Note: Descriptions are shown in the official language in which they were submitted.
TOPICAL COMPOSITION AND DELIVERY SYSTEM AND ITS USE
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of priority to U.S. Patent
Application
No.: 15/014,988 filed on February 3, 2016.
FIELD OF THE INVENTION
[0002] The described invention relates to topical formulations of a
pharmaceutical compositions containing an active therapeutic agent or
metabolite,
and a delivery system for administering the same topically, characterized in
that the
active(s) remain in the skin and penetration of the active(s) into the
bloodstream is
limited so as to reduce systemic side effects.
BACKGROUND OF THE INVENTION
1. Anatomy and Physiology of the Skin
[0003] The skin is the largest organ in the body consisting of several
layers and
plays an important role in biologic homeostasis, and is comprised of the
epidermis
and the dermis. The epidermis, which is composed of several layers beginning
with
the stratum corneum, is the outermost layer of the skin, and the innermost
skin layer
is the deep dermis. The skin has multiple functions, including thermal
regulation,
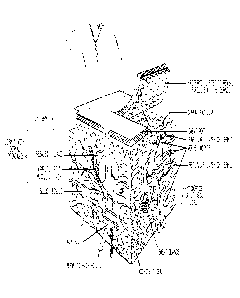
metabolic function (vitamin D metabolism), and immune functions. FIG. 1
presents a
diagram of skin anatomy.
[0004] In humans, the usual thickness of the skin is from 1-2 mm,
although
there is considerable variation in different parts of the body. The relative
proportions
of the epidermis and dermis also vary, and a thick skin is found in regions
where
there is a thickening of either or both layers. For example, on the
interscapular
(between the shoulder blades) region of the back, where the dermis is
particularly
thick, the skin may be more than 5 mm thick, whereas on the eyelids it may be
less
than 0.5 mm. Generally, the skin is thicker on the dorsal or extensor surfaces
of the
body than on the ventral or flexor surfaces; however, this is not the case for
the
hands and feet. The skin of the palms and soles is thicker than on any dorsal
1
Date Recue/Date Received 2022-04-07
surface except the intrascapular region. The palms and soles have a
characteristically thickened epidermis, in addition to a thick dermis.
[0005] The entire skin surface is traversed by numerous fine furrows,
which run
in definite directions and cross each other to bound small rhomboid or
rectangular
fields. These furrows correspond to similar ones on the surface of the dermis
so
that, in section, the boundary line between epidermis and dermis appears wavy.
On
the thick skin of the palms and soles, the fields form long, narrow ridges
separated
by parallel coursing furrows, and in the fingertips these ridges are arranged
in the
complicated loops, whorls (verticil) and spirals that give the fingerprints
characteristic
for each individual. These ridges are more prominent in those regions where
the
epidermis is thickest.
[0006] Where there is an epidermal ridge externally there is a
corresponding
narrower projection, called a "rete peg," on the dermal surface. Dermal
papillae on
either side of each rete peg project irregularly into the epidermis. In the
palms and
soles, and other sensitive parts of the skin, the dermal papillae are
numerous, tall
and often branched, and vary in height (from 0.05 mm to 0.2 mm). Where
mechanical demands are slight and the epidermis is thinner, such as on the
abdomen and face, the papillae are low and fewer in number.
Epidermis
[0007] The epidermis provides the body's buffer zone against the
environment.
It provides protection from trauma, excludes toxins and microbial organisms,
and
provides a semi-permeable membrane, keeping vital body fluids within the
protective
envelope. Traditionally, the epidermis has been divided into several layers,
of which
two represent the most significant ones physiologically. The basal-cell layer,
or
germinative layer, is of importance because it is the primary source of
regenerative
cells. In the process of wound healing, this is the area that undergoes
mitosis in
most instances. The upper epidermis, including stratum and granular layer, is
the
other area of formation of the normal epidermal-barrier function.
Stratum Corneum and the Acid Mantle
2
Date Recue/Date Received 2022-04-07
[0008] Stratum corneum is an avascular, multilayer structure that
functions as
a barrier to the environment and prevents transepidermal water loss. Recent
studies
have shown that enzymatic activity is involved in the formation of an acid
mantle in
the stratum corneum. Together, the acid mantle and stratum corneum make the
skin
less permeable to water and other polar compounds, and indirectly protect the
skin
from invasion by microorganisms. Normal surface skin pH is between 4 and 6.5
in
healthy people; it varies according to area of skin on the body. This low pH
forms an
acid mantle that enhances the skin barrier function.
Other layers of the Epidermis
[0009] Other layers of the epidermis below the stratum corneum include
the
stratum lucidum, stratum granulosum, stratum germinativum, and stratum basale.
Each contains living cells with specialized functions (FIG. 2). For example
melanin,
which is produced by melanocytes in the epidermis, is responsible for the
color of the
skin. Langerhans cells are involved in immune processing.
Dermal Appendages
[0010] Dermal appendages, which include hair follicles, sebaceous and
sweat
glands, fingernails, and toenails, originate in the epidermis and protrude
into the
dermis hair follicles and sebaceous and sweat glands contribute epithelial
cells for
rapid reepithelialization of wounds that do not penetrate through the dermis
(termed
partial-thickness wounds). The sebaceous glands are responsible for secretions
that
lubricate the skin, keeping it soft and flexible. They are most numerous in
the face
and sparse in the palm of the hands and soles of the feet. Sweat gland
secretions
control skin pH to prevent dermal infections. The sweat glands, dermal blood
vessels, and small muscles in the skin (responsible for goose pimples) control
temperature on the surface of the body. Nerve endings in the skin include
receptors
for pain, touch, heat, and cold. Loss of these nerve endings increases the
risk for
skin breakdown by decreasing the tolerance of the tissue to external forces.
[0011] The basement membrane both separates and connects the epidermis
and dermis. When epidermal cells in the basement membrane divide, one cell
remains, and the other migrates through the granular layer to the surface
stratum
corneum. At the surface, the cell dies and forms keratin. Dry keratin on the
surface
3
Date Recue/Date Received 2022-04-07
is called scale. Hyperkeratosis (thickened layers of keratin) is found often
on the
heels and indicates loss of sebaceous gland and sweat gland functions if the
patient
is diabetic. The basement membrane atrophies with aging; separation between
the
basement membrane and dermis is one cause for skin tears in the elderly.
Dermis
[0012] The dermis, or the true skin, is a vascular structure that
supports and
nourishes the epidermis. In addition, there are sensory nerve endings in the
dermis
that transmit signals regarding pain, pressure, heat, and cold. The dermis is
divided
into two layers: the superficial dermis and the deep dermis.
[0013] The superficial dermis consists of extracellular matrix
(collagen, elastin,
and ground substances) and contains blood vessels, lymphatics, epithelial
cells,
connective tissue, muscle, fat, and nerve tissue. The vascular supply of the
dermis
is responsible for nourishing the epidermis and regulating body temperature.
Fibroblasts are responsible for producing the collagen and elastin components
of the
skin that give it turgor. Fibronectin and hyaluronic acid are secreted by the
fibroblasts. The structural integrity of the dermis plays a role in the normal
function
and youthful appearance of the skin.
[0014] The deep dermis is located over the subcutaneous fat; it contains
larger
networks of blood vessels and collagen fibers to provide tensile strength. It
also
consists of fibroelastic connective tissue, which is yellow and composed
mainly of
collagen. Fibroblasts are also present in this tissue layer. The well-
vascularized
dermis withstands pressure for longer periods of time than subcutaneous tissue
or
muscle. The collagen in the skin gives the skin its toughness. Dermal wounds,
e.g.,
cracks or pustules, involve the epidermis, basal membrane, and dermis.
Typically,
dermal injuries heal rapidly.
2. Effects of application to the skin
4
Date Recue/Date Received 2022-04-07
[0015] Substances are applied to the skin to elicit one or more of four
general
effects: an effect on the skin surface, an effect within the stratum corneum;
an effect
requiring penetration into the epidermis and dermis; or a systemic effect
resulting
from delivery of sufficient amounts of a given substance through the epidermis
and
the dermis to the vasculature to produce therapeutic systemic concentrations.
One
example of an effect on the skin surface is formation of a film. Film
formation may
be protective (e.g., sunscreen) and/or occlusive (e.g., to provide a
moisturizing effect
by diminishing loss of moisture from the skin surface). One example of an
effect
within the stratum corneum is skin moisturization; which may involve the
hydration of
dry outer cells by surface films or the intercalation of water in the lipid-
rich
intercellular laminae; the stratum corneum also may serve as a reservoir phase
or
depot wherein topically applied substances accumulate due to partitioning
into, or
binding with, skin components.
[0016] It generally is recognized that short-term penetration occurs
through the
hair follicles and the sebaceous apparatus of the skin, while long term
penetration
occurs across cells. Penetration of a substance into the viable epidermis and
dermis
may be difficult to achieve, but once it has occurred, the continued diffusion
of the
substance into the dermis is likely to result in its transfer into the
microcirculation of
the dermis and then into the general circulation. It is possible, however, to
formulate
delivery systems that provide substantial localized delivery.
[0017] Percutaneous absorption is the absorption of substances from
outside
the skin to positions beneath the skin, including into the blood stream. The
epidermis of human skin is highly relevant to absorption rates. Passage
through the
stratum corneum marks the rate-limiting step for percutaneous absorption. The
major steps involved in percutaneous absorption of, for example, a drug,
include the
establishment of a concentration gradient, which provides a driving force for
drug
movement across the skin, the release of drug from the vehicle into the skin-
partition
coefficient and drug diffusion across the layers of the skin-diffusion
coefficient. The
relationship of these factors to one another is summarized by the following
equation:
[0018] J=Cveh x Km.D/x [Formula 1]
Date Recue/Date Received 2022-04-07
[0019] where J = rate of absorption; Cveh = concentration of drug in
vehicle;
Km = partition coefficient; and D = diffusion coefficient.
[0020] The many factors that affect the rate of percutaneous absorption
of a
substance include, without limitation, the following: (i) Concentration. The
more
concentrated the substance, the greater the absorption rate. (ii) Size of skin
surface
area. The wider the contact area of the skin to which the substance is
applied, the
greater the absorption rate. (iii) Anatomical site of application. Skin varies
in
thickness in different areas of the body. A thicker and more intact stratum
corneum
decreases the rate of absorbency of a substance. The stratum corneum of the
facial
area is much thinner than, for example, the skin of the palms of the hands.
The
facial skin's construction and the thinness of the stratum corneum provide an
area of
the body that is optimized for percutaneous absorption to allow delivery of
active
agents both locally and systemically through the body. (iv) Hydration.
Hydration
(meaning increasing the water content of the skin) causes the stratum corneum
to
swell which increases permeability. (v) Skin temperature. Increased skin
temperature increases permeability. (vi) Composition. The composition of the
compound and of the vehicle also determines the absorbency of a substance.
[0021] Most substances applied topically are incorporated into bases or
vehicles. The vehicle chosen for a topical application will greatly influence
absorption, and may itself have a beneficial effect on the skin. Factors that
determine the choice of vehicle and the transfer rate across the skin are the
substance's partition coefficient, molecular weight and water solubility. The
protein
portion of the stratum corneum is most permeable to water soluble substances
and
the lipid portion of the stratum corneum is most permeable to lipid soluble
substances. It follows that substances having both lipid and aqueous
solubility may
traverse the stratum corneum more readily. (See Dermal Exposure Assessment:
Principles and Applications, EPA/600/8-91/011b, January 1992, Interim Report -
Exposure Assessment Group, Office of Health and Environmental Assessment, U.S.
Environmental Protection Agency, Washington, D.C. 20460).
6
Date Recue/Date Received 2022-04-07
3. Wound healing
[0022] The term "wound healing" refers to the process by which the body
repairs trauma to any of its tissues, especially those caused by physical
means and
with interruption of continuity. The term "wound healing agent" refers to any
substance that facilitates the wound healing process.
[0023] A wound-healing response often is described as having three
distinct
phases-injury, inflammation and repair. Generally speaking, the body responds
to
injury with an inflammatory response, which is crucial to maintaining the
health and
integrity of an organism. If however it goes awry, it can result in tissue
destruction.
Phase I: Injury
[0024] Injury caused by factors including, but not limited to,
autoimmune or
allergic reactions, environmental particulates, infection or mechanical damage
often
results in the disruption of normal tissue architecture, initiating a healing
response.
Damaged epithelial and endothelial cells must be replaced to maintain barrier
function and integrity and prevent blood loss, respectively. Acute damage to
endothelial cells leads to the release of inflammatory mediators and
initiation of an
anti-fibrinolytic coagulation cascade, temporarily plugging the damaged vessel
with a
platelet and fibrin-rich clot.
[0025] Platelet recruitment, degranulation and clot formation rapidly
progress
into a phase of vasoconstriction with increased permeability, allowing the
extravasation (movement of white blood cells from the capillaries to the
tissues
surrounding them) and direct recruitment of leukocytes to the injured site.
The
basement membrane, which forms the extracellular matrix underlying the
epithelium
and endothelium of parenchymal tissue, precludes direct access to the damaged
tissue. To disrupt this physical barrier, zinc-dependent endopeptidases, also
called
matrix metalloproteinases (MM Ps), cleave one or more extracelluar matrix
constituents allowing extravasation of cells into, and out of, damaged sites.
Specifically, MMP-2 (gelatinase A, Type N collagenase) and MMP-9 (gelatinase
B,
Type IV collagenase) cleave type N collagens and gelatin, two important
constituents
of the basement membrane. Recent studies have found that MMP-2 and MMP-9 are
upregulated, highlighting that tissue-destructive and regenerative processes
are
7
Date Recue/Date Received 2022-04-07
common in fibrotic conditions. The activities of MMPs are controlled by
several
mechanisms including transcriptional regulation, proenzyme regulation, and
specific
tissue inhibitors of MMPs. The balance between MMPs and the various inhibitory
mechanisms can regulate inflammation and determine the net amount of collagen
deposited during the healing response.
Phase II: Inflammation
[0026] Once access to the site of tissue damage has been achieved,
chemokine gradients recruit inflammatory cells. Neutrophils, eosinophils,
lymphocytes, and macrophages are observed at sites of acute injury with cell
debris
and areas of necrosis cleared by phagocytes.
[0027] The early recruitment of eosinophils, neutrophils, lymphocytes,
and
macrophages providing inflammatory cytokines and chemokines can contribute to
local TG F13 and IL-13 accumulation. Following the initial insult and wave of
inflammatory cells, a late-stage recruitment of inflammatory cells may assist
in
phagocytosis, in clearing cell debris, and in controlling excessive cellular
proliferation, which together may contribute to normal healing. Late-stage
inflammation may serve an anti-fibrotic role and may be required for
successful
resolution of wound-healing responses. For example, a late-phase inflammatory
profile rich in phagocytic macrophages, assisting in fibroblast clearance, in
addition
to IL-10-secreting regulatory T cells, suppressing local chemokine production
and
TGF-13, may prevent excessive fibroblast activation.
[0028] The nature of the insult or causative agent often dictates the
character
of the ensuing inflammatory response. For example, exogenous stimuli like
pathogen-associated molecular patterns (PAMPs) are recognized by pathogen
recognition receptors, such as toll-like receptors and NOD-like receptors
(cytoplasmic proteins that have a variety of functions in regulation of
inflammatory
and apoptotic responses), and influence the response of innate cells to
invading
pathogens. Endogenous danger signals also can influence local innate cells and
orchestrate the inflammatory cascade.
[0029] The nature of the inflammatory response dramatically influences
resident tissue cells and the ensuing inflammatory cells. Inflammatory cells
8
Date Recue/Date Received 2022-04-07
themselves also propagate further inflammation through the secretion of
chemokines, cytokines, and growth factors. Many cytokines are involved
throughout
a wound-healing and fibrotic response, with specific groups of genes activated
in
various conditions.
Phase III: Tissue Repair and Contraction
[0030] The closing phase of wound healing consists of an orchestrated
cellular
re-organization guided by a fibrin (a fibrous protein that is polymerized to
form a
"mesh" that forms a clot over a wound site)-rich scaffold formation, wound
contraction, closure and re-epithelialization. The vast majority of studies
elucidating
the processes involved in this phase of wound repair have come from dermal
wound
studies and in vitro systems.
[0031] Myofibroblast-derived collagens and smooth muscle actin (a-SMA)
form
a provisional extracellular matrix, with macrophage, platelet, and fibroblast-
derived
fibronectin forming a fibrin scaffold. Collectively, these structures are
commonly
referred to as granulation tissues.
[0032] In addition to fibronectin, the provisional extracellular matrix
consists of
glycoproteins (such as PDGF), glycosaminoglycans (such as hyaluronic acid),
proteoglycans and elastin. Growth factor and TGF-P-activated fibroblasts
migrate
along the extracellular matrix network and repair the wound. Within skin
wounds,
TGF-13 also induces a contractile response, regulating the orientation of
collagen
fibers. Fibroblast to myofibroblast differentiation, as discussed above, also
creates
stress fibers and the neo-expression of a-SMA, both of which confer the high
contractile activity within myofibroblasts. The attachment of myofibroblasts
to the
extracellular matrix at specialized sites called the "fibronexus" or "super
mature focal
adhesions" pull the wound together, reducing the size of the lesion during the
contraction phase. The extent of extracellular matrix laid down and the
quantity of
activated myofibroblasts determines the amount of collagen deposition. To this
end,
the balance of matrix metalloproteinases (MMPs) to tissue inhibitor of
metalloproteinases (TIM Ps) and collagens to collagenases vary throughout the
response, shifting from pro-synthesis and increased collagen deposition
towards a
controlled balance, with no net increase in collagen. For successful wound
healing,
9
Date Recue/Date Received 2022-04-07
this balance often occurs when fibroblasts undergo apoptosis, inflammation
begins
to subside, and granulation tissue recedes, leaving a collagen-rich lesion.
From skin
studies, re-epithelialization of the wound site re-establishes the barrier
function and
allows encapsulated cellular re-organization. Several in vitro and in vivo
models,
using human or rat epithelial cells grown over a collagen matrix, or tracheal
wounds
in vivo, have been used to identify significant stages of cell migration,
proliferation,
and cell spreading. Rapid and dynamic motility and proliferation, with
epithelial
restitution from the edges of the denuded area occur within hours of the
initial
wound. In addition, sliding sheets of epithelial cells can migrate over the
injured area
assisting wound coverage. Several factors have been shown to regulate re-
epithelialization, including serum-derived transforming growth factor alpha
(TGF-a),
and matrix metalloproteinase-7 (MMP-7) (which itself is regulated by TIMP-1).
4. Delivery Systems for Topical Administration
[0033] Many active agents are administrated enterally or parenterally.
Enteral
routes of administration involve administration to any part of the
gastrointestinal tract,
typically via oral forms, e.g., pills, tablets, emulsions, and syrups, or via
rectal forms,
e.g., enemas, Murphy drips, and suppositories. Parenteral routes of
administration
involve administration by some means other than oral or rectal, typically via
injection.
