Note: Descriptions are shown in the official language in which they were submitted.
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
POLYMORPHIC CRYSTALLINE FORMS OF OBETICHOLIC ACID
SUMMARY OF THE INVENTION
The present invention relates to obeticholic acid, an agonist for FXR,
processes of
preparation for obeticholic acid, pharmaceutical formulations comprising
obeticholic
acid, and the therapeutic use of the same.
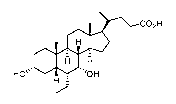
co2H
001 HI
HO"
H
obeticholic acid
(also known as INT-747)
The present invention relates to a process for the preparation of obeticholic
acid
using crystalline obeticholic acid as a synthetic intermediate. The
crystalline obeticholic
acid is selected from the group consisting of Forms A, C, D, F, G, and I. The
present
invention further relates to a process for the selective preparation of
crystalline
obeticholic acid.
The present invention relates to a crystalline obeticholic acid Form A
characterized by an X-ray diffraction pattern including characteristic peaks
at about 5.0
and 5.3 degrees 2-Theta. The crystalline obeticholic acid Form A is
characterized by an
X-ray diffraction pattern substantially similar to that set forth in Figure
41.
The present invention relates to a crystalline obeticholic acid Form C
characterized by an X-ray diffraction pattern including characteristic peaks
at about 4.2,
6.4, 9.5, 12.5, and 16.7 degrees 2-Theta. The crystalline obeticholic acid
Form C is
characterized by an X-ray diffraction pattern substantially similar to that
set forth in
Figure 5 and further characterized by a Differential Scanning Calorimetry
(DSC)
thermogram having an endotherm value at about 98 2 C.
The present invention relates to a crystalline obeticholic acid Form D
characterized by an X-ray diffraction pattern including characteristic peaks
at about 4.4,
5.2, and 7.5 degrees 2-Theta. The crystalline obeticholic acid Form D is
characterized by
an X-ray diffraction pattern substantially similar to that set forth in Figure
45.
The present invention relates to a crystalline obeticholic acid Form F
characterized by an X-ray diffraction pattern including characteristic peaks
at about 8.0,
1
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
13.2, and 13.8 degrees 2-Theta. The crystalline obeticholic acid Form F is
characterized
by an X-ray diffraction pattern substantially similar to that set forth in
Figure 49.
The present invention relates to a crystalline obeticholic acid Form G
characterized by an X-ray diffraction pattern including characteristic peaks
at about 12.9
and 13.4 degrees 2-Theta. The crystalline obeticholic acid Form G is
characterized by an
X-ray diffraction pattern substantially similar to that set forth in Figure
53.
The present invention relates to a crystalline obeticholic acid Form I
characterized
by an X-ray diffraction pattern including a characteristic peak at about 7.2
degrees 2-
Theta. The crystalline obeticholic acid Form I is characterized by an X-ray
diffraction
pattern substantially similar to that set forth in Figure 57.
The present invention relates to a process for preparing obeticholic acid Form
1,
comprising the step of converting crystalline obeticholic acid to obeticholic
acid Form 1.
The present invention relates to a process for preparing obeticholic acid Form
1,
comprising the steps of reacting 3a-hydroxy-6a-ethyl-7-keto-513-cholan-24-oic
acid with
NaBH4 to form crystalline obeticholic acid and converting crystalline
obeticholic acid to
obeticholic acid Form 1.
The present invention relates to a process for preparing obeticholic acid Form
1,
comprising the steps of reacting E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-513-
cholan-
24-oic acid with Pd/C and hydrogen gas to form 3a-hydroxy-6a-ethy1-7-keto-513-
cholan-
24-oic acid; reacting 3a-hydroxy-6a-ethy1-7-keto-513-cholan-24-oic acid with
NaBH4 to
form crystalline obeticholic acid; and converting crystalline obeticholic acid
to
obeticholic acid Form 1.
The present invention relates to a process for preparing obeticholic acid Form
1,
comprising the steps of reacting E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-
cholan-
24-oic acid methyl ester with NaOH to form E- or E/Z-3a-hydroxy-6-ethylidene-7-
keto-
513-cholan-24-oic acid; reacting E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-
cholan-24-
oic acid with Pd/C and hydrogen gas to form 3a-hydroxy-6a-ethy1-7-keto-5P-
cholan-24-
oic acid; reacting 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid with NaBH4
to
form crystalline obeticholic acid, and converting crystalline obeticholic acid
to
obeticholic acid Form 1.
The present invention relates to a process for preparing obeticholic acid Form
1,
comprising the steps of reacting 3a,7-ditrimethylsilyloxy-5P-chol-6-en-24-oic
acid
methyl ester with CH3CHO to form E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-5p-
2
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
cholan-24-oic acid methyl ester; reacting E- or E/Z-3a-hydroxy-6-ethylidene-7-
keto-513-
cholan-24-oic acid methyl ester with NaOH to form E- or E/Z-3a-hydroxy-6-
ethylidene-
7-keto-513-cholan-24-oic acid; reacting E- or E/Z-3a-hydroxy-6-ethylidene-7-
keto-513-
cholan-24-oic acid with Pd/C and hydrogen gas to form 3a-hydroxy-6a-ethy1-7-
keto-5P-
cholan-24-oic acid; reacting 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid
with
NaBH4 to form crystalline obeticholic acid, and converting crystalline
obeticholic acid to
obeticholic acid Form 1.
The present invention relates to a process for preparing obeticholic acid Form
1,
comprising the steps of reacting 3a-hydroxy-7-keto-5P-cholan-24-oic acid
methyl ester
1 0 with Li[N(CH(CH3)2)21 and Si(CH3)3C1 to form 3a,7-ditrimethylsilyloxy-
5p-chol-6-en-
24-oic acid methyl ester; reacting 3a,7-ditrimethylsilyloxy-5P-chol-6-en-24-
oic acid
methyl ester with CH3CHO to form E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-
cholan-24-oic acid methyl ester; reacting E- or E/Z-3a-hydroxy-6-ethylidene-7-
keto-5P-
cholan-24-oic acid methyl ester with NaOH to form E- or E/Z-3a-hydroxy-6-
ethylidene-
1 5 7-keto-5P-cholan-24-oic acid; reacting E- or E/Z-3a-hydroxy-6-
ethylidene-7-keto-5P-
cholan-24-oic acid with Pd/C and hydrogen gas to form 3a-hydroxy-6a-ethy1-7-
keto-5P-
cholan-24-oic acid; reacting 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid
with
NaBH4 to form crystalline obeticholic acid, and converting crystalline
obeticholic acid to
obeticholic acid Form 1.
20 The present invention relates to a process for preparing obeticholic
acid Form 1,
comprising the steps of reacting 3a-hydroxy-7-keto-5P-cholan-24-oic acid with
CH3OH
and H2SO4 to form 3a-hydroxy-7-keto-5P-cholan-24-oic acid methyl ester;
reacting 3a-
hydroxy-7-keto-5p-cholan-24-oic acid methyl ester with Li[N(CH(CH3)2)21 and
Si(CH3)3C1 to form 3a,7-ditrimethylsilyloxy-5P-chol-6-en-24-oic acid methyl
ester;
25 reacting 3a,7-ditrimethylsilyloxy-5P-chol-6-en-24-oic acid methyl ester
with CH3CHO to
form E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid methyl
ester;
reacting E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid methyl
ester
with NaOH to form E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic
acid;
reacting E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid with
Pd/C and
30 hydrogen gas to form 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid;
reacting 3a-
hydroxy-6a-ethy1-7-keto-5P-cholan-24-oic acid with NaBH4 to form crystalline
obeticholic acid, and converting crystalline obeticholic acid to obeticholic
acid Form 1.
3
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
The present invention relates to a process for preparing obeticholic acid Form
1,
wherein converting crystalline obeticholic acid Form A to obeticholic acid
Form 1
comprises the step of dissolving crystalline obeticholic acid Form A in
aqueous NaOH
solution and adding HC1.
The present invention relates to a process for preparing obeticholic acid Form
1,
wherein converting crystalline obeticholic acid Form C to obeticholic acid
Form 1
comprises the step of dissolving crystalline obeticholic acid Form C in
aqueous NaOH
solution and adding HC1.
The present invention relates to a process for preparing obeticholic acid Form
1,
1 0 wherein converting crystalline obeticholic acid Form D to obeticholic
acid Form 1
comprises the step of dissolving crystalline obeticholic acid Form D in
aqueous NaOH
solution and adding HC1.
The present invention relates to a process for preparing obeticholic acid Form
1,
wherein converting crystalline obeticholic acid Form F to obeticholic acid
Form 1
1 5 comprises the step of dissolving crystalline obeticholic acid Form F in
aqueous NaOH
solution and adding HC1.
The present invention relates to a process for preparing obeticholic acid Form
1,
wherein converting crystalline obeticholic acid Form G to obeticholic acid
Form 1
comprises the step of dissolving crystalline obeticholic acid Form G in
aqueous NaOH
20 solution and adding HC1.
The present invention relates to a process for preparing obeticholic acid Form
1,
wherein converting crystalline obeticholic acid Form I to obeticholic acid
Form 1
comprises the step of dissolving crystalline obeticholic acid Form I in
aqueous NaOH
solution and adding HC1.
The present invention relates to a process for preparing obeticholic acid Form
1,
wherein reacting 3a-hydroxy-6a-ethy1-7-keto-513-cholan-24-oic acid with NaBH4
to form
crystalline obeticholic acid is carried out at a temperature at about 85 C to
about 110 C
in a basic aqueous solution.
The present invention relates to a process for preparing obeticholic acid Form
1,
wherein reacting E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic
acid with
Pd/C and hydrogen gas to form 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid
is
carried out at a temperature at about 20 C to about 105 C and at a pressure
at about 0.5
4
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
to about 5 bars. In a separate embodiment, the hydrogenation is performed at a
temperature range from about 20 C to about 105 C, e.g. 30 C, 40 C, 50 C,
60 C, 70
C, 80 C, 90 C, and 100 C, as well as any increment in between. In another
embodiment, the hydrogenation is performed at a pressure at about 0.5 bar to
about 5.5
bar, e.g., 0.6 bar, 0.7 bar, 0.8 bar, 0.9 bar, 1.0 bar, 1.2 bar, 1.4 bar, 1.6
bar, 1.8 bar, 2.0
bar, 2.2 bar, 2.4 bar, 2.6 bar, 2.8 bar, 3.0 bar, 3.2 bar, 3.4 bar, 3.6 bar,
3.8 bar, 4.0 bar, 4.2
bar, 4.4 bar, 5.0 bar, 5.2 bar, 5.3 bar, and 5.4 bar, as well as any increment
in between.
The present invention relates to a process for preparing obeticholic acid Form
1, wherein
reacting E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid
methyl ester
1 0 with NaOH to form E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-513-cholan-
24-oic acid is
carried out at a temperature at about 20 C to about 60 C.
The present invention relates to a process for preparing obeticholic acid Form
1,
wherein reacting 3a,7-ditrimethylsilyloxy-513-chol-6-en-24-oic acid methyl
ester with
CH3CHO to form E- or E/Z-3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid
1 5 methyl ester is carried out in a polar aprotic solvent at a temperature
at about -50 C to
about -70 C in the presence of BF3. In one embodiment, the reaction is
carried out at a
temperature range of below -60 C to about -70 C in the presence of BF3. In
one
embodiment, the reaction is carried out at is carried out at an upper limit
temperature of
about -60 C in the presence of BF3.
20 The present invention relates to a process for preparing obeticholic
acid Form 1,
wherein reacting 3a-hydroxy-7-keto-5P-cholan-24-oic acid methyl ester with
Li[N(CH(CH3)2)21 and Si(CH3)3C1 to form 3a,7-ditrimethylsilyloxy-5P-chol-6-en-
24-oic
acid methyl ester is carried out in a polar aprotic solvent at a temperature
at about -10 C
to about -30 C.
25 The present invention relates to a process for preparing obeticholic
acid Form 1,
wherein reacting 3a-hydroxy-7-keto-5p-cholan-24-oic acid with CH3OH and H2S 04
to
form 3a-hydroxy-7-keto-5P-cholan-24-oic acid methyl ester is heated for about
3 hours
and the pH of the reaction mixture is adjusted with an aqueous basic solution
to a pH-
range of about 9.5 to about 10.
30 The present invention relates to a obeticholic acid, or a
pharmaceutically
acceptable salt, solvate or amino acid conjugate thereof, having a potency of
greater than
about 98%, greater than about 98.5%, greater than about 99.0%, or greater than
about
99.5%. The present invention relates to a pharmaceutical composition
comprising
5
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
obeticholic acid Form 1 produced by a process of the invention and a
pharmaceutically
acceptable carrier.
The present invention relates to a method of treating or preventing an FXR
mediated disease or condition in a subject comprise of administering an
effective amount
of obeticholic acid Form 1. The disease or condition is selected from biliary
atresia,
cholestatic liver disease, chronic liver disease, nonalcoholic steatohepatitis
(NASH),
hepatitis C infection, alcoholic liver disease, primary biliary cirrhosis
(PBC), liver
damage due to progressive fibrosis, liver fibrosis, and cardiovascular
diseases including
atherosclerosis, arteriosclerosis, hypercholesterolemia, and hyperlipidemia.
The present
1 0 invention relates to a method for lowering triglycerides in a subject
comprise of
administering an effective amount of obeticholic acid Form 1.
BRIEF DESCRIPTION OF THE DRAWINGS
1 5 Figure 1 is a HPLC-UV/MS chromatogram of crude compound 5 of
Step 4 of
Example 1 injected at 1 mg/mL, injection volume 3[11. The chromatogram
is obtained according to the method described in Example 2.
Figure 2 is a HPLC-UV/MS chromatogram of compound 5 of Step 4 of
Example 1,
purified reference injected at 1 mg/mL, injection volume 20 4. The
20 chromatogram is obtained according to the method described in
Example
2.
Figure 3 is a UV chromatogram of crude compound 5 of step 4 of Example
1 using
HPLC method. The chromatogram is obtained according to the method
described in Example 2.
25 Figure 4A is an accurate ion trace of m/z 850.61914 3ppm from
the main peak
fraction (RT 29.0 min) of compound 5 of Step 4 of Example 1, purely
isolated with HPLC method (see Example 2).
Figure 4B is an accurate ion trace of m/z 850.61914 3ppm from the
minor peak
fraction (RT 29.9 min) of compound 5 of Step 4 of Example 1, purely
30 isolated with HPLC method (see Example 2).
Figure 4C is an accurate ion trace of m/z 850.61914 3ppm from crude
compound 5
of Step 4 of Example 1 (see Example 2).
6
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Figure 4D is an accurate ion trace of m/z 850.61914 3ppm from compound
5 of
Step 4 of Example 1, purified reference (see Example 2).
Figure 5 is an XRPD diffractogram of crystalline obeticholic acid Form
C (see
Example 3).
Figure 6 shows TGA and DSC Thermograms of crystalline obeticholic acid Form
C
(see Example 3).
Figure 7 shows VT-XRPD diffractograms of crystalline obeticholic acid
at 25 C,
110 C, and 120 C (see Example 3).
Figure 8A is a GVS isotherm plot of crystalline obeticholic acid Form C
(see
Example 3).
Figure 8B is a GVS kinetic plot of crystalline obeticholic acid Form C
(see Example
3).
Figure 8C shows XRPD diffractograms of crystalline obeticholic acid Form
C before
and after GVS analysis (see Example 3).
Figure 9 shows XRPD diffractograms of crystalline obeticholic acid Form C
before
and after storage at 40 C/75% RH (see Example 3).
Figure 10 is an XRPD diffractogram of batch 1 of obeticholic acid Form
1(see
Example 5).
Figure 11 shows the XRPD diffractorgraphs for batches 1, 2, 3, 4, 5 and
6 of
obeticholic acid Form 1 (see Example 5).
Figure 12 is a NMR spectrum of batch 1 of obeticholic acid Form 1 in d6-
DMS0 (see
Example 5).
Figure 13 shows the 1H NMR spectra for batches 1, 2, 3, 4, 5 and 6 of
obeticholic
acid Form 1 (see Example 5).
Figure 14 is an expansion of 13C DEPTQ NMR spectrum of obeticholic acid
Form 1
from region 10-75 ppm (see Example 5).
Figure 15 is an expansion of 13C DEPT135 NMR spectrum of obeticholic
acid Form
1 supressing quaternary carbons from region 0-75 ppm (see Example 5).
Figure 16 is a quantitative 13C NMR of obeticholic acid Form 1 (see
Example 5).
Figure 17 is an expanded view of peaks at 32.3 ppm of Figure 16 (see
Example 5).
7
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Figure 18 is a FT-IR spectrum of batch 1 of obeticholic acid Form 1 (see
Example
5).
Figure 19 shows TGA and DSC thermograms of batch 1 of obeticholic acid
Form 1
(see Example 5).
Figure 20 shows modulated DSC thermograms of batch 1 of obeticholic acid
Form 1
(see Example 5).
Figure 21 shows the TGA traces of batches 1, 2, 3, 4, 5, and 6 of
obeticholic acid
Form 1 (see Example 5).
Figure 22 shows the DSC traces of batches 1, 2, 3, 4, 5, and 6 of
obeticholic acid
Form 1 (see Example 5).
Figure 23A is a picture of batch 1 of obeticholic acid Form 1 under polarized
light
microscopy. Figure 23B is a picture of batch 2 of obeticholic acid Form 1
under polarized light microscopy. Figure 23C is a picture of batch 3 of
obeticholic acid Form 1 under polarized light microscopy. Figure 23D is a
1 5 picture of batch 4 of obeticholic acid Form 1 under polarized
light
microscopy. Figure 23E is a picture of batch 5 of obeticholic acid Form 1
under polarized light microscopy. Figure 23F is a picture of batch 6 of
obeticholic acid Form 1 under polarized light microscopy.
Figure 24 shows GVS isotherm plot of batch 1 of obeticholic acid Form 1
(see
Example 5).
Figure 25 shows GVS kinetics plot of batch 1 of obeticholic acid Form 1
(see
Example 5).
Figure 26 shows XRPD diffractograms of batch 1 of obeticholic acid Form
1 before
and after GVS (see Example 5).
Figure 27 is a graph of the measurement of pKa at three different
methanol/water
ratios for obeticholic acid Form 1 (see Example 5).
Figure 28 is a Yasuda-Shedlovsky plot for obeticholic acid Form 1 (see
Example 5).
Figure 29 is a graph showing the distribution of the species depending
on pH for
obeticholic acid Form 1 (see Example 5).
8
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Figure 30 is a graph showing the difference curve obtained by
potentiometry for
obeticholic acid Form 1 (see Example 5).
Figure 31 shows the lipophilicity profile of obeticholic acid Form 1
(see Example 5).
Figure 32 shows the XRPD diffractograms of batch 1 of obeticholic acid
Form 1
after storage at 40 C/75% RH (see Example 5).
Figure 33 shows the XRPD diffractograms of batch 1 of obeticholic acid
Form 1
after storage at 25 C/97% RH (see Example 5).
Figure 34 shows a view of the molecule of obeticholic acid Form G from
the crystal
structure showing anisotropic atomic displacement ellipsoids for the non-
hydrogen atoms at the 50% probability level (see Example 6).
Figure 35 shows a view of the intermolecular hydrogen bonds of the
crystal structure
of obeticholic acid Form G where hydrogen bondings are shown in dashed
lines (See Example 6).
Figure 36 shows an XRPD overlay of the simulated powder pattern,
experimental
patterns of the collected crystal, and obeticholic acid Form G (see Example
6).
Figure 37 shows a graph of the plasma obeticholic acid profile vs. time
after oral
administration of 20 mg/kg of obeticholic acid Form 1 and crystalline
Form F (see Example 7).
Figure 38 shows a graph of the plasma concentration of tauro conjugate of
obeticholic acid Form 1 and crystalline Form F at different time interval
after the administration (see Example 7).
Figure 39 shows the DSC curve of Form 1 (see Example 7).
Figure 40 shows the DSC curve of Form F (see Example 7).
Figure 41 is an XRPD diffractogram of crystalline obeticholic acid Form A
(see
example 3).
Figure 42 is an overlay of TGA and DSC thermograms of crystalline
obeticholic acid
Form A (see example 3).
Figure 43 is an isotherm plot for the GVS experiment with Form A (see
example 3).
Figure 44 is a kinetics plot for the GVS experiment with Form A (see
example 3).
9
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Figure 45 is an XRPD diffractogram of crystalline obeticholic acid Form
D (see
example 9).
Figure 46 is an overlay of TGA and DSC thermograms of crystalline
obeticholic acid
Form D (see example 9).
Figure 47 is an isotherm plot for the GVS experiment with Form D (see
example 9).
Figure 48 is a kinetics plot for the GVS experiment with Form D (see
example 9).
Figure 49 is an XRPD diffractogram of crystalline obeticholic acid Form
F (see
example 9).
Figure 50 is an overlay of TGA and DSC thermograms of crystalline
obeticholic acid
Form F (see example 9).
Figure 51 is an isotherm plot for the GVS experiment with Form F (see
example 9).
Figure 52 is a kinetics plot for the GVS experiment with Form F (see
example 9).
Figure 53 is an XRPD diffractogram of crystalline obeticholic acid Form
G (see
example 9).
1 5 Figure 54 is an overlay of TGA and DSC thermograms of
crystalline obeticholic acid
Form G (see example 9).
Figure 55 is an isotherm plot for the GVS experiment with Form G (see
example 9).
Figure 56 is a kinetics plot for the GVS experiment with Form G (see
example 9).
Figure 57 is an XRPD diffractogram of crystalline obeticholic acid Form
I (see
example 9).
Figure 58 is an overlay of TGA and DSC thermograms of crystalline
obeticholic acid
Form I (see example 9).
Figure 59 is an isotherm plot for the GVS experiment with crystalline
obeticholic
acid Form I (see example 9).
Figure 60 is a kinetics plot for the GVS experiment with crystalline
obeticholic acid
Form I (see example 9).
DETAILED DESCRIPTION OF THE INVENTION
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
The present application is directed to obeticholic acid, a pharmaceutically
active
ingredient (also known as INT-747) having the chemical structure:
co2H
HO*s.00111H
H E
obeticholic acid
(also known as INT-747)
including, substantially pure obeticholic acid, a process for the preparation
of obeticholic
acid comprising crystalline obeticholic acid as a synthetic intermediate, and
analytical
methods for confirming the presence and purity of obeticholic acid and
synthetic
intermediates in the process to prepare obeticholic acid. The present
application also
describes pharmaceutical compositions and formulations of obeticholic acid and
uses for
such compositions.
Process to prepare obeticholic acid
The present application is directed to a process for preparing highly pure
obeticholic acid. The process of the present application is shown in Scheme 1.
The
process is a 6-step synthesis followed by one purification step to produce
highly pure
1 5 obeticholic acid.
11
CA 03009149 2018-06-19
WO 2017/111979 PCT/US2016/012651
Scheme 1
co2H co2cH3
Oke Step 1
HO" 0 HO" 411. 0
1
KLCA
I Step 2
0020H3
0020H3
e
(õ3c)3sioso s osi(cõ3)3.*. H
(H30)3SiO 0
3 H
2
Step 3 1
0020H3 002H
00.= Step 4
0.1111
0
H HO' , 0
H
4 5
Step 5 I
002H
FaSe
co2H
HO" OLH
Step 6 Oke
.00 H
H HO . 0
H =
crystalline obeticholic acid
6
Step 71
002H
000*
H =
obeticholic acid Form 1
The process of the present invention also includes a process according to
Scheme
1 where compounds 4 and 5 are each comprised of a mixture of the E and Z
isomers as
illustrated by the structures of compounds 4A and 5A below:
12
CA 03009149 2018-06-19
WO 2017/111979 PCT/US2016/012651
co2cH3 co2H
0.111 0.111
A .O.
H 0µµ 0
H 0
4A 5A
In one embodiment, the E/Z isomer ratio of E/Z-3a-hydroxy-6-ethylidene-7-keto-
5P-cholan-24-oic acid methyl ester (4A) is about 50%, greater than about 60%,
greater
than about 70%, greater than about 80%, greater than about 83%, greater than
about 85%,
greater than about 90%, greater than about 93%, greater than about 95%, or
greater than
about 99%. In one embodiment, the E/Z ratio is determined by HPLC. In one
embodiment, the ratio is greater than about 80%. In one embodiment, the ratio
is greater
than about 83%. In one embodiment, the ratio is greater than about 85%. In one
embodiment, the ratio is greater than about 90%. In one embodiment, the ratio
is greater
than about 93%. In one embodiment, the ratio is greater than about 95%. In one
embodiment, the ratio is greater than about 99%.
In one embodiment, the E/Z isomer ratio of E/Z-3a-hydroxy-6-ethylidene-7-keto-
5P-cholan-24-oic acid (5A) is about 50%, greater than about 60%, greater than
about
70%, greater than about 80%, greater than about 83%, greater than about 85%,
greater
than about 90%, greater than about 93%, greater than about 95%, or greater
than about
99%. In one embodiment, the E/Z ratio is determined by HPLC. In one
embodiment, the
ratio is greater than about 80%. In one embodiment, the ratio is greater than
about 83%.
In one embodiment, the ratio is greater than about 85%. In one embodiment, the
ratio is
greater than about 90%. In one embodiment, the ratio is greater than about
93%. In one
embodiment, the ratio is greater than about 95%. In one embodiment, the ratio
is greater
than about 99%.
The process of the present application has never been reported in the art. The
process is a 6-step synthesis followed by one purification step. Step 1 is the
esterification
of the C-24 carboxylic acid of 7-ketolithocholic acid (KLCA) using methanol in
the
presence of an acid catalyst and heat to produce the methyl ester compound 1.
Step 2 is
silylenol ether formation from compound 1 using chlorosilane in the presence
of a strong
base to produce compound 3. Step 3 is an acid mediated aldol condensation
reaction of
the silylenol ether compound 3 and acetaldehyde in the presence of an acid to
produce
compound 4 (or compound 4A). Step 4 is ester hydrolysis i.e., saponification
of the C-24
methyl ester of compound 4 (or compound 4A) to produce the carboxylic acid
compound
13
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
(or compound 5A). Step 5 is the hydrogenation of the 6-ethylidene moiety of
compound 5 (or compound 5A) followed by isomerization of the initially formed
643
ethyl group to produce compound 6. Step 6 is the selective reduction of the 7-
keto group
of compound 6 to a 7a-hydroxy group to produce crystalline obeticholic acid.
Step 7 is
5 the conversion of crystalline obeticholic acid to obeticholic acid Form
1.
The process of the present invention relates to a process for preparing
obeticholic
acid Form 1, where the process utilizes a crystalline form of obeticholic acid
as a
synthetic intermediate.
In one embodiment, the present invention relates to a process for preparing
obeticholic acid Form 1, comprising the step of converting crystalline
obeticholic acid to
obeticholic acid Form 1.
In one embodiment, the present invention relates to a process for preparing
obeticholic acid Form 1, comprising the steps of
1 5 reacting 3a-hydroxy-6a-ethyl-7-keto-513-cholan-24-oic acid (6) with
NaBH4 to
form crystalline obeticholic acid, and
converting crystalline obeticholic acid to obeticholic acid Form 1.
In one embodiment, the present invention relates to a process for preparing
obeticholic acid Form 1, comprising the steps of
reacting E/Z-3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid (5A) with
Pd/C and hydrogen gas to form 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid
(6),
reacting 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid (6) with NaBH4 to
form crystalline obeticholic acid, and
converting crystalline obeticholic acid to obeticholic acid Form 1.
In one embodiment, the present invention relates to a process for preparing
obeticholic acid Form 1, comprising the steps of
reacting E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid (5) with Pd/C
and hydrogen gas to form 3a-hydroxy-6a-ethyl-7-keto-51:3-cholan-24-oic acid
(6),
reacting 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid (6) with NaBH4 to
form crystalline obeticholic acid, and
converting crystalline obeticholic acid to obeticholic acid Form 1.
14
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
In one embodiment, the present invention relates to a process for preparing
obeticholic acid Form 1, comprising the steps of
reacting E/Z-3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid methyl
ester
(4A) with NaOH to form E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic
acid
(5A),
reacting E/Z-3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid (5A) with
Pd/C and hydrogen gas to form 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid
(6),
reacting 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid (6) with NaBH4 to
form crystalline obeticholic acid, and
converting crystalline obeticholic acid to obeticholic acid Form 1.
In one embodiment, the present invention relates to a process for preparing
obeticholic acid Form 1, comprising the steps of
reacting E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid methyl ester
(4) with NaOH to form E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid
(5),
reacting E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid (5) with Pd/C
and hydrogen gas to form 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid (6),
reacting 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid (6) with NaBH4 to
form crystalline obeticholic acid, and
converting crystalline obeticholic acid to obeticholic acid Form 1.
In one embodiment, the present invention relates to a process for preparing
obeticholic acid Form 1, comprising the steps of
reacting 3a,7-ditrimethylsilyloxy-5P-chol-6-en-24-oic acid methyl ester (3)
with
CH3CHO to form E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid methyl
ester (4A),
reacting E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid methyl ester
(4A) with NaOH to form E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic
acid
(5A),
reacting E/Z-3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid (5A) with
Pd/C and hydrogen gas to form 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid
(6),
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
reacting 3a-hydroxy-6a-ethy1-7-keto-513-cholan-24-oic acid (6) with NaBH4 to
form crystalline obeticholic acid, and
converting crystalline obeticholic acid to obeticholic acid Form 1.
In one embodiment, the present invention relates to a process for preparing
obeticholic acid Form 1, comprising the steps of
reacting 3a,7-ditrimethylsilyloxy-5P-chol-6-en-24-oic acid methyl ester (3)
with
CH3CHO to form E-3a-hydroxy-6-ethylidene-7-keto-5p-cholan-24-oic acid methyl
ester
(4),
reacting E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid methyl ester
(4) with NaOH to form E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid
(5),
reacting E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid (5) with Pd/C
and hydrogen gas to form 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid (6),
reacting 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid (6) with NaBH4 to
1 5 form crystalline obeticholic acid, and
converting crystalline obeticholic acid to obeticholic acid Form 1.
