Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 3
CONTENANT LES PAGES 1 A 244
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 3
CONTAINING PAGES 1 TO 244
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
84451174
SUBSTITUTED INDOLE MCL-1 INHIBITORS
RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. provisional application
serial number
62/304,124, filed March 4, 2016, and U.S. provisional application serial
number 62/402,903,
filed, September 30, 2016.
[0002]
TECHNICAL FIELD
[0003] The present disclosure pertains to compounds that inhibit the activity
of an anti-
apoptotic Bc1-2 family member Myeloid cell leukemia-1 (Mc1-1) protein,
compositions
containing the compounds, and methods of treating cancer involving over-
expressed or
dysregulated Mc1-1 protein.
BACKGROUND
[0004] Abnormal regulation of apoptosis is now recognized to play an important
role in the
development of cancer. The apoptosis pathway can be initiated by various
extracellular and
intracellular stresses, including growth factor deprivation, DNA damage,
oncogene induction,
and cytotoxic drugs (Dania', N. N. and Korsmeyer, SJ. Cell (2004) 116, 205-
219). The death
signal leads to the oligomerization of the pro-apoptotic proteins Box and Bak.
Upon activation,
they peimeabilize the mitochondrial outer membrane and release apoptogenic
factors into the
cytoplasm. This process is tightly regulated by both pro-apoptotic (Bax, Bak,
Bad, Bid, Bim,
Bmf, NOXA, PUMA) and anti-apoptotic (Bc1-2, Bc1-xL, Bel-w, Bc12-A1, Mel-1)
members of
the Bc1-2 family of proteins. Recent data suggests that the anti-apoptotic Bc1-
2 proteins function
to protect the cell from apoptotic insults, primarily by preventing disruption
of mitochondrial
outer membrane integrity by binding to the pro-apoptotic proteins as described
in Adams, J. M.
and Cory S. Oncogene (2007) 26, 1324-1337; Willis, S. N. et
1
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
al. Science (2007) 315,856-859. Because tumor cells are under stress,
alterations in their
apoptotic signaling pathways are believed to be crucial for survival. Recent
data implicates
down-regulated apoptosis in the onset of cancer. Research has shown, for
example, that anti-
apoptotic proteins, are over-expressed in many cancer cell types as described
in Beroukhim,
R. et al. Nature (2010) 463, 899-905; Zhang J. Y., Nature Reviews Drug
Discovery, (2002) 1,
101; Kirkin, V. et al. Biochirnica et Biophysica Acta (2004) 1644, 229-249;
and Amundson,
S.A. et al. Cancer Research (2000) 60, 6101-6110. This dysregulation results
in the survival
of cells that would otherwise have undergone apoptosis such as cancer cells.
This suggests
that neutralizing the function of anti-apoptotic Bc1-2 proteins may offer an
effective strategy
for the elimination of cancer cells. In addition, resistance to chemotherapy
which is a major
cause of treatment failure and poor prognosis in many cancers can be caused by
the
upregulation of anti-apoptotic Bc1-2 family proteins.
[0005] An important anti-apoptotic member of the Bc1-2 family is Myeloid cell
leukemia-1
(Mcl-1). Mc-1 is one of the most frequently amplified anti-apoptotic genes in
human
cancers including prostate, lung, pancreatic, breast, ovarian, and cervical
cancers, as well as
melanoma, B-cell chronic lymphocytic leukemia (B-CLL), acute myeloid leukemia
(AML)
and acute lymphoblastic leukemia (ALL) (Beroukhim et al. Nature (2010) 463,
899-905).
Moreover, its overexpression is implicated as a resistance factor for multiple
therapies
including widely prescribed microtubule-targeted agents for breast cancers,
such as paclitaxel
and vincristine as well as Gemcitabine, a first-line treatment option for
pancreatic cancer
(Wei et al. Cancer Chemother Pharmacol (2008) 62, 1055-1064 and Wertz et al.
Nature
(2011) 471, 110-114). These data suggest that Mc-1 is an important target for
a wide variety
of cancers.
[0006] In many cancer cell types, the cancer cell's survival is attributed to
the dysregulation
of the apoptotic pathway caused by the over-expression of one or more anti-
apoptotic Bc1-2
protein family members. Because of the important role for Bc1-2 family of
proteins in
regulating apoptosis in both cancerous and non-cancerous cells, and the inter-
cell variability
of Bc1-2 family protein expression, it could be advantageous to have a small
molecule
inhibitor that selectively targets and preferably binds to one type or a
subset of anti-apoptotic
Bc1-2 protein(s). A selective compound also may confer certain advantages in
the clinical
setting, by providing flexibility to select a dosing regimen to reduce on-
target toxic effects in
normal cells.
[0007] Because Mc-1 protein is an important Bc1-2 family member associated
with a
number of diseases, there is a need for compounds which bind to and inhibit
the activity of
2
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Mcl-1 protein.
SUMMARY
[0008] In some embodiments, the present disclosure provides compounds, and
pharmaceutically acceptable compositions thereof, that are effective as
inhibitors of Mcl-1.
[0009] Compounds of the present disclosure, and pharmaceutically acceptable
compositions
thereof, are useful for treating a variety of diseases, disorders or
conditions, associated with
MC1-1. Such diseases, disorders, or conditions include those described herein.
100101 Compounds provided by this disclosure are also useful for the study of
Mcl-1 in
biological and pathological phenomena and the comparative evaluation of new Mc-
1
inhibitors in vitro or in vivo.
DETAILED DESCRIPTION
1. General Description of Compounds of the Disclosure:
[0011] In one aspect, the present disclosure provides inhibitors of Mcl-1. In
some
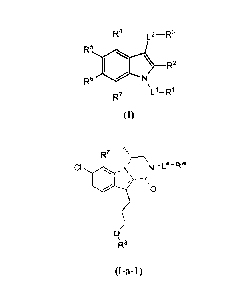
embodiments, such compounds include those of formula (I):
R4 L2¨R3
R5
\ R2
R6
R7
(I)
or a pharmaceutically acceptable salt thereof, wherein: LI is selected from a
covalent bond or
an optionally substituted bivalent straight or branched C1_6 hydrocarbon chain
wherein one or
more methylene units are optionally and independently replaced with ¨Cy¨; ¨Cy¨
is an
optionally substituted bivalent ring independently selected from phenylene, 3-
8 membered
saturated or partially unsaturated carbocyclylene, 5-6 membered heteroarylene
having 1-4
heteroatoms independently selected from nitrogen, oxygen, or sulfur, or 3-8
membered
saturated or partially unsaturated heterocyclylene having 1-4 heteroatoms
independently
selected from nitrogen, oxygen, or sulfur; L2 is an optionally substituted
bivalent straight or
branched C3_6 hydrocarbon chain wherein one or two methylene units of L2 are
optionally and
independently replaced with ¨0¨, ¨S¨, or ¨N(R')¨, and wherein two substituents
of L2 are
optionally taken together to form an optionally substituted bivalent ring
selected from 3-8
3
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
membered saturated or partially unsaturated carbocyclylene or 3-8 membered
saturated or
partially unsaturated heterocyclylene having 1-4 heteroatoms independently
selected from
nitrogen, oxygen, or sulfur; each R' is independently selected from hydrogen
or optionally
substituted C1_4 alkyl; RI is selected from hydrogen, halogen, R, -OR, -SR, -
S(0)R,
-S(0)2R, -S(0)2N(R)2, -N(R)2, -C(0)N(R)2, -C(0)R, -N(R)C(0)R, -N(R)C(0)0R,
-N(R)C(0)N(R)2, -N(R)S(0)2R, -N(R)S(0)2N(R)2, -C(0)0H, -C(0)Rx, -S(0)20H,
-S(0)R, or -S(0)2R'; R2 is selected from -C(0)-L3-1e, -C(0)N(R)-L3-12:,
-C(0)N(R)-C(R)2-L3-112, -C(0)0-L3-le or -C(0)S-L3-1e; L3 is selected from a
covalent
bond or an optionally substituted bivalent straight or branched C1_8
hydrocarbon chain
wherein one or more methylene units are optionally and independently replaced
with -Cy-,
-0-, -S-, -N(R)-, -N(R)C(0)-, -N(R)S(0)2-, -C(0)-, -C(0)N(R)-, -S(0)-, -S(0)2-
,
or -S(0)2N(R)-; Rz is selected from hydrogen, -Cy-R, R, -OR, -SR, -S(0)R, -
S(0)2R,
-S(0)2N(R)2, -N(R)2, -C(0)N(R)2, -C(0)R, -N(R)C(0)R, -N(R)C(0)0R,
-N(R)C(0)N(R)2, -N(R)S(0)2R, -N(R)S(0)2N(R)2, -C(0)0H, -C(0)Rx, -S(0)20H, or
-S(0)2RY, or is selected from:
H 0 H 0
0 N co.N...,r0
S 0 (271
g7FIN -0
H
0 N
r S
`2?4-NH
; Rx is selected from
-C(0)0R, -N(R)S(0)2CF3, -N(R)C(0)R, -N(R)C(0)0R, -N(R)C(0)N(R)2, or
-N(R)S(0)2R; RY is selected from -N(R)C(0)CF3, -N(R)C(0)R, or -N(R)C(0)N(R)2;
each
R is independently selected from hydrogen or an optionally substituted group
selected from
C1-12 aliphatic or a ring selected from a 3-10 membered saturated or partially
unsaturated
carbocyclic ring, phenyl, a 6-10 membered bicyclic saturated, partially
unsaturated or aryl
ring, a 3-8 membered saturated or partially unsaturated heterocyclic ring
having 1-2
heteroatoms independently selected from nitrogen, oxygen, or sulfur, a 5-6
membered
heteroaryl ring having 1-4 heteroatoms independently selected from nitrogen,
oxygen, or
sulfur, a 7-10 membered bicyclic saturated or partially unsaturated
heterocyclic ring having
1-5 heteroatoms independently selected from nitrogen, oxygen or sulfur, an 8-
10 membered
bicyclic heteroaryl ring having 1-5 heteroatoms independently selected from
nitrogen,
oxygen or sulfur, or a 12-15 membered tricyclic heteroaryl ring having 1-5
heteroatoms
independently selected from nitrogen, oxygen or sulfur; R3 is an optionally
substituted ring
4
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
selected from a 3-8 membered saturated or partially unsaturated monocyclic
carbocyclic ring,
phenyl, an 8-10 membered bicyclic aromatic carbocyclic ring, a 3-8 membered
saturated or
partially unsaturated monocyclic heterocyclic ring having 1-2 heteroatoms
independently
selected from nitrogen, oxygen, or sulfur, a 5-6 membered monocyclic
heteroaromatic ring
having 1-4 heteroatoms independently selected from nitrogen, oxygen, or
sulfur, or an 8-10
membered bicyclic heteroaromatic ring having 1-5 heteroatoms independently
selected from
nitrogen, oxygen, or sulfur; each of R4, R5, and R6 is independently selected
from R, halogen,
-CN, -NO2, -C(0)OR', -OR', -SR', -C(0)N(R')2 -N(R')2, -S(0)2N(R')2,
-N(R')S(0)2CF3, -C(0)R', -N(R')C(0)R', -S(0)R', -S(0)2R', -N(R')C(0)OR', and
-N(R')S(0)2R'; R7 is selected from hydrogen, halogen, -CN, -NO2, -C(0)0R, -
OCF3,
-OR, -SR, -S(0)20R, -P(0)(OH)2, -C(0)N(R)2, -N(R)2, -S(0)2N(R)2, -
N(R)S(0)2CF3,
-C(0)N(R)S (0)2R, -S(0)2N(R)C(0)0R, -
S(0)2N(R)C(0)N(R)2, -C(0)R,
-C(0)N(R)S(0)2CF3, -N(R)C(0)R, -OC (0)R, -
0C(0)N(R)2, -C(NR)N(R)2,
-N(R)C(NR)N(R)2, -S(0)R, -S(0)2R, -N(R)C(0)0R, or -N(R)S(0)2R, or an
optionally
substituted group selected from C1_6 aliphatic or a ring selected from a 3-8
membered
saturated or partially unsaturated carbocyclic ring, phenyl, a 3-8 membered
saturated or
partially unsaturated heterocyclic ring having 1-2 heteroatoms independently
selected from
nitrogen, oxygen, or sulfur, a 5-6 membered heteroaryl ring having 1-4
heteroatoms
independently selected from nitrogen, oxygen, or sulfur, an 8-14 membered
bicyclic or
polycyclic saturated, partially unsaturated or aryl ring, a 7-14 membered
bicyclic or
polycyclic saturated or partially unsaturated heterocyclic ring having 1-5
heteroatoms
independently selected from nitrogen, oxygen, or sulfur, or an 8-14 membered
bicyclic or
polycyclic heteroaryl ring having 1-5 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur; and optionally RI and R2, RI and R7, R4 and R5, R5 and R6
and/or R6 and
R7 are taken together with their intervening atoms to form an optionally
substituted ring
selected from a 3-8 membered saturated or partially unsaturated carbocyclic
ring, phenyl, a 3-
8 membered saturated or partially unsaturated heterocyclic ring having 1-4
heteroatoms
independently selected from nitrogen, oxygen, or sulfur, or a 5-6 membered
heteroaryl ring
having 1-4 heteroatoms independently selected from nitrogen, oxygen, or
sulfur.
10012] In other embodiments are compounds of formula (I), or a
pharmaceutically
acceptable salt, thereof, wherein I: is selected from a covalent bond or an
optionally
substituted bivalent straight or branched C1-6 hydrocarbon chain wherein one
or more
methylene units are optionally and independently replaced with -Cy-; -Cy- is
an optionally
substituted bivalent ring independently selected from phenylene, 3-8 membered
saturated or
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
partially unsaturated carbocyclylene, 5-6 membered heteroarylene having 1-4
heteroatoms
independently selected from nitrogen, oxygen, or sulfur, or 3-8 membered
saturated or
partially unsaturated heterocyclylene having 1-4 heteroatoms independently
selected from
nitrogen, oxygen, or sulfur; L2 is an optionally substituted bivalent straight
or branched C3-6
hydrocarbon chain wherein one or two methylene units of L2 are optionally and
independently replaced with -0-, -S-, or -N(R')-, and wherein two substituents
of L2 are
optionally taken together to form an optionally substituted bivalent ring
selected from 3-8
membered saturated or partially unsaturated carbocyclylene or 3-8 membered
saturated or
partially unsaturated heterocyclylene having 1-4 heteroatoms independently
selected from
nitrogen, oxygen, or sulfur; each R' is independently selected from hydrogen
or optionally
substituted C14 alkyl; RI is selected from hydrogen, halogen, R, -OR, -SR, -
S(0)R,
-S(0)2R, -S(0)2N(R)2, -N(R)2, -C(0)N(R)2, -C(0)R, -N(R)C(0)R, -N(R)C(0)0R,
-N(R)C(0)N(R)2, -N(R)S(0)2R, -N(R)S(0)2N(R)2, -C(0)0H, -C(0)1V, -S(0)20H,
or -S(0)2RY; R2 is selected from -C(0)-L3-Rz, -C(0)N(R)-L3-Rz,
-C(0)N(R)-C(R)2-L3-Rz, -C(0)0-L3-Rz or -C(0)S-L3--R; L3 is selected from a
covalent
bond or an optionally substituted bivalent straight or branched C1_8
hydrocarbon chain
wherein one or more methylene units are optionally and independently replaced
with -Cy-,
-0-, -S-, -N(R)-, -N(R)C(0)-, -N(R)S(0)2-, -C(0)N(R)-, -S(0)-, -S(0)2-,
or -S(0)2N(R)-; R' is selected from hydrogen, -Cy-R, R, -OR, -SR, -S(0)R, -
S(0)2R,
-S(0)2N(R)2, -N(R)2, -C(0)N(R)2, -C(0)R, -N(R)C(0)R, -N(R)C(0)0R,
-N(R)C(0)N(R)2, -N(R)S(0)2R, -N(R)S(0)2N(R)2, -C(0)0H, -C(0)R', -S(0)20H, or
-S(0)2RY, or is selected from:
H H
{N,r0
0 N =W) N =eC) Ni=11\1H
ic-4.1*(N-cf, "?.(N/
µ224-N H
; Rx is selected from
-C(0)0R, -N(R)S(0)2CF3, -N(R)C(0)R, -N(R)C(0)0R, -N(R)C(0)N(R)2, or
-N(R)S(0)2R; RY is selected from -N(R)C(0)CF3, -N(R)C(0)R, or -N(R)C(0)N(R)2;
each
R is independently selected from hydrogen or an optionally substituted group
selected from
C1-12 aliphatic or a ring selected from a 3-10 membered saturated or partially
unsaturated
carbocyclic ring, phenyl, a 6-10 membered bicyclic saturated, partially
unsaturated or aryl
ring, a 3-8 membered saturated or partially unsaturated heterocyclic ring
having 1-2
6
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
heteroatoms independently selected from nitrogen, oxygen, or sulfur, a 5-6
membered
heteroaryl ring having 1-4 heteroatoms independently selected from nitrogen,
oxygen, or
sulfur, a 7-10 membered bicyclic saturated or partially unsaturated
heterocyclic ring having
1-5 heteroatoms independently selected from nitrogen, oxygen or sulfur, or an
8-10
membered bicyclic heteroaryl ring having 1-5 heteroatoms independently
selected from
nitrogen, oxygen or sulfur; R3 is an optionally substituted ring selected from
a 3-8 membered
saturated or partially unsaturated monocyclic carbocyclic ring, phenyl, an 8-
10 membered
bicyclic aromatic carbocyclic ring, a 3-8 membered saturated or partially
unsaturated
monocyclic heterocyclic ring having 1-2 heteroatoms independently selected
from nitrogen,
oxygen, or sulfur, a 5-6 membered monocyclic heteroaromatic ring having 1-4
heteroatoms
independently selected from nitrogen, oxygen, or sulfur, or an 8-10 membered
bicyclic
heteroaromatic ring having 1-5 heteroatoms independently selected from
nitrogen, oxygen, or
sulfur; each of R4, R5, and R6 is independently selected from R, halogen, -CN,
-NO2,
-C(0)OR', -OR', -SR', -C(0)N(R')2 -N(R')2, -S(0)2N(R)2, -N(R')S(0)2CF3, -
C(0)R',
-N(R')C(0)R', -S(0)R', -S(0)2R', -N(R')C(0)OR', and -N(R')S(0)2R'; R7 is
selected
from hydrogen, halogen, -CN, -NO2, -C(0)0R, -0CF3, -OR, -SR, -S(0)20R,
-13(0)(OH)2, -C(0)N(R)2, -N(R)2, -S(0)2N(R)2, -N(R)S(0)2CF3, -C(0)N(R)S(0)2R,
-S(0)2N(R)C(0)0R, -S(0)2N(R)C(0)N(R)2, -C(0)R, -C(0)N(R)S(0)2CF3, -N(R)C(0)R,
-0C(0)R, -0C(0)N(R)2, -C(NR)N(R)2, -N(R)C(NR)N(R)2, -S(0)R, -S(0)2R,
-N(R)C(0)0R, or -N(R)S(0)2R, or an optionally substituted group selected from
C1-6
aliphatic or a ring selected from a 3-8 membered saturated or partially
unsaturated
carbocyclic ring, phenyl, a 3-8 membered saturated or partially unsaturated
heterocyclic ring
having 1-2 heteroatoms independently selected from nitrogen, oxygen, or
sulfur, a 5-6
membered heteroaryl ring having 1-4 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur, an 8-14 membered bicyclic or polycyclic saturated,
partially unsaturated or
aryl ring, a 7-14 membered bicyclic or polycyclic saturated or partially
unsaturated
heterocyclic ring having 1-5 heteroatoms independently selected from nitrogen,
oxygen, or
sulfur, or an 8-14 membered bicyclic or polycyclic heteroaryl ring having 1-5
heteroatoms
independently selected from nitrogen, oxygen, or sulfur; and optionally le and
R2, RI and R7,
R4 and R5, R5 and R6 and/or R6 and R7 are taken together with their
intervening atoms to form
an optionally substituted ring selected from a 3-8 membered saturated or
partially unsaturated
carbocyclic ring, phenyl, a 3-8 membered saturated or partially unsaturated
heterocyclic ring
having 1-4 heteroatoms independently selected from nitrogen, oxygen, or
sulfur, or a 5-6
7
84451174
membered heteroaryl ring having 1-4 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur.
In other embodiments, there is provided a compound having fonnula (I-a-1):
R7 ---'
CI Ni¨\N_La_Rw
/ 0
q
R3 (I-a-1), or
a pharmaceutically acceptable salt thereof,
wherein:
ci
R3 is or ;
1,4-Ir is
o
0 HO 0 0 0
0 OH
HO
HO HO 0 oH
0 0/
N N
HN
; ; .
; .
0 0 OH o o o
0 OH "0
HO HO HO
Oc'
----- \ ----- -
/
0 H 0
0
0
------ 0
71 N -----:---Nno r_Z
---- 0
\ /
\ / --- N -----
N N N
N N
= 7 ; .
7
0 0
0 HO OH OH
\¨
N)s. (NO
/--==NO --z---c__ \N-*.. ----- _____
=
, 2. ; 'z'z. =
,
8
Date Recue/Date Received 2023-06-16
84451174
\
N¨N
Ni 0 OH OH
OH
_/L
OH 0 OH
N
0
N \ N N N
N N N N
. . . .
r , , , ;
o/
\O 0
K/ 0 0H \ _ 0 0 OH HO
N
K
K __ / \04, _/....____ OH (:\ / 0 OH \
----
N N N
N N N
N
; .
9 ; .
2 9
0 I Ny 0 OH \
tr I o
OH
OH Ck.:4, OH
N \
/
0/
o/ . 0 0/
; ; ; \;
OH
o
I ....;\10 0 ,,õ,- ---- 1
OH N 0 OH HO NI I 0
\ / / OH
N \
0--
0
I
0 OH 0 0
OH
1
HON
\ N \
. * 0¨rN
r j-- ..---
--- 0 0H. ----/ 0 ¨0 0
OH = OH -
H Nr? 0 ---- OH HO 0 I ..... No
/ OH
0 OH
1,,N \ 0 0 0
N
N N N \
¨
N
* F-U*
2 2
8a
Date Regue/Date Received 2023-06-16
84451174
N OH
N-, I o OH
O TN .t OH
----- 0 OH
---- 0 N
N N
; ; ;
I N :
\ 0 u
OH ^HO ----"- 0
N
\N \ CH
Ni i
0
0
0 N N
OH
N NO
N N
N N N
; ; .
2 .
2
0
---1/` N ) HN
t....Nõ.0 ,,,,,,,0
N
r' N OH OH
0/ \r/47): OH I \N ---(- \N 0 OH
\ __ / \ __ < N \ N \
N N 111
; .
2 ; .
2
I O1
N 00 OH 1,,,,N,) 0
OH 1...Nõe00 . ,,,c
LN \ cN \ OH
N \ N
#
..---
110 / \ N 0
OH
;
8b
Date Regue/Date Received 2023-06-16
84451174
(0
N----1"r1,
I 0
HO 0 0 OH
,..- N 0
OH 0 OH CZ: OH
OH
-----N N.
N \
* *
F3C/ =
. = F,C ;
1 0 OH I
\ / OH 0.õ..) 0 OH
-........(N 0
N OH OH
(---Z____
_
N
FC)3 = 07.. 0 --- = 0. 0.--.. ;
a a
0 OH 0 OH
N...õ I 0
0
OH OH
N
OH
----14 N.
N \
o 0
b, N
a. c/N
0-- ; ',>= 0;
,,
HO 0
N.z.õ( 0 ...õ...(N 0
OH OH
N \
N \ N \
--- 0
\ / ¨
N
0 ======\
µ..... N
0 -===.µ 0 =====.\
\---
0 0
\ = O'''',.," = \ * \ *
, ,
HO 0
N \ H
OH OH
0
0 0
--___
--___ ---
0 -.===.µ .........¨N
"'il __ ........¨ N .........N
1.......¨N(
\--0
./L',.../
c).=""^,,/". ,
.
0===== ; 0 = 0 =
8c
Date Regue/Date Received 2023-06-16
84451174
HO 0 in
N---- 0 OH
OH
HO 0
N , 0
---N N, ---õ,
--"\-N/
\__/ * \ = \ ; \ =
, ,
HO 0
HO 0
HO 0
----ra OH
0
----
V...N/ ( N/
I
ON =
\ ; \ __ /.
HO 0
HO 0 HO
sN \ 0
-.,N \ N \ . OH o
¨
õ......21
--.¨
N
/ N ._
N
/ --\ . 0
\ - /N.-- = N.õ......,7, . (N) .
2 2
HO HO0
0
'N \ HO
0
HO HO 0
0
N \
* N \
(I---)
\--I 0
(NI---\
N7---\ N- Na
\---y = 0
- 0 ; \ =
,
8d
Date Regue/Date Received 2023-06-16
84451174
HO 0
=N \ I
HO 0 0 0 - NI
*
HO 0
N 1 OH OH
......N \ \ N \
Nao (1-)
*
\-----\ N 0 0 0
0-;
HO 0
HO 0 HO 0 HO 0 -....N \
* # 0
\----\
0 0
0 C---)
\Th 0 \---\
N---
.
0 --- = / - 0 ;
HO 0
HO 0 HO 0 HO
N \ 0
0
1\(--)., INIC-A N/Th 0
0 \__/, . 0 \N=-=-= 0 v.õ,../N1-- .
,
HO 0
HO
HO 0 o OH
0 0
NõN \ OH
-.N \ N(
0 IIP N \
0 ,N
OH
0 \INI¨. 0 = 0-- ; -
7 7 7
0 Ho
OH 0
HO
0
OH
----
-- o
------N N \
(A.õ.,. ai
----\_.¨N N N 0
HQ
\ . . .
8e
Date Regue/Date Received 2023-06-16
84451174
\ N
N
OH OH \ / i
OH .-,....... -*.--- \ N
/
N OH OH
N N
0 0 05 0 0
; ; .
; .
0
OH OH OH
OH OH
---__
,N HN
0 0; 0 0
F F CI O\;
; =
; = =
;
OH
I
0 n N (Y .......;N
..,....-
.....N \ 0 N....
0
N \
OH 0/ OH o/OH OH
/ / 0 0
. .
1
---n-
0
OH I N..... . N \ 0
OH
0
-"`N N N \ 0 OH
0 N \
a
OH OH
(:),-\
\---o
01 ; 0 , , = 0-- = 0¨ \ =
; ,
0.. N.,.- Nr
N \ 0 N \
OH OH OH
N .--= oH
0 0 ¨.\ 0¨\
0 \--
V-0 L 0 0
\ = \ = = \---- =
; ; ;
eNi= I
0
N} i
1,N \
0,1
0
OH
0
......N \
OH OH
OH HO
0
0
-..-_
0 --- \
0 (
CI / ---1 0 .....\ .......-N NI
./.........
L- 1.---. Ni
\--.N
)---- = \ ; \........./N-- . OH
5 N
\----- =
5
8f
Date Regue/Date Received 2023-06-16
84451174
o
=-=õ.N \ OH 0
OH 0
OH
........41 ---
N_....6 _.,..-N
HN---) tirõ......N. /
N;
0
......N \
OH
0
N \
o
HO
OH 0
0H HN---/,(Th
0
¨ ¨
.......-N
........-N
0 c.___N---)
.........,\,
0 HN
N
,,,,,
,.--. N
H
1 = HN''''N'''''''' = k===..../ = \
=
0 0 0 0 0 ,,N \ ,,N \ =-,,N \ N \ ..-,N \
OH OH OH OH OH
0 0
/
N¨e0 0
N
N --..0
N.... N -.-, / /1\1--,
/ / / / \¨
N \----- NO. N¨
\ - NO .
0
\ 0 OH
,,N \ 0
=-...N
OH OH o
CI
N * , Nf--\n 0 .
Nnv_
0_0
. \ __ , =
0
. 0
OH 0H 0
\N \ HO 0 HO
\/ N/ ...---
N N
= \ \ . 0
=
, ; =
, ,
HO ,O
0 o
HO HO .O
HO HO
(--0
/ N'''''''''''' / N '=''' V N''''''''''ts
O
\
. .
, ; ;
8g
Date Regue/Date Received 2023-06-16
84451174
HO 0
0
HO
N/
HO 0 HO
V N/
o/ 0
2 5
HO 0
0
0
0 HO
HO
N/ Ho
N/ N
0
0
OH.
/ =
0
HO
0
HO
HO 0
N V
HO
\ ;
HO 0 0
H
HO O
HO
N HO
0
N---.
N
N, =
N
0 OH
OH
HO
N N
=
HO
0 OH 0 OH
\
N
N F
NH2 . NTAM = F ;
8h
Date Recue/Date Received 2023-06-16
84451174
0 0 OH o oH
0 OH
HO
N5S) \ \ N
N \ _ni\ \ N\____O
NN; =
= ¨ \--=N =
,,
0 OH 0 OH
0 OH
0
HO
IN
\ N \
\ NNip,C)
N-Y
I = I =
0 OH 0 OH 0 OH
0 OH
\ N \ N \ \ N
"CNH
. \WO ,.õ.. N
\ = \µ
;
0 OH
.,
OH 0 HO
0
NC Ho
\ N /
N---
/ = .
0 OH
OH
OH 0 0
0 o HO
HO F
\ N CH
\
c_...N- N----
N
N\ .
,.
, ; Ns =
0
OH
0
0 0 HO OH
HO .
OH 0
HO
CI
\
0/ N
\ N 7/---N
\ N \ --.) =
, r , ,
8i
Date Regue/Date Received 2023-06-16
84451174
0
OH
o o
Me \N
\ NN,
Ni
I Me 0H OH
\
-----".-- N---__ N
==-..,,
',,... .,õ..:7õ,1=J . co 2H
;
HO
0 0
0 OH \ OH
\/N____ N /IN_ N
N
\ 011 \N OH r.--N
/
O / \
0 = \ =
0
o
/ \
HO
HO
0
/
N-----N N----N
HO
N-- -
N \
/N x
\ 0 \
N¨....._
N.
N \ \
0
0H
. . . .
; 5 5 5
0
0
N
HO
/
OH
HO
N¨N
1 0 N¨N
1
(:) 0 all
N---
'1111
\ N
= 0 H =
a ; ,
c., HO OH
OOH 0 OH
0
0 OH 0 OH
Niil
N / \
I N t......&N / N
I
L /
N I \ \ \ \
\ N N N N N
\ = \ = \ = \ - \ =
, , ,
HO
______________________________________________ o 0 OH
--
/
N N
--- / r.---
0 OH
OH 0
0 ....N
N ,,,,õ.õ."N / \
/
N OH 1 /
N
. 1 U. 0 '
,
8j
Date Regue/Date Received 2023-06-16
84451174
0
HO
HO 0 0
OH
HO -,.....
OH Ho 1
0 0
NI N
O;
N
NI
0
. CI
s s s s
HO 0
0 HO
0
N OH;
N
\ / N....... N
/ \ N
OH /
0 = = ---- .
,
0 OH
OH HO
OH 0
UH0 Ho
N
0
N . . = .
HO ;7 7 a 7
0 NH2
0
0
HO 0H
c N
OH
0
N........ 0
= i 0 = .
NH2
Nila NH2
NH2 0
0
-- N
--
N........-N 0
0
. . \ '
7 7 7
NH a NH2 0
0
___ HaN 0 0 NH2 H2N
1 0 /
N 0
. . = .
8k
Date Recue/Date Received 2023-06-16
84451174
0
0
0
H2N \ \N o
N
H
/ ON
----- N N
H
--- N--- N---
N---- N----
,...., 3 ....., ,......
. . .
5 5 5 5
\
0
0
?\N
/\ N
Q 0
N H
N
N--- N ----_
N-
,...õ..
. .
112N
0
C,
QH
0
N
H N
H
N ----
N..., -....õ. --N.,
. .
f / 5 1
0
NH, H2N 0 0
0 H 2N" H 0 HO\ N
N N
H \N H
H
\ N
N -
\-..õ ----Nr---\ N. = --..., N --_
,
N. =
, 1
0
0 0
0 0 -----
/ \N
HN-
''''N \
\ /
0
; .
; \ N
`,...... -
, ',.......
'
, ,
_õN
rl.,õ NW.- \\.\N
i PIN/NN II \ .,õ. =./..`
----N N
\N-
.5.,....õ,N
N
N----
N-- N---- N---
........ .--, ........ --õ,
. . .
a a a a a
81
Date Recue/Date Received 2023-06-16
84451174
\ I
(7, /N....:(N (N,,,,i4
N \I
/ \
\ ----/ N---7--- N\ ____/-----
,,,,
. .
V
1 \)
(N
\ \ /
N
0 N ---- N
\I
/ \
N--- \ +.-/ ----- -----
. ; . . .
2 2 ! 2
'''''''=N/N''''
0-----(11
_--- Nm- IN
0
.-"---- N
N---- N---- ---_
--_ I
; .
; .
; ;
,./.;H = /N,....N
C.:Pi
----0 \
N.--
N CI I
N---- N-- N-- N-- II--
\ .,,
. . . .
2 1 ; 2 2
0 0 H
)---0\N N
N N C) HN / 0 /
. ----(--,
N
N
0 ...".' N
...,.... ,N---.. 1 N--- --_ --
\ N \ N
2 ; ; ; 2
2
N
\
t..--
h II \
N /11 N / /N N / r \N ---c--1 ----------\N
2! t N
21--...õ. I 0
N ....,, 17¨\
N /N N N. 0 / )
'..""11
\ \ N \ N \ \ N \ N \ N \ N
N = N = N ' N = N ' N ' N = N
' 7 7 2 a 7 2
8m
Date Recue/Date Received 2023-06-16
84451174
.....-N
N--- \
4,,,,,,, N-
----- \\
N-,----N IN:=-.-. -- \
I 'N 1
1 0 N N. 0 N---__
N--õ, NI /7
N
, N
. N
/
\ \ N \ N NN
\ N
N\ = ; \ N \.= '''\ ; \ =
N/
ii-
FN/
N , N r-i- N , N
N , N N , N
/N \ 0 \
N'N 0 \
N\.....4
N
\
// \ N ----\
N¨N ro
ro .
\--\ N--
`,.
0 /
; , -- .
, .
frkl i ll--- /----C.0 N
/ F-N
N ,N UN N , N N , N
N ,N
N õ N
\ N \ \
NO
. \ 0
N
N
D N....--=\ \--µ,õ
0-- -- =
7 7 7
N
N/ C))
C IT- N
ND N ,N ii_Ni--/
FN/---/
0
N õ N Ni , N
N _-N
\ N
\ N N N
\---\
0¨ = 5 0¨ = 0¨ =
7 7
õ..... J.----.
i
r_cNr
i \
----
/F
uN.¨C N , N N ,I\1 N , il
N ,N
N _-N
N \......A
\---\ N¨' 14--
0.¨ = / = / = / \ =
7
8n
Date Regue/Date Received 2023-06-16
84451174
N\
0 -)
(
)---14 i õ,---.. N N N N
#-N
N , N I N , N NI' , N N /IV N õ,N --....
'N
I
/
\ N \ N \ N \ N \ N \ N \ N
\ = \ = \ \ = \ = \ = \ =
N ¨
\Q)
N _
"--N -N / N=0
FN
Kr,/ irN N õ I\1 Ni µ,õ N /
NõN NõN N N õ N
N N
\ = \ - \ - \ - \ =
, , , ,
N¨N ii¨N N--=µ HN¨N r;1
N'N' N_NI , 0
N N NI ,N N , N
N N)k... N N '=
' I I
..." 2,2
N \ N \ N \ N
\ = \ ; \ -, \ = \; \ -
, ,
\
//----
N µ
Y---"N 0
N N N N 0
!.
N õ, N N , i\J N õ N N õ, N
\ N \ N \ N \ N \ N
\ = \ = \
, ; \ = \ =
,
8o
Date Regue/Date Received 2023-06-16
84451174
0¨
0¨ c----/ Y---"N
NI FN/ FN
)=--N
) N ,N N , N
FN N
N , N N , N / ,
\ .
\ N \ N
CN
N.%... N..../Ss
\ ; \ ; 0 = 0 = ;
------C\ .
N
CN (ill Nrs\c) N 'N.,
V
.
2 ; or 0 =
2
and
1
---....----- ¨
¨ -----"---- \
N¨N ------(-- ----...---.
------n----- N¨N N¨N N¨N
N¨N
"N.
\
N¨N ----(Cr¨CN HO N N---
R7 iS \ = N¨NH = 0 = 0 = / ,,,N6 / =
, ; ;
J,11r
Jyy,
--A----- N ,- N
N¨N I N N-r
1 õ,v
N , N
. N N ,...-- Th\I
I = I =
; =
, =
,
i
i '= .'-').',/-
1
I N N
N , N N , N
,
N ,- N N N
N
-Nq N
N N.,õ- y
N__D .
II
OH; N . 0 ; 0 -
,
8p
Date Recue/Date Received 2023-06-16
84451174
N N N
NH N
N
N ,-N N N
o.. .
N
fµE'
c,,õN = OH ; =
;or
2. Compounds and Definitions:
[0013] Compounds of this disclosure include those described generally above,
and are further
illustrated by the classes, subclasses, and species disclosed herein. As used
herein, the following
definitions shall apply unless otherwise indicated. For purposes of this
disclosure, the chemical
elements are identified in accordance with the Periodic Table of the Elements,
CAS version,
Handbook of Chemistry and Physics, 75th Ed. Additionally, general principles
of organic
chemistry are described in "Organic Chemistry", Thomas Sorrell, University
Science Books,
Sausalito: 1999, and "March's Advanced Organic Chemistry", 5th Ed., Ed.:
Smith, M.B. and
March, J., John Wiley & Sons, New York: 2001.
[0014] The twit "aliphatic" or "aliphatic group", as used herein, means a
straight-chain (i.e.,
unbranched) or branched, substituted or unsubstituted hydrocarbon chain that
is completely
saturated or that contains one or more units of unsaturation, or a monocyclic
hydrocarbon or
bicyclic or polycyclic hydrocarbon that is completely saturated or that
contains one or more units
of unsaturation, but which is not aromatic (also referred to herein as
"carbocycle,"
"cycloaliphatic" or "cycloalkyl"), that has a single point of attachment to
the rest of the
molecule. Unless otherwise specified, aliphatic groups contain 1-6 aliphatic
carbon atoms. In
some embodiments, aliphatic groups contain 1-5 aliphatic carbon atoms. In
other embodiments,
aliphatic groups contain 1-4 aliphatic carbon atoms. In still other
embodiments, aliphatic groups
contain 1-3 aliphatic carbon atoms, and in yet other embodiments, aliphatic
groups contain 1-2
aliphatic carbon atoms. In some embodiments, "cycloaliphatic" (or
"carbocycle" or
8q
Date Recue/Date Received 2023-06-16
84451174
"cycloalkyl") refers to a monocyclic C3-C6 hydrocarbon that is completely
saturated or that
contains one or more units of unsaturation, but which is not aromatic, that
has a single point of
attachment to the rest of the molecule. Suitable aliphatic groups include, but
are not limited to,
linear or branched, substituted or unsubstituted alkyl, alkenyl, alkynyl
groups and hybrids thereof
such as (cycloalkyl)alkyl, (cycloalkenyl)alkyl or (cycloallcypalkenyl.
[0015] The term "lower alkyl" refers to a C1-4 straight or branched alkyl
group. Exemplary
lower alkyl groups are methyl, ethyl, propyl, isopropyl, butyl, isobutyl, and
tert-butyl.
[0016] The term "lower haloalkyl" refers to a C1-4 straight or branched alkyl
group that is
substituted with one or more halogen atoms.
8r
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
100171 The term "heteroatom" means one or more of oxygen, sulfur, nitrogen,
phosphorus,
or silicon (including, any oxidized form of nitrogen, sulfur, phosphorus, or
silicon; the
quaternized form of any basic nitrogen; or a substitutable nitrogen of a
heterocyclic ring, for
example N (as in 3,4-dihydro-2H-pyrroly1), NH (as in pyrrolidinyl) or NR+ (as
in N-
substituted pyrrolidinyl)).
[0018] The term "unsaturated," as used herein, means that a moiety has one or
more units of
unsaturation.
[0019] As used herein, the term "bivalent C1-8 (or C1-6) saturated or
unsaturated, straight
or branched, hydrocarbon chain", refers to bivalent allcylene, alkenylene, and
allcynylene
chains that are straight or branched as defined herein.
[0020] The term "allcylene" refers to a bivalent alkyl group. An "allcylene
chain" is a
polymethylene group, i.e., ¨(CH2)n¨, wherein n is a positive integer,
preferably from 1 to 6,
from 1 to 4, from 1 to 3, from 1 to 2, or from 2 to 3. A substituted alkylene
chain is a
polymethylene group in which one or more methylene hydrogen atoms are replaced
with a
substituent. Suitable substituents include those described below for a
substituted aliphatic
group.
[0021] The term "alkenylene" refers to a bivalent alkenyl group. A substituted
alkenylene
chain is a polymethylene group containing at least one double bond in which
one or more
hydrogen atoms are replaced with a substituent. Suitable substituents include
those described
below for a substituted aliphatic group.
[0022] The term "halogen" means F, Cl, Br, or I.
[0023] The term "aryl" used alone or as part of a larger moiety as in
"arallcyl," "aralkoxy,"
or "aryloxyalkyl," refers to monocyclic or bicyclic ring systems having a
total of five to
fourteen ring members, wherein at least one ring in the system is aromatic and
wherein each
ring in the system contains 3 to 7 ring members. The term "aryl" may be used
interchangeably with the term "aryl ring." In certain embodiments of the
present disclosure,
"aryl" refers to an aromatic ring system which includes, but not limited to,
phenyl, naphthyl,
anthracyl and the like, which may be optionally substituted. Also included
within the scope
of the term "aryl," as it is used herein, is a group in which an aromatic ring
is fused to one or
more non¨aromatic rings, such as indanyl, phthalimidyl, naphthimidyl,
phenanthridinyl, or
tetrahydronaphthyl, and the like.
[0024] The terms "heteroaryl" and "heteroar¨," used alone or as part of a
larger moiety,
e.g., "heteroarallcyl," or "heteroaralkoxy," refer to groups having 5 to 10
ring atoms, or 5 to
15 ring atoms, preferably 5, 6, or 9 ring atoms; having 6, 10, or 14 it
electrons shared in a
9
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
cyclic array; and having, in addition to carbon atoms, from one to five
heteroatoms.
Heteroaryl groups include, without limitation, thienyl, furanyl, pyrrolyl,
imidazolyl,
pyrazolyl, triazolyl, tetrazolyl, oxazolyl, isoxazolyl, oxadiazolyl,
thiazolyl, isothiazolyl,
thiadiazolyl, pyridyl, pyridazinyl, pyrimidinyl, pyrazinyl, indolizinyl,
purinyl, naphthyridinyl,
and pteridinyl. The terms "heteroaryl" and "heteroar¨", as used herein, also
include groups in
which a heteroaromatic ring is fused to one or more aryl, cycloaliphatic, or
heterocyclyl
rings, where the radical or point of attachment is on the heteroaromatic ring.
Non-limiting
examples include indolyl, isoindolyl, benzothienyl, benzofuranyl,
dibenzofuranyl, indazolyl,
benzimidazolyl, benzthiazolyl, quinolyl, isoquinolyl, cinnolinyl,
phthalazinyl, quinazolinyl,
quinoxalinyl, 4H¨quinolizinyl, carbazolyl, acridinyl, phenazinyl,
phenothiazinyl,
phenoxazinyl, tetrahydroquinolinyl, tetrahydroisoquinolinyl, pyrido[2,3¨b]-
1,4¨oxazin-
3(4H)¨one, 3,4-dihydro-1H41,4] oxazino[4,3 -a] indole, 3,4-dihydro-1H-
{1,4joxazino[4,3-
al indol- 1 -one, 1,2,3,4-tetrahydropyrazino[1,2-a]indole, and 3,4-
dihydropyrazino[1,2-a]indo1-
1(2H)-one. A heteroaryl group may be mono¨, bicyclic, or tricyclic. The term
"heteroaryl"
may be used interchangeably with the terms "heteroaryl ring," "heteroaryl
group," or
"heteroaromatic," any of which terms include rings that are optionally
substituted. The term
"heteroaralkyl" refers to an alkyl group substituted by a heteroaryl, wherein
the alkyl and
heteroaryl portions independently are optionally substituted.
[0025] As used herein, the terms "heterocycle," "heterocyclyl," "heterocyclic
radical," and
"heterocyclic ring" are used interchangeably and refer to a stable 5¨ to
7¨membered
monocyclic or 7-10¨membered bicyclic heterocyclic moiety that is either
saturated or
partially unsaturated, and having, in addition to carbon atoms, one or more,
preferably one to
four, heteroatoms. When used in reference to a ring atom of a heterocycle, the
term
"nitrogen" includes a substituted nitrogen. As an example, in a saturated or
partially
unsaturated ring having 1-3 heteroatoms selected from oxygen, sulfur or
nitrogen, the
nitrogen may be N (as in 3,1 dihydro-2H¨pyrroly1), NH (as in pyrrolidinyl),
or +NR (as in
N¨substituted pyrrolidinyl).
[0026] A heterocyclic ring can be attached to its pendant group at any
heteroatom or carbon
atom that results in a stable structure and any of the ring atoms can be
optionally substituted.
Examples of such saturated or partially unsaturated heterocyclic radicals
include, without
limitation, tetrahydrofuranyl, tetrahydrothiophenyl pyrrolidinyl, piperidinyl,
pyrrolinyl,
tetrahydroquinolinyl, tetrahydroisoquinolinyl,
decahydroquinolinyl, oxazolidinyl,
piperazinyl, dioxanyl, dioxolanyl, diazepinyl, oxazepinyl, thiazepinyl,
morpholinyl, and
quinuclidinyl. The terms "heterocycle," "het ero cy clyl," "heterocyclyl
ring," "heterocyclic
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
group," "heterocyclic moiety," and "heterocyclic radical," are used
interchangeably herein,
and also include groups in which a heterocyclyl ring is fused to one or more
aryl, heteroaryl,
or cycloaliphatic rings, such as indolinyl, 3H¨indolyl, chromanyl,
phenanthridinyl, or
tetrahydroquinolinyl, where the radical or point of attachment is on the
heterocyclyl ring. A
heterocyclyl group may be mono¨ or bicyclic. The term "heterocyclylalkyl"
refers to an alkyl
group substituted by a heterocyclyl, wherein the alkyl and heterocyclyl
portions
independently are optionally substituted.
[0027] As used herein, the term "partially unsaturated" refers to a ring
moiety that includes
at least one double or triple bond. The term "partially unsaturated" is
intended to encompass
rings having multiple sites of unsaturation, but is not intended to include
aryl or heteroaryl
moieties, as herein defined.
[0028] As described herein, compounds of the disclosure may contain
"optionally
substituted" moieties. In general, the term "substituted," whether preceded by
the term
"optionally" or not, means that one or more hydrogens of the designated moiety
are replaced
with a suitable substituent. Unless otherwise indicated, an "optionally
substituted" group
may have a suitable substituent at each substitutable position of the group,
and when more
than one position in any given structure may be substituted with more than one
substituent
selected from a specified group, the substituent may be either the same or
different at every
position. Combinations of substituents envisioned by this disclosure are
preferably those that
result in the formation of stable or chemically feasible compounds. The term
"stable," as
used herein, refers to compounds that are not substantially altered when
subjected to
conditions to allow for their production, detection, and, in certain
embodiments, their
recovery, purification, and use for one or more of the purposes disclosed
herein.
[0029] Suitable monovalent substituents on a substitutable carbon atom of an
"optionally
substituted" group are independently halogen; ¨(CH2)o-41Z ; ¨(CH2)o-40R ; -
0(CH2)04R ,
¨0¨(CH2)o-4C(0)0R ; ¨(CH2)04CH(OR )2; ¨(CF12)0-4SR ; ¨(CH2)o-4Ph, which may be
substituted with R"; ¨(CH2)0_40(CH2)0_1Ph which may be substituted with IV);
¨CH=CHPh,
which may be substituted with It"; ¨(CH2)0_40(CH2)0_1-pyridyl which may be
substituted
with R'; ¨NO2; ¨CN; ¨N3; ¨(CH2)o-4N(R )2; ¨(CH2)o-4N(R )C(0)R ; ¨N(R )C(S)R ;
¨(CH2)o-4N(R )C(0)NR 2; ¨N(R )C(S)NR 2;
¨(CH2)0-4N(R )C(0)0R ;
¨N(R )N(R )C(0)R ; ¨N(R )N(R )C(0)NR 2; ¨N(R )N(R )C(0)0R ; ¨(CH2)0-4C(0)R ;
¨(CH2)0-4C(0)OR'; ¨(CH2)o-4C(0)SR ;
¨(CH2)o-4C(0)0SiR 3;
¨(CH2)0-40C(0)R ; ¨0C(0)(CH2)0-45R¨; ¨(CH2)0-45C(0)R ; ¨(CH2)0-4C(0)NR 2;
11
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
¨(CH2)0-4C(0)N(W)S(0)21U; ¨C(S)NR 2; ¨C(S)SR ; ¨SC(S)SR , ¨(CH2)0-40C(0)NR 2;
¨C(0)N(OR )R ; ¨C(0)C(0)R ; ¨C(0)CH2C(0)R ; ¨C(NOR )R ; ¨(CH2)0-4SSR ;
¨(CH2)0-4S(0)R ; ¨(CH2)0-4S(0)2R ;
¨(CH2)0-4.S(0)20R ; .. ¨(CH2)0-40S(0)2R ;
¨S(0)2NR 2; ¨S(0)2N(R )C(0)R ; ¨(CF12)o-4S(0)R ; ¨N(R )S(0)2NR 2; ¨N(R )S(0)2R
;
¨N(OR )R ; ¨C(NH)NR 2; ¨P(0)2R ; ¨P(0)R 2; ¨0P(0)R 2; ¨0P(0)(OR )2; ¨SiR 3;
¨(C1-4 straight or branched alkylene)O¨N(R )2; or ¨(C1-4 straight or branched
alkylene)C(0)0¨N(R )2, wherein each R may be substituted as defined below and
is
independently hydrogen, an optionally substituted group selected from C1-12
aliphatic or a
ring selected from a 3-10 membered saturated or partially unsaturated
carbocyclic ring,
phenyl, a 6-10 membered bicyclic saturated, partially unsaturated or aryl
ring, a 3-8
membered saturated or partially unsaturated heterocyclic ring having 1-2
heteroatoms
independently selected from nitrogen, oxygen, or sulfur, a 5-6 membered
heteroaryl ring
having 1-4 heteroatoms independently selected from nitrogen, oxygen, or
sulfur, a 7-10
membered bicyclic saturated or partially unsaturated heterocyclic ring having
1-5
heteroatoms independently selected from nitrogen, oxygen or sulfur, or an 8-10
membered
bicyclic heteroaryl ring having 1-5 heteroatoms independently selected from
nitrogen,
oxygen or sulfur, ¨CH2Ph, ¨0(CH2)o-1Ph, or ¨CH2¨(5-6 membered heteroaryl
ring), or,
notwithstanding the definition above, two independent occurrences of R , taken
together with
their intervening atom(s), form a 3-12¨membered saturated, partially
unsaturated, or aryl
mono¨ or bicyclic ring having 0-4 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur, which may be substituted as defined below.
[0030] Suitable monovalent substituents on R (or the ring formed by taking
two
independent occurrences of R together with their intervening atoms), are
independently
halogen, ¨(CH2)o-21te, ¨(haloRe), (CH2)o-20H, ¨(CH2)o-201e, ¨(CH2)o-2CH(0R.)2;
¨0(haloR'), ¨CN, ¨N3, ¨(CH2)o-2C(0)R., ¨(012)o-2C (0)0H, ¨(CH2)0-2C(0)OR',
¨(CH2)0-2SRe, ¨(CH2)o-2S(0)R., ¨(CH2)o-25(0)21e, ¨(CH2)o-2SH, ¨(CH2)0-2NH2,
¨(CH2)o-2NHR", ¨(CH2)o-2NR"2, ¨NO2, ¨SiR'3, ¨0SiR"3, -C(0)SR", ¨(C1_4 straight
or
branched alkylene)C(0)0R", or ¨SSW' wherein each R* is unsubstituted or where
preceded
by "halo" is substituted with one or more halogens, and is independently
selected from an C
12 aliphatic or a ring selected from a 3-10 membered saturated or partially
unsaturated
carbocyclic ring, phenyl, a 6-10 membered bicyclic saturated, partially
unsaturated or aryl
ring, a 3-8 membered saturated or partially unsaturated heterocyclic ring
having 1-2
heteroatoms independently selected from nitrogen, oxygen, or sulfur, a 5-6
membered
12
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
heteroaryl ring having 1-4 heteroatoms independently selected from nitrogen,
oxygen, or
sulfur, a 7-10 membered bicyclic saturated or partially unsaturated
heterocyclic ring having
1-5 heteroatoms independently selected from nitrogen, oxygen or sulfur, or an
8-10
membered bicyclic heteroaryl ring having 1-5 heteroatoms independently
selected from
nitrogen, oxygen or sulfur, -CH2Ph, or -0(CH2)o-1Ph. Suitable divalent
substituents on a
saturated carbon atom of R include =0 and =S.
[0031] Suitable divalent substituents on a suitable carbon atom of an
"optionally
substituted" group include the following: =0, =S, =NNR*2, =NNHC(0)R*,
=NNHC(0)0R*,
=NNHS(0)2R*, =NW, =NOR*, -0(C(W2))2-30-, or -S(C(R*2))2_3S-, wherein each
independent occurrence of R* is selected from hydrogen, C1_6 aliphatic which
may be
substituted as defined below, or an unsubstituted 5-6-membered saturated,
partially
unsaturated, or aryl ring having 0-4 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur. Suitable divalent substituents that are bound to vicinal
substitutable
carbons of an "optionally substituted" group include: -0(CR*2)2-30-, wherein
each
independent occurrence of R* is selected from hydrogen, C1-6 aliphatic which
may be
substituted as defined below, or an unsubstituted 5-6-membered saturated,
partially
unsaturated, or aryl ring having 0-4 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur.
[0032] Suitable substituents on the aliphatic group of R* include halogen, -
R", -(haloRe), -
OH, -OR', -0(haloRe), -CN, -C(0)R", -C(0)0H, -C(0)01e, -C(0)NR"2, -SR", -
S(0)1e,
-S(0)2R", -NH2, -NHR", -NR"2, or -NO2, wherein each R" is unsubstituted or
where
preceded by "halo" is substituted only with one or more halogens, and is
independently C1_4
aliphatic, -CH2Ph, -0(CH2)0_11311, or a 5-6-membered saturated, partially
unsaturated, or aryl
ring having 0-4 heteroatoms independently selected from nitrogen, oxygen, or
sulfur.
[0033] Suitable substituents on a substitutable nitrogen of an "optionally
substituted" group
include -Rt, -NRt2, -C(0)Rt, -C(0)01e, -C(0)C(0)Rt, -C(0)CH2C(0)1e, -S(0)21e, -
S(0)2NRI2, -C(S)NW2, -C(NH)NR1-2, -C(NR)NW-2, or -N(Rt)S(0)2Rt; wherein each
Rt is
independently hydrogen, C 1_6 aliphatic which may be substituted as defined
below,
unsubstituted -0Ph, or an unsubstituted 5-6-membered saturated, partially
unsaturated, or
aryl ring having 0-4 heteroatoms independently selected from nitrogen, oxygen,
or sulfur, or,
notwithstanding the definition above, two independent occurrences of RI, taken
together with
their intervening atom(s) form an unsubstituted 3-12-membered saturated,
partially
unsaturated, or aryl mono- or bicyclic ring having 0-4 heteroatoms
independently selected
13
84451174
from nitrogen, oxygen, or sulfur.
[0034] Suitable substituents on the aliphatic group of Rt are independently
halogen,
- -(halon, ¨OH, ¨OR', ¨0(haloR'), ¨CN, ¨C(0)0H, ¨C(0)01e, ¨C(0)NR"2, ¨SR",
¨S(0)1e, ¨S(0)21e, ¨NH2, ¨NHR', ¨N1R"2, or ¨NO2, wherein each le is
unsubstituted or where
preceded by "halo" is substituted only with one or more halogens, and is
independently
C1-4 aliphatic, ¨CH2Ph, ¨0(CH2)0_11311, or a 5-6¨membered saturated, partially
unsaturated, or
aryl ring having 0-4 heteroatoms independently selected from nitrogen, oxygen,
or sulfur.
[0035] As used herein, the term "pharmaceutically acceptable salt" refers to
those salts which
are, within the scope of sound medical judgment, suitable for use in contact
with the tissues of
humans and lower animals without undue toxicity, irritation, allergic response
and the like, and
are commensurate with a reasonable benefit/risk ratio. Pharmaceutically
acceptable salts are well
known in the art. For example, S. M. Berge et al., describe pharmaceutically
acceptable salts in
detail in J. Pharmaceutical Sciences, 1977, 66, 1-19. Pharmaceutically
acceptable salts of the
compounds of this disclosure include those derived from suitable inorganic and
organic acids
and bases. Examples of pharmaceutically acceptable, nontoxic acid addition
salts are salts of an
amino group formed with inorganic acids such as hydrochloric acid, hydrobromic
acid,
phosphoric acid, sulfuric acid and perchloric acid or with organic acids such
as acetic acid,
oxalic acid, maleic acid, tartaric acid, citric acid, succinic acid or malonic
acid or by using other
methods used in the art such as ion exchange. Other pharmaceutically
acceptable salts include
adipate, alginate, ascorbate, aspartate, benzenesulfonate, benzoate,
bisulfate, borate, butyrate,
camphorate, camphorsulfonate, citrate, cyclopentanepropionate, digluconate,
dodecylsulfate,
ethanesulfonate, formate, fumarate, glucoheptonate, glycerophosphate,
gluconate, hemisulfate,
heptanoate, hexanoate, hydroiodide, 2¨hydroxy¨ethanesulfonate, lactobionate,
lactate, laurate,
lauryl sulfate, malate, maleate, malonate, methanesulfonate,
2¨naphthalenesulfonate, nicotinate,
nitrate, oleate, oxalate, palmitate, pamoate, pectinate, persulfate,
3¨phenylpropionate, phosphate,
pivalate, propionate, stearate, succinate, sulfate, tartrate, thiocyanate,
p¨toluenesulfonate,
undecanoate, valerate salts, and the like.
[0036] Salts derived from appropriate bases include alkali metal, alkaline
earth metal,
ammonium and N+(C1-4a1ky1)4 salts. Representative alkali or alkaline earth
metal salts include
sodium, lithium, potassium, calcium, magnesium, and the like. Further
pharmaceutically
acceptable salts include, when appropriate, nontoxic ammonium, quaternary
ammonium, and
amine cations formed using counterions such as halide
14
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
hydroxide, carboxylate, sulfate, phosphate, nitrate, lower alkyl sulfonate and
aryl sulfonate.
[0037] Unless otherwise stated, structures depicted herein are also meant to
include all
isomeric (e.g., enantiomeric, diastereomeric, and geometric (or
conformational)) forms of the
structure; for example, the R and S configurations for each asymmetric center,
Z and E
double bond isomers, and Z and E conformational isomers. Therefore, single
stereochemical
isomers as well as enantiomeric, diastereomeric, and geometric (or
conformational) mixtures
of the present compounds are within the scope of the disclosure. Unless
otherwise stated, all
tautomeric forms of the compounds of the disclosure are within the scope of
the disclosure.
Additionally, unless otherwise stated, structures depicted herein are also
meant to include
compounds that differ only in the presence of one or more isotopically
enriched atoms. For
example, compounds having the present structures including the replacement of
hydrogen by
deuterium or tritium, or the replacement of a carbon by a 13C- or 14C-enriched
carbon are
within the scope of this disclosure. Such compounds are useful, for example,
as analytical
tools, as probes in biological assays, or as therapeutic agents in accordance
with the present
disclosure.
3. Description of Exemplary Embodiments:
[0038] In one aspect, disclosed is a compound of formula (I):
R4 L2¨R3
R5
\ R2
R6
L1¨R1
R7
(I)
or a pharmaceutically acceptable salt thereof, wherein RI, R2; R3; R4; R5; R6;
R7; Li; and L2
are as defined above.
[0039] In certain embodiments, R2 is selected from ¨C(0)¨L3-1e,
¨C(0)N(R)¨L3¨Rz,
¨C(0)N(R)¨C(R)2¨L3¨Rz, or ¨C(0)S¨L3¨W.
[0040] In certain embodiments, L2 is selected from:
/--------0?-22; and
[0041] In certain embodiments, L2 is
[0042] In certain embodiments, R4 is hydrogen; R5 is hydrogen; and R6 is
halogen, C1-C6-
haloalkyl, or cyclopropyl.
[0043] In certain embodiments, R6 is chloro.
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
100441 In certain embodiments, the compound of formula (I) has formula (I-
a) or (I-b):
R11
, R8 R9
) R7
CI N N-L4-Rw CI N N-L41,e,
0 0
R3 (I-a); or R3 (I-b);
or a pharmaceutically acceptable salt thereof, wherein: L4 is independently
selected from a
covalent bond or an optionally substituted bivalent straight or branched C1.8
hydrocarbon
chain wherein one or more methylene units are optionally and independently
replaced with ¨
Cy'¨, ¨0¨, ¨S¨, ¨N(R)¨, ¨N(R)C(0)¨, ¨N(R)S(0)2¨, ¨C(0)¨, ¨C(0)N(R)¨, ¨S(0)¨,
¨S(0)2¨, or ¨S(0)2N(R)¨; ¨Cy'¨ is an optionally substituted bivalent ring
independently
selected from phenylene, 3-8 membered saturated or partially unsaturated
carbocyclylene, 5-6
membered heteroarylene having 1-4 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur, 3-8 membered saturated or partially unsaturated
heterocyclylene having 1-
4 heteroatoms independently selected from nitrogen, oxygen, or sulfur, an 8-10
membered
bicyclic arylene or heteroarylene having 1-4 heteroatoms independently
selected from
nitrogen, oxygen, or sulfur, or an 8-10 membered saturated or partially
unsaturated
heterocyclylene having 1-4 heteroatoms independently selected from nitrogen,
oxygen, or
sulfur; Rw is selected from hydrogen, ¨Cy'¨R, R, ¨OR, ¨SR, ¨S(0)R, ¨S(0)2R,
¨S(0)2N(R)2, ¨N(R)2, ¨C(0)N(R)2, ¨C(0)R, ¨N(R)C(0)R, ¨N(R)C(0)0R,
¨N(R)C(0)N(R)2, ¨N(R)S(0)2R, ¨N(R)S(0)2N(R)2, ¨C(0)0H, ¨C(0)0R, ¨C(0)R',
¨S(0)20H, or ¨S(0)2R, or is selected from:
H N H
HN
o
=Nr. 0
ONNsen "C) N NH
(7.<1µ1/
(-2(µ¨ N H
; le is selected from
¨C(0)0R, ¨N(R)S(0)2CF3, ¨N(R)C(0)R, ¨N(R)C(0)0R, ¨N(R)C(0)N(R)2, or
¨N(R)S(0)2R; RY is selected from ¨N(R)C(0)CF3, ¨N(R)C(0)R, or ¨N(R)C(0)N(R)2;
each
R is independently selected from hydrogen or an optionally substituted group
selected from
16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
C1_12 aliphatic or a ring selected from a 3-10 membered saturated or partially
unsaturated
carbocyclic ring, phenyl, a 6-10 membered bicyclic saturated, partially
unsaturated or aryl
ring, a 3-8 membered saturated or partially unsaturated heterocyclic ring
having 1-2
heteroatoms independently selected from nitrogen, oxygen, or sulfur, a 5-6
membered
heteroaryl ring having 1-4 heteroatoms independently selected from nitrogen,
oxygen, or
sulfur, a 7-10 membered bicyclic saturated or partially unsaturated
heterocyclic ring having
1-5 heteroatoms independently selected from nitrogen, oxygen or sulfur, or an
8-10
membered bicyclic heteroaryl ring having 1-5 heteroatoms independently
selected from
nitrogen, oxygen or sulfur; each of R5, R6, R8, R9, Rv.), RH, and K-12
is independently selected
from R, halogen, -CN, -NO2, -C(0)OR', -OR', -SR', -C(0)N(R')2-N(R')2, -
S(0)2N(R)2,
-N(R')S(0)2CF3, -C(0)R', -N(R')C(0)R', -S(0)R', -S(0)2R', -N(R')C(0)OR', and
-N(R')S(0)2R'; each R' is independently selected from hydrogen or optionally
substituted
C1_4 alkyl; and R7 is selected from hydrogen, halogen, -CN, -NO2, -C(0)0R, -
0CF3, -OR,
-SR, -S(0)20R, -P(0)(OH)2, -C(0)N(R)2, -N(R)2, -S(0)2N(R)2, -N(R)S(0)2CF3,
-C(0)N(R)S(0)2R, -S(0)2N(R)C(0)0R, -S(0)2N(R)C(0)N(R)2, -C(0)R,
-C(0)N(R)S(0)2CF3, -N(R)C(0)R, -0C(0)R, -0C(0)N(R)2, -C(NR)N(R)2,
-N(R)C(NR)N(R)2, -S(0)R, -S(0)2R, -N(R)C(0)0R, or -N(R)S(0)2R, or an
optionally
substituted group selected from Ci_6 aliphatic or a ring selected from a 3-8
membered
saturated or partially unsaturated carbocyclic ring, phenyl, a 3-8 membered
saturated or
partially unsaturated heterocyclic ring having 1-2 heteroatoms independently
selected from
nitrogen, oxygen, or sulfur, a 5-6 membered heteroaryl ring having 1-4
heteroatoms
independently selected from nitrogen, oxygen, or sulfur, an 8-14 membered
bicyclic or
polycyclic saturated, partially unsaturated or aryl ring, a 7-14 membered
bicyclic or
polycyclic saturated or partially unsaturated heterocyclic ring having 1-5
heteroatoms
independently selected from nitrogen, oxygen, or sulfur, or an 8-14 membered
bicyclic or
poly cyclic heteroaryl ring having 1-5 heteroatoms independently selected from
nitrogen,
oxygen, or sulfur.
100451 In
certain embodiments, of formula (I-a) or (I-b), or a pharmaceutically
acceptable
salt thereof, L4 is independently selected from a covalent bond or an
optionally substituted
bivalent straight or branched C1_8 hydrocarbon chain wherein one or more
methylene units
are optionally and independently replaced with -0-, -S-, -N(R)-, -N(R)C(0)-
,
-N(R)S(0)2-, -C(0)-, -C(0)N(R)-, -S(0)-, -S(0)2-, or -S(0)2N(R)-; -Cy'- is an
optionally substituted bivalent ring independently selected from phenylene, 3-
8 membered
saturated or partially unsaturated carbocyclylene, 5-6 membered heteroarylene
having 1-4
17
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
heteroatoms independently selected from nitrogen, oxygen, or sulfur, 3-8
membered saturated
or partially unsaturated heterocyclylene having 1-4 heteroatoms independently
selected from
nitrogen, oxygen, or sulfur, an 8-10 membered bicyclic arylene or
heteroarylene having 1-4
heteroatoms independently selected from nitrogen, oxygen, or sulfur, or an 8-
10 membered
saturated or partially unsaturated heterocyclylene having 1-4 heteroatoms
independently
selected from nitrogen, oxygen, or sulfur; Rw is selected from hydrogen, R, -
Cy'-R, -OR,
-SR, -S(0)R, -S(0)2R, -S(0)2N(R)2, -N(R)2, -C(0)N(R)2, -C(0)R, -N(R)C(0)R,
-N(R)C(0)0R, -N(R)C(0)N(R)2, -N(R)S (0)2R, -N(R)S(0)2N(R)2, -C(0)0H, -C(0)0R,
-C(0)R', -S(0)20H, or -S(0)2R', or is selected from:
H H
N
(7.24=i Le? i=i=" c7FIN-0
0 N C)N=eC) i=Nt
N NH
VC-NH
; Ir is selected from
-C(0)0R, -N(R)S(0)2CF3, -N(R)C(0)R, -N(R)C(0)0R, -N(R)C(0)N(R)2, or
-N(R)S(0)2R; RY is selected from -N(R)C(0)CF3, -N(R)C(0)R, or -N(R)C(0)N(R)2;
each
R is independently selected from hydrogen or an optionally substituted group
selected from
C1-12 aliphatic or a ring selected from a 3-10 membered saturated or partially
unsaturated
carbocyclic ring, phenyl, a 6-10 membered bicyclic saturated, partially
unsaturated or aryl
ring, a 3-8 membered saturated or partially unsaturated heterocyclic ring
having 1-2
heteroatoms independently selected from nitrogen, oxygen, or sulfur, a 5-6
membered
heteroaryl ring haying 1-4 heteroatoms independently selected from nitrogen,
oxygen, or
sulfur, a 7-10 membered bicyclic saturated or partially unsaturated
heterocyclic ring having
1-5 heteroatoms independently selected from nitrogen, oxygen or sulfur, an 8-
10 membered
bicyclic heteroaryl ring having 1-5 heteroatoms independently selected from
nitrogen,
oxygen or sulfur, or a 12-15 membered tricyclic heteroaryl ring having 1-5
heteroatoms
independently selected from nitrogen, oxygen or sulfur; each of R5, R6, R8,
R9, RR), and
R12 is independently selected from R, halogen, -CN, -NO2, -C(0)OR', -OR', -
SR',
-C(0)N(R')2 -N(R')2, -S(0)2N(R')2, -N(R')S(0)2CF3, -C(0)R', -N(R')C(0)R', -
S(0)R',
-S(0)2R', -N(R')C(0)OR', and -N(R')S(0)2R'; each R' is independently selected
from
hydrogen or optionally substituted C1-4 alkyl; and R7 is selected from
hydrogen, halogen,
-CN, -NO2, -C(0)0R, -0CF3, -OR, -SR, -S(0)20R, -P(0)(OH)2, -C(0)N(R)2, -N(R)2,
-N(R)S(0)2CF3, -C(0)N(R)S(0)2R, -
S(0)2N(R)C(0)0R,
18
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
¨S(0)2N(R)C(0)N(R)2, ¨C(0)R, ¨C(0)N(R)S(0)2CF3, ¨N(R)C(0)R, ¨0C(0)R,
¨0C(0)N(R)2, ¨C(NR)N(R)2, ¨N(R)C(NR)N(R)2, ¨S(0)R, ¨S(0)2R, ¨N(R)C(0)0R, or
¨N(R)S(0)2R, or an optionally substituted group selected from C1_6 aliphatic
or a ring
selected from a 3-8 membered saturated or partially unsaturated carbocyclic
ring, phenyl, a 3-
8 membered saturated or partially unsaturated heterocyclic ring haying 1-2
heteroatoms
independently selected from nitrogen, oxygen, or sulfur, a 5-6 membered
heteroaryl ring
haying 1-4 heteroatoms independently selected from nitrogen, oxygen, or
sulfur, an 8-14
membered bicyclic or polycyclic saturated, partially unsaturated or aryl ring,
a 7-14
membered bicyclic or polycyclic saturated or partially unsaturated
heterocyclic ring haying 1-
heteroatoms independently selected from nitrogen, oxygen, or sulfur, or an 8-
14 membered
bicyclic or polycyclic heteroaryl ring haying 1-5 heteroatoms independently
selected from
nitrogen, oxygen, or sulfur.
[0046] In certain embodiments, the compound of formula (I) has formula:
,
R' R7
)¨\ 2
CI N N¨L.-Rw CI N
0 0
R3 (I-a-1); R3 (I-a-2);
R7
( R7
CI 401 N/ CI N N¨L"-Rw
0 0
R3 (I-a-3); R3 (I-a-4);
R7 R7 R7
-;
CI 401 N N¨L--Rw CI 401 N N¨L4-R'' CI N NR'
0 0 0
R3 (I-a-5); R3 (I-a-6); R3
19
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
R7 CI r-Th
CI
N N-L4_Rw N N-L4_Rw
0 0
(I-a-7); R3 (I-b-1); R3 (I-b-2);
R7 r--L) R7 /-=-",r
N
CI CI N-L4_Rw N N-L4
-Fr
0 0
R3 (I-b-3); or R3 (I-b-4).
[0047] In certain embodiments, formula (I-a-1) is formula (I-a-7).
[0048] In certain embodiments, R3 is selected from:
CI CI
:=2?_
=%. =
[0049] In certain embodiments, R3 is selected from
CI
; and
[0050] In certain embodiments, R7 is an optionally substituted group
selected from
phenyl and a 5-6 membered heteroaryl ring having 1-4 heteroatoms independently
selected
from nitrogen. Exemplary optional substituents include, but are not limited
to, halogen, oxo
(=0), =S, cyano, nitro, fluoroallcyl, alkoxyfluoroallcyl, fluoroalkoxy, alkyl,
alkenyl, allcynyl,
haloalkyl, haloalkoxy, heteroalkyl, cycloalkyl, cycloalkenyl, aryl,
heteroaryl, heterocyclyl,
cycloalkylalkyl, cycloalkenylallcyl, heteroarylalkyl, arylalkyl,
heterocyclylalkyl, hydroxy,
hydroxy alkyl, alkoxy, alkylthio, alkoxy alkyl, alkylene, aryloxy, arylthio,
phenoxy,
benzyloxy, amino, alkylamino, dialkylamino, acylamino, aminoalkyl, arylamino,
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
diarylamino, sulfonylamino, sulfinylamino, sulfonyl, alkylsulfonyl,
arylsulfonyl,
aminosulfonyl, sulfinyl, -COOH, ketone, alkoxycarbonyl, aryloxycarbonyl,
amide,
carbamate, acyl, boronic acid, and boronic ester.
Rb Rb
N-N N s'N
[0051] In certain embodiments, R7 is ¨ ; ; or
; and Ra and
Rb are each independently hydrogen, hydroxy, alkyl, heteroalkyl, heterocyclyl,
or
heterocyclylalkyl, wherein said alkyl, heteroalkyl, heterocyclyl, and
heterocyclylalkyl are
independently substituted by 0, 1, 2, 3, 4, or 5 substituents independently
selected from the
group consisting of halogen, oxo (=0), =S, cyano, nitro, fluoroallcyl,
alkoxyfluoroalkyl,
fluoroalkoxy, alkyl, alkenyl, allcynyl, haloalkyl, haloalkoxy, heteroalkyl,
cycloallcyl,
cycloa1kenyl, aryl, heteroaryl, heterocyclyl, cycloalkylaBcyl,
cycloalkenyla1kyl,
heteroarylalkyl, arylalkyl, heterocyclylalkyl, hydroxy, hydroxyallcyl, alkoxy,
alkylthio,
a1koxyalkyl, alkylene, aryloxy, arylthio, phenoxy, benzyloxy, amino,
alkylamino,
dia1kylamino, acylamino, aminoalkyl, arylamino, diarylamino, sulfonylamino,
sulfinylamino,
sulfonyl, alkylsulfonyl, arylsulfonyl, aminosulfonyl, sulfinyl, -COOH, ketone,
alkoxycarbonyl, aryloxycarbonyl, amide, carbamate, acyl, boronic acid, and
boronic ester.
