
(biotherapeutic 4433-treated).
Bodyweights
Bodyweight data recorded from Day -14 until Day 0 and expressed as a
percentage of the initial (Day
-14) bodyweights were analysed by two-way ANOVA followed by Dunnett's post-
test for multiple
comparisons with Day -14 then for multiple comparison with the vehicle-treated
group. The data are
presented in Figure 19. Data from animals culled prior to the scheduled end of
the experiment were
excluded from the analyses.
When compared to Day -14, twice daily administrations by oral gavage induced a
significant
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
32
bodyweight loss in the vehicle-treated group on Day -9 and Day -7.
Bodyweight data recorded from Day 0 until Day 28 and expressed as a percentage
of the initial (Day
0) bodyweights were analysed by two-way ANOVA followed by Dunnett's post-test
for multiple
comparisons with Day 0 in the Vehicle group then for multiple comparison with
the vehicle-treated
group. The data are presented in Figure 20. Data from animals culled prior to
the scheduled end of the
experiment and from Satellite animals were excluded from the analyses. Day 28,
Day 35 and Day 42
data were further analysed by one-way ANOVA followed by Dunnett's post-test
for multiple
comparisons to the vehicle-treated group.
The onset of clinical signs of arthritis was associated with a significant
bodyweight loss on Day 26 and
Day 28 (p < 0.0001) when compared to Day 0 in the vehicle-treated group.
Clinical Observations
Clinical score data were analysed by two-way ANOVA followed by Dunnett's post-
test for multiple
comparisons between days in the vehicle-treated group then for multiple
comparisons between
experimental groups and the vehicle-treated group each day. The data are
presented in Figure 21. Data
recorded from animals culled prior to the end of the experiment were excluded
from the analysis. When
animals were culled due to the severity of the clinical signs of arthritis,
the last recorded score was
reported for the following days and used in the statistical analyses.
A significant increase of the clinical scores was observed in the vehicle-
treated group from Day 28
until Day 45 (p < 0.0001) when compared to Day 21.
Biotherapeutic #433 induced a reduction of the clinical scores when compared
to the vehicle-treated
group from Day 28 until Day 45, although the difference was non-significant.
Cell proliferation analysis
To validate the assay, splenocytes were cultured in the presence of soluble
anti-CD3 and anti-CD28
(anti-CD3/CD28) as positive control stimuli to confirm the proliferative
potential of the cells.
Strong proliferative responses to anti-CD3/CD28 were seen in all experimental
groups, showing cells
were healthy, viable and able to respond to activation signals.
To test the proliferative response in presence of Collagen II (CII),
splenocytes were cultured in the
presence of Cll at 50 ig/ml. Splenocyte proliferative response to CR were
analysed by two-way
ANOVA followed by Sydak's post-test for multiple comparisons between
unstimulated and CII-
stimulated splenocytes and one-way ANOVA followed by Dunnett's post-test for
comparison of CII-
stimulated response in different experimental groups with the vehicle-treated
group. The data are
presented in Figure 22.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
33
CH induced a highly significant increase of 31-1-thymidine incorporation (cpm)
when compared to the
unstimulated splenocytes in the vehicle-treated group (p < 0.0001).
The groups treated with biotherapeutic #433 demonstrated significantly lower
levels of CII-induced
splenocyte proliferation than the vehicle-treated group.
Cytokine levels in tissue culture supernatants
Levels of each cytokine were measured in tissue culture supernatants derived
from anti-CD3/CD28
stimulated cultures by luminex analysis. These showed robust responses for all
cytokines measured
(mean levels in vehicle group were as follows: IL-4 = 6,406 pg/ml; IL-6 = 306
pg/ml; IL-10 = 10,987
pg/ml; IL-17A = 11,447 pg/ml; IFN-y = 15,581 pg/ml; TNF-a = 76 pg/ml).
The following sections summarise the data obtained from the Collagen II-
stimulated cultures. Where
applicable, statistical analyses of the differences between cytokine levels in
supernatants of
unstimulated and CII-stimulated splenocytes were conducted using two-way ANOVA
followed by
Sidak's post-test for multiple comparisons, while one-way ANOVA followed by
Dunnett's post-test
was used for comparison of CII-stimulated response in biotherapeutic-treated
groups with the vehicle-
treated group. There was no significant difference in cytokine levels between
the groups in both cases.
This is likely due to the small sample size used (n=3).
