Note: Descriptions are shown in the official language in which they were submitted.
CI-1040 FOR THE TREATMENT OF VIRAL DISEASES
FIELD OF THE INVENTION
[0001] The present invention relates to a method of treating viral infections
comprising the
administration of the MEK inhibitor CI-1040 or a pharmaceutically acceptable
salt or derivative
thereof to a subject.
BACKGROUND OF THE INVENTION
[0002] Infections with RNA or DNA viruses are a significant threat for the
health of man and
animal. For instance, infections with influenza viruses do still belong to the
big epidemics of
mankind and cause year for year a big number of casualties. In terms of the
national
economies, they are an immense cost factor, for instance due to unfitness for
work. Infections
with the Borna disease virus (BDV), which mainly affects horses and sheep, but
which has
also been isolated for humans and is connected to neurological diseases,
equally have an
enormous economic importance.
[0003] The problem of controlling in particular RNA viruses is the
adaptability of the viruses
caused by a high fault rate of the viral polymerases, which makes the
production of suitable
vaccines as well as the development of antiviral substances very difficult.
Furthermore it has
been found that the application of antiviral substances immediately directed
against the
functions of the virus, shows a good antiviral effect at the beginning of the
treatment, but will
quickly lead to the selection of resistant variants based on mutation. An
example is the anti-
influenza agent amantadine and its derivatives directed against a
transmembrane protein of
the virus. Within a short time after the application, resistant variants of
the virus are generated.
Other examples are the new therapeutics for influenza infections inhibiting
the influenza-viral
surface protein neuraminidase. To these belong for instance Relenza. In
patients, Relenza-
resistant variants have already been found (Gubareva et al., 1998). Hopes
placed in this
therapeutical could therefore not be fulfilled.
[0004] Because of the very small genome and thus limited coding capacity for
functions being
necessary for the replication, all viruses are dependent to a high degree from
functions of their
host cells. By exertion of influence on such cellular functions being
necessary for the viral
replication, it is possible to negatively affect the virus replication in the
infected cell. Herein,
there is no possibility for the virus to replace the lacking cellular function
by adaptation, in
particular by mutations, in order to thus escape from the selection pressure.
This could already
be shown for the influenza A virus with relatively unspecific inhibitors
against cellular kinases
and methyl transferases (Scholtissek and Muller, 1991).
1
CA 2999670 2018-03-29
[0005] It is known in the art that cells have a multitude of signal
transmission paths, by means
of which signals acting on the cells are transmitted into the cell nucleus.
Thereby the cell is
capable to react to external stimuli and to react by cell proliferation, cell
activation,
differentiation, or controlled cell death. It is common to these signal
transmission paths that
they contain at least one kinase activating by phosphorylation at least one
protein
subsequently transmitting a signal. When observing the cellular processes
induced after virus
infections, it is found that a multitude of DNA and RNA viruses preferably
activate in the
infected host cell a defined signal transmission path, the so-called
Raf/MEK/ERK kinase signal
transmission path (Benn et al., 1996; Bruder and Kovesdi, 1997; Popik and
Pitha, 1998;
Rodems and Spector, 1998). This signal transmission path is one of the most
important signal
transmission paths in a cell and plays a significant role in proliferation and
differentiation
processes. Growth factor-induced signals are transmitted by successive
phosphorylation from
the serine/threonine kinase Raf to the dual-specific kinase MEK (MAP kinase
kinase/ERK
kinase) and finally to the kinase ERK (extracellular signal regulated kinase).
Whereas as a
kinase substrate for Raf, only MEK is known, and the ERK isoforms were
identified as the only
substrates for MEK, ERK is able to phosphorylate a whole number of substrates.
To these
belong for instance transcription factors, whereby the cellular gene
expression is directly
influenced (Cohen, 1997; Robinson and Cobb 1997; Treisman, 1996).
[0006] The drawback of prior art antiviral active substances is that they are
either directed
against a viral component and thus quickly lead to resistances (cf.
amantadine), or act in a too
broad and unspecific manner against cellular factors (for example methyl
transferase
inhibitors), and significant side effects are to be expected. Consequently,
none of the
substances being active against cellular factors is known to have been
developed to a
therapeutical for virus diseases. On the other hand, the inhibition of other
kinases, for instance
the inhibition of the kinase JNK of the MEKK/SEK/JNK signal transmission path,
can increase
the virus multiplication. Further it is known that the increased activation of
again other kinases,
for instance of the protein kinase C (PKC), inhibits the replication of
viruses (Driedger and
Quick, WO 92/02484).
[0007] With regard to the cellular processes induced after a virus infection,
it is found that a
multitude of DNA and RNA viruses activate, in the infected host cell, a
defined signal
transduction pathway, the so-called Raf/MEK/ERK kinase cascade.
[0008] This kinase cascade belongs to the most important signaling pathways in
the cell and
plays an essential role in proliferation and differentiation processes.
[0009] Growth-factor induced signals are transferred by successive
phosphorylation from the
serine/threonine kinase Raf to the dual specific kinase MEK (MAP kinase
kinase/ERK kinase)
and finally to the kinase ERK (extracellular signal regulated kinase). Whilst
as a kinase
2
CA 2999670 2018-03-29
substrate of Raf, only MEK is known, and the ERK isoforms have been identified
for MEK as
the only substrate, ERK can phosphorylate quite a number of substrates. Hereto
belong for
instance the phosphorylation of transcription factors, which leads to a direct
modification of the
cellular gene expression.
[0010] The investigation of this signaling pathway in cellular decision
processes has led to
the identification of several pharmacological inhibitors, which inhibit the
signaling pathway,
among other positions, on the level of MEK, i.e. at the 'bottleneck of the
cascade.
[0011] The MEK inhibitors 0I-1040, PD0325901, AZD6244, GDC-0973, RDEA119,
GSK1120212, AZD8330, R05126766, R04987655, TAK-733 and AS703026 are known in
the
.. art and, for example, shown in Figure 4 of Fremin and Meloche (2010).
100121 Neuraminidase (also known as sialidase, acylneuraminyl hydrolase, and
EC 3.2.1.18)
is an enzyme common among animals and a number of microorganisms. It is a
glycohydrolase
that cleaves terminal alpha-ketosidically linked sialic acids from
glycoproteins, glycolipids and
oligosaccharides. Many of the microorganisms containing neuraminidase are
pathogenic to
.. man and other animals including fowl, horses, swine and seals. These
pathogenic organisms
include influenza virus.
[0013] Neuraminidase has been implicated in the pathogenicity of influenza
virus. It is thought
to help the elution of newly synthesized virons from infected cells and assist
in the movement
of the virus (through its hydrolase activity) through the mucus of the
respiratory tract.
[0014] A class of specific anti-influenza agents, the neuraminidase
inhibitors, has
demonstrated inhibition of both influenza A and B viruses. Oseltamivir is used
for the treatment
of viral infections; however, it does not treat nasal congestion. Oseltamivir
is the ethyl ester
prodrug of the carbocyclic transition state sialic acid analog RO 64-0802
(GS4071), a potent
and selective inhibitor of influenza A and B virus neuraminidases. Oral
oseltamivir has been
approved for treatment of acute influenza in the United States in 1999. It has
demonstrated
efficacy both in treating and preventing influenza illness.
[0015] Oseltamivir phosphate is a prodrug of oseltamivir carboxylate
(oseltamivir), an inhibitor
of the neuraminidase glycoprotein essential for replication of influenza A and
B viruses.
Oseltamivir is available from Roche Pharma.TM. AG (Switzerland).
Alternatively, oseltamivir
can be prepared according to the methods described in U.S. Pat. No. 5,763,483
to
Bischofberger et al and U.S. Pat. No. 5,866,601 to Lew et al. About 10 - 15%
of patients taking
oseltamivir experience nausea and vomiting. Patients with kidney dysfunction
should take
lower doses.
