Note: Descriptions are shown in the official language in which they were submitted.
COMPOSITIONS AND METHODS RELATED TO THE PRODUCTION OF
ACRYLONITRILE
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This Application claims the benefit of U.S. Application No. 15/245,835,
filed
on August 24, 2016.
FIELD OF THE INVENTIONS
[0003] The compositions, articles, and methods disclosed herein relates to the
production of acrylonitrile and other useful chemicals.
BACKGROUND
[0004] The US Department of Energy and the industrial sector anticipate an 11-
18%
annual increase in the market for carbon fiber, specifically driven by
motivation to
reduce weight for vehicles (Global market opportunities for carbon fiber:
Carbon fiber
world conference, Washington DC 2011). Currently, carbon fibers that meet
specifications (250 ksi tensile strength and 25 Msi Young's modulus) for
automotive
applications are made from polyacrylonitrile (PAN), obtained from
acrylonitrile
(ACN), which is synthesized using propylene and ammonia. World ACN production
in 2010 was 5.7 million tons, and is highly dependent on volatility of
propylene
prices. Additionally, propylene production (a byproduct of naphtha cracking
for
ethylene) is reducing due to growth of the natural gas based process for
production of
ethylene. In light of these facts and increased demand for carbon fibers, US
DOE has
expressed interest in making ACN precursor that can meet specifications needed
for
production of carbon fibers from renewable non-food biomass with a goal of
$1.00/1b
cost.
[00051 There is a need for improved catalysts and methods for producing ACN
and
intermediates to make ACN. Such a catalytic composition and method are
described
herein.
1
CA 3033151 2019-09-18
SUMMARY OF THE INVENTION
[0006] Disclosed herein is a method comprising the steps of: a) separating at
least a portion of
ethylene glycol and propylene glycol from a first product comprising ethylene
glycol, propylene
glycol, and glycerol, thereby producing a second product comprising glycerol;
and b) contacting
the second product comprising glycerol with a first catalyst composition,
thereby producing a
third product comprising acrolein and hydroxyacetone, wherein the first
catalyst composition
comprises a first catalyst having the formula:
Ml M2,1\43yOz
wherein M1 is a metal promoting C-0 cleavage,
wherein M2 is a metal with acid sites promoting dehydration,
wherein M3 is an amphoteric catalyst support, with acid and base sites,
promoting
selective dehydration in conjunction with Ml,
wherein x is a molar ratio of M2 relative to Ml, from about 0.25 to about 4,
wherein y is a molar ratio of M3 relative to Ml, from about 0.25 to about 4,
wherein z is the total amount of oxygen bound to Ml, M2, and M3, and
corresponds to the sum of the oxidation states of M1, M2, and M3.
[0007] Also disclosed herein is a method comprising the step of: a) contacting
propylene glycol
with a third catalyst composition, thereby producing propanal, wherein the
third catalyst
composition consisting essentially of a third catalyst having the formula:
M4M5a0z
wherein M4 is a metal with acid sites promoting dehydration,
wherein M5 is an amphoteric catalyst support, with acid and base sites,
promoting
selective dehydration
wherein a is a molar ratio of M5 relative to M4, from about 0.25 to about 4,
wherein z is the total amount of oxygen bound to M4 and M5, and corresponds to
the sum of the oxidation states of M4 and M5.
[0008] Additional advantages will be set forth in part in the description
which follows, and in
part will be obvious from the description, or can be learned by practice of
the aspects described
below. The advantages described below will be realized and attained by means
of the chemical
compositions, methods, and combinations described herewith.
2
Date Recue/Date Received 2021-04-14
There is provided a method of preparing propanal from biomass, the method
comprising
the steps of: a) converting a first product comprising C5 and/or C6 sugars
from biomass to a
second product comprising ethylene glycol, propylene glycol, and glycerol in
the presence of a
multifunctional catalyst; b) separating ethylene glycol and glycerol from the
second product,
thereby producing a third product comprising propylene glycol; and c)
contacting the third
product comprising propylene glycol with a catalyst composition, thereby
producing a fourth
product comprising propanal, wherein the catalyst composition comprises a
catalyst having the
formula: M4M5a0z, wherein M4 is a metal with acid sites promoting dehydration,
wherein M5 is
an amphoteric metal catalyst support, with acid and base sites, wherein a is a
molar ratio of M5
relative to M4, wherein a is from about 0.25 to about 4, wherein z is the
total amount of oxygen
bound to M4, and M5, and corresponds to the sum of the oxidation states of M4,
and M5,
wherein metals M4 and M5 are different metals.
There is further provided a method of preparing propanal from biomass, the
method
comprising the steps of, a) converting a first product comprising C5 and/or C6
sugars from
biomass to a second product comprising ethylene glycol, propylene glycol, and
glycerol in the
presence of a multifunctional catalyst; b) separating ethylene glycol and
glycerol from the
second product, thereby producing a third product comprising propylene glycol;
and c)
contacting the third product comprising propylene glycol with a catalyst
composition, thereby
producing a fourth product comprising propanal, wherein the catalyst
composition comprises a
catalyst having the formula: M4M5aM6b0z wherein M4 is a metal with acid sites
promoting
dehydration, wherein M5 is an amphoteric metal catalyst support, with acid and
base sites,
promoting selective dehydration in conjunction with M6 when present, wherein
M6 is a metal
promoting C-0 cleavage, wherein a is a molar ratio of M5 relative to M4,
wherein a is from
about 0.25 to about 4, wherein b is a molar ratio of M6 relative to M4,
wherein b is from 0 to
about 4, wherein z is the total amount of oxygen bound to M4, M5, and M6, and
corresponds to
the sum of the oxidation states of M4, M5, and M6, wherein metals M4, M5, and
M6 are
different metals.
There is further provided a method of producing an acrylonitrile precursor
from biomass,
the method comprising the steps of: a)converting a first product comprising C5
and/or C6 sugars
from biomass to a second product comprising ethylene glycol, propylene glycol,
and glycerol in
the presence of a multifunctional catalyst; b) separating ethylene glycol and
propylene glycol
from the second product, thereby producing a third product comprising
glycerol; and c)
contacting the third product comprising glycerol with a catalyst composition,
thereby producing
a fourth product comprising acrolein and hydroxyacetone, wherein the catalyst
composition
comprises a catalyst having the formula: WO3Zr02 or WO3Si02.
2a
Date Recue/Date Received 2021-04-14
It is to be understood that both the foregoing general description and the
following
detailed description are exemplary and explanatory only and are not
restrictive.
DESCRIPTION OF THE FIGURES
[0009] The accompanying figures, which are incorporated in and constitute a
part of
this specification, illustrate several aspects and together with the
description serve to
explain the principles of the invention.
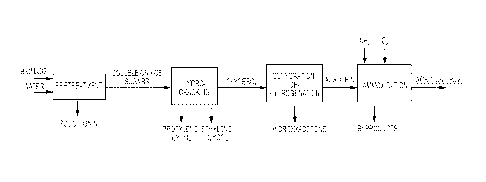
[0010] FIG. 1 shows the overall flow schematic of non-limiting aspects of the
method
disclosed herein for the process of producing acrylonitrile.
[0011] FIG. 2 shows the conversion of propylene glycol using a 20-W03/SiO2
catalyst.
[0012] FIG. 3 shows the influence of reaction condition for product
distribution of the
conversion of sugars to C2, C3 diols and triols, such as ethylene glycol,
propylene
glycol and glycerol.
[0013] FIG. 4 shows the single step process for hydrocracking reaction for
converting
sugars to C2, C3 diols and triols, such as ethylene glycol, propylene glycol
and
glycerol.
[0014] FIG. 5 shows the catalytic reaction pathway for propylene glycol to
acrolein
and propanal.
[0015] FIG. 6 shows the catalytic performance for propylene glycol conversion.
[0016] FIG. 7 shows the glucose conversion to glycols as a function of
temperature
for catalyst 1).
[0017] FIG. 8 shows the glucose conversion to glycols as a function of WHSV
for
catalyst 1).
[0018] FIG. 9 shows the glucose conversion to glycols as a function of
pressure for
catalyst 1).
[0019] FIG. 10 shows the stability as a function of time and reaction
conditions for
catalyst 3).
[0020] FIG. 11 shows the selectivity as a function of time and reaction
conditions for
catalyst 3).
3
CA 3033151 2019-09-18
[0021] FIG. 12 shows ASPEN simulations of a method disclosed herein.
[0022] FIG. 13 shows ASPEN simulations of a method disclosed herein.
[0023] FIG. 14 shows the effect of Bronsted-to-Lewis (B/L) site ration on
acrolein
selectivity.
[0024] Additional advantages of the invention will be set forth in part in the
description which follows, and in part will be obvious from the description,
or can be
learned by practice of the invention. It is to be understood that both the
foregoing
general description and the following detailed description are exemplary and
explanatory only and are not restrictive of the invention, as claimed.
DETAILED DESCRIPTION
[0025] The disclosed methods and articles can be understood more readily by
reference to the following detailed description.
[0026] Before the present compounds, compositions, articles, systems, devices,
and/or methods are disclosed and described, it is to be understood that they
are not
limited to specific articles or methods unless otherwise specified. It is also
to be
understood that the terminology used herein is for the purpose of describing
particular
aspects only and is not intended to be limiting. Although any methods and
materials
similar or equivalent to those described herein can be used in the practice or
testing of
the present invention, example methods and materials are now described.
[0027] The publications discussed herein are provided solely for their
disclosure prior
to the filing date of the present application. Nothing herein is to be
construed as an
admission that the present invention is not entitled to antedate such
publication by
virtue of prior invention. Further, the dates of publication provided herein
can be
different from the actual publication dates, which can require independent
confirmation.
4
CA 3033151 2019-09-18
CA 03033151 2019-02-05
WO 2018/038968 PCT/US2017/046905
1. Definitions
100281 As used herein, nomenclature for compounds, including organic
compounds,
can be given using common names, 1UPAC, IUBMB, or CAS recommendations for
nomenclature. When one or more siereochemical features are present, Cahn-
ingold-
Prelog rules for stemochernistry can be employed to designate stereochemical
priority, ElZ specification, and the like. One of skill in the art can readily
ascertain the
structure of a compound if given a name, either by systemic reduction of the
compound structure using naming conventions, or by commercially available
software, such as CHEMDRAWTM (Cambridgesoft Corporation, U.S.A.).
