Note: Descriptions are shown in the official language in which they were submitted.
DEMINERALIZATION AND UPGRADING OF PETROLEUM COKES
FIELD
[0001] The invention is in the field of chemical engineering,
particularly oxidative
processes for aqueous treatment of heavy hydrocarbon and metal-containing
solids, such as petroleum cokes.
BACKGROUND
[0002] In accordance with the IUPAC definition, "petroleum coke" is a
carbonization product of high-boiling hydrocarbon fractions obtained in
petroleum
processing ("petcoke"). It is the general term for all special petroleum coke
products
such as calcined and green petroleum coke (Fitzer et al., Pure & Appl. Chem.,
Vol.
67, No. 3, pp. 473-506, 1995). "Calcined coke" is generally defined as a
petroleum
coke obtained by heat treatment of green coke, for example to about 1600 K (or
between 1200 C and 1350 C), normally having a hydrogen content of less than
0.1
wt.% (see US Patent No. 4,022,569). "Green coke" (or raw coke) is defined as
the
primary solid carbonization product from high boiling hydrocarbon fractions,
obtained at temperatures below 900 K. It contains a fraction of matter that
can be
released as volatiles during subsequent heat treatment at temperatures up to
approximately 1600 K. This mass fraction, the so-called volatile matter, is in
the
case of green coke between 4 and 15 wt.%, depending in part on the heating
rate.
[0003] In bitumen upgrading, coking processes involve thermal cracking,
in
which the hydrogen to carbon (H/C) atomic ratio of the product is increased by
a
carbon rejection mechanism (Rana et al., A review of recent advances on
process
technologies for upgrading of heavy oils and residua, Fuel 86(9) (2007) 1216-
1231), which involves cracking and polymerization reactions (Dutta et al.,
Thermal
cracking of Athabasca bitumen: influence of steam on reaction chemistry,
Energy &
fuels 14(3) (2000) 671-676). Cracking reactions typically produce gas and
liquid
products, while radical polymerization reactions produce petroleum coke (Yoon
et
al., Thermogravimetric study of coal and petroleum coke for co-gasification,
Korean
Journal of Chemical Engineering 24(3) (2007) 512-517).
[0004] In petroleum refining, the residue from crude oil distillation
processes
may be further processed by what are termed "delayed coking" or "fluid coking"
processes, which both provide lighter liquids from the residual oil. Delayed
coking
1
CA 3012804 2018-07-27
commonly occurs at a temperature range of 415-450 C, while fluid coking
generally
uses higher temperatures ranging from 480 to 565 C (Wang et al., Clean and
efficient use of petroleum coke for combustion and power generation, Fuel
83(10)
(2004) 1341-1348). In a typical delayed coking process, coke drum reactors are
used to hold, or delay, a heated feedstock while thermal cracking takes place.
In
fluid coking, a portion of the coke formed in thermal cracking reactions is
burned as
a fluidized solid to provide heat for the cracking process. Delayed coke can
be
classified, based on its morphological characteristics, as shot, sponge or
needle
coke. Shot coke is a hard, spherical solid; sponge coke is generally dull and
black
with porous and amorphous structure; and needle coke is generally silver-gray,
having a crystalline structure (Birghila et al., "Study on physical-chemical
properties
of petroleum cokes", Romanian Journal of Physics 56(7-8) (2011) 976-82; Under,
"Everything you always wanted to know about petroleum coke: a handbook", Allis
Mineral Systems-Kenndy Van Saun, 1993; Small et al., Adsorption of acid
extractable oil sands tailings organics onto raw and activated oil sands
coke",
Journal of Environmental Engineering 138(8) (2012) 833-840).
[0005] Generally, petcoke has a high carbon content (80-85 wt%)
consisting of
polycyclic aromatic hydrocarbons with heteroatoms, such as sulfur, nitrogen,
and
oxygen, and some metals present (Lv et al., "Characterization of condensed
aromatics and heteroatomic species in Yanshan petroleum coke through ruthenium
ion-catalyzed oxidation using three mass spectrometers", RSC Advances 6(66)
(2016) 61758-61770). Disadvantageously, petcoke typically has relatively high
amounts of sulfur (4-8 wt%), vanadium (¨ 700 ppm) and has the potential to
impact
human and animal health (Caruso et al., "Petroleum coke in the urban
environment:
A review of potential health effects", International journal of environmental
research
and public health 12(6) (2015) 6218-6231).
SUMMARY
[0006] Processes are provided for the oxidative solubilization of metal-
containing petroleum cokes in a basic aqueous solution, so as to segregate a
solid
metal-containing residue from a solubilized and demineralized organics
fraction.
Oxidation conditions are provided that optimize the yield of soluble partial
oxidation
2
CA 3012804 2018-07-27
products and minimize the generation of CO2. In some embodiments, an oxidation
catalyst may be used. The pH of the solubilized organics fraction may be
reduced,
under conditions that precipitate an upgraded carbonaceous material, in some
embodiments comprising humic acid analogs, yielding a barren leachate
solution.
[0007] Methods are accordingly provided for processing petroleum cokes,
such
as solid green petroleum cokes comprising a carbonaceous component and a
transition metal component. The carbonaceous component may for example
include polycyclic aromatic hydrocarbons, and the petroleum coke may be
characterized by one or more of the elemental compositions set out in Table 1,
such as (in wt%): 80 carbon 598; hydrogen 58; .2.5 oxygen 510; nitrogen
0.5;
sulfur 5 10; 4.001 vanadium 50.8; 4.001 iron 50.5; ?Ø001 nickel 50.5; 4.001
molybdenum 50.1; and, 4.01 cobalt 51.
[0008] A soluble portion of the solid green petroleum coke may be
solubilized in
a basic subcritical aqueous solubilization liquid under solubilization
conditions that,
for example, involve a solubilization pressure of at least 500 psi (or 5 1000
psi, or
from 500 psi to1000 psi). The solubilization temperature may for example be
from
220 C to 240 C (or 220 or 225 or 230 or 235 C and/or 5_ 225 or 230 or 235
or
240 C). A solubilization base may be added, for example at a concentration
effective to maintain a desired solubilization pH such as pH 9, 10, 11 or
12
and/or is 5 9, 10, 11, 12 or 13. The added solubilization base may for example
be a
hydroxide, such as an alkali metal hydroxide, such as KOH or NaOH (the mass
ratio of solid green petroleum coke to KOH may for example be from about 0.5:1
to
about 5:1, such as about 1:1). The effective solubilization time, meaning the
period
during which a recite degree of solubilization takes place, may for example be
not
more than 1 or 2 hours. Solubilization may take place wholly or partially in
the
presence of an oxidizing atmosphere, such as an atmosphere enriched in oxygen,
for example comprising more than 21%, 25%, 30%, 35%, 40%, 45%, 50%, 55%,
60%, 65%, 70%, 75%, 80%, 85%, 90% or 95% oxygen. Agitation may take place
during the effective solubilization time, for example to generate a stirred
reaction
region in the solubilization liquid having a desired Reynolds number, for
example of
0,000. This may for example involve stirring, for example at 500 rpm (or from
500 rpm to 1000 rpm).
3
CA 3012804 2018-07-27
[0009] The solubilization conditions and effective solubilization time
may be
arranged so as to solubilize a desired proportion of the petcoke, for example
at
least 60%, 65%, 70% or 75% of the solid green petroleum coke. Dissolution
leaves
a leached solid residue and produces a pregnant solubilization solution that
includes a solubilized organics fraction. The solubilization conditions and
effective
solubilization time may be selected so that the solubilization reaction has a
selectivity for production of the solubilized organics fraction over a carbon
dioxide
gas fraction, for example of at least 50%, 60%, 70% ot 80%. Similarly,
conditions
may be provided so that at least some proportion, such as 10%, 15%, 20% or 25%
of the sulfur in the solid green petroleum coke reports to the leached solid
residue.
Similarly, conditions may be provided to limit the proportion of the
transition metal
component of the solid petcoke that reports to the pregnant solubilization
solution
as a dissolved transition metallic component, for example being no more than
40%,
50% or 60% of the transition metal component of the solid green petroleum
coke.
[0010] A recovered solids fraction may be precipitated from the pregnant
solubilization solution by lowering the pH, for example to a pH of 5 4, 5 or
6. The
recovered solids fraction may for example include a desired proportion of the
total
organic carbon (TOC) present in the pregnant solubilization solution as the
solubilized organics fraction comprises, for example 60c)/c), 70%, 80% or 90%.
The
recovered solids fraction may include an acid precipitated transition metal
component, for example making up a smaller weight percent fraction of the
recovered solids fraction than the weight percent fraction of the transition
metal
component in the solid green petroleum coke. In select embodiments, the
precipitated transition metal component may for example make up 5 1 % by
weight
of the recovered solids fraction.
[0011] The recovered solids fraction may for example have an ignition
temperature of not more than 450 or 420 C, and/or a volatile material content
of
more than 15 or 20 wt%, and/or a volatile material to fixed carbon ratio of at
least
0.2 or 0.25, and/or a heating value of at least 25 or 30 MJ/kg.
[0012] Catalytic method of processing petroleum cokes are also provided,
for
example involving the use of a copper tetrasilicate catalyst. For example
using a
solubilization temperature of from 150 C to 230 C, and an added
solubilization
base concentration effective to maintain a solubiliztion pH ..13, for an
effective
4
CA 3012804 2018-07-27
solubilization time of less than 6 hours, in the presence of an oxidizing
atmosphere
comprising more than 21% oxygen, and in the presence of with agitation to
generate a stirred reaction region in the solubilization liquid, so as to
solubilize at
least 90% of the solid green petroleum coke leaving a leached solid residue
and
producing a pregnant solubilization solution comprising a solubilized organics
fraction. The solubilization conditions and effective solubilization time
using a
catalyst may for example be selected so that the solubilization reaction has a
selectivity for production of the solubilized organics fraction over a carbon
dioxide
gas fraction of at least 80%, 85%, 90 or 95%. The copper-tetrasilicate
catalyst may
for example be a nanocrystalline material, for example a Gillespite group-type
solid
catalyst, belonging for example to the Gillespite group of minerals
(cuprorivaite
(CaCuSi4010); wesselsite (SrCuSi4010) and effenbergerite (BaCuSi4010) or
combinations thereof).
BRIEF DESCRIPTION OF THE DRAWINGS
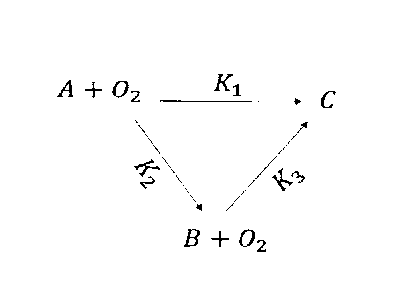
[0013] Figure 1 is a schematic illustration of a triangular reaction
scheme of
petcoke oxy-cracking, where A is the petcoke, B is the intermediates (desired
products, TOC), and C: CO2 in the gas phase (CG) + CO2 in the liquid phase
(carbonates IC).
[0014] Figure 2 includes three graphs showing concentrations of A, B, and C
as
a function of reaction time at different reaction temperature 200 C, 215 C,
and
230 C. The symbols represent experimental data, and the solid lines are the
kinetics model (Eqs.12-14).
[0015] Figure 3 is a graph showing Arrhenius plots of petcoke oxy-
cracking for
each reaction pathway.
[0016] Figure 4 is a plot illustrating the effect of mixing speed on the
conversion
of petcoke during oxy-cracking reaction (T = 215 C, P = 750 psi and t = 2 h).
[0017] Figure 5 is a bar graph illustrating the effect of petcoke
particle size on
reaction conversion of petcoke (T = 215 C, P = 750 psi and t = 2 h).
[0018] Figure 6 is a plot illustrating the effect of the reaction
temperature on the
selectivity and conversion of petcoke oxy-cracking (P = 750 psi and t = 1 h).
[0019] Figure 7 is a plot illustrating the effect of reaction time on
selectivity and
conversion of petcoke oxy-cracking (T = 180 C and P = 750 psi).
5
CA 3012804 2018-07-27
[0020] Figure 8 is a plot illustrating the effect of KOH amounts on the
selectivity
and conversion of petcoke oxy-cracking reaction (T = 230 C and P = 750 psi,
time
= 2 h).
[0021] Figure 9 is an FTIR spectra of the original petcoke, oxy-cracked
products
and residual petcoke at 230 C and 2 h residence time.
[0022] Figure 10 is an 1H NMR spectra for oxy-cracked petcoke ran with
D20
solvent. Signal frequencies for typical chemical structures are shown.
[0023] Figure 11 include two high-resolution XPS spectra of the
deconvoluted
Cis peak (a) before reaction, (b) after reaction.
[0024] Figure 12 includes two high-resolution XPS spectra of the
deconvoluted
01s peak (a) before reaction, (b) after reaction.
[0025] Figure 13 includes two high-resolution XPS spectra of the
deconvoluted
Nis peak (a) before reaction, (b) after reaction.
[0026] Figure 14 includes two high-resolution XPS spectra of the
deconvoluted
S2p peak (a) before reaction, (b) after reaction.
[0027] Figure 15 is a plot of XRD powder patterns of copper-silicate
cuprorivaite
(blue line), the vertical lines (black) are the reference data for the
cuprorivaite from
COD database.
[0028] Figure 16 is a schematic illustration of the unit cell of the
copper silicate
cuprorivaite framework drawn with BIOVIA structure module, a) Unit cell of
CaCuSi4010 b) Side view of the surface (001) of CaCuSi4010 and c) Top view of
the
surface (001) of CaCuSi4010. Blue spheres represent copper atoms, yellow
spheres
are silicon atoms, red spheres are oxygen atoms and green spheres are calcium
atoms.
[0029] Figure 17 is a plot illustrating nitrogen physisorption isotherms
for
copper-silicate.
[0030] Figure 18 includes 4 SEM images of copper-silicate material at
different
magnifications.
[0031] Figure 19 is a plot illustrating Infrared spectroscopy of the
prepared
copper-silicate material.
[0032] Figure 20 is a plot illustrating the effect of the reaction
temperature on
the selectivity and conversion of petcoke oxy-cracking (P = 750, t = 1 h, 1000
rpm
and 0.10 g of catalyst).
6
CA 3012804 2018-07-27
[0033] Figure 21 is a plot illustrating Reaction time effect on
selectivity and
conversion of petcoke oxy-cracking reaction (T = 200 C and P = 750 psi, 1000
rpm
and 0.10 g of catalyst).