[0016] Zanamivir (Relenza) is an orally inhaled powder currently approved in
19 countries for
the treatment of, and in two for the prophylaxis of influenza A and B.
Zanamivir is a competitive
3
CA 2999670 2018-03-29
inhibitor of the neuraminidase glycoprotein, which is essential in the
infective cycle of influenza
viruses. It closely mimics sialic acid, the natural substrate of the
neuraminidase. Over the last
few years, a number of events have resulted in changes to the zanamivir
prescribing
information which now contains warnings of bronchospasm, dyspnea, rash,
urticaria and
allergic type reactions, including facial and oropharyngeal oedema.
[0017] Peramivir is a neuraminidase inhibitor, acting as a transition-state
analogue inhibitor
of influenza neuraminidase and thereby preventing new viruses from emerging
from infected
cells.
[0018] It is known that neuraminidase inhibitors are not effective for all
influenza viruses and
a resistance can be developed by new generation of influenza virus strain.
[0019] As viruses and influenza virus in particular very often develop
resistances against
antiviral treatment, there is a need for new and improved therapy options.
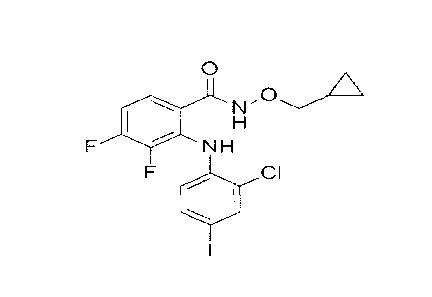
[0020] 0I-1040 is a clinically tested MEK inhibitor. The drug 0I-1040, also
known as 2-(2-
chloro-4-iodophenylamino)-N-(cyclopropylmethoxy)-3,4-difluorobenzamide, is an
ATP non-
competitive MEK1/2 inhibitor and was originally developed as an anti-tumor
drug where it
showed low toxicity (Barrett et al., 2008; Lorusso et al., 2005). It directly
inhibits MEK1 with a
50% inhibitory concentration (IC50) of 17 nM. The structure of 0I-1040 is
shown in formula (I):
0
N
NH
CI
1
[0021] Influenza is an acute respiratory disease caused by infection with
Influenza viruses
(IV). The disease affects the upper and/or lower respiratory tract and is
often accompanied by
systemic signs and symptoms such as fever, headache, myalgia, and weakness.
Outbreaks
of disease of variable extent and severity occur nearly every winter. They
result in significant
morbidity in the general population and increased mortality rates among
certain high-risk
patients mainly as a result of pulmonary complications.
[0022] The H1 N1 pandemic in 2009 clearly demonstrates that Influenza A virus
(IAV) has a
strong impact on global health systems (Mackey and Liang, 2012; Monto et al.,
2011;
Robertson and Inglis, 2011). Besides preventive vaccination, only a few
antiviral drugs are
approved. This highlights the urgent need for additional effective antivirals
to better control the
infection. Notably, in the early phase of a pandemic, when no vaccine is
available, antivirals
4
CA 2999670 2018-03-29
are the stand-alone treatment. Moreover, the appearances of IAV that are
resistant against
currently approved antivirals underline the urgent need for new and amply
available antiviral
drugs (Moss et al., 2010).
[0023] It is of great importance that antiviral therapy of viral infections is
started as soon as
.. possible after infection. E.g., the time window to start an influenza
therapy using oseltamivir is
only up to 24 h. Very often, a patient visits a physician after this period of
time rendering the
treatment with standard anti-viral treatments futile. Further, antiviral
treatments may be
contraindicated because of possible drug interactions, especially in elder
people, who often
have to take one or more medications continuously. Very often, it is not easy
to step the taking
of such medication. As a consequence, the time frame for starting an antiviral
therapy already
is passed before these patients are suited for antiviral therapy.
[0024] In view of the prior art, it is clear that there is the need of
compounds and compositions
effective in the treatment of virus diseases in particular in diseases caused
by influenza virus,
especially for late start of treatment. In addition, there is a need for
treatment of virus infections,
wherein the virus is resistant to antiviral therapy.
SUMMARY OF THE INVENTION
[0025] This need is obviated by using the MEK inhibitor CI-1040 or a
pharmaceutically
acceptable salt or derivative thereof for treating a viral infection.
[0026] The present invention relates to a method for treating a subject having
a viral infection,
comprising: administering to the subject the MEK inhibitor CI-1040 or a
pharmaceutically
acceptable salt or derivate thereof, wherein administering treats a viral
infection in the subject.
[0027] Preferably, the subject has been symptomatic for a viral infection for
at least 24 h, at
least 36 h or at least 48 h.
[0028] Preferably, the treating is started at least 24 hours and 48 hours post
onset of disease.
[0029] Preferably, the viral infection is caused by a negative RNA strand
virus. Preferably,
the virus is influenza virus, more preferably the virus is influenza A virus
(IAV) or influenza B
virus (IBV), even more preferably, the influenza A virus is selected from the
group consisting
according to H1N1, H2N2, H3N2, H5N6, H5N8, H6N1, H7N2, H7N7, H7N9, H9N2,
H1ON7,
N1ON8 or H5N1 or the Influenza B virus is selected from the group consisting
of IBV type
Yamagata and Victoria.
[0030] Preferably, the virus is resistant to antiviral treatment, wherein more
preferably the
antiviral treatment is administration of oseltamivir, zanamivir, peramivir,
amantadine,
rimantadine, favipiravir, baloxavir marboxil or pimodivir.
[0031] Preferably, the virus resistant to antiviral treatment is a Hi Ni H275Y
mutant.
5
CA 2999670 2018-03-29
[0032] Preferably, the subject is human.
FIGURES
[0033] Figure 1A shows the dose-dependent inhibition of A/Regensburg/06/2009
by CI-1040
(left panel), the determination of the ECK for CI-1040 (middle panel) and of
the CC50 (right
panel).
[0034] Figure 1B shows a broad antiviral range of CI-1040 at 10 pM against
different
influenza A viruses.
[0035] Figure 1C shows that CI-1040 can inhibit a Tamiflu (oseltamivir)-
resistant IAV strain.
[0036] Figure 2 shows immunofluorescence staining of human lung epithelial
cells.
Constituents of the cell nuclei with DAPI (first column) and of the viral RNP
complexes, PB1
(second column) and NP (third column) were stained. In the fourth column the
images were
merged. The Figure shows that CI-1040 blocks the export step of influenza A,
Hi NI, in human
lung epithelial cells (A549).
[0037] Figure 3A shows the efficacy of CI-1040 in the treatment of C57BL/6
mice infected
with influenza virus H1N1pdm09 and a timeline of the treatments. Results are
presented as
virus titer (10g10) pfu/ml (left lower panel) or cYo virus titer relative to
control, whereas control
was set to 100 % (right lower panel). The experiment was performed twice
independently.
[0038] Figure 3B shows the efficacy of CI-1040 in the treatment of C57BL/6
mice infected
with influenza virus H1N1pdm09 and proves the prolonged treatment window in
comparison
to oseltamivir (Tamiflue).
[0039] Figure 4 shows in vitro studies with the MEK-Inhibitor CI-1040 against
H5N1 influenza
virus.
[0040] Figure 5 shows results of the Oral treatment of influenza virus
infected mice with Cl-
1040 or PD-03250901.
DETAILED DESCRIPTION
[0041] Influenza viruses (IV) continue to pose an imminent threat to
human welfare.
Yearly re-occurring seasonal epidemic outbreaks and pandemics with high
mortality can occur.
Besides vaccination against a limited number of viral strains only a few
antiviral drugs are
available, which are losing their effectiveness as more and more IV strains
become resistant.
