Note: Descriptions are shown in the official language in which they were submitted.
METHODS AND COMPOSITIONS FOR DIRECT PRINT HAVING IMPROVED
RECYCLABILITY
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. patent application Ser.
No. 14/846,032,
filed September 4, 2015, which is a continuation-in-part of U.S. patent
application Ser. No.
14/528,324, filed Oct. 30, 2014, which is a divisional of U.S. patent
application Ser. No.
13/168,181, filed Jun. 24, 2011, and now issued as U.S. 8,876,979 and further
is a
continuation-in-part of U.S. patent application Ser. No. 12/581,952, filed
Oct. 20, 2009.
TECHNICAL FIELD
[0002] The present invention relates generally to ink compositions for
digital printing
on external surfaces of plastic articles. The present invention also relates
to plastic articles
having digital images printed thereon, including plastic containers having
digital images with
improved adhesion and/or recycling characteristics, as well as methods for
facilitating the
recycling thereof.
BACKGROUND
[0003] The plastic container industry continues to employ ever increasing
amounts of
recycling. Container manufacturers have recently started producing containers
with digitally
printed labels that are of a sufficient definition and quality to compete with
and potentially
replace prior conventional labeling techniques. Examples of such printing
techniques are
described in commonly-owned U.S. Pat. No. 7,625,059 and U.S. Pat No.
7,736,713.
[0004] Potential challenges arise when introducing containers with
digitally printed
labels into conventional container recycling processes. Some challenges have
been mentioned
and discussed in U.S. patent application Ser. No. 12/581,952. There is a clear
trend amongst
container manufacturers,
1
Date Recue/Date Received 2022-09-19
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
brand owners, end users, and governmental entities to improve and increase
plastic container
recycling efforts. Consequently, it is desirable to provide industry-
acceptable articles that are
sufficiently compatible with the current recycling infrastructure and
processes, or that provide
sufficient incentive and/or volumes to effectuate industry-wide changes. At
least initially, it
would be desirable to provide digitally printed articles that are recyclable
using current
industry standard processes¨i.e., processes that commonly include caustic high-
temperature
washing and grinding. As such, there is a desire for digitally printed plastic
articles, such as
containers, that have digital images that adhere to the article without
quality issues
throughout its useful life, but are more readily removable during plastic
recycling processes.
SUMMARY
[0005] In an exemplary aspect, an ink composition for digital printing
onto a plastic
article is disclosed. The ink composition generally comprises a base ink; an
ink removal-
promoting additive; and a carrier capable of dissolving at least a portion of
the ink removal-
promoting additive. The removal-promoting additive is configured or selected
to cause at
least a portion of cured droplets of ink to separate or loosen from the
external surface of a
plastic article when a printed digital image is exposed to a liquid-based
(e.g., water-based)
solution at or about a predetermined elevated temperature. Such predetermined
elevated
temperature may, without limitation, include those associated with
conventional recycling
processes.
[0006] In another aspect, a recyclable plastic article is disclosed that
has an external
surface with a digital image printed thereon by cured droplets of ink. The
droplets of ink
comprise a composition of ink as summarized above that includes a removal-
promoting
additive. Embodiments of methods for the removal of said ink from a plastic
container and
methods for facilitating such recycling are also disclosed.
[0007] Additional advantages of the invention will be set forth in part in
the
description which follows, and in part will be obvious from the description,
or can be learned
by practice of the invention. The advantages of the invention will be realized
and attained by
means of the elements and combinations particularly pointed out in the
appended claims. It is
to be understood that both the foregoing general description and the following
detailed
2
description are exemplary and explanatory only and are not restrictive of the
invention, as
claimed.
[0007a] There is provided ink composition for digital printing onto a plastic
article, the ink
composition comprising: (a) from about 65 to about 85 weight percent of an
ultraviolet-
curable (UV-curable) base ink; (b) from about 5 to about 20 weight percent of
an ink
removal-promoting additive having a glass transition temperature (Tg) of less
than about 70 C
; and (c) from about 5 to about 15 weight percent of a carrier capable of
dissolving at least a
portion of the ink removal-promoting additive.
BRIEF DESCRIPTION OF THE DRAWINGS
[0008] Embodiments of the invention will now be described, by way of
example, with
reference to the accompanying drawings, wherein:
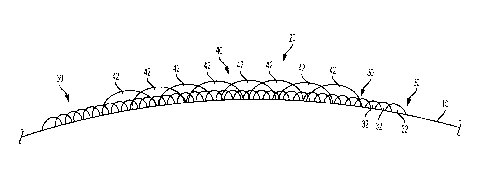
[0009] FIG. 1 generally illustrates a side view of a portion of a plastic
container with an
embodiment of a digital image printed thereon;
[0010] FIG. 2 is general representation of a quality review table/matrix
that may be used to
evaluate the acceptability of a printed image on a plastic article.
DETAILED DESCRIPTION
[0011] In various aspects, reference will now be made in detail to
embodiments of the
present invention, examples of which are described herein and illustrated in
the accompanying
drawings. While the invention will be described in conjunction with
embodiments, it will be
understood that they are not intended to limit the invention to these
embodiments. On the
contrary, the invention is intended to cover alternatives, modifications and
equivalents, which
may be included within the spirit and scope of the invention.
3
Date Recue/Date Received 2022-09-19
[0012] For general context, and without limitation, a portion of a surface
10 of a plastic
article (e.g., a container) with an embodiment of a printed image 20 is
generally illustrated in
FIG. 1. The illustrated embodiment of the image 20 includes a base coat 30
that may be
comprised of a plurality of base coat ink droplets 32, and may also include a
secondary coat 40
that can be comprised of a plurality of secondary coat ink droplets 42.
[0013] Containers (which include bottles) associated with the present
disclosure are
comprised of a plastic material or resin (e.g., acrylonitile-butadiene-styrene
(ABS),
polyethylene terephthalate (PET), polystyrene (PS), polyethylene
(PE)(including high-density
polyethylene (HDPE)), polypropylene (PP), polyvinyl chloride (PVC), etc.).
Further, the
containers may be mono-layer or multi-layer containers, and can be formed
using various
3a
Date Recue/Date Received 2022-09-19
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
conventional forming techniques including, without limitation, injection
molding, blow
molding, thermoforming, etc. In an embodiment, the outermost layer/surface may
be
comprised of a virgin plastic material. Although, it is noted that containers
in accordance with
the teachings of the disclosure may also include some percentage of recycled
content,
including a percentage of recycled content in the outer layer of the
container.
[0014] In embodiments, the article (e.g., container) may include a first
coat, or base
coat (e.g. base coat 30 shown in FIG. 1). The base coat 30 may be comprised of
a plurality of
base coat ink droplets 32 that are printed (e.g., digitally printed, such as
by a drop-on-demand
ink jet process) on an exterior surface of the article and are subsequently
cured or permitted
to cure. The ink may be UV curable ink, which is curable by UV radiation that
can be applied
by various known means, including but not limited to UV or LED lamps. The ink
droplets
may be monomer-based and comprised of an ink composition that serves to
improve the
application of the ink droplets (e.g., provides good processing
characteristics for printing)
and/or provides a visual characteristic (e.g., color or texture). For
embodiments, the base coat
30 may comprise white and/or colorless portions. Moreover, with disclosed ink
droplets, i.e.,
those that are monomer-based, curing can cause the ink droplets to polymerize.
Because the
inks that are employed are not solvent-based, the ink can be composed so that
during
recycling processes the ink does not bleed in solutions and give off
volatiles, as solvents
might. It is noted that the U.S. Environmental Protection Agency (EPA) has
promulgated
guidelines for solvents; however, polymers are solid and do not flash off or
give off volatiles.
That is, the separation of inks such as disclosed herein can be mechanical in
nature, as the ink
is in a cured polymer state and may be a physically removed (e.g., in the form
of flakes or
film), rather than being included as part of a chemical dissolution.
