Note: Descriptions are shown in the official language in which they were submitted.
CA 02999102 2018-03-19
BHC 153069 FC-Text
Individual blister pack for optimized stacking
The present invention relates to individual blister packs, methods for
production thereof, stack-like
arrangements containing individual blister packs of this type, and boxes
containing stack-like
arrangements of individual blister packs.
Blister packs have long been known. Medicinal product portions, such as
tablets and capsules, are
preferably supplied in blister packaging as primary packaging. In a blister
pack, the tablets or capsules
are present in an arrangement of individual indentations (bubbles). The
indentations are usually
sealed by an aluminium foil. The medicinal product portions can be removed
individually and are
protected against dirt and air moisture. A further advantage of medicinal
product portions in blister
packs lies in the easy identification of the remaining number of available
medicinal product portions.
A typical blister pack is illustrated in Figure la of the laid-open
application GB2184086A: 15 individual
medicinal product portions are provided in a planar arrangement of
approximately 6-8 cm x 4-6 cm.
Blister packs are typically introduced into yet a further packaging before
being passed on to the
patient. This secondary packaging is typically a folding box which, besides a
number of approximately
1 to 10 blister packs, usually also contains a package leaflet containing use
and safety information.
The secondary packaging prevents a shifting of blister packs stacked on top of
one another. It is
required essentially only for transport and is accrued by the user as waste.
On account of the bulky
and voluminous bubble structure of conventional blister packs, the secondary
packaging is also
comparatively voluminous.
Different approaches have been followed in order to simplify and make smaller
the secondary
packaging.
DE 10044118 Al discloses a blister pack, which, besides the indentations for
receiving medicinal
product portions, additionally has centring nubs, which extend beyond the
underside of the
indentations. The blister pack additionally has supporting nubs, which
likewise protrude beyond the
underside of the indentations. Perforations are disposed in the cover, in the
region of each centring
nub, such that, when the blister packs are stacked in alignment, each centring
nub of a blister strip
rests against a perforation of the blister strip disposed beneath. If a stack
of blister packs of this type
is pressed together, the centring nubs destroy the perforations disposed
beneath, and the centring
nubs protrude into the resultant openings. The blister packs are thus centred
relative to one another
- 1 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
V
in the horizontal plane in an interlocking manner. Here, the supporting nubs
rest against the cover
film of the blister strip disposed beneath, such that the blister strips are
supported against one
another. Since the blister packs stacked above one another cannot shift
relative to one another, it is
possible to dispense with a complex surrounding packaging, or such packaging
can be formed in a
very simple manner. However, it is disadvantageous that, as a result of the
described stacking, there
is no volume reduction of the surrounding packaging compared with a stacking
of similar blister
strips without centring nubs. Furthermore, an individual blister strip cannot
be easily removed from
the surrounding packaging; it must first be removed from the stack, which is
possible only in one
direction.
DE 19680564 Ti discloses a foldable blister strip. It has two parallel rows of
bubbles, which are offset
relative to one another in such a way that the bubbles in one row enter the
gaps between the
bubbles in the other row when the blister strip is folded. The folded blister
strip thus takes up less
space compared with when the rows of bubbles are stacked above one another in
the conventional
manner. However, for use, the folded blister strip must first be removed from
the surrounding
packaging and unfolded, which is comparatively laborious. A similar foldable
blister pack is also
disclosed in DE 29780456 U1.
The previously described blister packs contain more than one medicinal product
portion. However, a
patient generally takes just an individual medicinal product portion at the
time at which said
medicinal product portion is to be taken. If, for example, the patient must
take a medicinal product
portion every day, the patient will gradually empty the blister pack by
removing an individual
medicinal product portion each day. A patient often carries with them a
blister strip containing a
plurality of medicinal product portions, for example in order to have ready a
medicinal product
portion as required and/or in order to be reminded to take said medicinal
product portion regularly.
Generally, more medicinal product portions than are actually used are thus
generally carried with the
patient. There is thus a risk that the blister strip will be lost or damaged
and individual medicinal
product portions will be lost, will become broken, will become wet, or will
become otherwise
unusable.