Thus, new antiviral approaches that omit IV resistance are urgently needed.
Here, the
dependency on the cellular Raf/MEK/ERK signaling pathway for IV replication
opens a new
perspective. In consequence, the inventors studied the antiviral potential of
the MEK inhibitor
6
CA 2999670 2018-03-29
0I-1040 (PD184352) and show that CI-1040 surprisingly and significantly
reduces virus titers
in vitro via retention of viral RNP complexes in the cell nucleus.
Furthermore, CI-1040 is
surprisingly effective against a broad range of IV strains, including highly
pathogenic avian IV,
as well as against a Tamiflu -resistant IV strain. Using a mouse model, the
inventors
demonstrate that 0I-1040 can reduce IV lung titers in vivo. Surprisingly, the
treatment window
for CI-1040 expands to at least 48 h post infection when Tamiflu treatment
has no effect. In
conclusion, CI-1040 offers an interesting perspective for anti-IV approaches.
[0042] All
viruses depend on cellular factors and mechanisms for their replication and
so do Influenza Viruses (IV). They acquired the ability to highjack cellular
factors for its own
purpose (Ludwig et at., 2003). Given these dependencies, cellular virus-
supportive functions
are promising candidates for novel antiviral intervention (Ludwig, 2011;
Ludwig et al., 2003;
Planz, 2013). Besides directly targeting virus as shown for the approved
neuraminidase-
inhibitors, such as Tamiflu or the M2-ionchannel blockers like amantadine, a
new and
promising antiviral strategy to fight influenza is based on the fact that IV,
as intracellular
pathogen, is strongly dependent on the cellular signaling machinery (Gong et
al., 2009;
Ludwig, 2009).
[0043] IV
must pass cellular barriers during their intracellular replication. As such
the
viral ribonucleoproteins (RNPs), comprising the genome segments, are
transported from the
cytoplasm to the nucleus, where viral genome replication takes place, and back
to the cell
membrane later in the replication cycle, when progeny virus particles are
released from the
infected cell. Remarkably, the nuclear RNP export was shown to be strongly
dependent on the
virus-induced Raf/MEK/ERK signal transduction pathway (Pleschka et al., 2001;
Ludwig et al.,
2004). Hence, pathways that are required for the virus to cross intracellular
barriers, such as
the nuclear membrane, are most favorable for antiviral intervention.
[0044] A potential
advantage of antiviral strategies that target intracellular signaling
pathways is their reduced likeliness to induce viral resistance in comparison
to those that
directly target viral replication. This has already been shown for several
compounds (Ludwig
et al., 2004; Mazur et al., 2007). In contrast, potential adverse effects of
inhibitors of
intracellular signaling pathways must also be taken into consideration, since
they interfere with
the host cell machinery and with substantial cellular functions.
[0045] As described above, the MEK inhibitor of the invention is 0I-1040, as
well as
pharmaceutically acceptable salts and may also include derivatives and
metabolites thereof.
A derivative, also known as structural analog, is a compound having a
structure similar to that
of another compound, but differing from it in respect to a certain component.
It can differ in one
or more atoms, functional groups, or substructures, which are replaced with
other atoms,
groups, or substructures.
7
CA 2999670 2018-03-29
[0046] Surprisingly it has been found that the administration of 0I-1040 alone
is effective in
the treatment of viral infections as shown in the Examples 1, 2 and 4. CI-1040
is not directed
against the functions of the virus but selectively inhibits MEK, a cellular
enzyme and via this
selective effect inhibits the viral replication of virus. Hence, CI-1040 is
also effective against
viruses, which are resistant to standard antiviral treatment, which targets
the virus itself. As
mentioned above, for influenza, such treatments are neuraminidase inhibitors
such as
oseltamivir (Tamiflue) or zanamivir or M2 inhibitors such as amantadine or
rimantadine, etc.
Further antiviral treatments include the administration of peramivir,
favipiravir, baloxavir
marboxil and/or pimodivir.
[0047] The present invention relates to the treatment of a subject having a
viral infection,
comprising administering to the subject the MEK inhibitor 0I-1040 or a
pharmaceutically
acceptable salt or derivative thereof, wherein administering treats a viral
infection in the
subject. In addition, 0I-1040 can be administered to patients after treatment
with standard
antivirals has failed.
[0048] As shown in Example 1, CI-1040 can suppress replication of H1N1pdm09
influenza
virus (A/Regensburg/06/2009 (H1N1)) in vitro in human alveolar epithelia cells
(A549) at non-
toxic concentrations (0050 = >312.3pM). The concentration at which viral
propagation is
reduced by 50 % (effective concentration; EC50) was 0.026 pM, which is in the
same range
as oseltamivir for this virus (data not shown). The selectivity index is
12.012. EC50 value of
0I-1040 to inhibit 50% of tumor cell growth is ranging from 0.12 to 0.18 pM
(Sebolt-Leopold
et al., 1999). Thus, in cell culture an approximately 10-fold lower
concentration of 0I-1040
compared to anti-tumor activity is needed for antiviral activity. This may be
explained by the
fact that in tumor cells the Raf/MEK/ERK pathway is permanently activated due
to mutations.
In influenza virus infected cells the pathway is only activated for a short
time (Pleschka et al.
2001). Thus, Example 1 shows that 0I-1040 can inhibit an influenza virus at
non-toxic
concentrations in a comparable manner to oseltamivir.
[0049] This is confirmed in Example 3, where it was shown that 0I-1040
possesses anti-
influenza virus activity also in a mouse model. 0I-1040 treatment via the per
os route of
H1N1pdm09-infected mice (5xMLD50) resulted in more than 80% reduction of the
amount of
virus in the lung (Fig. 3A) at a time-point where the viral load in placebo-
treated mice is high
(24 hours post infection). This indicates that the metabolism of the compound
is sufficient to
reach the lung and that the antiviral activity found in vitro is also evident
during infection of an
organism. Example 4 supports this data.
[0050] Surprisingly, it has been found that CI-1040 is also effective if the
treatment is started
at 48 h after infection. By comparison, as shown in Example 3, oseltamivir is
only effective in
the treatment of influenza A in mice, if the treatment is started up to 24 h
post infection. By
8
CA 2999670 2018-03-29
using CI-1040, the inventors surprisingly found that the treatment window can
be prolonged to
at least 48 h, most likely more. This property of 0I-1040 could not be
expected. Hence, Cl-
1040 is especially useful in the treatment of viral diseases such as
influenza, if a proper
diagnosis could not be made timely, so that a standard viral therapy would be
without effect or
in cases where the standard antiviral treatment was found to be ineffective.
[0051] In
Example 3, the inventors investigated the "treatment window" ¨ the time of
the treatment start after infection. When treatment started with the infection
(left panel of Fig.
3B), the drop of bodyweight was comparable in all groups. All animals
developed disease and
all control mice (5/5) died. In the Tamiflu group 2/5 (40%) mice died whereas
3/5 mice, treated
with 01-1040, died (60%). In particular, the fact that Tamiflu -treated mice
died was not
expected since it was published that Tamiflu protects all mice when treatment
started with
the infection (Baranovich et al., 2014; Yen et al., 2005). Similar results
were observed, when
treatment started 24 h after infection (Fig. 3B, middle panel). When treatment
started 48 h after
infection, however, (Fig. 3B, right panel), all Tamiflu -treated mice died
(5/5, 100%). This
failure of protection if administered more than 48 hours post infection was
already described
for highly pathogenic strains (Leneva et al., 2000). However, it was
surprisingly found that if
the start of treatment was 48 h after the infection only 2/5 (40 c)/0) C1-1040-
treated mice died.