[0015] As summarized above, the ink composition that makes up the ink
droplets
includes a removal-promoting additive. In some aspects, the "removal-promoting
additive"
comprises at least one hydrophilic component or acidic component. Hydrophilic
components
may comprise one or more composition elements that exhibit hydrophilic (water-
loving)
properties. Acidic components may comprise one or more composition elements
that exhibit
acidic properties. For some embodiments, the removal-promoting additive will
comprise a
combination of hydrophilic and acidic components i.e., at least two removal
promoting
additives. For some ink droplet compositions a "dual" composition, in which
the removal-
4
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
promoting additive comprises both hydrophilic and acid components, a lesser
percentage of
weight (to total) may be used to provide comparable results to the use of
composition with
just a single additive.
[0016] Such hydrophilic components may include hydrophilic monomers,
hydrophilic
oligomers, and water dispersible monomers that provide the desired
functionality.
Hydrophilic monomers are often characterized as having oxygen or nitrogen
atoms, in
addition to halogens, in their backbone structure. Such monomers are commonly
prone to
attack by polar solvents such as water and ketones. Hydrophilic monomers also
tend to have a
lesser resistance to thermal degradation. Consequently, the inclusion of one
or more
hydrophilic components in the associated ink droplets can, at a later point
(such as the point
of post-use recycling), improve the separation of the image (i.e., the cured
ink droplets) from
the plastic structure of the article. By way of example and without
limitation, in a cured state,
the hydrophilic portion of a polymer will typically absorb water. When this
occurs, the water
may act as a plasticizer, increasing the mobility of the polymer's chains. The
increased
mobility can soften the polymer, making it more susceptible to removal. As
such, the
exposure of a plastic article, such as a container, including an image
comprised of an ink
composition with a removal-promoting additive (e.g., hydrophilic monomer) to a
liquid-
based solution (e.g., agitated water) at an elevated temperature will promote
the softening of
the ink composition (i.e., hydrophilic monomer) and the separation of the
image from the
plastic material. The elevated temperature may be predetermined, and may be a
temperature
that is above the temperature that the article will typically encounter in
normal use.
Moreover, to the extent desired, an adhesion-separation threshold may be
established such
that the softening and subsequent removal substantially only occurs at
elevated temperatures
(e.g., during a recycling process), and is substantially prevented during
normal intended use.
It is noted that aliphatic urethane acrylates are a general class of
hydrophilic monomers that
generally absorb water and may potentially be used as a hydrophilic component.
Moreover,
provided that they are appropriately viscous (i.e., not too viscous) for ink
jet/digital print
application, some hydrophilic oligomers may also be used as a hydrophilic
component.
[0017] Without limitation, for some embodiments, the removal-promoting
additive
may include hydrophilic monomers with a percent weight as to the total weight
(i.e., of the
associated ink and the removal-promoting additive) within the range of near 0%
to as much
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
as 20% by weight. For some embodiments, the percent weight of the removal-
promoting
additive will fall within the range of near 0 to 10% of the total weight.
Without limitation, an
example of a hydrophilic monomer that may be employed is methoxy polyethylene
glycol
monoacrylate (e.g., CD 553, commercialized by Sartomer USA, LLC (Sartomer)).
[0018] It is noted that some water dispersible monomers are also
hydrophilic and
appear to absorb water when cured. With such a composition, the water may act
as a
plasticizer and soften the cured ink composition (e.g., a cured ink film on
the surface of a
container), making it easier to remove the ink film during recycling
processing. With
embodiments of the disclosure, the inclusion of an appropriate hydrophilic
component to the
ink composition can add a hydrophilic quality to the ink, while maintaining
the jet-ability and
adhesion of the ink to the article substrate throughout the useful life of the
article.
[0019] Acidic components include acidic monomers that have a measurable
acidic
value. It is noted that the measurable quantity may be based on pH, acid
weight percentage,
or titrated value of an alkaline chemical (e.g., mg KOH/g [milligrams of
potassium hydroxide
per gram of monomer]). Further, for example, in a cured state, the acidic
portion of the
polymer may be vulnerable to a caustic solution. A reaction between the acidic
functionality
of the polymer chain and the alkalinity of the solution can yield a decrease
in adhesion to the
applied substrate, promoting the separation of the polymer. Without
limitation, examples of
acidic monomers that may be employed include acidic acrylate oligomer (e.g.,
CN 147,
commercialized by Sartomer) and monofunctional acid ester (e.g., CD 9050,
commercialized
by Sartomer). For instance, when a container involving an acidic component is
put into
contact with a wash (e.g., a conventional-type caustic wash), associated bonds
may be
broken, promoting an intended separation of the printed image (droplets of
ink) from the
plastic substrate.
[0020] In other aspects, the removal-promoting additive is configured or
otherwise
selected according to its desired properties that can facilitate the removal
of a cured ink
composition from the surface of a plastic article. For example, in one aspect,
it is desirable
for the removal-promoting additive to exhibit a relatively high solvent
solubility and a
relatively high aqueous solubility in combination with a glass transition
temperature that is
below that of the temperature typically utilized in conventional recycling
processes. To this
6
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
end, according to exemplary aspects, the removal-promoting additive exhibits a
glass
transition temperature (Tg) that is less than about 130 C. This can include
removal-
promoting additives that exhibit a glass transition temperature (Tg) that is
less than about
120T, less than about 110C, less than about 100 C, less than about 90 C, less
than about
80 C, less than about 70 C, less than about 60 C, or even less than about 50
C. In a further
aspect, the ink removal-promoting additive has a glass transition temperature
(Tg) in the
range of from about 50 C to about 130 C. In a still further aspect, the ink
removal-promoting
additive has a glass transition temperature (Tg) in the range of from about 55
C to about
125 C, or from about 65 C to about 105 C, or from about 55 C to about 95 C. In
a yet
further aspect, the ink removal-promoting additive can have a glass transition
temperature
(Tg) in any range derived from any of the above exemplary values.
[0021] As one of ordinary skill in the art will appreciate, the desired
level of aqueous
solubility can be characterized as a function of, for example, the acid number
of the removal-
promoting additive. The acid number can be expressed in terms of a titrated
value of an
alkaline chemical (e.g., mg KOH/g [milligrams of potassium hydroxide per gram
of removal-
promoting additive]). In these aspects, the removal-promoting additive can
exhibit an acid
number in the range of from about 100 mg KOH/ gm to about 250 mg KOH/ gm,
including
values of 110 mg KOH/ gm, 120 mg KOH/ gm,130 mg KOH/ gm, 140 mg KOH/ gm, 150
mg KOH/ gm, 160 mg KOH/ gm, 170 mg KOH/ gm, 180 mg KOH/ gm, 190 mg KOH/ gm,
200 mg KOH/ gm, 210 mg KOH/ gm, 220 mg KOH/ gm, 230 mg KOH/ gm, and 240 mg
KOH/ gm. In a further aspect, the ink removal-promoting additive has an acid
number in the
range of from about 150 mg KOH/ gm to about 205 mg KOH/ gm, including values
of 175
mg KOH/ gm, 185 mg KOH/ gm, and 195 mg KOH/ gm. In a still further aspect, the
ink
removal-promoting additive can have an acid number in any range derived from
any of the
above exemplary values.
[0022] In further aspects, the removal-promoting additive can exhibit a
glass
transition temperature (Tg) that is less than about 100 C and an acid number
in the range of
from about 100 mg KOH/ gm to about 250 mg KOH/ gm. In a still further aspect,
the ink
removal-promoting additive can exhibit a glass transition temperature (Tg) in
the range of
from about 50`C to about 90`C and an acid number in the range of from about
150 mg KOH/
gm to about 205 mg KOH/ gm. In a yet further aspect, the ink removal-promoting
additive
7
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
can have a glass transition temperature (Tg) and an acid number in any range
derived from
any of the above exemplary values.