In order to more accurately present the distribution of the data for the
cytokines with substantial spread
of the data, these are presented as scatter plots.
The group means of IL-4 in tissue culture supernatants after stimulation with
CII were <5pg/ml. These
are not considered biologically significant and not included here. The group
means of TNF-a in tissue
culture supernatants after stimulation with collagen were below limit of
quantitation.
Supernatant levels of IFN-y (Figure 23)
Along with IL-17, IFN-y is the major cytokine driving disease in the CIA
model. The scatter plot in
Figure 23 demonstrates IFN-y levels after CII stimulation, with group median
being higher for the
Vehicle-treated group compared to the biotherapeutic.
Supernatant levels of IL-17A (Figure 24)
Levels of IL-17A were 50pg/m1 in CII-stimulated cultures for the Vehicle-
treated group. The levels
of -this cytokine appeared to be lower in the biotherapeutic group compared to
the Vehicle-treated.
Supernatant levels of IL-10 (Figure 25)
Levels of IL-10 in Vehicle-treated group were 13 pg/ml and 2.1 pg/ml for CII-
stimulated, and media
control cultures, respectively. Higher levels of IL-10 (which is an anti-
inflammatory cytokine) for the
vehicle-treated group may be expected because inflammation and pro-
inflammatory cytokine
induction could be accompanied by an anti-inflammatory feedback mechanism.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
34
Supernatant levels of IL-6 (Figure 26)
Inflammatory cytokines such as IL-6 and TNF-ct are not typically produced at
high levels in anti-CII
cultures. However, their levels may be altered as a result of immune
modulation. Levels of IL-6 in CII-
stimulated cultures were modest, reaching 10pg/ml. Although higher than in
media control cultures,
these differences were too small to provide rationale for performing
statistical analyses.
Microbiological analysis
Bacterial growth was confirmed by measuring the optical density at 600 nm
using a spectrophotometer.
Bacterial identity was confirmed by comparing streaked plate pictures to
reference pictures.
Following the improved bacterial preparation method, consistently high doses
of bacterial strain were
administered from Day -2 and Day -3 as indicated by the high OD values
measured.
Faecal samples were collected and snap-frozen on Day -14, Day 0 and at
termination.
Hi sto p ath ology
The histopathology results are shown in Figures 65-69. As expected for this
model, intra-individual
and inter-individual variability was observed in terms of the presence/absence
of arthritis or the
severity of change present.
The nature of the pathology was as expected for this model, with extensive
mixed chronic-active
inflammation of the synovium and bursa extending to involve the peri-articular
soft tissues (muscle,
adipose tissue, dermal collagen). In the most severely affected joints there
was articular cartilage
degeneration and loss with intra-articular debris and inflammation and
disruption of the joint and bone
structure by fibrosis and inflammation.
The incidence of histopathological changes was: vehicle ¨ 80% (16/20);
Biotherapeutic #433 ¨ 55%
(12/22). Treatment with Biotherapeutic #433 reduced the incidence of
histopathological scores in
mouse hind limbs when compared to the vehicle-treated group (see Figures 65-
68). Histopathology
scores were analysed by one-way ANOVA for non-parametric data (Kruskal-Wallis
test) followed by
Dunn's post-test for multiple comparisons to the vehicle-treated group,
although the reduction
achieved by Biotherapeutic #433 was not statistically significant in this
analysis. Biotherapeutic #433
induced a reduction of the joint inflammation scores observed in
histopathology when compared to the
vehicle-treated group. Biotherapeutic #433 induced a reduction of the
cartilage damage scores
observed in histopathology when compared to the vehicle-treated group.
Biotherapeutic #433 induced
a reduction of the bone damage scores observed in histopathology when compared
to the vehicle-
treated group. Biotherapeutic #433 induced a reduction of the total
histopathology scores when
compared to the vehicle-treated group.
Summary
Increased clinical scores were observed from Day 28 after the first
administration of type 11 collagen,
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
as expected in this model of arthritis in DBA/1 mice. Biotherapeutic #433 was
shown to be effective
at treating arthritis in this model. Biotherapeutic #433 was effective for
reducing the severity of the
clinical scores and for reducing pathological disease in the joints, as
demonstrated in the
histopathological analysis.
5 Proliferative recall responses to Collagen II were seen in splenocyte
cultures from all experimental
groups. The collagen-specific response was significantly reduced following
treatment with
biotherapeutic #433 (Group 5).
