Note: Descriptions are shown in the official language in which they were submitted.
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 1 ¨
STRENGTH ENHANCERS AND METHOD OF ACHIEVING STRENGTH ENHANCEMENT IN
AN ELECTRONIC VAPOR DEVICE
The present invention relates generally to a pre-vaporization formulation for
e-vaping
devices. More particularly, the present invention relates to strength
enhancers in pre-
vaporization formulations.
Electronic vaping devices (or e-vaping devices) are used to vaporize a pre-
vaporization
formulation such as, for example, a liquid material, into a vapor in order for
an adult vaper to
inhale the vapor. E-vaping devices typically include a heater which vaporizes
the pre-
vaporization formulation to produce the vapor. An e-vaping device may include
several e-
vaping elements including a power source, a cartridge or e-vaping tank
including the heater,
and a reservoir holding the pre-vaporization formulation.
A tobacco-based smoking article produces a vapor known to create a desired
sensory
experience for adult smokers, including a low to moderate harshness response
in the throat and
a perceived warmth or strength in the chest of an adult smoker.
With respect to e-vaping devices, the harshness of the vapor, which is
typically
understood as the sensation experienced in the throat of an adult vaper, and
the strength of the
vapor, which is typically understood as the sensation experienced in the chest
of the adult
vaper, may vary based on the contents and concentrations of the pre-
vaporization formulation
used to form the vapor during vaping by an adult vaper. Typically, for a
similar amount of
nicotine in the pre-vaporization formulation, an e-vaping device is harsher
than a tobacco-based
product, but has a strength that is lower than the strength of the tobacco-
based product. Also,
as an amount of nicotine in the gas phase of the vapor generated by the e-
vaping device
increases, the harshness of the e-vaping device increases.
According to a first aspect the present invention relates to an e-vaping
device configured
to achieve a desirable balance of strength and harshness, where strength is
increased without
increasing harshness.
According to a second aspect the present invention relates to an e-vaping
device that is
configured to provide a perceived sensory experience for adult vapers that is
similar to the
sensory experience enjoyed while smoking a tobacco-based cigarette.
According to a third aspect the present invention relates to an e-vaping
device that is
configured to provide a sensory experience including levels of harshness in
the throat and
perceived strength or warmth in the chest that are similar to those
experienced when smoking a
tobacco-based product. In achieving a desirable balance of strength and
harshness, the
strength of the e-vaping product may be increased without increasing the
harshness thereof.
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 2 ¨
According to a fourth aspect of the present invention, a pre-vaporization
formulation for
an e-vaping device includes a mixture of a vapor former and one or more
strength enhancers or
additives.
The pre-vaporization formulation may include water, one or more acids, or both
water
and one or more acids.
The pre-vaporization formulation may be a liquid formulation.
The pre-vaporization formulation may comprise nicotine.
The one or more strength enhancers or additives may include at least one of
carvacrol,
thymol, monomenthyl succinate, N-(2-hydroxyethyl)-2,3-dimethy1-2-isopropyl
butanamide.
In at least one embodiment, the one or more strength enhancers or additives
may
include at least one of cinnamaldehyde, menthone, eugenol, zingerone or
vanillyacetone, and
gingerol.
The one or more strength enhancers or additives may include at least one of
capsicum,
allyl isothiocyanate, piperine, isoeugenol, carvacrol, thymol, menthol,
monomenthyl succinate
and N-(2-hydroxyethyl)-2,3-dimethy1-2-isopropyl butanamide.
In at least one embodiment, the above compounds as well as others can be found
in
extracts such as, for example, horseradish oil, garlic extract, onion oil,
black pepper, cayenne
pepper, ginger oil, thyme oil, cinnamon bark oil, turmeric, fenugreek,
cardamom, rosemary
extract, grapefruit oil and andrographis extract. The one or more strength
enhancers or
additives may include at least one of these extracts.
The one or more strength enhancers or additives may include one or more
compounds
that activate receptors in the respiratory system of an adult vaper such as
the transient receptor
potential cation channel, subfamily A, member 1 (TRPA1), the transient
receptor potential cation
channel subfamily V member 1 (TPRV1), or the nicotinic-acetylcholine receptors
in an adult
vaper, the agonists becoming part of the vapor and are carried in the
respiratory tract of the
adult vaper during vaping.
In at least one embodiment, the one or more strength enhancers or additives
may
include one or more of the above-listed compounds, among others, in a
composition range of
between about 0.0001 percent and about 1 percent, or in concentrations of up
to about 2
percent. Optionally, the concentration of the one or more strength enhancers
or additives may
be greater than about 2 percent when several strength enhancers are used in
combination. For
example, the total concentration of strength enhancers may be up to about 5
percent.
