Note: Descriptions are shown in the official language in which they were submitted.
I I
CA 03014400 2018-08-13
TITLE OF INVENTION:
OXOCARBON COMPOUND, RESIN COMPOSITION, AND INK COMPOSITION
TECHNICAL FIELD
[0001]
The present invention relates to an oxocarbon compound having a squarylium
skeleton or a croconium skeleton, and a resin composition and an ink
composition
containing the same.
BACKGROUND ART
[0002]
Oxocarbon compounds having a squarylium skeleton and a croconium skeleton
are known as dyes having an absorption region in a visible to near-infrared
range, and
can be synthesized by using squaric acid or croconic acid as a raw material
and
introducing heterocyclic rings on both sides of the raw material. As such
oxocarbon
compounds, the following compounds are known, for example.
[0003]
Patent Literature 1 discloses the following squarylium compound (see Example
2 of Patent Literature 1).
[0004]
[Chemical Formula 1]
N
N H
-0
[0005]
Patent Literature 2 discloses the following squarylium compound and
croconium compound (see Examples 14 and 17 of Patent Literature 2).
[0006]
[Chemical Formula 2]
1
I I
CA 03014400 2018-08-13
\N 0-
1101 N
-0
[0007]
[Chemical Formula 3]
0-
N 0 N
0 O-
S [0008]
Patent Literature 3 discloses the following squarylium compound (see Example
1 of Patent Literature 3).
[0009]
[Chemical Formula 4]
0- Bu
=
111
Bu -0
[0010]
Non-patent Literature 1 discloses the following squarylium compounds.
[0011]
[Chemical Formula 5]
0-
HN \
D N H
------
-0
[0012]
Non-patent Literature 2 discloses the following croconium compounds.
[0013]
2
CA 03014400 2018-08-13
[Chemical Formula 6]
0-
H H
N 0 N
R =
R
0 0-
[0014]
Dyes having an absorption region in a near-infrared region have been studied
for applying to various applications and are expected to be used as a security
ink, a
near-infrared cutoff filter and others.
[0015]
A security ink is used for printing encrypted information (e.g., barcode,
two-dimensional code, OCR letters, and the like) in banknotes, cash vouchers,
securities,
lottery tickets or the like for the purpose of forgery prevention, or used for
printing
delivery information or the like on letters and baggage for system efficiency.
As
means for reading the information printed by the security ink, light emitting
elements
such as various lasers and LEDs having emission wavelengths in the range of
850 nm to
1300 nm are usually used, and therefore, dyes used for the security ink are
required to
have a strong absorption in such a wavelength range and have excellent
invisibility
under visible light. For example, Patent Literatures 4 and 5 disclose an ink
containing
a phthalocyanine compound or a naphthalocyanine compound as a near-infrared
absorbing dye, and Patent Literature 6 discloses a phthalocyanine compound
having a
maximum absorption wavelength at around 900 run.
[0016]
A near-infrared cutoff filter is installed, for example, in an image sensing
device such as CCD (Charge Coupled Device) and CMOS (Complementary
Metal-Oxide Semiconductor), whereby optical noise (e.g., ghost and flare) can
be
removed. For example, as such an optical filter, Patent Literature 3 discloses
a
near-infrared cutoff filter containing a squarylium compound having an
absorption
maximum wavelength in a wavelength range of 660 rim to 710 nm. In an ordinary
image sensing device for visible light, an optical filter containing a dye
having a
3
I I
CA 03014400 2018-08-13
maximum absorption wavelength in a wavelength range of around 700 nm is
installed,
whereby incidence angle dependence of optical characteristics is reduced and
viewing
angle property can be improved.
[0017]
Meanwhile, some image sensing devices are required to be photographed under
night vision as in a surveillance camera, in contrast to general digital
cameras and video
cameras. Photographing under night vision, as in nighttime, is performed in a
state
invisible by human eyes, so it is performed by irradiating near-infrared light
and
receiving the reflected light with an image sensing device, for example. Also
in the
image sensing device for night vision, it is required to reduce the incidence
angle
dependence of optical characteristics, similarly to an ordinary image sensing
device for
visible light; however in the image sensing device for night vision, it is
difficult to
reduce the incident angle dependence by using a near-infrared cutoff filter
for visible
light and it is needed to provide a cutoff filter which absorbs light of a
wavelength
region within the range of 800 nm to 1000 nm, for example. In this case as
well, the
filter is required to contain a dye having a strong absorption in such a
wavelength region
and having a high light transmittance in a visible light region, whereby well
image
photographing both under visible light and under night vision is realized.
CITATION LIST
PATENT LITERATURE
[0018]
PATENT LITERATURE 1
Japanese Unexamined Patent Application Publication No. 2008-308602
PATENT LITERATURE 2
Japanese Unexamined Patent Application Publication No. HO6-25165
PATENT LITERATURE 3
Japanese Unexamined Patent Application Publication No. 2014-148567
PATENT LITERATURE 4
Japanese Unexamined Patent Application Publication No. H07-164729
4
PATENT LITERATURE 5
Japanese Unexamined Patent Application Publication No. 2002-309131
PATENT LITERATURE 6
Japanese Unexamined Patent Application Publication No. 2007-56105
NON-PATENT LITERATURE
[0019]
NON-PATENT LITERATURE 1
Miltsov et al., "New Cyanine Dyes: Norindosquarocyanines", Tetrahedron
Letters, 40 (1999): 4067-4068
NON-PATENT LITERATURE 2
Encinas et al., "Croconines: new acidochromic dyes for the near infrared
region", Tetrahedron Letters, 43 (2002): 8391-8393
SUMMARY OF INVENTION
TECHNICAL PROBLEM
[0020]
As described above, a near-infrared absorbing dye has been studied for
applying to a security ink, a near-infrared cutoff filter and the like, and in
order to
further expand its application, a dye capable of effectively absorbing light,
for
example, in a wavelength range exceeding 850 nm is required. However,
oxocarbon
compounds such as a squarylium compound and a croconium compound, being
capable of absorbing light in such a long wavelength region, have not been
known.
Meanwhile, as disclosed in Patent Literature 6, a phthalocyanine compound
which is
capable of absorbing light in a wavelength range around 900 nm is known,
however, a
phthalocyanine compound absorbs light in a visible light region and shows, for
example, green color, and so there was room for improvement in terms of
invisibility.
[0021]
The present invention has been achieved in view of the above circumstances,
and an object of the present invention is to provide an oxocarbon compound
which is
capable of absorbing near-infrared light on a longer wavelength side and
having high
light transmittance in a visible light region, and a resin composition and an
ink
composition containing the same.
CA 3014400 2020-02-20
CA 03014400 2018-08-13
SOLUTION TO PROBLEM
[0022]
The oxocarbon compound of the present invention which solves the above
problems is an oxocarbon compound represented by the following formula (1) or
(2).
[0023]
[Chemical Formula 7]
0- 0-
R1 0 R2 R3
0 R4
0- 0 0-
(1) (2)
In the formulas (1) and (2), R1 to R4 each independently represent a
structural
unit represented by the following formula (3).
[0024]
[Chemical Formula 8]
R5 R6 R7
A \
NH
(3)
In the formula (3), ring A represents an aromatic hydrocarbon ring optionally
having a substituent, an aromatic heterocyclic ring optionally having a
substituent, or a
condensed ring containing these ring structures optionally having a
substituent; R5 to R7
each independently represent hydrogen atom, an organic group or a polar
functional
group, or R5 and R6 are linked to each other to form a ring; * represents a
bonding site
to a 4-membered ring in formula (1) or a 5-membered ring in formula (2); and a
total
number oft electrons contained in the ring A and R7 is 12 or more.
[0025]
The oxocarbon compound of the present invention is composed so that it
6
CA 03014400 2018-08-13
electron system spreads from a squarylium skeleton or a croconium skeleton to
a
structural unit represented by the formula (3) and the total number of it
electrons
contained in the ring A and the substituent R7 in the structural unit of the
formula (3) is
12 or more, and therefore, it can effectively absorb near-infrared light on a
longer
wavelength side, and for example, absorb light in the wavelength range
exceeding 850
nm. Meanwhile, the oxocarbon compound itself has high light transmittance in a
visible light region, so that it is excellent in invisibility under visible
light.
[0026]
In the above formula (3), it is preferable that: R5 and R6 each independently
represent hydrogen atom, an alkyl group optionally having a substituent, an
aryl group
optionally having a substituent, an aralkyl group optionally having a
substituent, an
alkoxycarbonyl group optionally having a substituent or an aryloxycarbonyl
group
optionally having a substituent, or R5 and R6 are linked to each other to form
a
hydrocarbon ring optionally having a substituent ancUor a condensed ring
structure, or a
heterocyclic ring optionally having a substituent and/or a condensed ring
structure; and
R7 represents hydrogen atom, an alkyl group optionally having a substituent or
an aryl
group optionally having a substituent.
[0027]
The present invention also provides a resin composition comprising the
oxocarbon compound of the present invention and a resin component. The resin
composition may further comprise a liquid medium. The resin composition of the
present invention can be suitably applied to an optical filter such as an
image sensing
device for night vision by forming a film, and can be also suitably applied to
an image
sensing device used under visible light since it has high transmittance in a
visible light
region. The resin composition of the present invention can be also suitably
used for a
welding resin by a laser welding method, and it can be used to be contained in
a resin to
be welded or used as an absorber of laser light, for example.
[0028]
The present invention also provides an ink composition comprising the
oxocarbon compound of the present invention and a liquid medium. Since the ink
7
oxocarbon compound of the present invention and a liquid medium. Since the ink
composition of the present invention absorbs light in a near-infrared region
and is
excellent in invisibility under visible light, it can be suitably used as a
security ink.
[0029]
The present invention further provides a condensed heterocyclic compound
represented by the following formula (5) and a process for producing an
oxocarbon
compound comprising the step of reacting a condensed heterocyclic compound
represented by the following formula (5) with squaric acid or croconic acid to
obtain
the oxocarbon compound represented by the above formula (1) or the above
formula
(2). The condensed heterocyclic compound represented by the following formula
(5)
can be suitably used as a raw material for producing the oxocarbon compound of
the
present invention.
[0030]
[Chemical Formula 9]
R5 R6
CH2R7
A \
(5)
In the formula (5), ring A represents an aromatic hydrocarbon ring optionally
having a substituent, an aromatic heterocyclic ring optionally having a
substituent, or
a condensed ring containing these rings optionally having a substituent; 125
to R7 each
independently represent hydrogen atom, an organic group or a polar functional
group,
or R5 and R6 are linked to each other to form a ring; and a total number of TC
electrons
contained in the ring A and R7 is 12 or more.
[0030a]
In yet another aspect, the present invention provides an oxocarbon compound
represented by the following formula (1) or (2):
0- 0-
R1 0 R2 R3 0 R4
0- 0 0-
(1) (2)
8
CA 3014400 2020-02-20
wherein R1 to R4 each independently represent a structural unit represented by
the following formula (3):
R5 R6 R7
*
A \ NH
(3)
wherein ring A represents an aromatic hydrocarbon ring optionally having a
substituent, an aromatic heterocyclic ring optionally having a substituent, or
a
condensed ring containing these ring structures optionally having a
substituent,
wherein the substituent is selected from the group consisting of an alkyl
group, an
alkoxy group, an alkylthio group, an alkoxycarbonyl group, an alkylsulfonyl
group,
an aryl group, an aralkyl group, an aryloxy group, an arylthio group, an
aryloxycarbonyl group, an arylsulfonyl group, an arylsulfinyl group, a
heteroaryl
group, an amido group, a sulfonamido group, an ethylene-containing group, an
imine-
containing group, carboxy group, a benzothiazole group, a halogenoalkyl group,
cyano group, a halogeno group, hydroxyl group, nitro group, an amino group and
sulfo group,
R5 to R7 each independently represent hydrogen atom, an organic group or a
polar functional group, or R5 and R6 are linked to each other to form a ring,
wherein
the organic group is selected from the group consisting of an alkyl group, an
alkoxy
group, an alkylthio group, an alkoxycarbonyl group, an allcylsulfonyl group,
an aryl
group, an aralkyl group, an aryloxy group, an arylthio group, an
aryloxycarbonyl
group, an arylsulfonyl group, an arylsulfinyl group, a heteroaryl group, an
amido
group, a sulfonamido group, an ethylene-containing group, an imine-containing
group, carboxy group, a benzothiazole group, a halogenoalkyl group and cyano
group,
and the polar functional group is selected from the group consisting of a
halogeno
group, hydroxyl group, nitro group, an amino group and sulfo group,
* represents a bonding site to a 4-membered ring in formula (1) or a 5-
membered ring in formula (2), and
a total number of it electrons contained in the ring A and R7 is 12 or more.
8a
CA 3014400 2020-02-20
[0030b]
In yet another aspect, the present invention provides a condensed heterocyclic
compound represented by the following formula (5):
R5 R6
CH2R7
A \
(5)
wherein ring A represents an aromatic hydrocarbon ring optionally having a
substituent, an aromatic heterocyclic ring optionally having a substituent, or
a
condensed ring containing these rings optionally having a substituent,
R5 represents an alkyl group optionally having a substituent, an aryl group
optionally having a substituent, an aralkyl group optionally having a
substituent, an
alkoxycarbonyl group optionally having a substituent, or an aryloxycarbonyl
group
optionally having a substituent, R6 represents an aryl group optionally having
a
substituent, an aralkyl group optionally having a substituent, an
alkoxycarbonyl group
optionally having a substituent, or an aryloxycarbonyl group optionally having
a
substituent, or R5 and R6 are linked to each other to form a hydrocarbon ring
optionally having a substituent and/or a condensed ring structure or a
heterocyclic
ring optionally having a substituent and/or a condensed ring structure,
R7 represents hydrogen atom, an organic group or a polar functional group,
the substituent is selected from the group consisting of an alkyl group, an
alkoxy group, an alkylthio group, an alkoxycarbonyl group, an alkylsulfonyl
group, an
aryl group, an aralkyl group, an aryloxy group, an arylthio group, an
aryloxycarbonyl
group, an arylsulfonyl group, an arylsulfinyl group, a heteroaryl group, an
amido
group, a sulfonamido group, an ethylene-containing group, an imine-containing
group,
carboxy group, a benzothiazole group, a halogenoalkyl group, cyano group, a
halogeno group, hydroxyl group, nitro group, an amino group and sulfo group,
the organic group is selected from the group consisting of an alkyl group, an
alkoxy group, an alkylthio group, an alkoxycarbonyl group, an alkylsulfonyl
group,
an aryl group, an aralkyl group, an aryloxy group, an arylthio group, an
aryloxycarbonyl group, an arylsulfonyl group, an arylsulfinyl group, a
heteroaryl
group, an amido group, a sulfonamido group, an ethylene-containing group, an
imine-
8b
CA 3014400 2020-02-20
containing group, carboxy group, a benzothiazole group, a halogenoalkyl group
and
cyano group,
the polar functional group is selected from the group consisting of a halogeno
group, hydroxyl group, nitro group, an amino group and sulfo group, and
a total number of it electrons contained in the ring A and R7 is 12 or more.
[0030c]
In yet another aspect, the present invention provides a process for producing
an oxocarbon compound comprising the step of reacting a condensed heterocyclic
compound represented by the following formula (5):
R5 R6
CH2R7
A \
(5)
wherein ring A represents an aromatic hydrocarbon ring optionally having a
substituent, an aromatic heterocyclic ring optionally having a substituent, or
a
condensed ring containing these rings optionally having a substituent, wherein
the
substituent is selected from the group consisting of an alkyl group, an alkoxy
group,
an alkylthio group, an alkoxycarbonyl group, an alkylsulfonyl group, an aryl
group,
an aralkyl group, an aryloxy group, an arylthio group, an aryloxycarbonyl
group, an
arylsulfonyl group, an arylsulfinyl group, a heteroaryl group, an amido group,
a
sulfonamido group, an ethylene-containing group, an imine-containing group,
carboxy group, a benzothiazole group, a halogenoalkyl group, cyano group, a
halogeno group, hydroxyl group, nitro group, an amino group and sulfo group,
R5 to le each independently represent hydrogen atom, an organic group or a
polar functional group, or IV and R6 are linked to each other to form a ring,
wherein =
the organic group is selected from the group consisting of an alkyl group, an
alkoxy
= group, an alkylthio group, an alkoxycarbonyl group, an alkylsulfonyl
group, an aryl
group, an aralkyl group, an aryloxy group, an arylthio group, an
aryloxycarbonyl
group, an arylsulfonyl group, an arylsulfinyl group, a heteroaryl group, an
amido
group, a sulfonamido group, an ethylene-containing group, an imine-containing
group, carboxy group, a benzothiazole group, a halogenoalkyl group and cyano
group,
and the polar functional group is selected from the group consisting of a
halogeno
8c
CA 3014400 2020-02-20
group, hydroxyl group, nitro group, an amino group and sulfo group, and
a total number oft electrons contained in the ring A and R7 is 12 or more,
with squaric acid or croconic acid to obtain an oxocarbon compound
represented by the following formula (1) or the following formula (2):
0- 0-
R1 0 R2 R3 R4
0- 0 0-
(1) (2)
wherein RI to R4 each independently represent a structural unit represented by
the following formula (3):
R5 R6 R7
*
A \ NH
(3)
wherein ring A and R5 to R7 each have the same meaning as described above,
* represents a bonding site to a 4-membered ring in formula (1) or a 5-
membered ring in formula (2), and
a total number of it electrons contained in the ring A and R7 is 12 or more.
ADVANTAGEOUS EFFECTS OF INVENTION
[0031]
The oxocarbon compound of the present invention and the resin composition
and the ink composition containing the same can absorb near-infrared light on
a
longer wavelength side, and for example, absorb light in the wavelength range
exceeding 850
8d
CA 3014400 2020-02-20
CA 03014400 2018-08-13
nm, as well as they have high light transmittance in a visible light region,
so that they
are excellent in invisibility under visible light.
