Note: Descriptions are shown in the official language in which they were submitted.
CA 03030609 2019-01-11
WO 2018/011532
PCT/GB2017/000103
- 1 -
A CHLORINE DIOXIDE SOLUTION
GENERATING APPARATUS
The present invention relates to a chlorine dioxide solution generating
apparatus for use primarily in the supply of the solution for water treatment
and, in particular, to such an apparatus for use on site in the disinfection
of
water supplies.
Chlorine dioxide is used in water treatment as a disinfectant primarily
for eliminating water-borne pathogens such as Legionella bacteria and for
preventing the development of biofilms in water storage tanks. It is therefore
widely used in industrial applications for process water, for example as
washing water in the food industry, and for treating water supplies to large
buildings such as office blocks, hospitals and hotels where a water supply is
often provided via large header tanks and where air conditioning systems
have cooling towers, both of which are known breeding grounds for harmful
pathogens.
One of the most important qualities of chlorine dioxide is its high
water solubility, especially in cold water, and it is effective even at low
concentrations. It does not hydrolyse when it enters water and remains as a
dissolved gas in solution. However, chlorine dioxide is a compound that can
decompose extremely violently when separated from diluting substances. As
a result, preparation methods that involve the production of solutions of it
.. without going through a gas-phase stage are often preferred. Such methods
are generally used for its generation for water treatment as the solution
produced can then be dosed directly into a water supply. However, it is
essential that the chlorine dioxide solutions are handled in a safe manner to
retain the chlorine dioxide in solution as should the compound come out of
solution it can explosively decompose into chlorine and oxygen. It is for this
reason that chlorine dioxide solutions are produced in small batches on site
because such solutions cannot be stored for lengthy periods as they are
relatively unstable and deteriorate quickly. This not only adds to the risk of
CA 03030609 2019-01-11
WO 2018/011532
PCT/GB2017/000103
- 2 -
explosions occurring but also means that the dosing solution rapidly loses its
disinfecting capability.
Conventionally, a chlorine dioxide generating apparatus comprises a
reaction chamber in which either an aqueous sodium chlorite solution or an
aqueous sodium chlorate solution is mixed with a dilute acid such as
hydrochloric acid to produce an aqueous salt and chlorine dioxide solution.
Usually, for water treatment an aqueous sodium chlorite solution is mixed
with a dilute hydrochloric acid solution in the reaction chamber. The
concentration of the chlorine dioxide solution produced is typically between
1% and 5% by volume, as required, and is then further diluted into a motive
water stream which is added to the process water or water supply on site.
The acidic and sodium chlorite solutions are pumped into the reaction
chamber using calibrated dosing pumps. Usually it takes around 15 minutes
to produce a chlorine dioxide solution of the required concentration. This
chlorine dioxide solution is then either dosed directly from this tank into
the
process water or water supply or is pumped into a solution storage tank.
Hence, the generating apparatus may operate on a batch basis wherein the
level of the chlorine dioxide solution in the tank is controlled, a
predetermined low level turning on the generation process at a fixed rate.
The tank then fills up and stops at a predetermined high level. Metering
pumps dose the chlorine dioxide solution from the storage tank into the
water to be treated. Alternatively, the generating apparatus may operate on a
continuous basis with the chlorine dioxide solution produced in the reaction
chamber being injected directly into the process water or water supply at a
controlled rate, which is matched by the rate of production in the reaction
chamber. The dosing amounts required typically depend upon local
authority regulations and the application.
One of the main disadvantages of such conventional systems is that
the reaction chamber operates under pressure and is usually designed to
withstand pressures up to 15 bar. Potentially, this can cause several
problems. First, if the apparatus fails, leakage of dangerous chemicals can
CA 03030609 2019-01-11
WO 2018/011532 PCT/GB2017/000103
- 3 -
occur. It also means that all of the connections between the various parts of
the apparatus must be provided with adequate seals, which are themselves
open to failure. Second, should the dosing pumps malfunction, which they
are prone to do with age, the dosing chemicals may be overdosed to the
reaction chamber, which can result in a dangerous build-up of chlorine
dioxide gas in the chamber or in an incorrect dosage of the water to be
treated, in particular resulting in a surfeit of the dosing acid being added
to
the water.
