Note: Descriptions are shown in the official language in which they were submitted.
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
URANIUM RECOVERY
[0001] The process of extracting uranium from ore often involves leaching
the ore with
sulfuric acid to produce an acid leach solution. Often, the acid leach
solution is then passed
through an ion exchange resin (for example a strong base anion exchange
resin). The
uranium is thought to become loaded onto the resin in the form of the
lUO2(SO4)314- complex
anion. Often the uranium is removed from the resin by eluting with sulfuric
acid. The
sulfuric acid eluate produced in this elution is an acidic aqueous solution
that contains
sulfuric acid, uranium, and impurities such as iron.
[0002] Once this eluate is produced, the problem remains of how to recover
the uranium
from the sulfuric acid eluate and convert the uranium into a useful form such
as, for example,
sodium diuranate (SDU) or ammonium diuranate (ADU). In the past, a very common
method of solving this problem was a solvent extraction (SX) process performed
on the
sulfuric acid eluate, followed by a stripping step to remove uranium from the
solvent,
followed by treatment of the strip solution with aqueous sodium hydroxide or
aqueous
ammonium hydroxide to produce SDU or ADU. However, the use of SX to recover
uranium
from the sulfuric acid eluates has drawbacks such as solvent degradation,
organic losses, and
the use of flammable solvents.
[0003] It is desired to find a process that does not require treatment with
solvents for
recovering the uranium from the sulfuric acid eluate. WO 2015/135017 describes
a process
in which uranium is recovered from a Pregnant Strip Solution ("PSS",
optionally produced by
loading a leach solution onto an ion exchange resin and subsequently eluting
with sulfuric
acid) by loading the PSS onto a separate ion exchange resin that is a
chelating resin that
contains an amino phosphonic or phosphonic/sulfonic functional groups.
[0004] It is desired to provide a process for recovering uranium from
sulfuric acid eluate
that does not require solvent extraction or the use of chelating ion exchange
resins.
[0005] The following is a statement of the invention.
[0006] A first aspect of the present invention is a process for recovering
uranium
comprising
(a) bringing a solution (I) into contact with a resin (I) to produce a mixture
of a solution
(II) and a resin (II), wherein the solution (I) is an aqueous solution that
comprises 30
to 200 g/L sulfuric acid and that comprises 1 g/L to 50 g/L uranium, and
wherein
the resin (I) is a strong acid cation exchange resin, and
(b) separating the solution (II) from the resin (II).
1
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
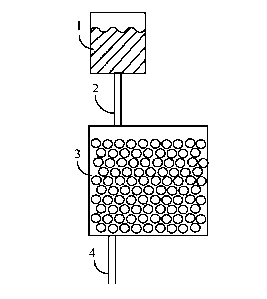
[0007] The following is a brief description of the drawings. Fig. 1 shows a
an
embodiment in which solution (I) passes through a bed to resin (I) to produce
solution (II)
and resin (II). Fig. 2 shows an embodiment in which further features have been
added to the
embodiment shown in Fig. 1; the further features added in Fig. 2 provide one
method of
recovering uranium from resin (II). Fig. 3 shows an embodiment in which
further features
have been added to the embodiment shown in Fig. 1; the further features added
in Fig. 3
provide a second method of recovering uranium from resin (II). Figure 4 shows
a flow sheet
for and embodiment of Option A as defined below. Figure 5 shows a flow sheet
for an
embodiment of Option B as defined below. Figure 6 shows an embodiment of
Option B that
incorporates a scrubbing step. Figure 7 shows uranium loading on a given
strong acid cation
exchange resin from various solutions containing different concentrations of
sulfuric acid and
uranium. Figure 8 shows uranium loading for various resins at different
uranium
concentrations at a given concentration of sulfuric acid.
[0008] The following is a detailed description of the invention.
[0009] As used herein, the following terms have the designated definitions,
unless the
context clearly indicates otherwise.
[0010] As used herein, an aqueous solution is a solution of one or more
compound
dissolved in a solvent, where the solvent contains water, and where the
solution contains 30%
or more water by weight.
[0011] "Resin" as used herein is a synonym for "polymer." A "polymer," as
used herein
is a relatively large molecule made up of the reaction products of smaller
chemical repeat
units. Polymers may have structures that are linear, branched, star shaped,
looped,
hyperbranched, crosslinked, or a combination thereof; polymers may have a
single type of
repeat unit ("homopolymers") or they may have more than one type of repeat
unit
("copolymers"). Copolymers may have the various types of repeat units arranged
randomly,
in sequence, in blocks, in other arrangements, or in any mixture or
combination thereof.
Polymers have weight-average molecular weight of 2,000 or more.
[0012] Molecules that can react with each other to form the repeat units of
a polymer are
known herein as "monomers." The repeat units so formed are known herein as
"polymerized
units" of the monomer.
[0013] Vinyl monomers have a non-aromatic carbon-carbon double bond that is
capable
of participating in a free-radical polymerization process. Vinyl monomers have
molecular
weight of less than 2,000. Vinyl monomers include, for example, styrene,
substituted
styrenes, dienes, ethylene, ethylene derivatives, and mixtures thereof.
Ethylene derivatives
2
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
include, for example, unsubstituted and substituted versions of the following:
vinyl acetate
and acrylic monomers. Acrylic monomers are monomers selected from substituted
and
unsubstituted (meth)acrylonitrile, (meth)acrylic acid, alkyl esters of
(meth)acrylic acid,
amides of (meth)acrylic acid, vinyl chloride, halogenated alkenes, and
mixtures thereof. As
used herein, the prefix "(meth)acryl-" means either acryl- or methacryl-.
