Note: Descriptions are shown in the official language in which they were submitted.
METHODS OF DETERMINING TISSUE VIABILITY
BACKGROUND
1. Technical Field
The present disclosure relates to medical procedures and, more particularly,
to
procedures for determining and monitoring characteristics of tissue in
preparation for
performing various surgical procedures.
2. Background of Related Art
Colorectal surgery sometimes requires anastomosis, which involves resecting a
piece of diseased bowel tissue and creating a new connection between two
presumably
healthy bowel segments. Typically, before performing the anastomosis, the
amount of
tissue to be resected is estimated using visual indicia of the bowel. The goal
is to
preserve as much healthy tissue as possible while at the same time removing
all of the
diseased tissue.
A risk involved in performing an anastomotic procedure is anastomotic leaks
typically caused by a failure to resect all of the diseased tissue. Current
methods used in
estimating the amount of tissue to be resected during an anastomotic procedure
are
sometimes inadequate in preventing all anastomotic leaks. Additionally, the
health and
viability of colon and bowel sections may be compromised by excessive tension
or
insufficient blood flow in the newly attached sections.
Accordingly, a need exists for surgical instruments that can sense and
visualize,
either sequentially or simultaneously, a multitude of parameters and factors
of the bowel
tissue to aid a surgeon in performing a more successful anastomotic surgical
procedure.
SUMMARY
Irt one aspect of the present disclosure, a method of performing a surgical
procedure is provided and includes generating an infrared image of subject
tissue using a
1
CA 2993198 2018-01-26
-I 41
k 1
thermographic camera, analyzing the infrared images to determine blood flow
characteristics of the subject tissue, and resecting a portion of the subject
tissue
determined to have abnormal blood flow characteristics.
In some embodiments, the method may further include stapling the subject
tissue
upon determining that the subject tissue has normal blood flow
characteristics.
In some embodiments, the method may further include comparing the infrared
image of the subject tissue with an infrared image of healthy tissue to
determine whether
the subject tissue exhibits normal blood flow characteristics.
In some embodiments, the method may further include generating an infrared
image of tissue known to be healthy using the thermographic camera, and
comparing the
infrared image of the tissue known to be healthy to determine whether the
subject tissue
exhibits normal blood flow characteristics.
In some embodiments, the thermographic camera may be a thermographic
endo s cope.
1 5 In another aspect of the present disclosure, a method of performing a
surgical
procedure is provided and includes applying pressure to a subject tissue,
removing the
pressure applied to the subject tissue, generating an infrared image of the
subject tissue
using a thermographic camera after removing the pressure applied to the
subject tissue,
observing a rate at which the subject tissue increases in temperature using
the infrared
image, and determining whether the subject tissue increases in temperature at
a rate
associated with healthy tissue.
In some embodiments, the method may further include resecting a portion of the
subject tissue that increases in temperature at a rate associated with
unhealthy tissue.
CA 2993198 2018-01-26 2
In some embodiments, the method may further include stapling the subject
tissue
upon determining that the subject tissue increases in temperature at the rate
associated
with healthy tissue.
In some embodiments, the method may further include reducing a temperature of
the subject tissue prior to generating the infrared image of the subject
tissue. The
pressure may be applied to the subject tissue using a tissue gasper and the
temperature of
the subject tissue is reduced by contacting the subject tissue with pre-
chilled tissue-
contacting surfaces of the tissue grasper.
In yet another aspect of the present disclosure, a method of performing a
surgical
procedure is provided and includes determining a local perfusion pressure of
subject
tissue of a patient, determining a systemic blood pressure of the patient,
calculating a first
index using the determined local perfusion pressure and the determined
systemic blood
pressure, generating an infrared image of the subject tissue using a
thermographic camera,
analyzing the infrared image to determine blood flow characteristics of the
subject tissue,
and determining viability of the subject tissue based on the first index and
the determined
blood flow characteristics of the subject tissue.
In some embodiments, the method may further include assigning a numerical
value to the tissue based on the determined blood flow characteristics, and
calculating a
second index using the first index and the assigned numerical value to the
determine the
viability of the subject tissue. The second index may be compared to a known
index
corresponding to viable tissue.
In some embodiments, determining the local perfusion pressure may include
applying pressure to the tissue using a tissue grasper. The method may further
include
reducing a temperature of the subject tissue prior to generating the infrared
image of the
CA 2993198 2018-01-26 3
1 A
I. .
subject tissue. The temperature of the subject tissue may be reduced by
contacting the
subject tissue with pre-chilled tissue-contacting surfaces of the tissue
grasper.
In some embodiments, the method may further include stapling the subject
tissue
upon determining that the subject tissue is viable.
In some embodiments, the method may further include observing a rate at which
the subject tissue increases in temperature using the infrared image, and
determining
whether the subject tissue increases in temperature at a rate associated with
healthy tissue.
In some embodiments, the method may further include comparing the infrared
image of the subject tissue with an infrared image of healthy tissue to
determine whether
the subject tissue exhibits normal blood flow characteristics.
In some embodiments, the method may further include generating an infrared
image of tissue known to be healthy using the thermographic camera, and
comparing the
infrared image of the tissue known to be healthy to determine whether the
subject tissue
exhibits normal blood flow characteristics.