In one embodiment, the present invention relates to a process for preparing
obeticholic acid Form 1, comprising the steps of
reacting 3a-hydroxy-7-keto-5P-cholan-24-oic acid methyl ester (1) with
Li[N(CH(CH3)2)21 and Si(CH3)3C1 to form 3a,7-ditrimethylsilyloxy-5P-chol-6-en-
24-oic
acid methyl ester (3),
reacting 3a,7-ditrimethylsilyloxy-5P-chol-6-en-24-oic acid methyl ester (3)
with
CH3CHO to form E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid methyl
ester (4A),
reacting E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid methyl ester
(4A) with NaOH to form E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic
acid
(5A),
reacting E/Z-3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid (5A) with
Pd/C and hydrogen gas to form 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid
(6),
reacting 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid (6) with NaBH4 to
form crystalline obeticholic acid, and
16
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
converting crystalline obeticholic acid to obeticholic acid Form 1.
In one embodiment, the present invention relates to a process for preparing
obeticholic acid Form 1, comprising the steps of
reacting 3a-hydroxy-7-keto-513-cholan-24-oic acid methyl ester (1) with
Li[N(CH(CH3)2)21 and Si(CH3)3C1 to form 3a,7-ditrimethylsilyloxy-513-chol-6-en-
24-oic
acid methyl ester (3),
reacting 3a,7-ditrimethylsilyloxy-513-chol-6-en-24-oic acid methyl ester (3)
with
CH3CHO to form E-3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid methyl
ester
(4),
reacting E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid methyl ester
(4) with NaOH to form E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid
(5),
reacting E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid (5) with Pd/C
and hydrogen gas to form 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid (6),
reacting 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid (6) with NaBH4 to
form crystalline obeticholic acid, and
converting crystalline obeticholic acid to obeticholic acid Form 1.
In one embodiment, the present invention relates to a process for preparing
obeticholic acid Form 1, comprising the steps of
reacting 3a-hydroxy-7-keto-5P-cholan-24-oic acid (KLCA) with CH3OH and
H2SO4 to form 3a-hydroxy-7-keto-5P-cholan-24-oic acid methyl ester (1),
reacting 3a-hydroxy-7-keto-5P-cholan-24-oic acid methyl ester (1) with
Li[N(CH(CH3)2)21 and Si(CH3)3C1 to form 3a,7-ditrimethylsilyloxy-5P-chol-6-en-
24-oic
acid methyl ester (3),
reacting 3a,7-ditrimethylsilyloxy-5P-chol-6-en-24-oic acid methyl ester (3)
with
CH3CHO to form E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid methyl
ester (4A),
reacting E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid methyl ester
(4A) with NaOH to form E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic
acid
(5A),
17
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
reacting E/Z-3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid (5A) with
Pd/C and hydrogen gas to form 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid
(6),
reacting 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid (6) with NaBH4 to
form crystalline obeticholic acid, and
converting crystalline obeticholic acid to obeticholic acid Form 1.
In one embodiment, the present invention relates to a process for preparing
obeticholic acid Form 1, comprising the steps of
reacting 3a-hydroxy-7-keto-5P-cholan-24-oic acid (KLCA) with CH3OH and
H2SO4 to form 3a-hydroxy-7-keto-5P-cholan-24-oic acid methyl ester (1),
reacting 3a-hydroxy-7-keto-5P-cholan-24-oic acid methyl ester (1) with
Li[N(CH(CH3)2)21 and Si(CH3)3C1 to form 3a,7-ditrimethylsilyloxy-5P-chol-6-en-
24-oic
acid methyl ester (3),
reacting 3a,7-ditrimethylsilyloxy-5P-chol-6-en-24-oic acid methyl ester (3)
with
1 5 CH3CHO to form E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid
methyl ester
(4),
reacting E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid methyl ester
(4) with NaOH to form E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid
(5),
reacting E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-oic acid (5) with Pd/C
and hydrogen gas to form 3a-hydroxy-6a-ethyl-7-keto-51:3-cholan-24-oic acid
(6),
reacting 3a-hydroxy-6a-ethyl-7-keto-5P-cholan-24-oic acid (6) with NaBH4 to
form crystalline obeticholic acid, and
converting crystalline obeticholic acid to obeticholic acid Form 1.
The present invention relates to a process for preparing obeticholic acid Form
1
using crystalline obeticholic acid as a synthetic intermediate. In one
embodiment, the
crystalline obeticholic acid is Form A. In one embodiment, the crystalline
obeticholic acid
Form A is characterized by an X-ray diffraction pattern similar to that set
forth in Figure
41. In one embodiment, the crystalline obeticholic acid Form A is crystallized
and
recrystallized from n-butyl acetate.
In another embodiment, the crystalline obeticholic acid is Form C. In one
embodiment, the crystalline obeticholic acid Form C is characterized by an X-
ray
diffraction pattern similar to that set forth in Figure 5. In another
embodiment, the
18
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
crystalline obeticholic acid is Form D. In one embodiment, the crystalline
obeticholic
acid Form D is characterized by an X-ray diffraction pattern similar to that
set forth in
Figure 45. In another embodiment, the crystalline obeticholic acid is Form F.
In one
embodiment, the crystalline obeticholic acid Form F is characterized by an X-
ray
diffraction pattern similar to that set forth in Figure 49. In another
embodiment, the
crystalline obeticholic acid is Form G. In one embodiment, the crystalline
obeticholic
acid Form G is characterized by an X-ray diffraction pattern similar to that
set forth in
Figure 53. In another embodiment, the crystalline obeticholic acid is Form I.
In one
embodiment, the crystalline obeticholic acid Form I is characterized by an X-
ray
diffraction pattern similar to that set forth in Figure 57.
Stet) 1
Step 1 is the reaction of 3a-hydroxy-7-keto-513-cholan-24-oic acid (KLCA) with
CH3OH and H2SO4 to form 3a-hydroxy-7-keto-513-cholan-24-oic acid methyl ester
(1).
In one embodiment of step 1, the reaction mixture is heated for about 3 hours
and the pH
of the reaction mixture is adjusted with an aqueous basic solution to a pH-
value of about
9.5 to about 10. In one embodiment, the isolation of 3a-hydroxy-7-keto-5P-
cholan-24-
oic acid methyl ester (1) further comprises treatment with activated carbon.
In one
embodiment, the isolation of 3a-hydroxy-7-keto-513-cholan-24-oic acid methyl
ester (1)
does not further comprise treatment with activated carbon. In one embodiment,
isolation
of 3a-hydroxy-7-keto-5P-cholan-24-oic acid methyl ester (1) without the
treatment with
activated carbon affords a higher yield. In one embodiment, reacting 3a-
hydroxy-7-keto-
5p-cholan-24-oic acid (1) with CH3OH and H2504 is carried out in methanol. In
one
embodiment, the basic solution is an aqueous NaOH solution. In one embodiment,
the
pH-value is about 9.5 to about 10.
In one embodiment, the methyl alcohol acts as the methylating reagent as well
as
the reaction solvent. In one embodiment, the solution containing the product
is treated
with activated carbon for about 30 minutes and filtered to remove the carbon
solids. In
one embodiment, the solution containing the product is not treated with
activated carbon.
To precipitate the product, water at about 5 C to about 20 C and seeding
material are
added. In another embodiment, the water is at about 10 C to about 15 C. In
one
embodiment, the product is isolated with a centrifuge and washed with a
mixture of
methanol and water. In one embodiment, the water content of the wet material
is
19
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
quantified by Karl Fischer (KF). In one embodiment, the material is dried in a
tumble
dryer before use in the next step. In one embodiment, the material is not
dried before use
in the next step. In one embodiment, the wet material is washed with heptane
to improve
drying.
Step 2
Step 2 is the reaction of 3a-hydroxy-7-keto-513-cholan-24-oic acid methyl
ester
(1) with Li[N(CH(CH3)2)21 and Si(CH3)3C1 to form 3a,7-ditrimethylsilyloxy-513-
chol-6-
en-24-oic acid methyl ester (3). In one embodiment, step 2 is carried out in a
polar
aprotic solvent at a temperature at about -10 C to about -30 C. In one
embodiment, the
polar aprotic solvent is tetrahydrofuran. In one embodiment, the temperature
is about -20
C to about -25 C. In one embodiment, reacting 3a-hydroxy-7-keto-5P-cholan-24-
oic
acid methyl ester (1) with Li[N(CH(CH3)2)21 and Si(CH3)3C1 is stirred for
about 2 hours.
In one embodiment, compound 1 is charged into the reactor under inert
conditions. In another embodiment, residual water and methanol are removed by
repeated
azeotropic distillation at about 65 C and ambient pressure to avoid water
hydrolysis of
chlorotrimethylsilane which is added later in this step. In another
embodiment, THF is
added to the residue as necessary and the distillation is repeated about 4
times. In another
embodiment, the distillation is repeated about 3 times, about 2 times, or
about 1 time. In
one embodiment, the remaining solution containing the product has a final
water content
of < 0.05% (Karl Fischer Titration). In one embodiment, the solution of the
product is
pre-cooled to about -10 C to about -30 C and then chlorotrimethylsilane is
added. In
another embodiment, the solution is pre-cooled to about -20 C to about -25
C. In one
embodiment, a strong base and THF are charged to a separate reactor and cooled
to about
-10 C to about -30 C. In one embodiment, the strong base is lithium
diisopropylamide.
In another embodiment, the reactor is inert, e.g., under a nitrogen or argon
atmosphere.
In another embodiment, the solution of base and THF is cooled to about -20 C
to about -
25 C. In one embodiment, the dry, cooled solution of 3a-hydroxy-7-keto-50-
cholan-24-
oic acid methyl ester, THF, and chlorotrimethylsilane is charged into the
basic solution at
about -10 C to about -30 C. In another embodiment, the temperature is about -
20 C to
about -25 C. In one embodiment, the reaction mixture is stirred for about 2
hours. In
one embodiment, for the workup, the reaction mixture is added to a pre-cooled
acidic
solution. In another embodiment, the acidic solution is an aqueous citric acid
solution. In
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
one embodiment, after the addition, the aqueous phase is separated and
discarded. In one
embodiment, the solvent is removed from the organic phase, by vacuum
distillation at
about 50 C. In one embodiment, the isolated residue is 3a,7a -
ditrimethy1si1y1oxy-50-
chol-6-en-24-oic acid methyl ester (3) is used 'as is' in the next step.
Alternatively,
compound 3 can be purified before Step 3.
Step 3
Step 3 is the reaction of 3a,7-ditrimethylsilyloxy-513-chol-6-en-24-oic acid
methyl
ester (3) with CH3CHO to form 3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic
acid
methyl ester (4). In one embodiment, step 3 is carried out in a polar aprotic
solvent at a
temperature at about -50 C to about -70 C in the presence of BF3. In one
embodiment,
the polar aprotic solvent is dichloromethane. In one embodiment, the BF3 is a
16% wt.
solution in acetonitrile. In another embodiment, the reaction is carried out
in the presence
of BF3 diethyl etherate. In one embodiment, the temperature is about -60 C to
about -65
C.
In one embodiment, compound 3 in a polar aprotic solvent is charged into an
inert
reactor. In another embodiment, the polar aprotic solvent is the residual
solvent from the
previous step (e.g., THF). In one embodiment, THF is added to help distill off
residual
water and diisopropylamine. At a maximum temperature of about 50 C, residual
amounts
of the polar aprotic solvent are distilled off under vacuum. The water content
in the
residue containing compound 3 is limited to < 0.5% (Karl Fischer titration).
The residue
containing compound 3 is then dissolved in a polar aprotic solvent and pre-
cooled to
about -50 C to about -70 C. The polar aprotic solvent is dichloromethane. In
another
embodiment, residue containing compound 3 in the polar aprotic solvent is pre-
cooled to
about -60 C to about -65 C. Acetaldehyde (CH3CHO) is added. A polar aprotic
solvent
and boron trifluoride (BF3) solvated complex are charged into a separate
reactor and then
cooled to about -50 C to about -70 C. In another embodiment, the polar
aprotic solvent
is dichloromethane. In another embodiment, the boron trifluoride solvated
complex is a
boron trifluoride acetonitrile complex. The temperature of the BF3 solution is
about -60
C to about -65 C. The solution containing compound 3 and acetaldehyde is
added to
the BF3 solution at about -60 C to about -65 C. In another embodiment, the
solution
containing compound 3 and acetaldehyde is dry. In one embodiment, the reaction
mixture is stirred for about two hours at about -60 C to about -65 C, heated
up to about
21
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
23 C to about 28 C, stirred for another about 2 hours and cooled to about 2
C to about
C for the hydrolysis/work-up. In one embodiment, the total time for addition
and
stirring is about 4 hours. In one embodiment, for the workup, the cooled
solution from
the reactor is added to a pre-cooled aqueous basic solution. In another
embodiment, the
5 aqueous basic solution is about 50% wt. sodium hydroxide (NaOH; caustic
soda). In one
embodiment, the phases are separated and the (lower) organic layer is
transferred to a
separate reactor. In one embodiment, from the organic layer, the solvent is
removed by
distillation at not more than (NMT) 50 C as far as possible. In one
embodiment, the
residue comprises 3a-hydroxy-6-ethylidene-7-keto-50-cho1an-24-oic acid methyl
ester (4)
10 and some remaining acetonitrile and dichloromethane. It is understood
that step 4 may
form E/Z-3a-hydroxy-6-ethy1idene-7-keto-50-cho1an-24-oic acid methyl ester
(4A). The
product of Step 3 is taken on directly to Step 4.
Step 4
Step 4 is the reaction of 3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic
acid
methyl ester (4) with NaOH to form E-3a-hydroxy-6-ethylidene-7-keto-513-cholan-
24-oic
acid (5). In one embodiment, prior to step 4, the residue from step 3 is
heated to about 45
C to about 60 C to remove residual amounts of solvent. In one embodiment, the
temperature is about 49 C to about 55 C. In one embodiment, the ester
hydrolysis
reaction reacting 3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid methyl
ester (4)
with NaOH is carried out at about 20 C to about 25 C in methanol, water, and
a NaOH
solution.
In one embodiment, reacting 3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic
acid methyl ester (4) is charged into a reactor. In another embodiment, the
reactor is
inert, e.g., under a nitrogen or argon atmosphere. At a temperature of NMT 50
C,
residual amounts of solvent are distilled off under vacuum. In one embodiment,
the
residue is heated up to about 45 C to about 60 C. In another embodiment, the
residue is
heated up to about 49 C to about 55 C. In another embodiment, the residue
from Step 3
(compound 4) is dissolved in methanol and water and an aqueous basic solution.
In
another embodiment, the aqueous basic solution is about 50% wt. sodium
hydroxide
(NaOH; caustic soda). The ester hydrolysis reaction of Step 4 is carried out
at about 20
C to about 60 C and stirred until the hydrolysis reaction is complete. In one
embodiment, the ester hydrolysis is carried out at about 20 C to about 25 C.
The pH of
22
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
the reaction mixture is checked to verify it is > 12. If the pH is < 12, then
additional
NaOH is added. The reaction mixture is diluted with water and the temperature
is
adjusted to about 20 C to about 35 C. In another aspect, the reaction
mixture is diluted
with water and the temperature is adjusted to about 25 C to about 35 C. In
one
embodiment, for the workup, the phases are separated and the lower product-
rich aqueous
layer is transferred into a separate reactor and the organic layer is
discarded. In one
embodiment, the pH of the product-rich aqueous phase is adjusted with aqueous
acid in
the presence of ethyl acetate. In another embodiment, the acid is an aqueous
citric acid
solution. In one embodiment, the phases are separated and the upper product-
rich organic
layer is retained and the lower aqueous layer is discarded. In one embodiment,
ethyl
acetate is distilled off from the organic layer and replaced with ethyl
acetate. In one
embodiment, the distillation is repeated until the water content of the
distillate is NMT
1% or until a constant boiling point is reached. In one embodiment, the
resulting product
suspension is cooled to about 10 C to about 30 C and isolated and washed
with ethyl
acetate. In one embodiment, the resulting product is isolated by filtration
(e.g. filter
nutche, centrifuge, etc). In another embodiment, the resulting product
suspension
containing compound 5 is cooled to about 20 C to about 25 C. In one
embodiment, the
drying of the resulting product is done under vacuum (e.g, cone dryer, paddle
dryer, tray
dryer, etc.) at about 60 C.
In one embodiment, crude E-3a-hydroxy-6-ethylidene-7-keto-513-cho1an-24-oic
acid (5) is crystallized using ethanol. In one embodiment, ethanol and crude
compound 5
are charged into reactor. In another embodiment, the reactor is inert. In one
embodiment,
to dissolve the crude compound 5, the mixture is heated to reflux. In one
embodiment,
mixture is cooled in a controlled cooling ramp to about 15 C to about 20 C.
In one
embodiment, the crystalline compound 5 is isolated using a centrifuge and then
washed
with ethyl acetate. In one embodiment, drying of crystalline compound 5 is
done under
vacuum (e.g, cone dryer, paddle dryer, tray dryer, etc.) and at about 60 C. A
sample can
be taken to measure assay, purity, and moisture of the purified compound 5. In
one
embodiment, purified compound 5 contains both E and Z isomers of 3a-hydroxy-6-
ethy1idene-7-keto-50-cho1an-24-oic acid. In one embodiment, the E to Z ratio
is about
99:1, about 98:2, about 95: 5, about 90:10, about 85:15, about 80:20, about
75:25, about
70:30, about 65:35, about 60:40, about 55:45, or about 50:50. See Example 2
for full
details regarding the identification and characterization of purified compound
5.
23
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Step 4 can also be carried out starting with a compound that is a mixture of
E/Z
isomer. For example, Step 4 is the reaction of E/Z-3a-hydroxy-6-ethylidene-7-
keto-513-
cholan-24-oic acid methyl ester (4A) with NaOH to form E/Z-3a-hydroxy-6-
ethylidene-
7-keto-513-cholan-24-oic acid (5A). In one embodiment, prior to step 4, the
residue from
step 3 is heated about 45 C to about 60 C to remove residual amounts of
solvent. In one
embodiment, the temperature is about 49 C to about 55 C. In one embodiment,
the ester
hydrolysis reaction involving reacting E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-
cholan-
24-oic acid methyl ester (4A) with NaOH is carried out at about 20 C to about
25 C in
methanol, water, and a NaOH solution. In one embodiment, the NaOH solution is
a 50%
wt. aqueous solution.
In one embodiment, reacting E/Z-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-
oic acid methyl ester (4A) is charged into a reactor. In another embodiment,
the reactor is
inert, e.g., under a nitrogen or argon atmosphere. At a temperature of NMT 50
C,
residual amounts of solvent are distilled off under vacuum. In one embodiment,
the
residue is heated up to about 45 C to about 60 C. In one embodiment, the
temperature is
about 49 C to about 55 C. In one embodiment, the residue from step 3
(compound 4A)
is dissolved in methanol and water and an aqueous basic solution. In another
embodiment, the aqueous basic solution is about 50% wt. sodium hydroxide
(NaOH;
caustic soda). The ester hydrolysis reaction of step 4 is carried out at about
20 C to about
60 C and stirred until the hydrolysis reaction is complete. In one
embodiment, the ester
hydrolysis is carried out at about 20 C to about 25 C. The pH of the
reaction mixture is
checked to verify it is > 12. If the pH is < 12, then additional NaOH is
added. The
reaction mixture is diluted with water and the temperature is adjusted to
about 25 C to
about 35 C. In one embodiment, for the workup, the phases are separated and
the lower
product-rich aqueous layer is transferred into a separate reactor and the
organic layer is
discarded. In one embodiment, the pH of the product-rich aqueous is adjusted
with
aqueous acid in the presence of ethyl acetate. In another embodiment, the acid
is an
aqueous citric acid solution. In one embodiment, the phases are separated and
the upper
product-rich organic layer is retained and the lower aqueous layer is
discarded. In one
embodiment, ethyl acetate is distilled off from the organic layer and replaced
with ethyl
acetate. In one embodiment, the distillation is repeated until the water
content of the
distillate is NMT 1% or until a constant boiling point is reached. In one
embodiment, the
resulting product suspension is cooled to about 10 C to about 30 C and
isolated and
24
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
washed with ethyl acetate. In one embodiment, the resulting product is
isolated by
filtration (e.g. filter nutche, centrifuge, etc.). In another embodiment, the
resulting
product suspension containing compound 5A is cooled to about 20 C to about 25
C. In
one embodiment, drying of the resulting product is done under vacuum (e.g,
cone dryer,
paddle dryer, tray dryer, etc.) at about 60 C. Compound 5A can be carried on
without
purification to Step 5.
In one embodiment, crude E/Z-3a-hydroxy-6-ethy1idene-7-keto-50-cho1an-24-oic
acid (5A) is crystallized using ethanol. In one embodiment, ethanol and crude
compound
5A are charged into reactor. In another embodiment, the reactor is inert. In
one
embodiment, to dissolve the crude compound 5A, the mixture is heated to
reflirc. In one
embodiment, mixture is cooled in a controlled cooling ramp to about 15 C to
about 20
C. In one embodiment, the crystalline compound 5A is isolated using a
centrifuge and
then washed with ethyl acetate. In one embodiment, drying of crystalline
compound 5A
is done under vacuum (e.g, cone dryer, paddle dryer, tray dryer, etc.) and at
about 60 C.
In one embodiment, the isolated crystalline product of step 4 is compound 5.
Alternative Step 4
Compound 5 can be prepared according to an alternative method. In one
embodiment, compound 4 is charged into the inert reactor. At about 50 C
(maximum)
residual amounts of solvent (e.g., acetonitrile, dichloromethane) may be
distilled off
under vacuum. The residue is dissolved in methanol and cooled. Water and
caustic soda
(50 % weight NaOH) are added. In one embodiment, the reaction mixture is
stirred for
about four hours at about 20 C to about 25 C. The solution is diluted with
water and
toluene is added. After stirring, the phases are separated and the lower
product-rich
aqueous layer is transferred into the inert reactor. The organic layer is
discarded. The pH
of the product rich aqueous layer is adjusted with aqueous citric acid in the
presence of
ethyl acetate. The phases are separated and the lower aqueous layer is
discarded. The
organic layer is transferred into the inert reactor. From the organic layer
ethyl acetate is
distilled off and replaced with ethyl acetate. In one embodiment, this
operation is repeated
until the water content of the distillate is not more than about 1 % or until
a constant
boiling point is reached. The resulting product suspension is cooled to about
20 C to
about 25 C, and compound 5 is isolated and washed with ethyl acetate in the
inert
centrifuge. Drying is done in the tumble dryer under vacuum and approximately
60 C.
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
This alternative Step 4 can also be carried out starting with a compound that
is a
mixture of E/Z isomer. In one embodiment, compound 4A is charged into the
inert
reactor. At about 50 C (maximum) residual amounts of solvent (e.g.,
acetonitrile,
dichloromethane) may be distilled off under vacuum. The residue is dissolved
in
methanol and cooled. Water and caustic soda (50%wt, NaOH) are added. In one
embodiment, the reaction mixture is stirred for approximately four hours at
about 20 C
to about 25 C. The solution is diluted with water and toluene is added. After
stirring, the
phases are separated and the lower product-rich aqueous layer is transferred
into the inert
reactor. The organic layer is discarded. The pH of the product rich aqueous
layer is
adjusted with aqueous citric acid in the presence of ethyl acetate. The phases
are
separated and the lower aqueous layer is discarded. The product-rich organic
layer is
transferred into the inert reactor. From the organic layer ethyl acetate is
distilled off and
replaced with ethyl acetate. In one embodiment, this operation is repeated
until the water
content of the distillate is not more than about 1 % or until a constant
boiling point is
reached. The resulting product suspension is cooled to 20 C to 25 C, and
compound 5A
is isolated and washed with ethyl acetate in the inert centrifuge. Drying is
done in the
tumble dryer under vacuum and approximately 60 C.
Step 5
Step 5 is the reaction of E-3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic
acid
(5) with Pd/C and hydrogen gas to initially form 3a-hydroxy-613-ethy1-7-keto-
513-cholan-
24-oic acid, which undergoes base-mediated isomerization to form 3a-hydroxy-6a-
ethy1-
7-keto-513-cholan-24-oic acid (6). Step 5 can be carried out in one phase
(hydrogenation
and isomerization together) or in two discrete phases (hydrogenation followed
by
isomerization). In one embodiment, Step 5 is carried out at a temperature at
about 90 C
to about 110 C and at a pressure at about 0.5 to about 5 bars. In one
embodiment, during
workup, the organic phase of the reaction mixture is treated with activated
carbon. In one
embodiment, the pressure is about 0.5 to about 5.5 bars. In another
embodiment, the
pressure is about 5 bars. In another embodiment, the hydrogenation is
performed at a
pressure of about 0.5 bar to about 5.5 bar, e.g 0.6 bar, 0.7 bar, 0.8 bar, 0.9
bar, 1.0 bar, 1.2
bar, 1.4 bar, 1.6 bar, 1.8 bar, 2.0 bar, 2.2 bar, 2.4 bar, 2.6 bar, 2.8 bar,
3.0 bar, 3.2 bar, 3.4
bar, 3.6 bar, 3.8 bar, 4.0 bar, 4.2 bar, 4.4 bar, 5.0 bar, 5.2 bar, as well as
any increment in
between. In one embodiment, the hydrogenation reaction mixture is allowed to
stir for
26
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
about 1 hour. In one embodiment, E-3a-hydroxy-6-ethylidene-7-keto-513-cholan-
24-oic
acid (5) in the presence of Pd/C and hydrogen gas is heated to about 100 C
and stirred
for about 2 hours to about 5 hours. In one embodiment, E-3a-hydroxy-6-
ethylidene-7-
keto-513-cholan-24-oic acid (5) in the presence of Pd/C and hydrogen gas is
heated to
about 100 C and stirred for about 3 hours.
In one embodiment, E-3a-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid (5)
in the presence of Pd/C and hydrogen gas is carried out in the presence of a
basic
solution. In one embodiment, the basic solution is water containing a 50% wt.
sodium
hydroxide (NaOH; caustic soda) solution. After the hydrogenation reaction, the
reaction
mixture is heated up to about 100 C (to carry out the isomerisation of the C-
6 position
from beta configuration to alpha configuration) and then cooled to about 40 C
to about
50 C. For the workup, the Pd/C is filtered off In one embodiment, to the
filtrate, n-
butyl acetate and an acid are added. In another embodiment, the acid is
hydrochloric acid
(HC1). The aqueous phase is separated and discarded after checking the pH-
value to
make sure that it was acidic. The product-rich organic phase is treated with
activated
carbon. In one embodiment, the activated carbon is filtered off and the
resulting product-
rich filtrate is concentrated by distillation and the resulting product
suspension is cooled
to about 10 C to about 30 C. In another embodiment, the product suspension
is cooled
to about 15 C to about 20 C. The suspension containing compound 6 is
isolated and
washed with n-butyl acetate. Compound 6 is filtered using a pressure filter.
In one
embodiment, drying is done in the pressure filter under vacuum at about 80 C.
In one embodiment in Step 5, E-3a-hydroxy-6-ethylidene-7-keto-5P-cholan-24-
oic acid (5), water, NaOH solution (e.g. 50% wt.), and Pd/C are mixed at about
5 bar of
H2 gas and at a temperature at about 100 C to about 105 C until H2 uptake
has ceased.
The reaction mixture is cooled to about 40 C to about 50 C and Pd/C is
filtered off
Then n-butyl acetate and HC1 are added to the solution containing compound 6.
In one
embodiment, the aqueous phase is separated and discarded. The organic phase
containing
compound 6 is treated with activated carbon. The carbon is filtered off and
the filtrate is
moved to another reactor where it is concentrated by distillation, and then
the suspension
is cooled to about 5 C to about 20 C. In one embodiment, compound 6 is
isolated via
filtration and the filtrate is dried on the pressure filter under vacuum at
about 80 C.
27
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Step 5 can also be carried out starting with a compound that is a mixture of
E/Z
isomer. For example, Step 5 is the reaction of E/Z-3a-hydroxy-6-ethylidene-7-
keto-513-
cholan-24-oic acid (5A) with Pd/C and hydrogen gas and heat to form 3ot-
hydroxy-6ot-
ethyl-7-keto-513-cholan-24-oic acid (6). Step 5 can be carried out in one
phase
(hydrogenation and isomerization together) or in two discrete phases
(hydrogenation,
followed by isomerization). In one aspect, step 5 is carried out at a
temperature at about
90 C to about 110 C and at a pressure at about 4 to about 5 bars. In one
embodiment,
during workup, the organic phase of the reaction mixture is treated with
activated carbon.
In one embodiment, the pressure is about 4.5 to about 5.5 bars. In another
embodiment,
the pressure is about 5 bars. In one embodiment, the hydrogenation reaction
mixture is
allowed to stir for about 1 hour. In one embodiment, reacting E/Z-3ot-hydroxy-
6-
ethylidene-7-keto-513-cholan-24-oic acid (5A) with Pd/C and hydrogen gas is
heated to
about 100 C and stirred for about 2 hour to about 5 hours. In one embodiment,
reacting
E/Z-3ot-hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid (5A) with Pd/C and
hydrogen gas is heated to about 100 C and stirred for about 3 hours.