100291 In this specification and in the claims which follow, reference will be
made to
a number of terms which shall be defined to have the following meanings:
100301 It must be noted that, as used in the specification and the appended
claims, the
singular forms "a," "an" and "the" include plural referents unless the context
clearly
dictates otherwise. Thus, for example, reference to "a therapeutic agent"
includes
mixtures of therapeutic agents, reference to "a pharmaceutical carrier"
includes
mixtures of two or more such carriers, and the like].
100311 "Optional" or "optionally" means that the subsequently described event
or
circumstance can or cannot occur, and that the description includes instances
where
the event or circumstance occurs and instances where it does not. For example,
the
phrase "optionally comprising an adhesive material" means that the adhesive
material
can or cannot be present and that the description includes both situations.
100321 Ranges can be expressed herein as from "about" one particular value,
and/or
to "about" another particular value. When such a range is expressed, a further
aspect
includes from the one particular value and/or to the other particular value.
Similarly,
when values are expressed as approximations, 1-). use of the antecedent
"about," it will
be understood that the particular value forms a further aspect. It will be
further
understood that the endpoints of each of the ranges are significant both in
relation to
the other endpoint, and independently of the other endpoint. It is also
understood that
there are a number of values disclosed herein, and that each value is also
herein
disdosed as "about" that particular value in addition to the value itself. For
example,
if the value "10" is disclosed, then "about 10" is also disclosed. It is also
understood
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
that each unit between two particular units are also disclosed. For example,
if 10 and
15 are disclosed, then 11, 12, 13, and 14 are also disclosed.
100331 A weight percent (wt. %) of a component, unless specifically stated to
the
contrary, is based on the total weight of the therapeutic composition or
composition or
material, in which the component is included.
100341 References in the specification and concluding claims to parts by
weight, of a
particular element or component in a composition or article, denote the weight
relationship between the element or component and any other elements or
components in the composition or article for which a part by weight is
expressed.
Thus, in a composition containing 2 parts by weight of component X and 5 parts
by
weight component Y, X and Y are present at a weight ratio 0172:5, and are
present in
such ratio regardless of whether additional components are contained in the
composition.
100351 Disclosed are the components to be used to prepare the compositions of
the
invention as well as the compositions themselves to be used within the methods
disclosed herein. These and other materials are disclosed herein, and it is
understood
that when combinations, subsets, interactions, groups, etc. of these materials
are
disclosed that while specific reference of each various individual and
collective
combinations and permutation of these compounds cannot be explicitly
disclosed,
each is specifically contemplated and described herein. For example, if a
particular
compound is disclosed and discussed and a number of modifications that can be
made
to a number of molecules including the compounds are discussed, specifically
contemplated is each and every combination and permutation of the compound and
the modifications that are possible unless specifically indicated to the
contrary. Thus,
if a class of molecules A, B, and C are disclosed as well as a class of
molecules D, E,
and F and an example of a combination molecule, A-D is disclosed, then even if
each
is not individually recited each is individually and collectively contemplated
meaning
combinations, A-F, B-D, B-E, B-F, C-D, C-E, and C-F are considered
disclosed.
Likewise, any subset or combination of these is also disclosed. Thus, for
example, the
sub-group of A-E, B-F, and C-E would be considered disclosed. This concept
applies
to all aspects of this application including, but not limited to, steps in
methods of
making and using the compositions of the invention. Thus, if there are a
variety of
additional steps that can be performed it is understood that each of these
additional
6
steps can be performed with any specific embodiment or combination of
embodiments of the
methods of the invention.
[0036] It is understood that the compositions disclosed herein have certain
functions.
Disclosed herein are certain structural requirements for performing the
disclosed functions,
and it is understood that there are a variety of structures that can perform
the same function
that are related to the disclosed structures, and that these structures will
typically achieve the
same result.
2. Production of intermediates to produce acrylonitrile
[0037] Micron-sized carbon fibers presently used are mostly produced by heat
treatment or
controlled pyrolysis of different precursor fibers. The most prevalent
precursors are PAN,
cellulose fibers (such as viscose, rayon, and cotton), petroleum and coal tar
pitch, and certain
phenolic fibers. Synthesis process involves heat treatment, oxidative
stabilization,
carbonization and graphitization to achieve desired mechanical strength. It is
well established
in the literature that strength of fibers is the function of crystallinity and
orientation, and by
reducing defects in the fiber. The best way to achieve this is to start with a
highly oriented
precursor and then maintain the initial high orientation during the process of
stabilization and
carbonization through tension.
[0038] PAN has highly polar nitrile groups which cause strong dipole-dipole
forces that act as
cross-links, making the polymer soluble only in highly ionizing solvents,
increasing its
melting point, and making it more suitable as a carbon fiber precursor. In
order to obtain PAN
which results in such fiber properties, precursor ACN is required which is
obtained from
ammoxidation of propylene (petrochemical). The production of ACN accounts for
approximately 70% of total cost, which is highly volatile with price currently
ranging between
$1,000 to 1,400/MT.
[0039] Recent advances have been made to produce ACN from glycerol (Olga, M.,
et al,
Chem. Sus. Chem. (2008) 1, 511-513; Liebig, C., et al, Applied Catalysis B:
Environmental
(2013) 132-133, 170-182; Ulgen et. al. Catalysis Letters. 2009, 131: 122-128;
Ulgen et. al.
Applied catalysis A. General 400 (2011), 34-38), which can be sourced as a
byproduct from
biodiesel plants. While glycerol is available from subsidized biodiesel
plants, for a long term
solution, suitable renewable feed stocks and conversion process are needed.
7
CA 3033151 2020-03-25
[0040] It is known that propylene ammoxidation proceeds through an allylie
intermediate
acrolein. This ammoxidation was studied as early as 1963 by Adams et al over
traditional
Bismuth molybdate catalysts (Adams et al, Journal of Catalysis. (1963) 2, 63-
68). A renewed
interest in acrolein ammoxidation has emerged due to high availability of
glycerol as a
byproduct from bio-diesel plants. Glycerol can be readily dehydrated to form
acrolein;
however biodiesel plants are fast disappearing due to lack of subsidies. Thus,
a constant
source and pure glycerol is not available nor is the process economically
attractive, ACN
produced from glycerol costs ¨$2200/MT.
[0041] Disclosed herein is a catalyst and method that derive acrolein from
glycerol. The
catalyst and method can be used with inexpensive starting materials, such as
sugars, e.g.
hemicellulose or cellulose sugars (cost around 5 to 40 cents/kg). The produced
acrolein can in
turn be converted to ACN. A schematic flow of the process to convert biomass
to ACN is
shown in FIG. 1.
[0042] Also disclosed herein is a catalyst that derives propanal from
propylene glycol.
Propanal can in turn be converted several other C3 chemicals, such as, for
example, propionic
acid.
[0043] A method of using sugars from sugars such as hemicellulose or cellulose
derived
sugars to produce polyols, e.g. ethylene glycol, propylene glycol, and
glycerol, to form
acrolein and hydroxyacetone (also known as acetol) is disclosed herein. The
method
disclosed herein includes the step of separating out ethylene glycol and
propylene glycol to
leave behind a product comprising glycerol. Such a method is desired because
of its low
carbon footprint and economic viability.
[0044] Also disclosed herein is a multifunctional catalyst capable of, in a
single step,
converting C5 and C6 sugars to ethylene glycol, propylene glycol, and
glycerol. In one
aspect, the, C5 and C6 sugars are hemicellulose or cellulose derived C5 and C6
sugars from
biomass.
a. First Catalyst Composition
[0045] The first catalyst disclosed herein can convert glycerol to a product
comprising
acrolein and hydroxyacetone. Such product mixture is desired because
8
=
CA 3033151 2020-03-25
hydroxyacetone can easily be separated from the acrolein by use of low energy
distillation or
flash vaporization. ACN can then be produced by ammoxidizing acrolein in
presence of
ammonia and oxygen (air).
[0046] Disclosed herein is a first catalyst composition for converting
glycerol to acrolein and
hydroxyacetone comprising a first catalyst having the formula:
M1M2xM3yOz
wherein MI is a metal promoting C-0 cleavage,
wherein M2 is a metal with acid sites promoting dehydration,
wherein M3 is an amphoteric catalyst support, with acid and base sites,
promoting
dehydration in conjunction with Ml,
wherein x is a molar ratio of M2 relative to Ml, from about 0.25 to about 4,
wherein y is a molar ratio of M3 relative to MI, from about 0.25 to about 4,
wherein z is the total amount of oxygen bound to Ml, M2, and M3, and
corresponds to
the sum of the oxidation states of MI, M2, and M3.
[0047] In the formula M1M2xM3y0z, the metals Ml, M2, and M3 are different
metals.
[0048] The catalyst composition comprises metals that advantageously C-0
cleavage (MI)
and then dehydrate (M2, M3) triols, such as, for example, glycerol. As such
the catalyst
composition can convert glycerol to acrolein to hydroxyacetone. These
conversions are
desired as hydroxyacetone can easily be separated from acrolein due to their
different boiling
temperatures.
[0049] MI is a metal promoting C-0 cleavage in molecules, such as, for
example, C-0
cleavage in triols, such as, for example, glycerol. In one aspect, MI is
selected from the
group consisting of Cu, Zn, and Sn. For example, Ml can be Cu. In another
example, Ml
can be Zn. In yet another example, Ml can be Sn.
[0050] M2 is a metal with acid sites promoting dehydration in molecules, such
as, for
example, dehydration in triols, such as, for example, glycerol. In one aspect,
M2 is selected
from the group consisting of W, Fe, and P. For example, M2 can be W. In
another example,
M2 can be Fe. In yet another example, M2 can be P.