Most of the T cell cytokines tested showed detectable increases between
Collagen II-stimulated and
media controls in the Vehicle-treated group. These increases were not as
obvious in the biotherapeutic-
10 treated group. This broadly supports the proliferative recall responses
to Collagen II described above.
There was evidence of suppression of the Th 1/Th17 axis, which is the
pathogenic response in this
model and in human RA. Correlation of reduced levels of cytokines with reduced
proliferation is
suggestive of immune modulation. There was no evidence that this modulation
resulted either from
enhanced levels of Th2 associated IL-4 or with increases in the immune
modulating cytokine, IL-10.
15 Example 4 ¨ Further analysis of the effect of bacterial inocula in the
mouse model of house dust
mite-induced asthma
The mice tested in Example 1 were subjected to further analyses to further
characterise the effect of
the compositions of the invention on the allergic asthma inflammatory
response.
Materials and methods
20 Blood withdrawal and serum preparation on day 14. Blood samples of
animals were collected via
cardiac puncture. Serum was isolated from the blood sample by centrifugation
for 5 min at 14000g and
stored at -20 C.
Organ removal on day 14. Collection of the left lung lobe in formalin for
follow-on histological
analysis. Collection of the right lung lobes (all remaining lobes) and removal
of serum for snap freezing
25 and follow-on analysis. Remaining BAL fluid was snap frozen for follow-
on analysis.
Measurement of antibody levels in serum and BAL fluid
Total IgE and house-dust-mite (HDM) specific IgG1 antibody production were
measured in the BAL
and serum by ELISA assay.
Isolation of lung and histological analysis
30 Left lung lobes were fixed in formalin followed by embedment in
paraffin, sectioning, and staining
with hematoxylin and eosin and PAS. Subsequent histological scoring was
performed blinded as
followed: Five random fields of view per sample were scored for inflammation
(peribronchial
infiltration and perivascular infiltration) and mucus production. Inflammatory
infiltration was scored
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
36
with the following grading system:
0 - normal
1 - mild inflammatory infiltrates
2 - moderate inflammatory infiltrates
3 - marked inflammatory infiltrates
4 - severe inflammatory infiltrates
5 - very severe inflammatory infiltrates
In each field of view, airways were measured in size and mucus cell numbers
were quantified/ um.
Measurement of inflammatory mediators in lung tissue
Right lung lobes (all remaining lobes) isolated for quantification of
inflammatory mediators were snap
frozen for subsequent measurement of CCL11, IFN-gamma, IL-1 alpha, IL-1 beta,
IL-4, IL-5, IL-9,
IL-17A, CXCL1, CCL3, CXCL2 and CCL5 by commercially available multiplex assay
(Merck-
Millipore). Analysis was performed according to the manufacturer's
instructions.
Results and analysis
The results of the experiments are shown in Figures 27-45.
In support of the findings described in Example 1, analysis of the cellular
infiltrates in the lung tissue
of mice treated with strain 433 showed a notable and statistically significant
reduction in mean
inflammation score (see Figures 31 and 33).
Antibody levels in the BAL fluid and serum were analysed (see Figures 27-30).
No clear effect of the
bacterial treatment on serum antibody levels was observed. This may reflect a
failure in the experiment,
because the spread of data and the error bars for each treatment are large,
and the positive and negative
controls do not appear to have behaved as would be expected. Also, the
baseline serum antibody levels
could have masked any changes.
Similarly, no clear effect of the bacterial treatment on cytokine levels in
lung tissue was observed (see
Figures 35-45). Again, this may reflect a failure in the experiment, because
the spread of data and the
error bars for each treatment are large, and the positive and negative
controls do not appear to have
behaved as would be expected. It is also possible that the mechanism of action
involved influences
earlier cytokine responses that were no longer detectable on day 4 post the
final I-1DM airway
challenge. Some care should be taken when interpreting the cytokine data in
the current study, due to
the variability in the levels detected. This variability could in part be
explained by the fact that the lung
tissue was separated for the different analyses, and thus one lung lobe might
not have been fully
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
37
representative or comparable to the same lobe in other mice due to patchy
distribution of the
inflammation.
Example 5 ¨ Further analysis of the effect of bacterial inocula in the mouse
model of severe
neutrophilic asthma
The mice tested in Example 2 were subjected to further analyses to further
characterise the effect of
the compositions of the invention on the neutrophilic response associated with
severe asthma.
