Note: Descriptions are shown in the official language in which they were submitted.
SINGLE PARTICLE ANALYSIS USING OPTICAL DETECTION
[001] PRIORITY APPLICATION
[002] This application is related to, and claims priority to and the benefit
of, U.S. Provisional
Application No. 62/596,812 filed on December 9, 2017 and to U.S. Application
No. 16/209,140
filed on December 4, 2018.
[001] TECHNOLOGICAL FIELD
[002] Certain configurations described herein are directed to analysis of
single particles. More
particularly, certain examples are described of single particle analysis using
inductively coupled
plasma optical detection.
[003] BACKGROUND
[004] It is often desirable to measure particulate matter in ambient
environments. Even though
the particulate matter may be analyzed, an origin of the particulate matter is
not necessarily
identifiable in all instances.
[005] SUMMARY
[006] Certain aspect, configurations, embodiments, examples and illustrations
are described of
methods and systems that can be used to identify one, two or more elements in
a particle. The
identified elements can then be used to identify a source of the particle if
desired.
[007] In some examples, a comprises simultaneously detecting an optical
emission from each
of an ionized first element and an ionized second element to identify at least
a first element in a
particle from a plurality of particles using the optical emission from the
ionized first element, and
to identify at least a second element in the particle from the plurality of
particles using the optical
emission from the second ionized element. The method may further comprise
using the
identified first element and the identified second element to identify a
source of the particle from
a plurality of particles. If desired, three, four or more elements within the
individual particle can
be identified and used to determine a source of the particle.
[008] In certain examples, the method comprises quantifying an amount of each
of the first
element and the second element in the particle. In some examples, the method
comprises
simultaneously detecting an optical emission from an ionized third element to
identify a third
1
CA 30266982018-12-06
element in the particle using the optical emission from the ionized third
element and identifying
the source of the particle using the identified first, second and third
elements. For example, the
method may also comprise quantifying an amount of each of the first element,
the second
element and the third element in the particle. If desired, one or more
clustering techniques for
separating particles from one source versus another source due to their
different elemental
compositions can be performed.
[009] In some examples, the method may comprise sampling air comprising the
particle and
providing the sampled air to an ionization device to ionize the first element
and the second
element in the sampled air.
[010] In other examples, the method may comprise sampling a hydrocarbon fluid
comprising
the particle and providing the sampled hydrocarbon fluid to an ionization
device to ionize the
first element and the second element in the sampled hydrocarbon fluid. In some
embodiments,
the method may comprise quantifying an amount of each of the first element and
the second
element in the particle and determining a vehicle site exhibiting wear using
the quantified
amount of the first element and the second element. In other instances, the
method may
comprise quantifying an amount of each of the first element and the second
element in the
particle and determining if the hydrocarbon fluid needs to be replaced using
the quantified
amount of the first element and the second element.
[011] In certain embodiments, the method comprises simultaneously detecting an
optical
emission from each ionized element from all elements in the particle to
identify all elements in
the particle. In other examples, the method may comprise quantifying each of
the identified
elements in the particle and determining a source of the particle using the
quantified elements.
[012] In some examples, the method comprises configuring the particle as a
nanoparticle. In
certain examples, the method comprises identifying the source of the
nanoparticle using the
identified first element and the identified second element.
[013] In some embodiments, the method may comprise ionizing the particle to
provide the
ionized first element and the ionized second element. In certain examples, the
method comprises
ionizing step comprises introducing the particle into an ionization source. In
other examples, the
method comprises introducing the particle into the ionization source using a
spray chamber or a
gas exchange device. In some embodiments, the method comprises configuring the
ionization
2
CA 3026698 2018-12-06
source as one of an inductively coupled plasma, a capacitively coupled plasma,
a glow discharge,
an arc or a spark.
[014] In some examples, the method comprises sampling a fluid comprising the
particle,
wherein the fluid is sampled in an inline process to monitor a state of the
fluid by periodically
sampling the fluid, identifying the first element and the second element in
the sampled fluid
using the optical emissions from the first ionized element and the second
ionized element, and
quantifying an amount of each of the first element and the second element in
the sampled fluid to
determine a source of particle in the inline process. In certain embodiments,
the method
comprises sampling a gas comprising the plurality of particles. In other
embodiments, the
method comprises sampling a gas used in a semiconductor manufacturing process.
In some
embodiments, each of the first element and the second element are inorganic
elements.
[015] In another aspect, a system for detecting elemental species present in a
particle of a
plurality of particles is described. In some examples, the system comprises a
sample introduction
device configured to provide an individual particle from the plurality of
particles, wherein the
provided individual particle comprises an average diameter of about 100 nm to
about 100
microns. The system may further comprise an ionization device fluidically
coupled to the
sample introduction device and configured to ionize elemental species present
in the provided
individual particle. The system may further comprise an optical detector
configured to
simultaneously detect an optical response from each of the ionized elemental
species from the
provided individual particle.
[016] In certain embodiments, the sample introduction device is configured as
a spray chamber
or a gas exchange device. In other embodiments, the sample introduction device
is configured to
directly inject the individual particle into the ionization device. In some
examples, the optical
detector comprises an optical spectrometer. In certain examples, the system
comprises a
processor electrically coupled to the optical detector and configured to
quantify an amount of
each element from a detected optical emission from each of the ionized
elemental species, e.g.,
the processor can be configured to execute instructions for quantifying an
amount of each
element from a detected optical emission from each of the ionized elemental
species. In some
examples, the processor is further configured to determine a source of the
particle using the
quantified amount of each element, e.g., can be configured to execute
instructions to determine a
source of the particle using the quantified amount of each element.
3
11 CA 3026698 2018-12-06
[017] In certain examples, the sample introduction device is configured to
provide an individual
particle to the ionization device. In other examples, the sample introduction
device is configured
to provide a hydrocarbon fluid comprising the particle to the ionization
device. In some
embodiments, the processor is configured to determine a wear location site
using the quantified
amount of the each of the elements in the particle.
[018] In some examples, the sample introduction device is configured to
periodically sample a
fluid present in an inline process and provide the sampled fluid to the
ionization device.
[019] In other examples, the sample introduction device is configured to
provide an air sample
comprising the particle to the ionization device. In some embodiments, the
sample introduction
device is configured to automatically sample an air space of a building and
provide the sampled
air space to the ionization device.
[020] In certain embodiments, the optical detector comprises at least one
grating to spatially
separate each optical emission wavelength from other optical emission
wavelengths to permit
simultaneous detection of each of the ionized elemental species.
[021] In some configurations, the ionization device comprises a torch and an
induction device
configured to sustain an inductively coupled plasma within the torch. In some
examples, the
induction device is configured as an induction coil, a plate electrode or a
radially finned
induction device.
[022] In some examples, the system may comprise a second ionization device
fluidically
coupled to the sample introduction device, the second ionization device and
the ionization device
configured to operate in parallel. The system may also comprise a second
detector fluidically
coupled to the second ionization device, the second detector configured to
simultaneously detect
optical emissions from each of the ionized elemental species present in the
second ionization
device. The system may further comprise a sampling device fluidically coupled
to the ionization
device in a first state and fluidically coupled to the second ionization
device in a second state.
The system may further comprise a processor electrically coupled to the
optical detector and
configured to execute instructions for quantifying an amount of each element
from a detected
optical absorption from each of the ionized elemental species, wherein the
processor is further
configured to determine a source of the particle using the quantified amount
of each element.