9
CA 3033151 2020-03-25
[0051] M3 is an amphoteric catalyst support, with acid and base sites,
promoting selective
dehydration in conjunction with M1 in molecules, such as, for example,
dehydration in
conjunction with M1 in triols, such as, for example, glycerol. In one aspect,
M3 is selected
from the group consisting of Zr, Al, Si, Mg, Ti, La, and Ce. For example, M3
can be Zr. In
another example, M3 can be Al. In yet another example, M3 can be Si. In yet
another
example, M3 can be Mg. In yet another example, M3 can be a Ti. In yet another
example,
M3 can be a La. In yet another example, M3 can be a Ce. Selection of M3 can be
is dictated
by the support's stability in steam phase conditions. For example, alumina can
be modified
using silica as described by Ravenelle et al (Ravenelle, R.M., et al, ACS
Catalysis (2011) 1,
552-561; Ravenelle, R.M., et al, ChemCatChem (2012) 4, 492-494).
[00521 In one aspect, M1 can be Cu, M2, can be W, and M3 is selected from the
group
consisting of Zr, Al, Si, Mg, Ti, La, and Ce. In another aspect, M1 can be Cu,
M2, can be
selected from the group consisting of W, Fe, and P, and M3 can be Zr. In yet
another aspect,
MI can be selected from the group consisting of Cu, Zn, and Sn, M2, can be W,
and M3 can
be Zr.
[0053] In one aspect, M1 can be Cu, M2 can be selected from the group
consisting of W, Fe,
and P, and M3 can be selected from the group consisting of Zr, Al, Si, Mg, Ti,
La, and Ce. In
another aspect, M1 can be selected from the group consisting of Cu, Zn, and
Sn, M2 can be
W, and M3 can be selected from the group consisting of Zr, Al, Si, Mg, Ti, La,
and Ce. In yet
another aspect, M1 can be selected from the group consisting of Cu, Zn, and
Sn, M2 can be
selected from the group consisting of W, Fe, and P, and M3 can be Zr.
[0054] In one aspect, x is a molar ratio of M2 relative to Ml, of from about
0.25 to about 4.
In another aspect, x is a molar ratio from about 0.8 to about 4. In yet
another aspect, x is a
molar ratio from about 1.8 to about 4. In yet another aspect, x is a molar
ratio from about 0.25
to about 3. In yet another aspect, x is a molar ratio from about 0.25 to about
2.2. In yet
another aspect, x is a molar ratio from about 0.8 to about 2.2.
[0055] In one aspect, y is a molar ratio of M3 relative to Ml, of from about
0.25 to about 4.
In another aspect, y is a molar ratio from about 0.8 to about 4. In yet
another aspect, y is a
molar ratio from about 1.8 to about 4. In yet another aspect, y is a molar
ratio from about 0.25
to
CA 3033151 2020-03-25
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
about 3. In yet another aspect, y is a molar ratio from about 0.25 to about
2.2. In yet
another aspect, y is a molar ratio from about 0.8 to about 2.2.
100561 In one aspect, z is the total amount of oxygen bound to MI, M2, and M3,
and
corresponds to the sum of the oxidation states of ME.. M2, and M3. It is known
in the
art how to determine z based on the oxidation state or Ml, M2, and M3.
100571 in one aspect, Ml and M2, together with their respective amounts of
oxygen
based on oxidation state, are present in. an. amount from I wt % to 40 wt% of
the
catalyst wherein the support M3 provides the balance wt %. In another aspect.
MI
and M2, together with their respective amounts of oxygen based on oxidation
state,
are present in an amount from l wt % to 30 wt% of the catalyst wherein the
support
M3 provides the balance wt %. Ml and M2, together with their respeeti ve
amounts of
oxygen based on oxidation state, are present in an amount from 1 wt % to 20
wt% of
the catalyst wherein the support M3 provides the balance wt %. Ml and M2,
together
with their respective amounts of oxygen based on oxidation state, are present
in an
amount from 5 wt 96 to 20 wt% of the catalyst wherein the support M3 provides
the
balance tvt %.
100581 In one aspect, the catalyst has the formula CuOW03Zr02. In another
aspect,
the catalyst has the formula CuOVv403Ti02. In another aspect, the catalyst has
the
formula CuOW038102.
100591 The catalyst can be produced using co-impregnation of metal salts on
acid
supports, followed by calcination, which is typically performed at a
temperature
between 300 C to 600 C, such as about 450 C.
b. Second and Third Catalyst
100601 The second and third catalyst disclosed herein can convert propylene
glycol to
a product comprising propanal or consisting essentially of propanal. Such
product
increases the by-product yield and thus helps in carbon conservation.
Furthermore it
monetizes the principle product being acrylonitrile. In addition, propanal is
an
intermediate that can be used to produce several C3 chemicals, such as, for
example,
propionic acid.
100611 Disclosed herein is a second catalyst composition for converting
propylene
glycol to propanal comprising a second catalyst having the formula:
I l
M4M5aM6b0z
wherein M4 is a metal with acid sites promoting dehydration,
wherein M5 is an amphoteric catalyst support, with acid and base sites,
promoting
selective dehydration in conjunction with M6 when present,
wherein M6 is a metal promoting C-0 cleavage,
wherein a is a molar ratio of M5 relative to M4, from about 0.25 to about 4,
- wherein b is a molar ratio of M6 relative to M4 from 0 to about 4,
wherein z is the total amount of oxygen bound to M4, M5, and M6, and
corresponds to
the sum of the oxidation states of M4, M5, and M6.
[0062] Also disclosed herein is a third catalyst composition for converting
propylene glycol to
propanal consisting essentially of a third catalyst having the formula:
M4M5a0z
wherein M4 is a metal with acid sites promoting dehydration,
wherein M5 is an amphoteric catalyst support, with acid and base sites,
promoting
selective dehydration,
wherein a is a molar ratio of M5 relative to M4, from about 0.25 to about 4,
wherein z is the total amount of oxygen bound to M4 and M5, and corresponds to
the
sum of the oxidation states of M4 and M5.
[0063] In the formula of the second catalyst M4M5aM6b0z, the metals M4, M5,
and M6 are
different metals.
[0064] In the formula of the third catalyst M4M5a0z, the metals M4 and M5 are
different
metals.
[0065] The catalyst composition comprises metals that advantageously dehydrate
(M4, M5)
and promote C-0 cleavage (M6) in diols, such as, for example, propylene
glycol. As such the
catalyst composition can convert propylene glycol to propanal. These
conversions are desired
as propanal can in turn be converted to acrolein.
[0066] M4 is a metal with acid sites promoting dehydration in molecules, such
as, for
example, dehydration in diols, such as, for example, propylene glycol. In one
aspect, M4 is
selected from the group consisting of W, Fe, and P. For example, M4
12
CA 3033151 2020-03-25
can be W. In another example, M4 can be Fe. In yet another example, M4 can be
P.
100671 M5 is an amphoteric catalyst support, with acid and base sites,
promoting dehydration
in conjunction with M6 when present in molecules, such as, for example, C-0
cleavage in
conjunction with M6 when present in diols, such as, for example, propylene
glycol. In one
aspect, M5 is selected from the group consisting of Zr, Al, Si, Mg, Ti, La,
and Ce. For
example, M5 can be Zr. In another example, M5 can be Al. In yet another
example, M5 can
be Si. In yet another example, M5 can be Mg. In yet another example, M5 can be
a Ti. In yet
another example, M5 can be a La. In yet another example, M5 can be a Ce.
Selection of M5
can be is dictated by the support's stability in steam phase conditions. For
example, alumina
can be modified using silica as described by Ravenelle et at (Ravenelle, R.M.,
et al, ACS
Catalysis (2011) 1, 552-561; Ravenelle, R.M., et al, ChemCatChem (2012) 4, 492-
494).
[0068] M6 is a metal promoting C-0 cleavage in molecules, such as, for
example, C-0
cleavage in diols, such as, for example, propylene glycol. In one aspect, M6
is selected from
the group consisting of Cu, Zn, and Sn. For example, M6 can be Cu. In another
example, M6
can be Zn. In yet another example, M6 can be Sn.
[0069] For the second catalyst of formula M4M5aM6b0z: In one aspect, M4 can be
W, M5 is
selected from the group consisting of Zr, Al, Si, Mg, Ti, La, and Ce, and M6
can be present
and be Cu. .In another aspect, M4 can be selected from the group consisting of
W, Fe, and P,
M5 can be Zr, and M6 can be present and be Cu. In yet another aspect, M4 can
be W, M5 can
be Zr, and M6 can be present and be selected from the group consisting of Cu,
Zn, and Sn.
[0070] For the second catalyst of formula M4M5aM6b0z: In one aspect, M4 can be
selected
from the group consisting of W, Fe, and P, M5 can be selected from the group
consisting of
Zr, Al, Si, Mg, Ti, La, and Ce, and M6 can be present and be Cu. In another
aspect, M4 can
be W, M5 can be selected from the group consisting of Zr, Al, Si, Mg, Ti, La,
and Ce, and
M6 can be selected from the group consisting of Cu, Zn, and Sn. In yet another
aspect, M4
can be selected from the group consisting of W, Fe, and P, M5 can be Zr, and
M6 can be
selected from the group consisting of Cu, Zn, and Sn.
13
CA 3033151 2020-03-25
[0071] For the third catalyst of formula M4M5a0z: In one aspect, M4 can be W,
and M5 is
selected from the group consisting of Zr, Al, Si, Mg, Ti, La, and Ce. In
another aspect, M4
can be selected from the group consisting of W, Fe, and P and M5 can be Zr. In
yet another
aspect, M4 can be W and M5 can be Zr.
[0072] In one aspect, a is a molar ratio of M5 relative to M4, of from about
0.25 to about 4.
In another aspect, a is a molar ratio from about 0.8 to about 4. In yet
another aspect, a is a
molar ratio from about 1.8 to about 4. In yet another aspect, a is a molar
ratio from about 0.25
to about 3. In yet another aspect, a is a molar ratio from about 0.25 to about
2.2. In yet
another aspect, a is a molar ratio from about 0.8 to about 2.2.
[0073] In one aspect, b is a molar ratio of M6 relative to M4, of from about
0.25 to about 4.
