Note: Descriptions are shown in the official language in which they were submitted.
CA 3000369
QUANTITATIVE HEART TESTING
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to and the benefit of U.S. Application
No. 62/235,309,
filed on September 30, 2015, U.S. Application No. 62/276,596, filed on January
8, 2016, U.S.
Application No. 62/276,639, filed on January 8, 2016, and U.S. Application No.
62/321,856,
filed on April 13, 2016.
TECHNICAL FIELD
[0002] The present disclosure relates generally to heart testing, and more
particularly to
systems, devices, and methods for quantifying and/or visualizing heart
condition.
BACKGROUND
[0003] Heart testing for coronary heart disease, myocardial ischemia, and
other abnormal heart
.. conditions is routinely performed using an electrocardiogram (ECG), which
represents
electrical potentials reflecting the electrical activity of the heart measured
via electrodes placed
on the patient's skin. The heart's electrical system controls timing of the
heartbeat by sending
an electrical signal through the cells of the heart. The heart includes
conducting cells for
carrying the heart's electrical signal, and muscle cells that contract the
chambers of the heart as
.. triggered by the heart's electrical signal_ The electrical signal starts in
a group of cells at the
top of the heart called the sinoatrial (SA) node. The signal then travels down
through the heart,
conducting cell to conducting cell, triggering first the two atria and then
the two ventricles.
Simplified, each heartbeat occurs by the SA node sending out an electrical
impulse. The
impulse travels through the upper heart chambers, called "atria", electrically
depolarizing the
atria and causing them to contract The atrioventricular (AV) node of the
heart, located on the
interatrial septum close to the tricuspid valve, sends an impulse into the
lower chambers of the
heart, called "ventricles," via the His-Purkinje system, causing
depolarization and contraction
of the ventricles. Following the subsequent repolarization of the ventricles,
the SA node sends
another signal to the atria to contract, restarting the cycle. This pattern
and variations therein
indicative of disease are detectable in an ECG, and allow medically trained
personnel to draw
inferences about the heart's condition. However, not every developing
abnormality is
Date Recue/Dede Received 2022-09-01
immediately visible in an ECG, and, consequently, many patients are
misdiagnosed as
healthy. Furthermore, although ECGs are nowadays typically recorded and
displayed
electronically, they often go little beyond the printed ECG traces of the past
in the type of
information they provide and the intuitiveness and convenience with which such
information
is presented.
10003A1 Various embodiments of the claimed invention relate to one or more non-
transitory
computer-readable media storing instructions for processing one or more
electrocardiograms
associated with a patient and with one or more respective leads, the
instructions, when
executed by one or more computer processors, causing the one or more computer
processors
to perform operations comprising: converting the one or more
electrocardiograms by time-
frequency transform into one or more respective two-dimensional time-frequency
maps;
identifying, within the one or more electrocardiograms, one or more points in
time associated
with a T wave; determining, for at least one of the one or more time-frequency
maps, one or
more repolarization measures corresponding to extrema across frequency of the
respective
time-frequency map at the one or more points in time associated with the T
wave; and
outputting at least one repolarization index based on the one or more
repolarization measures.
[0003B] Various embodiments of the claimed invention also relate to a method
comprising
using one or more electrodes placed on a patient, measuring one or more
electrocardiograms
associated with one or more respective leads. The method further comprises
using a computer-
implemented processing facility to perform operations comprising: converting
the one or more
electrocardiograms by time-frequency transform into one or more respective two-
dimensional
time-frequency maps; identifying, within the one or more electrocardiograms,
one or more
points in time associated with a T wave; determining, for at least one of the
one or more time-
frequency maps, one or more repolarization measures corresponding to extrema
of the
respective time-frequency map at the one or more points in time associated
with the T wave;
and outputting at least one repolarization index based on the one or more
repolarization
measures.
[0003C] Various embodiments of the claimed invention also relate to a heart
test system
comprising: an electrode interface configured to receive one or more
electrocardiogram
signals via one or more respective electrodes connectable to the electrode
interface; and a
la
Date Recue/Date Received 2023-08-10
processing facility communicatively coupled to the electrode interface, the
processing facility
comprising at least one computer processor and memory storing instructions for
execution by
the at least one computer processor. The instructions, when executed, cause
the at least one
computer processor to: generate, from the one or more electrocardiogram
signals, one or more
electrocardiograms for one or more respective leads; convert the one or more
electrocardiograms by time-frequency transform into one or more respective two-
dimensional
time-frequency maps; identify, within the one or more electrocardiograms, one
or more points
in time associated with a T wave; determine, for at least one of the one or
more time-
frequency maps, one or more repolarization measures corresponding to extrema
of the
respective time-frequency map at the one or more points in time associated
with the T wave;
and output at least one repolarization index based on the one or more
repolarization measures.
[0003D] Various embodiments of the claimed invention also relate to a method
comprising
receiving one or more electrocardiograms acquired for a patient for one or
more respective
leads. The method further comprises using one or more computer processors to
execute
instructions for processing the one or more electrocardiograms. The
instructions, when
executed, cause the one or more computer processors to perform operations
comprising:
converting the one or more electrocardiograms by time-frequency transform into
one or more
respective two-dimensional time-frequency maps; identifying, within the one or
more
electrocardiograms, one or more points in time associated with a T wave;
determining, for at
least one of the one or more time-frequency maps, one or more repolarization
measures
corresponding to extrema of the respective time-frequency map at the one or
more points in
time associated with the T wave; and outputting at least one repolarization
index based on the
one or more repolarization measures.
[0003E] Various embodiments of the claimed invention also relate to a heart
test system
comprising: one or more computer processors; and memory storing instructions
for execution
by the one or more computer processors. The instructions, when executed, cause
the one or
more computer processors to process one or more electrocardiograms acquired
for a patient
for one or more respective leads by performing operations comprising:
converting the one or
more electrocardiograms by time-frequency transform into one or more
respective two-
dimensional time-frequency maps; identifying, within the one or more
electrocardiograms,
lb
Date Recue/Date Received 2023-08-10
one or more points in time associated with a T wave; determining, for at least
one of the one
or more time-frequency maps, one or more repolarization measures corresponding
to extrema
of the respective time-frequency map at the one or more points in time
associated with the T
wave; and causing display to a user, on a display device or in a printable
report, of at least one
repolarization index determined based on the one or more repolarization
measures.
[0003F] Various embodiments of the claimed invention also relate to a heart
test system
comprising: one or more computer processors; and memory storing instructions
for execution
by the one or more computer processors, the instructions, when executed,
causing the one or
more computer processors to process one or more electrocardiograms acquired
for a patient
for one or more respective leads by performing operations comprising:
converting the one or
more electrocardiograms by time-frequency transform into one or more
respective two-
dimensional time-frequency maps; identifying, within the one or more
electrocardiograms,
points in time associated with a T wave, the points in time comprising at
least a first point in
time preceding a maximum of the T wave and a second point in time following
the maximum
of the T wave; determining, for at least one of the one or more time-frequency
maps,
repolarization measures corresponding to extrema of the respective time-
frequency map at the
one or more points in time associated with the T wave, the repolarization
measures comprising
a first repolarization measure corresponding to an extremum of the respective
time-frequency
map at the first point in time associated with the T wave and a second
repolarization measure
corresponding to an extremum of the respective time-frequency map at the
second point in
time associated with the T wave; and comparing the first repolarization
measure with the
second repolarization measure and determining a heart condition based on the
comparison.
[0003G] Various embodiments of the claimed invention also relate to a heart
test system
comprising: one or more computer processors; and memory storing instructions
for execution
by the one or more computer processors, the instructions, when executed,
causing the one or
more computer processors to process electrocardiograms acquired for a patient
for respective
leads, at least one of the electrocardiograms and the respective lead being
associated with a
left ventricle of a heart of the patient and at least one of the
electrocardiograms and the
respective lead being associated with a right ventricle of the heart of the
patient, the one or
more computer processors processing the electrocardiograms by performing
operations
lc
Date Recue/Date Received 2023-08-10
comprising: converting the electrocardiograms by time-frequency transform into
respective
two-dimensional time-frequency maps; identifying, within the
electrocardiograms, one or
more points in time associated with a T wave; determining one or more
repolarization
measures for the left ventricle from one or more time-frequency maps computed
from the at
least one of the electrocardiograms associated with the left ventricle, and
determining one or
more repolarization measures for the right ventricle from one or more time-
frequency maps
computed from the at least one of the electrocardiograms associated with the
right ventricle,
the one or more repolarization measures corresponding to extrema of the
respective time-
frequency map at the one or more points in time associated with the T wave;
comparing a left
.. ventricular repolarization index deteimined based on the one or more
repolarization measures
determined for the left ventricle with a right ventricular repolarization
index determined based
on the one or more repolarization measures determined for the right ventricle;
and determining
an abnormal heart condition based on the right ventricular repolarization
index being greater
than the left ventricular repolarization index.
10003111 Various embodiments of the claimed invention also relate to a heart
test system
comprising: one or more computer processors; and memory storing instructions
for execution
by the one or more computer processors. The instructions, when executed, cause
the one or
more computer processors to process one or more electrocardiograms acquired
for a patient
for one or more respective leads by performing operations comprising:
converting the one or
more electrocardiograms by time-frequency transform into one or more
respective two-
dimensional time-frequency maps; identifying, within the one or more
electrocardiograms,
one or more points in time associated with a T wave; determining, for at least
one of the one
or more time-frequency maps, one or more repolarization measures corresponding
to extrema
of the respective time-frequency map at the one or more points in time
associated with the T
wave; and determining a heart condition based on a comparison of at least one
repolarization
index determined based on the one or more repolarization measures against a
threshold.
id
Date Recue/Date Received 2023-08-10
BRIEF DESCRIPTION OF THE DRAWINGS
[0004] FIG. 1 is a block diagram illustrating an example system for
quantifying and
visualizing heart condition in accordance with various embodiments.
[0005] FIG. 2 is an example ECG, illustrating various segments and points in
time used in
accordance with various embodiments.
[0006] FIGS. 3A and 3B are graphs of an example ECG for a normal heart and a
scalogram
resulting from its wavelet transform, respectively, in accordance with one
embodiment.
[0007] FIGS. 3C and 3D are graphs of an example ECG for an abnormal heart and
a
scalogram resulting from its wavelet transform, respectively, in accordance
with one
embodiment.
[0008] FIG. 4 is a flow chart of methods for quantifying and visualizing heart
condition, in
accordance with various embodiments.
[0009] FIG. 5 is a perspective view of an example heart test device in
accordance with various
embodiments.