In at least one embodiment, harshness reduction compounds such as acids can be
included in the pre-vaporization formulation in order to achieve a desired
balance of increased
strength and decreased harshness in an e-vaping device.
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 3 ¨
In at least one embodiment, the one or more strength enhancers or additives
may be
used in a pre-vaporization formulation that does not include nicotine, the one
or more strength
enhancers or additives providing a level of strength that is similar to the
strength experienced by
an adult vaper when vaping a nicotine-containing vaping device or a tobacco-
based article. For
example, such strength enhancers or additives may be included in smoke
cessation inhalation
devices.
In at least one embodiment, the one or more strength enhancers or additives
increase
the strength of the e-vaping device without increasing the delivery of
nicotine and without
increasing the harshness of the e-vaping article.
The pre-vaporization formulation may comprise nicotine in an amount of between
about
0.5 percent and about 2 percent by weight or greater, or between about 0.5
percent and about 5
percent or greater. In at least one embodiment, the vapor formed by the vapor
former includes
a gas phase and a particulate phase, and the concentration of nicotine in the
vapor phase is
less than or equal to about 1 percent.
According to a fifth aspect of the present invention, a pre-vaporization
formulation of an
e-vaping device includes a mixture of a vapor former, nicotine in an amount of
between about
0.5 percent and about 2 percent by weight or greater, or between about 0.5
percent and about 5
percent or greater, and one or more strength enhancers or additives. In at
least one
embodiment, the vapor formed by the vapor former includes a gas phase and a
particulate
phase, and the concentration of nicotine in the vapor phase is less than or
equal to about 1
percent. The pre-vaporization formulation may include water, one or more
acids, or both water
and one or more acids. The pre-vaporization formulation may be a liquid
formulation. The one
or more strength enhancers or additives may comprise one or more of the
optional or preferred
strength enhancers or additives according to the fourth aspect of the present
invention.
The pre-vaporization formulation according to the fourth or fifth aspect of
the present
invention may include one or more acids. The pre-vaporization formulation may
include one or
more acids in a concentration of between about 0.1 percent and about 5
percent.
The one or more acids may have a boiling point of at least about 100 degrees
Celsius
and configured to volatilize when heated by a heater in the e-vaping device.
The pre-
vaporization formulation may be configured to form a vapor having a
particulate phase and a
gas phase when heated by the heater in the e-vaping device, the particulate
phase containing
protonated nicotine and the gas phase containing unprotonated nicotine. In
embodiments, the
vapor has a majority amount of the protonated nicotine and a minority amount
of the
unprotonated nicotine.
In at least one embodiment, the pre-vaporization formulation is configured to
form a
vapor having a nicotine gas phase component upon operation of the e-vaping
device. The acid
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 4 ¨
may be included in an amount sufficient to reduce the nicotine gas phase
component by about
70 percent or greater. In other embodiments, the addition of one or more acids
may reduce the
gas phase nicotine content by an amount in the range of between about 40
percent and about
70 percent by weight, or in the range of between about 40 percent and about 90
percent by
weight.
In at least one embodiment, the acid may be selected to have a liquid to vapor
transfer
efficiency of about 50 percent or greater. The acid may be present in an
amount sufficient to
reduce the nicotine gas phase component.
In at least one embodiment, the acid includes at least one of pyruvic acid,
formic acid,
oxalic acid, glycolic acid, acetic acid, isovaleric acid, valeric acid,
propionic acid, octanoic acid,
lactic acid, sorbic acid, malic acid, tartaric acid, succinic acid, citric
acid, benzoic acid, oleic acid,
aconitic acid, butyric acid, cinnamic acid, decanoic acid, 3,7-dimethy1-6-
octenoic acid, 1-
glutamic acid, heptanoic acid, hexanoic acid, 3-hexenoic acid, trans-2-
hexenoic acid, isobutyric
acid, lauric acid, 2-methylbutyric acid, 2-methylvaleric acid, myristic acid,
nonanoic acid, palmitic
acid, 4-pentenoic acid, phenylacetic acid, 3-phenylpropionic acid,
hydrochloric acid, phosphoric
acid and sulfuric acid.
In at least one embodiment, the acid includes pyruvic acid, lactic acid,
benzoic acid and
acetic acid.
The vapor former may include propylene glycol and glycerol or glycerin. The
vapor
former may include substantially equal concentrations of propylene glycol and
glycerol or
glycerin.
The pre-vaporization formulation may include water in a concentration of
between about
percent and about 20 percent.
According to a sixth aspect of the present invention an e-vaping device
includes a first
section and a second section. The first section includes a pre-vaporization
formulation reservoir
including a pre-vaporization formulation such as, for example, a liquid
material, a heater, a wick
in communication with the pre-vaporization formulation reservoir and in
communication with the
heater, a mouth-end piece, and a connector at a distal end of the first
section. The second
section includes a power supply, and a connector at a proximal end of the
second section
configured to matingly connect with the connector of the first section.