In another aspect, b is a molar ratio from about 0.8 to about 4. In yet
another aspect, b is a
molar ratio from about 1.8 to about 4. In yet another aspect, b is a molar
ratio from about 0.25
to about 3. In yet another aspect, b is a molar ratio from about 0.25 to about
2.2. In yet
another aspect, b is a molar ratio from about 0.8 to about 2.2.
[0074] In one aspect, z is the total amount of oxygen bound to M4. M5, and M6,
and
corresponds to the sum of the oxidation states of M4, M5, and M6. It is known
in the art how
to determine z based on the oxidation state of M4, M5, and M6.
[0075] In one aspect, z is the total amount of oxygen bound to M4 and M5, and
corresponds
to the sum of the oxidation states of M4 and M5. It is known in the art how to
determine z
based on the oxidation state of M4 and M5.
[0076] In one aspect, the second catalyst has the formula CuOW03Zr02. In
another aspect,
the second catalyst has the formula CuOW03T102. In another aspect, the second
catalyst has
the formula CuOWO3Si02.
[0077] In one aspect, the third catalyst has the formula WO3Zr02. In another
aspect, the third
catalyst has the formula WO3Ti02. In another aspect, the third catalyst has
the formula
WO3Si02.
[0078] The catalyst can be produced using co-impregnation of metal salts on
acid supports,
followed by calcination, which is typically performed at a temperature between
300 C to 600
C, such as about 450 C.
14
CA 3033151 2020-03-25
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
c. Method
100791 The method disclosed herein reduces the steps needed to convert biomass
to
useful products such as ethylene glycol, propylene glycol, glycerol, propanal,
acrolein.. and acrylonitrile. As a result, the method further reduces the
greenhouse gas
emission as compared to previously known processes.
I. Production of ethylene glycol, propylene glycol, and
glycerol
100801 Disclosed herein is a method that converts sugars, such as C5 andior C6
sugars
to ethylene glycol, propylene glycol, and glycerol.
100811 Disclosed herein is a method comprising the step of: a) in a single
step,
converting C5 and/or C6 sugars to a first product comprising ethylene glycol,
propylene glycol, and glycerol in the presence of a multifunctional catalyst
100821 In one aspect, the C5 and/or C6 sugars can be C5 and/or C6
hemicellulose and
cellulose derived sugars. The C5 and/or C6 sugars or C5 and/or C6
hemicellulose and
cellulose derived sugars can be produced from any type of biomass. Biomass is
known in the art and is biological material derived from living, or recently
living
organisms. The process of producing C5 and/or C6 sugars or C5 and/or C6
hemicellulose and cellulose derived sugars from biomass is known in the ad.
I00831 For example, hot water extraction of hemicellulose from biomass is a
self-
catalytic process, autohydrolysis. This mechanism of hydrolysis lies in
cleavage of 0-
acetyl and uronic acid substitutions that result in the formation of acetic
and other
organic acids, which makes it possible for further hydrolysis of
polysaccharides to
oligomers and monomers (Niemela, K., et al, 1999. Characterization of pulping
liquors, in: Analytical Methods in Wood Chemistry, Pulping and Papermaking.
Springer-Verlag, Berlin). The main degradation pathways of hetnicelluloses
under
acidic conditions liberate xylose, mannose, galactose, glucose, and acetic
acid. 'the
temperature and pressure and incubation time can be controlled to avoid the
degradation of xylltui to furfural (Palmqvist, E., et al, Bioresource
Technology (2000)
74,25-33) and of hexose to 5-hydroxymethylfurfural (HMF). FIG. 3 shows the
conversion of xylitol to ethylene glycol, propylene glycol, and glycerol and
other
products.
CA 03033151 2019-02-05
WO 2018/038968
FCT/US2017/046905
100841 The multifunctional catalyst is capable of converting the C5 and/or C6
sugars
in a single step to diols and triols. Thus, the there is no need for
separation steps or
the production of intermediates to produce the diols and triols. Such a method
saves
both time and resources as compared to methods requiring multiple steps.
(00851 The conversion of the C5 and/or C6 sugars to a first product comprising
ethylene glycol, propylene glycol, and glycerol in the presence of a
multifunctional
catalyst is performed in a solvent, such as, for example, water. In one
aspect, the
method further comprises the step of separating at least a portion of the
water from
the first product. hi one aspect, the concentration of the first pmduct is
from about 10
wt % to about 40 wt A), such as, for example, from about 20 wt% to about 35
wt %, in
the water after a portion of the water has been separated from the first
product.
100861 In one aspect, the multifunctional catalyst comprises one or more
metals
selected from the group consisting of Cu, Zn, Sn, Ni, Pt, Pd, Ru, and Re, and
a
support. In one aspect, the support is selected from the group consisting of
Al2O3,
SiO2, Carbon, TiO2, and MgO.
100871 In one aspect, the multifunctional catalyst comprises Ni and Cu and the
support comprises A1203. The selectivity of various products can be altered by
altering the amount of Ni and Cu and the support comprises Al2O3 as shown in
FIG.
5. Cu can serve for C-0 cleavage (Dasari, M.A., eta!, Applied Catalysis A:
General
(2005) 281 225-23), Ni for C-C cleavage and hydrogenation and Lewis acid
support
(A1203) for C-C cleavage and dehydration. Lower amount of copper and nickel
over
Lewis acid support favors formation of C3 polyols (less cracking), medium
nickel and
copper favors C2 diols (slight enhanced cracking), whereas even higher Ni and
Cu
favors formation of alcohols (high degree of both C-C and C-0 cleavage with
hydrogenation).
100881 In one aspect, the multifunctional catalyst comprises less than 10 wt %
of Cu
and less than 20 wt % of Ni, and the remainder being A1203. In another aspect,
the
multifunctional catalyst comprises from about I wt % to about 9 wt % of Cu and
from
about 1 vvt % to about 19 wt % of Ni, and the remainder being A1203.
Conventional
nickel catalyst supported on y-alumina are unstable due to structural loss
under
hydrothermal conditions (Ravenelle, R.M., et al, ACS Catalysis (2011) 1, 552-
561)
making metal sites unavailable. Effect of metal on support structure
(Ravenelle, R.M.,
16
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
et al, ChemCatChem (2012) 4, 492-494), role of co-adsorbed water on support
during
C2 and C3 polyol reactions (Copeland, J. R., et al, Langmuir (2013) 29, 581-
593) and
addition of silica to increase thermal stability has been studied.
Furthermore, addition
of Cu to Ni catalyst on such supports has known to increase their integrity
under
hydrothermal conditions (U.S. Patent 5,977,013).
[OM] In one aspect, the multifunctional catalyst can further comprise Pt, such
as less
than 0.5 wt % of Pt, less than 0.3 wt % of Pt, for example, about 0.1 wt % of
Pt. The
Pt facilitates long term activity and the ability of the catalyst to
regenerate.
100901 In one aspect, the first product comprises from about 10 wt % to about
40 wt
% of ethylene glycol, from about 10 wt % to about 50 WI % of propylene glycol,
and
from about 10 wt % to about 50 wt % or glycerol. in one aspect, the first
product
comprises from about 20 wt % to about 35 wt % of ethylene glycol, from about
30 wt
% to about 45 wt Woof propylene glycol, and from about 30 wt % to about 45 wt
% of
glycerol.
100911 In one aspect, at least 95 wt % of the C5 and/or C6 sugars in step a)
are
converted to the first product comprising ethylene glycol, propylene glycol,
and
glycerol. in another aspect, at least 98 wt % of the C5 and/or C6 sugars in
step a) are
converted to the first product comprising ethylene glycol, propylene glycol,
and
glycerol. In yet another aspect, at least 99 wt % of the C5 and/or C6 sugars
in step a)
are converted to the first product comprising ethylene glycol, propylene
glycol, and
glycerol.
100921 In one aspect, the step of converting C5 and/or C6 sugars to a first
product
comprising ethylene glycol, propylene glycol, and glycerol in the presence of
a
multifunctional catalyst is performed under mild conditions. For example, the
step of
converting C5 and/or C6 sugars to a first product comprising ethylene glycol,
propylene glycol, and glycerol in the presence of a multifunctional catalyst
is
performed under mild conditions can be performed at a temperature from about
130
C to about 200 C, and at a pressure from about 400 psig to about 1000 psig in
presence of hydrogen. In another example, the step of converting C5 and/or C6
sugars to a first product comprising ethylene glycol, propylene glycol, and
glycerol in
the presence of a multifunctional catalyst is performed under mild conditions
can be
17
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
performed at a temperature from about 130 C to about 180 C, and at a pressure
from
about 400 psig to about 800 psig in presence of hydrogen.
100931 The use of mild conditions during the conversion of the C5 and/or C6
sugars
to the first product allows the multifunctional catalyst to be active for a
longer period
of time as compared to the same catalyst used under harsher conditions.
Desired
conversion rates, for example above 95 wt %, of the C5 andior C6 sugars can be
achieved by the method for at least 100 hrs, such as, for example, from 100
hrs to 500
hrs.
(0094] In one aspect, the method can further comprise the step of contacting
the first
product comprising ethylene glycol, propylene glycol, and glycerol with the
catalyst
composition disclosed herein, thereby producing a second product comprising
acrolein and acetaldehyde. In one aspect, the method can further comprise the
steps
of separating at least a portion of the acetaldehyde from the second product;
and
converting at least a portion of the acrolein in the second product to
aciylonitrile.
100951 In one aspect, the method is performed on an industrial scale. For
example,
the method can produce at least 30 g,11/hr. such as, for example, at least 45
gil/hr. of
the first product per hour. In another example, the method can produce from
about 30
gil/lir to about 200 el/hr of the first product per hour.
100961 A schematic flow of the method disclosed herein is shown in FIG. 4.
11. Production of acrolein and hydroxyacetone
(00971 In FIG. 1, the second reaction step (Dehydration/dehydrogenation) of
the
multistep process involves selective conversion of C3 diols (i.e. propylene
glycol),
(rids (i.e. glycerol) to acrolein. in FIG. 1, the product from the first
reaction step
(hydrocracking of sugar) primarily contains glycerol and propylene glycol at
high
conversion (100%), selectivity (90%) and stability (>100hrs). Both propylene
glycol
and glycerol were tested as feed for the second reaction step to produce
acrolein.
