Note: Descriptions are shown in the official language in which they were submitted.
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
1
Method and apparatus for treating a leaching residue of a sulfur-
containing metal concentrate
The invention relates to a process and an apparatus for treating a leaching
residue generated in a leaching of a sulfur-containing metal concentrate,
where-
in the leaching residue is fed into a reactor, wherein a fluidizing gas is
injected
into the reactor to form a fluidized-bed containing at least a portion of the
leach-
ing residue, wherein the leaching residue is heated in the presence of inert
particles to a temperature between 600 and 900 C in an oxidizing atmosphere
to produce calcined particles and SO2.
Direct leaching of sulfuric zinc concentrate is a well-established applied
technol-
ogy for zinc production. The process generates a considerable amount of ele-
mental sulfur that is currently dumped due to lack of proven treatment
solutions.
With regard to the polymetallic contamination and the poor leachability re-
sistance it is increasingly difficult to obtain authority approval for long-
term
dumping of this sulfur residue.
A typical composition of the residue in dry state shows the following composi-
tion:
component range preferred range
Total S 40 - 70 wt-% 45 - 65 wt-%
Elementary S 30 - 60 wt-% 35 - 55 wt-%
Pb 1 - 20 wt-% 2 - 15 wt-%
Fe 3 - 20 wt-% 5 - 15 wt-%
5i02 3 - 20 wt-% 5- 15 wt-%
Zn 0,1 - 10 wt-`)/0 .. 1 - 5 wt-%
Ag 100 - 1000 g/t 200 - 600 g/t
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
2
The given composition is also taken as a basis for the current invention. The
sulfur is combusted to SO2 and the contained non-combustibles are separated
as a solid product, consisting mainly of silica, lead, zinc and iron as well
as up to
0,1 wt-% silver. The process allows the utilization of the solid product,
namely
Fe and Pb calcine in a lead smelter, silver recovery as well as the production
of
steam and sulphuric acid.
For a further utilization of the metallic compounds, especially silver, it is
neces-
sary to combust contained sulfur to SO2. Such roasting is e.g. performed in a
fluidized-bed like it is for example proposed in WO 2011/076995. Therein,
sulfur
containing leaching residue or part of it is fed to a fluidized-bed treatment
in
which the residue is burned into sulfuric dioxide and the valuable metals con-
tained in the leaching residue are recovered. To avoid agglomeration, sand is
added to the fluidized-bed.
However, later on it is difficult to separate sand particles from the valuable
me-
tallic compounds.
Therefore, it is the object of this current invention to provide a process and
a
corresponding apparatus for roasting sulfur-containing leaching residues and,
at
the same time, minimizing agglomeration as well as providing a complete sepa-
ration of the calcined material.
This problem is solved with a method according to current claim 1. A leaching
residue generated in a leaching of sulfur containing, preferably non-ferrous,
metal concentrate is fed into a reactor. Therein, the residue and inert
particles
are fluidized by a fluidizing gas which is injected from at least one nozzle,
pref-
erably from a nozzle grid of the reactor. Thereby, a fluidized-bed is formed,
which operates at temperatures between 500 and 900 C, preferably 600 to
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
3
900 C, most preferably between 650 and 850 C in an oxidizing atmosphere to
produce calcined particles and SO2. After spending a specific residence time,
the calcined particles produced out of the leaching residue are withdrawn from
the reactor while respective chemical reactions have been carried out.
Most important, the fluidized-bed is designed such that at least 60 wt-%,
prefer-
ably 80 wt-%, most preferably 90 wt-% (independent from the portion of re-
moved calcined particles) of the inert particles are removed from the
fluidized
bed while at least 60 wt-% preferably 80 wt-%, most preferably 90 wt-% (inde-
pendent from the portion of removed inert particles) of the calcined particles
are
removed together with a gas stream containing off-gases and the fluidizing
gas.
This effect can be achieved by different parameters being sensitive values for
the respective minimum fluidization velocities of the inert and the calcined
parti-
cles like particle diameters or densities.
Thereby, the different particles are already separated into the fluidizing-bed
reactor, which is why no further particle separation is needed afterwards. By
lifting the calcined particles above the fluidized-bed, it is possible to
withdraw at
least the main part of the calcined particles without any additional inert
material.
In addition, the specific during operation is beneficial for a further reason.
Should all particles lump together, the agglomerates will obtain a higher
effec-
tive particle size, enhance higher weight and, therefore, will sink down in
the
fluidized-bed. In the lower part of the fluidized bed, the inert particles,
due to
their higher concentration, prevent already lumped calcined particles from
sinter-
ing as it is well-known from the processes being state of the art.