[0052] In certain embodiments, Ra and Rb are selected from:
I
N / HO 0
NJ; N 0 ). Z
) . N) U
J
--... ...- ,. .õ+,.... j
H CH OH C NN N
+ = 3 = + = O2H = = . . )
;V .
I r(I ( )
0 I H I N I --y.0
N
I N N jNON N cl-)
0
N
. . H.; . (4,,N). ci. )(N). _Cry
.--) N
) N
,J
. AL .. . ALJN
-ci_ =
--..Na. 01 I
N,..-----\ ---
NH 0 J ,-'.'"Lsj ..) o
1
-µ . A. . --1- ; I ; or I .
[0053] In certain embodiments, R7 is selected from:
21
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
1
1
Nr,...- N
I
N N LN----)
1
41f.JV I
......<1 ..........cekr...... \,,,e,sy.,..^" '''.19srl -.......-
N¨N\ . N_NH . N..õ:õ..-.N . y . L.,..õ. N-. =
0 ;
,
1
Nõ..r.-- N
1
LN-1
0; and 0
[0054] In certain embodiments, R7 is selected from:
1
1
N ...-N
-......-
I
1 N ,- N
-..õ-
I
------ Hi.N
N,
N¨N N,,:,,, N . I .
\ = N¨NH = ===== 0 ; and
,
1
N,y4.N
LN11
0=
[0055] In certain embodiments, R7 is selected from:
1
1 1 1
1
---- ''.. = rt....:"."¨
N¨N. . N_NH . N_,..4..,-;.N ; and
,
[0056] In certain embodiments, R7 is
\
N N /
; or .
[0057] In certain embodiments, L4-R' is an optionally substituted 8-10
membered
bicyclic saturated, partially unsaturated or aryl ring, an optionally
substituted 8-10 membered
bicyclic saturated or partially unsaturated heterocyclic ring having 1-5
heteroatoms
22
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
independently selected from nitrogen, oxygen, or sulfur, or an optionally
substituted 8-10
membered bicyclic heteroaryl ring having 1-5 heteroatoms independently
selected from
nitrogen, oxygen, or sulfur.
[0058] In other embodiments, L4-1r is an optionally substituted 8-10
membered bicyclic
saturated, partially unsaturated or aryl ring, an optionally substituted 8-10
membered bicyclic
saturated or partially unsaturated heterocyclic ring having 1-5 heteroatoms
independently
selected from nitrogen, oxygen, or sulfur, an optionally substituted 8-10
membered bicyclic
heteroaryl ring having 1-5 heteroatoms independently selected from nitrogen,
oxygen, or
sulfur, an optionally substituted 1,2,3,4-tetrahydropyrazino[1,2-alindole, or
an optionally
substituted 3,4-dihydro-1H41,4]oxazino[4,3-a]indole.
[0059] In certain embodiments, L4-R' is
Y
COOH ¨X
x,N1 HOOC 1\1¨y
X \ X \
\ COOH
N'
COOH ; ; Y ; =
,
i' COOH COOH
HOOC N HOOC HOOC Xjj
X
sX
N:
' .
Y ;
Y A
1
HOOC 0 N B
)
0 X---- N N
COOH = Z Z = =
Y Y
i 1 Y
N N 1
N
\ \ \
COOH
B N N
\ N
/ ss
N
N IN N
Z = Rh ; RI ; RJ =
Y
1
N
\ Y
i
N
N \ Nc_...)
\
NH2 N
Rk ; 0 ; B CONH2 or Z ;
23
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
wherein
A and B are each independently hydrogen, halogen, Ci-Ca-alkyl, Ci-Ca-
haloallcyl, Ci-
Ca-alkoxy, CI-Ca-haloalkoxy, halogen, -OR', -CH2ORd,
r-----Nrs<
or ReN., .
,
X is C(H), C(CI-Ca-alkyl), C(Ci-Ca-haloalkyl) or N;
R23
R2,o ,R21 R2..so ,R21 RF,o ..õõL
N N N 0
r=-=-j <.LO )c.,..,,,Ø.õ,.
Y is hydrogen, Ci-Ca-alkyl, r ; ¨I- ; -nr ; or
\j=?
\ / __________________ \ / .=,-ri __\ N-R21
N \
Z is Rf ; Rf . O-R ; or FR =
,
RD) and - lc21
are each independently hydrogen or C1-C4-alkyl, or R2 and R21 together
with the nitrogen to which they are attached can form an optionally
substituted 4-8 membered
heterocyclic ring;
I ro
A.---,,,,, ,,, A---,,,.....,õ,1
Re is CI-Ca-alkyl, C2-Ca-alkenyl, , or
r---.N-
Rd is Ci-C4-alkyl; Re is Ci-Ca-alkyl; Rf is C i-Ca-alkyl, C2-C4-alkenyl, or
cyano; Rg is
I ro
N N.õ,..)
Ci-Ca-alkyl; Rh is CI-Ca-alkyl; Ri is CI-Ca-alkyl, ''' A''''''
, or
rN-
; Rj is CI-Ca-alkyl or Ci-Ca-alkoxy; and Rk is Ci-Ca-alkyl.
[0060] In certain embodiments, L4-R" is
Y _X
1 Y COOH
1 X HOOC 1\1-y ,N
1 X,N
,X
\ COOH \
N
COOH = V ; =
24
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
\/ COOH COON
HOOC N HOOC HOOC X X
sX
i
; .
; , ; ;
COOH
Fr COOH RI' HOOC N
...., ,..,
.-- i N COON
r'
N Nr
...-''
. ; ; = .
; ;
Y Y Y
t i 1
H000 0 0,) 1 \/ \
N
N
.........r. ,
;COOH; COOH' COOH
Y A
I COOH
N B B
00H I\
N N N
X¨
COON = Z - Z Z =
; ; ;
A COOH
\ \ XLIICOOH \ COOH
N Y N C i-C4a I kyl N
Z = , Z = Z
; ;
Y Y
1 t
N N
C1-C4alkyl ¨0
/N
X¨
C i-Colkyl ¨0 N N
\ COOH o / 0
N 11 Iti
61-C4alky1 ; Rh . Rh ;
,
Y
1
N Y Y Y
\ 1
N 1
N t
N
/
,,,N
N.,>--Ril X¨ N¨N X¨ X-
1:1 N¨N N ========. \\
Ni ,,.,.) . NI
==,,, =
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
Y
Y N
Y N Y
i
NI
NI \
......,---,
\ , \ x_ \ N
NI¨N Ril NI ),..TP
NI¨KI N,,,,,õ N NI N \
1 /.0 '''''-= R
R'1 N'= . R N
il - 0k_ - Rn =
, ,
Y
Y 1
Y NI Y N
1
NI
\
N \
\ \
N
N
Nc.2)
\:Z. \
/ N
;
N,N//'¨' N-----1
= RL Rk =
, , ;
Y
1
N COOH
T1I\ B 0 B 0
\ C0NH2 \ \
NH2 N ; N N-0,20 N 0
0 ; Z '
; or
;
wherein
A and B are each independently hydrogen, halogen, Ci-C4-alkyl, CI-C4-
haloalkyl, C1-
C4-alkoxy, CI-C4-haloalkoxy, halogen, -0R5, -CH2OR5, CH2CH2OC1-C4alkyl,
¨NHC(0)Ri, ¨
Cy''''A
N(C1-C4alkyl)C(0)Ri, ¨NRcRc, CH2N(R5)2, CH2CH2N(Rc)2, COOH,
0
--4,,
,------N--", 1----N 1 ReNyJ
Re,N,I?
CT'''',.'
Re- N,,,,,i
Re,N,õ,J
0 0 Re
, ,
0
rN'A.
W'''A rNArsxr r---N---,A .----N---,.),
0 0,..,õ) 0,.i ") ,or Re,Nõ,,,)
;
Xis C(H), C(Ci-C4-alkyl), C(Ci-C4-haloalkyl) or N;
R23
Rzy ,Rzi Rzo ,Rzi Rz_o ....,,L
N 'N N 0
r) (AO r)
Y is hydrogen, CI-C4-alkyl, I ; = "1"' - ¨7
; . --k------ --
;
26
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
.x.
_ ,µ 4,
RY1
.sj K¨\1\I j' (-)
\ / \ ______________________ \ /
\ // = N __ = N = RY1 - \----' \O = R26-N ;
'D \(i¨N
)
..,
I+ ;or 1R20 .
RY1, at each occurrence, is independently a halogen;
(C1-C4alkyl)
,NR N1 ("
N
Z is Ci-Ca-alkyl Rf ; Rf =
, (C1-C4alkyl)
,
R2.9 ..R21
N-(C1-C4alkyl) N-R21 K-0
\_g; FR`:' -1-
r0
r-----0 ,cyN,,,J
õNõ...)
..õ.r.õ_õ..0,.._.,..--,0..C1-C4alkyl .
,
R20 and R2'
are each independently hydrogen or CI-Ca-alkyl, or R2 and R21 together
with the nitrogen to which they are attached can form an optionally
substituted 4-8 membered
heterocyclic ring;
Re, at each occurrence, is independently hydrogen, CI-Ca-alkyl, C2-C4-alkenyl,
1 r0
,0,.. ),------0,1-Colkyl A--..,,,,O...,..õ--N-0,Ci-Colkyl
,
\
jz,,,,, = \ __ (,¨ r,\,-
N.,..N.,,i .
N ________________ , or
I 'N.1_1 _NIS> r0
Rd R is Ci-C4-alkyl
--. ..-----õ,,
Re is CI-Ca-alkyl, -OCI-Ca-alkyl, ce. 0 Ci-
Colkyl , C(0)C1-C4alkyl, or
\,0 0
R1. where Rel is halogen;
27
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
0
rNH rN)C
Rf is hydrogen, Ci-C4-alkyl, C2-C4-alkenyl, cyano, cz- , or
'-V.
Rg is hydrogen or CI-C4-alkyl;
Rh is hydrogen or C1-C4-alkyl;
Ri is hydrogen, C1-C4-alkyl,
N-
õõ,0 /õ.0
0
N)
Q
Ci-Colkyl
C1-Colkyl
1¨<\ Ci-Colkyl 14=)¨/ OCi-Colkyl
, or N __ =
RH is hydrogen, CI-C4-alkyl, CH2-0¨C1-C4allcyl;
Ri is hydrogen, Ci-C4-alkyl or CI-C4-alkoxy;
Rk is Ci-C4-alkyl;
le is hydrogen or Ci-C4alkyl;
R1' is hydrogen or Ci-C4alkyl;
R is hydrogen or C1-C4alkyl; and
RP is hydrogen, Ci-C4alkyl, CH2OCI-C4alkyl, or 4a Y
100611 In
certain embodiments, the optionally substituted 4-8 membered heterocyclic ring
formed by R2 and R21 together with the nitrogen to which they are attached is
selected from
s /--`c
NH N-01-C4alkyl N-C1-C4alkyl
0
the group consisting of \ , \__/ \
/
,and
28
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
100621 In certain embodiments, the compound of formula (I) is selected from
the group
consisting of the compounds in Table 1, or a pharmaceutically acceptable salt
thereof
Table I. Exemplary compounds.
O 0 HN \
".--...,..._A N-N N-N
N-N N. \ 0 \ N, \ 0
"'"'
..., = OH r-'1 OH
h \ CI N N CI N N
CI N N Br CI
I 0 I
/ 0 0
N N
0 0
0
4 40 di
CI I-1 CI 1-2 a 1-3
\ N-N \ \ N-N
N-N
"N. \ 0 '',. µ 0 N.. µ ..,
rTh OH r",µ OH r....õ) N \ =N
CI N N * CI N N * CI N N #
0 0 0
NN
WIN
/
O 0 0
4 4 ill
O 1-4 0 1-5 0 1-6
\ N-N 0 0
\ N-N HO--.f \ N-N H0_,,,,..õ)._
1 0
N.
OH 1---1 / \ N'-'N 0
CI N N # CI CI N N
/ N
0 / / N -II 0 0
/
O 0 0
4 411 4
CI 1-7 ci 1-8 0 1-9
0 \ \N-N
\ N-N H0.1...... N-N
1 0 \ µ 0
OH r...) OH
CI N N N N'---N L.,,,NH a N N * CI N N 40
, , , ,
0
0 \N
0 0 (le 0 kNTh
4 41
4 0
0 1-10 ci I-11 a 1-12
\ \
N-N N-N N-N
0 .,,, 14,44
OH CI r",1 OH
01 N \ \ ,
r-----, N_NH
N N # N N #
0 0 I 0
N-N
C_Nr-'0
0 0 0
.......i
4 * *
a 1-13 0 1-14 0 1-15
29
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
=
=N-N H$0.17 N-N
.--
CI N \
\ rTh OH
N-N N. N
, nN r-----, _ fl NP-1 _,,,0
HO CI
CI N N N µ-.../- A N N *
=
0 0 0 N. /
0 /
0 0
0 0 0
* *
4110
CI 1-16 a 1-17
a I-18
/ o / 0
N-N r_..v OH N-N r.) OH
/ /
, OH N-N r) $j)
CI N N 0 CI N N .
0 \ i
/ 0
O 0
0
4 111 4
01 1-19 1-20 a 1-21
i
N-N N-N re..... N-N )0
\ /
OH /
rTh
CI N N Cl / N N . 0 CI / r
N-CNH
/
0 0
0 --
O OH 0 0
0
4 4 41
a 1-22 a 1-23 CI 1-24 .
\N-N N-N/ 0 \ HC/17._,
OH N-N
r-Th OH *k---\ = .,() - Nflu 9
CI 0
N N * CI N N CI N N...../N)--' \--
/.71.....
/ 0 / / i 0
0 0 0
0
0
0 0
4 * 411
a 1-27
a 1-25 a 1-26
N-N
\N-N
N. \ N, rTh N.N
r---) _q.,, CI # /
0
/ 1 0
O 0
0 HO
HO 0 0
0
* ill *
a 1-28 a 1-29 a 1-30
o C \N-N / 0 ) N-N OH
N-N rTh N-s
N. t LI CI N N CI N
rTh N
a N
/ 0 0
HO'
HO
0 0
0
di 4 *
0 1-31 ci 1-32 a 1-33
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
O 'N-N H01,..\)_. N-N
CI /
t.- CI
CI N N Nit OH N N =
/ CI /
0 0 0
0
HO
0 0 0
*
* *
0 1-34
0 1-35 0 1-36
1 N-N 0 \ N-N
\N-N
CI H:,.....)._
, \
rTh H r-Th _ Nr-1 P rTh N,N
CI N N N V-.../N-<T CI N N N
N,,IN-IN
/
4113 /
0
0 OH 0 0
O HO
O 0 0
* * *
CI 1-38
0 1-37 0 1-39
\ N-N
N-N / 0 /
N-N r)
OH /
/
rTh N--\, rTh .p...1-N\
CI N N 0 \ CI N N = N/ CI
N N = PI
-
/
O 0 0
O OH
HO 0
0 0 0
* * #
CI 1-40 CI 1-41 0 1-42
O \ N-N \
"N-N HO--f N-N
N
N,
CI N N * iN CI
N N *
0 0 0
0 0
HO HO
0 0 0
* 0 0
CI 1-43 0 1-44 0 1-45
\ N-N /
\ F10 1_3_1 N-N
/-
/
N \ N µ / 7
/ \
N1, P i----\ ,
0 N N * N-N
N CI\--,-"Sc CI N N
/
0 o 0 0
HO4
0 0 HO
O 0
* *
*
0 1-47
0 1-46 0 1-48
/
\N-N
N-N (-0 /
N-N
/ N"-
N / 7/ / 7 0
N. (No )---\ ,
-N'5"
N N
CI N , m.N...-N,) CI N N CI N OH
/ 0 . / 0 * 0 / 0
O HO
O 0 0
HO
* * *
CI 1-49 0 1-50 0 1-51
31
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
/
N-N/ HO 1 OH
N-N /
0 / ---N "s"" 0 N-N ,N
111 \ / 0
N
i----= I'M., 1--- \N 4I
CI N N OH CI ". N * 1 CI
N. N
/
0 0 i 0
O 0 0
4 ill 4
CI 1-52 a 1-53 a 1-54
= /
N-11
HO HO
N-N N-N
1 / 0 0
/ ..õ,
s., f.........õµ ,...N \ / :
CI N N *
0 CI NiTh = 1
CI
NN
fk-
/
o 0
OH
1µ) 0
N
0 0 r .) ,
ii
4 4 L-0-
a 1-57
a 1-55 a 1-56
HN -N ,N OH
N-N/
HO \
1 N-N
',.
/1 0 N ' 0
i ^ \
1-Th ---o
ci N = ci N N * 0
/
0
0 0 0
N
0
0 0
4 4 111
a 1-58 ci 1-59 a 1-60
\ N-N (PH N-N / OH
\N-N
=. µ / ,, ,.
(..,µ0
N N CI
N N
/ 0
0 0
0 0
HO HO
O 0 0
411 401 411
a 1-61 a 1-62 a 1-63
\ N-N F
N. µ \-N
rNN-N
N-N N
N. \
CI N N * 0 rTh
i CI
N N
O I
/
0-N 0 0
O HO HO 0
0 0
= 4 di
a 1-64 a 1-65 a 1-66
\ Ci N-N l'OH / \ OH
N-N OH
N-N
--"-N '''' 0
o rTh CI 0 0
N N CI N N r \I II
/ / \
N
0
HO
O 0 0
4 4 4
a 1-67 a 1-68 a 1-69
32
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
N-NH \ N-NH N HN-N
/ N / ." '''. k
I
i"---\ /----\õj .'.- N-
CI N N CI N - CI N N *
0
HO HO
0
HO
0 0 0
* * 0
a 1-70 a 1-71 a 1-72
\N-N \
N-N N-N/
/ ,,, 4 OH
i---, rTh 0 i---\ o
a N 11 0 CI N N OH CI N N \ N
/
LJL
0 HN / / N 0 /
0 0
OH
OH
O 0 0
* * *
O 1-73 a 1-74 a 1-75
0 OH OH
i,
H01.1O , ..,70
N-N/
xi kt4
% ,..:11? 1
CI N N \ N CI N N \ N
/ 0 0 0
0 0 0
* * *
ci 1-76 a 1-77 a 1-78
N-14/ \N-N HO 0 \N-N
0
OH
N
CI 11---\N . \ a CI N N
/
/
N /
0 I 0 \ 0 0
HO
O 0 0
* * *
a 1-79 a 1-80 a 1-81
\N-N ====,
0 N-1\1/ N-N/ N _..-
4 OH / ...., 7 /
CI N -N-
0 im\N .
CI Ni *
/
O 0 0
HO
HO 0
O 0 0
* * *
CI 1-82 a 1-83 a 1-84
NN-N N-N/
(
`.. N NN
rm ci
= I
N NH CI N N * ''!si ci N1---N N
/ N /
O \
HO
0 OH
O 0 0
* * *
a 1-85 cl 1-86 a 1-87
33
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
HO \ N-N OH O___\. /
N-N/ 0 N-N _ \ r---
CI N \ N,
, \-N .
N CI Ni----\N *
N ili..,, CI
HO
0 \-iN-- =µ,)
O 0 0
0 0 *
CI 1-88 ci 1-89 a 1-90
\N-N N- NH µ
/ N.. N
I
rTh I
N ' \
(Th /¨ \õ,
CI
CI N N * //µ1 N " *
0 0 HO
0
O 0
HO HO
0 0
O * *
a 1-91 a 1-92 a 1-93
CI-N) HN-N
i
N-N r'0
/ N''''N ...-N,õ)
N-N/ CI rTh,
N N * iN
CI h\ *
N N
CI N N 0 / 0 0
0 /
HO HO
0 0
0
* *
a 1-94 ci 1-95 a 1-96
N-N/ I / \N-N N-N (
N. N CO21-I
\... \ F N
N
CI h \ *
N N CI
).-I ---\
N N */
= h \ = I
CI N N
0 0
HO HO
0 0
0
* * *
a 1-97 a 1-98 a 1-99
N-Ni N-N/
H N-N/
0
/ NH
CI h\ =
N N CI
N N I
CI /I"----\
N N fi OH
/ 0 / 0 /
0 0 0
HO HO
O 0 0
O * *
CI 1-100 a 1-101 a 1-102
OH HO HO
N-N/ 0
N-N/ 0
õ,
411 7-:., F N, \
CI N - CI N N \ N.,,--
/ 0 / 0 / 0
O 0 0
di di di
a 1-103 a 1-104 a I-105
34
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
N-N/ HO \N-N OH OH
1) N-NH
-N '` 0
i---= rTh 0 IV_
CI N N \ N CI N ilt
=,,, N N . / CINflN
0
HO
0 0 0
* ili 4
CI 1-106 a 1-107 a 1-108
HO
N-N/ 0
N-N/
1
0 /
N OH N/ \
N-NH
I¨ \.1 ..."
1 ..-= i * I
i¨\ , c i NN = CI N-4N 1 0
CI ,,, N - N N,
/ /
/ 0 0 OH
0
0 0
0
4 4 4
a 1-109 a 1-110 CI I-1.1.1.
0 OH 0
N-N/
/ NH/
/
/ F N-N ......31 / ..,, -_, OH
* N''..
/ /
0 / 0
0 OH 0
0 0 0
4 ill lit
a 1-112 ci 1-113 CI 1-114 ,
N-N/ 0 r 0
N-N/ HO
N-N/
0-N ,N / ,...,
i---= l* CI N / ,,, ,
INIµ / OH
N N
CI i---\ N lir CI N N
/
0 0
0 0 0
4 4 4
CI 1-115 a 1-116 a 1-117
o
N-N/ N N ' N N"'''' N
I I
...,
7"-N= ' OH .-." ,..
OH
r-Th N \ r---)
a )----\ =
N N CI N N . 0 CI N NH
/ 0 0
0
0 0 0
4 di 4
a 1-118 a 1-119 a 1-120
N-N/ \N-N \N-N
1
Nkm\N * I OH
CI CI
CI
0 / /
/ 0
0 0
0 0
HO HO
0 0 0
4 4 4
a 1-121 a 1-122 a 1-123
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
\N-N ..) N-N/ HO / HO
N _...
/ IV N-N N _.-=
/ st,1
i
" I 0
CI
\ . a N
i---\N .
CI N N * N N
/
0 0 0
0
HO
O 0 0
4 * *
CI 1-124 ci 1-125 a I-126
141 HO
N- no 0
,NN_ H01...1
I, 0
'.1 o N-NH
/ ...= 7,
01 Nt--N . N-N
OH / 0 ci NiTh 1, ,
/ 0
/ 0
0 0
o
4
/?-7'."- 4
CI 1-127 ci 1-128 ci 1-129
---,..
N ' N
N-N/
-1/ N-N/ HO 1
I N.
,, I 14,N / r 7 0 \ /
reTh N 1
01 N N * / CI r-N.,
N - * CI .1--\ *
N N
/
0 / 0
HO 0 HO
O 0 0
0 4 4
CI 1430 a 1-131 a 1-132 .
N-14/ HO 1 HO. /
N-N
N'N N-N/ /
Ci CI
N N
-"\ CI
N N--\ OH
Ni-NS N
/ 0 / 0 0
/ 0
0 0 0
111 4 411
a 1-133 a 1-134 a 1-135
o µ /
OH , q Z-
N-N
.." . /
N-N
rm
a - 0
/ HO
o / 0
0
O o
41 *
ci 1-136 1-137 a 1-138
N-ii 0
N-11 I
NN 0
OH
CI N NH CI N N *
/ / /
0 0 0
0 0 0
* 4 *
a 1-139 a 1-140 a 1-141
36
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
i
/ 0 0
N-N N-N Z
OH -OH
CI
N-N
N N . OH N ' \
/ / N CI
0
0 0
o
4 4 *
CI 1-142 a 1-143 ci 1-144
HO \N ....,,,
N ' N
N-N/ I
4 HO
rTh N
CI
0 CI 1N \ N,... ' .., /
Fe\N N \ ? N, 0
0 OH
o
0
0
o
*
. 4
ci 1-145 ci 1-146 a 1-147
--, .--,
N ' N HN-N N " N
I OH
rTh \
NrMN OH
,
0 CI
/ 0 0
0
0 0
0
4 iill 4
CI 1-148 a 1-150 a 1-151
---, HO
N ' N N ' N
I N''' N
.., \N I 0 I
...- --- rm 0/Th
--,
I = * 0
N
CI *
N N CI ,,, - \ Nõ CI N N *
/
0 0
HO 0
HO
0
0 0
* 4 le
CI 1-152 ci 1-153 a 1-154
...,
N ' N 0 OH
N-N/
I NN.., I I
rTh N iTh
0 N N * ;N CI N N 0 a o
/ / \
0 0 \ 0
0
HO
0 0 0
4 0 11
CI 1-155 ci 1-156 a 1-157
0 OH 0
H01.1
N-N/ HO
N-N/
-N -" 0 N-NH
*
N
h\ õ N - riTh,,
CI CI N - \ N. N- \--.
CI1.õ.õ"6.õ
/
/ 0 /
0 0
0
0 0
411 4 ill
a 1-158 a 1-159 a 1-160
37
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
µ OH 0
H017,µ
N-N/
N.'s
NN
/ -- 0
q HO
N-N CI N ,
/
4 :-H;s:
i"--\ / i---µõ, N -,
N \ N, 0 CI N m / 0
0 0
0
4 4
a 1-161 a 1-162 a 1-163
/
HO-5.% N-N Holi?
/ 4 OH
N-N
,,, N. a1 .. /
N-14/ / 7
:-....,,, .-\
CI N N 0
N /¨ N \ N,.,...,
/
I
/ 0
O /
O 0 0
* 4 4
a 1-164 a 1-165 a 1-166
/ NN OH HO._..::)
N-N
/
/
N-N
..,-- , ¨N '" 0
/¨\-
CI N N \ / Ci N)¨\N lit CI )---\ ¨
N N \ iN
/ 0
0 HO /0 / 0
o 0 0
4 * 4
a 1-167 a 1-168 a 1-169 .
/ o
N-N
N-N/ HO / HO
N-N
-b.
/ CI N N \ /N
0 /
0 OH 0 0
O 0 0
4 4 4
a 1-170 a 1-171 a 1-172
/ HO / / HO
N-N N-N
N-N
./ IC--.^^.
/ / F 0
N
CI i---\ 4
N N CI /--\ N \N = OH
N N \ N
/
0 0 0
O 0 0
4 4 4
a 1-173 a 1-174 a 1-175
...-, N 0
NN H ' N OH HO___.
7?
I '' N-N/
/ I
rTh 0 ...-
--1,1
CI N N "N
/ \
N a 1---\
N \ N
.,, CI N/- N
9 \I /
0
0 0
4 * *
a 1-176 el 1-177 a 1-178
38
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
HO / / HO
N-N/
N-N
N-N
0
,
/ i 0 4
CI N \
= 0 0
0
0 0
* *
*
CI I-180
ci 1-179 a 1-181
N-N/ HO OH HO
N-N/ 0
N-N/
/ \ N
i---\
/ CI N N
0 / 0
/ 0
0 0 0
di di *
c, 1-182 a 1-183 ci 1-184
/ HO HO
N-N
OH 0 0
N-11
a 1-Th 41
N N a l----\ .
N N
/ 0 / /
0 0
0
0 0
* 4 *
CI 1-185 ci 1-186 ci 1-187 .
N-N/
N-N/
N-N/
OH /
/ 0 OH
00
0 OH
0 0 0
111 411 4
a 1-188 a 1-189 cl 1-190
N-N/ HO HO 0 F3C
N-N/
N-N/
''
/
Ni¨NN \ N
CI i N'r"-NN lit 0 CI
/ /
0 / 0
0
0 0 0
4 4 di
a 1-191 a 1-192 0 1-193
HO /
N-N/ HO
N-N
N-N/ 0
a N -
= 0
0 0
0
* * *
CI 1-194 a 1-195 a 1-196
39
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
Ho),0 7;
N-N/ CF.
4
N-N/
/N N-N/ -.1", / N--t1)
/
F-Nõ i----\ CI N)- N \ N
CI N - CI N N
/ 0
0
0 0
* * 4
CI 1-197 a 1-198 a 1-199
FaC HO <,D
N-N/ CF.
N-N/
*
CI Ni-NN \ N iThõ CI N= /---\ N \N
a N -
O 0 0
4 4 0
a 1-200 a 1-201 I 1-202
.
N-N/ --*
N-N 0
0,1..\. \.- / 0
/ . 0
N-N/
/ 7 '''
CI i.--
N \ ip
N i ,=== 5
/----\' CI = 1---\ e
N N
/ 0 /
/ 0 0
O 0 0
0 4 ill
a 1-203 ci 1-204 a 1-205
N-N/ ON Cf.7...:
N-N/ NC
* N-N 1
/
,-- 5
/---\
CI N N \ N ra\. CI Nh\N N \
CI N N-dv,
/ 0 /
0 0
0 0 0
4 * 4
a 1-206 a 1-207 a 1-208
N-N/ N-N/
110_,05 FaC
NN
* ON
/-----\ õ,
= /-----\ CI N N \N .1----\õ, CI
N N
a N -
/ 0 / 0
/ 0 0
HO
0 0 0
4 4 411
CI 1-209 ci 1-210 0 1-211
m 1 H01,1 N'''''14
N-N/ 1 OH
0 iii4 --N ''' 0
,.,
/ rTh
CI N N CI N.r--\N .
/ 0 0
0
O 0 0
* * *
CI 1-212 a 1-213 CI 1-214
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
NI
k OH h .
0 i r= /1
)----\
CI N . CINH CI N NH
/
/ / 0
0 0
0 0 0
* * *
CI 1-215 ci 1-216 ci 1-217
HO HO 0
N'''''' N 0 HO
L.
N''= N Isl''N'''' =
I I
.., 0 ...." "... I
...'"
/õ....Th N \
.,
CI CI N N
CI N't-NN lit N N *
/ / \ / N
/
0 0 0
0 0
9
4 * *
CI 1-218 0 1-219 0 1-220
0 ,N..N,
H2N--;_i
/ 02N
N-N
N-N/ 0
CI Ni-N \ N,
/ /
14;'"...\ # '0 µ
, N \ 0
0 0 N\
0 0 0
* # *
ci 1-221 0 1-222 CI 1-223
NI '''''N kr, 0
I OH
....- / \ ,..." Ni \ N-N/ c,i)
N
=%. 0 ",õ 0 / ...,
)----\
CI N N OH CI N N
CI r-NN
o o
/ o
O o o
4 4 ill
0 1-224 0 1-225 0 1-226
H
NI
N'N O 0 0
I C ) --"(1
0,
, N'' \ N /N
I1/21)'^ N HO
"1
CI N N
/ 0
ci
/ \
0
0
0 0
4111
. 'Os--
CI 1-227 a 1-228 " 0 1-229
0 HOAI
HO
H017.1
, N-N/ /
r, N.%. , N/ \ ci N-N
tThN r- \
CI Ni--\
/ 0 0 C
N
CI
/ I /
0 CI
LI
1,
TMS
0 0 0
4 * *
CI 1-230 0 1-231 ci 1-232
41
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
HOg
N-N/ Hni-N.
N-N/
t-
N-N/
Th
i CI N r-s= N \ N
0
0 0
0
4 4 4
a 1-233 cl 1-234 01 1-235
11 N-N/ F40.1
NN
4 N"'"---N
---N '''=
I
% 1,4 / ....., 7
..--
N-N
t----\ --..
i ....= F 4 \ CI N N \ 0 ../.--\
).---s, CI N N
ci N N, / 0 /
/ 0 0
0 0 0
4 4 4
CI 1-236 a 1-237 a 1-238
O o
Hol,..1 ifib, FI0_,
/ HO
N-N/
N-N/ 711111
N-N i \ 0/ / ....., 7 N '''"
.1-"-\ k---= h\,,,
CI N N CI N N \ N CI N .
0 0 0
'CIO
0 / 0I 0
4 4 4
a 1-239 a 1-240
oci 1-241
7 0...\ ..ii? HO
N-N1 N-N/
HN N-N
/ ....., 7 ..."'
' .., 7 4 CN
CI N n,6; . CI N N N CI N N \ N,
/ 0 0 0õ / 0
I..
TNIS
0 0 0
4 4 4
a 1-242 a 1-243 a 1-244
/ (.........kiiN (3_..>:)
N-N/ -- i H0D
N-N / ...., 7
4
/ ....., , N ...."
''' i------\
. CI N N \ 0 i----\õ,
r"\N CI
0
0 0
0
0 0
4 411 4
a 1-245 a 1-246 a 1-247
N- / HO 0 /
N-N/
N-N/ \ N-N N I / -..,
/ ,õ-
".` '''
N) \N / -
Me
CI N õ, - CI N N / 0 * Me
/ 0 / 0
co2H
o
o 0
1111 4 1111P
el 1-248 ci 1-249 a 1-250
42
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
HN--N ..--..
N 'N / OH
ftl.:N I -, HO N-N
N-N/
0
CI Nr \N . CI
/ 0
0
/ 0
0 0 0
4 4 4
a 1-251 el 1-252 a 1-253
ill ilo i \s )::1 N ....",
' N N-N . 1-10;:g
\
N-N/ I ..., HO
, 0 ¨ \ ,,_. ,
\-N ,.. 0 7. N -
/¨\
CI
N - / 0 /
O 0 0
* * *
a 1-254 ci 1-255 0 1-256
/ HOA)
.--NH N¨N
N-N/
N-N/ 0 411 ,N, r,,JH
1---\ N.N o
CI N N \ N, im \
a N= I---- \14 \ N, CI N
N
/ 0 0
O 0 0
4 di 4
a 1-257 a 1-258 ci 1-259
0
N-N/
'.1 HOl
eli?
N'--- HO 4103
N-N
FN
i CI N \ N, 0 N N-c111,
0
0 0
4
/C)=-?---
CI 1-260 a 1-261 a /0---
1-262
0 HO C-7
0
S Os-
HOIC1 N-N "N-N Ni
CI
N-N
' / 7
N(-Th.N =¨/
¨NN---:?9
/ 0
/ 0
4
('¨ej
CI 1-263 a 1-264 1-265
11.2Na.:05 \ HO
,NH
/ / Oa N-N/ 0
N-N \N-N
CI 61--
* 1= ¨"A rr --\,,,, N. N CI
N n, CI - N -
I
O 0
0
4 41> 4
CI 1-266 ci 1-267 ci 1-268
43
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
,_, .. 0 / H2N 0
N NN "" r-^N R 14
I
--N N '''=
---,
r-s
CI N N
N-N CI N N
/ 0 / 0 / 0
0 0 0
4 4 4
a 1-269 a 1-270 a 1-271
O OH N'''''"N N/
NN I HO
I Ni
..-- co,
, --N ""^ ='='. 0 4 \N-N
...,
"tm\N ,
1-- \ CI ,.. E
CI ,,, N .= N - N,Nõ
a
0 0
O 0
* *
k
CI 1-272 ci 1-273 1-274
0 HA
HO rN .....? ( 4..õ.Ø...1
N
N-N/ 0
N-N/
N / ,
4
r--\,,, 1---= CI 1---\,, N \N
,...
0 0 0
O 0
/ 0
4 4 ill
CI 1-275 ci 1-276 a 1-277
c3L,
gN
NN
N,"=,^
0 I /
0.9
\ N-N N-N
% ...)::;) CI N N ' ,===
GI N .= '-N.,..
0 / 0
0
0
4
.5)jh
''-ill-C1 1-278 ci 1-279 a 1-280
c?
N-
N 1-1,N1?
N-N/ 24,1_,..?, .. / .. HN-,..5%
CI
1 , E N-N
N-N/
rm\N \ N CI tm\
N N \ N, CI ias\
, N ,, ---
0 0
.5)-4--- /1?--
a 1-281 a 1-282 a 1-283
Nal Q \ 0
HNT5....1
N-N
-- HN--__\) \ / HN-57 /
1
s
N-N/ ' , E
N-N
-...
..---Ni, CI N N- \1,.. / 0
/ 0 / 0
0 0 0
CI 1-284
a 1-285 *
a 1-286
44
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
N-N/ \ 0
" HN 0
* N-Ni /7:2
/
N-ry t----" \,,
01 N \N
CI N N \ N, / 0 / 0
/ 0
0 0
0
* 4
CI 1-287 a 1-288 a 1-289
o q HO
N-NI/ ON
/ 0
MI N-N
?
' r i N-N/
411
.z.
N " = Nõ 0 0
0 o 0
4
/St"?-- ill
ci 1-290 a 1-291 a 1-292
HO
0 / CZC:
N-N/ 0
N-N IN
N
-1,
HO = :
,X
/
0 Ci N N 0
N 1
/
0 0
0
4 111
/1)--
CI 1-293 a 1-294 a 1-295
NN q....N0 OH , ' HOI 0 HO 0
1 N j' Smic__
...-- 8/ N- N-N/-
'
:
N " / 0 0 0
0 0 0
4 * 4
a 1-296 a 1-297 ei 1-298
: Ho 0 NC
N-N/
N-N/ NC
,=:,
N N
CI /
'rThN * 0 CI
0 0
O 0 0
ip di 4
. 1-299 a1-300 a 1-301
NN (Zo
OH t 76 õNH 0
t'N N-14/
CI N . 0
N \ CI 1-- \
N N CI .= 1---\,,,
N .,
/
0 / 0 OH /
0
O 0 0
411 *
4
a 1-302 a 1-303 a 1-304
84451174
. õ.
P HN-N
N
/ ' NP". =
0 N-N/ ......&
N-N
CI ,,,iii.,
II / = =
,
/ 0
0
=
--?---
a 1-305 1 ? - --a 1-306 a 1-307
0
._ % HO =
N-
= S H.
0 / ..=
0 N-N '.õ ...,
N-N
N
CI N
,---\N \ N ., , ,
I =
/ 0
,. = 0
= *
a 1-308 GI 1-309 et 1-310
,....N Ho 0 HO....... --- HO =
N / ck..=
N-N
J.?... \
a N - a N N CI N N \
/
0 0
=
/4=^.-- 0
a 1-311 ai 1-312 CI 1-313
q."\4=NHO 0 N.;`,0 HO 0 HO 0
NN Fej\......14*.....A.õ N-N1
---N
CI NiTh4 a NrThll a i---\ / =
N N
0
4
CE 1-314 a 1-315 cr 1-316
HO (' HO 0
/ N-- /
N-N N-N
/ 7; / le
/ , / / F
0
/
0 0
/ a
N
1
0 0
4 ------- \--- *
a 1-317 a 1-318 ci 1-319
N 0 OH \ 0 OH
'Y 14 "),I / , -,
/ N-N. N
'' N-N \ õ.N
--N ...--
CIH /-\,,, It OH NrM4 ft CI N - CI
0 0
4 4
a 1-321 0 1-322
1-320
46
Date Regue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
Ho 0
(Q..7 H Ho 0
N-N/ /
N-N N-N/
''''
CI
1¨\õ, 0 F.\
ON¨ N N CI N11¨\N = ':;'
N ,,,
i
0 0
0
* * *
a 1-323 a 1-324 a 1-325
. .
Cc OH OA
N-N N-N/
/
HO 0 H
N ''= / N'''' N-N/
/ 7 1 ..==== i ' ...=== .7..
/---= i--- \ 4
CI N N CI N N
/ / / 0
0 (3 0
0
<>
0
0 0
0 .
4 4 4
a 1-326 a 1-327 ci 1-328
/
rii o OH I 1=II::0
NA.7 OH
N-N/
N-N
N-N/
N -
N
/-----s,
CI N N CI CI N N
N -
/ 0
0
0 N
4 0 4'
ci 1-329 a 1-330 a 1-331
O NH2 o OH HOA
N-N/
N-N/
N-N/
*--N --14 ..`"= / NI---
CI N . CI N N CI N IN
0 0
b 0
4 4 4
c, 1-332 a 1-333 a 1-334
. .
HN OH 0
OH N.---.4
N /
N-N/ '
N-N
H.1...,^-
4 H
N-N
ci IN/ CI
i
r---,
N =
/ o
O o
0
4 4
a 1-335 a 1-336 a 1-337
0 0 OH 0
N-N/ OH N-Nlf OH
*
0 0
61
I I
0
0 0
* 4 4
CI 1-338 a 1-339 a 1-340
47
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
..1)...
N-N/
:... 0
N-N/ ----N ..""
/ 7 N-N// 7 7
*
CI /----=
N N \ N
CI
/ 0 /
0
0 0 0
AP 411 di
c, 1-341 a 1-342 a 1-343
HO 0 0 OH N.7\
4,.... 0
/ N---
N-N/
N-I1 -N "." N-N/
-
/ / 7 / / ,..., .õ.
CI i-,., N it 0
N - CI Nis-sN IIP CI .
r--\
O 0 b 0
0
4 4 4
0, 1-344 a 1-345 ci 1-346
HO
..4P 0
tTh0 õ,..
HO
N-N/
N-N/ N-61/
õ,ts. ,.. ,,, F. iõ ...,. s
i_..... ,
...,-. .N...... CI Ni--"N
/ 0
0
0 0
4 di
a 1-347 01 1_348 ci 1-349
0 OH H7r;
N--
N-N/
N-N/
N.--\.C...,
.)----\õ,
CI Ni-N 0\
Ci N -
O 0 0
4'/?::?...--
0 1-350 a 1-351 a 1-352
7c; N H2N....g) HO-?
N-N/ / O,, / F
/ N'Th N-N
`--N "`. N-N
/ 7 ...
Ni----NN
a a el N .= N N,
0 0 0
O 0 0
4 di di
a 1-353 a 1-354 ci 1-355
0 OH NH2 N=N
N-N/ . 'N-
7
N-N"
N-N/
/ ...õ. ,.:
CI Ni---'N CI N N
/ 0
0
0 0
4 41 4
a 1-356 a 1-357 a 1-358
48
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
11 o
/ $:---.7 cm \io_c) o_.,A,Ni
.-
N-N N-N1 HO
N "-.. N-N \--N '''`
CI Nim'N a Ni¨'N CI k----=,,,
0
0 0
0
4 ),... 4
a 1-359 --?--ci 1-360 N-ri \o_.\:N C:I H 1-361
/
N-N
4.14 N /
i----\ 19-,-N N-N
411 / N
irTh9 CI NF'N
/ 0
/ 0
0
0
4 0
4
a 1-362 ilhci 1-363 a 1-364
HO 0
/ HO-f...e,
, H2N,Ii 0
N-N N-N N-N'' / Isr"\,..Ni
/¨= %---= h \
ci N NI-- =.".''' µN,õ CI N N \ N, CI N N
0 0 0
4 4 *
CI 1-365 a 1-366 a 1-367
a HOD I
HO / NN
/ N-N
/ N ,, -",Nr) / 1 gi
N-N N-N
/--=.2
CI Ni¨sN 0 CI N -
0 0
41 411 4
0 1-368 a 1-369 , a 1-370
,
.
0
/ ON OH ,
/
, N-N
µN--4,_ õ ., . ,N..õ
..-I, HO 0
/
N-N N `,. a N f"--\
N \ N /14-N N "-NI
-, ---
CI N N 0 CI N N \
/ 0 / 0 N=
0
0
111 0
* 4
OI 1-372
a 1-371 ci 1-373
OH
/ 0 54H2Na 0
NH2
/
N-N N "--. N-N NN
I
./
/¨\
a ,2 N .. a N N = / CI
N
/ /
0 0 0
0 0 0
4 4 *
a 1-374 a 1-375 a 1-376
49
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
0 NH2 "0
NH2
Nqo OH NN
I CZN 0
tr/I:.1/
/
...." T.
raN., 7-..i..! .,. N ,
r--- \
CI N .. CI N N
/am\
/
0 0
/ 0
O LQ
*
.?-7{).--- *
a 1-377 a 1-378 a 1-379
0 H 0/
/ 00C
4N N-N
0 OH
N-N N '",. N-N i \ O_gl
/
_ N "---
i--= im\
CI N N CI N N CI N N
O 0 0
4 *
.5)::?---
a 1-380 a 1-381 0 1-382
7 o....3H), o NH2
/ \--/N C:.*12.
N-N
N-N
/
...= , r
/--\... CI N
CI N .= r-N,¶ ...
O 0 0
* * *
a 1-383 a 1-384 a 1-385
_
/ \ /NO OH
N-N( N-N
NI?
/ ..... S'N .."'s N N-N"
/ ...., .7:
0 N N CI N N CI /"Thm
0 0
0
0 0
411
ill 4
0 1-386 0 1-388
a 1-387
...._
IV Cli HO ---$71
N-N/
N 'N N-N/
' ..., . I ..., ..: / ...... z.
:
i-----\,,,
CI N N \ N CI .N, CI Ni-aNN \ N
O 0 0
4 4 4
a 1-389 ci 1-390 a 1-391
-......r .2N,0 rs
....\i,
0 14--.
N-NS ol.INH2
N-N/ N-N/
/---\
C.I Ni¨NN \ N CI N hi-el:
0 Ni¨\N-erV,,,, ,
O 0 0
0 1-392 4
a 1-393 AP
cr 1-394
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
H HO_o
NN
..), O1?
NN
'
:
CI N :1(131- a N N iTh4
- N, Ci N .. , iThu , N,
/ 0 / ,,,
/ 0
0 0 0
CI 1-395 ci 1-396 a 1-397
0-)1 HO 0 0 OH
1õ NN
NI HO -.f.1 I N-NH
..,
le'^N
I CI
..... 7 N N = i----\
0 / 0
0
0 0
0
4 4 4
ci 1-398 a 1-399 ci 1-400
0 OH H2N ...mØ1,
/
N-N/ 0
N-N
4 N-le
i--\ "---\,õ
14 CI N N CI N i .= \ ,1
/
L.,-.// N 1
c.....0 0
0 0 0
4 4 ill
CI 1-401 ci 1-402 o 1-403
o o HO HO HO -'0 /
N-N1 /
N-N
N-N
/
a N N \ N ^,... ,...
/ / CI N - \ N
0
/
0
O o
"C? 1-404 * 1-405 o
\ 1-406
o o o
HO-17,1 HO H01/1
N-N/
N-N/ N-N/
CI N .', \ N a N .= \ N,
.... CI N t"\-õ, \ N
/ ====, /
O 0
0
0
ci 1-407 ö 1-408 a 1-409
. . .
o o NH2 0 NH2
H01,1
/
N-N N-N/
N-N/
N --"^
01 CI
i---= * 0
CI d N - \ N CI N - N N \
....
/ / /
0 0 0
O 0 0
0 ill 4
F 1-410 a 1-411 a 1-412
51
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
ciHN,õNI,N
,e, N"'"'
N-N N-N/ (R, .._.0
NH2
/
0I8,1 N
/
N-N
1 ,,, /-\ N ci N N
1---= CI N - \ /
CI N N'"I /
/ 00 0
0
0
a 1-413 illi *
ci 1-414 ci 1-415
Th
OH
(N --Irj7õ N
N1N HO 0
I ./e i ---N ""-= 0
NN/
cr
CI i-Th
F.
CI
0
0
0 0
4 4 4
a 1-416 a 1-417 a 1-418
rl-
lit 0,,, H
,õ
N-N/ 0 õN
N-N/
N-N/
CI Ni--NN \ N CI Nr-N = N
/
0
O 0
4 1101
./C5:
CI 1-419 ,, 1-420 ci 1-421
Ho 0
N-Ni A=TrA lc/
0/N_I
N-N/
''
N-N
CI N .= /-"Nd
..&1.9,.... ......
0 /
/ 0 0
0
0 0
4
/ 4
CI 1-422 ci 1-423 a 1-424
.....,fr.k.N ..N......,
1õr14.1
, : N- _ F=10.. 0 -HNNH
, ...;_.\= ,
I41--L-^N N-61/
N-N
N
1"-= 01 N N \ rN CI N N CI N
N \ N
O 0 0
/?:?--- 0--- ,?'"-St-l-
a 1-425 CI 1-426 a 1-427
O,1-1% 0
N..3"._1
/
N-N/
N-N N-N
i..., T.
CI Ni--- \N \ N, N CI isõ, Th -- CI
N .. \ N,
O 0
/?-1--?"--
/?'-"- a 1-428 ..5):?"--
ci 1-429 a 1-430
52
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
\N-N 0 0
N. µ HO 'N'UNI ---a--.----)
rTh 0 i,N
1 HO-A\ L"--NI F10171
N ' N
CI i---\
N 8- `...-Nõ.
N
0 / o
0
* o 0
CI 1-431 1-4321-433
H 0 OH OH
/N /
N-N
--"N '''' 0
N-N/
/
/--- \ ,,.,
tTho CI N .= Cl N ÷.
CI N - 0¨
/ 0 0
0
0 0
0
* * 4
.- 1-434 a 1-435 CI 1-436
r ,,A i OH
illN,,1
kõ1,1 N-N
/ õõ ., HI HN '' 0
f"-\,, F
N1N ? CI N .= 1 8011)
1 N _
I , , /
t--, o
o CI
0 o
*
: ? " - -c3 1 I-437 a 1-438 ? " - se 1 1-439
=
N-N
\ HO L.--- HO--5% HN...;_t
".
0 N 'N N-Ni
CI N N I ...-.
/
0 I CI rq N \- '''-iy 1 , , CI
N--\N - '-''';11.,
/N / 0 / 0
0
0
*
CI 1-440 ai 1-441 '--a 1-442
0----1 o..... H / OH
N-õN i__N 0 ss.., N ',.
b0 N 0
1,4) Hol% .
Nrj-N N-N
N
\
a N/¨\õ, - \ N,. / 0
CI
/ 0
0
0
0
4 4
a 1-444 a 1-445
/
N. N-N/ I
H_/ N ,N,
, HO______?
CI Ni¨NI ft F
N-N CI FN.,
N,I 'NI
I , ,
0 o
/ 0
o o
0
0 4
a 1-446 a 1-447 --?---a 1-448
53
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
01-1
1,1
<'\'''''\ I
õ.õ
/
N¨N HO-5%
r,I ,N
,L.
.." ; N 'N
N-N
=.
0 /
/ 0 0
0
0 0
4
S)."--.4
01 1-449 ci 1-450 a 1-451
o
¨I)
HoN
q HO 0 N-14/ CI,N
N-N
A I)I-N/
CI N N
CI
/ 0
0
0
4
./1).--
1-452 a 1-453 0 1-454
HO ;
(-A }:cy 0 -d- .L...., OH
N- .1õ9/
N-N N i = i
N-N/ '
''''
%-\,,,,
CI N - CI --
N -
0
0 0
*
.55::?"-- ..?:?*".-
CI 1-455 ci 1-456 ci 1-457
HO0 H:6 H0_6;/
)
N-N
CI N N/
N-N/
N-N
--N ."` ---1
CI N N CI N N
/ 0 \_i / 0
_a r-\0
N
o o (t3 0
4 4 4
a 1-458 a 1-459 1-460
Eic:
4") 0
N¨N/
N-N N C.__z.)
N1.2
^ rThu N-14/
/ 0 CI N N
/ 0
0
0
4
/0---
CI 1-461 , 0 1-462 a 1-463
µ 0/ N 0 NN2
N /N /
?'"--C,N N-N
N-N/ N ..-'
N-N/
7 , , CI i-NN e O\N
CI NiTh, \ N, CI ir-NN \ N, / 0
/ 0
/ 0
0
O 0
*
/S)-2.-- 4
I
o 1-464 0 1-465 C 1-
466
54
84451174
_A -- OH / OH HOD
/ 4... \ iN J
N-N N-N N-N
_ N .." 0 / õ 7 N
CI NiTh . CI i----\ *
N N CI N %----\
N
/ / /
o o o P
F.0
0 0 0
4 4
ci 1-467 a 1-468 , ci 1-469
1 HO 0
/ 0- 1 , =
\ OH
r _ rif. N- /
N-N N-Ni
'--N / _ N .." 0
N
CI i---\ * N a 'I a /---µ =
N N
0 0
H
O I o
*
4 . *
a 1-470 el 1-472
1-471
O H
74 , / N-Ni _ OH
N,
/ - N -**- 144..il
a
a 1-473 a 1-474 :, 1-475
o
\-1113)11 -1 \¨/No...1
N ' N NN
'` I N ''
\ ONN 0\ CI N N (3\
/ 0 / 0
i 0
/.._.._
ilhci 1-476 a 1-477 a 1-478
. .
O OH 0 OH
'VI N-Nõ, scis\,.. OH
N ''' 0
i----\,,
Ni-ThN . CI N -
ON
0 0
Fad / 0
bN
O 0 0
* * 4
a 1-479 a 1-480 a 1-481
. .
1 1
OH ...N N OH 0
\ CI N
N-N/ r----.._=N OH
N..)4'N
NN '
'"' 0
CI N a
,... E
i----\,d )---µN .
N ry ..4
/ /
O
o
*
.
a 1-482 a 1-483 a 1-484
Date Regue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
0 OH OH 0 OH
/
N-N
N-Ni N N/
¨"N ..'" 0 N¨
t ...., s
¨" ''''
CI N;¨\N . CI ., (---N
N .. CI N= ta-\N lip
/ 0 / 0 NH
/ 0
0
NO 0
9 <'¨NJ__ 0 0
* * 4
a 1-485 a 1-486 a 1-487
OH 0 OH
N-N/ OH
N-N/ ¨N 0
0
0 0
*
* CI 1-490
a 1-488 a 1-489
0 OH
/ /
OH 1",...--N r")õ.0 OH 0-,
IslN LN
N-N 1 \N /
N " 0 I
1 "N , t,i , .../.
CI im\ it
N N =i--\ CI Ni¨NN =
13\
CI H N 0\
/ / 0
0 / 0
0 0 0
*
.5\--:?-- *
CI 1-491 a 1-492 a 1-493
OH HO -.1%
Br N
N-N/ N
N-N/ I
OH
CI *
."^ 0 / " .., -
_
j----\
N N =
a 1--NN 0
CI
,-.
0 0
0
4 * 4
a 1-494 1-495 - 1-496
9 7-N= OH
-""N 0 C(Th
\_...;/N r4"-/. 0 H
0 HO -5% N-N
CI N N
FN.. / CI N -
0 HN--N)
/ 0 ¨N
\
0
0
* *
ks 1-497 a 1-498 , a 1-499
CN \ 0 OH
"-/N 0,1
\ -7,N
/
N-N N- 'N
N N-N N '" 0 I
"f
a ci Ni¨ \ N . CI N .= \ N
0 0 0
4 *
CI 1-500 0 1-501 a 1-502
56
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
crm ,
C"'--c.-- 0
L.,
N,i 41%
, , N OH /
N ')., 61 N-N N N-N
I / ..õ i N
,-- 5
ci N - \ Iv, CI N - CI N N
O 0 0
* * *
CI 1-503 ci 1-504 a 1-505
OH
/
--N ..." 0 , 17,N H c-L,
Cr) HO_b
i----\ N-N
/ N ''''' 0
N-N
CI N N ...- F
/ 0 OH
o _
i-N
/ 0
/ 0
0
0
4
*-- o
'C CI 1-506 al 1-507 i. 1-
508
1 0 1:
H711)
N .,
) \N-N FI:::?
\N-N
0 Ho.....1:3,..w N. \ i -.=, \ t
ir--"Nõ,
\ Nõ1
0 0
õ0---- 4 is
a 1-509 ci 1-510 ci 1-511
\ o OH
OH
N-19/ A__ N-N/
N `" 0
N= .."
CI
N N CI i--- N-6(\ it
N N
¨ CI N -
0¨Cli'l
0 0
/ 0
0 0 0
4 * *
CI 1-512 ci 1-513 ci 1-514
. _
C) (-Ni) ¨
q
N-N/
H N= N
N-N' N N-N1 iTh,
i---\ ,
CI N N
0
0
0
111
a 1-515 . 1-516 ci 1-517
/ ._<\: N 0 0H 0 3F;
/ qo OH
N-N IV /
7-....T N'''''' N
N N
!---\ CI -1
CI N N CI NF-\N . \
O 0 0
4 4 4
CI 1-518 CI 1-519 a 1-520
57
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
O OH 0 OH 0
N-N/
N-N/
N-N/
N.'.
0 9 HN-b
N
O 0 0
4 4 ill
a 1-521 a 1-522 a 1-523
1 I
Ii '= N rN.,.,,, 111µ
N-N N-N ,-7..\\
= \N-N
j"\õ,
N ...---1 CI CI
/ 0
Nc,..311,
O 0 0
C1 1-524 a 1-525 a 1-526
0õ
.,N
7-
5.... ri....,,,N
,,,.....t, 0
. \
N-N N-N
\ ,
r( -4 N H i----\
CI Ni-NN CI N N \ N
a :r-",
N N / 0 ICI 0 No 0
O 0
.5)---?"
GI 1-527 k 1-528 a 1-529
Nc \N-N
r\si ,ry
\
N-N 0
H01.1
\N-N tThu
CI N \ a
CI N\ / 0 N
\ /
N/ 0 ,
0 N 1
1,,,
0
0
0
11 411
--.\)-"--. 1-530 a 1-531 a 1-532
OH 0 0 OH
N-N \N-N
0
--1.._
r
r=-= , a .... =E
i=-=
N N
CI N N = N
0
0*...
NO 0 ,....7
O Ns, 0
0
411 411 411
a 1-533 a 1-534 a 1-535
--q_o_AN-1 (N-:_k_0 "N-N _6; (¨_,Z.....03H
\N-N i ..õ N-N N
N ,.., % 7 N ""-= \ _ N
"'"
r=-", i---\õ, 1---\
CI N N CI N - CI N N_
/ 0 / 0 / 0
O 0 0 0
\
illi 411 4
a 1-536 a 1-537 a 1-538
58
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
0 OH ... 0-OH
N-N/ OH
....N.
\N-N
N ' N
"--<r:-N
i--",
CI N N
/ 0 NH
0
O\,....\
N-
OThL 0 0 0\ 0 /
\
* * *
a 1-539 ci 1-540 ci 1-541
OH 0 0 OH
N-N
/ H01...st
N-N \
"N-N
/ õ.... ,
"-N --'=
CI N ,õ -
CI N N CI N N /-\
0
/ NH / N .=,-.
/
O&N/ 0 L..10 0 \-/
\
0 0 0
* * *
CI 1-542 1-543 ci 1-544
o N,
H0.1.1
il N-N' OH
\N-N "-N 0
N-1111
CI 0 1 , NiTh .
i.M,,
N ----1:1, CI N 19 i-µ N
/ 4 '' '0 0 CI
0 M111 ji,
0 0
CI 1-547
AO 1-545 ci 1-546
OH OH
N-N/
N-N/
N N
N-N/--N 0
N-
rTh..,
o
/ 0
/ 0 Nµ,.../
o Lo,
o o
o
.1:4--- 4 411
a 1-548 ci 1-549 a 1-550
OH cc cri
N-N/
0
CI(1....0 OH
N-N/
--/.
0 ritir(.:
/ 0 HN NiThq
01 Ni-N1 a
o
* N o
5:µ;µ--- /S.:
a 1-551 a 1-552 a ?--.* 1-
553
. . .
/ OH 0
HO.....?;). N
f4-N
/1 N-61/ -
N-11 14(:),
IN\.... j
0
'61
4 0
0
CI 1-554 4
..?:<S--
a 1-555 a 1-556
59
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
o....;
N
....*.
NN 1, N.,..
N-N1 4.4 OH I
--N .'" NN HO1:
E.
i---µ I
1--µ CI N N
CI , N N
c..
CI
O
0 N\_..,
I
O 0
0
..5)...*' 4
.3t{)---
a 1-557 a 1-558 a 1-559
"-N--ks 0
4 N
N-N/ H00 L OH11
N-N1 (õ
1)1-14/
/¨\õ, /---µN
CI N =====;:i CI N -
CI N)--NN-1.,,..
0 0
6
1
0 0 a
4 4 4
0, 1-560 a 1-561 ci 1-562
o OH
HO.....? N-N/
i ..., ..1 "-N 0 NN
..;H
CI NI N =
CI i¨NN \ N
O 0
0
4 0 4
a 1-563 a 1-564 or 1-565
C-14 C%
410 N N N-.5.,....t
N,-,N ,
N-N/
N -
i'
õ,
a N - CI N --::?..., CI N .---1:1õ,
4 4
CI 1-566 a 1-567 a 1-568
I
HI31) NI.N.N 0 0H
Ni-- -N
3 .
N
/ N
CI N - Nõ,,
0
N 1 0
0 0 0 0\
4 4 4
CI 1-569 a 1-570 a 1-571
7 OH N
r
¨ 0 OH N
N N ..,t. -<;/,
lek'N ("-Nli 61N
I I
-
CI N N CI N N CI
O 0 0
/ 0 c
O 0 0\ 0
4 4 4
a 1-572 a 1-573 a 1-574
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
0
HO.._
N-N/ --ds,_. 0N.....kOH
NN 1,1N
, , , N N
.- ..... N i N
i---µ,., :
CI N - N 1--",
..... CIdx N N CI N N
0 0 0
0 0 0
* 411
ci 1-575 a 1-576 a 1-577
_. 0 OH -....\ 0.....40H
L....N .)
N-N/ Thµ.....N N-N/ 0N
fel*N FICI,
0
0 IN'
I
0 'ThL0
0
4 4
..-::?'"---
a 1-578 ci 1-579 a 1-580
+..N....1,
01.'''IrksN
OH r:i N
7-N/ . N-Ni N N-N/
/ , N
CI NiL-NN N CI Ni¨\N it d 0, NI¨\N-b
0
1 1.),
0 0
!4)---- .51:-C)-- 4
a 1-581 a 1-582 a 1-583
H04) ....,1,,....,,
C-4...0
N-N/ ; 1N
N"...k.N .-
N
OH
N-N/
3
:
i
ci NrTh'' N¨tla
ci N N CI N N N
NO
¨N
\
0 0 0
4 4
a 1-584 a 1-585 a 1-586
k 0 OH
/ iq".0 OH
N)...IN
N-N
N-N/
/ ...., ,
/
CI N Ni--.\N
N - CI NILNN¨N,,
/
0
0
I
0 0 0
41 41
a 1-587 a 1-588 a 1-589
0 OH
\ q4::61
N-11 N N-N/ i \-4.1Ø...?
/
N-N
7,
N
i-- \ Nr-NN lit 7
N CI ri"\N
r"-NO CI
CI N
N -
/ 0 0--µ,.? / 0 Nµ...../
0 / 0 0---.>
¨N
0 0
/
a 1-590 a 1-591 a 1-592
61
84451174
N X 0 \_N OH Co OH
I N _ .""
N-N
N
N-N
v ;----= Ng_ CI [NI O r--\ r---\
N ciIj
N N
CI
/ 0
0
* *
GI 1-593 GI 1-594 ci 1-595 ,
/ON ...):.ozDH
N-re /14
CI
6(t
r.,,N.
CI N N N . H
. CI N N
0 = 0C;I:?,,,__.
-...
a 1-596 a 1-597 C 1-598
_ 0^)
N
N-N/ \ iNjli ¨ OH
N ''''
1"
i---= õõ/ . 1 I -
,
a N N
CI Nr- \N
/ 0
/ 0
0:?''..-- i("'t.-"-CI
. 1-599 . 1-600 4-
1-601
--N--.1 OH
/
is,....NN N-N
N-N
/
i---\N N-11 . =
'
= GI N
a
IV
(1
0
a
--?'"-- a
.
ci
1:4"ha 1-602 1-603 a 1-604
OH / fi-R__ N OH / o OH
/---\N
N-N N-N 0 N-N
CI
V 1
' r--\'
N ..,
CI N CI N N
/ 0 p / a o
k_o i o o
=
o a Y--- o
= * 4
ci 1-605 a 1-606 a 1-607
OH
/
N-N , q OH / (TR_
N--- OH
N-N
CI N N CI N N
/ 0 N-
0)¨ N/
c-0\ c-0
\
0 0 ():?__. )
* 4
CI 1-608 a 1-609 GI 1-610
62
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
OH OH OH
/ / /
N-N N-N N-N
0 -"N 0 / "'N *.'" 0
t ,., 7
CI rµN It CI NN4, a Nli--\14 It
/ N-.- / N-
0 -
CO
*._ /
N 0
NO 0
0*...
NO
\
0 0 0
4 4 111
a 1-611 a 1-612 a 1-613
0 OH a OH
: 012
/
N-N N-1P N-N1
g /
CI CI
N N N
/ 0 0,,
( a NI:i?
0 sl,
0 0
'10
0 NI-
t I 0
0
4
CI 1-614 0 1-615 a 1-616
/
Achl"'N'"-it,
A N
OH
N-N I R¨ OH
t / N 0 N-N N-11 fil
_ N 0
1
cr NiTh . ck a NiThq N.õ1 a 14 Illk (3%
Lo
I
o o o
111
a 1-617 a 1-618 a 1-619
OH
0
N-N/
/ /
q OH q,õ, OH
/ N-N N-N
N 0
r"-
CI N-+ N N.õ1 * 0\ 1...-\
lik O\
CI N N CI N N
0
0 L.NN2 0 0
0 0 0
ill
.5;µ)-----
CI 1-620 a 1-621 a 1-622
0 OH l
qa...rN / 0-.,, OH
N-N/ N-N
E L`
/ N ''''= 0
:
N 0 CI ,d1::? CI Nr-\N 41, 0
=
/
N - N. / 0
I
0 0 0
4 IS 4
CI 1-623 a 1-624 a 1-625
0 OH k 0 OH
f) OH /
N-N
N-N/ r
N
I , N
r \
CI N N 4, r-- =,,, a N N
/--% CI
/ 0 N N-
\--/N-
O
0
0 0
0
4 1* 4
CI 1-626 a 1-627 a 1-628
63
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
O OH 0..kH : (6
N-N/ N-N/ NN
--N CI N - CI N N CI N N
0-
0
)
0 r.c.
O 0 0 k
4 4
o¨
ill
a 1-629 a 1-630 a 1-631
Cl: OH
LI 0 H
N-N'
N-N/
µN-N C)-1,,......6;)
µõ
( / 0 0--\, CI N )-- -
0 0
o o
o
0.--- 4 o-
11111
a 1-632 a 1-633 ci 1-634
¨0 0
HO...
Ll 0 OH NNrN
NN ^N _..z, I
N-N
I .., . ''"'-hl
i--Nõ
CI Ni--\I CI N - N CI Nim \N isi.
0
9 0 eLle
I
0
0 0
/ *
a 1-635 el 1-636 o 1-637
crN irN .....0õ..rN
Oli)
leN H
I
1\1
rri N-Ni ....* .
j--\
CI ,,, N - N 0
0
0 coke 0 004LN,
I -I-
O 0 0
*--. 14%--- 4
a 1-638 o 1-639 el 1-640
ic.-$1...sN N144
4 N
NN
N-11 N N-141
7 ?, N _
r-s= 0 141--µ14--& N CI CI N N
0
0 0
0
0 0
a 1-641 II*
el 1-642 ,S):::?"-*
o 1-643
N....,..N HO--o/ ....\ H0-51..)1 CI
N-N/ N ivol.
1 4 N
.., 1, N 1, / zõ
CI rrN--t-( N-N/
i---µ
N N"i
CI N- _
N, ,frµ
CI ,. N -
0
F
0 0
4
F 0
*
.?:*;.\----
ei 1-644 a 1-645 ei 1-646
64
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
0 ON
Clirl, \N¨N
N/
N¨
N¨N/ CI :
f CI Ni¨NN *
CI N 1*--.. / Nao, &
0
. 0
0
a 1-647 a 1-648 ci 1-649
O ON 0
HO....
µN¨N IsIN ---q, OH
N.... \ N¨N/
N 0
CI N *
Naos,
0
( 0 0 .--,
4
O ,c,
0.
0
0
.4
., 1-650 ci 1-651 ci 1-652
o'N OH FIN-e%
Nv........, N¨N/ 4 N
N¨N/N 0 NN
=
CI N
CI i----\ 40
N -
^ N, / LiL'o 0--=,µ
O 0
Of
\
0
= 0
4 4 4
CI 1-653 ci 1-654 , ci 1-655
1. HØ..6.3 HO
N¨N FIC)¨VN 0
NN' OH 1
/ ,.... 3 L'N 0 E tii¨N/ N
CI it"µN = CI i----µN
N a i¨N
N N
/ 0 Th 0 0
0
\
0 0 0
4 4 4
a 1-656 ci 1-657 ci 1-658
CN 1 HO .,0 / OH
4*,N N¨N 1 \
N Nr. N A... / , , N
....= = N 0
tfp i----\ *
CI N N
el N N
CI Nfr'sN N / 0
0 /0
0
0
"--
/4 0
* 0
a 1-659 a 1-660 a 1-661
rksN 0010 / FIN
N=N=
4 N
N¨N/ . N¨N/
NN N-1.
CI Ni¨'4N-Psi, CI"--
N . N
O 0
0 0
0
4
ci 1-662 a 1-663 0 1-664
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
% HOA HO 0
HO
0- / 0 TS..1
N-N/ µ..N N-N N- N-11
i
"----µ ;,--
-=
CI N N CI N N CI N N N
O 0
O 0 0
4 4 4
a 1-665 a 1-666 a 1-667
rN.
iIN
N N-N/ n
N Wit.
N-N/
. 4 N
_
- CI Ni¨NN --IN NN
/
CI NI-NN N, / 0
0
HO ci ihN-e,
0
0
0
0
4 4
a 1-668 a 1-669 a 1-670
µ t µ
OH
CI N= CI OH OH
/ 0- \ N-N/ 0 N-11
N-N
µN 0
N im\n,
N == CI i----\,,
/ 0
/
cµ... i
N\
0 0 0
4 4 4
CI 1-671 a 1-672 a 1-673
x
,rµc1/4
N)/ 0--, OH
N-N \.:.4.
N....s..1
N)....t t N 0
N-N
Cl/ N-N1
i---
i N %8
..
\....../
0
O 0
,,--?---- 5::().--- 4
a 1-674 a 1-675 a 1-676
. . .
WN
N-N/ 0 OH N.,
N-N/ C3A,..N IrN/ . N
i----µ g
CI N N t--µ CI .frµ
/ 0
0 0 0
\
O 0 0
4 4 4
a 1-677 a 1-678 a 1-679
O OH r".> 0 OH
N-N/ ..._ N..N
N-N/
N \
N-Pli t
F
---N__
j.__õs!
CI CI N
CI N N'tZ
N N.
0 / 0
0
\
0 o o
*-- 4 4
01 1-680 ci 1-681 a 1-682
66
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
0_61 0
"*"*TrAl=N
N((õ
=
N-r/ .,
N-N/
CI N ÷. /--s= CI N .'d
/ 0
0
0 = 0
0--- 4 4
CI 1-683 a 1-684 a 1-685
--c. 0 OH HII*N14 ID OH
N-N/ /
LILN N-N
N-N
CI N N CI N N
/ 0 0
0 Os. I,
----,
0 0 0
4 4 4
ci 1-686 a 1-687 a 1-688
l'IN 0
H011 ,H
N-N/
,O-
N-N' . :p /
7
"'""
CI t¨µõ,
N -
CI N - N
/ :
0 0 ---INI3 0
0
\
O 0 0
/0"--- 4 4
a 1-689 a 1-690 ci 1-691
0Ai 0 OH 0
HOI.,, I
N-N/
71-N N-N/
/ ....pi
17 _
,õ
CI N .. CI N ÷. CI N .. ." ,.--;.11
0 `"`ISI
\.--0 \.....-,
0 0 0
4 40 4
a 1-692 a 1-693 , a 1-694
N-N/ 0 OH
CL-Q1, cir
N-N/ N-N' Ni--'
CI Ni--\N=
.. :
CI 4N' NI? CI r-Nim_4:7 4 N, , , / 0 0
= '0 ' HO
0
O 0
=?:4L /0"-- 4
a 1-695 a 1-696 a 1-697
-9õ
, N r.N
N-N/
N-Ni N-N
/ -
'11":;) ,
014 N N N 014 NrTh N dim, N
CI N- ' 0 '
O 0
.51?----
*---
a 1-698 ,
01 1-699 a 1-700
67
84451174
Hol_lo 0
H
N-N N-N O-1.1 CkNI
/ /
....N.....1
/
CI
/ µ,
C7 / 0
0 \ 0 N \
0
= 4
a 1-701 ci 1-702 --?---GI 1-703
. H010
N N..-1. 14.411 11' :,:l N-N
4 '3!..t i _
:
14 a
ci i---\,,,
N .=-\--"Ni.,
I ...
HO
I 0
0 0
411 1-704 :.. 0ci 1-706
1-705
r¨so- / µ0 HO
µc",si rrN;N
7N A....
N
NI, ii N ..
/
N-N/ _ N-N
CI N ,
j---\-
VI 0
N'Th 0
0
k1707
a 1-708 .
ci 1-709
µ 10 RC:. HC.: % HOA
N
--N -*-'
''.. S.
N "---µ,õ ,,
CI CI N ., a ,&,, N ,.
0 0 p 1.11 / '0 Co
\--0
\ \
0 0 0
*
a 1-710 a 1-711 a 1-712
0 OH
.....
L . CZ N-N
N/ ,.., 7 ---N .."=
)---\ fr\ rTh.
07
CI N N CI N N cl N ..
/ '0 0 / 0
N--\
* * 0
a 1-713 ci 1-714 ci 1-715
0 OH 0'...) \ 0,0H
7-....NH O
NN __.)H N-NH 0--\ ___,
LN
, --VN ,
I rThõ,
CI N .= i----\ CI N ÷.
CI N N
\
0 0
:?,_____O
* 0
CI 1-716 ci 1-717 ci 1-718
68
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
O OH +? 0 OH
Nse,
N-N/
N-NH
INR5 11_ 4.A, I, 1 "*N
/
CI N N CI
/ / 0-
0 CI N ^ N 0
O A 0
0 L
41111'
/1:14.--- 4
a 1-719 a 1-720 a 1-721
irN. - 0..._Dii
-N---1
µ10 OH
N-N/ N ool,,N,i
. N/? N-NH
CI N/N ---µ . 0
CI N N N, i 0 /
O 1 . o
NC ix,
0
o o
0
4 4
a 1-722 a 1-723 a 1-724
0 OH 0 OH 0 OH
N-N/ /
N-NH
/ l-.....N ^,...
: ? /
CI 111
i 0 0 / 0
N¨µ
0
* \
* *
CI 1-725 a 1-726 ci 1-727
11.1.7 OH ...N1 0
...-...
O'sj"N I 0 H \
N-NH N-N CN) 0
N -**"
- NA*N o...
) OH
CI NI¨ \N lik 0 CI N N *
a N H
0 0
0
O 0
0
4
a 1-728 ci 1-729 a 1-730
.
N-N (0 0 (a.)
N) \N-N c-}40H \N-N ks-N)
OH N
CI N lit a H (1)
/
N N CI N \N * 0
/ /
0 0 0 0
HO
O 0 0
.5):?--- 0
a 1-731 a 1-732 a 1-733
3
\N-N N-Nµ N OH
ris1:$
CI N 0 CI N 0 N-N/
ci II SN¨I
N \--N 0
0 )---0
0
0
* sN.--/f \OH
* * OH =
*
CI 1-734 ci 1-735 ci 1-736
69
84451174
N-N (0 N-N N-N/ c.Ø
CI N 0 CI M 0 a N 0
N-- \ 1(1._-)
-1-N c_NI8-0H
/ \ N
(-.In01-1
0
HO
ci 1-737 a 1-738 0 1-739
N-N1 / / ,...
.