[0034] Figure 22 is a schematic illustration of the square planar
configuration of
the copper atoms in the structure of CaCuSi4010, the blue spheres are copper
atoms and red ones are oxygen atoms.
[0035] Figure 23 is a graph showing Arrhenius plots of catalyzed petcoke
oxy-
cracking for each reaction pathway.
[0036] Figure 24 includes three graphs, illustrating concentration
profiles of A,
B, and C as a function of reaction time at different reaction temperatures
185, 200,
and 230 C under the presence of the Cu-silicate catalyst. The symbols
represent
experimental data, and the solid lines are the kinetics model.
[0037] Figure 25 is a bar graph illustrating the conversion and
selectivity of B
and C for three repeated cycles of Cu-silicates, 2 h, 200 C, 750 psi and 0.10
g of
catalyst.
[0038] Figure 26 is a plot illustrating overlays of the X-ray
diffraction patterns of
fresh and regenerated catalysts. The top pattern is the regenerated catalyst,
over
the fresh catalyst, above cuprorivaite and lastly wollastonite at the bottom.
[0039] Figure 27 is a plot showing FTIR spectra of the virgin petcoke,
oxy-
cracked products and the humic acid at 200 C and 2 h residence time.
[0040] Figure 28 is a plot showing the TG-DTA curve for the virgin
petcoke,
showing the ignition, peak and burnout temperatures.
[0041] Figure 29 is a plot showing the TG-DTA curve for oxy-cracked
petcoke
under air, showing the ignition, peak and burnout temperatures.
[0042] Figure 30 is a plot showing the heat flow of virgin and oxy-cracked
petcoke with temperature.
[0043] Figure 31 includes two plots, showing the conversion percent (a)
with
temperature at heating rates of 5, 10 and 20 C/min for a) virgin petcoke and
b)
oxy-cracked petcoke.
[0044] Figure 32 includes two plots showing thermogravimetric analysis of
the
virgin and oxy-cracked petcoke at a heating rate of 10 C/min, showing the M,
MV,
FC, and A.
7
CA 3012804 2018-07-27
DETAILED DESCRIPTION
[0045] In one aspect, an approach for petcoke conversion into valuable
products
is disclosed herein, using oxy-cracking reactions under relatively mild
operating
conditions of temperature and pressure in an aqueous alkaline medium. The
reaction conditions are exemplified in a batch reactor, with examples of
optimization
for high conversion rates and selectivity for water-solubilized products, with
minimal
amounts of CO2 emission. In select embodiments, the optimal reaction
temperature
and time were 230 C and 2 h, respectively. Reaction kinetics are disclosed,
at
residence times ranging between 0 and 2 h, and at different reaction
temperatures:
200, 215, and 230 C. The kinetics results illustrate that the petcoke is oxy-
cracked
simultaneously into water-soluble species and CO2, with the consecutive
reaction of
soluble species into CO2. The concentration of the oxy-cracked petcoke in the
liquid
phase was measured as a lumped TOC, while CO2 was determined in gas products
at the end of reaction using gas chromatography (GC) and inorganic carbon
(IC).
The oxygenated hydrocarbons (desired products) and the residual solids were
characterized using FTIR, NMR and XPS techniques. The results indicate that
the
main species solubilized in water were oxygenated hydrocarbons and some
organic
acids, such as carboxylic and sulfonic acids and their salts. The residual
solids
remaining after the reaction showed structures and functional groups similar
to the
original petcoke. Surprisingly, most of the metals contents reported to the
residual
petcoke, compared with the metals in the liquid phase. In accordance with the
disclosed embodiments, the present oxy-cracking technique can be used for
upgrading conversion and demineralization/desulfurization of petroleum cokes.
[0046] In an alternative aspect, aspects of which are described in
Example 2, a
copper-silicate nanocrystalline material belonging to the Gillespite group of
minerals
(cuprorivaite (CaCuSi4010); wesselsite (SrCuSi4010) and effenbergerite
(BaCuSi4010) or combinations thereof) was synthesized and used as a catalyst
for
petcoke oxy-cracking. In addition to the exemplified results using
cuprorivaite, a
cuprorivaite-wesselsite (50%-50%) solid solution was prepared by a
hydrothermal
method, and was tested with bituminous material, confirming efficacy of the
relevant catalytic activity across the Gillespite group of minerals, which
share the
same structural configuration of the copper-silicate active site for the
oxidation
reaction (differing only to the extent dictated by the distinct Ca, Sr or Ba
cations).
8
CA 3012804 2018-07-27
[0047] The catalyst activity and selectivity were illustrated in a batch
reactor
under a range of reaction conditions. A high reaction conversion rate and high
selectivity for water solubilized products, with almost zero emission of CO2,
were
exemplified even at high reaction temperatures. A triangular lumped kinetics
model
successfully describes the oxy-cracking reaction, based on the hydroxyl
radical
mechanism. In this model, the petcoke is oxy-cracked simultaneously into water
soluble species and CO2 with the consecutive reaction of soluble species into
002.
Surprisingly, the catalyst was found to be very stable enough in the aqueous
dissolution medium, with the leaching percentage being less than 3 wt% of the
whole Cu, even at elevated temperatures. After being reused three times, the
CaCuSi4010 catalyst retained its catalytic activity. The oxy-cracked compounds
solubilized in water during the reaction were characterized using FTIR and the
main
species were carboxylic, carbonyl, phenolic, and sulfonic functions - which
are
hunnic acid analog compounds. The excellent catalytic activity, selectivity,
stability
and environmentally benign nature of copper-silicates, under mild operating
conditions, provides an optimized oxy-cracking process.
[0048] In a further illustration of aspects of the oxy-cracking process,
as
described in Example 3, the use of oxy-cracked petcoke solids as a fuel is
exemplified. Characterizations are provided of the oxidation and combustion
.. properties of this fuel, as well as measuring calorific values.
Thermogravimetric
analysis was used to illustrate the thermal degradation behavior of the virgin
and
oxy-cracked petcoke. In the exemplified embodiments, the oxidation of oxy-
cracked
petcoke occurs at 475 C (which is lower than that of virgin petcoke where the
oxidation is occurred at 540 C). The heating values were estimated by
proximate
analysis using different correlations. The results indicate that oxy-cracked
products
contain a high proportion of volatile compounds and significantly high
calorific
heating value (-30 MJ/kg). These embodiments illustrate that the oxy-cracked
petcoke exhibits high reactivity, comparable to other fuels. The disclosed oxy-
cracking processes may accordingly be used to transform petcoke into a fuel
for
thermal applications, taking advantage not only of its combustion behavior,
but also
a low content of sulfur, nitrogen and metals.
9
CA 3012804 2018-07-27
[0049] For purposes of the present disclosure, green petcoke may be
defined by
compositional characteristics, for example in contrast to calcined petcoke, as
for
example set out in Table 1.
Table 1: Composition of Petroleum cokes
Composition wt%
Green Petcoke Exemplary Green Calcined Petcoke
Petcoke
Carbon Ã30 or 85 and/or 84.48 98
595 or 598
Hydrogen or 3.81 0.14
and/or
56, 57 or 58
Oxygen 2.5, 3, 4 or ?. 5.37* 0.02
5 and/or 56, 57,
58, 59 or 510
Nitrogen 0.5 1.55 0.22
Sulfur 1 or 2 or 3 or 4.46 1.2
4 and/or 5 4.5 or
5 5 or 5 10
Vanadium ?_ 0.001 or 0.01 0.08
and/or 5 0.1 or 5
0.5
Iron 0.001 or 0.01 0.06
and/or 5 0.1 or 5.
0.5
Nickel 0.001 or 0.01 0.03
and/or 5 0.1 or 5
0.5
Molybdenum 0.001 or 0.005 0.01
and/or .5_ 0.2 or 5
0.1
CA 3012804 2018-07-27
Cobalt ?Ø01 or 0.05 0.15
and/or 0.2 or 5
0.5 or 5_ 1
Ash 0.05 ¨ 0.5 0.19 ¨ 0.35 0.35
C/H 10:1 ¨ 50:1 18:1 ¨ 24:1 910:1
*Estimated by difference
[0050] Although various embodiments of the invention are disclosed
herein,
many adaptations and modifications may be made within the scope of the
invention
in accordance with the common general knowledge of those skilled in this art.
Such
modifications include the substitution of known equivalents for any aspect of
the
invention in order to achieve the same result in substantially the same way.
Terms
such as "exemplary" or "exemplified" are used herein to mean "serving as an
example, instance, or illustration." Any implementation described herein as
"exemplary" or "exemplified" is accordingly not to be construed as necessarily
preferred or advantageous over other implementations, all such implementations
being independent embodiments. Unless otherwise stated, numeric ranges are
inclusive of the numbers defining the range. The word "comprising" is used
herein
as an open-ended term, substantially equivalent to the phrase "including, but
not
limited to", and the word "comprises" has a corresponding meaning. As used
herein, the singular forms "a", "an" and "the" include plural referents unless
the
context clearly dictates otherwise. Thus, for example, reference to "a thing"
includes more than one such thing. Citation of references herein is not an
admission that such references are prior art to the present invention. Any
priority
document(s) and all publications, including but not limited to patents and
patent
applications, cited in this specification, and all documents cited in such
documents
and publications, are hereby incorporated herein by reference as if each
individual
publication were specifically and individually indicated to be incorporated by
reference herein and as though fully set forth herein. The invention includes
all
embodiments and variations substantially as hereinbefore described and with
reference to the examples and drawings.
11
CA 3012804 2018-07-27
EXAMPLES
Example 1: Conversion of petroleum coke into valuable products using oxy-
cracking
Materials and Setup
[0051] A sample of exemplary green petcoke was obtained from a commercial
source. This black-solid sample was characteristic of green petcoke,
comprising
complex hydrocarbons in a structure which includes polycyclic aromatic
hydrocarbons (3-7 rings), such as benzopyrene. The sample was ground and
sieved to a particle size ranging between 53 and 710 pm. Elemental analysis of
the
petcoke sample was carried out using a PerkinElmer 2400 CHN analyzer
(Waltham, Massachusetts, USA) for C, H, N contents and a Thermo Intrepid
inductively coupled plasma-atomic emission spectroscopy (ICP-AES) for sulfur
and
metal contents. The chemical composition of the selected petcoke sample is
listed
in Table 1, as the Exemplary Green Petcoke.
[0052] KOH (ACS reagent, 85 /0, pellets) purchased from Sigma-Aldrich
(Ontario, Canada) was used to adjust the pH of the reaction medium (deionized
water) and solubilize the petcoke in the aqueous basic medium. Oxygen 99.9%
ultrahigh purity purchased from Praxair (Calgary, Canada) was used as the
oxidant
gas.
[0053] The experimental setup made use of a 100 mL reactor vessel (model
number 4598, Parr Instrumental Company, Moline, II, USA), made of stainless
steel
SS-316 with 12 cm in length and 3.25 cm in diameter. The vessel was equipped
with a heating oven connected to a temperature control loop, a pressure gauge
and
a mechanical stirrer with a speed controller. The reactor vessel was capable
of
handling pressures up to 1700 psi and temperatures up to 270 C. The oxy-
cracking
experiments were carried out at temperatures from 150 to 250 C and pressures
up
to 1000 psi. In a typical experiment, 1.0 g of solid petcoke sample was
charged into
the reactor vessel containing 20 g of deionized water and a specified amount
of
KOH. The pH of the reaction medium was kept above 8.0 by adding 1.0 g KOH to
assist in solubilizing the petcoke and to ameliorate corrosion. Leak tests
were
performed by pressurizing the reactor with 02 up to 1200 psi prior to fixing
the
operating pressure. Then, the mixer was set to 1000 rpm to minimize the
interfacial
12
CA 3012804 2018-07-27
mass resistance between the gas and liquid phase and to ensure uniform
temperature and concentration profiles in the liquid phase. The reactor was
then
heated to the desired temperature. Once the desired pressure and temperature
are
attained, the zero-reaction time was set. The reaction was carried out at
different
residence times, namely 15, 30, 45, 60, 120, 180 and 240 min. Several
operating
parameters were varied to illustrate optimization of the oxy-cracking
reaction,
including temperature, reaction time, oxygen pressure, mixing speed, particle
size
and amount of KOH. At the end of the reaction, the reactor was cooled to room
temperature. Then, the gas phase was analyzed using gas chromatography, GC
(SRI 8610C, SRI Instruments). Afterwards, the liquid effluents were discharged
and
filtered for total organic carbon (TOC) analysis. A small amount of unreacted
solid
residue was collected at the bottom of the reactor vessel. The oxy-cracked and
insoluble (residual) petcoke fractions were recovered using an evaporator
(vacuum
oven) for further analysis by Fourier transformed infrared spectroscopy
(FTIR),
nuclear magnetic resonance (NMR) spectroscopy and X-ray photoelectron
spectroscopy (XPS) to illustrate the nature of the fractions. Elemental
analysis was
also performed on the dried recovered solids.
FTIR analysis
[0054] The functional groups within the chemical structure of the virgin
petcoke
sample, oxy-cracked (solubilized) and insoluble solid (residue) were
characterized
with a Shinnadzu IRAffinity-1S FTIR (Mandel, USA), provided with a smart
diffuse
reflectance attachment to carry out diffuse reflectance infrared Fourier
transform
spectroscopy (DRIFTS) analysis. Initially, the background was defined by
analyzing
about 500 mg of pure potassium bromide (KBr) powder; then, approximately 5 mg
of the petcoke sample dispersed in the 500 mg of KBr was analyzed. The IR
spectra were obtained in the wave number ranging from 400 to 4000 cm-1; all
the
spectra were acquired as averages of 50 scans with a resolution of 4 cm-1. In
the
case of the oxy-cracked (solubilized) sample, the solidified organic species
were
collected by drying the solubilized petcoke in water overnight at 65 C in a
vacuum
oven.