[023] In certain embodiments, a method of identifying a source of a material
comprises
simultaneously detecting an optical emission from each of an ionized first
element and an
4
CA 3026698 2018-12-06
ionized second element to quantify at least a first element in a particle of
the material using the
optical emission from the ionized first element and to quantify at least a
second element in the
particle of the material using the optical emission from the second ionized
element, and using the
quantified first element and the quantified second element to identify the
source of the material.
[024] Additional aspects, embodiments, configurations and examples are
described in more
detail below.
[025] BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
[026] Certain illustrations of the technology described herein are described
in more detail
below with reference to the drawings in which:
[027] FIG. 1 is an illustration showing two elemental species present in
different individual
particles, in accordance with certain examples;
[028] FIG. 2 is an illustration showing two elemental species present together
in individual
particles, in accordance with certain examples;
[029] FIG. 3 is another illustration showing two elemental species present
together in
individual particles, in accordance with certain embodiments;
[030] FIG. 4 is an additional illustration showing two elemental species
present together in
individual particles and individual particles where only one of the elemental
species is present by
itself, in accordance with certain examples;
[031] FIG. 5 is an additional illustration showing two elemental species
present together in
individual particles and individual particles where each of the elemental
species is present by
itself, in accordance with certain examples;
[032] FIG. 6 is another illustration showing two elemental species present
together in
individual particles as a core shell scenario, in accordance with certain
embodiments;
[033] FIG. 7 is an illustration of a process to detect two or more elemental
species present in an
individual particle, in accordance with certain examples;
[034] FIG. 8 is an illustration of one type of a sample introduction device
that can be used with
particles present in a liquid, in accordance with certain examples;
[035] FIG. 9 is an illustration of one type of a sample introduction device
that can be used with
particles present in a gas, in accordance with certain examples;
CA 3026698 2018-12-06
[036] FIG. 10 is an illustration of one type of ionization source, in
accordance with certain
examples;
[037] FIG. 11 is an illustration of another type of ionization source, in
accordance with certain
examples;
[038] FIG. 12 is an additional illustration of an ionization source, in
accordance with certain
examples;
[039] FIG. 13 is an illustration of an optical emission spectrometer, in
accordance with certain
embodiments;
[040] FIG. 14 is an illustration of an atomic absorption spectrometer, in
accordance with certain
embodiments;
[041] FIGS. 15A is an illustration of an optical response when two different
elements are
present in different individual particles, and FIG. 15B shows a graph of
signal intensity vs time,
in accordance with certain embodiments;
[042] FIG. 16 is an illustration of an optical response when two different
elements are present
in the same individual particle, in accordance with certain embodiments; and
[043] FIG. 17A is an illustration of an optical response when four different
elements (lithium,
manganese, cobalt and nickel) are present and measured in the sample, in
accordance with
certain examples;
[044] FIG. 17B shows a simulation where the nickel and cobalt are present in
the same particle,
in accordance with some examples;
[045] FIGS. 17C, 17D, 17E, 17F, 17G and 17H show various graphs correlating
different metal
measurements with each other, in accordance with certain embodiments; and
[046] FIGS. 18A, 18B, 18C, 18D and 18E are graphs showing the signal intensity
as a function
of time for iron and chromium, in accordance with some embodiments.
[047] It will be recognized by the person of ordinary skill in the art, given
the benefit of this
disclosure, that the representations in the drawings are provided merely for
illustration purposes.
The exact optical response, dimensions of the components and configuration of
the systems may
vary depending on the intended use of the methods and systems.
6
CA 3026698 2018-12-06
[048] DETAILED DESCRIPTION
[049] Certain illustrations of methods, systems and devices are provided below
to facilitate a
better understanding of the technology described herein. In some instances,
reference is made to
a single particle being analyzed for its elemental content or some portion
thereof. It will be
recognized by the person of ordinary skill in the art, given the benefit of
this disclosure, that
more than one particle can be analyzed, and the reference to single particle
does not mean that
only a single particle is or can be analyzed using the methods, systems and
devices described
herein.
[050] In some examples, the methods, systems and devices described herein can
be used to
detect one, two or more than two elements within a single individual particle.
The single particle
may be present as an individual particle or a stable aggregate or association
or system of
particles. Without wishing to be bound by this particular illustration, by
detecting two or more
elements within a single particle it can be possible to determine a source of
the particle. For
example, a single particle comprising copper and zinc can be linked to a brass
material, a single
particle comprising iron and chromium can be linked to a steel material, a
single particle
comprising two particular elements can be linked to a wear site in an engine,
a single particle
comprising two particular elements can be linked to a source of specific air
borne particles, a
single particle comprising two or three particular elements can be linked to
gun powder, etc. In
some examples, the exact size of the single particle, e.g., an average
particle diameter, may vary
from about 0.5 microns up to about 100 microns, more particularly about 0.5
microns to about 50
microns or about 0.5 microns to about 10 microns, though smaller or larger
particles can also be
analyzed using the methods and systems described herein. If desired, both the
identity of the
element(s) in a single particle and an amount of the element(s) in the single
particle can be
determined.
[051] In certain embodiments, the methods, systems and devices described
herein can be used,
for example, to determine a source of the analyzed particles. Referring to
FIG. 1, an illustration
is shown where an element A and an element B are present independently in
separate particles.
Optical signals 110-113, e.g., optical emission or optical absorption signals,
from first particles
comprising element A and optical signals 120-123 from second particles
comprising element B
are shown. As each particle (or the ionized products from each particle) is
provided to an optical
detector, the identity and amount of each of element A and element B can be
determined.
7
IT CA 3026698 2018-12-06
Because any one particle comprises only one of the elements (A or B), only a
single element is
detected for any one particle. As noted in more detail below, each of elements
A and B can emit
or absorb light at a characteristic wavelength that can be used to determine
the identity and
amount of each element present in the particle.
[052] In certain examples, it may be desirable to detect the presence of two
or more elements in
a single particle. An illustration is shown in FIG. 2 where the particles
provide optical signals
210-213 and 220-223. As each particle is introduced into an ionization source,
the elements A
and B are atomized and/or ionized and provided to a downstream optical
detector. Because both
elements are present in a single particle, both elements A and B are
simultaneously detected by
the optical detector. The illustration shown in FIG. 2 assumes the elements A
and B are present
in a fixed ratio in each of the individual particles and a fixed response is
observed for the
elements in any one particle.
[053] In some examples, the elements A and B may be present at variable ratios
in different
particles where some particles have higher amounts of one element than other
particles. An
illustration of a possible optical response is shown in FIG. 3 where elements
A and B exist
together in a variable ratio in individual particles. Different amounts of
elements A and B may be
detected in different individual particles, which can result in spreading out
of the optical signals.
In some instances, certain individual particles may comprise only a single
element whereas other
individual particles may comprise two or more elements. Referring to FIG. 4,
an illustration of
the optical response where certain individual particles only comprise element
B and other
individual particles comprise elements A and B. As each individual particle is
provided to the
ionization source, the elements may be ionized and provided to a downstream
optical detector so
that elements A and B can be simultaneously detected. Some particles may lack
element A
entirely, whereas other particles may comprise both elements A and B. In the
illustration of FIG.
4, elements A and B are generally present in a fixed or constant ratio, though
they could be
present in a variable ratio as well.