N-N Ix). N-N A.;)
CI 11 0 CI N 0 a N 0
/ /
CI 1-740 a 1-741 ci 1-742
/
N-N
N-N I
H
CI 11 = 0 11 0 a N N
N--
IN-)
--N8-(31-1 0
N'OH At
it ci 1-745
ci 1-743 GI 1-744
I I HOD
H H I
CI N HN-04 CI N HN-04 -,
/ OH / OH CI II HN
0 0 / o
0 0
0
411 4 #
ci 1-746 GI 1-747 CI 1-748
SYNTHESIS
[0063] The compounds of the present disclosure can be prepared in a number of
ways well
known to one skilled in the art of organic synthesis. The compounds of the
present disclosure
can be synthesized using the methods described below, together with synthetic
methods known
in the art of synthetic organic chemistry, or variations thereon as
appreciated by those skilled in
the art. Preferred methods include, but are not limited to, those described
below.
[0064] The compounds of the disclosure may be prepared using the exemplary
reactions and
techniques described in this section. The reactions are performed in solvents
appropriate to the
reagents and materials employed and are suitable for the transformations being
effective. Also,
in the description of the synthetic methods described below, it is to be
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
understood that all proposed reaction conditions, including solvent, reaction
atmosphere,
reaction temperature, duration of the experiment and workup procedures, are
chosen to be the
conditions standard for that reaction, which should be readily recognized by
one skilled in the
art. One having ordinary skill in the art may adjust one or more of the
conditions described
herein. One skilled in the art of organic synthesis understands that the
functionality present
on various portions of the edict molecule must be compatible with the reagents
and reactions
proposed. Not all compounds of the disclosure falling into a given class may
be compatible
with some of the reaction conditions required in some of the methods
described. Such
restrictions to the substituents, which are compatible with the reaction
conditions, will be
readily apparent to one skilled in the art and alternate methods can be used.
Scheme 1
CO2Et
R4 R4 COOH R4
R6
R5 5 R5
CO2Et
(1101 s NH2 R R6 N. NJ R6
R7 R7 CO2Et R7
1 2 3
OH OAr
R4 R4
R5 R5
CO2Et CO2Et
R6 R6
R7 R7
4 5
OAr OAr
R4 R4
R5 R5 _______________________________________________________ 0
__________ ===
COOH
R6 N R6 N-L3-
Rz
1,11
R7 R7
6 7
100651 In some embodiments, provided compounds of this invention may be
prepared as
shown in Scheme 1. Indole 3 can be assembled by using Japp-Klingemann reaction
described by, but not limited to, F. G. Salituro, et al. I Med. Chem. (1990)
33, 2944-2946 as
follows. Aniline 1 is converted to the corresponding benzenediazonium
intermediate
followed by condensation with ethyl 2-oxocyclopentanecarboxylate to give
hydrazone 2.
Intramolecular Fisher indole cyclization of the intermediate 2 is followed to
give indole 3.
71
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
The ethyl ester functional group at the flexible linker of indole 3 can be
selectively reduced
with excess BH3, and the resulting alcohol 4 can be condensed with phenols or
hydroxy-
heterocycles via Mitsunobu reaction to give the ether 5 using, but not limited
to, DEAD or
Dt-BuAD. Indole acid 6 can be generated by saponification of compounds 5 with
appropriate
bases, such as Cs2CO3, K7CO3, LiOH or NaOH, at a number of conditions that are
routine for
those skilled in the art of organic synthesis. Indole amides 7 can be produced
by coupling of
compounds 6 with suitable amines using coupling reagents, but not limited to,
PyBOP, DCC,
EDC, HBTU, or TBTU at a number of conditions that are routine for those
skilled in the art
of organic synthesis.
Scheme 2
OAr Ar¨B(OH)2 OAr
R4 9 R4
R5 R5
or
CO2Et CO2Et
R6 6
Ar¨B R
X 8 Ar
11
R5 R4 OAr
CO2Et Ar¨X
R6
13
B
0", 0
12
[0066] In some embodiments, compounds of Formula 11 containing Ar or
heteroaryl
substituents as R7 group may be synthesized by procedures illustrated in
Scheme 2.
Compounds of Formula 8, wherein X = Cl, Br, I, triflates or diazoderivatives,
can be prepared
as previously described in Scheme 1. A variety of boronic acids 9 or borates
10, which are
commercially available or can be prepared, can be coupled with intermediates 8
via e.g.,
Suzuki coupling protocol to afford biaryl adducts 11(Miyaura, N., Suzuki, A.,
Chem. Rev.
(1995), 2457). In some embodiments, one exemplary such procedure entails
treatment of the
aryl bromide or iodide 8 with an aryl boronic acid in the presence of a
catalytic Pd species,
such as Pd(PPh3)4., Pd(PPh3)2C12, Pd(OAc)2, Pd2(dba)3 and a suitable ligand
such as PPh3,
AsPh3, etc., or other such Pd catalyst, and a base such as Na2CO3, Cs2CO3,
K2CO3, Ba(OH)2
or Et3N. Alternatively, biaryl adducts 11 can be prepared from Pinacolborates
12 which can
be prepared from compounds 8 via Pd, such as Pd(PPh3)4, Pd(PPh3)2C12,
Pd(OAc)2,
72
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
Pd2(dba)3, catalyzed coupling of bis(pinacolato)diboron. Intermediates 12 can
be coupled
with a variety of aryl-halides or heteroaryl-halides 13 using Suzuki coupling
protocol
described above to give compounds 11. In some embodiments, a provided approach
allows
for great diversity in the subsequent coupling of indole boronic acids or
borates with
commercially available haloaromatic derivatives.
Scheme 3
R4 R3 R4 R3
R5 R5
\ COOR \ COOR __________
R6 R6
R7 15 R7 Lt
R1
14 16
R4 R3 R4 A3
R5 R5 0
\ COOH _________________________________ :11_L3_
R6
N
A7 Li R7 Ltõ R
R1
17 18
100671 In some embodiments, provided compounds of Formula 18 may be prepared
by
procedures outlined in Scheme 3. Compounds of Formula 14 can be reacted with
compounds
of Formula 15, wherein X is Cl, Br, I, OMs, or OTs with a base such as NaH,
K2CO3,
Cs7CO3, Et3N, or DIPEA in a suitable solvent such as DMF, THF, ether, DME, or
the like, to
give compounds of Formula 16. Applying the same reaction sequence as described
in
Scheme 1, compounds of Formula 16 can undergo saponification followed by
coupling
reaction to give compounds of Formula 18.
Scheme 4
R4 R3 R4 R3
R5
RR5
\ COOH NH 2
N H 2 Rg
6
H2N¨L3
R7LlR1 R7 us,.
R1
17 19 20
R4 A3
R5 0 0µ,0
)S.
Re N HN¨L3 N R
R7
21
100681 In some embodiments, compounds of Formula 21 can be synthesized by
procedures
depicted in Scheme 4 via selective sequential coupling reactions in one-pot.
An amino group
73
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
of compounds 19 can be coupled with compounds of Formula 17 as illustrated in
Scheme 1 to
afford inteiinediates 20. In the same pot, suitable carboxylic acids can be
coupled to the
sulfonamide group of compounds of Formula 20 using coupling reagents, but not
limited to,
PyBOP, DCC, EDC, HBTU, or TBTU at a number of conditions that are routine for
those
skilled in the art of organic synthesis, to yield acylsulfonamides of Formula
21.
Scheme 5
R
4 R4 R3 R3
R5
R5 0 0
\
R6 COOH 0 \
+
N
H2N¨L3)l'OR 1
1
R7 0.--R1 R7 Li\ Rl
17 22 23
R4 R3 R4 R3
R5 0 0 R5 0 0 Ow0
R6 N HN_____L3AOH Re N HN¨L3 N R
I I H
R7 Ll R7 L1
''.R1 ..R1
24 25
[0069] Alternatively, compounds of Formula 25 can be prepared by procedures
illustrated in
Scheme 5 by similar sequential coupling reactions. Compounds of Formula 17 can
undergo
coupling reactions with an amine functional group of compounds 22 as shown
Scheme 1 to
give intermediates 23. An ester group of Formula 23 can be saponificated using
aqueous
based, such as Cs2CO3, K2CO3, LiOH or NaOH, at a number of conditions that are
routine for
those skilled in the art of organic synthesis to generated compounds of
Formula 24.
Subsequent coupling reactions of acids 24 with suitable sulfonamides using
coupling reagents
at a number of conditions that are routine for those skilled in the art of
organic synthesis to
afford reverse acylsulfonamides of Formula 25.
Scheme 6
R4 R3 R4 R3
R5 0 0 R R5 0 0
\ + Brii_Nn
R6 N HN¨L3AOR Br R6 N
H \_p_AN¨L3 OR
27
R7 R7 R Vin
26 28
[0070] Exemplary method for preparing compounds of Formula 28, wherein the Ni
position
of indole and the amide NH is tethered to form rings, is described in Scheme 6
and proceeds
74
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
from compounds of Formula 26. Optionally substituted di-bromo alkanes 27 can
be used to
react with indole amides 26. The cyclization may be accomplished with a
variety of bases,
but not limited to, DBU, Et3N, DIPEA, Cs2CO3, K2CO3, NaH, or t-BuONa in a
suitable
solvent such as DMF, toluene, THF, DME, CH3CN, 1,4-dioxane or the like, to
afford
compounds of Formula 28 at a number of conditions that are routine for those
skilled in the
art of organic synthesis. Compounds of Formula 28 can be employed to
subsequent reactions
as depicted in above Schemes.
Scheme 7
R4 R3
R4 R3 00 R5 0
R5 ,\S', \
0 NBoc ¨..
\ COOR + ki_on R6 N NH
R7
H R
R7
14 29 o
or),0 ---------R 0
31
X-Ar-ILOR
34
R4 R3 R4 R3
R5 0 0 R5 0 Ck
\
A
R6 N NL3 ¨ ro.' . R6 N N-Ar
R7
k in R7
28 1 1 35
R4 R3 R4 R3
R5 0 0 R5 0 0
\ \ YOH
R6f N N_L3A0H R6 N N-Ar
R7 µ1+/)n R7 \I __ (/fl
R R
32 1 136
R4 R3 R4 R3 0
0."
R5 0 000 R5 0 0 -p-R.
RB N N¨L3 D, 1.1 ^ R6 N N-Ar
R7 VLA
k in R7 V-On
R R
33 37
[0071] An alternate route to substituted tricyclic indole amides is shown in
Scheme 7 and
described here. The tricyclic amide intermediates of Formula 30 can be
prepared by
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
alkylation of the indole NH of ester 14 with optionally substituted cyclic
sulfamidates 29
followed by cyclization upon removal of the Boc-protecting group (see, for
example, Richter
H. G. F. Bioorg. Med. Chem. Lett. 2010, 5713). The size and stereochemistry of
the newly
formed cyclic amide can be controlled by size and preset stereo-configuration
of the reagent
29. The NH group of Formula 30 can undergo allcylation reactions with
compounds of
Formula 31, wherein X is Cl, Br, I, OMs, or OTs with a base such as NaH,
K2CO3, Cs2CO3,
Et3N, or DIPEA in a suitable solvent such as DMF, THF, ether, DME, or the
like, to give
compounds of Formula 28. Corresponding compounds of Formulae 32 and 33 can be
prepared from the ester 28 by saponification and coupling of sulfonamides to
the carboxylic
acid functional group of compounds 32 as described in Scheme 5. Alternatively,
a variety of
aryl or heteroaryl halides of Formula 34, wherein X is Br, I, or OTf can be
coupled to the NH
group of Formula 30 in the presence of a catalytic Pd species, such as
Pd(OAc)2, Pd2(dba)3
and a suitable ligand such as Xantphos and a base such as Na2CO3, Cs2CO3, or
K2CO3 to
generate compounds of Formula 35. Same saponification and coupling of
sulfonamide
coupling protocols described above can also be applied to prepare
corresponding compounds
of Formulae 36 and 37.
Scheme 8
R4 R3 R4 R3
R5 0 R5 0 N
R-CN
________________________________ 11. N-NH
R6 N¨L3 R6
\.. N¨L3/EA
R7 R7 R in
38 39
[0072] In some embodiments, compounds of Formula 39 containing tetrazole
moiety can be
generated by the procedure depicted in Scheme 8. A nitrile group of compounds
38 can
undergo cyclization reation with NaN3 in the presence salt such as NH3C1,
Et3NHC1 or
catalytic amount of 12, A1C13 or TMSC1 in a suitable solvent such as DMF,
PhNO2 or NMP at
a number of conditions that are routine for those skilled in the art of
organic synthesis to give
tetrazoles of Formula 39.
Scheme 9
R4 R3 R4 R3
R5 0 R5 0
X-Cy¨Rw ¨11"
Rs NH 40 Rs N-Cy-R'
R7 n R7 \--On
30 41
76
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
100731 The NH group of Formula 30 can undergo cross-coupling reactions with a
variety
of aryl or heteroaryl halides of Formula 40, wherein X is Br, I, or OTf in the
presence of a
catalytic Pd species, such as Pd(OAc)2, Pd2(dba)3 and a suitable ligand such
as Xantphos and
a base such as Na2CO3, Cs2CO3, or K2CO3 to generate compounds of Formula 22.
Alternatively, compounds of Formula 30 can be produced using the Ullman
coupling
conditions in the presence of Cul and a suitable ligand such as (trans)-1,2-
N,N'-
dimethylaminocyclohexane or L-Proline and a base such as Cs2CO3, K2CO3 or
K2PO4 in a
suitable solvent such as toluene or DMF. The R7 and Rw of Formula 21 and 22
can be
further elaborated such as saponification, substitution, alkylation of NH or
OH, halogenation,
amidation and construction of heterocycles under a number of functional group
modification
conditions that are routine for those skilled in the art of organic synthesis.
ABBREVIATIONS
[0074] The following abbreviations are employed in the Examples and
elsewhere herein:
Dt-BuAD = di-tert-butyl azodicarboxylate
DCM = dichloromethane
EDC = 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide
TEA = triethylamine
DMAP = dimethylamino pyridine
HOBT = hydroxybenzotriazole
DBU = 1,8-Diazabicycloundec-7-ene
DMF = dimethy lformami de
DMSO = dimethylsulfoxide
THF = tetrahydrofuran
K2CO3= potassiumm carbonate
Cs2CO3 = cesium carbonate
DME = 1,2-di methoxy ethane
t-BuONa = sodium tert-butoxide
LDA = lithium di-isopropylamide
NaHMDS = sodium hexamethyldisilazide
LiHMDS = lithium hexamethyldisilazide
n-BuLi = n-butyl lithium
ether = diethyl ether
NaOH = sodium hydroxide
77
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
KOH = potassium hydroxide
Et0Ac = ethyl acetate
Na2CO3 = sodium carbonate
Na2SO4 = sodium sulfate
MgSO4 = magnesium sulfate
SiO2 = silicon dioxide
CH2C12 = methylene chloride
Me0H = methanol
Et0H = ethanol
Hex = hexanes
HCl = hydrochloric acid
Pd(dppf)C12 = [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium (II)
Pd2(dba)3 = tris(dibenzylideneacetone)dipalladium (0)
Pd(PPh3)4 = tetrakis(triphenylphosphine)palladium(0)
TFA = trifluoroacetic acid
Et3N = triethylamine
DIPEA = N,N-diisopropylethylamine
SnC12 = tin(II) chloride
DEAD = diethyl azodicarboxylate
TBAD = dit-butyl azodicarboxylate
NaH = sodium hydride
NaNO2 = sodium nitrite
CH3COONa = sodium acetate
NaN3 = sodium azid
MsC1 = methanesulfonyl chloride
TBAI = tetrabutylammonium iodide
EtI = ethyl iodide
EtBr = ethyl bromide
TBAF = tetrabutyl ammonium fluoride
NMM = N-Methylmorpholine
NMO = N-Methylmorpholine N-oxide
DBU = 1,8-Diazabicyclo(5.4.0)undec-7-ene
PCC = Pyridinium chlorochromate
BOP = (Benzotriazol-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate
78
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
MsC1 = mesyl chloride
PPA = polyphosphoric acid
mm = minute(s)
h or hr = hour(s)
mL or ml = milliliter
g = gram(s)
mg = milligram(s)
mmol = millimole(s)
RT or rt = room temperature
LRMS = low resolution mass spectrometry
NMR = nuclear magnetic resonance
EXAMPLES
[0075] The
following Examples are offered as illustrative as a partial scope and
particular
embodiments of the invention and are not meant to be limiting of the scope of
the invention.
Abbreviations and chemical symbols have their usual and customary meanings
unless
otherwise indicated. Unless otherwise indicated, the compounds described
herein have been
prepared, isolated and characterized using the Schemes and other methods
disclosed herein or
may be prepared using same.
General coupling procedure A:
[0076] A
mixture of lactam (1.0 eq), bromide (2,0 eq), Pd2(dba)3 (0.1 eq), Xantphos
(0.2
eq), and Cs2CO3 (2.5 eq) 1,4-Dioxane (1 mL) was sparged with Ar gas for 5
minutes. The
reaction mixture was sealed and heated to 110 C for 18 h. The reaction
mixture was cooled
to ambient temperature, poured to DCM/water (10 mL, 1:1). The organic layer
was separated
and the aqueous layer was extracted with DCM (2 x 5 mL). The combined organic
solution
was dried over MgSO4, filtered and concentrated in vacuo. The crude residue
was purified
by reverse phase HPLC or flash chromatography.
General coupling procedure B:
[0077] A
mixture of lactam (1.0 eq), bromide (2.0 eq), CuI (0.5 eq), (trans)-1,2-N,Ar-
dimethylaminocyclohexane (1.0 eq), and K2CO3 (2.5 eq) in toluene (1 mL) was
sparged with
Ar gas for 5 min. The reaction mixture was sealed and heated to 110 C for 18
h. Same work
up and purification protocols described in general coupling procedure A were
followed to
obtaine a desire product.
General coupling procedure C:
79
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
100781 A
mixture of lactam (1.0 eq), bromide (2.0 eq), Cul (0.5 eq), (trans)-1,2-N,N'-
dimethylaminocyclohexane (1.0 eq), and K3PO4 (2.5 eq) in DMF (1 mL) was
sparged with
Ar gas 5 minutes. The reaction mixture was sealed and heated to 120 C for 18
h. Same work
up and purification protocols described in general coupling procedure A were
followed to
obtaine a desire product.
General saponification procedure D:
[0079] To a solution of ester in a mixture of THF, Me0H, and H20 (3 mL, 0.5
mL, 0.5
mL), was added LiOH (5 mg). The reaction was heated to 50 C for 3 h then
cooled to
ambient temperature. The crude reaction mixture was concentrated in vacuo, and
the residue
was purified by reverse phase HPLC.
General peptide coupling procedure E:
[0080] To a
solution of (R)-3 -(7-chloro-10-(3-(4-chloro-3 ,5 -dimethy 1phenoxy)propy1)-4-
methyl-l-oxo-6-(1,3,5-trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-
a] ind ol-2(1H)-
y1)-1-methy1-1H-indole-5-carboxylic acid (10 mg, 0.014 mmol) and HATU (8 mg,
0.021
mmol) in DMF (1 mL) was added diisopropylethylamine (5 mg, 0.039 mmol), and
the
reaction was stirred at room temperature for 15 min. The appropriate amine was
added, and
the reaction was stirred at room temperature until complete by LCMS. Same work
up and
purification protocols described in general coupling procedure A were followed
to obtaine a
desire product.
General Procedure F: Headpiece Bromination & Alkylation
[0081] To a solution of indole or indazole (0.28 mmol) in DMF (2 mL) was added
NBS
(51 mg, 0.28 mmol) at 0 C, and the reaction was stirred 1 h. The reaction as
diluted into
DCM/H20 (20 mL, 1:1), the layers separated, and the aqueous layer was
extracted with DCM
(2 x 10 mL). The combined organic layers were washed with 10% aqueous Na2S203
(10
mL), dried over MgSO4, filtered, and concentrated in vacuo. The crude residue
was
dissolved in DMF (2 mL), sodium hydride was added (14 mg, 0.34 mmol), and the
reaction
was allowed to stir 5 min at room temperature. Alkyl iodide (0.34 mmol) was
added and the
reaction was allowed to stir for 1 h. The reaction was diluted with DCM (10
mL), quenched
with H20 (10 mL), the layers separated and the aqueous layer was extracted
with DCM (2 x
mL). The combined organic extracts were concentrated in vacuo. The crude
residue was
purified by flash chromatography.
General Procedure G: Headpiece Alkylation
[0082] To
asolution of substituted indole/indazole or naphthoic acid/quinolone
carboxylic
acid (0.20 mmol) in DMF (2 mL) was added NaH (10 mg, 0.24 mmol) at room
temperature
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
and stired for 5 min at room temperature. Alkyl iodide (0.24 mmol) was added,
and the
reaction was stirred for 1 h. The work up described in general coupling
procedure A was
followed. The crude residue was purified by flash chromatography.
General Procedure H: Alkylation of Indole Hydroxy Group
[0083] A
mixture of hydroxyl-indole (1.0 eq), Cs2CO3 (3.0 eq) and the appropriate
electrophile in DMF (0.05 M) was heated to 60 to 90 C until complete by LCMS.
The work
up described in general coupling procedure A was followed. The crude residue
was purified
by flash chromatography.
General Procedure I: Alkylation of Indole Nitrogen After Coupling to Tricyclic
Core
[0084] A
mixture of NH-indole (1.0 eq), Cs2CO3 (3.0 eq) and the appropriate
electrophile
in DMF (0.05 M) was heated to 60 to 90 C until complete by LCMS. The work up
described in general coupling procedure A was followed. The crude residue was
purified by
flash chromatography.
General Procedure J: Mesylation and SN2 Displacement of 2-Hydroxymethyl
Pyrimidine
[0085] To a
solution of hydroxymethyl-pyrimidine (1.0 eq) in DCM (0.03 M) was added
DIPEA (5.0 eq) followed by mesyl chloride (3.0 eq). The reaction mixture was
stirred at RT
for 2 h then concentrated in vacuo. The residue was dissolved in DMF (0.05 M),
and DIPEA
(5.0 eq) and the appropriate amine (5-10 eq) were added in sequence. The
reaction mixture
was stirred from 25 to 50 C until complete by LCMS. The work up described in
general
coupling procedure A was followed. The crude reaction mixture was used for
subsequent
saponification without further purification.
Example 1
Ethyl (R)-7-(7-chlo ro-1O-(3-(4-chlo ro-3,5-d imethylp henoxy)propyl)-4-methyl-
1-oxo-6-
(1,3,5-trimethyl-1H-py razo l-4-yl)-3,4-d ihy d ropy razino [1,2-a] ind ol-
2(1H)-yl)-1H-ind o le-
2-carboxylate
Step A. Preparation of 5-(2-(2-bromo-3-chlorophenyl)hydrazono)-6-ethoxy-6-
oxohexanoic acid
[0086] To a
stirring mixture of 2-bromo-3-chloroaniline (20 mmol) in 1M HC1 (25 mL)
and water (5 mL) at 0 C was added NaNO2 (1.38 g, 20 mmol) in water (20 mL),
CH3COONa (9.23 g, 112 mmol) in water (25 mL) and ethyl 2-oxocyclopentane
carboxylate
(3.0 mL, 20 mmol) in sequence. The reaction mixture was stirred for 15 min at
0 C then
warmed to 20 C over 2h and extracted with CH2C12, dried over MgSO4, filtered
and
81
84451174
concentrated in vacua to give the title compound as a red oil in 7.1 g (90%
crude).
Step B. Preparation of ethyl 7-bromo-6-chloro-3-(3-ethoxy-3-oxopropyl)-1H-
indole-2-
carb oxy late
[0087] To a solution of 5-(2-(2-bromo-3-chlorophenyl)hydrazono)-6-ethoxy-6-
oxohexanoic
acid (7.1 g, 18 mmol) in Et0H (30 mL) was added conc. H2SO4 (7.5 mL), slowly.
The reaction
mixture was refluxed for 1.5 h. The reaction was quenched by pouring into ice
then extracted
with CH2Cb. The combined organic layer was washed with sat. NaHCO3, water,
brine, dried
over MgSO4, filtered and concentrated in vacuo . The residue was purified by
flash
chromatography (Combi-flashTm Rf Hex/Et0Ac 25% gradient) to give the title
compound as an
off-white solid in 4.4 g (11 mmol). MS (ES) 402.0 (M+H).
Step C. Preparation of ethyl 7-bromo-6-chloro-3-(3-hydroxypropyl)-1H-indole-2-
carb oxylate
[0088] To a solution of ethyl 7-bromo-6-chloro-3-(3-ethoxy-3-oxopropy1)-1H-
indole-2-
carboxylate (1.9 g, 4.8 mmol) in THF (20 mmol) was added BH3 in THF (20 mL, 20
mmol) at
20 C. The reaction mixture was stirred for 15 h at 20 C and quenched by
addition of Me0H
then concentrated in vacua. The residue was purified by flash chromatography
(Combi-flash Rf
Hexane/Et0Ac gradient 0-50%) to give the title compound as a white solid (1.4
g, 3.9 mmol).
MS (ES) 360.1 (M+H).
Step D. Preparation of ethyl 7-
bromo-6-chloro-3-(3-(4-chloro-3,5-
dim ethylp hen oxy)p ropy1)-1H-in d ole-2-carb oxylate
[0089] To a solution of ethyl 7-bromo-6-chloro-3-(3-hydroxypropy1)-1H-
indole-2-carboxylate
(101 mg, 0.28 mmol), PPh3 (110 mg, 0.51 mmol) and 3,5-diMe-4-Cl-phenol (81 mg,
0.52 mmol)
in THF (3.5 mL) was added Dt-BuAD (99 mg, 0.51 mind) at 20 C. The reaction
mixture was
stirred for 15 h at 20 C then concentrated in vacua. The residue was purified
by flash
chromatography (Combi-flash Rf Hexane/Et0Ac gradient 0-10%) to give the title
compound
(105 mg, 0.21 mmol) as a colorless oil. MS (ES) 498.0 (M+H).
Step E. Preparation of ethyl 6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-7-(1,3,5-
trimethy1-1H-pyrazol-4-y1)-1H-in dole-2-carb oxylate
[0090] To a solution of ethyl 7-bromo-6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-
1H-indole-2-carboxylate (319 mg, 0.64 mmol) in dioxane (3.0 ml) and water (2.0
ml) at 20 was
added 1,3,5-trimethy1-4-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-1H-
pyrazole (168 mg,
0.71mmol), Pd(PPh3)4 (37 mg, 0.032 mmol) and K2CO3 (267 mg, 1.94 mmol). The
mixture was
degased then heated to 125 C in BiotageTM
82
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Initiator for 40 min. The reaction was quenched by addition of water,
extracted with Et0Ac,
dried over MgSO4, filtered and concentrated in vacuo. The residue was purified
by flash
chromatography (Combi-flash Rf Hexane/Et0Ac gradient 0-15%) to give the title
compound
(238 mg, 0.45 mmol) as a white solid. MS (ES) 528.2 (M+H).
Step F. Preparation of ethyl (R)-1-(1-((tert-butoxycarbonyl)amino)propan-2-y1)-
6-
chloro-3-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(1,3,5-trimethyl-1H-
pyrazol-4-y1)-
1H-indole-2-carboxylate
[0091] To a
solution of ethyl 6-chloro-3-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-1H-indole-2-carboxylate (360 mg, 0.68 mmol)
in
anhydrous DMF (2 mL) was added NaH (60%) (25 mg, 0.70 mmol) at 0 C and
stirred for 3
min. tert-Butyl (5)-5-methyl-1,2,3-ox athi azo li dine-3 -carboxylate 2,2-
dioxide (0.70 mmol)
was added. The reaction mixture was stirred in the ice bath for 20 min and
then at RT for
overnight. The reaction mixture was diluted with ethyl acetate (50 mL) and
washed with
brine (2 X 30 mL), dried with anhydrous MgSO4, filtered and concentrated in
vacuo. The
residue was purified by flash chromatography (Combi-flash Rf, Hex/acetone =
80:20) to give
the title compound (305 mg, 65%). MS (ES) 686.3 (M+H), Rf = 1.29.
Step G. Preparation of (R)-7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-
methy1-6-(1,3,5-trim ethy1-1H-pyrazol-4-y1)-3,4-dihyd ro pyrazin o[1,2-a] in d
01-1 (2H)-one
[0092] To a
solution of ethyl (R)-1-(1-((tert-butoxycarbonypamino)propan-2-y1)-6-chloro-
3-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(1,3,5-trimethyl-1H-pyrazol-4-y1)-
1H-indole-
2-carboxylate (218 mg, 0.32 mol) in anhydrous DCM (5.0 mL) at 0 C was added
TFA (1.5
mL) dropwise. The reaction mixture was warmed to RT and stirred for 2 h then
concentrated
in vacuo. The residue was dissolved in anhydrous ethanol (10 mL) then
anhydrous K2CO3
(829 mg, 1.92 mmols) was added. The reaction mixture was stirred at RT for
overnight then
diluted with ethyl acetate (60 mL) and washed with brine (2X 30 mL). The
organic layers
were dried over anhydrous MgSO4., filtered and concentrated. The residue was
purified by
flash chromatography (Combi-flash Rf, DCM/methanol = 0-10% gradient) to give
the title
compound (150 mg, 87%). MS (ES) 539.5 (M+H), Rf = 0.95.
Step H. Example 1
[0093] The title compound (210 mg, 52%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazino [1,2-a] ind ol-1 (211)-
one (300 mg, 0.55
mmol), ethyl 7-bromo-1H-indole-2-carboxylate (300 mg, 1.10 mmol), copper
iodide (50 mg,
83
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
0.26 mmol), (trans)-N1 ,N2 -di methylcyclohexane-1,2-diamine (80 mg, 0.56
mmol), and
K2C 03 (250 mg, 1.81 mmol). MS (ES) 726.3 (M+H).
Example 2
3-B romo-6-(8-chloro-11-(3-(4-chlo ro-3,5-d imethyl phenoxy)p ro py1)- 1-oxo-7-
(1,3,5-
trimethyl-1H- pyrazol-4-y1)-4,5-dihyd ro-1H-11,41diazepino [1,2-al indo1-2(3H)-
y1)-1-
methyl-1H-indole-4-carboxylic acid
Step A. Preparation of ethyl 1-(3-((tert-butoxycarbonyl)amino)propy1)-6-chloro-
3-(3-(4-
chloro-3,5-dimethylphenoxy)propy1)-7-(1,3,5-trimethyl-1H-pyrazol-4-y1)-1H-
indole-2-
carboxylate.
[0094] To a
solution of ethyl 6-chloro-3-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-1H-indole-2-carboxylate (360 mg, 0.683 mol)
in
anhydrous DMF (2 mL) was added NaH (60 %) (25 mg, 0.62 mol) at 0 C and
stirred for 3
min. tert-Butyl 2-oxo-1,3-oxazinane-3-carboxylate was added. The reaction
mixture was
stirred in the ice bath for 20 min then at RT overnight. The reaction mixture
was diluted with
ethyl acetate (50 mL) and washed with brine (2 x 30 mL), dried over anhydrous
MgSO4,
filtered and concentrated in vacuo. The residue was purified by flash
chromatography
(Combi-flash Rf, eluent 20% ethyl acetate in hexanes) to give the title
compound (305 mg,
65%). 11-1 NMR: 5 7.58 (d, J = 10.7 Hz, 1H), 7.23 (dõI = 10.7 Hz, 1H), 6.64
(s, 2H), 4.42-
4.35 (m, 3H), 4.32-4.24 (m, 1H), 4.18-4.08 (m, 1H), 3.98 (t, J= 7.5 Hz, 2H),
3.88 (s, 3H),
3.24 (t, J = 9.0 Hz, 2H), 2.76 (q, J = 7.0 Hz, 2H), 2.34 (s, 6H), 2.13 (t, J =
7,0 Hz, 2H), 2.09
(s, 3H), 2.03 (s, 3H), 1.48 (t, J = 7.0 Hz, 2H), 1.43 (t, J = 9.0 Hz, 3H),
1.42 (s, 9H); 13C
NMR: (125 MHz in CDC13) 6 162.3, 156.7, 155.6, 146.4, 138.0, 137.2, 136.9,
134.2, 126.8,
126.1, 126.0, 125.0, 121.7, 120.9, 116.4, 114.4, 113.5, 78.9, 67.4, 60.7,
42.8, 37.9, 36.2, 31.2,
30.5, 28.3, 21.8, 20.9, 14.3, 12.3, 10.3; LCMS: RT = 2.320 min; > 98% purity
at 215 nm and
254 nm; MS (ES) 685.0 (M+H).
Step B. Preparation of 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-2,3,4,5-tetrahyd ro- 1H- [1,4] diazep ino[1,2-a]
ind ol-1-one.
[0095] To a
solution of ethyl 1-(3-((tert-butoxycarbonyl)amino)propy1)-6-chloro-3-(3-(4-
chloro-3,5-dimethylphenoxy)propy1)-7-(1,3,5-trimethyl-lH-pyrazol-4-y1)-1H-
indole-2-
carboxylate (218 mg, 0.32 mol) in anhydrous CH2C12 at 0 C was added TFA (1.5
mL)
dropwise. The reaction mixture was warmed to RT, stirred for 2 h then
concentrated in vacua
The residue was dissolved in anhydrous ethanol (10 mL) then anhydrous K2CO3
(829 mg,
1.92 mmols) was added. The reaction mixture was stirred at RT for overnight.
The reaction
mixture was diluted with ethyl acetate (60 mL) and washed with brine (2 x 30
mL). The
84
84451174
organic layers were dried with anhydrous MgSO4, filtered and concentrated in
vacuo. The residue
was purified by flash chromatography (Combi-flash Rf, CH2C12/methanol = 0-10%
gradient) to give
the title compound (150 mg, 87%). NMR:
(500 MHz inCDC13) öLI7.62 (d, J = 10.7 Hz, 2H), 7.25
(d, J = 10.7 Hz, 2H), 6.65 (s, 2H), 6.23 (t, J = 7.5 Hz, 1H), 4.06-3.93 (m,
4H), 3.90 (s, 3H), 3.22-3.15
(m, 4H), 2.35 (s, 6H), 2.20 (quint., J= 8.7 Hz, 2H), 2.06 (s, 3H), 2.04 (s,
3H), 1.82-1.66 (m, 2H); 13C
NMR: (125 MHz in CDC13) ö 166.4, 156.8, 145.8, 138.1, 136.9, 135.8, 132.9,
131.6, 126.7, 125.9,
121.7, 121.2, 120.8, 115.1, 114.5, 113.5, 67.4, 41.0, 38.5 36.1, 30.2, 30.1,
20.9, 20.4, 12.1, 10.0;
LCMS: RT = 1.896 min., > 98% purity at 215 um and 254 urn; MS (ES) 539.0
(M+H).
Step C. Preparation of Methyl 3-(8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-1-oxo-
7-(1,3,5-trimethy1-1H-pyrazol-4-y1)-4,5-dihydro-lH-[1,41diazepino[1,2-alindo1-
2(311)-
Abenzoate
100961 The
title compound (66 mg, 76%) was prepared following General coupling procedure
B
using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(1,3,5-trimethyl-
1H-pyrazol-4-y1)-
2,3,4,5-tetrahydro-1H41,4]diazepino[1,2-alindol-1-one (70 mg, 0.13 mmol, 1.0
eq), methyl 3-
bromobenzoate (55 mg, 0.26 mmol, 2.0 eq), CuI (10 mg, 0.052 mmol, 0.40 eq),
rac-NX-
dimethylcyclohexane-1,2-diamine (15 mg, 0.105 mmol, 0.81 eq), and K2CO3 (55
mg, 0.40 mmol, 3.0
eq). 11-I NMR: (500 MHz in CDC13) 8 7.98-7.94 (m, H), 7.61 (d, J= 8.5 Hz, 1H),
7.51-7.46 (m, 2H),
7.24 (d, J= 8.5 Hz, 1H), 6.61 (s, 2H), 4.16-4.06 (m, 2H), 3.96 (t, J= 6.0,
2H), 3.91 (s, 3H), 3.85 (s,
3H), 3.76-3.67 (m, 2H), 3.16 (oct, J = 7.5 Hz, 2H), 2.29 (s, 6H), 2.23 -2.16
(m, 2H), 2.06 (s, 3H),
2.05 (s, 3H), 1.92-1.80 (m, 2H); 13C NMR: (125 MHz in CDC13) 8 166.2, 164.0,
156.8, 146.0, 142.3,
137.7, 136.9, 135.2, 133.0, 132.0, 131.4, 130.7, 129.3, 127.8, 126.7, 126.6,
125.9, 121.5, 121.2,
120.7, 115.5, 114.5, 113.2, 67.3, 52.2, 48.2, 40.7, 36.2, 30.2, 29.5, 20.8,
20.6, 12.3, 10.0; LCMS: RT
= 2.092 min, > 98% purity at 215 nm and 254 nm; MS (ES) 673.0 (M+H).
Step D. Example 2
100971 To a
solution of methyl 6-(8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-
oxo-
7-(1,3,5-trimethy1-1H-pyrazol-4-y1)-4,5-dihydro-1H- [1,41diazepi no [1,2-a]
indo1-2(3H)-y1)-1-methyl-
1H-indole-4-carboxylate (40 mg, 0.055 mmol) in THF (0.5 mL) was added NBS (10
mg, 0.058
mmol) then stirred for 15 h at RT. LiOH (0.14 ml, 0.28 mmol, 2N) was added,
and the mixtuer was
heated to 70 C for additional 6 h. The reaction was concentrated in vacuo,
and the crude residue
was dissolved in DMSO (1 mL) and filtered. The DMSO solution was purified by
reverse phase
HPLC (Phenomenex Gemini C18, H20/CH3CN gradient from 30-90% CH3CN, 0.1% 11-A)
to
yield the title compound (28 mg, 65%) as an
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
off-white solid. MS (ES) 790.2 (M+H).
Example 3
3-Chloro-6-(8-chloro-11-(344-chloro-3,5-dimethylphenoxy)propyl)-1-oxo-741,3,5-
trimethyl-1H-pyrazol-4-yl)-4,5-dihydro-1H-11,4] diazepino[1,2-al indo1-2(3H)-
yl)-1-
methyl- 1H-ind arb oxylic acid
[0098] The
title compound was prepared (22 mg, 0.029 mmol) as a white solid according
to procedures described in Example 2 Step D substituting NBS with NCS (7.4 mg,
0.055
mmol). MS (ES) 746.2 (M+H).
Example 4
6-18-Chloro-114344-chloro-3,5-dimethyl-phenoxy)propyl]-1-oxo-7-(1,3,5-
trimethylpy razol-4-y1)-4,5-d ihy d ro-3H- [1,4] d iazep ino [1,2-a] ind ol-2-
y1]-1-methyl-
benzotriazole-4-carboxylic acid
[0099] The
title compound (6.2 mg, 16%) was prepared following General coupling
procedure A using 8-chl oro-11 -(3 -(4-chl oro-3,5-dimethy 1phenoxy)p ro py1)-
7-(1,3,5 -trimethyl-
1H-py razol-4-y1)-2,3,4,5-tetrahydro-1H41,41diazepino[1,2-c]indol-l-one (30
mg, 0.056
mmol), methyl 6-bromo-1-methy1-1H-benzo [d][1,2,3]triazole-4-carboxylate (19
mg, 0.070
mmol), Pd2(dba)3 (8 mg, 0.0087 mmol), Xantphos (12 mg, 0.021 mmol), and Cs2CO3
(60 mg,
0.19 mmol) followed by saponification using General Procedure D. LCMS: RT =
1.288 min,
MS (ES) 714.1 (M+H).
Example 5
6-18-Chloro-1143-(4-chloro-3,5-dimethyl-phenoxy)propy1]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,41diazepino [1,2-a] ind o
benzotriazole-4-carb oxylic acid
[00100] The title compound (3.7 mg, 9.3%) was prepared following General
coupling
procedure A using 8-chl oro-11 -(3 -(4-chl oro-3,5-dimethy 1phenoxy)p ropy1)-7-
(1,3,5 -trimethyl-
1H-py razol-4-y1)-2,3,4,5-tetrahydro-1H41,41diazepino[1,2-c]indol-l-one (30
mg, 0.056
mmol), methyl 5-bromo-1-methy1-1H-benzo [d][1,2,3]triazole-7-carboxylate (25
mg, 0.092
mmol), Pd2(dba)3 (8 mg, 0.0087 mmol), Xantphos (12 mg, 0.021 mmol), and Cs2CO3
(60 mg,
0.19 mmol) followed by saponification using General Procedure D. LCMS: RT =
1.360 min,
MS (ES) 714.1 (M+H).
Example 6
7-18-Chloro-1143-(4-chloro-3,5-dimethyl-phenoxy)propy1]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihyd ro-3H-11,41diazepino indo1-
2-y1]-1-methyl-indole-
3-carbonitrile
86
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00101] The title compound (13 mg, 34%) was prepared following General
coupling
procedure A using 8-chl oro-11 -(3 -(4-chl oro-3,5-dimethy 1phenoxy)p ropy1)-7-
(1,3,5 -trimethyl-
1H-py razol-4-y1)-2,3,4,5-tetrahy dro-1H41,41diazepino [1,2-a] indol-1 -one
(30 mg, 0.056
mmol), 7-bromo-1-methy1-1H-indole-3-carbonitrile (30 mg, 0.14 mmol), Pd2(dba)3
(8 mg,
0.0087 mmol), Xantphos (12 mg, 0.021 mmol), and Cs2CO3 (60 mg, 0.19 mmol).
LCMS:
RT = 1.555 min, MS (ES) 693.1 (M+H).
Example 7
6-[8-chloro-11-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihyd ro-3H-11,41diazepino[1,2-a] ind 01-2-y1]-1-
methyl-
benzimidazole-4-carboxylic acid
Step A. Preparation of methyl 6-bromo-1-methyl-1H-benzo[d]imidazole-4-
carboxylate
[00102] To a solution of 6-bromo-1H-benzo[d]imidazole-4-carboxylic acid (200
mg, 0.84
mmol) in THF (5 mL) and Me0H (1 mL) at 0 C was added
trimethylsilyldiazomethane in
Et20 (2.0 M, 0.63 mL, 1.3 mmol). The reaction was stirred for 1 h at 0 C then
warmed to RT.
The reaction was quenched by addition of AcOH dropwise and concentrated in
vacuo. The
crude residue was diluted with DCM (20 mL) and washed with brine (10 mL). The
organic
layer was dried over MgSO4, filtered, and concentrated in vacuo. To a solution
of the crude
residue in DMF (2 mL) was added NaH (30 mg, 1.25 mmol) at RT and stirred for
30 min.
Methyl iodide (120 mg, 0.84 mmol) was added, and the reaction was stirred for
4 h at RT.
The reaction mixture was cooled to -78 C and quenched with Me0H. The mixture
was
diluted into DCM/H20 (20 mL, 1:1), the organic layer separated and the aqueous
layer was
extracted with DCM (2 x 5 mL). The combined organic extracts were dried over
MgSO4,
filtered, and concentrated in vacuo. The crude residue was purified by reverse
phase
preparative HPLC (Phenomenex Gemini C18, H20/CH3CN gradient to 20-80 % MeCN
0.1%
TFA) to afford the title compound (83 mg, 37%).
Step B. Example 7
[00103] The title compound (3 mg, 8%) was prepared following General coupling
procedure A using 8-chl oro-11 -(3 -(4-chl oro-3,5-dimethylphen oxy )p ro py1)-
7-(1,3,5 -trimethyl-
1H-py razol-4-y1)-2,3,4,5-tetrahydro-1H41,41diazepino [1,2-a] indol-1 -one (30
mg, 0.056
mmol), methyl 6-bromo-1-methy1-1H-benzo [d]imi d az ol e-4-carboxylate (35 mg,
0.13 mmol),
Pd2(dba)3 (8 mg, 0.0087 mmol), Xantphos (12 mg, 0.021 mmol), and Cs2CO3 (60
mg, 0.19
mmol) followed by saponification using General Procedure D. LCMS: RT = 1.048
min, MS
(ES) 713.0 (M+H).
Example 8
87
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
2-(4-(Tert-butoxycarb onyl)piperazin- 1-y1)-6-((8-chlo ro-11-(3-(4-chlo ro-3,5-
d imethylp henoxy)p ro py1)-1-oxo-7-(1,3,5-trimethyl-1H-pyrazol-4-y1)-4,5-
dihyd ro-1H-
11,41 diazepino 11,2-al ind ol-2(3H)-yl)methyl)isonicotinic acid
[00104] The title compound (67 mg, 76%) was prepared following General
coupling
procedure A using methyl 2-
chloro-6-((8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy )propy1)-1-oxo-7-(1,3,5-trimethy1-1H-py razol-4-y1)-4,5 -dihy
dro-1H-
[1,4]diazepino[1,2-a]indo1-2(311)-yl)methypisonicotinate (72 mg, 0.1 mmol),
tert-butyl
piperazine-l-carboxylate (22 mg, 0.12 mmol), Pd2(dba)3 (2 mg, 0.002 mmol),
Xantphos (3.5
mg, 0.006 mmol), and Cs2CO3 (49 mg, 0.15 mmol) followed by saponification
using General
Procedure D. LCMS: RT = 2.025 min, MS (ES) 858.3 (M+H).
Example 9
24(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-
trimethy1-1H-
pyrazol-4-y1)-4,5-dihyd ro-1H- [ 1,41 d iazepino [1,2-a] ind ol-2(3H)-
yl)methyl)-6-(4-(fu ran-2-
carbonyl)piperazin-1-yl)isonicotinic acid
Step A. Preparation of methyl 2-08-chloro-11-(3-(4-chloro-3,5-
d imethy 1phen oxy)p ro py1)-1-oxo-7-(1,3,5-trimethyl- 1H-py razol-4-y1)-4,5-d
ihyd ro-1H-
[1,4 diazepino [1,2-a] indo1-2(3H)-yl)methyl)-6-(piperazin-1-y1)isonicotinate
[00105] To a solution of tert-butyl 4-(6-((8-chloro-11-(3-(4-chloro-3,5-
di methylphenoxy )propy1)-1-oxo-7-(1,3,5-trimethy1-1H-py razol-4-y1)-4,5 -dihy
dro-1H-
[1,4] di azepino [ 1,2-a] indo1-2(3H)-y pmethyl)-4-(methoxy carbony Opyridin-2-
yOpiperazine-1-
carboxylate in CH2C12 (1 mL) was added TFA (0.5 mL) and the mixture was
stirred at room
temperature for 12 h. The reaction mixture was extracted in CH2C12 (3x15 mL),
dried (anhyd.
Na2SO4) and concentrated in vacuo to give the crude title compound. MS (ES)
772.2 (M+H).
Step B. Example 9
[00106] To a solution of methyl 2-((8-
chl oro-11 -(3-(4-chl oro-3,5-
d i methylphenoxy )propy1)-1-oxo-7-(1,3,5-trimethy1-1H-py razol-4-y1)-4,5 -
dihy dro-1H-
11,41diazepino[1,2-c]indo1-2(31)-yl)methyl)-6-(piperazin-1-ypisonicotinate (15
mg, 0.02
mmol), in CH2C12 (1 mL) at room temperature was added triethylamine (6 uL,
0.04 mmol)
followed by furan-2-carbonyl chloride (4 ut, 0.04 mmol) and the mixture was
stirred at room
temperature for 1 h. The mixture was diluted with water (2 mL) and extracted
in CH2C12 (3x5
mL), dried and concentrated in vacuo. The residue was dissolved in THF, Me0H,
and H20 (3
mL, 0.5 mL, 0.5 mL), and LiOH (5 mg) was added and the reaction was heated to
50 C for 3
h. The crude was purified by reverse phase HPLC (Phenomenex Gemini C18,
H20/CH3CN
88
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
gradient to 35-95 MeCN 0.1% TFA) to afford the title compound (16 mg, 94%).
LCMS:
RT = 1.785 mm, MS (ES) 852.4 (M+H).
Example 10
2-08-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-4,5-dihydro-1H-11,41diazepino11,2-al ind ol-2(3H)-yl)methyl)-6-
(piperazin-
1-yl)isonicotinic acid
[00107] Title compound (54 mg, 63%) was prepared according to General
saponification
procedure D using methyl 2-((8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-1-oxo-
7-(1,3,5-trimethyl-1H-pyrazol-4-y1)-4,5-dihy dro-1H-[1,4] diazepino [1,2-a]
indo1-2(3H)-
yl)methyl)-6-(piperazin-l-yDisonicotinate (87 mg, 0.11 mmol). LCMS: RT = 1.605
min, MS
(ES) 758.3 (M+H).
Example 11
4-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-4,5-dihyd ro-1H-11,41diazepino [1,2-a] in d I-2 (3H)-y1)-1-(2-
(dimethylamino)ethyl)-1H-indole-6-carboxylic acid
Step A. Preparation of methyl 4-amino-1-(2-(dimethylamino)ethyl)-1H-indole-6-
carboxylate
[00108] To a solution of methyl 4-amino-1H-indole-6-carboxylate (100 mg, 0.526
mmol)
in DMF (3 mL) was added NaH (63 mg, 1.6 mmol) and stirred at RT for 15 mm. 2-
Bromo-
N,N-dimethylethan-1 -amine (96 mg, 0.631 mmol) was added, and the resulting
minture was
stirred for 30 min then quenched by TFA (0.081 mL, 1.0 mmol). The reaction
mixture was
concentrated, and the residue was purified by reverse phase HPLC (Phenomenex
Gemini
C18, H20/CH3CN gradient to 0-50 MeCN 0.1% TFA) to give the title compound (50
mg,
36%) product. MS (ES) 262.3 (M+H).
Step B. Preparation of methyl 4-(6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-
7-(1,3,5-trimethyl-1H-pyrazol-4-y1)-1H-indole-2-carboxamido)-1-(2-
(dimethylamino)ethyl)-1H-indole-6-carboxylate
[00109] To a solution of 6-chloro-3-(3-(4-chloro-3,5-dimethylphenoxy )propy1)-
7-(1,3,5-
trimethy1-1H-pyrazol-4-y1)-1H-indole-2-carboxylic acid (70 mg, 0.14 mmol),
methyl 4-
amino-1-(2-(dimethylamino)ethyl)-1H-indole-6-carboxylate (40 mg, 0.15 mmol)
and DMAP
(34 mg, 0.28 mmol) in DCM (2 mL) was added EDC (54 mg, 0.28 mmol) and stirred
at RT
for 16 h. The reaction mixture was concentrated, and the residue was purified
by reverse
phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient to 0-50 % MeCN 0.1% TFA)
to give the title compound (24 mg, 23%). LCMS: RT = 1.695 min, MS (ES) 742.8
(M+H).
89
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Step C. Example 11
[00110] To a solution of methyl 4-(6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-
741,3,5 -trimethy1-1H-py razol-4-y1)-1H-indole-2-c arboxami do)-1-(2-
(dimethylamino)ethyl)-
1H-indole-6-carboxylate (24 mg, 0.032 mmol) and 1,3-dibromopropane (9.8 pl,
0.097 mmol)
in DMF (0.5 mL) was added Cs2CO3 (53 mg, 0.16 mmol) then stirred at rt for 20
h. The
reaction mixture was filtered, and the filterate was concentrated. The residue
was dissolved
in THF (1 mL) and LiOH (0.2 mL, 2N) and stirred at 60 C overnight. The
reaction mixture
was concentrated, and the residue was purified by reverse phase HPLC
(Phenomenex Gemini
C18, H20/CH3CN gradient to 30-80 % MeCN 0.1% TFA) to give the title compound
(9 mg,
36%). LCMS: RT = 1.671 min, MS (ES) 768.7 (M+H).
Example 12
6-18-Chloro-11- [3-(4-chloro-3,5-dimethyl-phenoxy)propy1]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihyd ro-3H-11,41diazepino11,2-al indol-2-yl]-2-(2-
morpholinoethypindazole-4-carboxylic acid
Step A. Preparaion of methyl 6-bromo-2-(2-morpholinoethyl)-2H-indazole-4-
carboxylate
[00111] To a solution of methyl 6-bromo-1H-indazole-4-carboxylate (100 mg,
0.392 mmol)
in DMF (5 mL) was added NaH (30 mg, 1.25 mmol) at RT. The reaction mixture was
stirred
for 30 min, followed by addition of 4-(2-bromoethyl)morpholine (120 mg, 0.619
mmol).
After 4 h, the reaction was quenched with water then concentrated in vacuo.
The residue was
purified by reverse phase preparative HPLC (Phenomenex Gemini C18, H20/CH3CN
gradient to 20-75 % MeCN 0.1% TFA) to separate the regioisomeric indazole
intermediates.
The desire region-isomer was dissolved in THF (5 mL) and Me0H (1 mL) and
trimethylsilyldiazomethane (2.0 M in Et20, 0.25 mL, 0.50 mmol) was added. The
reaction
was stired for 30 min at RT then quenched by addition of AcOH dropwise and
concentrated
in vacuo to afford the title compound (21 mg, 15%).
Step B. Example 12
[00112] The title compound (4.6 mg, 10%) was prepared following General
coupling
procedure A using 8-chl oro-11-(3 oro-
3,5 -dimethylph enoxy)propy1)-7-(1,3,5 -trimethyl-
1H-py razol-4-y 0-2,3,4,5-tetrahy dro-1H- [1,4]di azepino [1,2-a] indol-1 -one
(30 mg, 0.056
mmol), methyl 6-bromo-2-(2-morpholinoethyl)-2H-indazole-4-carboxylate (20 mg,
0.054
mmol), Pd2(dba)3 (8 mg, 0.0087 mmol), Xantphos (12 mg, 0.021 mmol), and Cs2CO3
(60 mg,
0.19 mmol) followed by . saponification using General Procedure D. LCMS: RT =
1.048
min, MS (ES) 812.1 (M+H).
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Example 13
7-18-Chloro-11- [3-(4-chloro-3,5-dimethyl-phenoxy)propyl]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,41diazepino [1,2-a] indol-2-yl]-1-
methyl-
indazole-3-carboxylic acid
[00113] The title compound (2.7 mg, 7%) was prepared following General
coupling
procedure A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethyl-
1H-pyrazol-4-y1)-2,3,4,5-tetrahydro-1H41,41diazepino[1,2-a]indol-1-one (30 mg,
0.056
mmol), methyl 7-bromo-l-methy1-1H-indazole-3-carboxylate (12 mg, 0.045 mmol),
Pd2(dba)3 (8 mg, 0.0087 mmol), Xantphos (12 mg, 0.021 mmol), and Cs2CO3 (60
mg, 0.19
mmol) followed by saponification using General Procedure D. LCMS: RT = 1.843
min, MS
(ES) 712.7 (M+H).
Example 14
6- [8-Chloro-11- [3-(4-chloro-3,5-dimethyl-phenoxy)p ropyl]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,41diazepino[1,2-al ind ol-2-yl] -142-
mo rp holinoethypind azole-4-ca rb oxylic acid
Step A.
Preparation of methyl 6-bromo-1-(2-morpholinoethyl)-1H-indazole-4-
carboxylate
[00114] The title compound (34 mg, 24%) was prepared following the same
procedure as
Example 12, Step A.
Step B. Example 14
[00115] The title compound (7.4 mg, 17%) was prepared following General
coupling
procedure A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethyl-
1H-pyrazol-4-y1)-2,3,4,5-tetrahydro-1H-[1,4] di azepino [1,2-a] ind 01-1 -one
(30 mg, 0.056
mmol), methyl 6-bromo-1-(2-morpholinoethyl)-1H-indazole-4-carboxylate (34 mg,
0.092
mmol), Pd2(dba)3 (8 mg, 0.0087 mmol), Xantphos (12 mg, 0.021 mmol), and Cs2CO3
(60 mg,
0.19 mmol) followed by saponification using General Procedure D. LCMS: RT =
1.661 min,
MS (ES) 811.8 (M+H).
Example 15
8-Chloro-11- [3-(4-chloro-3,5-dimethyl-phenoxy)propyl]-2-11-methyl-3-(2/1-
tetrazol-5-
yflindol-7-y1]-7-(1,3,5-trimethylpyrazol-4-y1)-4,5-dihyd ro-3H-
11,41diazepino11,2-al in dol-
1-one
[00116] To a solution of 7-(8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-1-oxo-
7-(1,3,5-trimethy1-1H-pyrazol-4-y1)-4,5-dihy dro-1H- [1,4] diazepino [1,2-a]
indo1-2(3H)-y1)-1 -
methy1-1H-indole-3-carbonitrile (10 mg, 0.014 mmol) in DMF (1 mL) was added
NaN3 (10
91
84451174
mg, 0.15 mmol) and ammonium chloride (10 mg, 0.19 mmol). The reaction was
capped and
heated to 130 C for 72 h then cooled to RT. The work up described in general
coupling
procedure A was followed then purified by reverse phase HPLC (Phenomenex
Gemini C18,
H20/CH3CN gradient 35-95 % MeCN 0.1% '11-A) to afford the title compound (2
mg, 19%).
LCMS: RT = 1.980 min, MS (ES) 735.9 (M+H).
Example 16
7-[8-Chloro-1143-(4-chloro-3,5-dimethyl-phenoxy)propy1]-1-oxo-7-(1,3,5-
trimethylpyrazol-
4-y1)-4,5-dihydro-3H41,4] diazepino [1,2-a] indo1-2-y1]-2-methyl-indazole-3-
carboxylic acid
[00117] The title compound (6.1 mg, 16%) was prepared following General
coupling
procedure A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethy1-
1H-pyrazol-4-y1)-2,3,4,5-tetrahydro-1H-[1,4]diazepino[1,2-a]indol-1-one (30
mg, 0.056 mmol),
methyl 7-bromo-2-methyl-2H-indazole-3-carboxylate (12 mg, 0.045 mmol),
Pd2(dba)3 (8 mg,
0.0087 mmol), Xantphos (29 mg, 0.11 mmol), and Cs2CO3 (60 mg, 0.19 mmol)
followed by
saponification using General Procedure D. LCMS: RT = 1.463 min, MS (ES) 713.0
(M+H).
Example 17
2-(4-acetylpiperazin-l-y1)-6-48-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propyl)-1-oxo-
7-(1,3,5-trimethyl-1H-pyrazol-4-y1)-4,5-dihydro-lH41,4]diazepino[1,2-alindol-
2(3H)-
yl)methyl)isonicotinic acid
[00118] To a solution of methyl 2-((8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-1-
oxo-7-(1,3,5-trimethy1-1H-pyrazol-4-y1)-4,5-dihydro-1H41,41diazepin0 [1,2-a]
indo1-2(3H)-
yl)methyl)-6-(piperazin- 1-ypisonicotinate (15 mg, 0.02 mmol), in CH2C12 (1
mL) at RT was
added triethylamine (6 L, 0.04 mmol) followed by acetyl chloride (3 L, 0.04
mmol) and the
mixture was stirred at room temperature for 1 h. The mixture was diluted with
water (2 mL) and
extracted in CH2C12 (3x5 mL), dried and evaporated. The crude material was
subjected to
saponification conditions following General Procedure D followed by
purification by reverse
phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient to 35-95% MeCN 0.1% TFA)
to
afford the title compound (14 mg, 88%). LCMS: RT = 1.721 min, MS (ES) 800.4
(M+H).
Example 18
92
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
5-18-chloro-11- [3-(4-chloro-3,5-dimethyl-phenoxy)p ropy11-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,4]diazepino[1,2-alind ol-2-yl] -2,3-
dihydrobenzofuran-7-carboxylic acid
[00119] The title compound (2 mg, 5%) was prepared following General coupling
procedure A using 8-chl oro-11 -(3 -(4-chl oro-3,5-dimethy 1phenoxy )p ro py1)-
7-(1,3,5 -trimethyl-
1H-pyrazol-4-y1)-2,3,4,5-tetrahydro-1H41,41diazepino[1,2-cdindol-1-one (30 mg,
0.056
mmol), methyl 5-bromo-2,3-dihydrobenzofuran-7-carboxylate (30 mg, 0.12 mmol),
Pd2(dba)3
(8 mg, 0.0087 mmol), Xantphos (29 mg, 0.11 mmol), and Cs2CO3 (60 mg, 0.19
mmol)
followed by saponification using General Procedure D. LCMS: RT = 1.825 min, MS
(ES)
700.8 (M+H).
Example 19
(R)-5-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-3-ethyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-d ihyd ropyrazino [1,2-a] ind ol-2(1H)-yl)qu
inoline-8-
carboxylic acid
[00120] The title compound (53% yield) was prepared following General coupling
procedure A using (R)-7-chl o ro-10-(3 -(4-chloro-3,5 -d
imethylphenoxy)propy1)-3 -ethy1-6-
(1,3,5-trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino ind ol-
1(21-1)-one and methyl 5 -
bromoquinoline-8-carboxylate followed by saponification using General
Procedure D. MS
(ES) 724.3 (M+H).
Example 20
(R)-6-47-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-3-ethyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindo1-2(1H)-
yl)methyl)nicotinic
acid
[00121] To a solution of (R)-7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-3-
ethy1-6-(1,3,5-trimethyl-1H-py razol-4-y1)-3,4-dihy d ropy razino indo1-
1(2H)-one (25 mg,
46 mmol) in anhydrous DMF (2 mL) was added NaH (60%) (3 mg, 78 mmol) at 0 C
under
N2 atmosphere and stirred for 10 min. Methyl 6-(bromomethyl)nicotinate (1.7
eq.) was
added to the reaction mixture and stirred for 10 min at 0 C and 1 h at RT.
The reaction
mixture was diluted with ethyl acetate (20 mL), washed with brine (2 X 10 mL),
dried over
anhydrous MgSO4 and concentrated in vacuo. The crude material was subjected to
saponification conditions following General Procedure D followed by
purification by reverse
phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient to 50-95 % MeCN 0.1%
TFA)
to give the title compound (68% yield). MS (ES) 688.3 (M+H) Rf = 0.79.
Example 21
93
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-3-ethyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihyd ropy razino [1,2-a] ind I-2 (1H)-yl)b
enzoic acid
[00122] The title compound (52% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethy 1phenoxy)propy1)-3 -
ethy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino indo1-
1(2H)-one and methyl 3 -
bromobenzoate followed by saponification using General Procedure D. MS (ES)
673.2
(M+H).
Example 22
2-(5-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-
trimethyl-
1H-pyrazol-4-y1)-4,5-dihydro-1H-11 ,4] diazepino 11,2-al indo1-2(31/)-y1)-1-
methy1-1H-
indo1-3-y1)acetic acid
[00123] The title compound (56 mg, 77%) was prepared following General
coupling
procedure A using 8-chloro-11 -(3 -(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5 -trimethyl-
1H-py razol-4-y1)-2,3,4,5-tetrahydro-1H-[1,41 di azepino [1,2-a] ind 01-1 -one
(54 mg, 0.1 mmol),
methyl 2-(5-bromo-1-methy1-1H-indo1-3-y1)acetate (34 mg, 0.12 mmol), Pd2(dba)3
(2 mg,
0.002 mmol), Xantphos (3.5 mg, 0.006 mmol), and Cs2CO3 (49 mg, 0.15 mmol)
followed by
saponification using General Procedure D. LCMS: RT = 1.756 min, MS (ES) 726.3
(M+H).
Example 23
(R)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-3-ethy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-yl)-3,4-dihyd ropyrazino[1,2-a]indo1-2(1H)-y1)benzoic
acid
[00124] The title compound (85% yield) was prepared following General coupling
procedure A using (R)-7-chl oro-10-(3 -(4-chloro-3,5 -dimethy 1phenoxy)propy1)-
3 -ethy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino indo1-
1(2H)-one and methyl 4-
bromobenzoate followed by saponification using General Procedure D. MS (ES)
673.2
(M+H); 1H-NMR (CDC13) 6 8.14 (d, 2H, J = 8 Hz), 8.10 (m, 1H), 7.47 (d, 1H, J =
8 Hz) 7.75
(m, 1H), 7.32 -7.28 ( m, 1H), 6.65 (s, 2H), 4.11 (tr, 2H, J = 8 Hz), 3.97
(multiple s, m, 5H),
3.52-3.34 (m, 2H), 2.33 (s, 6H), 2.30-2.09 (overlapped multiple s, tr, 10H),
1.64 (m, 2H),
0.57 (tr, 3H, J = 8 Hz).
Example 24
(R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-3-ethyl-6-(1,3,5-
trimethyl-
1H-pyrazol-4-y1)-3,4-dihydropyrazino 11,2-al indo1-1(21/)-one
[00125] To a solution of ethyl 6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-7-
(4,6-dimethylpyrimidin-5-y1)-1H-indole-2-carboxylate (1.25 g, 2.37 mmol) in
anhydrous
acetonitrile (20 mL) was added cesium carbonate (1.5 g, 4.74 mmol) follow by
tert-butyl (R)-
94
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
4-ethy1-1,2,3-oxathiazolidine-3-carboxylate 2,2-dioxide (3.8 mmols). The
reaction mixture
was stirred at 80 'C for overnight. Additional cesium carbonate (0.5 eq) and
tert-butyl (R)-4-
ethy1-1,2,3-oxathiazolidine-3-carboxylate 2,2-dioxide (0.5 eq) were added to
the mixture and
the reaction was heated for additional 6 h at 80 'C. The reaction mixture was
cooled to abient
temperature, diluted with ethyl acetate (100 mL). The solution was washed with
brine (3 X
50 mL), dried with anhydrous MgSO4, filtered and concentrated in vacuo. The
residue was
dissolved in anhydrous DCM (30 mL) and cooled to 0 C then TFA (10 eq) was
added
dropwise. The reaction mixture was stirred at room temperature for 4 h. The
reaction mixture
was concentrated to driness then re-dissolved in anhydrous Et0H (20 mL).
Anhydrous
K2CO3 (15 eq.) was added and the reaction mixture was stirred at RT for 2 h
then
concentrated to 1/3 of original volume and diluted with ethyl acetate (100
mL). The solution
was washed with brine (3 X 50 mL), dried over anhydrous MgSO4, filtered and
concentrated
in vacuo. The
residue was purified by flash chromatography (Combi-flash Rf,
DCM/methanol = 0-5% gradient) to give the title compound (80% for 3 steps). MS
(ES)
553.2 (M+H), Rf = 0.84; 11-I-NMR (CDC13) ö 7.66 (d, 1H, J = 8 Hz), 7.24 (d,
1H, J = 8 Hz),
6.65 (s, 2H), 5.77 (m, 1H), 4.03(tr, 2H, J = 8 Hz), 3.99 (s, 3H), 3.52-3.50
(m, 1H), 3.49-3.38
(m, 1H), 3.38-3.34 (m, 2H), 2.35 (s, 6H), 2.19 (tr, 2H, J = 8 Hz ), 2.09 (2s,
6H), 1.59 (m, 2H),
1.26 (tr, 3H, J = 8 Hz).
Example 25
7-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-
trimethy1-1H-
pyrazol-4-yl)-4,5-dihydro-1H-I1,41diazepino[1,2-alindol-2(31/)-y1)-2,3-
dihydrobenzo[b][1,41dioxine-5-carboxylic acid
[00126] The title compound was prepared following General coupling procedure A
using 8-
chlo ro-11-(3-(4-chl oro-3,5-d imethy 1phenoxy)propy1)-7-(1,3,5-trimethy1-1H-
py razol-4-y1)-
2,3,4,5-tetrahy dro-1H- [1,41diazepino [1,2-a] indol-1 -one and
methyl 7-bromo-2,3-
dihydrobenzo[b][1,4]dioxine-5-carboxylate followed by saponification using
General
Procedure D. MS (ES) 717.2 (M+H).
Example 26
(R)-8-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-4-methy1-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino11,2-alindol-2(11/)-y1)-2,3-
dihydrobenzo[b][1,4]dioxine-6-carboxylic acid
[00127] The title compound (45% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino[1,2-a] ind ol-1(2H)-
one and methyl 8-
84451174
bromo-2,3-dihydrobenzo[bi[1,41dioxine-6-carboxylate followed by saponification
using General
Procedure D. MS (ES) 717.2 (M+H).
Example 27
2-48-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-4,5-dihydro-1H-11,4] diazepino [1,2-a] indo1-2(31i)-y1)methyl)-6-
(4-((5-
methylfuran-2-y1)sulfonyl)piperazin-1-y1)isonicotinic acid
[00128] To a solution of methyl 2-((8-chloro-11 -(3 -(4-chl oro-3 ,5-
dimethylphenoxy )propy1)-1 -
oxo-7-(1,3 ,5-trimethy1-1H-py razol-4-y1)-4,5-dihy dro-1H- [1,4] di azepino
[1,2-a] indo1-2(3H)-
yl)methyl)-6-(piperazin-1-ypisonicotinate (15 mg, 0.02 mmol), in CH2C12 (1 mL)
at RT was
added Et3N (6 L, 0.04 mmol) followed by 5-methylfuran-2-sulfonyl chloride (6
ptL, 0.04 mmol)
and the mixture was stirred at room temperature for 1 h. The mixture was
diluted with water (2
mL) and extracted in CH2C12 (3x5 mL), dried and concentrated. The crude
material was
subjected to saponification conditions following General Procedure D and
purified by reverse
phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient to 35-95 % MeCN 0.1%
TFA) to
afford the title compound (13 mg, 69%). LCMS: RT = 2.019 min, MS (ES) 902.3
(M+H).
Example 28
4- [8-chloro-11- [3-(4-chloro-3,5-dim ethyl-phenoxy) propyl] -1-oxo-7-(1,3,5-
trimethylp yrazol-
4-y1)-4,5-dihydro-3H- [1,4] diazepino[1,2-a] indol-2-yl] -2- [2-(dim ethylamin
o)ethyl] indazole-
6-carboxylic acid
Step A. Preparation of methyl 4-bromo-2-(2-(dimethylamino)ethyl)-2H-indazole-6-
carboxylate
[00129] The title compound (28 mg, 22%) was prepared following General
Procedure G using
methyl 4-bromo-1H-indazole-6-carboxylate (100 mg, 0.393 mmol), NaH (15 mg,
0.625 mmol),
and 2-bromo-N,N-dimethylethan-1-amine (120 mg, 0.515 mmol).
Step B. Example 28
[00130] The title compound (22 mg, 51%) was prepared following General
coupling procedure
A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(1,3,5-
trimethyl-1H-pyrazol-
4-y1)-2,3,4,5-tetrahydro-1H-[1,4]diazepino[1,2-alindol-1-one (30 mg, 0.056
mmol), methyl 4-
bromo-242-(dimethylamino)ethyl)-2H-indazole-6-carboxylate (20 mg, 0.061 mmol),
Pd2(dba)3
(5 mg, 0.0054 mmol), Xantphos (8 mg, 0.013 mmol), and Cs2CO3 (60 mg, 0.19
mmol) followed
by saponification using General Procedure D using LiOH (5 mg, 0.21 mmol).
LCMS: RT
1.631 mm, MS (ES) 770.2 (M+H); 1H NMR (400 MHz,
96
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
DMSO-d6) 5 8.71 (s, 1H), 8.24 (d, J = 1.2 Hz, 1H), 7.73 (d, J = 8.6 Hz, 1H),
7.48 (d, J = 1.1
Hz, 1H), 7.27 (d, J= 8.6 Hz, 1H), 6.73 (s, 2H), 4.98 (t, J= 6.7 Hz, 2H), 4.13
(t, J = 6.8 Hz,
2H), 3.98 (t, J = 6.4 Hz, 2H), 3.78 (s, 3H), 3.76-3.74 (m, 4H), 3.09 ¨ 3.00
(m, 2H), 2.77 (s,
3H), 2.76 (s, 3H), 2.24 (s, 6H), 2.13 ¨ 1.99 (m, 5H), 1.93 (s, 3H), 1.75
(quint, J= 8.0 Hz, 2H).
Example 29
(R)-6-(7-chl o ro-10-(3-(4-ch loro-3,5-d imethyl p hen oxy) p ro py1)-3-ethy1-
1-ox 0-641,3,5-
trimethyl- 1H-pyrazol-4-y1)-3,4-dihyd ropyrazino [1,2-a] indo1-2(1H)-y1)-1-
methy1-1H-
ind ole-4-carboxylic acid
[00131] The title compound (45% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-3-
ethy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one
and methyl 6-
bromo-1-methy1-1H-indole-4-carboxylate followed by saponification using
General
Procedure D. MS (ES) 727.2 (M+H).
Example 30
6- 18-Chloro-11-13-(4-chloro-3,5-dimethyl-phenoxy)propy1]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,41diazepino11,2-al ind ol-2-yl] -142-
(dimethylamino)ethyl]indazole-4-carboxylic acid
Step A. Preparation of methyl 6-bromo-1-(2-(dimethylamino)ethyl)-1H-indazole-4-
carboxylate
[00132] The title compound (39 mg, 30%) was prepared following the procedure
described
Example 12, Step A using methyl 6-bromo-1H-indazole-4-carboxylate (100 mg,
0.392 mmol),
NaH (30 mg, 1.25 mmol), 2-bromo-N,N-dimethylethan-1-amine HBr salt (130 mg,
0.56
mmol), and trimethylsilyldiazomethane (2.0 M in Et20, 0.1 mL, 0.2 mmol).
Step B. Example 30
1001331 The title compound (17 mg, 40%) was prepared following General
coupling
procedure A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethyl-
1H-py razol-4-y1)-2,3,4,5-tetrahy dro-1H- [1,41diazepino[1,2-a] indol-1 -one
(30 mg, 0.056
mmol), methyl 6-bromo-1-(2-(dimethylamino)ethyl)-1H-indazole-4-carboxylate (20
mg,
0.061 mmol), Pd2(dba)3 (5 mg, 0.0054 mmol), Xantphos (8 mg, 0.013 mmol), and
Cs2CO3
(60 mg, 0.19 mmol) followed by saponification using General Procedure D using
LiOH (5
mg, 0.21 mmol). LCMS: RT = 1.656 min, MS (ES) 770.3 (M+H); NMR
(400 MHz,
DMSO-d6) 5 8.44 (d, J= 0.8 Hz, 1H), 8.25 (t, J= 1.3 Hz, 1H), 7.90 (d, J = 1.7
Hz, 1H), 7.73
(d, J= 8.6 Hz, 1H), 7.27 (d, J= 8.5 Hz, 1H), 6.74 (s, 2H), 4.97 (t, J= 6.7 Hz,
2H), 4.08 (t, J=
8.0 Hz, 2H), 3.98 (t, J= 6.3 Hz, 2H), 3.83 ¨3.70 (m, 5H), 3.62-3.59 (m, 2H),
3.06 (t, J = 8.0
97
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
Hz, 2H), 2.78 (s, 3H), 2.77 (s, 3H), 2.22 (s, 6H), 2.15 -2.00 (m, 5H), 1.96-
1.83 (m, 5H).