BRIEF DESCRIPTION OF THE DRAWINGS
[0032]
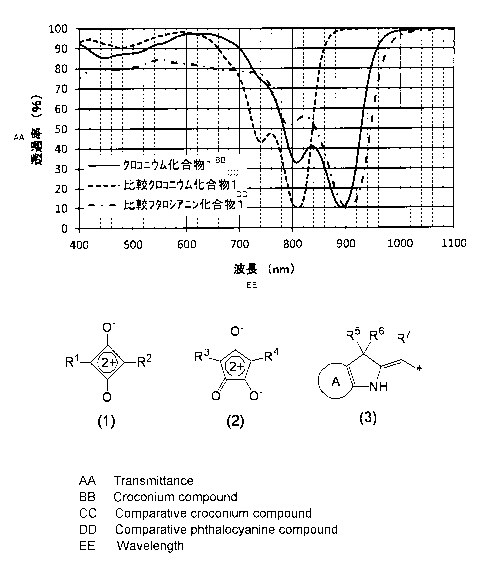
Fig. 1 shows transmittance spectra of a croconium compound 1, a comparative
croconium compound 1 and a comparative phthalocyanine compound 1 used in
Examples.
DESCRIPTION OF EMBODIMENTS
[0033]
[1. Oxocarbon compound]
An oxocarbon compound of the present invention is represented by the
following formula (1), which has a squarylium skeleton, or the following
formula (2),
which has a croconium skeleton.
[0034]
[Chemical Formula 101
0- 0-
R1 0 R2 R3
0 R4
0- 0 0-
(1) (2)
[0035]
In the formulas (1) and (2), R' to R4 each independently represent a
structural
unit represented by the following formula (3).
[0036]
[Chemical Formula 11]
9
CA 03014400 2018-08-13
R5 R6 R7
*
\
NH
(3)
[0037]
In the formula (3), ring A represents an aromatic hydrocarbon ring optionally
having a substituent, an aromatic heterocyclic ring optionally having a
substituent, or a
condensed ring containing these ring structures optionally having a
substituent; R5 to R7
each independently represent hydrogen atom, an organic group or a polar
functional
group, or R5 and R6 are linked to each other to form a ring; * represents a
bonding site
to a 4-membered ring in formula (1) or a 5-membered ring in formula (2); and a
total
number of it electrons contained in the ring A and R7 is 12 or more.
[0038]
In the formulas (1) and (2), RI and R2 may be the same or different from each
other, and R3 and R4 may be the same or different from each other. When RI and
R2
are different from each other or R3 and R4 are different from each other,
association or
aggregation between molecules of the oxocarbon compound are suppressed, and
improvement in solubility in solvents or resins is expected. Meanwhile, when
RI and
R2 are the same or when R3 and R4 are the same, improvement of durability
against heat
or light of the oxocarbon compound is expected.
[0039]
In some cases, there exists compounds having resonance relation to each other
in compounds having a squarylium skeleton (hereinafter referred to as
"squarylium
compound") and compounds having a croconium skeleton (hereinafter referred to
as
"croconium compound"). Examples of compound having resonance relation to the
squarylium compound of the formula (1) include, for example, compounds
represented
by the following formulas (la) and (lb). Examples of compound having resonance
relation to the croconium compound of the formula (2) include, for example,
compounds represented by the following formulas (2a) to (2c). The oxocarbon
t
CA 03014400 2018-08-13
compound of the present invention includes all these compounds having
resonance
relation; and specifically, the squarylium compound of the formula (1)
includes
compounds having resonance relation to that such as compounds represented by
the
following formulas (la) and (lb), and the croconium compound of the formula
(2)
includes compounds having resonance relation to that such as compounds
represented
by the following formulas (2a) to (2c).
[0040]
[Chemical Formula 12]
0 HN 0 g HN 0 0-
+HN
R5 R6
R6 R7 R7 R7 1
/ R6R5
R5 R6 / <I> R 6R5 R5 6
R7
/ = R7 R R5 R7
-õ,-
N NH+ 0- NH 0- ci NH 0
A A
(1 a) (1) (lb)
[0041]
[Chemical Formula 13]
P6 R7 0- R7 R6
R-g R5 R5 R6 R7 - R7
R6 R5
V a--
NH +HN 0 NH
0 0-
HN
0 0
(2a) (2)
R5 R6 R7 R7 R6 R5 R5 R6 R7 R7 R6 R5
/
*---
\
0 NH+ -
HN 0
NH -0 0 +HN
0 0
(2b) (2c)
[0042]
In the structural unit represented by the formula (3) that is bonded to the
squarylium skeleton or the croconium skeleton, * represents a bonding site to
a
4-membered ring of the squarylium skeleton represented by the formula (1) or a
5-membered ring of the croconium skeleton represented by the formula (2).
[0043]
In the oxocarbon compound of the present invention, the total number of 7t
11
CA 03014400 2018-08-13
electrons contained in the ring A and the substituent R7 is 12 or more. When
the It
electron system is formed in this manner, the oxocarbon compound can
effectively
absorb near-infrared light on a longer wavelength side, and for example,
absorb light in
the wavelength range exceeding 850 nm. Meanwhile, the oxocarbon compound shows
high light transmittance in a visible light region, so that it is excellent in
invisibility
under visible light. In the case where the oxocarbon compound of the present
invention absorbs light in a wavelength range exceeding 850 nm, the oxocarbon
compound does not necessarily absorb light in the entire wavelength range
exceeding
850 nm, and for example, it may absorb light in a part of the wavelength range
of 850
nm to 1300 nm in a certain degree.
[0044]
In the formula (3), the ring A represents an aromatic hydrocarbon ring, an
aromatic heterocyclic ring or a condensed ring containing these ring
structures, and
these rings may have a substituent. When the oxocarbon compound has the ring
A, the
It electron system spreads over a wide range from the squarylium skeleton or
the
croconium skeleton to the ring A via a pyrrole ring, whereby lengthening of
the
absorption wavelength can be realized.
[0045]
The aromatic hydrocarbon ring of the ring A is not particularly limited as
long
as it consists of carbon atom and hydrogen atom and has aromaticity; and
examples
thereof include, for example, a benzene ring, a naphthalene ring, a
phenanthrene ring, an
anthracene ring, a tetracene ring, a fluoranthene ring, a benzofluoranthene
ring, a
cyclotetradecaheptaene ring, and others. The aromatic hydrocarbon ring may
have
only one ring structure or condensed two or more ring structures. The aromatic
heterocyclic ring of the ring A is not particularly limited as long as it has
a ring structure
containing at least one atom selected from N (nitrogen atom), 0 (oxygen atom)
and S
(sulfur atom) and has aromaticity; and examples thereof include, for example,
a furan
ring, a thiophene ring, a pyrrole ring, a pyrazole ring, an oxazole ring, a
thiazole ring, an
imidazole ring, a pyridine ring, a pyridazine ring, a pyrimidine ring, a
pyrazine ring, a
purine ring, a pteridine ring, and others. The aromatic heterocyclic ring may
have only
12
CA 03014400 2018-08-13
one ring structure or condensed two or more ring structures. The condensed
ring
containing these ring structures has a structure in which an aromatic
hydrocarbon ring
and an aromatic heterocyclic ring are condensed, and examples thereof include,
for
example, an indole ring, an isoindole ring, a benzimidazole ring, a quinoline
ring, a
benzopyran ring, an acridine ring, a xanthene ring, a carbazole ring, and
others. These
ring structures may be condensed with the pyrrole ring in the formula (3) at
any
position.
[0046]
The number of It electrons contained in the ring A, that is, the number of it
.. electrons contained in the aromatic hydrocarbon ring, the aromatic
heterocyclic ring or
the condensed ring containing these ring structures is not particularly
limited, and may
be, for example, 4 or more or 6 or more. From the viewpoint of spreading the
it
electron system to a wider range in distance in the oxocarbon compound and
facilitating
lengthening the wavelength of the absorbing region, the number of it electrons
contained in the ring A is preferably 10 or more, more preferably 14 or more,
and even
more preferably 16 or more. Meanwhile, the upper limit of the number of it
electrons
contained in the ring A is not particularly limited; however, in consideration
of easily
production of the oxocarbon compound and solvent solubility thereof, the
number of it
electrons contained in the ring A is preferably 26 or less, more preferably 24
or less, and
even more preferably 22 or less. The number of It electrons contained in the
ring A is a
number including IC electrons of a carbon-carbon bond shared by the ring A and
the
pyrrole ring.
[0047]
As the substituent which the ring A may have (hereinafter referred to as
"substituent X"), organic groups or polar functional groups are shown.
Examples of
the organic group of the substituent X include, for example, an alkyl group,
an alkoxy
group, an alkylthiooxy group (an alkylthio group), an alkoxycarbonyl group, an
alkylsulfonyl group, an aryl group, an aralkyl group, an aryloxy group, an
arylthiooxy
group (an arylthio group), an aryloxycarbonyl group, an arylsulfonyl group, an
arylsulfinyl group, a heteroaryl group, an amido group, a sulfonamido group,
an
13
CA 03014400 2018-08-13
ethylene-containing group, an imine-containing group, carboxy group
(carboxylic acid
group), a benzothiazole group, a halogenoalkyl group, cyano group, and others.
Examples of the polar functional group of the substituent X include a halogeno
group,
hydroxyl group, nitro group, an amino group, sulfo group (sulfonic acid
group), and
others.
[0048]
Examples of the alkyl group include a linear or branched alkyl group such as
methyl, ethyl, propyl, isopropyl, n-butyl, isobutyl, t-butyl, pentyl, hexyl,
heptyl, octyl,
nonyl, decyl, undecyl, dodecyl, tridecyl, tetradecyl, pentadecyl, hexadecyl,
heptadecyl,
octadecyl, nonadecyl and icosyl; alicyclic alkyl groups such as cyclopropyl,
cyclobutyl,
cyclopentyl, cyclohexyl, cycloheptyl, cyclooctyl, cyclononyl and cyclodecyl;
and others.
The number of carbon atoms in the alkyl group is preferably 1 to 20, more
preferably 1
to 10, even more preferably 1 to 6, and in the case of an alicyclic alkyl
group, it is
particularly preferably 3 or more. The alkyl group may have a substituent, and
examples of the substituent, which the alkyl group may have, include a halogen
group,
hydroxyl group, carboxy group, an alkoxy group, cyano group, nitro group, an
amino
group, sulfo group, and others.
[0049]
Examples of the alkoxy group include, for example, methoxy, ethoxy, propoxy,
butoxy, pentyloxy, hexyloxy, heptyloxy, octyloxy, nonyloxy, decyloxy,
undecyloxy,
dodecyloxy, tridecyloxy, tetradecyloxy, pentadecyloxy, hexadecyloxy,
heptadecyloxy,
octadecyloxy, nonadecyloxy, icosyloxy, and others. The number of carbon atoms
in
the alkoxy group is preferably 1 to 20, more preferably 1 to 10, and even more
preferably 1 to 5. The alkyl group in the alkoxy group may be linear or
branched.
[0050]
Examples of the alkylthiooxy group (alkylthio group) include, for example,
methylthiooxy (methylthio), ethylthiooxy (ethylthio), propylthiooxy
(propylthio),
butylthiooxy (butylthio), pentylthio (pentylthio), hexylthiooxy (hexylthio),
heptylthiooxy (heptylthio), octylthiooxy (octylthio), nonylthiooxy
(nonylthio),
decylthiooxy (decylthio), undecylthiooxy (undecylthio), dodecylthiooxy
(dodecylthio),
14
CA 03014400 2018-08-13
tridecylthiooxy (tridecylthio), tetradecylthiooxy (tetradecylthio),
pentadecylthiooxy
(pentadecylthio), hexadecylthiooxy (hexadecylthio), heptadecylthiooxy
(heptadecylthio),
octadecylthiooxy (octadecylthio), nonadecylthiooxy (nonadecylthio),
icosylthiooxy
(icosylthio), and others. The number of carbon atoms in the alkylthiooxy group
is
preferably 1 to 20, more preferably 1 to 10, and even more preferably 1 to 5.
The alkyl
group in the alkylthiooxy group may be linear or branched.
[0051]
Examples of the alkoxycarbonyl group include, for example, an unsubstituted
alkoxycarbonyl group such as methoxycarbonyl, ethoxycarbonyl, propoxycarbonyl,
butoxycarbonyl, pentyloxycarbonyl, hexyloxycarbonyl, heptyloxycarbonyl,
octyloxycarbonyl, decyloxycarbonyl and octadecyloxycarbonyl, and a substituted
alkoxycarbonyl group such as trifluoromethyloxycarbonyl.
Examples of the
substituent include a halogeno group and others. The number of carbon atoms in
the
alkoxycarbonyl group is preferably 2 to 20, more preferably 2 to 10, and even
more
preferably 2 to 5. The alkyl group in the alkoxycarbonyl group may be linear
or
branched.
[0052]
Examples of the alkylsulfonyl group include, from example, a substituted or
unsubstituted alkylsulfonyl group such as methylsulfonyl, ethylsulfonyl,
propylsulfonyl,
isopropylsulfonyl, butylsulfonyl, hexylsulfonyl, cyclohexylsulfonyl,
2-ethylhexylsulfonyl, octylsulfonyl, methoxymethylsulfonyl,
cyanomethylsulfonyl and
trifluoromethylsulfonyl. The number of carbon atoms in the alkylsulfonyl group
is
preferably 1 to 20, more preferably 1 to 10, and even more preferably 1 to 5.
The alkyl
group in the alkylsulfonyl group may be linear, branched or cyclic.
[0053]
Examples of the aryl group include, for example, phenyl, biphenyl, naphthyl,
anthryl, phenanthryl, pyrenyl, indenyl, azulenyl, fluorenyl, terphenyl,
quarterphenyl,
pentalenyl, heptalenyl, biphenylenyl, indacenyl, acenaphthylenyl, phenalenyl
group, and
others. The number of carbon atoms in the aryl group is preferably 6 to 25,
and more
preferably 6 to 15. The aryl group may have a substituent, and examples of the
CA 03014400 2018-08-13
substituent, which the aryl group may have, include an alkyl group, an alkoxy
group, a
halogen group, a halogenoalkyl group, cyano group, nitro group, thiocyanate
group, an
acyl group, an alkoxycarbonyl group, an aryloxycarbonyl group, carbamoyl
group, sulfo
group, an alkylsulfinyl group, an arylsulfinyl group, an alkylsulfonyl group,
an
arylsulfonyl group, sulfamoyl group, and others.
[0054]
Examples of the aralkyl group include, for example, benzyl, phenethyl,
phenylpropyl, phenylbutyl, phenylpentyl, and others. The aralkyl group may
have a
substituent, and examples of the substituent, which the aralkyl group may
have, include
an alkyl group, an alkoxy group, a halogeno group, a halogenoalkyl group,
cyano group,
nitro group, thiocyanate group, an acyl group, an alkoxycarbonyl group, an
aryloxycarbonyl group, carbamoyl group, sulfo group, an alkylsulfinyl group,
an
arylsulfinyl group, an alkylsulfonyl group, an arylsulfonyl group, sulfamoyl
group, and
others. The number of carbon atoms in the aralkyl group is preferably 7 to 25,
and
more preferably 7 to 15.
[0055]
Examples of the aryloxy group include, for example, phenyloxy, biphenyloxy,
naphthyloxy, anthryloxy, phenanthryloxy, pyrenyloxy, indenyloxy, azulenyloxy,
fluorenyloxy, terphenyloxy, quaterphenyloxy, pentalenyloxy, heptalenyloxy,
biphenylenyloxy, indacenyloxy, acenaphthylenyloxy, phenalenyloxy, and others.
The
number of carbon atoms in the aryloxy group is preferably 6 to 25, and more
preferably
6 to 15.
[0056]
Examples of the arylthioxy group (arylthio group) include, for example,
phenylthioxy, biphenylthioxy, naphthylthioxy, anthrylthiooxy,
phenanthrylthiooxy,
pyrenylthiooxy, indenylthiooxy, azulenylthioxy, fluorenylthioxy,
terphenylthioxy,
quarterphenylthioxy, pentalenylthiooxy, heptalenylthiooxy, biphenylenylthioxy,
indacenylthioxy, acenaphthylenylthiooxy, phenalenylthioxy, and others. The
number
of carbon atoms in the arylthioxy group is preferably 6 to 25, and more
preferably 6 to
15.
16
CA 03014400 2018-08-13
=
[0057]
Examples of the aryloxycarbonyl group include, for example, a substituted or
unsubstituted phenyloxycarbonyl group such as
phenoxycarbonyl,
4-dimethylaminophenyloxycarbonyl, 4-
diethylaminophenyloxycarbonyl,
2-chlorophenyloxycarbonyl, 2-
methylphenyloxycarbonyl,
2-methoxyphenyloxycarbonyl, 2-
butoxyphen yloxycarbonyl,
3-chlorophenyloxycarbonyl, 3-
trifluoromethylphenyloxycarbonyl,
3-cyanophenyloxycarbonyl, 3-nitrophenyloxycarbonyl, 4-fluorophenyloxycarbonyl,
4-cyanophenyloxycarbonyl and 4-methoxyphenyloxycarbonyl; a substituted or
unsubstituted naphthyloxycarbonyl group such as 1-naphthyloxycarbonyl and
2-naphthyloxycarbonyl; and others. The
number of carbon atoms in the
aryloxycarbonyl group is preferably 7 to 25, and more preferably 7 to 15.
