Note: Descriptions are shown in the official language in which they were submitted.
84344055
- 1 -
PREVENTION AND TREATMENT OF HEART FAILURE
Technical Field
[0001]
The present invention relates to a medicament for
preventing or treating heart failure.
[0002]
[Background of the Invention]
Heart failure is a disease characterized by a
disease state where cardiac output decreases due to
dysfunction of cardiomyocytes and a disease state caused
by physical burden brought by a mechanism of maintaining
cardiac output. Cardiomyocytes play a role in
contraction and relaxation. For the contraction and
relaxation, Ca2+ ions are required. Contraction of
cardiomyocytes proceeds through stages of : an action
potential propagating to the transverse tubules to
depolarize the transverse tubule membrane; allowing Ca24
ions to flow into cells from a potential-dependent Type L
Ca2+ channel of the transverse tubules; the inflow Ca2+
ions binding to a Ca2+ release channel (ryanodine
receptor or RYR) of the sarcoplasmic reticulum to release
Ca2+ ions from the sarcoplasmic reticulum to the
cytoplasm; and the released Ca2+ ions within the cell
binding to troponin C to induce contraction of the
Date Regue/Date Received 2023-03-02
CA 03009609 2018-06-22
- 2 -
cardiomyocytes. Further, relaxation of cardiomyocytes
proceeds by taking Ca2+ ions into the sarcoplasmic
reticulum via a Ca2- release pump (SERCA) to reduce the
cytoplasmic level of Ca2+ ions and dissociate the Ca2+
ions from the troponin C. Accordingly, if something goes
wrong in any one of the stages and Ca2+ ions are not
released into the cytoplasm, cardiomyocytes fail to work,
with the result that heart failure occurs.
As a therapeutic drug for heart failure, e.g., a
cardiotonic drug such as p blocker, anti-aldosterone drug,
a diuretic drug and digitalis, an angiotensin converting
enzyme inhibitor and angiotensin II antagonist are used
in clinical sites for improvement of short-term symptoms
and hemodynamic stabilization. However, these drugs are
insufficient to improve the re-hospitalization rate and
long-term life prognosis. In recent years, it has been
desired to provide a novel heart-failure therapeutic drug
for improving re-hospitalization rate and long-term life
prognosis.
[0003]
Ca2+ ions (hereinafter also referred to simply as
calcium) play an essential role in maintaining/regulating
functions of various cells including not only nerve and
muscle but also endocrine cells and exocrine cells.
Therefore, the blood calcium level is precisely
controlled to fall within a narrow range. Parathyroid
hormone (PTH) plays a central role in maintaining the
1
, ,
CA 03009609 2018-06-22
i .
- 3 -
blood calcium level. Thus, secretion of PTH from the
parathyroid gland must be controlled sensitively in
response to a change in blood calcium level. Actually,
when blood calcium level changes, blood PTH level rapidly
changes in accordance therewith. Brown et al., pointed
out the possibility of the existence of the mechanism
where the extracellular calcium concentration is sensed
by parathyroid cells and the information thereof is
transmitted to the cells. In 1993, they successfully
cloned a calcium-sensing receptor (CaSR; hereinafter
simply referred to as a calcium receptor) from the bovine
parathyroid gland and determined the properties thereof
(Nature, 366, 575-580 (1993)).
The calcium receptor has the N terminal of 600 amino
acids in full length and is constituted of a large-
terminal extracellular region having 7 transmembrane
regions similarly to other G protein-coupled receptors
and an intracellular region having the C terminal
consisting of 200 or less amino acids.
If the extracellular calcium concentration increases,
phospholipase (PL)-C is activated and then the level of
inositol triphosphate (IP3) increases, with the result
that the intracellular calcium concentration increases.
In this mechanism, PTH secretion is presumably suppressed.
If the extracellular calcium concentration is maintained
at a high level, the intracellular calcium concentration
subsequently and continuously increases. Conceivably,
i
CA 03009609 2018-06-22
- 4 -
calcium flow-in from outside the cell is also promoted.
If the extracellular calcium concentration increases, PL-
A2 and D are activated. These are probably activated via,
e.g., protein kinase (PK)-C, which is simultaneously
activated via a calcium receptor. The calcium receptor
suppresses adenylyl cyclase via Gi protein or arachidonic
acid production through PL-A2 activation to reduce
intracellular cyclic AMP level (Bone, 20, 303-309 (1997)).
It is known that (5R)-N-[1-ethy1-1-(4-
ethylphenyl)propy1]-2,7,7-trimethyl-5-phenyl-4,5,6,7-
tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide
(hereinafter referred to also as "compound A"), (5R)-N-
[1-ethy1-].-(4-methoxyphenyl)propy1]-2,7,7-trimethyl-5-
pheny1-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-
carboxamide (hereinafter referred to also as "compound
B") and (5R)-N-[1-ethy1-1-(4-ethylphenyl)propy1]-5-(2-
fluoropheny1)-2,7,7-trimethyl-4,5,6,7-
tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide
(hereinafter referred to also as "compound C") are
involved in regulation of the activity of the calcium-
sensing receptor (CaSR) and regulation of parathyroid
hormone (PTH) (Patent literature 1 and Patent literature
2). Also, Non patent literature 1 discloses that
compound A serves as an antagonist to CaSR (Non patent
literature 1).
[0004]
CA 03009609 2018-06-22
4
- 5 -
In Non patent literatures 2 and 3, it is described
that cardiac function is improved by treatment with
parathyroid hormone; whereas, in Non patent literature 4,
it is described that cardiac function is exacerbated by
treatment with parathyroid hormone. Thus, the
relationship between a parathyroid hormone therapy and
improvement of cardiac function has not yet been
sufficiently revealed.