Example 31
6-(8-chlo ro- 11-(3-(4-chlo ro-3,5-d imethyl p henoxy)p ro py1)-1-o xo-7-
(1,3,5-trimethyl- 1H-
pyrazol-4-yl)-4,5-d ihyd ro-1H-11,41diazepino[1,2-al indo1-2(31/)-yl)-1-(2-
morpholinoethyl)-1H-indole-4-carboxylic acid
Step A. Preparation of methyl 6-bromo-1-02-(trimethylsilyl)ethoxy)methyl)-11-1-
indole-
4-carboxylate
[00134] To a solution of methyl 6-bromo-1H-indole-4-carboxylate (200 mg, 0.79
mmol) in
DMF (5 mL) was added NaH (38 mg, 0.95 mmol) and stirred at rt for 15 min. (2-
(Chloromethoxy)ethyl)trimethylsilane (197 mg, 1.2 mmol) was added, and the
resulting
minture was stirred for additional 30 min. The reaction was quenched by
addition of Me0H
(1 mL) then concentrated in vacuo. The residue was purified by flash
chromatography
(Combi-flash Rf, Hex/Et0Ac = 0-25% gradient) to give the title compound (270
mg, 89%) as
a colorless oil. LCMS: RT = 1.939 min, MS (ES) 384.1 (M+H)
Step B. Preparation of methyl 6-(8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propyl)-
1-oxo-7-(1,3,5-trimethy1-1H-pyrazol-4-y1)-4,5-dihydro-1H-11,41diazepino[1,2-
a[indol-
2(3H)-y1)-1-42-(trimethylsilypethoxy)methyl)-1H-indole-4-carboxylate
[00135] The title compound (187 mg, 80%) was prepared following General
coupling
procedure A using 8-chl oro-11 -(3 -(4-chl oro-3,5-di methylphen oxy)pro py1)-
7-(1,3,5 -tri methyl-
1H-py razol-4-y1)-2,3,4,5-tetrahy dro-1H-11,41di azepino[1,2-a] indol-1 -one
(150 mg, 0.278
mmol), methyl 6-bromo-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indole-4-
carboxylate (128
mg, 0.334 mmol), Pd2(dba)3 (5 mg, 0.0054 mmol), Xantphos (8 mg, 0.013 mmol),
and
Cs2CO3 (181 mg, 0.556 mmol). LCMS: RT = 2.461 min, MS (ES) 842.3 (M+H).
Step C. Preparation of methyl 6-(8-chloro-11-(3-(4-chloro-3,5-
d imethy 1p hen oxy)pro pyl)-1-oxo-7-(1,3,5-himethyl- 1H-py razol-4-yl)-4,5-d
ihyd ro-1H-
[1,4] diazepino 11,2-alindo1-2(3H)-yl)-1H-indole-4-carboxylate
[00136] A mixture of methyl 6-(8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-1-
oxo-7-(1,3,5-trimethy1-1H-pyrazol-4-y1)-4,5-dihy dro-1H-11,41 diazepino [1,2-
al indo1-2(3H)-
y1)-142-(trimethylsilypethoxy)methyl)-1H-indole-4-carboxylate (170 mg, 0.202
mmol),
TBAF (2,0 ml, 2.0 mmol) was heated to 100 C under microwave irradiation in
Biotage
Initiator for 20 min. The reaction was quenched by addition of H20 (5 mL) then
extracted
with DCM (10 mL x 2). The combined organic solution was concentrated and
purified by
reverse phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient to 35-95% MeCN
0.1%
TFA) to give the title compound (80 mg, 56%). LCMS: RT = 2.065 min, MS (ES)
712.3
98
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(M+H).
Step D. Example 31
[00137] To a solution of methyl 6-(8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-
1-oxo-7-(1,3,5-trimethy1-1H-py razol-4-y1)-4,5-dihy dro-1H41,41diazepino[1,2-
a]indol-2(3H)-
y1)-1H-indole-4-carboxylate (20 mg, 0.028 mmol) in DMF (0.5 mL) was added NaH
(4.5 mg,
0.11 mmol) then stirred for 20 min. 4-(2-Bromoethyl)morpholine (16 mg, 0.084
mmol) was
added, and the resulting mixture was stirred at rt for 20 h. The reaction was
quenched by
Me0H (0.5 mL), acidified with TFA and purified by reverse phase HPLC
(Phenomenex
Gemini C18, H20/CH3CN gradient to 30-80% MeCN 0.1% TFA) to give the title
compound
(9.8 mg, 43%). LCMS: RT = 1.670 min, MS (ES) 811.3 (M+H).
Example 32
4-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-4,5-dihydro-111-11,41 diazepino 11,2-al indo1-2(3H)-y1)-1-
(methylsulfony1)-
1H-indole-6-carboxylic acid
Step A. Preparation of methyl 4-bromo-1-42-(trimethylsilyDethoxy)methyl)-11-/-
indole-
6-carboxylate e
[00138] The title compounds were prepared according to procedures described in
Example
31 Step A using methyl 4-bromo-1H-indole-6-carboxylate. MS (ES) 384.1 (M+H).
Step B. Preparation of methyl 4-(8-chloro-11-(3-(4-chloro-3,5-
d imethylphen oxy)pro py1)-1-oxo-7-(1,3,5-trimethyl-1H-py razol-4-y1)-4,5-d
ihyd ro-1H-
[1 ,4] diazepino [1,2-al ind ol-2(3H)-y1)-1-02-(trimethylsilyDethoxy)methyl)-
1H-in dole-6-
carboxylate
[00139] The title compound was prepared according to the procedure in Example
31 Step B
substituting methyl 6-bromo-1 -((2-(trimethy lsily Deth oxy )methyl)-1H-ind ol
e-4-carboxy late
with methyl 4-bromo-1-((2-(trimethy lsilypethoxy)methyl)-1H-indole-6-carboxy
late. MS
(ES) 842.3 (M+H).
Step C. Preparation of methyl 4-(8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-trimethyl- 1H- pyrazol-4-y1)-4,5-d ihyd
ro-11-1-
[1,4] diazepino [1,2-a] ind ol-2(3H)-y1)-1H-indole-6-carboxylate
[00140] The title compound (50 mg, 59%) was prepared according to the
procedure in
Example 31 Step C using methyl 4-(8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-
1 -oxo-7-(1,3,5-trimethy1-1H-pyrazol-4-y1)-4,5-dihy dro-1H-[1,4] diazepino
[1,2-a] indo1-2(311)-
y1)-1-42-(trimethylsilypethoxy)methyl)-1H-indole-6-carboxylate (100 mg, 0.12
mmol) and
TBAF (0.593 mL, 0.593 mmol). LCMS: RT = 2.056 min, MS (ES) 712.2 (M+H).
99
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Step D. Example 32.
[00141] To a solution of methyl 4-(8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-
1-oxo-7-(1,3,54rimethy1-1H-py razol-4-y1)-4,5-dihy dro-1H41,41diazepino[1,2-a]
indo1-2(3H)-
y1)-1H-indole-6-carboxylate (25 mg, 0.035 mmol) in DMF (1 mL) was added NaH
(4.21 mg,
0.105 mmol) and stirred for 15 mm. MsC1 (8.20 ill, 0.11 mmol) was added and
the resulting
mixture was stirred overnight. The reaction mixture was concentrated and the
residue was
dissolved into THF (0.3 mL) and LiOH (0.2 mL, 2 N) then stirred at 60 C for 4
h. The
reaction mixture was concentrated, and the residue was purified by reverse
phase HPLC
(Phenomenex Gemini C18, H20/CH3CN gradient to 40-90 % MeCN 0.1% TFA) to give
the
title compound (4 mg, 15%) as an off-white solid. LCMS: RT = 1.979 min, MS
(ES) 775.9
(M+H).
Example 33
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-yl)-3,4-dihydropyrazino11,2-alindol-2(1H)-
yDbenzo[d]11,31dioxole-5-carboxylic acid
[00142] The title compound (42% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razin o[1,2-a] indol-1 (2H)-
one and methyl 7-
bromobenzo [d] [1,3]dioxole-5-carboxylate followed by saponification using
General
Procedure D. MS (ES) 703.2 (M+H).
Example 34
2-08-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-yl)-4,5-dihydro-1H-[1,4] diazepino 11,2-al indo1-2(3H)-yl)methyl)-6-
(4-
(dimethylcarbamoyDpiperazin-1-yDisonicotinic acid
[00143] To a solution of methyl 2-((8-
chloro-11-(3-(4-chloro-3,5-
di methylphenoxy )propy1)-1-oxo-7-(1,3,5-trimethy1-1H-py razol-4-y1)-4,5 -dihy
dro-1H-
[1,41diazepino[1,2-cdindo1-2(31)-yl)methyl)-6-(piperazin-l-ypisonicotinate (15
mg, 0.02
mmol), in CH2C12 (1 mL) at room temperature was added triethylamine (6 [IL,
0.04 mmol)
followed by dimethylcarbamic chloride (4 !AL, 0.04 mmol) then the mixture was
stirred at RT
for 1 h. The mixture was diluted with water (2 mL) and extracted in CH2C12
(3x5 mL), dried
and concentrated. The crude material was subjected to saponification
conditions following
General Procedure D and purified by reverse phase HPLC (Phenomenex Gemini C18,
100
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
H20/CH3CN gradient to 35-95 % MeCN 0.1% TFA) to afford the title compound (11
mg,
66%). LCMS: RT = 1.761 mm, MS (ES) 829.3 (M+H).
Example 35
(R)-2-chloro-4-(7-chloro-10-(3-(4-chloro-3,5-d imethylphenoxy)propy1)-4-methyl-
1-oxo-
6-(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]ind ol-2(1H)-
yl)benzoic
acid
[00144] The title compound (88% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazin o [1,2-a] indol-1 (211)-
one and methyl 4-
bromo-2-chlorobenzoate followed by saponification using General Procedure D.
MS (ES)
693.2 (M+H).
Example 36
4-18-Chloro-11-[3-(4-chloro-3,5-dimethyl-phenoxy)propy1]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,41diazepino [1,2-a] ind 01-2-y1]-2-
methyl-
indazole-6-carboxylic acid
[00145] The title compound (26 mg, 65%) was prepared following General
coupling
procedure A using 8-chl oro-11 -(3 -(4-chl oro-3,5-dimethylphen oxy )p ro py1)-
7-(1,3,5 -trimethyl-
1H-pyrazol-4-y1)-2,3,4,5-tetrahydro-1H41,41diazepino[1,2-c]indol-1-one (30 mg,
0.056
mmol), methyl 4-bromo-2-methyl-2H-indazole-6-carboxylate (20 mg, 0.074 mmol),
Pd2(dba)3 (5 mg, 0.0054 mmol), Xantphos (8 mg, 0.013 mmol), and Cs2CO3 (60 mg,
0.19
mmol) followed by saponification using General Procedure D using LiOH (5 mg,
0.21 mmol).
LCMS: RT = 1.873 min, MS (ES) 713.1 (M+H).
Example 37
5-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-4,5-dihydro-1H-11,41diazepino(1,2-alindo1-2(3H)-y1)-111-1,2,4-
triazole-3-
carboxylic acid
[00146] To a solution of 6-chloro-3-(3-(4-chloro-3,5-dimethylphenoxy )propy1)-
7-(1,3,5-
trimethy1-1H-pyrazol-4-y1)-1H-indole-2-carboxylic acid (50 mg, 0.1 mmol) in
DMF (2 mL)
were added methyl 5-amino-1H-1,2,4-triazole-3-carboxylate (15 mg, 0.1 mmol),
HBTU (76
mg, 0.2 mmol), triethylamine (42 pL, 0.3 mmol), and the mixture was stirred at
RT for 10 h.
Water (5 mL) was added to the mixture and extracted with CH2C12 (3 x 5 mL).
The organic
layer was dried (anhyd. Na2SO4) and concentrated. To the solution crude in DMF
(3 mL)
were added 1,3-dibromopropane (42 pt, 0.4 mmol) and Cs2CO3 (130 mg, 0.4 mmol).
The
mixture was stirred at 40 C for 6 h, filtered, diluted with water (5 mL) and
extracted with
101
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
CH2C12 (3 x 5 mL). The organic layer was dried (arihyd. Na2SO4) and
concentrated in vacuo.
The crude material was subjected to saponification conditions following
General Procedure D
and purified by reverse phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient
to 35-
95 % MeCN 0.1% TFA) to afford the title compound (34 mg, 52%). LCMS: RT =
2.088 min,
MS (ES) 650.2 (M+H).
Example 38
2-((8-Chlo ro-11-(3-(4-chloro-3,5-dimethyl phenoxy)p ro py1)- 1-oxo-7-(1,3,5-
trimethyl- 1H-
pyrazol-4-y1)-4,5-dihydro-1H-11,4] diazepino [1,2-a] ind ol-2(3H)-yl)methyl)-6-
(4-
(phenethylsulfonyl)piperazin-1-yl)isonicotinic acid
[00147] To a solution of methyl 2-((8-
chl oro-11 -(3 -(4-chl oro-3,5-
dimethy 1phenoxy)propy1)-1-oxo-7-(1,3,5-trimethy1-1H-py razol-4-y1)-4,5 -dihy
dro-1H-
[1,4]diazepino[1,2-c]indo1-2(3H)-yOmethyl)-6-(piperazin-l-yDisonicotinate (15
mg, 0.02
mmol), in CH2C12 (1 mL) at room temperature was added triethylamine (6 tL,
0.04 mmol)
followed by 2-phenylethane-1-sulfonyl chloride (8 mg, 0.04 mmol) and the
mixture was
stirred at RT for 1 h. The mixture was diluted with water (2 mL) and extracted
with CH2C12
(3x5 mL), dried and concentrated. The crude material was subjected to
saponification
conditions following General Procedure D and purified by reverse phase HPLC
(Phenomenex
Gemini C18, H20/CH3CN gradient to 35-95 % MeCN 0.1% TFA) to afford the title
compound (17 mg, 92%). LCMS: RT = 1.959 min, MS (ES) 926.3 (M+H).
Example 39
6-18-chloro-11-13-(4-chloro-3,5-d imethyl-phenoxy)p ropy11-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,41diazepino [1,2-al ind ol-2-yl] -1-
ethyl-ind azole-
4-carboxylic acid
Step A. Preparation of methyl 6-bromo-1-ethyl-1H-indazole-4-carboxylate
[00148] The title compound (44 mg, 39%) was prepared following the procedure
described
Example 12, Step A using methyl 6-bromo-1H-indazole-4-carboxylate (100 mg,
0.392 mmol)
and ethyl bromide (85 mg, 0.78 mmoL).
Step B. Example 39
[00149] The title compound (6 mg, 18%) was prepared following General
Procedure A
using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy )propy1)-7-(1,3,5-trimethy1-
1H-py razol-
4-y1)-2,3,4,5-tetrahy dro-1H-[1,4] di azepino [1,2-a] indol-1 -on e (25 mg,
0.046 mmol), methyl
6-bromo-1-ethy1-1H-indazole-4-carboxylate (20 mg, 0.071 mmol), Pd2(dba)3 (4
mg, 0.0044
102
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (60 mg, 0.19 mmol) followed by
saponification using General Procedure D. LCMS: RT = 1.977 min, MS (ES) 727.2
(M+H).
Example 40
4-18-Chloro-11- [3-(4-chloro-3,5-dimethyl-phenoxy)p ropyl]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,41diazepino[1,2-al ind ol-2-yl] -142-
(dimethylamino)ethyl] indazole-6-carboxylic acid
Step A. Preparation of methyl 4-bromo-1-(2-(dimethylamino)ethyl)-1H-indazole-6-
carboxylate
[00150] The title compound (70 mg, 55%) was prepared following the procedure
described
Example 12, Step A using methyl 4-bromo-1H-indazole-6-carboxylate (100 mg,
0.393 mmol)
and 2-bromo-N,N-dimethylethan- 1-amine (120 mg, 0.515 mmol).
Step B. Example 40
[00151] The title compound (26 mg, 60%) was prepared following General
coupling
procedure A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethy1-
1H-py razol-4-y1)-2,3,4,5-tetrahydro-1H41,41 diazepino[1,2-a] indol-l-one (30
mg, 0.056
mmol), methyl 4-bromo-1-(2-(dimethylamino)ethyl)-1H-indazole-6-carboxylate (20
mg,
0.061 mmol), Pd2(dba)3 (5 mg, 0.0054 mmol), Xantphos (8 mg, 0.013 mmol), and
Cs2CO3
(60 mg, 0.19 mmol) followed by saponification using General Procedure D. LCMS:
RT =
1.666 min, MS (ES) 770.3 (M+H).
Example 41
(R)-5-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyI)-3-ethyl-1-oxo-6-
(1,3,5-
trimethyl- 1H-pyrazol-4-y1)-3,4-dihyd ropyrazino [1,2-al indol-2(11/)-y1)- 1-
methyl-1H-
indole-7-carboxylic acid
[00152] The title compound (48% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethy 1phenoxy)propy1)-3 -
ethy1-6-
(1,3 ,5-trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropy razino ind ol-
1(211)-one and methyl 5 -
bromo-1-methy1-1H-indole-7-carboxylate followed by saponification using
General
Procedure D. MS (ES) 726.2 (M+H).
Example 42
6-18-chloro-11-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihyd ro-3H-11,41diazepino11,2-al ind ol-2-yl] -2-
12-
(dimethylamino)ethyll in dazole-4-carboxylic acid
Step A. Preparation of methyl 6-bromo-2-(2-(dimethylamino)ethyl)-2H-indazole-4-
carboxylate
103
CA 03016182 2018-08-29
WO 2017/152076 PCT/US2017/020699
[00153] The title compound (13 mg, 10%) was prepared following the procedure
described
Example 12, Step A using methyl 6-bromo-1H-indazole-4-carboxylate (100 mg,
0.392
mmol), sodium hydride (30 mg, 1.25 mmol), 2-bromo-N,N-dimethylethan-1-amine
HBr salt
(130 mg, 0.56 mmol).
Step B. Example 42
[00154] The title compound (12 mg, 28%) was prepared following General
coupling
procedure A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethyl-
1H-py razol-4-y1)-2,3,4,5-tetrahy dro-1H-[1,41di azepino[1,2-a] indol-1 -one
(30 mg, 0.056
mmol), methyl 6-bromo-2-(2-(dimethylamino)ethyl)-2H-indazole-4-carboxylate (13
mg,
0.039 mmol), Pd2(dba)3 (5 mg, 0.0054 mmol), Xantphos (8 mg, 0.013 mmol), and
Cs2CO3
(60 mg, 0.19 mmol) followed by saponification using General Procedure D. LCMS:
RT =
1.662 min, MS (ES) 770.2 (M+H); 11-1 NMR (400 MHz, DMSO-d6) 6 8.81 (s, 1H),
7.90 ¨
7.83 (m, 1H), 7.80 (d, J = 1.7 Hz, 1H), 7.72 (d, J= 8.6 Hz, 1H), 7.26 (d, J=
8.5 Hz, 1H), 6.73
(s, 2H), 5.03 (t, J = 6.6 Hz, 2H), 4.04 (t, J= 7.1 Hz, 2H), 3.96 (t, J= 6.3
Hz, 2H), 3.78 (s,
3H), 3.69 (dt, J= 25.3, 6.3 Hz, 4H), 3.06 (t, J= 7.4 Hz, 2H), 2.78 (s, 3H),
2.77 (s, 3H), 2.22
(s, 6H), 2.08 (q, J= 6.8 Hz, 2H), 2.02 (s, 3H), 1.91 (s, 3H), 1.79 (q, J= 7.9,
7.3 Hz, 2H).
Example 43
2-((8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-
trimethy1-1H-
pyrazol-4-y1)-4,5-dihydro-1H- [1,4] diazepino[1,2-al indo1-2(3H)-yl)methypis
nicotinic
acid
[00155] To a solution of 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-
7-(1,3,5-
trimethy1-1H-pyrazol-4-y1)-2,3,4,5-tetrahy dro-1H-[1,4] di azepino [1,2-a]
indol-1 -one (54 mg,
0.1 mmol) in DMF (2 mL) was added NaH (5 mg, 0.12 mmol) and the mixture was
stirred at
RT for 10 min. Methyl 2-(bromomethyl)isonicotinate (28 mg, 0.12 mmol, 1.2
eqv.) was
added to the mixture followed by TBAI (37 mg, 0.1 mmol) and stirred for
additional 2 h. The
mixture was diluted with water (5 mL) and extracted with CH2C12 (3 x 5 mL).
The organic
layer was dried over Na2SO4 and concentrated in vacuo. The crude material was
subjected to
saponification conditions following General Procedure D using 2M LiOH (1 mL)
and
purified by reverse phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient to
35-95 %
MeCN 0.1% TFA) to afford the title compound (59 mg, 88%). LCMS: RT = 1.849
min, MS
(ES) 674.2 (M+H).
Example 44
104
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
6-18-chlo ro-11- [3-(4-chlo ro-3,5-d imethyl-phenoxy)p ropy11-1-oxo-7-(1,3,5-
trimethylpyrazol-4-yl)-4,5-dihyd ro-3H-11,41diazepino [1,2-a] ind ol-2-y - 1H-
in d azole-4-
carboxylic acid
Step A. Preparation of methyl 6-bromo-1-42-(trimethylsilypethoxy)methyl)-11-/-
indazole-4-carboxylate
[00156] The title compound (154 mg, 93%) was prepared using the procedure
described in
Example 39 Step A using methyl 6-bromo-1H-indazole-4-carboxylate (110 mg, 0.43
mmol),
(2-(chloromethoxy)ethyl)trimethylsilane (110 mg, 0.65 mmol), and sodium
hydride (10 mg,
0.43 mmol) and purified by flash chromatography (Combi-flash Rf, Hex/Et0Ac = 0-
100%
gradient).
Step B. Preparation of methyl 6-(8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propyl)-
1-oxo-7-(1,3,5-trimethy1-1H-pyrazol-4-yl)-4,5-dihydro-1H- [1,4] diazepino 11,2-
a] ind ol-
2(3H)-y l)-14(2-(trimethylsilypethoxy)methyl)-11-/-ind azo le-4-carb oxylate
[00157] The title compound was prepared following General coupling procedure A
using 8-
chlo ro-11-(3-(4-chl oro-3,5-dimethylphenoxy)p ro py1)-7-(1,3,5-trimethy1-1H-
py razol-4-y1)-
2,3,4,5-tetrahydro-1H41,41diazepino[1,2-a]indol-1-one (30 mg, 0.056 mmol),
methyl 6-
bromo-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indazole-4-carboxylate (32 mg,
0.083
mmol), Pd2(dba)3 (8 mg, 0.0087 mmol), Xantphos (12 mg, 0.021 mmol), and Cs2CO3
(50 mg,
0.15 mmol).
Step C. Example 44
[00158] Methyl 6-(8-chloro-11-(3-(4-chloro-3,5-climethylphenoxy)propy1)-1-oxo-
7-(1,3,5-
trimethyl-1H-pyrazol-4-y1)-4,5-dihydro-1H41,41diazepino[1,2-a]indol-2(3H)-y1)-
1-((2-
(trimethylsilyl)ethoxy)methyl)-1H-indazole-4-carboxylate was dissolved in TBAF
(1.0 M in
THF, 3 mL). The reaction was irradiated in the microwave at 100 C for 1 h.
Water (0.5
mL) and LiOH (5 mg, 0.21 mmol) were added, and the reaction was heated to 50
C for
additional 1 h. The work up described in general coupling procedure A was
followed then
purified by reverse phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient 35-
95 %
MeCN 0.1% TFA) to afford the title compound (8 mg, 20% two Steps). LCMS: RT =
1.837
min, MS (ES) 699.3 (M+H).
Example 45
4-18-chloro-11-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,41diazepino[1,2-a]indol-2-y11-1-
methyl-
indazole-6-carboxylic acid
105
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00159] The title compound (21 mg, 52%) was prepared following General
coupling
procedure A using 8-chl oro-11 -(3 -(4-chl oro-3,5-dimethy 1phenoxy)p ropy1)-7-
(1,3,5 -trimethyl-
1H-py razol-4-y1)-2,3,4,5-tetrahy dro-1H-11,41diazepino[1,2-a] indol-1 -one
(30 mg, 0.056
mmol), methyl 4-bromo-1-methy1-1H-indazole-6-carboxylate (20 mg, 0.074 mmol),
Pd2(dba)3 (5 mg, 0.0054 mmol), Xantphos (8 mg, 0.013 mmol), and Cs2CO3 (60 mg,
0.19
mmol) followed by saponification using General Procedure D. LCMS: RT = 1.849
min, MS
(ES) 713.2 (M+H), IHNMR (400 MHz, DMSO-d6) 5 8.25 (s, 1H), 8.06 (d, J= 1.0 Hz,
1H),
7.73 (d, J= 8.6 Hz, 1H), 7.58 (d, J= 1.1 Hz, 1H), 7.27 (d, J= 8.6 Hz, 1H),
6.72 (s, 2H), 4.15
(s, 3H), 4.12 (t, J= 6.0 Hz, 2H) 3.97 (t, J=- 6.3 Hz, 2H), 3.78 (s, 3H), 3.76
(t, J= 3.8 Hz, 2H),
3.04 (d, J= 8.0 Hz, 2H), 2.23 (s, 6H), 2.13-2.00(m, 5H), 1.93 (s, 3H), 1.75
(m, 2H).
Example 46
2-(6-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-
trimethyl-
1H-pyrazol-4-y1)-4,5-dihydro-1H-11,41diazepino [1,2-a] indol-2(3H)-yl)-3,4-
dihyd roquinolin-1(2H)-yl)acetic acid
[00160] To a solution of 6-chloro-3-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-1H-indole-2-carboxylic acid (50 mg, 0.1 mmol) in
DMF (2 mL)
were added tert-butyl 6-amino-3,4-dihydroquinoline-1(2H)-carboxylate (25 mg,
0.1 mmol),
HBTU (76 mg, 0.2 mmol), triethylamine (42 gL, 0.3 mmol), and the mixture was
stirred at
RT for 10 h. Water (5 mL) was added to the mixture and it was extracted in
CH2C12 (3 x 5
mL). The organic layer was dried over Na2SO4 and concentrated. To the solution
of crude in
DMF (3 mL) were added 1,3-dibromopropane (42 L, 0.4 mmol), Cs2CO3 (130 mg,
0.4
mmol), and the mixture was stirred at 40 C for 6 h. The mixture was filtered,
diluted with
water (5 mL) and extracted with CH2C12 (3 x 5 mL). The organic layer was dried
over
Na2SO4 and concentrated. The crude residue was dissolved in CH2C12 (2 mL) and
TFA (2 mL)
was added. The mixture was stirred at room temperature for 6 h. Saturated
aqueous NaHCO3
(8 mL) was added to the mixture and extracted with DCM (3 x 5 mL). The organic
layer was
dried over Na2SO4, and concentrated. To a solution of crude in DMF (1 mL) was
added NaH
(5 mg, 0.12 mmol) and the mixture was stirred at room temperature for 10 min.
Ethyl
bromoacetate (14 gL, 0.12 mmol) was added to the mixture and stirred at RT for
2 h. The
reaction mixture was quenched with water (5 mL) and extracted with CH2C12 (3 x
5 mL). The
organic layer was dried over Na2SO4 and concentrated in vacuo. The crude
material was
subjected to saponification conditions following General Procedure D and
purified by reverse
phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient to 35-95 % MeCN 0.1%
TFA)
to afford the title compound (15 mg, 21%). LCMS: RT = 2.023 min, MS (ES) 728.2
(M+H).
106
84451174
Example 47
248-C hloro-11-(3-(4-ch loro-3,5-dim ethylphenoxy)pr opyl)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-yl)-4,5-dihy dro-1H-11,4] d iazep ino [1,2-a] indo1-2(3H)-yl)methyl)-
6-(4-
(cyclopropylsulfonyl)piperazin-1-yl)isonicotinic acid
1001611 The title compound (10 mg, 58%) was prepared following the procedure
described
Example 38 using methyl 2 -((8-chloro-11 -(3 -(4-chloro-3,5 -
dimethylphenoxy)propy1)-1- oxo-7-
(1,3,5-trimethyl- 1H-pyrazol-4 -y1)-4,5-dihy dro-1H-[1,4] di azepino [1,2-al
indo1-2(31/)-y pmethyl)-
6-(piperazin-1-ypisonicotinate (15 mg, 0.02 mmol) and cyclopropanesulfonyl
chloride (6 ttL,
0.04 mmol) followed by saponification conditions following General Procedure
D. LCMS: RT
= 1.931 min, MS (ES) 862.3 (M+H).
Example 48
(R)-3-(7-chloro-10-(3-(4-chlor o-3,5-dimethylph enoxy)p ropy1)4-methyl-l-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-yl)-3,4-dihyd ropyrazino [1,2-a] ind ol-2(1H)-yl)-1-
methyl-1H-
indazole-6-carboxylic acid
[00162] The title compound (40% yield) was prepared following General coupling
procedure
A using (R)-7-chloro-10-(3 -(4 -chloro-3,5 -dimethy 1phenoxy )propy1)-4-methy1-
6-(1,3,5-trimethyl-
1H-pyrazol-4-y1)-3,4-dihy dropy razino [1,2 -a] indo1-1(21/)- one and methyl 3
-bromo- 1-methyl-1H-
indazole-6-carboxylate followed by saponification using General Procedure D.
MS (ES) 713.2
(M+H).
Example 49
3-(8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-yl)-4,5-dihydro-1H41,4]diazepino[1,2-a]indo1-2(3H)-yl)-1-(2-
morpholinoethyl)-
1H-indazole-6-carboxylic acid
Step A. Preparation of methyl 3-bromo-1-(2-morpholinoethyl)-1H-indazole-6-
carboxylate
[00163] To a solution of methyl 3-bromo-1H-indazole-6-carboxylate (0.27 mmol)
in DMF (3
mL) was added sodium hydride (10 mg, 0.69 mmol), and the reaction was stirred
5 min at
RT. 4-(2-Chloroethyl)morpholine (1.1 mmol) was added, and the reaction was
stirred for
additional 1 h. The reaction was diluted with Et0Ac (10 mL), washed with H20
(3 x 10 mL),
dried over MgSO4 and concentrated in vacuo. The residue was purified by flash
chromatography
(Combi-flash Rf, Hex/Et0Ac = 0-40% gradient) to afford the title compound (76
mg, 75 %).
Step B. Example 49
107
Date Recue/Date Received 2023-06-16
84451174
1001641 A flame dried flask was charged with Pd2(dba)3 (1 mg, 0.5 mol%),
Xantphos (2 mg, 1
mol%), cesium carbonate (27 mg, 0.084 mmol), 8-chloro-11-(3-(4-chloro-3,5-
dimethy 1phenoxy)propy1)-7-(1,3,5-trimethy1-1H-pyrazol-4-y1)-2,3,4,5-
tetrahydro-1H-
[1,41diazepino[1,2-alindo1-1-one (30 mg, 0.056 mmol), methyl 3-bromo-1-(2-
morpholinoethyl)-
1H-indazole-6-carboxylate (24 mg, 0M67 mmol), and 1,4-dioxane (1 mL). The
reaction mixture
was degassed for 10 min under argon and stirred for 16 h at 110 C then
concentrated in vacuo.
The residue was filtered through silica pad with DCM/Me0H (3/1) then
concentrated in vacuo.
To a solution of crude methyl 3 -(8-chloro-11 -(3-(4-chloro -3,5-dimethy
1phenoxy)propy1)-1 -oxo-
7-(1,3,5-trimethyl -1H-pyrazol-4-y1)-4,5-dihy dro-1H - [1,4] diazepino [1,2-
ali ndo1-2(3H)-y1)-1 -(2 -
morpholinoethyl)-1H-indazole-6-carboxylate (0.056 mmol) in mixture of methanol
and dioxane
(1 mL / 2 mL) was added sodium hydroxide (0.2 mL, 2M solution). The reaction
mixture was
stirred for 3h at room temperature and then concentrated in vacuo. The residue
was purified by
reverse phase preparatory HPLC (Phenomenex Gemini C18, H20/CH3CN gradient to 5-
95%
CH3CN 0.1% TFA) to afford the title compound (17 mg, 36 %). LCMS: RT = 0.669
min (non
polar method, >99%, ELSD); MS (ES) 812.2 [M+111; 1H NMR (DMSO, 400 MHz) 8
(ppm) 8.41
(s, 1H), 7.79 (d, J= 8.4 Hz, 1H), 7.74 (d, J= 8.4 Hz, 1H), 7.69 (dd, J= 8.4,
1.2 Hz, 1H), 7.28 (d,
J= 8.8 Hz, 1H), 6.71 (s, 2H), 4.92-4.79 (m, 2H), 4.05 (t, J= 6.0 Hz, 2H), 3.99-
3.95 (m, 4H),
3.85 (t, Jr 6.0 Hz, 2H), 3.76 (s, 3H), 3.71-3.55(m, 6H), 3.26-3.10 (m, 2H),
3.07 (t, J= 6.8 Hz,
2H), 2.21 (s, 6H), 2.12-2.01 (m, 2H), 2.02 (s, 3H), 1.92 (s, 3H), 1.85-1.62
(m, 2H).
Example 50
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-l-oxo-6-
(1,3,5-
trimethy1-1H-pyraz ol-4-y1)-3,4-dihydropyrazin o [1,2-a] indo1-2(111)-yl)-1-(2-
morpholinoethyl)-1H-indazole-6-carboxylic acid
1001651 The title compound (40 mg, 87 %) was prepared according to procedures
described in
Example 49 Step B by substituting 8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-7-
(1,3,5-trimethyl- 1H-pyrazol-4 -y1)-2,3,4,5 -tetrahy dro-1H-[1,4] di azepino
[1,2-a] indol-1 -one with
(R)-7-chloro-10-(3-(4-chloro-3,5-dimethy 1phenoxy )propy1)-4-methy1-6-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a]indol-1(2H)-one (30 mg, 0.056 mmol).
LCMS: RT
0.697 min (non polar method, >99%, ELSD); MS (ES) 812.3 [M+111; 1H NMR (DMSO,
400
MHz) ö (ppm) 8.41 (s, 1H), 7.78 (d, J= 8.4 Hz, 1H), 7.75 (d, J= 8.4 Hz, 1H),
7.72 (dd, J= 8.4,
1.2 Hz, 1H), 7.31 (d, J= 8.4 Hz, 1H), 6.71 (s, 2H), 4.91-4.85 (m, 2H), 4.48-
4.43 (m, 2H), 4.25-
4.18 (m, 2H), 4.02-3.99
108
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(m, 4H), 3.96-3.92 (m, 1H), 3.77 (s, 3H), 3.75-3.51 (m, 4H), 3.39-3.16 (m,
4H), 3.23 (s, 6H),
2.10 (s, 1.5H), 2.10 (s, 1.5H), 2.10-1.97 (m, 2H), 1.89 (s, 1.5H), 1.02 (d, J=
6.0 Hz, 3H).
Example 51
(R)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-2(1H)-y1)-1-methyl-
1H-
indole-7-carboxylic acid
[00166] The title compound (84% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
and methyl 4-
bromo-1-methy1-1H-indole-7-carboxylate followed by saponification using
General
Procedure D. MS (ES) 712.3 (M+H); 1H-NMR (CDC13) 6 7.89 (d, 1H, J = 8 Hz),
7.79 (d, 1H,
J = 8 Hz), 7.34 (d, 1H, J = 8 Hz) 7.11 (m, 1H), 7.02 (m, 1H), 6.62 (s, 2H),
6.40 (m, 1H), 4.5
(m, 1H), 4.18 (tr, 2H, J = 8 Hz), 4.02 (s, 3H), 3.97 (s, 3H), 3.90-3.88 (m,
2H), 3.52-3.36 (m,
2H), 2.33 (s, 6H), 2.30-2.09 (overlapped multiple s, tr, 8H), 1.28 (two d, 3H,
J = 8 Hz).
Example 52
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino [1,2-a] indo1-2(11/)-y1)-1-
methyl-1H-
ind ole-4-carboxylic acid
[00167] The title compound (84% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one
and methyl 7-
bromo-1-methy1-1H-indole-4-carboxylate followed by saponification using
General
Procedure D. MS (ES) 712.3 (M+H); 1H-NMR (CDC13) 6 7.95 (d, 1H, J = 8 Hz),
7.75 (d, 1H,
J = 8 Hz), 7.35 (d, 1H, J = 8 Hz) 7.13 (m, 1H), 7.02 (m, 1H), 6.99 (m, 1H),
6.62 (s, 2H), 4.02
(s, 3H), 4.00 (tr, 2H, J = 8 Hz), 3.78 (s, 3H), 3.90-3.88 (m, 1H), 3.52-3.35
(m, 2H), 2.33 (s,
6H), 2.30-2.18 (overlapped multiple s, tr, m, 10H), 1.28 (two d, 3H, J = 8
Hz).
Example 53
(R)-6-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino [1,2-a] in d ol-2(1H)-y1)-1 -
methyl-1H-
indazole-4-carboxylic acid
[00168] The title compound (6.0 mg, 18%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(210-one
(25 mg, 0.046
mmol), methyl 6-bromo-1-methy1-1H-indazole-4-carboxylate (20 mg, 0.074 mmol),
109
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Pd2(dba)3 (4 mg, 0.0044 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (60 mg,
0.19
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.014
min, MS
(ES) 713.2 (M+H).
Example 54
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino [1,2-a] indo1-2(11-1)-y1)-2-
methy1-2H-
ind azole-3-carb oxylic acid
[00169] The title compound (7.3 mg, 22%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(25 mg, 0.046
mmol), methyl 7-bromo-2-methyl-2H-indazole-3-carboxylate (20 mg, 0.074 mmol),
Pd2(dba)3 (4 mg, 0.0044 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (60 mg,
0.19
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.066
min, MS
(ES) 713.2 (M+H).
Example 55
7-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-tri
methyl-1H-
pyrazol-4-y1)-4,5-dihyd ro-1H- [1,41diazepin o[1,2-al ind ol-2(31/)-y1)-1-
methyl-1H-indole-
4-carboxylic acid
[00170] The title compound (25mg, 62%) was prepared following General coupling
procedure A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethyl-
1H-py razol-4-y 0-2,3,4,5-tetrahy dro-1H-[1,41di azepin o [1,2-a] ind 01-1 -
one and methyl 7-
bromo-1-methy 1-1H-indol e-4-carboxylate followed by saponification using
General
Procedure D. MS (ES) 712.3 (M+H); 11-1 NMR (DMSO, 400 MHz) (ppm) 7.73 (s, 1H),
7.70 (s, 1H), 7.44 (d, J = 2.8 Hz, 1H), 7.26 (d, J= 8.4 Hz, 1H), 7.10 (dd, J=
12.0, 8.0 Hz,
1H), 7.02 (d, J= 3.2 Hz, 1H), 6.73 (s, 2H), 4.20-4.08 (m, 2H), 4.00 (t, J= 6.0
Hz, 2H), 3.79
(s, 3H), 3.77 (s, 3H), 3.75-3.68 (m, 1H), 3.53-3.45 (m, 1H), 3.16-3.08 (m,
1H), 2.99- 2.91 (m,
1H), 2.25 (s, 6H), 2.15-2.03 (m, 2H), 2.05 (s, 1.5H), 2.00 (s, 1.5H), 1.94 (s,
1.5H), 1.89 (s,
1.5H), 1.8-1.6 (m, 2H).
Example 56
(R)-6-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-al indo1-2(11/)-y1)-1-(2-
morpholinoethyl)-1H-indazole-4-carboxylic acid
Step A. Preparation of methyl 6-bromo-1-(2-morpholinoethyl)-1H-indazole-4-
carboxylate
110
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00171] The title compound (74 mg, 26%) was prepared following General
Procedure G
using methyl 6-bromo-1H-indazole-4-carboxylate (200 mg, 0.784 mmol), NaH (36
mg, 1.5
mmol), and 4-(2-bromoethyl)morpholine (225 mg, 1.16 mmol).
Step B. Example 56
[00172] The title compound (7.1 mg, 19%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razin o[1,2-a] indo1-1(211)-
one (25 mg, 0.046
mmol), methyl 6-bromo-1-(2-morpholinoethyl)-1H-indazole-4-carboxylate (30 mg,
0.081
mmol), Pd2(dba)3 (4 mg, 0.0044 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3
(60 mg,
0.19 mmol) followed by saponification using General Procedure D. LCMS: RT =
1.783 min,
MS (ES) 811.9 (M+H).
Example 57
(R)-6-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-yl)-3,4-dihydropyrazino 11,2-al indol-2(1H)-yl)-2-(2-
morpholinoethyl)-2H-indazole-4-carboxylic acid
Step A. Preparation of methyl 6-bromo-2-(2-morpholinoethyl)-2H-indazole-4-
carboxylate
[00173] The title compound (42 mg, 15%) was prepared following General
Procedure G
using methyl 6-bromo-1H-indazole-4-carboxylate (200 mg, 0.784 mmol), NaH (36
mg, 1.5
mmol), and 4-(2-bromoethyl)morpholine (225 mg, 1.16 mmol).
Step B. Example 57
[00174] The title compound (5.4 mg, 14%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino[1,2-c]indo1-1(210-
one (25 mg, 0.046
mmol), methyl 6-bromo-2-(2-morpholinoethyl)-2H-indazole-4-carboxylate (30 mg,
0.081
mmol), Pd2(dba)3 (4 mg, 0.0044 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3
(60 mg,
0.19 mmol) followed by saponification using General Procedure D. LCMS: RT =
1.726 min,
MS (ES) 812.3 (M+H).
Example 58
6-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(3,5-dimethyl-1H-
pyrazol-
4-y1)-1-oxo-4,5-dihydro-11/41,41diazepino[1,2-a[ind ol-2(31/)-y1)-1-methyl-1H-
indole-4-
carboxylic acid
Step A. Preparation of 3,5-dimethy1-444,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-
y1)-1-
((2-(trimethylsilyl)ethoxy)methyl)-1H-pyrazole
111
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00175] To a solution of 3,5-dimethy1-4-(4,4,5,5-tetramethy1-1,3,2-
dioxaborolan-2-y1)-1H-
pyrazole (6.456 g, 28.67 mmol) in DMF (57 ml) was added NaH (1.72 g, 43.0
mmol) and
stirred for 15 min. (2-(Chloromethoxy)ethyl)trimethylsilane (6.59 ml, 37.28
mmol) was
added to the reaction mixture and stirred at RT for additional 2 h. The
reaction was diluted
with DCM (50 mL) then quenched by H20 (50 mL). The layers were separated and
aqueous
phase was extracted with DCM (2 x 50 mL). The combined organic layer was dried
over
MgSO4 and concentrated in vacuo. The residue was purified by flash
chromatography
(Combi-flash Rf, DCM/Me0H = 0-10% gradient) to afford the title product (5.23
g, 52 %).
LCMS: RT = 1.318 min, MS (ES) 353.2 (M+H).
Step B. Preparation of ethyl 6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-7-
(3,5-dimethy1-1-42-(trimethylsilypethoxy)methyl)-1H-pyrazol-4-y1)-11-/-indole-
2-
carboxylate
[00176] A solution of ethyl 7-
bromo-6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-1H-indole-2-carboxylate (1.76 g, 3.55 mmol), 3,5-
dimethy1-4-
(4,4,5,5 -tetramethy1-1,3,2-di oxab orol an-2-y1)-1-((2-(trimethy ls
ilypethoxy)methyl)-1H-
pyrazole (1.50 g, 4.26 mmol), Pd(PPh3)4 (0.250 g, 0.178 mmol) and K2CO3 (1.47
g, 10.65
mmol) in 1,4-dioxane (12 mL) and water (4 mL) was irradiated at 140 C for 45
min under
microwave. The reaction mixture was concentrated in vacuo and purified by
flash
chromatography (Combi-flash Rf, Hex/Et0Ac = 0-70% gradient) to afford the
title as a white
solid (1.60 g, 70 %). LCMS: RT = 1.30 min, MS (ES) 644.2 (M+H).
Step C. Preparation of ethyl 1-(3-((tert-butoxycarbonyl)amino)propy1)-6-chloro-
3-(3-(4-
chlo ro-3,5-dimethylp hen oxy)p ropyl)-743,5-dimethyl- 1-02-
(trimethylsilypeth oxy)methyl)- 1H-pyrazol-4-yl)-1H-ind ole-2-ca rb oxylate
[00177] To a solution of ethyl 6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-7-
(3,5 -dimethy1-1-02-(trimethy ls ily Dethoxy)methyl)-1H-py razol-4-y1)-1H-
indole-2-
carboxylate (150 mg, 0.233 mmol) and tert-butyl 1,2,3-oxathiazinane-3-
carboxylate 2,2-
dioxide (66.2 mg, 0.279 mmol) in DMF (2 ml) was added NaH (12 mg, 0.30 mmol),
and the
resulting mixture was stirred at it for 2 h. Satureated aq. NH4C1 (20 mL) was
added to quench
the reaction. The mixture was extracted with DCM (20 mL x 3). The combined
organics were
concentrated, and the residue was purified by flash chromatography (Combi-
flash Rf,
Hex/Et0Ac = 0-30% gradient) to give the title product. LCMS: RT = 2.232 min,
MS (ES)
801.4 (M+H).
112
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Step D. Preparation of ethyl 1-(3-aminopropy1)-6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propyl)-7-(3,5-dimethyl-1-((2-(trimethylsilyl)ethoxy)methyl)-
1H-
pyrazol-4-yl)-1H-indole-2-carboxylate
[00178] To a stirred solution of ethyl 1-(3-((tert-butoxy
carbonyl)amino)propy1)-6-chloro-3-
(3 -(4-chl oro-3 ,5-dimethy 1phenoxy )propy1)-7-(3,5-d imethyl-1 -((2-
(trimethylsilyl)ethoxy )methyl)-1H-pyrazol-4-y1)-1H-indole-2-carboxylate (115
mg, 0.143
mmol) in DCM (3 mL) was added TFA (0.055 mL, 0.717 mmol), and the resulting
solution
was stirred at 40 C overnight. The reaction was quenched with saturated aq.
NaHCO3 (5
mL), the mixture was extracted with DCM (10 mL x 3). The combined organic
solution was
dried and concentrated to give the crude title compound, which was used for
the next step
without further purification. LCMS: RT = 1.288 min, MS (ES) 701.4 (M+H).
Step E. Preparation of 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-
(3,5-
d imethy l-1-42-(trimethy lsilypethoxy)methyl)-1H-pyrazo l-4-yl)-2,3,4,5-
tetrahy d ro- 1H-
[1,4] d iazepino[1,2-a] indol-1-one
[00179] To a solution of ethyl 1-(3-aminopropy1)-6-chloro-3-(3-(4-chloro-3,5-
di methylphenoxy )propy1)-7-(3,5-dimethy1-1-((2-(trimethylsilyl)ethoxy)methyl)-
1H-pyrazol-
4-y1)-1H-indole-2-carboxylate (100 mg, 0.142 mmol) in Me0H (2 mL) was added
K2CO3 (98
mg, 0.712 mmol), and the mixture was stirred for 2 h at 50 C then filtered.
The filtrate was
concentrated and purified by flash chromatography (Combi-flash Rf, Me0H/DCM =
0-10%
gradient) to give the title compound (74 mg, 79% two steps). LCMS: RT = 1.617
min, MS
(ES) 655.3 (M+H).
Step F. Preparation of methyl 6-(8-chloro-1143-(4-chloro-3,5-
dimethylphenoxy)propyl)-
7-(3,5-dimethyl-1-42-(trimethylsilypethoxy)methyl)-1H-pyrazol-4-yl)-1-oxo-4,5-
dihydro-11-/-11,41diazepino [1,2-alind 01-2(3H)-yl)-1-methyl-1H-ind ole-4-
carboxylate
[00180] The title compound (38 mg, 34%) was prepared following General
coupling
procedure A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(3,5-
dimethy1-1-
((2-(trimethylsilypethoxy)methyl)-1H-pyrazol-4-y1)-2,3,4,5-tetrahy dro-1H-
[1,4] di azepi no [1,2-a] ind 01-1 -one (74 mg, 0.113 mmol), methyl 6-bromo-1 -
methy 1-1H-indol e-
4-carboxylate (36.3 mg, 0.135 mmol), Pd2(dba)3 (2 mg, 0.0023 mmol), Xantphos
(3.9 mg,
0.0068 mmol), and Cs2CO3 (74 mg, 0.23 mmol). LCMS: RT = 1.807 min, MS (ES)
842.4
(M+H).
Step G. Example 58
[00181] A mixture of methyl 6-(8-chl oro-11 -(3-(4-chl oro-3,5 -
dimethylphenoxy )propy1)-7-
(3,5 -dimethy1-14(2-(trimethy ls ilypethoxy )methyl)-1H-py razol-4-y1)-1-oxo-
4,5-dihy dro-1H-
113
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[1,4]diazepino[1,2-a]indo1-2(3H)-y1)-1-methyl-1H-indole-4-carboxylate (32 mg,
0.038
mmol) and TBAF (380 1, 0.380 mmol) was heated to 100 C under microwave
irradiation in
Biotage Initiator for 30 min. The reaction mixture was concentrated, and the
residue was
purified by reverse phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient to
40-85
% MeCN 0.1% TFA) to give the title compound (16 mg, 60%). LCMS: RT = 1.852
min, MS
(ES) 698.2 (M+H).
Example 59
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino[1,2-a] indo1-2(1H)-y1)-1-
methy1-1H-
ind azole-3-carboxylic acid
[00182] The title compound (6.2 mg, 18%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino[1,2-a] ind ol-1 (2H)-
one (25 mg, 0.046
mmol), methyl 7-bromo-1-methy1-1H-indazole-3-carboxylate (20 mg, 0.074 mmol),
Pd2(dba)3 (4 mg, 0.0044 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (60 mg,
0.19
mmol) followed by saponification using General Procedure D. LCMS: RT = 1.984,
2.014
min, MS (ES) 713.2 (M+H); 1-1-1NMR (400 MHz, Chloroform-d) 5 8.22 ¨ 8.11 (m,
1H), 7.67
¨7.59 (m, 1H), 7.34¨ 7.23 (m, 2H), 7.19¨ 7.07 (m, 1H), 6.53 (s, 1.3H), 6.50
(s, 0.7H), 4.50-
4.32 (m, 1H), 4.28 ¨4.17 (m, 1H), 4.14 (d, J= 3.0 Hz, 1H), 4.06 (s, 1H), 4.01
(s, 1H), 3.96 ¨
3.82 (m, 5H), 3.58 ¨ 3.20 (m, 3H), 2.24 (s, 6H), 2.19- 2.15 (m, 3H), 2.14-2.07
(m, 2H), 2.03
(s, 0.7H), 2.02 (s, 0.3H), 1.98 (s, 2H), 1.25 (d, J = 6.4 Hz, 0.6H), 1.19 (d,
J = 6.4 Hz, 1.4H),
1.14 (d, J = 6.4 Hz, 0.3H), 1.08 (d, J6.4 Hz 0.7H).
Example 60
Methyl 8-(8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-
trimethyl-1H-pyrazol-4-y1)-4,5-dihydro-1H- [1,4] d iazep ino[1,2-al ind ol-
2(31/)-y1)-2,3-
dihyd robenzo [ b [1,4] dioxine-6-carboxylate
[00183] The title compound was prepared following General coupling procedure A
using 8-
chl oro-11-(3-(4-chl oro-3,5-dimethylphenoxy )propy1)-7-(1,3,5-trimethy1-1H-py
razol-4-y1)-
2,3,4,5 -tetrahy dro-1 H-[1,4] di azepino [1,2-a] indol-l-one, methyl
8-bromo-2,3-
dihydrobenzo[b][1,4]dioxine-6-carboxylate. MS (ES) 731.3 (M+H).
Example 61
5-18-chloro-1143-(4-chloro-3,5-dimethyl-phenoxy)propy11-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-I1,4]diazepino11,2-al indo1-2-y1]-4-
methy1-2,3-
dihydro-1,4-benzoxazine-7-carboxylic acid
114
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Step A. Preparation of methyl 5-bromo-3,4-dihydro-2H-benzo[b]11,41oxazine-7-
carboxylate
[00184] To a solution of methyl 4-amino-3-bromo-5-hydroxy benzoate (250 mg,
1.02
mmol) in DMF (5 mL) was added dibromoethane (350 mg, 1.9 mmol) and K2CO3 (300
mg,
2.2 mmol). The reaction was heated to 80 C for 20 h then cool to RT. The
reaction was
diluted with DCM/H20 (40 mL, 1:1), the organic layer separated, and the
aqueous layer was
extracted with DCM (2 x 20 mL). The combined organic layers were dried over
MgSO4,
filtered, and concentrated in vacuo. The crude reaction mixture was purified
by flash
chromatography (Combi-flash Rf, Hex/Et0Ac = 0-100% gradient) to afford the
title
compound (90 mg, 32%).
Step B. Preparation of methyl 5-bromo-4-methyl-3,4-dihydro-2H-
benzo[b][1,4]oxazine-
7-carboxylate
[00185] The title compound (35 mg, 74%) was prepared following General
Procedure G
using methyl 5-bromo-3,4-dihydro-2H-benzo [b][1,4]oxazine-7-carboxylate (45
mg, 0.17
mmol), sodium hydride (9 mg, 0.38 mmol), and methyl iodide (40 mg, 0.28 mmol).
Step C Example 69
[00186] The title compound (2.3 mg, 7%) was prepared following General
coupling
procedure A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethyl-
1H-py razol-4-y 0-2,3,4,5-tetrahy dro-1H-[1,41di azepino [1,2-a] indol-1 -one
(25 mg, 0.046
mmol), methyl 5-bromo-4-methyl-3,4-dihydro-2H-benzo[b][1,4]oxazine-7-
carboxylate (25
mg, 0.087 mmol), Pd2(dba)3 (4 mg, 0.0044 mmol), Xantphos (6 mg, 0.010 mmol),
and
Cs2CO3 (60 mg, 0.19 mmol) followed by saponification using General Procedure
D. LCMS:
RT = 1.953 mm, MS (ES) 729.9 (M+H).
Example 62
3-18-chloro-11-13-(4-chloro-3,5-d imethyl-phenoxy)p ropyl1-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,41diazepino [1,2-a] ind ol-2-yl] -4-
(2-
hydroxyethoxy)-5-methoxy-benzoic acid
Step A. Preparation of methyl 3-bromo-4-(2-((tert-
butyldimethylsilyl)oxy)ethoxy)-5-
meth oxybenzoate
[00187] The title compound (43 mg, 27%) was prepared following the same
procedure as
described in Example 66 Step A using methyl 3-bromo-4-hydroxy-5-
methoxybenzoate (100
mg, 0.381 mmol), Cs2CO3 (200 mg, 0.615 mmol) and (2-bromoethoxy)(tert-
butyl)dimethylsilane (130 mg, 0.543 mmol).
Step B. Example 62
115
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
1001881 Crude Methyl 4-(2-
((tert-butyldimethy lsily poxy)ethyl)-3-(8-chloro-11 -(3 -(4-
chlo ro-3,5-di methy 1phenoxy )propy1)-1-oxo-7-(1,3,5-trimethy1-1H-py razol-4-
y1)-4,5 -dihy d ro-
1H41,41diazepino[1,2-alindol-2(3H)-y1)-5-methoxybenzoate was prepared
following
General coupling procedure A using 8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-
7-(1,3,5-trimethy1-1H-py razol-4-y1)-2,3,4,5-tetrahy dro-1H41,41diazepino[1,2-
a] indol-1 -one
(20 mg, 0.037 mmol), methyl 3-bromo-4-(2-((tert-butyldimethylsilyl)oxy)ethoxy)-
5-
methoxybenzoate (30 mg, 0.068 mmol), Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos
(8 mg,
0.013 mmol), and Cs2CO3 (50 m, 0.153 mmol). To a solution of crude product in
THF (3
mL) was added TBAF (1.0 M, 0.05 mL, 0.05 mmol). The reaction was stirred at 40
C for 1
h then diluted into DCM/H20 (10 mL, 1:1), extracted with DCM (2 x 5 mL) and
concentrated
in vacuo, The residue was then subjected to General Procedure D to afford the
title
compound (2 mg, 7%). LCMS: RT = 1.858 min, MS (ES) 749.2 (M+H).
Example 63
(R)-547-Chloro-104344-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] in d ol-2(11/)-y1)-1-
nap hthoic acid
[00189] The title compound (31 mg, 87%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razin o[1,2-a] indol-1 (2H)-
one (27 mg, 0.05
mmol), methyl 5-bromo-1-naphthoate (16 mg, 0.06 mmol), Pd2(dba)3 (1 mg, 0.001
mmol),
Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification
using General Procedure D. LCMS: RT = 2.083 min, MS (ES) 709.2 (M+H).
Example 64
2-(1,2-benzoxazol-6-y1)-8-chloro-11- [3-(4-chloro-3,5-dimethyl-phenoxy)propy1]-
7-(1,3,5-
trimethylpy razol-4-y1)-4,5-dihydro-3H- [1,4] diazepino [1,2-a] ind ol-1-one
[00190] The title compound (13 mg, 53%) was prepared following General
coupling
procedure A using 8-chl oro-11 -(3-(4-chl oro-3,5-dimethy 1phenoxy )p ro py1)-
7-(1,3,5 -trimethyl-
1H-py razol-4-y1)-2,3,4,5-tetrahy dro-1H- [1,41diazepino[1,2-a] indol-1 -one
(20 mg, 0.037
mmol), methyl 6-bromobenzo[d]isoxazole-3-carboxy1ate (20 mg, 0.078 mmol),
Pd2(dba)3 (4
mg, 0.0043 mmol), Xantphos (8 mg, 0.013 mmol), and Cs2CO3 (50 m, 0.153 mmol).
LCMS:
RT = 1.995 min, MS (ES) 656.2 (M+H).
Example 65
3-18-Chloro-1143-(4-chloro-3,5-dimethyl-phenoxy)propy1]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,4]diazepino11,2-a]indol-2-y1]-4-12-(2-
fluorophenoxy)ethoxyl-5-methoxy-benzoic acid
116
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Step A. Preparation of methyl 3-bromo-4-(2-(2-fluorophenoxy)ethoxy)-5-
methoxybenzoate
[00191] The title compound (101 mg, 66%) was prepared following the procedure
described in Example 66 step 1 using methyl 3-bromo-4-hydroxy-5-
methoxybenzoate (100
mg, 0.381 mmol), 1-(2-bromoethoxy)-2-fluorobenzene (120 mg, 0.547 mmol), and
Cs2CO3
(200 mg, 0.615 mmol).
Step B. Example 65
[00192] The title compound (4 mg, 13%) was obtained following the procedures
described
in General Procedure A using 8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-7-
(1,3,5 -trimethy1-1H-py razol-4-y 0-2,3,4,5-tetrahy dro-1H-[1,4] di azepino
[1,2-a] in dol-l-one
(20 mg, 0.037 mmol), methyl 3-bromo-4-(2-(2-fluorophenoxy)ethoxy)-5-
methoxybenzoate
(30 mg, 0.074 mmol), Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (8 mg, 0.013
mmol), and
Cs2CO3 (50 m, 0.153 mmol) followed by General Procedure D. LCMS: RT = 2.051
min, MS
(ES) 842.8 (M+H).
Example 66
3-[8-Chloro-1143-(4-chloro-3,5-dimethyl-phenoxy)propyl]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H- [1,4] diazepino [1,2-al indol-2-y1]-5-
methoxy-4-(2-
phenylethoxy)benzoic acid
Step A. Preparation of methyl 3-bromo-5-methoxy-4-phenethoxybenzoate
[00193] To a solution of methyl 3-bromo-4-hydroxy -5 -methoxy benzoate (100
mg, 0.381
mmol) in DMF (3 mL) was added Cs2CO3 (200 mg, 0.615 mmol) followed by (2-
bromoethyl)benzene (100 mg, 0.541 mmol). The reaction was stirred at 50 C
until complete
as determined by LCMS. The reaction was then cooled to RT, diluted into
DCM/H20 (20
mL, 1:1). The layers were separated and the aqueous layer was extracted with
DCM (2 x 10
mL) and the combined organic extracts were dried over MgSO4, filtered, and
concentrated in
vacuo. The crude reaction mixture was purified by flash chromatography (Combi-
flash Rf,
Hex/Et0Ac = 0-100% gradient) to afford the title compound (80 mg, 57%).
Step B. Example 66
[00194] The title compound (8 mg, 27%) was obtained following the procedures
described
in General Procedure A using 8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-7-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-2,3,4,5-tetrahy dro-1H-[1,4] di azepino
[1,2-a] indol-l-one
(20 mg, 0.037 mmol), methyl 3-bromo-5-methoxy-4-phenethoxybenzoate (25 mg,
0.068
mmol), Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (8 mg, 0.013 mmol), and Cs2CO3
(50 m,
117
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
0.153 mmol) followed by saponification using General Procedure D. LCMS: RT =
2.230
min, MS (ES) 809.2 (M+H).
Example 67
3-18-Chloro-1143-(4-chloro-3,5-dimethyl-phenoxy)p ropy1]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,41diazepino[1,2-al ind ol-2-yl] -443-
hyd roxyp rop oxy)-5-methoxy-benzoic acid
Step A. Preparation of methyl 3-bromo-4-(3-((tert-
butyldimethylsilyl)oxy)propoxy)-5-
meth oxybenzoate
[00195] The title compound (107 mg, 64%) was prepared following the procedure
described in Example 66 Step A substituting (2-bromoethyl)benzene with (3-
bromopropoxy)(tert-butyl)dimethylsilane (130 mg, 0.514 mmol).
Step B. Example 67
[00196] The title compound (4 mg, 14%) was prepared following the same
procedure as
described in Example 62 Step B substituting methyl 3-bromo-4-(2-((tert-
buty ldimethylsilyl)oxy)ethoxy)-5-methoxy benzoate with methyl 3 -bro mo-4-(3-
((tert-
butyldimethylsilyl)oxy)propoxy)-5-methoxybenzoate (30 mg, 0.069 mmol). LCMS:
RT =
1.847 mm, MS (ES) 763.3 (M+H).
Example 68
3-(8-Chloro-11-(3-(4-chloro-3,5-di methyl phenoxy)p ropy1)-1-oxo-7-(1,3,5-
trimethy1-1H-
pyrazol-4-y1)-4,5-dihydro-1H-11,41diazepino[1,2-al ind ol-2(3H)-y1)-1-methyl-
1H-indole-
6-carboxylic acid
[00197] The title compound (32 mg, 90%) was prepared following General
coupling
procedure A using 8-chl oro-11 -(3 -(4-chl oro-3,5-dimethy 1phenoxy)p ropy1)-7-
(1,3,5 -trimethyl-
1H-py razol-4-y1)-2,3,4,5-tetrahy dro-1H41,41diazepino [1,2-a] indol-1 -one
(27 mg, 0.05
mmol), methyl 3 -bromo-l-methy1-1H-ind ole-6-carb oxylate (16 mg, 0.06 mmol),
Pd2(dba)3 (1
mg, 0.001 mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol)
followed
by saponification using General Procedure D. LCMS: RT = 1.992 min, MS (ES)
712.3
(M+H).
Example 69
(R)-7-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-2(1H)-y1)-1-methyl-
1H-
indole-3-carboxylic acid
[00198] The title compound (22 mg, 31%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
118
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-a] ind ol-1
(2H)-one (54 mg, 0.1
mmol), methyl 7-bromo-1-methy1-1H-indole-3-carboxylate (32 mg, 0.12 mmol),
Pd2(dba)3 (2
mg, 0.002 mmol), Xantphos (3.5 mg, 0.006 mmol), and Cs2CO3 (49 mg, 0.15 mmol)
followed by saponification using General Procedure D. LCMS: RT = 2.043 min, MS
(ES)
712.2 (M+H).
Example 70
(R)-6-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-dimethyl-1H-
pyrazol-4-y1)-4-methyl- 1-oxo-3,4-d ihyd ro pyrazino [1,2-a] ind ol-2(1H)-y1)-
1-methyl-1H-
ind ole-4-carboxylic acid
Step A. Preparation of (R)-7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-6-
(3,5-dimethyl-14(2-(trimethylsilypethoxy)methyl)-1H-pyrazol-4-y1)-4-methyl-3,4-
dihyd ropyrazino [1,2-al ind 01-1(2M-one
[00199] The title compound was prepared following the procedure described
Example 58
Step C to E using ethyl 6-chloro-3-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(3,5-
dimethy1-1-((2-(trimethy lsilypethoxy)methyl)-1H-pyrazol-4-y1)-1H-indole-2-
carboxylate,
and tert-butyl (S)-5-methyl-1,2,3-oxathiazolidine-3-carboxylate 2,2-dioxide.
MS (ES) 656.2
(M+H).
Step B.
Preparation of methyl (R)-6-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-6-(3,5-dimethyl-1-02-(trimethylsilyl)ethoxy)methyl)-1H-
pyrazol-4-y1)-4-methyl-1-oxo-3,4-dihydropyrazino [1,2-a] in d ol-2 (1H)-y1)-1-
methy1-1H-
indole-4-carboxylate
[00200] The title compound (30 mg, 47%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(3,5-
di methy1-1-((2-(trimethy lsi lypethoxy)methyl)-1H-py razol-4-y1)-4-methy1-3,4-
dihydropyrazino[1,2-a]indol-1(2H)-one (50 mg, 0.076 mmol), methyl 6-bromo-1-
methy1-1H-
indole-4-carboxylate (25 mg, 0.092 mmol), Pd2(dba)3 (1.4 mg, 0.0015 mmol),
Xantphos (2.7
mg, 0.0046 mmol), and Cs2CO3 (49 mg, 0.15 mmol). LCMS: RT = 2.011 min, MS (ES)
842.3 (M+H).
Step C. Example 70
[00201] The title compound (16 mg, 66%) was prepared following the procedure
described
Example 58 Step G using methyl (R)-6-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-6-(3,5-dimethy1-1-((2-(trimethylsily1)ethoxy)methyl)-
1H-pyrazol-
4-y1)-4-methyl-l-oxo-3,4-dihy dropyrazino [1,2-a] indo1-2(1H)-y1)-1-methy1-1H-
indole-4-
119
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
carboxylate (30 mg, 0.036 mmol) and TBAF (356 1, 0.356 mmol). LCMS: RT =
1.893 min,
MS (ES) 698.2 (M+H).
Example 71
(R)-4-(7-chloro-10-(3-(4-chloro-3,5-d imethylphenoxy)propy1)-6-(3,5-dimethy1-
1H-
pyrazol-4-y1)-4-methyl-1-oxo-3,4-d ihyd ropyrazino [1,2-a] ind ol-2(1H)-y1)-1-
methyl-1H-
ind ole-6-carboxylic acid
[00202] The title compound (29 mg, 59% two steps) was prepared according to
procedures
described in Example 70 B to C using (R)-7-chloro-10-(3-(4-chloro-3,5-
di methylph enoxy )propy1)-6-(3,5-dimethy1-1-((2-(trimethylsily1)ethoxy
)methyl)-1H-py razol-
4-y1)-4-methy1-3,4-dihy dropy razino [1,2-a] indo1-1(2H)-on e (50 mg, 0.076
mmol) and methyl
4-bromo-1-methy1-1H-indole-6-carboxylate (25 mg, 0.092 mmol). LCMS: RT = 1.895
min,
MS (ES) 698.2 (M+H).
Example 72
4-(8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)p ropy1)-7-(3,5-d imethy1-1H-
pyrazol-4-
y1)-1-oxo-4,5-dihyd ro-1H-11,4]d iazepino ind ol-
2(3H)-y1)-1-methyl-1H-ind ole-6-
carboxylic acid
[00203] The title compound was prepared according to procedures described in
Example 58
step F to G using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(3,5-
dimethy1-1-
((2-(trimethylsily1)ethoxy)methyl)-1H-pyrazol-4-y1)-2,3,4,5-tetrahy dro-1H-
[1,4] di azepino [1,2-a]indol- 1 -one and methyl 4-bromo-1-methy 1-1H-indole-6-
carboxy 1 ate.
LCMS: RT = 1.843 min, MS (ES) 698.2 (M+H).
Example 73
5-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-4,5-dihydro-1H-11,4]diazepino [1,2-al ind ol-2(31/)-y1)-3-hyd
roxy-1,4-
dihydropyrazine-2-carboxylic acid
[00204] The title compound (28 mg, 82%) was prepared following General
coupling
procedure A using 8-chl oro-11 -(3 -(4-chl oro-3,5-dimethy 1phenoxy )p ro py1)-
7-(1,3,5 -trimethyl-
1H-py razol-4-y1)-2,3,4,5-tetrahy dro-1H-[1,4] diazepino [1,2-a] ind ol-1 -one
(27 mg, 0.05
mmol), methyl 5-chloropyrazine-2-carboxylate (10 mg, 0.06 mmol), Pd2(dba)3 (1
mg, 0.001
mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification using General Procedure D. LCMS: = 1.841
min, MS (ES) 679.2 (M+H).
Example 74
120
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
3-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-yl)-4,5-dihydro-1H-11,4] diazepino [1,2-a] ind ol-2(31/)-yl)-1-
methyl-1H-indo le-
7-carboxylic acid
Step A. Preparation of methyl 3-bromo-1-methyl-1H-indole-7-carboxylate
[00205] The title compound (73 mg, 96%) was prepared following General
Procedure F
using methyl-indole-7-carboxylate (50 mg, 0.28 mmol), NBS (51 mg, 0.28 mmol),
NaH (14
mg, 0.34 mmol) and Mel (21 p.L, 0.34 mmol). LCMS: RT = 1.646 min, MS (ES)
268.1
(M+H).
Step B. Example 74
[00206] The title compound (23 mg, 65%) was prepared following General
coupling
procedure B using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethy1-
1H-pyrazol-4-y1)-2,3,4,5-tetrahydro-1H41,41diazepino[1,2-a]indol-l-one (27 mg,
0.05
mmol), methyl 3-bromo-1-methy1-1H-indole-7-carboxylate (16 mg, 0.06 mmol), Cul
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 p.L, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
2.038 min, MS (ES) 712.2 (M+H).
Example 75
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-d imethylp hen oxy)p rop y1)-4-methy1-1-
oxo-6-(1,3,5-
trimethyl-1H-pyrazol-4-yl)-3,4-dihyd ropyrazino[1,2-a] indo1-2(1H)-yl)-1-
methyl-1H-
ind ole-7-carboxylic acid
[00207] The title compound (31 mg, 87%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 3-bromo-1-methyl-1H-indole-7-carboxylate (16 mg, 0,06 mmol), CuI
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 viL, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =-
2.092 min, MS (ES) 712.2 (M+H); 11-1 NMR (400 MHz, DMSO-d6) 5 7.76 (d, J = 8.6
Hz,
1H), 7.62 - 7.53 (m, 2H), 7.49 (d, J= 2.8 Hz, 1H), 7.30 (d, J= 8.6 Hz, 1H),
7.09 (td, J= 7.7,
2.2 Hz, 1H), 6.72 (s, 2H), 4.48 (dd, J= 13.0, 3.8 Hz, 1H), 4.23 -4.08 (m, 1H),
3.98 (dt, J=
6.4, 3.4 Hz, 2H), 3.84 (s, 3H), 3.78 (s, 1.5H), 3.77 (s, 1.5H), 3.40 - 3.15
(m, 3H), 2.25 (s,
6H), 2.12 (s, 1.5H), 2.08 (d, J= 12.0 Hz, 2H), 2.04 (s, 1.5H), 1.98 (s, 1.5H),
1.89 (s, 1,5H),
1.08 (d, J= 6.4 Hz, 3H).
Example 76
121
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-l-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2(1H)-y1)- 1-
methy1-1H-
ind ole-5-carboxylic acid
[00208] The title compound (28 mg, 79%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-111-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 3-bromo-l-methyl-1H-indole-5-carboxylate (16 mg, 0.06 mmol), CuI
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 pL, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
2.003 min, MS (ES) 712.3 (M+H); 11-1 NMR (400 MHz, DMSO-d6) 6 8.11 (dd, J =
3.5, 1.5
Hz, 1H), 7.87 - 7.71 (m, 2H), 7.57 (dd, J= 9.4, 1.9 Hz, 2H), 7.30 (d, J= 8.6
Hz, 1H), 6.73 (s,
2H), 4.48 (dd, J= 13.0, 3.8 Hz, 1H), 4.24 -4.09 (m, 1H) 3.99 (tt, J = 6.4, 3.2
Hz, 2H), 3.85
(s, 3H), 3.79 (s, 1.5H), 3.77 (s, 1.5H), 3.72 - 3.59 (m, 1H), 3.23 (dt, J =
13.4, 7.4 Hz, 2H),
2.24 (s, 6H), 2.12 (s, 1.5H), 2.07 (dd, J= 12.7, 6.0 Hz, 2H), 2.03 (s, 1.5H),
1.98 (s, 1.5H),
1.89 (s, 1.5H), 1.19 -0.93 (m, 3H).
Example 77
(P, R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-
6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino11,2-al ind ol-2(1H)-y1)-
1-methyl-
1H-indole-5-carboxylic acid (separated atropisomer A)
[00209] Example 91 was separated using reverse phase HPLC (Phenomenex Gemini
C18,
H20/CH3CN gradient to 35-95 % MeCN 0.1% TFA, 10 mins gradient) to give the
title
compound as the first eluting atropisomer (13 mg, 37%). LCMS: RT = 1.970 min,
MS (ES)
712.0 (M+H).
Example 78
R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino 11,2-al ind 01-2( 1H)-
y1)-1-methyl-
1H-indole-5-carboxylic acid (separated atropisomer B)
[00210] Example 91 was separated using reverse phase HPLC (Phenomenex Gemini
C18,
H20/CH3CN gradient to 35-95 % MeCN 0.1% TFA, 10 mins gradient) to give the
title
compound as a second eluting atropisomer (11 mg, 31%). LCMS: RT = 1.989 min,
MS (ES)
712.0 (M+H).
Example 79
122
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-6-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2(1H)-y1)- 1-
methy1-1H-
ind ole-3-carboxylic acid
[00211] The title compound (32 mg, 90%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-c]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 6-bromo-l-methy1-1H-indole-3-carboxylate (16 mg, 0.06 mmol),
Pd2(dba)3 (1
mg, 0.001 mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol)
followed
by saponification using General Procedure D. LCMS: RT = 2.041 min, MS (ES)
712.3
(M+H); NMR
(400 MHz, DMSO-d6) 6 8.08 (d, J= 1.3 Hz, 1H), 8.00 (dd, J= 8.5, 2.7 Hz,
1H), 7.75 (d, J = 8.6 Hz, 1H), 7.56 (t, J = 2.5 Hz, 1H), 7.30 (d, J = 8.6 Hz,
1H), 7.21 (dt, J =
8.5, 2.2 Hz, 1H), 6.74 (s, 2H), 4.49 (dd, J= 13.0, 3.8 Hz, 1H), 4.21 ¨4.09 (m,
1H), 3.99 (t, J
= 6.6 Hz, 2H), 3.84 (s, 3H), 3.79 (s, 1.5H), 3.78 (s, 1.5H), 3.40 ¨ 3.13 (m,
3H), 2.26 (s, 6H),
2.12 (s, 1.5H), 2.07 (d, J= 6.9 Hz, 2H), 2.03 (s, 1.5H), 1.98 (s, 1.5H), 1.89
(s, 1.5H), 1.05 (d,
J = 6.4 Hz, 3H).
Example 80
3-(8-Chloro-11-(3-(4-chlo ro-3,5-d i methyl phenoxy)p ropy1)-1-oxo-7-(1,3,5-
trimethy1-11/-
pyrazol-4-y1)-4,5-dihydro-1H- [1,4]diazepin o 11,2-al indo1-2(311)-y1)-1-
methyl-1H-indole-
5-carboxylic acid
[00212] The title compound (28 mg, 79%) was prepared following General
coupling
procedure B using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethyl-
1H-pyrazol-4-y1)-2,3,4,5-tetrahy dro-1H-[1,4] diazepino[1,2-a] indol-1 -one
(27 mg, 0.05
mmol), methyl 3-bromo-1-methy1-1H-indole-5-carboxylate (16 mg, 0.06 mmol), CuI
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 lit, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
1.942 min, MS (ES) 712.3 (M+H).