An object of the present invention is to provide a chlorine dioxide
solution generating apparatus that overcome or substantially mitigates the
aforementioned problems.
According to the present invention there is provided chlorine dioxide
solution generating apparatus comprising
a reaction chamber that is supplied with metered quantities of an
acidic solution and a chlorite or chlorate solution from separate sources
whereby a reservoir of a chlorine dioxide solution is generated and retained
within the reaction chamber;
a conduit along which a pressurized flow of a motive fluid is fed and
with which the interior of the reaction chamber communicates via a dip tube;
a venturi located in the conduit adjacent an outlet from the dip tube
whereby suction is applied to the interior of the reaction chamber by the
pressurized flow of motive fluid to draw chlorine dioxide solution from the
reservoir whereby it is entrained into the flow of motive fluid in the
conduit;
and
a controller adapted to control the flow of motive fluid along the
conduit and the metering of the supply of said quantities of the acidic
solution and the chlorite or chlorate solution to said reaction chamber.
Preferably, the metered quantities of acid and chlorite or chlorate
solutions are supplied to the reaction chamber via respective first and second
supply pipes that each incorporate a volumetric flow meter and a valve, the
CA 03030609 2019-01-11
WO 2018/011532 PCT/GB2017/000103
- 4 -
opening and closing of the valve being controlled by the controller dependent
on the volume of solution that flows through and is measured by the
volumetric flow meter.
Preferably also, the conduit is fed with the motive fluid from a
pressurized supply of liquid to be disinfected by the chlorine dioxide
solution.
Preferably also, the pressurized flow of the motive fluid is metered
and controlled by a third volumetric flow meter and a third valve the opening
and closing of which is controlled by the controller.
Other preferred but non-essential features of the various aspects of
the present invention are described in the dependent claims appended
hereto.
The present invention will now be described by way of example with
reference to the accompanying drawings, in which:-
Fig. 1 is a diagram showing the general arrangement of a chlorine
dioxide solution generation apparatus according to the present invention;
Fig. 2 is a perspective schematic view of a set-up within a reaction
chamber forming part of the apparatus shown in Fig. 1; and
Fig. 3 is a view similar to Fig, 2 but of a modified set up of reaction
chamber for forming part of the apparatus shown in Fig. 1.
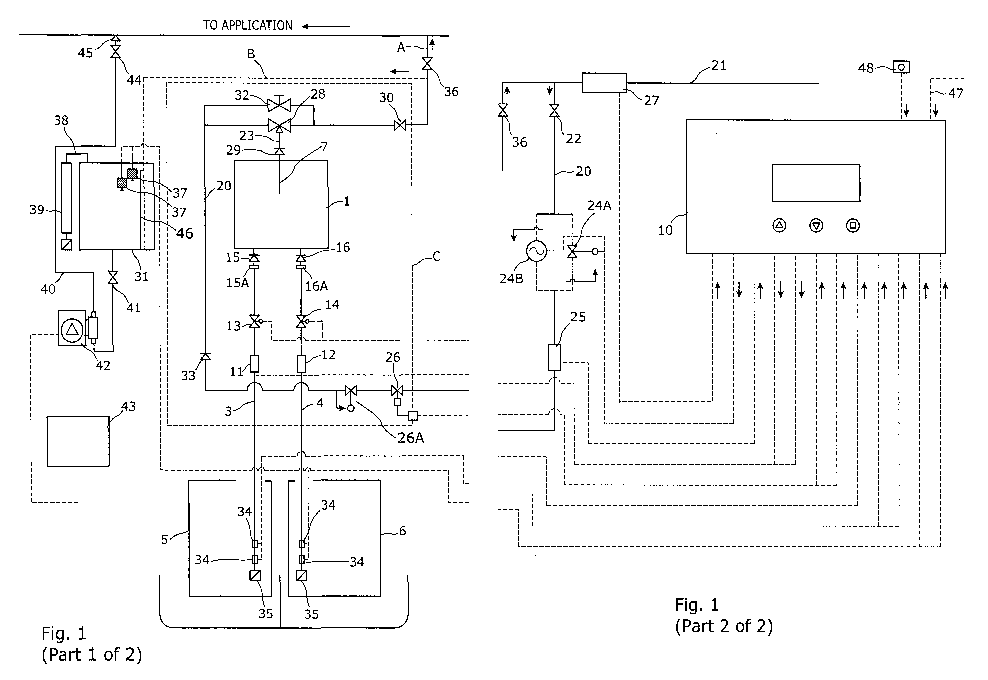
With reference to the drawings, a chlorine dioxide solution generation
apparatus comprises a reaction chamber 1 in which a reservoir 2 of a
chlorine dioxide solution is formed by a chemical reaction between metered
quantities of an acidic solution and a chlorite or chlorate solution that are
supplied to the reaction chamber 1 via respective first and second supply
CA 03030609 2019-01-11
WO 2018/011532 PCT/GB2017/000103
- 5 -
pipes 3 and 4 from tanks 5 and 6. As is described below, these solutions are
drawn into the reaction chamber 1 by negative pressure in the chamber 1 that
is created when quantities of the chlorine dioxide solution from the reservoir