According to a seventh aspect of the present invention an e-vaping device
includes a
first section and a second section. The first section may be a cartomizer. The
first section
includes a pre-vaporization formulation reservoir including a pre-vaporization
formulation, a
heater, and a mouth end piece. The heater is configured to heat the pre-
vaporization
formulation. The second section may be a power supply section. The second
section includes
a power source configured to supply power to the heater. The pre-vaporization
formulation may
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 5 ¨
be a pre-vaporization formulation according to the fourth or fifth aspect of
the present invention,
in accordance with any of the embodiments described herein.
The first section may comprise a wick in communication with the pre-
vaporization
formulation reservoir and in communication with the heater. The first section
may comprise a
connector at a distal end of the first section.
The second section may include a puff sensor configured to sense a puff taking
place at
the mouth-end piece. The second section may comprise a connector at a proximal
end of the
second section configured to matingly connect with the connector of the first
section.
The above and other features and advantages of example embodiments will become
more apparent by describing in detail, example embodiments with reference to
the attached
drawings. The accompanying drawings are intended to depict example embodiments
and
should not be interpreted to limit the intended scope of the claims. The
accompanying drawings
are not to be considered as drawn to scale unless explicitly noted.
Fig. 1 is a side view of an e-vaping device, according to an example
embodiment;
Fig. 2 is a cross-sectional view of an e-vaping device, according to an
example
embodiment; and
Fig. 3 is a cross-sectional view of another example embodiment of an e-vaping
device.
Some detailed example embodiments are disclosed herein. However, specific
structural
and functional details disclosed herein are merely representative for purposes
of describing
example embodiments. Example embodiments may, however, be embodied in many
alternate
forms and should not be construed as limited to only the embodiments set forth
herein.
Accordingly, while example embodiments are capable of various modifications
and
alternative forms, embodiments thereof are shown by way of example in the
drawings and will
herein be described in detail. It should be understood, however, that there is
no intent to limit
example embodiments to the particular forms disclosed, but to the contrary,
example
embodiments are to cover all modifications, equivalents, and alternatives
falling within the
scope of example embodiments. Like numbers refer to like elements throughout
the description
of the figures.
It should be understood that when an element or layer is referred to as being
"on,"
"connected to," "coupled to," or "covering" another element or layer, it may
be directly on,
connected to, coupled to, or covering the other element or layer or
intervening elements or
layers may be present. In contrast, when an element is referred to as being
"directly on,"
"directly connected to," or "directly coupled to" another element or layer,
there are no
intervening elements or layers present. Like numbers refer to like elements
throughout the
specification.
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 6 ¨
It should be understood that, although the terms first, second, third, and so
forth may be
used herein to describe various elements, components, regions, layers or
sections, these
elements, components, regions, layers, or sections should not be limited by
these terms. These
terms are only used to distinguish one element, component, region, layer, or
section from
another element, component, region, layer, or section. Therefore, a first
element, component,
region, layer, or section discussed below could be termed a second element,
component,
region, layer, or section without departing from the teachings of example
embodiments.
Spatially relative terms (for example, "beneath," "below," "lower," "above,"
"upper," and
the like) may be used herein for ease of description to describe one element
or feature's
relationship to another element or feature as illustrated in the figures. It
should be understood
that the spatially relative terms are intended to encompass different
orientations of the device in
use or operation in addition to the orientation depicted in the figures. For
example, if the device
in the figures is turned over, elements described as "below" or "beneath"
other elements or
features would then be oriented "above" the other elements or features.
Therefore, the term
"below" may encompass both an orientation of above and below. The device may
be otherwise
oriented (rotated 90 degrees or at other orientations) and the spatially
relative descriptors used
herein interpreted accordingly.
The terminology used herein is for the purpose of describing various
embodiments only
and is not intended to be limiting of example embodiments. As used herein, the
singular forms
"a," "an," and "the" are intended to include the plural forms as well, unless
the context clearly
indicates otherwise. It will be further understood that the terms
"includes," "including,"
"comprises," and "comprising," when used in this specification, specify the
presence of stated
features, integers, steps, operations, elements, or components, but do not
preclude the
presence or addition of one or more other features, integers, steps,
operations, elements,
components, or groups thereof.
Example embodiments are described herein with reference to cross-sectional
illustrations that are schematic illustrations of idealized embodiments (and
intermediate
structures) of example embodiments. As such, variations from the shapes of the
illustrations as
a result, for example, of manufacturing techniques or tolerances, are to be
expected.
