Note: Descriptions are shown in the official language in which they were submitted.
File No.: P4015CA00
Title: PROCESS FOR THE PRODUCTION OF BIO-BUTANOL BY FERMENTATION
OF GLYCOSIDIC WASTE MATTER
BACKGROUND
(a) Field
[0001] The subject matter disclosed relates to processes for the
extraction of
microbial inhibitors from hydrolyzed glycosidic waste matter containing free
reducing
sugars and for the production of bio-butanol from fermentation of detoxified
hydrolyzed
glycosidic waste matter containing free reducing sugars obtained therefrom.
Detoxification is achieved by solvent extraction of the hydrolyzed glycosidic
waste
matter containing free reducing sugars with a solvent selected from the group
consisting
of bis-(2-ethylhexyl) sebacate, 2-undecanone, or a combination thereof.
(b) Related Prior Art
[0002] Continuous global energy demand and concern over increasingly
expensive imported oil and diesel resources has led to the development of
renewable
energy sources that have driven research towards the utilization of
lignocellulosic food-
and agro-industrial wastes as feedstock for the production of biofuels Thus,
establishment of vigorous bio-based industry capable of producing bio-fuel is
compulsory to supplement petroleum as the main feedstock for fuel. However, as
the
demand for food resources increases, the search for renewable nonfood
resources to
displace substantial amounts of nonrenewable fossil fuels rests largely on low-
cost
lignocellulosics (Hu et al. Pretreatment and lignocellulosic chemistry.
Bioenergy
Research, Vol. 5(4), 2012: 1043-1066).
[0003] Bio-butanol, four carbons containing aliphatic alcohol has been
recently
considered as one of the emerging second generation liquid biofuel (Maiti et
al., Agro-
industrial wastes as feedstock for sustainable bio-production of butanol by
Clostridium
beijerinckii. Food and Bioproducts Processing, 98, 217-226). Butanol is
considered as a
superior bio-fuel due to its higher energy density (29.2 MJ/L), higher air
fuel ratio (11.2),
octane number (96), lower heat of evaporation (0.43 MJ/kg), and its ability to
blend with
1
CA 3013593 2018-08-08
File No.: P4015CA00
gasoline in higher percentage (80-85%) without any modification of
conventional Otto-
cycle engine compared to bioethanol, which has energy density (19.6 MJ/L),
higher air
fuel ratio (9), octane number (126) and lower heat of evaporation (0.92
MJ/kg). As a
liquid transportation fuel, butanol is superior to the first generation
biofuel due to higher
energy content, since butanol releases 96% of the energy of a gasoline volume
unit,
whereas ethanol only generates 73% of gasoline energy per unit volume and
other
properties such as low volatility, higher blending rate with gasoline without
engine
modification, decrease emission of NOx, octane improving power, convenient
distribution using current pipeline infrastructure, and better auto-emission
performance
(Cascone. Biobutanol: A Replacement for Bioethanol?. Chemical Engineering
Progress,
Vol. 104(8), 2008; Maiti etal., March 2016).
[0004] Bio-
butanol has been produced by anaerobic ABE (acetone-butanol-
ethanol) fermentation of different raw materials such as, monosaccharides
(e.g.
glucose, xylose etc.), poly-saccharides (e.g. starch), complex biomass (e.g.
lignocellulose) etc., using solventogenic Clostridia strains (Papoutsakis.
Engineering
solventogenic clostridia. Current opinion in biotechnology, Vol. 19(5), 2008:
420-429).
Techno-economic bio-butanol production evaluation showed that low cost
substrates
are required since the raw feedstock cost is the largest cost contributor to
the total
operating cost (60-70%). In an attempt to reduce the cost of butanol
production by
fermentation, the use of a variety of low cost feedstocks have been
investigated
including soy molasses, cracked corn, starch based packing peanuts,
maltodextrin, and
various other agricultural biomass products. Novel agricultural substrates
used for the
production of biofuels include wood (hardwood), by-products left over from the
corn
milling processes (corn fiber), residues from annual plants, whey permeate,
and waste
paper. While agricultural residues such as straws (wheat and rice) and corn
fiber are
economically available, these materials must first be subjected to
pretreatment and
enzymatic hydrolysis to produce hydrolysates for fermentation. The processes
used to
produce these hydrolysates often result in the generation of chemical
byproducts that
inhibit cell growth and fermentation. Such inhibitors include salts, furfural,
hydroxymethyl furfural (HMF), acetic, ferulic, glucuronic, and r-coumaric
acids, and
phenolic compounds. (Qureshi et al. Economics of butanol fermentation using
hyper-
2
CA 3013593 2018-08-08
File No.: P4015CA00
butanol producing Clostridium beijerinckii BA101. Food and bioproducts
processing,
Vol. 78(3), 2000: 139-144; Tao et al. Comparative techno-economic analysis and
reviews of n-butanol production from corn grain and corn stover. Biofuels,
Bioproducts
and Biorefining, Vol. 8(3), 2014: 342-361). Thus, the conversion of renewable
lignocellulosic waste biomass and their by-products (e.g. low value agro-
industrial
wastes, municipal organic etc.) to bio-butanol is the key step to provide an
affordable
and sustainable solution to the oil crisis and energy sector (Maiti et al.
Agro-industrial
wastes as feedstock for sustainable bio-production of butanol by Clostridium
beijerinckii.
Food and Bioproducts Processing, Vol. 98, 11 January 2016: 217-226).
[0005] Food
waste includes unconsumed food that is discarded by food
processing industries, retailers, restaurants, and consumers. Most of the food
industry
wastes find no current uses different from landfilling or first-generation
recycling
practices, such as animal feed, composting and incineration (Lin et al. Food
waste as a
valuable resource for the production of chemicals, materials and fuels.
Current situation
and global perspective. Energy Environmental Sci., Vol. 6(2), 2013: 426-464).
Dhillon,
G.S., Brar, S.K., Verma, M., Tyagi, R.D., 2011. Utilization of different agro-
industrial
wastes for sustainable bioproduction of citric acid by Aspergillus niger.
Biochemical
Engineering Journal 54, 83-92. Dhillon, G.S., Kaur, S., Brar, S.K., 2013.
Perspective of
apple processing wastes as low-cost substrates for bioproduction of high value
products: A review. Renewable and Sustainable Energy Reviews 27, 789-805.
(Dhillon
et al. Enhanced solid-state citric acid bio-production using appled pomace
waste
through surface response methodology. Journal of Applied Microbiology, Vol.
110,
2011: 1045-1055).Disposal of food waste in landfill or incineration can cause
severe
amount of greenhouse gases (CH4 and CO2) Composting is getting popular, as it
diverts
food waste from landfill and improves soil structure. However, this type of
practice is still
carried out at a relatively elevated cost, and has a potential problem of
pollution to
surface and underground water. In light of the above comments, effective
utilization of
food waste for fuels and chemicals will positively influence the energy and
environmental sustainability, and the economic competitiveness. Beer is the
most
appreciated and consumed beverage. Worldwide beer production process generated
in
thousands of tons of carbon rich organic wastes such as brewery spent grain
(BSG) and
3
CA 3013593 2018-08-08
File No.: P4015CA00
brewery liquid wastes (BLVV) in every year. (Maiti et al. Agro-industrial
wastes as
feedstock for sustainable bio-production of butanol by Clostridium
beijerinckii. Food and
Bioproducts Processing, Vol. 98, 11 January 2016: 217-226).
[0006] The
brewing sector holds a strategic economic position in the food
industry. The annual world beer production exceeding 1.34 billion hectolitres
was
reported in 2002 (Fillaudeau et al. Water, wastewater and waste management in
brewing industries. Journal of Cleaner Production, Vol. 14(5), 2006). Canada
produces
a large amount of beer (with an estimated 21.9 million hectoliters per year)
that leads to
an abundance of brewery wastes (Maiti et al., January 2016; Olajire.The
brewing
industry and environmental challenges. Journal of cleaner production, Vol. 30,
2012).
During beer production, three (bio) chemical reactions (mashing, boiling,
fermentation-
maturation) and three other solid-liquid separations (wort separation, wort
clarification
and rough beer clarification) are required, generating a large amount of solid
residues
(brewery spent grain ¨ BSG) and wastewater (brewery liquid waste ¨ BLVV)
(Fillaudeau
et al., 2006). About 3.5-4.4 L of water is required as brewing water for each
litre of beer
produced, while contaminated wastewater volume constitutes approximately 25%
of the
total volume of water consumed (Mielcarek et al. Biodegradability evaluation
of
wastewaters from malt and beer production. Journal of the Institute of
Brewing, Vol.
119(4), 2013: 242-250). Besides, brewer's spent grain from the fermentation
process is
the most abundant brewing by-product, corresponding to around 85% of the total
residues generated. Normally during beer production around 14 kg of spent
grain was
generated per hectolitres of beer (Olajire, 2012). Currently, it is only sold
or given free
as an animal feedstock (low value product) to reduce costs and provide added
revenue
(Mussatto et al. Techno-economic analysis for brewer's spent grains use on a
biorefinery concept: The Brazilian case. Bioresource technology, Vol. 148,
2013: 302-
310). Taking into account that Quebec holds 21.2% of the Canadian industry
breweries
(constitutes the 1.5% of gross domestic product (GDP) (IBIS World Industry
Report,
2015), the sanitation of wastewater effluent and efficient management of spent
grain or
trub (protein and hops left in kettle) accumulated during beer production is a
real
challenge of great social, environmental and economic value in this region.
4
CA 3013593 2018-08-08
File No.: P4015CA00
[0007] Solid waste as well as effluents from brewery industries also
threaten
environment as these are usually used in land filling. During brewery
fermentation
cellulose, hemicellulose, and protein components of biomass remain unutilized.
These
residual organic matters on microbial degradation cause foul smell, evolve
greenhouse
gases and increase acidity of soil. Thus loss of potential biomasses occurs
with adverse
impact on environment.
[0008] Agro-based industries are experiencing a surge in their growth
around the
globe (Dhillon et al., 2013). Worldwide statistical data on these highly
abundant agro-
industrial waste production have been reported previously (Maiti et al.,
January 2016).
Federation of Indian Chambers of Commerce and Industry report (2011) has
showed
that about 60-70% was discharged in the environment without any treatment and
the
rest was utilised for anaerobic digestion in Latin America, Eastern Europe,
Africa, and
Asia (except Japan) (Global Methane Initiative. (2011). Resource assessment
for
livestock and agro-industrial wastes¨India.).
[0009] Previously, (Gassara et al. Pomace waste management scenarios in
Quebec¨impact on greenhouse gas emissions. Journal of hazardous materials,
Vol.
192(3), 2011: 1178-1185) it was reported that in Canada the utilization of
agro-industrial
wastes, such as fruit wastes, to obtain high added value bio-products was the
least
polluting option in terms of GHG emissions in comparison with landfill
disposal,
incineration and composting. In this context, bio-butanol production based on
inexpensive agro-industrial waste is a promising renewable energy source for a
country
with abundant biomass resources, such as Canada in-order to reduce their
noxious
effect in the environment (Maiti etal., January 2016).
[0010] Nevertheless, even though research on bio-butanol upstream and
downstream processing has significantly progressed, the naturally abundant
Clostridia
are still not able to efficiently hydrolyse lignocellulosic based agro-
industrial waste (Ezeji
et al. Bioproduction of butanol from biomass: from genes to bioreactors.
Current opinion
in biotechnology, VoL 18(3), 2007: 220-227). The conversion of complex biomass
into
energy and biofuels requires effective utilization of C5 and C6 sugars present
in
hemicellulose, cellulose and starch by either processing these fractions
together or
=
CA 3013593 2018-08-08
File No.: P4015CA00
separating and processing them separately (GurbOz et al. Conversion of
hemicellulose
to furfural and levulinic acid using biphasic reactors with alkylphenol
solvents.
ChemSusChem, Vol. 5(2), 2012: 383-387). Unfortunately, the common industrial
pre-
treatment method, i.e. diluted Bronsted acid thermo-hydrolysis, generates a
complex
combination of microbial inhibitors, such as weak acids (acetic acid and
levulinic acid),
furan derivatives (e.g., furfural, hydroxymethyl furfural (HMF)) and a mixture
of phenolic
compounds (e.g., vanillin, vanillic acid, syringaldehyde, ferulic acid) which
inhibit and
thus diminish the bio-butanol production. Significant detrimental effects of
reported
microbial inhibitors and their modes of action are shown in Table 1.
[0011]
Individual inhibitory actions of these compounds and their potential
synergistic effects hinder bacterial growth and sugar conversion, as energy is
diverted
to maintenance and cell-repair by four main coping mechanisms (detoxification,
efflux,
repair and tolerance), making detoxification a compulsory step to enhance bio-
butanol
production (Jonsson et al. Bioconversion of lignocellulose: inhibitors and
detoxification.
Biotechnology for biofuels, Vol. 6, Issue 1, 2013: 1 ; Piotrowski et al. Death
by a
thousand cuts: the challenges and diverse landscape of lignocellulosic
hydrolysate
inhibitors. Frontiers in Microbiology, Vol 5, 2014: 90). Different
detoxification techniques
previously tested and enlisted in Table 2 could be expensive and laborious
processes
and may reduce titre of total fermentable sugars. In this context, development
of a
simple, rapid and highly selective detoxification method would be highly
desirable.
6
CA 3013593 2018-08-08
File No.: P4015CA00
0
LA.) Table 1. Inhibitory concentration of different hydrolysis process
by-products and their effect in ABE fermentation.
0
LA)
01
Inhibitor Concentration (g/L)
Effect of inhibitor in ABE production Effect in microbial cell
References
o
1. Adverse effect on enzymes required for
IA
co HMF
metabolism and long lag phase during cell
oi
growth
1. (<0.5 g/L) enhanced production and productivity
co
> 2-3 2. 2-3 g/L ) converted to other
less toxic acids by 2. Strong inhibition on ADH (anti-diuretic
(Zhang et al. New
hormone) led to accumulation of
Biotechnology, 1(3),
co specific microorganism
acetaldehyde (>0.5 mM), which inhibited DNA 2012)
Furfural 3. (>3 g/L) was deleterious for ABE
fermentation and protein synthesis
3. Membrane permeability decreased and
deactivated cell replication
1. (0.05 g/L) showed strong inhibition on cellulase
Syringaldehyde > 0.05 enzyme activity
2. (0.3-1.0 g/L) stopped ABE production
1. Disrupted electrochemical gradient by
transporting protons back across the
(Cho et al. Applied
Ferulic acid > 0.3 1 (>0.3 g/L) inhibited butanol
production mitochondrial membranes microbiology
and
2. Deteriorated cell membranes' ability to
biotechnology, 83(6),
1. (>1g/L) stopped bio-butanol production
2009)
serve as selective barriers and enzyme
Vanillic acid > 1 2. (>2.1 g/L) of total soluble
phenolic compounds had matrices, causing adverse effect in cell growth
strong negative effect in bio-butanol production
and sugar assimilation
Vanillic > 1 1. g/L) inhibited completely bio-
butanol production
(Liu et al. Bioresource
technology, 189, 2015;
1. (>0.74 g/L) Incomplete sugars utilization
Wang et a/. Process
2. (>0.92 g/L) Poor bio-butanol production (0.4 g/L);
1. Increased cell membrane fluidity, causing
Biochemistry, 46,
Soluble lignin >0.7-0.8 Accumulation of acetic and butyric
acid (-3-4 fold) in leakage of cellular contents, disrupting the cell 2011;
Zhang et al.
comparison with control samples
redox balance and causing acid crash Bioprocess and
biosystems
3. (>1.77 g/L) stopped fermentation process engineering, 37(5),
2014)
7
File No.: P4015CA00
0
w Table 2. Detoxification methods reported in the literature
Inhibitor reduction (%; FSC*** reduction
Substrate
Bacteria Ref.
o
[g/Lj) (%)
i-. Technique Reagent Temperature ( C) Time (min)
U)
(Lee et a/
01
Furfural (7; 0.03)
Journal of
to
Industrial
and
U) 5-HMF (7; .--
0.15)
0
Mixed softwood N.M.