While such administration routes allow for accurate and consistent dosing,
such
routes necessarily yield systemic effects, e.g., vestibular symptoms, headache
and
general malaise, and gastrointestinal symptoms, which in certain circumstances
are
not desirable.
[0034] Topical routes of administration involve administration to a body
surface, such as the skin, or mucous membranes. Many forms of topical
administration involve applying a therapeutic agent directly to the skin;
inhalable
mediations, eye drops, and ear-drops also are considered topical
administration
forms. Although topical administration generally provides a local effect, many
topically administered drugs likewise can exhibit systemic effects, such as
vestibular
symptoms (e.g., vertigo, dizziness or blurred vision), headache and general
malaise,
gastro-intestinal symptoms, such as diarrhea, nausea, gas, cramps, dry nose
and
dry mouth.
Date Recue/Date Received 2022-04-07
[0035] Formulations for topical application can take the compositional
form of a
liquid, a semisolid dosage form (e.g., a paste, a cream, a lotion, a powder,
an
ointment or a gel) or a patch.
[0036] Liquid formulations do not readily stay in place and can be
messy.
Semisolid formulations offer some advantages characteristic of topical
administration, such as ease of application, and increased local doses of
active
agent, with reduced systemic effects, but their potential disadvantages
include the
need for repeated application, difficulties in accurate dosing, and messy or
unattractive cosmetic attributes, all of which can lead to poor user
compliance, and
unintentional removal or transfer of active agent via contact with objects or
other
persons.
[0037] Topical patches, which are available in multiple forms including
single
and multi-layer drug-in-adhesive forms, matrix forms, and reservoir forms,
address
several of the shortcomings of semisolid formulations, for example, reducing
the
need for repeated application, providing accurate, and controlled release of
active
agent, and reducing the likelihood of unintentional removal or transfer of
drug or
active agent via contact with objects or other persons, but have a finite size
and
shape. Because topical patches have a finite size and shape, the application
area is
determined by the dimensions of the patch rather than the dimensions of the
affected
site. Accordingly, it may be necessary to use a number of patches in order to
cover
a large affected site. Furthermore, topical patches typically lack sufficient
flexibility to
be effectively administered to joints or other areas of skin subject to
significant
stretching movements. Topical patches can also lead to user discomfort,
particular
in warmer climates, and can be aesthetically unpleasing, which can also lead
to poor
user compliance.
[0038] A number of attempts have been made for delivering therapeutic
formulations topically. One common problem inherent to topical formulations
that
has been experienced thus far has been to control the therapeutic active
agent, as
well as the other composition components, such that they are specifically
confined in
the area of the skin in which they have been directly applied. This is turn
may result
in too fast release of the drug with the consequence of causing undesirable
spikes
11
Date Recue/Date Received 2022-04-07
and high levels of the drug in the bloodstream, thereby creating deleterious
effects
such as e.g. unwanted side-effects, wash-out or metabolism of the drug.
[0039] The described invention addresses and overcomes these
shortcomings.
The described composition and method provides a safe and effective topical
therapeutic drug delivery platform that can deliver drugs locally into the
skin. The
described invention is effective to deliver the components of the
pharmaceutical
formulation into the skin, to keep them in the skin, and to reduce the
potential of the
active therapeutic agent or its metabolites to enter the bloodstream.
Consequently,
the active therapeutic agent executes its effective biological function
locally at the
tissue of interest once being released from the skin.
SUMMARY OF THE INVENTION
[0040] According to one aspect, the described invention provides a
topical
delivery system comprising a pharmaceutical composition for application
directly to a
skin of a subject in need thereof comprising (a) a therapeutic amount of an
active
therapeutic agent to treat symptoms of a disease, disorder or condition; (b)
chemical
drivers comprising an amino benzoate local anesthetic, ethoxydiglycol and
methylsulfonyl methane, wherein the chemical drivers are effective at acting
synergistically to deliver the therapeutic agent and (c) a depot component
that is
effective to keep the active agent locally in the skin;to reduce distribution
of the
active agent to the blood stream; to encapsulate the pharmaceutical
composition and
to facilitate controlled or delayed type release of the active therapeutic
agent.
According to some embodiments, the active therapeutic agent has a molecular
weight below 500 Da. According to some embodiments, the active therapeutic
agent
is selected from the group consisting of a steroidal or non-steroidal
analgesic agent,
a wound healing agent, an antihistamine and an anti-neoplastic agent.
According to
some embodiments the amino benzoate local anesthetic is selected from the
group
consisting of benzocaine, lidocaine, tetracaine or a combination thereof.
According
to some embodiments, the pharmaceutical composition is in an administration
form
selected from the group consisting of a cream, gel, or a spray. According to
some
embodiments, the topical delivery system further comprises a vasoconstrictor.
According to some embodiments, the vasoconstrictor is nonirritating when
applied to
skin. According to some embodiments, the depot component is a liposome.
12
Date Recue/Date Received 2022-04-07
According to some embodiments, the liposome comprises a phosphatidyl choline,
cholesterol, and a pharmaceutically acceptable salt of an active therapeutic
agent
and at least one anionic or cationic phospholipid. According to some
embodiments,
the depot component comprises a polymer. According to some embodiments, the
depot component comprises a liposome and a polymer. According to some
embodiments, the depot component comprises a polymersome.
[0041] According to another aspect, the described invention provides a
method of delivering a pharmaceutical composition topically that is effective
to
reduce systemic side effects of the active agent comprising (a) applying a
pharmaceutical composition to a skin of a subject in need thereof, wherein the
pharmaceutical composition comprises: (i) a chemical driver effective to
penetrate
the stratum corneum of skin containing an active therapeutic agent, wherein
the
active therapeutic agent is an amino benzoate local anesthetic; ethoxydiglycol
and
methylsulfonyl methane (MSM); and (ii) a depot component that is effective to
keep
the pharmaceutical composition in the skin and to minimize distribution
systemically.
According to some embodiments, the active therapeutic agent has a molecular
weight below 500 Da. According to some embodiments, the active therapeutic
agent
does not get into the bloodstream. According to some embodiments, the depot
component of the composition is effective to facilitate controlled or delayed
type
release of the active therapeutic agent. According to some embodiments, the
depot
component is a polymer. According to other embodiments, the depot component is
a liposome. According to some embodiments, the liposome comprises a
phosphatidyl choline, cholesterol and a pharmaceutically acceptable salt of an
active
therapeutic agent and at least one anionic or cationic phospholipid. According
to
some embodiments, the depot component comprises a liposome and a polymer.
According to other embodiments, the depot component is a polymersome.
BRIEF DESCRIPTION OF THE DRAWINGS
[0042] FIG. 1 presents a diagram of skin anatomy. Taken from Stedman's
Medical Dictionary, 27th Ed., Lippincott, Williams & Wilkins, Baltimore, MD
(2000), at
1647.
[0043] FIG. 2 depicts layers of the epidermis.
13
Date Recue/Date Received 2022-04-07
[0044] FIG. 3 shows the study schema for the Phase I Clinical Study for
determining the uptake kinetics of NeuroMed 7Tmpain relief cream composed of
lidocaine HCI 4% w/w in base formula with dosing at 0 and 4 hours, and self-
reported visual analog scale (VAS); and venous blood draws at 0 (baseline), 1,
3, 5,
7, 9 and 11 hours.
[0045] FIG. 4 shows the percentage of pain reduction for the study
subjects.
[0046] FIG. 5 shows the average pain reduction for the study subjects.
[0047] FIG. 6 shows the maximal pain reduction for the study subjects.
[0048] FIG. 7 shows the time of maximum pain relief for the study
subjects.
DETAILED DESCRIPTION OF THE INVENTION
Glossary
[0049] The term "active therapeutic agent" as used herein refers to a
drug,
molecule, nucleic acid, protein, composition or other substance that provides
a
therapeutic effect. The term "active" as used herein refers to the ingredient,
component or constituent of the compositions of the described invention
responsible
for the intended therapeutic effect. The terms "therapeutic agent" and "active
agent"
are used interchangeably.
[0050] The term "administer" as used herein means to give or to apply.
The
term "administering" as used herein includes in vivo administration, as well
as
administration directly to tissue ex vivo.
[0051] The term "alginate" as used herein is an anionic biopolymers
produced
by a variety of microorganisms and marine algae. Alginate is a polysaccharide
that
comprises I3-D-mannuronic acid units and a-L-guluronic acid units. Some
alginate
polymers are block copolymers with blocks of the guluronic acid (or salt)
units
alternating with blocks of the mannuronic acid (or salt) units. Some alginate
molecules have single units of guluronic acid (or salt) alternating with
single units of
mannuronic acid (or salt). The ratio and distribution of the mannuronic and
guluronic
unit, along with the average molecular weight, affect the physical and
chemical
14
Date Recue/Date Received 2022-04-07
properties of the copolymer. See Haug, A. et al., Acta Chem. Scand., 183-90
(1966). Alginate polymers have viscoelastic rheological properties and other
properties that make it suitable for some medical applications. See Klock, G.
et al.,
"Biocompatibility of mannuronic acid-rich alginates," Biomaterials, Vol. 18,
No. 10,
707-13 (1997).
[0052] The term "analgesic" as used herein refers to any member of a
group of
drugs used to provide relief from pain. "Analgesic agents" act in various ways
on the
peripheral and central nervous systems, and are distinct from "anesthetic
agents..
[0053] The term "analog" as used herein refers to a compound having a
structure similar to another, but differing from it, for example, in one or
more atoms,
functional groups, or substructure.
[0054] The term "anesthetic agent" as used herein refers to an agent
that
reversibly produces a reduction or loss of sensation.
[0055] The term "anionic lipid" as used herein refers to a lipid which
has a
negative charge. Exemplary anionic lipids include, without limitation,
diacylglycerolhemisuccinates, e.g. DOGS, DMGS, POGS, DPGS, DSGS;
diacylglycerolhemimalonates , e.g. DOGM or DMGM; diacylglycerolhemiglutarates
,
e.g. DOGG, DMGG; diacylglycerolhemiadipates, e.g. DOGA, DMGA;
diacylglycerolhemicyclohexane-1, 4-dicarboxylic acids, e.g. DO-cHA, DM-cHA;
(2, 3-
Diacyl-propyl) amino}-oxoalkanoic acids e.g. DOAS, DOAM, DOAG, DOAA, DMAS,
DMAM, DMAG, DMAA; Diacyl-alkanoic acids, e.g. DOP, DOB, DOS, DOM, DOG,
DOA, DMP, DOB, DMS, DMM, DMG, DMA; Chemicals and derivatives thereof, e.g.
Chol-C2, Chol-C3, Chol-05, Chol-C6, Chol-C7 or Chol-C8; Chol-CI, CholC3N or
Cholesterolhemidicarboxylic acids and Cholesteryloxycarbonylaminocarboxylic
acids, e.g. Chol-C12 or CholC13N, fatty acids, e.g. Oleic acid, Myristic Acid,
Palmitic
acid, Stearic acid, Nervonic Acid, Behenic Acid; DOPA, DMPA, DPPA, POPA,
DSPA, Choi-504, DOPG, DMPG, DPPG, POPG, DSPG or DOPS, DMPS, DPPS,
POPS, DSPS or Cetyl-phosphate.
[0056] The aforementioned lipids may be formed with or without
cholesterol, or
with a derivative of cholesterol (e.g., cholesterol sulfate).
Date Recue/Date Received 2022-04-07
[0057] The terms "anti-neoplastic agent", "anticancer agent" or
"chemotherapeutic agent" are used interchangeably to refer to an agent that
inhibits
growth, proliferation, and spread of a neoplasm. Non-limiting examples of anti-
neoplastic agents include 5-fluorouracil, adriamycin, daunorubicin,
cytarabine,
vincristine, actinomycin D, mitomycin, bleomycin, acrarubicin, and
combinations
thereof. According to some embodiments, the anti-neoplastic agent can be
entrapped in a lysosome. For the antineoplastic agent to be entrapped in the
liposomes according to the invention, any such agent can be selected, provided
the
agent does not inhibit liposome formation.
[0058] The term "antihistamine agent" as used herein refers to any of
various
compounds that counteract histamine in the body and that are used for treating
allergic reactions (such as hay fever) and cold symptoms. Non-limiting
examples of
antihistamines usable in context of the described invention include
chlorpheniramine,
brompheniramine, dexchlorpheniramine, tripolidine, clemastine,
diphenhydramine,
promethazine, piperazines, piperidines, astemizole, loratadine and
terfenadine.
[0059] As used herein the term "anti-inflammatory agent" refers to a
therapeutic agent that counteracts and inhibits the process of inflammation
and
swelling. The term "non-steroidal anti-inflammatory agent" as used herein
refers to a
large group of agents that are aspirin-like in their action, including, but
not limited to,
ibuprofen (Advil ), naproxen sodium (Aleve0), and acetaminophen (Tylenol ).
Additional examples of non-steroidal anti-inflammatory agents that are usable
in the
context of the described invention include, without limitation, oxicams, such
as
piroxicam, isoxicam, tenoxicam, sudoxicam, and CP-14,304; disalcid,
benorylate,
trilisate, safapryn, solprin, diflunisal, and fendosal; acetic acid
derivatives, such as
diclofenac, fenclofenac, indomethacin, sulindac, tolmetin, isoxepac,
furofenac,
tiopinac, zidometacin, acematacin, fentiazac, zomepirac, clindanac, oxepinac,
felbinac, and ketorolac; fenamates, such as mefenamic, meclofenamic,
flufenamic,
niflumic, and tolfenamic acids; propionic acid derivatives, such as
benoxaprofen,
flurbiprofen, ketoprofen, fenoprofen, fenbufen, indopropfen, pirprofen,
carprofen,
oxaprozin, pranoprofen, miroprofen, tioxaprofen, suprofen, alminoprofen, and
tiaprofenic; pyrazoles, such as phenylbutazone, oxyphenbutazone, feprazone,
azapropazone, and trimethazone. Mixtures of these non-steroidal anti-
inflammatory
16
Date Recue/Date Received 2022-04-07
agents also may be employed, as well as the dermatologically acceptable salts
and
esters of these agents. One example is etofenamate, a flufenamic acid
derivative.
[0060] The term "anti-oxidant agent" as used herein refers to a
substance that
inhibits oxidation or reactions promoted by oxygen or peroxides. Non-limiting
examples of anti-oxidants include ascorbic acid (vitamin C) and its salts,
ascorbyl
esters of fatty acids, ascorbic acid derivatives (e.g., magnesium ascorbyl
phosphate,
sodium ascorbyl phosphate, ascorbyl sorbate), tocopherol (vitamin E),
tocopherol
sorbate, tocopherol acetate, other esters of tocopherol, butylated hydroxy
benzoic
acids and their salts, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
(commercially available under the tradename TroloxR), gallic acid and its
alkyl
esters, especially propyl gallate, uric acid and its salts and alkyl esters,
sorbic acid
and its salts, lipoic acid, amines (e.g., N,N-diethylhydroxylamine, amino-
guanidine),
sulfhydryl compounds (e.g., glutathione), dihydroxy fumaric acid and its
salts, glycine
pidolate, arginine pilolate, nordihydroguaiaretic acid, bioflavonoids,
curcumin, lysine,
methionine, proline, superoxide dismutase, silymarin, tea extracts, grape
skin/seed
extracts, melanin, and rosemary extracts.
[0061] The term "anti-static" refers to a compound used to treat
materials or
their surfaces in order to reduce or eliminate buildup of static electricity.
[0062] The term "apply" as used herein refers to placing in contact with
or to
lay or spread on.
[0063] The term "arabinogalactan" as used herein refers to a wood sugar
extracted from the Western Larch tree (also known as larch gum).
Arabinogalactans
are complex, highly branched polymers of arabinose and galactose in the ratio
of
from about 1:3 to about 1:10, i.e., 1:3, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9 or 1:10.
A
commercially available example is Laracare 200 from Lonza, Inc.
[0064] The term "bactericide" as used herein refers to a substance that
kills
bacteria. Bactericides may be disinfectants, antiseptics, antibiotics, etc.
[0065] The term "bio-distribution" as used herein refers to a method of
tracking
where drugs, active therapeutic agents, compounds of interest etc. travel in
the
subject in need thereof.
17
Date Recue/Date Received 2022-04-07
[0066] The term "buffer" refers to an aqueous solution consisting of a
mixture
of a weak acid and its conjugate base, or vice versa. The pH changes very
little
when a small or moderate amount of strong acid or base is added to it, and
thus it is
used to prevent changes in the pH of a solution.
[0067] The term "carrier" as used herein describes a material that does
not
cause significant irritation to an organism and does not abrogate the
biological
activity and properties of the compound of the composition of the described
invention. Carriers must be of sufficiently high purity and of sufficiently
low toxicity to
render them suitable for administration to the mammal being treated. The
carrier
can be inert, or it can possess pharmaceutical benefits. The terms
"excipient",
"carrier", or "vehicle" are used interchangeably to refer to carrier materials
suitable
for formulation and administration of pharmaceutically acceptable compositions
described herein.
The term "cationic lipid" as used herein refers to a lipid which has a
positive charge.
Exemplary cationic lipids include, without limitation, DOTAP, DMTAP, DPTAP,
DSTAP, POTAP, DODAP, PODAP, DMDAP, DPDAP, DSDAP, DODM HEAP or
DORI, PODMHEAP or PORI, DMDMHEAP or DMRI, DPDMHEAP or DPRI,
DSDMHEAP or DSRI, DOMDHEAP, POMDHEAP, DMMDHEAP, DPMDHEAP,
DSMDHEAP, DOMHEAP, POMHEAP, DMMHEAP, DPMHEAP, DSMHEAP,
DODHEAP, PODHEAP, DMDHEAP, DPDHEAP, DSDHEAP, DDAB, DODAC,
DOEPC, DMEPC, DPEPC, DSEPC, POEPC, DORIE, DMRIE, DOMCAP,
DOMGME, DOP5P, DOP6P, DC-Choi, TC-Chol, DAC-Chol, Chol-Betaine , N-methyl-
PipChol , CTAB, DOTMA, MoChol, HisChol, Chim, MoC3Chol, Choi-C3N-Mo3 ,
Chol-C3N-Mo2, Choi -C4N-Mo2 , Chol-DMC3N-Mo2 , CholC4Hex-Mo2 , DmC4Mo2,
DmC3Mo2, C3Mo2 , C3Mo3 , C5Mo2, C6Mo2, C8Mo2, C4Mo4 , PipC2-Chol,
MoC2Chol, PyrroC2Chol, ImC3Chol, PyC2Chol , MoDO, MoDP, DOIM or DPIM.