Materials and methods
Organ removal on day 18. Collection of the left lung lobe in formalin for
follow-on histological
analysis. Collection of the right lung lobes (all remaining lobes) and removal
of serum for snap freezing
and follow-on analysis. Remaining BAL fluid was snap frozen for follow-on
analysis.
Measurement of inflammatory mediators in lung tissue (follow-on analysis).
Right lung lobes (all
remaining lobes) isolated for quantification of inflammatory mediators were
snap frozen for
subsequent measurement of IFN-gamma, IL-1 alpha, IL-1 beta, CXCL1, CCL3,
CXCL2, CCL5, IL-
17A, TNF-alpha, IL-17F, IL-23 and IL-33 by commercially available multiplex
assay (Merck-
Millipore). Analysis was performed according to the manufacturer's
instructions.
Measurement of antibody levels in serum and BAL fluid (follow-on analysis).
House-dust-mite
(HDM) specific IgG1 and IgG2a antibody production were measured in the BAL and
serum by ELISA
assay.
Isolation of lung and histological analysis (follow-on analysis). Left lung
lobes were fixed in
formalin followed by embedment in paraffin, sectioning, and staining with
hematoxylin and eosin and
PAS. Subsequent histological scoring was performed blinded as followed: Five
random fields of view
per sample were scored for inflammation (peribronchial infiltration and
perivascular infiltration) and
mucus production. Inflammatory infiltration was scored with the following
grading system:
0 - normal
1 - mild inflammatory infiltrates
2 - moderate inflammatory infiltrates
3 - marked inflammatory infiltrates
4 - severe inflammatory infiltrates
5 - very severe inflammatory infiltrates
Results and analysis
The results of the experiments are shown in Figures 46-63.
Further analysis of antibody levels revealed that the efficacy of bacterial
strain 433 was also reflected
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
38
in reduced HDM-specific IgG1 levels in the BAL fluid and serum (see Figures 46
and 48). Firm
conclusions regarding an effect on IgG2a levels cannot be drawn. Overall, the
data from the antibody
analysis is suggestive of a reduction related to an overall reduced
inflammatory response, as opposed
to a selective effect on antibody isotype switching.
In relation to cytokine levels, as for Example 4, the spread of data and the
error bars for each treatment
are large, and the positive and negative controls do not appear to have
behaved as necessarily would
be expected. It is also possible that the mechanism of action involves
influencing earlier cytokine
responses that were no longer detectable on day 4 post the final HDM airway
challenge. Some care
should be taken when interpreting the cytokine data in the current study, due
to the variability in the
levels detected. This variability could in part be explained by the fact that
the lung tissue was separated
for the different analyses, and thus one lung lobe might not have been fully
representative or
comparable to the same lobe in other mice due to patchy distribution of the
inflammation. Despite this
variability, a clear anti-inflammatory effect on cytokine levels for strain
433 was shown, and the
positive control anti-IL-17 Ab generally behaved as expected.
With the above caveats, the data in Figure 55 suggests that treatment with
strain 433 may achieve a
reduction in the levels of IFNy, which may be indicative of a mechanism of
action related to influences
on chemokine release (and thus recruitment of cells) by stromal or innate
immune cells. IFNy is
involved in the Th17 pathway. Taking this dataset together, a clear conclusion
can be drawn that strains
433 was effective at protecting mice against inflammation in this mouse model
of severe neutrophilic
asthma.
Example 6 ¨ Efficacy of bacterial inocula in a mouse model of multiple
sclerosis
Summary
Mice were administered with compositions comprising bacterial strains
according to the invention and
the mice were subsequently immunised with myelin oligodendrocyte glycoprotein
to induce
experimental autoimmune encephalomyelitis (EAE). EAE is the most commonly used
experimental
model for human multiple sclerosis. The compositions of the invention were
found to have a striking
effect on disease incidence and disease severity.
Strain
433: bacteria deposited under accession number NCIMB 43283
Study design
Groups:
1. Negative control group. Treatment with vehicle control (per oral).
5. Treatment with therapeutic bacteria inoculum strain 433 (per oral).
9. Positive control group. Treatment with Dexamethasone (i.p.).
10. Untreated Control Group.
Number of mice per group = 10
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
39
Days -14 to day 27: Daily administration of vehicle control per oral (Group
1).
Days -14 to day 27: Daily administration of therapeutic bacteria inoculum per
oral (Group 5).