"Substituted" means
having at least one attached chemical group such as, for example, alkyl group,
alkenyl group,
vinyl group, hydroxyl group, alkoxy group, carboxylic acid group, other
functional groups,
and combinations thereof.
[0014] As used herein, vinyl aromatic monomers are vinyl monomers that
contain one or
more aromatic ring.
[0015] A monovinyl monomer is a vinyl monomer that has exactly one non-
aromatic
carbon-carbon double bond per molecule. A multivinyl monomer is a vinyl
monomer that
has two or more non-aromatic carbon-carbon double bonds per molecule.
[0016] Vinyl monomers are considered to form polymers through a process of
vinyl
polymerization, in which the carbon-carbon double bonds react with each other
to form a
polymer chain.
[0017] Another type of polymers are those formed by condensation reactions
between
monomers. For example, phenol and formaldehyde react with each other to form
"phenolic
resins" or "formophenolic resins."
[0018] A polymer in which 90% or more of the polymerized units, by weight
based on
the weight of the polymer, are polymerized units of one or more vinyl monomers
is a vinyl
polymer. A vinyl aromatic polymer is a polymer in which 50% or more of the
polymerized
units, by weight based on the weight of the polymer, are polymerized units of
one or more
vinyl aromatic monomer.
[0019] A resin is considered herein to be crosslinked if the polymer chain
has sufficient
branch points to render the polymer not soluble in any solvent. When it is
said herein that a
polymer is not soluble in a solvent, it means that less than 0.1 gram of the
resin will dissolve
in 100 grams of the solvent at 25 C.
[0020] A resin is considered herein to be a strong acid cation exchange
resin (SAC resin)
if 50 mole% or more of the polymerized units contain one or more sulfonate
group. The
sulfonate group may be attached to the monomer prior to polymerization or may
be added to
the polymerized unit after polymerization. The sulfonate group may be in
protonated form,
in a neutralized form involving one or more cations other than 1-1 , in ionic
form, or in a
3
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
mixture thereof. An SAC resin is said herein to be in "protonated form" if 90
mole % or
more of the sulfonate groups attached to the resin are in protonated form.
[0021] A resin is considered herein to be a strong base anion exchange
resin (SBA resin)
if 50 mole% or more of the polymerized units contain one or more quaternary
ammonium
group. The quaternary ammonium group may be attached to the monomer prior to
polymerization or may be added to the polymerized unit after polymerization.
The
quaternary ammonium group may be in hydroxide form, in a neutralized form
involving one
or more anions other than Off, in ionic form, or in a mixture thereof.
[0022] A resin is considered herein to be a weak base anion exchange resin
if 50 mole%
or more of the polymerized units contain one or more amine group. The amine
groups may
be primary, secondary, or tertiary, or a combination thereof. The amine group
may be
attached to the monomer prior to polymerization or may be added to the
polymerized unit
after polymerization. The amine group may be free base form, or in a
protonated form (i.e.,
an ammonium group) involving one or more anions or a mixture thereof.
[0023] A collection of particles is characterized by the diameters of the
particles. If a
particle is not spherical, the diameter of the particle is considered to be
the diameter of a
particle having the same volume as the particle. A collection of particles is
characterized
herein by the volume-average diameter of the collection.
[0024] Resins may be characterized by the average pore diameter, which is
measured by
the BET method. As used herein, a "gel" resin has average pore diameter of 10
nm or less.
As used herein, a "macroporous" resin has average pore diameter of greater
than 10 nm.
[0025] As used herein, "sulfuric acid" refers to pure H2504, or to a
mixture of H2504 and
water, or to a mixture of H2504 and sulfur trioxide, or to a mixture of H2504,
water, and
sulfur trioxide.
[0026] When it is stated herein that a solution contains a particular
dissolved ionic
species, it is to be understood that the solution may or may not contain one
or more ionic
species of the same charge as the particular ionic species, and it is to be
understood that the
solution will contain sufficient ionic species of the charge opposite to the
particular ionic
species in order to achieve balance of electrical charges.
[0027] It is to be understood herein that a statement that a solution
contains a particular
dissolved compound means that that particular compound dissolves in the
solution in the
normal way, whether that particular compound dissolves in the form of a
complete neutral
molecule or whether that particular compound dissolves by forming one or more
cation and
one or more anion, each of which dissolves separately.
4
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
[0028] As used herein, an organic solvent is a compound that contains
carbon atoms and
that is liquid over a temperature range that includes 15 C to 25 C.
[0029] When a ratio is said herein to be X:1 or greater, it is meant that
the ratio is Y:1,
where Y is greater than or equal to X. For example, if a ratio is said to be
3:1 or greater, that
ratio may be 3:1 or 5:1 or 100:1 but may not be 2:1. Similarly, when a ratio
is said herein to
be W:1 or less, it is meant that the ratio is Z:1, where Z is less than or
equal to W. For
example, if a ratio is said to be 15:1 or less, that ratio may be 15:1 or 10:1
or 0.1:1 but may
not be 20:1.
[0030] The process of the present invention involves the use of solution
(I). The solution
(I) may be formed by any process. Preferably, solution (I) is formed as
follows: leaching
uranium ore with sulfuric acid to produce an acid leach solution; then passing
the acid leach
solution through a strong base anion exchange resin to capture R502(504)314-
anions onto the
resin; then removing the uranium from the resin by eluting with sulfuric acid
to produce an
eluate that contains dissolved sulfuric acid (H2504) and dissolved U022+
cations. The eluate
may optionally be diluted with water prior to further use. The eluate or the
diluted eluate is
solution (I). Preferably the eluate is diluted prior to use as solution (I).
