Note: Descriptions are shown in the official language in which they were submitted.
CA 03024893 2018-11-20
WO 2017/202695
PCT/EP2017/062027
Title: Insole
Description
The invention relates to an insole for shoes, in particular as
a disposable product with an at least two-layer basic material
comprising a top layer facing the foot and a base layer that
faces the shoe bonded thereto, and with a foot surface facing
the foot and an opposite sole surface that faces the shoe.
A wide variety of insoles are known in the prior art. Among
other purposes, they serve to improve foot comfort or
cushioning in the shoe or to optimize the fit. Special
seasonal insoles are also known, e.g. for the purpose of
absorbing foot perspiration during the summer or providing
insulation against cold surfaces in winter. A general problem
in this case is that the foot perspires in the shoe, and
bacteria cause odor formation in shoes and in insoles as well.
Numerous variants have been proposed in the prior art in order
to prevent this odor formation or release, or at least to
control the odor.
For example, EP 0272690 Al describes a three-layer insole that
can be equipped with active substances on both the foot
surface and the sole surface.
Further multilayer insoles, in which active substances are
provided in order to alleviate odor, are described for example
in EP 1472945 A2 and EP 0414634 El.
Based on the known prior art, the object of the invention is
to provide in particular a disposable insole that provides
favorable liquid transport within the insole in order to
provide a sensation of foot dryness while simultaneously
providing favorable odor control.
The object is achieved by means of an insole as described
above, wherein the top layer and the base layer consist of a
CA 03024893 2018-11-20
WO 2017/202695 2
PCT/EP2017/062027
staple fiber nonwoven and bicomponent fibers, absorbent
cellulose-based fibers and/or hydrophilic synthetic fibers are
contained in the top layer and in the base layer, and the
insole contains a combination comprising at least two
different active substance groups, the active substance groups
being selected from the group of antimicrobial active
substances, odor-absorbing agents, and odor-masking
substances, and wherein both the top layer and base layer are
assigned at least one active substance group.
The bicomponent fibers should preferably be provided with a
percentage by weight of at least 10 wt%, preferably at least
15 wt%, and preferably at least 20 wt% based on the total
weight of the respective layer. More preferably, the
bicomponent fibers show a percentage of the total weight of
the respective layer of at most 60 wt%, preferably at most 55
wt%, further preferably at most 50 wt%, further preferably at
most 45 wt%, and further preferably at most 40 wt%.
Bicomponent fiber as a synthetic fiber is produced from two
polymers of different physical or chemical properties. The two
polymers or the two components of a bicomponent fiber can be
arranged inside the fiber in various ways, such as for example
adjacent to each other, or in particular in a sheath core-
arrangement.
The bicomponent fibers used for this invention preferably show
a low-melting-point component and a higher-melting-point
component, preferably in a sheath core-arrangement, wherein in
the case of such a sheath core-arrangement, the sheath then
comprises the low-melting-point component.
It was found that the use of bicomponent fibers makes it
possible to achieve a more "point-shaped" or partial bonding
of the two layers, as bonding takes place only via the lower-
melting-point component, in particular the sheath component.
The bicomponent fibers result in adhesive point-shaped bonding
of the fibers within and between the layers; in comparison to
this, in the case of true adhesion of the layers by means of
CA 03024893 2018-11-20
WO 2017/202695 3
PCT/EP2017/062027
adhesive agents or purely synthetic fibers that melt as a
whole, a more filmlike intermediate layer is achieved. The use
of bicomponent fibers therefore provides a certain channel
structure and thus improves the transfer of liquid from the
top layer to the base layer, and thus away from the foot into
the insole as well.
It was also found that the use of absorbent cellulosic fibers
and/or hydrophilic synthetic fibers, chiefly in the top layer,
advantageously improves the adjustability of liquid absorption
and binding properties. At the same time, this advantageously
contributes toward a dry microclimate of the foot of the user
or inside the shoe.
It was also found that the assignment of active substance
groups further advantageously contributes toward achievement
of a hygienic insole.
The at least two active substance groups are assigned to the
top layer and the base layer. This is understood to refer to
an arrangement both in the layers and on the layers, here in
particular on the foot or sole surface. The active substance
groups can both be evenly distributed over the surface and/or
thickness of the base layer or the top layer, or areas can be
provided that contain less or no active substance as compared
to areas that contain more or some of the active substance.
An arrangement on one of the layers is also understood to
refer to an arrangement in a coating.
Overall, by providing at least two layers of this basic
material, thus making it possible to adjust the liquid
absorption and binding properties by selecting the fibers in
connection with at least two active substance groups, an
improved hygienic insole can be provided.
As polymer materials for the bicomponent fibers, polyester
(PES), polyolefins, in particular PP, PE, and/or polyamides,
or combinations thereof are by all means conceivable.
CA 03024893 2018-11-20
WO 2017/202695 4
PCT/EP2017/062027
Particularly preferably, polyester-based fibers are used for
the insole bicomponent fibers. The polyester bicomponent
fibers comprise a low-melting-point component of copolyester
and a higher-melting-point component of polyester. The
polyester bicomponent fibers have a core of polyester (PBS)
and a sheath of copolyester. Particularly preferably,
identical bicomponent fibers are used in the top layer and/or
in the base layer.
