Note: Descriptions are shown in the official language in which they were submitted.
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
BIOCHAR AGGREGATE PARTICLES
CROSS-REFERENCE TO RELATED APPLICATIONS
[001] The application claims priority to U.S. Provisional Patent
Application Serial No.
62/290,026, filed on February 2, 2016, titled BIOCHAR AGGREGATE PARTICLES and
U.S. Provisional Patent Application Serial No. 62/293,160, filed on February
9, 2016, titled
BIOCHARS FOR USE IN COMPOSTS; this application is a continuation-in-part of
U.S.
Patent Application Serial No. 15/419,976, filed on January 30, 2017, titled
BIOCHAR FOR
USE WITH ANIMALS, which application claims priority to US Provisional Patent
Application Serial No. 62/288,068, filed January 28, 2016, titled BIOCHAR FOR
USE
WITH ANIMALS, U.S. Provisional Patent Application Serial No. 62/290,026, filed
on
February 2, 2016, titled BIOCHAR AGGREGATE PARTICLES, U.S. Provisional Patent
Application Serial No. 62/293,160, filed on February 9, 2016, titled BIOCHARS
FOR USE
IN COMPOSTS and U.S. Provisional Patent Application Serial No. 62/344,865
filed on June
2, 2016 titled MINERAL SOLUBILIZING MICROORGANISMS INFUSED BIOCHARS;
this application is also a continuation-in-part of U.S. Patent Application
Serial No.
15/393,176, filed on December 28, 2016, titled ADDITIVE INFUSED BIOCHAR, which
claims priority to U.S. Provisional Patent Application Serial No. 62/271,486
filed on
December 28, 2015 titled ADDITIVE INFUSED BIOCHARS; this application is also a
continuation-in-part of U.S. Patent Application Serial No. 15/393,214, filed
on December 28,
2016, titled BIOCHAR AS A MICROBIAL CARRIER, which claims priority to of U.S.
1
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
Provisional Patent Application Serial No. 62/271,486 filed on December 28,
2015 titled
ADDITIVE INFUSED BIOCHARS; this application is also a continuation-in-part of
U.S.
Patent Application No. 15/156,256, filed on May 16, 2016, titled ENHANCED
BIOCHAR,
which claims priority to United States Provisional Patent Application No.
62/162,219, filed
on May 15, 2015, titled ENHANCED BIOCHAR; this application is also a
continuation-in-
part of United States Patent Application No. 14/873,053 filed on October 1,
2015,
titled BIOCHARS AND BIOCHAR TREATMENT PROCESSES, which claims priority to
United States Provisional Patent Application No. 62/058,445, filed on October
1, 2014, titled
METHODS, MATERIALS AND APPLICATIONS FOR CONTROLLED POROSITY AND
RELEASE STRUCTURES AND APPLICATIONS and United States Provisional Patent
Application No. 62/058,472, filed on October 1, 2014, titled HIGH ADDITIVE
RETENTION BIOCHARS, METHODS AND APPLICATIONS.
FIELD OF INVENTION
[002] The invention relates to a biochar product and methods of producing a
biochar
aggregate particle.
BACKGROUND
[003] Biochar has been known for many years as a soil enhancer. It contains
highly porous,
high carbon content material similar to the type of very dark, fertile
anthropogenic soil found in
the Amazon Basin known as Terra Preta. Terra Preta has very high charcoal
content and is
made from a mixture of charcoal, bone, manure, among other substances. Biochar
is created by
the pyrolysis of biomass, which generally involves heating and/or burning of
organic matter, in
a reduced oxygen environment, at a predetermined rate. Such heating and/or
burning is stopped
when the matter reaches a charcoal like stage. The highly porous material of
biochar is perfectly
suited to host beneficial microbes, retain nutrients, hold water, and act as a
delivery system for
a range of beneficial compounds suited to specific applications.
[004] During the production of biochar, large portions of biochar fines or
dust particles are
created. Along with the loss of useful product, these dust particles can cause
problematic, or
even hazardous conditions for biochar manufacturing, packaging and in
application, including
2
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
in use through agricultural application equipment, in animal feed, or in
application to compost.
The various particle size distributions created during biochar manufacturing
lead to distribution
and application problems with equipment and cause the necessity of sizing
equipment and costly
capital expenditures. The low density of the biochar fines and dust particles
also makes mixing
of growth enhancers such as fertilizers or microbes difficult as it allows for
settling, separation,
and distribution problems.
[005] Given the known benefits of biochar, a need remains for: (i) a means
to produce
biochar in such a way that it has consistent granular particle sizes and
distributions and can meet
application needs in commercial agriculture, animal feed or maintenance, and
composting using
standard equipment and (ii) a method to utilize residual biochar dust or
biochar fines to create a
product with consistent size and physical/chemical properties that can be
uniformly distributed
in large and small scale applications to have the highest positive impact in
its application
including but not limited to agriculture, animal feed or maintenance, and
composting.
3
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
SUMMARY
[006] The present invention relates to a method for producing biochar
aggregate particles,
including, but not limited to agglomerates, extrudates, pellets, or granules,
from biochar using
starch or other binding material and/or additives to ease application, enhance
soil health, and
increase water retention in the soil.
[007] The method includes producing a biochar aggregate particle that may
contain
biochar, or a mixture of biochar, binders, fillers, and other additives such
as microbial products,
bacteria, plant nutrients, minerals, agricultural chemicals, fertilizers or
animal vitamins,
medications, or supplements.
[008] In one example, the method includes, collecting treated and/or
untreated biochar
particles, mixing said biochar particles with water and one or more binders,
such as a starch,
polymer, clay, or lignin, to create a slurry, filter pressing or de-watering
the slurry to create a
paste and extruding the paste through an extruder and creating biochar
aggregate particles.
Optionally, additives can be mixed with the slurry or paste. If collecting
treated biochar
particles, the particles may be treated in advance, for example pH adjusted or
treated to remove
deleterious substances.
[009] When extruding the paste, the paste may be cut into desired length
pieces and dried.
In certain applications, depending upon the extruder, the cutting of the
extrudate can be done in
conjunction with the extrusion process. Through this process, a specific
sized, dust free, biochar
aggregate particle is created that can be easily used in agricultural
distribution equipment.
[010] Using biochar aggregate particles allows for better application in
both the industrial
and individual sectors by allowing for the utilization of diverse processing
and distribution
equipment. For example, the application of biochar aggregate particles into
soil results in more
consistently fuller plants with unvarying vitality and longevity that can
ultimately be
maintained with less water.
[011] Other devices, apparatus, systems, methods, features and advantages
of the invention
are or will become apparent to one with skill in the art upon examination of
the following figures
and detailed description. It is intended that all such additional systems,
methods, features and
4
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
advantages be included within this description, be within the scope of the
invention, and be
protected by the accompanying claims.
BRIEF DESCRIPTION OF THE FIGURES
[012] The invention may be better understood by referring to the following
figures. The
components in the figures are not necessarily to scale, emphasis instead being
placed upon
illustrating the principles of the invention.
[013] Figure 1 illustrates a cross-section of one example of a raw biochar
particle.
[014] Figure 2a is a SEM (10KV x 3.00K 10.0m) of pore morphology of treated
biochar
made from pine.
[015] Figure 2b is a SEM (10KV x 3.00K 10.0m) of pore morphology of treated
biochar
made from birch.
[016] Figure 2c is a SEM (10KV x 3.00K 10.0m) of pore morphology of treated
biochar
made from coconut shells.
[017] Figure 3 is a chart showing porosity distribution of various
biochars.
[018] Figure 4 is a flow chart process diagram of one implementation of a
process for
treating the raw biochar in accordance with the invention.
[019] Figure 4a illustrates a schematic of one example of an implementation
of a biochar
treat processes that that includes washing, pH adjustment and moisture
adjustment.
[020] Figure 4b illustrates yet another example of an implementation of a
biochar
treatment processing that includes inoculation.
[021] Figure 5 is a schematic flow diagram of one example of a treatment
system for use in
accordance with the present invention.
[022] Figure 6 is a chart showing the water holding capacities of treated
biochar as compared
to raw biochar and sandy clay loam soil and as compared to raw biochar and
sunshine potting
soil.
[023] Figure 7 illustrates the different water retention capacities of raw
biochar versus
treated biochar measured gravimetrically.
[024] Figure 8 is a chart showing the retained water in vacuum impregnated
biochar over
other biochars after a seven week period.
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[025] Figure 9 is a chart showing the weight loss of treated biochars
versus raw biochar
samples when heated at varying temperatures using a TGA testing method.
[026] Figure 10 illustrates the plant available water in raw biochar,
versus treated biochar
and treated dried biochar.
[027] Figure 11 is a graph showing the pH of various starting biochars that
were made from
different starting materials and pyrolysis process temperatures.
[028] Figure 12 is a chart showing various pH ranges and germination for
treated biochars.
[029] Figure 13 is a Thermogravimetric Analysis (TGA) plot showing the
measurement of
water content, heavy organics and light organics in a sample.
[030] Figure 14 is a chart showing the impact of treatment on pores sizes
of biochar derived
from coconut.
[031] Figure 15 is a chart showing the impact of treatment on pores sizes
of biochar derived
from pine.
[032] Figure 16 is a chart showing the measured hydrophobicity index raw
biochar,
vacuum treated biochar and surfactant treated biochar.
[033] Figure 17 is a flow diagram showing one example of a method for
infusing biochar.
[034] Figure 18 illustrates the improved liquid content of biochar using
vacuum
impregnation as against soaking the biochar in liquid.
[035] Figure 19a is a chart comparing total retained water of treated
biochar after soaking
and after vacuum impregnation.
[036] Figure 19b is a chart comparing water on the surface, interstitially
and in the pores
of biochar after soaking and after vacuum impregnation.
[037] Figure 20 illustrates how the amount of water or other liquid in the
pores of vacuum
processed biochars can be increased varied based upon the applied pressure.
[038] Figure 21 illustrates the effects of NPK impregnation of biochar on
lettuce yield.
[039] Figure 22 is a chart showing nitrate release curves of treated
biochars infused with
nitrate fertilizer.
[040] Figures 23 and 24 are images that show how different sized bacteria
will fit in different
biochar pore size structures.
6
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[041] Figure 25 illustrates release rate data verse total pore volume data
for both coconut
shell and pine based treated biochars inoculated with a releasable bacteria.
[042] Figure 26 is a chart comparing examples of biochars.
[043] Figures 27a, 27b, 27c are charts comparing different examples of
biochars.
[044] Figure 28 is a chart comparing shoot biomass when the biochar added
to a soilless
mix containing soybean seeds is treated with microbial product containing
bradyrhizobium
japonicum. and when it is untreated.
[045] Figure 29 shows the comparison of root biomass in a treated verses an
untreated
environment.
[046] Figure 30 is a chart comparing the nitrogen levels when the biochar
is inoculated
with the rhizobial inoculant verses when it is not inoculated.
[047] Figure 31 illustrates the three day release rates of water infused
biochar compared to
other types of biochar.
[048] Figure 32a is a SEM (10KV x 3.00K 10.0m) of pore morphology of raw
biochar.
[049] Figure 32b is a SEM (10KV x 3.00K 10.0m) of pore morphology of raw
biochar
of Figure 32a after it has been infused with microbial species.
[050] Figure 32c is a SEM (10KV x 3.00K 10.0m) of a pore morphology of
another
example of raw biochar of Figure 17a after it has been infused with microbial
species.
[051] Figure 33 contains charts illustrating improved results obtained
through the use of
biochars.
[052] Figure 34 is an example of carbon dioxide production captured as a
continuous gas
bubble in BGB (left two tubes) and LTB (right two tubes) growth medium.
[053] Figures 35 and 36 illustrate improved growth rates of colonies of
Streptomyces
lydicus using biochars.
[054] Figures 37a is an image of biochar aggregate particles of the present
invention made
in the form pellets.
[055] Figure 37b is an image of biochar aggregate particles of the present
invention made in
the form an extrudates.
[056] Figure 37c is an image of the biochar aggregate particles made in the
form of biochar
sulfur prills.
7
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
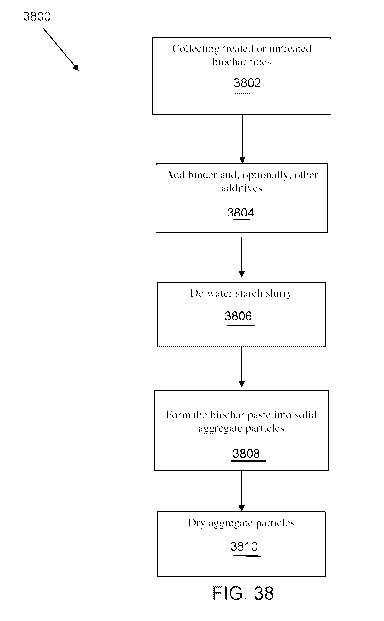
[057] Figure 38 is a flow diagram of one example of a method for producing
biochar
aggregate particles.
[058] Figures 39a-f illustrates the effects of size and grinding on
particle structure of a
biochar derived from a first biomass.
[059] Figures 40a-f illustrates the effects of size and grinding on
particle structure of a
biochar derived from a second biomass.
[060] Figure 41a shows the effect of size fraction on water holding
capacity of two
different biomass based treated biochars.
[061] Figure 41b shows the effect of size fraction on pH of two different
biomass based
treated biochars.
[062] Figure 41c shows the effect of size fraction on Cl- concentration of
two different
biomass based treated biochars.
[063] Figure 41d shows the effect of size fraction on electrical
conductivity of two
different biomass based treated biochars.
[064] Figure 42 is a diagram illustrating one example of the workflow for a
food
composting operation.
[065] Figure 43 is a chart showing the pH of compost as the percent of
lactic acid
increases.
[066] Figure 44 is a chart showing how pH is influenced in compost when
mixing greens,
woods and foods.
[067] Figure 45 is a chart showing the impact on composting temperatures
when treated
biochar is added to compost.
[068] Figure 46 is a chart showing the decrease of lactic acid production
in compost by
adding treated biochar.
[069] Figure 47 is a chart showing the increase in pH in compost by adding
treated biochar.
[070] Figure 48 is a chart showing the increase in oxygen levels in compost by
adding treated
biochar.
[071] Figure 49 is a chart showing the impact of the addition of both raw and
treated biochar in
a CASP compost environment to volatile fatty acids (VFAs).
8
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[072] Figure 50 is a chart showing the impact of the addition of both raw and
treated biochar in
a CASP compost environment to NH3 levels.
[073] Figure 51 is a chart showing the impact on volatile organic compounds
("VOC") by
adding treated and raw biochar to CASP compost.
[074] Figure 52 is a chart shows a test of evaporative water loss from control
compost against
blended treatments with raw or processed biochars at 1, 3 and 5% by volume.
[075] Figure 53 is a chart showing the effect that the addition of treated
biochar has on percent
mass water loss in a CASP compost environment.
[076] Figure 54 is a chart showing in impact of the addition of the inoculated
biochar to compost
on microbial abundance.
[077] Figure 55 is a chart showing in impact of the addition of the inoculated
biochar to compost
on VOCs.
[078] Figure 56 is a chart showing in impact of the addition of the inoculated
biochar to compost
on NH3
[079] Figure 57 is chart illustrating biochar capacity to absorb Cadmium.
9
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
DESCRIPTION OF THE INVENTION
[074] As illustrated in the attached figures, the present invention relates
to a method for
producing biochar aggregate particles that can be used in processing and
distribution
equipment for improved industrial application including but not limited to
agriculture, animal
health and maintenance, and compositing, when increased density or uniform
particle size,
composition or distribution is preferred or required in order to achieve the
highest positive
impact in its application.
[075] For the purposes of this application, prior treatment of the raw
biochar, as described
below, is not required as part of the production of the biochar aggregate
particles. However,
often treatment is preferred as the properties of the raw biochar can be
modified to significantly
increase the biochar' s ability to retain water and/or nutrients while also,
in many cases, creating
an environment beneficial to microorganisms. The processing of the biochar can
also ensure
that the pH of biochar used in the present application is suitable for its
application, which has
been a challenge for raw biochars. In certain application, it may be desirable
to produce the
biochar aggregate particles from treated biochars or the fines of treated
biochars.
[076] Biochars derived from different biomass or produced with differing
parameters, such
as higher or lower pyrolysis temperature or variations in residence time, will
have different
physical and chemical properties and can behave quite differently in different
applications. For
example, some chars will have a fairly uniform granular particle size and
shape with a high
density and relatively high crush strength that flows well, while others will
have a low density
and a low crush strength which means they breakdown easily creating many fines
and dust
particles and will also lead to poor flow characteristics. But these biochars
with poor particle
characteristics might be more economic or due to their other physical or
chemical characteristics
more effective in a specific application. Thus, turning these biochars into an
aggregate of the
present invention, allows them to be more useful and effective through
standard processing and
application equipment.
[077] A good example of aggregate need is when a biochar will be used as a
component of
an animal feed or be mixed with a granular fertilizer prior to application in
agriculture. Mixing
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
of particles that are significantly different in shape, size, or density will
generally lead to
segregation during shipping, handling, or application. Thus aggregating the
biochar into a similar
particle shape, size, or density of the rest of the mixture, say fertilizer or
animal feed pellet, will
allow for a uniform mix and rate to be achieved when fed to the animal or
applied to the soil.
[078] Currently biochar has mostly been a scientific curiosity, not found
in wide spread use
or large scale commercial applications, and instead has been relegated to
small niche
applications. It is, however, known, that biochar, having certain
characteristics can host
beneficial microbes, retain nutrients and supplements, hold liquids for
agricultural applications.
Accordingly, these same characteristics of biochar can be harnessed for other
application such as
composting, remediation, or animal maintenance, care and feeding.
[079] For purposes of this application, the term "biochar" shall be given
its broadest
possible meaning and shall include any solid carbonaceous materials obtained
from the
pyrolysis, torrefaction, gasification or any other thermal and/or chemical
conversion of a
biomass. For purposes of this application, the solid carbonaceous material may
include, but not
be limited to, BMF char disclosed and taught by U.S. Patent 8,317,891, which
is incorporated
into this application by reference. Pyrolysis is generally defined as a
thermochemical
decomposition of organic material at elevated temperatures in the absence of,
or with reduced
levels of oxygen. When the biochar is referred to as "treated" or undergoes
"treatment," it shall
mean raw, pyrolyzed biochar that has undergone additional physical,
biological, and/or
chemical processing.
[080] A s used herein, unless specified otherwise, the terms carbonaceous",
"carbon
based", "carbon containing", and similar such terms are to be given their
broadest possible
meaning, and would include materials containing carbon in various states,
crystallinities, forms
and compounds.
As used herein, unless stated otherwise, room temperature is 25 C. And,
standard
temperature and pressure is 25 C and 1 atmosphere. Unless stated otherwise,
generally,
the term "about" is meant to encompass a variance or range of 10%, the
experimental or
instrument error associated with obtaining the stated value, and preferably
the larger of
these.
A, Biochars
11
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[081] Typically, biochars include porous carbonaceous materials, such as
charcoal, that
are used as soil amendments or other suitable applications. Biochar most
commonly is created
by pyrolysis of a biomass. In addition to the benefits to plant growth, yield
and quality, etc.;
biochar provides the benefit of reducing carbon dioxide (CO2) in the
atmosphere by serving as
a method of carbon sequestration. Thus, biochar has the potential to help
mitigate climate
change, via carbon sequestration. However, to accomplish this important, yet
ancillary benefit,
to any meaningful extent, the use of biochar in agricultural applications must
become widely
accepted, e.g., ubiquitous. Unfortunately, because of the prior failings in
the biochar arts, this
has not occurred. It is believed that with the solutions of the present
invention may this level
of use of biochar be achieved; and more importantly, yet heretofore
unobtainable, realize the
benefit of significant carbon dioxide sequestration.
[082] In general, one advantage of putting biochar in soil includes long
term carbon
sequestration. It is theorized that as worldwide carbon dioxide emissions
continue to mount,
benefits may be obtained by, controlling, mitigating and reducing the amount
of carbon dioxide
in the atmosphere and the oceans. It is further theorized that increased
carbon dioxide emissions
are associated with the increasing industrial development of developing
nations, and are also
associated with the increase in the world's population. In addition to
requiring more energy,
the increasing world population will require more food. Thus, rising carbon
dioxide emissions
can be viewed as linked to the increasing use of natural resources by an ever
increasing global
population. As some suggest, this larger population brings with it further
demands on food
production requirements. Biochar uniquely addresses both of these issues by
providing an
effective carbon sink, e.g., carbon sequestration agent, as well as, an agent
for improving and
increasing agricultural output. In particular, biochar is unique in its
ability to increase
agricultural production, without increasing carbon dioxide emission, and
preferably reducing
the amount of carbon dioxide in the atmosphere. However, as discussed above,
this unique
ability of biochar has not been realized, or seen, because of the inherent
problems and failings
of prior biochars including, for example, high pH, phytotoxicity due to high
metals content
and/or residual organics, and dramatic product inconsistencies.
[083] Biochar can be made from basically any source of carbon, for example,
from
hydrocarbons (e.g., petroleum based materials, coal, lignite, peat) and from a
biomass (e.g.,
12
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
woods, hardwoods, softwoods, waste paper, coconut shell, manure, chaff, food
waste, etc.).
Combinations and variations of these starting materials, and various and
different members of
each group of starting materials can be, and are, used. Thus, the large number
of vastly different
starting materials leads to biochars having different properties.
[084] Many different pyrolysis or carbonization processes can be, and are
used, to create
biochars. In general, these processes involve heating the starting material
under positive
pressure, reduced pressure, vacuum, inert atmosphere, or flowing inert
atmosphere, through one
or more heating cycles where the temperature of the material is generally
brought above about
400 C, and can range from about 300 C to about 900 C. The percentage of
residual carbon
formed and several other initial properties are strong functions of the
temperature and time
history of the heating cycles. In general, the faster the heating rate and the
higher the final
temperature the lower the char yield. Conversely, in general, the slower the
heating rate or the
lower the final temperature the greater the char yield. The higher final
temperatures also lead
to modifying the char properties by changing the inorganic mineral matter
compositions, which
in turn, modify the char properties. Ramp, or heating rates, hold times,
cooling profiles,
pressures, flow rates, and type of atmosphere can all be controlled, and
typically are different
from one biochar supplier to the next. These differences potentially lead to a
biochar having
different properties, further framing the substantial nature of one of the
problems that the
present inventions address and solve. Generally, in carbonization most of the
non-carbon
elements, hydrogen and oxygen are first removed in gaseous form by the
pyrolytic
decomposition of the starting materials, e.g., the biomass. The free carbon
atoms group or
arrange into crystallographic formations known as elementary graphite
crystallites. Typically,
at this point the mutual arrangement of the crystallite is irregular, so that
free interstices exist
between them. Thus, pyrolysis involves thermal decomposition of carbonaceous
material, e.g.,
the biomass, eliminating non-carbon species, and producing a fixed carbon
structure.
[085] As noted above, raw or untreated biochar is generally produced by
subjecting
biomass to either a uniform or varying pyrolysis temperature (e.g., 300 C to
550 C to 750 C
or more) for a prescribed period of time in a reduced oxygen environment. This
process may
either occur quickly, with high reactor temperature and short residence times,
slowly with lower
reactor temperatures and longer residence times, or anywhere in between. To
achieve better
13
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
results, the biomass from which the char is obtained may be first stripped of
debris, such as
bark, leaves and small branches, although this is not necessary. The biomass
may further
include feedstock to help adjust the pH and particle size distribution in the
resulting raw biochar.
In some applications, it is desirous to have biomass that is fresh, less than
six months old, and
with an ash content of less than 3%. Further, by using biochar derived from
different biomass,
e.g., pine, oak, hickory, birch and coconut shells from different regions, and
understanding the
starting properties of the raw biochar, the treatment methods can be tailored
to ultimately yield
a treated biochar with predetermined, predictable physical and chemical
properties.
[086] In general, biochar particles can have a very wide variety of
particle sizes and
distributions, usually reflecting the sizes occurring in the input biomass.
Additionally, biochar
can be ground or crushed after pyrolysis to further modify the particle sizes.
Typically, for
agricultural uses, biochars with consistent, predictable particle sizes are
more desirable. By
way of example, the biochar particles can have particle sizes as shown or
measured in Table
lbelow. When referring to a batch having 1/4 inch particles, the batch would
have particles that
will pass through a 3 mesh sieve, but will not pass through (i.e., are caught
by or sit atop) a 4
mesh sieve.
Table 1
U.S. Mesh Inches Microns Millimeters
(i.e., mesh) (1-11111) (mm)
3 0.2650 6730 6.370
4 0.1870 4760 4.760
0.1570 4000 4.000
6 0.1320 3360 3.360
7 0.1110 2830 2.830
8 0.0937 2380 2.380
0.0787 2000 2.000
12 0.0661 1680 1.680
14 0.0555 1410 1.410
16 0.0469 1190 1.190
18 0.0394 1000 1.000
0.0331 841 0.841
0.0280 707 0.707
0.0232 595 0.595
0.0197 500 0.500
0.0165 400 0.400
14
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
45 0.0138 354 0.354
50 0.0117 297 0.297
60 0.0098 250 0.250
70 0.0083 210 0.210
80 0.0070 177 0.177
100 0.0059 149 0.149
120 0.0049 125 0.125
140 0.0041 105 0.105
170 0.0035 88 0.088
200 0.0029 74 0.074
230 0.0024 63 0.063
270 0.0021 53 0.053
325 0.0017 44 0.044
400 0.0015 37 0.037
[087] For most applications, it is desirable to use biochar particles
having particle sizes
from about 3/4 mesh to about 60/70 mesh, about 4/5 mesh to about 20/25 mesh,
or about 4/5
mesh to about 30/35 mesh. It being understood that the desired mesh size, and
mesh size
distribution can vary depending upon a particular application for which the
biochar is intended.
[088] Figure 1 illustrates a cross-section of one example of a raw biochar
particle. As
illustrated in Figure 1, a biochar particle 100 is a porous structure that has
an outer surface 100a
and a pore structure 101 formed within the biochar particle 100. As used
herein, unless
specified otherwise, the terms "porosity", "porous", "porous structure", and
"porous
morphology" and similar such terms are to be given their broadest possible
meaning, and would
include materials having open pores, closed pores, and combinations of open
and closed pores,
and would also include macropores, mesopores, and micropores and combinations,
variations
and continuums of these morphologies. Unless specified otherwise, the term
"pore volume" is
the total volume occupied by the pores in a particle or collection of
particles; the term "inter-
particle void volume" is the volume that exists between a collection of
particle; the term "solid
volume or volume of solid means" is the volume occupied by the solid material
and does not
include any free volume that may be associated with the pore or inter-particle
void volumes;
and the term "bulk volume" is the apparent volume of the material including
the particle
volume, the inter-particle void volume, and the internal pore volume.
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[089] The pore structure 101 forms an opening 121 in the outer surface 100a
of the biochar
particle 100. The pore structure 101 has a macropore 102, which has a
macropore surface 102a,
and which surface 102a has an area, i.e., the macropore surface area. (In this
diagram only a
single micropore is shown. If multiple micropores are present than the sum of
their surface
areas would equal the total macropore surface area for the biochar particle.)
From the
macropore 102, several mesopores 105, 106, 107, 108 and 109 are present, each
having its
respective surfaces 105a, 106a, 107a, 108a and 109a. Thus, each mesopore has
its respective
surface area; and the sum of all mesopore surface areas would be the total
mesopore surface
area for the particle. From the mesopores, e.g., 107, there are several
micropores 110, 111, 112,
113, 114, 115, 116, 117, 118, 119 and 120, each having its respective surfaces
110a, 111a, 112a,
113a, 114a, 115a, 116a, 117a, 118a, 119a and 120a. Thus, each micropore has
its respective
surface area and the sum of all micropore surface areas would be the total
micropore surface
area for the particle. The sum of the macropore surface area, the mesopore
surface area and the
micropore surface area would be the total pore surface area for the particle.