Example 81
6-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-
trimethy1-1H-
pyrazol-4-y1)-4,5-dihydro-11/41,41diazepin o[1,2-a] indo1-2(3H)-y1)-1-methy1-
1H-indole-
3-carboxylic acid
[00213] The title compound (20 mg, 70%) was prepared following General
coupling
procedure A using (8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-
trimethy1-1H-pyrazo1-4-y1)-2,3,4,5-tetrahydro-IH41 ,4] diazepino[1,2-a] indol-
1 -one (27 mg,
123
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
0.05 mmol), methyl 6-bromo-1-methy1-1H-indole-3-carboxylate (16 mg, 0.06
mmol),
Pd2(dba)3 (1 mg, 0.001 mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg,
0.075
mmol) followed by saponification using General Procedure D. LCMS: RT = 1.968
min, MS
(ES) 712.3 (M+H).
Example 82
648-Chloro-1143-(4-chloro-3,5-dimethyl-phenoxy)p ro pyl] -1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihyd ro-3H- [1,41d iazepino[1,2-al indo1-2-yl]-1-
(2-
methoxyethyl)indazole-4-carboxylic acid
Step A. Preparation of methyl 6-bromo-1-(2-methoxyethyl)-1H-indazole-4-
carboxylate
[00214] To a solution of methyl 6-bromo-1H-indazole-4-carboxylate (200 mg,
0.79 mmol)
in DMF (5 mL) was added NaH (40 mg, 1.63 mmol), and the reaction was stirred
for 30 min
at RT. 1-Bromo-2-methoxyethane (165 mg, 1.18 mmol) was added and the reaction
was
stirred overnight. The reaction was quenched with Me0H at -78 C, then diluted
with
DCM/H20 (60 mL, 1:1), the organic layer was separated, the aqueous layer was
extracted
with DCM (2 x 20 mL), the organic fractions combined, dried over MgSO4,
filtered, and
concentrated in vacuo. The crude reaction mixture was purified by flash
chromatography
(Combi-flash Rf, Hex/Et0Ac = 0-100% gradient) to afford the title compound (39
mg, 16%).
Step B. Example 82
[00215] The title compound (4 mg, 11%) was prepared following General coupling
procedure A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethyl-
1H-py razol-4-y 0-2,3,4,5-tetrahy dro-1H-[1,41di azepin o [1,2-a] ind 01-1 -on
e (25 mg, 0.046
mmol), methyl 6-bromo-1-(2-methoxyethyl)-1H-indazole-4-carboxylate (20 mg,
0.064
mmol), Pd2(dba)3(4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3
(50 mg,
0.153 mmol) followed by saponification using General Procedure D. LCMS: RT =
1.948
mm, MS (ES) 757.3 (M+H).
Example 83
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trim ethy1-1H-py razol-4-y1)-3,4-dihyd ro pyrazin o [1,2-al in d 01-2 (1H)-y1)-
1-ethyl-11/-
ind ole-7-carboxylic acid
Step A. Preparation of methyl 3-bromo-1-ethy1-11I-indole-7-carboxylate
[00216] The title compound (70 mg, 62%) was prepared following General
Procedure F
using methyl-indole-7-carboxylate (70 mg, 0.40 mmol), NBS (71 mg, 0.40 mmol),
NaH (20
124
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
mg, 0.48 mmol) and ethyl iodide (38 viL, 0.48 mmol). LCMS: RT = 1.731 mm, MS
(ES)
282.1 (M+H).
Step B. Example 83
[00217] The title compound (34 mg, 96%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropy razin o [1,2-a] ind 01-1
(211)-one (27 mg, 0.05
mmol), methyl 3-bromo-1-ethy1-1H-indole-7-carboxylate (16 mg, 0.06 mmol), CuI
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 1AL, 0.05 mmol), and
K2C 03
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
2.135 mm, MS (ES) 726.3 (M+H); NMR (400 MHz, DMSO-d6) 6 7.76 (d, J = 8.6 Hz,
1H),
7.65 ¨ 7.44 (m, 3H), 7.30 (d, J= 8.6 Hz, 1H), 7.15 ¨ 7.03 (m, 1H), 6.72 (s,
2H), 4.48 (dd, J-
13.0, 3.8 Hz, 1H), 4.43 ¨4.26 (m, 2H), 4.24 ¨ 4,09 (m, 2H), 3.99 (s, 3H), 3.78
(s, 1.5H), 3.77
(s, 1.5H), 3.68 ¨ 3,57 (m, 2H), 3.41 ¨3.14 (m, 2H), 2.25 (s, 6H), 2.12 (s,
1.5H), 2.11 ¨2.04
(m, 2H), 2.04 (s, 1.5H), 1.98 (s, 1,5H), 1.89 (s, 1.5H), 1.26 (td, J = 7.1,
1.4 Hz, 3H), 1.08 (d,
J = 6.4 Hz, 3H).
Example 84
(R)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-yl)-3,4-dihyd ropyrazino[1,2-a] indo1-2(1H)-yl)-1-
methyl-1H-
indazole-6-carboxylic acid
[00218] The title compound (13 mg, 39%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razin o[1,2-a] indol-1
(211)-one (25 mg, 0.046
mmol), methyl 4-bromo-1-methy1-1H-indazole-6-carboxylate (20 mg, 0,074 mmol),
Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (8 mg, 0.014 mmol), and Cs2CO3 (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.018
min, MS
(ES) 713.2 (M+H).
Example 85
5-18-Chloro-11-[3-(4-chloro-3,5-dimethyl-phenoxy)propyl]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihydro-3H-11,41diazepino [1,2-a] indol-2-yl]-1-
methyl-
indazole-7-carboxylic acid
[00219] The title compound (4 mg, 12%) was prepared following General coupling
procedure A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethyl-
1H-py razol-4-y1)-2,3,4,5-tetrahy dro-1H-[1,41di azepino [1,2-a] indol-1 -one
(25 mg, 0.046
125
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
mmol), methyl 5-bromo-1-methy1-1H-indazole-7-carboxylate (20 mg, 0.074 mmol),
Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (8 mg, 0.014 mmol), and Cs2CO3 (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT = 1.934
min, MS
(ES) 713.2 (M+H).
Example 86
8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(2,6-dimethylpheny1)-
2,3,4,5-
tetrahydro-11/41,41diazepino [1,2-a]indo1-1-one
Step A. Preparation of ethyl 6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-7-
(2,6-d imethyl pheny1)-1H-in d ole-2-c arb oxyl ate
[00220] A mixture of ethyl 7-bromo-6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-
1H-indole-2-carboxylate (25 mg, 0.050 mmoL), (2,6-dimethylphenyl)boronic acid
(10 mg,
0.068 mmol), Pd2(dba)3 (4 mg, 0.0043 mmol), dicyclohexyl(2-(phenanthren-9-
yl)phenyl)phosphane (6 mg, 0.014 mmol), and potassium phosphate (30 mg, 0.142
mmol)
was purged with Ar for 5 min then toluene (1 mL) was added. The reaction was
heated to
110 C for 16 h then cooled to RT. The reaction was diluted into DCM/H20 (10
mL, 1:1),
the layers were separated, and the aqueous layer was extracted with DCM (2 x
10 mL). The
combined organic extracts were dried by passage through a phase separator,
concentrated in
vacuo, and purified by flash chromatography (Combi-flash Rf, Hex/Et0Ac = 0-
100%
gradient) to afford the title compound (22 mg, 88%).
Step B. Preparation of ethyl 1-(3-((tert-butoxycarbonyl)amino)propy1)-6-chloro-
3-(3-(4-
chloro-3,5-dimethylphenoxy)propy1)-7-(2,6-dimethylpheny1)-1H-indole-2-
carboxylate
[00221] To a solution of ethyl 6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-7-
(2,6-dimethylpheny1)-1H-indole-2-carboxylate (96 mg, 0.18 mmol) and tert-butyl
1,2,3-
oxathiazinane-3-carboxylate 2,2-dioxide (50 mg, 0.21 mmol) in DMF (4 mL) was
added NaH
(6 mg, 0.25 mmol), and the reaction was stirred for16 h at RT. The work up
described in
general coupling procedure A was followed and purified by flash chromatography
(Combi-
flash Rf, Hex/Et0Ac = 0-100% gradient) to afford the title compound (72 mg,
57%).
Step C. Example 86
[00222] To a solution of ethyl 1 -(3 -((tert-butoxy carb onyl)amino)propy1)-6-
chl oro-3 -(3-(4-
chl oro-3,5-dimethylphen oxy )propy1)-7-(2,6-dimethy 1pheny1)-1H-indol e-2-
carboxylate (72
mg, 0.11 mmol) in dioxane (3 mL) was added HC1 (4.0 M in dioxanes, 0.5 mL, 2.0
mmol).
The reaction was stirred for 16 h at RT then concentrated in vacuo. The
residue was
dissolved in Me0H (5 mL), and K2CO3 (50 mg, 0.30 mmol) was added. The reaction
was
allowed to stir 20 h at 50 C. The work up described in general coupling
procedure A was
126
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
followed and purified by flash chromatography (Combi-flash RI', Hex/Et0Ac = 0-
100%
gradient) to afford the title compound (33 mg, 59%). LCMS: RT = 2.317 min, MS
(ES)
535.2 (M+H).
Example 87
(R)-6-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trim ethy1-11-/-py razol-4-y1)-3,4-dihyd ro pyrazin o [1,2-a] ind ol-2(111)-
y1)-1-ethyl-11-1-
indazole-4-carboxylic acid
[00223] The title compound (10 mg, 30%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(25 mg, 0.046
mmol), methyl 6-bromo-1-ethy1-1H-indazole-4-carboxylate (20 mg, 0.070 mmol),
Pd2(dba)3
(4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and cesium carbonate (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.036
min, MS
(ES) 727.2 (M+H).
Example 88
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-11-/-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-2(1H)-y1)-1-
methyl-1H-
indole-6-carboxylic acid
[00224] The title compound (30 mg, 84%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indo1-1(211)-one
(27 mg, 0.05
mmol), methyl 3-bromo-1-methy1-1H-indole-6-carboxylate (16 mg, 0.06 mmol), CuI
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 tL. 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
2.031 min, MS (ES) 712.3 (M+H); NMR
(400 MHz, DMSO-d6) 8.12 (s, 1H), 7.76 (d, J
= 8.6 Hz, 1H), 7.70- 7.60 (m, 2H), 7.48 (dd, J= 8.4, 4.4 Hz, 1H), 7.30 (d, J=
8.6 Hz, 1H),
6.72 (s, 2H), 4.48 (dd. J = 12.9, 18 Hz, 1H), 4.25 - 4.07 (m, 1H), 3.99 (td, J
= 6.4, 2.0 Hz,
2H), 3.88 (s, 3H), 3.78 (s, 1.5H), 3.77 (s, 1.5H), 3.39- 3.15 (m, 3H), 2.25
(s, 6H), 2.12 (s,
1.5H), 2.11 - 2.05 (m, 2H), 2.03 (s, 1.5H), 1.98 (s, 1.5H), 1.89 (s, 1.5H),
1.08 (d, J= 6.4 Hz,
3H).
Example 89
127
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
3-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-4,5-dihyd ro-1H-11,4] d iazepino 11,2-al in d I-2 (3H)-y1)-1-(2-
morpholinoethyl)-1H-indole-6-carboxylic acid
Step A. Preparation of methyl 3-bromo-1-(2-morpholinoethyl)-1H-indole-6-
carboxylate
[00225] The title compound (36 mg, 49%) was prepared following General
Procedure G
using methyl 3-bromo-1H-indole-6-carboxylate (51 mg, 0.20 mmol), sodium
hydride (10 mg,
0.24 mmol), 4-(2-chloroethyl)morpholine (36 mg, 0.24 mmol) and KI (40 mg, 0.24
mmol).
LCMS: RT = 1.515 min, MS (ES) 367.1 (M+H).
Step B. Example 89
[00226] The title compound (8 mg, 20%) was prepared following General coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-a] ind ol-1
(2H)-one (27 mg, 0.05
mmol), methyl 3-bromo-1-(2-morpholinoethyl)-1H-indole-6-carboxylate (19 mg,
0.06 mmol),
Cul (5 mg, 0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 tit, 0.05
mmol),
and K2CO3 (25 mg, 0.075 mmol) followed by saponification using General
Procedure D.
LCMS: RT = 1.719 and 1.745 min, MS (ES) 810.9 (M+H).
Example 90
(R)-5-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind 01-2 ( 1H)-y1)-4-
ethy1-3-oxo-3,4-
dihyd ro-2H- benzo [ b][1,41 oxazine-7-carboxylic acid
Step A. Preparation of methyl 5-bromo-4-ethyl-3-oxo-3,4-dihydro-2H-
benzo lb] [1,4] oxazine-7-carbo xylate
[00227] To a solution of methyl 4-amino-3-bromo-5-hydroxybenzoate (250 mg,
1.02
mmol) in THF (10 mL) was added NaliCO3 (150 mg, 1.8 mmol) and chloroacetyl
chloride
(170 mg, 1.5 mmol) at 0 C. The reaction was stirred for 2 h and warm to RT.
K2CO3 (150
mg, 1.1 mmol) was added, and the reaction was heated to 65 C then cooled to
RT. The
mixture was diluted with DCM/H20 (40 mL, 1:1) and extracted with DCM (2 x 20
mL). The
combined organic layers were dried over MgSO4, filtered, and concentrated in
vacua. The
crude reaction mixture was purified by flash chromatography (Combi-flash Rf,
Hex/Et0Ac =
0-100% gradient) to afford methyl 5-bromo-3-oxo-3,4-dihydro-2H-
benzo[b]11,4]oxazine-7-
carboxylate (86 mg, 30%). The product (40 mg, 0.14 mmol) was dissolved in DMF
(3 mL),
NaH (7 mg, 0.30 mmol) was added. The reaction was stirred at RT for 30 min,
and EtBr (30
mg, 0.28 mmol) was added. The reaction was stirred for 3 h at RT then diluted
with
128
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
DCM4120 (20 mL, 1:1). The organic layer was separated, and the aqueous layer
was
extracted with DCM (2 x 10 mL). The combined organic extracts were dried by
passage
through a phase separator and concentrated in vacuo. The crude reaction
mixture was
purified by flash chromatography (Combi-flash Rf, Hex/Et0Ac = 0-100% gradient)
to afford
the title compound (28 mg, 65%).
Step B. Example 90
[00228] The title compound (1 mg, 4%) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3.5-trimethyl-1H-pyrazol-4-y1)-3,4-dihy dropyrazino[1,2-a] indol-1 (211)-
one (20 mg, 0.037
mmol), methyl 5-bromo-4-ethyl-3-oxo-3,4-dihydro-2H-benzo[b] [1,4]oxazine-7-
carboxylate
(20 mg, 0.063 mmol), Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010
mmol), and
Cs2CO3 (35 mg, 0.107 mmol) followed by saponification using General Procedure
D.
LCMS: RT = 2.034 min, MS (ES) 757.8 (M+H).
Example 91
6-18-Chloro-11-13-(4-chloro-3,5-dimethyl-phenoxy)propy1]-7-(2,6-
dimethylpheny1)-1-
oxo-4,5-dihyd ro-3H-[1,4]diazepino[1,2-a]indo1-2-y11-1-methyl-indole-4-
carboxylic acid
[00229] The title compound (3 mg, 9%) was prepared following General coupling
procedure A using 8-chl
oro-11 -(3 -(4-chl oro-3 ,5-di methylphenoxy)propy1)-7-(2,6-
dimethylpheny1)-2,3,4,5-tetrahydro-1H41,41diazepino[1,2-alindol-1-one (25 mg,
0.037
mmol), methyl 6-bromo-1-methy1-1H-indole-4-carboxylate (20 mg, 0.063 mmol), P
d2(dba)3
(4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (35 mg, 0.107
mmol)
followed by saponification using General Procedure D. LCMS: RT = 1.531 min, MS
(ES)
708.3 (M+H).
Example 92
6- [8-Chloro-11- [3-(4-chloro-3,5-dimethyl-phenoxy)p ropyl]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-yl)-4,5-dihydro-3H-11,41diazepino[1,2-a1ind ol-2-yI]-1-
isopropyl-
indazole-4-carboxylic acid
Step A. Preparation of methyl 6-bromo-1-isopropyl-1H-indazole-4-carboxylate
[00230] The title compound (43 mg, 18%) was prepared following the procedure
described
in Example 82 Step A substituting 1-bromo-2-methoxyethane with 2-bromopropane
(190 mg,
1.54 mmol),
Step B. Example 92
[00231] The title compound (5 mg, 15%) was prepared following General coupling
procedure A using 8-chl oro-11 -(3 -(4-chl oro-3,5-dimethy 1phenoxy)p ropy1)-7-
(1,3,5 -trimethyl-
129
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
1H-py razol-4-y1)-2,3,4,5-tetrahy dro-1H- [1,4] diazepino [1,2-a] ind ol-1 -
one (25 mg, 0.046
mmol), methyl 6-bromo-1-isopropy1-1H-indazole-4-carboxy late (20 mg, 0.067
mmol),
Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.048
min, MS
(ES) 741.3 (M+H).
Example 93
(R)-6-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-dimethyl-1H-
pyrazol-4-y1)-4-methyl- 1-oxo-3,4-d ihyd ro pyrazino [1,2-a] ind ol-2(1H)-y1)-
1-methyl-1H-
ind azole-4-carb oxylic acid
[00232] The title compound (19 mg, 38% two steps) was prepared according to
procedures
described in Example 70 B to C using (R)-7-chloro-10-(3-(4-chloro-3,5-
di methy 1phenoxy)propy1)-6-(3,5-dimethy1-1-((2-(trimethylsily1)ethoxy)methyl)-
1H-py razol-
4-y1)-4-methy1-3,4-dihydropyrazino[1,2-a]indol-1(21-1)-one (50 mg, 0.076 mmol)
and methyl
6-bromo-1-methyl-1H-indazole-4-carboxylate (23 mg, 0.084 mmol). LCMS: RT =
1.859
min, MS (ES) 699.2 (M+H).
Example 94
(R)-6-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino indo1-2(1H)-
y1)-1-(2-
morpholinoethyl)-1H-indole-4-carboxylic acid
Step A.
Preparation of methyl (R)-6-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-methyl-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihydropyrazino [1,2-a] ind ol-2(1H)-y1)-1-02-(ttimethylsily1)ethoxy)methyl)-
1H-indole-4-
carboxylate
[00233] The title compound (138 mg, 88%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3 ,4-dihy dropyrazino[1,2-a]indol-1(211)-
one (100 mg,
0.184 mmol), methyl 6-bromo-1-((2-(tri methylsilyl)ethoxy )methyl)-1H-indol e-
4-carboxy I ate
(78 mg, 0.20 mmol), Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010
mmol), and
Cs2CO3 (121 mg, 0.37 mmol). MS (ES) 842.3 (M+H).
Step B.
Preparation of methyl (R)-6-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-methyl-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihydropyrazino[1,2-a]indol-2(1H)-y1)-1H-indole-4-carboxylate
[00234] A mixture of methyl (R)-6-
(7-chl oro-10-(3-(4-chl oro-3,5-
di methy 1phenoxy)propy1)-4-methy1-1-oxo-6-(1,3,5 -trimethy1-1H-py razol-4-y1)-
3,4-
130
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
dihy dropy razino 1,2-a] indo1-2(1H)-y1)-1-02-(trimethylsilypethoxy)methyl)-1H-
indol e-4-
carboxylate (138 mg, 0.164 mmol), TBAF (0.819 mL, 0.819 mmol) in THF (1 mL)
was
heated at 100 C under microwave irradiation in Biotage Initiator for 20 min.
The reaction
was quenched with H20 (5 mL) then extracted with DCM (10 mL x 2). The combined
organic solution was concentrated and the residue was purified by flash
chromatography
(Combi-flash Rf, Me0H/DCM = 0-5% gradient) to give the title compound (80 mg,
69%) as
a white solid. LCMS: RT = 2.115 min, MS (ES) 712.2 (M+H).
Step C. Example 94
[00235] To a solution of methyl (R)-6-
(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-methy1-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihydropyrazino[1,2-alindol-2(1H)-y1)-1H-indole-4-carboxylate (40 mg, 0.056
mmol) in
DMF (0.5 mL) was added NaH (4.5 mg, 0.11 mmol) and stirred for 20 min. 4-(2-
Bromoethyl)morpholine (22 mg, 0.11 mmol) was added, and the resulting mixture
was
heated to 50 C for 2 days. The reaction was quenched by Me0H (0.5 mL) and
acidified with
TFA then concentrated in vacuo. The residue was purified by reverse phase HPLC
(Phenomenex Gemini C18, H20/CH3CN gradient to 30-85 % MeCN 0.1% TFA) to give
the
title compound (14 mg, 30%). LCMS: RT = 1.714 min, MS (ES) 811.3 (M+H).
Example 95
6-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(3,5-dimethy1-1H-
pyrazol-
4-y1)-1-oxo-4,5-dihydro-11/- [1,4] diazepino[1,2-al ind ol-2(3H)-y1)-1-methyl-
1H-indazole-
4-carboxylic acid
[00236] The title compound (25 mg, 56% two steps) was prepared according to
procedures
described in Example 70 B to C using 8-chloro-11-(3-(4-chloro-3,5-
di methy 1phenoxy )propy1)-7-(3,5-dimethy1-1-02-(trimethylsily Dethoxy)methyl)-
1H-py razol-
4-y1)-2,3,4,5-tetrahydro-1H41,41diazepino[1,2-alindol-1-one (50 mg, 0.076
mmol) and
methyl 6-bromo-1-methy1-1H-indazole-4-carboxylate (23 mg, 0.084 mmol). LCMS:
RT =-
1.859 min, MS (ES) 699.2 (M+H).
Example 96
(R)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2 (1H)-y1)-1-(2-
morpholinoethyl)-1H-indole-6-carboxylic acid
[00237] The title compound (58% yield) was prepared according to procedures
described in
Example 94 Step A to C using (R)-7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-
methy1-6-(1,3,5-trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino1,2indol-1
(21I)-one and
131
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
methyl 4-bromo-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indole-6-carb oxy late.
LCMS: RT
= 1.735 min, MS (ES) 811.3 (M+H).
Example 97
(R)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-yl)-3,4-dihyd ropy razino[1,2-al indo1-2(1H)-yl)-2-
methyl-2H-
indazole-6-carboxylic acid
[00238] The title compound (6 mg, 18%) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -tri methy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazin o [1,2-a] indol-1
(211)-one (25 mg, 0.046
mmol), methyl 4-bromo-2-methy1-2H-indazole-6-carboxylate (20 mg, 0.074 mmol),
Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (8 mg, 0.014 mmol), and Cs2CO3 (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT = 1.949
min, MS
(ES) 713.2 (M+H).
Example 98
(R)-5-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-2(1H)-y1)-1-methyl-
1H-
indazole-7-carboxylic acid
[00239] The title compound (3 mg, 9%) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -tri methy1-1H-pyrazol-4-y1)-3,4-dihy dropy razin o [1,2-a] indol-1
(211)-one (25 mg, 0.046
mmol), methyl 5-bromo-l-methy1-1H-indazole-7-carboxylate (20 mg, 0.074 mmol),
Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (8 mg, 0.014 mmol), and Cs2CO3 (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT = 1.991
min, MS
(ES) 713.2 (M+H).
Example 99
(R)-6-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-11-/-pyrazol-4-yl)-3,4-dihydropyrazino11,2-alindol-2(1H)-y1)-1-ethyl-
111-
indole-4-carboxylic acid
[00240] The title compound (12 mg, 38%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazin o [1,2-a] in do1-1(2H)-
on e (40 mg, 0.074
mmol), methyl 6-bromo-l-ethy1-1H-indole-4-carboxylate (25 mg, 0.089 mmol),
Pd2(dba)3
(1.4 mg, 0.0015 mmol), Xantphos (2.6 mg, 0.0045 mmol), and Cs2CO3 (48 mg, 0.15
mmol)
132
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
followed by saponification using General Procedure D. LCMS: RT = 2.220 min, MS
(ES)
740.3 (M+H).
Example 100
(R)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino11,2-al indo1-2(111)-y1)-1H-ind
ole-6-
carboxylic acid
[00241] The title compound was prepared along with Example 96 as a byproduct.
MS (ES)
698.2 (M+H).
Example 101
(R)-6-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyI)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino11,2-a] indo1-2(1H)-y1)-1H-
indole-4-
carboxylic acid
[00242] The title compound was prepared along with Example 94 as a byproduct.
MS (ES)
698.2 (M+H).
Example 102
(R)-6-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino[1,2-a] ind ol-2(11/)-
yl)quinoline-2-
carboxylic acid
[00243] The title compound (21 mg, 59%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(21/)-one
(27 mg, 0.05
mmol), methyl 6-bromoquinoline-2-carboxylate (16 mg, 0.06 mmol), Pd2(dba)3 (1
mg, 0.001
mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification using General Procedure D. LCMS: RT = 2.025 min, MS (ES) 710.2
(M+H);
11-1 NMR (400 MHz, DMSO-d6) 8.51 (dd, J = 8.6, 1.7 Hz, 1H), 8.14 (t, J= 8.6
Hz, 2H),
8.09- 8.00 (m, 1H), 7.96 (ddd, J= 9.0, 2.4, 1.0 Hz, 1H), 7.78 (d, J= 8.6 Hz,
1H), 7.31 (d, J
= 8.6 Hz, 1H), 6.74 (s, 2H), 4.68 - 4.46 (m, 2H), 4.26 - 4.14 (m, 3H), 4.00
(s, 3H), 3.79 (s,
3H), 3.44 - 3.14 (m, 2H), 2.25 (s, 6H), 2.12 (s, 1.5H), 2.11 - 2.05 (m, 2H),
2.03 (s, 1.5H),
2.00 (s, 1.5H), 1.90 (s, 1.5H), 1.05 (d, J= 6.4 Hz, 3H).
Example 103
(R)-7-(7-Chloro-10-(3-(4-chl oro-3,5-d imethyl phenoxy)p ropy1)-4-methy1-1-oxo-
6-(1,3,5-
trim ethy1-1H-py razol-4-y1)-3,4-d ihyd ro py razin o [1,2-a] in d 01-2 (1H)-
y1)-1-ethy1-11/-
indole-3-carboxylic acid
Step A. Preparation of ethyl 7-bromo-1-ethyl-1H-indole-3-carboxylate
133
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00244] The title compound (58 mg, 68%) was prepared following General
Procedure G
using 7-bromo-1H-indole-3-carboxylic acid (70 mg, 0.29 mmol), NaH (26 mg, 0.64
mmol)
and Ell (51 tit, 0.64 mmol). LCMS: RT 1.803 min, MS (ES) 296.1 (M+H).
Step B. Example 103
[00245] The title compound (8 mg, 22%) was prepared following General coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazino [1,2-a] ind ol-1 (211)-
one (27 mg, 0.05
mmol), ethyl 7-bromo-1-ethyl-1H-indole-3-carboxylate (14 mg, 0.06 mmol), Cu!
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 tL, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
2.042 min, MS (ES) 726.3 (M+H).
Example 104
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-d imethyl phen oxy)p ropy1)-4-methyl-1-oxo-
6-(1,3,5-
trim ethy1-1H-py razol-4-y1)-3,4-dihyd ro pyrazin o [1,2-a] in d 01-2 (11/)-
y1)-1-ethyl-11/-
ind ole-6-carboxylic acid
Step A. Preparation of methyl 3-bromo-1-ethyl-1H-indole-6-carboxylate
[00246] The title compound (76 mg, 95%) was prepared following General
Procedure G
using methyl 3-bromo-1H-indole-6-carboxylate (72 mg, 0.28 mmol), sodium
hydride (14 mg,
0.34 mmol) and Ell (28 IAL, 0.34 mmol). LCMS: RT = 1.682 min, MS (ES) 282.1
(M+H).
Step B. Example 104
[00247] The title compound (35 mg, 96%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy0-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-a] ind ol-1
(2H)-one (27 mg, 0.05
mmol), methyl 3-bromo-1-ethy1-1H-indole-6-carboxylate (14 mg, 0.06 mmol), CuI
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 tit, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
2.064 min, MS (ES) 726.3 (M+H).
Example 105
(R)-8-(7-Chloro-10-(3-(4-chloro-3,5-d imethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino[1,2-a] ind 01-2( 1H)-yl)q u
inoline-3-
carboxylic acid
[00248] The title compound (34 mg, 96%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
134
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-allindol-1(2H)-one
(27 mg, 0.05
mmol), methyl 8-bromoquinoline-3-carboxylate (16 mg, 0.06 mmol), Pd2(dba)3 (1
mg, 0.001
mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification using General Procedure D. LCMS: RT = 2.010 min, MS (ES) 710.2
(M+H).
Example 106
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-11/-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] indo1-2(1H)-y1)-1-
methy1-1H-
ind ole-4-carboxylic acid
Step A. Preparation of methyl 3-bromo-1-methyl-1H-indole-7-carboxylate
[00249] The title compound (39 mg, 51%) was prepared following General
Procedure F
using methyl 1H-indole-4-carboxylate (50 mg, 0.28 mmol), NBS (51 mg, 0.28
mmol), NaH
(14 mg, 0.34 mmol), and Mel (21 1AL, 0.34 mmol). LCMS: RT = 1.414 min, MS (ES)
268.1
(M+H).
Step B. Example 106
[00250] The title compound (16 mg, 45%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 3-bromo-1-methy1-1H-indole-4-carboxylate (14 mg, 0.06 mmol), CuI
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 1AL, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
1.936 and 1.963 min, MS (ES) 712.3 (M+H).
Example 107
6-[8-Chloro-1143-(4-chloro-3,5-dimethyl-phenoxy)propy1]-1-oxo-7-(1,3,5-
trimethylpyrazol-4-y1)-4,5-dihyd ro-3H-11,41diazepino11,2-al indo1-2-yl] -142-
hyd roxyethyl)ind azole-4-carboxylic acid
Step A. Preparation of methyl 6-bromo-1-(2-((tert-
butyldimethylsilyl)oxy)ethyl)-11-/-
indazole-4-carboxylate
[00251] The title compound (73 mg, 23%) was prepared following the procedure
describe
in Example 82 Step A substituting 1-bromo-2-methoxyethane with (2-
bromoethoxy)(tert-
butypdimethylsilane (375 mg, 1.57 mmol).
Step B. Example 107
[00252] The title compound (3 mg, 9%) was prepared following General coupling
procedure A using 8-chl oro- 11 -(3 -(4-chl oro-3,5-dimethylphen oxy)pro py1)-
7-(1,3,5 -trimethyl-
135
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
1H-py razol-4-y1)-2,3,4,5-tetrahy dro-1H- [1,4] diazepino [1,2 -a] ind ol-1 -
one (25 mg, 0.046
mmol), methyl 6-bromo-1-(2-((tert-butyldimethylsilypoxy)ethyl)-1H-indazole-4-
carboxy late
(30 mg, 0.073 mmol), Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010
mmol), and
Cs2CO3 (50 mg, 0.153 mmol). Following isolation of the coupling product, the
crude residue
was dissolved in THF (3 mL) and TBAF (1.0 M, 0.1 mL, 0.1 mmol) was added and
the
reaction was allowed to stir overnight at RT. The crude reaction mixture was
concentrated
and carried forward to saponification following General Procedure D. LCMS: RT
= 1.786
min, MS (ES) 742.9 (M+H).
Example 108
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-d imethy1-
1H-
pyrazol-4-y1)-4-methyl-1-oxo-3,4-dihyd ropyrazino[1,2-a]indo1-2(1H)-y1)-1-
methyl-1H-
indole-3-carboxylic acid
[00253] The title compound was prepared according to procedures described in
Example 70
B to C using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-
dimethy1-1-
((2-(trimethylsilypethoxy)methyl)-1H-pyrazol-4-y1)-4-methyl-3,4-dihydropy
razino 111,2-
alindo1-1(2H)-one and methyl 7-bromo-1-methy1-1H-indole-3-carboxylate. MS (ES)
699.2
(M+H).
Example 109
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-dimethyl-1H-
pyrazol-4-y1)-4-methy1-1-oxo-3,4-dihyd ropyrazino [1,2-a] ind 01-2 (1H)-y1)-1-
methyl-1H-
indole-6-carboxylic acid
Step A. Preparation of methyl (R)-3-
(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-6-(3,5-dimethyl-14(2-(trimethylsilypethoxy)methyl)-1H-
pyrazol-4-y1)-4-methyl-1-oxo-3,4-dihydropyrazino[1,2-alindol-2(1H)-y1)-1-
methyl-1H-
indole-6-carboxylate
[00254] The title compound (88 mg, 69%) was prepared following General
Procedure B
using (R)-7-
chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-dimethy1-1-((2-
(trimethylsilyl)ethoxy)methyl)-1H-pyrazol-4-y1)-4-methyl-3,4-dihy dropy razino
[1,2-a] ind ol-
1(21-1)-one (100 mg, 0.15 mmol), methyl 3-bromo-1-methy1-1H-indole-6-
carboxylate (41 mg,
0.15 mmol), CuI (1.5 mg, 0.0078 mmol), (trans)-1,2-N,N'-
dimethylaminocyclohexane (2.2
mg, 0.015 mmol), and K2CO3 (42 mg, 0.31 mmol). MS (ES) 842.3 (M+H).
Step B. Example 109
[00255] The title compound was prepared according to procedures described in
Example 70
C using
methyl (R)-3-(7-chl oro-10-(3 -(4-chloro-3,5-dimethy 1phenoxy)propy1)-6-(3,5-
136
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
dimethy1-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-pyrazol-4-y1)-4-methyl-l-oxo-
3,4-
dihydropyrazino[1,2-a]indol-2(1H)-y1)-1-methyl-1H-indole-6-carboxylate. LCMS:
RT =
1.900 min, MS (ES) 698.2 (M+H).
Example 110
(R)-6-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyI)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] indo1-2(1H)-y1)-1-
methy1-1H-
ind ole-2-carboxylic acid
[00256] The title compound (28 mg, 79%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y 0-3,4-dihy dropy razino [1,2-a] ind 01-1
(211)-one (27 mg, 0.05
mmol), methyl 6-bromo-1-methy1-1H-indole-2-carboxylate (14 mg, 0.06 mmol), CuI
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 viL, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
2.091 and 2.114 min, MS (ES) 712.3 (M+H).
Example 111
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino11,2-a]ind ol-2(1H)-yl)imidazo
[1,5-
a]pyridine-1-carboxylic acid
[00257] The title compound (89% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino [1,2-a] ind 01-1
(211)-one and methyl 3-
bromoimidazo[1,5-alpyridine-1-carboxylate followed by saponification using
General
Procedure D. MS (ES) 701.3 (M+H); 1-1-1 NMR (DMSO, 400 MHz) ö (ppm) 8.08 (t,
J= 10.0
Hz, 2H), 7.79 (d, J= 8.4 Hz, 1H), 7.31 (d, J= 8.4 Hz, 1H), 7.29-7.26 (m, 1H),
6.93 (t, J= 6.8
Hz, 1H), 6.71 (s, 2H), 4.55-4.50 (m, 1H), 4.30-4.25 (m, 2H), 3.97 (t, J= 6.0
Hz, 2H), 3.77 (s,
3H), 3.33-3.15 (m, 2H), 2.23 (s, 6H), 2.11 (s, 1.5H), 2.09-2.01 (m, 2H), 2.02
(s, 1.5H), 1.97 (s,
1.5H), 1.88 (s, 1.5H), 1.09 (d, J= 5.6 Hz, 3H).
Example 112
(R)-2-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-d ihydro py razino[1,2-a] ind ol-2(1H)-
yl)imidazo [1,2-
a]pyridine-8-carboxylic acid
[00258] The title compound (49% yield) was prepared following General coupling
procedure A using (R)-7-chl oro-10-(3 -(4-chl oro-3,5-dimethy 1phenoxy
)propy1)-4-methy1-6-
137
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one
and methyl 2-
bromoimidazo[1,2-a]pyridine-8-carboxylate followed by saponification using
General
Procedure D. MS (ES) 701.4 (M+H).
Example 113
(R)-4-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-11/-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-2(1H)-y1)-1-methyl-
1H-
indole-2-carboxylic acid
[00259] The title compound (26 mg, 73%) was prepared following General
Procedure B
using (R)-7-
chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-6-(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-1(2H)-one (27 mg,
0.05 mmol),
methyl 4-bromo-l-methyl-1H-indole-2-carboxylate (14 mg, 0.06 mmol), Cul (5 mg,
0.025
mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 uL, 0.05 mmol), and K2CO3
(25 mg,
0.075 mmol) followed by saponification using General Procedure D. LCMS: RT =
2.076
min, MS (ES) 712.2 (M+H); NMR
(400 MHz, CDC13) 6 7.74 (d, J 3.1 Hz, 0.5H), 7.72
(d, J- 3.1 Hz, 0.5H), 7,49 - 7,37 (m, 2H), 7.31 (dd, J- 9.7, 5.1 Hz, 2H), 7.13
- 7.05 (m, IH),
6.70 - 6.55 (m, 2H), 4.57 -4.40 (m, 1H), 4.37 - 4.17 (m, 2H), 4.10 (s, 3H),
3.97 (s, 1.5H),
3.96(s, 1,5H), 3.62 (dd, J = 11.9, 4.2 Hz, 1H), 3.40 (ddt, J-= 42.4, 13.8, 7.3
Hz, 3H), 2.32(s,
6H), 2.26 (s, 1.5H), 2.25 (s, 1.5H), 2.22 (d, J = 7.0 Hz, 2H), 2.11 (s, 1.5H),
2.09 (s, 1.5H),
1.27 (d, J= 6.4 Hz, 1.5H), 1.20 (d, J= 6.5 Hz, 1.5H).
Example 114
(R)-5-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-2(1H)-y1)-1-methyl-
1H-
indole-2-carboxylic acid
[00260] The title compound (28 mg, 79%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indo1-1(2/)-one
(27 mg, 0.05
mmol), methyl 5-bromo-1-methy1-1H-indole-2-carboxylate (14 mg, 0,06 mmol), CuI
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 vit, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
2.084 min, MS (ES) 712.3 (M+H); 11-1NMR (400 MHz, DMSO-d6) 6 7.75 (d, J- 8.6
Hz, 1H),
7.66 - 7.54 (m, 2H), 7.36 - 7.26 (m, 2H), 7.23 (t, J- 0.9 Hz, 1H), 6.73 (s,
2H), 4.44 (dd, J
13.0, 3.8 Hz, 1H), 4.05 (s, 3H), 3.98 (tt, J- 6.3, 2.9 Hz, 3H), 3.78 (s,
1.5H), 3.77 (s, 1.5H),
138
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
3.63 (t, J= 13.5 Hz, 1H), 3.22 (dt, J= 13.2, 7.2 Hz, 2H), 2.26 (s, 6H), 2.12
(s, 1.5H), 2.10 ¨
2.04 (m, 2H), 2.03 (s, 1.5H), 1.97 (s, 1.5H), 1.88 (s, 1.5H), 1.04 (d, J= 6.4
Hz, 3H).
Example 115
(R)-2-(benzo ldl isoxazol-6-y1)-7-chlo ro-10-(3-(4-chloro-3,5-dimethylphenoxy)
propy1)-4-
methyl-6-(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino 11,2-al indo1-
1(21/)-one
[00261] The title compound (41% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropy razin o [1,2-a] indol-1
(211)-one and 6-
bromobenzordlisoxazole. MS (ES) 656.4 (M+H); 1H NMR (DMSO, 400 MHz) 6 (ppm)
7.73
(d, J = 8.8 Hz, 1H), 7.63 (dd, J = 8.4, 2.8 Hz, 1H), 7.28 (d, J= 8.4 Hz, 1H),
7.15 (s, 1H), 6.91
(d, J= 8.8 Hz, 1H), 6.72 (s, 2H), 4.45-4.41(m, 1H), 4.24-4.13 (m, 2H), 3.97
(t, J= 6.0 Hz,
2H), 3.76 (s, 3H), 3.32-3.15 (m, 2H), 2.24 (s, 6H), 2.08 (s, 1.5H), 2.07-2.02
(m, 2H), 1.98 (s,
1.5H), 1.94 (s, 1.5H), 1.85 (s, 1.5H), 0.94 (d, J= 6.4 Hz, 3H)
Example 116
(R)-5-(7-Chloro-10-(3-(4-chloro-3,5-d imethylp hen oxy) p rop y1)-4-methy1-1-
oxo-6-(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino [1,2-a] indo1-2(1H)-y1)-1-
methy1-1H-
indazole-3-carboxylic acid
[00262] The title compound (25 mg, 70%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-c]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 5-bromo-1-methy1-1H-indazole-3-carboxylate (14 mg, 0.06 mmol),
Cu! (5 mg,
0.025 mmol), (trans)-1,2-N,/V'-dimethylaminocyclohexane (8 viL, 0.05 mmol),
and K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
1.998 min, MS (ES) 713.2 (M+H).
Example 117
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2(1H)-y1)-2-
ethyl-2H-
ind azole-3-carboxylic acid
Step A. Preparation of methyl 7-bromo-2-ethyl-2H-indazole-3-carboxylate
[00263] The title compound (54 mg, 48%) was prepared following the procedure
described
in Example 82 Step A using methyl 7-bromo-1H-indazole-3-carboxylate (100 mg,
0.392
mmol), sodium hydride (18 mg, 0.75 mmol), and EtBr (80 mg, 0.74 mmol).
Step B. Example 117
139
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00264] The title compound (9.5 mg, 29%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino [1,2-a] ind 01-1
(210-one (25 mg, 0.046
mmol), methyl 7-bromo-2-ethyl-2H-indazole-3-carboxylate (20 mg, 0.071 mmol),
Pd2(dba)3
(4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg, 0.153
mmol)
followed by saponification using General Procedure D. LCMS: RT = 2.138 min, MS
(ES)
727.3 (M+H); 1H NMR (400 MHz, Chloroform-d) 7.86 (ddd, J= 8.4, 3.9, 0.9 Hz,
1H), 7.73
(dd, J= 8.6, 2.9 Hz, 1H), 7.43 ¨ 7.36 (in, 1H), 7.35 ¨ 7.24 (in, 2H), 6.68 ¨
6.62 (m, 2H), 4.84
(q, J = 7.2 Hz, 2H), 4.64 ¨ 4.49 (m, 1H), 4.36-4,20 (m, 1H), 4.04 (t, J = 6.4
Hz, 211), 3.96 (d,
J= 2.7 Hz, 3H), 3.79 (t, J= 13.5 Hz, 1H), 3.44 (ddt, J= 17.8, 11.7, 6.3 Hz,
2H), 2.33 (s, 6H),
2.28 ¨2.19 (m, 5H), 2.12 (s, 1.5H), 2.09 (s, 1.5H), 1.49 (td, J= 7.2, 5.8 Hz,
3H), 1.34 (d, J=
6.3 Hz, 1.5H), 1.26 (d, J = 6.4 Hz, 1.5H).
Example 118
(R)-7-(7-chloro-1043-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-11-/-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2(111)-y1)-
indazole-3-carboxylic acid
Step A. Preparation of methyl 7-bromo-1-ethyl-1H-indazole-3-carboxylate
[00265] The title compound (22 mg, 20%) was prepared in the same reaction as
described
in Example 117 Step A.
Step B. Example 118
[00266] The title compound (3 mg, 9%) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-a] ind ol-1
(210-one (25 mg, 0.046
mmol), methyl 7-bromo-1-ethy1-1H-indazole-3-carboxylate (20 mg, 0.071 mmol),
Pd2(dba)3
(4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg, 0.153
mmol)
followed by saponification using General Procedure D. LCMS: RT = 2.058 min, MS
(ES)
727.2 (M+H).
Example 119
7- [8-chloro-11-[3-(4-chloro-3,5-dimethyl-phenoxy)propy1]-7-(4,6-
dimethylpyrimidin-5-
y1)-1-oxo-4,5-dihydro-3H-11,41diazepino 11,2-a]indo1-2-y1]-1-methyl-indole-3-
carboxylic
acid
[00267] The title compound (1 mg, 3%) was prepared following General coupling
procedure A using 8-chl
oro-11 -(3 -(4-chloro-3 ,5-di methy 1phenoxy)propy1)-7-(4,6-
dimethy 1py rimi din-5-y1)-2,3,4,5-tetrahy dro-1H-[1,4] diazep ino [1,2-a]
indol-1 -one (25 mg,
140
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
0.046 mmol), methyl 7-bromo-1-methy1-1H-indole-3-earboxylate (20 mg, 0.074
mmol),
Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.032
min, MS
(ES) 710.2 (M+H).
Example 120
8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(4,6-dimethylpyrimidin-
5-y1)-
2,3,4,5-tetrahyd ro-1H-11,41diazepino [1,2-al indol-1-one
Step A. Preparation of ethyl 6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-7-
(4,6-d imethyl p yrimidin-5-y1)-1H-ind ole-2-carb oxylate
[00268] Ethyl 7-bromo-6-chl oro-3 -(3 -(4-chl oro-3,5-dimethylphenoxy)propy1)-
1H-indole-2-
carboxylate (1.647 g, 3.30 mmol) was combined with (4,6-dimethylpyrimidin-5-
yl)boronic
acid (1.2 g, 7.90 mmol), Pd2(dba)3 (0.30 g, 0.33 mmol), and dicyclohexyl(2',6'-
dimethoxy-
[1,1'-bipheny1]-2-yl)phosphine (SPhos) (0.406 g, 0.990 mmol) in 30 mL 1:1
THF/Toluene in
a pressure tube. The mixture was treated with K3PO4 (1.16 mL, 11.6 mmol) and
was stirred
minutes at RT under Ar flush. The reaction was heated to 110 C and stirred
for 24 h.
The mixture was cooled, poured in 150 rriL Et0Ac and stirred with 50 mL
saturated
ammonium chlroide. The organic layer was dried over anhydrous MgSO4 and was
concentrated in vacuo. The crude material was purified by flash chromatography
(Et0Ac/DCM = 0-50% gradient) to give the title compound (970 mg, 56 %) as an
amber
foam. 11-1 NMR (400 MHz, Chloroform-d) 6 9.09 (s, 1H), 8.93 (s, 1H), 7.68 (dd,
J = 8.6, 0.6
Hz, 1H), 7.25 (d, J = 8.7 Hz, 1H), 6.62 (s, 2H), 4.33 (q, J = 7.1 Hz, 2H),
3.97 (t, J = 6.1 Hz,
2H), 3.28 (dd, J = 8.1, 6.8 Hz, 2H), 2.32 (s, 6H), 2.20 (s, 6H), 2.21 -2.10
(m, 2H), 1.36 - 1.20
(m, 3H); 13C NMR (101 MHz, cdc13) 6 165.98, 161.85, 157.76, 156.71, 137.03,
134.32,
130.42, 127.16, 127.11, 126.11, 124.93, 124.67, 121.97, 118.44, 114.45, 67.35,
61.10, 30.24,
22.26, 21.21, 20.96, 14.32.
Step B. Preparation of ethyl 1-(3-((tert-butoxycarbonyl)amino)propy1)-6-chloro-
3-(3-(4-
chloro-3,5-dimethylphenoxy)propy1)-7-(4,6-dimethylpyrimidin-5-y1)-1H-indole-2-
carboxylate
[00269] To a solution of ethyl 6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-7-
(4,6-dimethylpyrimidin-5-y1)-1H-indole-2-carboxylate (500 mg, 0.952 mmol) and
tert-butyl
1,2,3-oxathiazinane-3-carboxylate 2,2-dioxide (300 mg, 1,26 mmol) in DMF (20
mL) was
added NaH (60 mg, 1.50 mmol), and the reaction was stirred at RT for 3 h. The
reaction was
diluted with DCM/H20 (60 mL, 1:1), and the layers were separated. The aqueous
layer was
extracted with DCM (2 x 30 mL), and the combined organic extracts were dried
over MgSO4,
141
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
filtered, and concentrated in vacuo. The crude reaction mixture was purified
by flash
chromatography (Combi-flash RI, Hex/Et0Ac = 0-100% gradient) to afford the
title
compound (525 mg, 81%).
Step C. Example 120
[00270] To a solution of ethyl 1-(3 -((tert-butoxy carbonyl)amino)propy1)-6-
chloro-3-(3-(4-
chloro-3,5-dimethylphenoxy )propy1)-7-(4,6-dimethylpyrimidin-5-y1)-1H-indole-2-
carboxylate in dioxane (20 mL) was added HC1 in dioxane (4.0 M, 3.0 mL, 12
mmol), and
the reaction was stirred for 72 h at RT. The reaction was concentrated in
vacuo, and the
crude residue was taken up in Me0H (30 mL). K2CO3 (300 mg, 2.17 mmol) was
added, and
the reaction was stirred for 6 h at 50 C. The reaction was concentrated in
vacuo, and the
residue extracted with DCM (3 x 30 mL) and washed with H20 (30 mL). The
combined
organic extracts were dried over MgSO4, filtered, and concentrated in vacuo.
The crude
reaction mixture was then purified by flash chromatography (Combi-flash Rf,
CH2C12/methanol = 0-10% gradient) to afford the title compound (243 mg, 58%).
LCMS:
= 1.947 mm, MS (ES) 537.3 (M+H).
Example 121
(R)-6-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] indo1-2(1H)-y1)-1-
methyl-1H-
indazole-3-carboxylic acid
[00271] The title compound (19 mg, 53%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-1(210-one
(27 mg, 0.05
mmol), methyl 6-bromo-1-methy1-1H-indazole-3-carboxylate (14 mg, 0.06 mmol),
Cu! (5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 jit, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =-
2.024 min, MS (ES) 713.2 (M+H); 11-1 NMR (400 MHz, DMSO-d6) 8 8.57 - 7.98 (m,
1H),
7.82- 7.69 (m, 1H), 7.63 (dd, J = 12.1, 7.1 Hz, 1H), 7.29 (dd, J = 8.6, 4.6
Hz, 2H), 6.74 (s,
2H), 4.74 - 4.45 (m, 1H), 4.45 - 4.32 (m, 1H), 4.25 - 4.08 (m, 2H), 4.01 (s,
3H), 4.00 - 3.95
(m, 1H), 3.79 (s, 1.5H), 3.78 (s, 1.5H), 3.74 -3.60 (m, 1H), 2.77 - 2.62 (m,
1H), 2.26 (s, 6H),
2.12 (s, 1.5H), 2.06 - 2.00 (m, 2H), 1.98 (s, 1.5H), 1.89 (s, 1.5H), 1.76 (s,
1.5H), 1.05 (d, J=
6.3 Hz, 3H).
Example 122
142
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
7-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-4,5-dihydro-111-11,4] diazepino [ 1,2-a] ind ol-2(3H)-y1)-1-
ethyl- 1H-in dazole-
5-carboxylic acid
Step A. Preparation of methyl 7-bromo-1-ethyl-1H-indazole-5-carboxylate
[00272] The title compound (42 mg, 37%) was prepared following the procedure
described
in Example 82 Step A using methyl 7-bromo-1H-indazole-5-carboxylate (100 mg,
0.392
mmol), Nal-1 (18 mg, 0.75 mmol), and EtBr (80 mg, 0.74 mmol).
Step B. Example 137
[00273] The title compound (3 mg, 9%) was prepared following General coupling
procedure A using 8-chl oro-11 -(3-(4-chl oro-3,5 -dimethy 1ph en oxy )p
ropy1)-7-(1,3,5 -trimethyl-
1H-pyrazol-4-y1)-2,3,4,5-tetrahy dro-1H-[1,4] diazepino[1,2-a] indol-1 -one
(25 mg, 0.046
mmol), methyl 7-bromo-1-ethyl-1H-indazole-5-carboxylate (20 mg, 0.074 mmol),
Pd2(dba)3
(4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg, 0.153
mmol)
followed by saponification using General Procedure D. LCMS: RT = 2.033 min, MS
(ES)
727.2 (Md-H).
Example 123
7-(8-Chloro-11-(3-(4-chlo ro-3,5-d i methyl phenoxy)p ropy1)-1-oxo-7-(1,3,5-
trimethy1-1H-
pyrazol-4-y1)-4,5-dihydro-1H-11,41diazepino [1,2-al indo1-2(3H)-y1)-1-methy1-
1H-
indazole-5-carboxylic acid
Step A. Preparation of methyl 7-bromo-1-methyl-1H-indazole-5-carboxylate
[00274] The title compound (64 mg, 61%) was prepared following the procedure
described
in Example 82 Step A using methyl 7-bromo-l-methyl-1H-indazole-5-carboxylate
(100 mg,
0.392 mmol), NaH (18 mg, 0.75 mmol), and Mel (75 mg, 0.52 mmol).
Step B. Example 123
[00275] The title compound (5 mg, 15%) was prepared following General coupling
procedure A using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(1,3,5-trimethy1-
1H-pyrazol-4-y1)-2,3,4,5-tetrahydro-1H41,41 diazepino[1,2-a] indol-1 -one (25
mg, 0.046
mmol), methyl 7-bromo-1-methy1-1H-indazole-5-carboxylate (20 mg, 0.074 mmol),
Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT = 1.926
min, MS
(ES) 712.9 (M+H).
Example 124
143
84451174
7-(8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-7-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-4,5-dihydro-11141,41diazepin o [1,2-a] indo1-2(3H)-y1)-2-ethyl-
211-in dazole-5-
carb oxylic acid
Step A. Preparation of methyl 7-bromo-2-ethyl-2H-indazole-5-carboxylate
[00276] The title compound (29 mg, 26%) was prepared in the same reaction as
described in
Example 122 Step A.
Step 2: Example 124
[00277] The title compound (3.9 mg, 12%) was prepared following General
Procedure A using
8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(1,3,5-trimethy1-1H-
pyrazol-4-y1)-
2,3,4,5-tetrahydro-1H- [1,4]diazepino[1,2-a]indol-l-one (25 mg, 0.046 mmol),
methyl 7-bromo-
2-ethy1-2H-indazole-5-carboxylate (20 mg, 0.074 mmol), Pd2(dba)3 (4 mg, 0.0043
mmol),
Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg, 0.153 mmol) followed by
saponification
using General Procedure D. LCMS: RT = 1.895 min, MS (ES) 726.9 (M+H).
Example 125
4- [(4R)-7-chlor o-10-[3-(4-chlor o-3,5-dim ethyl-ph enoxy)propyl] -4-methyl-l-
oxo-6-(1,3,5-
trime thylpyrazol-4-y1)-3,4-d ihydropyrazino[1,2-al ind ol-2-yl] -1-methyl-
indazole-3-
carboxylic acid
1002781 The title compound (4 mg, 11%) was prepared following General coupling
procedure
A using (R)-7-chloro-10-(3 -(4 -chloro-3,5 -dimethy 1phenoxy)propy1)-4-methy1-
6-(1,3,5-trimethyl-
1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-cdindol-1(2H)-one (27 mg, 0.050
mmol), methyl 4-
bromo-1-methy1-1H-indazole-3-carboxylate (20 mg, 0.074 mmol), Pd2(dba)3 (5 mg,
0.0055
mmol), Xantphos (7 mg, 0.012 mmol), and Cs2CO3 (50 mg, 0.153 mmol) followed by
saponification using General Procedure D. LCMS: RT = 1.944 min, MS (ES) 713.2
(M+H).
Example 126
4- [(4R)-7-chlor o-1043-(4-chlor o-3,5-dim ethyl-ph enoxy)prop yl] -4-methyl-l-
oxo-6-(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-2-y11-1-methyl-indazole-
3-
carboxylic acid (separated steroisomer A)
[00279] The title compound (8 mg, 20%) was prepared following General coupling
procedure
A using (R)-7-chl oro-10-(3 -(4 -chl oro-3,5 -dimethy 1ph enoxy)propy1)-4-
methy1-6-(1,3,5-trimethyl-
1H-pyrazol-4-y1)-3,4-dihy dropyrazino[1,2-c]indo1-1(2H)-one (30 mg, 0.052
mmol), methyl 4-
bromo- 1-methy1-1H-indazole-3-carboxylate (30 mg, 0.11 mmol), Pd2(dba)3 (5 mg,
0.0055 mmol),
Xantphos (7 mg, 0.012 mmol), and Cs2CO3 (50 mg, 0.153 mmol) followed by
saponification
using General Procedure D. LCMS: RT =
1.944 min, MS (ES)
144
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
713.2 (M+H). The title compound was separated using reverse phase HPLC
(Phenomenex
Gemini C18, H20/CH3CN gradient from 30-90% CH3CN, 0.1% TFA) during the final
purification step to give as a separated atropisomer (1st fraction, absolute
stereochemistry
undeterminded). MS (ES) 713.2 (M+H).
Example 127
4-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)pr0py11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2-yl] -1-methyl-ind
azole-3-
carboxylic acid (separated steroisomer B)
[00280] The title compound was separated along with Example 141 as a separated
atropisomer (2" fraction). MS (ES) 713.2 (M+H).
Example 128
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-dimethy1-1-
((tetrahyd ro-2H-p yran-4-yl)methyl)- 1H-p yrazol-4-y1)-4-methyl-1-oxo-3,4-
d ihyd ropy razino [1,2-a] indo1-2(1H)-y1)-1-methyl-1H-indole-3-carboxylic
acid
Step A. Preparation of methyl (R)-7-
(7-chloro-10-(3-(4-chloro-3,5-
d imethy 1p hen oxy)p ro py1)-6-(3,5-dimethyl-1-((tetrahyd ro-2H- py ran-4-
yl)methyl)-1H-
pyrazol-4-y1)-4-methyl-1-oxo-3,4-dihyd ropyrazino11,2-al indo1-2(1H)-y1)-1-
methyl-11/-
indole-3-carboxylate
[00281] To a solution of methyl (R)-7-
(7-chl oro-10-(3-(4-chl oro-3 ,5-
di methylph enoxy )propy1)-6-(3,5-dimethy1-1H-py razol-4-y1)-4-methy1-1 -oxo-
3,4-
dihy dropyrazino[1,2-a] indo1-2(1H)-y1)-1-methyl-1H-indole-3-carboxylate (20
mg, 0.028
mmol) in DMF (0.5 mL) was added NaH (1.4 mg, 0.034 mmol) and stirred for 15
min at RT.
4-(Bromomethyl)tetrahydro-2H-pyran (5.5 mg, 0.031 mmol) was added, and the
resulting
mixure was stirred overnight then concentrated in vacuo. The residue was
purified by flash
chromatography (Combi-flash Rf, Hex/Et0Ac = 0-70% gradient) to give the title
compound
(20 mg, 88%) as white foam. LCMS: RT = 2.107 min, MS (ES) 810.3 (M+H).
Step B. Example 128
[00282] The title compound (10 mg, 51%) was prepared following General
Procedure D for
saponification using methyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphen
oxy )propy1)-6-
(3,5-dimethy1-1-((tetrahy dro-2H-py ran-4-y pmethyl)-1H-py razol-4-y 0-4-
methy1-1-oxo-3,4-
dihy dropyrazino[1,2-a] indo1-2(1H)-y1)-1 -methyl-1H-indol e-3-carboxylate (20
mg, 0.025
mmol). LCMS: RT = 2.124 min, MS (ES) 796.3 (M+H).
Example 129
145
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-d imethylp henoxy)p ropy1)-6-(3,5-d
imethyl- 1H-
pyrazol-4-y1)-4-methyl-1-oxo-3,4-d ihyd ro pyrazin o [ 1,2-a] ind ol-2(1H)-y1)-
1-methyl-1H-
ind ole-5-carboxylic acid
[00283] The title compound was prepared following the procedure described in
Example
109 Step A and B using (R)-7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-6-(3,5-
di methy1-1-((2-(tri methylsilyl)ethoxy)methyl)-1H-py razol-4-y1)-4-methy1-3,4-
dihy dropy razino [1,2-a] indo1-1(211)-one and
methyl 3 -bromo-1-methyl- 1H-i ndol e-5-
carboxylate. LCMS: RT = 1.872 min, MS (ES) 698.2 (M+H).
Example 130
6-18-Chloro-11-[3-(4-chloro-3,5-dimethyl-phenoxy)propy1]-7-(4,6-
dimethylpyrimidin-5-
y1)-1-oxo-4,5-dihydro-3H-11,41diazepino 11,2-a] indo1-2-yl] -1-methyl-indole-4-
carboxylic
acid
[00284] The title compound (2 mg, 6%) was prepared following General coupling
procedure A using 8-
chloro-11-(3-(4-chloro-3,5-di methy 1phenoxy)propy1)-7-(4,6-
di methy 1py rimidin-5-y1)-2,3,4,5-tetrahy dro-1H-[1,4] diazepino [1,2-a]
indol-1 -one (25 mg,
0.046 mmol), methyl 6-bromo-1-methy1-1H-indole-4-carboxylate (20 mg, 0.067
mmol),
Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.012
min, MS
(ES) 710.2 (M+H).
Example 131
6-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihyd ropyrazino 11,2-al ind ol-2-y1]-1-iso p rop
yl-ind azole-4-
carboxylic acid
[00285] The title compound (8 mg, 23%) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -tri methy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino [1,2-a] indol-1
(21I)-one (25 mg, 0.046
mmol), methyl 6-bromo-1-isopropy1-1H-indazole-4-carboxylate (20 mg, 0.067
mmol),
Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.119
min, MS
(ES) 741.3 (M+H).
Example 132
4-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihyd ropyrazino[1,2-a] ind ol-2-yl] -2-methyl-
indazole-3-
carboxylic acid
146
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00286] The title compound (12 mg, 34%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino [1,2-a] ind 01-1
(210-one (27 mg, 0.050
mmol), methyl 4-bromo-2-methyl-2H-indazole-3-carboxylate (25 mg, 0.074 mmol),
Pd2(dba)3 (5 mg, 0.0055 mmol), Xantphos (7 mg, 0.012 mmol), and Cs2CO3 (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT = 1.940,
1.971
min, MS (ES) 713.2 (M+H)
Example 133
4-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihyd ropyrazino[1,2-a] indo1-2-yl] -2-methyl-
indazole-3-
carboxylic acid (separated steroisomer A)
[00287] The title compound was separated using reverse phase HPLC (Phenomenex
Gemini C18, H20/CH3CN gradient from 30-90% CH3CN, 0.1% TFA) during the final
purification step to give as a separated atropisomer (Pt fraction, absolute
stereochemistry
undeterminded). LCMS: RT = 1972 min, MS (ES) 713.2 (M+H).
Example 134
(R)-2-(4-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-
6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino11,2-al indo1-2(11/)-y1)-
1H-indol-1-
yDacetic acid
Step A. Preparation of tert-butyl (R)-4-(7-chloro-10-(3-(4-chloro-3,5-
dimethyl phen oxy)p ropy1)-4-methy1-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-
3,4-
dihyd ropy razino [1,2-a] ind ol-2(1H)-y1)-1H-ind ole-1-carb oxylate
[00288] The title compound (35 mg, 93%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino [1,2-a] ind 01-1
(2H)-one (27 mg, 0.05
mmol), tert-butyl 4-bromo-1H-indole-1-carboxylate (18 mg, 0.06 mmol),
Pd2(dba)3 (1 mg,
0.001 mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol).
LCMS: RT
= 2.453 min, MS (ES) 754.30 (M+H).
Step B. Example 134
[00289] To a solution of tert-butyl (R)-4-
(7-chl oro-10-(3-(4-chl oro-3,5 -
di methylph enoxy )propy1)-4-methy1-1-oxo-6-(1,3,5 -trimethy1-1H-pyrazol-4-y1)-
3,4-
dihydropyrazino[1,2-cdindo1-2(1H)-y1)-1H-indole-l-carboxylate (35 mg, 0.046
mmol) in
DCM (2 mL) was added TFA (2 mL), and the mixture was stirred at room
temperature for 6
h. Saturated aqueous NaHCO3 (8 mL) was added to the mixture and extracted in
DCM (3 x 5
147
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
mL). The organic layer was dried (anhyd. Na2SO4), evaporated. The residue was
dissolved in
DMF (1 mL), and NaH (5 mg, 0.12 mmol, 1.2 eqv.) was added then stirred at RT
for 10 min.
Ethyl bromoacetate (14 [IL, 0.12 mmol, 1.2 eqv.) was added to the mixture and
stirred at RT
for 2 h. The reaction was quenched with water (5 mL) and extracted with DCM
(3x5 mL).
The organic layer was dried (anhyd. Na2SO4) and concentrated. The residue was
subjected to
saponification using General Procedure D and purified by reverse phase HPLC
(Phenomenex
Gemini C18, H20/CH3CN gradient to 35-95 % MeCN 0.1% TFA) to afford the title
compound (16 mg, 45%). LCMS: RT = 1.748 min, MS (ES) 712.2 (M+H).
Example 135
(R)-2-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-111-pyrazol-4-y1)-3,4-dihydropyrazino11,2-alindol-2(11/)-y1)-11,2,41
triazolo[1,5-a]pyridine-7-carboxylic acid
1002901 The title compound (43% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-cdindo1-1(211)-one
and methyl 2-
bromo-[1,2,41triazo1o[1,5-alpyridine-7-carboxylate followed by saponification
using General
Procedure D. MS (ES) 700.4 (M+H); 1H NMR (DMSO, 400 MHz) 6 (ppm) 9.00 (dd, J
7.2,
3.6 Hz, 1H), 8.22 (d, J 8.0 Hz, 1H), 7.76 (d, J= 8.8 Hz, 1H), 7.58 (dd, J=
7.2, 1.2 Hz, 1H),
7.29 (d, J= 8.8, 1.2 Hz, 1H), 6.73 (s, 2H), 4.37-4.19(m, 3H), 4.13-3.95 (m,
2H), 3.76 (d, J
8.8 Hz, 3H), 3.36-3.15 (m, 2H), 2.24 (s, 6H), 2.09-2.05 (m, 2H), 2.08 (s,
1.5H), 1.99 (s, 1.5H),
1.97 (s, 1.5H), 1.87 (s, 1.5H), 0.95 (dd, J= 9.2, 6.8 Hz, 3H).
Example 136
2-(4-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-2-phenyl-
2,3,4,5-
tetrahydro-111-11,41diazepino [1,2-a] ind ol-7-yl)-3,5-d imethyl-1H-py razol-1-
yl)acetic acid
Step A. Preparation of 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-7-
(3,5-
dimethyl-111-pyrazol-4-y1)-2-pheny1-2,3,4,5-tetrahydro-1H-11,4] diazepino[1,2-
al ind ol-1-
one
1002911 The title compound was prepared according to procedures described in
Example 58
step F to G using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(3,5-
dimethy1-1-
02-06 methy ls ily Dethoxy )methyl)-1H-py razol -4-y 0-2,3,4,5-tetrahy dro-1H-
[1,41diazepino[1,2-a]indol-l-one and aniline. MS (ES) 601.2 (M+H)
Step B. Example 136
[00292] To a solution of 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-
7-(3,5-
dimethy1-1H-py razol-4-y 0-2-pheny1-2,3,4,5-tetrahy dro-1H-[1,4] di azepino
[1,2-a] indol-l-one
148
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(0.05 mmol) in DMF (1 mL) was added NaH (0.08 mmol) at RT, and the reaction
was stirred
for 30 min. Methyl 2-chloroacetate (0.1 mmol) was added, and the reaction was
stirred for 5
h at RT then diluted with DCM/H20 (20 mL, 1:1). The organic layer was
separated and the
aqueous layer was extracted with DCM (2 x 5 mL). The combined organic extracts
were
concentrated in vacuo. The crude reaction product was then saponified and
purified
following the procedure described in General Procedure D to afford the title
compound (71%
yield). MS (ES) 659.2 (M+H).
Example 137
(R)-7-(7-chloro-10-(3-(4-chlo ro-3,5-d imethyl phenoxy)p ro py1)-6-(3,5-d
imethy1-1-((1-
methyl piperidin-4-yl)methyl)-1H-pyrazol-4-y1)-4-methyl-1-oxo-3,4-
d ihydropyrazino [1,2-al indo1-2(1H)-y1)-1-methy1-1H-indole-3-carboxylic acid
Step A. Preparation of methyl (R)-7-
(7-chloro-10-(3-(4-chloro-3,5-
d imethy 1p hen oxy)propy1)-6-(3,5-dimethy1-1-((1-methylpiperidin-4-y1)methyl)-
1H-
pyrazol-4-y1)-4-methyl-1-oxo-3,4-dihyd ropyrazino[1,2-a] indo1-2(1H)-y1)-1-
methyl-1H-
ind o le-3-c arboxylate
[00293] To a solution of methyl (R)-7-
(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy )propy1)-6-(3,5-dimethy1-1H-pyrazol-4-y1)-4-methyl-l-oxo-3,4-
dihy dropy razino [1,2-a] i ndo1-2(1H)-y1)-1 -methyl- 1H-indol e-3-carboxy 1
ate (20 mg, 0.028
mmol) and (1-methylpiperidin-4-yl)methanol (7.25 mg, 0.056 mmol) in Toluene
(932 I) was
added 2-(tributy1-15-phosphanylidene)acetonitrile (140 1.1, 0.056 mmol) and
stirred at 50 C
for 20 h. The reaction mixture was concentrated, and the residue was purified
by flash
chromatography (Combi-flash Rf, DCM/Me0H = 0-10% gradient) to give the title
compound
(15 mg, 65%). LCMS: RT = 1.901 min, MS (ES) 823.3 (M+H).
Step B. Example 137
[00294] The title compound (5 mg, 34%) was prepared according to General
Procedure G
using methyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(3,5-dimethy1-
1-((1-methylpiperidin-4-y1)methyl)-1H-pyrazol-4-y1)-4-methyl-1-oxo-3,4-
dihy dropy razino [1,2-a] indo1-2(1H)-y1)-1-methy1-1H-indole-3-carboxylate (15
mg, 0.018) and
methyl 6-(bromomethyl)nicotinate. LCMS: RT = 1.740 min, MS (ES) 809.4 (M+H).
Example 138
(R)-6-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihyd ropy razino[1,2-a] indo1-2(1H)-
yl)quinoline-8-
carboxylic acid
[00295] The title compound (66% yield) was prepared following General coupling
149
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
and methyl 6-
bromoquinoline-8-carboxylate followed by saponification using General
Procedure D. MS
(ES) 710.2 (M+H); 11-1-NMR (Me0H-d3) 6 9.12 (dd, 1H, J1= 2 Hz, J1= 5 Hz), 9.90
(dd, 1H,
J1= 2 Hz, J1= 5 Hz), 8.81 (m, 1H), 8.33 (m, 1H) 7.93 (m, 1H), 7.78 (d, 1Hõ J =
8 Hz), 7.32
(d, 1Hõ J = 8 Hz), 6.63 (s, 2H), 4.46 (m, 2H), 4.35 (m, 1H), 4.01-3.96 (m,
2H), 3.87
(multiples, 3H), 3.77-3.74 (m, 1H), 3.49-3.38 (m, 2H), 3.11-3.08 (m, 1H), 2.27
(s, 6H), 2.23-
2.02 (multiples and in, 8H), 1.18 and 1.16 (two d, 3H, J = 8 Hz).
Example 139
(R)-6-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-11-1-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-2(1H)-y1)imidazo
11,2-
alpyridine-2-carboxylic acid
[00296] The title compound (78% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3 ,5-trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino[1,2-a]indo1-1(211)-
one and methyl 6-
bromoimidazo[1,2-a]pyridine-2-carboxylate followed by saponification using
General
Procedure D. MS (ES) 699.2 (M+H).
Example 140
(3R,4R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-3,4-dimethyl-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino [1,2-a] ind ol-1(21/)-one
[00297] The title compound (74% yield) was prepared following same procedures
described in Example 24 substituting tert-butyl (R)-4-ethy1-1,2,3-
oxathiazolidine-3-
carboxylate 2,2-dioxide with tert-butyl (4R,5S)-4,5-dimethy1-1,2,3-
oxathiazolidine-3-
carboxylate 2,2-dioxide. MS (ES) 553.4 (M+H); 11-1-NMR (CDC13) 6 7.70 (d, 1H,
J = 8 Hz),
7.29 (d, 1H, J = 8 Hz), 6.64 (s, 2H), 5.82 (m, 1H), 4.05-3.90 (m, 6H), 3.52-
3.50 (m, 1H),
3.47-3.31 (m, 3H), 2.35 (s, 6H), 2.17 (overlapped tr and multiple s, 8H),
1.21(d, 0.75H, J = 8
Hz), 1.19 (d, 0.75H, J = 8 Hz), 1.05 (d, 1.5H, J = 8 Hz), 1.03 (d, 1.5H, J = 8
Hz), 0.88 (d,
0.75H, J = 8 Hz), 0.82 (d, 0.75H, J = 8 Hz)
Example 141
3-03R,4R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-3,4-dimethy1-1-
oxo-6-
(1,3,5-trimethy1-11I-pyrazol-4-y1)-3,4-dihydropyrazino 11,2-al indo1-2(11-/)-
y1)benzoic acid
[00298] The title compound (62% yield) was prepared following General coupling
procedure A using (3R,4R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-
3,4-
dimethy1-6-(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-a]
indol-1 (2H)-one
150
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
and methyl 3-bromobenzoate followed by saponification using General Procedure
D. MS
(ES) 673.2 (M+H).
Example 142
4-03R,4R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-3,4-dimethyl-1-
oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino 11,2-al indo1-2(1H)-
yl)benzoic acid
[00299] The title compound (73% yield) was prepared following General coupling
procedure A using (3R,4R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy
)propy1)-3,4-
di methy1-6-(1,3,5 -trimethy1-1H-py razol-4-y 0-3,4-dihy dropy razino [1,2-a]
indo1-1(211)-one
and methyl 4-bromobenzoate followed by saponification using General Procedure
D. MS
(ES) 673.3 (M+H).
Example 143
6-03R,4R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-3,4-dimethyl-1-
oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino 11,2-al ind ol-2(1H)-y1)-
1-methyl-
1H-indole-4-carboxylic acid
[00300] The title compound (8% yield) was prepared following General coupling
procedure
A using
(3R,4R)-7-chl oro-10-(3-(4-chl oro-3,5-dimethy 1phenoxy)propy1)-3,4-di methy1-
6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino[1,2-a] ind ol-1(2H)-
one and methyl 6-
bromo-1-methy1-1H-indole-4-carboxylate followed by saponification using
General
Procedure D. MS (ES) 726.3 (M+H).