Therefore, example embodiments should not be construed as limited to the
shapes of regions
illustrated herein but are to include deviations in shapes that result, for
example, from
manufacturing. Therefore, the regions illustrated in the figures are schematic
in nature and their
shapes are not intended to illustrate the actual shape of a region of a device
and are not
intended to limit the scope of example embodiments.
Unless otherwise defined, all terms (including technical and scientific terms)
used herein
have the same meaning as commonly understood by one of ordinary skill in the
art to which
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 7 ¨
example embodiments belong. It will be further understood that terms,
including those defined
in commonly used dictionaries, should be interpreted as having a meaning that
is consistent
with their meaning in the context of the relevant art and will not be
interpreted in an idealized or
overly formal sense unless expressly so defined herein.
When the terms "about" or "substantially" are used in this specification in
connection with
a numerical value, it is intended that the associated numerical value include
a tolerance of 10
percent around the stated numerical value. Moreover, when reference is made to
percentages
in this specification, it is intended that those percentages are based on
weight, that is, weight
percentages. The expression "up to" includes amounts of zero to the expressed
upper limit and
all values therebetween. When ranges are specified, the range includes all
values
therebetween such as increments of 0.1 percent.
As used herein, the term "vapor former" describes any suitable known compound
or
mixture of compounds that, in use, facilitates formation of a vapor and that
is substantially
resistant to thermal degradation at the operating temperature of the e-vaping
device. Suitable
vapor-formers consist of various compounds such as, for example, polyhydric
alcohols including
at least one of propylene glycol, and glycerol or glycerin. In at least one
embodiment, the vapor
former is propylene glycol.
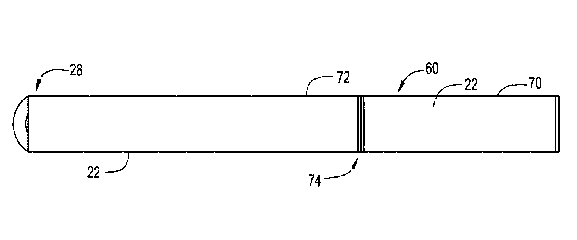
Fig. 1 is a side view of an e-vaping device 60, according to an example
embodiment. In
Fig. 1, the e-vaping device 60 includes a first section or cartomizer 70 and a
second section 72,
which are coupled together at a threaded joint 74 or by other connecting
structure such as one
or more of a snug-fit, snap-fit, detent, clamp or clasp or the like. In one
embodiment, the first
section or cartomizer 70 may be a replaceable cartridge and the second section
72 may be a
reusable section. Alternatively, the first section or cartomizer 70 and the
second section 72 may
be integrally formed in one piece.
Fig. 2 is a cross-sectional view of an example embodiment of an e-vaping
device. As
shown in Fig. 2, the first section or cartomizer 70 can house a mouth-end
insert 20, a capillary
vapor generator including a capillary tube 18, a heater 19 to heat at least a
portion of the
capillary tube 18, and a reservoir 14.
The second section 72 can house a power supply 12, a control circuitry 11
configured to
control the power supply 12, and a puff sensor 16. The puff sensor is
configured to sense when
an adult vaper is puffing on the e-vaping device 60, which triggers operation
of the power supply
12 via the control circuitry 11 to activate the heater 19 to heat the pre-
vaporization formulation
housed in the reservoir 14 and form a vapor. A threaded portion 74 of the
second section 72
can be connected to a battery charger, when not connected to the first section
or cartomizer 70,
to charge the battery or power supply 12.
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 8 ¨
As shown in Fig. 3, in other example embodiments, a valve 40 can be a two-way
valve,
and the reservoir 14 can be pressurized. For example, the reservoir 14 can be
pressurized
using a pressurization arrangement 405 configured to apply constant pressure
to the reservoir
14. As such, emission of vapor formed via heating of the pre-vaporization
formulation housed in
the reservoir 14 is facilitated.
In example embodiments, the capillary tube 18 is formed of or includes a
conductive
material, and therefore acts as its own heater 19 by passing current through
the tube. The
capillary tube 18 may be any electrically conductive material capable of being
resistively heated,
while retaining the necessary structural integrity at the operating
temperatures experienced by
the capillary tube 18, and which is non-reactive with the pre-vaporization
formulation. Suitable
materials for forming the capillary tube 18 are one or more of stainless
steel, copper, copper
alloys, porous ceramic materials coated with film resistive material, nickel-
chromium alloys, and
combinations thereof. For example, the capillary tube 18 is a stainless steel
capillary tube 18
and serves as a heater 19 via electrical leads 26 attached thereto for passage
of direct or
alternating current along a length of the capillary tube 18. Therefore, the
stainless steel
capillary tube 18 is heated by resistance heating. Alternatively, the
capillary tube 18 may be a
non-metallic tube such as, for example, a glass tube. In such an embodiment,
the heater 19 is
formed of or includes a conductive material capable of being resistively
heated, such as, for
example, stainless steel, nichrome or platinum wire, arranged along the glass
tube. When the
heater arranged along the glass tube is heated, pre-vaporization formulation
in the capillary
tube 18 is heated to a temperature sufficient to at least partially volatilize
pre-vaporization
formulation in the capillary tube 18.
