Note: Descriptions are shown in the official language in which they were submitted.
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
CO-FORMULATION COMPRISING A PLANT GROWTH REGULATOR AND AN OIL,
AND METHODS OF PREPARING AND USING SAID CO-FORMULATION
FIELD OF THE INVENTION
[0001] The invention generally relates to the co-formulation of at least one
plant growth
regulator, or at least one plant growth regulator and an additional
biologically active
ingredient, or mixtures thereof, with various oils to form agricultural oil-
based dispersions
or oil dispersions. More particularly, the present invention relates to an oil-
dispersion
containing a plant growth regulator, or a mixture of a plant growth regulator
and an
herbicide, which may subsequently be used as a defoliant. It will be
convenient to hereinafter
describe the invention in relation to the use of the oil dispersion as a
defoliant, particularly
upon cotton crops. It should be appreciated, however, that the present
invention is not limited
to that application only.
BACKGROUND TO THE INVENTION
100021 The defoliation of cotton crops is a well-established practice that has
been undertaken
commercially for many years. Once defoliation has been induced, the harvesting
of the
cotton crop by mechanical means is greatly facilitated. In the past, most of
the chemicals
used in this practice achieved defoliation by subjecting the plant to
"biological shock", which
resulted in chlorosis and freezing of the leaves and resultant burning of the
plant. However,
this treatment adversely affected both the yield and quality of the cotton
crop.
[0003] It is a far more preferable practice for pure defoliation to occur,
where only the leaves
of the plant are affected and the remainder of the plant remains unharmed. One
example of
where this was first outlined was in 1967 in United States Patent No.
3,321,293, which
described the use of purine derivatives of the kinetin type, the most
preferred being 6-
anilinopurine. This compound was shown to be particularly efficacious in small
quantities
without exhibiting any herbicidal side-effects.
[0004] The utility of plant growth regulators, specifically those which
retarded specific
vegetative growth characteristics, was later discovered as being useful in the
field of
defoliation. For example, the application of thidiazole-urea derivatives for
use as a plant
growth regulator, or as a defoliant used in combination with one other plant
growth regulator,
was first described in 1981 by Schering AG in United States Patent No.
4,261,726. Building
1
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
upon this, in 1986, Schering AG described the use of thidiazuron in
combination with diuron
as further improved defoliant compositions in United States Patent No.
4,613,354.
100051 Additional improvements in the efficiency of defoliation of the base
mixtures of
thidiazuron, or thidiazuron in combination with diuron, where the improved
performance is
only achieved when these active ingredients are combined with additional
pesticidal
components, was described in, for example, United States Patent No. 6,274,535.
100061 Not surprisingly, even further efficiencies or improvements are
continually sought
so that modern-day agrochemicals meet the demand for improved ecological
profiles,
biological efficacy, and/or economics. As such, there is a corresponding need
to develop
formulations and/or delivery systems that better satisfy at least one of these
requirements
and continue to provide benefits or advantages over existing technology.
100071 One common formulation design strategy for achieving such requirements
is the
combination or co-formulation of agrochemicals and/or specific formulation
auxiliaries,
which might otherwise be applied individually. The primary logic is that one
of the more
effective methods for fulfilment of the aforementioned requirements, for
example, the co-
formulation of agrochemicals and/or specific formulation auxiliaries, which
previously
might have been applied as separate, individual delivery systems, could
potentially be
applied as one formulation. This would result in reduced waste, reduced labour
inputs,
reduced application complexity, as well as possible synergistic benefits with
respect to
efficacy.
100081 One particular formulation type that is well suited to this delivery
strategy is the
dispersion of an agriculturally active solid in oil, forming either an oil
dispersion (hereinafter
"OD") concentrate, or an oil-miscible flowable (hereinafter "OF") concentrate,
as defined
by CropLife International, an international trade association of agribusiness
companies that
was founded in 2001. The key advantage in this instance is that an oil or
solvent, which may
typically be applied in a customary fashion as an individual or stand-alone
spraying oil as an
adjuvant, is included in the composition itself.
100091 This type of formulation is particularly relevant to the field of
defoliation. For
example, in United States Patent No. 4,613,354 the possibility of formulating
thidiazuron,
or mixtures of thidiazuron and diuron is discussed, but this document also
discloses that the
nature and rate of action of a given composition may be increased through the
addition of
additives including organic solvents, wetting agents or oils. In many
countries, this stated
effect is already being exploited, not through modification of the specific
defoliating
composition via addition of these components, but through application of these
components
2
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
to the pre-application pre-broadcast mixture in the form of commercially
available spray
oils, for example. This is known to enhance the efficiency of defoliation.
100101 While it would therefore seem to be reasonably intuitive to attempt co-
formulation
of an oil and defoliating agent to form an oil-based dispersion or OD, it is
however known
to those skilled in the art that non-aqueous dispersions of agrochemically
active ingredients
are notoriously difficult to stabilise. Given the major difficulty associated
with the
development of robust OD and OF formulations of systems with commercially
feasible
stability horizons, these often require complex preparative methodologies and
specialty
functional additives. It is therefore not completely obvious, and nor is it an
intuitive process,
to develop such a formulation, which contrasts with the relative ease of
successfully
formulating the most common delivery systems that are typically chosen for the
application
of existing defoliants. Such common delivery systems may include, but are not
limited to,
wettable powders, soluble powders, water dispersible granules, water soluble
granules,
dusts, suspension concentrates, emulsifiable concentrates, and emulsions.
100111 The complexity attributed to the development of any OD or OF
formulation versus
traditional delivery methods will no doubt extend to commercial
considerations, which will
generally lead to strong assertions of economic impracticality. For example,
commercial
manufacture of an OD formulation might be difficult and expensive, while the
cost of the
specialty formulation components might also be comparatively high versus those
used in
aqueous delivery systems. Therefore, when weighing up the foreseeable benefit
for the use
of an OD or an OF formulation versus the use of existing aqueous formulations
used in
conjunction with oil-based additives, one would likely only foresee a small
labour benefit
i.e., the need to handle one product rather than two. This leads to a sense of
impracticality
and even disadvantage.
100121 Additionally, for an OD or an OF formulation to be optimally
efficacious, it will also
preferably contain a significant concentration of non-aqueous media,
preferably an oil,
where the concentration of the oil relative to the remaining components that
facilitate
effective delivery of the formulation, such as one or more emulsifying agent
co-solvent, is
significantly higher. However, high concentrations of oil, particularly
mineral or base oils,
are generally detrimental to formulation stability, owing to their low-
polarity and the
resultant impact upon the performance of functional formulation auxiliaries,
such as
dispersants and rheology modifiers, for example. In addition, one of the key
dilemmas faced
when developing OD formulations is that surfactants, particularly surfactant
emulsifying
agents, which allow for suitable stable dilution in water prior to
application, are often
3
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
antagonistic to stability. This leads to additional impracticality where
development of
sufficiently stable OD formulations, that promise effective efficacy traits
through
incorporation of oil at, or close to, the allowable limit, can be extremely
difficult.
100131 Indeed, the present inventor has encountered exceptional difficulty in
stabilising
thidiazole-urea plant growth regulators, such as thidiazuron, when suspended
in the desired
oil-rich carrier, where an array of destabilisation phenomena tends to result
in compositions,
which are not fit for consideration. The present inventor therefore sought to
prepare useful
compositions comprising a plant growth regulator. Additionally, in the past,
thidiazuron, or
mixtures of thidiazuron with diuron, appear to have been inefficiently applied
and more than
was actually required for optimal efficacy has been used, resulting in
wastage. This has had
disadvantageous consequences from an environmental and an economical
perspective.
100141 Surprisingly, the present inventor has now discovered a method to
afford
substantially stable and homogeneous co-formulations, which have allowed for a
significant
reduction in total agrochemical input, as well as a reduction in the amount of
active
compound required for efficacy, particularly when used in defoliation
applications. It had
been assumed that there would be little difference in defoliation rate and
efficiency
regardless of whether an oil was applied as part of a co-formulation diluted
into a spray
liquor, or as an individual spray oil composition jointly applied into a spray
liquor with
another composition containing a defoliation agent. Such an improved
synergistic effect was
not foreseeable from the above-described prior art. This reduction provides
advantages
relating to a reduced agrochemical footprint.
100151 The present invention seeks to overcome, or at least substantially
ameliorate, some
of the disadvantages and shortcomings of the prior art.
SUMMARY OF THE INVENTION
100161 According to the present invention, there is provided an agricultural
co-formulation
comprising:
i) an effective amount of at least one plant growth regulator, or at least
one plant
growth regulator and an additional biologically active ingredient, in a
comminuted form having an average particle size in the range of from 1 to 12
microns;
ii) at least one oil;
iii) at least one oil-soluble surfactant dispersing agent; and
4
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
iv) at least one emulsifying agent,
wherein the final concentration of the at least one plant growth regulator, or
the at least
one plant growth regulator and the additional biologically active ingredient,
is optionally
adjusted by adding additional oil and one or more theology modifiers and/or
activation
agents as required to substantially stabilise the co-formulation; and wherein
the efficacy
of the plant growth regulator is substantially maintained or improved.
100171 The form of the co-formulation of the present invention may be selected
from an oil
dispersion ("OD") concentrate, an oil-miscible flowable ("OF") concentrate, an
oil-based
suspension concentrate ("SC"), or an oil-based suspoemulsion ("SE"). When used
for
defoliation purposes, the co-formulation is an oil-based dispersion
concentrate, i.e. an OD
formulation.
[0018] The term "effective amount" means an amount of a component of the co-
formulation
according to the invention that is sufficient for enhancing the plant growth,
yield and/or
vigour and that does not entail any appreciable symptom of phytotoxicity for
the plant or
crop. Such an amount can vary within a wide range depending on the type of
plant or crop,
the climatic conditions and the components included in the co-formulation
according to the
invention. This amount can be determined by systematic field trials that are
within the
capabilities of a person skilled in the art.
100191 As used herein, the term "plant growth regulator" includes compounds
eliciting a
response in terms of plant organ number modulation in a dose-dependent manner.
Plant
organ number modulation refers to the enhancement or inhibition of plant organ
growth or
development. Inhibition may be complete blockage or partial blockage. For
instance, plant
organ number modulation can relate to inhibition of shoot branching or
enhancement of root
formation. Shoot branching means the process of outgrowth of axillary or
adventitious buds,
resulting in the formation of vegetative shoots, flowers or inflorescences.
Inhibition means
to permanently or temporarily suppress the growth of buds or inhibit the
formation of roots.
The inhibition can be complete, by affecting all axillary/adventitious buds,
or partial,
affecting only a subset of axillary/adventitious buds.
