Note: Descriptions are shown in the official language in which they were submitted.
CA 03010857 2018-07-06
Description
Title of Invention
PHARMACEUTICAL COMPOSITION COMPRISING NEBIVOLOL
WITH IMPROVED DISSOLUTION RATE
Technical Field
The present invention relates to a pharmaceutical composition having an
improved dissolution rate of nebivolol and a method for preparing the same.
More
io specifically, the present invention relates to a pharmaceutical
composition with an
improved dissolution rate, comprising nebivolol or a pharmaceutically
acceptable salt
thereof as an active ingredient, an alkalizing agent and a pharmaceutically
acceptable
additive.
Background Art
A beta-blocker is used for the treatment of hypertension, angina pectoris,
myocardial infarction, cardiac disorders, migraine or essential tremor, etc.
Nebivolol, a beta-blocker, which is a 2,2'-iminobisethanol derivative
represented by the following Formula 1, has a centrosymmetric structure with
four
chiral centers.
[Formula 11
F
0 0
1
OH OH
Nebivolol, a mixture of D- and L-form enantiomers, is a 13¨receptor blocking
agent, which is highly selective for 131 -adrenergic receptor, and has a
vasodilation
activity related to an effect on endothelial nitrogen oxide, which makes
nebivolol
CA 03010857 2018-07-06
different from other conventional 13-blockers. It is believed that nebivolol
increases the
concentration of nitrogen oxide via L-arginine-nitrogen oxide pathway in
vascular
endothelium, and it is found that nebivolol improves endothelial dysfunction
and
vascular elasticity. Furthermore, it is known that nebivolol has an
antioxidant effect
which is beneficial to vascular endothelial functionality, and therefore,
nebivolol is used
as an effective antihypertensive agent having a beneficial effect on vascular
endothelium and cardiovascular system. It is also found that nebivolol is
effective on
cardiovascular disorders such as hypertension, congestive heart failure,
arteriosclerosis
and endothelial dysfunction, etc.
Generally, a pharmaceutical composition is provided as a solid formulation
suitable for oral administration, i.e., tablets, etc., in consideration of
convenient use, in
which case bioavailability is significantly required. A critical factor
affecting
bioavailability is a dissolution process in vivo, which may be determined by a
dissolution rate via a dissolution test in a laboratory. In this regard,
nebivolol has a
IS problem of a very low dissolution rate at a relatively high pH.
In order to solve the problem of nebivolol, KR Patent No. 0361636 discloses a
method of micronizing nebivolol. However, micronized nebivolol still has a
problem
of a low dissolution rate, and also has a great deviation of bioavailability
under fasting
or fed condition. EP Patent No. 0145067 discloses a method of improving a
dissolution rate of nebivolol by adding a wetting agent to micronized
nebivolol. KR
Patent No. 896266 discloses a nebivolol hydrochloride form with improved
dissolution
rate without using a wetting agent, and KR Patent Publication No. 2011-0130872
discloses a pharmaceutical composition using crystalline form nebivolol with
starch in
order to improve a dissolution rate of nebivolol.
In the conventional methods, however, nebivolol should be milled and sieved to
be micronized, which is very inconvenient and uneconomical due to increased
process
time and cost. Addition of a wetting agent also contributes to rise of
manufacturing
cost. In addition, a great deviation of bioavailability under fasting or fed
condition
2
CA 03010857 2018-07-06
could decrease therapeutic effect.
Furthermore, in the conventional methods, only the dissolution rate of
nebivolol
at pH 1.2 to 4.0 was confirmed, and the dissolution rate in water was not
verified.
According to the preliminary experiment performed previously to the present
invention,
it was confirmed that all the nebivolol preparations showed a low dissolution
rate of
70% or less when tested in water. When the preparations with decreased
dissolution
rate of drug are administered, oral absorption and bioavailability of drug may
decrease,
which may lead to decreased efficacy of drug in a human body. Accordingly,
there
has been a compelling need for development of a nebivolol preparation ensuring
a
satisfactory dissolution rate without a deviation in vivo.