In one embodiment, reacting E/Z-3ot-hydroxy-6-ethylidene-7-keto-5P-cholan-24-
oic acid (5A) with Pd/C and hydrogen gas is carried out in the presence of a
basic
solution. In one embodiment, the basic solution is water and a 50% wt. sodium
hydroxide
(NaOH; caustic soda) solution. After the hydrogenation reaction, the reaction
mixture is
heated up to about 100 C (to carry out the isomerisation of the C-6 position
from beta
configuration to alpha configuration) and then cooled to about 40 C to about
50 C. For
the workup, the Pd/C is filtered off In one embodiment, to the filtrate, n-
butyl acetate
and an acid are added. In another embodiment, the acid is hydrochloric acid
(HC1). The
aqueous phase is separated and discarded after checking the pH-value to make
sure that it
was acidic. The product-rich organic phase is treated with activated carbon.
In one
embodiment, the activated carbon is filtered off and the resulting product-
rich filtrate is
concentrated by distillation and the resulting product suspension is cooled to
about 10 C
to about 30 C. In another embodiment, the product suspension is cooled to
about 15 C
to about 20 C. The suspension containing compound 6 is isolated and washed
with n-
butyl acetate. Compound 6 is filtered using a pressure filter. In one
embodiment, drying
is done in the pressure filter under vacuum at about 80 C.
In one embodiment in Step 5, E/Z-3ot-hydroxy-6-ethylidene-7-keto-5P-cholan-24-
oic acid (5A), water, NaOH solution (e.g. 50% wt.), and Pd/C are mixed at
about 5 bar of
28
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
H2 gas and at a temperature at about 100 C to about 105 C until H2 uptake
has ceased.
The reaction mixture is cooled to about 40 C to about 50 C and Pd/C is
filtered off
Then n-butyl acetate and HC1 are added to the solution containing compound 6.
In one
embodiment, the aqueous phase is separated and discarded. The product rich
organic
phase is treated with activated carbon. The carbon is filtered off and the
filtrate is moved
to another reactor where it is concentrated by distillation, and then the
product suspension
is cooled to about 5 C to about 20 C. In one embodiment, compound 6 is
isolated via
filtration and the filtrate is dried on the pressure filter under vacuum at
about 80 C.
In another embodiment, the hydrogenation/isomerization reactions described
above to prepare compound 6 are carried out in two phases (starting from
compound 5 or
compound 5A). First, the hydrogenation is carried out at about 4 to 5 bars and
at about 20
C to about 40 C and then second, the reaction mixture is heated to about 95
C to about
105 C. Heating the reaction mixture isomerizes the ethyl group at the 6-
position from
the initially formed 6-beta configuration to the desired 6-alpha
configuration. The
reaction mixture is heated until the isomerization is complete.
Step 6
Step 6 is the reaction of 3a-hydroxy-6a-ethy1-7-keto-513-cholan-24-oic acid
(6)
with NaBH4 to form crystalline obeticholic acid. In one embodiment, Step 6 is
carried
out at a temperature at about 85 C to about 110 C in a basic aqueous
solution. In one
embodiment, the temperature is about 90 C to about 95 C. In one embodiment,
the basic
aqueous solution is an aqueous NaOH solution. In one embodiment, the basic
aqueous
solution is a mixture of 50% wt. NaOH solution and water. In one embodiment,
the
reaction mixture of compound 6 and NaBH4 was stirred for about 3 hours to
about 5
hours. In another embodiment, the reaction mixture was stirred for about 4
hours.
For the workup, after the reaction is complete, the mixture is cooled to about
80
C and transferred to a cooled reactor. In one embodiment, at about 20 C to
about 60 C,
n-butyl acetate and an acid are added. In one embodiment, the temperature is
about 40 C
to about 45 C. In another embodiment, the acid is citric acid. The aqueous
phase is
separated and discarded after checking the pH-value to make sure that it was
acidic. The
product-rich organic phase is concentrated by distillation. In one embodiment,
n-butyl
acetate is added to the residue and distilled off again. In one embodiment, n-
butyl acetate
is added again to the residue and then is slowly cooled down. In another
embodiment the
residue is seeded at about 50 C. In another embodiment, after crystallization
has
29
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
occurred, the mixture is heated to 52 C and then slowly cooled down to about
15 C to
about 20 C. In another embodiment, the residue is cooled to about 15 C to
about 20 C.
In one embodiment, the resulting obeticholic acid is washed with n-butyl
acetate. In one
embodiment, the obeticholic acid is isolated and washed with n-butyl acetate
(e.g, in a
pressure filter). In another embodiment, the pressure filter is inert. The
crystalline
product is dried under vacuum at about 60 C and is identified as Form A
characterized
by an X-ray diffraction pattern substantially similar to that set forth in
Figure 41 including
characteristic peaks at about 5.0 and 5.3 degrees 2-Theta. In one embodiment,
the
resulting crystalline obeticholic acid is isolated from organic solvent (e.g.,
heptane) and is
characterized by an X-ray diffraction pattern including characteristic peaks
at about 5.0
and 5.3 degrees 2-Theta. In one embodiment, the crystalline obeticholic acid
Form A is
characterized by an X-ray diffraction pattern substantially similar to that
set forth in
Figure 41. See example 3 for full details regarding the identification and
characterization
of crystalline obeticholic acid Form A.
Step 7
Step 7 is the conversion of crystalline obeticholic acid Form A to obeticholic
acid
Form 1. In one embodiment, Step 7 comprises the step of dissolving crystalline
obeticholic acid Form A in aqueous NaOH solution and adding HC1.
In one embodiment, crystalline obeticholic acid is dissolved in water and
caustic
soda solution (50% wt.) at about 20 C to about 50 C. In one embodiment, the
temperature is about 30 C to about 40 C. In one embodiment, the crystalline
obeticholic acid is Form A. In one embodiment, the resulting solution of
crystalline
obeticholic acid Form A is added to diluted acid at about 20 C to about 50
C. In
another embodiment, the temperature is about 30 C to about 40 C. In another
embodiment, the acid is hydrochloric acid (e.g., 37%). In one embodiment, the
37%
hydrochloric acid solution is diluted with water to less than about 1% by
volume. In one
embodiment, the 37% hydrochloric acid solution is diluted with water to about
0.7% by
volume. In one embodiment, the suspension of product in the diluted acid is
stirred for
about 30 minutes at about 20 C to about 50 C. In another embodiment, the
temperature
is about 30 C to about 40 C. In one embodiment, obeticholic acid Form 1 is
isolated
and washed with water (e.g., in the pressure filter) at NMT about 20 C. In
one
embodiment, obeticholic acid Form 1 is isolated and washed with water (e.g.,
in the
pressure filter) at NMT about 20 C. In another embodiment, the pressure
filter is inert.
CA 03009149 2018-06-19
WO 2017/111979 PCT/US2016/012651
The product is dried on the pressure filter under vacuum at a temperature of
NMT about
50 C.
The process of the present application utilizes a crystalline intermediate in
the
preparation of obeticholic acid Form 1, which unexpectedly led to significant
improvements in the overall preparation and purity of the final product.
Specifically,
Step 6 of the synthesis produces a novel crystalline form of obeticholic acid.
The
production of this crystalline form leads to substantially pure obeticholic
acid Form 1.
The process of the present application is an improvement over the processes
disclosed in the prior art. The preparation of obeticholic acid is disclosed
in U.S.
Publication No. 2009/0062526 Al (herein referred to as the ¨526 publication"),
U.S.
Patent No. 7,138,390 (referred to herein as the ¨390 patent"), and WO
2006/122977
(referred to herein as the ¨977 application").
The process to prepare obeticholic acid in the '390 patent (referred to herein
as the
¨390 process") is depicted in Scheme 3 (R is ethyl):
Scheme 3
HO1CO2H
C61\
CO2H
HU"
co2Et
co2H
HO'" '40H
HO".**OH
H
Even though this process comprises a few steps, it presents a series of
drawbacks.
In all of the steps, the reaction products are purified on a chromatographic
column,
namely a very expensive separation method that cannot be used on an industrial
scale.
Moreover, the reaction yield in step 2 is extremely low (12-13%) with a
considerable
decrease in the global yield, which is lower than 3.5%. This process also uses
hexamethylenphosphonamide as reactant, which is a known carcinogenic agent.
31
CA 03009149 2018-06-19
WO 2017/111979 PCT/US2016/012651
The process to prepare obeticholic acid in the '977 application is depicted in
Scheme 4.
Scheme 4
O
O
,õ..
OH
HO". 0
step 1
CH3OH/H*'
HO
0 2A
1A Istep 2
triethylamine/
trimethylchlorosilane
,õ 0 ,,,,. 0
..
Step 3
=
'11111111 o_ lithium diisopropylamide/
trimethylchlorosilane
-. ___________________________
... I 0-
-.. I
-S'== sl- Si"O
CIS15.-N-1(
--- s 0
0 ,,
4A-1 3A
Istep 4
acetaldehyde/
boron trifluoride
diethyl etherate
0
0 ,õ..
,,,,.
ee 0-
step 5 .
O.
1110* OH
Se
HO o 1) NaOH HO"' 0
"
I 2)H*
6A
5A-1
Step 6 I
H2/cat.
OH-
0 ,õ.. 0
ONa
1) heat
2) 11*
HO" L"----Lf---LO
----#7A -
8A
1 step 7
purification
0 0
. ,õ..
C613.--N-l<
HO"
step 8
1) sodium boronhydride .
2)H* HOs .c61 ''OH:50H
'
---; 9A .--; crude obeticholic acid
I step 9
purification
0
0
,õ..
HO'.cig:ri(OH
step 10
purification HO' i ...tDH
. "OH ----' crude obeticholic acid
----'
purified obeticholic acid
The '977 process to prepare obeticholic acid is an 8-step synthetic process
which
includes one purification step (step 7) followed by 2 additional purification
steps. There
32
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
are a significant number of differences between the '977 process and the
process of the
present application. Table A below describes at least some of the differences
between the
two processes:
33
CA 03009149 2018-06-19
WO 2017/111979 PCT/US2016/012651
Table A: Differences Between '977 Process and Process of the Application
Changes
Synthetic Step Advantages of the
Change
'977 Process Process of the application
Scale and safety
Methanesulfonic acid Sulfuric acid
(mesylate)
Step 1 30% ammonia (aqueous) NaOH
(aqueous) Scale-up
No Use of activated carbon
Improve purity/color
purification/treatment treatment
LDA is a suitable
Lithium diisopropylamide
Triethylamine alternative reagent
for this
(LDA)
Step 2 step
(Process of application THF is a suitable
step 2 combines '977 Toluene Tetrahydrofuran (THF) alternative
reagent for this
Process Steps 2 and 3) step
Quench into citric acid
No acidic quench Scale-up
solution
Step 3 Safety concerns of
(Process of application Boron trifluoride diethyl Boron trifluoride
handling etherate (T4
step 3 same as '977 etherate acetonitrile complex emission
controls not
Step 4) needed)
Toluene Methanol Safety (toluene);
scale
Step 4
Phosphoric acid Citric acid (aqueous)
(Process of application Scale-up
step 4 same as '977 (aqueous) quench quench
Step 5) No Crystallization step is part
Improve purity
purification/treatment of workup
Phosphoric acid Hydrochloric acid
Scale-up
Step 5 (aqueous) quench (aqueous) quench
(Process of application No Use of activated carbon
Improve purity/color
step 5 combines '977 purification/treatment treatment
Process steps 6 and 7) Purification carried out Crystallization step is
part
Scale-up
as Step 7 of workup
Dichloromethane n-Butylacetate Safety
(dichloromethane)
Phosphoric acid Citric acid (aqueous)
Step 6 Scale-up
(aqueous) quench quench
(Process of application
step 6 combines '977 Purification carried out
Crystallization step is part
as Step 9 ¨ using Scale and safety
Process steps 8 and 9) of workup ¨ using n-
dichloromethane /ethyl (dichloromethane)
butylacetate
acetate
Step 7 Ammonia solution NaOH solution Scale-up
(Process of application
Phosphoric acid Hydrochloric acid
step 7 same as '977 Scale-up
step 10) (aqueous) quench (aqueous) quench
The differences in the process of the present application as compared to the
'977
process result in significant improvements to the process, including
improvements related
to scale-up optimization, safety, as well as purity and improvements in the
overall
34
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
process. The purity of obeticholic acid produced by the processes of the
present
application is substantially pure. Specifically, obeticholic acid produced by
the processes
of the present application is substantially more pure than obeticholic acid
produced by
processes in the prior art, including the '390 process and the '977 process.
For example,
a comparison of the results presented in the Certificate of Analysis of
obeticholic acid
produced by a process of the present application and obeticholic acid produced
by the
'977 process are shown in the Table B below. The percentages of impurities
were
determined using HPLC methods.
Table B: Comparison of Impurities of Obeticholic Acid Generated from Process
of
the Application and '977 Process
l'i?arameter,,
Water (I(F) NMT 4.5')/0 1.0% 2.1%
Impurity 1 and Impurity 4 NMT 0.15% <0.05% <0.05%
Impurity 2 NMT 0.15% <0.05% <0.1%
Impurity 3 NMT 0.15% <0.05% <0.1%
Impurity 5 NMT 3.0% 0.2% 1.0%
Impurity 6 NMT 0.15% <0.05% <0.05%
Impurity 1 is 6-ethylursodeoxycholic acid.
Impurity 2 is 3a-hydroxy-6a-ethyl-7-cheto-513-cholan-24-oic acid.
Impurity 3 is 6p-ethylchenodeoxycholic acid.
Impurity 4 is 3a,7a-dihydroxy-6-ethyliden-5P-cholan-24-oic acid.
Impurity 5 is chenodeoxycholic acid.
Impurity 6 is 3a(3a,7a-dihydroxy-6a-ethy1-513-cholan-24-oyloxy)-7a-hydroxy-6a-
ethy1-513-cholan-24-oic
acid (6ECDCA dimer).
NMT refers to "not more than".
Crystalline Obeticholic Acid as a Synthetic Intermediate
Obeticholic acid is currently being developed as an active pharmaceutical
ingredient as a non-crystalline solid. In order to facilitate the development
of obeticholic
acid, an initial crystallization and polymorphism study was carried out in
order to
determine if crystalline forms were accessible and if so, if they were
suitable for
development. After a preliminary solubility screen designed to give a better
understanding of the behavior of the material in various solvents, it appeared
that the
material had a tendency to form gels and could possibly be crystallized. An
extensive
polymorph screen was then carried out, exposing the material to a large range
of solvents
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
and crystallization conditions in order to identify and characterize as many
relevant
polymorphs as possible. Five different solid forms were found during this
screen.
Three forms (A, C, and D) of obeticholic acid were mixed hydrates/solvates
containing 0.25 mol eq of water and varying amounts of a range of organic
solvents. On
heating, these solids lost crystallinity and the solvent at the same time and
unfortunately,
these solvated forms were not suitable for further development as a
pharmaceutical
ingredient due to their low melting temperatures and high solvent content. It
is also noted
that similar "unsuitable" forms of this type exist. For example, a low-melting
solvated
form was found in later experiments, as well as single crystals of another
form, which
was shown to be a monohydrate/anisole solvate by SCXRD (Single crystal X-ray
diffraction).
The two remaining forms were higher melting and potentially more promising,
but
one of them (Form G) could not be reproduced on scale-up, nor repeated despite
many
attempts. The difficulty in producing this form alone makes it unsuitable for
development. The remaining non-solvated Form F was reproducibly prepared, but
it
required extensive recrystallization procedures and the use of nitromethane,
which is a
toxic solvent and may detonate if sensitized by amines, alkalis, strong acids,
or high
temperatures or adiabatic compression. Concerns about the residual levels of
nitromethane deemed Form F also to be unsuitable for development. Crystalline
obeticholic acid Form I was also identified.
The overall results of the initial crystallization and polymorph study
revealed that
the material could form various forms of crystalline materials, but none of
the crystalline
materials or forms were considered suitable for development.
It was not until much later that it was discovered the importance of producing
crystalline obeticholic acid as an intermediate in the penultimate step of the
process of the
present application. Crystalline obeticholic acid could readily be isolated on
large scale
using the process of the application. This crystalline obeticholic acid was
determined to
be consistent with Form A from the initial crystallization and polymorph
study. The
formation, ease of isolation, and highly pure crystalline obeticholic acid
produced as a
synthetic intermediate in step 7 in the process of the present application is
indeed critical
to the preparation of substantially pure obeticholic acid.
In one embodiment, the present invention relates to a crystalline obeticholic
acid
Form A characterized by an X-ray diffraction pattern including characteristic
peaks at
about 5.0 and 5.3 degrees 2-Theta. In one embodiment, the X-ray diffraction
pattern
36
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
includes characteristic peaks at about 5.0, 5.3 and 7.7 degrees 2-Theta. In
one
embodiment, the X-ray diffraction pattern includes characteristic peaks at
about 5.0, 5.3,
7.7, and 10.0 degrees 2-Theta. In one embodiment, the X-ray diffraction
pattern includes
characteristic peaks at about 5.0, 5.3, 7.7, 10.0, and 11.0 degrees 2-Theta.
In one
embodiment, the X-ray diffraction pattern includes characteristic peaks at
about 5.0, 5.3,
7.7, 10.0, 11.0, and 12.4 degrees 2-Theta. In one embodiment, the X-ray
diffraction
pattern includes characteristic peaks at about 5.0, 5.3, 7.7, 10.0, 11.0,
12.4, and 14.9
degrees 2-Theta. In one embodiment, the present invention relates to a
crystalline
obeticholic acid Form A characterized by an X-ray diffraction pattern
substantially
1 0 similar to that set forth in Figure 41. In one embodiment, the X-ray
diffraction pattern is
collected on a diffractometer using Cu Ka radiation (40 kV, 40 mA).
In one embodiment, the present invention relates to a crystalline obeticholic
acid,
wherein said crystalline obeticholic acid is Form A and has a purity greater
than about
90%. In one embodiment, the purity of said crystalline obeticholic acid Form A
is
1 5 determined by HPLC. In one embodiment, the present invention relates to
a crystalline
obeticholic acid Form A, or a pharmaceutically acceptable salt, solvate or
amino acid
conjugate thereof In one embodiment, the solvate is a n-butyl solvate. In one
embodiment, the purity is greater than about 92%. In one embodiment, the
purity is
greater than about 94%. In one embodiment, the purity is greater than about
96%. In one
20 embodiment, the purity is greater than about 98%. In one embodiment, the
purity is
greater than about 99%.
In one embodiment, the present invention relates to a crystalline obeticholic
acid,
wherein said crystalline obeticholic acid is Form A and has a potency greater
than about
90%. In one embodiment, the purity of said crystalline obeticholic acid Form A
is
25 determined by HPLC and/or other analytical procedures known in the art.
In one
embodiment, the present invention relates to a crystalline obeticholic acid
Form A, or a
pharmaceutically acceptable salt, solvate or amino acid conjugate thereof In
one
embodiment, the solvate is a hydrate. In one embodiment, the potency is
greater than
about 92%. In one embodiment, the potency is greater than about 94%. In one
30 embodiment, the potency is greater than about 96%. In one embodiment,
the potency is
greater than about 98%. In one embodiment, the potency is greater than about
99%.
In one embodiment, the present invention relates to a crystalline obeticholic
acid
Form A that contains a total of less than about 4% of one or more impurities
selected
37
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
from 6-ethylursodeoxycholic acid, 3a-hydroxy-6a-ethy1-7-cheto-513-cholan-24-
oic acid,
6P-ethylchenodeoxycholic acid, 3a,7a-dihydroxy-6-ethyliden-513-cholan-24-oic
acid,
chenodeoxycholic acid, and 3a(3a,7a-dihydroxy-6a-ethy1-5P-cholan-24-oyloxy)-7a-
hydroxy-6a-ethy1-5P-cholan-24-oic acid. In one embodiment, the total
impurities is less
than about 3.8%. In one embodiment, the total impurities is less than about
3.6%.
In one embodiment, the present invention relates to a crystalline obeticholic
acid
Form C characterized by an X-ray diffraction pattern including characteristic
peaks at
about 4.2, 6.4, 9.5, 12.5, and 16.7 degrees 2-Theta. In one embodiment, the X-
ray
diffraction pattern includes characteristic peaks at about 4.2, 6.4, 9.5,
12.5, 12.6, 15.5,
15.8, 16.0, 16.7 and 19.0 degrees 2-Theta. In one embodiment, the X-ray
diffraction
pattern includes characteristic peaks at about 4.2, 6.4, 8.3, 9.5, 11.1, 12.2,
12.5, 12.6,
15.5, 15.8, 16.0, 16.3, 16.7, 18.6 and 19.0 degrees 2-Theta. In one
embodiment, the X-ray
diffraction pattern includes characteristic peaks at about 4.2, 6.4, 8.3, 9.5,
11.1, 12.2,
12.5, 12.6, 15.5, 15.8, 16.0, 16.3, 16.7, 17.0, 17.8, 18.6, 18.8, 19.0, 20.5
and 20.9 degrees
2-Theta. In one embodiment, the present invention relates to a crystalline
obeticholic acid
Form C characterized by an X-ray diffraction pattern substantially similar to
that set forth
in Figure 5. In one embodiment, the X-ray diffraction pattern is collected on
a
diffractometer using Cu Ka radiation (40 kV, 40 mA). In one embodiment, the X-
ray
diffraction pattern includes characteristic peaks at about 12.0 to about 12.8
and about 15.4
to about 21Ø
In one embodiment, the present invention relates to a crystalline obeticholic
acid
Form C characterized by a Differential Scanning Calorimetry (DSC) thermogram
having
an endotherm value at about 98 2 C, as measured by a Mettler DSC 823e
instrument. In
one embodiment, the Differential Scanning Calorimetry (DSC) thermogram has an
endotherm value at about 98 2 C, as measured by a Mettler DSC 823e
instrument.
In one embodiment, the present invention relates to a crystalline obeticholic
acid,
wherein said crystalline obeticholic acid is Form C and has a purity greater
than about
90%. In one embodiment, the purity of said crystalline obeticholic acid Form C
is
determined by HPLC. In one embodiment, the present invention relates to a
crystalline
obeticholic acid Form C, or a pharmaceutically acceptable salt, solvate or
amino acid
conjugate thereof In one embodiment, the solvate is a hydrate. In one
embodiment, the
purity is greater than about 92%. In one embodiment, the purity is greater
than about
94%. In one embodiment, the purity is greater than about 96%. In one
embodiment, the
38
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
purity is greater than about 98%. In one embodiment, the purity is greater
than about
99%.
In one embodiment, the present invention relates to a crystalline obeticholic
acid,
wherein said crystalline obeticholic acid is Form C and has a potency greater
than about
90%. In one embodiment, the purity of said crystalline obeticholic acid Form C
is
determined by HPLC and/or other analytical procedures known in the art. In one
embodiment, the present invention relates to a crystalline obeticholic acid
Form C, or a
pharmaceutically acceptable salt, solvate or amino acid conjugate thereof In
one
embodiment, the solvate is a hydrate. In one embodiment, the potency is
greater than
1 0 about 92%. In one embodiment, the potency is greater than about 94%. In
one
embodiment, the potency is greater than about 96%. In one embodiment, the
potency is
greater than about 98%. In one embodiment, the potency is greater than about
99%.
In one embodiment, the present invention relates to a crystalline obeticholic
acid
Form C that contains a total of less than about 4% of one or more impurities
selected
from 6-ethylursodeoxycholic acid, 3a-hydroxy-6a-ethy1-7-cheto-513-cholan-24-
oic acid,
6P-ethylchenodeoxycholic acid, 3a,7a-dihydroxy-6-ethyliden-513-cholan-24-oic
acid,
chenodeoxycholic acid, and 3a(3a,7a-dihydroxy-6a-ethy1-5P-cholan-24-oyloxy)-7a-
hydroxy-6a-ethy1-5P-cholan-24-oic acid. In one embodiment, the total
impurities is less
than about 3.8%. In one embodiment, the total impurities is less than about
3.6%.
Example 3 of the application provides full characterization of these
crystalline
forms of obeticholic acid.
The single crystal X-ray structure of obeticholic acid was obtained and the
absolute stereochemistry assigned. For example, the single crystal X-ray
structure of
crystalline obeticholic acid Form G was determined from a crystal obtained
from the
recrystallization of obeticholic acid from an acetonitrile solution after
cooling to 5 C at
0.1 C/min followed by maturation at RT/50 C 8 h cycles for 1 week.
The structure is orthorhombic, space group P212121, and contains one molecule
of
obeticholic acid in the asymmetric unit. Final R1 [I>2o-(01= 3.22 %. The
absolute
stereochemistry of the molecule was determined as shown below with a Flack
parameter
= -0.01 (13). The structure had no disorder.
39
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
COOH
P
S
õ.== i<
HO ===,,
% /OH
H
A bioavailability study of obeticholic acid Form 1 (non-crystalline) vs.
crystalline
obeticholic acid Form F was carried out (Example 7). The results of the study
show that
that physical state of a solid obeticholic acid can play a role in the
bioavailability of the
molecule when administered orally to a subject. The plasma kinetics after oral
administration and the efficiency of the intestinal absorption and the
pharmacokinetics of
solid obeticholic acid Form 1 (non-crystalline) and crystalline Form F were
evaluated
according to methods known in the art. Example 8 of the present invention
shows the
1 0 profiles of obeticholic acid plasma concentration vs time, the tmax,
Cmax and AUC after
administration of Form 1 or Form F of obeticholic acid (see Figures 37-38).
Crystalline
Form F has a higher bioavailability than obeticholic acid Form 1 (non-
crystalline). The
plasma profiles show that the Form F is absorbed more efficiently (higher AUC)
and even
the kinetics is more regular, reflecting an optimal distribution of the drug
in the intestinal
content.
The water solubility of obeticholic acid Form 1 (non-crystalline) is slightly
higher
than that of Form F. Form F appears to be stable as the thermo gravimetric
analysis
(TGA) did not show any weight loss in the temperature range studied.
Substantially Pure Obeticholic Acid
The present application provides substantially pure obeticholic acid and
pharmaceutically acceptable salts, solvates, or amino acid conjugates thereof:
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
CO2H
Ho"' $4rOH
H E
obeticholic acid
(also known as INT-747)
Other names for the pharmaceutically active ingredient obeticholic acid are
INT-747,
3a,7a-dihydroxy-6a-ethyl-50-cho1an-24-oic acid, 6a-ethyl-chenodeoxycholic
acid, 6-
ethyl-CDCA, 6ECDCA, and cholan-24-oic acid,6-ethy1-3,7-dihydroxy-
,(3a,50,6a,7a)-.
The present application provides compositions comprising obeticholic acid
Form 1 and processes for the synthesis of highly pure obeticholic acid Form 1
which are
safe and which produce obeticholic acid on a large scale. In one aspect,
obeticholic acid
Form 1 is produced on a commercial scale process. The term "commercial scale
process"
refers to a process which is run as a single batch of at least about 100
grams. In one
aspect, the process of the present application produces obeticholic acid Form
1 in high
yield (>80%) and with limited impurities.
The term "purity" as used herein refers to the amount of obeticholic acid
based on
HPLC. Purity is based on the "organic" purity of the compound. Purity does not
include
a measure of any amount of water, solvent, metal, inorganic salt, etc. In one
aspect, the
purity of obeticholic acid is compared to the purity of the reference standard
by
comparing the area under the peak. In another aspect, the known standard for
purity is an
obeticholic acid reference standard. In one aspect, obeticholic acid has a
purity of greater
than about 96%. In one aspect, obeticholic acid has a purity of greater than
about 98%.
For example, the purity of obeticholic acid Form 1 is 96.0%, 96.1%, 96.2%,
96.3%,
96.4%, 96.5%, 96.6%, 96.7%, 96.8%, 96.9%, 97.0%, 97.1%, 97.2%, 97.3%, 97.4%,
97.5%, 97.6%, 97.7%, 97.8%, 97.9 %, 98.0%, 98.1%, 98.2%, 98.3%, 98.4%, 98.5%,
98.6%, 98.7%, 98.8%, 98.9%, 99.0%, 99.1%, 99.2%, 99.3%, 99.4%, 99.5%, 99.6%,
99.7%, 99.8%, or 99.9%. For example, the purity of obeticholic acid Form 1 is
98.0%,
98.1%, 98.2%, 98.3%, 98.4%, 98.5%, 98.6%, 98.7%, 98.8%, 98.9%, 99.0%, 99.1%,
99.2%, 99.3%, 99.4%, 99.5%, 99.6%, 99.7%, 99.8%, or 99.9%. For example, the
purity
of obeticholic acid is 98.0%, 98.5%, 99.0%, 99.5%, 99.6%, 99.7%, 99.8%, or
99.9%. For
example, the purity of obeticholic acid is 98.5%, 99.0%, or 99.5%. In one
embodiment,
the obeticholic acid is obeticholic acid Form 1.
41
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
In one embodiment, the present invention relates to obeticholic acid having a
purity greater than about 98%. In one embodiment, the purity is determined by
HPLC. In
another embodiment, the present invention relates to obeticholic acid, or a
pharmaceutically acceptable salt, solvate or amino acid conjugate thereof In
one
embodiment, the purity is greater than about 98.5%. In one embodiment, the
purity is
greater than about 99.0%. In one embodiment, the purity is greater than about
99.5%. In
one embodiment, the obeticholic acid is obeticholic acid Form 1.
The term "potency" as used herein is a measure of the amount of obeticholic
acid
based on that of a known standard (e.g., acceptance criteria of about 95% to
about 102%).
Potency takes into account all possible impurities including water, solvents,
organic, and
inorganic impurities. In one aspect, the known standard is obeticholic acid.
