Note: Descriptions are shown in the official language in which they were submitted.
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
TISSUE EXCISION DEVICE WITH ANCHOR
STABILITY ROD AND ANCHOR STABILITY ROD
RELATED APPLICATIONS
100011 The present application claims the benefit of U.S. Non-Provisional
Patent Application
Serial No. 14/967,020; filed December 11, 2015, which claims priority to U.S.
Provisional
Patent Application Serial No. 62/211,256, filed August 28, 2015; this
application also claims
the benefit of U.S. Non-Provisional Patent Application Serial No. 14/967,032;
filed
December 11, 2015, which claims priority to U.S. Provisional Patent
Application Serial No.
62/211,544, filed August 28, 2015; this application also claims the benefit of
U.S. Non-
Provisional Patent Application Serial No. 14/967,038; filed December 11, 2015,
which
claims priority to U.S. Provisional Patent Application Serial No. 62/211,264,
filed August 28,
2015; and also claims the benefit of U.S. Non-Provisional Patent Application
Serial No.
14/967,058; filed December 11, 2015, which claims priority to U.S. Provisional
Patent
Application Serial No. 62/211,549, filed August 28, 2015, hereby incorporated
by reference
in their entirety.
FIELD OF THE INVENTION
100021 The present invention relates generally to surgical instruments, and
more particularly,
to a tissue excision device.
BACKGROUND OF THE INVENTION
100031 Generally, to date there have been two coring type, excisional breast
biopsy devices
developed and marketed. These devices are described in the following U.S.
Patent Nos.
5,111,828; 5,197,484; 5,353,804; 6,080,113; 6,267,732; 6,383,145; 6,551,253;
5,782,775;
5,817,034; 5,857,982; 6,036,657; 6,077,231; 6,165,1406; and 6,213,957, all of
which are
hereby incorporated by reference.
100041 These devices were originally developed for use with stereotactic
imaging equipment.
Generally, these devices use the same basic technology. The typical biopsy
device includes a
localization needle with a guide wire preloaded into the device. The
localization needle and
guide wire are used to locate and localize the target area. The methodology of
their usage can
be summarized as follows:
1
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
1. Localize the target area with needle/wire hook;
2. Translate device up to the target area using a bladed stylet;
3. Core out the target specimen using a bladed cannula; and
4. Transect the tissue using a garrote wire to release the specimen.
100051 The device can either by a handheld device or may be a fixed device.
The below
more detailed description of the method of using a prior art device is
described with respect
to a handheld device.
100061 First, a localization needle is placed at the center of the target
tissue. A localization
wire is used to fix the handheld device to the tissue. After the localization
wire is deployed, a
stylet is manually advanced to a point just proximal of the target.
100071 One problem associated with the current device is that the localization
hook has very
little holding power. Another issue related to the prior art devices is the
potential of the stylet
to push andlor compress, i.e., the tissue in front of the stylet, i.e.,
"snowplow'.
100081 After the stylet reaches the target tissue, the cannula is manually
advanced over the
target tissue. With the cannula advanced over the target tissue, a mechanism,
such as a
garrote wire is activated to sever the target tissue from the breast. With the
target tissue
severed from the breast, the device, along with the target tissue with the
cannula, may be
removed.
100091 Generally, these prior art devices are purely mechanical devices, i.e.,
in other words,
the coring cannula is advanced by hand. The surgeon or user rotates a knob
that activates a
gear system to rotate and advances the coring cannula. This results in a
relatively slow,
intermittent advance of the cannula due to the start/stop motion of the
surgeon. The start/stop
motion can increase patient discomfort, as well as produce an undesirable
irregular specimen
shape.
100101 As discussed above, once the cannula has been advanced over the target
tissue, a
garrote wire may be used to cut the sample tissue (which is inside the
cannula) from the
breast so that it may be removed. The garrote wire has several limitations.
Typically, the
garrote wire traverse (at least partially) along the length of the device,
then is bent at a 90
degree angle, after which it encircles an inner surface of the coring cannula.
The right angle
in the garrote wire results in requiring a large amount of force to pull on
the garrote wire to
transect the tissue sample. Additionally, the garrote wire is generally
located a distance
behind the cutting edge of the coring cannula This results in a core of tissue
which is cored
2
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
by the coring cannula, which is not transected by the garrote wire, and thus
remains in the
breast. Furthermore, the garrote wire may tear the tissue rather than cutting
the tissue.
Additionally, dense tissue can be pushed aside rather than cut.
100111 Another issue related to prior art designs is the size of the cutting
edge of the cannula
with respect to the stylet. Prior to entry of the device into the breast, a
skin incision is made
using a scalpel. This incision is generally just slightly wider than the
diameter of the cannula.
Once the incision is made, the stylet is advanced in the breast, up to the
point where the
coring blade is ready to enter the incision. At this point, the surgeon will
use nerve hooks to
grab the skin and open the incision to allow the cutting edge of the cannula
to enter the
breast. However, the process of using the nerve hooks to grab the skin to make
the incision
wider can be cumbersome and inefficient and can cause patient discomfort.
[0012] The current devices use a stylet with integral cutting blades. The flat
stOet blades are
fixed to the stylet which may result in several adverse conditions. First, the
close proximity
of the cutting edge of the stylet blades to the ramp or stylet tip results in
the pushing or
compression or other inadvertent movement of the tissue by the stylet. The
prior designs also
results in a fixed minimal proximal margin equal to the length of the stylet
system.
[0013] Improved designs for tissue excision devices and related components
include those
described in U.S. Patent Nos. 8,597,200, 8,597,201, 8,597,202, 8,597,203,
8,597,204,
8,597,504, 8,529,467, 8,535,240, 8,444,573, 8,529,466, 8,484,988, and
8,740,809.
[0014] In particular, U.S. Patent No. 8,597,204 and U.S. Patent Application
Serial No.
14/062,519 disclose use of an independent needle device that is used to place
a tissue anchor
at a target area in the breast. Once the tissue anchor is in place, it
provides the means to guide
the excision device to the target area and perform the excision of a specimen.
This method of
performing the biopsy enables the user (surgeon) to place the tissue anchor
using different
methods of visualization, such as MRI, PET, Tomography or ultrasound. The
surgeon can
choose the best method of visualization based upon the size and type of target
tissue. After
the tissue anchor has been placed using the preferred method of visualization,
the tissue
excision device is advanced to the target area using ultrasound guidance.
[0015] The density and consistency of the breast tissue that the tissue anchor
is placed into
varies greatly. Due to this variety in the tissue, the tissue anchor may
migrate or move within
the breast during the introduction of the excision device. It is a critical
requirement of this
excision procedure that the tissue anchor remain at the target area once it is
placed.
3
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
100161 Another key consideration during the breast excision procedure is the
size of the
initial incision that is made into the breast. Great efforts are made to
minimize the incision
size. Reduction to the incision size can benefit patient health with reduced
recovery time as
well as improved cosmetic results. Currently, the procedure requires that the
surgeon make a
skin incision that is slightly larger than the diameter of the device cannula.
A cannula with a
15 min diameter, for example, would require an initial skin incision of
approximately 17-18
mm. There is a need for improved devices and methods for excising an intact
tissue
specimen of an adequate size through the smallest possible incision.
100171 An additional key consideration during the breast excision procedure is
the size of the
initial incision that is made into the breast. Great efforts are made to
minimize the incision
size. Reduction to the incision size can benefit patient health with reduced
recovery time as
well as improved cosmetic results. Currently, without using an incision
expander, the
procedure requires that the surgeon make a skin incision that is slightly
larger than the
diameter of the device cannula. A cannula with a 15 mm diameter, for example,
would
require an initial skin incision of approximately 17-18 mm. There is a need
for improved
devices and methods for excising an intact tissue specimen of an adequate size
through the
smallest possible incision.
100181 The procedure for excising a breast tissue sample with a handheld
excision device is
performed with the patient lying on her back. In certain cases, it may be
beneficial for the
surgeon to compress and support the breast from which the tissue sample is
being excised.
Compressing the breast may help to immobilize the tissue prior to and during
excision of the
tissue sample, which may result in a more accurate tissue excision.
100191 The present invention is aimed at one or more of the problems
identified above.
SUMMARY OF THE INVENTION
100201 A device is disclosed for allowing a surgeon to maintain control of a
tissue anchor and
prevent movement of the tissue anchor during introduction of an excision
device into breast
tissue for purposes of tissue excision.
100211 in a first aspect of the present invention, an excision device is
disclosed. The excision
device includes a housing coupled to a coring cannula, a stylet with a blade,
and a guide rod
assembly. A hollow central passageway extends through the center of the
excision device.
The guide rod assembly is coupled to the housing and includes a guide element
having first
4
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
and second ends and comprised of a guide rod and a locking member. The guide
rod is
slidably disposed within the central passageway. The locking member is fixed
to the second
end of the guide rod. The guide rod assembly further includes an anchor
stabilization rod
slidably disposed within the central passageway. A coupling device, a first
portion of which
is fixed to the guide rod and a second portion fixed to the anchor
stabilization rod, removably
couples the anchor stabilization rod to the guide rod.
100221 In a second aspect of the present invention, a guide rod assembly is
disclosed. The
guide rod assembly is coupled to the housing and includes a guide element
having first and
second ends and comprised of a guide rod and a locking member. The guide rod
is slidably
disposed within a central passageway of the excision device. The locking
member is fixed to
the second end of the guide rod. The guide rod assembly further includes an
anchor
stabilization rod slidably disposed within the central passageway. A coupling
device, a first
portion of which is fixed to the guide rod and a second portion fixed to the
anchor
stabilization rod, removably couples the anchor stabilization rod to the guide
rod.
100231 in a third aspect of the present invention, a guide rod assembly is
disclosed. The guide
rod assembly is coupled to the housing and includes a guide element having
first and second
ends and comprised of a guide rod and a locking member. The guide rod is
slidably disposed
within a central passageway of the excision device. The locking member is
fixed to the
second end of the guide rod. The guide rod assembly further includes an anchor
stabilization
rod slidably disposed within the central passageway. A coupling device, a
first portion of
which is fixed to the guide rod and a second portion fixed to the anchor
stabilization rod,
removably couples the anchor stabilization rod to the guide rod. The guide rod
assembly
further includes a fixed arm support removably coupled to the second end of
the anchor
stabilization rod.
100241 In a fourth aspect of the present invention, a method for preventing
movement of a
tissue anchor of an excision device during tissue excision is disclosed. The
method comprises
advancing a first end of a guide rod into a target tissue, introducing a first
end of an anchor
stabilization rod into an excision device, coupling the first end of the
anchor stabilization rod
to a second end of the guide rod inside the excision device, and controlling
movement of the
guide rod within the target tissue with a knob coupled to a second end of the
anchor
stabilization rod.
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[0025] A device is also disclosed for allowing a surgeon to excise a larger
tissue sample
through a smaller skin incision than would be possible with traditional
excision devices.
[0026] In a fifth aspect of the present invention an excision device is
disclosed. The excision
device includes a housing, a stylet coupled to the housing and having a tip
containing at least
one blade, a hollow central passageway extending through the center of the
housing and the
stylet, a localization needle coupled to the stylet and being slidably
disposed within a central
passageway, and an expanding cannula. The expanding cannula includes a body
having a first
end and a second end, the first end coupled to the housing, and an expanding
coring
mechanism coupled to the second end of the body. The expanding coring
mechanism
includes an annular spring a plurality of formed fingers extending between the
annular spring
and the second end of the body, and a retractable flexible blade extending
from the second
end of the body and over the annular spring.
[0027] In a sixth aspect of the present invention, an expanding cannula is
disclosed. An
expanding cannula includes a body having first and second ends, the first end
coupled to the
excision device. The expanding cannula further comprises an expanding coring
mechanism
coupled to the second end of the body, the mechanism including an annular
spring, a plurality
of formed fingers extending between the annular spring and the second end of
the body, and a
retractable flexible blade extending from the second end of the body and over
the annular
spring.
[0028] In a seventh aspect of the present invention, a method for excising
tissue using an
expanding cannula is disclosed. The method comprises introducing a bladed
stylet coupled to
the excision device into a target tissue, retracting the bladed stylet to
expose a distal end of an
expanding cannula, rotating the expanding cannula using a motor drive to
advance the
expanding cannula into the target tissue, and collecting a tissue sample.
[0029] In an eighth aspect of the present invention, a method for excising
tissue using an
expanding cannula is disclosed. The method comprises introducing a bladed
stylet coupled to
the excision device into a target tissue, retracting the bladed stylet to
expose a distal end of an
expanding cannula, rotating the expanding cannula using a motor drive to
advance an
expanding coring mechanism into the target tissue, collecting a tissue sample
with the
expanding coring mechanism, and retracting the expanding cannula from the
target tissue.
6
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[0030] Devices and methods are disclosed for allowing a surgeon to introduce
an excision
device with a larger diameter through a smaller skin incision than would
otherwise be
possible with traditional excision devices.
[0031] In a ninth aspect of the present invention, an incision expander with
removable stylet
is disclosed. An incision expander includes a housing, an expanding cannula
coupled to the
housing, a mechanism to expand the expanding cannula coupled to the housing
and the
expanding cannula, and a removable bladed stylet extending through the center
of housing
and the expanding cannula.