The hydrophilic synthetic fibers provided in addition to the
bicomponent fibers in the nonwoven composition of the base
layer and/or top layer preferably all have a higher melting
temperature than the low-melting-point component of the
bicomponent fibers. Because the melting point of the
hydrophilic synthetic fibers further provided in the top layer
and/or base layer, and if applicable, of optional further
fibers, is higher than the melting point of the at least one
component of the bicomponent fibers, these hydrophilic
synthetic fibers themselves do not melt. In any case, the
absorbent cellulosic fibers have no melting point. By means of
this fiber configuration, the layers, in particular the top
layer facing the foot, can produce a textile feel and a dry
microclimate.
By means of exerting pressure on the layer arrangement of the
top layer and base layer and heating the layer arrangement so
that the surface of the bicomponent fibers melts, fusion bonds
can be formed between the fibers of the top layer and the base
layer that bond the layers to one another, as well as fusion
bonds within the individual layers, thus advantageously
contributing toward partial consolidation of the layers, in
particular of the top layer, and therefore positively
affecting their abrasion resistance. Strong consolidation by
means of the large number of point-shaped bonding sites
obtained by using bicomponent fibers can reduce or even
eliminate detachment of individual fibers from the fiber
composite, and this manifests itself in favorable abrasion
resistance.
CA 03024893 2018-11-20
WO 2017/202695 5
PCT/EP2017/062027
Advantageously, the density of the bicomponent fibers is 1.0
to 6.5 dtex, in particular 1.2 to 4.0 dtex, and preferably 1.5
to 3.0 dtex. It is particularly advantageous to select fine
fibers, as fineness of the fibers can allow a large number of
point-shaped bonding sites to be obtained, which has a
positive effect on the cohesion of the fibers and layers and
thus provides improved abrasion resistance.
The selected length of the bicomponent fibers is
advantageously 10 to 80 mm, in particular 20 to 70 mm, and
preferably 40 to 50 mm. This advantageously allows penetration
of the respective layer and production of bonding sites on the
fiber surfaces.
As absorbent cellulosic fibers, fluff pulp, cotton, viscose or
combinations thereof can be used in particular.
As hydrophilic synthetic fibers, polymer-based fibers composed
of polyolefins, in particular PP or PE, or polymer-based
fibers composed of polyester, polymer-based polyamides, or
combinations thereof are preferably used, wherein the
synthetic fibers can be provided with a hydrophilic bright
finish.
Moreover, the use of polyester-based fibers, either as
bicomponent fibers and/or as hydrophilic synthetic fibers, has
also been found to be particularly advantageous because of
their structurally elastic behavior. These fibers have a
bulking property that is attributable to their resilience.
According to a preferred embodiment, it can be provided that
the base layer and the top layer differ in at least one
property. The property can be selected from the group of fiber
composition, weight per unit area, thickness, density, and
water retention capacity. In this manner, the properties of
the layers can better be tailored to one another by using
layers that complement one another. For example, the top layer
can be configured in such a way that it absorbs perspiration
CA 03024893 2018-11-20
WO 2017/202695 6
PCT/EP2017/062027
from the boot as quickly as possible and transfers it to the
base layer.
It is particularly preferred if the top layer comprises 25-35
wt% bicomponent fibers, in particular polyester-based, at
least 20 wt% and preferably at least 30 wt% hydrophilic
synthetic fibers, in particular polyester fibers, and if
applicable/optionally, also up to 50 wt%, in particular up to
40 wt% cellulose-based fibers, in particular viscose.
Furthermore, it can be provided that the base layer comprises
35-60 wt% bicomponent fibers, preferably polyester-based, at
least 40 wt% cellulose-based fibers, in particular cotton
fibers, and if applicable/optionally, also up to 10 wt%
synthetic fibers, in particular polyester fibers.
It is particularly preferable here if the top layer and/or the
base layer are subjected to a consolidation process prior to
laminate formation. Known consolidation processes such as
thermal consolidation methods, calendering, mechanical
needling, water jet needling, etc. can be used. Preferably,
combinations of the above-mentioned consolidation processes
are used. Particularly preferably, water jet needling or
mechanical needling is carried out together with subsequent
thermal consolidation. In this manner, both the stability of
the layer or layers and that of the product as a whole can be
improved, which also results in improved tread properties. In
addition, for example, by means of mechanical needling, in
particular of the top layer, the porosity of the top layer can
be improved, and thus its capacity to allow liquid to pass
through.
In order to allow perspiration to be drawn off in the most
effective manner possible, it can preferably be provided that
the insole has an infiltration time of at most 20 sec, in
particular at most 15 sec, and most particularly at most 10
sec. Together with coordination of the structure of the top
and base layer, this makes it possible to achieve a dry
microclimate of the foot in a particularly favorable manner.
CA 03024893 2018-11-20
WO 2017/202695 7
PCT/EP2017/062027
The infiltration time is determined according to the following
method:
The infiltration time describes the capacity of the insole to
keep liquid away from the product surface nearest to the body,
i.e. from the foot surface of the insole.
The following test equipment is required:
- a stopwatch graduated in sec,
- a liquid supply device.