[0068] The term "chemical driver" as used herein refers to a component
or
components of the formulation of the described invention that provides the
driving
force for a drug to diffuse from the vehicle, into and through the stratum
corneum of
the skin. According to some embodiments, the chemical drivers of the described
invention synergistically cooperate to deliver the therapeutic agent.
18
Date Recue/Date Received 2022-04-07
[0069] The term "cholesterol" as used herein refers to a monohydric
secondary
alcohol of the cyclopentenophenantrene (4-ring fused) system containing one
double
bond. According to some embodiments, cholesterol is a liposome component.
According to some embodiments, it is useful to enhance incorporation and
emulsification of medicinal products in oils or fats.
[0070] The term "colorant" as used herein refers to a substance used to
impart
a color on a composition to improve the attractiveness of the composition
and/or to
enable easy product identification. Non-limiting examples of colorants include
oil-
soluble dyes, oil dispersible dyes, water-soluble dyes, e.g. acid blue 3, acid
blue 104,
acid green 1, acid green 25, acid yellow 3, acid yellow 73 sodium salt, D&C
green
No. 5, 6, & 8, D&C yellow No. 7, 8, 10, & 11, D&C violet No. 2, FD&C blue No.
1 & 2,
FD&C green No. 3, FD&C yellow No. 5 & 6, and mixtures thereof.
[0071] The term "compatible" as used herein refers to a property of
components of a composition whereby the components are capable of being
combined with each other in a manner such that there is no interaction that
would
substantially reduce the efficacy of the composition under ordinary use
conditions.
[0072] The term "component" as used herein refers to a constituent part,
element or ingredient.
[0073] The terms "composition" and "formulation" are used
interchangeably
herein to refer to a product of the described invention that comprises all
active and
inert ingredients.
[0074] The term "condition" as used herein, refers to a variety of
health states
and is meant to include disorders or diseases caused by any underlying
mechanism
or disorder, injury, and the promotion of healthy tissues and organs.
[0075] The term "consequence" as used herein refers to an effect, result
or
outcome of something that occurred earlier.
[0076] The term "contact" and all its grammatical forms as used herein
refers to
a state or condition of touching or of immediate or local proximity.
19
Date Recue/Date Received 2022-04-07
[0077] The term "controlled release" as used herein refers to a drug-
containing
formulation in which the manner and profile of drug release from the
formulation are
controlled. This includes immediate as well as non-immediate release
formulations,
with non-immediate release formulations including, but not limited to,
sustained
release and delayed release formulations. The term "sustained release" (also
referred to as "extended release") is used herein in its conventional sense to
refer to
a drug formulation that provides for gradual release of a drug over an
extended
period of time, and that may result in substantially constant levels of a drug
over an
extended time period. The term "delayed release" is used herein in its
conventional
sense to refer to a drug formulation in which there is a time delay between
administration of the formulation and the release of the drug therefrom.
"Delayed
release" may or may not involve gradual release of drug over an extended
period of
time, and thus may or may not be "sustained release." The term "long-term"
release,
as used herein, means that the drug formulation is constructed and arranged to
deliver therapeutic levels of the active ingredient for at least: 2 hours, 3
hours, 4
hours, hours, 6 hours, 7 hours, 8 hours, 9 hours, 10 hours, 11 hours, 12
hours, 13
hours, 14 hours, 15 hours, 16 hours, 17 hours, 18 hours, 19 hours, 20 hours,
21
hours, 22 hours, 23 hours, 24 hours, 25 hours, 26 hours, 27 hours, 28 hours,
29
hours, 30 hours, 31 hours, 32 hours, 33 hours, 34 hours, 35 hours, 36 hours,
37
hours, 38 hours, 39 hours, 40 hours, 41 hours, 42 hours, 43 hours, 44 hours,
45
hours, 46 hours, 47 hours, 48 hours, 49 hours, 50 hours, 51 hours, 52 hours,
53
hours, 54 hours, 55 hours, 56 hours, 57 hours, 58 hours, 59 hours, 60 hours,
61
hours, 62 hours, 63 hours, 64 hours, 65 hours, 66 hours, 67 hours, 68 hours,
69
hours, 70 hours, 71 hours, 72 hours, 73 hours, 74 hours, 75 hours, 76 hours,
77
hours, 78 hours, 79 hours, 80 hours, 81 hours, 82 hours, 83 hours, 84 hours,
85
hours, 86 hours, 87 hours, 88 hours, 89 hours, 90 hours, 91 hours, 92 hours,
93
hours, 94 hours, 95 hours, 96 hours, 97 hours, 98 hours, 99 hours, 100 hours,
101
hours, 102 hours, 103 hours, 104 hours, 105 hours, 106 hours, 107 hours, 108
hours, 109 hours, 110 hours, 111 hours, 112 hours, 113 hours, 114 hours, 115
hours, 116 hours, 117 hours, 118 hours, 119 hours, or 120 hours.
[0078] The term "copolymer" as used herein refers to a polymer derived
from
more than one species of monomer. The term "polymer" refers to a large
molecule,
Date Recue/Date Received 2022-04-07
or macromolecule, composed of many repeated subunits. The term "monomer"
refers to a molecule that may bind chemically to other molecules to form a
polymer.
[0079] The term "derivative" as used herein means a compound that may be
produced from another compound of similar structure in one or more steps. A
derivative of a compound retains at least a degree of the desired function of
the
compound. Accordingly, an alternate term for "derivative" may be "functional
derivative." Derivatives can include chemical modifications, such as
alkylation,
acylation, carbamylation, iodination or any modification that derivatizes the
compound. Such derivatized molecules include, for example, those molecules in
which free amino groups have been derivatized to form amine hydrochlorides, p-
toluene sulfonyl groups, carbobenzoxy groups, t-butyloxycarbonyl groups,
chloroacetyl groups or formal groups. Free carboxyl groups can be derivatized
to
form salts, esters, amides, or hydrazides. Free hydroxyl groups can be
derivatized
to form 0-acyl or 0-alkyl derivatives. See, e.g., Methods and Analytical
Procedures,
Elsevier Biomedical Press, New York (1975).
[0080] The term "disease" or "disorder" as used herein refers to an
impairment
of health or a condition of abnormal functioning.
[0081] The term "drug" as used herein refers to a substance intended for
use in
the diagnosis, cure, mitigation, treatment or prevention of disease or
disorder, or to
affect the structure or function of the body.
[0082] The terms "effective therapeutic amount", an "amount effective",
or
"pharmaceutically effective amount" of one or more of the active agents is
used
interchangeably to refer to an amount that is sufficient to provide the
intended benefit
of treatment. An effective amount of an active agent that can be employed
according to the described invention generally ranges from about 0.01 mg/kg
body
weight to about 100 g/kg body weight. However, dosage levels are based on a
variety of factors, including the type of injury, the age, weight, sex,
medical condition
of the patient, the severity of the condition, the route of administration,
and the
particular active agent employed. Thus the dosage regimen may vary widely, but
can be determined routinely by a physician using standard methods.
21
Date Recue/Date Received 2022-04-07
[0083] The terms "emollient" or "moisturizer" as used herein are used
interchangeably to refer to complex mixtures of chemical agents specially
designed
to make the external layers of the skin (epidermis) softer and more pliable.
An
emollient increases the skin's hydration (water content) by reducing
evaporation.
[0084] The term "emulsifier" as used herein refers to an additive that
help two
liquids mix. For example, water and oil separate in a glass, but adding an
emulsifier
will help the water and oil to mix together.
[0085] The term "excipient" as used herein refers to any inactive
ingredient that
is added to the composition of the described invention and that is not
intended to
exert therapeutic effects at the intended dosage, although it may act to
improve
product delivery. Additional characteristics of excipients can be found in the
Guidance for Industry Nonclinical Studies for the Safety Evaluation of
Pharmaceutical Excipients issued by the US Food and Drug Administration Center
for Drug Evaluation and Research (May, 2005).
[0086] The term "flocculant" as used herein refers to a substance that
promotes the clumping of particles.
[0087] The term "fragrant" as used herein refers to an aroma compound,
also
known as odorant, or flavorant, which is a chemical compound that has a smell
or
odor r when it is sufficiently volatile to be transported to the olfactory
system in the
upper part of the nose. Generally molecules meeting this specification will
have
molecular weights of <300 g/mole. Flavors affect both the sense of taste and
smell,
whereas fragrances affect only smell. Generally, flavors tend to be naturally
occurring, while fragrances tend to be synthetic. Aroma compounds can be found
in
food, wine, spices, perfumes, fragrance oils, and essential oils.
[0088] The term "hydrogel' as used herein refers to a network of
polymer
chains that are hydrophilic, sometimes found as a colloidal gel in which water
is the
dispersion medium. Hydrogels are highly absorbent (they can contain over 90%
water) natural or synthetic polymeric networks. Hydrogels also possess a
degree of
flexibility very similar to natural tissue, due to their significant water
content.
22
Date Recue/Date Received 2022-04-07
[0089] The term "hydrophilic" as used herein refers to a material or
substance
having an affinity for polar substances, such as water.
[0090] The term "impregnate" as used herein in its various grammatical
forms
refers to causing to be infused or permeated throughout, or to fill
interstices with a
substance.
[0091] As used herein the term "inflammation" refers to a physiologic
response
to infection and injury in which cells involved in detoxification and repair
are
mobilized to the compromised site by inflammatory mediators. The term "acute
inflammation" as used herein, refers to inflammation, usually of sudden onset,
characterized by the classical signs, with predominance of the vascular and
exudative processes. The term "chronic inflammation" as used herein refers to
inflammation of slow progress and marked chiefly by the formation of new
connective tissue; it may be a continuation of an acute form or a prolonged
low-
grade form, and usually causes permanent tissue damage.
[0092] The term "lipid" as used herein refers to a group of naturally
occurring
molecules that include fats, waxes, sterols, fat-soluble vitamins (e.g.
vitamins A, D,
E, and K), mono-di or triglycerides phospholipids, and others. The main
biological
functions of lipids include storing energy, signaling, and acting as
structural
components of cell membranes. Exemplary lipids include natural phospholipids
(e.g.,
egg yolk lecithin (phosphatidyl choline), soybean lecithin, lysolecithin,
sphingomyelin,
phosphatidic acid, phosphatidyl serine, phosphatidyl glycerol, phosphatidyl
inositol,
phosphatidyl ethanol amine, di phosphatidyl glycerol, cardioli pin and
plasmalogen);
synthetic lipids (e.g., dicetyl phosphate, distearoyl phosphatidyl choline,
dioleoylphosphatidyl ethanol amine, dipalmitoyl phosphatidyl choline,
diphalmitoyl
phosphatidyl ethanol amine, diphalmitoyl phosphatidyl serine, eleostearoyl
phosphatidyl choline, eleostearoyl phosphatidyl ethanol amine and eleostearoyl
phosphatidyl serine); hydrogenated products that may be obtained from the
natural
phospholipids or synthetic lipids; derivatives of the natural phospholipids or
synthetic
lipids; and fatty acid mixtures that may be obtained by hydrolysis of the
natural
phospholipids or synthetic lipids.
23
Date Recue/Date Received 2022-04-07
[0093] The term "lipophilic" as used herein refers to preferring or
possessing an
affinity for a non-polar environment compared to a polar or aqueous
environment.
[0094] The term "liposome" as used herein refers to a man-made spherical
vesicle containing at least one lipid bilayer. The liposome can be used as a
vehicle
for administration of components, such as, but not limited to, pharmaceutical
compositions and pharmaceutical formulations, active therapeutic agents,
drugs,
enzymes, other proteins and peptides, and DNA and RNA fragments, etc.
[0095] The terms "local anesthetic" or "analgesic agents" are used
interchangeably herein to refer to any drug that provides local numbness or
moderation of painful signals sthat although still perceived are no longer
painful, or
any drug that provides a regional blockage of nociceptive pathways (afferent
and/or
efferent). "Local anesthetic" as used herein also encompasses drugs not
traditionally associated with local anesthetic properties but which have a
local
anesthetic effect, for example, non-narcotic analgesics, such as,
acetylsalicylic acid,
ketoprofen, piroxicam, diclofenac, indomethacin, ketorolac, rofecoxib, and
celecoxib,
and pharmaceutically acceptable salts thereof, or mixtures thereof.
[0096] The phrase "localized administration", as used herein, refers to
administration of a therapeutic agent in a particular location in the body.
[0097] The phrase "localized pharmacologic effect", as used herein,
refers to a
consequence of treatment or a therapeutic effect limited to a certain
location, i.e. in
proximity to a certain location, place, area or site. The phrase
"predominantly
localized pharmacologic effect", as used herein, refers to a therapeutic
effect of a
drug that is limited to a certain location by at least 1 to 3 orders of
magnitude, which
is achieved by a localized administration as compared to a systemic
administration.
[0098] The term "lubricant" as used herein refers to a substance
introduced to
reduce friction between surfaces in mutual contact, which ultimately reduces
the heat
generated when the surfaces move. It may also have the function of
transmitting
forces, transporting foreign particles, or heating or cooling the surfaces.
[0099] The term "matrix" as used herein refers to a three dimensional
network
of fibers that contains voids (or "pores") where the fibers intersect. The
structural
24
Date Recue/Date Received 2022-04-07
parameters of the pores, including the pore size, porosity, pore
interconnectivity/tortuosity and surface area, affect how substances (e.g.,
fluid,
solutes) move in and out of the matrix.
[00100] The term "maximum tolerated dose" as used herein refers to the
highest
dose of a drug that does not produce unacceptable toxicity.
[00101] The terms "minimum effective concentration," "minimum effective
dose,"
or "MEC" are used interchangeably to refer to the lowest concentration of a
drug
required to produce a desired pharmacological effect in most patients.
[00102] The terms "neoplasm" or "tumor" as used herein are used
interchangeably to refer to an abnormal mass of tissue that results when cells
divide
more than they should or do not die when they should. Neoplasms may be benign
(not cancer) or malignant (cancer). For example, a benign neoplasm (or benign
tumor) is a tumor that stops growing by itself, does not invade other tissues
and does
not form metastases
[00103] The term "neutral lipid" as used herein refers to a lipid which
has
neither a positive or negative charge. Exemplary neutral lipids include,
without
limitation, cholesterol, cholesterol esters, triglycerides and fatty acids.
[00104] The term "non-cellulosic copolymer" as used herein refers to a
copolymer not containing or derived from cellulose. The term "cellulose" as
used
herein refers to a natural carbohydrate high polymer (polysaccharide)
consisting of
anhydroglucose untis joined by an oxygen linkage to form long molecular chains
that
are essentially linear that can be hydrolyzed to glucose.
[00105] The term "pain" as used herein refers to a distressing feeling
often
caused by intense or damaging stimuli. As such, the term "pain" is
characterized by
an unpleasant sensory detected and signaled by the nerves and emotional
experience associated with actual or potential tissue damage.
[00106] The terms "penetration enhancer" and "permeation enhancer" are
used
interchangeably to refer to natural or synthetic molecules that facilitate the
transport
of co-administered active agents across biological membranes.
Date Recue/Date Received 2022-04-07
[00107] The term "pharmaceutical composition" is used herein to refer to
a
composition that is employed to prevent, reduce in intensity, cure or
otherwise treat a
target condition or disease.
[00108] The term "pharmaceutically acceptable salt" as used herein refers
to
those salts which are, within the scope of sound medical judgment, suitable
for use
in contact with the tissues of humans and lower animals without undue
toxicity,
irritation, allergic response and the like and are commensurate with a
reasonable
benefit/risk ratio. When used in medicine the salts should be pharmaceutically
acceptable, but non-pharmaceutically acceptable salts may conveniently be used
to
prepare pharmaceutically acceptable salts thereof. Such salts include, but are
not
limited to, those prepared from the following acids: hydrochloric,
hydrobromic,
sulphuric, nitric, phosphoric, maleic, acetic, salicylic, p-toluene sulphonic,
tartaric,
citric, methane sulphonic, formic, malonic, succinic, naphthalene-2-sulphonic,
and
benzene sulphonic. Also, such salts may be prepared as alkaline metal or
alkaline
earth salts, such as sodium, potassium or calcium salts of the carboxylic acid
group.
By "pharmaceutically acceptable salt" is meant those salts which are, within
the
scope of sound medical judgment, suitable for use in contact with the tissues
of
humans and lower animals without undue toxicity, irritation, allergic response
and the
like and are commensurate with a reasonable benefit/risk ratio.
Pharmaceutically
acceptable salts are well-known in the art. For example, P. H. Stahl, et al.
describe
pharmaceutically acceptable salts in detail in "Handbook of Pharmaceutical
Salts:
Properties, Selection, and Use" (Wiley VCH, Zurich, Switzerland: 2002). The
salts
may be prepared in situ during the final isolation and purification of the
compounds
described within the described invention or separately by reacting a free base
function with a suitable organic acid. Representative acid addition salts
include, but
are not limited to, acetate, adipate, alginate, citrate, aspartate, benzoate,
benzenesulfonate, bisulfate, butyrate, camphorate, camphorsufonate,
digluconate,
glycerophosphate, hemisulfate, heptanoate, hexanoate, fumarate, hydrochloride,
hydrobromide, hydroiodide, 2-hydroxyethansulfonate (isethionate), lactate,
maleate,
methanesulfonate, nicotinate, 2-naphthalenesulfonate, oxalate, pamoate,
pectinate,
persulfate, 3-phenylpropionate, picrate, pivalate, propionate, succinate,
tartrate,
thiocyanate, phosphate, glutamate, bicarbonate, p-toluenesulfonate and
undecanoate. Also, the basic nitrogen-containing groups may be quaternized
with
26
Date Recue/Date Received 2022-04-07
such agents as lower alkyl halides such as methyl, ethyl, propyl, and butyl
chlorides,
bromides and iodides; dialkyl sulfates like dimethyl, diethyl, dibutyl and
diamyl
sulfates; long chain halides such as decyl, lauryl, myristyl and stearyl
chlorides,
bromides and iodides; arylalkyl halides like benzyl and phenethyl bromides and
others. Water or oil-soluble or dispersible products are thereby obtained.
Examples
of acids which may be employed to form pharmaceutically acceptable acid
addition
salts include such inorganic acids as hydrochloric acid, hydrobromic acid,
sulphuric
acid and phosphoric acid and such organic acids as oxalic acid, maleic acid,
succinic
acid and citric acid. Basic addition salts may be prepared in situ during the
final
isolation and purification of compounds described within the invention by
reacting a
carboxylic acid-containing moiety with a suitable base such as the hydroxide,
carbonate or bicarbonate of a pharmaceutically acceptable metal cation or with
ammonia or an organic primary, secondary or tertiary amine. Pharmaceutically
acceptable salts include, but are not limited to, cations based on alkali
metals or
alkaline earth metals such as lithium, sodium, potassium, calcium, magnesium
and
aluminum salts and the like and nontoxic quaternary ammonia and amine cations
including ammonium, tetramethylammonium, tetraethylammonium, methylamine,
dimethylamine, trimethylamine, triethylamine, diethylamine, ethylamine and the
like.