These and other objects will be more clearly illustrated below by the
description
of the drawings and the detailed description of the preferred embodiments.
BRIEF DESCRIPTION OF THE DRAWINGS
The accompanying drawings, which are incorporated in and constitute a part of
this specification, illustrate embodiments of the present disclosure and,
together with the
detailed description of the embodiments given below, serve to explain the
principles of
the disclosure.
FIG. 1 is a front, perspective view of a surgical system including a surgical
instrument and a thermographic camera, in accordance with the present
disclosure;
FIG. 2 is a flow chart depicting a method of using the thermographic camera of
the surgical system of FIG. 1 to assess tissue viability;
CA 2993198 2018-01-26
4
..-
FIG. 3 is a flow chart depicting another method of using the thermographic
camera of the surgical system of FIG. 1 to assess tissue viability;
FIG. 4 is a flow chart depicting a method of using the thermographic camera in
conjunction with the surgical instrument of the surgical system of FIG. 1 to
assess tissue
viability;
FIG. 5 is a rear, perspective view of the surgical instrument of the surgical
system
of FIG. 1;
FIG. 6 is an enlarged view of a handle portion of the surgical instrument of
FIG. 5
illustrating a display of the surgical instrument;
FIG. 7 is an enlarged view of the display of the surgical instrument of FIG.
5;
FIG. 8 is an enlarged view of a jaw assembly of the surgical instrument of
FIG. 5
illustrating sensors incorporated therein;
FIG. 9 is a top view of the surgical instrument of FIG. 5 illustrating the jaw
assembly grasping tissue; and
FIG. 10 is a flow chart depicting another method of using the thermographic
camera of FIG. 1 in conjunction with the surgical instrument of FIG. 5 to
assess tissue
viability.
DETAILED DESCRIPTION
Embodiments of the presently disclosed surgical systems and methods of use
will
now be described in detail with reference to the drawing figures wherein like
reference
numerals identify similar or identical elements. As used herein and as is
traditional, the
term "distal" will refer to that portion which is further from the clinician
while the term
"proximal" will refer to that portion which is closer to the clinician.
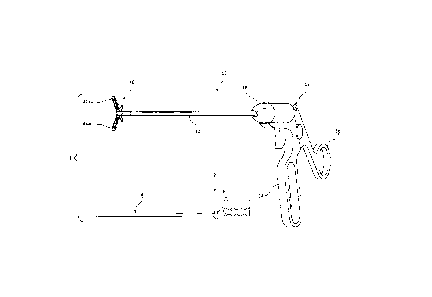
With reference to FIG. 1, a medical diagnostic system 1 is illustrated, which
generally includes a thermographic camera for taking infrared images of a
surgical site,
CA 2993198 2018-01-26
5
=
and a surgical instrument, such as, for example, a tissue grasper 10 for
grasping and
sensing a multiplicity of biological parameters of grasped tissue to assist a
surgeon in
performing a surgical procedure, for example, an anastomotic surgical
procedure. The
thermographic camera may be a thermographic endoscope 2 capable of being used
in a
minimally invasive manner. A detailed description of an exemplary
thermographic
endoscope may be found in U.S. Patent No. 5,445,157, filed on February 20,
1992, the
entire contents of which are incorporated by reference herein.
The thermographic endoscope 2 includes a shaft portion 4 and a display 6 in
communication (e.g., electrical or wireless) with the shaft portion 4. The
shaft portion 4
is dimensioned for insertion through a surgical incision in a patient and to
be positioned
adjacent the tissue to be imaged. Thermographic endoscope 2 also includes an
infrared
image forming device (not shown) disposed in a distal portion of the shaft 4
for forming
an infrared image of the tissue to be visualized, and a device (not shown) for
transmitting
the infrared image formed by the infrared image forming device to a device
(not shown)
for converting the infrared image, which is transmitted by the infrared image
transmitting
device into a visible image for displaying the visible image on display 6. In
some
embodiments, rather than using a thermographic endoscope, the surgical system
1 may
utilize a portable thermographic camera not intended for use in a minimally
invasive
manner.
Thermographic endoscope 2 is configured to produce an image of tissue under
inspection by taking advantage of the differences in the amount of infrared
radiation
emitted from different areas of the tissue. Different parts of tissue may
exhibit unique
blood flow characteristics based on various parameters of the tissue, for
example, the
health of the tissue (e.g., healthy, diseased, or necrosed), the amount and
type of
vasculature in the tissue, and the particular type of tissue (e.g., colorectal
tissue). The
CA 2993198 2018-01-26
6
blood flow characteristics of tissue include the rate at which tissue recovers
to a baseline
temperature after having been cooled to a selected temperature below the
baseline
temperature, and the rate at which a normal blood flow returns to the tissue
after having
been blocked (i.e., a perfusion rate).