[0020] Examples of plant growth regulators, which may be used in accordance
with the
invention include, but are not limited to, antiauxins, such as clofibric acid
or 2,3,5-tri-
iodobenzoic acid; auxins, such as 4-CPA, 2,4-D, 2,4-DB, 2,4-DEP, dichlorprop,
fenoprop,
IAA, I BA, naphthaleneacetamide, a-naphthaleneacetic acid, 1 -naphthol,
naphthoxyacetic
acid, potassium naphthenate, sodium naphthenate, 2,4,5-T, cytokinins, such as
2iP,
benzyladeine kinetin, zeatin; defoliants, such as calcium cyanamide,
dimethipin, endothal,
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
ethephon, merphos, metoxuron, pentachlorophenol, thidiazuron, tribufos;
ethylene
inhibitors, such as aviglycine and 1-methylcyclopropene; ethylene releasers,
such as ACC,
etacelasil, ethephon and glyoxime; growth inhibitors, such as abscisic acid,
ancymidol,
butralin, carbaryl, chlorphonium, chlorpropham, dikegulac, flumetralin,
fluoridamid,
fosamine, glyphosine, isopyrimol, jasmonic acid, maleic hydrazide, mepiquat,
piproctanyl,
prohydrojasmon, propham, 2,3,5- tri-iodobenzoic acid; morphactins, such as
chlorfluren,
chlorflurenol, dichlorflurenol, flurenol; growth retardants/modifiers, such as
chlormequat,
daminozide, flurprimidol, mefluidide, paclobutrazol, cyproconazole,
tetcyclacis,
uniconazole, ancymidol, trinexapac-ethyl, and progexadione-CA; growth
stimulators, such
as brassinolide, forchlorfenuron, hymexazol, 2-amino-6-oxypurine derivatives,
indolinone
derivatives, 3,4-disubstituted maleimide derivatives and fused azepinone
derivatives. The
term also includes other active ingredients such as benzofluor, buminafos,
carvone,
ciobutide, clofencet, cloxyfonac, cyclanilide, cycloheximide, epocholeone,
ethychlozate,
ethylene, fenridazon, heptopargil, holosulf, inabenfide, karetazan, lead
arsenate,
methasulfocarb, prohexadione, pydanon, sintofen, triapenthenol, and
trinexapac. Plant
growth regulators, such as indolinone derivative plant stimulators, described
in WO
2005/107466; 3,4-disubstituted maleimide derivatives described in WO
2005/107465; fused
azepinone derivatives described in WO 2005/107471; and 2-amino-6-oxypurine
derivatives
described in WO 2005/1 07472 are also included in the term.
[0021] The term "plant" or "crop" as referred to herein may be any plant or
crop in plantation
or in culture, especially agricultural crops, horticultural crops or
silvicultural crops, and more
preferably, cotton plants, including transgenic cotton plants.
[0022] According to one preferred aspect of this invention, the plant growth
regulator is
thidiazuron. In a particularly preferred embodiment of the co-formulation,
thidiazuron is
present in a concentration range of from 1 to 500g/L, or more preferably, from
1 to 250g/L,
or more preferably, from 1 to 200g/L, or more preferably, from 1 to 150g/L, or
more
preferably, from 1 to 120g/L, or more preferably, from 1 to 100g/L, or more
preferably, from
1 to 80g/L, or more preferably, from 1 to 50g/L, or more preferably, from 1 to
30g/L, or
more preferably, from 1 to 20g/L, or more preferably, from 50 to 100g/L, or
more preferably,
from 50 to 150g/L, or more preferably, from 100 to 150g/L, or more preferably,
from 100 to
200g/L, or more preferably, from 150 to 250g/L. In a most preferred form,
thidiazuron is
present in a concentration range of from 60 to 120g/L and most preferably, in
a concentration
of 100g/L. Where the plant growth regulator is other than thidiazuron, the
plant growth
regulator is present in a concentration range of from 1 to 500g/L, or more
preferably, from
6
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
1 to 200g/L, or more preferably, from 1 to 120g/L, or further more preferably,
from 1 to
100g/L.
100231 In another preferred form of the invention, the co-formulation further
comprises at
least one additional biologically active ingredient selected from a fungicide;
an insecticide;
an herbicide; a miticide; a nematocide; a molluscicide; an algicide; or a
pesticide; or any
mixture thereof. While any co-formulation comprising a combination of
thidiazuron and any
one or more of the above-listed additional biologically active ingredient/s is
included within
the scope of the present invention, in one preferred form of the invention,
the co-formulation
comprises thidiazuron in combination with a fungicide. In another preferred
form, the co-
formulation comprises thidiazuron in combination with an insecticide. In yet
another
preferred form, the co-formulation comprises thidiazuron in combination with a
miticide. In
yet another preferred form, the co-formulation comprises thidiazuron in
combination with a
nematocide. In yet another preferred form, the co-formulation comprises
thidiazuron in
combination with a molluscicide. In a most preferred form of the invention,
the co-
formulation comprises thidiazuron in combination with an herbicide.
100241 In a particularly preferred embodiment of the co-formulation, the
additional
biologically active ingredient is present in a concentration range of from 1
to 250g/L, or
more preferably, from 1 to 100g/L, or more preferably, from 1 to 80g/L, or
more preferably,
from 1 to 60g/L, or more preferably, from 1 to 50g/L, or more preferably, from
1 to 30g/L,
or more preferably, from 1 to 20g/L, or more preferably, from 80 to 100g/L, or
more
preferably, from 50 to 100g/L, or more preferably, from 50 to 80g/L, or more
preferably,
from 30 to 50g/L, or more preferably, from 20 to 30g/L. The concentration of
the additional
biologically active ingredient is most preferably selected from 48, 30 or
15g/L, respectively.
In the most preferred form, the additional biologically active ingredient is
present in a
concentration of 30g/L.
100251 Where the additional biologically active ingredient is preferably at
least one
herbicide, it may be selected from, but is not to be taken as being limited
to: a dinitroaniline
herbicide; a diphenylether herbicide; a phenoxypropionate herbicide; and
including atrazine,
nicosulfuron, carfentrazone, naptalam, 2,4-D, quizalofop, benefin, bentazon,
prometryn,
mesotrione, flumioxazin, clomazone, ethalfluralin, napropamide, diquat, s-
metolachlor,
ametryn, dimethenamid, fluazifop, oxyfluorfen, paraquat, topramezone, diuron,
pronamide,
alachlor, tembotrione, linuron, rimsulfuron, sethoxydim, bensulide,
pendimethalin, pyrazon,
cycloate, glyphosate, maleic hydrazide, halosulfuron, pelargonic acid,
clethodim,
metribuzin, rimsulfuron, terbacil, ethalfluralin, phenmedipham, clopyralid, a
combination of
7
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
clomazone and ethalfluralin, MCPB, pebulate, trifluralin, or any mixtures
thereof. While any
co-formulation comprising a combination of thidiazuron and any of the above-
listed
herbicides is included within the scope of the present invention, in a most
preferred form,
the herbicide is diuron.
100261 When the co-formulation comprises a combination of thidiazuron and
diuron,
thidiazuron is preferably present in a concentration range of from 1 to
250g/L, or more
preferably, from Ito 200g/L, or more preferably, from 1 or 100g/L, or more
preferably, from
1 to 80g/L, or more preferably, from 1 to 50g/L, or more preferably, from 1 to
30g/L, or
more preferably, from 1 to 20g/L and most preferably, in a concentration of 60
g/L; and
diuron is preferably present in a concentration range of from 1 to 250 g/L, or
more preferably,
from 1 to 100g/L, or more preferably, from 1 to 80 g/L, or more preferably,
from 1 to 60g/L,
or more preferably, from 1 to 50g/L, or more preferably, from 1 to 30g/L, or
more preferably,
from 1 to 20g/L, or more preferably, from 50 to 80g/L, or more preferably,
from 30 to 50g/L,
or more preferably, from 20 to 30g/L. The concentration of diuron is most
preferably selected
from 48, 30 or 15g/L, respectively. In the most preferred form, diuron is
present in a
concentration of 30g/L.
[0027] Where thidiazuron is present in the co-formulation of the present
invention on its
own, or when combined with diuron as the biologically active ingredient, the
co-formulation
has been found to display surprisingly synergistic defoliation efficacy on
crops, when
compared to existing defoliants used in accordance with existing defoliation
practices.
[0028] It is essential that the plant growth regulator and/or the active
ingredient be finely
divided via, for example, comminution or other means for the purposes of
ensuring that the
dispersion concentrate is maintained in a substantially stable form. For
comminution of even
finer particle size ranges, machines like the ball mill, vertical roller mill,
hammer mill, roller
press or high compression roller mill, vibration mill, jet mill and the like
can be used. For
yet finer particle sizes, which are sometimes referred to as "ultrafine
grinding", specialist
mills can be used. The comminution of the at least one plant growth regulator,
or at least one
plant growth regulator and an additional biologically active ingredient, of
the present
invention results in an average particle size of the components in the range
of from 1 to 12
microns, or more preferably, of from 1 to 8 microns, or even more preferably,
of from 1 to
6 microns. In a most preferred embodiment of the present invention,
comminution may be
carried out using wet grinding via a horizontal mill, for example, one
supplied by Engineered
Mills, Inc. of Grayslake, Illinois, to produce a co-formulation with an
average (d0.5) particle
8
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
size of less than 5 microns, with the particle size analysis being determined
by microscopic
approximation, or by the use of an acceptable Grind Gauge or Hegman Gauge.
100291 The at least one oil, which forms the continuous phase of the co-
formulation, is
preferably selected from a paraffin oil, such as a kerosene, for example, one
of the
EXXSOLR D range available from ExxonMobil Chemical Company of Spring, Texas;
YUBASE 3' available from SK Corporation of Seoul, Republic of Korea; PROPAR'12
available from Caltex; JEFFSOI? AG 1555 solvent available from Huntsman
Corporation;
a seed oil, such as methyl and ethyl oleate, methyl and ethyl soyate and their
corresponding
fatty acids; an aromatic hydrocarbon, such as an alkyl benzene and an alkyl
naphthalene,
such as SOLVESSOB 150 available from ExxonMobil Chemical Company; a
polyalkylene
glycol ether; a fatty acid diester; a fatty alkylamide or diamide; a
dialkylene carbonate; a
ketone; an alcohol; or any mixtures thereof. In a most preferred form of the
invention, the at
least one oil is selected from any Group 2 or 3 Base Oil, as defined by
corresponding
Viscosity Indexes. In a preferred embodiment, the at least one oil is used in
a concentration
range of from 1 to 700g/L, or more preferably, of from 100 to 700g/L, or more
preferably,
of from 100 to 450g/L, or more preferably, of from 100 to 300g/L, or more
preferably, of
from 1 to 450g/L, or more preferably, of from 300 to 450g/L, or even more
preferably, of
from 450 to 700g/L. In the most preferred embodiments, the at least one oil is
used in a
concentration of greater than 500g/L.