[0058]
Examples of the arylsulfonyl group include, for example, a substituted or
unsubstituted phenylsulfonyl group such as phenylsulfonyl, 1-naphthylsulfonyl,
2-naphthylsulfonyl, 2-chlorophenylsulfonyl, 2-methylphenylsulfonyl,
2-methoxyphenylsulfonyl, 2-butoxyphenylsulfonyl, 2-
fluorophenylsulfonyl,
3-methylphenylsulfonyl, 3-chlorophenylsulfonyl, 3-
trifluoromethylphenylsulfonyl,
3-cyanophenylsulfonyl, 3-nitrophenylsulfonyl, 3 -
fluorophenylsulfonyl,
4-methylphenylsulfonyl, 4-fluorophenylsulfonyl, 4-cyanophenylsulfonyl,
4-methoxyphenylsulfonyl and 4-dimethylaminophenylsulfonyl; a substituted or
unsubstituted naphthylsulfonyl group such as 1-naphthylsulfonyl and
2-naphthylsulfonyl; and others. The number of carbon atoms in the arylsulfonyl
group
is preferably 6 to 25, and more preferably 6 to 15.
[0059]
Examples of the arylsulfinyl group include, for example, a substituted or
unsubstituted phenylsulfinyl group such as phenylsulfinyl, 2-
chlorophenylsulfinyl,
2-methylphenylsulfinyl, 2-methoxyphenylsulfinyl, 2-
butoxyphenylsulfinyl,
2-fluorophenylsulfinyl, 3-methylphenylsulfinyl, 3-chlorophenylsulfinyl,
3-trifluoromethylphenylsulfinyl, 3-cyanophenylsulfinyl, 3-nitrophenylsulfinyl,
17
CA 03014400 2018-08-13
4-methylphenylsulfinyl, 4-fluorophenylsulfinyl, 4-
cyanophenylsulfinyl,
4-methoxyphenylsulfinyl and 4-dimethylaminophenylsulfinyl; a substituted or
unsubstituted naphthylsulfinyl group such as 1-naphthylsulfinyl and 2-
naphthylsulfinyl;
and others. The number of carbon atoms in the arylsulfinyl group is preferably
6 to 25,
and more preferably 6 to 15.
[0060]
Examples of the heteroaryl group include, for example, thienyl, thiopyranyl,
isothiocromenyl, pyrrolyl, imidazolyl, pyrazolyl, pyridyl, pyralidinyl,
pyrimidinyl,
pyridazinyl, thiazolyl, isothiazolyl, furanyl, pyranyl, and others. The number
of
carbon atoms in the heteroaryl group is preferably 2 to 20, and more
preferably 3 to 15.
[0061]
Examples of the amide group include those represented by the formula:
-NHCORal, wherein Rai represents an alkyl group, an aryl group, an aralkyl
group or a
heteroaryl group. Specific examples of the alkyl group, the aryl group, the
aralkyl
group and the heteroaryl group are shown as groups mentioned above, and a part
of
hydrogen atoms may be replaced by a halogen atom.
[0062]
Examples of the sulfonamide group include those represented by the formula:
-NHS021e, wherein le2 represents an alkyl group, an aryl group, an aralkyl
group or a
heteroaryl group. Specific examples of the alkyl group, the aryl group, the
aralkyl
group and the heteroaryl group are shown as groups mentioned above, and a part
of
hydrogen atoms may be replaced by a halogen atom.
[0063]
Examples of the ethylene-containing group include those represented by the
formula: -CRa3=CRa4-Ra5, wherein Ra3 to Ra5 represent hydrogen atom, an
aliphatic
hydrocarbon group, an aryl group, a heteroaryl group (particularly pyridyl
group), cyano
group, an alkoxycarbonyl group, carboxy group, and others, and these groups
may have
a substituent. The aliphatic hydrocarbon groups of Ra3 to le5 may be saturated
or
unsaturated, and are preferably unsaturated. As such aliphatic hydrocarbon
group, a
group having a repeating unit represented by -(CH=CH)k- (k is an integer of 1
to 10,
18
CA 03014400 2018-08-13
preferably an integer of 1 to 5) is preferable, and a vinyl group is
exemplified. In
addition, 1,1-dicyanoethylene group and 1-cyanoethylene group are also
preferable.
As the aliphatic hydrocarbon group, for example, those having 1 to 20 carbon
atoms are
preferable, and those having 1 to 10 carbon atoms are more preferable.
Examples of
the aryl group, heteroaryl group and alkoxycarbonyl group of Ra3 to Ras
include the
above-mentioned aryl group, heteroaryl group and alkoxycarbonyl group.
[0064]
Examples of the imine-containing group include those represented by the
formula: -CH=N-le6, wherein le6 represents an amino group optionally having a
substituent. The amino group of Rao may be substituted or unsubstituted.
Examples
of the amino group having a substituent include a monoalkylamino group, a
dialkylamino group, a monoarylamino group, a diarylamino group, a
monoalkylmonoarylamino group, and others. Examples of the alkyl group or aryl
group bonded to the amino group of Ra6 include the above-mentioned alkyl group
and
aryl group.
[0065]
Examples of the halogenoalkyl group include, for example, a
monohalogenoalkyl group such as fluoromethyl, 3-fluoropropyl, 3-chloropropyl,
6-fluorohexyl and 4-fluorocyclohexyl; a dihalogenoalkyl group such as
dichloromethyl;
an alkyl group having a trihalometyl unit such as 1,1-dihydro-perfluoroethyl,
1,1-dihydro-perfluoro-n-propyl, 1,1-
dihydro-perfluoro-n-butyl,
2,2-bis(trifluoromethyl)propyl and 2,2,2-trichloroethyl; a perhalogenoalkyl
group such
as trifluoromethyl, perfluoroethyl, perfluoro-n-pentyl and perfluoro-n-hexyl;
and others.
The number of carbon atoms in the halogenoalkyl group is preferably 1 to 20,
more
preferably 1 to 10, and even more preferably 1 to 5. The halogen of the
halogenoalkyl
group is preferably a fluorine atom, a chlorine atom or a bromine atom, and
particularly
preferably a fluorine atom.
[0066]
Examples of the halogeno group include fluoro group, chloro group, bromo
group, and iodo group.
19
CA 03014400 2018-08-13
[0067]
As the substituent X, among the above, an alkyl group, an alkoxy group, an
alkylthiooxy group, an alkoxycarbonyl group, an aryl group, an aryloxycarbonyl
group,
cyano group, a halogeno group and nitro group are preferable, and an alkyl
group, an
alkoxy group, an alkylthiooxy group, a halogeno group and an aryl group are
more
preferable, whereby increasing solvent solubility of the oxocarbon compound
and finely
adjusting the maximum absorption wavelength of the oxocarbon compound to a
desired
wavelength range are facilitated. In addition, the effect of easily production
of the
oxocarbon compound is also obtained. In this case, the number of carbon atoms
in the
alkyl group, the alkoxy group and the alkylthiooxy group is preferably 1 to 5,
more
preferably 1 to 3, even more preferably 1 to 2, the number of carbon atoms in
the
alkoxycarbonyl group is preferably 2 to 6, more preferably 2 to 4, even more
preferably
2 to 3, and the number of carbon atoms in the aryl group and the
aryloxycarbonyl group
is preferably 6 to 12, more preferably 6 to 10. The ring A may not have the
substituent
X. In the case where the ring A has the substituent X, the number thereof is
preferably
1 to 5, more preferably 1 to 3, and even more preferably 1 to 2. In the case
where the
ring A has the plurality of substituents X, the plurality of substituents X
may be the
same or different from each other.
[0068]
In the formula (3), R5 to R7 each independently represent hydrogen atom, an
organic group or a polar functional group, or R5 and R6 are linked to each
other to form
a ring. Examples of the organic group and the polar functional group of R5 to
R7
include the groups exemplified as the organic group and the polar functional
group of
the substituent X. Examples of the ring structure formed from R5 and R6
include a
hydrocarbon ring and a heterocyclic ring, and these ring structures may have
aromaticity or may not have aromaticity.
[0069]
In the case where R5 and R6 are not linked to each other and are independent
groups, it is preferable that R5 and R6 each independently represent hydrogen
atom, an
alkyl group optionally having a substituent, an aryl group optionally having a
CA 03014400 2018-08-13
substituent, an aralkyl group optionally having a substituent, an
alkoxycarbonyl group
optionally having a substituent, or an aryloxycarbonyl group optionally having
a
substituent. Specific examples of these groups are shown as groups exemplified
as the
substituent X. When R5 and R6 are such groups, increasing solvent solubility
of the
oxocarbon compound and finely adjusting the maximum absorption wavelength of
the
oxocarbon compound to a desired wavelength range are facilitated. In addition,
the
effect of easily production of the oxocarbon compound is also obtained.
[0070]
In the case where R5 or R6 is an alkyl group or an alkoxycarbonyl group, the
number of carbon atoms (the number of carbon atoms excluding the substituent)
of the
alkyl group contained in these groups is preferably 1 to 20, more preferably 1
to 12,
even more preferably 1 to 10 in a linear or branched alkyl group, and
preferably 4 to 7,
more preferably 5 to 6 in an alicyclic alkyl group. Preferable examples of the
alkyl
group include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, t-butyl,
pentyl, hexyl,
heptyl, octyl, cyclopentyl, cyclohexyl, and others. Note that, in the case
where R5 or
R6 is an alkyl group, the substituent which the alkyl group may have does not
include an
aryl group. In the case where R5 or R6 is an aryl group or an aryloxycarbonyl
group,
the number of carbon atoms (the number of carbon atoms excluding the
substituent) of
the aryl group contained in these groups is preferably 6 to 10. Preferable
examples of
the aryl group include phenyl, naphthyl, and others. In the case where R5 or
R6 is an
aralkyl group, the number of carbon atoms (the number of carbon atoms
excluding the
substituent) is preferably 7 to 20, more preferably 7 to 15. Examples of the
aralkyl
group include benzyl, phenethyl, phenylpropyl, and others. Preferable examples
of the
substituent which each of R5 and R6 may have include an alkoxy group, a
halogeno
group, halogenoalkyl group, cyano group, a nitro group, and others.
[0071]
In the case where R5 and R6 are linked to each other to form a ring, the
structural unit of the above formula (3) is represented by the following
formula (4).
The ring structure formed by R5 and R6, that is, the ring B in the following
formula (4)
may be any ring structure, and the ring B may have a substituent. The ring B
is
21
CA 03014400 2018-08-13
a
preferably a hydrocarbon ring optionally having a substituent and/or a
condensed ring
structure, or a heterocyclic ring optionally having a substituent and/or a
condensed ring
structure.
[0072]
[Chemical Formula 14]
R7
A
(4)
[0073]
Examples of the hydrocarbon ring of the ring B include, for example, a
monocyclic cycloalkane having 3 to 10 carbon atoms such as cyclopentane,
cyclohexane and cycloheptane; a monocyclic cycloalkene having 3 to 10 carbon
atoms
such as cyclopentene, cyclopentadiene, cyclohexene, cyclohexadiene (for
example,
1,3-cyclohexadiene), cycloheptene and cycloheptadiene; and others. As the
heterocyclic ring of the ring B, a ring structure of the above-mentioned
hydrocarbon
ring in which one or more of carbon atoms constituting the ring of the
hydrocarbon ring
is replaced by at least one atom selected from N (nitrogen atom), S (sulfur
atom) and 0
(oxygen atom); and examples thereof include, for example, a furan ring, a
tetrahydrofuran ring, a thiophene ring, a tetrahydrothiophene ring, a pyrrole
ring, a
pyrrolidine ring, a pyrazole ring, an oxazole ring, a thiazole ring, an
imidazole ring, a
pyridine ring, a piperidine ring, a pyridazine ring, a pyrimidine ring, a
pyrazine ring, a
pyran ring, a tetrahydropyran ring, and others. These hydrocarbon ring and
heterocyclic ring may have a condensed ring structure condensed with another
ring, and
examples of such ring structure include, for example, an indene ring, a
naphthalene ring,
an anthracene ring, a fluorene ring, a benzofluorene ring, an indole ring, an
isoindole
ring, a benzimidazole ring, a quinoline ring, a benzopyran ring, an acridine
ring, a
xanthene ring, a carbazole ring, a purine ring, a pteridine ring, and others.
[0074]
22
CA 03014400 2018-08-13
The hydrocarbon ring and the heterocyclic ring of the ring B may have a
substituent, and examples of such substituent include an alkyl group, an
alkoxy group,
an aryl group, a thioalkoxy group, an aryloxy group, a thioaryloxy group, an
alkylamido
group, a halogeno group, a halogenoalkyl group, cyano group, nitro group, an
alkoxycarbonyl group, an aryloxycarbonyl group, an alkylsulfinyl group, an
arylsulfinyl
group, an alkylsulfonyl group, an arylsulfonyl group, and others. The
halogenoalkyl
group may be one in which at least a part of hydrogen atoms of the alkyl group
is
substituted with a halogen atom, and is preferably a perhalogenoalkyl group in
which all
of hydrogen atoms of the alkyl group are substituted with halogen atoms. As
the
halogenoalkyl group, a fluoroalkyl group is more preferable, and a
perfluoroalkyl group
is even more preferable. The number of carbon atoms in the halogenoalkyl group
is
preferably 1 to 5, more preferably 1 to 3, and even more preferably 1 to 2.
[0075]
From the viewpoint of lengthening the absorption wavelength of the oxocarbon
compound, the ring B preferably contains it electrons, and examples of such
ring
structure include, for example, a cyclohexadiene ring, a pyrrole ring, a pyran
ring, an
indene ring, an indole ring, an isoindole ring, a benzopyran ring, a fluorene
ring, a
xanthene ring, and ohters. As the structural unit represented by the above
formula (4)
in this case, structural units represented by the following formulas (4-1) to
(4-12) are
shown, for example.
[0076]
[Chemical Formula 15]
23
i 1
CA 03014400 2018-08-13
,
R8,/, \ R9
R8
R8 R8 / -1-
k R8 ,R8 V
I \ \ 'N I I )\ N I. /
R7 R7 R7 ' R7
a NH * 0 NH * = NH * 0 N H *
(4-1) (4-2) (4-3) (4-4)
R8 R8 R9
- \
R8-C. - NI\ N I R7 i N R7 N R8 fr A i
R7
l \--- i N
' R7
a NH * = NH * = NH *
0 N H *
(4-5) (4-6) (4-7) (4-8)
v R8
0, R8 0., R8 R9
0 --/,
11 8 11 9 ,\/
R9--/L,_ \ N 1 ----FR \-
R7 R7 "'-, R7 ---- R7
= NH *
0 NH * CIV NH * A \ - *
¨ NH
(4-9) (4-10) (4-11) (4-12)
[0077]
In the formulas (4-1) to (4-12), R8 and R9 represent a group or an atom bonded
to the ring structure formed by R5 and R6, and it is preferable that R8 and R9
each
independently represent a group or an atom selected from the group consisting
of
hydrogen atom, an alkyl group, an alkoxy group, a halogeno group, a
halogenoalkyl
group, cyano group, nitro group, an alkoxycarbonyl group, an aryloxycarbonyl
group,
an alkylsulfinyl group, an arylsulfinyl group, an alkylsulfonyl group and an
arylsulfonyl
group. Details of the preferred embodiment and others of the halogenoalkyl
group are
as described above. The number of each of R8 and R9 bonded to the ring varies
depending on the ring structure, and in the case where plural R8 or plural R9
are bonded,
plural R8 or plural R9 may be the same or different from each other. Among
them, it is
preferable that R8 and R9 are each independently a group or an atom selected
from the
group consisting of hydrogen atom, an alkyl group, an alkoxy group, a halogeno
group,
a halogenoalkyl group and cyano group, and more preferably a group or an atom
24
I I
CA 03014400 2018-08-13
=
selected from the group consisting of hydrogen atom, a halogen group, a
halogenoalkyl group and cyano group. In this case, it is preferable that all
of R8 and
R9, which are bonded to the ring structure formed by R5 and R6, are not
hydrogen atoms.
That is, a substituent selected from the group consisting of an alkyl group,
an alkoxy
gruop, a halogen group, a halogenoalkyl group and cyano group is preferably
bonded
to the ring structure formed by R5 and R6, and more preferably, a substituent
selected
from the group consisting of a halogeno group, a halogenoalkyl group and cyano
group
is bonded to the ring structure formed by R5 and R6. Thereby, lengthening the
absorption wavelength of the oxocarbon compound can be realized.
[0078]
It is preferable that the ring B is a hydrocarbon ring having a condensed ring
structure which may have a substituent, or a heterocyclic ring having a
condensed ring
structure which may have a substituent, whereby further lengthening the
absorption
wavelength of the oxocarbon compound can be realized. The hydrocarbon ring or
the
heterocyclic ring having a condensed ring structure is preferably formed so
that TE
electron conjugated system spreads to the condensed ring, and from such a
viewpoint,
the number of it electrons contained in the ring B is preferably 6 or more,
more
preferably 8 or more, even more preferably 10 or more, and still even more
preferably
12 or more. The upper limit of the number of it electrons contained in the
ring B is not
particularly limited; however, considering ease of production and solvent
solubility of
the oxocarbon compound, it is preferably 20 or less, more preferably 18 or
less, and
even more preferably 16 or less. The number of IC electrons contained in the
ring B
means a number of TE electrons contained in the ring structure of the ring B,
and in the
case where the ring B has a condensed ring structure, it includes it electrons
contained
in the condensed ring structure, and it does not include TE electrons
contained in a
substituent which the ring B may have.
[0079]
Preferable examples of the ring B include an indene ring, an indole ring, an
isoindole ring, a benzopyran ring, a fluorene ring and a xanthene ring. In the
structural
units represented by the above formulas (4-1) to (4-12), the structural units
represented
CA 03014400 2018-08-13
by the formulas (4-2), (4-3), (4-4), (4-6), (4-8), (4-9), (4-11) and (4-12)
are preferable.
