Note: Descriptions are shown in the official language in which they were submitted.
PHARMA-EN FO RMATI CS SYSTEM
10
BACKGROUND
The present Invention relates generally to medical apparatus and
methods. More specifically, the present invention relates to apparatus
and methods for automatic identification of ingestion or other actual,
physical
administration of a pharmaceutical material.
Prescription medications are effective remedies for many patients when
taken properly, e.g., according to instructions. However, studies have shown
that,
on average, about 50% of patients do not comply with prescribed medication
regimens. A low rate of compliance with medication regimens results in a large
number of hospitalizations and admissions to nursing homes every year. In the
United States -alone, it has recently been estimated that the. cost to the
resulting
from patient non-compliance is reaching $100 billion annually.
Consequently, various methods and apparatus have been made available
to improve patient compliance with prescribed regimens in efforts to improve
patient health. To date, many different types of "smart" packaging devices
have
been developed. In some cases, such devices automatically dispense the
appropriate pill. In other cases,. there are electronic controls that detect
and
record when the pill is taken out of the box.
CA 3041518 2019-04-26
2
While devices and protocols have been developed for improving patient
compliance, there is continued interest in the development of new ways of
monitoring patient compliance.
SUMMARY
The present invention allows, for the first time, the specific identification
of
pharmaceutical pills and other types of pharmaceutical delivery systems, such
as
skin diffusion patches, so that the actual, physical.delivery of the
pharmaceutical
into the body can be automatically detected and this information stored.
Because the inventive automatic reporting of physical drug administration does
not require patient or clinician input, it avoids many of the inaccuracies
which
introduce uncertainty in current drug administration monitoring systems. These
inventive features are particularly critical when a patient's compliance or
mental
capacity are a consideration, such as in the administration of psychotropic
drugs.
The present invention also allows for the identification of sources of illicit
drugs
for law enforcement purposes.
Embodiments af the invention include compositions having: an active
agent; an identifier and a pharmaceutically acceptable _carrier. In one
embodiment of the present invention, an ingestible pill is made identifiable
by
providing an electronic microchip as part of the pill structure. In some
aspects,
the electronic microchip is completely encased within the pill. In this
embodiment,
the pill broadcasts a signal when it is dissolved in an ionic solution such as
stomach fluids. The broadcasted signal is received by another device, e.g., a
receiver, either inside or near the body. In turn, the receiver then records
that the
pill has in fact reached the stomach and is in the process of being dissolved.
In certain of these embodiments, the signal is an oscillating signal which is
picked up by an implanted or topically applied receiver. The implant has one
or
two electrode(s) that sense the varying signal. The implant is configured so
that it
can identify the code and record that a specific pill has been ingested at a
specific time.
BRIEF DESCRIPTION OF THE FIGURES
FIG. 1 provides a diagrammatic, exemplary representation of the
pill embodiment of the present invention.
CA 3041518 2019-04-26
=
3
FIGS. 2A and 2B provide a more detailed view of the pill composition
shown in FIG. 1.
FIGS. 3A to 3C provide views of different embodiments of signal
generation elements of the invention.
FIG-. 4 shows diagrammatically the effects of the pill ingestion
where some of the pill has eroded away.
FIG. 6 provides a similar arrangement to FIG. 4, with a coil rather
than two electrodes as the output.
FIGS. 6A to 6D provide detail of certain implementations of
electronic circuits of various embodiments of the invention.
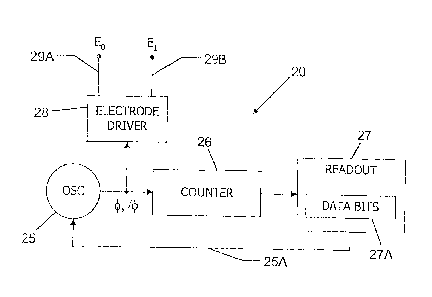
FIG. 7 provides an oscillator and a counter implementation
according to an embodiment of the invention.
FIG. 8 is an additional embodiment of an oscillator where V
control modulates the amount of voltage driving the oscillator.
FIG. 9 is an additional embodiment with a simple trickle or
asynchronous counter.
FIG. 10 provides a schematic representation of a three terminal,
monopole signal generation element according to an embodiment Of the
invention.
FIGS. 11A to 13B are diagrams showing a method for fabricating
an identifier according to an embodiment of the invention.
FIG. 14 shows the multiplexer and the addressing system.
FIG. 15 shows a detail of the 4 bit mux of the system shown in FIG. 14.
FIG. 16 shows the 1 bit mux in detail that makes up the 4 bit mux.
FIG. 17 is an additional monopole embodiment of a signal generation
element.
FIG. 18A is an exemplary schematic diagram of a signal-transmission
driver circuit that transmits a signal at a fixed frequency, in accordance
with one
embodiment of the present invention.
FIG. 18B1-18B2 provides an exemplary schematic diagram of a receiver
circuit, in accordance with one embodiment of the present invention.
FIG. 19 shows one exemplary split (i.e., segmented) battery design, in
accordance with one embodiment of the present invention.
CA 3041518 2019-04-26
4
FIG. 20 shows one exemplary design of the driver circuit that uses split
battery electrodes for transmission, in accordance with one embodiment of the
present invention.
FIG. 21 shows one exemplary split battery design with a split cathode, in
=
accordance with one embodiment of the present invention.
FIG. 22 shows one exemplary design where the battery electrodes for the
driver circuit are coupled to the driver circuit via two external wires, in
accordance
with one embodiment of the present invention.
FIG. 23 shows the principle of an experiment with a split battery
configuration.
FIG. 24 shows the performance of a pair of split batteries.
DETAILED DESCRIPTION
The present invention provides the clinician an important new tool in their
therapeutic armamentarium: automatic detection and identification of
pharmaceutical agents actually delivered into the body. The applications of
this
new information device and system are multi-fold. By 'example, when used in
concert with other medical sensing devices, correlation between drug delivery,
batch and dosage can be correlated to a physiological response. In this
manner,
optimal pharma-therapeutic regimens may be formulated by the clinician. By
example, cardiac stimulating drugs can be titrated to the most appropriate
dosages, minimizing side effects such as cardiac muscle exhaustion and rebound
effects among others, and optimizing both dosage and timing for each,
individual
patient.
Assessment of a range of alternate medications is made possible by the
present invention without resort to awaiting overt clinical sequel of
treatment,
many of which can be seriously adverse. By example, positive effects would be
quickly ascertainable without being obscured by more random factors. Negative
responses, such as changes in blood pressure, would become clearly evident as
drug related or independent above background physiologic variation.
The ability to document the ingestion of a drug or other actual exposure of
the body to a medication has many important clinical applications. In the
simplest
form, this technique provides accurate data of when a pill has been taken and
CA 3041518 2019-04-26
5
which pill has been taken. This allows the precise determination of which pill
was
taken at a specific point in time. Such monitoring capability assures patients
are
taking the prescribed medication correctly. This information avoids the
potential
for over prescription of medications that are not actually being taken. By
example,
if pain killers are intended to be administered to a patient, it is possible
to verify
that the patient did in fact take those pain killers in a certain period of
time. This is
an important tool in limiting the illicit sale of unconsumed drugs to an
unintended
party. In the case of cardio vascular pills, the clinician or care giver is
able to
verify that the amount of the drug was taken has been taken at approximately
the
right point and time. Thus, the true efficacy of the drug can be accurately
evaluated. Proper administration and patient compliance is especially critical
in
Alzheimer's, psychiatric, and alcohol aversion drugs, and in the treatment of
rest
home residents. In the case of accidental and other overdoses situations, the
intervening clinician will be able to discern how far the ingestion has
proceeded,
and how many pills are involved.
In one clinical arena, the present invention allows, in concert with other
sensing device developed by some of the present inventors, the measurement
and assessment of the cardiac response to those medications. These co-
employed sensing devices can be those enumerated below, among others.
Other sensing technology developed by some of the present inventors allows
measurement of heart health and cardiac efficiency. Using these tools in
concert
with the present inventive device, the clinician will be able to compare the
response of the heart and body to the administered pharmaceutical.
The data provided by the present invention can optionally be recorded
over time. The recording system records synchrony or conduction velocity of a
signal going through cardiac tissue and how that is mediated by the presence
of
a certain medication. This unique data is made possible by the present
invention
since it can determine electronically exactly when the pill or other
medication was
being absorbed into the body.
From this innovative data, the present invention provides the clinician an
accurate dose response curve showing the response to that medication and the
timing of the digestion of the pill. Such innovative data has many
applications.
For instance, the clinician now has the ability to determine which patients
have no
= response to the medicine in the pill. In a study situation, such patients
can be
CA 3041518 2019-04-26
6
removed from a study or a test of the clinical utility of a certain
medication. This
provides that only people who have a beneficial response to a certain
medication
are retained in the trail. This feature will improve the efficacy of
medications and
to reduce the amount of medications that people take that are not being
useful. It
may also be used in trials to determine which patients actually consumed the
medicine, and which did not.
In more standard clinical .environments, this unique data allows careful
selection and titration of drug administration without resort to more Vert
physical
symptoms to ascertain contraindications, efficacy, and optimal dosage levels.
The present invention provides a record for emergency room technicians or
. doctors
when a patient is admitted to a hospital so that the patient's status can be
accurately ascertained. Dosage events within the last hour or day prior to
admission, and the identity of the last medication, will be immediately
available.
The clinician obtains this information through simple interrogation of the
implanted or portable device. This device would tell them without any
uncertainty
what pills have been taken. As the inventive technology becomes more wide
spread, this data will become more regularly available. The present inventive
microchips described below are Sufficiently inexpensive when put into standard
production that most or all pharmaceuticals will be fitted with them as a
matter of
course.
In other embodiments of the inventive microchips, the chips can be fitted
with coils, susceptible of interrogation without being dissolved in the body.
This is
accomplished by transmitting RF energy into the coil in such a way that the
inquirer will be apprised of the presence and identity of a pill before it is
ingested.
In an additional embodiment of the present invention, a "smart box" is
provided that can interrogate each pill and ascertain its address. The box can
write a distinctive product number or product code so that every single pill
ever
made is provided with a unique identifier. Fuses, for example, may be
selectively
destroyed so the addresses may be detected electrically or optically.
Particularly
in the case of controlled substances, such as a narcotic, this will be
important in
limiting the illegal used of previously legitimate medicines. The present
invention
makes it possible to identify precisely who bought such a pill from the
authorized
pharmacist. This use of the present invention will rein in the number of
illicit uses
of controlled substances on the market place.
CA 3041518 2019-04-26
7
In further describing the invention in greater detail, embodiments of the
compositions are reviewed first, followed by a discussion of systems including
the
subject compositiOns, methods of using the subject compositions and systems
and various illustrative applications in which the compositions and methods
find
use. Also reviewed in greater detail below are kits that include the subject
compositions.
COMPOSITIONS
Embodiments of the invention include active agent compositions having an
identifier stably associated therewith. In certain embodiments, the
compositions
are disrupted upon administration to a subject. As such, in certain
embodiments, the compositions are physically broken, e.g., dissolved,
degraded,
eroded, etc., following delivery to a body, e.g., via ingestion, injection,
etc. The
compositions of these embodiments are distinguished from devices that are
configured to be ingested and survive transit through the gastrointestinal
tract
substantially, if not completely, intact.' While the compositions of these
embodiments are themselves disrupted upon administration, components of the
composition, e.g., the identffier, may survive transit of the gastrointestinal
tract,
e.g., as described in greater detail below.
In certain embodiments, the compositions include an active agent/carrier
component and an identifier. Each of these different components are reviewed
separately in greater detail below.
Active Agent/Carrier Component
The subject compositions include an active agent/carrier component. By
"active agent/carrier component" is meant a composition, which may be a solid
or
fluid (e.g., liquid), which has an amount of active agent, e.g., a dosage,
present in
a pharmaceutically acceptable carrier. The active agent/carrier component may
be referred to as a "dosage formulation."
Active Agent
"Active agent" includes any compound or mixture of compounds which
produces a physiological result, e.g., a beneficial or useful result, upon
contact
CA 3041518 2019-04-26
8
with a living organism, e.g., a mammal, such as a human. Active agents are
distinguishable from such components as vehicles, carriers, diluents,
lubricants,
binders and other formulating aids, and encapsulating or otherwise protective
components. The active agent may be any molecule, as well as binding portion
or
fragment thereof, that is capable of modulating a biological process in a
living
subject. In certain embodiments, the active agent may be a substance used in
the diagnosis, treatment, or prevention of a disease or as a component of a
medication. In certain embodiments, the active agent may be a chemical
substance, such as a narcotic or hallucinogen, which affects the central
nervous
system and causes changes in behavior.
The active agent (i.e., drug) is capable of interacting with a target in a
living subject. The target may be a number of different types of naturally
occurring structures, where targets of interest include both intracellular and
extracellular targets. Such targets may be proteins, phospholipids, nucleic
acids
and the like, where proteins are of particular interest. Specific
proteinaceous
targets of interest include, without limitation, enzymes, e.g. kinases,
phosphatases, reductases, cyclooxygenases, proteases and the like, targets
comprising domains involved in protein-protein interactions, such as the SH2,
SH3, FIB and PDZ domains, structural proteins, e.g. actin, tubulin, etc.,
membrane receptors, immunoglobulins, e.g. IgE, cell adhesion receptors, such
as integrins, etc, ion channels, transmembrane pumps, transcription factors,
signaling proteins, and the like.
The active agent (i.e., drug) may include one or more functional groups
necessary for structural interaction with the target, e.g., groups necessary
for
hydrophobic, hydrophilic, electrostatic or even covalent interactions,
depending
on the particular drug and its intended target. Where the target is a protein,
the
drug moiety may include functional groups necessary for structural interaction
with proteins, such as hydrogen bonding, hydrophobic-hydrophobic interactions,
electrostatic interactions, etc., and may include at least an amine, amide,
sulfhydryl, carbonyl, hydroxyl or carboxyl group, such as at least two of the
functional chemical groups.
Drugs of interest may include cyclical carbon or heterocyclic structures
and/or aromatic or polyaromatic structures substituted with one or more of the
above functional groups. Also of interest as drug moieties are structures
found
CA 3041518 2019-04-26
9
among biomolecules, including peptides, saccharides, fatty acids, steroids,
purines, pyrimidines, derivatives, structural analogs or combinations thereof.
Such compounds may be screened to identify those of interest, where a variety
of
different screening protocols are known in the art.
The drugs may be derived from a naturally occurring or synthetic
compound that may be obtained from a wide variety of sources, including
libraries of synthetic or natural compounds. For example, numerous means are
available for random and directed synthesis of a wide variety of organic
compounds and biomolecules, including the preparation of randomized
oligonucleotides and oligopeptides. Alternatively, libraries of natural
compounds
in the form of bacterial, fungal, plant and animal extracts are available or
readily
produced. Additionally, natural or synthetically produced libraries and
compounds
are readily modified through conventional chemical, physical and biochemical
means, and may be used to produce combinatorial libraries. Known
pharmacological agents may be subjected to directed or random chemical
modifications, such as acylation, alkylation, esterffication, amidification,
etc. to
produce structural analogs.
As such, the drug may be obtained from a library of naturally occurring or
synthetic molecules, including a library of compounds produced through
combinatorial means, i.e., a compound diversity combinatorial library. When
obtained from such libraries, the drug moiety employed will have demonstrated
some desirable activity in an appropriate .screening assay for the activity.
Combinatorial libraries, as well as methods for producing and screening such
libraries, are known in the art and described in US Patent Nos: 5,741,713;
5,734,018; 5,731,423; 5,721 ,099; 5,708,153; 5,698,673; 5,688,997; 5,688,696;
5,684,711; 5,641 ,862; 5,639,603; 5,593,853; 5,574,656; 5,571,698; 5,565,324;
5,549,974; 5,545,568; 5,541 ,061 ; 5,525,735; 5,463,564; 5,440,016; 5,438,119;
5,223,409.
Broad categories of active agents of interest include, but are not limited to:
cardiovascular agents; pain-relief agents, e.g., analgesics, anesthetics, anti-
inflammatory agents, etc.; nerve-acting agents; chemotherapeutic (e.g., anti-
neoplastic) agents; etc.
In certain embodiments, the active agent is a cardiovascular agent, i.e., an
agent employed in the treatment of cardiovascular or heart conditions. In
certain
CA 3041518 2019-04-26
o
embodiments, the active agent is a cardiovascular agent, Le., an agent
employed
in the treatment of cardiovascular OF heart conditions. Cardiovascular agents
of
interest include, but are not limited to: cardioprotective agents, e.g.,
Zinecard
(dexrazoxane); blood modifiers, including anticoagulants (e.g., coumadin
(warfarin sodium), fragmin (dalteparin sodium), heparin, innohep (tinzaparin
sodium), lovenox (enoxaparin sodium), orgaran (danaparoid sodium))
antiplatelet
agents (e.g., aggrasta (tirofiban hydrochloride), aggrenox (aspirin/extended
release dipyridamole), agrylin (anagrelide hydrochloride), ecotrin
(acetylsalicylic
acid), folan (epoprostenol sodium), halfprin (enteric coated aspirin),
integrlilin
(eptifibatide), persantine (dipyridamole USP), plavix (clopidogrel bisulfate),
pieta!