2 are sucked out through a dip tube 7 which is inserted into the chamber 1,
its inlet 8 being located at the upper level 9 of the reservoir 2. The
quantities
of the solutions supplied to the chamber 1 are controlled by an electronic,
preferably programmable controller 10 that is operationally linked to first
and second volumetric flow meters ii and 12 and first and second valves 13
and 14, typically solenoid valves, which are respectively located in the first
and second supply pipes 3 and 4. Downstream of the valves 13 and 14 in the
supply pipes 3 and 4 are first and second non-return valves 15 and 16
respectively that may include restrictors 15A and 16A, if necessary,
dependent on the flow conditions in the pipes 3 and 4.
A first set-up within the reaction chamber 1 is shown in Fig. 2. Here,
the outlets from the supply pipes 3, 4 are merged by means of a mixing tee 17
that is connected to a blending column 18 of inverted U-shape. The mixing
tee 17 and the blending column 18 ensure that the solutions from the tanks 5,
6 are blended and well mixed prior to egressing from the column 18 via an
outlet 19 that is located close to the base of the reaction chamber 1 and
below
the lower level of the dip tube 7. Usually, it takes around 15 minutes to
achieve the maximum production of chlorine dioxide from a mixture of an
aqueous sodium chlorite solution and a dilute hydrochloric acid solution.
Hence, the requirement for a sufficient reservoir 2 in the reaction chamber 1
to ensure a constant supply of a suitable strength chlorine dioxide solution
and also good mixing of the reacting solutions as they flow into the chamber
1. For reasons that are explained below, the level of the inlet 8 into the dip
tube 7 is always located at the upper level 9 of the reservoir 2 and therefore
the height of the inlet 8 above the base of the reaction chamber 1 determines
the size of the reservoir 2, that is the quantity of solution retained within
the
reaction chamber 1. This quantity should be an appropriate quantity
dependent on the rate at which it will be used for disinfection purposes,
which in turn is dependent on the application, and the time taken for the
CA 03030609 2019-01-11
WO 2018/011532 PCT/GB2017/000103
- 6 -
solutions from the tanks 5 and 6 to react fully. As the reaction time is
typically of the order of 15 minutes, it is necessary for the dwell time of
the
solutions in the reaction chamber 1 to be this long. Using this data and
dependent on the application the optimum size of the reservoir 2 can be
predetermined by a person skilled in the art. In turn, this governs the
position of the inlet 8 of the dip tube 7 in the reaction chamber 1.
An advantage of this set-up is that the size of the reaction chamber 1
is not dependent on the required size of the reservoir 2 provided that it can
be accommodated in the chamber 1. Instead, the level of the inlet 8 into the
dip tube 7 is the critical factor that determines the size of the reservoir 2
and
this level can be varied to suit the application. In some embodiments, the
dip tube 7 is fitted to the reaction chamber 1 in such a way that the level of
its
inlet 8 can be adjusted on site as required and only one size of reaction
chamber 1 needs to be supplied as it can be used for a wide range of
applications where the rate of production of chlorine dioxide solution may
vary widely.