[054] In other instances, elements A and B may be present in individual
particles together but
there may also be particles which only comprise one of element A and one of
element B. In such
instances, an optical response similar to that shown in FIG. 5 may be
encountered. In this
illustration, some particles only comprise element A (and provide no optical
response when
element B is being detected), some particles only comprise element B (and
provide no optical
8
CA 3026698 2018-12-06
response when element A is being detected), and some particles comprise both
elements A and
element B (and provide an optical response when both elements A and B are
simultaneously
being detected).
[055] In some examples, the exact nature of the particles where two elements
are present
together may vary. In some examples, the elements A and B may be bound or
coordinated to
different groups of the particle, whereas in other instances one or more of
the elements A or B
can be present in a different structure of the particle that forms a portion
of the particle. For
example, one of the elements may be present in a core of a core shell particle
configuration and
the other element can be present in the shell of a core shell particle
configuration. An illustration
is shown in FIG. 6 where elements A and B exist together with element A being
in the core of
the particle (with variable core size) and element B being in the shell of the
particle (with fixed
shell size). An illustration of a possible optical response is shown, which
shows that the
presence of the core-shell configuration can provide a different response than
when the elements
are present in a common structural component of a single particle (see FIG.
2).
[056] In certain examples, two or more elements within a single particle can
simultaneously be
detected using an optical response or signal from each of an ionized first
element and an ionized
second element to identify at least the first element in a particle and to
identify at least the second
element in the particle. The identified first element and the identified
second element can be
used to identify a source of the particle from a plurality of particles. If
desired, an amount of
each of the ionized first element and the ionized second element can also be
determined to
quantitate how much of each of the first element and the second element is
present in the
individual particle. A third element, fourth element, etc. can also be
identified and/or quantified
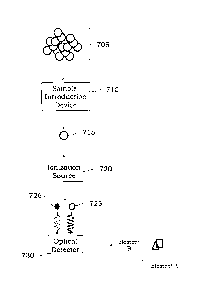
in the individual particle. A generalized illustration of a process for
identifying at least a first
element and at least a second element is shown in FIG. 7. A plurality of
individual particles
(collectively 705) can be introduced into a sample introduction device 710 to
select or provide an
individual particle 715 from the plurality of individual particles 705. The
provided individual
particle 715 can then be provided to an ionization source 720 to ionize the
elemental species in
the provided individual particle 715. While not shown, each individual
particle of the plurality
of individual particles can be provided separately to the ionization source
720 to detect the first
element and the second element in each provided individual particle, e.g.,
sequentially.
Depending on the exact ionization source selected, the organic elements
present in the individual
9
CA 30266982018-12-06
particle 715 are atomized/ionized and generally do not emit light (or absorb
light) at similar
wavelengths as any ionized inorganic elemental species. After ionization of
the individual
particle 715 in the ionization source 720, the elemental species 725, 726 can
be provided to an
optical detector 730 for detection, or light emitted from or absorbed by the
species 725, 726 can
be detected by the optical detector 730. The optical detector 730 can be
configured to
simultaneously detect an optical response for each of the elemental species
725, 726 as shown in
the graph in FIG. 7. The detected optical signals for each of elements A and B
in each of the
individual particles from the plurality of particles 705 can be used to
determine a source of the
particles. In some examples, the exact optical response or optical signal from
the first element
and second element can vary. As noted below, in some instances, an optical
emission from each
of the first element and the second element can simultaneously be detected and
used to determine
the identity of the elements and how much of each element is present in each
individual particle.
While the exact emission wavelength varies from element to element and certain
elements can
emit light at more than a single wavelength, different emission wavelengths
can be monitored so
that minimal spectral emission wavelength overlap is observed during the
simultaneous detection
of the two or more elements. In other instances, light absorption by the first
element and second
element can be used to identify the elements present and/or the amount of the
elements that are
present in the individual particle.
[057] In some examples, the exact sample introduction device used may depend,
at least in part,
on the environment of the particles. For example, where the particles are
present in a liquid
sample the sample introduction device may comprise or use a nebulizer, an
injector, capillary
tubing, etc. Where a nebulizer is used, the nebulizer can take many forms
including crossflow
nebulizers, concentric nebulizers and microflow nebulizers. The nebulizer can
be used by itself
or in combination with one or more spray chambers as noted below. Where
injectors are used,
the injector may take the form of a needle, capillary or other tubing with a
small orifice. In some
examples, the sample introduction device can be configured to provide
individual particles from
a plurality or particles introduced into the sample introduction device. For
example, a spray
chamber similar to an Asperonrm spray chamber commercially available from
PerkinElmer
Health Sciences, Inc. (Waltham, MA) or other suitable spray chambers can be
used. The
dimensions and/or configuration of the spray chamber can be selected to permit
particles as large
CA 3026698 2018-12-06
as 100 microns to be provided from the sample introduction device to the
downstream ionization
source.
[058] One illustration of a spray chamber is shown in FIG. 8. The spray
chamber 800 generally
comprises an outer chamber or tube 810 and an inner chamber or tube 810. The
outer chamber
810 comprises dual makeup gas inlets 812, 814 and a drain 818. The makeup gas
inlets 812, 814
are typically fluidically coupled to a common gas source, though different
gases could be used if
desired. While not required, the makeup gas inlets 812, 814 are shown as being
positioned
adjacent to an inlet end 811, though they could instead be positioned
centrally or toward an
outlet end 813. The inner chamber or tube 820 is positioned adjacent to a
nebulizer tip 805 and
may comprise two or more microchannels 822, 824 configured to provide a makeup
gas flow to
reduce or prevent particle droplets from back flowing and/or depositing on the
inner tube 820.
The configuration and positioning of the inner tube 820 provides laminar flow
at areas 840, 842
which acts to shield inner surfaces of the outer chamber 810 from any droplet
deposition. The
tangential gas flow provided by way of gas introduction into the spray chamber
800 through the
inlets 812, 814 acts to select particles of a certain size range. The
microchannels 822, 824 in the
inner tube 820 also are designed to permit the gas flows from the makeup gas
inlets 812, 814 to
shield the surfaces of the inner chamber or tube 820 from droplet deposition.
In certain
examples, the microchannels 822, 824 can be configured in a similar manner,
e.g., have the same
size and/or diameter, whereas in other configurations the microchannels 822,
824 may be sized
or arranged differently. In some instances, at least two, three, four, five or
more separate
microchannels can be present in the inner chamber or tube 820. The exact size,
form and shape
of the microchannels may vary and each microchannel need not have the same
size, form or
shape. In some examples, different diameter microchannels may exist at
different radial planes
along a longitudinal axis Li of the inner tube to provide a desired shielding
effect. Illustrative
spray chambers are described, for example, in U.S. Patent Application No.
15/597,608 filed on
May 17, 2017, the entire disclosure of which is hereby incorporated herein by
reference for all
purposes.
[059] In certain embodiments, the exact dimensions of the spray chamber 800
may vary. In
certain configurations, a longitudinal length from the nebulizer tip 805 to
the end of the spray
chamber 800 may be about 10 cm to about 15 cm, e.g., about 12 or 13 cm. The
diameter of the
outer tube 810 may vary from about 1 cm to about 5 cm, e.g., about 3 cm or 4
cm. The largest
11
CA 3026698 2018-12-06
diameter of the inner tube 820 may vary from about 0.5 cm to about 4 cm, and
the distance
between outer surfaces of the inner tube 820 and inner surfaces of the outer
tube 810 can be
selected to provide a desired laminar flow rate, e.g., the distance may be
about 0.1 cm to about
0.75 cm. In certain examples, the inner tube 820 is shown as having a
generally increasing
internal diameter along the longitudinal axis of the outer chamber 810, but
this dimensional
change is not required. Some portion of the inner tube 810 may be "flat" or
generally parallel
with the longitudinal axis Li to enhance the laminar flow, or in an
alternative configuration,
some portion of the inner tube 820 may generally be parallel to the surface of
the outer tube 810,
at least for some length, to enhance laminar flow. The inner diameter of the
outer chamber
increases from the inlet end 811 toward the outlet end 813 up to a point and
then decreases
toward the outlet end 813 such that the inner diameter of the outer chamber
810 is smaller at the
outlet end 813 than at the inlet end 811. If desired, the inner diameter of
the outer chamber 810
may remain constant from the inlet end 811 toward the outlet end 813 or may
increase from the
inlet end 811 toward the outlet end 813. If desired, two or more different
spray chambers which
are the same or different can be fluidically coupled to each other to assist
in selection of
individual particles from a plurality of particles.
[060] In some examples, it may be desirable to sample an air space with
particles present in
gaseous form. For example, in many industrial settings, it may be desirable to
keep a level of
certain air borne particles below a threshold level to ensure worker safety
and/or increase air
quality. The air space can be sampled to extract some of the gaseous particles
to test whether or
not certain types of particles are present. For example, particulate matter
can be analyzed to
determine selected particles of a desired size, e.g., similar to PM10, PM2.5
and PM1 monitoring,
and determine whether or not any particularly hazardous particles are present
in the air sample.
In some examples where gaseous particles are analyzed, a gas exchange device
(GED) may be
present as or part of the sample introduction device. The exact form of the
gas exchange device
used may vary based on the air sampled and the desired output from the gas
exchange device. In
one example, a gas exchange device may comprise two or more tubes or chambers
where an
inner tube or chamber comprises a porous membrane or pores of a desired size.
A simplified gas
exchange device 900 is shown in FIG. 9 as comprising an outer tube 910 and an
inner tube 920.
A sampled gas comprising gaseous particles can be introduced into the inner
tube 920 through an
inlet 912. A gas such as argon or other inert gases may be introduced into the
outer tube 910
12
CA 3026698 2018-12-06
through an inlet (not shown) and used as a sweep gas. Pressure differences
between the sweep
gas and the sampled gas can act to permit the sweep gas to diffuse into the
inner tube 920 and
permit non-particle species to diffuse from the inner tube 920 into the outer
tube 910. The
particles within the air sample are generally large (compared to the size of
ambient gases in the
sampled air) and diffuse to little or no degree through the porous membrane of
the inner tube
920. The sweep gas flows into the inner tube 910 and can be used to carry the
gaseous particles
in the inner tube 920 to a downstream ionization source through an outlet 914
of the gas
exchange device 900. If desired, a gas exchange device can be coupled to a
different sample
introduction device such as an injector, capillary tube, spray chamber,
another GED, etc. The
pore size present in the inner tube 920 can vary depending on the desired
particle size to be used
and may act to filter out smaller particles from the air sample if desired.
While not shown, an
additional outlet may be present and fluidically coupled to the outer tube 910
to permit the sweep
gas and non-particulate material extracted from the sampled air to exit from
the outer tube 910.
[061] In some embodiments, the ionization source 720 may take many different
forms and is
generally effective to ionize the elemental species present in each individual
particle. In some
examples, the ionization source may be a high temperature ionization source,
e.g., one with an
average temperature of about 4000 Kelvin or more, such as, for example, a
direct current plasma,
an inductively coupled plasma, an arc, a spark or other high temperature
ionization sources. The
exact ionization source used may vary depending on the particular elements
and/or particles to be
analyzed, and illustrative ionization sources include those which can atomize
and/or ionize the
elemental species to be detected, e.g., those ionization sources which can
atomize and/or ionize
metals, metalloids and other inorganic species or organic species.
[062] In certain examples, the ionization source may comprise one or more
torches and one or
more induction devices. Certain components of an ionization source are shown
in FIGS. 10-12.
Illustrative induction devices and torches are described, for example, in U.S.
Patent Nos.
9,433,073 and 9,360,403, the entire disclosure of which is hereby incorporated
herein by
reference for all purposes. Referring to FIG. 10, a device comprising a torch
1010 in
combination with an induction coil 1020 is shown. The induction coil 1020 is
typically
electrically coupled to a radio frequency generator (not shown) to provide
radio frequency
energy into the torch 1010 and sustain an inductively coupled plasma 1050
within some portion
of the torch 1010. A sample introduction device (not shown) can be used to
introduce individual
13
CA 3026698 2018-12-06
particles into the plasma 1050 to ionize and/or atomize the elemental species
present in the
individual particle. The ionized and/or atomized elemental species may be
detected within the
torch using axial or radial detection or can be provided to a downstream
chamber or other device
for detection. In some instances, optical emissions from each of the two or
more elemental
species within the torch 1010 can be simultaneously detected using a detector
optically coupled
to the torch 1010.
[063] In an alternative configuration, the induction coil 1020 in FIG. 10
could be replaced with
one or more plate electrodes. For example and referring to FIG. 11, a first
plate electrode 1120
and a second plate electrode 1121 are shown as comprising an aperture that can
receive a torch
1110. For example, the torch 1110 can be placed within some region of an
induction device
comprising plate electrodes 1120, 1121. A plasma or other
ionization/atomization source 1150
such as, for example, an inductively coupled plasma can be sustained using the
torch 1110 and
inductive energy from the plates 1120, 1121. A radio frequency generator 1130
is electrically
coupled to each of the plates 1120, 1121. If desired, only a single plate
electrode could be used
instead. A sample introduction device can be used to introduce individual
particles into the
plasma 1150 to ionize and/or atomize species in the sample. In a typical
configuration, a
nebulizer is fluidically coupled to a spray chamber to provide liquid sample
to the spray
chamber. The spray chamber can select and aerosolize individual particle and
provide them to
the plasma 1150. Alternatively, a gas exchange device can be used to provide
individual gaseous
particles into the plasma 1150. Two or more elemental species in the
introduced individual
particle can be ionized or atomized and detected using optical techniques to
identify and/or
quantitate the elemental species present in the individual particle.
[064] In other configurations, an induction device comprising one or more
radial fins could
instead be used in methods and systems described herein. Referring to FIG. 12,
a device or
system may comprise an induction coil 1220 comprising at least one radial fin
and a torch 1210.
A plasma or other ionization/atomization source (not shown) such as, for
example, an
inductively coupled plasma can be sustained using the torch 1210 and inductive
energy from the
radially finned induction device 1220. A radio frequency generator (not shown)
can be
electrically coupled to the induction device 1220 to provide radio frequency
energy into the torch
1210. A sample introduction device (not shown) can be used to introduce
individual particles
into the torch 1210. Elemental species in the introduced individual particle
can be ionized or
14
CA 3026698 2018-12-06
atomized and detected using optical techniques to identify and/or quantitate
the elemental species
present in the individual particle. In other instances, one or more capacitive
device such as, for
example, capacitive coils or capacitive plates can be used in an ionization
source. Further two or
more induction devices, capacitive devices or other devices which can provide
energy into the
torch to sustain an atomization/ionization source such as a plasma can also be
used.
[065] In certain embodiments, the two or more elemental species present in the
individual
particle may be detected by measuring an optical signal or response. The exact
type of optical
signal or response can vary and optical emission or optical absorption are
typically used to
identify and/or quantify the elemental species. In some examples, a suitable
detector which can
simultaneously detect two or more wavelengths of light can be used to measure
the presence of
an optical signal from each of the elemental species. For example, when the
individual particle
is introduced into an ionization source and is ionized, each elemental species
in the particle can
emit light as it is excited by energy from the ionization source. The detector
can receive the
emitted optical signals simultaneously from the different elemental species
and can determine an
identity of the elemental species based on the emitted wavelength and/or can
determine an
amount of the identified elemental species using an optical emission
intensity. Illustrative
detectors include, but are not limited to, a detector comprising one or more
complementary
metal-oxide-semiconductor (CMOS) devices, a detector comprising one or more
charge-coupled
devices (CCDs) and other detectors that may comprise individually addressable
arrays or pixels
that can be used to simultaneously detect two or more light emissions with
different
wavelengths. If desired, the detector may be configured as a two dimensional
detector array
which can be used to detect and/or image the various wavelengths of light
emitted from the
elemental species. In some embodiments, the detector may comprise a
photomultiplier tube,
gratings, lenses, etc. to be able to detect one, two or more different
wavelengths of emitted light.
[066] In certain configurations, the elemental species present in the
individual particle can be
detected using optical emission spectroscopy (OES). Referring to FIG. 13, an
OES device or
system 1300 includes a sample introduction device 1310, an ionization source
or device 1320
and a detector or detection device 1330. The sample introduction device 1310
may comprise a
spray chamber, gas exchange device or may take other forms. The ionization
device 1320 may
comprise, for example, one or more components as illustrated in FIGS. 10-12 or
other devices
and components which can provide or sustain an ionization source. The detector
or detection
CA 3026698 2018-12-06
device 1330 may take numerous forms and may be any suitable device that may
simultaneously
detect optical emissions from two or more elemental species, such as optical
emissions 1325,
1326. If desired, the detection device 1330 may include suitable optics, such
as lenses, mirrors,
prisms, windows, band-pass filters, etc. The detection device 1330 may also
include outings,
such as echelle outings, to provide a multi-channel OES device. Gratings such
as echelle
gratings may allow for simultaneous detection of multiple emission
wavelengths. The gratings
may be positioned within a monochromator or other suitable device for
selection of one or more
particular wavelengths to monitor. In some examples, the OES device 1300 may
be configured to
implement Fourier transforms to provide simultaneous detection of multiple
emission
wavelengths. The detection device 1330 may be configured to monitor emission
wavelengths
over a large wavelength range including, but not limited to, ultraviolet,
visible, near and far
infrared, etc. The OES device 1300 may further include suitable electronics
such as a
microprocessor and/or computer and suitable circuitry to provide a desired
signal and/or for data
acquisition. Suitable additional devices and circuitry are known in the art
and may be found, for
example, on commercially available OES devices such as Optima 2100DV series,
Optima 5000
DV series OES devices or Optima 8000 or 8300 series OES devices commercially
available from
PerkinElmer Health Sciences, Inc. An optional display 1340, which may be a
readout, screen,
printer, computer, etc. may be present to monitor detection of the elemental
species. The OES
devices may further include autosamplers, such as AS90 and AS93 autosamplers
commercially
available from PerkinElmer Health Sciences, Inc. or similar devices available
from other
suppliers. The OES device 1300 can be calibrated, for example, using standard
concentration
of elements and particles of known size to provide a calibration curve for
each element which
can be used to quantify each element. If desired, peak height, peak area or
both can be used to
determine the amount of each of the elements present in the individual
particle.
[067] In certain embodiments, the exact wavelengths of emitted light which are
detected can be
used to identify the particular elemental species that are present in the
individual particle. Many
elements can emit light at more than a single wavelength. Atomic species may
also emit light at a
different wavelength than ionized species. Illustrative optical emissions
wavelengths for some
different elemental species include, but are not limited to, 328.066 nm or
338.288 nm for silver,
396.151 nm or 308.212 nm for aluminum, 188.980 nm or 193.696 nm for arsenic,
249.772 nm or
249.676 nm for boron, 455.402 nm or 233.524 nm for barium, 313.104 nm or
313.042 nm for
16
11 CA 3026698 2018-12-06
beryllium, 317.932 nm or 422.673 nm for calcium, 226.502 nm or 214.434 nm for
cadmium,
228.615 rim or 230.785 nm for cobalt, 205.560 nm or 267.711 nm for chromium,
324.754 nm or
327.393 nm for copper, 238.201 nm or 239.568 nm for iron, 766.490 nm for
potassium, 670.784
nm for lithium, 285.212 nm or 279.076 nm for magnesium, 257.607 nm or 293.305
nm
manganese, 202.032 nm or 203.846 nm for molybdenum, 589.587 nm or 330.237 nm
for
sodium, 231.604 nm for sodium, 213.617 nm or 178.224 nm for phosphorous,
220.354 nm for
lead, 180.671 nm or 181.975 nm for sulfur (as sulfate), 206.834 nm or 217.582
nm for antimony,
196.029 nm for selenium, 251.609 nm or 221.663 nm for silicon, 421.549 nm or
460.733 nm for
strontium, 283.730 nm or 401.913 nm for thorium, 334.943 nm or 368.519 rim for
titanium,
190.801 nm for thallium, 292.402 nm or 290.880 nm for vanadium, 409.014 nm for
uranium,
207.912 nm or 239.708 nm for tungsten, 213.858 nm or 206.199 nm for zinc and
291.138 nm
for lutetium. Additional suitable elemental emission wavelengths will be
selected by the person
of ordinary skill in the art, given the benefit of this disclosure, and
depending on the detector
selected, the use of radial detection, the use of axial detection, etc.
[068] In certain examples, the elemental species present in the individual
particle can be
detected using an atomic absorption spectrometer (AAS) to measure light
absorbed by the
different elemental species. Referring to FIG. 14, a single beam AAS device
1400 comprises a
light source 1410, a sample introduction device 1420, an ionization device
1430, and a detection
device 1440. The sample introduction device 1420 may be any one or more of
those described
herein or other suitable sample introduction devices. A power source (not
shown) may be
configured to supply power to the light source 1410, which provides one or
more wavelengths of
light 1412 for absorption by atoms and ions in the ionization device 1430.
Suitable light sources
include, but are not limited to mercury lamps, cathode ray lamps, lasers, etc.
The light source
1410 may be pulsed using suitable choppers or pulsed power supplies, or in
examples where a
laser is implemented, the laser may be pulsed with a selected frequency, e.g.
5, 10, or 20
times/second. The exact configuration of the light source 1410 may vary. For
example, the light
source 1410 may provide light axially along a torch of the ionization device
1430 or may provide
light radially along the torch of the ionization device 1430. The example
shown in FIG. 14 is
configured for axial supply of light from the light source 1410. There can be
signal-to-noise
advantages using axial viewing of signals. If desired, the light source can
provide light to a
chamber separate from the ionization device 1430, e.g. a chamber positioned
downstream of the
17
CA 3026698 2018-12-06
ionization device 1430. For example, the elemental species can be provided
from the ionization
device 1430 to a downstream chamber that is optically coupled to the light
source 1410.
Notwithstanding that many different configurations are possible, the detection
device 1440 is
optically coupled to the light source 1410 so that an amount of light absorbed
by a particular
elemental species is detected. In some examples, the light source 1410 can
provide light of at
least two different wavelengths with one wavelength being absorbed by a first
elemental species
and the other wavelength of light being absorbed by a second elemental
species. If desired, a
spectrometer can be present between the light source 1410 and the ionization
device 1430 (or
secondary chamber) to provide a plurality of different individual light
wavelengths for
absorption by the elemental species from the individual particle. The
ionization device 1430
may comprise one or more components as illustrated in FIGS. 10-12 or other
devices and
components which can provide or sustain an ionization source. As sample is
atomized and/or
ionized in the ionization device 1430, the incident light 1412 from the light
source 1410 may
excite atoms. That is, some percentage of the light 1412 that is supplied by
the light source 1410
may be absorbed by the atoms and ions in the ionization device 1430. The
remaining percentage
of the light may be transmitted to the detection device 1440 as wavelengths
1432, 1433. The
detection device 1440 may provide one or more suitable wavelengths using, for
example, prisms,
lenses, gratings and other suitable devices such as those discussed above in
reference to the OES
devices, for example. To account for the amount of absorption by sample in the
ionization
device 1430, a blank, such as water or particles lacking any elemental
species, may be introduced
prior to sample introduction to provide a 100% transmittance reference value.
The amount of
light transmitted once sample is introduced into the ionization device 1430
may be measured,
and the amount of light transmitted with sample may be divided by the
reference value to obtain
the transmittance. The negative logio of the transmittance is equal to the
absorbance. AAS
device 1400 may further include suitable electronics such as a microprocessor
and/or computer
and suitable circuitry to provide a desired signal and/or for data
acquisition. Suitable additional
devices and circuitry may be found, for example, on commercially available AAS
devices such
as AAnalyst series spectrometers or PinAAcle spectrometers commercially
available from
PerkinElmer Health Sciences, Inc. The AAS devices may further include
autosamplers known in
the art, such as AS-90A, AS-90p1us and AS-93p1us autosamplers commercially
available from
PerkinElmer Health Sciences, Inc. Where the ionization device 1430 is
configured to sustain an
18
CA 3026698 2018-12-06
inductively coupled plasma, a radio frequency generator electrically coupled
to an induction
device may be present. In certain embodiments, a double beam AAS device,
instead of a single
beam AAS device could instead be used.
[069] In some examples, the wavelength of light absorbed can be used to
identify the elemental
species present in an individual particle. Many elements may absorb light at
two or more
different wavelengths. In addition, atomic species may absorb different
wavelength of light than
ionized species. It may be desirable to select monitoring wavelengths that do
not overlap one
another when two or more wavelengths of light are being provided to the
ionized elemental
species. Further, the wavelength selected may differ when using axial
detection and radial
detection. Illustrative absorption wavelengths for some different elemental
species include, but
are not limited to, 328.1 nm for silver, 309.3 nm for aluminum, 193.7 nm for
arsenic, 242.8 nm
for gold, 249.7 nm for boron, 553.6 for barium, 234.9 nm for beryllium, 223.1
nm for bismuth,
422.7 nm for calcium, 228.8 nm for cadmium, 240.7 nm for cobalt, 357.9 nm for
chromium,
852.1 nm for cesium, 324.8 nm for copper, 404.6 nm for dysprosium, 400.8 nm
for erbium,
459.4 nm for europium, 248.3 nm for iron, 287.4 nm for gallium, 368.4 nm for
gadolinium,
265.1 nm for germanium, 286.6 nm for hafnium, 253.7 nm for mercury, 410.4 nm
for holmium,
303.9 nm for indium, 264.0 nm for iridium, 766.5 nm for potassium, 550 nm for
lanthanum,
670.8 for lithium, 336.0 nm for lutetium, 285.2 nm for magnesium, 279.5 nm for
manganese,
313.3 nm for molybdenum, 589 nm for sodium, 334.4 nm for niobium, 492.4 nm for
neodymium, 232.0 nm for nickel, 290.9 nm for osmium, 213.6 nm for phosphorous,
283.3 nm
for lead, 244.8 nm for palladium, 495.1 nm for praseodymium, 265.1 nm for
platinum, 780.0 nm
for rubidium, 346.9 nm for rhenium, 343.5 nm for rhodium, 349.9 nm for
ruthenium, 217.6 nm
for antimony, 391.2 nm for scandium, 196.0 nm for selenium, 251.16 nm for
silicon, 429.7 nm
for samarium, 286.3 nm for tin, 460.7 nm for strontium, 271.5 nm for tantalum,
432.6 nm for
thorium, 261.4 nm for technetium, 214.3 nm for tellurium, 364.3 nm for
titanium, 267.8 nm for
thallium, 371.8 nm for thulium, 351.5 nm for uranium, 318.4 nm for vanadium,
255.1 nm for
tungsten, 410.2 nm for yttrium, 398.8 nm for ytterbium, 213.9 nm for zinc, and
360.1 nm for
zirconium.
[070] In certain examples, the methods and systems herein may comprise or use
a processor,
which can be part of the system or instrument or present in an associated
device, e.g., computer,
laptop, mobile device, etc. used with the instrument. For example, the
processor can be used to
19
CA 3026698 2018-12-06
provide or construct an image representative of the optical emissions from the
various elemental
species. Such processes may be performed automatically by the processor
without the need for
user intervention. For example, the processor can use emission signal
intensities along with one
or more calibration curves to determine how much of each elemental species is
present in the
individual particle. In certain configurations, the processor may be present
in one or more
computer systems and/or common hardware circuity including, for example, a
microprocessor
and/or suitable software for operating the system, e.g., to control the sample
introduction device,
ionization device, detector, etc. In some examples, the detection device
itself may comprise its
own respective processor, operating system and other features to permit
detection of various
elemental species. The processor can be integral to the systems or may be
present on one or
more accessory boards, printed circuit boards or computers electrically
coupled to the
components of the system. The processor is typically electrically coupled to
one or more
memory units to receive data from the other components of the system and
permit adjustment of
the various system parameters as needed or desired. The processor may be part
of a general-
purpose computer such as those based on Unix, Intel PENTIUM-type processor,
Motorola
PowerPC, Sun UltraSPARC, Hewlett-Packard PA-RISC processors, or any other type
of
processor. One or more of any type computer system may be used according to
various
embodiments of the technology. Further, the system may be connected to a
single computer or
may be distributed among a plurality of computers attached by a communications
network. It
should be appreciated that other functions, including network communication,
can be performed
and the technology is not limited to having any particular function or set of
functions. Various
aspects may be implemented as specialized software executing in a general-
purpose computer
system. The computer system may include a processor connected to one or more
memory
devices, such as a disk drive, memory, or other device for storing data.