100321 In a tenth aspect of the present invention, an incision expander with
tapered stylet is
disclosed. An incision expander includes a housing, a cannula coupled to the
housing, and a
tapered stylet extending through the center of housing and the cannula.
100331 In an eleventh aspect of the present invention, a method for
introducing an excision
device through an incision is disclosed. A bladed stylet is introduced into a
target tissue. The
bladed stylet extends through the center of an incision expander. The incision
expander
comprises a housing and an expanding cannula. A mechanism to expand the
expanding
cannula is actuated. The bladed stylet is retracted from the incision
expander. An excision
device is introduced into the target tissue through the center of the incision
expander.
[0034] In a twelfth aspect of the present invention, a tissue excision system
is disclosed. The
system includes an excision device having a housing coupled to a coring
cannula, a bladed
stylet coupled to the housing, a hollow central passageway extending through
the center of
the housing, the coring cannula, and the stylet, and a guide rod assembly
removably coupled
to the housing. The system further includes an incision expander coupled to
the excision
device and having a housing, an expanding cannula coupled to the housing, a
mechanism
coupled to the housing and the expanding cannula and being configured to
expand the
expanding cannula, wherein the bladed stylet extends through the center of the
housing of the
excision expander and the expanding cannula.
[0035] Devices and methods are disclosed for allowing a surgeon to compress
and support a
breast from which a tissue sample may be excised.
[0036] In a thirteenth aspect of the present invention, a breast compression
device is
disclosed. The breast compression device comprises a back plate, a front
paddle comprising
two or more fingers, and an adjuster to adjust the space between the back
plate and the front
paddle.
7
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
100371 In a fourteenth aspect of the present invention; a breast compression
device is
disclosed. The breast compression device comprises a back plate, a front
paddle comprising
two or more fingers, and a bar connecting the back plate and the front paddle.
100381 In a fifteenth aspect of the present invention, a method for
compressing breast tissue
with a breast compression device is disclosed. The breast compression device
is wrapped
around the breast tissue from any angle. The breast compression device
includes a back plate,
a front paddle comprising two or more fingers, and a bar connecting the back
plate and the
front paddle. The breast compression device is adjusted to provide enough
compression to
render the breast tissue immobile.
100391 In a sixteenth aspect of the present invention, a tissue excision
system is disclosed.
The system includes an excision device and a breast compression device. The
excision device
includes a housing coupled to a coring cannula, a stylet coupled to the
housing and having a
bladed tip, a hollow central passageway extending through the center of the
housing, the
coring cannula, and the stylet, and a guide rod assembly removably coupled to
the housing.
The breast compression device includes a back plate, a front paddle with two
or more fingers,
and an adjuster to adjust the space between the back plate and the front
paddle. The bladed
stylet is advanced to a target tissue between the two or more fingers of the
front paddle of the
compression device.
BRIEF DESCRIPTION OF 'THE DRAWINGS
100401 Other advantages of the present invention will be readily appreciated
as the same
becomes better understood by reference to the following detailed description
when
considered in connection with the accompanying drawings wherein:
100411 Figure 1 is a drawing of a biopsy device with an integrated needle,
according to an
embodiment of the present invention;
100421 Figures 2A-2D include a series of views of the biopsy device of Figure
1 illustrating
operation thereof;
100431 Figure 3 is an isometric drawing of a biopsy device with an independent
needle
assembly, according to an alternative embodiment of the present invention;
100441 Figure 4A is a drawing of the independent needle assembly of Figure 3;
100451 Figure 4B is another drawing of the independent needle assembly of
Figure 3;
8
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[0046] Figure 5A is a drawing of a guide element of the independent needle
assembly of
Figures 3 and 4;
[0047] Figure 5B is a larger view of a portion of Figure 5A;
[0048] Figure 6 is an isometric illustration of a handheld breast biopsy
device having a
housing and a handle, according to an embodiment of the present invention;
[0049] Figure 7 is a cut-away view of the handle of a breast biopsy device,
according to an
embodiment of the present invention;
[0050] Figure 8 is a first cut-away view of a breast biopsy device
illustrating the drivetrain
components, according to an embodiment of the present invention;
[0051] Figure 9 is a second cut-away view of the breast biopsy device of
Figure 8;
[0052] Figure 10 is a drawing of the housing, according to an embodiment of
the present
invention:
[0053] Figure 11 is a drawing of the stylet, according to an embodiment of the
present
invention;
[0054] Figure 12 a drawing of the cannula, according to an embodiment of the
present
invention;
[0055] Figure 13A is an illustration of a guide element of an independent
needle assembly,
according to a first embodiment of the present invention;
[0056] Figure 13B is an illustration of a partial view of the independent
needle assembly of
Figure 13A, in the unlocking configuration;
[0057] Figure 13C is an illustration of a partial view of the independent
needle assembly of
Figure 13A, in the locking configuration;
[0058] Figure 14 is an illustration of a guide element according to a second
embodiment of
the present invention, in the unlocking configuration;
[0059] Figure 15 is an illustration of the guide element of Figure 14A in the
locking
configuration;
100601 Figure 16A is an illustration of a guide element according to a fourth
embodiment of
the present invention;
[0061] Figure 16B is a front view of the guide element of Figure 16A in the
unlocking
configuration;
[0062] Figure 16C is a side view of the guide element of Figure 16A in the
unlocking
configuration;
9
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
100631 Figure 16D is a side view of the guide element of Figure 16A in the
locking
configuration;
100641 Figure 17 is a side view of a guide element according to a fifth
embodiment of the
present invention;
100651 Figure 18 is a side view of a guide element according to a sixth
embodiment of the
present invention;
100661 Figure 19A is an illustration of a partial view of an integrated needle
assembly with a
guide element in the unlocking configuration, according to an embodiment of
the present
invention;
100671 Figure 19B is an illustration of the integrated needle assembly of
Figure 19A with the
guide element in the locking configuration;
100681 Figure 19C is an illustration of the integrated needle assembly of
Figure 19A with two
three wires of the guide element retracted into the localization needle;
100691 Figure 19D is an illustration of a part of the guide element with a
single wire which
remains in the target tissue to provide orientation of the sample;
100701 Figure 20A is a partial side view of a coring cannula in an initial
position and a final
position, according to an embodiment of the present invention;
100711 Figure 20B is a front view of the coring cannula of Figure 20A;
100721 Figure 21A is a partial side view of the coring cannula of Figure 20B
during initial
advanced of a flexible transection blade;
100731 Figure 21B is a front view of the coring cannula and flexible
transection blade of
Figure 21A;
100741 Figure 22A is a partial side view of the coring cannula and flexible
transection blade
in a second blade position;
100751 Figure 22B is a front view of the coring cannula flexible transection
blade in the
second blade position;
100761 Figure 23A is a view of the flexible transection blade in an initial
configuration,
according to an embodiment of the present invention;
100771 Figure 23B is a top view of the flexible transection blade in a cutting
configuration,
according to an embodiment of the present invention;
100781 Figures 23C is a front view of the flexible transection blade in the
cutting
configuration showing a cutting edge, according to an embodiment of the
present invention;
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[0079] Figure 24 is a graphical representation of the drivetrain of the device
and the flexible
transaction blade in the initial position, according to a first embodiment of
the present
invention;
[0080] Figure 25A is a graphical representation of the drivetrain of the
device and the
flexible transaction blade, of Figure 24, in the final position;
[0081] Figure 25B is a front view of the graphical representation of the
drivetrain of the
device and the flexible transaction blade, of Figure 24, in the final
position;
100821 Figure 26 is a graphical representation of the drivetrain of the device
and the flexible
transaction blade in the initial position, according to a second embodiment of
the present
invention:
100831 Figure 27 is a graphical representation of the drivetrain of the device
and the flexible
transaction blade, of Figure 24, in the final position;
100841 Figure 28A is a first cut away view of the graphical representation of
the drivetrain of
the device and the flexible transaction blade, of Figure 24, in the final
position;
[0085] Figure 28B is a second cut away view of the graphical representation of
the drivetrain
of the device and the flexible transaction blade, of Figure 24, in the final
position;
[0086] Figure 29 is a graphical representation of an alternative drivetrain,
according to an
embodiment of the present invention;
[0087] Figure 30 is a graphical representation of a second alternative
drivetrain in an initial
position, according to an embodiment of the present invention;
[0088] Figure 31 is a graphical representation of the second alternative
drivetrain in a final
position;
100891 Figure 32A is a graphical representation of a flexible transection
blade and a coring
cannula with a circular cutting ring, according to an embodiment of the
present invention;
100901 Figure 32B is a front view of the flexible transection blade and coring
cannula of
Figure 32A;
[0091] Figure 33A is a graphical representation of a flexible transection
blade and a coring
cannula with a partial cutting ring, according to an embodiment of the present
invention;
[0092] Figure 33B is a front view of the flexible transection blade and coring
cannula of
Figure 33A;
[0093] Figure 34A is a graphical representation of a flexible transection
blade which forms
the cutting edge of the coring cannula, according to an embodiment of the
present invention;
11
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[0094] Figure 34B is a front view of the flexible transection blade and coring
cannula of
Figure 34A;
[0095] Figure 35 is a graphical representation of a prior art coring cannula
with an internal
cutting ring;
[0096] Figure 36A is a graphical representation of a coring cannula with an
angled cutting
ring, according to an embodiment of the present invention;
[0097] Figure 36B is a front view of the coring cannula and cutting ring of
Figure 36A;
[0098] Figure 406A is a first view of a garrote wire for use with the cutting
ring of Figures
36A and 36B;
[0099] Figure 406B is a second view of a garrote wire for use m ith the
cutting ring of Figures
36A and 36B;
1001001 Figure 406C is a third view of a garrote wire for use with the
cutting ring of
Figures 36A and 36B;
1001011 Figure 38A is a graphical representation of a coring cannula with
an external
cutting ring, according to an embodiment of the present invention;
[00102] Figure 38B is a view of a portion of the coring cannula and
external cutting
ring of Figure 38A;
[00103] Figure 39 is a graphical representation of a coring cannula with an
external
cutting ring, according to an other embodiment of the present invention;
[00104] Figure 40 is a graphical representation of a prior art coring
cannula with a
cutting ring;
[00105] Figure 41 is a graphical representation of a coring cannula with a
cutting ring,
according to an embodiment of the present invention;
1001061 Figure 42A is a side view of a prior art coring cannula and stylet;
1001071 Figure 42B is a front view of the prior art coring cannula and
stylet of Figure
42A;
1001081 Figure 43A is a side view of a coring cannula and a collapsible
stylet in an
initial configuration, according to an embodiment of the present invention;
1001091 Figure 43B is a first front view of the coring cannula and stylet
of Figure 43A;
1001101 Figure 43C is a side view of the coring cannula and stylet of
Figure 43A in a
contracted configuration;
12
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
1001111 Figure 43D is a second front view of the coring cannula and stylet
of Figure
43A with the stylet in the contracted configuration;
1001121 Figure 44 is a view of a prior art stylet;
1001131 Figure 45 is a view of a stylet including an independent stylet
mechanism,
according to an embodiment of the present invention;
[00114] Figure 46A is a first view of an expanding localization needle,
according to an
embodiment of the present invention;
1001151 Figure 46B is a second view of the expanding localization needle of
Figure
46A;
[00116] Figure 46C is a view of the expanding localization needle of Figure
46A with
an actuation mechanism, according to a first embodiment of the present
invention;
[00117] Figure 46D is a view of the expanding localization needle of Figure
46A with
an actuation mechanism, according to a second embodiment of the present
invention;
[00118] Figure 47A is a first view of an expanding localization needle,
according to an
other embodiment of the present invention;
1001191 Figure 47B is a partial view of the expanding localization needle
of Figure
47A:
1001201 Figure 48A is a first view of a stylet with a rotating blade.
according to an
embodiment of the present invention;
[00121] Figure 48B is a second view of the stylet with the rotating blade
of Figure
48A;
[00122] Figure 49 is an illustration of a stylet with multiple rotating
blades, according
to an embodiment of the present invention;
[00123] Figure 50A is a graphical representation of a portion of a breast
biopsy device
with a garrote wire and a trigger mechanism includes a pair of cleats;
1001241 Figure 50B is a second graphical representation of the breast
biopsy device of
Figure 5A;
[00125] Figure 50C is a third graphical representation of the breast biopsy
device of
Figure 50A;
1001261 Figure 50D is a fourth graphical representation of the breast
biopsy device of
Figure 50A;
13
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
1001271 Figure 51A is a graphical representation of another embodiment of
the trigger
mechanism of Figure 50A;
1001281 Figure 51B is a graphical representation of a further embodiment of
the trigger
mechanism of Figure 50A;
1001291 Figure 52A is a top view of a graphical representation of a top
view of a breast
biopsy device having a rotatable trigger, according to an embodiment of the
present
invention;
1001301 Figure 52B is a side view of the breast biopsy device of 'Figure
52A;
1001311 Figure 52C is a second view of the breast biopsy device of Figure
52A; and
1001321 Figure 52D is a third view of the breast biopsy device of Figure
52A.