Also, a benefit in bodyweight, clinical score and overall survival was still
found in CI-1040-
treated mice. This antiviral effect was found using a concentration of 0I-1040
that was below
the concentrations used in anti-tumor mouse models, indicating that a lower
amount of Cl-
1040 is required to achieve an antiviral effect compared to an anti-tumor
effect.
[0052]
According to the FDA label, oseltamivir (Tamiflu ) is currently indicated for
the
treatment of influenza in patients who have been symptomatic for no more than
2 days. This
corresponds to what was observed in the mouse model in Example 3, where
oseltamivir is no
longer effective if it is administered more than 48 h post infection with
influenza virus. In
contrast, 0I-1040 was effective even 48 h post infection. Hence, it has been
shown that Cl-
1040 has a longer treatment window than oseltamivir. For this reason, it is
likely that the effects
observed in the mouse model can be extrapolated to human patients, meaning
that CI-1040
or a pharmaceutically acceptable salt or a derivative thereof can be
administered to patients
more than 2 days after onset of symptoms of a viral infection. This also makes
sense when the
method of action of 0I-1040 is compared to that of oseltamivir. As mentioned
above,
oseltamivir is thought to block the elution of virions from the infected
cells. For this reason, it is
only effective if the virions have not yet left the cell. It was found that CI-
1040, by contrast,
blocks the MEK pathway that is necessary for viral replication, so that the
viral RNP complexes
are retained in the cell nucleus. Regarding the molecular mode of action of
MEK inhibition, the
inventors have previously shown that inhibition of the kinase with the
prototype inhibitor U0126
leads to a blockade of nuclear cytoplasmic transport of viral RNP complexes
(Pleschka et al.,
9
CA 2999670 2018-03-29
2001). This could be confirmed for CI-1040 (see also Example 2). While, RNPs
are present in
the nucleus at 4 h post infection in control and CI-1040 treated cells (Fig.
2, upper two lanes),
they translocate to the cytoplasm at 6 hours post infection to be packaged
into new virions in
control cells (Fig. 2, third lane). This export step is readily blocked in the
presence of CI-1040
as evidenced by predominant nuclear staining of the viral NP, the major
constituent of the
RNPs, as well as the PB1 polymerase, which is associated with RNPs (Fig. 2,
lower lane).
[0053] In
this context and in light of the data in Examples 2 and 3, discussed above, it
has been shown that treatment window for CI-1040 in human patients is larger
than for
oseltamivir.
[0054] Accordingly, the method of the invention also relates to treating a
viral infection in
subjects that have been symptomatic for at least 24 hours, at least 36 hours,
or at least
48 hours. These times of course include all time frames in between, such as
25, 26, 27, 28,
29, 30, 31, 32, 33, 34, 35, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46 or 47 hours
for the subject
being symptomatic. In a preferred embodiment, the subject has been symptomatic
for at least
48 hours before treatment. For example, treatment can be started between 24
and 48 hours
after onset of disease. In this context it should be noted that "being
symptomatic" and "onset
of disease" can be used interchangeably. However, the treatment could also be
started at least
60 h, at least 72 h or at least 96 h post infection. In cases where CI-1040 or
a pharmaceutically
acceptable salt or derivative thereof is administered when a different
antiviral was already
found to be ineffective, CI-1040 or a pharmaceutically acceptable salt or
derivative thereof can
be administered after 24 hours, when the treatment with the previous antiviral
has been
completed.
[0055] The expression "a patient or subject who is symptomatic" for a viral
infection is defines
as a patient or subject showing symptoms of a viral infection. In case of an
influenza virus
infection, the symptoms include fever, cough, nasal congestion, runny nose,
sneezing, sore
throat, fatigue, headache or muscle aches. Not all of these symptoms may occur
after an
influenza virus infection. Typically, influenza starts suddenly chills or a
chilly sensation, which
accompanies the sudden onset of (high) fever. This point in time may be
defined as the
beginning of the period of being symptomatic.
[0056] As already described above, the MEK inhibitor of the invention is CI-
1040, as well as
its pharmaceutically acceptable salts, derivatives and metabolites thereof.
[0057] In one aspect, the method of the invention is for the treatment of a
viral disease which
is an infection caused by negative RNA strand virus. More preferably, the
viral disease is
caused by an influenza virus; even more preferably is caused by influenza A or
B viruses.
Influenza A viruses are for example H1N1, H2N2, H3N2, H5N6, H5N8, H6N1, H7N2,
H7N7,
CA 2999670 2018-03-29
H7N9, H9N2, H1ON7, N1ON8 or H5N1. Influenza B viruses are for example the
Yamagata or
Victoria type.
[0058] Besides the H1N1pdm09 subtype the H3N2 subtype is circulating among the
human
population and contributes to the annual epidemics (Broberg et al., 2015).
This strain originally
caused the 1968 pandemic outbreak or the so-called "Hong Kong-Flu" and has
then adapted
to cause "only" seasonal epidemics. There are occasional infections of humans
with "bird flu"
viruses of the subtypes H5 and H7 displaying a very high mortality (Morens et
al., 2013;
Osterholm et al., 2012). H5N1 viruses first appeared in humans in 1997 and
since then have
caused numerous infections of humans. Furthermore, during an outbreak of an
H7N7 virus of
avian origin in the Netherlands in 2003 several people were infected and one
person died
(Fouchier et al., 2004). In 2013, a novel "bird flu" virus of the H7N9 subtype
emerged in China
that already killed more than 400 (428 of 1230 confirmed cases as of Feb. 22,
2017) people
(Ref.: FAO). Moreover, besides IAV also influenza B virus is found to cause
epidemics. Thus,
the activity of 10 pM CI-1040 against prototype strains of these aggressive
virus subtypes was
tested and results are shown in Figure 1B (see also Example 1). A consistently
strong antiviral
activity could be demonstrated. CI-1040 can inhibit several IAV strains,
indicating a broad
activity of CI-1040 against human IAV and influenza B virus causing seasonal
epidemics as
well as against avian IAV that represent an imminent threat to the human
population as they
have the potential to become pandemic.
[0059] The emergence of viral resistance to licensed influenza drugs is a
rising issue. High
frequency of resistance to the M2-ion channel blocker amantadine in clinical
isolates in the
US have led to the conclusion that M2 inhibitors should not be used for the
treatment and
prophylaxis of influenza until susceptibility to these drugs has been
reestablished among
circulating influenza A isolates (Bright et al., 2006). In contrast, it was
reported that the
resistance to neuraminidase inhibitors (Tamiflu and Relenza ) is generally
low. During
clinical trials of Tamiflu in seasonal influenza only a low percentage of
resistance has been
reported (Aoki et al., 2007). However, more worrying rates of resistance have
been first
detected in a smaller study in Japanese children where 18% of all isolates
were resistant (Kiso
et al., 2004). Since then, the number of reports on viral resistance to
Tamiflu has rapidly
increased, including findings on the emergence of influenza B viruses as well
as A/ H5N1,
A/H7N9 and pandemic A/H1N1 type viruses that are insensitive against the
compound. During
the 2007-2008 influenza season Tamiflu -resistant variants of seasonal
influenza Hi Ni
emerged, leading to a global subtype wide resistance in the following years
(Lackenby et al.,
2008; Thorlund et al., 2011). Besides the problem of resistance, recent
studies also
questioned the clinical benefit of Tamiflu based on a critical evaluation of
clinical trial data
(Jefferson et al., 2009; Jefferson et al., 2014).