Furthermore, the lifting of the calcined particles above the fluidized bed
results
in a more uniform reaction of the oxygen contained within the fluidization gas
and the sulfur within the residue. This occurs since the oxygen concentration
is
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
4
maximum at the inner circuit, while the sulfur concentration follows the exact
opposite trend, i.e. it is minimum or zero at the inner circuit, is higher in
the first
zone and highest in the second zone due to the concentration of the residue
particles. As a result of the more uniform sulfur oxidation, hotspot formation
which is a main cause of sintering is avoided. In this context, the inert
substanc-
es, preferably located next to the bottom in a particular high concentration,
operates like a form of an isolating layer, while the term "inert" is used to
de-
scribe a substance that is not commonly reactive during the partial roasting.
It is preferred that the claimed process features two zones being arranged
above each other with respect to the reactors' height. In this option, at
least
60 wt-%, preferably 80 wt-%, most preferably 90 wt-% (independent from the
portion of calcined particles) of the inert particles are found in a first
zone of the
fluidized-bed, while at least 60 wt-%, preferably 80 wt-%, most preferably 90
wt-
% (independent from the portion of inert particles)of the residue particles or
calcined residue particles are found in a second zone above the first zone.
The
formation of these two zone occurs during steady state reactor operation and
is
particularly apparent during fluidizing gas ramp down (in case of controlled
shut
downs or planned trips).
Fine particles found in the first zone are removed from the fluidized-bed
while
fine particles from the second zone are removed together with the gas stream
containing off-gases and the fluidizing gas. The existence of these different
zones can be adjusted by different parameters or different densities of the
resi-
due particles and/or the inert particles as particle diameters and densities
are
sensitive values for the respective minimum fluidization velocities. Moreover,
the
formation of these two zones becomes more apparent by ramping down the
supply of the fluidization gas to the reactor, which results into a "safe" in
terms
of sintering potential short or long reactor shut down as explained in the
para-
graphs below.
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
Additionally, such mode of operation protects the reactor from sintering also
during planned or unexpected operational stops. Gradual ramp down of the
fluidization gas supply results to a more distinct formation of the two
fluidization
5 zones since the reactive necessity comes close to or falls below the
minimum
fluidization velocity of the inert particles (during fluidization gas ramp
down)
while still being above the corresponding minimum fluidization velocity of the
residue/calcined particles. Further gas ramp down to the point where inert
parti-
cles are no longer fluidized with a subsequent abrupt stop of the fluidization
gas
supply results to a shut-down where the first zone contains a maximized inert
amount (higher than during steady state operation) so no sintering can occur
during the period where the reactor is not in operation. Moreover, the reactor
sintering potential during start-up is also minimized in the vicinity of some
noz-
zles to the first zone which exhibits a maximized inert content. Any sintering
processes taking place in the second zones are reversed during start-up due to
the momentum and resulting movement of the first zone.
Another option of the current invention is that at least 60 wt-%, preferably
80 wt-
%, most preferably 90 wt-% (independent from the calcined particles) of the
inert
particles and at least 60 wt-%, preferably 80 wt-%, most preferably 90 wt-%
(independent from the portion of inert particles) of the leaching residue
and/or
the calcined particles are found in a common mixing zone. Preferably, they are
homogeneous mixed. Thereby, a dilution of the residue is achieved, whereby
agglomeration is prevented.
Preferably, the diameter of at least 70 wt-%, preferably at least 80 wt-%, of
the
calcined particles is below 60 pm or the diameter of at least 70 wt-%
preferably
80 wt-% of the inert particles is between 0,05 to 3 mm, preferably 0,1 to 2
mm.
Thereby, the separate withdrawing is possible. Furthermore, the latter values
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
6
are typical size for calcined particles SiO2 particles in the form of sand,
which is
why no further pretreatment is necessary.
It is especially cost effective to use SiO2 particles as inert particles since
sand is
cheap, regularly available and easy to handle. Further, SiO2 is also contained
in
a typical leaching residue as mentioned above.
Moreover, a preferred design of the claimed process uses 0,01 to 1 t inert
mate-
rial, preferably sand, per ton of dried leaching residue. Thereby, it is
possible to
achieve that the injected leaching residue does not reach the reactor bottom,
but
is combusted in the upper part of the bed and transported thereof as calcined
particles by the off-gas stream.
