Note: Descriptions are shown in the official language in which they were submitted.
CA 03037723 2019-03-20
WO 2018/057954
PCT/US2017/053052
AGENTS AND METHODS FOR MODULATING THE SENSORY
IMPACT OF TOBACCO OR HERBAL SMOKE
BACKGROUND
[0001] Tobacco smoke is a causal risk factor for cancer (especially but not
exclusively
lung cancer), cardiovascular disease, and lung dysfunction, including chronic
obstructive
pulmonary disease (COPD). Despite the recognized and widely known hazards of
tobacco
smoke, the addictive properties of cigarettes and other tobacco products have
been a barrier
to smoking cessation or reduction. There are a number of proposed strategies
for reducing
the harm associated with smoking. One attempted strategy has been to produce
cigarettes
with low tar and nicotine delivery during smoking, intended to be below the
threshold for
establishment or maintenance of nicotine addiction. Such "light" cigarettes
have had little
impact on smoking-related illnesses, in part due to low consumer satisfaction,
but also
because smokers tend to compensate for low nicotine, tar, and flavor component
delivery by
increasing the number and volume of puffs per cigarette, to titrate nicotine
delivery or
airway sensations to a level that provides relief from acute tobacco craving
symptoms.
Another attempted strategy has been to develop methods for nicotine delivery
without
combustion of tobacco, including "heat-not-burn" technology that seeks to
release nicotine
from the tobacco via volatilization or formation of an aerosol without many of
the toxicants
in combusted tobacco smoke, but which still delivers the highly addictive drug
nicotine.
[0002] Smoking remains one of the leading causes of preventable morbidity
and
mortality throughout the world. The World Health Organization estimates there
are around
1.3 billion tobacco users world-wide, and notes that tobacco use causes nearly
6 million
deaths per year, with an estimated 8 million deaths a year by 2030, should
current trends
continue. The Centers for Disease Control and Prevention has stated that
smoking is the
leading cause of preventable death in the U.S., where there are an estimated
540,000
premature deaths per year due to cigarette smoking, and the economic cost of
smoking is
estimated to exceed S300 billion per year. In addition to providing smokers
with effective
smoking cessation treatment options, new approaches are needed to substitute
for the
rewarding effects of smoking using less harmful alternatives.
[0003] While tobacco is the leading cause of preventable disease and death,
nicotine is
the fundamental cause of addiction among tobacco users. Even by removing
harmful toxins
and carcinogens from combusted tobacco smoke, nicotine is well known to have
serious side
effects across all systems of the body including cardiovascular, respiratory,
renal and
CA 03037723 2019-03-20
WO 2018/057954
PCT/US2017/053052
reproductive systems. The US Surgeon General has concluded that nicotine is as
addictive
as cocaine or heroin and thus is one of the most addicting agents known.
Studies have
consistently demonstrated the carcinogenic potential of nicotine. The US Food
and Drug
Administration (FDA) recently announced its intention to place nicotine at the
center of its
regulation of tobacco products, believing that reducing nicotine in tobacco
products can
significantly reduce tobacco-related disease and death. There is a growing
acceptance that
isolating nicotine use from smoking is only a partial solution and that
finding safer
alternatives to nicotine should be a priority.
BRIEF SUMMARY
[0004] In an example, a device includes a wrapper, tobacco disposed in the
wrapper, and
a first agent that activates a TRPA1 channel disposed in the wrapper.
[0005] The first agent may include at least one of grains of paradise,
galangal and 6-
paradol.
[0006] The device may be at least partially combustible.
[0007] The device may include a filter disposed toward an end of the device
and coupled
to the wrapper.
[0008] The device may include a capsule operable to be broken by finger
pressure.
[0009] The first agent may be disposed in the tobacco, and a second agent
that activates
at least one of a TRPM8 channel, a TRPV3 channel, and a TRPV1 channel may be
disposed
in the capsule.
[0010] The second agent may activate the TRPM8 channel and be operable to
provide a
cooling sensation.