Similar problems can occur in hospitals, care homes or comparable
establishments in which
medicinal product portions are provided to the patients/residents by the care
staff. In an
establishment of this type, the member of care staff often removes individual
medicinal product
portions from the blister packs, which contain a plurality of medicinal
product portions, and gives the
individual product portions to the patients in small containers when said
portions are to be taken. It
- 2 -
CA 02999102 2018-03-19
a
BHC 153069 FC-Text
is conceivable that mix-ups can occur here. It is also conceivable that a
container of this type
accidentally tips over and the content falls to the ground. It is conceivable
that the spilled medicinal
product portions therefore can no longer be used for hygiene reasons. However,
it is also
conceivable that the spilled medicinal product portions can no longer be
assigned to the patients. In
some establishments it is also usual for medicinal product portions to be
removed from blister packs
and stored in day or week pots/boxes, where they are exposed to light and
moisture (in particular
when the rations are stored in a bathroom). In this respect, it would be
advantageous if the
medicinal product portions remained packaged in a blister pack until just
before their removal by the
patient, said blister pack containing the necessary labelling in order to
clearly identify the content.
In order to solve the specified problems, blister strips are offered which
have perforations so as to be
able to separate off individual bubbles containing medicinal product portions
(see, for example,
EP 679587 Al). It is thus possible for the patient to carry with them only the
amount of medicinal
product portions actually used. It is also possible to give packaged medicinal
product portions to a
patient in a hospital or a resident in a care home. However, these blister
units generally do not have
the complete information concerning the content, since the labelling on the
rear side of a blister strip
is usually destroyed by separating off individual blister units. In addition,
when separating off
individual blister units, there is the risk that blister strips and/or blister
units will be damaged; by way
of example, the cover film can tear. By separating off individual units, sharp
edges and corners are
additionally often produced, on which a person can injure themselves, or as a
result of which damage
to materials can be caused, for example when blister units or remainders of
blister strips without
surrounding packaging are stored in pockets or coats.
In addition, blister strips having a fixed number of a plurality of medicinal
product portions limit
flexibility. By way of example, if a blister strip contains 10 medicinal
product portions, only packs
containing this number or a multiple thereof (20, 30, etc.) can be offered.
The problem forming the basis of the present invention is therefore that of
providing medicinal
product portions in packaged form that allows a greater degree of flexibility
and reliability when it
comes to provision and handling, and at the same time is space-saving and
resource-saving.
This problem is solved in accordance with the invention by the subjects of
independent Claims 1, 12,
20 and 21. Preferred embodiments can be found in the dependent claims.
- 3 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
A first subject of the invention is a stack-like arrangement of N individual
blister packs, where N is an
integer greater than 1,
each single individual blister pack comprising
- a planar main body with a bubble,
- one or more medicinal product portions in the bubble, and
- a cover film, which closes the bubble,
the height hs of the stack-like arrangement being less than N-times the sum of
the height hB of a
bubble, the thickness dG of a planar main body and the thickness d0 of a cover
film:
hs< N = (h8+ dG+ dD).
A medicinal product portion is understood to mean a solid administration form
of a medicinal
product that can be taken by a patient as an individual unit. Examples of
medicinal product portions
are tablets, pills, lozenges and capsules.
An individual blister pack is understood to mean a combination of an
individual blister packaging and
a medicinal product portion or a plurality of medicinal product portions. One
or more medicinal
product portions is/are packaged in the individual blister packaging and
together with this individual
blister packaging form an individual blister pack containing one or more
medicinal product portions,
or an individual blister for short. The term "individual" relates to the
number of bubbles. An
individual blister is therefore a unit having an individual bubble. Synonymous
terms that are used in
the prior art instead of the term bubble are cavity and indentation, for
example.
The individual blister packaging comprises a planar main body. The planar main
body usually consists
of a film layer. A further film layer ¨ referred to here as the cover film ¨
serves to seal the bubble.
A planar body is understood to mean a body that, in two of the three spatial
directions in a Cartesian
coordinate system, has a greater extent than in the third spatial direction.
If it is imagined that the
bubble is not there and if it is assumed that the main body does not have any
curvature, the extent in
the third spatial direction (z-direction, thickness of the film layer) is only
a fraction of the extent in
the two other spatial directions (x- and y-direction). The thickness of the
film layer dG (extent in the z-
direction) is preferably less than a tenth of the extent in the orthogonal
spatial directions extending
perpendicularly to the thickness (x- and y-direction), particularly preferably
less than a twentieth.