[0023] By selecting a removal promoting additive that exhibits a desired
combination
of aqueous solubility and glass transition temperature, it is possible to
provide a curable ink
that exhibits desired adhesion, scratch resistance, and solvent resistance
while also facilitating
the removal process of the ink as a precursor to a recycling process. When
subjected to a
caustic aqueous environment at a temperature that exceeds the Tg of the
removal-promoting
additive, a softening of additive can result thus leading to a relatively
cleaner detachment of
the- cured ink layer from the surface of the plastic it was printed on.
[0024] These removal-promoting additives can be present in the ink
compositions in
an amount from about 1 to about 20 weight percent of the ink composition. This
can further
include specific exemplified amounts of 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12,
13, 14, 15, 16, 17, 18,
and 19 weight percent. In a further aspect, the ink removal-promoting additive
can be present
in an amount any range of amounts derived from any of the above exemplary
values. For
example, the removal-promoting additives can be present in the ink
compositions in an
amount from about 5 to about 20 weight percent of the ink composition.
[0025] In an exemplary aspect, ink removal-promoting additives that
exhibit the
above combination of aqueous solubility and relatively low glass transition
temperature
include the styrene maleic anhydride resins. In a still further aspect, the
styrene maleic
anhydride resins can be a copolymer of styrene maleic anhydride copolymer. In
a yet further
aspect, the styrene maleic anhydride is esterified styrene maleic anhydride.
Exemplary non-
limiting commercially available styrene maleic anhydride resins that are
suitable for use as a
removal-promoting additive are the Cray Valley SMA*3840 and SMA 1440F resins
from
Total.
[0026] In further aspects, the ink composition that makes up the ink
droplets also
comprises a carrier capable of solubilizing and dissolving at least a portion
of the ink
removal-promoting additive. The carrier can be monomer based or solvent based.
Exemplary monomers that can be used as a carrier include the class of acrylic
monomers. In
an exemplary aspect, suitable acrylic monomers can include 1,3-butylene glycol
diacrylate,
1,6-hexanediol diacrylate, 1,3-butylene glycol dimethacrylate, isobomyl
acrylate, or isodecyl
8
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
methacrylate, or a combination thereof. In some aspects, the acrylic monomer
is selected
from 1,3-butylene glycol diacrylate, 1,6-hexanediol diacrylate, and 1,3-
butylene glycol
dimethacrylate. As noted above, in aspects the carrier capable of solubilizing
the removal-
promoting additive can be solvent based. Suitable solvents can, in some
aspects, be aprotic
solvents or aqueous based solvents. Specific but non-limiting examples of
suitable solvent
carriers include N-methyl-2-pyrrolidone (NMP) and acetone.
[0027] The carrier component can be present in the ink compositions in an
amount
from about 1 to about 20 weight percent of the ink composition. This can
further include
specific exemplified amounts of 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14,
15, 16, 17, 18, and 19
weight percent. In a further aspect, the carrier can be present in an amount
any range of
amounts derived from any of the above exemplary values. For example, the
carrier can be
present in the ink compositions in an amount from about 5 to about 15 weight
percent of the
ink composition.
[0028] The base ink portion of the ink composition can be any
conventionally known
curable ink, such as conventional UV curable ink. The base ink can be present
in the ink
composition in an amount in the range of about 50 to about 85 weight percent
of the ink
composition, including exemplary weight percent values of 51, 52, 53, 54, 55,
56, 57, 58, 59,
60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78,
79, 80, 81, 82, 83, and
84 weight percent. In a further aspect, the base ink can be present in any
range of amounts
that is derived from any of the above exemplary values. For example, base ink
can be present
in the ink compositions in an amount from about 65 to about 85 weight percent
of the ink
composition.
[0029] In one exemplary aspect, disclosed herein is a ink composition,
comprising:
(a) from about 65 to about 85 weight percent of the base ink; (b) from about 5
to about 20
weight percent of the ink removal-promoting additive having a glass transition
temperature
less than about 100 C ; and (c) from about 5 to about 15 weight percent of the
carrier capable
of dissolving at least a portion of the ink removal-promoting additive.
According to this
exemplary aspect the base ink can be a base UV white ink, the ink removal
promoting
additive can be styrene maleic anhydride resin, and the carrier can be solvent
based and can
comprise N-methyl-2-pyrrolidone (NMP), or acetone, or other acceptable
solvent.
9
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
[0030] As described herein, disclosed are recyclable plastic articles
comprising
digitally-printed image printed directly on the curved external surface, the
image comprising
cured droplets of an ink composition as previously described herein. In a
further aspect, an
exemplary recyclable plastic article comprises a curved external surface with
a digitally-
printed image printed directly on the curved external surface, the image
comprising cured
droplets of ink, and the droplets of ink comprising a composition including an
ink removal-
promoting additive having a glass transition temperature less than about 100
C. In a still
further aspect, the digitally-printed image has an adhesion score of at least
6.0 and up through
9.0, and the removal-promoting additive is configured to cause the cured
droplets of ink to
separate or loosen from the curved external surface of the article when the
digitally-printed
image is exposed to the basic solution at an elevated temperature of at least
about 70 C.
[0031] In a further aspect, the article comprises a plastic container. In
a still further
aspect, the plastic container is a bottle. In a yet further aspect, the
plastic container is
comprised of one or more of the following materials: polyethylene,
polyethylene
terephthalate, high density polyethylene, and polypropylene.
[0032] In a further aspect, the removal-promoting additive present in the
ink
composition is configured to cause the cured droplets of ink to separate or
loosen from the
curved external surface of the article when the digitally-printed image is
exposed to an a
liquid based solution, such as an aqueous or water based solution, at a
predetermined elevated
temperature. For example, the cured droplets of ink can separate or loosen
from the curved
external surface of the article when the digitally-printed image is exposed to
a basic solution
having a pH of at least about 10, 11, 12, or 13 and an elevated temperature in
the range of
from about 70 to 90 C. In a further aspect the basic solution has a pH in the
range of from
about 12-13. According to aspects, after exposure to the basic solution at the
elevated
temperature for a sufficient period of time, at least about 90 weight percent
of the cured ink
droplets separate from the curved external surface of the article. In a yet
further aspect, the
digitally-printed image includes a base coat including a plurality of cured
base coat ink
droplets, and a secondary coat including a plurality of cured secondary coat
ink droplets, the
secondary coat being applied to at least a portion of the base coat.
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
[0033] By way of example, and without limitation, an embodiment of a
polyethylene
terephthalate (PET) container including UV-cured printed ink having a blended
removal-
promoting additive was subjected to ink-removal testing. The blended removal-
promoting
additive of the tested containers included acidic monomers of the type noted
above, i.e.,
acidic acrylate oligomer and/or monofunctional acid ester. The containers may
be subjected
to a bath solution comprising a pH level of at least 9.0, which can assist
label removal as
generally known in the art of plastic container recycling, with the solution
being heated to
approximately 85 C. Various samples were exposed to such conditions for 12
minutes, and
were evaluated on a scale of 0 to 5 as follows:
[0034] 0¨No improvement in removal relative to the control sample
[0035] 1 __ Minimal improvement observed, removal obtained in only some
areas
through abrasion with a metal object
[0036] 2 __ Some improvement observed, the entire film could be removed by
scratching with a metal object
[0037] 3¨Cured ink film was removable by scratching with a fingernail
[0038] 4¨Cured ink film was easily removable simply by wiping with a clean
wipe
[0039] 5 __ Cured ink film fell off during wash test.