In Non patent literature 5, it is described that
cardiac hypertrophy is improved by a CaSR inhibitor, i.e.,
Calhex231, in a transverse aortic constriction (TAO)
model; however, it is not elucidated that the drug is
effective or not after cardiac function deteriorates.
Non patent literature 6 discloses that, if Calhex231 is
administered, more specifically, if the drug is
administered after the cardiac load is given, cardiac
hypofunction is not improved. Further, in Non patent
literature 7, it is described that a CaSR antagonist
invalidates a cardioprotective effect in an ischemia
preconditioning model. Thus, the relationship of CaSR
inhibition with improvement of cardiac hypofunction or
improvement of survival rate has not yet been
sufficiently elucidated.
Citation List
Patent Literature
[0005]
CA 03009609 2018-06-22
=
- 6 -
Patent literature 1: W02004/017908
Patent literature 2: Japanese Patent Laid-Open No.
2005-239611
Non patent literature
[0006]
Non patent literature 1: Bioorganic & Medicinal
Chemistry 19: 1881-1894, 2011
Non patent literature 2: Cardiovascular research,
77: 722-731, 2008
Non patent literature 3: Cardiovascular research,
93: 330-339, 2012
Non patent literature 4: Experimental and molecular
medicament 42, 61-68, 2010
Non patent literature 5: Cell Physiol. Biochem., 36:
1597-1612, 2015
Non patent literature 6: Cell Physiol. Biochem., 33:
557-568, 2014
Non patent literature 7: Am. J. Physiol. Heart Circ.
Physiol., 299: H1309-H1317, 2010
Summary of Invention
Technical Problem
[0007]
The present invention provides a medicament for
preventing or treating heart failure.
CA 03009609 2018-06-22
- 7 -
Solution to Problem
[0008]
The present inventors found that a compound selected
from the group consisting of compound A, compound B,
compound C and salts thereof is effective in preventing
and/or treating heart failure. The present invention was
achieved based on the finding.
[0009]
More specifically, according to the present
invention, the following inventions are provided.
[1] A medicament for preventing or treating heart
failure, comprising a compound selected from the group
consisting of (5R)-N-[1-ethy1-1-(4-ethylphenyl)propy1]-
2,7,7-trimethyl-5-phenyl-4,5,6,7-tetrahydropyrazolo[1,5-
a]pyrimidine-3-carboxamide, (5R)-N-[1-ethy1-1-(4-
methoxyphenyl)propy1]-2,7,7-trimethyl-5-pheny1-4,5,6,7-
tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide, (5R)-
N-[1-ethy1-1-(4-ethylphenyl)propy11-5-(2-fluoropheny1)-
2,7,7-trimethy1-4,5,6,7-tetrahydropyrazolo[1,5-
a]pyrimidine-3-carboxamide and salts thereof.
[2] The medicament according to the above [1], for
treating heart failure.
[3] The medicament according to the above [1],
wherein heart failure is acute decompensated heart
failure.
[la] A method for preventing or treating heart
failure in a mammal characterized by administering, to a
CA 03009609 2018-06-22
- 8 -
mammal, a compound selected from the group consisting of
(5R)-N-[1-ethy1-1-(4-ethylphenyl)propy1]-2,7,7-trimethyl-
5-pheny1-4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-
carboxamide, (5R)-N-[1-ethy1-1-(4-methoxyphenyl)propy1]-
2,7,7-trimethyl-5-phenyl-4,5,6,7-tetrahydropyrazolo[1,5-
a]pyrimidine-3-carboxamide, (5R)-N-[1-ethy1-1-(4-
ethylphenyl)propy1]-5-(2-fluoropheny1)-2,7,7-trimethyl-
4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide
and salts thereof.
[2a] The method according to the above [la], for
treating heart failure.
[3a] The method according to the above [la], wherein
the heart failure is acute decompensated heart failure.
[lb] A compound for preventing or treating heart
failure, selected from the group consisting of (5R)-N-[1-
ethy1-1-(4-ethylphenyl)propy1]-2,7,7-trimethyl-5-phenyl-
4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide,
(5R)-N-[1-ethy1-1-(4-methoxyphenyl)propy1]-2,7,7-
trimethy1-5-pheny1-4,5,6,7-tetrahydropyrazolo[1,5-
a]pyrimidine-3-carboxamide, (5R)-N-[1-ethy1-1-(4-
ethylphenyl)propyll-5-(2-fluoropheny1)-2,7,7-trimethyl-
4,5,6,7-tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide
and salts thereof.
[2h] The compound according to the above [lb], for
treating heart failure.
CA 03009609 2018-06-22
A
- 9 -
[3h] The compound according to the above [lb],
wherein the heart failure is acute decompensated heart
failure.
[lc] Use of a compound selected from the group
consisting of (5R)-N-[1-ethy1-1-(4-ethylphenyl)propy1]-
2,7,7-trimethyl-5-phenyl-4,5,6,7-tetrahydropyrazolo[1,5-
a)pyrimidine-3-carboxamide, (5R)-N-[1-ethy1-1-(4-
methoxyphenyl)propy1]-2,7,7-trimethy1-5-phenyl-4,5,6,7-
tetrahydropyrazolo[1,5-a]pyrimidine-3-carboxamide, (5R)-
N-[1-ethy1-1-(4-ethylphenyl)propyl]-5-(2-fluoropheny1)-
2,7,7-trimethy1-4,5,6,7-tetrahydropyrazolo[1,5-
a]pyrimidine-3-carboxamide and salts thereof, for
producing a prophylactic or therapeutic agent for heart
failure.
[2c] Use according to the above [lc], for treating
heart failure.
[3c] Use according to the above [lc], wherein the
heart failure is acute decompensated heart failure.