100301 The addition of at least one oil-soluble surfactant dispersing agent,
which assists with
maintaining the dispersion concentrate in a substantially stable form, is
preferably selected
from a fatty acid-polyalkylene glycol condensate, such as TERSPERSER 2510
dispersant;
or a polyamine-fatty acid condensate, such as TERSPERSE 4850 dispersant or
TERSPERSE' 4890 dispersant; a random polyester condensate, such as TERSPERSE'
2520 dispersant; and a salt of a polyolefin condensate, such as TERSPERSE'
2422
dispersant, which are all products of Huntsman Corporation. More preferably,
the at least
one dispersing agent is a condensation product of the reaction of polyalkylene
glycol or
polyalkylene glycol ether and a fatty acid, such as TERSPERSER 2510 dispersant
or a random
polyester condensate, such as TERSPERSER 2520 dispersant. The surfactant
dispersing
agent is used in a concentration range of from 1 to 70g/L, or more preferably,
of from 1 to
40g/L, or more preferably, of from 40 to 70g/L, or more preferably, of from 1
to 15g/L. The
term "condensate" as used herein refers to the reaction product of a
condensation reaction
following the elimination of water or a small molecule, or to the reaction
product of an
addition reaction.
9
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
[0031] The co-formulation of the present invention further preferably
comprises at least one
rheology modifier to overcome the strong tendency of the finely dispersed
plant growth
regulator when on its own or when in combination with the active ingredient,
or the dispersed
phase, to settle or consolidate over time. The theology modifier is used in an
amount of from
1 to 20g/L, or more preferably, of from 1 to 15g/L, or more preferably, of
from 1 to 10g/L,
or more preferably, of from 1 to 5g/L. If required, the at least one rheology
modifier is
preferably selected from fumed silica, both hydrophobic and hydrophilic
variants, such as
one provided by the AEROSIL1' range from EVONIK; a gelling clay, such as one
provided
by the hydrophobic members of the BENTONE range from ELEMENTISR, and more
particularly, either BENTONEB 38, a hydrophobically modified hectorite clay,
or
BENTONE' SD-1, a hydrophobically modified organic derivative of bentonite; a
triglyceride or other fatty acid ester of glycerol; a rubber-type co-polymer,
particularly one
containing styrene residues, such as styrene-butadiene co-polymers, such as
KRATOW
G1701, available from KRATON Corporation; and a co-polymer, block or
otherwise, such
as a polyester and/or a polyamides. More preferably, the rheology modifier is
BENTONEO
SD-1, or even more preferably, the combination of BENTONE SD-1, a
hydrophobically
modified organic derivative of bentonite, which is used in an amount of from 1
to 20g/L, or
more preferably, of from 1 to 15g/L, or more preferably, of from 1 to 10g/L;
and AEROSIL'
200, a hydrophilic fumed silica, which is used in an amount of from 1 to
40g/L, or more
preferably, of from 1 to 20g/L, or more preferably, of from 1 to 10g/L, or
more preferably,
of from 1 to 5g/L.
100321 Where a rheology modifier is used, the co-formulation may further
comprise at least
one secondary activation agent to yield optimal performance. Where the
secondary
activation agent comprises a gelling clay, the addition of polar solvents
including water,
methanol, ethanol, propylene carbonate, or any mixtures thereof may be
required. Further,
where a rheology modifier is used, the co-formulation may further preferably
comprise at
least one secondary activation agent to facilitate highly improved rheological
modification
performance.
[0033] The co-formulation preferably further comprises at least one inert
solid filler,
including, but not limited to, titanium dioxide, such as TIONA 625 available
from CRISTAL
Global and one or more rheologically inactive phyllosilicates, which can also
act as
activation agents that assist in stabilising the co-formulation.
[0034] Since the co-formulation of the present invention exists as a
continuous phase in oil/s,
the choice of an emulsifying agent is somewhat governed by the type of oil/s
used as the
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
continuous phase. Generally, an emulsifying agent having a low hydrophobic-
lipophobic
balance ("HLB") is most suitable. The HLB required for most oil phases used in
the present
co-formulation is usually below 10. Such an emulsifying agent is preferably
selected from
one or more alkoxylated fatty alcohol/s, sorbitan ester/s and their
corresponding ethoxylate/s,
ethoxylated fatty acid/s, ethoxylated castor oil/s, calcium and ammonium and
alkylammonium salts of alkylbenzene sulphonate, alkylsulphosuccinate salt/s,
ethylene
oxide-propylene oxide block copolymer/s, ethoxylated alkylamine/s and
ethoxylated alkyl
phenol/s, or any mixtures thereof.
[0035] When the co-formulation comprises thidiazuron, or thidiazuron in
combination with
diuron, the at least one emulsifying agent is most preferably selected from
the group of castor
oil ethoxylates, in particular, TERMULR 3201 emulsifier, TERMUL 3512
emulsifier;
alcohol ethoxylates, in particular, TERI(' 12A3N, 12A4N, 13A7, 13A9, 17A2 and
SURFONIC TDA-6; alcohol alkoxylates, such as TERMULR 5429, 5459 and 5500;
fatty
acid ethoxylates, such as TERIC 0F6; sorbitan ester ethoxylates, such as
ECOTERICR
T85; a sulphosuccinate, such as TERMULB 3665 emulsifier, all of which are
available from
Huntsman Corporation; and amine and calcium salts of dodecylbenzene
sulphonate, such as
the NANSA EVM range of products available from INNOSPEC Inc.. The most
preferred
emulsifying agent comprises any one or any combination of calcium salt of
dodecylbenzene
sulphonate, at least one alcohol alkoxylate, and sorbitan ester ethoxylate in
an amount of
from 1 to 300g/L, or more preferably, of from 1 to 250g/L, or more preferably,
of from Ito
175g/L, or more preferably, of from 1 to 150g/L, or more preferably, of from
175 to 250g/L,
or more preferably, of from 150 to 175g/L, or more preferably, of from 100 to
150g/L, or
more preferably, of from 1 to 100g/L.
[0036] Since the co-formulation of the present invention exists as a
continuous phase in oil/s,
which may further be diluted in water as facilitated by the presence of an
emulsifying agent/s,
the invention may further comprise water-soluble surfactant dispersing agents
that help
maintain the pre-broadcast aqueous dispersion in a substantially stable form.
If required, the
at least one water-soluble surfactant dispersing agent is preferably selected
from, but not
limited to, sodium or ammonium salts of alkyl naphthalene sulphonate
formaldehyde
condensates, such as TERSPERSEO 2020; sodium, calcium and ammonium salts of
lignosulphonates; sodium or ammonium salts of co-polymers, such as TERSPERSE
2700;
sulfonates of cumene or xylene, such as the ELTESOL SC or SX range of products
available
from INNOSPEC Inc.
11
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
[0037] The scope of the present invention further extends to a method of
preparing the co-
formulation of the invention comprising the following steps, wherein steps c)
and d) may be
carried out in any order or simultaneously:
a) comminuting at least one plant growth regulator, or at least one plant
growth regulator
and an additional biologically active ingredient, to achieve an average
particle size in
the range of from Ito 12 microns;
b) adding the comminuted at least one plant growth regulator, or at least
one plant growth
regulator and an additional biologically active ingredient, to at least one
oil either by
stirring or high-shear mixing to create an oil dispersion;
c) adding at least one oil-soluble surfactant dispersing agent to the oil
dispersion;
d) adding at least one emulsifying agent to the oil dispersion; and
optionally
e) adjusting the temperature of the oil dispersion to between about 60 C
and 70 C with
stirring; and optionally
0 adjusting the final concentration of at least one plant growth regulator,
or at least one
plant growth regulator and an additional biologically active ingredient, in
the dispersion
by adding additional oil and one or more rheology modifiers and/or activation
agents as
required to substantially stabilise the co-formulation.
[0038] The scope of the present invention further extends to an alternative
method of
preparing the co-formulation of the invention comprising the following steps
wherein steps
e) and 0 may be carried out in any order or simultaneously:
a) combining at least one rheology modifying agent/s with at least one oil;
b) adding at least one plant growth regulator, or at least one plant growth
regulator and an
additional biologically active ingredient, to the oil from step a) either by
stirring or high-
shear mixing to create an oil dispersion;
c) adding at least one activation agent to further develop a desired
stability behaviour of
the oil dispersion;
d) comminuting the oil dispersion of step c) to achieve an average particle
size of the at
least one plant growth regulator, or at least one plant growth regulator and
an additional
biologically active ingredient, in the range of from 1 to 12 microns;
e) adding at least one oil-soluble surfactant dispersing agent to the oil
dispersion;
0 adding at least one emulsifying agent to the oil dispersion; and
optionally
g) adjusting the temperature of the oil dispersion to between about 60 C
and 70 C with
stirring; and optionally
12
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
h) adjusting the final concentration of at least one plant growth
regulator, or at least one
plant growth regulator and an additional biologically active ingredient, in
the dispersion
by adding additional oil and one or more additional rheology modifiers and/or
activation
agents as required to substantially stabilise the co-formulation.
100391 The scope of the present invention extends to a further preferred
method of preparing
the co-formulation of the invention comprising the following steps:
a) adding at least one oil-soluble surfactant dispersing agent to at least one
oil;
b) adding at least one plant growth regulator, or at least one plant growth
regulator and an
additional biologically active ingredient, to the oil either by stirring or
high-shear mixing
to create an oil dispersion;
c) adding at least one activation agent to further develop a desired
stability behaviour of
the oil dispersion;
d) comminuting the oil dispersion of step c) to achieve an average particle
size of the at
least one plant growth regulator, or at least one plant growth regulator and
an additional
biologically active ingredient, in the range of 1 to 12 microns; and
optionally
e) adjusting the temperature of the oil dispersion to between about 60 C
and 70 C with
stirring; and optionally
0 adjusting the final concentration of at least one plant growth regulator,
or at least one
plant growth regulator and an additional biologically active ingredient, in
the dispersion
by adding additional oil, at least one emulsifying agent, and one or more
rheology
modifiers and/or at least one other activation agents as required to
substantially stabilise
the co-formulation.