Under the circumstance, the present inventors have intensively studied for
preparing a nebivolol preparation with a stable and enhanced dissolution rate
without a
deviation at the pH change in vivo and found that an alkalizing agent added to
a
pharmaceutical composition comprising nebivolol results in a significant
enhancement
of a dissolution rate even at pH 5 to 7 or in water as well as at a low pH to
complete the
present invention.
Disclosure of Invention
Technical Problem
An object of the present invention is to provide a pharmaceutical composition
with an improved dissolution rate of nebivolol and a method for preparing the
same.
Solution to Problem
In accordance with one aspect of the present invention, there is provided a
pharmaceutical composition for preventing or treating cardiovascular diseases,
comprising nebivolol or a pharmaceutically acceptable salt thereof, an
alkalizing agent
and a pharmaceutically acceptable additive.
In an embodiment, the pharmaceutically acceptable salt of nebivolol is
3
CA 03010857 2018-07-06
preferably a nebivolol hydrochloride, and may be comprised in an amount of 0.2
to 10
parts by weight based on 100 parts by weight of the composition.
The alkalizing agent is preferable to show a pH of 8 to 12 when suspended or
dissolved in water, and may be selected from the group consisting of magnesium
oxide,
magnesium carbonate and magnesium aluminum silicate. Furthermore, the
alkalizing
agent may be comprised in an amount of 0.02 to 10 parts by weight based on 100
parts
by weight of the composition.
In another embodiment, the pharmaceutical composition may further comprise
an additional active ingredient for preventing or treating cardiovascular
diseases. The
additional active ingredient may be at least one selected from the group
consisting of
hydrochlorothiazide, ramipril, enalapril, lercanidipine, nisoldipine,
amlodipine, losartan,
eprosartan, candesartan, telmisartan, valsartan, olmesartan, rosuvastatin,
atorvastatin,
pravastatin, fluvastatin, lovastatin, simvastatin and pharmaceutically
acceptable salts
thereof, and rosuvastatin calcium is preferred.
In accordance with another aspect of the present invention, there is provided
a
method for preparing a pharmaceutical composition, which comprises the steps
of:
a) mixing nebivolol or a pharmaceutically acceptable salt thereof with a
pharmaceutically acceptable additive to form a mixture; and
b) formulating the mixture,
wherein an alkalizing agent is added in step a) or b).
Advantageous Effects of Invention
The pharmaceutical composition of the present invention does not cause a
decrease in the dissolution rate of nebivolol and can significantly improve
the
dissolution rate, not only at a low pH, such as pH 1.2, but also at a
relatively high pH
of 5 to 7, so that an improved therapeutic effect can be expected.
Furthermore, the
pharmaceutical composition may reduce the manufacturing cost since the
micronization of nebivolol or the addition of a wetting agent is not required,
and can
4
CA 03010857 2018-07-06
minimize the occurrence of a deviation of bioavailability under fasting or fed
condition
since there is little deviation of dissolution rate due to the pH change,
thereby
maintaining a constant pharmacological action, and therefore, the
pharmaceutical
composition is very useful in the preparation of a nebivolol preparation or a
composite
preparation containing an additional active ingredient, such as rosuvastatin
calcium.
Brief Description of Drawings
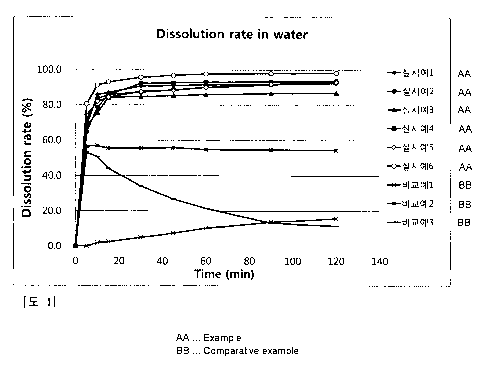
Fig. I is a graph showing a dissolution rate of nebivolol in water over time
for
the preparations of Examples 1 to 6 and Comparative Examples 1 to 3, measured
i() according to paddle method.