Advantageous Effects of Invention
[0010]
According to the present invention, it is possible
to prevent or treat heart failure.
Brief Description of Drawings
[0011]
CA 03009609 2018-06-22
- 10 -
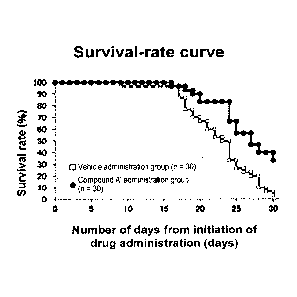
[Figure 1] Figure 1 shows the Kaplan-Meier curve of a
heart failure model animal to which compound A' or a
vehicle was administered.
Description of Embodiments
[0012]
In the specification, the term "subject" refers to a
mammal, for example, a human. In the specification, a
"subject with heart failure" refers to a subject
suffering from heart failure. If the subject is a human,
the subject is referred to as a "patient". If the
subject with heart failure is a human, the subject is
referred to as "heart failure patient".
[0013]
In the specification, the term "pharmaceutically
acceptable salt" refers to an acid addition salt or base
addition salt, which is an acceptable salt if it is
administered in a living body.
[0014]
In the specification, the term "heart failure"
refers to a state where output of blood from the heart
(hereinafter referred to as "cardiac output")
deteriorates and/or a symptom produced by regulatory
mechanism of suppressing deterioration in cardiac output
and means a physical condition where a sufficient blood
circulation volume cannot be ensured. In the
specification, the term "treatment of heart failure"
CA 03009609 2018-06-22
- 11 -
refers to improving the state where cardiac output
deteriorates and/or the symptom produced by a regulatory
mechanism of suppressing deterioration in cardiac output.
As a heart failure treatment for improvement of the
short-term symptoms and hemodynamics stabilization, e.g.,
a p blocker and an anti-aldosterone drug are used;
however, in the present invention, a decrease of ejection
fraction can be suppressed and a survival rate can be
improved without using these drugs. Thus, in the present
invention, heart failure (more specifically, e.g.,
deterioration in cardiac output) can be prevented or
treated.
[0015]
In the specification, the term "ejection fraction"
(EF), which is a cardiac function evaluation index, is a
value obtained by dividing blood volume (ejection volume)
fed by the heart per beat by the left ventricular volume
at the time of cardiac dilatation. In a subject with
heart failure (heart failure patient), a decrease of
ejection fraction is observed. Thus, improvement of
ejection fraction is one of the goals for heart failure
treatment.
[0016]
The clinical condition of heart failure is
classified into four stages depending on the severity
thereof by the New York Heart Association (NYHA).
[Table 1]
CA 03009609 2018-06-22
= =
- 12 -
Table 1: NYHA Heart Failure Classification
Class Patient's symptom
Patients have cardiac disease but without
resulting limitation of physical activity.
Class I Ordinary physical activity does not cause undue
fatigue, palpitations, dyspnea, or anginal
pain.
Patients have cardiac disease resulting in
slight limitation of physical activity. They
Class II are comfortable at rest. Ordinary physical
activity results in fatigue, palpitations,
dyspnea, or anginal pain.
Patients have cardiac disease resulting in
Cl marked limitation of physical activity. They
ass
are comfortable at rest. Less than ordinary
physical activity results in fatigue,
palpitations, dyspnea, or anginal pain.
Patients have cardiac disease resulting in an
inability to carry on any physical activity
without discomfort. Symptoms of cardiac
Class IV
insufficiency or of the anginal syndrome may be
present even at rest. If any physical activity
is undertaken, discomfort is increased.
Class IIs: slight limitation of physical activity
Class IIm: Medium limitation of physical activity
[0017]
According to the AHA/ACC stage classification
(American Heart Association/American College of
Cardiology), the clinical condition of heart failure is
classified into four stages depending on the severity
thereof.
[Table 2]
Table 2: AHA/ACC stage classification of heart failure
Stage Definition
A Risk factor is present; however, no cardiac
dysfunction is observed
Cardiac contractile dysfunction of left
CA 03009609 2018-06-22
- 13 -
ventricle with no symptom
Symptomatic heart failure
Intractable heart failure
[0018]
The correspondence relationship between the NYHA
classification and the AHA/ACC stage classification is
roughly as follows.
[Table 3]
Table 3: Correspondence relationship between the NYHA
classification and the AHA/ACC stage classification
NYHA class AHA/ACC stage class
A
Class I
Class II
Class III
Class IV
Class Iv
[0019]
In the specification, the term "heart failure" is a
disease state different from that of myocardial
infarction, and the type of medicament to be used for
treatment differs from that of myocardial infarction.
More specifically, myocardial infarction refers to
ischemic condition (necrosis) of heart muscle, which is
caused by a decrease in blood supply to cardiomyocytes
due to, e.g., emboli, produced within blood vessel.
Because of this, myocardial infarction is treated or
prevented by removing emboli or inhibiting embolization;
CA 03009609 2018-06-22
= =
- 14 -
more specifically, by administration of a thrombolytic
agent or an antithrombocytic agent, an anti-coagulation
method, an anti-ischemic therapy or a hyperlipemia
treatment. In contrast, heart failure refers to a state
where cardiac output deteriorates and/or the symptom
produced by a regulatory mechanism of suppressing
deterioration in cardiac output, and treated by e.g., a
cardiotonic agent and/or a diuretic agent. Likewise,
since the disease states differ, therapies differ. To
reiterate, heart failure is a different disease from
myocardial infarction. Note that, in the specification,
deterioration in cardiac output in a subject with
myocardial infarction is included in "heart failure" and
can be treated by the present invention. More
specifically, some of the subjects with myocardial
infarction presumably have heart failure other than
myocardial infarction. In the present invention, a
disease state caused by heart failure in subjects with
myocardial infarction can be prevented or treated.