However, such a set-up has some disadvantages, in particular the
requirement to create negative pressure within the reaction chamber 1 when
air is present above the upper level of the reservoir. Also, leakage from the
reservoir 1 is apt to occur eventually around inlets for the supply pipes 3, 4
that pass through the bottom of the reaction chamber 1. Hence, an
alternative set-up for the chamber 1 is shown in Fig. 3. Here, the supply
pipes 3, 4 enter the top of the reaction chamber 1 and terminate just above
the base of the chamber 1 in a lower region that in use will contain an inert
aggregate, such as ceramic beads, up to a predetermined level marked X.
This inert aggregate facilitates blending of the two reagents. Preferably, the
ends of the supply pipes 3, 4 are chamfered to prevent their hitting the
bottom of the chamber 1 and becoming blocked. In this arrangement the size
of the reaction chamber 1 is dependent on the required size of the reservoir 2
and is factory set as it is a requirement for the chamber 1 to be virtually
CA 03030609 2019-01-11
WO 2018/011532
PCT/GB2017/000103
- 7 -
always full of the chlorine dioxide solution. Hence, the inlet 8 of the dip
tube
7i5 very close to the top of the reaction chamber 1.
In both set-ups chlorine dioxide solution is drawn from the reservoir 1
through the dip tube 7, which is connected to a conduit 20 that is fed with a
pressurized flow of a motive fluid as an offshoot from a pressurized supply
pipe 21, which may be a water mains supply or similar pressurized supply of
liquid to be disinfected by the chlorine dioxide solution. An isolating valve
22 is provided in the conduit 20 to enable the apparatus to be isolated from
the mains supply pipe 21 if necessary. Between the valve 22 and an outlet
pipe 23 from the dip tube 7 is a pressure regulating valve 24A, a third
volumetric flow meter 25, a valve 26, which is typically a solenoid valve, and
a pressure regulating valve 26A that provides stable flow characteristics
along the conduit 20. The valves 24A, 26 and 26A and the flow meter 25 are
all operationally linked to the controller 10. In addition, a fourth
volumetric
flow meter 27 is located in the mains supply pipe 21 upstream of the conduit
and is operationally connected to the controller 10.
In some embodiments where the pressure in the mains supply pipe 21
20 is low,
the pressure regulating valve 24A may be replaced by a circulating
pump 24B, the operation of which is also controlled by the controller 10.
Both the valve 24A and the pump 24B are shown in parallel in Fig. 1 but in
practice only one or the other of them would be used.
A venturi 28 is located in the conduit 20 adjacent the outlet from then
outlet pipe 23 from the dip tube 7. The motive flow of pressurized liquid
through the venturi 28 over the end of the outlet pipe 23 applies suction to
the interior of the dip tube 7 and therefore to the interior of the reaction
chamber 1. This draws chlorine dioxide solution from the reservoir 2,
through a non-return valve 29 and into the venturi 28 where it is entrained
into the flow of motive fluid in the conduit 20 and conducted via a second
isolating valve 30 either back to the mains supply pipe 21 through a pipe A or
to a batch storage tank 31 along a pipe B dependent on the application. The
CA 03030609 2019-01-11
WO 2018/011532 PCT/GB2017/000103
- 8 -
pipe B terminates inside the batch storage tank 31 at a low level to ensure
that there is always a liquid/gas trap between the batch storage tank 31 and
the chamber 1 to retain chlorine dioxide gas within the chamber 1. The
prevention of the escape of chlorine dioxide gas to the batch storage tank 31
and to atmosphere improves the efficiency of the reaction process by
retaining the gas in the chamber 1 so that it can be homogenised in the
venturi 28 during any subsequent batch sequences.
Optionally, the operation of the venturi 28 may be controlled by the
setting of by-pass valve 32 that is located in parallel with the venturi 28 in
the conduit 20. In addition, immediately upstream of the venturi 28 in the
conduit 20 is a non-return valve 33.
The levels of the solutions in the supply tanks 5 and 6 are monitored
by float switches 34 which are linked to the controller 10. In addition, the
ends of the supply pipes 3 and 4 within the tanks 5 and 6 are connected to
non-return foot valves 35, the inlets of which are covered by fine mesh to
prevent contaminating particles of other debris from being drawn into the
apparatus from the tanks 5, 6.