(cilostazol), reopro (abciximab), ticlid (ticlopidine hydrochloride)),
thrombolytic
agents (activase (alteplase), retavase (reteplase), streptase
(streptokinase));
adrenergic blockers, such as cardura (doxazosin mesylate), dibenzyline
(phenoxybenzamine hydrochloride), hytrin (terazosin hydrochloride), minipress
(prazosin hydrochloride), minizide (prazosin hydrochloride/polythiazide);
adrenergic stimulants, such as aldoclor (methyldopa ¨ chforothiazide), aldomet
(methyldopa, methyldopate HCO, aldoril (methyldopa ¨ hydrochlorothiazide),
catapres (clonidine hydrochloride USP, clonidine), clorpres (clonidine
hydrochloride and chlorthalidone), combipres (clonidine hydrochloride/
chlorthalidone), tenex (guanfacine hydrochloride); alpha/bet adrenergic
blockers,
such as coreg (carvedilol), normodyne (labetalol hydrochloride); angiotensin
converting enzyme (ACE) inhibitors, such as accupril (quinapril
hydrochloride),
aceon (perindopril erbumine), altace (ramipril), captopril, lotensin
(benazepril
hydrochloride), mavik (trandolapril), monopril (fosinopril sodium tablets),
prinivil
(lisinopril), univasc (moexipril hydrochloride), vasotec (enalaprilat,
enalapril
maleate), zestril (lisinopril); angiotensin converting enzyme (ACE) inhibitors
with
calcium channel blockers, such as lexxel (enalapril maleate ¨ felodipine ER),
lotrel (amlodipine and benazepril hydrochloride), tarka
(trandolapril/verapamil
hydrochloride ER); angiotensin converting enzyme (ACE) inhibitors with
diuretics,
such as accuretic (quinapril HC1/hydroclorothiazide), lotensin (benazepril
hydrochloride and hydrochlorothiazide USP), prinizide
(lisinopril¨
hydrochlorothiazide), uniretic (moexipril hydrochloride/hydrochlorothiazide),
vaseretic (enalapril maleate ¨ hydrochlorothiazide), zestoretic (lisinopril
and
hydrochlorothiazide); angiotensin 11 receptor antagonists, such as atacand
CA 3041518 2019-04-26
11
(candesartan cilexetil), avapro (irbesartan), cozaar (Josartan potassium),
diovan
(valsartan), micardis (telmisartan), teveten (eprosartan rnesylate);
angiotensin 11
receptor antagonists with diuretics, such as avalide (irbesartan
hydrochlorothiazide), diovan (valsartan and hydrochlorothiazide), hyzaar
.. (losartan potassium ¨ hydrochlorothiazide); antiarrhythmics, such as Group
(e.g., mexitil (mexiletine hydrochloride, USP), norpace (disopyramide
phosphate),
procanbid (procainamide hydrochloride), quinaglute (quinidine gluconate),
quinidex (quinidine sulfate), quinidine (quinidine gluconate injection, USP),
rythmol (propafenone hydrochloride), tambocor (flecainide acetate), tonocard
.. (tocainide HCI)), Group II (e.g., betapace (sotalol HCI), brevibloc
(esmolol
hydrochloride), inderal (propranolol hydrochloride), sectral (acebutolol
hydrochloride)), Group III (e.g., betapace (sotalol
cordarone (amiodarone
hydrochloride), corvert (ibutilide fumarate injection), pacerone (amiodarone
HCI),
tikosyn (dofetilide)), Group IV (e.g., calan (verapamil hydrochloride),
cardizem
.. (diltiazem NCI), as well as adenocard (adenosine), lanoxicaps (digoxin),
lanoxin
(digoxin));
antilipemic acids, including bile acid sequestrants (e.g., colestid
(micronized colestipol hydrochloride), welchol (colesevelam hydrochloride));
fibric
acid derivatives (e.g., atromid (clofibrate), lopid (gemfibrozal tablets,
USP), tricor
(fenofibrate capsules)), FINIG-CoA red uctase inhibitors (e.g., baycol
(cerivastatin
.. sodium tablets), lescol (fluvastatin sodium), lipitor (atorvastatin
calcium), mevacor
(lovastatin), pravachol (pravastatin sodium), zocor (simvastatin)), Nicotinic
Acid
(e.g., Niaspan (niacin extended release tablets)); beta adrenergic blocking
agents, e.g., betapace (sotalol NCI), blocadren (timolol maleate), brevibloc
(esmolol hydrochloride), cartrol (carteolol hydrochloride), inderal
(propranolol
hydrochloride), kerlone (betaxolol hydrochloride), nadolol, sectral
(acebutolol
hydrochloride), tenormin (atenolol), toprol (metoprolol succinate), zebeta
(bisoprolol fumarate); beta adrenergic blocking agents with diuretics, e.g.,
corzide
(nadolol and bendroflumethiazide tablets), inderide (propranolol hydrochloride
and hydroclorothiazide), tenoretic (atenolol and chlorthalidone), timolide
(timolol
.. maleate ¨ hydrochlorothiazide), ziac (bisoprolol fumarate and hydrochloro-
thiazide); calcium channel blockers, e.g., adalat (nifedipine), calan
(verapamil
hydrochloride), cardene (nicardipine hydrochloride), cardizem (diltiazem NCI),
covera (verapamil hydrochloride), isoptin (verapamil hydrochlnride), nimotop
= (nimodipine), norvasc (amlodipine besylate), plendil (felodipine),
procardia
CA 3041518 2019-04-26
12
(niredipine), sular (nisoldipine), tiazac (diltiazem hydrochloride), vascor
(bepridil
hydrochloride), verelan (verapiimil hydrochloride); diuretics, including
carbonic
anhydrase inhibitors (e.g., daranide (dichlorphenamide)), combination
diuretics
(e.g., aldactazide (spironolactone with hydrochlorothiazide), dyazide
(triamterene
arid hydrochlorothiazide), maxzicle (triamterene and hydrochlorothiazide),
moduretic (amiloride HCI hydrochlorothiazide)), loop diuretics (demadex
(torsemide), edecrin (ethacrynic acid, ethacrynate sodium), furosemide),
potassium-sparing 'diuretics (aldactone (spironolactone), dyrenium
(triamterene),
midamor (amiloride NCI)), thiazides & related diuretics (e.g., diucardin
(hydroflumethiazide), diuril (chlorothiazide, chlorothiazide sodium), enduron
(methyclothiazide), hydrodiuril hydrochlorothiazide), indapamide, microzide
(hydrochlorothiazide) mykrox (metolazone tablets), renese (polythi-azide),
thalitone (chlorthalidone, USP), zaroxolyn (metolazone)); inotropic agents,
e.g.,
. digitek (digoxin), dobutrex (dobutamine), lanoxicaps (digoxin), lanoxin
(digoxin),
primacor (milrinone'lactate); activase (alteplase recombinant); adrenaline
chloride
(epinephrine injection, USP); demser (metyrosine), inversine (mecamylamine
HCI), reopro (abciximab), retavase (reteplase), streptase (streptokinase),
tnkase
(tenecteplase); vasodilators, including coronary vasodilators (e.g., imdur
(isosorbide mononitrate), ismo (isosorbide mononitrate), isordil (isosorbide
dinitrate), nitrodur (nitroglycerin), nitrolingual (nitroglycerin lingual
spray), nitrostat
(nitroglycerin tablets, USP), sorbitrate (isosorbide dinitrate)), peripheral
vasodilators & combinations (e.g., corlopam (fenoldopam mesylate), fiolan
(epoprostenol sodium), primacor (milrinone lactate)), vasopressors, e.g.,
aramine
(metaraminol bitartrate), epipen (EpiPen 0.3 mg brand of epinephrine auto
injector, EpiPen Jr. 0.15 mg brand of epinephrine auto injector), proamatine
(midodrine hydrochloride); etc.
In certain embodiments, specific drugs of interest include, but are not
limited to: psychopharmacological agents, such as (1) central nervous system
depressants, e.g. general anesthetics (barbiturates, benzodiazepines,
steroids,
cyclohexanone derivatives, and miscellaneous agents), sedative-hypnotics
(benzodfazepines, barbiturates, piperidinediones and triones, quinazoline
derivatives, carbamates, aldehydes and derivatives, amides, acyclic ureides,
benzazepines and related drugs, phenothiazines, etc.), central voluntary
muscle
tone modifying drugs (anticonvulsants, such as hydantoins, barbiturates,
CA 3041518 2019-04-26
13
oxazolidinediones, succinimides, acylureides, glutarimides, benzodiazepines,
secondary and tertiary alcohols, dibenzazepine derivatives, valproic acid and
derivatives, GABA analogs, etc.), analgesics (morphine and derivatives,
oripavine
derivatives, morphinan derivatives, phenylpiperidines, 2,6-methane-3-
.. benzazocaine derivatives, diphenylpropylamines and isosteres, salicylates,
p-
aminophenol derivatives, 5-pyrazolone derivatives, arylacetic acid
derivatives,
fenamates and isosteres, etc.) and antiemetics (anticholinergics,
antihistamines,
antidopaminergics, etc.), (2) central nervous system stimulants, e.g.
analeptics
(respiratory stimulants, convulsant stimulants, psychomotor stimulants),
narcotic
antagonists (morphine derivatives, oripavine derivatives, 2,6-methane-3-
benzoxacine derivatives, morphinan derivatives) nootropics, (3)
psychopharmacologicals, e.g. anxiolytic sedatives (benzodiazepines,
propanediol
carbamates) antipsychotics (phenothiazine derivatives, thioxanthine
derivatives,
other tricyclic compounds, butyrophenone derivatives and isosteres,
diphenylbutylamine derivatives, substituted benzam ides,
arylpiperazine
derivatives, indole derivatives, etc.), antidepressants (tricyclic compounds,
MAO
inhibitors, etc.), (4) respiratory tract drugs, e.g. central antitussives
(opium
alkaloids and their derivatives);
pharmacodynamic agents, such as (1) peripheral nervous system drugs,
e.g. local anesthetics (ester derivatives, amide derivatives), (2) drugs
acting at
synaptic or neuroeffector junctional sites, e.g. cholinergic agents,
cholinergic
blocking agents, neuromuscular blocking agents, adrenergic agents,
antiadrenergic agents, (3) smooth muscle active drugs, e.g. spasmolytics
(anticholinergics, musculotropic spasmolytics), vasodilators, smooth muscle
Stimulants, (4) histamines and antihistamines, e.g. histamine and derivative
thereof (betazole), antihistamines (1-11-antagonists, H2-antagonists),
histamine
metabolism drugs, (5) cardiovascular drugs, e.g. cardiotonics (plant extracts,
butenolides, pentadienolids, alkaloids from erythrophleum species, ionophores,
-
adrenoceptor stimulants, etc), antiarrhythmic drugs, antihypertensive agents,
antilipidemic agents (clofibric acid derivatives, nicotinic acid derivatives,
hormones and analogs, antibiotics, salicylic acid and derivatives),
antivaricose
drugs, hemostyptics, (6) blood and hemopoietic system drugs, e.g. antianemia
drugs, blood coagulation drugs (hemostatics, anticoagulants, antithrombotics,
thrombolytics, blood proteins and their fractions), (7) gastrointestinal tract
drugs,
CA 3041518 2019-04-26
14
e.g. digestants (stomachics, choleretics), antiblcer drugs, antidiarrhcal
agcnts, (8)
locally acting drugs;
chemotherapeutic agents, such as (1) anti-infective agents, e.g.
ectoparasiticides (chlorinated hydrocarbons, pyrethins, sulfurated compounds),
anthelmintics, antiprotozoal agents, antimalarial agents, antiamebic agents,
antileiscmanial drugs, antitrichomonal agents, antitrypanosomal agents,
sulfonamides, antimycobacterial drugs, antiviral chemotherapeutics, etc., and
(2)
cytostatics, i.e. antineoplastic agents or cytotoxic drugs, such as alkylating
agents, e.g. Mechlorethamine hydrochloride (Nitrogen Mustard, Mustargen,
HN2), Cyclophosphamide (Cytovan, Endoxana), lfosfamide (IFEX), Chlorambucil
(Leukeran), Melphalan (Phenylalanine Mustard, L-sarcolysin, Alkeran, L-PAM),
Busulfan (Myleran), Thiotepa (Triethylenethiophosphoramide), Carmustine
(BiCNU, BCNU), Lomustine (CeeNU, CCNU), Streptozocin (Zanosar) and the
like; plant alkaloids, e.g. Vincristine (Oncovin), Vinblastine (Velban,
Velbe),
Paclitaxel (Taxol), and the like; antimetabolites, e.g. Methotrexate (MTX),
Mercaptopbrine (Purinethol, 6-MP), Thioguanine (6-TG), Fluorouracil (5-FU),
Cytarabine (Cytosar-U, Ara-C), Azacitidine (Mylosar, 5-AZA) and the like;
antibiotics, e.g. Dactinomycin (Actinomycin D, Cosmegen), Doxorubicin
(Adriamycin), Daunorubicin (duanomycin, Cerubidine), Idarubicin (Idamycin),
Bleomycin (Blenoxane), Picamycin (Mithramycin, Mithracin), Mitomycin
(Mutamycin) and the like, and other anticellular proliferative agents, e.g.
Hydroxyurea (Hydrea), Procarbazine (Mutalane), Dacarbazine (DTIC-Dome),
Cisplatin (Platinol) Carboplatin (Paraplatin), Asparaginase (Elspar) Etoposide
(VePesid, VP-16-213), Amsarcrine (AMSA, m-AMSA), .Mitotane (Lysodren),
Mitoxantrone (Novatrone), and the like;
antibiotics, such as: aminoglycosides, e.g. amikacin, apramycin,
arbekacin, bambermycips, butirosin, dibekacin, dihydrostreptomycin,
fortimicin,
gentamicin, isepamicin, kanamycin, micronomcin, neomycin, netilmicin,
paromycin, ribostamycin, sisomicin, spectinomycin, streptomycin, tobramycin,
trospectomycin; amphenicols, e.g. azidamfenicol, chloramphenicol, florfenicol,
and theimaphenicol; ansamycins, e.g. rifamide, rifampin, rifamycin,
rifapentine,
rifaximin; b-lactams, e.g. carbacephems, carbapenems, cephalosporins,
cehpamycins, monobactams, oxaphems, penicillins; lincosam ides, e.g.
clinamycin, lincomycin; macrolides, e.g. clarithromycin, dirthromycin,
CA 3041518 2019-04-26
15
erythrolnycin, etc.; polypeptides, e.g. amphornycin, bacitracin, capreomycin,
etc.;
tetracyclines, e.g. apicycline, chlortetracycline, clomocycline, etc.;
synthetic
antibacterial agents, such as 2,4-diaminopyrimidines, nitrofurans, quinolones
and
analogs thereof, sulfonamides, sulfones;
antifungal agents, such as: polyenes, e.g. amphotericin B, candicidin,
dermostatin, filipin, fungichromin, hachimycin, hannycin, lucensomycin,
mepartricin, natamycin, nystatin, pecilocin, perimycin; synthetic antifungals,
such
as allylamines, e.g. butenafine, naftifine, terbinafine; imidazoles, e.g.
bifonazole,
butoconazole, chlordantoin, chlormidazole, etc., thlocarbamates, e.g.
tolciclate,
triazoles, e.g. fluconazole, itraconazole, terconazole;
anthelmintics, such as: arecoline, aspidin, aspidinol, dichlorophene,
embelin, kosin, napthalene, niclosamide, pelletierine, quinacrine,
alantolactone,
amocarzine, amoscanate, ascaridole, bephenium, bitoscanate, carbon
tetrachloride, carvacrol, cyclobendazole, diethylcarbamazine, etc.;
antimalarials, such as: acedapsone, amodiaquin, arteether, artemether,
artemisinin, artesunate, atovaquone, bebeerine, berberine, chirata,
chlorguanide,
chloroquine, chlorprogaunil, cinchona, cinchonidine, cinchonine, cycloguanil,
cientiopicrin, halofantrine, hydroxychloroquine, mefloquine hydrochloride, 3-
methylarsacetin, pamaquine, plasmocid, primaquine, pyrimethamine, quinacrine,
.. quinidine, quinine, quinocide, quinoline, dibasic sodium arsenate;
antiprotozoan agents, such as: acranil, tinidazole, ipronidazole,
ethylstibamine, pentamidine, acetarsone, aminitrozole, anisomycin, nifuratel,
tinidazole, benzidazole, suramin, and the like.
Name brand drugs of interest include, but are not limited to: RezulinO,
LovastatinTM, EnalaprilTM, RrozacTM, PrilosecTM, LipotorTM, ClaritinTM,
ZocorTM,
CiprofloxacinTM, ViagraTM, CrixivanTM, RitalinTM, and the like.
Drug compounds of interest are also listed in: Goodman & Gilman's, The
Pharmacological Basis of Therapeutics (9th Ed) (Goodman et al. eds) (McGraw-
Hill) (1996); and 2001 Physician's Desk Reference.
