Note: Descriptions are shown in the official language in which they were submitted.
Synthesis and Characterization of Lithium Nickel Manganese Cobalt
Phosphorous Oxide
This is a divisional application of Canadian Patent Application
Serial No. 2,956,032 filed on July 24, 2014.
TECHNICAL FIELD
[0002] The present disclosure is generally concerned with processing
techniques for materials synthesis for lithium ion batteries.
It should be understood that the expression "the invention" and the
like used herein may refer to subject matter claimed in either the parent or
the divisional applications.
BACKGROUND
[0003] Conventional phosphate material (e.g., LiFePO4, LiMnPO4)
materials are structurally stable materials that do not exhibit decomposition
of
the material when charged to high voltages (e.g., higher than 4.5V). The
structure stability is also reflected by the fact that very small or no
exothermic
reactions are observed when heated to high temperatures without the
presence of lithium residing in the structure. However, the phosphate
materials do exhibit smaller theoretical capacity (around 170mAh/g) and lower
electrical conductivity. As a result, conventional phosphate material is
restrictive or picky on the synthesis conditions and electrode preparation
methods for lithium ion battery applications.
1
CA 3002650 2018-04-25
BRIEF DESCRIPTION OF THE DRAWINGS
[0004] Many aspects of the disclosure can be better understood with
reference to the following drawings. The components in the drawings are not
necessarily to scale, emphasis instead being placed upon clearly illustrating
the principles of certain embodiments of the present disclosure. Moreover, in
the drawings, like reference numerals designate corresponding parts
throughout the several views.
[0005] FIG. 1(a) is an illustrative view of a crystal structure of a
conventional structured LiNiO2.
[0006] FIG. 1(b) is a diagram of an X-Ray Diffraction pattern for LiNiP02
in accordance with embodiments of the present disclosure.
[0007] FIG. 1(c) is a diagram of an X-Ray Diffraction pattern for L13Ni2P06
in accordance with embodiments of the present disclosure.
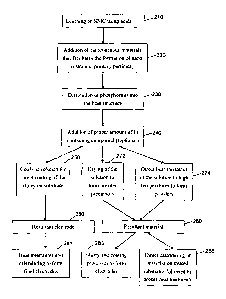
[0008] FIG. 2 is a flow chart diagram depicting an exemplary synthesis
process for phosphate material in accordance with embodiments of the
present disclosure.
[0009] FIGS. 3(a)-3(b) are diagrams illustrating results of an examination
of synthesized materials using X-ray diffraction in accordance with
embodiments of the present disclosure.
[00010] FIG. 4(a) is a diagram illustrating results of an examination of
synthesized materials using X-ray diffraction in accordance with embodiments
of the present disclosure.
[00011] FIGS. 4(b)-4(d) are diagrams illustrating results of an examination
of synthesized materials and precursor materials using scanning electron
microscope for comparison analysis.
2
CA 3002650 2018-04-25
[00012] FIG. 5 is a diagram showing phase evolution data for synthesized
materials during varying heat treatments in accordance with an exemplary
embodiment of the present disclosure.
[00013] FIGS. 6(a)-(c) are diagrams showing electrochemical properties of
exemplary electrodes in accordance with embodiments of the present
disclosure.
[00014] FIG. 7 is a diagram of an exemplary embodiment of a furnace and
a heat treatment environment for the synthesis of materials in accordance
with the present disclosure.
DETAILED DESCRIPTION
[00015] Disclosed herein are certain embodiments of a novel chemical
synthesis route for lithium ion battery applications. Accordingly, various
embodiments are focused on the synthesis of a new active material using
NMC (Lithium Nickel Manganese Cobalt Oxide) as the precursor for a
phosphate material having a layered crystal structure. Partial phosphate
generation in the layer structured material stabilizes the material while
maintaining the large capacity nature of the layer structured material.