[090] Macropores are typically defined as pores having a diameter greater
than 300 nm,
mesopores are typically defined as diameter from about 1-300 nm, and
micropores are typically
defined as diameter of less than about 1 nm, and combinations, variations and
continuums of
these morphologies. The macropores each have a macropore volume, and the sum
of these
volumes would be the total macropore volume. The mesopores each have a
mesopore volume,
and the sum of these volumes would be the total mesopore volume. The
micropores each have
a micropore volume, and the sum of these volumes would be the total micropore
volume. The
sum of the macropore volume, the mesopore volume and the micropore volume
would be the
total pore volume for the particle.
[091] Additionally, the total pore surface area, volume, mesopore volume,
etc., for a batch
of biochar would be the actual, estimated, and preferably calculated sum of
all of the individual
properties for each biochar particle in the batch.
[092] It should be understood that the pore morphology in a biochar
particle may have
several of the pore structures shown, it may have mesopores opening to the
particle surface,
it may have micropores opening to particle surface, it may have micropores
opening to
macropore surfaces, or other combinations or variations of interrelationship
and structure
16
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
between the pores. It should further be understood that the pore morphology
may be a
continuum, where moving inwardly along the pore from the surface of the
particle, the pore
transitions, e.g., its diameter becomes smaller, from a macropore, to a
mesopore, to a
micropore, e.g., macropore 102 to mesopore 109 to micropore 114.
[093] In general, the biochars have porosities that can range from 0.2
cm3/cm3 to about
0.8 cm3/cm3 and more preferably about 0.2 cm3/cm3 to about 0.5 cm3/cm3.
(Unless stated
otherwise, porosity is provided as the ratio of the total pore volumes (the
sum of the micro
+ meso + macro pore volumes) to the solid volume of the biochar. Porosity of
the biochar
particles can be determined, or measured, by measuring the micro-, meso-, and
macro pore
volumes, the bulk volume, and the inter particle volumes to determine the
solid volume by
difference. The porosity is then calculated from the total pore volume and the
solid volume.
[094] As noted above, the use of different biomass potentially leads to
biochars having
different properties, including, but not limited to different pore structures.
By way of example,
Figures 2A, 2B and 2C illustrate Scanning Electron Microscope ("SEM") images
of various
types of treated biochars showing the different nature of their pore
morphology. Figure 2A is
biochar derived from pine. Figure 2B is biochar derived from birch. Figure 2C
is biochar
derived from coconut shells.
[095] The surface area and pore volume for each type of pore, e.g., macro-,
meso- and
micro- can be determined by direct measurement using CO2 adsorption for micro-
, N2
adsorption for meso- and macro pores and standard analytical surface area
analyzers and
methods, for example, particle analyzers such as Micrometrics instruments for
meso- and micro
pores and impregnation capacity for macro pore volume. Mercury porosimetry,
which
measures the macroporosity by applying pressure to a sample immersed in
mercury at a pressure
calibrated for the minimum pore diameter to be measured, may also be used to
measure pore
volume.
[096] The total micropore volume can be from about 2% to about 25% of the
total pore
volume. The total mesopore volume can be from about 4% to about 35% of the
total pore
volume. The total macropore volume can be from about 40% to about 95% of the
total pore
volume. By way of example, Figure 3 shows a bar chart setting out examples of
the pore
volumes for sample biochars made from peach pits 201, juniper wood 202, a
first hard wood
17
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
203, a second hard wood 204, fir and pine waste wood 205, a first pine 206, a
second pine
207, birch 208 and coconut shells 209.
[097] As explained further below, treatment can increase usable pore
volumes and, among
other things, remove obstructions in the pores, which leads to increased
retention properties and
promotes further performance characteristics of the biochar. Knowing the
properties of the
starting raw biochar, one can treat the biochar to produce controlled,
predictable and optimal
resulting physical and chemical properties.
[098] B. Treatment
[099] The rationale for treating the biochar after pyrolysis is that given
the large pore
volume and large surface are of the biochars, it is most efficient to make
significant changes in
the physical and chemical properties of the biochar by treating both the
internal and external
surfaces and internal pore volume of the char. Testing has demonstrated that
if the biochar is
treated, at least partially, in a manner that causes the forced infusion
and/or diffusion of liquids
into and/or out of the biochar pores (through mechanical, physical, or
chemical means), certain
properties of the biochar can be altered or improved over and above simply
contacting these
liquids with the biochar. By knowing the properties of the raw biochar and the
optimal desired
properties of the treated biochar, the raw biochar can then be treated in a
manner that results in
the treated biochar having controlled optimized properties.
[0100] For purposes of this application, treating and/or washing the
biochar in accordance
with the present invention involves more than a simple wash or soak, which
generally only
impacts the exterior surfaces and a small percentage of the interior surface
area. "Washing" or
"treating" in accordance with the present invention, and as used below,
involves treatment of
the biochar in a manner that causes the forced, accelerated or assisted
infusion and/or diffusion
of liquids and/or additivities into and/or out of the biochar pores (through
mechanical, physical,
or chemical means) such that certain properties of the biochar can be altered
or improved over
and above simply contacting these liquids with the biochar or so that
treatment becomes more
efficient or rapid from a time standpoint over simple contact or immersion.
[0101] In particular, effective treatment processes can mitigate
deleterious pore surface
properties, remove undesirable substances from pore surfaces or volume, and
impact anywhere
from between 10% to 99% or more of pore surface area of a biochar particle. By
modifying the
18
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
usable pore surfaces through treatment and removing deleterious substances
from the pore
volume, the treated biochars can exhibit a greater capacity to retain water
and/or other nutrients
as well as being more suitable habitats for some forms of microbial life.
Through the use of
treated biochars, agricultural applications can realize increased moisture
control, increased
nutrient retention, reduced water usage, reduced water requirements, reduced
runoff or
leaching, increased nutrient efficiency, reduced nutrient usage, increased
yields, increased yields
with lower water requirements and/or nutrient requirements, increases in
beneficial microbial
life, improved performance and/or shelf life for inoculated bacteria, and any
combination and
variation of these and other benefits.
[0102] Treatment further allows the biochar to be modified to possess
certain known
properties that enhance the benefits received from the use of biochar. While
the selection of
feedstock, raw biochar and/or pyrolysis conditions under which the biochar was
manufactured
can make treatment processes less cumbersome, more efficient and further
controlled, treatment
processes can be utilized that provide for the biochar to have desired and
generally sustainable
resulting properties regardless of the biochar source or pyrolysis conditions.
As explained
further below, treatment can (i) repurpose problematic biochars, (ii) handle
changing biochar
material sources, e.g., seasonal and regional changes in the source of
biomass, (iii) provide for
custom features and functions of biochar for particular soils, regions or
agricultural purposes;
(iv) increase the retention properties of biochar, (v) provide for large
volumes of biochar having
desired and predictable properties, (vi) provide for biochar having custom
properties, (vii)
handle differences in biochar caused by variations in pyrolysis conditions or
manufacturing of
the "raw" biochar; and (viii) address the majority, if not all, of the
problems that have, prior to
the present invention, stifled the large scale adoption and use of biochars.
[0103] Treatment can wash both the interior and exterior pore surfaces,
remove harmful
chemicals, introduce beneficial substances, and alter certain properties of
the biochar and the
pore surfaces and volumes. This is in stark contrast to simple washing which
generally only
impacts the exterior surfaces and a small percentage of the interior surface
area. Treatment can
further be used to coat substantially all of the biochar pore surfaces with a
surface modifying
agent or impregnate the pore volume with additives or treatment to provide a
predetermined
feature to the biochar, e.g., surface charge and charge density, surface
species and distribution,
19
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
targeted nutrient addition, magnetic modifications, root growth facilitator,
and water
absorptivity and water retention properties. Just as importantly, treatment
can also be used to
remove undesirable substances from the biochar, such as dioxins or other
toxins either through
physical removal or through chemical reactions causing neutralization.
[0104] Figure 4 is a schematic flow diagram of one example treatment
process 400 for use
in accordance with the present invention. As illustrated, the treatment
process 400 starts with
raw biochar 402 that may be subjected to one or more reactors or treatment
processes prior to
bagging 420 the treated biochar for resale. For example, 404 represents
reactor 1, which may
be used to treat the biochar. The treatment may be a simple water wash or may
be an acid wash
used for the purpose of altering the pH of the raw biochar particles 402. The
treatment may
also contain a surfactant or detergent to aid the penetration of the treatment
solution into the
pores of the biochar. The treatment may optionally be heated, cooled, or may
be used at ambient
temperature or any combination of the three. For some applications, depending
upon the
properties of the raw biochar, a water and/or acid/alkaline wash 404 (the
latter for pH
adjustment) may be the only necessary treatment prior to bagging the biochar
420. If, however,
the moisture content of the biochar needs to be adjusted, the treated biochar
may then be put
into a second reactor 406 for purposes of reducing the moisture content in the
washed biochar.
From there, the treated and moisture adjusted biochar may be bagged 420.
[0105] Again, depending upon the starting characteristics of the raw
biochar and the
intended application for the resale product, further processing may still be
needed or desired.
In this case, the treated moisture adjusted biochar may then be passed to a
third reactor 408 for
inoculation, which may include the impregnation of biochar with beneficial
additives, such as
nutrients, bacteria, microbes, fertilizers or other additives. Thereafter, the
inoculated biochar
may be bagged 420, or may be yet further processed, for example, in a fourth
reactor 410 to
have further moisture removed from or added to the biochar. Further moisture
adjustment may
be accomplished by placing the inoculated biochar in a fourth moisture
adjustment reactor 410
or circulating the biochar back to a previous moisture adjustment reactor
(e.g. reactor 406).
Those skilled in the art will recognize that the ordering in which the raw
biochar is processed
and certain processes may be left out, depending on the properties of the
starting raw biochar
and the desired application for the biochar. For example, the treatment and
inoculation
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
processes may be performed without the moisture adjustment step, inoculation
processes may
also be performed with or without any treatment, pH adjustment or any moisture
adjustment.
All the processes may be completed alone or in the conjunction with one or
more of the others.
[0106] For example, Figure 4a illustrates a schematic of one example of an
implementation
of biochar processing that includes washing the pores and both pH and moisture
adjustment.
Figure 4b illustrates yet another example of an implementation of biochar
processing that
includes inoculation.
[0107] As illustrated in Figure 4a, raw biochar 402 is placed into a
reactor or tank 404. A
washing or treatment liquid 403 is then added to a tank and a partial vacuum,
using a vacuum
pump, 405 is pulled on the tank. The treating or washing liquid 403 may be
used to clean or
wash the pores of the biochar 402 or adjust the chemical or physical
properties of the surface
area or pore volume, such as pH level, usable pore volume, or VOC content,
among other things.
The vacuum can be applied after the treatment liquid 403 is added or while the
treatment liquid
403 is added. Thereafter, the washed/ adjusted biochar 410 may be moisture
adjusted by
vacuum exfiltration 406 to pull the extra liquid from the washed/moisture
adjusted biochar 410
or may be placed in a centrifuge 407, heated or subjected to pressure gradient
changes (e.g.,
blowing air) for moisture adjustment. The moisture adjusted biochar 412 may
then be bagged
or subject to further treatment. Any excess liquids 415 collected from the
moisture adjustment
step may be disposed of or recycled, as desired. Optionally, biochar fines may
be collected
from the excess liquids 415 for further processing, for example, to create a
slurry, cakes, or
biochar extrudates.
[0108] Optionally, rather than using a vacuum pump 405, a positive pressure
pump may be
used to apply positive pressure to the tank 404. In some situations, applying
positive pressure
to the tank may also function to force or accelerate the washing or treating
liquid 403 into the
pores of the biochar 402. Any change in pressure in the tank 404 or across the
surface of the
biochar could facilitate the exchange of gas and/or moisture into and out of
the pores of the
biochar with the washing or treating liquid 403 in the tank. Accordingly,
changing the pressure
in the tank and across the surface of the biochar, whether positive or
negative, is within the
scope of this invention.
21
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0109] As illustrated Figure 4b, the washed/ adjusted biochar 410 or the
washed/ adjusted
and moisture adjusted biochar 412 may be further treated by inoculating or
impregnating the
pores of the biochar with an additive 425. The biochar 410, 412 placed back in
a reactor 401,
an additive solution 425 is placed in the reactor 401 and a vacuum, using a
vacuum pump, 405
is applied on the tank. Again, the vacuum can be applied after the additive
solution 425 is added
to the tank or while the additive solution 425 is being added to the tank.
Thereafter, the washed,
adjusted and inoculated biochar 428 can be bagged. Alternatively, if further
moisture
adjustment is required, the biochar can be further moisture adjusted by vacuum
filtration 406 to
pull the extra liquid from the washed/moisture adjusted biochar 410 or may be
placed in a
centrifuge 407 for moisture adjustment. The resulting biochar 430 can then be
bagged. Any
excess liquids 415 collected from the moisture adjustment step may be disposed
of or recycled,
as desired. Optionally, biochar particulates or "fines" which easily are
suspended in liquid may
be collected from the excess liquids 415 for further processing, for example,
to create a slurry,
biochar extrudates, or merely a biochar product of a consistently smaller
particle size. As
described above, both processes of the Figure 4a and 4b can be performed with
a surfactant
solution in place of, or in conjunction with, the vacuum 405.
[0110] While known processes exist for the above described processes,
research associated
with the present invention has shown improvement and the ability to better
control the
properties and characteristics of the biochar if the processes are performed
through the infusion
and diffusion of liquids into and out of the biochar pores. One such treatment
process that can
be used is vacuum impregnation and vacuum and/or centrifuge extraction.
Another such
treatment process that can be used is the addition of a surfactant to infused
liquid, which infused
liquid may be optionally heated, cooled, or used at ambient temperature or any
combination of
the three.
[0111] Since research associated with the present invention has identified
what physical and
chemical properties have the highest impact on plant growth and/or soil
health, the treatment
process can be geared to treat different forms of raw biochar to achieve
treated biochar
properties known to enhance these characteristics. For example, if the pH of
the biochar needs
to be adjusted to enhance the raw biochar performance properties, the
treatment may be the
infusion of an acid solution into the pores of the biochar using vacuum,
surfactant, or other
22
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
treatment means. This treatment of pore infusion through, for example, the
rapid, forced
infusion of liquid into and out the pores of the biochar, has further been
proven to sustain the
adjusted pH levels of the treated biochar for much longer periods than biochar
that is simply
immersed in an acid solution for the same period of time. By way of another
example, if the
moisture content needs to be adjusted, then excess liquid and other selected
substances (e.g.
chlorides, dioxins, and other chemicals, to include those previously deposited
by treatment to
catalyze or otherwise react with substances on the interior or exterior
surfaces of the biochar)
can be extracted from the pores using vacuum and/or centrifuge extraction or
by using various
heating techniques. The above describes a few examples of treatment that
result in treated
biochar having desired performance properties identified to enhance soil
health and plant life.
[0112] Figure 5 illustrates one example of a system 500 that utilizes
vacuum impregnation
to treat raw biochar. Generally, raw biochar particles, and preferably a batch
of biochar
particles, are placed in a reactor, which is connected to a vacuum pump, and a
source of treating
liquid (i.e. water or acidic/basis solution). When the valve to the reactor is
closed, the pressure
in the reactor is reduced to values ranging from 750 Torr to 400 Torr to 10
Torr or less. The
biochar is maintained under vacuum ("vacuum hold time") for anywhere from
seconds to 1
minute to 10 minutes, to 100 minutes, or possibly longer. By way of example,
for about a 500
pound batch of untreated biochar, a vacuum hold time of from about 1 to about
5 minutes can
be used if the reactor is of sufficient size and sufficient infiltrate is
available to adjust the
necessary properties. While under the vacuum the treating liquid may then be
introduced into
the vacuum chamber containing the biochar. Alternatively, the treating liquid
may be
introduced into the vacuum chamber before the biochar is placed under a
vacuum. Optionally,
treatment may also include subjecting the biochar to elevated temperatures
from ambient to
about 250 C or reduced temperatures to about -25 C or below, with the
limiting factor being
the temperature and time at which the infiltrate can remain flowable as a
liquid or semi-liquid.
[0113] The infiltrate or treating liquid is drawn into the biochar pore,
and preferably drawn
into the macropores and mesopores. Depending upon the specific doses applied
and pore
structure of the biochar, the infiltrate can coat anywhere from 10% to 50% to
100% of the total
macropore and mesopore surface area and can fill or coat anywhere from a
portion to nearly all
(10% - 100%) of the total macropore and mesopore volume.
23
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0114] As described above, the treating liquid can be left in the biochar,
with the batch being
a treated biochar batch ready for packaging, shipment and use in an
agricultural or other
application. The treating liquid may also be removed through drying,
subsequent vacuum
processing, centrifugal force (e.g., cyclone drying machines or centrifuges),
with the batch
being a treated biochar batch ready for packaging, shipment and use in an
agricultural
application. A second, third or more infiltration, removal, infiltration and
removal, and
combinations and variations of these may also be performed on the biochar with
optional drying
steps between infiltrations to remove residual liquid from and reintroduce
gasses to the pore
structure if needed. In any of these stages the liquid may contain organic or
inorganic
surfactants to assist with the penetration of the treating liquid.
[0115] As illustrated in Figure 5, a system 500 for providing a biochar,
preferably having
predetermined and uniform properties. The system 500 has a vacuum infiltration
tank 501. The
vacuum infiltration tank 501 has an inlet line 503 that has a valve 504 that
seals the inlet line
503. In operation, the starting biochar is added to vacuum infiltration tank
501 as shown by
arrow 540. Once the tank is filled with the starting biochar, a vacuum is
pulled on the tank, by
a vacuum pump connected to vacuum line 506, which also has valve 507. The
starting biochar
is held in the vacuum for a vacuum hold time. Infiltrate, as shown by arrow
548 is added to the
tank 501 by line 508 having valve 509. The infiltrate is mixed with the
biochar in the tank 501
by agitator 502. The mixing process is done under vacuum for a period of time
sufficient to
have the infiltrate fill the desired amount of pore volume, e.g., up to 100%
of the macropores
and mesopores.
[0116] Alternatively, the infiltrate may be added to the vacuum
infiltration tank 501 before
vacuum is pulled on the tank. In this manner, infiltrate is added in the tank
in an amount that
can be impregnated into the biochar. As the vacuum is pulled, the biochar is
circulated in the
tank to cause the infiltrate to fill the pore volume. To one skilled in the
art, it should be clear
that the agitation of the biochar during this process can be performed through
various means,
such as a rotating tank, rotating agitator, pressure variation in the tank
itself, or other means.
Additionally, the biochar may be dried using conventional means before even
the first
treatment. This optional pre-drying can remove liquid from the pores and in
some situations
may increase the efficiency of impregnation due to pressure changes in the
tank.
24
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0117] Pressure is then restored in the tank 501 and the infiltrated
biochar is removed, as
shown by arrow 541, from the tank 501 to bin 512, by way of a sealing gate 511
and removal
line 510. The infiltrated biochar is collected in bin 512, where it can be
further processed in
several different ways. The infiltrated biochar can be shipped for use as a
treated biochar as
shown by arrow 543. The infiltrated biochar can be returned to the tank 501
(or a second
infiltration tank). If returned to the tank 501 the biochar can be processed
with a second
infiltration step, a vacuum drying step, a washing step, or combinations and
variations of these.
The infiltrated biochar can be moved by conveyor 514, as shown by arrow 542,
to a drying
apparatus 516, e.g., a centrifugal dryer or heater, where water, infiltrate or
other liquid is
removed by way of line 517, and the dried biochar leaves the dryer through
discharge line 518
as shown by arrow 545, and is collected in bin 519. The biochar is removed
from the bin by
discharge 520. The biochar may be shipped as a treated biochar for use in an
agriculture
application, as shown by arrow 547. The biochar may also be further processed,
as shown by
546. Thus, the biochar could be returned to tank 501 (or a second vacuum
infiltration tank) for
a further infiltration step. The drying step may be repeated either by
returning the dry biochar
to the drying apparatus 516, or by running the biochar through a series of
drying apparatus, until
the predetermined dryness of the biochar is obtained, e.g., between 50% to
less than 1%
moisture.
[0118] The system 500 is illustrative of the system, equipment and
processes that can be
used for, and to carry out the present inventions. Various other
implementations and types of
equipment can be used. The vacuum infiltration tank can be a sealable off-axis
rotating vessel,
chamber or tank. It can have an internal agitator that also when reversed can
move material
out, empty it, (e.g., a vessel along the lines of a large cement truck, or
ready mix truck, that can
mix and move material out of the tank, without requiring the tank's
orientation to be changed).
Washing equipment may be added or utilized at various points in the process,
or may be carried
out in the vacuum tank, or drier, (e.g., wash fluid added to biochar as it is
placed into the drier
for removal). Other steps, such as bagging, weighing, the mixing of the
biochar with other
materials, e.g., fertilized, peat, soil, etc. can be carried out. In all areas
of the system referring
to vacuum infiltration, optionally positive pressure can be applied, if
needed, to enhance the
penetration of the infiltrate or to assist with re-infusion of gaseous vapors
into the treated char.
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
Additionally, where feasible, especially in positive pressure environments,
the infiltrate may
have soluble gasses added which then can assist with removal of liquid from
the pores, or
gaseous treatment of the pores upon equalization of pressure.
[0119] As noted above, the biochar may also be treated using a surfactant.
The same or
similar equipment used in the vacuum infiltration process can be used in the
surfactant treatment
process. Although it is not necessary to apply a vacuum in the surfactant
treatment process, the
vacuum infiltration tank or any other rotating vessel, chamber or tank can be
used. In the
surfactant treatment process, a surfactant, such as yucca extract, is added to
the infiltrate, e.g.,
acid wash or water. The quantity of the surfactant added to the infiltrate may
vary depending
upon the surfactant used. For example, organic yucca extract can be added at a
rate of between
0.1 - 20%, but more preferably 1-5% by volume of the infiltrate. The
infiltrate with surfactant
is then mixed with the biochar in a tumbler for several minutes, e.g., 3-5
minutes, without
applied vacuum. Optionally, a vacuum or positive pressure may be applied with
the surfactant
to improve efficiency, but is not necessary. Additionally, infiltrate to which
the surfactant or
detergent is added may be heated or may be ambient temperature or less.
Similarly, the mixture
of the surfactant or detergent, as well as the char being treated may be
heated, or may be ambient
temperature, or less. After tumbling, excess free liquid can be removed in the
same manner as
described above in connection with the vacuum infiltration process. Drying,
also as described
above in connection with the vacuum infiltration process, is an optional
additional step. Besides
yucca extract, a number of other surfactants may be used for surfactant
treatment, which
include, but are not limited to, the following: nonionic types, such as,
ethoxylated alcohols,
phenols- lauryl alcohol ethoxylates, Fatty acid esters- sorbitan, tween 20,
amines, amides-
imidazoles; anionic types, such as sulfonates- arylalkyl sulfonates and
sulfate- sodium dodecyl
sulfate; cationic types, such as alkyl- amines or ammoniums- quaternary
ammoniums; and
amphoteric types, such as betaines- cocamidopropyl betaine.
[0120] Optionally, the biochar may also be treated by applying ultrasonics.
In this treatment
process, the biochar may be contacted with a treating liquid that is agitated
by ultrasonic waves.
By agitating the treating liquid, contaminants may be dislodged or removed
from the biochar
due to bulk motion of the fluid in and around the biocarbon, pressure changes,
including
26
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
cavitation in and around contaminants on the surface, as well as pressure
changes in or near
pore openings (cavitation bubbles) and internal pore cavitation.
[0121] In this manner, agitation will cause contaminants of many forms to
be released from
the internal and external structure of the biochar. The agitation also
encourages the exchange
of water, gas, and other liquids with the internal biochar structure.
Contaminants are transported
from the internal structure to the bulk liquid (treating fluid) resulting in
biochar with improved
physical and chemical properties. The effectiveness of ultrasonic cleaning is
tunable as bubble
size and number is a function of frequency and power delivered by the
transducer to the treating
fluid
[0122] In one example, applying ultrasonic treatment, raw wood based
biochar between 10
microns to 10 mm with moisture content from 0% to 90% may be mixed with a
dilute mixture
of acetic acid and water (together the treating liquid) in a processing vessel
that also translates
the slurry (the biochar/treating liquid mixture). During translation, the
slurry passes near an
ultrasonic transducer to enhance the interaction between the fluid and
biochar. The biochar
may experience one or multiple washes of dilute acetic acid, water, or other
treating fluids. The
biochar may also make multiple passes by ultrasonic transducers to enhance
physical and
chemical properties of the biochar. For example, once a large volume of slurry
is made, it can
continuously pass an ultrasonic device and be degassed and wetted to its
maximum, at a rapid
processing rate. The slurry can also undergo a separation process in which the
fluid and solid
biochar are separated at 60% effectiveness or greater.
[0123] Through ultrasonic treatment, the pH of the biochar, or other
physical and chemical
properties may be adjusted and the mesopore and macropore surfaces of the
biochar may be
cleaned and enhanced. Further, ultrasonic treatment can be used in combination
with bulk
mixing with water, solvents, additives (fertilizers, etc.), and other liquid
based chemicals to
enhance the properties of the biochar. After treatment, the biochar may be
subject to moisture
adjustment, further treatment and/or inoculation using any of the methods set
forth above.
[0124] C. Benefits of Treatment
[0125] As illustrated above, the treatment process, whether using vacuum,
surfactant or
ultrasonic treatment, or a combination thereof, may include two steps, which
in certain
applications, may be combined: (i) washing and (ii) inoculation of the pores
with an additive.
27
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
When the desired additive is the same and that being inoculated into the
pores, e.g., water, the
step of washing the pores and inoculating the pores with an additive may be
combined.
[0126] While not exclusive, washing is generally done for one of three
purposes: (i) to
modify the surface of the pore structure of the biochar (i.e., to allow for
increased retention of
liquids); (ii) to modify the pH of the biochar; and/or (iii) to remove
undesired and potentially
harmful compounds or gases.
[0127] 1. Increases Water Holding Capacity/Water Retention Capacity
[0128] As demonstrated below, the treatment processes of the invention
modify the surfaces
of the pore structure to provide enhanced functionality and to control the
properties of the
biochar to achieve consistent and predicable performance. Using the above
treatment processes,
anywhere from at least 10% of the total pore surface area up to 90% or more of
the total pore
surface area may be modified. In some implementations, it may be possible to
achieve
modification of up to 99% or more of the total pore surface area of the
biochar particle. Using
the processes set forth above, such modification may be substantially and
uniformly achieved for
an entire batch of treated biochar.
[0129] For example, it is believed that by treating the biochar as set
forth above, the
hydrophilicity of the surface of the pores of the biochar is modified,
allowing for a greater water
retention capacity. Further, by treating the biochars as set forth above,
gases and other substances
are also removed from the pores of the biochar particles, also contributing to
the biochar particles'
increased water holding capacity. Thus, the ability of the biochar to retain
liquids, whether water
or additives in solution, is increased, which also increases the ability to
load the biochar particles
with large volumes of inoculant, infiltrates and/or additives.
[0130] A batch of biochar has a bulk density, which is defined as weight in
grams (g) per cm3
of loosely poured material that has or retains some free space between the
particles. The biochar
particles in this batch will also have a solid density, which is the weight in
grams (g) per cm3 of
just particles, i.e., with the free space between the particles removed. The
solid density includes
the air space or free space that is contained within the pores, but not the
free space between
particles. The actual density of the particles is the density of the material
in grams (g) per cm3 of
material, which makes up the biochar particles, i.e., the solid material with
pore volume removed.