The planar main body preferably has no curvature or only a slight curvature.
In a preferred
embodiment the radius of curvature is at least 10 times the extent in the x-
and/or y-direction. In a
- 4 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
particularly preferred embodiment the radius of curvature is at least 20 times
the extent in the x-
and/or y-direction. In an even more particularly preferred embodiment the
radius of curvature is at
least 50 times the extent in the x- and/or y-direction.
It is clear to a person skilled in the art of plastics processing that the
planar main body always has a
certain curvature, even if the objective is to form the main body (with the
exception of the bubble) as
flat as possible. The introduction of the bubble into the main body, which is
usually implemented by
thermoforming, can cause stresses in the main body, which lead to a curvature.
Furthermore,
curvatures can be introduced into the main body by the mechanical processing,
such as printing,
cutting, gripping (for example during transport), etc. The application of the
cover film also constitutes
a mechanical loading, which can lead to a deformation. For the sake of
simplicity, an ideal, uncurved
main body is assumed in the present description. However, this is only so as
to be able to better
present the invention and is not to be understood as a limitation of the
invention.
The planar main body can assume a wide variety of forms. It can assume a
circular, oval, triangular,
quadrangular (for example rectangular or square), pentagonal, hexagonal and
further basic forms in
the xy plane, and the corners can be rounded in each case.
The planar main body preferably has a square, rectangular or angular basic
form in the xy plane,
however the corners can be rounded. The term "angular" is explained in greater
detail further below.
The corners of the planar main body are preferably rounded in order to avoid
injury to the individuals
handling the individual blisters and/or in order to avoid damage to materials
with which the
individual blisters come into contact.
The main body must at least be large enough for a bubble for receiving a
medicinal product portion
to be introduced and for the bubble to be closed by a cover film. A typical
size is, for example, an
extent in the x- and y-direction from 0.6 cm to 6 cm and in the z-direction
from 0.2 mm to 2 mm.
However, the main body can also be larger or smaller.
Information regarding the medicinal product situated in the bubble is
preferably applied to the cover
film. Information of this type can be, for example, the name of the active
substance, the name of the
manufacturer and/or distributor, a batch number, the expiry date, a dosage,
and other information.
Some information is requested by authorities monitoring a circulation of
medicinal products, and
- 5 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
. .
other information can be useful for the individuals encountering the packaged
medicinal product, for
example patients, doctors, care staff, transport and logistics companies,
packers, etc.
Information regarding the time at which the medicinal product is to be taken
is also useful. For
example, specific colourings can be used; for example a red colour for taking
the medicinal product in
the morning and a blue colour for taking the medicinal product in the
evening/at night is
conceivable.
It is also conceivable for information to be applied to the cover film, which
information provides
access to further information, for example an optical code which provides
information via the
Internet regarding risks and side effects.
The provision of medicinal product portions and individual blisters thus
provides a high flexibility
with regard to individual design possibilities. The individualization of the
individual blisters, for
example by an individual optical code, is also conceivable.
Information can additionally or alternatively also be applied to the main body
on the belly side. The
belly side is the side on which the bubble protrudes from the main body and
the side arranged
opposite the side on which the cover film is applied.
The main body therefore has a minimum size, which makes it possible to apply
at least the legally
required information to the main body and/or the cover film.
A particular minimum size additionally offers the advantages that the
individual blister will not be so
quickly lost, and that it can be more easily handled and opened, in particular
for older individuals.
Of course, information can also be introduced for example as an engraving into
the main body
and/or the cover film.
The bubble is formed in the main body. It usually serves to receive an
individual medicinal product
portion. However, it is also conceivable for a plurality of medicinal product
portions to be situated in
a bubble. For example, it is conceivable for the active substance quantity
that must be taken by a
patient at a defined moment in time to be too large for an individual
medicinal product portion
because problems can occur during swallowing. It can therefore be expedient to
divide the active
substance between a plurality of smaller medicinal product portions. The
bubble preferably contains
- 6 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
at the most four medicinal product portions; it particularly preferably
contains a single medicinal
product portion.
The bubble can be formed in the main body centrally or in a decentralized
manner. The terms
"centrally" and "decentralized" will be explained in greater detail further
below.