[0020]
In the specification, the term "compensated heart
failure" generally refers to a state produced in vivo by
a regulatory mechanism (compensation mechanism) for
maintaining blood circulation in response to
deterioration in cardiac output caused by heart failure.
Accordingly, compensated heart failure can be prevented
or treated by the present invention. Examples of the
CA 03009609 2018-06-22
- 15 -
compensation include compensation according to the Frank-
Starling law, compensation by myocardial remodeling and
nervous humoral compensation.
(1) Compensation according to the Frank-Starling law
In the compensation according to the Frank-Starling
law, compensation mechanism functions such that
deterioration in cardiac output is improved by increasing
preload, and increased cardiac output per beat thereby.
However, the compensation effect is limited by an
increase of arterial pressure. As a result, preload
further increases, symptoms such as lung congestion and
peripheral edema, are developed.
(2) Compensation by myocardial remodeling
When pressure load is applied to cardiomyocytes, the
wall thickness of the heart increases and the diameter of
the heart ventricle decreases, that is, concentric
hypertrophy develops. Concentric hypertrophy herein is a
compensation mechanism of maintaining normal
contractility against an increase in afterload. However,
when the heart muscle of the left ventricle is reduced in
retractility due to concentric hypertrophy, even if the
circulating blood volume is low, ventricular diastolic
pressure increases and congestion occurs.
Due to volume overload on cardiomyocytes, the inner
cavity of the heart is enlarged to develop eccentric
hypertrophy. In the eccentric hypertrophy, retractility
CA 03009609 2018-06-22
- 16 -
of the ventricle is enhanced and preload decreases and
the cardiac output comes to deteriorate.
As described above, if compensation by myocardial
remodeling is excessive, heart failure may exacerbate.
(3) Nervous humoral compensation
When cardiac output of blood deteriorates due to
heart failure, arterial pressure decreases. If so, the
sympathetic nerve is activated to release a catecholamine.
The catecholamine increases heart rate and cardiac
contractility and induces vasoconstriction and renin
secretion. When arterial pressure decreases, the
pressure of afferent glomerular arteriole of kidney
decreases, with the result that secretion of renin from
juxtaglomerular cells is accelerated. Renin promotes
production of angiotensin II, contracts the artery and
increase afterload. Angiotensin II also promotes
resorption of sodium ions and water in the kidney, and
thus, blood volume increases and preload increases.
Angiotensin II further promotes secretion of a
catecholamine. This reaction transiently improves blood
circulation in a subject with heart failure; however, if
sympathetic stimulation lasts for a long term,
responsiveness to stimulation becomes poor and the
compensation system will not work.
[0021]
In the present invention, the term "acute heart
failure" generally refers to a disease state where an
1
CA 03009609 2018-06-22
. .
- 17 -
organic and/or functional abnormality occurs in the
heart; compensation mechanism by a cardiac pump
(function) immediately fails to work; ventricular end-
diastolic pressure rises; and perfusion into major organs
fails, with the result that symptoms and signs abruptly
emerge from these phenomena or are exacerbated. The
"acute heart failure" herein includes an acute
exacerbation period of chronic heart failure. The acute
heart failure is roughly divided into the following six
disease states: acute decompensated heart failure,
hypertensive acute heart failure, acute cardiogenic
pulmonary edema, cardiogenic shock, high-output heart
failure and acute right heart failure. In the present
invention, acute heart failure can be prevented or
treated.
[0022]
In the present invention, the term "chronic heart
failure" generally refers to a state where the cardiac
output deteriorates by a chronic myocardial damage and a
blood volume satisfying oxygen demand by peripheral
organs is neither absolutely nor relatively pumped out,
with the result that the lung, systemic venous system or
both of them are congested and a problem occurs in daily
life. Some of the subjects with chronic heart failure
have both heart failure and chronic myocardial damage in
combination. Of them, heart failure can be prevented or
treated in the present invention.
CA 03009609 2018-06-22
- 18 -
[0023]
In the specification, the term "acute decompensated
heart failure" generally refers to a new-type acute heart
failure, which is associated with mild signs and symptoms
of heart failure and fails to satisfy diagnostic criteria
such as cardiogenic shock, pulmonary edema and
hypertensive acute heart failure, or referred to one with
acute exacerbation of chronic heart failure.
[0024]
In the specification, the term "hypertensive acute
heart failure" refers to a disease state caused by
hypertension and associated with signs and symptoms of
heart failure, acute lung congestion or pulmonary edema.
[0025]
In the specification, the term "acute cardiogenic
pulmonary edema" refers to a disease state where dyspnea
and orthotic respiration are observed and rale and
pulmonary edema are associated.
[0026]
In the specification, the term "cardiogenic shock"
refers to a serious disease state where microcirculation
of the peripheral and systemic major organs is
significantly damaged by heart pump failure and
sequentially tissue hypoperfusion is developed.
[0027]
In the specification, the term "high output heart
failure" refers to a disease state caused by e.g.,
CA 03009609 2018-06-22
- 19 -
thyrotoxicosis, anemia, shunt disease, beriberi heart,
Paget's disease and iatrogenic factors and associated
with lung congestion although the limbs are warm.
[0028]
In the specification, the term "acute right heart
failure" refers to a disease state of low blood pressure
or low cardiac output associated with elevated venous
pressure or hepatomegaly.
[0029]
In the present invention, acute decompensated heart
failure can be prevented or treated.
[0030]
Heart failure includes ischemic heart failure and
non-ischemic heart failure.
Ischemic heart failure refers to heart failure
caused by losing balance between oxygen demand and supply
in the heart muscle. Ischemic heart failure includes
those caused by angina pectoris and arteriosclerosis. In
the present invention, ischemic heart failure can be
prevented or treated.