11
CA 2999670 2018-03-29
[0060] With the present MEK inhibitor C1-1040 which, as has been shown
herein,
targets an intracellular protein, unresponsiveness of the drug against
influenza virus might be
excluded. Mutations in viral proteins cannot be excluded, but to escape the
influence of MEK-
inhibitor treatment the virus need to change its dependency on the Raf/MEK/ERK
signaling
pathway for nuclear export of its RNP-complexes. This would result in a change
of the biology
of the virus. In the present invention, it could be demonstrated that CI-1040
is effective against
a neuraminidase inhibitor (oseltamivir)-resistant IAV strain
A/Mississippi/3/2001 (H1N1). The
inventors took advantage of the fact that this virus exists as an oseltamivir-
resistant Hi Ni
variant, which differs from the wild type only in the H275Y resistance
mutation in the
neuraminidase. As shown in Figure 1C oseltamivir and CI-1040 are very potent
to inhibit the
growth of the A/Mississippi/3/2001 wild type strain (left part of Figure 1C).
No virus was
detectable after oseltamivir treatment and only 1.5% of the virus was found
after CI-1040
treatment. In contrast, when the antiviral effect was investigated using the
mutant strain with
the H275Y mutation (right part of Figure 1C) oseltamivir had lost most of its
effectiveness (only
.. 43% reduction), while CI-1040 showed a comparable antiviral effect as
against the wild type
strain (99.9% reduction).
[0061] As further shown in Example 1, CI-1040 is also effective against
influenza viruses,
which are resistant to antiviral treatment. This is a feature of CI-1040 that
solves a problem of
many standard antiviral treatments and allows CI-1040 to be administered after
treatment with
standard antivirals has failed. Standard antiviral treatment as used herein is
defined as a
treatment with a drug that has been approved for use as an antiviral and which
is effective in
inhibiting the development of the viral pathogen at any step of its life
cycle. Examples include
entry inhibitors, uncoating inhibitors, inhibitors of reverse transcription,
polymerase inhibitors,
endonuclease inhibitors, protein maturation inhibitors, integrase inhibitors,
transcription
inhibitors, translation inhibitors, protease inhibitors, virion assembly
inhibitors or virion release
inhibitors. In the context of influenza viruses, there are two different
standard approaches of
antiviral treatment known: neuraminidase inhibitors and M2 protein inhibitors.
[0062] Neuraminidase inhibitors (NAls) are a class of drugs which block the
neuraminidase
enzyme. They are commonly used as antiviral drugs because they block the
function of viral
neuraminidases of the influenza virus, by preventing its reproduction by
budding from the host
cell. Oseltamivir (Tamiflu) a prodrug, Zanamivir (Relenza), Laninamivir
(Inavir), and Peramivir
belong to this class. Unlike the M2 inhibitors, which work only against the
influenza A,
neuraminidase inhibitors act against both influenza A and influenza B. The
neuraminidase
inhibitors oseltamivir and zanamivir were approved in the US and Europe for
treatment and
.. prevention of influenza A and B.
12
CA 2999670 2018-03-29
[0063] The antiviral drugs amantadine and rimantadine inhibit a viral ion
channel (M2 protein),
thus inhibiting replication of the influenza A virus. These drugs are
sometimes effective against
influenza A if given early in the infection but are ineffective against
influenza B viruses, which
lack the M2 drug target. Influenza viruses readily develop resistances against
M2 inhibitors.
[0064] The antiviral drug favipiravir is an inhibitor of RNA viral
polymerases. The antiviral drug
baloxavir marboxil is an inhibitor of the influenza virus endonuclease PA.
Both drugs are
licensed for influenza treatment in Japan. The antiviral drug pimodivir is a
blocker of the
influenza viral PB2 protein and has been shown to be efficient against
uncomplicated influenza
A in clinical trials. All three drugs, favipiravir, baloxavir marboxil and
pimodivir have been shown
to provoke drug resistant variants in experimental models or clinical
testings.
[0065] Accordingly, in one embodiment the virus is resistant to antiviral
treatment. As outlined
herein, the antiviral treatment may relate to the administration of
neuraminidase inhibitors,
such as oseltamivir, zanamivir, laninamivir and peramivir, and/or
administration of M2 inhibitors
such as amantadine and rimantadine. Accordingly, the antiviral treatment may
be the
administration of oseltamivir, zanamivir, amantadine and/or rimantadine. In
another
embodiment, the antiviral treatment relates to the administration of
oseltamivir, zanamivir,
peramivir, amantadine, rimantadine, favipiravir, baloxavir marboxil and/or
pimodivir. As shown
in Example 1, 0I-1040 is also effective against the Influenza A strain H1N1
A/Mississippi/3/2001 H275Y mutant, which is resistant to oseltamivir.
Accordingly, in one
embodiment, the virus is H1N1 A A/Mississippi/3/2001 H275Y mutant and in
another
embodiment, the virus resistant to antiviral treatment is H1N1
A/Mississippi/3/2001 H275Y
mutant.
[0066] In one embodiment, the virus is H1N1 A/Mississippi/3/2001 wild type or
H1N1pdm09,
for which CI-1040 is also effective as shown in Example 1.
[0067] In the method of the invention, the MEK inhibitor 0I-1040 may be
administered orally,
intravenously, intrapleurally, intramuscularly, topically or via inhalation.
Preferably, the MEK
inhibitor is administered via nasal inhalation or orally.
[0068] The subject or patient of the invention can be a mammal or a bird.
Examples of suitable
mammals include, but are not limited to, a mouse, a rat, a cow, a goat, a
sheep, a pig, a dog,
a cat, a horse, a guinea pig, a canine, a hamster, a mink, a seal, a whale, a
camel, a
chimpanzee, a rhesus monkey and a human. Examples of suitable birds include,
but are not
limited to, a turkey, a chicken, a goose, a duck, a teal, a mallard, a
starling, a Northern pintail,
a gull, a swan, a Guinea fowl or water fowl, to name a few. Human patients are
a particular
embodiment of the present invention.
13
CA 2999670 2018-03-29
[0069] In particular embodiments, the subject is a human subject, which
optionally is more
than 1 year old and less than 14 years old, between the ages of 50 and 65,
between the ages
of 18 or 50, or older than 65 years of age. In other embodiments the subject
is a human subject,
selected from the group consisting of subjects who are at least 50 years old,
subjects who
reside in chronic care facilities, subjects who have chronic disorders of the
pulmonary or
cardiovascular system, subjects who required regular medical follow-up or
hospitalization
during the preceding year because of chronic metabolic diseases, renal
dysfunction,
hemoglobinopathies, or immunosuppression.
[0070] In the method of the invention, the MEK inhibitor 0I-1040 or a
pharmaceutically
acceptable salt or derivative thereof is administered in a therapeutically
effective amount.
[0071] The therapeutically effective amount for each active compound can vary
with factors
including but not limited to the activity of the compound used, stability of
the active compound
in the patient's body, the severity of the conditions to be alleviated, the
total weight of the
patient treated, the route of administration, the ease of absorption,
distribution, and excretion
of the active compound by the body, the age and sensitivity of the patient to
be treated, adverse
events, and the like, as will be apparent to a skilled artisan. The amount of
administration can
be adjusted as the various factors change over time.
[0072] The pharmaceutical composition comprising the MEK CI-1040 inhibitor or
a
pharmaceutically acceptable salt or derivative thereof may be in the form of
orally
administrable suspensions or tablets; nasal sprays, sterile injectable
preparations
(intravenously, intrapleurally, intramuscularly), for example, as sterile
injectable aqueous or
oleaginous suspensions or suppositories. When administered orally as a
suspension, these
compositions are prepared according to techniques available in the art of
pharmaceutical
formulation and may contain microcrystalline cellulose for imparting bulk,
alginic acid or sodium
alginate as a suspending agent, nnethylcellulose as a viscosity enhancer, and
sweeteners/flavoring agents known in the art. As immediate release tablets,
these
compositions may contain microcrystalline cellulose, dicalcium phosphate,
starch, magnesium
stearate and lactose and/or other excipients, binders, extenders,
disintegrants, diluents, and
lubricants known in the art. The injectable solutions or suspensions may be
formulated
according to known art, using suitable non-toxic, parenterally acceptable
diluents or solvents,
such as mannitol, 1,3-butanediol, water, Ringer's solution or isotonic sodium
chloride solution,
or suitable dispersing or wetting and suspending agents, such as sterile,
bland, fixed oils,
including synthetic mono- or diglycerides, and fatty acids, including oleic
acid.