As can be appreciated, due to the heat carried by blood, the blood flow
characteristics of a particular tissue are closely correlated with the thermal
profile
exhibited by the tissue. As such, the differences in blood flow
characteristics of a
selected portion of tissue may be visualized using infrared imaging
techniques, for
example, the thermographic endoscope 2 of surgical system 1. Providing a
clinician with
a visual image or a live feed of infrared images of tissue (e.g., colorectal
tissue) indicative
of the blood flow characteristics of the tissue prior to performing an
anastomosis may
assist the clinician in determining whether the selected tissue is viable, as
will be
described in detail below.
In operation, thermographic endoscope 2 may be used prior to, during, or after
a
surgical procedure, for example, an anastomotic surgical procedure, to provide
a clinician
with enhanced visual data about the subject tissue. In an anastomotic surgical
procedure,
unhealthy or diseased bowel tissue is resected and the ends of the remaining
healthy
segments of bowel are reconnected using one of a variety of anastomosis
techniques,
including, but not limited to, end to end stapler anastomosis, hand sewn
anastomosis, and
linear stapled anastomosis. Prior to reconnecting the ends of the separate
bowel segments
to one another using any of the above-noted anastomosis techniques, the
viability of the
ends of the separate bowel segments should be assessed in order to predict the
likelihood
of post-surgery anastomotic leaks or other adverse outcomes. To aid in making
this
viability assessment, a clinician may make use of thermographic endoscope 2 of
the
present disclosure.
CA 2993198 2018-01-26
7
With reference to FIG. 2, a use of thennographic endoscope 2 will be
described.
In use, each of the two ends of the presumably healthy bowel segments are
imaged using
the thermographic endoscope 2 by positioning the infrared image forming device
(not
shown) thereof adjacent the bowel segments. In step 102, the infrared image
forming
device forms an infrared image of the tissue, which is then converted and
transmitted into
a visible image or live feed of infrared images "IR" displayed on display 14.
In step 104,
the clinician analyzes the video feed of infrared images "IR" to determine
whether the
tissue exhibits nonual heat distribution characteristics (e.g., a uniform heat
profile
throughout the imaged tissue). If the clinician determines that the tissue
exhibits normal
heat distribution characteristics, the clinician continues to step 106, which
includes
stapling the two ends of the bowel segments, thereby completing the
anastomosis.
Alternatively, if the clinician determines that the tissue exhibits abnormal
heat
distribution characteristics (e.g., a non-uniform heat profile throughout the
imaged tissue),
the clinician continues to step 108, which includes resecting a portion of the
tissue
exhibiting abnormal heat distribution characteristics. This process will have
to be
repeated until all of the tissue is shown to exhibit normal heat distribution
characteristics.
An experienced clinician may be able to determine whether the tissue is
healthy
based on the infrared images "IR" alone, as described with reference to the
flow chart of
FIG. 2. However, with reference to FIG. 3, additional steps may be taken to
assist the
clinician in detennining whether the subject tissue exhibits normal heat
distribution
characteristics.
In particular, in step 202, tissue that is known to be healthy may be
simultaneously
imaged using the then-nographic endoscope 2, or in some embodiments, any
suitable
thermographic camera, to display infrared images of the known-to-be healthy
tissue on
the display 14 in juxtaposed relation to the infrared images "IR" of the
subject tissue. In
CA 2993198 2018-01-26
8
= = =
this way, in step 204, the clinician may compare the infrared images of the
known-to-be
healthy tissue and the subject tissue to determine the degree of similarity
between their
heat profiles. In step 206, the clinician determines whether the subject
tissue is healthy
based on the visual comparison of the infrared images "IR" of the subject
tissue with the
infrared images of the known-to-be healthy tissue.
In one embodiment, upon assessing the infrared images "IR" of the subject
tissue,
the clinician may assign a numerical value to the subject tissue based on how
close the
heat distribution of the subject tissue corresponds to the heat distribution
found in the
known-to-be healthy tissue. For example, based on the infrared images "IR,"
the
clinician may assign the tissue a numerical value ranging from 1 to 100, 1 to
50, 1 to 10,
or 1 to 5, wherein the lowest numerical value (e.g., 1) equates to
necrosed/diseased tissue,
and the highest numerical value (e.g., 100) equates to ideally healthy/viable
tissue. As
such, a clinician can use this number, determined using the infrared images
"IR" of the
subject tissue, to make a determination on whether the two ends of the bowel
segments
are healthy enough to be reconnected or whether more tissue needs to be
resected. If the
clinician determines that the tissue exhibits normal heat distribution
characteristics, the
clinician continues to step 208, which includes reconnecting the two ends of
the bowel
segments, thereby completing the anastomosis. Alternatively, if the clinician
determines
that the tissue exhibits abnormal heat distribution characteristics compared
to the known-
to-be healthy tissue, the clinician continues to step 210, which includes
resecting a
portion of the tissue exhibiting abnormal heat distribution characteristics.
This process
will have to be repeated until all of the tissue is shown to exhibit normal
heat distribution
characteristics compared to the known-to-be healthy tissue.