Preferably, the ratio
of dilution water to eluate is, by weight, 0.4:1 or more; more preferably
0.6:1 or more; more
preferably 0.8:1 or more. Preferably, the ratio of dilution water to eluate
is, by weight, 8:1 or
less; more preferably 6:1 or less; more preferably 4:1 or less.
[0031] Solution (I) is an aqueous solution that contains uranium and
dissolved sulfuric
acid. Preferably, the concentration of uranium in solution (I), as elemental
uranium, is
preferably 1 g/L or more; more preferably 2 g/L or more. Preferably, the
concentration of
uranium in solution (I), as elemental uranium, is 50 g/L or less; more
preferably 20 g/L or
less; more preferably 10 g/L or less. Preferably, solution (I) contains
dissolved sulfuric acid
in an amount of 30 g/L or more; more preferably 40 g/L or more. Preferably,
solution (I)
contains dissolved sulfuric acid in an amount of 200 g/L or less; more
preferably 100 g/L or
less. Preferably the pH of solution (I) is 2 or less.
[0032] In the process of the present invention, solution (I) is brought
into contact with
resin (I). Resin (I) is a strong acid cation exchange resin. The mole percent
of polymerized
units of resin (I) that contains one or more sulfonate groups is 50% or more;
preferably 60%
or more; more preferably 70% or more; more preferably 80% or more; more
preferably 90%
or more. Preferably, The mole percent of polymerized units of resin (I) that
contains one or
more nitrogen-containing groups is 5% or less; more preferably 2% or less;
more preferably
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
1% or less; more preferably zero. Preferably, The mole percent of polymerized
units of resin
(I) that contains one or more phosphorous-containing groups is 5% or less;
more preferably
2% or less; more preferably 1% or less; more preferably zero. Preferably, The
mole percent
of polymerized units of resin (I) that contains one or more carboxyl groups is
5% or less;
more preferably 2% or less; more preferably 1% or less; more preferably zero.
[0033] Some examples of commercial resins that are suitable as resin (I)
are
AMBERJETTm 1600H, AMBERLITETm 200, AMBERSEPTm 200, AMBERLYSTTm 35 Wet,
and AMBERLYSTTm 40 Wet; among these three resins, AMBERLYSTTm 35 Wet is
preferred.
[0034] Preferably, resin (I) is a vinyl aromatic polymer. Preferred vinyl
aromatic
monomers are styrene and divinyl benzene. Preferably, the amount of
polymerized units of
one or more vinyl aromatic monomer is, by weight based on the weight of the
polymer, 75%
or more; more preferably 90% or more; more preferably 95% or more. Preferably,
resin (I)
contains polymerized units of one or more multivinyl monomer. Preferably, the
amount of
polymerized units multivinyl monomer is, by weight based on the weight of
resin (I), 2% or
more; more preferably 4% or more; more preferably 8% or more; more preferably
10% or
more; more preferably 12% or more; more preferably 14% or more. Preferably,
the amount
of polymerized units multivinyl monomer is, by weight based on the weight of
resin (I), 30%
or less; more preferably 25% or less. Preferably, resin (I) is made by a
process that includes
polymerizing a monomer or mixture of monomers that contains one or more
monomers that
are vinyl aromatic monomers that contain only carbon and hydrogen atoms, and
then, after
completion of the polymerization, performing one or more chemical reactions to
attach one or
more sulfonate groups to the aromatic rings in the polymer.
[0035] Preferably the resin (I) is in the form of a collection of
particles. Preferably the
particles contain crosslinked polymer. Preferably the volume-average diameter
of the
collection of particles is 50 um or more; more preferably 100 um or more.
Preferably the
volume-average diameter of the collection of particles is 1,000 um or less.
[0036] Preferably, before resin (I) is brought into contact with solution
(I), the amount of
uranium in any form, characterized as grams of elemental uranium per liter of
resin, in resin
(I) is 5 g/L or less; more preferably 1 g/L or less; more preferably 0.2 g/L
or less.
[0037] Preferably, before resin (I) is brought into contact with solution
(I), resin (I) is in
protonated form.
[0038] While the present invention is not limited to any particular theory,
it is
contemplated that when solution (I) is brought into contact with resin (I),
some or all of the
U022+ cations in solution (I) will become resident on resin (I), associated
with the sulfonate
6
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
anions attached to the resin (I). The ion exchange reaction of loading uranium
on the SAC
resin is believed to be the following:
2 R-S03-1-1+ + U022+ <=> (R-503-)2 U022+ + 2H+
where R is the resin matrix.
[0039] Solution (I) and resin (I) are brought into contact with each other
to make a
mixture. It is contemplated that some alterations in the compositions of
solution (I) and resin
(I) will take place, for example by transfer of U022+ cations from solution
(I) to resin (I).
When the mixture is separated into a liquid portion and a solid portion, the
liquid portion will
be the altered solution (I), now labeled solution (II); and the solid portion
will be the altered
resin (I), now labeled resin (II).
[0040] It is noted that resin (I) and resin (II) normally contain some
water. Each of resin
(I) and resin (II) each independently preferably contains water in an amount,
by weight based
on the total weight of the resin, 1% to 60%.
[0041] The steps of bringing solution (I) into contact with resin (I) and
then separating
solution (II) from resin (II) may be accomplished by any method. A preferred
method is to
provide a fixed bed of particles of resin (I) and then pass solution (I)
through the fixed bed of
particles of resin (I). The solution that exits from the fixed bed will be
solution (II).