28
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0131] In general, as bulk density increases the pore volume is expected to
decrease. When
the pore volume is macro or mesoporous, the ability of the material to hold
infiltrate, e.g.,
inoculant is directly proportional to the pore volume, thus it is also
expected to decrease as bulk
density increases. With the infiltration processes, the treated biochars can
have impregnation
capacities that are larger than could be obtained without infiltration, e.g.,
the treated biochars
can readily have 30%, 40%, 50%, or most preferably, 60%-100% of their total
pore volume
filled with an infiltrate, e.g., an inoculant. The impregnation capacity is
the amount of a liquid
that a biochar particle, or batch of particles, can absorb. The ability to
make the pores surface
hydrophilic, and to infuse liquid deep into the pore structure through the
application of positive
or negative pressure and/or a surfactant, alone or in combination, provides
the ability to obtain
these high impregnation capabilities. The treated biochars can have
impregnation capacities,
i.e., the amount of infiltrate that a particle can hold on a volume held/total
volume of a particle
basis, that is greater than 0.2 cm3/cm3 to 0.8 cm3/cm3. Resulting bulk
densities of treated biochar
can range from 0.1- 0.6 g/cm3 and sometimes preferably between 0.3 - 0.6 g/cm3
and can have
solid densities ranging from 0.2 ¨ 1.2 g/cm3.
[0132] Accordingly, by using the treatment above, the water retention
capacity of biochar can
be greatly increased over the water retention capacities of various soil types
and even raw biochar,
thereby holding water and/or nutrients in the plant's root zone longer and
ultimately reducing the
amount of applied water (through irrigation, rainfall, or other means) needed
by up to 50% or
more. Figure 6 is a chart showing the water retention capacities of soils
versus raw and treated
biochar. The soils sampled are loam and sandy clay soil and a common
commercial horticultural
mix. The charts show the retained water as a function of time.
[0133] In chart A, the bottom line represents the retained water in the
sandy claim loam soil
over time. The middle line represents the retained water in the sandy clay
soil with 20% by
volume percent of unprocessed raw biochar. The top line represents the
retained water in the
sandy clay loam soil with 20% by volume percent of treated biochar (adjusted
and inoculated
biochar). Chart B represents the same using a soilless potting mix rather than
sandy clay loam
soil.
[0134] As illustrated in Figure 7, testing showed a treated biochar had an
increased water
retention capacity of approximately 1.5 times that of the raw biochar from the
same feedstock.
29
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
Similar results have been seen with biochars derived from various feedstocks.
With certain
biochar types, the water retention capacity of treated biochar could be as
great as three times that
of raw biochar.
[0135] "Water holding capacity," which may also be referred to as "Water
Retention
Capacity," is the amount of water that can be held both internally within the
porous structure and
in the interparticle void spaces in a given batch of particles. While a
summary of the method of
measure is provided above, a more specific method of measuring water holding
capacity/water
retention capacity is measured by the following procedure: (i) drying a sample
of material under
temperatures of 105 C for a period of 24 hours or using another
scientifically acceptable
technique to reduce the moisture content of the material to less than 2%, less
than 1%; and
preferably less than .5% (ii) placing a measured amount of dry material in a
container; (iii) filling
the container having the measured amount of material with water such that the
material is
completely immersed in the water; (iv) letting the water remain in the
container having the
measured amount of material for at least ten minutes or treating the material
in accordance with
the invention by infusing with water when the material is a treated biochar;
(v) draining the water
from the container until the water ceases to drain; (vi) weighing the material
in the container (i.e.,
wet weight); (vii) again drying the material by heating it under temperatures
of 105 C for a period
of 24 hours or using another scientifically acceptable technique to reduce the
moisture content of
the material to less than 2% and preferably less than 1%; and (viii) weighing
the dry material
again (i.e., dry weight) and, for purposes of a volumetric measure,
determining the volume of the
material.
[0136] Measured gravimetrically, the water holding/water retention capacity
is determined
by measuring the difference in weight of the material from step (vi) to step
(viii) over the weight
of the material from step (viii) (i.e., wet weight-dry weight/dry weight).
Figure 8 illustrates the
different water retention capacities of raw biochar versus treated biochar
measured
gravimetrically. As illustrated, water retention capacity of raw biochar can
be between 100-
200%, whereas treated biochar can have water retention capacities measured
gravimetrically
between 200-400%.
[0137] Water holding capacity can also be measured volumetrically and
represented as a
percent of the volume of water retained in the biochar after gravitationally
draining the excess
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
water/volume of biochar The volume of water retained in the biochar after
draining the water can
be determined from the difference between the water added to the container and
water drained
off the container or from the difference in the weight of the wet biochar from
the weight of the
dry biochar converted to a volumetric measurement. This percentage water
holding capacity for
treated biochar may be 50-55 percent and above by volume.
[0138] Given biochar' s increased water retention capacity, the application
of the treated
biochar and even the raw biochar can greatly assist with the reduction of
water and/or nutrient
application. It has been discovered that these same benefits can be imparted
to agricultural
growth.
[0139] Treated biochar of the present invention has also demonstrated the
ability to retain
more water than raw biochar after exposure to the environment for defined
periods of time. For
purposes of this application "remaining water content" can be defined as the
total amount of water
that remains held by the biochar after exposure to the environment for certain
amount of time.
Exposure to environment is exposure at ambient temperature and pressures.
Under this definition,
remaining water content can be measured by (i) creating a sample of biochar
that has reached its
maximum water holding capacity; (ii) determining the total water content by
thermogravimetric
analysis (H20 (TGA)), as described above on a sample removed from the output
of step (i) above,
(iii) exposing the biochar in the remaining sample to the environment for a
period of 2 weeks (15
days, 360 hrs.); (iv) determining the remaining water content by
thermogravimetric analysis (H20
(TGA)); and (v) normalizing the remaining (retained) water in mL to 1 kg or 1
L biochar. The
percentage of water remaining after exposure for this two-week period can be
calculated by the
remaining water content of the biochar after the predetermined period over the
water content of
the biochar at the commencement of the two-week period. Using this test,
treated biochar has
shown to retain water at rates over 4x that of raw biochar. Testing has
further demonstrated that
the following amount of water can remain in treated biochar after two weeks of
exposure to the
environment: 100-650 mL/kg; 45-150 mL/L; 12-30 gal/ton; 3-10 gal/yd3 after 360
hours (15 days)
of exposure to the environment. In this manner, and as illustrated in Figure
8, biochar treated with
a process consistent with those described in this disclosure can increase the
amount of retained
water in biochar about 3 times compared to other methods even after seven
weeks. In general,
the more porous and the higher the surface area of a given material, the
higher the water retention
31
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
capacity. Further, it is theorized that by modifying the
hydrophilicity/hydrophobicity of the pore
surfaces, greater water holding capacity and controlled release may be
obtained. Thus, viewed
as a weight percent, e.g., the weight of retained water to weight of biochar,
examples of the present
biochars can retain more than 5% of their weight, more than 10% of their
weight, and more than
15% of their weight, and even more than 50% of their weight compared to an
average soil which
may retain 2% or less, or between 100-600 ml/kg by weight of biochar.
[0140] Tests have also shown that treated biochars that show weight loss of
> 1% in the
interval between 43-60 C when analyzed by the Thermal Gravimetric Analysis
(TGA) (as
described below) demonstrate greater water holding and content capacities over
raw biochars.
Weight loss of > 5%-15% in the interval between 38-68 C when analyzed by the
Thermal
Gravimetric Analysis (TGA) using sequences of time and temperature disclosed
in the following
paragraphs or others may also be realized. Weight percentage ranges may vary
from between >
1% - 15% in temperature ranges between 38-68 C, or subsets thereof, to
distinguish between
treated biochar and raw biochar.
[0141] Figure 9 is a chart 900 showing the weight loss of treated biochars
902 versus raw
biochar samples 904 when heated at varying temperatures using the TGA testing
described below.
As illustrated, the treated biochars 902 continue to exhibit weight loss when
heated between 40-
60 C when analyzed by the Thermal Gravimetric Analysis (TGA) (described
below), whereas
the weight loss in raw biochar 804 between the same temperature ranges levels
off. Thus, testing
demonstrates the presence of additional moisture content in treated biochars
902 versus raw
biochars 904.
[0142] In particular, the treated biochars 902 exhibit substantial water
loss when heated in
inert gas such as nitrogen following treatment. More particularly, when heated
for 25 minutes at
each of the following temperatures 20, 30, 40, 50 and 60 C the treated
samples lose about 5-%
to 15% in the interval 43 - 60 C and upward of 20-30% in the interval between
38- 68 C. The
samples to determine the water content of the raw biochar were obtained by
mixing a measured
amount of biochar and water, stirring the biochar and water for 2 minutes,
draining off the water,
measuring moisture content and then subjecting the sample to TGA. The samples
for the treated
biochar were obtained by using the same measured amount of biochar as used in
the raw biochar
32
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
sample and using treatment process consistent with those described in this
disclosure. .The
moisture content is then measured and the sample is subjected to TGA described
above.
[0143] The sequences of time and temperature conditions for evaluating the
effect of biochars
heating in inert atmosphere is defined in this application as the "Bontchev-
Cheyne Test" ("BCT").
The BCT is run using samples obtained, as described above, and applying
Thermal Gravimetric
Analysis (TGA) carried out using a Hitachi STA 7200 analyzer under nitrogen
flow at the rate of
110 mL/min. The biochar samples are heated for 25 minutes at each of the
following
temperatures: 20, 30, 40, 50 and 60 C. The sample weights are measured at the
end of each dwell
step, at the beginning and at the end of the experiment. The analyzer also
continually measures
and records weight over time. Biochars treated with a process consistent with
those described in
this disclosure to enhance water holding or retention capacities typically
exhibit weight loss of >
5% in the interval between 38-68 C, > 1% in the interval between 43-60 C.
Biochars with
greater water holding or retention capacities can exhibit > 5% weight loss in
the interval between
43-60 C measured using the above described BCT.
[0144] Lastly, as illustrated in Figure 10, plant available water is
greatly increased in treated
biochar over that of raw biochar. Figure 10 illustrates the plant available
water in raw biochar,
versus treated biochar and treated dried biochar and illustrates that treated
biochar can have a
plant available water percent of greater than 35% by volume.
[0145] "Plant Available Water" is the amount of unbound water in a material
available for
plants to uptake. This is calculated by subtracting the volumetric water
content at the permanent
wilting point from the volumetric water content at field capacity, which is
the point when no water
is available for the plants. Field capacity is generally expressed as the bulk
water content retained
in at ¨33 J/kg (or ¨0.33 bar) of hydraulic head or suction pressure. Permanent
wilting point is
generally expressed as the bulk water content retained at -1500J/kg (or -15
bar) of hydraulic head
or suction pressure. Methods for measuring plant available water are well-
known in the industry
and use pressure plate extractors, which are commercially available or can be
built using well-
known principles of operation.
[0146] 2. Adjusts pH
[0147] With regard to treatment for pH adjustment, the above described
vacuum infiltration
processes and/or surfactant treatment processes have the ability to take raw
biochars having
33
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
detrimental or deleterious pHs and transform those biochars into a treated
biochar having pH that
is in an optimal range for most plant growth, and soil health. Turning to
Figure 11, a graph 1100
is provided that shows the pH of various starting raw biochars that were made
from different
starting materials and pyrolysis process temperatures, including coconut
shells 1104, pistachio
shells 1101, corn at 500 C 1105, corn at 900 C 1102, bamboo 1103, mesquite
1106, wood and
coffee 1108, wood (Australia) 1109, various soft woods 1110, 1111, 1112, 1113,
1114, 1115,
1116, 1117, red fir at 900 C 1107, various grasses at 500 C 1118, 1119, 1120,
grass 1121, and
grass at 900 C 1123. The treatment processes described in this disclosure, can
be used to alter
the pH from the various undesirable pH levels and bring the pH into the
preferred, optimal range
1124 for most plant growth, soil health and combinations of these. Figure 12
is a chart 1200
showing percentage of germination for lettuce plants for particular pHs, and a
desired germination
range 1201. A control 1204 is compared with an optimal pH range 1202, and a
distribution 1203
of growth rates across pHs is shown.
[0148] If treated for pH adjustment, the treated biochar takes a few days
after treatment for
the pH to normalize. Once normalized, tests have proven that pH altered
biochar remains at a
stable pH, typically the treatment is used to lower the stable pH to below
that of the raw biochar,
for up to 12 months or more after treatment. Although in certain situations,
the pH could be
altered to be higher than the raw biochar when needed.
[0149] For example, the treatment process of the present invention can
remove and/or
neutralize inorganic compounds, such as the calcium hydroxide ((CaOH)2),
potassium oxide
(K20K20K20), magnesium oxide (MgO), magnesium hydroxide (Mg(OH)2), and many
others
that are formed during pyrolysis, and are fixed to the biochar pore surfaces.
These inorganics, in
particular calcium hydroxide, adversely affect the biochar's pH, making the pH
in some instances
as high as 8.5, 9.5, 10.5 and 11.2. These high pH ranges are deleterious,
detrimental to crops, and
may kill or adversely affect the plants, sometimes rendering an entire field a
loss.
[0150] The calcium hydroxide, and other inorganics, cannot readily and
quickly be removed
by simple washing of the biochar, even in an acid bath. It cannot be removed
by drying the
biochar, such as by heating or centrifugal force. It is theorized that these
techniques and
methodologies cannot reach or otherwise affect the various pore surfaces,
e.g., macro-, meso- and
34
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
micro- in any viable or efficacious manner; and thus cannot remove or
otherwise neutralize the
calcium hydroxide.
[0151] Upon modification of the pore surface area by removal and/or
neutralization of
deleterious substances, such as calcium hydroxide, the pH of the biochar can
be reduced to the
range of about pH 5 to about pH 8, and more preferably from about pH 6.4 to
about 7.2, and still
more preferably around 6.5 to 6.8, recognizing that other ranges and pHs are
contemplated and
may prove useful, under specific environmental or agricultural situations or
for other applications.
Thus, the present treated biochars, particles, batches and both, have most,
essentially all, and more
preferably all, of their pore surfaces modified by the removal, neutralization
and both, of the
calcium hydroxide that is present in the starting biochar material. These
treated biochars have
pHs in the range of about 5 to about 8, about 6.5 to about 7.5, about 6.4 to
about 7, and about 6.8.
Prior to and before testing, biochar is passed through a 2mm sieve before pH
is measured. All
measurements are taken according to Rajkovich et. al, Corn growth and nitrogen
nutrition after
additions of biochars with varying properties to a temperate soil, Biol.
Fertil. Soils (2011), from
which the International Biochar Initiative (IBI) method is based.
[0152] There are a wide variety of tests, apparatus and equipment for
making pH
measurements. For example, and preferably when addressing the pH of biochar,
batches, particles
and pore surfaces of those particles, two appropriates for measuring pH are
the Test Method for
the US Composting Council ("TMCC") 4.11-A and the pH Test Method promulgated
by the
International Biochar Initiative. The test method for the TMCC comprises
mixing biochar with
distilled water in 1: 5 [mass : volume] ratio, e.g., 50 grams of biochar is
added to 250 ml of pH
7.0 0.02 water and is stirred for 10 minutes; the pH is then the measured pH
of the slurry. The
pH Test Method promulgated by the International Biochar Initiative comprises 5
grams of biochar
is added to 100 ml of Aiate pH= 7.0 0.02 and the mixture is tumbled for 90
minutes; the pH of
the slurry is measured at the end of the 90 minutes of tumbling.
[0153] 3. Removing/Neutralizing Deleterious Materials
[0154] Further, the treatment processes are capable of modifying the pore
surfaces to remove
or neutralize deleterious materials that are otherwise difficult, if not for
all practical purpose,
impossible to mitigate. For example, heavy metals, transition metals, sodium
and phytotoxic
organics, polycyclic aromatic hydrocarbons, volatile organic compounds (VOCs),
and perhaps
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
other phytotoxins. Thus, by treating the biochar in accordance with the
treatment processes set
forth and described above, the resulting treated biochar has essentially all,
and more preferably
all, of their pore surfaces modified by the removal, neutralization and both,
of one or more
deleterious, harmful, o r potentially harmful material that is present in the
starting biochar
material.
[0155] For example, treatment can reduce the total percentage of residual
organic
compounds (ROC), including both the percentage of heavy ROCs and percentage of
VOCs.
Through treatment, the total ROC can be reduced to 0-25% wt.%, percentage
heavy ROC
content can be reduced to 0-20% wt.% and VOC content can be reduced to less
than 5% wt.%.
For purposes of this application, "Residual organic compounds" (ROCs) are
defined as
compounds that burn off during thermogravimetric analysis, as defined above,
between 150
degrees C and 950 degrees C. Residual organic compounds include, but are not
limited to,
phenols, polyaromatic hydrocarbons, monoaromatic hydrocarbons, acids,
alcohols, esters, ethers,
ketones, sugars, alkanes and alkenes. Of the ROCs, those that burn off using
thermogravimetric
analysis between 150 degrees C and 550 degrees are considered light organic
compounds
(volatiles or VOCs), and those that burn off between 550 degrees C and 950
degrees C are heavy
residual organic compounds. It should be noted that there may be some
inorganic compounds
which also are burned off during TGA analysis in these temperature ranges, but
these are
generally a very low percentage of the total emission and can be disregarded
in the vast majority
of cases as slight variations. In any of these measurements, a gas
chromatograph / mass
spectrometer may be used if needed for higher degrees of precision.
[0156] The percent water, total organic compounds, total light organic
compounds (volatiles
or VOC) and total heavy organic compounds, as referenced in this application
as contained in a
biochar particle or particles in a sample may all be measured by
thermogravimetric analysis.
Thermogravimetric analysis is performed by a Hitachi STA 7200 analyzer or
similar piece of
equipment under nitrogen flow at the rate of 110 mL/min. The biochar samples
are heated for
predetermined periods of time, e.g., 20 minutes, at a variety of temperatures
between 100 and 950
C. The sample weights are measured at the end of each dwell step and at the
beginning and at
the end of the experiment. Thermogravimetric analysis of a given sample
indicating percentage
water in a sample is determined by % mass loss measured between standard
temperature and 150
36
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
degrees C. Thermogravimetric analysis of a given sample indicating percentage
of residual
organic compounds is measured by percentage mass loss sustained between 150
degrees C and
950 degrees C. Thermogravimetric analysis of a given sample indicating
percentage of light
organic compounds (volatiles) is measured by percentage mass loss sustained
between 150
degrees C and 550 degrees C. Thermogravimetric analysis of a given sample
indicating
percentage of heavy organic compounds is measured by percentage mass loss
sustained between
550 degrees C and 950 degrees C. Figure 13 is an example of a
Thermogravimetric Analysis
(TGA) plot outlining the above explanation and the measure of water, light
organics and heavy
organics.
[0157] As noted above, treatment can remove or neutralize heavy metals,
transition
metals, sodium and phytotoxic organics, polycyclic aromatic hydrocarbons,
volatile organic
compounds (VOCs), other phytotoxins, and even dioxins. Thus, by treating the
biochar in
accordance with the treatment processes set forth and described above, the
resulting treated
biochar has essentially all, and more preferably all, of their pore surfaces
modified by the
removal, neutralization or both, of one or more deleterious, harmful, o r
potentially harmful
material that is present in the starting biochar material.
[0158] Dioxins may also be removed through the treatment processes of the
present
invention. Dioxins are released from combustion processes and thus are often
found in raw
biochar. Dioxins include polychlorinated dibenzo-p-dioxins (PCDDs) (i.e., 75
congeners (10
are specifically toxic)); polychlorinated dibenzofurans (PCDFs) (i.e., 135
congeners (7 are
specifically toxic)) and polychlorinated biphenyls (PCBs) (Considered dioxin-
like compounds
(DLCs)).
[0159] Since some dioxins may be carcinogenic even at low levels of
exposure over
extended periods of time, the FDA views dioxins as a contaminant and has no
tolerances or
administrative levels in place for dioxins in animal feed. Dioxins in animal
feed can cause
health problems in the animals themselves. Additionally, the dioxins may
accumulate in the fat
of food-producing animals and thus consumption of animal derived foods (e.g.
meat, eggs, milk)
could be a major route of human exposure to dioxins. Thus, if biochar is used
in animal
applications, where the animals ingest the biochar, the ability to remove
dioxins from the raw
biochar prior to use is of particular significance.
37
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0160] Results have proven the removal of dioxins from raw biochar by
applying the
treatment process of the present invention. To demonstrate the removal of
dioxins, samples of
both raw biochar and biochar, treated within the parameters set forth above,
were sent out for
testing. The results revealed that the dioxins in the raw biochar were removed
through treatment
as the dioxins detected in the raw biochar sample were not detected in the
treated biochar
sample. Below is a chart comparing the test results of measured dioxins in the
raw verses the
treated biochar.
Dioxins Amount Detected in Raw Biochar Amount Detected in Treated
Biochar
Sample Sample
Tetradioxins 26.4 ng/Kg-dry Not detectable
Pentadioxins 5.86 ng/Kg-dry Not detectable
Hexadioxins 8.41 ng/Kg-dry Not detectable
[0161] A number of different dioxins exist, several of which are known to
be toxic or
undesirable for human consumption. Despite the test results above, it is
possible that any
number of dioxins could be present in raw biochar depending on the biomass or
where the
biomass is grown. It is shown, however, in the above testing, that the
treatment process of the
present invention can be used to eliminate dioxins present in raw biochar.
[0162] Seventeen tetra-octo dioxins and furan congeners are the basis for
regulatory
compliance. Other dioxins are much less toxic. Dioxins are generally regulated
on toxic
equivalents (TEQ) and are represented by the sum of values weighted by Toxic
Equivalency
TEQ = X TEFi
Factor (TEF)
[0163] 2,3,7,8-TCDD has a TEF of 1 (most toxic). TEQ is measured as ng/kg
WHO-
PCDD/F-TEQ//kg NDs are also evaluated. Two testing methods are generally used
to determine
TEQ values: EPA Method 8290 (for research and understanding at low levels (ppt-
ppq); and
EPA Method 1613B (for regulatory compliance). Both are based on high
resolution gas
chromatography (HRGC)/high resolution mass spectrometry (HRMS).
38
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0164] The required EU Feed Value is equal to or less than 0.75 ng/kg WHO-
PCDD/F-
TEQ//kg. Treated biochar, in accordance with the present invention, has shown
to have TEQ
dioxins less than 0.5 ng/kg WHO-PCDD/F-TEQ//kg, well below the requirement for
EU Feed
limits of 0.75 ng/kg WHO-PCDD/F-TEQ//kg. As further set forth above, treatment
can reduce
the amount of detectable dioxins from raw biochar such that the dioxins are
not detectible in
treated biochar. Two methods are used: EPA Method 8290 (for research and
understanding
at low levels (ppt-ppq); and EPA Method 1613B (for regulatory compliance).
Both are based
on high resolution gas chromatography (HRGC)/high resolution mass spectrometry
(HRMS).
[0165] 4. Pore Volume
[0166] Generally, a treated biochar sample has greater than 50% by volume
of its porosity
in macropores (pores greater than 300 nanometers). Further, results indicate
that greater than
75% of pores in treated biochar are below 50,000 nanometers. Also, results
indicate that greater
than 50% by volume of treated biochar porosity are pores in the range of 500
nanometers and
100,000 nanometers. Bacterial sizes are typically 500 nanometers to several
thousand
nanometers. Bacteria and other microbes have been observed to fit and colonize
in the pores of
treated biochar, thus supporting the pore size test results.
[0167] Macropore volume is determined by mercury porosimetry, which
measures the meso
and/or macro porosity by applying pressure to a sample immersed in mercury at
a pressure
calibrated for the minimum pore diameter to be measured (for macroporosity
this is 300
nanometers). This method can be used to measure pores in the range of 3 nm to
360,000 nm.
Total volume of pores per volumetric unit of substance is measured using gas
expansion method.
[0168] Depending upon the biomass from which the biochar is derived,
mercury
porosimetry testing has shown that washing under differential pressure, using
the processes
described above, can increase the number of both the smallest and larger pores
in certain biochar
(e.g., pine) and can increase the number of usable smaller pores. Treatment of
biochar using
either vacuum or surfactant does alter the percentage of total usable pores
between 500 to
100,000 nanometers and further has varying impact on pores less than 50,000
nanometers and
less than 10,000 nanometers.
[0169] Figure 14 is a chart 1400 showing the impact of treatment on pores
sizes of biochar
derived from coconut. The majority of the coconut based biochar pores are less
than 10 microns.
39
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
Many are less than 1 micron. Vacuum processing of the biochar results in small
reduction of
to 50 micron pores, with increase of smaller pores on vacuum processing. The
mercury
porosimetry results of the raw biochar are represented by 1402 (first column
in the group of
three). The vacuum treated biochar is represented by 1404 (second column in
the group of
three) and the surfactant treated biochar is 1406 (third column in the group
of three).
[0170] Figure 15 is a chart 1500 showing the impact of treatment on pores
sizes of biochar
derived from pine. The majority of the pine based biochar pores are 1 to 50
microns, which is
a good range for micro-biologicals. Vacuum processing results in significant
reduction of the
10 to 50 micron pores, with an increase of smallest and largest pores. The
mercury porosimetry
results of the raw biochar are represented by 1502 (first column in the group
of three). The
vacuum treated biochar is represented by 1504 (second column in the group of
three) and the
surfactant treated biochar is 3006 (third column in the group of three).
[0171] 5. Electrical Conductivity
[0172] The electrical conductivity (EC) of a solid material-water mixture
indicates the
amount of salts present in the solid material. Salts are essential for plant
growth. The EC
measurement detects the amount of cations or anions in solution; the greater
the amount of ions,
the greater the EC. The ions generally associated with salinity are Ca2 , Mg2
, K , Nat, fl+ ,
NO3-, 5042-, Cl-, HCO3, OH-. Electrical conductivity testing of biochar was
done following the
method outlined in the USDA's Soil Quality Test Kit Guide and using a
conventional EC meter.
The biochar sample is mixed with DI water in a 1:1 biochar to water ratio on a
volume basis.
After thorough mixing, the EC (dS/m) is measured while the biochar particles
are still suspended
in solution. Treatment, as outlined in this disclosure can be used to adjust
the ions in the char.
Testing of treated biochar shows its EC is generally greater than 0.2 dS/m and
sometimes greater
than 0.5 dS/m.
[0173] 6. Cation Exchange Capacity
[0174] One method for cation exchange capacity ("CEC") determination is the
use of
ammonium acetate buffered at pH 7.0 (see Schollenberger, C.J. and Dreibelbis,
ER. 1930,
Analytical methods in base-exchange investigations on soils, Soil Science, 30,
161-173). The
material is saturated with 1M ammonium acetate, (NH40Ac), followed by the
release of the
NH4 + ions and its measurement in meq/100 g (milliequivalents of charge per
100 g of dry soil)
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
or cmolc/kg (centimoles of charge per kilogram of dry soil). Instead of
ammonium acetate
another method uses barium chloride according to Mehlich, 1938, Use of
triethanolamine
acetate-barium hydroxide buffer for the determination of some base exchange
properties and
lime requirement of soil, Soil Sci. Soc. Am. Proc. 29:374-378. 0.1 M BaC12 is
used to saturate
the exchange sites followed by replacement with either Mg504 or MgC12.
[0175] Indirect methods for CEC calculation involves the estimation of
extracted Ca2 ,
Mg2 , I( , and Na + in a standard soil test using Mehlich 3 and accounting for
the exchangeable
acidity (sum of 1-1 , Al3 , Mn2 , and Fe2 ) if the pH is below 6.0 (see
Mehlich, A. 1984, Mehlich-
3 soil test extractant: a modification of Mehlich-2 extractant, Commun. Soil
Sci. Plant Anal.