However, although both propylene glycol and glycerol indeed can be converted
to
acrolein, the conversion of propylene glycol also produces propanal. It is
difficult to
separate propanal from acrolein. Accordingly, it can be difficult and
expensive to
obtain a pure acrolein product if the product contains large amounts of
propanal.
18
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
Thus, it is desired to avoid that propanal and acrolein be produced in the
same
reaction step.
100981 Furthermore, compared to glycerol, propylene glycol conversion
selectivity to
acrolein (KO% versus 42%) was lower at high conversion (1()()%).
10099] It was discovered that the overall mechanism of the formation of
acrolein from
propylene glycol was composed of a number of dehydration and dehydrogenation
reactions. Two major intermediates were identified to be responsible for
acrolein
production, which were allyl alcohol and propanal. However, subsequent
production
of hydrogen via dehydrogenation further hydrogenated acrolein and reduced its
overall selectivity. The produced hydrogen also results in the formation of
ethylene
glycol, which was found to significantly deactivate the first catalyst
disclosed herein.
Accordingly, the success of propylene glycol conversion to acrolein will hinge
on the
effective removal of the produced hydrogen.
100100] As dictated by the product composition from the first reaction
step,
wherein sugars are converted to ethylene glycol, propylene glycol, and
glycerol,
propylene glycol constitutes about half of the feed to the following reaction
step,
wherein about the other half is glycerol. With high acrolein selectivity from
glycerol,
the overall selectivity for acrolein production in the ed reaction step would
thus be
-,62%. Combined with the potential to produce hydroxyacetone as co-product
from
glycerol, and the ability to conveniently separate acrolein from
hydroxyacetone, the
overall efficiency of this process is high. However, such use of mixed feed
(propylene glycol and glycerol) also produces propanal and acetaldehyde (low
boiling
point products) from the propylene glycol and when mixed with acrolein would
be
difficult to separate and adds impurity to the final product. Accordingly, the
method
disclosed herein separates propylene glycol from glycerol prior to conversion
of the
glycerol to the acrolein and co-products. This results in that the product
contains no
to minimal amounts of propanal, which is undesired in a product mixture with
acrolein and increases overall selectivity on carbon basis to 67..5%
1001011 The separated propylene glycol can in turn be converted to
propanal.
100102] Disclosed herein is a method comprising the step of. a)
separating at
least a portion of ethylene glycol and propylene glycol from a first product
comprising ethylene glycol, propylene glycol. and glycerol, thereby producing
a
19
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
second product comprising glycerol; and b) contacting the second product
comprising
glycerol with a first catalyst composition disclosed herein, thereby producing
a third
product comprising acrolein and hydroxy acetone, wherein the first catalyst
composition comprises a first catalyst disclosed herein.
[00103] In one aspect, the method further comprises the steps of c)
separating
at least a portion of the hydroxyacetone from the third product, thereby
forming a
fourth product comprising acrolein; and; and d) following step c) converting
at least a
portion of the acrolein in the fourth product to acrylonitrile.
[00104] In it known in the art how to convert acrolein to acrylonitrile.
For
example, acrylonitrile can be produced from acrolein ammoxidation, which is a
simultaneous oxidation of an organic group (R) and ammonia, such a mutual
reaction
results in oxidative condensation product to form R'-CN molecule such as
acrylonitrile. Such a reaction is not possible if a separate oxidation of R
and ammonia
are conducted. Hadley et al (Hadley, D. J., Chemy Ind. (1961) 238) has
proposed a
two-stop reaction mechanism, dehydration duo to reaction with ammonia (fast
stop),
and oxidative-dehydration of intermediate (rate limiting step). Therefore, as
partial
pressure of oxygen increases, the higher surface concentration of oxygen on
catalyst
leads to higher selectivity to acrylonitrile and acetonitrile (by-product)
with net
decrease in CO,.
[00105] In one aspect, step b) is performed at atmospheric pressure.
[00106] In one aspect, the first product is present in a solvent, such
as, for
example, water.
[00107] In one aspect, the second product is present in a solvent, such
as, for
example, water.
[001081 In one aspect, the third product is present in diluent, such as,
for
example, water. In one aspect, the method can further comprise removing at
least a
portion of the diluent, thereby concentrating the third product.
[00109] The mechanism for the conversion of propylene glycol to acrolein
and
propanal with various types of catalysts is shown in FIG. 5.
[00110] The first product contains high boiling point molecules;
propylene
glycol (188 C), ethylene glycol (197.3 C) and glycerol (290 C). Propylene
glycol
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
and ethylene glycol can be separated from the first product via distillation
to form a
second product comprising glycerol. In one aspect, at least about 60 wt % of
the
propylene glycol is separated from the first product. For example, at least
about 70 wt
%, 80 wt %, 90 wt % or 95 wt % of propylene glycol can be separated from the
first
product. In another aspect, from about 60 wt % to about 98 wt % of the
propylene
glycol is separated from the first product. For example, from about 70 wt %,
80 wt
%, 90 wt % or 95 t % to about 98 wt % of the propylene glycol can be separated
from the first product.
100111] In one aspect, the second product comprises at least 2 times
more
glycerol than propylene glycol by weight. For example, the second product can
comprise at least 5, 10, 20, 30, 50, of 75 times more glycerol than propylene
glycol by
weight. In another aspect, the second product comprises from about 2 times to
about
90 times more glycerol than propylene glycol by weight. For example, the
second
product comprises from about 5 firms to about 50 times more glycerol than
propylene
glycol by weight.
1001121 In one aspect, from about 50 wt % to about 99 wt % of the
glycerol in
the second product can be converted to acrolein. For example, from about 60 wt
% to
about 95 wt % of the glycerol in the second product can be converted to
acrolein. In
another example, from about 70 wt % to about 95 wt % of the glycerol in the
second
product can be converted to acrolein.
100113] In one aspect, the third product comprises at least about 50 wt
% of
acrolein. For example, the third product comprises at least about 60 wt %, 70
wt %,
or 80 wt % of acrolein.
1001141 In one aspect, the third product comprises from about 50 wt % to
about
95 wt % of acrolein and from about 50 wt % to about 5 wt % of hydroxy acetone.
For
example, third product comprises from about 60 wt % to about 95 wt % of
acrolein
and from about 40 wt % to about 5 wt % of hydroxyacetone. In another example,
third product comprises from about 70 wt % to about 95 wt % of acrolein and
from
about 30 wt % to about 5 wt 13/0 of hydroxy acetone. The third product can be
present
in a diluent, which is not a part of the third product.
10011.51 In one aspect, the first product is produced from C5 and/or C6
sugars
in a single step using a multifunctional catalyst, as described elsewhere
herein. In one
21
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
aspect, the C5 and/or C6 sugars can be C5 and/or C6 hemicellulose and
cellulose
derived sugars. The process of producing C5 and/or C6 sugars or C5 and/or C6
hemicellulose and cellulose derived sugars from biomass is also described
herein.
1001161 It was surprisingly shown that ethylene glycol deactivates the
first
catalyst composition and first catalyst such that the selectivity and
conversion rates of
glycerol are significantly decreased. Thus, a method wherein the ethylene
glycol is
present during conversion of glycerol to acrolein and hydroxyacetone cannot be
performed continuously for an extended period of time without losing
significant
(more than 10% or 20%) selectivity and conversion rates of glycerol.
Accordingly,
the method disclosed herein avoids this issue by separating ethylene glycol
from the
first product, thereby minimizing its presence during the conversion of
glycerol to
acrolein and hydroxyacetone. Therefore, the method disclosed herein can be
performed continuously for an extended time without significant (less than
10%) loss
in selectivity and conversion rate of glycerol to acrolein and hydroxyacetone.
1001171 In one aspect, thc method comprising the step of: a) separating
at least
a portion of ethylene glycol and propylene glycol from a first product
comprising
ethylene glycol, propylene glycol, and glycerol, thereby producing a second
product
comprising glycerol; and b) contacting the second product comprising glycerol
with a
first catalyst composition disclosed herein, thereby producing a third product
comprising acrolein and hydroxyacetone, wherein the first catalyst composition
comprises a first catalyst disclosed herein can be performed for at least 5
hours
without losing more than 10 % of the conversion rate of the second product to
the
third product. In one aspect, the method comprising the step of: a) separating
at least
a portion of ethylene glycol and propylene glycol from a first product
comprising
ethylene glycol, propylene glycol, and glycerol, thereby producing a second
product
comprising glycerol: and b) contacting the second product comprising glycerol
with a
first catalyst composition disclosed herein, thereby producing a third product
comprising acrolein and hydroxyacetone, wherein the first catalyst composition
comprises a first catalyst disclosed herein can be performed for at least 24
hours
without losing more than 10 % of the conversion rate of the first product to
the second
product. In one aspect, the method comprising the step of: a) separating at
least a
portion of ethylene glycol and propylene glycol from a first product
comprising
ethylene glycol, propylene glycol, and glycerol, thereby producing a second
product
22
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
comprising glycerol; and b) contacting the second product comprising glycerol
with a
first catalyst composition disclosed herein, thereby producing a third product
comprising acrolein and hydroxyacetone, wherein the first catalyst composition
comprises a first catalyst disclosed herein can be performed from about 5
hours to
about 50 hours without losing more than 10 % of the conversion rate of the
first
product to the second product.
100118] In one aspect, the method is performed on an industrial scale.
For
example, the method can produce at least 75 WI of the third product per hour.
100119] A schematic flow of the method disclosed herein is shown in FIG.
1.
100120] In one aspect, the method further comprises contacting at least
a
portion of the separated propylene glycol with a second catalyst composition
disclosed herein, thereby producing propane!, wherein the second catalyst
composition comprises a second catalyst disclosed herein.
100121] In one aspect, the second catalyst composition converts at least
about
60 wt c,vo of the separated propylene glycol to propanal. For example, the
second
catalyst composition can convert at least about 70 wt %, 80 wt %, or 90 wt %
of the
separated propylene glycol to propanal. In another aspect, the second catalyst
composition converts from about 60 wt % to about 98 wt % of the separated
propylene glycol to propane]. For example, the second catalyst composition can
convert from about 70 wt %, 80 wi %, or 90 wt % to about 98 wt % of the
separated
propylene glycol to propanal.