Detailed Description
Hereinafter, the present invention will be further explained.
Nebivolol, an active ingredient of the pharmaceutical composition according to
the present invention, which is represented by the following Formula 1, is a
highly
selective p 1 -blocker and known to be efficacious in the control of
hypertension.
[Formula 11
0 0
OH OH
In the composition according to the present invention, nebivolol may be used
as various forms such as a free base or a pharmaceutically acceptable salt
thereof, as
long as an equivalent pharmacological activity of nebivolol is maintained.
As for the pharmaceutically acceptable salt, since nebivolol is basic, it may
be
treated with acid to be converted into a pharmaceutically acceptable acid
addition salt
thereof. Examples of a suitable acid include an inorganic acid such as a
hydrohalogenic acid (e.g., hydrochloric acid, hydrobromic acid), sulfuric
acid, nitric
acid, phosphoric acid, etc.; or an organic acid such as acetic acid, propanoic
acid,
5
CA 03010857 2018-07-06
hydroxyacetic acid, 2-hydroxypropanoic acid, 2-oxopropanoic acid, ethanedioic
acid,
propanedioic acid, butanedioic acid, (Z)-2-butenedioic acid, (E)-2-butenedioic
acid, 2-
hydroxybutanedioic acid, 2,3-dihydroxybutanedioic acid, 2-hydroxy-1,2,3-
propanetricarboxylic acid, methanesulfonic acid, ethanesulfonic acid,
benzenesulfonic
acid, toluenesulfonic acid, cyclohexanesulfamic acid, 2-hydroxybenzoic acid, 4-
amino-
2-hydroxybenzoic acid, etc. According to the present invention, a preferred
acid
addition salt is hydrochloride.
The amount of nebivolol or a pharmaceutically acceptable salt thereof in the
pharmaceutical composition may be adjusted in an acceptable range if needed,
and
preferably, 0.2 to 10 parts by weight, more preferably, 1 to 4 parts by weight
based on
100 parts by weight of the composition. According to an embodiment of the
present
invention, the amount of nebivolol is 1.5 to 3.6 parts by weight, as nebivolol
hydrochloride form, based on 100 parts by weight of the composition.
The pharmaceutical composition according to the present invention is
characterized in that an alkalizing agent is comprised in the composition in
order to
solve the problem that the dissolution rate of nebivolol or a pharmaceutically
acceptable
salt thereof decreases at pH 5.0 to 7.0, e.g., in water, thereby resulting in
reduced
absorption and bioavailability. It is generally known that since nebivolol is
weak base,
its solubility and dissolution rate would reduce with the increase of pH.
Surprisingly,
the present inventors found that the dissolution rate of nebivolol
significantly increased
with the addition of an alkalizing agent, which would be quite unexpected by
those
skilled in the art and confirmed for the first time by the present inventors.
In a preferred embodiment of the present invention, the alkalizing agent
showing a pH of 8 to 12 when suspended or dissolved in water may be selected.
For
example, magnesium oxide, magnesium carbonate or magnesium aluminum silicate
is
preferred. According to the present invention, an excellent dissolution rate
of
nebivolol may be obtained at a low pH such as pH 1.2 as well as at pH 5 to 7,
e.g., in
water, by the addition of an alkalizing agent to nebivolol or a
pharmaceutically
6
CA 03010857 2018-07-06
acceptable salt thereof, thereby maintaining a high dissolution rate of drug
even with the
pH change when administered to a living body. Therefore, the problem of a low
dissolution rate of nebivolol and the consequential sudden change in
dissolution rate in
vivo may be settled.