From the viewpoint of lengthening the absorption wavelength, a fluorene ring
optionally having a substituent or a xanthene ring optionally having a
substituent are
particularly preferable as the ring B, and in the structural units represented
by the above
formulas (4-1) to (4-12), the structural units represented by formulas (4-4)
and (4-12)
are particularly preferable. In these structural units, it is preferable that
all of R8
bonded to the benzene ring contained in the fluorene ring or the xanthene ring
are not
hydrogen atoms and all of R9 are not hydrogen atoms, and at least one of the
above-mentioned substituent (particularly, a halogeno group, a halogenoalkyl
group or
cyano group) is bonded to each of the benzene ring.
[0080]
It is preferable that R7 is hydrogen atom, an alkyl group optionally having a
substituent or an aryl group optionally having a substituent, and details of
the alkyl
group optionally having a substituent and the aryl group optionally having a
substituent
are made reference to the explanation on Rs and R6 of the above. When R7 is an
aryl
group optionally having a substituent, the it electron system of the oxocarbon
compound
expands and the absorption wavelength can be shifted to a longer wavelength,
that are
preferable. In this case, the number of it electrons contained in R7 is
preferably 6 to 10
from the viewpoint of ease of production of the oxocarbon compound.
Specifically, as
the aryl group of R7, phenyl or naphthyl whcih may have a substituent is
preferable, and
phenyl is more preferable. Despite of that, R7 may not have a it electron and
may be
hydrogen atom or an alkyl group (particularly an alkyl group having 1 to 4
carbon
atoms). In this case, it is preferable in that solvent solubility of the
oxocarbon
compound can be easily enhanced and production of the oxocarbon compound is
facilitated. That the number of It electrons contained in R7 means a number of
it
electrons contained in R7 of the it electron system continuously spreading
from the
squarylium skeleton or the croconium skeleton to R7.
[0081]
In the oxocarbon compound, the total number of it electrons contained in the
rings A and R7 is 12 or more, preferably 14 or more, and more preferably 16 or
more,
26
I
CA 03014400 2018-08-13
=
whereby near-infrared light on a longer wavelength side (for example, light in
a
wavelength range exceeding 850 nm) can effectively absorb. In the oxocarbon
compound of the present invention, it is more preferable that the number of it
electrons
contained in the ring A is in such a range, whereby the it electron system
spreads over a
wider range in distance and lengthening the absorption wavelength is easily
achieved.
Examples of the ring A in this case include a phenanthrene ring, an anthracene
ring, a
tetracene ring, a fluoranthene ring, a benzofluoranthene ring, a
cyclotetradecaheptaene
ring, an acridine ring, a carbazole ring, and others. Meanwhile, the upper
limit of the
total number of it electrons contained in the rings A and R7 is not
particularly limited;
however, in consideration of ease of production and solvent solubility of the
oxocarbon
compound, it is preferably 30 or less, more preferably 28 or less, and even
more
preferably 26 or less.
[0082]
Among the structural units represented by the above formula (4), examples of
the structural units which are relatively easy to produce and which can give
oxocarbon
compounds capable of absorbing light on a longer wavelength side are shown
below.
The structural units shown in the following formulas (4-21) to (4-26) are
specific
examples of the structural unit of the above formula (4-4), and the structural
units
shown in the following formulas (4-27) to (4-32) are specific examples of the
structural
unit of the above formula (4-12). In the following formulas (4-21) to (4-32),
the
explanations on R7 to R9 in the following formulas (4-21) to (4-32) are made
reference
to the above description. A fluoranthene ring is provided as the ring A in the
formulas
(4-21), (4-22), (4-27) and (4-28), a phenanthrene ring is provided as the ring
A in the
formulas (4-23), (4-24), (4-29) and (4-30), and an anthracene ring is provided
as the
ring A in the formulas (4-25), (4-26), (4-31) and (4-32). The binding
(condensed ring)
form of these ring structures to the pyrrole ring is not limited to
embodiments shown in
the following formulas. Further, as the ring A, other ring structures may be
provided.
[0083]
[Chemical Formula 16]
27
CA 03014400 2018-08-13
J
1
=
R9 R9 R9
R8 -+ R8 + R9 -1-
N R7 N
R7 N R7
*
NH * NH * NH
(4-21) (4-22) (4-23)
R9 R9
-1-
R8 + R8 R9 R8 --I-
-V
R7
N R7
NH * *
NH
(4-24) (4-25) NH *
(4-26)
[0084]
[Chemical Formula 17]
R9 R9 R9
R8 0 ---/., RB 0 v,
NH * NH *
NH *
(4-27) (4-28) (4-29)
R9 R9
R9
X LJ-\
7R7
-, R7
*
NH * * NH
NH
(4-30) (4-31) (4-32)
[0085]
28
CA 03014400 2018-08-13
As the oxocarbon compound having the structural unit represented by the
above formula (4), a croconium compound represented by the formula (2) is
preferable
from the viewpoint of absorbing light on a longer wavelength side. Further, in
the
formula (2), the rings A in R3 and R4 preferably have the same ring structure,
and the
rings B in R3 and R4 preferably have the same ring structure. More preferably,
in the
formula (2), the rings A in R3 and R4 have the same structure, wherein the
structure
including a ring structure of the ring A and a structure of a substituent
which may bond
thereto, and the rings B of R3 and R4 have the same structure, wherein the
structure
including a ring structure of the ring B and a structure of a substituent
which may bond
thereto.
[0086]
In the oxocarbon compound of the present invention, the maximum absorption
wavelength is preferably 835 rim or longer, more preferably 840 rim or longer,
even
more preferably 845 nm or longer, from the viewpoint of increasing an
absorption rate
.. of light in the wavelength range exceeding 850 nm. The upper limit of the
maximum
absorption wavelength is not particularly limited, and may be, for example,
1300 nm or
shorter, or 1100 rim or shorter.
[0087]
As to visible light transmittance, an average transmittance in a wavelength
range of 400 nm to 700 nm is preferably 83% or more, more preferably 84% or
more,
and even more preferably 85% or more when the transmittance at the maximum
absorption wavelength is set to 10% (or 10% or less).
[0088]
As described above, the oxocarbon compound of the present invention is
capable of absorbing near-infrared light on the longer wavelength side,
specifically
effectively absorbing light in the wavelength range exceeding, for example,
850 nm,
since the it electron system spreads from the squarylium skeleton or the
croconium
skeleton to the ring A (or further to the substituent R7) and the total number
of it
electrons contained in the ring A and the substituent R7 is 12 or more.
Meanwhile,
since the oxocarbon compound itself has high light transmittance in a visible
light
29
CA 03014400 2018-08-13
region, it is excellent in invisibility under visible light. For
example, some
phthalocyanine compounds have the absorption maximum wavelength in a
wavelength
range exceeding 850 tun, but the phthalocyanine compounds have an absorption
peak
derived from the soret band in a wavelength range around 400 nm and may cause
to
show green color; meanwhile, the oxocarbon compound of the present invention
is
excellent in invisibility while functioning as a near-infrared absorbing dye.
[0089]
The oxocarbon compound of the present invention can be produced by reacting
a condensed heterocyclic compound represented by the following formula (5)
with
squaric acid or croconic acid. In the following formula (5), ring A and R5 to
R7 each
represent the same meaning as that in the above formula (3), and the total
number of 7(
electrons contained in the ring A and R7 is 12 or more. Preferred embodiments
of the
ring A and R5 to R7 are also as described above.
[0090]
[Chemical Formula 18]
R5 R6
CH2R7
A \ NJ/
(5)
[0091]
The condensed heterocyclic compound represented by the formula (5) is
suitably used as a raw material of the oxocarbon compound of the present
invention,
whereby the oxocarbon compound of the present invention can be easily
produced.
That is, the oxocarbon compound of the present invention can be produced by a
process
comprising the step of reacting the condensed heterocyclic compound
represented by
the formula (5) with squaric acid or croconic acid to obtain the oxocarbon
compound
represented by the formula (1) or (2). Specifically, the squarylium compound
represented by the formula (1) can be produced by reacting the condensed
heterocyclic
compound of the formula (5) with squaric acid, and the croconium compound
.1
CA 03014400 2018-08-13
represented by the formula (2) can be produced by reacting the condensed
heterocyclic
compound of the formula (5) with croconic acid.
[0092]
The amount of the condensed heterocyclic compound used in reacting the
condensed heterocyclic compound with squaric acid or croconic acid is
preferably equal
molar or more, more preferably 1.5 times molar or more, even more preferably 2
times
molar or more, and preferably 5 times molar or less, more preferably 4 times
molar or
less, even more preferably 3 times molar or less, relative to squaric acid or
croconic
acid.
[0093]
The reaction of the condensed heterocyclic compound with squaric acid or
croconic acid is preferably carried out in the presence of a solvent. Examples
of usable
solvents include, for example, chlorinated hydrocarbons such as chloroform and
methylene chloride; aromatic hydrocarbons such as benzene, toluene, xylene and
trimethylbenzene; chlorinated aromatic compounds such as chlorotoluene and
dichlorobenzene; ethers such as tetrahydrofuran (THF), dioxane, cyclopentyl
methyl
ether, diisopropyl ether and diethyl ether; alcohols such as methanol,
ethanol, propanol,
isopropanol, butanol, tert-butanol and tert-amyl alcohol; and others. These
solvents
may be used alone, or two or more of them may be used in combination. When an
alcohol is used as the reaction solvent, it is preferable to use a tertiary
alcohol. The
amount (total amount) of the solvent used is preferably equal mass or more,
more
preferably 5 times mass or more, even more preferably 10 times mass or more,
and
preferably 100 times mass or less, relative to squaric acid or croconic acid.
[0094]
In the reaction of the condensed heterocyclic compound with squaric acid or
croconic acid, the reaction temperature may be appropriately set, and for
example, is
preferably 30 C or higher, more preferably 60 C or higher, even more
preferably 80 C
or higher, and preferably 170 C or lower, more preferably 140 C or lower. The
reaction is preferably carried out under reflux condition. The reaction time
is not
particularly limited and may be appropriately set depending on the progress of
the
31
CA 03014400 2018-08-13
reaction, and for example, it is preferably 0.5 hour or longer, more
preferably 1 hour or
longer, and preferably 48 hours or shorter, more preferably 24 hours or
shorter. The
atmosphere during the reaction is preferably an inert gas (e.g., nitrogen,
argon, and the
like) atmosphere.
[0095]
The squarylium compound can be synthesized by appropriately employing a
known synthesis method of reacting the condensed heterocyclic compound with
squaric
acid. For example, the squarylium compound can be synthesized by the synthesis
process described in the following article: Serguei Miltsov et at., "New
Cyanine Dyes:
Norindosquarocyanines", Tetrahedron Letters, Vol.40, Issue 21, p.4067-4068
(1999).
[0096]
A process for synthesizing the croconium compound is not particularly
restricted, and it can be synthesized by appropriately adopting a known
synthesis
method of reacting the condensed heterocyclic compound with croconic acid. For
example, the croconium compound can be synthesized by the methods described in
Japanese Unexamined Patent Application Publication Nos. 2002-286931, 2007-
31644,
2007-31645, and 2007-169315.
[0097]
The condensed heterocyclic compound of the formula (5) can be synthesized
by appropriately employing a known synthesis method, and produced, for
example,
according to the following reaction formula. In the following reaction
formulas, ring A
and R5 to R7 each represent the same meaning as in the above formula (3).
[0098]
[Chemical Formula 19]
0 R5 R5
A \ R5 C H2R7
N HN H2 = HCI + R7 --a-- A \ N
R5
(6) (7) (5)
[0099]
32
i
CA 03014400 2018-08-13
=
For example, the condensed heterocyclic compound in which the ring A is a
fluoranthene ring, R5 and R6 are methyl groups and R7 is hydrogen atom can be
synthesized by reacting fluoranthenylhydrazine
hydrochloride with
3-methyl-2-butanone. Thus, the condensed heterocyclic compound of the formula
(5)
can be synthesized by reacting hydrazine hydrochloride having an aromatic
hydrocarbon ring structure, an aromatic heterocyclic ring structure or a
condensed ring
structure containing these rings (that is, the compound of the above formula
(6)) with a
dimethylketone derivative (that is, the compound of the above formula (7)).
Synthesis
of the condensed heterocyclic compounds can be also referred to the following
article:
Sajjadifar et al., "New 3H-Indole Synthesis by Fischer's Method. Part I",
Molecules,
Vol.15, p.2491-2498 (2010).
[0100]
The oxocarbon compound obtained by reacting the condensed heterocyclic
compound of the formula (5) with squaric acid or croconic acid can be
appropriately
purified by known purification means such as filtration, silica gel column
chromatography, alumina column chromatography, sublimation, recrystallization,
and
crystallization. The chemical structure of the obtained oxocarbon compound can
be
analyzed by known analytical methods such as mass spectrometry, single crystal
X-ray
structural analysis, fourier transform infrared spectroscopy, and nuclear
magnetic
resonance spectroscopy.
[0101]
[2. Resin composition]
The oxocarbon compound of the present invention may be mixed with a resin
component to give a resin composition. The resin composition contains at least
the
oxocarbon compound of the present invention and a resin component. Since the
resin
composition of the present invention can effectively absorb light in a near-
infrared
region, for example, light in a wavelength range exceeding 850 nm, it can be
suitably
applied to an optical filter such as an image sensing device for night vision
by forming a
resin molding such as a film. In thus formed optical filter, incident angle
dependence
of optical characteristics on the short wavelength side of a near-infrared
region is
33
CA 03014400 2018-08-13
= =
g
reduced, the viewing angle is improved, and further transmittance in a visible
light
region is high, and therefore, thus formed optical filter can be suitably
applied to an
image sensing device used also under visible light. The resin molding can be
also
applied to a near-infrared absorbing film or a near-infrared absorbing plate
that shields a
heat ray for energy saving, a material for a solar cell utilizing visible
light and
near-infrared light, a specific wavelength absorption filter for a plasma
display panel
(PDP) or CCD, and the like.
[0102]
The resin composition of the present invention can be also suitably used for
laser welding applications. Joining a resin by a laser welding method can be
performed by placing a light transmitting resin that transmits laser light on
a light
absorbing resin that absorbs laser light and irradiating laser light from the
light
transmitting resin side. The irradiated laser light passes through the light
transmitting
resin and energy is absorbed at the surface of the light absorbing resin to
generate heat,
whereby the light absorbing resin melts, the light transmitting resin also
melts due to
thermal conduction, and the both resins are joined. In some cases, as the
light
absorbing resin, a colored resin containing carbon black, a black dye or the
like is used;
however, since laser light having a wavelength of 800 nm to 1300 nm (for
example,
semiconductor laser, YAG laser, fiber laser) is used in the laser welding,
forming the
light absorbing resin from the resin composition of the present invention
enables laser
welding between transparent resins. Thus, the oxocarbon compound of the
present
invention can function as an absorber for laser light, that is, as a heat
generation source.
In the laser welding method, the resin composition of the present invention
also can be
used as a laser light absorber disposed between two light transmitting resins.
[0103]
The oxocarbon compound contained in the resin composition may be a
squarylium compound, a croconium compound, or both. The oxocarbon compound
contained in the resin composition may be only one kind or may be two kinds or
more.
[0104]
Two or more kinds of the squarylium compounds represented by the above
34
CA 03014400 2018-08-13
formula (1) may be contained in the resin composition, or two or more kinds of
the
croconium compounds represented by the above formula (2) may be contained in
the
resin composition. The thus formed resin composition can effectively absorb
the light
in a near-infrared region, improves solubility of the oxocarbon compound in
the resin,
and facilitates forming the resin composition containing the oxocarbon
compound at a
high concentration. In this case, from the viewpoint of increasing the
solubility of the
oxocarbon compound in the resin, the resin composition preferably contains at
least
squarylium compounds having a structure in which RI and R2 are different from
each
other in the above formula (1) or preferably contains at least croconium
compounds
having a structure in which R3 and R4 are different from each other in the
above formula
(2). More preferably, the resin composition contains three or more kinds of
the
oxocarbon compounds, and examples of such resin composition include, for
example, a
resin composition containing a squarylium compound in which RI and R2 are both
Rx in
the above formula (1), a squarylium compound in which RI and R2 are both Ry in
the
above formula (1) and a squarylium compound in which RI is Rx and R2 is Ry in
the
above formula (1), and a resin composition containing a croconium compound in
which
R3 and R4 are both Rx in the above formula (2), a croconium compound in which
R3 and
R4 are both Ry in the above formula (2) and a croconium compound which is R3
is Rx
and R4 is Ry in the above formula (2). Such resin composition is preferable
since it
contains plural oxocarbon compounds and is easy to produce. Here, the
above-described Rx and Ry represent any structural unit represented by the
above
formula (3), and Rx and Ry are not the same.
[0105]
The oxocarbon compound of the present invention can be regarded as one kind
of dye, and the resin composition of the present invention may contain other
dyes
together with the oxocarbon compound of the present invention as long as the
desired
performance according to an application is secured. Examples of the dye which
may
be contained in the resin composition include, for example, squarylium dyes or
croconium dyes other than the oxocarbon compound of the present invention,
cyclic
tetrapyrrole dyes which may have copper (e.g., Cu(II)), zinc (e.g., Zn(II)) or
the like as a
CA 03014400 2018-08-13
central metal (e.g., porphyrins, chlorins, phthalocyanines and cholines),
cyanine dyes,
quaterrylene dyes, naphthalocyanine dyes, nickel complex dyes, copper ion
dyes,
diimmonium dyes, subphthalocyanine dyes, xanthene dyes, azo dyes,
dipyrromethene
dyes, and others. These other dyes may be used alone or two or more of them
may be
used in combination.