In addition, the average residence time for the material fed as leaching
residue
and removed as calcined particles is between 20 to 200 min, preferably between
30 to 180 min. Thereby a complete turnover can be achieved.
The average residence time for the inert particles is in the range of several
hours, preferably 2 to 10 hours, most preferably between 3 and 7 hours which
is
why it is possible to keep this solid stream small.
Preferably, the inlet velocity of the fluidizing gas is between 0,2 to 2 m/s,
prefer-
ably 0,5 to 1,5 m/s. Due to this parameter, it is possible that after drying
and/or
reducing the sulfur content of the calcined particles these particles were
lifted
above the second zone in a so called free-board zone. Therefrom, the particles
can be withdrawn together with the fluidizing gas stream and separated, e.g.
with a cyclone. Fluidizing velocity in the sense of the invention is the
velocity of
the gas phase, generated in the furnace at operating conditions related to the
empty furnace.
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
7
The oxidizing atmosphere is preferably adjusted such that A as the oxygen-fuel
equivalence ratio (defined as the ratio between the oxygen mass stream enter-
ing the fluid bed furnace divided by the minimum oxygen needed to achieve
complete stochiometric combustion of the introduced sulfur residue) is between
1,1 and 1,8, preferably 1,1 and 1,5, most preferably 1,3 and 1,5 to ensure a
complete turnover. Therefore, it is proposed to use air or oxygen enriched air
as
fluidizing gas, since air is a cheap source for the oxygen needed for complete
sulfur combustion. However, it is possible to use nitrogen or any other inert
gas
as fluidizing gas, whereby a gas with oxygen content is separately introduced.
The sulfur residue is mostly available as filter cake. To feed the material ho-
mogenously into the reactor, it is proposed to mix the material with water,
pref-
erably by means of an intensive agitator in order to disintegrate lumps or ag-
glomerates. In this scenery, the solid content is adjusted to 30 to 65 wt-%,
de-
pending on the content of the non-combustibles in the residue.
Further, it is also possible to mix the leaching residue with any inert
material,
preferably the inert material used in the fluidized-bed, whereby most
preferably
also material already used in the fluidized-bed is admixed to the residue.
There-
by, it is possible to form granules and feed the residue in form of a more ho-
mogenized particles into the reactor. As a result, it is easier to adjust the
fluidiz-
ing parameters like fluidization velocity so that most of the calcined
particles can
be withdrawn together with the off-gas stream. Further, this option has a
benefit
of a higher steam production out of the off-gases since no water is added to
the
feed material. Concluding, the overall energy balance is improved.
It is also preferred to separate the particles removed from the fluidized-bed
into
inert and calcined particles, so valuable metallic compounds being removed
together with the inert particles can be regained.
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
8
In this context, it is also preferred to use a mill in this separation stage
to liber-
ate conglomerates of calcine and inert material to optimize the process and
reach a better separation.
Furthermore, it is also preferred to separate the particles out of the second
zone
from the off-gases, so the particles can be cooled and the energy consumption
of the process is improved by recycling at least parts of the particles'
energy.
The off-gases are fed to a post combustion chamber, a boiler, a hot gas clean-
ing as well as a wet gas cleaning stage.
As already addressed, the energy balance of the process can be optimized by
recycling energy gained in the cooling of inert particles as well as the
cooling of
calcined particles. Thereby, the inert and/or the calcined particles are
cooled by
preheating a gas stream and the preheated gas stream is recycled to a process
stage before the reactor, preferably a mixer for the granules and/or to the
reac-
tor itself. Most preferably, the cooling of the inert and/or calcined
particles can
be used to preheat the fluidized gas and/or the oxygen source.
Moreover, the invention is directed to an apparatus with the features of claim
14.
Such an apparatus for treating a leaching residue generated in a leaching of
sulfur-containing, preferably non-ferrous metal concentrate comprises a mixing
tank to form a slurry out of the residues. Alternatively the leaching residue
is
treated together with inert material/sand in a high intensity mixer to
generate
granules. Further, it comprises a reactor in which during operation a
fluidized-
bed is formed. The reactor features at least one feeding conduit for feeding
the
residue into the reactor, with at least one conduit for feeding for treating a
leach-
ing residue generated in a leaching of a sulphur-containing non-ferrous metal
concentrate into the reactor, a supply conduit for feeding a fluidized gas
into the
reactor, at least one offtake line for withdrawing calcined particles produced
out
of the leaching residue together with the off-gas from the reactor and with an
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
9
outlet line for withdrawing inert particles from the fluidized bed, wherein
the
outlet line is positioned such that at least 60 wt-% of the inert particles
are with-
drawn through this outlet line. With this arrangement, agglomerations in the
reactor are avoided and, simultaneously, calcined particles are withdrawn with-
out being substantially mixed with the inert particles, preferably at least 10
wt-%
of inert particles are entrained in the off-gas together with the calcined
particles.