Other representative organic amines useful for the formation of base addition
salts
include ethylenediamine, ethanolamine, diethanolamine, piperidine, piperazine
and
the like. Pharmaceutically acceptable salts also may be obtained using
standard
procedures well known in the art, for example by reacting a sufficiently basic
compound such as an amine with a suitable acid affording a physiologically
acceptable anion. Alkali metal (for example, sodium, potassium or lithium) or
alkaline earth metal (for example calcium or magnesium) salts of carboxylic
acids
may also be made.
[00109] As used
herein the phrase "pharmaceutically acceptable carrier" refers
to any substantially non-toxic carrier useable for formulation and
administration of
the composition of the described invention in which the product of the
described
invention will remain stable and bioavailable. The pharmaceutically acceptable
carrier must be of sufficiently high purity and of sufficiently low toxicity
to render it
suitable for administration to the mammal being treated. It further should
maintain
the stability and bioavailability of an active agent. The pharmaceutically
acceptable
27
Date Recue/Date Received 2022-04-07
carrier can be liquid or solid and is selected, with the planned manner of
administration in mind, to provide for the desired bulk, consistency, etc.,
when
combined with an active agent and other components of a given composition.
[00110] The term "pharmacologic effect", as used herein, refers to a
result or
consequence of exposure to an active agent.
[00111] The term "pharmacokinetics" as used herein describes how the body
affects a specific drug after administration through the mechanisms of
absorption
and distribution, as well as the chemical changes of the substance in the body
(e.g.
by metabolic enzymes such as cytochrome P450 or glucuronosyltransferase
enzymes), and the effects and routes of excretion of the metabolites of the
drug.
[00112] The term "plasticizer" as used herein refers to an additive that
increases
the plasticity or fluidity of a material.
[00113] The term, "polyethylene glycol" as used herein is used to refer
to a
condensation polymer of ethylene glycol with the general formula
HOCH2(CH2OCH2)nCH2OH or H(OCH2CH2)n0H. Average molecular weights range
from 200 to 6000. Polyethylene glycols can be used as medicaments for topical
application in the treatment of wounds, for the treatment of inflammatory skin
disease, for the prevention of scar formation and/or for enhancing the repair
of
damaged skin or mucosa.
[00114] The term "polymer" as used herein refers to a molecule formed by
the
chemical union of two or more monomer or oligomer units. The chemical units
are
normally linked together by covalent linkages. The two or more combining units
in a
polymer can be all the same, in which case the polymer is referred to as a
homopolymer. They can also be different and, thus, the polymer will be a
combination of the different units. Such polymers are referred to as
copolymers. The
relationship between the polymer subunits may be oriented head-to-head or head-
to-
tail relative to each subunit. Polymers can be divided into two broad groups:
synthetic (non-natural polymers) and natural polymers.
[00115] Examples of non-natural polymers include, but are not limited to
polyalcohols such as ethylene vinyl alcohol (EVAL), hydroxyethyl acrylate,
28
Date Recue/Date Received 2022-04-07
poly(ethylene glycol), poly(vinyl alcohol), poly(hydroxypropyl
methacrylamide),
poly(propylene glycol); polyamines (such as polyvinylamine, polyallylamine,
tetramethyleneamine, pentamethyleneamine, hexamethyleneamine, bis(2-
hydroxyethyl)amine, bis(2-aminoethyl)amine, tris(2-aminoethyl)amine, branched
or
linear polyethyleneimine e.g., LubrasolsTM - and salts thereof, and
derivatives of
polyethyleneimine such as acylated polyethyleneimine); dendrimers (such as
polyamidoamine (PAMAM) Starburst dendrimers); polyalkylene glycol derivatives
(such as amine-substituted polyethylene and polypropylene glycols);
polyacrylates
(such as amine-substituted and alcohol-substituted polyacrylates); multi-amino
PEG;
polymers where the backbone polymeric structure is substituted with the
following
pendant nucleophilic or electrophilic groups such as PEG substituted with
amines,
hydroxylamine, hydrazines, thiols, xanthates, amides, hydrazides,
sulfonamides,
oximes, malonates, imides, aldehydes, succinimidyl, isocyanates,
vinylsulfones,
oxiranes, arylhalides, allylhalides, alkyl halides, esters, ethers or
anhydrides.
[00116] Examples of anionic biopolymers include carboxymethylcellulose
and
salts thereof, salts of carboxymethyl and carboxymethylhydroxyethyl starches,
and
other glucoaminoglycans such as chondroitin sulfate, dermatan sulfate, heparin
and
heparin sulfate and keratin sulfates.
[00117] Examples of natural polymers include, without limitation,
hyaluronic
acid, chondroitin sulfate, alginate, guar gum, fructan, arabinogalactan and
any
corresponding salt or derivative of thereof.
[00118] Hyaluronic acid is a linear polysaccharide (long-chain biological
polymer) formed by repeating disaccharide units consisting of D-glucuronic
acid and
N-acetyl-D- glucosamine linked by 8(1-3) and 8(1-4) glycosidic linkages.
Hyaluronic
acid is distinguished from other glycosaminoglycans in that is free from
covalent links
to protein and sulphonic groups. Hyaluronic acid is ubiquitous in animals,
with the
highest concentration found in soft connective tissue. The viscoelastic
properties of
hyaluronic acid, that is, hard elastic under static conditions though less
viscous
under small shear forces, enables hyaluronic acid to basically function as a
shock
absorber for cells and tissues. Hyaluronic acid also has a relatively large
capacity to
absorb and hold water. These properties of hyaluronic acid are dependent on
the
molecular weight, the solution concentration, and physiological pH. At low
29
Date Recue/Date Received 2022-04-07
concentrations, the individual chains entangle and form a continuous network
in
solution, which gives the system pronounced viscoelasticity and
pseudoplasticity that
is unique for a water-soluble polymer at low concentration.
[00119] As used herein, the term "fructan" refers to all oligosaccharides
and
polysaccharides that have a majority of anhydro fructose units and derivatives
thereof. The fructan can have a polydisperse chain length distribution and can
be
straight-chain or branched. The fructans include primarily 13-2,6 bonds as in
levan, or
13-2,1 bonds as in a carboxyl modified fructant, e.g., inulin.
[00120] Examples of synthetic polymers include, without limitation,
polyethylene,
polystyrene, polyester, polyvinyl chloride, polyamide, polypropylene, and
nylon.
[00121] The term "polymersome" as used herein refers to a class of
artificial
vesicles, tiny hollow spheres that enclose a solution. Polymersomes are made
using
amphiphilic synthetic block copolymers to form the vesicle membrane, and have
radii
ranging from 50 nm to 5 pm or more. Most reported polymersomes contain an
aqueous solution in their core and are useful for encapsulating and protecting
sensitive molecules, such as but not limited to pharmaceutical compositions
and
pharmaceutical formulations, active therapeutic agents, drugs, enzymes, other
proteins and peptides, and DNA and RNA fragments, etc. The polymersome
membrane provides a physical barrier that isolates the encapsulated material
from
external materials, such as those found in biological systems.
[00122] The term "potency" as used herein refers to efficacy,
effectiveness, or
strength of a drug. The potency of a drug is the reciprocal of dose, and has
the units
of persons/unit weight of drug or body weight/unit weight of drug. Relative
potency
compares the relative activity of drugs in a series relative to some
prototypic member
of the series. "Efficacy" connotes the property of a drug to achieve the
desired
response, and maximum efficacy denotes the maximum achievable effect.
[00123] The terms "povidone" "2-pyrrolidinone", "polyvinylpyrrolidone"
and PVP
are used interchangeably to refer to a synthetic polymer consisting of linear
1-vinyl-
2-pyrrolidinone groups. PVP is produced commercially as a series of products
having mean molecular weights ranging from about 10,000 to about 700,000. The
viscosity of solutions containing 10% or less PVP is essentially the same as
that of
Date Recue/Date Received 2022-04-07
water; solutions more concentrated than 10% become more viscous, depending on
the concentration and molecular weight of the polymer used.
[00124] The term "preservative" as used herein refers to a substance that
is
added to a product to prevent decomposition by microbial growth or by
undesirable
chemical changes.
[00125] The term "reduced" or "to reduce" as used herein refers to a
diminution,
a decrease, an attenuation or abatement of the degree, intensity, extent,
size,
amount, density or number.
[00126] The term "release" as used herein and its various grammatical
forms,
refers to dissolution of an active drug component and diffusion of the
dissolved or
solubilized species. According to some embodiments, this occurs by a
combination
of the following processes: (1) hydration of a matrix, (2) diffusion of a
solution into
the matrix; (3) dissolution of the drug; and (4) diffusion of the dissolved
drug out of
the matrix.
[00127] The term "similar" is used interchangeably with the terms
analogous,
comparable, or resembling, meaning having traits or characteristics in common.
[00128] The terms "soluble" and "solubility" refer to the property of
being
susceptible to being dissolved in a specified fluid (solvent). The term
"insoluble"
refers to the property of a material that has minimal or limited solubility in
a specified
solvent. In a solution, the molecules of the solute (or dissolved substance)
are
uniformly distributed among those of the solvent. A "suspension" is a
dispersion
(mixture) in which a finely-divided species is combined with another species,
with the
former being so finely divided and mixed that it doesn't rapidly settle out.
In
everyday life, the most common suspensions are those of solids in liquid.
[00129] The terms "solubility enhancer" or "solubilizing agent" are used
interchangeably to refer to any chemical and/or biological agent able to
improve the
solubility of an agent in a solvent. Exemplary solubility enhancers include
povidone,
cholesterol, cyclodextrins, and polyethylene glycols. Exemplary solubility
enhancers
also include surfactants, which act as solubilizing agents by forming
micelles. The
HLB system is used to describe the characteristics of a surfactant. It is an
arbitrary
31
Date Recue/Date Received 2022-04-07
scale to which HLB values are experimentally determined and assigned. If the
HLB
value is low, the number of hydrophilic groups on the surfactant is small,
which
means it is more lipophilic (oil soluble) than hydrophilic (water soluble).
Conversely,
if the HLB value is high, there are a large number of hydrophilic groups on
the
surfactant, which makes it more hydrophilic (water soluble) than oil soluble.
An HLB
value of 10 or higher means that the agent is primarily hydrophilic.
[00130] The term "solvent" as used herein refers to a substance capable
of
dissolving another substance (termed a "solute") to form a uniformly dispersed
mixture (solution).
[00131] The term "stabilizer" as used herein refers to a chemical which
tends to
inhibit the reaction between two or more other chemicals.
[00132] As used herein the term "steroidal anti-inflammatory agent",
refers to
any one of numerous compounds containing a 17-carbon 4-ring system and
includes
the sterols, various hormones (as anabolic steroids), and glycosides.
Representative examples of steroidal anti-inflammatory drugs include, without
limitation, corticosteroids such as hydrocortisone, hydroxyltriamcinolone,
alpha-
methyl dexamethasone, dexamethasone-phosphate, beclomethasone dipropionates,
clobetasol valerate, desonide, desoxymethasone, desoxycorticosterone acetate,
dexamethasone, dichlorisone, diflucortolone valerate, fluadrenolone,
fluclorolone
acetonide, flumethasone pivalate, fluosinolone acetonide, fluocinonide,
flucortine
butylesters, fluocortolone, fluprednidene (fluprednylidene) acetate,
flurandrenolone,
halcinonide, hydrocortisone acetate, hydrocortisone butyrate,
methylprednisolone,
triamcinolone acetonide, cortisone, cortodoxone, flucetonide, fludrocortisone,
difluorosone diacetate, fluradrenolone, fludrocortisone, diflorosone
diacetate,
fluradrenolone acetonide, medrysone, amcinafel, amcinafide, betamethasone and
the balance of its esters, chloroprednisone, chlorprednisone acetate,
clocortelone,
clescinolone, dichlorisone, diflurprednate, flucloronide, flunisolide,
fluoromethalone,
fluperolone, fluprednisolone, hydrocortisone valerate, hydrocortisone
cyclopentylpropionate, hydrocortamate, meprednisone, paramethasone,
prednisolone, prednisone, beclomethasone dipropionate, triamcinolone, and
mixtures thereof.
32
Date Recue/Date Received 2022-04-07
[00133] The terms "subject" or "individual" or "patient" are used
interchangeably
to refer to a member of an animal species of mammalian origin an animal
species of
mammalian origin, including but not limited to, mouse, rat, cat, goat, sheep,
horse,
hamster, ferret, pig, dog, platypus, guinea pig, rabbit and a primate, such
as, for
example, a monkey, ape, or human.
[00134] The phrase "subject in need thereof" as used herein refers to a
subject
that (i) will be administered a topical composition of the described
invention; (ii) is
applying the topical composition of the described invention; or (iii) has
applied the
topical composition of the described invention, unless the context and usage
of the
phrase indicates otherwise.
[00135] When using the terms "substantial", "substantially", "essential"
or
"essentially" herein it is intended that the feature which is described by
these terms is
present in an amount or has an impact which provides for a technical effect
with
relevance for the exercise of the presently claimed invention. For instance, a
"substantial amount" of a substance in a composition is an amount which
provides
for a technical effect exhibited by the substance to a degree which provides
for a
technical effect in terms of the described invention. Likewise, if a
composition is
indicated as comprising "substantially no" with respect to a substance, this
means
that the composition is allowed to include insignificant amounts of the
substance, as
long as these amounts do not have any technical impact on the other
ingredients in
the composition and does not in itself "make a difference" or put in other
words,
"substantially no" and "essentially no" means that e.g. trace amounts or
effects may
be present as long as they do not have an overall technical influence.
[00136] The term "surfactant" as used herein refers to a compound that
lowers
the surface tension (or interfacial tension) between two liquids or between a
liquid
and a solid.
[00137] The term "susceptible" as used herein refers to being at risk
for.
[00138] The term "synergistic effect" as used herein, refers to a
combined effect
of two chemicals, which is greater than the sum of the effect of each agent
given
alone.
33
Date Recue/Date Received 2022-04-07
[00139] The phrase "systemic administration", as used herein, refers to
administration of a therapeutic agent with a pharmacologic effect on the
entire body.
Systemic administration includes enteral administration (e.g. oral) through
the
gastrointestinal tract and parenteral administration (e.g. intravenous,
intramuscular,
etc.) outside the gastrointestinal tract.
[00140] The terms "therapeutic amount", "therapeutic effective amount" or
an
"amount effective" of one or more of the therapeutic agents is an amount that
is
sufficient to provide the intended benefit of treatment. Combined with the
teachings
provided herein, by choosing among the various active compounds and weighing
factors such as potency, relative bioavailability, patient body weight,
severity of
adverse side-effects and preferred mode of administration, an effective
prophylactic
or therapeutic treatment regimen may be planned which does not cause
substantial
toxicity and yet is effective to treat the particular subject. Generally, a
maximum
dose should be used, that is, the highest safe dose according to some medical
judgment. However, dosage levels are based on a variety of factors, including
the
type of injury, the age, weight, sex, medical condition of the patient, the
severity of
the condition, the route of administration, and the particular therapeutic
agent
employed. Thus the dosage regimen may vary widely, but can be determined
routinely by a surgeon using standard methods. "Dose" and "dosage" are used
interchangeably herein. Additionally, the terms "therapeutically effective
amounts"
and "pharmaceutically effective amounts" include prophylactic or preventative
amounts of the compositions of the described invention. In prophylactic or
preventative applications of the described invention, pharmaceutical
compositions or
medicaments are administered to a patient susceptible to, or otherwise at risk
of, a
disease, disorder or condition in an amount sufficient to eliminate or reduce
the risk,
lessen the severity, or delay the onset of the disease, disorder or condition,
including
biochemical, histologic and/or behavioral symptoms of the disease, disorder or
condition, its complications, and intermediate pathological phenotypes
presenting
during development of the disease, disorder or condition. Topical
administration, in
contrast to transdermal administration, generally provides a local rather than
a
systemic effect.
34
Date Recue/Date Received 2022-04-07
[00141] The term "therapeutic component" as used herein refers to a
therapeutically effective dosage (Le., dose and frequency of administration)
that
eliminates, reduces, or prevents the progression of a particular disease
manifestation in a percentage of a population. An example of a commonly used
therapeutic component is the ED50 which describes the dose in a particular
dosage
that is therapeutically effective for a particular disease manifestation in
50% of a
population.
[00142] The term "therapeutic effect" as used herein refers to a
consequence of
treatment, the results of which are judged to be desirable and beneficial. A
therapeutic effect may include, directly or indirectly, the arrest, reduction,
or
elimination of a disease manifestation. A therapeutic effect may also include,
directly
or indirectly, the arrest reduction or elimination of the progression of a
disease
manifestation.
[00143] The term "thickening agent" refers to a substance that can
increase the
viscosity of a liquid without substantially changing its other properties.
[00144] The term "thinning agent" as used herein refers to a substance
that
reduces the viscosity of a liquid making it easier to apply.
[00145] The term "topical" refers to administration of a pharmaceutical
composition at, or immediately beneath, the point of application. The terms
"topically", "topical administration" and "topically applying" are used
interchangeably
to refer to delivering a pharmaceutical composition of the described invention
onto
one or more surfaces of a tissue or cell, including epithelial surfaces. The
composition may be applied by pouring, dropping, or spraying, if a liquid;
rubbing on,
if an ointment, lotion, cream, gel, or the like; dusting, if a powder;
spraying, if a liquid
or aerosol composition; or by any other appropriate means. Topical
administration
generally provides a local rather than a systemic effect.
[00146] The term "treat" or "treating" includes abrogating, substantially
inhibiting,
slowing or reversing the progression of a disease, condition or disorder,
substantially
ameliorating clinical or esthetical symptoms of a condition, substantially
preventing
the appearance of clinical or esthetical symptoms of a disease, condition, or
disorder, and protecting from harmful or annoying symptoms. The term "treat"
or
Date Recue/Date Received 2022-04-07
"treating" as used herein further refers to accomplishing one or more of the
following:
(a) reducing the severity of the disorder; (b) limiting development of
symptoms
characteristic of the disorder(s) being treated; (c) limiting worsening of
symptoms
characteristic of the disorder(s) being treated; (d) limiting recurrence of
the
disorder(s) in patients that have previously had the disorder(s); and (e)
limiting
recurrence of symptoms in patients that were previously symptomatic for the
disorder(s).
[00147] As used herein the term "vasoconstrictor" is used to describe an
active
therapeutic agent that causes a narrowing of blood vessels resulting from
contraction
of the muscular wall of the vessels, in particular the large arteries and
small
arterioles. The process is the opposite of vasodilation, the widening of blood
vessels.
[00148] The term "vitamin" as used herein, refers to any of various
organic
substances essential in minute quantities to the nutrition of most animals act
especially as coenzymes and precursors of coenzymes in the regulation of
metabolic
processes.