[0010] FIG. 6 is a user interface diagram for an example home screen in
accordance with
various embodiments.
[0011] FIG. 7 is a user interface diagram showing an example report screen in
accordance
with various embodiments.
[0012] FIGS. 8A-8C show the energy icon contained in the report screen of FIG.
7 in three
different states, corresponding to high myocardial energy, moderate myocardial
energy, and
low myocardial energy in accordance with various embodiments.
[0013] FIG. 9 is a user interface diagram showing a portion of the example
report screen of
FIG. 7 in a different scrolling position, in accordance with various
embodiments.
[0014] FIG. 10 is a user interface diagram showing an example report screen
including a user-
input control for lead selection in accordance with various embodiments.
[0015] FIG. 11A and 11B is a flow chart illustrating an electrocardiography
workflow in
accordance with various embodiments.
2
Date recue/Date received 2023-03-06
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
[0016] FIG. 12 is a block diagram of an example computer system as may serve
as
processing facility in accordance with various embodiments.
DESCRIPTION
[0017] Described herein, in various embodiments, are systems, devices, and
methods for
enhancing the diagnostic capabilities and utility of ECGs through advanced
signal processing
and through the presentation of data in a meaningful, user-friendly way.
[0018] In accordance with various embodiments, ECGs __ i.e., time-domain
signals
reflecting the electric potential of the heart throughout one or more cardiac
cycles ¨ are
computationally converted, by a suitable time-frequency transform, into
respective two-
dimensional time-frequency maps. In a "time-frequency map," as the term is
herein broadly
understood, the signal value (corresponding, e.g., to a measured electric
potential) is provided
as a function of two independent variables: time, and a measure of the
spectral components
of the signal such as, e.g., frequency (in the narrower sense) or a scaling
factor. For example,
in some embodiments, short-time Fourier transform is used to convert the ECGs
into
spectrograms, where the signal value is a function of time and frequency. In
other
embodiments, the ECGs are converted by (continuous or discrete) wavelet
transform into so-
called scalograms, where the signal value is a function of time and a scaling
factor. More
generally, a filter bank may be used to transform the ECG into a time-
frequency
representation. For ease of reference, the dimension of the time-frequency map
that
corresponds to the frequency or scaling factor (or any other measure of the
spectral
components) is herein generally referred to as the frequency dimension or
simply frequency.
[0019] The time-frequency maps, by themselves or in conjunction with the ECGs
from which
they are derived, may be displayed to a physician (or other clinical
personnel) for
interpretation, and/or analyzed automatically to derive quantitative metrics
of heart condition
and function therefrom, By spreading out the spectral components of the
measured ECG
signals, the time-frequency maps can visualize information not discernible
from the ECGs
themselves, which can help detect conditions traditionally not diagnosed based
on ECGs,
such as, e.g., myocardial ischemia.
[0020] It has been found that the signal portion associated with the T wave
within the ECG,
which represents the repolarization of the ventricles, is a particularly
suitable indicator of
heart condition. Accordingly, in various embodiments, repolarization measures
associated
with one or more points in time (or ranges in time) within the T wave are
determined. More
3
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
specifically, in some embodiments, the T wave, and one or more relevant points
in time
therein, are identified within an ECG, and the time-frequency map derived from
the ECG is
then analyzed at the one or more points in time to determine one or more
extrema (i.e.,
maxima or minima) of the signal value of the time-frequency map across
frequency. (The
phrase "across frequency," in this context, means that the maximum or minimum
is
determined for a fixed point in time from the signal value of the time-
frequency map as a
(one-dimensional) function of frequency only. By contrast, an extremum
determined "across
time and frequency" is the maximum or minimum signal value within the two-
dimensional
time-frequency map (or a two-dimensional portion thereof, e.g., if the time
dimension is
limited to an interval)) In certain embodiments, repolarization measures are
determined for
the point in time where the T wave peaks (i.e., assumes its maximum), and/or
for "early"
and/or "late" points in time within the T wave, that is, for points in time
preceding and/or
following the maximum of the T wave and being in the vicinity of that maximum
(e.g., points
falling within a time interval defined by two points bracketing the T wave
maximum at which
the T wave assumes half its maximum value). In certain embodiments, the early
and late
times are selected as close as possible to the peak while still being
distinguishable. A
"repolarization peak measure (RPM)," a "repolarization early measure (REM),"
and a
"repolarization late measure (RLM)" are defined herein as the maximum or
minimum signal
value of the time-frequency map at the time where the T wave peaks, the early
time, and the
late time, respectively.
10021] From one or more repolarization measures (e.g., corresponding to
extrema across
frequency at various points in time associated with the T wave) determined for
a patient, one
or more repolarization indices may be computed. For example, repolarization
measures (such
as, e.g., REMs, RLMs, and/or RPMs) may be determined from time-frequency maps
computed from the ECGs of different leads (signals acquired by different
electrodes or
combinations thereof), and may be averaged across multiple leads, multiple
cardiac cycles
within each time-frequency map, or both. Left and right ventricular
repolarization indices,
indicative of the condition of the left or right ventricles of the heart, may
be derived from one
or more repolarization measures associated with leads associated with the left
and right
ventricle, respectively, optionally in conjunction with age- and/or gender-
dependent
adjustment factors and/or a measured heart rate. The repolarization indices
may be displayed
or otherwise communicated to a physician (or other clinically trained person)
to facilitate an
assessment of the ventricles' condition, or may be output to an automatic
diagnosis
algorithm. In some embodiments, repolarization measures or repolarization
indices are
4
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
compared against each other, or against a threshold, to assess whether, and/or
to which
degree, heart function is impaired. For example, an RLM exceeding an REM, a
right
ventricular repolarization index exceeding a left ventricular repolarization
index, or an RLM
or REM falling below a specified threshold may all indicate an abnormality in
heart function.
[0022] The overall signal level in ECGs, and consequently also the time-
frequency maps
derived therefrom, can show significant variations between measurements (e.g.,
taken at
different times) and between leads within a measurement that are unrelated to
the heart's
condition and function and, thus, do usually not possess clinical
significance. Therefore, to
render the data (including ECGs, time-frequency maps, repolarization measures,
and
repolarization indices) comparable across measurements, leads, or even
patients, the time-
frequency map is normalized, in various embodiments, prior to display and/or
to the
determination of the repolarization measures. Normalization may be applied to
the signed
time-frequency map as it results directly from time-frequency transform, that
is, a time-
frequency map generally having both positive and negative signal values, or to
the unsigned
time-frequency map as it results by taking the absolute value of the time-
frequency transform.
Further, the normalization may be uniformly applied to the entire time-
frequency map, or
separately to different portions thereof (e.g., portions corresponding to
individual heartbeats.)
The normalization may be based on the difference between the maximum and the
minimum
of the time-frequency map (in its entirety) or a portion thereof (e.g., a
portion limited, in the
time dimension, to a time interval corresponding to an integer number of
heartbeats, a single
heartbeat, or even only a segment of the ECG signal within a heartbeat). For
example, the
time-frequency map may be shifted up in signal value by the negative of its
minimum value
(such that the minimum of the shifted map equals zero), and thereafter scaled
based on the
(new) maximum value. Typically, the maximum of the time-frequency transform
corresponds to the R peak or the S peak within the QRS complex (which are not
always
clearly identifiable in each lead), but maxima falling outside the time
interval corresponding
to the QRS complex are also possible. In various embodiments, the portion of
the time-
frequency map across which a maximum and minimum for normalization are
identified is
chosen to encompass at least the RS segment.
[0023] In accordance with various embodiments, the ECGs, time-frequency maps,
and/or
repolarization indices resulting from heart testing are assembled into a user
interface for
display to, e.g., a physician. The time-frequency maps may be shown as color
maps (which,
if based on normalized signal values, may each span the full color range from
red to blue).
Since, in general, not all ECGs and associated time-frequency maps always fit
simultaneously
5
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
within the display of the heart test device, the user interface may provide
user-input control
elements that allow an operator to select the leads for which ECGs and/or time-
frequency
maps are to be displayed, and in which order. The user interface may further
enable the
operator to scroll through all available leads and, within the ECG and/or time-
frequency map
for a given lead, to scroll along the time axis to different portions; in some
embodiments,
such scrolling can be accomplished via a swiping gesture on a touchscreen
display. During
scrolling along the time axis, an ECG and its corresponding time-frequency map
may be
locked so as to both display the same limited time range. In some embodiments,
the user
interface further displays a Glasgow-analysis summary (as known to those of
ordinary skill in
the art), and/or a graphic icon generated based on the numerical
repolarization indices to
provide an intuitive visual indicator of overall heart condition. The icon
may, for instance, be
or include a segmented waveform symbol that signifies, via a number of greyed-
out segments
within an otherwise colored symbol, a degree of impairment of heart function
(e.g., whether
the heart condition is normal, abnormal, or suspect). The results of ECG
testing may be
displayed within a report screen of a multiple-screen user interface
configured to guide
clinical personnel through the electrocardiography process, from patient
selection and
connection of the patient electrodes through the performance of an
electrocardiography test to
the presentation of the test results.
10024] The foregoing will be more readily understood from the following more
detailed
description, which references the accompanying drawings.
10025] FIG. 1 illustrates, in block-diagram form, various functional
components of an
example system for quantifying and visualization heart condition in accordance
with various
embodiments. The system 100 includes one or more electrodes 102 for acquiring
ECG
signals (e.g., 10 electrodes for a traditional 12-lead ECG), a processing
facility 104 for
processing the ECG signals, e.g., to obtain time-frequency maps and
repolarization indices,
and an electrode interface 106 connecting the electrodes 102 to the processing
facility 104.
The electrode interface 106 includes circuitry that outputs electrical signals
suitable as input
to the processing facility 104, e.g., by digitally sampling analog input
signals. The system
100 further includes a display device 108 for outputting the ECG test results
(including, e.g.,
the ECGs, time-frequency maps, and/or repolarization indices), and optionally
other
input/output devices 109, such as a keyboard and mouse and/or a printer, for
instance. The
display device 108 may be a touchscreen doubling as a user-input device. The
processing
facility 104, electrode interface 106, display 108, and input/output devices
109 may be
implemented as a single, stand-alone device implementing all computational
functionality for
6
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
ECG signal processing and presentation. Alternatively, they may be provided by
the
combination of multiple communicatively coupled devices. For example, an ECG
test device
with limited functionality for recording and/or processing ECG signals
received from one or
more electrodes 102 via an electrode interface 106 of the device may outsource
certain
computationally intense processing tasks to other computers with which it is
communicatively coupled via a wired or wireless network. Thus, the
functionality of the
processing facility 104 may be distributed between multiple computational
devices that
communicate with each other. Whether provided in a single device or
distributed, the
processing facility 104 may be implemented with dedicated, special-purpose
circuitry (such
as, e.g., a digital signal processor (DSP), field-programmable gate array
(FPGA), analog
circuitry, or other), a suitably programmed general-purpose computer
(including at least one
processor and associated memory), or a combination of both.