15(12): 1409-1416). When treated using the above methods, including but not
limited by
washing under a vacuum, treated biochars generally have a CEC greater than 5
millieq/1 and
some even have a CEC greater than 25 (millieq/l). To some extent, treatment
can be used to
adjust the CEC of a char.
41
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0176] 7. Anion Exchange Capacity
[0177]
Similar to CEC measurements, anion exchange capacity ("AEC") may be calculated
directly or indirectly- saturated paste extraction of exchangeable anions, Cl-
, NO3-, S042-, and
P043- to calculate anion sum or the use of potassium bromide to saturate
anions sites at different
pHs and repeated washings with calcium chloride and final measurement of
bromide (see
Rhoades, J.D. 1982, Soluble salts, p. 167-179. In: A. L. Page et al. (ed.)
Methods of soil
analysis: Part 2: Chemical and microbiological properties; and Michael
Lawrinenkoa and David
A. Laird, 2015, Anion exchange capacity of biochar, Green Chem., 2015, 17,
4628-4636).
When treated using the above methods, including but not limited by washing
under a vacuum,
treated biochars generally have an AEC greater than 5 millieq/1 and some even
have an AEC
greater than 20 (millieq/l). To some extent, treatment can be used to adjust
the CEC of a char.
[0178] 8. Hydrophilicity/Hydrophobicity
[0179]
The ability to control the hydrophilicity of the pores provides the ability to
load the
biochar particles with larger volumes of inoculant. The more hydrophilic the
more the biochars
can accept inoculant or infiltrate. Tests show that biochar treated in
accordance with the above
processes, using either vacuum or surfactant treatment processes increase the
hydrophilicity of
raw biochar. Two tests may be used to test the hydrophobicity/hydrophilicity
of biochar: (i)
the Molarity of Ethanol Drop ("MED") Test; and (ii) the Infiltrometer Test.
[0180]
The MED test was originally developed by Doerr in 1998 and later modified by
other researchers for various materials. The MED test is a timed penetration
test that is noted
to work well with biochar soil mixtures. For 100% biochar, penetration time of
different
mixtures of ethanol/water are noted to work better. Ethanol/Water mixtures
verses surface
tension dynes were correlated to determine whether treated biochar has
increased hydrophilicity
over raw biochar. Seven mixtures of ethanol and deionized water were used with
a sorption
time of 3 seconds on the biochar.
[0181]
Seven solutions of deionized ("DI") water with the following respective
percentages of ethanol: 3, 5, 11, 13, 18, 24 and 36, were made for testing.
The test starts with a
mixture having no DI. If the solution is soaked into the biochar in 3 seconds
for the respective
solution, it receives the corresponding Hydrophobicity Index value below.
42
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
. = , =
a.aiiiiiiiiiiiiiiiiiiil-
1111111111111111111111111111111111111111111111111111111?Wry Hydrophc
=
3%
5%
====================
...................
'11W
13%
18%
24%
A .* Itt94.0)Orppiwkik
[0182] To start the test the biochar ("material/substrate") is placed in
convenient open
container prepared for testing. Typically, materials to be tested are dried
110 C overnight and
cooled to room temperature. The test starts with a deionized water solution
having no ethanol.
Multiple drips of the solution are then laid onto the substrate surface from
low height. If drops
soak in less than 3 seconds, test records substrate as "0". If drops take
longer than 3 seconds or
don't soak in, go to test solution 1. Then, using test solution 1, multiple
drops from dropper are
laid onto the surface from low height. If drops soak into the substrate in
less than 3 seconds,
test records material as "1". If drops take longer than 3 seconds, or don't
soak in, go to test
solution 2. Then, using test solution 2, multiple drops from dropper laid onto
the surface from
low height. If drops soak into the substrate in less than 3 seconds, test
records material as "2".
If drops take longer than 3 seconds, or don't soak in, go to test solution 3.
Then, using test
solution 3, multiple drops from dropper laid onto the surface from low height.
If drops soak
into the substrate in less than 3 seconds, test records material as "3". If
drops take longer than
3 seconds, or don't soak in, go to solution 4.
[0183] The process above is repeated, testing progressively higher numbered
MED
solutions until the tester finds the solution that soaks into the substrate in
3 seconds or less. The
substrate is recorded as having that hydrophobicity index number that
correlates to the solution
number assigned to it (as set forth in the chart above).
[0184] Example test results using the MED test method is illustrated below.
MATERIAL HYDROPHOBICITY INDEX
Raw Biochars 3 to 5
43
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
Treated Biochars 1 to 3
[0185] Another way to measure and confirm that treatment decreases
hydrophobicity and
increases hydrophilicity is by using a mini disk infiltrometer. For this test
procedure, the bubble
chamber of the infiltrometer is filled three quarters full with tap water for
both water and ethanol
sorptivity tests. Deionized or distilled water is not used. Once the upper
chamber is full, the
infiltrometer is inverted and the water reservoir on the reserve is filled
with 80 mL. The
infiltrometer is carefully set on the position of the end of the mariotte tube
with respect to the
porous disk to ensure a zero suction offset while the tube bubbles. If this
dimension is changed
accidentally, the end of the mariotte tube should be reset to 6 mm from the
end of the plastic
water reservoir tube. The bottom elastomer is then replaced, making sure the
porous disk is
firmly in place. If the infiltrometer is held vertically using a stand and
clamp, no water should
leak out.
[0186] The suction rate of 1 cm is set for all samples. If the surface of
the sample is not
smooth, a thin layer of fine biochar can be applied to the area directly
underneath the
infiltrometer stainless steel disk. This ensures good contact between the
samples and the
infiltrometer. Readings are then taken at 1 min intervals for both water and
ethanol sorptivity
test. To be accurate, 20 mL water or 95% ethanol needs to be infiltrated into
the samples.
Record time and water/ethanol volumes at the times are recorded.
[0187] The data is then processed to determine the results. The data is
processed by the
input of the volume levels and time to the corresponding volume column. The
following
equation is used to calculate the hydrophobicity index of R
I = at + bA5
a: Infiltration Rate, cm/s
b: Sorptivity, cm/s1/2
R = 1.95 * bethanolL
"water
[0188] Figure 16 illustrates one example of the results of a hydrophobicity
test performed
on raw biochar, vacuum treated biochar and surfactant treated biochar. As
illustrated, both the
44
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
vacuum treated and surfactant treated biochar are more hydrophilic than the
raw biochar based
upon the lower Index rating. In accordance with the test data in Figure 16,
the hydrophobicity
of raw biochar was reduced 23% by vacuum processing and 46% by surfactant
addition.
[0189] As an example, raw biochar and treated biochar were tested with
ethanol and water,
five times for each. The results below show that the hydrophobicity index of
the treated biochar
is lower than the raw biochar. Thus, tests demonstrate that treating the
biochar, using the
methods set forth above, make the biochar less hydrophobic and more
hydrophilic.
MATERIAL HYDROPHOBICITY INDEX
Dried Raw Biochar 12.9
Dried Vacuum Treated Biochar 10.4
Dried Surfactant Treated Biochar 7.0
As Is Raw Biochar 5.8
As Is Vacuum Treated Biochar 2.9
[0190] Further, through the treatment processes of the present invention,
the biochar can
also be infused with soil enhancing agents. By infusing liquid into the pore
structure through
the application of positive or negative pressure and/or a surfactant, alone or
in combination,
provides the ability to impregnate the macropores of the biochar with soil
enhancing solutions
and solids. The soil enhancing agent may include, but not be limited to, any
of the following:
water, water solutions of salts, inorganic and organic liquids of different
polarities, liquid
organic compounds or combinations of organic compounds and solvents, mineral
and organic
oils, slurries and suspensions, supercritical liquids, fertilizers, plant
growth promoting
rhizobacteria, free-living and nodule-forming nitrogen fixing bacteria,
organic decomposers,
nitrifying bacteria, phosphate solubilizing bacteria, biocontrol agents,
bioremediation agents,
saprotrophic fungi, ectomycorrhizae and endomycorrhizae, among others.
[0191] 9. Impregnation and/or Inoculation with Infiltrates or Additives
[0192] In addition to mitigating or removing deleterious pore surface
properties, by treating
the pores of the biochar through a forced, assisted, accelerate or rapid
infiltration process, such as
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
those described above, the pore surface properties of the biochar can be
enhanced. Such
treatment processes may also permit subsequent processing, may modify the pore
surface to
provide predetermined properties to the biochar, and/or provide combinations
and variations
of these effects. For example, it may be desirable or otherwise advantageous
to coat
substantially all, or all of the biochar macropore and mesopore surfaces with
a surface
modifying agent or treatment to provide a predetermined feature to the
biochar, e.g., surface
charge and charge density, surface species and distribution, targeted nutrient
addition,
magnetic modifications, root growth facilitator, and water absorptivity and
water retention
properties.
[0193] By infusing liquids into the pores of biochar, it has been
discovered that additives
infused within the pores of the biochar provide a time release effect or
steady flow of some
beneficial substances to the environment, e.g. root zones of the plants, and
also can improve and
provide a more beneficial environment for microbes which may reside or take up
residence within
the pores of the biochar. In particular, additive infused biochars placed in
the soil prior to or after
planting can dramatically reduce the need for high frequency application of
additives, minimize
losses caused by leaching and runoff and/or reduce or eliminate the need for
controlled release
fertilizers. They can also be exceptionally beneficial in animal feed
applications by providing an
effective delivery mechanism for beneficial nutrients, pharmaceuticals,
enzymes, microbes, or
other substances.
[0194] For purposes of this application, "infusion" of a liquid or liquid
solution into the
pores of the biochar means the introduction of the liquid or liquid solution
into the pores of the
biochar by a means other than solely contacting the liquid or solution with
the biochar, e.g.,
submersion. The infusion process, as described in this application in
connection with the present
invention, includes a mechanical, chemical or physical process that
facilitates or assist with the
penetration of liquid or solution into the pores of the biochar, which process
may include, but
not be limited to, positive and negative pressure changes, such as vacuum
infusion, surfactant
infusion, or infusion by movement of the liquid and/or biochar (e.g.,
centrifugal force and/or
ultrasonic waves) or other method that facilitates, assists, forces or
accelerates the liquid or
solution into the pores of the biochar.
46
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0195] Prior to infusing the biochar, the biochar, as described in detail
above, may be
washed and/or moisture adjusted. Figure 17 is a flow diagram 1700 of one
example of a method
for infusing biochar with an additive. Optionally, the biochar may first be
washed or treated at
step 1702, the wash may adjust the pH of the biochar, as described in more
detail above, or may
be used to remove elemental ash and other harmful organics that may be
unsuitable for the
desired infused additive. Optionally, the moisture content of the biochar may
then be adjusted
by drying the biochar at step 1704, also as described in further detail above,
prior to infusion of
the additive or inoculant at step 1706.
[0196] In summary, the infusion process may be performed with or without
any washing,
prior pH adjustment or moisture content adjustment. Optionally, the infusion
process may be
performed with the wash and/or the moisture adjustment step. All the processes
may be
completed alone or in the conjunction with one or more of the others.
[0197] Through the above process of infusing the additive into the pores of
the biochar, the
pores of the biochar may be filled by 25%, up to 100%, with an additive
solution, as compared
to 1-20% when the biochar is only submerged in the solution or washed with the
solution for a
period of less than twelve hours. Higher percentages may be achieved by
washing and/or drying
the pores of the biochar prior to infusion.
[0198] Data have been gathered from research conducted comparing the
results of soaking
or immersion of biochar in liquid versus vacuum impregnation of liquid into
biochar. These
data support the conclusion that vacuum impregnation provides greater benefits
than simple
soaking and results in a higher percentage volume of moisture on the surface,
interstitially and
in the pores of the biochar.
[0199] In one experiment, equal quantities of pine biochar were mixed with
equal
quantities of water, the first in a beaker, the second in a vacuum flask. The
mixture in the
beaker was continuously stirred for up to 24 hours, then samples of the
suspended solid were
taken, drained and analyzed for moisture content. The mixture in the vacuum
flask was
connected to a vacuum pump and negative pressure of 15" was applied. Samples
of the treated
solid were taken, drained and analyzed for moisture content. Figure 18 is a
chart illustrating
the results of the experiment. The lower graph 1802 of the chart, which shows
the results of
soaking over time, shows a Wt.% of water of approximately 52%. The upper graph
1804 of
47
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
the chart, which shows the results of vacuum impregnation over time, shows a
Wt.% of water
of approximately 72%.
[0200] Figures 19a and 19b show two charts that further illustrate that the
total water
and/or any other liquid content in processed biochar can be significantly
increased using
vacuum impregnation instead of soaking. Figure 19a compares the mL of total
water or other
liquid by retained by 1 mL of treated biochar. The graph 1902 shows that
approximately .17
mL of water or other liquid are retained through soaking, while the graph 1904
shows that
approximately .42 mL of water or other liquid are retained as a result of
vacuum impregnation.
Figure 19b shows that the retained water of a biochar subjected to soaking
consists entirely of
surface and interstitial water 1906, while the retained water of a biochar
subjected to vacuum
impregnation consists not only of surface and interstitial water 1908a, but
also water
impregnated in the pores of the biochar 1908b.
[0201] In addition, as illustrated by Figure 20, the amount of moisture
content impregnated
into the pores of vacuum processed biochars by varying the applied (negative)
pressure during
the treatment process. The graphs of four different biochars all show how the
liquid content
of the pours of each of them increase to 100% as the vacuum is increased.
[0202] The pores may be substantially filled or completely filled with
additives to provide
enhanced performance features to the biochar, such as increased plant growth,
nutrient
delivery, water retention, nutrient retention, disadvantageous species
control, e.g., weeds,
disease causing bacteria, insects, volunteer crops, etc. By infusing liquid
deep into the pore
structure through the application of positive or negative pressure, surfactant
and/or ultrasonic
waves, alone or in combination, provides the ability to impregnate the
mesopores and
macropores of the biochar with additives, that include, but are not limited
to, soil enhancing
solutions and solids. It should be noted that using these infusion techniques
allows for
impregnating the pores with additives that are more fragile. For example,
since heating is not a
requirement for these infusion techniques, microbes, chemicals, or compounds
can be infused
without risk of destroying the microbes or changing chemicals or compounds due
to high
temperatures. Also the process can be done at low temperatures to infuse
chemicals that have
low boiling points to keep them a liquid.
48
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0203] The additive may be a soil enhancing agent that includes, but is not
be limited to, any
of the following: water, water solutions of salts, inorganic and organic
liquids of different
polarities, liquid organic compounds or combinations of organic compounds and
solvents,
mineral and organic oils, slurries and suspensions, supercritical liquids,
fertilizers, PGPB
(including plant growth promoting rhizobacteria, free-living and nodule-
forming nitrogen fixing
bacteria, organic decomposers, nitrifying bacteria, and phosphate solubilizing
bacteria),
enzymes, biocontrol agents, bioremediation agents, saprotrophic fungi,
ectomycorrhizae and
endomycorrhizae, among others.
[0204] Fertilizers that may be infused into the biochar include, but are
not limited to, the
following sources of nitrogen, phosphorous, and potassium: urea, ammonium
nitrate, calcium
nitrate, sulfur, ammonium sulfate, monoammonium phosphate, ammonium
polyphosphate,
potassium sulfate, or potassium chloride.
[0205] Similar beneficial results are expected from other additives, such
as: bio pesticides;
herbicides; insecticides; nematicides; plant hormones; plant pheromones;
organic or inorganic
fungicides; algicides; antifouling agents; antimicrobials; attractants;
biocides, disinfectants and
sanitizers ; miticides ; microbial pesticides; molluscicides ; bacteriacides;
fumigants; ovicides ;
repellents; rodenticides, defoliants, desiccants; insect growth regulators;
plant growth
regulators; beneficial microbes; and, microbial nutrients or secondary signal
activators, that may
also be added to the biochar in a similar manner as a fertilizer.
Additionally, beneficial macro-
and micro- nutrients such as, calcium, magnesium, sulfur, boron, zinc, iron,
manganese,
molybdenum, copper and chloride may also be infused into the biochar in the
form of a water
solution or other solvent solution.
[0206] Examples of compounds, in addition to fertilizer, that may be
infused into the pores
of the biochar include, but are not limited to: phytohormones, such as,
abscisic acid (ABA),
auxins, cytokinins, gibberellins, brassinosteroies, salicylic acid,
jasmonates, planet peptide
hormones, polyamines, karrikins, strigolactones; 2,1,3-Benzothiadiazole (BTH),
an inducer of
systemic acquired resistance that confers broad spectrum disease resistance
(including soil borne
pathogens); signaling agents similar to BTH in mechanism or structure that
protects against a
broad range or specific plant pathogens; EPSPS inhibitors; synthetic auxins;
photosystem I
inhibitors photosystem II inhibitors; and HPPD inhibitors.
49
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0207] In one example, a 1000 ppm NO3- N fertilizer solution is infused
into the pores of
the biochar. As discussed above, the method to infuse biochar with the
fertilizer solution may
be accomplished generally by placing the biochar in a vacuum infiltration tank
or other sealable
mixing vessel, chamber or tank. When using vacuum infiltration, a vacuum may
be applied to
the biochar and then the solution may be introduced into the tank.
Alternatively, the solution
and biochar may both be introduced into the tank and, once introduced, a
vacuum is applied.
Based upon the determined total pore volume of the biochar or the incipient
wetness, the amount
of solution to introduce into the tank necessary to fill the pore of the
biochar can be determined.
When infused in this manner, significantly more nutrients can be held in a
given quantity of
biochar versus direct contact of the biochar with the nutrients alone.
[0208] When using a surfactant, the biochar and additive solution may be
added to a tank
along with 0.01 - 20% of surfactant, but more preferably 1-5% of surfactant by
volume of
fertilizer solution. The surfactant or detergent aids in the penetration of
the wash solution into
the pores of the biochar. The same or similar equipment used in the vacuum
infiltration process
can be used in the surfactant treatment process. Although it is not necessary
to apply a vacuum
in the surfactant treatment process, the vacuum infiltration tank or any other
mixing vessel,
chamber or tank can be used. Again, while it is not necessary to apply a
vacuum, a vacuum
may be applied or the pressure in the vessel may be changed. Further, the
surfactant can be
added with or without heat or cooling either of the infiltrate, the biochar,
the vessel itself, or
any combination of the three.
[0209] The utility of infusing the biochar with an additive is that the
pores in biochar create
a protective "medium" for carrying said additive to the environment. As an
example when
the additive is a fertilizer the nutrient infused biochar provides a more
constant supply of
available nutrients to the soil and plants and continues to act beneficially,
potentially sorbing
more nutrients or nutrients in solution even after introduction to the soil.
By infusing the
nutrients in the pores of the biochar, immediate oversaturation of the soil
with the nutrients is
prevented and a time released effect is provided. This effect is illustrated
in connection with
Figures 18 and 19 below. As demonstrated in connection with Figures 18 & 19
below, biochars
having pores infused with additives, using the infusion methods described
above, have been
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
shown to increase nutrient retention, increase crop yields and provide a
steadier flow of fertilizer
to the root zones of the plants.
[0210] Figure 21 is a chart showing improved mass yield in lettuce with
fertilizer infused
biochar using vacuum impregnation. Figure 21 compares the mass yield results
of lettuce grown
in different environments. One set of data measurements represents lettuce
grown in soil over
a certain set time period with certain, predetermined amounts of fertilizer
infused into the
biochar. A second set of data represents lettuce grown in soil over a certain
set period of time
with the same amount of unimpregnated biochar added at the beginning of the
trial and certain
predetermined amounts of NPK solution added to the soil over time. Growth
comparisons were
made between the same amount of fertilizer solution infused into the biochar
as added directly
to the soil, using the same watering schedule. As illustrated, the test
results demonstrated a 15%
yield increase in growth when infusing approximately 750 mg/pot of NPK into
the biochar than
when applying it directly to the soil. Similarly, the same mass yield of
lettuce is achieved at
400 mg NPK/pot with infused biochar than at 750 mg/pot when adding the
fertilizer solution
directly to the soil.
[0211] Figure 22 is a chart illustrating the concentration of nitrate (N)
found in distilled
water after washing differentially treated biochar. In the illustrated
example, two biochar
samples (500 ml each) mixed with 1000 ppm NO3- N fertilizer solution were
washed with
distilled water. The resulting wash was then tested for the presence of
nitrate (N), measured
in ppm. In one sample, the biochar was submerged in and mixed with the
nutrient solution.
In the other example, the biochar was mixed or washed with a nutrient solution
augmented
with 1% surfactant by volume (i.e., 1 ml of surfactant per 100 ml of
fertilizer solution) in a
tumbler. In both examples, the biochar was not dried completely before
infusion with the
NO3- N fertilizer solution, but used as received with a moisture content of
approximately 10-
15%. In both examples, the biochar was mixed with solution and/or surfactant
(in the case of
a second sample) with a bench scale tumbler, rotating the drum for four (4)
minutes without
vacuum. The results demonstrate that the biochar treated with the 1%
surfactant increases the
efficiency of infiltrating nitrate fertilizer into biochar and then
demonstrates the release of the
nutrient over time. To yield the above data, the test was repeated six times
for each treatment
sample, with 10 washes for each sample per repeat test.
51
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0212] The above are only a few examples of how additive infused biochar
may be
produced for different uses. Those skilled in the art will recognize that
there may be other
mechanisms for infusing fertilizer or other soil additives into the pores of
the biochar without
departing from the scope of the invention. Those skilled in the art will
further recognize that
the present invention can be used on any type of soil application, including,
but not limited to,
the following: crops, turf grasses, potted plants, flowering plants, annuals,
perennials,
evergreens and seedlings, as will be further described below.
[0213] For example, in another implementation, additive infused biochar may
be produced
for use for consumption by animals and/or humans. Biochar may be infused in
the same
manner as described above with nutrients (such as carbohydrates, minerals,
proteins, lipids),
vitamins, drugs and/or other supplements (such as enzymes or hormones, to name
a few), or
a combination of any of the foregoing, for consumption by either humans and/or
animals.
Coloring, flavor agents and/or coating may also be infused into the pores of
the biochar or
applied to the surface. The foregoing may be included to enhance the
performance of the
substance in the digestive tract or to ease or facilitate the ingestion of the
biochar.
[0214] D. Biochar as a Habitat for Microorganisms
[0215] Biotechnology, specifically the use of biological organisms, usually
microorganisms,
to address chemical, industrial, medical, or agricultural problems is a
growing field with new
applications being discovered daily. To date, much research has focused on
identifying,
developing, producing and deploying microbes for various uses. However,
despite much work
on the microbes themselves, relatively little work has been performed on how
to carry, deliver,
and encourage the successful establishment of these microbes in their targeted
environment. Most
current technology for microbial carriers in agriculture is based on
technologies or products that
are highly variable and, in many cases, lead to highly unpredictable
performance of microbes in
the field. For example, many commercial microbes in agricultural settings are
delivered on peat,
clay, or other carriers derived from natural sources, accompanied by limited
engineering or
process control.
[0216] Biochar have a proclivity to interact positively with many microbes
relevant to plant
health, animal health, and human public health applications. In fact, there
has been a level of
initial research focused on inoculating biochar with microbes and/or using
biochar in conjunction
52
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
with microbes or materials with microbes, e.g. compost. See co-owned US Patent
8,317,891
Method for Enhancing Soil Growth Using Bio-char and Fischer et al., and
Synergisms between
compost and biochar for sustainable soil amelioration
2012
http://www .intechopen. com/s ource/pdfs/27163/InTech-
Synergisms between compost and biochar for sustainable soil amelioration.pdf.
[0217] However, biochars, especially in raw form, often suffer from many
characteristics
which make their interaction with microbial organisms extremely unpredictable.
Key among
these undesirable characteristics is a high degree of variability. Because of
this and other factors,
biochar has been, to date, unused in large scale commercial biotechnology
applications. There
are several methods by which this variability can be ameliorated. At a high
level, the methods to
overcome these challenges fall into two categories: (i) making the biochar a
more favorable
habitat for the microbes ¨ either by modifying its properties, adding
materials beneficial to
microbes, or removing materials deleterious to microbes, or (ii) inoculating,
applying, or
immobilizing the microbes on the biochar in ways that mitigate the underlying
variability in the
material. Both of these high-level methods can be used independently or in
conjunction and have
been shown to have a significant impact on the suitability of biochar in many
biotechnology
applications.
[0218] Before delving into the varying treatment methods that will turn the
biochar into a
microbial carrier or co-deploying with microbes, it is important to be able to
view biochar as a
habitat for microbes. Biochar, especially treated biochar, has many physical
properties that
make it interesting as a microbial habitat. The most obvious of these is its
porosity (most
biochars have a surface area of over 100 m2/g and total porosity of 0.10
cm3/cm3 or above).
Furthermore, many biochars have significant water holding and nutrient
retention characteristics
which may be beneficial to microbes. Previous disclosure has outlined how
these characteristics
can be further improved with treatment, e.g., US Patent Application Serial No.
15/156,256, filed
on May 16, 2016, and titled Enhanced Biochar.
[0219] However, recent data indicates that the Earth may be home to more
than one trillion
independent species of microbes (See Kenneth J. Locey and JayT. Lennon,
Scaling laws predict
global microbial diversity, Proceedings of the National Academy of Science,
vol. 113 no. 21
(see full text at littp://www.pnas.orgicontenti113/21/5970Sull). Clearly, each
of these
53
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
microbial species does not require an identical habitat. In fact, many have
evolved in different
condition.s and thrive in different environments. Biochar, due to its organic
origins, porosity,
and amenability to treatment seems to be an extremely desirable base product
to be used in the
construction of microbial carriers or co-deployment of microbes. If the
properties of the biochar
can be made to match the properties expected by particular microbes, or groups
of microbes,
empirical data has shown that a much greater impact can be delivered in many
applications
whether the targeted biochar is used as a carrier, substrate, co-deployed
product, or merely is
introduced into the same environment at a separate time. It stands to reason,
as many real-world
environments are composed of very complex microbial ecosystems, that giving
certain microbes
in these ecosystems a more favorable habitat, can ultimately help those
microbes to become
more successfully established, and potentially shift the entire ecosystem
based on their improved
ability to compete for resources. Clearly this is a very desirable
characteristic when the
successful deployment and establishment of a targeted microbe into a new
environment is a
desired outcome.
[0220] There are many properties of a habitat which may be important to
certain microbes,
but some of the most important are: pH, hydrophobicity or hydrophilicity,
ability to hold
moisture, ability to retain and exchange certain types of nutrients, ion
exchange capacity (cationic
and anionic), physical protection from predatory or competitive microbes or
protozoa (usable and
inhabitable porosity), presence or absence of nutrients, micronutrients, or
sources of metabolic
carbon, ability to host other symbiotic microbes or plant systems (such. as
plant root tissue), or
others which may be important to various types or species of microbes. Ability
to either enhance
or suppress the availability of certain enzymes can also be an extremely
important factor in
building a viable habitat.. This invention focuses on methods and systems that
can be used to
consistently produce biochar which has these targeted characteristics, methods
that can be used
to effectively create a particular formulation of biochar targeted to match. a
particular microbe or
group of microbes, and techniques for deploying the desired microbes along
with this targeted
biochar, through inoculation, co-deployment, integrated growth / fermentation,
or other methods.
[0221] By using treatment properties disclosed previously, proper feedstock
selection, and
control of the pyrolysis process, the following are some, but not all, of the
properties that can be
consistently targeted and controlled at production scales to improve the
biochar for use with
54
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
microbes or as a microbial carrier. Examples of those properties include (1)
pH, (2)
hydrophobicity, (3) sodium levels, (4) usable pore size distribution and
usable pore volume, (5)
particle size and distribution, (6) exterior and interior surface geometry,
(7) nutrient exchange,
(8) exterior and interior surface geometry, (9) useable carbon or energy
source, (10) toxic
materials or compounds, (11) surface structure/crystals/tortuosity, (12)
compatibility with biofilm
formations, and (13) enzyme activity.