[0106]
In the case where the resin composition of the present invention also contains
other dyes, the content of other dyes is preferably 60 mass% or less, more
preferably 40
mass% or less, even more preferably 20 mass% or less, relative to 100 mass% of
the
total of the oxocarbon compound of the present invention and other dyes; and
it is
particularly preferable that any other dye is not substantially contained.
[0107]
From the viewpoint of developing desired performance, the content of the
oxocarbon compound of the present invention in the resin composition is
preferably
0.01 mass% or more, more preferably 0.3 mass% or more, and even more
preferably 1
mass% or more, based on 100 mass% of the solid content of the resin
composition. In
addition, from the viewpoint of enhancing formability or film-forming property
of the
resin composition, the content of the oxocarbon compound of the present
invention in
the resin composition is preferably 25 mass% or less, more preferably 20 mass%
or less,
and even more preferably 15 mass% or less, based on 100 mass% of the solid
content of
the resin composition. In the case where the resin composition also contains
other
dyes, the total content of the oxocarbon compound of the present invention and
other
dyes is preferably within the above-mentioned range. The mass of the solid
content of
the resin composition means mass obtained by removing a liquid medium from the
resin
composition.
[0108]
As the resin component contained in the resin composition, a known resin can
be used. As the resin component, those having high transparency and capable of
dissolving or dispersing the oxocarbon compound of the present invention are
preferable. In the case where other dyes are used in combination, the resin
component
36
CA 03014400 2018-08-13
which is capable of dissolving or dispersing also other dyes is preferable. By
employing such resin component, it becoms possible to achieve both high
transmittance
in a wavelength range to be transmitted and high absorption in a wavelength
range to be
blocked.
[0109]
As the resin component, not only a resin which has been completely
polymerized but also a resin raw material (including a precursor of the resin,
a raw
material of the precursor, a monomer constituting the resin, and the like)
which is to be
polymerized or crosslinked to be incorporated into the resin at the time of
molding the
resin composition can be used. In the present invention, both are the resin
component.
However, in the latter case, a part or all of the structure of the oxocarbon
compound
may be decomposed due to unreacted substances, reactive terminal functional
groups,
ionic groups, catalysts, acid/basic groups, and others present in the reaction
solution
obtained by the polymerization reaction. Therefore, in the case that there is
such a
concern, it is desirable to form the resin composition by blending the
oxocarbon
compound in a resin which has been completely polymerized.
[0110]
Examples of the resin component include, for example, a (meth)acrylic resin, a
(meth)acrylic urethane resin, a polyvinyl chloride resin, a polyvinylidene
chloride resin,
a polyolefin resin (e.g., polyethylene resin, polypropylene resin, and the
like), a
cycloolefin resin (including cycloolefin copolymer, norbornene resin, and the
like), a
petroleum resin, a rosin resin, a rosin ester resin, an urea resin, a melamine
resin, an
urethane resin, a styrene resin, a styrene-acryl resin, a styrene-maleic acid
resin,
polyvinyl acetate, an ethylene-vinyl acetate resin, a vinyl acetal resin, a
polyamide resin
(e.g., nylon and the like), an aramid resin, a polyimide resin, a polyamide-
imide resin,
an alkyd resin, a phenol resin, an epoxy resin, a polyester resin (e.g.,
polybutylene
terephthalate resin, polyethylene terephthalate resin, polyarylate resin, and
the like), a
polysulfone resin, a butyral resin, a polycarbonate resin, a polyether resin,
an
acrylonitrile butadiene styrene resin, an acrylonitrile-styrene copolymer, a
cellulose
derivative (e.g., ethyl cellulose, cellulose acetate, nitrocellulose, and the
like), a silicone
37
CA 03014400 2018-08-13
=
=
resin, a modified silicone resin (e.g., (meth)acrylic silicone resin, alkyl
polysiloxane
resin, silicone urethane resin, silicone polyester resin, silicone acrylic
resin, and the like),
a fluorine resin (e.g., fluorinated aromatic polymer, polytetrafluoroethylene,
perfluoroalkoxy fluorine resin, fluorinated polyaryl ether ketone, fluorinated
polyimide,
fluorinated polyamic acid, fluorinated polyether nitrile, and the like), a
diallyl phthalate
resin, a coumarone-indene resin, a terpene phenol resin, a xylene resin, an
alkyd resin, a
maleic acid resin, a fumaric acid resin, and others
[0111]
The resin component preferably has high transparency, that makes it easy to
suitably apply the resin composition to optical applications and laser welding
applications. For example, a total light transmittance of the resin component
at a
thickness of 0.1 mm is preferably 75% or higher, more preferably 80% or
higher, and
even more preferably 85% or higher. The upper limit of the total light
transmittance of
the resin component is not particularly limited, and the total light
transmittance may be
100% or lower, or 95% or lower, for example. The total light transmittance is
determined according to JIS K 7105.
[0112]
It is preferable that the resin component has a high glass transition
temperature
(Tg), whereby heat resistance of the resin composition and various molded
products
obtained therefrom can be enhanced. The glass transition temperature of the
resin
component is preferably, for example, 50 C or higher, more preferably 70 C or
higher,
and even more preferably 80 C or higher. The upper limit of the glass
transition
temperature of the resin component is not particularly limited, and from the
viewpoint
of securing molding processability of the resin composition, it is preferably,
for example,
.. 380 C or lower.
[0113]
The resin composition may be a thermoplastic resin composition that can be
used for molding such as injection molding and extrusion molding, or may be a
paintable resin composition that can be applied by a spin coating method, a
solvent
casting method, a roll coating method, a spray coating method, a bar coating
method, a
38
CA 03014400 2018-08-13
dipping coating method, a screen printing method, a flexographic printing
method, an
inkjet method, or the like.
[0114]
In the case where the resin composition is a thermoplastic resin composition,
a
molded product can be obtained from the resin composition by injection
molding,
extrusion molding, vacuum molding, compression molding, blow molding or the
like.
In this method, a thermoplastic resin is used as the resin component, and the
oxocarbon
compound and the thermoplastic resin are blended and heat-molded, thereby
obtaining a
molded product. For example, the oxocarbon compound may be added to a powder
or
pellet of a base resin, heated to about 150 C to 350 C to be melted, and then
molded.
The shape of the molded product is not particularly limited, and examples
thereof
include a plate, a sheet, a granule, a powder, a block, a particle
agglomerate, a sphere,
an ellipsoid, a cube, a column, a rod, a cone, a cylinder, a spicular, a
fibrous, a hollow
filamentous, a porous, and others. The molded product may have any deformed
shape.
In kneading the resin, additives such as an ultraviolet absorber and a
plasticizer, that are
used for ordinary resin molding, may be added.
[0115]
In the case where the resin composition is a paintable resin composition, a
liquid or paste-like resin composition containing the oxocarbon compound is
coated on
a transparent substrate (for example, a resin plate, a film, a glass plate or
the like),
whereby a film having a thickness of 200 pm or less or a planar molded product
such as
a plate having a thickness of more than 200 p.m can be formed. The paintable
resin
composition comprises the oxocarbon compound, a resin component and a liquid
medium, and can be obtained, for example, by dissolving the oxocarbon compound
in a
solvent containing a resin component, or dispersing the oxocarbon compound in
a
dispersion medium containing a resin component.
[0116]
In the case where the resin composition is a paintable resin composition, a
solvent-soluble resin that is soluble in an organic solvent is preferably used
as the resin
component. The solvent-soluble resin means a resin soluble in an organic
solvent, and
39
CA 03014400 2018-08-13
a resin which is soluble in 1 part by mass or more based on 100 parts by mass
of an
organic solvent is preferable. When the resin component is a solvent-soluble
resin, a
thin film having a smaller thickness can be easily produced by forming a film
using, for
example, a spin coating method, a solvent casting method, or the like. The
solvent-soluble resin may have a reactive group capable of undergoing a
crosslinking
reaction (curing reaction), wherein the reactive group may be, for example, a
ring-opening polymerizable group such as an epoxy group, an oxetane ring and
an
ethylene sulfide group, or a radically curable group and/or an addition
curable group
such as an acrylic group, a methacryl group and a vinyl group. Examples of the
solvent-soluble resin include, for example, a polyimide resin, a polyamide-
imide resin, a
fluorinated aromatic polymer, a (meth)acrylic resin, a polyamide resin, an
aramid resin,
a polysulfone resin, a cycloolefin resin, an urethane resin, a phenolic resin,
an epoxy
resin, a polyarylate resin, a polycarbonate resins, and others.
[0117]
As the resin, a polyimide resin, a polyamide-imide resin, a fluorinated
aromatic
polymer, a (meth)acrylic resin, a polysulfone resin, a cycloolefin resin, an
epoxy resin, a
polyarylate resin, and a polycarbonate resin are preferable from the viewpoint
of
excellent transparency and heat resistance.
[0118]
The polyimide resin is a polymer having an imide bond in the repeating unit of
the main chain, and can be obtained, for example, by polymerizing a
tetracarboxylic
acid dianhydride and a diamine to obtain a polyamic acid, and dehydrating and
cyclizing (namely, imidizing) it. As the polyimide resin, an aromatic
polyimide in
which aromatic rings are linked by an imide bond is preferably used. As the
polyimide
resin, for example, Kapton (registered trademark) available from Du Pont,
Aurum
(registered trademark) available from Mitsui Chemicals, Meldin (registered
trademark)
available from Saint-Gobain, TPS (registered trademark) T13 000 series
available from
Toray Plastics Precision Co., Ltd., and others can be used.
[0119]
The polyamide-imide resin is a polymer having an amide bond and an imide
CA 03014400 2018-08-13
bond in the repeating unit of the main chain. As the polyamide-imide resin,
for
example, TorIon (registered trademark) available from Solvay Advanced
Polymers,
Vylomax (registered trademark) available from Toyobo Co., Ltd., TPS
(registered
trademark) TI5000 series available from Toray Plastic Precision Co., Ltd., and
others
can be used.
[0120]
The fluorinated aromatic polymer is polymer having a repeating unit
containing an aromatic ring having one or more fluorine atoms and at least one
bond
selected from the group consisting of an ether bond, a ketone bond, a sulfone
bond, an
amide bond, an imide bond and an ester bond. Among them, a polymer essentially
having a repeating unit containing an aromatic ring having one or more
fluorine atoms
and an ether bond. As the fluorinated aromatic polymer, for example, those
described
in Japanese Unexamined Patent Application Publication No. 2008-181121 can be
used.
[0121]
The (meth)acrylic resin is a polymer having a repeating unit derived from
(meth)acrylic acid or a derivative thereof, and for example, a resin having a
repeating
unit derived from a (meth)acrylic acid ester such as a poly(meth)acrylic ester
resin is
preferably used. The (meth)acrylic resin also preferably has a ring structure
in the
main chain, and examples of the ring structure include, for example, carbonyl
group-containing ring structures such as a lactone ring structure, a glutaric
anhydride
structure, a glutarimide structure, a maleic anhydride structure and a
maleimide ring
structure; and carbonyl group-free ring structures such as an oxetane ring
structure, an
azetidine ring structure, a tetrahydrofuran ring structure, a pyrrolidine ring
structure, a
tetrahydropyran ring structure and a piperidine ring structure. The
carbonyl
group-containing ring structure may also include a structure containing a
carbonyl
derivative group such as an imide group. As the (meth)acrylic resin having a
carbonyl
group-containing ring structure, those disclosed in, for example, Japanese
Unexamined
Patent Application Publication Nos. 2004-168882, 2008-179677, WO 2005/54311,
Japanese Unexamined Patent Application Publication No. 2007-31537, and others
can
be used.
41
CA 03014400 2018-08-13
[0122]
The polysulfone resin is a polymer having a repeating unit containing an
aromatic ring, a sulfonyl group (-SO2-), and an oxygen atom. As the
polysulfone resin,
for example, Sumika Excel (registered trademark) PES3600P or PES4100P
available
from Sumitomo Chemical Co., Ltd., UDEL (registered trademark) P-1700 available
from Solvay Specialty Polymers Co., Ltd., or the like can be used.
[0123]
The cycloolefin resin is a polymer obtained by polymerizing a cycloolefin as
at
least a part of the monomer component and is not particularly limited as long
as it has
an alicyclic structure in a part of the main chain. As the cycloolefin resin,
for example,
Topas (registered trademark) available from Polyplastics Co., Ltd., Apel
(registered
trademark) available from Mitsui Chemicals, Zeonex (registered trademark) and
Zeonor
(registered trademark) available from Zeon Corporation, Arton (registered
trademark)
available from JSR Corporation, and others can be used.
[0124]
The epoxy resin is a resin that can be cured by crosslinking epoxy compounds
(prepolymer) in the presence of a curing agent or a curing catalyst. Examples
of the
epoxy compound include aromatic epoxy compounds, aliphatic epoxy compounds,
alicyclic epoxy compounds, hydrogenated epoxy compounds, and others. As the
epoxy resin, for example, fluorene epoxy (Ogsol (registered trademark) PG-100)
available from Osaka Gas Chemicals Co., Ltd., a bisphenol A epoxy compound
(JER
(registered trademark) 828EL) and a hydrogenated bisphenol A epoxy compound
(JER
(registered trademark) YX8000) available from Mitsubishi Chemical Corporation,
an
alicyclic liquid epoxy compound (Celloxide (registered trademark) 2021P)
available
from Daicel Corporation, and others can be used.
[0125]
The polyarylate resin is a polymer obtained by polycondensation of a dihydric
phenol compound and a dibasic acid (for example, an aromatic dicarboxylic acid
such
as phthalic acid), and has a repeating unit containing an aromatic ring and an
ester bond
in the main chain. As the polyarylate resin, for example, Vectran (registered
42
CA 03014400 2018-08-13
=
trademark) available from Kuraray Co., Ltd., U polymer (registered trademark)
and
Unifiner (registered trademark) available from Unitika Ltd., and others can be
used.
[0126]
The polycarbonate resin is a polymer containing a carbonate group
(-0-(C=0)-0-) in the repeating unit of the main chain. As the polycarbonate
resin, for
example, Panlite (registered trademark) available from Teijin, Iupilon
(registered
trademark), Novarex (registered trademark) and Xantar (registered trademark)
available
from Mitsubishi Engineering-Plastics Corporation, SD polyca (registered
trademark)
available from Sumika Styron Polycarbonate, and others can be used.
[0127]
The resin composition may contain a liquid medium such as an organic solvent;
and for example, in the case where the resin composition is a paintable resin
composition, the resin composition can be easily coated since it contains a
liquid
medium. The liquid medium may function as a solvent of the oxocarbon compound
or
may function as a dispersion medium. Examples of the liquid medium include,
for
example, ketones such as methyl ethyl ketone, methyl isobutyl ketone and
cyclohexanone; glycol derivetives (an ether compound, an ester compound, an
ether
ester compound, and the like) such as PGMEA (2-acetoxy-1 -methoxypropane),
ethylene
glycol monobutyl ether, ethylene glycol monoethyl ether and ethylene glycol
ethyl ether
acetate; amides such as N,N-dimethylacetamide; esters such as ethyl acetate,
propyl
acetate and butyl acetate; pyrrolidones such as N-methyl-pyrrolidone
(concretely,
1-methy1-2-pyrrolidone and the like); aromatic hydrocarbons such as toluene
and
xylene; aliphatic hydrocarbons such as cyclohexane and heptane; ethers such as
tetrahydrofuran, dioxane, diethyl ether and dibutyl ether; and others. These
liquid
media may be used alone, or two or more of them may be used in combination.
[0128]
The content of the liquid medium is preferably 50 mass% or more, more
preferably 70 mass% or more, and preferably less than 100 mass%, more
preferably 95
mass% or less, based on 100 mass% of the resin composition. By adjusting the
content of the liquid medium within such a range, it becomes easy to obtain
the resin
43
CA 03014400 2018-08-13
=
composition having a high concentration of the oxocarbon compound.
[0129]
Incidentally, amides such as N,N-dimethylacetamide may decompose the
oxocarbon compound, so that the use amount thereof is preferably small.
Therefore,
the content of amides is preferably 60 mass% or less, more preferably 40 mass%
or less,
even more preferably 20 mass% or less, still even more preferably 5 mass% or
less, and
particularly preferably 0 mass% (that is, amides are not contained).
[0130]
The resin composition may contain, for example, a compound having an
absorption ability in a wavelength range of 350 nm to 400 nm (that is,
ultraviolet
absorber). The existance of these compounds suppresses deterioration of the
resin
composition due to the light in the wavelength range of 350 nm to 400 nm. In
the case
where a compound having an absorption ability in the wavelength range of 350
nm to
400 nm is used in combination, TINUVIN (registered trademark) series available
from
BASF Co., Ltd., Zislizer (registered trademark) series available from Sankyo
Kasei Co.,
Ltd., Adk Stab (registered trademark) series available from Adeka Corporation,
Sumisorb (registered trademark) series available from Sumitomo Chemical Co.,
Ltd.,
Biosorb (registered trademark) series available from Kyodo Chemical Co., Ltd.,
Seesorb (registered trademark) series available from Shipro Kasei Kaisha, and
others
can be used as the compound having an absorption ability in the wavelength
range of
350 nm to 400 nm.
[0131]
The resin composition may contain a surface conditioner, which suppresses
appearance defects such as striation and dents in a resin layer when the resin
composition is cured to form the resin layer. The kinds of the surface
conditioner are
not particularly limited, and a siloxane surfactant, an acetylene glycol
surfactant, a
fluorine surfactant, an acrylic leveling agent, or the like can be used. As
the surface
conditioner, for example, BYK (registered trademark) series available from BYK
Chemie and KF series available from Shin-Etsu Chemical Co., Ltd., and others
can be
used.