Example 144
2-(4-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-2-(quinolin-8-
y1)-
2,3,4,5-tetrahydro-1H-11,41diazepino 11,2-al ind o1-7-y1)-3,5-d imethy1-1H-py
razol-1-
yl)acetic acid
[00301] The title compound (55% yield) was prepared according to procedures
described in
Example 136 step A and B by substituting aniline with 8-bromoquinoline. MS
(ES) 710.2
(M+H)
Example 145
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind 01-2 (1H)-y1)-1,7-d
imethyl- 1H-
ind ole-5-carboxylic acid
Step A. Preparation of ethyl 1,7-dimethy1-1H-indole-5-carboxylate
[00302] A mixture of ethyl 7-bromo- 1 -methy1-1H-indole-5-carboxylate (200 mg,
0.71
mmol), methylboronic acid (57.9 mg, 0.97 mmol), dicyclohexyl(21,61-dimethoxy-
[1,1'-
biphenyl]-2-yOphosphane (79 mg, 0.193 mmol), K3PO4 (253 mg, 1.29 mmol) and
Pd0Ac2
151
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(22 mg, 0.097 mmol) was degased for 10 min under Ar. Toluene (1.5 ml) was
added and the
resulting mixure was heated at 100 C for 3 h. The reaction mixture was
concentrated, and
the residue was purified by flash chromatography (Combi-flash Rf, Hex/Et0Ac =
0-20%
gradient) to give the title compound (140 mg, 99%). LCMS: RT = 1.558 min, MS
(ES) 218.2
(M+H).
Step B. Preparation of ethyl 3-bromo-1,7-dimethyl-1H-indole-5-carboxylate
[00303] To a stirred solution of ethyl 1,7-dimethy1-1H-indole-5-carboxylate
(140 mg, 0.64
mmol) in DMF (2 mL) was added NBS (115 mg, 0.64 mmol) at 0 C. After 1 h, the
reaction
was quenched by addition of H20 (10 mL). The mixture was extracted with DCM
(10 mL X
2). The combined organic solution was washed with H20 (20 mL), dried and
concentrated.
The residue was purified by flash chromatography (Combi-flash Rf, Hex/Et0Ac =
0-10%
gradient) to give the title compound (176 mg, 92%) as a yellow solid.
Step C. Example 145
[00304] The title compound (33 mg, 50%) was prepared following General
coupling
procedure B using R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-1(2H)-one
(50 mg, 0.093
mmol), ethyl 3-bromo-1,7-dimethy1-1H-indole-5-carboxylate (33 mg, 0.11 mmol),
Cul (0.9
mg, 0.005 mmol), ans)-1,2-N,N'-dimethylaminocyclohexane (1.3 mg, 0.0093 mmol),
and
K2CO3 (26 mg, 0.19 mmol) followed by saponification using General Procedure D.
LCMS:
RT = 2.018 min, MS (ES) 726.3 (M+H).
Example 146
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-dimethyl-
14(1-
methylpiperidin-4-yl)methyl)-1H-pyrazol-4-y1)-4-methyl-1-oxo-3,4-
dihydropyrazino11,2-al indol-2(1H)-yl)-1-methyl-1H-indole-5-carboxylic acid
[00305] The title compound (14 mg, 60%) was prepared according to procedures
described
in Example 137 Step A and B using methyl (R)-3-(7-chloro-10-(3-(4-chloro-3,5-
di methylphenoxy )propy1)-6-(3,5-dimethy1-1H-pyrazol-4-y1)-4-methyl-l-oxo-3,4-
dihy dropyrazino[1,2-a]indo1-2(1H)-y1)-1-methy1-1H-indole-5-carboxylate (20
mg, 0.028
mmol) and (1-methylpiperidin-4-yl)methanol (18 mg, 0.14 mmol). MS (ES) 698.2
(M+H).
Example 147
5-18-Chloro-11- 13-(4-chloro-3,5-dimethyl-phenoxy)propyll-7-(4,6-
dimethylpyrimidin-5-
y1)-1-oxo-4,5-dihydro-3H-11,41diazepino11,2-alindo1-2-yll quinoline-8-
carboxylic acid
[00306] The title compound (12.7 mg, 38%) was prepared following General
coupling
procedure A using 8-chl
oro-11 -(3 -(4-chloro-3 ,5-di methy 1phenoxy)propy1)-7-(4,6-
152
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
dimethylpyrimidin-5-y1)-2,3,4,5-tetrahydro-1H-[1,4]diazepino[1,2-a]indol-1-one
(25 mg,
0.046 mmol), methyl 5-bromoquinoline-8-carboxylate (20 mg, 0.075 mmol),
Pd2(dba)3 (4 mg,
0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg, 0.153 mmol)
followed by
saponification using General Procedure D. LCMS: RT = 2.008 min, MS (ES) 708.3
(M+H);
NMR (600 MHz, Chloroform-d) 6 9.20 (s, 1H), 9.00 (dd, J = 4.4, 1.6 Hz, 1H),
8.87 (d, J =
7.8 Hz, 1H), 8.32 (dd, J = 8.6, 1.6 Hz, 1H), 7.81 (d, J = 8.6 Hz, 1H), 7.64
(dd, J = 8.6, 4.3 Hz,
1H), 7.56 (d, J= 7.8 Hz, 1H), 7.39 (d, J= 8.6 Hz, 1H), 6.61 (s, 2H), 3.99 (h,
J= 6.4 Hz, 5H),
3.77 (dt, J= 14.2, 5.7 Hz, 1H), 3.20 (dt, J= 12.5, 7.4 Hz, 2H), 2.42 (s, 3H),
2.39 (s, 3H), 2.33
(s, 6H), 2.21 (quint, J = 6.0 Hz, 2H), 1.95-1,92(m, 1H), 1.83-1.78 (m, 1H).
Example 148
5-[(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-6-(4,6-
dimethy 1pyrimid in-5-y1)-4-methyl- 1-oxo-3,4-d ihyd ro py razino [1,2-a] ind
01-2-
yllnaphthalene-1-carboxylic acid
[00307] The title compound (8.3 mg, 25%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
dimethylpyrimidin-5-y1)-4-methy1-3,4-dihydropyrazino[L2-a]indol-1(211)-one (25
mg, 0.046
mmol), methyl 5-bromo-1-naphthoate (20 mg, 0.075 mmol), Cul (5 mg, 0.026
mmol),
(trans)-1,2-NX-dimethylaminocyclohexane (8 mg, 0.056 mmol), and K2CO3 (20 mg,
0.14
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.107
min, MS
(ES) 707.2 (M+H); NMR
(500 MHz, Chloroform-d) 6 9.14 (s, 1H), 9.05 (t, J = 9.7 Hz,
1H), 8.33 (dd, J = 7.3, 4.3 Hz, 1H), 8.17 (d, J = 10.0 Hz, 0.5H), 7.91 (d, J=
10.0 Hz, 0.5H),
7.81 (dd, J = 8.7, 1.7 Hz, 1H), 7.63 (dt, J = 15.9, 8.0 Hz, 1H), 7.52 (q, J=
8.5 Hz, 1H), 7.44
(d, J = 10.0 Hz, 0.5H), 7.36 (dd, J = 11.5, 8.0 Hz, 1.5H), 6.60 (d, J= 9.0 Hz,
2H), 4.42 (dd, J
= 12.9, 3.8 Hz, 1H), 4.01 (q, J= 6.0 Hz, 2H), 3.83-3.75 (m, 1H), 3.49 - 3.33
(m, 3H), 2.48 (s,
1.5H), 2.46 (s, 1.5H), 2.31 (s, 9H), 2.22 (h, J= 7.5 Hz, 2H), 1.31 (d, J= 3.9
Hz, 1.5H), 1.29
(d,J= 3.9 Hz, 1.5H).
Example 150
8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(3,5-dimethyl-1H-
pyrazol-4-
y1)-2-(quinolin-8-y1)-2,3,4,5-tetrahydro-1F141,41diazepino [1,2-a]ind ol-1-one
[00308] The title compound was prepared following the procedure described in
Example
109 Step A and B using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(3,5-
dimethy1-1-((2-(trimethylsily1)ethoxy)methyl)-1H-pyrazol-4-y1)-2,3,4,5-
tetrahydro-1H-
[1,4]diazepino[1,2-a]indol-1-one and 8-bromoquinoline. MS (ES) 698.2 (M+H).
Example 151
153
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
5-18-Chloro-11- 13-(4-chloro-3,5-dimethyl-phenoxy)p ropyl]-7-(4,6-
dimethylpyrimidin-5-
yl)-1-oxo-4,5-dihydro-3H-11,41diazepino[1,2-al indo1-2-yl[naphthalene-1-
carboxylic acid
[00309] The title compound (6.3 mg, 19%) was prepared following General
coupling
procedure A using 8-chl
oro-11 -(3 -(4-chloro-3 ,5-di methy 1phenoxy)propy1)-7-(4,6-
dimethy 1py rimidin-5-y1)-2,3,4,5-tetrahy dro-1H-[1,41 diazepino[1,2-a] indol-
1 -one (25 mg,
0.046 mmol), methyl 5-bromo-1-naphthoate (20 mg, 0.074 mmol), Pd2(dba)3 (4 mg,
0.0043
mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg, 0.153 mmol) followed by
saponification using General Procedure D. LCMS: RT = 2.079 min, MS (ES) 707.2
(M+H).
Example 152
6-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-6-(4,6-
dimethylpyrimidin-5-y1)-4-methyl-1-oxo-3,4-dihydropyrazino11,2-al in do1-2-yl]
-1-
methyl-indole-4-carboxylic acid
[00310] The title compound (8.1 mg, 25%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
di methy 1py rimidin-5-y1)-4-methy1-3,4-dihy dropyrazino [1,2-a] indo1-1(21-1)-
one (25 mg, 0.046
mmol), methyl 6-bromo-1-methy1-1H-indole-4-carboxylate (20 mg, 0.075 mmol),
CuI (5 mg,
0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2CO3
(20 mg, 0.14 mmol) followed by saponification using General Procedure D. LCMS:
RT =
2.075 min, MS (ES) 710.2 (M+H); 1H NMR (500 MHz, Chloroform-d) 5 9.15 (s, 1H),
7.82 ¨
7.72 (m, 2H), 7.64 (s, 1H), 7.33 (d, J= 8.6 Hz, 1H), 7.24 (d, J= 3.1 Hz, 1H),
7.14 (d, J= 3.2
Hz, 1H), 6.62 (s, 2H), 4.41 (dd, J= 13.0, 3.6 Hz, 1H), 4.00 (t, J= 6.2 Hz,
2H), 3.83 (s, 3H),
3.73 (d, J = 6.0 Hz, 1H), 3.60 (d, J = 12.7 Hz, 1H), 3.47-3.32 (m, 2H), 2.46
(s, 3H), 2.31 (s,
6H), 2.28 (s, 3H), 2.22 (q, J= 6.9 Hz, 2H), 1.20 (d, J= 6.4 Hz, 3H).
Example 153
3- [(4R)-7-chlo ro-10-13-(4-chloro-3,5-dimethyl-phenoxy)p ro pyl]
dimethylpyrimidin-5-yl)-4-methyl-1-oxo-3,4-dihyd ropyrazino11,2-al indo1-2-y11-
1-
methyl-indole-6-carboxylic acid
[00311] The title compound (16.3 mg, 49%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
di methylpy ri mi din-5-y1)-4-methy1-3,4-dihy dropy razino [1,2-a] indol -
1(211)-one (25 mg, 0.046
mmol), methyl 3-bromo-1-methyl-1H-indole-6-carboxylate (20 mg, 0.075 mmol),
CuI (5 mg,
0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2CO3
(20 mg, 0.14 mmol) followed by saponification using General Procedure D. LCMS:
RT =
2.061 mm, MS (ES) 710.2 (M+H); 11-1 NMR (600 MHz, Chloroform-d) 6 9.25 (s,
1H), 7.79
154
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(d, J = 8.6 Hz, 1H), 7.63 (d, J = 1.9 Hz, 1H), 7.58 ¨ 7.51 (m, 1H), 7.34 (d,
J= 8.6 Hz, 1H),
6.65 (s, 2H), 4.35 ¨ 4.30 (m, 4H), 4.01 (t, J= 6.2 Hz, 2H), 3.88 (s, 2H), 3.66
(s, 2H), 3.18 (t,
J= 7.5 Hz, 2H), 2.45 (s, 6H), 2.34 (s, 6H), 2.23 (dd, J= 10.0, 4.5 Hz, 2H),
1.73 (s, 2H).
Example 154
5-18-Chloro-1143-(4-chloro-3,5-dimethyl-phenoxy)propy11-7-(4,6-
dimethylpyrimidin-5-
y1)-1-oxo-4,5-dihydro-3H-11,41diazepino [1,2-a] ind ol-2-y1]-2,3-dihydro-1,4-
benzodioxine-
7-carboxylic acid
[00312] The title compound (5.7 mg, 17%) was prepared following General
coupling
procedure A using 8-chl
oro-11 -(3 -(4-chl oro-3 ,5-di methylphen oxy)propy1)-7-(4,6-
dimethylpyrimidin-5-y1)-2,3,4,5-tetraby dro-1H-[1,41 diazepino[1,2-a] indol-1 -
one (25 mg,
0.046 mmol), methyl 8-bromo-2,3-dihydrobenzo [b][1,4]dioxine-6-carboxylate (20
mg, 0.074
mmol), Pd2(dba)3(4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3
(50 mg,
0.153 mmol) followed by saponification using General Procedure D. LCMS: RT =
1.975
mm, MS (ES) 715.2 (M+H); 1HNMR (600 MHz, Chloroform-d) 6 9.25 (s, 1H), 7.79
(d, J=
8.6 Hz, 1H), 7.63 (d, J= 1.9 Hz, 1H), 7.58 ¨ 7.51 (m, 1H), 7.34 (d, J= 8.6 Hz,
1H), 6.65 (s,
2H), 4.35 ¨4.30 (m, 4H), 4.01 (t, J= 6.2 Hz, 2H), 3.88 (s, 2H), 3.66 (s, 2H),
3.18 (t, J= 7.5
Hz, 2H), 2.45 (s, 6H), 2.34 (s, 6H), 2.23 (dd, J = 10.0, 4.5 Hz, 2H), 1.73 (s,
2H).
Example 155
6-[8-Chloro-11- [3-(4-chloro-3,5-dimethyl-phenoxy)propy11-7-(4,6-
dimethylpyrimidin-5-
y1)-1-oxo-4,5-dihydro-3H- [1,41diazepino[1,2-al ind ol-2-yl]-1-methyl-ind
azole-4-
carboxylic acid
[00313] The title compound (9 mg, 27%) was prepared following General coupling
procedure A using 8-
chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(4,6-
dimethylpyrimidin-5-y1)-2,3,4,5-tetrahy dro-1H-[1,41 diazepino[1,2-a] indol-1 -
one (25 mg,
0.046 mmol), methyl 6-bromo-1-methy1-1H-indazole-4-carboxylate (20 mg, 0.074
mmol),
Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT -= 1.960
min, MS
(ES) 711.3 (M+H); 1-H NMR (600 MHz, Chloroform-a) 6 9.22 (s, 1H), 8.51 (d, J=
0.9 Hz,
1H), 7.83 ¨ 7.76 (m, 2H), 7.74 ¨ 7.70 (m, 1H), 7.36 (d, J = 8.6 Hz, 1H), 6.65
(s, 2H), 4.11 (s,
3H), 4.02 (t, J= 6.1 Hz, 2H), 3.89 (t, J= 6.4 Hz, 2H), 3.83 (t, J = 6.0 Hz,
2H), 3.22 (dd, J=
8.3, 6.5 Hz, 2H), 2.40 (s, 6H), 2.32 (s, 6H), 2.20 (s, 2H), 1.90¨ 1.85 (m,
2H).
Example 156
155
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
3-1[8-Ch lo ro-11- [3-(4-chloro-3,5-dimethyl-phenoxy)p ropy1]-7-(4,6-
dimethylpyrimidin-5-
y1)-1-oxo-4,5-dihydro-3H-11,41diazepino [1,2-alindo1-2-y1[4-methyl-indole-5-
carboxylic
acid
[00314] The title compound (7.5 mg, 23%) was prepared following General
coupling
procedure A using 8-
chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(4,6-
dimethylpyrimidin-5-y1)-2,3,4,5-tetrahydro-1H41,4]diazepino[1,2-a]indol-1-one
(25 mg,
0.046 mmol), methyl 3-bromo-l-methyl-1H-indole-5-carboxylate (20 mg, 0.074
mmol),
Pd2(dba)3 (4 mg, 0.0043 mmol), Xantphos (6 mg, 0.010 mmol), and Cs2CO3 (50 mg,
0.153
mmol) followed by saponification using General Procedure D. LCMS: RT = 1.964
min, MS
(ES) 710.2 (M+H); NMR
(600 MHz, Chloroform-d) 6 9.21 (s, 1H), 8.25 (s, 1H), 8.02
(ddd, J = 8.8, 4.1, 1.5 Hz, 1H), 7.79 (dd, J = 8.6, 6.2 Hz, 1H), 7.37 (dd, J =
24.7, 8.6 Hz, 2H),
7.16 (s, 1H), 6.65 (d, J= 4.6 Hz, 2H), 4.01 (t, J= 6.1 Hz, 2H), 3.90 (s, 2H),
3.84 (s, 3H), 3.79
(t, J= 6.0 Hz, 2H), 3.21 (t, J= 9.0 Hz, 2H), 2.40 (s, 6H), 2.32 (s, 6H), 2.25
(quint, J = 6.0 Hz,
2H), 1.84-1.80 (m, 2H).
Example 157
(R)-2-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino[1,2-al ind ol-2(11/)-
yl)quinoline-5-
carboxylic acid
[00315] The title compound (22 mg, 62%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 2-chloroquinoline-5-carboxylate (16 mg, 0.06 mmol), Pd2(dba)3 (1
mg, 0.001
mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification using General Procedure D. LCMS: RT = 1.323 min, MS (ES) 710.2
(M+H).
Example 158
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-d imethylp hen oxy) p rop y1)-4-methy1-1-
oxo-6-(1,3,5-
trimethyl- 111-py razol-4-y1)-3,4-d ihyd ro pyrazino [1,2-a] in d ol-2(1H)-y1)-
1-p ro pyl-1H-
indole-5-carboxylic acid
Step A. Preparation of propyl 3-bromo-1-propy1-1H-indole-5-carboxylate
[00316] The title compound (56 mg, 61%) was prepared following General
Procedure F
using 1H-indole-5-carboxylic acid (46 mg, 0.28 mmol), NBS (51 mg, 0.28 mmol),
NaH (25
mg, 0.62 mmol) and 1-iodopropane (61 L, 0.48 mmol). LCMS: RT = 1.761 min, MS
(ES)
324.2 (M+H).
156
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Step B. Example 158
[00317] The title compound (29 mg, 78%) was prepared following General
coupling
procedure B using (R)-7-chl oro-10-(3-(4-chl oro-3,5 -dimethy 1phenoxy)propy1)-
4-methyl-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino [1,2-a] ind 01-1
(21/)-one (27 mg, 0.05
mmol), propyl 3-bromo-1-propy1-1H-indole-5-carboxylate (15 mg, 0.06 mmol), CuI
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 [IL, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
2.104 min, MS (ES) 740.2 (M+H); 11-1 NMR (400 MHz, DMSO-d6) 6 8.11 (d, J = 1.6
Hz,
1H), 7.84 - 7.69 (m, 2H), 7.62 (dd, J= 8.2, 1.7 Hz, 2H), 7.29 (d, J = 8.6 Hz,
1H), 6.73 (s,
2H), 4.48 (dt, J= 12.7, 3.2 Hz, 1H), 4.28 -4.10 (m, 4H), 3.98 (s, 3H), 3.78
(s, 1.5H), 3.77 (s,
1.5H), 3.71 -3.56 (m, 2H), 3.44 - 3.10 (m, 2H), 2.24 (s, 6H), 2.12 (s, 1.5H),
2.11 -2.04 (m,
2H), 2.03 (s, 1.5H), 1.98 (s, 1.5H), 1.89(s, 1.5H), 1.80 (h, J = 7.4 Hz, 2H),
1.09 (d, J = 6.4
Hz, 3H), 0.88 (td, J = 7.4, 2.7 Hz, 3H).
Example 159
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind 01-2 (1H)-y1)-1,5-d
imethyl-1H-
indole-3-carboxylic acid (separated stereoisomer A)
Step A. Preparation of 7-bromo-5-methyl-1H-indole-3-carboxylic acid
To a stirred solution of 7-bromo-5-methyl-1H-indole (300 mg, 1.43 mmol) in DMF
(1 mL)
was added TFAA (0.24 mL, 1.71 mmol) dropwiseat at 0 C. The reaction solution
was
warmed up to rt and stirred overnight. The reaction was quenched with H20 (10
mL) then
extracted with DCM (10 mL X 2). The combined organic solution was dried and
concentrated in vacuo. The residue was dissolved in H20 (1 mL) and NaOH (200
mg, 5.00
mmol) was added. The reaction mixture was refluxing for 3 h then neutralized
with HC1 (6
N). The resulting mixture was extracted with Et0Ac (20 mL X 2). The combined
organic
solution was dried, concentrated and purified by reverse phase HPLC
(Phenomenex Gemini
C18, H20/CH3CN gradient to 20-80 % MeCN 0.1% TFA) to give the title compound
(100
mg, 30%). LCMS: RT = 1.252 min, MS (ES) 254.1 (M+H).
Step B. Preparation of methyl 7-bromo-1,5-dimethy1-1H-indole-3-carboxylate
[00318] To a stirred solution of 7-bromo-5-methyl-1H-indole-3-carboxylic acid
(100 mg,
0.394 mmol) in DMF (1 mL) was added NaH (38 mg, 1.57 mmol) at 0 C. After 15
mm, Mel
(0.098 mL, 1.574 mmol) was added, and the resulting mixture was stirred for 1
h. The
reaction was quenched with H20 (3 mL) then extracted with DCM (10 mL X 2). The
157
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
combined organic solution was dried, concentrated to give the title compound.
It was used
for next step without further purification. MS (ES) 282.1 (M+H)
Step C. Example 159
[00319] The title compound (70 mg, 60%) was prepared following General
coupling
procedure A using (R)-7-chl oro-10-(3 -(4-chl oro-3,5-dimethy 1phenoxy
)propy1)-4-methy1-6-
(1,3,5 -trimethy1-11-1-py razol-4-y1)-3,4-dihy dropy razino [1,2-a] indo1-
1(2H)-one (200 mg, 0.37
mmol), methyl 7-bromo-1,5-dimethy1-1H-indole-3-carboxylate (115 mg, 0.41
mmol),
Pd2(dba)3 (20 mg, 0.022 mmol), Xantphos (43 mg, 0.074 mmol), and Cs2CO3 (242
mg, 0.74
mmol) followed by saponification using General Procedure D. During the final
purification
step, the title compound was isolated as a separated atropisomer (lst
fraction, absolute
stereochemistry undeterminded). LCMS: RT = 2.044 min, MS (ES) 726.3 (M+H).
Example 160
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-6-(3,5-dimethyl-
111-
pyrazol-4-yl)-4-methyl-1-oxo-3,4-dihyd ro pyrazin o [1,2-a] ind ol-2(1H)-y1)-
1,7-dimethyl-
1H-indole-5-carboxylic acid
[00320] The title compound was prepared following the procedure described in
Example
109 Step A and B using (R)-7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-6-(3,5-
dimethy1-1-((2-(trimethylsily1)ethoxy)methyl)-1H-pyrazol-4-y1)-4-methyl-3,4-
dihy dropy razino [1,2-a] indo1-1(211)-on e and ethyl 3-bromo-1,7-dimethy1-1H-
indole-5-
carboxylate. MS (ES) 712.2 (M+H).
Example 161
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-dimethyl-
14(1-
methylpiperidin-4-yl)methyl)-1H-pyrazol-4-y1)-4-methyl-1-oxo-3,4-
dihydropyrazino [1,2-a]indol-2(1H)-yl)-1,7-dimethyl-1H-ind ole-5-carboxylic
acid
[00321] The title compound (25 mg, 74%) was prepared according to procedures
described
in Example 137 Step A and B using ethyl (R)-3-(7-chloro-10-(3-(4-chloro-3,5-
di methylphenoxy )propy1)-6-(3,5-dimethy1-1H-pyrazol-4-y1)-4-methyl-l-oxo-3,4-
dihy dropyrazino[1,2-a]indol-2(1H)-y1)-1,7-dimethyl-1H-indole-5-carboxylate
(34 mg, 0.046
mmol) and (1-methylpiperidin-4-yl)methanol (30 mg, 0.23 mmol). LCMS: RT =
1.767 min,
MS (ES) 823.3 (M+H).
Example 162
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyI)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-2(1H)-y1)-1,5-
dimethyl-111-
indole-3-carboxylic acid (separated stereoisomer B)
158
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00322] The title compound was isolated along with Example 159 as a separated
atropisomer (2nd fraction, absolute stereochemistry undeterminded). LCMS: RT =
2.074
mm, MS (ES) 726.3 (M+H).
Example 163
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] indo1-2(1H)-y1)-1-
methy1-1H-
ind azole-5-carb oxylic acid
[00323] The title compound (20 mg, 56%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y 0-3,4-dihy dropy razino [1,2-a] ind 01-1
(211)-one (27 mg, 0.05
mmol), methyl 3-bromo-1-methy1-1H-indazole-5-carboxylate (17 mg, 0.06 mmol),
Pd2(dba)3
(1 mg, 0.001 mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075
mmol)
followed by saponification using General Procedure D. LCMS: RT = 1.994 min, MS
(ES)
713.2 (M+H).
Example 164
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydro pyrazino [1,2-al indo1-2(11/)-y1)-1-
ethyl-11-1-
ind ole-5-carboxylic acid
Step A. Preparation of ethyl 3-bromo-1-ethy1-1H-indole-5-carboxylate
[00324] The title compound (51 mg, 61%) was prepared following General
Procedure F
using 1H-indole-5-carboxylic acid (46 mg, 0.28 mmol), NBS (51 mg, 0.28 mmol),
NaH (25
mg, 0.62 mmol) and EtI (53 tit, 0.62 mmol). LCMS: RT = 1.756 mm, MS (ES) 296.1
(M+H).
Step B. Example 164
[00325] The title compound (34 mg, 94%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5 -tri methy1-1H-pyrazol-4-y1)-3,4-dihy dropy razin o [1,2-a] ind 01-1
(211)-one (27 mg, 0.05
mmol), ethyl 3-bromo-1-ethy1-1H-indole-5-carboxylate (15 mg, 0.06 mmol), Cut
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 viL, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
1.155 mm, MS (ES) 726.3 (M+H).
Example 165
159
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-l-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-2(1H)-y1)-1-methyl-
W-
indazole-7-carboxylic acid
1003261 The title compound (27 mg, 76%) was prepared following General
coupling
procedure B using (R)-7-chl oro-10-(3-(4-chl oro-3,5 -dimethy 1phenoxy
)propy1)-4-methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-a] ind ol-1
(211)-one (27 mg, 0.05
mmol), methyl 3-bromo-1-methy1-1H-indazole-7-carboxylate (15 mg, 0.06 mmol),
CuI (5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 pL, 0.05 mmol), and
K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
2.058 min, MS (ES) 713.2 (M+H).
Example 166
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-l-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino11,2-alindol-2(1H)-y1)-1-
isopropyl-1H-
ind ole-5-carboxylic acid
Step A. Preparation of isopropyl 3-bromo-1-isopropy1-1H-indole-5-carboxylate
[00327] The title compound (28 mg, 30%)was prepared following General
Procedure F
using 1H-indole-5-carboxylic acid (46 mg, 0.28 mmol), NBS (51 mg, 0.28 mmol),
NaH (25
mg, 0.62 mmol) and 2-iodopropane (61 :at, 0.62 mmol). LCMS: RT = 1.765 mm, MS
(ES)
324.2 (M+H).
Step B. Example 166
[00328] The title compound (13 mg, 37%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropy razin o [1,2-a] indol-1
(211)-one (27 mg, 0.05
mmol), isopropyl 3-bromo-1-isopropy1-1H-indole-5-carboxylate (15 mg, 0,06
mmol), CuI (5
mg, 0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 L, 0.05 mmol),
and
K2CO3 (25 mg, 0.075 mmol) followed by saponification using General Procedure
D. LCMS:
RT = 2.087 min, MS (ES) 740.2 (M+H).
Example 167
(R)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] indo1-2(1H)-y1)-1-
methy1-1H-
pyrrolo 12,3-b] pyridine-6-carboxylic acid
[00329] The title compound (87% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
160
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(210-one
and methyl 4-
bromo-1-methy1-1H-pyrrolo[2,3-13]pyridine-6-carboxylate followed by
saponification using
General Procedure D. MS (ES) 713.3 (M+H); IHNMR (DMSO, 400 MHz) 6 (ppm) 7.83
(d,
J = 4.8 Hz, 1H), 7.78 (d, J = 8.8 Hz, 1H), 7.75 (d, J = 3.2 Hz, 1H), 7.29 (d,
J = 8.8 Hz, 1H),
6.70 (s, 2H), 6.44 (dd, J = 8.4, 3.2 Hz, 1H), 4.71-4.64(m, 1H), 4.20-4.10 (m,
2H), 3.99-3.95
(m, 2H), 3.89 (s, 3H), 3.73 (s, 3H), 3.33-3.20 (m, 2H), 2.23 (s, 6H), 2.09 (s,
1.5H), 2.09-2.03
(m, 2H), 2.00(s, 1.5H), 1.98(s, 1.5H), 1.89(s, 1.5H), 1.00 (dd, = 6.4, 3.6 Hz,
3H).
Example 168
7-[(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-6-(4,6-
dimethylpyrimidin-5-y1)-4-methy1-1-oxo-3,4-dihydropyrazino[1,2-al in d ol-2-
yl] -1-
methyl-indole-3-carboxylic acid
[00330] The title compound (3.8 mg, 12%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
di methy 1py rimidin-5-y1)-4-methy1-3,4-dihy dropy razino [1,2-allindo1-1(2H)-
one (25 mg, 0.046
mmol), methyl 7-bromo-1-methyl-1H-indole-3-carboxylate (20 mg, 0.075 mmol),
CuI (5 mg,
0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2CO3
(20 mg, 0.14 mmol) followed by saponification using General Procedure D. LCMS:
RT =
2.056 min, MS (ES) 710.1 (M+H); 1-1-1 NMR (500 MHz, Chloroform-a) 6 9.12 (s,
1H), 8.25
(d, J 7.9 Hz, 0.7H), 8.21 (d, J 7.9 Hz, 0.3H), 7.79 (s, 0.7H), 7.77 (s, 1.3H),
7.36 (d, J
8.9 Hz, 1.3H), 7.32 (d, J = 7.8 Hz, 0.7H), 7.05 (d, J = 7.6 Hz, 1.3H), 7.00
(d, J = 7.6 Hz,
0.7H), 6.62 (s, 1.3H), 6.57 (s, 0.7H), 4.16 (dd, J= 13.5, 3.5 Hz, 1H), 4.08
¨3.96 (m, 2H),
3.93 (s, 1H), 3.84-3.71 (m, 3H) 3.54 (dd, J= 23.0, 13.2 Hz, 1H), 3.37 (dq, J=
14.4, 7.2 Hz,
2H), 2.44 (s, 3H), 2.34-2.28 (m, 6H), 2.26 (s, 3H), 2.23-2.16 (m, 2H), 1.30
(d, J = 6.5 Hz,
2H), 1.19 (d, J = 6.5 flz, 1H).
Example 169
(R)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino[1,2-a] indo1-2(1H)-y1)-1-
methy1-1H-
pyrrolo [2,3-b] pyridine-2-carboxylic acid
[00331] The title compound (90% yield) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
and methyl 4-
bromo-1-methy1-1H-pyrrolo[2,3-b]pyridine-2-carboxylate followed by
saponification using
General Procedure D. MS (ES) 713.3 (M+H); IFINMR (DMSO, 400 MHz) 6 (ppm) 8.47
(d,
J = 4.8 Hz, 1H), 7.76 (d, J = 8.8 Hz, 1H), 7.29 (d, J = 8.4 Hz, 1H), 7.15 (d,
J = 5.2 Hz, 1H),
161
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
7.08 (s, 1H), 6.70 (s, 2H), 4.68-4.63(m, 1H), 4.18-4.09 (m, 2H), 4.06 (s, 3H),
4.02-3.95 (m,
2H), 3.76 (s, 3H), 3.37-3.16 (m, 2H), 2.25 (s, 3H), 2.22 (s, 6H), 2.06 (s,
1.5H), 2.03-1.99 (m,
2H), 1.98 (s, 1.5H), 1.89 (s, 1.5H), 0.99 (d, J= 6.4 Hz, 3H).
Example 170
2-1(4R)-7-chloro-1043-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methyl-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihyd ropy razino 11,2-al indo1-2-y11-1-methyl-ind
ole-4-
carboxylic acid
Step A. Preparation of methyl 2-bromo-1H-indole-4-carboxylate
[00332] To a solution of methyl 2-oxoindoline-4-carboxylate (100 mg, 0.523
mmol) in
DCE (5 mL) were added POBr3 (880 mg, 3.05 mmol) and imidazole (140 mg, 2.06
mmol).
The reaction was heated to 80 C and stirred for 16 h. The reaction was cooled
to RT,
quenched with H20 (20 mL) and extracted with DCM (3 x 20 mL). The combined
organic
extracts were dried over MgSO4, filtered, and concentrated in vacuo. The crude
reaction
mixture was purified by flash chromatography (Combi-flash PS, Hex/Et0Ac = 0-
100%
gradient) to afford the title compound (27 mg, 20%).
Step B. Preparation of methyl 2-bromo-1-methyl-1H-indole-4-carboxylate
[00333] The title compound (21 mg, 75%) was prepared following General
Procedure F
using methyl 2-bromo-1H-indole-4-carboxylate, NaH (6 mg, 0.25 mmol) and Mel
(30 mg,
0.211 mmol)..
Step C. Example 170
[00334] The title compound (9.7 mg, 29%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-1(210-one
(25 mg, 0.046
mmol), methyl 2-bromo-1-methy1-1H-indole-4-carboxylate (20 mg, 0.075 mmol),
CuI (5 mg,
0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2CO3
(20 mg, 0.14 mmol) followed by saponification using General Procedure D. LCMS:
RT =
2.116 min, MS (ES) 712.1 (M+H); NMR
(600 MHz, Chloroform-d) 6 7.84 (dt, J= 7.7,
1.4 Hz, 1H), 7.56 (dd, 8.6,
5.8 Hz, 1H), 7.41 (t, J= 7.4 Hz, 1H), 7.22 ¨ 7.14 (m, 2H), 6.88
(s, 1H), 6.46 (d, J= 1.5 Hz, 2H), 4.29-4.09 (m, 2H), 3.87-3.83 (m, 2H), 3.79
(s, 3H), 3.52 (s,
1.5H), 3.51 (s, 1.5H), 3.47 ¨ 3.37 (m, 1H), 3.27 (dtd, J= 12.5, 7.3, 5.0 Hz,
1H), 3.19 (dt, J=
116, 7.4 Hz, 1H), 2.17 (s, 6H), 2.10 (s, 1.5H), 2.09 (s, 1.5H), 2.05-2.02 (m,
2H), 1.93 (s,
1.5H), 1.91 (s, 1.5H), 1.07 (br. s, 1.5H), 1.03 (br. S, 1.5H).
Example 171
162
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-5-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-l-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2(1H)-y1)- 1-
methyl- 1H-
ind ole-3-carboxylic acid
[00335] The title compound (30 mg, 84%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino[1,2-a] ind ol-1 (2H)-
one (27 mg, 0.05
mmol), methyl 5-bromo-l-methy1-1H-indole-3-carboxylate (16 mg, 0.06 mmol),
Pd2(dba)3 (1
mg, 0.001 mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol)
followed
by saponification using General Procedure D. LCMS: RT = 2.018 min, MS (ES)
712.2
(M+H); NMR
(400 MHz, DMSO-d6) 6 8.10 (d, J= 0.9 Hz, 1H), 7.94 (t, J= 2.1 Hz, 1H),
7.74 (d, J = 8.6 Hz, 1H), 7.56 (d, J = 8.8 Hz, 1H), 7.29 (d, J = 8.6 Hz, 1H),
7.23 (td, J = 8.9,
2.1 Hz, 1H), 6.74 (s, 2H), 4.49 (dd, J= 12.9, 3.8 Hz, 1H), 4.21 -4.10 (m, 1H),
4.01 - 3.94 (m,
3H), 3.88 (s, 3H), 3.78 (s, 1.5H), 3.77 (s, 1.5H), 3.23 (h, J= 6.9 Hz, 2H),
2.25 (s, 6H), 2.12 (s,
1.5H), 2.07 (q, J= 7.1, 6.5 Hz, 2H), 2.03 (s, 1.5H), 1.98 (s, 1.5H), 1.88 (s,
1.5H), 1.05 (dd, J
= 6.5, 3.8 Hz, 3H).
Example 172
(R)-4-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethyl-111-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] indo1-2( 1H)-
yl)quinoline-6-
c arb oxyli c acid
[00336] The title compound (26 mg, 73%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-1(2H)-one
(27 mg, 0.05
mmol), methyl 4-bromoquinoline-6-carboxylate (16 mg, 0.06 mmol), Pd2(dba)3 (1
mg, 0.001
mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification using General Procedure D. LCMS: RT = 1.816 min, MS (ES) 710.2
(M+H).
Example 173
4-1(4R)-7-chloro-1043-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] in d ol-2-y11-1-ethyl-ind
ole-3-carb oxylic
acid
Step A. Preparation of methyl 4-bromo-1-ethy1-1H-indole-3-carboxylate
[00337] To a solution of 4-brorno-1H-indole-3-carboxylic acid (700 mg, 2.92
mmol) in
THF (20 mL) and Me0H (1 mL) was added trimethylsilyldiazomethane (2.0 M
solution in
Et20, 1.6 mL, 3.2 mmol) was added at 0 C, and the reaction mixture was
stirred for 2 h then
warmed to RT. The reaction was quenched with AcOH, concentrated in vacuo, and
the crude
163
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
residue was purified by flash chromatography (Combi-flash Rf, Hex/Et0Ac = 0-
100%
gradient) to give methyl 4-bromo-1H-indole-3-carboxylic acid (513 mg, 69%
yield). The
title compound (52 mg, 93%) was prepared following General Procedure F using
methyl 4-
bromo-1H-indole-3-carboxylic acid (50 mg, 0.20 mmol), NaH (9 mg, 0.375 mmol)
and EtBr
(40 mg, 0.367 mmol).
Step B. Example 173
[00338] The title compound (11.6 mg, 35%) was prepared following General
coupling
procedure B using (R)-7-chl oro-10-(3-(4-chl oro-3,5 -dimethylph enoxy)propy1)-
4-methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazin o [1,2-a] indol-1 (211)-
one (25 mg, 0.046
mmol), methyl 4-bromo-1-ethy1-1H-indole-3-carboxylate (20 mg, 0.071 mmol), Cu!
(5 mg,
0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2CO3
(20 mg, 0.14 mmol) followed by saponification using General Procedure D. LCMS:
RT =
1.996, 2.029 min, MS (ES) 726.1 (M+H).
Example 174
(4R)-7-chloro-10-13-(4-chloro-3,5-d imethyl- phenoxy)p ropy11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2-y1]-1-methyl-ind
ole-5-
carboxylic acid
Step A. Preparation of methyl 2-bromo-1-methy1-1H-indole-5-carboxylate
[00339] The title compound (33 mg, 23%) was prepared following the procedures
described in Example 170 Step A using methyl 2-oxoindoline-5-carboxylate (100
mg, 0.52
mmol), POBr3 (400 mg, 1.4 mmol), imidazole (100 mg, 1.47 mmol), and Step B
using NaH
(6 mg, 0.25 mmol) and Mel (30 mg, 0.21 mmol).
Step B. Example 174
[00340] The title compound (8.9 mg, 27%) was prepared following General
coupling
procedure B using (R)-7-chl oro-10-(3-(4-chl oro-3,5 -dimethy 1phenoxy)propy1)-
4-methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino [1,2-a] indol-1
(211)-one (25 mg, 0.046
mmol), methyl 2-bromo-1-methy1-1H-indole-5-carboxylate (20 mg, 0.074 mmol),
Cu! (5 mg,
0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2C 03
(20 mg, 0.14 mmol) followed by saponification using General Procedure D. LCMS:
RT =-
2.097 min, MS (ES) 712.2 (M+H); 11-1 NMR (600 MHz, Chloroform-d) 5 8.38 (s,
1H), 8.00
(ddd, J = 8.6, 3.0, 1.6 Hz, 1H), 7.72 (dd, J = 8.6, 4.6 Hz, 1H), 7.39¨ 730 (m,
2H), 6.62 (s,
2H), 6.41 (d, J = 2.4 Hz, 1H), 4.44-4.24 (m, 2H), 4.03-4.00 (m, 2H), 3.96 (s,
3H), 3.69 (s,
1.5H), 3.68 (s 1.5H), 3.62 ¨ 3.53 (m, 1H), 3.44 (dt, J= 14.1, 7.3 Hz, 1H),
3.35 (dt, J= 13.1,
164
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
7.5 Hz, 1H), 2.33 (d, J= 1.3 Hz, 6H), 2.26 (s, 1.5H), 2.24 (s, 1.5H), 2.23-
2.18 (m, 2H), 2.09
(s, 1.5H), 2.08 (s, 1.5H), 1.23 (d, J= 6.2 Hz, 1.5H), 1.22 (d, J= 6.2 Hz,
1.5H).
Example 175
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyI)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-2(1H)-y1)-1-methyl-
1H-
indazole-4-carboxylic acid
[00341] The title compound (9 mg, 25%) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazino[1,2-a] indol-1 (211)-
one (27 mg, 0.05
mmol), methyl 3-bromo-1-methy1-1H-indazole-4-carboxylate (17 mg, 0.06 mmol),
Pd2(dba)3
(1 mg, 0.001 mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075
mmol)
followed by saponification using General Procedure D. LCMS: RT = 1.948 min, MS
(ES)
713.2 (M+H).
Example 176
3-18-Chloro-11-13-(4-chloro-3,5-dimethyl-phenoxy)p ropyl1-7-(4,6-
dimethylpyrimidin-5-
y1)-1-oxo-4,5-dihydro-3H-11,41diazepino 11,2-a]indol-2-y11-1-methyl-indole-6-
carboxylic
acid
[00342] The title compound (5.4 mg, 20%) was prepared following General
coupling
procedure B using 8-chl
oro-11 -(3 -(4-chl oro-3,5-di methylphen oxy)propyI)-7-(4,6-
dimethy 1pyrimidin-5-y1)-2,3,4,5-tetrahy dro-1H-[1,4] diazepino [ 1,2-a] indol-
1 -one (20 mg,
0.037 mmol), methyl 3-bromo-1-methy1-1H-indole-6-carboxylate (20 mg, 0.074
mmol), Cu!
(5 mg, 0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056
mmol), and
K2CO3 (40 mg, 0.29 mmol) followed by saponification using General Procedure D.
LCMS:
RT = 2.020 min, MS (ES) 710.2 (M+H).
Example 177
3-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propyl1-6-(4,6-
dimethylpyrimidin-5-yl)-4-methyl-1-oxo-3,4-dihydropyrazino11,2-alindol-2-y11-1-
methyl-indole-5-carboxylic acid
[00343] The title compound (11.2 mg, 43%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
dimethylpyrimidin-5-y1)-4-methy1-3,4-dihydropyrazino[1,2-a]indo1-1(211)-one
(20 mg, 0.037
mmol), methyl 3-bromo-1-methy1-1H-indole-5-carboxylate (20 mg, 0.074 mmol),
CuI (5 mg,
0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2CO3
(40 mg, 0.29 mmol) followed by saponification using General Procedure D. LCMS:
RT =
165
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
2.018 min, MS (ES) 710.2 (M+H); 11-1 NMR (500 MHz, Chloroform-a) 8 9.16 (s,
1H), 8.30 (s,
1H), 8.00 (d, J = 10.0 Hz, 1H), 7.77 (d, J = 8.5 Hz, 1H), 7.36 (d, J = 5.0 Hz,
1H), 7.35 (d, J =
10.0 Hz, 1H), 6.62 (s, 2H), 4.38 (dd, J = 13.1, 3.7 Hz, 1H), 4.00 (s, 2H),
3.83 (s, 3H), 3.74 (s,
1H), 3.62 (d, J= 12.7 Hz, 1H), 3.39 (m, 2H), 2.44 (s, 3H), 2.31 (s, 6H), 2.29
(s, 3H), 2.25-
2.18 (m, 3H), 1.24 (d, J= 6.5 Hz, 3H).
Example 178
(R)-7-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] indo1-2(1H)-y1)-1-
methy1-1H-
ind ole-2-carboxylic acid
Step A. Preparation of ethyl 7-bromo-1-methyl-1H-indole-2-carboxylate
[00344] The title compound (45 mg, 80%) was prepared following General
Procedure G
using ethyl 7-bromo-1H-indole-2-carboxylate (54 mg, 0.20 mmol), NaH (10 mg,
0.24 mmol)
and Mel (15 L, 0.24 mmol). LCMS: RT = 1.882 min, MS (ES) 282.1 (M+H).
Step B. Example 178
[00345] The title compound (16 mg, 45%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazino[1,2-a] indol-1 (211)-
one (27 mg, 0.05
mmol), ethyl 7-bromo-1-methy1-1H-indole-2-carboxylate (14 mg, 0.06 mmol), Cul
(5 mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 1.1L, 0.05 mmol),
and K2CO3
(25 mg, 0.075 mmol) followed by saponification using General Procedure D.
LCMS: RT =
2.059 min, MS (ES) 712.2 (M+H).
Example 179
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-al indo1-2(1H)-y1)- 1-
methyl-1H-
indazole-4-carboxylic acid (separated stereoisomer, 2" eluting atropisomer)
[00346] The title compound (absoluted configuration unknown, 6 mg, 17%) was
isolated
from Example 175 using reverse phase HPLC (Phenomenex Gemini C18, H20/CH3CN
gradient to 35-95 % MeCN 0.1% TFA, 10 mins gradient) as the second eluting
stereoisomer.
LCMS: RT = 1.961 min, MS (ES) 713.2 (M+H).
Example 180
2-1(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methyl-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] in d ol-2-y1]-1-methyl-ind
ole-6-
carboxylic acid
166
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Step A. Preparation of methyl 2-bromo-1-methyl-1H-indole-6-carboxylate
[00347] The title compound (74 mg, 53%) was prepared following the procedures
described in Example 170 Step A using methyl 2-oxoindoline-6-carboxylate (100
mg, 0.52
mmol), POBr3 (400 mg, 1.39 mmol), and imidazole (100 mg, 1.47 mmol) and Step B
using
NaH (15 mg, 0.63 mmol) and Mel (60 mg, 0.42 mmol).
Step B. Example 180
[00348] The title compound (11.9 mg, 36%) was prepared following General
coupling
procedure B using (R)-7-chl oro-10-(3-(4-chl oro-3,5 -dimethylph enoxy)propy1)-
4-methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazin o [1,2-a] indol-1 (211)-
one (25 mg, 0.046
mmol), methyl 2-bromo-1-methy1-1H-indole-6-carboxylate (20 mg, 0.074 mmol),
CuI (5 mg,
0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2CO3
(20 mg, 0.14 mmol) followed by saponification using General Procedure D. LCMS:
RT =
2.096 min, MS (ES) 712.2 (M+H); IFINMR (600 MHz, Chloroform-a) 6 7.96 (d, J=
3.0 Hz,
1H), 7.66 (dt, J= 8.3, 1.8 Hz, 1H), 7.52 (dd, J= 8.6, 4.6 Hz, 1H), 7.41 (dd, J
= 8.3, 1.5 Hz,
1H), 7.13 (dd, J= 8.6, 6.0 Hz, 1H), 6.42 (d, J= 2.2 Hz, 2H), 6.16 (s, 1H),
4.28 - 4.02 (m,
2H), 3.90 - 3.78 (m, 2H), 3.74 (s, 3H), 3.51 (s, 1.5H), 3.50 (s, 1.5H), 3.41-
3.31 (m, 1H), 3.29
-3.22 (m, 1H), 3.14 (m, 1H), 2.13 (s, 6H), 2.05 (s, 1.5H), 2.03 (s, 1.5H),
2.02- 1.97 (m, 2H),
1.88 (d, J = 6.2 Hz, 3H), 1.03 (d, J= 3.9 Hz, 1.5H), 0.97 (d, J.-- 5.6 Hz,
1.5H).
Example 181
4-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methyl-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino 11,2-al indo1-2-yl] -1-(2-
methoxyethyl)indole-
3-carboxylic acid
Step A. Preparation of methyl 4-bromo-1-(2-methoxyethyl)-1H-indole-3-
carboxylate
[00349] The title compound (58 mg, 94%) was prepared following the procedure
described
in Example 173 Step A using methyl 4-bromo-1H-indole-3-carboxylate (50 mg,
0.20 mmol),
NaH (9 mg, 0.38 mmol), and 1-bromo-2-methoxyethane (50 mg, 0.36 mmol).
Step B. Example 181
[00350] The title compound (13.7mg, 39%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropy razin o [1,2-a] indol-1
(211)-one (25 mg, 0.046
mmol), methyl 4-bromo-1-(2-methoxyethyl)-1H-indole-3-carboxylate (25 mg, 0.080
mmol),
Cul (5 mg, 0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056
mmol),
and K2CO3 (20 mg, 0.14 mmol) followed by saponification using General
Procedure D.
LCMS: RT = 1.956, 1.908 min, MS (ES) 756.1 (M+H).
167
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Example 182
4- [(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)pr0py11-4-methyl-1-oxo-
6-(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino indo1-2-y11-1-methyl-ind
carboxylic acid
Step A. Preparation of methyl 4-bromo-1-methyl-1H-indole-3-carboxylate
[00351] The title compound (51 mg, 97% yield) was prepared following the
procedure
described in Example 173 Step A using methyl 4-bromo-1H-indole-3-carboxylate
(50 mg,
0.20 mmol), NaH (9 mg, 0.38 mmol), and Mel (50 mg, 0.35 mmol).
Step B. Example 182
[00352] The title compound (11 mg, 34%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino[1,2-a] ind ol-1 (2H)-
one (25 mg, 0.046
mmol), methyl 4-bromo-1-methy1-1H-indole-3-carboxylate (20 mg, 0.074 mmol),
CuI (5 mg,
0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2CO3
(20 mg, 0.14 mmol) followed by saponification using General Procedure D. LCMS:
RT =
1.952, 1.988 min, MS (ES) 712.00 (M+H).
Example 183
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethyl- 1H-py razol-4-y1)-3,4-d ihyd ro pyrazino [1,2-a] ind 01-2 (1H)-y1)-
1,5-d imethy1-11/-
ind ole-3-carboxylic acid
[00353] The title compound was prepared following General coupling procedure A
using
(R)-7-chl oro-10-(3-(4-chl oro-3,5 -dimethy 1phenoxy)propy1)-4-methyl-6-(1,3,5-
trimethyl-1H-
py razol-4-y hy
dropy razino [1,2-a] indo1-1(211)-one and methyl 7-bromo-1,5 -dimethyl-
1H-indole-3-carboxylate followed by saponification using General Procedure D.
MS (ES)
726.3 (M+H).
Example 184
(R)-5-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl4H-pyrazol-4-y1)-3,4-dihyd ro pyrazino[1,2-al ind ol-2(1H)-
yl)quinoline-2-
carboxylic acid
[00354] The title compound was prepared following General coupling procedure A
using
(R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-6-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one and methyl 5-
bromoquinoline-2-
carboxylate followed by saponification using General Procedure D. MS (ES)
710.2 (M+H);
IFINMR (Me0D, 400 MHz) 6 (ppm) 8.56 (d, J= 8.4 Hz, 1H), 8.22 (d, J = 8.8 Hz,
1H), 8.08
168
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(dd, J = 7.6, 1.2 Hz, 1H), 7.83 (s, 1H), 7.79 (d, J= 7.6 Hz, 1H), 7.76 (d, J=
8.4 Hz, 1H), 7.29
(d, J = 7.6 Hz, 1H), 6.70 (s, 2H), 4.33-4.29 (m, 1H), 3.97 (t, J = 6.0 Hz,
2H), 3.85 (s, 3H),
3.72-3.62 (m, 2H), 3.51-3.31 (m, 2H), 2.28 (s, 6H), 2.24 (s, 1.5H), 2.22-2.15
(m, 2H), 2.17 (s,
1.5H), 2.09 (s, 1.5H), 2.09 (s, 1.5H), 2.02 (s, 1.5H), 1.31 (s, 3H).
Example 185
(R)-6-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1 ,2-a] in d ol-2(1H)-y1)-2-
naphthoi c acid
[00355] The title compound (28 mg, 79%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5-trimethyl-1H-pyrazo1-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 6-bromo-2-naphthoate (16 mg, 0.06 mmol), Pd2(dba)3 (1 mg, 0.001
mmol),
Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification
using General Procedure D. LCMS: RT = 2.120 min, MS (ES) 709.0 (M+H).
Example 186
(R)-5-(7-Chloro-10-(3-(4-chloro-3,5-d imethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] in d ol-2(1H)-y1)-2-nap
hthoic acid
(separated stereoisomer, eluting atropisomer)
[00356] The title compound was prepared following General coupling procedure A
using
(R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-6-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-3,4-dihydropyrazino[1,2-c]indol-1(2H)-one (27 mg, 0.05 mmol),
methyl 5-
bromo-2-naphthoate (16 mg, 0.06 mmol), Pd2(dba)3 (1 mg, 0.001 mmol), Xantphos
(2 mg,
0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by saponification using
General
Procedure D and purified by reverse phase HPLC (Phenomenex Gemini C18,
H20/CH3CN
gradient to 35-95 % MeCN 0.1% TFA) to afford the title compound as the first
eluting
stereoisomer (absoluted configuration unknown). LCMS: RT = 2.047 mm, MS (ES)
709.0
(M+H); 1-H NMR (400 MHz, CDC13) ö 8.84 - 8.65 (m, 1H), 8.16 - 7.94 (m, 2H),
7.86 - 7.67
(m, 2H), 7.68 - 7.56 (m, 1H), 7.49 (td, J = 21.2, 7.8 Hz, 1H), 7.37 - 7.31 (m,
1H), 6.71 -6.49
(m, 2H), 4.50 (qd, J = 12.8, 4.0 Hz, 1H), 4.42 - 4.20 (m, 1H), 4.07 - 3.96 (m,
2H), 3.93 (s,
0.75H), 3.92 (s, 1.5H), 3.91 (s, 0.75H), 3.54- 3.35 (m, 3H), 2.32 (s, 6H),
2.29 - 2.24 (m, 2H),
2.21 (s, 0.75H), 2.20 (s, 1.5H), 2.19 (s, 0.75H), 2.11 (s, 0.75H), 2.10 (s,
0.75H), 2.09 (s,
1.5H), 1.40 - 1.21 (m, 3H).
Example 187
169
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-5-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] in d ol-2(1H)-y1)-2-
naphthoic acid
(separated stereoisomer, 2" eluting atropisomer)
[00357] The title compound was isolated along with Example 186 as the second
eluting
stereoisomer (absoluted configuration unknown). MS (ES) 709.0 (M+H).
Example 188
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] in d ol-2(1H)-y1)-1-
naphth oi c acid
[00358] The title compound (29 mg, 82%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-cdindol-1(211)-one
(27 mg, 0.05
mmol), methyl 3-bromo-1-naphthoate (16 mg, 0.06 mmol), Pd2(dba)3 (1 mg, 0.001
mmol),
Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification
using General Procedure D. LCMS: RT = 2.123 and 2.140 mm, MS (ES) 709.0 (M+H);
1H
NMR (400 MHz, DMSO-d6) 6 8.84 (dd, J= 8.1, L8 Hz, 1H), 8.25 (t, J= 1.9 Hz,
1H), 8.12 (s,
1H), 8.05 - 7.94 (m, 1H), 7.77 (d, J= 8.6 Hz, 1H), 7.71 -7.56 (m, 1H), 7.31
(d, J= 8.5 Hz,
1H), 7.11 (s, 0.5H), 6.98 (s, 0.5H), 6.74 (s, 2H), 4.68 - 4.48 (m, 1H), 4.27 -
4.13 (m, 1H),
4.08 - 3.92 (m, 2H), 3.79 (s, 3H), 3.29 (ddt, J= 41.8, 13.5, 6.4 Hz, 3H), 2.24
(s, 6H), 2.13 (s,
1.5H), 2.09 (s, 1.5H), 2.08 - 2.03 (m, 2H), 2.00 (s, 1.5H), 1.90 (s, 1.5H),
1.05 (dd, J= 6.5,
1.6 Hz, 3H),
Example 189
(R)-4-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] in d ol-2(1H)-y1)-1-
naphthoic acid
[00359] The title compound (21 mg, 59%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-c]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 4-bromo-1-naphthoate (16 mg, 0.06 mmol), Pd2(dba)3 (1 mg, 0.001
mmol),
Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification
using General Procedure D. LCMS: R-r = 2.091 min, MS (ES) 709.0 (M+H); 1H NMR
(400
MHz, DMSO-d6) 6 8.91 (dd, J= 8.9, 3.3 Hz, 1H), 8.27- 8.12 (m, 1H), 7.92 (t, J
= 9.3 Hz,
1H), 7.82 - 7.76 (m, 1H), 7.75 - 7.47 (m, 3H), 7.40 - 7.27 (m, 1H), 6.70 (d,
J= 4.6 Hz, 2H),
4.77 - 4.52 (m, 1H), 4.36 - 4.09 (m, 1H), 4.04 - 3.86 (m, 2H), 3.78 (s, 1.5H),
3.75 (s, 1.5H),
3.67 - 3.54 (m, 2H), 3.26- 3.10 (m, 1H), 2.24 (s, 6H), 2.14 (s, 1.5H), 2.12-
2.06 (m, 2H),
2.05 (s, 1.5H), 2.01 (s, 1.5H), 1.92 (s, 1.5H), 1.24 - 1.08 (m, 3H).
170
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Example 190
(R)-1-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethyl-11-1-pyrazol-4-y1)-3,4-dihyd ropyrazino [1,2-a] in d ol-2(1H)-y1)-2-
nap hthoic acid
[00360] The title compound (18 mg, 51%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-114-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-c]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 1-bromo-2-naphthoate (16 mg, 0.06 mmol), Pd2(dba)3 (1 mg, 0.001
mmol),
Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification
using General Procedure D. LCMS: RT = 2.065 and 2.092 min, MS (ES) 709.0
(M+H); 1-1-1
NMR (400 MHz, DMSO-d6) 6 8.20 - 8.05 (m, 2.5H), 8,01 (dd, J= 8.7, 2.1 Hz, 1H),
7.91 -
7.85 (m, 0.5), 7.78 (dd, J= 8.6, 2.7 Hz, 1H), 7.74- 7.60 (m, 1.5H), 7.58 (d,
J= 7.6 Hz, 0.5H),
7.33 (dd, J = 8.6, 2.1 Hz, 1H), 6.70 (d, J = 4.8 Hz, 2H), 4.40 (dq, J = 10.5,
6.2, 5.2 Hz, 1H),
4.29 - 4.14 (m, 1H), 3.95 (t, J= 6.5 Hz, 2H), 3.78 (s, 1.5H), 3.75 (s, 1.5H),
3.30- 3.14 (m,
3H), 2.22 (s, 6H), 2.16 (s, 1H), 2.13 (s, 0.5H), 2.10 (s, 0.5H), 2.07 (s, 1H),
2.05 (s, 0.5H),
2.04- 1.98 (m, 2H), 1.96 (s, 1H), 1.92 (s, 0.5H), 1.87 (s, 1H), 1.32- 1.10 (m,
3H).
Example 191
(R)-8-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino11 ,2-a] in d ol-2(1H)-y1)-1-
naphth oi c acid
[00361] The title compound (7 mg, 20%) was prepared following General coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 8-bromo-1-naphthoate (16 mg, 0.06 mmol), Pd2(dba)3 (1 mg, 0.001
mmol),
Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification
using General Procedure D. LCMS: RT = 2.012 mm, MS (ES) 709.0 (M+H);IFINMR
(400
MHz, DMSO-d6) 6 8.13 (dd, J= 7.5, 2.1 Hz, 1H), 8.04 (dd, J= 8.3, 1.2 Hz, 1H),
7.74 (d, J =
8.6 Hz, 1H), 7.68 (t, J = 7.8 Hz, 1H), 7.63 - 7.53 (m, 2H), 7.52 - 7.42 (m,
1H), 7.30 (d, J =
8.6 Hz, 1H), 6.75 (s, 2H), 4.49 (dd, J= 13.8, 3.9 Hz, 1H), 4.29 - 4.10 (m,
1H), 3.95 (q, J
6.4 Hz, 2H), 3.80 (s, 1.5H), 3.78 (s, 1.5H), 3.26 - 3.04 (m, 3H), 2.24 (s,
6H), 2.13 (s, 1.5H),
2.11 - 2.04 (m, 2H), 2.03 (s, 1.5H), 2.00(s, 1.5H), 1.91 (s, 1.5H), 1.17 (d, J
= 6.5 Hz, 3H).
Example 192
(R)-7-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-al ind ol-2(11/)-y1)-4-
meth oxy-1-
methy1-1H-ind ole-2-carboxylic acid
171
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00362] The title compound (11 mg, 30%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino [1,2-a] ind 01-1
(211)-one (27 mg, 0.05
mmol), methyl 7-bromo-4-methoxy -1 -methy 1-1H-indol e-2-carboxy late (18 mg,
0.06 mmol),
Pd2(dba)3 (1 mg, 0.001 mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg,
0.075
mmol) followed by saponification using General Procedure D. LCMS: RT = 1.094
min, MS
(ES) 742.0 (M+H).
Example 193
(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy1]-4-methy1-2-[1-
methyl-5-
(trifluoromethyl)indol-3-y11-6-(1,3,5-trimethylpyrazol-4-y1)-3,4-
dihydropyrazino 11,2-
alindo1-1-one (separated stereoisomer, rd eluting atropisomer)
Step A. Preparation of 3-bromo-1-methy1-5-(trifluoromethyl)-1H-indole
[00363] The title compound (61 mg, 51%) was prepared following General
procedure F
using 5-(trifluoromethyl)-1H-indole (80 mg, 0.43 mmol), NBS (100 mg, 0.561
mmol), NaH
(18 mg, 0.75 mmol), and Mel (90 mg, 0.63 mmol).
Step B. Example 193
[00364] The title compound (7.6 mg, 29%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y 0-3,4-dihy dropy razin o [1,2-a] indol-1
(211)-one (20 mg, 0.037
mmol), 3-bromo-1-methy1-5-(trifluoromethyl)-1H-indole (20 mg, 0.072 mmol), Cul
(5 mg,
0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2CO3
(20 mg, 0.14 mmol) followed by saponification using General Procedure D. The
title
compound was separated using reverse phase HPLC (Phenomenex Gemini C18,
H20/CH3CN
gradient from 30-90% CH3CN, 0.1% TFA) during the final purification step to
give as a
separated atropisomer (2nd fraction, absolute stereochemistry undeterminded).
LCMS: RT =
2.293. min, MS (ES) 736.0 (M+H).
Example 194
(R)-7-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] indo1-2(111)-
yl)benzofuran-2-
carboxylic acid
[00365] The title compound (19 mg, 54%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 7-bromobenzofuran-2-carboxylate (13 mg, 0.06 mmol), Cul (5 mg,
0.025
172
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 ttL, 0.05 mmol), and K2CO3
(25 mg,
0.075 mmol) followed by saponification using General Procedure D. LCMS: RT =
1.103 min,
MS (ES) 699.0 (M+H).
Example 195
(R)-7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-2-(3-oxo-2,3-
dihydro-1H-inden-4-y1)-6-(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-
dihydropyrazino[1,2-
a]indol-1(2H)-one
[00366] The title compound (8 mg, 24%) was prepared following General coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indo1-1(210-one
(27 mg, 0.05
mmol), 7-bromo-2,3-dihydro-1H-inden-1-one (11 mg, 0.06 mmol), Cul (5 mg, 0.025
mmol),
(trans)-1,2-NX-dimethylaminocyclohexane (8 [tL, 0.05 mmol), and K2CO3 (25 mg,
0.075
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.069
min, MS
(ES) 669.1 (M+H).
Example 196
(R)-5-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino[1,2-a] indo1-2(1H)-
yl)benzofuran-2-
carboxylic acid
[00367] The title compound (29 mg, 83%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5-trimethyl-11-1-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one
(27 mg, 0.05
mmol), methyl 5-bromobenzofuran-2-carboxylate (14 mg, 0.06 mmol), Cul (5 mg,
0.025
mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 1.11õ 0.05 mmol), and
K2CO3 (25 mg,
0.075 mmol) followed by saponification using General Procedure D. LCMS: RT =
1.130 min,
MS (ES) 699.0 (M+H); '1-1NMR (400 MHz, DMSO-d6) 6 7.76 (d, J= 2.2 Hz, 2H),
7.74 (d, J
= 2.5 Hz, 1H), 7.68 (d, J= 1.0 Hz, 1H), 7.49 (ddd, J= 8.9, 3.6, 2.2 Hz, 1H),
7.30 (d, J= 8.6
Hz, 1H), 6.73 (s, 2H), 4.47 (d, J= 11.4 Hz, 2H), 4.19 - 4.11 (m, 1H), 3.99 (s,
3H), 3.78 (s,
1.5H), 3.77 (s, 1.5H), 3.74 - 3.61 (m, 2H), 3.22 (dt, J= 13.4, 7.4 Hz, 2H),
2.26 (s, 6H), 2.12
(s, 1.5H), 2.08 (d, J= 13.1 Hz, 2H), 2.03 (s, 1.5H), 1.97 (s, 1.5H), 1.88 (s,
1.5H), 1.04 (d, J-
6.4 Hz, 3H),
Example 197
173
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-4-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl- 1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-yl)-3,4-dihyd ropy razino [1,2-a] ind ol-2(11/)-yl)-1-
is op ro pyl-1H-
indole-2-carboxylic acid
Step A. Preparation of methyl 4-bromo-1-isopropyl-1H-indole-2-carboxylate
[00368] The title compound (35 mg, 59%) was prepared following General
Procedure G
using methyl 4-bromo-1H-indole-2-carboxylate (51 mg, 0.20 mmol), NaH (10 mg,
0.24
mmol) and 2-iodopropane (15 [IL, 0.44 mmol). LCMS: RT = 1.901 min, MS (ES)
296.0
(M+H).
[00369] Step B. Example 197
[00370] The title compound (21 mg, 57%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino [1,2-a] indol-1
(211)-one (27 mg, 0.05
mmol), methyl 4-bromo-1-isopropy1-1H-indole-2-carboxylate (15 mg, 0.06 mmol),
Pd2(dba)3
(1 mg, 0.001 mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075
mmol)
followed by saponification using General Procedure D. LCMS: RT = 1.192 and
1.257 min,
MS (ES) 740.1 (M+H).
Example 198
(R)-4-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyI)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a1 ind ol-2(1H)-y1)-1-
((tetrahyd ro-
2H-pyran-4-yl)methyl)-1H-indole-2-carboxylic acid
Step A. Preparation of methyl 4-bromo-1-((tetrahydro-2H-pyran-4-yl)methyl)-1H-
indole-2-carboxylate
[00371] The title compound (53 mg, 75%) was prepared following General
Procedure G
using methyl 4-bromo-1H-indole-2-carboxylate (51 mg, 0.20 mmol), NaH (10 mg,
0.24
mmol) and 4-(brornomethyl)tetrahydro-2H-pyran (32 [iL, 0.24 mmol). LCMS: RT =
1.776
min, MS (ES) 352.0 (M+H).
Step B. Example 198
[00372] The title compound (31 mg, 78%) was prepared following General
coupling
procedure A using (R)-7-chl oro-10-(3 -(4-chl oro-3,5-dimethy 1phenoxy
)propy1)-4-methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3 ,4-dihy dropyrazino [1,2-a] indol-1
(21/)-one (27 mg, 0.05
mmol), methyl 4-bromo-1 -((tetrahy dro-2H-py ran-4-y Dmethyl)-1H-ind ol e-2-
carboxylate (21
mg, 0.06 mmol), Pd2(dba)3 (1 mg, 0.001 mmol), Xantphos (2 mg, 0.003 mmol), and
Cs2CO3
174
84451174
(25 mg, 0.075 mmol) followed by saponification using Procedure D. LCMS: RT =
2.079 min,
MS (ES) 796.0 (M+H).
Example 199
(4R)-7-chloro-1043-(4-chloro-3,5-dimethyl-phenoxy)propy1]-4-methy1-241-methyl-
6-
(trifluoromethyl)indol-3-y1]-6-(1,3,5-trimethylpyrazol-4-y1)-3,4-
dihydropyrazino [1,2-
a]indo1-1-one (separated stereoisomer, 2" eluting atropisomer)
Step A. Preparation of 3-bromo-1-methyl-6-(triflu oromethyl)-1H-indole
[00373] The title compound (115 mg, 38%) was prepared following General
procedure F using
6-(trifluoromethyl)-1H-indole (200 mg, 1.08 mmol), NBS (240 mg, 1.35 mmol),
NaH (70 mg,
2.92 mmol), and Mel (220 mg, 1.55 mmol).
Step B. Example 199
[00374] The title compound (7.4 mg, 28%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(20 mg, 0.037
mmol), 3-bromo-1-methy1-6-(trifluoromethyl)-1H-indole (20 mg, 0.072 mmol), Cul
(5 mg, 0.026
mmol), (trans)-1,2-N,/V'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2CO3 (20 mg,
0.14 mmol) followed by saponification using General Procedure D. The title
compound was
separated using reverse phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient
from 30-
90% CH3CN, 0.1% 11-A) during the final purification step to give as a
separated atropisomer
(2nd fraction, absolute stereochemistry undeterminded). LCMS: RT = 2.312 min,
MS (ES) 736.0
(M+H); 1H NMR (400 MHz, Chloroform-d) 8 7.74 (dd,J= 8.6, 3.5 Hz, 1H), 7.64 (d,
Jr 8.8 Hz,
1H), 7.55 (d, J= 8.4 Hz, 1H), 7.41 ¨7.34 (m, 1H), 7.34 ¨ 7.28 (m, 2H), 6.61
(s, 2H), 4.41 (d, J=
11.9 Hz, 1H), 4.22 (s, 1H), 4.05 (s, 3H), 4.00 (tt, J= 6.2, 3.1 Hz, 2H), 3.88
(s, 2H), 3.85 (s, 1H),
3.64 (cl, J = 12.6 Hz, 1H), 3.39 (m, 2H), 2.31 (cl, J = 2.0 Hz, 6H), 2.27 (s,
3H), 2.22 (m, 5H),
1.23 (d, J= 5.5 Hz, 1H), 1.19 (d, J= 5.5 Hz, 2H).
Example 200
(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propyl] -4-methyl-241-
methyl-5-
(triflu oromethyl)indo1-3-yl] -6-(1,3,5-trimethylpyrazol-4-y1)-3,4-
dihydropyrazino [1,2-
a] ind ol-1-one (separated stereoisomer, 1st eluting atropisomer)
[00375] The title compound (6.9 mg, 26%) was separated as the Pt eluting
atropisomer with
Example 193. LCMS: RT = 2.267 min, MS (ES) 736.0 (M+H).
Example 201
175
Date Recue/Date Received 2023-06-16
84451174
(R)-7-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] indo1-2(1H)-y1)-1,5-dim
ethyl-1H-
indole-2-carboxylic acid
Step A. Preparation of methyl 7-bromo-1,5-dimethy1-1H-indole-2-carboxylate
[00376] The title compound (24 mg, 43%) was prepared following General
Procedure G using
7-bromo-5-methyl-1H-indole-2-carboxylic acid (51 mg, 0.20 mmol), NaH (16 mg,
0.40 mmol)
and Mel (26 !IL 0.40 mmol). LCMS: RT = 1.761 min, MS (ES) 282.1 (M+H).
Step B. Example 201
[00377] The title compound (22 mg, 61%) was prepared following General
coupling procedure
A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethy 1phenoxy)propy1)-4-methy1-6-
(1,3,5-trimethyl-
1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one (27 mg, 0.05 mmol),
methyl 7-
bromo-1,5-dimethy1-1H-indole-2-carboxylate (17 mg, 0.06 mmol), Pd2(dba)3 (1
mg, 0.001
mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification using General Procedure D. LCMS: RT = 1.163 min, MS (ES) 726.1
(M+H).
Example 202
(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propyl]-4-methyl-241-methyl-
6-
(frith oromethyl)ind ol-3-yl] -6-(1,3,5-trimethylp yrazol-4-y1)-3,4-
dihydropyrazin o [1,2-
a]indo1-1-one (separated stereoisomer, 1st eluting atropisomer)
[00378] The title compound (6.5 mg, 24%) was separated as the Pt eluting
atropisomer with
Example 199. LCMS: RT = 2.285 min, MS (ES) 736.0 (M+H); 1H NMR (400 MHz,
Chlorofoun-d) 8 7.75 (d, J= 8.6 Hz, 1H), 7.64 (d, J= 9.1 Hz, 1H), 7.55 J=
8.4 Hz, 1H), 7.37
(d, J = 8.3 Hz, 1H), 7.32 (dd, J = 8.8, 3.8 Hz, 2H), 6.61 (s, 2H), 4.40 (s,
1H), 4.09 (s, 4H), 4.03 ¨
3.93 (m, 2H), 3.89 (s, 2H), 3.86 (s, 1H), 3.66 (s, 1H), 3.52 ¨ 3.27 (m, 2H),
2.35 (s, 3H), 2.32 (s,
6H), 2.23-2.11 (m, 5H), 1.26 (s, 3H).
Example 203
(R)-7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-2-(1-oxo-2,3-
dihydro-
11/-ind en-4-y1)-6-(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino [1,2-
a] indo1-1(211)-
one
[00379] The title compound (24 mg, 72%) was prepared following General
coupling procedure
B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethy 1phenoxy)propy1)-4-methy1-6-
(1,3,5-trimethyl-
1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one (27 mg, 0.05 mmol),
4-bromo-2,3-
dihydro-1H-inden-1-one (11 mg, 0.06 mmol), Cu! (5 mg, 0.025 mmol),
176
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(trans)-1,2-N,N'-dimethylaminocyclohexane (8 uL, 0.05 mmol), and K2CO3 (25 mg,
0.075
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.104
min, MS
(ES) 669.1 (M+H).
Example 204
Methyl (R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-
oxo-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2(1H)-y1)-
1-methyl-
1H-indole-5-carboxylate (separated stereoisomer, 1st eluting atropisomer)
[00380] The title compound (12 mg, 33%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy0-4-
methyl-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(210-one
(27 mg, 0.05
mmol), methyl 3-bromo-1-methyl-1H-indole-5-carboxylate (16 mg, 0.06 mmol), CuI
(5 mg,
0.025 mmol), (trans)-1,2-N,Ar -dimethylaminocyclohexane (8 IAL, 0.05 mmol),
and K2 C 03
(25 mg, 0.075 mmol). The title compound was isolated as the first eluting
stereoisomer from
reverse phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient to 35-95 % MeCN
0.1% TFA). LCMS: RT = 2.146 min, MS (ES) 726.1 (M+H); 1HNMR (400 MHz, CDC13)
8.29 (dd, J= 1.6, 0.6 Hz, 1H), 7.98 (dd, J= 8.7, 1.6 Hz, 1H), 7.72 (d, J= 8.6
flz, 1H), 7.38
(dd, J= 8.8, 0.7 Hz, 1H), 7.34 ¨ 7.30 (m, 2H), 6.64 (s, 2H), 4.47 (dd, J=
12.6, 3.8 Hz, 1H),
4.13 (s, 1H), 4.02 (t, J= 6.3 Hz, 2H), 3.96 (s, 3H), 3.92 (s, 3H), 3.85 (s,
3H), 3.71 (dd, J=
12.5, 1.7 Hz, 1H), 3.41 (ddt, J= 37.4, 13.2, 7.4 Hz, 2H), 2.33 (s, 6H), 2.26
(s, 3H), 2.25 ¨
2.17 (m, 2H), 2.09(s, 3H), 1.27 (d, J= 6.5 Hz, 3H).