[00149] The term "wetting agent" as used herein refers to a substance
that
reduces the surface tension of water in order to allow it to spread drops onto
a
surface, thereby increasing the spreading abilities of a liquid.
[00150] The term "wound healing agent" as used herein refers to an agent
that
promotes an intricate process where the skin or other body tissue repairs
itself after
injury. In normal skin, the epidermis (surface layer) and dermis (deeper
layer) form a
protective barrier against the external environment. As such, the term "wound
healing agent" refers to any substance that facilitates the wound healing
process.
[00151] The term "zwitterion" is a neutral molecule with a positive and a
negative electrical charge.
[00152] According to one aspect, the described invention provides a
topical
delivery system comprising a pharmaceutical composition formulated for
application
directly to a skin of a subject in need thereof comprising (a) a therapeutic
amount of
an active therapeutic agent that is effective to treat symptoms of a disease,
disorder
36
Date Recue/Date Received 2022-04-07
or condition; (b) a chemical driver comprising an amino benzoate local
anesthetic,
ethoxydiglycol and methylsulfonylmethane (MSM), wherein the chemical drivers
are
effective to deliver the therapeutic agent to the skin; and (c) a depot
component that
is effective to keep the active agent locally in the skin and to reduce
distribution of
the active to the blood stream.
Depot components for keeping the pharmaceutical formulation in the skin and
facilitating controlled release of the active agent
[00153] According to some embodiments, the composition of the described
invention contains a depot component that is effective for keeping the active
agent
concentrated locally in the skin. According to some such embodiments, the
depot
component is effective to facilitate controlled or delayed type release of the
active
therapeutic agent. According to some embodiments, the depot component reduces
the potential of the active agent, active metabolite, the chemical drivers, or
a
combination thereof to enter the bloodstream. According to some embodiments,
the
chemical driver component that remains in the skin is effective to allow the
active
agent and/or the active metabolite to further diffuse away from the skin, such
that the
active agent can execute its biological function at the specific tissue of
interest.
According to some embodiments, the depot component comprises a liposome.
According to some embodiments, the depot component comprises a polymer.
According to some embodiments, the depot component comprises a complex of a
liposome and a polymer or a polymersome. Other examples of depot components
include, without limitation, micelles, reverse micelles, emulsions,
microemulsions,
etc.
[00154] Liposomes are generally known as sub-micron spherical vesicles
comprised of phospholipids and cholesterol that form a hydrophobic bilayer
surrounding an aqueous core. These structures have been used with a wide
variety
of therapeutic agents and allow for a drug to be entrapped within the liposome
based
in part upon its own hydrophobic (e.g. bilayer entrapment) or hydrophilic
properties
(e.g. entrapment in the aqueous compartment). Liposomes are generally used for
controlled release and for drug targeting of lipid-capsulated compounds
(Betageri et
al, Liposome Drug Delivery Systems, Technomic Publishing Co., Inc., Lancaster,
PA,
1993).
37
Date Recue/Date Received 2022-04-07
[00155] Typically, encapsulating a drug, an active therapeutic agent or a
pharmaceutical composition in a liposome can alter the pattern of bio-
distribution and
the pharmacokinetics for the drugs. In certain cases, liposomal encapsulation
has
been found to lower drug toxicity. For example, long circulating liposomal
formulations can avoid uptake by organs of the mononuclear phagocyte system,
primarily in the liver and spleen. According to some embodiments, such long-
circulating liposomes may include a surface coat of flexible water soluble
polymer
chains that act to prevent interaction between the liposome and plasma
components
that play a role in liposome uptake. According to some embodiments, such
liposomes can be made of saturated, long-chain phospholipids and cholesterol,
without this coating.
[00156] Exemplary liposomes may comprise a lipid layer comprising
liposome
forming lipids. The lipid may include at least one phosphatidyl choline which
provides the primary packing/entrapment/structural element of the liposome.
The
phosphatidyl choline comprises mainly C16 or longer fatty-acid chains. Chain
length
provides for both liposomal structure, integrity, and stability. Optionally,
one of the
fatty-acid chains may have at least one double bond. As used herein, the term
"phosphatidyl choline" includes, without limitation, soy PC, egg PC dielaidoyl
phosphatidyl choline (DEPC), lecithin, dioleoyl phosphatidyl choline (DOPC),
distearoyl phosphatidyl choline (DS PC), hydrogenated soybean phosphatidyl
choline
(HSPC), dipalmitoyl phosphatidyl choline (DPPC), 1-palmitoy1-2-oleo
phosphatidyl
choline (POPC), dibehenoyl phosphatidyl choline 30 (DBPC), and dimyristoyl
phosphatidyl choline (DMPC).
[00157] As used herein, the term "Soy-PC" refers to phosphatidyl choline
compositions including a variety of mono-, di-, tri-unsaturated, and saturated
fatty
acids. Soy-PC may include palmitic acid present in an amount of about 12% to
about
33% (i.e., about 12%, about 13%, about 14%, about 15%, about 16%, about 17%,
about 18%, about 19%, about 20%, about 21%, about 22%, about 23%, about 24%,
about 25%, about 26%, about 27%, about 28%, about 29%, about 30%, about 31%,
about 32%, or about 33%) by weight; stearic acid present in an amount of about
3%
to about 8% (i.e., about 3%, about 4%, about 5%, about 6%, about 7%, or about
8%)
by weight; oleic acid present in an amount of about 4% to about 22% (i.e.,
about 4%,
38
Date Recue/Date Received 2022-04-07
about 5%, about 6%, about 7%, about 8%, about 9%, about 10%, about 11%, about
12%, about 13%, about 14%, about 15%, about 16%, about 17%, about 18%, about
19%, about 20%, about 21%, or about 22%), by weight; linoleic acid present in
an
amount of about 60% to about 66% (i.e., about 60%, about 61%, about 62%, about
63%, about 64%, about 65%, or about 66%) by weight; and linolenic acid present
in
an amount of about 5% to about 8% (i.e., about 5%, about 6%, about 7%, or
about
8%) by weight.
[00158] As used herein, the term "Egg-PC" refers to a phosphatidyl
choline
composition including, but not limited to, a variety of saturated and
unsaturated fatty
acids. For example, Egg-PC may comprise palmitic acid present in an amount of
10
about 34% (i.e., about 10%, about 11%, about 12%, about 13%, about 14%, about
15%, about 16%, about 17%, about 18%, about 19%, about 20%, about 21%, about
22%, about 23%, about 24%, about 25%, about 26%, about 27%, about 28%, about
29%, about 30%, about 31%, about 32%, about 33% or 3 about 4%) by weight;
stearic acid present in an amount of about 10% by weight; oleic acid present
in an
amount of about 31% by weight; and linoleic acid present in an amount of about
18%
by weight.
[00159] According to some embodiments, the liposome comprises
cholesterol.
The ratio of phosphatidyl choline to cholesterol may be, for example, from
about
0.5:1 to about 4:1 by mole ratio. According to some embodiments, the ratio of
phosphatidyl choline to cholesterol may be from about 1:1 to about 2:1 by mole
ratio,
e.g., about 1.1; about 1.1:1; about 1.2:1: about 1.3:1; about 1.4:1; about
1.5:1; about
1.6:1; about 1.7:1; about 1.8:1; about 1.9:1, or about 2:1. According to some
embodiments, the ratio of phosphatidyl choline to cholesterol may be about 2:1
by
mole ratio.
[00160] As used herein the term "total lipid" includes phosphatidyl
cholines and
any anionic phospholipid present in the liposome membrane.
[00161] The liposome may also comprise physiologically acceptable salts
to
maintain proper isotonicity. Any pharmaceutically acceptable salt that
achieves
isotonicity is acceptable, including, without limitation, for example, e.g.
NaCI.
39
Date Recue/Date Received 2022-04-07
[00162] The liposomes of the described invention may comprise a lipid
layer of
phospholipids and cholesterol. According to some embodiments, the ratio of
phospholipid to cholesterol is sufficient to form a liposome that will not
dissolve or
disintegrate once administered to the animal. The phospholipids and
cholesterol
may be dissolved in suitable solvent or solvent mixtures. After a suitable
amount of
time, the solvent is removed via vacuum drying and/or spray drying. The
resulting
solid material can be stored or used immediately. Subsequently, the resulting
solid
material is hydrated in an aqueous solution containing an appropriate
concentration
of the therapeutic agent at an appropriate temperature, resulting in
multilamellar
vesicles (MLV). The solutions containing MLV can be size-reduced via
homogenization to form Small Unilameller Vesicles (SUVs) with the drug
passively
entrapped within the formed SUVs. The resulting liposome solution can be
separated from unencapsulated therapeutic agent, for example by chromatography
or filtration, and then filtered for use.
[00163] According to some embodiments, an anionic liposome may also be
used. According to some embodiments, an anionic liposome provides a Coulombic
character to the liposomes. According to some embodiments, anionic lipids can
help
stabilize the system upon storage, can prevent fusion or aggregation or
flocculation,
and can facilitate or enable freeze drying. Exemplary anionic lipids include,
without
limitation, phospholipids in the phosphatidic acid, phosphatidylglycerol, and
phosphatidylserine classes (PA, PG, and PS). Further examples include Cm or
larger fatty-acid chains. Further exemplary anionic phospholipid include,
without
limitation, Egg-PG (Egg Phosphatidyglycerol), Soy-PG (Soy-
Phosphatidylglycerol),
DSPG 20 (Distearoyl Phosphatidyglycerol), DPPG (Dipalmitoyl
Phosphatidyglycerol),
DEPG (Dielaidoyl Phosphatidyglycerol), DOPG (Dioleoyl Phosphatidyglycerol),
DSPA (Distearoyl Phosphatidic Acid), DPPA (Dipalmitoyl Phosphatidic Acid),
DEPA
(Dielaidoy Phosphatidic Acid), DOPA (Dioleoyl Phosphatidic Acid), DSPS
(Distearoyl
Phosphatidylserine), DPPS (Dipalmitoyl Phosphatidylserine), 25 DEPS (Dielaidoy
Phosphatidylserine), and DOPS (Dioleoyl Phosphatidylserine), or any mixtures
thereof.
[00164] According to some embodiments, a cationic liposome may be used.
Exemplary cationic lipids include, without limitation, stearylamine (SA),
Date Recue/Date Received 2022-04-07
lauryltrimethylammonium bromide; cetyltrimethylammonium bromide, myristyl
trimethylammonium bromide, dimethyldioctadecylammonium bromide (DDAB), 313-
[N-(N1',N1-dimethylaminoethane)-carbamoyl]cholesterol (DC-Cholesterol), 1,2-
ditetradecanoy1-3-trimethylammonium-propane (DMTAP), 1,2-dioctadecanoy1-3-
trimethylammonium-propane (DOTAP) and DOTAP derivatives such as 1,2-di-(9Z-
octadecenoy1)-3-trimethylammonium-propane and 1,2-dihexadecanoy1-3-
trimethylammonium-propane, 1,2-di-(9Z-octadecenoyI)-3-dimethylammonium-
propane (DODAP) and DODAP derivatives such as 1,2-ditetradecanoy1-3-
dimethylammonium-propane, 1,2-dihexadecanoy1-3-dimethylammonium-propane,
and 1,2-dioctadecanoy1-3-dimethylammonium-propane, 1,2-di-O-octadeceny1-3-
trimethylammonium propane (DOTMA), 1,2-dioleoyl-c-(4'-trimethylammonium)-
butanoyl-sn-glycerol (DOTB), dioctadecylamide-glycylspermine, SAINT-2,
polycationic lipid 2,3-dioleyloxy-N42(spermine-carboxamido)ethyll-N,N-dimethyl-
1-
propanaminiumtrifluoroacetate (DOSPA), and GL67TM. The cationic lipids may
also
constitute derivatives of the foregoing. Additional examples of cationic
lipids and
lipid components may be found in or made according to US 4,804,539 issued to
Guo
et al. According to some embodiments, the liposomes may contain about 10-40
(i.e.,
about 10, about 11, about 12, about 13, about 14, about 15, about 16, about
17,
about 18, about 19, about 20, about 21, about 22, about 23, about 24, about
25,
about 26, about 27, about 28, about 29, about 30, about 31, about 32, about
33,
about 34, about 35, about 36, about 37, about 38, about 39, or about 40) mole
percent of an amine-derivatized lipid component in which a charged amine group
is
spaced from a lipid polar head region by a carbon-containing spacer arm at
least 3
atoms in length. According to some embodiments, the liposomes have a close
packed lipid structure produced by inclusion of between 20-50 (i.e., about 20,
about
21, about 22, about 23, about 24, about 25, about 26, about 27, about 28,
about 29,
about 30, about 31, about 32, about 33, about 34, about 35, about 36, about
37,
about 38, about 39, about 40, about 41, about 42, about 43, about 44, about
45,
about 46, about 47, about 48, about 49, or about 50) mole percent of
cholesterol or
an amine-derivatized cholesterol, and/or phospholipids with predominantly
saturated
acyl chain moieties. According to some embodiments, the liposomes may be
suspended in an aqueous medium containing a high-viscosity polymer, formulated
in
paste form, or embedded in a polymer matrix, to further enhance liposome
retention.
41
Date Recue/Date Received 2022-04-07
[00165] Polymers can also be used for controlled or delayed type release
procedures (Langer, Accounts Chem. Res. 26:537, 1993). For example, the block
copolymer, polaxamer 407 exists as a viscous yet mobile liquid at low
temperatures
but forms a semisolid gel at body temperature. It has been shown to be an
effective
vehicle for formulation and sustained delivery of recombinant interleukin-2
and
urease (Johnston et al, Pharm. Res. 9:425, 1992; Pec, J. Parent. Set Tech.
44(2):58,
1990). Alternatively, hydroxyapatite has been used as a microcarrier for
controlled
release of proteins (ljntema et al, Int. J. Pharm. 112:215, 1994). Other
illustrative
and exemplary polymers utilized either alone, in combination, in association
with a
liposome, or as a polymersome, may include for example, Poly(ethylene glycol)
(PEG/PEO), Poly(2-methyloxazoline), Polydimethylsiloxane (PDMS),
Poly(caprolactone (PCL), Poly(lactide) (P LA), Poly(methyl methacrylate) (PM
MA),
povidone, cellulose acetate phthalate (CAP), hydroxypropyl methylcellulose
phthalate (HPMCP), polyvinyl acetate phthalate (PVAP), hydroxypropyl
methylcellulose acetate succinate (HPMCAS), cellulose acetate trimellitate,
hydroxypropyl methylcellulose succinate, cellulose acetate succinate,
cellulose
acetate hexahydrophthalate, cellulose propionate phthalate, copolymer of
methylmethacrylic acid and methyl methacrylate, copolymer of methyl acrylate,
methylmethacrylate and methacrylic acid, copolymer of methylvinyl ether and
maleic
anhydride (Gantrez ES series), ethyl methyacrylate-methylmethacrylate-
chlorotrimethylammonium ethyl acrylate copolymer, natural resins such as zein,
shellac and copal collophorium, and several commercially available enteric
dispersion systems (e.g., EUDRAGIT L30D55, EUDRAGITO FS30D, EUDRAG IT
L100, KOLLICOAT EMM30D, ESTACRYL 30D, COATERIC , and
AQUATERICO). The foregoing is not a comprehensive and exhaustive list, and
there are other polymeric materials that would meet the objectives of the
described
invention of providing for a controlled or delayed type release profile of the
active
therapeutic agent from the skin.
Delivery system containing pharmaceutical compositions
[00166] According to some embodiments, the therapeutic agent and/or
active
metabolite remains in the skin and does not enter the bloodstream.
42
Date Recue/Date Received 2022-04-07
[00167] According to some embodiments, the chemical drivers
methylsulfonyl methane (MSM), an amino benzoate local anesthetic, and
ethoxydiglycol work together cooperatively and synergistically to deliver the
active
therapeutic agent.
[00168] MSM (formula (CH3)2S02), also known as DMS02, methyl sulfone, and
dimethyl sulfone. CAS Registry Number 67-71-0) is an organosulfur compound,
and
as shown in Formula (I), is a polar molecule having two oxygen atoms that can
readily interact with positively charged atoms or molecules.
....00" 4.
H3C CH3
[00169] (I)
[00170] According to some embodiments, MSM can be administered in a
maximum daily dose of up to 6 g/day; aaccording to some embodiments MSM is
present as from 1-10 % w/w of the pharmaceutical composition, i.e., 1%, 2%,
3%,
4%, 5%, 6%, 7%, 8%, 9%, or 10% w/w of the pharmaceutical composition.
[00171] Research in animal models indicates MSM has a very low toxicity
when
administered topically (see Liu, P. et al., "Metal Chelator combined with
permeability
enhancer ameliorates oxidative stress-associated neurodegeneration in rat eyes
with
elevated intraocular pressure," Free Radic. Biol. Med. 69: 289-99 (2014)).
[00172] Zhang and coworkers determined that MSM functions as a
permeability
enhancer and an excipient to facilitate transport of the chelator EDTA
(Mw=292,24
g/mol) across biologic membranes, and to make possible localized and regional
chelation. Topical application of MSM with C14 EDTA onto the rat cornea led to
an
uptake of the C" EDTA in all tested ocular tissues. Without MSM, EDTA did not
penetrate the eye. Additionally, Zhang and co-workers suggested that MSM could
also be an adjuvant for delivering ciprofloxacin and other chemical compounds
to
43
Date Recue/Date Received 2022-04-07
specific, local tissue sites (See "Assessment of methylsulfonylmethane as a
permeability enhancer for regional EDTA chelation therapy"; Drug Delivery;
Vol. 16;
Pages 243-248, 2009).
0 0
\\Q//1
H3C CH3
[00173] (I)
[00174] Ethoxydiglycol (also known as diethylene glycol monoethyl ether
having
formula CH3CH2OCH2CH2OCH2CH2OH) 2-(2-ethoxyethyoxy)ethanol, CAS Registry
Number 111-90-0) is a low molecular weight cosmetic grade synthetic solvent
and
viscosity decreasing agent used in cosmetics and personal care products to
ensure
even distribution of the ingredients throughout a product. Glycols are a class
of
alcohols that contain two hydroxyl groups, and are also called a diols.
According to
some embodiments, ethoxydiglycol is present in a range of 0.10-5% w/w of the
pharmaceutical composition, i.e., 0.1%, 0.2%, 0.3%, 0.4%, 0.5%, 0.6%, 0.7%,
0.8%,
0.9%, 1.0%, 1.1%, 1.2%, 1.3%, 1.4%, 1.5%, 1.6%, 1.7%, 1.8%, 1.9%, 2.0%, 2.1%,
2.2%, 2.3%, 2.4%, 2.5%, 2.6%, 2.7%, 2.8%, 2.9%, 3.0%, 3.1%, 3.2%, 3.3%, 3.4%,
3.5%, 3.6%, 3.7%, 3.8%, 3.9%, 4.0%, 4.1%, 4.2%, 4.3%, 4.4%, 4.5%, 4.6%, 4.7%,
4.8%, 4.9% or 5% w/w of the pharmaceutical composition.