100401 In a most preferred fomi, the present invention provides a method of
preparing a
substantially homogenous and stable co-formulation comprising the following
steps, wherein
steps a) to d) are carried out before or after steps e) to g) and before step
h):
a) adding at least one oil-soluble surfactant dispersing agent to at least
one first oil;
b) adding an effective amount of at least one plant growth regulator, or at
least one plant
growth regulator and an additional biologically active ingredient, in the oil
with stirring
or high-shear mixing to create an oil dispersion;
c) adding at least one activating agent to further develop a desired
stability behaviour of
the oil dispersion;
d) comminuting the oil dispersion of step c) to produce a substantially
homogeneous first
dispersion concentrate wherein at least one plant growth regulator, or at
least one plant
13
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
growth regulator and an additional biologically active ingredient, has an
average particle
size in the range of 1 to 12 microns;
e) dispersing at least one rheology modifier in at least one second oil by
stirring or high-
shear mixing to form a second substantially homogeneous dispersion concentrate
in the
form of a gel;
0 adding at least one solvent to the second dispersion concentrate with
continued stirring or
high-shear mixing to form an improved gel;
g) dispersing at least one oil-soluble surfactant dispersing or emulsifying
agent into the
improved gel by stirring or high-shear mixing to form a second substantially
homogeneous
dispersion concentrate;
h) adding at least one other rheology modifier to the gel of step g) under
low-shear mixing to
form a pre-mix carrier; and
i) adding an amount of the first dispersion concentrate to an amount of the
pre-mix carrier to
obtain the substantially homogenous and stable co-formulation.
100411 It is noteworthy that the improved gel in step 0 occurs once the
emulsifying agent
has been added. The gel is improved in that while it maintains similar
rheological traits, it is
easier to handle having a lower viscosity which allows for better mixing in
step (i). It is also
thermodynamically preferable to form a stable second dispersion concentrate,
which assists
in substantially avoiding the unwanted side-effects of adding concentrated
emulsifiers to the
millbase pre-mix.
100421 The scope of the present invention also extends to a method for
applying the present
co-formulation to a crop requiring defoliation, whereby it is expected that
the synergistic
benefit of co-formulating at least one plant growth regulator, or at least one
plant growth
regulator in combination with an additional biologically active ingredient,
with at least one
oil, may yield benefits with respect to improved formulation efficiency and
biological
efficacy. More particularly, the present inventor has found that the
application of the co-
formulation of the present invention when used as a defoliant maintains or
yields
improvements in defoliation efficiency in crops and in particular, in cotton
crops.
100431 It has been found that, due to the improved delivery or application of
the co-
formulation of the present invention, less of the plant growth regulator on
its own, or in
combination with the additional biologically active ingredient, is/are
required. In addition,
the co-formulation of the present invention removes the need to apply or mix
the at least one
plant growth regulator and/or the at least one active ingredient separately as
has been done
in the past, which is advantageous from a handling and ease-of-use
perspective. Due to the
14
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
synergism that exists between at least one plant growth regulator, or at least
one plant growth
regulator in combination with an additional biologically active ingredient and
the at least
one oil, the present invention therefore results in substantially improved
efficiency of
defoliation.
100441 The scope and findings of this invention is not to be limited solely to
the use of oil-
based dispersion co-formulations for defoliation purposes on, for example,
cotton crops. It
is expected that the synergism shown by the compositions described may also
transfer to
broader and less specific applications of pesticides. These include, but are
not limited to, the
application of oil-based suspension co-formulations containing a plant growth
regulator, a
fungicide, an insecticide, an herbicide, a miticide, a nematocide, a
molluscicide, an algicide,
or other pesticide, or any mixtures thereof.
[0045] The following descriptions relate only to specific embodiments of the
present invention
and are in no way intended to limit the scope of the present invention to
those specific
embodiments. In particular, the following description is exemplary rather than
limiting in
nature. Variations and modifications to the disclosed methods that do not
necessarily depart
from the essence of this invention may become apparent to those skilled in the
art.
BRIEF DESCRIPTION OF THE FIGURES
100461 In order that the present invention can be understood and put into
practical effect,
Figure 1 illustrates the results of flow assessments conducted on particular
examples, which
show rheological features attributed to dispersion instability. This
instability has been linked to
further catastrophic destabilisation phenomena exhibited by complete co-
formulation
examples, and highlights the significant improvements afforded by the present
invention which
surprisingly manage to diminish the unwanted effects of these challenges.
DETAILED DESCRIPTION OF THE INVENTION
[0047] The invention will now be described with reference mainly to oil-based
co-formulations
comprising thidiazuron, as the plant growth regulator, or thidiazuron, as the
plant growth
regulator, and diuron, as the biologically active ingredient. It is
anticipated that similar results
can be found for suspension formulations in oil of other solid plant growth
regulators or
biologically active ingredients, which are not soluble in the continuous oil
phase.
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
[0048] When the co-formulation comprises thidiazuron on its own in oil,
thidiazuron is most
preferably present in a concentration range of 1 to 250g/L, or more
preferably, from 1 to
200g/L, or more preferably, from 1 or 100g/L, or more preferably, from 1 to
80g/L, or more
preferably, from 1 to 50g/L, or more preferably, from 1 to 30g/L, or more
preferably, from
1 to 20g/L and most preferably, in a concentration of 60 g/L.
[0049] When the co-formulation comprises a combination of thidiazuron and
diuron,
thidiazuron is most preferably present in a concentration range of 1 to
250g/L, or more
preferably, from 1 to 200g/L, or more preferably, from 1 or 100g/L, or more
preferably, from
1 to 80g/L, or more preferably, from 1 to 50g/L, or more preferably, from 1 to
30g/L, or
more preferably, from 1 to 20g/L and most preferably, in a concentration of 60
g/L; and
diuron is preferably present in a concentration range of from 1 to 250 g/L, or
more preferably,
from 1 to 100g/L, or more preferably, from 1 to 80 g/L, or more preferably,
from 1 to 60g/L,
or more preferably, from 1 to 50g/L, or more preferably, from 1 to 30g/L, or
more preferably,
from 1 to 20g/L, or more preferably, from 50 to 80g/L, or more preferably,
from 30 to 50g/L,
or more preferably, from 20 to 30g/L. The concentration of diuron is most
preferably selected
from 48, 30 or 15g/L, respectively. In the most preferred form, diuron is
present in a
concentration of 30g/L.
[0050] In a most preferred form of the invention, the at least one oil is a
Group 3 Base Oil
with a Viscosity Index of between 110 and 120, which is used in a
concentration range of
450 to 700g/L.
100511 The at least one oil-soluble surfactant dispersing agent is most
preferably a
condensation product of the reaction of polyalkylene glycol or polyalkylene
glycol ether and a
fatty acid, such as TERSPERSE" 2510 dispersant, or a random polyester
condensate, such
as TERSPERSEB 2520 dispersant, which is used in a concentration range of from
1 to 70g/L,
or more preferably, of from 1 to 40g/L, or more preferably, of from 40 to
70g/L, or more
preferably, of from 1 to 15g/L.
[0052] The at least one emulsifying agent required for the co-formulation
comprising
thidiazuron, or thidiazuron and diuron, is most preferably selected from the
group of sorbitan
ester ethoxylates, in particular, ECOTERIC1M T85 fatty acid ethoxylate;
alcohol alkoxylates,
such as TERMUL 3201, 5429, 5459 and 5500 emulsifiers; alcohol ethoxylates, in
particular, TERI(' 12A3N and TERIC 13A7 fatty acid ethoxylates, most of which
are
available from Huntsman Corporation; and amine and calcium salts of
dodecylbenzene
sulphonate, such as the NAN SA EVM range of surfactant products, and more
preferably,
NAN SA" EVM 70/2E surfactant. The most preferred emulsifying agent is the
combination
16
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
of at least one or any calcium salt of dodecylbenzene sulphonate, at least one
alcohol
alkoxylate, and sorbitan ester ethoxylate, each in amount of from 1 to 175g/L.
[0053] Where a rheology modifier is required, the most preferred is a
hydrophobically
modified organic derivative of bentonite, namely, BENTONE SD-1, or more
preferably the
combination of a hydrophobically modified organic derivative of bentonite,
namely,
BENTONE' SD-1, which is used in an amount of from 1 to 20g/L, or more
preferably, of
from 1 to 15g/L, or more preferably, of from 1 to 10g/L; and a hydrophilic
fumed silica,
AEROS112 200, which is most preferably used in an amount of from 1 to 20g/L.
[0054] The secondary activation agent, which may be required to yield further
improved
performance, is most preferably selected from a gelling clay, which may also
require the
addition of polar solvents including water, methanol, ethanol, propylene
carbonate, or any
mixtures thereof.
BRIEF DESCRIPTION OF THE EXAMPLES
[0055] A typical oil dispersion formulation known in the art has a composition
as described in
Table A below.
Table A - Typical Components required for an Oil Dispersion Formulation:
Typical amount, %
Component Purpose
w/w*
Active ingredient <60 As a toxicant
To prevent particle
Dispersant (oil-soluble) <8
aggregation
To emulsify the oil phase
Emulsifier 5 - 20 when the formulation is
added to water
Anti-settling and 0 . 5 - To prevent sedimentation
structuring agents and syneresis
Oil balance To form a continuous phase
* where the total amount of all the components adds up to 100%.
17
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
EXAMPLE 1 ¨ Comparative Example A.
100561 The below Table 1 provides an example of a typical commercial aqueous
suspension
concentrate ("SC") containing thidiazuron:
Table 1
Components g/L
Thidiazuron 500
Dispersing Agent/s 10 ¨ 50
Wetting Agent/s 10 ¨ 20
Humectant 50 ¨ 100
Antifoam 1 ¨ 10
Xanthan gum 0.1 ¨ 0.5
Biocidal agent 0.05 ¨ 0.25
Water To volume.
Such a formulation would be prepared in a manner familiar to those skilled in
the art.
EXAMPLE 2¨ Comparative Example B.
100571 Table 2 below provides an example of a commercial aqueous suspension
concentrate
("SC") containing both diuron and thidiazuron:
Table 2
Components g/L
Diuron 60
Thidiazuron 120
Dispersing Agent/s 10 ¨ 50
Wetting Agent/s 10 ¨ 20
Humectant 50 ¨ 100
Antifoam 1 ¨ 10
X anthan gum 0.1 ¨ 0.5
Biocidal agent 0.05 ¨ 0.25
18
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
Water To volume.
Such a formulation would be prepared in a manner familiar to those skilled in
the art.
EXAMPLE 3 ¨ Initial example of the co-formulation of the present invention and
as
exemplified in-field.
100581 An oil-based suspension co-formulation of diuron and thidiazuron
according to the
present invention was prepared as follows:
Table 3
Components g/L
Diuron 15.00
Th idiazuron 30.00
TERSPERSE'' 2510 dispersant 30.00
TERMUL" 3201 emulsifier 100.00
NANSA EVM 70/2E 20.00
surfactant
TERMULR 5459 emulsifier 20.00
BENTONE' 38 7.50
JEFFSOL" AG 1555 solvent 2.47
YUBASE 3 To volume.
100591 Firstly, a pre-mix `masterbatch' of activated hydrophobically-modified
hectorite was
prepared via the dispersion of lOg BENTONE' 38 (ELEMENTIS) in 86.66g of YUBASE
via a SILVERSON high-shear mixer. To the resultant homogeneous dispersion,
3.34g
JEFFSOLR AG 1555 was added with continued shear until a homogeneous gel-like
substance
was afforded. This was set aside.