[00016] For comparison, conventional phosphate material (e.g., LiFePO4,
LiMnPO4) materials are structurally stable materials that do not exhibit
decomposition of the material when charged to high voltages (e.g., higher
than 4.5V). The structure stability is also reflected by the fact that very
small
or no exothermic reactions are observed when heated to high temperatures
without the presence of lithium residing in the structure. However, the
phosphate materials do exhibit smaller theoretical capacity (around
3
CA 3002650 2018-04-25
170mAh/g) and lower electrical conductivity. In contrast, the layer structured
materials exhibit higher theoretical capacity (around 270mAh/g) with better
materials intrinsic electrical conductivity.
[000171 In accordance with an embodiment of the present disclosure, a
targeted phosphate material is Li3Ni2P06 (1/3 of the transition metal sites
are
replaced by phosphorous) and its derivatives (less than 1/3 of transition
metal
sites are replaced by phosphorous). This material has a higher theoretical
capacity of 305mAh/g. Meanwhile, this new class of material can be modified
to stabilize the layer structured material by incorporating a different amount
of
phosphate (or phosphorous oxide) that renders this new class of material as
exhibiting high capacity and safety dual characteristics. As an example, the
crystal structure (only 1 unit cell) of conventional layer structured LiNi02
is
shown for illustration in FIG. 1(a). A total of 12 atom layers are repeated in
the Li-O-Ni-0 order. If 1/3rd of the Ni sites are replaced by the phosphorous
atoms, the material will be Li3N12P06 as mentioned earlier. If 1/6th of the Ni
sites are replaced by the phosphorous atoms, the material will become
Li3Ni2.5P0.506 (i.e., Li6Ni6P012), and so on. For simplicity, a general
formula
may be given as LiNi(1_x)Px02for this new class of material. The phosphorous
will range from 0.33 to 0.01. The simulated XRD (X-Ray Diffraction) patterns
for LiNiP02 and Li3Ni2P06 are shown in FIG. 1(b) and FIG. 1(c), respectively,
for illustrating the iso-structural nature of the phosphorous replaced
material.
Further, from the enclosed experimental results, it is also apparent to
synthesize materials with general formula of LixX2/3+yR1/3-y02,
0.0010.33, where X can be Ni or a combination of transition metal
elements, such as Cobalt, Nanganese, Nickel, etc.
4
CA 3002650 2018-04-25
[00018] For one embodiment, NMC material (Lithium Nickel Manganese
Cobalt oxide, LiNi1i3Mn1r3C01/302) is particularly chosen as the synthesis
starting material (i.e., precursor). A reason, among others, is to leach out
Manganese in the solution state that could expedite the diffusion of
Phosphorous ions (or phosphate ions) to the original positioned residing Mn
ions. Furthermore, the leached Mn can be re-grown on the surface of the
skeletal material (the material being leached) and ensure good electrical
conductivity of the synthesized material.
[00019] FIG. 2 shows the general synthesis steps utilized in an exemplary
embodiment of the present disclosure. To begin, NMC is leached (210) using
acids. Next, the addition of carbonaceous materials facilitates (220) the
formation of nano materials (primary particles). Then, phosphorous is
dissolved (230) into the host structure, and a proper amount of lithium (Li)
containing compound may be optionally added (240). To form (260) a
resultant electrode, the resulting solution is cooled (250) for direct coating
of
the slurry on a substrate. After which, heat treatments and calendaring may
be applied to form (265) final electrodes.
[00020] Alternatively, to form a resultant material (280), the resulting
solution may be dried (272) to form powder precursors, or direct heat
treatment (to high temperatures) may be applied (274) to the resulting
solution to form powders. After which, slurry and coating processes may be
applied to form (285) electrodes. Alternatively, direct calendaring of the
resultant material on treated substrates followed by proper heat treatments
may be performed (288).
CA 3002650 2018-04-25
[00021] For clarity, exemplary synthesis routes are described using the
following examples, in accordance with embodiments of the present
disclosure.
EXAMPLE 1
Characterization of the occurrence of the phosphorous replaced layer
structured material with general formula of LixNliimMnimC0imPom-002
[00022] 1. Initially, dissolve oxalic acid (22.5g (0.25 mole)) in CMC
(carboxymethyl cellulose 1wt% solution) (40g) at 80 C.