- The structure of this supply device is discussed in
further detail with reference to Fig. 6a and Fig. 6b,
wherein Fig. 6a is a side view and Fig. 6b a top view.
The supply device 300 is composed of steel with a weight
of 500-510 g and has a base plate 302 with an extension L
of 100 mm x 100 mm and a total height hl of 8 mm. In the
middle of this base plate 302, an opening 304 is made
from which a cylinder 306 having an outer diameter D1 of
25 mm, an inner diameter D2 of 20 mm, and a total height
h3 of 41 mm extends. The lower end of the cylinder, which
is oriented toward the base plate, has a sieve-like
structure 308. In this structure are arranged 25 holes
310 with a diameter of 1 mm. The sieve structure or the
holes have a height h2 of 2 mm.
- A piston stroke pipette, e.g. from Eppendorf, with an
accuracy of 0.5-5 ml, adjustable to a pipetting volume of
2 ml, with a corresponding pipette tip
- Test solution: demineralized water.
In order to carry out the test procedure, the test pieces must
be laid out flat. Optionally, in the case of curved test
products, the edges must be cut in such a way that the test
samples can be laid flat.
CA 03024893 2018-11-20
WO 2017/202695 8
PCT/EP2017/062027
The test pieces must be conditioned for at least 2 h in a
standard climate at 23 C 2 C and 50% 2% humidity. The
samples must not be kinked or folded in the area provided for
placement of the opening cylinder, and other changes and
impurities are to be avoided.
The entire insole is used as a test piece.
For the test, the insole is spread out flat, with the upper
side facing the foot, i.e. the foot surface, facing upward.
The above-described liquid supply device is placed on the
spread out test pieces in such a way that the opening of the
supply cylinder is positioned on the middle of the insole. 2
ml of test is dispensed onto the test pieces through the
opening cylinder. For this purpose, the measuring pipette,
with the pipette tip at the level of the upper edge of the
opening cylinder and inside the opening cylinder in the
middle, is pressed down in one movement, and the 2 ml of test
liquid is released. The infiltration time ends and is measured
as soon as no more test liquid is visible in the opening
cylinder.
For the test, 5 measurements are carried out on 5 insoles.
The infiltration time is given as a mean value in sec without
decimal places.
The foot feel can preferably be further improved if the top
layer and/or the base layer has/have a water retention
capacity of at least 1 g/g and at most 15 g/g (g of liquid per
g of nonwoven layer). More preferably, the water retention
capacity of the top layer and/or base layer is at least 2 g/g,
more particularly at least 4 g/g, and further preferably at
least 5 g/g, wherein a particularly preferred embodiment has a
maximum value of 12 g/g.
Preferably, the water retention capacity of the base layer is
greater than the water retention capacity of the top layer.
CA 03024893 2018-11-20
WO 2017/202695 9
PCT/EP2017/062027
The water retention capacity of the insole in particular is
between 1 g/g and 15 g/g.
The water retention capacity is determined by the following
method.
The following test equipment is used for this purpose:
- An envelope-like dihedral wire mesh between which the
subsequent samples are inserted. The wire mesh has
external dimensions of 120 mm x 120 mm, with a mesh width
of 1.5-2 mm and a mass of approx. 23 2 g.
- A beaker or vessel having dimensions suitable for
accommodating the wire mesh
- a precision balance showing values to two decimal places,
- a stopwatch graduated in sec,
- a punch cutter measuring 100 x 100 mm,
- demineralized water.
In order to prepare the samples, sample test strips measuring
100 mm x 100 mm are punched out of the layer to be tested or
the product as a whole. If the samples are to be narrower,
narrower test strips are punched out and placed next to one
another so that an area of 100 x 100 mm can be measured.
In order to determine the water retention capacity of the
individual layers, the layers are to correspondingly separated
from the product as a whole.
Before the test, the samples are conditioned for at least 24 h
at 23 C 2 C and 50% 2% relative humidity. The test pieces
are uniformly clamped into the wire mesh between the covering
surfaces thereof with a total weight of at least 1 g
(precisely weighed to 0.01 g (M1)). In cases where an
individual sample weighs less than 1 g, multiple samples are
layered together to form a sample stack, which should weigh at
least 1 g. The wire mesh, together with the sample, is
immersed in demineralized water and left therein for 60 sec.
CA 03024893 2018-11-20
WO 2017/202695 10
PCT/EP2017/062027
After this, the wire mesh, together with the sample, is
removed from the liquid, and the liquid is allowed to drip off
one corner for a period of 120 sec. The samples are then
removed from the wire mesh and again precisely weighed to 0.01
g (M2).
The water absorption capacity is calculated according to the
equation (M2-M1)/M1 and then indicated in g/g. The value is
obtained as the mean value of 3 determinations rounded off to
one decimal place.
It is particularly advantageous in this case if the water
retention capacity of the base layer in particular is higher
than the water retention capacity of the top layer, allowing
the foot perspiration to be stored near the sole and thus at a
distance from the foot.