Days 0-28: administration of Dexamethasone (i.p.) three times a week (Group 9)
Day 0: M0G35-55 (myelin oligodendrocyte glycoprotein - 2mg/m1) and CFA (2mg/m1
MTB) were
mixed 1:1 resulting in lmg/m1 solutions. 100 1 of the peptide-CFA mixture was
injected
subcutaneously into each hind leg. Administration of pertussis toxin
intraperitoneally (300ng).
Day 1: Administration of pertussis toxin intraperitoneally (30Ong).
Days 7-onwards: Measurement of disease incidence and weight three times a
week.
Endpoints and analysis
Mice were analysed for disease incidence and disease severity three times a
week. Scoring was
performed blind. Disease severity was assessed using a clinical score ranging
from 0 to 5, with 5
indicating a dead mouse (see clinical scoring system below).
Monitoring
On the indicated days mice were weighed and observed for disease activity
score and disease incidence.
Disease activity score observations:
0 - No obvious changes in motor function compared to non-immunized
mice.
0.5 - Tip of tail is limp.
1.0 - Limp tail.
1.5 - Limp tail and hind leg inhibition.
2.0 - Limp tail and weakness of hind legs.
OR - There are obvious signs of head tilting when the walk is observed. The
balance is poor.
2.5 - Limp tail and dragging of hind legs.
OR - There is a strong head tilt that causes the mouse to occasionally fall
over.
3.0 - Limp tail and complete paralysis of hind legs.
3.5 - Limp tail and complete paralysis of hind legs.
In addition to: Mouse is moving around the cage, but when placed on its side,
is unable to right
itself.
Hind legs are together on one side of body.
4.0 - Limp tail, complete hind leg and partial front leg paralysis.
Mouse is minimally moving around the cage but appears alert and feeding
4.5 - Complete hind and partial front leg paralysis, no movement around the
cage.
Mouse is immediately euthanized and removed from cage.
5.0 Mouse is euthanized due to severe paralysis.
When an animal has equal or greater disease activity score of 1, it is
considered to have a positive
disease incidence score.
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
Results
The results of the study are shown in Figures 70 and 71.
Disease induction in the negative control groups was successful with high
scores shown by the vehicle
control and the untreated control. The effect of treatment with strain 433 was
striking and the mice
5 treated with strain 433 exhibited notably reduced disease incidence and
disease severity.. These data
indicate the strain 433 may be useful for treating or preventing multiple
sclerosis.
Example 7 ¨ Stability testing
A composition described herein containing at least one bacterial strain
described herein is stored in a
sealed container at 25 C or 4 C and the container is placed in an atmosphere
having 30%, 40%, 50%,
10 60%, 70%, 75%, 80%, 90% or 95% relative humidity. After 1 month, 2
months, 3 months, 6 months,
1 year, 1.5 years, 2 years, 2.5 years or 3 years, at least 50%, 60%, 70%, 80%
or 90% of the bacterial