[0222]
[0223] It is well known that various microbes prefer varying levels of
acidity or alkalinity.
For example, a.cidophiles have evolved to inhabit extremely acidic
environments. Likewise,
alkaliphiles prefer more basic (alkali) environments. it has been clearly
shown that the methods
outlined for treating biochars can product targeted pH values that can be
sustained over long
periods of time.
[0224] 2, Hydrophobicity
[0225] There are several common sources of hydrophobicity in porous
carbonaceous
materials. One of them is the occurrence of hydrophobic organic compounds on
the surface of
the char ¨ typically residual from the pyrolysis process. Targeted removal of
these compounds is
a method to improve the hydrophobicity of porous carbonaceous substances.
These compounds
can be removed in a non-selective way by increasing the pyrolysis temperature
of the biomass to
a level at which the compounds will disassociate with the material and become
gaseous. This
method, while useful, is very broad, and can also remove other desirable
compounds as well as
changing the surface chemistry of the residual carbon, increasing ash
percentages, or reducing
carbon yield by reacting and removing more carbon than is necessary. These
compounds can also
be selectively removed by the application of a targeted solvent using the
mechanisms previously
disclosed to infiltrate liquids into the pore volume of the material. This
method is also effective,
and has shown. to be much more predictable in the removal of certain
compounds. Since the vast
majority of microbes rely heavily on water for both transport and life, the
easy association of
water with a material has a large bearing on. its ability to sustain microbial
life.
[0226] 3. Sodium Levels
[0227] Differing types of microbes have varying proclivities for the
presence of sodium.
Some microbes Halobacierium spp.õS'alinibacter ruber, Wallemia ichthyophaga
prefer high
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
levels of salinity, while others prefer moderate or limited levels of sodium.
Sodium can be
removed from biochar by either simple washing, or more preferably and
effectively, treatrnent
methods which infuse a solvent (most commonly water, although others may be
used) into the
pores of the material. Sodium can be added, by using the same methods except
instead of using
a solvent, the liquid -bein.g washed with or infused is a solution high in
sodium content.
Additionally, since sodium usually manifests itself as a cation in solution,
temporary or permanent
adjustment of the cationic exchange capacity (CEC) of the material through
treatment which
impacts the ability of the material to exchange cations. Lowering the CEC of
the material will in
many cases reduce its ability to exchange sodium cations, while raising the
CEC will typically
enhance the ability of the material to exchange sodium cations, with
exceptions occurring if other
cations are present in quantities that cause them to preferentially exchange
instead of the sodium
cations present. Finally, differing biomass feedstock contains differing
levels of sodium ¨
selecting an appropriate feedstock prior to pyrolysis will result in a raw or
untreated biochar with
reasonably controlled levels of sodium. For example, pine wood, when
pyrolyzed, results in a
raw char with lower levels of sodium, while coconut shells result in char with
higher levels of
sodium after pyrolysis
ASH Composition Untreated Coconut Untreated Pine Untreated Pine
Shell Biochar Biochar #1 Biochar #2
Ultimate Analysis
Moisture free results
Ash 6.7% 9.2% 3.6%
Ash Composition
Sodium Oxide, as 5.7 % 1.2% 0.8%
N a20
Regardless, it should be clear that there are various methods available to
produce final product
with a targeted sodium level, making it suitable for various microbes
depending on their
preference for an environment with a certain sodium level.
[0228] 4. Usable Pore Size Distribution and Usable Pore Volume
[0229] One very important quality of a microbial habitat is the
availability of shelter from
environmental or biological hazards. A few examples of environmental hazards
are high
temperature, UV radiation, or low moisture, while an example of a biological
hazard is the
existing of predatory multicellular microbes such as protozoa, including both
flagellates and
36
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
ciliates. In order for a particle or material to provide shelter for microbes,
at least two conditions
must be present: (i) The material must consist of pores or openings of a size
which can be
inhabited by the microbe in question (ii) but prevent the hazard from entering
(e.g. pore size
smaller than the size of predators, such as protozoa., or deep enough to be
shaded from UV rays)
and, (iii) the pores mentioned previously must be usable ¨ namely, they should
not be occupied
by solid matter (clogged) and/or they should not contain substances that are
toxic or undesirable
for the microbe in question. In some cases, the pore size distribution of a
biochar can be adjusted
by the selection of the biomass feedstock to be pyrolyzed and the conditions
of the pyrolysis
process itself. For example, pine wood has a relatively narrow pore size
distribution, with most
pores falling in the range from l0-70 p.m. Coconut shells, on the other hand,
have a much wider
size distribution, with many pores below 111 in, and also a high percentage of
porosity above
1.00p.rn. It is theorized that materials with pores of a single size or where
most pores are of similar
size can potentially be good carriers or habitats for certain, targeted
microbes, while materials
consisting of broader ranges of pore sizes may be better habitats for
communities, consortia or
groups of microbes, where each microbe may prefer a slightly different pore
size. Furthermore,
the pore size of a material may also be controlled during the pyrolysis
process by increasing
temperature or performing "activation" or other steps common in activated
carbon production to
react or remove carbon, leaving larger pores, or exposing availability of
pores that were once
inaccessible from the exterior surface of the material. Adjusting the particle
size of the material
may also change the pore size distribution in at least two ways: (i) exposing
pores that, were not
available or accessible previously, or (ii.) destroying larger pores by
fracturing, splitting, or
dividing them. In many cases, raw biochar may contain a proper pore size
distribution, but for
one reason or another, the pores are not usable by the microbes in question.
In other cases, the
pore size distribution provided by the natural feedstock may be undesirable.
Both properties may
also be impacted through treatment of the raw biochar itself. Larger pores can
be created using
strong acids or other caustic substances either by simple washing or through
forced or rapid
infusion into the pores. Conversely, a material with fewer usable pores may be
created by
intentionally "clogging" or filling the larger pores with either solids, gums,
or liquids designed to
stay resident in the pores themselves. This treatment may be done in a
controlled way to only
partially fill the pores. For example, one could infuse a limited amount of
heated liquid, such as
57
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
a resin, that will become solid at normal atmospheric temperatures. If the
volume of liquid used
is less than the available pore volume of the material being infused, some of
the porosity of the
material will be left untreated and available for use. Most importantly, and
most commonly,
usable pore volume may be increased through the act of simply removing
contaminants (physical
or chemical) from the pores. Rapid infusion and extraction of liquids may be
used to accomplish
this. As discussed previously, appropriate solvents may be infused or
extracted to remove
chemical contaminants. Additionally, gasses or liquids may be driven into or
out of the pores to
force the removal of many solid obstructions, such as smaller particles of ash
or simply smaller
particles of raw biochar which may have become lodged in the pore in question.
Regardless of
the mechanism used, it has been shown that the available, uncontaminated,
usable pore volume
and pore size has a major role in determining the efficacy of biochars in
microbial roles.
[0230] Figures 23 and 24 are images that show how different sized bacteria
will fit in different
biochar pore size structures. Figure 23 is rod-shaped gram-positive bacteria,
Bacillus
thuringiensis israelensis, in a treated pine biochar, with pore openings of
¨10-20 um and bacteria
of ¨2-5 um. Figure 24 is rod-shaped gram-negative bacteria, Serratia
liquefaciens, in a treated
coconut shell biochar, with pore openings of ¨2-10 pm and bacteria of ¨ 1-2
um.
[0231] In addition, total pore volume in the size of 5-50 t,tm has been
shown to correlate with
microbial release rate after inoculation on treated biochar. Figure 25
illustrates release rate data
verse total pore volume data for both coconut shell and pine based treated
biochars inoculated
with a releasable bacteria. As illustrated in Figure 25, the data was plotted
in a graph, and clearly
shows that as pore volume increases so does the release rate.
[0232] 5. Exterior and Interior Surface Geometry
[0233] Two important properties of microbial carriers are: (i) their
ability to release
microbes from their surfaces and (ii) their ability to immobilize or stabilize
microbes on their
surfaces. Depending on the final application or use of the carrier, one or
both of these properties
may be desired. For example, for carriers designed to quickly release a
microbe into a targeted
domain such as a lake, river, or other waterway, the release characteristics
of the material are
paramount. For other applications, such as applications of certain symbiotic
microbes in
agriculture, rapid release may be undesirable, rather it may be important to
sustain the microbes
within the porosity of the material until plant tissue, such as root biomass,
is nearby to provide
58
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
nutrition for the microbes in question. The surface and pore geometry of the
material used as a
carrier can be critical to determine this behavior. For example, material with
generally smooth,
uniform surfaces will typically release many microbes much more effectively,
while material
with more rugged, varied, tortuous pore surfaces and geometry will typically
retain and
immobilize microbes more effectively. The biomass used in the production of
the final material
is one of the most important factors in surface geometry. However, even this
quality can be
altered through treatment. Specifically, smooth surfaces may be etched by
implementing the
treatment and infusion processes previously disclosed with strong acids,
rendering them
rougher. Conversely, rough surfaces may be treated with either organic or
inorganic compounds
to coat and remove contour. Mechanical means may also be used to affect
changes in particle
geometry. Many forms of charred material have relatively low crush strength
and are relatively
brittle. The method used to grind, or size particles can have a large impact
on the geometry of
the final particles. For example, particles milled using a ball mill or other
type of grinding
technology will typically have a smoother exterior geometry after the milling
is complete and
may lose a good amount of their porosity through the simple mechanical
crushing of pores.
However, particles sized using ultrasonic vibrations or even simple physical
vibrations to
shatter, rather than crush larger particles into smaller ones, will typically
retain their geometry,
or sometimes result in smaller particles with more rugged geometries than the
particles at the
beginning. It should be apparent to one skilled in the art that there are
various mechanical
mechanisms available to effect these changes, but the resulting particles can
be tailored to meet
a particular microbial release or immobilization outcome.
[0234] 6. Particle Size and Distribution
[0235] It i.s well known that the particle size and particle size
distribution of a material has
a key impact on its formulation as a microbial carrier. In many cases, these
factors are very
different for porous carbonaceous materials than they are for other common
microbial carriers.
In standard carriers, typically the reduction of particle size is a method
used to increase surface
area, and thus the area available to support, immobilize, and carry microbes.
However, in porous
materials, specifically materials with a large volume of usable interior
porosity, sometimes a
reduction in particle size does not cause a large increase in the usable
surface area --- specifically
because the interior surfaces of the material were already exposed, and
reducing the size of the
59
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
particle does not change that fact. This leads to a somewhat counterintuitive
behavior in some
cases in which the reduction of the particle size of a porous material
actually degrades its
performance as a microbial carrier, due to the phenomena that surfaces that
were once sheltered
inside the material are exposed as exterior surfaces when the material is
split or crushed, making
the material less desirable as a habitat for microbes that require shelter
from the sunounding
environment. Additionally, at times the actual distribution of particle sizes
can be a key factor
in performance. As a simple example, imagine an aggregated material which
consists of only
two particle sizes: I MITI and I m. Furthermore, imagine that 50% of the mass
of the aggregate
resided in the lanni particles with the remainder in the 1 pm particles.
Lastly, imagine that the
imm particles were porous carbonaceous particles with an average pore size of
approximately
50m. It should be clear that if this aggregate was placed in a container and
agitated, that a.
good portion of the lum particles would end up inhabiting the pore volume of
the imm particles,
impacting their usability. In fact, this is the behavior that we see in
practice. Therefore, for
certain microbial applications, it is desirable to remove extremely small
particles, often referred
to as fines, from the aggregate. This has the additional benefit of reducing
dust during
application, which is particularly important in aerial applications, and
reducing the -level of
surface runoff for applications in water, which also is important in certain
microbial
applications. The small particles may be removed through several methods such
as sieving,
blowing or aerodynamic removal, separation with either stationary or moving
liquids
(hydrostatic or hydrodynamic separation) of various viscosities, temperatures,
flow rates, etc.
However, at times, having a mixture of smaller and larger particles can be
desirable. The most
common cases are when communities of microbes are to be deployed, or the
aggregate is to
remain generally intact for a period of time (fermentation applications, long
term storage
applications, or preparation for other formulation uses such as
palletization), in which case, the
interparticle void space is also an important factor and can be optimized for
a particular microbe
or set of microbes by providing a range of particle sizes and geometries.
[0236] 7, Nutrient Exchange
[0237] The ability of a material to hold or exchange nutrients is an
incredibly important
characteristic, not only for microbial, but also for general agricultural
applications. There are
two primary mechanisms that porous carbonaceous materials can exchange
nutrients: (i)
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
sorption or retention of the nutrients on the interior and exterior surfaces
of the material, and (ii)
retention of the nutrients either in suspension or solution in liquid or
gasses residing in the pore
volume of the material. Both mechanisms are very useful, but also very
different in function.
Surface sorption or retention is driven by two main properties, among others:
(i) ion. exchange
capacity of the material and. (ii.) reactivity or electrical charge of
compounds present on or
coating the surfaces of the material. Retention of nutrients in solution or
suspension are
impacted by other, different characteristics of the material, such as
hydrophilicity, oil sorption
capacity, usable pore volume and pore size distribution, and interior pore
geometry and
tortuosity. The surface retention of nutrients can be targeted by selecting
the feedstock biomass
(some materials render a char after pyrolysis with vastly differing ionic
exchange capacities
(C.EC and AEC) than others). It can also be impacted by adjusting pyrolysis
conditions. Higher
pyrolysis temperatures tend to reduce CEC and nutrient adsorption capability.
See Gal, Xiapu
et at "Effects of Feedstock and Pyrolysis Temperature on Biochar Adsorption of
Ammonium
and Nitrate." Ed. Jonathan A. Coles. PLoS ONE 9.12 (2014): e113888. PMC. Web.
19 Nov.
2016. In addition, the surface retention of nutrients can be impacted by
treating the surfaces of
the material with substances targeted towards adjusting the ionic exchange
characteristics. For
example, using the previously disclosed treatment methods to infuse H202 into
the pores of the
carbonaceous material and then evaporating the liquid can increase the
cationic exchange
properties of the material.
[0238] Furthermore, another way to exchange nutrients more efficiently is
to use the pore
volume rather than, or in addition to, the pore surfaces ¨ namely keeping the
nutrients in solution
or liquid or gaseous form and placing them in the volume of the pores rather
than attempting to
sorb them on the surfaces of the material. This can be an incredibly useful
technique not only
for plant life and soil health, but also for microbes. The food sources can
vary from simple to
complex such as glucose, molasses, yeast extract, kelp meal, or bacteria media
(e.g.
MacConkey, Tryptic Soy, Luria-Bertani). When using the pore volume to exchange
nutrients
in this way, it should be clear that a wide variety of nutrients may be used,
and targeted
combinations of pore volume, size, and nutrition can be produced to assist in
the delivery,
establishment, or successful colonization of targeted microorganisms or groups
of
microorganisms. It should be clear by this point that merely immersing the
biochar or porous
61
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
carbonaceous material in a liquid nutrient broth may be partiaUy effective in
filling the pore
volume or coating the pore surfaces with these nutrients and should be
considered within the
scope of this invention, however using the treatment techniques outlined in
this and related
disclosures is much more effective at both coating the surfaces and infusing
nutrition into the
pore volume of the material itself. Since many microbes rely on liquid for
mobility, placing
liquid into the pore volume of the material is in many cases a prerequisite
for successfully
infusing, carrying, or delivering microbes.
[0239] 8. Usable Carbon or Energy Sources
[0240] Related to the ability to improve nutrient exchange is the ability
to treat the pore
volume, pore surfaces, exterior surfaces, or any combination of these with not
only custom
broths or growth media, but also other forms of carbon known. to be beneficial
to microbes and
plant life. Some examples of this are carbohydrates (simple and complex),
humic substances,
plant macro and micronutrients such as nitrogen (in many forms, such as
ammonium and
nitrates), phosphorous, potassium, iron, magnesium, calcium, and sulfur and
trace elements such
as manganese, cobalt, zinc, copper, molybdenum. These nutrients may either be
infused in liquid
or gaseous form, or even as a suspended solid in liquid. The liquid may be
left in the pores, or
may be removed. if removed through evaporation, nutrients in solution or
suspended solids
may be left. behind, while if removed by mechanical or physical means, a.
portion of the liquid
may be left behind as well as some solids. It should be noted that the various
forms of removal
have differing advantages and disadvantages and that many energy sources may
be added either
at the same time or in sequence, with one, or many, removal steps in between
treatment or
infusion steps.
[0241] 9, Toxic Materials or Compounds
[0242] The selective addition or removal of materials or substances known
to be toxic to a.
certain microbe or lifeform is a key step in preparation of biochar for use
a.s a microbial habitat
or carrier. It has been shown, that through treatment, potentially toxic
compounds can be
removed with much greater effectiveness than through simple pyrolysis alone.
Some examples
of the potentially deleterious compounds that may be removed are: volatile
organic compounds
(VOCs), monoaromatics, polycyclic aromatic hydrocarbons (PAHs), heavy metals,
and
chlorinated compounds (e.g. dioxins and furans). A
proven approach to remove these
62
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
substances is to wash the exterior surfaces with and/or rapidly infuse a
solvent into the pore
volume of the material targeted to remove these substances. Following the
infusion with either
mechanical extraction, drying, or other methods to remove the solvent, laden
with the substances
in question, from the pores and interparticle spaces is a desirable, but not.
strictly necessary step
to further reduce the levels of toxicity. For example, the following data
shows removal of
dioxins using the treatment process of the present invention.
Raw coconut Treated coconut Raw pine. Treated pine.
shell biochar shell biochar biochar biochar
TEQ ng,/kg 0.7 0.4 9.6 0.4
(method
8290A)
[0243] Another approach for some toxic compounds (benzene as one example)
is, rather
than removing the compounds in question, to react them in place with other
compounds to
neutralize the toxicant. This approach can be used either with washing, or
forced / assisted
infusion, and in these cases a removal step is less necessary although it
still can be used to
prepare the material for another, subsequent phase of treatment.
[0244] Much attention is given to the removal of toxic compounds, but it
should be also be
noted that at times, it can be extremely beneficial to actually add or treat
the material with toxic
compounds. A primary example of this is sterilization, or preparation for
selective infusion.
Even after pyrolysis, residual biological life has been found to potentially
establish itself in
biochars given the right conditions. Treating, washing, or infusing the
material with antiseptics
such as methanol, ethanol, or other antibacterial or antiviral substances can
be a key step in
removing contamination and preparing the material for use in microbial
applications. .A
variation on this approach is to infuse, treat, or wash the material with a
selectively toxic
compound, such as a targeted antibiotic or pharmaceutical targeted towards
interrupting the
lifecycle of a specific set of microorganisms or organisms, thereby giving
other microbes, either
through infusion or merely contact in situ the opportunity to establish. Some
examples of this
treatment would be the use of antifungals such as cycloheximide to suppress
fungal growth and
provide an environment more well suited toward the establishment of bacteria.
As has been
stated previously, the methods may be used alone, or in. combination with one
another.
Specifically, a toxic compound such as ethanol, may be infused, removed, and
then steps may
63
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
be taken to remove other toxic compounds, followed by steps to add carbon
sources or growth
medi a
[0245] 10. Surface Structures / Crystals / Tortuosity
[0246] The physical surface and pore structure of the material is
critically important to its
suitability as a microbial habitat. There are many factors that contribute to
the surface structure
of the material. The most notable of these factors is the biomass used to
produce the
carbonaceous material ¨ the cellular structure of the biomass dictates the
basic shape of many
of the pores. For example, pyrolyzed coconut shells typically have less
surface area, and a more
diverse distribution of pore sizes than pyrolyzed pine wood., which., when
pyrolyzed at the same
temperature, has greater surface area, but a more uniform (less diverse) pore
size distribution.
Tortuosity, or the amount of curvature in a given path through a selected pore
volume is also an
extremely important characteristic of engineered porous carbonaceous
materials.
[0247] Figure 26 shows the total fungi/bacteria ratio for two biochars
derived from
different biochar starting materials, e.g., feedstocks. Each biochar was
loaded with different
levels of moisture, and the total fungi/bacteria ratio was monitored during
the first week.
Biochar A 2301 showed a constant total fungi/bacteria ratio of 0.08 across
moisture levels
rang5000ng from 15% to 40%, while Biochar B 2302 showed a constant total
fungi/bacteria
ratio of 0.50 for moisture levels ranging from 30% to 40%. It is theorized
that, a fungi/bacteria
ratio between 0.05 and 0.60 is an effective prescription for a stable biochar
composition. This
composition allows a commercially viable product, which has sufficient shelf
life that it can
be delivered to storage houses waiting for the proper planting window.
[0248] It is theorized that the difference in the observed total
fungi/total bacteria ratios
of may also be explainable by the structures of the biochars. Biochar' s
having an open pore
structure, e.g., more interconnected pores, promotes more bacteria formation;
while closed
pores, e.g., relatively non-connected nature of the pores, tends to promote
fungi formation.
Biochars with differing microbial communities may be beneficial for specific
applications in
commercial agriculture. Thus, custom or tailored loading of the microbial
population may
be a desired implementation of the present invention.
[0249] For example, as shown in Figures 27a, 27b and 27c, Biochar A 2701
shows that it
has a greater population of, i.e., is inhabited by, more gram negative, gram
positive and
64
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
actinomycetes than Biochar B 2702. Thus, for example, Biochar A would be more
applicable
for use with certain agricultural crops in which Plant Growth Promoting
Bacteria (PGPB)
species in the actinomycetes, gram (-) pseudomonas, and bacillus groups are
used for
nutrient utilization and uptake.
[0250] It should be noted that both pyrolysis and post-treatment can be
used to further modify
the shape of these pores and structures. Pyrolyzing at higher temperatures,
injecting select gasses
or liquids during pyrolysis, or both typically will increase the pore volume
and surface area of the
material in question. Steam is the most readily available gas to cause this
effect, but hydrogen
sulfide, carbon dioxide, carbon monoxide, as well as other reactive gasses can
be used. Prior art
has clearly shown that the surface area of a biochar changes based on
feedstock and pyrolysis
temperature. Post treatment focused on a forced infusion of a strong acid, or
other reactive
substance into the pore space of the carbonaceous material can also be used to
modify the pore
size and pore volume of material by removing or breaking down the carbon
matrix which forms
the structure of the biochar, or other porous carbonaceous material. Acid
etching or infusion can
also be used to make smoother surfaces rougher. Rough surfaces can be very
useful in the
attachment and immobilization of microbes. Smooth surfaces can be useful for
the easy release
of carried microbes. Coating the surface area with materials such as starches
is a technique to
make rough surfaces smoother. Ultrasound., with or without a transmission
media (gel, liquid,
oil, or other) can also be used to rupture interpore divisions and create more
pore space. Flash
gasification, either at atmospheric pressure, or under negative or positive
pressure, of liquid
infused into the pores by the methods previously disclosed can also be used to
crack, disrupt, or
fracture solid material separating adjacent pores.
[0251] While much attention is given to modifying the pore structure by
removing
carbonaceous material, it should be noted that the pore structure can also be
modified by the
coating, forced infusion, and/or addition of materials which will bond to the
carbon and consume
pore volume, smooth surfaces, add tortuosity, change the exterior surfaces, or
all of these. in the
most simple form, it should be clear that materials may be added to coat
surfaces or fill pore
volume either through forced infusion, simple contact, or other means.
However, if the material
is infused or even simply contacted with a super saturated solution of a
substance that will
crystalize, such as sucrose, sodium chloride, or other common or uncommon
substances known
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
to form crystals. It should be noted that the crystals or substances used to
create them do not need
to be water soluble, and in fact in many cases it is desirable if they are
not. The crystals may also
be composed of nutrients or substances which may be beneficial to microbial or
plant life.
Examples of this are sucrose and mor3oammonium phosphate, both known for their
ability to
easily crystalize and be beneficial for microbial and plant life respectively.
By adding material
or even growing crystals on the carbon, a hybrid material is formed which can
have many
properties that are exceptionally useful for the delivery and establishment,
of microbial systems.
Crystallization is also way to add tortuosity to a carbonaceous material and
typically is much
more effective in this aspect than coating with solids alone.
[0252] ii. Compatibility with Biofilm Formation
[0253] Biofihns can be an important factor in the survival of a microbe in.
extreme or challenging
conditions. Bacterial communities can shift their morphology to increase
nutritional access and
decrease predation. One such modification is that the bacteria may attach to
surfaces, such as
those found in biochar, in a densely compacted community. In this compacted
form, they may
form an extracellular polymeric substance (EPS) matrix called a biofilm. These
communities
can contain hundreds o f different species which find shelter under the
protective EPS coating
from predatory protozoa, pathogens, contaminants, and other environmental
stressors. In some
cases, usually related to public health or healthcare, biofilms are
undesirable as they typically
allow pathogenic microbes to survive exposure to antiseptics, antibiotics,
predatory microbes
such as protozoa., or other agents which may eliminate them or negatively
impact their prospects
for survival. But in agricultural settings, encouraging target biofilm
establishment could lead to
improved microbe survival and thus improved agricultural or crop benefits.
[0254] As outlined in the article titled The Effect of Environmental
Conditions on Biofilm
Formation of Burkholderia psudomallei Clinical Isolates, it can be seen that
certain bacteria
require certain environmental factors, among them surface pH, for the creation
of biofilms. See
Ranh, et al., The Effect of Environmental Conditions on Biofilm Formation of
Burkholderia
psudomallei Clinical Isolates (September 6,
2012)
(http://dx.doi.org/10.1371/journal.pone.0044104) It is believed that other
surface characteristics
(rugged vs. smooth surfaces, surface charge, and more), along with moisture
levels and relative
humidity also play a large role in biotin-1i formation.
66
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0255] But for certain microbes requiring deployment into environments
known to present
survival challenges, optimizing a delivery material to encourage the formation
of these protective
biofilms can provide the targeted microbes with a significant advantage. Also,
many vegetable
and short cycle row crops such as tomatoes, lettuce, and celery form
mutualistic relationships
with bacteria that lead to the formation of biofilms on root hairs that
function not only in
nutrient uptake but also in plant pathogen resistance.
[0256] A.s outlined in previous disclosure, treatment of raw biochar can be
used to adjust the
surface pH to a level suitable for biofilm formation. Similarly, adjusting the
humidity by
selectively leaving a measured or controlled amount of water resident in the
pore V01.11MC, of the
material can also provide benefit. Lastly, the techniques outlines for
modifying the physical
surface properties of the material either by smoothing or roughening, can be
key factors also.
[0257] It should be clear that these factors can also be reversed to create
an environment that
is unsuitable for biofilin formation in applications where the formation of
biofilms on the carrier
is not desirable ¨ e.g. delivery or applications where quick relea.se of
microbes from the carrier is
important.
[0258] 12. Surface Char2e.
[0259] The surface charge of a porous carbonaceous material can be crucially
important in the
association and establishmer3t of targeted microbes with or on the material.
For example, most
bacteria have a net negative surface charge and in certain conditions a
specific bacterium may
favor attachment to positively charged surfaces. In some biological
applications, this attachment
may be preferred, in others, attachment may not be preferred. However,
modifying the surface
charge of the material is clearly a way to impact the suitability for
attachment of certain microbes.
There are many ways in which the surface charge of a carbonaceous material may
be changed or
modified. One way to accomplish this is by treating the surface area of the
material with a solution
containing a metal, such as Mn. Zr3. Fe, or Ca. This can be performed either
by doping the
material with these metals prior to or during pyrolysis, or more preferably,
by using a forced
infusion or treatment technique after pyrolysis to deposit these substances on
the interior and/or
exterior suifaces of the carbonaceous material. By controlling the amount and
or types of
substances infused, the surface charge of the material can be modified by
encouraging loading of
02- or other anions, or conversely, N , NI-12+, or other cations. This
modification of surface charge
67
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
can have a profound impact on the ability of certain microorganism to be
immobilized on the
interior and exterior surfaces of the material.