The cover film is applied to what is referred to as the rear side of the main
body and closes the
bubble, which protrudes from the main body on the opposite belly side. Figure
1 illustrates this.
Figure 1 shows an example of an individual blister 1 in plan view and from two
sides. The individual
blister 1 comprises a planar main body 2, in which a bubble 3 is formed. In
the bubble 3 there is
situated a single medicinal product portion (not illustrated in Figure 1). The
bubble is closed by a
cover film 4. The bubble 3 is formed centrally in the planar main body 2. The
basic shape of the main
body is rectangular - the corners being rounded.
The cover film extends usually, but not necessarily, over the entire rear side
of the main body and
terminates flush with the edges of the main body. The cover film thus
preferably likewise has the
basic shape of the main body and in the ideal case is flat (without
curvature).
In the stack according to the invention, the cover films preferably lie in
planes extending parallel to
one another.
There are various possibilities for stacking which, in the case of cover films
extending in parallel, are
provided by the orientation of the cover films with respect to the direction
of the force of gravity. In
the "simplest case" the cover films extend horizontally to the force of
gravity and the individual
blister packs are stacked "on top of one another". In another case the cover
films extend vertically to
the force of gravity and the individual blister packs are stacked
"adjacently". In addition, the cover
films can be brought into any other orientation between "vertical" and
"horizontal" by rotation of
the stack. The present invention is not limited to any orientation of the
stack with respect to the
force of gravity. The term "stack" in the present description is interpreted
more broadly than in
colloquial language, in which case it is usually understood to mean only an
"arrangement on top of
one another". The term "height hs of the stack-like arrangement" also is not
to be understood to
mean that only individual blisters stacked "on top of one another" are the
subject of the present
invention. The term "height" is thus interpreted here more broadly than is
usually understood
thereby. These considerations, which are assumed of cover films extending in
parallel, also are not
- 7 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
' intended such that they apply only to stacks with cover films extending in
parallel. By way of
example, the present invention also includes stacks in which the cover films
of the individual blisters
do not lie in planes extending parallel to one another.
For the following consideration, a stack is assumed in which the cover films
of the individual blisters
are arranged horizontally to the force of gravity, and the individual blisters
are therefore arranged
"on top of one another". This therefore results in an individual blister
disposed at the very bottom in
the stack and an individual blister disposed at the very top in the stack.
Further individual blisters can
be situated therebetween. In a stack of this type an adjacent individual
blister is to be understood to
mean the individual blister that is in contact with the individual blister in
question. Here, the adjacent
individual blister can be situated above or below the individual blister in
question. The individual
blister at the very bottom has only a single adjacent individual blister; this
lies above the individual
blister situated at the very bottom. Similarly, the individual blister at the
very top also has just one
adjacent individual blister; this lies below the individual blister situated
at the very top. Should
further individual blisters be provided between the individual blisters at the
very bottom and at the
very top, these each have two adjacent individual blisters, one of the
adjacent individual blisters
being situated above the individual blister in question, and the other of the
adjacent individual
blisters being situated below the individual blister in question. Similar
considerations apply to a stack
in which the individual blisters are arranged "adjacently".
Typical numbers N of individual blisters in a stack-like arrangement are 2, 3,
4, 5, 6, 7, 8, 9, 10, 12, 14,
16, 18, 20, 21, 28, 30 and 31. However, other quantities are also conceivable.
In a preferred embodiment, N is an integer in the range from 2 to 31.
In a further preferred embodiment, N is 7.
In a further preferred embodiment, N is 28.
In the stack according to the invention the bubbles preferably point in the
same direction. However,
it is also conceivable for the individual blisters to be arranged rear side to
rear side and belly side to
belly side in alternation. Mixed forms of the specified stack forms are also
conceivable.