With reference to FIG. 4, manipulation of the subject tissue prior to and/or
during
an analysis of the infrared images "IR" of the subject tissue may further
assist the
CA 2993198 2018-01-26
9
= L..
clinician in gathering information about the subject tissue. For example, the
infrared
images "IR" generated using the thennographic endoscope 2 may be taken after
perfonning a grasp-then-release method on the tissue to assess tissue
perfusion. In
particular, in step 302, the bowel segments may be grasped, either separately
or together,
using any suitable device (e.g., tissue grasper 10) capable of applying a
clamping pressure
on the tissue sufficient to prevent or substantially prevent blood from
flowing through the
grasped tissue. Clamping the tissue in this way removes a substantial amount
of blood
from each of the two ends of bowel segments, thereby bringing each portion of
the
subject tissue to the substantially same temperature, in effect calibrating
the tissue. In
step 304, in the instance where tissue grasper 10 is used to clamp the tissue,
the tissue
may be cooled by pre-chilling the tissue-contacting surfaces 54, 56 of tissue
grasper 10
and grasping the tissue between the pre-chilled tissue contacting-surfaces 54,
56 of tissue
grasper 10.
Upon the ceasing of blood flow through the grasped tissue and/or the cooling
of
the grasped tissue from the pre-chilled tissue-contacting surfaces 54, 56 of
tissue grasper
10, thennographic endoscope 2 is positioned so that the infrared image forming
device of
then-nographic endoscope 2 is facing the grasped tissue. In step 306, the
clamping
pressure on the tissue is removed to allow for blood flow to return to the
tissue to
gradually restore the temperature of the tissue to its initial value or
baseline temperature.
This grasp-and-release step allows for a more accurate deten-nination of the
heat profile of
the subject tissue.
In step 308, a video feed of infrared images taken by thennographic endoscope
2
of the subject tissue will be displayed on display 14 beginning at the time
the clamping
pressure on the tissue is released. As the blood begins to return to the
tissue, the amount
of infrared radiation emitted by the tissue will increase at a rate correlated
with the health
CA 2993198 2018-01-26
of the tissue. For example, if the tissue is healthy, tissue perfusion should
occur at a rate
that is known for healthy tissue of the type being studied. Accordingly, the
infrared
images "IR" taken by thennographic endoscope 2 would indicate that the tissue
increased
in temperature (due to blood flowing therethrough) at a rate substantially
similar or the
same as that known for healthy tissue. Alternatively, if the tissue is not
healthy or not
viable, tissue perfusion should occur at a rate less than expected for
otherwise healthy
tissue of the particular type being studied. Accordingly, the infrared images
"IR" taken
by thennographic endoscope 2 would indicate that the tissue increased in
temperature
(due to blood flowing therethrough) at a rate less than that known for
otherwise healthy
tissue. As such, in step 310, the clinician visually observes the rate at
which the subject
tissue increases in temperature using the infrared images "IR" to determine
whether the
subject tissue increases in temperature at a normal rate.
An experienced clinician may be able to determine whether the tissue is
healthy
based solely on the rate of thermal change of the tissue visually observed
from the
infrared images "IR." However, to assist the clinician in determining whether
the subject
tissue exhibits a normal rate of thermal change, a measurement may be taken of
the time
it takes for the tissue to return to its initial, baseline temperature. This
measured time
may be compared to an index containing a list of known times each
corresponding to a
different degree of viability of tissue of the type being studied. For
example, if healthy
bowel tissue is known to typically take approximately 5 seconds to warm up
naturally to
its baseline temperature after being chilled to 5 degrees Fahrenheit below its
baseline
temperature, and the bowel tissue being studied was measured to take
approximately 10
seconds, this information can be used by the clinician to determine that the
subject tissue
may not be viable. As such, a clinician can use this information, determined
using the
infrared images "IR" of the subject tissue, to make a determination on whether
the two
CA 2993198 2018-01-26
11
ends of the bowel segments are healthy enough to be reconnected or whether
more tissue
needs to be resected. If the clinician determines that the tissue returns to
its baseline
temperature at a normal rate, the clinician continues to step 312, which
includes stapling
the two ends of the bowel segments, thereby completing the anastomosis.
Alternatively,
if the clinician determines that the tissue returns to its baseline
temperature at an
abnormal rate (e.g., faster or slower than that expected), the clinician
continues to step
314, which includes resecting a portion of the tissue exhibiting an abnormal
rate of
thermal change. This process will have to be repeated until all of the tissue
is shown to
exhibit normal rates of thermal change.
As will be described below, the above-described methods of using
thermographic imaging to assess tissue viability may be used in combination
with other
measurements taken of the patient, for example, a local perfusion pressure
reading and/or
a systemic blood pressure reading, to provide an even more accurate assessment
of tissue
vi ability.
With reference to FIGS. lA and 5-10, surgical system 1 may further include
tissue
grasper 10, which is configured to grasp tissue and sense a multiplicity of
biological
parameters of the grasped tissue to assist a surgeon in performing a surgical
procedure,
for example, an anastomotic surgical procedure. The tissue grasper 10
generally includes
a handle portion 12, an elongated shaft 30, and a jaw assembly 40.
The handle portion 12 of the tissue grasper 10 includes a stationary handle 14
and
a pivoting or movable handle 16 pivotably coupled to the stationary handle 14.
Manipulation of the pivoting handle 16 relative to the stationary handle 14
effects a
closing of the jaw assembly 40 to grasp tissue. The handle portion 12 of the
tissue
gasper 10 includes a longitudinal body portion 18 formed with the stationary
handle 14.