[0011] The second agent may include at least one of menthol, physcool
(monomenthyl
succinate), icilin, geraniol, linalool, hydroxycitronellal, WS-3, WS-23,
PMD38, Cool-actP,
FrescolatMGA, FrescolatMA and PMD38.
[0012] The second agent may activate the TRPV3 channel and be operable to
provide a
warming sensation.
[0013] The second agent may include at least one of carvacrol, thymol,
eugenol,
eucalyptol, incensol, borneol, camphor, dihydrocarveol.
[0014] The second agent may activate the TRPV1 channel.
[0015] The second agent may include at least one of Szechuan pepper, all
spice, mustard
and rosemary.
[0016] The device may include a second agent that activates a TRPM8 channel
and is
operable to provide a cooling sensation.
2
CA 03037723 2019-03-20
WO 2018/057954 PCT/US2017/053052
[0017] The device may include a second agent that activates a TRPV3 channel
and is
operable to provide a warming sensation.
[0018] The device may include a second agent that activates the TRPV1
channel.
[0019] The tobacco may be a low nicotine tobacco.
[0020] The device may include at least one of herb and tea leaves.
[0021] In another example, a device includes a wrapper, at least one of
herb and tea
leaves disposed in the wrapper, and a first agent that activates a TRPA1
channel disposed in
the wrapper.
[0022] The first agent may include at least one of grains of paradise,
galangal and 6-
paradol.
[0023] The device may include a capsule operable to be broken by finger
pressure. The
first agent may be disposed in the at least one of herb and tea leaves. A
second agent that
activates at least one of a TRPM8 channel, a TRPV3 channel, and a TRPV1
channel may be
disposed in the capsule.
[0024] The second agent may activate the TRPM8 channel and be operable to
provide a
cooling sensation.
[0025] The second agent may activates the TRPV3 channel and be operable to
provide a
warming sensation.
[0026] The second agent may activate the TRPV1 channel.
BRIEF DESCRIPTION OF THE DRAWINGS
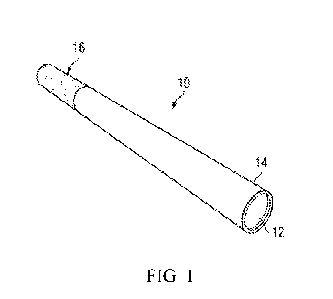
[0027] Figure 1 is a perspective view of an exemplary device for delivering
compositions of the present disclosure.
[0028] Figure 2 is an exploded schematic side view of an exemplary
embodiment of the
device of Figure 1.
[0029] Figure 3 is a schematic side view of an exemplary cellulose acetate
tip of the
device of Figure 1.
DETAILED DESCRIPTION
[0030] Embodiments described herein relate generally to an improved
composition to
modulate the sensory effects of low nicotine or non-nicotine tobacco or
tobacco substitute
products and providing relief from acute nicotine craving symptoms.
[0031] Sensory aspects of smoking contribute to smoking behavior and relief
of cigarette
craving both through conditioned or learned association with nicotine
delivery, but also
through direct autonomic and sensory-metabolic reflexes independent of
nicotine entry into
3
CA 03037723 2019-03-20
WO 2018/057954 PCT/US2017/053052
the brain. In particular, tracheal stimulation (sometimes referred to as
"throat scratch,"
"throat impact" or "throat hit") is reported as a component of smoking
satisfaction and relief
of cigarette craving. Isolated reduction of throat sensations with local
anesthetics reduce the
relief of smoking craving induced by nicotine-containing cigarette smoke;
conversely,
maintenance of throat scratch while disabling olfaction and oral taste
sensation with an
anesthetic mouthwash can alleviate the negative affect associated with
cigarette craving.
The "throat scratch" can affect perceived taste qualities and more subtle
throat and airway
autonomic sensory nerve stimulation, which can have effects beyond taste
sensations.
[0032] An understanding of actions of nicotine that permit improvements in
mimicry of
its subjective and physiological effects in a non-combustible simulated
cigarette is that its
initial perceived actions are upon sensory receptors in the respiratory tract.