- 8 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
In a conventional blister stack, in which N blisters are stacked rear side to
belly side, the height hs of
the stack-like arrangement is N times the sum of the height hB of a bubble,
the thickness dG of a flat
main body and the thickness dr, of a cover film:
Conventional blister stack: hs= N = (hB + dG + do)
This is illustrated in Figure 2, where, for schematic reasons, the thickness D
of the film composite
consisting of the flat main body and the cover film is specified. The
following is true: D = dG+ dp =
The stack height is reduced in accordance with the invention:
blister stack according to the invention: hs< N = (FIB+ dG + dp)
In a preferred embodiment the height of the stack-like arrangement is:
hs= N = (dG + dD)+ hB
In a particularly preferred embodiment, the following is true for the stack
height:
N = (dG + do) + hB 5. hs < N = (hB + dG + dD)
in a further preferred embodiment the height of the stack-like arrangement is:
hs= (N -1) = (dG + dD+ hN) + (hB + dG + dD), where hN < hB
hN denotes the height of the supporting structures, which will be described in
greater detail further
below.
In a further preferred embodiment the height of the stack-like arrangement is:
h=% = (N+1) = (hB + dG + do)
In one embodiment of the present invention the individual blisters are stacked
such that the cover
film of an individual blister rests on the main body of an adjacent individual
blister in a region that
lies beside the bubble. Figure 3 illustrates this embodiment.
- 9 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
Figure 3 shows an example of an arrangement according to the invention in plan
view (bottom) and
from a side (top). Seven examples (1-1, 1-2, 1-3, 1-4, 1-5, 1-6, 1-7) of the
individual blister 1 from
Figure 1 are arranged on top of one another in a stack-like manner. All
bubbles point in the same
direction. The individual blister 1-1 is arranged at the very bottom in the
stack. The individual blister
1-2 rests on the individual blister 1-1. Here, the cover film of the
individual bubble 1-2 rests on the
main body of the adjacent individual bubble 1-1, more specifically in a region
beside the bubble. For
the individual blister 1-7, it is specified which side is the belly side B and
which side is the rear side R.
The belly side B is therefore the side on which the bubble 3 protrudes from
the main body 2. The rear
side R is the side on which the cover film 4 is applied that closes the bubble
3.
The height of the stack-like arrangement shown in Figure 3 is:
fts = N = (dG + dD) + hB = 7 = (dG + de)+ he
If the stack from Figure 3 is projected onto the plane extending parallel to
the basic shapes (rectangle
with rounded corners) of the flat main body (xy plane), the following area
requirement Fs results:
Fs =7 = FE - 6 = Fo
FE is, here, the area content of a single individual blister in the projection
onto the plane disposed
parallel to the basic shape of the planar main body (xy plane), and Fo is the
overlap region of two
adjacent individual blisters in the same projection plane.
If N individual blisters are disposed adjacently in alignment on a table,
their area requirement is N
times the area requirement FE of an individual blister:
Fs= N = FE
The following is generally true for a stack-like arrangement according to the
invention:
Fs< N = FE
The area requirement of a stack-like arrangement according to the invention is
smaller than the area
requirement of adjacently arranged individual blisters, since the individual
blisters of the stack-like
arrangement overlap in the xy projection plane.
-10-
CA 02999102 2018-03-19
BHC 153069 FC-Text
For example, the following is true for a stack as shown in Figure 3:
F5= N = FE - (N -1) = Fo
Preferred embodiments of stacks according to the invention or those that have
a greater relative
overlap region Fore!:
For el = Fo / FE 5.1
Preferably, Fore is from 0.3 to 1, particularly preferably from 0.5 to 1.
The size of the relative overlap region can be maximized in different ways,
for example by a
decentralized arrangement of the bubbles and/or by recesses in the main body,
as will be explained
in greater detail hereinafter.
In a further embodiment of the present invention the bubbles of the individual
blisters are arranged
in a decentralized manner with respect to the basic shape of the planar main
body.
The centre of the basic shape of the planar main body is determined either by
its centre of symmetry
or, if the basic shape has no centre of symmetry, by the centre of gravity of
the basic shape. The
bubble also has a centre, which is determined either by the centre of symmetry
or, if the bubble has
no centre of symmetry, by the centre of gravity of the bubble.
A decentralized arrangement of the bubble is to be understood to mean an
arrangement in which
the centre of the bubble does not coincide with the centre of the basic shape
of the planar main
body.
Due to the decentralized arrangement, there is a greater bubble-free area on
the main body beside
the bubble, on which area an adjacent individual blister can rest via its
cover film. The adjacent
individual blisters can thus "move closer to one another" in the xy plane,
such that not only a denser
packing of the individual blisters in the stack direction, but also
perpendicularly thereto, results.