1001331 Figure 53 is an isometric view of an exemplary guide rod assembly;
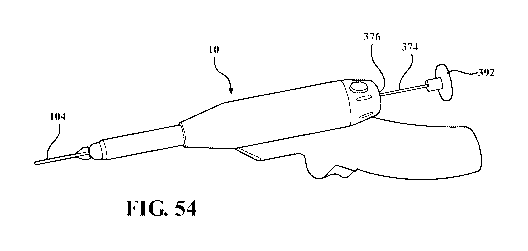
1001341 Figure 54 is a side view of an exemplary excision device with
anchor
stabilization rod;
1001351 Figure 55 is a partial isometric view of an anchor stabilization
rod and a guide
rod including a hook-and-loop coupling device;
1001361 Figure 56 is a partial isometric view of an anchor stabilization
rod and a guide
rod including a notched coupling device;
1001371 Figure 57 is a partial isometric view of an anchor stabilization
rod and a guide
rod including a wireform attachment coupling device;
1001381 Figure 58 is an isometric view of an exemplary excision device with
an anchor
stabilization rod and fixed support arm is shown;
1001391 Figure 59 is a partial isometric view of an expanding cannula;
[001401 Figure 60 is a partial isometric view of a partially expanded
expanding
cannula;
1001411 Figure 61 is a partial isometric view of a fully expanded expanding
cannula;
1001421 Figure 62 is an isometric view of an assembled incision expander
according to
a first embodiment;
1001431 Figure 63 is an isometric view of a disassembled incision expander;
and
1001441 Figure 64 is an isometric view of an incision expander with bladed
stylet
removed and expanded cannula in an expanded state;
1001451 Figure 65 is an isometric view of an assembled incision expander
according to
a second embodiment.
14
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[00146] Figure 66 is an isometric view of a breast compression device;
[00147] Figure 67 is a top view of a breast compression device according to
a second
embodiment:
1001481 Figure 68 is a top view of a breast compression device according to
FIG. 54
illustrating compression of a breast;
1001491 Figure 69 is a front view of a breast compression device according
to FIG. 54
illustrating compression of a breast; and
[00150] Figure 70 is a top view of a breast compression device with a
partial side view
of a biopsy device.
DETAILED DESCRIPTION OF INVENTION
1001511 Referring to the Figures, wherein like numerals indicate like or
corresponding
parts throughout the several views, the present invention provides a device
and method for
allowing a surgeon to maintain control of a tissue anchor and prevent movement
of the tissue
anchor during introduction of an excision device into breast tissue for
purposes of tissue
excision.
1001521 An exemplary breast biopsy device 10 and a method of operating the
breast
biopsy device 10 are disclosed herein. With reference to Figure 1, in one
aspect of the
present invention, the breast biopsy device 10 is embodied in a handheld
device 12. It should
be noted that the present invention may be embodied in a fixed device (not
shown).
1001531 The handheld device 12 may include a housing 14 (see Figure 10) and
a
handle 16. In one aspect, the housing 14 is removable from the handle 16. The
handle 16 is
reusable. The housing 14 (and all parts contained therein) are disposable and
generally
provided sterile. In one aspect of the present invention, the device 10 may
include an
integrated needle assembly 18 (described below). In another aspect of the
present invention,
the device 10 may include an independent needle assembly 18' (described
below).
1001541 With particular reference to Figures 10, 11 and 12, the housing 14
may include
an inner passage 22 (see Figure 10). A coring cannula 20 is slidably mounted
within the
inner passage 22 of the housing 14. The coring cannula 20 has a longitudinal
axis 24 and
may include a shaft 26 centered on the axis 24.
1001551 In one embodiment, the coring cannula 20 is coupled to the housing
such that
rotational movement of the coring cannula 20 about the axis 24 results in
linear movement of
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
the coring cannula 20 along the axis 24. As discussed more fully below, the
coring cannula
20 has a cutting edge allowing it to cut through tissue as it is rotated and
advanced.
[00156] It
should be noted that in other embodiments, the coring cannula 20 may
simply rotate within the housing 14. Linear movement of the coring cannula 20
(to advance
the device 10 and the coring cannula 20 into the breast) may be provided by
external
mechanical means or by the user.
[00157] The
breast biopsy device 10 includes a stylet 28, which includes a stylet
housing 38. With particular reference to Figure 11, the stylet includes a tip
30. The tip 30
includes at least one blade 32 and a central passage 34. The tip 30 may also
include a slot 31
for the at least one blade 32.
[00158] In one
embodiment of the present invention, the stylet 28 is mounted within
the coring cannula 20. The stylet 28 includes first and second blades 32A, 32B
integrated
between two half portions 38A, 38B of a stylet housing 38. The stylet 28
transects, dilates,
and separates tissue as the device 10 is inserted or advanced towards the
biopsy site.
[00159] A drive
assembly 40 mounted within the housing 14 and the handle 16 (see
Figures 7 and 9) rotates the cannula 20 and controllably rotates the cannula
20. In one
embodiment, the drive assembly 40 also moves the cannula 20 in a direction
parallel to (and
along) the axis 24. In one aspect, the coring cannula 20 has a predetermined
linear
advancement per revolution of the coring cannula 20. In one embodiment, the
predetermined
linear advancement is 0.050 inches per revolution. In an other embodiment, the
predetermined linear advancement is 0.084 inches per revolution.
[00160] The
drive assembly 40 may include a motor assembly comprised of a DC
motor and step down transmission 42 and a drivetrain 44. The DC motor and step
down
transmission 42 is coupled to the drivetrain 44 (Figure 9). The drive assembly
40 and the
drivetrain 44 are explained more fully below.
[00161] In one
aspect of the present invention, the drive assembly 40 rotates the coring
cannula 20 as a single speed, for example, at or around 80 revolutions per
minute.
Alternatively, the drive assembly 40 rotates the coring cannula 20 at a
variable speed (see
below).
1001621 The
needle assembly 18, 18' may include a localization needle 54. The
localization needle 54 has an inner channel 56 and is slidably removable from
the central
passage of the stylet 28. The needle assembly 18, 18' further includes a guide
element 52
16
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
(see Figures 4 and 5A). The guide element 52 is used to secure the tissue
while the coring
cannula 20 is advanced.
1001631 In one
embodiment of the present invention, the guide element 52 has a first
end 58 and a second end 60. The first end 58 of the guide element 52 is
slidably disposed
within the channel 56 of the localization needle 54.
1001641 In one
embodiment, the guide element 52 is composed, at least in part, of a
metal alloy. In one embodiment, the metal alloy is composed of nickel and
titanium. In one
embodiment, the metal alloy is nitinol.
1001651 A
locking member 62 is formed at the second end 60 of the guide element 52.
The locking member 62 has an unlocking configuration and a locking
configuration. The
locking member 62 is in the unlocking configuration when the guide element 52
is in the first
position, i.e., fully contained within the localization needle 54 (see Figure
13B). The locking
member 62 is in the locking configuration when the guide element 52 is in the
second
position, i.e., then the locking member 62 is outside of the localization
needle 54 (see Figure
13C). In the illustrated embodiment, the locking wire 62 is formed of multiple
wires 64, e.g.,
two, which are predisposed toward the locking configuration. When the guide
element 52 is
slid back into the localization needle 54, the inner channel 56 of the
localization needle 54
constrains and confines the wires 64 in the unlocking configuration. Once the
guide element
52 is slid towards and into the second position, the wires 64 are freed from
the constraints on
the localization needle 54 and allowed to move toward and into the locking
configuration.
1001661 In one
aspect of the present invention, the locking configuration is defined by
a predefined shape of the wires 64. In one embodiment, the predefined shape is
a hook
shape.
[00167] In one
embodiment, wires (not shown) may be wrapped around the wires 64 to
provide rigidity to allow the guide element 52 to be moved within the
localization needle 54.
The number of wires 64, as well as the diameter of the wires 64 (and wires
used to provide
rigidity) is optimized to provide maximize holding and as a function of the
type of targeted
tissue, e.g., hard or soft tissue.
[00168] With
reference to Figures 14 and 15, in another embodiment of the present
invention, the locking member 62 may include a twisted pair of wires 66. The
guide element
52 is shown in the first position in Figure 14 with the twisted pair of wires
66 in the
17
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
unlocking configuration. The guide element 52 is shown in the second position
in Figure 15
with the twisted pair of wires 66 in the locking configuration.
[00169] With
reference to Figures 16A, 16B, 16C, and 16D, in another embodiment of
the present invention, the locking member 62 is formed from braided wire or
cable 68. As
shown, in one embodiment, the distal ends of the cables may be straightened
and then formed
into a predetermined shape, such as a hook shape. The number of wires or
cables may vary,
e.g., the braided wire or cable may include 4, 7 or any number of individual
wires or cables.
The guide element 52 is shown in the first position in Figure 16C with the
braided cable 68 in
the unlocking configuration. The guide element 52 is shown in the second
position in Figure
16D with the braided cable 68 in the locking configuration.
[00170] With
reference to Figures 17 and 18, in another embodiment, the guide
element 52 may include a pushrod 70 and at least two flexible fingers 72A,
72B. In one
embodiment, the pushrod 70 and flexible fingers 72A, 72B are unitarily formed
(Figure 17).
In another embodiment, the flexible fingers 72A, 72B are affixed to the
pushrod 70 (Figure
18).
1001711 In one
aspect, the flexible fingers 72A, 72B may be predisposed towards the
locking configuration through a heat treat process.
[00172]
Returning to Figures 7 and 9, in one embodiment of the present invention, the
drive assembly 40 may include a variable speed circuit 74 electrically coupled
to the motor
assembly 42, 44. A forward/reverse switch 76 is electrically coupled to the
variable speed
circuit 74. A speed control trigger 78 is electrically coupled to the variable
speed circuit 74.
The forward/reverse switch 76 controls the direction of the DC motor 42, and
thus, the
direction of movement (forward/reverse) of the cannula 20 along the axis 24.
[00173] The
variable speed circuit 74 controls the speed and rotation of the cannula 20
as a function of the forward/reverse switch 76 and actuation of the speed
control trigger 78.
In one aspect, the variable speed circuit 74 has a predetermined speed range,
for example 0-
100 revolutions per minute.
[00174] The DC
motor and transmission 42 is powered by a rechargeable battery 46,
which may be charged via an external power source (not shown) through
recharging port 48.
In one embodiment, the rechargeable battery 46 is a lithium ion battery.
18
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[001751 The DC
motor and transmission 42 is used to provide low speed and high
torque to the drivetrain 44. A drive gear 50 is directly coupled between the
motor 42 and the
drivetrain 44.
[00176] With
specific reference to Figures 7, 8, and 9, the drivetrain 44 may include a
spline gear 80, a spline gear support 82, a lead screw 84, a shaft 86, and a
ring gear
transmission 88. The spline gear 80 is contained with the housing 14 and is
supported by the
spline gear support 82. The drive gear 50 engages the spline gear 82 to
transfer power to the
drivetrain 44, and thus, the coring cannula 20.
[00177] The
speed of the DC motor 42 is controlled by user actuation of the speed
control trigger 78. The variable speed circuit 74 enables variable speed ramp
up and slow
down. In one embodiment, a speed range of approximately 0 -100 rpm at the
cannula may be
provided.
[00178] The
drivetrain 44 is contained within the housing 14, which is removable
coupled to the handle 16. When the device 10 is assembled, the spline gear 80
engages the
drive gear 50 within the handle 16. Power transferred through the drive gear
50 causes
rotation of the spline gear 80. The spline gear 80 is attached to the spline
gear support 82. The
spline gear support 82 is keyed to the shaft 86. The spline gear support key
82 provides
rotation to the shaft 86 while allowing it to move axially (along axis 24).
The lead screw 84,
which is fixed to the housing 14, is engaged with threads at the proximal end
of the shaft 86.
The coring cannula 20 is attached to the shaft 86. As the shaft 86 is rotated,
the threaded
engagement with the lead screw 84 creates axial movement of the coring cannula
20.
[00179] As the
cannula 20 rotates, it continues to move forward for a distance
detennined by the thread length on the lead screw 84. As the shaft 86 reaches
the end of the
threads, it will continue to rotate, but will no longer move forward. The
timing is designed
such that when the shaft 86 reaches the end of the threaded section of the
lead screw 84, the
transmission lockout (lock out button 90) engages. With the lock out button 90
engaged, the
ring gear assembly 88 is activated and begins to advance a drive dog 92
forward along the
drive screw 50.
[00180] The
drive dog 92 is coupled to a severing mechanism 94 which is used to
sever the tissue contained within the coring cannula 20, which is described
more fully below.
[00181] The
general process of utilization of the device will now be described. First,
the localization needle 54 is advanced into the breast under ultrasound
guidance. In one
19
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
aspect of the present invention, this is performed manually. For instance,
with the handheld
device 10, the user manually inserts the needle 54 by positioning and manually
moving the
device 10. When the needle 54 reaches the target area, the tissue anchor or
locking member
62 is advanced to secure the tissue prior to advancement of the device 10.
Next, the
localization needle 54 is released allowing the device 10 to move
independently of the needle
54 The device 10 is now advanced into the breast, with the stylet blades 32
separating the
tissue up to the target area. When the device 10 has reached the target area,
the coring
cannula 20 is advanced. The cannula 20 is advanced by depressing the speed
control trigger
78 on the handle 16, with the forward/reverse switch 76 in a forward position.