[0040] The testing was intended to, among other things, identify solutions
that
provided a removal performance level of 4 or 5. However, a level of at least 4
is not
necessarily a requirement, and for some applications a lower removal level may
also be
acceptable. The results of the testing indicated that the inclusion of the
removal-promoting
(acidic) additives blended into the ink composition provided a significant
improvement in the
removal of the cured ink from the plastic (PET) material. While both forms of
acidic
additives exhibited an improvement promoting ink removal, with the instant
test
configuration, a somewhat lesser compositional percentage of the acidic
acrylate oligorner, as
opposed to the monofunctional acid ester (10% versus 15%, respectively), was
used to obtain
at least a level 4 removal level.
11
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
[0041] In embodiments, the ink composition may optionally include a
hydrophobic
component. That is, the ink composition may, for example, include hydrophilic
and
hydrophobic components. Hydrophobic components may include hydrophobic (water-
hating)
monomers. The hydrophobic properties generally relate to polarity. A number of
hydrophobic
monomers are characterized as having an organic backbone structure composed
primarily of
hydrogen and carbon; and thus, such monomers tend to be non-polar and offer
resistance to
polar solvents (such as water and alcohols), as well as to acids and bases.
For such dual-
component embodiments, the ink composition should strike a balance between the
hydrophilic and hydrophobic components (e.g., hydrophilic monomers and
hydrophobic
monomers). The hydrophobic monomers may help keep the ink drops adhered to the
article
substrate, and the hydrophilic monomers may help soften (and even separate)
when the image
is exposed to a recycling process/bath (e.g., agitated water at an elevated
temperature).
[0042] For an embodiment of a container with an image printed thereon, at
least a
first base coat 30 (which may include a hydrophilic component) may be applied
to a surface
of a container at a first time ti and at a first temperature Ti. The
temperature Ti will be in the
range of temperatures that are appropriate for application of the associated
base coat. In an
embodiment, the base coat 30 may be curable (e.g., ultraviolet (UV) curable),
and further
may be cured prior to an application of a secondary coat.
[0043] For some embodiments, a secondary coat 40 may be comprised of a
plurality
of secondary coat ink droplets 42 that are distributed on at least a portion
of the base coat 30.
Further, if desired, additional layers of "secondary" coats (e.g., a tertiary
layer, etc.) may also
be applied on the secondary coat 40. With embodiments of the disclosure, the
secondary coat
ink droplets 42 may also be comprised of an ink composition including a
hydrophilic
component.
[0044] A plurality of secondary coat ink droplets 42 may collectively form
a part of
an application pattern which, in turn, may form all or a portion of an image.
Furthermore, as
generally illustrated in FIG. 1, portions of one or more adjacent secondary
coat ink droplets
42 may overlap or intermix with each other. The secondary coat 40, and the
constituent
secondary coat ink droplets 42, may comprise various known colors, including
without
limitation, primary printing colors such as cyan, magenta, and yellow.
Further, controlling the
12
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
overlapping of or combinations of certain colors in certain areas can provide
additional
"process" colors. Additionally, the secondary coat ink droplets 42 may be
curable. For
example, UV curable secondary coat ink droplets may comprise all or a portion
of the
intended image. Where curing is accomplished by radiation, the ink composition
may include
a photo initiator. It is additionally noted that cured ink on a container
surface may be
primarily held by two bonds¨i.e., a polar bond between the ink polymer and the
plastic, and
a mechanical bond due to the plastic surface having an uneven surface at a
microscopic level
(e.g., microstructures). For a number of embodiments of the present
disclosure, it is best to
attack both bonds. For some applications, a hydrophilic component may work
better on
mechanical bonds, while an acidic component may work better on polar bonds.
Depending
upon the application, the secondary coat ink droplets can vary in diameter,
which can range,
for instance, from about 10 microns to about 200 microns. The secondary coat
40 may be
applied to a surface 10 of a container at a second time t2 and at a second
temperature T2,
wherein the second temperature T2 at which the secondary coat 40 is applied is
typically less
than the first temperature T1 at which the base coat 30 is applied.
[0045] In embodiments of the invention, the time between application of a
base coat
and the application of a secondary coat (e.g., t2 minus ti) may be reduced
for example, to as
little as ten seconds or less. For some embodiments, the application time
differential will be
within two to six seconds. Moreover, in embodiments, the application
temperature
differential between the temperature at which the base coat 30 is applied and
the temperature
at which a secondary coat 40 is applied to a portion of the base coat 30,
i.e., T1 minus T2, may
be controlled to be equal to or less than about 10 F. For some embodiments,
the application
temperature differential will be within about 5 F. to about 10 F. Moreover,
for some
applications, it may be desirable to modify the temperatures associated with
the application of
the base coat 30 and the secondary coat 40 so that the respective application
temperatures are
closer together _______________________________________________________ i.e.,
so that the temperature differential between the applied coats is reduced
or minimized. This can be accomplished, for instance, by (a)
lowering/decreasing T1, (b)
raising/increasing T2, or (c) a combination of (a) and (b). Such
aforementioned time and/or
temperature control with respect to the base coat and secondary coat can
provide for better
adhesion of the resulting printed image with respect to the article.
13
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
[0046] It is noted that in addition to time and temperature, irradiance is
a factor that
can also affect the effective cure rate for a printed image. That is, with
certain times (e.g., ti
and t2) and temperatures (e.g., T1 and T2), there may be an associated
Irradiance¨i.e., ci and
62. For example, in embodiments a base coat may be cured at irradiance Ci, and
an associated
secondary coat may be cured at irradiance E2. Further, in embodiments, the
effective cure
rate¨which may be based on a combination of time, temperature, and
irradiance¨the
irradiance may generally be provided by the following equation:
Irradiance (E)=(d0/dA)
where, 41)=irradiant flux (measured in watts), and A¨area (cm 2)
[0047] For example, without limitation, the range of irradiance for some
embodiments will be between about 0.1 watts/cm2and about 10.0 watts/cm2.
[0048] For some applications, such as where a curable ink (e.g., a UV-
curable or
radiation-curable ink) is used, the relevant coat or ink may be cured after
each respective
print station. For example, without limitation, an embodiment of a process
may, at least in
part, comprise: application of base coat; cure step; application of secondary
coat; and cure
step. Alternatively, also by way of example and without limitation, the
process may, at least
in part, comprise: application of base coat; cure step; application of base
coat; cure step;
application of secondary coat, and cure step.
[0049] Moreover, for embodiments of the invention, it can be desirable for
the
production/subsequent handling rate of containers/bottles to match or
substantially match the
flow/processing rates of the associated printing machine(s).
[0050] Further, it has been found that the quality of printed images may
be, at least in
part, controlled and/or improved through one or more of the following
techniques:
[0051] (a) selection and/or calibration of ink sets;
[0052] (b) control of substrate (i.e., container surface) temperature;
and/or
[0053] (c) timing control.
14
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
[0054] With respect to the selection and/or calibration of ink sets, this
is
accomplished, at least in part, by the selection and/or calibration of the
inks comprising the
base and secondary coats. It has been discovered that the inks used can be
selected to provide
desired time and/or temperature characteristics, including relative to one
another in
combination. For example, selection of certain inks having given viscosities
can exhibit or
provide certain desired temperature related effects.
[0055] With regard to the control of the substrate (i.e., container
surface) temperature,
the temperature of a relevant portion of a sidewall (or other portion of a
container) can be
treated or controlled to some measure. For example a given portion of the
container can be
pre-treated. Such pre-treatment can be facilitated using various known
techniques that may
include, without limitation, flame, corona, and plasma treatment. However, the
invention is
not limited to those specific pre-treatment techniques.
[0056] With respect to timing control, the time associate with the
movement of
containers, for instance through a production machine, as well as the timing
of the
applications of the base coat and/or secondary coat, can be controlled. It can
be desirable for
the production/subsequent handling rate of containers/bottles to match or
substantially match
the flow/processing rates of the associated printing machine(s).