Preferably the ratio of the concentration of uranium in solution (I) to the
concentration of
uranium in solution (II) is 10:1 or more; more preferably 50:1 or more.
Preferably, the
process of passing solution (I) through the fixed bed of resin (I) is
continued until the time
when the uranium concentration in solution (II) begins to rise, for example
until the ratio of
the concentration of uranium in solution (I) to the concentration of uranium
in solution (II)
falls below 10:1. At that time, the flow of solution (I) is preferably halted.
At that time, resin
(II) is considered to be "loaded" with U022+.
[0042] Preferably, the amount of dissolved compounds in solution (II) other
than H2504
is, by weight based on the weight of solution (II), 5% or less; more
preferably 2% or less;
more preferably 1% or less. In some embodiments, solution (II) may be used as
a source of
sulfuric acid, for example as the sulfuric acid that is mixed with uranium ore
to produce an
acid leach solution.
[0043] Uranium may be recovered from resin (II) by any method It is
preferred to
recover uranium from resin (II) and convert the uranium to the form of a
precipitated
diuranate salt. Two preferred methods are herein called "Option A" and "Option
B.
[0044] The following is a description of Option A.
7
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
[0045] Solution (III) is brought into contact with resin (II) to form a
mixture. Solution
(III) is an aqueous solution that contains dissolved HC1. Preferably the
amount of HC1
dissolved in solution (III) is, by weight based on the weight of solution
(III), 10% or more;
more preferably 15% or more. Preferably the amount of HC1 dissolved in
solution (III) is,
by weight based on the weight of solution (III), 30% or less; more preferably
20% or less.
Preferably, the total amount of solutes in solution (III) other than HC1 is,
by weight based on
the weight of solution (III), 5% or less; more preferably 2% or less; more
preferably 1% or
less.
[0046] While the present invention is not limited to any particular theory,
it is
contemplated that when solution (III) is brought into contact with resin (II),
some or all of the
U022+ in resin (II) will become converted to UO2C13- and will become dissolved
in the water
that is present.
[0047] Solution (III) and resin(II) are brought into contact with each
other to make a
mixture. It is contemplated that some alterations in the compositions of
solution (III) and
resin (II) will take place, for example by conversion of uranium from U022+
cations resident
on resin (II) to UO2C13" dissolved in the water that is present. When the
mixture is separated
into a liquid portion and a solid portion, the liquid portion will be the
altered solution (III),
now labeled solution (IV); and the solid portion will be the altered resin
(II), now labeled
resin (III). It is contemplated that solution (IV) will contain dissolved
UO2C13- anions.
[0048] The steps of bringing solution (III) into contact with resin (II)
and then separating
solution (IV) from resin (III) may be accomplished by any method. A preferred
method is to
provide a fixed bed of particles of resin (II) and then pass solution (III)
through the fixed bed
of particles of resin (II). The solution that exits from the fixed bed will be
solution (IV).
Preferably, the previous step of mixing solution (I) with resin (I) is
performed by passing
solution (I) through a fixed bed of resin (I); then, preferably, the resulting
resin (II) stays in
the same fixed bed, and solution (III) is then passed through the fixed bed of
resin (II).
Preferably, the process of passing solution (III) through the fixed bed of
resin (II) is
continued until the time when the uranium concentration in solution (II)
begins to fall. For
example, the instantaneous concentration of uranium may be measured as a
function of time
as solution (IV) exits the fixed bed, and the maximum concentration may be
noted. The time
may be noted when the ratio of the instantaneous concentration of uranium in
solution (IV) as
it exits the fixed bed to the maximum concentration is 0.1:1 or lower. At that
time, the flow
of solution (III) is preferably halted. At that time, resin (II) is considered
to be depleted of
8
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
U022+, and the depleted resin (II) is known herein as resin (III). It is
contemplated that
solution (IV) contains dissolved UO2C13- ions.
[0049] Preferably, the amount of uranium, as elemental uranium, in resin
(III) is 5 gram
per liter of resin (g/L) or less; more preferably 1 g/L or less; more
preferably 0.2 g/L or less.
Preferably, resin (III) could be used as resin (I) in a subsequent performance
of the process of
the present invention.
[0050] In further steps of Option A, solution (IV) is brought into contact
with resin (IV).
Resin (IV) is a strong base anion exchange resin or a weak base anion exchange
resin,
preferably a strong base anion exchange resin. When a strong base anion
exchange resin is
used, the resin is preferably in chloride form. When a weak bas anion exchange
resin is used,
the resin is preferably in HC1 form. Preferably the resin (IV) is in the form
of a collection of
particles. Preferably the particles contain crosslinked polymer. Preferably
the volume-
average diameter of the collection of particles is 50 um or more; more
preferably 100 um or
more. Preferably the volume-average diameter of the collection of particles is
1,000 um or
less.
[0051] Resin (IV) is preferably a strong base anion exchange resin (SBA
resin). Resin
(IV) is preferably a gel type resin. Some examples of commercial resins that
are suitable as
resin (IV) are AMBERLITETm IRA400, AMBERLITETm IRA402, AMBERJETTm 4200,
AMBERJETTM 4400, AMBERSEPTm 400, DOWEXTM MARATHONTm A, and DOWEXTM
MONOSPHERETM 550A; among these six resins, preferred is AMBERLITETm IRA400.
[0052] Preferably, before resin (IV) is brought into contact with solution
(IV), the amount
of uranium in any form in resin (IV), characterized as grams of elemental
uranium per liter of
resin, is 1 g/L or less; more preferably 0.3 g/L or less; more preferably 0.1
g/L or less.