In the composition of the present invention, the amount of alkalizing agent is
preferably 0.02 to 10 parts by weight, more preferably, 0.04 to 4 parts by
weight based
on 100 parts by weight of the composition. If the amount of alkalizing agent
is less
than 0.02 parts by weight based on 100 parts by weight of the composition, the
effect on
improvement of dissolution rate may be week, whereas an excessive amount over
10
parts by weight based on 100 parts by weight of the composition may lead to a
decreased dissolution rate of nebivolol. In the Examples of the present
invention,
compositions containing nebivolol with each of magnesium carbonate, magnesium
oxide and magnesium aluminum silicate as an alkalizing agent (Examples 1 to 5)
and
composite preparations of nebivolol and rosuvastatin containing an alkalizing
agent
(Examples 6 to 9) were compared with a preparation similar to commercial
product
containing no alkalizing agent (Comparative Example 1) and preparations of
Comparative Examples 2 and 3, and it was confirmed that the compositions of
Examples showed significantly high dissolution rates of nebivolol in water.
The pharmaceutical composition according to the present invention further
comprises a pharmaceutically acceptable additive, in addition to an active
ingredient
and an alkalizing agent, within a range having no influence on the effect of
the present
invention. Specifically, the pharmaceutically acceptable additive may be at
least one
selected from the group consisting of a filler, a binder, a disintegrant, a
glidant and a
lubricant.
Examples of the filler which may be used in the present composition include
cellulose derivatives such as microcrystalline cellulose, low-substituted
hydroxypropyl
cellulose, methyl cellulose, etc., starches such as potato starch, corn
starch, etc., lactose,
fructose, etc.
7
CA 03010857 2018-07-06
Examples of the binder include polyvinyl pyrrolidone, hydroxypropylmethyl
cellulose, hydroxypropyl cellulose, ethyl cellulose, methyl cellulose,
poloxamer,
polyethylene oxide, polymethylacrylate, natural gums, synthetic gums,
copovidone,
gelatin, etc.
Examples of the disintegrant include sodium starch glycolate, sodium
carboxymethyl cellulose, croscarmellose sodium, low-substituted hydroxypropyl
cellulose, pre-gelatinized starch, crospovidone, etc.
Examples of the glidant include colloidal silicon dioxide, magnesium aluminum
metasilicate, etc.
Examples of the lubricant include magnesium stearate, stearic acid, sodium
stearyl fumarate, glyceryl palmitostearate, etc.
In a preferred embodiment, the pharmaceutical composition of the present
invention may comprise, as pharmaceutically acceptable additives, 40 to 95
parts by
weight of a filler, 1 to 10 parts by weight of a binder, 1 to 10 parts by
weight of a
disintegrant, 0.1 to 2 parts by weight of a glidant, 0.1 to 2 parts by weight
of a lubricant
based on 100 parts by weight of the composition.
The type of administration for the pharmaceutical composition with improved
dissolution rate according to the present invention is not specifically
limited, and may
be any one suitable for various administration route such as oral, parenteral
(i.e.,
intramuscular, subcutaneous, intravenous, etc.). Oral administration is
especially
preferred for the present composition. Examples of
formulations for oral
administration include tablets, capsules, granules, troches, emulsions,
suspensions, etc.
Preferably, the present composition may be provided as a solid formulation for
oral
administration such as tablets or capsules in consideration of convenient use.
The
composition according to the present invention may be effectively used since
it has no
decrease in the dissolution rate regardless of the administered formulations
and also has
little deviation of dissolution rate due to the pH change in vivo.
The composition according to the present invention uses an alkalizing agent
8
CA 03010857 2018-07-06
such as magnesium carbonate, magnesium oxide and magnesium aluminum silicate,
and
therefore, without going through a complicated and high-priced process such as
micronization of nebivolol or addition of a wetting agent, it may ensure an
excellent
dissolution rate of 80% or higher within 15 minutes after starting dissolution
with no
decrease of dissolution rate in water, as confirmed in Experimental Example.