[00023] 2. Add LiNiir3Mnu3Co1i302 (97g (1 mole)) to the solution. At this
time, purplish foam evolves implying the dissolution of Mn into the solution.
Keep the solution at 80 C for two more hours until reaction is completed.
[00024] Remarks: Step 1 and 2 are used for leaching Mn from
LiNiimMnii3Coi/302. The acid used in step 1 is not limited to oxalic acid.
Formic acid, acetic acid, hydrochloric acid, or nitric acid may also be used.
However, organic acids are preferred in certain embodiments.
[00025] 3. Add proper amount of carbonaceous materials. In this case,
sucrose (67.5g) was added into the solution. React for 2 more hours.
[00026] Remarks: Step 3 is used in facilitating the formation of nano
crystalline materials. The carbonaceous material is not limited to sucrose.
Methyl cellulose (MC), Methylcarboxylmethyl cellulose (CMC), Cellulose
acetate, starch, or styrene butadiene rubber may be used in achieving the
same goal.
[00027] 4. Then, titrate phosphoric acid (38.3g (0.33 mole, 85% in H3PO4
content)) to the solution slowly (in half an hour).
[00028] 5. Cool down the solution. At this moment, the solution is good
6
CA 3002650 2018-04-25
,
for direct coating on Al foil or can be dried to form powders for later on
reaction. That is, the solution can be used in making the electrode directly
or
can be used in making powder materials.
[00029] In the case of direct coating process, since manganese oxalate
(transition metal source), phosphate ions (phosphorous source), and
aluminum substrates (aluminum source) are all present, the coated solution
can adhere to the substrate when heat treated to high temperatures, as
described in U.S. Patent No. 9,343,743, entitled "Methods and System for
Making an Electrode Free from a Polymer Binder."
[00030] In the case of powder formation process, the methods in drying the
solution can be flexible. This is usually conducted at 150 C for several
hours.
FIG. 3(a) and FIG. 3(b) show the XRD data for the as-prepared powder (dried
at 150 C) being heat treated at 250 C and 330 C separately for 4 hours in air.
From FIG. 3(a) it can be seen that the resultant material consists of two
layer
structured materials with different lattice parameters. With the sample being
heat treated at 330 C, the two (003) peaks merged into only one broadened
peak as a new material. This new material can be described with the
following reactions:
LiNiiaMnii3C0-1/302 + 1/3 H3PO4 ---*
4/3Li3/4NiimMnimCo-imPii402+ balanced H and 0,
with the creation of 25% Li vacancies or
LiNili3MminCoir302+ 1/3 H3PO4 --+
Li0Ni113Mnii3Co11302 + 1/3 L13PO4+ balanced H and 0,
with the creation of 100% Li vacancies.
7
CA 3002650 2018-04-25
[00031] Since trace Li3PO4 is observed in the resultant material, it may be
concluded that the resultant material is between the two extreme cases (i.e.,
partial phosphorous incorporated layer structured material) with residual
Li3PO4.
[00032] It should be mentioned that no olivine structured materials were
observed from the XRD data. So, the occurrence of layer structured, partial
replacement of transition metal sites with phosphorous ions can be concluded
as the structure of the resultant material. If a
microscopic view is
implemented in this example, one can also conclude that the resultant
material is comprised of layer structured materials with different lattice
parameters.
EXAMPLE 2
Characterization of the occurrence of nano crystalline formation during the
transformation of NMC to LixNiimMnimCoimP(lm-y)02
[00033] 1. Initially, dissolve oxalic acid (22.5g (0.25 mole)) in CMC
(carboxymethyl cellulose 1wt% solution) (40g) at 80 C.
[00034] 2. Add LiNiv3Mnii3Cov302 (97g (1 mole)) to the solution. At this
time, purplish foam evolves implying the dissolution of Mn into the solution.
Keep the solution at 80 C for two more hours until reaction is completed.
[00035] Remarks: Step 1 and 2 are used for leaching Mn from
LiNiv3Mni/3Con302. The acid used in step 1 is not limited to oxalic acid.
Formic acid, acetic acid, hydrochloric acid, or nitric acid may also be used.
However, organic acids are preferred in certain embodiments.
8
CA 3002650 2018-04-25
[00036] 3. Add proper amount of carbonaceous materials. In this case,
methyl cellulose (MC) (67.5g) was added into the solution. React for 1 hour.
[00037] 4. Add 30g of n-Butanol for 3 more hours of reaction.
[00038] Remarks: Step 3 and 4 are used in facilitating the formation of
nano crystalline materials. The carbonaceous material is not limited to
sucrose. Methyl cellulose (MC), Methylcarboxylmethyl cellulose (CMC),
Cellulose acetate, starch, or styrene butadiene rubber may be used in
achieving the same goal.
[00039] 5. Then, titrate phosphoric acid (38.3g (0.33 mole, 85% in H3PO4
content)) to the solution slowly (in an hour).
[00040] Remarks: Steps 5 was utilized in dissolving phosphorous into the
structure. Then, the resultant slurry was transferred to a metallic aluminum
boat and heat treated to 300 C for 4 hours in air in a box furnace. The heat
treated material's XRD data is shown in FIG. 4(a). Meanwhile, FIG. 4(b) and
FIG. 4(c) are the SEM (scanning electron microscope) pictures representing
the heat treated material (20kX) and the original NMC material (10kX) (before
any treatment) for comparisons. It can be seen that the morphology of the
heat treated materials is nano particles (primary particle) in nature, which
is
very different from the original NMC materials morphology.
[00041] FIG. 4(d) is an additional SEM picture conducted by cross
sectioning the heat treated material. It can be seen that the heat treated
material is pretty much porous in nature which can be reflected by the
physical data shown in Table 1 as the surface area has been increased from
0.4 to 6 m2/g.
9
CA 3002650 2018-04-25
Table 1
Particle size Data (urn) Surface
Area Data
D10 D50 , D100 (BET) (m2/g)
LiNi113Mn1i3Co1n02 6.06 11.84 38.88 0.3867
Heat Treated 6.08 12.84 42.69 6.0663
Material t
Physical properties of the precursor NMC (LiNiiaMninC01/302) and the
Example 2 heat treated material (300 C for 4 hours).
t Heat treated material was obtained after heat treating the sample at 300 C
for 4 hours.
EXAMPLE 3
Synthesis and characterization of LixNiimMnimCov4P(l/4-y)02 with the addition
of Li content
[00042] 1. Initially, dissolve formic acid (47g (1 mole)) in MC (Methyl
cellulose 1wt% water solution) (40g) at 80 C.
(00043] 2. Add LiNiir3MninCoi/302 (97g) (1 mole) to the solution. At this
time, purplish foam evolves implying the dissolution of Mn into the solution.
Keep the solution at 80 C for two more hours until reaction is completed.
[00044] Remarks: Step 1 and 2 are used for leaching Mn from
LiNiv3MninC01/302. The acid used in step 1 is not limited to formic acid.
Oxalic acid, acetic acid, hydrochloric acid, or nitric acid may also be used.
However, organic acids are preferred in certain embodiments.
[00046] 3. Add proper amount of carbonaceous materials. In this case,
methyl cellulose (MC) (20g) was added into the solution. React for 1 hour.
[00046] 4. Add 30g of n-Butanol for 1 more hour of reaction.
[00047] Remarks: Step 3 and 4 are used in facilitating the formation of
nano crystalline materials.
CA 3002650 2018-04-25
[00048] 5. Then, titrate phosphoric acid (28.89 (0.25 mole, 85% in H3PO4
content)) to the solution slowly (in an hour).
[00049] 6. Prepare the solution containing lithium on the side by dissolving
Li2CO3 (18.5g) in formic acid/water (ratio 30g/60g) solution. 18.5g L12CO3 is
equivalent to 0.5 mole of Li content.
[00050] 7. Add the solution prepared in step 6 to the solution resulted from
step 5.
[00051] Remarks: Step 5 was utilized in dissolving phosphorous into the
structure and step 6 was used in increasing the lithium content (e.g.,
decreasing Li vacancies in the structure as mentioned in Example 1).
[00052] 8. Then, increase the solution temperature from 80 C (kept from
step 1 to 6) to 110 C for drying the solution. The dried xerogel was crushed
into powder form to be ready for the following heat treatments.
[00053] FIG. 5 shows the phase evolution data for the as-prepared powder
and the samples being heat treated at 300 C, 380 C, 450 C, 550 C, and
650 C separately for 4 hours in oxygen. Original NMC precursor is also
placed for comparisons. From FIG. 5, it can be seen that the heat treated
materials showed broadened peaks implying the formation of phosphorized
material with new nano crystallines formed on the surface of the resultant
materials. Before the heat treatments, the as-prepared powder shows a
mixture of manganese formate (hydrated and non-hydrated) and the leached
NMC materials. So, it is apparent that the newly formed nano crystalline
materials can result from the formation of LiMn204, or nano (amorphous)
LiMnPO4 during the heat treatment processes. Other impurities such as
L13PO4 can be a consequence of excess or non-reacted lithium and
11
CA 3002650 2018-04-25
phosphate ions.
[00054] From the data described in Examples 1, 2, and 3, several new
findings may be mentioned. First, it can be concluded that during the
processes disclosed in the present disclosure, the NMC material can be
phosphorized. Second, during the formation of the phosphorized layer
structured material, new nano crystallines can be formed on the surface of
the precursor material with the presence of the porous structure of the final
material. It is apparent that the porous structure is formed during the
leaching
process, and the leached material can re-grow onto the parent material in the
form of nano crystalline materials. The broadening of the peaks can be
comprehended as the result of the existence of phosphorized layer structured
material and the newly formed nano materials. The newly formed nano
materials are originated mainly from the presence of leached manganese
(formate). Next, heat treatments to elevated temperatures (please refer to
the phase evolution study shown in FIG. 5) does not change the peak
broadening nature of the material implying the stability of the phosphorized
phase can be maintained with the increase of temperature.
[00055] Accordingly, from the aforementioned examples, occurrence of
phosphate material was observed corresponding to the general formula:
L1,Ni1mMn1/4C01/4P(j/4-y)02, .
[00056] The following examples may be used in characterizing the
materials described above. Two exemplary methods used in making
exemplary electrodes in accordance with the present disclosure are
described.
12
CA 3002650 2018-04-25
Method 1
Conventional method in making the slurry and coating on the aluminum
substrate
[00057] Example electrode preparation: Active material (5g), Super PV
(1g) and SBR (Styrene-Butadiene Rubber) (0.3g) were used in the slurry
making. After coating using doctor blade, the coated electrode was dried at
110 C for 3 hours followed by punching of the electrode. After vacuum drying
again at 110 C for overnight, the electrodes were transferred to the glove box
for test cell assembly. The test cell was three-electrode design with Li as
the
reference electrode.
Method 2
Direct formation of the material on the substrate
[00058] For example electrode preparation:
[00059] 1. Load the active battery material on top of the as made substrate
by spreading the active material powder through a 250 mesh sieve of a
calendaring machine.
[00060] 2. Pass the as made (active material loaded) electrode through the
calendaring machine again for compacting the electrode.
[00061] 3. Send the as made electrode to the box furnace for various heat
treatments.
[00062] 4. Punch the heat treated electrode and vacuum dry the samples
at 110 C for overnight. The dried electrodes were then transferred to the
glove box for test cell assembly.
[00063] For substrate preparation:
13
CA 3002650 2018-04-25
[00064] i. Prepare 5M Phosphoric acid/n-Butanol solution (dissolve 23g
phosphoric acid and add n-Butanol to 40m1 in volume).
[00065] ii. Soak a substrate (Al plate) in the prepared solution that was
kept at 50 C for 2 minutes. Then, transfer the substrate to 100m1 n-Butanol
for rinsing. After rinsing, keep the substrate upright and dry at 50 C.
[00066] iii. Allow Mn02 powders to pass through a 250 mesh sieve and
spread on the substrate. Then, take the loaded substrate for calendaring
followed by a gentle heat treatment at 330 C for 2 hours in air.
EXAMPLE 4
[00067] Electrochemical characterizations for the electrodes were made
using the as-prepared powders described in Example 3, followed by heat
treating the electrode at 330 C for 4 hours in air. The electrode was made
using the method 2 described above in which an average of 5.3mg of active
material was loaded on the substrate.
[00068] For the exemplary electrode, a charge capacity of 251mAh/g was
observed. The first discharge capacity was calculated to be 334mAh/g with
two plateaus observed (please refer to FIG. 6(a)). The extremely high first
discharge capacity could be attributed to the new nano crystalline material
(oxides) formed on the surface of the skeletal material.
EXAMPLE 5
[00069] Electrochemical characterizations for the electrodes were made
using the as-prepared powders described in Example 3, followed by heat
14
CA 3002650 2018-04-25
. .
treating the electrode at 700 C for 4 hours in oxygen. The electrode was
made using the method 2 described above.
[00070] For the exemplary electrode, it was observed that the aluminum
substrate was able to sustain a heat treatment of 700 C under oxygen
atmosphere. It should be noted that if the aluminum substrate is coated with
active material on two sides, the aluminum substrate will be even stronger
due to the strong oxidizing environment. In this case, an electrode with
2.1mg loading of active material was tested.
[00071] A charge capacity of 231.7mAh/g was observed. The first
discharge capacity was calculated to be 114.7mAh/g with no obvious
plateaus observed (please refer to FIG. 6(b)). The loss of charge capacity
could be a result from the presence of the impurity phase observed shown in
the phase evolution study.
EXAMPLE 6
[000721 Electrochemical characterizations for the material synthesized
using the as-prepared powders described in Example 3 were made by heat
treating the as-prepared powders to 700 C for 4 hours in oxygen. The
electrode was made using the conventional slurry making and coating method
as described in method 1.
[00073] In this example, an electrode with 2.8mg loading (using the recipe
described in method 1, active material is 81%) was tested. A charge capacity
of 108.5mAh/g was observed. The first discharge capacity was calculated to
be 52.6mAh/g with no obvious plateaus observed (please refer to FIG. 6(c)).
CA 3002650 2018-04-25
The shortage in capacity can be attributed to too small sample size used for
the test. A corresponding c-rate of about c/3 was used for testing the sample.
[00074] Any process descriptions should be understood as representing
steps in an exemplary process, and alternate implementations are included
within the scope of the disclosure in which steps may be executed out of
order from that shown or discussed, including substantially concurrently or in
reverse order, depending on the functionality involved, as would be
understood by those reasonably skilled in the art of the present disclosure.
[00075] FIG. 7 shows the design of a furnace and a heat treatment
environment for the synthesis of the materials presently disclosed. FIG. 7
shows reaction vessel 1, which is open to air in furnace 2. The furnace is
open to the atmosphere at 3a and 3b so as to maintain substantially
atmospheric pressure in the furnace. Flow of gases into or out of the furnace
is dependent on heating and cooling cycles of the furnace and chemical
reactions taking place with materials in the furnace. Air is free to enter the
furnace, and air and/or products of a chemical reaction of materials 4 in the
reaction vessel 1 are free to exit the furnace. Materials 4 in vessel 1 react
chemically during heating steps to form cathode materials in accordance with
the present disclosure. Materials 4 in vessel 1, which face air found in the
furnace, are covered by a layer of a high temperature inert blanket 5, which
is
porous to air and escaping gases caused by the heating step. Heating coils
of the furnace are indicated at 6.
[00076] It should be emphasized that the above-described embodiments
are merely possible examples of implementations, merely set forth for a clear
understanding of the principles of the disclosure. Many variations and
16
CA 3002650 2018-04-25
modifications may be made to the above-described embodiment(s) without
departing substantially from the spirit and principles of the disclosure. All
such modifications and variations are intended to be included herein within
the scope of this disclosure and protected by the following claims.
17
CA 3002650 2018-04-25