[0057] The present disclosure may also include a system for assessing or
evaluating
the "acceptability," such as the commercial acceptability, of a container
having a printed
image ____________________________________________________________ such as a
digitally printed label. That is, for embodiments of the invention, the
system for assessing or evaluating can provide an "adhesion score." FIG. 2
generally
represents a quality review table/matrix that can be used to assess or
evaluate the
acceptability of a printed image on a container. As generally shown, the Y-
axis may involve
numbers associated with an overall pass-or-fail score. In the illustrated
embodiment, numbers
1 through 5 indicate that the containers are not acceptable, while numbers 6
through 9
indicate that the associated containers are acceptable. It is important to
note that while a score
of at least a 6 will "pass" as acceptable with respect to the instant
table/matrix, the invention
is not limited to the specific table/matrix shown and, altematively, more
scores could be
provided for and/or the passing score could be raised or lowered as desired or
necessary. A
plurality of tests _______________________________________________ which may
include various standard tests, including those previously
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
noted¨are represented in the columns provided on the X-axis. For example,
without
limitation, Test I may include a "Sutherland Rub Test," Test 2 may comprise a
"3M #610
Tape Test," Test 3 may include a "Simulated Ship Test," and Test 4 may
comprise a "3M
#810 Tape Test." As generally shown in the table, various pass-or-fail
designations may be
represented on the table in connection with each noted Test. With respect to
several of the
aforementioned "standard" tests, the tests may be modified as appropriate for
use in
connection with a printed image as opposed to a traditionally applied label.
For instance, with
various "tape" tests, which may follow the ASTM D 3359-08 standards, the tests
associated
with the table/matrix may or may not involve the cutting of the image portion
with a cutting
tool prior to applying a pressure sensitive tape. That is, in an embodiment,
"Test 2" may
involve a "modified" 3M #610 Tape Test in so far as the portion of the image
portion of a
container that is subjected to testing may not be cross-cut or otherwise
separated from the
container. Moreover, with the table set forth in FIG. 2, an indication of a
"pass," with respect
to tape tests directly practicing the modified ASTM standard (i.e., the test
does not involve
cross-cutting/separation) would generally be represented by any removed
portions being no
larger than 2.0 mm2. With respect to tape tests directly practicing the ASTM
standard, an
indication of "pass" would generally be a classification "4B" or "5B" (under
the ASTM FIG.
I Classification of Adhesion Test Results), or would involve less than 5% of
the printed area
removed.
100581 In an embodiment, it is desirable to provide a container with a
printed image
(e.g., digitally printed image) that, at a minimum, passes a modified 3M #610
tape test and is
nonetheless "recyclable." A digital image that is printed on a container is
considered to be
"recyclable" if it would achieve less than a "4B" classification (i.e., 5% of
more of the area is
removed) employing an ASTM D 3359 standard #810 tape test. A container with a
digital
image that passes Test 2 (modified 3M #610 Tape Test) and Test 3 (Simulated
Ship Test), yet
fails Test 4 (3M #810 Tape Test), would achieve an adhesion score of either
6.0 or 7Ø Such
a container with a printed image having an adhesion score of 6.0 or 7.0 is
commercially
suitable for shipment (i.e., passing a Simulated Ship Test) while providing an
adhesion
associated with the printed image that is sufficient for normal/intended use
but is favorably
separable for subsequent recycling. Stated differently, the adhesion
associated with the
16
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
configured digital image is sufficiently strong for intended use but does not
impede
separation during recycling.
[0059] With respect to such a table/matrix, each test may be conducted on
an
adequate (e.g., statistically significant or representative) sampling of
containers. After all
tests are completed, results may be tabulated and entered into the
table/matrix, to provide an
"adhesion" score. The associated score outcomes can then be correlated.
[0060] Among other things, the teaching of the present disclosure can
provide for
improved recyclability. Recycling inks printed on various articles in an
effective manner can
provide a number of cost and efficiency benefits, as well as providing
benefits to the
environment. For example, without limitation, containers with digitally
printed images
(which may be formed by cured UV or radiation curable ink) that are comprised,
at least in
part, of an ink composition including a hydrophilic and/or an acidic component
can be
conveniently removed in connection with conventional plastic recycling
processes. Industry
standard recycling process of plastic containers conventionally include
grinding containers
into granulated plastic flakes, subjecting these flakes to a high-heat caustic
wash process,
drying the cleaned flakes, sorting, and extruding into resin pellets for
resale. With
embodiments that embody aspects of the disclosed teachings, digital image on
the container
may remain with the resin flakes after the grinding process, the digital image
will be
substantially separated from the resin flakes during the high-heat caustic
wash process, which
may be agitated, and thereby not contaminating the clean resin flakes to be
formed into resin
pellets.
[0061] With separation techniques, there are at least four methodologies
that can be
Employed¨alone or in various combinations¨to attack polar and/or mechanical
bonds to
promote the removal of the ink from the article. The techniques include those
using: (1) water
or liquid-based solutions (e.g., for additives with hydrophilic components or
having the
desired Tg and acid numbers as described herein); (2) caustic components
(e.g., for additives
with acidic components or the desired combination of Tg and acid number as
described
herein)¨i.e., chemical reactions may be used to release polar bonds; (3) heat
or temperature;
and/or (4) mechanical force (e.g., high pressure spray (psi)).; or any
combinations thereof.
17
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
[0062] The disclosure envisions a number of recycling processes that may
be used to
remove the ink from the article. For example, one embodiment of a method for
recycling
plastic containers comprises: providing a plastic container having a digital
image, the ink
composition including a removal-promoting additive (e.g., a hydrophilic or
acidic
component); and exposing the digital image to a liquid-based solution¨e.g.,
water with or
without a caustic component)¨at an elevated temperature; optionally agitating
the solution.
Before or after the exposure, the container may be subject to a grinding
operation. Similar
embodiments may be said to be along the lines of including dry grind,
elutriate, wash, dry,
and elutriate.
[0063] Another process, which may be more similar to conventional
industrial
recycling and is commercially available (for example, from SOREMA (Italy)),
may be said to
involve a single wash, wet grinding, centrifuge, sorting, and float
tank/separation. A "single
wash" may, for instance, be employed by having bottles conveyed (e.g. by a set
screw or
multi-screw system) through a high-pressure wash (e.g., a high-temperature
caustic wash).
This can add a mechanical force component to assist with label removal.
Typically, the
bottles are whole that is, not ground prior to such a wash. Materials,
including polymerized
inks, may be washed away and fall into a collection or grating system below
the conveying
mechanism.
[0064] In a further aspect, the present disclosure provides a method for
removing
cured ink from a plastic container, the method comprising: (a) providing a
plastic container
having a curved external surface with a digital image printed directly on the
curved external
surface of the plastic container by a drop-on-demand ink jet printing process,
the image
comprising cured droplets of ink applied directly to the curved external
surface of the plastic
container and having an adhesion score of at least 6.0 and up through about
9.0, and the
droplets of ink comprising a composition including an ink removal-promoting
additive
having a glass transition temperature less than about 100 C; (b) exposing at
least a portion of
the digitally printed image of the plastic container to a basic solution at an
elevated
temperature of at least about 70 C; and (c) removing at least a portion of
the digitally printed
image from the curved external surface on which the image was printed.
18
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
[0065] In a further aspect, the plastic container is a bottle. In a still
further aspect, the
plastic container is comprised of one or more of the following materials:
polyethylene,
polyethylene terephthalate, high density polyethylene, and polypropylene.
[0066] In a further aspect, the ink removal-promoting additive comprises a
styrene
maleic anhydride copolymer. In a still further aspect, the ink removal-
promoting additive has
a glass transition temperature (Tg) in the range of from about 50 C to about
130 C. In a yet
further aspect, the ink removal-promoting additive has a glass transition
temperature (Tg) in
the range of from about 65 C to about 110 C. In a still further aspect, the
ink removal-
promoting additive has a glass transition temperature (Tg) in the range of
from about 55'C to
about 65'C. In an even further aspect, the ink removal-promoting additive has
an acid
number in the range of from about 150 mg KOH/ gm to about 205 mg KOH/ gm. In a
still
further aspect, the ink removal-promoting additive has an acid number in the
range of from
about 165 mg KOH/ gm to about 205 mg KOH/ gm.