[0053] While the present invention is not limited to any particular theory,
it is
contemplated that when solution (IV) is brought into contact with resin (IV),
some or all of
the UO2C13- in solution (IV) will become resident on resin (IV), associated
with the
ammonium groups attached to the resin (IV).
[0054] Solution (IV) and resin (IV) are brought into contact with each
other to make a
mixture. It is contemplated that some alterations in the compositions of
solution (IV) and
resin (IV) will take place, for example by transfer of UO2C13- anions from
solution (IV) to
resin (IV). When the mixture is separated into a liquid portion and a solid
portion, the liquid
portion will be the altered solution (IV), now labeled solution (V); and the
solid portion will
be the altered resin (IV), now labeled resin (V). It is contemplated that
solution (V) contains
dissolved HC1. The ion exchange reaction is believed to be the following:
9
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
RI\T C1- + [UO2C131 - <=> RNI R502C131- + C1
[0055] where R represents the resin matrix together with the alkyl groups
on the
quaternary ammonium group.
[0056] It is noted that resin (IV) and resin (V) normally contain some
water. Each of
resin (IV) and resin (V) each independently preferably contains water in an
amount, by
weight based on the total weight of the resin, 1% to 60%.
[0057] The steps of bringing solution (IV) into contact with resin (IV) and
then
separating solution (V) from resin (V) may be accomplished by any method. A
preferred
method is to provide a fixed bed of particles of resin (IV) and then pass
solution (IV) through
the fixed bed of particles of resin (IV). The solution that exits from the
fixed bed will be
solution (V). Preferably the ratio of the concentration of uranium in solution
(IV) to the
concentration of uranium in solution (V) is 10:1 or more; more preferably 50:1
or more.
Preferably, the process of passing solution (IV) through the fixed bed of
resin (IV) is
continued until the time when the uranium concentration in solution (V) begins
to rise, for
example until the ratio of the concentration of uranium in solution (IV) to
the concentration
of uranium in solution (V) falls below 10:1. At that time, the flow of
solution (IV) is
preferably halted. At that time, resin (V) is considered to be "loaded" with
UO2C13-,
associated with the ammonium groups on resin (V).
[0058] Preferably, solution (V) is an aqueous solution that contains
dissolved HC1. The
preferred characteristics of solution (V) are the same as those of solution
(III), though the
characteristics of the two solutions may be chosen independently. In some
embodiments,
solution (V) is recycled and used as source for all or part of solution (III).
[0059] Preferably, solution (V) qualifies for use as solution (III).
[0060] It is contemplated that resin (V) contains uranium in the form of
UO2C13-. This
uranium may be removed from resin (V) by any method. A preferred method is to
bring
solution (VI) into contact to form a mixture. Preferably, solution (VI)
contains water in an
amount, by weight based on the weight of solution (VI), 95% or more; more
preferably 97%
or more; more preferably 99% or more.
[0061] While the present invention is not limited to any particular theory,
it is
contemplated that when solution (VI) is brought into contact with resin (V),
some or all of the
UO2C13- in resin (V) will become converted to UO2C12 that is dissolved in the
water that is
present. The ion exchange reaction is believed to be
RNI R502C131- <=> RI\T C1- + U022+ +2 Cl
-
where R represents the resin matrix together with the alkyl groups on the
ammonium group.
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
[0062] Solution (VI) and resin(V) are brought into contact with each other
to make a
mixture. It is contemplated that some alterations in the compositions of
solution (VI) and
resin (V) will take place, for example by conversion of uranium from UO2C13-
anions resident
on resin (V) to UO2C12 dissolved in the water that is present. When the
mixture is separated
into a liquid portion and a solid portion, the liquid portion will be the
altered solution (VI),
now labeled solution (VII); and the solid portion will be the altered resin
(V), now labeled
resin (VI). It is contemplated that solution (VII) contains dissolved UO2C12.
[0063] The steps of bringing solution (VI) into contact with resin (V) and
then separating
solution (VII) from resin (VI) may be accomplished by any method. A preferred
method is to
provide a fixed bed of particles of resin (V) and then pass solution (VI)
through the fixed bed
of particles of resin (V). The solution that exits from the fixed bed will be
solution (VII).
Preferably, the previous step of mixing solution (IV) with resin (IV) was
performed by
passing solution (IV) through a fixed bed of resin (IV); then, preferably, the
resulting resin
(V) stays in the same fixed bed, and solution (VI) is then passed through the
fixed bed of
resin (V). Preferably, the process of passing solution (VI) through the fixed
bed of resin (V)
is continued until the time when the uranium concentration in solution (II)
begins to fall. For
example, the instantaneous concentration of uranium may be measured as
solution (VII) exits
the fixed bed, and the maximum concentration may be noted. The time may be
noted when
the ratio of the instantaneous concentration of uranium in solution (VII) as
it exits the fixed
bed to the maximum concentration is 0.1:1 or lower. At that time, the flow of
solution (VI)
is preferably halted. At that time, resin (V) is considered to be depleted of
UO2C13-, and the
depleted resin (V) is known herein as resin (VI).
[0064] Preferably, the amount of uranium, as elemental uranium, in resin
(VI) is 1 gram
per liter of resin (g/L) or less; more preferably 0.3 g/L or less; more
preferably 0.1 g/L or
less. Preferably, resin (VI) could be used as resin (IV) in a subsequent
performance of the
Option A process of the present invention.