Non-ischemic heart failure is heart failure except
ischemic heart failure. Also in the present invention,
non-ischemic heart failure can be treated.
[0031]
In the specification, "congestive heart failure"
generally refers to a disease state where cardiac output
deteriorates with swelling in pulmonary and peripheral
1
CA 03009609 2018-06-22
- 20 -
tissue. Congestive heart failure includes heart failures
caused by, for example, arrhythmia, ischemic heart
disease, acute myocardial infarction, hypertension,
cardiac myopathy, myocarditis, congenital heart disease,
and other diseases. Congestive heart failure includes
acute heart failure and chronic heart failure.
Congestive heart failure can be also prevented or treated
by the present invention.
[0032]
Examples of the symptoms of chronic heart failure
include increased fatigue, shortness of breath with
motion, anorexia, hypanakinesia, cough, palpitation,
lower-leg edema, swelling, body weight gain with swelling,
and dyspnea during sleep. The present invention can
treat at least one state selected from the above states.
Examples of the symptoms of acute heart failure include
sudden onset of symptoms of chronic heart failure as
mentioned above, dyspnea and muzziness. The present
invention can treat at least one state selected from e.g.,
these states.
[0033]
In the present invention, a disease state of heart
failure in a subject can be prevented or treated by
administering a compound selected from the group
consisting of compound A, compound 13, compound C and
salts thereof to the subject.
[0034]
1
CA 03009609 2018-06-22
- 21 -
According to the present invention, there is
provided a medicament comprising a compound selected from
compound A, B and C and salts thereof, for use in
preventing or treating heart failure.
[0035]
Compound A is represented by the following formula:
[Formula 1]
WAX)
HN
0
[0036]
Compound B is represented by the following formula:
[Formula 2]
N-.
'1401 HN
0
0
[0037]
Compound C is represented by the following formula:
[Formula 3]
CA 03009609 2018-06-22
= =
- 22 -
II 1.
0
[0038]
In the present invention, compound A, B and C and
salts thereof can be used for preventing or treating
heart failure. According to the present invention, there
is provided a medicament for use in preventing or
treating heart failure and particularly a medicament for
use in increasing cardiac output or preventing or
treating decrease of cardiac output in a subject with
heart failure, comprising a compound selected from the
group consisting of compound A, B and C and salts thereof.
[0039]
In the present invention, compound A, B and C and
salts thereof can be used for preventing or treating
deterioration in cardiac output in a subject with heart
failure. Accordingly, the medicament of the present
invention may be a cardioprotective drug comprising a
compound selected from compound A, B and C and salts
thereof.
[0040]
In a specific embodiment of the present invention,
the medicament of the present invention can be used in
CA 03009609 2018-06-22
- 23 -
preventing or treating ischemic heart failure or non-
ischemic heart failure. In a specific embodiment of the
present invention, the medicament of the present
invention can be used in preventing or treating non-
ischemic heart failure. In a further specific embodiment
of the present invention, the medicament of the present
invention can be used in treating non-ischemic heart
failure.
[0041]
In a specific embodiment of the present invention,
the medicament of the present invention can be used in
preventing or treating decompensated heart failure. In a
specific embodiment of the present invention, the
medicament of the present invention can be used in
preventing or treating acute heart failure. In a
specific embodiment of the present invention, the
medicament of the present invention can be used in
preventing or treating acute decompensated heart failure.
In a further specific embodiment of the present invention,
the medicament of the present invention can be used in
preventing or treating acute decompensated heart failure.
[0042]
In the present invention, compound A, B and C and
salts thereof improve a decrease of ejection fraction in
a subject with heart failure or increases ejection
fraction. The medicament of the present invention may be
a medicament (improving drug) comprising a compound
CA 03009609 2018-06-22
- 24 -
selected from compound A, B and C and salts thereof, for
improving a decrease of ejection fraction and death
caused by a decrease of ejection fraction in a subject
with heart failure.
[0043]
In the present invention, compound A, B and C and
salts thereof can suppress exacerbation of cardiac
function or further exacerbation thereof in a subject
with heart failure. The medicament of the present
invention may be a medicament comprising a compound
selected from compound A, B and C and salts thereof for
use in suppressing exacerbation of cardiac function or
further exacerbation thereof in a subject with heart
failure. The medicament of the present invention may be
a medicament comprising a compound selected from compound
A, B and C and salts thereof for protecting the heart in
a subject with heart failure. In the present invention,
compound A, B and C and salts thereof can reduce cardiac
load and suppress cardiac hypertrophy, interstitial
fibrosis and an increase of apoptosis in a subject with
heart failure. The medicament of the present invention
may be a medicament comprising a compound selected from
compound A, B and C and salts thereof, for use in
treating at least one (disease) state selected from an
increase of cardiac load, cardiac hypertrophy,
interstitial fibrosis and/or an increase of cardiomyocyte
apoptosis, in a subject with heart failure.
CA 03009609 2018-06-22
- 25 -
[0044]
In a specific embodiment of the present invention,
the medicament of the present invention can be used in
preventing or treating Class I, II, III or IV-stage heart
failure according to the NYHA classification. In a
specific embodiment of the present invention, the
medicament of the present invention can be used in
preventing or treating A, B, C or D-stage heart failure
according to the AHA/ACC stage classification
[0045]
Compound A, B and C and salts thereof may be a
solvate or not. The solvate may be a solvent such as
ethanol and water. If the solvent to be comprised is
water, the solvate is a hydrate. The hydrate includes
not only a stoichiometric hydrate but also hydrates
different in water content.
[0046]
Compound A, B and C and salts thereof may be labeled
with an isotope (e.g., 3H, 13C, 14C, 18F, 35S, 1251).