Specific compounds of interest also include, but are not limited to:
antineoplastic agents, as disclosed in U.S. Patent no.'s 5880161,
5877206, 5786344, 5760041, 5753668, 5698529, 5684004, 5665715, 5654484,
5624924, 5618813, 5610292, 5597831, 5530026, 5525633, 5525606, 5512678,
CA 3041518 2019-04-26
_
16
5508277, 5463181, 5409893, 5358952,5318965, 5223503, 5214068, 5196424,
5109024, 5106996, 5101072, 5077404, 5071848, 5066493, 5019390, 4996229,
4996206, 4970318, 4968800, 4962114, 4927828, 4892887, 4889859, 4886790,
4882334, 4882333, 4871746, 4863955, 4849563, 4845216, 4833145, 4824955,
4785085, 476925, 4684747, 4618685, 4611066, 4550187, 4550186, 4544501,
4541956, 4532327, 4490540, 4399283, 4391982, 4383994, 4294763, 4283394,
4246411, 4214089, 4150231, 4147798, 4056673, 4029661, 4012448;
psycopharmacologicalipsychotropic agents, as disclosed in U.S. Patent
no.'s 5192799, 5036070, 4778800, 4753951, 4590180, 4690930, 4645773,
4427694, 4424202, 4440781, 5686482, 5478828, 5461062, 5387593, 5387586,
5256664, 5192799, 5120733, 5036070, 4977167, 4904663, 4788188, 4778800,
4753951, 4690930, 4645773, 4631285, 4617314, 4613600, 4590180, 4560684,
4548938, 4529727, 4459306, 4443451, 4440781, 4427694, 4424202, 4397853,
.4358451, 4324787, 4314081, 4313896, 4294828, 4277476, 4267328, 4264499,
4231930, 4194009, 4188388, 4148796, 4128717, 4062858, 4031226, 4020072,
4018895, 4018779, 4013672, 3994898, 3968125, 3939152, 3928356, 3880834,
3668210;
cardiovascular agents, as disclosed in U.S. Patent no.'s 4966967,
5661129, 5552411, 5332737, 5389675, 5198449, 5079247, 4966967, 4874760,
4954526, 5051423, 4888335, 4853391, 4906634, 4775757, 4727072, 4542160,
4522949, 4524151, 4525479, 4474804, 4520026, 4520026, 5869478, 5859239,
5837702, 5807889, 5731322, 5726171, 5723457, 5705523, 5696111, 5691332,
5679672, 5661129, 5654294, 5646276, 5637586, 5631251, 5612370, 5612323,
5574037, 5563170, 5552411, 5552397, 5547966, 5482925, 5457118, 5414017,
5414013, 5401758, 5393771, 5362902, 5332737, 5310731, 5260444, 5223516,
5217958, 5208245, 5202330, 5198449, 5189036, 5185362, 5140031, 5128349,
5116861, 5079247, 5070099, 5061813, 5055466, 5051423, 5036065, 5026712,
5011931, 5006542, 4981843, 4977144, 4971984, 4966967, 4959383, 4954526,
4952692, 4939137, 4906634, 4889866, 4888335, 4883872, .4883811, 4847379,
4835157, 4824831, 4780538, 4775757, 4774239, 4771047, 4769371, 4767756,
4762837, 4753946, 4752616, 4749715, 4738978, 4735962, 4734426, 4734425,
4734424, 4730052, 4727072, 4721796, 4707550, 4704382, 4703120, 4681970,
4681882, 4670560, 4670453, 4668787, 4663337, 4663336, 4661506, 4656267,
4656185, 4654357, 4654356, 4654355, 4654335, 4652578, 4652576, 4650874,
CA 3041518 2019-04-26
17
4650797, 4649139, 4647585, 4647573, 4647565, 4647561, 4645836, 4639461,
4638012, 4638011, 4632931, 4631283, 4628095, 4626548, 4614825, 4611007,
4611006, 4611005, 4609671, 4608386, 4607049, 4607048, 4595692, 4593042,
4593029, 4591603, 4588743, 4588742, 4588741, 4582854, 4575512, 4568762,
4560698, 4556739, 4556675, 4555571, 4555570, 4555523, 4550120, 4542160,
4542157, 4542156, 4542155, 4542151, 4537981, 4537904, 4536514, 4536513,
4533673, 4526901, 4526900, 4525479, 4524151, 4522949, 4521539, 4520026,
4517188, 4482562, 4474804, 4474803, 4472411, 4466979, 4463015, 4456617,
4456616, 4456615, 4418076, 4416896, 4252815, 4220594, 4190587, 4177280,
4164586, 4151297, 4145443, 4143054, 4123550, 4083968, 4076834, 4064259,
4064258, 4064257, 4058620, 4001421, 3993639, 3991057, 3982010, 3980652,
3968117, 3959296, 3951950, 3933834, 3925369, 3923818, 3898210, 3897442,
3897441, 3886157, 3883540, 3873715, 3867383, 3873715, 3867383, 3691216,
3624126;
antimicrobial agents as disclosed in U.S. Patent no.'s 5902594, 5874476,
5874436, 5859027, 5856320, 5854242, 5811091, 5786350, 5783177, 5773469,
5762919, 5753715, 5741526, 5709870, 5707990, 5696117, 5684042, 5683709,
5656591, 5643971, 5643950, 5610196, 5608056, 5604262, 5595742, 5576341,
5554373, 5541233, 5534546, 5534508, 5514715, 5508417,.5464832, 5428073,
5428016, 5424396, 5399553, 5391544, 5385902, 5359066, 5356803, 5354862,
5346913, 5302592, 5288693, 5266567, 5254685, 5252745, 5209930, 5196441,
5190961, 5175160, 5157051, 5096700, 5093342, 5089251, 5073570, 5061702,
5037809, 5036077, 5010109, 4970226, 4916156, 4888434, 4870093, 4855318,
4784991, 4746504, 4686221, 4599228, 4552882, 4492700, 4489098, 4489085,
4487776, 4479953, 4477448, 4474807, 4470994, 4370484, 4337199, 4311709,
4308283, 4304910, 4260634, 4233311, 4215131, 4166122, 4141981, 4130664,
4089977, 4089900, 4069341, 4055655, 4049665, 4044139, 4002775, 3991201,
3966968, 3954868, 3936393, 3917476, 3915889, 3867548, 3865748, 3867548,
3865748, 3783160, 3764676, 3764677;
anti-inflammatory agents as disclosed in U.S. Patent no.'s 5872109,
5837735, 5827837, 5821250, 5814648, 5780026, 5776946, 5760002, 5750543,
5741798, 5739279, 5733939, 5723481, 5716967, 5688949, 5686488, 5686471,
5686434, 5684204, 5684041, 5684031, 5684002, 5677318, 5674891, 5672620
5665752, 5656661, 5635516, 5631283, 5622948, 5618835, 5607959, 5593980,
CA 3041518 2019-04-26
18
5593960, 5580888, 5552424, 5552422 5516764, 5510361, 5508026, 5500417,
5498405, 5494927 5476876 5472973 5470885, 5470842, 5464856, 5464849
54629527 5459151, 5451686, 5444043 5436265, 5432181, RE034918, 5393756,
5380738, 5376670, 5360811, 5354768, 5348957, 5347029, 5340815, 5338753,
5324648, 5319099, 5318971, 5312821, 5302597, 5298633, 5298522, 5298498,
5290800, 5290788, 5284949, 5280045, 5270319, 5266562, 5256680, 5250700,
5250552, 5248682, 5244917, 5240929, 5234939, 5234937, 5232939, 5225571,
5225418, 5220625, 5212189, 5212172, 5208250, 5204365, 5202350, 5196431,
5191084, 5187175, 5185326, 5183906, 5177079, 5171864, 5169963, 5155122,
5143929, 5143928, 5143927, 5124455, 5124347, 5114958, 5112846, 5104656,
5098613, 5095037, 5095019, 5086064, 5081261, 5081147, 5081126, 5075330,
5066668, 5059602, 5043457, 5037835, 5037811, 5036088, 5013850, 5013751,
5013736õ 500654, 4992448, 4992447, 4988733, 4988728, 4981865, 4962119,
4959378, 4954519, 4945099, 4942236,4931457, 4927835, 4912248, 4910192,
4904786, 4904685, 4904674, 4904671, 4897397, 4895953, 4891370, 4870210,
4859686, 4857644, 4853392, 4851412, 4847303, 4847290, 4845242, 4835166,
4826990, 4803216, 4801598, 4791129, 4788205, 4778818, 4775679, 4772703,
4767776, 4764525, 4760051, 4748153, 4725616, 4721712, 4713393, 4708966,
4695571, 4686235, 4686224, 4680298,4678802, 4652564, 4644005, 4632923,
4629793, 4614741, 4599360, 4596828, 4595694, 4595686, 4594357, 4585755,
4579866, 4578390, 4569942, 4567201, 4563476, 4559348, 4558067, 4556672,
4556669, 4539326, 4537903, 4536503, 4518608, 4514415, 4512990, 4501755,
4495197, 4493839, 4465687, 4440779, 4440763, 4435420, 441.2995, 4400534,
4355034, 4335141, 4322420, 4275064, 4244963, 4235908,4234593, 4226887,
4201778, 4181720, 4173650, 4173634, 4145444, 4128664, 4125612, 4124726,
4124707, 4117135, 4027031, 4024284, 4021553, 4021550, 4018923, 4012527,
4011326, 3998970, 3998954, 3993763, 3991212, 3984406, 3978227, 3978219,
3978202, 3975543, 3968224, 3959368, 3949082, 3949081, 3947475, 3936450,
3934018, 3930005, 3857955, 3856962, 3821377, 3821401, 3789121, 3789123,
3726978, 3694471, 3691214, 3678169, 3624216;
immunosuppressive agents, as disclosed in U.S. Patent no.'s 4450159,
4450159, 5905085, 5883119, 5880280, 5877184, 5874594, 5843452, 5817672,
5817661, 5817660, 5801193, 5776974, 5763478, 5739169, 5723466, 5719176,
5696156, 5695753, 5693648, 5693645, 5691346, 5686469, 5686424, 5679705,
CA 3041518 2019-04-26
19
5679640, 5670504, 5665774, 5665772, 5648376, 5639455, 5633277, 5624930,
5622970, 5605903, 5604229, 5574041, 5565560, 5550233, 5545734, 5540931,
5532248, 5527820, 5516797, 5514688, 5512687, 5506233, 5506228, 5494895,
5484788, 5470857, 5464615, 5432183, 5431896, 5385918, 5349061, 5344925,
5330993, 5308837, 5290783, 5290772, 5284877, 5284840, 5273979, 5262533,
5260300, 5252732, 5250678, 5247076, 5244896, 5238689, 5219884, 5208241,
5208228, 5202332, 5192773, 5189042, 5169851, 5162334, 5151413, 5149701,
5147877, 5143918, 5138051, 5093338, 5091389, 5068323, 5068247, 5064835,
5061728, 5055290, 4981792, 4810692, 4410696, 4346096, 4342769, 4317825,
4256766, 4180588, 4000275, 3759921;
analgesic agents, as disclosed in U.S. Patent no.'s 5292736, 5688825,
5554789, 5455230, 5292736, 5298522, 5216165, 5438064, 5204365, 5017578,
4906655, 4906655, 4994450, 4749792, 4980365, 4794110, 4670541, 4737493,
4622326, 4536512, 4719231, 4533671, 4552866, 4539312, 4569942, 4681879,
4511724, 4556672, 4721712, 4474806, 4595686, 4440779, 4434175, 4608374,
4395402, 4400534, 4374139, 4361583, 4252816, 4251530, 5874459, 5688825,
5554789, 5455230, 5438064, 5298522, 5216165, 5204365, 5030639, 5017578,
5008264, 4994450, 4980365, 4906655, 4847290, 4844907, 4794110, 4791129,
4774256, 4749792, 4737493, 4721712, 4719231, 4681879, 4670541, 4667039,
4658037, 4634708, 4623648, 4622326, 4608374, 4595686, 4594188, 4569942,
4556672, 4552866, 4539312, 4536512, 4533671, 4511724, 4440779, 4434175,
4400534, 4395402, 4391827, 4374139, 4361583, 4322420, 4306097, 4252816,
4251530, 4244955, 4232018, 4209520, 4164514 4147872, 4133819, 4124713,
4117012, 4064272, 4022836, 3966944;
cholinergic agents, as disclosed in U.S. Patent no.'s 5219872, 5219873,
5073560, 5073560, 5346911, 5424301, 5073560, 5219872, 4900748, 4786648,
4798841, 4782071, 4710508, 5482938, 5464842, 5378723, 5346911, 5318978,
5219873, 5219872, 5084281, 5073560, 5002955, 4988710, 4900748, 4798841,
4786648, 4782071, 4745123, 4710508;
adrenergic agents, as disclosed in U.S. Patent no.'s 5091528, 5091528,
4835157, 5708015, 5594027, 5580892, 5576332, 5510376, 5482961, 5334601,
5202347, 5135926, 5116867, 5091528, 5017618, 4835157, 4829086, 4579867,
4568679, 4469690, 4395559, 4381309, 4363808, 4343800, 4329289, 4314943,
4311708, 4304721, 4296117, 4285873, 4281189, 4278608, 4247710, 4145550,
CA 3041518 2019-04-26
20
4145425, 4139535, 4082843, 4011321, 4001421, 3982010, 3940407, 3852468,
3832470;
antihistamine agents, as disclosed in U.S. Patent no.'s 5874479, 5863938,
5856364, 5770612, 5702688, 5674912, 5663208, 5658957, 5652274, 5648380,
5646190, 5641814, 5633285, 5614561, 5602183, 4923892, 4782058, 4393210,
4180583, 3965257, 3946022, 3931197;
steroidal agents, as disclosed in U.S. Patent no.'s 5863538, 5855907,
5855866, 5780592, 5776427, 5651987, 5346887, 5256408, 5252319, 5209926,
4996335, 4927807, 4910192, 4710495, 4049805, 4004005, 3670079, 3608076,
5892028, 5888995, 5883087, 5880115, 5869475, 5866558, 5861390, 5861388,
5854235, 5837698, 5834452, 5830886, 5792758, 5792757, 5763361, 5744462,
5741787, 5741786, 5733899, 5731345, 5723638, 5721226, 5712264, 5712263,
5710144, 5707984, 5705494, 5700793, 5698720, 5698545, 5696106, 5677293,
.
5674861, .5661141, 5656621, 5646136, 5637691, 5616574, 5614514, 5604215,
5604213, 5599807, 5585482, 5565588, 5563259, 5563131, 5561124, 5556845,
5547949, 5536714, 5527806., 5506354, 5506221, 5494907, 5491136, 5478956,
5426179, 5422262, 5391776, 5382661, 5380841, 5380840, 5380839, 5373095,
= 5371078, 5352809, 5344827, 5344826, 5338837, 5336686, 5292906, 5292878,
5281587, 5272140, 5244886, 5236912, 5232915, 5219879, 5218109, 5215972,
5212166, 5206415, 5194602, 5166201, 5166055, 5126488, 5116829, 5108996,
5099037, 5096892, 5093502, 5086047, 5084450, 5082835, 5081114, 5053404,
5041433, 5041432, 5034548, 5032586, 5026882, 4996335, 4975537, 4970205,
4954446, 4950428, 4946834, 4937237, 4921846, 4920099, 4910226, 4900725,
4892867, 4888336, 4885280, 4882322, 4882319, 4882315, 4874855, 4868167,
4865767, 4861875, 4861765, 4861763, 4847014, 4774236, 4753932, 4711856,
4710495, 4701450, 4701449, 4689410, 4680290, 4670551, 4664850, 4659516,
4647410, 4634695, 4634693, 4588530, 4567000, 4560557, 4558041, 4552871,
4552868, 4541956, 4519946, 4515787, 4512986, 4502989, 4495102.
Also of interest are analogs of the above compounds.
For all of the above active agents, the active agents may be present as
pharmaceutically acceptable salts.
As indicated above, the active agent of the compositions are typically
present in a pharmaceutically acceptable vehicle or carrier, e.g., as
described
CA 3041518 2019-04-26
21
below. In certain embodiments, the active agent is present in an amount of
from
about 0.1% to about 90% by weight, e.g., from about 1% to about 30% by weight
of the active compound.
Pharmaceutically Acceptable Carrier
As summarized above, the compositions of the invention further include a
pharmaceutically acceptable vehicle (i.e., carrier). Common carriers and
excipients, such as corn starch or gelatin, lactose, dextrose, sucrose,
microcrystalline cellulose, kaolin, mannitol, dicalcium phosphate, sodium
chloride,
and alginic acid are of interest. Disintegrators commonly used in the
formulations
of the invention include croscarmellose, microcrystalline cellulose, corn
starch,
sodium. starch glycolate and alginic acid.
A liquid composition may comprise a suspension or solution of the
compound or pharmaceutically acceptable salt in a suitable liquid carrier(s),
for
example, ethanol, glycerine, sorbitol, non-aqueous solvent such as
polyethylene
glycol, oils or water, with a suspending agent, preservative, surfactant,
wetting
agent, flavoring or coloring agent. Alternatively, a liquid formulation can be
prepared from a reconstitutable powder. For example, a powder containing
active
compound, suspending agent, sucrose and a sweetener can be reconstituted
with water to form a suspension; and a syrup can be prepared from a powder
containing active ingredient, sucrose and a sweetener.
A composition in the form of a tablet or pill can be prepared using any
suitable pharmaceutical carrier(s) routinely used for preparing solid
compositions.
Examples of such carriers include magnesium stearate, starch, lactose,
sucrose,
microcrystalline cellulose and binders, for example, polyvinylpyrrolidone. The
tablet can also be provided with a color film coating, or color included as
part of
the carrier(s). In addition, active compound can be formulated in a controlled
release dosage form as a tablet comprising a hydrophilic or hydrophobic
matrix.
"Controlled release", "sustained release", and similar terms are used to
denote a mode of active agent delivery that occurs when the active agent is
released from the delivery vehicle at an ascertainable and controllable rate
over a
period of time, rather than dispersed immediately upon application or
injection.
Controlled or sustained release may extend for hours, days or months, and may
vary as a function of numerous factors. For the pharmaceutical composition of
CA 3041518 2019-04-26
22
the present invention, the rate of release will depend on the type of the
excipient
selected and the concentration of the excipient in the composition. Another
determinant of the rate of release is the rate of hydrolysis of the linkages
between
and within the units of the polyorthoester. The rate of hydrolysis in turn may
be
controlled by the composition of the polyorthoester and the number of
hydrolysable bonds in the polyorthoester. Other factors determining the rate
of
release of an active agent from the present pharmaceutical composition include
particle size, acidity of the medium (either.internal or external to the
matrix) and
physical and chemical properties of the active agent in the matrix.
A composition in the form of a capsule can be prepared using routine
encapsulation procedures, for example, by incorporation of active compound and
excipients into a hard gelatin capsule. Alternatively, a semi-solid matrix of
active
compound and high molecular weight polyethylene glycol can be prepared and
filled into a hard gelatin capsule; or a solution of active compound in
polyethylene
glycol or a suspension in edible oil, for example, liquid paraffin or
fractionated
coconut oil can be prepared and filled into a soft gelatin capsule.
Tablet binders that can be included are acacia, methylcellulose, sodium
carboxymethylcellulose, poly-vinylpYrrolidone (Povidone), hydroxypropyl methyl-
cellulose, sucrose, starch and ethylcellulose. Lubricants that can be used
include
magnesium stearate or other metallic stearates, stearic acid, silicone fluid,
talc,
waxes, oils and colloidal silica.
Flavoring agents such as peppermint, oil of wintergreen, cherry flavoring
or the like can also be used. Additionally, it may be desirable to add a
coloring
agent to make the dosage form more attractive in appearance or to help
identify
the product.
The compounds of the invention and their pharmaceutically acceptable
salts that are active when given parenterally can be formulated for
intramuscular,
intrathecal, or intravenous administration.
A typical composition for intramuscular or intrathecal administration will be
of a suspension or solution of active ingredient in an oil, for example,
arachis oil
or sesame oil. A typical composition for intravenous or intrathecal
administration
will be a sterile isotonic aqueous solution containing, for example, active
ingredient and dextrose or sodium chloride, or a mixture of dextrose and
sodium
chloride. Other examples are lactated Ringer's injection, lactated Ringer's
plus
CA 3041518 2019-04-26
23
dextrose injection, Normosol-M and dextrose, lsolyte E, acylated Ringer's
injection, and the like. Optionally, a co-solvent, for example, polyethylene
glycol,
a chelating agent, for example, ethylenediamine tetraacetic acid, and an anti-
oxidant, for example, sodium metabisulphite may be included in the
formulation.
Alternatively, the solution can be freeze dried and then reconstituted with a
suitable solvent just prior to administration..
The compounds of the invention and their pharmaceutically acceptable
salts which are active on rectal administration can be formulated as
suppositories. A typical suppository formulation will generally consist of
active
ingredient with a binding and/or lubricating agent such as a gelatin or cocoa
butter or other low melting vegetable or synthetic wax or fat.