In one
aspect, obeticholic acid has a potency of greater than about 96%. In one
aspect,
obeticholic acid has a potency of greater than about 98%. In one aspect, the
known
standard is obeticholic acid. In another aspect, potency is 100% minus the
amounts of
water, sulphated ash, residual solvents, and other impurity contents such as 6-
ethylursodeoxycholic acid, 3a-hydroxy-6a-ethy1-7-cheto-513-cholan-24-oic acid,
613-
ethylchenodeoxycholic acid, 3a,7a-dihydroxy-6-ethyliden-513-cholan-24-oic
acid,
chenodeoxycholic acid, and 3a(3a,7a-dihydroxy-6a-ethy1-5P-cholan-24-oyloxy)-7a-
hydroxy-6a-ethyl-5P-cholan-24-oic acid. In another embodiment, potency
accounts for
impurities due to water, solvent, metals, inorganic salts, and other inorganic
or organic
impurities. For example, the potency of obeticholic acid Form 1 is 96.0%,
96.1%, 96.2%,
96.3%, 96.4%, 96.5%, 96.6%, 96.7%, 96.8%, 96.9%, 97.0%, 97.1%, 97.2%, 97.3%,
97.4%, 97.5%, 97.6%, 97.7%, 97.8%, 97.9 %, 98.0%, 98.1%, 98.2%, 98.3%, 98.4%,
98.5%, 98.6%, 98.7%, 98.8%, 98.9%, 99.0%, 99.1%, 99.2%, 99.3%, 99.4%, 99.5%,
99.6%, 99.7%, 99.8%, or 99.9%. In one aspect, the potency of obeticholic acid
Form 1 is
98.0%, 98.1%, 98.2%, 98.3%, 98.4%, 98.5%, 98.6%, 98.7%, 98.8%, 98.9%, 99.0%,
99.1%, 99.2%, 99.3%, 99.4%, 99.5%, 99.6%, 99.7%, 99.8%, or 99.9%. For example,
the
potency of obeticholic acid is 98.0%, 98.5%, 99.0%, 99.5%, 99.6%, 99.7%,
99.8%, or
99.9%. For example, the potency of obeticholic acid is 98.5%, 99.0%, or 99.5%.
In one
embodiment, the obeticholic acid is obeticholic acid Form 1.
In one embodiment, the present invention relates to obeticholic acid
containing a
total of less than about 2% of one or more impurities selected from 6-
42
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
ethylursodeoxycholic acid, 3a-hydroxy-6a-ethy1-7-cheto-513-cholan-24-oic acid,
613-
ethylchenodeoxycholic acid, 3a,7a-dihydroxy-6-ethyliden-513-cholan-24-oic
acid,
chenodeoxycholic acid, and 3a(3a,7a-dihydroxy-6a-ethy1-5P-cholan-24-oyloxy)-7a-
hydroxy-6a-ethy1-5P-cholan-24-oic acid. In one embodiment, the total of
impurities is
less than about 1.5%. In one embodiment, the total of impurities is less than
about 1.4%.
In one embodiment, the obeticholic acid is obeticholic acid Form 1.
In one embodiment, obeticholic acid contains less than about 10% of water,
less
than about 9% of water, less than 8% of water, less than 7% of water, less
than 6% of
water, less than 5% of water, less than 4% of water, less than 3% of water,
less than 2%
1 0 of water, or less than 1% of water. In one embodiment, obeticholic acid
contains less than
about 1.2% of water. In one embodiment, obeticholic acid contains less than
about 1.0%
of water. In one embodiment, the obeticholic acid is obeticholic acid Form 1.
In another embodiment, obeticholic acid contains not more than (NMT) 0.15 %
of 6-ethylursodeoxycholic acid and 3a,7a-dihydroxy-6-ethyliden-513-cholan-24-
oic acid.
1 5 In another embodiment, obeticholic acid contains a total of less than
about 0.07% of 6-
ethylursodeoxycholic acid and 3a,7a-dihydroxy-6-ethyliden-513-cholan-24-oic
acid. In
one embodiment, obeticholic acid contains a total of less than about 0.06% of
6-
ethylursodeoxycholic acid and 3a,7a-dihydroxy-6-ethyliden-5P-cholan-24-oic
acid. In
one embodiment, obeticholic acid contains a total of less than about 0.05% of
6-
2 0 ethylursodeoxycholic acid and 3a,7a-dihydroxy-6-ethyliden-5P-cholan-24-
oic acid. In
one embodiment, the obeticholic acid is obeticholic acid Form 1.
In one embodiment, obeticholic acid contains not more than (NMT) 0.15 % of 3a-
hydroxy-6a-ethy1-7-cheto-5P-cholan-24-oic acid. In one embodiment, obeticholic
acid
contains less than about 0.07% of 3a-hydroxy-6a-ethyl-7-cheto-5P-cholan-24-oic
acid.
25 In one embodiment, obeticholic acid contains less than about 0.06% of 3a-
hydroxy-6a-
ethy1-7-cheto-5P-cholan-24-oic acid. In one embodiment, obeticholic acid
contains less
than about 0.05% of 3a-hydroxy-6a-ethy1-7-cheto-5P-cholan-24-oic acid. In one
embodiment, the obeticholic acid is obeticholic acid Form 1.
In one embodiment, obeticholic acid contains not more than (NMT) 0.15% of 613-
30 ethylchenodeoxycholic acid. In one embodiment, obeticholic acid contains
less than
about 0.07% of 6P-ethylchenodeoxycholic acid. In one embodiment, obeticholic
acid
contains less than about 0.06% of 613-ethylchenodeoxycholic acid. In one
embodiment,
43
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
obeticholic acid contains less than about 0.05% of 6P-ethylchenodeoxycholic
acid. In one
embodiment, the obeticholic acid is obeticholic acid Form 1.
In one embodiment, obeticholic acid contains no more than (NMT) 3% of
chenodeoxycholic acid (CDCA). In one embodiment, obeticholic acid contains
less than
about 1% of CDCA. In one embodiment, obeticholic acid contains less than about
0.5%
of CDCA. In one embodiment, obeticholic acid contains less than about 0.3% of
CDCA.
In one embodiment, obeticholic acid contains less than about 0.2% of CDCA. In
one
embodiment, the obeticholic acid is obeticholic acid Form 1.
In one embodiment, obeticholic acid contains no more than (NMT) 4% of CDCA
1 0 and 6-ethylursodeoxycholic acid.
In one embodiment, obeticholic acid contains no more than (NMT) 1.5 % of
3a(3a,7a-dihydroxy-6a-ethy1-513-cholan-24-oyloxy)-7a-hydroxy-6a-ethy1-513-
cholan-
24-oic acid. In one embodiment, obeticholic acid contains less than about 1%
of
3a(3a,7a-dihydroxy-6a-ethy1-513-cholan-24-oyloxy)-7a-hydroxy-6a-ethy1-513-
cholan-
1 5 24-oic acid. In one embodiment, obeticholic acid contains less than
about 0.07% of
3a(3a,7a-dihydroxy-6a-ethy1-513-cholan-24-oyloxy)-7a-hydroxy-6a-ethy1-513-
cholan-
24-oic acid. In one embodiment, obeticholic acid contains less than about
0.06% of
3a(3a,7a-dihydroxy-6a-ethy1-513-cholan-24-oyloxy)-7a-hydroxy-6a-ethy1-513-
cholan-
24-oic acid. In one embodiment, obeticholic acid contains less than about
0.05% of
20 3a(3a,7a-dihydroxy-6a-ethy1-5P-cholan-24-oyloxy)-7a-hydroxy-6a-ethyl-5P-
cholan-
24-oic acid. In one embodiment, the obeticholic acid is obeticholic acid Form
1.
Oral Formulation and Administration
Obeticholic acid is for oral administration. In one embodiment, the
formulation is
25 oral administration for the prevention and treatment of FXR mediated
diseases and
conditions. In one embodiment, the formulation comprises of obeticholic acid
Form 1. In
another embodiment, the formulation comprises of substantially pure
obeticholic acid.
Formulations suitable for oral administration may be provided as discrete
units,
such as tablets, capsules, cachets (wafer capsule used by pharmacists for
presenting a
30 drug), lozenges, each containing a predetermined amount of obeticholic
acid; as powders
or granules; as solutions or suspensions in aqueous or non-aqueous liquids; or
as oil-in-
water or water-in-oil emulsions.
44
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Formulations of the invention may be prepared by any suitable method,
typically
by uniformly and intimately admixing obeticholic acid with liquids or finely
divided solid
carriers or both, in the required proportions and then, if necessary, shaping
the resulting
mixture into the desired shape.
For example a tablet may be prepared by compressing an intimate mixture
comprising a powder or granules of obeticholic acid and one or more optional
ingredients,
such as a binder, lubricant, inert diluent, or surface active dispersing
agent, or by
moulding an intimate mixture of powdered active ingredient and inert liquid
diluent.
For example, one or more tablets may be administered to get to a target dose
level
based on the subject's weight, e.g., a human between about 30 kg to about 70
kg.
In one embodiment, the subject is a child and the formulation is used to treat
biliary atresia. Biliary atresia, also known as "extrahepatic ductopenia" and
"progressive
obliterative cholangiopathy" is a congenital or acquired disease of the liver
and one of the
principal forms of chronic rejection of a transplanted liver allograft. In the
congenital
form, the common bile duct between the liver and the small intestine is
blocked or absent.
The acquired type most often occurs in the setting of autoimmune disease, and
is one of
the principal forms of chronic rejection of a transplanted liver allograft.
Infants and children with biliary atresia have progressive cholestasis with
all the
usual concomitant features: jaundice, pruritus, malabsorption with growth
retardation, fat-
soluble vitamin deficiencies, hyperlipidemia, and eventually cirrhosis with
portal
hypertension. If unrecognized, the condition leads to liver failure¨but not
kernicterus, as
the liver is still able to conjugate bilirubin, and conjugated bilirubin is
unable to cross the
blood¨brain barrier. The cause of the condition is unknown. The only effective
treatments
are certain surgeries such as the Kasai procedure, or liver transplantation
In one embodiment, the human child has had a Kasai procedure, where the Kasai
procedure effectively gives them a functional bile duct when they born either
without a
bile duct or one that is completely blocked at birth.
In addition to the ingredients specifically mentioned above, the oral
formulations
of the present invention may include other agents known to those skilled in
the art of
pharmacy, having regard for the type of formulation in issue. Oral
formulations suitable
may include flavoring agents.
In one embodiment, the present invention relates to a pharmaceutical
formulation
of obeticholic acid or a pharmaceutically acceptable salt, solvate, or amino
acid conjugate
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
thereof, wherein obeticholic acid is produced by a process of the invention
(obeticholic
acid Form 1). In another embodiment, the formulation is administered orally.
In one embodiment, the formulation is in tablet form. In another embodiment,
the
formulation comprises obeticholic acid and one or more components selected
from
microcrystalline cellulose, sodium starch glycolate, magnesium stearate,
coating material,
or colloidal silicon dioxide. In one embodiment, the coating material is an
Opadry0
coating material.
In another embodiment, the formulation comprises about 0.1 mg to about 1500 mg
of obeticholic acid per tablet. In another embodiment, the formulation
comprises about 1
mg to about 100 mg. In another embodiment, the formulation comprises about 1
mg to
about 50 mg. In another embodiment, the formulation comprises about 1 mg to
about 30
mg. In another embodiment, the formulation comprises about 4 mg to about 26
mg. In
another embodiment, the formulation comprises about 5 mg to about 25 mg. In
one
embodiment, the formulation comprises about 1 mg to about 2 mg. In one
embodiment,
the formulation comprises about 1.2 mg to about 1.8 mg. In one embodiment, the
formulation comprises about 1.3 mg to about 1.7 mg. In one embodiment, the
formulation
comprises about 1.5 mg.
In one embodiment, the formulation comprises of about 1 mg to about 25 mg of
obeticholic acid per tablet. In one embodiment, the formulation comprises
about 1 mg of
obeticholic acid, about 180 to about 190 mg of microcrystalline cellulose,
about 10 to
about 15 mg of sodium starch glycolate, about 1 to about 3 mg of magnesium
stearate,
and about 5 mg to about 10 mg of coating material. In one embodiment, the
coating
material is an Opadry0 coating material.
In one embodiment, the formulation comprises of about 1 mg to about 25 mg of
obeticholic acid per tablet. In one embodiment, the formulation comprises
about 1 mg of
obeticholic acid, about 185.0 mg of microcrystalline cellulose, about 12.0 mg
of sodium
starch glycolate, about 2.0 mg of magnesium stearate, and about 8.0 mg of
coating
material. In one embodiment, the coating material is an Opadry0 coating
material.
In one embodiment, the formulation comprises of about 1 mg to about 25 mg of
obeticholic acid per tablet. In one embodiment, the formulation comprises
about 5 mg of
obeticholic acid, about 175 to about 190 mg of microcrystalline cellulose,
about 10 to
about 15 mg of sodium starch glycolate, about 1 to about 3 mg of magnesium
stearate,
and about 5 mg to about 10 mg of coating material. In one embodiment, the
coating
material is an Opadry0 coating material.
46
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
In one embodiment, the formulation comprises of about 1 mg to about 25 mg of
obeticholic acid per tablet. In one embodiment, the formulation comprises
about 5 mg of
obeticholic acid, about 181.0 mg of microcrystalline cellulose, about 12.0 mg
of sodium
starch glycolate, about 2.0 mg of magnesium stearate, and about 8.0 mg of
coating
material. In one embodiment, the coating material is an Opadry0 coating
material.
In one embodiment, the formulation comprises of about 1 mg to about 25 mg of
obeticholic acid per tablet. In one embodiment, the formulation comprises
about 10 mg of
obeticholic acid, about 170 mg to about 180 mg of microcrystalline cellulose,
about 10
mg to about 15 mg of sodium starch glycolate, about 1 mg to about 3 mg of
magnesium
stearate, and about 5 mg to about 10 mg of coating material. In one
embodiment, the
coating material is an Opadry0 coating material.
In one embodiment, the formulation comprises of about 1 mg to about 25 mg of
obeticholic acid per tablet. In one embodiment, the formulation comprises
about 10 mg of
obeticholic acid, about 176.0 mg of microcrystalline cellulose, about 12.0 mg
of sodium
starch glycolate, about 2.0 mg of magnesium stearate, and about 8.0 mg of
coating
material. In one embodiment, the coating material is an Opadry0 coating
material.
In one embodiment, the formulation comprises of about 1 mg to about 25 mg of
obeticholic acid per tablet. In one embodiment, the formulation comprises
about 25 mg of
obeticholic acid, about 150 mg to about 160 mg of microcrystalline cellulose,
about 10
mg to about 15 mg of sodium starch glycolate, about 1 mg to about 3 mg of
magnesium
stearate, about 5 to about 10 mg of coating material, and about 1 to about 10
mg of
colloidal silicon dioxide. In one embodiment, the coating material is an
Opadry0 coating
material.
In one embodiment, the formulation comprises of about 1 mg to about 25 mg of
obeticholic acid per tablet. In one embodiment, the formulation comprises
about 25 mg of
obeticholic acid, about 157.0 mg of microcrystalline cellulose, about 12.0 mg
of sodium
starch glycolate, about 2.0 mg of magnesium stearate, about 8.0 mg of coating
material,
and about 4.0 mg of colloidal silicon dioxide. In one embodiment, the coating
material is
an Opadry0 coating material.
All percentages and ratios used herein, unless otherwise indicated, are by
weight.
The percent dimeric impurity is on an area percent basis, typically as
quantified by
analytical HPLC.
Throughout the description, where compositions are described as having,
including, or comprising specific components, it is contemplated that
compositions also
47
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
consist essentially of, or consist of, the recited components. Similarly,
where methods or
processes are described as having, including, or comprising specific process
steps, the
processes also consist essentially of, or consist of, the recited processing
steps. Further, it
should be understood that the order of steps or order for performing certain
actions is
immaterial so long as the invention remains operable. Moreover, two or more
steps or
actions can be conducted simultaneously.
Formulation of Tablets
Film Coated Tablet
Reference to
Component Quantity per Tablet Function
Standard
1 mg tablet
Obeticholic acid 1.0 mg* API HSE
Microciystalline cellulose 185.0 mg* Filler/Binder USP-
NF/EP/JP
Sodium starch glycolate 12.0 mg Disintegrant USP-
NF/EP/JP
Magnesium stearate 2.0 mg Lubricant USP-
NF/EP/JP
Opadiy0 II green, white, or
8.0 mg Coating Material HSE
yellow
Total weight 208.0 mg
5 mg tablet
Obeticholic acid 5.0 mg* API HSE
Microciystalline cellulose 181.0 mg* Filler/Binder USP-
NF/EP/JP
Sodium starch glycolate 12.0 mg Disintegrant USP-
NF/EP/JP
Magnesium stearate 2.0 mg Lubricant USP-
NF/EP/JP
Opadiy0 II green, white, or
8.0 mg Coating Material HSE
yellow
Total weight 208.0 mg
mg tablet
Obeticholic acid 10.0 mg* API HSE
Microciystalline cellulose 176.0 mg* Filler/Binder USP-
NF/EP/JP
Sodium starch glycolate 12.0 mg Disintegrant USP-
NF/EP/JP
Magnesium stearate 2.0 mg Lubricant USP-
NF/EP/JP
Opadiy0 II green, white, or
8.0 mg Coating Material HSE
yellow
Total weight 208.0 mg
25 mg tablet
Obeticholic acid 25.0 mg* API HSE
Microciystalline cellulose 157.0 mg* Filler/Binder USP-
NF/EP/JP
Sodium starch glycolate 12.0 mg Disintegrant USP-
NF/EP/JP
Magnesium stearate 2.0 mg Lubricant USP-
NF/EP/JP
Collodial silicon dioxide 4.0 mg Glidant USP-
NF/EP/JP
48
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Opadiy0 II green, white, or
8.0 mg Coating Material HSE
yellow
Total weight 208.0 mg
API: Active pharmaceutical ingredient
HSE = In house specification
USP-NF = US Pharmacopeia National Formulary
Ph Eur = European Pharmacopeia
JP =Japanese Pharmacopeia
* obeticholic acid quantity presented assumes API is anhydrous and 100% pure;
actual amount is adjusted based on the
potency of the drug substance Lot used, and amount of microcrystalline
cellulose is correspondingly decreased.
1 0 In one embodiment, the tablet comprises yellow Opadry0. In another
embodiment, the tablet comprises white Opadry0. In another embodiment, the
tablet
comprises green Opadry0.
Pharmaceutical Compositions
1 5 Obeticholic acid, including obeticholic acid Form 1, substantially pure
forms of
obeticholic acid and crystalline forms of obeticholic acid, or a
pharmaceutically
acceptable salt, solvate, or amino acid conjugate thereof is useful for a
variety of
medicinal purposes. Obeticholic acid may be used in methods for the prevention
or
treatment of FXR mediated diseases and conditions. In one embodiment, the
disease or
20 condition is selected from biliary atresia, cholestatic liver disease,
chronic liver disease,
nonalcoholic steatohepatitis (NASH), hepatitis C infection, alcoholic liver
disease,
primary biliary cirrhosis (PBC), liver damage due to progressive fibrosis,
liver fibrosis,
and cardiovascular diseases including atherosclerosis, arteriosclerosis,
hypercholesterolemia, and hyperlipidemia. In one embodiment, obeticholic acid
Form 1
25 may be used in methods for lowering triglycerides. In one embodiment,
crystalline
obeticholic acid may be used in methods for lowering triglycerides.
Obeticholic acid
Form 1 or crystalline obeticholic acid may increase HDL. Other effects of
obeticholic
acid Form 1 or crystalline obeticholic acid include lowering of alkaline
phosphatase
(ALP), bilirubin, ALT, AST, and GGT.
30 In one embodiment, the present invention relates to a pharmaceutical
composition
comprising obeticholic acid and a pharmaceutically acceptable carrier, wherein
the
obeticholic acid is produced by a process of the invention, e.g., obeticholic
acid Form 1.
In one embodiment, the pharmaceutical composition comprises of substantially
pure
obeticholic acid and a pharmaceutically acceptable carrier. In another
embodiment, the
35 pharmaceutical composition comprises of crystalline obeticholic acid and
a
49
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
pharmaceutically acceptable carrier. In another embodiment, the crystalline
obeticholic
acid is the Form A. In another embodiment, the crystalline obeticholic acid is
the Form
C. In one embodiment, the crystalline obeticholic acid is Form D. In one
embodiment,
the crystalline obeticholic acid is Form F. In one embodiment, the crystalline
obeticholic
acid is Form G. In one embodiment, the crystalline obeticholic acid is Form I.
In one embodiment, the present invention relates to a method of treating or
preventing an FXR mediated disease or condition in a subject comprising
administering
an effective amount of obeticholic acid Form 1 produced by a process of the
invention or
a pharmaceutical composition thereof In one embodiment, the present invention
relates
to a method of treating or preventing an FXR mediated disease or condition in
a subject
comprising administering an effective amount of substantially pure obeticholic
acid
produced by a process of the invention or a pharmaceutical composition thereof
In one
embodiment, the present invention relates to a method of treating or
preventing an FXR
mediated disease or condition in a subject comprising administering an
effective amount
of crystalline obeticholic acid or a pharmaceutical composition thereof In
another
embodiment, the crystalline obeticholic acid is Form C. In one embodiment, the
crystalline obeticholic acid is Form A. In one embodiment, the crystalline
obeticholic
acid is Form C. In one embodiment, the crystalline obeticholic acid is Form D.
In one
embodiment, the crystalline obeticholic acid is Form F. In one embodiment, the
crystalline obeticholic acid is Form G. In one embodiment, the crystalline
obeticholic
acid is Form I.
In another embodiment, the disease or condition is cardiovascular disease or
cholestatic liver disease and for lowering triglycerides. In another
embodiment, the
cardiovascular disease is atherosclerosis or hypercholesterolemia. In another
embodiment,
the subject is a mammal. In another embodiment, the mammal is human.
In another embodiment, the compound or pharmaceutical composition is
administered orally, parenterally, or topically. In another embodiment, the
compound or
pharmaceutical composition is administered orally.
In one embodiment, the present invention relates to a method for inhibiting
fibrosis in a subject who is suffering from a cholestatic condition, the
method comprising
the step of administering to the subject an effective amount of obeticholic
acid or a
pharmaceutical composition thereof, wherein obeticholic acid is produced by
the process
of the invention. In one embodiment, the present invention relates to a method
for
inhibiting fibrosis in a subject who is not suffering from a cholestatic
condition, the
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
method comprising the step of administering to the subject an effective amount
of
obeticholic acid or a pharmaceutical composition thereof, wherein obeticholic
acid is
produced by the process of the invention. In embodiment, the fibrosis to be
inhibited
occurs in an organ where FXR is expressed.
In one embodiment, the cholestatic condition is defined as having abnormally
elevated serum levels of alkaline phosphatase, 7-glutamyl transpeptidase
(GGT), and 5'
nucleotidase. In another embodiment, the cholestatic condition is further
defined as
presenting with at least one clinical symptom. In another embodiment, the
symptom is
itching (pruritus). In another embodiment, the fibrosis is selected from the
group
consisting of liver fibrosis, kidney fibrosis, and intestinal fibrosis. In
another
embodiment, the cholestatic condition is selected from the group consisting of
primary
biliary cirrhosis, primary sclerosing cholangitis, drug-induced cholestasis,
hereditary
cholestasis, and intrahepatic cholestasis of pregnancy. In another embodiment,
the subject
is not suffering from a cholestatic condition associated with a disease or
condition
selected from the group consisting of primary liver and biliary cancer,
metastatic cancer,
sepsis, chronic total parenteral nutrition, cystic fibrosis, and granulomatous
liver disease.
In another embodiment, the subject has liver fibrosis associated with a
disease
selected from the group consisting of hepatitis B; hepatitis C; parasitic
liver diseases;
post-transplant bacterial, viral and fungal infections; alcoholic liver
disease (ALD); non-
alcoholic fatty liver disease (NAFLD); non-alcoholic steatohepatitis (NASH);
liver
diseases induced by methotrexate, isoniazid, oxyphenistatin, methyldopa,
chlorpromazine, tolbutamide, or amiodarone; autoimmune hepatitis; sarcoidosis;
Wilson's
disease; hemochromatosis; Gaucher's disease; types III, IV, VI, IX and X
glycogen
storage diseases; ai-antitrypsin deficiency; Zellweger syndrome; tyrosinemia;
fructosemia; galactosemia; vascular derangement associated with Budd-Chian
syndrome,
veno-occlusive disease, or portal vein thrombosis; and congenital hepatic
fibrosis.
In another embodiment, the subject has intestinal fibrosis associated with a
disease
selected from the group consisting of Crohn's disease, ulcerative colitis,
post-radiation
colitis, and microscopic colitis.
In another embodiment, the subject has renal fibrosis associated with a
disease
selected from the group consisting of diabetic nephropathy, hypertensive
nephrosclerosis,
chronic glomerulonephritis, chronic transplant glomerulopathy, chronic
interstitial
nephritis, and polycystic kidney disease.
51
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Definitions
For convenience, certain terms used in the specification, examples and
appended
claims are collected here.
As used herein the term "obeticholic acid" or "OCA" refers to a compound
having
the chemical structure:
co2H
Ore
õ. O.HO' /0 H
H
. Other chemical names for obeticholic acid
include: 3a,7a-dihydroxy-6a-ethy1-5(3-cho1an-24-oic acid, 6a-ethyl-
chenodeoxycholic
acid, 6-ethyl-CDCA, 6ECDCA, cholan-24-oic acid,6-ethy1-3,7-dihydroxy-,(3a,5(3,
1 0 6a,7a)- and INT-747. The CAS registry number for obeticholic acid is
459789-99-2.
This term refers to all forms of obeticholic acid, e.g., non-crystalline,
crystalline and
substantially pure.
As used herein the term "crystalline obeticholic acid" refers to any
crystalline
form of a compound having the chemical structure:
CO2 H
HO'" 1/0H
H E
1 5 .
Crystalline obeticholic acid means that the
compound is crystallized into a specific crystal packing arrangement in three
spatial
dimensions or the compound having external face planes. The crystalline form
of
obeticholic acid (or a pharmaceutically acceptable salt, amino acid conjugate,
solvate
thereof) can crystallize into different crystal packing arrangements, all of
which have the
20 same elemental composition of obeticholic acid. Different crystal forms
usually have
different X-ray diffraction patterns, infrared spectral, melting points,
density hardness,
crystal shape, optical and electrical properties, stability and solubility.
Recrystallization
52
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
solvent, rate of crystallization, storage temperature, and other factors may
cause one
crystal form to dominate. Crystals of obeticholic acid can be prepared by
crystallization
under different conditions, e.g., different solvents, temperatures, etc.
As used herein, the term "crystalline obeticholic acid Form A" refers to a
crystalline form of obeticholic acid with an X-ray diffraction pattern that is
substantially
similar to that set forth in Figure 41, e.g., one of the crystalline forms as
characterized in
Example 3.
As used herein, the term "crystalline obeticholic acid Form C" refers to a
crystalline form of obeticholic acid with an X-ray diffraction pattern that is
substantially
similar to that set forth in Figure 5, e.g., one of the crystalline forms as
characterized in
Example 3.
As used herein, the term "substantially pure obeticholic acid" refers to
obeticholic
acid that has a potency of greater than about 95%. The potency of the
obeticholic acid
takes into account impurities including e.g., water, solvents, and other
organic and
inorganic impurities that are in a sample of obeticholic acid. In another
embodiment, the
known standard for potency is 100% obeticholic acid, and the potency is
determined by
subtracting percentages of impurities such as solvent, water, and other
organic and
inorganic impurities from 100% of the known standard. In one aspect, the
inorganic
impurities include e.g., inorganic salts and sulphated ash. In one aspect, the
organic
impurities include 6-ethylursodeoxycholic acid, 3a-hydroxy-6a-ethy1-7-cheto-
513-cholan-
24-oic acid, 613-ethylchenodeoxycholic acid, 3a,7a-dihydroxy-6-ethyliden-513-
cholan-24-
oic acid, chenodeoxycholic acid, and 3a(3a,7a-dihydroxy-6a-ethy1-513-cholan-24-
oyloxy)-7a-hydroxy-6a-ethyl-513-cholan-24-oic acid. The amounts of the
impurities can
be determined by procedures known in the art, e.g., HPLC, NMR, or methods from
US
Pharmacopeia, or European Pharmacopeia, or a combination of two or more of
these
methods.
As used herein, the term "purity" refers to a chemical analysis of a compound
obtained from e.g., HPLC. In one embodiment, the purity of a compound is
compared to
the purity of the reference standard, e.g., obeticholic acid, via the area
under their
respective peak for comparisons. In one embodiment, purity accounts for the
organic
impurities in a sample.
As used herein, the term "reaction mixture" refers a mixture of one or more
substances combined together. In one embodiment, the mixing or combining of
the
53
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
substances causes a chemical transformation or change in one or more of the
original
substances.
As used herein, the term "obeticholic acid Form 1" refers to non-crystalline
obeticholic acid. In one embodiment, this form of obeticholic acid is produced
via a
crystalline obeticholic acid as a synthetic intermediate. For example, this
form of
obeticholic acid is produced by the process of the application via crystalline
obeticholic
acid Form A as the synthetic intermediate. In one embodiment, obeticholic acid
Form 1
is the form that it used as the pharmaceutically active ingredient. See
Example 5 for more
details.
1 0 "Treating", includes any effect, e.g., lessening, reducing, modulating,
or
eliminating, that results in the improvement of the condition, disease,
disorder, etc.
"Treating" or "treatment" of a disease state includes: inhibiting the disease
state, i.e.,
arresting the development of the disease state or its clinical symptoms; or
relieving the
disease state, i.e., causing temporary or permanent regression of the disease
state or its
1 5 clinical symptoms.
"Preventing" the disease state includes causing the clinical symptoms of the
disease state not to develop in a subject that may be exposed to or
predisposed to the
disease state, but does not yet experience or display symptoms of the disease
state.
"Disease state" means any disease, disorder, condition, symptom, or
indication.
20 The term "effective amount" as used herein refers to an amount of
obeticholic
acid (e.g., an FXR-activating ligand) that produces an acute or chronic
therapeutic effect
upon appropriate dose administration. The effect includes the prevention,
correction,
inhibition, or reversal of the symptoms, signs and underlying pathology of a
disease/condition (e.g., fibrosis of the liver, kidney, or intestine) and
related
25 complications to any detectable extent.