[0073] The pharmaceutical compounds in the method of present invention can be
administered in any suitable unit dosage forms. Suitable oral formulations can
be in the form
of tablets, capsules, suspension, syrup, chewing gum, wafer, elixir, and the
like.
14
CA 2999670 2018-03-29
Pharmaceutically acceptable carriers such as binders, excipients, lubricants,
and sweetening
or flavoring agents can be included in the oral pharmaceutical compositions.
If desired,
conventional agents for modifying tastes, colors, and shapes of the special
forms can also be
included.
[0074] For injectable formulations, the pharmaceutical compositions can be in
lyophilized
powder in admixture with suitable excipients in a suitable vial or tube.
Before use in the clinic,
the drugs may be reconstituted by dissolving the lyophilized powder in a
suitable solvent.
Definitions
[0075] Throughout this specification and the claims which follow, unless the
context requires
otherwise, the word "comprise", and variations such as "comprises" and
"comprising", will be
understood to imply the inclusion of a stated integer or step or group of
integers or steps but
not the exclusion of any other integer or step or group of integer or step.
When used herein
the term "comprising" can be substituted with the term "containing" or
sometimes when used
herein with the term "having".
[0076] When used herein "consisting of" excludes any element, step, or
ingredient not
specified in the claim element. When used herein, "consisting essentially of"
does not exclude
materials or steps that do not materially affect the basic and novel
characteristics of the claim.
In each instance herein any of the terms "comprising", "consisting essentially
of" and
"consisting of" may be replaced with either of the other two terms.
[0077] As used herein, the conjunctive term "and/or" between multiple recited
elements is
understood as encompassing both individual and combined options. For instance,
where two
elements are conjoined by "and/or", a first option refers to the applicability
of the first element
without the second. A second option refers to the applicability of the second
element without
the first. A third option refers to the applicability of the first and second
elements together. Any
one of these options is understood to fall within the meaning, and therefore
satisfy the
requirement of the term "and/or" as used herein. Concurrent applicability of
more than one of
the options is also understood to fall within the meaning, and therefore
satisfy the requirement
of the term "and/or" as used herein.
[0078] As used herein, the term "pharmaceutically acceptable salts" refers to
the relatively
non-toxic, organic or inorganic salts of the active compounds, including
inorganic or organic
acid addition salts of the compound.
EXAMPLES
[0079] CI-
1040 is a white powder with a molecular weight (MW) of 478.67 and is
soluble in DMSO, slightly soluble in ethanol and is practically insoluble in
water. CI-1040 was
CA 2999670 2018-03-29
dissolved in DMSO for the in vitro investigations. The final concentration of
DMSO in culture
media was 1%. For in vivo experiments CI-1040 was also dissolved in DMSO.
Then, 50 pl
DMSO containing CI-1040 was mixed with 150 pl Cremophor EL and 800 pl PBS. CI-
1040 was
purchased from either Active Biochem or Selleckchem. Previously it was shown
that two weeks
oral dosing of 0I-1040 at a daily dose of 200 mg/kg was not toxic to mice and
reduced tumor
growth. MEK was inhibited for nine hours in tumors of mice single dosed orally
with CI-1040
at 150 mg/kg (Sebolt-Leopold et al., 1999).
Example 1: CI-1040 suppresses replication of H1N1pdm09 virus in vitro
[0080]
Human lung epithelial cells (A549) were infected with either H1N1pdm09
A/Regensburg/06/2009 (M01 = 0.005) or were used to determine the E050 and
0050 values (Fig. 1 A, B). Human lung epithelial cells (A549) were infected
with either H3N2
A/Victoria/03/1975 (M01 = 0.05), H5N1 A/mallard/Bavaria/1/2006 (M01 = 0.001),
H7N7
A/FPV/Bratislava/79 (M01 = 0.01) or H7N9 A/Anhui/1/2013 (M01 = 0.005) (Fig.
1B); or with
A/Mississippi/3/2001 wild-type or A/Mississippi/3/2001 carrying a specific
mutation in the
neuraminidase (H275Y) (M01 = 0.005) (Fig. 1C). Samples were cultured at 37 C.
After 30 min
the inoculum was removed, cells were rinsed with PBS and supplemented with 500
pi IMDM
or DMEM/BA-Medium (0.2 % BA, 1 mM MgCl2, 0.9 mM CaC12, 100 U/m1 penicillin,
0.1 mg/ml
streptomycin) and 0.6 pl TPCK-treated Trypsin (only for H1N1pdm09, H3N2, H7N9)
in
presence of different concentrations of CI-1040, 1 pM Tamiflu (oseltamivir)
or solvent control.
After an incubation period of 18 h (H7N7) or 24 h (H1N1pdm09, H3N2, H5N1,
H7N9) at
37 C viral titers were determined by standard plaque assay. Viral titers are
depicted as plaque
forming units/ml (PFU/ml) and calculated to percent virus titer compared to
solvent control,
which was set to 100%. Data represent the means of one (H7N9) or two
independent
experiments with two (H7N9) or three (all other) biological replicates and two
(H7N9) or three
(all other) technical replicates. (Fig. 1A) Dose-dependent inhibition of
A/Regensburg/06/2009
by CI-1040 (left panel) determination of the ECK, for 01-1040 (middle panel)
and CC50 (right
panel). Note: a concentration of 100 pM didn't lead to a cytotoxic effect and
therefore a
sigmoidal curve could not be designed. Thus, the C050 value is an
extrapolation by the
Graphpad Prism software. (Fig. 1B) 0I-1040 (10 pM) shows a broad antiviral
range against
different 1AV. (Fig. 1C) 01-1040 can inhibit a Tamiflu&resistant IAV strain.
Example 2: CI-1040 blocks the nuclear cytoplasmic transport of viral RNP
complexes
[0081]
Human lung epithelial cells (A549) were pre-treated with DMSO or 20 pM Cl-
10401 h before infection with 1AV A/PR8/34 (H1N1) at a multiplicity of
infection (M01) of 5.30
min after the viral attachment at 4 C, attempts were rinsed with PBS and
incubated 30 min at
37 C with fresh medium for viral internalization. Cells were rinsed with PBS
and supplemented
500 pl DMEM-Medium (6% BA, 1 mM MgCl2, 1 mM CaCl2, 100 Wm! penicillin, 0.1
mg/ml
16
CA 2999670 2018-03-29
streptomycin) containing DMSO or CI-1040 were given to the cells. After the
specific infection
periods, samples were prepared for immunofluorescence staining of constituents
of the viral
RNP complexes, PB1 (second column) and NP (third column) and. Anti-NP (BioRad)
and anti-
PB1 (Santa Cruz) were used to detect these viral proteins. Cell nuclei were
stained with DAPI
(first column).
100821 The
results in Figure 2 show that CI-1040 blocks the export step of influenza A,
H1N1, in human lung epithelial cells (A549).
Example 3: CI-1040 is effective in an in vivo model of influenza infection and
has a
prolonged therapy window in comparison to Tamiflu
[0083] 8 weeks old
C57BL/6 mice (five per group) were infected with 1.5 x 105 PFU (5
x MLD50) of the influenza virus strain A/Regensburg/D6/2009, (RBI, H1N1pdm09)
on day 0
(anesthetized with ketamine/rompun). Starting 8 h prior to infection mice were
treated per os
every 8 h with 125 mg/kg CI-1040 (CI-1040 dissolved in a combination of 50 pl
DMSO/150 pl
Cremophor/800 pl PBS) (see Fig. 3A, upper panel). Mice were sacrificed 24 h
post infection
and lungs were weighed, transferred into a Lysing Matrix D tube (MP Bio) and
subsequently a
10-fold volume of the lung of BSS (buffered salt solution) was applied to the
samples. Organs
were shredded using the FastPrep FP 120 (Savant). To remove the cell debris
the
homogenates were centrifuged for 15 min at 2000 rpm and the supernatant
collected.