Accordingly, the composition according to the present invention may reduce the
manufacturing cost since the micronization of nebivolol or the addition of a
wetting
agent is not required, and may minimize the occurrence of a deviation of
bioavailability
under fasting or fed condition since there is little deviation of the
dissolution rate due to
the pH change, thereby ensuring a constant pharmacological action and an
improved
therapeutic effect.
The pharmaceutical composition of the present invention may be prepared
according to a conventional manufacturing process of a solid oral dosage form,
for
example, direct compression, wet granulation, dry granulation, etc. Preferred
method
Is for preparing a pharmaceutical composition according to the present
invention
comprises the steps of: a) mixing nebivolol or a pharmaceutically acceptable
salt thereof
with a pharmaceutically acceptable additive to form a mixture; and b)
formulating the
mixture, wherein an alkalizing agent is added in step a) or b).
Preferably, the mixing step may comprise the step of mixing an active
ingredient
comprising an effective amount of nebivolol or a pharmaceutically acceptable
salt
thereof with a pharmaceutically acceptable additive to form a mixture and
compressing
the mixture to form granules. More preferably, granules may be prepared by a
wet
granulation method.
Preferably, the formulating step may comprise the step of mixing the granules
prepared from the mixture with the remains of a pharmaceutically acceptable
additive
and compressing into tablets. In the manufacturing method according to the
present
invention, an alkalizing agent may be mixed with an active ingredient and an
additive to
be granulated in the mixing step, or post-mixed with granules prepared in the
mixing
9
CA 03010857 2018-07-06
step and the remains of a pharmaceutically acceptable additive in the
formulating step.
In a further embodiment, the manufacturing method may further comprise the
step of coating the formulated pharmaceutical composition by a conventional
method.
The pharmaceutical composition of the present invention may further comprise
an additional active ingredient for preventing or treating cardiovascular
diseases such as
hypertension, congestive heart failure, arteriosclerosis, metabolic disorder,
endothelial
dysfunction, etc. in addition to nebivolol or a pharmaceutically acceptable
salt thereof.
Examples of the additional active ingredient for preventing or treating
cardiovascular
diseases include, but not limited to, hydrochlorothiazide, ramipril,
enalapril,
lo lercanidipine, nisoldipine, amlodipine, losartan, eprosartan,
candesartan, telmisartan,
valsartan, olmesartan, rosuvastatin, atorvastatin, pravastatin, fluvastatin,
lovastatin,
simvastatin or pharmaceutically acceptable salts thereof. In a preferred
embodiment,
the further active ingredient is rosuvastatin or a pharmaceutically acceptable
salt thereof,
more preferably, rosuvastatin calcium.
Mode for the Invention
Hereinafter, the present invention is explained in detail by Examples. The
following Examples are intended to further illustrate the present invention
without
limiting its scope.
Examples 1 to 3
In accordance with the composition as described in Table 1, nebivolol
hydrochloride was mixed with additives, and purified water with Tween 80
dissolved
therein was added thereto. The mixture was used to prepare granules according
to a
wet granulation method. Subsequently, magnesium oxide, magnesium carbonate or
magnesium aluminum silicate, as an alkalizing agent, and the remains of
additives were
post-mixed with the granules, and colloidal silicon dioxide and magnesium
stearate, as a
lubricant, were added thereto. The resultant was compressed into tablets
(239.2
CA 03010857 2018-07-06
mg/tablet) using a tablet press (STP Machinery Co., Ltd., ZP198 Model) with a
round
punch having a diameter of 8.6 mm.
Table 1 below shows the amount of component per tablet.