"A therapeutically effective amount" means the amount of obeticholic acid
that,
when administered to a mammal for treating a disease, is sufficient to effect
such
treatment for the disease. The "therapeutically effective amount" will vary
depending on
obeticholic acid, the disease and its severity and the age, weight, etc., of
the mammal to
30 be treated.
A therapeutically effective amount of obeticholic acid can be formulated with
a
pharmaceutically acceptable carrier for administration to a human or an
animal.
Accordingly, obeticholic acid or its formulations can be administered, for
example, via
oral, parenteral, or topical routes, to provide an effective amount of the
compound. In
54
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
alternative embodiments, obeticholic acid prepared in accordance with the
present
invention can be used to coat or impregnate a medical device, e.g., a stent.
"Pharmacological effect" as used herein encompasses effects produced in the
subject that achieve the intended purpose of a therapy. In one embodiment, a
pharmacological effect means that primary indications of the subject being
treated are
prevented, alleviated, or reduced. For example, a pharmacological effect would
be one
that results in the prevention, alleviation or reduction of primary
indications in a treated
subject. In another embodiment, a pharmacological effect means that disorders
or
symptoms of the primary indications of the subject being treated are
prevented, alleviated,
or reduced. For example, a pharmacological effect would be one that results in
the
prevention or reduction of primary indications in a treated subject.
The invention also comprehends isotopically-labeled obeticholic acid, or
pharmaceutically acceptable salts, solvate, or amino acid conjugates thereof,
which are
identical to those recited in formulae of the invention and following, but for
the fact that
1 5 one or more atoms are replaced by an atom having an atomic mass or mass
number
different from the atomic mass or mass number most commonly found in nature.
Examples of isotopes that can be incorporated into obeticholic acid, or
pharmaceutically
acceptable salts, solvate, or amino acid conjugates thereof include isotopes
of hydrogen,
carbon, nitrogen, fluorine, such as 1-1, nc, 14c and 18F.
Obeticholic acid, or pharmaceutically acceptable salts, solvates, or amino
acid
conjugates thereof that contain the aforementioned isotopes and/or other
isotopes of other
atoms are within the scope of the present invention. Isotopically-labeled
obeticholic acid,
or pharmaceutically acceptable salts, solvates, or amino acid conjugates
thereof, for
example those into which radioactive isotopes such as 3H, 14C are
incorporated, are useful
in drug and/or substrate tissue distribution assays. Tritiated, i.e., 3H, and
carbon-14, i.e.,
14µ,u,
isotopes are particularly preferred for their ease of preparation and
detectability.
Further, substitution with heavier isotopes such as deuterium, i.e., 2H, can
afford certain
therapeutic advantages resulting from greater metabolic stability, for example
increased
in vivo half-life or reduced dosage requirements and, hence, may be preferred
in some
circumstances, isotopically labeled obeticholic acid, or pharmaceutically
acceptable salts,
solvates, or amino acid conjugates thereof can generally be prepared by
carrying out the
procedures disclosed in the Schemes and/or in the Examples of the invention,
by
substituting a readily available isotopically labeled reagent for a non-
isotopically labeled
reagent. In one embodiment, obeticholic acid, or pharmaceutically acceptable
salts,
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
solvates, or amino acid conjugates thereof are not isotopically labelled. In
one
embodiment, deuterated obeticholic acid is useful for bioanalytical assays. In
another
embodiment, obeticholic acid, or pharmaceutically acceptable salts, solvates,
or amino
acid conjugates thereof are radiolabelled.
"Geometric Isomers" means the diastereomers that owe their existence to
hindered
rotation about double bonds. These configurations are differentiated in their
names by the
prefixes cis and trans, or Z and E, which indicate that the groups are on the
same or
opposite side of the double bond in the molecule according to the Cahn-Ingold-
Prelog
rules.
1 0 "Solvates" means solvent addition forms that contain either
stoichiometric or non
stoichiometric amounts of solvent. Obeticholic acid may have a tendency to
trap a fixed
molar ratio of solvent molecules in the crystalline solid state, thus forming
a solvate. If
the solvent is water the solvate formed is a hydrate, when the solvent is
alcohol, the
solvate formed is an alcoholate. Hydrates are formed by the combination of one
or more
molecules of water with one of the substances in which the water retains its
molecular
state as H20, such combination being able to form one or more hydrate.
Additionally, the
compounds of the present invention, for example, the salts of the compounds,
can exist in
either hydrated or unhydrated (the anhydrous) form or as solvates with other
solvent
molecules. Nonlimiting examples of hydrates include monohydrates, dihydrates,
etc.
Nonlimiting examples of solvates include ethanol solvates, acetone solvates,
etc.
"Tautomers" refers to compounds whose structures differ markedly in
arrangement of atoms, but which exist in easy and rapid equilibrium. It is to
be
understood that obeticholic acid may be depicted as different tautomers. It
should also be
understood that when obeticholic acid and synthetic intermediates of the
invention have
tautomeric forms, all tautomeric forms are intended to be within the scope of
the
invention, and the naming of obeticholic acid does not exclude any tautomer
form.
Obeticholic acid and synthetic intermediates of the invention can exist in
several
tautomeric forms, including the keto-enol. For example, in keto-enol
tautomerism a
simultaneous shift of electrons and a hydrogen atom occurs. Tautomers exist as
mixtures
of a tautomeric set in solution. In solid form, usually one tautomer
predominates. Even
though one tautomer may be described, the present invention includes all
tautomers of the
present compounds.
It is to be understood accordingly that the isomers arising from asymmetric
carbon
atoms (e.g., all enantiomers and diastereomers) are included within the scope
of the
56
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
invention, unless indicated otherwise. Such isomers can be obtained in
substantially pure
form by classical separation techniques and by stereochemically controlled
synthesis.
Furthermore, the structures and other compounds and moieties discussed in this
application also include all tautomers thereof Alkenes can include either the
E- or Z-
geometry, where appropriate. Obeticholic acid and synthetic intermediates may
exist in
stereoisomeric form, and therefore can be produced as individual stereoisomers
or as
mixtures.
A "pharmaceutical composition" is a formulation containing obeticholic acid in
a
form suitable for administration to a subject. In one embodiment, the
pharmaceutical
composition is in bulk or in unit dosage form. It is can be advantageous to
formulate
compositions in dosage unit form for ease of administration and uniformity of
dosage.
Dosage unit form as used herein refers to physically discrete units suited as
unitary
dosages for the subject to be treated; each unit containing a predetermined
quantity of
active reagent calculated to produce the desired therapeutic effect in
association with the
required pharmaceutical carrier. The specification for the dosage unit forms
of the
invention are dictated by and directly dependent on the unique characteristics
of the
active reagent and the particular therapeutic effect to be achieved, and the
limitations
inherent in the art of compounding such an active agent for the treatment of
individuals.
The unit dosage form is any of a variety of forms, including, for example, a
capsule, an IV bag, a tablet, a single pump on an aerosol inhaler, or a vial.
The quantity
obeticholic acid (e.g., a formulation of obeticholic acid, or a
pharmaceutically acceptable
salt, solvate, or amino acid conjugate thereof) in a unit dose of composition
is an effective
amount and is varied according to the particular treatment involved. One
skilled in the art
will appreciate that it is sometimes necessary to make routine variations to
the dosage
depending on the age and condition of the patient. The dosage will also depend
on the
route of administration. A variety of routes are contemplated, including oral,
pulmonary,
rectal, parenteral, transdermal, subcutaneous, intravenous, intramuscular,
intraperitoneal,
inhalational, buccal, sublingual, intrapleural, intrathecal, intranasal, and
the like. Dosage
forms for the topical or transdermal administration of a compound of this
invention
include powders, sprays, ointments, pastes, creams, lotions, gels, solutions,
patches and
inhalants. In one embodiment, obeticholic acid is mixed under sterile
conditions with a
pharmaceutically acceptable carrier, and with any preservatives, buffers, or
propellants
that are required.
57
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
The term "flash dose" refers to obeticholic acid formulations that are rapidly
dispersing dosage forms.
The term "immediate release" is defined as a release of obeticholic acid from
a
dosage form in a relatively brief period of time, generally up to about 60
minutes. The
term "modified release" is defined to include delayed release, extended
release, and
pulsed release. The term "pulsed release" is defined as a series of releases
of drug from a
dosage form. The term "sustained release" or "extended release" is defined as
continuous
release of obeticholic acid from a dosage form over a prolonged period.
A "subject" includes mammals, e.g., humans, companion animals (e.g., dogs,
cats,
birds, and the like), farm animals (e.g., cows, sheep, pigs, horses, fowl, and
the like) and
laboratory animals (e.g., rats, mice, guinea pigs, birds, and the like). In
one embodiment,
the subject is human. In one embodiment, the subject is human child (e.g.,
between about
30 kg to about 70 kg). In one embodiment, the human child has had a Kasai
procedure,
where the Kasai procedure effectively gives them a functional bile duct when
they born
either without a bile duct or one that is completely blocked at birth.
As used herein, the phrase "pharmaceutically acceptable" refers to those
compounds, materials, compositions, carriers, and/or dosage forms which are,
within the
scope of sound medical judgment, suitable for use in contact with the tissues
of human
beings and animals without excessive toxicity, irritation, allergic response,
or other
problem or complication, commensurate with a reasonable benefit/risk ratio.
"Pharmaceutically acceptable excipient" means an excipient that is useful in
preparing a pharmaceutical composition that is generally safe, non-toxic and
neither
biologically nor otherwise undesirable, and includes excipient that is
acceptable for
veterinary use as well as human pharmaceutical use. A "pharmaceutically
acceptable
excipient" as used in the specification and claims includes both one and more
than one
such excipient.
While it is possible to administer obeticholic acid directly without any
formulation, obeticholic acid is usually administered in the form of
pharmaceutical
formulations comprising a pharmaceutically acceptable excipient and
obeticholic acid.
These formulations can be administered by a variety of routes including oral,
buccal,
rectal, intranasal, transdermal, subcutaneous, intravenous, intramuscular, and
intranasal.
Oral formulation of obeticholic acid are described further herein under the
section entitled
"Oral Formulation and Administration".
58
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
In one embodiment, obeticholic acid can be administered transdermally. In
order
to administer transdermally, a transdermal delivery device ("patch") is
needed. Such
transdermal patches may be used to provide continuous or discontinuous
infusion of a
compound of the present invention in controlled amounts. The construction and
use of
transdermal patches for the delivery of pharmaceutical agents is well known in
the art.
See, e.g., U.S. Patent No. 5,023,252. Such patches may be constructed for
continuous,
pulsatile, or on demand delivery of pharmaceutical agents.
In one embodiment of the present invention, there is provided a pharmaceutical
formulation comprising at least obeticholic acid as described above in a
formulation
adapted for buccal and/or sublingual, or nasal administration. This embodiment
provides
administration of obeticholic acid in a manner that avoids gastric
complications, such as
first pass metabolism by the gastric system and/or through the liver. This
administration
route may also reduce adsorption times, providing more rapid onset of
therapeutic benefit.
The compounds of the present invention may provide particularly favorable
solubility
profiles to facilitate sublingual/buccal formulations. Such formulations
typically require
relatively high concentrations of active ingredients to deliver sufficient
amounts of active
ingredients to the limited surface area of the sublingual/buccal mucosa for
the relatively
short durations the formulation is in contact with the surface area, to allow
the absorption
of the active ingredient. Thus, the very high activity of obeticholic acid,
combined with
its high solubility, facilitates its suitability for sublingual/buccal
formulation.
Obeticholic acid is preferably formulated in a unit dosage form, each dosage
containing from about 0.1 mg to about 1500 mg. In another embodiment, the
formulation
comprises about 1 mg to about 100 mg. In another embodiment, the formulation
comprises about 1 mg to about 50 mg. In another embodiment, the formulation
comprises about 1 mg to about 30 mg. In another embodiment, the formulation
comprises about 4 mg to about 26 mg. In another embodiment, the formulation
comprises
about 5 mg to about 25 mg. In one embodiment, the formulation comprises about
1 mg to
about 2 mg. In one embodiment, the formulation comprises about 1.2 mg to about
1.8
mg. In one embodiment, the formulation comprises about 1.3 mg to about 1.7 mg.
In one
embodiment, the formulation comprises about 1.5 mg. The term "unit dosage
form" refers
to physically discrete units suitable as unitary dosages for human subjects
and other
mammals, each unit containing a predetermined quantity of active material
calculated to
produce the desired therapeutic effect, in association with a suitable
pharmaceutical
excipient as described above.
59
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Obeticholic acid is generally effective over a wide dosage range. For
examples,
dosages per day normally fall within the range of about 0.0001 to about 30
mg/kg of body
weight. In the treatment of adult humans, the range of about 0.1 to about 15
mg/kg/day,
in single or divided dose, is especially preferred. In embodiment, the
formulation
comprises about 0.1 mg to about 1500 mg. In another embodiment, the
formulation
comprises about 1 mg to about 100 mg. In another embodiment, the formulation
comprises about 1 mg to about 50 mg. In another embodiment, the formulation
comprises about 1 mg to about 30 mg. In another embodiment, the formulation
comprises about 4 mg to about 26 mg. In another embodiment, the formulation
comprises
1 0 about 5 mg to about 25 mg. In one embodiment, the formulation comprises
about 1 mg to
about 2 mg. In one embodiment, the formulation comprises about 1.2 mg to about
1.8
mg. In one embodiment, the formulation comprises about 1.3 mg to about 1.7 mg.
In one
embodiment, the formulation comprises about 1.5 mg. However, it will be
understood
that the amount of obeticholic acid actually administered will be determined
by a
1 5 physician, in the light of the relevant circumstances, including the
condition to be treated,
the chosen route of administration, the form of obeticholic acid administered,
the age,
weight, and response of the individual patient, and the severity of the
patient's symptoms,
and therefore the above dosage ranges are not intended to limit the scope of
the invention
in any way. In some instances dosage levels below the lower limit of the
aforesaid range
20 may be more than adequate, while in other cases still larger doses may
be employed
without causing any harmful side effect, provided that such larger doses are
first divided
into several smaller doses for administration throughout the day.
"Process of the invention" refers to a method for preparing obeticholic acid
as
described herein, wherein the method comprises of crystalline obeticholic
acid.
25 "Fibrosis" refers to a condition involving the development of excessive
fibrous
connective tissue, e.g., scar tissue, in a tissue or organ. Such generation of
scar tissue
may occur in response to infection, inflammation, or injury of the organ due
to a disease,
trauma, chemical toxicity, and so on. Fibrosis may develop in a variety of
different
tissues and organs, including the liver, kidney, intestine, lung, heart, etc.
30 The term "inhibiting" or "inhibition," as used herein, refers to any
detectable
positive effect on the development or progression of a disease or condition.
Such a
positive effect may include the delay or prevention of the onset of at least
one symptom
or sign of the disease or condition, alleviation or reversal of the symptom(s)
or sign(s),
and slowing or prevention of the further worsening of the symptom(s) or
sign(s).
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
As used herein, a "cholestatic condition" refers to any disease or condition
in
which bile excretion from the liver is impaired or blocked, which can occur
either in the
liver or in the bile ducts. Intrahepatic cholestasis and extrahepatic
cholestasis are the two
types of cholestatic conditions. Intrahepatic cholestasis (which occurs inside
the liver) is
most commonly seen in primary biliary cirrhosis, primary sclerosing
cholangitis, sepsis
(generalized infection), acute alcoholic hepatitis, drug toxicity, total
parenteral nutrition
(being fed intravenously), malignancy, cystic fibrosis, and pregnancy.
Extrahepatic
cholestasis (which occurs outside the liver) can be caused by bile duct
tumors, strictures,
cysts, diverticula, stone formation in the common bile duct, pancreatitis,
pancreatic tumor
or pseudocyst, and compression due to a mass or tumor in a nearby organ.
Clinical symptoms and signs of a cholestatic condition include: itching
(pruritus),
fatigue, jaundiced skin or eyes, inability to digest certain foods, nausea,
vomiting, pale
stools, dark urine, and right upper quadrant abdominal pain. A patient with a
cholestatic
condition can be diagnosed and followed clinically based on a set of standard
clinical
laboratory tests, including measurement of levels of alkaline phosphatase, y-
glutamyl
transpeptidase (GGT), 5' nucleotidase, bilirubin, bile acids, and cholesterol
in a patient's
blood serum. Generally, a patient is diagnosed as having a cholestatic
condition if serum
levels of all three of the diagnostic markers alkaline phosphatase, GGT, and
5'
nucleotidase, are considered abnormally elevated. The normal serum level of
these
markers may vary to some degree from laboratory to laboratory and from
procedure to
procedure, depending on the testing protocol. Thus, a physician will be able
to determine,
based on the specific laboratory and test procedure, what is an abnormally
elevated blood
level for each of the markers. For example, a patient suffering from a
cholestatic
condition generally has greater than about 125 IU/L alkaline phosphatase,
greater than
about 65 IU/L GGT, and greater than about 17 NIL 5' nucleotidase in the blood.
Because
of the variability in the level of serum markers, a cholestatic condition may
be diagnosed
on the basis of abnormal levels of these three markers in addition to at least
one of the
symptoms mentioned above, such as itching (pruritus).
The term "organ" refers to a differentiated structure (as in a heart, lung,
kidney,
liver, etc.) consisting of cells and tissues and performing some specific
function in an
organism. This term also encompasses bodily parts performing a function or
cooperating
in an activity (e.g., an eye and related structures that make up the visual
organs). The
term "organ" further encompasses any partial structure of differentiated cells
and tissues
61
CA 03009149 2018-06-19
WO 2017/111979 PCT/US2016/012651
that is potentially capable of developing into a complete structure (e.g., a
lobe or a section
of a liver).
All publications and patent documents cited herein are incorporated herein by
reference as if each such publication or document was specifically and
individually
indicated to be incorporated herein by reference. Citation of publications and
patent
documents is not intended as an admission that any is pertinent prior art, nor
does it
constitute any admission as to the contents or date of the same. The invention
having
now been described by way of written description, those of skill in the art
will recognize
that the invention can be practiced in a variety of embodiments and that the
foregoing
1 0 description and examples below are for purposes of illustration and not
limitation of the
claims that follow.
In the specification, the singular forms also include the plural, unless the
context
clearly dictates otherwise. Unless defined otherwise, all technical and
scientific terms
used herein have the same meaning as commonly understood by one of ordinary
skill in
1 5 the art to which this invention belongs. In the case of conflict, the
present specification
will control.
All percentages and ratios used herein, unless otherwise indicated, are by
weight.
EXAMPLES
EXAMPLE 1: Synthesis of obeticholic acid
The compound numbers referred to in this synthetic procedure refer to those
found
in Scheme 1 and the reaction that correspond to each of the steps.
Step 1 ¨ Preparation of 3a-hydroxy-7-keto-50-cho1an-24-oic acid methyl ester
(1):
1. Me0H, H2SO4
CO2H reflux (62 C) CO2CH3
2. NaOH, Act. Carbon
3. Filtration
* 4. H20/Precipitation (10-15 C) 000111
0 5. Isolation
HO"s' 0 HO'" 0
3a-hydroxy-7-keto-50-cholan-24-oic acid 3a-hydroxy-7-keto-5p-cholan-24-oic
acid methyl ester
KLCA 1
Reaction 1: Esterification of C-24 carboxylic acid of 7-keto lithocholic acid
(KLCA)
62
CA 03009149 2018-06-19
WO 2017/111979 PCT/US2016/012651
3a-hydroxy-7-keto-50-cho1an-24-oic acid (KLCA; 500.0 g, 1.28 mol) was
esterified using methyl alcohol (2500 mL), in the presence of acidic catalysis
(sulfuric
acid, 1.0 mL) and was heated up to 62 C to 64 C for approximately 3 hours,
to yield 3a-
hydroxy-7-keto-50-cho1an-24-oic acid methyl ester (1). In this reaction, the
methyl
alcohol acts as the methylating reagent as well as the reaction solvent. For
the work-up,
the pH-value was adjusted with sodium hydroxide solution (2N) to pH 9.5 to 10.
The
solution was treated with activated carbon (25 g) for approximately 30 minutes
and
filtered to remove the carbon solids. Alternatively, the solution was not
treated with
activated carbon. To precipitate the product, water (625 mL) at 10 C to 15 C
was added
1 0 over 15 minutes and seeding material was added. The reaction mixture is
stirred for 1
hour at 10 C to 15 C. Another portion of water (1875 mL) was added over
about 20 to
25 minutes. The product suspension was stirred for 30 minutes at 10 C to 15
C. The
product was isolated with a centrifuge and washed with a mixture of methanol
and water
(1:1, 350 mL). The water content of the wet material was quantified by Karl
Fischer
1 5 (KF). The material was dried in a tumble dryer under vacuum at NMT 70
C. The
material can also be used in the next step without drying. The yield
(calculated on dried
product) is 501.4 g (1.24 mol, 96.8%).
Step 2 ¨ Preparation of 3a,7a -ditrimethy1si1y1oxy-513-cho1-6-en-24-oic acid
methyl ester
20 (3):
1. THF/LDA, -25 C
CO2CH3 H3 2. Si(CH3)3CI
CO2CH3
tp= 3. aq. Citric Acid/
Phase Separation
4. Removal of Solvent,
Ho**". o1:1 (H3c)3siol
3a-hydroxy-7-keto-56-cholan-24-oic acid methyl ester 3a-trimethylsilyloxy-7-
keto-56-cholan-24-oic acid methyl ester
1 2 (not isolated)
CO2CH3
0
(H3c)3sies' H OSi(CH3)3
3a,7-ditrimethylsilyloxy 513 chol 6 en 24 oic acid methyl ester
3
63
CA 03009149 2018-06-19
WO 2017/111979 PCT/US2016/012651
Reaction 2: Silylenol ether formation from 7-keto lithocholic methyl ester
Compound 1 (60.69 g, 150 mmol, calculated as dry substance), containing
residual water and methanol, was charged into the reactor under inert
conditions and was
dissolved in tetrahydrofuran (THF, 363 mL). Water and methanol were removed by
repeated azeotropic distillation at approximately 65 C and ambient pressure.
THF was
added to the residue as necessary and the distillation was repeated
approximately 4 times.
The remaining solution must have a final water content of < 0.05% (Karl
Fischer
Titration). This solution was pre-cooled to -20 C to -25 C and then
chlorotrimethylsilane (73.33 g, 675 mmol, 4.5 equivalents) was added in about
30 to 45
minutes. Under nitrogen atmosphere, lithium diisopropyl amide (28% LDA
solution, 900
mmol) and THF (504 mL) were charged to a separate inert reactor and cooled to -
20 C to
-25 C. The dry, cooled solution of compound 1, THF (84 mL), and
chlorotrimethylsilane was charged into the LDA solution at -20 C to
1 5 -25 C. Then, the reaction mixture was stirred for approximately 2
hours. For the
workup, the reaction mixture was added to a pre-cooled aqueous solution of
citric acid
(34.6 g in 300 mL) at 2 C to 8 C. After the addition, the aqueous phase was
separated
and discarded. From the organic phase, the liquid was removed by vacuum
distillation at
maximum 50 C. The isolated residue contained compound 3 and some residual
solvents
and was used 'as is' in the next step.
Step 3 ¨ Preparation of 3a-hydroxy-6-ethy1idene-7-keto-5(3-cho1an-24-oic acid
methyl
ester (4):
co2cH3 1i BCFFI2acHN2C6C0H006 -60 C
co2cH3
2
0111 3. Stirring at 25 C
4. NaOH/H20/Phase Separation 0111
*01 5. Removal of Solvent, 50 oiy,
(H3C)3SiOµs OSi(CH3)3
Hl
3a,7-ditrimethylsilyloxy-513-chol-6-en-24-oic acid 3a-
hydroxy-6-ethylidene-7-keto-5p-cholan-24-oic acid
methyl ester methyl ester
3 4
Reaction 3: Aldol condensation of the silylenol ether and acetaldehyde
64
CA 03009149 2018-06-19
WO 2017/111979 PCT/US2016/012651
Compound 3 (164.68 g, 300 mmol, calculated as dried substance) solution in THF
was charged into an inert reactor. At a maximum temperature of 50 C, residual
amounts
of THF were distilled off under vacuum. The water content in the residue was
limited to
< 0.5% (Karl Fischer titration) in order to proceed. The residue was then
dissolved in
dichloromethane (200 mL) and pre-cooled to -60 C to -65 C. Acetaldehyde
(33.8 mL,
600 mmol) was then added. Under nitrogen atmosphere, dichloromethane (700 mL)
and
boron trifluoride (16 wt% solution in acetonitrile, 318 g, 750 mmol)
acetonitrile complex
were charged into a separate reactor and then cooled to -60 C to -65 C. At -
60 C to -65
C, the dry compound 3 solution was added. The reaction mixture was stirred for
1 0 approximately two hours at -60 C to -65 C, heated up to 23 C to 28
C, stirred for
another approximately 3 hours and cooled to approximately 2 C to 10 C for
the
hydrolysis/work-up. For the workup, the cooled solution from the reactor was
added to a
pre-cooled aqueous solution of 50% wt. caustic soda (40 mL) and 660 mL of
water. After
about 10 minutes of intensive stirring, the phases were separated and the
(lower) organic
1 5 layer was transferred to a separate reactor. From the organic layer,
the solvent was
removed by distillation at NMT 50 C as far as possible. The residue,
consisting of
compound 4 and some remaining acetonitrile and dichloromethane, was discharged
into
drums. Compound 4A, a mixture of E/Z-isomers can also be prepared by the
procedure
described above for Step 3.
Step 4 ¨ Preparation of 3a-hydroxy-6-ethy1idene-7-keto-5(3-cho1an-24-oic acid
(5):
1. Me0H, NaOH, H20, 50 C
CO2CH3 2. Phase separation CO2H
3. Citric acid, AcOEt,
Phase Separation
4. Distillation with AcOEt _________________ P.11111
___________________________________________ v.-
5. Crystallization from Et0H
HOµs..4IFHO"."P
H I 6. Dried under vacuum (60 C) H I
3a-hydroxy-6-ethylidene-7-keto-5p-cholan-24-oic acid 30c-
hydroxy-6-ethylidene-7-keto-513-cholan-24-oic acid
methyl ester 5
4
Reaction 4: Saponification of C-24 ester
Compound 4 (258.37 g, 600 mmol, calculated as dried substance) was charged
into an inert reactor. At a temperature of NMT 50 C, residual amounts of
solvent were
distilled off under vacuum. The residue was dissolved in methanol (360 mL) and
water
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
(54 mL) and caustic soda 50% wt. (54 mL) were added. The reaction mixture was
heated
up to 49 C to 53 C and stirred at this temperature for at least 2 hours. The
pH of the
reaction mixture is checked and verified to be > 12. If the pH is < 12,
additional NaOH is
added and the 2 hour reaction time is repeated. The solution was diluted with
water
(1000 mL) and the temperature was adjusted to 25 C to 35 C. For the workup,
reaction
mixture was allowed to rest for at least 30 minutes. The phases were separated
and the
lower aqueous layer was transferred into a separate reactor and the organic
layer was
discarded. Ethyl acetate (1400 mL) and aqueous citric acid (244 g in 480 mL)
were
added with intensive stirring to the aqueous layer. The reaction mixture was
stirred at 25
C to 35 C for 10 minutes. The phases were separated and the lower aqueous
layer was
discarded. Ethyl acetate was distilled off from the organic layer and replaced
with ethyl
acetate (800 mL). This operation was repeated until the water content of the
distillate was
NMT 1% or until a constant boiling point was reached. The suspension was
cooled to 20
C to 25 C, stirred for 30 minutes, and then the product was isolated and
washed with
ethyl acetate (100 mL, 3 to 4 times). Drying was done in a tumble dryer under
vacuum at
approximately 60 C. The yield is 118.71 g (47.5% from KLCA) of crude compound
5.
Compound 4A, a mixture of E/Z isomers also can be used as starting material to
produce
compound 5A, a mixture of E/Z isomers.
Crude compound 5 was then crystallized using ethanol. The crude compound for
crystallization can also be a mixture of E/Z isomers, compound 5A. Ethanol
(390 to 520
mL) and crude compound 5 (130 g) were charged into an inert reactor. To
dissolve the
crude compound 5, the reaction mixture was heated to reflux. Then, the
reaction mixture
was cooled in a controlled cooling ramp to 15 C to 20 C within 3 to 5 hours
by a linear
profile. The crystalline compound 5A was isolated using a centrifuge and then
washed
with ethyl acetate (50-100 mL, 2 times). Drying was done in the tumble dryer
under
vacuum and at approximately 60 C. This leads to 85.8 g (66%) yield. A sample
was
taken to measure assay, purity, and moisture of the purified compound 5.
Purified
compound 5 is the E isomer of 3a-hydroxy-6-ethy1idene-7-keto-50-cho1an-24-oic
acid.
See example 2 for full details regarding the identification and
characterization of purified
compound 5. Isolation of the purified compound 5, the E isomer, can be
optional. The E
isomer and Z isomers have different solubilities. The E isomer is less
soluable and
crystallizes such that the Z isomer can be washed away.
An alternative method to prepare compound 5 is as follows. Compound 4 (111.96
g) was charged into the inert reactor. At maximum 50 C residual amounts of
solvent
66
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
(e.g., acetonitrile, dichloromethane) were distilled off under vacuum. The
residue was
dissolved in methanol (156 mL) and cooled to about 10 C. Tap-water (23.4 mL)
and
caustic soda 50 % (23.4 mL) were added. The reaction mixture was stirred for
about four
hours at about 20 C to about 25 C. The solution was diluted with tap-water
(433 mL)
and toluene (144 mL) was added. After stirring, the phases were separated and
the lower,
aqueous layer was transferred into the inert reactor. The organic layer was
discarded.
