Note: Descriptions are shown in the official language in which they were submitted.
CA 03001086 2018-04-05
WO 2017/064369 PCT/F12016/050713
1
DIFFERENTIAL FLOTATION OF SULFIDE ORES FOR RECOVERING
REFRACTORY GOLD
Field of the invention
The present invention relates to recovery of gold from materials
comprising refractory gold-containing minerals and more particularly to a met-
hod of beneficiating gold-containing materials comprising refractory gold-
containing sulfidic minerals prior to gold leaching for increasing
concentration of
leachable gold in the gold-containing material.
Background of the invention
Currently gold-containing materials such as gold ores and concentra-
tes are almost exclusively subjected to cyanide leaching for recovery of gold.
Ho-
wever, in non-free-milling gold-containing materials such as refractory ores
and
concentrates gold particles are in locked within a matrix, most commonly
sulfidic
minerals, and cyanide solution cannot break up the sulfide structures such as
py-
rite (FeS2) and/or arsenopyrite (FeAsS). Thus pretreatment is necessary to de-
compose the mineral structure to liberate gold for subsequent recovery.
Typically
gold-containing refractory sulfidic materials is preprocessed before leaching
by
pressure oxidation (PDX) to facilitate the recovery of gold at economic scale.
The-
re are also processes recovering gold using halide media from sulfidic or
other
gold-containing materials. However, presence of refractory sulfidic materials
dec-
reases gold recovery also in these processes.
In pressure oxidation sulfidic minerals are oxidized exposing encapsu-
lated gold and liberating it into solution for subsequent recovery by
leaching. The
PDX process takes place in an autoclave in harsh conditions at high
temperature
and high pressure. The cost of such autoclave equipment is high and often the
au-
toclave capacity limits the processing capacity of the gold processing plant.
To maintain the heat and acid balance the PDX process also requires
certain sulfur content in the treated material. The sulfur content can be
adjusted
before the PDX processing by processing all or part of the material in a
sulfide flo-
tation process where material having low sulfur content is removed as
flotation
tailings and the flotation concentrate is reported to the PDX.
W02013110757A1 discloses a method for enrichment of metal sulfide
ores in desired minerals in cases where the ores have sulfide-containing
gangues
by addition of an oxidant to slurries prepared from the ores during or
immediate-
ly prior to froth flotation without any conditioning of the pulp.
2
US6210648B1 discloses a method for flotation of refractory auriferous
sulfides using an oxygen-deficient flotation gas for a non-selective flotation
of dif-
ferent iron-containing sulfide mineral species prior to PDX. The method
promotes
the flotation of the refractory auriferous sulfides and claims to enhance
separation of sulfide minerals, including refractory auriferous sulfides, from
non-
sulfide gangue material. The flotation concentrates are recovered from the
flotation froth enriched in sulfide minerals. However, the method does not
allow
selective separation of sulfidic minerals from each other.
Brief description of the invention
It is thus an object to provide a method and an apparatus for
implementing the method so as to alleviate the above disadvantages. The
surface
modifying froth flotation is based on selective modification of the surface of
the
reactive sulfidic mineral particles having high gold content. This may be
achieved
by adjusting the electrochemical potential of the froth flotation process to a
level
where the surfaces of the reactive sulfidic mineral particles are chemically
modified rendering the reactive sulfidic mineral particles having high gold
content hydrophilic and non-floatable while the non-reactive sulfidic mineral
particles having low gold content remain hydrophobic. Modification of the
surface
of the particle can be attained by addition of a surface modifying chemical
such as
oxidizing or reducing agent or by other chemical means.
It is an advantage of the present method is that when material
comprising mostly gold-containing sulfidic minerals is processed the PDX
processing capacity is increased and/or capex costs relating to PDX process is
reduced.
Brief description of the drawings
In the following the invention will be described in greater detail by
means of preferred embodiments with reference to the drawings, in which
Figure 1 illustrates as a first example a process flow of a process for
recovery of gold from gold-containing raw materials comprising refractory gold-
containing minerals; and
Figure 2 illustrates as a second example a process flow of a process for
recovery of gold from gold-containing raw materials comprising refractory gold-
containing minerals;
Date Regue/Date Received 2023-01-19
CA 03001086 2018-04-05
WO 2017/064369 PCT/FI2016/050713
3
Figure 3 illustrates as a third example a process flow of a process for
recovery of gold from gold-containing raw materials comprising refractory gold-
containing minerals;
Figure 4 illustrates flotation recovery rate of different types of sulfidic
pyrite minerals as a factor to electropotential of the flotation pulp.