When the
cannula 20 reaches its full core length, the severing mechanism 94 is
actuated, separating the
tissue core from surrounding tissue. In one embodiment, the severing mechanism
94 may
include a flexible blade (see below) will automatically advance from the
distal end of the
coring cannula 20. After the core of tissue has been cut free, the device 10
is removed from
the breast. With the device out of the breast, the forward/reverse switch 76
is placed in a
reverse position and the flexible blade is retracted using the speed control
trigger 78 allowing
the tissue sample to be retrieved from the coring cannula.
[001821 As
stated above, in one aspect of the present invention, an integrated needle
assembly 18, as shown in Figure 1 and demonstrated in Figures 2A, 2B, 2C, and
2D, may be
provided. With the integrated needle assembly 18, the needle assembly 18 and
the coring
cannula are integrated into a single unit 18, 20 (see Figure 2A). With the
integrated needle
assembly 18, the needle assembly 18 is inserted within the central passage 34
of the stylet
housing 38 when the localization needle 54 is inserted into the breast (Figure
2B). Once the
localization needle 54 reaches the target tissue, a locking member actuation
button 96,
located on the top of the housing 14 is slid forward. The actuation button 96
is linked to the
locking member 62 resulting in the locking member 62 being slid out of the
localization
needle 54 securing the target tissue.
1001831 Once the
target tissue is secured, the localization needle 54 and locking
member 82 are released from the housing 14 by actuation of one of the
localization needle
release button(s) 98 located thereon. This allows the device 10 to be slid up
localization
needle 54 (the stylet blades 32 separating the tissue allowing the stylet 28
and coring cannula
20 to pass. Once the coring cannula 20 is adjacent the target tissue, the
process proceeds as
above.
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
1001841 Then,
the device 10 would be fed down the guide rod 104 and the process
would proceed as above (see Figure 5A).
1001851 With
respect Figure 5B, the central passage 34 is formed by the stylet tube 36.
The stylet tube 36 includes an opening 106 which allows the needle assembly
18, 18B' to
pass into the central passage 34. The stylet tube 36 also may include a guide
portion 108
which extends past the opening 106 to assist in the placement of the guide rod
into the central
passage 34.
[00186] In
another aspect of the present invention, an independent needle assembly 18'
may be provided (see Figures 3, 4A, 4B, 5A, 5B). The independent needle
assembly 18' is
separate from the coring cannula 20. The independent needle assembly 18'
includes a
localization needle 54', an independent needle handle 100, and a plunger 102.
The
localization needle 54' is inserted into the breast tissue using the handle
100. Once the target
tissue is reached, the plunger 102 is pushed forward. The locking member 62 is
pushed
forward by the plunger 102, pushing the wires 64 into the target tissue,
thereby securing the
target tissue. The localization needle 54' (and handle 100), may thereafter be
removed,
leaving the locking member 62 within the breast with a guide rod 104 extending
out of the
breast (see Figure 4B).
1001871 The
localization needle 54' has a first end 54A' and a second end 54'. The
localization needle includes an internal channel or bore 56. The handle 100
has first and
second ends 100A, 100B and an internal bore 268. The first end 54A of the
needle 54' is
fixed to the second end of the handle 100B. The internal bore 56 of the needle
54' and the
internal bore 268 of the handle 100 form an assembly bore 270 therethrough. In
the
illustrated embodiment, the guide element 52 has a guide rod 104 and a locking
member 62.
The guide element 104 has first and second ends 58, 60 and is removably
contained within
the assembly bore 270. The locking member 62 is fixed to the second end 60 of
the guide rod
104. The plunger 102 includes a pushrod 102B and an actuation element 102A
coupled to
the pushrod 102B. The plunger 102A is movable from a first state (Figure 4A)
to a second
state (Figure 4B) One end of the pushrod 102B acts on the first end 58 of the
guide rod 104,
forcing the locking member 62 out of the needle 18 as the pushrod 102 is moved
from the
first state to the second state.
1001881 With
reference to Figures 19A-19D, in one embodiment one of the wires 64'
from the locking member 62 is detachable from the pushrod 70. It should be
noted that
21
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
although Figures 19A-19D illustrated this feature with respect to the
independent needle
assembly 18, the detachable wire 64' concept may also be used with the
integrated needle
assembly 18'.
1001891 As shown
in Figure 19A, the locking member 62 is contained within the
localization needle 54 when the localization needle is initially inserted into
the breast tissue.
When the localization needle 54 reaches the target tissue, the locking member
62 is deployed
as discussed above (Figure 19B) to secure the target tissue. Then the cannula
20 is advanced
over the target tissue and severed using the severing mechanism 94 (see
above). Once the
device 10 has been removed from the breast, the localization needle 18' may be
used to push
the severed tissue from the cannula 20. The tissue anchors or wires 64 may
then be retracted.
The third hook 64' may either not be attached to the pushrod 70 or may be
detachable
therefore. The third hook or wire 64' remains attached or secured to the
tissue to provide an
orientation marker for the sample during pathology (see Figure 19D).
1001901 In
another aspect of the present invention, the guide rod 104 may include scale
markings 110 to provide an indication to the user the depth of the
anchor/guide element 52
µ\ 'thin the breast, as shown in Figures 13A and 19B.
1001911 In
another aspect of the present invention, the biopsy device 12 includes a
flexible transection blade 112. The flexible transection blade 112 is a flat
metal blade with
one end sharpened is formatted to the required radius (see below). The blade
thickness and
material properties as such that the formed flexible transection blade 112 can
be flattened out
will "spring" back to its formed shape. The blade 112 will be held in a flat
position along the
side of the coring cannula.
[001921 The
coring cannula 20 will use an angled or non-continuous cutting ring (see
below) at the completion of the coring process. As shown in Figures 20A and
20B, the
coring cannula 20 is movable along the axis 24 from an initial cannula
location 118 (shown in
dotted lines) to a final cannula location 120 in response to rotation of the
coring cannula 20
about the axis 24 in a first direction.
1001931 In one
embodiment, once the coring cannula 20 reaches the final cannula
location 120, it will continue to rotate but will not advance axially forward.
A mechanism
122 will be engaged to drive the flexible transection blade 112 forward. The
flexible
transection blade 112 exits the coring cannula 20 at a point slightly distal
to the cutting edge
of the coring cannula 20 (see Figures 21A and 21B). As the flexible blade 112
is driven out
22
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
of the cannula 20, it will begin to return to its pre-formed curvature. Since
this advancement
is taking place while the coring cannula 20 is rotating, the result will be a
curved, complete
cut through the tissue. The path of the blade 112 is designed to intersect
with the distal end
of the cutting path from the coring cannula 20, resulting in complete
transection and release
of the tissue specimen.
1001941 With
reference to Figures 23A, 23B, and 23C, in one embodiment the flexible
transection blade 112 consists of a thin strip 124 of spring steel or nitinol.
The flexible
transection blade 112 has a first end 126 and a second or distal end 128. The
flexible
transection blade 112 is coupled to the coring cannula 20 at the first end
126. The distal end
128 of the flexible transection blade 112 is cut to an optimized angle and
sharpened to a
cutting edge 114 (see Figure 23C). A hole mount 130 may be provided for
mounting the
blade 112 to the drive assembly 40.
1001951 In one
embodiment as shown in Figures 8 and 9, the flexible transection blade
112 is stored in a channel 116 built into the coring cannula 20. The flexible
transection blade
112 is held flat in this stored position. At the completion of the coring
process, the coring
cannula 20 will cease axial advancement, but will continue to rotate. During
this rotation the
flexible blade 112 is driven forward, advancing past the coring cannula 20. As
the flexible
blade 112 advances, it will assume its pre-formed, curved position. Rotation
causes the
flexible blade 112 to create a semi-circular cut in the tissue. When the
flexible transection
blade 112 advances past the center of rotation, a complete cut results,
releasing the tissue
core. The curved blade 112 holds the tissue core inside the cannula 20 until
removed from
the breast.
100196) The
flexible transection blade 112 has a first blade position and a second blade
position. The flexible transection blade 112 is in the first blade position
while the coring
cannula 20 is between the initial and final cannula locations 118, 120. As
shown in Figure
20A, in one embodiment, when the flexible transection blade 112 is in the
first blade position
it is contained within the coring cannula 20, and thus, not visible. Rotation
of the coring
cannula 20 in the first direction while the coring cannula 20 is at the final
cannula location
120 rotates the flexible transection blade 112 about the axis 24, moving the
flexible
transection blade 112 from the first blade position to the second blade
position (shown in
Figure 22B).
23
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
1001971 Testing
has revealed a few key elements of the invention. First, the most
efficient cutting of tissue is accomplished by creating relative motion
between cutting
surface, i.e., the cutting edge 114, and tissue. Second, the relationship
between the cutting
edge 114 and the rate of advancement of the length and angle of the cutting
edge 114 must
result in a cutting surface that is greater in length than the linear
advancement per revolution.
Further, the rate of advancement per revolution should be optimized to
minimize cutting
forces. This approach will ensure that a thin, flexible blade 112 will follow
the desired
cutting path.
1001981 With
particular reference to Figures 24, 25A, and 25B, in one embodiment the
mechanism 122 may include a friction wheel transmission 132. The friction
wheel
transmission 132 includes friction wheel 134 which is force fit over the shaft
26. The shaft
26 is directly coupled to the cannula 20 through a drive ring 20 which is
fixed to the housing
14. A drive screw 136 is fixed to the friction wheel 134, which is coupled to
the flexible
transection blade 112. As the shaft 26 is advanced by the drive assembly 40,
the friction
wheel 134, and thus the flexible transection blade 116 is also advanced. The
friction wheel
134 is force fit over the shaft 26 such that the transmission force may be
controlled. The
relationship between the friction wheel 134 and the drive ring 136 and/or the
relationship
between the friction wheel 134 and the shaft 26 can be adjusted so that if the
force
encountered by the flexible transection blade 112 increases to a certain
point, the friction
wheel 134 will slip on the shaft 26 preventing further advancement of the
blade 112. The
blade 112 will continue to rotate until the sample tissue has been cut and the
forces reduced.
Blade advancement will then automatically resume.
1001991 With
particular reference to Figures 26, 27, and 28, in another embodiment the
mechanism 122 may include a gear drive transmission 140. The gear drive
transmission 140
provides continuous drive with maximum power transfer. In one aspect of the
present
invention, the gears within the gear drive transmission 140 remain meshed but
do not rotate
during axial advancement of the coring cannula 20. When advancement of the
coring
cannula 20 is complete, the gear drive transmission 140 automatically engages
and begins to
drive the flexible transection blade 112.
1002001 The gear
drive transmission 140 may include a gear housing 146, a ring gear
150, and a drive gear 152. A plunger 144 is slidably coupled to the gear
housing 146 and is
spring biased in an outward direction. While the coring cannula 20 is between
the initial
24
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
cannula location 118 and the final cannula location 120, plunger 144 is
pressed inwardly by
the inner wall of the housing 20 such that one end is inserted a receiving
slot 148 on the shaft
26. Thus, the gear housing 146 is locked relative to the shaft 26. The shaft
gear housing 146
thereby rotates with the shaft 26, and there is no relative motion between the
gears 140, 152.
When the gear housing 146 reaches a release slot 154 in the housing 14, the
spring biased
plunger 144 slides into the release slot 154, thereby releasing the shaft 26,
the ring gear 150 is
fixed relative to the housing 14 and the drive gear 152 rotates with the shaft
26, thereby
driving the flexible transection blade 112 forward.
1002011 With
particular reference to Figure 29, in still another embodiment a lead
screw 156 is used to enable the shaft 26 to advance and rotate, stop advancing
but continue
rotating and then retract to its original position. The shaft 24 includes an
opening 158 leading
to a shaft threaded section 160. The lead screw 156 is rotatably fixed to the
housing 14 and
includes a first end portion 162, a second end portion 164, and a lead screw
threaded section
166, which meshes with the shaft threaded section 160.
1002021 The
shaft 26 is driven forward (to the right in Figure 29) until the back edge of
shaft 174 reaches the front edge of lead screw threads 178. At this position,
the shaft 26 will
continue to rotate but no longer advances. The shaft threaded portion 160 is
supported by
shoulder 180. A first spring 168 makes contact with surface 172 exerting a
slight backward
force on the shaft 26.
1002031 When the
drive assembly 40 is reversed, the force exerted on surface 172 by
the first spring 168 urges re-start of threads between the shaft 26 and the
lead screw 156. The
shaft 26 will then move backward until the contact surface 176 clears the
surface 172. A
second spring 170 now provides force to urge restart in the forward direction.
1002041 This
configuration may also be adopted to drive the flexible transection blade
112.
1002051 With
particular reference to Figure 30 in still another embodiment, a drive
screw 184 may be used to drive motion of the flexible transection blade 112. A
drive gear
182 is fixed to the drive screw 184 which is threadably coupled to the drive
dog 186. During
forward motion of the coring cannula 20, the drive dog 186 is allowed to slip
relative to the
drive screw 184. During activation of the flexible transection blade 112, the
drive gear 182,
and thus, the drive screw 184 rotate. The drive dog 186 has an internal
threaded bore (not
shown) which is mated with the drive screw 184. As the drive screw 184
rotates, the drive
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
dog 186 advances (or retracts) along the screw 184, thereby advancing the
flexible
transection blade 112.