13
CA 3012804 2018-07-27
Total organic carbon (TO C) analysis
[0055] A Shimadzu total organic carbon analyzer (TOC-L CPH/CPN) was used
to determine the carbon content of the solubilized organic and inorganic
species
present in the water. The TOC samples were prepared by centrifuging the
solubilized species (Eppendorf centrifuge 5804) at 5000 rpm and 15 min to
separate the remaining solid (i.e., unreacted and insoluble species). The
total
carbon (TC), total organic carbon (TOC), and inorganic carbon (IC) of the
aqueous
phase were measured. Both TC and IC measurements were calibrated using
standard solutions of potassium hydrogen phthalate and sodium hydrogen
carbonate. Fifteen milliliters of the centrifuged solutions were placed in
standard
TOC vails. Using the TOC software to control the system, the TC was
automatically
measured. After that, an acid was added to evolve CO2 from the sample to
measure the remaining organic compounds, which were considered as TOC. All the
measurements were taken three times, and the average was used for the
calculations with a 5% relative standard deviation.
1H Nuclear magnetic resonance (NMR) spectroscopy
[0056] The NMR spectrum of the oxy-cracked sample was determined with a
Bruker 600 MHz spectrometer (4 mm BL4 liquid probe, cross-polarization
program,
and spin rate of 8k). The 1H NMR spectrum was taken at 298 K using a D20
solvent with a pulse sequence zg30, a relaxation time of 2 s, and averaging
160
scans/run. The NMR spectrum was analyzed using the commercial NMR simulator
software (Mnova NMR) helping the assignment of most structure types available
at
different frequencies.
Gas chromatography (GC) analysis
[0057] Compositional analysis of produced gases was carried out with a
GC
(SRI 8610C Multiple Gas #3 gas chromatograph SRI Instruments, Torrance, CA).
The GC was provided with a thermal conductivity detector (TCD) and two packed
.. columns connected in parallel (3' molecular sieve/6' Hayesep-D columns).
The
molecular sieve column is used for permanent gases, while the Hayesep-D column
allows analysis for hydrocarbons up to C5. The gas analysis was carried out
after
the oxy-cracking reaction is completed and cooled down to room temperature.
The
14
CA 3012804 2018-07-27
GC measurements were repeated 5 times for each sample, and the average
relative error was lower than 3%.
X-ray photoelectron spectroscopy (XPS)
[0058] The XPS analysis was conducted on the petcoke sample before and after
reaction using an XPS PHI VersaProbe 5000 spectrometer to provide information
about the distribution of different atoms on the sample surface based on their
binding energy. The oxy-cracked sample was collected after drying it in a
vacuum
oven at 65 C overnight. The spectra were taken using a monochromatic Al source
.. (1486.6 eV) at 50 W and a beam diameter of 200.0 pm with a take-off angle
of 45 .
The samples were pressed on double-sided tape and the spectra were taken with
double neutralization. The sample sputtering protocol involved 20 min of Argon
sputtering at 45 , 2 kV, 1.5 pA 2 x 2 (less than 10.5 nm/min). Calibration was
performed with a SiO2/Si wafer having a SiO2 layer of 100 nnn.
Elemental analysis
[0059] A combustion method using a PerkinElmer 2400 CHN analyzer
(Waltham, Massachusetts, USA) was used for analyzing carbon, hydrogen, and
nitrogen contents after and before oxy-cracking reaction. Both sulfur and
nitrogen
contents for organic materials were determined with an Antek 9000 system
(Houston, TX, USA) by running toluene solutions (10 wt %/vol.). Calibration
was
performed with Accustandard IS-17368 (N) and Accustandard SCO-500x (S)
standards. For metal analysis (Fe, Ni, Co, Mo and V), the microwave assisted
acid
digestion procedure was used in a commercial unit model MARS 6 from GEM
Corporation (Matthews, NC, USA) for digesting the solid residual samples. The
system is provided with UltraPrep vessels of 100 mL capacity and a MARSXpress
DuoTemp controller which was operated at a frequency of 2.45 GHz at 100% of
full
power (maximum of 1600 W). Sulfur and metal concentrations in the oxy-cracked
and residual samples were determined by ICP-AES.
Reaction Kinetics
[0060] While not being bound to a particular mechanism, the oxy-cracking
mechanism of petcoke may be conceptualized as a triangular reaction pathway,
as
CA 3012804 2018-07-27
depicted in Figure 1, where petcoke solubilized in water with small quantities
of
produced CO2 at early stages of the reaction. Under certain reaction
conditions,
longer residence time and higher temperature, the solubilized petcoke in water
starts reacting with oxygen to produce CO2.
[0061] Petcoke has a complex structure, and many soluble and insoluble
intermediates were accordingly produced during the reaction. The concentration
of
the intermediates (desired products) in the liquid phase was calculated based
on
carbon mass as the lumped total organic carbon (TOC) concentrations. However,
the carbon content of initial feedstock was calculated using elemental
analysis;
carbon content before reaction (feedstock) = (mass of petcoke) x (carbon A in
feed). The produced gas, primarily CO2, was analyzed online using GC. Other
determined gas concentrations were very small. The reaction conversion based
on
carbon mass was calculated based on the following equation:
CAO-CR
Conversion, X =(1)
CAO
where CAO is the carbon concentration of virgin petcoke before the reaction,
CR is
the residual carbon concentration (unreacted petcoke) that remains after the
reaction. The numerator term (CAo-CR) in Eq (1) represents the amount of
carbon in
the liquid phase as total carbon (TC = TOC + IC) and the amount of carbon in
the
gas phase CO2 (CG). Hence,
CAO - CR = (TOC) + (IC) + CG (2)
considering CO2 gas as obeying ideal gas behavior, then the carbon content in
the
gas phase (CG) may be calculated as follows:
PV
CG = 12 x -RT (3)
where, P and T are the pressure and temperature at the end of reaction,
respectively. V is the volume of the gas phase in the reactor vessel and R is
the
ideal gas constant.
[0062] The selectivity for production of the desired products (B) and
CO2 (C)
may be calculated as follows:
(TOC)
Selectivity to product B - (TOC)+1C+ CG (4)
Select" . to product C (5)
(TOC + CG
(ICCG)
)+I
16
CA 3012804 2018-07-27
[0063] The kinetic rate equations for the oxy-cracking reaction in a
batch reactor
can be expressed by the set of the following three differential equations:
dcA _
¨ - -rA = (Ki
+ K2) cr (6)
dt
dCB
¨= Trg= K2 - K3 Cr (7)
dt
dCc
= +rc = + K3 Cr (8)
dt
where,
= e-Ei/RT con2 (9)
K2 = k'2 e-E2/ RT cgt2 (10)
K3 = e-E3/RT cort2 (11)
where CA, CB, and Cc are the carbon concentrations of original petcoke,
desired
products, and CO2, respectively. CO2 is the concentration of oxygen, n1 ,n2
and m
are the reaction order of A, B and 02, respectively. t is the reaction time,
and Ki, K2,
and K3 are the reaction rate constants. The reaction orders are experimentally
determined to be first order for A and B, i.e., n1 = n2 = 1. Typically, the
order of
oxygen is either near zero (m = 0) or excess oxygen is used to reduce its
effect on
the reaction kinetics and enable hydrocarbon species (A and B) to be the
limiting
reactant. Therefore, the oxygen terms will be considered as a constant, hence,
Equations 6 to 8 can be expressed as follows:
dcA
¨dt (Ki + K2)CA
(12)
dCB rs jz
¨ = m2k..A - 1µ3%...E5 (13)
dt
dCc _ v
¨ - 1\3l013
(14)
dt
[0064] The kinetic parameters, i.e., Ki, K2, and K3 were estimated using
the
Mathematica software (V10.2) by fitting the experimental data to the
differential
equations (3.12-3.14) under the following initial conditions: at t=0, CA= GAO,
and GB
= Cc = 0. The proportional weighed sum-of-squares was minimized using the
Mathematica until all values of the correlation coefficient (R2) were very
close to
1Ø The kinetics data were collected at three different temperatures of 200,
215,
and 230 C and reaction times varying from 0 to 1 h. However, other important
parameters, such as the operating partial pressure (750 psi), the mass ratio
of
petcoke to KOH, and the impeller speed (1000 rpm) were all kept fixed. At
these
17
CA 3012804 2018-07-27
temperatures and reaction, times, an optimized range of conditions was
selected to
make the reaction favorable to the desired products. Indeed, at high
temperatures
(>250 C) and residence times (>2 h), combustion reaction becomes more
favorable than oxy-cracking and more CO2 was produced. However, low reaction
conversions were obtained at low temperatures (<180 C). The oxy-cracking
reaction was not significantly affected by the oxygen partial pressure beyond
750
psi. Also, the oxy-cracking reaction rate was found to be independent of the
impeller speed above 500 rpm, indicating there is no mass transfer limitation
beyond this speed limit. The estimated reaction constants of the petcoke oxy-
cracking are presented in Table 2.
Table 2: Determined values of non-catalyzed oxy-cracking reaction constants.
T ( C) Ki (s-1) K2 (s-1) K3 (S-1)
200 2.27 x 10-5 1.84 x 10-4 1.71 x 10-5
215 6.99 x 10-5 3.46 x 10-4 2.42 x 10-5
230 2.43 x 104 87.37 x 10-4 3.67 x 10-5
[0065] Consequently, the activation energies and frequency factors were
estimated using Arrhenius equation based on the temperature and reaction
constants as follows:
-Ei
K = k'ie RT (15)
where k'i is the frequency factor for each step of the reaction, E is the
activation
energy, i is the reaction step pathway (1, 2, and 3), R is the ideal gas
constant, and
T is the temperature.
[0066] Figure 2 compares the experimental data with the kinetic model
for
concentration profiles of petcoke (A), intermediate compounds (B), and CO2 (C)
at
three different temperatures of 200, 215, and 230 C as a function of time.
Error
bars shown in the figure represent the calculated standard deviation based on
the
TOC and GC measurements. Noticeably, the kinetic model showed an excellent
agreement with the experimental results and described the proposed triangular
reaction kinetics scheme accurately. It is clear that the reaction temperature
is
acting as a key parameter in the oxy-cracking reaction. Thus, at a higher
18
CA 3012804 2018-07-27
temperature (i.e., 230 C), the solubilization of oxy-cracked compounds in
water is
increased and reached to the maximum concentration faster than at lower
temperatures. Moreover, at a high reaction temperature, the produced CO2 in
the
gas phase is detected at the early stage of the reaction. Even at low reaction
time,
i.e., 15 min, the amount of produced CO2 is determinable by GC. This indicates
that
a direct reaction may be occurring between oxygen and petcoke to form CO2.
[0067] Figure 3 shows the Arrhenius plot of petcoke oxy-cracking
reaction at
three different reaction temperatures. By plotting In(k) against 1/T, a good
fitting
was accomplished between Arrhenius equation and the experimental data,
indicated by R2 values closed to 1. From the slope and intercept of the best-
fit-line
at each temperature, the values of activation energies and frequency factors
of
petcoke oxy-cracking were calculated and summarized in Table 3.
Table 3: Estimated activation energies and frequency factors of non-catalyzed
petcoke oxy-cracking.
Activation energy (kcal.mo1-1) Frequency factor (s-1)
El 39.46 0.495 1.74 x 1012
E2 21.87 0.532 2.19 x 106
E3 11.95 0.981 5.75
[0068] At the beginning of the reaction, an induction period is found in
which
there is small amount of CO2 released. This small amount of CO2 is consistent
with
the presence of short alkyl chains in the petcoke structure, as confirmed by
FTI R.
This is also consistent with the fact that the highest activation energy value
(E1=39.46 kcal/mol) in the first reaction pathway. The activation energy value
may
be attributed to the complexity of aggregated structures in petcoke. As a
result,
petcoke aggregates require more oxygen penetration during the oxy-cracking
reaction to achieve the desired conversion.
[0069] These results are indicative of a mechanism whereby petcoke
particles
are solubilized as oxygenated hydrocarbon analogs of carboxylic acids and the
like.
These findings are consistent with a second reaction pathway having an
activation
19
CA 3012804 2018-07-27
energy of E2=21.87 kcal/mol and a high value of the frequency factor 2.19 x
106s-1.
Consequently, CO2 may be produced in the third reaction pathway, E3=11.98
kcal/mol, by further reaction between solubilized aromatic moieties and
oxygen.
Although the activation energy for deep oxidation of petcoke to produce CO2 in
the
first reaction pathway (39.46 kcal/mol) is much higher than the one obtained
in the
third pathway (partial oxidation) (11.98 kcal/mol), the frequency factor in
the first
pathway (1.74 x1012s-1) is also higher than the third pathway (5.75 s-1).
These
findings provide evidence that process conditions may be arranged so that the
rate
of conversion of petcoke into CO2 at the beginning of the reaction is roughly
equivalent to the rate of CO2 production from the oxidation of organic
compounds
solubilized in water. In effect, surprisingly, conditions may be arranged so
that the
rate of forming and producing intermediate compounds (desired products) is
more
favorable than the rate of CO2 production via both reaction pathways. This is
evident from the finding that the activation energy E2 was lower than E1 and
the
frequency factor k'2 is higher than k'3. The overall result is accordingly to
provide a
process in which the production of CO2 is minimized.
Effects of operating conditions on petcoke oxy-cracking reaction
[0070] In this section, the effects of operating conditions such as
temperature,
residence time, oxygen partial pressure, amount of KOH, petcoke particle size
and
impeller speed are exemplified. These parameters are optimized not only to
maximize the reaction conversion and selectivity to produce the water-
solubilized
hydrocarbons (desired products) but also to minimize the amount of CO2
produced
during the oxy-cracking reaction.
[0071] Optimization of the oxygen partial pressure revealed that the
reaction
was not significantly affected by oxygen partial pressures beyond 750 psi.
Within
the relevant pressure and temperature range (180-250 C), the water exists only
as
a subcritical liquid. In this Example, at the given pressures, oxygen was
present in
an excess amount.
[0072] The effect of mixing was investigated during the petcoke oxy-
cracking
reaction. High mixing speeds may be used to minimize the interfacial mass
resistance between the gas and liquid phase, enhancing the transfer of oxygen
from the gas phase to the liquid phase. Additionally, mixing helps to maintain
CA 3012804 2018-07-27
relatively uniform temperature and concentration profiles in the liquid phase.
The
reaction conversion was evaluated by varying the mixing speed from 0 to 1000
rpm
while fixing other parameters such as temperature (215 C), oxygen pressure
(750
psi) and reaction time (2 h). As seen in Figure 4, when the mixing speed is
below
500 rpm, a significant reduction in the reaction conversion occurred,
evidencing
mass transfer as a controlling step. However, above 500 rpm, the effect is
drastically reduced and there was practically no effect on the reaction
conversion,
i.e., the reaction region is the controlling step. Therefore, an appropriately
high
mixing speed may advantageously be applied to the aqueous phase. For example,
to ameliorate mass transfer resistance, the reaction may take place in a
turbulent
region (i.e., Reynolds numbers, Re > 10000).