Memory is typically used
for storing programs, calibration curves, emission or absorption wavelengths,
and data values
during operation of the OES or AAS instrument. Components of the computer
system may be
coupled by an interconnection device, which may include one or more buses
(e.g., between
components that are integrated within a same machine) and/or a network (e.g.,
between
components that reside on separate discrete machines). The interconnection
device provides for
communications (e.g., signals, data, instructions) to be exchanged between
components of the
system. The computer system typically can receive and/or issue commands within
a processing
CA 3026698 2018-12-06
time, e.g., a few milliseconds, a few microseconds or less, to permit rapid
control of the system.
For example, computer control can be implemented to control sample
introduction, detector
parameters, etc. The processor typically is electrically coupled to a power
source which can, for
example, be a direct current source, an alternating current source, a battery,
a fuel cell or other
power sources or combinations of power sources. The power source can be shared
by the other
components of the system. The system may also include one or more input
devices, for example,
a keyboard, mouse, trackball, microphone, touch screen, manual switch (e.g.,
override switch)
and one or more output devices, for example, a printing device, display
screen, speaker. In
addition, the system may contain one or more communication interfaces that
connect the
computer system to a communication network (in addition or as an alternative
to the
interconnection device). The system may also include suitable circuitry to
convert signals
received from the various electrical devices present in the systems. Such
circuitry can be present
on a printed circuit board or may be present on a separate board or device
that is electrically
coupled to the printed circuit board through a suitable interface, e.g., a
serial ATA interface, ISA
interface, PCI interface or the like or through one or more wireless
interfaces, e.g., Bluetooth,
Wi-Fi, Near Field Communication or other wireless protocols and/or interfaces.
[071] In certain embodiments, the storage system used in the systems described
herein typically
includes a computer readable and writeable nonvolatile recording medium in
which codes of
software can be stored that can be used by a program to be executed by the
processor or
information stored on or in the medium to be processed by the program. The
medium may, for
example, be a hard disk, solid state drive or flash memory. The program or
instructions to be
executed by the processor may be located locally or remotely and can be
retrieved by the
processor by way of an interconnection mechanism, a communication network or
other means as
desired. Typically, in operation, the processor causes data to be read from
the nonvolatile
recording medium into another memory that allows for faster access to the
information by the
processor than does the medium. This memory is typically a volatile, random
access memory
such as a dynamic random access memory (DRAM) or static memory (SRAM). It may
be
located in the storage system or in the memory system. The processor generally
manipulates the
data within the integrated circuit memory and then copies the data to the
medium after
processing is completed. A variety of mechanisms are known for managing data
movement
between the medium and the integrated circuit memory element and the
technology is not limited
21
CA 3026698 2018-12-06
thereto. The technology is also not limited to a particular memory system or
storage system. In
certain embodiments, the system may also include specially-programmed, special-
purpose
hardware, for example, an application-specific integrated circuit (ASIC) or a
field programmable
gate array (FPGA). Aspects of the technology may be implemented in software,
hardware or
firmware, or any combination thereof. Further, such methods, acts, systems,
system elements
and components thereof may be implemented as part of the systems described
above or as an
independent component. Although specific systems are described by way of
example as one
type of system upon which various aspects of the technology may be practiced,
it should be
appreciated that aspects are not limited to being implemented on the described
system. Various
aspects may be practiced on one or more systems having a different
architecture or components.
The system may comprise a general-purpose computer system that is programmable
using a
high-level computer programming language. The systems may be also implemented
using
specially programmed, special purpose hardware. In the systems, the processor
is typically a
commercially available processor such as the well-known Pentium class
processors available
from the Intel Corporation. Many other processors are also commercially
available. Such a
processor usually executes an operating system which may be, for example, the
Windows 95,
Windows 98, Windows NT, Windows 2000 (Windows ME), Windows XP, Windows Vista,
Windows 7, Windows 8 or Windows 10 operating systems available from the
Microsoft
Corporation, MAC OS X, e.g., Snow Leopard, Lion, Mountain Lion or other
versions available
from Apple, the Solaris operating system available from Sun Microsystems, or
UNIX or Linux
operating systems available from various sources. Many other operating systems
may be used,
and in certain embodiments a simple set of commands or instructions may
function as the
operating system. Further, the processor can be designed as a quantum
processor designed to
perform one or more functions using one or more qubits.
[072] In certain examples, the processor and operating system may together
define a platform
for which application programs in high-level programming languages may be
written. It should
be understood that the technology is not limited to a particular system
platform, processor,
operating system, or network. Also, it should be apparent to those skilled in
the art, given the
benefit of this disclosure, that the present technology is not limited to a
specific programming
language or computer system. Further, it should be appreciated that other
appropriate
programming languages and other appropriate systems could also be used. In
certain examples,
22
11 CA 3026698 2018-12-06
the hardware or software can be configured to implement cognitive
architecture, neural networks
or other suitable implementations. If desired, one or more portions of the
computer system may
be distributed across one or more computer systems coupled to a communications
network.
These computer systems also may be general-purpose computer systems. For
example, various
aspects may be distributed among one or more computer systems configured to
provide a service
(e.g., servers) to one or more client computers, or to perform an overall task
as part of a
distributed system. For example, various aspects may be performed on a client-
server or multi-
tier system that includes components distributed among one or more server
systems that perform
various functions according to various embodiments. These components may be
executable,
intermediate (e.g., IL) or interpreted (e.g., Java) code which communicate
over a communication
network (e.g., the Internet) using a communication protocol (e.g., TCP/IP). It
should also be
appreciated that the technology is not limited to executing on any particular
system or group of
systems. Also, it should be appreciated that the technology is not limited to
any particular
distributed architecture, network, or communication protocol.
[073] In some instances, various embodiments may be programmed using an object-
oriented
programming language, such as, for example, SQL, SmallTalk, Basic, Java,
Javascript, PHP,
C++, Ada, Python, i0S/Swift, Ruby on Rails or C# (C-Sharp). Other object-
oriented
programming languages may also be used. Alternatively, functional, scripting,
and/or logical
programming languages may be used. Various configurations may be implemented
in a non-
programmed environment (e.g., documents created in HTML, XML or other format
that, when
viewed in a window of a browser program, render aspects of a graphical-user
interface (GUI) or
perform other functions). Certain configurations may be implemented as
programmed or non-
programmed elements, or any combination thereof. In some instances, the
systems may
comprise a remote interface such as those present on a mobile device, tablet,
laptop computer or
other portable devices which can communicate through a wired or wireless
interface and permit
operation of the systems remotely as desired.
[074] In certain examples, the processor may also comprise or have access to a
database of
information about elemental species and the like, which can include optical
emission
wavelengths, optical absorption wavelengths and other common information. For
example, a
collection of calibration curves for different elemental species can be stored
in the database and
used to estimate elemental concentrations in the individual particle without
the need for the user
23
if CA 3026698 2018-12-06
to perform calibration curves for each of the elements. Such methods may be
particularly
desirable where the amount of sample is limited. The instructions stored in
the memory can
execute a software module or control routine for the system, which in effect
can provide a
controllable model of the system. The processor can use information accessed
from the database
together with one or software modules executed in the processor to determine
control parameters
or values for different components of the systems, e.g., different gas flow
rates, different light
wavelengths to be monitored, etc. Using input interfaces to receive control
instructions and
output interfaces linked to different system components in the system, the
processor can perform
active control over the system. For example, the processor can control the
detection device,
sample introduction devices, ionization devices, etc.