The body portion 18 has a display 20, for example, an LED display or liquid-
crystal
CA 2993198 2018-01-26
12
A j. . 1..,
display ("LCD") for displaying various tissue parameters measured by various
sensors
42a-c, 44a-c of the tissue grasper 10.
With reference to FIGS. 6 and 7, the body portion 18 of the handle portion 12
defines a longitudinal axis "X" that is coaxial with a longitudinal axis
defined by the
elongated shaft 30. The body portion 18 has a planar upper surface 22 on which
the
display 20 is situated. It is contemplated that the display 20 may be disposed
at any
suitable location of the body portion 18 or on any other component of the
tissue grasper
10.
The display 20 may have multiple display sections, for example, three display
sections 20a, 20b, 20c, arranged in a linear array along the longitudinal axis
"X." It is
contemplated that the display 20 may include more or less than three discrete
display
sections. A first display section 20a of the display 20 is configured to
display a visual
indication of a measured tissue perfusion of tissue grasped by the tissue
grasper 10. A
second display section 20b of the display 20 is configured to display a visual
indication of
a measured amount of pressure being applied to tissue grasped by the tissue
grasper 10.
A third display section 20c of the display 20 is configured to display a
measured
thickness of the tissue grasped by the tissue grasper 10.
The visual indication displayed by each of the display sections 20a, 20b, 20c
incudes a range of colors increasing in brightness from a proximal portion to
a distal
portion thereof For example, when viewing each of the first and second display
sections
20a, 20b in a proximal to distal direction, the first and second display
sections 20a, 20b
may each be green, yellow, orange, and then red, with each color portion
gradually
blending into the adjacent color portion. Other colors and color arrangements
for the first
and second display sections 20a, 20b are contemplated. The tissue grasper 10
may be
configured such that when the green of the first display section 20a is lit,
this may
CA 2993198 2018-01-26
13
A
indicate a relatively high tissue perfusion being measured by the tissue
grasper 10,
whereas when the red of the first display section 20a is lit, this may
indicate a relatively
low tissue perfusion being measured by the tissue grasper 10. The information
related to
the tissue perfusion may be useful when making a determination on the
viability of the
tissue being grasped, as will be described in more detail below.
With reference to the second display section 20b of the display 20, when the
green
of the second display section 20b is lit, this may be indicative of a
relatively low grasping
or clamping pressure being applied to tissue by the tissue gasper 10, whereas
when the
red of the second display section 20b is lit, this may be indicative of a
relatively high
grasping or clamping pressure being applied to tissue by the tissue grasper
10. The
clamping pressure measured by the tissue grasper 10 and displayed by the
second display
section 20b of the tissue grasper 10 is used in conjunction with the tissue
perfusion
displayed by the first display section 20a of the tissue grasper 10 to
determine the
perfusion pressure of the grasped tissue.
The perfusion pressure is measured by applying a sufficient amount of clamping
pressure on the subject tissue until there is no perfusion (i.e., no blood
flow) through the
tissue, and then slowly reducing the clamping pressure until perfusion through
the
grasped tissue restarts. The pressure at which the perfusion restarts is the
tissue perfusion
pressure. For a detailed description of a method of measuring surface
perfusion pressure,
reference may be made to U.S. Patent No. 7,618,376, the entire contents of
which are
incorporated by reference herein.
The information related to the amount of pressure being applied to tissue by
the
tissue grasper 10 may also be useful in preventing over-compression of tissue
to prevent
damage to healthy tissue.
CA 2993198 2018-01-26
14
In viewing the third display section 20c from a proximal to distal direction,
the
third display section 20c may be grey, brown, violet, and black. In some
embodiments,
third display section 20c may be grey, white, blue, purple, tan, green, and
black. Other
colors and color arrangements for the third display section 20c are
contemplated. When
the grey (proximal portion) of the third display section 20c is lit, this may
be an indication
that tissue grasped by the tissue grasper 10 has a relatively small thickness
(i.e., the tissue
is too thin to withstand being reconnected), whereas when the black (distal
portion) of the
third display section 20c is lit, this may be an indication that tissue
grasped by the tissue
gasper 10 has a relatively large thickness (i.e., the tissue is too thick to
be reconnected).
The information related to tissue thickness may be useful for making a
determination on
the type of reconnection technique to be used. Where stapling is selected, the
information
can be useful in selecting the size of the staples to be used when performing
a surgical
anastomosis on the gasped tissue.
In some embodiments, instead of display 20 utilizing ranges of colors or
brightness to illustrate the measured tissue parameters (e.g., tissue
perfusion, tissue
compression, and tissue thickness), the display 20 may display ranges of
numbers or
various numeral outputs. In particular, the first, second, and third display
sections 20a,
20b, 20c may display the number ranges 0 to 3, 0 to 5, 0 to 10, 0 to 100, or
any other
suitable range, to illustrate information about the tissue being grasped by
the tissue
grasper 10. For example, when the first display section 20a displays the
number 0, this
may be an indication that the grasped tissue has very little or no perfusion
(i.e., no blood
flow), whereas when the first display section 20a displays the number 100,
this may be an
indication that the grasped tissue has a high perfusion (i.e., ideal blood
flow).