Deuterides of compound A, B and C and salts thereof
obtained by converting 1H with 2H (D) are also included.
Compound A, B and C and salts thereof may be in the
form of a pharmaceutically acceptable co-crystal or co-
crystalline salt. The co-crystal or co-crystal salt
herein refers to a crystalline substance constituted of
two types or more solids distinctive at room temperature
and having mutually different physical properties (for
CA 03009609 2018-06-22
- 26 -
example, structure, melting point, heat of fusion,
hygroscopicity, solubility and stability). The co-
crystal or co-crystalline salt can be produced by a co-
crystallization method known per se.
[0047]
Examples of the salts of compound A, B and C include
a salt with an inorganic base, an ammonium salt, a salt
with an organic base, a salt with an inorganic acid, a
salt with an organic acid and a salt with a basic or
acidic amino acid.
Preferable examples of the salt with an inorganic
base include an alkali metal salt such as a sodium salt
and a potassium salt; an alkaline earth metal salt such
as a calcium salt, a magnesium salt, a barium salt; and
an aluminum salt.
Preferable examples of the salt with an organic base
include salts with trimethylamine, triethylamine,
pyridine, picoline, ethanolamine, diethanolamine,
triethanolamine, dicyclohexylamine and N, N'-
dibenzylethylenediamine.
Preferable examples of the salt with an inorganic
acid include salts with hydrochloric acid, hydrobromic
acid, nitric acid, sulfuric acid and phosphoric acid.
Preferable examples of the salt with an organic acid
include salts with formic acid, acetic acid,
trifluoroacetic acid, fumaric acid, oxalic acid, tartaric
acid, maleic acid, citric acid, succinic acid, malic acid,
CA 03009609 2018-06-22
- 27 -
methanesulfonic acid, benzenesulfonic acid and p-
toluenesulfonic acid.
Preferable examples of the salt with a basic amino
acid include salts with arginine, lysine and ornithine.
Preferable examples of the salt with an acidic amino
acid include salts with aspartic acid and glutamic acid.
Of these salts, a pharmaceutically acceptable salt
is preferable.
[0048]
In a specific embodiment of the present invention, a
salt of compound A can be a p-toluenesulfonate (tosylate)
(hereinafter referred to also as "compound A'"). In a
specific embodiment of the present invention, a salt of
compound B can be a hydrochloride (hereinafter referred
to also as "compound B'"). In a specific embodiment of
the present invention, a salt of compound C can be a p-
toluenesulfonate (tosylate) (hereinafter referred to also
as "compound C'").
[0049]
In a specific embodiment of the present invention, a
compound selected from compound A, compound A', compound
B, compound B', compound C and compound C' can be used
for preventing or treating heart failure. In a specific
embodiment of the present invention, compound A or
compound A' can be used for preventing or treating heart
failure. In a specific embodiment of the present
84344055
- 28 -
invention, compound A' can be used for preventing or
treating heart failure.
[0050]
Compound A, B and C and salts thereof can be
prepared by a method known per se (for example, method
described in W02004/017908 and Yoshida M. et al., Bioorg.
Med. Chem., 19: 1881-1894, 2011.
[0051]
The dosage varies depending on e.g., the subject to
be administered, administration route, disease and
symptoms. For example, in the case of oral
administration to a human (body weight about 50 kg), the
dosage falls within the range of about 0.1 mg to about
500 mg in terms of compound A, B or C and preferably
about 1 mg to about 100 mg. In the case of parenteral
administration, the dosage can be selected from the range
of about 0.01 mg to about 100 mg and preferably about 0.1
mg to about 10 mg. The dosage is administered in a
single dose or several doses (for example, one to three
per day).
[0052]
The medicament of the present invention may comprise
one compound selected from compound A, B and C and salts
thereof, and a pharmaceutically acceptable carrier.
Date Regue/Date Received 2023-03-02
CA 03009609 2018-06-22
- 29 -
As the pharmaceutically acceptable carrier, various
organic or inorganic carrier substances routinely used as
a drug substance are used and blended as an excipient, a
lubricant, a binder or a disintegrator in a solid
preparation; a solvent, a solubilizer, a suspending agent,
a tonicity agent, a buffer or a soothing agent in a
liquid preparation. If necessary, a formulation additive
such as a preservative, an antioxidant, a stabilizer,
colorants or a sweetener can be used.
[0053]
In an embodiment, the medicament of the present
invention can be a medicament for parenteral
administration or oral administration. The medicament of
the present invention can be a medicament for oral
administration.
[0054]
The medicament of the present invention can be used
in combination with another drug such as a drug for heart
failure, for example, at least one of the following
drugs:
(1) Therapeutic agent for heart failure
(i) p receptor antagonist (p blocker)
Carvedilol, metoprolol, atenolol, etc.
(ii) Diuretic agent
Hydrochlorothiazide, spironolactone, furosemide,
indapamide, bendrofluazide, cyclopenthiazide, bumetanide,
ethacrynic acid, etc.
CA 03009609 2018-06-22
. .
- 30 -
(iii) Cardiotonic drug
Digitalis, digoxin, dobutamine, etc.
(iv) Anti-aldosterone drug
Spironolactone, eplerenone
(v) Heart rate lowering drug
Ivabradine, etc.
(vi) Intravenous cardiotonic drug
h-ANP, etc.
(vii) Angiotensin-converting enzyme inhibitor
Captopril, enalapril, delapril, etc.
(viii) Angiotensin II antagonist
Candesartan cilexetil, candesartan, losartan,
eprosartan, valsartan, telmisartan, irbesartan,
tasosartan, olmesartan, olmesartan medoxomil, azilsartan,
azilsartan medoxomil, etc.
(ix) Others
Relaxin, etc.