Example 205
Methyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-
oxo-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino 11,2-al indo1-2(1H)-y1)-
1-methy1-
1H-indole-3-carboxylate
[00381] The title compound (26 mg, 36%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5 -tri methy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-a] ind ol-1
(210-one (54 mg, 0.1
mmol), methyl 7-bromo-1-methyl-1H-indole-3-carboxylate (32 mg, 0.12 mmol),
Pd2(dba) 3 (2
mg, 0.002 mmol), Xantphos (3.5 mg, 0.006 mmol), and Cs2CO3 (49 mg, 0.15 mmol).
LCMS:
RT = 2.162 and 2.193 min, MS (ES) 726.3 (M+H).
Example 206
177
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2(1H)-y1)-1-
methy1-1H-
indole-6-carbonitrile
[00382] The title compound (34 mg, 98%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-111-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(27 mg, 0.05
mmol), 3-bromo-1-methy1-1H-indole-6-carbonitrile (14 mg, 0.06 mmol), Cul (5
mg, 0.025
mmol), (trans)-1,2-N,N'-dimethylarninocyclohexane (8 pL, 0.05 mmol), and K2CO3
(25 mg,
0.075 mmol). LCMS: RT = 2.165 min, MS (ES) 693.1 (M+H).
Example 207
Methyl (R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-l-
oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-al indo1-2(1H)-y1)-1-
methyl-
1H-indole-5-carboxylate (separated stereoisomer, rd eluting atropisomer)
[00383] The title compound (10 mg, 27%) was isolated as the second eluting
stereoisomer
along with Example 204 from reverse phase HPLC (Phenomenex Gemini C18,
H20/CH3CN
gradient to 35-95 % MeCN 0.1% TFA). LCMS: RT = 2.167 min, MS (ES) 726.1 (M+H);
11-1
NMR (400 MHz, CDC13) ö 8.29 (d, J = 1.5 Hz, 1H), 7.98 (dd, J= 8.7, 1.6 Hz,
1H), 7.71 (d, J
= 8.6 Hz, 1H), 7.38 (d, J= 8.7 Hz, 1H), 7.33 ¨ 7.28 (m, 2H), 6.64 (s, 2H),
4.43 (dd, J= 12.8,
3.8 Hz, 1H), 4.34 ¨ 4.24 (m, 1H), 4.02 (tt, J= 6.2, 3.3 Hz, 2H), 3.94 (s, 3H),
3.92 (s, 3H),
3.85 (s, 3H), 3.70 (dd, J= 12.8, 1.6 Hz, 1H), 3.54 ¨ 3.23 (m, 2H), 2.33 (s,
6H), 2.24 (s, 3H),
2.23 ¨2.15 (m, 2H), 2.09 (s, 3H), 1.24 (d, J= 6.5 Hz, 3H).
Example 208
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-al ind ol-2(1H)-y1)-1-
methy1-1H-
indole-5-carbonitrile
[00384] The title compound (33 mg, 95%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(27 mg, 0.05
mmol), 3-bromo-l-methyl-1H-indole-5-carbonitrile (14 mg, 0.06 mmol), Cul (5
mg, 0.025
mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 L, 0.05 mmol), and K2CO3
(25 mg,
0.075 mmol). LCMS: RT = 1.214 min, MS (ES) 693.0 (M+H).
Example 209
178
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl- 1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind o l-2(1H)-y1)-
indole-7-carbonitrile
[00385] The title compound (26 mg, 75%) was prepared following General
coupling
procedure B using (R)-7-chl oro-10-(3-(4-chl oro-3,5 -dimethy 1phenoxy
)propy1)-4-methy1-6-
(1,3,5 -trimethy1-111-py razol-4-y1)-3,4-dihy dropy razino [1,2-a] ind ol-1
(211)-one (27 mg, 0.05
mmol), 3-bromo-l-methyl-1H-indole-7-carbonitrile (14 mg, 0.06 mmol), Cul (5
mg, 0.025
mmol), (trans)-1,2-N,N'-dimethylarninocyclohexane (8 1.1L, 0.05 mmol), and
K2CO3 (25 mg,
0.075 mmol). MS (ES) 693.2 (M+H).
Example 210
4- [(4R)-7-chloro-10- [3-(4-chloro-3,5-dimethyl-phenoxy)p ro pyl]
dimethylpyrimidin-5-yl)-4-methyl-1-oxo-3,4-dihyd ropyrazino [1,2-a] indo1-2-
y11-1-
methyl-indole-2-carboxylic acid
[00386] The title compound (2 mg, 8%) was prepared following General coupling
procedure B using ((R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
di methylpy ri mi din-5-y 0-4-methy1-3,4-dihy dropy razino [1,2-a] indo1-1(2H)-
one (20 mg, 0.037
mmol), methyl 4-bromo-1-methy1-1H-indole-2-carboxylate (20 mg, 0.074 mmol),
CuI (5 mg,
0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2CO3
(20 mg, 0.14 mmol) followed by saponification using General Procedure D. LCMS:
RT =
2.071 min, MS (ES) 710.1 (M+H).
Example 211
4-[(4R)-7-chloro-10- [3-(4-chloro-3,5-dimethyl-phenoxy)p ropyl1-4-methyl-1-oxo-
6-(1,3,5-
trimethylpyrazol-4-yl)-3,4-dihydropyrazino11,2-al indol-2-yl]-1-methyl-2-
(trifluoromethyl)ind ole-6-carboxylic acid
Step A. Preparation of methyl 4-bromo-1-methy1-2-(trifluoromethyl)-1H-indole-6-
carboxylate
[00387] A mixture of methyl 4-bromo-1H-indole-6-carboxylate (125 mg, 0.49
mmol), 1-
(trifluoromethyl)-1X3-benzo[d][1,2]iodaoxo1-3(11/)-one (100 mg, 0.54 mmol),
and copper (I)
acetate (5 mg, 0.04 mmol) were dissolved in Me0H (5 mL). The reaction mixture
was
purged with Ar for 5 min, sealed, and stirred at RT for 4 h. The reaction was
then heated to
40 C for 36 h. The reaction was cooled to RT, diluted with DCM/H20 (20 mL,
1:1), the
layers separated, and the aqueous layer was extracted with DCM (2 x 10 mL).
The
combined organic extracts were dried over MgSO4, filtered, and concentrated in
vacuo. The
179
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
crude reaction mixture was purified by flash chromatography (Combi-flash RI,
Hex/Et0Ac =
0-100% gradient) to afford methyl 4-bromo-2-(trifluoromethyl)-1H-indole-6-
carboxylate (44
mg, 28% yield). The resultant product was methylated following General
coupling procedure
G using NaH (6 mg, 0.25 mmol) and Mel (40 mg, 0.28 mmol) to afford the title
compound
(28 mg, 61% two steps).
Step B. Example 211
[00388] The title compound (3 mg, 8%) was prepared following General coupling
procedure B using (R)-7-chl oro-10-(3-(4-chl oro-3,5 -dimethylph enoxy)propy1)-
4-methy1-6-
-trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazin o [1,2-a] indol-1 (211)-one
(25 mg, 0.046
mmol), methyl 4-bromo-1-methy1-2-(trifluoromethyl)-1H-indole-6-carboxylate (28
mg, 0.083
mmol), Cu! (5 mg, 0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8
mg, 0.056
mmol), and K2CO3 (20 mg, 0.14 mmol) followed by saponification using General
Procedure
D. LCMS: RT = 2.187 min, MS (ES) 780.0 (M+H).
Example 212
(S)-3-(7-chloro-10-(3-(4-chlo ro-3,5-d imethylp henoxy) prop yl)-4-methyl- 1-
oxo-6-(1,3,5-
trimethyl-1H- pyrazol-4-y1)-3,4-d ihyd ropyrazino [1,2-a] ind ol-2(1H)-y1)-1-
methyl-1H-
ind ole-5-carboxylic acid
[00389] The title compound (8% yield) was prepared following General coupling
procedure
A using (5)-7-
chi oro-10-(3 -(4-chl oro-3,5-dimethylphenoxy)pro py1)-4-methy1-6-(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one and
methyl 3-bromo-
1 -methyl-1H-indole-S-carboxylate followed by saponification using General
Procedure D.
MS (ES) 712.2 (M+H).
Example 213
5-18-Chloro-11- [3-(4-chloro-3,5-d imethyl-phenoxy)p ro pyl] -7-(4,6-d
imethylpy rimid in-5-
yl)-1-oxo-4,5-dihydro-3H-11 ,41diazepino 11,2-al indol-2-yl] naphthalene-2-
carboxylic acid
[00390] The title compound (4.8 mg, 18%) was prepared following General
coupling
procedure B using 8-chl
oro-11 -(3 -(4-chl oro-3 ,5-dimethylphenoxy)propy1)-7-(4,6-
dimethy 1py rimidin-5-y1)-2,3,4,5-tetrahy dro-1H-[1,41 diazepino[1,2-a] indol-
1 -one (20 mg,
0.037 mmol), methyl 5-bromo-2-naphthoate (15 mg, 0.056 mmol), Cul (5 mg, 0.026
mmol),
(trans)-1,2-NX-dimethylaminocyclohexane (8 mg, 0.056 mmol), and K2CO3 (20 mg,
0.14
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.065
min, MS
(ES) 707.0 (M+H).
Example 214
180
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-l-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-2(1H)-y1)-1-methyl-
1H-
indole-3-carboxylic acid (separated stereoisomer, 1" eluting atropisomer)
[00391] The title compound was isolated as a separated atropisomer (1st
fraction, absolute
stereochemistry undeterminded) from mixture of atropisomers. MS (ES) 712.2
(M+H).
Example 215
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino11,2-alindol-2(1H)-y1)-1-methyl-
1H-
indole-3-carboxylic acid (separated stereoisomer, 2" eluting atropisomer)
[00392] The title compound was isolated as a separated atropisomer (211d
fraction, absolute
stereochemistry undeterminded) from mixture of atropisomers. MS (ES) 712.2
(M+H).
Example 216
(P,R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-6-(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino11,2-alindol-1(2H)-one
[00393] The title compound was separated from diasteromeric (R)-7-chloro-10-(3-
(4-
chloro-3,5-dimethylphenoxy)propy1)-4-methy1-6-(1,3,5-trimethyl-1H-pyrazol-4-
y1)-3,4-
dihydropyrazino[1,2-Mindol-1(21-1)-one using reverse phase HPLC. Absolute
stereochemistry
was determined by single crystal X-ray structure. [a]d25 = -138.7.
Example 217
(M,R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-6-(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(21/)-one
[00394] The title compound was separated from diasteromeric (R)-7-chloro-10-(3-
(4-
chloro-3,5-dimethylphenoxy)propy1)-4-methy1-6-(1,3,5-trimethy1-1H-pyrazol-4-
y1)-3,4-
dihydropyrazino[1,2-a]indol-1(2H)-one using reverse phase HPLC along with
Example 216.
Absolute stereochemistry was determined by single crystal X-ray structure.
[4125= -182.4.
Example 218
5-[(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propy11-6-(4,6-
dimethylpyrimidin-5-y1)-4-methyl-l-oxo-3,4-dihydropyrazino[1,2-alindol-2-
yl[naphthalene-2-carboxylic acid
[00395] The title compound (5.8 mg, 22%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
dimethylpyrimidin-5-y1)-4-methy1-3,4-dihydropyrazino[1,2-alindol-1(211)-one
(20 mg, 0.037
mmol), methyl 5-bromo-2-naphthoate (15 mg, 0.057 mmol), Cul (5 mg, 0.026
mmol),
(trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and K2CO3 (20
mg, 0.14
181
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.037
min, MS
(ES) 706.9 (M+H); NMR
(400 MHz, Chloroform-d) 6 9.10 (s, 1H), 8.63 (d, J= 1.6 Hz,
1H), 8.00 (dt, J= 8.9, 1.1 Hz, 1H), 7.89 (dt, J= 17.6, 8.8 Hz, 1.5H), 7.74
(dd, J= 8.6, 2.1 Hz,
1H), 7.67 (d, J= 8.8 Hz, 0.5 Hz), 7.52 (ddd, J = 13.5, 8.3, 7.3 Hz, 1H), 7.44
(dd, J = 7.3, 1.2
Hz, 0.5H), 7.34 (dd, J= 7.4, 1.1 Hz, 0.5H), 7.30 (s, 1H), 7.19 (s, 1H), 6.53
(s, 1H), 6.51 (s,
1H), 4.39 (dd, J= 26.2, 11.3 Hz, 1H), 3.92 (q, J= 5.9 Hz, 2H), 3.70 (s, 1H),
3.32 (dtt, J
27 .9 , 13.5, 7.1 Hz, 3H), 2.43 (s, 1.5H), 2.42 (s, 1.5H), 2.27 (s, 3H), 2.23
(s, 3H), 2.22 (s, 3H),
2.13 (q, J = 6.9 Hz, 2H), 1.23 (d, J= 6.8 Hz, 1.5H), 1.21 (d, J= 6.8 Hz,
1.5H).
Example 219
7-18-Chloro-11-[3-(4-chloro-3,5-dimethyl-phenoxy)propy1]-7-(4,6-
dimethylpyrimidin-5-
yl)-1-oxo-4,5-dihydro-3H-11,41diazepino 11,2-a] indo1-2-yl] -1-methyl-indole-2-
carboxylic
acid
[00396] The title compound (2.5 mg, 9%) was prepared following General
coupling
procedure B using 8-
chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(4,6-
dimethylpyrimidin-5-y1)-2,3,4,5-tetrahydro-IH41,41diazepino[1,2-a]indol-1-one
(20 mg,
0.037 mmol), ethyl 7-bromo-1-methy1-1H-indole-2-carboxylate (15 mg, 0.053
mmol), CuI (5
mg, 0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol),
and
K2CO3 (20 mg, 0.14 mmol) followed by saponification using General Procedure D.
LCMS:
RT = 2.039 min, MS (ES) 710.1 (M+H).
Example 220
4-18-Chloro-1143-(4-chloro-3,5-dimethyl-phenoxy)pr0py11-7-(4,6-
dimethylpyrimidin-5-
y1)-1-oxo-4,5-dihydro-3H-11,41diazepino11,2-alindol-2-yllquinoline-6-
carboxylic acid
[00397] The title compound (4.5 mg, 17%) was prepared following General
coupling
procedure B using 8-
chloro-11-(3-(4-chloro-3,5-climethylphenoxy)propy1)-7-(4,6-
dimethylpyrimidin-5-y1)-2,3,4,5-tetrahydro-1H41,41diazepino[1,2-a]indol-1-one
(20 mg,
0.037 mmol), methyl 4-bromoquinoline-6-carboxylate (10 mg, 0.037 mmol), CuI (5
mg,
0.026 mmol), (trans)-1,2-N,/V'-dimethylaminocyclohexane (8 mg, 0.056 mmol),
and K2CO3
(20 mg, 0.14 mmol) followed by saponification using General Procedure D. LCMS:
RT =
1.786 min, MS (ES) 708.0 (M+H); NMR
(400 MHz, Chloroform-a) 6 9.19 (s, 1H), 9.11 (s,
1H), 8.65 (s, 1H), 8.41 (q, J = 8.8 Hz, 2H), 7.79 (d, J = 8.6 Hz, 1H), 7.38
(d, J = 8.6 Hz, 1H),
7.32 (d, J = 4.5 Hz, 1H), 6.62 (s, 2H), 4.11 -3.96 (m, 4H), 3.90 (s, 2H), 3.22
(t, J= 7.4 Hz,
2H), 2.36 (s, 6H), 2.30 (s, 6H), 2.24 (quint, J= 7.1, 6.6 Hz, 2H), 1.86 (br s,
2H).
Example 221
182
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
3- 1(4R)-7-chloro-10- [3-(4-chloro-3,5-dimethyl-phenoxy)p ropy1]-4-methyl-1-
oxo-6-(1,3,5-
trimethylpy razol-4-y1)-3,4-dihyd ropyrazino 11,2-al ind ol-2-y11- 1-methyl-id
ole-5-
carboxamide
[00398] The title compound (5.2 mg, 52%) was prepared following General
Procedure E
employing (R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-
1-oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-2(1H)-y1)-1-
methyl-1H-
indole-5-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(10 mg,
0.078 mmol), and 7M ammonia in Me0H (0.05 mL, 0.35 mmol). LCMS: RT = 1.924
min,
MS (ES) 711.0 (M+H).
Example 222
(R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-2-(1-methy1-
6-
(methylsulfony1)-1H-indol-3-y1)-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihydropyrazino[1,2-alindol-1(21frone
Step A. Preparation of 3-bromo-1-methyl-6-(methylsulfony1)-1H-indole
[00399] To a solution of 6-(methylsulfony1)-1H-indole (50 mg, 0.26 mmol) in
DMF (3 mL)
was added Nal-1 (14 mg, 0.58 mmol) and stirred at RT for 30 min. Mel (70 mg,
0.49 mmol)
was added and the reaction was stirred at RT for 3 h, diluted into DCM (10
mL), quenched
with H20 (10 mL). The layers were separated and the aqueous layer was
extracted with
DCM (2 x 10 mL). The combined organic extracts were dried and concentrated in
vacuo.
The residue was purified by flash chromatography (Combi-flash Rf, Hex/Et0Ac =
0-100%
gradient) to give 1-methyl-6-(methylsulfony1)-1H-indole. The product was
dissolved in
DMF (3 mL), cooled to 0 C, and NBS (60 mg, 0.34 mmol) was added. After 30
min, the
reaction was diluted into DCM (20 mL), washed with 10% aqueous Na2S203, dried
over
MgSO4, filtered, and concentrated. The crude residue was purified by flash
chromatography
(Combi-flash Rf, Hex/Et0Ac = 0-100% gradient) to afford the title compound (52
mg, 70%).
Step B. Example 222
[00400] The title compound (18.3 mg, 65%) was prepared following General
coupling
procedure B using (R)-7-chl oro-10-(3-(4-chloro-3,5-di methylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropyrazino[1,2-a]indo1-1(21-1)-
one (20 mg, 0.037
mmol), 3-bromo-1-methy1-6-(methylsulfony1)-1H-indole (20 mg, 0.069 mmol), CuI
(5 mg,
0.026 mmol), (trans)-1,2-N,/V'-dimethylaminocyclohexane (8 mg, 0.056 mmol),
and K2CO3
(25 mg, 0.18 mmol). LCMS: RT = 2.015, 2.034 min, MS (ES) 745.9 (M+H); 1H NMR
(400
MHz, Chloroform-d) 6 8.04 (t, J= 1.0 Hz, 1H), 7.75 ¨7.60 (m, 3H), 7.39 (d, J=
6.0 Hz, 1H),
7.31 (dd, J = 8.0, 4.0 Hz, 1H), 6.62 (d, J = 1.6 Hz, 2H), 4.42 (dt, J= 12.2,
4.2 Hz, 1H), 4.35-
183
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
4.18 (m, 1H), 4.01 (td, J= 5.4, 4.5, 2.5 Hz, 2H), 3.94 (s, 3H), 3.92 (s, 3H),
3.66 ¨ 3.56 (m,
1H), 3.51 ¨3.29 (m, 2H), 3.10 (s, 3H), 2.33 (s, 6H), 2.28 ¨2.14 (m, 5H), 2.09
(s, 1.5H), 2.07
(s, 1.5H), 1.26 (d, J = 6.4 Hz, 1.5H), 1.19 (d, J= 6.4 Hz, 1.5H).
Example 223
(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propy1]-4-methy1-2-(1-
methy1-5-
nitro-indol-3-y1)-6-(1,3,5-trimethylpyrazol-4-y1)-3,4-dihydropyrazino [1,2-al
indol-1-one
[00401] The title compound (17.4 mg, 67%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazino[1,2-a] indol-1 (211)-
one (20 mg, 0.037
mmol), 3-bromo-1-methy1-5-nitro-1H-indole (20 mg, 0.078 mmol), Cul (5 mg,
0.026 mmol),
(trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and K2C 03 (25
mg, 0.18
mmol). LCMS: RT = 2.136, 2.159 min, MS (ES) 712.9 (M+H); 11-1 NMR (400 MHz,
Chloroform-a) 8 8.50 (dd, J= 6.2, 2.2 Hz, 1H), 8.18 (dd, J= 9.1, 2.2 Hz, 1H),
7.71 (dd, J=
8.6, 2.2 Hz, 1H), 7.41 (d, J = 9.2 Hz, 1H), 7.37 (s, 0.5H), 7.32 ¨ 7.29 (m,
1.5H), 6.66 ¨ 6.61
(m, 2H), 4.49-4.23 (m, 2H), 4.06¨ 3.98 (m, 2H), 3.92 (s, 3H), 3.87 (s, 3H),
3.65 (dd, J = 12.3,
7.2 Hz, 1H), 3.53 ¨ 3.29 (m, 2H), 2.33 (s, 6H), 2.28 ¨ 2.16 (m, 5H), 2.08 (d,
J= 1.4 Hz, 3H),
1.26 (d, J = 6.4 Hz, 1.5H), 1.24 (d, J = 6.4 Hz, 1.5H).
Example 224
8- [(4R)-7-chloro-10- [3-(4-chloro-3,5-dimethyl-phenoxy)pro pyl]
dimethylpyrimid in-5-y1)-4-methy1-1-oxo-3,4-d ihyd ropy razino [1,2-a] indo1-2-
yl]quinoline-5-carboxylic acid
[00402] The title compound (11.8 mg, 45%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
dimethylpyrimidin-5-y1)-4-methy1-3,4-dihydropyrazino[1,2-a]indo1-1(211)-one
(20 mg, 0.037
mmol), methyl 8-bromoquinoline-5-carboxylate (20 mg, 0.075 mmol), CuI (5 mg,
0.026
mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and K2CO3
(25 mg,
0.18 mmol) followed by saponification using General Procedure D. LCMS: RT =
1.990 min,
MS (ES) 707.8 (M+H).
Example 225
8- [(4R)-7-chloro-10- [3-(4-chloro-3,5-dimethyl-phenoxy)pro pyl]
dimethylpyrimid in-5-y1)-4-methy1-1-oxo-3,4-d ihyd ropyrazino [1,2-a] indo1-2-
yl]quinoline-4-carboxylic acid
[00403] The title compound (13.9 mg, 52%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
184
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
dimethylpyrimidin-5-y1)-4-methy1-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(20 mg, 0.037
mmol), methyl 8-bromoquinoline-4-carboxylate (20 mg, 0.075 mmol), CuI (5 mg,
0.026
mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and K2CO3
(25
mg, 0.18 mmol) followed by saponification using General Procedure D. LCMS: RT
= 2.012
min, MS (ES) 707.8 (M+H).
Example 226
(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propy1]-4-methy1-2-(2-
methyl-1-
oxo-3,4-dihydropyrazino[1,2-a]indol-6-y1)-6-(1,3,5-trimethylpyrazol-4-y1)-3,4-
dihydropyrazino[1,2-alindol-1-one
Step A. Preparation of 6-bromo-3,4-dihydropyrazino[1,2-a]indo1-1(21/)-one
[00404] The title compound (71 mg, 70%) was prepared following the procedure
described
in Example 86 Steps B and C using ethyl 7-bromo-1-methy1-1H-indole-2-
carboxylate (100
mg, 0.56 mmol), tert-butyl 1,2,3-oxathiazolidine-3-carboxylate 2,2-dioxide
(180 mg, 0.81
mmol), NaH (24 mg, 1.0 mmol), HC1 (4.0 M in dioxanes, 2 mL, 8.0 mmol), and
K2CO3 (150
mg, 1.09 mmol).
Step B. Preparation of 6-bromo-2-methy1-3,4-dihydropyrazino[1,2-alindol-1(211)-
one
[00405] The title compound (18 mg, 86%) was prepared following General
Procedure G
using 6-bromo-3,4-dihydropyrazino[1,2-alindo1-1(21)-one (20 mg, 0.075 mmol),
Nal-I (3
mg, 0.13 mmol), and Mel (20 mg, 0.14 mmol).
Step C. Example 226
[00406] The title compound (6.1 mg, 18%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(21)-one
(25 mg, 0.046
mmol), 6-bromo-2-methyl-3,4-dihydropyrazino[1,2-alindo1-1(210-one (18 mg,
0.064 mmol),
Cu! (5 mg, 0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056
mmol),
and K2CO3 (20 mg, 0.15 mmol). MS (ES) 736.9 (M+H).
Example 227
8-1(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propy11-6-(4,6-
dimethylpyrimidin-5-y1)-4-methyl-l-oxo-3,4-dihydropyrazino[1,2-a]indo1-2-
yl]quinoline-3-carboxylic acid
[00407] The title compound (15.4 mg, 58%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
dimethylpyrimidin-5-y1)-4-methyl-3,4-dihydropyrazino[1,2-a]indol-1(21)-one (20
mg, 0.037
mmol), methyl 8-bromoquinoline-3-carboxylate (20 mg, 0.075 mmol), Cu! (5 mg,
0.026
185
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and K2CO3
(25 mg,
0.18 mmol) followed by saponification using General Procedure D. LCMS: RT =
2.002 mm,
MS (ES) 707.9 (M+H); NMR
(400 MHz, Chloroform-a) 6 9.39 (d, J= 1.9 Hz, 1H), 9.22
(s, 1H), 8.88 (d, J= 2.0 Hz, 1H), 7.96 (d, J= 8.1 Hz, 1H), 7.88 ¨ 7.77 (m,
2H), 7.72 (t, J=
7.8 Hz, 1H), 7.36 (d, J= 8.6 Hz, 1H), 6.62 (s, 2H), 4.71 (s, 1H), 4.00 (t, J=
6.1 Hz, 2H), 3.75
(s, 1H), 3.52 (s, 1H), 3.39 (m, 2H), 2.53 (s, 3H), 2.36 (s, 3H), 2.31 (s, 6H),
2.23 (quint, J-
7.0, 6.3 Hz, 2H), 1.33 (d, J= 5.9 Hz, 3H).
Example 228
3-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(4,6-dimethyl-2-(4-
methylpiperazin-1-y1)pyrimidin-5-y1)-1-oxo-4,5-dihydro-1H- [1,4] diazepino[1,2-
a]indo1-
2(31/)-y1)-1-methy1-1H-ind ole-5-carboxylic acid
Step A. Preparation of 4,6-dimethy1-2-(4-methylpiperazin-1-yl)pyrimidine
[00408] A mixture of 2-chloro-4,6-dimethylpyrimidine (1.0 g, 7.0 mmol), 1-
methylpiperazine (750 mg, 7.5 mmol), and K2CO3 (1.5 g, 10.9 mmol) in MeCN (10
mL) was
heated to 50 C for 72 h. Reaction was diluted with DCM/H20 (60 mL, 1:1), the
organic
layer was separated, and the aqueous layer was extracted with DCM (2 x 30 mL).
The
combined organic layers were dried over MgSO4, filtered, and concentrated in
vacuo to
afford the title compound (1.39 g, 96%).
Step B. Preparation of 5-b rom o-4,6-d imethy1-2-(4-methyl pi perazin- 1-y1)
pyrimi d ine
[00409] To a solution of 4,6-dimethy1-2-(4-methylpiperazin-1-yOpyrimidine
(1.39 g, 6.75
mmol) in CHC13 (10 mL) and NBS (1.25 g, 7.02 mmol) was added. The reaction was
heated
to 65 C for 10 min then cooled to RT. The reaction mixture was diluted with
DCM/H20 (60
mL, 1:1) and extracted with DCM (2 x 30 mL). The combined organic layers were
dried
over MgSO4, filtered, and concentrated in vacuo. The residue was purified by
flash
chromatography (Combi-flash Rf, DCM/Me0H = 0-10% gradient) to afford the title
compound (1.66 g, 86%).
Step C. Preparation of (4,6-dimethy1-2-(4-methylpiperazin-1-yl)pyrimidin-5-
yl)boronic
acid
[00410] To a solution of 5-bromo-4,6-dimethy1-2-(4-methylpiperazin-1-
y1)pyrimidine (250
mg, 0.88 mmol) in toluene/THF (4 mL/1 mL) was added triisopropyl borate (250
mg, 1.33
mmol), and the reaction was cooled to -40 C. n-Butyllitium (2.5 M, 0.48 mL,
1.20 mmol)
was added over 1 h and stirred for 30 min, then warmed to -20 C over 1 h. The
reaction was
quenched with 1M HC1, the organic layer was removed, and the aqueous layer was
adjusted
to pH 7 with 1M NaOH. The aqueous layer was extracted with 10% iPrOH in CHC13
(3 x 30
186
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
mL), and the combined organic layers were dried over MgSO4, filtered, and
concentrated in
vacuo to afford the title compound (140 mg, 56%).
Step D. Preparation of ethyl 6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-7-
(4,6-d imethy1-2-(4-methyl p perazin- 1-y1) pyrimid in-5-y1)-1H-ind ole-2-
carboxylate
[00411] A mixture of ethyl 7-bromo-6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-
1H-indole-2-carboxylate (100 mg, 0.20 mmol), (4,6-dimethy1-2-(4-
methylpiperazin-1-
yl)pyrimidin-5-yl)boronic acid (75 mg, 0.32 mmol), Pd2(dba)3 (20 mg, 0.022
mmol), S-Phos
(25 mg, 0.061 mmol), and potassium phosphate (130 mg, 0.61 mmol) in THF (3 mL)
and
toluene (3 mL) was sparged with Ar for 5 min. The reaction was heated to 110
C for 24 h
then cooled to RT and diluted with DCM/H20 (20 mL, 1:1). The layers were
separated, and
the aqueous layer was extracted with DCM (2 x 10 mL). The combined organic
layers were
dried over MgSO4, filtered, and concentrated in vacuo. The residue was
purified by flash
chromatography (Combi-flash Rf, Hex/Et0Ac = 0-100% gradient) to give the title
compound
(52 mg, 42%).
Step E. Preparation of 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-
(4,6-
dimethy1-2-(4-methylpiperazin-1-y1)pyrimidin-5-y1)-2,3,4,5-tetrahydro-1H-
11,41diazepino[1,2-alindol-1-one
[00412] The title compound (31 mg, 58%) was prepared following the procedures
described in Example 120 Steps B and C using ethyl 6-chloro-3-(3-(4-chloro-3,5-
dimethy 1phenoxy)propy1)-7-(4,6-dimethy 1-2-(4-methy 1piperazin-l-yl)py
rimidin-5-y1)-1H-
indole-2-carboxy late (52 mg, 0.083 mmol), tert-butyl 1,2,3-oxathiazinane-3-
carboxylate 2,2-
dioxide (30 mg, 0.13 mmol), NaH (8 mg, 0.20 mmol), HCl in dioxanes (4.0 M, 0.5
mL, 2.0
mmol), and K2CO3 (30 mg, 0.22 mmol).
Step F. Example 228
[00413] The title compound (13 mg, 33%) was prepared following General
coupling
procedure B using 8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(4,6-
dimethy1-2-
(4-methylpiperazin-1-yppyrimidin-5-y1)-2,3,4,5-tetrahy dro-1H-[1,4] diazepino[
1,2-ajindol- 1-
one (31 mg, 0.049 mmol), methyl 3-bromo-1-methy1-1H-indole-5-carboxylate (30
mg, 0.11
mmol), CuI (5 mg, 0.026 mmol), (trans)-1,2-N,/V'-dimethylaminocyclohexane (8
mg, 0.056
mmol), and K2CO3 (25 mg, 0,18 mmol) followed by saponification using General
Procedure
D. LCMS: RT = 1.760 min, MS (ES) 808.0 (M+H); NMR (400 MHz, Chloroform-d) 8
8.28 (s, 1H), 8.00 (d, J= 8.8 Hz, 1H), 7.69 (d, J = 8.8 Hz, 1H), 7.38 (d, J =
8.8 Hz, 1H), 7.29
(d, J = 8.6 Hz, 1H), 7.18 (s, 1H), 6.64 (s, 2H), 5.04 (d, J = 11.2 Hz, 2H),
4.11 (t, J = 6.4 Hz,
2H), 4.00 (t, J= 6.1 Hz, 2H), 3.83 (s, 3H), 3.77 (t, J= 6.2 Hz, 4H), 3.56 (s,
2H), 3.21 (t, J =
187
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
7.4 Hz, 2H), 2.92 (s, 5H), 2.31 (s, 6H), 2.28 -2.19 (m, 2H), 2.12 (s, 6H),
1.91 (d, J= 6.0 Hz,
2H).
Example 229
(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)p ropy1]-2-(2-isopenty1-1-
oxo-3,4-
dihydropyrazino [1,2-al ind ol-6-y1)-4-methyl-6-(1,3,5-trimethyl pyrazol-4-y1)-
3,4-
dihyd ropyrazino [1,2-al indol-1-one
Step A. Preparation of 6-bromo-2-isopenty1-3,4-dihydropyrazino[1,2-a] indo1-
1(2H)-one
[00414] The title compound (15 mg, 59%) was prepared following General
Procedure G
using 6-bromo-3,4-dihydropyrazino[1,2-alindo1-1(210-one (20 mg, 0.075 mmol),
NaH (3
mg, 0.13 mmol), and isovaleryl bromide (25 mg, 0.17 mmol).
Step B. Example 229
[00415] The title compound (4.7 mg, 13%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(25 mg, 0.046
mmol), 6-bromo-2-isopenty1-3,4-dihydropyrazino[1,2-a]indo1-1(21)-one (15 mg,
0.045
mmol), Cul (5 mg, 0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8
mg, 0.056
mmol), and K2CO3 (25 mg, 0.18 mmol) followed by saponification using General
Procedure
D. LCMS: RT = 2.177 min, MS (ES) 793.0 (M+H).
Example 230
(R)-4-Chloro-8-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-
1-oxo-
6-(1,3,5-trimethyl-11I-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-al ind ol-2(1H)-
yl)quinoline-
2-carboxylic acid
[00416] The title compound (11 mg, 30%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 8-bromo-4-chloroquinoline-2-carboxylate (19 mg, 0.06 mmol),
Pd2(dba)3 (1
mg, 0.001 mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol)
followed
by saponification using General Procedure D. LCMS: RT = 2.089 min, MS (ES)
743.9
(M+H); 11-1NMR (400 MHz, DMSO-d6) 5 8.31 (dt, J= 7.8, 1.5 Hz, 1H), 8.26 (d, J=
1.8 Hz,
1H), 8.04 - 7.90 (m, 2H), 7.77 (d, J = 8.6 Hz, 1H), 7.31 (d, J = 8.6 Hz, 1H),
6.72 (s, 2H),
4.77 - 4.55 (m, 1H), 4.27 - 4.12 (m, 1H), 3.96 (t, J = 6.5 Hz, 2H), 3.78 (s,
1.5H), 3.76 (s,
1.5H), 3.35 - 3.12 (m, 3H), 2.23 (s, 6H), 2.13 (s, 1.5H), 2.11 -2.04 (m, 2H),
2.02 (s, 1.5H),
1.98 (s, 1.5H), 1.89 (s, 1.5H), 1.37- 1.16 (m, 3H).
Example 231
188
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl- 1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-al ind ol-2(11/)-y1)-1-((2-
(trimethylsilypethoxy)methyl)-1H-indole-5-carboxylic acid
Step A. Preparation of methyl 3-bromo-1-42-(trimethylsilypethoxy)methyl)-1H-
indole-
5-carboxylate
[00417] The title compound (96 mg, 88%) was prepared following General
Procedure F
using methyl 1H-indole-5-carboxylate (50 mg, 0.28 mmol), NBS (51 mg, 0.28
mmol), NaH
(14 mg, 0.34 mmol) and (2-(chloromethoxy)ethyl)trimethylsilane (60 H.L, 0.34
mmol).
LCMS: RT = 1.979 mm, MS (ES) 384.0 (M+H).
Step B. Example 231
[00418] The title compound (30 mg, 72%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one
(27 mg, 0.05
mmol), methyl 3-bromo-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indole-5-
carboxylate (23
mg, 0.06 mmol), Cul (5 mg, 0.025 mmol), (trans)-1,2-N,N'-
dimethylaminocyclohexane (8
pL, 0.05 mmol), and K2CO3 (25 mg, 0.075 mmol) followed by saponification using
General
Procedure D. LCMS: RT = 2.257 min, MS (ES) 828.0 (M+H).
Example 232
(R)-5-Chloro-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-
1-oxo-
6-(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2(11/)-
yl)benzofu ran-2-carboxylic acid (separated stereoisomer, 1st eluting
atropisomer)
[00419] The title compound (11 mg, 30%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazino[1,2-a] indol-1 (211)-
one (27 mg, 0.05
mmol), methyl 7-bromo-5-chlorobenzofuran-2-carboxylate (19 mg, 0.06 mmol),
Pd2(dba)3 (1
mg, 0.001 mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol)
followed
by saponification using General Procedure D The title compound was isolated as
a separated
atropisomer (1st fraction, absolute stereochemistry undeterminded) by reverse
phase HPLC
(Phenomenex Gemini C18, H20/CH3CN gradient to 35-95 % MeCN 0.1% TFA). LCMS:
RT = 2.138 min, MS (ES) 732.9 (M+H).
Example 233
189
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
7- 1(4R)-7-chloro-10- 13-(4-chloro-3,5-dimethyl-phenoxy)propy1]-4-methyl-1-oxo-
6-(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino11,2-a]indol-2-y11-1H-ind ole-2-
carboxylic
acid (separated stereoisomer, 1" eluting atropisomer)
Step A. Preparation of ethyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-methyl-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihydropyrazino[1,2-a]indol-2(1H)-y1)-1H-indole-2-carboxylate
[00420] The title compound (210 mg, 52%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(300 mg, 0.55
mmol), ethyl 7-bromo-1H-indole-2-carboxylate (300 mg, 1,10 mmol), CuI (50 mg,
0.26
mmol), (trans)-N1,N2-dimethylcyclohexane-1,2-diamine (80 mg, 0.56 mmol), and
K2CO3
(250 mg, 1.81 mmol).
Step B. Example 233
[00421] The title compound (6.8 mg, 35%) was obtained following General
Procedure D
using ethyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-1-oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-cdindol-2(11/)-y1)-
1H-indole-2-
carboxylate (20 mg, 0.0 27 mmol) and isolated as a separated atropisomer (15t
fraction,
absolute stereochemistry undeterminded) by reverse phase HPLC (Phenomenex
Gemini C18,
H20/CH3CN gradient from 30-90% CH3CN, 0.1% 11- A). LCMS: RT = 1.954 min, MS
(ES)
697.9 (M+H).
Example 234
(R)-7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-2-(1-methyl-
1H-
indo1-7-y1)-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-
a[indol-1(2H)-
one
Step A. Preparation of (R)-7-chloro-10-(344-chloro-3,5-dimethylphenoxy)propy1)-
2-
(1H-indol-7-y1)-4-methyl-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihydropyrazino11,2-
a[indol-1(211)-one
[00422] The title compound was prepared following General coupling procedure B
using
(R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-6-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one (27 mg, 0,05 mmol), 7-
bromo-1H-
indole (12 mg, 0.06 mmol), CuI (5 mg, 0,025 mmol), (trans)-1,2-N,N'-
dimethylaminocyclohexane (8 uL, 0.05 mmol), and K2CO3 (25 mg, 0.075 mmol).
Step B. Example 234
190
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00423] The title compound (17 mg, 51%) was prepared following General
procedure G
using (R)-7-
chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-2-(1H-indol-7-y1)-4-
methy1-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropy razino [1,2-a] indol-
1 (21I)-one,
NaH (5 mg, 0.12 mmol) and Mel (8 tiL, 0.12 mmol). LCMS: RT = 2.169 and 2.194
min, MS
(ES) 667.9 (M+H).
Example 235
(R)-7-Chlo ro-10-(3-(4-chl oro-3,5-dimethylp hen oxy)p ro py1)-4-methy1-2-(1-
methyl-5-(1H-
tetrazol-5-y1)-1H-ind ol-3-y1)-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihydropyrazino 11,2-alindo1-1(211)-one (separated stereoisomer, 2"d eluting
atropisomer)
[00424] To a solution of (R)-3-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-
methyl-l-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-
a]indo1-2(1H)-
y1)-1-methyl-1H-indole-5-carbonitrile (69 mg, 0.1 mmol) in DMF (2 mL) were
added sodium
azide (33 mg, 0.5 mmol), ammonium chloride (27 mg, 0.5 mmol) and the mixture
was heated
at 120 C for 12 h then cooled to ambient temperature. The reaction mixture
was quenched
with H20 (5 mL), extracted with CH2C12 (3x15 mL), dried (anhyd. Na2SO4), and
concentrated. The residue was purified by reverse phase HPLC (Phenomenex
Gemini C18,
H20/CH3CN gradient to 35-95 % MeCN 0.1% TFA) to afford the title compound (25
mg,
34%) as the second eluting atropisomer (absolute configuration undetermined).
LCMS: RT =
2.007 min, MS (ES) 736.0 (M+H).
Example 236
(R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-2-(1-methy1-
6-(2H-
tetrazol-5-y1)-1H-indol-3-y1)-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihyd ropyrazino[1,2-a]ind 01-1(211)-one
[00425] The title compound (13 mg, 80%) was prepared according to the
procedure for
Example 235 using (R)-3 -(7-chl oro-10-(3-(4-chl oro-3,5-dimethy 1phenoxy)p
ropy1)-4-methy I-
1-oxo-6-(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-al
indo1-2(1H)-y1)-1-
methy1-1H-indole-6-carbonitrile (15 mg, 0.022 mmol). LCMS: RT = 2.045 min, MS
(ES)
736.0 (M+H).
Example 237
(R)-2-(Benzofu ran-3-y1)-7-chl oro-10-(3-(4-chlo ro-3,5-di methylphenoxy)p
ropy1)-4-
methyl-6-(1,3,5-trimethyl- 1H-py razol-4-y1)-3,4-d ihyd ro pyrazino 11,2-al
indo1-1(21/)-one
(separated stereoisomer, 1" eluting atropisomer)
[00426] The title compound (9 mg, 27%) was prepared following General coupling
procedure B as the first eluting atropisomer (absolute configuration
undetermined) using (R)-
191
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
7-chloro-10-(3 -(4-chl oro-3,5 -dimethy 1phenoxy )propy1)-4-methy1-6-(1,3,5-
trimethyl-1H-
py razol-4-y hy
dropy razino [1,2-a] ind ol-1 (2H)-one (27 mg, 0.05 mmol), 3-
bromobenzofuran (13 mg, 0.06 mmol), Cu! (5 mg, 0.025 mmol), (trans)-1,2-N ,N' -
dimethylaminocy clohexane (8 uL, 0.05 mmol), and K2CO3 (25 mg, 0.075 mmol).
LCMS:
RT = 2.260 min, MS (ES) 655.0 (M+H).
Example 238
7-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-6-(4,6-
dimethylpyrimidin-5-y1)-4-methy1-1-oxo-3,4-dihydropyrazino[1,2-alindo1-2-y11-1-
methyl-indole-2-carboxylic acid
[00427] The title compound (23 mg, 58%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
dimethylpyrimidin-5-y1)-4-methy1-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(30 mg, 0.056
mmol), ethyl 7-bromo-1-methy1-1H-indole-2-carboxylate (30 mg, 0.11 mmol),
Pd2(dba)3 (10
mg, 0.011 mmol), Xantphos (15 mg, 0.026 mmol), and Cs2CO3 (80 mg, 0.246 mmol)
followed by saponification using General Procedure D. LCMS: RT = 1.957 min, MS
(ES)
709.9 (M+H).
Example 239
(R)-8-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-d ihyd ropy razino [ 1,2-al ind ol-2(1H)-y1)-4-
methoxyquinoline-2-carboxylic acid
[00428] The title compound (16 mg, 44%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(27 mg, 0.05
mmol), methyl 8-bromo-4-chloroquinoline-2-carboxylate (19 mg, 0.06 mmol),
Pd2(dba)3 (1
mg, 0.001 mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol)
followed
by saponification using General Procedure D. LCMS: RT = 2.019 and 2.034 min,
MS (ES)
739.9 (M+H); 1H NMR (400 MHz, DMSO-d6) (3 8,20 (dd, J= 8.4, 1.3 Hz, 1H), 7.83
(ddd, J=
7.4, 3.1, 1.5 Hz, 114), 7.78 - 7.68 (m, 2H), 7.59 (d, J = 1.6 Hz, 1H), 7.30
(d, J = 8.6 Hz, 1H),
6.73 (s, 2H), 4.88 - 4.49 (m, 1H), 4.31 -4.18 (m, 1H), 4.15 (s, 3H), 3.97 (t,
J= 6.4 Hz, 2H),
3.78 (s, 1.5H), 3.75 (s, 1.5H), 3.34- 3.15(m, 3H), 2.24 (s, 6H), 2.13 (s,
1.5H), 2.11 - 2.04
(m, 2H), 2.02(s, 1.5H), 1.98 (s, 1.5H), 1.89(s, 1.5H), 1.36- 1.15 (in, 3H).
Example 240
192
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino 11,2-al ind ol-2(1H)-y1)-1-(2-
(tetrahyd ro-
2H-pyran-4-yl)ethyl)-1H-indole-5-carboxylic acid
[00429] To a solution of methyl (R)-3-
(7-chloro-10-(3-(4-chloro-3,5-
di methylphenoxy )propy1)-4-methy1-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-
3,4-
dihydropyrazino[1,2-c]indol-2(1H)-y1)-142-(trimethylsilypethoxy)methyl)-1H-
indole-5-
carboxylate (15 mg, 0.019 mmol) in DCM (1 mL) was added TFA (1 mL), and the
mixture
was stirred at RT for 6 h. Saturated aqueous NaHCO3 (8 mL) was added to the
mixture and
extracted with DCM (3 x 5 mL), The organic layer was dried (anhyd. Na2SO4) and
concentrated in vacuo. The crude product was allcylated following General
procedure G using
NaH (5 mg, 0.12 mmol) and 4-(2-bromoethyl)tetrahydro-2H-pyran (6 mg, 0.031
mmol)
followed by saponification using General Procedure D to afford the title
compound (8 mg,
52%). LCMS: RT = 2.168 min, MS (ES) 810.3 (M+H).
Example 241
1-Benzy1-7-[(4R)-7-chloro-10- [3-(4-chloro-3,5-dimethyl-phenoxy)p ropy11-4-
methyl-1-
oxo-6-(1,3,5-trimethylpyrazol-4-y1)-3,4-dihyd ropy razino[1,2-al indo1-2-yl]
indole-2-
carboxylic acid
[00430] To a solution of ethyl (R)-7-
(7-chl oro-10-(3-(4-chl oro-3,5-
di methylphenoxy )propy1)-4-methy1-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-
3,4-
dihydropyrazino[1,2-a]indo1-2(1H)-y1)-1H-indole-2-carboxylate (10 mg, 0,014
mmol) in
DMF (1 mL) was added NaH (2 mg, 0.08 mmol), and the reaction was stirred at RT
for 30
min. Benzyl bromide (5 mg, 0.029 mmol) was added, and the reaction was stirred
for 16 h at
RT then diluted with DCM/H20 (20 mL, 1:1). The mixture was extracted with DCM
(2 x 5
mL), dried by passing through a phase separator and concentrated in vacuo. The
crude
reaction product was then saponified and purified following General Procedure
D to afford
the title compound (6.9 mg, 64% yield). LCMS: RT = 2.084 min, MS (ES) 787.8
(M+H).
Example 242
7- [(4R)-7-chloro-10- [3-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-
6-(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino [1,2-al in d ol-2-y1]- 1H-ind ole-2-
carb oxylic
acid (separated stereoisomer, 2' eluting atropisomer)
[00431] The title compound (8,1, 42% yield) was prepared in the same procedure
as
described in Example 233 as the rd fraction. LCMS: RT = 1.953 min, MS (ES)
697.9
(M+H).
Example 243
193
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Methyl (R)-3-(7-chlo ro-10-(3-(4-chlo ro-3,5-d imethylphen oxy)p ropyl)-4-
methyl-l-oxo-6-
(1,3,5-trimethy1-1H-py razol-4-yl)-3,4-d ihyd ropy razino I 1,2-ajind ol-2(1H)-
yl)-1-02-
(trimethyls ilyl)ethoxy)methyl)-1H-ind ole-5-carbo xy late
[00432] The title compound (37 mg, 88%) was prepared following General
coupling
procedure B using (R)-7-chl oro-10-(3-(4-chl oro-3,5 -dimethy 1phenoxy
)propy1)-4-methy1-6-
(1,3,5 -trimethy1-11-1-py razol-4-y1)-3,4-dihy dropy razino [1,2-a] ind ol -1
(21-1)-one (27 mg, 0.05
mmol), methyl 3-bromo-1-42-(trimethylsi ly Deth oxy)methyl)-1H-indol e-5-carb
oxy I ate (23
mg, 0.06 mmol), Cul (5 mg, 0.025 mmol), (trans)-1,2-N,/V'-
dimethylaminocyclohexane (8
pL, 0.05 mmol), and K2CO3 (25 mg, 0.075 mmol). LCMS: RT = 2.451 min, MS (ES)
841.9
(M+H).
Example 244
(R)-3-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyI)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino [1,2-al indol-2(11/)-y1)-7-
cyano-1-
methyl4H-indole-5-carboxylic acid
Step A. Preparation of ethyl 3-bromo-7-cyano-1-methyl-1H-indole-5-carboxylate
[00433] To a solution of ethyl 7-bromo-1H-indole-5-carboxylate (97 mg, 0.36
mmol) in
DMF (2 mL) was added NaH (17 mg, 0.43 mmol) and the mixture was stirred at RT
for 10
min. Methyl iodide (27 gL, 0.43 mmol) was added to the mixture and stirred at
ambient
temperature for 2 h. The mixture was diluted with water (5 mL) and was
extracted in CH2C12
(3 x 5 mL). The organic layer was dried (anhyd. Na2SO4) and concentrated. The
crude was
subjected to General Procedure B using sodium cyanide (22 mg, 0.43 mmol), CuI
(7 mg, 0.04
mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (57 uL, 0.36 mmol), and K2C
03 (120
mg, 0.72 mmol) and purified by flash chromatography (Combi-flash Rf, Hex/Et0Ac
= 0-20%
gradient). To a solution of resulted ethyl 7-cyano-1-methy1-1H-indole-5-
carboxylatehe in
DMF (2 mL) was added and NBS (51 mg, 0.28 mmol) at 0 C and stirred for 1 h.
Same work
up and purification protocols described in general coupling procedure A were
followed to
afford the title compound (52 mg, 47%). LCMS: RT = 1.672 min, MS (ES) 307.0
(M+H).
Step B. Example 244
[00434] The title compound (25 mg, 68%) was prepared following General
coupling
procedure B using (R)-7-chl oro-10-(3-(4-chl oro-3,5 -dimethy 1phenoxy
)propy1)-4-methy1-6-
(1,3,5 -tri methy1-1H-py razol-4-y1)-3,4-dihy dropyrazino [1,2-a] ind ol-1
(211)-one (27 mg, 0.05
mmol), ethyl 3-bromo-7-cyano-1-methyl-1H-indole-5-carboxylate (19 mg, 0.06
mmol), Cul
(5 mg, 0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 pL, 0.05
mmol), and
194
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
K2CO3 (25 mg, 0.075 mmol) followed by saponification using General Procedure
D. LCMS:
RT = 2.006 min, MS (ES) 736.9 (M+H).
Example 245
7-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino1,2indo1-2-y11-1-(3- pyrid
ylmethyl)ind ole-
2-carboxylic acid
[00435] The title compound (7.3 mg, 84%) was prepared following the procedure
described
Example 241 using ethyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-
methyl-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-
a]indo1-2(1H)-
y1)-1H-indole-2-carboxylate (8 mg, 0.011 mmol), NaH (2 mg, 0.05 mmol) and 3-
chloromethylpyridine HC1 salt (5 mg, 0.031 mmol). LCMS: RT = 1.760, 1.786 min,
MS
(ES) 789.0 (M+H).
Example 246
(R)-2-(Benzofuran-3-y1)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino 11,2-a] indo1-
1(21/)-one
(separated stereoisomer, 2" eluting atropisomer)
[00436] The title compound (11 mg, 33%) was prepared following General
coupling
procedure B as the second eluting atropisomer (absolute configuration
undetermined) along
with Example 237. LCMS: RT = 2.238 min, MS (ES) 655.0 (M+H).
Example 247
7-1(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propy1]-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihy d ropy razino 11,2-al ind ol-2-y11-1-(2-py rid
ylmethypind ale-
2-carboxylic acid
[00437] The title compound (4 mg, 37%) was prepared following the procedure
described
Example 241 using ethyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-
methy1-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-
a]indo1-2(1H)-
y1)-1H-indole-2-carboxylate (10 mg, 0.014 mmol) NaH (2 mg, 0.05 mmol) and 2-
bromomethylpyridine HBr salt (5 mg, 0.020 mmol). LCMS: RT = 1.839 min (major),
1.867
min (minor), MS (ES) 789.0 (M+H).
Example 248
7- [(4R)-7-chloro-10-13-(4-chloro-3,5-d imethyl-phenoxy)p ropyl] -4-methyl-1-
ox 0-641,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino[1,2-al indo1-2-yl] -1-(4-
pyridylmethyl)indole-
2-carboxylic acid
195
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00438] The title compound (5.7 mg, 53%) was prepared following the same
procedure as
described for Example 247, substituting 3-bromomethylpryidine HBr with 4-
bromomethylpyridine HBr salt (5 mg, 0.020 mmol). LCMS: RT = 1.817 min, MS (ES)
788.8
(M+H).
Example 249
7-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino 11,2-al ind ol-2-yl] - 1-(2-pyrid
ylmethyl)ind ole-
2-carboxylic acid (separated stereoisomer, et eluting atropisomer)
[00439] The title compound (2.1 mg, 19%) was separated from Example 247 by
reverse
phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient 35-95 % MeCN 0.1% TFA)
as the 1st eluting atropisomer (absolute stereochemistry undetermined). LCMS:
RT = 1.839
mm, MS (ES) 789.0 (M+H).
Example 250
(R)-3-(7-Chlo ro-10-(3-(4-chloro-3,5-d imethylp hen oxy)p rop y1)-4-methy1-1-
oxo-6-(1,3,5-
trimethy1-111-py razol-4-y1)-3,4-d ihyd ro py razino11,2-al in d I-2 (1H)-y1)-
1,7-d imethyl- 1H-
ind ole-6-carboxylic acid
Step A. Preparation of methyl 3-bromo-1,7-dimethy1-11-1-indole-6-carboxylate
[00440] The title compound (65 mg, 81%) was prepared following General
Procedure F
using methyl 7-methyl-1H-indole-6-carboxylate (50 mg, 0.28 mmol), NBS (51 mg,
0.28
mmol), NaH (14 mg, 0.34 mmol) and Mel (22 L, 0.34 mmol). LCMS: RT = 1.602
min, MS
(ES) 282.1 (M+H).
Step B. Example 250
[00441] The title compound (27 mg, 74%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-c]indol-1(2H)-one
(27 mg, 0.05
mmol), methyl 3-bromo-1,7-dimethy1-1H-indole-6-carboxylate (15 mg, 0.06 mmol),
CuI (5
mg, 0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 1.tL, 0.05
mmol), and
K2CO3 (25 mg, 0.075 mmol) followed by saponification using General Procedure
D. LCMS:
RT = 2.043 min, MS (ES) 726.0 (M+H); 11-1 NMR (400 MHz, DMSO-d6) 6 7.75 (d, J=
8.6
Hz, 1H), 7.49 (d, J= 3.3 Hz, 1H), 7.39 (dd, J= 8.4, 2.7 Hz, 1H), 7.29 (d, J=
8.6 Hz, 1H),
7.21 (dd, J= 8.4, 4.7 Hz, 1H), 6.72 (s, 2H), 4.44 (dd, J= 13.0, 3.9 Hz, 2H),
4.11 (s, 3H), 3.98
(tt, J= 6.4, 2.7 Hz, 2H), 3.78 (s, 1.5H), 3.77 (s, 1.5H), 3.58 (t, J= 11.2 Hz,
1H), 3.39 - 3.16
196
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(m, 2H), 2.95 (s, 3H), 2.25 (s, 6H), 2.12 (s, 1.5H), 2.11 ¨ 2.04 (m, 2H), 2.04
(s, 1.5H), 1.97
(s, 1.5H), 1.88 (s, 1.5H), 1.07 (d, J= 6.5 Hz, 3H).
Example 251
(R)-7-Chloro-10-(3-(4-chlo ro-3,5-d imethyl pheno xy)p ro py1)-4-methyl-2-(1-
methyl-5-
(1H- tetrazol-5-y1)-1H-ind ol-3-y1)-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihyd ropy razino [1,2-a] ind ol-1(2H)-one (separated stereoisomer, lst
eluting atropisomer)
[00442] The title compound (28 mg, 38%) was isolated along with Example 235 as
the first
eluting atropisomer (absolute configuration undetermined). LCMS: RT = 1.981
min, MS (ES)
736.0 (M+H).
Example 252
4-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-6-(4,6-
dimethylpyrim id in-5-y1)-4-methyl- oxo-3,4-dihy d rop yrazino [ 1,2-a] in d
ol-2-y11 -1-
methyl-indole-3-carboxylic acid
[00443] The title compound (11.5 mg, 42%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
di methylpy rimi din-5-y1)-4-methy1-3,4-d ihy dropy razino [1,2-a] indo1-1(2H)-
one (20 mg, 0.037
mmol), methyl 4-bromo-1-methy1-1H-indole-3-carboxylate (20 mg, 0.075 mmol),
Cul (5 mg,
0.026 mmol), (trans)-N1,N2-dimethylcyclohexane-1,2-diamine (8 mg, 0.056 mmol),
and
K2CO3 (25 mg, 0.18 mmol) followed by saponification using General Procedure D.
LCMS:
RT = 1.954 min, MS (ES) 709.9 (M+H); NMR
(400 MHz, Chloroform-d) (3 9.13 (s, 1H),
7.81 (d, J = 8.6 Hz, 1H), 7.45 ¨ 7.30 (m, 3H), 7.16 (dd, J= 5.4, 3.0 Hz, 1H),
7.06 (d, J= 4.4
Hz, 1H), 6.56 (s, 2H), 4.38 (dd, J= 12.3, 4.0 Hz, 1H), 3.92 (qd, J = 7.8, 6.3,
4.7 Hz, 2H),
3.80 (s, 3H), 3.71 (s, 1H), 3.49 ¨ 3.17 (m, 3H), 2.52 (s, 3H), 2.33 (d, J= 8.0
Hz, 1H), 2.29 ¨
2.19 (m, 8H), 2.17 ¨ 2.04 (m, 2H), 1.28 (d, J= 6.4 Hz, 3H).
Example 253
(R)-4-(7-Chloro-10-(3-(4-chloro-3,5-d imethylp henoxy) p rop y1)-4-methy1-1-
oxo-6-(1,3,5-
trimethy1-11/- pyrazol-4-y1)-3,4-d ihyd ro py razino[1,2-a] ind ol-2(1H)-
yl)quinoline-8-
carboxylic acid
[00444] The title compound (20 mg, 56%) was prepared following General
coupling
procedure A using (R)-7-chl oro-10-(3 -(4-chl oro-3,5-dimethylph enoxy)propy1)-
4-methy1-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazin o[1,2-a] ind 01-1 (211)-
one (27 mg, 0.05
mmol), methyl 4-bromoquinoline-8-carboxylate (16 mg, 0.06 mmol), Pd2(dba)3 (1
mg, 0.001
mmol), Xantphos (2 mg, 0.003 mmol), and Cs2CO3 (25 mg, 0.075 mmol) followed by
saponification using General Procedure D. LCMS: RT = 2.030 min, MS (ES) 709.9
(M+H);
197
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
1HNMR (400 MHz, DMSO-d6) 6 9.21 (d, J = 5.0 Hz, 1H), 8.70 ¨ 8.48 (m, 1H), 8.19
(dt, J =
8.5, 1.8 Hz, 1H), 7.83 (ddd, J= 15.6, 8.6, 4.4 Hz, 3H), 7.34 (d, J = 8.6 Hz,
1H), 6.70 (d, J =
5.4 Hz, 2H), 4.92 ¨ 4.62 (m, 1H), 4.40 ¨ 4.12 (m, 2H), 3.97 (t, J= 6.4 Hz,
2H), 3.79 (s, 3H),
3.39 - 3.13 (m, 2H), 2.23 (s, 6H), 2.14 (s, 1.5H), 2.04 (s, 3H), 2.02 ¨ 1.97
(m, 2H), 1.92 (s,
1.5H), 1.15 (dd, J= 6.5, 3.1 Hz, 3H).
Example 254
7- [(4R)-7-chloro-10- [3-(4-chloro-3,5-dimethyl-phenoxy)pr0py11-4-methy1-1-oxo-
6-(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-2-y1]-1-
(cyclopropylmethyl)indole-2-carboxylic acid
[00445] The title compound (7.6 mg, 92%) was prepared following the procedure
described
Example 241 using ethyl (R)-7-(7-chl oro-10-(3-(4-chl oro-3,5 -
dimethylphenoxy)propy1)-4-
methyl-1 -oxo-6-(1,3,5-trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-
a] indo1-2(1H)-
y1)-1H-indole-2-carboxylate (8 mg, 0.011 mmol), NaH (2 mg, 0.08 mmol) and
bromomethylcyclopropane (5 mg, 0.075 mmol). LCMS: RT = 2.096 min, MS (ES)
751.9
(M+H).
Example 255
8- [(4R)-7-chloro-10- [3-(4-chloro-3,5-dimethyl-phenoxy)propy11-6-(4,6-
dimethylpyrimidin-5-y1)-4-methyl-1-oxo-3,4-dihydropyrazino [1,2-a] indo1-2-
yl]naphthalene-1-carboxylic acid
[00446] The title compound (1.3 mg, 5%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
dimethylpyrimidin-5-y1)-4-methyl-3,4-dihydropyrazino[1,2-alindol-1(211)-one
(20 mg, 0.037
mmol), methyl 8-bromo-1-naphthoate (20 mg, 0.075 mmol), copper iodide (5 mg,
0.026
mmol), (trans)-NI,N2-dimethylcyclohexane-1,2-diamine (8 mg, 0.056 mmol), and
K2CO3 (25
mg, 0.18 mmol) followed by saponification and purification following General
Procedure D.
LCMS: RT = 1.976 min, MS (ES) 706.9 (M+H).
Example 256
7- [(4R)-7-chloro-10- [3-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-
6-(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2-yl] -1-(2-meth
oxyethyl)ind ole-
2-carboxylic acid
[00447] The title compound (2.6 mg, 25% yield) was prepared following the same
procedure as described in Example 245 substituting 3-chloromethylpyridine HC1
with 1-
bromo-2-methoxyethane (5 mg, 0.036 mmol). LCMS: RT = 2.016 mm, MS (ES) 755.8
(M+H).
198
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Example 257
N- [3-1(4R)-7-chloro-10-p-(4-chloro-3,5-dimethyl-phenoxy)propyl]-4-methy14-oxo-
6-
(1,3,5-trimethylpyrazol-4-y1)-3,4-dihyd ropy razino [1,2-alindol-2-y11-1-
methyl-indol-5-
yl[acetamide
Step A. Prepaation of N-(3-bromo-1-methyl-1H-indo1-5-y1)acetamide
[00448] To a solution of 1-methyl-1H-indo1-5-amine (60 mg, 0.41 mmol) in DCM
(3 mL)
was added DIPEA (100 mg, 0.78 mmol) followed by acetyl chloride (40 mg, 0.513
mmol) at
RT, and the reaction was stirred for 10 min. The reaction was quenched by the
addition of
Me0H, diluted into DCM/H20 (20 mL, 1:1), the layers were separated, and the
aqueous layer
was extracted with DCM (2 x 10 mL). The combined organic extracts were dried
concentrated in vacuo. The crude residue was dissolved in DMF (3 mL), and NBS
(75 mg,
0.42 mmol) was added. The reaction was stirred for 1 h at RT and determined to
be complete
by LCMS. The reaction was diluted into DCM (20 mL) and washed with 10% aqueous
Na2S203 solution. The organic layer was dried, concentrated, and purified by
flash
chromatography (Combi-flash Rf, Hex/Et0Ac = 0-100% gradient) to afford the
title
compound (40 mg, 36%).
Step B. Example 257
[00449] The title compound (15.2 mg, 75%) was prepared following General
Procedure B
using (R)-7-
chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-6-(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one (15 mg,
0.028
mmol), N-(3-bromo-l-methy1-1H-indo1-5-yl)acetamide (20 mg, 0.075 mmol), CuI (5
mg,
0.026 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), and
K2CO3
(30 mg, 0.22 mmol). MS (ES) 724.9 (M+H).
Example 258
(R)-7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-4-methyl-2-(1-methyl-
7-(1H-
tetrazol-5-yl)-1H-indol-3-yl)-6-(1,3,5-trimethyl-11-/-pyrazol-4-yl)-3,4-
dihydropyrazino[1,2-a]indol-1(2H)-one
[00450] The title compound (15 mg, 93%) was prepared according to the
procedure for
Example 235 using (R)-3 -(7-chl oro-10-(3-(4-chl oro-3,5-di methylphenoxy)pro
py1)-4-methyl-
1 -oxo-6-(1,3,5-trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-a]
indo1-2(1H)-y1)-1-
rnethy1-1H-indole-7-carbonitrile (15 mg, 0.022 mmol) and sodium azide (7 mg,
0.11 mmol),
LCMS: RT = 2.004 and 2.028 min, MS (ES) 736.0 (M+H).
Example 259
199
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-5-Chloro-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-
l-oxo-
6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino11,2-alindo1-2(1H)-
y1)benzofuran-2-carboxylic acid (separated stereoisomer, 2" eluting
atropisomer)
[00451] The title compound (12 mg, 33%) was isolated along with Example 232 as
the 2"d
eluting atropisomer (absolute configuration undetermined). LCMS: RT = 2.245
min, MS
(ES) 732.9 (M+H).
Example 260
(R)-2-(5-Acety1-1-methy1-1H-indo1-3-y1)-7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propyl)-4-methyl-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihyd ropyrazino [1,2-a]ind 01-1(211)-one
[00452] The title compound (21 mg, 59%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -tri methy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-a] ind ol-1
(21I)-one (27 mg, 0.05
mmol), 1-(3-bromo-l-methyl-1H-indo1-5-ypethan-1-one (13 mg, 0.06 mmol), CuI (5
mg,
0.025 mmol), (trans)-1,2-N,N'-dimethylaminocyclohexane (8 4, 0.05 mmol), and
K2CO3
(25 mg, 0,075 mmol). LCMS: RT = 2.078 and 2.099 min, MS (ES) 710.0 (M+H).
Example 261
3-44R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-dimethyl
methy 1p yrro lid in-3-yl)methyl)-1H-pyrazol-4-y1)-4-methyl-1-oxo-3,4-d ihyd
ro
pyrazino[1,2-alindo1-2(1H)-y1)-1-methyl-1H-indole-5-carboxylic acid
Step A. Preparation of methyl (R)-3-
(7-chloro-10-(3-(4-chloro-3,5-
d imethy 1p hen oxy)pro py1)-6-(3,5-dimethy1-1H-py razol-4-y1)-4-methyl-1-oxo-
3,4-
d ihyd ropyrazino [1,2-a] ind ol-2(1H)-y1)-1-methyl-1H-indole-5-carboxylate
[00453] To solution of methyl (R)-3 -
(7-chl oro-10-(3-(4-chl oro-3,5-
di methylphenoxy )propy1)-6-(3,5-di methy1-1-02-(trimethylsi ly Dethoxy
)methyl)-1H-py razol-
4-y1)-4-methy1-1 -oxo-3,4-dihy dropyrazino [1,2-a] indo1-2(1H)-y1)-1-methy1-1H-
indole-5-
carboxylate (100mg, 0,12 mmol) in THF (2 mL) was added TBAF (0.59 mL, 0.59
mmol).
The reaction mixture was heated to 100 C for 20 min under microwave. The
solvent was
removed in vacuo and residue was purified by flash chromatography (Combi-flash
Rf,
DCM/Me0H = 0-10% gradient) to afford the title compound as brown oil (75 mg,
88 %).
LCMS: RT = 0.984 min, MS (ES) 712.2 (M+H).
Step B. Example 261
200
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00454] To a solution of methyl (R)-3 -
(7-chl oro-10-(3-(4-chl oro-3,5-
di methy 1phenoxy )propy1)-6-(3,5-dimethy1-1H-py razol-4-y1)-4-methy 1- 1 -oxo-
3,4-
dihy dropyrazino1,2indo1-2(1H)-y1)-1 -methyl-1H-indole-5-carboxy late (41 mg,
0.057
mmol) in DMF (1.5 mL) was added NaH (5 mg, 0.11 mmol). After 15 min, 3-
(bromomethyl)-1-methylpyrrolidine (20 mg, 0.11 mmol) was added to reaction
mixture and
stirred overnight. The solvent was concentrated in vacuo and residue was
filtered through
silica pad with DCM/Me0H (3/1) then solvent was removed in vacuo. The residue
was re-
dissolved in mixture of methanol and dioxane (1 mL / 2 mL), and sodium
hydroxide (0.2 mL,
2M solution) was added. The reaction mixture was stirred for 3h at room
temperature and
then concentrated in vacuo. The residue was purified by reverse phase HPLC
(Phenomenex
Gemini C18, H20/CH3CN gradient to 35-95 % MeCN 0.1% TFA) to afford the title
compound (18 mg, 49 %). LCMS: RT = non polar method: 0.712 min, MS (ES) 795.3
(M+H).
Example 262
(R)-3-(7-ch loro-10-(3-(4-chloro-3,5-dimethyl p hen oxy) p ro pyI)-6-(3,5-d
imethy 1-1-
((tetrahy d ro-2H-py ran-4-yl)methyl)- 1H-py razol-4-y I)-4-methyl-1-oxo-3,4-d
ihyd ro
pyrazino indo1-2(1H)-yI)-1-methyl-1H-indole-5-carboxylic acid
[00455] The title compound (15 mg, 41 %) was prepared according to the
procedure used in
Example 261 Step B by substituting 3-(bromomethyl)-1-methylpyrrolidine for 4-
(bromomethyl)tetrahydro-2H-pyran. LCMS: RT = non polar method: 0.976 min, MS
(ES)
796.3 (M+H).
Example 263
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-dimethy1-1-
(2-
(pyrro lid in-1-ypethyl)-11-/-pyrazol-4-y1)-4-methyl-l-oxo-3,4-d ihyd rop
yrazino [1,2-
alindol-2(11/)-yl)-1-methyl-1H-indole-5-carboxylic acid
[00456] The title compound (13 mg, 40 %) was prepared according to the
procedure used in
Example 261 Step B by substituting 3-(bromomethyl)-1-methylpyrrolidine for 1-
(2-
bromoethyDpyrrolidine. LCMS: RT = non polar method: 0.766 min, MS (ES) 795.3
(M+H);
1-H NMR (Me0D, 400 MHz) 5 (ppm) 8.27 (s, 1H), 7.95 (d, J = 8.4 Hz, 1H), 7.78
(d, J = 8.8
Hz, 1H), 7.53 (d, J= 8.8 Hz, 1H), 7.39 (d, J= 3.6 Hz, 1H), 7.30 (d, J= 8.4 Hz,
1H), 6.64 (s,
2H), 4.62-4.47 (m, 3H), 4.32-4.30 (m, 1H), 4.02- 3.95 (m, 2H), 3.89 (s, 2H),
3.78-3.68 (m,
4H), 3.52-3.30 (m, 2H), 3.20-3.13 (m, 2H), 2.68 (s, 2H), 2.29 (s, 6H), 2.25-
2.03 (m, 4H), 2.20
(s, 3H), 2.04 (s, 3H), 1.25 (t, J= 6.0 Hz, 3H).
Example 264
201
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
1-1(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propyl]-4-methyl-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino11,2-a]indol-2-yl]isoquinoline-6-
carboxylic
acid
[00457] The title compound (14 mg, 53%) was prepared following General
coupling
procedure C using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-111-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one
(20 mg, 0.037
mmol), methyl 1-chloroisoquinoline-6-carboxylate (20 mg, 0.90 mmol), CuI (5
mg, 0.026
mmol), (trans)-N1,N2-dimethylcyclohexane-1,2-diamine (8 mg, 0.056 mmol), and
K3PO4 (30
mg, 0.14 mmol) followed by saponification using General Procedure D. LCMS: RT
= 2.299
min, MS (ES) 709.9 (M+H).
Example 265
(R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-2-(1-methyl-
5-
(piperazin-1-ylsulfony1)-1H-indol-3-y1)-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-
3,4-
dihydropyrazino[1,2-alindo1-1(2H)-one
Step A. Preparation of tert-butyl 44(3-bromo-1-methyl-1H-indo1-5-
ypsulfonyppiperazine-1-carboxylate
[00458] To a solution of tert-butyl 4-((1-methy1-1H-indo1-5-
y1)sulfonyl)piperazine-1-
carboxylate (65 mg, 0.17 mmol) in DMF (2 mL) was added NBS (35 mg, 0.20 mmol),
and
the reaction was stirred at RT until complete as determined by LCMS. The
reaction was
diluted into DCM (20 mL), washed with 10% aqueous Na2S203 (10 mL), dried, and
concentrated in vacuo to afford the title compound (66 mg, 85%).
Step B. Preparation of tert-butyl (R)-44(3-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-methyl-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihydropyrazino[1,2-a]indo1-2(1H)-y1)-1-methyl-1H-indol-5-
y1)sulfonyl)piperazine-1-
carboxylate
[00459] The title compound (25 mg, 49% crude) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one
(20 mg, 0.037
mmol), ter t-butyl 4-((3-bromo-l-methy1-1H-indo1-5-yOsulfonyl)piperazine-1-
carboxylate (30
mg, 0.066 mmol), Cul (5 mg, 0.026 mmol), (trans)-N1,N2 -di methylcy clohexane-
1,2-diamine
(8 mg, 0.056 mmol), and K2CO3.
Step C. Example 265
[00460] To a solution of the crude reaction product from Step B in dioxane (2
mL) was
added HC1 in dioxane (4.0 M, 0.5 mL, 2.0 mmol), and the reaction was stirred
for 16 h at RT.
202
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
The reaction mixture was concentrated in vacuo, and half of the material was
purified by
reverse phase HPLC (Phenomenex Gemini C18, H20/CH3CN gradient 35-95 MeCN 0.1%
TFA) to afford the title compound (7.3 mg, 48% yield two steps). LCMS: RT =
1.848 min,
MS (ES) 816.0 (M+H).