CA 2993198 2018-01-26
In some embodiments, the display 20 may illustrate information about grasped
tissue utilizing any suitable indicia, for example, words such as poor,
satisfactory, or
good.
In some embodiments, tissue grasper 10 may not include display 20, and
instead,
tissue grasper 10 may be configured to be connected to or be in communication
with
another type of display, for example, display 6 of thermographic endoscope 2,
a tablet, a
cell phone, a computer monitor, a laptop, or any suitable display device.
Tissue grasper
may be connected to any of the aforementioned display devices via USB wires,
Wi-Fi,
or the like.
10 With
reference to FIGS. 1, 5, and 8, the elongated shaft 30 of tissue grasper 10
extends distally from the handle portion 12 and houses an actuation rod (not
explicitly
shown). The elongated shaft 30 may be rotatable with respect to the handle
portion 12 to
rotate the jaw assembly 40 about the longitudinal axis "X." The elongated
shaft 30 has
two prongs 32, 34 formed at its distal end 30b. A pivot pin 36 extends
transversely
through the prongs 32, 34 and the jaw assembly 40 to provide a point about
which the jaw
members 42, 44 of the jaw assembly 40 pivot. The actuation rod has a proximal
end (not
shown) operably coupled to the movable trigger 16 (FIGS. 1, 5), and a distal
end (not
shown) extending between the prongs 32, 34 and which is operably coupled to
the jaw
assembly 40.
With reference to FIGS. 8 and 9, the jaw assembly 40 includes first and second
juxtaposed jaw members 42, 44, respectively, which are simultaneously movable
between
a substantially approximated position, in which the jaw members 42, 44 are in
relatively
close relation to one another, and a spaced position, in which the jaw members
42, 44 are
separated at least a sufficient distance to receive tissue therebetween, for
example, tissue
"T." The jaw assembly 40 includes a first proximal portion or a first flange
46 extending
CA 2993198 2018-01-26
16
proximally from the first jaw member 42, and a second proximal portion or a
second
flange 48 extending proximally from the second jaw member 44. The flanges 46,
48 each
include an elongated angled cam slot 50, 52 defined therethrough configured to
engage
the distal end of the actuation rod (not explicitly shown) that drives the
opening and
closing of the jaw members 42, 44 as the actuation rod moves through the cam
slots 50,
52. As such, manipulation of the pivoting handle 16 (FIGS. 1, 5) relative to
the stationary
handle 14 actuates the actuation rod to effect an opening or a closing of the
jaw assembly
40 about tissue "T."
With continued reference to FIGS. 8 and 9, the jaw members 42, 44 each have a
tissue contacting surface 54, 56 such that when the jaw assembly 40 is in the
closed
configuration, tissue "T" is grasped between the tissue contacting surfaces
54, 56. The
jaw assembly 40 may be coupled to a cooling system (not shown) configured to
pass
coolant through jaw member 42, 44 to cool tissue contacting surfaces 54, 56
thereof
Alternatively, tissue contacting surfaces 54, 56 may include a thermoelectric
cold plate
for reducing the temperature of tissue contacting surfaces 54, 56. Tissue
contacting
surfaces 54, 56 are cooled so that when tissue contacting surfaces 54, 56 are
applied to
tissue, the tissue contacting surfaces 54, 56 reduce the temperature of the
tissue, as will be
described in detail below.
The jaw members 42, 44 each have a first sensor 42a, 44a, a second sensor 42b,
44b, and a third sensor 42c, 44c, disposed on respective tissue contacting
surfaces 54, 56.
The sensors 42a-c, 44a-c are arranged on respective tissue contacting surfaces
54, 56 in a
linear array along a longitudinal axis defined by respective jaw members 42,
44. In some
embodiments, the sensors 42a-c, 44a-c may be arranged on respective tissue
contacting
surfaces 54, 56 in any suitable manner.
CA 2993198 2018-01-26
17
The first sensors 42a, 44a of the jaw assembly 40 are perfusion sensors, for
example, Doppler flow sensors, configured to measure local perfusion (i.e.,
blood flow)
through tissue grasped between the jaw members 42, 44. The first sensors 42a,
44a may
measure perfusion of the grasped tissue on the basis of known techniques, such
as Laser-
Doppler Flowmetry ("LDF"), measuring light scattering, and/or measuring
absorption of
light from one or more LED's or other light sources. For a detailed
description of LDF
technology, reference may be made to U.S. Patent Nos. 4,109,647 and 4,862,894,
the
entire contents of each of which are incorporated by reference herein.
In some embodiments, only one of the tissue contacting surfaces 54, 56 of the
jaw
assembly-40 has a perfusion sensor. The first sensors 42a, 44a are in
communication, via
lead wires or wireless connection, with the first display section 20a of the
display 20 such
that upon the first sensors 42a, 44a measuring the perfusion in grasped
tissue, the first
sensors 42a, 44a transmit the measurement data to the first display section
20a, which
displays the measurement in any of the manners described above with reference
to FIGS.
2 and 3 (e.g., a range of colors, brightness levels, numbers, words.)