[0260] Another application of surface charge can be found by temporarily
charging the
carbonaceous material during inoculation with microbes. Carbon is used as a
cathode or anode
in many industrial applications. Because of its unique electrical properties,
carbon, or more
specifically porous carbonaceous materials, may be given a temporary surface
charge by the
application of a difference in electrical potential. One application of this
mechanism, is to create
a temporarily positively charged surface to encourage microbial attachment.
Then, while the
charge is maintained, allowing the microbes to attach themselves to and
colonize the carrier.
Once the colonization is complete, the charge can be released and the carrier,
laden with microbes
can either be deployed as is, or can undergo further treatment to stabilize
the microbes such as
lyophilizati on, or freeze drying.
[0261] 13. Enzyme Activity
[0262] For some types of microbes, enzyme activity, or the presence of certain
enzymes is every
bit as important as the availability of energy or nutrition. Enzymes can be
critical in the ability
of microbes to metabolize nutrition, which in turn can be a key element of
reproduction, survival,
and effective deployment. There are six main types of enzymes: hydrolases,
isomerases, ligases,
lyases, oxidoreductases, and transferases. These enzymes can be important in
microbial
applications. Through treatment or even simple contact, enzymes, like
nutrients and energy
sources, can be deposited on the surfaces or within the pore volume of porous
carbonaceous
materials, either as solids, or in solution/suspension, ensuring the enzymes
are not degraded
through the process. However, forced infusion of enzymes through the treatment
processes
previously outlined allows for much greater storage capacity and much greater
levels of contact
with the interior surfaces of the biochar, and as such, is preferable to
simple contact. In some
cases, the carbonaceous material can be used to deliver enzymes alone into an
environment where
both a habitat and enzymes are needed to promote or encourage the growth of
certain indigenous
microbes.
[0263] Another important aspect of enzyme activity is that some bacteria
make extra-cellular
enzymes which could be bound by the biochar or either reduce or even stop
biochemical reactions.
Thus, in certain situations when application is appropriate the carbonaceous
material can be used
68
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
to inhibit or make certain enzymes ineffective. For example, if the biochar is
being used as a
carrier for food or certain chemicals that are vulnerable to breakdown by
enzymatic degradation
and these specific enzymes would be bound by the biochar, then using the
carbonaceous material
as the carrier would provide for greater shelf-life and viability of the
product versus traditional
carriers.
[0264] 14. Sterilization
[0265] in many cases, it is desirable to remove potential unwanted microbes
from the surfaces
and pore volume of the material through sterilization. At outlined above,
infusion with antiseptics
or antibiotics are a. way to accomplish this. Boiling, or more preferably,
forced infusion of steam
is also a technique that can be used to remove resident microbial life.
Heating to a temperature
above 100 degrees C, and preferably between 100 and 150 degrees C is also
effective for
removing some microbial life. Heating may be required for ideally 30 minutes
or more,
depending on volume, method, and extent (temperature, radiation). Autoclaving
can also be used
30 minutes, 121 degrees C, 20 psig. For applications requiring a high level of
sterility, gamma
irradiation can be used, with dosages adjusted for the level of sterility
needed in ranges of 5 to 10
kGy or even 50 to 100 kGy or even higher dosage levels. For all sterilization
methods, the extent
of treatment required will depend on the volume of material and the required
level of sterilization.
in general, sterilization, using heat, should be done for at least 30 minutes,
but should he adjusted
as needed.
[0266] At this point, it, should be clear that all of these properties can
be controlled and
modified to create a treated, controlled biochar that is suitable for use as a
microbial carrier,
delivery system, habitat, fermentation substrate, or environmental (soil,
water or other)
enhancement. By contro-iling these properties and producing a material matched
to the application
and the microbe(s) in question, effectiveness can be dramatically improved
over both traditional
biological carriers, and many -forms of raw, untreated, uncontrolled biochar.
Furthermore,
varying materials, with varying properties, may be aggregated to provide
delivery systems or
habitats targeted towards consortia, communities, or groups of microbes.
[0267] E. Inoculating, Applying, or Immobilizing the Microbes on the
Biochar
[0268] Typically, the prior art teaches either placing biochar on soils
alone or combining
the biochar with compost and using this mixture as a soil amendment. The
nature of the
69
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
microbial population in this compost mixture is poorly disclosed by the prior
art. Thus using
more targeted methods to get the desired microbes into the suitable habitat
created by the raw
biochar, or more preferably treated or controlled biochar is desired. The
following are some but
not all, methods and systems that can be used to inoculate, deploy, or
otherwise associate
microbial life with a treated or untreated biochar:
[0269] 1. Co-deployment
[0270] This method focuses on deploying the microbes at the same time as
the biochar. This
can be done either by deploying the biochar into the environment first,
followed by microbes or
by reversing the order, or even deploying the two components simultaneously.
An example of
this would be the deployment of a commercial brady rhizobium inoculant
simultaneously with
the introduction of a treated biochar into the soil media. The system here is
the combination use
of a biochar and microbes in the environment, and more preferably a char
treated to have suitable
properties for a target microbe or group of microbes which it is used with in
a targeted
application for a specified purpose, for example a symbiotic crop of said
microbe(s).
[0271] In one experiment, various biochar feedstocks with various post-
treatments were
added to a soilless mix containing soybean seeds that had been treated with a
commercial
microbial product containing bradyrhizobium japonicum. and compared to both a
control with
microbe inoculant and one without. Some of the treated biochars co-deployed
with the inoculant
increased seed germination rates, one by 29%. Others increased nodulation
measured at 10
weeks, one more than doubled the number of nodules. The use of the microbial
inoculant
increased shoot biomass in all treatments. Figure 28 is a chart comparing
shoot biomass when
the biochar added to a soilless mix containing soybean seeds is treated with
microbial product
containing bradyrhizobium japonicum. and when it is untreated. As illustrated
in Figure 28,
shoot biomass increased with the biochar was treated.
[0272] Figure 29 shows the comparison of root biomass in a microbial
inoculated
environment versus one without inoculation. As illustrated in Figure 29, when
inoculated, root
biomass decreased with the inoculant alone yet increased with the use of all
the treated biochars
with or without inoculant.
[0273] In addition leaf tissue analysis was done which showed some of the
treated biochars
co-deployed with the rhizobial inoculant showed a significant increase in
nitrogen uptake.
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
Figure 30 is a chart comparing the nitrogen levels when the biochar is
inoculated with the
rhizobial inoculant verses when it is not inoculated. Statistical significance
in the chart in Figure
30 is marked with a star. In all cases, nitrogen levels increase with
inoculation.
[0274] As outlined in these results, the addition of a treated biochar
suitable for co-
deployment with this particular microbe increased nodulation, increased
nitrogen
fixation/availability, and resulted in substantially increased root mass. It
should be noted that
to demonstrate the differing performance of varying formulations, two
formulations were tested,
each showing different interactions with the microbe in question, along with
significant
variations in performance. This is just one example to demonstrate the
invention of how the
specific combination of biochar feedstock, biochar treatment, co-deployed
microbe, and
application (this case plant species) can lead to improved microbial
effectiveness and thus
improved results (this case plant vigor), versus no treatment, applying the
microbe alone, or
applying the biochar alone. Another example of co-deployment benefit could be
using a biochar
that has strong absorption properties in combination with fertilizer (or
infused with fertilizer)
and microbes in an agricultural setting. The biochar properties that help
retain and then slowly
release nutrients and ions will also help the targeted microbe population to
establish and grow
without being impacted by the high levels of fertilizer salts or nutrients
which can often impede
and sometimes kill the microbes being deployed.
[0275] 2. Basic Inoculation
[0276] A more advanced method of inoculation centers on mixing the microbe or
microbes in
question with the treated or untreated biochar before deployment. In some
cases, the biochar in
question can be treated, produced, or controlled to assist with this
deployment, making this case
slightly different than merely inoculating a microbe on untreated biochar. In
one form, microbes
suspended in liquid (either water, growth media, or other liquids) are
deposited on the biochar
and mixed together until both materials are well integrated and then the
material is deployed as
a granular solid. It has been shown that materials that have been treated to
be more hydrophilic
typically accept this inoculation more readily than hydrophobic materials ¨
demonstrating yet
another way in which the treatment of biochar can enhance performance. In
another form of
basic inoculation, the biochar is delivered in suspension in the liquid also
carrying the microbes.
This biochar/liquid/microbe slurry is then deployed as a liquid. In this form,
sizing the biochar
71
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
particles in such a way that their surface properties and porosity is
maintained is a key element
of effectiveness. Additionally, ensuring that the pores are treated to allow
easy association of
both liquid and microbes with the surfaces of the biochar is important. An
example of a basic
inoculation method of biochar for a bacteria in lab scale is as follows:
1) Isolate Pseudomonas protegens on a plate with 1.5% w/v Tryptic Soy Broth
solidified
with 1.5% w/v agar and incubate at 30 C for 12h
2) Take an isolated colony of Pseudomonas protegens and grow up in a 1.5% w/v
TSB
solution (90 ml) along with 10 g sterile biochar (sterilized at 110C in small
batches for
15-20 min) and combine both in a sterile 250 ml Erlenmeyer flask
3) Shake contents of flask at 150 rpm at 30 C for 12h, or greater
4) Transfer contents of flask into a sterilized ultracentrifuge tube (250 ml)
and spin at
10,000 x g for 10 min
5) Carefully remove supernatant liquid fraction by filtering through a Whatman
No 4 filter
with a vacuum filtration system to separate out the bulk liquid from biochar.
After basic inoculation, the material and the microbes may be deployed
immediately, stored for
future use, or stabilized using technology such as lyophilization.
[0264] 3. Assisted Inoculation
[0265] Another form of inoculation, which appears to have greater efficacy
with some
microbial systems, is assisted inoculation. Assisted inoculation involves
providing mechanical,
chemical, or biological assistance to move the targeted microbe either into
the pore volume of
the carrier or onto interior surfaces of the material that normally may not be
accessible.
Realizing that many microbes require liquid, and preferably water, for
mobility, the most
straightforward method of assisted inoculation requires infiltrating the pore
volume of the
material with water prior to contact with the targeted microbes. This water
infusion can be done
using the treatment methods described previously in this disclosure. It has
been shown that,
with certain microbes, making this change alone will have a positive impact on
the ability of
microbes to associate with and infiltrate the material. In one experiment, it
was shown that
water infusion improved release rate on both a treated pine biochar with
granular particles and
with a coconut biochar powder. Figure 31 illustrates the three-day release
rates of water infused
72
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
biochar compared to other types of biochar. As illustrated, results vary
depending upon the
biomass.
[0266] Changes can also be made in the media to reduce surface tension and
increase
flowability through the addition of a surfactant to the water, either into the
liquid used to carry
the microbes, or into the pores of the material itself, through simple
contact, or preferably forced
infusion.
[0267] Additionally, the microbes themselves may be assisted into the pores
using the
treatment techniques previously outlined. Care needs to be taken to match the
microbe to the
technique used, but many microbes are capable of surviving vacuum infiltration
if performed at
relatively gentle, lower pressure differentials (+/- 10% of standard
temperature and pressure).
Some microbes, and many spores however are capable of surviving vacuum
infiltration even at
relatively large pressure differentials (+/- 50, 75, or even 90 or 95% or more
variation from
standard temperature and pressure). When this technique is used, a liquid
mixture is constructed
containing both liquid to be infused and the microbe or microbes in question.
The liquid is then
used as the "infiltrant" outlined in previous disclosure related to placing
liquid into the pore
volume of the material. The final material, infiltrated with microbes, may
then be heated to
incubate the microbes, cooled to slow development of the microbes or stabilize
the microbes, or
have other techniques applied such as lyophilization. The material may then be
delivered in
solid granular form, powdered, further sized downward by grinding or milling,
upward by
agglomerating, aggregating, or bonding, or suspended in a liquid carrier. A
clear advantage to
this assisted infusion approach is that the material can be processed or
handled after inoculation
with more microbial stability because the targeted microbes are inhabiting the
interior pore
volume of the material and are less prone to degradation due to contact with
exterior surfaces,
or other direct physical or environmental contact. This method may be applied
repeatedly, with
one or more microbes, and one to many moisture removal steps. It may also be
combined with
the other inoculation methods disclosed here either in whole or in part.
[0268] Figures 32a, 32b and 32c show scanning electron microscopy (SEM)
images of
raw biochar compared to ones that have been processed by being infused under
vacuum with
bio-extract containing different microbial species.
73
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0269] Figure 32a is a SEM (10KV x 3.00K 10.0m) of pore morphology of raw
biochar.
Figure 32b is a SEM (10KV x 3.00K 10.0m) of pore morphology of raw biochar of
Figure 32a
after it has been infused with microbial species. Figure 32c is a SEM (10KV x
3.00K 10.0m)
of a pore morphology of another example of raw biochar of Figure 32a after it
has been infused
with microbial species. The images confirm the ability to incorporate
different microbes into
the pores of biochar by treatment. In turn, these beneficial microbes can
interact with and
enhance the performance of the environment they are deployed into, for example
the plants'
root systems when the inoculated biochar is mixed with the soil in the root
zone.
[0270] Compared to a biochar that has immersed in a compost tea, which may
have a
relatively short, e.g., a few days for the life of the microbes, the
impregnated populations of
examples of the present treated biochars, are stable over substantially longer
periods of time,
e.g., at least an 8 week period and in some cases 1 year or more as measured
by
PLFA(Phospholipid-derived fatty acids) analysis. PLFA analysis extracts the
fatty acid side
chains of phospholipid bilayers and measures the quantity of these biomarkers
using GC-
MS. An estimate of the microbial community population can thus be determined
through
PLFA analysis. The microbial activity may also be inferred through PLFA
analysis by
monitoring the transformation of specific fatty acids. Thus, the impregnation
of the biochar
with a microbial population provides for extended life of the microbes by at
least 5x, 10x,
or more over simple contact or immersion. In fact, some microbes may be better
suited to
surfactant infiltration versus vacuum infiltration and vice versa and this may
impact the shelf
life, penetration, viability, or other characteristics of the microbes.
[0271] As used herein, unless stated otherwise, the stable shelf life of an
example of a
biochar product having a microbial population is the period of time over which
the product
can be stored in a warehouse, e.g., dry environment, temperature between 40 F-
90 F, with a
less than 50% decrease in microbial population.
[0272] 4. Integrated Growth / Deployable Substrate
[0273] With many microbes, especially fungi, it can be helpful to develop
or "grow" the
microbes on the material itself. With porous materials, rather than
mechanically or chemically
assisting the infiltration of the microbes, it can be beneficial to allow the
microbes themselves to
inhabit the pore volume of the material prior to deployment. In fact, with
materials constructed
74
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
to effectively immobilize microbes, this can be the most efficient technique
to stabilize, store, and
ultimately deploy the microbes in question.
[0274] An example of this method involves preparing the biochar material
for the microbes,
sometimes through thorough cleansing, other times through addition of either
enzymes or energy
sources needed by the microbe in question, preferably using the treatment
techniques described
previously in this disclosure. Once the material is prepared, the microbes are
placed onto the
material, or infused into the material and then incubated for a period of
time. In the case of many
microbial systems, the microbes themselves will inhabit the material and form
close affiliations
with available surfaces and pore volume. At this point, the material can be
deployed with the
microbes actively attached and affiliated. With many microbes, especially
fungi, this is a
preferred method of deployment and shows many advantages over co-deployment,
or basic
inoculation because of the tight integration of biological life with the
material itself.
[0275] An example of an integrated growth inoculation method of biochar for
a fungus in lab
scale is as follows :
1) Make petri dishes containing corn meal agar (17 g/L), glucose (10 g/L), and
yeast extract
(1 g/L)
2) Inoculate plates with Sordaria fimicola and incubate between 22-30C for at
least 1 day
to produce hyphae
3) Sterilize an inoculating loop and slice "plugs" of the hyphae and agar
generating cubes
that are agar and hyphal mass
4) Inoculate a sterile plate with a "plug" in the center of the plate, around
perimeter have
sterile biochar
5) Incubate plate for at least a day and remove biochar (that are now covered
with grown
over hyphae)]
[0276] It should be noted that because of this effect, biochars, and
specifically treated
biochars can also be extremely effective substrates for solid state
fermentation ¨ particularly when
growth media or energy sources are added to the pore volume of the material.
So, once incubation
is ongoing, the material can either be removed, with the integrated microbes,
and deployed, or it
can be stabilized for long term storage, or it can be left in situ and used as
a fermentation or growth
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
substrate to develop or grow more microbes ¨ especially those that require a
solid to propagate
and develop.
[0277] 5. Media and/or Enzyme Infiltration
[0278] As mentioned previously, growth media, energy sources, enzymes, or
other beneficial
/ necessary components for microbial growth may be infused into the pore
volume or coated onto
the surfaces of the material in question. This method can be combined with any
of the other
inoculation techniques disclosed here. It has been shown that with certain
microbes and certain
types of material, inoculation with growth media or enzymes can significant
impact the
effectiveness of the biochar material as a carrier.
[0279] 6. Habitat Pre-Establishment (Enhanced Rhizosphere)
[0280] There are certain microbes which, because of symbiotic associations
with host
organisms, such as plants, prefer to develop in the vicinity of the organism,
such as the active root
or other plant tissue. An effective method for deploying these organisms can
be to develop and
deploy the plant/microbe/habitat (biochar) system together as a unit.
[0281] An example of this is germinating seed or transplanting a seedling
or developing
juvenile plant in the presence of treated or untreated biochar, and the
targeted microbes. Biochar
that has been treated to encourage hydrophilicity and neutral pH typically
allows for easier
affiliation of plant root tissue with the material. As this affiliation
occurs, a habitat for symbiotic
organisms is developed within the material itself due to the proximity of
active plant tissue to
microbes reliant on the tissue for energy. As this symbiosis continues, the
number, activity, and
colony size of the targeted microbes will continue to grow. At this point, the
plant and biochar
can be deployed together into the target environment, acting as a pre-
established habitat and
carrying the microbes along with them.
[0282] Another option is to develop and then remove the biochar from the
"incubation"
system either by stripping the biochar material from the symbiotic organism,
such as the root
mass, or by sieving or sifting the media used to grow the plant. At this
point, the microbes can
either be deployed directly or stabilized for storage.
[0283] Thus, through more controlled inoculation of the biochar particles,
one can achieve
a predetermined and controllable amount of a microbial community, e.g.,
population, into the
soil. This integration of a microbial community with a biochar particle, and
biochar batches
76
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
provides the ability to have controlled addition, use and release of the
microbes in the
community. In agricultural applications, this integration c an further
enhance, promote and
facilitate the growth of roots, e.g., micro- roots, in the biochar pores,
e.g., pore morphology,
pore volume.
[0284] Other methods than those listed above exist for integrating a
microbial community
with an untreated or previously infused biochar particle. Different manners
and methods would
be preferred depending on needs to minimize contamination, encourage biochar
pore
colonization/infiltration, minimize labor and cost and producing a uniform, or
mostly uniform,
product.
[0285] Other methods for integrating a microbial community with a biochar
particle may
include, but are not be limited to the following : while under vacuum, pulling
the microbial
solution through a treated biochar bed that is resting on a membrane filter;
spraying a microbial
solution on top of a treated biochar bed; lyophilizing a microbial solution
and then blending said
freeze dried solution with the treated biochar; again infusing, as defined
previously, the treated
biochar with a microbial solution; adding treated biochar to a growth medium,
inoculating with
the microbe, and incubating to allow the microbe to grow in said biochar
containing medium;
infusing, as defined previously, the biochar with a food source and then
introducing the substrate
infused biochar to a microbe and incubating to allow the microbes to grow;
blending
commercially available strains in dry form with treated biochar; adding the
treated biochar to a
microbial solution and then centrifuging at a high speed, potentially with a
density gradient in
order to promote the biochar to spin down with the microbes; densely packing a
column with
treated biochar and then gravity flowing a microbial solution through the
column and possibly
repeating this multiple times; or adding the microbe to a solution based
binder that is well known
to enter the treated biochar pores and then adding said solution to the
treated biochar. In order
to insure the proper microbial community the treated biochar may need to be
sterilized prior to
these methods for integrating a microbial community. All or parts of the above
manners and
methods may be combined to create greater efficacy. In addition, those skilled
in the art will
recognize that there may be additional manners or methods of infusing biochars
with
microbials, including those created by the combination of one or more of the
manners and
methods listed above, without departing from the scope of the present
invention.
77
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0286] F. Using Microbial Inoculated Biochars
[0287] Thus, treated biochar can have a microbial community in its pores
(macro-, meso-,
and combinations and variations of these), on its pore surfaces, embedded in
it, located on its
surface, and combinations and variations of these. The microbial community can
have several
different types, e.g., species, of biologics, such as different types of
bacteria or fungi, or it
may have only a single type. For example, a preferred functional biochar, can
have a preferred
range for bacterial population of from about 50-5000000 micrograms/g biochar;
and for fungi,
from about 5 to 500000 micrograms/g biochar. A primary purpose in agricultural
settings,
among many purposes, in selecting the microbial population is looking toward a
population
that will initiate a healthy soil, e.g., one that is beneficial for, enhances
or otherwise advance
the desired growth of plants under particular environmental conditions. Two
types of
microbial infused biochars will be discussed further for agricultural
settings: bacteria and
fungi. However, the microbes may also be used in other applications, including
but not limited
to animal health, either directly or through interactions with other microbes
in the animals'
digestive tract and public health applications, such as microbial larvicides
(e.g. Bacillus
thuringiensis var. israelensis (Bl-0) and Bacillus sphaericus used to fight
Malaria).
[0288] G. Bacteria Inoculated Biochars
[0289] PGPB include, for example, plant growth promoting rhizobacteria,
free-living and
nodule-forming nitrogen fixing bacteria, organic decomposers, nitrifying
bacteria, phosphate
solubilizing bacteria, biocontrol agents, bioremediation agents, archea,
actinomycetes,
thermophilic bacteria, purple sulfur bacteria, cyanobacteria, and combinations
and variations
of these.
[0290] PGPB may promote plant growth either by direct stimulation such as
iron chelation,
phosphate solubilization, nitrogen fixation and phytohormone production or by
indirect
stimulation, such as suppression of plant pathogens and induction of
resistance in host plants
against pathogens. In addition, some beneficial bacteria produce enzymes
(including chitinases,
cellulases, -1,3 glucanases, proteases, and lipases) that can lyse a portion
of the cell walls of
many pathogenic fungi. PGPB that synthesize one or more of these enzymes have
been found
to have biocontrol activity against a range of pathogenic fungi including
Botrytis cinerea,
78
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
Sclerotium rolfsii, Fusarium oxysporum, Phytophthora spp., Rhizoctonia solani,
Pythium
ultimum.
[0291] Currently the most economic conventional solid carrier used to
deliver microbes is
peat. A solid carrier allows for a relatively long shelf life and a more
direct application to a
plant's root system compared to a microbial liquid solution, which would be
sprayed directly.
[0292] Research has shown a substantial increase in PGPB growth and
distribution resulting
from being infused in biochar. For example, data resulting from research
conducted to compare
the effects upon CO2 production (an indicator of bacterial growth) using peat
and biochars show
the beneficial effects of using various biochars in promoting PGPB growth. As
illustrated in
the left-hand chart in Figure 33, peat results in CO2 production of between
approximately 10%
and 30% (depending upon the grown medium), whereas biochars result in CO2
production of
approximately 48% and 80%. Replicated experimental results using different
biochars confirm
CO2 production of approximately 30% to 70% (depending on the grown medium), as
compared
to approximately 10% to 20% for the peat control.
[0293] The method developed for determining this CO2 production as an
indicator of
bacterial growth consists of the following. The substrate, here biochar or
peat, is sterilized by
heating at 110C for 15 hours. A bacterial stock solution is then created, here
Tryptic Soy Broth
was solidified with agar at 1.5% w/v in petri plates to isolate the gram
negative non-pathogenic
organism Escherichia coli ATCC 51813 (15h growth at 37 C). Then an isolated
colony is
captured with an inoculating loop and suspend in 10 ml sterile buffer
(phosphate buffer saline
or equivalent) to create the bacterial stock solution. Lactose containing
assays are then used,
here, test tubes that contain 13 ml of either Lauryl Tryptose Broth (LTB) or
Brilliant Green
Broth (BGB) that also contain a Durham tube. A negative control is generated
by adding 10 i.t.L
of sterile buffer to triplicate sets of LTB and BGB tubes. A positive control
is generated by
adding 10 i.t.L of bacterial stock solution to triplicate sets of LTB and BGB
tubes. A negative
substrate is generated by adding 1.25 ml (-1% v/v) of sterile substrate to
triplicate sets of LTB
and BGB tubes. A positive substrate is generated by adding 1.25 ml (-1% v/v)
of sterile
substrate and 10 i.t.L of bacterial stock solution to triplicate sets of LTB
and BGB tubes. The
tubes of the four treatments are then incubated statically in a test tube rack
at 37 C for at least
15h. The tubes are then carefully observed and any gas bubbles captured by the
Durham tube
79
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
within respective LTB or BGB tubes are closely measured with a ruler. Small
bubbles <0.2 mm
should not be considered. A continuous bubble as shown in individual tubes in
Figure 34 are
what are observed and quantified. Figure 34 is an example of carbon dioxide
production
captured as a continuous gas bubble in BGB (left two tubes) and LTB (right two
tubes) growth
medium. The percent carbon dioxide production is then calculated by dividing
the recorded
bubble length by the total Durham tube length and multiplying by 100.
[0294] Further tests were conducted using the Streptomyces lidicus WYEC 108
bacterium
found in one of the commercially available products sold under the Actinovate
brand.
Actinovate products are biofungicides that protect against many common foliar
and soil-borne
diseases found in outdoor crops, greenhouses and nurseries. The formulations
are water-soluble.
[0295] Figure 35 illustrates the effects upon the growth of Streptomyces
lidicus using
conventional peat versus biochars. In the test illustrated by the photograph
on the left of Figure
35, an Actinovate powder was blended with peat, placed in an inoculated media
and incubated
at 25 C. The photograph shows the distribution and density of white colonies
after 3 days. In
the test illustrated by the photograph on the right of Figure 35, an
Actinovate powder was
blended with the treated biochar, placed in an inoculated media and incubated
at 25 C. The
photograph also shows the distribution and density of white colonies after 3
days, the
distribution and density of which are significantly greater than those
achieved with peat.
[0296] Figure 36 further illustrates the improved growth of the Actinovate
bacterium using
biochar versus peat. The left photograph shows only limited and restricted
growth away from
the peat carrier. The right photograph shows abundant growth of the bacterium
spread much
farther out from the biochar carrier.
[0297] Another application of using biochar inoculated with bacteria would
be in the biofuel
industry, where methanotroph inoculated biochar could be used to create
methanol.
Methanotrophic bacteria are proteobacteria with diverse respiration
capabilities, enzyme types,
and carbon assimilation pathways. However, Methylosinus trichosporium OB3b is
one of the
few methanotrophs that can be manipulated by environmental conditioning to
exclusively
produce methanol from methane. M. trichosporium OB3b is one of the most well
studied aerobic
Ci degraders and can be grown in either batch or continuous systems. As
mentioned earlier, the
large pore volume and surface area of biochar is suitable for bacterial
colonization and should
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
subsequently increase substrate access to enzyme activation sites. To improve
the conversion
rate, copper, nitrate, and phosphate should be included in the system. The use
of biochar as a
support material for the aerobic bioconversion of methane to methanol provides
a pore
distribution suitable for both adsorptions of methane and impregnation with
bacteria. By
providing biological and adsorptive functionality the biochar can intensify
the bacteria in the
biochar and increases the conversion rate.