The compounds of this invention and their pharmaceutically acceptable
salts which are active on topical administration can be formulated as
transdermal
compositions or transdermal delivery devices ("patches"). Such compositions
include, for example, a backing, active compound reservoir, a control
Membrane,
liner and contact adhesive. Such transdermal patches may be used to provide
continuous or discontinuous infusion of the compounds of the present invention
in
controlled amounts. The construction and use of transdermal patches for the
delivery of pharmaceutical agents is well known in the art. For example, see
U.S.
Pat. No. 5,023,252, Such patches
may be constructed for continuous, pulsatile, or on demand delivery of
pharmaceutical agents.
Optionally, the pharmaceutical composition may contain other
pharmaceutically acceptable components, such a buffers, surfactants,
antioxidants, viscosity modifying agents, preservatives and the like. Each of
these
components is well-known in the art. For example, see U.S. Pat. No. 5,985,310.
Other components suitable for use in the formulations of the present
invention can be found in Remington's Pharmaceutical Sciences, Mace
Publishing Company, Philadelphia, Pa., 17th ed. (1985).
Identifiers
= Also present in the subject compositions is an identifier. The identifier
may
vary depending on the particular embodiment and intended application of the
CA 3041518 2019-04-26
24
composition. In certain embodiments, the identifier is a component that emits
a
signal upon activation by a stimulus, e.g., by interrogation, .upon contact
with a
target physiological location, etc. As such, the identifier may be an
identifier that
emits a signal when it contacts a target body (i.e., physiological) site. In
addition
or alternatively, the identifier may be an identifier that emits a signal when
interrogated.
In yet other embodiments, the identifier is an inert, but identifiable marker,
e.g., an engraved identifier (such as one that is fabricated from a material
or
materials that survive digestion). This marker may then be identified, for
example,
following an autopsy or forensic examination. It is possible to provide a more
internal device within a pill to determine both that its surface has partially
been
subject to digestion, but also that the inner pill material has also been
digested.
This application is particularly useful in experimental pharmacological
settings.
The identifier of these embodiments is one that does not necessarily emit a
signal, but which can be optically inspected, e.g., visually or machine read,
to
obtain information about the composition with which it was associated prior to
administration.
While the identifier may be an identifier that does not emit a signal, in
certain embodiments (as summarized above) the identifier is one that does emit
a
signal. Depending on the needs of a particular application, the signal may be
a
generic signal, e.g., a signal that merely identifies that the composition has
contacted the target site, or a unique signal, e.g., a signal which in some
way
uniquely identifies that a particular composition from a group or plurality of
different compositions in a batch has contacted a target physiological site.
As
such, the identifier may be one that, when employed in a batch of unit
dosages,
e.g., a batch of tablets, emits a signal which 'cannot be distinguished from
the
signal emitted by the identifier of any other unit dosage member of the batch.
In
yet other embodiments, the identifier emits a signal that uniquely identifies
a
given unit dosage, even from other identical unit dosages in a given batch.
Accordingly, in certain embodiments the identifier emits a unique signal that
distinguishes a given type of unit dosage from other types of unit dosages,
e.g., a
given medication from other types of medications. In certain embodiments, the
identifier emits a unique signal that distinguishes a given unit dosage from
other
unit dosages of a defined population of unit dosages, e.g., a prescription, a
batch
CA 3041518 2019-04-26
25
or a lifetime production run of dosage formulations. In certain embodiments,
the
. identifier emits a signal that is unique, Le., distinguishable, from a
signal emitted
by any other dosage formulation ever produced, where such a signal may be
viewed as a universally unique signal (e.g., analogous to a human fingerprint
which is distinct from any other fingerprint of any other individual and
therefore
uniquely identifies an individual on a universal level). In one embodiment,
the
signal may either directly convey information about the composition, or
provide
an identifying code, which may be used to retrieve information about the
composition from a database, i.e., a database linking identifying codes with
compositions.
The identifier may be any component or device that is capable of
generating a detectable signal following activation in response to a stimulus.
In
certain embodiments, the stimulus activates the identifier to emit a signal
once
the composition comes into contact with a physiological target site, e.g., as
summarized above. For example, a patient may ingest a pill that upon contact
with the stomach fluids, generates a detectable signal. Depending on the
embodiment, the target physiological site or location may vary, where
representative target physiological sites of interest include, but are not
limited to:
a location in the gastrointestinal tract (such as the mouth, esophagus,
stomach,
small intestine, large intestine, etc.); another location inside the body,
such as a
parental location, vascular location, etc.; or a topical location; etc.
In certain embodiments the stimulus that activates the identifier is an
interrogation signal, such as a scan or other type of interrogation. In these
embodiments, the stimulus activates the identifier, thereby emitting a signal
which
is then received and processed, e.g., to identify the composition in some
manner.
In certain of these embodiments, the identifier may include a power source
that transduces broadcast power and a signal generating element that modulates
the amount of transduced power, such that a signal is not emitted from the
identifier but instead the amount of broadcast power transduced by the
identifier
is detected and employed as the "signal." Such embodiments are useful in a
variety of applications, such as applications where the history of a given
composition is of interest, e.g., as reviewed in greater detail below.
In certain embodiments, the identifier is dimensioned to be complexed with
the active agent/pharmaceutically acceptable carrier component of the
CA 3041518 2019-04-26
26
composition so as to produce a composition that can be readily adminitored to
a
subject in need thereof. As such, in certain embodiments, the identifier
element is
dimensioned to have a width ranging from about 0.05 mm to about 1 mm, such
as from about 0.1 mm to about 0.2 mm, a length ranging from about 0.05 mm to
about 1 mm, such as from about 0.1 mm to about 0.2 mm and a height ranging
from about 0.1 mm to about 1 mm, such as from about 0.05 mm to about 0.3 mm,
including from about 0.1 mm to about 0.2 mm. In certain embodiments the
identifier is 1 mm3 or smaller, such as 0.1 mm3 or &bailer, including 0.2 mm3
or
smaller. The identifier element may take a variety of different
configurations, such
as but not limited to: a chip configuration, a cylinder configuration, a
spherical
configuration, a disc configuration, etc, where a particular configuration may
be
selected based on intended application, method of manufacture, etc.
The identifier may generate a variety of different types of signals, including
but not limited, RF, magnetic, conductive (near field), acoustic, etc.
As is known in the art (see, e.g., JD. Jackson, Classical
Electrodynamics, 2nd Edition, pp. 394-396 (1975)), the electric (E) and
magnetic
(B) fields for radiation of an oscillating electric dipole antenna with an
angular
frequency a) and corresponding wave number k (where k = a)/c, with c being the
speed of light in the relevant medium) are given by the equations:
etkr ( 1
B k2 (n.xp) 1 ;and (1)
r ikr
.
E = k2 xp) xn ______________________ + [3n (Et = p) ¨ pi - ,
(2)
r
where n is a unit vector in the direction from the center of the dipole source
to a
location x at a distance r from the source, and p is a space-integrated
density of
electric charge given by p = fx'p(x) cr,x' .
As can be seen from Eqs. (1) and (2), in the "far field" region, where r>>
A. (where the wavelength
27c/k), the electric and magnetic fields are dominated
by terms that decrease with distance as 1/r. In
this region, mutually
perpendicular electric and magnetic fields feed off one another to propagate
the
signal through space. Where r,
the 1/r2 ("induction") terms in Eqs. (1) and (2)
become significant, and where 2\, r, an
additional quasi-electrostatic term that
varies as itr3 also becomes significant.
CA 3041518 2019-04-26 = =
27
Conventional RF communication takes place at distances r¨ 2 to r>>
For instance, implantable medical devices such as pacemakers typically
communicate in the 405-MHz frequency band, corresponding to wavelengths of
0.75 meters, somewhat smaller than the scale of a human body. As is known in
the art, higher frequencies are advantageously not used because structures
within the body begin to absorb radiation, leading to undesirable signal loss;
substantially lower frequencies (longer wavelengths) are generally regarded as
undesirable because much of the energy is redirected into the induction and/or
quasi-static field components rather than the far-field component that can be
sensed using conventional antennas. It should also be noted that RFID
applications with a transponder and a base unit typically use wavelengths such
that r¨ A, and generally rely on magnetic induction to transmit power from the
transponder to the base unit. In certain embodiments, these RF signals are
employed.
In contrast to these approaches, certain embodiments of the present
invention advantageously operate at wavelengths much larger than the human
body (2.>> 1 meter) to communicate information within the patient's body.
For instance, in some embodiments,
frequencies on the order of 100 kHz, corresponding to wavelengths of around 3
km (in air), are advantageously used. At distances rthat are short as compared
to the wavelength X, the quasi-static electric field term in Eqs. (1) and (2)
dominates, and thus the propagating signal is predominantlY electrical rather
than
electromagnetic. Such signals readily propagate in a conductive medium such as
the human body. For instance, at a frequency of 100 kHz and distances on the
order of 1-2 meters, the quasi-static (1/r3) component of Eq. (2) is estimated
to be
on the order of 106 times stronger than the far-field (1/r) component. Thus,
long-wavelength signaling using near-field coupling is efficient. Further,
because
the signals are required to travel relatively short distances (typically 2
meters OF
less), detectable signals can be transmitted using very small antennas.
A wide range of frequencies may be used for transmission of signals. In
some embodiments, the transmission frequency is within the "LF" band (low
frequency, defined as 30-300 kHz) of the RF spectrum, below the frequency
CA 3041518 2019-04-26 =
28
range of AM radio (around 500 to 1700 kHz). Within the LE band, the range from
160-190 kHz has been designated by the FCC for experimental use, with
specified upper limits on external signal strength. In embodiments of the
present
invention where the signals are largely confined within the patient's body as
described below, this experimental band can be used.
However, the invention is not limited to the 160-190 kHz band or to the
LF (30-300 kHz band). Lower bands may also be used; for instance, in the VLF
band (3-30 kHz, wavelengths of 10-100 km in air), signals can penetrate water
to=
a distance of 10-40 meters. Since the electrical properties of the human body
are
similar to those of salt water, it is expected that signals in this band would
also
readily propagate through the body. Thus, any frequency band corresponding to
a wavelength that is at least an order of magnitude larger than the human body
¨ e.g., 2\, ¨ 10 m or longer, or frequencies on the order of 30 MHz or below ¨
can be used. =
While there is no necessary lower limit on the frequency of signals used,
several practical considerations may affect the choice of frequency. For
instance,
it is well known that the human body carries low-level oscillating signals
induced
by nearby AC-powered devices, which operate at 60 Hz (US) or similar
frequencies in other parts of the world. To avoid interference caused by AC
electrical power systems, frequencies near 60 Hz are advantageously not used.
In addition, as is known in the art, longer wavelengths correlate with lower
information transfer rates, and the information-transfer capacity at long
wavelengths (e.g., below the 3 kHz-30kHz VLF band) may be too small for the
amount of information that is to be transferred in a particular system.
Further,
longer wavelengths generally require longer dipole antennas to produce a
detectable signal, and at some point the antenna size may become a limiting
factor in frequency selection.
' According to some embodiments of the invention, given a suitable choice
of frequency, a signal strong enough to travel to a receiver within the body
can be
.. generated using a very small antenna. For instance, 100 kHz signals
generated
by a dipole antenna just a few millimeters long can be propagated to a
receiver
antenna placed 1-2 meters away. This quasi-electrostatic transmission is
believed to be aided by the fact that the implanted antenna is directly in
contact
CA 3041518 2019-04-26
29
with a conductive medium, for example, the patient's tissues. For purposes of
analyzing electrical properties, human tissue can be approximated as an
electrolyte solution with electrical properties comparable to those of salt
water.
Thus, as in an electrolyte bath, the quasi-electrostatic field created by an
oscillating dipole antenna induces an oscillating current in the body. As a
result
of the inherent electrical resistivity of the body (comparable to salt water),
the
oscillating current creates oscillating potential variations within the body
that can
be sensed using a suitable receiver. (See, e.g., L.D. Landau et al. Electro-
dynamics of Continuous Media, Ch. 3 (1960)). Examples of suitable receivers
include the leads of a pacemaker, which create a dipole with an axis of about
20
cm or any other implanted wires with length from 10-100 cm.
It should be noted that these currents are undesirable in the context of
=
conventional RF communication, in which current flow in the near field leads
to
power loss in the far-field. In fact, many RF transmitters include devices
designed to minimize near-field current leakage. In near-field transmitters of
these embodiments of the present invention, maximizing such currents is
desirable.
Further, for quasi-electrostatic signals, the patient's skin advantageously
acts as a conductive barrier, confining the signals within the patient's body.
This
confines the signals within the body and also makes it difficult for stray
external
= signals to penetrate the body and create noise or interference in the
transmitted
signals. Confinement of the signals can mitigate, to some extent, the 1/r3
falloff of
the near-field signal, further reducing power requirements. Such effects have
been observed in the laboratory, e.g., in a salt water bath, in which the
water/air
interface acting as a conductive barrier. Similar effects have been observed
in
communicating with submarines via RF transmission in the ELF (3-30Hz) and
SLF (30-300 Hz) bands. These effects, have also been observed in sonar
communications;" although sonar uses acoustic., rather than electrical or
electromagnetic, fields to transmit information, the surface of the water acts
as a
conductive barrier for acoustic energy and mitigates the fall-off of signal
intensity
=
with distance.
As a result of these phenomena, a transmitter with a very small antenna
and a small power source are sufficient to create a near-field signal that is
detectable within the patient's body. For instance, the antenna can be formed
by
CA 3041518 2019-04-26
30
a pair of electrodes a few millimeters or less in length, spaced apart by a
few
millimeters, with oscillating voltages of opposite phase applied to create an
oscillating electric dipole. Such antennas can be disposed almost anywhere
within the body.
Further, in some embodiments, the frequency, transmitter antenna
length, and receiver antenna length are selected such that only microwatts of
power are required to produce a detectable signal, where conventional RF
communication (e.g., at around 405 MHz) would require at least milliwatts.
Accordingly, very compact power supplies that produce only small amounts of
power can be used; examples are described in Section IV below.
As such, depending on the particular embodiment of interest, the
frequency may range from about .1 Hz or lower to about 100 mHz or higher,
e.g.,
from about 1 kHz to about 70 mHz, including from about 5 kHz to about 200 kHz.
In certain embodiment, the signal that is emitted by the identifier is an
acoustic signal. in these embodiments, any convenient acoustic signal
generation
element may be present in the identifier, e.g., a piezoelectric element, etc.
The transmission time of the identifier may vary, where in certain
embodiments the transmission time may range from about 0.1 p.sec to about 4
hours or longer, such as from about 1 sec to about 4 hours. Depending on the
given embodiment, the identifier may transmit a signal once or transmit a
signal
two or more times, such that the signal may be viewed as a redundant signal.
In certain embodiments, the identifier may be one that is programmable
- following manufacture, in the sense that the signal generated by the
identifier
may be determined after the identifier is produced, where the identifier may
be
field programmable, mass programmable, fuse programmable, and even
reprogrammable. Such embodiments are of interest where uncoded identifiers
are first produced and following incorporation into a composition are then
coded
to emit an identifying signal for that composition. Any convenient programming
technology may be employed. In certain embodiments, the programming
technology employed is RFID technology. RFID smart tag technology of interest
that may be employed in the subject identifiers includes, but is not limited
to: that
described in U.S. Patent Nos. 7,035,877; 7,035,818; 7,032,822; 7,031,946, as
well as published application no. 20050131281, and the like,
CA 3041518 2019-04-26
31
With RFID or other smart tag
technology, a manufacturer/vendor may associate a unique ID code with a given
identifier, even after the identifier has been incorporated into the
composition. In
certain embodiments, each individual or entity involved in the handling of the
composition prior to use may introduce information into the identifier, e.g.,
in the
form of programming with respect to the signal emitted by the identifier,
e.g., as
described in U.S. Patent No. 7,031,946.
The identifier of certain embodiments includes a memory element, where
the memory element may vary with respect to its capacity. In certain
embodiments, the memory element has a capacity ranging from about 1 bit to 1
gigabyte or more, such as 1 bit to 1 megabyte, including from about 1 bit to
about
128 bit. The particular capacity employed may vary depending on the
application,
e.g., whether the signal is a generic signal or coded signal, and where the
signal
may or may not be annotated with some additional information, e.g., name of
active agent, etc.
Identifier components of embodiments of the invention have: (a) an
activation component and (b) a signal generation component, where the signal
generation component is activated by the activation component to produce an
identifying signal, e.g., as described above.
Activation Component
The activation component is a component that activates the signal
generation element to emit a signal upon experience of a stimulus, e.g.,
contact
of the .composition with a target physiological site of interest, such as the
stomach. The activation component may be configured to be activated in a
number of different ways. The following sections detail certain different ways
in
'which the identifier may be activated. As can be seen from the following
review,
the activation component may or may not be integrated with a power source,
e.g.,
a battery. Illustrative activation approaches include, but are not limited to:
Battery
Completion, e.g., Battery activated by electrolyte addition and Battery
activated
by cathode or anode addition; Battery connection, e.g., Battery activated by
= conductor addition; Transistor-mediated Battery Connection, e.g., Battery
activated by transistor gate, Geometry Modification, Detection of Geometry
CA 3041518 2019-04-26
32
Modification by Resonant Structure, Pressure Detection, Resonant Structure
Modification; etc. Each of these illustrative activation approaches is now
reviewed
in greater detail.
Battery Completion
Battery activated by electrolyte addition
In these embodiments, the battery includes, when completed, a cathode,
an anode, and an electrolyte. When the composition (e.g., pill) is
administered,
e.g., ingested, and travels through the esophagus, it proceeds to enter the
stomach. The cathode and anode provided within the composition do not
, constitute a full battery. However, as the composition dissolves to expose
the
cathode and anode, the stomach fluid acts as the electrolyte component of the
battery. The added component of the stomach fluid thus completes the battery.
Therefore, as the composition contacts the target site, e.g., by entering the
stomach and dissolving to the point of cathode and anode exposure, a power
source is provided which activates the identifier, e.g., in chip
configuration. The
data signal is then transmitted. This configuration is described in greater
detail
below, e.g., in terms FIG. 4.
Battery activated by cathode or anode addition
In an extension of this approach, the system is activated by having the
triggering event add a cathode or anode component, with the electrolyte being
intrinsic in the partial, pre-battery configuration. The battery is completed,
producing power and activating the composition, although not necessarily at
the
identical point of time.