Cigarette smoke
constituents, including but not limited to nicotine, act on sensory and
autonomic afferent
nerve terminals sending sensory information to the brain from the mouth,
throat and
respiratory tract via the vagus, trigeminal and other cranial nerves, and
induce autonomic
reflexes and effects in the central nervous system that contribute to the
subjective experience
of cigarette smoking, including "throat scratch," and to relief of craving,
and also to
subjective reflex-mediated experiences such as "head rush." Cigarette smoking
causes EEG
changes and corresponding subjective experiences in the brain via sensory
reflexes even
before nicotine is absorbed and transported to the brain.
[0033] The pharmacological actions of nicotine include actions on nicotinic
cholinergic
receptors in the central and peripheral nervous systems. A class of sensory
receptors distinct
from cholinergic receptors can be exploited by compositions and devices of
this disclosure to
provide mimicry of the sensation of inhalation of cigarette smoke to a degree
sufficient to
provide satisfaction and attenuation of craving for nicotine-containing
cigarettes. Simulation
of nicotine's actions on sensory receptors on nerve endings in the respiratory
tract is
achieved with selected volatile agents acting on the chemesthetic sensory
modality mediated
by transient receptor potential channels, which are pharmacologically distinct
from nicotine-
responsive cholinergic or acetylcholine receptors.
[0034] The chemical senses comprise taste, olfaction, and "chemesthesis"
(also known
as the "common chemical sense," or trigeminal chemosensation). Chemesthesis
refers to
actions of chemicals, e.g. food constituents, on nerve endings mediating
sensations of pain or
temperature, including the pungent, hot or cool sensations elicited by
specific constituents of
many ingested or inhaled substances including hot peppers (capsaicin), black
pepper
(piperine), menthol, garlic (allicin), horseradish and wasabi (allyl
isothiocyanate), camphor,
wintergreen, cinnamon (cinnamaldehyde), carbonated beverages (carbon dioxide)
and some
4
CA 03037723 2019-03-20
WO 2018/057954
PCT/US2017/053052
air pollutants. Such sensations in the oral and nasal cavities are mediated
via the trigeminal
nerve, and are elements of the somatosensory system, distinguishing them from
olfaction
and taste, although chemesthesis is an integral component of characteristic
sensory
information about foods or airborne chemicals.
[0035] In the throat, respiratory tract and lungs, chemesthetic signals are
transmitted to
the brain via the glossopharyngeal and vagus nerves. Chemesthetic signals can
thereby
directly affect brain activity in the somatosensory cortex and other brain
regions, including
appetitive circuits involved in craving for particular foods or other
chemesthetic stimuli
including tobacco smoke.
[0036] Crossover of chemesthetic signaling provided by compounds of the
present
disclosure with chemesthetic signaling pathways involved in perception and
physiological
responses to tobacco smoke contributes to the ability of devices and
compositions of the
present disclosure to reduce craving or negative affect or mood associated
with delay or
withdrawal of tobacco smoke.
[0037] During tobacco smoking, chemosensory signals from the respiratory
tract inform
the brain that smoke, likely containing nicotine based on experience, has been
inhaled,
thereby triggering alterations of activity in appetitive circuits involved in
converting absence
of central nicotinic receptor activation into negative affect or dysphoria
associated with
cigarette craving. Activation of chemosensory neurons that anatomically or
functionally
overlap with neurons terminating in the airways that respond to nicotine and
other smoke
constituents provides signals to the brain that similarly trigger neural
reflex-mediated relief
of nicotine-withdrawal dysphoria without actually delivering nicotine or
potentially toxic
smoke constituents resulting from combustion of tobacco. Similarly, mimicry of
tactile,
kinesthetic, organoleptic aspects of cigarette smoking, in addition to
chemosensory mimicry,
reinforces the ability of devices and compositions of the present disclosure
to relieve
symptoms of nicotine withdrawal or cigarette craving.