Electro-dialysis - N.M.* N.M.
Acetic acid (100; 1.84)
Engineering
n.)
o
TPC** (70; 2) Chemistry, 19(6),
--.
i-.
2013)
co
(Wu
at al.
O
Journal of
co Formic acid (100;
6.9) 17 Red algae K. marxianus microbiology and
1 Electro-dialysis - N.M. N.M.
o
Levulinic acid (100; 6.1)
biotechnology,
co
24(9), 2014)
(Telli-Okur et al.
Furfural + 5-HMF (42;
Sunflower seed Bioresource
Neutralization CaO 60 30
9 Pichia stipites
0.33)
hull technology,
99(7), 2008)
Neutralization N.M. N.M. N.M. Levulinic acid
(12; 0.73) 25 Red Algae K. marxianus (Wu et al , 2014)
Furfural + 5-HMF (41;
Sunflower seed (Telli-Okur et al.,
12
Pichia stipites
Overliming CaO 60 30 0.32)
hull 2008)
(Larsson et al.
Saccharomyces
Applied
Overliming Ca(OH)2 N.M. 60
Furfural (20; 0.20)
0
Spruce biochemistry and
5-HMF (22; 1.30)
cerevisiae
biotechnology,
77(1-3), 1999)
Formic acid (52; 3.59)
42
Red Algae K. marxianus (Wu etal., 2014)
Overliming Ca(OH)2 60 30
Levulinic acid (48; 2.93)
(Andary et a/.,
Overliming Ca(OH)2 60 60 p-Coumeric acid
(34; 0.35) N.M. Olive stones N.M.
2013)
Furfural (93; 0.75)
Glucose 25 Saccharomyces
Overliming NFLOH 80 180
5-HMF (89; 2.76)
Mannose 16 Spruce
cerevisiae
(Larsson et a/.,
1999)
Na2S03 in helium Furfural (53;
0.53) Saccharomyces
Overliming N.M. 60
0 Spruce
atmosphere 5-HMF (52; 3.07)
cerevisiae
(Miyafuji et a/.
Treated charcoal Furfural (100;
0.26)
Enzyme
and
Absorption (prepared at Room >300 5-HMF (100;
0.490) Saccharomyces
Vanillic acid (100; 0.33)
0 Spruce chips
cerevisiae
Microbial
Technology,
600 C)
Vanillin (100; 0.36)
32(3), 2003)
Overliminig + Furfural + 5-HMF
(68: Sunflower seed (Telli-Okur et a/.,
CaO + charcoal 30 24
11 Pichia stipites
Absorption 0.53)
hull 2008)
(Ge et a/. African
Overliming + Overliming (15)
Glucose 18 Journal of
CaO + Overliming (100)
+ absorption Acetic acid
(28; 3.0) Corn cob C. shehatae
Filtration +
Xylose 28 Microbiology
Activated charcoal + absorption (40) TPC (97; 0.95)
hemicellulose ACCC 20335
Absorption (60)
Arabinose 9 Research, 5(10),
2011)
Furfural (100; 1.0)
Until 90% of 5-HMF (4; 0.24)
Saccharomyces (Larsson at a/.,
Evaporation - N.M.
0 Spruce
evaporation Acetic acid (65;
1.56) cerevisiae 1999)
Formic acid (74; 1.18)
8
File No.: P4015CA00
0
Furfural (30; =-0.15)
(Dhamole et al.
Non-toxic thermo- 5-HMF (=-10%; =-
0.1) Journal of
co Surfactant-based
p
separating -Coumeric acid
(90; 0.45) Chemical
O cloud point N.M. N.M.
0 Corn stover -
i-. copolymer (L62D Vanillin (100;
=0.5) Technology and
t.t.) extraction (CPE)
5%) Ferulic acid
(100; --Ø5) Biotechnology,
01
to Syringaldehyde
(100; =0.5) 88(9), 2013)
co
(Wilson et al.
n.) Furfural (100;
0.28) Applied
Extraction + Ethyl acetate (1:1)
Glucose 6 Aspenwood Pichiastipitis -- oN.M.HBA (100; 1.07) --
microbiology and
E N.M.N
i-. rotoevaporation
- 4 times Xylose 19 chips CBS 5776 -- biotechnology,
co Vanillin (100;
0.21)
I
31(5-6), 1989)
o
co
ol *NM: Not mentioned; **TPC: Total phenolic compounds; "*FSC:
FermenTable sugar concentration
co
9
File No.: P4015CA00
SUMMARY
[0012] According to an embodiment, there is provided a process for
extracting a
microbial inhibitor from a hydrolysate of glycosidic waste matter containing
free reducing
sugars prior to a fermentation reaction, the process comprising the step of:
solvent extraction of the hydrolysate with a solvent selected from the group
consisting of
bis-(2-ethylhexyl) sebacate, 2-undecanone, and a combination thereof, over a
period of
sufficient length to extract the microbial inhibitor therefrom, thereby
obtaining an extract
containing the microbial inhibitor and reducing the level of the microbial
inhibitor in the
hydrolysate.
[0013] According to another embodiment, there is provided a process for the
production of bio-butanol by fermentation of a detoxified hydrolysate of
glycosidic waste
matter containing free reducing sugars, the process comprising the step of:
fermentation of the detoxified hydrolysate with a solventogenic microorganism
over a
period of sufficient length and at a temperature sufficient to produce the bio-
butanol,
wherein the detoxified hydrolysate is obtained from a solvent extraction of a
hydrolysate
of glycosidic waste matter containing free reducing sugars with a solvent
selected from
the group consisting of bis-(2-ethylhexyl) sebacate, 2-undecanone, and a
combination
thereof, over a period of sufficient length to extract a microbial inhibitor
therefrom,
thereby obtaining an extract containing the microbial inhibitor and the
detoxified
hydrolysate.
[0014] The process may further comprise the step of:
solvent extraction of a hydrolysate of glycosidic waste matter containing free
reducing
sugars with a solvent selected from the group consisting of bis-(2-
ethylhexyl) sebacate,
2-undecanone, and a combination thereof, over a period of sufficient length to
extract a
microbial inhibitor therefrom, thereby obtaining an extract containing the
microbial
inhibitor and the detoxified hydrolysate.
[0015] The hydrolysate of glycosidic waste matter containing free reducing
sugars may be a hydrolysate of cellulosic waste matter containing free
reducing sugars,
CA 3013593 2018-08-08
File No.: P4015CA00
a hydrolysate of amylosic waste matter containing free reducing sugars, or a
combination thereof.
[0016] The hydrolysate of glycosidic waste matter containing free reducing
sugars may be obtained from cellulosic waste matter, amylosic waste matter, or
a
combination thereof.
[0017] The cellulosic waste matter may be obtained from brewery liquid
waste,
brewery spent grain, apple pomace ultrafiltration sludge, apple pomace solid
waste, or
combinations thereof.
[0018] The amylosic waste matter may be from starch industry wastewater.
[0019] The solvent may be bis-(2-ethylhexyl) sebacate.
[0020] The solvent may be 2-undecanone.
[0021] The ratio of hydrolysate of glycosidic waste matter containing free
reducing sugars to solvent (Vaqueous:Vorganic) may be from 5:1 to 1:2.
[0022] The ratio may be 2:1.
[0023] The ratio may be 5:1.
[0024] The ratio may be 3:1.
[0025] The ratio may be 1:1.
[0026] The ratio may be 1:2.
[0027] The process may comprise mixing of the hydrolysate and the solvent
during solvent extraction.
[0028] The mixing may be performed using a propeller impeller.
[0029] The mixing may be performed by providing an input of energy from
0.02 to
0.12 W.h/L.
[0030] The period of sufficient length to extract the microbial inhibitor
may be
from 15 to 60 minutes.
[0031] The time sufficient to extract the microbial inhibitor may be 30
minutes.
11
CA 3013593 2018-08-08
File No.: P4015CA00
[0032] The solvent extraction may be performed at a temperature from 15 C
to
30 C.
[0033] The temperature sufficient for the solvent extraction may be room
temperature (25 C).
[0034] The solvent extraction may comprise separating the obtained
hydrolysate
from the solvent using at least one of a funnel separation, a centrifugal
force-assisted
separation, and a combination thereof.
[0035] The obtained hydrolysate may be produced by hydrolysis of
glyosidic
waste matter, the hydrolysis comprising at least one of a chemical hydrolysis,
a thermal
hydrolysis, an enzymatic hydrolysis, a mechanical hydrolysis, and combinations
thereof.
[0036] The obtained hydrolysate may be produced by the thermal hydrolysis
of
the glycosidic waste matter, the thermal hydrolysis comprising at least one of
a
microwave-assisted hydrolysis and an autoclave-assisted hydrolysis.
[0037] The hydrolysis may be performed under a pressure of 89 kPa to 110
kPa.
[0038] The hydrolysis may be performed at a pH of about 0.32 to about 10.
[0039] The hydrolysis may be performed at a temperature greater than 100
C.
[0040] The hydrolysis may be performed at pH 0.76 in H2SO4, at 121 C, 16
psi
(110.3 kPa), for 40 mins.
[0041] The thermal hydrolysis may be a Bronsted acid catalyzed
pressurized
thermal hydrolysis.
[0042] The Bronsted acid catalyzed pressurized thermal hydrolysis may be
performed using H2SO4, HCI, betaine hydrochloride, H202, or combinations
thereof.
[0043] The acid concentration may be from about 2 N to about 8.7 N.
[0044] The thermal hydrolysis may be an alkali catalyzed hydrolysis.
[0045] The alkali may be NaOH.
[0046] The alkali concentration may be from about 1 N to about 2 N.
[0047] The mechanical hydrolysis may be an ultra-sonication.
12
CA 3013593 2018-08-08
File No.: P4015CA00
[0048] The fermentation may be performed at a temperature from 30 C to 40
C.
[0049] The fermentation may be performed at a temperature of 37 C.
[0050] The fermentation may be performed in batch mode for at least 48
hours.
[0051] The fermentation may be performed in batch mode for 72 hours.
[0052] The fermentation may be performed under agitation.
[0053] The solventogenic microorganism may comprise a clostridia
bacteria.
[0054] The clostridia bacteria may comprise at least one of Clostridium
acetobutylicum NRRL B-582, Clostridium beijerinckii NRRL B-466 and a
combination
thereof.
[0055] The following defines some of the terms used throughout the
specification.
Where the provided definition of a term departs from the commonly used meaning
of the
term, applicant intends to use the provided definition in the absence of an
explicit
indication to the contrary.
[0056] The term "bio-butanol" is intended to mean butanol that has been
produced from biomass. Bio-butanol is produced by a microbial fermentation,
similar to
ethanol and can be made from the same range of sugar, starch or cellulosic
feedstocks.
According to the present invention it is generated from hydrolyzed glycosidic
(e.g.
cellulosic or amylosic) waste matter.
[0057] The term "glycosidic waste matter" or "glycosidic matter" is
intended to
mean matter that comprises carbohydrate molecules that contain glycosidic
bonds of
natural origins, such as for example cellulosic and/or amylosic material from
plants.
Preferably, the matter is chosen from brewery liquid waste (BLVV), brewery
spent grain
(BSG), apple pomace ultrafiltration sludge (APUS), apple pomace solid waste
(APS),
starch industry wastewater (SIVV) or combinations thereof.
[0058] The term "bioreactor" is intended to mean an apparatus in which a
biological reaction or process is carried out. This includes small, medium and
large
(industrial) scale apparatuses.
13
CA 3013593 2018-08-08
File No.: P4015CA00
[0059] The term "fermentation medium" is intended to mean a growth medium
in
which fermentation by suitable microorganism such as bacteria, yeast and fungi
to
make useful products can take place. In some embodiments, the fermentation
medium
may be supplemented with several different kinds of additives (see below).
[0060] The term "fermentation mixture" is intended to mean a combination
of the
fermentation medium and the microorganisms.
[0061] The term "solventogenic microorganism" is intended to mean a
microorganism that is capable of producing a solvent, such as ethanol,
butanol, or
other, and include for example Clostridia bacteria such as Clostridium
acetobutylicum
NRRL B-582, Clostridium beijerinckii NRRL B-466, Clostridium acetobutylicum,
Clostridium beijerinckii, Clostridium saccharobutylicum, and Clostridium.
saccharoperbutylacetonicum, Clostridium Bezrinckii BA101, Clostridia strain TU-
103,
yeasts, genetically engineered Pseudomonas putida DOT-TI E, genetically
engineered
Bacillus subtilis GRSW2-131, genetically engineered B. subtilis 168 and B.
subtilis
KS438, as well as other solventogenic recombinant bacteria and microorganisms,
or
combinations thereof.
[0062] The term "solventogenic production conditions" is intended to mean
fermentation conditions that are suitable for the production of solvents (e.g.
bio-butanol)
by the selected solventogenic microorganism. Such conditions include for
example the
appropriate temperature, pH, nutrient and salt condition, agitation, pressure
as well as
any other suitable and/or necessary condition required to achieve hydrogen
production
under fermentative conditions.
[0063] The terms "detoxification", or "detoxified" is intended to mean
the removal
or the decrease of their levels in a hydrolysate of glycosidic waste matter so
as to lift
their inhibitory effect on the fermentation of the matter.
[0064] Features and advantages of the subject matter hereof will become
more
apparent in light of the following detailed description of selected
embodiments, as
illustrated in the accompanying figures. As will be realized, the subject
matter disclosed
and claimed is capable of modifications in various respects, all without
departing from
the scope of the claims. Accordingly, the drawings and the description are to
be
14
CA 3013593 2018-08-08
File No.: P4015CA00
regarded as illustrative in nature, and not as restrictive and the full scope
of the subject
matter is set forth in the claims.
BRIEF DESCRIPTION OF THE DRAWINGS
[0065] Further features and advantages of the present disclosure will
become
apparent from the following detailed description, taken in combination with
the
appended drawings, in which:
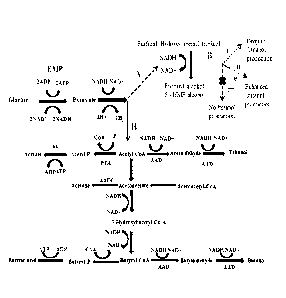
[0066] FIG. 1 illustrates biochemical pathways for fermentation and
inhibition
thereof according to an embodiment of the present invention. A: Effects of
furan
derivatives inhibitors during ABE fermentation. B: Pathway for anaerobic
(acetone-
ethanol-butanol) ABE fermentation. I: Furfura1/5-HMF (<0.5 g/L) enhanced
production
and productivity. II: Furfura1/5-HMF (2-3 g/L) dropped in production and
productivity.
III: Furfural/ 5-HMF (>3 g/L) was deleterious for ABE fermentation.
[0067] FIG. 2 shows graphs that illustrate the kinetics of metabolites
production
during ABE fermentation using brewery industry liquid waste (BLW): (A)
control; (B)
charcoal mediated detoxified BLW; (C) over-lime detoxified BLW; (D) two-phase
partitioning bioreactor systems (TPPB) detoxified BLW.