[00206] With
particular reference to Figure 31, in a further embodiment, a
modification is shown. In the illustrated embodiment, the drive dog 186' is
fixed to an end of
the drive screw 184'. The drive gear 182' has in internal threaded bore (not
shown) which is
mated with the drive screw 184'. As the drive gear 182' rotates, the drive
screw 184' and the
drive dog 186' advances or retracts.
[002071 As
discussed more fully below, the cutting edge 114 of the cutting cannula 20
may be formed by a cannula insert 188 and may have different configurations.
[00208] With
particular reference to Figures 32A and 32B, the cannula insert 188
forms a circular coring blade 190. As shown the flexible transection blade 112
advances past
the circular coring blade 190. The flexible blade 112 transects tissue distal
to the front edge
of cutting ring 190.
[00209] With
particular reference to Figures 33A and 33B, in another embodiment the
cannula insert 188 forms a partial cutting ring 192. The partial cutting ring
192 forms a
partial cutting face 194 with an angled edge 196. As the coring cannula 20
rotates, the angled
edge 196 cuts through the tissue. As shown, with the partial cutting ring 192,
the flexible
transection blade 112 does not extend past the furthermost edge of the partial
cutting rung
192. Thus, the tissue sample is confined within the cutting ring 192.
[00210] With
particular reference to Figures 34A and 34B, in still another
embodiment, the cutting edge 114 of the flexible transection blade 112 is used
to core the
sample tissue (Figure 34A). The flexible transection blade 112 is then
advanced to transect
tissue (Figure 34B).
[00211] With
particular reference to Figure 35, a prior art cutting ring 200 is shown.
The prior art cutting ring 200 is nestled within a bore 202 of the distal end
of the cutting
cannula 20. As shown, the coring cannula 20 has an inner diameter of dl and
the cutting ring
200 has an inner diameter of d2. In the
prior art device shown in Figure 35, di is
substantially equally to d2. The outer surface of the coring cannula 205 has a
ramping surface
206 from the outer dimension of the coring cannula 205 to the distal end of
the coring
cannula 205. As shown, the outer diameter of the coring cannula d4 is greater
than the outer
dimension, d3, of the prior art cutting ring 200.
26
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
1002121 In the
prior art cutting ring 200 of Figure 35, the mechanism for transecting
the tissue sample is a garrote wire 204 which transverses the outer wall of
the coring cannula
20. At a
location near the distal end of the coring cannula 205 the garrote wire 204
forms a
right angle and encircles the inner diameter of the coring cannula 20. As
shown, this occurs
at a substantial distance, d5, from the distal end of the cutting ring 200.
This arrangement
presents two problems. First, the 90 degree bend in the garrote wire 204
significantly
increases the force required to pull the garrote wire and transect the tissue.
Second, the large
distance, d5. between the garrote wire 204 and the cutting edge of the cutting
ring 200,
results in a core of tissue, or tissue plug, which is cored by the coring
cannula 205, but not
transected by the garrote wire. This cored tissue thus remains in the breast.
1002131 With
particular reference to Figures 36A and 36B, in one embodiment a
partial cutting ring 208 may be provided. The illustrated partial cutting ring
208 may include
a face cutting surface 208A, which has a cutting edge perpendicular to the
axis 24, and a side
cutting surface 208B. The use of the side cutting surface 208B introduces side
cutting. Side
cutting is less likely to result in unwanted pushing or movement of tissue.
Additionally, the
blade angle allows the garrote wire 210 to be installed outside of the coring
blade and then
clear of the cutting ring when retracting. This also limits the tissue plug
problem identified
above. Furthermore, the angle in the garrote wire 210 may be increased (as
shown), reducing
the required transection forces (see Figures 406A, 406B, 406C).
1002141 With
reference to Figures 38A, 38B, and 39 in an other aspect of the present
invention, the coring cannula 20 may include an external cutting ring 198. The
external
cutting ring 198 has an interior bore 212 within an interior diameter, d2. The
coring cannula
20 has a reduced diameter portion 214 at its distal end. As shown, the
external cutting ring
198 is fitted over the reduced diameter portion of the coring cannula 214. As
shown, the
outer diameter (d4) of the coring cannula 20 is substantially equal to the
outer diameter of the
external cutting ring, d3.
1002151 As shown, the transecting mechanism 122 may include a garrote wire
210.
1002161 With
specific reference to Figures 38A and 38B, in one embodiment the
garrote wire 210 is removably coupled to the coring cannula 20 by one or more
bent tabs 216
formed integrally with the coring cannula 20. The one or more bent tabs 216
may be
integrally formed with the coring cannula 20. The mechanism 122 is located at
the distal end
of the coring cannula 20. The distal end of the coring cannula 20 is within a
minimal
27
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
distance of the distal end of the external cutting ring 198. This minimizes
the tissue plug
problem discussed above. In one embodiment, the minimal distance is < 0.25
inches.
1002171 With
specific reference to Figure 39, the distal end of the external cutting ring
198 is spaced from the distal end of the coring cannula 20. In the illustrated
embodiment, the
mechanism 122 is located at the distal end of the external cutting ring 198.
The mechanism
122 is within the minimal distance of the distal end of the external cutting
ring 198. The
garrote wire may be removably held in place by one or more tabs 218 which may
be formed
integrally with the cutting ring 198.
100218J With
particular reference to Figure 41, in one aspect of the present invention,
the coring cannula 20 may be provided with a cutting ring in which the inner
diameter of the
coring cannula 20 has an inner diameter which is smaller than the inner
diameter of the
cutting ring. Tissue is flexible, malleable and compressible. With the inner
diameter of the
coring cannula 20 being smaller than the inner diameter of the cutting ring,
the tissue sample
is compressed as it enters the coring cannula 20 (behind the cutting ring).
Compression of
the tissue sample results in better retention of the tissue sample in the
cannula 20.
Additionally, with the reduced inner diameter of the cannula 20, the outer
diameter of the
cannula 20 may also be reduced, until it is the equal to or nearly equal to
the outer diameter
of the cutting ring. This results in (1) a smaller enti),, incision and (2)
reduction of the
required coring cutting force.
1002191 With
particular reference to Figure 40, a prior art cannula 222 is shown. The
prior art cannula 222 has a cutting ring 224. The prior art coring cannula 222
has an inner
diameter (dl) which is equal to the inner diameter (d2) of the cutting ring
224. Additionally,
the outer diameter (d4) of the prior art coring cannula 222 is greater than
the outer diameter
(d3) of the cutting ring 224.
1002201 With
particular reference to Figure 41, a coring cannula 226 according to an
embodiment of the present invention is shown. The coring cannula 226 has a
distal end 228
and is centered on the axis 24 and is coupled to the housing 14. The coring
cannula 226
having an inner surface 230 forming a cannula bore 232. The cannula bore 232
has an inner
diameter (dl) and is rotatable about the axis. A cutting ring 234 has an inner
surface 236
which forms a cutting ring bore 238 and is located at the distal end of the
coring cannula 226.
The cutting ring bore 238 has an interior diameter (d2). A tapered wall 240 is
coupled
between the coring cannula 226 and the cutting ring 234. The tapered wall 240
provides a
28
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
ramped surface between the inner surface 236 of the cutting ring 234 and the
inner surface
236 of the coring cannula 226.
[00221] As
discussed, the inner diameter, dl. of the cannula bore 232 is less than the
inner diameter, d2, of the cutting ring 234. Furthermore, the outer diameter,
d4, of the coring
cannula 226 is equal to, or only slightly larger than, the outer diameter, d3,
of the cutting ring
234.
1002221 In one
embodiment, the coring cannula 226 and the cutting ring 234 are
unitarily formed. In an other embodiment, the coring cannula 226 and the
cutting ring 234 are
formed separately. In one embodiment (as described above), the coring cannula
226 may
have a reduced diameter portion formed at the distal end 228. The cutting ring
234 is an
external cutting ring which is fitted over the reduced diameter portion of the
coring cannula.
The distal end of the cutting ring 234 forms a coring cannula cutting edge
238.
1002231 With
reference to Figures 42A, 42B, 43A, 43B, 43C, in another aspect of the
present invention a collapsible stylet may be provided (see below). With
particular reference
to Figures 42A and 42B, a prior art stylet 242 is shown. The prior art stylet
242 is contained
within the coring cannula 244. The prior art stylet 242 has a slot 243 for the
stylet blades
246. The diameter, di, of the prior art stylet 242 is fixed, and smaller than
the diameter, d2,
of the cutting edge 248 of the coring cannula 244. Thus, in use, after the
stylet 242 has cut
into the tissue, the skin may need to be opened further to allow the coring
cannula 244 to
enter the tissue.
1002241 With
particular reference to Figures 43A, 43B, and 43C, a collapsible stylet
250 according to one embodiment of the present invention is illustrated. The
coring cannula
20 has a distal end 252, a longitudinal axis 24 and is centered on the axis 24
(see above). The
collapsible stylet 250 has a tip 254, which contains at least one blade 256,
and a central
passage 258 and is coupled to the coring cannula 20. The tip 254 has a recess
260 located
near a proximal end 262 thereof. The tip 254 is movable between an initial
configuration
(shown in Figures 43A and 43B) and a contracted configuration (shown in
Figures 43C and
43D). When the tip 254 is in the initial configuration, the cutting edge 264
of the coring
cannula 20 is within the recess 260. This allows the coring cannula 20 to
enter the incision
with the stylet 250, prior to the coring process, without the need to widen or
open the incision
any further. The tip 250 remains in the initial configuration as the coring
cannula 20 is
moved from the initial cannula location towards the final cannula location.
Once the coring
29
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
cannula 20 reaches the final cannula location, the tip may be moved into the
contracted
configuration (Figure 43C). In the contract configuration, the cutting edge
264 of the coring
cannula 20 is exposed when the tip 254 is in the contracted configuration.
1002251 In the
illustrated embodiment, the tip 254 has a first half portion 254A and a
second half portion 254B. As shown in the illustrated embodiment, the first
and second half
portions 254A, 254B have a semi-circular cross-section (see Figures 43B and
43C) and an
inner surface 268A, 268B. The inner surface 266A of the first portion 254A
faces the inner
surface 266B of the second portion 254B. The first and second half portions
254A, 254B
have a first part 268A, 268B and a second part 270A, 270B. The second parts
270A, 270B
are sloped and curved forming an entry segment 272. The first and second parts
268A, 268B
form a linear segment 274. The linear segment 274 has an associated first
diameter, (dl),
when the tip is in the initial configuration (Figure 43B). The first diameter
associated with
the linear segment 274 is greater than or equal to a diameter associated with
the cutting edge
248 of the coring cannula 20. Thus, the cutting edge 248 can sit within the
recess 260 prior
to the coring process (see above).
100226) As shown
in Figure 43C, when the tip 254 is in the contracted configuration
the linear segment 274 has a second diameter, (d2). The second diameter is
less than
diameter associated with the cutting edge 248 of the coring cannula 20. This
allows the
coring cannula 20 to be rotated and moved forward (over the stylet) to perform
the coring
process.
1002271 In one
embodiment, the first and second half portions 254A, 254B are biased
towards the initial configuration. In the illustrated embodiment, the
collapsible stylet 250
includes a collet tube 251 and a collet closer 253. As shown, the tube 251
includes a ramping
portion 251A and a distal end 251B. The distal end 251B is fitted between the
first and
second half portions 254A, 254B and bias the first and second half portions
254A, 254B into
the initial configuration. A collet closer 253 is provided which is movable
between a first
position (shown in Figure 43A) and a second position (shown in Figure 43C).
The collet
closer 253 acts on the ramping portion 251A of the collet tube 251 to compress
the distal end
251B. This allows the first and second half portions 254A, 254B of the
collapsible stylet 250
to collapse to the contract position. The collet closer 253 may be movable
from the first
position to the second position by the user through actuation of a button
provided on the
housing 14 (not shown).
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[00228] With
specific reference to Figures 44 and 45, in another aspect of the present
invention, an independent stylet mechanism 276 is provided. With particular
reference to
Figure 44, a prior art stylet 278 is shown. The prior art stylet 278 includes
integral cutting
blades 280. Since the integral cutting blades 280 are fixed relating to the
stylet tip, the
distance between the blades 280 and the stylet 278 is fixed at a minimal
distance. This
increases the chances of inadvertent movement or compression of the tissue,
i.e.,
"snowplowing".
[00229] With
specific reference to Figure 45, the independent/retractable stylet
mechanism 276 includes a tube 282 and at least one stylet blade 284 affixed to
the tube 282.
A stylet 286 includes a stylet tip 288 with a central passage 290. The tube
282 is slidably
disposed within the central passage 290 of the stylet 286.
1002301 In the
illustrated embodiment, the stylet mechanism 276 includes first and
second blades 284A, 284B.
[00231] In one
aspect, the tube 282 may include an internal bore 292 for receiving the
guide element 52 (see above).