Determination of virus titer in the homogenates was performed using the AVICEL
plaque
assay. The experiment was performed twice independently. Figure 3A shows the
efficacy of
CI-1040 in the treatment of C57BL/6 mice infected with influenza virus
H1N1pdm09.
[0084] In
another set of experiments, 8 weeks old C57BL/6 mice (five per group) were
infected with 1.5 x 105 PFU (5 x MLD50) of the influenza virus strain
A/Regensburg/D6/2009,
(RB1, H1N1pdm09). Starting either with the infection (Fig. 3B, left panel) or
24 h after infection
(Fig. 3B, middle panel) or 48 h after infection (Fig. 3C, right panel) mice
were treated with either
150 mg/kg/daily BID CI-1040 solved in 50 pl DMSO/150 pl Cremophor/800 pl PBS;
or 4
mg/kg/daily BID Tamiflu0 solved in H20 or solvent (placebo) control. All
animals received a
volume of 200 pl per os and were monitored twice daily and bodyweight was
measured.
Bodyweight prior to infection was set as 100 % and increase/decrease of
bodyweight was
given in percentage. When an animal died, or had to be sacrificed according to
the rules of the
German animal protection law the bodyweight at this day was noted throughout
the end of the
experiment (day 14 p.i.). The overall clinical score was divided into four
categories (score for
breathing, surrounding, posture and grooming). For each category, an
individual score from 0
to 3 is given resulting in an overall maximal score of 12. Nevertheless, mice
were sacrificed
when a total score of nine was reached or when a score of three was reached in
two categories.
Clinical score at the day of death was noted throughout the end of the
experiment. The results
in Figure 3B show the efficacy of CI-1040 in the treatment of C57BL/6 mice
infected with
17
CA 2999670 2018-03-29
influenza virus H1N1pdm09 and proves the prolonged treatment window in
comparison to
oseltamivir (Tamiflu ).
Example 4: Further in vitro and in vivo studies
[00851 In vitro studies with the MEK-Inhibitor CI-1040 against H5N1 influenza
virus
[0086] MDCK II cells were infected with A/Mallard/Bavaria/01/2006 (MB1, H5N1)
at a MOI of
0,001. After 30 min. cells were treated with CI-1040 for 24h.
[0087] From Figure 4 it can be seen that The MEK-Inhibitor CI-1040 is highly
potent against
different influenza virus strains in cell culture. CI-1040 is also potent
against panH1N1 (RBI)
and FPV.
[0088] Oral treatment of influenza virus infected mice with CI-1040 or PD-
03250901
[0089] Eight hours prior infection BL/6 mice were treated (per os) with CI-
1040, PD-03250901
(25mg/kg) or solvent. At the time point of infection (A/Regensburg/D6/09,
H1N1pdm09, RB1,
5-fold MLD50) mice were treated again, afterwards all eight hours (4 in
total). Twenty-four
hours after infection lung virus titer were detected. (n=5)
[0090] From Figure 5 it can be seen that the MEK-Inhibitors CI-1040 and PD-
03250901 are
potent in reducing virus titer in the lung of H1N1pdm09 infected mice.
REFERENCES
Aoki, F.Y., Boivin, G., Roberts, N., 2007. Influenza virus susceptibility and
resistance to
oseltamivir. Antivir Ther 12, 603-616.
Benn et al., J Virol 70, 4978-4985, 1996.
Bruder and Kovesdi, J Virol 71, 398-404, 1997.
Appiah, G.D., Blanton, L., D'Mello, T., Kniss, K., Smith, S., Mustaquim, D.,
Steffens, C., Dhara,
R., Cohen, J., Chaves, S.S., Bresee, J., Wallis, T., Xu, X., Abd Elal, Al.,
Gubareva, L.,
Wentworth, D.E., Katz, J., Jernigan, D., Brammer, L., Centers for Disease, C.,
Prevention,
2015. Influenza activity - United States, 2014-15 season and composition of
the 2015-16
influenza vaccine. MMWR Morb Mortal Wkly Rep 64, 583-590.
Baranovich, T., Burnham, A.J., Marathe, B.M., Armstrong, J., Guan, Y., Shu,
Y., Peiris, J.M.,
Webby, R.J., Webster, R.G., Govorkova, E.A., 2014. The neuraminidase inhibitor
oseltamivir
is effective against A/Anhui/1/2013 (H7N9) influenza virus in a mouse model of
acute
respiratory distress syndrome. J Infect Dis 209, 1343-1353.
Barrett, S.D., Bridges, A.J., Dudley, D.T., Saltiel, A.R., Fergus, J.H.,
Flamme, C.M., Delaney,
A.M., Kaufman, M., LePage, S., Leopold, W.R., Przybranowski, S.A., Sebolt-
Leopold, J., Van
Becelaere, K., Doherty, A.M., Kennedy, R.M., Marston, D., Howard, W.A., Jr.,
Smith, Y.,
Warmus, J.S., Tecle, H., 2008. The discovery of the benzhydroxamate MEK
inhibitors CI-1040
and PD 0325901. Bioorg Med Chem Lett 18, 6501-6504.
18
CA 2999670 2018-03-29
Bright, R.A., Shay, D.K., Shu, B., Cox, N.J., Klinnov, Al., 2006. Adamantane
resistance among
influenza A viruses isolated early during the 2005-2006 influenza season in
the United States.
Jama 295, 891-894.
Broberg, E., Snacken, R., Adlhoch, C., Beaute, J., Galinska, M., Pereyaslov,
D., Brown, C.,
Penttinen, P., Region, W.H.O.E., the European Influenza Surveillance, N.,
2015. Start of the
2014/15 influenza season in Europe: drifted influenza A(H3N2) viruses
circulate as dominant
subtype. Euro Surveill 20.
Cohen, Trends in Cell Biol 7,353-361, 1997.
Droebner, K., Pleschka, S., Ludwig, S., Planz, 0., 2011. Antiviral activity of
the MEK-inhibitor
U0126 against pandemic H1N1v and highly pathogenic avian influenza virus in
vitro and in
vivo. Antiviral Res 92, 195-203.
Fouchier, R.A., Schneeberger, P.M., Rozendaal, F.W., Broekman, J.M., Kemink,
S.A.,
Munster, V., Kuiken, T., Rimmelzwaan, G.F., Schutten, M., Van Doornum, G.J.,
Koch, G.,
Bosman, A., Koopmans, M., Osterhaus, A.D., 2004. Avian influenza A virus
(H7N7) associated
with human conjunctivitis and a fatal case of acute respiratory distress
syndrome. Proc Natl
Acad Sci USA 101, 1356-1361.
Fremin and Meloche (2010), J. Hematol. Oncol. 11;3:8.
Gong, J., Fang, H., Li, M., Liu, Y., Yang, K., Liu, Y., Xu, W., 2009.
Potential targets and their
relevant inhibitors in anti-influenza fields. Curl Med Chem 16, 3716-3739.
Gubareva et al., J Infect Dis 178, 1257-1262, 1998.
Jefferson, T., Jones, M., Doshi, P., Del Mar, C., 2009. Neuraminidase
inhibitors for preventing
and treating influenza in healthy adults: systematic review and meta-analysis.
BMJ 339, b5106.