Further, an apparatus according to the invention is equipped with at least one
feeding device to transport a slurry or granules into the reactor.
In a preferred embodiment of the invention, a fluidizing gas is supplied to a
fluidizing-bed reactor to a so called nozzle grid, a plate containing nozzles
with
1 to 300 holes per m2 furnace area. The nozzles may be of several types includ-
ing the following: (i) not extending from the nozzle grid and having one
orifice in
the upward direction, (ii) extending above the nozzle shaft having one or more
than one orifices at angles between 0 and 180 and (iii) equipped nozzles same
as a latter with an added characteristic of a cap to further protect blocking
of the
orifices.
Preferably, the apparatus also features a first cooler connected to the outlet
for
the inert particles, and a second cooler connected to a cyclone, wherein the
calcined particles are separated from the off-gas for a separate handling of
both
sorts of particles
Further developments, advantages and possible applications of the invention
can also be taken from the following description of the drawings. All features
described and/or illustrated form the subject matter of the invention per se
or in
any combination, independent of their inclusion in the claims or their back
refer-
ence.
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
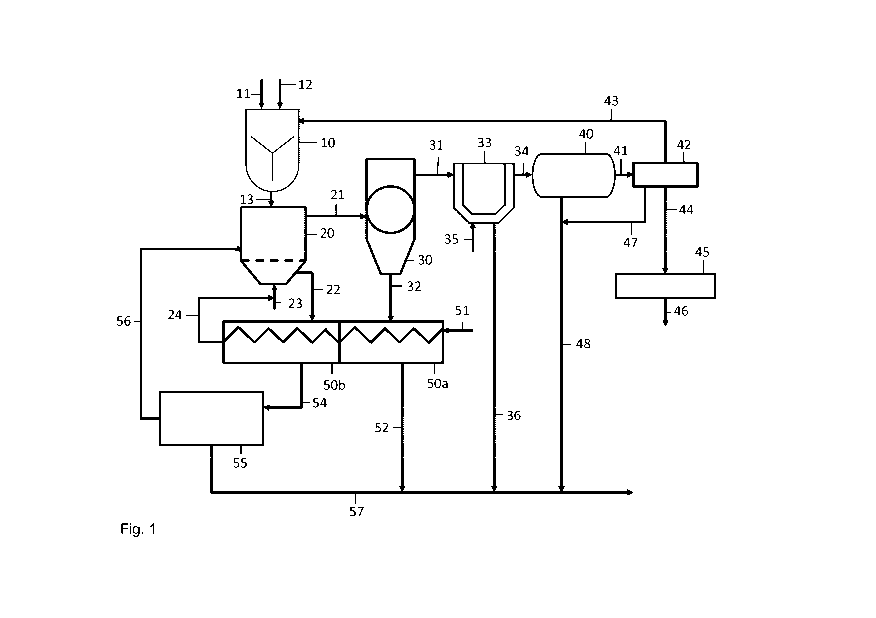
Fig. 1 shows schematically a process according to the invention using
a
slurry and
Fig. 2 shows schematically a process according to the invention using
5 granules.
Fig. 1 shows a slurry tank 10, in which the sulfur-containing residue formed
in a
direct leaching process is filled in via conduit 11. The sulfur residue is
mostly
available as filter cake. The material is mixed with water through conduit 12
by
10 means of an intensive agitator in order to disintegrate lumps and
agglomerates.
The solid content is adjusted to 30 to 65 wt-%, depending on the content of
non-
combustibles in the sulfur residue.
The slurry is injected into the fluidizing-bed reactor 20 by means of conduit
13.
The fluidizing-bed reactor 20 contains a bed of fluidized sand. The sand
serves
two purposes, namely at first providing a stable bed of fluidized solids into
which
sulfur residue can be injected and where all reactions shall take place and
sec-
ond preventing sintering of the non-combustibles by separation of individual
PbSO4/Pb0 grains.