[0298] In general, bacteria communicate via the distribution of signaling
molecules which
trigger a variety of behaviors like swarming (rapid surface colonization),
nodulation
(nitrogen fixation), and virulence. Biochars can bind signaling molecules and
in particular it
is believed can bind a major signaling molecule to their surface. This binding
ability can be
dependent upon many factors including on the pyrolysis temperature. This
dependency on
pyrolysis temperature and other factors can be overcome, mitigated, by the use
of examples
of the present vacuum infiltration techniques. For example, a signaling
molecule that is
involved in quorum sensing- multicellular-like cross-talk found in prokaryotes
can be bound
to the surface of biochars. Concentration of biochars required to bind the
signaling molecule
decreased as the surface area of biochars increased. These signaling molecules
may be added
to the surface of a biochar and may be used to manipulate the behavior of the
bacteria. An
example of such a use would be to bind the molecules which inhibit cell-to-
cell
communication and could be useful in hindering plant pathogens; using
techniques in the
present invention signaling molecules may be added to the surface of a biochar
to engineer
specific responses from various naturally occurring bacteria.
[0299] H. Fungi Inoculated Biochars
[0300] Beneficial fungi include, for example, saprotrophic fungi,
biocontrol fungi,
ectomycorrhizae, endomycorrhizae, ericoid mycorrhizae, and combinations and
variations of
these. It is further theorized that, in general, biochars with greater fungal
development may
be better suited for perennial crops such as grapes, almonds, blueberries, and
strawberries
in which symbiotic relationships with arbuscular mycorrhizal fungi (AMF) are
favored over
PGPBs. The presence of high concentrations of AMF spores in biochars can
therefore rapidly
promote fungal colonization of plant root hairs leading to extensive mycelial
development.
81
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
Increased plant root associations with mycelial filaments would consequently
increase
nutrient and water uptake.
[0301] Mycorrhizal fungi, including but not limited to Endomycorrhizae and
Ectomycorrhizae, are known to be an important component of soil life. The
mutualistic
association between the fungi and the plant can be particularly helpful in
improving plant
survivability in nutrient-poor soils, plant resistance to diseases, e.g.
microbial soil-borne
pathogens, and plant resistance to contaminated soils, e.g. soils with high
metal concentrations.
Since mycorrhizal root systems significantly increase the absorbing area of
plant roots,
introducing mycorrhizal fungi may also reduce water and fertilizer
requirements for plants.
[0302] Typically mycorrhizae are introduced into soil as a liquid
formulation or as a solid
in powder or granular form and contain dormant mycorrhizal spores and/or
colonized root
fragments. Often the most economic and efficient method is to treat the seeds
themselves, but
dealing with traditional liquid and powder inoculums to coat the seed can be
difficult. In
accordance with the present invention, inoculated biochar may be used to coat
the seeds by, for
example, using a starch binder on the seeds and then subjecting the seeds to
inoculated biochar
fines or powder. Another method is by placing the mycorrhizae inoculum in the
soil near the
seeding or established plant but is more costly and has delayed response as
the plants initial
roots form without a mycorrhizal system. This is because the dormant
mycorrhizae are only
activated when they come close enough to living roots which exude a signaling
chemical. In
addition if the phosphorus levels are high in the soil, e.g. greater than 70
ppm, the dormant
mycorrhizae will not be activated until the phosphorus levels are reduced.
Thus applying
inoculant with or near fertilizers with readily available phosphorus levels
can impede the desired
mycorrhizal fungi growth. A third option is to dip plant roots into an
inoculant solution prior to
replanting, but this is costly as it is both labor and time intensive and only
applicable to
transplanting.
[0303] If the colonization of mycorrhizae can be quickened and the density
of the
mycorrhizae' s hyphal network can be increased then the beneficial results of
mycorrhizal root
systems, e.g. increased growth, increased survivability, reduced water, and
reduced fertilizer
needs, can be realized sooner. Prior art shows that compost, compost teas,
humates, and fish
fertilizers can improve microbial activities and in more recent studies have
shown physically
82
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
combining arbuscular mycorrhizal fungi (AMF) inoculant with raw biochar has
resulted in
additional plant yield compared to each alone. See Hammer, et. al. Biochar
Increases
Arbuscular Mycorrhizal Plant Growth Enhancement and Ameliorates Salinity
Stress, Applied
Soil Ecology Vol 96, Nov 2015 (pg. 114-121).
[0304] An ideal carrier for the mycorrhizae would have moisture, air, a
neutral pH, a
surface for fungi to attach, and a space for the roots and fungi to meet. Thus
a previously
infused biochar created by the method disclosed above would be a better
carrier than raw
biochar alone. The infused biochar could be physically mixed with a solid
mycorrhizal fungi
inoculant or sprayed with a liquid mycorrhizal inoculant prior to or during
application at
seeding or to established plants. Additionally, the infused biochar and
mycorrhizal fungi
inoculant could be combined to form starter cubes, similar to Organo-Cubes,
rockwool, oasis
cubes, and peat pots.
[0305] The infused biochar could be further improved upon by initially
infusing or further
infusing the biochar with micronutrients for mycorrhizal fungi, for example
but not limited to
humic acid, molasses, or sugar. The growth nutrient infused biochar would
further expedite
the colonization of the mycorrhizal fungi when physically combined with the
inoculant and
applied to seeds or plants.
[0306] Another improvement to the infused biochar would be to initially
infuse or further
infuse the biochar with the signaling molecules of mycorrhizal fungi. The
signaling molecule
infused biochar would further expedite the colonization of the mycorrhizal
fungi when
physically combined with the inoculant and applied to seeds or plants, as it
would bring the
mycorrhizae out of dormancy quicker and thus establish the mycorrhizal root
system quicker.
[0307] Another method for establishing and improving mycorrhizal fungi
colonies would
be by growing mycorrhizae into the infused biochar and then applying the
mycorrhizal fungi
inoculated biochar to seeds or plants. Similar to a sand culture (Ojala and
Jarrell 1980
http://jhbiotech.com/docs/Mycorrhizae-Article.pdf), a bed of infused biochar
is treated with a
recycled inoculated nutrient solution by passing it through the bed multiple
times.
[0308] I. Batch Treatment/Bulk Production
[0309] As demonstrated above, the treatment processes described above are
particularly
well suited for large scale production of biochar. The processes and biochars
of the present
83
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
invention provides a unique capability to select starting materials and
pyrolysis techniques
solely on the basis of obtaining a particular structure, e.g., pore size,
density, pore volume,
amount of open pores, interconnectivity, tortuosity, etc. Thus, these starting
materials and
processes can be selected without regard to adverse, harmful, phytotoxic side
effects that
may come from the materials and processes. This is possible, because the
infiltration steps
have the capability of mitigating, removing or otherwise address those adverse
side effects.
In this manner, a truly custom biochar can be made, with any adverse side
effects of the
material selection and pyrolysis process being mitigated in later processing
steps.
[0310] Further, the processes are capable of treating a large, potentially
variable, batch of
biochar to provide the same, generally uniform, predetermined customized
characteristics for
which treatment was designed to achieve, e.g., pH adjustment. Treatment can
result in treated
biochar batches in which 50% to 7 0 % to 80% to 99% of the biochar particles
in the batch
have same modified or customized characteristic, e.g., deleterious pore
surface materials
mitigated, pore surface modified to provide beneficial surface, pore volume
containing
beneficial additives.
[0311] Accordingly, the ability to produce large quantities of biochar
having a high level
of consistency, predictability and uniformity, provides numerous advantages in
both large
and small agricultural applications, among other things. For example, the
ability to provide
large quantities of biochar having predetermined and generally uniform
properties will find
applications in large scale agriculture applications. Thus, treated biochar
batches from about
1001bs up to 50,000+lbs and between may have treated biochar particles with
predetermined,
uniform properties.
[0312] As the treated biochar batches are made up of individual biochar
particles, when
referring to uniformity of such batches it is understood that these batches
are made up of tens
and hundreds of thousands of particles. Uniformity is thus based upon a
sampling and testing
method that statistically establishes a level of certainty that the particles
in the batch have
the desired uniformity.
[0313] Thus, when referring to a treated batch of biochar as being
"completely uniform"
or having "complete uniformity" it means that at least about 99% of all
particles in the batch
have at least one or more property or feature that is the same. Same being
within appropriately
84
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
set tolerances for said property. When a treated batch of biochar is referred
to as "substantially
uniform" or having "substantial uniformity" it means that at least about 95%
of all particles
in the batch have at least one or more property or feature that is the same.
When a treated
batch of biochar is referred to as "essentially uniform" or having "essential
uniformity" it
means that at least about 80% of all particles in the batch have at least one
or more property
or feature that is the same. The batches can have less than 25%, 20% to 80%,
and 80% or
more particles in the batch that have at least one or more property or feature
that is the same.
Further, the batches can have less than 25%, 20% to 80%, and 80% or more
particles in the
batch that have at one, two, three, four, or all properties or features that
are the same.
[0314] J. Aggregate Biochar Particles
[0315] It has been discovered that the same benefits can be achieved
through the production
and application of biochar aggregate particles as biochar particles that have
not been aggregated.
The creation of biochar aggregate particles, however, allows for easier
product distribution
for in various applications including industrial agricultural equipment, and
provides a highly
beneficial use for the biochar dust and fines, which are generally discarded.
In this same
manner, biochar aggregate particles may be produced for use for consumption by
animals or
use in composting.
[0316] The biochar, prior to being formed into a solid aggregate (e.g.,
through
agglomeration, extrusion, or pelletization), may be raw or treated, as
described above. If the
biochar is treated, not only can various characteristics including pH be
adjusted as needed, but
fertilizers, nutrients, vitamins, supplements, microbes or other additives may
be infused into the
biochar prior to aggregation. (as further described below). However,
regardless of whether the
biochar is raw or treated, the present application for biochar aggregate
particles can be utilized
for both.
[0317] There are various types of aggregation methods and resulting
aggregate particles.
Figures 37a, 37b and 37c shows three resulting aggregate examples. Figure 37a
shows pellets,
Figure 37b shows extrudates and Figure 37c shows biochar sulfate prills.
[0318] As an example, one method to produce biochar aggregate particles is
depicted in the
flow diagram shown in Figure 38. The flow diagram 3800 of Figure 38 is an
example of one
method that may be used for producing biochar aggregate particles. In general,
the method of
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
producing biochar aggregate particles from biochar may be accomplished by
first collecting
the treated or untreated biochar fines at step 3802. The fines may be
collected by washing the
biochar media, which may cause the biochar fines and dust to be placed in
suspension in the
liquid solution used to wash and/or treat the biochar. The biochar fines can
also be produced
by grinding, crushing, sieving, or otherwise resizing biochar of a larger
particle size to one
better suited for extrusion, compression, coagulation, or other forms of
pelletization.
[0319] For example, the biochar fines may be separated from larger biochar
particles by
dry-sieving to remove the fine particles followed by wet-sieving with
deionized water to remove
fine fractions that remained. To separate particles of 0.5 mm or less of
equivalent diameter,
both the dry-sieving and wet-sieving may be carried out with a US size 35 mesh
sieve. Biochar
fines or dust may also be created by mechanical means such as grinding cutting
or crushing the
raw or treated biochar particles. These mechanically created small particles
can be separated as
set forth above through sieving or may be collected by washing or treating the
material and
using the resulting solution to recover the smaller particles. The recovery of
small biochar
particles from the solution can be accomplished by using chemical or physical
means of
separation or even a combination of multiple chemical and physical separation
methods or steps.
[0320] While the biochar particles or fines may be treated in advance of
collection, it is also
possible to treat them once collected or as part of the collection process.
Optionally, other
physical and chemical properties may be adjusted during the treating step, as
needed, or may
be adjusted prior to, or during the fines collection process. For example, the
biochar fines
may be collected during treatment of the biochar media (e.g., to adjust the
pH). The fines may
then be collected in the treating solution by adding a flocculent and/or
coagulant to the treating
liquid, which creates a biochar slurry (the "flocculent slurry").
[0321] Given the application, it may be necessary to de-water the
flocculent slurry before
further treatment, as part of the collection process. The flocculent slurry is
de-watered,
typically using a belt filter press to create a biochar paste. Those skilled
in the art will recognize
that other de-watering systems, besides a belt filter press may be used to de-
water the biochar
slurry and that mechanisms other than a flocculent, such as filtration,
settling, or other separation
technology, may be used to separate the biochar from the minerals, inorganic
compounds, and
other substances found in the slurry that remain in the washing or treating
solution.
86
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0322] Once the biochar fines are collected, a binder is then added to the
biochar particles
at step 3804. The binder solution used to coagulate the fines may be prepared
by mixing a
starch, polymer, lignin, clay, or other binder with water or appropriate
solvents. The addition
of the binder solution creates a biochar slurry or a paste (the "binder
slurry"). The binder
solution may be prepared by mixing, for example, enough corn starch with
deionized H20 to
create a solution. For example, the starch may be approximately 2% by weight,
but may range
from 0.5% to 10% by weight. Those skilled in the art will recognize that
another material,
besides corn starch may be used as a binder. Additionally, other binders may
be used with the
restriction that they must be appropriate for the application they will be
used in. So for example,
they may not be toxic in the quantities used in agriculture or animal feed and
must be suitable
for introduction into whatever application without profound ill effect. Some
examples of other
generally non-toxic binders that may be used are gelatins, cellulose, sugars,
or combinations
thereof. While the above describes adding the binder after the flocculent
slurry is dewatered,
the binder may also be added to the flocculent slurry before de-watering.
[0323] Like the flocculent slurry, the binder slurry is also de-watered
before further
treatment, step 3806. The binder slurry may be de-watered using a belt filter
press to create a
biochar paste. Those skilled in the art will recognize that other de-watering
systems, besides a
belt filter press may be used to de-water the biochar.
[0324] Optionally, other growth or beneficial additives may also be added
to the slurry at
step 3706. The binder and the growth additives may be added together or at
separate stages,
before or after the de-watering step 3806, with or without de-watering
between, depending
upon the application, the binder and the additives. In either event, the
biochar is de-watered
at step 3806 prior to further treatment.
[0325] Such growth enhancing additives may include, but are not limited to,
fertilizers and
beneficial microbes that can withstand the biochar aggregation process. For
certain additives,
the temperature of the process may need to be adjusted to avoid, for example,
the denaturing
of the proteins. Such additives can be added to the biochar particles (either
with or after de-
watering the starch slurry) through mixing. If a fertilizer is desired, the
fertilizer may be
pulverized to prepare for addition. The fertilizer may be pulverized to an
average particle size
of < lmm before dispensing. Liquid fertilizers may also be used in solution.
For example, 1000
87
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
ppm NO3- N fertilizer solution may be used. Examples of fertilizers that may
be added to the
paste, include, but are not limited to the following: ammonium nitrate,
ammonium sulfate,
monoammonium phosphate, ammonium polyphosphate, Cal- Mag fertilizers or
micronutrient
fertilizers. Other additives, such as fungicides, insecticides, nematicides,
plant hormones,
beneficial microbial spores, secondary signal activators, vitamins,
medications, supplements, or
sensory enhancers may also be added to the paste in a similar manner as a
fertilizer, the inclusion
of which does not depart from the scope of the invention. Additionally,
beneficial macro- and
micro- nutrients such as nitrogen, phosphorous, potassium, calcium, magnesium,
sulfur, boron,
zinc, iron, manganese, molybdenum, copper and chloride can be added to the
mixture at this
time.
[0326] Examples of compounds, in addition to fertilizer, that may be
blended with, infused
into the pores of or coated on the surface of the biochar include, but are not
limited to: 2,1,3-
Benzothiadiazole (BTH), an inducer of systemic acquired resistance that
confers broad spectrum
disease resistance (including soil borne pathogens); signaling agents similar
to BTH in
mechanism or structure that protects against a broad range or specific plant
pathogens;
biopesticides; herbicides; and fungicides.
[0327] As noted above, all the above additives may also be added at various
steps in the
described processes, including with the flocculant or coagulant, with the
binder, or prior to
the creation of the slurry or biochar paste. Such additives may be added
through a pre-
treatment process, such as those treatment processes described above (e.g.,
vacuum infiltration
or surfactant treatment), or other treatment processes that result in the
infusion of liquids
and/or vapors into the pores of the biochar. It may also be possible to
contact the biochar
aggregate particles, once they are produced, with additives. Such contact or
coating after
production of the biochar aggregate particles is within the scope of the
present invention.
[0328] Once de-watered, at step 3806, the biochar particles become a
thicker slurry or
paste (the "biochar paste"). The biochar paste, now including a binder and
possibly other
additives, is then formed into solid shapes, at step 3808 and then dried, at
step 3810. To form
the biochar paste into solids, alternative forms of processing may be used.
For example, the
paste may be passed through an extruder, a pelletizer, a briquetter, a
granulator and/or other
heating, cooling, dehydration, or pressure system capable of forming the paste
into solid
88
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
shapes. Alternatively, the biochar may be mixed with the binder, both in a dry
form, and then
fed into the equipment used to form the solid shaped biochar aggregates while
adding moisture
and/or other additives.
[0329] In one example of an implementation, the biochar paste is shaped
through an
extruder that is heated at a temperature of 25-120 C in order to adequately
activate the starch
or other binder. The extruder may be specifically set depending on the
appointed application
to produce an extrudate size, ranging from 1 to 5 mm in diameter. At step
3810, the resulting
extrudates are dried using a hot air, tunnel oven dryer, or other dryer known
to the art. For
some application, e.g., when microbes are added to or inoculated into the
biochar particles, it
may not be desirable to use heat to activate the binder. Alternatively, lipids
or other binders that
bind at cold temperatures may be used, with the substitution of cooling
equipment in place of
heating equipment to activate said binder.
[0330] The biochar aggregate particles from the extruder may be cut into
predetermined
specific sized particles, which may take the form of pellets. The steps of
extruding and cutting
may be performed together by the extruder, or separately, again depending upon
the
application and equipment capabilities. In addition larger extrudates can be
formed creating
a biochar spike, which can be applied by pushing them into soil near existing
plants or trees.
[0331] In one example of an implementation, the biochar aggregate particles
may be created
from pyrolyzed wood or cellulosic biomass, as described above. The resulting
biochar fines or
dust are then removed from the other biochar particles at step 3802. As part
of the collection
process, the fines may optionally be washed with a treatment solution, as
described in detail
above. The treatment solution may, for example, be added to neutralize the
biochar pH levels,
as needed, depending upon the pH of the biochar fines. A neutralized biochar
slurry is then
exposed to a de-watering station and a flocculent is added to coagulate the
fines or dust for de-
watering. To dewater the flocculent slurry, a belt filter press or other
equipment known to the
art may be used. Once dewatered, a starch or another suitable binder is added
to the biochar
particles, at step 3804. Other additives may also be added to the biochar
particles during this
step. The biochar particles are again de-watered at step 3806 and the slurry
becomes a thicker
slurry or paste. The de-watered biochar paste may then be formed into
aggregate solids at step
3808, by, for example, the use of an extruder. The aggregate particles are
then dried using a hot
89
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
air, tunnel oven dryer, or other dryer known to the art, at step 3810. The
aggregate particles
could also be freeze dried (e.g., lyophilized) and/or allowed to air dry, at
step 3810. Such drying
can be done before or after the biochar paste is subjected to a forming
processes.
[0332] Treatment of biochar fines or dust is optional, but may be desired
for pH adjustment
and/or removal of elemental ash and other harmful organics or materials, as
described in more
detail above. Depending on the chemical properties of the biochar dust or
fines, either water or
acidic acid can be used to adjust the pH to neutral levels, and obtain a
neutralized biochar slurry.
The wash may also contain a surfactant or detergent to aid in the penetration
of the wash
solution into the pores of the char. Those skilled in the art will recognize
that other pH adjusting
agents, besides acidic acid may be used to neutralize the biochar pH levels.
Additionally, other
binders may be used with the restriction that they must be suitable for
introduction into their
particular application, for example not phytotoxic for use in soil or toxic to
animals or humans
for use in animal feed or maintenance. Some examples of these other pH
adjustment agents
include, but are not limited to gypsum, sulfur, lime, or combinations thereof.
As set forth
earlier, treatment can be performed on the fines or on the larger biochar
media from which the
fines are collected.
[0333] The above illustrated example details only one method of how biochar
aggregate
particles may be produced. As noted above, alternate forming processes may
also be used
besides passing the biochar paste through an extruder, such as a pelletizer, a
briquetter, a
granulator and/or other heat, cold, evaporation and/or pressure system capable
of forming the
paste into solid shapes.
[0334] Further, in another implementation, raw or treated biochar fines
and/or larger
biochar particles may be dried and ground to a smaller particle size or
powder. The biochar
powder can then be mixed with a binder in a rotary drum to create reasonably
uniform
spherical biochar aggregate particles.
[0335] Further, in another implementation, the biomass, prior to pyrolysis,
may be formed
into solids shape aggregates, such as pellets, by equipment designed to create
pellets, granules
and/or briquettes. Further, these pellets may be stabilized by mixing a dry
binder or a binder
solution with biomass prior to pelletizing to improve the mechanical stability
of the formed
pellet. These binders may include but are not limited to starches, polymers,
clays, or lignins.
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
By shaping the biomass prior to pyrolysis, the biomass may retain the solid
shape. Depending
upon the biomass, the biomass aggregate may need to be treated prior to
pyrolysis to maintain
the original shape with, for example, a binder solution. Wet formed, or
solution treated pellets
may require drying before handling and pyrolysis. This drying may be done
using hot air, a
tunnel oven dryer, or other dryer known to the art.
[0336] In creating biochar aggregates, it is critical to determine the
proper biochar particle
size and the proper method to use to create said particles for the biochar
aggregate production.
Setting the correct size limits and method will ensure the aggregates maintain
the physical and
chemical characteristics that make the specific biochar effective in the
target application.
Figures 39 and 40 show SEM photos from two different biomass based treated
biochars.
Figure 39 shows the effect of size and grinding on particle structure for
three different particle
size ranges: 0.1-0.3 mm, 0.05-1mm, and <0.05 mm. These particles were
collected using two
different methods: (i) sieving the as is treated biochar and (ii) grinding the
as is treated biochar
and then sieving. Figure 39 shows one treated biochar ("treated biochar 1")
and Figure 40
shows a second treated biochar ("treated biochar 2"). In the treated biochar 1
SEM photos
(Figures 39a, b, c, d, e and f), it is clear that the two methods of
collection show no substantial
difference in pore structure. It is also clear that the particle structure is
destroyed once the
particle sizes are less than 0.05 mm. In the treated biochar 2 SEM photos
(Figures 40a, b, c,
d, e and f), a different observation is noted, when the material is just
sieved to 0.3-0.5mm
range, the biochar particle has retained its porous structure, but when the as
is treated biochar
2 is ground using a medium grind or a fine grind and then sieved to 0.3-0.5mm
range, then the
porous structures have been mostly destroyed. Figures 40d, 40e and 40f are
zoomed images
of Figures 40a, 40b and 40c.
[0337] In addition, various particle size ranges from the two treated
biochars were further
tested to see how biochar characteristics changed with particle size. Figures
41 a, b, c and d
show the effect of size fraction on four properties, water holding capacity,
pH, Cl-
concentration, and electrical conductivity of two different biomass based
treated biochars. For
treated biochar 1, these properties, except pH, were stable across particle
sizes except when
the particles were smaller than 0.1mm. This is likely due to the loss of pore
structure
somewhere below 0.1 mm for this treated biochar. For treated biochar 2, some
properties,
91
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
electrical conductivity and chloride concentration, seemed to correlate to
particle size in a
similar way to that of treated biochar 1. But decreasing particle size of
treated biochar 2 had
the opposite effect on water holding capacity and pH versus treated biochar 1.
[0338] These observations show how particle size and the method used to
create them can
have significant impact on both the pore structure of the particles and the
biochars properties.
Thus an aggregate's properties and effectiveness can be maintained, adjusted,
or harmed based
on the method for creating and collecting sized biochar particles in addition
to the method of
aggregation and may differ based on the biochar feedstock and pyrolysis
method.
[0339] Further in another implementation, the biomass may be sized prior to
pyrolysis so
that the aggregate can be made with the as is biochar particles or treated
biochar particles
without additional sizing. Eliminating the need to size the biochar further,
may help to
maintain the biochar properties when aggregating as biochar pore structures
that are
susceptible to being destroyed during sizing post pyrolysis will not be harmed
using this
method.
[0340] As noted above, the biochar aggregate particles can be created with
either raw
biochar or treated biochar that is treated in the manner or method further
described below.
Biochar aggregate particles can be applied through a wide range of devices,
including
agricultural equipment including but not limited to broadcast spreaders, drop
spreaders and/or
hand distribution means. The application of biochar aggregate particles can be
used for trees,
row crops, vines, turf grasses, potted plants, flowering plants, annuals,
perennials, evergreens
and seedlings. The biochar aggregate particles may also be applied to animal
pens, bedding,
and/or other areas where animal waste is present to reduce odor and emission
of unpleasant or
undesirable vapors. Furthermore it may be applied to compost piles to reduce
odor, emissions,
and temperature or even to areas where fertilizer or pesticide runoff is
occurring to slow or
inhibit leaching and runoff. The aggregates may also be integrated with animal
feed and/or
other substances beneficial to animal health, either whole (biochar pellets
mixed with separate
feed pellets to form an aggregate, for example), or with animal feed or other
beneficial
substances mixed into the biochar slurry or paste prior to extrusion. Biochar
aggregate particles
may be incorporated into or around the root zone of a plant. As most trees,
rows, and specialty
crops extract greater than 90% of their water from the first twenty-four
inches below the soil
92
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
surface, the above applications will generally be effective incorporating the
biochar around the
root zone from the top surface of the soil and up to a depth of 24" below the
top surface of the
soil, depending on the plant type and species, or alternatively, within a 24"
radius surrounding
the roots regardless of root depth or proximity from the top surface of the
soil. When the plant
roots are closer to the surface, the incorporation of the biochar within the
top 2-6" inches of the
soil surface may also be effective. Greater depths are more beneficial for
plants having larger
root zones, such as trees.
[0341] Biochar aggregates are particularly useful, when they will be put
into an application
that requires mixing with other solid granular products. This is because the
aggregates can be
designed and created to be similar in shape, size, or density to that which it
will be mixed with.
When the aggregates are physically similar to the material particles they will
be mixed with then
the final mixture will stay more uniformly mixed and have better flow
properties. When a
specific rate of each material in the mixture is needed, say in agriculture or
animal feed, then a
uniform mixture is critical to ensure the soil or animal consistently gets the
correct rate.
[0342] K. Biochars for Use In Composting
[0343] In addition to the use of treated biochar in connection with
agriculture and animal
applications for human consumption, treated biochar can also be used
throughout the world, in
numerous composting applications. The biochar used in composting applications
can be all
treated biochar, in accordance with the treatment processes set forth above,
or may be mixed with
raw, untreated biochar.
[0344] Fig. 42 is a diagram illustrating one example of the work flow for a
commercial food
composting operation. As illustrated in the diagram, compost material is first
dropped at a
weigh station, where clients are paid various rates for the compost materials.
The materials
then released to a tipping floor and segmented by types. Green waste/woods are
cleaned and
ground down on the production floor. Foods are slowly received and stored.
Screening of
green waste/woods creates various sized inputs. Stored food is blended with
green waste/woods
via screening to remove inerts from food.
[0345] When composted using covered aerated static piles ("CASP"), piles of
the materials
are placed over porous pipes. Tarps are laid over the pipes. Negative pressure
aerates the piles
and pulls odor into a biofilter. The CASPs run for approximately 30 days. When
the piles are
93
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
composted using woodrow (mechanical turning), the piles are kept in the
woodrow for
approximately 15 days.