[00232] The
independent/retractable stylet mechanism 276 is adjustable within/along
the central passage 290 of the stylet 286. Thus, the user can adjust the
distance between the
blades 284 and the stylet tip 288 to reduce the chance of snowplowing
occurring.
[00233] With
reference to Figures 46A-46D and 47A-47C, in another aspect of the
present invention, a localization needle with an integral locking member 294
is provided. In
one embodiment, the localization needle 294 includes a needle portion 296 and
a locking
member 304. The needle portion 296 having a proximal end 298, a distal end 300
and a
channel 302 formed therein.
1002341 The
locking member 304 is formed integrally with the needle portion 296. As
shown, the locking member 304 may be formed at the distal end 300 of the
needle portion
296 and has an unlocking configuration (shown in Figure 46A) and a locking
configuration
(shown in Figure 46B).
[00235] In the
illustrated embodiment, in the unlocking configuration, the localization
needle 294 is straight, i.e., without bends or kinks. In the locking
configuration, bends, or
barbs, as shown in Figure 46B have been introduced into the localization
needle 294. These
bends, barbs, are introduced into the localization needle 294 after the
localization needle 294
31
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
has been inserted into the breast, thereby locking the localization needle 294
relative to the
target tissue (see above).
[00236] In one
embodiment, the localization needle 294 includes an actuation device
306. The actuation device 306 is coupled to the distal end 300 and is used to
apply a force
thereto (see Figure 46B). The force acts to bring the distal end 300 closer to
the proximal end
298. With the proximal end 298 fixed to, for example, the housing 14 of the
biopsy device
10, the localization needle 294 collapses at the locking member 304 creating
the barbs, or
extensions, as shown, thereby controllably moving the locking member from the
unlocking
configuration to the locking configuration.
[00237] In one
aspect of the present invention, the actuation device 306 includes a
member 308 coupled to an inner surface of the distal end 300 of the needle
portion 296.
[00238] With
particular reference to Figure 46C, in one embodiment the member 308
may include a wire 310 fixed to the inner diameter of the localization needle
294. The wire
310 may be attached to a lever (not shown) on the housing, or some other
suitable
mechanism, which pulls the wire 310 back toward the proximal end 298.
[00239] In
another embodiment, the member 308 is a threaded rod 312 which is
received by a threaded receiving member 314 which is coupled to the inner
surface of the
distal end 300 of the needle portion 296. This arrangement allows the
localization needle 294
to be moved back into the unlocking configuration if the placement needs to be
corrected.
[00240] In
another aspect of the present invention, the locking member 304 is formed
by at least one pair of opposed slots 316 within the needle portion 296. In
one embodiment,
the slots 316 may be laser cut from the needle portion 296. As shown in
Figures 46A-46D, in
one embodiment, the slots 316 may have a general rectangular shape with
rounded ends. The
slots 316 may include one or may directional cutouts 317 which assist in
forming the
extensions or barbs. The directional cutouts 317 may be triangular shaped.
[00241] In
another aspect of the present invention the slots 316 may have a general
diamond shape, as shown in Figures 47A-47C.
[00242] With
particular reference to Figures 48A, 48B, and 49, in another aspect of the
present invention, one or more rotating circular blades 318, 318A, 318B may be
used. The
use of the rotating circular blades 318, 318A, 318B improves the efficiency of
the stylet and
reduces the risk of compression and/or tearing of the tissue as the stylet in
pushed into the
breast.
32
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[00243] The
rotating circular blade(s) 318, 318A, 318B may be powered (see below)
or may rotating freely. The rotation of the blade (s) 318, 318A, 318B whether
from an
external source or as a result of friction between the blade 318, 318A, 318B
and the tissue,
creates relative motion therebetween.
[00244] With
particular reference to Figure 48A, in one embodiment a stylet 320 is
provided with a single rotating circular blade 318. The stylet 320 is coupled
to a coring
cannula 322. The coring cannula 322 has a longitudinal axis 324 and is
centered on the axis
324. The stylet 320 is coupled to the coring cannula 322. The stylet 320
includes a stylet
tube 326. The rotating circular blade 318 is rotatably coupled to a distal end
328 of the stylet
tube 326.
1002451 With
particular reference to Figure 48B, in another embodiment, the rotating
circular blade 318 is not mechanically driven, but is allowed to freely
rotate. As the device
is advanced into the tissue, force exerted by the tissue will tend to rotate
the circular blade
318, eliminating the tendency to push/tear tissue and improving cutting
efficiency.
1002461 In both
embodiments, the singular rotating circular blade 318 is mounted on
its center point 334. As shown, the center point 334 is centered over the
stylet tube 326.
[00247] The
rotating circular blade 318 defines a first plane which is parallel to the
axis 324. The axis defines a second plane. The first and second planes
intersect at a right
angle. The center point 334 of the rotating circular blade 318 is located on
both the first and
second planes.
[00248] A blade
drive mechanism 330 is coupled to the rotating circular blade 318 for
controllably rotating the circular blade 318. In one embodiment, the blade
drive mechanism
330 may include a motor (not shown) and drive cable 332. Alternatively, the
blade drive
mechanism 330 may include a rod and gearing system (not shown).
[00249] With
particular reference to Figure 49, the stylet 320 may include a pair of
offset blades 318A, 318B. The second blade 318B defines a third plane which is
parallel to
the first plane. As shown in Figure 39, the center 334A, 334B of the blades
318A, 318B are
offset a predetermined distance. The first and second blades 318A, 318B may be
mechanically driven or may be allowed to rotate freely.
[00250]
Returning to Figures, 2A-2D, 5A and 5B, in another aspect of the present
invention the biopsy device 10 includes at least one retractable stylet blade
336. The at least
33
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
one retractable stylet blade 336 is part of a stylet blade mechanism 338. The
stylet blade
mechanism 338 may include first and second retractable blades 336A, 336B, as
shown.
1002511 The
stylet blade mechanism 338 is coupled to the coring cannula 20 via the
stylet tip 30. In one embodiment. the stylet blade mechanism 338 includes the
stylet tube 36.
The at least one retractable stylet blade 336 is fixed to the stylet tube 36.
The stylet tube 36 is
slidably disposed within the style( housing 38. The central passage 34 is
formed by the stylet
tube 36.
1002521 The
stylet blade mechanism 338 is movable between a cutting position and a
retracted position. In the cutting position, the at least one stylet blade is
located a distance in
front of the style( tip 30 (as shown). In the retracted position, the at least
one stylet blade 336
is located within the stylet tip 30.
1002531 In one
embodiment, the stylet blade mechanism 338 may be manually moved
from the retracted position to the cutting position. In one embodiment, the
stylet blade
mechanism 338 is spring biased towards the cutting position.
1002541 As
discussed above, the biopsy device 10 may further comprise a guide
portion 108 formed at the end of the stylet tube 30. The guide portion 108
extends past an
opening of the stylet tube 30. The guide portion 108 having an interior curved
surface 340.
The interior curved surface 340 assists in guiding the end of the guide
element 52 into the
central passageway 108.
1002551 It
should be noted that the stylet blade mechanism 338 and the retractable
stylet blades 336 may be used with either integrated localization needle or
the independent
needle (see above). With respect to Figures 5A and 5B, the stylet blade
mechanism 338 is
used with the independent needle handle assembly 18'. As discussed above, the
independent
needle handle assembly 18' is inserted into the breast, the guide element 52
is extended
outside of the needle 54 and the locking member 62 is affixed to the target
tissue. Once the
locking member 62 is locked into the target tissue, the guide element 52 is
removed from the
needle 54. The guide element 52 is then inserted into central passageway 108.
1002561 With the
guide element 52 within the central passageway 108 and the stylet
blade mechanism 338 in the cutting position, the biopsy device 10 is slid up
the guide
element 52, the stylet blades 336 cutting the tissue and allowing the device
10 to reach the
target tissue. Once the target tissue is reached, the stylet blade mechanism
338 can be
34
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
retracted such that the blade(s) 336 are contained within the tip 30. The
coring cannula 20
can then be advanced over the target tissue.
1002571 With
reference to Figures 50A, 50B, 50C, 50D, 51A and 51B, in still another
aspect of the present invention, a garrote wire 210 is used to transect the
tissue sample.
1002581 The
prior art devices, which employ a garrote wire, use a linear pull "trigger"
system to activate the garrote wire. A limitation of the design is the travel
required to fully
pull the garrote wire. This limitation becomes an issue for larger cannula
sizes. As the
cannula diameter increases, the length of garrote wire required to transect
tissue increases
resulting in an increase in required travel. The travel length is limited by
the overall length of
the device. Continuing to increase the device length is not a viable option.
1002591 As
discussed below, the breast biopsy device 10 may include a trigger
mechanism 342 which includes a trigger 344 (shown diagrammatically in Figures
50A-51B).
The trigger 344 is generally pulled backward to pull garrote wire 210
backward, thereby
transecting the tissue sample within the coring cannula 20.
1002601 As shown
in Figure 50A, the breast biopsy device 10 includes a pair of
rotatable cleats 346 which are coupled to the housing 14 (through a trigger
body 350) and are
rotatable between a first cleat position (shown in Figure 50A) and a second
cleat position
(shown in Figure 50B). As shown, in one embodiment, the rotatable cleats 346
include a
plurality of teeth 348 which grip the garrote wire 210. The cleats 346 are
coupled to the
trigger mechanism 342 and when the trigger mechanism 342 is actuated, i.e.,
pulled
backward relative to the housing 14. Friction causes the cleats 346 to rotate,
thereby
engaging the teeth 348 into the garrote wire 210. Then, as the trigger
mechanism 342 is
pulled backward, the cleats 346 move therewith, pulling the garrote wire 210
as well.
1002611 With
specific reference to Figure 50A, when the garrote wire 210 is in a first
position, the wire 210 forms a loop 352 which is external to the coring
cannula 20. After the
coring cannula 20 is extended and surrounds the sample tissue, the trigger
mechanism 342 is
used to complete separate the sample tissue from the breast.
1002621 In one
embodiment, a single actuation of the trigger mechanism 342, e.g., a
single pull of the trigger 342, moves the garrote wire 210 from the first wire
position to a
second wire position in which the garrote wire 210 is within the coring
cannula 20 (and the
sample completely separated from the breast).
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[00263] In
another embodiment, multiple actuations of the trigger mechanism 342, or
multiple pulls of the trigger 344, are required. In the illustrated
embodiment, two pulls of the
trigger 344 are required. Each pull of the trigger 344, moving the garrote
wire a distance
defined by the distance between X1 and X2.
[00264] Figure
50A shows the garrote wire 210 in an initial position with the loop 352
in its largest configuration. Figure 50B shows the garrote wire 210 in an
intermediate
location, after the first pull of the trigger 344 (the trigger 344 and trigger
body 350 are shown
at full travel).
[00265] Figure
50C shows the garrote wire 210 at the intermediate location, with the
trigger body 350 returned to the initial position. In one aspect, the trigger
body 350 is spring
biased back to the initial position. In another aspect, the trigger body 350
may be manually
moved back to the initial position.
1002661 Figure
50D shows the garrote wire 210 at the final location, fully actuated and
within the coring cannula 20. At this point, the sample is completely severed
from the breast.
[00267] This
improvement to the linear pull system will enable the use of larger
cannula sizes to provide for multiple pulls of the trigger 344 on the garrote
wire 210.
Multiple pulls can be accomplished using the breakaway cleat system. The cleat
system
works as follows: When the trigger 348 is pulled, cleats 346 with separated
edges or teeth
348 grip the garrote wire 210, allow the trigger 344 to pull the wire 219 the
full length of
travel. At the end of travel, the trigger 344 is pushed forward back to the
start position.
When the trigger 344 is moved in this direction, the cleat 346 (cam)
disengages the wire so
that the trigger 344 slides forward without affecting the wire 210. As the
trigger 344 is pulled
back, the cleats 346 re-engage the wire 210, pulling it to further transect
tissue. This process
is repeated until transection is completed.
[00268] With
reference to Figure 51A in a further embodiment, a second pair rotatable
cleats 354 may be fixed directly to the device 10, e.g., directly to the
housing 14. The second
pair of rotatable cleats 354 are not fixed to the trigger body 350. The second
pair of cleats
354 prevented undesirable forward motion of the garrote wire 210.
[00269] With
reference to Figure 51B in an other embodiment, the garrote wire 210
may have a number of beads 356 fixed thereto (crimped or welded thereon) to
assist in
grabbing of the wire by the cleats 346, 354.
36
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[002701 As
discussed above, the prior art utilizes a linear pull trigger system, in which
the trigger is pulled straight back to actuate the garrote wire. The trigger
rides in a track and
is supported by guide rods to maintain the desired linear pull. When the
trigger is pulled back
it engages a support ring attached to the garrote wire. This support ring
moves backward
with the trigger, pulling the garrote wire across the cannula, transecting the
core of tissue at
the distal end. However, there are a significant number of cases which
encounter "tough"
breast tissue. When tough tissue is encountered, transection force increases
significantly, at
times resulting in incomplete transection. The user cannot provide enough
input force to
fully actuate the trigger system. Occurrences of this problem increases as
cannula diameter
increases.