44
CA 03014400 2018-08-13
[0132]
The resin composition may contain a dispersant, which stabilizes
dispersibility
of the oxocarbon compound in the resin composition, thereby suppressing
reaggregation.
The kinds of the dispersant is not particularly limited, and EFKA series
available from
Efka Additives, BYK (registered trademark) series available from BYK Chemie,
Solspers (registered trademark) series available from Lubrizol Japan, Disparon
(registered trademark) series available from Kusumoto Chemicals, Ajisper
(registered
trademark) series available from Ajinomoto Fine-Techno Co., Ltd., KP series
available
from Shin-Etsu Chemical Co., Ltd., Polyflow series available from Kyoeisha
Chemical
Co., Ltd., Megafac (registered trademark) series available from DIC
Corportaion,
Disper Aid series available from San Nopco Limited, and others can be used.
[0133]
The resin composition of the present invention may contain various additives
such as a plasticizer, a surfactant, a viscosity modifier, an antifoaming
agent, an
antiseptic agent, a specific resistance adjusting agent, and an adhesion
improver, as
needed.
[0134]
[3. Optical filter (Application example of resin composition)]
The resin composition of the present invention can be preferably used as a
resin
composition for preparing filter used in various applications such as optical
device
applications, display device applications, mechanical parts,
electric/electronic parts, and
others. As a filter for optical applications, it can be suitably applied to an
optical filter
of an image sensing device for particularly night vision, whereby an optical
filter
suitable for an image sensing device for night vision, of which the incident
angle
dependence of optical characteristics in a near-infrared region is reduced and
the
viewing angle is improved, can be obtained. The filter may be formed from a
single
resin layer or a plurality of resin layers, or may be integrated with a
support.
[0135]
The filter integrated with a support can be formed, for example, by coating
the
resin composition on a surface of the support (or in the case of having
another layer
CA 03014400 2018-08-13
such as a binder layer between the support and the resin layer, the surface of
the another
layer) using a spin coating method or a solvent casting method, and drying or
curing.
Or, the filter may be formed by thermocompression bonding a planar molded
product
formed from the resin composition to the support.
[0136]
The resin layer formed from the resin composition may be provided on only
one side of the support or may be provided on both sides thereof. The
thickness of the
resin layer is not particularly limited, and is preferably, for example, 0.5
gm or more,
more preferably 1 gm or more, and preferably 10 mm or less, more preferably 5
mm or
.. less, even more preferably 3 mm or less, particularly preferably 1 mm or
less, from the
viewpoint of securing desired near-infrared light cutoff performance.
[0137]
As the support, it is preferable to use a transparent substrate such as a
resin
plate, a resin film, a glass plate or the like. As the resin plate or the
resin film used for
the support, for example, one formed from the above-described resin component
is
preferably used. When a glass plate is used as the support, it is preferable
to provide a
binder layer formed from, for example, a silane coupling agent between the
support and
the resin layer. Thereby, adhesion between the resin layer and the glass
support can be
enhanced. A silane coupling agent may be contained in the resin composition
forming
the resin layer as an adhesion improver, and also in this case, adhesion
between the resin
layer and the glass support can be enhanced.
[0138]
On the resin layer formed from the resin composition, a protective layer
composed of the same or different resin as the resin layer, as a second resin
layer, may
be laminated. By providing the protective layer, durability (resistance to
decomposition) of the oxocarbon compound contained in the resin layer can be
enhanced. The protective layer may be provided on only one side of the resin
layer or
may be provided on both sides thereof. In the case where the resin layer is
provided
on the support, the protective layer is preferably provided on the surface of
the resin
layer opposite to the support.
46
CA 03014400 2018-08-13
[0139]
From the viewpoint of suppressing decomposition of the oxocarbon compound
contained in the resin layer, the protective layer is preferably formed to
have a low
oxygen permeability, for example, have an oxygen permeability of 100 cc/m2/day
or
lower, more preferably 70 cc/m2/day or lower, and even more preferably 50
cc/m2/day
or lower, at 23 C under dry condition, measured according to JIS K 7126-2
method.
The lower limit of the oxygen permeability is not particularly limited, and
may be 0
cc/m2/day. The oxygen permeability is measured by introducing oxygen gas into
a
chamber on one side of the test piece and introducing nitrogen gas into a
chamber on the
other side thereof. From the viewpoint of suppressing decomposition of the
oxocarbon
compound, the resin layer and/or the protective layer may contain an
ultraviolet
absorber.
[0140]
In the case of forming an optical filter from the resin composition of the
present invention, the optical filter may comprise a layer having
antireflection property
or anti-glare property for reducing glare of fluorescent lamp or the like
(antireflection
film), a layer having scratch prevention property, a transparent substrate
having other
functions, or the like.
[0141]
The optical filter may comprise a near-infrared reflection film (for example,
a
reflection film in the wavelength range of 700 nm to 800 nm) on the resin
layer. The
near-infrared reflection film is preferably provided on a light incident side
of the resin
layer. As the near-infrared reflection film, an aluminum vapor deposition
film, a noble
metal thin film, a resin film which contains indium oxide as a main component
and in
which metal oxide fine particles containing a small amount of tin oxide are
dispersed, a
dielectric multilayer film in which high refractive index material layers and
low
refractive index material layers are alternately laminated, or the like can be
employed.
Providing the near-infrared reflection film in the optical filter makes it
possible to cut
near-infrared light from transmitted light of the optical filter. The near-
infrared
reflection film may also have an ultraviolet ray reflective function.
47
CA 03014400 2018-08-13
[0142]
The near-infrared reflection film and the antireflection film (visible light
antireflection film) can be composed of a dielectric multilayer film in which
high
refractive index material layers and low refractive index material layers are
alternately
laminated. As a material constituting the high refractive index material
layer, a
material having a refractive index of 1.7 or more can be used, and a material
having a
refractive index in the range of 1.7 to 2.5 is usually selected. Examples of
the material
constituting the high refractive index material layer include, for example,
oxides such as
titanium oxide, zinc oxide, zirconium oxide, lanthanum oxide, yttrium oxide,
indium
oxide, niobium oxide, tantalum oxide, tin oxide, and bismuth oxide; nitrides
such as
silicon nitride; mixtures of the above oxides and nitrides, and the above
oxides or
nitrides with which a metal such as aluminum and copper or carbon is doped
(e.g.,
tin-doped indium oxide (ITO), antimony-doped tin oxide (ATO)), and others. As
a
material constituting the low refractive index material layer, a material
having a
refractive index of 1.6 or less can be used, and a material having a
refractive index in
the range of 1.2 to 1.6 is usually selected. Examples of the material
constituting the
low refractive index material layer include, for example, silicon dioxide
(silica),
alumina, lanthanum fluoride, magnesium fluoride, sodium aluminum hexafluoride,
and
others.
[0143]
The optical filter can be used as one of the constituents of the image sensing
device. The image sensing device is also called a solid-state image sensing
device or
an image sensor chip, and is an electronic component which converts light of a
subject
into an electrical signal or the like and outputs it. The image sensing device
usually
comprises a detection element (sensor) such as CCD (Charge Coupled Device) or
CMOS (Complementary Metal-Oxide Semiconductor) and may comprise a lens. The
image sensing device can be used for a mobile phone camera, a digital camera,
an
in-vehicle camera, a surveillance camera, a display element (e.g., LEDs and
the like),
and the like. In particular, the image sensing device including the optical
filter formed
from the resin composition of the present invention can be suitably applied to
a
48
CA 03014400 2018-08-13
surveillance camera for night vision or the like. The image sensing device
comprises
one or more of the above-described optical filters, and may further comprise
another
filter (for example, a visible light cutoff filter, an infrared cutoff filter,
an ultraviolet
cutoff filter, or the like), as needed.
[0144]
The resin composition of the present invention can be used for applying to any
base material, in addition to the filter for optical use described above. The
base
material is not particular limited, as long as a resin layer can be formed by
applying the
resin composition, and examples thereof include, for example, steels,
nonferrous metals
(light metals, noble metals, rare metals, rare earths, copper, zinc, lead,
tin, and the like),
wood, glass, concrete, stone, ceramics, resin, rubber, leather, paper, cloth,
hair, skin and
others. The shape of the base material is also not particularly limited, and
examples
thereof include a granular, a powder, a block, an aggregate of them, a plate,
a sheet, a
sphere, an ellipsoid, a cubic, a column, a rod, a cone, a cylinder, a
spicular, a fibrous, a
hollow filamentous, a porous, and others.
[0145]
[4. Ink composition]
The oxocarbon compound of the present invention can be applied to an ink
composition. The ink composition contains at least the oxocarbon compound of
the
present invention and a liquid medium. The ink composition of the present
invention
can effectively absorb light in a wavelength range exceeding, for example, 850
nm,
have high visible light transmittance, and be excellent in invisibility, so it
can be
suitably used as a security ink.
[0146]
The oxocarbon compound contained in the ink composition may be a
squarylium compound, a croconium compound, or both. The oxocarbon compound
contained in the ink composition may be only one kind or may be two kinds or
more.
[0147]
Two or more kinds of the squarylium compounds represented by the above
formula (1) may be contained in the ink composition, or two or more kinds of
the
49
CA 03014400 2018-08-13
croconium compounds represented by the above formula (2) may be contained in
the
ink composition. The thus formed ink composition can effectively absorb the
light in a
near-infrared region, improves solubility of the oxocarbon compound in the
liquid
medium, and facilitates forming the ink composition containing the oxocarbon
compound at a high concentration. In this case, from the viewpoint of
increasing the
solubility of the oxocarbon compound in the liquid medium, the ink composition
preferably contains at least squarylium compounds having a structure in which
RI and
R2 are different from each other in the above formula (1) or preferably
contains at least
croconium compounds having a structure in which R3 and R4 are different from
each
other in the above formula (2). More preferably, the ink composition contains
three or
more kinds of the oxocarbon compounds, and examples of such a ink composition
include, for example, a ink composition containing a squarylium compound in
which RI
and R2 are both Rx in the above formula (1), a squarylium compound in which RI
and
R2 are both Ry in the above formula (1) and a squarylium compound in which RI
is R.,
and R2 is Ry in the above formula (1), and a ink composition containing a
croconium
compound in which R3 and R4 are both Rõ in the above formula (2), a croconium
compound in which R3 and R4 are both Ry in the above formula (2) and a
croconium
compound which is R3 is Rõ and R4 is Ry in the above formula (2). Such ink
composition is preferable since it contains plural oxocarbon compounds and is
easy to
produce. Here, the above-described Rx and Ry represent any structural unit
represented
by the above formula (3), and Rx and Ry are not the same.
[0148]
The ink composition may contain other dyes together with the oxocarbon
compound of the present invention, as long as the desired performance
according to an
application is secured. Details of other dyes is made reference to the
descriptions of
other dyes which may be included in the resin composition. In the application
of a
security ink or the like, the content of other dyes is preferably 50 mass% or
less, more
preferably 20 mass% or less, and even more preferably 5 mass% or less,
relative to 100
mass% of the total of the oxocarbon compound of the present invention and the
other
dyes.
CA 03014400 2018-08-13
[0149]
The content of the oxocarbon compound of the present invention in the ink
composition is preferably 0.1 mass% or more, more preferably 1 mass% or more,
and
preferably 40 mass% or less, more preferably 30 mass% or less, even more
preferably
20 mass% or less, based on 100 mass% of the ink composition. In the case where
the
ink composition also contains other dyes, the total content of the oxocarbon
compound
of the present invention and other dyes is preferably within the above-
mentioned range.
Adjusting to such a range makes it easier to obtain a printed surface of
sufficient density
and to ensure stability of the oxocarbon compound and other dyes in the ink
composition.
[0150]
The liquid medium may function as a solvent of the oxocarbon compound or
may function as a dispersion medium. Examples of the liquid medium include,
for
example, ketones such as acetone, methyl ethyl ketone, methyl butyl ketone,
methyl
isobutyl ketone, diisobutyl ketone and cyclohexanone; alcohols such as
methanol,
ethanol, n-propyl alcohol, isopropyl alcohol, n-butyl alcohol, sec-butyl
alcohol,
tert-butyl alcohol, tert-amyl alcohol and n-hexanol; glycol derivetives (an
ether
compound, an ester compound, an ether ester compound, and the like) such as
ethylene
glycol, diethylene glycol, triethylene glycol, propylene glycol, ethylene
glycol
monomethyl ether, ethylene glycol monoethyl ether, ethylene glycol monobutyl
ether,
diethylene glycol monomethyl ether, diethylene glycol monoethyl ether,
diethylene
glycol monobutyl glycol, propylene glycol monomethyl ether, and propylene
glycol
monomethyl ether acetate (PGMEA); esters such as ethyl acetate, propyl
acetate, butyl
acetate, ethyl lactate, and 3-ethoxypropionate ester; aromatic hydrocarbons
such as
toluene and xylene; aliphatic hydrocarbons such as cyclohexane,
methylcyclohexane,
and heptane; cyclic ethers such as tetrahydrofuran and dioxane; and others.
These
liquid media may be used alone, or two or more of them may be used in
combination.
[0151]
The content of the liquid medium is preferably 30 mass% or more, more
preferably 40 mass% or more, even more preferably 50 mass% or more, and
preferably
51
CA 03014400 2018-08-13
95 mass% or less, more preferably 90 mass% or less, even more preferably 80
mass%
or less, based on 100 mass% of the ink composition. By adjusting the content
of the
liquid medium within such a range, solubility and dispersibility of the
oxocarbon
compound can be increased and it becomes easy to obtain the ink composition
having a
high concentration of the oxocarbon compound.
[0152]
The ink composition may comprise a resin component in addition to the liquid
medium. By blending the resin component, viscosity, adhesiveness or the like
of the
ink composition can be improved. As the resin component, a known resin used
for an
ink composition can be used, and examples thereof include a (meth)acrylic
resin, a
(meth)acrylic urethane resin, a polyvinyl chloride resin, a polyvinylidene
chloride resin,
a polyolefin resin (e.g., polyethylene resin, polypropylene resin, and the
like), a
cycloolefin resin (including cycloolefin copolymer, norbomene resin, and the
like), a
petroleum resin, a rosin resin, a rosin ester resin, an urea resin, a melamine
resin, an
urethane resin, a styrene resin, a styrene-acrylic resin, a styrene-maleic
acid resin,
polyvinyl acetate, an ethylene-vinyl acetate resin, a vinyl acetal resin, a
polyamide resin
(e.g., nylon and the like), an aramid resin, a polyimide resin, a polyamide-
imide resin,
an alkyd resin, a phenolic resin, an epoxy resin, a polyester resin (e.g.,
polybutylene
terephthalate resin, polyethylene terephthalate resin, polyarylate resin and
the like), a
polysulfone resin, a butyral resin, a polycarbonate resin, a polyether resin,
an
acrylonitrile butadiene styrene resin, an acrylonitrile-styrene copolymer, a
cellulose
derivative (e.g., ethyl cellulose, cellulose acetate, nitrocellulose, and the
like), a silicone
resin, a modified silicone resin (e.g., (meth)acrylic silicone resin, alkyl
polysiloxane
resin, silicone urethane resin, silicone polyester resin, silicone acrylic
resin, and the like),
a fluorine resin (e.g., fluorinated aromatic polymer, polytetrafluoroethylene,
perfluoroalkoxy fluorine resin, fluorinated polyaryl ether ketone, fluorinated
polyimide,
fluorinated polyamic acid, fluorinated polyether nitrile, and the like), a
diallyl phthalate
resin, a cumarone-indene resin, a terpene phenol resin, a xylene resin, an
alkyd resin, a
maleic acid resin, a fumaric acid resin, and others. The blending amount of
the resin
component in the case of blending the resin component is preferably 1 mass% or
more,
52
CA 03014400 2018-08-13
more preferably 5 mass% or more, and preferably 60 mass% or less, more
preferably 50
mass% or less, based on 100 mass% of the ink composition.
[0153]
The ink composition may contain various additives such as an ultraviolet
absorber, a surface conditioner, a dispersant, a conductivity regulator, a
polymerization
inhibitor, a leveling agent, an antioxidant and the like, as needed. Details
of these
additives is made reference to the descriptions of additives in the resin
composition.
[0154]
The ink composition can be produced by a known method. For example, the
ink composition can be prepared by mixing a dye including the oxocarbon
compound of
the present invention, a liquid medium and, if necessary, a polymerizable
monomer, a
polymerization initiator and various additives, using a mixer such as a sand
mill. If
necessary, the thus obtained mixture may be filtered with a filter having a
pore diameter
of 3 gm or less or 1 i.tm or less.
[0155]
The ink composition of the present invention can be applied to various inks
such as an ordinary solvent-based ink used for a screen printing ink, a
gravure printing
ink, an offset printing ink, a flexographic printing ink, an ink jet recording
ink or the
like, a light (ultraviolet light, visible light, infrared light) curable ink,
and a water-based
ink.
[0156]
The ink composition can be printed (applied) on any material, thereby forming
a printed matter. A base material to be printed may be paper, cloth, wood,
glass,
concrete, stone, ceramics, resin, rubber, leather, steel, nonferrous metal
(light metal,
precious metal, rare metal, rare earth, copper, zinc, lead, tin, and the
like), hair, skin or
the like, for example. The shape of the base material to be printed is also
not
particularly limited, and examples thereof include a sheet, a plate, a sphere,
an ellipsoid,
a cubic, a column, a rod, a cone, a cylinder, a granular, a powder, a block, a
particle
agglomerate, a spicular, a fibrous, a hollow filamentous, a porous and others.
[0157]
53
[5. Others]
= In addition to the resin composition, the resin molding, the photothermal
conversion material for laser welding, and the ink composition as described
above, the
oxocarbon compound of the present invention can be applied to photofixing
materials
utilizing light which is less likely to cause troubles due to pressurization
or heating
(e.g., electrostatic charge development toner for a flash fixing method),
cosmetic
materials having a near-infrared light absorbing or cutting function,
materials for light
detection and distance measurement (LIDAR) system, and the others.