It is particularly favorable if the top layer is configured in
such a way that it favorably absorbs liquid, in particular
perspiration, and transfers it to the base layer in order to
quickly transport the liquid away from the foot. Moreover, it
can be favorable if the top layer is capable of distributing
the liquid in a lateral direction, i.e. in the surface of the
top layer, in order to utilize the capacity of the base layer
to absorb liquid in the most uniform manner possible and thus
optimally.
In order to ensure favorable bonding between the base layer
and the top layer, it is preferable if the two layers are
bonded to each other substantially over their entire
overlapping surfaces. Particularly preferred is partial
bonding or bonding at points distributed over the entire
overlapping surface. More preferably, bonding can be carried
out by means of embossed patterns. Particularly preferably,
the base layer and the top layer are bonded to each other by
means of a pattern produced by calendering. By introducing
embossed patterns into the layers and bonding of the layers
thereby, the capillarity can be increased, thus improving
CA 03024893 2018-11-20
WO 2017/202695 11
PCT/EP2017/062027
liquid transport. The percentage of embossing points on the
entire surface of the insole is preferably 5-15%.
According to the invention, the active substance groups are
preferably provided on the foot surface and on the sole
surface.
In particular, the odor-absorbing agent is advantageously
provided on the sole surface. In this manner, one can prevent
odor that is emitted downward from the sole and may become
fixed in the shoe as well. In particular, odors that are
already present in the shoe can be bound. Moreover, it can
also be provided that an odor-absorbing agent is provided in
the base layer and not only on the sole surface in order to
reduce or prevent odor formation in the layer that stores
perspiration.
Odor absorption is to be understood in particular as also
referring to an adsorption mechanism.
Furthermore and advantageously, it can be provided that the
odor-masking substance is provided on the foot surface and/or
in the top layer in order to counteract odor occurring
directly on the foot.
In order to further combat odor formation, it can be provided
that an antimicrobial active substance is provided on the foot
surface and/or in the top layer and/or on the sole surface
and/or in the base layer. In this manner, and optionally also
by providing the antimicrobial active substance in the layers,
i.e. in the top layer and/or in the base layer, odor formation
due to bacteria can be counteracted, as the growth of bacteria
that decompose perspiration is inhibited or completely
prevented. When the antimicrobial active substance is used on
the sole surface, it combats microorganisms on the sole side,
and thus also combats odor development in the shoe. When the
antimicrobial active substance is used on the foot surface
and/or in the top layer, it can advantageously contribute
toward preventing the occurrence of new odors.
CA 03024893 2018-11-20
WO 2017/202695 12
PCT/EP2017/062027
In order to ensure that odors are effectively counteracted, it
can be provided that at least one of the active substance
groups is not mixed into the base layer and/or the top layer,
but remains substantially on the sole and/or foot surface.
Particularly preferably, at least two active substance groups
are provided exclusively on the sole surface and/or foot
surface. It is particularly preferred in this case for at
least one active substance group to be provided in particle-
bound and/or polymer-bound form on the sole and/or foot
surface of the basic material. Preferably, at least two of the
active substance groups are provided in particle-bound and/or
polymer-bound form on the sole and/or foot surface. In this
manner, one can ensure that active substances remain at the
desired location and exert their effect there.
Alternatively, it can be provided that at least one active
substance group, in particular the antimicrobial active
substance, is not provided in particle-bound and/or polymer-
bound form on the sole and/or foot surface of the basic
material and can thus infuse or is infused into the interior
of the respective layer, in particular also into the adjacent
layer. This can already be provided by the manufacturer, or it
can be the case that at the time of use, the perspiration
carries the active substance into the top layer and/or the
base layer that together form the basic material. In
particular, when the base layer is the main layer that binds
perspiration, it is advantageous to have the antimicrobial
active substance present in the interior of the layer in order
to combat and/or prevent bacterial growth.
It is conceivable to provide an active substance group, e.g.
an antimicrobial agent, in the top layer and/or on the top
layer, i.e. the foot surface, in order to counteract odor
formation due to additional fresh perspiration, and to arrange
an odor-absorbing active substance in or on the base layer in
order to combat old odors. For example, one can use activated
carbon that e.g. can be introduced in a coating applied to the
sole surface.
CA 03024893 2018-11-20
WO 2017/202695 13
PCT/EP2017/062027
Alternatively, odor-masking substances on or in the top layer
can be combined with an odor-absorbing active substance on the
sole surface or in the base layer in order to mask newly-
occurring odors.
In addition, combinations of odor-masking substances in or on
the top layer with antimicrobial active substances in or on
the base layer are also conceivable in order to counteract
odor formation by inhibiting bacterial growth and mask new
odor formation.
It is particularly preferable and effective for combatting
odor if a combination of three different active substance
groups is provided. The combination thus comprises at least
one antimicrobial active substance, an odor-absorbing agent,
and an odor-masking substance.
In a particularly preferred embodiment, at least two active
substance groups are arranged on one of the surfaces, the sole
surface or foot surface, and at least one active substance
group is arranged on the other surface, the foot surface or
sole surface.
The insole can have a coating on the sole surface that imparts
to the sole surface increased frictional force compared to the
uncoated sole surface. The coating can consist of point-
shaped, line-shaped, and/or sheet-shaped coating elements or
combinations thereof. In particular, a coating composed of
coating lines is preferably provided.