1002711
Constriction and transection of breast tissue by the garrote wire can best be
described by separating it into two phases. Phase 1 includes 0% to 70-95%
constriction of
the tissue by the garrote wire. The 70-95% range is dependent on cannula size
and tissue
density. The requirements of Phase 1 are long travel and low/medium input
force. The
current linear pull system works well during Phase 1. Phase 2 covers up to the
final 30% of
tissue constriction and eventual transection. The requirements of Phase 2 are
limited travel
with potentially high input forces required. The linear pull system does not
always meet
these requirements.
1002721 With
reference to Figures 52A, 52B, 52C, and 52D, in another aspect of the
present invention, the garrote wire 210 actuation by a trigger mechanism 358.
The trigger
mechanism 358 is coupled to the housing 14 and the garrote wire 210 (via
support ring 4060).
In the illustrated embodiment, the trigger mechanism 358 includes a trigger
360 slidably
mounted in a trigger channel 362 in the housing 14. In the illustrated
embodiment the trigger
channel 362 is formed by a linear support track 368 within the housing 14. The
trigger 360 is
movable from a first trigger position (shown in Figure 52A and 52B) to an
intermediate
trigger position (shown in Figure 52C) within the trigger channel 362.
1002731 The
garrote wire 210 is coupled directly to the trigger 360. In response to the
trigger 360 being moved from the first trigger position to the intermediate
trigger position,
the garrote wire is moved from the first wire position to an intermediate wire
position. In the
illustrated embodiment, the triggers 360 drops into a cam channel 366 once it
reaches the
intermediate trigger position.
37
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
1002741 Once the
trigger 360 reaches the intermediate trigger position it can move no
further within the trigger channel 362. The trigger 360 is further rotatably
movable about a
trigger axis 364 from the intermediate trigger position to a second trigger
position (shown
dotted lines in Figures 52D). In response to movement of the trigger 360 to
the second
trigger position, the garrote wire 210 is moved from the intermediate wire
position to the
second wire position in response thereto.
1002751 The
addition of a rotational cam mechanism, i.e., the rotatable trigger 360, to
the trigger mechanism 358 will address Phase 2. The rotational cam provides a
mechanical
advantage to the user allowing greater input force with limited travel. The
concept described
here is a "hybrid" system, using the linear pull system for the first 70-95%
wire travel and
then switching to the rotational cam system for the final phase of
transection.
1002761 In use,
the user will pull the trigger 360 along the linear track. At an
optimized position, the trigger 360 will reach the end of the trigger channel
and engage the
cam activation system. In this position the trigger will no longer translate,
but will now rotate
so that the input force is transferred through the cam to the support ring
4060.
1002771 A
stabilization assembly may be used in conjunction with a tissue excision
device to allow a surgeon to maintain control of a tissue anchor and prevent
movement of the
tissue anchor during introduction of an excision device into breast tissue.
1002781
Referring now to FIG. 53, an isometric view of an exemplary guide rod
assembly according to one embodiment of the present invention is shown. Guide
rod 104
includes a hook 400 that extends from the proximal end of the guide rod 104.
1002791
Referring now to FIG. 54, a side view of an exemplary excision device 10
with an anchor stabilization rod 402 is shown. Due to the soft nature of some
breast tissue, it
is virtually impossible to securely an anchor it into position, regardless of
the anchor design.
Anchor stabilization rod 402 extends through and slides freely within the
central passageway
404 of the excision device 10.
1002801 In one
aspect of the present invention, the excision device 10 includes a
coupling device 406 that may be used to controllably couple and decouple the
anchor
stabilization rod 402 with the guide rod 104.
1002811
Referring now to FIG. 55, a partial isometric view of an anchor stabilization
rod 402 and a guide rod 104 including a hook-and-loop coupling device 406 is
shown. In one
embodiment of the present invention, the distal end of the anchor
stabilization rod 402
38
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
includes a loop 408. The loop 408 is designed to engage with the hook 400 on
the proximal
end of the guide rod 104. The hook-and-loop configuration provides an easy
method for the
surgeon to initially engage and secure the guide rod 104.
1002821
Referring now to FIG. 56, a partial isometric view of an anchor stabilization
rod 402 and a guide rod 104 including a coupling device 406 according to a
second
embodiment of the present invention is shown. In the second embodiment, the
distal end of
the anchor stabilization rod 402 and the proximal end of the guide rod 104
have notches 410
that engage with one another to connect the two rods 104, 402 to one another.
100283J
Referring now to FIG. 57, a partial isometric view of an anchor stabilization
rod 402 including a coupling device 406 according to a third embodiment of the
present
invention is shown. In the third embodiment, the distal end of the anchor
stabilization rod 402
includes a wireform attachment 412. The wireform attachment 412 includes
convex curves
386. The proximal end of guide rod 104 is hollow and includes a plurality of
apertures 388
and an opening 390. The wireform attachment 412 is inserted into the opening
390. The
wireform attachment 412 compresses as it is inserted into guide rod 104. When
the convex
curves 386 reach the plurality of apertures 388, the wireform attachment 412
expands slightly
such that the convex curves 386 engage with the apertures 388, forming a cross-
hold with the
guide rod 104 and connecting the two rods 104, 402 to one another.
1002841
Referring again to FIG. 54, once the rods have been connected by the coupling
device 406, the surgeon (or an assistant) maintains control over the position
of the tissue
anchor by holding onto a knob 392 at the proximal end of the anchor
stabilization rod 402. As
the surgeon advances the device 10 fonvard, the joint (not shown) where the
coupling device
406 connects the two rods 104, 402 is contained within the central passageway
404 of the
excision device 10. Once the joint is contained, the only motion allowed of
the rods 104, 402
will be forward or backward along the axis of the central passage 404. In
addition, the size of
the tube that creates the central passageway 404 in the excision device 10 is
designed to
compress the coupling device 406, so that the two rods 104, 402 cannot become
disengaged
when inside the central passageway 404.
1002851
Referring now to FIG. 58, an isometric view of an exemplary excision device
with an anchor stabilization rod and fixed support arm is shown. In addition
to the control
provided to the surgeon or an assistant by the knob 392, a fixed support arm
394 may be
attached to the proximal end of the anchor stabilization rod 402 for
additional support. After
39
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
the two rods 104, 402 have been connected and entered into the central
passageway 404 of
the excision device 10, the proximal end of the anchor stabilization rod 402
may be attached
to the fixed support arm 396, which would hold the rods 104, 402 securely in
position. The
fixed support arm 394 may be flexible in its unattached state to enable it to
be attached easily
to the anchor stabilization rod 402. After the fixed support arm 394 is
attached to the anchor
stabilization rod 402, the anchor stabilization rod 402 is locked in position.
The fixed support
arm 394 may attach to the side of a surgical bed, a floor stand, or any other
stationary object
or surface.
1002861 In one
embodiment of the present invention, an expanding cannula 400
provides a means to enable the surgeon to excise a larger tissue sample and
remove it from
the breast through a smaller skin incision. By way of example and not
limitation, the
exemplary expanding cannula described herein allows for the excision of a 15
mm diameter
tissue sample through a 10 mm skin incision.
1002871
Referring now to FIG. 59, a partial isometric view of an expanding cannula
400 according to an embodiment of the present invention is shown. In a
preferred
embodiment, expanding cannula 400 is approximately 8 mm in diameter is
designed to enter
the breast through a 10 mm skin incision. The expanding cannula 400 is
introduced into the
breast using a bladed stylet with a flexible transaction blade (not shown in
FIG. 59). The
initial configuration of the expanding cannula 400 with blade may be similar
to the
configuration shown in FIG. 34A. The flexible transection blade 112 is exposed
as the
expanding cannula 400 of FIG. 59 is inserted into the breast.
1002881 When the
expanding cannula 400 reaches the target area, the stOet is retracted,
exposing the distal end 402 of the expanding cannula 400. After the stylet has
been retracted,
the expanding cannula 400 is rotated. The expanding cannula 400 may rotate in
a manner
similar to the coring cannula 20 of FIG. 20A, when the flexible transection
blade 112 is in the
first blade position it is contained within the coring cannula 20, and thus,
not visible.
However, the expanding cannula 400 of FIG. 53 rotates but does not advance,
whereas the
coring cannula 20 of FIG. 20A both rotates and advances.
1002891 In one
embodiment, after the stylet has been retracted, the expanding cannula
400 may alternatively be rotated using a motor drive, such as the exemplary
motor drive
described in FIG. 24.
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
1002901
Referring now to FIG. 60, a partial isometric view of a partially expanded
expanding cannula 400 is shown. As the expanding cannula 400 rotates, an
expanding coring
mechanism 404 begins to advance out of the distal end 402 of the expanding
cannula 400.
The expanding coring mechanism 404 includes a flexible blade 406. The flexible
blade 406 is
in the retracted position, with only an exposed edge exposed. The exposed edge
of the
flexible blade 406 begins to core a cylinder of tissue as it advances forward
through the
tissue. The expanding coring mechanism 404 is configured such that the
diameter of the
shape formed by the exposed edge of the flexible blade 406 increases as the
expanding coring
mechanism 404 advances.
1002911 In the
illustrated embodiment, a ring 408 at the distal end of the expanding
coring mechanism 404 is formed by an annular spring 410 initially in the
compressed
position. As the annular spring 410 advances axially, it begins to expand.
1002921
Referring now to FIG. 61, a partial isometric view of a fully expanded
expanding cannula is shown. The rate and amount of expansion of the annular
spring 410 is
controlled by a plurality of formed fingers 412 that define the shape of the
cavity. During the
initial phase of advancement, the size of the annular spring 410 advances to
its full diameter.
After the full diameter of the annular spring 410 has been reached, the formed
fingers 412
continue to advance axially to a pre-determined distance that has been
established to define
the length of the excised tissue specimen (not shown). When the formed fingers
412 are fully
extended, the flexible blade 406 begins to advance, transecfing the distal end
of the tissue
specimen. The advancement of the flexible blade 406 may be similar to the
advancement of
flexible transection blade 112 as shown in FIGS. 21A and 22A.
1002931 After
completion of the procedure, the excision device with the tissue
specimen contained is removed from the breast.
1002941 In one
embodiment of the present invention, an incision expander 414
provides a means to enable the surgeon to introduce an excision device 10 with
a larger
diameter through a smaller skin incision than would otherwise be possible. By
way of
example and not limitation, the exemplary incision expander described herein
allows for the
excision of a 15 mm diameter tissue sample through a 10 mm skin incision.
1002951
Referring now to FIG. 62, an isometric view of an assembled incision
expander 400 according to a first embodiment is shown. Incision expander 414
includes a
housing 416, an expanding cannula 418, a mechanism to expand the cannula, such
as levers
41
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
420, and a removable bladed stylet 422. In general, the housing 416 is
cylindrical and the
diameter may be increased. The removable bladed stylet 422 is housed within
the housing
416 when the housing 416 has a reduced diameter. The bladed stylet 422 (within
the housing)
is inserted into the breast to create an incision. The bladed stylet 422 is
removed and the
diameter of the housing 416 is increased to allow for introduction of a large
excision device
through the incision, as described below.
[00296] In one
embodiment, the incision expander 414 is initially introduced into the
breast through an incision that is approximately lOmm long. After the initial
10mm incision
is made by the surgeon, the incision expander 414 is pushed into the breast.
The flat blades
on the front edge of the bladed stylet 422 cut and separate the tissue.
[00297]
Referring now to FIG. 63, an isometric view of a disassembled incision
expander 414 is shown. After the incision expander 414 has been fully inserted
into the
breast, one or more levers 420 are actuated to increase the diameter of the
expanding cannula
418.
[00298] The
expanding cannula 418 may be comprised of a thin sheet of material that
is formed into a cylinder. The expanding cannula 418 may be comprised of a
metal, a
polymer, or any material that has the ability to maintain its cylindrical form
as it expands and
contracts. The wall of the expanding cannula 418 overlaps itself, which allows
it to maintain
its cylindrical configuration as it expands. The one or more levers 420 are
connected to the
housing 416 and the expanding cannula 418. Actuating the one or more levers
420 expands
the diameter of the housing 416 and the expanding cannula 414. After the
cylinder has been
expanded, the bladed stylet 422 can be removed from the incision expander 414.
[00299]
Referring now to FIG. 64, an isometric view of an incision expander 414 with
bladed stylet 422 removed and expanded cannula 418 in an expanded state is
shown. The
incision expander 414 then remains in the breast in its expanded position
after the bladed
stylet 422 is removed (not shown). In this configuration, the opening through
the center of the
incision expander 414 (via the housing 416 and the expanding cannula 418) is
large enough
to allow the introduction of a larger excision device, such as the exemplary
excision device
described herein.
[00300] After
the procedure has been completed (i.e., the tissue sample has been
excised using the excision device 10), and the excision device is removed from
the breast
tissue, the one or more levers 420 are moved back to their initial position
(as shown in FIG.
42
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
62), which reduces the diameter of the cylinder of the expanding cannula 418
and the
diameter of the housing 416. The incision expander 414 is then removed from
the breast.