[00175] According to some embodiments, the molecular weight of the
therapeutic agent of the described invention is less than 500 Da.
[00176] The cumulative effect of these three components together is more
than
additive. As such, lower amounts of each component can be used than would
normally be used alone to deliver an active therapeutic agent. According to
some
embodiments, the chemical drivers enhance delivery of active therapeutic
agents
having a molecular weight of less than 500 Da. According to some embodiments,
44
Date Recue/Date Received 2022-04-07
the chemical drivers may also be able to enhance delivery of active
therapeutic
agents having a molecular weight higher than 500 Da.
[00177] According to some embodiments, the synergistic effect of the MSM,
amino benzoate local anesthetic, and ethoxydiglycol, is effective to provide
increased speed to anesthesia, and a reduction of the amino benzoate local
anesthetic and the therapeutic agent concentration because of improved
penetration
of the stratum corneum resulting in effective analgesia.
[00178] According to some embodiments, the amino benzoate local
anesthetic
blocks nerve signals where applied. According to some embodiments, the
chemical
drivers are effective to increase percutaneous perfusion wherein heat, pH and
the
polarity of the chemical drivers are factors that affect percutaneous
perfusion.
[00179] Exemplary amino benzoate local anesthetics include, without
limitation,
lidocaine (1-10%, Le., 1%, 2%, 3%, 4%, 5%, 6%, 7%, -%,
0/ 9%, or 10%) w/w of the
composition), benzocaine (5-20% (Le., 5%, 6%, 7%, , -%
0 / 9%, 10%, 11%, 12%, 13%,
14%, 15%, 16%, 17%, 18%, 19% or 20%) w/w of the composition), and tetracaine
(2% w/w of the composition). Alternatively, any other suitable local
anesthetic can
be used including, without limitation, ambucaine, amolanone, amylcaine,
benoxinate,
benzocaine, betoxycaine, biphenamine, bupivacaine, butacaine, butamben,
butanilicaine, butethamine, butoxycaine, carticaine, chloroprocaine,
cocaethylene,
cocaine, cyclomethycaine, dibucaine, dimethisoquin, dimethocaine, diperodon,
dyclonine, ecogonidine, ecogonine, euprocin, fenalcomine, formocaine,
hexylcaine,
hydroxyteteracaine, isobutyl p-aminobenzoate, leucinocaine, levoxadrol,
lidocaine,
mepivacaine, meprylcaine, metabutoxycaine, methyl chloride, myrtecaine,
naepaine,
octacaine, orthocaine, oxethazaine, parenthoxycaine, phenacaine, phenol,
piperocaine, piridocaine, polidocanol, pramoxine, prilocaine, procaine,
propanocaine,
proparacaine, propipocaine, propoxycaine, pseudococaine, pyrrocaine,
ropivacaine,
salicyl alcohol, tetracaine, tolycaine, trimecaine, zolamine, or a
pharmaceutically
acceptable salt thereof, or a mixture thereof. Amide type local anesthetics
are
characterized by an amide functionality, while ester type local anesthetics
contain an
ester functionality. Exemplary amide type local anesthetics include
bupivacaine,
prilocaine, mepivacaine, etidocaine, ropivacaine, dibucaine, and mixtures
thereof.
Date Recue/Date Received 2022-04-07
Exemplary ester type local anesthetics include procaine, chloroprocaine, their
pharmaceutically acceptable salt, or a mixture thereof.
[00180] According to some embodiments, the amino benzoate local
anesthetic
is lidocaine (or lidocaine HCl), also known as 2-(diethylamino)-/V-(2,6-
dimethylphenyl)acetamide shown in Formula (II).
Ali CH3
CH3
0
CH3
[00181] CH3 (II)
[00182] Lidocaine can be administered in amounts of 0.5 to 4.5 mg/kg/dose
(i.e.,
0.5, 1.0, 1.5, 2.0, 2.5, 3.0, 3.5, 4.0, or 4.5 mg/kg/dose). The lidocaine can
be in the
form of viscous lidocaine 2% w/w generally used to treat sore throat,
teething, mouth
or esophageal sores, or swelling inside the mouth. Viscous lidocaine can also
be
used to prevent gagging during dental procedures. Lidocaine spray 4% w/w can
be
used under "crash" circumstances, where speed is of the essence. Lidocaine
spray
is generally used when a breathing tube is inserted down the larynx during
intubation
to numb the gag reflex. Combined with the other components of the topical
composition for fast anesthesia, time to perform intubation can be decreased
where
even a few seconds reduced can save a life. Lidocaine spray can also be used
during childbirth. Lidocaine spray is commercially available as a 10% w/w
solution,
and the maximum dose per day is 30 mg within 30 minutes.
[00183] Other amino benzoate local anesthetics with similar dosing to
lidocaine
include tetracaine (2-(dimethylamino)ethyl 4-(butylamino)benzoate), shown in
Formula (III), and benzocaine (ethyl 4-aminobenzoate), shown in Formula (IV).
46
Date Recue/Date Received 2022-04-07
N
..,CH3
O. CH3
[00184] (III)
CH3
H2N
[00185] (IV)
[00186] Exemplary active therapeutic agents may include without
limitation,
analgesic agents, wound healing agents, anti-inflammatory agents (steroidal
and
non-steroidal); anti-oxidant agents; antihistamines or anti-neoplastics either
singly or
as a combination thereof.
[00187] According to some embodiments, the active agent of the topical
composition of the described invention can be an analgesic agent. Exemplary
analgesics may include the following molecules but not limited to non-
steroidal anti-
inflammatory drugs (NSAIDS), e.g., paracetamol (acetaminophen), ibuprofen,
naproxen, and, COX-2 inhibitors, opioids, flupirtine, and specific agents
including,
but not limited to tricyclic antidepressants, such as amitriptyline, nefopam,
and
anticonvulsants, including carbamazepine, gabapentin, and pregabalin.
[00188] According to some embodiments, the active agent of the topical
composition of the described invention is an antineoplastic agent. Exemplary
anti-
neoplastics may include, without limitation 5-fluorouracil, temozolomide,
busulfan,
ifosamide, melphalan, carmustine, lomustine, mesna, capecitabine, gemcitabine,
floxuridine, decitabine, mercaptopurine, pemetrexed disodium, methotrexate,
vincristine, vinblastine, vinorelbine tartrate, paclitaxel, docetaxel,
ixabepilone,
47
Date Recue/Date Received 2022-04-07
daunorubicin, epirubicin, doxorubicin, idarubicin, amrubicin, pirarubicin,
mitoxantrone, etoposide, etoposide phosphate, teniposide, mitomycin C,
actinomycin
D, colchicine, topotecan, irinotecan, gemcitabine cyclosporin, verapamil,
valspodor,
probenecid, biricodar, terfenadine, quinidine, and pervilleine A.
[00189] According to some embodiments, the active agent of the topical
composition of the described invention comprises an anti-inflammatory agent.
Non-
limiting examples of non-steroidal anti-inflammatory agents include, ibuprofen
(Advil ), naproxen sodium (Alevee), and acetaminophen (Tylenol ), oxicams,
such
as piroxicam, isoxicam, tenoxicam, sudoxicam, and CP-14,304; disalcid,
benorylate,
trilisate, safapryn, solprin, diflunisal, and fendosal; acetic acid
derivatives, such as
diclofenac, fenclofenac, indomethacin, sulindac, tolmetin, isoxepac,
furofenac,
tiopinac, zidometacin, acematacin, fentiazac, zomepirac, clindanac, oxepinac,
felbinac, and ketorolac; fenamates, such as mefenamic, meclofenamic,
flufenamic,
niflumic, and tolfenamic acids; propionic acid derivatives, such as
benoxaprofen,
flurbiprofen, ketoprofen, fenoprofen, fenbufen, indopropfen, pirprofen,
carprofen,
oxaprozin, pranoprofen, miroprofen, tioxaprofen, suprofen, alminoprofen, and
tiaprofenic; pyrazoles, such as phenylbutazone, oxyphenbutazone, feprazone,
azapropazone, and trimethazone. Mixtures of these non-steroidal anti-
inflammatory
agents also may be employed, as well as the dermatologically acceptable salts
and
esters of these agents. For example, etofenamate, a flufenamic acid
derivative, can
be used for topical application.
[00190] Non-limiting examples of steroidal anti-inflammatory agents
include
corticosteroids such as hydrocortisone, hydroxyltriamcinolone, alpha-methyl
dexamethasone, dexamethasone-phosphate, beclomethasone dipropionates,
clobetasol valerate, desonide, desoxymethasone, desoxycorticosterone acetate,
dexamethasone, dichlorisone, diflucortolone valerate, fluadrenolone,
fluclorolone
acetonide, flumethasone pivalate, fluosinolone acetonide, fluocinonide,
flucortine
butylesters, fluocortolone, fluprednidene (fluprednylidene) acetate,
flurandrenolone,
halcinonide, hydrocortisone acetate, hydrocortisone butyrate,
methylprednisolone,
triamcinolone acetonide, cortisone, cortodoxone, flucetonide, fludrocortisone,
difluorosone diacetate, fluradrenolone, fludrocortisone, diflorosone
diacetate,
fluradrenolone acetonide, medrysone, amcinafel, amcinafide, betamethasone and
48
Date Recue/Date Received 2022-04-07
the balance of its esters, chloroprednisone, chlorprednisone acetate,
clocortelone,
clescinolone, dichlorisone, diflurprednate, flucloronide, flunisolide,
fluoromethalone,
fluperolone, fluprednisolone, hydrocortisone valerate, hydrocortisone
cyclopentylpropionate, hydrocortamate, meprednisone, paramethasone,
prednisolone, prednisone, beclomethasone dipropionate, triamcinolone, and
mixtures thereof.
[00191] According to some embodiments, the active agent of the topical
composition of the described invention comprises an anti-oxidant agent.
Exemplary
anti-oxidants may include ascorbic acid (vitamin C) and its salts, ascorbyl
esters of
fatty acids, ascorbic acid derivatives (e.g., magnesium ascorbyl phosphate,
sodium
ascorbyl phosphate, ascorbyl sorbate), tocopherol (vitamin E), tocopherol
sorbate,
tocopherol acetate, other esters of tocopherol, butylated hydroxy benzoic
acids and
their salts, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid
(commercially
available under the tradename TroloxR), gallic acid and its alkyl esters,
especially
propyl gallate, uric acid and its salts and alkyl esters, sorbic acid and its
salts, lipoic
acid, amines (e.g., N,N-diethylhydroxylamine, amino-guanidine), sulfhydryl
compounds (e.g., glutathione), dihydroxy fumaric acid and its salts, glycine
pidolate,
arginine pilolate, nordihydroguaiaretic acid, bioflavonoids, curcumin, lysine,
methionine, proline, superoxide dismutase, silymarin, tea extracts, grape
skin/seed
extracts, melanin, and rosemary extracts.
[00192] According to some embodiments, the antioxidant may be alpha
tocopherol (Vitamin-E), ascorbic acid, ascorbic acid esters, glutathione,
lipoic acid,
uric acid, carotenes, propyl gallate, sodium bisulfite, sodium sulfite,
butylated
hydroxytoluene (BHT), butylated hydroxyanisole (BHA), or cysteine.
[00193] According to some embodiments, the active agent of the topical
composition of the described invention comprises an antihistamine. Non-
limiting
examples of antihistamines include, without limitation, chlorpheniramine,
brompheniramine, dexchlorpheniramine, tripolidine, clemastine,
diphenhydramine,
promethazine, piperazines, piperidines, astemizole, loratadine and
terfenadine.
[00194] According to some embodiments, the topical composition of the
described invention further contains a topical vasoconstrictor as an
additional active
49
Date Recue/Date Received 2022-04-07
agent. Non-limiting examples of topical vasoconstrictors include, for example,
oxymetazoline, isoproterenol, phenylephrine, norepinephrine, ephedrine,
epinephrine, dobutamine, droxidopa, vasopressin, pseudoephedrine. According to
some embodiments, the vasoconstrictor is not a substance that causes a
dermatitis
or other irritation, e.g., epinephrine, synephrine, or ephedrine.
[00195] According to some embodiments, the topical composition is
characterized by controlled release or delayed release of locally sustained
levels of a
minimum effective concentration (MEC) of the active agent.
[00196] The intensity of effect of a drug (y-axis) can be plotted as a
function of
the dose of drug administered (X-axis). Goodman & Gilman's The Pharmacological
Basis of Therapeutics, Ed. Joel G. Hardman, Lee E. Limbird, Eds., 10th Ed.,
McGraw
Hill, New York (2001), p. 25, 50). These plots are referred to as dose-effect
curves.
Such a curve can be resolved into simpler curves for each of its components.
These
concentration-effect relationships can be viewed as having four characteristic
variables: potency, slope, maximal efficacy, and individual variation.
[00197] The location of the dose-effect curve along the concentration
axis is an
expression of the potency of a drug. Id. If the active therapeutic agent is to
be
administered by transdermal absorption, a highly potent active therapeutic
agent is
required, since the capacity of the skin to absorb active therapeutic agents
is limited.
[00198] The slope of the dose-effect curve reflects the mechanism of
action of a
drug. The steepness of the curve dictates the range of doses useful for
achieving a
clinical effect.
[00199] Maximal or clinical efficacy refers to the maximal effect that
can be
produced by a drug. Maximal efficacy is determined principally by the
properties of
the drug and its receptor-effector system and is reflected in the plateau of
the curve.
In clinical use, a drug's dosage may be limited by undesired effects.
[00200] Biological variability may exist. An effect of varying intensity
may occur
in different individuals or subjects at a specified concentration or a drug.
It follows
that a range of concentrations may be required to produce an effect of
specified
intensity in all subjects.
Date Recue/Date Received 2022-04-07
[00201] Lastly, different individuals may vary in the magnitude of their
response
to the same concentration of a drug when the appropriate correction has been
made
for differences in potency, maximal efficacy and slope.
[00202] The duration of a drug's action is determined by the time period
over
which concentrations exceed the MEC. Following administration of a dose of
drug,
its effects usually show a characteristic temporal pattern. A plot of drug
effect vs.
time illustrates the temporal characteristics of drug effect and its
relationship to the
therapeutic window. A lag period is present before the drug concentration
exceeds
the minimum effective concentration (MEC) for the desired effect. Following
onset of
the response, the intensity of the effect increases as the drug continues to
be
absorbed and distributed. This reaches a peak, after which drug elimination
results
in a decline in the effect's intensity that disappears when the drug
concentration falls
back below the MEC. The therapeutic window reflects a concentration range that
provides efficacy without unacceptable toxicity. Accordingly another dose of
drug
should be given to maintain concentrations within the therapeutic window.
[00203] According to some embodiments, the potency of the active
therapeutic
agent in the claimed pharmaceutical composition is maintained within a range
of
from 3 to 5% w/w of the composition i.e., at least 2% w/w of the composition
when
the local anesthetic is lidocaine; from 10 to 20% (10%, 11%, 12%, 13%, 14%,
15%,
16%, 17%, 18%, 19%, or 20%) w/w of the composition i.e., at least 5% w/w of
the
composition when the local anesthetic is benzocaine and from 1 to 2 A (i.e.,
1.0%,
1.1%, 1.2%, 1.3%, 1.4%, 1.5%, 1.6%, 1.7%, 1.8%, 1.9% or 2.0%) w/w of the
composition i.e., at least 1% w/w of the composition when the local anesthetic
is
tetracaine.
[00204] According to some embodiments, the concentration of the active
therapeutic agent is at least 1% w/w of the composition, at least 2% w/w of
the
composition, at least 3% w/w of the composition, at least 4% w/w of the
composition,
at least 5% w/w of the composition, at least 6% w/w of the composition, at
least 7%
w/w of the composition, at least 8% w/w of the composition, at least 9% w/w of
the
composition, at least 10% w/w of the composition; at least 11% w/w of the
composition; at least 12% w/w of the composition; at least 13% w/w of the
composition; at least 14% w/w of the composition; at least 15% w/w of the
51
Date Recue/Date Received 2022-04-07
composition; at least 16% w/w of the composition; at least 17% w/w of the
composition; at least 18% w/w of the composition; at least 19% w/w of the
composition; at least 20% w/w of the composition, at least 30% w/w of the
composition, at least 40% w/w of the composition, at least 50% w/w of the
composition, or at least 60% w/w of the composition. According to some
embodiments, the concentration of the active agent is from about 1% to about
10%
w/w of the composition, i.e., at least 1%, at least 2%, at least 3%, at least
4%, at
least 5%, at least 6%, at least 7%, at least 8%, at least 9%, or 10% w/w of
the
composition when the local anesthetic is lidocaine. According to some
embodiments, the concentration of the active agent is from about 5% to about
20%
w/w of the composition, i.e., at least 5%, at least 6%, at least 7%, at least
8%, at
least 9%, at least 10%, at least 11%, at least 12%, at least 13%, at least
14%, at
least 15%, at least 16%, at least 17%, at least 18%, at least 19% or 20% w/w
of the
composition when the local anesthetic is benzocaine. According to some
embodiments, the concentration of the active agent is from about 1% to about
2%
w/w of the composition, i.e. at least 1%, or 2% w/w of the composition when
the local
anesthetic is tetracaine.
[00205] According to some embodiments, the content of the active agent
retained on skin and its permeation/flux into the skin can be measured as a
function
of time. According to some embodiments, flux is determined using one of many
available artificial membranes attached to a Franz diffusion cell. According
to some
embodiments, permeation and retention are determined using human cadaver skin
attached to a Franz diffusion cell. According to some embodiments, the
retained
concentration is correlated to the minimum effective concentration.
[00206] According to some embodiments, the pharmaceutical composition can
be applied directly to the skin.
[00207] The pharmaceutical composition may further include auxiliary
agents,
e.g., lubricants, preservatives, stabilizers, wetting agents, emulsifiers,
salts for
influencing osmotic pressure, buffers, colorants, flavorants and/or fragrances
and the
like which are compatible with the active compounds, carriers, excipients,
flocculants, penetration enhancers, plasticizers, pH balancers, moisturizers,
52
Date Recue/Date Received 2022-04-07
emollients, surfactants and emulsifiers, bactericides, thickening agents,
softening
agents, etc.