The fluidized sand bed in the fluidized-bed reactor 20 holds the non-
combustible
compounds from the injected sulfur residue mainly in the upper part of the
bed,
while the bottom is largely depleted of non-combustibles. The combus-
tion/roasting process is conducted in such a way that the injected slurry is
dis-
tributed homogenously across the fluidized sand bed. Most of the injected mate-
rial does not reach the furnace bottom but is combusted in the upper part of
the
bed. The fluidizing velocity is adjusted such that the very fine non-
combustibles
(x80 < <40 pm) are entrained in the process gas for the most part and leave
the
furnace with the off-gas via offtake conduit 21.
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
11
A smaller part of the non-combustibles form agglomerates and are discharged
with some portion of the sand through an outlet conduit 22, like e.g. an
overflow
weir, as it is common practice in roasting of zinc concentrates and pyrite.
Another part of the non-combustible forms relatively coarse agglomerates
(> 1 mm) segregating to the bottom of the fluidized-bed. This material is dis-
charged in intervals of several hours through a not shown bottom discharge.
In the fluidized bed reactor, the different separation from inert and calcined
particles by different withdrawing positions is achieved by proper adjustment
of
the sand ratio (0,01 to 1 t per dried sulfur residue), sand particle size (0,1
to 2
mm) and fluidization velocity (0,5 to 1,5 m/sec) in combination with the
utilization
of the outlet during continuous operation as well as a fine granulometry of
the
non-combustibles (x80 < <40 pm) which is inherent to the process.
The stream of calcined particles and the off-gas is fed through offtake
conduit 21
into a cyclone 30, wherein the gas stream is separated from the calcined parti-
cles. The separated particles are fed via conduit 32 into a cooler 50a, like a
cooling drum. As a heat transfer medium, gas is fed into the cooler via line
51.
The cooled cyclone discharge is fed via line 52 into line 57.
The off-gas stream containing mainly non-combustibles together with small sand
particles is further fed via conduit 31 into a post-combustion 33 to oxidize
sulfur
fumes with additional air or any oxygen containing gas. Dust generated therein
is fed via conduit 36 into collecting conduit 57.
The off-gas is further directed via conduit 34 into a waste heat boiler 40 for
heat
recovery by steam production. Solids separated in the waste heat boiler are
also
combined via line 48 with all product streams in conduit 57. In special cases,
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
12
e.g. when the capacity of the envisaged unit is too small, the waste heat
boiler
40 may be replaced through an evaporative cooler.
Moving downstream the gas path, solids are fed into a standard hot gas clean-
ing system 42 via conduit 41. The off-gas in the hot gas cleaning 44 is
further
used for sulfuric acid production after cleaning in a standard wet gas
cleaning
system 45.
Particles separated in the hot gas cleaning system 43 may be also mixed
through conduit 47 and 48 with the total product stream in conduit 57.
However,
in the case that the solid stream (or any other solid stream) contains still a
sig-
nificant amount of sulfide sulfur then the stream is recirculated, preferably
to the
slurry tank via conduit 43. Also, even it is not shown it is possible to
recycle the
solid stream into the reactor 20. Further, also not shown recycling conduits
from
the post combustion 33 and/or the boiler 40 are possible.
Inert particles withdrawn from the fluidized-bed of the fluidized-bed reactor
20
are fed into the cooler 50b where they are also cooled with air or any other
gas.
This hot gas stream can be used as fluidizing gas and fed via line 24 into the
fluidizing bed reactor via line 23. It is possible, that both coolers are
designed as
cooling sections and use the same heat transfer medium. Also, even if it is
not
shown, it is possible to have separate lines for the heat transfer medium in
both
cooling sections.
The cooled sand is fed into an optional mill and separation unit 55 via
conduit
54, wherein the metallurgical particles are separated from sand. The
metallurgi-
cal particles are collected in conduit 57, wherein also all other product
lines will
feed in.
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
13
The sand or any other inert particles can be recycled into the fluidized-bed
reactor 20 directly via conduit 56.
Fig. 2 shows a nearly identical process. The only difference is that not a
slurry,
but granules are fed into the fluidized-bed reactor 20. This option has a
benefit
of a higher steam production since no water is added to the feed material. The
sticky sulfur residue filter cake needs to be disintegrated in order to permit
a
controlled feeding into the furnace 20. This is achieved by means of mixing
the
sulfur residue with sand in a mixer 14, preferably a high shear mixer. Therein
the sulfur residue is fed in via conduit 11 as well as additional sand is fed
in via
conduit 15. It was found that an addition of 1,5 to 4 t of sand per ton of
dried
sulfur residue is required to achieve a free flowing feed mixture. The grain
size
of the sand is preferably 0,1 to 1 mm. The intensive mixing generates granules
with a suitable grain size for the fluidized-bed roasting (the bulk of the
solids
being between 300 and 600 pm, while solids between 0,1 and 3 mm will still be
present).