100122] In one aspect, the method further comprises converting the
propane' to
acrolein.
1001231 Also disclosed herein is a method comprising the step of: a)
contacting
propylene glycol with a third catalyst composition disclosed herein, thereby
producing propanal, wherein the third catalyst composition comprises a third
catalyst
disclosed herein.
100124] In one aspect, the third catalyst composition converts at least
about 60
wt % of the propylene glycol to propane]. For example, the third catalyst
composition
can convert at least about 70 wt %, 80 wt %, or 90 wt % of the propylene
glycol to
propanal. In another aspect, the third catalyst composition converts from
about 60 wt
% to about 98 wt % of the propylene glycol to propanal. For example, the third
23
catalyst composition can convert from about 70 wt %, 80 wt %, or 90 wt % to
about 98 wt %
of the propylene glycol to propanal.
[00125] In one aspect, the method further comprises converting the propanal to
acrolein.
3. Aspects
[00126] In view of the disclosure herein below are described certain more
particularly
described aspects of the inventions. These particularly recited aspects should
not however be
interpreted to have any limiting effect on any different claims containing
different or more
general teachings described herein, or that the "particular" aspects are
somehow limited in
some way other than the inherent meanings of the language and formulas
literally used
therein.
[00127] Aspect 1: A method comprising the steps of: a) separating at least a
portion of
ethylene glycol and propylene glycol from a first product comprising ethylene
glycol,
propylene glycol, and glycerol, thereby producing a second product comprising
glycerol; and
b) contacting the second product comprising glycerol with a first catalyst
composition,
thereby producing a third product comprising acrolein and hydroxyacetone,
wherein the first
catalyst composition comprises a first catalyst having the formula:
M1 M2,1\43yOz
wherein M1 is a metal promoting C-0 cleavage,
wherein M2 is a metal with acid sites promoting dehydration,
wherein M3 is an amphoteric catalyst support, with acid and base sites,
promoting selective dehydration in conjunction with Ml,
wherein xis a molar ratio of M2 relative to Ml, from about 0.25 to about 4,
wherein y is a molar ratio of M3 relative to Ml, from about 0.25 to about 4,
wherein z is the total amount of oxygen bound to Ml, M2, and M3, and
corresponds to the sum of the oxidation states of Ml, M2, and M3.
[00128] Aspect 2: The method of aspect 1, wherein the method further comprises
the steps
of: c) separating at least a portion of the hydroxyacetone from the third
product, thereby
forming a fourth product comprising acrolein; and d) following step c)
converting at least a
portion of the acrolein in the fourth product to acrylonitrile.
24
Date Recue/Date Received 2021-04-14
[00129] Aspect 3: The method of aspects 1 or 2, wherein the method further
comprises prior
to step a), in a single step, converting CS and/or C6 sugars to the first
product comprising
ethylene glycol, propylene glycol, and glycerol in the presence of a
multifunctional catalyst.
[00130] Aspect 4: The method of any one of aspects 1-3, wherein the method
further
comprises contacting at least a portion of the separated propylene glycol with
a second
catalyst composition, thereby producing propanal, wherein the second catalyst
composition
comprises a second catalyst having the formula:
M4M5aM6b0z
wherein M4 is a metal with acid sites promoting dehydration,
wherein M5 is an amphoteric catalyst support, with acid and base sites,
promoting selective dehydration in conjunction with M6 when present,
wherein M6 is a metal promoting C-0 cleavage,
wherein a is a molar ratio of M5 relative to M4, from about 0.25 to about 4,
wherein b is a molar ratio of M6 relative to M4, from 0 to about 4,
wherein z is the total amount of oxygen bound to M4, M5, and M6, and
corresponds to the sum of the oxidation states of M4, MS, and M6.
[00131] Aspect 5: The method of any one of aspects 1-4, wherein M1 is selected
from the
group consisting of Cu, Zn, and Sn.
[00132] Aspect 6: The method of any one of aspects 1-5, wherein M2 is selected
from the
group consisting of W, Fe, and P.
[00133] Aspect 7: The method of any one of aspects 1-6, wherein M3 is selected
from the
group consisting of Zr, Al, Si, Mg, Ti, La, and Ce.
[00134] Aspect 8: The method of any one of aspects 1-7, wherein MI is Cu.
[00135] Aspect 9: The method of any one of aspects 1-8, wherein M2 is W.
[00136] Aspect 10: The method of any one of aspects 1-9, wherein M3 is Zr.
[00137] Aspect 11: The method of any one of aspects 1-10, wherein the catalyst
has the
formula CuOW03Zr02, or CuOW03Ti02, or CuOWO3Si02.
[00138] Aspect 12: The method of any one of aspects 3-11, wherein the CS
and/or C6 sugars
is C5 and/or C6 hemicellulose and cellulose derived sugars.
CA 3033151 2020-03-25
[00139] Aspect 13: The method of any one of aspects 3-12, wherein the
multifunctional
catalyst comprises one or more metals selected from the group consisting of
Cu, Zn, Sn, Ni,
Pt, Pd, Ru, and Re, and a support.
[00140] Aspect 14: The method of aspect 15, wherein the support is selected
from the group
consisting of A1203, SiO2, carbon, TiO2, and MgO.
[00141] Aspect 15: The method of any one of aspects 1-14, wherein the method
is performed
continuously for at least 5 hours.
[00142] Aspect 16: The method of any one of aspects 1-15, wherein at least 60
wt % of the
propylene glycol is separated from the first product.
[00143] Aspect 17: The method of any one of aspects 1-16, wherein the third
product
comprises at least 50 wt % of acrolein.
[00144] Aspect 18: The method of any one of aspects 1-18, wherein the second
product
comprises at least 2 times more glycerol than propylene glycol by weight.
[00145] Aspect 19: A method comprising the step of: a) contacting propylene
glycol with a
third catalyst composition, thereby producing propanal, wherein the third
catalyst composition
consisting essentially of a third catalyst having the formula:
M4M5a0,
wherein M4 is a metal with acid sites promoting dehydration,
wherein M5 is an amphoteric catalyst support, with acid and base sites,
promoting selective dehydration,
wherein a is a molar ratio of M5 relative to M4, from about 0.25 to about 4,
wherein z is the total amount of oxygen bound to M4 and M5, and corresponds
to the sum of the oxidation states of M4 and M5.
[00146] Aspect 20: The method of aspect 19, wherein M4 is selected from the
group
consisting of W, Fe, and P, and M5 is selected from the group consisting of
Zr, Al, Si, Mg, Ti,
La, and Ce.
4. Examples
[00147] The following examples are put forth so as to provide those of
ordinary skill in the art
with a complete disclosure and description of how the compounds, compositions,
articles,
devices and/or methods claimed herein are made and evaluated, and are intended
to be purely
exemplary and are not intended to limit the
26
CA 3033151 2020-03-25
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
disclosure. Efforts have been made to ensure accuracy with respect to numbers
(e.g.,
amounts, temperature, etc.), but some errors and deviations should be
accounted for.
Unless indicated otherwise, parts are parts by weight, temperature is in C or
is at
ambient temperature, and pressure is at or near atmospheric. The Examples are
provided herein to illustrate the invention, and should not be construed as
limiting the
invention in any way.
1. EXPERIMENTAL PARAMETERS AND TESTING OF CATALYSTS
100148] Preparation of CuOW03Zr02: A Tungsten Hexachloride Solution in
Ethanol (0.056g/m1 of solution) was prepared (other salts of tungsten such
ammonium
paratunestate (APT) and ammonium metatwigstate (AMT) dissolved in water were
also used). It is noted that other tungsten salts may also be iced in this
process. ZrO2
was obtained from Alfa Aesar (catalog number 43815) with a surface area of 90
m2/g.
This was crushed to the required size (-20, + 35 mesh) and the required amount
of
Tungsten Hexa.chloride solution for 20 wt ')/0 resultant W03 was added (61.6
ml, the
salt has poor solubiiity and thus excess solution was added), along with 10 ml
of
water to achieve better dissolution and retrieve traces of salt. The solution
was
impregnated on crushed ZrO2 and allowed to thy at room temperature for 4 to 5
hrs. A
second solution of Copper nitrate hexahydrate (0.043 g/m1) was prepared; this
solution was impregnated on top of previously obtained tungsten hexachloride
on
ZrO2 sample. The sample was allowed to dry at 100 C overnight. Dried sample
was
calcined in a high temperature oven under air using following program, room
temperature to 100 C at 1 C/min and held at 1.10 C for 1 hour, then 110 C to
450
C at 5 C/min, and held at 450 C for 3 hours.
100149] Preparation of WO3Si02 and CuOWO3SiG2 catalysts: A 75 ml
aqueous solution of ammonium paratungstate (APT) salt (0.014g/m1 of solution)
was
prepared at 80 C under continuous stirring for 4-6 hours. An excess volume
and an
elevated temperature were used as this salt is poorly soluble in water. 4g of
SiO2,
obtained from Degussa (Aerosil 200, catalog number 132138) as fumed silica,
with
a surface area of 200 m2/g, was subsequently added to the APT solution. The
excess
water was evaporated by overnight stirring at 60 C. The sample was then
allowed to
dry at 100 C over night. The dried sample was calcined in a high temperature
oven
under static air using following program: room temperature to 100 C at 1
C/min and
27
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
held at 110 C for 1 hour, then 110 C to 450 C at 2.5 C/min and held at 450
C for
4 hours. The prepared catalyst contained 20 wt % W03.
1001501 For the preparation of CuOWO3SiO2 catalyst, 0.99g of copper
nitrate
(Cu(NO3)2.3H20) salt dissolved in 10 ml water was additionally impregnated to
the
calcined WO3SiO2 catalyst via incipient wetness impregnation. The resulting
sample
was dried at 100 C over night and then calcined using the same high
temperature
calcination protocol as the WO3Si02 sample. The CuO loading on the resulting
catalyst was 5 wt %. After calcination both the catalysts were pelletized and
sieved to
(-20,+35 mesh).
