Note: Descriptions are shown in the official language in which they were submitted.
PROCESSING OF PRODUCED FLUIDS FROM A SUBTERANNEAN FORMATION IN
A NEAR-AZEOTROPIC INJECTION PROCESS
CROSS-REFERENCE TO RELATED APPLICATION
[0001] This application claims the benefit of U.S. Provisional Patent
Application 62/607,073
filed 18 December 2017 entitled PROCESSING OF PRODUCED FLUIDS FROM A
SUBTERRANEAN FORMATION IN A NEAR-AZEOTROPIC INJECTION PROCESS, U.S.
Provisional Patent Application 62/607,077 filed 18 December 2017 entitled
PROCESSING OF
PRODUCED FLUIDS FROM A SUBTERRANEAN FORMATION IN A NEAR-AZEOTROPIC
INJECTION PROCESS and U.S. Provisional Patent Application 62/607,081 filed 18
December
2017 entitled PROCESSING OF PRODUCED FLUIDS FROM A SUBTERRANEAN
FORMATION IN A NEAR-AZEOTROPIC INJECTION PROCESS.
BACKGROUND
Field of Disclosure
[0002] The present disclosure relates to production of oil from a
subterranean reservoir in a
near-azeotropic solvent-based oil recovery process and processing of related
process fluids.
Description of Related Art
[0003] This section is intended to introduce various aspects of the art.
This discussion is
believed to facilitate a better understanding of particular aspects of the
present techniques.
Accordingly, it should be understood that this section should be read in this
light, and not
necessarily as admissions of prior art.
[0004] Modern society is greatly dependent on the use of hydrocarbon
resources for fuels and
chemical feedstocks. Subterranean rock formations that can be termed
"reservoirs" may contain
resources such as hydrocarbons that can be recovered. Removing hydrocarbons
from the
subterranean reservoirs depends on numerous physical properties of the
subterranean rock
formations, such as the permeability of the rock containing the hydrocarbons,
the ability of the
hydrocarbons to flow through the subterranean rock formations, and the
proportion of
hydrocarbons present, among other things.
- 1 -
CA 3026716 2019-09-26
[0005] Easily produced sources of hydrocarbons are dwindling, leaving
less conventional
sources to satisfy future needs. As the costs of hydrocarbons increase, less
conventional sources
become more economical. One example of less conventional sources becoming more
economical
is that of oil sand production. The hydrocarbons produced from less
conventional sources may
have relatively high viscosities, for example, ranging from 1000 centipoise
(cP) to 20 million cP
with American Petroleum Institute (API) densities ranging from 8 degree ( )
API, or lower, up to
20 API, or higher. The hydrocarbons recovered from less conventional sources
may include
heavy oil. However, the hydrocarbons produced from the less conventional
sources may be
difficult to recover using conventional techniques. For example, the heavy oil
may be sufficiently
viscous that economical production of the heavy oil from a subterranean
formation (also referred
to as a "subterranean reservoir" herein) is precluded.
[0006] Several conventional recovery processes, such as but not limited
to thermal recovery
processes, have been utilized to decrease the viscosity of the heavy oil.
Decreasing the viscosity
of the heavy oil may decrease a resistance of the heavy oil to flow and/or
permit production of the
heavy oil from the subterranean reservoir by piping, flowing, and/or pumping
the heavy oil from
the subterranean reservoir. While each of these recovery processes may be
effective under certain
conditions, each possess inherent limitations.
[0007] One of the conventional recovery processes utilizes steam
injection. The steam
injection may be utilized to heat the heavy oil to decrease the viscosity of
the heavy oil. Water
and/or steam may represent an effective heat transfer medium, but the pressure
required to produce
saturated steam at a desired temperature may limit the applicability of steam
injection to high
pressure operation and/or require a large amount of energy to heat the steam.
[0008] Another of the conventional recovery processes utilizes cold
and/or heated solvents.
Cold and/or heated solvents may be injected into a subterranean reservoir as
liquids and/or vapors
to decrease the viscosity of heavy oil present within the subterranean
reservoir. Solvent-based
recovery processes may include, but are not limited to, solvent assisted
cyclic steam stimulation
(SA-CSS), solvent assisted steam assisted gravity drainage (SA-SAGD), solvent
assisted steam
flood (SA-SF), vapor extraction process (VAPEX), heated vapor extraction
process (H-VAPEX),
cyclic solvent process (CSP), heated cyclic solvent process (H-CSP), solvent
flooding, heated
solvent flooding, liquid extraction process, heated liquid extraction process,
solvent-based
-2-
2747922
CA 3026716 2018-12-06
extraction recovery process (SEP), thermal solvent-based extraction recovery
processes (TSEP),
and any other such recovery process employing solvents either alone or in
combination with steam.
In particular is also a near-azeotropic heated VAPEX (AH-VAPEX) as described
in Patent No.
CA 2,915,571 C to Boone et al., wherein a process is described for injecting
steam and a vaporized
hydrocarbon solvent as near-azeotropic conditions to improve the overall
recovery process cost,
efficiencies, and/or hydrocarbon recovery.
[0009] However, the AH-VAPEX process described only includes the
conditions under which
the steam and the vaporized hydrocarbon solvent should be injected to improve
the overall
recovery processes in a subterranean reservoir. A need exists in the industry
for improved
technology, including technology for methods and other process required for
further improvement
of the AH-VAPEX process, including, but not limited to, recovery, processing
and re-use of fluids
recovered from produced heavy oil stream resulting from the AH-VAPEX process.
SUMMARY
[0010] It is an object of the present disclosure to provide improved
systems and methods for
the efficient and cost effective operation of near-azeotropic heated VAPEX (AH-
VAPEX)
processes.
[0011] In a preferred embodiment herein is a method for the processing of
the produced fluids
by AH-VAPEX process in a main processing facility to obtain a heavy oil
product and to generate
near-azeotropic vapor injection mixture. In this processing facilities
embodiment, the azeotropic
evaporation phenomena is utilized to separate the produced water and solvent
compounds from
the production mixture. Hence, the produced water and hydrocarbons are
generally processed
together in the main processing facility prior to preparation of the near-
azeotropic vapor injection
mixture.
[0012] In a preferred embodiment herein is a method for recovering
viscous hydrocarbons
from a subterranean reservoir, the method comprising:
a) drilling at least one well pair in a subterranean reservoir, wherein the
well pair is
comprised of an injection well and a production well, and the injection well
is located at an
elevation above the production well within the subterranean reservoir;
-3-
2747922
CA 3026716 2018-12-06
b) injecting a near-azeotropic reservoir injection mixture comprising steam
and a solvent
mixture into the subterranean reservoir via the injection well, wherein the
first near-azeotropic
reservoir injection mixture is injected in the vapor phase;
c) producing a reservoir product stream comprising a reduced-viscosity
hydrocarbons,
water, and a condensate; wherein the condensate comprises at least a portion
of the solvent
mixture;
d) sending at least a portion of the reservoir product stream to a primary
separation unit,
and producing a primary water stream, a primary gas vapor stream, and a
primary hydrocarbon
phase stream;
e) sending at least a portion of the primary hydrocarbon phase stream to a
solvent
separation unit; and producing a solvent vapor stream, and a heavy oil product
stream;
0 sending at least a portion of the primary water stream to a water treatment
unit and
producing a treated water stream;
g) sending at least a portion of the solvent vapor stream to a gas separation
unit, and
producing an off gas stream and a recovered solvent stream; and
h) vaporizing at least a portion of the recovered solvent stream and at least
a portion of
the treated water stream in at least one vapor generation unit;
wherein the near-azeotropic reservoir injection mixture comprises at least a
portion of the
recovered solvent stream and at least a portion of the treated water stream.
[0013] In another preferred embodiment herein is a method for recovering
viscous
hydrocarbons from a subterranean reservoir, the method comprising:
a) drilling at least one well pair in a subterranean reservoir, wherein the
well pair is
comprised of an injection well and a production well, and the injection well
is located at an
elevation above the production well within the subterranean reservoir;
b) injecting a near-azeotropic reservoir injection mixture comprising steam
and a solvent
mixture into the subterranean reservoir via the injection well, wherein the
near-azeotropic
reservoir injection mixture is injected in the vapor phase;
-4-
2747922
CA 3026716 2018-12-06
c) producing a reservoir product stream comprising a reduced-viscosity
hydrocarbons,
water, and a condensate; wherein the condensate comprises at least a portion
of the solvent
mixture;
d) optionally adding a primary make-up solvent stream, a primary make-up water
stream
or a combination thereof to the reservoir product stream to produce a primary
separation unit
feedstream;
e) sending at least a portion of the primary separation unit feedstream to a
primary
separation unit, and producing a primary water stream, a primary gas vapor
stream, and a heavy
oil product stream;
0 sending at least a portion of the primary gas vapor stream to a gas
separation unit; and
producing a recovered solvent/water stream and an off gas stream;
g) optionally adding a final make-up solvent stream, a final make-up water
stream or a
combination thereof to the recovered solvent/water stream to produce a final
tailored reservoir
solvent/water mixture, wherein a hydrocarbon solvent and water in the final
tailored reservoir
solvent/water mixture is compositionally at a near-azeotropic mixture at the
subterranean
reservoir operating conditions; and
h) vaporizing at least a portion of the final tailored reservoir solvent/water
mixture in at
least one vapor generation unit;
wherein the near-azeotropic reservoir injection mixture comprises at least a
portion of the
vaporized final tailored reservoir solvent/water mixture.
[0014] In yet another preferred embodiment herein is a method for
recovering viscous
hydrocarbons from a subterranean reservoir, the method comprising:
a) drilling at least one well pair in a subterranean reservoir, wherein the
well pair is
comprised of an injection well and a production well, and the injection well
is located at an
elevation above the production well within the subterranean reservoir;
b) injecting a near-azeotropic reservoir injection mixture comprising steam
and a solvent
mixture into the subterranean reservoir via the injection well, wherein the
near-azeotropic
reservoir injection mixture is injected in the vapor phase;
-5-
2747922
CA 3026716 2018-12-06
c) producing a reservoir product stream comprising a reduced-viscosity
hydrocarbons,
water, and a condensate; wherein the condensate comprises at least a portion
of the solvent
mixture;
d) sending at least a portion of the reservoir product stream to a primary
separation unit,
and producing a primary water stream, a primary vapor stream, and a primary
hydrocarbon phase
stream;
e) sending at least a portion of the primary hydrocarbon phase stream to a
solvent
separation unit; and producing a solvent vapor stream, and a heavy oil product
stream;
0 sending at least a portion of the primary vapor stream and at least a
portion of the
solvent vapor stream to a primary gas separation unit;
g) sending at least a portion of the primary water stream to the primary gas
separation
unit;
h) producing a primary gas separation vapor stream and a primary gas
separation liquid
stream from the primary gas separation unit;
i) sending at least a portion of the primary gas separation liquid stream to a
secondary gas
separation unit;
j) sending at least a portion of the primary gas separation gas stream to a
tertiary gas
separation unit;
k) producing an off gas stream and a recovered solvent/water stream from the
tertiary gas
separation unit;
1) optionally adding a final make-up solvent stream, a final make-up water
stream, or a
combination thereof to the recovered solvent/water stream to form a final
tailored reservoir
solvent/water mixture; wherein the solvent and water in the final tailored
reservoir solvent/water
mixture are at a near-azeotropic mixture; and
m) vaporizing the final tailored reservoir solvent/water mixture in at least
one vapor
generation unit;
wherein the near-azeotropic reservoir injection mixture comprises at least a
portion of the
vaporized final tailored reservoir solvent/water mixture.
-6-
2747922
CA 3026716 2018-12-06
[0015] The foregoing has broadly outlined the features of the present
disclosure so that the
detailed description that follows may be better understood. Additional
features will also be
described herein.
DESCRIPTION OF THE DRAWINGS
[0016] These and other features, aspects and advantages of the present
disclosure will become
apparent from the following description and the accompanying drawings, which
are briefly
discussed below.
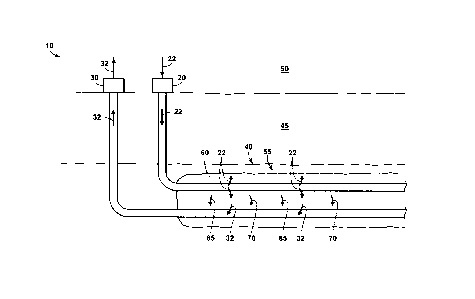
[0017] Figure 1 is a simplified schematic representation of an example of
a well system that
may be utilized in an AH-VAPEX process for in-situ heavy oil recovery.
[0018] Figure 2 illustrates the collective dew point curves of vapor
mixtures of n-alkanes
solvents with water at 1.5 MPa pressure.
[0019] Figure 3 is a simplified illustration of an AH-VAPEX main
processing facility
according to an embodiment of the systems and processes disclosed herein.
[0020] Figure 4 is a simplified illustration of an AH-VAPEX main processing
facility
according to an embodiment of the systems and processes disclosed herein.
[0021] Figure 5 is a simplified illustration of an AH-VAPEX main
processing facility
according to an embodiment of the systems and processes disclosed herein.
DETAILED DESCRIPTION
[0022] For the purpose of promoting an understanding of the principles of
the disclosure,
reference will now be made to the features illustrated in the drawings and
specific language will
be used to describe the same. It will nevertheless be understood that no
limitation of the scope of
the disclosure is thereby intended. Any alterations and further modifications,
and any further
applications of the principles of the disclosure as described herein, are
contemplated as would
normally occur to one skilled in the art to which the disclosure relates. It
will be apparent to those
skilled in the relevant art that some features that are not relevant to the
present disclosure may not
be shown in the drawings for the sake of clarity.
-7-
2747922
CA 3026716 2018-12-06
[0023] At the outset, for ease of reference, certain terms used in this
application and their
meanings as used in this context are set forth. To the extent a term used
herein is not defined
below, it should be given the broadest definition persons in the pertinent art
have given that term
as reflected in at least one printed publication of issued patent. Further,
the present techniques are
not limited by the usage of the terms shown below, as all equivalents,
synonyms, new
developments, and terms or processes that serve the same or a similar purpose
are considered to
be within the scope of the present disclosure.
[0024] A "hydrocarbon" is an organic compound that primarily includes the
elements
hydrogen and carbon, although nitrogen, sulfur, oxygen, metals, or any number
of other elements
may be present in small amounts. Hydrocarbons generally refer to components
found in heavy oil
or in oil sands. However, the techniques described herein are not limited to
heavy oils, but may
also be used with any number of other subterranean reservoirs. Hydrocarbon
compounds may be
aliphatic or aromatic, and may be straight chained, branched, or partially or
fully cyclic.
[0025] "Bitumen" is a naturally occurring heavy oil material. Generally,
it is the hydrocarbon
component found in oil sands. Bitumen can vary in composition depending upon
the degree of
loss of more volatile components. It can vary from a very viscous, tar-like,
semi-solid material to
solid forms. The hydrocarbon types found in bitumen can include aliphatics,
aromatics, resins,
and asphaltenes. A typical bitumen might be composed of:
19 weight (wt.)% aliphatics (which can range from 5 wt.% - 30 wt.%, or
higher);
19 wt.% asphaltenes (which can range from 5 wt.% - 30 wt.%, or higher);
wt.% aromatics (which can range from 15 wt.% - 50 wt.%, or higher);
32 wt.% resins (which can range from 15 wt.% - 50 wt.%, or higher); and
some amount of sulfur (which can range in excess of 7 wt.%).
100261 The percentage of the hydrocarbon types found in bitumen can vary.
In addition
25 bitumen can contain some water and nitrogen compounds ranging from less
than 0.4 wt.% to in
excess of 0.7 wt.%. The metals content, while small, may be removed to avoid
contamination of
synthetic crude oil. Nickel can vary from less than 75 ppm (parts per million)
to more than 200
ppm. Vanadium can range from less than 200 ppm to more than 500 ppm.
-8-
2747922
CA 3026716 2018-12-06
[0027] The term "heavy oil" includes bitumen, as well as lighter
materials that may be found
in a sand or carbonate reservoir. "Heavy oil" includes oils that are
classified by the American
Petroleum Institute (API), as heavy oils, extra heavy oils, or bitumens. Thus
the term "heavy oil"
includes bitumen. Heavy oil may have a viscosity of about 1000 centipoise (cP)
or more, 10,000
cP or more, 100,000 cP or more or 1,000,000 cP or more. In general, a heavy
oil has an API
gravity between 22.3 API (density of 920 kilograms per meter cubed (kg/m3) or
0.920 gams per
centimeter cubed (g/cm3)) and 10.0 API (density of 1,000 kg/m3 or 1 g/cm3).
An extra heavy oil,
in general, has an API gravity of less than 10.00 API (density greater than
1,000 kg/m3 or greater
than 1 g/cm3). For example, a source of heavy oil includes oil sand or
bituminous sand, which is
a combination of clay, sand, water, and bitumen. The recovery of heavy oils is
based on the
viscosity decrease of fluids with increasing temperature or solvent
concentration. Once the
viscosity is reduced, the mobilization of fluids by steam, hot water flooding,
or gravity is possible.
The reduced viscosity makes the drainage quicker and therefore directly
contributes to the recovery
rate. A heavy oil may include heavy end components and light end components.
[0028] The term "asphaltenes" or "asphaltene content" refers to pentane
insolubles (or the
amount of pentane insoluble in a sample) according to ASTM D3279. Other
examples of standard
ASTM asphaltene tests include ASTM test numbers D4055, D6560, and D7061.
[0029] "Heavy end components" in heavy oil may comprise a heavy viscous
liquid or solid
made up of heavy hydrocarbon molecules. Examples of heavy hydrocarbon
molecules include,
but are not limited to, molecules having greater than or equal to 30 carbon
atoms (C30+). The
amount of molecules in the heavy hydrocarbon molecules may include any number
within or
bounded by the preceding range. The heavy viscous liquid or solid may be
composed of molecules
that, when separated from the heavy oil, have a higher density and viscosity
than a density and
viscosity of the heavy oil containing both heavy end components and light end
components. For
example, in Athabasca bitumen, about 70 weight (wt.) % of the bitumen contains
C30+ molecules
with about 18 wt. % of the Athabasca bitumen being classified as asphaltenes.
The heavy end
components may include asphaltenes in the form of solids or viscous liquids.
[0030] "Light end components" in heavy oil may comprise those components
in the heavy oil
that have a lighter molecular weight than heavy end components. The light end
components may
include what can be considered to be medium end components. Examples of light
end components
-9-
2747922
CA 3026716 2018-12-06
and medium end components include, but are not limited to, light and medium
hydrocarbon
molecules having greater than or equal to 1 carbon atom and less than 30
carbon atoms. The
amount of molecules in the light and medium end components may include any
number within or
bounded by the preceding range. The light end components and medium end
components may be
composed of molecules that have a lower density and viscosity than a density
and viscosity of
heavy end components from the heavy oil.
[0031] A "fluid" includes a gas or a liquid and may include, for example,
a produced or native
reservoir hydrocarbon, an injected mobilizing fluid, hot or cold water, or a
mixture of these among
other materials. "Vapor" refers to steam, wet steam, and mixtures of steam and
wet steam, any of
which could possibly be used with a solvent and other substances, and any
material in the vapor
phase.
[00321 "Facility" or "surface facility" is a tangible piece of physical
equipment through which
hydrocarbon fluids are either produced from a subterranean reservoir or
injected into a
subterranean reservoir, or equipment that can be used to control production or
completion
operations. In its broadest sense, the term facility is applied to any
equipment that may be present
along the flow path between a subterranean reservoir and its delivery outlets.
Facilities may
comprise production wells, injection wells, well tubulars, wellbore head
equipment, gathering
lines, manifolds, pumps, compressors, separators, surface flow lines, steam
generation plants,
processing plants, and delivery outlets. In some instances, the term "surface
facility" is used to
distinguish from those facilities other than wells.
[0033] "Pressure" is the force exerted per unit area by the gas on the
walls of the volume.
Pressure may be shown in this disclosure as pounds per square inch (psi),
kilopascals (kPa) or
megapascals (MPa). "Atmospheric pressure" refers to the local pressure of the
air. "Absolute
pressure" (psia) refers to the sum of the atmospheric pressure (14.7 psia at
standard conditions)
plus the gauge pressure. "Gauge pressure" (psig) refers to the pressure
measured by a gauge,
which indicates only the pressure exceeding the local atmospheric pressure
(i.e., a gauge pressure
of 0 psig corresponds to an absolute pressure of 14.7 psia). The term "vapor
pressure" has the
usual thermodynamic meaning. For a pure component in an enclosed system at a
given pressure,
the component vapor pressure is essentially equal to the total pressure in the
system. Unless
otherwise specified, the pressures in the present disclosure are absolute
pressures.
- 10 -
2747922
CA 3026716 2018-12-06
[0034] A "subterranean reservoir" or "subterranean formation" is a
subsurface rock or sand
reservoir from which a production fluid, or resource, can be harvested. A
subterranean reservoir
may interchangeably be referred to as a subterranean formation. The
subterranean formation may
include sand, granite, silica, carbonates, clays, and organic matter, such as
bitumen, heavy oil (e.g.,
bitumen), oil, gas, or coal, among others. Subterranean reservoirs can vary in
thickness from less
than one foot (0.3048 meters (m)) to hundreds of feet (hundreds of meters).
The resource is
generally a hydrocarbon, such as a heavy oil impregnated into a sand bed.
[0035] "Thermal recovery processes" include any type of hydrocarbon
recovery process that
uses a heat source to enhance the recovery, for example, by lowering the
viscosity of a
hydrocarbon. The processes may use injected mobilizing fluids, such as but not
limited to hot
water, wet steam, dry steam, or solvents alone, or in any combination, to
lower the viscosity of the
hydrocarbon. Any of the thermal recovery processes may be used in concert with
solvents. For
example, thermal recovery processes may include cyclic steam stimulation
(CSS), steam assisted
gravity drainage (SAGD), steam flooding, in-situ combustion and other such
processes.
100361 "Solvent-based recovery processes" include any type of hydrocarbon
recovery process
that uses a solvent, at least in part, to enhance the recovery, for example,
by diluting or lowering a
viscosity of the hydrocarbon. Solvent-based recovery processes may be used in
combination with
other recovery processes, such as, for example, thermal recovery processes. In
solvent-based
recovery processes, a solvent is injected into a subterranean reservoir. The
solvent may be heated
or unheated prior to injection, may be a vapor or liquid and may be injected
with or without steam.
Solvent-based recovery processes may include, but are not limited to, solvent
assisted cyclic steam
stimulation (SA-CSS), solvent assisted steam assisted gravity drainage (SA-
SAGD), solvent
assisted steam flood (SA-SF), vapor extraction process (VAPEX), heated vapor
extraction process
(H-VAPEX), near-azeotropic heated vapor extraction process (AH-VAPEX), cyclic
solvent
process (CSP), heated cyclic solvent process (H-CSP), solvent flooding, heated
solvent flooding,
liquid extraction process, heated liquid extraction process, solvent-based
extraction recovery
process (SEP), thermal solvent-based extraction recovery processes (TSEP), and
any other such
recovery process employing solvents either alone or in combination with steam.
A solvent-based
recovery process may be a thermal recovery process if the solvent is heated
prior to injection into
the subterranean reservoir. The solvent-based recovery process may employ
gravity drainage.
-11-
2747922
CA 3026716 2018-12-06
[0037] "Azeotrope" or "Azeotropic" (or similar), as used herein, means
the thermodynamic
azeotrope of a mixture when the mixture is a two (2) component mixture of
water (steam) and a
single component solvent at a specified pressure. When the solvent is a multi-
component solvent
mixture, the terms "Azeotrope" or "Azeotropic" (or similar), as used herein,
means the minimum
boiling point (at a specified pressure) of water (steam) and the multi-
component solvent mixture.
The term "Near-Azeotropic" (or similar), as used herein, means within a
certain range (as specified
in its individual context where utilized) of the azeotrope point as defined
herein.
[0038] A "wellbore" is a hole in the subsurface made by drilling or
inserting a conduit into the
subsurface. A wellbore may have a substantially circular cross section or any
other cross-sectional
to shape, such as an oval, a square, a rectangle, a triangle, or other
regular or irregular shapes. The
term "well," when referring to an opening in the formation or reservoir, may
be used
interchangeably with the term "wellbore." Further, multiple pipes may be
inserted into a single
wellbore, for example, as a liner configured to allow flow from an outer
chamber to an inner
chamber.
[0039] "Permeability" is the capacity of a structure to transmit fluids
through the
interconnected pore spaces of the structure. The customary unit of measurement
for permeability
is the milliDarcy (mD).
[0040] "Reservoir matrix" refers to the solid porous material forming the
structure of the
subterranean reservoir. The subterranean reservoir is composed of the solid
reservoir matrix,
typically rock or sand, around pore spaces in which resources such as heavy
oil may be located.
The porosity and permeability of a subterranean reservoir is defined by the
percentage of volume
of void space in the rock or sand reservoir matrix that potentially contains
resources and water.
[0041] A "solvent extraction chamber" is a region of a subterranean
reservoir containing heavy
oil that forms around a well that is injecting solvent into the subterranean
reservoir. The solvent
extraction chamber has a temperature and a pressure that is generally at or
close to a temperature
and pressure of the solvent injected into the subterranean reservoir. The
solvent extraction
chamber may form when heavy oil has, due to heat from the solvent, dissolution
within the solvent,
combination with the solvent, and/or the action of gravity, at least partially
mobilized through the
pore spaces of the reservoir matrix. The mobilized heavy oil may be at least
partially replaced in
the pore spaces by solvent, thus forming the solvent chamber. The solvent
chamber may contain
- 12 -
2747922
CA 3026716 2018-12-06
liquid solvent, vapor solvent, condensed solvent, residual heavy oil, water,
gas, non-condensable
gas and/or a combination and/or mixture of them. In practice, layers in the
subterranean reservoir
containing heavy oil may not necessarily have pore spaces that contain 100
percent (/0) heavy oil
and may contain only 70 - 80 volume (vol.) % heavy oil with the remainder
possibly being water.
A water and/or gas containing layer in the subterranean reservoir may comprise
100% water and/or
gas in the pore spaces, but generally contains 5 - 70 vol.% gas and 20 - 30
vol.% water with any
remainder possibly being heavy oil.
[0042] A "vapor chamber" is a solvent extraction chamber that includes a
vapor, or vaporous
solvent. The vapor chamber may contain other gases including vapor water,
and/or non-
condensable gases. The vapor chamber may also contain vapor mixtures of water
and solvent. The
vapor chamber may also contain near-azeotropic or azeotropic vapor mixtures of
water and
solvent. Thus, when the solvent is injected into the subterranean reservoir as
a vapor, a vapor
chamber may be formed around the well.
[0043] A "compound that has five or more carbon atoms" or "C5+"may
include any suitable
single chemical species that may include five or more carbon atoms. A
"compound that has five
or more carbon atoms" also may include any suitable mixture of chemical
species. Each of the
chemical species in the mixture of chemical species may include five or more
carbon atoms and
each of the chemical species in the mixture of chemical species also may
include the same number
of carbon atoms as the other chemical species in the mixture of chemical
species. For example, a
compound that has five carbon atoms may include a pentane, n-pentane, a
branched pentane,
cyclopentane, a pentene, n-pentene, a branched pentene, cyclopentene, a
pentyne, n-pentyne, a
branched pentyne, cyclopentyne, methylbutane, dimethylpropane, ethylpropane,
and/or any other
hydrocarbon with five carbon atoms. A compound with six carbon atoms, seven
carbon atoms, or
eight carbon atoms may include a single chemical species with six carbon
atoms, seven carbon
atoms, or eight carbon atoms, respectively, and/or may include a mixture of
chemical species that
each include six carbon atoms, seven carbon atoms, or eight carbon atoms,
respectively.
[0044] The terms "approximately," "about," "substantially," and similar
terms are intended to
have a broad meaning in harmony with the common and accepted usage by those of
ordinary skill
in the art to which the subject matter of this disclosure pertains. It should
be understood by those
of skill in the art who review this disclosure that these terms are intended
to allow a description of
- 13 -
2747922
CA 3026716 2018-12-06
certain features described and claimed without restricting the scope of these
features to the precise
numeral ranges provided. Accordingly, these terms should be interpreted as
indicating that
insubstantial or inconsequential modifications or alterations of the subject
matter described and
are considered to be within the scope of the disclosure. These terms when used
in reference to a
quantity or amount of a material, or a specific characteristic of the
material, refer to an amount that
is sufficient to provide an effect that the material or characteristic was
intended to provide. The
exact degree of deviation allowable may in some cases depend on the specific
context.
[0045] The articles "the", "a" and "an" are not necessarily limited to
mean only one, but rather
are inclusive and open ended so as to include, optionally, multiple such
elements.
[0046] As used herein, the phrase "at least one," in reference to a list of
one or more entities
should be understood to mean at least one entity selected from any one or more
of the entity in the
list of entities, but not necessarily including at least one of each and every
entity specifically listed
within the list of entities and not excluding any combinations of entities in
the list of entities. This
definition also allows that entities may optionally be present other than the
entities specifically
identified within the list of entities to which the phrase "at least one"
refers, whether related or
unrelated to those entities specifically identified. Thus, as a non-limiting
example, "at least one of
A and B" (or, equivalently, "at least one of A or B," or, equivalently "at
least one of A and/or B")
may refer, to at least one, optionally including more than one, A, with no B
present (and optionally
including entities other than B); to at least one, optionally including more
than one, B, with no A
present (and optionally including entities other than A); to at least one,
optionally including more
than one, A, and at least one, optionally including more than one, B (and
optionally including other
entities). In other words, the phrases "at least one," "one or more," and
"and/or" are open-ended
expressions that are both conjunctive and disjunctive in operation. For
example, each of the
expressions "at least one of A, B and C," "at least one of A, B, or C," "one
or more of A, B, and
C," "one or more of A, B, or C" and "A, B, and/or C" may mean A alone, B
alone, C alone, A and
B together, A and C together, B and C together, A, B and C together, and
optionally any of the
above in combination with at least one other entity.
[0047] As used herein, the term "and/or" placed between a first entity
and a second entity
means one of (1) the first entity, (2) the second entity, and (3) the first
entity and the second entity.
Multiple entities listed with "and/or" should be construed in the same manner,
i.e., "one or more"
- 14 -
2747922
CA 3026716 2018-12-06
of the entities so conjoined. Other entities may optionally be present other
than the entities
specifically identified by the "and/or" clause, whether related or unrelated
to those entities
specifically identified. Thus, as a non-limiting example, a reference to "A
and/or B," when used
in conjunction with open-ended language such as "comprising" may refer to A
only (optionally
including entities other than B); to B only (optionally including entities
other than A); to both A
and B (optionally including other entities). These entities may refer to
elements, actions,
structures, steps, operations, values, and the like.
[0048] As used herein the terms "adapted" and "configured" mean that the
element,
component, or other subject matter is designed and/or intended to perform a
given function. Thus,
the use of the terms "adapted" and "configured" should not be construed to
mean that a given
element, component, or other subject matter is simply "capable of" performing
a given function
but that the element, component, and/or other subject matter is specifically
selected, created,
implemented, utilized, programmed, and/or designed for the purpose of
performing the function.
It is also within the scope of the present disclosure that elements,
components, and/or other recited
subject matter that is recited as being adapted to perform a particular
function may additionally or
alternatively be described as being configured to perform that function, and
vice versa.
[0049] As used herein, the phrase, "for example," the phrase, "as an
example," and/or simply
the term "example," when used with reference to one or more components,
features, details,
structures, embodiments, and/or methods according to the present disclosure,
are intended to
convey that the described component, feature, detail, structure, embodiment,
and/or method is an
illustrative, non-exclusive example of components, features, details,
structures, embodiments,
and/or methods according to the present disclosure. Thus, the described
component, feature,
detail, structure, embodiment, and/or method is not intended to be limiting,
required, or
exclusive/exhaustive; and other components, features, details, structures,
embodiments, and/or
methods, including structurally and/or functionally similar and/or equivalent
components,
features, details, structures, embodiments, and/or methods, are also within
the scope of the present
disclosure. Any of the ranges disclosed may include any number within and/or
bounded by the
range given.
[0050] In the illustrative figures herein, in general, elements that are
likely to be included are
illustrated in solid lines, while elements that are optional may be
illustrated in dashed lines.
- 15 -
2747922
CA 3026716 2018-12-06
=
However, elements that are shown in solid lines may not be essential. Thus, an
element shown in
solid lines may be omitted without departing from the scope of the present
disclosure.
[0051] Figures 1-5 provide illustrative, non-exclusive examples of
systems according to the
present disclosure, components of systems, data that may be utilized to select
a composition of a
hydrocarbon solvent mixture and or a reservoir injection mixture that may be
utilized with systems,
and/or methods, according to the present disclosure, of operating and/or
utilizing systems.
Elements that serve a similar, or at least substantially similar, purpose are
labeled with like
numbers in each of Figures 1-5, and these elements may not be discussed in
detail herein with
reference to each of Figures 1-5. Similarly, all elements may not be labeled
in each of Figures 1-5,
but associated reference numerals may be utilized for consistency. Elements,
components, and/or
features that are discussed herein with reference to one or more of Figures 1-
5 may be included in
and/or utilized with any of Figures 1-5 without departing from the scope of
the present disclosure.
[0052] The inventions disclosed herein are related to process
improvements to a Near-
Azeotropic Heated VAPEX process for in-situ recovery of heavy oil products
from a subterranean
reservoir. The Near-Azeotropic Heated VAPEX may also be referred to herein by
such terms as
"Near-Azeotropic H-VAPEX", "Azeotropic H-VAPEX", "Azeo. H-VAPEX", or "AH-
VAPEX",
all of which should be construed to have the same meaning in the context of
this disclosure.
100531 AH-VAPEX is a variation of a heated VAPEX (H-VAPEX) process in
which an
optimum volume of steam is co-injected with hydrocarbon solvent in vapor
phase. The optimum
volume of steam for given solvent is determined according to the phase
behavior of solvent and
water mixture at the operation pressure, and is the exact or near
azeotropic/minimum boiling point
concentration of water in vapor phase. In both AH-VAPEX and H-VAPEX processes,
the well
configuration is typically similar to a steam-assisted gravity drainage (or
"SAGD") process in
which two substantially horizontal wells (or "well pair") are installed
substantially one above the
other in the hydrocarbon-containing subterranean reservoir, wherein the upper
well is utilized as
an injection well and the lower well is utilized as a production well. The AH-
VAPEX process
injects a specific solvent and steam ratio in the vapor phase through the
injection well and utilizes
a gravity drainage oil recovery mechanism of the mobilized heavy oil due to
reduced in-situ
viscosity by increased temperature and dilution/mixing with the condensed
solvent compounds.
[0054] Figure 1 is a non-limiting schematic representation of a well
configuration that may
- 16 -
2747922
CA 3026716 2018-12-06
utilize the AH-VAPEX process which is supplied for the purpose of illustrating
an embodiment of
an AH-VAPEX process that may be utilized with, or may be included in the
systems and methods
according to the present disclosure. Figure 1 is utilized only to assist in
explaining details related
to the present disclosure, and is not meant to be limiting in any manner,
including any limitations
on reservoir or well configurations, solvent or steam usage or requirements,
or overall recovery
system and/or oil processing requirements.
[0055] Figure 1 illustrates a simplified, and non-limiting description of
well system 10 that
may be utilized in an AH-VAPEX process which includes an injection well 20 and
a production
well 30 that extend within a subterranean reservoir 40 that is present within
a subsurface region 45
and/or that extend between a surface region 50 and the subterranean reservoir
40.
[0056] In the AH-VAPEX process, a reservoir injection mixture 22
comprising steam and a
hydrocarbon-containing solvent mixture (or "solvent mixture" herein) wherein
the reservoir
injection mixture 22 is injected substantially in the vapor phase into the
subterranean reservoir 40
via an injection well 20. As noted, the solvent mixture is comprised of
hydrocarbons, and in
preferred embodiments, the solvent mixture is substantially comprised of
hydrocarbons, or even
essentially comprised of hydrocarbons. The term hydrocarbon-containing solvent
mixture herein
is preferably a mixture or range of boiling point hydrocarbon compounds, but
as utilized herein,
may additional additionally consist essentially of a single hydrocarbon
compound. It has been
discovered that, in a VAPEX type heavy oil recovery process, the optimum
volume of steam for
given solvent is determined according to the phase behavior of solvent and
water mixture at the
operation pressure, and is the exact or near azeotropic/minimum boiling point
concentration of
water in vapor phase.
[0057] Continuing with Figure 1, the AH-VAPEX comprises of injecting the
vapor stream into
the subterranean reservoir 40 via the injection well 20 and producing a
reservoir product stream
32 from the subterranean reservoir 40 via the production well 30. The
reservoir injection mixture
22 utilized in the process includes steam and a solvent mixture. Preferably
the solvent mixture is
comprised essentially of hydrocarbons. In a preferred embodiment, the steam
and solvent mixture
is within 30%+/-, 20%+/-, or 10%+/- of the azeotropic solvent molar fraction
of the steam and the
solvent mixture as measured at the reservoir operating pressure.
Alternatively, molar fraction of
solvent mixture in the solvent and steam injection mixture is 70-100%, 80-
100%, or 90 to 100%
- 17 -
2747922
CA 3026716 2018-12-06
of the azeotropic solvent molar fraction of the steam and the solvent mixture
as measured at the
reservoir operating pressure. Alternatively, the molar fraction of solvent
mixture in the solvent and
steam injection mixture is 70-110% of the azeotropic solvent molar fraction of
the steam and the
solvent mixture as measured at the reservoir operating pressure. In preferred
embodiments, the
reservoir injection mixture 22 is comprised of at least 75%, 90%, 95, or
substantially 100% by
weight of the steam and the solvent mixture.
[0058] In preferred embodiments, at least 90%, at least 95%, or
essentially all (by weight) of
the reservoir injection mixture is injected into the subterranean reservoir in
vapor form. In other
embodiments, at least 5 wt%, 10 wt%, 20 wt%, 40 wt%, 60 wt%, 75 wt%, 85 wt%,
90 wt%, or 95
wt% of the solvent mixture is hydrocarbon compounds. The solvent mixture may
include a
hydrocarbon fraction that comprises, consists of, or consists essentially of
C4 to C12 hydrocarbons,
or C5 to C9 hydrocarbons. The solvent mixture may include a hydrocarbon
fraction that comprises,
consists of, or consists essentially of at least one of alkanes, iso-alkanes,
naphthenic hydrocarbons,
aromatic hydrocarbons, and olefin hydrocarbons. In preferred embodiments,
these compositions
will also apply to the reservoir injection mixtures produced by the facilities
and associated
processes described herein.
[0059] The reservoir injection mixture 22 may be injected into the
subterranean reservoir at an
injection temperature and an injection pressure. The injection temperature may
be at, or near, a
saturation temperature for the heated solvent at the injection pressure. When
more than one solvent
.. is utilized, the extraction process may be referred to as a multi-solvent-
based recovery process
and/or a multi-component solvent-based recovery process, which, at elevated
temperatures, may
be referred to as a high temperature multi-component solvent-based recovery
process, which may
be a high temperature multi-component vapor extraction process.
[0060] Available solvents and solvent mixtures that may be utilized in
the AH-VAPEX
.. processes described herein may range from light hydrocarbon mixtures such
as NGLs, and LPG
to heavy fractions such as different refinery streams. Preferably, these
mixtures are mainly
composed of hydrocarbon compounds with 3 to 12 carbon atoms and beyond. In the
processes
herein, these compounds form vapor mixtures with steam wherein the vapor
mixture exhibits
azeotropic behavior as the collective dew point curves of Figure 2
demonstrates for a range of C3
to Ci2 alkanes at 1.5 MPa pressure. Hence, after injection, these compositions
of hydrocarbon
- 18 -
2747922
CA 3026716 2018-12-06
vapor and water vapor tend to co-condense at the azeotropic temperature in the
reservoir at given
reservoir pressure to form two immiscible liquid phases. Also, a mixture of
liquid water and these
liquid hydrocarbon compounds tends to evaporate to a single vapor phase at
these azeotropic
conditions at given reservoir pressure and temperature. In general, the
azeotropic temperature for
vapor mixtures of water and the hydrocarbon compounds at the given reservoir
pressure is equal
to or less than the saturation temperature of pure water vapor and pure
hydrocarbon compounds.
For the purpose of the AH-VAPEX processes herein, depending on the solvent
type, a near
azeotropic/minimum boiling point mixture of solvent and steam contains 15-98
vol. % solvent and
2-85 vol. % steam, in cold liquid equivalents, calculated at standard
temperature and pressure.
[0061] Returning to Figure 1, once provided to subterranean reservoir 40,
the reservoir
injection mixture 22 may combine with a bituminous hydrocarbon deposit 55
within a solvent
extraction chamber 60, may dilute the bituminous hydrocarbon deposit 55, may
dissolve in the
bituminous hydrocarbon deposit 55, and/or may dissolve the bituminous
hydrocarbon deposit 55,
thereby decreasing the viscosity of the bituminous hydrocarbon deposit. In an
AH-VAPEX
process, a solvent extraction chamber 60, which may also be referred as a
vapor chamber, is
created. The vaporous hydrocarbon solvent mixture may condense within the
solvent extraction
chamber 60. When reservoir injection mixture 22 condenses, the hydrocarbon
solvent mixture
may release latent heat (or latent heat of condensation), transfer thermal
energy to the subterranean
reservoir, and/or generate a condensate 65. Condensation of the reservoir
injection mixture 22
may heat a bituminous hydrocarbon deposit 55 that may be present within the
subterranean
reservoir, thereby decreasing a viscosity of the bituminous hydrocarbon
deposit. In embodiments,
the subterranean reservoir operating temperature may be 30-250 C or 80-150 C.
In further
embodiments, the subterranean reservoir operating pressure may be 5-95% of a
fracture pressure
of the reservoir, or 0.2 to 4 MPa, or 1 to 2.5 MPa. Conversely, the
subterranean reservoir operating
pressure may be equal to the pressure of a gas cap in the subterranean
reservoir, the pressure of a
gas zone within the subterranean reservoir, the pressure of a bottom water
zone in the subterranean
reservoir, or the pressure of a mobile water zone within the subterranean
reservoir.
[0062] The bituminous hydrocarbon deposit 55 may include bitumen, gaseous
hydrocarbons,
asphaltenes, and/or water. The reservoir injection mixture 22 and/or
condensate 65 also may
combine with, mix with, be dissolved in, dissolve, and/or dilute bituminous
hydrocarbon deposit
- 19 -
2747922
CA 3026716 2018-12-06
55, further decreasing the viscosity of the bituminous hydrocarbon deposit.
[0063] The energy transfer between the reservoir injection mixture 22 and
bituminous
hydrocarbon deposit 55 and/or the mixing of reservoir injection mixture 22
and/or condensate 65
with bituminous hydrocarbon deposit 55 may generate reduced-viscosity
hydrocarbons 70, which
.. may flow to production well 30. The reduced-viscosity hydrocarbons 70 may
flow to production
well 30 due to gravity and/or pressure drop. After flowing to production well
30, a reservoir
product stream 32 containing heavy oil is produced from the subterranean
reservoir. The reduced-
viscosity hydrocarbons 70 may have a lower viscosity than the hydrocarbons
within the
subterranean reservoir 45 had before the reservoir injection mixture 22 was
injected into the
subterranean reservoir 40. The reservoir product stream 32 may comprise the
reduced-viscosity
hydrocarbons 70 and condensate 65 in any suitable ratio and/or relative
proportion. The reservoir
product stream 32 may also contain asphaltenes, gaseous hydrocarbons, water,
water soluble
minerals and salts, solids, and/or other materials or contaminants. The
reservoir product stream
32 is generally comprised of a hydrocarbon liquid phase (including reduced-
viscosity heavy oil
.. and a condensed portion of injected solvent compounds, i.e., condensate), a
gas phase mixture
(including in-situ native solution gas compounds such as CH4, process gaseous
by-products such
as CO2 and H2S, water vapor and a portion of injected solvent compounds) and a
water liquid
phase (including a portion in-situ formation water with dissolved minerals and
a condensed portion
of injected steam or water vapor). The reservoir product stream 32 may also
carry some suspended
minerals and solid particles (including sand, silt and clay from the
subterranean formation).
Detailed Embodiment 1
[0064] Figure 3 depicts a schematic flow diagram of the main processing
facility 300 for an
embodiment of a process herein for managing the reservoir product stream 32,
producing product
streams, and producing a tailored reservoir solvent mixture 345 for reuse for
injection as and/or
co-injection with another stream as a component of the reservoir injection
mixture 22. In the
currently disclosed embodiment of this process, the reservoir product stream
32, or a portion
thereof, is sent to a primary separation unit 301. In this primary separation
unit 301, a major
portion of the produced liquid water is separated from the reservoir product
stream 32 to produce
a primary water stream 302. The primary water stream 302 may also contain some
amount of
- 20 -
2747922
CA 3026716 2018-12-06
hydrocarbons and solids. Preferably, the primary separation unit 301 utilizes
a gravity settling
phenomena, for instance, a device such as a gravity settler. The pressure and
temperature of the
primary separation unit 301 may be chosen in such a way to evolve and isolate
a vapor phase,
mainly composed of solution gas compounds, process gaseous by-products, water
vapor and may
contain a portion of compounds from the solvent mixture of reservoir injection
mixture 22, to
produce a primary gas vapor stream 304. The primary separation unit 301 also
produces a third
stream which is designated as a primary hydrocarbon phase stream 305 which
contains the
majority of the reduced-viscosity hydrocarbons 70, as well as the majority of
the solvent mixture
that is a component of the reservoir injection mixture 22, that are present in
the reservoir product
stream 32, and which has been produced and recovered from the AH-VAPEX
processes described
herein. In preferred embodiments, the primary hydrocarbon phase stream 305
recovers at least
70%, at least 80%, at least 90%, at least 95%, or at least 99% by weight of
the reduced-viscosity
hydrocarbons 70 present in the portion of the reservoir product stream 32 that
is sent to the primary
separation unit 301. In preferred embodiments, the primary hydrocarbon phase
stream 305
recovers at least 70%, at least 80%, at least 90%, at least 95%, or at least
99% by weight of the
solvent mixture present in the portion of the reservoir product stream 32 that
is sent to the primary
separation unit 301. The primary hydrocarbon phase stream 305 may also contain
some water and
solids.
100651 In the currently disclosed processes, the primary hydrocarbon
phase stream 305 is sent
to a solvent separation unit 310. In the solvent separation unit 310, the
majority of the solvent
boiling range compounds are separated from the primary hydrocarbon phase
stream 305. In
embodiments, the solvent separation unit 310 can be comprised of a single
stage flash unit, a
multiple-stage flash unit, a distillation/stripping column and/or a
combination thereof. Depending
on the temperature and pressure of the primary hydrocarbon phase stream 305, a
primary
.. hydrocarbon phase heater 307 may be used to heat the stream to a certain
process temperature and
provide the required thermal energy to vaporize the solvent compounds. In
embodiments, this
temperature on the hydrocarbon outlet of the primary hydrocarbon phase heater
307 is maintained
to vaporize at least 70%, at least 80%, at least 90%, at least 95%, or at
least 99% by weight of the
solvent boiling range compounds present in the primary hydrocarbon phase
stream 305 at the
operating pressure of the solvent separation unit 310. The operating pressure
of the solvent
separation unit 310 will be taken at the inlet to the flash unit or
distillation column deployed within
-21 -
2747922
CA 3026716 2018-12-06
the solvent separation unit 310. The thermal energy for the primary
hydrocarbon phase heater 307
may be provided by any direct or indirect heating method (for example: fired
heater, hot oil loop,
by heat exchange with other process streams, such as waste heat recovery, or
by direct steam
heating).
[0066] In embodiments, a solvent separation unit controller 309, may be
used to control the
flow and/or pressure drop in the primary hydrocarbon phase stream 305. In
embodiments, the
solvent separation unit controller 309 is adjusted to maintain the primary
hydrocarbon phase
stream 305 upstream of the controller substantially in the liquid phase. In
embodiments, the
solvent separation unit controller 309 is further adjusted such as to flash
the solvent boiling range
compounds of the primary hydrocarbon phase stream 305 substantially to the
vapor phase entering
the solvent separation unit 310. Chemicals may be added to the reservoir
product stream 32 and/or
the primary hydrocarbon phase stream 305 to prevent foaming, fouling, scaling,
and/or other
similar operation phenomena in the associated process equipment. In addition,
special mechanical
designs may be utilized in the equipment associated with the primary
separation unit 301 and/or
the solvent separation unit 310 to prevent the aforementioned phenomena.
[0067] A heavy oil product stream 312 is produced from the solvent
separation unit 310 from
which, in embodiments, most of the solvent boiling range compounds have been
removed. The
heavy oil product stream 312 may be of sufficient composition to meet
necessary pipeline
specifications in which case the heavy oil product stream 312 may be sent to a
pipeline as a pipeline
product stream 320. In embodiments herein, the solvent separation unit 310 may
be operated such
that a sufficient amount of solvent, for example C4 to C12 hydrocarbons,
remains in the heavy oil
product stream 312 to allow the heavy oil product stream to meet pipeline
specifications or reduces
the amount of a diluent 314 that needs to be added to meet pipeline
specifications.
[0068] Alternatively, in embodiments herein, the heavy oil product stream
312 may be too
viscous or have to low an API gravity to meet specifications for pipeline
transportation. In these
instances, a diluent 314 may be added to the heavy oil product stream 312 to
produce the pipeline
product stream 320. A diluent treating unit 315 may additionally be utilized
to further control the
amount of diluent added and provide proper mixing of the diluent 314 and heavy
oil product stream
312 to meet the specifications of the produced pipeline product stream 320.
Additionally, or
optionally, excess solids and water present in the heavy oil product stream
312 can be removed in
- 22 -
2747922
CA 3026716 2018-12-06
the diluent treating unit 315 to meet the pipeline specifications. The diluent
treating unit 315 may
utilize electrostatic mechanisms to separate excess solids and water from the
heavy oil product
stream. When a diluent treating unit 315 is utilized, the diluent 314 may be
added to the heavy oil
product stream 312 and/or optionally directly into the diluent treating unit
315. In embodiments,
the design and operation pressure and temperature of the solvent separation
unit 310 produces the
heavy oil product stream 312 such that it contains a portion of the solvent
boiling range compounds
in order to meet the pipeline specification requirements.
[0069] The solvent separation unit 310 also produces a solvent vapor
stream 325. In preferred
embodiments, this stream comprises solvent boiling range compounds recovered
from the
reservoir product stream 32 which are within the boiling point ranges of the
solvent mixture
utilized in the reservoir injection mixture 22. The solvent vapor stream 325
may also include other
gaseous compounds that were part of the reservoir product stream 32 and not
removed in the
primary gas vapor stream 304 of the primary separation unit 301.
[0070] In embodiments, each the heavy oil product stream 312 and the
solvent vapor stream
325 may be composed of a single stream each (as shown in simplified Figure 3)
or they may be
composed of a combination of multiple streams produced in the solvent
separation unit 310 which
are grouped and/or combined to form the heavy oil product stream 312 and the
solvent vapor
stream 325, respectively.
[0071] As noted prior, some of the solvent boiling range compounds may be
recovered in the
primary gas vapor stream 304 from the primary separation unit 301. In this
case, in preferred
embodiments, at least a portion of the primary gas vapor stream 304 and at
least a portion of the
solvent vapor stream 325 can be combined into stream 327 and sent to the gas
separation unit 330
for further processing. Alternatively, only the solvent vapor stream 325, or a
portion thereof, is
sent via stream 327 to the gas separation unit 330. Prior to the gas
separation unit 330, stream 327
is sent through a solvent cooler 328, where the stream is cooled so that at
least portion of the
solvent boiling range compounds are condensed to a liquid. The solvent cooler
328 may be
designed and operated to provide thermal energy to other process streams main
processing facility
300. Alternatively, at least a portion of the primary gas vapor stream 304 and
at least a portion of
the solvent vapor stream 325 may independently undergo similar gas separation
processes and the
final streams then may be combined with the corresponding streams.
- 23 -
2747922
CA 3026716 2018-12-06
100721 In preferred embodiments, the gas separation unit 330 produces an
off gas stream 331,
a recovered solvent stream 333, and a gas separator water stream 335 as
illustrated in Figure 3.
The off gas stream 331 will generally be comprised of light hydrocarbon gases,
such as methane
and ethane, but generally will also include some non-hydrocarbon gases such as
CO2 and H2S.
Optionally, special provisions and designs may be utilized in the gas
separation units to separate
some of the light hydrocarbon gases from the entrained CO2 and/or H2S for
proper treatment and/or
disposal prior to sending the off gas stream 331 to a fuel gas system or for
direct use in fired heating
equipment that may be part of the main processing facility 300. Optionally,
the off gas stream 331
may be added directly to a make-up fuel gas stream 332 prior to use in the
fired heating equipment.
[0073] The gas separation unit 330 is operated under conditions to produce
a recovered solvent
stream 333 that is generally be free of impurities and ready for near-
azeotropic vapor generation.
The gas separator water stream 335 will recover the condensed water from the
water gas separation
unit 330. The gas separator water stream 335 should be substantially free of
impurities and require
little, if any, further treatment prior to uses such as boiler feedwater
supplied to fired heating
equipment that may be part of the main processing facility 300. In
embodiments, the gas separation
unit 330 can be comprised of a single stage flash unit, a multiple-stage flash
unit, a
distillation/stripping column and/or a combination thereof
100741 In the processes herein, the recovered solvent stream 333 is
tailored in composition for
use as a solvent mixture for use in the AH-VAPEX process, or if needed, a make-
up solvent stream
.. 340 can be added prior to final use to produce a tailored reservoir solvent
mixture 345 for use in
the reservoir injection mixture 22. Figure 3 shows an optional solvent storage
system 342 which
may be utilized as a surge buffer and/or mixing step in the process. The make-
up solvent stream
340 can be alternatively introduced to the recovered solvent stream 333
upstream and/or
downstream of the optional solvent storage system 342 if utilized. The make-up
solvent stream
340 may be used to add additional solvent to the process to make up for
solvent losses from the
AH-VAPEX process to the subterranean reservoir as well as from the main
processing facility 300
and/or may be used to tailor the recovered solvent stream 333 to compositional
specifications for
a tailored reservoir solvent mixture 345 required for the near-azeotropic
formulation of the
reservoir injection mixture 22.
[0075] Much of the water required to generate the steam for the AH-VAPEX
process can be
- 24 -
2747922
CA 3026716 2018-12-06
recovered from the reservoir product stream 32 as illustrated in the disclosed
processes herein. As
shown in Figure 3, at least a portion of the primary water stream 302 may be
sent to a water
treatment unit 350. In the water treatment unit 350, the primary water stream
302 is treated to
boiler feed-water specifications by means of conventional water treatment
methods. The water
treatment unit 350 may utilize any of physical-chemical water treatment
methods such as hot lime
water softening kits and/or mechanical methods such as reverse osmosis, water
vapor compression
evaporators, evaporative water treatment methods, or any other water treating
method. Additional
make-up water 352, if required, may be added before or after or during water
treatment processes
depending on its source and required treatment to meet boiler feed-water
specifications. In general,
a portion of the water utilized in the water treatment unit 350 will be
disposed (not recovered and
recycled) to carry-over all impurities such as solids, salts and minerals
which can be sent to a water
disposal facilities 354 where the disposed water will undergo the extra
processing to remove
hydrocarbons and other contaminants to meet environmental regulations.
[0076] The water treatment unit 350 will produce a treated water stream
354, which as noted,
meets boiler feed-water specifications. The gas separator water stream 335 may
be added as shown
to the treated water stream 354 if of sufficient quality to meet boiler feed-
water specifications.
Alternatively, all, or a portion, of the gas separator water stream 335 may be
sent to the water
treatment unit 350. Optionally, the treated water stream 354 may be sent to a
water storage tank
355 prior to be utilized in the AH-VAPEX process.
[0077] The treated water stream from the water storage tank 355 (designated
as stored treated
water stream 358) and the tailored reservoir solvent mixture 345 (or a portion
of each thereof) are
supplied to a vapor generation unit 360 in required proportions to generate
the near-
azeotropic/minimum boiling point vapor mixture for use in the AH-VAPEX
process. The vapor
generation unit 360 may be comprised of heat exchanger, a steam heat
exchanger, a hot oil heat
exchanger, a fired heater, or any other suitable vaporizer design. The water
and solvent mixture
may be combined together and evaporated simultaneously to the corresponding
dew-point
temperature at the unit operation pressure. Excess heat generated in the vapor
generation unit 360
may be utilized to superheat the combined stream. Alternatively, stored
treated water stream 358
and the tailored reservoir solvent mixture 345 may be vaporized separately at
the corresponding
saturation temperature at the unit operation pressure, and subsequently
combined prior to use in
- 25 -
2747922
CA 3026716 2018-12-06
the AH-VAPEX process. A reservoir injection mixture 22 is produced (see Figure
3) from the
process which is subsequently injected into the injection well 20 under the
near-azeotropic
conditions herein (see Figure 1). The use of superheat will result in some
degrees of superheat of
the final vapor mixture which can assist in providing excess heat to the
solvent extraction chamber
60 thereby improving the hydrocarbon recovery of the AH-VAPEX process. In an
embodiment,
wherein the near-azeotropic reservoir injection mixture is injected into the
subterranean reservoir
at 1 to 50 C of superheat, measured with respect to the saturation
temperature of the near-
azeotropic reservoir injection mixture at the subterranean reservoir operating
pressure. In
preferred embodiments, at least 75 wt%, at least 90 wt% at least 95 wt%, or at
least 99 wt% of the
near-azeotropic reservoir injection mixture consists of the recovered solvent
stream and the treated
water stream as obtained from the processes herein.
100781 The main processing facility 300 may additionally include a feed
pump 362 and/or
mixing unit 365 upstream of the vapor generation unit 360. Either the feed
pump 362 and/or
mixing unit 365 may be utilized to provide proper mixing of the stored treated
water stream 358
and the tailored reservoir solvent mixture 345 prior to entering the vapor
generation unit 360. In
preferred embodiments, the feed pump 362 is utilized to raise the unit
operation pressure to
sufficient enough pressure to transport the near-azeotropic/minimum boiling
point vapor mixture
(i.e., the reservoir injection mixture 22) to the injection well 20 wellheads
in order to facilitate
injection of the reservoir injection mixture 22 into the subterranean
reservoir 40. Although the
processes disclosed herein have been illustrated with a single main processing
facility and a single
subterranean well pair, a main processing facility may be built and dedicated
to each
injection/production well-pair, or to a group of injection/production wells,
or to all of the
injection/production well-pairs associated with a particular reservoir.
Detailed Embodiment 2
100791 Figure 4 depicts a schematic flow diagram of the main processing
facility 400 for an
embodiment of a process herein for managing the reservoir product stream 32,
producing product
streams, and producing a tailored reservoir solvent mixture 445 for reuse for
injection as and/or
co-injection with another stream as a component of the reservoir injection
mixture 22. In the
currently disclosed embodiment of this process, the reservoir product stream
32, or a portion
- 26 -
2747922
CA 3026716 2018-12-06
thereof, is sent to a primary separation unit 401. Here an optional production
mixture make-up
solvent stream 403a and/or an optional production mixture water stream 403b is
added to the
reservoir product stream 32 as will be further described as follows to form a
primary separation
unit feedstream 406. The primary separation unit feedstream 406 is optionally
sent through a
primary feedtream heater 407 and a primary feedtream controller 409. The
primary functions of
the primary feedtream heater 407 and a primary feedtream controller 409 will
be further described
as follows.
[0080] In this primary separation unit 401, a portion of the produced
liquid water is separated
from the reservoir product stream 32 to produce a primary water stream 402.
The primary water
stream 402 may also contain some amount of hydrocarbons and solids. In an
embodiment, the
primary separation unit 401 utilizes a gravity settling phenomena, for
instance, a device such as a
gravity settler. However, in other embodiments, the primary separation unit
401 can be comprised
of a single stage flash unit, a multiple-stage flash unit, a
distillation/stripping column and/or a
combination thereof. Here, the pressure and temperature of the primary
separation unit 401, as
well as the composition of the primary separation unit feedstream 406, is
controlled in such a
manner as to evolve a vapor phase and produce a primary gas vapor stream 404
wherein the
primary gas vapor stream 404 comprises a hydrocarbon solvent/water mixture at
near-azeotropic
conditions. The primary gas vapor stream 404 may also comprise solution gas
compounds, process
gaseous by-products, and other compounds other that the hydrocarbon
solvent/water compounds
that are produced at near azeotropic conditions.
[0081] The separation of near-azeotropic hydrocarbon solvent/water
mixture in the primary
separation unit 401 from the primary separation unit feedstream 406 is
achieved through a
volatility-based separation process utilizing the properties that: 1) the
water and solvent
compounds of the tailored primary separation unit feedstream 406 are more
volatile than the heavy
oil compounds in the reservoir product stream 32, and 2) the water and solvent
compounds tailored
primary separation unit feedstream 406 will tend to evaporate simultaneously
and to form an
azeotropie vapor mixture. Since an important goal of this process is to
compositionally tailor and
control the process such that a hydrocarbon solvent/water mixture at near-
azeotropic conditions
can be produced in and recovered from the primary separation unit 401, certain
equipment and
controls, although some which will not be required in all installations or at
all times during the
-27-
2747922
CA 3026716 2018-12-06
=
process, will generally be employed as on the front end of, and in, the
primary separation unit 401
as will be further described.
[0082] In most instances, the reservoir product stream 32 will not be
produced from the
subterranean reservoir 40 under process conditions, or with a physical
composition, to optimize
the processes as described herein. Here an optional primary make-up solvent
stream 403a and/or
an optional primary water stream 403b is added to the reservoir product stream
32 in order to
modify the solvent and water composition of the primary separation unit
feedstream 406 as
necessary to improve or optimize the processing in the primary separation unit
401. Here, the
primary make-up solvent stream 403a is added to bring the hydrocarbon solvent
concentration of
the primary separation unit feedstream 406 to proper levels for processing in
the primary
separation unit 401 to produce a primary gas vapor stream 404 comprising a
hydrocarbon
solvent/water mixture at near-azeotropic conditions. Likewise, the primary
water stream 403b is
added to bring the water concentration of primary separation unit feedstream
406 to proper levels
for processing in the primary separation unit 401 to produce a primary gas
vapor stream 404
comprising a hydrocarbon solvent/water mixture at near-azeotropic conditions.
It should be noted
here that the concentration of the hydrocarbon solvent and water in the
primary separation unit
feedstream 406 may be, but is likely not to be, the same relative
concentrations hydrocarbon
solvent/water mixture produced as the primary gas vapor stream 404. This due
to the fact that
some water in the primary separation unit feedstream 406 will be withdrawn
from the primary
separation unit 401 as a primary water stream 402 for reasons, such as removal
of entrained solids
and contaminants, as will be discussed further herein. Additionally, in
embodiments, some of the
hydrocarbon solvent in the primary separation unit feedstream 406 may be
withdrawn as part of a
heavy oil product stream 412 for reasons as discussed later herein.
[0083] As noted, in most instances, the reservoir product stream 32 will
not be produced under
process conditions to optimize the processes as described herein. To address
this, as shown in
Figure 4, the primary separation unit feedstream 406 is optionally sent
through a primary
feedstream heater 407 and a primary feedtream controller 409. The optional
primary feedtream
heater 407 is operated under conditions to provide the thermal energy for
vaporization and to heat
the primary separation unit feedstream 406 to the temperature in the primary
separation unit 401
necessary to produce the primary gas vapor stream 404 such that the
hydrocarbon solvent/water
-28-
2747922
CA 3026716 2018-12-06
mixture within the primary gas vapor stream 404 is produced at near-azeotropic
conditions. In a
similar manner, the optional primary feedtream controller 409 is operated
under conditions to
maintain the pressure in the primary separation unit 401 at a pressure
necessary to produce the
primary gas vapor stream 404 such that the hydrocarbon solvent/water mixture
within the primary
gas vapor stream 404 is produced at near-azeotropic conditions.
[0084] The thermal energy for the primary feedtream heater 407 may be
provided by any direct
or indirect heating method. These methods may include the use of fired heater,
a hot oil loop, by
heat exchange with other process streams (e.g., waste heat recovery streams),
or by direct steam
heating. Optionally, chemicals may be added to the tailored primary separation
unit feedstream
to 406 to prevent foaming, fouling, scaling, and/or other similar
operational phenomena in the
primary separation unit 401. In addition, special mechanical designs and
provisions may be
utilized in the primary separation unit 401 to prevent the aforementioned
operational phenomena.
[0085] The primary separation unit 401 also produces a heavy oil product
stream 412 which
contains the majority of the reduced-viscosity hydrocarbons 70 recovered from
the subterranean
reservoir 40, and are present in the reservoir product stream 32, and which
has been produced and
recovered from the AH-VAPEX processes described herein. In preferred
embodiments, the heavy
oil product stream 412 recovers at least 70%, at least 80%, at least 90%, at
least 95%, or at least
99% of the reduced-viscosity hydrocarbons 70 present in the portion of the
reservoir product
stream 32 that is sent to the primary separation unit 401. In preferred
embodiments, the heavy oil
product stream 412 recovers at least 70%, at least 80%, at least 90%, at least
95%, or at least 99%
of the solvent mixture present in the portion of the reservoir product stream
32 that is sent to the
primary separation unit 401. The heavy oil product stream 412 may also contain
some water and
solids. In embodiments, each the heavy oil product stream 412 and the primary
gas vapor stream
404 may be composed of a single stream each (as shown in simplified Figure 4)
or they may be
composed of a combination of multiple streams produced in the primary
separation unit 401 which
are grouped and/or combined to form the heavy oil product stream 412 and the
primary gas vapor
stream 404, respectively.
[0086] The heavy oil product stream 412 is produced from the solvent
separation unit 410
from which, in embodiments, most of the solvent boiling range compounds have
been removed.
The heavy oil product stream 412 may be of sufficient composition to meet
necessary pipeline
- 29 -
2747922
CA 3026716 2018-12-06
specifications in which case the heavy oil product stream 412 may be sent to a
pipeline as a pipeline
product stream 420. In embodiments herein, as noted, the primary separation
unit 401 may be
operated such that a sufficient amount of solvent, for example C4 to C12
hydrocarbons, remains in
the heavy oil product stream 412 to allow the heavy oil product stream to meet
pipeline
specifications or reduces the amount of a diluent 414 that needs to be added
to meet pipeline
specifications.
[0087] Alternatively, in embodiments herein, the heavy oil product stream
412 may be too
viscous or have to low an API gravity to meet specifications for pipeline
transportation. In these
instances, a diluent 414 may be added to the heavy oil product stream 412 to
produce the pipeline
to product stream 420. A diluent treating unit 415 may additionally be
utilized to further control the
amount of diluent added and provide proper mixing of the diluent 414 and heavy
oil product stream
412 to meet the specifications of the produced pipeline product stream 420.
Additionally, or
optionally, excess solids and water present in the heavy oil product stream
412 can be removed in
the diluent treating unit 415 to meet the pipeline specifications. The diluent
treating unit 415 may
utilize electrostatic mechanisms to separate excess solids and water from the
heavy oil product
stream. When a diluent treating unit 415 is utilized, the diluent 414 may be
added to the heavy oil
product stream 412 and/or optionally directly into the diluent treating unit
415. In embodiments,
the design and operation pressure and temperature of the primary separation
unit 401 produces the
heavy oil product stream 412 such that it contains a portion of the solvent
boiling range compounds
in order to meet the pipeline specification requirements.
[0088] Continuing with Figure 4, in the primary separation unit 401, the
majority of the solvent
boiling range compounds are separated from the heavy oil product stream 412.
In embodiments,
this temperature and pressure of the primary separation unit 401 is maintained
to vaporize at least
70%, at least 80%, at least 90%, at least 95%, or at least 99% of the solvent
boiling range
compounds present in the primary separation unit feedstream 406. The operating
pressure of the
primary separation unit 401 will be taken at the inlet to the flash unit or
distillation column
deployed within the primary separation unit 401.
[0089] As noted, the primary separation unit 401 also produces a primary
gas vapor stream
404 which comprises a hydrocarbon solvent/water mixture at near-azeotropic
conditions. The
.. primary gas vapor stream 404 may also comprise solution gas compounds,
process gaseous by-
- 30 -
2747922
CA 3026716 2018-12-06
products, and other compounds other than the hydrocarbon solvent/water
compounds. Here, at
least a portion of the primary gas vapor stream 404 that is produced from the
primary separation
unit 401 is sent to a gas separation unit 430 for further processing. Prior to
the gas separation unit
430, the primary gas vapor stream 404 is sent through a gas separation cooler
428, where the
stream is cooled so that at least portion of the solvent boiling range
compounds and the water in
the primary gas vapor stream 404 are condensed to a liquid.
[0090] In preferred embodiments, at least 80%, 90%, 95% or substantially
all of the solvent in
the primary gas vapor stream 404 is condensed and removed as part of the
recovered solvent/water
stream 433. In preferred embodiments, at least 80%, 90%, 95% or substantially
all of the water in
the primary gas vapor stream 404 is condensed and removed as part of the
recovered solvent/water
stream 433. In preferred embodiments, the solvent/water mixture in the
recovered solvent/water
stream 433 is at a near-azeotropic composition. The gas separation cooler 428
may be designed
and operated to provide thermal energy to other process streams of the main
processing facility
400.
[0091] In preferred embodiments, the gas separation unit 430 produces an
off gas stream 431
and the recovered solvent/water stream 433 as illustrated in Figure 4. The off
gas stream 431 will
generally be comprised of light hydrocarbon gases, such as methane and ethane,
but generally will
also include some non-hydrocarbon gases such as CO2 and H2S. Optionally,
special provisions
and designs may be utilized in the gas separation units to separate some of
the light hydrocarbon
gases from the entrained CO2 and/or H2S for proper treatment and/or disposal
prior to sending the
off gas stream 431 to a fuel gas system or for direct use in fired heating
equipment that may be
part of the main processing facility 400. Optionally, the off gas stream 431
may be added to a
make-up fuel gas stream 432 prior to use in the fired heating equipment.
[0092] The gas separation unit 430 is operated under conditions to
produce a recovered
solvent/water stream 433 that is generally be free of impurities and ready for
near-azeotropic vapor
generation. In embodiments, the gas separation unit 430 can be comprised of a
single stage flash
unit, a multiple-stage flash unit, a distillation/stripping column and/or a
combination thereof.
[0093] In the processes herein, an optional final make-up solvent stream
440a and/or an
optional final water stream 440b may be added to the recovered solvent/water
stream 433. The
optional final make-up solvent stream 440a and/or an optional final water
stream 440b, may be
-31 -
2747922
CA 3026716 2018-12-06
used to make a final compositional modification to the recovered solvent/water
stream 433 prior
to use of the mixture in the AH-VAPEX processes herein. However, since the
primary separation
unit 401 and the gas separation unit 430 will normally be operated under
conditions to produce the
recovered solvent/water stream 433 at a near-azeotropic mixture of hydrocarbon
solvent and water,
the final make-up solvent stream 440a and/or the final water stream 440b may
be added primarily
for the purpose of making up for volume loses of the solvent and/or water
utilized in the
subterranean reservoir during the AH-VAPEX heavy oil recovery processes and/or
volume losses
in the processes described herein for recover and preparation of the reservoir
injection mixture 22.
As such, the recovered solvent/water stream 433 may be furthered tailored in
composition and/or
volume for use in the AH-VAPEX process for use in the reservoir injection
mixture 22. The final
make-up solvent stream 440a and the final water stream 440b, may be provided
from the same
source as the primary make-up solvent stream 403a and/or the primary water
stream 403b. In
embodiments, the primary make-up solvent stream, the final make-up solvent
stream, or both may
be comprised of hydrocarbons that have been produced by a source separate from
the subterranean
IS reservoir. The primary make-up solvent stream, the final make-up solvent
stream, or both may be
comprised of a natural gas liquid, a natural gas condensate, a liquefied
petroleum gas, or a crude
oil refinery naphtha.
[0094] Figure 4 also shows an optional solvent/water storage system 442
which may be
utilized as a surge buffer and/or mixing step in the process. The final make-
up solvent stream 440a
and/or the final water stream 440b can be alternatively introduced to the
recovered solvent/water
stream 433 upstream and/or downstream of the optional solvent/water storage
system 442 if
utilized.
[0095] As shown in Figure 4, at least a portion of the primary water
stream 402 may be sent
to a water disposal treatment unit 450. In the water disposal treatment unit
450, the primary water
stream 402 is treated to allow for the proper disposal of the primary water
stream 402. The primary
water stream 402 will contain impurities from the primary separation unit
feedstream 406 such as
solids, salts and minerals. The dissolved minerals in the primary water stream
402 will not
vaporize in the primary separation unit 401, and will tend to stay in the
liquid portion of the water
produced from the primary separation unit 401. Hence, a relatively
concentrated liquid solution
of minerals in water will form in the primary separation unit 401. The design
of the primary
- 32 -
2747922
CA 3026716 2018-12-06
separation unit 401 will not allow the full vaporization of all of the water
in the primary separation
unit feedstream 406 and this excess water will be utilized in the liquid phase
as the carrier media
for the disposal of the impurities. In addition, a gravity settling vessel may
be used at the inlet of
the primary separation unit 401 to remove some of the suspended particles
which are heavy enough
to settle. The primary water stream 402 will undergo the extra processing in a
disposal water
treatment unit 450 to remove hydrocarbons and other contaminants necessary to
meet
environmental regulations and be sent to final disposal to water disposal
facilities 454.
[0096] A final tailored reservoir solvent/water mixture 445 is supplied
to a vapor generation
unit 460 to generate the near-azeotropic/minimum boiling point vapor mixture
for use in the AH-
VAPEX process. The vapor generation unit 460 may be comprised of heat
exchanger, a steam
heat exchanger, a hot oil heat exchanger, a fired heater, or any other
suitable vaporizer design. The
water and solvent of the final tailored reservoir solvent/water mixture 445 is
evaporated to the
corresponding dew-point temperature at the unit operation pressure. Excess
heat generated in the
vapor generation unit 460 may be utilized to superheat the stream. A reservoir
injection mixture
22 is produced (see Figure 4) from the process which is subsequently injected
into the injection
well 20 under the near-azeotropic conditions herein (see Figure 1). A
reservoir injection mixture
22 is produced is in the vapor phase and preferably additionally contains some
amount of
superheat. The use of a superheater (which may be part of the vapor generation
unit 460) will
result in some degrees of superheat of the final vapor mixture which can
assist in providing excess
heat to the solvent extraction chamber 60 thereby improving the hydrocarbon
recovery of the AH-
VAPEX process. In an embodiment, wherein the near-azeotropic reservoir
injection mixture is
injected into the subterranean reservoir at 1 to 50 C of superheat, measured
with respect to the
saturation temperature of the near-azeotropic reservoir injection mixture at
the subterranean
reservoir operating pressure.
[0097] The main processing facility 400 may additionally include a feed
pump 462 and/or
mixing unit 465 upstream of the vapor generation unit 460. Either the feed
pump 462 and/or
mixing unit 465 may be utilized to provide proper mixing the final tailored
reservoir solvent
mixture 465 prior to entering the vapor generation unit 460. In preferred
embodiments, the feed
pump 462 is utilized to raise the unit operation pressure to sufficient enough
pressure to transport
the near-azeotropic/minimum boiling point vapor mixture (i.e., the reservoir
injection mixture 22)
- 33 -
2747922
CA 3026716 2018-12-06
to the injection well 20 wellheads in order to facilitate injection of the
reservoir injection mixture
22 into the subterranean reservoir 40. Although the processes disclosed herein
have been
illustrated with a single main processing facility and a single subterranean
well pair, a main
processing facility may be built and dedicated to each injection/production
well-pair, or to a group
.. of injection/production wells, or to all of the injection/production well-
pairs associated with a
particular reservoir.
Detailed Embodiment 3
[0098] Figure 5 depicts a schematic flow diagram of the main processing
facility 500 for an
embodiment of a process herein for managing the reservoir product stream 32,
producing product
streams, and producing a tailored reservoir solvent mixture 545 for reuse for
injection as and/or
co-injection with another stream as a component of the reservoir injection
mixture 22. In the
currently disclosed embodiment of this process, the reservoir product stream
32, or a portion
thereof, is sent to a primary separation unit 501. In this primary separation
unit 501, a major
portion of the produced liquid water is separated from the reservoir product
stream 32 to produce
a primary water stream 502. The primary water stream 502 may also contain some
amount of
hydrocarbons and solids. Preferably, the primary separation unit 501 utilizes
a gravity settling
phenomena, for instance, a device such as a gravity settler. The pressure and
temperature of the
primary separation unit 501 may be chosen in such a way to evolve and isolate
a vapor phase,
mainly composed of solution gas compounds, process gaseous by-products, water
vapor and may
contain a portion of compounds from the solvent mixture of reservoir injection
mixture 22, to
produce a primary vapor stream 504. The primary separation unit 501 also
produces a third stream
which is designated as a primary hydrocarbon phase stream 505 which contains
the majority of
the reduced-viscosity hydrocarbons 70, as well as the majority of the solvent
mixture that is a
component of the reservoir injection mixture 22, that are present in the
reservoir product stream
32, and which has been produced and recovered from the AH-VAPEX processes
described herein.
In preferred embodiments, the primary hydrocarbon phase stream 505 recovers at
least 70%, at
least 80%, at least 90%, at least 95%, or at least 99% of the reduced-
viscosity hydrocarbons 70
present in the portion of the reservoir product stream 32 that is sent to the
primary separation unit
501. In preferred embodiments, the primary hydrocarbon phase stream 505
recovers at least 70%,
- 34 -
2747922
CA 3026716 2018-12-06
at least 80%, at least 90%, at least 95%, or at least 99% of the solvent
mixture present in the portion
of the reservoir product stream 32 that is sent to the primary separation unit
501. The primary
hydrocarbon phase stream 505 may also contain some water and solids.
[0099] In the currently disclosed processes, the primary hydrocarbon
phase stream 505 is sent
to a solvent separation unit 510. In the solvent separation unit 510, the
majority of the solvent
boiling range compounds are separated from the primary hydrocarbon phase
stream 505. In
embodiments, the solvent separation unit 510 can be comprised of a single
stage flash unit, a
multiple-stage flash unit, a distillation/stripping column and/or a
combination thereof. Depending
on the temperature and pressure of the primary hydrocarbon phase stream 505, a
primary
hydrocarbon phase heater 507 may be used to heat the stream to a certain
process temperature and
provide the required thermal energy to vaporize the solvent compounds. . In
embodiments, this
temperature on the hydrocarbon outlet of the primary hydrocarbon phase heater
507 is maintained
to vaporize at least 70%, at least 80%, at least 90%, at least 95%, or at
least 99% of the solvent
boiling range compounds present in the primary hydrocarbon phase stream 505 at
the operating
pressure of the solvent separation unit 510. The operating pressure of the
solvent separation unit
510 will be taken at the inlet to the flash unit or distillation column
deployed within the solvent
separation unit 510. The thermal energy for the primary hydrocarbon phase
heater 507 may be
provided by any direct or indirect heating method (for example: fired heater,
hot oil loop, by heat
exchange with other process streams, such as waste heat recovery, or by direct
steam heating).
[00100] In embodiments, a solvent separation unit controller 509, may be used
to control the
flow and/or pressure drop in the primary hydrocarbon phase stream 505. In
embodiments, the
solvent separation unit controller 509 is adjusted to maintain the primary
hydrocarbon phase
stream 505 upstream of the controller substantially in the liquid phase. In
embodiments, the
solvent separation unit controller 509 is further adjusted such as to flash
the solvent boiling range
compounds of the primary hydrocarbon phase stream 505 substantially to the
vapor phase entering
the solvent separation unit 510. Chemicals may be added to the reservoir
product stream 32 and/or
the primary hydrocarbon phase stream 505 to prevent foaming, fouling, scaling,
and/or other
similar operation phenomena in the associated process equipment. In addition,
special mechanical
designs may be utilized in the equipment associated with the primary
separation unit 501 and/or
the solvent separation unit 510 to prevent the aforementioned phenomena.
- 35 -
2747922
CA 3026716 2018-12-06
[00101] A heavy oil product stream 512 is produced from the solvent separation
unit 310 from
which, in embodiments, most of the solvent boiling range compounds have been
removed. The
heavy oil product stream 512 may be of sufficient composition to meet
necessary pipeline
specifications in which case the heavy oil product stream 512 may be sent to a
pipeline as a pipeline
product stream 520. In embodiments herein, the solvent separation unit 510 may
be operated such
that a sufficient amount of solvent, for example C4 to C12 hydrocarbons,
remains in the heavy oil
product stream 512 to allow the heavy oil product stream to meet pipeline
specifications or reduces
the amount of a diluent 514 that needs to be added to meet pipeline
specifications.
[00102] Alternatively, in embodiments herein, the heavy oil product stream 512
may be too
.. viscous or have to low an API gravity to meet specifications for pipeline
transportation. In these
instances, a diluent 514 may be added to the heavy oil product stream 512 to
produce the pipeline
product stream 520. A diluent treating unit 515 may additionally be utilized
to further control the
amount of diluent added and provide proper mixing of the diluent 514 and heavy
oil product stream
512 to meet the specifications of the produced pipeline product stream 520.
Additionally, or
optionally, excess solids and water present in the heavy oil product stream
512 can be removed in
the diluent treating unit 515 to meet the pipeline specifications. The diluent
treating unit 515 may
utilize electrostatic mechanisms to separate excess solids and water from the
heavy oil product
stream. When a diluent treating unit 515 is utilized, the diluent 514 may be
added to the heavy oil
product stream 512 and/or optionally directly into the diluent treating unit
515. In embodiments,
the design and operation pressure and temperature of the solvent separation
unit 510 produces the
heavy oil product stream 512 such that it contains a portion of the solvent
boiling range compounds
in order to meet the pipeline specification requirements.
[00103] The solvent separation unit 510 also produces a solvent vapor stream
525. In preferred
embodiments, this stream comprises solvent boiling range compounds recovered
from the
reservoir product stream 32 which are within the boiling point ranges of the
solvent mixture
utilized in the reservoir injection mixture 22. The solvent vapor stream 525
may also include other
gaseous compounds that were part of the reservoir product stream 32 and not
removed in the
primary vapor stream 504 of the primary separation unit 501.
[00104] In embodiments, each of the heavy oil product stream 512 and the
solvent vapor stream
525 may be composed of a single stream each (as shown in simplified Figure 5)
or they may be
- 36 -
2747922
CA 3026716 2018-12-06
=
composed of a combination of multiple streams produced in the solvent
separation unit 510 which
are grouped and/or combined to form the heavy oil product stream 512 and the
solvent vapor
stream 525, respectively.
[00105] As noted prior, some of the solvent boiling range compounds may be
recovered in the
primary vapor stream 504 from the primary separation unit 501. In this case,
in preferred
embodiments, at least a portion of the primary vapor stream 504 and at least a
portion of the solvent
vapor stream 525 can be combined into a primary gas separation feed stream 527
and sent to a
primary gas separation unit 570 for further processing. The further processing
may include use of
the primary gas separation feed stream 527 to treat the primary produced water
stream 502.
to Alternatively, only the solvent vapor stream 525, or a portion thereof,
is sent via a primary gas
separation feed stream 527 to the primary gas separation unit 570. In the
primary gas separation
unit 570, at least a portion of the thermal energy for water phase change from
liquid to vapor may
be provided by condensation of some of the solvent compounds in the vapor
phase from the gas
separation feed stream 527. Additional make-up water 552, if required, may be
added before or
after or during water treatment processes depending on its source. The amount
of make-up water
552, added to the process may adjusted in order to produce a primary gas
separation vapor stream
571 containing a solvent and water at near-azeotropic conditions. The primary
gas separation
vapor stream 571 may also be comprised of light hydrocarbon gases, such as
methane and ethane,
as well as some non-hydrocarbon gases such as CO2 and H2S.
[00106] Alternatively or additionally, the amount of make-up water 552, added
to the process
may adjusted in order to produce a primary gas separation vapor stream 571
wherein the quality
of the water in the primary gas separation vapor stream 571 is of sufficient
quality to meet boiler
feed-water specifications. The primary gas separation unit 570 also produces a
primary gas
separation liquid stream 572 which comprises condensed solvents and water. A
gas treating make-
up solvent stream 573 may be added to the primary gas separation liquid stream
572 to form a
secondary gas separation feed stream 574. In preferred embodiments, the
secondary gas separation
feed stream 574 is passed through a gas separation stage heater 575 which
vaporizes at least a
portion of the solvent and water in the secondary gas separation feed stream
574 before being set
to a secondary gas separation unit 580.
[00107] In the secondary gas separation unit 580, most of the solvent and some
of the water is
-37-
2747922
CA 3026716 2018-12-06
separated in a vapor phase to form a secondary gas separation vapor stream
581. In preferred
embodiments, the secondary gas separation vapor stream 581 comprises a solvent
and water
mixture at near-azeotropic conditions. In preferred embodiments, essentially
all of the secondary
gas separation unit 580 is vaporized and leaves the secondary gas separation
unit 580 as part of
the secondary gas separation vapor stream 581. In preferred embodiments, the
secondary gas
separation liquid stream 535 leaving the secondary gas separation unit 580 is
comprised of less
than 10 wt%, 5 wt% or 2 wt% hydrocarbons.
[00108] Continuing with Figure 5, the primary gas separation vapor stream 571
and the
secondary gas separation vapor stream 581 are combined to form a tertiary gas
separation feed
stream 582 and sent to a tertiary gas separation unit 585. Prior to the
tertiary gas separation unit
585 the tertiary gas separation feed stream 582 is passed through a gas
separation stage cooler 583.
While, for simplification purposes, the combined tertiary gas separation feed
stream 582 is
illustrated as passing through the gas separation stage cooler 583, in an
alternative a portion of the
tertiary gas separation feed stream 582 and/or a portion of any of the
separate streams making up
the tertiary gas separation feed stream 582 may be passed through a cooler.
Also, while one cooler
is shown for exemplary purposes, multiple separate coolers may be utilized in
the step and may be
associated with different component streams to the tertiary gas separation
unit 585.
[00109] In preferred embodiments, the tertiary gas separation feed stream 582
produces an off
gas stream 531 and the recovered solvent/water stream 533 as illustrated in
Figure 3. The off gas
stream 531 will generally be comprised of light hydrocarbon gases, such as
methane and ethane,
but generally will also include some non-hydrocarbon gases such as CO2 and
H2S. Optionally,
special provisions and designs may be utilized in the gas separation units to
separate some of the
light hydrocarbon gases from the entrained CO2 and/or H2S for proper treatment
and/or disposal
prior to sending the off gas stream 531 to a fuel gas system or for direct use
in fired heating
equipment that may be part of the main processing facility 500. Optionally,
the off gas stream 531
may be added directly to a make-up fuel gas stream 532 prior to use in the
fired heating equipment.
[00110] The tertiary gas separation unit 585 is operated under conditions to
produce a recovered
solvent/water stream 533 that is generally be free of impurities and ready for
near-azeotropic vapor
generation. In embodiments, the primary gas separation unit 570, the secondary
gas separation
unit 580, and/or the tertiary gas separation unit 585 can be comprised of a
single stage flash unit,
- 38 -
2747922
CA 3026716 2018-12-06
a multiple-stage flash unit, a distillation/stripping column and/or a
combination thereof
[00111] In the processes herein, an optional final make-up solvent stream 540a
and/or an
optional final make-up water stream 540b may be added to the recovered
solvent/water stream
533. The optional final make-up solvent stream 540a and/or an optional final
make-up water
stream 540b, may be used to make a final compositional modification to the
recovered
solvent/water stream 533 prior to use of the mixture in the AH-VAPEX processes
herein.
However, since the primary separation unit 501 and the gas separation units
570, 580, and 585 will
normally be operated under conditions to produce the recovered solvent/water
stream 533 at a
near-azeotropic mixture of hydrocarbon solvent and water, the final make-up
solvent stream 540a
and/or the final make-up water stream 540b may be added primarily for the
purpose of making up
for volume loses of the solvent and/or water utilized in the subterranean
reservoir during the AH-
VAPEX heavy oil recovery processes and/or volume losses in the processes
described herein for
recover and preparation of the reservoir injection mixture 22. As such, the
recovered solvent/water
stream 533 may be furthered tailored in composition and/or volume for use in
the AH-VAPEX
process for use in the reservoir injection mixture 22. In embodiments, the
final make-up solvent
stream may be comprised of hydrocarbons that have been produced by a source
separate from the
subterranean reservoir. The final make-up solvent stream may be comprised of a
natural gas liquid,
a natural gas condensate, a liquefied petroleum gas, or a crude oil refinery
naphtha.
[00112] Figure 5 also shows an optional solvent/water storage system 542 which
may be
utilized as a surge buffer and/or mixing step in the process. The final make-
up solvent stream 540a
and/or the final make-up water stream 540b can be alternatively introduced to
the recovered
solvent/water stream 533 upstream and/or downstream of the optional
solvent/water storage
system 542 if utilized.
[00113] As shown in Figure 5, at least a portion of the secondary gas
separation liquid stream
535 leaving may be sent to a water disposal treatment unit 550. In the water
disposal treatment
unit 550, the secondary gas separation liquid stream 535 is treated to allow
for the proper disposal
of the primary water stream 502. The secondary gas separation liquid stream
535 will contain
impurities from the reservoir product stream 32 such as solids, salts and
minerals. The dissolved
minerals in the secondary gas separation liquid stream 535 will not vaporize
in the secondary gas
separation unit 580, and will tend to stay in the liquid portion of the water
produced secondary gas
-39-
2747922
CA 3026716 2018-12-06
separation unit 580. Hence, a relatively concentrated liquid solution of
minerals in water will form
in secondary gas separation unit 580. The design of the secondary gas
separation unit 580 will not
allow the full vaporization of all of the water in the secondary gas
separation feed stream 574 and
this excess water will be utilized in the liquid phase as the carrier media
for the disposal of the
impurities. The secondary gas separation liquid stream 535 will undergo the
extra processing in a
disposal water treatment unit 550 to remove hydrocarbons and other
contaminants necessary to
meet environmental regulations and be sent to final disposal to water disposal
facilities 554.
[00114] A final tailored reservoir solvent/water mixture 545 is supplied to a
vapor generation
unit 560 to generate the near-azeotropic/minimum boiling point vapor mixture
for use in the AH-
process. The vapor generation unit 560 may be comprised of heat exchanger, a
steam
heat exchanger, a hot oil heat exchanger, a fired heater, or any other
suitable vaporizer design. The
water and solvent of the final tailored reservoir solvent/water mixture 545 is
evaporated to the
corresponding dew-point temperature at the unit operation pressure. Excess
heat generated in the
vapor generation unit 560 may be utilized to superheat the stream. A reservoir
injection mixture
22 is produced (see Figure 5) from the process which is subsequently injected
into the injection
well 20 under the near-azeotropic conditions herein (see Figure 1). A
reservoir injection mixture
22 is produced is in the vapor phase and preferably additionally contains some
amount of
superheat. The use of a superheater (which may be part of the vapor generation
unit 560) will
result in some degrees of superheat of the final vapor mixture which can
assist in providing excess
heat to the solvent extraction chamber 60 thereby improving the hydrocarbon
recovery of the AH-
VAPEX process. In an embodiment, wherein the near-azeotropic reservoir
injection mixture is
injected into the subterranean reservoir at 1 to 50 C of superheat, measured
with respect to the
saturation temperature of the near-azeotropic reservoir injection mixture at
the subterranean
reservoir operating pressure.
[00115] The main processing facility 500 may additionally include a feed pump
562 and/or
mixing unit 565 upstream of the vapor generation unit 560. Either the feed
pump 562 and/or
mixing unit 565 may be utilized to provide proper mixing the final tailored
reservoir solvent
mixture 545 prior to entering the vapor generation unit 560. In preferred
embodiments, the feed
pump 562 is utilized to raise the unit operation pressure to sufficient enough
pressure to transport
the near-azeotropic/minimum boiling point vapor mixture (i.e., the reservoir
injection mixture 22)
- 40 -
2747922
CA 3026716 2018-12-06
to the injection well 20 wellheads in order to facilitate injection of the
reservoir injection mixture
22 into the subterranean reservoir 40. Although the processes disclosed herein
have been
illustrated with a single main processing facility and a single subterranean
well pair, a main
processing facility may be built and dedicated to each injection/production
well-pair, or to a group
of injection/production wells, or to all of the injection/production well-
pairs associated with a
particular reservoir.
[00116] In the present disclosure, several examples have been discussed and/or
presented in the
context of flow diagrams, or flow charts, in which the methods are shown and
described as a series
of blocks, or steps. Unless specifically set forth in the accompanying
description, the order of the
blocks may vary from the illustrated order in the flow diagram, including with
two or more of the
blocks (or steps) occurring in a different order and/or concurrently.
[00117] In the event that any patents, patent applications, or other
references are referenced
herein and (1) define a term in a manner that is inconsistent with and/or (2)
are otherwise
inconsistent with, either a portion of the present disclosure or any of the
other references referenced
herein, the portion of the present disclosure shall control.
Industrial Applicability
[00118] The systems and methods disclosed in the present disclosure are
applicable to the oil
and gas industry.
[00119] It is believed that the following claims particularly point out
certain combinations and
subcombinations that are novel and non-obvious. Other combinations and
subcombinations of
features, functions, elements and/or properties may be claimed through
amendment of the present
claims or presentation of new claims in this or a related application. Such
amended or new claims,
whether different, broader, narrower, or equal in scope to the original
claims, are also regarded as
included within the subject matter of the present disclosure.
- 41 -
CA 3026716 2019-09-26