[0026] The processing facility 104 may include various functionally distinct
modules, such as
an ECG-signal-processing module 110 that prepares the (e.g., digitally
sampled) electrical
potentials for display (e.g., by filtering, smoothing, scaling, etc.) and
analysis; a time-
frequency transform module 112 that converts each ECG signal into a two-
dimensional time-
frequency map (signed or unsigned) and, optionally, normalizes the time-
frequency map; an
index-builder module 114 that analyzes the ECGs and/or time-frequency maps to
determine
repolarization measures and/or repolarization indices (which may involve,
e.g., identifying
delimiters between successive cardiac cycles, determining certain features
(such as the QRS
complex, T wave, and other segments) within the ECUs, selecting points in time
within the T
wave, determining repolarization measures (such as, e.g., REMs, RLMs, and/or
RPMs) from
the time-frequency maps, reading in any other relevant parameters (such as
gender- or age-
based adjustment factors, heart rate, etc.), and computing the ventricular
indices and/or any
functions thereof); an analysis module 116 that derives further metrics and/or
determines
heart condition from the repolarization indices; and a user-interface 118
module that
generates graphic representations of the data provided by the other modules
and assembles
them into a screen for display. The ECG-signal-processing module 110 may be a
conventional processing module as used in commercially available heart
monitors and/or as is
capable of straightforward implementation by one of ordinary skill in the art.
The time-
frequency transform module 112, index-builder module 114, analysis module 116,
and user-
interface module 118 implement algorithms and provide functionality explained
in detail
below, and can be readily implemented by one of ordinary skill in the art
given the benefit of
the present disclosure.
7
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
[0027] As will be readily appreciated, the depicted modules reflect merely one
among several
different possibilities for organizing the overall computational functionality
of the processing
facility 104. The modules may, of course, be further partitioned, combined, or
altered to
distribute the functionality differently. The various modules may be
implemented as
hardware modules, software modules executed by a general-purpose processor, or
a
combination of both. For example, it is conceivable to implement the time-
frequency
transform module 112, which generally involves the same operations for each
incoming ECG
signal, with special-purpose circuitry to optimize performance, while
implementing the
index-builder module 114 and the analysis module 116 in software to provide
flexibility for
adjusting parameters and algorithms, e.g., in response to new medical data.
[0028] While the quantification of heart function in accordance herewith is,
in general, not
limited to any particular number of electrodes, the system 100 includes, in
various
embodiments, ten electrodes 102 to facilitate obtaining a standard twelve-lead
ECG, as is
routinely used in the medical arts. In accordance with the standard
configuration, four of the
ten electrodes (conventionally labeled LA, RA, LL, RL) are placed on the
patient's left and
right arms and legs; two electrodes (labeled VI and V2) are placed between the
fourth and
fifth ribs on the left and right side of the sternum; a further, single
electrode (labeled V3) is
placed between V2 and V4 on the fourth intercostal space; one electrode
(labeled V4) is
placed between the fifth and sixth ribs at the mid-clavicular line (the
imaginary reference line
that extends down from the middle of the clavicle), and, in line therewith,
another electrode
(labeled V5) is positioned in the anterior axillary line (the imaginary
reference line running
southward from the point where the collarbone and arm meet), and the tenth
electrode
(labeled V6) is placed on the same horizontal line as these two, but oriented
along the mid-
axillary line (the imaginary reference point straight down from the patient's
armpit). The
electric potentials measured by electrodes V1 through V6 correspond to six of
the twelve
standard leads; the remaining six leads correspond to the following
combinations of the
signals measured with the individual electrodes: I= LA ¨ RA; IT = LL ¨ RA; In
= LL ¨ LA;
a'VR = RA ¨ (LA+LL); aVL = LA ¨1/2 (FtA+LL); and aVF = LL ¨ 1/2 (RA+LA).
[0029] FIG. 2 schematically shows an example ECG 200 for a single cardiac
cycle,
illustrating the P wave 202, QRS complex 204 (which includes the RS segment
206), and T
wave 208. As depicted, the electric potential usually reaches its maximum 210
at R during
the QRS complex 204. However, the polarity of the signal may be inverted (such
that the R
peak has a negative value). Further, in some ECG signals, the S peak has a
greater absolute
value than the R peak. In fact, not every ECG unambiguously exhibits the
features shown in
8
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
the (rather typical) example ECG 200. This uncertainty can cause difficulty in
attempts to
normalize the signal based on a discrete feature of the ECG such as, e.g., the
R peak. To
circumvent this difficulty, various embodiments base normalization, instead,
on a signal
maximum and minimum identified across a time range, such as the time interval
encompassing at least the RS segment 206 (and thus including both the R and
the S peak if
they are, in fact, clearly represented in the signal), irrespective of the
feature to which that
maximum or minimum corresponds (if any). FIG. 2 also illustrates certain
points in time at
which data is evaluated in accordance with various embodiments, such as the
time 212 at
which the T wave 208 assumes its maximum, and example early and late times
214, 216
bracketing the maximum of the T wave. In general, the early and late times
214, 216 may be
anywhere on the rising edge and falling edge, respectively, of the T wave. In
various
embodiments, they are selected within ranges between the T wave maximum and
points in
time preceding and following the T wave maximum, respectively, at which the T
wave
assumes some specified fraction, e.g., half, of its maximum value.
[0030] In accordance herewith, the measured ECGs are transformed into two-
dimensional
time-frequency maps by a suitable mathematical transform, such as, for
instance, wavelet
transform. For a given continuous ECG signal x(t), the continuous wavelet
transform is
given by:
W (a, b) = f (¨t-b) x(t)dt,
va ¨oo a
where is a selected wavelet, b corresponds to a shifted position in time and a
to a scaling
factor, and W (a, b) is the two-dimensional function of position in time and
scale resulting
from the transform, also called wavelet coefficients. Similarly, for a
discretized ECG signal
x(k) (where k is an integer), the continuous wavelet transform is given by:
(.11(k.0+1)T (t¨b) sikT t¨b _,,)
W (a, b) = x(k) ¨ L ) I UL
a ¨00 st" k. ( a y
where T is the sampling period. The wavelet selected for processing may be,
for example, a
Mexican hat wavelet, Morlet wavelet, Meyer wavelet, Shannon wavelet, Spline
wavelet, or
other wavelet known to those of ordinary skill in the art. Other well-known
time-frequency
transforms that may be used alternatively to continuous or discrete wavelet
transform include,
e.g., the short-term Fourier transform.
9
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
[0031] The time-frequency maps (such as, e.g., scalograms) generally include
both positive
and negative values. For an intuitive interpretation of the signal value of
the time-frequency
map as a measure of the electrical energy of the heart, however, the sign is
not relevant
(since, in a measure of the energy, the electrical potential is squared).
Accordingly, in some
embodiments, the absolute value of the signal value (or the square of the
signal value) is
taken at each time-frequency point, resulting in an unsigned time-frequency
map. The
unsigned time-frequency map may be advantageous, in particular, for display in
a user
interface (e.g., to a physician) since it avoids presenting information that
is not of immediate,
intuitively discernible clinical significance and is potentially distracting.
On the other hand,
since the signed time-frequency map contains generally more information than
the unsigned
time-frequency map, the computation of repolarization measures and indices may
(but need
not) be based on the signed map.
[00321 FIGS. 3A and 3B illustrate an example ECG for a normal heart and an
unsigned
scalogram resulting from its wavelet transform (followed by taking the
absolute),
respectively. In the scalogram, the position b corresponding to time is along
the abscissa and
the scale a (corresponding to frequency) along the ordinate, and the signal
value W is
encoded by color or intensity (e.g., gray-scale value). As can be seen, the
various peaks of
the normal ECG are reflected in relatively high intensity in the scalogram,
allowing
identification of the different ECG segments. For comparison, FIGS. 3C and 3D
show an
example ECG and associated scalogram, respectively, for an abnormal heart.
Here, features
that are prominent in the normal scalogram (e.g., the T wave) have rather low
intensity.
While this lower intensity generally tracks the lower values of the T wave in
the ECG, it will
be appreciated that the scalogram may provide better visual clues.
Accordingly, the
scalogram can aid a physician or other skilled clinician to assess heart
functioning.
[0033] To facilitate meaningful comparisons between time-frequency maps
derived from
ECGs obtained simultaneously for different leads, the time-frequency maps may
be
normalized. Normalization may involve scaling and/or shifting signal values in
the time-
frequency map to map the range of signal values in the map (or at least a
portion of the map,
as explained below) to a specified numerical range (hereinafter "target
range"), e.g., 0 to 255
or -128 to +127 (as are convenient ranges for binary representations, and can,
in turn, be
straightforwardly mapped onto color or gray-scale values for display). Using a
particular
normalization and the associated target range consistently not only across
leads, but also
across measurements taken at different times and/or even for different
patients may also serve
to improve comparability of data over time and across the patient population,
as it eliminates
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
or at least reduces overall signal-level variations, which are often not
attributable to different
heart conditions, allowing physicians to focus on the clinically relevant
relative signal levels
within a time-frequency map.
[0034] The normalization may be based on a regional maximum and minimum
defined as the
maximum and minimum of the time-frequency map across frequency and across time
within
a selected interval, and may then be applied to a second selected interval
that may or may not
be the same as the first selected interval. The maximum and minimum of the
time-frequency
map across frequency and across time within that second selected interval are
hereinafter
called the absolute maximum and minimum, and they may, but need not, coincide
with the
regional maximum and minimum. The first selected interval is typically, but
not required to
be, shorter than the second selected interval. In some embodiments, the
regional maximum
and minimum are determined across the entire time-frequency map, corresponding
to the
entire measurement time of the ECG from which it is derived, and the
normalization is
applied over that same range (such that the first and second selected
intervals are equal). In
other embodiments, the regional maximum and minimum are identified within a
portion of
the time-frequency map that is limited in its time dimension, e.g., to an
integer number of
heartbeats (e.g., disregarding partial heartbeats) or only a single heartbeat.
A time-frequency
map encompassing multiple heartbeats may, for instance, be broken up into
portions
corresponding to individual heartbeats, and each portion may be normalized
separately
(potentially resulting in some discontinuity of the signal values in the
normalized time-
frequency map); in this case, first and second selected intervals are likewise
equal to each
other. Normalization may even be based on a time interval encompassing only
part of a
heartbeat, selected to likely (but not certainly) include the absolute maximum
and minimum.
For instance, in some embodiments, regional maximum and minimum are determined
within
a portion of a time-frequency map that encompasses at least the RS segment.
Note, however,
that it is possible for, e.g., the T wave maximum to exceed the maximum in the
QRS
complex. In cases where the absolute maximum and minimum of the time-frequency
map lie
outside the portion of the map across which the regional maximum and minimum
are
determined, the normalization will result in signal values exceeding the
target range.
(Normalization may also be applied in the time domain. In this case, the
regional minimum
and maximum are across time over the selected time interval.)
[0035] Normalization may be applied according to the following equation:
n (d ,.,min) * (nmax¨nmin)
(dmax¨dmin)
11
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
where
fl is the normalized data point;
nmin is the normalized target-range minimum;
nmax is the normalized target-range maximum;
d is the data point to be normalized;
dmin is the regional minimum; and
dmax is the regional maximum.
For example, to map onto the target range from 0 to 255, nmin is 0 and nmax is
255; in
effect, this normalization shifts the time-frequency map to a minimum equal to
zero and
thereafter scales the shifted map based on its shifted regional maximum. More
generally, the
normalization shifts the time-frequency map to a minimum equal to nmin and
then scales the
values of the shifted time-frequency map (taken relative to the minimum value)
by the ratio
of the difference between maximum and minimum of the target range to the
difference
between the regional maximum and minimum.
[0036] Normalization can be applied to signed as well as unsigned time-
frequency maps. As
will be appreciated, the result of the normalization will vary depending on
whether the
underlying time-frequency map is signed or unsigned. For example, when mapping
a signed
time-frequency map with a positive R peak and a negative S peak onto the
target range from
0 to 255, several of the frequencies at the point in time corresponding to the
S peak will map
to or near zero. However, when the normalization is applied to the absolute
value of the
otherwise same time-frequency map, some frequencies at points in time between
R and S will
now map to or near zero whereas several of the frequencies at the point in
time corresponding
to the S peak will map onto a relatively larger positive number within the
target range.
[0037] The time-frequency maps (optionally following normalization) may be
displayed to a
physician for evaluation. Alternatively or additionally, they may be further
analyzed, in
accordance with various embodiments, to determine various quantitative
indicators of heart
condition and function. To that end, various measures of the electric activity
of the heart can
be obtained, e.g., by determining extrema (i.e., maximum and/or minimum
values) across
frequency of the (normalized) time-frequency map (W or I WI) at certain points
(or ranges) in
time corresponding to distinctive features of the underlying ECGs, in
particular, certain
points (or ranges) in time associated with the T wave. Measures associated
with the T wave
12
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
are herein referred to as "repolarization measures" and include, for example,
the maximum
value at an early time within the T wave (REM), the maximum value at a late
time within the
T wave (RLM), or the maximum value at the peak of the T wave (RPM). Additional
repolarization measures, e.g., including an integral over a time interval
within the T wave,
may also be defined and used to quantify heart condition.
[0038] From the repolarization measures determined in the time-frequency map,
one or more
repolarization indices may be derived, e.g., by averaging or based on
information external to
the ECG or time-frequency map. For example, if the repolarization measures are
obtained
based on ECGs covering multiple cardiac cycles, the individually determined
maxima may be
averaged over these cycles. Further, the various repolarization measures can
generally be
derived separately from different time-frequency maps obtained by transform of
ECGs
measured for different respective leads, and repolarization measures of the
same type (e.g.,
the REMs) may be averaged across multiple leads. In particular, ventricular
repolarization
indices may be derived by averaging only across leads associated with the same
(i.e., left or
right) ventricle. For example, a ventricular index early measure for the right
ventricle
(VIEM RV) may be calculated by (e.g., arithmetically) averaging over the REMs
of leads V1
and V2, a ventricular index late measure for the right ventricle (VILM RV) may
be
calculated by averaging over the RLMs of leads V1 and V2, and a ventricular
index peak
measure for the right ventricle (VIF'M RV) may be calculated by averaging over
the RPMs
of leads V1 and V2. Similarly, VIEM, VILM, and/or VIPM for the left ventricle
(VIEM_LV,
VILM LV, and VIPM_RV) may be calculated by averaging over the REMs, RLMs, and
RPMs, respectively, of leads V4, V5, and V6. In certain embodiments, further
indices are
derived from the preceding ones. For instance, a ventricular index average
measure for the
right ventricle (VIAM_RV) may be calculated as the sum of VIEM RV and VILM RV,
divided by the heart rate (measured in beats per minute). Similarly, a
ventricular index
average measure for the left ventricle (VIAM_RV) may be calculated as the sum
of
VIEM LV and VILM LV, divided by the heart rate. Further, in some embodiments,
an
index for the heart as a whole is computed from respective indices for the
left and right
ventricles, e.g., by forming the ratio, difference, or some other function of
left and right
ventricular indices.
[0039] Further, while the repolarization measures are generally indicators of
how well the
heart functions, they can also be affected by age and gender, independently of
any abnormal
heart condition. To eliminate or at least reduce differences that do not
result from heart
abnormalities, the repolarization measures may be adjusted, when computing
repolarization
13
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
indices, with suitable age- and/or gender-dependent factors. In one
embodiment, the
adjustment distinguishes merely between male and female patients, using an
adjustment
factor of 1 for males (i.e., keeping the measures as is) and an adjustment
factor smaller than
one (e.g., 1/1.24) for females. In some embodiments, further refinements are
made to
.. distinguish between patients up to forty years old and patients older than
forty years. For
example, for females older than forty years, the adjustment factor may be
decreased to 1/1.26.
Other age-based classifications and adjustment factors may be implemented as
well.
[0040] FIG. 4 is a flow chart summarizing methods 400 for quantifying and
visualizing heart
condition in accordance with various embodiments. The method 400 involves
measuring one
or more ECGs associated with one or more respective leads (action 402), using
one or more
electrodes placed on a patient. In some embodiments, ten electrodes are used
to obtain
twelve leads. (The phrase "measuring electrocardiograms" is intended to
encompass both the
acquisition of electrocardiogram signals with the electrodes, and the
digitization and/or initial
processing of these signals to generate an electrocardiogram for each lead,
which may include
.. combining multiple electrocardiogram signals to obtain an electrocardiogram
for a single
lead, as described above.) In action 404, the ECG(s) are converted by time-
frequency
transform (e.g., wavelet transform) into one or more respective two-
dimensional time-
frequency maps (e.g., scalograms). The time-frequency map(s) may be used in
the original
signed form, or converted to unsigned map(s) by taking the absolute value at
each time-
.. frequency point (optional action 406), or both. Further, the time-frequency
map(s) may be
normalized (action 408), as described above. In some embodiments, for example,
the time-
frequency map is normalized based on the maximum and minimum identified in the
time-
frequency map across frequency and across a time interval encompassing at
least the RS
segment (and, in some embodiments, encompassing a fill cardiac cycle (or
heartbeat),
multiple (an integer number of) cardiac cycles, or the entirety of the
measurement time).
[0041] To visualize the heart condition, a user interface displaying the ECGs
and/or
corresponding time-frequency maps may be generated (action 410). The signal
values in the
time-frequency maps may be, e.g., color-coded or represented according to a
gray scale. To
focus the user's (e.g., an interpreting physician's) attention on the
electrical energy of the
heart, it may be beneficial, as indicated above, to present unsigned (i.e.,
absolute-value) time-
frequency maps. Due to spatial constraints, the user interface may, at any
given time, display
only portions of the ECGs and time-frequency maps corresponding to time
intervals smaller
than the total measurement time. For example, out of data for a twelve-second
interval, the
display may be limited to a three-second subset. In addition, the number of
ECGs and time
14
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
frequency maps displayed at any given time may be limited, e.g., to three out
of twelve ECGs
and corresponding time-frequency maps. The displayed selection of ECGs and
time-
frequency map and the displayed time-range may depend on, and be adjusted
based on, user
input (received at 412). For example, a drop-down menu displayed next to each
screen
portion allocated to an ECG and time-frequency map may facilitate selection of
any of the
available leads. Further, the user may have the ability to scroll through the
entire
measurement time, e.g., with a conventional scrollbar, or with a swiping
gesture performed,
with a mouse-controlled cursor or on a touchscreen, in a region displaying an
ECG or time-
frequency map. In order to enable features within an ECG to be properly
correlated with
features in the corresponding time-frequency map, the displayed portions are
temporally
aligned (and, usually, temporally coextensive), and the alignment is retained
(or, in other
words, "locked in") as the user scrolls through the ECG or time-frequency map.
Further, if
ECGs and time-frequency maps are displayed for multiple leads, they may
likewise be
temporally aligned and temporally coextensive, and locked-in in their
alignment as the user
scrolls through any one of them.
[0042] To quantify heart condition, the time-frequency maps are analyzed in
conjunction
with the respective ECGs. Specifically, in action 414, one or more points in
time associated
with the T wave (e.g., early and late times and/or the time where the T wave
peaks) are
identified within an ECG. The corresponding time-frequency map is then
analyzed at these
points in time to determine, separately at each point in time (or within a
small time interval
surrounding the respective points in time), a maximum and/or minimum across
frequency
(action 416). The one or more extrema across frequency determined in the time-
frequency
maps at one or more points in time identified in respective ECGs constitute
repolarization
measures. Based on these repolarization measures, one or more repolarization
indices can be
determined in action 418. A repolarization index may be based on (and in the
simplest case
be equal to) a single repolarization measure or combine multiple
repolarization measures
(e.g., by averaging repolarization measures over leads or cardiac cycles). In
addition, the
repolarization index may include an adjustment factor that is based on the age
or gender of
the patient, or on some other characteristic of the patient or circumstance of
the measurement.
The computed repolarization indices may be output (in action 420) in various
ways. For
example, they may be included in the user interface (e.g., along with the ECGs
and time-
frequency maps) for display on-screen or in a printable report, communicated
to the user in
some other manner, or provided as input to another algorithm.
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
[0043] In some embodiments, the repolarization measures and/or repolarization
indices are
automatically analyzed (action 422), based on heuristics or empirical data, to
obtain a
qualitative assessment of heart condition. For example, based on an
expectation that the early
repolarization measure is greater than the late repolarization measure,
observation of a late
repolarization measure exceeding the early repolarization measure (for the
same ECG and
time-frequency map) may be taken as a sign of abnormal or impaired heart
function, and
communicated as such to the user. Similarly, since the left ventricular
repolarization index
should be greater than the right ventricular repolarization index for a
healthy heart, the
reverse relationship (i.e., a right ventricular repolarization index greater
than the left
repolarization index) indicates an abnormality or impairment that may be
communicated to
the user. Accordingly, comparisons between repolarization measures and
repolarization
indices may be used to assess heart function. Alternatively or additionally,
repolarization
measures and indices (properly normalized or computed from normalized time-
frequency
maps) may be compared against empirical thresholds. For example, with a
normalization of
the time-frequency maps to a range from 0 to 255, an early or late
repolarization measure for
the left or right ventricle that falls below a threshold in the range from 55-
75 has been found
to correlate strongly with some problem in heart function. In some
embodiments, one or
more repolarization indices are used to determine a myocardial energy
category, e.g.,
distinguishing between high energy (corresponding to no or low functional
impairment),
moderate energy (corresponding to moderate functional impairment), and low
energy
(corresponding to high functional impairment). Comparisons of repolarization
measures
and/or repolarization indices against each other or against specified
thresholds in various
combinations may also serve to categorize heart function as normal, suspect,
or abnormal.
[0044] Various modifications of the method 400 may be implemented. For
example, as
noted above, ventricular indices may be computed based on repolarization
measures
determined from values of the time-frequency map at one or more points in time
other than
early or late times, and/or over one or more ranges of time. Further, not
every action of the
depicted method 400 need be implemented in every embodiment. Accordingly, the
depicted
method 400 is to be understood as one example embodiment only.
[0045] FIG. 5 shows an example heart test device 500 in perspective view. The
depicted
device takes the form of a tablet computer 500 including a touchscreen display
502 as well as
a control panel 504 with physical buttons (e.g., to power the tablet 500
on/off). In some
embodiments, as shown, the display 502 presents a multi-tab user interface,
explained in
more detail below with respect to FIGS. 6-10. Some of the tabs (shown along
the right edge
16
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
of the display 502) may be duplicated by the physical buttons of the control
panel 504,
allowing an operator to navigate between different screens and associated
device functions in
different ways. Electrodes for acquiring the ECG signals may be hooked up to
the tablet
computer 500 via a suitable connector 506 (e.g., a DB15 connector). The tablet
500 contains
a general-purpose processor and volatile as well as non-volatile memory
storing instructions
for implementing the functional processing modules 110, 112, 114, 116, 118. Of
course, in
various alternative embodiments, the heart test device may take different form
factors, such
as that of a desktop computer, laptop computer, or smartphone (to name just a
few), each
with a suitable electrode interface, which may include custom circuitry for
converting the
electrode signals into digital signals suitable for further processing with
software.
Furthermore, an electrocardiography system providing the functionality
described herein
need not necessarily be implemented in a single device, but can be provided by
multiple
devices used in combination, e.g., a conventional ECG monitor connected to a
general-
purpose computer running software to implement the processing functionality
described
herein,
[0046] Turing now to the user interface, FIG. 6 depicts an example home screen
of the user
interface as it may appear, e.g., when an operator first turns on the ECG test
device 500. The
home screen may, for example, provide links to reference materials such as a
quick-start
guide and a more comprehensive user manual. In accordance with one embodiment,
as
shown, the user interface includes multiple tabs, corresponding to multiple
respective screens,
that are visible in each screen (e.g., on the right hand side), allowing easy
navigation between
the screens. The tabs may be arranged in an order that corresponds to the
natural workflow
through the electrocardiography process, described further below. For example,
in addition
to the general tabs for the home screen and a settings screen, the tabs may
include, in this
order, a patient tab, a test tab, and a report tab.
[0047] FIG. 7 illustrates an example report screen in accordance with various
embodiments.
As shown, the report screen may be partitioned into multiple screen portions
arranged in an
intuitive manner so as to allow the viewer to quickly locate the desired
information. At the
top of the screen, patient information, such as a unique patient identifier
and the patient's
name, as well as patient-specific parameters affecting the interpretation of
the ECGs, such as
age and gender, may be displayed, along with a record identifier composed of,
e.g., a date and
time stamp for the test. In a left panel, ECGs and time-frequency maps for one
or more leads
may be displayed, e.g., in a vertical arrangement. The time-frequency maps can
visualize
information not discernible from the ECGs from which they are derived, e.g.,
by providing a
17
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
picture of the electrical energy of the heart during various stages within the
cardiac cycle, and
can be useful in detecting conditions such as myocardial ischemia, which are
traditionally not
diagnosed based on ECGs. ECGs are included in the display because of their
familiarity to
physicians and other medical practitioners and for the purpose of identifying
temporally
defined features of the signal, such as the QRS complex and T wave. In
accordance with
various embodiments, the signal value of the time-frequency map (e.g., the
electrical
potential or voltage that is plotted as a function of time and frequency) is
encoded in a color
scale (or, alternatively, as shown in the black-and-white drawings, in a grey
scale). While the
signal value itself, as resulting from the time-frequency (e.g., short-time
Fourier or wavelet)
transform applied to the ECG, may be a signed value (generally resulting in
both positive and
negative values across the map), the color-coded depicted value may be
unsigned, as obtained
from a signed value by computing the absolute value. Using unsigned signal
values in the
color-coded maps serves to represent the energy level of the time-dependent
frequency
content, independent of the phase of those frequencies, thus allowing the
energy of either
positive or negative phase to appear at the same point (along frequency) on
the time-
frequency map.
[0048] As described above, the ECGs and time-frequency maps may be analyzed,
in
accordance with various embodiments, to provide quantitative indices
indicative of heart
health and/or a qualitative assessment or categorization. The results of the
analysis may be
presented, as shown in the right panel of FIG. 7, in numerical, textual,
and/or graphic form.
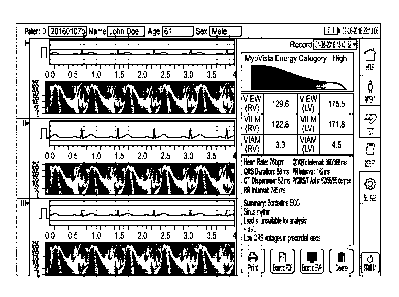
For example, as shown, the right panel may include an "energy icon"
representing the
patient's overall heart health, a number of numerical indices (e.g.,
repolarization indices as
described above) providing a more detailed picture underneath the icon, and a
conventional
Glasglow-analysis textual summary underneath the numerical indices. The
Glasgow-analysis
summary portion may display such metrics, derived from the ECGs, as the
patient's heart rate
and durations of certain ECG features (such as the QRS complex). In addition,
it may
summarize the quality and reliability of the test, e.g., based on signal-to-
noise levels of
various leads. Glasgow analysis is known to those of ordinary skill in the
art, and will not be
further elaborated upon herein.
[0049] FIGS. 8A-8C show the energy icon of FIG. 7 in isolation in three
different states,
corresponding to high myocardial energy, moderate myocardial energy, and low
myocardial
energy, respectively. (These three states may be interpreted as normal,
suspect, and abnormal
conditions, respectively.) In the depicted embodiment, the energy icon is a
segmented
waveform symbol including three segments 800, 802, 804, which are filled, for
a healthy
18
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
patient (FIG. 8A), with a color gradient (shown, due to the conversion to
black-and-white
drawings, with variations in the grayscale value) mirroring the color scale of
the time-
frequency maps. For a patient with moderately impaired heart function or
suspect heart
condition (FIG. 7B), the first, left-most segment is greyed-out (shown by a
uniform grey
filling as distinguished from the previous variation). For a patient with
strongly impaired or
abnormal heart function (FIG. 6C), both the left segment and the middle
segment are greyed
out, symbolizing the much lower myocardial energy. The energy icon, thus,
provides a
clinician with an immediate visual clue as to the patient's heart health. As
will be readily
appreciated, the energy icon is amenable to finer gradation of the diagnostic
assessment if
modified to include more than three segments.
[0050] For a large number of leads, e.g., for a full twelve-lead ECG, it is
generally
impractical to display all twelve ECGs and associated time-frequency maps at
once on the
display. Accordingly, in various embodiments, the user is given the ability to
scroll vertically
through the ECG (left) panel to view different ones of the leads. For
illustration, compare
FIGS. 7 and 9, for example. While, in FIG. 7, ECGs and time-frequency maps for
leads I, II,
and HI are shown, FIG. 9 illustrates the screen in a different scrolling
position where, instead,
ECGs and time-frequency maps for leads aVL and aVF can be seen.
[0051] Alternatively or additionally to being able to scroll through all
leads, the user may be
given the opportunity to select leads for display for each of the (sub-
)portions of the left panel
and thereby specify the order in which the leads are displayed. In various
embodiments, the
user-input controls for lead selection are drop-down menus displayed,
initially in their closed
state, adjacent the screen portions for respective ECGs and time-frequency
maps. Each drop-
down menu lists, once activated and opened by the operator, all twelve leads,
facilitating user
selection of any one of the leads for display within the current screen
portion; FIG. 10
illustrates an opened drop-down menu for the first displayed lead. In some
embodiments,
once a new lead is selected, its position is swapped with the lead that
previously occupied the
respective screen portion. For example, if lead V1 is changed to lead V5 in
the drop-down
menu, the vertical positions of the respective ECGs and time-frequency maps
will be
swapped, and V1 will appear where V5 was previously located.
[0052] As shown in the report screens depicted in FIGS. 7, 9, and 10, the ECGs
may be
displayed with the time axis extending horizontally (as is customary). In
accordance with
various embodiments, the corresponding time-frequency maps are likewise
oriented with
their time axes in the horizontal direction, and are temporally aligned with
the ECGs,
meaning that ECG and corresponding time-frequency map both show a given point
in time at
19
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
the same horizontal position. In addition to the temporal alignment within a
screen portion
showing an ECG and time-frequency map derived therefrom, the various screen
portions
displaying different ECGs (and corresponding time-frequency maps) may likewise
be
temporally aligned. Further, the left panel (and, indeed, the screen) may not
be wide enough
.. to display the ECGs and time-frequency maps in their entirety, covering the
full acquisition
period. Instead, the ECGs and time-frequency maps may be displayed partially,
for a limited
time range. The user interface may facilitate, however, a horizontal scroll by
the operator
through the ECG and/or time-frequency map to affect a temporal shift of the
limited time
range being displayed. During such a scroll, the ECG and corresponding time-
frequency map
.. may be "locked" so as to maintain their temporal alignment. Analogously,
the other ECGs
and time-frequency maps within the report screen may be locked to the screen
portion being
scrolled through, and thus move along with the scrolled through ECG/time-
frequency map.
A scroll can be effected in various ways, such as by a traditional scroll bar.
In various
embodiments, however, touchscreen capability of the display of the heart
monitor device is
exploited to allow scrolling via a swiping gesture performed on-screen in a
direction
substantially horizontal (and thus parallel to the time axis of the ECG),
within a screen
portion displaying the ECG and corresponding time-frequency map. From the
swiping
gesture, a shifted limited time range may be determined and applied to the
shifting of the
displayed ECG/time-frequency map portions. (As will be readily appreciated by
one of
ordinary skill in the art, the features of temporal alignment and temporal
locking described
above are not contingent upon the horizontal orientation of the time axis.
Rather, it is
conceivable that ECGs and/or corresponding time-frequency maps be displayed in
a
horizontal arrangement with their time axes pointing downward, in which case
the temporal
alignment would be vertical.)
10053] FIGS. 11A and 11B provide a flowchart illustrating an
electrocardiography workflow
1100 supported by the depicted user interface, in accordance with various
embodiments. The
medical professional performing this workflow is, in a typical clinical
setting (but not
necessarily), a nurse (rather than a physician). Herein, the person operating
the heart monitor
device (e.g., to perform the workflow depicted in FIGS. 11A and 11B, or to
subsequently
view the results) is generically called the "operator." In FIGS. 11A and 11B,
actions of the
operator are shown on the right, and operations performed by the heart test
device are
depicted on the left. Referring to FIG. 11A, the operator, being presented
with a multi-tab
user interface (action 1102), generally starts by selecting the patient tab
(action 1104). On
the patient screen displayed as a result (action 1106), the operator can
either select an existing
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
patient from a list (optionally in conjunction with filtering based on
operator-supplied search
tokens), or create a new patient, e.g., by pressing a "New" button displayed
on the screen and
entering the relevant patient information (action 1108). Once a patient has
been selected, a
pop-up message may briefly appear on the patient screen to confirm the
selection (action
.. 1110).
[0054] The operator then navigates to the test screen (action 1112). If the
patient has not
already been prepped for the test (e.g., electrodes have been attached to
patient, patient cable
has been attached to heart monitor device and electrodes, etc.), the operator
can do so at this
stage (action 1114). Once available, the test screen displays real-time traces
of the ECG
.. signals (action 1116). The operator usually views the real-time ECG traces
to assess whether
all the electrodes are connected and the ECG signals are adequate to proceed
with the
test. Once the operator is satisfied with the quality of the real-time traces,
he can initiate a
test (action 1118) by pressing, e.g., a "Test" button provided on the test
screen. Upon
activation, this button may be replaced by a "Stop/Countdown Timer" button
(action 1120)
that displays the remaining test duration while the test is running, and also
facilitates operator
abortion of the test.
[0055] When the ECG test is complete, the user interface automatically
navigates the
operator to the reports screen (action 1122). The reports screen may initially
(e.g., during the
first 15-20 seconds), while the indices and energy icon are being computed,
display merely
the ECGs and corresponding time-frequency maps as well as a Glasgow analysis
summary
for viewing by the operator (action 1124). Optionally, a "Calculating..." or
similar text
message may alert the operator that additional information is forthcoming. An
operator may
simply wait for the computation of the icon and indices to complete. Once the
reports screen
is updated with the computed indices and icon (action 1126), the operator may
view the
results (action 1128). The operator may also be given the option to print or
export the report
(e.g., to an external USB drive) (action 1130). If the operator chooses to
print the results (at
1128), a print-preview window may allow the operator to navigate the possibly
multiple
pages of the report as well as send the report to a network or physically
attached printer.
Printing is useful to allow a physician (who is not the operator) to view the
test results offline
before coming back into the exam room to discuss the results with the patient.
[00561 The embodiments describe hereinabove relate to the quantification and
visualization
of heart condition based on ECGs in conjunction with time-frequency maps
derived
therefrom. Some of the features described with reference to time-frequency
maps may,
however, also apply to, and be advantageous in the context of, the ECGs
themselves. For
21
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
example, for better comparability of the ECGs across leads, measurements, and
patients, the
ECGs may be normalized based on the absolute maximum and minimum of the ECG
(in its
entirety) or a portion thereof. Values of the normalized ECGs at certain
points in time
associated with the T wave may serve as repolarization measures for
quantification of heart
condition.
[0057] Certain embodiments are described herein as including a number of logic
components
or modules. Modules may constitute either software modules (e.g., code
embodied on a non-
transitory machine-readable medium or in a signal transmitted over a network)
or hardware-
implemented modules. A hardware-implemented module is a tangible unit capable
of
.. performing certain operations and may be configured or arranged in a
certain manner. In
example embodiments, one or more computer systems (e.g., a standalone, client
or server
computer system) or one or more processors may be configured by software
(e.g., an
application or application portion) as a hardware-implemented module that
operates to
perform certain operations as described herein.
.. [0058] In various embodiments, a hardware-implemented module may be
implemented
mechanically or electronically. For example, a hardware-implemented module may
comprise
dedicated circuitry or logic that is permanently configured (e.g., as a
special-purpose
processor, such as a field programmable gate array (FPGA) or an application-
specific
integrated circuit (ASIC)) to perform certain operations. A hardware-
implemented module
may also comprise programmable logic or circuitry (e.g., as encompassed within
a general-
purpose processor or other programmable processor) that is temporarily
configured by
software to perform certain operations. It will be appreciated that the
decision to implement a
hardware-implemented module mechanically, in dedicated and permanently
configured
circuitry, or in temporarily configured circuitry (e.g., configured by
software) may be driven
.. by cost and time considerations.
[0059] Accordingly, the term "hardware-implemented module" should be
understood to
encompass a tangible entity, be that an entity that is physically constructed,
permanently
configured (e.g., hardwired) or temporarily or transitorily configured (e.g.,
programmed) to
operate in a certain manner and/or to perform certain operations described
herein.
Considering embodiments in which hardware-implemented modules are temporarily
configured (e.g., programmed), each of the hardware-implemented modules need
not be
configured or instantiated at any one instance in time, For example, where the
hardware-
implemented modules comprise a general-purpose processor configured using
software, the
general-purpose processor may be configured as respective different hardware-
implemented
22
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
modules at different times. Software may accordingly configure a processor,
for example, to
constitute a particular hardware-implemented module at one instance of time
and to constitute
a different hardware-implemented module at a different instance of time.
[0060] Hardware-implemented modules can provide information to, and receive
information
from, other hardware-implemented modules. Accordingly, the described hardware-
implemented modules may be regarded as being communicatively coupled. Where
multiple
of such hardware-implemented modules exist contemporaneously, communications
may be
achieved through signal transmission (e.g., over appropriate circuits and
buses) that connect
the hardware-implemented modules. In embodiments in which multiple hardware-
implemented modules are configured or instantiated at different times,
communications
between such hardware-implemented modules may be achieved, for example,
through the
storage and retrieval of information in memory structures to which the
multiple hardware-
implemented modules have access. For example, one hardware-implemented module
may
perform an operation, and store the output of that operation in a memory
device to which it is
communicatively coupled. A further hardware-implemented module may then, at a
later
time, access the memory device to retrieve and process the stored output.
Hardware-
implemented modules may also initiate communications with input or output
devices, and
can operate on a resource (e.g., a collection of information).
[0061] The various operations of example methods described herein may be
performed, at
least partially, by one or more processors that are temporarily configured
(e.g., by software)
or permanently configured to perform the relevant operations. Whether
temporarily or
permanently configured, such processors may constitute processor-implemented
modules that
operate to perform one or more operations or functions. The modules referred
to herein may,
in some example embodiments, comprise processor-implemented modules.
[0062] Similarly, the methods described herein may be at least partially
processor-
implemented. For example, at least some of the operations of a method may be
performed by
one or processors or processor-implemented modules. The performance of certain
of the
operations may be distributed among the one or more processors, not only
residing within a
single machine, but deployed across a number of machines. In some example
embodiments,
the processor or processors may be located in a single location (e.g., within
a home
environment, an office environment or as a server farm), while in other
embodiments the
processors may be distributed across a number of locations.
[0063] The one or more processors may also operate to support performance of
the relevant
operations in a "cloud computing" environment or as a "software as a service"
(SaaS). For
23
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
example, at least some of the operations may be performed by a group of
computers (as
examples of machines including processors), these operations being accessible
via a network
(e.g., the Internet) and via one or more appropriate interfaces (e.g.,
Application Program
Interfaces (APIs).)
[0064] Example embodiments may be implemented in digital electronic circuitry,
or in
computer hardware, firmware, software, or in combinations of them. Example
embodiments
may be implemented using a computer program product, e.g., a computer program
tangibly
embodied in an information carrier, e.g., in a machine-readable medium for
execution by, or
to control the operation of, data processing apparatus, e.g., a programmable
processor, a
computer, or multiple computers.
[0065] A computer program can be written in any form of programming language,
including
compiled or interpreted languages, and it can be deployed in any form,
including as a stand-
alone program or as a module, subroutine, or other unit suitable for use in a
computing
environment. A computer program can be deployed to be executed on one computer
or on
multiple computers at one site or distributed across multiple sites and
interconnected by a
communication network.
[0066] In example embodiments, operations may be performed by one or more
programmable processors executing a computer program to perform functions by
operating
on input data and generating output. Method operations can also be performed
by, and
apparatus of example embodiments may be implemented as, special purpose logic
circuitry,
e.g., a field programmable gate array (FPGA) or an application-specific
integrated circuit
(ASIC).
[0067] The computing system can include clients and servers. A client and
server are
generally remote from each other and typically interact through a
communication network.
The relationship of client and server arises by virtue of computer programs
running on the
respective computers and having a client-server relationship to each other. In
embodiments
deploying a programmable computing system, it will be appreciated that that
both hardware
and software architectures require consideration. Specifically, it will be
appreciated that the
choice of whether to implement certain functionality in permanently configured
hardware
(e.g., an ASIC), in temporarily configured hardware (e.g., a combination of
software and a
programmable processor), or a combination of permanently and temporarily
configured
hardware may be a design choice.
[0068] FIG. 12 is a block diagram of a machine in the example form of a
computer system
1200 within which instructions for causing the machine to perform any one or
more of the
24
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
methodologies discussed herein may be executed. In alternative embodiments,
the machine
operates as a standalone device or may be connected (e.g., networked) to other
machines. In
a networked deployment, the machine may operate in the capacity of a server or
a client
machine in server-client network environment, or as a peer machine in a peer-
to-peer (or
distributed) network environment. While only a single machine is illustrated,
the term
"machine" shall also be taken to include any collection of machines that
individually or
jointly execute a set (or multiple sets) of instructions to perform any one or
more of the
methodologies discussed herein. The example computer system 1200 includes one
or more
processors 1202 (e.g., a central processing unit (CPU), a graphics processing
unit (GPU) or
both), a main memory 1204 and a static memory 1206, which communicate with
each other
via a bus 1208. The computer system 1200 may further include a video display
unit 1210
(e.g., a liquid crystal display (LCD) or a cathode ray tube (CRT)). The
computer system
1200 also includes an alphanumeric input device 1212 (e.g., a keyboard), a
user interface (UT)
navigation device 1214 (e.g., a mouse), a disk drive unit 1216, a signal
generation device
1218 (e.g., a speaker), a network interface device 1220, and a data interface
device 1228
(such as, e.g., an electrode interface 106).
[0069] The disk drive unit 1216 includes a machine-readable medium 1222
storing one or
more sets of instructions and data structures (e.g., software) 1224 embodying
or utilized by
any one or more of the methodologies or functions described herein. The
instructions 1224
may also reside, completely or at least partially, within the main memory 1204
and/or within
the processor 1202 during execution thereof by the computer system 1200, the
main memory
1204 and the processor 1202 also constituting machine-readable media.
[0070] While the machine-readable medium 1222 is shown in an example
embodiment to be
a single medium, the term "machine-readable medium" may include a single
medium or
multiple media (e.g., a centralized or distributed database, and/or associated
caches and
servers) that store the one or more instructions or data structures. The term
"machine-
readable medium" shall also be taken to include any tangible medium that is
capable of
storing, encoding, or carrying instructions for execution by the machine and
that cause the
machine to perform any one or more of the methodologies of the present
invention, or that is
capable of storing, encoding or carrying data structures utilized by or
associated with such
instructions. The term "machine-readable medium" shall accordingly be taken to
include, but
not be limited to, solid-state memories, and optical and magnetic media.
Specific examples
of machine-readable media include non-volatile memory, including by way of
example
semiconductor memory devices, e.g., Erasable Programmable Read-Only Memory
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
(EPROM), Electrically Erasable Programmable Read-Only Memory (EEPROM), and
flash
memory devices; magnetic disks such as internal hard disks and removable
disks; magneto-
optical disks; CD-ROM and DVD-ROM disks, or other data-storage devices.
Further, the
term "machine-readable medium" shall be taken to include a non-tangible signal
or
transmission medium, including an electrical signal, a magnetic signal, an
electromagnetic
signal, an acoustic signal and an optical signal.
[0071] The following numbered examples are illustrative embodiments:
[0072] 1. A method comprising: using one or more electrodes placed on a
patient,
measuring one or more electrocardiograms associated with one or more
respective leads;
converting the one or more electrocardiograms by time-frequency transform into
one or more
respective two-dimensional time-frequency maps; identifying, within the one or
more
electrocardiograms, one or more points in time associated with a T wave;
determining, for at
least one of the one or more time-frequency maps, one or more repolarization
measures
corresponding to extrema across frequency of the respective time-frequency map
at the one
or more points in time associated with the T wave; and outputting at least one
repolarization
index based on the one or more repolarization measures.
[0073] 2. The method of example 1, further comprising normalizing each of the
one or more
time-frequency maps based at least in part on a difference between a maximum
and a
minimum identified in the respective time-frequency map across time in an
interval
encompassing an RS segment and across frequency, wherein the one or more
repolarization
measures are determined from the normalized time-frequency maps.
[0074] 3. The method of example 2, wherein normalizing the one or more time-
frequency
maps comprises shifting each time-frequency map to a minimum equal to zero and
thereafter
scaling the respective time-frequency map based on the maximum.
[0075] 4. The method of example 1 or example 2, wherein the time interval
across which the
maximum and minimum are identified in the time-frequency map encompasses at
least one
heartbeat.
[0076] 5. The method of example 1 or example 2, wherein the time interval
across which the
maximum and minimum are identified in the time-frequency map encompasses a
measurement time of the associated electrocardiogram in its entirety.
[0077] 6. The method of example 1 or example 2, wherein the time interval
across which the
maximum and minimum are identified in the time-frequency map corresponds to an
integer
number of heartbeats.
26
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
[0078] 7. The method of any one of examples 1-6, wherein the one or more
points in time
associated with the T wave fall within a time interval defined by points
preceding and
following a maximum of the T wave at which the T wave assumes half of its
maximum
value.
[0079] 8. The method of example 7, wherein the one or more points in time
associated with
the T wave comprise a first point in time preceding the maximum of the T wave
and a second
point in time following the maximum of the T wave.
[0080] 9. The method of example 8, wherein a first repolarization measure
corresponding to
an extremum at the first point in time and a second repolarization measure
corresponding to
an extremum at the second point in time are determined, the method further
comprising
comparing the first and second repolarization measures.
[0081] 10. The method of example 9, further comprising determining a heart
condition based
on the comparison.
[0082] 11. The method of example 10, further comprising communicating the
heart condition
to a user.
[0083] 12. The method of example 10 or example 11, wherein an abnormal heart
condition
is determined based on the second repolarization measure being greater than
the first
repolarization measure.
[0084] 13. The method of any one of examples 1-12, wherein electrocardiograms
are
measured and respective repolarization measures are determined for at least
one lead
associated with a left ventricle of the patient's heart and at least one lead
associated with a
right ventricle of the patient's heart, and wherein a left ventricular
repolarization index based
on the at least one repolarization measure determined for the left ventricle
is compared with a
right ventricular repolarization index based on the at least one
repolarization measure
determined for the right ventricle.
[0085] 14. The method of example 13, further comprising determining a heart
condition
based on the comparison.
[0086] 15. The method of example 14, further comprising communicating the
heart
condition to a user.
[0087] 16. The method of example 14 or example 15, wherein an abnormal heart
condition
is determined based on the right ventricular repolarization index being
greater than the left
ventricular repolarization index.
[0088] 17. The method of any one of examples 13-16, wherein the left
ventricular
repolarization index comprises an average over multiple repolarization
measures,
27
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
corresponding to extrema at a selected one of the points in time, determined
based on
electrocardiograms measured for multiple respective leads associated with the
left ventricle,
and the right ventricular repolarization index is determined by averaging over
multiple
repolarization measures, corresponding to extrema at the selected point in
time, determined
based on electrocardiograms measured for multiple respective leads associated
with the right
ventricle.
[0089] 18. The method of any one of examples 1-17, wherein the at least one
repolarization
index comprises an average over two or more repolarization measures.
[0090] 19. The method of example 18, wherein the average is taken over two or
more heart
beats.
[0091] 20. The method of example 18 or example 19, wherein the average is
taken over two
or more leads.
[0092] 21. The method of any one of examples 1-20, wherein the at least one
repolarization
index comprises an adjustment factor that is based on at least one of an age
or a gender of the
patient.
[0093] 22. The method of any one of examples 1-21, wherein the at least one
repolarization
index is computed from the at least one repolarization measure and a heart
rate of the patient.
[0094] 23. The method of any one of examples 1-22, wherein the time-frequency
transform
comprises a wavelet transform and the time-frequency map comprises a
scalogram.
[0095] 24. The method of example 23, wherein the time-frequency transform
comprises a
continuous wavelet transform.
[0096] 25. The method of any one of examples 1-24, wherein the time-frequency
maps are
absolute-value maps.
[0097] 26. The method of any one of examples 1-25, further comprising
determining a heart
condition based on a comparison of the at least one repolarization index
against a threshold.
[0098] 27. The method of example 26, further comprising communicating the
heart
condition to a user.
[0099] 28. The method of example 26 or example 27, wherein an abnormal heart
condition
is determined based on the at least one repolarization index being below the
threshold.
[0100] 29. The method of any one of examples 1-28, wherein the outputting
comprises
displaying the at least one repolarization index in a user interface.
[0101] 30. A heart test system comprising: an electrode interface configured
to receive one
or more electrocardiogram signals via one or more respective electrodes
connectable to the
electrode interface; and a processing facility communicatively coupled to the
electrode
28
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
interface and configured to: generate, from the one or more electrocardiogram
signals, one or
more electrocardiograms for one or more respective leads; convert the one or
more
electrocardiograms by time-frequency transform into one or more respective two-
dimensional
time-frequency maps; identify, within the one or more electrocardiograms, one
or more
points in time associated with a T wave; determine, for at least one of the
one or more time-
frequency maps, one or more repolarization measures corresponding to extrema
of the
respective time-frequency map at the one or more points in time associated
with the T wave;
and output at least one repolarization index based on the one or more
repolarization
measures.
[0102] 31. The system of example 30, wherein the electrode interface and the
processing
facility are integrated into a single heart test device.
[0103] 32. The system of example 30 or example 31, wherein the processing
facility is
configured to implement the method of any one of examples 2-29.
[0104] 33. One or more computer-readable media storing instructions for
processing one or
more electrocardiograms associated with one or more respective leads, the
instructions, when
executed by one or more computer processors, causing the one or more computer
processors
to: convert the one or more electrocardiograms by time-frequency transform
into one or more
respective two-dimensional time-frequency maps; identify, within the one or
more
electrocardiograms, one or more points in time associated with a T wave;
determine, for at
least one of the one or more time-frequency maps, one or more repolarization
measures
corresponding to extrema of the respective time-frequency map at the one or
more points in
time associated with the T wave; and output at least one repolarization index
based on the one
or more repolarization measures.
[0105] 34. The one or more computer-readable media of example 33, storing
instructions
which, when executed by the one or more computer processors, cause the one or
more
computer processors to carry out the method of any one of examples 2-29.
[0106] 35. A method comprising: using one or more electrodes placed on a
patient,
measuring one or more electrocardiograms associated with one or more
respective leads;
converting the one or more electrocardiograms by time-frequency transform into
one or more
corresponding two-dimensional time-frequency maps; and generating a user
interface
displaying, for at least one of the one or more electrocardiograms, at least a
portion of the
electrocardiogram and, in temporal alignment therewith, a temporally
coextensive portion of
the corresponding time-frequency map.
29
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
[0107] 36. The method of example 35, further comprising: identifying, within
the one or
more electrocardiograms, one or more points in time associated with a T wave;
determining
at least one repolarization index from values of the one or more time-
frequency maps at the
one or more points in time associated with the T wave; and causing the at
least one
repolarization index to be displayed in the user interface.
[0108] 37. The method of example 36, wherein, for multiple leads, multiple
respective
electrocardiograms are measured and transformed into multiple corresponding
time-
frequency maps, and wherein the generated user interface displays only a
subset comprising
fewer than all of the multiple electrocardiograms and corresponding time-
frequency maps,
the at least one repolarization index being independent from a selection of
electrocardiograms
and time-frequency maps for inclusion in the displayed subset.
[0109] 38. The method of example 36 or 37, wherein generating the user
interface comprises
representing unsigned values of the one or more time-frequency maps based on a
color scale,
and wherein the at least one repolarization index is determined from signed
values of the one
or more time-frequency maps at the one or more points in time associated with
the T wave.
[0110] 39. The method of any one of examples 36-38, further comprising
determining a
heart condition based on the at least one repolarization index and generating,
for display
within the user interface, an icon indicative of the heart condition.
[0111] 40. The method of example 39, wherein the icon comprises a segmented
waveform
symbol signifying, via a number of greyed-out segments within the otherwise
colored
waveform symbol, a degree of impairment of heart function.
[0112] 41. The method of any one of examples 35-40, wherein the displayed
portions of the
electrocardiogram and the corresponding time-frequency map encompass less than
an entire
measurement time of the electrocardiogram, the method further comprising
temporally
shifting, responsive to user input, the displayed portions of the
electrocardiogram and the
corresponding time-frequency map.
[0113] 42. The method of example 41, wherein the displayed portions are
temporally shifted
based on user input comprising a scrolling action associated with at least one
of a screen
portion displaying the electrocardiogram or a screen portion displaying the
corresponding
time-frequency map.
[0114] 43. The method of example 42, wherein the scrolling action comprises a
swiping
gesture performed within a screen portion displaying the electrocardiogram or
the
corresponding time-frequency map and in a direction substantially parallel to
a time axis of
the electrocardiogram and the corresponding time-frequency map.
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
[0115] 44. The method of example 43, wherein the scrolling action is performed
on a
touchscreen.
[0116] 45. The method of any one of examples 35-44, wherein the generated user
interface
displays at least portions of multiple electrocardiograms and corresponding
time-frequency
maps for multiple respective leads, the portions of the electrocardiograms and
time-frequency
maps for different ones of the leads being temporally coextensive and
temporally aligned
with each other.
[0117] 46. The method of example 45, further comprising temporally shifting,
responsive to
a scrolling action associated with one of the electrocardiograms or the
corresponding time-
frequency map, the displayed portions of all of the multiple
electrocardiograms and
corresponding time-frequency maps.
[0118] 47. The method of any one of examples 35-46, wherein, for multiple
leads, multiple
respective electrocardiograms are measured and transformed into multiple
corresponding
time-frequency maps, and wherein the generated user interface displays only a
subset
comprising fewer than all of the multiple electrocardiograms and corresponding
time-
frequency maps, the subset being selectable via one or more user-input control
elements
included in the user interface.
[0119] 48. The method of example 47, wherein the user interface comprises
multiple screen
portions, each facilitating, via an associated one of the user-input control
elements, user
selection of one of the measured electrocardiograms and the corresponding time-
frequency
map for display in the screen portion.
[0120] 49. The method of example 48, wherein each of the user-input control
elements
comprises a drop-down menu displaying, upon activation, user-selectable
symbols for all of
the leads.
[0121] 50. A heart test system comprising: an electrode interface configured
to receive one
or more electrocardiogram signals via one or more respective electrodes
connectable to the
electrode interface; a display device; and a processing facility configured to
generate a user
interface screen based at least in part on the received one or more
electrocardiogram signals
and to cause display of the user interface screen on the display device,
wherein generating
.. and causing display of the user interface screen comprises: generating,
from the one or more
electrocardiogram signals, one or more electrocardiograms for one or more
respective leads;
converting the one or more electrocardiograms by time-frequency transform into
one or more
corresponding two-dimensional time-frequency maps; generating a user interface
displaying,
for at least one of the one or more electrocardiograms, at least a portion of
the
31
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
electrocardiogram and, in temporal alignment therewith, a temporally
coextensive portion of
the corresponding time-frequency map.
[0122] 51. The system of example 50, wherein the electrode interface, the
display device,
and the processing facility are integrated into a single heart test device.
[0123] 52. The system of example 50 or example 51, wherein the display device
comprises a
touchscreen.
[0124] 53. The system of any one of examples 50-52, wherein the processing
facility is
configured to implement the method of any one of examples 36-49.
[0125] 54. One or more computer-readable media storing instructions for
processing one or
more electrocardiograms associated with one or more respective leads, the
instructions, when
executed by one or more computer processors, causing the one or more
processors to:
convert the one or more electrocardiograms by time-frequency transform into
one or more
corresponding two-dimensional time-frequency maps; and generate a user
interface
displaying, for at least one of the one or more electrocardiograms, at least a
portion of the
electrocardiogram and, in temporal alignment therewith, a temporally
coextensive portion of
the corresponding time-frequency map.
[0126] 55. The one or more computer-readable media of example 54, storing
instructions
which, when executed by the one or more computer processors, cause the one or
more
processors to carry out the method of any one of examples 35-49.
[0127] 56. A heart test device comprising: an electrode interface configured
to receive a
plurality of electrocardiogram signals via a plurality of respective
electrodes connectable to
the electrode interface; a display device; and a processing facility
comprising circuitry
configured to generate a user interface screen based at least in part on the
received
electrocardiogram signals and to cause display of the user interface screen on
the display
device, wherein generating and causing display of the user interface screen
comprises:
generating for display, based on the electrocardiogram signals, a plurality of
one-dimensional
time-dependent electrocardiograms for a plurality of respective leads; causing
at least partial
display of a subset of the electrocardiograms, corresponding to a subset of
the leads, in
multiple respective screen portions of the user interface screen; causing
display, within each
of the screen portions adjacent the electrocardiogram at least partially
displayed therein, of a
user-input control element facilitating user selection of any one of the
plurality of leads; and
in response to user selection of one of the leads via the user-input control
elements, causing at
least partial display, within the corresponding screen portion, of the
electrocardiogram for the
selected lead.
32
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
[0128] 57. The device of example 56, wherein the user-input control element
comprises a
drop-down menu displaying, upon activation, user-selectable symbols for all of
the leads.
[0129] 58. The device of example 56 or example 57, wherein the at least
partially displayed
electrocardiograms are temporally aligned.
.. [0130] 59. The device of any of examples 56-58, wherein generating and
causing display of
the user interface screen further comprises: generating for display, from each
of the one-
dimensional time-dependent electrocardiograms for the plurality of leads, a
corresponding
two-dimensional time-frequency map; causing at least partial display of a
subset of the two-
dimensional time-frequency maps, corresponding to the subset of the leads,
each time-
.. frequency map of the subset being displayed along with the corresponding
electrocardiograms within the corresponding screen portion; and, in response
to user selection
of one of the leads via the user-input control elements, causing at least
partial display of the
time-frequency map for the selected lead in the corresponding screen portion
along with the
corresponding electrocardiogram.
.. [0131] 60. A method comprising: measuring a plurality of electrocardiogram
signals using a
plurality of respective electrodes placed on a patient; using a processing
facility to generate a
user interface screen based at least in part on the received electrocardiogram
signals and to
cause display of the user interface screen on a display device, wherein
generating and causing
display of the user interface screen comprises: generating for display, based
on the
.. electrocardiogram signals, a plurality of one-dimensional time-dependent
electrocardiograms
for a plurality of respective leads; causing at least partial display of a
subset of the
electrocardiograms, corresponding to a subset of the leads, in multiple
respective screen
portions of the user interface screen; causing display, within each of the
screen portions
adjacent the electrocardiogram displayed therein, of a user-input control
element facilitating
user selection of any one of the plurality of leads; and in response to user
selection of one of
the leads via the user-input control elements, causing at least partial
display, within the
corresponding screen portion, of the electrocardiogram for the selected lead.
[0132] 61. A heart test device comprising: an electrode interface configured
to receive one
or more electrocardiogram signals via one or more respective electrodes
connectable to the
electrode interface; a display device; and a processing facility comprising
circuitry
configured to generate a user interface screen based at least in part on the
received one or
more electrocardiogram signals and to cause display of the user interface
screen on the
display device, wherein generating and causing display of the user interface
screen
comprises: generating, from the one or more electrocardiogram signals, for
each of one or
33
CA 03000369 2019-03-28
WO 2017/058578
PCT/US2016/052717
more leads, a one-dimensional time-dependent electrocardiogram; using a time-
frequency
transform to compute, from each of the one or more electrocardiograms, a
corresponding
two-dimensional time-frequency map representing an unsigned signal value as a
function of
time and frequency; causing, for at least one of the leads, display of
temporally aligned
portions of the electrocardiogram and the corresponding time-frequency map,
the unsigned
signal value of the time-frequency map being color-coded.
[0133] 62. A method comprising: presenting, on a display of an electronic
heart monitor
device, a multi-tab user interface configured to guide an operator of the
device through a
electrocardiography workflow, the multi-tab user interface comprising at least
a patient tab, a
test tab, and a report tab; in response to operator selection of the patient
tab, presenting a
patient screen comprising one or more first user-input control elements
facilitating operator
selection of a patient among a list of existing patients and one or more
second user-input
control elements facilitating operator entry of patient information for a new
patient; in
response to operator selection of the test tab and following connection of one
or more
electrodes to the heart monitor device, presenting a test screen comprising
one or more real-
time traces of one or more respective electrocardiogram signals measured by
the one or more
connected electrodes and further presenting a third user-input control element
facilitating
operator initiation of an electrocardiogram test; upon operator selection of
the third user-input
control element, causing acquisition of one or more electrocardiogram signals
throughout a
specified test duration and presenting, within the test screen, a fourth user-
input control
element displaying a countdown timer based on the specified test duration and
facilitating
operator abortion of the electrocardiogram test; upon completion of the
electrocardiogram
test, automatically presenting a reports screen associated with the reports
tab, the reports
comprising report information including at least one electrocardiogram
computed based on
the one or more electrocardiogram signals and one or more fifth user-input
control elements
facilitating operator initiation of at least one of printing or exporting the
report information.
[0134] Although the invention has been described with reference to specific
example
embodiments, it will be evident that various modifications and changes may be
made to these
embodiments without departing from the broader scope of the invention.
Accordingly, the specification and drawings are to be regarded in an
illustrative rather than a
restrictive sense.
34