Battery connection
Battery activated by conductor addition
In another embodiment of the present invention, the battery is connected
to the circuitry when it enters the stomach. The battery becomes
connected,.and
thus activates the identifier, by conductor addition. In this case, there is a
physically complete battery and a complete chip. When these two components
are awash in physiological fluid, such as in the stomach, they become
electronically connected. This triggering event electrically connects the
battery to
- the signaling microchip, thus activating the smart pill.
Transistor-mediated Battery Connection
CA 3041518 2019-04-26
33
Battery activated by transistor gate
Another design allowing the battery and the chip together to activate the
smart pill has the feature of a transistor gate between the battery and the
reporting chip. Once the transistor gate is switched on, such as by activation
with
the stomach, the reporting signal is transmitted.
There are numerous methods well known to the ordinary skilled artisan for
turning on a transistor gate. Most of them involve activating the gate by
closing a
switch, which can include a transistor switch or other types of switches.
The gate can be activated by applying a small gate current. This is how,
for xample, transistors are typically activated. The gate current can be
, generated in any number of ways well known to the ordinary skilled artisan .
= Any
circuitry which detects the presence of the pill in the environment of
interest, such
as the stomach, generates the gate current and turns the system on.
The gate current can be turned on by detecting a conductivity variation.
For instance, a circuit can be provided that detects a small change in the
conductivity of the stomach. While the stomach is conductive, the pill might
not
be. As a result, when the conductivity variation is detected, the transistor
gate is
activated, turning the smart pill on and generating a reporting signal.
The conductivity can be modulated by a .change in the solution
concentration. By example, the system detects a different solution
concentration
in the stomach in contrast to areas outside the stomach. The solution pH is
detected, by a modulation of the conductivity, which turns on the gate, and
turn
on the pill generating a reporting signal.
The stomach contains ionic conductive fluids. Those ionic conductive
fluids can be employed to modulate the conductivity of the gate and turn on
the
smart pill, generating a reporting signal. Individual enzymes can be detected
in
the stomach. For instance, a chem-FET can be employed that looks for the
pepsin content in the stomach, turning the pill on, thus reporting the
presence of
= the enzyme. =
Temperature change can also be detected using the innovations of the
present invention. The stomach is typically a steady 37 C. Areas outside the
stomach are more typically 20 C or less. When the pill enters the stomach and
becomes heated up, the pill is so designed that this adjusts the conductivity
and
turn the identifier on, generating a reporting signal.
CA 3041518 2019-04-26
34
The conductivity of the transistor can be modified by a microscopic
property called carrier mobility. A detection approach using this property
uses
the transistor itself as a detector. The carrier mobility is modulated by
temperature, a well-known phenomenon. In this manner, the transistor is used
as a temperature sensor by using that transistor to turn on the smart pill,
generating a reporting signal.
Another approach is to change the charge on the gate of a MOSFET
transistor. The gate charge can be modulated by the factors to be detected.
This
is again a configuration using the transistor to turn on the circuit,
generating a
reporting signal.
In another configuration, the gate charge is modulated by a material to be
detected in the solution. A specific ion would preferentially change the gate
charge. This system is modulated by a crystal potential. A crystal potential
occurs when crystals generate electric fields under certain circumstances.
The electric field can change the charge on the gate, turning on the
transistor and generating a reporting signal. This change may be modulated by
a
chemical potential, resulting from an osmotic or ionic process. This causes
charge to accumulate on the gate, thereby, turning it on and generating a
reporting signal.
A change in the electrical potential can also cause a reporting signal using
a variety potentials. For example, a gravitational potential can detect the
change
in height of the detector. In the case of a patient swallowing the pill, the
change
in pill height would indicate ingestion. -
In another embodiment, a transistor gate has associated with it a
capacitance. That capacitance is then modulated by certain properties peculiar
to the target site, e.g., the stomach.
In one case, the capacitance is changed by being enveloped in the
stomach. This effect on the capacitance is then detected. The gate charge is
modulated by change in the carrier concentration. The carrier concentration is
modulated by temperature. This approach provides a slightly different
approach,
but similar in concept to using the transistor as a temperature sensor as
above.
Geometry Modification
A transistor structure is also provided that has a geometry that changes.
Gate capacitance is determined by a change in geometry which occurs in the
CA 3041518 2019-04-26
35
stomach, detecting a change in capacitance. These changes can take place in a
variety of ways, for example, as further described below_
A variety of physiologic factors change the geometry. Pressure in the
stomach different than pressure outside occurs with the natural squeezing
during
the production of chyme, as well as at other times. This changes the gate
capacitance. The change is detected by having a dielectric on the gate. In
this
case, the gate consists of a number of layers, one of which is the dielectric.
In an additional embodiment, the enzymes of the stomach dissolve the
dielectric, changing the gate capacitance, which is then detected. Various
physical and chemical conditions within the stomach dissolve that gate
dielectric,
thereby activating the circuit.
Detection of Geometry Modification by Resonant Structure
A resonant structure on the gate is provided in other variants. In this case,
a mechanical structure is provided that has a characteristic frequency. This
frequency is excited by the triggering event, and measured. Various
interactions
with the stomach will cause a change in that resonance.
Pressure Detection
Gate capacitance and resonance with modulation source can also be
utilized for detection. In this case, an excitation is provided to the
resonance
structure from a modulation source, such as a sound wave. The gate
capacitance of that resonance structure can be used to detecting pressure
waves. A resonance structure sits out in the stomach and is hooked up to a
detection circuit on a transistor. In the stomach, the resonance circuit
detects
pressure waves.
Of pressure sound waves within the body, there are particular sounds that
are characteristic, such as the heart beat and respiration. These sounds are
detected and used to turn the circuit on.
Pressure waves are also detected by resonant Q factor modulation. Q
factor modulation can be accomplished in a number of different manners. The
resonance structure has two .components, a frequency and a Q factor. The Q
factor is modulated by detecting some environmental change.
Resonant Structure Modification
By example, the structure has a very different Q factor in air than it does in
the fluid of the stomach. Thus, the dampening can be detected by .the fluid
CA 3041518 2019-04-26
36
viscosity. Additionally, the structure can be configured to be eaten away by
the
acid or some of the enzymes in the stomach, which changes the cue.
Degradation by stomach acid or enzymes also changes the resonant
frequency. It is simple to detect the frequency shift of such a structure. The
frequency is shifted as this structure is changed in the stomach. There are
two
approaches to modifying the structure. A catabolic process can occur where the
structure gets dissolved, which is easily detectable. Also, an anabolic
process
would occur where an enzyme from the stomach binds to this structure, making
it
larger. This effect will also modify the resonance structure. The resonance
modification is detected either as a frequency change or a Q factor
modulation.
Battery Power Sources
As reviewed above, in certain embodiments, the activation element is a
power source that is turned on upon contact of the power source with a target
site, e.g., a physiological target site, such as the stomach, e.g., stomach
acid. In
certain embodiments, the power source is a battery that is turned on to
provide
power upon contact with the physiological target site, where the battery is
coupled to the signal generation component such that when the battery is
turned
on, the signal generation component emits the identifying signal.
In certain embodiments, the battery that is employed is one that comprises
two dissimilar materials which constitute the two electrodes of the battery.
In
certain embodiments, these two materials are shielded from the surrounding
environment by an additional layer of material. When the shielding material
(e.g.,
active agent/carrier matrix), is dissolved or eroded by the surrounding fluid,
the
electrode materials are exposed and come in contact with the body fluid, such
as
stomach acid or other types of electrolyte fluid. A potential difference, that
is, a
voltage, is generated between the electrodes as a result of the respective
oxidation and reduction reactions incurred to the two electrode materials. A
voltaic cell, or battery, can be thereby formed. Accordingly, in embodiments
of the
invention, such batteries are configured such that when the two dissimilar
materials are exposed to the target site, e.g., the stomach, the digestive
tract,
etc., during the physical and chemical erosion of the composition in which the
signal generation element is present, a voltage is generated. In such
embodiments, the power source described above is not a "battery" in the
CA 3041518 2019-04-26
= _
37
common sense of the word, but rather as defined in the discipline of physics.
The two dissimilar materials in an electrolyte are at different potentials,
similar to
the physics model of a 'potato battery'. As an example, copper and zinc when
put
into a cell have different potentials. Similarly, gold and magnesium have
different
.. potentials. As a result, a potential difference between the two dissimilar
materials
is generated.
Various battery-activation configurations are possible. Representative
types of cell-activation approaches include, but are not limited to:
activation by
presence of electrolyte, activation by presence of a cathode material,
activation
by presence of a conductive material.
After the battery is activated, further activation configurations can be
employed to activate the signal generation component. For example, the signal
generation component can be activated through the activation of the gate of a
metal oxide semiconductor (MOS) circuit, such as a CiVIOS switch. Activation
of
the gate of the MOS circuit can be based on one or more parameters, which
include but are not limited to: gate current, gate charge, and gate
capacitance.
The gate current, for activation purposes, can be a function of the
conductivity of surrounding body 'fluids or tissues. Such conductivity can
further
be a function of one or more parameters, which include but are not limited to:
solution concentration, solution pH value, ionic content of solution,
enzymatic
content of solution, temperature, and carrier mobility. Carrier mobility can
also be
a function of temperature. =
Similarly, the gate charge can be a function of one or more parameters,
which include but are not limited to: solution composition, crystal potential,
electrical potential, gravitational potential, gate capacitance, and carrier
concentration. The carrier concentration can also be a function of
temperature.
The gate capacitance can be a function of the capacitive geometry of the
gate, which can further be a function of pressure, a resonant input, Or the
characteristics of a dielectric material coupled to the gate. The
characteristics of
the dielectric material can vary with one or more parameters, which include
but
are not limited to: chemical contents of a digestive tract, chemical character
of a
physiological location, and amount of dissolution of the dielectric material
in body
fluids.
CA 3041518 2019-04-26
38
In certain embodiments, the battery is one that is made up of active
electrode materials, electrolyte, and inactive materials, such as current
collectors,
packaging, etc. The active materials are any pair of materials with different
electrochemical potentials. Suitable materials are not restricted to metals,
and in
certain embodiments the paired materials are chosen from metals and non-
metals, e.g., a pair made up of a metal (such as Mg) and a salt (such as Cu!).
With respect to the active electrode materials, any pairing of substances ¨
metals, salts, or intercalation compounds - with suitably different
electrochemical
potentials (voltage) and low interfacial resistance are suitable.
A variety of different materials may be employed as the battery electrodes.
In certain embodiment, electrode materials are chosen to provide for a voltage
upon contact with the target physiological site, e.g., the stomach, sufficient
to
drive the signal generation element of the identifier. In certain embodiments,
the
voltage provided by the electrode materials upon contact of the metals of the
power source with the target physiological site is 0.001 V or higher,
including 0.01
V or higher, such as 0.1 V or higher, e.g., 0.3 V or higher, including 0.5
volts or
higher, and including 1.0 volts or higher, where in certain embodiments, the
voltage ranges from about 0.001 to about 10 volts, such as from about 0.01 to
about 10 V.
Materials and pairings of interest include, but are not limited to those
reported in Table 1 below.
TABLE 1
Anode Cathode
Metals Magnesium, Zinc
Sodium (t), Lithium (t)
Iron
Salts Copper salts: iodide,
chloride, bromide,
sulfate, formate, (other anions possible)
Fe3+ salts: e.g. orthophosphate,
pyrophosphate, (other anions possible)
Oxygen (if) on platinum, gold or other
catalytic surfaces
Intercalation Graphite with Li, K, Ca, Vanadium oxide
compounds Na, Mg Manganese oxide
= CA 3041518 2019-04-26
39
t Protected anodes: tiertain high energy anode material such qs Li, Na, and
other alkali
metals are unstable in their pure form in the presence of water or oxygen.
These may
however be used in an aqueous environment if stabilized. One example of this
stabilization is
the so-called "protected lithium anode" developed by Polyplus Corporation
(Berkeley, CA),
where a polymer film is deposited on the surface of lithium metal to protect
it from rapid
oxidation and allow its use in aqueous environment or air ambient (Polyplus
has IP pending
on this).
ilDissolved oxygen can also serve as a cathode. In this case, the dissolved
oxygen in the
bodily fluids would be reduced to OH- at a suitable catalytic surface such at
Pt or gold. Other
catalysts are also possible.
in certain embodiments, one or both of the metals may be doped with a
non-metal, e.g., to enhance the voltage output of the battery. Non-metals that
may be used as doping agents in certain embodiments include, but are not
limited to: sulfur, iodine and the like.
In certain embodiments, the electrode materials are copper iodine (Cul) as
the anode and magnesium (Mg) as the cathode. Embodiments of the present
invention use electrode materials that are not harmful to the human body.
In certain embodiments, the batteries have a small form factor. Batteries
may be 10 mm3 or smaller, such as 1.0 mm3 or smaller, including 0.1 mm3 or
smaller, including 0.02 mm3 or smaller. As such, in certain embodiments, the
battery element is dimensioned to have a width ranging from about 0.05 mm to
about 1 mm, such as from about 0.1 mm to about 0.2 mm; a length ranging from
about 0.05 mm to about 1 mm, such as from about 0.1 mm to about 0.2 mm and
a height ranging from about 0.1 mm to about 1 mm, such as from about 0.05 mm
to about 0.3 mm, including from about 0.1 mm to about 0.2 mm.
As reviewed below, in certain embodiments the battery has a split or
segmented configuration.
In certain embodiments, the battery is one which is free of packaging. As
such, the electrodes are exposed and not protected by any protecting or
sealing
structure. As such, following removal of the active agent/carrier matrix
material
with which the battery may be associated, the battery per se does not itself
include an protective packaging such that the electrodes are free to contact
the
electrolyte at the target physiological location.
In certain of these embodiments, the battery power source may be viewed
as a power source that exploits reverse electrolysis in an ionic solution such
as
gastric fluid, blood, or other bodily fluids and some tissues. FIG. 4
illustrates an
identifier 30 having a signal generation element 40 powered by reverse
CA 3041518 2019-04-26
40
electrolysis. Signal generation element 40 is electrically connected to metal
electrodes 32 and 33, which are made of two different materials and are
electrically insulated from each other. When metal electrodes 32 and 33 are
immersed in an ionic solution 39, a potential difference develops between
them;
for instance, electrode 33 rises to a higher potential V+ while electrode 32
falls to
6 lower potential V.-. This potential difference can be used to power
circuitry 40.
Electrodes 32 and 33 can be implemented in various ways; for instance,
areas on opposing surfaces of an integrated circuit chip can be coated with
two
different metals, and the entire chip can be placed in the ionic solution.
Alternatively, electrodes 32 and 33 may extend away from element 40 as shown.
Other arrangements may also be used.
As illustrated above, electrodes 32 and 33 can be made of any two
materials appropriate to the environment in which the identifier 30 will be
operating. For
instance, in some embodiments where ionic solution 39
.. comprises stomach acids, electrodes 32 and 33 may be made of a noble metal
(e.g., gold, silver, platinum, palladium or the like) so that they do not
corrode
prematurely. Alternatively, the electrodes can be fabricated of aluminum or
any
other conductive material whose survival time in the applicable ionic solution
is
long enough to allow identifier 30 to perform its intended function.
Where the power s6urce is a battery, the battery may be fabricated in a
number of different ways. In certain embodiments, fabrication protocols which
may be categorized as "planar" processing protocols are employed, as developed
in greater detail below.
Additional Power Sources
Other sources, internal or external to the remote device, may also be
employed in addition to or instead of those described above. For example,
chemical or radioisotope batteries with a suitable form factor may be used to
power some remote devices. Recently-developed fuel cells that use blood as an
energy source can be miniaturized and used to provide electrical energy for a
low-power microchip. Piezoelectric crystals that convert mechanical energy
(e.g.,
compression) to electrical energy can be employed for remote devices disposed
where suitable mechanical forces can be brought to bear, such as in or around
the heart, stomach, joints, or other moving parts of the body. In yet other
CA 3041518 2019-04-26
41
=
embodiments, a power source modeled on the cellular energy factory, with power
being extracted from ATP in the blood so that blood, in ellect, "nourishes"
the
identifier,
employed_ In other embodiments, acoustic energy (e_g., ultrasound)
can be coupled into a remote device through piezoelectric or similar
converters.
In yet other embodiments, the activation element is not an on board power
source, but an element that is powered from a separate power source and
provides an activation signal to the signal generation component upon contact
of
the composition with the target site. For example, the activation element may
be
coupled to a power receiver which is configured to receive broadcast power and
transduce the broadcast power into a form suitable for driving the signal
generation element. In certain embodiments, the power receiver may be a coil.
Alternatively, the activator component may be powered by a distinct power
source, e.g., a sealed battery, a power element that converts mechanical
energy
of the pill into electrical power, e.g., a piezoelectric power element, etc.
As such,
the activator may or may not itself be the power source, and in those
embodiments where it is not the power source, the identifier may include a
distinct power source, such as receiver or power generator.
Signal Generation Component
The signal generation component of the identifier element is a structure
that, on
activation by the activation component, emits a detectable signal, e.g.,
that can be received by a receiver, e.g., as described in greater detail
below. The
signal generation component of certain embodiments can be any convenient
device that is capable of producing a detectable signal and/or modulating
transduced broadcast power, upon activation by the activation component.
Detectable signals of interest include, but are not limited to: conductive
signals,
acoustic signals, etc. As -reviewed above, the signals emitted by the signal
generator may be generic or unique signals, where representative types of
signals of interest include, but are not limited to: frequency shift coded
signals;
amplitude modulation signals; frequency modulation signals; etc.
In certain embodiments, the signal generation element includes circuitry,
as developed in more detail below, which produces or generates the signal. The
type of circuitry chosen may depend, at least in part, on the driving power
that is
supplied by the power source of the identifier. For example, where the driving
CA 3041518 2019-04-26
42
power is 1.2 volts or above, standard CMOS circuitry may be employed. In other
embodiments where the driving power ranges from about 0.7 to about 1.2 V, sub-
threshold circuit designs may be employed. For driving powers of about 0.7 V
or
less, zero-threshold transistor designs may be employed.
In certain embodiments, the signal generation component includes a
voltage-controlled oscillator (VCO) that can generate a digital clock signal
in
response to activation by the activation component. The VCO can be controlled
by a digital circuit, which is assigned an address and which can control the
VCO with a control voltage. This digital control circuit can be embedded onto
a
chip that includes the activation component and oscillator. Using amplitude
modulation or phase shift keying to encode the address, an identifying signal
is
transmitted.