[0065] Preferably, solution (VII) is brought into contact with a hydroxide
to form a
mixture, and the corresponding diuranate salt precipitates. The diuranate salt
is considered to
be a useful form of uranium that is appropriate for various uses. Preferred
hydroxides are
sodium hydroxide and ammonium hydroxide, which produce precipitates of sodium
diuranate
(SDU) and ammonium diuranate (ADU), respectively.
[0066] The following is a summary of a preferred embodiment of Option A and
some of
the contemplated ion exchange reactions and expected benefits of performing
Option A.
Thus, the SAC resin is eluted with HC1, and the uranium is eluted as anionic
chloride
11
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
complex. This eluate passes through a strong or weak base anion exchange resin
in the Cl
-
form where uranium is fixed while HC1 comes out and is recovered. Uranium is
then eluted
from the anion exchanger with water. In this way only a small quantity of
chemicals is
consumed, which is the HC1 that is eluted along with the uranium in the water
elution step.
From the water eluate, uranium is recovered by precipitation with NaOH or
ammonia as SDU
or ADU. The flow sheet is shown in figure 4.
[0067] The following is a description of Option B, for removing uranium
from resin (II)
and converting the uranium to sodium diuranate.
[0068] In Option B, solution (X) is brought into contact with resin (II).
[0069] Solution (X) is brought into contact with resin (II) to form a
mixture. Solution (X)
is an aqueous solution that contains dissolved Na2SO4 or dissolved (NH4)2504;
preferably
dissolved Na2SO4. Preferably the amount of dissolved Na2SO4 or dissolved
(NH4)2504 in
solution (X) is, by weight based on the weight of solution (X), 1% or more;
more preferably
2% or more; more preferably 5% or more. Preferably the amount of dissolved
Na2SO4 or
dissolved (NH4)2504 in solution (X) is, by weight based on the weight of
solution (X), 25%
or less; more preferably 20% or less; more preferably 15% or less. Preferably,
the total
amount of solutes in solution (X) other than Na2SO4 or (NH4)2504, by weight
based on the
weight of solution (X), is 5% or less; more preferably 2% or less; more
preferably 1% or less.
[0070] While the present invention is not limited to any particular theory,
it is
contemplated that when solution (X) is brought into contact with resin (II),
some or all of the
U022+ in resin (II) will become converted to neutral complex or to an anionic
complex such
as (U02150413)4- and will become dissolved in the water that is present. It is
expected that
the SAC resin will not firmly fix the neutral or anionic complex of uranium.
It is expected
that uranium will elute first, followed by iron, thus achieving a first
separation of uranium
from iron.
[0071] Solution (X) and resin(II) are brought into contact with each other
to make a
mixture. It is contemplated that some alterations in the compositions of
solution (X) and
resin (II) will take place, for example by conversion of uranium from U022+
cations resident
on resin (II) to U025042- dissolved in the water that is present. When the
mixture is
separated into a liquid portion and a solid portion, the liquid portion will
be the altered
solution (X), now labeled solution (XI); and the solid portion will be the
altered resin (II),
now labeled resin (XI). It is contemplated that solution (XI) will contain
dissolved
1UO2(504)312-=
12
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
[0072] The steps of bringing solution (X) into contact with resin (II) and
then separating
solution (XI) from resin (XI) may be accomplished by any method. A preferred
method is to
provide a fixed bed of particles of resin (II) and then pass solution (X)
through the fixed bed
of particles of resin (II). The solution that exits from the fixed bed will be
solution (XI).
Preferably, the previous step of mixing solution (I) with resin (I) was
performed by passing
solution (I) through a fixed bed of resin (I); then, preferably, the resulting
resin (II) stays in
the same fixed bed, and solution (X) is then passed through the fixed bed of
resin (II).
Preferably, the process of passing solution (X) through the fixed bed of resin
(II) is continued
until the time when the uranium concentration in solution (XI) begins to fall.
For example,
the instantaneous concentration of uranium may be measured as solution (XI)
exits the fixed
bed, and the maximum concentration may be noted. The time may be noted when
the ratio of
the instantaneous concentration of uranium in solution (XI) as it exits the
fixed bed to the
maximum concentration is 0.1:1 or lower. At that time, the flow of solution
(X) is preferably
halted. At that time, resin (II) is considered to be depleted of U022+, and
the depleted resin
(II) is known herein as resin (XI). It is contemplated that solution (XI)
contains dissolved
Na2SO4 and dissolved R502(S0,)3l2 =
[0073] Preferably, the amount of uranium, as elemental uranium, in resin
(XI) is 1 gram
per liter of resin (g/L) or less; more preferably 0.3 g/L or less; more
preferably 0.1 g/L or
less. Preferably, resin (XI) could be used as resin (I) in a subsequent
performance of the
process of the present invention.
[0074] Preferably, solution (XI) is brought into contact with a hydroxide
salt to form a
mixture, and the corresponding diuranate salt precipitates. The diuranate salt
is considered to
be a useful form of uranium that is appropriate for various uses. Preferred
hydroxide salts are
sodium hydroxide and ammonium hydroxide, which produce, respectively,
precipitate of
sodium diuranate (SDU) and ammonium diuranate (ADU).
[0075] Optionally, after precipitation of diuranate salt, the remaining
liquid, which
contains dissolved Na2SO4, may be used as all or part of solution (X).
[0076] Optionally, resin (XI) could be subjected to an additional step in
order to remove
residual Na2SO4 that may be present.
[0077] Optionally, Solution (XII) is brought into contact with resin (XI)
to form a
mixture. Solution (XII) is an aqueous solution that contains dissolved H2504.
Preferably the
amount of dissolved H2504 in solution (XII) is, by weight based on the weight
of solution
(XII), 1% or more; more preferably 2% or more; more preferably 5% or more.