100151] 2. Experimental Parameters and Testing of Catalysts for Glycerol
Conversion to Acrolein
1001521 W032r02, CuOW03Zr02 and W03/SiO2 catalyst were tested for the
conversion of glycerol to acrolein. 5 wt % glycerol was used as a reactant and
a co
flow of N2 of 50 SCCM This reaction was conducted in fixed bed reactor. The
catalyst was loaded in the center of heated zone, with inert low surface glass
beads as
filler material on top and bottom of reactor. At the bottom of reactor a 5
micron metal
grid was used as support for catalyst material. At the inlet of reactor, feed
was
preheated to desired temperature. The gas phase reaction was studied at 280 C
and 1
atm using W03/ZrO2, CuO/W03/Zr02, and W03/SiO2 as dehydrating catalysts, with
a
flow of lehr and 1 grams of each catalyst for a WliSV of 1/hr. The gas
effluent was
connected to an online GC-FID which was pre-calibrated for acrolein, by-
products
and feed component. The presence of acrolein in the product was confirmed
using a
standard on GC-F1D and GC-MS.
28
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
Table 1
Catalyst W03/Z102 CuOAV03/Zr02 W03/SiO2
Temperature - C 280 280 280
Pressure - psig 14.7 14.7 14.7
Glycerol conversion 100% 100% 100%
¨%
Selectivity to 82% 35% 71% to 82 %
Acrolein 1
100153] As shown in Table 1,
a CuO/W03/ZrO:thave intermediate selectivity
and WO;/SiO2 catalyst have comparable selectivity for the production of
acrolein
from glycerol as compared to a W0.3/Z102 catalyst.
100154] The role of acid
sites in the catalyst In order to determine effect of
acid sites in the catalyst, two types of measurements were conducted ¨ total
acidity
using NH3-TPD and Bronsted-to-Lewis acidity ratio using FTIR-Pyridine. The
role of
acidity was determined, as follows:
1. Table 2 shows evidence of high Bronsted to Lewis acid site ratio (B/L) in
20-
W03/SiO2 (APT salt) similar to the 10.45-W03/ZrOz (APT salt) catalyst;
2. B/L ratio correlates with selectivity of acrolein, as shown in FIG. 14;
3. AMT catalyst have lower total acid site density and as well as 13/1.,
ratio.
Therefore, most AMT catalysts performed poorly as compared to APT salt
catalyst as described herein;
4. The addition of CuO increased the total acidity of the catalyst and
resulted in
the formation of hydroxyacetone and lower selectivity to acrolein, see Table
2.
29
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
Table 2
Catalyst Total acidity B/L ratio
(mmoles/g)
10.45-W03/Zr02 (APT) 2.01
11 -W03.2r02 (AMT) 0.145 0.87
5-Cu0/11-W03/Zr02 (AMT) 0.463
20-W03/Si 02 (Al'!) 0.117 1.57
5-Cu0/20-W03/Si02 (APT) 0.250
100155] Role of support and surface area: Two types of support were
studied- i) ZrO2 an amphoteric support with both acid and base sites; and ii)
SiO2, a
weak acid with no basic sites.
100156] Table 3 shows that the net catalyst surface area for W03/SiO2
catalyst
is significantly higher than the ZrO2 supported catalyst. Both catalysts show
a high
initial activity (100% conversion). However, the Si0i catalyst shows a much
longer
life time under the tested conditions.
Table 3
Support surface area Catalyst surface area after W03
Catalyst (In2/gm) deposition (m2/gram)
10.45 wt%
WOar02 90 37
20 wt% WO/SiO2 250 150
1001571 Lifetime of Catalyst: Table 4 shows that W03/S102 catalyst has
longer life (51 hours v. 15-20 hours) as compared to W03/ZrO2 catalyst.
1001581 Since ZrO2 is a known amphoteric oxide, 10.45-W03/Zr02 is
expected
to retain significant amount of basic sites. Surprisingly, 20-\V03/SiO2 does
not retain
such significant basicity as SiO2 is inherently neutral and with introduction
of W03
attains a handful of acid sites and but no basic sites. It is possible, that
due to lack of
W03 loading on ZrO2 support, which is conducted to induce weak acid sites,
basic
sites are still active and leading to secondary dehydration/condensation
reactions
responsible for catalyst deactivation.
100159] Co-feeding the reaction with air improved ar..-rolein
selectivity for the
20-W03/SiO2 catalysts to 82% (from 71% without air) (see Table 1).
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
1001601 Although the selectivity to acrolein obtained from these two
catalysts
were similar (see Table 4, the lifetime of the acidic 20-W03/S102 catalyst was
higher
than the amphoteric 10.45-W03/Zr02 catalyst.
Table 4
Catalyst Composition Feed Selectivity to Catalyst life
Conversion acrolein ( %) (hrs)
Glycerol (%)
1 10.45w-t% 100 80 15-20
wo3ar02
2 20 wi.%W03/S102 100 71 to 82 --51
1001611 .. Both the WO3Si02 and CuOW03.Zr02 catalysts were also tested for
the conversion of propylene glycol. 5 wt % propylene glycol with a co flow of
N2 of
50 SCCM was used as reactant and the reaction was conducted in a fixed bed
reactor.
The catalyst was loaded in the center of heated zone, with inert low surface
glass
beads as filler mateial on top and bottom of reactor. At the bottom of reactor
a 5
micron metal grid was used as support for catalyst material. At the inlet of
reactor,
feed was preheated to desired temperature. The gas phase reaction was studied
at 280
C and 1 atm with a weight hourly space velocity (WHSV) of lihr using lg
catalyst.
At these conditions, WO3Si02 catalyst underwent dehydration and
dehydrogenation
reactions which resulted in the formation of propanal, acrolein and ethylene
glycol
(EG). The results using this catalyst appear in Table 5, FIG 2 and FIG 6. On
the other
hand, on the CuOWO;SiO, catalyst only dehydration mechanism was active which
resulted in very selective production of propanal (no acrolein or F.G. The
results have
been listed in Table 5 and plotted in FIG 6. The gas effluent was connected to
an
online GC-FID which was pre-calibrated for acrolein and feed component. The
presence of listed products were confirmed using a standard on GC-F1D and GC-
MS.
31
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
Table 5
Catalyst W03/SiO2 CuO/W03/Si02
Temperature - C 280 280
Pressure - psig 14.7 14.7
Propylene glycol 100%* 100%
conversion ¨ %
Selectivity:
Acrolein - % 11 0
Propanal - /. 33 82
EG - % 30 1
*Conversion dropped to 0 within 8h due to catalyst deactivation
2. EXPERIMENTAL PARAMETERS AND TESTING OF CATALYSTS FOR
SUGAR CONVERSION TO GLYCOLS AND GLYCEROL
100162] Catalyst for conversion of sugar to glycols was prepared using
following methods and materials: catalyst 1) 0.1 wt% 0we/oNi/A1203 - Nickel
Hexhydrate salt was dissolved in water for a resultant concentration of
0.04036 grams
of Ni/gram of solution. 0.25 grams of Tetraamineplatinum (II) nitrate solution
was
added to this solution. Commercial Alumina obtained from Alfa Aesar (catalog
number 43855) with a surface area of 220 m2/g and pore size of 70 A was used.
This
support was crushed and sieved to (-20, +35 mesh). Salt solution was
impregnated on
the solid catalyst support to obtain desired metal concentration ranging from
1 to 20
wt% of metal (Ni) and 0.1 wt% of Pt. The sample was allowed to dry at room
temperature for 3 his and then at 60 C on a hot plate overnight Dried sample
was
calcined under air in a conventional high temperature oven using the following
program. Room temperature to 100 C in 1.5 hours, held at 11:10 C for 1 hour,
100 to
120 C in 30 minutes, held at 120 C for 1 hour, 120 to 450 C at 10 'Chitin and
held
at 450 C for 3 hours.
100163] Catalyst 2): For a 0.1 wt%Pt/I 0 wt%Ni/ 10 wt%Cu/A1203 catalyst -
Nickel Hexhydrate salt was dissolved in water for a resultant concentration of
0.04036 grams of Ni/gram of solution. 0.25 grams of Tetraamineplatinum (H)
nitrate
32
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
solution was added to this solution. Commercial Alumina obtained from Alfa
Aesar
(catalog number 43855) with a surface area of 220 ni2/g and pore size of 70 A
was
used. This support was crushed and sieved to (-20, +35 mesh). Salt solution
was
impregnated on the solid catalyst support to obtain desired metal
concentration
ranging from 1 to 20 wt % of metal (Ni) and 0.1 wt% of Pt. The sample was
allowed
to chy at room temperature for 3 his and then at 60 C on a hot plate
overnight.
Similarly, copper solution was prepared using Copper nitrate hexahydrate in
water for
a concentration of (0.0789 grams of copper/gram of solution). This solution
was
impregnated on top of the obtained sample, dried at room temp for 3 hours and
then at
60 C overnight.
1001641 Catalyst 3): For a 0.1 wt9OPt/10 wt%Ni/ 10 wt%Cu/A1203
catalyst (Catalyst Al2, as shown in FICis. 10 and 11)¨ Copper Nitrate
trihydrate salt
was dissolved in water for a resultant concentration of 0.078 grams of Cu/gram
of
solution. Commercial Alumina obtained from Alfa Aesar (catalog number 43855)
with a surface area of 220 inz/g and pore size of 70 A was used. This support
was
crushed and sieved to (-20, +35 mesh). Salt solution was impregnated on the
solid
catalyst support to obtain desired metal concentration ranging from 1 to 20 wt
% of
metal (Cu). The sample was allowed to dry at room temperature for 3 his and
then at
60 C on a hot plale overnight. Dried sample was calcined under air in a
conventional
high temperature oven using the following program. The temperature was
increased
from room temperature to 60 C at a rate of 12 C/min and held at 60 C for 5
minutes. The temperature was increased from 60 C to 100 C at 14 C/min, and
held
at 100 C for I hour. The temperature was then increased from 100 C to 120 C
at 7
C/min, and held at 120 C for 1.5 hours. The temperature was then increased
from
120 C to 450 C at 10 C/min and held at 450 C for 3 hours. Following
calcination,
the catalyst sample was allowed to cool down to room temperature. A second
metal
impregnation was then conducted. Nickel solution was prepared using nickel
nitrate
hexahydrate in water for a concentration of (0.05 grams of nickel/gram of
solution).