The signal generation component may include a distinct transmitter
component that serves to transmit the generated signal to a remote receiver,
which may be internal or external to the patient, as reviewed in greater
detail
below. The transmitter component, when present, may take a number of different
configurations, e.g., depending on the type of signal that is generated and is
to be
emitted. In certain embodiments, the transmitter component is made up of one
or
more electrodes. In certain embodiments, the transmitter component is made up
of one or more wires, e.g., in the form of antenna(e). In certain embodiments,
the
transmitter component is made up of one or more coils. As such, the signal
transmitter may include a variety of different tranSmitters, e.g., electrodes,
antennas (e.g., in the form of wires) coils, etc. In certain embodiments, the
signal
is transmitted either by one or two electrodes or by one or two wires. A two-
electrode transmitter is a dipole; a one electrode transmitter forms a
monopole. In
certain embodiments, the transmitter only requires one diode drop of power.
In some embodiments, the transmitter unit uses an electric dipole or
electric monopole antenna to transmit signals. FIG. 6A illustrates a dipole
antenna. Oscillator 504 provides driving signals (c and an inverted signal
denoted herein as 1(1)) to an electrode driver 506. FIG. 6C is a circuit
diagram
showing details of a dipole electrode driver 600 implemented using
conventional
CMOS driver circuits. Electrode 602 is driven to a potential E0 by transistors
604,
606 in response to driving signal 4) while electrode 608 is driven to a
potential El
CA 3041518 2019-04-26
= ,
43
by transistors 610, 612 in response to inverted driving signal /4). Since
driving
signals 4 and 4 oscillate with opposite phase, potentials E0 and El also
oscillate
with opposite phase. It will be appreciated that driver 600 and all other
electronic
circuits described herein can be implemented using sub-micron CMOS
processing technologies known in the art; thus, the size of the circuitry is
not a
limiting factor on the size of a remote device.
In some embodiments, a monopole antenna can be substituted for the
dipole antenna of FIG. 6A. FIG. 6D illustrates a driver circuit for a monopole
antenna that can be implemented in conventional CMOS integrated circuits. This
antenna driver is generally similar to one half of the driver circuit of FIG.
6C, with
driver transistors 702, 704 driving a single electrode 706 to a potential Em
in
=
response to driving signal ch.
In either the dipole or monopole case, the driver circuit is powered by a
potential difference (AV) between terminals V+ and V-. This potential
difference,
which can be constant or variable, as desired.
FIG. 6A is a block diagram of a transmitter signal generation element
500 for an identifier according to an embodiment of the present invention. In
this
embodiment, generation element 500 receives a signal M from the activation
component which activates the signal generation element to produce and emit a
signal. Signal generation element 500 includes control logic 502, an
oscillator
504, an electrode driver 506, and an antenna 608 (in this instance, a pair of
electrodes operated as an electric dipole antenna). In operation, 'oscillator
504
generates an oscillating signal (waveform) in response to signals from control
logic 502. The signals from control logic 502 can start or stip the oscillator
and
in some embodiments can also shape one or more aspects of the oscillatory
signal such as amplitude, frequency, and/or phase. Oscillator 504 provides the
waveform to electrode driver 506, which drives current or voltage on antenna
508
to transmit a signal into the conductive medium of body tissues or fluids.
Depending on a given embodiment, the signal may or may not be
modulated. For example, in certain embodiments the frequency of the signal may
be held constant. In yet other embodiments, the signal may be modulated in
some manner, e.g., via carrier based modulate schemes, ultra-wide band (or
time
domain based) modulation schemes, etc.
CA 3041518 2019-04-26
44
Referring again to FIG. GA., in some embodiments, oscillator 504
operates at a constant frequency. The receipt of a constant-frequency signal
in
and of itself can provide useful information, e.g., that a remote device is
present
and operational. In some embodiments, oscillator 504 rnOdulates its signal to
encode additional information.
Information can be encoded in various ways, generally by modulating
(varying) some property of the transmitted signal, such as frequency,
amplitude,
phase, or any combination thereof. Modulation techniques known in the art may
be employed.
In general, information can be transmitted using analog or digital
techniques. "Analog techniques" refers generally to instances in which the
modulated property is varied in different degrees, with the degree of
variation
being correlated to a value representing the information to be transmitted.
For
instance, suppose that element 500 is transmitting a signal. Oscillator 504
can
be designed to operate over some range of frequencies. "Digital techniques"
refers generally to instances in which the information to be transmitted is
represented as a sequence of binary digits (bits), and the signal is modulated
based on the bit stream. For instance, suppose again that transmitter 500 is
transmitting a signal using digital techniques. Oscillator 504 can be designed
to
operate at at least two different frequencies, with one frequency
corresponding to
bit value 0 and another frequency corresponding to bit value 1. In embodiments
of the present invention, either analog techniques, digital. techniques, or a
combination thereof can be used to transmit information. In addition, various
types of modulation may be implemented.
For instance, in one embodiment, frequency modulation is used.
Oscillator 504 can be a voltage-controlled oscillator (VCO), an oscillator
circuit in
which the oscillation frequency depends on an applied voltage. Control logic
502
supplies an appropriate voltage (e.g., reflecting the value of the measurement
data, M), and the frequency of the signal indicates the value of the data. In
another embodiment, amplitude modulation is used; for instance, the amplitude
of
the driving signals (I) and /(1), can be varied, or the positive and negative
rails of the
driver circuit (e.g., V+ and V-) can be varied to control the amplitude. In
another
embodiment, phase modulation is used. For
instance, in digital signal
CA 3041518 2019-04-26
45
transmission, one phase corresponds to bit value 0, an opposite phase
corresponds to bit value 1, and the phase shifts represent transitions.
Oscillator
504 can include a switch circuit that either directly connects or cross-
connects the
driving signals (I) and 4 to the inputs of a driver circuit. Combinations of
frequency modulation, amplitude modulation, and/or phase modulation may also
be used as desired.
In some embodiments, the transmitter may transmit a "packet" that
includes a unique identifier for the identifier, which in turn is for the
composition
with which the identifier is associated. The unique identifier may also
provide
information from the remote device (e.g., the identity Of the active agent
(i.e.,
annotation information)). Other techniques for distinguishing different
signals
may also be used, including: operating different transmitters in different
frequency
bands, allowing each transmitter to be identified by its frequency and/or
configuring different transmitters to transmit at different (and known) times,
allowing the transmitter to be identified by when it transmits.
Additional Components
Depending on the particular embodiment, the identifier may include a
number of different additional components. Some components of interest
include,
but are not limited, those reviewed below.
Power Enhancers
Where the activator is a power source that is turned on upon contact with
a target physiological site, in certain embodiments, circuits for enhancing or
boosting voltage output of the power source, e.g., battery, are provided,
e.g.,
charge pumping circuits, charge doublers, etc. Such voltage enhancing elements
may enhance the voltage output by at about 2-fold or more, such as by about 5-
fold or more.
Power Storage
In certain embodiments, the activation component includes a power
storage element. For example, a duty cycle configuration may be employed,
e.g.,
where slow energy production from a battery is stored in a power storage
element, e.g., in a capacitor, which then provides a burst of power that is
deployed to the signal generation component. In certain embodiments, the
CA 3041518 2019-04-26
46
activation component includes a timing element which modulates, e.g., delays,
delivery of power to the signal generation element, e.g., so signals from
different
compositions, e.g., pills, that are administered at substantially the same
time are
produced at different times and are therefore distinguishable.
Additional Features
In certain embodiments, the compositions are characterized by having one
or more of the following features. In certain embodiments, the compositions
include an identifier which employs a conductive near-field mode of
communication in which the body itself is employed as a conductive medium. In
such embodiments, the compositions include circuitry that, when freed from the
composition upon disruption of the composition (e.g., as described above) the
circuitry comes into direct contact with the .body and does not remain
encapsulated or protected in some manner. In these embodiments, the signal is
not a magnetic signal or high frequency (RE) signal. In certain embodiments,
the
systems are ones that include a receiver which is stably associated with the
body, e.g., implanted or topically applied to an external location, such that
the
systems are distinguished from those in which an external device that is not
stably associated with the body is employed to collect data. In certain
embodiments, the compositions do not include an imaging system, e.g., camera
or other visualization .or imaging element, or components thereof, e.g., CCD
element, illumination element, etc. In certain embodiments, the compositions
do
not include a sensing element, e.g., for sensing a physiological parameter,
beyond the activator which detects contact with the targeted physiological
site. In
=
certain embodiments, the compositions do. not include a propulsion element. In
certain embodiments, the compositions do not include a sampling element, such
as a fluid retrieval element. In certain embodiments, the compositions do not
include an actuatable active agent delivery element, such as an element that
= retains an active agent with the composition until a signal is received
that causes
the delivery element to release the active agent.
IDENTIFIER FABRICATION
In certain embodiments of interest, the identifier element includes a
semiconductor support component. Any of a variety of different protocols may
be
CA 3041518 2019-04-26
47
employed in manufacturing the identifier structures and components thereof_
For
example, molding, deposition and material removal, e.g., planar processing
techniques, such as Micro-Electro-Mechanical Systems (MEMS) fabrication
techniques, including surface micromachining and bulk micromachining
techniques, may be employed. Deposition techniques that may be employed in
certain embodiments of fabricating the structures include, but are not limited
to:
electroplating, cathodic arc deposition, plasma spray, sputtering, e-beam
evaporation, physical vapor deposition, chemical vapor deposition, plasma
enhanced chemical vapor deposition, etc. Material removal techniques included,
but are not limited to: reactive ion etching, anisotropic chemical etching,
isotropic
chemical etching, planarization, e.g., via chemical mechanical polishing,
laser
ablation, electronic discharge machining (EDM), etc. Also of interest are
lithographic protocols. Of interest in certain embodiments is the use of
planar
processing protocols, in which structures are built up and/or removed from a
surface or surfaces of an initially planar substrate using a variety of
different
material removal and deposition protocols applied to the substrate in a
sequential
manner.
FIGS. 11A to 13B are diagrams showing a method for fabricating an
identifier according to an embodiment of the invention. Fig. 11A depicts a
cross-
section of a semiconductor wafer, 121, processed by silicon foundry such as
IBM
or Taiwan Semiconductor Manufacturing Company. The top surface of the wafer,
122, contains numerous electrical contact pads, 123, and an insulating
dielectric
layer, 124. The contact pads can be Al but could also be Cu, Ti, or similar
metal;
the dielectric may be a combination of SiO2 and Si3N4, but could be other
insulators. In the first process step, shown in FIG. 11B, wafer 121 has been
thinned from the back side via grinding or chemical/mechanical polishing to
reduce thickness to a desired thickness. A final thickness might be about 300
pm
but it can range from about 10 to about 1000 p.m such as from about 50 ¨ about
500 p.m.
FIG. 12A shows the second process step, in which a layer of corrosion
resistant metal, 125, has been added to the front side of the wafer to cover
the
electrical contacts, 123. The typical metal is platinum but one could also use
other corrosion resistant metals such as Au, Ti, In, or another phtinum group
. CA 3041518 2019-04-26
48
metal. The corrosion resistant metal may be deposited by physical vapor
deposition, For example, and may be from about 0.05 to about 100 um thick,
such
as from about 0.5 to about 5 ti..m thick. The metal 125 is formed into a
desired
pattern via photolithography and etching which are standard semiconductor
processing techniques.
FIG. 12B shows the deposition of the cathode material 126. Cathode
materials of interest include, but are not limited to: Cu or Cul, e.g., as
described
above. They are deposited by physical vapor deposition, electrodeposition, or
plasma deposition, among other protocols. The cathode may be from about 0.05
to about 500 pm thick, such as from about 5 to about 100 p.m thick. The
cathode
shape is controlled by shadow mask deposition, or photolithography and
etching.
Each chip may contain two or more regions, 127 and 127A, of cathode material
as desired.
Next anode material 128A is deposited as shown in FIG. 12C. Anode
materials of interest include, but are not limited: Mg, Zn, or other
electronegative
metals. Adhesion layer 128B may be necessary to help anode material to adhere
to the silicon. Typical adhesion layers for the anode are Ti, TiVV, Cr or
similar
material. Anode material and the adhesion layer may deposited by physical
vapor
deposition, electrodeposition or plasma deposition. the cathode may be from
about 0.05 to about 500 p.m thick, such as from about 5 to about 100 p.m
thick.
FIG. 13A shows the optional protection layer 129A which is deposited and
patterned. In some applications it may be advantageous to control the rate of
anode or cathode exposure to the electrolyte environment, so an insulating
layer
may be deposited and patterned in such a way that it has openings, 129B, of
limited size. This way the solution reaches the anode or cathode material at a
controlled rate. Fig. 13A illustrates the protection layer on the front
(cathode) side
of the wafer but it could be also deposited on backside (anode side) of wafer.
Typical materials for the protection layer are polyimide, or other photo
definable
polymer any of which may be spin coated or spray coated. Alternatively a
dielectric like SiO2, SIC, SiN may be deposited by physical vapor deposition
or
chemical vapor deposition. -
The wafer is then singulated into individual die 115, 116, 117 as shown in
FIG. 13B. Dicing can be accomplished by dicing with a diamond blade saw or by
CA 3041518 2019-04-26
49
reactive ion etching. These are standard silicon semiconductor processing
techniques. As reviewed above, the chip dimensions may vary. As such, in
certain embodiments, the chip (i.e., identifier) element is dimensioned to
have a
width ranging from about 0.05 mm to about 1 mm, such as from about 0.1 mm to
.. about 0.2 mm; a length ranging from about 0.05 mm to about 1 mm, such as
from
about 0.1 mm to about 0.2 mm and a height ranging from about 0.1 mm to about
1 mm, such as from about 0.05 mm to about 0.3 mm, including from about 0.1
mm to about 0.2 mm.
SPECIFIC PILL EMBODIMENTS
In further describing various embodiments of the compositions of the
invention, specific embodiments are now described in greater detail in view of
the
figures. FIG. 1 provides a diagrammatic, exemplary representation of a
pill/capsule embodiment of the present invention, in which the composition is
configured as an orally ingestible pharmaceutical formulation in the form of a
pill
or capsule. The stomach 12 of the patient 10 who ingests the composition 14 is
shown. This "smart pill" is shown as it has traveled from the mouth 16 to
inside 18
the patient's stomach. Upon reaching the stomach, the pill/capsule undergoes a
dissolving process with both the mechanical action of the stomach and the
various chemical materials in the stomach fluids, such as hydrochloric acid
and
other digestive agents.
FIGS. 2A and 2B provide a more detailed view of the pill composition
shown in FIG. 1. FIG. 2A illustrates an identifier 20 disposed inside a pill
14.
Identifier 20 is present as an integrated circuit (IC). The backside (bottom)
of
circuit 20 is at least partially coated with a first metal 21, and a portion
of the front
(top) of circuit 20 is coated with a different metal 22, allowing circuit 20
to be
powered by reverse electrolysis, e.g., as described above connection with FIG.
4.
Also on the top surface are two transmitter electrodes 23, 24.
When pill 14 is fabricated, the integrated circuit 20 is surrounded by at
least one external layer that may include pharmacologically active and/or
inert
materials in any combination. The external layer dissolves in the stomach
through
a combination of the mechanical action of the stomach and the action of
various
chemical constituents (e.g., hydrochloric acid) in stomach fluids.
CA 3041518 2019-04-26
50
As pill 14 is dissolved, areas of integrated circuit 20 become exposed to
the stomach contents, which for present purposes can be regarded as an
electrolyte solution. As dissolution of the pill exposes metal layers 21and
22,
power is supplied to circuit 20, which begins to operate and continues to
operate
until metal layers' 21and 22 or the circuit itself are sufficiently dissolved
by
digestive processes and acids to become non-functional. Eventually, the
remains
of the chip are excreted from the body.
In an alternative embodiment, the integrated circuit 20 is attached to,
rather than encapsulated in, the pill 14. For instance, circuit 20 might be
placed
at one end of the pill as the pill is being prepared, in a soluble coating on
the
surface of the pill, or the like. In embodiments where circuit 20 is wholly or
partially exposed, integrated circuit 20 begins to operate sooner after the
pill
enters the stomach rather than after the pill dissolves. =
. In one embodiment, circuit 20 transmits a signal identifying pill 14. The
identifier may indicate the type (active ingredient(s), brand, etc.) and/or
dosage of
pill 14 and may also provide a lot number, serial number, or similar
identifying
information that would allow particular pills to be traced, e.g., as reviewed
above.
FIG. 2B is a block diagram of one embodiment .of electronic circuit 20. In
this embodiment, circuit 20 is a transmitter unit that sequentially transmits
a
.. predetermined series of address (identifier) bits using frequency shift
keying, with
a first oscillation frequency corresponding to bit value 0 and a second
oscillation
frequency corresponding to bit value 1. As described above, metal layers 21
and
22 supply power to circuit 20. The power (not explicitly shown in FIG. 2B) is
supplied to an oscillator 25, a counter 26, a readout circuit 27, and an
electrode
driver 28 that drives transmitter electrodes 29A, 29B to transmit the signal.
Oscillator 25 may be of generally conventional design (e.g., a ring
oscillator) and
is advantageously configured to operate in the quasi-electrostatic frequency
region as described above. Oscillator 25 generates a driving signal (I) that
oscillates between high and low voltage levels and an inverted driving signal
/4)
that is opposite in phase to driving signal 4). In one embodiment, oscillator
25 is a
voltage-controlled oscillator (VCO) with an oscillation frequency that depends
on
a control voltage provided on a signal path 25A. Counter 26 counts the
oscillations of driving signals f',o and /ep and provides the current count to
readout
CA 3041518 2019-04-26
51
circuit 27. In one embodiment, counter 26 is an eight-bit counter of generally
conventional design; other types of counters (including counters with
different
widths) may also be used. Readout circuit 27 is configured with a set of
address
(identifier) bits 27A that are advantageously fixed, e.g., at the time circuit
20 is
fabricated. As noted above, the bits can be unique to a particular instance of
pill
14 or common to a lot of pills fabricated under the same conditions or common
to
all pills containing a particular pharmacological agent. Address bits 14 can
be
stored in nonvolatile storage circuits of generally conventional design, and
any
number of address bits (e.g., 8, 16, 32, .48, etc.) may be provided. Readout
.. circuit 27 generates an oscillator control signal (e.g., a voltage) on line
25A that
controls the frequency of VCO 25. In one embodiment, readout circuit 27 is
configured to select a current address bit, e.g., based on the current count
provided by counter 26, and to generate a control signal on signal line 25A
that
selects a frequency corresponding to the value of that bit. After some number
of
.. cycles (as determined by counter 26), readout circuit 27 selects the next
address
bit and generates the corresponding control voltage on signal line 25k Various
frequencies may be used to represent the address bit values "1" and "0." In
one
embodiment, frequencies of 100 kHz and 200 kHz may be used to represent
values "0" and "1," respectively. Other values (e.g., 1 MHz and 2 MHz or 1 kHz
and 5 kHz) may also be used. The chosen frequencies advantageously are well
below the absorption modes of human tissues, which are typically above 400
MHz. As described above, VCO 25 generates complementary signals 4), /4) that
oscillate at a frequency determined by the control signal on signal line 25A.