Preferably, at least one of the active substance groups is
inserted into the coating and/or bonded thereto. Particularly
preferably, the odor-absorbing agent and/or the antimicrobial
active substance is inserted into the coating and/or bonded
thereto.
The coating on the sole surface can be composed of a plurality
of individual patterns formed by coating lines. The individual
CA 03024893 2018-11-20
WO 2017/202695 14
PCT/EP2017/062027
patterns are preferably more than a solely point-shaped
pattern. In the configuration of a pattern as a line, the line
preferably does not extend exclusively as a straight line in
only one vector direction, but this pattern of lines has at
least one curve and/or at least one kink.
The result is that the coating lines do not run solely in one
preferred direction.
Individual patterns can show arrangements as pattern groups in
which at least two pattern elements are arranged adjacent to
one another and in contact, or in particular also pattern
groups in which one pattern element at least partially
surrounds or encloses another pattern element, such as for
example concentric arrangements, or geometric figures of any
kind that lie one inside the other and are in contact at one
point.
In contrast to a full-surface coating application, it is also
possible by means of a non-full-surface coating on the sole
surface, such as in particular a line-shaped coating, to
maintain further desired properties of the basic material of
the insole, such as for example air permeability and/or
breathing activity and/or flexural strength of the insole, at
a high level, while simultaneously achieving favorable
ergonomic adaptation to the foot of a wearer or on the surface
contours of the shoe.
Particularly preferably, the sole surface can be covered by
the coating with a coverage ratio of at least 6%, in
particular at least 8%, in particular at least 10%, more
particularly at least 20% and most particularly of at most
50%, even more particularly at most 40%, and most particularly
preferably at most 30%.
It is provided in particular that the coating elements cover
the sole surface substantially in its entire extension, i.e.
not only in special areas such as the heel and/or the area of
the ball of the foot. It is therefore preferably provided that
. . CA 03024893 2018-11-20
WO 2017/202695 15
PCT/EP2017/062027
the coating extends over the entire sole surface, wherein
depending on the pattern provided, individual areas of the
sole surface, such as for example the toe/ball area and/or
heel area, can have a higher density of coating elements, and
other areas, such as for example the area of the arch of the
foot, can have a lower density. In particular, in the
embodiment in which at least one active substance group is
introduced into the coating or bonded thereto, the areas of
the shoe that are susceptible to perspiration and thus odor,
such as the areas of the toes/ball of the foot in particular,
can be taken into account.
In line-shaped coating elements, the line width can be at
least 0.2 mm, in particular at least 0.4 mm, in particular at
least 0.5 mm, and more particularly at least 0.6 mm. The line
width should preferably be at most 2 mm, more particularly at
most 1.6 mm, more particularly at most 1.2 mm, and more
particularly at most 1.0 mm. The ratio of line length to line
width should be at least 5 times, preferably at least 6 times,
further preferably at least 8 times, and even more preferably
at least 10 times the line width.
The height of the coating elements should be at least 0.1 mm,
in particular at least 0.2 mm. The height of the coating
elements should be at most 0.8 mm, more particularly at most
0.6 mm, and more particularly at most 0.4 mm. Measurement of
the height can be carried out using a microscope having a
corresponding magnification, specifically as the difference
between a determined upper edge of the basic material and the
upper edge of the coating element.
With these preferred heights of the coating elements,
unpleasant haptic effects on the foot are advantageously
prevented.
The weight per unit area of the coating on the sole surface
can be at least 5 g/m2, in particular at least 10 g/m2, more
particularly at least 15 g/m2, and more particularly at least
20 g/m2. Towards the top, the weight per unit area should
CA 03024893 2018-11-20
WO 2017/202695 16
PCT/EP2017/062027
preferably be limited to 50 g/m2, and more particularly to at
most 30 g/m2.
The coating is in particular polymer-based, and more
particularly based on a polymer from the group comprising
polyolefins (in particular PE, PP), acetates, in particular
ethylene vinyl acetate (EVA), polyamides (PA), styrene
polymers, or combinations thereof.
As materials for the coating, materials with a Shore A
hardness of at least 30, in particular at least 40, in
particular at least 50, more particularly at least 60 and most
particularly of at most 90, more particularly at most 80, and
even more particularly at most 70 are preferably used.
Measurement of Shore A hardness is carried out according to
the standards DIN 53505:2000-08 and ISO 868:2003(E). A Shore A
hardness testing device is used.
The sole side with the coating can have a dynamic friction
coefficient measured according to ASTM D 1894-01 of at least
0.6, in particular at least 0.8, and more particularly at
least 1Ø The maximum values reached should be at most 2.0,
more particularly at most 1.5, and more particularly at most
1.2.
According to the invention, the insole comprises at least two
different active substance groups.
The odor-absorbing agent can be selected from the group of the
carbons, in particular activated carbon, zeolite, starch,
diatomaceous earth, or combinations thereof.
It can be provided that the antimicrobial active substance can
be selected from the group of the antimicrobial metals, in
particular silver, or the polysaccharides, in particular
chitosan, or combinations thereof. Antimicrobial metals are
advantageous in that the active substance is first released in
the form of ions on entry of liquid, thus making it possible
to achieve the required dosing over a long period and to at
CA 03024893 2018-11-20
WO 2017/202695 17
PCT/EP2017/062027
least limit the amount of the odor-producing bacteria. Silver
can preferably be used in the form of silver particles. The
silver particles can preferably be composed of a matrix, in
particular a glass ceramic matrix having silver bound to its
surface and/or bound in its interior.