In some embodiments, the first sensors 42a, 44a may also be in communication,
via lead wires or wireless connection, with a computing device or processor
(not shown)
such as a laser Doppler monitor, which processes the information collected by
the first
sensors 42a, 44a to calculate the tissue perfusion. The computing device may
also be in
communication, via lead wires or wireless connection, with the first display
section 20a to
send the processed information related to the tissue perfusion to the first
display section
20a so that the first display section 20a can display the tissue perfusion in
any of the
manners described above with reference to FIGS. 6 and 7 (e.g., colors,
brightness levels,
numbers, words.)
CA 2993198 2018-01-26
18
With continued reference to FIGS. 8 and 9, the second sensors 42b, 44b of the
jaw
assembly 40 are pressure sensors or pressure measuring devices, for example,
MEMS
devices. For a detailed description of various MEMS devices, reference may be
made to
U.S. Patent No. 8,808,311, the entire contents of which are incorporated by
reference
herein. The second sensors 42b, 44b are configured to measure the amount of
pressure
applied by the tissue grasper 10 to the grasped tissue (i.e., the clamping
pressure). In
some embodiments, only one of the tissue contacting surfaces 54, 56 has a
pressure
sensor. The second sensors 42b, 44b are in electrical communication, via lead
wires of
wireless connection, with the second display section 20b of the display 20
such that upon
the second sensors 42b, 44b measuring the clamping pressure applied to the
grasped
tissue, the second sensors 42b, 44b transmit the measurement data to the
second display
section 20b, which displays the measurement in any of the manners described
above with
reference to FIGS. 6 and 7 (e.g., colors, brightness levels, numbers, words.)
Additionally
or alternately, the second sensors 42b, 44b may send the measured clamping
pressure to
the computing device for processing, which then sends the information to the
display 20.
The third sensors 42c, 44c of the jaw assembly 40 are gap determination
elements,
for example, slide potentiometers, rotational potentiometers, devices used to
measure
impedance between the contacting surfaces 54, 56, or Linear Variable
Differential
Transformers ("LVDT"), configured to measure the thickness of the grasped
tissue by
measuring the gap distance between the jaw members 42, 44. For a detailed
description
of various sensors capable of measuring tissue thickness, reference may be
made to U.S.
Patent No. 8,002,795, the entire contents of which are incorporated by
reference herein.
In some embodiments, only one of the tissue contacting surfaces 54, 56 has a
gap
determination element. The third sensors 42c, 44c are in electrical
communication, via
wire leads or wireless connection, with the third display section 20c of the
display 20
CA 2993198 2018-01-26
19
such that upon the third sensors 42c, 44c measuring the tissue thickness of
the grasped
tissue, the third sensors 42c, 44c transmit the measurement data to the third
display
section 20e, which displays the measurement in any of the manners described
above with
reference to FIGS. 6 and 7 (e.g., colors, brightness levels, numbers, words.)
Additionally
or alternately, the third sensors 42c, 44c may be in communication, via lead
wires or
wireless connection, to the computing device, which receives the tissue
thickness
measurements from the third sensors 42c, 44c, and processes the measurement
data and
then sends the information to the display 20.
In some embodiments, the display 20 may include a fourth display section (not
shown) configured to display an index representative of the ratio of the
surface perfusion
pressure measured by first sensors 42a, 44a and second sensors 42b, 44b of the
tissue
grasper 10, and a systemic blood pressure measured by a blood pressure cuff
(not shown),
as will be described in detail below.
In operation, the tissue grasper 10 may be used prior to, during, or after a
surgical
procedure, for example, an anastomotic surgical procedure, to gather various
data about
the subject tissue. In an anastomotic surgical procedure, unhealthy or
diseased bowel
tissue is resected and the ends of the remaining healthy segments of bowel are
reconnected. Prior to reconnecting the ends of the separate bowel segments to
one
another, the viability of the ends of the separate bowel segments should be
assessed in
order to predict the likelihood of post-surgery anastomotic leaks or other
adverse
outcomes. To aid in making this viability assessment, a surgeon may make use
of the
tissue grasper 10 in conjunction with the then-nographic endoscope 2 (FIG. l).
In use of the tissue grasper 10, each of the two ends of the presumably
healthy
bowel segments are grasped, either separately or together, between the tissue
contacting
surfaces 54, 56 of the jaw assembly 40. While monitoring the perfusion reading
(i.e.,
CA 2993198 2018-01-26
blood flow) displayed on the first display section 20a of the tissue grasper
10, the pivoting
handle 16 is manipulated to gradually increase the clamping pressure until the
perfusion
reading indicates that no blood flow or virtually no blood flow is moving
through the
grasped tissue. While continuously monitoring both the first and second
display sections
20a, 20b, the movable handle 16 of the tissue grasper 10 is manipulated to
gradually
reduce the amount of clamping pressure being applied to the tissue. The
clamping
pressure is reduced until first display section 20a displays a perfusion
reading indicating
that blood flow has returned to the grasped tissue. At the moment that the
perfusion
reading indicates that the blood flow is returned, the clamping pressure
reading displayed
by the second display section 20b is noted, which is the local perfusion
pressure of the
grasped tissue. The measured local perfusion pressure may be used to assess
the viability
of the grasped tissue. Additionally or alternately, the measured local
perfusion pressure
may be used in combination with other measurements and observations, for
example, a
systemic blood pressure reading and infrared images of the tissue, to aid in
making the
determination of the viability of the tissue.