[0068] FIG. 3 is a graph that illustrates the effect of time during
optimization of
rotational speed of impeller propellant using 250 rpm.
DETAILED DESCRIPTION
[0069] In one embodiment there is disclosed a process for extracting a
microbial
inhibitor from a hydrolysate of glycosidic waste matter containing free
reducing sugars
prior to a fermentation reaction, the process comprising the step of:
solvent extraction of the hydrolysate with a solvent selected from the group
consisting of
bis-(2-ethylhexyl) sebacate, 2-undecanone, and a combination thereof, over a
period of
sufficient length to extract the microbial inhibitor therefrom, thereby
obtaining an extract
containing the microbial inhibitor and reducing the level of the microbial
inhibitor in the
hydrolysate.
CA 3013593 2018-08-08
File No.: P4015CA00
[0070] In another embodiment, there is disclosed a process for the
production of
bio-butanol by fermentation of a detoxified hydrolysate of glycosidic waste
matter
containing free reducing sugars, the process comprising the step of:
fermentation of the detoxified hydrolysate with a solventogenic microorganism
over a
period of sufficient length and at a temperature sufficient to produce the bio-
butanol,
wherein the detoxified hydrolysate is obtained from a solvent extraction of a
hydrolysate
of glycosidic waste matter containing free reducing sugars with a solvent
selected from
the group consisting of bis-(2-ethylhexyl) sebacate, 2-undecanone, and a
combination
thereof, over a period of sufficient length to extract a microbial inhibitor
therefrom,
thereby obtaining an extract containing the microbial inhibitor and the
detoxified
hydrolysate.
[0071] The process may further comprise the step of:
solvent extraction of a hydrolysate of glycosidic waste matter containing free
reducing
sugars with a solvent selected from the group consisting of bis-(2-
ethylhexyl) sebacate,
2-undecanone, and a combination thereof, over a period of sufficient length to
extract a
microbial inhibitor therefrom, thereby obtaining an extract containing the
microbial
inhibitor and the detoxified hydrolysate
[0072] In another embodiment, there is disclosed a process for the
production of
bio-butanol from fermentation of a hydrolyzed glycosidic waste matter
containing free
reducing sugars, the process comprising the step of:
solvent extraction of a hydrolysate of a glycosidic waste matter containing
free reducing
sugars with a solvent selected from the group consisting of bis-(2-ethylhexyl)
sebacate,
2-undecanone, and a combination thereof, over a period of sufficient length to
extract a
microbial inhibitor therefrom, thereby obtaining an extract containing the
microbial
inhibitor and a hydrolyzed glycosidic waste matter containing free reducing
sugars; and
fermentation of the hydrolyzed glycosidic waste matter containing free
reducing sugars
with a solventogenic microorganism for over a period of sufficient length and
at a
temperature sufficient to produce the bio-butanol.
16
CA 3013593 2018-08-08
File No.: P40150A00
[0073] Waste matter
[0074] In embodiments of the process of the present invention, the
hydrolyzed
glycosidic waste matter containing free reducing sugars may be a hydrolyzed
cellulosic
waste matter containing free reducing sugars, a hydrolyzed amylosic waste
matter
containing free reducing sugars, or a combination thereof. In embodiments, the
hydrolyzed glycosidic waste matter containing free reducing sugars may be
obtained
from a cellulosic waste matter, an amylosic waste matter, or a combination
thereof. The
cellulosic waste matter may be from, for example, brewery liquid waste,
brewery spent
grain, apple pomace ultrafiltration sludge, apple pomace solid waste, or
combinations
thereof. The amylosic waste matter may be from, for example, starch industry
wastewater.
[0075] Extraction solvents and conditions
[0076] According to these embodiments, the solvent may be selected from
the
group consisting of bis-(2-ethylhexyl) sebacate, 2-undecanone, and a
combination
thereof. In the process, the ratio of hydrolyzed glycosidic waste matter
containing free
reducing sugars (the aqueous phase) to solvent (the organic phase)
(Vaqueous:Vorganic) is
from 5:1 to 1:2, or from 5:1 to 1:1, or from 5:1 to 2:1, or from 5:1 to 3:1,
or 3:1 to 1:2, or
from 3:1 to 1:1, or from 3:1 to 2:1, or 2:1 to 1:2, or from 2:1 to 1:1, or
from 5:1, or from
3:1, or from 2:1, or from 1:1, or from 2:1, and preferably 2:1..
[0077] According to an embodiment, the processes of the present invention
comprise mixing of the hydrolyzed glycosidic waste matter containing free
reducing
sugars and the solvent during solvent extraction, in order to increase
extraction of the
inhibitor found in the hydrolyzed glycosidic waste matter. In embodiments, the
mixing
may be effected with a propeller impeller, although any suitable device to mix
the
components will be adequate. The input of energy for mixing may be from 0.02
W.h/L to
0.12 W.h/L, or from 0.04 W.h/L to 0.12 W.h/L, or from 0.06 W.h/L to 0.12
W.h/L, or from
0.08 W.h/L to 0.12 W.h/L, or from 0.1 W.h/L to 0.12 W.h/L, or from 0.02 W.h/L
to 0.10
W.h/L, or from 0.04 W.h/L to 0.10 W.h/L, or from 0.06 W.h/L to 0.10 W.h/L, or
from 0.08
W.h/L to 0.10 W.h/L, or from 0.02 W.h/L to 0.08 W.h/L, or from 0.04 W.h/L to
0. 08
W.h/L, or from 0.06 W.h/L to 0. 08 W.h/L, or from 0.02 W.h/L to 0.06 W.h/L, or
from 0.04
17
CA 3013593 2018-08-08
File No.: P40150A00
W.h/L to 0. 06 W.h/L, from 0.02 W.h/L to 0.04 W.h/L. The time sufficient to
extract the
microbial inhibitor is from 15 to 90 minutes, or from 30 to 90 minutes, or
from 45 to 90
minutes, or from 60 to 90 minutes, or from 15 to 60 minutes, or from 30 to 60
minutes,
or from 45 to 60 minutes, or from 15 to 45 minutes, or from 30 to 45 minutes,
or from 15
to 30 minutes, or 15, 30, 45, 60, or 90 minutes.
[0078] In embodiments, the process of the present invention may be
performed
at a temperature sufficient for the solvent extraction. Suitable temperatures
may include
temperatures from 15 C to 30 C. In a preferred embodiment, the temperature
sufficient
for the solvent extraction is room temperature (25 C).
[0079] According to embodiments, the extraction is completed by the
separation
of the aqueous and organic phases from one another. Separation may be a funnel
separation, a centrifugal force assisted separation, or a combination thereof,
such that
the hydrolyzed glycosidic waste matter containing free reducing sugars and the
solvent
are separated.
[0080] Hydrolysis conditions
[0081] According to an embodiment, in the process of the present
invention, the
hydrolyzed glycosidic waste matter containing free reducing sugars may be
produced
by a chemical hydrolysis, a thermal hydrolysis, an enzymatic hydrolysis, a
mechanical
hydrolysis, or a combination thereof, of the glycosidic waste matter.
According to an
embodiment, the thermal hydrolysis may be a microwave hydrolysis.
[0082] In embodiments, the hydrolysis may be performed under a pressure of
89
kPa to 110 kPa.
[0083] In embodiments, the hydrolysis is performed at a pH of about 0.32
to
about 10
[0084] In embodiments, the hydrolysis is performed at a temperature of
greater
than 100 C.
[0085] In embodiments, the hydrolysis is performed at pH 0.76 in H2SO4, at
121 C, 16 psi (110.3 kPa), for 40 mins.
18
CA 3013593 2018-08-08
File No.: P4015CA00
[0086] The thermal hydrolysis may also be a Bronsted acid catalyzed
pressurized
thermal hydrolysis. The Bronsted acid catalyzed pressurized thermal hydrolysis
may be
performed with H2SO4, HCI, betaine hydrochloride, H202, or combinations
thereof. The
acid concentration may be from about 2 N to about 8.7 N.
[0087] In embodiments, the thermal hydrolysis may be an alkali catalyzed
hydrolysis. The alkali may be NaOH. The alkali concentration may be from about
1 N to
about 2 N.
[0088] In embodiments, the mechanical hydrolysis may be an ultra-
sonication.
[0089] In embodiments, the fermentation is performed at a temperature
from
30 C to 40 C.
[0090] In embodiments, the fermentation is performed at a temperature of
37 C.
[0091] In embodiments, the fermentation is performed for in batch mode
for at
least 48 hours.
[0092] In embodiments, the fermentation is performed in batch mode for 72
hours.
[0093] In embodiments, the fermentation is performed under agitation.
[0094] Solventogenic microorganisms
[0095] In embodiments, the solventogenic microorganism is a clostridia
bacteria.
According to preferred embodiments, the clostridia bacteria is Clostridium
acetobutylicum NRRL B-582, Clostridium beijerinckii NRRL B-466 Clostridium
acetobutylicum, Clostridium beijerinckii, Clostridium saccharobutylicum, and
Clostridium. saccharoperbutylacetonicum, Clostridium Bezrinckii BA101,
Clostridia
strain TU-103, yeasts, genetically engineered Pseudomonas putida DOT-T1E,
genetically engineered Bacillus subtilis GRSW2-B1, genetically engineered B.
subtilis
168 and B. subtilis KS438, as well as other solventogenic recombinant bacteria
and
microorganisms, or a combination thereof.
[0096] In these examples, a two-phase partitioning extraction was
considered as
an efficient alternative to reduce microbial inhibitors harmful effects. Two-
phase
19
CA 3013593 2018-08-08
File No.: P4015CA00
partitioning bioreactor systems (TPPBs) were devised in the early 1990s for
off-gas
biological-treatment in order to increase the mass transfer of low hydrophobic
volatile
organic compounds (VOC) from the gas phase to the microorganisms and to reduce
the
microbial inhibition due to the presence of high VOC or toxic metabolite
concentrations
(Munoz et al. Recent advances in two-phase partitioning bioreactors for the
treatment of
volatile organic compounds. Biotechnology advances, Vol. 30(6), 2012: 1707-
1720).
Hence, the objectives of these examples were: (1) to investigate the capacity
of organic
solvents to remove typical lignocellulosic hydrolysate microbial inhibitors,
(2) to identify
the best solvent with higher removal efficiency with no toxic effect on
solventogenic
Clostridia bacteria, (3) to optimize different process parameters to enhance
inhibitors
removal efficiency from a real brewery industry hydrolysate as glycosidic
material in
order to improve bio-butanol production, (4) to estimate the power consumption
for cost-
effective production scale-up, (5) to investigate alternative glycosidic
materials, and (6)
to compare microwave and other alternative pretreatment techniques for
hydrolysis.
[0097] The present invention will be more readily understood by referring
to the
following examples which are given to illustrate the invention rather than to
limit its
scope.
EXAMPLE 1
PRODUCTION OF BIO-BUTANOL FROM BREWERY INDUSTRIAL WASTES
[0098] 1.1 Microorganism and inoculums development
[0099] Clostridium acetobutylicum NRRL B-582 (ATCC-824) (CA) and
Clostridium beijerinckii NRRL B-466(CB) were considered for current
investigation to
produce bio-butanol from different brewery industry wastes. They were kindly
provided
by Agricultural Research Station, USDA (USA). The microorganisms were grown
and
maintained in peptone-yeast extract-glucose (PYG) media under anaerobic
condition
(vegetative growth) at 37 1 C and 150 rpm for 24h and 17 h, respectively,
before
being transferred into the fermentation medium. The medium (g L-1) comprised:
glucose
(10); yeast extract (10); peptone (5); tryptone (5); cysteine-HCI (0.5);
K2HPO4 (2.04);
KH2PO4 (0.04); FeSO4,7H20 (1.1x 10-3); CaCl2 (8x 10-3); MgSO4,7H20 (0.0192);
NaCI
CA 3013593 2018-08-08
File No.: P4015CA00
(0.08); and NaHCO3 (0.4). 125 mL serum bottles (working volume 50 mL) were
used for
both butanol production and inoculum development (Maiti et al., January 2016,
Maiti et
al., August 2015). Anaerobic conditions were maintained within the bottle by
sparging
N2 for 10 minutes and immediately sealed by an aluminum crimp seal containing
silicone septum (Fisher scientific, Canada) by means of a hand operated
crimper (E-Z
CrimperTM, VWR, Ontario, Canada). Prior to culture development, the medium was
sterilized for 20 min at 121 1 C. About 10% (v/v) (dry cell weight 35-50
mg/mL) of
microbial culture in its exponential phase of growth (0D600nm = 1.3-1.5) was
used as
inoculum for all the experiments conducted in this investigation.
[00100] 1.2 Chemicals and other materials
[00101] Chemicals, such as glucose, urea, MgSO4-7H20, NaOH, FeSO4.7H20,
CaCl2, cysteine, NaCI, NaHCO3, Ca(OH)2, Na2S03, H2SO4, n-butanol, acetone,
acetic
acid, butyric acid, ethano1,2-undecanone (98%) and bis-(2-
ethylhexyl)sebacate(98%)
were purchased from Fisher Scientific (Ontario, Canada and New Jersey, USA).
Vanillin, vanillic acid, feluric acid, furfural, HMF, acetic acid, levulinic
acid,
syringaldehyde, glucose, xylose, galactose and fructose were purchased from
Sigma
Aldrich (USA). All standards were of analytical grade. Casein peptone,
tryptone,
K2HPO4 and KH2PO4 were purchased from VWR (Ontario, Canada) and the yeast
extract was a kind gift from Lallemand Inc. (Montreal, Canada). The substrates
used in
this example (i.e. brewery liquid waste (BLVV) and brewery industry spent
grains (BSG))
were generously provided by La Barberie Microbrasserie Cooperative de Travail
(Quebec, Canada).
[00102] 1.3 Organic-solvents tested for efficient extraction of
microbial
inhibitors from an agro-waste hydrolysate mimic
[00103] 2-undecanone and bis-(2-ethylhexyl) sebacate were tested for
efficient
extraction of a mixture of relevant inhibitors (furfural, 5-HMF, vanillic
acid, vanillin,
syringaldehyde and ferulic acid) from a synthetic media simulating a real agro-
waste
hydrolysate composed of 50-52 g/L glucose; 3.0-3.2 g/L of both furfural and
HMF, 0.5
g/L of the other compounds (vanillic acid, vanillin, syringaldehyde and
feluric acid).
Media composition was designed according to the inhibitory compound limit
21
CA 3013593 2018-08-08
File No.: P4015CA00
concentration reported in the literature for bio-butanol producing
solventogenic
Clostridia (Baral et al. Microbial inhibitors: formation and effects on
acetone-butanol-
ethanol fermentation of lignocellulosic biomass. Applied microbiology and
biotechnology, Vol. 98(22), 2014: 9151-9172; Qureshi et al. Production of
butanol (a
biofuel) from agricultural residues: Part I¨Use of barley straw hydrolysate.
Biomass and
bioenergy, Vol. 34(4), 2010: 559-565).Different volume ratio of the
hydrolysate mimic
and organic solvents, such as (5:1), (3:1), (2:1), (1:1) and (1:2)
(vaqueous:vorganic) were
tried in order to minimize extractant expenditure. Organic and aqueous phase
mixing
was carried out by a propeller impeller. Rotation rate (100, 200, 250, 300 and
400 rpm)
and operation-time (15, 30, 45, 60 and 90 min) were tested at room temperature
(25
C). A comparative study between settling through separating funnel vs.
centrifugal
force assisted separation was also made to guarantee an optimal recovery of
the broth
phase.