[Table 1]
Components Process Example 1 Example 2 Example 3
mg/Tab % mg/Tab % mg/Tab %
Nebivolol HC1 Wet granulation 5.45 2.3 5.45 2.3 5.45
1.6
Lactose monohydrate 168.99 70.6 170.49 71.3 185.35
53.0
Microcrystalline cellulose 46.00 19.2 46.00 19.2 80.0
22.9
Hypromellose 2910 4.60 1.9 4.60 1.9 12.0 3.4
Tween 80 0.46 0.2 0.46 0.2 -
Butylated hydroxyanisole
Microcrystalline cellulose Post-mixing
Sodium starch glycolate 10.00 4.2 10.00 4.2 10.00
4.2
Croscarmellose Na
Magnesium oxide 20.00 0.8 0.50 0.2 0.10 0.04
Magnesium aluminum
silicate
Magnesium carbonate
Rosuvastatin Ca
Colloidal silicon dioxide Lubricant 0.50 0.2 0.50 0.2
0.50 0.2
Magnesium stearate mixing 1.20 0.5 1020 0.5 1.20 0.5
Ethanol
Water 35.00 35.00 35.00
Opadry 03B28796
Subtotal for wet granules 225.50 227.00 227.40
Total 239.20 100.0 239.20 100.0 239.20 100.0
Examples 4 and 5
In accordance with the composition as described in Table 2, nebivolol
hydrochloride was mixed with additives, and purified water was added thereto.
The
mixture was used to prepare granules according to a wet granulation method.
Subsequently, magnesium oxide, magnesium carbonate or magnesium aluminum
silicate, as an alkalizing agent, and the remains of additives were post-mixed
with the
granules, and colloidal silicon dioxide and magnesium stearate, as a
lubricant, were
1
CA 03010857 2018-07-06
added thereto. The resultant was compressed into tablets (350.0 mg/tablet)
using a
tablet press (STP Machinery Co., Ltd., ZP198 Model) with a round punch having
a
diameter of 10 mm.
Examples 6 and 7
In accordance with the composition as described in Table 2, nebivolol
hydrochloride was mixed with additives, and a mixture of purified water and
ethanol
with BHA and Tween 80 dissolved therein was added thereto. The mixture was
used
to prepare granules according to a wet granulation method. Subsequently,
rosuvastatin
calcium, magnesium aluminum silicate, as an alkalizing agent, and the remains
of
additives were post-mixed with the granules, and colloidal silicon dioxide and
magnesium stearate, as a lubricant, were added thereto. The resultant was
compressed
into tablets (350.0 mg/tablet: Example 6; 147.0 mg/tablet: Example 7) using a
tablet
press (STP Machinery Co., Ltd., ZP198 Model) with a round punch having a
diameter
Of 10 MM or 7.5 mrn.
The tablets thus obtained were coated by spray coating with Opadry 03B28796
(Colorcon, Inc.) dissolved in 80% ethanol and water to produce film-coated
tablets.
Table 2 below shows the amount of component per tablet.
[Table 2]
Components Process Example 4 Example 5 Example 6
Example 7
mg/Tab % mg/Tab % mg/Tab % mg/Tab %
Nebivolol HC1 Wet 5.45 1.6 5.45 1.6 5.45 1.5 5.45
3.6
Lactose monohydrate granulation 185.35 53.0 189.35 54.1
162.32 44.8 68.33 45.0
Microcrystalline 80.00 22.9 80.00 22.9 80.00 22.1
33.70 22.2
cellulose
Hypromellose 2910 12.00 3.4 12.00 3.4 12.00 3.3
5.00 3.3
Tween 80 0.20 0.06 0.10 0.07
Butylated 0.03 0.0 0.02 0.0
hydroxyanisole
Microcrystalline Post- 40.00 11.4 40.00 11.4 42.00 11.6