1003011
Referring now to FIG. 65, an isometric view of an assembled incision
expander 424 according to a second embodiment is shown. The incision expander
424
includes a long, tapered, bladed stylet 426. The tapered section of the stylet
426 gradually
expands the diameter of the incision as it is introduced into the breast. When
the incision
expander 424 is fully introduced, the stylet 426 is removed, leaving the
larger diameter
expanding cannula 418 in place in the breast. In one embodiment, cannula 418
is an
expanding cannula. The incision is partially expanded by the tapered end of
stylet 424 and
then further expanded by expanding cannula 418, as described above. In an
alternate
embodiment, cannula 418 is not an expanding cannula and the expansion of the
incision is
performed solely by the tapered end of stylet 424.
1003021 A
compression device described below compresses and supports the breast
during a breast biopsy procedure. Although designed for use in conjunction
with the
exemplary excision devices described herein, the compression device can be
used with any
excisional or incisional biopsy device.
1003031
Referring now to FIG. 66, an isometric view of a breast compression device
430 according to a first embodiment is shown. Breast compression device 430
includes a
back plate 432 to support the breast 439. Breast compression device 430
further includes a
front paddle 434. The breast 439 is compressed by front paddle 434 when the
breast 439 is
placed between back plate 432 and front paddle 434. Front paddle 434 is
comprised of at
least two fingers 436, with a U-shaped space in between that provides access
to the breast
tissue by a biopsy device. The fingers 436 are independently adjustable to
allow fine
adjustment of the compression as necessary. Breast compression device 430
further includes
an adjuster 438 to adjust the interior spacing of the device, shown in FIG. 66
as adjustment
sleeve 440.
1003041
Referring now to FIG. 67, a top view of a breast compression device 442
according to a second embodiment is shown. In one embodiment, breast
compression device
442 does not include an adjuster to adjust the interior spacing of the device.
Rather, breast
compression device 442 includes a bar 444 that connects back plate 432 and
front paddle 434.
Breast compression device 442 may be comprised of a single sheet of material
that has elastic
43
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
or spring-like properties. In the second embodiment, the material itself
provides the
compressive force against the breast 439.
[00305] In a third embodiment, breast compression device 442 includes both
bar 444
and adjuster 438 to adjust the interior spacing of the device. In the third
embodiment, breast
compression device 442 may be comprised of a single sheet of material that has
elastic or
spring-like properties to provide the compressive force against the breast
439.
[00306] Referring now to FIG. 68, a top view of a breast compression device
442
according to FIG. 67 illustrating compression of breast 439 is shown. The
breast compression
device 442 may be wrapped around the breast 439 from any direction or angle
and allow for
access of both an ultrasound probe and a biopsy device, as needed for a biopsy
procedure.
[00307] Referring now to FIG. 69, a front view of a breast compression
device 442
according to FIG. 67 illustrating compression of a breast 439 is shown. In
this illustration, the
breast compression device 442 has been wrapped around the breast 439 from the
front of the
breast 439.
[00308] Referring now to FIG. 70, a top view of a breast compression device
442 with
a partial side view of a biopsy device 446 is shown. The biopsy device 446 has
access to the
breast 439 through the U-shaped space in between fingers 436. The open space
in the area
above the breast 439 and the breast compression device 442 provides
unrestricted access for
an ultrasound probe (not shown).
1003091 The invention will now be discussed with reference to the clauses
listed
below. It should be noted that the present invention may comprise the
following clauses in
any combination, even if not specifically listed.
1003101 1. An excision device comprising:
a housing;
a stylet coupled to the housing and having a tip containing at least one
blade;
a hollow central passageway extending through the center of the housing and
the
stylet;
a localization needle coupled to the stylet and being slidably disposed within
a central
passageway; and
an expanding cannula comprising:
a body having a first end and a second end, the first end coupled to the
housing, and
44
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
an expanding coring mechanism coupled to the second end of the body, the
expanding coring mechanism including:
an annular spring,
a plurality of formed fingers extending between the annular spring and the
second end
of the body, and
a retractable flexible blade extending from the second end of the body and
over the
annular spring.
1003111 2. The
excision device of clause 1, wherein the annular spring is
configured in a compressed state.
[00312] 3. The
excision device of clause 1, wherein the annular spring is
configured in an expanded state.
1003131 4. The
excision device of clause 1, wherein the body is 8 mm in diameter.
1003141 5. The
excision device of clause 1, wherein the expanding coring
mechanism is advanced by a motor drive.
[00315] 6. The
excision device of clause 1, wherein the formed fingers define a
cavity between the body and the annular ring for collecting a tissue sample
from a target
tissue
1003161 7. The
excision device of clause 1, wherein the retractable flexible blade
has at least one exposed edge for cutting the tissue sample as the expanding
coring
mechanism is advanced into the target tissue
1003171 8. An
expanding cannula of an excision device, the expanding cannula
comprising:
a body having a first end and a second end, the first end being coupled to the
excision
device; and
an expanding coring mechanism coupled to the second end of the body, the
expanding
coring mechanism including:
an annular spring,
a plurality of formed fingers extending between the annular spring and the
second end of the body, and
a retractable flexible blade extending from the second end of the body and
over the annular spring.
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
1003181 9. The
expanding cannula of clause 8, wherein the annular spring is
configured in a compressed state.
1003191 10. The
expanding cannula of clause 8, wherein the annular spring is
configured in an expanded state.
1003201 11. The
expanding cannula of clause 8, wherein the body is 8 mm in
diameter.
1003211 12. The
expanding cannula of clause 8, wherein the expanding coring
mechanism is advanced by a motor drive.
1003221 13. The
expanding cannula of clause 8, wherein the formed fingers define
a cavity between the body and the annular ring for collecting a tissue sample
from a target
tissue
1003231 14. The
expanding cannula of clause 8, wherein the retractable flexible
blade has at least one exposed edge for cutting the tissue sample as the
expanding coring
mechanism is advanced into the target tissue
1003241 15. A method
for excising tissue using an expanding cannula of an
excision device, the method comprising:
introducing a bladed stylet coupled to the excision device into a target
tissue:
retracting the bladed stylet to expose a distal end of an expanding cannula
coupled to
the excision device;
rotating the expanding cannula using a motor drive to advance the expanding
cannula
into the target tissue; and
collecting a tissue sample with the expanding cannula.
1003251 16. The
method of clause 15, further comprising retracting the expanding
cannula from the target tissue.
1003261 17. The
method of clause 15, wherein the expanding cannula includes an
expanding coring mechanism.
1003271 18. The
method of clause 17, wherein the expanding coring mechanism
comprises an annular spring that expands in diameter as the expanding cannula
is advanced
into the target tissue.
1003281 19. The
method of clause 18, wherein the expanding coring mechanism
includes a flexible blade that rotates axially to cut a core of tissue from
the target tissue as the
expanding cannula is advanced into the target tissue.
46
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
1003291 20. A method for excising tissue using an expanding cannula of
an
excision device, the method comprising:
introducing a bladed stylet coupled to the excision device into a target
tissue;
retracting the bladed stylet to expose a distal end of an expanding cannula
coupled to
the excision device;
rotating the expanding cannula using a motor drive to advance an expanding
coring
mechanism into the target tissue;
collecting a tissue sample with the expanding coring mechanism; and
retracting the expanding cannula from the target tissue.
1003301 21. The method of clause 20, wherein the expanding coring
mechanism
comprises an annular spring that expands in diameter as the expanding cannula
is advanced
into the target tissue.
1003311 22. The method of clause 21, wherein the expanding coring
mechanism
includes a flexible blade that rotates axially to cut a core of tissue from
the target tissue as the
expanding cannula is advanced into the target tissue.
1003321 23. An incision expander comprising:
a housing;
an expanding cannula coupled to the housing;
a mechanism coupled to the housing and the expanding cannula and being
configured
to expand the expanding cannula; and
a removable bladed stylet extending through the center of housing and the
expanding
cannula.
1003331 24. The incision expander of clause 23, wherein the housing is
cylindrical.
1003341 25. The incision expander of clause 23, wherein the mechanism
to expand
the expanding cannula comprises one or more levers.
1003351 26. The incision expander of clause 23, wherein the expanding
cannula is
cylindrical.
1003361 27. The incision expander of clause 23, wherein the actuation
of the
mechanism to expand the expanding cannula increases the diameter of the
expanding
cannula
1003371 28. The incision expander of clause 23, wherein the expanding
cannula is
comprised of a metal.
47
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[00338] 29. The incision expander of clause 23, wherein the expanding
cannula is
comprised of a polymer.
[00339] 30. An incision ex pander comprising:
a housing;
a cannula coupled to the housing; and
a tapered stylet extending through the center of housing and the expanding
cannula.
1003401 31. The incision expander of clause 30, wherein the housing is
cylindrical.
1003411 32. The incision expander of clause 30, wherein the expanding
cannula is
cylindrical.
[00342] 33. The incision expander of clause 30. wherein the expanding
cannula is
comprised of a metal.
[00343] 34. The incision expander of clause 30, wherein the expanding
cannula is
comprised of a polymer.
[00344] 35. A method for introducing an excision device through a skin
incision,
the method comprising:
introducing a bladed stylet into a target tissue, the bladed stylet extending
through the
center of an incision expander, the incision expander comprising a housing and
an expanding
cannula;
actuating a mechanism to expand the expanding cannula, the mechanism being
coupled to the housing and the expanding cannula;
retracting the bladed stylet from the incision expander; and
introducing an excision device into the target tissue through the center of
the incision
expander.
[00345] 36. The method of clause 35, wherein the mechanism to expand
the
expanding cannula comprises one or more levers.
[00346] 37. The method of clause 35, wherein the actuation of the
mechanism to
expand the expanding cannula increases the diameter of the expanding cannula.
[00347] 38. The method of clause 35, further comprising collecting a
tissue sample
with the excision device from the target tissue.
[00348] 39. The method of clause 38, further comprising retracting the
excision
device from the target tissue.
48
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
[00349] 40. A tissue excision system, the system comprising:
an excision device comprising:
a housing coupled to a coring cannula,
a stylet coupled to the housing and having a tip containing at least one
blade,
a hollow central passageway extending through the center of the housing, the
coring
cannula, and the stylet,
a guide rod assembly removably coupled to the housing: and
an incision expander coupled to the excision device, the incision expander
comprising:
a housing,
an expanding cannula coupled to the housing,
a mechanism coupled to the housing and the expanding cannula and being
configured to expand the expanding cannula, and
wherein the stylet extends through the center of the housing of the incision
expander and the expanding cannula.
1003501 41. A breast compression device comprising:
a back plate;
a front paddle comprising two or more fingers; and
an adjuster to adjust the space between the back plate and the front paddle.
1003511 42. The breast compression device of clause 41, further
comprising a U-
shaped space between the two or more fingers.
1003521 43. The breast compression device of clause 41, wherein the
two or more
fingers are independently adjustable.
[00353] 44. The breast compression device of clause 41, wherein the
adjuster
comprises an adjustment sleeve.
[00354] 45. A breast compression device comprising:
aback plate;
a front paddle comprising two or more fingers; and
a bar connecting the back plate and the front paddle.
1003551 46. The breast compression device of clause 45, further
comprising a U-
shaped space between the two or more fingers.
49
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
1003561 47. The breast compression device of clause 45, wherein the
two or more
fingers are independently adjustable.
[003571 48. The breast compression device of clause 45, wherein the
breast
compression device comprises a single sheet of material.
1003581 49. The breast compression device of clause 45, wherein the
breast
compression device is comprised of a spring-like material.
[00359] 50. A method for compressing breast tissue with a breast
compression
device, the method comprising:
wrapping the breast compression device around the breast tissue from any
angle, the
breast compression device including:
a back plate,
a front paddle comprising two or more fingers, and
a bar connecting the back plate and the front paddle; and
adjusting the breast compression device to provide enough compression to
render the
breast tissue immobile.
1003601 51. The method of clause 50, wherein adjusting the breast
compression
device comprises adjusting at least one of the two or more fingers.
[00361] 52. The method of clause 50, wherein the breast compression
device
further comprises an adjuster.
[00362] 53. The method of clause 52, wherein adjusting the breast
compression
device comprises adjusting at the adjuster.
[00363] 54. The method of clause 53, wherein the adjuster comprises an
adjustment sleeve.
[00364] 55. The method of clause 50, wherein the breast compression
devices
further includes a U-shaped space between the two or more fingers.
[00365] 56. The method of clause 55, further comprising introducing a
biopsy
device to the breast tissue through the U-shaped space.
[00366] 57. The method of clause 50, wherein the breast compression
device
comprises a single sheet of material.
[00367] 58. The method of clause 50, wherein the breast compression
device is
comprised of a spring-like material.
[00368] 59. A tissue excision system, the system comprising:
CA 02997004 2018-02-28
WO 2017/040064
PCT/US2016/047720
an excision device comprising:
a housing coupled to a coring cannula,
a stylet coupled to the housing and having a tip containing at least one
blade,
a hollow central passageway extending through the center of the housing. the
coring
cannula, and the stylet,
a guide rod assembly removably coupled to the housing: and
a breast compression device comprising:
a back plate,
a front paddle comprising two or more fingers, wherein the stylet of the
excision
device is advanced to a target tissue between the two or more fingers, and
an adjuster to adjust the space between the back plate and the front paddle.
1003691 Any
modifications and variations of the present invention are possible in light
of the above teachings. The invention may be practiced otherwise than as
specifically
described within the scope of the appended claims.
51