The
signals (I), /(1) are used to control an electrode driver 28, which may be
implemented, &g., as shown in FIG. 6D. It should be noted that since
electrodes
21 and 22 are in contact with stomach fluids when circuit 20 is operative, the
near-field component is coupled directly into the conductive medium of the
patient's body and can be detected by a suitably configured data collector,
e.g.,
as described below. In one embodiment, the collector is configured to log the
received address (identifier) and the time of receipt. The data collector can
also
be configured to retransmit this information to an external device, either in
real
time or while the patient is in a medical facility. It will be appreciated
that the
transmitter described herein is illustrative and that variations and
modifications
CA 3041518 2019-04-26
52
are possible. For instance, other encoding schemes could be used to transmit
the data; in one such embodiment, phase shift keying rather than frequency
keying is used. In some embodiments, multiple address bits can be encoded into
a single symbol that is transmitted using various keying schemes known in the
art.
FIG. 3A provides a detailed depiction of an embodiment of a signal
generation element 30 which labels the pharmaceutical material and is
encapsulated in the center of the composition. Signal generation element 30 is
in
the form of IC constructed from a silicon chip where various functional
elements,
e.g., in the form of one or more layers of circuits, are disposed on a silicon
substrate 31. The chip can be fabricated using standard integrated circuit
techniques. An example of such a fabrication approach is a 0.54 CIVIOS process
made available by AMI Semiconductor in Idaho, USA. Shown on the backside of
the substrate, the bottom of the chip 31 is metal 1 32 which functions as one
battery electrode and on the topside of the chip is metal 2 33 which functions
as
the other battery electrode. Also on the top side of the chip 31 are electrode
1 34
and electrode 2 35, which constitute a pair of signal-transmission electrodes.
In certain embodiments, electrode 1 34 and electrode 2 35 are fabricated
from a material that does not readily corrode in the stomach environment,
e.g.,
they are fabricated from noble metals. Alternatively, in some cases the
electrodes can be fabricated of a standard aluminum, such as that available
from
AMI Semiconductor. The criteria for electrode material selection will be
readily
ascertainable by the ordinary skilled -artisan. That is, if the survival time
of the
electrode is long enough for detection, it is suitable for use. Standard
aluminum
metals or other lower cost metals if used for electrodes 1 and 2 (34 and 35)
in
appropriate applications allow a lower cost for the device. In some cases
dissolution of the electrodes, and thus extinction of the reporting signal,
can
provide a secondary indication of the full dissolution of the pill and
incorporated
devices.
Metal 1 and metal 2 (32 and 33), in distinction to material selection for the
electrode component of the inventive device, are two different metals. Metal 1
and metal 2 are, selected so that the potential applied to the silicon is a
positive
voltage on the top surface and a negative voltage on the bottom surface. In
this
CA 3041518 2019-04-26
53
way the substrate is essentially at the same potential as the cathode, which
can
be the ground reference for the circuits, and the top surface, with a Si02
insulation layer, is coupled to a positive voltage, referenced to that ground
on the
bottom side.
FIG. 3B provides a view of an alternative signal generation element
according to an embodiment of the invention. Instead of electrodes, the signal
generation element 30 depicted in FIG. 3B includes two antennae 36 and 37
attached to silicon chip 31. Also shown are metals 1 and 2 (32 and 33). The
assembly 30 includes circuitry on a silicon chip 31 with either two or four
metal
structures (32, 33, 36 and 37) attached to it. In embodiments where two
different
metals are employed, the two metal structures serve as battery metals, that is
metal 1 and metal 2 (32 and 33). These metal structures can be provided in a
variety of forms. For instance, in one embodiment, metal 1. and metal 2 are
very
thick plated elements on the surface of the chip, front and back (e.g., as
shown in
FIG. 3C described below). . In another embodiment, metal 1 and metal 2 are
relatively long wires that are simply bonded to the chip at some point, e.g.,
as
shown in FIG. 3B. Metal 1 and metal 2 in some cases are insulated. In this
case,
the erosion occurs at the tip and then propagates towards the chip 31. The
erosion as it dissolves in the solution starts at the end of the wire and
gradually
work its way toward the chip 31. This configuration improves battery life. In
another configuration, a metal is plated up on the front and back of the chip,
and
then the surface disappears. The two wires can also be employed as antennae.
In one configuration, a perpendicular pair of antennae (36 and 37) are
provided.
In this implementation, there would be two other metal structures which are
typically of the same material. This material can be selected from a variety
of
metals, such as platinum or gold. These metal structures are attached to the
chip
and extend some dimension away from:the chip. Typically these structures are
on the order of a millimeter to a centimeter combined length. In some
configurations, a significant portion of the metal structures are insulated so
that
the dipole created is of maximum dimension. In other configurations, just the
battery metals perform that dipole function, e.g., as described below in
connection with FIG. 3C, or a separate antenna is provided.
In certain embodiments, the signal generation element does not include
antennae and instead uses battery components as antennae, such as shown in
CA 3041518 2019-04-26
54
FIG. 30. In FIG. 30, signal generation element 30 includes silicon support
layer
31 positioned between metal 1 layer 32 and metal 2 layer 33. Also shown is
circuitry layer 38. In such embodiments, when a switch on the chip, e.g., in
the
circuitry layer, is closed, a current is produced between the two metals of
the
battery, which is then detected. In certain embodiments, a membrane larger
then
chip which defines a path for the current to travel is provided.
Yet another embodiment of a battery which is activated upon contact with
a physiological fluid is shown in FIGS. 3D and 3E. In the structure shown in
these
figures, the battery comprises top and bottom portions each supporting an
eletrode, where the top and bottom portions can be brought together to produce
a
structure comprising a volume bounded by opposing first and second electrodes,
where the volume may be filled with an electrolyte, e.g., physiological fluid,
when
active. FIG. 3D provides a representation of a bottom portion 31A of the
battery
in which material 1 32A is deposited into a recessed chamber 33A on top of a
substrate (e.g., silicon chip) 34A. Recessed chamber 33A has one or more ends
open to allow electrolyte to enter. Material 2 35A is deposited on a separate
substrate 36A to produce a second portion 37A, which is then bonded, e.g. by
bonds 38A and 38B, to the chip in a "flip chip" type process. All processing
can
be done at the wafer scale. Where desired, the openings of the recessed
chamber are filled with a degradable material, e.g., with a polymer, to
control how
quickly the battery is activated. Substrates 34A and 36A for materials 34A and
35A can be silicon, metal, or polymer/plastic. In certain embodiments, the
structure shown in FIGS. 3D and 3E is a battery where the first electrode is
deposited into a recessed chamber on the top of the chip. The 'recessed space
has one or two open ends to allow electrolyte flow. The second electrode is
deposited on a separate substrate (e.g. a silicon wafer, a metal film or a
polymer
film), then bonded on top of the wafer with the chips on it in a "flip-chip"
type
process. The processing is done at 'a wafer scale and the cells diced as
usual.
The advantages of this configuration include: protection of the electrode
surfaces
from being blocked by components present in the stomach or the stomach lining
itself; prevention of contact between any species generated on the battery
(e.g.,
Cu) and the stomach lining that could have toxicity risks; 3) provision of
uniform
consumption of electrode materials across the electrode surface and more
uniform current distribution between the electrodes.
CA 3041518 2019-04-26
55
FIG. 4 provides a diagrammatic representation of the events which occur
when the pill is ingested and dissolved to the point that some of the pill has
been
chemically and/or physically eroded away. Metal 1 and metal 2 (32 and 33) are
now in an ionic solution 39. This creates a low voltage (V-) and a high
voltage
(V+) as applied to an electronic circuit 40. The two outputs of that
electronic
circuit 40 are EO 41 and El 42, which are the signal-transmission electrodes
on
the top surface. In an alternate embodiment no shown in FIG. 4 where the
signal
generation element 30 includes a single electrode, the output is E0 41.
FIG. 5 shows a similar arrangement as in FIG. 4. However, instead of
having two electrodes as the output, a coil is provided. Metal 1 and metal 2
(32
and 33) are applied to the electronic circuit 40 of signal generation element
30.
The outputs of the electronic circuit 40 are coupled to a coil 43. This
configuration provides that a battery is created by metal 1 and metal 2 (32
and
33) when exposed to ionic solution. This battery drives the circuit 40, which
creates an oscillating frequency. This oscillating current goes through the
coil and
generates a RE magnetic signal. Unlike near-field quasi-static electrical
signals,
which may suffer from significant attenuation through body tissues, the RF
magnetic signal can be transmitted through body tissues with less attenuation.
The RE magnetic signal is then picked up by an external or internal receiver
device that has a magnetic-signal detection mechanism. If a broadcast is
provided at a high enough frequency, a pager-like device that is worn by the
patient will detect whenever a pill is ingested.
FIG. 6B shows the detail of one implementation of an electronic circuit that
can be employed in a signal generation element. On the left side are the two
battery electrodes, metal 1 and metal 2 (32 and 33). These metals, when in
contract with an electrolyte, form a battery and provide power to an
oscillator 61,
in this case shown as a schematic. The metal 1 32 provides a low voltage,
(ground) to the oscillator 61. Metal 2 33 provides a high voltage (Vhigh) to
the
oscillator 61. As the oscillator 61 becomes operative, ifgenerates a clock
signal
.. 62 and an inverted clock signal 63, which are opposites of each other.
These two
clock signals go into the counter 64 which simply counts the number of clock
cycles and stores the count in a number of registers. In the example shown
here,
an 8 bit counter is employed. Thus, the output of counter 64 begins with a
value
of "00000000," changes to "00000001" at the first clock cycle, and continues
up
CA 3041518 2019-04-26
56
to "1 1 1 1 .1 1 1 1 ." The 8-bit output of counter 64 is coupled to the input
of an
address multiplexer (mux) 65. In one embodiment, mux 65 contains an address
interpreter, which can be hard-wired in the circuit, and generates a control
voltage to control the oscillator 61. Mux 66 uses the output of counter 64 to
reproduce the address in a serial bit stream, which is further fed to the
signal-
transmission driving circuit. Mux 65 can also be used to control the duty-
cycle of
the signal transmission. In one embodiment, mux 65 turns on, signal
transmission
only one sixteenth of the time, using the clock counts generated by counter
64.
Such a low duty cycle conserves power and also allows other devices to
transmit
without jamming their signals. The address of a given chip can be 8 bits, 16
bits
or 32 bits. Typically, more than 8 bits will be used in a product because
there are
so many different types of pharmaceuticals. Each pharmaceutical will have its
own specific address.
The present invention also allows the possibility that, where appropriate,
each pharmaceutical batch can be provided with a batch specific address. This
allows identification of where the pill was made, when the pill was made, and
in
what batch it was made. In some cases, each pill will have a unique
identifier.
This would be particularly useful when drugs are more likely to be
subsequently
stolen or used illicitly, and thus should be tracked, or where questions of
contamination may arise.
According to one embodiment, mux 65 produces a control voltage, which
encodes the address serially and is used to vary the output frequency of
oscillator
61. By example, when the control voltage is low, that is, when the serial
address
bit is at a 0, a 1 megahertz signal is generated by the oscillator. When the
control
voltage is high, that is, when the address bit is a 1, a 2 megahertz signal is
generated the oscillator. Alternately, this can be 10 megahertz and 20
megahertz,
or a phase shift keying approach where the device is limited to modulating the
phase. The purpose of mux 65 is to control the frequency of the oscillator or
an
AC alternative embodiment of the amplified signal of oscillation.
The outputs of mux 65 are coupled to electrode drive 66 which can drive
the electrodes to impose a differential potential to the solution, drive an
oscillating
current through a-coil to generate a magnetic signal, or drive a single
electrode to
push or pull charge to or from the solution.
CA 3041518 2019-04-26
57
In this manner, the device broadcasts the sequence of O's and l's which
constitute the address stored in mux 65. That address would be broadcast
repeatedly, and would continue broadcasting until metal I or metal 2 (32 and
33)
is consumed and dissolved in the solution, when the battery no longer
operates.
FIG. 7 is an alternate embodiment of the present invention. This
implementation of the circuit 70 shows the oscillator 71 and a counter 72. The
mux 73 takes 5 bits from counter 72 as its input. On the upper right corner of
FIG. 7 is an exemplary circuit diagram for the signal-transmission electrode
driver. Two CMOS invertors respectively take the clock and inverted clock
signals
as their inputs, and drives electrodes e0 and el.
FIG. 8 provides one implementation of an oscillator 80. In this case, Vcontrol
81 basically controls the amount of voltage driving the oscillator 80. When
Vcontrol
is low, a 20,000 ohm resistor 82 separates Vlow 83, which is the low power-
supply
voltage, and the oscillator control line, Vosc control 84. When Vcontrol is
high, the
Voso_control goes to VI., putting the maximum voltage across the oscillator
circuitry
and resulting in a higher frequency coming out of the clock signal and the
inverted clock signal (85 and 86).
FIG. 9 shows a simple trickle or asynchronous counter which has in this
case four flip flops with some simple inverters that simply count all the way
up
and then start over again back to zero, and start counting all the way up
again. In
one embodiment, a multiplexer can take AO and Al, A2, A3, as its address
inputs
and can compare these inputs with a stored address, and then have the stored
address output as the oscillator control signal.
As indicated above, in certain embodiments the signal generation element
may include a single electrode, and therefore have a monopole configuration.
In
one embodiment of the present invention, as shown in FIG. 10, a three
terminal,
monopole signal generation element 100 is provided. In this embodiment, the
signal generation element 100 of the pill has one electrode 101 which is
capacitively coupled to chip 107. Two metal electrodes 103 and 102 constitute
the electrodes for the battery, which provides power for the signal generation
element 100. Electrodes 102 and 103 are coupled to the chip 107 through two
resistors 104 and 105, and an optional storage capacitor 106. In one
embodiment, electrode 102 is the ground and electrode 103 provides Vhigh for
the
signal generation element. Electrode 101 is the output of the mono pole signal
CA 3041518 2019-04-26
58
generation element. During operation, electrode 101 will push current into and
out of body's fluid at a high frequency. A receiver will detect the pushing
and
pulling of that charge out of the body's fluids. Note that the biggest
difference
between this configuration and the configuration described previously is that
this
configuration provides a mono pole. When chip 107's output changes, capacitor
108 forces the potential on electrode 101 to change instantly, which result "
a
corresponding change in the potential of the body. A receiver that is in
contact of
the body can thereby detect a large transient voltage change.
This inventive design produces an alternating current into and out of the
body which is detected by a receiver (not shown). The output coupling
capacitors
may be optional. However, the presence of these capacitors prevents any DC
currents and forces an AC signal. =
FIG. 14 shows the multiplexer and the addressing system 73 of the
circuitry of the signal generation element of FIG. 7. In this case, there are
two 4
bit muxes (141 and 142) and a 1 bit mux 143, wherein the 1 bit mux 143 takes
the outputs of the two 4 bit muxes 141 and 142 as its input. Each input port
of
muxes 141 and 142 is coupled to either the high voltage Vhigh or the low
voltage
V. This configuration of the present invention will allow for a 32 bit number,
which is hard-wired to the 32 inputs of the two muxes, to be converted to a
multiplexed serial output 144. As the counter goes through the 5 bits of
counting,
the output of mux 144 sequentially selects the inputs of muxes 141 and 142.
When the 5 bit counter reaches "11111," the sequence will start over from the
beginning again. This way the 16 bit address is repeatedly sent. An
alternative
approach is to send 16 bits of zeros and 16 bits of address alternatively, so
that
the receiving circuitry can be woken up and synchronized.
FIG. 15 shows a detail of the 4 bit mux 141 of the system shown in FIG.
14. The 4 bit mux is constructed from 4 levels of 1 bit muxes.
Fig. 16 shows the 1 bit mux in detail that makes up the 4 bit mux 141.
FIG. 17 is an additional mono pole embodiment 170 of a signal generation
element. The biggest difference from the prior described embodiments is that a
current source 171 is placed in series with the power supply created by MI 172
and M2 173. This creates a DC current between 11A1 172 and M2 173. This DC
current does not compete with the AC signal generated by the electrode 174.
This DC current will then go to one or another capacitor (175 and 176) and
would
CA 3041518 2019-04-26
59
either charge up the electrode or charge up another capacitor. The concept
behind this embodiment is to have a DC current created between M1 and M2 and
an AC signal generated at the single electrode. Coupling capacitor 176 is
optional.
FIG. 18A is an exemplary schematic diagram of a signal-transmission
driver circuit. This circuit is based on an 8-pin 555 timer chip. As is shown
on
FIG. 18A, the pin designations of the 555 timer chip are as follows: pin 1 is
the
ground; pin 2 is the trigger, pin 3 is the output, pin 4 is reset, pin 5 is
the control
voltage, pin 6 is the threshold, pin 7 is discharge, and pin 8 is the power
supply to
the chip Vdd. The output pin and the ground pin are capacitively coupled to
two
transmission electrodes, respectively. During operation, this circuit
transmits a
signal at a fixed frequency.
FIG. 18B1 to 18B2 is an exemplary schematic diagram of a receiver
circuit. Shown on the upper left portion of the diagram is a front-end
amplification
stage, which receives the signal through a pair of electrodes and performs
differential amplification to the signal using an instrumentation amplifier.
In the
middle portion of the diagram is a cascaded four-stage filter. In one
embodiment,
the first two stages are high-pass filters with a cut-off frequency higher
than 1
KHz, such as a cut-off frequency at approximately 10 KHz. The high-pass filter
removes the low-frequency noises and interferences, such as the 60 Hz power-
line noise. The last two stages are low-pass filters with a cut-off frequency
lower
than 500 KHz, such as a cut-off frequency at approximately 200 KHz. The low-
pass filters can remove high-frequency noises and interferences. The filtered
and amplified signal is fed to an LED, as is shown on the lower left portion
of the
diagram. When a signal is detected, the LED is lit indicating presence of the
signal.