Example 266
4-[(4R)-7-chloro-1043-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino [1,2-al in d ol-2-y1]- 1-methyl-ind
ole-2-
carboxamide
[00461] The title compound (4.8 mg, 48%) was prepared following General
Procedure E
employing (R)-4-(7-chl oro-10-(3-(4-chl oro-3,5-dimethylphenoxy )propy 0-4-
methy1-1-ox o-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihy dropyrazino[1,2-a] indo1-2(1H)-y1)-
1-methy 1-1H-
indole-2-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(10 mg,
0.078 mmol), and ammonia in Me0H (7.0 M, 0.05 mL, 0.35 mmol). LCMS: RT = 2.061
min, MS (ES) 711.0 (M+H).
Example 267
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] in d ol-2 (1H)-y1)-N,1-
d imethyl-1H-
indole-5-sulfonamide
Step A. Preparation of 3-bromo-N,1-dimethy1-1H-indole-5-sulfonamide.
[00462] To a solution of 1-methyl-1H-indole-5-sulfonyl chloride (40 mg, 0.17
mmol) in
DCM (2 mL) was added DIPEA (40 mg, 0.31 mmol), Methylamine hydrochloride (30
mg,
0.45 mmol) was added, and the reaction was stirred at RT until complete by
LCMS then
concentrated in vacuo. The crude residue was then brominated following the
procedure
described in Example 265 Step A using NBS (35 mg, 0.20 mmol) to afford the
title
compound (37 mg, 71%).
Step B. Example 267
[00463] The title compound (9 mg, 32%) was prepared following General coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropyrazino[1,2-a]indol-1(2H)-one
(20 mg, 0.037
mmol), 3-bromo-N,1-dimethy1-1H-indole-5-sulfonamide (25 mg, 0.083 mmol), Cul
(5 mg,
0.026 mmol), (trans)-N1,N2-dirnethylcyclohexane-1,2-diamine (8 mg, 0.056
mmol), and
K2CO3 (25 mg, 0.18 mmol). LCMS: RT = 2.059 min, MS (ES) 760.9 (M+H); IFINMR
(400
MHz, Chloroform-d) 6 8.09 (d, J= 11.2 Hz, 1H), 7.73 (ddd, J= 9.3, 7.6, 1.8 Hz,
2H), 7.45
(d, J= 8.7 Hz, 1H), 7.40 ¨ 7.28 (m, 2H), 6.63 (s, 2H), 4.51 (dd, J= 40.0, 11.5
Hz, 1H), 4.20
203
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(d, J = 21.5 Hz, 1H), 4.08 (s, 1.5H), 4.05 (s, 1.5H), 3.99 (t, J= 6.2 Hz, 2H),
3.86 (s, 3H), 3.70
(t, J= 12.6 Hz, 1H), 3.51 - 3.28 (m, 2H), 2.62 (s, 1.5H), 2.60 (s, 1.5H), 2.34
(s, 1.5H), 2.32
(s, 6H), 2.28 (s, 1.5H), 2.24- 2.09 (m, 5H), 1.23 (d, J= 5.9 Hz, 3H).
Example 268
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihyd ropyrazino [1,2-a] in d 01-2 (1H)-y1)-1-
(2-
methoxyethyl)- 1H-in d ole-5-carb oxylic acid
Step A. Preparation of methyl 3-bromo-14(2-(trimethylsilypethoxy)methyl)-1H-
indole-
5-carboxylate
[00464] The title compound (107 mg, 71%) was prepared following General
Procedure G
using methyl 3-bromo-1H-indole-5-carboxylate (100 mg, 0.39 mmol), NaH (24 mg,
0.59
mmol), and (2-(chloromethoxy)ethyl)trimethylsilane (0.11 mL, 98.5 mg, 0.59
mmol).
Step B.
Preparation of methyl (R)-3-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-methyl-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihyd ropy razino [1,2-a] ind ol-2(1H)-y1)-1-02-(trimethylsilypetho xy)methyl)-
1H-ind ole-5-
carboxylate
[00465] The title compound (93 mg, 82%) was prepared following General
Procedure B
using methyl 3-bromo-1-((2-(trimethylsilyl)ethoxy)methyl)-1H-indole-5-
carboxylate (78 mg,
0.20 mmol), (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-
6-(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one (61 mg,
0.11 mmol),
(1R,2R)-N1,N2-dimethylcyclohexane-1,2-diamine (14 mg, 0.098 mmol), CuI (9.4
mg, 0.049
mmol) and K2CO3 (54.7 mg, 0.40 mmol). MS (ES) 842.3 (M+H).
Step C. Preparation of methyl (R)-3-
(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-methyl-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihyd ropy razino [1,2-a] ind o1-2(1H)-y1)-1H-ind ole-5-carb o xylate
[00466] To a solution of methyl (R)-3-
(7-chloro-10-(3-(4-chloro-3,5-
di methylphenoxy )propy1)-4-methy1-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-
3,4-
dihy dropyrazino[1,2-c]indo1-2(1H)-y1)-142-(trimethylsilypethoxy )methy 1)-1H-
indole-5-
carboxylate (110 mg, 0.13 mmol) in THF (0.5 mL) was added TBAF (1.18 mL, 1.17
mmol,
1.0 M in THF), and the reaction mixture was stirred as 40 C for 24 h. The
reaction was
cooled to RT, quenched with saturated NH4C1 aq, solution and extracted with
Et0Ac (3 x 5
mL). The combined organic layer was washed with brine, dried over MgSO4,
filtered, and
concentrated in vacuo. The residue was purified by flash chromatography (Combi-
flash Rf,
204
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Hex/Et0Ac = 0-100% gradient followed by DCM/Me0H = 0-10% gradient) to afford
the
title compound (40 mg, 43%). MS (ES) 712.3 (M+H).
Step D. Example 268
[00467] The title compound (5.1 mg, 21%, 2 steps) was prepared following
General
Procedure G using methyl (R)-3-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-
methyl-1-oxo-6-(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]
indo1-2(1H)-
y1)-1H-indole-5-carboxylate (23 mg, 0.032 mmol), NaH (1.9 mg, 0.048 mmol), 1-
bromo-2-
methoxyethane (30 mg, 0.275 mmol) followed by saponification and purification
following
General Procedure D. LCMS: RT = 1.947 min, MS (ES) 756.3 (M+H).
Example 269
3- 1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)p ro pyl]
dimethylpyrim idin-5-yl)-4-methyl-l-oxo-3,4-dihy d rop yrazino [1,2-a] ind ol-
2-y l] -1-
methyl-ind ole-5-carb oxamid e
[00468] The title compound (5.6 mg, 55%) was prepared following General
Procedure E
using ((R)-3-
(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(4,6-
dimethylpyrimidin-5-y1)-4-methyl-l-oxo-3,4-dihydropyrazino[1,2-a]indol-2(1H)-
y1)-1-
methyl-1H-indole-5-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026
mmol),
DIPEA (10 mg, 0.078 mmol), and ammonia in Me0H (7.0 M, 0.05 mL, 0.35 mmol).
LCMS:
RT = 2.015 min, MS (ES) 708.9 (M+H).
Example 270
7-1(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methyl-1-oxo-6-
(1,3,5-
trimethylpy razol-4-y D-3,4-dihyd ropy razino 11,2-al indo1-2-y11-1-methyl-
indole-2-
carboxamide
[00469] The title compound (6.8 mg, 68%) was prepared following General
Procedure E
using (R)-7-
(7-chl oro-10-(3-(4-chl oro-3,5-dimethy 1phenoxy )pro py1)-4-methy1-1-oxo-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3 ,4-dihy dropyrazino[1,2-a] indo1-2(1H)-
y1)-1-methy1-1H-
indole-2-carboxyl ic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(10 mg,
0.078 mmol), and ammonia in Me0H (7.0 M, 0.05 mL, 0.35 mmol). LCMS: RT = 2.001
min, MS (ES) 711.0 (M+H).
Example 271
(R)-7 -(10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(4,6-dimethylpyrimidin-5-
y1)-4,7-
dimethyl-l-oxo-3,4-dihydropyrazino[1,2-al indo1-2(1H)-y1)-1-(pyridin-2-
ylmethyl)-1H-
indole-2-carboxylic acid (partially separated the 1" eluting atropisomer)
205
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Step A. Preparation of ethyl (R)-7-
(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-6-(4,6-dimethylpyrimidin-5-y1)-4-methy1-1-oxo-3,4-
dihyd ropyrazino[1,2-al indo1-2(11/)-y1)-1H-indole-2-carboxylate
[00470] The title compound (14 mg, 13%) was prepared following General
Procedure B
using ethyl 7-bromo-1H-indole-2-carboxylate (80 mg, 0.30 mmol), (R)-7-chloro-
10-(3-(4-
chloro-3,5-dimethylphenoxy )propy1)-6-(4,6-dimethylpy rimi din-5 -y1)-4-methy1-
3,4-
dihy dropy razino [1,2-a] indol -1 (211)-one (80 mg, 0.15
mmol), (1R,2R)-N1,N2-
dimethylcyclohexane-1,2-diamine (47 p.L, 42.4 mg, 0.30 mmol), CuI (28.4 mg,
0.15 mmol)
and K2CO3 (61.8 mg, 0.45 mmol). MS (ES) 704.3 (M+H).
Step B. Example 271
[00471] The title compound was prepared following General Procedure G using
ethyl (R)-
7-(10-(3-(4-chloro-3,5-dimethylphenoxy )propy1)-6-(4,6-dimethylpy rimidin-5-
y1)-4,7-
dimethy 1-1-oxo-3,4-dihy dropyrazino[1,2-a]indo1-2(111)-y1)-1H-indole-2-
carboxylate (14 mg,
0.019 mmol), NaH (4.6 mg, 0.11 mmol), 2-(bromomethyl)pyridine hydrobromide
(14.4 mg,
0.058 mmol) followed by saponification using General Procedure D. The title
compound
was isolated as a partially separated the first eluting isomer (2.2 mg, 15%,
absolute
configuration undetermined). LCMS: RT = 1.877 min, MS (ES) 767.3 (M+H).
Example 272
7-[(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-6-(4,6-
dimethylpyrimidin-5-y1)-4-methyl-1-o xo-3,4-dihyd ro pyrazin o [1,2-a] ind o1-
2-y1]-1,5-
d imethyl-ind ole-2-carb oxylic acid
[00472] The title compound (14.9 mg, 37%) was prepared following General
coupling
procedure A using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
dimethylpyrimidin-5-y1)-4-methy1-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one (30
mg, 0.056
mmol), methyl 7-bromo-1,5-dimethy1-1H-indole-2-carboxylate (25 mg, 0.089
mmol),
Pd2(dba)3 (6 mg, 0.0065 mmol), Xantphos (8 mg, 0.013 mmol), and Cs2CO3 (60 mg,
0.19
mmol) followed by saponification using General Procedure D. LCMS: RT = 2.092,
2.119
min, MS (ES) 723.9 (M+H); II-1 NMR (400 MHz, Chloroform-d) ö 9.19 (s, 1H),
7.82 (dd, J =
8.7, 1.1 Hz, 1H), 7.48 (s, 0.7H), 7.44 (s, 0.3H) 7.39 - 7.34 (m, 2H), 6.95 (d,
J = 1.5 Hz, 1H),
6.63 (s, 1.3H),6.61 (s, 0.7H), 4.20 (d, J= 21.5 Hz, 2H), 4.06 ¨ 3.93 (m, 4H),
3.85 ¨3.71 (m,
1H),3.53 (dd, J= 12.6, 5.4 Hz, 1H), 3.45 ¨ 3.32 (m, 2H), 2.52 (s, 1H), 2.50
(s, 2H), 2.43 (s,
2H), 2.40 (s, 1H), 2.34 (s, 1H), 2.33 (s, 2H), 2.32 (s, 6H), 2.25 ¨ 2.18 (m,
2H),1.32 (d, J= 6.4
Hz, 2H), 1.22 (d, J = 6.5 Hz, 1H).
206
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Example 273
3-[(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-6-(4,6-
dimethylpyrimidin-5-y1)-4-methyl-1-oxo-3,4-dihydropyrazino[1,2-al indo1-2-y11-
1-
methyl-indazole-4-carboxylic acid
[00473] The title compound (13.2 mg, 39%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-
(4,6-
dimethylpyrimidin-5-y1)-4-methy1-3,4-dihydropyrazino[1,2-a]indo1-1(2H)-one (25
mg, 0.046
mmol), methyl 3-bromo-1-methy1-1H-indazole-4-carboxylate (20 mg, 0.075 mmol),
CuI (5
mg, 0.026 mmol), (trans)-N1,N2-dimethylcyclohexane-1,2-diamine (8 mg, 0.056
mmol), and
K2CO3 (30 mg, 0.22 mmol) followed by saponification using General Procedure D.
LCMS:
RT = 1.935 min, MS (ES) 711.0 (M+H); NMR
(400 MHz, Chloroform-a) 6 9.17 (s, 1H),
7.75 (d, J= 8.7 Hz, 1H), 7.59 (d, J= 7.7 Hz, 1H), 7.34 (dd, J= 7.8, 5.4 Hz,
3H), 6.51 (s, 2H),
4.36 (s, 1H), 4.07 (s, 3H), 3.92 (s, 2H), 3.74 (s, 2H), 3.36 (s, 1H), 3.21
(dt, J = 13.7, 7.4 Hz,
1H), 2.49 (s, 3H), 2.26 (s, 6H), 2.24 - 2.10 (m, 5H), 1.27 (d, J= 5.7 Hz, 3H).
Example 274
(R)-7-chloro-10-(3-(4-chlo ro-3,5-d imethylp henoxy)p ro py1)-4-methy1-2-(1-
methyl-5-((4-
methyl pi perazin-1-yl)s ulfony1)-1H-ind ol-3-y1)-6-(1,3,5-trimethyl-11/-
pyrazol-4-y1)-3,4-
d ihyd ropyrazino [1,2-al indo1-1(2H)-one
Step A. Preparation of 3-bromo-1-methyl-5-((4-methylpiperazin-1-yl)sulfony1)-
1H-
indole
[00474] The title compound (49 mg, 75%) was prepared following the procedure
described
in Example 265 Step A using 1-methy1-5-((4-methylpiperazin-1-y1)sulfony1)-1H-
indole (80
mg, 0.17 mmol) and NBS (35 mg, 0.20 mmol).
Step B. Example 274
[00475] The title compound (22.6 mg, 73%) was prepared following General
coupling
procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(21-1)-one
(20 mg, 0.037
mmol), 3-bromo-1-methy1-5-((4-methylpiperazin-1-yOsulfony1)-1H-indole (30 mg,
0.081
mmol), Cul (5 mg, 0.026 mmol), (trans)-N1,N2-dimethylcyclohexane-1,2-diamine
(8 mg,
0.056 mmol), and K2CO3 (25 mg, 0.18 mmol) followed by saponification using
General
Procedure D. LCMS: RT = 1.868 min, MS (ES) 829.9 (M+H); NMR
(400 MHz,
Chloroform-d) 6 7.97 (d, J= 1.6 Hz, 0.5 H), 7.90 (d, J= 2.0 Hz, 0.5H) 7.75
(dd, J= 8.6, 5.5
Hz, 1H), 7.63 (ddd, J = 13.7, 8.7, 1.7 Hz, 1H), 7.51 (s, 0.5H), 7.49 (s,
0.5H), 7.37 (s, 1H),
7.32 (dd, J = 8.6, 1.9 Hz, 1H), 6.65 (s, 1H), 6.64 (s, 1H), 4.50 (dd, J= 12.8,
4.0 Hz, 0.5H),
207
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
4.28 (d, J= 12.4 Hz, 1.5H), 4.11 ¨ 3.96 (m, 5H), 3.94¨ 3.70 (m, 7H), 3.64 ¨
3.27 (m, 3H),
3.15 ¨2.87 (m, 4H), 2.82 (s, 1.5H), 2.79 (s, 1.5H), 2.34 (s, 3H), 2.33 (s,
3H), 2.32 (s, 1.5H),
2.28 (s, 1.5H), 2.25 - 2.19 (m, 2H), 2.14 (d, J= 2.6 Hz, 3H), 1.32 (d, J= 6.1
Hz, 1.5H), 1.18
(d, J = 6.3 Hz, 1.5H).
Example 275
3-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)pr0py11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihyd ropyrazino 11,2-al indo1-2-yl] -1,2-dimethyl-
ind ole-5-
c arb oxyli c acid
Step A. Preparation of methyl 3-bromo-1,2-dimethy1-1H-indole-5-carboxylate
[00476] The title compound (98 mg, 81%) was prepared following General
Procedure F
using 2-methyl-1H-indole-5-carboxylic acid (75 mg, 0.43 mmol), NBS (90 mg,
0.51 mmol),
NaH (42 mg, 1.75 mmol), and Mel (200 mg, 1.41 mmol).
Step B. Example 275
[00477] The title compound (12.5 mg, 35%) was prepared following General
coupling
procedure B using (R)-7-chl oro-10-(3-(4-chl oro-3,5 -dimethy 1phenoxy
)propy1)-4-methy1-6-
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino[1,2-a] ind ol-1
(211)-one (25 mg, 0.046
mmol), methyl 3-bromo-1,2-dimethy1-1H-indole-5-carboxylate (30 mg, 0.11 mmol),
Cul (5
mg, 0.026 mmol), (tr ans)-N1 ,N2 -di methylcy clohexane-1,2-diamine (8 mg,
0.056 mmol), and
K2CO3 (30 mg, 0.22 mmol) followed by saponification using General Procedure D.
LCMS:
RT = 2.109 min, MS (ES) 726.0 (M+H).
Example 276
(R)-7 -(10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(4,6-dimethylpyrimidin-5-
y1)-4,7-
dimethyl-l-oxo-3,4-dihydropyrazino [1,2-a] indo1-2(1H)-y1)-1-(pyri d in-2-
ylmethyl)-111-
indole-2-carboxylic acid (partially separated the rd eluting atropisomer)
[00478] The title compound was isolated by reverse phase HPLC (Phenomenex
Gemini
C18, H20/CH3CN gradient to 35-95 % MeCN 0.1% TFA, 10 mins gradient) as a
partially
separated the 2' eluting isomer (absolute configuration undetermined). LCMS:
RT = 1.897
min, MS (ES) 767.3 (M+H).
Example 277
3-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihyd ropyrazino 11,2-al in d ol-2-y11-1-methyl-ind
ole-6-
carboxamide
[00479] The title compound (3.2 mg, 32%) was prepared following General
Procedure E
employing (R)-3 -(7-chl oro-10-(3-(4-chl oro-3,5-dimethy 1phenoxy)propy1)-4-
methy1-1-oxo-6-
208
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(1,3,5 -trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-a] ind I-2
(1H)-y1)-1-methy 1-1H-
indole-6-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(10 mg,
0.078 mmol), and ammonia in Me0H (7.0 M, 0.05 mL, 0.35 mmol). LCMS: RT = 2.011
mm, MS (ES) 711.0 (M+H).
Example 278
(R)-2-(5-((4-acetylpiperazin- 1-yl)sulfony1)-1-methyl-1H-ind ol-3-y1)-7-chloro-
10-(3-(4-
chloro-3,5-dimethylphenoxy)propy1)-4-methyl-6-(1,3,5-trimethyl-1H-pyrazol-4-
y1)-3,4-
dihydropyrazino [1,2-a]indo1-1(21-/)-one
1004801 To a solution of (R)-7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-
methy1-2-(1-methyl-5-(piperazin-1-ylsulfonyl)-1H-indol-3-y1)-6-(1,3,5-
trimethyl-1H-pyrazol-
4-y1)-3,4-dihydropyrazino[1,2-alindo1-1(2H)-one (15 mg, 0.018 mmol) in DCM (3
mL) was
added DIPEA (10 mg, 0.078 mmol) followed by acetyl chloride (5 mg, 0.037
mmol). The
reaction was stirred at RT for 10 min. The reaction was quenched with Me0H,
diluted with
DCM/H20 (10 mL, 1:1) and extracted with DCM (2 x 5 mL). The combined organic
extracts
were dried, concentrated in vacuo, and purified by reverse phase HPLC
(Phenomenex Gemini
C18, H20/CH3CN gradient 35-95 % MeCN 0.1% TFA) to afford the title compound
(12.1
mg, 77%). LCMS: RT = 2.245, 2.262 min, MS (ES) 858.0 (M+H).
Example 279
7-(8-Chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-7-(4,6-
dimethylpyrimidin-5-
y1)-1-oxo-4,5-dihydro-1H-11,41 diazepino [1,2-a] in d ol-2(31/)-y1)-1-(pyridin-
2-ylmethyl)-
1H-indole-2-carboxylic acid
Step A. Preparation of ethyl 7-(8-chloro-11-(3-(4-chloro-3,5-
dimethylphenoxy)propyl)-
7-(4,6-dimethylpyrimidin-5-yl)-1-oxo-4,5-dihydro-1H-(1,4] diazepino [1,2-a]
ind ol-2(31/)-
y1)-1H-ind ole-2-carboxylate
1004811 The title compound (21 mg, 20% yield) was prepared following General
Procedure
B using ethyl 7-bromo-1H-indole-2-carboxylate (79.8 mg, 0.30 mmol), 8-chloro-
11-(3-(4-
chloro-3,5-dimethylphenoxy )propy1)-7-(4,6-dimethy 1py rimi din-5 -y1)-2,3,4,5
-tetrahy dro-1H-
[1,4]diazepino[1,2-a_lindol-1 -one (80 mg, 0.15 mmol), (1R,2R)-N1,/V2-
dimethylcyclohexane-
1,2-diamine (47 uL, 42.4 mg, 0.30 mmol), Cu! (28.4 mg, 0.15 mmol) and K2CO3
(61.8 mg,
0.45 mmol). MS (ES) 724.3 (M+H).
Step B. Example 279
1004821 The title compound (5.7 mg, 32%, 2 steps) was prepared following
General
Procedure G using ethyl 7-(8-chl oro-11-(3 -(4-chl oro-3,5-
dimethylphenoxy)propy1)-7-(4,6-
209
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
dimethylpyrimidin-5-y1)-1-oxo-4,5-dihy dro-1H41,4]diazepino[1,2-c]indol-2(3H)-
y1)-1H-
indole-2-carboxylate (17 mg, 0.023 mmol), NaH (2.8 mg, 0.069 mmol), 2-
(bromomethyl)pyridine hydrobromide (8.9 mg, 0.035 mmol) followed by
saponification
using General Procedure D. LCMS: RT = 1.845 min, MS (ES) 787.1 (M+H). NMR
(400
MHz, CDC13) 6 9.18 (s, 1H), 8.59 (s, 1H), 8.01-7.97 (m, 1H), 7.78 (d, J= 8.0
Hz, 1H), 7.65
(d, J = 8.6 Hz, 1H), 7.59 (s, 1H), 7.39-7.34 (m, 1H), 7.30-7.23 (m, 2H), 7,06
(d, J= 7.3 Hz,
1H), 6.84 (d, J= 8.0 Hz, 1H), 6.59 (s, 2H), 6.41 (d, J= 17.8 Hz, 1H), 6.12 (d,
J= 17.0 Hz,
1H), 4.09-3.97 (m, 2H), 3.84-3.79 (m, 1H), 3.70-3.59 (m, 2H), 3.38-3.36 (m,
1H), 2.89-2.84
(m, 1H), 2.42 (s, 3H), 2.32 (s, 6H), 2.28 (s, 3H), 2.16-2.12 (m, 1H), 2.05-
1.94 (m, 2H), 1.88-
1.83 (m, 1H), 1.64-1.57 (m, 1H).
Example 280
3-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methyl-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino11,2-alindol-2-y11-1-methyl-N-(2-
pyridyl)indole-5-carboxamide
[00483] The title compound (3.5 mg, 31%) was prepared following General
Procedure E
employing (R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-
l-oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indo1-2(1H)-y1)-1-
methyl-1H-
indole-5-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(20 mg,
0.155 mmol), and 2-aminopyridine (5 mg, 0.053 mmol). The reaction was stirred
for 16 h at
room temperature followed by 8 h at 50 C. LCMS: RT = 1.994 min, MS (ES) 787.9
(M+H).
Example 281
3-1(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propyl1-4-methyl-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino11,2-ailindol-2-y1]-1-methyl-N-(2-
pyridylmethyl)indole-5-carboxamide
[00484] The title compound (6.6 mg, 58%) was prepared following General
Procedure E
employing (R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-
1-oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-2(1H)-y1)-1-
methyl-1H-
indole-5-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(20 mg,
0.155 mmol), and pyridin-2-ylmethanamine (5 mg, 0.046 mmol). After 16 hr the
reaction
was complete. LCMS: RT = 1.861 min, MS (ES) 802.0 (M+H).
Example 282
210
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
3-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy1]-4-methyl-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino[1,2-alind ol-2-yll-N,1-dimethyl-N-
(3-
pyridylmethyl)indole-5-carboxamide
[00485] The title compound (8.5 mg, 75%) was prepared following General
Procedure E
employing (R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-
1-oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-c]indol-2(1H)-y1)-1-
methy1-1H-
indole-5-carboxylic acid (10 mg, 0,014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(20 mg,
0.155 mmol), and N-methyl-1-(pyridin-3-yOmethanamine (5 mg, 0,041 mmol). After
16 h
the reaction was complete. LCMS: RT = 1.860 min, MS (ES) 816.0 (M+H).
Example 283
3-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methyl-1-oxo-6-
(1,3,5-
trimethylp yrazol-4-y1)-3,4-d ihydropy razino [ 1,2-al indo1-2-y11-1-methyl-N-
(4-
pyridyl)indole-5-carboxamide
[00486] The title compound (6.6 mg, 58%) was prepared following General
Procedure E
employing (R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-
1-oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihy dropyrazino[1,2-a] indo1-2(1H)-y1)-
1-methy 1-1H-
indole-5-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(20 mg,
0.155 mmol), and 4-aminopyridine (5 mg, 0.053 mmol). The reaction was stirred
for 16 h at
room temperature followed by 8 h at 50 C. LCMS: RT = 1.875 min, MS (ES) 787.9
(M+H).
Example 284
3-1(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methyl-1-oxo-6-
(1,3,5-
trimethylp yrazol-4-y1)-3,4-d ihydropy razino [ 1,2-a] ind ol-2-y1]-1-methyl-N-
(4-
p yridylmethyl)ind ole-5-c arb oxamide
[00487] The title compound (8.3 mg, 73%) was prepared following General
Procedure E
employing (R)-3 -(7-chl oro-10-(3-(4-chl oro-3,5-dimethy 1phenoxy )pro py1)-4-
methy1-1-oxo-6-
(1,3,5 -trimethy1-1H-pyrazol-4-y1)-3 ,4-dihy dropyrazino[1,2-a] indo1-2(1H)-
y1)-1-methy1-1H-
indole-5-carboxyl ic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(20 mg,
0.155 mmol), and pyridin-4-ylmethanamine (5 mg, 0.046 mmol). After 16 h the
reaction was
be complete, LCMS: RT = 1.825 min, MS (ES) 802.0 (M+H).
Example 285
3-[(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino[1,2-al indo1-2-y1]-1-methyl-N-
phenyl-indole-
5-carboxamide
211
84451174
[00488] The title compound (6.3 mg, 57%) was prepared following General
Procedure E
employing (R)-3-
(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1 -oxo-6-
(1,3,5-irimethy1-1H-pyrazol-4 -y1)-3 ,4-dihydropyrazino [1,2 -a] indo1-2(1H)-
y1)-1-methy1-1H-
indole-5-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(20 mg,
0.155 mmol), and aniline (5 mg, 0.053 mmol). The reaction was stirred for 16 h
at room
temperature followed by 8 h at 50 C. LCMS: RT = 2.234 min, MS (ES) 787.0
(M+H).
Example 286
(R)-3-(7-chlor o-10-(3-(4-chlo ro-3,5-dimethylphenoxy)prop y1)-4-methy1-1-oxo-
6-(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazin o [1,2-a] ind ol-2(1H)-y1)-N,1-
dim ethyl-1H-
indole-5-carboxamide
[00489] The title compound (6.8 mg, 67%) was prepared following General
Procedure E
employing (R)-3-
(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2 -a] indo1-2(11/)-
y1)- 1-methyl-1H-
indole-5-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(20 mg,
0.155 mmol), and methylamine hydrochloride (5 mg, 0.074 mmol). After 16 h the
reaction was
complete. LCMS: RT = 2.029 min, MS (ES) 725.0 (M+H).
Example 287
3- [(4R)-7-chlor o-10-[3-(4-chlor o-3,5-dim ethyl-ph enoxy)propy1]-4-methyl-l-
oxo-6-(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino[l,2-a[indol-2-y1]-1-methyl-N-(3-
pyridylmethyl)indole-5-carboxamide
[00490] The title compound (8.8 mg, 78%) was prepared following General
Procedure E
employing (R)-3-
(7-chloro-10-(3-(4-chloro-3 ,5-dimethylphenoxy)propy1)-4-methyl-1 -oxo-6-
(1,3,5-trimethyl- 1H-pyrazol-4-y1)-3 ,4-dihy dropyrazino [1,2 -a] indo1-2(1H)-
y1)- 1-methyl- 1H-
indole-5-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(20 mg,
0.155 mmol), and pyridin-3-ylmethanamine (5 mg, 0.046 mmol). After 16 h the
reaction was
complete. LCMS: RT = 1.841 min, MS (ES) 802.0 (M+H).
Example 288
(R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-2-(1-methy1-
1H-indo1-
3-y1)-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-
1(2H)-one
[00491] The title compound (14.6 mg, 78%) was prepared following General
Procedure B
using (R)-7-chloro-10-(3 -(4 -chloro-3,5-dimethy 1phenoxy)propy1)-4-methy1-6-
(1,3,5-trimethyl-
1H-pyrazol-4-y1)-3,4-dihy dropyrazino[1,2-cdindol-1(211)-one (15 mg, 0.028
mmol), 3-bromo-1-
methy1-1H-indole (11.7 mg, 0.056 mmol),
(1R,2R)-N1,N2 -
212
Date Recue/Date Received 2023-06-16
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
dimethylcyclohexane-1,2-diamine (8.9 lat, 8.0 mg, 0.056 mmol), CuI (5.3 mg,
0.028 mmol)
and K2CO3 (11.6 mg, 0.084 mmol). LCMS: RT = 2.151 min, MS (ES) 667.9 (M+H);
NMR (400 MHz, CDC13) 8 7.59 (dd, J = 8.6, 2.3 Hz, 1H), 7.40 (dd, J = 7,9, 3,8
Hz, 1H),
7.28-7.26 (m, 1H), 7.22-7.21 (m, 1H), 7.18-7.16 (m, 1H), 7.10 (d, J= 5.3 Hz,
1H), 7.05 (t, J
= 7.5 Hz, 1H), 6.54 (s, 2H), 4.31-4.26 (m, 1H), 4.24-4.10 (m, 1H), 3.95-3.90
(m, 2H), 3.80
(d, J = 3.2 Hz, 3H), 3.73 (s, 3H), 3.56 (ddd, J = 12.5, 7.9, 1.5 Hz, 1H), 3.40-
3.33 (m, 1H),
3.31-3.24 (m 1H), 2.24 (s, 6H), 2.12 (s, 3H), 1.95 (d, J= 2.9 Hz, 3H), 1.19-
1.15 (m, 2H),
1.10 (d, J= 6.4 Hz, 3H).
Example 289
3-[(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-2-y11-N,IV,1-
trimethyl-ind ole-5-
carboxamide
1004921 The title compound (8.0 mg, 76%) was prepared following General
Procedure E
employing (R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-
l-oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-2(1H)-y1)-1-
methyl-1H-
indole-5-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(20 mg,
0.155 mmol), and dimethylamine hydrochloride (5 mg, 0.061 mmol). After 16 h
the reaction
was complete. LCMS: RT = 2.085 min, MS (ES) 739.0 (M+H).
Example 290
(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propyli-4-methyl-2-R-methyl-
5-
(pyrrolidine-1-carbonyl)indol-3-y11-6-(1,3,5-trimethylpyrazol-4-y1)-3,4-
dihydropyrazino[1,2-a]indo1-1-one
1004931 The title compound (7,8, 72%) was prepared following General Procedure
E
employing (R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-
l-oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-2(1H)-y1)-1-
methyl-1H-
indole-5-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(20 mg,
0.155 mmol), and pyrrolidine (5 mg, 0.070 mmol). After 16 hr the reaction was
complete.
LCMS: RT = 2.193 min, MS (ES) 765.0 (M+H); IFINMR (400 MHz, Chloroform-d) .5
7.85
¨ 7.69 (m, 2H), 7.48 ¨ 7.28 (m, 4H), 6.62 (d, J= 1.8 Hz, 2H), 4.53-4.36 (m,
1H), 4.15-4.09
(m, 4H), 3.99 (t, J= 6.1 Hz, 2H), 3.88 ¨ 3.79 (m, 3H), 3.74-3.49 (s, 4H), 3.37
(ddt, J = 34.7,
13.6, 7.1 Hz, 2H), 2.34 (s, 1.5H), 2.32 (s, 3H), 2.31 (s, 3H), 2.28 (s, 1.5H),
2.22-2.13 (m, 6H),
2.05-1.81 (br. s, 4H), 1.24 (m, 3H).
Example 291
213
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
3- [(4R)-7-chloro-10- 13-(4-chloro-3,5-dimethyl-phenoxy)p ropyl] -4-methyl-1-
oxo-6-(1,3,5-
trimethylpyrazol-4-y1)-3,4-d ihyd ropy razino [1,2-al ind 01-2-y1F 1-methyl-N-
(3-
pyridyl)indole-5-carboxamide
[00494] The title compound (6.7 mg, 60%) was prepared following General
Procedure E
employing (R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-
1-oxo-6-
(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-2(1H)-y1)-1-
methyl-1H-
indole-5-carboxylic acid (10 mg, 0.014 mmol), HATU (10 mg, 0.026 mmol), DIPEA
(20 mg,
0.155 mmol), and 3-aminopyridine (5 mg, 0.053 mmol). The reaction was stirred
for 16 h at
room temperature followed by 8 h at 50 C. LCMS: RT = 1.882 min, MS (ES) 787.9
(M+H).
Example 292
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-py razol-4-y1)-3,4-d ihy d ropy razino [1,2-a] indo1-2(1H)-y1)-1-
(2-
morpholinoethyl)-1H-indole-6-carboxylic acid (separated the 2' eluting
atropisomer)
Step A. Preparation of methyl 3-bromo-1-(2-morpholinoethyl)-1H-indole-6-
carboxylate
[00495] The title compound (27 mg, 19%) was prepared following General
Procedure G
using methyl 3-bromo-1H-indole-6-carboxylate (100 mg, 0.39 mmol), Nall (94.6
mg, 2.36
mmol) and 4-(2-chloroethyl)morpholine hydrochloride (220 mg, 1.2 mmol). MS
(ES) 367.0
(M+H).
Step B. Example 292
[00496] The title compound (2.5 mg, 6%, 2 steps) was prepared following
General
Procedure B using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-6-
(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one
(30 mg, 0.056
mmol), methyl 3-bromo-1-(2-morpholinoethyl)-1H-indole-6-carboxylate (27 mg,
0.074
mmol), (1R,2R)-N1,N2-dimethylcyclohexane-1,2-diamine (7.0 L, 6.4 mg, 0.044
mmol), Cul
(4.3 mg, 0.022 mmol) and K2CO3 (27.0 mg, 0.21 mmol) followed by saponification
using
General Procedure D. The title compound was isolated as the 2nd eluting isomer
from the
final purification by reverse phase HPLC (absolute configuration
undetermined). LCMS: RT
= 1.837 min, MS (ES) 811.3 (M+H).
Example 293
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino 11,2-al indo1-2(1H)-y1)-1-(2-
morpholinoethyl)-1H-ind ole-6-carb oxylic acid (separated the 1" eluting
atropisomer)
214
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00497] The title compound (11.5 mg, 25%, 2 steps) was isolated as the
eluting isomer
from the final reverse phase HPLC along with Example 292. LCMS: RT = 1.815
min, MS
(ES) 811.3 (M+H).
Example 294
3-17- [2-(benzyloxymethyl)-4,6-d imethy 1- py rimid in-5-yl] -8-ch loro-11-
[344- chloro-3,5-
dimethyl- phenoxy)pro py11-1-oxo-4,5-d ihyd ro-3H-11,41diazepino 11,2-al ind
ol-2-yl] -1-
methyl-ind ole-5-carboxylic acid
Step A. Preparation of ethyl 7-(2-((benzyloxy)methyl)-4,6-dimethylpyrimidin-5-
y1)-1-(3-
((tert-butoxycarbonyl)amino)propy1)-6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propyl)-1H-indole-2-carboxylate
[00498] A mixture of ethyl 7-(2-((benzyloxy)methyl)-4,6-dimethylpyrimidin-5-
y1)-6-
chloro-3-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1H-indole-2-carboxylate (180
mg, 0.278
mmol), tert-butyl 1,2,3-oxathiazinane-3-carboxylate 2,2-dioxide (100 mg, 0.42
mmol), and
Cs2CO3 (200 mg, 0.61 mmol) in MeCN (10 mL) was heated to 80 C for 16 h. After
which
time, an additional portion of tert-butyl 1,2,3-oxathiazinane-3-carboxylate
2,2-dioxide (30
mg, 0Ø13 mmol) was added. The reaction was heated an additional 6 h, cooled
to RT then
diluted with Et0Ac/H20 (40 mL, 1:1). The organic layer was separated, and the
aqueous
layer was extracted with Et0Ac (2 x 20 mL). The combined organic layers were
dried over
MgSO4, filtered, and concentrated in vacuo. The crude reaction mixture was
purified by
flash chromatography (Combi-flash Rf, Hex/Et0Ac = 0-100% gradient) to afford
the title
compound (194 mg, 87%).
Step B. Preparation of 7-(2-((benzyloxy)methyl)-4,6-dimethylpyrimidin-5-y1)-8-
chloro-
11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-2,3,4,5-tetrahyd ro-1H- [ 1,4] d
iazepino [1,2-
a] ind ol-1- one
[00499] To a solution of ethyl 7-(2-((benzyloxy)methyl)-4,6-dimethylpyrimidin-
5-y1)-1-(3-
((tert-butoxy carbonyl)amino)propy1)-6-chloro-3-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-
1H-indole-2-carboxylate (194 mg, 0.24 mmol) in 1,4-dioxane (20 mL) was added a
solution
of 4M HC1 in dioxanes (1 mL, 4 mmol). The reaction was stirred overnight then
concentrated in vacuo. The crude residue taken up in Me0H (10 mL) then K2CO3
(300 mg,
2.17 mmol) was added. The reaction was heated to 50 C for 5 h, concentrated
then
dissolved in DCM/H20 (60 mL, 1:1). The organic layer was separated, and the
aqueous layer
was extracted with DCM (2 x 20 mL). The combined organic layers were dried
over MgSO4,
filtered, and concentrated in vacuo. The crude reaction mixture was purified
by flash
215
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
chromatography (Combi-flash RI, Hex/Et0Ac = 0-100% gradient) to afford the
title
compound (117 mg, 74% 2 steps).
Step C. Preparation of methyl 3-(7-(2-((benzyloxy)methyl)-4,6-
dimethylpyrimidin-5-yl)-
8-chloro-11-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-1-oxo-4,5-dihydro-1H-
11,41diazepino[1,2-alindol-2(3H)-y1)-1-methyl-1H-indole-5-carboxylate
[00500] The title compound (78 mg, 53%) was prepared following General
coupling
procedure B using 7-(2-((benzyloxy)methyl)-4,6-dimethylpyrimidin-5-y1)-8-
chloro-11-(3-(4-
chloro-3,5-dimethylphenoxy)propy1)-2,3,4,5-tetrahydro-1H41,4]diazepino[1,2-
alindol-1-one
(115 mg, 0.17 mmol), methyl 3-bromo-1-methy1-1H-indole-5-carboxylate (100 mg,
0.37
mmol), Cu! (10 mg, 0.052 mmol), (trans)-1,2-N,/V'-dimethylaminocyclohexane (15
mg, 0.10
mmol), and K2CO3 (75 mg, 0.54 mmol).
Step D. Example 294
[00501] The title compound (4 mg, 80%) was prepared following General
Procedure D
using methyl 3-(7-(2-((benzyloxy)methyl)-4,6-dimethylpyrimidin-5-y1)-8-chloro-
11-(3-(4-
chloro-3,5-dimethylphenoxy)propy1)-1-oxo-4,5-dihydro-lH-11,41diazepino[1,2-
alindol-
2(3H)-y1)-1-methyl-1H-indole-5-carboxylate (5 mg, 0.0059 mmol) and LiOH (2 mg,
0.083
mmol). LCMS: RT = 2.155 min, MS (ES) 829.9 (M+H).
Example 295
7-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methyl-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino11,2-alindol-2-y1]-5-methyl-1-(2-
pyridylmethyBindole-2-carboxylic acid
Step A. Preparation of methyl (R)-7-
(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propyl)-4-methyl-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihydropyrazino[1,2-a[indol-2(1H)-yl)-5-methyl-1H-indole-2-carboxylate
[00502] The title compound was prepared following General Procedure C using
(R)-7 -
chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-6-(1,3,5 -tri
methyl-1 H-
pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(2H)-one (30 mg. 0.055 mmol),
methyl 7-
bromo-5-methyl-1H-indole-2-carboxylate (30 mg, 0.112 mmol), Cul (5 mg, 0.026
mmol),
(trans)-1,2-NX-dimethylaminocyclohexane (8 mg, 0.056 mmol), K3PO4 (40 mg, 0.19
mmol).
Step B. Example 295
[00503] The title compound (9.3 mg, 21%, 2 steps) was prepared following the
same
procedure as described for Example 247 using methyl (R)-7-(7-chloro-10-(3-(4-
chloro-3,5-
dimethylphenoxy)propy1)-4-methy1-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
216
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
dihydropyrazino[1,2-allindol-2(1H)-y1)-5-methyl-1H-indole-2-carboxylate NaH (6
mg, 0.25
mmol) and 2-(Bromomethyl)pyridine HBr salt (30 mg, 0.119 mmol) followed by
saponification using General Procedure D. LCMS: RT = 2.096, 2.121 min, MS (ES)
802.9
(M+H).
Example 296
7-[(4R)-7-chloro-10- [3-(4-chloro-3,5-dimethyl-phenoxy)propy11-6-(4,6-
dimethylpyrimidin-5-yl)-4-methyl- 1-oxo-3,4-dihydropyrazino 11,2-al indo1-2-
yl] -5-
methyl-1-(2-pyridylmethyl)indole-2-carboxylic acid
Step A. Preparation of methyl (R)-7-
(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-6-(4,6-dimethylpyrimidin-5-y1)-4-methyl-1-oxo-3,4-
dihydropyrazino[1,2-a]indol-2(11/)-y1)-5-methyl-11-/-indole-2-carboxylate
[00504] The title compound was prepared following General Procedure C using
(R)-7-
chloro-10-(3-(4-chloro-3,5-dimethy 1phenoxy)propy1)-6-(4,6-dimethy 1py rimi
din-5 -y1)-4-
methy1-3,4-d ihy dropy razino [1,2-a] ind ol-1(2H)-one (30 mg. 0.056 mmol),
methyl 7-bro mo-5-
methy1-1H-indole-2-carboxy late (30 mg, 0.112 mmol), Cul (5 mg, 0.026 mmol),
(trans)-1,2-
N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), K3PO4. (40 mg, 0.19 mmol).
Step B. Example 296
[00505] The title compound (5.7 mg, 13%, 2 steps) was prepared following the
same
procedure as described for Example 247 using methyl methyl (R)-7-(7-chloro-10-
(3-(4-
chloro-3,5-dimethylphenoxy)propy1)-6-(4,6-dimethylpyrimidin-5-y1)-4-methyl-l-
oxo-3,4-
dihydropyrazino[1,2-a]indol-2(1H)-y1)-5-methyl-1H-indole-2-carboxylate, NaH (6
mg, 0.25
mmol) and 2-(Bromomethyl)pyridine HBr salt (30 mg, 0.119 mmol) followed by
saponification using General Procedure D. LCMS: RT = 1.956 min, MS (ES) 801.0
(M+H).
Example 297
(P, R)-6-(benzylo xy)-4-(7-chlo ro-10-(3-(4-c hloro-3,5-dimethylphenoxy)p ro
py l)-4-methy
1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-yl)-3,4-dihyd ropy razino11,2-al indo1-
2(1H)-yl)-1-
methyl-1H-indole-2-carboxylic acid
[00506] The title compound was prepared following General coupling procedure B
using
(P, R)-7-chl oro-10-(3-(4-chl oro-3,5 -di methylphenoxy)propy1)-4-methy1-6-
(1,3,5-tri methyl-
1H-py razol-4-y 0-3,4-dihy dropyrazino [1,2-a] in do1-1(210-on e, methyl
6-(b enzy loxy)-4-
bromo-1-methyl- 1H-ind ol e-2-carboxylate, CuI
(trans)-1,2-N,N'-
dimethylaminocyclohexane, and K2CO3 followed by saponification and
purification
following General Procedure D. LCMS: RT = 2.337 min, MS (ES) 818.2 (M+H).
Example 298
217
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
7- 1(4R)-7-chloro-10- 13-(4-chloro-3,5-dimethyl-phenoxy)p ropy1]-4-methyl-1-
oxo-6-(1,3,5-
trimethylpy razol-4-y1)-3,4-d ihyd ro py razino [1,2-a] ind ol-2-yl] -1-
(thiazol-2-
ylmethy 1)ind ole-2-carb oxyli c acid
[00507] To a solution of ethyl (R)-7-
(7-chloro-10-(3-(4-chloro-3,5-
di methylphenoxy )propy1)-4-methy1-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-
3,4-
dihydropyrazino[1,2-c]indol-2(1H)-y1)-1H-indole-2-carboxylate (20 mg, 0.028
mmol) in
DMF (2 mL) was added Cs2CO3 (40 mg, 0.123 mmol), KI (20 mg, 0.12 mmol), and 2-
(chloromethyl)thiazole (25 mg, 0.19 mmol), and the reaction was heated to 90
C for 4 h.
The reaction was then cooled to RT and diluted with DCM/H20 (20 mL, 1:1). The
organic
layer was separated, and the aqueous layer was extracted with DCM (2 x 5 mL).
The
combined organic extracts were dried and concentrated in vacuo. The crude
product was
saponified and purified following the procedure described in General Procedure
D to afford
the title compound (2.2 mg, 10%, 2 steps). LCMS: RT = 2.067 min, MS (ES) 795.0
(M+H).
Example 299
(P, R)-7-(benzyloxy)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy1-
1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] ind ol-
2(1H)-y1)-1-
methyl-1H-indole-2-carboxylic acid
[00508] The title compound was prepared following General coupling procedure B
using
(P, R)-7-chl oro-10-(3-(4-chl oro-3,5 -dimethylph en oxy)propy1)-4-methy1-6-
(1,3,5-trimethyl-
1H-py razol-4-y1)-3,4-dihy dropyrazino [1,2-a] in do1-1(210-on e, methyl 7-(b
enzy loxy )-4-
bromo-l-methy 1-1H-indol e-2-carboxylate, CuI , (trans)-1,2-N,N'-
dimethylaminocy clohexane, and K2CO3 followed by saponification and
purification
following General Procedure D. LCMS: RT = 2.329 min, MS (ES) 817.9 (M+H).
Example 300
7- [(4R)-7-chloro-10- 13-(4-chloro-3,5-dimethyl-phenoxy)p ropyl] -4-methyl-1-
oxo-6-(1,3,5-
trimethylpy razol-4-y1)-3,4-dihyd ro py razin o [1,2-a] ind ol-2-y11-1-methyl-
ind ole-2-
carbonitrile
[00509] The title compound (11.7 mg, 32%) was prepared following General
Procedure C
using (R)-7-
chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-6-(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(210-one (25 mg.
0,046 mmol),
7-bromo-1-methy1-1H-indole-2-carbonitrile (17 mg, 0.068 mmol), Cut (5 mg,
0.026 mmol),
(trans)-1, 2-N ,1µ1" -dimethylaminocyclohexane (8 mg, 0.056 mmol), K3PO4 (30
mg, 0.14
mmol). LCMS: RT = 2.221 min, MS (ES) 693.0 (M+H).
Example 301
218
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
4- 1(4R)-7-chloro-10- 13-(4-chloro-3,5-dimethyl-phenoxy)p ropy1]-4-methyl-1-
oxo-6-(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino [1,2-alindo1-2-y11-1-methyl-ind ole-
2-
carbonitrile
Step A. Preparation of 4-bromo-1-methyl-1H-indole-2-carboxamide
[00510] The title compound (47 mg, 47%) was prepared following General
Procedure E
using 4-bromo-1-methyl-1H-indole-2-carboxylic acid (100 mg, 0.39 mmol), HATU
(200 mg,
0.526 mmol), DIPEA (200 mg, 1.55 mmol), and ammonia in methanol (7.0 M, 0.25
mL, 1.75
mmol).
Step B. Preparation of 4-bromo-1-methy1-1H-indole-2-carbonitrile
[00511] A solution of 4-bromo-1-methy1-1H-indole-2-carboxamide (47 mg, 0.17
mmol) in
POC13 (2mL) was heated to 100 C for 2 h. The reaction was cooled to RT and
poured into
ice/aqueous NaHCO3. The aqueous layer was extracted with DCM (3 x 10 mL), the
combined organic reactions were dried over MgSO4, filtered, and concentrated
in vacuo. The
crude reaction mixture was purified by flash chromatography (Combi-flash Rf,
Hex/Et0Ac =
0-100%) to afford the title compound (32 mg, 73% yield).
Step C. Example 301
[00512] The title compound (25 mg, 65%) was prepared following General
Procedure C
using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-6-
(1,3,5-
trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indol-1(211)-one (30 mg.
0.056
mmol), 4-bromo-1-methy1-1H-indole-2-carbonitrile (26 mg, 0.11 mmol), CuI (5
mg, 0.026
mmol), (trans)-1,2-N,/V'-dimethylaminocyclohexane (8 mg, 0.056 mmol), K3PO4
(50 mg,
0.24 mmol). LCMS: RT = 2.276 min, MS (ES) 693.0 (M+H); 1H NMR (400 MHz,
Chloroform-d) 8 7.74 (dd, J= 8.6, 1.7 Hz, 1H), 7.53 - 7.43 (m, 1H), 7.40 -
7.27 (m, 2H),
7.13 - 7.02 (m, 2H), 6.65 - 6.60 (m, 2H), 4.48 (d, J= 11.6 Hz, 1H), 4.32-4.14
(m, 2H), 4.05
- 4.00 (m, 4H), 3.95 (s, 3H), 3.59 (d, J= 12.4 Hz, 1H), 3.46 (dt,J= 14.2, 7.5
Hz, 1H), 3.33
(dt, J= 14.0, 7.5 Hz, 1H), 2.33 (s, 5H), 2.29 (s, 1.5H), 2.26 (1.5H), 2.22-
2.17 (m, 3H), 2.15
(s, 1.5H), 2.10 (s, 1.5H), 1.24 (d, J= 4.8 Hz, 1.5H), 1.18 (d, J= 6.4 Hz,
1.5H).
Example 302
7-1(4R)-7-chloro-10-[3-(4-chloro-3,5-dimethyl-phenoxy)propy11-6-(4,6-
dimethylpyrimidin-5-y1)-4-methyl-1-oxo-3,4-dihydropyrazino11,2-alindo1-2-y1]-4-
methoxy-1-(2-pyridylmethyl)indole-2-carboxylic acid
Step A. Preparation of methyl (R)-7-
(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-6-(4,6-dimethylpyrimidin-5-y1)-4-methy1-1-oxo-3,4-
dihydropyrazino[1,2-a[indo1-2(1H)-y1)-4-methoxy-1H-indole-2-carboxylate
219
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00513] The title compound (15 mg, 36%) was prepared following General
Procedure C
using (R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(4,6-
dimethylpy rimi di n-
5-y1)-4-methy1-3 ,4-dihy dropy razino [1,2-a] indo1-1(2H)-one (30 mg. 0.056
mmol), methyl 7-
bromo-4-methoxy-1H-indole-2-carboxylate (30 mg, 0.11 mmol), CuI (5 mg, 0.026
mmol),
(trans)-1,2-N,N'-dimethylaminocyclohexane (8 mg, 0.056 mmol), K3PO4 (40 mg,
0.19
mmol).
Step B. Example 302
[00514] The title compound (7.2 mg, 43%) was prepared following the procedure
described
Example 247 using methyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-6-
(4,6-dimethylpy rimidin-5-y1)-4-methyl-l-oxo-3,4-dihy dropyrazino [1,2-a]
indo1-2(1H)-y1)-4-
methoxy-1H-indole-2-carboxylate, NaH (6 mg, 0.25 mmol) and 2-
(bromomethyl)pyridine
HBr salt (30 mg, 0.12 mmol). LCMS: RT = 2.061 min, MS (ES) 816.9 (M+H).
Example 303
(P, R)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-1-oxo-
6-
(1,3,5-trimethyl-1H-pyrazol-4-yl)-3,4-dihydropyrazino ind ol-
2(1H)-yl)-6-hyd roxy-
1-methyl-1H-indole-2-carboxylic acid
[00515] To a solution of (P, R)-6-(benzyloxy)-4-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy )propy1)-4-methy1-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-
3,4-
dihydropyrazino[1,2-a]indol-2(1H)-y1)-1-methy1-1H-indole-2-carboxylic acid (6
mg, 0.0072
mmol) in Me0H (3 mL) was added Pd/C (10% wt, 2 mg, 0.0018 mmol). The reaction
was
flushed with hydrogen then stired under H2 for 6 h at RT. The reaction was
filtered,
concentrated in vacuo, and purified by reverse phase HPLC (Phenomenex Gemini
C18,
H20/CH3CN gradient 35-95 MeCN 0.1% TFA) to afford the title compound (4 mg,
72%
yield). LCMS: RT = 2.007 min, MS (ES) 727.9 (M+H).
Example 304
7-1 (4R)-7-chloro-10- [3-(4-chloro-3,5-dimethyl-phenoxy)p ropyl1-4-methyl-1-
oxo-6-(1,3,5-
trimethylpyrazol-4-yl)-3,4-dihyd ro pyrazino 11,2-al indol-2-yl]-1-(thiazol-4-
ylmethyl)indole-2-carboxylic acid
[00516] The title compound (6.3 mg, 58%) was prepared following the procedure
described
Example 247 using ethyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-
rnethyl-l-oxo-6-(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]
indo1-2(1H)-
y1)-1H-indole-2-carboxylate (10 mg, 0.014 mmol), NaH (5 mg, 0.12 mmol) and 4-
(chloromethyl)thiazole (10 mg, 0.075 mmol) followed by saponification using
General
Procedure D. LCMS: RT = 2.099, 2.129 min, MS (ES) 794.9 (M+H).
220
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
Example 305
(M, R)-4-(7-chloro-10-(3-(4-chloro-3,5-d imethylphenoxy)p ropyl)-4-methyl-1-
oxo-6-
(1,3,5-trimethyl-1H-py razol-4-yl)-3,4-dihyd ropy razino [1,2-a] ind ol-2(1H)-
yl)-6-hyd roxy-
1-methyl-1H-ind ole-2-carboxylic acid (singlr atropisomer)
[00517] The title compound (3.3 mg, 59%) was prepared following the same
procedure as
Example 303 using (M, R)-6-
(benzyloxy)-4-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy )propy1)-4-methy1-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-
3,4-
dihydropyrazino[1,2-a]indo1-2(1H)-y1)-1-methyl-1H-indole-2-carboxylic acid (6
mg, 0.0072
mmol) and Pd/C (10% wt, 2 mg, 0.0019 mmol). LCMS: RT = 2.020 min, MS (ES)
727.9
(M+H).
Example 306
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-dimethyl-1-
(2-
(pyrro lid in-1-ypethyl)-11-1-pyrazol-4-y1)-4-methyl- 1-oxo-3,4-d ihyd rop
yrazino [ 1,2-
alindol-2(11/)-yl)-1-methyl-1H-indole-6-carboxylic acid
[00518] The title compound was prepared according to the procedure used in
Example 261
Step B by substituting 3-(bromomethyl)-1-
methy 1py rrol i dine with 1-(2-
bromoethyl)pyrrolidine. LCMS: RT = non polar method: 0.976 min, MS (ES) 795.3
(M+H).
Example 307
(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy1]-4-methyl-241-methyl-
2-
(2H-tetrazol-5-yl)indo1-4-yl]-6-(1,3,5-trimethylpyrazol-4-y1)-3,4-
dihydropyrazino [1,2-
a]indo1-1-one
[00519] The title compound (4 mg, 21%) was prepared according to the procedure
for
Example 235 using (R)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-
4-methyl-
1 -oxo-6-(1,3,5-trimethy1-1H-py razol-4-y1)-3,4-dihy dropy razino [1,2-a]
indo1-2(1H)-y1)-1 -
methy1-1H-indole-2-carbonitrile (18 mg, 0.026 mmol) and sodium azide (10 mg,
0.15 mmol).
LCMS: RT = 2.145, 2.172 min, MS (ES) 736.0 (M+H).
Example 308
(R)-3-(7-chlo ro-10-(3-(4-chloro-3,5-d imethyl phen oxy) p ro py1)-6-(3,5-d
imethyl-1-
((tetrahyd ro-2H-p yran-4-yl)methyl)- yrazol-4-y1)-4-methyl- 1 -oxo-3,4-
d ihyd ropy razino[1,2-al indo1-2(11/)-y1)-1-methyl-1H-indole-6-carboxylic
acid
[00520] The title compound was prepared according to the procedure used in
Example 261
Step B by substituting 3 -
(bromomethyl)-1-methy 1py rrolidine with 4-
(bromomethyl)tetrahydro-2H-pyran. MS (ES) 796.3 (M+H); 11-1-NMR (Me0H-d3) 5
8.19 (d,
1H, J = 8 Hz), 7.79-7.75 (m, 2H), 7.487.47 (m, 2H) 7.31 (d, 1H, J = 8 Hz),
6.62 (s, 2H),
221
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
4.50-4.47 (m, 1H), 4.30-4.28 (m, 1H) 4.06 (tr, 2H, J = 8 Hz), 3.99 (s, 3H),
3.92 (s, 3H), 3.36-
3.60 (m, 2H), 3.50-3.40 (m, 4H), 2.33 (s, 6H), 2.29- 2.16 ( multiples s (3H),
tr ( 2H), total
5H), 2.08 (s, 1.5H), 2.01 (s, 1.5H), 1.54-1.46 (m, 2H), 1.45 -1.40 (m, 2H),
1.31 (m, 1H),
1.23-1.19 (multiple d, 3H).
Example 309
(R)-3-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(1-(2-
(dimethylamino)ethyl)-3,5-dimethy1-1H-pyrazol-4-y1)-4-methyl-1-oxo-3,4-
dihydropyrazino [1,2-a] in d ol-2(1H)-y1)-1-methyl-1H-ind ole-6-c arb oxylic
acid
[00521] The title compound was prepared according to the procedure used in
Example 261
Step B by substituting 3-(bromomethyl)-1-methylpyrrolidine with 2-bromo-N,N-
dimethylethan-l-amine. MS (ES) 769.3 (M+H)
Example 310
(M, R)-6-(benzyloxy)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methyl-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropyrazino (1,2-al
ind ol-
2(1H)-y1)-1-methyl-1H-indole-2-carboxylic acid
[00522] The title compound (5.5 mg, 92%) was prepared following General
Procedure D
using methyl 04, R)-6-
benzyloxy -4- [7-chl oro-1043 -(4-chl o ro-3,5 -dimethyl-
phenoxy )propyl] -4-methyl- 1 -oxo-6-(1,3,5-trimethylpy razol-4-y1)-3,4-dihy
dropy razin o [1,2-
alindo1-2-y1]-1-methyl-indole-2-carboxylate (6 mg, 0.0072 mmol). LCMS: RT =
2.349 min,
MS (ES) 817.9 (M+H).
Example 311
7- 1(4R)-7-ch loro-10-13-(4-chloro-3,5-d imethyl-phenoxy)p ropyl] -4-methyl-1-
oxo-6-(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihyd ro py razino [ 1,2-a] ind ol-2-yl[ -1-(pyrimi
d in-5-
ylmethypind ole-2-carb oxyli c acid
[00523] The title compound (2 mg, 31%) was prepared according to the procedure
used in
Example 298 using ethyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-
methy 1-1 -oxo-6-(1,3,5-trimethy1-1H-py razol-4-y1)-3,4-dihydropy razino[1,2-
a] indo1-2(1H)-
y1)-1H-indole-2-carboxylate (6 mg, 0.0083 mmol), Cs2CO3 cesium carbonate (20
mg, 0.061
mmol), KI (10 mg, 0Ø060 mmol), and 5-(chloromethyl)pyrimidine (20 mg, 0.15
mmol)
followed by saponification using General Procedure D. LCMS: RT = 2.006 min, MS
(ES)
790.0 (M+H).
Example 312
222
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(M, R)-7-(benzyloxy)-4-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propyl)-4-
methyl-1-oxo-6-(1,3,5-trimethyl-1H-py razol-4-yl)-3,4-d ihyd ropy razino[1,2-
al ind ol-
2(1H)-yl)-1-methyl-1H-ind ole-2-carboxylic acid
[00524] The title compound (4 mg, 81%) was prepared following General
Procedure D
using methyl (M, R)-7-
benzyloxy -4- [7-chlo ro-1043 -(4-chlo ro-3,5 -dimethyl-
phenoxy )propyl] -4-methyl- 1 -oxo-6-(1,3,5-trimethylpyrazol-4-y1)-3,4-
dihydropyrazino[1,2-
alindo1-2-y1]-1-methyl-indole-2-carboxylate (5 mg, 0.0060 mmol). LCMS: RT =
2.192 min,
MS (ES) 817.9 (M+H).
Example 313
7-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy1]-4-methyl-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino11,2-a] ind ol-2-yl] -4-meth oxy-1-
(2-
py ri dylmethyBind ole-2-ca rb oxylic acid
Step A. Preparation of
methyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-
d imethylp hen oxy)propyl)-4-methyl-1-oxo-6-(1,3,5-trimethyl-11-/-p yrazol-4-
yI)-3,4-
dihyd ropyrazino[1,2-a]indol-2(1H)-yl)-4-methoxy-1H-indole-2-carboxylate
[00525] The title compound was prepared following General Procedure C using
(R)-7-
chloro-10-(3-(4-chl oro-3,5-dimethylphenoxy )propy1)-4-methy1-6-(1,3,5 -tri
methyl-1H-
pyrazol-4-y1)-3,4-dihydropyrazino[1,2-c]indol-1(2H)-one (30 mg. 0.056 mmol),
methyl 7-
bromo-4-methoxy-1H-indole-2-carboxylate (30 mg, 0.11 mmol), CuI (5 mg, 0.026
mmol),
(trans)-1,2-NX-dimethylaminocyclohexane (8 mg, 0.056 mmol), K3PO4 (40 mg, 0.19
mmol).
Step B. Example 313
[00526] The title compound (12.8 mg, 28% 3 steps) was prepared following the
procedure
described Example 241 using methyl
(R)-7-(7-chloro-10-(3-(4-chloro-3,5-
di methy 1phenoxy )propy1)-4-methy1-1-oxo-6-(1,3,5 -trimethy1-1H-py razol-4-
y1)-3,4-
dihy dropyrazino[1,2-alindo1-2(1H)-y1)-4-methoxy-1H-indole-2-carboxy late
(from Step A),
NaH (6 mg, 0.25 mmol) and 2-(bromomethyl)pyridine HBr salt (30 mg, 0.12 mmol)
followed
by saponification using General Procedure D. LCMS: RT = 1.941, 1.965 min, MS
(ES)
819.0 (M+H).
Example 314
7- [(4R)-7-chloro-10-13-(4-ch loro-3,5-d imethyl-phenoxy)p ropyl] -4-methyl-1-
ox 0-641,3,5-
trimethylpyrazol-4-y1)-3,4-dihyd ropyrazino11,2-al indo1-2-y1]-1- [(5-methyl-
1,2,4-
oxadiazol-3-yl)methyl]indole-2-carboxylic acid
223
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
[00527] The title compound (4.2 mg, 19%) was prepared following the procedure
described
Example 298 using ethyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-4-
methyl-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-dihydropy razino[1,2-
cdindo1-2(1H)-
y1)-1H-indole-2-carboxylate (20 mg, 0.028 mmol), Cs2CO3 (40 mg, 0.123 mmol),
KI (20 mg,
0.12 mmol), and 3-(chloromethyl)-5-methyl-1,2,4-oxadiazole (25 mg, 0.186 mmol)
followed
by saponification using General Procedure D to afford the title compound.
LCMS: RT =
2.063 min, MS (ES) 793.9 (M+H).
Example 315
7-1(4R)-7-chloro-10-13-(4-chloro-3,5-dimethyl-phenoxy)propy11-4-methy1-1-oxo-6-
(1,3,5-
trimethylpyrazol-4-y1)-3,4-dihydropyrazino 11,2-al indo1-2-y1]-1-1(5-methy1-
1,3,4-
oxadiazol-2-y1)methyl]ind ole-2-carboxylic acid
[00528] The title compound (4.9 mg, 45%) was prepared following the procedure
described
Example 241 using ethyl (R)-7-(7-chloro-10-(3-(4-chloro-3 ,5 -dimethy
1phenoxy)propy1)-4-
methyl-1 -oxo-6-(1,3,5-trimethy1-1H-py razol-4-y1)-3,4-dihydropy razino [1,2-
a] indo1-2(1H)-
y1)-1H-indole-2-carboxylate (10 mg, 0.014 mmol), NaH (3 mg, 0.12 mmol), and 2-
(chloromethy1)-5-methy1-1,3,4-oxadiazole (10 mg, 0.076 mmol) followed by
saponification
using General Procedure D. LCMS: RT = 2.072 min, MS (ES) 793.9 (M+H).
Example 316
(R)-7-(7-Chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methy1-1-oxo-6-
(1,3,5-
trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino [1,2-a] in d ol-2 (1H)-y1)-1,3-
d imethyl-1H-
indole-2-carboxylic acid
Step A. Preparation of methyl 7-bromo-3-methyl-1H-indole-2-carboxylate
[00529] To a solution of (2-bromophenyl)hydrazine hydrochloride (223 mg, 1.0
mmol) in
Me0H (5 mL) was added ethyl pyruvate (0.1 mL, 116 mg 1.0 mmol) followed by
conc. HC1
(5 mL). The reaction mixture was stirred at 70 C for 4 h then cooled to RT,
quenched with
sat. NaHCO3 aq. solution and extracted with DCM (3 x 5 mL). The combined
organic layer
was washed with brine, dried over MgSO4, filtered, and concentrated in vacuo.
The residue
was purified by flash chromatography (Combi-flash Rf, Hex/Et0Ac = 0-100%
gradient) to
afford the title compound (227 mg, 85% yield). LCMS: RT = 1.618 min, MS (ES)
268.0
(M+H).
Step B. Preparation of methyl 7-b romo- 1,3-d imethy1-1H-ind ole-2-carb
oxylate
[00530] The title compound (38 mg, 37% yield) was prepared following General
Procedure
G using methyl 7-bromo-3-methyl-1H-indole-2-carboxylate (100 mg, 0.37 mmol).
LCMS:
RT = 1.828 mm, MS (ES) 282.0 (Md-H); 11-1 NMR (400 MHz, CDC13) 8 7.50 (d, J =
7.9 Hz,
224
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
1H), 7.42 (d, J= 7.5 Hz, 1H), 6.87 (t, J= 7.8 Hz, 1H), 4.25 (s, 3H), 3.87 (s,
3H), 2.44 (s, 3H).
Step C. Preparation of methyl (R)-7-
(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propyl)-4-methyl-1-oxo-6-(1,3,5-trimethyl-1H-pyrazol-4-y1)-3,4-
dihyd ropyrazino [1,2-a] ind ol-2(1H)-yl)-1,3-d imethyl-1H-ind ole-2-carb
oxylate
[00531] The title compound (20 mg, 33 %) was prepared following General
Procedure B,
using methyl 7-bromo-1,3-dimethy1-1H-indole-2-carboxylate (35 mg, 0.124 mmol)
and (R)-
7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-methyl-6-(1,3,5-
trimethyl-1H-
pyrazol-4-y1)-3,4-dihydropyrazino[1,2-c]indol-1(2H)-one (44.6 mg, 0.083 mmol).
LCMS:
RT = 2.244, 2.267 min, MS (ES) 739.9 (M+H).
Step D. Example 316
[00532] The title compound (3.1 mg, 15%) was prepared following General
procedure D
using methyl (R)-7-(7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-4-
methy 1-1-oxo-
6-(1,3,5-trimethy1-1H-pyrazol-4-y1)-3,4-dihydropyrazino[1,2-alindol-2(1H)-y1)-
1,3-
dimethyl-1H-indole-2-carboxylate (20 mg, 0.027 mmol). LCMS: RT = 2.096 min, MS
(ES)
726.0 (M+H); 11-1 NMR (400 MHz, CDC13) 5 7.78 (d, J = 8.0 Hz, 1H), 7.74-7.68
(m, 1H),
7.36-7.33 (m, 1H), 7.23-7.10 (m, 2H), 6,64-6.59 (m, 2H), 4.52-4.46 (m, 1H),
4.30-4.25 (m,
2H), 4.13-4.11 (m, 4H), 4.01-3.97 (m, 4H), 3.42-3.36 (m, 2H), 2.63-2.61 (m,
3H), 2.39-2.32
(m, 9H), 2.24-2.15 (m, 5H), 1.31-1.28 (m, 3H).
Example 317
(R)-4-(7-Chloro-10-(3-(4-chloro-3,5-d imethylp henoxy)p ropyl)-4-methyl-1-oxo-
6-(1,3,5-
trimethyl-1H-py razol-4-yl)-3,4-d ihyd ropy razino [1,2-a] indol-2(1H)-yl)-1,6-
dimethyl-1H-
indole-2-carboxylic acid
[00533] The title compound was prepared according to General Procedure A using
(R)-7-
chloro-10-(3-(4-chl oro-3,5-dimethylphenoxy)propy1)-4-methy1-6-(1,3,5 -tri
methyl-1H-
pyrazol-4-y1)-3,4-dihydropyrazino[1,2-a]indo1-1(2H)-one and methyl 4-bromo-1,6-
dimethyl-
1H-indole-2-carboxylate followed by saponification using General Procedure D.
LCMS: RT
= 2.71 min, MS (ES) 726.2 (M+H) (LC method III). 11-1 NMR (400 MHz, Chloroform-
d)
7.68 (d, J = 8.8 Hz, 1H), 7.28 (d, J = 8.8 Hz, 1H), 7.26 (s, 1H), 7.22 (s,
1H), 6.92 (s, 1H),
6.60 (s, 2H), 4.40 (dd, J= 12.4, 3.6 Hz, 1H), 4.19 (d, J= 4.8 Hz, 1H), 4.03
¨3.96 (m, 5H),
3.90 (s, 3H), 3.58 (d, J = 12.4 Hz, 1H), 3.49 ¨ 3.31 (m, 2H), 2.51 (s, 3H),
2.30 (s, 6H), 2.21 ¨
2.17 (m, 5H), 2.04 (s, 3H), 1.24 (d, J= 6.4 Hz, 3H).
Example 318
225
CA 03016182 2018-08-29
WO 2017/152076
PCT/US2017/020699
(R)-4-(7-C hlo ro-10-(3-(4-chloro-3,5-d imethylp henoxy)propy1)-6-(3,5-
dimethyl-1-(2-
(pyrro lid in-1-ypethyl)-111-pyrazol-4-y1)-4-methyl-1-oxo-3,4-d ihy d
ropyrazino [1,2-
alindo1-2(1H)-y1)-1-methyl-1H-indole-2-carboxylic acid.
Step A. Preparation of methyl (R)-4-
(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-6-(3,5-dimethyl-1-02-(trimethylsily1)ethoxy)methyl)-1H-
pyrazol-4-y1)-4-methyl-1-oxo-3,4-dihydropyrazino[1,2-alindol-2(1H)-y1)-1-
methyl-11/-
indole-2-carboxylate
[00534] The title compound was prepared following General coupling procedure B
using
(R)-7-chloro-10-(3-(4-chloro-3,5-dimethylphenoxy)propy1)-6-(3,5-dimethy1-1-42-
(trimethylsilypethoxy)methyl)-1H-pyrazol-4-y1)-4-methyl-3,4-
dihydropyrazino[1,2-a]indol-
1(2H)-one and methyl 4-bromo-1-methy1-1H-indole-2-carboxylate. MS (ES) 842.3
(M+H).
Step B. Preparation of methyl (R)-4-
(7-chloro-10-(3-(4-chloro-3,5-
d imethy 1p hen oxy)propy1)-6-(3,5-dimethy1-1H-py razol-4-y1)-4-methy l-1-oxo-
3,4-
dihyd ropy razino [1,2-a] indo1-2(1H)-y1)-1-methyl-1H-indole-2-carboxylate
[00535] The title compound (77 mg, 90 %) was prepared following the procedure
described
Example 261 Step A using methyl (R)-4-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy )propy1)-6-(3,5-dimethy1-142-(trimethylsilypethoxy)methyl)-1H-
pyrazol-
4-y1)-4-methyl-l-oxo-3,4-dihy dropy razino[1,2-a] indo1-2(1H)-y1)-1-methy1-1H-
indole-2-
carboxylate (100mg, 0.12 mmol) and l'BAF (0.59 mL, 0.59 mmol). MS (ES) 712.2
(M+H).
Step C. Example 318
[00536] The title compound (17 mg, 38% 2 steps) was prepared following the
procedure
described Example 261 Step B using methyl (R)-4-(7-chloro-10-(3-(4-chloro-3,5-
dimethylphenoxy)propy1)-6-(3,5-dimethyl-1H-pyrazol-4-y1)-4-methyl-l-oxo-3,4-
dihydropyrazino[1,2-c]indol-2(1H)-y1)-1-methyl-1H-indole-2-carboxylate (41 mg,
0.057
mmol), NaH (5 mg, 0.113 mmol) and 1-(2-bromoethyl)pyrrolidine (20 mg, 0.113
mmol)
followed by saponification using General Procedure D. LCMS: RT = 0.863 min (LC
method
IV), MS (ES) 795.8 (M+H); NMR
(DMSO, 400 MHz) 15 (ppm) 7.79 (d, J= 8.4 Hz, 1H),
7.55 (d, J= 8.4 Hz, 1H), 7.38 (t, J= 8.4 Hz, 1H), 7.30 (d, J= 8.4 Hz, 1H),
7.06 (s, 2H), 6.70
(s, 2H), 4.59-4.40 (m, 2H), 4.20-4.11 (m, 1H), 4.04 (s, 3H), 3.97-3.93 (m,
2H), 3.65-3.60 (m,
2H), 3.55-3.50 (m, 2H), 3.12-2.90 (m, 2H), 2.53 (s, 3H), 2.22 (s, 6H), 2.18
(s, 2H), 2.05 (d, J
= 4.0 Hz, 3H), 103-1.90 (m, 2H), 2.05 (s, 3H), 1.85-1.82(m, 2H), 1.08-1.05 (m,
4H).
Example 319
226
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 3
CONTENANT LES PAGES 1 A 244
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 3
CONTAINING PAGES 1 TO 244
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: