Note: Descriptions are shown in the official language in which they were submitted.
CA 03007100 2018-06-01
- 1 -
Method and system for the catalytic methanization of reactant gases
The invention relates to a method for the catalytic methanization of reactant
gases,
namely carbon dioxide and/or carbon monoxide, using hydrogen in a reactor,
having
a first step in which hydrogen gas is produced electrolytically from water,
wherein in
a second step the catalytic methanization of carbon dioxide and/or carbon
monoxide
is carried out using the obtained hydrogen, and a system for this purpose.
Renewable electricity, in particular from wind and photovoltaic systems,
fluctuates
greatly, both in terms of time and space. It is therefore necessary to strike
a balance
between supply and demand in the context of the expansion of renewable energy
production. In this case, it may selectively be necessary to store large
amounts of
energy over several days or weeks. A suitable form of energy storage for this
purpose
is the so-called "power-to-gas" technology in which hydrogen is produced by
means
of water electrolysis, wherein the necessary electrical energy comes from a
renewable
energy source. The hydrogen thus produced is reacted in a heterogeneous gas-
catalytic reaction with carbon dioxide in particular to methane, wherein the
methane,
optionally after appropriate treatment, is then fed into the existing natural
gas
infrastructure. In this way, the primary electrical energy is stored outside
the grid in
the form of natural gas. This natural gas can then be converted back into
electrical
energy as needed or used in the form of heat by combustion or as fuel, for
example,
to drive vehicles.
Such a "power-to-gas" method can be found in DE 10 2013 102 669 A1. The
production of predominantly liquid hydrocarbons from carbon dioxide, water and
regenerative electrical energy is described here.
The methanization of carbon dioxide with hydrogen has usually been used
commercially for years in the gasification of coal or biomass. Another area of
application is the purification of gas mixtures in production plants of the
chemical
industry, for example in the removal of small amounts of carbon monoxide
and/or
carbon dioxide from the synthesis gas of ammonia plants. These processes are
usually
carried out in fixed bed or fluidized bed reactors, wherein these are usually
operated
continuously, so that no or only very small load changes are present. Such a
method
and a system for this purpose are disclosed in WO 2014/154250 A1.
CA 03007100 2018-06-01
,
- 2 -
Since the methanization is a highly exothermic reaction, the catalysts used,
usually
pellet-shaped bulk catalysts in the case of fixed bed reactors, must be
protected from
excessive temperature increase in the bed. This is usually realized by product
gas
recirculation and intermediate cooling.
US 2012/0063963 A1 describes the preparation of a methanization catalyst,
wherein
honeycomb-like support structures are provided for the catalyst. This support
structure may be either a ceramic oxide or also a metal. A similar catalyst is
also
described in EP 2 893 977 AL
In the case of power-to-gas processes, the amount of the reaction gas produced
by
electrolysis, namely hydrogen gas, is obtained as a function of the available
electrical
current, namely a time-limited current surplus. Since this can of course vary
greatly,
the conventional methods for methanization are not or only poorly suited,
because
such load fluctuations can only be compensated for by means of very large
hydrogen
buffer stores.
It is therefore the object of the invention to provide a method which
eliminates the
disadvantages of the prior art and in particular allows continuous
methanization of
carbon dioxide and/or carbon monoxide with hydrogen largely independent of
load
fluctuations of the electrical energy used for the electrolysis of water.
This object is achieved by a method for the catalytic methanization of
reactant gases,
namely carbon dioxide and/or carbon monoxide, using hydrogen in a reactor,
wherein
in a first step hydrogen gas is produced electrolytically from water, and the
electrical
current required for the electrolysis is drawn from a renewable energy source,
e.g.
wind energy. The catalytic methanization itself takes place in a subsequent
second
step, wherein it is provided according to the invention that the catalyst used
for the
methanization is arranged on a carrier structure preferably designed as a
honeycomb
structure with a high heat storage capacity, preferably with a heat storage
capacity
greater than 700 J/(kgK), said carrier structure being used as a storage
compound
for the reaction heat produced during the methanization process.
Since the methanization represents an exothermic reaction, the resulting heat
is
stored in the carrier structure of the catalyst. As a result, the reactor
space is kept at
the reaction temperature through the catalyst arranged in the reactor for
longer
periods, even if the methanization is interrupted in the reactor due to load
fluctuations
and the resulting lack of hydrogen.
CA 03007100 2018-06-01
- 3 -
Particularly preferably, it is provided that the reactor has at least two
chambers which
are fed in series, in parallel or alternately with reactant gas. The chambers
preferably
contain at least two switchable compartments in which the catalysts are
arranged. As
already described above, this has the advantage that at partial load operation
only at
least one chamber and/or compartment is charged with reaction gas, namely
hydrogen and carbon dioxide and/or carbon monoxide for carrying out the
methanization reaction, while at least one second chamber is kept in standby
mode,
wherein the reaction heat stored in the carrier structure maintains that
second
chamber and/or compartment at reaction temperature.
For purposes of this disclosure, the terms "reactor chamber", "chamber" and
"reactor
space" are used interchangeably. The same applies to the terms "compartment"
and
"section".
During the methanization, at least one heat exchanger device particularly
preferably
ensures the removal of excess reaction heat which, if required, can be used
for
controlling the temperature of further reaction chambers.
To carry out the method according to the invention, it is particularly
preferable to use
a plant having at least one reactor in which the catalyst is arranged, wherein
the at
least one reactor has at least one gas feed line and preferably at least one
heat
exchanger device, characterized in that the catalyst used for the
methanization has a
carrier structure having a high heat storage capacity.
In a particularly preferred embodiment of the invention, the carrier structure
of the
catalyst has a honeycomb structure with honeycomb-shaped base bodies, wherein
advantageously the honeycomb-shaped base bodies have a rectangular, square,
uniform triangular or uniform hexagonal cross-section. These cross-sections
allow the
construction of catalyst layers within a reactor space, wherein preferably the
honeycombs are arranged along their side edges to each other. Honeycombs with
a
side length of 0.05 m to 0.3 m and a honeycomb height of 0.1 m to 0.6 m have
proved to be particularly suitable for the construction of catalyst layers.
The carrier structure of the catalyst is advantageously made of a material
selected
from the group consisting of ceramic oxides such as silicon oxide, titanium
oxide,
aluminum oxide, cerium oxide, zirconium oxide, or mixtures thereof. Due to the
reaction heat liberated during the methanization and the resulting high
temperatures,
carrier structures have proven their worth in particular which are made of
cordierite,
mullite or aluminum oxide compounds fired at 1300 C to 1600 C. In particular,
these
CA 03007100 2018-06-01
- 4 -
materials withstand the high temperature stresses and temperature changes
associated with methanization.
In this case, it is provided according to the invention that a catalytically
active
material, preferably as a washcoat, is applied at least partially to the
surface of the
carrier structure, wherein the catalyst contains at least one element of the
group VIII
elements, selected in particular from the nickel group, the cobalt group
and/or the
iron group.
For the production of the washcoat, an acidic metal oxide suspension, for
example
aluminum oxide or zirconium oxide, is usually applied to the carrier
structure, and the
carrier structure coated in this way is subsequently dried and fired. Finally,
this layer
is impregnated with a salt solution of the chosen catalyst, and the catalyst
is fixed on
the carrier structure by further drying and optionally firing.
In a particularly preferred embodiment of the invention, a reactor system with
at least
two reactor chambers is provided, in which the catalyst, in particular ceramic
honeycomb catalysts, are arranged, wherein advantageously each reactor chamber
has at least one gas line and preferably at least one heat exchanger device.
As a
result, the partial load behavior of the reactor system, which is particularly
preferably
designed as a tray reactor system, is considerably improved.
In a further preferred embodiment, each chamber is additionally subdivided
into at
least two compartments or sections, wherein an inflow with reactant gas to
each
compartment independently can be provided. This provides an additionally
higher
load flexibility.
In the case of low reactant gas streams, in particular with regard to the
hydrogen
produced by electrolysis, only a first compartment of a first chamber can be
provided
with an inflow, for example, while the other compartments remain in the idle
state.
Due to the large ceramic mass of the carrier structure, in particular in
honeycomb
catalysts, the heat generated during the reaction is stored, wherein the
compartment
or compartments remain in the idle state at reaction temperature by the heat
emitted
from the carrier structure. The supply of reactant gas can be alternately
controlled
between the individual compartments or sections of the tray reactor such that
in all
compartments alternately exothermic reaction heat is released and stored, and
thus
the compartments are each kept at reaction temperature. In continuous
operation,
the heat exchanger devices in the respective reaction chambers allow removal
of
excess heat, in order to avoid overheating of the catalyst and/or the reactor
space.
CA 03007100 2018-06-01
- 5 -
The system according to the invention can be adapted in size to the respective
throughputs, in particular in the case of tray reactors it is possible to
provide an
extension of the system when using honeycomb catalysts in a simple manner. In
this
case, only the number of honeycombs used has to be adapted to the maximum
expected reactant gas flow.
In an alternative embodiment of the invention, a reactor system with at least
two
fixed bed reactors is provided, in which the catalyst is arranged, wherein
advantageously each reactor has at least one gas supply line and preferably at
least
one heat exchanger device.
Particularly preferably, it is provided that the carrier structure of the
catalyst is
arranged within the respective reactors or reaction chambers in layers,
wherein the
layer structure preferably has 4 to 30 channels per square centimeter, thus a
cell
density of 25 cpsi to 200 cpsi. These channels allow the respective reaction
gases to
flow through the respective layers without significant pressure drop while
providing a
sufficiently high contact area of the gases with the active sites of the
catalyst.
The storage capacity in the respective reaction chamber can be improved by the
layer
structure having at least one catalytically active layer or area and
additionally at least
one catalytically inactive layer or area, wherein the inactive layer is
preferably formed
from the carrier structure for the catalyst. In this case, the respective
layers
particularly preferably have the honeycomb structure already described,
wherein the
honeycomb-shaped base bodies of the catalytically inactive layer have no
catalytically
active constituents and/or coating and are used exclusively for heat storage.
It can
likewise be provided that catalytically active and catalytically inactive
honeycomb
bodies are mixed in any ratio within a layer.
The invention is explained in more detail below with reference to non-limiting
exemplary embodiments with associated figures, wherein:
Fig. 1 shows a schematic view of a first embodiment of the device
according
to the invention;
Fig. 2 shows a schematic cross-section of a reaction chamber of the
device
from Fig. 1;
Fig. 3 shows a schematic view of a second embodiment of the device
according to the invention; and
CA 03007100 2018-06-01
,
- 6 -
Fig. 4 shows a schematic view of a third embodiment of the device according
to the invention.
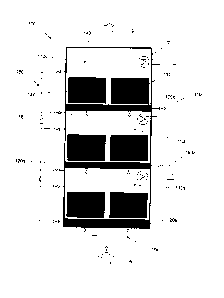
Fig. 1 shows a schematic representation of a reactor 100 according to the
invention,
which is designed as a tray reactor in this embodiment of the invention. This
tray
reactor 100 has three reactor chambers 110a, 110b, 110c, each having a gas
distribution layer 120a, 120b, 120c, usually of porous material, which serves
to
uniformly distribute the gas within the reactor chambers 110a, 110b, 110c.
In each reactor chamber 110a, 110b, 110c, in this embodiment of the invention,
catalyst material 140 in the form of honeycomb catalysts is arranged in two
compartments 131, 132 (Fig. 2).
The feed of reactant gases (arrow A) is carried out via a gas distribution
system 150a,
150b, 150c, which is cyclically switchable, so that inflow into the respective
compartments 131, 132 can occur independently of each other. This cyclic
switching
allows a time-staggered sequence of methanization reactions in the individual
compartments 131, 132 or in the catalyst layers located therein. In this case,
the
temporal sequence is selected such that exothermic reaction heat is released
in the
individual compartments 131, 132 in order to keep the compartments 131, 132
and,
as a consequence, the reactor chambers 110a, 110b, 110c at the operating
temperature. The reaction heat is stored here in the honeycomb base bodies of
the
carrier structure of the catalyst 140.
A bypass system 160 permits a cyclical switching of the reactant gas in the
individual
reactor chambers 110a, 110b, 110c, wherein shut-off valves 161 provided for
this
purpose in the gas distribution system 150a, 150b, 150c optionally prevent the
gas
supply into the gas distribution layers 120a, 120b, 120c. If the reactant gas
streams
are available in an insufficient quantity for operation of all reactor
chambers 110a,
110b, 110c or compartments 131, 132, this cyclic switching permits a time-
staggered
sequence of methanization reactions in the individual reactor chambers 110a,
110b,
110c or compartments 131, 132.
Furthermore, each reactor chamber 110a, 110b, 110c is equipped with heat
exchanger devices 170, which allow a dissipation of excess heat and/or
temperature
of the respective reactor chamber 110a, 110b, 110c.
The removal of the product gas, namely the raw methane, is preferably carried
out
at the top of the tray reactor 100 (arrow B).
CA 03007100 2018-06-01
,
- 7 -
Fig. 2 shows, by way of example, the reactor chamber 110a in a plan view,
wherein
catalyst material 140 is arranged in each of the two compartments 131, 132.
The
catalyst layer 140 consists of a plurality of honeycomb-shaped base bodies,
which
have a catalytically active salt, for example in the form of a washcoat,
wherein the
base bodies are arranged along their side edges to one another.
The honeycomb base bodies of this catalyst material 140 have channels (not
shown)
extending parallel to the longitudinal axis of the tray reactor 100, which
allow a flow
through the catalyst material 140 in the compartments 131, 132.
Fig. 3 shows a further embodiment of the invention, wherein the tray reactor
100
shown therein has substantially the same structure as shown in Fig. 1. In this
variant,
the compartments 131, 132 are filled with catalyst material 140, wherein the
catalyst
material 140 is penetrated by heat storage layers 141. These heat storage
layers 141
are catalytically inactive, and are particularly preferably formed from the
honeycomb
base bodies of the carrier structure of the catalytically active layer 140,
but in contrast
to this have no catalytically active equipment. The arrangement of these heat
storage
layers 141 may surround or pass through the catalyst layer 140.
A third embodiment of the device 200 according to the invention can be seen
from
Fig. 4. In this case, three fixed bed reactors 210 are provided, which in turn
are filled
with catalyst material 140, which consists according to the invention of
honeycomb
base bodies. In turn, a gas distribution layer 220 is provided which effects a
uniform
distribution of the gas flowing in via a gas distribution system 250 (arrow A)
within
the reactor chamber. If necessary, the incoming gas can be tempered by means
of a
heat exchanger device 270.
Furthermore, shut-off valves 261 are provided in the gas distribution system
250,
which allow a cyclic and/or alternating supply of reactant gases to the
respective fixed
bed reactors 210.
It is understood that the present invention is not limited to the embodiments
shown
above. In particular, different types of fixed bed reactors may be used, which
are
suitable for receiving the catalyst layers described above. It is essential to
the
invention that these catalyst layers have a high heat storage capacity and/or
further
catalytically inactive layers with a high heat storage capacity are provided.
This heat
storage capacity allows a partial load operation of the reactor or of a system
comprising several reactors according to the invention, which has at least two
independently operable reactor chambers and/or reactors, which can be operated
CA 03007100 2018-06-01
- 8 -
either simultaneously or in a time-staggered manner depending on the load. An
essential advantage of the system according to the invention is that it can be
expanded as required by further reactor chambers and/or reactors with
associated
catalyst layer in a simple manner. Furthermore, this design allows a very
flexible
operation that tolerates highly fluctuating load changes due to the coupling
of the
methanization method with the electrolysis used to produce the hydrogen gas
with
electrical energy from renewable energy sources.