[0038] Transient receptor potential ion channels ("TRP channels" or, simply
"TRP") are
a class of receptors on sensory nerves that trigger chemesthetic nerve
activation. TRP
channels mediate pungent, hot or cool sensations of food or air. TRP
activation on sensory
nerve endings can induce local effects in addition to sensation per se (e.g.
focal
vasodilatation via axon reflex) or may trigger sensory-autonomic reflexes (e.g
changes in
bronchial tone, coughing, sneezing or changes in body temperature regulation,
or subjective
sensations) mediated through the reflex arcs involving the spinal cord or
brain. While TRP
activation can provide warning of potentially noxious environmental or dietary
factors, TRP
stimulation can also elicit pleasure or satisfaction, as is implied by the
examples of common
CA 03037723 2019-03-20
WO 2018/057954 PCT/US2017/053052
TRP activators above. A feature of TRP activation is that chemesthetic
sensations (or TRP
sensations), which are generally more sensitive to temperature sensations than
to smell
sensations provide less inherent discrimination between different agonists
than is the case for
olfactory sensation. Sensory nerve endings associated with the trigeminal,
glossopharyngeal
and vagus nerves may contain multiple types of chemosensory receptors,
providing a basis
for chemesthetic mimicry by chemically diverse agents.
[0039] TRP channels can be activated by concentrations of compounds below
those that
cause actual physical changes in the respiratory tract, acting as sensitive
sentinels of possible
irritation or damage. TRP channel activators therefore provide a potential
mode of action for
volatile constituents to have smoke-mimetic chemosensory or chemesthetic
effects without
delivery of otherwise bioactive quantities of the sensory agents into the
body.
[0040] Referring to Fig. 1, in some embodiments, an exemplary cigarette
device 10 may
be approximately the size and shape of a conventional cigarette. Volatile
constituents are
dispersed within a matrix 12 encased within a wrapper 14 resembling the outer
wrap of a
conventional cigarette. In some embodiments, the wrapper is relatively
nonporous, liquid
impermeable wrapper 14. The matrix 12 may be provided by a porous material
such as
cellulose or it may also be provided by tobacco or tobacco substitute product.
The end 16 of
the device 10 may be constructed to resemble a conventional filter rod in
appearance and/or
feel. In some embodiments, a filter rod is used at the end 16 of the device
10. The present
disclosure is not restricted to cigarette-like devices, but also includes
substitute cigars, pipes,
e-cigarettes, inhalers, and other devices used to smoke tobacco or mimic
smoking.
[0041] Referring to Fig. 2, an exemplary embodiment of a device 10 includes
a substrate
122 enclosed in a wrapper 123 resembling the outer wrap of a cigarette. The
substrate 122
may include a channel 124. The channel 124 may include a tobacco or substitute
tobacco
product 126 and a porous substrate 128 to contain constituents described
herein. The
substrate 128 may also be omitted and the constituents applied directly to the
tobacco or
tobacco substitute product. The tobacco or substitute tobacco product 126 may
be separated
from the substrate 128 by a substantially non-porous or liquid impermeable
barrier to
provide separation between the tobacco or substitute tobacco product and the
constituents of
the disclosure. This may protect the constituents from the heat of burning the
tobacco or
substitute tobacco product.
[0042] An untreated region 130 may be included at a second end 132 of the
device 10,
which is subjected to mouth-applied suction by a user. The untreated region
130 may be
untreated cellulose or other filtering material. In some embodiments, the
untreated region
6
CA 03037723 2019-03-20
WO 2018/057954 PCT/US2017/053052
130 may be a filter plug. In some embodiments, the untreated region 130 may be
a
cellulose acetate tip with a micro capsule (discussed in more detail with
respect to Fig. 3).
[0043] The region 130 and part of the channel 124 may be covered with a
wrap 134.
The wrap 134 enhances visual similarity between the device 10 and a cigarette.