17.70 11.6
cellulose mixing
Sodium starch glycolate 15.00 4.3 15.00 4.1 6.30
4.1
12
Croscarmellose Na 15.00 4.3
Magnesium oxide
Magnesium aluminum 7.00 2.0 7.00 1.9 3.00 2.0
silicate
Magnesium carbonate - 3.00 0.9
Rosuvastatin Ca - _____________________________ 20.80 5.7 5.20
3.4
Colloidal silicon Lubricant 1.70 0.5 1.70 0.5 1.70 0.5
0.70 0.5
dioxide mixing
Magnesium stearate 3.50 , 1.0 3.50 1.0 3.50 1.0 1.50
1.0
Ethanol 3.00 2.00 __
Water 45.00 45.00 41.00 18.00
Opadry 031128796 12.0 3.3 5.0 3.3
Subtotal for wet granules 282.80 286.80 260.00 112.60 ,
Total 350.00 100.0 350.00 100.0 362.00
, 100.0 -- 152.00 -- 100.0
Examples 8 and 9
In accordance with the composition as described in Table 3, nebivolol
hydrochloride was mixed with additives, and a mixture of purified water and
ethanol
with Tween 80 dissolved therein was added thereto. The mixture was used to
prepare
granules according to a wet granulation method. Subsequently, rosuvastatin
calcium,
magnesium carbonate, as an alkalizing agent, and the remains of additives were
post-
mixed with the granules, and colloidal silicon dioxide and magnesium stearate,
as a
lubricant, were added thereto. The resultant was compressed into tablets
(350.0
mg/tablet) using a tablet press (STP Machinery Co., Ltd., ZP198 Model) with a
round
punch having a diameter of 10 mm.
The tablets thus obtained were coated by spray coating with Opadry 031;680010
(Colorcon, Inc.) dissolved in 80% ethanol to produce film-coated tablets.
[Table 3]
Components Process Example 8 Example 9
mg/Tab % mg/Tab %
Nebivolc11-1C1 Wet granulation 5.45 1.5 5.45 1.5
I.actose monohydrate 162.35 44.8 162.35 44.8
Mierocrystalline cellulose 80.00 22.1 80.00 22.1
Hypromellose 2910 12.00 3.3 12.00 3.3
Tween 80 0.20 0.06 0.20 0.06
13
CA 3010857 2018-07-26
CA 03010857 2018-07-06
Microcrystalline cellulose Post-mixing 48.00 13.3 63.60 17.6
Sodium starch glycolate 15.00 4.1 15.00 4.1
Magnesium carbonate 1.00 0.3 1.00 0.3
Rosuvastatin Ca 20.80 5.7 5.20 1.4
Colloidal silicon dioxide Lubricant 1.70 0.5 1.70 0.5
Magnesium stearate mixing 3.50 1.0 3.50 1.0
Opadry 03B28796 12.00 3.3 12.00 3.3
Total 362.00 100.0 362.00 100.0
Comparative Examples 1 to 3
In accordance with the composition as described in Table 4, ncbivolol
hydrochloride was mixed with additives, and purified water with Tween 80
dissolved
therein (Comparative Examples l and 2) or purified water (Comparative Example
3)
was added thereto. The mixture was used to prepare granules according to a wet
granulation method. Subsequently, the remains of additives were post-mixed
with the
granules, and colloidal silicon dioxide and magnesium stearate, as a
lubricant, were
added thereto. The resultant was compressed into tablets (239.2 mg/tablet:
lo Comparative Example 1 similar to a commercial product; 350.0 mg/tablet:
Comparative
Examples 2 and 3) using a tablet press (STP Machinery Co., Ltd., ZP198 Model)
with a
round punch having a diameter of 8.6 mm or 10.0 mm.