In at least one embodiment, at least two electrical leads 26 are bonded to the
metallic
capillary tube 18. In at least one embodiment, one electrical lead 26 is
coupled to a first,
upstream portion 101 of the capillary tube 18 and a second electrical lead 26
is coupled to a
downstream, end portion 102 of the capillary tube 18.
In operation, when an adult vaper puffs on the e-vaping device, the puff
sensor 16
detects a pressure gradient caused by the puffing of the adult vaper, and the
control circuitry 11
activates the heater 19 to heat the pre-vaporization formulation located in
the reservoir 14.
Once the capillary tube 18 is heated, the pre-vaporization formulation
contained within a heated
portion of the capillary tube 18 is volatilized and expressed out of the
outlet 63, where the pre-
vaporization formulation expands and mixes with air and forms a vapor in
mixing chamber 240.
The power supply 12 of example embodiments can include a battery arranged in
the
second section 72 of the e-vaping device 60. The power supply 12 is configured
to apply
voltage across the heater 19, and the heater 19 volatilizes the pre-
vaporization formulation
housed in the reservoir 14.
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 9 ¨
In at least one embodiment, the electrical contacts or connection between the
heater 19
and the electrical leads 26 are substantially conductive and temperature
resistant while the
heater 19 is substantially resistive so that heat generation occurs primarily
along the heater 19
and not at the contacts.
The power supply or battery 12 may be rechargeable and include circuitry
allowing the
battery to be chargeable by an external charging device. In this case, the
circuitry, when
charged, provides power for a pre-determined number of puffs, after which the
circuitry may
have to be re-connected to an external charging device.
In at least one embodiment, the e-vaping device 60 may include control
circuitry which
can be on a printed circuit board 11. The control circuitry 11 may also
include a heater
activation light 27 that is configured to glow when the heater 19 is
activated. In at least one
embodiment, the heater activation light 27 comprises at least one LED and is
at a distal end 28
of the e-vaping device 60 so that the heater activation light 27 illuminates a
cap which takes on
the appearance of a burning coal during a puff. Moreover, the heater
activation light 27 can be
configured to be visible to the adult vaper. The light 27 may also be
configured such that the
adult vaper can activate, deactivate, or activate and deactivate the light 27
when desired, such
that the light 27 would not activate during vaping if desired.
In at least one embodiment, the e-vaping device 60 further includes a mouth-
end insert
20 having at least two off-axis, diverging outlets 21. In at least one
embodiment, the mouth-end
insert 20 includes at least two diverging outlets 21 (for example, 3 to 8
outlets or more). In at
least one embodiment, the outlets 21 of the mouth-end insert 20 are located at
ends of off-axis
passages 23 and are angled outwardly in relation to the longitudinal direction
of the e-vaping
device 60 (that is, divergently). As used herein, the term "off-axis" denotes
an angle to the
longitudinal direction of the e-vaping device. Also, the mouth-end insert (or
flow guide) 20 may
include outlets uniformly distributed around the mouth-end insert 20 so as to
substantially
uniformly distribute vapor in an adult vaper's mouth during use.
In at least one embodiment, the e-vaping device 60 is about the same size as a
conventional cigarette. In some embodiments, the e-vaping device 60 may be
about 80
millimeters to about 110 millimeters long, for example about 80 millimeters to
about 100
millimeters long and about 7 millimeters to about 10 millimeters in diameter.
The outer cylindrical housing 22 of the e-vaping device 60 may be formed of or
include
any suitable material or combination of materials. In at least one embodiment,
the outer
cylindrical housing 22 is formed at least partially of metal and is part of
the electrical circuit
connecting the control circuitry 11, the power supply 12, the puff sensor 16
and the heater 19.
Fig. 3 is a cross-sectional view of an e-vaping device according to an example
embodiment. As shown in Fig. 3, the e-vaping device 60 can also include a
middle section
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 10 ¨
(third section) 73, which can house the liquid pre-vaporization formulation
reservoir 14 and the
heater 19. The middle section 73 can be configured to be fitted with a
threaded joint 74' at an
upstream end of the first section or cartomizer 70 and a threaded joint 74 at
a downstream end
of the second section 72. In this example embodiment, the first section or
cartomizer 70 houses
the mouth-end insert 20, while the second section 72 houses the power supply
12 and the
control circuitry 11 that is configured to control the power supply 12.