0.25 grams of Telraamineplatintun (II) nitrate solution was added to this
solution.
Resultant salt solution was impregnated on the calcined sample to obtain
desired
metal concentration ranging from 1 to 20 wt /0 of metal (Ni) and 0.1 wt% of
Pt. Sample was dried at room temp for 3 hours and then at 60 C overnight. The
dried
sample was calcined under air in a conventional high temperature oven using
the
33
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
following program. The temperature was increased from room temperature to 60
C at
a rate of 12 C/min and held at 60 C for 5 minutes. The temperature was
increased
from 60 C to 100 C at 14 C/min, and held at 100 C for 1 hour. The
temperature
was then increased from 100 C to 120 C at 7 C/min and held at 120 C for 1.5
hours. The temperature was then increased from 120 C to 450 C at 10 C/min
and
held at 450 C for 3 hours. Following calcination, catalyst sample was allowed
to
cool down to room temperature and stored for testing.
1001651 Catalyst Testing: The Pt/Ni/A1203 sample was tested as a
catalyst for
the conversion of glucose to ethylene glycol, propylene glycol and glycerol.
10 wt%
glucose in water was used as feed with Hydrogen as co feed at 50 SCCM. The
reaction was conducted in a fixed bed reactor, in trickle flow, top down
approach.
Prior to reactor entrance a preheating zone was included to heat feed to
desired
temperature. The catalyst was filled in the center of reactor with inert glass
beads as
filler materials on top and bottom of the reactor. A 5 micron metal frit was
used at the
bottom of reactor to secure catalyst in place. 5 grams of catalyst was loaded
with a
flow rate of liquid 10 we/0 glucose in water varying from 2.5 to 10 ml/hr. The
temperature was varied from 150 to 200 C and pressure from 450 to 750 PSIG.
100166] The results of the experiments described above are shown in
FIGs. 7-9
are for catalyst 1), described above. FIG. 7 shows the glucose conversion to
glycols
as a function of temperature for catalyst 1). The data in FIG. 7 was produced
using the
following conditions: WHSV - GHSV - 600/hr, Pressure 600 PSIG, Feed of 10
wt% glucose and Hz. The selectivity for the production of ethylene glycol,
propylene
glycol, and glycerol was highest between 160 C and 180 C.
100167] FIG. 8 shows the glucose conversion to glycols as a function of
WHSV
for catalyst 11. The data in FIG. 8 was produced using the following
conditions:
GHSV ¨ 600/hr, Pressure 600 PSIG, Temperature 170 'C. Feed of 10 wi% glucose
and H2. The selectivity for the production of ethylene glycol, propylene
glycol, and
glycerol was highest at a WHSV of 1.5/hr.
100168] FIG. 9 shows the glucose conversion to glycols as a function of
pressure for catalyst 1). The data in FIG. 9 was produced using the following
conditions: WHSV - GHSV - 600/hr, Temperature 170 C. Feed of 10 wt%
34
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
glucose and H7. The selectivity for the production of ethylene glycol,
propylene
glycol, and glycerol was highest at a pressure between 550 and 650 psig.
1001691 FIG. 10 shows that Catalyst 3) was stable for more than 100
hours
with a 100% conversion in a reaction under the following conditions: WHSV ¨
1/hr
(0 to about 50 his), 0.5 hr -1 (from about 50 his to about 115 his), 0.25 hr -
1 (from
about 115 his to about 120 hrs), GHSV ¨ 600/hr, Temperature 170 C. Feed of 10
wt% glucose in water and H2. Pressure 600 psig. FIG. 10 also shows the
selectivity
for the production of ethylene glycol, propylene glycol, and glycerol over the
course
of the reaction.
100170] FIG. 11 shows the selectivity of Catalyst 3) for the production
of
ethylene glycol, propylene glycol, and glycerol, as compared to the
selectivity of
sorbitol and unknowns over the course of the reaction as described for FIG.
10. The
selectivity for ethylene glycol, propylene glycol, and glycerol, as compared
to the
selectivity of sorbitol and unknowns was stable over the course of the
reaction.
3. FIRST CATALYST DEACTIVATION BY ETHYLENE GLYCOL
1001711 Polyols (diols and triols) were produced selectively from
biomass
derived sugar as described herein at high conversion. However, as shown in
FIG. 2,
the first catalyst disclosed herein used in the subsequent dehydration
reaction
(production of acrolein and by products from first product comprising ethylene
glycol,
propylene glycol, and glycerol) deactivate rapidly in presence of ethylene
glycol. FIG.
2 shows conversion of propylene glycol on 20-W03/SiO2 (APT) caudyst with re
lion
time and corresponding selectivity to ethylene glycol formation. The
conversion
drops with formation of ethylene glycol due to catalyst deactivation.
Accordingly, the
method disclosed herein separates ethylene glycol and propylene glycol from
the first
product prior to conversion of the glycerol to acrolein and hydroxyacetone.
100172] Furthermore, glycerol can be converted to acrolein at a high
conversion and selectivity using the first catalyst disclosed herein. The
catalytic
performance was correlated with higher ratio of 13ronsted to Lewis acidity of
the
catalysts used. However, same catalysts were shown to be less selective for
propylene glycol conversion to acrolein, as shown in FIG. 6.
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
4. ASPEN SIMULATIONS
100173] Aspen simulations were performed to compare: 1) a method where
propylene glycol and ethylene glycol are separated from the first product
prior to
conversion of glycerol to acrolein and hydroxyacetone (case a); and 2) a
method
where propylene glycol and ethylene glycol are not separated from the first
product
prior to conversion of the first product to acrolein and by products (case b),
see FIG.
12.
[001741 The detail specifications of the products produced in FIG. 12
are
shown in Table 6 below.
Table 6
Property case a Case b
Desired product Glycerol Glycerol, PG
Selectivity (mot%) Glycerol : PG= 1:1, EG Glycerol:PG = 1:1, EG
<5% <5%
Separation unit & spec Flash (I): 160 C, 4bar Flash (1): 100 C, ibar
Separation (R1 S1-V) loss H20 (97%), Glycerol H20 (43%), PG (2%)
(3%), PG (73%, to
propanal)
Recovered stream (RIS 1 -L) 79% Glycerol 53% Glycerol
composition (dry basis) 19% PG 40% PG
Desired product Acrolein Acrolein
Selectivity 82% Glycerol 4 Acrolein 82% Glycerol 4
18% Glycerol 4 Acetol Acrolein
40% PG 4 Acrolein 18% Glycerol 4 Acetol
33% PG -3 Propanal 40% PG -3 Acrolein
10% PG 4 Acetol 33% PG 4 Propanal
10% PG -3 Acetol
Separation unit & spec Flash (1): 90 C, lbar Flash (1): 97 C, lbar
Separation (R2S2L) loss 1420 (59%), Acrolein Water (72%), Acrolein
(3.7%), Acetol (87%) (3%), Acetol (93%)
Recovered stream (R2S2V) 87% Acrolein 74% acrolein
36
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
composition (dry basis) 3% Acetol 2% Acetol
8% propanal 22% propanal
100175.1 Post-reaction separation was set to be conducted using flash
evaporation. The specification of the flash column has been set to ensure
maximum
propylene glycol, ethylene glycol and H20 separation (case a) or maximum H20
separation (case b) from the top stream (R1-Sly). In case a this top stream
will be
used to produce propanal from propylene glycol using using the second or third
catalyst composition disclosed herein.
100176] In case a, however, both the produced PG and glycerol remain
unseparated and are passed on to the next reaction step for acrolein
conversion. Here
significant difference arises in flash separation between case a and b. In
case a, energy
requirement is significantly higher due to heating all the water emerging out
of
Reactor 1 (R-I) at a higher temperature (conservative approach) and
potentially in
two separate steps and flash drums.
100177] FIG. 12 also shows ASPEN diagrams of units around Reactor 2 (R-
2).
Here the difference between case a and b becomes obvious in the product stream
(R2). For case a, acrolein is produced only from glycerol (0.27 lb acrolein/lb
of sugar)
whereas in case b it is produced both from propylene glycol and glycerol (0.36
lb/lb
glycerol). However, considerable formation of propanal is noticeable in case b
compared to case a (0.10 lb vs. 0.031b1 lb sugar). As described herein, the
separation
of propanal from acrolein can be difficult due to similar boiling points and
would
require a multistage distillation column.
Table 7
Desired product Acrvlonitrile Acnilonitrile
Selectivity 90% Acrolein -3 ACN 90% Acrolein 4 ACN
75% Propanal 4 CO2 75% Propanal CO2
25% Propanal 4 25% Propane' 4
Propionitrile Propionitrile
10% Acrolein 4 CO2 10% Acrolein 4 CO2
Separation unit & spec HE (1), Stripper (1), HE (1), Stripper (1),
Distillation (1), Distillation (1), Settling
Settling drum (1) drum (1)
Separation loss ACN (9%) ACN (11%)
Recovered stream (ACN-F) 98% ACN 98% ACN
composition (dry basis)
37
CA 03033151 2019-02-05
WO 2018/038968
PCT/US2017/046905
1001781 ASPEN calculations of the Ammoxidation step of acrolein in case
a
and case b is shown in FIG. 13. : This reaction step is currently being
studied. The
performance of the catalyst is estimated from literature, and the separation
and
purification of ACN is also obtained from commercial process (Langvardt, P. W.
2011. Acrylonitrile. Ullmann's Encyclopedia of Industrial Chemistry). After
purification the production of acrylonitrile is 0.201h1b sugar for case a and
0.271bilb
sugar for case b). Table 7 above summarizes the ASPEN simulations for this
process.
(001791 Various modifications and variations can be made to the
compounds,
composites, kits, articles, devices, compositions, and methods described
herein. Other
aspects of the compounds, composites, kits, articles, devices, compositions,
and
methods described herein will be apparent from consideration of the
specification and
practice of the compounds, composites, kits, articles, devices, compositions,
and
methods disclosed herein. It is intended that the specification and examples
be
considered as exemplary.
38