The non-return valves 15, 16 and 29 control the flow of solutions into
and out of the reaction chamber 1 and it is important that they operate
efficiently. For this reason, these valves 15, 16, 29 are preferably sprung
non-
return valves with titanium springs being used so that their operation is
unaffected by the corrosive and oxidative nature of the solutions passing
through them.
In those embodiments wherein the chlorine dioxide solution from the
reaction chamber 1 is injected directly into the mains water supply pipe 21
along pipe A, that is the apparatus will operate in a "continuous mode", a
further isolating valve 36 may be provided downstream of the valve 30 so
that a unit C, comprising the components within the rectangle shown in
dashed lines in Fig. 1, may be completely isolated from the mains water
CA 03030609 2019-01-11
WO 2018/011532 PCT/GB2017/000103
- 9 -
supply. In other embodiments wherein the chlorine dioxide solution from
the reaction chamber 1 is stored in a batch storage tank 31, that is the
apparatus will operate in a "batch mode", one or more high and low level
sensors 37 may be provided in the tank 30 and linked to the controller 10.
The sensors 37 may be used by the controller 10 to start and stop operation
of the apparatus to ensure that there is always an adequate supply of chlorine
dioxide solution in the tank 31.
The unit C along with the controller 10 may be supplied separately
from the other components of the apparatus described above as it is suitable
for retro-fitment to an existing water supply installation.
In the embodiments that use a batch storage tank 31, it will be
appreciated that the storage tank 30 may retain the chlorine dioxide solution
for some time and consequently it is possible for the chlorine dioxide start
to
come out of solution and create a pressure build-up within the tank 31. This
would be a dangerous, potentially explosive hazard. To prevent this from
happening, the tank 31 is maintained at atmospheric pressure via vent 38 in
which is located a scrubber 39 that removes the chlorine dioxide from the
gas passing through it.
The chlorine dioxide solution in the tank 31 is dosed into mains
supply pipe 21 through a pipe 40 via an isolating valve 41 and dosing pump
42, the operation of which is controlled by a pump controller 43.
Alternatively, the dosing pump 42 could be connected to the controller 10
and the separate pump controller 43 dispensed with. A further isolating
valve 44 and non-return valve 45 are preferably also included in the pipe 40
downstream of the dosing pump 42. Also a weir 46 may be provided in the
tank 31, isolating the inlet from pipe B from the bulk of the chlorine dioxide
solution stored in the tank 31 from a supply is drawn via the pipe 40.
The controller 10 preferably comprises a dedicated, programmable
device that may include a remote control option 47, for example via the
CA 03030609 2019-01-11
WO 2018/011532 PCT/GB2017/000103
- 10 -
Internet or Wi-Fi. For safety, the controller lo also incorporates an
emergency stop button 48 that is set up to cause the immediate cessation of
chlorine dioxide generation by the apparatus and the closure of the valves
13, 14 and the valve 24A or pump 24B.
In use, all of the isolating valves 22, 30, 36, 41 and 44 are open and
the controller 10 is powered up. After running through a series of checks to
ensure that alarm conditions can be safely monitored the controller 10
commences operation of the apparatus. First, in the case that the apparatus
is newly commissioned the reaction chamber 1 will be empty so that the
reservoir 2 must first be created by filling the reaction chamber 1 with the
required volume of chlorine dioxide solution. This is a known predetermined
quantity, as indicated above, that has already been catered for by the
position of the dip tube 7 in the reaction chamber 1. Hence, the controller 10
opens the motive fluid flow valve 26 and the valves 13 and 14. The valve 26 is
opened for a predetermined volume as measured by the third flow sensor 25
prior to the valve 13 and in turn the valve 14 being opened sequentially, to
establish and maintain a controlled vacuum in the outlet pipe 23. The valve
26 is retained open sufficient for the predetermined volume as measured by
the flow sensor 25 to flow through after the valves 13 and 14 close, which is
governed by the volume passing through the flow sensors 11 & 12. This is in
order to deliver remaining water required to maintain a correct ratio of water
and chlorine dioxide solution entering the batch tank 31 and also to act as a
water purge to remove any remaining chlorine dioxide solution from the
venturi 28 and send it to the batch tank 31.