In at least one embodiment, the first section or cartomizer 70, the second
section 72 and
the third section 73 include a common outer cylindrical housing 22 extending
in a longitudinal
direction along the length of the e-vaping device 60. Moreover, in at least
one embodiment, the
middle section 73 is disposable and one or both of the first section or
cartomizer 70 and the
second section 72 are reusable. The sections 70, 72, 73 can be attached by
threaded
connections or connectors 74 and 74' whereby the middle section 73 can be
replaced when the
reservoir 14 is used up. In another embodiment, the first section or
cartomizer 70 is replaceable
so as to avoid the need for cleaning one or both of the capillary tube 18 and
the heater 19. In at
least one embodiment, the first section or cartomizer 70, the second section
72 and the middle
section 73 may be integrally formed without threaded connections to form a
disposable e-vaping
device.
In the example embodiment illustrated in Fig. 3, the reservoir 14 is a
tubular, elongated
body formed of or including an elastomeric material so as to be one or both of
flexible and
compressible when squeezed. In at least one embodiment, the elastomeric
material can be one
of silicone, plastic, rubber, latex, and combinations thereof.
In at least one embodiment, the reservoir 14 is in fluid communication with a
capillary
tube 18 so that when squeezed, the reservoir 14 can deliver a volume of a pre-
vaporization
formulation such as a liquid material to the capillary tube 18.
Contemporaneously to delivering
pre-vaporization formulation to the capillary, the power supply 12 is
activated upon the
application of the manual pressure on the reservoir 14, and the capillary tube
18 is heated to
form a heated section wherein the pre-vaporization formulation is volatilized.
Upon discharge
from the heated capillary tube 18, the volatilized material expands, mixes
with air and forms a
vapor.
As shown in Fig. 3, the reservoir 14 includes a valve 40 configured to
maintain the liquid
pre-vaporization formulation within the reservoir 14 and to open when the
reservoir 14 is
squeezed and pressure is applied to the reservoir 14. In at least one
embodiment, the valve 40
opens when a critical, minimum pressure is reached so as to avoid inadvertent
dispensing pre-
vaporization formulation from the reservoir 14 or activating the heater 19. In
at least one
embodiment, the pressure required to press the pressure switch 44 is high
enough such that
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 1 1 ¨
accidental heating is avoided. Such arrangement avoids activation of the
heater 19 in the
absence of pre-vaporization formulation being pumped through the capillary.
Once pressure upon the reservoir 14 is relieved, the valve 40 closes and the
heated
capillary tube 18 discharges any pre-vaporization formulation remaining
downstream of the
valve 40.
In at least one embodiment, the strength enhancers or additives may include
cinnamaldehyde, menthone, eugenol, zingerone or vanillyacetone, and gingerol.
The strength
enhancers may also include capsicum, allyl isothiocyanate, piperine,
isoeugenol, carvacrol,
thymol, menthol, monomenthyl succinate and N-(2-hydroxyethyl)-2,3-dimethy1-2-
isopropyl
butanamide. The strength enhancers include one or more compounds that activate
receptors in
the respiratory system of an adult vaper such as the transient receptor
potential cation channel,
subfamily A, member 1 (TRPA1), the transient receptor potential cation channel
subfamily V
member 1 (TPRV1), or the nicotinic-acetylcholine receptor agonists in an adult
vaper, the
receptors becoming part of the particulate phase and are carried in the
respiratory tract of the
adult vaper with the particulate phase during vaping.
In at least one embodiment, the above compounds as well as others can be found
in
extracts such as, for example, horseradish oil, garlic extract, onion oil,
black pepper, cayenne
pepper, ginger oil, thyme oil, cinnamon bark oil, turmeric, fenugreek,
cardamom, rosemary
extract, grapefruit oil and andrographis extract.
In at least one embodiment, the pre-vaporization formulation may include one
or more
strength enhancers in a composition range of between about 0.0001 percent and
about 1
percent, in concentrations of up to about 2 percent, or in concentrations of
more than about 2
percent and less than about 5 percent when the strength enhancer is a
combination of a
plurality of strength enhancers. These strength enhancers may activate
receptors in the
respiratory system of an adult vaper.
In at least one embodiment, a pre-vaporization formulation such as, for
example, a liquid
formulation, of an e-vaping device includes a mixture of one or more of a
vapor former, one or
more strength enhancers, nicotine, one or more acids, and water.
The pre-vaporization formulation optionally includes water. Water may be
included in an
amount ranging from about 5 percent by weight based on the weight of the pre-
vaporization
formulation to about 40 percent by weight based on the weight of the pre-
vaporization
formulation. For example, water may be included at about 20 percent by weight
based on the
weight of the pre-vaporization formulation.
The following examples describe various combinations and corresponding
concentrations of the strength enhancers. The amount of nicotine in the
various formulations
may be between about 0.5 percent and about 1.5 percent or between about 0.5
percent and
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 12 ¨
about 2 percent. The various strength enhancers are discussed above. The acid
discussed
below includes at least one of pyruvic acid, formic acid, oxalic acid,
glycolic acid, acetic acid,
isovaleric acid, valeric acid, propionic acid, octanoic acid, lactic acid,
sorbic acid, malic acid,
tartaric acid, succinic acid, citric acid, benzoic acid, oleic acid, aconitic
acid, butyric acid,
cinnamic acid, decanoic acid, 3,7-dimethy1-6-octenoic acid, 1-glutamic acid,
heptanoic acid,
hexanoic acid, 3-hexenoic acid, trans-2-hexenoic acid, isobutyric acid, lauric
acid, 2-
methylbutyric acid, 2-methylvaleric acid, myristic acid, nonanoic acid,
palmitic acid, 4-pentenoic
acid, phenylacetic acid, 3-phenylpropionic acid, hydrochloric acid, phosphoric
acid and sulfuric
acid. The following examples of pre-vaporization formulations are discussed:
EXAMPLE 1: A pre-vaporization formulation solution includes about a few ppm or
about
0.001 percent to about 0.1 percent of a strength enhancer or additive, about
60 percent to about
80 percent of a vapor former (for example, propylene glycol and glycerol in
substantially equal
concentrations), about 10 percent to about 20 percent water, about 0.5 percent
to about 2
percent nicotine by weight (NBW), and up to about 5 percent of an acid.
EXAMPLE 2: A pre-vaporization formulation solution includes about 1 percent of
a
strength enhancer or additive, about 60 percent to about 80 percent vapor
former (for example,
propylene glycol and glycerol), about 15 percent to about 20 percent water,
and about 1 percent
to about 2 percent nicotine by weight (NBW), and up to about 3 percent of an
acid.
EXAMPLE 3: A pre-vaporization formulation solution includes about 1.5 percent
of a
strength enhancer or additive, about 60 percent to about 80 percent vapor
former (for example,
propylene glycol and glycerol), about 15 percent to about 20 percent water,
about 0.5 percent to
about 1.5 percent nicotine by weight (NBW), and up to about 1 percent of an
acid.
EXAMPLE 4: A pre-vaporization formulation solution includes about 2 percent of
a
strength enhancer or additive, about 60 percent to about 80 percent vapor
former (for example,
propylene glycol and glycerol), about 15 percent to about 20 percent water,
about 0.5 percent to
about 2 percent nicotine by weight (NBW), and substantially no acid or about
0.1 percent of the
acid.
EXAMPLE 5: A pre-vaporization formulation solution includes about 2 percent to
about 5
percent of a combination of a plurality of strength enhancers or additives,
about 60 percent to
about 80 percent vapor former (for example, propylene glycol and glycerol),
about 15 percent to
about 20 percent water, about 0.5 percent to about 2 percent nicotine by
weight (NBW), and
substantially no acid or about 0.1 percent of the acid.
EXAMPLE 6: A pre-vaporization formulation solution includes about 0.1 percent
of one
of cinnamaldehyde, menthone, eugenol, zingerone or vanillyacetone, and
gingerol, about 60
percent to about 80 percent vapor former (for example, propylene glycol and
glycerol), about 15
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 13 ¨
percent to about 20 percent water, about 0.5 percent to about 2 percent
nicotine by weight
(NBW), and up to about 5 percent of an acid.
EXAMPLE 7: A pre-vaporization formulation solution includes about 1 percent of
one or
more of cinnamaldehyde, menthone, eugenol, zingerone or vanillyacetone, and
gingerol, about
60 percent to about 80 percent vapor former (for example, propylene glycol and
glycerol), about
15 percent to about 20 percent water, about 0.5 percent to about 2 percent
nicotine by weight
(NBW), and up to about 3 percent of an acid.
EXAMPLE 8: A pre-vaporization formulation solution includes about 1.5 percent
of one
or more of cinnamaldehyde, menthone, eugenol, zingerone or vanillyacetone, and
gingerol,
about 60 percent to about 80 percent vapor former (for example, propylene
glycol and glycerol),
about 15 percent to about 20 percent water, about 0.5 percent to about 2
percent nicotine by
weight (NBW), and up to about 1 percent of an acid. Alternatively, the pre-
vaporization
formulation includes about 1.5 percent of a combination of two or more of
cinnamaldehyde,
menthone, eugenol, zingerone or vanillyacetone, and gingerol.