[00104] 1.4 Bio-compatibility study of selected organic-solvents for
efficient extraction of microbial inhibitors and improved bio-butanol
production
[00105] The main constraint of the extracting phase is that it must perform
the
inhibitor removal with preservation of the cell viability. In solvent toxicity
tests, various
sets of experiments were developed for both microorganisms as follows: set-1
:(control-
1) 52 g/L of glucose; set-2:control-1 + (5-15)% 2-undecanone; set-3:control-1
+ (5-
15)% bis-(2-ethylhexyl) sebacate; set-4:(control-2): 52 g/L glucose +
bacterial inhibitory
solution (BIS) (3.0-3.2 g/L of both furfural and HMF; 0.5 g/L of the other
compounds
(vanillic acid, vanillin, syringaldehyde and ferulic acid)); set-5: (control-
3): (5-15)% of
each organic extractant without glucose and BIS; set-6:52 g/L glucose + 5
times diluted
BIS*; set-7: 52 g/L glucose + 10 times diluted BIS*; set-8: 52 g/L glucose +
50 times
diluted BIS*, set-9: control-2 + extraction (2:1 = agro-waste hydrolysate
mimic : organic
solvent); set-10: Set-7 + extraction (2:1 = agro-waste hydrolysate mimic :
organic
solvent); set-11: Set-8 + extraction(2:1= agro-waste hydrolysate mimic :
organic
solvent). Set-1, set-4 and set-5 were included as control-1, control-2 and
control-3
respectively. All the experiments were carried out in P2 nutrient media as
described later
in batch fermentation section.
22
CA 3013593 2018-08-08
File No.: P4015CA00
[00106] 1.5 Pretreatment of real agro-industrial wastes:brewery industry
liquid waste (BLW)and brewery spent grain (BSG)
[00107] Brewery liquid waste (BLVV) was received in a semi-solid
heterogeneous
sludge state; while brewery spent grain (BSG) was a solid residue. In order to
compare
their performance, all the biomass was dried at 65 1 C for 72 h prior to
hydrolysis.
Additionally, dried BSG was grinded to obtain a particle size below 20 mm. The
composition of the dry feedstock is reported in Table 3. Both dried residues
were pre-
treated by means of a Bronsted acid-catalysed thermal hydrolysis in an
autoclave
(sulfuric acid (H2SO4) at a temperature of 121 1 C for 40 minutes, pressure of
16 psi (
110.3 kPa) and pH of 0.76) in order to allow cellulolytic enzymes access to
the
polysaccharide matrices.
23
CA 3013593 2018-08-08
File No.: P4015CA00
Table 3. Physicochemical characterization of brewery industry wastes (dry
weight basis).
n
Brewery Liquid Waste
Components Brewery spent grain
Surplus yeast
Spent hops
co
o pH 5.2
0.1 5.4 0.1 5.1 0.1
i-.
co Total Solid (g/L) 229.4
1.5 -
01 Ash content (%) 7.8 0.7 8.9
1.4 -
to Extractive (%) 3.5 0.4 5.7
0.6 -
co
Carbohydrates (%) 36.4
1.5 40.0 0.5
n.) Crude fiber (%) - 3.0
1.5 26.5 2.4
o
i-. Cellulose (%) 17.1 1.0 -
93 Hemicellulose (%) 32.5 1.5 -
o
Lignin ( /0) 13.4 1.9 -
03 Free reducing sugar (g/kg) 22.7
5.3 102.8 4.7 -
oi
Glucose (g/kg) 1.6 0.1 55.8
1.3 -
co Fructose (g/kg) -
-
Galactose (g/kg) - 5.9
0.9 -
Xylose (g/kg) - 5.7
0.9 -
Micronutrients (mg/kg)
Cd 7.3 0.3 4.2
0.7 -
Al 1099 135 8677
105 -
Mn 4464 145 1551
112 -
Al 1450 186 8915
256 -
As 13.9 2.6 68.8
5.0
Ca 243347 124
310589 156 -
Co 2.6 0.0 25.2
0.2 -
Cr 55.6 1.6 49.8
1.6 -
Cu 1120 36 2126
56 -
Fe 12069 134 12077
114 -
K 52330 75 95476
89 -
Mg 2096 156 1878
123 -
Na 11154 107 23141
92 -
Ni 87.5 3.8 263.7
23.6 -
P 69459 145 10533
178 -
Pb 3.9 1.2 4.7
0.5 -
Se 100.4 32.1 123.3
12.2 -
Zn 7312 45 10527
156 -
24
File No.: P4015CA00
[00108] 1.6 Detoxification of BLW and BSG samples
[00109] Detoxification of BLW and BSG samples was carried out using two
different already reported methods and the alternative method proposed herein.
A
modified version of the over liming method described by Martinez et al. (2001)
was
employed to detoxify both hydrolysates (Martinez et al. Detoxification of
dilute acid
hydrolysates of lignocellulose with lime. Biotechnology progress, Vol. 17(2),
2001: 287-
293). The pH of the hydrolysate was adjusted to 10 with Ca(OH)2 and later
stored
overnight at 30 C . The hydrolysate was mixed with 1 g/L Na2S03 and the
mixture was
heated at 90 1 C for 1 h. Subsequently, the precipitate of metal hydroxides
was
separated by centrifugation at 7650 x g (30 1 C) for 30 min. The precipitate
so formed
was discarded. The supernatant was neutralized to pH 6.7 0.1 with 1 M H2504
and
centrifuged at 30 1 C for 30 min at 7650 x g to separate the precipitate.
[00110] In the detoxification method proposed by Ge et al. (2011), over
liming with
CaO (pH 7.0 and 100 C for 15 min) + filtration + powdered activated charcoal
(3% at 40
C for 1h and 200 rpm) was implemented (Ge et al. Comparison of different
detoxification methods for corn cob hemicelluose hydrolysate to improve
ethanol
production by Candida shehatae ACCC 20335. African Journal of Microbiology
Research, Vol. 5(10), 2011: 1163-1168). The precipitate form composed of metal
hydroxides and charcoal was separated by centrifugation at 7650 x g (30 1 C)
for 30
min. The supernatant was neutralized to pH 6.7 0.1 with 1 M H2SO4 and
centrifuged at
30 1 C for 30 min at 7650 x g to separate the precipitate. In both already
reported
detoxification methods, the clear supernatant was used as the carbon source
for the
following fermentation studies. The alternative detoxification method proposed
in this
example consisted in an ex-situ organic extraction by means of bis-(2-
ethylhexyl)
sebacate ((2:1) vhydrolysate:vsoivent) at 25 C (room temperature), using a
propeller impeller
at 250 rpm as mixing mechanism followed by broth recovery through a separating
funnel for 1h. A propeller-type impeller was selected as it is used for low-
viscosity liquid
and it has been widely applied in vessels ranging from portable type to large
tanks.
CA 3013593 2018-08-08
File No.: P4015CA00
[00111] 1.7 Batch fermentation
[00112] Batch fermentation was performed in 125 mL serum vials (working
volume
of 50 mL) at pH 6.7 0.1. The P2 medium was sterilized at 121 1 C for 20
min. Prior to
fermentation, anaerobic conditions for each bottle were maintained by sparging
the
medium with N2 for 10 minutes and immediately closing with an aluminum crimp
seal
containing silicon septum (Fisher scientific, Canada) by means of a hand-
operated
crimper (E-Z CrimperTM, VWR, Ontario, Canada). A modified P2 medium, with the
following composition, was used for fermentation experiments: Buffer 4KH2PO4
50 g/L,
K2HPO4 50 g/L, ammonium acetate 220 g/L; Minerals 4MgSO4.7H20 20 g/L,
MnSO4.H20 1 g/L, FeSO4-7H20 1 g/L, NaCI 1 g/L; Vitamins 4 thiamin 0.1 g/L,
biotin
0.001 g/L). Since brewery industry wastes were already enriched with yeast
protein, no
additional yeast protein nor peptone were supplemented.
[00113] The fermentation was initiated by inoculating the P2 medium with
the seed
cultures at a ratio of 10% (v:v). CA and CB bacterial strains were used for
the
experiments related to the bio-compatibility tests and CB was selected to
carry out
detoxification tests with real brewery industry hydrolysate wastes (BLW and
BSG).
Fermentation experiments were performed at 37 1 C with shaking at 150 rpm
for 72 h
in duplicates. 1 mL of culture broth from each batch assay was used for
metabolite
analysis. Data described here present average values from duplicate runs for
duplicate
samples.
[00114] 1.8 Total reducing sugars determination
[00115] Total reducing sugars concentration (TRS) was determined by the di-
nitro-
salicylic acid method using glucose as the standard (Miller, Use of
dinitrosalicylic acid
reagent for determination of reducing sugar. Analytical chemistry, Vol. 31(3),
1959: 426-
428). The amount of TRS extracted from hydrolyzed substrates was determined by
UV-
visible spectrophotometer (Cary-50, Varian) using 3,5-dinitrosalicylic acid as
the reagent
(DNS method) at 540 nm.
26
CA 3013593 2018-08-08
File No.: P4015CA00
[00116] 1.9 Microbial inhibitors and reduced sugar compounds
determination
[00117] A complex mixture of several reduced sugar compounds (e.g. as
glucose,
fructose, and xylose) and microbial inhibitors (furfural, HMF, acetic acid,
levulinic acid,
vanillin, vanillic acid, feluric acid and syringaldehyde) were produced during
the pre-
treatment step (hydrolysis). In order to analyse these compounds, liquid
samples were
collected and analyzed using liquid chromatography-mass spectrometry (LC-MS)
and
liquid chromatography-tandem mass spectrometry (LC/MS-MS) equipped with a
ZORBAX Carbohydrate column (5pm, 150 x 4.6 mm ID column) (Agilent
Technologies,
USA) with D6 glucose as the internal standard and a Biobasic-18 column (5pm,
250 x
4.6 mm ID) (Agilent Technologies, USA) with phenylethanol-D5 as the internal
standard.
Before injecting the sample, it was centrifuged for 5 minutes at 7650 x g and
the
supernatant was filtered by 0.45 pm syringe filter. Methanol water (8:2) and
acetonitrile
water (8.5:1.5) were used to dilute the sample before inhibitor and
carbohydrate
analysis.
[00118] 1.10 ABE (acetone, butanol, ethanol) fermentation products
determination
[00119] To determine the different metabolites produced during ABE
fermentation
(i.e. butanol, ethanol, acetone, butyric acid, and acetic acid) liquid samples
from each
fermentation broth were collected and analyzed using gas chromatography (GC)
(G07890B, Agilent Technologies, USA) equipped with a FID detector and a HP-
INNOWaxTM column (30m, 0.25mm ID, 0.25pm df). lsobutanol was used as the
internal
standard. Before injecting the sample (1 mL) in the GC for products analysis,
the
sample was centrifuged for 5 minutes at 7650 x g and the supernatant was
filtered by
0.45 pm syringe filter. The GC conditions comprised: helium carrier gas at a
flow rate of
1 mL/min with a temperature ramp from the initial temperature of 50 C to 150
C (10
C/min) and from 150 C to 250 C (20 C/min) for a 16-min method run time at
11.421
psi (78.75 kPa). Removal of inhibitors from hydrolysate was calculated as (Eq.
1):
27
CA 3013593 2018-08-08
File No.: P4015CA00
Initial concentration of inhibitor (g/L)¨ final concentration of inhibitors
after removal (g/L)
Removal (%) = 100 X
Initial concentration of inhibitor (g/L)
(Eq. 1)
[00120] 1.11 Results and Discussion
[00121] 1.11.1 Organic-solvent used for efficient extraction of microbial
inhibitors from an agro-waste hydrolysate mimic
[00122] Presence of microbial inhibitors in agro-industrial waste
hydrolysates
favors lower production of bio-butanol (as shown in Table 1), even in presence
of
sufficient amount of reducing sugar compounds necessary to enable the
exponential
growth of cells (Maiti et al., January 2016, Ranjan et al. Biobutanol:
science,
engineering, and economics. International Journal of Energy Research, Vol.
36(3),
2012: 277-323). Conversion of inhibiting aromatic homo-cyclic phenolic
compounds and
heterocyclic furan derivatives to their corresponding less toxic substances
leads to
solventogenic (ABE) losses due to higher utilization of NADH in reduction of
inhibitors
instead of desired biosynthetic pathway of bio-butanol production (Ujor et al.
Glycerol
supplementation of the growth medium enhances in situ detoxification of
furfural by
Clostridium beijerinckii during butanol fermentation. Applied microbiology and
biotechnology, Vol. 98(14), 2014: 6511-6521). To overcome the limitation
encountered
in biochemical production of bio-butanol due to the presence of microbial
inhibitory
compounds, a two-phase partitioning extraction was considered in the current
example,
a solution based on the addition of a non-aqueous phase, either a liquid
solvent or a
solid polymer, to a biological process .Thus, this detoxification method
relies on the
adequate selection of the extractant, which mainly depends on the
characteristics of the
microbial community present in the process and the characteristics of the
inhibitors to
be treated. Based on several requirements, such as higher affinity for the
target
pollutant, availability in bulk quantities (low cost), biocompatibility with
Clostridia bacteria
in order not to poison subsequent fermentation step, resistance to autoclaving
and non-
biodegradability, 2-undecanone and bis-(2-ethylhexyl) sebacate were selected
for highly
selective extraction of inhibitors from the aqueous phase.
28
CA 3013593 2018-08-08
File No.: P4015CA00
[00123] The preliminary results about the optimum organic-aqueous phase
volume
ratio showed that increasing ratios implied higher extraction of inhibitory
compounds for
both organic solvents. In order to make the method more cost effective, the
ratio (2:1)
(vaqueous:vorganic) was considered for further investigation since the results
obtained were
adequate enough for detoxification purpose (Table 4). In Table 4, the results
for four
consecutive extractions with each organic solvent are described. Inhibitor
removal by
bis-(2-ethylhexyl) sebacate was lower in comparison with 2-undecanone's
capacity,
whose extraction efficiency towards furan derivatives was about (87-90) (:)/0
and higher
than 95% for each phenolic compounds since the first stage. The selectivity of
extraction with bis-(2-ethylhexyl) sebacate is slightly lower towards furan
derivatives
which was about 78-79% and phenolic compounds >93% Palmqvist et al., (2000),
has
previously reported that depending on the nature of lignocellulosic biomass,
around
about- (4-5 g/L of furan derivative could be produced upon diluted acid
hydrolysis
(Palmqvist et al. Fermentation of lignocellulosic hydrolysates. II: inhibitors
and
mechanisms of inhibition. Bioresource technology, Vol. 74(1), 2000: 25-33).
Thus the
slightly incomplete extraction of furan derivatives recorded in the case of
bis-(2-
ethylhexyl) sebacate (78-79%) might even have a positive impact on anaerobic
ABE
fermentation, since the presence of furfural and HMF in the aqueous media in
the range
from ¨ (0.5 to 2.0) g/L has been proven to enhance bio-butanol production and
productivity as they might act as fermentation precursors (Qureshi et al.
Effect of
cellulosic sugar degradation products (furfural and hydroxymethyl furfural) on
acetone¨
butanol¨ethanol (ABE) fermentation using Clostridium beijerinckii P260. Food
and
Bioproducts Processing, Vol. 90(3), 2012: 533-540; Zhang et al.
Biotransformation of
furfural and 5-hydroxymethyl furfural (HMF) by Clostridium acetobutylicum ATCC
824
during butanol fermentation. New Biotechnology, Vol. 1(3), 2012: 345-351).