strain shall remain as measured in colony forming units determined by standard
protocols.
Sequences
SEQ ID NO:1 (Roseburia hominis strain A2-181 16S ribosomal RNA gene, partial
sequence -
15 AY804148)
1 taaaggttga tcctggctca ggatgaacgc tggaggcgtg cttaacacat gcaagtcgaa
61 cgaagcactt taattgattt cttcggaatg aagtttttgt gactgagtgg cggacgggtg
121 agtaacgcgt gggtaacctc gctcatacag ggggataaca gttggaaacg actgctaata
181 ccgcataagc gcacaggatt gcatgatcca gtgtgaaaaa ctccggtggt atgagatgga
20 241
cccgcgtctg attagccagt tggcggggta acggcccacc aaagcgacga tcagtagccg
301 acctgagagg gtgaccggcc acattgggac tgagacacgg cccaaactcc tacgggaggc
361 agcagtgggt aatattgcac aatgggggaa accctgatgc agcgacgccg agtgagcgaa
421 gaagtatttc ggtatgtaaa gctctatcag caggaagaag aatgacggta cctgactaaa
481 aagcaccggc taaatacgtg ccagcagccg cggtaatacg tatggtgcaa gcgttatccg
25 541
gatttactgg gtgtaaaggg agcgcaggcg gtacggcaag tctgatgtga aatcccgggg
601 ctcaaccccg gtactgcatt ggaaactgtc ggactagggt gtctgagggg taagtggaat
661 tcctagtgta gcggtgaaat gcgtagatat taggaggaac accagtggcg aaggcggctt
721 actggacgat tactgacgct gaggctcgaa agcgtgggga gcaaacagga ttagataccc
781 tggtagtcca cgccgtaaac gatgaatact aggtgtcggg gagcattgct cttcggtgcc
30 841
gcagcaaacg caataagtat tccacctggg gagtacgttc gcaagaatga aactcaaagg
901 aattgacggg gacccgcaca agcggtggag catgtggttt aattcgaagc aacgcgaaga
961 accttaccaa gtcttgacat cccactgaca aagtatgtaa tgtactttct cttcggagca
1021 gtggtgacag gtggtgcatg gttgtcgtca gctcgtgtcg tgagatgttg ggttaagtcc
1081 cgcaacgagc gcaaccccta ttcttagtag ccagcggttt ggccgggcac tctagggaga
35 1141
ctgccaggga taacctggag gaaggtgggg atgacgtcaa atcatcatgc cccttatgac
1201 ttgggctaca cacgtgctac aatggcgtaa acaaagggaa gcaatcccgc gagggggagc
1261 aaatctcaaa aataacgtct cagttcggac tgtagtctgc aactcgacta cacgaagctg
1321 gaatcgctag taatcgcgaa tcagaatgtc gcggtgaata cgttcccggg tcttgtacac
1381 accgcccgtc acaccatggg agttggtaat gcccgaagtc agtgacccaa ccgcaaggag
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
41
1441 ggagctgccg aagcaggact gataactggg gtgaagtcgt aacaagt
SEQ ID NO:2 (Roseburia hominis A2-183 16S rRNA gene, type strain A2-183T -
AJ270482)
1 gatcctggct caggatgaac gctggcggcg tgcttaacac atgcaagtcg aacgaagcac
61 tttaattgat ttcttcggaa tgaagttttt gtgactgagt ggcggacggg tgagtaacgc
121 gtgggtaacc tgcctcatac agggggataa cagttggaaa cgactgctaa taccgcataa
181 gcgcacagga ttgcatgatc cagtgtgaaa aactccggtg gtatgagatg gacccgcgtc
241 tgattagcca gttggcgggg taacggccca ccaaagcgac gatcagtagc cgacctgaga
301 gggtgaccgg ccacattggg actgagacac ggcccaaact cctacgggag gcagcagtgg
361 ggaatattgc acaatggggg aaaccctgat gcagcgacgc cgcgtgagcg aagaagtatt
421 tcggtatgta aagctctatc agcagggaag aagaatgcgg tacctgacta agaagcaccg
481 gctaaatacg tgccagcagc cgcggtaata cgtatggtgc aagcgttatc cggatttact
541 gggtgtaaag ggagcgcagg cggtacggca agtctgatgt gaaatcccgg ggctcaaccc
601 cggtactgca ttggaaactg tcggactaga gtgtcggagg ggtaagtgga attcctagtg
661 tagcggtgaa atgcgtagat attaggagga acaccagtgg cgaaggcggc ttactggacg
721 attactgacg ctgaggctcg aaagcgtggg gagcaaacag gattagatac cctggtagtc
781 cacgccgtaa acgatgaata ctaggtgtcg gggagcattg ctcttcggtg ccgcagcaaa