100601 Then, as per Table 3, the required amount of TERSPERSE 2510 dispersant
was
dissolved in an amount of YUBASE 3 that would allow for a thidiazuron
concentration in the
vicinity of 7% w/w. Diuron and thidiazuron technical were then dispersed in
the resultant
mixture under high-shear using a SILVERSON mixer. This was continued until a
homogeneous
mixture was afforded.
19
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
[0061] This slurry was then comminuted using an horizontal mill (Engineered
Mills, Inc.) to
produce a concentrate containing components having an average (d0.5) particle
size of less than
p.m. Particle size analysis was determined by microscopic approximation.
[0062] To the concentrate, TERMULR 3201 emulsifier, NANSA" EVM 70/2E
surfactant,
TERMUL" 5459 emulsifier and the pre-prepared rheology modifier, prepared as
described
above, were added, followed by the remaining quantity of YUBASE IV'. The
resulting oil-based
suspension was then homogenised via low to moderate shear mixing. The
composition was
then observed as displaying satisfactory stability and dilution behaviour.
This is also an example
of the methodology of adding a rheology modifier post-comminution.
EXAMPLE 4 - Higher-loading variant of EXAMPLE 3 and exemplified in-field.
100631 An oil-based suspension co-formulation of diuron and thidiazuron
according to the
present invention was prepared as follows:
Table 4
Components g/L
Diuron 30.00
Thidiazuron 60.00
TERSPERSE" 2510 dispersant 40.00
TERMUL" 3201 emulsifier 100.00
NANSA EVM 70/2E 20.00
surfactant
BENTONE" 38 0.57
JEFFSOL' AG 1555 solvent 0.19
YUBASE 3 To volume.
[0064] As per Table 4, the required amount of TERSPERSER 2510 dispersant was
dissolved
in an amount of YUBASE 3' that would allow for a thidiazuron concentration in
the vicinity
of 12% w/w. Diuron and thidiazuron technical were then dispersed in the
resultant mixture
under high-shear using a SILVERSON mixer. This was continued until a
homogeneous mixture
was afforded.
[0065] This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 um, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopic approximation.
100661 To the concentrate, TERMUL" 3201 emulsifier, and NANSA" EVM 70/2E
surfactant
and the pre-prepared rheology modifier (described in EXAMPLE 3) were added,
followed by
the remaining quantity of YUBASE 3. The resulting oil-based suspension was
then
homogenised via low to moderate shear mixing. The composition was then shown
to display
satisfactory stability and dilution behaviour. This is another example of the
methodology of
adding rheology modifiers post-comminution.
EXAMPLE 5 - Thidiazuron-only example and exemplified in-field.
100671 An oil-based suspension co-formulation of thidiazuron according to the
present
invention was prepared as follows:
Table 5
Components g/L
Th idiazuron 100.00
TERSPERSE 2510 dispersant 40.00
TERMULR 3201 emulsifier 100.00
NANSA EVM 70/2E 20.00
surfactant
BEN TON ER 38 0.50
JEFFSOL' AG 1555 solvent 0.17
YUBASE 3 To volume.
100681 As per Table 5, the required amount of TERSPERSE 2510 dispersant was
dissolved
in an amount of YUBASE 3 that would allow for a thidiazuron concentration in
the vicinity
21
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
of 17 to 18% w/w. Diuron and thidiazuron technical were then dispersed in the
resultant mixture
under high-shear using a SILVERSON mixer. This mixing was continued until a
homogeneous
mixture was afforded.
[0069] This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 p.m, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopy, where the dispersion displayed weak to moderate
flocculation of
active ingredient crystals.
[0070] To the concentrate, TERMUL 3201 emulsifier, NAN SA" EVM 70/2E
surfactant and
the pre-prepared rheology modifier as described in EXAMPLE 3 were added,
followed by the
remaining quantity of YUBASE 3 . The resulting oil-based suspension was then
homogenised
via low to moderate shear mixing. The composition was then observed as
displaying
satisfactory stability and dilution behaviour. This is another example of the
methodology of
adding rheology modifiers post-comminution.
EXAMPLE 6
[0071] A millbase concentrate for use in the preparation of a complete oil-
based suspension co-
formulation of thidiazuron according to the present invention was prepared as
follows:
Table 6
Components % w/w
Thidiazuron 20.00
TERSPERSEP 4850 dispersant 4.00
SOLVESSO 150 2.00
YUBASE 3 Balance
22
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
100721 As per Table 6, the required amount of TERSPERSER 4850 dispersant was
dissolved
in an amount of pre-mixed YUBASE 3" and SOLVESSO 150. Thidiazuron technical
was then
dispersed in the resultant mixture under high-shear using a SILVERSON mixer.
This was
continued until a homogeneous mixture was afforded.
100731 This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Heguian Gauge. Particle size
analysis was additionally
determined by microscopy, where the dispersion displayed weak to moderate
flocculation of
active ingredient crystals.
EXAMPLE 7
100741A millbase concentrate for use in the preparation of a complete oil-
based suspension co-
formulation of thidiazuron according to the present invention was prepared as
follows:
Table 7
Components % w/w
Thidiazuron 20.00
TERSPERSE" 2510 dispersant 4.00
SOLVESSO 150 2.00
YUBASE 3 To volume.
100751 As per Table 7, the required amount of TERSPERSE 2510 dispersant was
dissolved
in an amount of pre-mixed YUBASE 3" and SOLVESSO 150. Thidiazuron technical
was then
dispersed in the resultant mixture under high-shear using a SILVERSON mixer.
This was
continued until a homogeneous mixture was afforded.
23
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
[0076] This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopy, where the dispersion displayed weak to moderate
flocculation of
active ingredient crystals.
EXAMPLE 8
[0077] A millbase concentrate for use in the preparation of a complete oil-
based suspension co-
formulation of thidiazuron according to the present invention was prepared as
follows:
Table 8
Components % w/w
Thidiazuron 20.00
TERSPERSEP 2520 dispersant 4.00
SOLVESSO 150 2.00
YUBASE 3 Balance
[0078] As per Table 8, the required amount of TERSPERSER 2520 dispersant was
dissolved
in an amount of pre-mixed YUBASE 3 and SOLVESSO 150. Thidiazuron technical was
then
dispersed in the resultant mixture under high-shear using a SILVERSON mixer.
This was
continued until a homogeneous mixture was afforded.
100791 This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
24
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopy, where the dispersion displayed weak to moderate
flocculation of
active ingredient crystals.
EXAMPLE 9
[0080] A millbase concentrate for use in the preparation of a complete oil-
based suspension co-
formulation of thidiazuron according to the present invention was prepared as
follows:
Table 9
Components % w/w
Thidiazuron 20.00
TERSPERSE 4890 4.00
SOLVESSO 150 2.00
YUBASE 3 Balance
[0081] As per Table 9, the required amount of TERSPERSE" 4890 dispersant was
dissolved
in an amount of pre-mixed YUBASE 3' and SOLVESSO 150. Thidiazuron technical
was then
dispersed in the resultant mixture under high-shear using a SILVERSON mixer.
This was
continued until a homogeneous mixture was afforded.
[0082] This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
determined by microscopy, where the dispersion displayed weak to moderate
flocculation of
active ingredient crystals.
EXAMPLE 10
[0083] A millbase concentrate for use in the preparation of a complete oil-
based suspension co-
formulation of thidiazuron according to the present invention was prepared as
follows:
Table 10
Components % w/w
Thidiazuron 15.08
TERMUL 3201 15.08
NANSA EVM70/2E 3.77
TERIC 13A7 5.66
SOLVESSO 150 4.61
YUBASE 3 Balance
[0084] As per Table 10, the required amounts of TERM UL 3201, NANSAO EVM70/2E
and
TER1C1zD 13A7 were added to an amount of YUBASE 3. Thidiazuron technical was
then
dispersed in the resultant mixture under high-shear using a SILVERSON mixer.
This was
continued until a homogeneous mixture was afforded.
[0085] This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopy, where the dispersion displayed weak to moderate
flocculation of
active ingredient crystals.
26
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
EXAMPLE 11 ¨ Comparative Rheological Assessment
100861 Fundamental rheological examination of Examples 6 to 10 illustrates the
exceptional
difficulties associated with the stabilisation of thidiazuron through analysis
of these simple
millbase concentrates. This data highlights the existence of inherent
instability of the finely
divided disperse phase when suspended in the preferred concentrations of the
oil-based carrier,
regardless of the presence of various, common surfactant dispersing agent/s,
which should
typically assist with maintaining the dispersion concentrate in a
substantially stable form.
The present inventor has concluded that this underlying instability observed
in millbase
concentrates is the primary source of instability in complete co-formulations
comprising
thidiazuron and the desired oil.
Flow Assessment
100871 Basic flow measurements, performed upon a Malvern Kinexus Pro Rheometer
using
40mm plate geometry, 150 m gap, 25 C, 0.1 ¨ 1.0s-1, (logarithmic table),
illustrated in Figure
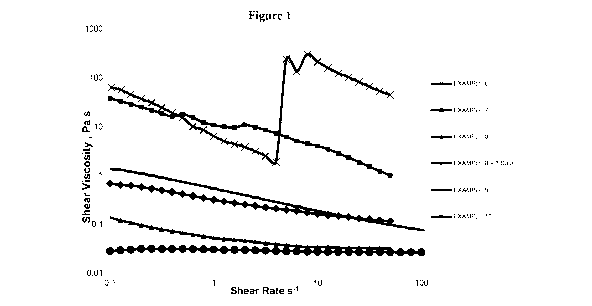
1 below, show that the co-formulations described in EXAMPLES 6, 7 and to a
lesser extent
EXAMPLE 10, display rheological features attributed to dispersion instability,
for example,
low-shear viscosities in the region of 1 - 100 Pa.
100881 EXAMPLE 8 shows significant improvement with initial measurements
displaying
only minor thixotropy and viscosity readings of << 1 Pa across the measured
shear rate range.
After 6 days however, EXAMPLE 8 is shown to display onset of the same de-
stabilising
phenomena with viscosity approaching 1 Pa, or a nearly 1000-fold increase in
shear viscosity.
100891 EXAMPLE 10 is an anomalous example, which highlights further improved
dispersion
stability, where a lack of thixotropy and time-dependent rheological changes
are displayed.
However, this further improvement unexpectedly shows no benefit when utilised
in the
preparation of a complete composition, as exemplified by EXAMPLE 20, thus
highlighting
further unprecedented complexity.
EXAMPLE 12- Improvement upon EXAMPLE 4.
[0090] An oil-based suspension co-formulation of diuron and thidiazuron
according to the
present invention was prepared as follows:
27
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
Table 12
Components g/L
Diuron 30.00
Thidiazuron 60.00
TERSPERSER 2510 dispersant 40.00
TERMUL" 3201 emulsifier 100.00
NAN SA EVM 70/2E 20.00
surfactant
AEROSILB 200 4.00
BENTONE' 38 0.25
JEFFSOLR-AG 1555 solvent 0.20
YUBASE 3 To volume.