[0067] In a further aspect, the basic solution is an aqueous solution
having a pH in the
range of about 12-13 and wherein the elevated temperature is in the range of
from about 70 to
90 C. In a still further aspect, the step of removing includes scratching or
wiping the ink
from the container after exposure of the image to the basic solution. In a yet
further aspect,
the ink is mechanically removed in the form of flakes or film.
[0068] The foregoing descriptions of specific embodiments of the present
invention
have been presented for purposes of illustration and description. They are not
intended to be
exhaustive or to limit the invention to the precise forms disclosed, and
various modifications
and variations are possible in light of the above teaching. The embodiments
were chosen and
described in order to explain the principles of the invention and its
practical application, to
thereby enable others skilled in the art to utilize the invention and various
embodiments with
various modifications as are suited to the particular use contemplated. It is
intended that the
scope of the invention be defined by the claims appended hereto and their
equivalents.
A. EXPERIMENTAL
[0069] The following examples are put forth so as to provide those of
ordinary skill in
the art with a complete disclosure and description of how the compounds,
compositions,
19
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
articles, devices and/or methods claimed herein are made and evaluated, and
are intended to
be purely exemplary and are not intended to limit the disclosure. Efforts have
been made to
ensure accuracy with respect to numbers (e.g., amounts, temperature, etc.),
but some errors
and deviations should be accounted for. Unless indicated otherwise, parts are
parts by weight,
temperature is in C or is at ambient temperature, and pressure is at or near
atmospheric.
[0070] In the following exemplary examples, various ink compositions
according to
the present disclosure were prepared and tested for use in direct printing on
an external
surface of a plastic article. The ink compositions can be prepared by first
dissolving the ink
removal-promoting additive in the carrier, and then adding the carrier
solution to a base ink.
The prepared ink composition can then be used in direct printing on the
external surface of
the plastic article.
[0071] Performance and recyclability of the various ink compositions on
plastic
articles were also evaluated. The ink compositions, including UV-curable ink
having a
removal-promoting additive and a carrier, were direct printed on the surface
of a polyethylene
terephthalate (PET), polyethylene (PE), and/or high density polyethylene
(HDPE) samples
and cured.
[0072] The cured ink of the sample was then subjected to ink-removal
testing, which
can include testing of one or more of: adhesion, scratch resistance and
solvent resistance
properties. The samples can be subjected to various bath solutions which can
assist label
removal as generally known in the art of plastic container recycling, for
example, a bath
solution comprising a pH level of at least 9.0 and with the solution being
heated to
approximately 75 C or 85 C. The testing was performed at time periods
including
immediately after treatment (imm) and at 1 hour (1hr) after treatment.
[0073] The materials shown in Table 1 were used to prepare the
compositions
described and evaluated herein.
CA 02997185 2018-02-28
WO 2017/040654 PCT/US2016/049690
Table 1.
Item Description Supplier
Luwax S Montanic acid wax BASF
Luwax V Polyether wax BASF
Joncryl 538A Acrylic emulsion BASF
Joncryl 611 Acrylic resin BASF
Joncryl 682 Acrylic resin BASF
Michem Flex R 1924 Water based print receptive primer Michelman, Inc
BYK111 Acidic copolymer additive Altana
CITRIC Acid Citric acid Sigma Aldrich
DURACOTE Cold End Coating Sun Chemical
Ceraflour 998 PTFE modified polyethylene wax Altana
SMA1440 styrene maleic anhydride copolymer Cray Valley USA, LLC
SMA3840 Styrene maleic anhydride copolymer Cray Valley USA, LLC
NMP N-methyl-2-pyrrolidone Sigma Aldrich
1. Exipi1.
100741 For the
following example, the removal-promoting additives of the tested
samples were waxes and resins. The carrier was acetone or N-methyl-2-
pyrrolidone (NMP)
(commercially available from Sigma Aldrich). The inventive ink formulations
and
performance data are provided in Tables 2-5 below. For the following data,
"pass" indicates
that no ink removal was observed, and "fail" indicates that significant and/or
complete ink
removal was observed. Any % value listed corresponds to the observed % ink
removal.
Table 2.
imm 1 hr 1 hr
ambient water soak
Formulation Caustic Test Score scratch 610 tape 810 tape scratch scratch 610
tape
Luwax S (A) NA fail fail * fail * fail fail fail
Luwax S (B) NA fail fail * fail * fail fail fail
Luwax V (A) NA fail fail * fail * fail fail
fail
Luwax V (B) NA fail fail * fail * fail fail
fail
Joncryl 538A (A) NA pass pass fail fail pass fail
21
CA 02997185 2018-02-28
WO 2017/040654 PCT/US2016/049690
imm 1 hr 1 hr
ambient water soak
Formulation Caustic Test Score scratch 610 tape 810 tape scratch scratch 610
tape
Joncryl 538A (B) NA pass pass fail fail pass 20% fail
Joncryl 611(A) NA fail pass 50% fail fail pass 40% fail
Joncryl 611 (B) NA fail pass 50% fail pass fail fail
Joncryl 682 (A) NA pass pass 70% fail fail fail fail
Joncryl 682 (B) NA fail pass 70% fail fail pass fail
Michem (A) NA pass pass pass fail pass pass
Michem (B) NA pass pass pass fail pass pass
Control 0 fail fail fail fail fail fail
Luwax S (A) NA fail fail fail fail fail 50% fail
Luwax V (A) 3 fail fail fail fail fail fail
Luwax V (B) 3 slight 50% fail fail fail fail
fail
Joncryl 538A (A) 1 slight pass Fail fail pass fail
Joncryl 538A (B) NA slight pass pass fail pass fail
Joncryl 611 (A) 1 slight pass pass fail pass fail
Joncryl 611 (B) 1 fail pass pass fail 50% fail fail
Joncryl 682 (A) 1 slight pass fail fail fail fail
Joncryl 682 (B) 2 pass pass 50% fail fail pass fail
Michem (A) 0 pass pass pass fail pass pass
Michem (B) 0 pass pass pass fail pass pass
Control (A) 0 slight pass 50% fail pass pass fail
Control (B) 0 slight pass 50% fail fail 50% fail fail
*fail=partial fail due to inadequate tape adhesion to surface; ink easily
removed
Table 2. (cont.)
imm 1 hr 1 hr imm 1 hr imm imm 1 hr imm
ice water soak 75C water soak 75C Caustic soak
Formulation scratch scratch 610tape scratch scratch 610tape scratch scratch
610 tape
Luwax S (A) fail fail fail fail fail part fail NA NA
NA
Luwax S (B) fail fail fail fail fail part fail NA NA
NA
Luwax V (A) fail fail fail fail fail part fail NA NA
NA
Luwax V (B) fail fail fail fail fail part fail NA NA
NA
Joncryl 538A pass pass 50% fail fail fail NA NA NA
(A) fail
Joncryl 538A pass pass 50% fail pass fail NA NA NA
(B) fail ,
Joncryl 611 (A) fail fail 30% fail fail 60% NA NA
NA
fail fail
22
CA 02997185 2018-02-28
WO 2017/040654 PCT/US2016/049690
imm 1 hr 1 hr imm 1 hr
imm imm 1 hr imm
ice water soak 75C water soak 75C Caustic soak
Formulation scratch scratch 610tape scratch scratch 610tape scratch scratch
610 tape
Joncryl 611 (B) fail fail 50% fail pass 40% NA NA
NA
fail fail
Joncryl 682 (A) pass pass pass pass fail fail NA NA NA
Joncryl 682 (B) pass pass 30% fail fail fail NA NA NA
fail
Michem (A) pass pass pass pass pass 30% 'NA NA 'NA
fail
Michem (B) pass pass pass pass pass pass NA NA NA
Control fail fail fail fail fail fail NA NA NA
Luwax S (A) fail fail fail fail fail fail NA NA NA
Luwax V (A) fail fail fail fail fail 20% fail fail
fail
fail
Luwax V (B) pass slight fail fail slight fail fail
fail fail
fail fail
Joncryl 538A fail pass 50% fail fail fail fail slight
fail
(A) fail
Joncryl 538A pass pass 50% fail fail fail NA NA NA
(B) fail
Joncryl 611(A) pass pass pass fail fail fail slight fail
fail
Joncryl 611(B) fail pass pass fail pass fail slight fail
fail
Joncryl 682 (A) pass slight 20% fail fail fail fail
slight fail
fail fail
Joncryl 682 (B) fail slight fail slight slight fail fail
slight fail
fail fail fail
Michem (A) pass pass pass slight pass 10% fail pass
pass
fail fail
Michem (B) pass pass pass pass pass pass fail pass
pass
Control (A) pass pass 50% pass pass fail slight slight
50%
fail
Control (B) pass pass -50% pass pass fail slight slight fail
fail
Table 3.