Moreover, it can be provided that the odor-masking substance
is a synthetic and/or naturally-based fragrance-producing
substance. Essential oils, as well as perfume oils, are
conceivable for this purpose. Particularly preferably, the
odor-masking substance is an at least partially bound and/or
complexed fragrance, such as for example a perfume embedded in
cyclodextrin structures and/or in particular a
microencapsulated fragrance that in particular comprises a
perfume oil. Microencapsulated fragrances are advantageous in
that they can allow delayed release of an active substance.
For example, it can be provided that the microcapsules are
destroyed by pressure or shearing, i.e. by the weight of the
person, for example, thus causing the perfume to be released
in a controlled manner.
It is advantageously provided that the antimicrobial active
substance is provided in a weight per unit area of 0.001-2
g/m2, in particular 0.01-2 g/m2, more particularly 0.05-1.5
g/m2, more particularly 0.1-1.0 g/m2 , and more particularly
0.1-0.5 g/m2.
It is advantageously provided that the insole is equipped with
an antimicrobial active substance in an amount based on the
entire insole of 0.0001-2 wt%, in particular 0.001-2 wt%, more
particularly 0.002-1.5 wt%, and even more particularly 0.002-
1.0 wt%.
The odor-absorbing agent can preferably be provided with a
weight per unit area of 0.2-30 g/m2, in particular 1-20 g/m2,
and more particularly 2.5-15 g/m2.
It is advantageously provided that the insole is equipped with
an odor-absorbing agent in an amount based on the entire
CA 03024893 2018-11-20
WO 2017/202695 18
PCT/EP2017/062027
insole of 0.1-6 wt%, in particular 0.5-5 wt%, and more
particularly 1.0-4 wt%.
Finally, it can be provided that the odor-masking substance is
provided in a weight per unit area of 0.1-5 g/m2, in particular
0.5-3 g/m2, and more particularly 1-2 g/m2.
It is advantageously provided that the insole is equipped with
an odor-masking substance in an amount based on the entire
insole of 0.05-1 wt%, in particular 0.1-0.8 wt%, and more
particularly 0.2-0.6 wt%.
It is particularly preferred in this case if the combination
of the active substance groups of antimicrobial active
substances and odor-absorbing agents are introduced in a
weight ratio of 1 : 2 to 1 : 500 and/or the combination of the
active substance groups of antimicrobial active substances and
odor-masking substances are introduced in a weight ratio of 1
: 0.5 to 1 : 150.
The basic material comprises, in particular also in the case
of a multilayer basic material, a base layer with a weight per
unit area preferably of at least 180 g/m2, further preferably
at least 200 g/m2, further preferably at least 220 g/m2,
further preferably at most 300 g/m2, further preferably at
most 280 g/m2, and further preferably at most 250 g/m2. The
top layer has in particular a weight per unit area of at least
g/m2, further preferably at least 15 g/m2, further
preferably at least 20 g/m2, further preferably at most 100
g/m2, further preferably at most 90 g/m2, and further
preferably at most 80 g/m2. Depending on the intended purpose
of use of the insole, such as e.g. as a variant for the summer
or the winter, the top layer can preferably have lower maximum
limits for the summer of in particular at most 50 g/m2 in
weight per unit area, while the top layer for the winter can
have higher minimum values of in particular at least 50 g/m2
weight per unit area.
CA 03024893 2018-11-20
WO 2017/202695 19
PCT/EP2017/062027
Preferably, the thickness of the insole, including a coating
on the sole surface, is 1-3 mm, and preferably 1-2 mm.
The thickness of an insole (including a coating) is determined
using a specific measuring pressure of 0.5 kPa on a scanner
area of 25 cm2. In particular, the DMT thickness gauge from the
firm Schroder may be used. Moreover, the thickness is
determined in accordance with DIN EN ISO 9073-2: 1995.
The insole is preferably a disposable product, i.e. a single
use product. In principle, however, insoles that can be washed
or cleaned are also conceivable.
Further advantages of the invention can be found in the
further documents. The features can be essential to the
invention either individually or in combination.
The invention is described in the following with reference to
a drawing. The figures show the following:
Fig. 1: an insole according to the invention,
Fig. 2: an insole according to Fig. 1 with an additional
coating on the sole surface,
Fig. 3a: a schematic section through the basic material of an
insole according to the invention,
Fig. 3b: a schematic diagram of a section of a fiber layer
with "adhesion" points of bicomponent fibers,
Fig. 4: diagrams a) to b) show enlarged sections of an
insole according to the invention,
Fig. 5: diagrams a) to c) show enlarged sections of an
insole according to the invention,
Fig. 6: a schematic diagram of the test device for measuring
infiltration time.