[0073] The effect of petcoke particle size on the oxy-cracking reaction
was also
demonstrated. Petcoke particle sizes ranging from 53 to 710 pm were
exemplified
to illustrate the effect on petcoke solubilization or mass transfer
limitations. Figure
5 shows the reaction conversion of petcoke oxy-cracking evaluated at different
petcoke particle sizes, constant temperature (215 C), mixing speed (1000 rpm),
oxygen pressure (750 psi) and reaction time (2 h). Effective reactions were
demonstrated over a wide range of particle sizes. As exemplified, total
petcoke
conversion to desired products and CO2 was approximately constant (about
78.5%), and independent of particle size.
Effect of the temperature
[0074] The effect of the temperature on the conversion and selectivity
of the
oxy-cracking reaction was illustrated between 180 and 250 C. Other parameters
were constant, such as the oxygen partial pressure set to 750 psi (to ensure
the
water was present in a subcritical state), mixing rate was 1000 rpm to prevent
liquid
phase interfacial mass transfer resistance, and the residence time was 1 h. As
demonstrated, the reaction performance improved with increasing temperature.
Accordingly, in select embodiments, the reaction temperature may be optimized
to
facilitate the oxy-cracking reaction. For example, by increasing temperature
(i.e., up
to 250 C) the solubilization of oxy-cracked compounds in water is increased.
Although the solubilization of oxygenated hydrocarbons is increased at a high
temperature, the selectivity of producing CO2 gas is also increased. Hence,
under
21
CA 3012804 2018-07-27
longer reaction times the oxygenated intermediates further decomposed
oxidatively
to CO2 and H20. Figure 6 shows the conversion and selectivity of oxy-cracking
reaction at different reaction temperatures. It is clear that as the
temperature
increased the petcoke conversion to produce solubilized-hydrocarbons (B) is
increased with a slight increase in CO2. However, the selectivity to produce
the
desired products (B) is slightly decreased with a further increase in
temperature
(250 C). Moreover, no reaction occurred at temperatures lower than 150 C with
the
considered residence time. For instance, the reaction conversion was less than
30% when the temperature ranged from 150 to 180 C at 1 h residence time. In a
select embodiment, the highest conversion was obtained when the temperature
ranged from 220 to 240 C. Based on that, in some embodiments, the optimum
reaction temperature which provides the highest conversion and selectivity to
synchronized with a minimal amount of CO2 centers around 230 C, as presented
in
Figure 6.
[0075] These results illustrate that temperature has a significant effect
on the
overall conversion rate, and a meaningful effect on the selectivity for water
solubilized products. Accordingly, the oxy-cracking temperature is an
optimizable
parameter. The reaction temperature will affect not only the conversion rate
and
selectivity, but also the acidity of the products formed. Even as reaction
rates
increase with temperature, the final TOC values of the desired products (6)
for
temperatures higher than about 230 C are effectively constant after 1 h. In
effect,
more acidic functional groups were produced at higher temperatures (200-250
C),
and this is confirmed by lowering the values of pH for neutralization
reactions to
about 8.5. This evidences the ability to use temperature to select the nature
of
products produced by the oxy-cracking process.
Effect of reaction times
[0076] The effect of reaction time was demonstrated by varying the time
from 15
min to 4 h under a constant pressure (750 psi), mixing speed (1000 rpm) and
operating temperature (180 C). The effect of reaction time on the conversion
and
selectivity of the oxy-cracking reaction is shown in Figure 7. It is evident
that the
conversion of petcoke to oxy-cracked hydrocarbons (6) and CO2 significantly
increases with time. However, beyond an optimum, the selectivity for product B
is
22
CA 3012804 2018-07-27
slightly decreased with further increases in time, and simultaneously the
selectivity
to product C slightly increased with time. Reaction time is accordingly an
optimizable parameter in the context of overall conversion and selectivity for
B, in
the exemplified embodiment being optimized at a residence time of about 2 h.
Moreover, reaction time has an effect on the acidity of products formed. By
increasing the reaction time, the pH of the liquid phase decreased, thus more
acidic
compounds were produced.
Effect of KOH
[0077] Alternative embodiments of the oxy-cracking reaction were
exemplified
by changing the dosage of KOH from 0 to 2.5 g at constant temperature (230 C),
oxygen pressure (750 psi), reaction time (2 h) and mixing speed (1000 rpm).
Figure 8 shows the effect of KOH on the reaction conversion and selectivity to
both
B and C. As illustrated, the conversion as well as the selectivity to B,
significantly
increased by increasing the amount of KOH and then slightly decreased by
further
increase of KOH dosage. However, the selectivity to C decreased by increasing
the
KOH amount. Thus, the optimal amount of KOH was found to be (1 g KOH/ 1 g
petcoke) where the highest values of the reaction conversion and selectivity
to B
were achieved and the lowest amount of CO2 was produced. KOH also ameliorated
corrosion caused by high acidity species generated during the early oxidation
stages of the process. These results illustrate that KOH is an optimizable
parameter
for enhancing the solubilization of oxy-cracked materials, increasing the
conversion
rate, as well as the selectivity for the desired products.
Characterization of Products
FTIR analysis for petcoke and oxy-cracking products
[0078] The FTIR spectrum of the original petcoke was compared with the
oxy-
cracked product and the non-converted residue as well. Figure 9 shows the
infrared spectra of the original petcoke, residual petcoke (non-soluble solid)
and
oxy-cracked petcoke solubilized fraction isolated from the reaction carried
out at
230 C and 2 h (i.e., the exemplary optimized conditions). It is evident from
the
figure that FTIR spectra of original petcoke and the oxy-cracked one are
distinctly
different.
23
CA 3012804 2018-07-27
[0079] The spectrum of the original petcoke shows IR bands that can be
assigned to the alkyls/aliphatic (2850-3000 cm-1) and aromatic (-3040 cm-1 and
930-750 cm-1) regions. The presence of C-H bonds vibration out-of-plane in
aromatics can be assigned to the 748, 804, and 860 cm-1 bands. The
corresponding C=C aromatic stretching vibration appears near 1580 cm-1,
slightly
below the typical 1600 frequency, thus believed conjugated with other groups
such
as in the C=C region. However, for the oxy-cracked sample, the noticeable
lower
contribution from aromatic out plane bands is observed (930-750 cm-1). The
transmittance at 3040 cm-1 due to aromatic C-H stretching vibrations can be
found
in the spectra for both the original and the oxy-cracked petcoke; however,
much
less important in the latter.
[0080] In the aliphatic region, the presence of alkyl groups in the
petcoke
sample such as -CH3, =CH2 and -CH2CH3 is evidenced by the bands around
2940 cm-1 and 1380 cm-1 which can be assigned to asymmetric and symmetric -C-
H stretching and bending vibrations, respectively. The weak band at around
3500
cm-1 observed for the original petcoke can be assigned to free 0-H stretching
vibration mode of hydroxyl functional groups. The broad-band spanning from
about
2700 to 2000 cm-1 possibly corresponds to hydrogen bonded -OH functionalities.
The presence of sulfoxide species in the original petcoke is assigned at the
small
band -1031 cm-1.
[0081] The FTIR spectrum of the insoluble petcoke (solid residue after
reaction)
is also shown in Figure 9. The structures of insolubilized solid material
(residue)
was found to be very similar to the original petcoke according to the IR
spectra, with
some features changed due to the contribution of oxygenated functions. It is
clear
from the spectrum that at 3300-3700 cm-1 there is a higher contribution of OH
groups in the remaining insolubilized solid compared with the original
petcoke. Also,
the C-O-C contributions (1363 cm-1) in the remaining solids was found less
intense
compared to the original petcoke which showed a broad-band spanning from about
1360-1100 cm-1. This later band can also be derived from the contribution of
sulfones (centered in 1130 cm-1), in addition to other S-oxidized forms
(sulfoxide at
1030 cm-1) with higher intensity compared with the original and oxy-cracked
samples.
24
CA 3012804 2018-07-27
[0082] The FTIR spectrum of the oxy-cracked petcoke is dramatically
different
from that of raw petcoke (Figure 9). It is worth noticing that a new
significant band,
appearing as an intense and broad peak in the range between 3300 and 3600 cm-1
corresponds to -0-H stretching vibration mode of hydroxyl functional groups.
This is
evidence that the organic species of petcoke are oxy-cracked to oxygenated
species bearing alcoholic, carboxylic and phenolic functional groups.
Interestingly,
the presence of carboxylate anion is observed as a doublet band centered at
1580
cm-1, indicating the presence of carboxylic salts. Free acids presence is also
evidenced by the C=0 band appearing at 1700 cm-1, thus some of the ¨OH
observed in 3300-3600 cm-1 can be assigned to these free acids. Another
important
feature is the disappearance of most aromatic moieties in the region of out-of-
plane
bands (930-750 cm-1), together with the important reduction of the aromatic C-
H
stretching at 3030 cm-1. Alkyl groups are visible in the range of 3000-2850 cm-
1,
less contributing to the spectrum in comparison with the original sample and
the
unreacted solid. Moreover, the presence of esters (-1,850 cm-1) and aldehyde
functions (-2700 cm-1) are also evident. Carboxyl, esters, aromatic esters and
ketones C=0 functionalities could appear between 1600 and 1800 cm-1, thus all
are
feasible and not easily discriminated by the bands within this region of the
spectrum. The C-O-C and/or sulfonic bands (1360-1100 cm-1) in the oxy-cracked
products are less intense compared to the original sample, as occurred with
the
insoluble solid. One of the most important features of the oxy-cracked sample
is the
broad band spanning from about 2300-2800 cm-1; this is evidence of a
contribution
of -CO3 (carbonates) to the sample which was isolated under basic conditions.
[0083] From the FTIR results, it is evident that the oxidized organic
functional
groups such as hydroxyl (-OH), carboxylic salts (0=C-0-), carboxylic acids (R-
CO2H) and minor amounts of aldehyde/esters are formed during the oxy-cracking
reaction. The functionalities identified by IR spectra of oxy-cracked petcoke
are in
accordance with the compounds found using XPS and NMR techniques.
1H NMR analysis of the oxy-cracked petcoke
[0084] Nuclear magnetic resonance (NMR) analysis of oxy-cracked product
was
performed on a Bruker CFI 600 MHz spectrometer by dissolving the sample in
deuterated water. The 1H NMR spectrum of the oxy-cracked sample produced at
CA 3012804 2018-07-27
230 C and 2 h reaction time is shown in Figure 10. The NMR spectrum indicates
that the oxy-cracked sample contains a significant quantity of aliphatic
groups with
chemical shifts in the range of 0-3 ppm. Methylene moieties (1.8 ppm) and
methylenes bonded to the aromatic groups (2-2.7 ppm) can be present in the oxy-
cracked sample as also confirmed by the FTIR results. However, terminal methyl
groups (at about 0.8 ppm) are not detectable as important signals in the oxy-
cracked petcoke. Moreover, the presence of the oxygenated functional groups
such
as alkoxy groups (probably methoxy, based on the sharp signals determined) are
observed in the 3.7-4 ppm region. This is a strong indication, again in
agreement
with the FTIR and XPS results, that the oxy-cracked products are oxidized,
producing typical oxygenated hydrocarbon compounds including ethers, acids and
their salts. On the other hand, aromatic protons span chemical shifts in the
range
6-9 ppm. These compounds could be diaromatic carboxylate salts molecules as
assigned in the strong signal appearing around 8.5 ppm and methoxy-phenol type
molecules (6.5 ppm) as well. The presence of carboxyl groups from carboxylic
acids is supported by the small signals appearing around 10 ppm. From these
results, it is evident that carboxyl derivatives and oxygenated hydrocarbons
produced during oxy-cracking are the most significant fractions solubilized in
water.
These findings match well with the ones derived from the FTIR spectroscopy and
XPS.
XPS results of petcoke oxy-cracking
[0085] As shown in the FTIR analysis, the chemical functionalities of
petcoke
before and after the reaction were identified. By XPS analysis, the atomic
composition of selected elements and group functionalities on the surface of
original and oxy-cracked products was determined. Based on the FTIR and the
elemental analysis, the deconvolution of Cis, 01s, Nis and S2p signals along
their
positions was carried out. Table 4 shows the atomic concentration (%) of the
main
components, types and quantities of functional groups in both samples (i.e.,
petcoke after and before reaction).
26
CA 3012804 2018-07-27
Table 4
Before Reaction After Reaction
Atomic Bond Bond Atomic Bond Bond
Conc. assignment Conc. Conc. assignment Conc.
(%) (%) (%) (%)
C=C 70.66 C=C 16.18
C-C/C-H 16.05 C-C/C-H 43.09
C-0 8.91 C-0
Cis 88.75 C=0 2.48 28.60 O-C=0 40.5
C-0 8.91 C-0, 0=C
CO 2.48 OH
C=0 I" 40.5
Ols 8.65 67.70 C-OH
C-N=C 1.02 C-N=C 0.05
Nis 1.05 2.90
C-S-C 0.90 C-S-C --
S2p 1.55 S-0 0.80 S-0
[0086] It is evident from the results that the original petcoke is
mainly composed
of carbon (88.75 at%), and a minor amount of heteroatoms such as oxygen (8.65
at%), nitrogen (1.05 at%) and sulfur (1.55 at%). However, the oxy-cracked
sample
showed a higher oxygen percentage (67.70 at%) and much lower carbon (28.60
at%) and sulfur percentage (0.80 at%) compared with the original petcoke
sample.