100911 As per Table 12, the required amount of BENTONE' 38 was added to an
amount of
YUBASE 3, where the latter equated to an amount that would allow for a
thidiazuron
concentration in the vicinity of 20%. The resultant suspension was then
subject to mixing at
high-shear (SILVERSON) for a specific duration, followed by drop-wise addition
of
JEFFSOL' AG 1555 solvent with continued shear to develop the appropriate
rheology. The
rate of shear was then decreased, and the required mass of AEROSIL 200
(EVONIK) was
added followed by continued shear for a specific duration. TERSPERSER 2510
dispersant was
then dissolved in the mixture ensuring homogeneity, followed by dispersion of
diuron and
thidiazuron technical.
100921 This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopic approximation.
28
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
[0093] To the concentrate, TERMUL1 3201 emulsifier and NANSA1 EVM 70/2E
surfactant
were added, followed by the remaining quantity of YUBASE 31. The resulting oil-
based
suspension was then homogenised via low-shear (overhead stirring). The
composition was
shown to display satisfactory stability and dilution behaviour. This is an
example of a
methodology, whereby the rheology modifiers were added prior to comminution.
EXAMPLE 13- Improvement upon EXAMPLE 5.
[0094] An oil-based suspension co-formulation of thidiazuron according to the
present
invention was prepared as follows:
Table 13
Components g/L
Thidiazuron 100.00
TERSPERSE1 2510 dispersant 30.00
TERMUL1 3201 emulsifier 100.00
NAN SA' EVM 70/2E 20.00
surfactant
AEROSIL 200 1.50
BENTONE' 38 0.25
JEFFSOL1 AG 1555 solvent 0.20
SOLVESSOR 150 5.00
YUBASE 3 To volume.
[0095] As per Table 13, the required amount of BENTONER 38 was added to an
amount of
YUBASE 3, which equated to roughly 50% of the total requirement, and SOLVESSO'
150
(EXXON Chemical). The resultant suspension was then subject to mixing at high-
shear
(SILVERSON) for a specific duration, followed by drop-wise addition ofJEFFSOL1
AG 1555
solvent with continued shear to develop the appropriate rheology. The rate of
shear was then
decreased, and the required mass of AEROSIL1 200 (EVONIK) was added followed
by
continued shear for a specific duration. TERSPERSER 2510 dispersant was then
dissolved in
the mixture ensuring homogeneity, followed by dispersion of thidiazuron
technical.
29
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
[0096] This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopic approximation.
[0097] To the concentrate, TERMUL 3201 emulsifier and NANSA' EVM 70/2E
surfactant
were added, followed by the remaining quantity of YUBASE 3. The resulting oil-
based
suspension was then homogenized via low-shear overhead stirring. The
composition was
shown to display satisfactory stability and dilution behaviour. This is an
example of a
methodology, whereby rheology modifiers were added prior to comminution.
EXAMPLE 14
[0098] An oil-based suspension co-formulation of thidiazuron according to the
present
invention was prepared as follows:
Table 14
Components g/L
Thidiazuron 100.00
TERSPERSE' 2520 dispersant 20.00
TERM U L' 3201 emulsifier 100.00
NAN SA EVM 70/2E 25.00
surfactant
T ERIC' 13A7 emulsifier 37.50
AEROSIL' 200 4.23
BENTONER SD-1 14.81
SOLVESSOR 150 14.94
YUBASE 3 To volume.
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
[0099] A pre-mix "masterbatch" gel was prepared via the dispersion of 14.81g
BENTONE'
SD-1 (ELEMENTIS) in 80.25g of YUBASE 3" via a SILVERSON high-shear mixer. To
the
resultant homogeneous dispersion, 4.94g SOLVESSO 150 was added with continued
shear
until a gel-like substance was afforded. This was set aside.
[0100] A pre-mix "masterbatch" gel was prepared via the addition of 4.23g
AEROSIL" 200 to
95.77g of YUBASE" 3 under high shear until a gel-like substance was afforded.
This was set
aside.
[0101] As per Table 14 above, the required amount of TERSPERSER 2520
dispersant was
dissolved in a mixture comprising the remaining amount of SOLVES SO 150 and
YUBASE
3, where the latter equated to an amount that would allow for a thidiazuron
concentration in
the vicinity of 20%. Thidiazuron technical was then dispersed in the resultant
mixture under
high-shear using a SILVERSON mixer. This was continued until a homogeneous
mixture was
afforded.
[0102] This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 tm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopic approximation.
[0103] To the concentrate, TERMUL' 3201 emulsifier, NANSA" EVM 70/2E
surfactant,
TERIC1 13A7 emulsifier and the pre-prepared masterbatch gels were added,
followed by the
remaining quantity of YUBASE 3 . The resulting oil-based suspension was then
homogenised
via low-shear mixing. The composition was then shown to display satisfactory
stability and
further improved dilution behaviour, which meets the desired commercial
performance criteria.
This is another example of the methodology of adding rheology modifiers post-
comminution.
EXAMPLE 15- Further Improvement upon EXAMPLE 14.
[0104] An oil-based suspension co-formulation of thidiazuron according to the
present
invention was prepared as follows:
31
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
Table 15
Components g/L
Th idiazuron 100.00
TERSPERSE'' 2520 dispersant 20.00
TERMULR 3201 emulsifier 100.00
NANSA EVM 70/2E 25.00
surfactant
TERICR 13A7 emulsifier 37.50
AEROSIL 200 4.23
BENTONE' SD-1 14.81
SOLVESSOR 150 29.49
YUBASE 3 To volume.
[0105] A pre-mix "masterbatch" gel was prepared via the dispersion of 14.81g
BENTONE'
SD-1 (ELEMENTIS) in 83.19g of YUBASE IR via a SILVERSON high-shear mixer. To
the
resultant homogeneous dispersion, 2.00g SOLVESSO' 150 was added with continued
shear
until a gel-like substance was afforded. This was set aside.
[0106] A further pre-mix "masterbatch" gel was prepared via the addition of
4.23g AEROSIL"'
200 to 95.74g of YUBASE' 3 under high shear until a gel-like substance was
afforded. This
was set aside.
[0107] As per Table 15 above, the required amount of TERSPERSER 2520
dispersant was
dissolved in a mixture comprising the remaining amount of SOLVES so' 150 and
YUBASE
where the latter equated to an amount that would allow for a thidiazuron
concentration in
the vicinity of 20%. Thidiazuron technical was then dispersed in the resultant
mixture under
high-shear using a SILVERSON mixer. This was continued until a homogeneous
mixture was
afforded.
[0108] This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
32
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopic approximation.
101091 To the concentrate, TERMULR 3201 emulsifier, NANSA" EVM 70/2E
surfactant,
TERIC 13A7 emulsifier and the pre-prepared masterbatch gels were added,
followed by the
remaining quantity of YUBASE 3*. The resulting oil-based suspension was then
homogenised
via low shear mixing. The composition was then shown to display satisfactory
stability and
further improved dilution behaviour which meets the desired commercial
performance criteria.
This is another example of the methodology of adding rheology modifiers post-
comminution.
EXAMPLE 16¨ Further Improvement upon EXAMPLE 15.
Table 16
Components g/L
Thidiazuron 100.00
TERSPERSEP 2520 dispersant 10.00
TERMUL" 3201 emulsifier 100.00
NANSA EVM 70/2E 25.00
surfactant
TERI(' 13A7 emulsifier 37.50
AEROSILB 200 4.24
BENTONE* SD-1 14.21
SOLVESSO' 150 40.00
YUBASE To volume.
[0110] A pre-mix "masterbatch" gel was prepared via the dispersion of 14.21g
BENTONE'
SD-1 (ELEMENTIS) in 76.39g of YUBASE 3" via a SILVERSON high-shear mixer. To
the
resultant homogeneous dispersion, 9.40g SOLVESSO' 150 was added with continued
shear
until a gel-like substance was afforded. This was set aside.
[01111A further pre-mix "masterbatch" gel was prepared via the addition of
4.24g AEROSILR
200 to 95.76g of YUBASE* 3 under high shear until a gel-like substance was
afforded. This
was set aside.
33
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
[0112] As per Table 16 above, the required amount of TERSPERSE" 2520
dispersant was
dissolved in a mixture comprising the remaining amount of SOLVES SO 150 and
YUBASE
3, where the latter equated to an amount that would allow for a thidiazuron
concentration in
the vicinity of 20%. Thidiazuron technical was then dispersed in the resultant
mixture under
high-shear using a SILVERSON mixer. This was continued until a homogeneous
mixture was
afforded.
[01131 This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 lam, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopic approximation. Particle size analysis was
additionally determined
by microscopy, where the dispersion displayed weak to moderate flocculation of
active
ingredient crystals.
[0114] To the required amount of pre-mix carrier, TERMULR 3201 emulsifier,
NANSA'. EVM
70/2E surfactant, TERIC' 13A7 emulsifier was added with stirring, followed by
the slow
addition of the millbase concentrate and the remaining quantity of YUBASE 3.
Stirring was
continued until a homogeneous suspension was afforded. This composition was
shown to
display further improved stability behaviour. This is another example of the
methodology of
adding rheology modifiers post-comminution.
EXAMPLE 17¨ Further Improvement upon EXAMPLE 12.
Table 17
Components g/L
Diuron 30.00
Thidiazuron 60.00
TERSPERSE 2520 dispersant 10.00
TERM U Lk 3201 emulsifier 100.00
34
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
NAN SA R EVM 70/2E 25.00
surfactant
TERICR 13A7 emulsifier 40.00
AEROSIL' 200 4.23
BENTONER SD-1 14.21
SOLVESSO* 150 50.00
YUBASE 3 To volume.
101151 A pre-mix carrier was prepared via the dispersion of 14.21g BENTONER SD-
1
(ELEMENTIS) in 200g of YUBASE 3" via a SILVERSON high-shear mixer. To the
resultant
homogeneous dispersion, 9.40g SOLVESSOR 150 was added with continued shear
until a gel-
like consistency was afforded. Finally, 4.23g AEROSIL' 200 was added under low-
shear
mixing until a homogeneous albeit flowable gel was afforded. The mixture was
set aside.
10116] As per Table 17 above, the required amount of TERSPERSE' 2520
dispersant was
dissolved in a mixture comprising the remaining amount of SOLVESSOR 150 and
YUBASE
IR, where the latter equated to an amount that would allow for a thidiazuron
concentration in
the vicinity of 20%. Diuron and thidiazuron technical was then dispersed in
the resultant
mixture under high-shear using a SILVERSON mixer. This was continued until a
homogeneous
mixture was afforded.