The systemic blood pressure may be taken using any suitable device, for
example,
a blood pressure cuff, applied to any suitable body portion of the patient,
for example, the
arm of the patient. With reference to FIG. 10, in step 402, an index may be
calculated by
taking a ratio of the local perfusion pressure measured by the tissue grasper
10 and the
systemic blood pressure taken using the blood pressure cuff. The index may be
calculated by the computing device and displayed as a number on the display 20
of the
tissue grasper 10. The calculated index is predictive of whether an
anastomotic leak may
occur and/or the grade of an anastomotic leak. As such, a surgeon can use the
index to
make a determination on whether the two ends of the presumed healthy bowel
segments
are healthy enough to be reconnected or whether more tissue needs to be
resected. For a
CA 2993198 2018-01-26
21
detailed description of a method of calculating a perfusion index, reference
may be made
to U.S. Patent No. 7,618,376, the entire contents of which were incorporated
by reference
above.
To provide a more precise assessment of tissue viability, the thermographic
endoscope 2 may be used in conjunction with the measurements taken by the
tissue
grasper 10 and the blood pressure cuff. In step 404, infrared images of the
subject tissue
are generated using thermographic endoscope 2 and in step 406, the infrared
images of
the subject tissue are analyzed to observe the heat distribution
characteristics of the
subject tissue. In some embodiments, prior to step 404, tissue grasper 10 may
be used to
initiate the grasp-then-release steps 302, 304, 306 of FIG. 4. In step 408,
the tissue may
be assigned a numerical value by the clinician based on the heat distribution
characteristics observed using the infrared images of the subject tissue
displayed on
display 6. In step 410, this numerical value may be compared with the local
perfusion
pressure/systemic blood pressure index to provide a second index. In step 412,
this
second index may be compared to a known index corresponding to viable tissue
to
determine whether the tissue is viable. If the calculated second index is the
same or
substantially the same as the known index corresponding to viable tissue, the
clinician
continues to step 414, which includes reconnecting the two ends of the bowel
segments,
thereby completing the anastomosis. Alternatively, if the calculated second
index
deviates a threshold amount from the known index corresponding to viable
tissue, the
clinician continues to step 416, which includes resecting more of the tissue.
This process
will have to be repeated until all of the tissue is determined to be viable.
In some embodiments, the tissue grasper 10 may be pre-programmed to clamp
tissue at a predetermined clamping pressure that is known to result in
perfusion through
the grasped tissue to stop. The tissue grasper 10 may also be pre-programmed
to reduce
CA 2993198 2018-01-26
22
. .. ,
.
the clamping pressure at a predetermined rate and automatically send the
pressure reading
to the computing device at the moment when perfusion through the grasped
tissue
restarts. The perfusion pressure reading may also be displayed on the display
20. This
automated process eliminates human error in operating the tissue grasper 10 by
controlling the amount of clamping pressure being applied to the tissue
instead of the
surgeon.
The tissue grasper 10 may also be used to determine the size of the staples to
be
used in stapling the bowel segments together. The staple size may be
determined by
viewing the third display section 20c of the tissue grasper 10, which
indicates the
thickness of the grasped tissue. The leg height of the staples selected for
the stapling
procedure will correspond to the thickness of the tissue. It is contemplated
that the tissue
thickness measured by the third sensors 42c, 44c may be sent to the computing
device,
which has stored therein a look-up table that contains a staple size
corresponding to each
tissue thickness measurement.
In addition to the thermographic endoscope 2 and the tissue grasper 10 being
used
to ensure that the tissue is in condition for reconnecting (e.g., stapling) or
acceptable for
reconnecting, the thermographic endoscope 2 and the tissue grasper 10 may also
be used
as a check after the staples have been fired to ensure that the tissue is
healthy (e.g., has
good blood flow, is healing properly, etc.).
The thermographic endoscope 2 and the tissue grasper 10 may also be configured
to be incorporated into a robotic surgical system (not shown). The robotic
surgical
system is powered locally or remotely, and has electronic control systems
localized in a
console or distributed within or throughout the robotic surgical system. The
robotic
surgical system permits a surgeon to remotely manipulate the thermographic
endoscope 2
and the tissue grasper 10 to more precisely control the movement thereof The
surgical
CA 2993198 2018-01-26
23
. .. .
.
system 1 may be configured to send the infrared images generated by
thermographic
endoscope 2 and the measurements gathered by the sensors 42a-c, 44a-c of
tissue grasper
to an interface of the robotic surgical system on which the images and
measurements
may be displayed for the surgeon to read.
5 Although the illustrative embodiments of the present disclosure have
been
described herein, it is understood that the disclosure is not limited to those
precise
embodiments, and that various other changes and modifications may be affected
therein
by one skilled in the art without departing from the scope or spirit of the
disclosure. All
such changes and modifications are intended to be included within the scope of
the
10 disclosure.
CA 2993198 2018-01-26
24