[075] In some examples, the methods and systems described herein can be used
to detect
simultaneous optical signals from two or more different elements present in an
individual
particle. Quantitation of each of the elements in the individual particle
introduced into the
ionization device can be performed if desired. In some instances, the method
comprises
separating each emitted wavelength in the simultaneous optical emissions to
permit detection of
each elemental species over wavelength range of about 165 nm to about 790 nm
and optionally
to permit quantitation of an amount of each elemental species present in the
individual particle.
[076] Referring to FIG. 15A, a scenario is shown where an optical emission
signal from two
different elements is monitored. In this example, elements A and B are present
in different
particles. As the particle comprising element A arrives at the ionization
source, it is ionized and
emits an optical signal that can be recorded as values 1510. Element A
generally emits light
continuously. As the second particle comprising element B arrives at the
ionization source,
optical signals 1520 can be monitored. The actual emission wavelength can be
used to identify
the element in the particle as noted herein. Because the elements A and B are
present in different
particles, monitoring the signals as a function of time causes the element B
signals 1520 to occur
after the element A signals 1510. Even though elements A and B are present on
the same graph
the signals for the two elements would typically be obtained by simultaneously
monitoring two
different emission wavelengths. In comparison, FIG. 15B shows a graph of
signal intensity vs
time where elements A and B are present in the same individual particle. As
the particle enters
the ionization source and is ionized, both elements A and B emit
simultaneously. A curve may
be fit to the signals for each element to determine a curve peak height an
area under the curve or
24
11 CA 3026698 2018-12-06
both to use these values for quantifying the amount of each of elements A and
B present in the
individual particle. Each particle of a plurality of particles may be measured
in a similar way to
determine an elemental composition of each individual particle. If desired,
more than two
elements in any one particle may be identified and/or quantified. For example
and referring to
FIG. 16, a signal intensity versus time graph is shown where optical emissions
from three
different elements species (color coded to show the presence of different
elements) are shown. A
curve can be generated to the optical signals for each element and used to
quantify an amount of
each element present in the individual particle. Depending on the particular
elements identified,
the source of the particle may be traced. For example, the presence and amount
of the specific
elements in the individual particle can be linked back to the source of the
particles. The source
of the particles may be, for example, an air sample, a specific site or
component in an engine or
transmission, a contaminant generated during in-line production of chemicals,
hydrocarbon
fluids, petroleum products or other industrial materials and the like. For
example, a fluid used in
an inline process can be sampled periodically to monitor a state of the fluid.
A first element and
a second element in a particle of the sampled fluid can be identified and
quantified to determine
a source of the particle in the inline process. In other instances, a gas
could be sampled
intermittently or automatically to monitor an ambient environment of a
facility.
[077] In some examples, different particles may have similar elemental
compositions which can
render it difficult to determine the exact source of the particle. Use of a
clustering technique for
separating the particles from one source versus another due to their different
elemental
compositions may be performed. For example, elemental composition of different
particles may
be aggregated to assist in identifying the particular source of those
particles and whether the
source is the same or is different.
[078] Certain specific examples are described in more detail below to
facilitate a better
understanding of the technology described herein.
[079] Example 1 ¨ Engine Oil analysis
[080] A used engine oil analysis (UOA) may be performed to measure elemental
species
present in an individual particle. A used engine oil sample may be obtained
and particulate
matter can be separated from any fluids. The particular type of elements in
each particle can be
11 CA 3026698 2018-12-06
used to identify a wear site. For example, the presence of iron and chromium
in the same
particle may indicate wear of a steel component present in the engine.
[081] Example 2 ¨ Transmission fluid analysis
[082] A transmission fluid analysis may be performed to measure elemental
species present in
an individual particle. A sample of transmission fluid may be removed through
a fill hole or
through a drain, and particulate matter can be separated from any fluids. The
particular type of
elements can be used to identify a wear site. For example, the presence of
copper and zinc the
same particle may indicate wear of brass synchronizer rings of the
transmission.
[083] Example 3 ¨ Air Monitoring
[084] Particulate species in air may be monitored using the techniques
described herein. For
example, particles of a certain size may be sampled from the air and the
elements composition of
each particle may be used to identify the source of the particles.
[085] Example 4¨ Water Monitoring
[086] A water sample may be obtained, e.g., from a well, pond, river, stream,
lake, municipal
feed, etc. to monitor particulate levels in the water. The particles can be
separated from the
water by filtration, centrifugation or other means. The elements present in
each particle can be
detected and identified to determine a source of the particulate matter in the
water sample. For
example, the identified elements can be used to trace a contaminant sample to
a source.
[087] Example 5 ¨ Inline Sampling
[088] An inline chemical process to produce hydrocarbon fluids may be
monitored by sampling
particulate matter from one or more of the fluid lines. The elemental
composition of each
individual particle in the sampled particulate matter may be determined and
used to identify a
contaminant source, catalyst degradation or wear of the fluid lines in the
system.
26
CA 3026698 2018-12-06
[089] Example 6
[090] To test the measurement of two or more elements in a sample, a metal
alloy that included
lithium, manganese, nickel and chromium was analyzed using an Optima 8300 OES
instrument.
The levels of the four metals that were measured are shown in FIG. 17A.
[091] Several possible scenarios may exist for the metals measured in FIG.
17A. Referring to
FIG. 17B, a Spotfire analysis and linear regression is shown assuming that the
nickel and cobalt
coexist in the same particles. The graph of FIG. 17B includes cobalt particle
events on the x-axis
and nickel particle events on the y-axis. There is a highly linear response
which is indicative of
the metals being present in the sample particle.
[092] FIGS. 17C-17H show graphs were different pair of metals are correlated.
FIG. 17C
shows correlation of manganese (y-axis) and lithium (x-axis). FIG. 17D shows
correlation of
nickel (y-axis) and manganese (x-axis). FIG. 17E shows correlation of nickel
(y-axis) and
lithium (x-axis). FIG. 17F shows correlation of cobalt (y-axis) and manganese
(x-axis). FIG.
17G shows correlation of cobalt (y-axis) and lithium (x-axis). FIG. 17H shows
correlation of
cobalt (y-axis) and nickel (x-axis). The various curves can be used to better
understand whether
two, three or all four of the metals are present in the same particle.
[093] Example 7
[094] A used engine oil sample was analyzed using an Optima 8300 OES
instrument to
measure the levels of iron and chromium to confirm the presence of steel in
the used engine oil.
The results are shown in FIGS. 18A-18E. FIGS. 18A and 18C shows the signal
intensities for
iron as a function of time. FIGS. 18B, 18D and 18E show the signal intensities
for chromium as
a function of time. Both iron and chromium were observed confirming the
presence of steel in
the used engine oil sample.
[095] When introducing elements of the examples disclosed herein, the articles
"a," "an," "the"
and "said" are intended to mean that there are one or more of the elements.
The terms
"comprising," "including" and "having" are intended to be open-ended and mean
that there may
be additional elements other than the listed elements. It will be recognized
by the person of
ordinary skill in the art, given the benefit of this disclosure, that various
components of the
examples can be interchanged or substituted with various components in other
examples.
27
CA 30266982018-12-06
[096] Although certain aspects, examples and embodiments have been described
above, it will
be recognized by the person of ordinary skill in the art, given the benefit of
this disclosure, that
additions, substitutions, modifications, and alterations of the disclosed
illustrative aspects,
examples and embodiments are possible.
28
CA 3026698 2018-12-06