101171 This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hepnan Gauge. Particle size analysis
was additionally
determined by microscopy, where the dispersion displayed weak to moderate
flocculation of
active ingredient crystals.
101181 To the required amount ofpre-mix carrier, TERMULR 3201 emulsifier,
NANSAR EVM
70/2E surfactant, TERI(' 13A7 emulsifier was added with stirring, followed by
the slow
addition of the millbase concentrate and the remaining quantity of YUBASE 3.
Stirring was
continued until a homogeneous suspension was afforded. This composition was
shown to
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
display further improved stability behaviour. This is another example of the
methodology of
adding rheology modifiers post-comminution.
EXAMPLE 18- Further Improvement upon Example 16.
Table 18
Components g/L
Thidiazuron 100.00
TERSPERSE" 2520 dispersant 10.00
TERMUL" 3201 emulsifier 100.00
NAN SA EVM 70/2E 25.00
surfactant
T ERIC 13 A7 emulsifier 37.50
AEROSILR 200 4.24
BENTONE' SD-1 14.21
SOLVESSOR 150 40.00
YUBASE 3 To volume.
[0119] A pre-mix carrier was prepared via the dispersion of 14.21g BENTONEk SD-
1
(ELEMENTIS) in 200g of YUBASE 3". via a SILVERSON high-shear mixer. To the
resultant
homogeneous dispersion, 9.40g SOLVESSO' 150 was added with continued shear
until a gel-
like consistency was afforded. Finally, 4.24g AEROSIL' 200 was added under low-
shear
mixing until a homogeneous albeit flowable gel was afforded. The mixture was
set aside.
[0120] As per Table 18 above, the required amount of TERSPERSER 2520
dispersant was
dissolved in a mixture comprising the remaining amount of SOLVES SO' 150 and
YUBASE
3, where the latter equated to an amount that would allow for a thidiazuron
concentration in
the vicinity of 20%. Thidiazuron technical was then dispersed in the resultant
mixture under
high-shear using a SILVERSON mixer. This was continued until a homogeneous
mixture was
afforded.
[0121] This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
36
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopic approximation. This concentrate was briefly set
aside.
101221 To the required amount of pre-mix carrier, TERMUL 3201 emulsifier,
NANSAR EVM
70/2E surfactant, TERIC 13A7 emulsifier was added with stirring. The resultant
mixture was
then heated to approximately 60 C to 70 C, and with continued stirring, the
millbase
concentrate was slowly added followed by the remaining quantity of YUBASE 3.
Stirring was
continued while allowing the composition to cool, affording a homogeneous
suspension. This
composition was shown to display improved stability behaviour. This is another
example of the
methodology of adding rheology modifiers post-comminution.
EXAMPLE 19¨ Alternative to Example 15 ¨ Addition of Titanium dioxide.
Table 19
Components g/L
Thidi azuron 100.00
TERSPERSE'' 2520 dispersant 10.00
TERMULR 3201 emulsifier 100.00
NAN SA" EVM 70/2E 25.00
surfactant
TERICR 13A7 emulsifier 37.50
TIONA 625 10.00
AEROSIL' 200 4.21
BENTONER SD-1 14.21
SOLVESSO 150 40.00
YUBASE 3 To volume.
101231 A pre-mix carrier was prepared via the dispersion of 3.90g BENTONE SD-1
(ELEMENTIS) in 47.83g of YUBASE 3' via a SILVERSON high-shear mixer, yielding
a very
fine suspension. To the resultant homogeneous dispersion, 2.57g SOLVESSO 150
was added
with continued shear until a gel-like consistency was afforded. 27.41g TERMULO
3201, 6.85g
37
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
NANSA EVM70/2E, and 10.28g TERICCD 13A7 were then added to the mixture
followed
by low-speed mixing to homogenise. Lastly, 1.15g AEROSIL 200 was added slowly
under
low-shear mixing until a homogeneous, weakly gelled mixture was afforded. The
mixture was
set aside.
101241 As per Table 19 above, the required amount of TERSPERSE" 2520
dispersant was
dissolved in a mixture comprising the remaining amount of SOLVES SO 150 and
YUBASE
3, where the latter equated to an amount that would allow for a thidiazuron
concentration in
the vicinity of 20%. Thidiazuron technical and TIONA 625 were then dispersed
in the resultant
mixture under high-shear using a SILVERSON mixer. This was continued until a
homogeneous
mixture was afforded.
101251 This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6inm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopic approximation.
101261 To the required amount of pre-mixed carrier, the millbase concentrate
was slowly added
followed by the remaining quantity of YUBASE 3. Stirring was continued until a
homogeneous suspension was afforded. This composition was shown to display
further
improved stability behaviour. This is another example of the methodology of
adding rheology
modifiers post-comminution.
EXAMPLE 20¨ Alternative to Example 16 ¨ No dispersant required.
Table 20
Components g/L
Thidiazuron 100.00
TERMUL" 3201 emulsifier 200.00
38
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
NAN SA R EVM 70/2E 50.00
surfactant
TERICR 13A7 emulsifier 75.00
AEROSIL' 200 4.24
BENTONER SD-1 14.21
SOLVESSO* 150 40.00
YUBASE 3 To volume.
101271 A pre-mix "carrier-base" was prepared via the dispersion of 14.21g
BENTONE SD-1
(ELEMENTIS) in 200g of YUBASE 3R via a SILVERSON high-shear mixer. To the
resultant
homogeneous dispersion, 9.40g SOLVESSOR 150 was added with continued shear
until a gel-
like consistency was afforded. Finally, 4.24g AEROSIL' 200 was added under low-
shear
mixing until a homogeneous albeit flowable gel was afforded. The mixture was
set aside.
101281 As per Table 20 above, half the required amounts of TERMUL 3201, NANSA
EVM70/2E and TERIC 13A7 were added to a mixture comprising the remaining
amount of
SOLVESSO' 150 and YUBASE 3, where the latter equated to an amount that would
allow
for a thidiazuron concentration in the vicinity of 15%. Thidiazuron technical
was then dispersed
in the resultant mixture under high-shear using a SILVERSON mixer. This was
continued until
a homogeneous mixture was afforded.
101291 This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Heghian Gauge. Particle size
analysis was additionally
determined by microscopic approximation.
101301 To the required amount of pre-made "carrier-base", the remaining TER UL
3201
emulsifier, NANSAR EVM 70/2E surfactant, TERI(' 13A7 emulsifier was added with
stirring,
followed by the slow addition of the millbase concentrate and the remaining
quantity of
YUBASE 3. Stirring was continued until a homogeneous suspension was afforded.
This
39
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
composition was shown to display further improved stability behaviour. This is
another
example of the methodology of adding rheology modifiers post-comminution.
EXAMPLE 21 ¨ Alternative to Example 16.
Table 21
Components g/L
Thidi azuron 100.00
TERSPERSE" 2520 dispersant 15.00
ELTESOLO SC93 15.00
TERMUL 3201 emulsifier 100.00
NANSA"; EVM 7012E 25.00
surfactant
T ERIC" 13A7 emulsifier 37.50
AEROSIC 200 4.24
BENTONE" SD-1 14.21
SOLVESSO"' 150 40.00
YUBASE 3 To volume.
101311 A pre-mix carrier was prepared via the dispersion of 3.90g BENTONE SD-1
(ELEMENTIS) in 47.83g of YUBASE 3' via a SILVERSON high-shear mixer, yielding
a very
fine suspension. To the resultant homogeneous dispersion, 2.57g SOLVESSO 150
was added
with continued shear until a gel-like consistency was afforded. 27.41g TERMULO
3201, 6.85g
NANSA EVM70/2E, and 10.28g TERICCD 13A7 were then added to the mixture
followed
by low-speed mixing to homogenise. Lastly, 1.15g AEROSIL"' 200 was added
slowly under
low-shear mixing until a homogeneous, weakly gelled mixture was afforded. The
mixture was
set aside.
101321 As per Table 21 above, the required amount of TERSPERSE 2520
dispersant was
dissolved in a mixture comprising the remaining amount of SOLVES SO 150 and
YUBASE
3 , where the latter equated to an amount that would allow for a thidiazuron
concentration in
the vicinity of 15%. Thidiazuron technical and TIONA 625 were then dispersed
in the resultant
mixture under high-shear using a SILVERSON mixer. This was continued until a
homogeneous
mixture was afforded.
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
[0133] This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopic approximation.
101341 To the required amount of pre-mixed carrier, the millbase concentrate
was slowly added
followed by the remaining quantity of YUBASE 3. Stirring was continued until a
homogeneous suspension was afforded. This composition was shown to display
further
improved stability behaviour. This is another example of the methodology of
adding rheology
modifiers post-comminution.
101351 It is anticipated that the methods and compositions improving upon
EXAMPLE 16 will
also further improve the stability characteristics of EXAMPLE 17.
EXAMPLE 22¨ Alternative to Example 16.
Table 22
Components g/L
Thidiazuron 100.00
TERSPERSE" 4890 dispersant 20.00
TERM U LR 3201 emulsifier 100.00
NAN SA " EVM 70/2E 25.00
surfactant
T ERIC 13A7 emulsifier 37.50
AEROSIL' 200 4.21
BENTONER SD-1 14.21
SOLVESSOR 150 19.39
YUBASE 3 To volume.
41
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
[0136] A pre-mix carrier was prepared via the dispersion of 3.90g BENTONE SD-1
(ELEMENTIS) in 47.83g of YUBASE 3" via a SILVERSON high-shear mixer, yielding
a very
fine suspension. To the resultant homogeneous dispersion, 2.57g SOLVESSO 150
was added
with continued shear until a gel-like consistency was afforded. 27.41g
TERMULED 3201, 6.85g
NANSA EVM70/2E, and 10.28g TERIC 13A7 were then added to the mixture
followed
by low-speed mixing to homogenise. Lastly, 1.15g AEROSIL' 200 was added slowly
under
low-shear mixing until a homogeneous, weakly gelled mixture was afforded. The
mixture was
set aside.
[0137] As per Table 22 above, the required amount of TERSPERSE' 4890
dispersant was
dissolved in a mixture comprising the remaining amount of SOLVESSOR 150 and
YUBASE
3-", where the latter equated to an amount that would allow for a thidiazuron
concentration in
the vicinity of 15%. Thidiazuron technical was then dispersed in the resultant
mixture under
high-shear using a SILVERSON mixer. This was continued until a homogeneous
mixture was
afforded.
[0138] This slurry was then comminuted using a horizontal mill (Engineered
Mills, Inc.). The
process involved slowly feeding the concentrate into the milling apparatus
operating at a low
rotational speed of from 500 to 1000 RPM, wherein the mill grinding chamber
had been pre-
loaded with from 1 to 1.6mm diameter glass, or more preferably, zirconium
silica media from
60 to 80% of total volume capacity, and the jacketed coolant temperature was
pre-set and
maintained at an externally controlled temperature of from 15 to 25 C.