Figures 4 and 5 illustrate this embodiment.
Figure 4 shows an example of an individual blister 1 in plan view and from two
sides. The individual
blister 1 comprises a planar main body 2, in which a bubble 3 is formed. In
the bubble 3 there is
- 11 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
situated an individual medicinal product portion (not illustrated in Figure
4). The bubble is closed by
a cover film 4. The bubble 3 is formed in the planar main body 2 in a
decentralized manner. The basic
shape of the main body is rectangular - the corners being rounded.
Figure 5 shows an example of an arrangement according to the invention in a
plan view (bottom) and
from a side (top). Seven examples (1-1, 1-2, 1-3, 1-4, 1-5, 1-6, 1-7) of the
individual blister 1 from
Figure 4 are arranged on top of one another in a stack-like manner. All
bubbles point in the same
direction.
The height of this stack-like arrangement is:
hs= N = (dG + dp)+ hi3= 7 = (dG do)+ he
In a further embodiment the base area of each individual blister has a recess,
within which the
bubble of an adjacent individual blister can be placed. This also results in a
denser packing both in
the stack direction and perpendicularly thereto. Figures 6 and 7 illustrate
this embodiment.
The height of this stack-like arrangement is:
hs= N = (dG+ dr))+ he
The term "recess" is to have a broad meaning here. Under consideration of the
main body of
conventional blister packs (for example see Figure la of the laid-open
application GB2184086A), the
edges of the basic shape have a convex contour. By way of example, a circular,
a square, and a
rectangular basic shape always have a convex contour (see Figure 6, for
example). If, by contrast, a
semi-circular recess is punched out in the edge region of a square basic
shape, the contour of the
basic shape is no longer convex at all points, but is concave in the region of
the recess.
Accordingly, the expression "basic areas with recess" is to be understood to
mean all basic areas
where the contour, besides convex regions, also comprises at least one concave
region.
The recess is preferably round, semi-circular, ellipsoidal, semi-ellipsoidal,
or rectangular.
In a preferred embodiment two different individual blisters, which behave like
an image and mirror
image relative to one another, are stacked in alternation. Figure 6 shows an
example. The two
individual blisters 1 and 1 behave like an image and mirror image relative to
one another. Both have
- 12 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
a planar main body 2, in which a bubble 3 for receiving a medicinal product
portion is formed. The
bubble is arranged in a decentralized manner with respect to the main body.
Both main bodies have
a semi-circular recess 5. Figure 7 shows how two of the individual blisters 1
and 1' are arranged on
top of one another in alternation in a stack-like manner. The stack height is:
hs= 4 = (dG + dD) + hB
In a further embodiment the main body of each individual blister has
supporting structures. The
supporting structures can be formed in the main body, similarly to the bubble.
It is also conceivable
for the supporting structures to be applied to the main body. In accordance
with the invention, the
supporting structures have a lower height than the bubble: hN < hB.
In a stack of individual blisters with supporting structures, the cover film
of an individual blister rests
against the supporting structures of the adjacent individual blister. Such
supporting structures lead
to a stabilization of the stack, but possibly at the cost of the packing
density in the stack direction.
The height of the stack-like arrangement is:
hs = (N -1) = (dG + dD + hN) + (hB + dG + dD ) = N = (dG + dD) + (N-1) = hN +
hB
Figure 8 shows an example. A bubble 3 is arranged in a decentralized manner in
the main body 2 of
the individual blister 1. A circular recess 5 is formed in the main body 2, in
which recess a bubble of a
further individual blister in a stack-like arrangement can be placed. The main
body also has
supporting structures in the form of two supporting nubs 7, of which the
height hN is less than the
height of the bubble hB.
In Figure 9, two of the individual blisters from Figure 8 are stacked on top
of one another. Here, the
lower individual blister is rotated through 180 relative to the upper
individual blister, such that the
bubble of the lower individual blister falls precisely into the recess in the
upper individual blister. The
main body of the upper individual blister rests on the supporting nubs of the
lower individual blister.