Detailed description of the invention
The present invention is directed to recovery of gold form gold-
containing raw materials refractory gold-containing sulfidic minerals. Gold in
ref-
ractory gold-containing minerals is poorly amenable to leaching making the
reco-
very process utilizing direct cyanidation uneconomical. Typical gold-
containing
material comprising refractory gold-containing minerals has a gold recovery
rate
less than 80% when subjected to direct cyanidation. The term "refractory gold-
containing material" refers to the presence of gold locking or cyanide
consuming
materials, particularly sulfidic minerals such as iron sulfides (e.g. pyrite,
mar-
ls casite, pyrrhotite), arsenic sulfides (e.g. arsenopyrite, orpiment,
realgar), copper
sulfides (e.g. chalcopyrite), antimony sulfides (e.g. aurostibnite, stibnite),
telluri-
des, elemental sulfur, or any mixture thereof.
Said gold-containing raw material typically comprises less than 80%
w/w, in particular less than 50% w/w, preferably less than 35% w/w, more pref-
erably less than 25% w/w, even more preferably 10 to 0% w/w native gold of the
total gold in the said raw material, the total gold comprising native gold and
gold
locked in the raw material, in particular in sulfidic minerals. The term
"native
gold" refers to free-milling gold as opposite to gold locked in the material,
in par-
ticular sulfidic minerals such as iron sulfides (e.g. pyrite). Typically said
refracto-
ry gold-containing raw material is or is derived from ore or concentrate
wherein
the main mineral is pyrite or arsenopyrite. In addition to gold and sulfur,
the said
raw material may further comprise other elements such as silver, copper,
nickel,
cobalt, zinc, iron, lead, aluminum, and/or silicon.
Such gold-containing refractory raw material comprises 1) a first type
of refractory sulfidic minerals having high gold content and 2) a second type
of
refractory sulfidic minerals having low gold content. It has been surprisingly
rea-
lized that there is marked difference the chemical reactivity of said first
type of
refractory sulfidic minerals having high gold content and said second type of
ref-
ractory sulfidic minerals having low gold content although said first type and
said
second type of sulfidic minerals are to be classified within a single species
of ref-
ractory sulfidic minerals, e.g. pyrite. The first type of refractory sulfidic
minerals
CA 03001086 2018-04-05
WO 2017/064369 PCT/FI2016/050713
4
having high gold content appears to have reactive surface while the second
type
of refractory sulfidic minerals having low gold content appears to have non-
or
less-reactive surface. As gold is in particular associated with the reactive
sulfidic
minerals it is desirable to be able to separate the different types of the
refractory
sulfidic minerals to improve the quality of the PDX processes material. The
pre-
sent process is based on selective modification of the surfaces of the
reactive ref-
ractory sulfidic mineral particles having high gold content thus making the
said
particles non-floatable.
For example gold bearing pyrite varies in mineral morphology, in par-
ticular surface area of the sulfidic mineral. Reactive pyrite having high gold
con-
tent is typically present in the raw material as microcrystalline pyrite,
whereas
non-reactive pyrite having low gold content is present as coarse pyrite.
The gold content of the reactive sulfidic minerals having high gold con-
tent is higher than the gold content of the non-reactive sulfidic minerals
having
low gold content. In particular the gold content of the reactive sulfidic
minerals
having high gold content is above 5 pm, preferably above 8 ppm, more
preferably
between 10 to 100 ppm. Further in particular the gold content of the non-
reactive
sulfidic minerals having low gold content is below 5 ppm, preferably below 1
ppm, more preferably between 0 to 0.1 ppm.
Accordingly provided herein is a process for recovery of gold form
gold-containing raw materials comprising refractory gold-containing minerals,
in
particular gold-containing refractory sulfidic minerals, comprising
(a) obtaining gold-containing raw material comprising refractory gold-
containing sulfidic minerals comprising first type of refractory sulfidic
mineral
having high gold content and second type of sulfidic mineral having low gold
con-
tent;
(b) forming a mineral pulp comprising first type of refractory sulfidic
mineral particles having high gold content and second type of sulfidic mineral
particles having low gold content by suspending ground gold-containing
material
in water and optionally further milling the material;
(c) conditioning the mineral pulp by addition of a surface modifying
chemical for modifying the surface of the first type of refractory sulfidic
mineral
particles having high content thus making the said particles non-floatable to
ob-
tain a conditioned pulp;
CA 03001086 2018-04-05
WO 2017/064369 PCT/FI2016/050713
(d) subjecting the conditioned pulp to a froth flotation process to sepa-
rate first type of refractory sulfidic mineral particles having high gold
content
from the second type of sulfidic mineral particles having low gold content;
(e) recovering the non-floatable first type of refractory sulfidic mineral
5 particles having high cold content as flotation tailings;
(f) pressure oxidizing (PDX) the flotation tailings recovered in step (e)
to obtain a discharge slurry comprising liberated gold; and
(g) recovering gold from the discharge slurry obtained in step (1).
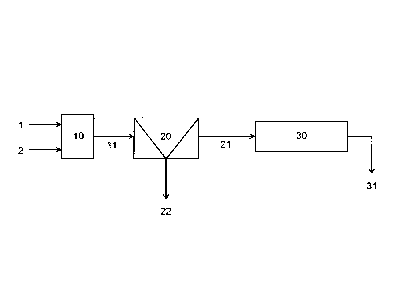
Figure 1 is illustrates a first example of a process for the recovery of
gold from gold-containing raw materials comprising refractory gold-containing
minerals. Referring to Figure 1, gold-containing refractory raw material comp-
rising 1) a first type of refractory sulfidic mineral having high gold content
i.e.
reactive refractory sulfidic mineral" and 2) a second type of refractory
sulfidic
mineral having low gold content i.e. "non-reactive refractory sulfidic
mineral", ty-
pically provided as ground ore, is mixed with water to form a mineral pulp 1.
Said
mineral pulp comprising reactive refractory sulfidic mineral particles having
high
gold content and non-reactive sulfidic mineral particles having low gold
content is
subjected to a conditioning phase 10 wherein the reactive refractory sulfidic
min-
eral particles are deactivated i.e. rendered aerophobic and non-reactive to
flota-
tion chemicals by addition of a surface modifying chemical 2, which modifies
the
surface of the reactive sulfidic mineral particles. Deactivation of the
reactive ref-
ractory sulfidic mineral particles thus renders said particles non-floatable
while
the non-reactive sulfidic mineral particles which do not interact or react
with the
surface modifying chemical 2 remain floatable. The deactivation of the first
type
of sulfidic mineral particles having high gold content accordingly prevents en-
richment of gold in froth in the following selective sulfidic mineral forth
flotation
phase 20, and allows selective removal of second type of sulfidic mineral
particles
having low gold content.
For allowing deactivation of the reactive sulfidic mineral particles be-
fore the flotation phase, the conditioning phase 10 is performed before
selective
sulfidic mineral froth flotation phase 20. Preferably the surface modifying
chemi-
cal 2 is added at least 2 minutes before initiation of the selective sulfidic
mineral
flotation phase 20. For enhanced selectivity, the surface modifying chemical 2
is
added at least 5 minutes before initiation of the flotation phase 20. As under
ex-
tended exposure to the surface modifying chemical 2 also some of the second
type
of sulfidic mineral particles having low gold content may become deactivated
and
CA 03001086 2018-04-05
WO 2017/064369 PCT/FI2016/050713
6
thus made less readily floatable, the duration of the conditioning phase 10
prefe-
rably does not exceed 30 min, more preferably 15 min, even more preferably 10
min. However, the desirable duration of the conditioning phase 10 is dependent
on the relative nature of the first type and second type of sulfidic mineral
parti-
des and even longer duration of the condition phase 10 may be tolerable.
The conditioning phase 10 is preferably performed in an agitated reac-
tor referred herein as "a deactivation reactor".
The surface modification of the reactive pyrite minerals having high
gold content is attained in the conditioning phase 10 by introduction of a
surface
modifying chemical 2. Desirable amount of the surface modifying chemical is de-
pendent on the surface area of reactive pyrite and the amount of other
minerals
consuming the surface modifying chemical 1. Preferably the amount of the
surface
modifying chemical 1 is 50 to 500 g/t of treated ore. Preferably the surface
modi-
fying chemical 2 is selected from a group consisting of nitrogen, oxidizing
agents,
reducing agents, complexing agents and any mixtures thereof.
In a particular example, modifying the surface of the reactive sulfidic
mineral particles making the said particles non-floatable is accomplished by
oxi-
dizing the surfaces of the reactive sulfidic mineral particles by dosing of an
oxidi-
zing agent. Preferably said oxidizing agent is selected from a group
consisting of
hydrogen peroxide, oxygen, ozone, alkali permanganate, chlorine, bromine,
sulfu-
ric acid, and any mixtures thereof. More preferably said oxidizing agent is
hydro-
gen peroxide. The suitable amount of the oxidizing agent is dependent on the
sur-
face area of reactive pyrite and the amount of other minerals consuming the
oxi-
dizing agent in the selected condition such as electrochemical potential.
Prefera-
bly the amount of the oxidizing agent is 50 to 500 g/t of treated ore.
In an alternative example, modifying the surface of the reactive sulfidic
mineral particles making the said particles non-floatable is accomplished by
re-
ducing the surfaces of the reactive sulfidic mineral particles by dosing of a
redu-
cing agent. Preferably said reducing agent is selected from a group consisting
of
hydrogen, carbon monoxide, sodium sulfide, sodium hydrosulfide, sodium dit-
hionite, sulfur dioxide, ferrous sulfate, Fe powder, Zn powder, and any
mixtures
thereof. The suitable amount of the reducing agent is dependent on the surface
area of reactive pyrite and the amount of other minerals consuming the
reducing
agent in the selected condition such as electrochemical potential. Preferably
the
amount of the reducing agent is 50 to 500 g/t of treated ore.
CA 03001086 2018-04-05
WO 2017/064369 PCT/FI2016/050713
7
In an alternative example, modifying the surface of the reactive sulfidic
mineral particles making the said particles non-floatable is accomplished by
mo-
difying the surfaces of the reactive sulfidic mineral particles by dosing of a
comp-
lexing agent. Preferably said complexing agent is selected from a group
consisting
of ethylenediaminetetraacetic acid (EDTA), diethylenetriamine (DETA), alkali
cy-
anide, ammonia, alkali chloride, and any mixtures thereof. The suitable amount
of
the complexing agent is dependent on the surface area of reactive pyrite and
the
amount of other minerals consuming the complexing agent in the selected condi-
tion such as electrochemical potential. Preferably the amount of the
complexing
agent is 50 to 500 g/t of treated ore.
After conditioning phase 10 has been finished the conditioned pulp 11
comprising deactivated first type of refractory mineral particles is subjected
to
the selective sulfidic mineral froth flotation phase 20. The conditioned pulp
11 is
introduced to tanks known as flotation cells that are aerated to produce
bubbles.
Any aerophilic and/or hydrophobic particles attach to the gas bubbles, which
rise
to the surface forming a froth. In the selective sulfidic mineral froth
flotation
phase 20 the non-reactive sulfidic mineral particles remaining or are rendered
aerophilic by optional addition of one or more of a surfactant, a frother, or
a col-
lector chemical and separation is achieved by passing air bubbles through the
conditioned slurry. The undesirable second type of refractory sulfidic mineral
particles having low gold content adhere to the air bubbles forming a froth
floa-
ting on the surface of the pulp.
The forth is removed as a flotation overflow 22 and the first type of
refractory sulfidic mineral particles having high remain in the pulp together
with
any gangue mineral unresponsive to the froth flotation and can be recovered in
as
the flotation underflow 21 the form of a mineral pulp, i.e. flotation
tailings.
The flotation tailings 21 are then subjected to a pressure oxidation
phase 30, wherein under conditions known to a skilled person, the sulfidic
mine-
rals are oxidized exposing encapsulated gold and liberating it into solution.