Rotational speed was
slowly increased from 2000 to 2500 RPM for a period of from 30 to 45 minutes,
producing a
concentrate with an average (d0.5) particle size of roughly 5 gm, determined
by approximation
using a 0 to 100 Grind Gauge, or a 8 to 0 Hegman Gauge. Particle size analysis
was additionally
determined by microscopic approximation.
[0139] To the required amount of pre-mixed carrier the millbase concentrate
was slowly added
followed by the remaining quantity of YUBASE 3 . Stirring was continued until
a
homogeneous suspension was afforded. Despite the improved dispersion
characteristics of the
millbase concentrate (EXAMPLE 10), this composition was shown to display a
significant
reduction in stability behaviour. This is another example of the methodology
of adding rheology
modifiers post-comminution.
EXAMPLE 23 ¨ Formulation Stability.
42
CA 03017030 2018-09-07
WO 2018/090072
PCT/AU2017/000216
101401 The critical measure of stability for the aforementioned oil-based
suspension co-
formulations of thidiazuron, according to the present invention, were simple
visual observations
of samples stored at ambient, room temperature over an extended period.
Table 23
Formulation Days after Stability Observations Stored at Ambient
Stability
preparation Temperature, Days after Preparation. Rating
EXAMPLE 12 35 Thickened suspension, poor flowability. Fail
EXAMPLE 13 19 Thickened suspension, poor flowability. Fail
EXAMPLE 14 54 Partly flowable suspension Fail
EXAMPLE 15 48 Thickened suspension Fail
EXAMPLE 16 48 Partly flowable suspension Fail
EXAMPLE 17 98 Flowable suspension Pass
EXAMPLE 18 69 Flowable suspension Pass
EXAMPLE 19 56 Flowable suspension Pass
EXAMPLE 20 97 Flowable suspension Pass
EXAMPLE 21 80 Flowable suspension Pass
EXAMPLE 22 8 Thickened, barely flowable suspension Fail
EXAMPLE 24.1
Table 24.1: Mean Percentage Defoliation, Locale A
4 7 14
Treatment Rate Rate
DAT DAT DAT
mL
g ai/ha
product/ha
1. Untreated Control 0.4 6.5 19.9
2. EXAMPLE 1 + D-C TRON* 150 + 1000 75' + 827' 8.0
26.7 52.5
-
3. EXAMPLE 1 + D-C TRON* 200 + 1000 100 a 827' 6.9
43.6 68.7
4. EXAMPLE 2 + D-C TRON* 250 + 1000 30" +15b
+ 827' 6.3 32.4 58.8
5. EXAMPLE 2 + D-C TRON* 400 + 1000 48" + 24b
+ 827' 12.5 45.7 74.2
6. EXAMPLE 2 + D-C TRON* 800 + 1000 96" + 48b
+ 827' 12.4 41.6 67.2
43
CA 03017030 2018-09-07
WO 2018/090072
PCT/AU2017/000216
7. EXAMPLE 3 _1000
30' + 15b + 675c 12.8 41.9 62.3
8. EXAMPLE 3 1600
48' + 246 + 1080c 13.1 42.5 71.8
9. EXAMPLE 3 3200
96 + 486 + 2160c 22.0 52.9 79.9
10. EXAMPLE 4 _500
30' + 15b + 330c 24.4 37.9 60.7
11. EXAMPLE 4 _800
48" + 24b + 528c 28.9 52.0 71.4
12. EXAMPLE 4 1600
96" + 48b + 1056c 36.9 61.6 72.5
13. EXAMPLES 750 75' +
488c 12.1 43.7 64.5
14. EXAMPLES 1000 100' +
650c 45.8 48.9 68.2
a = rate g ai/ha of thidiazuron
b = rate g ai/ha of diuron
c = rate g ai/ha of oil
* DC-TRON is a spray oil which contains 827g/L petroleum oil and between 80-
100g/L of
emulsifying agent/s.
EXAMPLE 24.2
Table 24.2: Mean Percentage Defoliation, Locale B
Treatment Rate Rate 8 DAT
14 DAT
mL product/ha g ai/ha
1. Untreated 26.2 40.5
2. EXAMPLE 1 + D-C TRON* 150+ 1000 75' + 827c 29.2
50.6
3. EXAMPLE 1 + D-C TRON* 200 + 1000 100' + 827` 31.3
65.6
4. EXAMPLE 2 + D-C TRON' 250 + 1000 30" +15b + 827c 30.6
56.7
5. EXAMPLE 2 + D-C TRON* 400 + 1000 48' + 24b + 827c
31.2 _62.7
6. EXAMPLE 2 + D-C TRON* 800 + 1000 96a 48b 827c
30.6 _62.4
7. EXAMPLE 3 1000 30' + b+
675c 35.8 66.5
48' +
8. EXAMPLE 3 1600 80 46.3
77.8
10c24b +
96a
9. EXAMPLE 3 3200 60 46.5
67.2
21c48b
10. EXAMPLE 4 500 30" + 15b
+ 330c 36.7 70.4
11. EXAMPLE 4 800 48' + 24b
+ 528c 40.1 71.9
96' +
12. EXAMPLE 4 1600 48b + 48.5
73.4
1056c
13. EXAMPLE 5 750 75a 488c 29.6
65.7
14. EXAMPLE 5 1000 100' + 650c 27.1
62.7
a = rate g ai/ha of thidiazuron
b = rate g ai/ha of diuron
c = rate g ai/ha of oil
44
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
* DC-TRON is a spray oil which contains 827g/L petroleum oil and between 80-
100g/L of
emulsifying a gent/s.
RESULTS
10141 The results obtained following the use of the co-formulations of the
present invention
and their efficacy are as illustrated above. In all cases, synergy is
demonstrated and shown by
the retention or improvement in defoliation performance, when compared to the
use of
formulations of the prior art, as is shown in Examples 24.1 and 24.2 above and
despite
reductions in total agrochemical inputs.
[0142] In Tables 24.1 and 24.2 above, when assessing the synergism of the co-
formulation of
thidiazuron, or thidiazuron and diuron, with an oil, the skilled person must
be conscious of
application rates.
[0143] In this particular instance, it is suggested that the synergism
afforded by the preparation
of a formulation described in EXAMPLES 3 to 5, when used at a given rate, is
shown by
defoliation performance, which is equivalent to or better than formulations of
thidiazuron, or
thidiazuron and diuron, as described by EXAMPLES 1 and 2, applied at
equivalent rates based
on active ingredientper hectare, and a spray oil, applied at a typical rate,
individually. It is likely
that there is an enhanced effect achieved by co-formulating thidiazuron, or
thidiazuron and
diuron, with an oil, whereby essentially equivalent or better performance is
achieved through
significantly reduced agrochemical input. Alternatively, the enhanced effect
may elicit the use
of less formulation, or that thidiazuron, or thidiazuron and diuron, is
displaying improved
defoliation efficiency facilitated by co-formulation with oil.
[0144] EXAMPLE 24.1, as illustrated in Table 24.1 above, highlights the
synergistic effects
afforded by co-formulation of an oil with either a plant growth regulator on
its own, or with a
mixture of a plant growth regulator and an herbicide, in comparison to the
customary use of a
plant growth regulator or a mixture of a plant growth regulator and an
herbicide in combination
with a formulated spraying oil.
[0145] With respect to the co-formulation of an oil and a plant growth
regulator used on its
own, the synergism is best represented by Treatment 13 in Table 24.1, where
thidiazuron is
applied as an oil-based co-formulation or OD formulation. At 4, 7 and 14 days
after treatment
("DAT"), this treatment consistently shows statistically improved defoliation
when compared
to Treatment 2, where thidiazuron is applied alongside a spraying oil
formulation in a customary
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
fashion. These results have been achieved through a 34.8% reduction in total
agrochemical
input per hectare. Additionally, Treatment 13 may be viewed as comparable to
Treatment 3 in
terms of defoliation performance, whereby the total agrochemical input and
biologically active
defoliant applied per hectare have been reduced by 37.5% and 25%,
respectively.
[0146] With respect to the co-formulation of an oil and a mixture of plant
growth regulator and
an herbicide, the synergism is best represented by Treatment 10 in Table 24.1.
At 4, 7 and 14
DAT, this treatment consistently shows improved defoliation performance when
compared to
Treatment 4 in Table 24.1, which uses a spray oil formulation and using the
equivalent plant
growth regulator and herbicide rates. These results have been achieved through
a 60% reduction
in total agrochemical input per hectare.
[0147] Example 24.2, as illustrated in Table 24.2 above, highlights the
synergistic effects
afforded by co-formulation of an oil with a plant growth regulator, or with a
mixture of a plant
growth regulator and an herbicide, in comparison to the use of a traditional
plant growth
regulator or a mixture of plant growth regulator and herbicide in combination
with a spray oil
as shown in Examples 1 and 2.
[0148] With respect to the co-formulation of an oil with a plant growth
regulator on its own,
synergism is again best represented by Treatment 13 in Table 24.2. At 8 days
after treatment
("DAT"), this treatment shows statistically equivalent defoliation when
compared to Treatment
2 in Table 24.2. At 14 DAT, Treatment 13 shows statistically improved
defoliation
performance when compared to Treatment 2. These results have been achieved
through a 34.8%
reduction in the total agrochemical input per hectare. Again, Treatment 13 may
even be viewed
as comparable to Treatment 3 in terms of defoliation performance, whereby the
total
agrochemical input and biologically active defoliant appliedper hectare have
been reduced by
37.5% and 25%, respectively.
[0149] With respect to the co-formulation of an oil and a mixture of a plant
growth regulator
and an herbicide, the synergism is best represented by Treatment 10 in Table
24.2. At 8 DAT,
this treatment shows marginally improved defoliation performance when compared
to
Treatment 4. At 14 DAT, Treatment 10 is statistically superior. These results
have been
achieved through a 60% reduction in total agrochemical input per hectare.
[0150] Further advantages and improvements may very well be made to the
present
invention without deviating from its scope. Although the invention has been
shown and
described in what is conceived to be the most practical and preferred
embodiment, it is
recognized that departures may be made therefrom within the scope and spirit
of the
invention, which is not to be limited to the details disclosed herein, but is
to be accorded the
46
CA 03017030 2018-09-07
WO 2018/090072 PCT/AU2017/000216
full scope of the claims, so as to embrace any and all equivalent devices and
apparatus. Any
discussion of the prior art throughout the specification should in no way be
considered as an
admission that such prior art is widely known or forms part of the common
general
knowledge in this field.
101511 Where the terms "comprise", "comprises", "comprised" or "comprising" or
the terms
"include", "includes", "included" or "including" are used in this
specification, they are to be
interpreted as specifying the presence of the stated features, integers, steps
or components
referred to, but not to preclude the presence or addition of one or more other
feature, integer,
step, component or group thereof.
47