By controlling the total batch water volume using the flow sensor 25
in relation to the volume of chemical reagents passing through the flow
sensors ii and 12, the controller 10 can be programmed to produce a
predetermined strength of chlorine dioxide solution that is delivered to the
batch tank 31 by controlling operation of the regulating valves 11, 12 and 26.
CA 03030609 2019-01-11
WO 2018/011532 PCT/GB2017/000103
- 11 -
The motive flow through the venturi 28 sucks air out of the reaction
chamber 1, which in turn sucks in the solutions from the tanks 5 and 6. The
quantities of each solution from the tanks 5 and 6 is monitored by the
volumetric flow meters ii and 12 and when each of them has passed the
required volume of solution therethrough, the controller 10 closes the
relevant valve 13, 14 to ensure that the correct quantity of that solution has
entered the reaction chamber 1. Similarly, when the required volume of
motive fluid has passed through the volumetric flow meter 25, the valve 26 is
also closed. The non-return valves 15 and 16 ensure that the solutions are
retained within the reaction chamber 1 and the non-return valve 33 ensures
that the motive fluid is retained in the conduit 20.
The efficiency of the venturi 28 and its repeatable performance is
governed by its pressurized water stream 20 operating at a predetermined
pressure set by the pressure regulating valve 26A and the inherent back
pressure downstream that is governed by the height of the weir 46 in the
batch tank 31.
The motive fluid which has been used to draw the initial quantity of
the solutions into the reaction chamber 1 can either be allowed to flow
directly back into the mains flow in pipe 21 via the valves 30 and 36 or to
flow into the batch storage tank 31 where it be immediately pumped back
into the mains flows in the pipe 21 by the dosing pump 42 in order not to
dilute the dosing solution which will subsequently be stored in the tank 31.
Once the reaction chamber 1 contains the requisite quantities of the
solutions from the tanks 5, 6 then the controller 10 will wait for a reaction
time of around 15 minutes for generation of the chlorine dioxide solution in
the chamber 1 before initiating a steady state operation of the apparatus. If
the apparatus is operating in a batch mode to produce a batch of chlorine
dioxide solution in the batch storage tank 31 via pipe B the following
sequence is used.
a. The motive fluid flow valve 26 is opened.
CA 03030609 2019-01-11
WO 2018/011532 PCT/GB2017/000103
- 12 -
b. The acid and chlorite/chlorate valves 13 and 14 are opened
sequentially.
c. Once the volumetric flow meters 11, 12 have signalled to the
controller that predetermined volumes of the acid solution and the
chlorite or chlorate solution have passed through them the
controller closes the valves 13,14,
e. When the volumetric flow meter 25 has signalled to the controller
that a correct predetermined volume of motive fluid has passed
through it, the controller closes the valve 26.
d. The controller io waits for a predetermined cycle time, typically
between 1 to 2 minutes, and then repeats the cycle from point a.
above.
This sequence of operation produces a concentrated supply of
chlorine dioxide solution in the batch storage tank 31, which is subsequently
dosed into the mains water supply 21 by the dosing pump 42 under the
control of the pump controller 43. The level sensors 37 in the tank 31 allow
the controller to stop the aforementioned sequence when the level of solution
in the tank 31 is high and to commence it again once the level drops below a
predetermined lower level.
It should be appreciated that once the reservoir 2 has been created in
the reaction chamber 1, its upper level 9 remains substantially constant
because the same quantity of liquid leaves the reaction chamber 1 as is
sucked into it by the action of the venturi 28. Should the level of the
solutions in the tanks 5, 6 fall to a low level, this is also signalled to the
controller 10 by the float switches 34 and the controller 10 will also act to
close the valves 13 and 15 to cease the aforementioned sequence until the
tank 5, 6 is replenished or replaced. Also, should either or both of the
volumetric flow sensors ii and 12 detect an abnormal disturbance, for
example if air rather than liquid is passing through suggesting that there may
be an obstruction in pipes 3, 4 or that the tanks 5, 6 are empty, for more
than a predetermined time, typically a few seconds, then the controller 10
CA 03030609 2019-01-11
WO 2018/011532 PCT/GB2017/000103
- 13 -
will act to close the valves 13, 14 and 26 and to stop operation of the
apparatus.