Preferably the
amount of dissolved H2504 in solution (XII) is, by weight based on the weight
of solution
13
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
(XII), 20% or less; more preferably 15% or less; more preferably 10% or less.
Preferably, the
total amount of solutes in solution (XII) other than H2SO4, by weight based on
the weight of
solution (XII), is 5% or less; more preferably 2% or less; more preferably 1%
or less.
[0078] Solution (XII) may contain a freshly prepared solution, or solution
(XII) may
contain material obtained from solution (II), or solution (XII) may contain a
mixture thereof.
[0079] While the present invention is not limited to any particular theory,
it is
contemplated that when solution (XII) is brought into contact with resin (XI),
some or all of
the Na + ions in resin (XI) will become dissolved in the water that is
present.
[0080] Solution (XII) and resin(XI) are brought into contact with each
other to make a
mixture. It is contemplated that some alterations in the compositions of
solution (XII) and
resin (XI) will take place, for example by transfer of Na + ions from resin
(XI) to becoming
dissolved in the water that is present. When the mixture is separated into a
liquid portion and
a solid portion, the liquid portion will be the altered solution (XII), now
labeled solution
(XIII); and the solid portion will be the altered resin (XI), now labeled
resin (XII). It is
contemplated that solution (XIII) will contain dissolved Na2SO4. It is further
contemplated
that resin (XII) is suitable for use as resin (I).
[0081] The steps of bringing solution (XII) into contact with resin (XI)
and then
separating solution (XIII) from resin (XII) may be accomplished by any method.
A preferred
method is to provide a fixed bed of particles of resin (XI) and then pass
solution (XII)
through the fixed bed of particles of resin (II). The solution that exits from
the fixed bed will
be solution (XIII). Preferably, the previous step of mixing solution (X) with
resin (II) was
performed by passing solution (X) through a fixed bed of resin (II); then,
preferably, the
resulting resin (XI) stays in the same fixed bed, and solution (XII) is then
passed through the
fixed bed of resin (XI). Preferably, the process of passing solution (XII)
through the fixed
bed of resin (XI) is continued until the time when the sodium concentration in
solution (XI)
begins to fall. For example, the instantaneous concentration of sodium may be
measured as
solution (XIII) exits the fixed bed, and the maximum concentration may be
noted. The time
may be noted when the ratio of the instantaneous concentration of sodium in
solution (XIII)
as it exits the fixed bed to the maximum concentration is 0.1:1 or lower. At
that time, the
flow of solution (XII) is preferably halted. At that time, resin (XI) is
considered to be
depleted of sodium, and the depleted resin (XI) is known herein as resin
(XII). It is
contemplated that solution (XIII) contains dissolved Na2SO4.
[0082] Preferably, solution (XIII) is an aqueous solution that contains
dissolved Na2SO4.
The preferred characteristics of solution (XIII) are the same as those of
solution (X), though
14
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
the characteristics of the two solutions may be chosen independently. In some
embodiments,
solution (XIII) is recycled and used as source for all or part of solution
(X).
[0083] In order to improve the efficiency of the process, a step can
optionally be included
where the resin is oversaturated with part of the solution (XI).
[0084] In the practice of the present invention, liquid solutions are
conveyed from one
location to another. In each case, the liquid solutions may be moved by the
force of gravity
or may be driven by one or more pumps. The liquid solutions may be conveyed
through
pipes or tubes of any shape of cross section or may be conveyed by any other
object capable
of conveying liquid from one location to another.
[0085] Some specific embodiments of the present invention are shown in the
Figures. In
Figure 1, a source 1 supplies solution (I). The source may be any vessel or
container.
Solution (I) passes through a pipe 2 into a container 3 that holds resin (I)
but allows liquid
solution to pass through, after making intimate contact with resin (I).
Solution (II) exits from
container 3 via pipe 4.
[0086] Figure 2 shows the same features as Figure 1, and Figure 2 also
shows the features
of an embodiment of Option A. After solution (I) has passed through container
3 for a time
until resin (I) is loaded, the flow of solution (I) is halted. Then, as shown
in Figure 2, the
flow of solution (III) is begun, from a source 5. The source may be any vessel
or container.
Solution (III) passes through a pipe 6 into container 3 that holds resin (II)
but allows liquid
solution to pass through. Solution (IV) exits from container 3 via pipe 7.
Solution (IV) then
enters container 8, which contains resin (IV). Solution (IV) passes over resin
(IV), and
solution (IV) becomes solution (V) and resin (IV) becomes resin (V). Solution
(V) exits
container 8 via pipe 9. Then the flow of solution (III) is halted, thus also
halting the flow of
solutions (IV) and (V). Then the flow of solution (VI) is begun, from a source
10. The
source may be any vessel or container. Solution (VI) passes through a pipe 11
into container
8. Solution (VI) exits from container 8 via pipe 12. Solution (VI) passes over
resin (V), and
solution (VI) becomes solution (VII), and resin (V) becomes resin (VI).
Solution (VII) exits
container 8 via pipe 12.
[0087] Figure 3 shows the same features as Figure 1, and Figure 3 also
shows the features
of an embodiment of Option B. After solution (I) has passed through container
3 for a time
until resin (I) is loaded, the flow of solution (I) is halted. Then, as shown
in Figure 3, the
flow of solution (X) is begun, from a source 13. The source may be any vessel
or container.
Solution (X) passes through a pipe 14 into container 3. Solution (X) passes
over resin (II),
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
and solution (X) becomse solution (XI) while resin (II) becomes resin (XI).