The height of the stack-like arrangement is:
hs = (N -1) = (dG + dD+ hN) + (hB + dG + dD) = 2 = (dG + dD) + hB+ hN
The size of the relative overlap region is Forel = 1
- 13 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
. ,
, . A special form of a "base area with recess" is the angular base area,
which constitutes a particularly
preferred embodiment. Here, the recess is rectangular. An example of an
angular base area is shown
in Figure 10. An angular base area is understood to mean an area in which two
area elements extend
in different directions starting from a common area and delimit a free region
between the area
elements. In the preferred embodiment discussed here of a stack-like
arrangement according to the
invention, the bubble of an individual blister falls into the free region
between the area elements of
the main body of an adjacent individual blister. The area elements extending
in different directions
enclose an angle of less than 1800. The angle preferably lies in a range from
1200 to 60 , particularly
preferably in a range from 100 to 80 ; and the angle is most preferably 900.
The area elements
extending in different directions are preferably of the same shape; they are
preferably rectangular.
They can be the same size or different sizes. In a preferred embodiment they
are the same size; in
another preferred embodiment they are different sizes.
In Figure 10 an individual blister having an angular main body is illustrated.
The main body 2
comprises two rectangular area elements 6 and 6', which are arranged at an
angle of 90 to one
another and delimit a free region, in which the bubble of a further individual
blister in a stack-like
arrangement can be placed. The main body 2 has supporting structures in the
form of supporting
nubs 7, of which the height hN is less than the height 178 of the bubble 3.
Figure 11 shows, in a plan
view (bottom) and in a side view (top), how two of the individual blisters
from Figure 10 are stacked
on top of one another.
The stack height is:
hs= (N -1) = (dG + dp+ hN)+ (hB + dG + do) = 2 = (dG + do) + (hN + h)
The size of the relative overlap region is Fo''' = 2/3.
In a further embodiment, the bubble of each individual blister of a stack
according to the invention is
arranged in a decentralized manner with respect to the basic shape of the main
body, and the main
body additionally has a recess, in which the bubble of an adjacent individual
blister can be placed.
Figures 12 and 13 illustrate this embodiment. In Figure 12, an individual
blister 1 is illustrated, of
which the main body 2 has two rectangular area elements 6 and 6', which are
arranged at an angle of
90 to one another and delimit a free region, in which the bubble of a further
individual blister can be
placed in a stack-like arrangement. The main body 2 also has supporting
structures in the form of a
supporting rib 8. Twice the height hN of the supporting rib added to the
thickness dG of the main body
- 14 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
and the thickness dD of the cover film corresponds in the present example to
exactly the height 178 of
the bubble: 2 = hN + dG + dD = he
Figure 13 shows how three of the individual blisters from Figure 12 are
stacked on top of one
another in a spiralled manner.
The stack height is:
hs = (N -1) = (dG + + hN) + (hB + dD + dD) =1/2 = (N+1) = (hB + dG + dD) = 2 =
(hB + dG + dD)
The size of the relative overlap region is Forel = 1
Figures 14, 15, 16 and 17 show further embodiments of the present invention,
in which the medicinal
product portion and accordingly also the bubble have an elongate form.
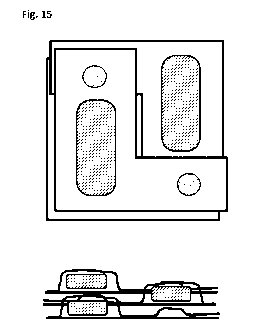
In Figure 14 an individual blister 1 is illustrated, of which the main body 2
has two rectangular area
elements 6 and 6', which are arranged at an angle of 90 to one another and
delimit a free region, in
which the bubble of a further individual blister in a stack-like arrangement
can be placed. The area
elements 6 and 6' are of different sizes.
In Figure 15 three of the individual blisters from Figure 15 are stacked on
top of one another.
Figure 16 shows an individual blister 1, in the main body 2 of which a bubble
3 is arranged in a
decentralized manner. A recess 5 is introduced into the main body 2, into
which recess a bubble of a
further individual blister in a stack-like arrangement can be placed. The main
body also has
supporting structures in the form of four supporting nubs 7, of which the
height hN is less than the
height of the bubble N.
In Figure 17 two of the individual blisters from Figure 16 are stacked on top
of one another. Here, the
lower individual blister is rotated through 180 relative to the upper
individual blister, such that the
bubble of the lower individual blister falls precisely into the recess in the
upper individual blister. The
main body of the upper individual blister rests on the supporting nubs of the
lower individual blister.
A further subject of the present invention is a box containing a stack-like
arrangement according to
the invention of individual blisters. The box serves as secondary packaging.
It usually also contains a
package leaflet containing information regarding taking the medicinal product
portions. The box
- 15 -
CA 02999102 2018-03-19
, =
BHC 153069 FC-Text
serves to stabilize the stack-like arrangement according to the invention. It
consists for example of
card, plastic, metal or also a composite material. In a preferred embodiment
the box is a folding box
made of card, as is also used for conventional blister strips.
In a preferred embodiment the box contains a viewing window, via which the
remaining amount of
individual blisters provided in the box can be determined.
In a further preferred embodiment the box has a lateral opening in the lower
region of the box, via
which a single individual blister can be removed from the box. On account of
the force of gravity, the
individual blisters remaining in the box move down when the lower individual
blister is removed and
can be individually removed in succession until the box is empty.
In Figure 18 three boxes according to the invention are illustrated. The right-
hand box contains seven
individual blisters, the middle box contains fourteen individual blisters, and
the left-hand box
contains 28 individual blisters. The left-hand box has a viewing window on its
side, via which viewing
window the remaining number of individual blisters can be determined. The left-
hand box also has
an opening in the lower region, via which the individual blisters can be
removed laterally.
A further subject of the present invention is constituted by individual
blisters embodied in a
particular manner in order to enable a stacking according to the invention.
In a preferred embodiment the individual blisters are characterized in that
the bubble is arranged in
a decentralized manner with respect to the main body. Particularly preferred
embodiments are
illustrated in Figures 4, 6, 8, 12, 14 and 16.
In a further preferred embodiment the individual blisters have a recess, in
which the bubble of an
adjacent individual blister in a stack-like arrangement according to the
invention can be placed.
Particularly preferred embodiments are illustrated in Figures 6, 8, 10, 12, 14
and 16.
In a further preferred embodiment the individual blisters have supporting
structures, of which the
height hN is less than the height of the bubble hB. In a further preferred
embodiment, the height h8 of
the bubble corresponds to the sum of twice the height hN of the supporting
structures, the thickness
dG of the main body and the thickness dp of the cover film: hB= 2 = hN + dG +
dr,. Particularly preferred
embodiments are illustrated in Figures 8, 10, 12, 14 and 16.
- 16 -
CA 02999102 2018-03-19
BHC 153069 FC-Text
. ,
' In a further preferred embodiment, the main body of the individual
blister according to the invention
has a rectangular or square basic shape, in which the corners are optionally
rounded. Particularly
preferred embodiments are illustrated in Figures 4, 6, 8 and 16.
In a further preferred embodiment the main body of the individual blister
according to the invention
has an angular basic shape, in which the corners are optionally rounded.
Particularly preferred
embodiments are illustrated in Figures 10, 12 and 14.
In a further preferred embodiment the individual blister exists in two
different copies, which behave
like an image and mirror image relative to one another. A particularly
preferred embodiment is
illustrated in Figure 6.
In a further preferred embodiment, the planar main bodies have one or more
basic shapes which,
apart from optionally rounded corners, allows/allow a tesselation of a
rectangular area.
Tesselation (also referred to as tiling, paving or scrap-free blanking) is
understood to mean the
covering of an area by smaller areas of identical shape, with no gaps and no
overlaps. The basic
shapes of the planar main bodies form the sub-areas here. The advantage of
tesselation lies in the
fact that the main body can be obtained by dividing a larger blister sheet,
wherein division leftovers
(cutting leftovers, waste) are reduced to a minimum.
By way of example, the individual blisters shown in Figures 1, 4, 8, 10, 12,
14 and 16 allow a
tesselation.
A further subject of the present invention is a method for producing
individual blisters according to
the invention.
The method according to the invention is characterized in that the individual
blisters are separated
off from a sheet or a web comprising a multiplicity of individual blisters.
The separation can be
implemented by conventional methods, such as laser cutting, mechanical
cutting, punching, etching,
electron beam machining, ultrasound and water jet. These and further methods
are described, for
example, in DIN standards 8588, 8589 and 8590.
In a particularly preferred embodiment, the individual blisters are produced
from a macro blister
pack, which is described in greater detail in EP15182316.8.
- 17 -