CA 03024893 2018-11-20
WO 2017/202695 20
PCT/EP2017/062027
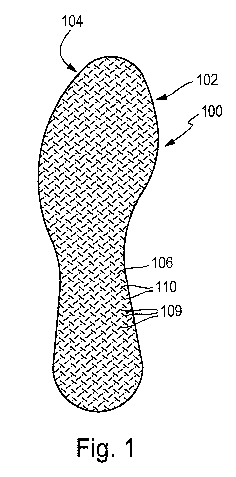
Fig. 1 shows a top view of the sole surface of an insole
according to the invention 100, wherein the sole surface 102
during use of the insole faces the interior of a shoe and the
surface opposite the sole surface 102 faces the foot as a
foot-side surface 104. The insole 100 is composed of a two-
layer basic material, specifically with a top layer 200 facing
the foot and a base layer 202 facing the shoe, as shown in
Fig. 3a as a schematic section through the insole 100
according to Fig. 1. The top layer 200 and the base layer 202
are composed of a staple fiber nonwoven. The nonwoven material
comprises bicomponent fibers in the top layer and the base
layer, indicated by the reference number 150, and absorbent
cellulose-based fibers and/or hydrophilic synthetic fibers,
indicated by the reference number 152.
In both the top layer 200 and the base layer 202, the total
weight of the respective layer preferably comprises at least
wt% bicomponent fibers, and an amount of 60 wt% is
preferably not exceeded. With their low-melting-point
component, preferably the sheath component, the bicomponent
fibers contribute toward point-shaped bonding 160 of the
fibers in the layers and between the layers, as shown
schematically in Fig. 3b. The top layer and the base layer
advantageously show a water absorption capacity of at least 2
g/g. The insole shows an infiltration time of at most 20 sec.
The basic material is consolidated by being pre-calendered,
i.e., passed between a heated calendar roller with protruding
embossing projections and a counterpressure roller. In this
manner, the surface structure 106 that can be seen in Fig. 1,
in this case with point-shaped and rib-shaped embossing
structures 109, is formed. The engraving depth achieved by
means of the calendering, which is 0.7 mm in the present case,
can, however, be set as desired by the person having ordinary
skill in the art based on his or her technical expertise. In
the embossed area, high-density embossed areas 109 are formed
in addition to less dense areas 110. The percentage of the
entire surface area accounted for by the high-density areas
109 is 5-15%.
CA 03024893 2018-11-20
WO 2017/202695 21
PCT/EP2017/062027
The basic material of the insole has a base layer with a
preferred basis weight of 200-300 g/m2 and a top layer with a
preferred basis weight of 20-100 g/m2, and depending on the
seasonal use of the insole, a higher weight per unit area of
the top layer of 50-100 g/m2 can be favored for winter insoles
and a lower weight per unit area of 20-50 g/m2 can be favored
for summer insoles.
As shown in Fig. 2, a coating 112 of coating lines 114 is
provided on the sole surface 102 of the insole 100 facing away
from the sole of the foot and toward the inside of a shoe.
This prevents sliding of the insole 100 inside a shoe. The
coating lines 114 are polymer-based and preferably composed of
EVA (ethylene vinyl acetate). The material preferably has a
Shore A hardness of at least 30, preferably 60-80, and
preferably at most 90. The coating is applied by means of an
engraving process, wherein the insole 100 is passed between a
gravure roller and a counter-roller. The width of the coating
lines is preferably 0.5-0.7 mm. The height of the coating
lines is preferably 0.2-0.3 mm so that application of the
coating pattern will not result in any unpleasant haptic
effects on the foot.
The coating shown in Fig. 2 has a plurality of individual
patterns 120 that are formed by the coating lines 114. In the
case shown, each individual pattern 120 is preferably formed
by pattern groups 124, wherein the pattern groups are composed
of at least three pattern elements 126, here concentrically
arranged circles, and no coating mass is applied between the
individual circles of each pattern group forming an individual
pattern, thus leaving an uncoated area 116 therein. In this
manner, by means of the coating lines 114, a total coverage
ratio on the sole surface of approx. 20-25% is achieved. This
relatively low coverage ratio of the coating has no
substantial effect on the further attributed and desired
properties of the basic material of the insole, such as for
example air permeability and/or breathing activity.
CA 03024893 2018-11-20
WO 2017/202695 22
PCT/EP2017/062027
The dynamic friction coefficient of the coated sole surface,
measured in accordance with ASTM D 1894-01, is between 0.8 and
1.4.
In Fig. 4 diagrams a) and b) and Fig. 5 diagrams a) to c), a
section is shown through an insole according to the invention
100 comprising a basic material with two layers, specifically
a top layer 200 and a base layer 202. The base layer 202 faces
a shoe and comprises the sole surface 102. The top layer 200
faces a foot and comprises the foot surface 104. It can be
seen in the diagrams that a coating 112 is provided on the
sole surface that can be configured analogously to the coating
shown in Fig. 2.
In the insole 100, active substances for preventing or
alleviating odor are assigned to the base layer 202, in
particular the sole surface 102, and to the top layer 200, in
particular the foot surface 104 respectively. These active
substances are selected from the active substance groups of
antimicrobial active substances, odor-absorbing agents, and
odor-masking substances.
Examples for the arrangement of two active substance groups
are shown in Figs. 4a and 4b.