[0087] Figure ha and 11 b show the deconvoluted Cis spectra of petcoke
before and after oxy-cracking. The deconvolution of Cis signals was performed
through centering the peaks for different functional groups at specific
binding
energy levels. It is evident that the distribution of carbon species in the
original
petcoke is dramatically different than the oxy-cracked sample. The Cis
spectrum of
original petcoke (Figure 11a), contains mainly four bond types (C=C), (C-C),
(C-
O) and (C=0) set to 283.79 eV, 284.80 eV, 286.34 eV, and 289.21 eV,
respectively. The abundance of the 283.79 eV band (C=C) evidences that the
petcoke sample contained a high amount of aromatic compounds and lower
amount of oxygenated functionalities, as revealed by the FTIR and NMR analyses
as well. However, the C1s spectrum of oxy-cracked sample (Figure 11b) shows
the
presence of similar signals as in the original petcoke with completely
different
intensities. Hence, the signal intensity attributed to the aromatic bonds
(C=C) is
27
CA 3012804 2018-07-27
much decreased, while the abundance of oxygenated functions (0-C=0) was found
very important. Figure 12 (oxygenates XPS) confirms the presence of carboxyl
functions, as well as new C-OH, formed functionalities. The signal at 530.32
eV in
both samples (i.e., original and oxy-cracked) attributed to the oxygen in C-
0/0-C=0
bonds which is higher by almost three times in oxy-cracked sample compared to
the original petcoke. A distinctive signal at 532.77 eV in oxy-cracked sample
(Figure 12b) is observed and attributed to oxygen in alcoholic groups (C-OH).
This
can be explained by the high degree of oxidation in petcoke during the oxy-
cracking
reaction. These findings are in agreement with the results obtained from the
FTIR
and NMR analyses (Figures 9 and 10). The presence of heteroatoms such as
nitrogen and sulfur are evidenced in Figures 13 and 14, respectively. Figure
13a
and b show the Nis spectra for both samples. The spectra indicate the presence
of
pyridines (C-N=C) at 397.89 eV, which are naturally occurring. Similarly, the
S2p
doublet of petcoke was observed at 163.68 and 164.28 eV (Figure 14),
indicating
the presence of sulfur-containing functional groups such as thiophenics,
sulphonic
species (166.7 eV) and low contribution of sulphates (168 eV). Lower
contributions
from thiophenics were evident in the oxy-cracked sample (Figure 14b) which is
indicated by the relatively lower intensity of the S2p doublet. However, the
sulphate
contribution was found to be higher and particularly sulphonic species (166.7
eV)
were found much more important in the oxy-cracked sample. This is evidence
that
sulfur compounds exist in the oxy-cracked sample, however, with a relatively
low
contribution (25 % reduction), as discussed below.
Sulfur and metal analysis
[0088] The content of sulfur and metals in petcokes depends on the nature
of
the crude and the particulars of the coking process, and these constituents
may for
example be found as a variety of organic and inorganic compounds. The sulfur
compounds, for example, one of the most significant impurities in petcoke, may
be
attached to the carbon skeleton as thiophenes or to aromatic naphthenic
molecules
or between the aromatic sheets. Metals, mainly nickel and vanadium, may occur
as
metal chelates or porphyrines as in the asphaltenes. Other metals may not be
chemically bonded, but intercalated in the petcoke structure, for example as
mineral
salts.
28
CA 3012804 2018-07-27
[0089] In this Example, the elemental analysis of petcoke was undertaken
to
illustrate the ability of the present process to demineralize the petcoke.
Table 5
shows the elemental analysis for the original petcoke sample (1000 mg), water
solubilized products (oxy-cracked sample) and the remaining solids (residue)
after
the reaction that was carried out at 230 C for 2 h, but before acid
precipitation of
solids. For comparison, Table 9 shows the chemical composition of the virgin
and
oxy-cracked petcoke sample after acid treatment to precipitate solids.
Table 5: Elemental content in the virgin, oxy-cracked and residue petcoke at
temperature 230 C, pressure 750 psi and time 2 h.
m Original Residual Residual Liquid Liquid Gas Gas Total
cT
3 (mg) (mg) (%) (mg) (%) (mg) (%) mass
cc.
proport
ion %
C 844.750 40.710 4.82 747.350 88.47 22.00 2.60 95.89
H 38.100 2.280 5.98 33.850 88.85 0.00 0.00 94.82
N 15.500 2.850 18.39 6.650 42.90 5.00* 32.26 93.54
S 44.600 11.740 26.32 27.450 61.55 0.00 0.00 87.87
V 0.785 0.114 14.52 0.576 73.38 0.00 0.00
87.89
Ni 0.255 0.097 38.04 0.203** 79.61 0.00 0.00 117.64
Fe 0.568 1.088 191.55 0.018** 3.17 0.00 0.00 194.72
Mo 0.012 0.011 91.67 0.004 33.33 0.00 0.00
125.00
Co 0.051 0.044 86.27 0.018 35.29 0.00 0.00
121.56
Total 944.621 58.934 6.249 816.119 86.39 27.00 2.86 95.49
* Some of N2 gas (0.878 vol%) were detected in the gas phase by GC.
**The relatively high value is due to the leaching from the reactor wall and
impeller.
[0090] These results were obtained under conditions that favoured high
reaction
conversion rates, with 95% of petcoke being oxy-cracked and solubilized in
water.
As seen in the Table 5, under these conditions more carbon, hydrogen and
29
CA 3012804 2018-07-27
nitrogen can be found in the liquid phase compared with residual solid.
Moreover,
the primary heteroatoms and metals present in the original petcoke sample are
sulfur and metals such as vanadium, nickel, iron, cobalt and molybdenum. The
liquid phase does contain some amounts of sulfur and metals. However, more
iron,
nickel, cobalt and molybdenum content can be observed in the residual solid
compared with the liquid phase (oxy-cracked products). Also, more iron, and
nickel
were found in the residual solid compared with original petcoke. Surprisingly,
around 26% of sulfur remained in the residual solids, presumably as highly-
fused
sulfur aromatic rings and possible coprecipitated sulfates. It is evident from
these
findings that the nonsolublized solids (residue) contain a higher amount of
metals
than the oxy-cracked petcoke (solubilized). These findings illustrate that the
oxy-
cracking process may be adapted for petcoke demineralization and
desulfurization.
Example 2: Nanocrystalline copper silicate for catalytic oxy-cracking of
petroleum coke
[0091] In this Example, a nanocrystalline copper-silicate (CaCuSi4010)
material
belonging to the Gillespite group of minerals was introduced to enhance the
selectivity and conversion of the oxy-cracking reaction of petroleum coke.
This
exemplified embodiment is accordingly demonstrative of the efficacy of the
Gillespite group of minerals, or combinations thereof, which in addition to
cuprorivaite (CaCuSi4010), includes wesselsite (SrCuSi4010) and effenbergerite
(BaCuSi4010). The nanocrystalline material was characterized using BET, SEM,
FTIR and XRD techniques. The catalytic activity of the nanocrystalline
material was
illustrated by cracking the residual feedstock (petcoke) in the liquid phase.
The
results showed that the catalyst enables the reaction to occur at a lower
temperature with higher conversion as compared with the non-catalyzed
reaction.
An insignificant amount of CO2 was formed in the gas and liquid phases at high
temperature as confirmed by GC and TOC analyses, respectively. The triangular
lump kinetics model was used to describe the reaction pathways. The oxy-
cracked
products were found to be humic acid analogs with different contributions of
functional groups such as carboxylic, carbonyl, and sulfonic acids as
confirmed by
FTIR analysis.
CA 3012804 2018-07-27
[0092] The Cu-silicate (CaCuSi4010) catalyst of the present example was
prepared using a co-precipitation synthetic route and thermal treatment. In
alternative embodiments, a range of methods, such as co-precipitation-thermal
or
hydrothermal methods, may be used to obtain one or more nanocrystalline copper
silicates of the Gillespite group of minerals for use as a catalyst in methods
of the
invention ((Cuprorivaite (CaCuSi4010); Wesselsite (SrCuSi4010) and
Effenbergerite
(BaCuSi4010) or combinations thereof). The prepared catalyst was characterized
before and after oxy-cracking reaction using XRD, SEM, BET, and FTIR. The
activity of the catalyst was investigated through the oxy-cracking process
which
was carried out in a batch reactor under aqueous alkaline medium and mild
operating conditions for maximum solubility and selectivity of petcoke.
[0093] For purposes of this Example, minimal emission of CO2 was an
objective
for the proposed oxy-cracking process. The oxy-cracking conversion and
selectivity
were measured using the total organic carbon analysis (TOC) while the gas
emissions were characterized using gas chromatography (GC). The catalytic oxy-
cracking reaction mechanism was developed based on the radical mechanism. The
oxy-cracked products were characterized using the FTIR. The present study
illustrates that using the nanocrystalline copper-silicate materials, of the
Gillespite
group of minerals, for the petcoke oxy-cracking provides an efficient catalyst
for
converting petcoke into commodity chemicals like humic acid analogs.
Materials and Methods
Chemicals and reagents
[0094] As an example of preparation of the copper-silicate Cuprorivaite
(CaCuSi4010) nanocrystalline material, the following chemicals and reagents
were
used: 70 wt% purity nitric acid (HNO3, Sigma Aldrich, Ontario, Canada);
copper(II)
acetate (Cu(00CCH3)2.H20, Sigma Aldrich, Ontario, Canada); sodium silicate (27
wt% SiO2, 10.8 wt.% Na2O, Sigma Aldrich, Ontario, Canada), calcium hydroxide
(Ca(OH)2, Sigma Aldrich, Ontario, Canada); 99% purity sodium hydroxide (NaOH,
VWR, Ontario, Canada); and sulfuric acid (95-98% purity, Sigma Aldrich,
Ontario,
Canada) was used for the catalyst regeneration. For the oxy-cracking reaction,
green petcoke sample (as in Example 1) was ground and sieved to a particle
size
of 53 to 710 pm and used as the source of feedstock. Potassium hydroxide (KOH,
31
CA 3012804 2018-07-27
ACS reagent, Sigma-Aldrich, Ontario, Canada) was used to adjust the
pH of
the reaction medium. Ultra-high purity oxygen (99.9%, Praxair, Calgary,
Canada)
was used as the oxidant gas. Potassium bromide (KBr, Sigma-Aldrich, Ontario,
Canada) was used for the infrared analysis. Ultra-high purity nitrogen (99.9%,
Praxair, Calgary, Canada) was used for the surface area measurements of the
prepared material. The carrier gas for the GC was helium (99.9% ultra-high
purity,
Praxair, Calgary, Canada). Commercial humic acid (53680 humic acid, Sigma-
Aldrich, Ontario, Canada) was used and characterized for comparison purposes,
with oxy-cracked products. All chemicals and reagents were used as received
without any further purification.
Synthesis of nanociystalline copper-silicate material
[0095] The copper-silicate (CaCuSi4010) material for the present example
was
synthesized using a simple co-precipitation method followed by a thermal
.. treatment; however, other methods that produce nanocrystalline materials,
such as
hydrothermal methods, are suitable. An acidic solution was prepared by
dissolving
12 ml of nitric acid into 600 ml deionized water with magnetic stirring (300
rpm)
followed by the addition of 10.254 g copper(II) acetate. After complete
dissolution of
the copper in the acid solution, ¨ 45.492 g of sodium silicate was carefully
added to
the solution with agitation for 5 min until a homogenized solution was
achieved.
Subsequently, a blue gel formed when the pH was increased to 8.0-8.5 by the
addition of NaOH pellets under magnetic stirring (300 rpm). The blue gel was
allowed to stand for 10 min in order to ensure that pH was stable in the range
of
8.0-8.5. The solution was then filtered and washed using copious amounts of de-
ionized water under vacuum at room temperature in order to remove excess
salts.
After thorough washing, the filtered product was allowed to stand at room
temperature by passing air through it for ¨15 min under vacuum suction.
Approximately 3.762 g of calcium hydroxide was added to the wet cake and mixed
gently until a homogeneous and pale blue smooth paste was obtained. The pale
blue paste was dried in an oven overnight at 200 C. The dried product was
ground
using a mortar and pestle, and calcined at 850 C for 3 h in a muffle furnace
with a
heating ramp of 10 C/min. The furnace was then cooled down to room
32
CA 3012804 2018-07-27
temperature, and the powdered Cu-silicate with nanocrystalline domain sizes
was
obtained.
Catalyst characterizations
[0096] The crystalline phases of the prepared and spent catalysts were
characterized using X-ray diffraction (XRD) Ultima III Multi-Purpose
Diffraction
System (Rigaku Corp., The Woodlands, TX) with Cu Ka radiation operating at 40
kV and 44 mA. The scan range was 3-90 28 using a 0.05 degree and a counting
time of 0.2 degree/min. The crystalline domain sizes of the prepared materials
were
determined using the Scherrer equation as implemented in the PDXL software.
The textural properties and surface areas of the prepared catalyst were
measured
using the Brunauer-Emmett-Teller (BET) method. This was accomplished by
performing nitrogen physisorption at -196 C using TriStar II 3020,
Micromeritics
Corporate, Norcross, GA. The test sample was previously outgassed at 150 C
.. under N2 flow overnight before analysis to remove the moisture. Scanning
electron
microscopy (SEM) was used to visualize the surface morphology of the prepared
materials. A field emission Quanta 250 SEM manufactured by FEI was used, with
an accelerating voltage of 20 kV and a spot size of 3.0 to view the morphology
of
the samples. The tested sample was prepared by taping a very small quantity of
the
powder over a carbon tape holder and releasing the excess and loose particles.
Finally, the molecular bonds in the prepared catalyst were identified using a
Shimadzu IRAffinity-1S FTIR (Mandel, USA).
Catalytic oxy-cracking of petcoke sample
[0097] Petcoke oxy-cracking examples were carried out in a 100 ml stainless
steel vessel (model number 4598, Parr Instrumental Company, Moline, II, USA).
The vessel was equipped with a heating oven connected to a temperature control
loop, a pressure gauge and a mechanical stirrer with a speed controller. In a
typical
experiment, 1.0 g of solid petcoke sample and a predetermined amount of
catalyst
(0.10 g CaCuSi4010) were charged into the reactor vessel containing 20.0 g of
deionized water and 1.0 g of KOH before heating up the reaction vessel to the
required temperature. KOH is required in an amount that is adequate to
increase
the pH of the solution, and thus enhance the solubility of petcoke (and may
also
33
CA 3012804 2018-07-27
serve to avoid potential corrosion problems). Prior to heating, the reaction
vessel
was leak tested by sealing and pressurizing the vessel with 02. The reaction
solution was then heated to the desired temperature, with the stirring speed
set at
1000 rpm. A high mixing speed was used here in order to minimize the
interfacial
mass transfer resistance between the gas and liquid phase and to ensure
uniform
temperature and concentration profiles in the liquid phase. Once the set
temperature was reached, reaction time zero is defined. The reaction
experiments
were carried out at different times (15, 30, 60, 90 and 120 min), temperatures
(150-
250 C) and at a constant pressure (750 psi).
Characterization of oxy-cracking products and the spent catalyst
[0098] At the end of the reaction, the reactor was cooled down and
connected to
the GC (SRI 8610C, Torrance, CA) to analyze the released gases. The GC is
provided with a thermal conductivity detector (TCD) and two packed columns
connected in parallel (3' molecular sieve/6' Hayesep-D columns). Afterwards,
the
liquid phase was carefully withdrawn and filtered for total organic carbon
(TOC)
analysis using a Shimadzu Total Organic Carbon Analyzer (TOC-L CPH/CPN). All
the measurements in TOC and GC were taken respectively three and five times,
and the average was used for the calculations with a 5% relative standard
deviation.