Jefferson, T., Jones, M.A., Doshi, P., Del Mar, C.B., Hama, R., Thompson,
M.J., Spencer, E.A.,
Onakpoya, I., Mahtani, K.R., Nunan, D., Howick, J., Heneghan, C.J., 2014.
Neuraminidase
inhibitors for preventing and treating influenza in healthy adults and
children. The Cochrane
database of systematic reviews 4, CD008965.
Jernigan, D.B., Cox, N.J., 2015. H7N9: preparing for the unexpected in
influenza. Annu Rev
Med 66, 361-371.
Kiso, M., Mitamura, K., Sakai-Tagawa, Y., Shiraishi, K., Kawakami, C., Kimura,
K., Hayden,
F.G., Sugaya, N., Kawaoka, Y., 2004. Resistant influenza A viruses in children
treated with
oseltamivir: descriptive study. Lancet 364, 759-765.
Lackenby, A., Thompson, Cl., Democratis, J., 2008. The potential impact of
neuraminidase
inhibitor resistant influenza. Curr Opin Infect Dis 21, 626-638.
Leneva, I.A., Roberts, N., Govorkova, E.A., Goloubeva, 0.G., Webster, R.G.,
2000. The
neuraminidase inhibitor GS4104 (oseltamivir phosphate) is efficacious against
A/Hong
Kong/156/97 (H5N1) and A/Hong Kong/1074/99 (H9N2) influenza viruses. Antiviral
Res 48,
101-115.
19
CA 2999670 2018-03-29
Lorusso, P.M., Adjei, A.A., Varterasian, M., Gadgeel, S., Reid, J., Mitchell,
D.Y., Hanson, L.,
DeLuca, P., Bruzek, L., Piens, J., Asbury, P., Van Becelaere, K., Herrera, R.,
Sebolt-Leopold,
J., Meyer, M.B., 2005. Phase land pharmacodynamic study of the oral MEK
inhibitor CI-1040
in patients with advanced malignancies. J Olin Oncol 23, 5281-5293.
.. Ludwig, S., 2009. Targeting cell signalling pathways to fight the flu:
towards a paradigm change
in anti-influenza therapy. J Antimicrob Chemother 64, 1-4.
Ludwig, S., 2011. Disruption of virus-host cell interactions and cell
signaling pathways as an
anti-viral approach against influenza virus infections. Biol Chem 392, 837-
847.
Ludwig, S., Planz, 0., Pleschka, S., Wolff, T., 2003. Influenza-virus-induced
signaling
cascades: targets for antiviral therapy? Trends Mol Med 9, 46-52.
Ludwig, S., Wolff, T., Ehrhardt, C., Wurzer, W.J., Reinhardt, J., Planz, 0.,
Pleschka, S., 2004.
MEK inhibition impairs influenza B virus propagation without emergence of
resistant variants.
FEBS Lett 561, 37-43.
Mackey, T.K., Liang, B.A., 2012. Lessons from SARS and H1N1/A: employing a WHO-
WTO
forum to promote optimal economic-public health pandemic response. J Public
Health Policy
33, 119-130.
Marjuki, H., Yen, H.L., Franks, J., Webster, R.G., Pleschka, S., Hoffmann, E.,
2007. Higher
polymerase activity of a human influenza virus enhances activation of the
hemagglutinin-
induced Raf/MEK/ERK signal cascade. Virol J 4, 134.
Mazur, I., Wurzer, W.J., Ehrhardt, C., Pleschka, S., Puthavathana, P.,
Silberzahn, T., Wolff,
T., Planz, 0., Ludwig, S., 2007. Acetylsalicylic acid (ASA) blocks influenza
virus propagation
via its NF-kappaB-inhibiting activity. Cell Microbiol 9, 1683-1694.
Monto, AS., Black, S., Plotkin, S.A., Orenstein, W.A., 2011. Response to the
2009 pandemic:
effect on influenza control in wealthy and poor countries. Vaccine 29, 6427-
6431.
Morens, D.M., Taubenberger, J.K., Fauci, A.S., 2013. H7N9 avian influenza A
virus and the
perpetual challenge of potential human pandemicity. MBio 4.
Morrison, J., Josset, L., Tchitchek, N., Chang, J., Belser, J.A., Swayne,
D.E., Pantin-Jackwood,
M.J., Tumpey, T.M., Katze, M.G., 2014. H7N9 and other pathogenic avian
influenza viruses
elicit a three-pronged transcriptomic signature that is reminiscent of 1918
influenza virus and
is associated with lethal outcome in mice. J Virol 88, 10556-10568.
Moss, R.B., Davey, R.T., Steigbigel, R.T., Fang, F., 2010. Targeting pandemic
influenza: a
primer on influenza antivirals and drug resistance. J Antimicrob Chemother 65,
1086-1093.
Olschlager, V., Pleschka, S., Fischer, T., Rziha, H.J., Wurzer, W., Stitz, L.,
Rapp, U.R., Ludwig,
S., Planz, 0., 2004. Lung-specific expression of active Raf kinase results in
increased mortality
of influenza A virus-infected mice. Oncogene 23, 6639-6646.
Osterholm, M.T., Kelley, N.S., Sommer, A., Belongia, E.A., 2012. Efficacy and
effectiveness
of influenza vaccines: a systematic review and meta-analysis. Lancet Infect
Dis 12, 36-44.
CA 2999670 2018-03-29
Planz, 0., 2013. Development of cellular signaling pathway inhibitors as new
antivirals against
influenza. Antiviral Res 98, 457-468.
Planz, 0., Pleschka, S., Ludwig, S., 2001. MEK-specific inhibitor U0126 blocks
spread of
Borna disease virus in cultured cells. J Virol 75, 4871-4877.
Pleschka, S., Wolff, T., Ehrhardt, C., Hobom, G., Planz, 0., Rapp, U.R.,
Ludwig, S., 2001.
Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK
signalling cascade.
Nat Cell Biol 3, 301-305.
Popik and Pitha, Virology 252, 210-217, 1998.
Robertson, J.S., Inglis, S.C., 2011. Prospects for controlling future
pandemics of influenza.
Virus Res 162, 39-46.
Robinson and Cobb, Curr. Opin. Cell Biol 9, 180-186, 1997.
Rodems and Spector, J Virol 72, 9173-9180, 1998.
Scholtissek and Muller, Arch Virol 119, 111-118, 1991.
Sebolt-Leopold, J.S., Dudley, D.T., Herrera, R., Van Becelaere, K., Wiland,
A., Gowan, R.C.,
Tecle, H., Barrett, S.D., Bridges, A., Przybranowski, S., Leopold, W.R.,
Saltiel, A.R., 1999.
Blockade of the MAP kinase pathway suppresses growth of colon tumors in vivo.
Nat Med 5,
810-816.
Spence, J.K., Bhattachar, S.N., Wesley, J.A., Martin, P.J., Babu, S.R., 2005.
Increased
dissolution rate and bioavailability through comicronization with
microcrystalline cellulose.
Pharm Dev Technol 10, 451-460.
Thorlund, K., Awad, T., Boivin, G., Thabane, L., 2011. Systematic review of
influenza
resistance to the neuraminidase inhibitors. BMC Infect Dis 11, 134.
Treisnnan, Curr. Opin. Cell Biol 8, 205-215, 1996.
Yen, H.L., Monto, A.S., Webster, R.G., Govorkova, E.A., 2005. Virulence may
determine the
necessary duration and dosage of oseltamivir treatment for highly pathogenic
A/Vietnam/1203/04 influenza virus in mice. J Infect Dis 192, 665-672.
York, I., Donis, R.O., 2013. The 2009 pandemic influenza virus: where did it
come from, where
is it now, and where is it going? Curr Top Microbiol Immunol 370, 241-257.
21
CA 2999670 2018-03-29