[00208] The composition can also include agents that assist in
maintaining the
molecular structure integrity of the therapeutic or help deliver the
therapeutic agent
through the skin, such as but not limited to solvents that break down
lipophilic
therapeutics or adjust ionic charge for easier delivery into skin, detergents
such as
but not limited to anionic detergents (e.g., alkylbenzenesulfonates), cationic
detergents, non-ionic detergents (e.g., ethoxylates, PEGylates), or
zwitterionic
detergents (3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate)),
cyclodextrins that readily complex with lipophilic therapeutics like steroids,
including
cc-cyclodextrin, 8-cyclodextrin, and y-cyclodextrin, other complexing agents
(i.e.
chelating agents with two or more separate coordinate bonds between a multiple
bonded ligand and a central atom (metal), such as, but not limited to,
glutamic acid,
histidine, malate, phytochelatin, hemoglobin, chlorophyll,
ethylenediaminetetraacetic
acid (EDTA), amino acid chelates, and dimercaprol), and other amphipathic
chemicals.
[00209] Exemplary plasticizers include, without limitation, phthalic
anhydride
esters, esters of adipic acid, epoxidized esters, trimellitic esters,
triacetin, N-methy1-
2-pyrrolidone, glycerol formaldehyde, triethyl citrate (T EC),
acetyltributylcitrate,
ethanol, and polyethylene glycol.
[00210] Non-limiting examples of penetration enhancers include propylene
glycol (PG), dimethylsulfoxide (DMSO), dimethyl formamide (DMF), allantoin,
urazole, N,N-dimethylacetamide (DMA), decylmethylsulfoxide (C10 MSO),
polyethylene glycol monolaurate (PEGML), propylene glycol monolaurate (PGML),
glycerol monolaurate (GM L), lecithin, the 1-substituted azacycloheptan-2-
ones, e.g.,
1-n-dodecylcyclazacycloheptan-2-one (available under the trademark Azone from
Whitby Research Incorporated, Richmond, Va.), alcohols, and the like. The
penetration enhancer may also be a vegetable oil, for example, safflower oil,
cottonseed oil and corn oil. Additional penetration enhancers may generally be
found in Remington's Pharmaceutical Sciences, 18th or 19th editions, published
by
the Mack Publishing Company of Easton, Pennsylvania.
53
Date Recue/Date Received 2022-04-07
[00211] Exemplary anti-oxidants may include the following, but not
limited to,
ascorbic acid and glutathione (GSH) etc. According to some embodiments, the
antioxidant agent is present at a concentration from 10%-20% (i.e., 10%, 11%,
12%,
13%, 14%, 15%, 16%, 17%, 18%, 19% or 20%) w/w of the composition for ascorbic
acid and from 2%-5% (i.e., 2%, 2.1%, 2,2%, 2.3%, 2.4%, 2.5%, 2.6%, 2.7% 2.8%,
2.9%, 3%, 3.1%, 3.2%, 3.3%, 3.4%, 3.5%, 3.6%, 3.7%, 3.8%, 3.9%, 4%, 4.1%,
4,2%, 4.3%, 4.4%, 4.5%, 4.6%, 4.7%, 4.8%, 4.9%, or 5%) for glutathione.
[00212] Surfactants are organic compounds that are amphiphatic,
containing
both hydrophobic groups and hydrophilic groups. Surfactants include, but are
not
limited to anionic surfactants, cationic surfactants and non-ionic
surfactants.
[00213] Anionic surfactants include fatty acid soaps (including sodium
oleate,
sodium palmitate, sodium myristate, sodium sterate, potassium oleate and
triethanolamine oleate); alkyl sulfates (including sodium dodecyl sulfate,
ammonium
lauryl sulfate, triethanolamine lauryl sulfate and sodium alkyl sulfate);
alkyl lactylates
(including calcium stearoxy1-2-lactylate), alkyl lactates (including sodium-0-
stearyllactate and sodium stearoyllactylate) alkyl benzenesulfonates
(including
calcium dodecyl benzene sulfonate); alkyl sulfonates (including alkyl aryl
sulfonate);
alkyl phosphates; alkyl oleates; alkyl stearates (including self-emulsifying
glycerol
monostearate); alkyl esters (including dioctyl ester of sodium sulphosuccininc
acid
(AOT, Aerosol OT); acyl sulfates; or acyl sulfosuccinates.
[00214] Cationic surfactants include alkyl primary, secondary, tertiary,
or
quaternary amines; high-molecular-weight amine and fatty amine blends;
polyoxyethylene fatty amines (including tallow amine); alkyl sulfates
(including N-
cetyl-N-ethyl morpholinium ethyl sulfate(35 /0)); alkyl pyridinium and
quaternary
ammonium salts.
[00215] Non-ionic surfactants include alcohol ethoxylate, alkylphenol
ethoxylate,
fatty acids (such as oleic acid), lanolin alcohols (such as polyoxyethylene
(5) lanolin
alcohol (ether and ester), polyoxyethylene (50) lanolin (ether and ester),
acetylated
polyoxyethylene (10) lanolin, polyoxyethylene (16) lanolin alcohol, acetylated
polyoxyethylene (9) lanolin), alkyl polyglycosides, mono-, di- or glyceride
esters
(such as diglycerine sesquioleate), acetylated monoglycerides, polyglycerols,
54
Date Recue/Date Received 2022-04-07
polyglycerol esters (such as decaglycerol decaoleate, decaglycerol octaoleate,
decaglycerol tetraoleate), phospholipids (such as lecithin), mono- or
diglyceride
esters of citric acid, tartaric acid and lactic acid, sorbitan fatty acid
esters (such as
sorbitan monostearate (Span 60, Crill 3), sorbitan monooleate (Arlacel 80,
Span 80,
Crill 4), sorbitan isosterate (Grill 6), sorbitan monolaurate (Arlacel 20,
Span 20, Crill
1), sorbitan trioleate (Span 85, Crill 45), sorbitan tristearate (Span 65),
sorbitan
sesquioleate (Arlacel 83, Crill 43), sorbitan monopalmitate (Span 40, Crill
2)), polyol
fatty acid esters (such as ethylene glycol distearate, ethylene glycol
monostearate,
diethylene glycol monostearate, propylene glycol monostearate, propylene
glycol
monolaurate, polyoxyethylene (1.5) nonylphenol, polyoxyethylene (4)
nonylphenol,
polyoxyethylene (5) nonylphenol, polyoxyethylene (6) nonylphenol,
polyoxyethylene
(8) nonylphenol, polyoxyethylene (20) nonylphenol, polyoxyethylene (30)
nonylphenol, polyoxyethylene (10) nonylphenol, poly(ethylene glycol) 200
distearate,
poly(ethylene glycol) 300 dilaurate, poly(ethylene glycol) 400 distearate,
polyoxyethylene octylphenol, poly(ethylene glycol) 400 dilaurate,
poly(ethylene
glycol) 400 monostearate, poly(ethylene glycol) 400 monolaurate, poly(ethylene
glycol) 4000 distearate, polyoxyethylene (10) octylphenol, poly(ethylene
glycol) 600
monostearate, Polyoxyethylene (14) nonylphenol, polyoxyethylene (24)
cholesterol,
polyoxyethylene (25) soyasterol, poly(ethylene glycol) 1000 monooleate,
polyoxyethylene (25) propylene glycol monostearate, poly(ethylene glycol) 1000
monolaurate, polyoxyethylene (70) dinonylphenol), glycerol fatty acid esters
(such as
glycerol dioleate, glycerol monoleate, glycerol monostearate, glycerol
monolaurate,
polyoxyethylene (20) glycerol monostearate), sucrose fatty acid esters (such
as
sucrose distearate, sucrose monolaurate), polyoxyethylene sorbitan fatty acid
esters
(polysorbates) (such as polyoxyethylene (4) sorbitan monolaurate,
polyoxyethylene
(5) sorbitan monooleate, polyoxyethylene (20) sorbitan monooleate (Tween 80),
polyoxyethylene (40) sorbitol hexaoleate, polyoxyethylene (50) sorbitol
hexaoleate,
polyoxyethylene (20) sorbitan tristearate, polyoxyethylene (20) sorbitan
trioleate,
polyoxyethylene (20) sorbitan monostearate, polyoxyethylene (20) sorbitan
monopalmitate, polyoxyethylene (20) sorbitan monolaurate (Tween 20),
polysorbate
20 NF, EP, JP, poly(ethylene glycol)-20 sorbitan isostearate, poly(ethylene
glycol)
(20) sorbitan trioleate (Crillet 45), poly(ethylene glycol) (20) sorbitan
stearate (Crillet
3 Super, Polysorbate 60), poly(ethylene glycol) (20) sorbitan oleate (Crillet
4 Super,
Polysorbate 80), poly(ethylene glycol) (20) sorbitan laurate (Crillet 2 Super,
Date Recue/Date Received 2022-04-07
Polysorbate 40)), monoesters (such as polyoxyethylene (4) stearic acid,
polyoxyethylene (8) stearic acid, polyoxyethylene (8) lauric acid,
polyoxyethylene
(40) stearic acid, polyoxyethylene (50) stearic acid), polyethoxylated esters
of acyl
acids (such as polyoxyethylene (2) octyl alcohol, polyoxyethylene (4) tridecyl
alcohol,
polyoxyethylene (6) tridecyl alcohol, polyoxyethylene (8) tridecyl alcohol),
copolymers of polyethylene oxide and polypropylene oxide, polyoxyethylene
fatty
ethers (such as polyoxyethylene fatty ethers derived from lauryl, cetyl,
stearyl and
oleyl alcohols, polyoxyethylene (4) lauryl ether, polyoxyethylene (23) lauryl
ether (Brij
35), polyoxyethylene (2) cetyl ether (Brij 52), polyoxyethylene (10) cetyl
ether,
polyoxyethylene (20) cetyl ether (Brij 58), polyoxyethylene (2) stearyl ether
(Brij 72),
polyoxyethylene (10) stearyl ether, polyoxyethylene (20) stearyl ether,
polyoxyethylene (2) oleyl ether, polyoxyethylene (10) oleyl ether (Brij 97),
polyoxyethylene (20) oleyl ether, polyoxyethylene (21) stearyl ether,
polyoxyethylene
(12) lauryl ether), fatty amides (such as N,N,-Dimethylstearamide),
Polyethylene
glycol ether of linear alcohol, polyoxyethylene (15) tall oil fatty acids
(ester),
acetylated sucrose diesters, isopropyl ester of lanolin fatty acids,
polyoxyethylene
sorbitol beeswax derivative, Polyoxypropylene/Polyoxyethylene condensate,
sodium
oleate, polyoxyethylene (20) castor oil (ether, ester), glycerol oleate &
propylene
glycol (Arlacel 186) and Cremophor.
[00216] Exemplary pharmaceutical carriers also include starch, glucose,
lactose,
sucrose, gelatin, saline, gum acacia, keratin, urea, malt, rice flour, chalk,
silica gel,
sodium stearate, glycerol monostearate, talc, sodium chloride, dried skim
milk,
glycerol, propylene, glycol, water, and ethanol. If desired, the carrier can
also
contain minor amounts of wetting or emulsifying agents, or pH buffering
agents.
[00217] Formulations for topical application can take the compositional
form of a
liquid, a semisolid dosage form (e.g., a paste, a cream, a lotion, a powder,
an
ointment or a gel), a patch or a spray. According to some embodiments, the
topical
composition may be a cream or gel that can be applied to an affected area of
the
skin of a subject in need thereof. Different release profiles can be achieved
with
different forms, such as but not limited to controlled release, delayed
release,
extended release, or sustained release. The topical pharmaceutical composition
may be applied multiple times a day, once per day, or as often as needed.
56
Date Recue/Date Received 2022-04-07
[00218] According to some embodiments, an exemplary pharmaceutical cream
formulation may include: Lidocaine (local anesthetic agent), MSM (chemical
driver),
ethoxydiglycol (chemical driver), deionized water, polyacrylamide (a
flocculant), C13-
14 isoparaffin (an emollient), laureth-7 (surfactant and emulsifier),
propylene glycol
(penetration enhancer), triethanol amine (pH balancer), emu oil (antifungal
agent),
tea tree oil (antifungal agent), arnica Montana extract (anti-inflammatory
agent);
ethylhexylglycerin (fragrant), phenoxyethanol (bactericide), isopropyl
palmitate
(emollient, moisturizer, thickening agent, anti-static), stearic acid
(surfactant and
softening agent), 5-fluorouracil (anti-neoplastic). Any suitable excipients in
these
categories also can be used in accordance with the embodiments of the
described
invention.
[00219] Lidocaine is a widely used local anesthetic that was first
synthesized by
Lofgren in 1943 (Lofgren N, Lundqvist B (1946). Svensk Kemisk Tidskrift 58:206-
17). Its IUPAC name is: 2-(diethylamino)-N-(2,6-dimethylphenyl) acetamide, and
its
CAS number is: 137-58-6 / 73-78-9 (hydrochloride). Lidocaine is used as a
topical
pain reliever/numbing agent in both prescription and over the counter (OTC)
forms
(Drug Bank. (2013, February 08). Lidocaine. http:// drugbank.ca/drugs/DB00281,
accessed 28 Aug 2013), may be used as an injected local anesthetic during
various
surgical procedures, and also is used intravenously in certain circumstances,
such
as in cardiac arrest. Lidocaine also is a first line anti-arrhythmic drug when
used at
high doses (Sleight PJ (1990). Cardiovasc Pharmacol 16: S113-119);
(Collinsworth,
K. Circulation 50: 1217-30 (1974). In addition, lidocaine is often the local
anesthetic
of choice during intubation, minimally invasive surgery, and many dental
procedures
(Mehta P, Caiazzo A, Maloney P (1998). Anesth Prog 45: 38-41).
[00220] The effectiveness of lidocaine as a local anesthetic is
distinguished by
its accelerated onset of action and intermediate duration. As a result,
lidocaine is
suitable for infiltration, block and surface anesthesia (Alabdalla J, Hoffart
L.
Lidocaine. http://www.lidocaine.weebly.com/references.htm, accessed 27 Aug
2013). While lidocaine's mechanism of action is favorable for multiple modes
of
anesthesia, the ability of a formulation to allow adequate dermal penetration
has
limited the utility of topical lidocaine treatments. The study of cutaneous
barriers to
topical absorption suggests that hydrophobicity has little impact on the
ability of a
57
Date Recue/Date Received 2022-04-07
topically applied drug to reach interstitial fluid (Fortenbach CR, Modjtahedi
BS,
Maibach HI (2008). Skin Pharmacol Physiol 21: 294-299); (Hansen S, Lehr CM,
Schaefer UF (2013). Adv Drug Deliv Rev 65: 251-264). However, it has also been
noted that greater lipid solubility results in increased diffusion through
cell
membranes, and thereby slowing the onset of anesthesia (in the case of
anesthetics)
(Becker DE, Reed KL (2012). Anesth Prog 59: 90-102). In the case of drugs
bearing charged groups, as with the tertiary amines of lidocaine and related
substances, transfer efficiency into circulation is related to the pKa of the
charged
group, where a pKa of 7.4 or slightly below providing greater entry into
neuronal cell
membranes and thus greater anesthetic efficiency. Other studies have indicated
that
dosing with other agents such as epinephrine, can increase the concentration
of
lidocaine in the brain (Takahashi R, Oda Y, Tanaka K, Morishima HO, Inoue K,
Asada A (2006). Anesthesiol 105: 984-989). Because there appears to be a
strong
linear correlation between the concentration of a drug in serum and in
interstitial
fluid, the ability to provide efficient transdermal drug delivery has
significant clinical
implications (Jepps OG, Dancik Y, Anissimov YG, Roberts MS (2013). Adv Drug
Deliv Rev 65: 152-168). Indeed, several groups have reported on formulations
intended to enhance topical drug delivery (Lee PJ, Ahmad N, Langer R,
Mitragotri S,
Shastri VP (2006). Intl J Pharmaceut 308: 33-39); (Roberts MS, Cross SE
(1999).
Inflammopharmacol 7:339-50); (Osborne DW (2011). J Cosmet Dermatol 10: 324-
9); (Otto A, Wiechers JW, Kelly CL, Hadgraft J, du Plessis J (2008). Skin
Pharmacol
Physiol 21: 326-334). To take advantage of the potential benefits from topical
drug
application, a unique, proprietary formulation of lidocaine has been developed
by
Sambria Pharmaceutical, focusing on agents that act as the "drivers" of
cutaneous
penetration. Our studies suggest that this formulation for lidocaine provides
excellent results in providing anesthetic effects for local, acute pain.
[00221] Along with providing direct delivery to interstitial fluid, there
are a
number of advantages and disadvantages to using a topical pain relief cream.
Advantages include, but are not limited to, avoidance of hepatic first-pass
metabolism, convenience and ease of application, and the ability to target a
specific
site of pain. Disadvantages may include skin irritation, and also may include
poor or
variable permeability through the skin, which can result in insufficient
therapeutic
58
Date Recue/Date Received 2022-04-07
effect for the patient (Moody ML (2010). Topical Medications in the Treatment
of
Pain. New York City: McMahon Publishing).
[00222] As shown in Table 1, the amount of used deionized water would
then
accordingly be chosen in the pharmaceutical formulation such that the final
amount
w/w % will be equal to 100%:
[00223] Table 1: Exemplary cream formulation.
Specific Ingredient Amount (w/w %) Range (w/w %)
Deionized water Can be varied 1-50%
Lidocaine 4.00% 1-20%
MSM 3.00% 1-10%
Ethoxydiglycol 1.00% 0.10-5%
Polyacrylamide 6.50% 1-20%
C13-14 isoparaffin 6.50% 1-20%
Laureth-7 6.50% 1-20%
Propylene Glycol 1.00% 0.10-5%
Triethanolamine 0.90% 0.10-5%
Emu Oil 0.25% 0.10-5%
Tea Tree Oil 0.20% 0.10-5%
Arnica Montana Extract 0.50% 0.10-5%
Ethylhexylglycerin 0.40% 0.10-5%
Phenoxyethanol 0.40% 0.10-5%
Isopropyl PaImitate 0.20% 0.10-5%
59
Date Recue/Date Received 2022-04-07
Specific Ingredient Amount (w/w %) Range (w/w %)
Stearic Acid 0.15% 0.05%-5%
5-fluorouracil 1.00% 1.00%-5%
[00224] According to some embodiments, formulations and doses can be
tailored to a subject's fat content, as some therapeutic can be lost to the
fat layer
(the rate and extent of the diffusion of the therapeutic and amino benzoate
local
anesthetic can vary).
[00225] The pharmaceutical composition of the described invention is
administered and dosed in accordance with Good Medical Practice's (GMP's) and
guidelines provided and approved by the Food and Drug Administration (FDA),
taking into account the clinical condition of the individual subject, the site
and method
of administration, scheduling of administration, patient age, sex, body
weight,
whether or not the subject is on other medication and other factors known to
medical
practitioners. The pharmaceutically "effective amount" for purposes herein is
thus
determined by such considerations as are known in the art. The amount must be
effective to achieve improvement including but not limited to improved
survival rate
or more rapid recovery, or improvement or elimination of symptoms and other
indicators as are selected as appropriate measures by those having ordinary
skill in
the art.