[0099] The oxy-cracked products were recovered by drying in a vacuum oven
overnight at 65 C and characterized using FTIR. A Shimadzu IRAffinity-1S FTIR
(Mandel, USA), provided with a smart diffuse reflectance attachment to carry
out
diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS) analysis,
was
used. Initially, the background was defined by analyzing ¨500 mg of potassium
bromide (KBr), then ¨ 5 mg of the sample dispersed into the 500 mg of KBr and
analyzed together. The IR spectra were obtained in the wavenumber ranging from
400 to 4000 cm-1, then the spectra were acquired as averages of 50 scans with
a
resolution of 4 cm-1. A small amount of presumably unreacted solid residue and
the
used catalyst were collected at the bottom of the reactor vessel. The residual
materials (e.g., spent catalyst, minerals and insolubilized petcoke) were
recovered
and dried using vacuum oven at 65 C for XRD analysis. Additionally, the metal
analysis was performed for the liquid phase to detect any leaching from the
34
CA 3012804 2018-07-27
catalyst. The leached metal concentrations in the samples (Cu) was analyzed at
ALS Environmental Laboratories (Alberta, Canada) using Inductively Coupled
Plasma Mass Spectroscopy (Dissolved Metals in Water method by CRC ICPMS).
The carbon, hydrogen, and nitrogen contents after the oxy-cracking reaction
were
-- analysed using a PerkinElmer 2400 CHN analyzer (Waltham, Massachusetts,
USA). The oxy-cracking reaction conversion and selectivity were determined
from
the carbon mass using Equations (1) to (5) from Example 1.
Stability tests
[00100] The stability of the copper-silicate catalyst in the heterogeneous
oxy-
cracking reaction is exemplified as follows. A small amount of the residual
material
which contains the spent catalyst and the insolubilized petcoke was collected
at the
bottom of the reactor vessel. The residual materials were recovered by
filtering the
solution after the catalytic tests and drying at 65 C overnight in a vacuum
oven to
-- remove the residual water. The dried sample was then washed with a diluted
sulfuric acid (<3%) solution to remove unwanted metals such as K, Ni, and Fe
that
remained in residual materials after reaction, and filtered again. The
filtrate sample
was calcined at 600 C for 6 h in order to remove any organic species that may
have been adsorbed on the material. The spent catalyst was then reused for
-- several cycles of oxy-cracking reaction after further analysis by XRD.
Results and discussion
Characterization study of the prepared catalyst
[00101] The structure of the prepared copper-silicate material was
defined by
-- a variety of techniques. XRD was employed to identify the framework
structure. As
shown in Figure 15, the good intensity of the signals in the XRD patterns
implies a
well crystallized material. The XRD pattern of this material was matched
perfectly
with the pdf card 01-085-0158 (cuprorivaite) of the Crystallographic Open
Database
(COD database) included within the PDXL software (Integrated X-ray powder
-- diffraction software). Additionally, the broad signals clearly indicate the
formation of
a nanocrystalline copper silicate.
[00102] As displayed in Figure 16, the CuCaSi4010 has a tetragonal
crystal
structure (a = p = y=90 ) with space group P4/ncc where its lattice constants
are: a
CA 3012804 2018-07-27
= b =7.3017 A , c =15.1303 A accordingly with a unit cell volume equal to
806.7
A 3. The metal and ligand oxygen atoms lie in the (001) crystal plane (the XY
plane)
along with the [001] Z molecular direction. The Si-centered tetrahedra are
parallel to
(001) and linked to form two Si8020 sheets within the height of one cell and
each
tetrahedron has one unshared corner. The presence of Cu atom sites has a
centrosymmetric, planar ligand environment of (Do) symmetry, which is formed
by
four oxygens from the unshared corners; such coordination is super-stable and
characteristic of divalent Cu. On the other hand, the Ca atoms are found to be
situated in 8-fold coordination midway between sheets. Moreover, the
crystalline
domain sizes of the prepared material were estimated from the most intense
peaks
using Scherrer's equation which is implemented in the PDXL software where the
average domain size was -93 nm. The positions and relative intensities of the
diffraction peaks of the synthesized pure-phase sample are in good agreement
with
previously reported studies on copper silicate, also known as Egyptian blue.
[001031 The textural properties of the prepared catalyst were illustrated
by N2
physisorption at -196 C using the BET analysis. Figure 17 shows the nitrogen
physisorption isotherms of the CaCuSi4010 which can be classified as Type II
curves based on the I UPAC classification. The curve indicates the absence of
any
microporosity in the prepared materials as the isotherm starts from zero
without any
sudden jump in the Y-axis at p/p - 0. Additionally, the estimated specific
surface
area was 0.63 m2/g by applying the BET method in the range of relative
pressures
(p/p ) between 0.03 and 0.3 and assuming a value of 0.162 nm2 for the cross-
section of adsorbed nitrogen molecules at -196 C.
[00104] SEM was used to analyze the surface morphology of the
catalyst.
Figure 18 shows SEM images for the surface of copper-silicates at different
magnifications. This analysis indicated that the prepared copper-silicate
material
has characteristic steps, ridges, and terraces on the surface of the prepared
nanocrystalline material. The powders were made up of coarse crystals of
cuprorivaite with different shapes and sizes and the size of these particles
are in
the scale of a few microns (10 and 30 pm). The SEM images revealed that the
synthesized CaCuSi4010 has a nonporous structure with large grains of fused
micronic-crystals.
36
CA 3012804 2018-07-27
[00105] Figure 19 shows the infrared spectrum of the prepared copper-
silicate material. The IR-spectrum displays characteristic bands lying mainly
in the
region between 1400 and 400 cm-1 which are attributed to the asymmetric and
symmetric stretching vibrations of Si-O-Si and Si-O-Cu, and the bending
vibration
of 0-Si-0 and 0-Cu-0. Additionally, the silicate band 1085 cm-, was clearly
shifted down which indicates the formation of Si-O-Cu bond and provides
evidence
for the incorporation of copper metal in the silicate framework structure. The
presence of water molecules bound to the surface of copper-silicates is
evident
here by the -OH stretching bands at 3637 cm-1 and -OH bending band at 1640 cm
1. However, the small band centered at around 665 cm-1 is related to the
bending
vibration of -OH that may be located in the tetrahedral position shared by
four Cu
atoms.
Catalytic activity and selectivity
[00106] The catalytic activity and selectivity for oxy-cracking of petcoke
over
CaCuSi4010 catalyst is illustrated in a batch reaction. Based on wet air
oxidation
studies, the reaction rate depends on many factors such as temperature,
catalyst
loading and solution pH. The results in terms of petcoke oxy-cracking
conversion
and selectivity to produce both of intermediates (desired products, B) and CO2
(C)
are presented in Figures 20 and 21. The batch reactions were carried out by
varying the temperature from 150 to 250 C while keeping the rest of the
reaction
conditions constant (oxygen partial pressure 750 psi, stirring speed 1000 rpm,
residence time 1 h, and 0.10 g of catalyst). As seen in Figure 20, the rate of
the
oxy-cracking reaction conversion is significantly increased upon raising the
reaction
temperature. Thus, at 250 C, -97% petcoke conversion was reached after 1 h
over the CaCuSi4010 catalyst. The reaction conversion was more than 45 % even
at low temperatures of 175 C, which is promising when compared to the non-
catalytic oxy-cracking of petcoke as no reaction has been observed to occur at
that
temperature. Although a high reaction temperature (250 C) may not be optimal
in
the oxy-cracking process without a catalyst, as illustrated in Example 1, in
the
presence of the Cu-silicate catalyst no unfavourable amount of CO2 was
observed.
Surprisingly, the selectivity to produce the desired products (B) was 99% even
at
the low reaction temperature of 150 C and reaction time of 15 min.
37
CA 3012804 2018-07-27
[00107] The effect of reaction times on catalytic activity and
selectivity was
illustrated by varying the time from 15 to 120 min keeping the temperature at
200
C as shown in Figure 21. The rate of reaction conversion is significantly
increased
with time. Full reaction conversion was obtained after 2 h at 185 C, whereas
only 1
h was required at 200 C and 0.5 h at 250 C. Surprisingly, the selectivity
for
product B is almost constant and reaches 99% with time. Even at the longest
reaction time of 120 min, the amount of CO2 produced was not detectable by GC.
Produced CO2 may be present in the aqueous basic solution (pH >8) in the form
of
carbonates and bicarbonates. This Example illustrates that the Cu-silicate
catalyst,
used under the exemplified conditions, possesses superior activity and
selectivity
compared with other catalysts that might be considered for wet oxidation
reactions
such as Mn02/ Ce02, Ru, Pt and RufTi02, Mn-Ce-oxide and Perovskite catalysts
LaB03 (B= Cu, Fe, Mn, Co, Ni).
[00108] The activity of Cu-silicates in the oxy-cracking reaction is
indicative of
activity provided by catalysts having analogous Cu+2characteristic, for
example in
silicate frameworks. As shown in Figure 22, the Cu atom in the silicate
catalyst has
four coordinated atoms of oxygen as for Si. Thus, the square planar
configuration
allows d-orbitals to take part in the reaction. Hence, anchoring Cu+2 in the
silicate
framework the present synthetic method leads to a material with
nanocrystalline
domain size, and thereby increases the number of active sites which have a
benefit
in activating the petcoke. Surprisingly, the activity of oxy-cracking reaction
over
CaCuSi4010 was shown to be higher than the activity of alternative high
surface
area catalysts for wet air oxidation. This indicates that beyond surface area,
the
types of active site on the surface of present catalyst contribute to the
catalytic
activity in oxy-cracking. In some embodiments, involvement of a calcium ion in
the
cuproravite structure may act as a basic aid to assist the active site of the
catalyst
surface, for example by attracting reactant molecules, thus enhancing the
performance of catalyst.
[00109] The influence of pH on the conversion and selectivity of oxy-
cracking
reaction is illustrated in Example 1, which evidenced embodiments in which an
elevated pH is advantageous for high reaction rates. An aspect of such
embodiments may be that, under alkaline conditions (pH >8), hydroxyl radical
formation is increased, and more produced CO2 will be dissolved in solution.
In
38
CA 3012804 2018-07-27
embodiments of Example 1, 1.0 g of KOH per 1.0 g of petcoke was shown to be an
optimum value for select embodiments. Collectively, the present Examples
illustrate
embodiments in which pH can have a significant effect not only on the
catalytic
activity but also on the stability and leaching of the active phase from the
catalyst.
Reaction kinetics and mechanism
[00110] The catalytic performance of the Cu-silicate material on the
petcoke
oxy-cracking was illustrated in the presence of oxygen as an oxidant. The
kinetic
experimental data was collected at temperatures of 185 C, 200 C, and 230 C
and
reaction times varying from 0 to 2 h. It was shown in Example 1 that under
relatively
severe reaction conditions (i.e., temperatures > 250 C and residence times >2
h),
the complete oxidation reaction may be favoured over partial oxy-cracking, so
that
the production of CO2 may be significant. Additionally, in Example 1, the
reaction
conversions were found to be low at temperatures less than 185 C. In a
typical
oxy-cracking reaction, the solubility of oxygen in the aqueous solution is
increased
with pressure, which favors oxy-cracking. However, oxygen partial pressure
beyond
750 psi did not significantly affect the reaction; and hence was kept constant
at that
value for a range of exemplary embodiments. In select embodiments, stirring
was
shown to be important, for example to favor the interaction between oxygen and
petcoke. In the present Example, no mass transfer limitation was observed when
the impeller speed operated above 500 rpm. Therefore, the impeller speed was
fixed to 1000 rpm during all the reaction runs. The mass ratio of petcoke to
KOH
was fixed to 1:1, this is where the highest conversion and selectivity were
obtained
in embodiments of Example 1.
[00111] The triangular reaction pathway, as depicted in Figure 1, and as
described in Example 1, may similarly be used to describe the mechanism of a
catalyzed reaction. For the catalyzed reaction, Figure 23 represents the
Arrhenius
plot of a catalyzed petcoke oxy-cracking reaction at the three reaction
temperatures. The three curves are approximately linear with the correlation
coefficient values close to 1. Table 6 sets out determined values of catalyzed
oxy-
cracking reaction constants, and Table 7 set out estimated activation energies
and
frequency factors of catalyzed petcoke oxy-cracking.
39
CA 3012804 2018-07-27
[00112] Comparisons between kinetic results from Example 1, without a
catalyst, and Example 2, with a catalyst, indicate that the reaction rate in
the
second pathway proceeds favorably towards the intermediates which are the
desired products (oxy-cracked products). Using the catalyst, this rate is much
faster
than without a catalyst. This is also reflected in the low value of activation
energy in
the presence of a catalyst which is 25% less than that in the absence of a
catalyst.
Surprisingly, the reaction rate for forming CO2 in either reaction pathways (1
or 3)
with presence of catalyst is lower than that without it. As shown in Figure
24, the
concentration profiles of petcoke (A), oxy-cracked compounds (B), and CO2 (C)
at
the three reaction temperatures as a function of time fit well with the
exemplified
kinetic model. The error bars in Figure 24 represent the calculated standard
deviation based on the TOC and GC measurements. Petcoke was not directly
oxidized to CO2 but partially oxidized to intermediates as phenolic and
carboxylic
substances produced through hydroxyl radical (.0H) attacks. Insignificant
amounts
.. of CO2 were detected at the beginning of the reaction, presumably due to
the deep
oxidation of the short alkyl chains left over on the petcoke structures after
the
coking process. Thus, a low activation energy (Ei=15.40 kcal/mol) in the first
reaction pathway is accounted for. The insignificant amount of CO2 may be
related
to a short induction period, which may in some embodiments be required to
reach a
sufficiently high concentration of catalyst in the liquid phase in order to
incorporate
oxygen into the hydrocarbon molecules.