Ethyl acetate (607 mL) and a solution of citric acid (105.7 g in 208 mL of
water) were
added during intensive stirring to the aqueous layer. The phases were
separated and the
lower, aqueous layer was discarded. The organic layer was transferred into the
inert
1 0 reactor. From the organic layer ethyl acetate was distilled off and
replaced with ethyl
acetate (347 mL). In one embodiment, this operation was repeated with ethyl
acetate
(173 mL) until the water content of the distillate was not more than about 1 %
or until a
constant boiling point was reached. The present suspension was cooled to 20 C
to 25 C.
Compound 5 was isolated and washed with ethyl acetate (3 to 4 times each 43
mL) with
1 5 inert centrifuge. Drying was done in the tumble dryer under vacuum and
approximately
60 C (64.8% yield based on compound 1). Compound 4A (a mixture of E/Z
isomers)
can also be used as starting material for Step 4 to produce Compound 5A (a
mixture of
E/Z isomers).
20 Step 5 ¨ Preparation of 3a-hydroxy-6a-ethy1-7-keto-5(3-cho1an-24-oic
acid (6):
1. 50% NaOH, H20,
Pd/C, H2, 25 C/5 bar
2. Release H2
CO2H a Stirring at 100 C CO2H
4. Filtration of catalyst
diae 5. Conc. HCl/nBuOAc, 40 C
Phase Separation
. 41P
000
6. Act Carbon, 40 C
7. Condense by distillation
8. Crystallization from nBuOAc
HO"- 9. Dried at 60 C H 0
H I H E
3cc-hydroxy-6-ethylidene-7-keto-5p-cholan-24-oic acid 3a-
hydroxy-6a-ethyl-7-keto-513-cholan-24-oic acid
5 6
Reaction 5: Hydrogenation of the 6-ethylidene moiety
A mixture of purified compound 5 (110 g, 264 mmol), water (1100 mL), caustic
soda solution (35.8 mL, 682 mmol) at 50% and palladium catalyst (Pd/C, 11 g)
were
charged to a hydrogenation reactor. The temperature was adjusted to 25 C to
35 C and
the reactor was flushed three times with nitrogen (2 bar) and three times with
hydrogen (1
67
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
bar). These pressure values were given relative to ambient pressure (= 0 bar).
A
hydrogen pressure of 5 bar was applied and the reaction mixture was heated up
to 100 C
(for isomerisation to the alpha position) over a period of 1.5 hours and then
stirred for 3
hours while maintaining the hydrogen pressure at 4.5 to 5 bar. The reaction
mixture is
then cooled to 40 C to 50 C. For the workup, the Pd/C is filtered off To the
filtrate, n-
butyl acetate (1320 mL) and hydrochloric acid (67.8 mL, 815 mmol, 37%) were
added.
The aqueous phase was separated and discarded. The organic phase was treated
with
activated carbon (5.5 g) for about 10 minutes at 40 to 50 C. The activated
carbon was
filtered off and the filtrate was condensed by distillation and the resulting
suspension was
1 0 cooled to 15 C to 20 C within 2 to 3 hours. The precipitated compound
6 was isolated
and washed with n-butyl acetate (160 mL). The product was filtered using a
pressure
filter. Drying was done in the pressure filter under vacuum at approximately
60 C. This
leads to 89.8 g (81.2%) of Compound 6. Compound 5A, a mixture of E/Z isomers,
can be
used in step 5 to prepare Compound 6.
Step 6 ¨ Preparation of 3a,7a-dihydroxy-6a-ethy1-5(3-cho1an-24-oic acid
(obeticholic
acid):
co2H co2H
1. H20, NaOH, Reflux (90 C)
2. Addition of NaBH4, 90 C (reflux)
3. Citric acid, nBuOAc2, 40 C 011
4. Distillation from nBuOAc
5. Crystallization from nBuOAc
HON'.7 1E01 6. Recrystallization from nBuOAc HOµ'= 100.,
H /OH
7. Dried under vacuum at 80 C
3a-hydroxy-6a-ethyl-7-keto-5p-cholan-24-oic acid 3a,7a-
dihydroxy-6a-ethyl-513-cholan-24-oic acid
6 crystalline obeticholic acid
Reaction 6: Selective reduction of 7-keto group to 7a-hydroxy group
A mixture of compound 6 (86 g, 205.4 mmol), water (688 mL) and 50% sodium
hydroxide solution (56.4 mL) was reacted with sodium borohydride (7.77 g,
205.4 mmol)
in a mixture of 50% wt. sodium hydroxide solution (1.5 mL) and water (20 mL)
at 90 C
to 105 C. The reaction mixture was heated to reflux and stirred for at least
3 hours. For
the workup, after the reaction was complete, the reaction mixture was cooled
to
approximately 80 C and transferred to a cooled reactor. At 30 C to 50 C, n-
butyl
acetate (860 mL) and citric acid (320.2 g, anhydrous) in water (491 mL) were
added. The
68
CA 03009149 2018-06-19
WO 2017/111979 PCT/US2016/012651
aqueous phase was separated and discarded after checking the pH-value to make
sure that
it was acidic. The organic phase was transferred and distilled. The residue is
diluted with
n-butyl acetate and was slowly cooled to 15 C to 20 C and the crude
obeticholic acid
was filtered using a centrifuge. The wet product was crystallized from n-butyl
acetate.
The product obeticholic acid was isolated and washed with n-butyl acetate (43
mL, 4
times) in an inert pressure filter. Drying was done in the pressure filter
under vacuum at
approximately 80 C. This led to 67.34 g (77.9%) of crystalline obeticholic
acid
characterized by an X-ray diffraction pattern including characteristic peaks
at about 5.0
and 5.3 degrees 2-Theta. The crystalline obeticholic acid Form A is
characterized by an
1 0 X-ray diffraction pattern substantially similar to that set forth in
Figure 41. See example
3 for full details regarding the identification and characterization of
crystalline obeticholic
acid.
Step 7 ¨ Preparation of obeticholic acid Form 1:
co2H co2H
1 H20, NaOH, Dissolution, 30 C
101
2 HCI, Precipitation, H20
3 Dried under vacuum at 50 C
EWI
HiVs."0:11H = 'OH
3a,7a-dihydroxy-6a-ethyl-5P-cholan-24-oic acid obeticholic acid Form 1
cystalline obeticholic acid
Reaction 7: Preparation of obeticholic acid Form 1 from crystalline
obeticholic acid Form
A
Crystalline obeticholic acid Form A characterized by an X-ray diffraction
pattern
including characteristic peaks at about 5.0 and 5.3 degrees 2-Theta (58 g) was
dissolved
in water (870 mL) and caustic soda solution (50%, 8.7 mL, 166 mmol) at 30 C
to 40 C.
The mixture was stirred until all solid has dissolved. The product was
precipitated using
the following workup. The obeticholic acid solution was slowly added via a
filter to
diluted hydrochloric acid (37%, 16.05 mL, 193 mmol) in water (870 mL) at 30 C
to 40
C. The suspension was stirred for approximately 30 minutes at 30 C to 40 C
and then
cooled to not more than (NMT) 20 C. The product was isolated and washed with
water
(465 mL, 6 times) in the inert pressure filter. Drying was done in the
pressure filter under
69
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
vacuum at a temperature of NMT 50 C. This led to 53.2 g (91.7%) of obeticholic
acid
Form 1.
EXAMPLE 2: Characterization of E-3a-hydroxy-6-ethylidene-7-keto-5(3-cho1an-24-
oic
acid (5)
Compound 5 is the key intermediate for the process of the application. The
compound was isolated from ethyl acetate and was then crystallized from
ethanol. The
highly pure compound 5 allows for efficient and high yielding production of
compound 6
and subsequently crystalline obeticholic acid Form A characterized by an X-ray
diffraction pattern including characteristic peaks at about 5.0 and 5.3
degrees 2-Theta and
obeticholic acid Form 1, including substantially pure obeticholic acid.
The structure of compound 5 from step 4 in example 1 was confirmed using 1H
NMR, 13C NMR, and mass spec. Crude product from step 4 resulted in a major
product
at retention time (RT) 27.457 min and a minor product at RT 28.078 min in the
UV
chromatogram generated by quality control method 1 by means of LC/MS-coupling.
The
two products are the E/Z isomers of compound 5:
0
411-1
Ht.151744101.4
H I HO 0
H I
Exact Mass ,,418.2027
Molecular Formula =C264-14004
These two isomers show the same accurate mass and the same fragmentation in
the
MS/MS spectrum. They cannot be distinguished by the mass spectrometric data.
Using a semi-preparative method to isolate the E/Z isomer peaks, the
structures of
the E/Z isomers were confirmed using a two stage approach. The HPLC quality
control
method 1 used a non-volatile phosphoric acid buffer and thus, direct LC/MS
coupling
with the non-volatile buffer was not possible. Preliminary tests for
adjustment of the
method showed that only a UPLC method allowed for very high plate numbers for
adequate separation of the E/Z isomers. The two stage approach was the
following: Step
A was identification of the E/Z isomers in two samples with the new developed
UPLC/MS method and Step B was isolation of the fraction of the E/Z isomer
peaks with
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
the HPLC method 2 and subsequent identification with the UPLC/MS method 1. The
experimental details of the methods were as follows:
Table C
1. MS compatible UPLC method (method n
Instrument: Accela UPL Ccoupling with LTQ FTSpectrometer
(ThermoScientific)
Column: 200 x 2mm Hypersil Gold 1.9 gm
Eluent: A: Water + 10 mM Ammoniumformiat + 0.1% Formic acid
B: Acetonitrile
Gradient: 45% B in 20 minutes to 60% B (10 min isocratic)
Flow: 0.4 ml/min, 40 C column temperature
Detection: MS: ESI positive and negative ions; UV: PDA 200.600 nm
Mass resolution: R=100000 ICR
Sample: 1 mg / ml in water/acetonitrile (1:1), 3 1/20 1 injected
2. HPLC (method 2)
Agilent1100 HPLC (Agilent Technologies)
Instrument:
125 x 4mm Purospher STAR C18 5 Lm
Column:
Eluent: A: Water pH 2.6 with phosphoric acid
B: Acetonitrile
30 % B in 10 minutes to 35% B in 30 minutes to 60% B
Gradient:
In 1 minutes to 90% B (9 min isocratic)
1 ml/min, 35 C column temperature
Flow:
UV: DAD 200 ¨ 400 nm (UVA 200 nm)
Detection:
mg / ml in water/acetonitrile (9:1), 25 1 injected
Sample:
5
The results are shown in Figures 1 and 2. Figures 1 and 2 are UPLC UV/MS
chromatograms for "crude compound 5" (Figure 1) and compound 5 "purified
reference"
(Figure 2) obtained on a high performance UPLC column. For Figure 1, the
sample was
dissolved at 1 mg/mL in ACN/H20 1:1; 200 x 2mm Hypersil GOLD R122; LMA:H20 +
10 10 mM AF + 0.1% HFo; LMB:ACN; 45%-20-60%(10); 0.4mL/min; 40 C; UVA=200
nm; 3 [IL injection volume. For Figure 2, the sample was dissolved at 1 mg/ml
in
ACN/H20; 200 x 2 mm Hypersil GOLD R122; A: 10 mM AF + 0.1%HFo; B:ACN; 45%-
20-60%B(10); 0.4 mL/min; 20 [IL injection volume. In both samples, the
molecular
weight of the main component (RT 9.92 min) and of the minor component (RT
10.77
1 5 min) was the same as expected and the accurate masses of the two
compounds were
71
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
consistent with the structures provided as shown in Tables D and E of data of
the positive
and negative ion measurement show below:
Table D: Data of the positive ion measurement
Ri(min) Ion miz Formula Structure proposal
Cal HAI 04
9.98 4730008 AM 0.35 ppm M H E isomer
C;52 Fis: 05
833.59381 AM 1.45 ppm 2M +H E Isomer
Ca2 F13,0 N
850.61938 LM 028 ppm 2M NH4 E isomer
10.77 417.30023 C.w, H41 04 M H Z Isomer
833 59409 C52 Hal 08 2M +H Z isomer
= 850.61984 Ca2 Hs4 0,3 N 2M + NH4 Z isomer
Table E: Data of the negative ion measurement
RT(min) Ion nalz Formula Structure proposal
Cm Fin 04
9.98 415.28520 AM -0.44 ppm M-H Z isomer
CZ7 H41 0e
461 29051 AM -0.76 ppm + Form4t Z isomer
Ca2 Fim
831.57683 AM -1.46 ppm 2M - H Z isomer
1017 41528545 Cra Ha; 04 M-H E isomer
461.29069 C27 H41 06 M Formiat E isomer
- -
831.57739 Ca2 F1.79 2M - H E isomer
- -
To ensure the portability of the quality control HPLC method 2, the original
separation was repeated exactly under the prescribed conditions. The main peak
and the
minor peak were isolated as semipreparative. The resulting UV chromatogram
with the
1 0 marked positions of the trapped fractions is shown in Figure 3. Figure
3 is a UV
chromatogram of crude compound 5 using HPLC method 2; 125 x 4mm Purospher STAR
C18 5 lam AG; LMA:H20 pH 2.6 with H3PO4; LMB:ACN; 30%B-10-35%-30-60%-1-
90%(9); 1 mL/min; 35 C; UVA=200nm; ohne MS; 25 mL. Subsequently, the isolated
fractions were separately analyzed with the newly developed UPLC/MS method.
For the
1 5 evaluation of the accurate ion trace of the quasimolecular ion [2M+NH41
at
850.61914 3ppm was used. The resulting chromatograms of the main peak
fraction, the
minor peak fraction and of the two samples are shown in Figure 4 (A-D). The MS
studies
showed that the two peaks generated by quality control method 2 at RT 27.457
min and at
RT 28.078 min are two isomers with the formula C26H4004. This formula is
consistent
20 with the structure proposed for the E/Z isomers. Thus, the development
of the UPLC-MS
72
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
method has shown that the E/Z isomers of 3a-hydroxy-ethyliden-7-keto-513-
cholic-24
acid are chromatographically separable with high resolution. The accurate MS
data from
the FR-ICR mass spectrometer are consistent with the structure proposed for
the E/Z
isomers. For both isomers, the same formula C26H4004 was derived.
Due to the semi-preparative isolation of the E/Z-isomer peaks with HPLC method
2 and subsequent identification with the UPLC-MS method we can show that the
two
peaks generated by the quality control method 2 (RT 27.457 minutes and RT
28.078
minutes, see figure 1) are the two isomers with the formula C26H4004. This
formula is
consistent with the structure proposal of the E/Z-isomers. In conjunction with
the NMR
results described below the following assignments were obtained: RT 27.457
minutes
belongs to the E-isomer and RT 28.078 minutes to the Z-isomer.
The assignment of the 1H and 13C shifts for the E isomer of 3a-hydroxy-
ethyliden-
7-keto-5P-cholic-24 acid are shown below. Shifts were estimated according to
"L.
Bettarello et al., II Farmaco 55 (2000), 51-55 (substance 3a-hydroxy-7-keto-
513-cholan-
24-oic acid).
0
1-i3C22241
õ
OH
11
13
16
H
2 1
5 7
HO 0
4 H 1
Table F: 1H Shift Assignment (1H-NMR, 500 MHz, 303K, CD30D)
Chemical shift ippinj Intensity [Hi Multipty Assignment
6.10 1 25
3
2.69 1 DD 5
2.28 2 IDT 23
1.72 3 26
1.05 3 S19
0.99 3 21
0,70 3 S18
Table G: 13C Shift Assignment (13C-NMR, 125 MHz, 303K, CD30D)
73
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Chemical shift [ppm] = Multiplicity Assignment
207.5 S 7
17Þ S 24
145.3 S 6
130.4 25
71.0 3
56.0 S17
52.0 and 50.1 D each 8 and 14
46.9 EI 5
44.7 S 13
40.7 EI 9
40.3 T 12*
38.3 T 4*
36.5 EI 20
35.8 S 10
35.4 T 1
32.3 and 32.0 T each 22 and 23
30.5
29.4 T16*
27.0 T 15*
23.2 19
22.4 T 11
18.9 Q 21
12.7 Q 26
12.5 Q 18
S = singlet
D = doublet
T = triplet
Q = quartet
M = multiplet
DD = doublet of doublets
DT = doublet of triplets
1 0 EXAMPLE 3: Characterization of crystalline obeticholic acid
I. Solid-state characterization of the product from step 6 of
Scheme 1 and
Example 1 showed that the obeticholic acid is crystalline. This crystalline
form is labeled
Form A and is substantially similar to the X-ray Diffractogram in Figure 41
and is
characterized by peaks at about 5.0 and 5.3 degrees 2-Theta. The corresponding
data for
the X-ray Diffractogram of Form A are presented in Table 4 below.
Table 4: X-ray Diffractogram Data of Crystalline Obeticholic Acid Form A
Angle Intensity %
2-Theta
5.0 53.8
74
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
5.3 34.6
6.3 54.0
7.3 15.6
7.7 23.3
8.2 10.5
8.3 11.8
8.4 11.8
8.9 35.9
9.4 20.4
10.0 63.0
10.5 11.7
11.0 50.8
12.4 83.2
14.5 19.7
14.9 39.9
15.3 36.9
16.0 75.7
16.5 69.1
16.8 100.0
17.9 39.2
18.7 35.6
19.0 34.4
19.6 12.1
20.6 16.2
21.3 22.8
Small Scale Synthesis of Crystalline Obeticholic Acid Form A
Amorphous obeticholic acid (approximately 30 mg) was weighed into a glass vial
and warmed to 50 C. n-Butyl acetate (60 uL) was added and observations were
made.
The sample was stirred at 50 C for 1 hour and was cooled to 5 C using a
linear cooling
rate of 0.1 C/min. The sample was stirred at 5 C for 2 days. Solids were
filtered, air
dried and analyzed by XRPD. The solution was stored in the freezer for -48
hours, and
then left to evaporate at ambient conditions to afford Form A. Additional
methods of
preparing Form A are provided in Example 9.
Scale-up Synthesis of Crystalline Obeticholic Acid Form A
Amorphous obeticholic acid (approximately 1 g) was weighed into a large glass
vial and warmed to 50 C. Ethyl acetate (1.5 ml, 1.5 volumes) was added at 50
C,
forming a clear solution. Anti-solvent (heptane, 2.0 ml, 2 volumes) was added,
initially
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
forming a cloudy precipitate, which converted to an oil on stirring. After
stirring at 50 C
for 1 hour the oil remained so an additional 0.5 ml (0.5 volumes) ethyl
acetate was added,
which dissolved the oil, forming a cloudy liquid. The sample was seeded with
approximately 1 mg of sample from the small scale experiment directly above.
The
sample was stirred at 50 C for another hour then was cooled to -20 C using a
linear
cooling rate of 0.1 C/min. The sample was stirred at -20 C overnight, after
which a
small amount of the solid was filtered, air dried and analyzed by XRPD. The
remainder
of the sample was filtered through a 0.45 p.m PTFE filter and was washed with
2 x 2 ml
portions of heptane. The sample was air dried for approximately 30 minutes
then was
dried in a vacuum oven overnight at RT. The resulting white solid (898.9 mg)
was
characterized as Form A having an X-ray Diffractogram substantially similar to
that of
Figure 41. Figure 42 is an overlay of the TGA and DSC thermograms of
crystalline
obeticholic acid Form A. Figure 43 is an isotherm plot for the GVS experiment
with
Form A. Figure 44 is a kinetics plot for the GVS experiment with Form A.
11. Below is a table that summarizes the characterization of
crystalline
obeticholic acid Form C:
Table G: Summary of Crystalline Obeticholic Acid Form C Characteristics
Technique Crystalline Obeticholic Acid Form C
appearance White powder
NMR Consistent with supplied structure ca. 3.5% w/w
heptane
XRPD Crystalline
TGA Weight losses between r.t. to 85 C (0.4%) and
85-
115 C (4.1%)
DSC Endotherm with onset of 97.9 C
GVS Slightly hygroscopic, 1.2 % water uptake at 90%
RH
Karl Fisher Water 1.5 w/w
Determination
Stability at 40 C/75% RH No change in form or crystallinity
Thermal Analysis
DSC (Differential Scanning Calorimetry) data were collected on a Mettler DSC
823e equipped with a 34 position auto-sampler. The instrument was calibrated
for energy
and temperature using certified indium. Typically 0.5-1 mg of each sample, in
a pin-holed
aluminium pan, was heated at 10 C=min-1 from 25 C to 350 C. A nitrogen
purge at 50
76
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
ml=min-1 was maintained over the sample. The instrument control and data
analysis
software was STARe v 9.20.
TGA (Thermo-Gravimetric Analysis) data were collected on a Mettler
TGA/SDTA 851e equipped with a 34 position auto-sampler. The instrument was
temperature calibrated using certified indium. Typically 5-10 mg of each
sample was
loaded onto a pre-weighed aluminium crucible and was heated at 10 C=min-1
from
ambient temperature to 300 C. A nitrogen purge at 50 ml=min-1 was maintained
over the
sample. The instrument control and data analysis software was STARe v 9.20.
Two weight loss steps were observed by TGA of crystalline obeticholic acid
Form
C. The first took place between room temperature (r.t.) and 85 C (0.41 %) and
the
second occurred between 85 C-115 C (4.10 %). The first weight loss step can
be
attributed to water loss with the second step being attributed to the loss of
the remaining
water (water responsible for around 1.2 % weight loss) and the loss of bound
heptane (ca.
3.4 % weight loss). Crystalline obeticholic acid Form C contained between 0.15
and 0.2
moles solvent (heptane) and ca. 1.5 % w/w (0.3 moles). The DSC thermogram of
crystalline obeticholic acid Form C contained one endotherm. This was fairly
sharp and
had an onset of around 98 C. See Figure 6. Different solvents would have
different
boiling points and therefore would evaporate at different temperatures within
the DSC
and TGA experiments.
X-Ray Powder Diffraction (XRPD) Analysis
Bruker AXS C2 GADDS
X-Ray Powder Diffraction patterns were collected on a Bruker AXS C2 GADDS
diffractometer using Cu Ka radiation (40 kV, 40 mA), automated XYZ stage,
laser video
microscope for auto sample positioning and a HiStar 2-dimensional area
detector. X-ray
optics consisted of a single Gael multilayer mirror coupled with a pinhole
collimator of
0.3 mm. A weekly performance check was carried out using a certified standard
NIST
1976 Corundum (flat plate).
The beam divergence, i.e. the effective size of the X-ray beam on the sample,
was
approximately 4 mm. A 0-0 continuous scan mode was employed with a sample -
detector
distance of 20 cm which gives an effective 20 range of 3.2 ¨ 29.7 .
Typically the
sample was exposed to the X-ray beam for 120 seconds. The software used for
data
collection was GADDS for WNT 4.1.16 and the data were analyzed and presented
using
Diffrac Plus EVA v 9Ø0.2 or v 13Ø0.2.
77
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Ambient conditions: Samples run under ambient conditions were prepared as flat
plate specimens using powder as received without grinding. Approximately 1-2
mg of the
sample was lightly pressed on a glass slide to obtain a flat surface.
Non-ambient conditions: Samples run under non-ambient conditions were
mounted on a silicon wafer with heat-conducting compound. The sample was then
heated
to the appropriate temperature at ca. 10 C= min-1 and subsequently held
isothermally for
ca. 1 minute before data collection was initiated.
Bruker AXS/Siemens D5000
1 0 X-Ray Powder Diffraction patterns were collected on a Siemens D5000
diffractometer using Cu Ka radiation (40 kV, 40 mA), 0-0 goniometer,
divergence of V20
and receiving slits, a graphite secondary monochromator and a scintillation
counter. The
instrument is performance checked using a certified Corundum standard (NIST
1976).
The software used for data collection was Diffrac Plus XRD Commander v2.3.1
and the
1 5 data were analyzed and presented using Diffrac Plus EVA v 11,0Ø2 or v
13Ø0.2.
Samples were run under ambient conditions as flat plate specimens using powder
as received. Approximately 20 mg of the sample was gently packed into a cavity
cut into
polished, zero-background (510) silicon wafer. The sample was rotated in its
own plane
during analysis. The details of the data collection are:
20 = Angular range: 2 to 42 20
= Step size: 0.05 20
= Collection time: 4 s=sterl
Bruker AXS D8 Advance
25 X-Ray Powder Diffraction patterns were collected on a Bruker D8
diffractometer
using Cu Ka radiation (40 kV, 40 mA), 0-20 goniometer, and divergence of V4
and
receiving slits, a Ge monochromator and a Lynxeye detector. The instrument is
performance checked using a certified Corundum standard (NIST 1976). The
software
used for data collection was Diffrac Plus XRD Commander v 2.5.0 and the data
were
30 analyzed and presented using Diffrac Plus EVA v 11Ø0.2 or v 13Ø0.2.
Samples were run under ambient conditions as flat plate specimens using powder
as received. Approximately 5 mg of the sample was gently packed into a cavity
cut into
polished, zero-background (510) silicon wafer. The sample was rotated in its
own plane
during analysis. The details of the data collection are:
78
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
= Angular range: 2 to 42 20
= Step size: 0.05 20
= Collection time: 0.5 s=sterl
The corresponding data for the X-ray diffractogram is presented in the table
below. The software used for data collection was Diffrac Plus XRD Commander
v2.6.1
and the data were analyzed and presented using Diffrac Plus EVA v13Ø0.2 or
v15Ø0Ø
Samples were run under ambient conditions as flat plate specimens using powder
as
received. The sample was gently packed into a cavity cut into polished, zero-
background
(510) silicon wafer. The sample was rotated in its own plane during analysis.
The details
of the data collection are:
= Angular range: 2 to 42 20
= Step size: 0.05 20
= Collection time: 0.5 s=sterl
1 5 Table H: X-ray Diffractogram Data of Crystalline Obeticholic Acid Form
C
peak Angle 2-Theta (deg) d value (Angstrom)
1 4.2 21.0203
2 6.35 13.90839
3 8.298 10.64718
4 9.5 9.30229
5 11.05 8.00042
6 12.246 7.22192
7 12.498 7.07692
8 12.647 6.99367
9 15.497 5.71337
10 15.843 5.5895
11 15.998 5.53561
12 16.346 5.41836
13 16.695 5.30601
14 16.996 5.21251
17.849 4.96547
16 18.593 4.76844
17 18.798 4.71689
18 19.047 4.65579
19 20.493 4.33041
20.894 4.24808
VT-XRPD (Variable Temperature-X-ray Diffraction) revealed that the endotherm
seen in the DSC thermogram corresponded to the desolvation of the sample as no
form
changes were observed on heating. A temperature difference exists between the
DSC and
20 the VT-XRPD data as the VT-XRPD experiment was carried out in a large
space with the
79
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
sample exposed whereas the DSC experiment was carried out in a confined,
closed space.
This difference is around 20 C and explains why the sample melted at a much
lower
temperature in the DSC experiment and the sample still appears crystalline at
110 C in
the VT-XRPD experiment. VT-XRPD shows that drying of the solvent from the
material
resulted in loss of crystallinity which is consistent with the material being
in a solvated
form. See Figure 7.
Gravimetric Vapor Sorption (GVS)
Sorption isotherms were obtained using a SMS DVS Intrinsic moisture sorption
1 0 analyzer, controlled by DVS Intrinsic Control software v 1Ø0.30. The
sample
temperature was maintained at 25 C by the instrument controls. The humidity
was
controlled by mixing streams of dry and wet nitrogen, with a total flow rate
of 200
ml=min-1. The relative humidity was measured by a calibrated Rotronic probe
(dynamic
range of 1.0-100% RH), located near the sample. The weight change, (mass
relaxation) of
the sample as a function of % RH (relative humidity) was constantly monitored
by the
microbalance (accuracy 0.005 mg).
5 to 20 mg of sample was placed in a tared mesh stainless steel basket under
ambient conditions. The sample was loaded and unloaded at 40% RH and 25 C
(typical
room conditions). A moisture sorption isotherm was performed as outlined below
(2
scans giving 1 complete cycle). The standard isotherm was performed at 25 C
at 10 %
RH intervals over a 0.5-90 % RH range. Data analysis was undertaken in
Microsoft Excel
using DVS Analysis Suite v6Ø0.7. Method Parameters for SMS DVS Intrinsic
Experiments are as follows:
Parameters V Ales
Adsorption, - Scan
Desorption ,/ Adsorption - Scan 2 90 - 0, 0 ¨ 40 %
Intervals (% R 10
Number of Scans ,
Flow rate (mi'm.in) 200
Temperature CC) 25
Stability CCinin.1) 0.2
Sorption Time (hours) 6 hour time out
The sample was recovered after completion of the isotherm and re-analyzed by
XRPD.
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Analysis of crystalline obeticholic acid Form C showed that the sample was
slightly hygroscopic as a mass increase of 1.18% was noted between 0-90% RH.
This
uptake of water was steady throughout the analysis and equilibrium was reached
for all
steps. The hysteresis of the curve was small indicating that the sample
readily lost the
water it had taken up. XRPD analysis after the GVS analysis showed that the
sample was
unchanged. See Figures 8A, 8B, and 8C.
Water Determination by Karl Fischer Titration (KF)
The water content of each sample was measured on a Mettler Toledo DL39
Coulometer using Hydranal Coulomat AG reagent and an argon purge. Weighed
solid
samples were introduced into the vessel on a platinum TGA pan which was
connected to
a subaseal to avoid water ingress. Approximately 10 mg of sample was used per
titration
and duplicate determinations were made.
Karl Fischer analysis showed that crystalline obeticholic acid Form C
contained
1.5% water which corresponds to about 0.3 moles water.
One Week Stability at 40 C and 75% RH
The stability of obeticholic acid at 40 C and 75% RH (relative humidity) was
determined as follows. A sample of obeticholic acid was stored in a humidity
chamber for
one week at 40 C/75 % RH. The sample was re-analyzed by XRPD and was found to
have been unchanged.
The solid state study has shown that the presence of a relatively large amount
of
organic solvent is required to crystallize obeticholic acid Form C. It is
highly unlikely
that a sample of obeticholic acid Form 1 will spontaneously crystallize to
form crystalline
obeticholic acid Form C on storage.