841 cgcaataagt attccacctg gggagtacgt tcgcaagaat gaaactcaaa ggaattgacg
901 gggacccgca caagcggtgg agcatgtggt ttaattcgaa gcaacgcgaa gaaccttacc
961 aagtcttgac atcccactga cagagtatgt aatgtacttt ctcttcggag cagtggtgac
1021 aggtggtgca tggttgtcgt cagctcgtgt cgtgagatgt tgggttaagt cccgcaacga
1081 gcgcaacccc tattcttagt agccagcggt tcggccgggc actctaggga gactgccagg
1141 gataacctgg aggaaggtgg ggatgacgtc aaatcatcat gccccttatg acttgggcta
1201 cacacgtgct acaatggcgt aaacaaaggg aagcaatccc gcgaggggga gcaaatctca
1261 aaaataacgt ctcagttcgg actgtagtct gcaactcgac tacacgaagc tggaatcgct
1321 agtaatcgcg aatcagaatg tcgcggtgaa tacgttcccg ggtcttgtac acaccgcccg
1381 tcacaccatg ggagttggta atgcccgaag tcagtgaccc aaccgcaagg agggagctgc
1441 cgaaggcagg actgataact ggggtgaagt cgtaacaagg gtacg
SEQ ID NO:3 (consensus 16S rRNA sequence for Roseburia hominis strain 433)
AAGAGTTT GGGHCAGGCT CAGGAT GAACGCT GGCGGCGT GCTTAACACAT GCAAGT
CGAACGAAGCACTTTAATT GA
TTTCTTCGGAATGAAGTTTTTGTGACTGAGTGGCGGACGGGTGAGTAACGCGTGGGTAACCTGCCTCATACAGGGGG
ATAACAGTTGGAAACGACTGCTAATACCGCATAAGCGCACAGGATTGCAT GATCCAGT GT
GAAAAACTCCGGTGGTA
TGAGAT GGACCCGCGT CT GATTAGCCAGTT GGCGGGGTAACGGCCCACCAAAGCGACGAT
CAGTAGCCGACCTGAGA
GGGTGACCGGCCACATTGGGACTGAGACACGGCCCAAACTCCTACGGGAGGCAGCAGTGGGGAATATTGCACAATGG
GGGAAACCCTGATGCAGCGACGCCGCGTGAGCGAAGAAGTATTTCGGTATGTAAAGCTCTATCAGCAGGGAAGAAGA
AT GACGGTACCT GACTAAGAAGCACCGGCTAAATACGT GCCAGCAGCCGCGGTAATACGTAT
GGTGCAAGCGTTAT C
CGGATTTACT GGGT GTAAAGGGAGCGCAGGCGGTACGGCAAGT CTGAT GT GAAAT CCCGGGGCT
CAACCCCGGTACT
GCATTGGAAACT GT CGGACTAGAGTGT CGGAGGGGTAAGT GGAATT CCTAGT GTAGCGGT
GAAATGCGTAGATATTA
GGAGGAACACCAGTGGCGAAGGCGGCTTACTGGACGATTACTGACGCTGAGGCTCGAAAGCGTGGGGAGCAAACAGG
ATTAGATACCCTGGTAGTCCACGCCGTAAACGATGAATACTAGGTGTCGGGGAGCATTGCTCTTCGGTGCCGCAGCA
AACGCAATAAGTATNCCACCTGGGGAGTACGTTCGCAAGAATGAAACTCAAAGGAATTGACGGGGACCCGCACAAGC
CA 02988693 2017-12-07
WO 2016/203221
PCT/GB2016/051774
42
GGTGGAGCNTGTGGTTTAATTCGAAGCAACGCGAAGAACCTTACCAAGTCTTGACATCCCACTGACAGAGTATGTAA
TGTACTTTCTCTTCGGAGCAGTGGTGACAGGTGGTGCATGGTTGTCGTCAGCTCGTGTCGTGAGATGTTGGGTTAAG
TCCCGCAACGAGCGCAACCCCTATTCTTAGTAGCCAGCGGTTTGGCCGGGCACTCTAGGGAGACTGCCAGGGATAAC
CTGGAGGAAGGTGGGGATGACGTCAAATCATCATGCCCCTTATGACTTGGGCTACACACGTGCTACAATGGCGTAAA
CAAAGGGAAGCAATCCCGCGAGGGGGAGCAAATCTCAAAAATAACGTCTCAGTTCGGACTGTAGTCTGCAACTCGAC
TACACGAAGCTGGAATCGCTAGTAATCGCGAATCAGAATGTCGCGGTGAATACGTTCCCGGGTCTTGTACACACCGC
CCGTCACACCATGGGAGTTGGTAATGCCCGAAGTCAGTGACCCAACCGCAAGGAGGGAGCTGCCGAAGGCAGGACTG
ATAACTGGGGTGAAGTCTACRSAGGGTAGCCGTRMMC
SEQ ID NO:4 (strain 433 genome sequence) - see electronic sequence listing.
REFERENCES
[1] Spor et al. (2011) Nat Rev Microbiol. 9(4):279-90.
[2] Eckburg et al. (2005) Science. 10;308(5728):1635-8.
[3] Macpherson et al. (2001)Microbes Infect. 3(12):1021-35
[4] Macpherson et al. (2002) Cell Mal Life Sci. 59(12):2088-96.
[5] Mazmanian et al. (2005) Cell 15;122(1):107-18.
[6] Frank et al. (2007) PNAS 104(34):13780-5.
[7] Scanlan et al. (2006)J Clin Microbiol. 44(11):3980-8.
[8] Kang et al. (2010)Inflamm Bowel Dis. 16(12):2034-42.
[9] Machiels et al. (2013) Gut. 63(8):1275-83.