[00113] The oxidation of the hydrocarbons over copper-silicate
catalyst
demonstrates that a complex reaction takes place in the liquid phase, which
can be
attributed to the complexity of petcoke aggregates. In select embodiments, the
dissociation of the carbon bonds adjacent to heteroatoms such as sulfur,
oxygen
and nitrogen may take place. This is supported by the low value of the
activation
energy in the second reaction pathway (E2=17.00 kcal/mol) and high value of
frequency factor (2.36 x 104s-1). The low activation energy (E2) value
supports an
understanding that polymerization reactions may in some embodiments be
involved, and that the formation of (.0H) radicals over the catalyst may be a
rate
limiting step. In some embodiments, an insignificant amount of CO2 as
carbonates
and bicarbonates (pH-8.5-9.8) may be formed in the third reaction pathway, for
example due to the further reaction between the solubilized hydrocarbons and
CA 3012804 2018-07-27
oxygen. However, this reaction pathway would generally require higher
activation
energy (E3=28.10 kcal/mol) for producing the CO2 compared to the first
reaction
pathway.
[00114] These findings suggest that the path of conversion of petcoke
into
CO2 is favored at the beginning of the reaction, which is associated with a
higher
rate of reaction compared with the oxidation of solubilized organic compounds
in
water. Moreover, these results also show that, in some embodiments, even
though
the reaction rates are increased with temperature, the final TOC values of oxy-
cracked compounds (B) for a temperature higher than 200 C are practically
constant after 1 h. The reason for this is putatively due to the ability of
these formed
short-chain organic species to resist the oxidation process. Another
explanation for
this observation is the relatively short life of free radicals in some
embodiments
(due to scavenging effects), for example where the presence of strong basic
solution, e.g. KOH, destroys some of the free radicals which would otherwise
directly attack organic compounds.
Table 6: Determined values of catalyzed oxy-cracking reaction constants.
T ( C) K1 (s-1) 1<2 (S-1) K3 (8-1)
185 8.21 x105 1.67 x 10-4 1.63 x 10-6
200 1.07 x 10-4 3.85 x 10-4 9.61 x 10-6
230 3.56x 10-4 9.25x 10-4 2.69x 10-5
Table 7: Estimated activation energies and frequency factors of catalyzed
petcoke
oxy-cracking.
Activation energy (kcal.mo1-1) Frequency factor (s-1)
15.40 0.235 1.65x 103
E2 17.01 0.632 2.36 x 104
E3 28.10 0.781 4.56 x 107
[00115] Without being bound to a particular theory, the mechanism of
oxy-
cracking reaction over the produced Cu-silicate catalyst may be understood to
41
CA 3012804 2018-07-27
follow a wet air oxidation mechanism, undergoing several mechanistic steps. In
this
context, it is relevant that solid petcoke particles generally float in water
as chunks
and masses due to hydrophobicity effects. KOH in the aqueous medium plays a
role in dispersing the petcoke particles through a saponification-like
reaction. After
the petcoke particles are dispersed in the alkaline medium, and in the
presence of
oxygen, the oxy-cracking reaction takes place in several steps on the catalyst
surface. Oxygen molecules may diffuse to the surface of the catalyst suspended
in
the liquid phase. The role of the catalyst is accordingly to activate the
reactants,
and thus transfer electrons to initiate free radicals. Subsequently, the
adsorbed
oxygen may oxy-crack the petcoke at an active site and convert it to
oxygenated
hydrocarbons that are soluble in water, due to the polar functionalization of
aromatic edges and paraffinic terminal carbons via oxygen incorporation. In
select
embodiments, copper in the prepared nanocrystalline CaCuSi4010 may have a
buffering capacity for the oxygen on its surface in which the alternation
between
.. oxidation states (Cu+2/Cu+1) and formation of oxygen vacancies can occur
under
select reaction conditions. These oxygen vacancies have the potential to
transfer
more oxygen through the lattice. Eventually, reaction termination takes place
when
the generated radicals are consumed by reacting with K ions from
bicarbonate/carbonate (-110 ppm at 230 C) that are formed during the reaction
or
by recombining themselves. In the result, at the end of the reaction, three
phases
are obtained. The gas phase remained as predominantly oxygen, with an
insignificant amount of CO2, while the liquid phase contained oxygenated
hydrocarbons, such as humic analogs. Finally, the residue (solid phase)
consisted
of minerals, spent catalyst and some unreacted residue.
Leaching and stability tests of copper silicate
[00116] Metal analysis for copper before and after the reaction is
presented in
Table 8. The data is reported in terms of the percentage and concentration of
copper leached with respect to the initial amount present in the catalyst
(CaCuSi4010) at various reaction temperatures.
42
CA 3012804 2018-07-27
Table 8: Estimated leached active metal (Cu) from the catalyst at different
oxy-
cracking reaction temperatures. Experimental conditions: catalyst dose, 0.10
g;
reaction time, 1 h.
Active Metal Leaching at reaction temperature ( C)
metals (%) in 170 200 230
the wt% ppm wt% ppm wt% ppm
catalyst
Cu 16.90 1.75 15.30 2.45 21.50 2.98 26.14
[00117] The percentage of active metal (Cu) leached to the aqueous
solution
increased with reaction temperature. The leaching of copper from the catalyst
was
detected in the range 2 to 3 wt% of the original total amount of Cu, during
the oxy-
cracking reaction. This illustrates the stability of the CaCuSi4Olocatalyst in
the
aqueous leaching solution, in that the maximum leaching percentage was less
than
3% (26 ppm) from the original Cu amount at elevated temperatures. In an
exemplary embodiment, an excess of KOH (i.e., pH >10) was added to the
aqueous solution at the beginning of the reaction. In this embodiment, even at
the
end of the reaction, the pH was still > 8.5, with the reduction being due to
the
formation of acidic functional groups that consume a portion of the original
KOH. In
select embodiments, the effect of KOH is not only to enhance the solubility of
oxy-
cracked materials, but also to maintain the basic pH of the solution, thus,
preventing the leaching of copper.
[00118] The stability and reusability of the present catalyst are
exemplified
herein. To illustrate the long-term stability of the catalyst, a number of
successive
cycles of petcoke oxy-cracking were conducted. The catalyst was separated from
the reaction mixture at the end of the reaction and washed. The activity of
the
recycled catalyst was determined by carrying out oxy-cracking at a temperature
of
200 C with a residence time of 2 h. Other operating conditions were kept
constant.
The catalyst activity in terms of reaction conversion and selectivity for both
B and C
for the three consecutive experiments is presented in Figure 25. As shown, the
selectivity to produce B reached nearly 98% after three cycles of reaction,
with the
third cycle showing an insignificant downward trend as compared with the first
run,
43
CA 3012804 2018-07-27
within the experimental error. The selectivity for C is slightly increased
with each
run; however, the trend is within the range of experimental error.
Surprisingly, even
after three runs, the reaction conversion was stable and maintained at 92%,
90%
and 87% for three consecutive runs, respectively, which evidences the
successful
reusability and stability of the CaCuSi4019 catalyst (Egyptian Blue, which
shows
resistance to fading even under strong light and can still be observed in
Egyptian
historical relics which have been exposed for thousands of years without
losing
their color).
[00119] These findings were confirmed by XRD analysis of the spent
catalyst
as shown in Figure 26. The figure shows that the XRD patterns following
regeneration after the first cycle and the fresh catalyst (compared to
cuprorivaite
and wollastonite). The main catalyst structure remained unchanged; however,
amorphous material can be observed together with traces of wollastonite. The
XRD
pattern of the regenerated catalyst matches perfectly with the pdf card no. 01-
085-
0158 (cuprorivaite) of the Crystallographic Open Database (COD). However, some
small traces of wollastonite (Ca3Si309) was present based on the pdf card no.
1011227. Egyptian Blue pigment is understood to consist of CaCuSi4010 with
variable amounts of wollastonite (CaSiO3), high amounts of Cu oxides and
cuprite
(Cu2O). A difference in crystalline domain sizes were observed between the
fresh
(93 nm) and regenerated (44 nm) catalyst, which may be due to the
disaggregation
of some crystallites and/or a new ordering of crystalline matrix under the
reaction
conditions. Additionally, the intensities of the crystallographic phase in the
regenerated catalyst are lower than for the fresh catalyst.
FTIR analysis of the oxy-cracking products
[00120] The infrared spectra of the original petcoke and the oxy-
cracked
compounds (i.e., solubilized fractions) isolated from a reaction that was
carried out
at 200 C for 2 h are shown in Figure 27. As seen, for the original petcoke
spectrum, the alkyls/aliphatic and aromatic regions assigned at (2850-3000 cm-
1)
and (-3040 cm-1 and 930-742 cm-1), respectively. The aromatic stretching
vibration
of C=C appears at around 1600 cm-1, which could be conjugated with other
groups.
The out-of-plane C-H bonds vibration in the aromatic range is assigned at 804,
and
860 cm-1 bands. The C-H stretching vibration due to the aromatic appears at
3040
44
CA 3012804 2018-07-27
cm-1. Moreover, the presence of alkyl groups such as -CH3, =CH2 and -CH2CH3 is
evidenced by the bands around 2910 cm-1 and 1380 cm-1 which can be assigned to
asymmetric and symmetric -C-H stretching and bending vibrations, respectively.
The possibility of -OH functionalities (3500 cm-1) is present, which seems to
be
interacting through hydrogen bonding as the signals are very broad spanning
from
about 2700 to 2000 cm-1. The presence of heteroatoms such as sulfur in form of
sulfoxide species can be assigned to the small band -1031 cm-1. It can be
concluded that the petcoke has a high contribution of polynuclear aromatic
hydrocarbons and a relatively small contribution of aliphatic chains with some
heteroatoms such as sulfur, nitrogen and oxygen.
[00121] The FTIR spectrum of the oxy-cracked products is dramatically
different than the original petcoke. The oxy-cracked products obtained at 200
C
were compared with a sample of commercial humic acid as shown in Figure 27. As
seen, the IR spectrum of oxy-cracked products with the presence of the
catalyst
resembles the one obtained for the commercial humic acids. The broad band
spanning from 3700 to 2500 cm-1 indicates the presence of OH groups in both
samples (i.e., oxy-cracked and commercial humic acid). An important feature is
the
intense and broad peak appearing between 3318 and 3503 cm-1 which correspond
to -0-H stretching vibration mode of hydroxyl functional groups. These
functionality
groups are formed due to the presence of oxygen in the aqueous phase and are
related to oxygenated species such as carboxylic functional groups. The
presence
of carboxylic acids (C=0) are evident as indicated by the double band centred
at
1710 cm-1 in both samples; however, they are more observed in the oxy-cracked
products as compared with the commercial humic acid. Interestingly, a complete
cracking in the aromatic species is evidenced in the oxy-cracked sample not
only
by the disappearance of aromatic moieties in the region of out-of-plane bands
(930-750 cm-1) but also the reduction of the aromatic C-H stretching at 3030
cm-1.
On the other hand, the alkyl groups are no longer visible in the range of 3000-
2850
cm-1 in the oxy-cracked sample indicating the complete oxy-cracking of petcoke
at
that reaction temperature. Moreover, another important feature present in the
oxy-
cracked sample is the sharp band at -1842 cm-1 indicating a possibility of
carbonyl
compounds such as lactones and esters; this band did not appear in the
commercial humic acid sample. Another difference between the oxy-cracked
CA 3012804 2018-07-27
sample and the commercial humic acid is the band at 1215 cm-1 corresponding to
the presence of sulfur as sulfone compounds, this is due to the high
concentration
of sulfur in the sample (-6% sulfur). These results illustrate that the oxy-
cracking
product characteristics are similar to humic analogs, but with some sulfur
content.
The product consists of primarily oxidized organic functional groups such as
hydroxyl (-OH), carboxylic salts (0=C-0-), carboxylic acids (R-CO2H) and minor
amounts of esters. These functionalities identified by IR spectra over the
catalyst
are in accordance with the compounds found in Example 1, derived from
processes
carried out without a catalyst, although no humic acids analogs were found in
the
absence of the catalyst. These findings illustrate that the copper-silicate
catalyst is
more selective toward producing humic acid analogs under the exemplified
conditions, than reactions carried out without a catalyst.
Example 3: Comparative thermal properties and heating values of oxy-
cracked and virgin petroleum coke.
[00122] This Example compares the heating value and thermo-oxidative
behaviour of the oxy-cracked and virgin petcoke using thermogravimetric
analysis
(TGA). For this purpose, a sample of petcoke was oxy-cracked at 200 C and 750
psi in a Parr reactor, in keeping with the description set out in Example 1.
TGA
analysis illustrated that the oxy-cracked petcoke is oxidized with improved
kinetics
compared to virgin petcoke. There was also a significant improvement in the
combustion performance parameters of the oxy-cracked petcoke such as ignition,
peak and burnout temperatures. In the result, the heating value of oxy-cracked
petcoke is similar to virgin petcoke, whereas the nitrogen and sulfur content
in the
.. oxy-cracked petcoke is much lower than that of virgin petcoke.
[00123] A sample of oxy-cracked petcoke, was prepared as generally
described in Example 1, to provide solubilized organic species, collected from
the
alkaline solubilization solution and solidified after washing with acid and
drying in a
vacuum oven overnight at 65 C. HCI (37%, ACS reagent, Sigma Aldrich, Ontario,
.. Canada) was used for washing the oxy-cracked sample. In brief, the reaction
was
carried out in a PARR batch reactor by mixing 1.0 g of original petcoke with
20 ml
of deionized water under alkaline conditions (pH-13). The petcoke sample was
oxy-cracked at 200 C, with oxygen pressure of 750 psi, for 120 min. The mixer
46
CA 3012804 2018-07-27
speed was set at 1000 rpm. At the end of the reaction, the liquid effluent was
discharged and filtered in a centrifuge (Eppendorf centrifuge 5804) at 5000
rpm for
15 min to separate the remaining solid (i.e., unreacted and/or insolubilized
species).
The pH of the obtained liquid solution after reaction ranged between 8 and 10
depending on reaction conditions. The pH was measured using a Mettler Toledo
pH meter (Mississauga, Canada). Afterwards, few drops of HCI (37%) was added
to the black liquid solution (solubilized petcoke in water) until the pH of
the solution
decreased to ¨6. Following acidification, the solid particles were allowed to
settle
for 3 h and then separated by centrifuging and decanting the supernatant
solution.