EXAMPLES
[0159]
The present invention will be hereinafter described more specifically with
reference to examples; however, the present invention is not restricted by
these
examples, and can be certainly put into practice after appropriate
modifications within
in a range meeting the gist of the above and the below, all of which are
included in the
technical scope of the present invention
[0160]
(1) Synthesis of Dye Compounds
(1-1) Synthesis Example 1 (Synthesis of Croconium Compound 1)
60 mL of hydrochloric acid was added into a 300 mL four-necked flask, the
temperature in the flask was cooled to -10 C or lower, and while keeping the
temperature in the flask not exceed 0 C, 5.13 g (0.024 mol) of 3-
aminofluoranthene
was added thereto and dissolved. After the exotherm subsided, a solution of
1.63 g
(0.024 mol) of sodium nitrite in 11 g of distilled water was added dropwise
over 1
hour while keeping the temperature in the flask at -10 C or lower. After
completion
of the
54
CA 3014400 2020-02-20
CA 03014400 2018-08-13
dropwise addition, a solution of 26.63 g (0.118 mol) of tin chloride dihydrate
dissolved
in 27 mL of hydrochloric acid was added dropwise over 1 hour while keeping the
temperature in the flask not exceed 0 C, thereby proceeding the reaction.
After
completion of the reaction, cake obtained by filtration was dried at 60 C for
12 hours
using a vacuum dryer, thereby obtaining 5.5 g of fluoranthene-3-hydrazine
hydrochloride. The yield based on 3-aminofluoranthene was 87.2 mol%.
[0161]
Subsequently, in a 50 mL four-necked flask, 3.01 g (0.011 mol) of
fluoranthene-3-hydrazine hydrochloride obtained above, 0.96 g (0.011 mol) of
3-methyl-2-butanone, and 20 g of 1-butanol as a solvent were placed and
reacted at
60 C for 4 hours under nitrogen flow (5 mL/min) while stirring using a
magnetic stirrer,
thereby obtaining a condensed heterocyclic compound. After completion of the
reaction, the solution containing the condensed heterocyclic compound was
cooled to
room temperature, filtered, and the obtained filtrate was transferred to a 200
mL
four-necked flask. 0.78 g (0.006 mol) of croconic acid and 20 g of toluene
were added
thereto, and the mixture was stirred using a magnetic stirrer under nitrogen
flow (5
mL/min), and the reaction was carried out under reflux condition for 6 hours
while
removing water eluted using a Dean-Stark apparatus. After completion of the
reaction,
thus obtained reaction solution was concentrated with an evaporator, the
obtained solid
was purified by a column chromatography (developing solvent: chloroform), and
the
purified isolate was further recrystallized in methanol, thereby obtaining
0.45 g of a
croconium compound 1, the objective product. The yield based on croconic acid
was
12.2 mol%.
[0162]
The obtained compound was identified by a mass spectrometer ("LCMS-2020"
manufactured by Shimadzu Corporation, M/Z=50-2000, positive/negative
simultaneous
scanning). Specifically, about 1 mg of the obtained compound was applied to a
glass
rod and adhered thereto, ionized with a direct ionization unit (DART) ("DART-
OS"
manufactured by Shimadzu Corporation, heater temperature: 500 C), and
introduced
into the mass spectrometer, whereby the mass spectrum of the compound was
measured.
i
CA 03014400 2018-08-13
[0163]
[Chemical Formula 20]
0 NH2 1. NaNO2aq
N HNH2HCI
2. SnC12.2H20 v.
Conc HCI aq ohi 1 11 o
APO butanol
411*.
0 0-
o) ro
HO OH , , 0
___________________________ N. 11 NH HN / \
toluene
butanol 011 0 0- \¨i110110
Croconium Compound 1
[0164]
(1-2) Synthesis Example 2 (Synthesis of Croconium Compound 2)
A croconium compound 2 shown in Table 1 was obtained in the same manner
as in the Synthesis Example 1, except that ethyl 2-methylacetoacetate was used
instead
of 3-methyl-2-butanone in the Synthesis Example 1. The yield based on croconic
acid
was 5.8 mol%.
[0165]
(1-3) Synthesis Example 3 (Synthesis of Croconium Compound 3)
4.45 g (0.022 mol) of 3-(trifluoromethyl)phenylacetone was placed in a 300 mL
four-necked flask under nitrogen flow, temperature in the flask was cooled to -
10 C or
lower, and 19.06 g of hexamethyldisilazane lithium (1.3M tetrahydrofuran
solution),
8.46 g (0.066 mol) of N,N'-dimethylpropylene urea, 2.77 g (0.022 mol) of
manganese
chloride and 4.14 g (0.024 mol) of benzyl bromide were sequentially added
thereto
while keeping the temperature in the flask not exceed 0 C, followed by
stirring
overnight without controlling the temperature. The resulting reaction solution
was
quenched with dilute hydrochloric acid, extracted with ethyl acetate, and
washed three
times with brine. The obtained organic phase was dehydrated with sodium
sulfate,
concentrated using an evaporator, and then the obtained solid was purified by
a column
chromatography (developing solvent: chloroform), thereby obtaining 5.0 g of
56
CA 03014400 2018-08-13
4-phenyl-3-(3-(trifluoromethyl)phenyl)butan-2-one. The yield based on
3-(trifluoromethyl)phenylacetone was 78.1 mol%. The synthesis procedures
thereafter
were conducted in the same manner as in the Synthesis Example 1, except that
4-phenyl-3-(3-(trifluoromethyl)phenyl)butan-2-one was used
instead of
3-methyl-2-butanone in the Synthesis Example 1, thereby obtaining a croconium
compound 3 shown in Table 1. The yield based on croconic acid was 10.1 mol%.
[0166]
(1-4) Synthesis Example 4 (Synthesis of Croconium Compound 4)
5-Phenyl-3-(3-(trifluoromethyl)phenyl)pentan-2-one was obtained in the same
manner as in the Synthesis Example 3, except that (2-iodoethyl)benzene was
used
instead of benzyl bromide in the Synthesis Example 3. The yield based on
3-(trifluoromethyl)phenylacetone was 86.6 mol%. The synthesis procedures
thereafter
were conducted in the same manner as in the Synthesis Example 1, except that
5-phenyl-3-(3-(trifluoromethyl)phenyl)pentan-2-one was used instead of
3-methyl-2-butanone in the Synthesis Example 1, thereby obtaining a croconium
compound 4 shown in Table 1. The yield based on croconic acid was 8.3 mol%.
[0167]
(1-5) Synthesis Example 5 (Synthesis of Croconium Compound 5)
3-(3,4-Dimethoxypheny1)-5-phenylpentan-2-one was obtained in the same
manner as in the Synthesis Example 3, except that 3,4-dimethoxyphenylacetone
was
used instead of 3-(trifluoromethyl)phenylacetone and (2-iodoethyl)benzene was
used
instead of benzyl bromide in the Synthesis Example 3. The yield based on
3,4-dimethoxyphenylacetone was 95.1 mol%. The synthesis procedures thereafter
were conducted in the same manner as in the Synthesis Example 1, except that
3-(3,4-dimethoxypheny1)-5-phenylpentan-2-one was used instead of
3-methyl-2-butanone in the Synthesis Example 1, thereby obtaining a croconium
compound 5 shown in Table 1. The yield based on croconic acid was 15.4 mol%.
[0168]
(1-6) Synthesis Example 6 (Synthesis of Croconium Compound 6)
3-(3-(Trifluoromethyl)phenyl)decan-2-one was obtained in the same manner as
57
CA 03014400 2018-08-13
in the Synthesis Example 3, except that 1-iodoheptane was used instead of
benzyl
bromide in the Synthesis Example 3. The
yield based on
3-(trifluoromethyl)phenylacetone was 89.1 mol%. The synthesis procedures
thereafter
were conducted in the same manner as in the Synthesis Example 1, except that
3-(3-(trifluoromethyl)phenyl)decan-2-one was used instead of 3-methyl-2-
butanone in
the Synthesis Example 1, thereby obtaining a croconium compound 6 shown in
Table 1.
The yield based on croconic acid was 9.2 mol%.
[0169]
(1-7) Synthesis Example 7 (Synthesis of Croconium Compound 7)
A croconium compound 7 shown in Table I was obtained in the same manner
as in the Synthesis Example 1, except that 1,1-diphenylacetone was used
instead of
3-methyl-2-butanone in the Synthesis Example 1. The yield based on croconic
acid
was 1.8 mol%.
[0170]
(1-8) Synthesis Example 8 (Synthesis of Croconium Compound 8)
Into a 300 mL four-necked flask placed in a water bath, 6.73 g (0.060 mol) of
potassium tert-butoxide, 35 g of super dehydrated tetrahydrofuran, 3.32 g
(0.020 mol)
of fluorene, 3.52 g (0.040 mol) of ethyl acetate were sequentially added under
nitrogen
flow, while paying attention to heat generation, and then the mixture was
stirred under
reflux condition for 3 hours while heating by a hot water bath. After cooling
the
obtained reaction solution and quenching with dilute hydrochloric acid, the
resultant
was extracted with ethyl acetate and washed three times with brine. The
obtained
organic phase was dehydrated with sodium sulfate, concentrated with an
evaporator, and
the obtained solid was purified by a column chromatography (developing
solvent:
chloroform), thereby obtaining 4.1 g of 9-acetyl-9H-fluorene. The yield based
on
fluorene was 97.6%. The synthesis procedures thereafter were conducted in the
same
manner as in the Synthesis Example 1, except that 9-acetyl-9H-fluorene was
used
instead of 3-methyl-2-butanone and tert-amyl alcohol was used instead of 1-
butanol as a
solvent in the Synthesis Example 1, thereby obtaining a croconium compound 8
shown
in Table 1. The yield based on croconic acid was 30.5 mol%.
58
[0171]
(1-9) Synthesis Example 9 (Synthesis of Croconium Compound 9)
2-Bromo-9-acetyl-9H-fluorene was obtained in the same manner as in the
Synthesis Example 8, except that 2-bromo-9H-fluorene was used instead of
fluorene
in the Synthesis Example 8. The yield based on 2-bromo-9H-fluorene was 97.1
mol%. The synthesis procedures thereafter were conducted in the same manner as
in
the Synthesis Example 1, except that 2-bromo-9-acetyl-9H-fluorene was used
instead
of 3-methyl-2-butanone in the Synthesis Example 1, thereby obtaining a
croconium
compound 9 shown in Table 1. The yield based on croconic acid was 10.4 mol%.
[0172]
(1-10) Synthesis Example 10 (Synthesis of Croconium Compound 10)
2-Iodo-9-acetyl-9H-fluorene was obtained in the same manner as in the
Synthesis Example 8, except that 2-iodo-9H-fluorene was used instead of
fluorene in
the Synthesis Example 8. The yield based on 2-iodo-9H-fluorene was 95.8 mol%.
The synthesis procedures thereafter were conducted in the same manner as in
the
Synthesis Example 1, except that 2-iodo-9-acetyl-9H-fluorene was used instead
of 3-
methy1-2-butanone in the Synthesis Example 1, thereby obtaining a croconium
compound 10 shown in Table 1. The yield based on croconic acid was 18.9 mol%.
[0173]
(1-11) Synthesis Example 11 (Synthesis of Croconium Compound 11)
2-Trifluoromethy1-9H-fluorene was synthesized using the method described in
Gonda et al., "Efficient Copper-Catalyzed Trifluoromethylation of Aromatic and
Heteroaromatic Iodides: The Beneficial Anchoring Effect of Boranes", Organic
Letters, 16 (2014): 4268-4271. Then, 2-trifluoromethy1-9-acetyl-9H-fluorene
was
obtained in the same manner as in the Synthesis Example 8, except that 2-
trifluoromethy1-9H-fluorene was used instead of fluorene in the Synthesis
Example 8.
The yield based on 2-trifluoromethy1-9H-fluorene was 91.4 mol%. The synthesis
procedures thereafter were conducted in the same manner as in the Synthesis
Example
1, except that 2-trifluoromethy1-9-acetyl-9H-fluorene was used instead of 3-
methy1-2-
butanone in the Synthesis Example 1, thereby obtaining a croconium compound 11
shown in Table 2. The yield based on croconic acid was 10.1 mol%.
59
CA 3014400 2020-02-20
CA 03014400 2018-08-13
[0174]
(1-12) Synthesis Example 12 (Synthesis of Croconium Compound 12)
2-Cyano-9H-fluorene was synthesized using the method described in the
Journal of Organic Chemistry, vol.69, p.987-990 (2004). Then,
2-cyano-9-acetyl-911-fluorene was obtained in the same manner as in the
Synthesis
Example 8, except that 2-cyano-9H-fluorene was used instead of fluorene in the
Synthesis Example 8. The yield based on 2-eyano-9H-fluorene was 95.9 mol%. The
synthesis procedures thereafter were conducted in the same manner as in the
Synthesis
Example 1, except that 2-cyano-9-acetyl-9H-fluorene was used instead of
3-methyl-2-butanone in the Synthesis Example 1, thereby obtaining a croconium
compound 12 shown in Table 2. The yield based on croconic acid was 12.4 mol%.
[0175]
(1-13) Synthesis Example 13 (Synthesis of Croconium Compound 13)
9-Acetyl-9H-2,7-dibromofluorene was obtained in the same manner as in the
Synthesis Example 8, except that 2,7-dibromofluorene was used instead of
fluorene in
the Synthesis Example 8. The yield based on 2,7-dibromofluorene was 95.9 mol%.
The synthesis procedures thereafter were conducted in the same manner as in
the
Synthesis Example 1, except that 9-acetyl-9H-2,7-dibromofluorene was used
instead of
3-methyl-2-butanone in the Synthesis Example 1, thereby obtaining a croconium
compound 13 shown in Table 2. The yield based on croconic acid was 18.5 mol%.
[0176]
(1-14) Synthesis Example 14 (Synthesis of Croconium Compound 14)
2-Bromo-7-iodo-9-acetyl-9H-fluorene was obtained in the same manner as in
the Synthesis Example 8, except that 2-bromo-7-iodo-911-fluorene was used
instead of
fluorene in the Synthesis Example 8. The yield based on 2-bromo-7-iodo-9H-
fluorene
was 95.9 mol%. The synthesis procedures thereafter were conducted in the same
manner as in the Synthesis Example 1, except that
2-bromo-7-iodo-9-acetyl-9H-fluorene was used instead of 3-methyl-2-butanone in
the
Synthesis Example 1, thereby obtaining a croconium compound 14 shown in Table
2.
The yield based on croconic acid was 19.3 mol%.
CA 03014400 2018-08-13
[0177]
(1-15) Synthesis Example 15 (Synthesis of Croconium Compound 15)
2,7-Di-tert-butyl-9-acetyl-9H-fluorene was obtained in the same manner as in
the Synthesis Example 8, except that 2,7-di-tert-butyl-9H-fluorene was used
instead of
fluorene in the Synthesis Example 8. The yield based on 2,7-di-tert-butyl-9H-
fluorene
was 97.3 mol%. The synthesis procedures thereafter were conducted in the same
manner as in the Synthesis Example 1, except that
2,7-di-tert-butyl-9-acetyl-9H-fluorene was used instead of 3-methyl-2-butanone
in the
Synthesis Example 1, thereby obtaining a croconium compound 15 shown in Table
2.
.. The yield based on croconic acid was 9.3 mol%.
[0178]
(1-16) Synthesis Example 16 (Synthesis of Croconium Compound 16)
9-Acetyl-9H-fluorene was obtained by the method described in the Synthesis
Example 8 and 9-acetyl-9H-2,7-dibromofluorene was obtained by the method
described
in the Synthesis Example 13. The synthesis procedures thereafter were
conducted in
the same manner as in the Synthesis Example 1, except that 9-acetyl-9H-
fluorene and
9-acety1-911-2,7-dibromofluorene were used in a molar ratio of 1:1 instead of
3-methy1-2-butanone in the Synthesis Example 1, thereby obtaining a croconium
compound 16 (mixture) shown in Table 2. The yield based on croconic acid was
12.1
mol%.
[0179]
(1-17) Synthesis Example 17 (Synthesis of Croconium Compound 17)
1-(1H-inden-1 -yl)ethan-l-one was obtained in the same manner as in the
Synthesis Example 8, except that indene was used instead of fluorene in the
Synthesis
Example 8. The yield based on indene was 74.8 mol%. The synthesis procedures
thereafter were conducted in the same manner as in the Synthesis Example 1,
except
that 1-(1H-indene-1-yl)ethan-1-one was used instead of 3-methyl-2-butanone in
the
Synthesis Example 1, thereby obtaining a croconium Compound 17 shown in Table
2.
The yield based on croconic acid was 1.5 mol%.
[0180]
61
CA 03014400 2018-08-13
=
(1-18) Synthesis Example 18 (Synthesis of Croconium Compound 18)
9-Acetyl-9H-xanthene was obtained in the same manner as in the Synthesis
Example 8, except that xanthene was used instead of fluorene in the Synthesis
Example
8. The yield based on xanthene was 84.2 mol%. The synthesis procedures
thereafter
were conducted in the same manner as the Synthesis Example 1, except that
9-acetyl-91-1-xanthene was used instead of 3-methyl-2-butanone in the
Synthesis
Example 1, thereby obtaining a croconium compound 18 shown in Table 2. The
yield
based on croconic acid was 11.7 mol%.