[10] WO 2013/050792
[11] W003/046580
[12] WO 2013/008039
[13] WO 2014/167338
[14] Goldin and Gorbach (2008) Clin Infect Dis. 46 Suppl 2:S96-100.
[15] Azad et al. (2013) BAH. 347:f6471.
[16] Stanton and Savage (1983)Appl Environ Microbiol. 45(5):1677-84
[17] Duncan et al. (2006) Int. i Syst. Evol. Microbiol. 56: 2437-2441
[18] Masco et al. (2003) Systematic and Applied _Microbiology, 26:557-563.
[19] Sriitkova et al. (2011)J. Microbiol. Methods, 8'7(1):10-6.
[20] Ye et al. (2015) PLoS One. 10(1):e0117704.
[21] Fabro et al. (2015) Immunobiology. 220(1):124-35.
[22] Yin et al. (2014) Immunogenetics. 66(3):215-8.
[23] Cheluvappa et al. (2014) Clin Exp ImmunoL 175(2):316-22.
[24] Schieck et al. (2014)J Allergy Clin ImmunoL 133(3):888-91.
[25] Balato et al. (2014)J Eur Acad Dermatol VenereoL 28(8):1016-24.
[26] Monteleone et al. (2011) MC Medicine. 2011, 9:122.
[27] Fahy (2009) Proc Am Thorac Soc 6.256-259
[28] Miossec and Kolls (2012) Nat Rev Drug Discov. 11(10):763-76.
[29] Yang et al. (2014) Trends Pharmacol Sci. 35(10):493-500.
[30] Koenders et al. (2006) J. Immunol. 176:6262-6269.
[31] Amedei et al. (2012) Int J Mol Sci. 13(10): 13438-60.
[32] Shabgah et al. (2014) Postepy. Dermatot Alergol. 31(4):256-61.
[33] Miyamoto-Shinohara et al. (2008)J Gen. Appl. Microbiol, 54, 9-24.
[34] Cryopreservation and Freeze-Drying Protocols, ed. by Day and McLellan,
Humana Press.
[35] Leslie et al. (1995)Appl. Environs Microbiol. 61, 3592-3597.
[36] Mitropoulou et al. (2013)J Nutr Metab. (2013) 716861.
[37] Kailasapathy et al. (2002) Curr Issues Intest Microbiol. 3(2):39-48.
[38] Handbook of Pharmaceutical Excipients, 2nd Edition, (1994), Edited by A
Wade and PJ Weller
[39] Remington's Pharmaceutical Sciences, Mack Publishing Co. (A. R. Gennaro
edit. 1985)
[40] Handbook ofMicrobiological Media, Fourth Edition (2010) Ronald Atlas, CRC
Press.
[41] Maintaining Cultures for Biotechnology and Industry (1996) Jennie C.
Hunter-Cevera, Academic Press
[42] Strobel (2009)Methods Mol Biol. 581:247-61.
[43] Gennaro (2000) Remington: The Science and Practice of Pharmacy. 20th
edition, ISBN: 0683306472.
CA 02988693 2017-12-07
WO 2016/203221 PCT/GB2016/051774
43
[44] Molecular Biology Techniques: An Intensive Laboratory Course, (Ream et
al., eds., 1998, Academic Press).
[45] Methods In Enzymology (S. Colowick and N. Kaplan, eds., Academic Press,
Inc.)
[46] Handbook of Experimental Immunology, Vols. I-IV (D.M. Weir and C.C.
Blackwell, eds, 1986, Blackwell
Scientific Publications)
[47] Sambrook et al. (2001) Molecular Cloning: A Laboratory Manual, 3rd
edition (Cold Spring Harbor Laboratory
Press).
[48] Handbook of Surface and Colloidal Chemistry (Birdi, K.S. ed., CRC Press,
1997)
[49] Ausubel et al. (eds) (2002) Short protocols in molecular biology, 5th
edition (Current Protocols).
[50] PCR (Introduction to Biotechniques Series), 2nd ed. (Newton & Graham
eds., 1997, Springer Verlag)
[51] Current Protocols in Molecular Biology (F.M. Ausubel et al., eds., 1987)
Supplement 30
[52] Smith & Waterman (1981) Adv. Appl. Math. 2: 482-489.
[53] Brand et al. (2007) Nature Protocols. 2(5):1269-1275
[54] Jiao et al. (2014) Immunopathology and Infectious Diseases. 184(4):1085-
93.