Gold
can then be recovered from the thus obtained discharge slurry 31 comprising li-
berated gold by conventional methods known to a person skilled in the art, for
example by leaching.
Gold containing raw materials typically comprise also silicates in addi-
tion to gold bearing minerals. If desired, these silicates may be removed from
the
mineral pulp prior to pressure oxidation phase 30. Removal of the silicates
can be
performed either before separation of the first type and second type of
sulfidic
CA 03001086 2018-04-05
WO 2017/064369 PCT/FI2016/050713
8
minerals or after it.
Figure 2 illustrates as a second example a process for recovery of gold
from gold-containing raw materials comprising refractory gold-containing mine-
rals wherein silicates are removed by flotation after separation of the
reactive
and non-reactive sulfidic minerals by conditioning 10 and selective sulfide
froth
flotation 20. In Figure 2 like components are designated by the same reference
numerals as used in Figure 1.
As already discussed in connection of Figure 1, mineral pulp 21 comp-
rising second type of sulfidic minerals having high gold content has been
recove-
red as flotation tailings from the selective sulfidic mineral flotation phase
20. In
the example presented in Figure 2, the mineral pulp 21 is subsequently
subjected
to a silicate flotation phase 40 before it is subjected to pressure oxidation
phase
30.
The silicate flotation phase 40 can be performed by any conventional
method known to a person skilled in art. In silicate flotation phase 40
silicates are
floated rendering a silicate-depleted mineral pulp 41 comprising sulfidic
minerals
having high gold content depleted of silicates as the flotation tailing i.e.
flotation
underflow and the silicates are removed as the overflow 42 of the flotation
phase.
After this further beneficiation phase, the obtained silicate-depleted mineral
pulp
41 is then subjected to pressure oxidation as discussed in context of Figure
1.
Figure 3 illustrates as a third example a process for recovery of gold
from gold-containing raw materials comprising refractory gold-containing mine-
rals wherein the silicates are removed by bulk sulfide flotation of sulfidic
mineral
particles before separation of the first type and the second type of sulfidic
mineral
particles by conditioning 10 and selective sulfide froth flotation 20. In
Figure 3
like components are designated by the same reference numerals as used in
Figure
1 and/or Figure 2.
Referring to Figure 3, gold-containing raw material, typically ground
ore, is mixed with water to form a mineral pulp 1. Said mineral pulp is
subjected
to a bulk sulfide flotation phase 50, wherein sulfidic minerals are floated
and thus
separated from silicates.
The bulk sulfide flotation phase 50 can be performed by any conven-
tional method known to a person skilled in art, rendering a pre-treated
mineral
pulp 51 depleted of silicates and comprising both first type of refractory
sulfidic
mineral particles having high gold content and second type of refractory
sulfidic
mineral particles having low gold content as the overflow. The silicates are
dep-
CA 03001086 2018-04-05
WO 2017/064369 PCT/FI2016/050713
9
ressed and removed as the underflow 52 of the silicate flotation phase 50.
After
this bulk sulfide flotation phase 50, the pre-treated mineral pulp 51 is then
sub-
jected to a conditioning phase 10 and a selective sulfidic mineral flotation
phase
20, followed by a pressure oxidation phase 30, as discussed in context of
Figure 1.
Figure 4 illustrates exemplary flotation recovery rate of different types
of sulfidic pyrite minerals as a factor to electropotential of the flotation
slurry. Py-
rite 1 illustrates recovery of reactive pyrite minerals having high gold
content and
Pyrite 2 illustrates recovery of non-reactive pyrite minerals having low gold
con-
tent. As can be seen from the Figure 2, when the conditions of the flotation
slurry
are kept at the desired range mainly Pyrite 1 is recovered indicating that in
these
conditions only the surfaces of the reactive sulfidic mineral particles are
modified
thus rendering the said particles non-floatable while surfaces of Pyrite 2
remain
unmodified and can be removed by flotation.
It will be obvious to a person skilled in the art that, as the technology
advances, the inventive concept can be implemented in various ways. The inven-
tion and its embodiments are not limited to the examples described above but
may vary within the scope of the claims.