imm 1 hr 1 hr
ambient water soak
Formulation Caustic Test scratch 610 810 scratch scratch 610
Score tape tape tape
BYK111 + J 682 (A) 1 pass pass 10% fail fail fail
BYK111 + J 682 (B) 1 fail pass 50% fail fail fail
23
CA 02997185 2018-02-28
WO 2017/040654 PCT/US2016/049690
imm 1 hr 1 hr
ambient water soak
Formulation Caustic Test scratch 610 810
scratch scratch 610
Score tape tape tape
BYK111 + J 682 (C) 2 fail pass 20% fail fail fail
CITRIC + J 682 (A) 2 fail pass 100% fail fail
fail
CITRIC + J 682 (B) 1 fail pass 60% fail fail fail
CITRIC + J 682 (C) 1 fail fail 20% fail pass fail
BYKI 11 + J538A (A) 1 pass pass 20% fail pass fail
BYKI 11 + J538A (B) 2 pass pass 20% fail slight fail
BYKI 11 + J538A (C) 1 pass pass 100% fail slight fail
CITRIC + J538A (A) 0 pass pass 60% fail slight fail
CITRIC + J538A (B) 0 pass pass 20% fail fail fail
CITRIC + J538A (C) 1 pass pass pass moderate pass
fail
BYK111 + DURACOTE 1 slight pass pass fail fail
fail
(A)
BYK111 + DURACOTE 2 slight pass pass fail fail
fail
(B)
BYK1 11 + DURACOTE 1 slight pass pass fail fail
fail
(C)
CI _______________ IRIC + DURACOTE 0 slight pass 20% pass
pass fail
(A)
CITRIC + DURACOTE 0 slight pass pass slight pass
fail
(B)
CITRIC + DURACOTE 0 slight pass pass moderate slight
fail
(C)
BYK111 + MICHEM (A) 1 slight pass 50% fail pass
pass
BYK111 + MICHEM (B) 1 pass pass pass fail pass pass
BYKI II + MICHEM (C) 1 slight pass pass fail pass
20%
CONTROL (A) 0 slight pass pass slight pass fail
CONTROL (B) 0 slight pass pass slight slight fail
Table 3. (cont.)
imm 1 hr 1 hr imm 1 hr imm
ice water soak 75C Caustic soak
Formulation scratch scratch 610 tape scratch scratch 610 tape
BYK111 + J 682 (A) fail _fail pass fail fail fail
BYK111 + J 682 (B) fail _slight pass fail _fail fail
BYK111 + J 682 (C) fail _slight pass fail fail fail
CITRIC + J 682 (A) fail slight fail fail fail fail
24
CA 02997185 2018-02-28
WO 2017/040654 PCT/US2016/049690
imm 1 hr 1 hr imm 1 hr imm
ice water soak 75C Caustic soak
Formulation scratch
scratch 610 tape scratch scratch 610 tape
CITRIC + J 682 (B) fail slight pass fail fail fail
CITRIC + J 682 (C) fail fail pass fail fail fail
BYK111 + J538A (A) fail pass fail fail fail fail
BYK111 + J538A (B) fail pass fail fail fail fail
BYK111 + J538A (C) fail slight fail fail fail fail
CITRIC + J538A (A) fail fail fail fail slight fail
CITRIC + J538A (B) fail pass fail fail slight fail
CITRIC + J538A (C) fail pass pass .. fail .. fail .. fail
BYK111 + DURACOTE (A) fail fail pass fail fail fail
BYK111 + DURACOTE (B) fail fail fail fail fail fail
BYK111 + DURACOTE (C) fail fail 50% fail slight fail
CITRIC + DURACOTE (A) pass slight fail slight slight fail
CITRIC + DURACOTE (B) pass slight fail slight slight fail
CITRIC + DURACOTE (C) pass slight fail slight slight fail
BYK111 + MICHEM (A) pass pass pass pass slight 50%
BYK111 + MICHEM (B) pass slight pass pass slight 50%
BYK111 + MICHEM (C) pass pass pass slight slight fail
CONTROL (A) pass pass fail pass slight fail
CONTROL (B) pass pass pass slight slight fail
Table 4.
imm 1 hr 1 hr
ambient water soak
Formulation Caustic Test scratch 610 810 scratch scratch 610
Score tape tape tape
Ceraflour 998 (A) 0 pass pass pass pass pass pass
Ceraflour 998 (B) 1 pass pass pass pass pass pass
SMA1440 (50% solids) 50% fail
4 (A) pass pass pass pass
SMA1440 (50% solids) 50% 50%
5 (B) pass pass pass pass
SMA1440 (50% solids) fail 50%
4 (C) pass pass pass pass
SMA1440, 50% (25% pass
4 solids) (A) pass pass pass pass .. pass
SMA1440, 50% (25% pass 50%
3 pass pass pass pass
solids) (B)
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
imm 1 hr 1 hr
ambient water soak
Formulation Caustic Test scratch 610 810
scratch scratch 610
Score tape tape tape
SMA1440, 50% (25% pass 50%
solids) (C) 4 pass pass pass pass
SMA3840 (A) 2 pass pass pass pass pass pass
SMA3840 (B) 2 pass pass pass pass pass pass
SMA3840 (C) 1 pass pass pass pass pass pass
J682, 50% (A) 0 pass pass pass pass pass pass
J682, 50% (B) 1 pass pass pass fail fail fail
SMA1440/1% 998 (A) 4 pass pass pass pass pass pass
SMA1440/1% 998 (B) 4 pass pass pass pass pass pass
SMA1440/1% 998 (C) 4 pass pass pass fail pass pass
Control (A) 0 pass pass slight pass pass pass
Control (B) 0 pass pass pass pass pass pass
Table 4. (cont.)
imm 1 hr 1 hr imm 1 hr 1 hr
ice water soak 75C Caustic Soak
Formulation scratch
scratch 610 tape scratch scratch 610 tape
Ceraflour 998 (A) pass pass pass pass pass Pass
Ceraflour 998 (B) pass pass pass pass pass Pass
SMA1440 (50% solids) (A) pass pass 10% fail fail fail
SMA1440 (50% solids) (B) pass pass fail fail fail fail
SMA1440 (50% solids) (C) pass pass .. fail .. fail .. fail .. fail
SMA1440, 50% (25% solids) (A) pass pass pass fail fail fail
SMA1440, 50% (25% solids) (B) pass fail pass fail fail fail
SMA1440, 50% (25% solids) (C) pass pass pass fail NI fail
SMA3840 (A) pass pass pass pass pass 50%
SMA3840 (B) pass pass pass pass pass 20%
SMA3840 (C) pass pass pass pass pass Pass
J682, 50% (A) pass pass pass pass pass Pass
J682, 50% (B) fail pass pass fail fail fail
26
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
imm 1 hr 1 hr imm 1 hr 1 hr
ice water soak 75C Caustic Soak
Formulation scratch scratch 610 tape scratch scratch 610 tape
SMA1440/1% 998 (A) pass pass pass fail fail fail
SMA1440/1% 998 (B) pass pass pass fail fail fail
SMA1440/1% 998 (C) pass pass fail fail fail fail
Control (A) pass pass pass pass pass pass
Control (B) pass pass pass pass pass pass
Table 5.