[0181]
(1-19) Synthesis Example 19 (Synthesis of Comparative Croconium
Compound 1)
Into a 50 mL four-necked flask, 0.73 g (0.004 mol) of
2,3,3-trimethy1-4,5-benzo-314-indole, 0.25 g (0.002 mol) of croconic acid, and
10 g of
1-butanol and 10 g of toluene as solvents were added, and the mixture was
reacted
under reflux condition for 6 hours, while stirring under nitrogen flow (5
mL/min) using
a magnetic stirrer and removing water eluted using a Dean Stark apparatus.
After
completion of the reaction, the obtained reaction solution was concentrated
with an
evaporator, the obtained solid was purified by a column chromatography
(developing
solvent: chloroform), and the purified isolate was further recrystallized in
methanol,
whereby 0.30 g of a comparative croconium compound 1 shown in Table 2 was
obtained. The yield based on croconic acid was 32.7 mol%.
[0182]
(1-20) Synthesis Example 20 (Synthesis of Comparative Phthalocyanine
Compound 1)
A comparative phthalocyanine compound 1 shown in Table 3 was obtained by
the synthesis method described in Example 2 of Japanese Unexamined Patent
Application Publication No. 2007-56105.
[0183]
[Table 1]
62
il
CA 03014400 2018-08-13
'
Croconium Compound 1 Croconium Compound 2
o- --\ o o- o /---
o o
.
- 0 -
, 0 ---
/ \ NH HN / \
7 , ¨ 0 0- HN 41
I I 7 od NH Q 0-
...
W II"
Croconium Compound 3 Croconium Compound 4 .
F3C CF3 F3C CF3
= . 'a \ / . 41 lik li 41
0-
--- 0 --.._
4111 NH HN . it NH HN 41,
OS 0 0-
It. SS 0 a
al.
Croconium Compound 5 Croconium Compound 6
Me0 OMe Me0 OMe F3C CF3
. = 0-
C7- 11 = 15 IP cr 1,
C7H15
111 0 0-
NH HN 110fi. Olip 11 NH 0 a HN
OS =
=
II
110
_
Croconium Compound 7 Croconium Compound 8
SO e 0- IP 0 000 0- %to
AI NH HN . = NH HN 110,
OS 0 0- 10. .11. 0 0-
IIP
010
Croconium Compound 9 Croconium Compound 10
0110, o- liPalli &IP o- AO
Br Br I I
11 NH HN . . NH 0 0_ HN
OS 0 0-
00 OS 10
[0184]
[Table 2]
63
1 CA 03014400 2018-08-13
Croconium Compound 11 Croconium Compound 12
00 0- 1116.1 400. 0- 1Peop
cF3 CF3 . CN CN
ON"
AI NH HN . NH HN 410, 0 a
11010 1.10. 0 0'
10.
Croconium Compound 13 Croconium Compound 14
411, Br Br 11,
di I 0-1 Oa
Ole 0-
4110 41-.. ..-101
Br 0 Br Br 0 Br
. NH HN 41 41 NH 0 0_ HN
OS 0,
0 0'
lik
00 sell
11/
110
Croconium Compound 15 Croconium Compound 16
4. R x R y ii p
fe 41. 00 t_Bru ip
0011 0-
0-
10 Rx 0 --- Ry
t-Bu --- 0 --- t-Bu . NH HN
.41 .
LA 0 0-
. NH HN 1141 0-
OS 0 1101
Rx=H or Br Ry=H or Br
. ,
Croconium Compound 17 Croconium Compound 18
41011, 0-
- 0 - .
. NH HN 41
OP Ole
ilk NH 0 0.
411.4I 0 0-
11/ HN 4I
11/ 1/4111.
, _________________________________________________________
Comparative Croconium Compound 1
0-
2 \ NH CP HN / p
0 0_
[0185]
[Table 3]
64
CA 03014400 2018-08-13
Comparative Phthalocyanine Compound 1
Q /H C H
s * N N
N *
S
N-Cu-N
N
* *
H3_co)
os
H3C 4
[0186]
(2) Preparation of Resin
(2-1) Preparation of Fluorinated Aromatic Resin
Into a reactor equipped with a thermometer, a condenser tube, a gas inlet tube
and a stirrer, 16.74 parts by mass of 4,4'-bis(2,3,4,5,6-
pentafluorobenzoyl)diphenyl
ether, 10.5 parts by mass of 9,9-bis(4-hydroxyphenyl)fluorene, 4.34 parts by
mass of
potassium carbonate and 90 parts by mass of dimethylacetamide were placed. The
mixture placed in the reactor was heated to 80 C and reacted for 8 hours.
After
completion of the reaction, the reaction solution was poured into 1% acetic
acid aqueous
solution while vigorously stirring the reaction solution with a blender.
The
precipitated reaction product was separated by filtration, washed with
distilled water
and methanol, and then dried under reduced pressure, thereby obtaining a
fluorinated
aromatic resin. The fluorinated aromatic resin had a glass transition
temperature (Tg)
of 242 C and a number average molecular weight (Mn) of 70,770. The number
average molecular weight was determined by a gel permeation chromatography and
expressed by a polystyrene conversion value.
[0187]
(2-2) Preparation of Acrylic Resin
Into a reactor equipped with a thermometer, a condenser tube, a gas inlet tube
and a stirrer, 21.0 parts by mass of methyl a-allyloxymethyl acrylate (AMA),
9.0 parts
by mass of N-phenylmaleimide and 45.0 parts by mass of ethyl acetate as a
CA 03014400 2018-08-13
polymerization solvent were placed, and the temperature thereof was raised
while
stirring under a flow of nitrogen gas. After the temperature inside the
reactor was
stabilized at 70 C, 0.03 part by mass of an azo-based radical polymerization
initiator
(ABN-V available from Nippon Finechem Co., Ltd.) was added to start
polymerization
reaction. The reaction was carried out for 3.5 hours while maintaining the
temperature
inside the reactor at a range of 69 C to 71 C, and then cooled to room
temperature.
Tetrahydrofuran was added thereto as a diluent solvent, and reprecipitation
treatment
was carried out using n-hexane as a poor solvent, and the precipitate was
separated by
suction filtration. The obtained precipitate was dried at 80 C under reduced
pressure
for 2 hours by using a reduced pressure drier, thereby obtaining an acrylic
resin. A
weight average molecular weight of the acrylic resin measured by a gel
permeation
chromatography was 31,600. A glass transition temperature (Tg) of the acrylic
resin
measured by a differential scanning calorimeter was 152 C.
[0188]
(3) Production Example of Filter (Resin Laminated Substrate)
(3-1) Production Example 1
2 parts by mass of a polycarbonate resin (Iupilon (registered trademark) E-
2000,
available from Mitsubishi Engineering-Plastics Corporation), 0.02 parts by
mass of the
croconium compound 7 and 18 parts by mass of chloroform were mixed to obtain a
dye
containing-resin composition. About 1 cc of this resin composition was dropped
onto
a glass substrate (D263Teco, available from SCHOTT AG, 60 mm x 60 mm x 0.3 mm,
average transmittance 91%), and then coated thereon using a spin coater (1H-
D7,
available from Mikasa Co., Ltd.). The glass substrate on which a film of the
resin
composition was formed was dried at 100 C for 30 minutes under a nitrogen
atmosphere by using an inert oven (available from Yamato Scientific Co., Ltd.,
DN
6101), thereby obtaining a resin laminated substrate 1 in which a resin layer
was formed
on a glass substrate. The thickness of the resin layer was about 2 pm.
[0189]
(3-2) Production Example 2
A resin laminated substrate 2 was produced in the same manner as in the
66
CA 03014400 2018-08-13
Production Example 1, except that a cycloolefin resin (Zeonor (registered
trademark)
141OR available from Zeon Corporation) was used instead of the polycarbonate
resin as
a resin used and dichlorobenzene was used instead of chloroform in the
Production
Example I.
[0190]
(3-3) Production Example 3
A resin laminated substrate 3 was produced in the same manner as in the
Production Example 1, except that a norbomene resin (Arton (registered
trademark)
RX4500 available from JSR Corporation), which is one kind of the cycloolefin
resin,
was used instead of the polycarbonate resin as a resin used, the croconium
compound 8
was used instead of the croconium compound 7, and toluene was used instead of
chloroform in the Production Example 1.
[0191]
(3-4) Production Example 4
A resin laminated substrate 4 was produced in the same manner as in the
Production Example 1, except that a cycloolefin copolymer (Topas (registered
trademark) 5013 available from Polyplastics Co., Ltd.), which is one kinds of
the
cycloolefin resin, was used instead of the polycarbonate resin as a resin
used, the
croconium compound 8 was used instead of the croconium compound 7, and methyl
.. cyclohexane was used instead of chloroform in the Production Example 1.
[0192]
(3-5) Production Example 5
A resin laminated substrate 5 was produced in the same manner as in the
Production Example 1, except that the fluorinated aromatic resin prepared in
the above
(2-1) was used instead of the polycarbonate resin as a resin used, the
croconium
compound 8 was used instead of the croconium compound 7, and toluene was used
instead of chloroform in the Production Example I.
[0193]
(3-6) Production Example 6
A resin laminated substrate 6 was produced in the same manner as in the
67
CA 03014400 2018-08-13
Production Example 1, except that the acrylic resin prepared in the above (2-
2) was
used instead of the polycarbonate resin as a resin used, the croconium
compound 8 was
used instead of the croconium compound 7, and toluene was used instead of
chloroform
in the Production Example 1.
[0194]
(3-7) Comparative Production Example 1
A comparative resin laminated substrate 1 was produced in the same manner as
in Production Example 1, except that the comparative phthalocyanine compound 1
was
used instead of the croconium compound 7 in the Production Example 1.
[0195]
(4) Evaluation
(4-1) Spectroscopic Measurement of Dye Compounds and Resin Laminated
Substrates
A chloroform solution of each of the dye compounds obtained in the Synthesis
Examples 1 to 20 was prepared, and an absorption spectrum (a transmittance
spectrum)
in a wavelength range of 400 nm to 1100 nm was measured. The concentration of
the
chloroform solution of the dye compound was adjusted so that the transmittance
at the
maximum absorption wavelength was 10% ( 0.05%), the wavelength transmittance
was
measured with a spectrophotometer (UV-1800 manufactured by Shimadzu
Corporation)
at a pitch of 1 nm, and a wavelength at which the absorption was maximum in
the
wavelength range of 400 nm to 1100 nm (that is referred to as "maximum
absorption
wavelength 2,,max"), a wavelength at which the transmittance was 30% on the
longer
wavelength side of the maximum absorption wavelength (that is referred to as
"%T30"),
an average transmittance at the wavelength range of 400 run to 700 nm (that is
referred
to as "visible light transmittance"), and a transmittance at the wavelength of
400 nm
(that is referred to as "%T(400nm)") were determined. In addition, with
respect to
each of the resin laminated substrates produced in the Production Examples 1
to 6 and
the Comparative Production Example 1, an absorption spectrum (a transmittance
spectrum) in a wavelength range of 400 nm to 1100 nm was measured in the same
manner as the above and the maximum absorption wavelength Xmax, %T30, visible
68
CA 03014400 2018-08-13
light transmittance, %T(400nm) under the condition that the transmittance at
the
maximum absorption wavelength was 10% ( 0.05%) were determined.
[0196]
Results of the spectroscopic measurement of the respective dye compounds in a
chloroform solution are summarized in Table 4, and transmittance spectra of
the
croconium compound 1, the comparative croconium compound 1, and the
comparative
phthalocyanine compound 1 are shown in Fig. 1. In the croconium compounds 1 to
18,
a total number of it electrons in the ring A and the substituent R7 was 16,
the maximum
absorption wavelength Xmax was 892 nm to 934 nm, %T30 was 918 nm to 963 nm,
the
visible light average transmittance was 85.2% to 92.2%. Meanwhile, in the
comparative croronium compound 1, a total number of it electrons in the ring A
and the
substituent R7 was 10, the maximum absorption wavelength Xmax was 810 nm, %T30
was 830 nm, and the visible light average transmittance was 92.8%. Linear
interpolation of the relationship between the number of it electrons and the
wavelength
of %T30 in the croconium compound 1 (the croconium compound having the lowest
wavelength %T30 among the croconium compounds 1 to 18) and the comparative
croconium compound 1 leads to the fact that the wavelength of %T30 becomes 850
nm
or higher and light in the wavelength range exceeding 850 nm can be
effectively
absorbed when the number of it electron is 12 or more. When the wavelength of
%T30
is 850 nm or higher, it can be suitably used for security inks or laser
welding
applications, for example. Though the comparative phthalocyanine compound 1
had
the maximum absorption wavelength Xmax of 904 nm and was capable of absorbing
light in the wavelength range more than 850 nm, it had a low visible light
average
transmittance of 80.8%, which was somewhat inferior in terms of invisibility.
[0197]
[Table 4]
69
i
CA 03014400 2018-08-13
=
,
,
Visible Light
2,max %130 Average %T(400nm)
Transmittance
_
Croconium Compound 1 892 nm 918 nm 91.9% 92.2%
Croconium Compound 2 916 nm 948 nm 88.2% 81.3%
_
Croconium Compound 3 921 nm 946 nm 91.7% 91.6%
Croconium Compound 4 915 nm 944 nm 89.2% 85.3%
-
Croconium Compound 5 909 nm 937 nm 86.5% 80.2%
Croconium Compound 6 913 nm 938 nm 89.4% 82.5%
Croconium Compound 7 923 nm 950 nm 92.0% 90.9%
Croconium Compound 8 921 nm 949 nm 91.3% 90.2%
Croconium Compound 9 . 927 nm 956 nm 90.0% 87.7%
Croconium Compound 10 , 928 nm 957 nm 90.5% 89.1%
Croconium Compound 11 927 nm 956 nm 89.6% 86.8%
_
Croconium Compound 12 931 nm 961 nm 90.1% 88.0%
Croconium Compound 13 934 nm 963 nm 90.2% 88.9%
_
Croconium Compound 14 932 nm 963 nm 87.2% 83.0%
,
Croconium Compound 15 920 nm 950 nm 85.3% 80.1%
_
Croconium Compound 16 926 nm 956 nm 90.7% 88.5%
-
Croconium Compound 17 918 nm 949 nm 85.2% 80.9%
,
Croconium Compound 18 922 nm 951 nm 92.2% 90.3%
,
Comp. Croconium Compound 1 , 810 nm 830 nm 92.8% 93.0%
,..
Comp. Phthalocyanine Compound 1 904 nm 934 nm 80.8%
75.5%
-
[0198]
Table 5 summarizes the spectroscopic measurement results of the resin
laminated substrates. The resin laminated substrates 1 and 2 contained the
croconium
compound 7 as a dye, the resin laminated substrates 3 to 6 contained the
croconium
compound 8 as a dye, and the comparative resin laminated substrate 1 contained
the
CA 03014400 2018-08-13
comparative phthalocyanine compound 1 as a dye. Regardless of the kind of
resin, the
value of the maximum absorption wavelength Xmax of the resin laminated
substrate was
almost equal to that in chloroform solution. The visible light transmittances
of the
resin laminated substrates 1 to 6 were higher than that of the comparative
resin
laminated substrate 1. The resin laminated substrates 1 to 6 can be suitably
applied to,
for example, an optical filter of an image sensing device for night vision.
[0199]
[Table 5]
Visible Light
Amax %130 Average %T(400nm)
Transmittance
Resin Laminated Substrate 1 930 nm 962 nm 82.1% 80.7%
Resin Laminated Substrate 2 939 nm 977 nm 77.1% 75.6%
Resin Laminated Substrate 3 926 nm 958 nm 85.0% 83.6%
Resin Laminated Substrate 4 934 nm 957 nm 79.6% 79.4%
Resin Laminated Substrate 5 928 nm 952 nm 84.2% 83.3%
Resin Laminated Substrate 6 923 nm 984 nm 74.9% 71.3%
Comp. Resin Laminated Substrate 1 902 nm 940 nm 69.2%
61.0%
[0200]
(4-2) Storage Stability of Ink Composition
5 mg of the croconium compound 7 obtained in the Synthesis Example 7 was
weighed and diluted with chloroform to adjust 100 mL. 5 mL of the obtained
chloroform solution was taken out using a whole pipette and further diluted
with
chloroform to adjust 50 mL, whereby an ink composition 1 was prepared. The
obtained ink composition 1 was stored for 1 month under shading at room
temperature.
With respect to the ink composition 1 immediately after preparation and after
storage
for one month, an absorption spectrum (a transmittance spectrum) in wavelength
range
of 400 nm to 1100 nm was measured in the same manner as the above, and the
transmittance at the maximum absorption wavelength and the visible light
transmittance
71
CA 03014400 2018-08-13
were determined respectively. Degradation on the spectroscopic characteristics
of the
ink composition 1 was not recognized even after one month storage, and it was
confirmed that the ink composition 1 was excellent in storage stability.
[0201]
[Table 6]
Transmittance Visible Light
Storage Period (at max. absorption Average
wavelength) Transmittance
Immediately
8.6% 92.0%
after Preparation
Ink Composition 1
One-month Storage 8.6% 92.0%
INDUSTRIAL APPLICABILITY
[0202]
The oxocarbon compound of the present invention is useful as, for example, an
optical filter for a semiconductor light receiving element having a near-
infrared light
absorbing or cutting function, a near-infrared absorbing film or a near-
infrared
absorbing plate that shields a heat ray for energy saving, an information
indication
material such as a security ink and an invisibnle bar code ink, a material for
a solar cell
utilizing visible light and near-infrared light, a specific wavelength
absorption filter for
a plasma display panel (PDP) or CCD, a photothermal conversion material for
laser
welding, a photofixing material utilizing light which is less likely to cause
troubles due
to pressurization or heating (e.g., electrostatic charge development toner for
a flash
fixing method), a cosmetic material having a near-infrared light absorbing or
cutting
function, a material for light detection and distance measurement (LIDAR)
system, and
the like.
72