Use of the disclosed compositions
[00226] According to some embodiments, the topical delivery system of the
described invention can be used in the manufacture of a medicament for
treating a
plurality of skin conditions, disorders or diseases. Non-limiting examples of
diseases
or disorders that can be treated with the pharmaceutical composition of the
described invention include, without limitation, pruritus, atopic dermatitis,
psoriasis,
acne, skin infections, skin infestations, skin neoplasms, wounds to the skin,
pain
causing disorders and skin manifestations of autoimmune disorders or uses for
Date Recue/Date Received 2022-04-07
anesthesia prior to procedures including, but not limited to, for example
superficial
dermal instrumentation.
[00227] According to another aspect, the described invention provides a
method
for treating a disease, disorder or condition susceptible to treatment
topically
comprising administering the topical composition described herein to skin.
[00228] According some embodiments, the pharmaceutical composition of the
described invention can be administered as the pharmaceutical formulation
alone, or
as an active ingredient in combination with pharmaceutically acceptable
carriers,
diluents, adjuvants and other auxiliary vehicles. According to some
embodiments,
the subject is for example, a warm-blooded animal, for example a mammal,
including
man. The pharmaceutically acceptable carriers, diluents, adjuvants and
vehicles, as
well as implant carriers generally refer to inert, non-toxic solid or liquid
fillers, diluents
or encapsulating material not reacting with the active ingredients of the
invention.
[00229] The doses given may be as a single dose, or as multiple doses
over a
predetermined period stretching a plurality of days, months or years. As used
herein
the term "plurality" refers to an event characterized by more than one.
According to
some embodiments, the pharmaceutical composition is administered multiple
times
at a plurality of treatment dates, or as needed in the judgment of a treating
physician.
[00230] According to some embodiments, treatment can be continuous or
discontinuous. As used herein, the term "continuous" refers to an activity
that is
unbroken and without interruption. As used herein, the term "discontinuous"
refers to
an activity that is broken and with interruption for a predetermined amount of
time as
judged by the treating physician. As such, the treatment may advantageously be
conducted continuously over a period of days, months, or years or
discontinuously
over a period of days, months, or years.
[00231] Where a range of values is provided, it is understood that each
intervening value, to the tenth of the unit of the lower limit unless the
context clearly
dictates otherwise, between the upper and lower limit of that range and any
other
stated or intervening value in that stated range is encompassed within the
invention.
The upper and lower limits of these smaller ranges which can independently be
included in the smaller ranges is also encompassed within the invention,
subject to
61
Date Recue/Date Received 2022-04-07
any specifically excluded limit in the stated range. Where the stated range
includes
one or both of the limits, ranges excluding either both of those included
limits are
also included in the invention.
[00232] Unless defined otherwise, all technical and scientific terms used
herein
have the same meaning as commonly understood by one of ordinary skill in the
art
to which this invention belongs. Although exemplary methods and materials have
been described, any methods and materials similar or equivalent to those
described
herein can also be used in the practice or testing of the described invention.
[00233] As used herein and in the appended claims, the singular form "a,"
"and,"
"the" include plural referents unless the context clearly dictates otherwise.
The terms
"comprises," "comprising," "includes," "including," "having" and their
conjugates mean
"including but not limited to." Terms and phrases used in this application,
and
variations thereof, unless otherwise expressly stated, should be construed as
open
ended as opposed to limiting. As examples of the foregoing, the term,
"including"
should be read as meaning "including, without limitation" or the like. The
term,
"example" is used to provide exemplary instances of the item in discussion,
not an
exhaustive or limiting list thereof. Adjectives such as e.g., "conventional,"
"traditional," "known" and terms of similar meaning should not be construed as
limiting the item described to a given time period, or to an item available as
of a
given time, but, instead these terms should be read to encompass conventional,
traditional, normal, or standard technologies that may be available, known
now, or at
any time in the future. Likewise, a group of items linked with the conjunction
"and"
should not be read as requiring that each and every one of those items be
present in
the grouping, but rather should be read as "and/or" unless expressly stated
otherwise. Similarly, a group of items linked with the conjunction "or" should
not be
read as requiring mutual exclusivity among that group, but rather should also
be read
as "and/or" unless expressly stated otherwise. The presence of broadening
words
and phrases such as "one or more," "at least," "such as but not limited to,"
or other
like phrases in some instances shall not be read to mean that the narrower
case is
intended or required in instances, wherein such broadening phrases may be
absent.
[00234] Additionally, for example any sequence(s) and/or temporal order
of
sequence of the system and method that are described herein this disclosure
are
62
Date Recue/Date Received 2022-04-07
illustrative and should not be interpreted as being restrictive in nature.
Accordingly, it
should be understood that the process steps may be shown and described as
being
in a sequence or temporal order, but they are not necessarily limited to being
carried
out in any particular sequence or order.
[00235] Although the described invention has been described and
illustrated
herein with referred to some embodiments, it will be apparent to those of
ordinary
skill in the art that other embodiments may perform similar functions and/or
achieve
like results. Thus, it should be understood that various features and aspects
of the
disclosed of the disclosed embodiments can be combined with, or substituted
for one
another in order to form varying modes of the disclosed invention. Many
different
embodiments such as variations, adaptations, modifications, and equivalent
arrangements thus fall within the scope and spirit of the described invention.
Although a specific composition has been described, broader invention that
would
include some elements are also contemplated herein this disclosure.
[00236] The publications discussed herein are provided solely for their
disclosure prior to the filing date of the described invention. Nothing herein
should
be construed as an admission that the described invention is not entitled to
antedate
such publication by virtue of prior invention. Further, the dates of
publication
provided may be different from the actual publication dates, which may be
independently confirmed.
EXAMPLES
[00237] The following examples are put forth so as to provide those of
ordinary
skill in the art with a complete disclosure and description of how to make and
use the
described invention, and are not intended to limit the scope of what the
inventors
regard as their invention nor are they intended to represent that the
experiments
below are all or the only experiments performed. Efforts have been made to
ensure
accuracy with respect to numbers used (e.g., amounts, temperatures, etc.) but
some
experimental errors and deviations should be accounted for. Unless indicated
otherwise, parts are by weight, molecular weight is weight average molecular
weight,
temperature is in degrees Centigrade, and pressure is at or near atmospheric.
Example 1 Phase 1 Clinical study
63
Date Recue/Date Received 2022-04-07
Objectives of the Study
[00238] The primary objective of this Phase Clinical I study was to
determine the
safety of topically applied NeuroMed7Tm 4% lidocaine, as indicated by its
uptake into
blood and clearance rate and also by the occurrence of latent adverse events.
[00239] Secondary objectives included:
= estimating the effectiveness of a proprietary cream formulation;
= determining the effect of dosage frequency on blood levels and clearance
rate;
= among patients reporting acute pain, determining the effectiveness of the
topical cream in reducing pain, as expressed by self-reported visual analog
scale (VAS); and
= estimating rates of absorption and distribution, and also of metabolism
by
simultaneous determination of lidocaine and of monoethylglycinexylidide
(MEGX), the primary metabolite of lidocaine.
Materials and Methods
Ethics:
[00240] The study was conducted by the clinical research unit of Insight
Institute
of Neurosurgery & Neuroscience (Flint, MI), in accordance with the guidelines
on
International Conference on Harmonisation Guidelines for Good Clinical
Practice
(E6[R1]) (International Conference on Harmonisation Guidelines for Good
Clinical
Practice (E6[R1]). ich.org/products/guidelines/efficacy/efficacy-
single/article/good-
clinical-practice.html. Accessed 13 Nov 2013), the Code of Federal Regulations
for
Good Clinical Practice (21 CFR Parts 50 and 56) (US Food and Drug
Administration.
FDA regulations relating to Good Clinical Practice and clinical trials.
http://www.fda.gov/scienceresearch/specialtopics/runningclinicaltrials-
/ucm114928.htm. Accessed 13 Nov 2013), and the Declaration of Helsinki
regarding
the treatment of human study subjects (WMA Declaration of Helsinki ¨ Ethical
principles for medical research involving human subjects.
64
Date Recue/Date Received 2022-04-07
http://wma.net/en/20activities/10ethics/10helsinki/. Accessed 13 Nov 2013).
The
clinical study protocol and informed consent form were reviewed and approved
(July
30, 2013) by the institutional review board at Western Institutional Review
Board
(WIRB, Olympia, WA, study 20131169). All subjects signed informed consent
forms
prior to enrolling in the study, and were interviewed for latent adverse
effects within
24 h after completion of study activities. All study procedures were performed
between Aug 15 and Sept 22, 2013. All subjects who registered into the study
met
the eligibility criteria that had been approved by WIRB.
[00241] Inclusion Criteria included:
= Healthy individuals of both genders and any ethnic background;
= Age 18 years;
= Willing to submit to venipuncture at study intervals; and
= Ability to understand and the willingness to sign a written informed
consent.
[00242] Exclusion Criteria included:
= Cardiac, hepatic, renal, pulmonary, neurological, gastrointestinal and
hematological diseases, psychiatric disorders, and allergy to local
anesthetics;
= History of chronic disease;
= Pregnancy;
= Active local skin infection or skin pathological condition at the site of
administration;
= Tattoo, surgical scar or skin condition at the site of administration
that might
interfere with penetration of agent into the skin; and
= Currently using lidocaine or any related amide-containing agent that
might
provide a false positive result in the clinical analysis of lidocaine.
Study drug:
Date Recue/Date Received 2022-04-07
[00243] The lidocaine preparation marketed as NeuroMed7Tm was obtained
from SambriaTM Pharmaceuticals at 4% (w/w) in a cream that includes
methylsulfonyl methane (MSM) and ethoxydiglycol in accordance with the
formulation
disclosed in Table 1 above.
Drug application and safety sample analysis:
[00244] The 4% lidocaine cream was provided by Sambria Pharmaceuticals
(Woodstock, GA). Whole blood (10 mL per bleed) was collected using standard
venipuncture into serum Vacutainer tubes (Franklin Lakes, IL). Following
centrifugation at 1000 x g for 30 min, serum was transferred to 5 mL
polypropylene
tubes and snap-frozen in a dry ice/ethanol slurry. Frozen samples were shipped
overnight on dry ice to NMS Labs, Willow Grove, PA. The samples were analyzed
by gas chromatography, using forensic standards with a detection limit of 0.1
mcg/mL for both lidocaine and MEGx, ie, 15 ¨ 20-fold below the therapeutic
reference range (NMS Labs, Willow Grove, PA)
(http://www.pathology.med.umich.edu/handbook/, accessed 6 Sept 2013). This
analytical method was selected to allow quantitation of sub-clinical levels of
lidocaine
and MEGx that are not captured using standard clinical laboratory methods
(typically, fluorescence polarization immunoassay). The selected method also
will
detect levels of drug that fall within the clinical range, which is necessary
to verify the
relationship between dosage and physiological response.
Clinical study schema:
[00245] The general study plan required 2 equivalent, 1 mL doses of
NeuroMed7Tm 4% lidocaine to a subject's selected region of acute pain and drug
application at 0 and 4 h, with schema presented in Figure 3. As indicated, 10
mL of
venous blood was collected throughout the study period, at time intervals
likely to
capture peak and trough levels (Greenblatt DJ et al. (1985). Arch Otolaryngol
111:
2988-3000); (Baumann LS, et al. (2010). J Drugs Dermatol 9: 1500-1504). Each
subject provided self-rated pain evaluations at those same time intervals,
focusing
on pain at the identified site of acute pain and of drug application, using
the 1-10
Visual Analog Scale (VAS) (Meier T, et al. (2003). Pain 106: 151-158).
66
Date Recue/Date Received 2022-04-07
Results and discussion
Safety study:
[00246] The primary goal of this Phase I study was to investigate the
extent to
which lidocaine enters circulation following topical application of NeuroMed7
4%
lidocaine cream, to indicate drug safety. Blood levels frequently are used as
an
index of toxicity, particularly in the absence of physiological signs. There
are clinical
signs that indicate the presence of adverse effects due to relatively high
levels of
lidocaine, and also of some physiologically active degradation products, in
particular,
MEGx. This study used both blood measurements, and questions posed to study
subjects on specific side effects, as indicators of NeuroMed7Tm safety.
[00247] Venous blood samples were taken prior to the initial dosing at 0
h, and
1, 3, 5, 7, 9 and 11 hours following the initial (1 h) drug application, which
for the last
4 samples (indicated on Figure 3. Study Schema) also corresponds to 1, 3, 5
and 7
hours after the second (4 h) drug application. Lidocaine levels were below the
detection limit of 0.1 mcg/mL ( g/mL) in all blood samples. Among all samples
analyzed for MEGx, only one, sample #4 for subject 16, the level of MEGx was
at the
detection limit, i.e., 0.1 mcg/mL. Given that the anticipated peak levels in
blood
occur between 1 and 2 hours after administration, and the unlikelihood of
consenting
subjects to more frequent venipuncture, the study was designed to approximate
the
peak drug levels resulting from each lidocaine dose.
[00248] For these blood analytes, among 239 serum samples that were
analyzed, only 1 displayed a measurable result, despite our use of an
analytical
method that is 15 ¨ 20-fold more sensitive than typical clinical laboratory
methods
that are calibrated for therapeutic levels ranging from 1.5 to 5.0 mcg/mL
(http://www.mayomedicallaboratories.com/test-
catalog/Clinical+and+Interpretive/8382. Accessed 19 Nov 2013); (Becker DE,
Reed
KL (2012). Anesth Prog 59: 90-102). The one detectable sample was collected 1
h
after the second lidocaine administration, and results from the additive
effect of the
subject's initial dose along with the 4 h dose. It is concluded from
toxicology studies
that the doses of lidocaine in NeuroMed7Tm, used as indicated in the FDA
monograph, are well below the levels of concern.
67
Date Recue/Date Received 2022-04-07
[00249] Toxic levels of lidocaine typically occur at levels greater than
6.0
mcg/mL, with symptoms including central nervous excitation, lightheadedness,
dizziness, tinnitus, confusion, and blurred or double vision (Valdes R et al.
(1998).
Clin Chem 44(5): 1096-1099). Within 24 h after concluding the study, as well
as
during their immediate participation, subjects were contacted with questions
regarding side effects. There were no such reports of adverse effects related
to drug
activity. Three subjects did experience slight lightheadedness that appeared
to
result from venipuncture, as they were felt after each blood draw. It is
concluded
from the measured blood levels, as well as clinical signs, that the subjects
in this
study expressed no symptoms of lidocaine toxicity.
Studies of efficacy:
[00250] The central data used to describe efficacy was the 10-point VAS.
While
this measure is subjective, the study subjects were requested specifically to
be
consistent in their pain estimates. The mean initial pain score at 0 h (+/-
SD) was 4.0
(1.3). The reported pain reduction for all subjects and at all time points was
significant and transient, as expected (Figure 4). The subjective nature self-
scored
pain scales, noted with VAS, as well with other pain scales, results in
considerable
subject-to-subject variation. This is particularly dramatic when looking at
the
variation (expressed as standard deviation) in the average pain reduction
within the
study cohort (Figure 5). Having such a broad range results from the inherent
subjectivity of the initial pain score, compounded by the subjective estimate
in
efficacy. Regardless of specific numbers, NeuroMed7Tm had a positive effect in
reducing acute pain.
[00251] Related to the individual reduction in pain is the extent of pain
reduction,
based on the initial score. This analysis groups the cohort by percent of pain
reduction, asking how many subjects experienced various level of pain relief.
As
indicated in Figure 6, many subjects experienced a high level of pain relief,
with a
total of 28 of 34 subjects having relief at 50% or better.
[00252] Effect latency, or the time before maximum pain relief, was
another
measure of interest. Again, there are inter-subject variables that may impact
the
time before maximum pain effect. Indeed, having 2 doses of lidocaine imposes
on
68
Date Recue/Date Received 2022-04-07
each subject 2 pharmacokinetic curves that may, in some individuals and
depending
on the time between dosing, be additive. Nonetheless, it is instructive that 9
subjects
expressed maximum pain relief at the 1 hour point, with the extent of relief
presenting an almost exponential decay following that point (Figure 7).
Conclusion:
[00253] Thirty-four subjects were enrolled (20 women, 14 men). Prior to
drug
administration, neither lidocaine nor MEGx was found in the serum of any of
the
subjects. Serum concentrations for both analytes were below the limit of
detection
for the analytical method (0.1 mcg/mL), with the exception of one male
subject,
whose 5-hour MEGx level was reported as detectable at the detection limit of
0.1
mcg/mL. Possible adverse reactions among study subjects, which included
central
nervous system and cardiovascular effects, were not reported. Initial self-
reported
acute pain levels by VAS ranged from 1 to 8, with a mean (+/-SD) prior to drug
administration of 4.33(1.72); pain levels subsequent to lidocaine application
were at
lh: 2.33(1.8); 3h: 2.14(2.16); 5h: 1.88(2.09); 7h: 1.73(2.29); 9h: 1.67(2.23);
and 11h:
2.07(1.69). The study population achieved reductions in the initial level of
acute pain
of 41% (1 h), 54% (3 h), 59% (5 h), 64% (7 h), 62% (9 h) and 57% (11 h). Pain
reduction was 50% or greater among 82% of subjects (28 of 34), with the time
elapsed to reach maximal pain reduction being 1 h for 27% of subjects,
followed by
21% (3 h), 18% (5 h), 9% (7 h), 12% (9 h), and 6% (11 h).
[00254] This Phase 1 study provides direct evidence demonstrating the
safety of
NeuroMed7Tm 4% lidocaine cream when used as indicated in the OTC monograph,
ie, 1 mL dosing at least 4 h apart, with a maximum of 2 doses per day. No
sufficiently high blood levels of lidocaine or MEGx that would indicate
toxicity was
detected, and there were no reported clinical signs of overdose. Our analysis
of
efficacy demonstrated positive responses whose broad variation is attributed
to the
locus of pain and pain history, as well as other uncontrolled variables.
Despite the
spread in response, the use of NeuroMed7Tm as indicated provides effective
topical
pain relief, while presenting little in the way of secondary adverse effects.
Lidocaine
is an agent that can be physiologically damaging at doses much higher than
used in
this study. However, when used as indicated, NeuroMed7Tm provides a broad
margin of safety to the user.
69
Date Recue/Date Received 2022-04-07
[00255] Thus the lidocaine formulation presented no measurable safety
issues,
either in measureable serum levels (since the highest measurable level was
0.10
mcg/mL, whereas toxicity is indicated at >5 mcg/mL), or in physiological
response,
and was effective among the majority of these subjects.
[00256] While the present invention has been described with reference to
the
specific embodiments thereof it should be understood by those skilled in the
art that
various changes may be made and equivalents may be substituted without
departing
from the true spirit and scope of the invention. In addition, many
modifications may
be made to adopt a particular situation, material, composition of matter,
process,
process step or steps, to the objective spirit and scope of the present
invention. All
such modifications are intended to be within the scope of the claims appended
hereto.
Date Recue/Date Received 2022-04-07