[00124] While evaluating a detoxification treatment, the degradation of
fermentable
sugars is a major drawback that must be taken into account. Most of the
methods
reported so far in the literature involve a destruction of a certain
percentage (up to 42
%) of the total reducing sugar content (Table 2), which definitely has
negative effect in
ABE pathway as sugar is the main source of ATP to develop the ABE fermentation
(Ranjan et al., 2012). No sugar degradation was observed for the current
organic
29
CA 3013593 2018-08-08
File No.: P4015CA00
solvent extraction method (Table 4), which indicates the suitability of the
detoxification
treatment proposed.
CA 3013593 2018-08-08
File No.: P4015CA00
0
co Table 4. Organic-solvent screening for efficient
extraction of microbial inhibitors from an agro-waste hydrolysate mimic.
o
I-
(A)
Ca Extraction with 2-undecanone (V.I.e..: VØ. =
2:1)
to
t....)
n.)
o HMF (g/L) Furfural (g/L)
Vanillin (mg/L) Vanillic acid (mg/L) Syringaldehyde (mg/L) Ferulic
acid (mg/L) TRS* (g/L)
i-.
co
O Standard 2.723 2.956 565.9 565.9
300.2 485.6 52.00
co
O
co
Extraction 1 0.267(90%) 0.392 (87%) 10.3 (98%)
9.8(95%) 7.6 (97%) 0.7 (99%) 52.00 (0%)
Extraction 2 0.077(97%) 0.082 (97%) ND (100%) ND
(100%) ND (100%) ND (100%) 52.00 (0%)
Extraction 3 ND (100%) ND (100%) ND (100%) ND (100%)
ND (100%) ND (100%) 52.00(0%)
Extraction 4 ND (100%) ND (100%) ND (100%) ND (100%)
ND (100%) ND (100%) 52.00 (0%)
Extraction with bis-(2-ethylhexyl)sebacate (vNue..: vorgw. = 2:1)
HMF (g/L) Furfural (g/L)
Vanillin(mg/L) Vanillic acid (mg/L) Syringaldehyde (mg/L)
Ferufic acid (mg/L) TRS (g/L)
Standard 2.723 2.956 565.9 565.9
300.2 485.6 52.00
Extraction 1 0.595 (78%) 0.620 (79%) 35.2 (95%) 38.6
(93%) 20.1 (93%) 12.2 (97%) 52.00(0%)
Extraction 2 0.097(96%) 0.106(96%) 16.3 (97%) 16.3
(97%) 2.3(99%) 1.5 (99%) 52.00(0%)
Extraction 3 ND (100%) ND (100%) ND (100%) ND (100%)
ND (100%) ND (100%) 52.00 (0%)
Extraction 4 ND (100%) ND (100%) ND (100%) ND (100%)
ND (100%) ND (100%) 52.00 (0%)
*TRS = Total reducing sugar
31
File No.: P4015CA00
[00125] 1.11.2 Bio-compatibility study of selected organic-solvents for
efficient extraction of microbial inhibitors and improved bio-butanol
production
[00126] Bio-compatibility of the organic solvent used for extraction with
solventogenic Clostridia is another important point to be considered, as ¨5-10
% of the
organic compound could remain after phase separation. No data appears
available
regarding bis-(2-ethylhexyl) sebacate and 2-undecanone organic solvents'
toxicity on
Clostridia. In these cases, the octanol-water partition coefficient value (log
Kow) is
usually cited as a toxicity parameter for the non-aqueous phase selection.
Liquid
organic solvent with log Kow values higher than 4 are generally considered as
non-toxic
for Gram-negative bacteria commonly found in off-gas treatment bioreactors
(e.g.
Pseudomonas putida) (Ramos et al. Mechanisms of solvent tolerance in gram-
negative
bacteria. Annual Reviews in Microbiology, Vol. 56(1), 2002: 743-768). However,
species
of the genus Clostridium are all Gram-positive.
[00127] In order to check the biocompatibility of these solvents considered
for
current investigation different sets of experiments as described in section
1.4 were
subjected to batch fermentation. The results of the current investigation are
presented in
Table 5. When 52 g/L of glucose were provided to the P2 medium without any
inhibitor
(set-1), about 12.85 0.2 g/L and 11.27 0.3 g/L of ABE were recorded for CA
and CB
strains after 72 h of fermentation with an average utilization of 35.3 1.2
g/L and 36.42
2.3 g/L of the available glucose respectively (Table 5). Set-2 and set-3
demonstrated
that when (5-15%) of each solvent (2-undecanone and bis-(2-ethylhexyl)
sebacate )
were supplemented with set-1, bio-butanol generation in presence of 2-
undecanone
solvent was reduced by 70% and 67% for CA and CB respectively, in comparison
with
results obtained from set-1. However, bio-butanol production was remaining
almost
same with the control experiment upon same percentage of supplementation of
bis-(2-
ethylhexyl) sebacate as shown in Table 5. Thus, bio-compatibility experiments
proved
that bis-(2-ethylhexyl) sebacate was in fact more suitable for detoxification
purposes as
compared to 2-undecanone, in term of bio-butanol production. While 2-
undecanone was
retained as an alternative solvent, bis-(2-ethylhexyl) sebacate was preferred
and
considered for other investigation.
32
CA 3013593 2018-08-08
File No.: P4015CA00
0
ta
o
1-=
ta
01
to
ta Table 5. Bio-compatibility study of bis-(2-ethylhexyl sebacate
for efficient extraction of microbial inhibitors and improved bio-butanol
production.
r..) CA
CB
o
1-=
co N Set Composition
oI Butanol (g/L)
ABE (g/L) Butanol (g/L) ABE (g/L)
co
oi
1 Control-1 52 g/L of glucose 9.0 0.5
12.85 0.2 8.4 0.5 11.27 0.3
co
2 Control-1 + (5-15)% 2-undecanone 2.77 0.12
3.82 0.2 3.11 0.3 4.28 0.4
3 Control-1 + (5-15)% bis-(2-ethylhexyl) sebacate
9.13 0.2 12.5 0.2 8.62 0.3 11.48 0.4
Control-2 52 g/L glucose + bacterial inhibitory solution
(BIS) (3.0-3.2 g/L of both furfural and HMF; 0.5 g/Lof the
4 other compounds (vanillic acid, vanillin, syringaldehyde
ND ND ND ND
and
ferulic acid))
Control-3 (5-1 5)% of each organic extractant without
ND ND ND ND
glucose and BIS
6 52 g/L glucose + 5 times diluted BIS* ND ND
ND ND
7 52 g/L glucose + 10 times diluted BIS* 2.1 0.1
2.98 0.4 1.1 0.2 1.9 0.3
8 52 g/L glucose + 50 times diluted BIS* 7.12 0.12
10.0 0.2 6.13 0.6 8.9 0.3
Control-2 + extraction (2:1= agrowaste hydrolysate mimic: 10.25 + 0.5 9
13.96 0.3 9.58 0.2 13.15 0.3
organic solvent)
Set-7 + extraction(2:1 = agro-waste hydrolysate mimic:
9.13 0.2 12.5 0.2 8.62 0.3 11.48 0.4
organic solvent)
Set-8 + extraction(2:1 = agro-waste hydrolysate mimic:
11 9.1 0.3 12.2 0.3 8.4 0.2 11.0 0.3
organic solvent)
*BIS = bacterial inhibitory solution composed of 2.8-3.0 g/L of both furfural
and HMF; 0.5 g/L of the other compounds (vanillic acid, vanillin,
syringaldehyde and feluric acid).
All the experiments were carried out in P2 nutrient
33
File No.: P4015CA00
[00128] Phenolic compounds, furan derivatives (furfural, 5-HMF) etc. have
their
detrimental effect in ABE fermentation depending on their concentration (Table
1).
Phenolic compounds in waste hydrolysate, during ABE fermentation, interfere
with the
living cell by changing protein-to-lipid ratio in cell membrane and furan
derivatives by
utilizing NADH cofactor or NADPH for reduction instead of ABE production as
shown in
FIG. 1 (Jonsson et al. Bioconversion of lignocellulose: inhibitors and
detoxification.
Biotechnology for biofuels, Vol. 6(1), 2013:1). Thus, in order to check the
effect of
inhibition and detoxification using previously selected extracting solvent
[bis-(2-
ethylhexyl) sebacate], additional experiments were carried out and
corresponding
results were recorded in Table 5. About 9.5 g/L and 8.4 g/L of butanol
production was
observed from the control 1 conditions (52 g/L glucose) after 72 h of ABE
fermentation
(Table 5). Control 2 conditions [52 g/L glucose + bacterial inhibitory
solution (BIS)]
demonstrated that no production of butanol was achieved in any case (CA and
CB) in
the presence of typical toxic concentrations of each inhibitor (Table 1 & FIG.
1),
corroborating the findings of (Baral etal., 2014).
[00129] Furthermore, to determine if the detrimental effect of inhibitory
substrates
was dose dependent, set-6, containing 52 g/L glucose + 5x diluted BIS was
subjected to
fermentation and no desired metabolites were recorded for both cases. Thus,
strong
synergistic inhibitory effect below individual inhibitory level could be
possible. Likewise,
times diluted BIS (set-7) solution resulted in (2.98 0.4) g/L and (1.9
0.3) g/L of
ABE and production of bio-butanol increased up to 7.12 0.12 g/L for CA and
6.13
0.6 g/L for CB (31-32 % 4, compared to control 1) when 50 times diluted
inhibitor
solution was used (set-8). In this dilution range, bio-butanol and ABE
production did not
reach the maximum values (set-1) probably also due to synergistic effects
created
between inhibitors, rather than the influence of each inhibitory compound
concentration,
as all the hydrolysis by-products were below their corresponding inhibition
limit (Table
1). Thus, detoxification must be performed before fermentation to reduce the
inhibitory
effect due to presence of inhibitory substrates.
[00130] Production of butanol in set-9 after extraction of control 2 was
higher than
in previous experimental tests as it successfully removed the inhibitors from
the
fermentation broth. It was also evident that the production was even higher
compared to
34
CA 3013593 2018-08-08
File No.: P4015CA00
control 1. Presence of lower concentration of furan derivative was reported to
help in
enhanced butanol production via a free, electron-mediated mechanism as shown
in
FIG. 1 (Baral et al., 2014). Thus, set-9 results suggested that the presence
of furan
derivatives traces in the media (incomplete removal as seen in Table 4) were
utilized by
the culture, and this might be the reason for improved yields in comparison
with (control
1/ set-1), achieving an average increase in the bio-butanol production (8-10)%
for both
bacterial strains. Experiments from set-10 and set-11 demonstrated that
butanol
productions were almost similar with the control 1 as the extraction process
helped to
remove (for both > 99%) the inhibitors from the mimic synthetic media in this
dilution
range. Furthermore, control 3 (5 -15% of each organic extractant without
glucose and
BIS) resulted in no butanol production which implied that the organic solvent
was not
utilized by CA and CB as a carbon source (in the presence or not of an
additional easily
assimilable carbon source like glucose), discarding its potential
biodegradability. Thus,
bis-(2-ethylhexyl) sebacate was chosen as the preferred solvent for removing
microbial
inhibitors from a real agro-industrial hydrolysate (BLW and BSG) as well as
for
optimizing further process operation parameters.
[00131] 1.11.3 Brewery industry wastes and
physicochemical
characterization of waste biomass
[00132] The physiochemical characterization of brewery industry spent
grains
(BSG) and brewery industry liquid waste (BLW) are presented in Table 3. In
brewery
industry after separation of wort, the residue left is called brewery spent
grains (BSG)
(Olajire, 2012; Macheiner et al. Pretreatment and hydrolysis of brewer's spent
grains.
Engineering in life sciences, Vol. 3(10), 2003: 401-405). Brewery industry
liquid waste is
a complex mixture of surplus yeast and plant residues (remaining fine particle
BSG,
spent hops), is semi-solid in nature, comes at the final stage after second
fermentation,
collected during tank wash (Fillaudeau et al., 2006, Olajire, 2012). Analysis
of raw agro-
industrial wastes revealed the presence of free reducing sugar in BLW: 15 g/L
(10.5 g/L
glucose, 2.5 g/L xylose) (Maiti etal., January 2016)
CA 3013593 2018-08-08
File No.: P4015CA00
[00133] 1.11.4 Enhanced bio-butanol production using BLW and BSG as
feedstock
[00134] Since one of the objectives of the present example is to utilize
BLW and
BSG hydrolysates as carbon sources to produce bio-butanol and improve bio-
butanol
production, un-hydrolyzed waste biomass were subjected to batch fermentation
in P2
media. Due to lack of sufficient reducing sugars no butanol production was
observed
like previously reported (Maiti et al., January 2016). In order to enhance
butanol
production, each biomass was subjected to hydrolysis. The resulting
hydrolysates
included sugars (TRS content of 52 g/L) and undesired microbial inhibitors as
follows:
furfural (0.642 g/L), HMF (3.123 g/L), levulinic acid (0.236 g/L) and total
phenolic
compounds (0.512) g/L in BLW hydrolysate and furfural (2.340 g/L), HMF (1.564
g/L)
and total phenolic compounds (0.845 g/L) in BSG hydrolysate. Prior to
detoxification,
batch fermentation was run in P2 media with both hydrolysates (TRS content of
52 g/L).
As expected, the presence of different microbial inhibitors in the hydrolysate
solutions
(section 1.1) had strong synergistic effect on ABE fermentation inhibition and
no bio-
butanol production was attained. Accordingly, the two-phase extraction method
of the
present example and two previously known detoxification methods to enhance bio-
butanol production from each hydrolysate were tested for comparison purposes
as
shown in Table 6.
Table 6. Bio-butanol production achieved by means of Clostridium beijerinckii
after application of
different detoxification methods.
CaO overliming + powdered
activated charcoal Ca(OH)2 overliming Two-phase extraction
Substrates ________________________________________________________________
Butanol (g/L) Time (h) Butanol (g/L) Time (h) Butanol (g/L) Time
(h)
BLW 4.3 0.3 120 6.2 0.1 72 8.0 0.12 72
BSG 4.8 0.2 120 5.8 0.3 72 7.2 0.1 72
[00135] A reduced bio-butanol production and productivity of charcoal
mediated
detoxification was observed compared to Ca(OH)2 overliming and two-phase
extraction
36
CA 3013593 2018-08-08
File No.: P4015CA00
method. Presence of suspended charcoal particles in the fermentation broth
could be a
reason for a longer lag-phase as shown in Table 6 and FIG. 2(B). In a similar
way,
Lopez-Linares et al. (2015) were not able to produce bio-ethanol or detect any
consumption of glucose after 144 h when rape straw hydrolysate was detoxified
by
activated charcoal (Lapez-Linares et al. Cofermentation of pentoses and
hexoses by
Escherichia coli. Spanish Journal of Agricultural Research, Vol. 13(3), 2015:
213). To a
lesser extent, Gupta etal. (2015) observed a reduced bio-ethanol production
rate for the
first 2 h of fermentation when 2.0% (wv-1) activated charcoal was used as
detoxification
method (Gupta et al. Scale-up of abatement of fermentation inhibitors from
acid
hydrolysates for efficient conversion to ethanol as biofuel. Journal of
Chemical
Technology and Biotechnology, Vol. 91(6), 2016: 1826-1834). The currently
developed
detoxification method achieved an average increase of 46% and 25% in bio-
butanol
concentration in comparison with CaO overliming + powdered activated charcoal
and
Ca(OH)2 over-liming, respectively, from BLW. The kinetics of metabolites
production
using different detoxification method mediated ABE production using BLW has
been
presented in FIG. 2. FIG. 2: (A), (B), (C) and (D) presents the kinetics of
metabolites
formation from control, charcoal mediated detoxification followed by
fermentation using
BLW, overliming followed by fermentation and TPPB detoxification followed by
fermentation using BLW respectively. Likewise, results obtained from BSG after
fermentation was also recorded in Table 6. About 33% and 20% enhancement in
butanol production was observed using the currently developed detoxification
method
compared to the other two (Table 6). The TRS loss observed with the known
detoxification methods might be a main reason to explain this difference
(Meinita et al.,
2012). Additionally, these two methods were based on the conversion, to some
extent,
of the microbial inhibitors to their corresponding less toxic forms, unlike
the currently
developed method where all the phenolic compounds as well as soluble lignin
were
effectively extracted from the broth medium, ensuring no inhibition of the
processes due
to these compounds. Thus, Clostridium beijerinckii NRRL B-466 can successfully
utilize
the mixed free reducing sugars from the detoxified brewery industry waste
hydrolysates
to convert it into the desired products of ABE fermentation.