(2) Others
(x) Ca-sensitivity enhancing agent
MCC-135, etc.
(xi) Ca channel antagonist
Nifedipine, diltiazem, verapamil, lomerizine
hydrochloride, amlodipine besylate, etc.
(xii) Antiplatelet drug, anticoagulant
Heparin, aspirin, warfarin, dabigatran, rivaroxaban,
pixaban, edoxaban, etc.
(xiii) HMG-CoA reductase inhibitor
,
CA 03009609 2018-06-22
- 31 -
Atorvastatin, simvastatin, etc.
(xiv) Uric acid lowering drug
Probenecid, allopurinol, febuxostat, etc.
(xv) Alpha blocking agent
Doxazosin, etc.
(xvi) Oral adsorbent
Kremezin, etc.
(xvii) Hyperkalemia therapeutic agent
Calcicol, etc.
(xviii) Hyperphosphatemia therapeutic agent
Sevelamer, lanthanum carbonate, etc.
(xix) Metabolic acidosis ameliorating drug
Sodium bicarbonate, etc.
(xx) Activated vitamin
[0055]
The medicament (drug) to be blended or used in
combination with the medicament of the present invention
includes both a medicament which is formulated as a
single preparation comprising a compound selected from
compound A, B and C and salts thereof and a drug used in
combination (combination drug), and a medicament which is
formulated as separate preparations: a preparation
comprising a compound selected from compound A, B and C
and salts thereof and a preparation comprising a
combination drug (for example, combined medicament).
Hereinafter, these will be collectively referred to
simply as a combined medicament of the present invention.
CA 03009609 2018-06-22
=
- 32 -
[0056]
The combined medicament of the present invention can
be formulated by a method similar to that in the above-
mentioned medicament comprising a compound selected from
compound A, B and C and salts thereof by separately or
simultaneously mixing a compound selected from compound A,
B and C and salts thereof and a combination drug with or
without a pharmaceutically acceptable carrier. The
dosage of the combined medicament of the present
invention per day varies depending on, e.g., the symptom;
the age, sex, body weight and difference in sensitivity
of the subject to be administered; timing and interval of
administration, the feature, prescription and type of the
medicament; and type of active ingredient and is not
particularly limited.
[0057]
In administering a combined medicament of the
present invention, a compound selected from compound A, B
and C and salts thereof and a combination drug may be
administering at the same timing. Further, a combination
drug is first administered, and then, a compound selected
from compound A, B and C and salts thereof may be
administered. Alternatively, a compound selected from
compound A, B and C and salts thereof is first
administered, and then, a combination drug may be
administered. In the case of administering them at time
intervals, the time interval varies depending on the
CA 03009609 2018-06-22
- 33 -
active ingredient, dosage form and the administration
method. For example, if a combination drug is first
administered, a method of administering a compound
selected from compound A, B and C and salts thereof
within one minute to three days, preferably 10 minutes to
one day, more preferably 15 minutes to 1 hour after a
combination drug is administered, is mentioned as an
approach. If a compound selected from compound A, B and
C and salts thereof is first administered, a method of
administering a combination drug within one minute to one
day, preferably 10 minutes to 6 hours, more preferably 15
minutes to one hour after the compound is administered,
is mentioned as an approach.
[0058]
In combined medicament of the present invention
comprising a compound selected from compound A, B and C
and salts thereof concomitantly with a combination drug,
the individual contents of the compound selected from
compound A, B and C and salts thereof and the combination
drug vary depending on the dosage form of the preparation
of the combined medicament; however, the contents usually
fall in the range of about 0.01 to 90 wt% relative,
preferably about 0.1 to 50 wt% and further preferably
about 0.5 to 20 wt% to the total amount of the
preparation.
[0059]
CA 03009609 2018-06-22
- 34 -
The content of the carrier in the combined
medicament is usually about 0 to 99.8 wt%, preferably
about 10 to 99.8 wt% and further preferably about 10 to
90 wt% relative to the total amount of the preparation.
[0060]
In the case of a combined medicament, comprising a
compound selected from compound A, B and C and salts
thereof and a combination drug as separate preparations,
the preparation comprising a combination drug can be
produced in the same manner as in the compound selected
from compound A, 13 and C and salts thereof, and put in
use.
[0061] .
The medicament of the present invention may be
either a solid preparation such as a powder, a granule, a
tablet or a capsule, or a liquid such as a syrup or an
emulsion.
The medicament of the present invention can be
produced in accordance with a routine method including
mixing, kneading, granulating, tableting, coating,
sterilizing and emulsifying, depending on the dosage form
of the preparation. Here, as to the production of the
preparation, each section of General Rules for
Preparations of the Japanese Pharmacopoeia, for example,
can be referred. The medicament of the present invention
may be formed as a sustained release agent comprising an
active ingredient and a biodegradable polymer compound.
CA 03009609 2018-06-22
- 35 -
[0062]
In an embodiment of the present invention, there is
provided a medicament for preventing or treating heart
failure in the subject in need thereof, comprising a
compound selected from compound A, B and C and salts
thereof and to be used in combination with a combination
drug.
[0063]
In another aspect of the present invention, there is
provided a method for preventing or treating heart
failure in a subject in need thereof, including
administering a compound selected from compound A, B and
C and salts thereof to the subject. In the method of the
present invention, a heart failure to be treated can be
any one of the diseases or disease states already
described as those to be treated by the medicament of the
present invention. In the method of the present
invention, in the case where a compound selected from
compound A, B and C and salts thereof is administered, a
medicament comprising a compound selected from compound A,
B and C and salts thereof may be administered.
[0064]
In another aspect of the present invention, there is
provided use of a compound selected from compound A, B
and C and salts thereof for producing a medicament for
preventing or treating heart failure in a subject in need
thereof. Heart failure to be treated by the medicament
CA 03009609 2018-06-22
- 36 -
can be any one of the diseases or conditions already
described as those to be treated by the medicament of the
present invention.