On the other hand, if the apparatus is operating in a continuous mode
to inject the chlorine dioxide solution from the reaction chamber 1 directly
into the mains water supply pipe 21 via pipe A, then the following sequence
may be followed.
a. The motive fluid flow valve 26 is opened.
b. The acid and chlorite/chlorate valves 13 and 14 are opened.
c. Once the volumetric flow meters 11, 12 have signalled to the
controller that predetermined volumes of the acid solution and the
chlorite or chlorate solution have passed through them the
controller closes the valves 13, 14,
d. When the volumetric flow meter 25 has signalled to the controller
that a correct predetermined volume of motive fluid has passed
through it, the controller closes the valve 26.
e. The controller 10 waits for a predetermined cycle time, and then
repeats the cycle from point b. above.
In this mode, typically the volume of the reservoir 2 will be larger and
the predetermined cycle time much shorter than in the batch mode. The
motive fluid flow along the conduit 30 will be continuous so that it is
constantly entraining chlorine dioxide solution from the reaction chamber 1.
However, the quantity of liquid sucked out of the chamber 1 will be matched
by the quantities of liquid flowing in through the pipes 3, 4 as soon as the
valves 13, 14 open owing to the negative pressure build up within the
chamber 1. However, it is necessary to program the controller 10 such that
the volumes of liquid passing through the volumetric flow meters 11, 12 can
match the quantity being drawn off through the dip tube 7 so that the upper
level 9 of the reservoir remains substantially constant.
CA 03030609 2019-01-11
WO 2018/011532 PCT/GB2017/000103
- 14 -
The volumetric flow meter 25 is an important part of the apparatus as
it controls the volume of motive fluid passing along conduit 20 and therefore
the dilution of the solution being produced by the apparatus. If for any
reason the flow of motive fluid is interrupted or for any reason the correct
volume of motive fluid passing through the flow meter 25 in a given time
cannot be maintained or is too high, then the controller 10 will act to close
the valves 13, 14 and 26, in order to stop operation of the apparatus. The
controller 10 will also signal an alarm so that the problem can be
investigated. The volumetric flow meter 25 therefore acts as a "watchdog",
monitoring that the apparatus is operating correctly.
The apparatus according to the invention has several advantages over
conventional apparatus, as follows.
1. The quantities of acid solution and the chlorite or chlorate solution
supplied to the reaction chamber 1 are measured by the volumetric
flow meters ii and 12, which accurately measure the volume of
liquid passing through them, rather than via dosing pumps. This is
a significantly more precise way of measuring small quantities of
liquid as dosing pumps tend to become less accurate over time as
their constituent parts wear.
2. Unlike conventional apparatus wherein the reaction chamber 1
operates under pressure, in the present invention the reaction
chamber 1 is open to atmosphere or subject to negative pressure.
This means that the chamber 1 is less likely to leak and if a seal
fails, the chlorine dioxide solution is less likely to be expelled from
the chamber 1.
3. As the reaction chamber 1 is not subject to high pressures it can be
made of a durable plastics material, such as polyvinylidene
fluoride, or polyvinylidene difluoride (PVDF), as can much of the
rest of the apparatus. This makes the apparatus less expensive to
CA 03030609 2019-01-11
WO 2018/011532 PCT/GB2017/000103
- 15 -
produce and increases the life of the apparatus, which otherwise
tends to suffer from degradation by the acidic and oxidative
solutions used.
4. In order for the apparatus to produce the chlorine dioxide solution
in the reaction chamber 1 there has to be a pressurized flow of
motive fluid in the conduit 20. Hence, should the flow of fluid in
the mains supply pipe 21 be interrupted for any reason, such as a
burst pipe, then the production of chlorine dioxide solution in the
reaction chamber 1 will cease. This is safer than conventional
arrangements wherein the production of the chlorine dioxide
solution is unaffected by problems in the mains water supply,
which can lead to an unacceptable overdosing of the supply when
reconnected.
5. After initial set-up, the reaction chamber 1 always contains the
correct solution and cannot be contaminated by an over-supply of
either the acid solution or the chlorite or chlorate solution.