37
CA 3013593 2018-08-08
File No.: P4015CA00
[00136] 1.11.5 Translation of flask scale data to commercial-scale TPPB
extraction
[00137] The transfer rate of the toxic compounds from the aqueous phase to
the
organic phase is proportional to the interfacial area, which itself is
determined by the
percentage of the organic solvent and the stirring conditions in the reactor.
Once the
identity and amount of a suitable organic solvent was determined, the stirring
rate
constituted the most important operational variable. Ascon-Cabrera and
Lebeault (1995)
found a fourfold linear increase in the interfacial area when increasing the
agitation rate
from 200 to 800 rpm (Ascon-Cabrera et al. Interfacial area effects of a
biphasic
aqueous/organic system on growth kinetic of xenobiotic-degrading
microorganisms.
Applied microbiology and biotechnology, Vol. 43(6), 1995: 1136-1141). However,
agitation rates over 500 rpm were not recommended in a context of full-scale
application due to the high energy consumptions and to the technical
difficulties
associated to their implementation (Gardin et al. Biodegradation of xylene and
butyl
acetate using an aqueous-silicon oil two-phase system. Biodegradation, Vol.
0(3), 1999:
193-200). In this example with increase in rotational speed from 100 to 250
rpm there
was increase in extraction and no significant enhancements in microbial
inhibitors
extraction were observed with stirring rates above 250 rpm (Table 7).
Centrifugal
assisted separation performed equally well as a separating funnel, which
nevertheless
remains simpler and cheaper (Table 7). Furthermore, time is another important
factor in
this context. From FIG. 3, it is evident that using rotational speed 250 rpm,
the
extraction of different inhibitory compound were a lower after 15 min of
extraction.
However, when extraction time was increased to 30 minutes or more, the
extraction
levels were almost the same. Thus, rotational speed of 250 rpm and extraction
time of
30 min appear to be suitable parameters.
38
CA 3013593 2018-08-08
File No.: P4015CA00
0
LA.) Table 7. Optimization of different base line parameter to
scale up
0
1-=
LA.)
01 Phase separation by centrifugal force (%)
Phase separation by separating funnel (%)
to
ta
n.)
o Inhibitor Bi-phasic system homogenizer rotational
speed (rpm) Bi-phasic system homogenizer rotational speed (rpm)
1-=
co
o1
100 200 250 300 400
100 200 250 300 400
co
O
co
Furfural 68 69 78 78 80
65 68 75 76 78
HMF 71 74 79 82 84
71 73 78 80 80
Vanillin 95 95 97 97 96
93 93 95 97 97
Vanillic acid 92 93 94 94 96
90 91 93 93 93
Syringaldehyde 100 100 100 100 100
100 100 100 100 100
Ferulic acid 97 96 97 95 97
94 94 93 94 96
39
File No.: P4015CA00
[00138] The amount of power consumption is another determining factor to
evaluate
the suitability of the method proposed. In order to make a preliminary
estimation of this
value, the correlation proposed by Furukawa et al. (2012) for a propeller
impeller in an
unbaffled mixing vessel was applied taking into account several design
parameters, such
as liquid mixture viscosity, density and depth, vessel diameter, impeller
diameter, angle and
height of impeller blade, number of impeller blades, rotational speed and
friction factor
(Furukawa et al., 2012). Accordingly, the power consumption may be obtained by
the
following equation (Nix, = Power Number):
P= Npc, x bulk density = (revolution per second)3 = (impeller diameter)5
[00139] The conditions used for the calculation of the power consumption
were: an
agitation speed of 250 rpm, an impeller diameter of 42 mm, a liquid mixture
density of
955.15 kg/m3 and a viscosity of 0.0091 Pa-s. Power inputs per unit volume for
the reactor
were therefore estimated to be 0.072 W/L, which can be considered a low value
in
comparison with other two-phase partitioning reactor cases described in the
literature
(Quijano et al. KLa measurement in two-phase partitioning bioreactors: new
insights on
potential errors at low power input. Journal of chemical technology and
biotechnology, Vol.
85(10), 2010: 1407-1412).
[00140] 1.12 Conclusion
[00141] In order to minimize environmental pollution and add positive
momentum in
bioenergy, carbon pool management of different brewery-industrial wastes such
as brewery
industry liquid waste (BLW) and spent grain (BSG) have been considered. An
efficient,
rapid ex-situ detoxification has been developed to reduce inhibitor
concentration in the
hydrolysate and enhance bio-butanol production. More than 80% extraction of
furan
derivatives and more than 95% extraction of phenolic compounds and almost no
extraction
of reducing sugar from simulated synthetic media as well as waste hydrolysate
has made
this method more interesting compared to literature reports. Ex-situ
extraction of microbial
inhibitors using bis-(2-ethylhexyl) sebacate as solvent leads to higher
production of 8.0 g/L
of bio-butanol from BLW compare to the use of literature reported methods such
as over
liming (6.2 g/L) and charcoal (4.3 g/L) detoxification. The currently
developed detoxification
method increased bio-butanol production potential of BLW because reasonable
production
CA 3013593 2018-08-08
File No.: P4015CA00
of ABE was possible without sugar supplement. Extension of this solvent
extraction
detoxification method to BSG produced 7.2 g/L of bio-butanol. Lower power
consumption
and reuse of the extraction solvent make this detoxification technique useful
for improved
production of bio-butanol from agro-industrial waste hydrolysate.
[00142]
Typically, in liquid-liquid extractions, the fermentation broth is extracted
(in-
situ or ex-situ) with a suitable hydrophobic, high boiling organic liquid to
adsorb ABE, and
the solvent unloaded broth is recycled to the fermenter. Bio-butanol is
subsequently
concentrated in a higher-boiling solvent and removed from the extractant in a
recovery-
regeneration unit (usually distillation), and the solvent is reused (Kraemer
et al. Separation
of butanol from acetone¨butanol¨ethanol fermentation by a hybrid
extraction¨distillation
process. Computers & Chemical Engineering, Vol. 35(5), 2011: 949-963). In
contrast, in the
current case, the second phase is added in order to detoxify the hydrolysate
before
proceeding with the fermentation. Thus, maintaining an optimum and stable
reuse
efficiency of the organic solvent phase is even more important for commercial-
scale
extraction. As solvent extraction efficiency decreased in the range of 5-15%
due to
progressive saturation, organic liquid biological clean-up could be performed,
so that the
organic solvent would be reused without any energy intensive process. Phenolic
compounds with similar characteristics to those found in lignocellulosic
biomass
hydrolysates have been previously efficiently degraded in two phase
partitioning
bioreactors (Table 8) developed for off-gases treatment by means of bacterial
mixed
cultures and specific strains.
41
CA 3013593 2018-08-08
File No.: P4015CA00
0
Table 8. Biodegradation rates of different phenolic compounds typically found
in off-gases by means of two-phase partitioning bio-reactors.
LA)
LA.) Contaminant/Inhibitor Bacteria Solvent
Aqueous-organic Concentration
phase ratio (v:v)
(mg/L) Time to biodegrade (h) Ref.
(Dumont et
LA.)
al.
o Biotechnique
Mixed culture wastewater
co Styrene
plant Silicone oil 9:1
230 5 s for Air
Pollution
co
Control II,
2007)
co
(Tomei et a/.
Water
Mixed culture wastewater
4-nitrophenol 2-undecanone 9:1 350 7 Science and
plant
Technology,
62(4), 2010)
(Collins et al.
Biotechnolog
Pseudomonas
4000 (168 in the 12 h lag-phase; y and
Phenol 2-undecanone 2:1
putida ATCC 11172
aqueous phase) Total consumption in 60 hours bioengineeri
ng, 55(1);
1997)
(Guieysse et
al.. Applied
microbiology
Phenanthrene and Pseudomonas sp. and
72 h for phenanthrene;
Silicone oil 2:1
100 and
pyrene Sphingomonas sp.
Pyrene was consumed as cosubstrate
biotechnolog
y, 56(5-6),
2001)
(Flamed et
a/.
Phenol (+ benzene + Pseudomonas
Biochemical
2-undecanone 16:1
400 28
toluene) putida Fl
Engineering
Journal,
19(2), 2004)
(Guieysse et
a/. Water
2-Undecanone,
24 h lag-phase, science and
Phenol Pseudomonas mandelii 3:1
4000-5000
diethyl sebacate
Total consumption in 75 h technology,
52(10-11),
2005)
(Zilouei of a/.
Chemospher
Pentachlorophenol Sphingobiumchloropheno
Dioctylsebacate 2:1
10000-11000 60 h
licum DSM 8671
e, 72(11),
2008)
42
File No.: P4015CA00
EXAMPLE 2
ALTERNATIVE GLYCOSIDIC MATERIAL
[00143] Brewery liquid waste (BLW), starch industry wastewater (SIVV),and
apple
pomace ultrafiltration sludge (APUS) were pre-treated using diluted H2SO4 at
121 1 C for
40 min to enhance total reducing sugar. Hydrolysates were kept overnight in 65
C to raise
the reducing sugar concentration, to around 60 g/L. The pH of the concentrated
hydrolysate
solution was adjusted to 10.5 0.1 by Ca(OH)2 and was kept overnight at 40 C
to remove
the excess metal ions as metal hydroxides and reduce the effect of process
inhibitors.
Hydroxide precipitate was centrifuged at 7650 x g (10,000 rpm).
[00144] Prior to fermentation of agro-industrial wastes as well as
hydrolysates,
investigations were made for characterization of the complex agro-industrial
waste biomass
to assess carbohydrate pool and presence of micronutrients. The physiochemical
characterization including total solids and free reducing sugars,
carbohydrates, pH and
micronutrients of three wastes, such as APUS, BLW and SIW were thoroughly
investigated
and is reported in Table 9. Analysis of raw agro-industrial wastes revealed
the presence of
free reducing sugar in BLW: 15 g/L (10.5 g/L glucose, 2.5 g/L xylose), APUS:15
g/L (8 g/L
glucose and 6.4 g/L fructose) and SIW (0.65 g/L).
43
CA 3013593 2018-08-08
File No.: P4015CA00
0
LA)
0 Table 9: Chemical composition of waste biomass used for
butanol production before and after hydrolysis
1-.
LA)
01
to
w
n.) Components BLW APUS SIW
BLWH APUSH SIWH
o
1-. pH 5.4 0.1 3.4 0.1 3.3 0.2 -
- -
co
o1 Total Solid (g/L) 129.4 1.5 98.5 2.4 15.9 0.15
- - -
co
oi Free reducing sugar (g/L) 14.8 1.4 13.0 2.5
0.65 0.15 30.0 2.52 30.0 2.68 30.0 1.46
co Micronutrients (mg/L)
(Element with wavelength)
Al (396.152) 0.135 0.01 0.66 0.03 0.07 0.01
0.05 0.013 0.138 0.02 0.01 0.0
As (188.980) - - 0.06 0.01
- 0.01 0.02 -
Ca (396.847) 112.91 2.5 104.36 4.61 36.40 3.5
7.6272 1.72 5.93 1.05 1.10 0.54
Co (238.892) - - - -
- -
Cr (267.716) 0.045 0.02 0.145 0.04 0.01 0.0
0.02 0.04 0.03 0.02 0.02 0.0
Cu (324.754) 0.43 0.05 0.35 0.05 0.08 0.02
0.05 0.06 0.01 0.01 0.029 0.01
Fe (238.204) 2.74 0.43 29.086 0.14 2.67 0.05
0.05 0.02 2.398 0.05 0.09 0.0
K (769.897) 785.36 8.32 1332.69 12.5
253.41 6.28 714.315 6.5 1298.78 16.3 258.33 4.2
Mg (279.553) 92.39 2.54 67.78 3.5 62.41 2.48
32.37 1.21 1.28 0.04 0.198 0.08
Na (589.592) 19.96 1.71 554.94 3.42 310.44
5.62 1567.67 12.5 1586.32 5.81 1511.33 25.6
Ni (222.486) 0.08 0.01 0.06 0.02 0.015 0.03
0.042 0.05 0.02 0.01 0.01 0.0
P (213.618) 461.141 2.5 368.81 3.51 124.28
4.21 427.88 1.21 240.77 2.87 51.18 2.5
Pb (220.353) - - - -
- -
S (181.972) 179.26 4.6 225.012 1.63 79.13
3.23 175.058 4.31 149.44 2.67 62.08 5.8
Se (196.026) 0.10 0.01 0.12 0.05 0.07 0.06
0.05 0.02 0.05 0.03 0.02 0.0
Zn (206.200) 0.52 0.01 0.45 0.03 1.083 0.02
0.02 0.04 0.02 0.02 0.05 0.01
SIW: Starch Industry wastewater
APUS: Apple Pomace Ultrafiltration Sludge
BLW: Suspended Brewery Liquid Waste (BLW),
SIWH: Starch Industry Wastewater Hydrolysate
APUSH: Apple Pomace Ultrafiltration Sludge Hydrolysate
=
BLWH: Suspended Brewery Liquid Waste Hydrolysate
44
File No.: P4015CA00
[00145] It is important to note that in the present application, the
microbial
inhibitors are considered from the point of view of waste. However, it should
be noted
that these inhibitors are also for the most part molecules of interest having
a good
market value (ferulic acid, 200-300 $/kg, Vanillin 150 $/kg, etc.) and which
could have
applications other than that of being a simple waste. In fact, in some
embodiments, if in
one liter of hydrolysate there is 3 g/L of a furfural type inhibitor and 100
ml of solvent are
added to recover it, one will effectively end up with this 3g of furfural in
100 ml of
solvent, which represents a 10-fold concentration. Therefore, another
embodiment of
the present invention may be the use of these solvents as extraction tools for
phenolic
compounds to concentrate the microbial inhibitors. Put another way, the target
product
are phenolic compounds and the waste would be the remaining sugars.
EXAMPLE 3
MICROWAVE ASSISTED HYDROLYSIS
[00146] 3.1 Substrate procurement and preparation
[00147] The five agro-industrial residues (BLW, BSG, SIW, APS and APUS)
selected for this example are nutrient-rich organic wastes generated in
thousands of
tones worldwide every year (Dhillon et al., 2011). Additionally, they have
proven to be
valid candidates for the production of higher value bio-products (Table 10).
Three of the
feedstock (BLW, SIW and APUS) were received as semi-solid substrates, while
BSG
and APS were in solid state. In order to compare their performance, all the
biomasses
were dried at 60 for 72 h prior to hydrolysis. The composition of the dry
feedstocks is
reported in Table 11.