[0065]
According to a specific embodiment of the present
invention, the compound to be administered or the
compound to be compriseed in the medicament is a tosylate
of compound A.
Examples
[0066]
Compound A' was prepared in accordance with the
method known in the art and described in W02004/017908
and Yoshida M. et al., Bioorg. Med. Chem., 19: 1881-1894,
2011.
[0067]
Calsequestrin (CSQ) cardiac specific transgenic mice
(CSQ-Tg mice), which were reported in Larry R. Jones et
al., J. Clin. Invest. 101: 1385-1393, 1998, were obtained
from University of Pennsylvania, breeded in our company
and put in use. In tests, using male and female mice,
administration of a medicament was started on and after
the mice reached 5 weeks old. The animals were raised in
the conditions: room temperature: 20 to 26 c, humidity:
40 to 70%, illumination time: 12 hours/day (7:00-19:00)
by giving a solid feed (CE-2, manufactured by CLEA Japan,
Inc.) and tap water. In the CSQ-Tg mice, as already
CA 03009609 2018-06-22
- 37 -
reported, Ca2+ intracellular release was suppressed;
myocardial contraction declined and cardiac output
decreased. Cardiac hypertrophy and heart failure
developed.
[0068]
Example 1: Atrial weight reduction effect of compound A'
in heart failure model animal
In this example, using CSQ-Tg mice as a heart
failure model animal, the atrial weight reduction effect
of compound A' was evaluated.
[0069]
Compound A' was suspended (10 mL/kg) in a 0.5 %
aqueous methylcellulose solution (hereinafter in Examples,
sometimes referred to as a "vehicle") and orally
administered to CSQ-Tg male mice of 5 weeks old with
heart failure in a dosage of 30 mg/kg body weight/day
once per day (QD) for 14 days (n = 7). To negative
control (vehicle administration group) mice, a 0.5 %
aqueous methylcellulose solution was administered (n = 9).
Thereafter, the atrial weight of the mice was measured.
The results were as shown in Table 4.
[0070]
[Table 4]
Average value Standard
Left atrial weight
(mg) deviation (mg)
Vehicle administration
12.6 4.4
group
Compound A'
8.4 1.0
administration group
,
CA 03009609 2018-06-22
= .
- 38 -
[0071]
As shown from Table 4, it was found that, in the
Compound A' administration group, the left atrial weight
(mg) decreases, and significant difference was confirmed
by the t-test (p < 0.05). From the results, it was
demonstrated that compound A' has an improvement effect
(atrial weight reduction effect) on heart remodeling in
heart failure.
[0072]
Example 2: Improvement effect of compound A' on left
ventricular hypertrophy and lung weight increase
In this example, the effect of compound A' in
improving left ventricular hypertrophy and lung weight
increase caused by heart failure was examined.
[0073]
Compound A' was suspended in a 0.5 % aqueous
methylcellulose solution (10 mL/kg) and orally
administered to CSQ-Tg female mice of 5 weeks old with
heart failure in a dosage of 30 mg/kg body weight/day
once per day (QD) for 14 days (n = 10). To negative
control (vehicle administration group) mice, a 0.5 %
aqueous methylcellulose solution was administered (n =
10). Thereafter, lung weight (Lung), left-ventricle
weight (LV) and body weight (BW) were individually
measured and were standardized with the BW. The results
were as shown in Table 5.
[0074]
1
CA 03009609 2018-06-22
=
- 39 -
[Table 5]
Average value standard
LV/BW Lung/BW
deviation
Vehicle administration group 9.3 1.6 8.1 1.3
Compound A' administration
7.9 1.0 6.8 1.3
group
[0075]
As shown in Table 5, in the Compound A'
administration group, the lung weight and left
ventricular weight both statistically significantly
decreased (t-test, both p < 0.05). From the results, it
was demonstrated that compound A' improves left
ventricular hypertrophy and lung weight increase.
[0076]
Example 3: Effect of compound A' on survival rate of
heart failure model animals
In this example, effect of compound A' on survival
rate of heart failure model animals was examined.
[0077]
Compound A' was orally administered to CSQ-Tg female
mice (n - 30) of 5 weeks old in a dosage of 30 mg/kg body
weight/day once per day (QD) for 30 days. To negative
control (vehicle administration group) mice, a 0.5 %
aqueous methylcellulose solution was administered (n =
30). The results were as shown in Figure 1.
[0078]
As shown in Figure 1, the survival rate of the
compound A' administration group was significantly
84344055
- 40 -
improved compared to the vehicle group (log-rank test, p
< 0.001). From this, it was demonstrated that compound
A' improves the condition of heart failure. This is the
effect of compound A' in improving death of decreased
cardiac function by overexpression of Calsequestrin.
[0079]
From the above results of Examples, it is understood
that compound A' improves ejection fraction in a subject
with heart failure and can treat heart failure in this
mechanism. It was found that compound A' at least has an
effect of reducing cardiac load of the heart in failure
and an effect of improving cardiac function or inhibiting
exacerbation of cardiac function by suppressing
myocardial hypertrophy, interstitial fibrosis, apoptosis
and the like. Based on this, it is interpreted that
compound A' has not only a therapeutic effect but also a
preventive effect on heart failure.
Industrial Applicability
[0080]
According to the present invention, there is
provided a medicament for preventing or treating heart
failure. Thus, the invention is useful.
Date Regue/Date Received 2023-03-02
84344055
- 41 -
(0081]
This application claims for the priority based on
Japan Japanese Patent Application No. 2015-253809 (filed
on December 25, 2015).
Date Regue/Date Received 2023-03-02_