In some
cases, the substrate 128 may be disposed inside the region of the device 10
covered by the
wrap 134. A portion 136 of the wrap 134 may include trade dress for
identifying the device
or the maker thereof.
[0044] In an embodiment, the device 10 may have a length of approximately
100 mm
and a diameter of approximately 7.9 mm in diameter. The channel 124 may be
approximately 80 mm in length and the region 130 may be approximately 20 mm in
length.
The substrate 128 may be 10 mm or more in length. The wrap 134 may be
approximately 30
mm in length.
[0045] Referring to Fig. 3, the region 130a is an exemplary cellulose
acetate tip with a
pellet or capsule 140, such as a micro bead, that is stable and does not
release the active
agents therein until crushed or broken by finger pressure. The micro capsule
140 may be of
various sizes such as 3.5-5mm in diameter. The micro capsule 140 may be
arranged with its
center at approximately 15 mm from the second end 132.
[0046] The present disclosure provides a selection of volatile activators
of TRP channels
and acid sensitive ion channels which, when incorporated into a low nicotine
or non-nicotine
combustion or heating device, provide improved chemosensory mimicry of the
experience of
nicotine delivery versus prior low nicotine or non-nicotine delivery devices.
Importantly,
the compositions of this disclosure may impart chemosensory activity without
also providing
undesirable flavors.
[0047] Compositions of the disclosure include ingredients that are co-
delivered with
tobacco or botanical tobacco substitute smoke, or with vapor from tobacco or
botanical
tobacco substitute materials. There are two primary methods described in this
disclosure: 1)
incorporation of sensory agents in the tobacco or tobacco substitutes prior to
forming them
into cigarettes or heat-not-burn products ; or 2) inclusion of sensory agents
into the filter of a
cigarette such that they are released into the mainstream smoke during
inhalation. In this
latter embodiment, filter elements are optionally pellets or capsules that are
stable and do not
release the active agents until crushed or broken by finger pressure, or by
incorporating
segmented filter technology to embed a section to co-deliver ingredients.
[0048] Agents of the disclosure for simulating or amplifying throat or
airway sensations
associated with cigarette and herbal smoking or aerosolizing include sensate
constituents of
aframomum malagueta, including 6-paradol, of galangal root, including galangal
acetate and
7
CA 03037723 2019-03-20
WO 2018/057954 PCT/US2017/053052
its analogs, of hydroxy-alpha sanshool which is found in extracts of Szechuan
peppers, of
thymol which is found in extracts of Thyme, and of camphor and its analogs
which is found
in extracts of rosemary.
[0049] In one embodiment, a sensory agent, which optionally comprises
pulverized
aframomum melegueta seeds, ethanolic or methanolic extracts of the seeds, or
purified,
semi-purified or synthetic compounds found in the seeds, including 6-paradol,
are applied to,
or mixed with, tobacco or botanical tobacco substitute. The treated tobacco or
tobacco
substitute is then formed into a cigarette by standard manufacturing methods.
Similarly,
powdered galangal root, or ethanolic galangal extracts or purified or semi-
purified chemical
constituents are applied to tobacco or tobacco substitute and formed into
cigarettes.
[0050] In one embodiment, 6-paradol or galangal is combined in a cigarette,
which may
include the tobacco or botanical tobacco substitute, with an agent that
provides a cooling
effect by acting on TRPM8, a transient receptor potential channel mediating a
cooling
sensation evoked by agonists, including but not limited to menthol, physcool
(monomenthyl
succinate), icilin, geraniol, linalool, hydroxycitronellal, WS-3, WS-23,
PMD38, Cool-actP,
FrescolatMGA, FrescolatMA and PMD38. The cooling agent is optionally added to
the
tobacco and is delivered by combustion or volatilization in a heat-not-burn
device. In
another embodiment the cooling agent is incorporated into the cigarette
filter, either by
application to the filter itself, or by encapsulation in a crushable pellet or
capsule that
releases volatile cooling agent into mainstream smoke when broken by finger
pressure.