CA 3013593 2018-08-08
File No.: P4015CA00
Table 10 - nutrient-rich organic wastes candidates for the production of
higher value
bio-products
Feedstock Biorefinery products
Substrate for microbial fuel cells
BLW
Bio-ethanol production
Nutraceuticals-rich solution production
BSG Biogas production
Xylanase production
a-amylase and g-galactosidase production
siw Single-cell protein production with high lysine content
Lactic acid production
Water soluble pigments production
Exo-pectinase production
APS
Immobilization carrier for solid-state fermentation
Lacase production
APUS Citric acid production
46
CA 3013593 2018-08-08
File No.: P4015CA00
Table 11 - Physicochemical characterization of agrc-industrial wastes.
Components BLW BSG APS APUS SIW
pH 5.4 0.1 5.2 0.1 3.2 0.1 3.4 0.1 3.3 0.2
Total Solid (g/L) 229.4 1.5 - 384.5 2.4
16.4 0.15
% Ash Content 8.947 1.34 7.785 0.65 4.705 0.53 2.549 0.78
3.549 0.94
% Extractive 5.733 0.56 3.526 0.42 3.115 0.78 2.850 0.23
1.243 0.74
Starch % 5.6 1.2 12.5 0.85 - 30 1.56
(W/dry weight)
Cellulose % 19.8 1.67 17.1 0.97 23.2 1.3 21.8 1.78 -
(W/dry weight)
Hemicellulose 16.5 2.87 32.5 1.45 5.4 0.67 - -
% (W/dry
weight)
Lignin (W/dry 8.5 1.78 13.4 1.9 23.5 2.13 20.56 2.56 -
weight)
Free reducing 102.844 4.67 22.660 5.34 155.064 2.12
175.360 5.89 21.567 0.98
sugar (g/kg) ,
Glucose(g/kg) 55.768 1.34 1.567 0.078 35.552 0.98 40.345
1.76 1.245 0.09
Fructose(g/kg) - - 32.678 1.67 30.678 2.67 -
Galactose(g/kg) 5.946 0.89 - 3.876 0.67 - -
Xylose(g/kg) 5.678 0.92 - 3.145 0.98 - -
Micronutrients(mg/kg)
Cd (214.439) 4.155 0.65 7.315 0.25 - - 1.305
0.05
Al (308.215) 8677.31 105 1098.8 135 653.24 121 3905.985 142
5678.9 95
Mn (257.610) 1550.84 112 4464.25 145 2147.695 139 1080.025
89 6.4 1.45
Al (396.152) 8914.725 256 1450.06 186 971.1 126 4106.59 155
-
As (188.980) 68.805 5.04 13.88 2.64 27.875 3.67 34.08 1.64
30.40 1.24
Ca (315.887) 310588.5 156 243347 124 75017.45 136 42284.8
256 10950.6 180
Co (230.786) 25.15 0.23 2.655 0.02 6.85 0.86 4.825 1.23
8.9 2.04
Cr (267.716) 49.77 1.56 55.59 1.56 36.82 1.56 131.245 1.56
2.67 1.56
Cu (327.395) 2125.56 56.23 1119.57 36.14 582.78 156.67
471.185 126.05 253.41 112.28
Fe (238.204) 12077.45 114 12068.8 134 4326.76 123 12652.3
104 5341 78.67
K (766.491) 95475.75 88.56 52329.85 75.06 27466.95 64.56 44156.25 198.04
26241 108.56
Mg (280.270) 1877.84 123 2095.73 156 2769.27 101 2752.28
132 3104.4 121.67
Na (588.995) 23141.1 92 11154.2 106.90 2662.86
86.78 7835.53 167.89 2141.5 78.67
Ni (222.486) 263.67 23.56 87.47 3.78 101.53 17.50 161.28
20.65 -
P(213.618) 10532.95 178 69458.75 145 84583.65 128 17234.4 278
Pb (220.353) 4.66 0.46 3.87 1.23 - - 7.5 1.64
Se (196.026) 123.335 12.23 100.435 32.09 66.315 10.20 46.1
8.23 108 22.11
Zn (213.857) 10526.6 156.36 7311.895 45.06 684.825 66.30 239.93 16.09
256.789 23.16
47
CA 3013593 2018-08-08
File No.: P4015CA00
[00148] 3.2 Substrate selection
The pre-screening process was developed by microwave-assisted Bronsted acid-
catalysed hydrolysis at 161 C for 25 minutes with a feedstock mass
concentration of 40
g/L. The microwave (MARSTm microwave extractor, CEM Corporation, North
Carolina,
USA) was applied at 1000 W and hydrochloric acid (HCl) (2 N) was employed as
homogeneous mineral acid solution. HCI (36.5-38 w/w %) was obtained from
Fisher
Scientific (USA). Each run was performed in triplicate. Reaction parameters
were
chosen as an approximate guide based on known examples of renewable
lignocellulosic biomasses and agro-industrial wastes tested as raw materials
for
levulinic acid (LA) production as summarized in Table 12. Nevertheless, choice
of
hydrolysis treatment and its severity might differ hinging on the
heterogeneity and
complexity of the substrate (Morone et al. Levulinic acid production from
renewable
waste resources: bottlenecks, potential remedies, advancements and
applications.
Renewable and Sustainable Energy Reviews, Vol. 51, 2015: 548-565). The
substrate(s) achieving a higher production of the sum of LA were used for
further
parameter optimisation by means of RSM for hyper-production of LA. Glucose and
5-
HMF content was also going to be taken into account since they are starting
molecules
for LA synthesis via one-pot acid catalyst from lignocellulosic biomass.
48
CA 3013593 2018-08-08
File No.: P4015CA00
0
Table 12 - Hydrolysis reaction parameters for various substrates
u)
0
1-.
u)
cri
ko
u.)
Substrate
Substrate Hydrolysis strategy Acid
(concentration) Temp (K) Time (min) LA yield ( /0)*
I'.)
concentration
o Pretreatment: [EMIIVINI]
i-. Empty fruit bunch H2504
(8.1 %wt) 439 44.5 10%wt 31.6
co Bronsted acid catalyzed pressurized
thermal hydrolysis
ol Pretreatment: [EMIM][CI]
Kenaf H2SO4
(7.8 %wt) 416 66.2 10%wt 39.5
co Bronsted acid catalyzed pressurized
thermal hydrolysis
I Pretreatment: soxhlet extraction
o
co Rice husk Bronsted acid catalyzed pressurized (56
bar) thermal HCI (4.5% (v/v)) 443 60 100 g/L 59.4
hydrolysis
Cicer arietinum Bronsted acid catalyzed pressurized
thermal hydrolysis HCI (1 M) 423 -- 120 -- 50 g /L -- 32.6
Pinus radiata Bronsted acid catalyzed pressurized
thermal hydrolysis HCl (1 M) 423 120 50 g /L 19.0
Sugarcane bagasse Bronsted acid catalyzed pressurized
thermal hydrolysis HCI (1 M) 423 120 50 g /L 36.5
Sugarcane bagasse Bronsted acid catalyzed pressurized
thermal hydrolysis HCI (4.45 %wt) 493 45 10.5%wt 22.8
Paddy straw Bronsted acid catalyzed pressurized
thermal hydrolysis HCI (4.45 %wt) 493 45 10.5%wt 23.7
Wheat straw Bronsted acid catalyzed pressurized
thermal hydrolysis H2SO4 (3.5%) 482 37.6 6%wt 19.86
Bronsted acid catalysed, pressurized (30 bar, N2) thermal
Paper mill sludge H2SO4
(98%) -8.3 meq 473 60 72.9 g/L 15.4
hydrolysis
Lewis acid catalyzed pressurized (20 bar, N2) thermal
Corncob residue 4:1 (weorr,cob:wAia3) 453 120 40 g/L 20.9
hydrolysis; NaCI acted as promoter
Post-harvest tomato plant Microwave assisted (1000W) flash
pressurized (40 bar, N2) HCI (1 M) 498 2 100 g/L 63**
waste thermal hydrolysis
Microwave-assisted (250W) Bronsted acid-catalysed thermal
BetaIne hydrochloride 453***
Wheat straw
60 1.3%wt 23.1
hydrolysis (40
%wt)
Chitin
Microwave-assisted (250 W) Bronsted acid catalyzed thermal
H2SO4 (2 M)
463 30 50 g/L 21.6
hydrolysis
Microwave-assisted (250 W) Bronsted acid catalyzed
Poplar sawdust HCI
(37%) - 11.5 meq 473 15 72.9 g/L 29.3
pressurized (30 bar, N2) thermal hydrolysis
Microwave-assisted (250 W) Bronsted acid catalyzed
Olive tree pruning HCI (37%) - 11.5 meq 473 15 72.9 g/L
20.1
pressurized (30 bar, N2) thermal hydrolysis
Chitosan Microwave-assisted Lewis acid-catalysed
thermal hydrolysis SnC14.5H20 (0.06 M) -- 473 -- 30 -- 25 g/L -- 23.9
* Levulinic acid yield was defined as: "Yield of levulinic acid (wt%) =
100x(levulinic acid content after reaction (g))/(Initial biomass content (g))"
"*Tabasso et al (2014) defined levulinic acid yield as: "Yield of levulinic
acid (wt%) = 100x(organic content in soluble fraction (g))/(Organic content in
the biomass
(g yr
*** The reaction was first conducted at 150 C to produce furfural and then at
180 C to produce levulinic acid.
49
File No.: P4015CA00
[00149] 3.3 LA production during screening study
[00150] During HCI catalysed thermo-hydrolysis, cellulose and hemicellulose
are
degraded into hexoses (e.g. glucose and fructose), the key intermediates in
the
production of LA. Hexoses are primarily dehydrated to 5-HMF, which is
accelerate by
Bronsted acid catalysts, and thereupon 5-HMF is rehydrated into LA with a
theoretical
yield of 64.5 wt% due to formic acid conjoint formation (Tarabanko et al.
Sodium
hydrosulfate as the catalyst for carbohydrate conversion into the levulinic
acid and 5-
hydroxymetylfurfural derivatives. Journal of Siberian Federal University, Vol.
1, 2008:
35-49). Pentoses produced via hemicellulose hydrolysis, such as xylose, can
also be
transformed in LA, but several separation steps are compulsory. This multi-
process
includes xylose dehydration to furfural, which is converted to furfuryl
alcohol (via gas
phase hydrogenation step) and finally to LA by means of a hydrolytic ring
opening
reaction (Hu et al. One-pot synthesis of levulinic acid/ester from C5
carbohydrates in a
methanol medium. ACS Sustainable Chemistry Engineering, Vol. 1, 2013: 1593-
1599).
[00151] LA production during the screening of the five agro-industrial
residues is
shown in Table 13. LA production of 204.4, 159.7, 66.4, 49.5 and 12.0 g/kg
were
recorded for BLW, BSG, APS, APUS and SIW, respectively. Accordingly, BSG and
BLW were selected for further optimisation tests of reaction time, HCI
concentration,
and feedstock concentration making use of RSM for higher LA production. Both
substrates exhibited the highest LA generation compared with the other
feedstock.
Moreover, they also contained some starch in their composition (Table 11),
which is
known to reach higher LA yields with milder treatment conditions in comparison
to pure
lignocellulosic biomasses (Morone et al, 2015). Owing to the content of TRS
(especially
glucose) and 5-HMF, LA production was expected to enhance with the improved
parameters.
CA 3013593 2018-08-08
File No.: P4015CA00
0
w Table 13. Characterization of the hydrolysis products
0
1-.
w
Levulinic acid
cri Feedstock TRS (g/kg) Glucose (g/kg) Xylose (g/kg) 5-
HMF (g/kg) Furfural (g/kg)
to
(g/kg)
w
n)
0 APS 258.8 53.9 9.4 6.7
24.3 66.4
1-.
co
1
0 APUS 336.8 143.9 Nd
12.6 8.4 49.5
co
1
0
co
BLW 123.1 34.1 Nd 7.6
6.5 204.4
SIW 253.3 146.2 Nd
35.8 6.1 12.0
BSG 141.2 32.7 Nd 8.2
48.2 159.7
Xylose depletion was attributed to the presence of HCI, since Br-misted acids
were recognised as relevant factor for the selective conversion of
xylose into furfural (Channnakid etal., 2014).
51
File No.: P4015CA00
[00152] 3.4 LA production enhancement utilizing BSG and BLW as
substrates
[00153] Table 14 represents the results of central composite design which
consist
of experimental data for studying the effect of three independent variables
(reaction time
(A), acid concentration (B) and feedstock concentration (C)) on LA production
from BSG
and BLW samples. LA production ranged from about 38.2 g/kg to a maximum 341.1
g/kg for BSG residue, while it oscillated from 45.9 g/kg to 409.3 g/kg for BLW
waste.
[00154] During beer production, three (bio)chemical reactions (mashing,
boiling,
fermentation and maturation) and other three solid-liquid separations (wort
separation,
wort clarification and rough beer clarification) are required, generating a
large amount of
solid residues (BSG) and wastewater (BLW), which management constitutes a
relevant
problem for the brewing industry (Fillaudeau et al., 2006). As shown in Table
14, the
present example demonstrates the potential of BLW and BSG for high LA
production
(409 g/kg and 341 g/kg) by means of microwave-assisted HCI-catalysed thermal
hydrolysis without prior special pretreatment, offering at the same time a
solution to the
prevailing environmental problem and a chance for balancing the books. A
comparison
with the results obtained by other authors using other alternative agro-
industrial wastes
and forestry residues (Table 12) place BLW and BSG in a highly ranked
position.
[00155] Regarding the optimized parameters, the utilization of microwave
heating
allowed to halve the time process from >1 h to less than 30 min. Apart from
process
time reduction, heating method has not offered additional improvements (e.g.
acid
concentration reduction) in the literature (Szabolcs et al. Microwave-assisted
conversion
of carbohydrates to levulinic acid: an essential step in biomass conversion.
Green
Chemistry, Vol. 15, 2013). With respect to HCI concentration, LA production
achieved a
maximum at HCI 4.5 N for both substrates and then decreased rapidly with the
further
rise of the acid concentration. A substrate concentration of 85 g/L resulted
in maximum
LA production from BSG and BLW samples, which could be considered a high
substrate
concentration in comparison with substrate concentrations as compared to other
samples, as shown in Table 12.
52
CA 3013593 2018-08-08
File No.: P4015CA00
Table 14 - Experimental design and the responses of BSG and BLW feedstock
obtained for the 20 different
experiments proposed
Factor 1 Factor 2 Factor 3 Response 1
Response 2
B: Acid
Levulinic acid
A: Time C: Substrate Levulinic acid from BSG
Test concentration from
BLW
(min) concentration (g/L) (g/kg)
(N) (g/kg)
1 15.00 2.00 50.00 112.6 133.1
2 27.50 4.50 143.86 132.5 156.0
3 27.50 4.50 85.00 341.1 409.3
4 27.50 4.50 85.00 341.1 409.3
27.50 4.50 85.00 341.1 409.3
6 27.50 4.50 85.00 341.1 409.3
7 15.00 7.00 50.00 87.7 102.2
8 27.50 0.30 85.00 205.4 236.4
9 48.52 4.50 85.00 96.1 105.3
40.00 2.00 50.00 107.9 119.5
11 6.48 4.50 85.00 91.5 105.7
12 40.00 2.00 120.00 147.2 165.7
13 27.50 4.50 85.00 341.1 409.3
14 27.50 4.50 85.00 341.1 409.3
40.00 7.00 120.00 103.7 114.4
16 15.00 7.00 120.00 96.1 105.9
17 27.50 8.70 85.00 94.1 110.9
18 27.50 4.50 26.14 38.2 45.9
19 15.00 2.00 120.00 85.4 98.4
40.00 7.00 50.00 56.1 77.3
EXAMPLE 4
PRETREATMENT OF WASTE BIOMASS
[00156] Brewery liquid waste (BLW), starch industry waste (SIVV), and
apple
pomace ultrafiltration sludge (APS) were semi-solid in nature while brewery
industry
spent grains (BSG), apple pomace solid waste (APS) were solid. To compare the
efficiency of the of different hydrolysis techniques to produce fermentable
sugars and
inhibitors, the dried weight of the all biomass was determined. All the
biomass was dried
at 60 C for 72 h prior to hydrolysis.