The settled black solid contained most of the organic products based on TOC
measurements (-90% of total TOC). However, small amount of hydrocarbons
(<10% of total TOC) remained soluble in the supernatant. Remaining solubilized
hydrocarbons could be recovered by allowing the solution to settle for 48 h.
The
settled-solid hydrocarbons were centrifuged and washed twice with 5% HCI
solution
to remove the excess K left over after the oxy-cracking reaction. The
collected
solidified organic species were dried in a vacuum oven overnight at 65 C for
TGA
analysis. The elemental analysis for this sample (after acid treatment) was
measured and compared with virgin petcoke and the chemical composition
summarized in Table 9.
Table 9. The chemical composition of the virgin and oxy-cracked petcoke
sample.
Composition (wt%) Virgin petcoke Oxy-cracked petcoke
84.48 62.31
3.81 2.68
1.55 1.10
4.46 1.32
V 0.08 0.04
Fe 0.06 0.01
Ni 0.03 0.01
Mo 0.01 0.00
Co 0.051 0.00
0.00 3.54
47
CA 3012804 2018-07-27
0* 5.47 28.99
* Estimated by the difference
Thermogravimetric analysis
[00124] The virgin petcoke and the washed oxy-cracked petcoke were
subjected to thermal oxidation using a thermogravimetric TGA/DSC analyzer (SDT
Q600, TA Instruments, Inc., New Castle, DE). As for the oxidation study,
samples
of ¨5 mg of both materials were heated up from room temperature to 800 C with
a
heating rate of 10 C/min under the air flow of 100 cm3/ min. The TGA results,
weight loss (TG) and weight loss rate (DTG) profiles, were analyzed to
determine
the combustion performance parameters (i.e., ignition, peak, and burnout
temperatures). These parameters can be calculated by the intersection method.
The ignition temperature is calculated at the point where the TG peak, which
is the
point of initial devolatilization after the sample was dried, and the tangent
line of the
mass loss profile are intersected. The peak temperature is determined at the
.. maximum DTG peak. Eventually, the burnout temperature is calculated at the
intersection point between the two tangent lines; the first line is tangent to
the mass
loss profile at the point where the DTG peak occurs and the second line is
tangent
to the point where the weight loss is unchanged. It is also approximated by
the
temperature where weight loss of the sample reaches to ¨1%/min at the terminal
phase of the DTG profile.
Heating value measurements
[00125] The high heating value (HHV) of virgin and oxy-cracked petcoke
was
determined by proximate analysis using TGA. The moisture (M) content and
volatile
matter (VM) were estimated by heating up the sample from room temperature to
500 C under nitrogen atmosphere flowing at a rate of 100 ml/min. The fixed
carbon
(FC) and the ash (A) content (residue) were obtained by continuing heating the
sample from 500 to 800 C at a heating rate of 10 C/min under air flow,
passing at
a flow rate of 100 ml/min over the sample. The change in the sample weight was
monitored until there was no further change in weight. After estimating the
values
for of the aforementioned properties (i.e., M, VM, FC, and A), the heating
value was
calculated using alternative correlations (Schuster et at., Brennst Chem, 32
(1951)
48
CA 3012804 2018-07-27
19-20; KeicOkbayrak et al., Fuel, 70 (1991) 979-981; Cordero et al., Fuel, 80
(2001)
1567-1571; Parikh et al., Fuel, 84 (2005) 487-494; Majumder et al., Fuel,
87(2008)
3077-3081).
Elemental analysis
[00126] A PerkinElmer 2400 CHN analyzer (Waltham, Massachusetts, USA)
was used for analyzing carbon, hydrogen, and nitrogen contents for virgin and
oxy-
cracked petcoke samples using combustion method. The sulfur content was
determined with an Antek 9000 system (Houston, TX, USA) calibrated with
Accustandard SCO-500x (S) standards and running toluene solutions (10 wt.
%/vol.). The metal contents in the virgin and oxy-cracked petcoke samples were
analyzed at ALS Environmental Laboratories (Alberta, Canada) using Inductively
Coupled Plasma Mass Spectroscopy (Dissolved Metals in Water method by CRC
ICPMS).
Results and discussion
Thermo-oxidative decomposition of virgin and oxy-cracked petcoke
[00127] Thermo-oxidative decomposition of virgin and oxy-cracked
petcoke
was performed to illustrate the thermal degradation behavior under air.
Figures 28
and 29 show the rate of mass loss (TG) and the derivative of rate of mass loss
(DTG) profiles under oxidation by air from room temperature to 800 C at a
heating
rate of 10 C/min for the virgin and oxy-cracked petcoke, respectively. It is
evident
from the profiles (Figure 28) that the oxidation of petcoke sample occurs at a
temperature around 540 C which is evidenced by the presence of an exothermic
symmetric peak beyond 540 C as shown in Figure 30. There is an initial
increase
in mass loss for the virgin petcoke sample (Figure 28) which may be due to the
adsorption of oxygen. The oxy-cracked sample lost a higher percentage of its
original weight and more quickly than the virgin petcoke sample at the early
oxidation stage, which may be explained by the high content of volatile matter
present in the oxy-cracked sample. As shown in Figure 29, the oxy-cracked
sample
is completely oxidized with maximum rate at 475 C, which is lower than that
of the
virgin petocke. This shows that the oxy-cracked petcoke can be oxidized
earlier
than the virgin petcoke under similar oxidation conditions. This is also
evident in the
49
CA 3012804 2018-07-27
heat flow profiles of the two samples shown in Figure 30, from which it is
evident
that the oxidation of the oxy-cracked sample occurs earlier than the virgin
petcoke.
This shift to lower oxidation temperatures in oxy-cracked petcoke may be
related to
the presence of low molecular weight compounds, present as volatile matter
formed
during the oxy-cracking reaction. This is evidence of an enhanced reactivity
of the
oxy-cracked petcoke, whereas the virgin petcoke has a relatively low
reactivity.
[00128] Figure 31 shows the plot of conversion degree (a) against
temperature for non-isothermal oxidation at three heating rates (5, 10, and 20
C/min). The degree of conversion (a) is the fraction of reactant decomposed at
a
specific temperature and is defined in terms of the mass change or the mass of
volatile generated. The conversion percent ratio or the extent of reaction of
petcoke
and oxy-cracked samples was estimated by Eq. (15):
o int
a = m- (15)
mo- moo
where mo is the initial sample mass, mt is the sample mass at any time and m.
is
the final sample mass.
[00129] Figure 31 illustrate that as the heating rate decreased, the
thermo-
oxidative decomposition is shifted gradually to the lower temperature for both
samples. Surprisingly, the decomposition temperature of the oxy-cracked sample
is
much lower than virgin petcoke at any heating rate. At low heating rate (5
C/min),
for example, to obtain a 50% conversion of virgin petcoke a temperature of 498
C
is required while a temperature of 445 C is needed for oxy-cracked one to
obtain
the same conversion. This significant decrease in reaction temperature again
shows that the oxy-cracked sample is more easily oxidized as compared to the
virgin petcoke. At a temperature lower than 430 C, about 30% conversion is
obtained for the oxy-cracked sample whereas no conversion is observed in
petcoke
at that temperature. This high conversion in the oxy-cracked sample at that
temperature may for example be attributed to vaporization of volatile matter
that
was formed during the oxy-cracking reaction, in select embodiments.
[00130] The slope of the oxy-cracked sample changes during the first
half of
.. the reaction process, as shown in Figure 31b, indicating that multiple
reaction
mechanisms are taken place during the oxidation reaction. This is in contrast
to the
CA 3012804 2018-07-27
slope profile of the virgin petcoke which shows that the oxidation is
happening by
one mechanism.
[00131] The ignition (TiG), peak (Tm) and burnout (TB) temperatures
for a fuel
are important parameters related to combustion performance. The ignition
temperature, the temperature at which a sudden decrease in mass loss on the
DTG
curves, indicates how easily the fuel is ignited. The peak temperature and its
corresponding rate of mass loss are determined at the maximum rate of mass
loss.
These parameters (i.e., Tm and its mass loss) indicate the combustibility and
reactivity of the fuel, where fuel with low value of Tm temperature can easily
ignite
and react. Burnout temperature, on the other hand, is defined as the
temperature at
which the mass of the sample remains constant without any change during the
combustion process. Table 10 shows the determined values of these key
combustion parameters extracted from Figures 29 and 30 for both virgin and oxy-
cracked samples, respectively.
Table 10: Thermal properties of the virgin and oxy-cracked petcoke
Ignition Temp., Peak Temp., Burnout Temp.,
(TIG), C (T,77), C (TB), C
Virgin (green) 480 535 590
petcoke
Oxy-cracked 420 475 508
petcoke
[00132] The exemplified combustion parameters were shown to be low in
the
case of oxy-cracked sample. In particular, the initial degradation temperature
(ignition temperature) of the oxy-cracked sample is significantly reduced by
13% as
compared to the virgin petcoke. This is putatively due to the high content of
the
volatile matters in the oxy-cracked sample. The higher ignition temperature of
petcoke sample can putatively be attributed to the higher nitrogen content,
which
retards ignition of volatiles and reactions at the material surface. The
reactivity of a
fuel is usually evaluated by the peak temperature; the higher the temperature
indicating the lower the reactivity. Interestingly, the peak temperature was
found to
51
CA 3012804 2018-07-27
be low for the oxy-cracked sample, thus exhibiting the presence of more
reactive
compounds. A significant difference was observed in burnout temperature
between
virgin petcoke and oxy-cracked, one reducing the burnout times of the fuel.
Based on these results, it is evident that the oxy-cracked products are more
reactive, efficient, less pollutant and more easily oxidized than the virgin
petcoke.
This can be ascribed to the high content of volatile matter (VM) formed in the
oxy-
cracked sample.
Heating values of virgin and oxy-cracked petcoke
[00133] Heating values (HHV) were experimentally determined based on the
amount of volatile matter (VM), moisture (M), ash (A) and fixed carbon (FC)
contents in oxy-cracked and virgin petcoke samples extracted from Figure 32.
Figure 32 shows the profiles for the % mass loss with the increase in the
temperature for virgin petcoke as well as for oxy-cracked petcoke obtained up
to
750 C. As shown in the Figure, during the heating of the samples, the first
stage in
mass loss corresponds to the drying step (-200 C) where the moisture is
evaporated from the samples. The second region is the devolatilization stage
(200-
500 C) where the volatiles are removed. It is worth noting that the first two
stages
are obtained under pyrolysis process where the (M) and (VM) contents are
determined. Moreover, the combustion stage is taking place between 500-630 C,
where the loss of heavier hydrocarbons (total carbon) occurs. The final stage
relates to the residual combustion stage (ash, >630 C) where the combustion
process has nearly ended. The combustion and residual combustion stages were
obtained under oxidation with air where the total fixed carbon (FC) and ash
(A)
content are estimated.
[00134] Typical proximate and ultimate analysis of petcoke and oxy-
cracked
samples are summarized in Table 11.
Table 11: Proximate and ultimate analysis of samples
Proximate analysis (wt%) Virgin (green) petcoke Oxy-cracked petcoke
Volatile material (VM) 1.99 20.79
Moisture (M) 0.007 5.38
52
CA 3012804 2018-07-27
Ash (A) 2.68 3.60
Fixed Carbon (FC) 95.32 70.23
VM/FC ratio 0.021 0.29
Ultimate analysis (wt%)
84.40 62.31
3.81 2.68
1.55 1.10
4.46 1.32
[00135] The VM content of the oxy-cracked sample is significantly
higher than
the virgin petcoke sample. The ash content (A) was found to be less than 4% in
oxy-cracked sample which was found to contain mainly potassium (K) left over
after
reaction while the ash content in the virgin petcoke contained metals such as
iron,
nickel, vanadium and cobalt. The high ratio of VM/FC observed in the oxy-
cracked
sample indicates high availability of energy in the fuel. As for the ultimate
analysis,
the oxy-cracked sample compared to virgin petcoke has a lower than average
carbon, sulfur, hydrogen and nitrogen content and a higher content of oxygen.
[00136] The HHV for the samples was estimated using proximate
correlations
(Equations 16-20) and presented in Table 12.
Schuster et al., op cit:
HHV = 4.183 x 10-3 x (800 + Vm x (70 ¨ 1.65 x Vm)) (16);
Kligukbayrak et al., op. cit.:
HHV = 76.56¨ 1.3(Vm + A) + 7.03 x 10-3(Vm +A)2 (17);
Cordero et al., op. cit.:
HHV = 354.3Fc + 170.8Vm (18);
Parikh et al., op. cit.:
HHV = 0.3536Fc + 0.1559Vm ¨ 0.0078A (19);
Majumder et al., op. cit.:
HHV = 0.35Fc + 0.33 Vm ¨ 0.11M ¨ 0.03A (20).
Table 12: The heating values (HHV) for virgin and oxy-cracked petcoke samples.
53
CA 3012804 2018-07-27
Correlation Heating value (MJ/kg)
Virgin (green) petcoke Oxy-cracked petcoke
Equation 16 34.02 36.57
Equation 17 32.80 27.88
Equation 18 34.11 28.43
Equation 19 34.00 28.05
Equation 20 33.94 30.74
[00137] The estimated HHV of the virgin petcoke sample is in range of
(30-
37MJ/kg) using any of these correlations which are in good agreement with the
reported values of petcoke. The HHV values of oxy-cracked products are in the
range of (28-31 MJ/kg; ignoring a very high value by Schuster equation (16)
which
represents an emphasis on the value of VM). The reduction in HHV of oxy-
cracked
petcoke reflects a relatively high oxygen content, in the form of carboxyl and
phenolic compounds. A higher FC content and lower VM content were observed in
the virgin petcoke sample as compared to the oxy-cracked sample. Even though
the HHV of petcoke is higher than the oxy-cracked sample, the nitrogen and
sulfur
content of the oxy-cracked sample is much lower. This represents the potential
for
a fuel relatively low in NOX and SOX emissions. Surprisingly, the HHVs of the
oxy-
cracked products were found to be higher than that for ranked-coals (9.50 ¨ 27
MJ/kg). These results reflect the potential for the oxy-cracked products to be
used
as fuel, for example for power generation, for example by co-firing, pyrolysis
or
gasification.
54
CA 3012804 2018-07-27