[0051] In one embodiment, 6-paradol or galangal is combined in a cigarette,
which may
include the tobacco or botanical tobacco substitute, with agents that provide
an additional
trigeminal sensory effect by acting on TRPA1 and TRPV1, transient receptor
potential
channels evoked by agonists, including but not limited to hydroxy-alpha
sanshool which is
found in extracts of Szechuan peppers, isothiocyanates which are found in
extracts of
Mustard Seed, Yellow Mustard, or combinations thereof. The TRPV1 and TRPA1
agonists
are optionally incorporated into the tobacco, as either a purified or semi-
purified compound,
a semi-purified extract, or as a simple ethanolic or methanolic extract of a
botanical material
of which the TRPV1 and TRPA1 agent is an endogenous constituent.
[0052] In one embodiment, 6-paradol or galangal is combined in a cigarette,
which may
include the tobacco or botanical tobacco substitute, with an agent that
provides an additional
trigeminal sensory effect by acting on TRPV3, a transient receptor potential
channel
mediating a warming sensation evoked by agonists, including but not limited to
carvacrol,
thymol, eugenol, eucalyptol, incensol, borneol, camphor, dihydrocarveol or
combinations
thereof. The TRPV3 agonist is optionally incorporated into the tobacco or
herbal cut rag, as
8
CA 03037723 2019-03-20
WO 2018/057954
PCT/US2017/053052
either a purified or semi-purified compound, a semi-purified extract, or as a
simple ethanolic
extract of a botanical material of which the TRPV3 agent is an endogenous
constituent.
[0053] In another embodiment, the TRPV1 and/or TRPV3 agents are
incorporated into
the cigarette filter, either by application to the filter itself, or by
encapsulation in a crushable
pellet or capsule that releases volatile cooling agent into mainstream smoke
when broken by
finger pressure. Many TRPV1 and/or TRPV3 agents, such as terpenoid compounds
are
volatile and therefore advantageous for delivery in the filter, entering
mainstream smoke
during its passage through the filter. Examples of TRPV1 agents that may be
included in the
filter and/or capsule include Szechuan pepper, all spice, mustard and
rosemary. Examples
of TRPV3 agents that may be included in the filter and/or capsule include
camphor,
carvacrol, thymol, and incensole acetate.
[0054] In one embodiment, a cooling agent and a TRPV3 activating agent are
combined
in a pellet or capsule in the filter of a cigarette, while a TRPA1 agonist,
including but not
limited to aframomum malagueta extract (or 6-paradol) or a galangal extract or
galangal
acetate) is incorporated into tobacco and released into mainstream smoke
during
combustion.
[0055] In one embodiment, a cooling agent and a TRPV1 activating agent are
combined
in a pellet or capsule in the filter of a cigarette, while a TRPA1 agonist,
including but not
limited to aframomum malagueta extract (or 6-paradol) or a galangal extract or
galangal
acetate) is incorporated into tobacco and released into mainstream smoke
during
combustion.
[0056] TRPV3 and TRPA1 activating agents may be more expensive and more
volatile
than TRPV1 activating agents. Therefore, inclusion of the TRPV3 and TRPA1
agents in a
pellet or capsule offers advantages in using less of the agent (more cost
effective) and
improving the shelf life of the product.
[0057] Example 1
[0058] Aframomum melegueta seeds (200 grams), and powdered galangal root,
or
ethanolic galangal extracts, were pulverized in a coffee grinder and extracted
through a
heated extraction process with ethanol for 3 hours. The extract was filtered
in three separate
steps. Using a syringe and needle, 0.1 ml was injected into a light cigarette,
withdrawing the
needle while dispensing the extract from the syringe down the length of the
tobacco column.
After being allowed to dry, the cigarette displayed an intensified throat
sensation, mimicking
a key element of the sensory impact of a stronger cigarette.
[0059] Example 2
9
CA 03037723 2019-03-20
WO 2018/057954 PCT/US2017/053052
[0060] Aframomum melegueta seeds (2.4 kg), thyme leaves (7.4 kg), rosemary
(6.6 kg),
and powdered galangal root (8.1 kg), were pulverized in a coffee grinder and
extracted
through a heated extraction process with 106 liters of methanol for 3 hours.