53
CA 3013593 2018-08-08
File No.: P4015CA00
[00157] 4.1 Different hydrolysis techniques to produce fermentable sugars
[00158] Dried waste biomass was pre-treated by using 11 different
hydrolysis
techniques:
[00159] (a) chemical: (1) Bronsted acid catalyzed using autoclave: 1 (M)
H2SO4 at
121 1 C for 40 minutes, 13 psi (89.6 kPa); (2) Alkali catalyzed using
autoclave: 1 (N)
NaOH at 121 1 C for 40 minutes, 13 psi (89.6 kPa), (pH= 10.0 0.1); (3) H202
catalyzed acid hydrolysis in autoclave: H202(30 v/v, 0.05mL) at 121 1 C for 40
minutes,
13 psi (89.6 kPa), (pH = 3-3.1 with H2SO4); Microwave assisted: (4) Bronsted
acid
catalyzed using microwave digester: 1 (M) H2SO4 at 121 1 C for 25 minutes,
1000W;
(5) Alkali catalyzed using microwave digester: 1 (N) NaOH at 121 1 C for 40
minutes,
(pH= 10.0 0.1), 1000W
[00160] (b) Nano spray drier particle catalyzed : (6) Fe nano particles
(NPs)
catalyzed inert condition acidic (pH = 3-3.1) at 121 1 C for 40 minutes, 13
psi (89.6
kPa) in autoclave; (7) Ca NPs catalyzed inert condition alkaline (pH= 10.0
0.1) at
121 1 C for 40 minutes, 13 psi (89.6 kPa) in autoclave; (8) Both Ca and Fe NPs
catalyzed inert at 121 1 C for 40 minutes, 13 psi (89.6 kPa) in autoclave;
[00161] (c) Hydrothermal: (9) Neutral pH at 121 1 C for 40 minutes, 13 psi
(89.6
kPa) in autoclave (10) Neutral pH at 121 1 C for 25 minutes, 1000W in
microwave
digester;
[00162] (d) Mechanical: (11) ultra-sonication.
[00163] Prior to carrying out agro-industrial waste hydrolysate (AWH)
fermentation
as sole substrate, investigations were made to perform characterization of the
complex
agro-industrial waste biomass to ascertain the ability of Clostridium
beijerinckii B-466 to
ferment representative sugars present in the AWH. The physicochemical
characterization components which are really important for bio-butanol
production have
been thoroughly investigated and reported in Table 11 above for all the
considered
waste biomass. It can be seen that unlike BSG and SIW, APUS, APS and BLW are
already enriched with free reducing sugars.
54
CA 3013593 2018-08-08
File No.: P4015CA00
[00164] 4.2 Comparisons of different hydrolysis techniques in the
production of fermentable sugars and fermentation inhibitors from different
agro-
industrial wastes
[00165] Different hydrolysis techniques such as chemical, hydrothermal,
mechanical and nanoparticles catalyzed techniques have been explored to
enhance the
fermentable reducing sugars, and the results of a comparative study of
reducing sugar
producing efficiency of these different hydrolysis techniques from agro-
industrial wastes
are presented in Table 15.
[00166] 4.3 Brewery industry wastes
[00167] In brewery industry after separation of wort, the residue left is
called
brewery spent grains (BSG) (Olajire, 2012; Macheiner et al., 2003;). It
contains on dry
weight basis about (50-62) % polysaccharide (Table 11). Brewery industry
liquid waste
(BLVV), which is semi-solid in nature, came at the final stage after second
fermentation
(Olajire, 2012). It is enriched with free reducing sugar and yeast proteins
additional with
higher polysaccharide content.
[00168] Depending on the process condition cellulose and hemicellulose have
been degraded in different reducing sugars such as glucose, galactose, xylose
etc. Due
to their typical lignocellulosic composition where outer lignin entirely
covers and bounds
inner polysaccharides, acid catalyzed hydrolysis is more promising over other
techniques employed, as shown in the results of Table 15. Solubilization of
hemicellulose was favored in lower pH compared to higher, alkali catalyzed
hydrolysis,
as acid catalyzed hydrolysis facilitated the breakdown of glycosidic bonds.
However,
hydrothermal, NPs catalyzed and mechanical techniques were proved to be less
efficient in hemicellulose rich biomass. Acid catalyzed hydrolysis
significantly reduced
recalcitrance of lignocellulosic biomass. Moreover, microwave assisted acid
catalyzed
and acid catalyzed-autoclave conditioned hydrolysis have been analyzed with
different
results. Same acid strength 1 N catalyzed hydrolysis in autoclave proved to be
more
promising than microwave assisted one due to less further conversion of
polysaccharides to reducing sugars and to fermentation inhibitors as shown in
Table 15,
CA 3013593 2018-08-08
File No.: P4015CA00
which presents the results of a comparative study of fermentation inhibitors
production
with different hydrolysis techniques applied to agro-industrial wastes.
[00169] 4.4 Apple industry wastes
[00170] Both apple pomace solid (APS) and apple pomace ultrafiltration
sludge
(APUS) are rich sources of carbohydrates, minerals, vitamins and dietary
fibers. These
can be exploited for the production of biobutanol. Easily biodegradable, high
organic
load containing apple industry wastes are produced worldwide in huge amount
and
these wastes must be managed in a right way to avoid noxious environmental
effects
(Dhillon et al., 2013). However, unlike brewery industry wastes these wastes
were
enriched with fructose. As shown in Table 11, APS is mainly composed of
cellulose as
polysaccharide, with more aldohexose compared to aldopentose. There was no
hemicellulose analyzed in APUS.
[00171] The degradation of cellulose is thermally accelerated and acid
catalyzes
chain scission mechanism (Hu et al., 2012). NPs catalyzed hydrolysis, which
has been
previously reported to be successful in hydrolysis of crystalline cellulose
(Feng et al.
Solid-and nano-catalysts pretreatment and hydrolysis techniques. Pretreatment
Techniques for Biofuels and Biorefineries, Green Energy and Technology Series,
Springer, 2013), was not effective here. Since the susceptibility of cellulose
over
different pH range has been reported to be different and more efficient in
lower pH, thus
acid catalyzed hydrolysis proved more promising over other methods. Microwave
assisted hydrothermal method has also proved to be near as effective as acid
catalyzed
hydrolysis for total reducing sugar production as shown in Table 15. Moreover,
as
shown in Table 16, microwave assisted hydrothermal method has also proved to
produce less fermentation inhibitors as compared with acid catalyzed
hydrolysis.
[00172] 4.5 Starch industry wastes
[00173] Starch, second largest compound produced by plant next to
lignocellulose,
is found in waste materials produced from the processing of plant raw
materials (Jin et
al. Utilisation of starch processing wastewater for production of microbial
biomass
protein and fungal a-amylase by Aspergillus oryzae. Bioresource technology,
Vol. 66(3),
1998: 201-206; Rakshit. Utilization of starch industry wastes. Bioconversion
of Waste
56
CA 3013593 2018-08-08
File No.: P4015CA00
Materials to Industrial Products, Springer, 1998). Unlike cellulose (13 1-4
glycosidic
linkage polysaccharide), the starch (a 1-4 glycosidic linkage polysaccharide)
is reported
to be hydrolyzed very easily (Rakshit, 1998). The physicochemical
characterization of
starch industry waste (SIVV) is shown in Table 11.
[00174] As
compared to acid catalyzed methods, other hydrolysis methods proved
to be less effective as shown in Table 15. However, microwave assisted acid
catalyzed
hydrolysis method proved to be more promising as compared to acid-autoclave
hydrolysis method. Moreover, as shown in Table 16, except for alkali-autoclave
hydrolysis method, all other hydrolysis methods generated a low concentration
of
inhibitors.
57
CA 3013593 2018-08-08
File No.: P4015CA00
Table 15- Comparative study of fermentable reducing sugars producing
efficiency of different hydrolysis techniques from agro-industrial
0
wastes
ua
o
1-.
(A)
01 BLW(g/kg) BSG(g/kg)
APS(g/kg) APUS(g/kg) SIW(g/kg)
to
ta u)
I'.) *2 S' c a)
u) (i) a) a)
(1)
Cl) a) Cl) a) Cl) a)
o 2.N. .2' 2 a)
Cl) o o
1-. 2 f -.'ct 0 - t - a) X 8 - a3 - t -
c:C u) a) X (i) X cn
v) ---,)
o --- cn --- 0-6)-
0 (3) 03 0) TO C?) 0 CI 0
C3) a, C3) TO 'E'7) 0 0) CO TO r.:Y-') 0 CO
= 0 CO -0 0 Cl) -2-3
0 .y ....
I >.a) ----. 3,-
o I H H H cr) 5 :62 0 ,!_:.-') x 27 1-2
.c.:1) 5 2.2 >><" 22 0 22
co
cl) a.)
co >
as 375.12 175.56 128.11
329.90 196.97
197.502 148.502 19.536 468.214
52.945 375.122 94.502 19.536 611.011 185.218
="0-- 78 2 2 2
6 3
<((3
..
a)
>
co 290.91
56038
+ µ`-' T5 85.61 85.61 1.0412 53.895 2.328
0.3225 1.524 290.911 85.61 1.0412 378.490 190.868
. 2.07
7
7 O21
(7) N 5
2 < i as
C
_
a.) . _
_
E
ri
El_) 244.36
152.49
78.348 78.348 0.266 37.493 2.158 ND ND 244.365 78.348 0.266 628.062 202.827
2.842
"ru = ,
03 D
8
.0 ..
E .TC
a) _ -
_c
0
a)
>
co 360.73
359.31 246.20
+ ,-, 122.18 122.18 ND 413.400 146.91 97.45 26.789
360.730 122.18 ND 336.844 143.893
:r) 2 u
3 2
r.) .,2
<
-
a)
>
+ a)
199.65 62.010
.12 59.866 2.475 ND ND 199.657 ND 1.12
299.638 78.127 1.901
0 2 7
0
co .,2
Z 2
, -o
a) co
(i) N a-
255.38 17179
EL >, u) 1.603 1.603 0.522 122.182 10.428 ND ND
255.389 1.603 0.522 335.234 56.235 . 3.318
z 03 z
9 0
CO co
O 0
58
File No.: P4015CA00
295.19
264.57
0 0, 4.183 4.183 1.736 57.876 8.7175 1.965 0.078
295.198 4.183 1.736 353.651 63.503 17.733
8
6
a) cr)
0
I-
(A) .
01
l0
(A) CO
0- 131.98
138.25
7.79 7.79 0.233 36.134 0.681 0.1575 ND
131.981 7.79 0.233 256.567 43.671 2.253
o z 1
9
1-. a)
CO Li-
0- I _
,
CO
oI TO
.0 C
CO E o 333.84
100.48 120.56
. -
OS a) 3 100.486 100.486 ND 180.567
22.545 ND ND 333.843 ND 520.118 197.102 2.545
6
7
0 _co . ,02 .-
a.) - c
, a)
>
104.53
co4904.51
104.532 104.532 ND 208.235 33.168 ND ND 404.519
ND 631.277 186.781 128 3.168
2 S' "
a) 2
z
>)
To -='= ca 7 229.97
E E () 71.308 71.308 0.3575 32.469 0.658 ND ND
229.973 71.308 0.3575 597.899 199.557 32.469 0.658
(D = -
H Z<
59
File No.: P4015CA00
0
Table 16 - Comparative study of fermentation inhibitors production with
different hydrolysis techniques applied to agro-industrial wastes
w
0
1-.
ta
cri
to BLW(g/kg) BSG(g/kg)
APS(g/kg) APUS(g/kg) SIW(g/kg)
ta
.
in
n.) =/9-) S' c -:_f T2. .0
.0 .0 .0 .0
c u_ 7,2
c u_ Tli c u_ 7S? c
E u_ (s c
=
= =
co to 2 7 7 1- 7 t- 2
..?. t- 2 = z 2 7 7
oI
>Q) 2 't
a) ?..-) 't
7
a) (7) '5
> :0 =
a) 0
't
= > :0
a) 0
= Lt
m > 70
I 1-- I-- in u_ _..1 co IA u_ _1 co 1.1) U..
...-I CD 1.2) LL J (13 6 LL J CD
CO
oI .
.
CO a)
co> T, 21.346 1.65 9.525 3.17 11.45 2.75 45.503.125 13.85 37.575 1.263 19.25
2.689 1.561 0.007
-5
< C13
a)
as>
+ - T.) 16.927 0.725 0.375 0.168 0.375
0.375 2.875 0.625 0.375 2.608 0.421 0.375 ND 0.725
0.198
7 d i 12
l-J N =
0 < 1 0
C
a)
E
'cis
(2
:= 0.145 0.375 0.375 0.218 0.375 0.375 0.312 0.375
0.375 0.92 2.825 0.375 120.401 3.240 12.290
To co
0
._
(V.) ;FC
_ .
C..) co
c>
+ g 29.736 1.780
4.008 13.589 48.592.921 17.41 4.601 0.375 0.307 0.120 ND 3.582
6.094 0.201
:0 t5 2
(7) -
< 2
a)
>
+ fl3
i 6 ND 0.057 0.066 0.299 0.205 0.018 0 0.431
0.139 0 0.430 0.138 ND 0.162 ND
Cc)o t)
z 2
. .
0
a) ca
cn N CL
a. ND 0.081 ND 0.597 2.366 0.062 0.135 <0.375
0.375 0.215 <0.375 <0.375 ND 1.062 0.285
z vo z
co co
0 (..)
File No.: P4015CA00
0
0 o) 0.782 0.656 ND 0.695 0.475 0.375 0.147
<0.375 0.95 0.354 <0.375 0.45 ND 5.603 ND
U.)
0
Fn
U.) _
01
l0
a. 048
I'.) u) 0.491 0.375 0.375 0.3818 0.
0.0790 0.126 <0.375 2.2 <0.375 <0.375 0.1345 ND 0.128 ND
o z 2
1-= a)
co L.L.
o1 .
co 713
oI .0
C C
CO _c CO 0
.=. ND 0.239 0.375 ND 0.239 0.375 ND 0.089 ND
3.810 2.275 0.375 ND 0.239 0.019
cu
o
M
Fii
_
-
-
a)
>
==-. La
E 8 ND 0.259 0.375 ND 0.259 0.375 1.698 6.747 ND
12.445 1.246 ND ND 0.259 ND
Ta a) 2
z
E
-c
- >
ND ND ND ND ND ND
0.312 0.375 0.375 NF 0.047 ND ND ND ND
- o
c
a) "5
z<
61
File No.: P4015CA00
[00175] While
preferred embodiments have been described above and illustrated
in the accompanying drawings, it will be evident to those skilled in the art
that
modifications may be made without departing from this disclosure. Such
modifications
are considered as possible variants comprised in the scope of the disclosure.
62
CA 3013593 2018-08-08