Solution (XI)
exits from container 3 via pipe 15.
[0088] Figure 3 also shows an optional further step in Option B, in which,
after the flow
of solution (X) is halted, the flow of solution (XII) is begun. An additional
source supplies
solution (XII) to container 3. The source may be any vessel or container.
Solution (XII)
passes through a pipe 17 into container 3. Solution (XII) passes over resin
(XI), and solution
(XII) becomes solution (XIII) while resin (XI) becomes resin (XII). Solution
(XIII) exits
from container 3 via pipe 18.
[0089] Figure 4 shows a flow sheet for an embodiment of Option A.
[0090] Figure 5 shows a flow sheet for an embodiment of Option B. In order
to minimize
the amount of chemicals consumed, scrubbing of the loaded resin can be done
with part of
the concentrated eluate (Figure 6) where the resin is overloaded/saturated
with uranium and
less with H+ which then can be recovered.
[0091] Preferably the process of the present invention does not involve the
use of any
organic solvent.
[0092] Regardless of the specific method used for removing uranium from
resin (II), it is
contemplated that several advantages arise because the method of the present
invention relies
upon the use of ion exchange resins and aqueous solutions, without the need
for the use of
organic solvent. One advantage is that the safety and environmental issues
associated with
organic solvents are avoided.
[0093] Second, compared to SX methods, relatively small amounts of
materials are
consumed in the process of the present invention. The resins, when saturated,
are capable of
being regenerated and then re-used. The various solutions that elute from the
resins can be
put to use within the process of the present invention or else can be recycled
for use in other
industrial processes. For example, solution (II) is expected to be a solution
of sulfuric acid in
water, and it is expected that that solution can be recycled to make use of
the sulfuric acid.
[0094] The following are examples of the present invention.
[0095] Example 1A Loading the SAC resin
[0096] A solution containing 42 g/L H2504 and 2.5 g U/L was allowed to pass
through a
commercial SAC resin (AMBERLYSTTm 35 WET, a macroporous strong acid cation
exchange resin from the Dow Chemical Company) in the IT form at 1 BV/h (BV =
volume
of solution / volume of resin) and ambient temperature. After 9 hours (after 9
BV), the
effluent concentration was <5 ppm U while the acid was at the feed solution
concentration.
The resin became saturated after 17 BY where the resin loading was 39 g U/LR
(39 g
16
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
uranium per liter of resin). The resin is considered to be saturated when the
ratio of the
concentration of uranium in the effluent to the concentration of uranium in
solution (I) is
0.95:1 or higher. This is the loading capacity of the head column in a three
column merry-
go-round configuration (two on loading and one on regeneration).
[0097] Example 1B: Option A
[0098] Elution was performed with a 20% HC1 solution at 1 BV/h. After 3 BY
more than
90% of uranium had been eluted. The eluate was then allowed to pass through a
commercial
SBA resin (AMBERLITETm IRA-400, a strong base anion exchange gel resin from
the Dow
Chemical Company) in Ci form at 1 BV/h. No uranium was detected in the
effluent. Then
the resin was eluted with water. After 2 BY of water more than 90% of the
uranium had been
eluted.
[0099] It is contemplated that the lost HC1 quantity could be reduced by
for example,
draining the resins before the water elution.
[0100] Example 2 (Option B)
[0101] An SAC resin was loaded with uranium as in example 1A. This resin
was then
eluted with a 10% Na2SO4 solution at 1 BV/h. After 4 BY, 90% of the uranium
had been
eluted from the resin. The resin was subsequently put back to the H form using
2 BY of 5%
H2SO4
[0102] It is contemplated that this quantity of H2504 lost could be
decreased by
oversaturating (scrubbing) the loaded resin with part of the H2504
concentrated eluate.
[0103] Example 3: Comparison of acid concentrations
[0104] Using AMBERLYSTTm 35Wet, the uranium loading capacity was measured
as
follows. An initial solution of uranium of uranium and sulfuric acid was
prepared and a
volume Vs of this solution was mixed with a given volume of resin YR. Uranium
concentration of the initial solution is labeled Uo and is reported below in
grams of uranium
per liter of solution. Three different concentrations of sulfuric acid were
used. When
equilibrium was established, the uranium concentration was determined and
labeled Uf. The
amount of uranium adsorbed on the resin was calculated by _Q=Vs*(Uo-Uf)/VR
where Q is
the equilibrium loading capacity of the resin. The equilibrium concentration
of uranium
adsorbed on the resin is reported in grams of uranium per liter of resin (g
U/LR). The results
are shown in Figure 7. The lowest concentration of sulfuric acid led to the
highest
equilibrium loading of uranium on the resin. This result demonstrates the
benefit of having
the optimum level of sulfuric acid dissolved in solution (I).
[0105] Example 4: Comparison of resins
17
CA 03017957 2018-09-14
WO 2017/209828
PCT/US2017/022399
[0106] The method of Example 3 was repeated, using 4% sulfuric acid and
three different
resins. The properties of the resins were as follows. "DVB" is the amount of
polymerized
units of divinylbenzene in weight % based on the weight of resin.
DVB
Resin Number type
(%)
R1 gel 16.0
R2 gel 8.4
R3 macroporous 16.5
[0107] The results are shown in Fig. 8. R3 shows the best uranium loading
capacity,
followed closely by R1, then followed by R2. This result demonstrates that, as
the DVB
level increases from 8.4% to 16.5%, the loading capacity also increases.
[0108] Overall, the operating capacities of the SAC resins were 20-50 g
U/LR (grams of
uranium per liter of resin).
18