The extract was
filtered in three separate steps. This extract was applied to tea leaves as a
tobacco substitute.
Application was by fine spray over the non-tobacco herbal cut-rag which was
then mixed
and tumbled to coat evenly. The treated cut-rag was dried in a commercial
grade oven for 4
hours. Following the drying process, the cut-rag was sprayed with propylene
glycol to reach
a moisture level of approximately 18%. The non-tobacco herbal cut-rag was
manufactured
into cigarettes on a Hauni Protos high speed cigarette making line. The
cigarettes displayed
an intensified throat sensation, mimicking a key element of the sensory impact
of a nicotine
containing tobacco cigarette.
[0061] Example 3
[0062] Aframomum melegueta seeds (200 grams), and powdered galangal root,
or
ethanolic galangal extracts, were pulverized in a coffee grinder and extracted
through a
heated extraction process with methanol for 3 hours. The extract was filtered
in three
separate steps. This extract was applied to a very low nicotine (VLN) tobacco
cut-rag with
less than 0.04% nicotine. Application was by fine spray over the cut-rag which
was then
mixed and tumbled to coat evenly. The treated cut-rag was dried in a
commercial grade
oven for 4 hours. Following the drying process, the cut-rag was sprayed with
propylene
glycol to reach a moisture level of approximately 18%. The VLN cut-rag was
hand-rolled
into cigarettes, which displayed an intensified throat sensation, mimicking a
key element of
the sensory impact of a tobacco cigarette with regular level of nicotine
content.
[0063] Example 4
[0064] Aframomum melegueta seeds (200 grams), and powdered galangal root,
or
ethanolic galangal extracts, were pulverized in a coffee grinder and extracted
through a
heated extraction process with methanol for 3 hours. The extract was filtered
in three
separate steps. This extract was applied to a blend of herb and tea leaves as
a tobacco
substitute. Application was by fine spray over the cut-rag which was then
mixed and
tumbled to coat evenly. The treated cut-rag was dried in a commercial grade
oven.
Following the drying process, the cut-rag was cut and ground finely and
sprayed with
vegetable glycerin to reach a moisture level of approximately 18%. The moist
cut-rag was
then placed into a herbal vaporizer device, which upon heating displayed an
intensified
throat sensation, mimicking a key element of the sensory impact of a tobacco
vaporizer. The
composition of Example 4 is particularly advantageous for use in non-
combustion heat not
burn technology in which the composition is heated to release vapor or aerosol
but burning
CA 03037723 2019-03-20
WO 2018/057954
PCT/US2017/053052
and combustion is not required. The device may be a heat stick, for example a
shorter
cigarette type device including a tobacco blend. The device may also be a
reusable device
that accepts any type of cut rag or material in a heating chamber.
[0065] It will be appreciated that the described exemplary constituents are
not limiting
and the described constituents include equivalents such as synthetic
alternatives. It will also
be appreciated that description of constituents in compositions as by weight
or by volume is
merely exemplary and is not limiting. Constituents may be measured using any
of a variety
of available methods.
[0066] In addition, it will be appreciated that the above described devices
and
compositions are not limited to cigarette-like rods, but are also applicable
to other devices
such as inhalers that may be used to deliver the constituent(s). Also, it will
be appreciated
that the above described devices are applicable to applications beyond smoking
substitution
and smoking cessation.
[0067] While various embodiments in accordance with the disclosed
principles have
been described above, it should be understood that they have been presented by
way of
example only, and are not limiting. Thus, the breadth and scope of the
invention(s) should
not be limited by any of the above-described exemplary embodiments, but should
be defined
only in accordance with the claims and their equivalents issuing from this
disclosure.
Furthermore, the above advantages and features are provided in described
embodiments, but
shall not limit the application of such issued claims to processes and
structures
accomplishing any or all of the above advantages.