EXAMPLE 4: Obeticholic Acid Tablet formulation
The table below shows the quantitative composition of obeticholic acid
tablets. The 5 mg,
10 mg, and 25 mg formulations have been used as phase 3 clinical trial
material.
Table I: Film Coated Tablet
Film Coated Tablet
81
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Reference to
Component Quantity per Tablet Function
Standard
1 mg tablet
Obeticholic acid 1.0 mg* API HSE
Microclystalline cellulose 185.0 mg* Filler/Binder USP-NF/EP/JP
Sodium starch glycolate 12.0 mg Disintegrant USP-NF/EP/JP
Magnesium stearate 2.0 mg Lubricant USP-NF/EP/JP
Opacity II green or white 8.0 mg Coating Material HSE
Total weight 208.0 mg
mg tablet
Obeticholic acid 5.0 mg* API HSE
Microclystalline cellulose 181.0 mg* Filler/Binder USP-NF/EP/JP
Sodium starch glycolate 12.0 mg Disintegrant USP-NF/EP/JP
Magnesium stearate 2.0 mg Lubricant USP-NF/EP/JP
Opacity II green or white 8.0 mg Coating Material HSE
Total weight 208.0 mg
mg tablet
Obeticholic acid 10.0 mg* API HSE
Microclystalline cellulose 176.0 mg* Filler/Binder USP-NF/EP/JP
Sodium starch glycolate 12.0 mg Disintegrant USP-NF/EP/JP
Magnesium stearate 2.0 mg Lubricant USP-NF/EP/JP
Opacity II green or white 8.0 mg Coating Material HSE
Total weight 208.0 mg
25 mg tablet
Obeticholic acid 25.0 mg* API HSE
Microclystalline cellulose 157.0 mg* Filler/Binder USP-NF/EP/JP
Sodium starch glycolate 12.0 mg Disintegrant USP-NF/EP/JP
Magnesium stearate 2.0 mg Lubricant USP-NF/EP/JP
Collodial silicon dioxide 4.0 mg Glidant USP-NF/EP/JP
Opacity II green or white 8.0 mg Coating Material HSE
Total weight 208.0 mg
API: Active pharmaceutical ingredient
HSE = In house specification
USP-NF = US Pharmacopeia National Formulary
5 Ph Eur = European Pharmacopeia
JP =Japanese Pharmacopeia
* obeticholic acid quantity presented assumes API is anhydrous and 100% pure;
actual amount is adjusted based on the
potency of the drug substance Lot used, and amount of microcrystalline
cellulose is correspondingly decreased.
EXAMPLE 5: Characterization of Obeticholic Acid Form 1
Obeticholic acid Form 1 refers to the non-crystalline form of obeticholic
acid.
This form of obeticholic acid can be produced via a crystalline obeticholic
acid as a
82
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
synthetic intermediate. Obeticholic acid Form 1 can be used as the
pharmaceutically
active ingredient. Obeticholic acid Form 1 was characterized and analyzed as
follows.
Batch 1 of obeticholic acid form 1 was characterized using the following
techniques: assessment by X-ray powder diffraction (XPRD) for crystallinity,
1H and 13C
nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy (FT-
IR),
optical assessment (e.g., particle shape/size), thermal properties (e.g.,
differential
scanning calorimetry (DSC) and thermo-gravimetric analysis (TGA)), water
determination by Karl Fischer (KF), storage at 40 C and 75 %RH and reanalysis
after 2
weeks by XRPD, pKa by potentiometric method, Log P/D (octanol/water) by
potentiometry, and stability to moisture using gravimetric vapor sorption
(GVS; e.g.,
complete sorption-desorption cycle with analysis of solid collected by XRPD).
Five other
batches (e.g., batch 2, 3, 4, 5, and 6) of obeticholic acid Form 1 were also
characterized
and compared using the following techniques: assessment by XRPD and comparison
to
main batch 1 pattern, 1H and 13C NMR, FT-IR, optical assessment (e.g.,
particle
shape/size), thermal properties (e.g., DSC, TGA, and hot-stage microscopy),
and water
determination by KF.
X-Ray Powder Diffraction (XRPD) Analysis
X-Ray Powder Diffraction patterns were collected on a Bruker AXS C2 GADDS
diffractometer using Cu Ka radiation (40 kV, 40 mA), automated XYZ stage,
laser video
microscope for auto-sample positioning and a HiStar 2-dimensional area
detector. X-ray
optics consists of a single Gael multilayer mirror coupled with a pinhole
collimator of
0.3 mm. The beam divergence, i.e. the effective size of the X-ray beam on the
sample,
was approximately 4 mm. A 0-0 continuous scan mode was employed with a sample
¨
detector distance of 20 cm which gives an effective 20 range of 3.2 ¨ 29.7
. Typically
the sample was exposed to the X-ray beam for 120 seconds. The software used
for data
collection was GADDS for WNT 4.1.16 and the data were analyzed and presented
using
Diffrac Plus EVA v 9Ø0.2 or v 13Ø0.2.
Samples run under ambient conditions were prepared as flat plate specimens
using
powder as received without grinding. Approximately 1-2 mg of the sample was
lightly
pressed on a silicon wafer to obtain a flat surface. The diffractograms show
that
obeticholic acid Form 1 is non-crystalline (See, Figure 10 and Figure 11).
NMR Characterization
83
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
NMR spectra were collected on a Bruker 400 MHz instrument equipped with an
auto-sampler and controlled by a DRX400 console. Automated experiments were
acquired using ICONNMR v4Ø4 (build 1) running with Topspin v 1.3 (patch
level 8)
using the standard Bruker loaded experiments. For non-routine spectroscopy,
data were
acquired through the use of Topspin alone. Samples were prepared in d6-DMSO,
unless
otherwise stated. Off-line analysis was carried out using ACD SpecManager v
9.09 (build
7703).
Figure 12 shows the 1H NMR spectrum for batch 1. 1H NMR spectra of batches 2-
6 were also recorded and compared with the spectrum of batch 1. See Figure 13.
The
1 0 spectra are all similar, but with varying amounts of water. Some
differences are noted in
the integration of the large group of protons between 0.75 ppm and 2 ppm,
where peaks
overlap and cannot be integrated separately. Table J shows the total number of
protons
integrated in the spectra of batches 1-6, taking into account the variation in
the 0.75 ¨ 2
ppm region.
Table J
Batch number Number of H by integration
(excluding COOH)
1 43
2 42
3 40
4 41
5 42
6 41-42
The carboxylic acid proton has been excluded, so the number of protons should
be
43, but it actually varies from 40 to 43 between the 6 spectra. However, the
area where
the variation comes from (0.75-2 ppm) is quite wide, and due to the quality of
the
baseline, this integration cannot be relied upon.
As the spectrum could not be fully assigned and the integration varied, a 13C
NMR
spectrum of batch 2 was recorded. Figure 14 shows the DEPTQ spectrum, where
CH2 and
quaternary carbons peaks point up, while CH3 and CH groups point down. There
are
thirteen peaks pointing down, which correspond to nine CHs and four CH3
groups. This is
consistent with the structure. The peak of the carbon of the carboxylic acid
was seen at
175 ppm. It has been excluded from this expanded view for clarity of the area
of interest.
However, there are only eleven peaks pointing up, whereas there should be
twelve, as
there are ten CH2 groups and two quaternary carbons in the molecule (excluding
the
84
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
carbonyl). One carbon appears to be overlapping with another signal.
Therefore, a
DEPT135 spectrum was collected, suppressing the quaternary carbon signals,
which
could show whether the overlapping signal is quaternary. See Figure 15. A
comparison of
the DEPT135 spectrum with the DEPTQ spectrum shows that one peak (at 42.5 ppm)
disappears. There are two quaternary carbons in the molecule, which should
correspond
to two peaks disappearing. Therefore the overlapping carbon signal is a
quaternary one.
Further, an experiment to determine the relaxation time of the carbons was
carried
out to determine where the missing quaternary carbon signal is overlapping
with another
carbon signal. See Figure 16. This 13C spectrum contains peaks that were
integrated. This
1 0 showed that peak at 32.3 ppm accounts for two carbons. See Figure 17
for an expanded
view of the peak at 32.3 ppm. Thus, twenty-six carbons are now accounted for
by
integrations (including the carboxylic acid), which is consistent with the
structure.
FT-IR by ATR
1 5 Data were collected on a Perkin-Elmer Spectrum One fitted with a
Universal ATR
sampling_accessory. The data were collected and analyzed using Spectrum v5Ø1
software. See Figure 18.
Thermal Analysis by Differential Scanning Calorimetry (DSC) and Thermo-
Gravimetric
20 Analysis (TGA)
DSC data were collected on a TA Instruments Q2000 equipped with a 50 position
autosampler. The instrument was calibrated for energy and temperature
calibration using
certified indium. Typically 0.5-3 mg of each sample, in a pin-holed aluminum
pan, was
heated at 10 C.min-1 from 25 C to 300 C. A nitrogen purge at 50 ml.min-1
was
25 maintained over the sample. The instrument control software was
Advantage for Q Series
v2.8Ø392 and Thermal Advantage v4.8.3 and the data were analyzed using
Universal
Analysis v4.3A. For modulated DSC, the sample was prepared as before, and the
pan was
heated at 2 0C min from 25 C to 200 C. Modulator conditions were an
amplitude of
0.20 C and a periodicity of 40 s. The sampling interval was 1 sec/pt.
30 TGA data were collected on a TA Instruments Q500 TGA, equipped with a 16
position autosampler. The instrument was temperature calibrated using
certified Alumel.
Typically 5-10 mg of each sample was loaded onto a pre-tared platinum crucible
and
aluminum DSC pan, and was heated at 10 C.min-lfrom ambient temperature to 350
C.
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
A nitrogen purge at 60 ml.min-1 was maintained over the sample. The instrument
control
software was Advantage for Q Series v2.8Ø392 and Thermal Advantage v4.8.3.
Thermal analysis of batch 1 was performed by DSC and TGA. The TGA trace
(see Figure 19) shows a weight loss of 1.7% between ambient temperature and
121 C,
which is likely to be loss of water. The DSC trace (see Figure 19) shows a
broad low
temperature endotherm, probably corresponding to the loss of water, followed
by a small
endotherm with onset at 94 C.
This second endotherm might indicate a glass transition and was further
investigated by modulated DSC (see Figure 20). This technique enables
reversible events,
1 0 such as a glass transition, to be separated from irreversible ones,
such as loss of solvent or
a melt of a crystalline form. The reversible heat flow trace in modulated DSC
shows the
glass transition as a step with an inflexion point (Tg) at 95 C. This is high
for a glass
transition and suggests that Form 1 is stable. The small endotherm with onset
at 89 C on
the non-reversible heat flow trace corresponds to molecular relaxation of the
bulk
1 5 material at the glass transition temperature.
The DSC trace (see Figure 19) shows decomposition starting around 220 C,
which also corresponds to the TGA trace curving down.
The TGA traces of batches 1, 2, 3, 4, 5, and 6 are of similar shape (Figure
21).
The weight losses measured between ambient and 120 C are shown in Table K.
They are
20 consistent with the varying amounts of water observed by NMR. These
amounts were
further quantified by Karl Fischer (KF) water titration. See water
determination by KF.
Table K: Summary of TGA weight losses of received samples
Batch number Weight loss by TGA
1 1.7%
2 0.6%
3 1.2%
4 0.9%
5 1.5%
6 1.6%
Figure 22 shows the DSC traces of the six batches for comparison. The traces
are
25 similar, with a broad low temperature endotherm of varying size,
consistent with varying
amounts of water, followed by a small endotherm around the glass transition
temperature
as seen in section DSC and TGA. The results are summarized in Table L.
Table L: Summary of DSC results of received samples
86
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Batch 1st endotherm, broad 2nd endotherm, small Start of
number decomposition
1 28.3J/g, Tmax = 64 C 1.2J/g, Tonset =94 C 220 C
2 7.4J/g, Tmax =48 C 1.4J/g, Tonset =94 C 220 C
3 none 2.0J/g, Tonset =89 C 175 C
4 14.5J/g, Tmax = 58 C 1.3J/g, Tonset =94 C 200 C
12.2J/g, Tmax = 59 C 1.2J/g, Tonset =94 C 175 C
6 28.7J/g, Tmax =59 C 1.5J/g, Tonset =94 C 200 C
Polarized Light Microscopy (PLM)
Samples were studied on a Leica LM/DM polarized light microscope with a
digital video camera for image capture. A small amount of each sample was
placed on a
5 glass slide, mounted in silicone oil and covered with a glass slip, the
individual particles
being separated as well as possible. The sample was viewed with appropriate
magnification and partially polarized light, coupled to a)\, false-color
filter.
Figures 23A-23F show that batches 1, 2, 3, 4, 5, and 6 are material made up of
large hard agglomerates of small irregular particles. Batches 1, 2, 3, 4, 5,
and 6 all look
similar. No birefringence was observed under plane polarized light, which is
consistent
with the material being non-crystalline. Particle size ranges from less than
l[tm to 3 p.m.
The small size of these particles suggests that they have been precipitated
out very
quickly.
Gravimetric Vapor Sorption (GVS)
Sorption isotherms were obtained using a SMS DVS Intrinsic moisture sorption
analyzer, controlled by SMS Analysis Suite software. The sample temperature
was
maintained at 25 C by the instrument controls. The humidity was controlled by
mixing
streams of dry and wet nitrogen, with a total flow rate of 200 ml.min-1. The
relative
humidity was measured by a calibrated Rotronic probe (dynamic range of 1.0-100
%
RH), located near the sample. The weight change, (mass relaxation) of the
sample as a
function of % RH was constantly monitored by the microbalance (accuracy
0.005 mg).
Typically 5-20 mg of sample was placed in a tared mesh stainless steel basket
under ambient conditions. The sample was loaded and unloaded at 40% RH and 25
C
(typical room conditions). A moisture sorption isotherm was performed as
outlined below
(2 scans giving 1 complete cycle). The standard isotherm was performed at 25
C at 10%
RH intervals over a 0.5-90 % RH range.
Table M
87
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
P arameters Values
Adsotption - Scan 1 40 - 90
Desorption Adsorption - Scan 2 85 - Dry, Dry - 40
Intervais a RH) I 0
Number of Scans
Flow rate (mi.min-') 200
Temperature r'(7 25
Saiiìii aì
Sorption Tim rs) hoar time out
The Gravimetric Vapor Sorption (GVS) isotherm was obtained for batch 1 at 25
C and is shown in Figure 24. The sample appears to be moderately hygroscopic,
with a
total weight change of 3.8% from 0 to 90% relative humidity (RH). The
hysteresis (area
between adsorption and desorption curves) is small, indicating that the solid
releases quite
readily the water adsorbed. No formation of hydrate is observed. There was no
significant
weight change after the whole experiment (0.3%).
The kinetics plot of the GVS (Figure 25) shows that the adsorption of the
water
occurred mostly at very high humidities and the desorption at very low
humidities. On the
adsorption phase, the sample reached equilibrium quite quickly up to 80% RH
and took
longer to equilibrate at 90% RH. On desorption, the mass stabilized at all
steps.
After completion of the GVS, the sample was recovered and reanalyzed by
XRPD, which showed that the material was still non-crystalline (Figure 26).
Water Determination by Karl Fischer (KF)
The water content of each sample was measured on a Mettler Toledo DL39
Coulometer using Hydranal Coulomat AG reagent and an argon purge. Weighed
solid
samples were introduced into the vessel on a platinum TGA pan, which was
connected to
a subaseal to avoid water ingress. Approximately 10 mg of sample was used per
titration
and duplicate determinations were made.
Titration of water by coulometric Karl Fischer gave a result of 2.4 wt% water.
This is slightly higher than the weight loss observed by TGA. It could mean
that some of
the water is not released from the material on heating, but it is likely to be
due to the
different experimental procedures for these two techniques.
The water content of each batch was determined by coulometric Karl Fischer.
Table N shows these results and compares them with earlier Karl Fischer
results obtained
and with the weight losses observed by TGA. Data are consistent as the trend
is the same
in all three analyses. The Karl Fischer data obtained earlier show lower
amounts of water
88
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
than the results obtained here. This is consistent with the material being
hygroscopic,
although some samples have taken up more water than others. TGA weight loss is
consistently lower than the results obtained by Karl Fischer titration, which
might mean
that some water stays trapped in the material and is not released on heating
but might also
be due to the experimental procedure.
Table N: Karl Fischer (KF) results and summary of water content data
Batch number KF water content Earlier KF results TGA weight loss
1 2.4% 2.1% 1.7%
2 1.9% 0.4% 0.6%
3 2.5% 1.4% 1.2%
4 2.2% 0.92% 0.9%
5 2.3% 0.53% 1.5%
6 2.8% 2.1% 1.6%
pKa Determination and Prediction
pKa determination data were collected on a Sirius GlpKa instrument with a D-
PAS attachment. Measurements were made at 25 C in aqueous solution by UV and
in
methanol water mixtures by potentiometry. The titration media was ionic-
strength
adjusted (ISA) with 0.15 M KC1 (aq). The values found in the methanol water
mixtures
were corrected to 0 % co-solvent via a Yasuda-Shedlovsky extrapolation. The
data were
refined using Refinement Pro software v1Ø Prediction of pKa values was made
using
ACD pKa prediction software v9.
HC
9 Ac c
- Predicted 4.76
OH Measured (Pat): 4.82
C H3 Using ACD )
,-;
The pKa of obeticholic acid was measured by potentiometry using methanol as a
cosolvent (Figure 27) and extrapolated to 0% co-solvent using a Yasuda-
Shedlovsky
extrapolation (Figure 28). The pKa enables determination of the proportion of
the neutral
and the ionized form of the compound at a given pH. Figure 29 shows the
distribution of
89
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
the species depending on pH.
Log P Determination
Data were collected by potentiometric titration on a Sirius GlpKa instrument
using
three ratios of octanol: ionic-strength adjusted (ISA) water to generate Log
P, Log Pion,
and Log D values. The data were refined using Refinement Pro software v1Ø
Prediction
of Log P values was made using ACD v9 and Syracuse KOWWIN v1.67 software.
Table 0: Predicted and measured LogP
ACID (V9) Predicted LogP
Measured LogP 5.54
Me ZAS ured Log Pion 1,58
Measured Log-D7.4 2,98
LogP was predicted using ACD software then measured by potentiometry. Three
titrations were performed at three different octanol / ISA water ratios,
giving the
difference curve plotted in Figure 30. The black curve is the pure aqueous pKa
titration
and the 3 colored curves correspond to the three octanol / ISA water ratios.
The shifts in
pKa enable determination of LogP.
The lipophilicity curve (logD as a function of pH) is shown in Figure 31. Log
D is
the distribution coefficient, representing the combined lipophilicity of all
species present
at a specific pH. LogP is a compound constant, which corresponds to the
partition
coefficient of the pure neutral species, while LogPion is that of the pure
ionized species.
LogP and LogPion can be determined from the lipophilicity curve, as the
intersection of
the Y axis with respectively the tangent at the start of the pH scale (when
the molecule is
purely in its neutral form) and the tangent at the end of the pH scale (when
the molecule
is completely ionized).
Two Weeks Stability at 40 C & 75% RH and 25 C & 97% RH
A sample of batch 1 was stored at 40 C and 75% relative humidity (RH) in an
accelerated stability testing of the solid form. Another sample was stored at
25 C and
97% relative humidity to check the effect of very high humidity. Both samples
were re-
analyzed by XRPD after five days and after two weeks. Both samples remained
non-
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
crystalline under the two storage conditions for up to two weeks, showing that
Form 1 is
stable to these conditions. See Figure 32 and Figure 33.
The six batches analyzed were all non-crystalline. The glass transition
temperature
was measured at 95 C with a modulated DSC experiment. The six batches appeared
very
similar with all analytical techniques used, the only difference between them
being their
water content, which varied from 1.9% to 2.8% by Karl Fischer titration.
Thermal
analysis showed the varying amount of water and indicated decomposition
starting
around 175-220 C. Measured pKa was 4.82 and LogP is 5.54. Microscopic
evaluation
showed large hard agglomerates of very small irregular particles.
Stability testing showed that the material was still non-crystalline after two
weeks
under accelerated conditions (40 C / 75% RH) or under high humidity (25 C /
97% RH).
Gravimetric Vapor Sorption (GVS) analysis showed the material is only
moderately
hygroscopic, with a total weight gain of 3.8% from 0 to 90% relative humidity
(RH). No
hydrate formation was observed under GVS. The sample re-analyzed by XRPD after
GVS was still non-crystalline. The high glass transition temperature and the
stability
testing results suggest that the non-crystalline form is stable.
EXAMPLE 6: Single Crystal X-ray Structure and Absolute Stereochemistry
The single crystal X-ray structure of obeticholic acid was determined from a
crystal
obtained from the recrystallization of obeticholic acid from an acetonitrile
solution after
cooling to 5 C at 0.1 C/min followed by maturation at RT/50 C 8 h cycles for 1
week
(see Figure 34). The structure is consistent with Form G and a simulated XRPD
pattern
has been generated as a reference pattern for this material. Form G can be
prepared by
cooling a solution of obeticholic acid in e.g., acetonitrile.
The structure is orthorhombic, space group P212121, and contains one molecule
of
obeticholic acid in the asymmetric unit. Final R1 [I>2o-(01 = 3.22 %. The
crystal
exhibited prism morphology of approximate dimensions 0.4 x 0.4 x 0.3mm. The
absolute
stereochemistry of the molecule was determined as S at chiral centers C5, C9,
C10 and
C14 and R at chiral centers C3, C6, C7, C8, C13, C17 and C22 with a Flack
parameter = -
0.01 (13). For the inverted structure with chiral centers C5, C9, C10 and C14
in the R
configuration and chiral centers C3, C6, C7, C8, C13, C17 and C22 in the S
91
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
configuration, the Flack parameter = 1.01(13), confirming the assignation
mentioned
above.
Overall, the structure had a strong data set and no disorder.
COOH
H
HO% 'OH
H
CH3
The software used to assign the stereochemistry (PLATON) determines the chiral
center (C8) as an R stereocenter, whereas ACD software (and the Cahn-Ingold-
Prelog)
assignment for (C8) is S. However, the assignment of the trans ring junction
for B/C ring
system is absolutely defined from the crystal structure.
Determination of the absolute structure using Bayesian statistics on Bijvoet
differences, (Hooft et al., i Appl. Cryst, (2008), 41, 96-103), reveals that
the probability
of the absolute structure as presented being correct is 1.000, while the
probabilities of the
absolute structure being a racemic twin or false are 0.000 and 0.000
respectively. The
Flack equivalent and its uncertainty are calculated through this program to be
-0.019(17).
The structure of obeticholic acid contains one 5 membered ring and 3 six
membered rings which are fused together. Conformational analysis on the 5
membered
ring (C13, C14, C15, C16 and C17)) reveals that the closest puckering
descriptor for this
ring is a half-chair. Conformational analysis on the three 6 membered rings
(C1, C2, C3,
C4, C5 and C10); (C5, C6, C7, C8, C9 and C10) and (C 8, C9, C11, C12 C13 and
C14)
reveals that the closest puckering descriptor for these rings is a chair.
Two unique intermolecular hydrogen bonds are observed in the crystal
structure.
Each molecule of obeticholic acid forms a hydrogen bond to two different
symmetry
related molecules of obeticholic acid, with the oxygens, 01 and 04, acting as
donors to
the oxygens, 03 and 01 respectively, acting as acceptors, 01¨ H1C---03 [D= =
=A =
2.7419(12)A1 and 04¨ H4C---01 [D= = =A = 2.6053(13)A (see Figure 35). These
interactions result in a complex 3 dimensional hydrogen bonded network. The
final
92
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Fourier difference map shows maximal and minimal electron densities of 0.402
and -
0.176 eA-3, respectively.
An overlay of the calculated XRPD pattern for the structure with the
experimental
batches shows that the crystal is consistent with the bulk and is obeticholic
acid Form G
(see Figure 36).
Table 1. Crystal data for obeticholic acid Form G
Crystallization solvents Acetonitrile
Crystallization method Maturation at RT/50 C
Empirical formula C26 H44 04
1 0 Formula weight 420.63
Temperature 100(2) K
Wavelength 1.54178 A
Crystal size 0.40 x 0.40 x 0.30 mm
Crystal habit Colourless Prism
1 5 Crystal system Orthorhombic
Space group P212121
Unit cell dimensions a = 8.72510(10) A a = 90
b = 12.69860(10) A (3 = 90
c = 22.5408(2) A y = 90
20 Volume 2497.44(4) A3
4
Density (calculated) 1.119 Mg/m3
Absorption coefficient 0.574 mm-1
F(000) 928
Table 2. Data collection and structure refinement for obeticholic acid Form G
Diffractometer SuperNova, Dual, Cu at zero, Atlas
Radiation source SuperNova (Cu) X-ray Source, CuKa
Data collection method omega scans
Theta range for data collection 9.15 to 74.49
Index ranges
Reflections collected 50001
Independent reflections 5073 [R(int) = 0.02201
Coverage of independent reflections 99.4 %
Variation in check reflections N/A
93
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Absorption correction Semi-empirical from equivalents
Max. and min. transmission 1.00000 and 0.78605
Structure solution technique direct
Structure solution program SHELXTL (Sheldrick, 2001)
Refinement technique Full-matrix least-squares on F2
Refinement program SHELXTL (Sheldrick, 2001)
Function minimized D4,(F02_Fc2)2
Data / restraints / parameters 5073 / 0 / 286
Goodness-of-fit on F2 1.060
A/Gmax 0.001
Final R indices
5039 data; I>13(I) R1 = 0.0320, wR2 = 0.0859
all data R1 = 0.0322, wR2 = 0.0861
Weighting scheme calc w=1 / [c52 (F02)+(
0.0503P)2+0.5520131
1 5 where P=(F02+2Fc2)/3
Absolute structure parameter -0.01(13)
Largest diff peak and hole 0.402 and -0.176 eA-3
Refinement summary of the structure is as follows:
Ordered Non-H atoms, XYZ Freely refining
Ordered Non-H atoms, U Anisotropic
H atoms (on carbon), XYZ Idealized positions riding on attached atoms
H atoms (on carbon), U Appropriate multiple of U(eq) for bonded atom
H atoms (on heteroatoms), XYZ Freely refining
H atoms (on heteroatoms), U Isotropic
Disordered atoms, OCC No disorder
Disordered atoms, XYZ No disorder
Disordered atoms, U No disorder
Example 7: Bioavailability Difference Between Obeticholic Acid Form 1 (non-
crystalline) and Crystalline (Form F) Forms
The physical state of a solid obeticholic acid can play a role in the
bioavailability
of the molecule when administered orally to a subject (e.g., rats). The study
described
below was carried out to evaluate the plasma kinetics after a single oral
administration
and the efficiency of the intestinal absorption and the pharmacokinetics of
solid non-
crystalline and crystalline forms of obeticholic acid. The profiles of
obeticholic acid
94
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
plasma concentration vs time, the tmax, Cmax and AUC after administration of
obeticholic
acid Form 1 (non-crystalline) or Form F were compared (see Figures 37-38)
Obeticholic acid Form 1 (non-crystalline) and Form F were administered to rats
and in each animal blood was collected at different period of times for at
least 3 hours.
Six animals were studied for each form of obeticholic acid.
Experimental protocol:
The test substance used was obeticholic acid Form 1 (non-crystalline) and
crystalline Form F. Form F can be prepared by maturation from acetonitrile or
nitromethane. The formulation was prepared as a suspension in water at pH 4.
The study
model is adult male Sprague Dawley rats about 225 to about 250 g (Harlan
Laboratories).
Six animals were used per dosage route. The dosage is PO 20 mg/kg/5 mL. The
animals
were fasted overnight before treatment with the formulation of obeticholic
acid. Oral
administration was performed by gastric gavage.
On day one animals were be fitted with a cannula implanted in the left jugular
vein (SOP VIVO/SAF6), anaesthesia was obtained by Isoflurane. The experiment
was
started after one day of recovery from surgery. About 500 L of blood (250 L
of
plasma) was taken via cannula in a heparinised syringe (Na Heparin) and
collected
immediately in microtubes in an ice/water bath. Within 1 hour, samples were
centrifuged
at 10000 x g for 5 minutes at 4 C. Plasma was immediately transferred in
microtubes and
stored at ¨20 C. Samples of blood were collected 30 minutes, 1 hour, 1.3
hour, 2 hours,
and 3 hour post-dose. Plasma samples were analyzed using the HPLC-ES/MS/MS
quantitative method. Pharmacokinetics study was preformed using non-
compartmental
methods.
Results:
The mean plasma concentrations of obeticholic after 20 mg/Kg b.w oral single
dose administration of the two solid forms are reported in Figure 37. Values
are the mean
of six set of experiments for each formulation. The standard deviations are
reported in the
graph.
After administration of the crystalline form the Cmax is achieved after 1.5
hours and the
plasma obeticholic acid concentration follows a regular kinetics with one
maximum value
and after 3 hours the dose is almost half of the Cmax.
The kinetics profile after the administration of obeticholic acid Form 1 (non-
crystalline) Form 1 is different from that of the crystalline Form F. An early
plasma
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
concentration peak is obtained after 30 minutes and a second one after 2
hours. The
variability of the data in the 6 rats is very low and this behaviour is
statistically different
from that of the crystalline form. The AUC for the three hours studied is
higher for the
crystalline form. The kinetics suggest that the obeticholic acid is still
present in plasma
after 3 hours. It has previously been demonstrated that the passage of
obeticholic acid
through the liver produce the hepatic metabolite tauro conjugate, which is
secreted into
bile and accumulate in the enterohepatic circulation. Thus, the measurement of
the tauro
conjugate can be used to determine the passage of the amount of obeticholic
acid through
the liver. The rate of tauro conjugate formation is reported in Figure 38,
which shows
that the tauro conjugate formation is faster and a higher concentration is
achieved after
administration of the crystalline form.