In the design of Fig. 4a, an antimicrobial active substance
204, in this case not in a polymer- or particle-bound
configuration, is applied to the foot surface 104 and
partially also to the top layer 202, and on the sole surface
102, an odor-absorbing agent 206 is bound into the coating
112. In the design of Fig. 4b, an odor-masking substance 208
is provided on the foot surface and an antimicrobial active
substance 210 is provided in the coating 112 on the sole side
102 as active substances.
Examples of the arrangement of the three active substance
groups are shown in Figs. 5a-5b.
CA 03024893 2018-11-20
WO 2017/202695 23
PCT/EP2017/062027
In all of the configurations in Fig. 5a) to c), the active
substances are distributed in such a way that the odor-
absorbing agent 206 is provided in the coating 112 on the sole
surface 102, wherein a highly-porous, fine granular carbon
such as activated carbon is preferably used. The odor-
absorbing agent is thus in direct contact with the shoe and
odors present therein. In this manner, the odors are directly
and rapidly absorbed.
In all three configurations of Fig. 5 as well, the odor-
masking substance is provided on the foot surface in the form
of microencapsulated fragrances that contain perfume oils,
said fragrances being indicated by the reference number 208.
The fragrances are released in a dosed and controlled manner
by pressure and shearing on use of the insole, which causes
the capsules to be destroyed.
In Fig. 5a), a polysaccharide, indicated here in particular by
the reference number 204, is used as an antimicrobial active
substance, specifically both on the foot surface 104 and in
the nonwoven material of the top layer 200, and partially in
the nonwoven material of the base layer 202. This active
substance serves to control the odor of fresh foot
perspiration in that its antimicrobial activity inhibits the
growth of bacteria.
A similar design is shown in Fig. 5b), wherein the
antimicrobial active substance here is in the form of silver
particles 210 on the foot surface 104. The silver particles
are preferably composed of silver bound to a glass-ceramic
matrix.
An alternative design is shown in Fig. 5c), wherein the same
silver particles 210 as in Fig. 5b) and the odor-absorbing
agent 206 are present in the coating 112 on the sole surface
102. In this arrangement, the antimicrobial active substance
210 can serve to control bacterial growth on the sole side,
and thus also to control odor in the shoe. New perspiration
and the formation of new odors are prevented by the fragrances
CA 03024893 2018-11-20
WO 2017/202695 24
PCT/EP2017/062027
contained in the odor-masking substances 208 and the
perspiration-absorbing action of the top layer 200 and base
layer 202.
A measurable antimicrobial action can be achieved by selecting
an antimicrobial active substance from the active substance
groups and using it in the insole. As examples of this, the
embodiments of the insole according to the diagram of Fig. 5a
as example 1 and the diagram of Fig. 5b as example 2 are
described, and the antimicrobial action thereof is measured.
The insole has a basic material composed of a base layer of
200-300 g/m2 and a top layer of 50-100 g/m2. The base layer has
a fiber composition of 35-60 wt% PES bicomponent fibers and
further a mixture of hydrophilic synthetic PES fibers and
absorbent cellulosic fibers; the top layer comprises 25-35 wt%
of PES bicomponent fibers and further hydrophilic synthetic
PES fibers. The top layer is laminated onto the base layer by
means of pressure, temperature, and embossing.
In example 1, chitosan is applied to the foot surface as an
antimicrobial active substance together with a
microencapsulated perfume oil as an odor-masking substance.
The amount of chitosan used is 0.05-0.06 wt%, and the amount
of the encapsulated perfume oil used is 0.5-0.65 wt%, based
respectively on the total weight of the insole. As an odor-
absorbing agent, activated carbon is applied to the sole side
in a line-shaped polymer coating comprising a content of 1-2%
based on the total weight of the insole.
In example 2, as a variant of example 1, particle-bound silver
with a content of 0.003-0.005 wt% based on the total weight of
the insole is applied to the foot surface as an antimicrobial
active substance.
The antimicrobial action is measured in accordance with DIN EN
ISO 20743A:2013-12. Staphylococcus epidermidis ATCC 14990 is
used as a test microbe. The test is conducted based on the
absorption method, and the plate count method is used for
CA 03024893 2018-11-20
WO 2017/202695 25
PCT/EP2017/062027
quantitative measurement. Changes are made such that NaC1 0.9%
+ 0.05% Tween 80 is used as an inoculation medium and NaCl
0.9% + 0.20% Tween 80 is used as an elution medium. Microbial
growth over 18 h on the sample is calculated compared to the
control or reference material according to the following
formula:
A = (log10 Ct-logio Co) - (log10 Tt-log10 To) =
where C denotes the control fabric. T denotes the test sample.
logio Ct or logn Tt = general logarithm of the arithmetic mean
for the bacterial count after incubation for 18 h in control
fabric C or test sample T.
log10 C0 or logn To= logarithm of the arithmetic mean for the
bacterial count immediately after inoculation in control
fabric C or test sample T.
A simplified calculation is taken as a basis, with the
modification of equating microbial growth log10 Coandlogn To.
For both example 1 and example 2, strong antibacterial
activity was detected, i.e. with a microbial reduction 3 log
CFU.
Such an insole provides good tread comfort, combined with a
feeling of foot dryness and reduced odor formation.