EXAMPLE 9: A pre-vaporization formulation solution includes about 2 percent of
a
combination of cinnamaldehyde, menthone, eugenol, zingerone or vanillyacetone,
and gingerol,
about 60 percent to about 80 percent vapor former (for example, propylene
glycol and glycerol),
about 15 percent to about 20 percent water, about 0.5 percent to about 2
percent nicotine by
weight (NBW), and substantially no acid.
EXAMPLE 10: A pre-vaporization formulation solution includes more than about 2
percent of more than one of cinnamaldehyde, menthone, eugenol, zingerone or
vanillyacetone,
and gingerol, about 60 percent to about 80 percent vapor former (for example,
propylene glycol
and glycerol), about 15 percent to about 20 percent water, about 0.5 percent
to about 2 percent
nicotine by weight (NBW), and substantially no acid. Alternatively, the pre-
vaporization
formulation includes more than about 2 percent of a combination of two or more
of
cinnamaldehyde, menthone, eugenol, zingerone or vanillyacetone, and gingerol.
EXAMPLE 11: A pre-vaporization formulation solution includes about 0.1 percent
to
about 2.5 percent of a combination of horseradish oil, garlic extract, onion
oil, black pepper,
cayenne pepper, ginger oil, thyme oil, cinnamon bark oil, turmeric, fenugreek,
cardamom,
rosemary extract, grapefruit oil and andrographis extract, about 60 percent to
about 80 percent
vapor former (for example, propylene glycol and glycerol), about 15 percent to
about 20 percent
water, about 0.5 percent to about 2 percent nicotine by weight (NBW), and an
optional amount
of acid of up to about 5 percent.
EXAMPLE 12: A pre-vaporization formulation solution for a smoke cessation
device
includes about 0.1 percent to about 2 percent of a strength enhancer or
additive, about 60
percent to about 80 percent vapor former (for example, propylene glycol and
glycerol), about 10
CA 03001780 2018-04-12
WO 2017/103136 PCT/EP2016/081469
- 14 ¨
percent to about 20 percent water, about 0.5 percent to about 2 percent
nicotine by weight
(NBW), and between about 0.1 percent to about 5 percent of an acid.
In at least one embodiment, the acid is operative upon the vapor generated
from the pre-
vaporization formulation upon operation of the e-vaping device so as to reduce
the amount of
perceived throat harshness, while the strength enhancer is operative upon the
vapor to increase
the perceived strength of the vapor.
According to at least one example embodiment, the acid has the capacity to
transfer into
a vaporized formulation. Liquid to vapor transfer efficiency of an acid is the
ratio of the mass
fraction of the acid in the vaporized formulation to the mass fraction of the
acid in the pre-
vaporization formulation. In at least one embodiment, the acid has a pre-
vaporization
formulation such as a liquid to vapor transfer efficiency of about 50 percent
or greater, for
example about 60 percent or greater. For example, pyruvic acid, lactic acid,
oxalic acid, acetic
acid and glycolic acid have a liquid to vapor transfer efficiency of about 50
percent or greater.
In at least one embodiment, the pre-vaporization formulation includes an acid
having a liquid to
vapor transfer efficiency of about 50 percent or greater. In another
embodiment, the pre-
vaporization formulation excludes any acid having a liquid to vapor transfer
efficiency of about
25 percent or less.
According to at least one example embodiment, the acid has a boiling point of
at least
about 100 degrees Celsius, and may be included in the pre-vaporization
formulation in an
amount sufficient to adjust the pH of the pre-vaporization formulation in the
range of about 3 to
about 8.
According to at least one example embodiment, the pre-vaporization formulation
includes one or more of pyruvic acid, formic acid, oxalic acid, glycolic acid,
acetic acid,
isovaleric acid, valeric acid, propionic acid, octanoic acid, lactic acid,
sorbic acid, malic acid,
tartaric acid, succinic acid, citric acid, benzoic acid, oleic acid, aconitic
acid, butyric acid,
cinnamic acid, decanoic acid, 3,7-dimethy1-6-octenoic acid, 1-glutamic acid,
heptanoic acid,
hexanoic acid, 3-hexenoic acid, trans-2-hexenoic acid, isobutyric acid, lauric
acid, 2-
methylbutyric acid, 2-methylvaleric acid, myristic acid, nonanoic acid,
palmitic acid, 4-pentenoic
acid, phenylacetic acid, 3-phenylpropionic acid, hydrochloric acid, phosphoric
acid, sulfuric acid,
and combinations thereof. The acid also may be incorporated in the form of a
salt. The pre-
vaporization formulation also includes a vapor former, optionally water,
nicotine, and flavorants.
Example embodiments having therefore been described, it will be obvious that
the same
may be varied in many ways. Such variations are not to be regarded as a
departure from the
intended scope of example embodiments, and all such modifications as would be
obvious to
one skilled in the art are intended to be included within the scope of the
following claims.