imm 1 hr
1 hr
ambient water soak
Formulation Caustic scratch 610 810 scratch scratch 610
Test Score tape tape tape
Control A 0 pass fail fail pass pass fail
Control B 0 pass fail fail pass pass fail
Control C 0 pass fail fail pass pass fail
Control D 0 pass pass fail pass pass fail
Control E 0 pass pass pass pass pass fail
Control F 0 pass pass fail pass pass pass
SMA1440 (in acetone) as primer
A 2 pass pass pass pass pass pass
SMA1440 (in acetone) as primer
3 B pass pass pass pass pass pass
SMA1440 (in acetone) as primer
C 3 pass pass pass pass pass pass
SMA1440 (in acetone) as primer
4 D pass pass pass pass pass pass
SMA1440 (in acetone) as primer E 4 pass pass pass pass pass
pass
SMA1440 (in acetone) as primer F 3 pass pass pass pass pass
pass
SMA1440 (in acetone) as primer
A pass pass pass pass pass pass
SMA1440 (in acetone) as primer
5 B pass pass pass pass pass pass
SMA1440 (in acetone) as primer
4 C pass pass pass pass pass fail
SMA1440 (in acetone) as primer
4 D pass pass pass pass pass pass
27
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
imm 1 hr
1 hr
ambient water soak
Formulation Caustic scratch 610 810 scratch scratch 610
Test Score tape tape tape
SMA1440 (in acetone) as primer E 5 pass pass pass pass
pass pass
SMA1440 (in acetone) as primer F 5 pass pass pass pass
pass fail
SMA1440 (&10% acetone) in ink
pass pass pass pass pass pass
A
SMA1440 (&10% acetone) in ink
4 pass pass pass pass pass pass
SMA1440 (&10% acetone) in ink
3 pass pass fail pass pass pass
SMA1440 (&10% acetone) in ink
4 pass pass pass pass pass fail
SMA1440 (&10% acetone) in ink
4 pass fail pass pass pass fail
SMA1440 (&10% acetone) in ink
5 pass pass pass pass pass fail
SMA1440 (& 3% acetone) in ink
4 pass pass fail pass pass pass
A
SMA1440 (& 3% acetone) in ink
3 pass pass pass pass pass fail
SMA1440 (& 3% acetone) in ink
4 pass pass fail pass pass pass
SMA1440 (& 3% acetone) in ink
4 pass pass pass pass pass pass
SMA1440 (& 3% acetone) in ink
4 pass pass pass pass pass pass
SMA1440 (& 3% acetone) in ink
3 pass pass pass pass pass pass
Table 5. (cont.)
imm 1 hr 1 hr imm 1 hr 1 hr
ice water soak 75C Caustic Soak
Formulation scratch scratch 610 tape scratch scratch 610
tape
Control A pass pass pass fail -- fail -- fail
Control B pass pass pass fail -- fail -- fail
Control C pass pass fail fail -- fail -- fail
Control D pass pass fail fail -- fail -- fail
Control E pass pass pass fail -- fail -- fail
Control F pass pass pass fail -- fail -- fail
28
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
imm 1 hr 1 hr imm 1 hr 1 hr
ice water soak 75C Caustic Soak
Formulation scratch
scratch 610 tape scratch scratch 610 tape
SMA1440 (in acetone) as primer A pass pass pass fail fail fail
SMA1440 (in acetone) as primer B pass pass pass fail fail fail
SMA1440 (in acetone) as primer C pass pass pass fail fail fail
SMA1440 (in acetone) as primer D pass pass pass fail fail fail
SMA1440 (in acetone) as primer E pass pass pass fail fail fail
SMA1440 (in acetone) as primer F pass pass pass fail fail fail
SMA1440 (in acetone) as primer A pass pass pass NA* NA* NA*
SMA1440 (in acetone) as primer B pass pass pass NA* NA* NA*
SMA1440 (in acetone) as primer C pass pass pass fail fail fail
SMA1440 (in acetone) as primer D pass pass pass fail fail fail
SMA1440 (in acetone) as primer E pass pass pass NA* NA* NA*
SMA1440 (in acetone) as primer F pass pass pass NA* NA* NA*
SMA1440 (&10% acetone) in ink A pass pass pass NA* NA* NA*
SMA1440 (&10% acetone) in ink B pass pass pass fail fail fail
SMA1440 (&10% acetone) in ink C pass pass pass fail fail fail
SMA1440 (&10% acetone) in ink D pass pass pass fail fail fail
SMA1440 (&10% acetone) in ink E pass pass pass fail fail fail
SMA1440 (&10% acetone) in ink F pass pass pass NA* NA* NA*
SMA1440 (& 3% acetone) in ink A pass pass pass fail fail fail
SMA1440 (& 3% acetone) in ink B pass pass pass fail fail fail
SMA1440 (& 3% acetone) in ink C pass pass pass fail fail fail
SMA1440 (& 3% acetone) in ink D pass pass pass fail fail fail
SMA1440 (& 3% acetone) in ink E pass pass pass fail fail fail
SMA1440 (& 3% acetone) in ink F pass pass pass fail fail fail
NA*=ink removal too great to test (all ink removed)
100751 As the
data show, the SMA3840 and SMA1440F ink formulation samples
exhibited improved performance. The SMA3840 ink formulation showed some signal
of
improvement, which indicates an improvement in ink removal when compared to
control.
The SMA1440F ink formulation demonstrated no significant signal during normal
use ("in
use") tests, but strong removal during caustic wash testing. Testing was
repeated several
times on PET, EPET, and PE, all showing good removal in the caustic test.
29
CA 02997185 2018-02-28
WO 2017/040654
PCT/US2016/049690
2. EXAMPLE 2.
100761 For this example, ink compositions, including white UV-curable ink
having a
removal-promoting additive and a carrier, were direct printed on the surface
of a polyethylene
terephthalate (PET) and high density polyethylene (HDPE) samples and cured.
The removal-
promoting additive of the tested samples was styrene maleic anhydride
copolymer
(SMA1440F, commercially available from Cray Valley USA, LLC), and the carrier
was N-
methy1-2-pyrrolidone (NMP, commercially available from Sigma Aldrich). The ink
formulations and performance data are provided in Table 6 below.
Table 6.
Ink Formulation Test 1 Test 2 Test 3
Ink 1 ¨ commercially available UV inkjet
white ink ¨50% ¨30% ¨55%
Ink 2 - Ink 1 + removal promoting additive &
¨85% ¨95% ¨98%
carrier
% ink removed/clean flakes (based on visual analysis)
[0077] As the data show, near 100% removal of the inventive ink
compositions were
observed compared with only 50-60% removal of the standard ink controls. On
HDPE
samples, the inventive ink compositions exhibited greater than 50% ink removal
compared to
only approximately 10% ink removal for the standard ink controls. The
inventive ink
compositions results satisfy the current recycle guidelines published by The
Association of
Postconsumer Plastic Recyclers.
[0078] It will be apparent to those skilled in the art that various
modifications and
variations can be made in the present invention without departing from the
scope or spirit of
the invention. Other embodiments of the invention will be apparent to those
skilled in the art
from consideration of the specification and practice of the invention
disclosed herein. It is
intended that the specification and examples be considered as exemplary only,
with a true
scope and spirit of the invention being indicated by the following claims.