[Table 4]
Components Process Comparative Comparative Comparative
Example 1 Example 2 Example 3
mg/Tab % mg/Tab % mg/Tab %
Nebivolol HCI Wet granulation 5.45 2.3 5.45 1.6 5.45
1.6
Lactose monohydrate 141.75 59.3 191.89 54.8 192.35
55.0
Microcrystalline cellulose 46.00 19.2 80.00 22.9 120.00
34.03
Hypromellose 2910 4.60 1.9 12.00 3.4 12.00 3.4
Tween 80 0.46 0.2 0.46 0.1
Butylated hydroxyanisole
Microcrystalline cellulose Post-mixing 23.00 9.6 40.00 11.4
Sodium starch glycolate - 15.00 4.3
Croscarmellose Na 16.10 6.7 15.00 4.3
Magnesium oxide
Magnesium aluminum
14
CA 03010857 2018-07-06
silicate
Magnesium carbonate
Rosuvastatin Ca
Colloidal silicon dioxide Lubricant 0.69 0.3 1.70 0.5
1.70 0.5
Magnesium stearate mixing 1.15 0.5 3.50 1.0 3.50 1.0
Ethanol
Water 35.00 35.00 35.00
Opadry 03B28796
Subtotal for wet granules 198.26 289.80 329.80
Total 239.20 100.0 350.00 100.0 350.00 100.0
Experimental Example: Dissolution test in water
Tablets prepared in Examples 1 to 6, 8 and 9 and Comparative Examples 1 to 3
were subjected to a drug dissolution test to determine the dissolution rate of
nebivolol.
Dissolution test was performed at 50 rpm according to paddle method using
water as a dissolution medium. 5 mL of each test solution taken at 5, 10, 15,
30, 45, 90
and 120 minutes after starting the test was filtered through a membrane filter
and
analyzed using HPLC under the following condition to calculate dissolution
rate against
the standard solution. Table 5 and Fig. 1 show the results.
- Analytical conditions of HPLC -
Detector: UV 225 nm
Column: Xbridge C18, 4.6 x 150 mm, 5 pm
Mobile phase: buffer/methanol (40/60)
(Buffer: 3.4 g of tetrabutyl ammonium hydrogen sulfate dissolved in 1L of
water)
Flow rate: 1.0 mL/min
Injection volume: 20 pL
[Table 5]
Samples 0 5 10 15 30 45 60 90 120
Example 1 0.0 68.4 85.9 87.1 90.7 91.1 91.5 91.7
92.4
Example 2 0.0 64.7 78.7 86.0 87.2 88.7 90.0 91.5
93.3
Example 3 0.0 68.1 75.8 84.2 84.8 85.4 86.0 86.6
87.1
Example 4 0.0 71.8 83.7 85.8 92.3 92.6 93.0 93.3
93.7
CA 03010857 2018-07-06
Example 5 0.0 74.2 81.9 84.4 87.8 88.4 90.0 91.8
93.0
Example 6 0.0 80.6 91.3 93.0 95.6 96.9 97.6 98.0
98.3
Example 8 0.0 68.3 88.8 91.9 93.8
Example 9 0.0 68.0 78.8 83.5 87.8
Comparative Example 1 0.0 0.0 1.9 2.4 4.9 7.4 10.2
13.7 15.7
Comparative Example 2 0.0 56.6 57.0 55.4 55.5 55.9 54.7
54.6 54.54
Comparative Example 3 0.0 53.1 50.7 44.1 34.1 26.8 21.5
13.2 11.5
As confirmed in Table 5 and Fig. 1, all of the nebivolol preparations and the
composite preparations comprising nebivolol and rosuvastatin according to the
present
invention showed excellent dissolution rates of 83% to 93% within 15 minutes
after
starting the test, while the tablets of Comparative Examples 1 to 3 prepared
without
adding alkalizing agents showed very low dissolution rates even at 2 hours
after starting
the test.
Furthermore, it was confirmed that the composite preparations of nebivolol and
rosuvastatin using magnesium carbonate as an alkalizing agent (Examples 8 and
9) as
well as those using magnesium aluminum silicate (Examples 6 and 7) showed
dissolution rates of 80% or higher at 15 minutes after starting the test.
The above results demonstrates that nebivolol preparations as well as
composite
preparations comprising nebivolol and an additional active ingredient for
preventing or
treating cardiovascular diseases may have the effect of improving dissolution
rate of
nebivolol in water of relatively high pH, regardless of the type of alkalizing
agent.
16