Note: Descriptions are shown in the official language in which they were submitted.
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
COMBINATIONAL COMPOSITIONS AND METHODS OF USE THEREOF
FIELD OF THE DISCLOSURE
[01] The disclosure is directed towards the field of medicine and, more
specifically, the
use of a combination of compounds to treat disease and regulate cholesterol
levels.
BACKGROUND
[02] There is no cure for diseases and disorders based on autoimmune and
inflammatory
mechanisms. Existing therapies suppress the immune system to such an extent
that the
treatments expose patients to additional disorders or require additional
therapy for the side
effects of the first treatment. Many medicaments prescribed for a disease not
related to either
autoimmunity or inflammation can induce these conditions as a side effect.
There is a long
felt yet unmet need in the art for an efficacious treatment for autoimmune and
inflammatory
conditions that do not inhibit the ability of the immune system to function
but rather resets
the level of activity of the immune system back to a healthy homeostatic
level.
SUMMARY
[03] The compositions and methods of the disclosure reduce autoimmune and
inflammatory activity in a subject in need thereof by establishing an
immunological
homeostasis through the administration of a combination of a fatty acid and a
cholesterol
lowering compound (e.g. a statin).
[04] The disclosure provides a pharmaceutical composition for the treatment of
a disease
or disorder comprising i) at least one fatty acid, and ii) at least one
cholesterol lowering
compound.
[05] The disclosure provides a pharmaceutical composition for reducing total
cholesterol
and maintaining total cholesterol homeostasis comprising i) at least one fatty
acid, and ii) at
least one cholesterol lowering compound. In certain embodiments, maintaining
total
cholesterol homeostasis comprises maintaining a total cholesterol percent
relative range of
about 15%, of about 10%, or of about 5% between a maximum total cholesterol
level and a
minimum total cholesterol level. In certain embodiments, maintaining total
cholesterol
homeostasis comprises maintaining a total cholesterol range of about 5 mg/dL
to about 25
- 1 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
mg/dL or of about 10 mg/dL to about 15 mg/dL between a maximum total
cholesterol level
and a minimum total cholesterol level. In certain embodiments, a maximum total
cholesterol
level and a minimum total cholesterol level are maintained below 200 mg/dL,
below 175
mg/dL, or below 150 mg/dL. In certain embodiments, total cholesterol is
reduced at least 5%,
at least 10%, at least 15%, at least 20% or at least 40%.
[06] Pharmaceutical compositions of the disclosure comprise at least one fatty
acid.
Exemplary fatty acids include, but are not limited to, a fatty oil, a fatty
acid ethyl ester, a fatty
acid triglyceride, a saturated acid, an oil, an ester or a triglyceride, or a
combination thereof
Exemplary fatty acids may further include, but are not limited to, an Omega-3
fatty acid, an
Omega-6 fatty acid, an Omega-9 fatty acid, or a derivative thereof
[07] Pharmaceutical compositions of the disclosure comprise at least one
cholesterol
lowering compound. Exemplary cholesterol lowering compounds include, but are
not limited
to, an HMG-CoA reductase inhibitor or statin, Ezetimibe, a fibrate, a
carboxylic acid,
Benezafibrate, Ciprofibrate, Gemfbroizil, Fenofibrate, Clinofibrate, niacin,
bile acid
sequestrants, Colestipol, Cholestyramine, Endur-Acin, Colesevelam, a PCSK9
enzyme
inhibitor, or any combination thereof In certain embodiments, the at least one
HMG-CoA
reductase inhibitor or statin may be Atorvastatin, Fluvastatin, Lovastatin,
Pitavastatin,
Pravastatin, Rosuvastatin, Simvastatin, or any combination thereof
[08] Pharmaceutical compositions of the disclosure may comprise at least one
vitamin or
hormone. Exemplary vitamins include, but are not limited to, vitamin B,
vitamin C or vitamin
D, or a combination thereof In certain embodiments, the at least one vitamin D
may be
vitamin D2 or vitamin D3. In certain embodiments, the at least one vitamin C
may be
ascorbate or ascorbic acid.
[09] Pharmaceutical compositions of the disclosure may comprise at least one
organic
compound, herb or derivative thereof Exemplary organic compounds, herbs or
derivatives
thereof include, but are not limited to, bacopa, vinpocetine, an alkaloid,
reserpine,
reserpinine, akuammicine, maj dine, vinerine, ervine, vineridine, tombozine,
vincananine,
vincanidine, vincamore, apovincamine, vincamore, apovincamine, or vincaminol.
[010] Pharmaceutical compositions of the disclosure may be used in the
treatment of a
disease or disorder including, but not limited to, an autoimmune disease, an
inflammatory
disorder, a neurodegenerative disorder, a bacterial infection or a viral
infection. In certain
- 2 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
embodiments, the disease or disorder may be psoriasis, keratosis, spongy gum
and bleeding
gum disease, atherosclerosis, heart disease, myopathy, neuropathy, common
cold, myositis,
arthritis, dementia, Parkinson's disease, Alzheimer's disease. In certain
embodiments, the
keratosis may be actinic, pilaris or seborrheic. In certain embodiments, the
pharmaceutical
composition may be used in the treatment of the common cold. As used herein,
the "common
cold" comprises a viral infection of a portion of the upper respiratory tract.
[011] The disclosure provides a pharmaceutical composition comprising at least
600 mg of
a fish oil concentrate, at least 600 mg of a fish oil, at least 180 mg of an
eicosapentaenoic
acid (EPA), at least 120 mg of a docosahexaenoic acid (DHA) and at least 10 mg
of a
cholesterol lowering composition. In certain embodiments, the composition may
be
formulated for daily oral administration for use in the treatment of
psoriasis.
[012] The disclosure provides a pharmaceutical composition comprising at least
1000 mg of
a fish oil, at least 667 mg of a EPA, at least 333 mg of a DHA and at least 10
mg of a
cholesterol lowering composition. In certain embodiments, the composition may
be
formulated for daily oral administration for use in the treatment of
keratosis, atherosclerosis,
heart disease, or any combination thereof
[013] The disclosure provides a pharmaceutical composition comprising at least
600 mg of
a fish oil concentrate, at least 600 mg of a fish oil, at least 180 mg of an
eicosapentaenoic
acid (EPA), at least 120 mg of a docosahexaenoic acid (DHA), at least 10 mg of
a cholesterol
lowering composition and at least 1000 mg of vitamin C. In certain
embodiments, the
composition may be formulated for daily oral administration for use in the
treatment of
spongy gum and bleeding gum disease.
[014] The disclosure provides a pharmaceutical composition comprising at least
600 mg of
a fish oil concentrate, at least 600 mg of a fish oil, at least 180 mg of an
eicosapentaenoic
acid (EPA), at least 120 mg of a docosahexaenoic acid (DHA), at least 10 mg of
a cholesterol
lowering composition and at least 3000 mg of vitamin C. In certain
embodiments, the
composition may be formulated for daily oral administration for use in the
treatment of a
bacterial infection, viral infection or the common cold.
[015] The disclosure provides a pharmaceutical composition comprising at least
600 mg of
a fish oil concentrate, at least 600 mg of a fish oil, at least 180 mg of an
eicosapentaenoic
acid (EPA), at least 120 mg of a docosahexaenoic acid (DHA), at least 10 mg of
a cholesterol
- 3 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
lowering composition and at least 2000 IU of vitamin D. In certain
embodiments, the
composition may be formulated for daily oral administration for use in the
treatment of
myopathy or neuropathy.
[016] The disclosure provides a pharmaceutical composition comprising at least
600 mg of
a fish oil concentrate, at least 600 mg of a fish oil, at least 180 mg of an
eicosapentaenoic
acid (EPA), at least 120 mg of a docosahexaenoic acid (DHA), at least 10 mg of
a cholesterol
lowering composition, and at least 250 mg of bacopa. In certain embodiments,
the
composition may further comprise at least 10 mg of vinpocetine. In certain
embodiments, the
composition may be formulated for daily oral administration for use in the
treatment of
dementia, Parkinson's disease or Alzheimer's disease.
[017] The disclosure provides a method for the treatment of a disease or
disorder in a
subject in need thereof comprising administering a therapeutically effective
amount of a
pharmaceutical composition comprising i) at least one fatty acid, and ii) at
least one
cholesterol lowering compound, such that said disease or disorder is treated.
[018] The disclosure provides a method for reducing total cholesterol and
maintaining total
cholesterol homeostasis in a subject in need thereof comprising administering
a
therapeutically effective amount of a pharmaceutical composition comprising i)
at least one
fatty acid, and ii) at least one cholesterol lowering compound, such that
total cholesterol is
reduced and total cholesterol homeostasis is maintained. In certain
embodiments, maintaining
total cholesterol homeostasis comprises maintaining a total cholesterol
percent relative range
of about 15%, of about 10%, or of about 5% between a maximum total cholesterol
level and a
minimum total cholesterol level while the pharmaceutical composition is
administered. In
certain embodiments, maintaining total cholesterol homeostasis comprises
maintaining a total
cholesterol range of about 5 mg/dL to about 25 mg/dL or of about 10 mg/dL to
about 15
mg/dL between a maximum total cholesterol level and a minimum total
cholesterol level
while the pharmaceutical composition is administered. In certain embodiments,
a maximum
total cholesterol level and a minimum total cholesterol level are maintained
below 200
mg/dL, below 175 mg/dL, or below 150 mg/dL while the pharmaceutical
composition is
administered. In certain embodiments, total cholesterol is reduced at least
5%, at least 10%, at
least 15%, at least 20% or at least 40%. In certain embodiments, reducing
total cholesterol
levels comprises reducing triglyceride levels.
- 4 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[019] In certain embodiments of the methods of the disclosure, the
pharmaceutical
composition may comprise at least one fatty acid. Exemplary fatty acids
include, but are not
limited to, a fatty oil, a fatty acid ethyl ester, a fatty acid triglyceride,
a saturated acid, an oil,
an ester or a triglyceride, or a combination thereof Exemplary fatty acids may
further
include, but are not limited to, an Omega-3 fatty acid, an Omega-6 fatty acid,
an Omega-9
fatty acid, or a derivative thereof
[020] In certain embodiments of the methods of the disclosure, the
pharmaceutical
composition may comprise at least one cholesterol lowering compound. Exemplary
cholesterol lowering compounds may include, but are not limited to, an HMG-CoA
reductase
inhibitor or statin, Ezetimibe, a fibrate, a carboxylic acid, Benezafibrate,
Ciprofibrate,
Gemfbroizil, Fenofibrate, Clinofibrate, niacin, bile acid sequestrants,
Colestipol,
Cholestyramine, Endur-Acin, Colesevelam, a PCSK9 enzyme inhibitor, or any
combination
thereof In certain embodiments, the at least one HMG-CoA reductase inhibitor
or statin may
be Atorvastatin, Fluvastatin, Lovastatin, Pitavastatin, Pravastatin,
Rosuvastatin, Simvastatin,
or any combination thereof
[021] In certain embodiments of the methods of the disclosure, the
pharmaceutical
composition may comprise at least one vitamin or hormone. The at least one
vitamin may
include vitamin B, vitamin C, vitamin D or a combination thereof In certain
embodiments,
the vitamin D may be vitamin D2 or vitamin D3. In certain embodiments, the
vitamin C is
ascorbate or ascorbic acid.
[022] In certain embodiments, the methods of the disclosure include
administering a
pharmaceutical composition and at least one organic compound, herb or
derivative thereof
The at least one organic compound, herb or derivative thereof may include, but
is not limited
to, bacopa, vinpocetine, an alkaloid, reserpine, reserpinine, akuammicine, maj
dine, vinerine,
ervine, vineridine, tombozine, vincananine, vincanidine, vincamore,
apovincamine,
vincamore, apovincamine, and vincaminol.
[023] Compositions and methods of the disclosure may be used in the treatment
of a disease
or disorder, including, but not limited to, an autoimmune disease, an
inflammatory disorder, a
neurodegenerative disorder, a bacterial infection or a viral infection. In
certain embodiments,
the disease or disorder may be psoriasis, keratosis, spongy gum and bleeding
gum disease,
atherosclerosis, heart disease, myopathy, neuropathy, common cold, myositis,
arthritis,
- 5 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
dementia, Parkinson's disease, Alzheimer's disease. In certain embodiments,
the keratosis
may be actinic, pilaris or seborrheic.
[024] The disclosure provides a method for treating psoriasis in a subject in
need thereof
comprising administering a therapeutically effective amount of a
pharmaceutical composition
comprising at least 600 mg of a fish oil concentrate, at least 600 mg of a
fish oil, at least 180
mg of an eicosapentaenoic acid (EPA), at least 120 mg of a docosahexaenoic
acid (DHA) and
at least 10 mg of a cholesterol lowering composition.
[025] According to the compositions and methods of the disclosure,
pharmaceutical
compositions may be formulated for oral delivery.
[026] According to the compositions and methods of the disclosure,
pharmaceutical
compositions may be administered daily or weekly.
[027] The disclosure provides a method for treating keratosis,
atherosclerosis, heart disease,
or any combination thereof, in a subject in need thereof comprising
administering a
therapeutically effective amount of a pharmaceutical composition comprising at
least 1000
mg of a fish oil, at least 667 mg of a EPA, at least 333 mg of a DHA and at
least 10 mg of a
cholesterol lowering composition. In certain embodiments, the pharmaceutical
composition
may be formulated for oral delivery. In certain embodiments, the
pharmaceutical composition
may be administered daily or weekly.
[028] The disclosure provides a method for treating spongy gum and bleeding
gum disease
in a subject in need thereof comprising administering a therapeutically
effective amount of a
pharmaceutical composition comprising at least 600 mg of a fish oil
concentrate, at least 600
mg of a fish oil, at least 180 mg of an eicosapentaenoic acid (EPA), at least
120 mg of a
docosahexaenoic acid (DHA), at least 10 mg of a cholesterol lowering
composition and at
least 1000 mg of vitamin C. In certain embodiments, the pharmaceutical
composition may be
formulated for oral delivery. In certain embodiments, the pharmaceutical
composition may be
administered daily or weekly.
[029] The disclosure provides a method of treating a bacterial infection,
viral infection or
the common cold in a subject in need thereof comprising administering a
therapeutically
effective amount of a pharmaceutical composition comprising at least 600 mg of
a fish oil
concentrate, at least 600 mg of a fish oil, at least 180 mg of an
eicosapentaenoic acid (EPA),
at least 120 mg of a docosahexaenoic acid (DHA), at least 10 mg of a
cholesterol lowering
- 6 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
composition and at least 3000 mg of vitamin C. In certain embodiments, the
pharmaceutical
composition may be formulated for oral delivery. In certain embodiments, the
pharmaceutical
composition may be administered daily or weekly.
[030] The disclosure provides a method of treating myopathy or neuropathy in a
subject in
need thereof comprising administering a therapeutically effective amount of a
pharmaceutical
composition comprising at least 600 mg of a fish oil concentrate, at least 600
mg of a fish oil,
at least 180 mg of an eicosapentaenoic acid (EPA), at least 120 mg of a
docosahexaenoic acid
(DHA), at least 10 mg of a cholesterol lowering composition and at least 2000
IU of vitamin
D. In certain embodiments, the pharmaceutical composition may be formulated
for oral
delivery. In certain embodiments, the pharmaceutical composition may be
administered daily
or weekly.
[031] The disclosure provides a method of treating dementia, Parkinson's
disease or
Alzheimer's disease in a subject in need thereof comprising administering a
therapeutically
effective amount of a pharmaceutical composition comprising at least 600 mg of
a fish oil
concentrate, at least 600 mg of a fish oil, at least 180 mg of an
eicosapentaenoic acid (EPA),
at least 120 mg of a docosahexaenoic acid (DHA), at least 10 mg of a
cholesterol lowering
composition, and at least 250 mg of bacopa. In certain embodiments, the
pharmaceutical
composition may be formulated for oral delivery. In certain embodiments, the
pharmaceutical
composition may be administered daily or weekly.
BRIEF DESCRIPTION OF THE DRAWINGS
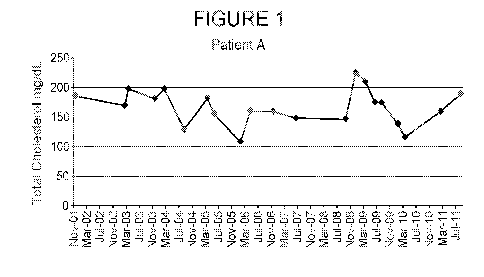
[032] Figure 1 is a graph depicting total cholesterol (mg/dL) as a function of
time for
Patient A (data points provided in Table 2) over first time period (November
2001 to July
2011).
[033] Figure 2 is a graph depicting total cholesterol (mg/dL) as a function of
time for
Patient A (data points provided in Table 2) over second time period (August
2011 to August
2015).
[034] Figure 3 is a graph depicting total cholesterol (mg/dL) as a function of
time for
Patient B (data points provided in Table 3) over first time period (November
1998 to May
2013).
- 7 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[035] Figure 4 is a graph depicting total cholesterol (mg/dL) as a function of
time for
Patient B (data points provided in Table 3) over second time period (August
2013 to May
2015).
[036] Figure 5 is a schematic diagram depicting an exemplary timeline for
administration of
a composition of the disclosure with exemplary data points following
collection of a sample
and measurement of total cholesterol from, for example, circulating blood.
DETAILED DESCRIPTION
[037] The disclosure provides a pharmaceutical composition for the treatment
of a disease
or disorder comprising i) at least one fatty acid, and ii) at least one
cholesterol lowering
compound. Compositions and methods of the disclosure reduce total cholesterol
and maintain
total cholesterol homeostasis.
[038] Some cholesterol lowering medicaments may establish a homeostatic
cholesterol
level. For example the combination of Lipitor and TriCor (fenofibrate) or
Vytorin (a
combination of Zetia and Simvastatin) as a monotherapy may establish a
homeostatic
cholesterol level. In contrast, neither Lipitor nor Zetia, when administered
as a monotherapy,
will establish a homeostatic cholesterol level. Both Lipitor and Zetia need an
Omega-3 fish
oil to establish a homeostatic cholesterol level, as illustrated in Patient
A's Experiments 5-15.
[039] Importantly, cholesterol lowering agents administered as a monotherapy
will not treat
autoimmune or inflammatory diseases. Cholesterol lowering agents require a
coinitiator,
including, but not limited to, a fatty acid, a vitamin, a hormone, a
flavanoid, a carotenoid, a
retinoid, a mineral, or any combination thereof Likewise, the co-initiator
requires a statin, a
cholesterol lowering agent, a lipoprotein lowering agent, a triglyceride
lowering agent (e.g.
statin(s), fibrate(s), PCSK9, monoclonal antibody inhibitors, ApoA-1 milano,
succinofuco
(AG1-1067), and Apoprotein-B inhibitor Mipomersen) or any combination thereof
[040] For example, Omega-3 fatty acids (triglyceride or ethyl ester) or
vitamin D may serve
as a co-initiator with a statin. This combination of a fatty acid, a vitamin
and a statin may be
used to efficaciously treat autoimmune and/or inflammatory diseases,
including, but not
limited to, myopathy, myositis, psoriasis, keratosis, atherosclerosis,
neuropathy and other
related diseases such as neurodegenerative diseases.
- 8 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[041] Compositions and methods of the disclosure may be used to treat a number
of
conditions having a common underlying feature of increased or unregulated
fatty acids and/or
cholesterol levels, either as result of the disease or disorder itself, or as
a side effect of a
treatment for that disease or disorder. The compositions and methods of the
disclosure
establish a homeostatic cholesterol level that is independent from the
absolute level (or,
magnitude) of the homeostatic cholesterol value. Thus, the homeostatic
cholesterol level is
described as a relative level compared to an initial level present either
during a disease state
or as a consequence of treatment of an underlying disease with another
therapy.
[042] Compositions of the disclosure may comprise one or more of a fatty acid,
a fat
(unsaturated or saturated fat), a vitamin, a flavanoid, a carotenoid, a
retinoid, or a hormone in
combination with a statin. Compositions of the disclosure may be used to treat
any
autoimmune or inflammatory disease.
[043] Many autoimmune diseases are MHC-Class 1 mediated diseases. Statins
repress T-
lymphocyte activation, however, statins are specific for MHC-Class II and do
not involve
MI-IC-Class 1 expression. Cholesterol lowering medicaments have little or no
direct
association with autoimmune diseases or other diseases. Thus, treatment with a
statin alone
would not be efficacious to treat an autoimmune or inflammatory disease.
[044] Thus, the compositions and methods provide a combination of a
cholesterol-lowering
medicament (e.g. a statin) with a fatty acid to treat disease with
unexpectedly superior
efficacy, to reduce cholesterol levels and to establish a healthy homeostatic
cholesterol level.
Unexpectedly, the compositions and methods of the disclosure are efficacious
for treating
autoimmune and inflammatory disorders.
[045] Compositions and methods of the disclosure may include any statin, and,
therefore,
are not reliant on the use of any one particular statin.
[046] Provided herein is a description of the treatment of eight diseases by
the compositions
and methods of the invention that are further illustrated by working examples.
Psoriasis
[047] Psoriasis is a common autoimmune disease of the skin characterized by
scaly patches,
papules and plaques. There are 5 prevalent forms of psoriasis: plaque,
pustular, guttate,
erythrodermic and invesse. Psoriasis may occur over small areas of the body or
over the
entire body. Areas of the more common plaque Psoriases are small (e.g. 0.5 in.
in diameter)
- 9 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
or large (e.g. 1.5-2.0 in. in diameter) patches of skin characterized by a
silvery-white
epidermal cell morphology.
[048] In a subject suffering from psoriasis, the subject's immune system
attacks skin cells
because the epidermal cells of the skin cells are recognized, improperly, as a
rapidly growing
pathogen (similar to the way the immune system would recognize cancer).
Consequently,
psoriasis may be considered an autoimmune disease.
[049] A subject having psoriasis may also have one or more mutations in the
PSOR1 gene.
This PSOR1 gene is located on chromosome 6 in the major histocompatibility
complex
(MHC). The MHC is a complex responsible in part for distinguishing self-
antigens from
non-self-antigens and preventing the development of autoimmune conditions.
Psoriasis
vulgaris may be associated with additional mutations in human leukocyte
antigen (HLA)-C
variant, including HLA-CW6. Psoriasis may be associated with an increased
abundance of
CD8+T cells, HLA-CW6, IL-12b, IL-23b, TNF alpha and NF-KB.
Keratosis
[050] Keratosis often presents as an excessive accumulation of keratin on the
epidermis or
mucous membranes. Some of the more common types of keratosis include actinic,
pilaris
and seborrheic keratosis. Actinic keratosis is a precancerous skin condition.
Actinic keratosis
is characterized by usually thick, scaly and rough patches of skin. If
untreated, actinic
keratosis cells may transform into squamous-cell carcinoma. Keratosis pilaris
is
characterized by an excess of keratin that surrounds the hair follicles in the
pore. The
entrapped hair continues to grow causing a slightly red raised bump.
Seborrheic keratosis is a
non-cancerous growth of discolored skin that usually appears brown, black or
light.
Spongy gum and bleeding gum disease
[051] Spongy gum and bleeding gum disease are associated with gingivitis and
subsequent
periodontal disease. Both diseases are associated with bacterial gum
infections. Without
treatment, the bacterial gum infections may destroy gum tissue and auxiliary
bone.
[052] Gingivitis is a minor bacterial gum inflammation that causes the gums to
be soft and
bleed. As gingivitis progresses it advances to periodontitis, a severe
inflammation around the
tooth. Over time the gums pull away from the teeth and form pockets that
become infected.
If the subject's immune system cannot eliminate the bacterial infection, the
gum tissue and
bone surrounding the tooth or teeth may be damaged or destroyed.
- 10 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[053] Patient A suffered severe spongy gum and bleeding gum disease, because
he was
taking both blood thinning and high blood pressure medication. The
compositions and
methods of the disclosure may be used to treat the severe spongy gum and
bleeding gum
disease that results from taking both blood thinning and high blood pressure
medications.
Myopathy
[054] Myopathy is term used to describe a wide variety of muscular conditions.
Myopathies
may be inherited or acquired. Inherited myopathies include dystrophies,
myotonic,
congenital myopathies, mitochondrial myopathy, familial periodic paralysis,
and
inflammatory and metabolic myopathies. Acquired myopathies include substance
induced
myopathy, glucocorticoid myopathy, dermatomyositis, inclusion body myositis,
polymyositis, myositis ossificans, rhabdomyolysis and myoglobinurias.
[055] Statin induced myopathy (i.e. an acquired myopathy) often takes one of
three forms:
myalgia, myositis and rhabdomyolysis. Myalgia, or muscle pain, is a symptom of
many
diseases. Myositis, or muscle inflammation, may result in an elevation of
creatine kinase; a
muscle enzyme found in the blood. Rhabdomyolysis is condition in which damaged
muscle
(skeletal striated muscle) breaks down. Subjects having rhabdomyolysis often
have muscle
protein in their bloodstream that may cause severe kidney damage and eventual
death.
[056] The pain associated with myositis (myopathy) and neuropathy (e.g. nerve
decay,
particularly in the context of gum disease) may be treated with a composition
of the
disclosure comprising fish oil, Vitamin D3 (a hormone) and a statin. When
administered with
fish oil, Vitamin D, in part, also controls the amount of cholesterol
manufactured in the liver
without negatively influencing the concentration of active statins.
Atherosclerosis
[057] Cardiovascular disease is the number one cause of death worldwide.
Atherosclerosis
(i.e., a hardening of the arteries that happens within blood vessels) is often
caused by the
oxidation of unsaturated fats in the bloodstream. Unsaturated fats cannot pass
through the
endothelial cell lining of the arterial and venial cell walls; however,
oxidized fats can
transport themselves across the endothelial cell membrane. The transport of
oxidized fats
across the walls of the blood vessels triggers an autoimmune reaction in which
the subject's
immune system recognizes the oxidized fat as a pathogen and attacks it. Non-
oxidized fats
and non-oxidized cholesterols do not cause heart disease.
- 11 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[058] Oxidized fats and oxidized cholesterol attract white blood cells called
T-lymphocytes
and macrophages. As white blood cells ingest the oxidized fats and
cholesterol, they form the
plaque that is associated with atherosclerosis, stroke and heart attacks.
Macrophages form
foam cells that release harmful molecules into the bloodstream. Foam cells are
found in
atherosclerosis and heart disease.
[059] Peroxides and free radicals oxidize fat and cholesterol, inducing
oxidative stress.
Oxidative stress damages any and all components of a cell, including, but not
limited to,
proteins, lipids and DNA.
[060] Atherosclerosis is characterized by the build-up of stable and unstable
plaque. A hard
fibrous sheath may encase the plaque formed by the autoimmune reaction. When
the body
successfully encases autoimmune reaction, the plaque formed is called stable
plaque. When
the body fails to encapsulate the autoimmune reaction (i.e. the formation of
an unstable
plaque), the unstable plaque may break loose. Consequently, the inflammatory
cells from the
reaction spread within the blood causing the blood to coagulate. The
coagulation is called a
blood clot. Blood clots that block the flow of blood through a blood vessel
may cause a heart
attack.
[061] The combination of Omega-3 oils (fatty acids) and statin(s) stabilize
the cholesterol in
lipoproteins (fat deposits). Moreover, Omega-3 oils and the associated high
concentration of
EPA, DHA and DPA are responsible for the vasodilatory effects found in blood
vessels. As a
result the blood vessels widen; thereby, relaxing the smooth muscles and
lowering the blood
pressure.
[062] By preventing the autoimmune response that underlies the development of
atherosclerosis, the compositions and methods of the disclosure lower the risk
of
atherosclerosis and lower the risk of future heart attacks and strokes.
Neuropathy
[063] Neuropathy is a term describing damage or disease of the nerves.
Neuropathy is used
more often to describe damage or disease of the peripheral nervous system
(PNS) as opposed
to the central nervous system (CNS). Neuropathy may be caused by systemic
disease,
medicaments, injury, inflammation, autoimmune diseases, bacterial infection
and/or viral
infection.
- 12 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[064] Neuropathy and myopathy share a common feature in that damage to the
nerves and
muscles, respectively, leads to an inflammatory response. In certain
circumstances, damage
to the nerves and muscles, respectively, may be caused by an autoimmune
reaction to a self-
antigen. Compositions and methods of the disclosure may be used to treat
neuropathy, at least
in part, because the compositions and methods of the disclosure reduce
autoimmune and
inflammatory responses.
Common Cold
[065] The common cold comprises a viral infectious disease of the upper
respiratory tract
which affects a subject's nose, sinus and throat. There are over 200 different
viral strains that
cause the common cold and each strain has a distinct set of symptoms. Usually
a person will
recover in about 1-2 weeks with the exception that a small number of the
population will
need 3-4 weeks before the symptoms abate. Infants and preschool children are
susceptible to
colds because they have not developed an immune system to many viruses. Older
children
and adults develop immunity to many of the viruses that cause the common cold.
However,
one can still have a cold when infected with a cold virus, because of a
weakened immune
system (through, for example, disease, immunosuppressive therapy, or age
(either very young
or old)).
[066] Compositions of the disclosure may be used to treat the common cold. In
particular,
those compositions of the disclosure comprising a statin, an Omega-3 fatty
acids or
polyunsaturated oils and vitamin C may be used to treat the common cold.
Patient A (see
Examples) contracted the common cold. In this study the cold was gone within 3
days of
initiating treatment with a composition of the disclosure. As the cold may
otherwise have
lasted several weeks, treatment according to the compositions and methods of
the disclosure
demonstrate a significant improvement over conventional therapies.
Dementia
[067] Dementia (also known as senility) represents a broad range of brain
diseases that
cause short term mild cognitive impairment (MCI) and eventually, severely
impair a subject's
basic cognitive functions. A chromic decline in memory with at least one
additional
cognitive function signals the onset of dementia. In addition to memory,
cognitive functions
including, but not limited to, aphasia (communication), apartia (motor
execution), agnosiax
(recognition) and executive function (synthesis) may be affected.
- 13 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[068] Dementia may be classified as vascular dementia, Alzheimer's disease,
progressive
supranuclear palsy, corticobasal degeneration, frontotemporal dementia and
Lewy body
dementia. Compositions and methods of the disclosure preserve a subject's
cognitive
functions and prevent further deterioration of memory.
[069] Patient A's long standing use of statins has had a dramatic negative
effect on his
ability to remember names and organize daily routines. Patient A had taken
bacopa, an
ayuryeda herb used in India to improve mental alertness, comprehension, memory
and
recollection. At the time, prior to his heart attack and after his quadruple
bypass, bacopa had
no effect on memory or recollection; however, after Patient A took Omega-3
fish oil and
statin(s) the effect of bacopa was amplified. He had no problem with memory
loss or
organizing daily routines.
[070] In combination with Omega-3 fish oil and a statin, Patient A was
administered
vinpocetine. Vinpocetine is an extract of the periwinkle plant that is an
effective vasodilator
and a nootropic for improving memory and cerebral metabolism.
[071] Patient B started to have seizures in October, 2004 and continued to
have minor
seizures until September 2010 when the seizure intensity and duration began to
increase.
Consequently, her anti-seizure medication was increased to its maximum dosage.
In January,
2015 she experienced a few auras or preictal events that immediately proceed
the onset of a
seizure. Until April 2015, the preictal events occurred every other day. In
May 2015, she
was taken off Crestor as a possible cause of the preictals, but the events
still occurred. In
June 2015, she was given vinpocetine. Since taking vinpocetine, the auras
ceased and have
not returned.
[072] Kindling models in rats as well as Patient B's preictals demonstrate
that vinpocetine
exhibits anticonvulsant properties. Furthermore, vinpocetine may inhibit the
upregulation of
NF-kB by TNF2, thereby preventing inflammation.
[073] Compositions of the disclosure, and, in particular, those comprising
vinpocetine also
significantly improved memory retention and muscular coordination in Patient
A.
[074] In addition to the diseases and disorders described above, compositions
and methods
of the disclosure may be used to treat any one or more of the following
diseases and
conditions.
[075] Table 1: Diseases and Disorders
- 14 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
Acute disseminated encephalomyelitis (ADEM)
Addison's disease
Agammaglobulinemia
Alopecia areata
Amyotrophic lateral sclerosis (Also Lou Gehrig's disease; Motor Neuron
Disease)
Ankylosing Spondylitis
Antiphospholipid syndrome
Antisynthetase syndrome
Atopic allergy
Atopic dermatitis
Autoimmune aplastic anemia
Autoimmune cardiomyopathy
Autoimmune enteropathy
Autoimmune hemolytic anemia
Autoimmune hepatitis
Autoimmune inner ear disease
Autoimmune lymphoproliferative syndrome
Autoimmune peripheral neuropathy
Autoimmune pancreatitis
Autoimmune polyendocrine syndrome
Autoimmune progesterone dermatitis
Autoimmune thrombocytopenic purpura
Autoimmune urticaria
Autoimmune uveitis
Balo disease/Balo concentric sclerosis
Behcet's disease
Berger's disease
Bickerstaffs encephalitis
Blau syndrome
Bullous pemghigoid
Cancer
Castleman's disease
Celiac disease
Chagas disease
Chronic inflammatory demyelinating polyneuropathy
Chronic recurrent multifocal osteomyelitis
Chronic obstructive pulmonary disease
Churg-Strauss syndrome
Cicatricial pemphigoid
Cogan syndrome
Cold agglutinin disease
Complement component 2 deficiency
Contact dermatitis
Cranial arteritis
CREST syndrome
Crohn's disease
Cushing's Syndrome
Cutaneous leukocytoclastic angiitis
Dego's disease
Dercum's disease
Dermatitis heretiformis
Dermatomyositis
Diabetes mellitus type I
Diffuse cutaneous systemic sclerosis
- 15 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
Dressler's syndrome
Drug-induced lupus
Discoid lupus erythematosus
Eczema
Endometriosis
Enthesitis-related arthritis
Eosinophilic fasciitis
Eosinophilic gastroenteritis
Eosinophilic pneumonia
Epidermolysis bullosa acquisita
Erythema nodosum
Erythroblastosis fetalis
Essential mixed cryoglobulinemia
Evan's syndrome
Fibrodysplasia ossificans progressive
Fibrosing alveolitis (or Idiopathic pulmonary fibrosis)
Gastritis
Gastrointestinal pemphigoid
Glomerulonephritis
Goodpasture's syndrome
Granulomatosis with polyangiitis
Graves disease
Guillain-Barre syndrome (GBS)
Hashimoto's encephalopathy
Hashimoto's thyroiditis
Henoch-Schonlein purpura
Herpes gestationis aka Gestational Pemphigoid
Hidradenitis suppurative
Hughes-Stovin syndrome
Hypogammaglobulinemia
Idiopathic inflammatory demyelinating diseases
Idiopathic pulmonary fibrosis
Idiopathic thrombocytopenic purpura (see Autoimmune thrombocytopenic purpura)
IgA nephropathy
Inclusion body myositis
Interstitial cystitis
Juvenile idiopathic arthritis aka Juvenile rheumatoid arthritis
Kawasaki's disease
Lambert-Eaton myasthenic syndrome
Leukocytoclastic vasculitis
Lichen planus
Lichen sclerosis
Linear IgA disease (LAD)
Lupoid hepatitis aka autoimmune hepatitis
Lupus erythematosus
Majeed syndrome
Meniere's disease
Microscopic polyangiitis
Miller-Fisher syndrome (see Guillain-Barre Syndrome)
Mixed connective tissue disease
Morphea
Mucha-Habermann disease aka Pityriasis lichenoides et varioliformis acuta
Multiple sclerosis
Microscopic colitis
- 16 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
Myasthenia gravis
Myositis
Narcolepsy
Neuromyelitis optica (also Devic's disease)
Neuromyotonia
Ocular cicatricial pemphigoid
Opsoclonus myoclonus syndrome
Ord's thyroiditis
Palindromic rheumatism
PANDAS (pediatric autoimmune neuropsychiatric disorders associated with
streptococcus)
Paraneoplastic cerebellar degeneration
Paroxysmal nocturnal hemoglobinuria (PNH)
Parry Romberg syndrome
Parsonage-Turner syndrome
Pars planitis
Pemphigus vulgaris
Pernicious anaemia
Perienus encephalomyelitis
POEMS syndrome
Polyareteritis nodosa
Polymyalgia rheumatica
Polymyositis
Primary sclerosing cholangitis
Primary biliary cirrhosis
Progressive inflammatory neuropathy
Psoriasis
Psoriatic arthritis
Pyoderma gangrenosum
Pure red cell aplasia
Rasmussen's encephalitis
Raynaud phenomenon
Relapsing ploychondritis
Reiter's syndrome
Restless leg syndrome
Retroperitoneal fibrosis
Rheumatoid arthritis
Rheumatic fever
Sarcoidosis
Schizophrenia
Schmidt syndrome another form of APS
Schnitzler syndrome
Scleritis
Scleroderma
Serum Sickness
Sjogren's syndrome
Spondyloarthropathy
Still's disease see Juvenile Rheumatoid Arthritis
Stiff person syndrome
Subacute bacterial endocarditis (SBE)
Susac's syndrome
Sweet's syndrome
Sydenham chorea (see PANDAS)
Sympathetic ophthalmia
Systemic lupus erythematosus (see Lupus erythematosus)
- 17 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
Takayasu's arteritis
Temporal arteritis (also known as "giant cell arteritis")
Thrombocytopenia
Tolosa-Hunt syndrome
Transverse myelitis
Ulcerative colitis (one of two types of idiopathic inflammatory bowel disease
"IBD")
Undifferentiated connective tissue disease different from Mixed connective
tissue disease
Undifferentiated spondyloarthropathy
Urticarial vasculitis
Vasculitis
Vitiligo
Definitions
[076] Omega fatly acids and Omega oils: Omega-3 fatty acids include natural,
manufactured or pharmaceutical fatty acids and fatty acid esters as well as
other derivatives
such as natural oils, congregates or salts. The fatty acid, fatty acid esters
or fatty acid oils or
ethyl-fatty acids or fatty acid alcohols or fatty acid glycols or fatty acid
triglycerides that
include, but are not limited to: Omega-3 polyunsaturated short-chain fatty
acids with an
aliphatic tail less than 6 carbon atoms; polyunsaturated medium-chain fatty
acids with an
aliphatic tail of 6-12 carbon atoms; polyunsaturated long-chain fatty acids
with an aliphatic
tail of 13-21 carbon atoms and very-long chain fatty acids with an aliphatic
tail greater than
22 carbon atoms. The short chain and very-long chain fatty acids or esters
include:
myristoleic, palmitoleic, sapienic, oleic, elaidic, vaccenic, linoleic,
linolelaidic, alpha-
linolenic, arachidonic, eicosapentaenoic (EPA), alpha-linolenic, erucic and
docosahexaenoic
(DHA) acids. Also included are the saturated fatty acids that include:
caprylic, capric, lauric,
myristic, palmitic, stearic, arachidic, lignoceric and cerotic acid. The Omega-
3 fatty acids
with a statin(s) have a profound effect on the autoimmune diseases. Likewise
Omega-6 fatty
acids and Omega-9 fatty acids with a statin(s) have a similar effect on
autoimmune diseases
as well as the Omega-3 fatty acids. The Omega-6 fatty acids include
arachidonic acid and
alpha-linoleic acid. The Omega-9 fatty acids include oleic, erucic, elaidic,
mead and
nervonic acid.
[077] Omega-3, Omega-6 and Omega-9 are effective in treating autoimmune
diseases when
administered with a statin. The ratio of different fatty acids is usually at a
minimum of 1:1.
That is the ratio of EPA to DHA is preferably 1:1; however, the ratio could be
equally a 1:0
or 0:1 or 2:1. Most over the counter Omega-3 fatty acids are 2:1; however,
other Omega-3
fatty acids are 1:2, EPA to DHA. For example, psoriasis required in Patient A
an EPA of 180
- 18 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
mg and a DHA of 120 mg with a fish oil concentration of 600 mg and a
statin(s). However,
to effectively treat the various forms of keratosis, Patient A needed 1000 mg
of Omega-3
fatty acids that included 667 mg of EPA and 333 mg of DHA. That was the
minimum for
Patient A. Increasing the concentration of fatty acids or fatty oils or fatty
esters or fatty
triglycerides or fatty acid alcohols did not relieve the symptoms of the
disease, because the
disease was treated at the lower concentration. The higher concentration just
lowered the
total cholesterol. The total cholesterol's homeostasis was established when an
Omega-3 or
Omega-6, or Omega-9 was combined with a statin(s). The omega fatty acids alone
did not
effectively treat the diseases rather it was the combination of omega fatty
acids or mega oils
and statin(s) that effectively treated the diseases.
[078] Cholesterol lowering medicaments: Statins are also called HMG-CoA
reductase
inhibitors. They are drugs used to lower cholesterol levels by inhibiting the
enzyme HMG-
CoA reductase that is responsible for the preparation of cholesterol in the
liver. The statins
used herein as well as others include Atorvastatin (Lipitor or Torvast),
Fluvastatin (Lescol),
Lovastatin (Mevacor, Altocor, or Altoprev), Pitavastatin (Livalo or Pitava),
Pravastatin
(Provachol, Selektine, or Lipostat), Rosuvastatin (Crestor) and Simvastatin
(Zocor or Lipex).
There are several combinations of statins that are extremely effective, one
such combination
includes Ezetimibe/Simvastatin (Vytorin) or combinations of statins and
vitamins such as
Lovastin/Niacin (Advicor), Simvastatin/Niacin (Simcor) or combinations of 2
different
functional drugs such a Atorvastatin/Amlodipine hesylate (Caduet). There are
other
cholesterol lowering drugs that act by decreasing cholesterol in the small
intestine such a
drug is Ezetimibe (Zetia or Ezetrol). Any drug that lowers cholesterol is a
prime candidate
for treating diseases with another medicament. The cholesterol lowering
drug(s) or
medicament(s) cannot effectively treat diseases as a monotherapy; they need
another
medicament to aid in treating the disease.
[079] Other cholesterol lowering medicaments include fibrates, amphipathic
carboxylic
acids, such as Bezafibrate (Bezalip), Ciprofibrate (Modalim), Gemfbroizil
(Lopid),
Fenofibrate (Tr Cor) or Clinofibrate (Lipoclin). Other cholesterol lowering
medications as
well as vitamins include niacin and bile acid sequestrants such as Colestipol
(Colestid),
Cholestyramine (Questron), Endur-Acin and Colesevelam (Welchol).
- 19 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[080] There is another cholesterol lowering medicament that inhibits synthesis
of an
enzyme PCSK9 that binds to the receptors of low density lipoprotein (LDL)
cholesterol
resulting in making the LDL receptor more effective in removing LDL from the
blood. The
new drugs are Alirocumab and Evolocumab. Any cholesterol lowering medicament
in
combination with a fatty acid(s), fatty acid ester(s), fatty acid
triglycerides(s) or the like will
treat diseases. It is proven herein that the concentration of cholesterol
lowering
medicament(s) or fatty acid(s) is not significant and a minimum amount is only
needed. Also
the type of cholesterol lowering medicament or fatty acid is not significant
because the level
of total cholesterol homeostasis will be at that level necessary to treat the
disease.
[081] Vitamins: Vitamins are essential organic compounds that an organism
requires for
survival, but cannot manufacture the organic compound in sufficient
quantities; as is the case
of vitamin D that requires sun exposure. There are 13 vitamins that are
classified by their
biological and chemical activity. Vitamins have many diverse biochemical
functions. As
shown herein the functions of the vitamins are not well understood.
[082] For example, vitamin D may be classified as a hormone and functions as a
regulator
of mineral metabolism, treats rickets in children and osteoporosis in the
elderly; but, herein, it
has been shown to treat myopathy and neuropathic pain. With the new
combination of fatty
acid(s), cholesterol lowering medicament(s) and vitamin D the range of
disease(s) treated is
extended to additional and various inflammatory diseases.
[083] Vitamin D is a secosteroid comprising vitamin D3 (Cholecalciferol) and
vitamin D2
(Ergocalciferol). Either forms of vitamin D are beneficial in treating
myopathy, myositis and
neuropathy. The vitamin D used herein was vitamin D3 and the minimum
concentration was
2000 IU that resulted in a blood plasma vitamin D3 25-0H level greater than 30
ng/ml.
However, at a vitamin D3 intake of 12,000 IU, the total cholesterol level
increased, but
myopathy, myositis or neuropathy did not return; rather, the diseases were
still abated.
[084] Vitamin C like vitamin D is an essential in human vertebrates. There are
2 forms
found in nature for vitamin C, ascorbate as L-ascorbate and ascorbic acid as L-
ascorbic acid.
Both are present in human vertebrates and are conformational isomers. Herein,
vitamin C was
used in the form of L-ascorbic acid. By itself vitamin C has a fluctuating
effect in biological
reactions. Treating a particular disease requires a certain concentration
while treating
- 20 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
another disease requires a different concentration and, likewise, treating a
viral infection, the
common cold, requires a very different concentration.
[085] The connection between treating diseases and viral infections that
specifically require
the use of vitamin C, also require fatty acid(s) and a cholesterol lowering
medicament.
Controlling the total cholesterol is a cause and effect result of combining a
fatty acid and a
cholesterol lowering medicament. Adding vitamin C to the matrix adds to the
enhanced
treating of autoimmune diseases and viral infections. The spongy gum disease
requires at
least 1000 mg/day of vitamin C; whereas, the common cold, a viral disease,
requires 8000
mg/day.
[086] Organic Compounds: Bacopa is an aquatic plant that belongs to the family
Plantaginaceae. It is an herb used in Indian culture as an ayurvedic medicine
to treat anxiety
and memory disorders. Bacopa monnieri was used exclusively herein; however,
any
kingdom of Plantal is contemplated. The minimum is 250 mg/day; however, for a
stronger
cognitive awakening, 500 mg/day is preferred.
[087] Vinpocetine has been reported to have vasodilation and nootropic
improvement in
cognitive functions and cerebral metabolism. Also it has been reported as a
dominant anti-
inflammatory agent in the treatment of Parkinson's disease and Alzheimer's
disease.
[088] Vinpocetine in Patient B at 30 mg/day inhibited preictals in a patient
accustomed to
seizures. In Patient A, the bacopa expanded memory and learning capabilities
and the
vinpocetine improved the cognitive functions as well as cerebral metabolism.
All of this was
accomplished with the addition of concurrent administration of fatty acid(s)
and a cholesterol
lowering medicament(s). As a semi synthetic derivative alkaloid of vincamine
or any other
alkaloid derivative including, but is not limited to, reserpine, reserpinine,
akuammicine,
maj dine, vinerine, ervine, vineridine, tombozine, vincananine, vincanidine,
vincamone,
apovincamine, vincaminol and perivincine are examples of cognitive enhancing
medicaments.
[089] Excipients: Excipients are substances used along with the active
ingredient of a
medication. Their purposes are many from such important enhancements as
absorption and
solubility of the active ingredient to viscosity reduction, to shelf life of
the overall medication
and to enhancement of manufacturing processes. The types of excipients
include, but are not
limited to: anti-adherents, binders, coatings, color, disintegrants,
encapsulants, flavors,
- 21 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
glidants, lubricants, preservatives, sorbents and sweeteners. Compositions and
methods of
the disclosure may be administered as a solid tablet or gel capsule or
injection or vaporizer.
[090] The term "total cholesterol percent relative range" is meant to
describe:
[(the maximum total cholesterol level ¨ the minimum total cholesterol level)/
((the maximum
total cholesterol level + the minimum total cholesterol level)/2)1 x 100.
[091] The term "total cholesterol relative range" is meant to describe:
(the maximum total cholesterol level ¨ the minimum total cholesterol level)/
((the maximum
total cholesterol level + the minimum total cholesterol level)/2).
[092] At least two data points are collected to determine a total cholesterol
value. A first
data point may be collected prior to administration of a composition of the
disclosure and a
second data point may be collected after administration of the composition of
the disclosure.
Alternatively, or in addition, a third or subsequent data point may be
collected following a
treatment change in the components of the composition, one or more
dosages/dosing
schedules of the components of the composition or a change of formulation of
one or more
components of the composition. As treatment with a composition of the
disclosure may
decrease the subject's cholesterol level between measurements, the first or
earlier data point
may represent the maximum total cholesterol and the second, third, subsequent
or latest data
point may represent the minimum total cholesterol. Alternatively, should
treatment with a
composition of the disclosure may increase the subject's cholesterol level
between
measurements, the first or earlier data point may represent the minimum total
cholesterol and
the second, third, subsequent or latest data point may represent the maximum
total
cholesterol.
[093] In certain embodiments, a first data point may be collected after daily
administration
of a composition of the disclosure for at least three months and additional
(second, third, and
subsequent) data points may be collected thereafter weekly or monthly for a
period of at least
2, 3, 4, 5, 6, 7, 8, 9, 10, 11 or 12 months. Figure 5 provides an exemplary
timeline illustrating
these embodiments. From collected and analyzed samples, a maximum total
cholesterol (or
highest cholesterol measurement) is determined and from the same sample, a
minimum total
cholesterol value (or lowest cholesterol measurement) is determined.
[094] In certain embodiments, the at least two data points should be
measured/collected
monthly for a period of at least 2, 3, 4, 5, 6, 7, 8,9, 10, 11 or 12 months.
In certain
- 22 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
embodiments, the at least two data points should be measured/collected two
months apart. In
certain embodiments, the last of the at least two data points should be
measured/collected
after all changes to the medication have been made during, for example, a
treatment schedule
or clinical trial. In the context of a clinical trial, individual subject data
may be kept separate
or may be pooled prior to data analysis.
[095] As used throughout the disclosure, the singular forms "a," "and," and
"the" include
plural referents unless the context clearly dictates otherwise. Thus, for
example, reference to
"a method" includes a plurality of such methods and reference to "a dose"
includes reference
to one or more doses and equivalents thereof known to those skilled in the
art, and so forth.
[096] The term "about" or "approximately" means within an acceptable error
range for the
particular value as determined by one of ordinary skill in the art, which will
depend in part on
how the value is measured or determined, e.g., the limitations of the
measurement system.
For example, "about" can mean within 1 or more standard deviations.
Alternatively, "about"
can mean a range of up to 20%, or up to 10%, or up to 5%, or up to 1% of a
given value.
Alternatively, particularly with respect to biological systems or processes,
the term can mean
within an order of magnitude, preferably within 5-fold, and more preferably
within 2-fold, of
a value. Where particular values are described in the application and claims,
unless otherwise
stated the term "about" meaning within an acceptable error range for the
particular value
should be assumed.
[097] The term "comprising" is intended to mean that the compositions and
methods include
the recited elements, but do not exclude others. "Consisting essentially of"
when used to
define compositions and methods, shall mean excluding other elements of any
essential
significance to the combination when used for the intended purpose. Thus, a
composition
consisting essentially of the elements as defined herein would not exclude
trace contaminants
or inert carriers. "Consisting of shall mean excluding more than trace
elements of other
ingredients and substantial method steps. Embodiments defined by each of these
transition
terms are within the scope of this invention.
[098] As used herein, "expression" refers to the process by which
polynucleotides are
transcribed into mRNA and/or the process by which the transcribed mRNA is
subsequently
being translated into peptides, polypeptides, or proteins. If the
polynucleotide is derived from
genomic DNA, expression may include splicing of the mRNA in a eukaryotic cell.
- 23 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[099] "Gene expression" refers to the conversion of the information, contained
in a gene,
into a gene product. A gene product can be the direct transcriptional product
of a gene (e.g.,
mRNA, tRNA, rRNA, antisense RNA, ribozyme, shRNA, micro RNA, structural RNA or
any other type of RNA) or a protein produced by translation of an mRNA. Gene
products
also include RNAs which are modified, by processes such as capping,
polyadenylation,
methylation, and editing, and proteins modified by, for example, methylation,
acetylation,
phosphorylation, ubiquitination, ADP-ribosylation, myristilation, and
glycosylation.
[0100] "Modulation" or "regulation" of gene expression refers to a change in
the activity of a
gene. Modulation of expression can include, but is not limited to, gene
activation and gene
repression.
[0101] The terms "treating" and "treatment" as used herein refer to reduction
in severity
and/or frequency of symptoms, elimination of symptoms and/or underlying cause,
and
improvement or remediation of damage. The phrase "administering to a subject"
refers to the
process of introducing a composition or dosage form of the invention into the
subject (e.g., a
human or other mammalian subject) via an art-recognized means of introduction.
[0102] By the terms "effective amount" and "therapeutically effective amount"
of an agent,
compound, drug, composition or combination of the invention which is nontoxic
and
effective for producing some desired therapeutic effect upon administration to
a subject or
patient (e.g., a human subject or patient).
Pharmaceutical Formulations
[0103] Compositions of the disclosure comprising at least one fatty acid and
at least one
cholesterol lowering compound may be provided in any formulation or any route
of
administration. In certain embodiments, compositions of the disclosure are
formulated for
oral administration as, for example, a liquid, a tablet, a capsule, a caplet,
or a particulate
(either in a liquid suspension, a solid dosage form, or an encapsulated form).
[0104] Nonlimiting examples of formulations are provided below.
[0105] Tablets may be manufactured using standard tablet processing procedures
and
equipment. Direct compression and granulation techniques are preferred. In
addition to the
active agent, tablets will generally contain inactive, pharmaceutically
acceptable carrier
materials such as binders, lubricants, disintegrants, fillers, stabilizers,
surfactants, coloring
agents, and the like.
- 24 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[0106] Capsules are also preferred oral dosage forms, in which case the active
agent-
containing composition may be encapsulated in the form of a liquid or solid
(including
particulates such as granules, beads, powders or pellets). Suitable capsules
may be either hard
or soft, and are generally made of gelatin, starch, or a cellulosic material,
with gelatin
capsules preferred. Two-piece hard gelatin capsules are preferably sealed,
such as with
gelatin bands or the like. See, for example, Remington: The Science and
Practice of
Pharmacy, cited earlier herein, which describes materials and methods for
preparing
encapsulated pharmaceuticals.
[0107] Oral dosage forms, whether tablets, capsules, caplets, or particulates,
can, if desired,
be formulated so as to provide for controlled release and/or sustained
release, i.e., gradual,
release of a composition of the disclosure, from the dosage form to the
patient's body over an
extended time period, typically providing for a substantially constant blood
level of the at
least one fatty acid or the at least one cholesterol lowering compound over a
specific time
period (e.g. 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 16, 24, 36, or 72 hours or
any time period in
between). Release of the composition may also be delayed; that is, there is a
time lag between
administration and the start of release of the composition.
[0108] Generally, as will be appreciated by those of ordinary skill in the
art, sustained release
dosage forms can be formulated by dispersing the active agent within a matrix
of a gradually
hydrolyzable material such as a hydrophilic polymer, or by coating a solid,
drug-containing
dosage form with such a material. Hydrophilic polymers useful for providing a
sustained
release coating or matrix include, by way of example: cellulosic polymers such
as
hydroxypropyl cellulose, hydroxyethyl cellulose, hydroxypropyl methyl
cellulose, methyl
cellulose, ethyl cellulose, cellulose acetate, and carboxymethylcellulose
sodium; acrylic acid
polymers and copolymers, preferably formed from acrylic acid, methacrylic
acid, acrylic acid
alkyl esters, methacrylic acid alkyl esters, and the like, e.g. copolymers of
acrylic acid,
methacrylic acid, methyl acrylate, ethyl acrylate, methyl methacrylate and/or
ethyl
methacrylate; and vinyl polymers and copolymers such as polyvinyl pyrrolidone
e.g.,
Povidone K30, polyvinyl acetate, and ethylene-vinyl acetate copolymer.
Preferred sustained
release polymers herein include those available as "Methocel" polymers from
Dow Chemical,
particularly the methylcellulose ether polymers in the Methocel TM A group,
having a
- 25 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
viscosity grade of about 4,000 cps and a methoxyl content of about 27.5% to
31.5%, e.g.,
MethocelTM A 15LV, MethocelTM A15C, and MethocelTM A4M.
[0109] When sustained release preparations are prepared, tablets, granules,
powder, capsules,
and the like can be produced according to a conventional method after adding
excipient, and
as necessary, binder, disintegrating agent, lubricant, coloring agent, taste-
modifying agent,
flavoring agent, and the like. These additives may be ones generally used in
the field, and for
example, lactose, sodium chloride, glucose, starch, microcrystalline
cellulose, and silicic acid
as the excipient, water, ethanol, propanol, simple syrup, gelatin solution,
hydroxypropyl
cellulose, methyl cellulose, ethyl cellulose, shellac, calcium phosphate, and
polyvinylpyrrolidone as the binder, agar powder, sodium hydrogen carbonate,
sodium lauryl
sulfate, and stearic acid monoglyceride as the disintegrating agent, purified
talc, stearic acid
salt, borax, and polyethylene glycol as the lubricant, 13-carotene, yellow
iron sesquioxide, and
caramel as the coloring agent, and saccharose and orange peel as the taste-
modifying agent
can be listed as examples. It should be noted that various grades of
microcrystalline cellulose
are preferred fillers herein, e.g., Avice10 PH101, Avice10 PH 102, and Avice10
PH200
(FMC), with particle sizes of about 50 microns, 100 microns, and 190 microns,
respectively.
Microcrystalline cellulose having a particle size in the range of about 50
microns to 200
microns is preferred herein.
[0110] The dosage forms may also be provided with a delayed release coating,
e.g.,
composed of an acrylate and/or methacrylate copolymers. Examples of such
polymers are
those available under the trade name "Eudragit" from Rohm Pharma (Germany).
The
Eudragit series E, L, S, RL, RS, and NE copolymers are available as
solubilized in organic
solvent, in an aqueous dispersion, or as a dry powder. Preferred acrylate
polymers are
copolymers of methacrylic acid and methyl methacrylate, such as the Eudragit L
and Eudragit
S series polymers. Other preferred Eudragit polymers are cationic, such as the
Eudragit E,
RS, and RL series polymers. Eudragit E100 and E PO are cationic copolymers of
dimethylaminoethyl methacrylate and neutral methacrylates (e.g., methyl
methacrylate),
while Eudragit RS and Eudragit RL polymers are analogous polymers, composed of
neutral
methacrylic acid esters and a small proportion of trimethylammonioethyl
methacrylate.
[0111] Preparations according to this invention for parenteral administration
include sterile
aqueous and nonaqueous solutions, suspensions, and emulsions. Injectable
aqueous solutions
- 26 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
contain the active agent in water-soluble form. Examples of nonaqueous
solvents or vehicles
include fatty oils, such as olive oil and corn oil, synthetic fatty acid
esters, such as ethyl
oleate or triglycerides, low molecular weight alcohols such as propylene
glycol, synthetic
hydrophilic polymers such as polyethylene glycol, liposomes, and the like.
Parenteral
formulations may also contain adjuvants such as solubilizers, preservatives,
wetting agents,
emulsifiers, dispersants, and stabilizers, and aqueous suspensions may contain
substances that
increase the viscosity of the suspension, such as sodium carboxymethyl
cellulose, sorbitol,
and dextran. Injectable formulations are rendered sterile by incorporation of
a sterilizing
agent, filtration through a bacteria-retaining filter, irradiation, or heat.
They can also be
manufactured using a sterile injectable medium. The active agent may also be
in dried, e.g.,
lyophilized, form that may be rehydrated with a suitable vehicle immediately
prior to
administration via injection.
[0112] Compositions of the disclosure may also be administered through the
skin using
conventional transdermal drug delivery systems, wherein the active agent is
contained within
a laminated structure that serves as a drug delivery device to be affixed to
the skin. In such a
structure, the drug composition is contained in a layer, or "reservoir,"
underlying an upper
backing layer. The laminated structure may contain a single reservoir, or it
may contain
multiple reservoirs. In one embodiment, the reservoir comprises a polymeric
matrix of a
pharmaceutically acceptable contact adhesive material that serves to affix the
system to the
skin during drug delivery. Alternatively, the drug- containing reservoir and
skin contact
adhesive are present as separate and distinct layers, with the adhesive
underlying the reservoir
which, in this case, may be either a polymeric matrix as described above, or
it may be a liquid
or hydrogel reservoir, or may take some other form. Transdermal drug delivery
systems may
in addition contain a skin permeation enhancer.
[0113] In addition to the formulations described previously, the composition
may be
formulated as a depot preparation for controlled release of the at least one
fatty acid or the at
least one cholesterol lowering compound, preferably sustained release over an
extended time
period. These sustained release dosage forms are generally administered by
implantation
(e.g., subcutaneously or intramuscularly or by intramuscular injection).
[0114] Although the present compositions will generally be administered
orally, parenterally,
transdermally, or via an implanted depot, other modes of administration are
suitable as well.
- 27 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
For example, administration may be transmucosal, e.g., rectal or vaginal,
preferably using a
suppository that contains, in addition to the active agent, excipients such as
a suppository
wax. Formulations for nasal or sublingual administration are also prepared
with standard
excipients well known in the art. The pharmaceutical compositions of the
invention may also
be formulated for inhalation, e.g., as a solution in saline, as a dry powder,
or as an aerosol.
[0115] The term "dosage form" denotes any form of a pharmaceutical composition
that
contains an amount of active agent sufficient to achieve a therapeutic effect
with a single
administration. When the formulation is a tablet or capsule, the dosage form
is usually at least
one such tablet or capsule. The frequency of administration that will provide
the most
effective results in an efficient manner without overdosing will vary with the
characteristics
of the particular active agent, including both its pharmacological
characteristics and its
physical characteristics, such as hydrophilicity.
[0116] By "pharmaceutically acceptable" is meant a material that is not
biologically or
otherwise undesirable, i.e., the material may be incorporated into a
pharmaceutical
composition administered to a patient without causing any undesirable
biological effects or
interacting in a deleterious manner with any of the other components of the
composition in
which it is contained. When the term "pharmaceutically acceptable" is used to
refer to a
pharmaceutical carrier or excipient, it is implied that the carrier or
excipient has met the
required standards of toxicological and manufacturing testing or that it is
included on the
Inactive Ingredient Guide prepared by the U.S. Food and Drug administration.
"Pharmacologically active" (or simply "active") as in a "pharmacologically
active" (or
"active") derivative or analog, refers to a derivative or analog having the
same type of
pharmacological activity as the parent compound and approximately equivalent
in degree.
[0117] As used herein, "subject" or "individual" or "patient" refers to any
subject for whom
or which therapy is desired, and generally refers to the recipient of the
therapy to be practiced
according to the invention. The subject can be any vertebrate, but will
typically be a mammal.
If a mammal, the subject will in many embodiments be a human, but may also be
a domestic
livestock, laboratory subject or pet animal.
[0118] All percentages and ratios are calculated by weight unless otherwise
indicated.
[0119] All percentages and ratios are calculated based on the total
composition unless
otherwise indicated.
- 28 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[0120] Every maximum numerical limitation given throughout this disclosure
includes every
lower numerical limitation, as if such lower numerical limitations were
expressly written
herein. Every minimum numerical limitation given throughout this disclosure
will include
every higher numerical limitation, as if such higher numerical limitations
were expressly
written herein. Every numerical range given throughout this disclosure will
include every
narrower numerical range that falls within such broader numerical range, as if
such narrower
numerical ranges were all expressly written herein.
[0121] The values disclosed herein are not to be understood as being strictly
limited to the
exact numerical values recited. Instead, unless otherwise specified, each such
value is
intended to mean both the recited value and a functionally equivalent range
surrounding that
value. For example, a value disclosed as "20 mg/dL" is intended to mean "about
20 mg/dL."
[0122] Every document cited herein, including any cross referenced or related
patent or
application, is hereby incorporated herein by reference in its entirety unless
expressly
excluded or otherwise limited. The citation of any document is not an
admission that it is
prior art with respect to any invention disclosed or claimed herein or that it
alone, or in any
combination with any other reference or references, teaches, suggests or
discloses any such
invention. Further, to the extent that any meaning or definition of a term in
this document
conflicts with any meaning or definition of the same term in a document
incorporated by
reference, the meaning or definition assigned to that term in this document
shall govern.
[0123] While particular embodiments of the disclosure have been illustrated
and described,
various other changes and modifications can be made without departing from the
spirit and
scope of the disclosure. The scope of the appended claims includes all such
changes and
modifications that are within the scope of this disclosure.
EXAMPLES
[0124] In order that the invention disclosed herein may be more efficiently
understood,
examples are provided below. It should be understood that these examples are
for illustrative
purposes only and are not to be construed as limiting the invention in any
manner.
Throughout these examples, molecular cloning reactions, and other standard
recombinant
DNA techniques, were carried out according to methods described in Maniatis et
al.,
- 29 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
Molecular Cloning - A Laboratory Manual, 2nd ed., Cold Spring Harbor Press
(1989), using
commercially available reagents, except where otherwise noted.
EXAMPLE 1: Patient Demographics.
[0125] The data presented herein were collected over a 14 year period from a
69 year old,
Caucasian male (Patient A) and a 72 year old Caucasian female (Patient B).
[0126] Patient A had several heart attacks in 1991, 1995 and 2001. The heart
attack in 1991
resulted in administering a balloon angioplasty. The attack in 1995 resulted
in the placement
of a stent in the coronary artery. In 2001, the heart attack resulted in a
quadruple bypass.
After years without a heart incident, Patient A suffered arterial fibrillation
in March 2010.
Patient A was implanted with a defibrillator in the upper left chest along
with cardiac
ablation.
[0127] There were a number of statins prescribed for Patient A beginning in
2001 with
Lipitor, Vytorin, Provachol and Zetia. Statins were changed continually
because the
insurance provider no longer listed a particular statin on its formulary. The
only non-statin
was Tricor, a fenofibrate, which reduces cholesterol and triglycerides.
[0128] After suffering arterial fibrillation, Patient A was prescribed a
series of medicaments
to control the fibrillation, blood pressure and water retention. The
prescribed medications
included Lisinopril, Digoxin, Warfarin, Metroprolo and Lasix.
[0129] The medicament Metroprolol alone is known to cause psoriasis and a
combination of
Metoprolol and statin are known to cause psoriasis and other autoimmune
diseases.
[0130] A 72 year old female Caucasian, Patient B, does not have a heart
disease or any
autoimmune disease; however, she has had seizures beginning in 2004. Magnetic
resonance
imaging (MRI) showed a small scar in the hippocampus region and is believed to
cause her
seizures. The seizures are controlled by two drugs, Depakote and Keppra.
[0131] The reason to include Patient B in this study is because Patient B and
Patient A are
spouses and work and eat meals together. Whatever Patient A consumes so does
Patient B.
The vitamins, hormones, fatty acids and minerals are taken by both Patients
under the same
conditions. Patient B becomes a baseline (negative control) to measure the
effects of
vitamins, hormones, fatty acids, organic compounds and minerals taken without
statins.
- 30 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[0132] The effect of taking a fatty acid, hormone, vitamin or organic compound
while
taking a statin or other cholesterol lowering medicine(s) was measured in
Patient A using
Patient B as a negative control. Both Patients A and B took the same fatty
acids, hormones,
vitamins, minerals, organic compounds and antioxidants. Also they had the same
meals each
day, 7 days a week. A comparison of Figure 1 (Patient A) and Figure 3 (Patient
B) reveals
striking results provided herein.
[0133] The concentration of medicaments will vary depending on numerous
factors, such
as, for example, the seriousness of the disease; use of other medications; the
subjects
physiology; other comorbidities; medicament preparation, for example, pill
versus gel
capsule; medicament administration and the type of disease being treated.
EXAMPLE 2: Experiment 1
[0134] Patient B's medical history began on July, 2001. Her total cholesterol
was 217
mg/dL. The total cholesterol increased to 259 mg/dL in October, 2004. But in
December,
2004, the total cholesterol decreased to 174 mg/dL, a total decrease of 32.8%.
At the time,
Patient B was under the care and guidance of a naturopathic doctor who advised
Patient B to
undergo a bodily detoxification. The detoxification process is described in
Table 6. The goal
was to take for 2 weeks: greens and acidophilus (2 times daily), Ultra Clear
or Plus (3 times
daily) and Meta fiber or flaxseed (2 times daily). The purpose was to rid the
body's cells of
waste and toxins. What resulted was a lowering of the total cholesterol by
32.8%, a very
impressive decrease. Patient B was given 3 months to recuperate from the
detoxification
before she went through the detoxification program again for another 2 weeks.
[0135] In April, 2005, Patient B's total cholesterol level was measured. From
October,
2004 her total cholesterol value decreased by 38%. That is from the December,
2004, her
total cholesterol decreased by another 5.2%. This is a substantial decrease in
total
cholesterol, lipoproteins and hormones just by taking a few greens,
acidophilus, minerals,
vitamins, flax seed and fiber.
[0136] Once the detoxification was over, the blood's total cholesterol,
lipoproteins and
hormones, returned to a normal value. This oscillatory cholesterol value
lasted from May,
2005 through June, 2012, more than 7 years as illustrated in Figure 3 and
Figure 4.
- 31 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
EXAMPLE 3: Experiment 2
[0137] Table 3 has 2 interesting data points that will be used later in
establishing the
meaning of vitamin D. Those data points are September, 2010 and June, 2012.
The total
cholesterol increases and the blood plasma concentration of total vitamin D
increases. From
2 data points and the high blood plasma concentration of vitamin D, one can
conclude that
very high concentrations of vitamin D in blood serum will increase total
cholesterol.
[0138] For the next year, August, 2013, the cholesterol level dropped to 179
mg/dL when
Patient B took fish oil, Omega-3 fatty acid ethyl ester with a concentration
of 2100 mg of fish
oil, containing EPA-800 mg and DHA-400 mg. In the mixture taken on August,
2013 were
the ingredients of vitamin C, vitamin E, calcium, magnesium and vitamin D.
[0139] As shown in Table 3, the lower cholesterol is due to the Omega-3 fatty
acids and
low concentration of vitamin D. From August, 2013, Figure 4, through
September, 2014, the
total cholesterol constantly oscillates from a maximum of 237 mg/dL to a
minimum of 179
mg/dL a total change of 32.4%. Such a large fluctuation is not a homeostatic
control limit.
EXAMPLE 4: Experiment 3.
[0140] From August, 2013 through September, 2014, there were no signs of
treating
diseases, autoimmune or otherwise. Patient B was given Crestor, (20 mg)
resulting in a
significant decrease in cholesterol. The total cholesterol was homeostatic at
a maximum of
179 mg/dL and a minimum of 173 mg/dL for a percentage relative range of 3.4%,
a very tight
range for total cholesterol.
EXAMPLE 5: Experiment 4
[0141] Experiment 4 is a subset of Experiment 3. Patient B was given Crestor
(20 mg) and
her cholesterol level was measured from January, 2015 through April, 2015.
During the
experiment, she experienced auras (preictals), brief moments where she was
unable to form a
vocal sentence. It is usual that her preictals occur before a grand mal
seizure. At first her
auras occurred monthly. In April they increased to every other day without a
grand mal
seizure. In May, she discontinued Crestor, but the auras continued with the
same frequency.
The following month, June, she was given vinpocetine and Coenzyme Q10 (trans-
form). The
auras discontinued the next day.
[0142] In summary from Patient B's data illustrated in Figure 3-4 and Table 3,
we conclude
that: (1) total cholesterol oscillates continuously over a broad range; (2) a
drastic change in
- 32 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
diet, for example, detoxification for one week, drastically reduces total
cholesterol and
subdues total cholesterol for a number of years, in this case approximately 7
years; (3) an
addition of Omega-3 fatty acids initially shocks the human system similar to
detoxification,
but continual administration of the fatty acids (triglycerides or ethyl ester
fatty acids) or
hormones or vitamins does not stabilize the total cholesterol; (4) an addition
of a statin to the
Omega-3 fish oil and hormone or vitamin, now called a medicament, stabilizes
the total
cholesterol within very narrow limits.
EXAMPLE: Experiment 5.
[0143] Patient A sheds much of the insight into treating autoimmune diseases
and other
diseases. The data was collected over a 14 year period. Experiment 5 begins in
January,
2001 and ends November, 2009. The statins administered during this time were
modified,
added, changed, substituted, eliminated and adjusted according to health
insurance
formularies. The additions and modifications were graphically represented in
Figure 1-2 and
illustrated in Table 2.
[0144] Patient A underwent a quadruple bypass in May, 2001. He was prescribed
Lipitor
and Lopressor shown in Table 2. That changed in March, 2003 to Lipitor and
Metoprolol. In
an attempt to lower Patient's A total cholesterol, the Lipitor concentration
was increased from
40 mg/day to 80 mg/day on February, 2004.
[0145] The blood work done in August, 2004 gave a total cholesterol of 129
mg/dL a 69%
decrease from the present February reading of 198 mg/dL; however, continuing
with the
same statin concentration did nothing to maintain the same or similar total
cholesterol level.
In fact the total cholesterol increased drastically to 183 mg/dL. Increased
levels of statins
give a drastic decrease in total cholesterol levels, but the change is not
permanent and
conditions revert to their previous state.
[0146] In May, 2005, the medication was enhanced by the inclusion of Tricor.
The total
cholesterol decreased to 156 mg/dL.
[0147] In January, 2006, Patient A started a 2 week detoxification program as
illustrated in
Table 6 (the same program started by Patient B in December 2004 and April
2005). Results
for Patient A and Patient B were similar; a drastic reduction in total
cholesterol. For Patient
A whose total cholesterol was 187 mg/dL, ingesting Lipitor at 40 mg per day,
the
- 33 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
detoxification lowered the total cholesterol to 108 mg/dL, a change of -42.2%.
Total
cholesterol decreased by 42% just by detoxifying the body.
[0148] Without taking any statins, Patient B had a similar reaction to the
detoxification.
The purpose of discussing detoxification is the drastic effect it has on
overall hormone
synthesis. Whether or not detoxification is useful in treating autoimmune
diseases; its effect
on the human body is what is important. The same effect was observed with
respect to
Patient A and Patient's B's consumption of Omega-3 fatty acids. Total
cholesterol will
drastically decrease with the consumption of an Omega-3 fatty acid(s) or
oil(s) and will
decrease with the consumption of Omega-3 fatty acid(s) and a statin(s), but in
the case of an
Omega-3 fatty oil(s) and a statin(s) the result is to lower the total
cholesterol and maintain
that level for a considerable time within a narrow tolerance range
establishing total
cholesterol homeostasis.
[0149] The combination of an oil and a statin results in homeostasis. Another
consequence
of combining Omega-3 fatty acid(s) or oil(s) and a statin(s) are to treat
autoimmune diseases.
As will be discussed, other vitamin(s), hormone(s), organic compound(s),
enzyme(s),
protein(s) and antibodies will treat other autoimmune diseases or other
diseases and in the
process establish a total cholesterol homeostasis.
[0150] Also, total cholesterol (TC) will affect high density lipoproteins
(HDL), low density
lipoproteins (LDL), triglycerides (TG) through the Friedwald equation
TC=HDL+LDL+
(0.2TG). Controlling the total cholesterol regulates very low density
lipoproteins (VLDL),
apolipoprotein Al, apolipoprotein B, an intermediate density lipoprotein
(IDL),
apolipoproteins, chylomicrons, enzymes, vitamins, hormones, fats, proteins,
lipids, glycerol,
etc.
[0151] After detoxification, Lipitor and Tricor administration returned the
total cholesterol
to the previous level of May, 2005.
[0152] In November, 2006 Patient A was put on Vytorin. The result stabilized
total
cholesterol. Again, as previous, Patient A was taken off Vytorin due to
insurance formularies
and he was put on Provachol (80 mg) and Zetia (10 mg). Total cholesterol was
reduced
slightly.
- 34 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[0153] In August, 2009, Tricor was added to Lipitor in an attempt to reduce
total cholesterol
and triglycerides more specifically to reduce LDL and VLDL as well as increase
HDL. The
Lipitor and Tricor combination was a dismal failure.
101541 A new combination was tried in January, 2010 with excellent results.
Total
cholesterol dropped to 138 mg/dL from its 175 mg/dL a total change of -21.1%.
In March,
2010 the total cholesterol dropped further to 116 mg/dL. Again comparing
August data to
March data, there was a total change of -33.7%. The result showed a drastic
lowering of the
total cholesterol.
Example 7: Experiment 6
[0155] April, 2010, Patient A's heart went into arterial fibrillation. It was
decided to
implant a defibrillator in the left chest.
[0156] Thereafter Patient A was prescribed a number of medications in addition
to Lipitor
and Zetia. The additional medications included Lisinopril, Digoxin, Warfarin,
Metoprolol
and later Lasix. Table 4 lists the medication and their concentrations in
milligrams per day
(mg/day).
[0157] After the implant, taking Lipitor and Zetia did not perform as stellar
as in January,
2010. The total cholesterol values oscillated from a high in August, 2011 of
190 mg/dL to a
low in September, 2012 to 139 mg/dL a percentage relative range of 164.5%.
This value was
not within the range of homeostasis.
[0158] Patient A was prescribed a number of medications. One of which was
Metoprolol.
As an adverse side effect, Metoprolol is known to cause psoriasis.
[0159] Ten months after taking Metoprolol, Patient A suffered from psoriasis
on the left and
right calves. By August, 2011 the psoriasis included the calves and the entire
back.
Warfarin, taken daily as a blood thinner, caused the psoriasis on the calves
to bleed
excessively due to the dermal skin layer cracking and separating from the
epidermal layer.
[0160] By March, 2012, the psoriasis had progressed to the scalp. The
psoriasis measured
1-1.5 inches in diameter and as the epidermal layer was removed by scratching
the scalp or
vigorously rubbing the scalp would remove large areas of hair.
[0161] By September, 2012, the ears were next affected resulting in excessive
skin loss.
Each epidermal layer would peel off from the ear. Later in February, 2013,
psoriasis
appeared on the face, 3 dots as precursors to plaque psoriasis.
- 35 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
EXAMPLE 8: Experiment 7
[0162] In March, 2013, Patient A took Omega-3 fish oil, vitamin D3, vitamin C
and a
vitamin B complex. The itching from psoriasis was gone in 2 weeks. There was
no calf
bleeding and no gum bleeding. During the same time, myositis disappeared.
Within the next
2 weeks, one month in all, all psoriasis had disappeared. The total
cholesterol in September,
2012 was 139 mg/dL.
[0163] September's cholesterol reading was a result of taking Lipitor (80 mg)
and Zetia (10
mg). The same concentration taken back in January, 2010 through September,
2012 resulted
in average total cholesterol of 149 mg/dL. Comparing the average total
cholesterol data
calculated above at 149 mg/dL with March, 2013 data of 116 mg/dL, one observes
a total
cholesterol change of -22.1%, a very substantial decrease.
[0164] Total cholesterol was lowered significantly on a number of occasions.
They were
the detoxification of Patient B and Patient A and when Patient A was taking a
high
concentration of a new statin or multiple statins; however, the total
cholesterol reverted to its
previous high values and continued thereafter to oscillate. But, from May,
2013 through July,
2013, the total cholesterol's range was 5.0 mg/dL and the percentage relative
range was
3.9%, a very tight range. A similar observation was made in Experiment 3 with
Patient B.
EXAMPLE 9: Experiment 8
[0165] It is important to understand that Patient A took the medicaments 30
days prior to
his blood specimen collected and a comprehensive metabolic panel and lipid
panel evaluated
in March, 2013. Seven days after the medicaments were ingested, the itching on
the dermal
and epidermal skin surface ceased. Another 7 days and the psoriasis on the
face disappeared.
[0166] Within 14 days thereafter, all the psoriasis on the calves, back, scalp
and ears was
gone; hence, a 30 day period is all that is necessary for Patient A to be
evaluated for effective
remedies attributed to a medicament. Blood work is evaluated approximately
every 30 days
after March, 2013.
[0167] After March, 2013, vitamin C was the first medicament to be removed.
Taking
vitamin C for 2 months was enough to stop the gum bleeding and strengthen the
gum tissue.
Removal of vitamin C consequently had no effect. Also, there was no effect on
the total
cholesterol.
- 36 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[0168] The vitamin C was replaced and Omega-3 fish oil removed. Within 2 weeks
in
August, 2013, psoriasis returned as well as another skin disease known as
keratosis, both
pilaris and seborrheic. Patient A was given 2000 mg of Omega-3 fish oils as
outlined in
Table 2 and cross-referenced in Table 4. Within 2 weeks the psoriasis and
pilaris keratosis
were gone. The seborrheic keratosis took 4-6 months to disappear.
[0169] Throughout the time interval May, 2013 through October, 2013, the total
cholesterol
remained within a very narrow range even when the Omega-3 fish oils were
removed for 2
weeks. The total cholesterol high at 138 mg/dL was not included in Experiment
7's percent
relative range, because the Omega-3 fish oils have a strong influence on the
total cholesterol.
This will be evident in later Experiments.
[0170] There was one exception to Experiment 8. In September, 2013, the total
cholesterol
was 158 mg/dL. Table 2 illustrates that the only significant change was the
vitamin D3
dosage. It was 12,000 IU units.
[0171] From the previous months, the dosage of vitamin D3 ranged from 3000 IU
to 5000
IU and to 6000 IU with no apparent effect on the total cholesterol level. At a
vitamin D3
consumption of 5000 IU, the total vitamin D, 25-0H in the blood serum measured
44.6
ng/mg; however, at a very high concentration of vitamin D3, 12,000 IU; total
vitamin D, 25-
OH measured was 89.3 ng/ml; the total cholesterol level increases, but there
was no change
in autoimmune disease characteristics or other disease characteristics.
[0172] Vitamin D3 may lower active statin metabolites. Thus, increasing the
vitamin D3
concentration may lower the efficacy of Atorvastatin.
[0173] Patient A went back to his normal medicament concentrations in October,
2013 and
returned to a total cholesterol value of 132 mg/dL.
[0174] In November and December, 2013, the vitamin D3 was split into a vitamin
D3, 1000
IU power tablet and a vitamin D3, 1000 IU gel capsule. The total cholesterol
increased
slightly to 150 mg/dL. Psoriasis and keratosis did not return. Myopathy was
slight. On a
scale of 0-10, 0 being no pain and 10 being severe pain, Patient A's pain was
about 1. Patient
A has had myositis since he started taking statins in June, 2001. The level of
pain increased
progressively over the years reaching a level of 4 in March, 2013. Thereafter
the myositis
disappeared.
- 37 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[0175] The indication was that powder vitamin D3 does not have an effect on
total
cholesterol nor an effect on myopathy; also it is indicated that more than
1000 IU of vitamin
D3 as a liquid gel is needed to control myopathy for Patient A.
EXAMPLE 10: Experiment 9
[0176] In January, 2014, Patient A was taken off Lipitor and given Simvastatin
at 40 mg.
Zetia (10 mg) and Simvastatin (40 mg) is known also as Vytorin. Simultaneously
Patient A
discontinued taking vitamin D3. The first night after taking the
Zetia/Simvastatin
combination minus the vitamin D3, he experienced severe myositis at a pain
level of 8 out of
10. Pain persisted throughout the early morning. Exercise lowered the pain
level to 2
throughout the day.
[0177] It was the Simvastatin that cause a high level of pain. The next day he
was given
another 40 mg of Simvastatin along with Zetia and without vitamin D. Patient A
had another
painful night. The pain level was again 8. Patient A was given vitamin D3 1000
IU in solid
tablet and vitamin D3 1000 IU added to the Omega-3 fish oil. The pain
persisted. The
vitamin D3 powder provided little to no benefit and the vitamin D3 1000 IU
added to the
Omega-3 fish oils was not enough. Total vitamin D, 25-0H was 35 ng/ml.
[0178] Due to the high pain level, much larger vitamin D3 concentration was
administered.
Vitamin D3 was increased to 3000 IU. Again the same statins were administered
as before
without any myositis (myopathy). For 3 months, Patient A had no psoriasis, no
keratosis, no
bleeding gums and no myopathy. However, because the statin combination of
Zetia/Simvastatin is not as efficacious as the statin combination
Zetia/Lipitor, the total
cholesterol increased, but remained in a narrow range. The total cholesterol
range was 23
mg/dL and the percent relative range was 13.1% that is higher than the
Lipitor/Zetia
combination.
[0179] There were two regions in Figure 2 that illustrate the relationship
between Omega-3
fish oil and statin(s). Region 1 begins May, 2013 through October, 2013.
Region 2 begins
January, 2014 through March, 2014. Region 1 was influenced by Lipitor/Zetia
and Omega-3
fish oil. Region 2 was influenced by Simvastatin/Zetia and Omega-3 fish oil.
Each region
had a different total cholesterol range. Lipitor had a lower range than
Simvastatin, but
Lipitor was at twice the concentration as Simvastatin. At the same
concentrations each statin
would perform in a similar manner. Region 1 had average total cholesterol of
132 mg/dL.
- 38 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[0180] Region 2's average total cholesterol was 175 mg/dL. Both regions' total
cholesterol
differed by 43 mg/dL, a considerable amount given that the total cholesterol
is less than 200
mg/dL for a patient with coronary heart disease. The same analogy holds
through later
experiments with different statins and different Omega-3 fish oil(s).
[0181] Whatever the level of total cholesterol, Patient A had no recurrence of
psoriasis, no
recurrence of keratosis, no recurrence of bleeding gums, no recurrence of
neuropathy and no
recurrence of myositis. The level of total cholesterol was indifferent to the
treating of an
autoimmune disease. The Omega-3 fatty oils are responsible for the narrow
total cholesterol
range and responsible along with a statin(s) for treating autoimmune diseases
and other
diseases.
[0182] Other organic compounds will treat other diseases aside from autoimmune
diseases.
Also, other organic compounds will narrow and still lower again the total
cholesterol.
Vitamin D3 was an active ingredient for treating myopathy that required the
combination of
Omega-3 fish oil(s) and statin(s) to attain a superior efficacy of treatment.
EXAMPLE 11: Experiment 10
[0183] In April, 2014, Patient A was administered Crestor (40 mg) as a
replacement for
Lipitor (80 mg). The difference in total cholesterol for either statin was
very slight; however,
the concentration of Crestor was half that of Lipitor. Likewise the autoimmune
diseases were
suppressed with Omega-3 fish oil and Crestor and Omega-3 fish oil, Crestor and
vitamin D3.
[0184] Patient A was administered Omega-3 fish oil(s) at a concentration of
3000 mg. The
fish oil(s) EPA (eicosapentaenoic acid) and DHA (docosahexaenoic acid) were
now 3 times
what they were in previous months. The result was a drastic decrease in total
cholesterol.
The percentage change was -20%; it represented the total cholesterol for
April, 2014 of 137
mg/dL to May, 2014 whose total cholesterol was 109 mg/dL. There was no change
in the
autoimmune diseases.
EXAMPLE 12: Experiment 11
[0185] Experiment 11 was designed to check the results found in Experiment 10.
Patient A
changed his Crestor (40 mg) to Lipitor (80 mg). The total cholesterol
increased substantially,
because Crestor is a more efficacious inhibitor of the enzyme HMG-CoA
reductase. The
Lipitor/Zetia statins maintained a very tight total cholesterol range from
July, 2014 through
October, 2014.
- 39 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[0186] In November, 2014, Patient A was administered Crestor (40 mg) as a
substitute for
Lipitor (80 mg) and administered 3000 mg of Omega-3 fish oil(s). The total
cholesterol
decreased drastically to 103 mg/dL from a previously total cholesterol value
of 136 mg/dL, a
decrease of approximately 24%. The previous medicaments were the same as
illustrated in
Table 2. Also, Patient A experienced a root canal extraction. There was no
problem in the
repair of the root canal; however, Patient A experienced no pain for months
while the roots
were decaying, a result of vitamin D therapy.
EXAMPLE 13: Experiment 12
[0187] In December, 2014, Patient A continued his administration of Crestor
and Zetia, but
he added to his medicaments Curcumin, quercetin, bacopa, bioperine and
phosphatidylcholine. There was a definite effect on dementia, short and long
term memory.
EXAMPLE 14: Experiment 13
[0188] Attempting to find the lower concentration limit necessary to treat the
autoimmune
diseases psoriasis, keratosis and myopathy; vitamin D3 was lowered to 1000 IU
and Omega-3
fish oil(s) was lowered to 600 mg. During January, 2015, Patient A noticed a
slight muscle
pain in the buttocks and lower back. Vitamin D3 measured in the blood as total
vitamin D,
25-0H was 31.4 ng/ml.
[0189] Vitamin D3 was increased to 2000 IU per day in February, 2015. The pain
associated with myositis soon disappeared; however, due to the low
concentration of Omega-
3 (s) a small skin rash or seborrheic keratosis appeared. Early May, 2015 the
Omega-3 fish
oil(s) was increased to 1600 mg (600+1000 mg) whose EPA concentration was 847
mg and
whose DHA concentration was 453 mg. After the medicament Omega-3 oil(s) was
administered, the skin condition disappeared.
EXAMPLE 15: Experiment 14
[0190] At the end of April, 2015, Patient A's Omega oil(s) were increased to
2980 mg, but
this time Omega 6 and Omega 9 oils were added as shown in Table 2.
[0191] Omega-3 oils were broken-down as: EPA-800 mg, DHA-400 mg, Omega 6-276
mg
and Omega 9-170 mg. Within the next month, July, 2015, seborrheic keratosis
returns and is
believed due to the Omega 6 oil. Vitamin C and vitamin B complex were removed
without
any adverse effects to the disease patterns. The soft gum and bleeding gums
were repaired
where the gums were hard and no bleeding occurred.
- 40 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[0192] For September, 2015, Omega 6 and Omega 9 were eliminated as medicaments
and
the seborrheic keratosis disappeared. Also vinpocetine at 30 mg was added as a
medicament
in June, 2015. The total cholesterol decreased by approximately ten percent,
not a substantial
amount; however, the total cholesterol tolerance interval is very narrow from
June through
August; hence, vinpocetine stabilizes cholesterol as well as lowers the total
cholesterol. A
result observed with Omega-3 fish oils. Again another organic compound found
effective
with a statin(s).
[0193] At a similar time, Patient B was given vinpocetine when she was having
minor
preictals every other day. Vinpocetine eliminated the minor auras. Patient A
reported an
enhancement of long and short memory with bacopa included.
EXAMPLE 16: Experiment 15
[0194] Later in August, 2015 and early September, Omega-3 oil(s) were removed
as a
medicament for Patient A. Within a few days, Patient A was covered entirely on
the front
right chest and on the right upper and lower back with pilaris keratosis. The
Omega-3 oil(s)
were introduced at a concentration of 2100 mg and the pilaris keratosis
disappeared within 2
weeks; hence, vinpocetine will not treat the autoimmune disease of keratosis;
however, it has
a strong effect on controlling the total cholesterol after Omega-3 oil(s) has
been administered
as well as controlling dementia.
Data Tables
[0195] Table 2 is an accumulation of Patient A's data for total cholesterol
illustrated as total
cholesterol (TC), high density lipoprotein (HDL), low density lipoprotein
(LDL),
triglycerides (TG), drug type and their concentrations and the comments
associated with the
drugs, vitamins, hormones, oils, fish oils, statins, organic compounds,
vitamin D, vitamin D3
blood concentration, and adsorption parameters.
[0196] Table 2: Patient A Data
DATE TC HDL LDL TG DRUG CONC. COMMENTS
(mg/dL) (mg/dL) (mg/dL) (mg/dL) type (mg/day)
5/2001 quadrupel by-pass
surgery
6/2001 1, 34 40, 100 Lipitor, Lopressor
11/2001 187 47 123 105 1,9 40,50 Lipitor, Metoprolol
2/2003 170 53 90 134 1, 9, 16 40, 50, 1000 Niacin
3/2003 198 51 128 95 1,9 40,25
11/2003 182 62 107 65 1,9 40,25
2/2004 198 51 128 95 1 80 Lipitor
8/2004 129 54 63 60 1 80
- 41 -
CA 03020087 2018-10-04
WO 2017/180127 PCT/US2016/027524
3/2005 183 37 62 417 1 80
5/2005 156 51 92 64 1,2 80, 145 Lipitor,
Tricor
1/2006 108 39 57 59 1, 2 80, 145 Detoxification
cleaning
4/2006 161 55 91 75 1,2 80,145
11/2006 160 54 90 78 3 10,40 Vytorin
(Zetia/Simuvastatin)
6/2007 149 50 86 63 3 10,40
9/2008 147 35 80 161 3 10,40
12/2008 225 40 166 94 4 80 Provacol
3/2009 211 50 137 118 4,5 80,10 Provachol, Zetia
6/2009 176 38 110 140 4 80 Provachol
8/2009 175 42 114 97 1,2 40, 145 Lipitor,
Tricor
1/2010 138 51 76 56 1, 5, 10 A, B Lipitor,
Zetia,
Lisinopril, Digoxin,
Warfarin, Metoprolo
3/2010 116 40 63 76 1, 5, 10 A, B
4/2010 23, 35 A, B, 240 Atrial fibrillation,
defibrillator implanted
2/2011 160 43 92 125 1, 5, 10 A, B psoriasis
on legs
8/2011 190 42 107 203 3, 10 10, 40, B psoriasis on
legs, back,
11/2011 Lasik
3/2012 151 40 85 129 23,19 A, B, 80 psoriasis on
legs, back,
scalp
9/2012 139 36 73 148 23, 19 A, B, 80 psoriasis on
legs, back,
scalp, ears and on
2/2013 appears on face
3/2013 116 33 61 112 22, 11, 12, C, 3000 IU,
2-weeks itching gone,
13, 14 125,1000, no bleeding from skin,
1000 no psoriasis, no
myopathy, 2-weeks
no gum bleeding, EPA-
667, DHA-333
5/2013 133 36 73 118 22, 11, 12, C, 3000 IU,
13, 14 125,1000,
1000
6/2013 127 36 56 176 22, 11, 12, C, 3000 IU,
no psoriasis, no
13,14 125,1000,0 myopia, no vitamin C
7/2013 132 36 77 96 22, 11a, 12, C, 6000 IU,
no psoriasis, no
13,14 125,1000,0 myopia, no vitamin C
8/2013 138 36 75 134 22, 11a, 12, C, 5000 IU,
psoriasis returns, there
13, 14 125, 0, 1000 is a new skin disease,
Keratosis, small pilaris
keratosis on back, left
head, Total vitamin D,
25-0H is 44.6ng/m1
9/2013 158 41 88 143 22, 11a, 12, C, 12,000 IU, no
psoriasis, seborrheic
13, 14 125, keratosis takes months
2000,1000 before it will go away.
Seen on rt. arm. Old
skin defect. Everything
back to normal. Total
vitamin D, 25-0H is
89.3ng/m1 EPA-1334,
DHA-666
- 42 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
10/2013 132 40 72 98 22, 11, 12, C, 4000 IU,
13, 14 125, 2000,
1000
11/2013 150 43 79 139 22, 15, 12, C,1000 IU D3-
Nature's Made
13, 14 powder and powder myopathy
1000 IU from returned slightly, no
Omega-3, psoriasis, no keratosis.
125, 1000, total vitamin D, 25-0H
1000 is 64.1 ng/ml
12/2013 151 40 87 120 22, 15, 12, C,1000 IU no
psoriasis, no
13, 14 powder and keratosis, total vitamin
1000 IU from D, 25-0H is 35 ng/ml
Omega-3,
125, 1000,
1000
1/2014 176 39 96 207 3, 10, 15, 10/40, B, no
psoriasis, no
12, 13, 14 1000 IU from keratosis, have split
Omega-3, vytorin to10/40
125, 1000, Ezetimbe/Simvastatin;
1000 myopathy
returns-very
high pain level, 8 of 10
2/2014 186 48 105 164 3, 10, 11, 10/40, B, no
psoriasis, no
12, 13, 14 3000 IU,
keratosis, no myopathy,
125,1000, niacin lowers
1000 cholesterol, total
vitamin D, 25-0H is
33.2 ng/ml
3/2014 163 41 90 160 3,10,11,12, 10/40,B, no
psoriasis, no
13,14,16 3000 IU, keratosis, no myopathy,
125,1000, niacin lowers
1000, 500 cholesterol, total
vitamin D, 25-0H
is 33.2 ng/ml
4/2014 137 46 60 157 17,5,10,11, 40,10,B,
12,13,14 3000 IU,125,
1000, 1000
5/2014 109 40 44 124 17,5,10,11, 40,10,B,
conc. of fish oil 3X and
12,13,14 5000 IU,125, EPA and DHA is 3X
3000,1000
6/2014 116 43 52 103 17,5,10,11, 40,10,B,
12,13,14 5000 IU,125,
3000,1000
7/2014 142 46 68 138 22, 11, 12, C, 3000 IU, total
vitamin D, 25-0H
13, 14 125, 1000, is 47.3 ng/ml
1000
8/2014 150 40 70 199 22, 11, 12, C, 5000 IU,
13, 14, 18 125, 3000,
1000, 20
9/2014 157 42 85 148 22, 11, 12, C, 5000 IU,
13, 14, 18 125, 3000,
1000, 20
10/2014 136 42 72 110 22, 11, 12, C, 3000 IU,
13, 14, 18 125, 1000,
1000, 20
- 43 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
11/2014 103 38 45 99 17,5,10,11, 40,10,
B, bioperine has no effect
12,13,14 5000 IU, 125, on cholesterol or
3000, 1000 psoriasis, keratosis,
gum disease or
myopathy, root canal,
no pain
12/2014 118 36 48 171 17,5,10,11, 40,10,B, taking
curcumin,
12,13,14 5000 IU,125, quercetin, bacopa,
3000,1000 bioperine
and
phosphatidyl cholin had
no effect on cholesterol,
but a definite effect on
dementia, short and
long term memory
observed by Patient A
1/2015 123 44 55 121 17, 5, 10, 40,
10,B, total vitamin D, 25-0H
11,12, 32, 1000 IU, 125, is 31.4
ng/ml, slight
14 600, 1000 muscle
pain in buttocks
& lower back, EPA-
180, DHA-120
2/2015 135 45 65 123 17, 5, 10, 40, 10,B, pain in
lower back is
11,12, 32, 2000 IU,
125, gone however dry skin
14 600, 1000 or keratosis
returned
4/2015 134 46 56 162 17, 5, 10, 40,
10,B, common cold-increase
11, 12, 13 30001U, 125, vitamin C
to
and 32, 14 1600mg,
8000mg/day - (3) days,
1000 EPA-847,
DHA-453 no
pain, no skin condition
4/2015 121 43 53 127 17, 5, 10, 40, 10,
B, EPA-1247, DHA-653
11, 12, 13, 30001U, 125,
32 and 33, 2650mg,
14 1000
6/2015 108 43 45 100 17, 5, 10, 40, 10, B, Omega-3
1484, EPA
11, 27 and 20001U, 465, DHA
252, Omega
33, 25, 26 1484, 30, 6-276, Omega 9-170,
1200
vinpocetine,
phosphatidyl choline,
no vitamin C, no
vitamin B
7/2015 113 43 45 124 17, 5, 40, 10, B, Seborrheic
keratosis
10,11, 33, 2000 IU, returned
due to Omega
25,26 1050, 30, 6 or Omega 9..
1200
8/2015 107 33 44 150 17,5, 40, 10,B,
elimination of Omega 6
10,11,33, 20001U, and Omega
9,
25,26 1050, 30, eliminated seborrheic
1200 keratosis, vinpocetine,
stabilizes cholesterol
and lowers it as well as
inhibits preictal events,
Patient B, and enhances
long and short term
memory with Bacopa
included
- 44 -
CA 03020087 2018-10-04
WO 2017/180127 PCT/US2016/027524
[0197] Table 3 is an accumulation of Patient B's data for total cholesterol
illustrated as total
cholesterol (TC), high density lipoprotein (HDL), low density lipoprotein
(LDL),
triglycerides (TG), drug type and their concentrations and the comments
associated with the
drugs, vitamins, hormones, oils, fish oils, statins, organic compounds,
vitamin D, vitamin D3
blood concentration, and adsorption parameters.
[0198] Table 3: Patient B Data
DATE TC HDL LDL TG DRUG CONC. COMMENTS
(mg/dL) (mg/dL) (mg/dL) (mg/dL) type (mg/day)
11/1998 218 101 99 106
7/2001 217 105 104 48
6/2003 246 97 137 57 5/23/03
Santa Fe
trip - memory
loss
10/2004 259 110 136 64 1 9/26/04
seizure
hospital, 10/4/04
seizure
12/2004 174 91 72 53 1 500 11/31/04
seizure
hospital,
detoxification
4/2005 156 87 61 41 1 750 1/3/05
seizure,
detoxification
5/2005 207 84 110 64 1 1000
3/2006 199 96 90 64 1 1000
6/2006 190 78 100 60 1 1000
11/2006 218 86 119 63 1 1500 10/30/06
seizure
45 minutes
3/2007 211 95 103 66 1,2 1500, 1000 7/24/07
strange
feeling on & off
all day
1/2008 209 90 100 95 1, 2 1500, 1500 9/14/07
auras,
5/5/08 speech
impaired,
11/25/09 seizure,
9/2010 208 107 83 108 1, 2, 12 1500,
2000, 1/21/09 seizure,
D 8/29/09
speech
impaired, 3/2010
calcuim and
vitamin D. Total
vitamin D, 25-
OH is 37 ng/ml
6/2012 240 103 121 79 1, 2, 10 1500, 2500,
1/6/10 (4)
1200,600, seizures,
1000 IU
hospital, 2/28/11
seizures, 8/1/12
speech impaired,
6/27/2012 total
vitamin D, 25-
OH is 79 ng/ml
8/2013 179 88 80 55 1, 2, 3, 4, 1500,
3000, Omega-3 at 2100
5, 10 A, E mg,
vitamin D3
at 1000 IU
- 45 -
CA 03020087 2018-10-04
WO 2017/180127 PCT/US2016/027524
9/2013 198 94 85 93 13 F Omega-3
at 2100
mg, vitamin D3
at 3000 IU
10/2013 195 96 86 66 13 F
12/2013 205 116 72 83 13 F
2/2014 221 128 79 70 13 F 2/28/14
aura,
can't speak,
lasted 10 mm.
3/2014 195 118 69 41 13 F
4/2014 237 132 94 54 13 F
5/2014 231 112 105 69 13 F
6/2014 212 116 86 50 10, 11, G, E,100
14
7/2014 179 85 70 120 10, 11, G, E,100
14
8/2014 227 134 81 61 10, 11, G, E,100
14
9/2014 229 117 92 101 10, 11, G, E,100
14
1/2015 188 144 33 57 8, 10, 11, G, E,100, 20 Crestor
20 mg.
14 aura
2/2015 179 134 32 66 8, 10, 11, G, E,100, 20 aura.
total
14 vitamin
D, 25-
OH is 56.6ng/m1
4/2015 173 105 57 56 8, 10, 11, G, E,100,
aura
14, 15, 20, H
16, 17
4/2015 179 135 34 50 8, 10, 11, G, E,100,
increase
14, 15, 20, H frequency
of
16, 17 auras,
every
other day
5/2015 10, 11, G, E,100, H no
Crestor,
14, 15, increase
16, 17 frequency
of
auras, every
other day
6/2015 10, 11, G, E,100, I total
vitamin D,
14, 15, 25-0H is
44.2
16, 17, ng/ml,
no auras,
18, 19
vinpocetine and
coenzyme Q10
(trans form)
inhibits preictals
[0199] Table 4 describes the drugs and organic compounds administered to
Patient A as
well as the concentrations of each drug and organic compound.
[0200] Table 4: Patient A Drug Types and Doses
Number/Letter Drug Organic Compounds
Concentrations
(mg/day)
1 Lipitor
2 Tricor 145
3 Vytorin 10/40
- 46 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
4 Provachol 80
(Pravastatin)
Zetia 10
6 Lisinopril 5
7 Digoxin 0.25
8 Warfarin Sodium 3.04
9 Metoprolol 200
combination of 6, 7, 5, 0.25, 3.04, 200
8 & 9
11 vitamin 03, 2000 IU, 20001U
Carlson (soft gels)
11 a vitamin 03, 5000 IU, 50001U
Nature's Bounty
12 B mega B-125, Ultra 1 capsules
Plan
13 Omega-3 fish oil 1000 1000 mg
EPA-667/DHA-
with vitamin 03 1000 333
IU, Optim Nutrition
14 vitamin C, Vitacost 1000
vitamin 03, 1000 IU, 1000 IU
Nature's Made (tablets)
16 Niacin (B-3), Twin Lab 500
17 Crestor 40
18 Bioperine 20
19 Lasix 80
Simvastatin 40
21 combination of 10 & 19 5, 0.25, 3.04,
200, 80
22 combination of 1, 5 & 21 80, 10, 5, 0.25,
3.04,
200, 80
23 combination of 1, 5 &10 80, 10, 5, 0.25,
3.04,
200
24 Aspirin, Kirkland 325
Vinpocetine, Vitacost 30
26 Phosphatidyl Choline 1200
complex, Country Life
27 Triple Omega, Omega- Omega-3-
434, Omega-
3-6-9, Nature Made 6-276, Omega-9-170
28 Curcumin 2K, Stop 1330
Aging Now
29 Quercetin, Swanson 650
Bacopa, Nature's 500
Answer
31 Max-Q10, Stop Aging 200
Now
32 Omega-3 fish oils with 600 fish
oil EPA
vitamin E, Ultra Plan 180/DHA120
33 Mega EFA, Omega-3 2100 mg fish oil, EPA
EPA & DHA, Vitacost 800/DHA 400
34 Lopressor 100
Diltiazem 240
A concentration of 1 & 5 80 & 10
concentration of 10 5,0.25,
3.04, 200
concentration of A & 21 80, 10 ,5, 0.25, 3.04,
200, 80
- 47 -
CA 03020087 2018-10-04
WO 2017/180127 PCT/US2016/027524
[0201] Table 5 describes the drugs and organic compounds administered to
Patient B as
well as the concentrations of each drug and organic compound.
[0202] Table 5: Patient B Drug Types and Doses
Number/Letter Drug Organic Compounds Concentrations
(mg/day)
1 Depakote
2 Keppra
3 Mega EFA, Omega -3 2100 mg
fish oil, EPA-
EPA & DHA, Vitacost 800, DHA-400
4 vitamin C, 1000, Vitacost 2000
vitamin E-400 IU, Nature 400 IU
Bounty
6 vitamin 03, 2000 IU, 2000 IU
Vitacost
7 vitamin 03, 5000 IU, 5000 IU
Vitacost
8 Crestor 20
9 Mega-B-125, Ultra Plan 125
Calcium Magnesium 1200, 600, 1000 IU
Citrate with vitamin 03,
So!gar
11 Liquid CoQ10, Qunol 100
12 Calcium, Magnesium, 1600, 800, 400 IU
vitamin D, Complete
Bone, Rx Formulations
13 concentration of 1, 2, 3, 1500, 3000,
2100,
4, 5,6, 9, 10 2000,
400 IU, 2000 IU,
125, 1200, 600,
1000 IU
14 combination of 1, 2, 3, 4, 1500, 3000,
2100,
5, 7, 9 2000,
400 IU, 5000 IU,
125
Curcumin 2K with 1330, 20
BioPerine, Stop Aging
Now
16 Quercetin, Swanson 650
17 Bacopa, Nature's Answer 500
18 Vinpocetine, Vitacost 30
19 Coenzyme Q10, Stop 200
Aging Now
A concentration of 3, 4, 5 2100, 2000,
400 IU
concentration of 3, 4, 5, 4200, 2000, 400 IU,
6,9 2000 IU, 125
concentration of 3, 4, 5, 4200, 2000, 400 IU,
7,9 5000 IU, 125
concentration of 12 1600 Ca, 800Mg,
400 IU, 0-3
concentration of 10 1200, 600, 1000 IU
- 48 -
CA 03020087 2018-10-04
WO 2017/180127 PCT/US2016/027524
concentration of 13 1500, 3000, 2100,
2000, 400 IU, 2000 IU,
125, 1200, 600,
1000 IU
concentration of 14 1500, 3000, 2100,
2000, 400 IU, 5000 IU,
125
concentration of 15, 16, 1330, 20, 650, 500
17
concentration of 15, 16, 1330,
20, 650, 500, 30,
17, 18, 19 200
[0203] Table 6 describes the Detox Schedule for Patient A (see also Figure 1).
[0204] Table 6: Detox Schedule
Hour Product Amount In Liquid
7:00 AM Greens First 1-2 scoops + HMF 8 oz water
(acidophilus)
powder/superpowder 1/2-
1 tsp
8:00 AM Ultra Sustaine 2 scoops 8 oz Rice Dream or
Cranberry with water
or Permavite 1-1/2 Tbls 8 oz Rice Dream or
Cranberry with water
8:30 AM water 2 cups (16 oz) water
MetaFiber 2 scoops 8 oz Cranberry with
water
or Ground Flax 1/4 cup 8 oz Cranberry with
Seeds water
9:00 AM water 2 cups (16 oz) water
Ultra Clear or Plus 2 scoops 80z Rice Dream
12:00 PM Greens First 1-2 scoops + HMF at least 8 oz water
(acidophilus)
powder/superpowder 1/2-
1 tsp
1:30 PM water water 2 cups (16 oz)
Ultra Clear or Plus 2 scoops 8 oz Rice Dream
3:30 PM water 2 cups (16 oz) water
Ultra Sustaine 2 scoops 8 oz Rice Dream
or Permavite 1-1/2 Tbls 8 oz Rice Dream
5:30 PM water 2 cups (16 oz) water
Ultra Clear or Plus 2 scoops 8 oz Rice Dream
8:00 PM MetaFiber 2 scoops 8 oz Cranberry with
water
or Ground Flax 1/4 cup 8 oz Cranberry with
Seeds water
8:30 PM Smooth Move Tea 1 tea bag 2 cups boiling water
or Herbatox 3-4 capsules
* Ultra Meal can be used throughout day when you feel hungry and to pick up
your energy (2 scoops) to 8 oz
liquid
- 49 -
CA 03020087 2018-10-04
WO 2017/180127
PCT/US2016/027524
[0205] Table 7 is a list of autoimmune diseases and the cellular and cytokine
characteristics/markers that may be used to identify a patient having one or
more of these
conditions.
[0206] Table 7: Autoimmune Disease Markers
Autoimmune Disease Markers
Psoriasis 008+ T cells, HLA-0W6, IL-12b, IL-23b,
TNF-
alpha, NF-kB
Ankylosing Spondylitis 008, HLA-B27
Antiphospholipid syndrome HLA-DR7, HLA-B8, GKA-DR2, HLA-DR3
Celiac HLA-DQ8, DQ 2.5
Crohn's Th17, Thl , ATG16L1, CARD15, XBP1
Diabetes mellitus I HLA-DR3, HLA-0R4
Mixed connective tissue HLA-0R4
Michan-Habermann T cells
Multiple sclerosis HLA-0R2, PECAM-1
Myastlienia gravis HA-B8, HLA-0R3, HLA-DR1
Myositis, dermatomyositis Polymyositis IFN-gama, IL-1, TNF-alpha
Psoriatic arthritis HLA-B27
Rheumatoid arthritis HLA-0R4, PTPN22, B cells, TNF alpha, IL-
17,
IL-1, IL-6, IL-15
Spondyloarthropathy HLA-B27
undifferentiated connective tissue HLA-0R4
autoimmune Uveitis HLA-B27
[0207] Table 8 illustrates the various fatty acids and their concentrations as
fatty acid
triglycerides and fatty acid ethyl esters measured from the blood serum of
Patient A. Also
shown are the concentrations of vitamin D3, as cholecalciferol D3 in its
powder and gel forms
as well as the total vitamin D and 25-0H.
[0208] Table 8: Patient A Treatment Data
DATE TC (mg/dL) EPA (mg) DHA (mg) 25-OF COMMENTS
(ng/m1)
2/2013 116 667 333 triglyceride - Omega-3
5/2013 133 667 333 triglyceride - Omega-3
6/2013 127 667 333 triglyceride - Omega-3
7/2013 132 667 333 triglyceride - Omega-3
8/2013 138 0 0 44.6 triglyceride - Omega-
3
9/2013 158 1334 666 89.3 triglyceride - Omega-
3
10/2013 132 1334 666
11/2013 150 667 333 64.1 triglyceride - Omega-
3
12/2013 151 667 333 35 triglyceride - Omega-3
1/2014 176 667 333 triglyceride - Omega-3
1000 IU from Omega-3
significant pain from
vytorin
- 50 -
CA 03020087 2018-10-04
WO 2017/180127 PCT/US2016/027524
2/2014 186 667 333 33.2 3000 IU, no myopathy,
vytorin
3/2014 163 667 333 33.2 3000 IU, no myopathy,
vytorin
4/2014 137 667 333 3000 IU, Crestor, Zetia
5/2014 109 2001 999 5000 IU, Crestor, Zetia
6/2014 116 2001 999 5000 IU, Crestor, Zetia
7/2014 142 667 333 47.3 3000 IU, Lipitor,
Zetia
8/2014 150 2001 999 5000 IU, Lipitor, Zetia
9/2014 157 2001 999 5000 IU, Lipitor, Zetia
10/2014 136 667 333 3000 IU, Lipitor, Zetia
11/2014 103 2001 999 5000 IU, Crestor, Zetia
12/2014 118 2001 999 50001U
1/2015 123 180 120 31.4 1000 IV, slight pain
lower back
2/2015 135 180 120 2000 IU, pain gone
4/2015 134 847 453 3000 IU, triglyceride
fatty acids & fatty acid
ethyl esters, 3000 IU,
combination fatty acids
4/2015 121 1247 653 Omega 6-276, Omega
9-170, vinpocetine,
phosphatidyl choline
6/2015 108 465 252 Seborrheic keratosis
returned
7/2015 113 667 333 Omega-3 fatty acid
ethyl ester
8/2015 107 667 333 eliminate Omega 6 and
Omega 9, eliminated
keratosis
[0209] Table 9: A copy of the Lipid-modifying Effects 10 mg and 20 mg Crestor
(AstraZeneca Pharmaceuticals) in Primary Dysbetalipoproteinemia (Type III
hyperlipo
proteinemia) Change (95% Cl) from Baseline (N=32).
Marker Median at Baseline Median percent change
Median percent change
(mg/dL) from baseline (95 % Cl from baseline (95 % Cl
CRESTOR 10 mg) CRESTOR 20 mg
Total-C 342.5 -43.3 -47.6
Triglycerides 503.5 -40.1 -43
NonHDL-C 294.5 -48.2 -56.4
VLDL + IDL-C 209.5 -46.8 -56.2
LDL-C 112.5 -54.4 -57.3
HDL-C 35.5 10.2 11.2
RLP-C 82 -56.4 -64.9
Apo-E 16 -42.9 -42.5
- 51 -