[0346] Biochar can be applied to composting environments to allow for the
control of
temperature, moisture, pH levels, odors and bacterial cultures. As illustrated
below, applying
biochar in composting environments has been shown to significantly reduce
water loss, control
temperatures, reduce odors and control acidic pH issues. The present treatment
processes for
biochar allow for the capability of custom-manufacturing biochar for use in
composting for a
particular climate, environment, geographical area, or by more precisely
controlling key
characteristics of the biochar.
[0347] The method of the present invention for applying biochar to composts
includes
blending low, affordable rates of treated biochar (1% - 5% v/v) with feedstock
high in food
residuals (40% v/v). Treated biochar may also be blended with other materials,
such as raw and/or
processed biochar, processed differently than the treatment processes
described above, and with
compost having other compositions than feedstock high in food residuals.
Blending various rates
of treated biochar, by itself, or with raw and/or processed biochar, in
various composting
environments may produce different desired results.
[0348] One of the recurring problems in composting environments is to control
the acidity
levels and the lowering of pH in the compost. Food residuals contain high
levels of organic
acids like lactic acid. Low pH shifts the microbial community to more acid
tolerant microbes
that stimulate a feedback loop wherein lactobacilli produce more lactic acid.
Figure 43 is a
chart showing the pH of compost as the percent of lactic acid increases. As
illustrated in Figure
43, the more lactic acid by percent, the lower the pH in compost. Figure 43
shows the general
pH of compost materials, before commencing the composting process. Figure 44
demonstrates
how pH is influenced in compost when mixing green wastes, woods and foods. As
illustrated,
the addition of foods and woods to compost lower the pH of the compost. Green
waste provides
the highest pH, while the combination of foods, green waste and wood, produce
the lowest pH.
[0349] In composting, different microbial communities degrade the organic
acids to raise the
pH. Generally, the starting point for feedstock composting is a pH of >6Ø In
CASP methods,
feedstock compost may remain acidic to a pH of <5Ø Acidic compost is not
ideal for plant
94
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
nutrient uptake or other uses of the compost. Raising the pH in the compost is
desired for a
number of reasons.
[0350] Adding treated biochar to compost has been shown to increase aeration
and lower
and/or control the temperatures in the compost, leading to higher, less acidic
pH levels. Lower
temperatures are critical in the early stages of composting to stimulate the
mesophilic ("cool-
loving") microbes to outcompete the thermophilic ("heat-loving") microbes
inherent to food
residuals. Lactic acid bacteria are thermophiles that generally reduce the pH
levels in compost.
Adding treated biochar to compost appears to reduce lactic acid bacteria and
generally increase
the pH levels in compost. Despite lower temperatures, pathogen reduction still
occurs. These
reduced composting temperatures also means less air and water will be
required.
[0351] Figure 45 is a chart showing the impact on composting temperatures when
1% and
3% treated biochar are added to the compost (control). The control represents
the compost with
0% added biochar. As shown by Figure 45, adding treated biochar to compost in
a windrow
environment generally decreases the temperature in the compost. It was shown
that adding 1-
3% treated biochar to the compost in a windrow environment generally lowered
the temperature
in the compost between 5-20 F.
[0352] Figure 46 is a chart showing the decrease of lactic acid production
in compost by
adding treated biochar. As shown by Figure 46, adding treated biochar to
compost in a windrow
environment generally decreases the lactic acid in the compost. The addition
of 1% treated
biochar in the compost reduced the lactic acid by 0.5-0.6 %DM and the addition
of 3% treated
biochar in the compost reduced the lactic acid by as much as 1.0-1.1 %DM. The
control
compost is represented by 0% added treated biochar.
[0353] Figure 47 is a chart showing the increase in pH in compost by adding
treated biochar.
As shown by Figure 47, adding treated biochar to compost in a windrow
environment generally
increases the pH level in the compost. The addition of 1% treated biochar in
the compost
increased the basicity from between 4.7-4.8 pH to approximately 5.1 pH. The
addition of 3%
treated biochar in the compost increased the basicity from between 4.7-4.8 pH
to approximately
5.3 pH. The control compost is represented by 0% added treated biochar.
[0354] Figure 48 is a chart showing the increase in oxygen levels in compost
by adding
treated biochar. As shown by Figure 48, adding treated biochar to compost in a
windrow
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
environment generally increases the oxygen level in the compost. The addition
of 1% treated
biochar in the compost increased the oxygen level from approximately 18.4% to
approximately
19.8%. The control compost is represented by 0% added treated biochar. The
increased oxygen
levels show the increased aeration in the compost and may explain the lowered
temperatures
also observed.
[0355] Figures 49 and 50 show the impact of the addition of both raw and
treated biochar in
a CASP compost environment to volatile fatty acids (VFAs) and ammonia (NH3)
levels,
respectively. When comparing raw biochar to treated biochar in CASP
environments, it was
generally shown that raw biochar has no effect on volatile fatty acids (VFAs)
and increases NH3
levels. Treated biochar on the other hand was shown to reduce both VFAs and
NH3 levels and
indicative of reducing air emissions. VFAs and NH3 levels are known to be odor
indicating
compounds. Reducing the amount of VFAs and NH3 levels in the compost should
indicate a
reduction in the odor produced by the compost. Additionally, if NH3 levels are
reduced, then
the nitrogen is more likely staying in the form of ammonium (NH4) and
eventually turning into
nitrates, which improves the quality of the resulting compost product.
[0356] As shown by Figure 49, adding treated biochar to compost in a CASP
environment
generally decreases VFAs while the addition of raw biochar has no visible
effect. The control
compost has 0% added biochar. As shown by Figure 50, adding treated biochar to
compost in
a CASP environment generally decreases NH3 while the addition of raw biocarbon
increases
NH3. The control compost has 0% added biochar.
[0357] Figure 51 is a chart showing the impact on volatile organic compounds
("VOC") by
adding treated and raw biochar to CASP compost. As shown, the addition of raw
or treated
biochar has variable effects on VOCs and can increase or decrease volatile
organic compounds.
The measurements were taken from negative pressure system of compost from a
CASP
environment tapped into a summa canister to capture gases generated by the
compost. The
addition of treated biochar to compost, compared to the control compost (0%
biochar added),
decreased the percentage of methyl-iso-butyl ketone (MB K), ethanol and
methanol, while it
increased the percentage of 2-propanol, propene, 2-butanone and acetone. The
addition of raw
biochar, compared to the control compost, decreased the percentage of propene,
2-butanone,
acetone and methanol, while it increased the percentage of MBK and 2-propanol.
96
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0358] Figure 52 is a chart shows a test of evaporative water loss from
control compost
(Control 100) against blended treatments with raw or processed biochars at 1,
3 and 5% by
volume. Treated biochar at 1 or 3% outperformed raw treatments by as much as
10%. Treated
biochar added to control compost at 3% v/v showed a dramatic 17.5% reduction
in evaporative
loss. The control compost in Figure 52 is without raw and/or treated biochar.
As shown, the
evaporative loss of water in compost decreased as much as 10% if the compost
is mixed with
1-3% processed biochar. Mixing the compost with 3% treated biochar has shown
to maintain
moisture levels in the compost essential for a climate similar to California.
[0359] Figure 53 is a chart showing the effect that the addition of treated
biochar has on
percent mass water loss in a CASP compost environment. Mass was determined by
pile volume
and bulk density. As shown in Figure 53, adding % treated biochar to the piles
of control
compost reduced the water mass loss by 10%. The control compost in Figure 53
is without raw
and/or processed biochar.
[0360] All biochar treatments of compost have shown reductions in water loss
and mixing
various levels of treated biochar into the compost can assist to control
essential moisture levels
for various climates and assist in optimizing the composting process. Similar
effects are seen
windrow compost environments. As treated biochar controls the pile
temperatures (see Figure
45), despite the lower temperatures pathogen reduction still occurs. Lower
pile temperature can
reduce water demand up to 1,000 gallons of water added every 3-4 days.
[0361] Figures 54, 55 and 56 all demonstrate the impact of inoculating the
biochar with
specialized microbes. In all cases, the compost includes 2.6 % biochar. The
biochar added to the
control is raw biochar. The biochar B2XNA and B2XA are inoculated with
bacillus. Bacillus
spp. was chosen for their ability to form endospores that allow the microbes
to survive harsh
temperature found during composting. Relative Percent Abundance of Bacillus
spp. is as follows:
Bacillus licheniformis (25%); Bacillus szutsauensis (5%); Bacillus
amyloliquefaciens (15%);
Bacillus subtilis (18%); Bacillus velezensis (26%); and Bacillus pumilus
(33%). The types of
Bacillus used were selected for the following purposes: nutrient cycling (B.
licheniformis and B.
subtilis), nitrogen fixation (B. pumilus), biocontrol of plant pathogens (B.
velezensis and B.
subtilis), and plant growth promotion (B. pumilus and B. subtilis). B2XA was
pH adjusted,
97
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
whereas B2XNA was not pH adjusted. B4XA is inoculated with twice as much
bacillus as the
B2XA and was also pH adjusted.
[0362] Figure 54 is a chart showing in impact of the addition of the
inoculated biochar to
compost on microbial abundance. Figure 55 is a chart showing in impact of the
addition of the
inoculated biochar to compost on VOCs. Figure 56 is a chart showing in impact
of the addition
of the inoculated biochar to compost on NH3
[0363] Figure 54 shows that compost piles having 2.6% inoculated biochar had
elevated
populations of gram positive bacteria. As illustrated, compost piles mixed
with biochar
inoculated with bacteria are shown to have elevated populations of gram
positive bacteria. This
suggests that thermotolerant endospore forming bacterial inoculated into
biocarbon can survive
native competition in composting systems and may have a positive effect on the
composting
process.
[0364] Regarding Figure 55, it was generally determined that inoculated
biocarbon decreases
VOC levels. However, inoculated biochar, B4XA, treatment of biocarbon
increased the VOC
levels, possibly due to elevated bacillus populations.
[0365] Regarding Figure 56, it was generally determined that inoculated
biocarbon decreases
NH3 levels.
[0366] In general, in the application of biochar to compost, it was shown
that treated biochar
is able to raise the pH levels in composting with food waste, improves
aeration, lowers
temperature of compost piles, and can reduce odor indicating compounds like
ammonia, VFAs
and other volatile organics. Compared to raw biochar, treated biochar
outperforms with the
control of most of the odor indicating compounds and, at lower doses, with the
ability to reduce
evaporative loss. Treated biochar helps reduce overall water loss that occurs
during composting
and helps reduce water inputs regarding temperature control.
[0367] In addition to the composting benefits seen by adding treated biochar,
the value of the
resulting compost is also increased. Since the treated biochar helped retain
nitrogen during the
composting (as seen by reduced NH3), the compost itself will have higher
nutrients when applied
in agriculture usage. Also, the treated biochar remains in the compost and
continues to display
the benefits outlined in this invention, including but not limited to water
and nutrient retention.
98
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
Thus when the resulting compost is used in agriculture the compost will show
similar
improvement trends as when treated biochar itself is applied.
[0368] In another implementation or this invention, treated biochar could be
added directly to
animal bedding to control odors. Then once used, the bedding could be recycled
via composting
and still get the benefits of the treated biochar in composting. And finally
the resulting compost
which still has treated biochar could be used in agriculture and still
continue to provide additional
benefits to plants as well.
[0369] L. Animal Applications
[0370] Generally, treated biochar of the present inventions can be used with
numerous animal
species, large and small scale farming, and in a variety of animal management
applications and
systems, and combinations and variations of these. In fact, this particular
solution provides the
capability to custom-manufacture biochar for a particular species, physiology,
nutritional need,
pathogen susceptibility, illness, environment, geographical area or other
application by more
precisely controlling key characteristics.
[0371] The fundamental benefit of treated biochar use in animal
applications is the fact that
deleterious characteristics can be adjusted and toxic materials left over from
the biomass and its
pyrolysis can be removed. For example, pH can be adjusted, and undesirable
ash, inorganic
compounds, toxins or heavy metals, and organic compounds such as acids,
esters, ethers, ketones,
alcohols, sugars, phenyls, alkanes, alkenes, phenols, polychlorinated
biphenyls or poly or mono
aromatic hydrocarbons, can be removed. As described previously, one major
concern with
charcoals or raw biochars used in animal applications is the potential for
dioxins which are
released from combustion processes and are an example of toxic material that
the treatment of the
present invention can remove. Thus, a treated biochar can be used in animal
applications where
ingestion may be possible such as bedding, or specifically as a feed additive,
whether it be for
general purpose such as color, manure odor control, or roughage replacement or
as a technical
additive as a binding agent or carrier as it can be made without toxins,
specifically dioxins,
consistently with various feedstocks and various pyrolysis methods without
risk of harm to the
animals or humans that consume the animal products/meat from said animals.
[0372] Through the use of detoxified treated biochars, the other benefits
of biochar
qualities can be realized in applications related to the care, maintenance and
feeding of
99
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
animals. These benefits can include increase in animals' uptake of foodstuffs
and the energy
contained within them; reduction in the amount of nutrients lost into
excrement and manure;
detoxification of the animal and enrichment of the beneficial microbes in the
digestive track that
are key to maintaining an animal's metabolism and helping it to resist
dangerous pathogens;
reduction in methane production; better odor control of stalls, pens, cages,
lagoons and other
animal enclosures; and any combination and variation of these and other
benefits. The results
are increased growth rates for animals consuming treated biochar, as well as
better overall health
of the animals that consume it, greater efficiencies in animal care and
maintenance, and
improved odor. As an additional benefit, manure produced by an animal that
consumes biochar
contains biochar, making this manure better for agricultural purposes than
ordinary manure.
[0373]
For animal applications, in the same way that biochars are known to bind
organic
contaminants in soil environments due to hydrophobic-hydrophobic interactions,
treated
biochar may bind organic toxins as they pass through an animal's digestive
system, for example,
when cattle are suffering from botulism or diarrhea. Another toxin binding
application could
be with commercial farm pollinating bee hives. Bee species have been on the
decline in the
US and this year, the first species of bee in the continental US was placed on
the endangered
species list. Bee species' decline appears to be in part due to fungicides,
and insecticides,
including neonicotinoids, leading to bees becoming more susceptible to
disease. See Pettis
et al., Crop Pollination Exposes Honey Bees to Pesticides Which Alters Their
Susceptibility
to the Gut Pathogen Nosema ceranae, PLOS, July 24, 2013
(http ://journals .plo s .org/plo sone/article?id=10 .1371/
journal.pone.0070182).
[0374]
Adding small particle treated biochar to a commercial hive feed patty, which
generally consist of sugar and protein and may have additional vitamins or
probiotics, may
allow the insects to ingest said treated biochar and allow for it to help bind
the pesticide
toxins and help lessen their sub-lethal effects to keep the bees more
resistant to pathogens
even when they have been and continue to be exposed to these pesticides.
[0375] In another example, biochars were shown to absorb Cadmium, a heavy
metal, but the
absorption capacity was depended on the biochar properties including the
biomass feedstock
type. Figure 57 is chart illustrating biochar capacity to absorb Cadmium. Thus
a specific treated
100
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
biochar formulation can be developed for each toxin binding animal application
to ensure
optimum results for the specific toxin(s) of concern.
[0376] Another application is to use the treated biochar as bedding in
order to reduce
odors, absorb ammonia, and absorb toxins and thus lead to an environment that
will lead to
healthier animals and also lead to a better secondary product of quality
manure by reduced
nutrient leaching. A bedding trial was conducted with broiler chickens. After
decaking the
houses, the test houses had treated biochar spread evenly over the entire
house at various
rates while one was left as a control. The flock produced over the two sets of
trials was
above normal. After the first trial, manure samples were tested from each
house for nutrient
content and estimated 1st year availability of said nutrients. The estimated
manure value was
then calculated off of the estimated 1st year availability. Results showed
higher value for
the bedding with treated biochar, as seen in table below :
14 Year Availability (lbs/ton)
Control With Treated Biochar
Total N 37 35
P (P205) 31 34
K (K20) 56 61
Total Est. Value $56.40 $58.90
[0377] As was discussed previously, mixing treated biochar in while
composting can reduce
odors, these same mechanisms can be used to reduce odors when treated biochar
is mixed with
animal bedding, manure, swine lagoons, etc.
[0378] Typically, the prior art teaches mixing raw biochar with animal feed
without
`precharging' with nutrients, microbes, etc. Through impregnation of the
biochar particles, one
can achieve a predetermined and controllable amount of a particular nutrient,
medication,
foodstuff, microbial community, etc. being ingested by the animal. Once in the
rumen, data
indicates that these infused additives will also be released more slowly over
time, yielding yet
another benefit over additives mixed directly into the feed. This integration
of a beneficial
additive with a biochar particle and biochar batches provides the ability to
have controlled
addition, use and release of the additive or additives. This integration may
further enhances,
101
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
promotes and facilitate animal growth and health, aid in digestion and
digestibility of food,
improvement hygiene, increase intestinal health, reduces the amount of
nutrients lost into
excrement and manure and reduces methane discharge.
[0379] Enhancing treated biochar with an additive, including infusing
liquids into the pores
of biochar, can provide additional benefits in animal applications, by making
it an effective
delivery mechanism for beneficial nutrients, pharmaceuticals, enzymes,
microbes, or other
substances. Additionally a sensory enhancer, such as a smell or flavor (e.g.
salt), could be infused
to increase the animal's desire to ingest said biochar.
[0380] The additive may include, but not be limited to, water, water
solutions of salts,
inorganic and organic liquids of different polarities, liquid organic
compounds or combinations
of organic compounds and solvents, vitamins, supplements and/or medications,
nutrients,
minerals, oils, amino acids, fatty acids, supercritical liquids, growth
promotants, proteins and
enzymes, phytogenics, carbohydrates, antimicrobial additives and sensory
additives (e.g. flavor
enhancers salt or sweeteners or smell enhancers), among others, to provide
nutrition, promote the
overall health of the animal, and increase the animal's desire to ingest said
biochar. Vitamins,
supplements, minerals, nutritional and/or medications may be used to prevent,
treat or cure animal
illnesses and diseases and/or control the nutritional value of the animals
overall diet.
[0381] For example, dietary supplementation with certain nutrients (e.g.,
arginine, glutamine,
zinc, and conjugated linoleic acid) can regulate gene expression and key
metabolic pathways to
improve fertility, pregnancy outcome, immune function, neonatal survival and
growth, feed
efficiency, and meat quality. Such additives in the biochar can help provide
the proper balance
of protein, energy, vitamins and nutritionally important minerals in animal
diets. Additionally,
for poultry, the additive may include, for example, coccidiostats and/or
histomonostats, which are
both shown to control the health of the poultry. The present invention can be
used to help correct
deficiencies in basal diets (e.g., corn- and soybean meal-based diets for
swine; milk replacers for
calves and lambs; and available forage for ruminants).
[0382] The treated biochar can also have a microbial community infused in
its pores (macro-
, meso-, and combinations and variations of these), on its pore surfaces,
embedded in it, located
on its surface, and combinations and variations of these. The microbial
community can have
several different types, e.g., species, of biologics, such as different types
of bacteria or fungi, or
102
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
it may have only a single type. A primary purpose, among many purposes, in
selecting the
microbial population is looking toward a population that will promote animal
health either
directly or through interactions with other microbes in the animals digestive
tract. These types of
beneficial microbes are essential to a functional gastrointestinal tract and
immune system in many
types of animals, serving many functional roles, including degradation of
ingesta, pathogen
exclusion, production of short-chain fatty acids, compound detoxification,
vitamin
supplementation, and immunodevelopment.
Beneficial bacteria include Lactobacillus
acidophilus LA1 (which decreases adhesion of diarrheagenic Escherichia coli to
Caco-2 cells by
85% and prevents invasion of the same cells by E. coli (95%), Yersinia pseudo-
tuberculosis (64%)
and Salmonella enterica serovar Typhimurium) and Lactobacillus rhamnosus GG to
prevent E.
coli 0157:H7-induced lesions in Caco-2 cells.
[0383]
Further, biochar may be impregnated with probiotic bacteria to treat diseases
in farm-
raised fish. Infectious diseases pose one of the most significant threats to
successful aquaculture.
The maintenance of large numbers of fish crowded together in a small area
provides an
environment conducive for the development and spread of infectious diseases.
In this crowded,
relatively unnatural environment, fish are stressed and more susceptible to
disease. Moreover, the
water environment, and limited water flow, facilitates the spread of pathogens
within crowded
populations. There is thus an urgent need in aquaculture to develop microbial
control strategies,
since disease outbreaks are recognized as important constraints to aquaculture
production and
trade and since the development of antibiotic resistance has become a matter
of growing concern.
One alternative disease control relies on the use of probiotic bacteria as
microbial control agents.
Another implementation of the invention therefore involves the impregnation of
biochar for
consumption by aquatic animals as a treatment or preventative for disease.
[0384]
Additionally, biochar may be infused with bacteria which prove helpful in
methane
reduction. An example of this is to infuse the biochar with methanotrophic
bacteria (bacteria
which are able to metabolize methane as a source of carbon and energy).
Bacteria which
metabolize methane are useful in two regards ¨ they can reduce the
environmental methane
emissions from the rumen and they (the bacteria) also serve as nutrition for
the animal itself,
leading to increased weight gain. Infusing biocarbon with microbes such as
these can lead to
103
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
methane reduction in cattle applications that exceeds the methane reduction of
solely untreated
biochar itself.
[0385] Additive infused biochars may be mixed with the animals regular
feeds or may be
included within a salt or mineral block and made available for animals to self-
feed or self-
administer the additives.
[0386] While this application focuses mainly on applications of infused
biochars in
connection with farm-raised animals, those skilled in the art will also
recognize that the invention
could also be applied more generally for veterinary purposes for many types of
animals other than
livestock, poultry, fish or horses, including pets, as well as in a wide
variety of environments and
contexts, for example, for zoo or aquarium animals or for other penned or
caged animals, insects
such as bees, or for wild animals.
[0387] Furthermore, the treated or additive infused biochar can be sized,
agglomerated, or
suspended in solution to optimize its use in a specific animal application.
For example, if using
as a feed additive with smaller animals or very young animals, small particles
will be required
and being able to suspend these small particles in a solution will make for an
easier application.
[0388] In addition, if the treated or additive infused biochar is being
used to deliver its specific
benefit in a targeted location in the animals' digestive tract, it can be
mixed with an additive or
coated to allow for a slower release or a targeted release in said location.
So, for example if the
additive or biochar is being targeted for use in the intestines or after
rumens a specific coating
substance and thickness can be chosen so as to degrade at the required
specified rate leading to
the biochar or additive being available after the stomach or rumens. This
could be specifically
useful for getting beneficial microbes to targeted organs in the digestive
tract. If the microbe is
infused into the pores to a significant depth of at least approximately 10 to
20 microns, then both
the biochar structure itself and a coating could be used to protect the
microbe through harsh
conditions, such as stomach acid, prior to getting to the targeted organ
location.
[0389] Treated biochar and additive infused treated biochar can be used in
promoting growth
and health in livestock (dairy and beef cattle, sheep, goats and swine);
poultry; farm-raised wild
animals (e.g. bison, deer and elk); farm-raised fish and other aquatic
animals; horses and other
members of the horse family; for controlling levels of certain pathogens, e.g.
salmonella in
poultry; for veterinary uses, such as delivery systems for medications,
supplements and/or
104
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
vitamins; for maintenance and welfare of zoo animals or other caged, penned or
contained
animals; for pets; for zoos and aquaria; for wild animals; for insects, such
as bees, and for
combinations and variations of these.
[0390] Treated biochars and practices and methods provide for healthier
animals, increase
food intake efficiency, promote better digestion and reduce methane emissions,
and combinations
and variations of these, and other features that relate to the increased
holding, retention and time
discharge features of the present biochars and processes.
[0391] Treated biochar may also be used in other applications, for example,
such mixing with
manure in holding ponds to potentially reduce gaseous nitrogen losses, soil
remediation (for
example absorption and capture of pesticide, contaminates, heavy metals, or
other undesirable,
disadvantageous soil components), ground water remediation, other
bioremediations, storm water
runoff remediation, mine remediation and mercury remediation.
[0392] In summary, the treatment processes of the present information may
be used to clean
the pores of the biochar, ridding the pores of dioxins or other detrimental
substances, or
infiltrating the pores of biochar with a variety of substances, for a number
of purposes, including
but not limited to, infiltrating the pores of biochar with nutrients,
vitamins, drugs, microbes,
and/or other supplements, or a combination of any of the foregoing, for
consumption by
animals. The treated biochar may also be applied to animal pens, bedding,
and/or other areas
where animal waste is present to reduce odor and emission of unpleasant or
undesirable vapors.
Furthermore it may be applied to compost piles to reduce odor, emissions, and
temperature to
enable the use of the food waste and animal feed in composting. Biochar can
also be applied to
areas where fertilizer or pesticide runoff is occurring to slow or inhibit
leaching and runoff. The
biochar may also be treated with additives which make it easier to dispense or
apply, such as non-
toxic oils, anti-clumping/binding additives, surface drying agents, or other
materials.
[0393] While the above teaches a treatment process for biochar that
increases the amount of
additives that can be retained within the pores of the biochar, it is within
the scope of the present
invention to contact raw or treated biochar with additives (e.g. by
submersion) for purposes of
creating a delivery system for additives useful for animal health and
consumption.
[0394] As set forth above, the treated biochar of the present invention may
be used in various
agriculture activities, as well as other activities and in other fields.
Additionally, the treated
105
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
biochar may be used, for example, with: farming systems and technologies,
operations or
activities that may be developed in the future; and with such existing
systems, operations or
activities which may be modified, in part, based on the teachings of this
specification. Further,
the various treated biochar and treatment processes set forth in this
specification may be used
with each other in different and various combinations. Thus, for example, the
processes and
resulting biochar compositions provided in the various examples provided in
this specification
may be used with each other; and the scope of protection afforded the present
inventions
should not be limited to any particular example, process, configuration,
application or
arrangement that is set forth in a particular example or figure.
[0395] Although this specification focuses on applications related to the
maintenance, care,
feeding and health of animals, it should be understood that the materials,
compositions, structures,
apparatus, methods, and systems, taught and disclosed herein, may have
applications and uses for
many other activities in addition to agriculture for example, as filters,
additives, and in
remediation activities, among other things.
[0396] It is understood that one or more of these may be preferred for one
application, and
another of these may be preferred for a different application. Thus, these are
only a general
list of preferred features and are not required, necessary and may not be
preferred in all
applications and uses.
[0397] It is noted that there is no requirement to provide or address the
theory underlying the
novel and groundbreaking functionality, performance or other beneficial
features and properties
that are the subject of, or associated with, implementations of the present
inventions.
Nevertheless, to the extent that various theories are provided in this
specification it is done to
further advance the art in this important area. These theories put forth in
this specification, unless
expressly stated otherwise, in no way limit, restrict or narrow the scope of
protection to be
afforded the claimed inventions. These theories many not be required or
practiced to utilize the
present inventions. It is further understood that the present inventions may
lead to new, and
heretofore unknown theories to explain the functionality, performance or other
beneficial features
and properties that are the subject of, or associated with, embodiments of the
methods, articles,
materials, and devices of the present inventions; and such later developed
theories shall not limit
the scope of protection afforded the present inventions.
106
CA 03013588 2018-08-02
WO 2017/136611 PCT/US2017/016306
[0398] Those skilled in the art will recognize that there are other methods
that may be used
to treat biochar in a manner that forces the infusion of liquids into the
pores of the biochar without
departing from the scope of the invention. The foregoing description of
implementations has
been presented for purposes of illustration and description. It is not
exhaustive and does not limit
the claimed inventions to the precise form disclosed. Modifications and
variations are possible
in light of the above description or may be acquired from practicing the
invention. The claims
and their equivalents define the scope of the invention.
107