Prior to feeding into the fluidized-bed reactor 20, the granules are passed
via
conduit 16 into an optional drier 17 in order to increase their stability.
Removed
water is withdrawn via conduit 18. The dryer 17 may utilize preheated air
which
is passed by directly or indirectly to another process stage and/or preheated
water or other liquid through a heat source inherent or external to the
process.
Moreover, the dryer 17 may also be electrically heated or designed as a fluid-
ized-bed. The necessity of the drying stage depends on the characteristics of
the sulfur residue. It is well possible that not at all sulfur residues will
require
drying prior to roasting.
The combustion/roasting in the fluidized-bed reactor 20 differs from the
process
in Fig. 1 in so far that the entire bed volume of the fluidized-bed reactor 20
is
used for the combustion/roasting process. Sintering is tackled by optimal sepa-
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
14
ration of sticky particles (lead sulfates, -oxides) in a sand matrix.
Combustion
takes place at 650 to 850 C with a A factor of 1,1 to 1,5 as in the process
ac-
cording to Fig. 1. In balance of the fluidized-bed reactor 20 exhibits a heat
defi-
cit, a carbonaceous fuel may be combusted in the fluidized-bed reactor 20 to
maintain the desired operation temperature. This is also possible for process
according to Fig. 1. However, the process shall be self-sustaining to be
attrac-
tive.
The fluidization velocity is in the typical range of stationary roasting,
namely 0,5
to 1,5 m/sec. The major calcined portion is discharged via the outflow weir.
Small sand and calcine particles are entrained in the roaster off-gas. The
fluidiz-
ing bed reactor 20 has a bottom discharge for internal discharging or eventual
coarse agglomerates. Calcine discharged from the fluidized-bed reactor 20 is
cooled in a cooling drum 50 (a,b). Separation of sand and silver containing
valuable components is achieved by treatment in an attrition stage and subse-
quent classifying by screening or air classification.
The off-gas contains mainly non-combustibles together with some small portion
of sand. It has to be noted that the solid particle contains most of the
silver as
well as lead sulfates/oxides, zinc sulfates/oxides, iron mainly as hematite
and
silica and shall be sold and further treated in a lead smelter.
In an additional process stage clean sulfur can be separated from the sulfur
residue in a vacuum distillation stage that is operated with steam being
generat-
ed in the waste heat boiler at 250 to 300 C. The non-combustible fraction is
thus enriched up to 60 wt-% and exists as very fine (x80 < < 40 pm) suspended
solids in a liquid sulfur phase. This sulfur phase is atomized to fine
particles (x80
< 80 pm) and can be used for combustion as described for both figures. Evapo-
rated sulfur is condensed in a bath of liquid sulfur at a temperature short
below
the evaporation temperature of sulfur. Evaporated impurieties as mercury are
CA 03012028 2018-07-19
WO 2017/129341 PCT/EP2016/082772
thus kept in gas phase and can be separated from the sulfur. The off gas is
further cleaned in state of the art gas cleaning stages. The condensed sulfur
is
pure elemental sulfur and can be sold as product.
5 The sand (or any other inert particles) removed in the mill and
separation unit
are at least partly recycled to the mixer for forming granules via a conduit
61.
However, it is also possible to recycle at least parts of these particles into
fluid-
ized bed reactor 20.
10 Pre-heated air (or any other gas) from at least one cooler 50a, 50b are
fed at
least partly into the dryer 17 via conduits 62, 63, where they are used for
drying
and/or pre-heating the granules.
Further, at least parts of the pre-heated gas from at least one cooler 50a,
50b in
15 the fluidized bed reactor 20 via conduits 62, 64 , where it can be used
as flu-
idzing gas and/or oxygen source.
CA 03012028 2018-07-19
WO 2017/129341
PCT/EP2016/082772
16
List of references
mixer
11 -13 conduit
5 14 mixer
15, 16 conduit
17 dryer
18, 19 conduit
fluidized-bed reactor
10 21 offtake conduit
22 outlet conduit
23, 24 conduit
cyclone
31,32 conduit
15 33 post combustion stage
34 - 36 conduit
boiler
41 conduit
42 hot gas cleaning
20 43, 44 conduit
wet gas cleaning
46 - 48 conduit
50a, 50b cooler
51 - 54 conduit
25 55 mill and separation unit
56, 57 conduit
61 - 64 conduit