Melting point and glass transition
The melting point of obeticholic acid Form 1 (non-crystalline) Form 1 and
crystalline Form F were measured using a conventional method. The melting
point of
Chenodeoxycholic acid and Ursodeoxycholic acid were measured as reference
compounds. Measurements have been performed in triplicate. For the crystalline
form the
transition from the solid to liquid state is defined as melting
temperature(Tm) while for the
non-crystalline form is defined as glass temperature transition (Tg), In the
table are
reported the measured values expressed in both Celsius C and Kelvin K.
Table 3: Melting points of obeticholic acid (Form 1 and Form F) and CDCA and
UDCA
Experimental data Literature data
Compound T( C) Tg ( C) T( C) Tg ( C)
119
CDCA 136-140 143 98
163
UDCA 203-207 203 105
Obeticholic 120-124
108-112
acid 235-237
Experimental data Literature data
96
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Compound Tm ( K) Tg ( K) Tg/Tm ( K) Tm ( K) Tg ( K) Tg/Tm ( K)
392
CDCA 409-413 416 371 0,85
436
UDCA 476-480 477 378 0,79
Obeticholic 393-397
381-385 0,75 0,75
acid 508-510
Results:
The values obtained for CDCA and UDCA agree with those previously reported,
where the melting point of UDCA is higher than that of CDCA. The transition
glass
temperature Tg of Form 1 (102-112 C) is lower than the melting point
temperature Tm
of Form F (120-124 C). This observed pattern agrees with previous reported
data when
the two solid state forms are compared. Form F has an additional transition at
a higher
temperature (235-237 C).
1 0 The ratio between the highest melting point temperature and the glass
transition
temperature expressed in Kelvin degree is quite similar to other drugs and
other bile
acids. (J. Kerc et al. Thermochim. Acta, 1995 (248) 81-95).
Differential scanning calorimetry analysis
1 5 Differential scanning calorimetry (DSC) analysis was carried out to
better define
the melting points and the physical state of obeticholic acid crystalline and
non-crystalline
forms. The instrument used was a Mettler Toledo DSC model 821e. Approximately
4-5
mg of each Form 1 and Form F were submitted to analysis. The compounds were
exposed
to the temperature range of 30-300 C at 10 C/min heating rate.
20 Figure 39 shows the DSC curve obtained for obeticholic acid crystalline
Form F.
One endothermic transition at 120.04 C was detected corresponding to the
melting point
of the compound. This result was confirmed also by hot stage microscopy (HSM);
in the
range 30 -240 C the solid-liquid transition observed was at 122-124 C. In
the DSC
trace, the peak shape and intensity obtained for Form F are in agreement with
typical
25 behaviour showed by crystalline forms. However, the peak width is rather
broad; this can
be due to not homogeneous crystals. Thermo gravimetric analysis (TGA) did not
show
any weight loss in the 30-300 C temperature range.
97
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Figure 40 shows the DSC curve obtained for obeticholic acid non-crystalline
Form 1. One endothermic transition at 79.95 C was observed. Peak shape and
intensity
are in agreement with behaviour expected for non-crystalline compounds. For
these
substances energy required for solid-liquid transition (glass transition) is
less than for
crystalline compounds. The thermogram did not show any weight loss in the 30-
300 C
temperature range.
Water solubility
The water solubility of obeticholic acid Form 1 (non-crystalline) and
crystalline
Form F was measured following procedures known in the art. Briefly, the solid
was
suspended in water at a low pH (HC1 0.1 mol/L) and left to equilibrate at 25
C for one
week under slightly mixing. The saturate solution was filtered and the
concentration of
the compound in solution measured by HPLC-ES-MS/MS.
1 5 Results:
Water solubility (pmol/L)
Form 1 17.9
Form F 9.1
Form 1 present a higher solubility 17.9 p,mol/L vs. 9.1 p,mol/L for Form F.
According to the bioavailability data of the obeticholic acid, crystalline
Form F is
higher than the obeticholic acid Form 1 (non-crystalline). Despite an earlier
plasma
concentration peak after administration of the Form 1, the plasma profiles
show that the
Form F is absorbed more efficiently (higher AUC) and even the kinetics is more
regular,
reflecting an optimal distribution of the drug in the intestinal content. Form
1 shows this
early peak then a later second one with a Cmax lower that than of Form F.
The water solubility of the Form 1 is higher than that of Form F. Form F
appears
to be stable as the thermo gravimetric analysis (TGA) did not show any weight
loss in the
temperature range studied.
According to these results, Form F when administered orally appears more
efficiently absorbed by the intestine and taken up by the liver. The rate of
formation of
the main hepatic metabolite tauro conjugate is almost twice for Form F
compared to Form
1, suggesting a more efficient transport and accumulation in the enterohepatic
circulation
and the plasma concentration after 3 hours.
98
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Example 8: Preparation of Radiolabeled Obeticholic Acid
Radiolabeled obeticholic acid was prepared according to the scheme below.
Scheme 5
OMe OMe
LDA, TMSCI
THF, -780C .40
TMSOss' 0 TMSOµ$ S
' OTM
1X 2X
0
0
0
OMe 5 OH
* H
11
NaOH
BF3 OEt2
DC-toluene 55 Me0H 55o*
HO". 0 45 C õn 0
3X 4X
0
1) H2, Pd/C
OH NaBH4 OH
Na0H, H20 NaOH
HO5
.00 H20
0 100 C H0`µµ
5X [ethyl-1-14C]obeticholic acid
*=14c
NMR spectra were recorded in CDC13 and Me0D-d4 solution in 5-mm o.d. tubes
(Norell, Inc. 507-HP) at 30 C and were collected on Varian VNMRS-400 at 400
MHz
for The chemical shifts (6) are relative to tetramethylsilane (TMS =
0.00 ppm) and
expressed in ppm. LC-MS/MS was taken on Ion-trap Mass Spectrometer on Accela-
Thermo Finnigan LCQ Fleet operating EST (-) ionization mode. HPLC was taken on
Agilent 1200 series (Column: Xterra MS C8, 250 x 4.6 mm, 5 p.m, 40 C) in line
(3-Ram.
Specific activity was taken on LSA (Liquid Scintillation Analyzer, Perkin
Elmer, Tri-
Carb 2900TR).
Preparation of compound 2X
99
CA 03009149 2018-06-19
WO 2017/111979 PCT/US2016/012651
0 0
OMe
OMe
LDA, TMSCI
.0w
THF, -78 C s.O.1
TMSO's TMS0µ OTMS
1X 2X
To a solution of diisopropylamine (1.59 g, 15.8 mmol) in dry THF (6.0 mL) was
added n-BuLi (6.30 mL, 2.5 M, 15.8 mmol) at -20 C. After stirring the
reaction mixture
for 1 h at -20 C, cooled to -78 C and TMSC1 (1.72 g, 15.8 mmol) was added
followed
by compound 1X (3.00 g, 6.29 mmol) in dry THF (6.0 mL). The reaction mixture
was
stirred for 1 h at -78 C, quenched by addition of NaHCO3 and stirred for 30
min at room
temperature. The organic layer was separated and concentrated in vacuo to give
the
compound 2X (3.29 g, 95%) and used for next step without further purification.
1 0 Preparation of compound 3X
0
0 0
OMe
* H
____________________________________________ 00
,0111111 OMe
BF3 OEt2
DCM-toluene
.OW HO's 0
TMSO OTMS
2X * = 14c
3X
The [1-14Clacetaldehyde (330 mCi, 5.63 minol) (prepared from [14C1BaCO3,
SA = 58.6 mCi/mmol) in toluene (1.0 mL) and acetaldehyde (130 mg, 2.95 mmol)
in
DCM (2.0 mL) were mixed at -78 C and then transferred to a solution of
compound 2X
1 5 (3.29 g, 6.00 mmol) in DCM (13.0 mL) followed by addition of BF3.0Et2
(1.05 g, 7.40
mmol) at -78 C. After stirring for 1 h at -78 C, the reaction mixture was
allowed to
warm up to 35 C and stirred for 1 h at the above temperature. The reaction
was quenched
by addition of water (10 mL), the aqueous layer was extracted with DCM, the
combined
organic layer was dried over anhydrous Na2SO4, filtered and concentrated in
vacuo The
20 residue was purified by column chromatography on Si02 (Hexane: Et0Ac =
5:1 to 3:1) to
give the compound 3X (102 mCi, 31%, SAW 37.0 mCi/mmol) as a white solid.
1H-NMR (CDC13, Varian, 400 MHz): 8 0.65 (3H, s); 0.93 (3H, d, J= 6.0 Hz), 1.01
(3H,
s), 1.06-1.49 (12H, m), 1.62-2.04 (7H, m), 1.69 (3H, d, J= 6.8 Hz), 2.18-2.28
(2H, m),
100
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
2.32-2.43 (2H, m), 2.58 (1H, dd, J= 12.8, 4.0 Hz), 3.62-3.70 (1H, m), 3.67
(3H, s), 6.18
(1H, q, J= 6.8 Hz).
Preparation of compound 4X
0
0
NaOH Olt OH
H0"
o
.** OMe
HO% Me0H ,OO
45 C HON'
I
* = 14c
3X 4X
To a solution of compound 3X (102 mCi, 2.75 mmol) in Me0H (6.0 mL) was
added NaOH (220 mg, 5.50 mmol) in H20 (3.0 mL) at room temperature. After
stirring
the reaction mixture for 1 h at 45 C, cooled to room temperature, Me0H was
removed
under reduced pressure and diluted with H20 (12 mL). The aqueous layer was
acidified
1 0 with H3PO4, extracted with DCM and the organic layer was concentrated
in vacuo. The
residue was suspended in Et20 and the precipitate was collected by filtration
to give the
compound 4X (86.3 mCi, 85%) as a white solid.
1H-NMR (CDCI3, Varian, 400 MHz); 8 0.63 (3H, s), 0.92 (3H, d, J= 6.0 Hz), 0.99
(3H, s),
1.04-1.50 (13H, m), 1.61-2.01 (7H, m), 1.67 (3H, d, J= 7.2 Hz), 2.21-2.28 (2H,
m), 2.35-
1 5 2.41 (2H, m), 2.56 (1H, dd, J= 12.8, 4.0 Hz), 3.58-3.69 (1H, m), 6.16
(1H, q,J= 7.2 Hz).
Preparation of compound 5X
0 0
OH 1) H2, Pd/C
OH
NaOH, H20
OO 2) 100 C 0*
HO" I CI HO"' . 0
* = 14c
4X 5X
The mixture of compound 4X (86.3 mCi, 2.35 mmol) and 5% - Pd/C (100 mg) in
20 aq. 0.5 M NaOH (10 mL, 5.0 mmol) was stirred for 10 h at room
temperature under H2
atmosphere (balloon) and then stirred for 14 h at 100 C. The catalyst was
removed by
filtration, washed with water and the filtrate was acidified with H3PO4. The
precipitates
101
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
was collected by filtration, the solid was dissolved in Et0Ac, washed with
brine, filtered
through a short pad of Si02 and concentrated in vacuo. The residual solid was
recrystallization with Et0Ac to give the compound 5X (67.7 mCi, 78%) as a
white solid.
1H-NMR (Me0D-d4, Varian, 400 MHz): 8 0.71 (311, s), 0.75-0.84 (1H, m), 0.81
(3H, t,
J = 7.4 Hz), 0.921.01 (1H, m), 0.96 (3H, d, J= 6.4 Hz), 1.06-1.38 (7H, m),
1.25 (3H, s),
1.41-1.96 (1211, m), 2.01-2.05 (1H, m), 2.11-2.24 (2H, m), 2.30-2.37 (1H, m),
2.50 (1H,
t, J= 11.4 Hz), 2.80-2.85 (1H, m), 3.42-3.49 (1H, m).
Preparation of [ethyl-1-14Clobeticholic acid
0 0
OH NaBH4
OH
NaOH
.00 0 H20
HOµ' .00.
1000c HO's
.
*
[ethyl-1-14C]obeticholic acid
5X * = 14c
To a solution of compound 5X (67.7 mCi, 1.83 mmol) in aq. 2 M NaOH (4.50
mL, 9.00 mmol) was added a solution of NaBH4 (416 mg, 11.0 mmol) in 1120 (2.0
ml) at
80 C. After stirring the reaction mixture for 2 h at 100 C, water (6.0 mL)
was added at
room temperature and acidified with H3PO4. The aqueous layer was extracted
with DCM,
dried over anhydrous Na2SO4, filtered through a short pad of Si02 and
concentrated in
vacuo, The residue was purified by column chromatography on 5i02 (Hexane:Et0Ac
=
1:1 to 1:3) to give the product (44.0 mCi, 65%) as a white solid. The product
(44.0 mCi,
1.19 mmol) and obeticholic acid (120 mg, 0.285 mmol) were dissolved in Et0Ac
(4 mL),
the solution was stirred for 2 h at 50 C and then concentrated in vacuo. The
residual oil
suspended in Et20, the precipitate was collected by filtration to give the
[ethy1-1-
14Clobeticholic acid (560 mg, 38.5 mCi, SA = 29 mCi/ mmol) as a white solid.
(CDC13, Varian, 400 MHz): 8 0.66 (3H, s), 0.88 (311, s), 0.93 (3H, t, J = 7.2
Hz), 0.93 (3H, d, / = 6.4 Hz), 0.96-1.04 (1H, m), 1.08-1.52(14H, m), 1.51-
1.60(1011,
m), 2.22-2.30 (111, m), 2.36-2.44 (1H, m), 3.38-3.45 (111, m), 3.71 (IH, s).
LC-MS/MS (MS: LCQ Fleet): MS Calcd.: 421.56; MS Found: 421.07 [M-Ht.
Radio TLC: TLC plate of silica 60 F254, and mobile phase is Et0Ac.
Radiochemical purity is
98.90%, Rf= 0.675
102
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
HPLC (Agilent 1200 series): Mobile phase; acetonitrile: 5 mM Phosphate buffer
(pH =
3):Me0H = 450:450:100. Radiochemical purity is 98.19% (0-ram), Rt = 20.00 min.
[Ethyl-1-14Clobeticholic acid has a molecular formula of 14C1C25H4404 and a
molecular
weight of 421.46 at the specific activity of 29 mCi/mmol by LSC.
Example 9. Synthesis of Crystalline Obeticholic Acid
The procedures for the selective synthesis of Forms A, C, D, F, G, and I were
identified and listed below. The results of these experiments are provided in
Table 5.
1 0 Procedure 1: Cooling then Maturation
Amorphous obeticholic acid (approximately 30 mg) was weighed into a glass vial
and warmed to 50 C. 2 volume (60 ill) portions of acetonitrile were added at
50 C.
Initially the sample formed a gum, which dissolved after the addition of 14
volumes of
solvent. On stirring flakes of solid precipitate were observed. The sample was
cooled to
5 C using a linear cooling rate of 0.25 C/min. The resulting suspension was
analyzed
by XRPD and was then matured (shaken in 8 hour cycles at 50 C/RT). After 5
days of
maturation the sample was equilibrated to room temperature and was re-analyzed
by
XRPD.
Procedure 2: Cooling
Amorphous obeticholic acid (approximately 30 mg) was weighed into glass vials
and warmed to 50 C. Solvent was added (as per Table 5 and observations were
made.
Samples were stirred at 50 C for 1 hour then were cooled to 5 C using a
linear cooling
rate of 0.1 C/min. Samples were stirred at 5 C for 2 days. Any solids were
filtered, air
dried and analyzed by XRPD. Solutions were stored in the freezer for ¨48
hours, and
then were left to evaporate at ambient conditions. Solids were analyzed by
XRPD.
Procedure 3: Anti-Solvent Addition then Cooling
Amorphous obeticholic acid (approximately 30 mg) was weighed into glass vials
and warmed to 50 C. Solvent was added (as per Table 5) to dissolve the
amorphous
material and then anti-solvent (heptane, volumes as per Table 5) was added and
observations were made. Samples were stirred at 50 C for 1 hour then were
cooled to 5
C using a linear cooling rate of 0.1 C/min. Samples were stirred at 5 C for
2 days.
Solids were filtered, air dried and analyzed by XRPD.
103
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
Procedure 4: Maturation
Amorphous obeticholic acid (approximately 30 mg) was weighed into glass vials
and warmed to 50 C. Solvent was added (as per Table 5) to dissolve the
amorphous
material. Samples were stirred at 50 C for 1 hour then were matured (shaken
in 8 hour
cycles at 50 C/RT). After 3 days samples containing solids were filtered, air
dried and
analyzed by XRPD. Samples that were solutions were matured with a cooler upper
temperature (stirred in 4 hour cycles at 40 C/RT, Procedure 4b) and any that
formed
solids were analyzed by XRPD after 1 and 2 days.
Procedure 5: Anti-solvent then Cooling
Amorphous obeticholic acid (approximately 30 mg) was weighed into a glass vial
and warmed to 50 C. MTBE (90 [1.1, 3 volumes) was added to dissolve the
amorphous
material and then anti-solvent (heptane, 90 [1.1, 3 volumes) was added and
observations
were made. The sample was stirred at 50 C for 1 hour then was cooled to 25 C
using a
linear cooling rate of 0.2 C/min. After ¨ 30 minutes at 25 C the resulting
solid was
filtered, air dried and analyzed by XRPD.
Procedure 6: Maturation
Amorphous obeticholic acid (approximately 30 mg) was weighed into a glass vial
and warmed to 50 C. Anisole (90 [1.1, 3 volumes) was added to dissolve the
amorphous
material. The sample was matured (shaken in 4 hour cycles at 40 C/RT) for a
total of 17
days.
Procedure 7: Cold Slurry
Amorphous obeticholic acid (approximately 30 mg) was weighed into a glass vial
and cooled to 5 C. Acetonitrile/water mixtures (as per, pre-cooled to 5 C)
were added
and samples were stirred at 5 C overnight. Observations were made and solids
were
filtered, air dried and analyzed by XRPD. Some solids were large lumps which
were
crushed prior to XRPD analysis. One sample was a sticky solid so was left to
stir at 5 C
for a total of 17 days, forming a hard lump of solid, which was crushed and
analyzed by
XRPD.
Procedure 8: Cooling
Amorphous obeticholic acid (approximately 30 mg) was weighed into glass vials
and warmed to 50 C. Solvent (see Table 5) was added to dissolve the amorphous
material the samples were stirred at 50 C for 1 hour. The samples were cooled
to -20 C
using a linear cooling rate of 0.1 C/min. After stirring at -20 C overnight
the samples
104
CA 03009149 2018-06-19
WO 2017/111979 PCT/US2016/012651
were equilibrated to room temperature and resulting solids were filtered, air
dried and
analyzed by XRPD.
Table 5: Summary of Methods to Synthesize Crystalline obeticholic acid and
Results
Volume AntiSol Volume Crystalline
Procedure Solvent
( L) vent ( L) Form
1 Acetonitrile 60 n/a n/a G
2 n-Butyl acetate 60 n/a n/a A
2 Diisopropyl ether 60 n/a n/a C*
Methyl isobutyl
2 90 n/a n/a D*
ketone
2 Nitromethane 1800 n/a n/a F
2 Acetonitrile 300 n/a n/a G
Methyl isobutyl
3 90 Heptane 90 A
ketone
3 n-Butyl acetate 90 Heptane 60 A
3 Ethyl acetate 60 Heptane 60 A
3 Isopropyl acetate 60 Heptane 60 A
3 Methyl t-butyl ether 90 Heptane 90 A
4 Acetonitrile 150 n/a n/a G
4 Nitromethane 450 n/a n/a F
4b Diisopropyl ether 60 n/a n/a C*
Methyl t-butyl ether 90 Heptane 90 C
6 Anisole 90 n/a n/a oil
Acetonitrile/Water
7 60 n/a n/a I
90/10
Acetonitrile/Water
7 60 n/a n/a I
80/20
Acetonitrile/Water
7 60 n/a n/a I
50/50
Acetonitrile/Water
7 90 n/a n/a I
25/75
8 Dichloromethane 60 n/a n/a C*
8 Isopropylacetate 90 n/a n/a C*
5
* Partially Crystalline
105
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
A. Small Scale Synthesis of Crystalline Obeticholic Acid Form D
In one method, amorphous obeticholic acid (approximately 30 mg) was weighed
into a glass vial and warmed to 50 C. Methyl isobutyl ketone (90 L) was
added and
observations were made. The sample was stirred at 50 C for 1 hour then was
cooled to 5
C using a linear cooling rate of 0.1 C/min. The sample was stirred at 5 C
for 2 days.
Any solids were filtered, air dried and analyzed by XRPD. Solutions were
stored in the
freezer for -48 hours, and then were left to evaporate at ambient conditions.
Solids were
analyzed by XRPD and the peaks are provided in Table 6.
1 0 Table 6: Peak listings for Form D
Angle Intensity %
2-Theta
4.4 16.9
5.2 18.8
6.3 34.7
7.5 22.4
7.8 9.0
8.4 10.8
9.0 19.6
9.5 70.1
10.1 47.5
10.4 24.4
11.0 24.6
11.3 27.5
11.5 28.4
11.8 11.4
12.3 87.2
12.7 42.8
13.4 8.4
13.7 10.1
14.5 63.7
14.9 39.3
15.4 19.7
15.9 63.9
16.5 80.0
16.8 100.0
17.5 20.4
17.8 26.1
18.7 32.1
19.1 32.5
106
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
19.8 13.9
20.2 13.9
20.8 18.2
21.2 25.0
B. Scale-up Synthesis of Crystalline Obeticholic Acid Form D
In one method, amorphous obeticholic acid (approximately 1 g) was weighed into
a large glass vial and warmed to 50 C. MIBK (2.0 ml, 2 volumes) was added at
50 C,
forming a clear solution on stirring. The sample was stirred at 50 C for 2
hours then was
cooled to -20 C using a linear cooling rate of 0.1 C/min. The sample was
stirred
at -20 C overnight, after which a small amount of the solid was filtered, air
dried and
analyzed by XRPD. The remainder of the sample was filtered through a 0.45 tm
PTFE
filter. The sample was air dried for approximately 30 minutes then was dried
in a vacuum
1 0 oven overnight at RT. The resulting white solid was characterized as
Form D. Figure 45
is an XRPD diffractogram of crystalline obeticholic acid Form D. Figure 46 is
an overlay
of TGA and DSC thermograms of crystalline obeticholic acid Form D. Figure 47
is an
isotherm plot for the GVS experiment with crystalline obeticholic acid Form D.
Figure
48 is a kinetics plot for the GVS experiment with crystalline obeticholic acid
Form D.
C. Small Scale Synthesis of Crystalline Obeticholic Acid Form F
In one method, amorphous obeticholic acid (approximately 30 mg) was weighed
into glass vials and warmed to 50 C. Nitromethane was added to dissolve the
amorphous
material. Samples were stirred at 50 C for 1 hour then were matured (shaken
in 8 hour
cycles at 50 C/RT). After 3 days samples containing solids were filtered, air
dried and
analyzed by XRPD. Solids were analyzed by XRPD and the peaks are provided in
Table
7.
Table 7: Peak listings for Form F
Angle Intensity %
2-Theta
7.9 78.2
8.0 78.9
8.4 4.7
9.4 6.1
9.9 82.8
10.5 100.0
11.4 19.5
12.0 11.2
107
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
12.5 7.0
13.2 56.2
13.8 26.9
14.1 32.4
15.0 37.9
15.6 44.1
15.7 44.2
16.0 53.8
16.5 23.8
16.8 20.4
17.5 47.5
18.1 18.0
19.2 17.8
19.6 19.2
20.3 3.4
21.1 23.7
21.6 9.5
22.7 7.1
23.3 5.9
24.5 14.9
D. Scale-up Synthesis of Crystalline Obeticholic Acid Form F
In one method, obeticholic acid (approximately 1 g) was weighed into a large
glass vial and warmed to 50 C. Nitromethane (15.0 ml, 15 volumes) was added
at 50 C,
resulting in the sample forming balls of gummy solid. The sample was matured
(shaken
in 8 hour cycles at 50 C/RT) overnight after which the sample was a
suspension of large
hard lumps of solid and needle shaped particles. Some of the solid was crushed
into a
white powder and was analyzed by XRPD. The lumps of solid were broken up
manually
and the sample was matured for a further 24 hours. A small amount of the
resulting
suspension was filtered, air dried and analyzed by XRPD. The remainder of the
sample
was filtered through a 0.45 p.m PTFE filter. The sample was air dried for
approximately
40 minutes then was dried in a vacuum oven overnight at 40 C. The resulting
white
solid was characterized as Form F. Figure 49 is an XRPD diffractogram of
crystalline
obeticholic acid Form F. Figure 50 is an overlay of TGA and DSC thermograms of
crystalline obeticholic acid Form F. Figure 51 is an isotherm plot for the GVS
experiment with Form F. Figure 52 is a kinetics plot for the GVS experiment
with Form
F.
108
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
E. Small Scale Synthesis of Crystalline Obeticholic Acid Form G
In one method, amorphous obeticholic acid (approximately 30 mg) was weighed
into a glass vial and warmed to 50 C. 2 volume (60 ill) portions of
acetonitrile were
added at 50 C. Initially the sample formed a gum, which dissolved after the
addition of
14 volumes of solvent. On stirring flakes of solid precipitate were observed.
The sample
was cooled to 5 C using a linear cooling rate of 0.25 C/min. The resulting
suspension
was analyzed by XRPD and was then matured (shaken in 8 hour cycles at 50
C/RT).
After 5 days of maturation the sample was equilibrated to room temperature and
was re-
analyzed by XRPD and the peaks are provided in Table 8.
Table 8: Peak listings for Form G
Angle Intensity %
2-Theta
7.9 40.7
10.9 20.8
12.3 39.4
12.8 80.2
12.9 91.4
13.4 12.5
13.8 4.5
14.3 48.6
14.5 65.9
15.4 4.5
16.9 100.0
17.2 2.0
17.6 10.5
18.1 4.5
18.5 15.2
18.8 7.4
19.8 4.4
20.4 20.3
20.7 24.1
21.1 21.4
21.6 1.9
21.8 8.5
23.0 14.6
23.1 30.5
23.5 4.5
23.8 2.3
109
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
24.5 7.8
24.6 7.5
F. Scale-up Synthesis of Crystalline Obeticholic Acid Form G
Amorphous obeticholic acid (approximately 100 mg) was weighed into glass vials
and warmed to 50 C. Ten 100 mg scale experiments were carried out in
parallel.
Acetonitrile (0.5 ml, 5 volumes, pre-warmed to 50 C) was added to each sample
forming
a colorless gum which mostly dissolved on standing at 50 C. Each sample was
seeded
with ¨1 mg of the product in the small scale experiment and the samples were
matured
(shaken in 8 hour cycles at 50 C/RT) for 3 days. After maturation the samples
contained
crystals on the walls of the vial and a hard solid on the base of the vial
which formed a
1 0 white powder on scratching. Each sample was analyzed by XRPD and the
samples were
combined by filtering them all through a 0.45 tm PTFE filter. The sample was
air dried
for approximately 30 minutes then was dried in a vacuum oven overnight at RT.
The
resulting white solid (795.8 mg) was characterized and identified as Form G.
Figure 53
is an XRPD diffractogram of crystalline obeticholic acid Form G. Figure 54 is
an overlay
1 5 of TGA and DSC thermograms of crystalline obeticholic acid Form G.
Figure 55 is an
isotherm plot for the GVS experiment with Form G. Figure 56 is a kinetics plot
for the
GVS experiment with Form G.
G. Small Scale Synthesis of Crystalline Obeticholic Acid Form I
20 In one method, amorphous obeticholic acid (approximately 30 mg) was
weighed
into a glass vial and cooled to 5 C. Acetonitrile/water mixtures (50/50, 60
uL) were
added and samples were stirred at 5 C overnight. Observations were made and
solids
were filtered, air dried and analyzed by XRPD. Some solids were large lumps
which were
crushed prior to XRPD analysis. One sample was a sticky solid so was left to
stir at 5 C
25 for a total of 17 days, forming a hard lump of solid, which was crushed
and analyzed by
XRPD and the peaks are provided in Table 9.
Table 9: Peak listings for Form I
Angle Intensity %
2-Theta
7.2 79.8
10.5 8.9
12.2 6.1
110
CA 03009149 2018-06-19
WO 2017/111979
PCT/US2016/012651
12.7 38.7
13.9 92.6
14.5 100.0
15.7 16.9
15.7 16.6
16.5 14.1
17.4 38.5
17.9 10.2
18.5 19.6
18.8 13.4
19.0 13.0
19.2 28.4
19.5 20.6
22.8 28.4
23.8 2.6
24.2 8.4
25.3 4.3
H. Scale-up Synthesis of Crystalline Obeticholic Acid Form I
Amorphous obeticholic acid (approximately 1 g) was weighed into a large glass
vial and cooled to 5 C. Acetonitrile/water mixture (2 ml, 2 volumes of a
50/50 v/v pre-
mixed mixture, pre-cooled to 5 C) was added and the sample formed a large
lump of
gummy off-white solid. The sample was stirred at 5 C overnight after which it
consisted
of a large lump of hard white solid. The solid lump was broken up into a white
powder
and a small amount was filtered, air dried and analyzed by XRPD. The sample
was stirred
at 5 C for a further 4 days then a small amount of the resulting suspension
was filtered,
air dried and analyzed by XRPD. The remainder of the sample was filtered
through a 0.45
p.m PTFE filter. The sample was air dried for approximately 40 minutes then
was dried in
a vacuum oven overnight at RT. The resulting white solid (955.4 mg) was
characterized.
Figure 57 is an XRPD diffractogram of crystalline obeticholic acid Form I.
Figure 58 is
an overlay of TGA and DSC thermograms of crystalline obeticholic acid Form I.
Figure
59 is an isotherm plot for the GVS experiment with Form I. Figure 60 is a
kinetics plot
for the GVS experiment with Form I.
111