The device described above generally includes two circuits: one is a logic
circuit that generates the address bit sequence, and one is a driver circuit
that
drives the transmission electrodes based on the address bit sequence. The
power-consumption 'characteristics of these two circuits are different.
Typically,
the logic circuit requires a high voltage power supply, e.g., a 1.2 V power
supply,
to switch the CMOS circuits. However, the current drawn through the logic
circuit
CA 3041518 2019-04-26
60
is relatively small. For example, in one embodiment, the current drawn through
the logic circuits is approximately 5 p,A.
On the other hand, the driver circuit may draw a much larger current,
because of the power it requires to transmit a sufficiently detectable signal.
Consequently, the voltage of the power supply can be pulled down to a lower
level. For example, the driver circuit can draw 100 A and pull the battery
voltage down to 0.5 V.
Because the area of the battery electrodes can be limited due to the size
constraint of the device, the interference between the two circuits with
regard to
power supply may be significant. As a result, the driver circuit could pull
the
battery Voltage down to a point that makes the logic circuit inoperable. One
embodiment of the present invention uses a split battery configuration to
decouple the power supplies for the logic and driver circuits.
FIG. 19 shows one exemplary split (Le., segmented) battery design. Two
battery electrodes 193 and 194, which are made from copper iodine, constitute
the battery anodes for the logic circuit '191 and driver circuit 192,
respectively.
Effectively, electrodes 193 and 194 form two separate batteries with a shared
common magnesium cathode 195. In this way, the driver circuit 192 can draw
sufficient current to drive transmission electrodes 196 without significantly
impairing the power supply for the logic circuit 191.
During operation, driver circuit '192 draws a current from the battery
formed by electrodes 194 and 195, and pushes this current through transmission
electrodes 196 into the body. In a further embodiment, the device can avoid
the
use of separate transmission electrodes by using the battery electrodes for
transmission. FIG. 20 shows such a configuration. The driver circuit 206
essentially contains a switch coupled between the anode 204 and the cathode.
This switch can be turned on or off by the address signal from the logic
circuit
201. When the switch is turned on, the battery for the driver circuit is
effectively
short-circuited within the chip. Consequently, a current 207 flows through the
body from the cathode to anode 204. The resistance of the body tissue can
thereby generate a voltage difference, which can be readily detected by, for
example, a differential amplifier.
CA 3041516 2019-04-26
63.
In some cases, the size of the cathode could be limited, resulting in
coupling between the power supplies for the logic and driver circuits even
with
split anodes. According to one embodiment, as is shown in FIG. 21, the cathode
can also be split to further decouple the two power supplies. Here, two
separate
magnesium electrodes 211 and 212 serve as separate cathodes for the two
batteries respectively serving the logic and driver circuits. The coupling
between
the two circuits can thus be minimized.
In a further embodiment, the battery electrodes for the driver circuit can be
detached from the chip and coupled to the driver circuit through two external
wires, as is shown in FIG. 22. The battery electrodes for the logic circuit,
on the
other hand, can still be deposited on the chip to provide high-voltage power
supply to the logic. circuit. The external wires 221 and 222, which can be
approximately 1 cm long each, form a long dipole and can provide attendant
signal amplification. As a result, the effectiveness of the transmission is
not
limited by the size of the chip. In one embodiment, the wires are initially
folded
within a pill and can unfold when the pill is digested.
METHODS OF MAKING COMPOSITIONS
A variety of manufacturing protocols may be employed to produce
compositions according to the invention. In manufacturing the subject
compositions, a signal generation element is stably associated with the
pharmaceutical dosage from in some manner. By stably associated is meant that
the signal generation element and the dosage form to do separate from each
other, at least until administered to the subject in need thereof, e.g., by
ingestion.
The signal generation element may be stably associated with the pharmaceutical
carrier/active agent component of the composition in a number of different
ways.
In certain embodiments, where the carrier/active agent component is a solid
structure, e.g., such as a tablet or pill, the carrier/active agent component
is
produced in a manner that provides a cavity for the signal generation element.
The signal generation element is then placed into the cavity and the cavity
sealed, e.g., with a biocompatible material, to produce the final composition.
For
example, in certain embodiments a tablet is produced with a die that includes
a
feature which produces a cavity in the resultant compressed tablet. The signal
generation element is placed into the cavity and the cavity sealed to produce
the
CA 3041518 2019-04-26
62
final tablet. In a variation of this embodiment, the tablet is compressed with
a
removable element, e.g., in the shape of a rod or other convenient shape. The
removable element is then remoVed to produce a cavity in the tablet. The
signal
generation element is placed into the cavity and the cavity sealed to produce
the
final tablet. In another variation of this embodiment, a tablet without any
cavity is
first produced and then a cavity is produced in the tablet, e.g., by laser
drilling.
The signal generation element is placed into the cavity and the cavity sealed
to
produce the final tablet. In yet other embodiments, a tablet is produced by
combining the signal generation element with subparts of the tablet, where the
subparts may be pre-made subparts or manufactured sequentially. For example,
in certain embodiments tablets are produced by first making a bottom half of
the
tablet, placing the signal generation element on a location of the bottom half
of
the tablet, and then placing top portion of the tablet over the bottom half
and
signal generation element to produce the final desired composition. In certain
embodiments, a tablet is produced around a signal generation element such that
the signal generation element is located inside of the produced tablet. For
example, a signal generation element, which may or may not be encapsulated in
a biocompatible compliant material, e.g., gelatin (to protect the signal
generation
element), is combined with carrier/active agent precursor, e.g., powder, and
compressed or molded into a tablet in a manner such that the signal generation
element is located at an internal position of the tablet. Instead of molding
or
compressing, the carrier/active agent component is, in certain embodiments,
sprayed onto the signal generation element in a manner that builds up the
tablet
structure. In yet another embodiment, the active agent/carrier component
precursor may be a liquid formulation which is combined with the signal
generation element and then solidified to produce the final composition. In
yet
other embOdiments, pre-made tablets may be fitted with the signal generation
element by stably attaching the signal generation element to the tablet. Of
interest are protocols that do not alter the properties of the tablet, e.g.,
dissolution
etc. For example, a gelatin element that snap fits onto one end of a tablet
and
has the chip integrated with it is employed in certain embodiments. The
gelatin
element is colored in certain embodiments to readily identify tablets that
have
been fitted with the signal generation element. Where the composition has an
active agent/carrier composition filled capsule configuration, e.g., such as a
CA 3041518 2019-04-26
=
63
gelatin capsule filled configuration, the signal generation element may be
integrated with a capsule component, e.g., top or bottom capsule, and the =
capsule filled with the active agent/carrier composition to produce the final
composition. The above reviewed methods of manufacture are merely illustrative
of the variety of different ways in which the compositions of the invention
may be
manufactured.
SYSTEMS
Also provided are systems that include the subject compositions. Systems
of the subject invention include, in certain embodiments, one or more active
agent containing compositions, e.g., as reviewed above, as well as a signal
detection component, e.g., in the form of a receiver. The signal detection
component may vary significantly depending on the nature of the signal that is
generated by the signal generation element of the composition, e.g., as
reviewed
above.
In certain embodiments, the signal detection component is an implantable
component. By implantable component is Meant that the signal* detection
component is designed, i.e., configured, for implantation into a.subject,
e.g., on a
semi-permanent or permanent basis. In these embodiments, the signal detection
component is in vivo during use. In yet other embodiments, the signal
detection
component is ex vivo, by which is meant that the detection component is
present
outside of the body during use. In certain of these embodiments, as developed
in
greater detail below, either separate from or integrated with the ex vivo
detection
component may be a dosage dispenser element, e.g., for dispensing dosages of
the compositions based on signal detected from the signal generation element
of
the detector. Such features may also be present in implantable detection
components, e.g., to provide a closed loop administration system that
administers
a subsequent dosage based on input about ingestion of a previous dosage.
As reviewed above, in certain embodiments the signal generation element
of the composition is activated upon contact with a target body site. In
certain of
these embodiments, the signal detection component is activated upon detection
of a signal from the signal generation element. In certain of these
embodiments,
the composition generates an intermittent signal. In certain of these
CA 3041518 2019-04-26
64
embodiments, the detection element is capable of simultaneously detecting
multiple compositions.
The signal detection component may include a variety of different types of
signal receiver elements, where the nature of the receiver element necessarily
varies depending on the nature of the signal produced by the signal generation
element. In certain embodiments, the signal detection component may include
one or more electrodes for detecting signal emitted by the signal generation
element. In certain embodiments, the receiver device will be provided with two
electrodes that are dispersed at some distance. This distance allows the
electrodes to detect a differential voltage. In certain embodiments, the first
electrode is in contact with an electrically conductive body element, e.g.,
blood,
and the second electrode is in contact with an electrically insulative body
element
relative to said conductive body element, e.g., adipose tissue (fat). In an
alternative embodiment, a receiver that utilizes a single electrode is
employed. In
certain embodiments, the signal detection component may include one or more
coils for detecting signal emitted by the signal generation element. In
certain
embodiments, the signal detection component includes an acoustic detection
element for detecting signal emitted by the signal generation element
For those embodiments where the signal generated by the identifier is a
near-field conductive signal, e.g., as reviewed above, the receiver of the
present
systems may also be viewed as "data collectors." As used herein, a "data
collector" is any device equipped with receiving antenna to detect the
potential
differences created in the body by a transmitter as described above, thus
receiving the information transmitted. A data collector may handle received
data
in various ways. In some embodiments, the collector simply retransmits the
data
to an external device (e.g., using conventional RF communication). In other
embodiments, the data collector processes the received data to determine
whether to take some action such as operating an effector that is under its
control, activating a visible or audible alarm, transmitting a control signal
to an
effector located elsewhere in the body, or the like. In still other
embodiments, the
data collector stores the received data for subsequent retransmission to an
external device or for use in processing of subsequent data (e.g., detecting a
change in some parameter over time). It is to be understood that data
collectors
CA 3041518 2019-04-26
65
may perform any combination of these and/or other operations using received
data.
While the receiving antenna is advantageously inside the patient or in
contact with the patient's skin, it is not required that data collector be
entirely
internal to the patient. For instance, a watch or belt worn externally and
equipped
with suitable receiving electrodes can be used as a data collector in
accordance
with one embodiment of the present invention. The data collector may provide a
further communication path via which collected data can be extracted by a
patient
or health care practitioner. For instance, an implanted collector may include
conventional RF circuitry (operating, e.g., in the 405-MHz medical device
band)
with which a practitioner can communicate, e.g., using a data retrieval
device,
such as a wand as is known in the art. Where the data collector includes an
external component, that component may have output devices for providing,
e.g.,
audio and/or visual feedback; examples include audible alarms, LEDs, display
screens, or the like. The external component may also include an interface
port
via which the component can be connected to a computer for reading out data
stored therein.
In some embodiments, the data collector is implanted. For instance, as
noted above, pacemaker leads provide a suitably sized receiving antenna.
Typical pacemakers include a control unit (referred to as a "can") that
incorporates logic circuits configured to perform various data collection and
processing operations. The can is also connected to RF transmitter/receiver
circuitry that allows communication between the pacemaker and an external
wand operated by a health care practitioner. Thus, where the patient has a
.pacemaker, leveraging the existing unit as a data collector may be an
efficient
choice.
In certain embodiments, the system further includes an element for storing
data, i.e., a data storage element. Typically, the data storage element is a
computer readable medium. The term "computer readable medium" as used
herein refers to any storage or transmission medium that participates in
providing
instructions and/or data to a computer for execution and/or processing.
Examples
of storage media include floppy disks, magnetic tape, CD-ROM, a hard disk
drive,
a ROM or integrated circuit, a magneto-optical disk, or a computer readable
card
such as a PCMCIA card and the like, whether or not such devices are internal
or
CA 3041518 2019-04-26
66
external to the computer. A file containing information may be "stored" on
computer readable medium, where "storing" means recording information such
that it is accessible and retrievable at a later date by a computer. With
respect to
computer readable media, "permanent memory" refers to memory that is
permanent. Permanent memory is not erased by termination of the electrical
supply to a computer or processor. Computer hard-drive ROM (Le. ROM not
used as virtual memory), CD-ROM, floppy disk and DVD are all examples of
permanent memory. Random Access Memory (RAM) is an example of non-
permanent memory. A file in permanent memory may be editable and re-writable.
In certain embodiments, the data that is recorded on the data storage
element includes at least one of, if not all of, time, date, and an identifier
of each
composition administered to a patient, where the identifier may be the common
name of the composition or a coded version thereof. In certain embodiments,
the
data of interest includes hemodynamic measurements. In certain embodiments,
the data of interest includes cardiac tissue properties. In certain
embodiments,
the data of interest includes pressure or volume measurements.
The invention also provides computer executable instructions (i.e.,
programming) for performing the above methods. The computer executable
instructions are present on a computer readable medium. Accordingly, the
invention provides a computer readable medium containing programming for use
in detecting and processing a signal generated by a composition of the
invention,
e.g., as reviewed above.
As such, in certain embodiments the systems include one or more of: a
data storage element, a data processing element, a data display element, data
transmission element, a notification mechanism, and a user interface. These
additional elements may be incorporated into the receiver and/or present on an
external device, e.g., a device configured for processing data and making
decisions, forwarding data to a remote location Which provides such
activities,
etc.
In certain embodiments, the signal detection component includes a cardiac
monitoring- element, such as shown in the system of FIG. 1. FIG.1 shows a
human 10 who has an implanted cardiovascular device "can" 8 and a lead 6,
which components are employed to monitor and detect the signal emitted from
pill 14. The monitoring device can be positioned in other locations as well,
such
CA 3041518 2019-04-26
67
as subcutaneously, in the heart, or in the waist near the stomach, for
example.
Positioning may he suggested by a particular application.
The inventive monitoring system can also be positioned as an external
device. By example, it could be positioned by a harness that is worn outside
the
body and has one or more electrodes that attach to the skin at different
locations_
The inventive construct can be linked to a portable device, for example a
watch
that has one or two electrodes dispersed on the wrist. There are many places
where such a receiving electrode system could be placed and created such as,
hearing aids that beep, necklace, belt, shoes (PZT ¨ powered), or earrings.
As indicated above, in certain embodiments the systems include an
external device which is distinct from the receiver (which may be implanted or
topically applied in certain embodiments), where this external device provides
a
- number
of functionalities. Such an apparatus can include the capacity to provide
feedback and appropriate clinical regulation to the patient. Such a device can
take any of a number of forms. By example, the device can be configured to sit
on the bed next to the patient. The device can read out the information
described
in more detail in other sections of the subject patent application, both from
pharmaceutical ingestion reporting and from psychological sensing devices,
such
as is produced internally by a pacemaker device or a dedicated implant for
detection of the pill. The purpose of the external apparatus is to get the
data out
of the patient and into an external device. One feature of external apparatus
is its
ability to provide pharmacologic and physiologic information in a form that
can be
transmitted through a transmission medium, such as a telephone line, to a
remote location such as a clinician or to a central monitoring agency.
In certain embodiments, the cardiac = monitoring element includes a
conduction velocity measurement element. In certain embodiments, the cardiac
monitoring element includes a pressure sensor. In certain embodiments, the
cardiac monitoring element includes a dimension sensor.
Additional physiological sensors with various designs have been described
in additional applications by some of the present inventors. These sensors can
be used jointly with the present inventive systems. In addition, other
applications
by some of the present inventors describe multiplexing systems with which the
present invention can be very usefully employed in an interactive, synergistic
manner.
CA 3041518 2019-04-26
68
This prior work by some of the present inventors describes the use of
dimension sensors to determine heart parameters in order to facilitate
appropriate therapy intervention, such as resynchronization therapy. Using the
present invention to determining the time of blood-stream absorption of
cardiac
treatment pharmaceutical and correlating this with changes produced in heart
function sensed by those devices provides highly valuable information for the
clinician in titrating medications and providing synergy between
pharmacological
and'electrophysiological treatment.
Embodiments of the present invention can be used in various systems.
Such systems may include various types of sensors. Such sensors and systems
have been described in various applications by some of the present inventors.
These applications also describe multiplexing systems previously developed by
some of the present inventors with which the present invention can be
employed.
These applications include: U.S. Patent Application No. 10/734490 published as
20040193021 titled: "Method And System For Monitoring And Treating
Hemodynamic Parameters"; U.S. Patent Application No. 11/219,305 published as
20060058588 titled: "Methods And Apparatus For Tissue Activation And
Monitoring"; International Application No. PCT/US2005/046815 titled:
"Implantable Addressable Segmented Electrodes"; U.S. Patent Application No.
11/324,196 titled "Implantable Accelerometer-Based Cardiac Wall Position
Detector"; U.S. Patent Application No. 10/764,429, entitled "Method and
Apparatus for Enhancing Cardiac Pacing," U.S. Patent Application No.
10/764,127, entitled "Methods and Systems for Measuring Cardiac Parameters,"
U.S. Patent Application No.10/764,125, entitled "Method and System for Remote
Hemodynamic Monitoring"; International Application No. PCT/ US2005/046815
titled: "Implantable Hermetically Sealed Structures"; U.S. Application No.
11/368,259 "
Fiberoptic Tissue Motion Sensor"; International Application
No. PCT/U52004/041430 titled: "Implantable Pressure Sensors,"; U.S. Patent
Application No. 11/249,152 entitled "Implantable Doppler Tomography System,"
International Application Serial No. PCT/USUS05/39535 titled "Cardiac Motion
Characterization by Strain Gauge".
CA 3041518 2019-04-26
69
Some of the present inventors have developed a variety of display and
software tools to coordinate multiple sources of sensor information. Examples
of
these can be seen in PCT application serial no. PCT/US2006/12246 titled:
"Automated Optimization of Multi-Electrode Pacing for Cardiac
Resynchronization
" and filed on March 31, 2006.
The above described systems are reviewed in terms of communication
between an identifier on a pharmaceutical composition and a receiver. However,
the systems are not so limited. In a broader sense, the systems are composed
of
two or more different modules that communicate with each other, e.g., using
the
transmitter/receiver functionalities as reviewed above, e.g., using the
monopole
transmitter (e.g., antenna) structures as described above. As such, the above
identifier elements may be incorporated into any of a plurality of different
devices,
. e.g., to provide a communications system between two self-powered devices in
= the body, Where the self-powered devices may be sensors, data receivers
and
storage elements, effectors, etc. in an exemplary system, one of these devices
may be a sensor and the other may be a communication hub for communication
to the outside world. This inventive embodiment may take a number of forms.
There can be many sensors, many senders and one receiver. They can be
transceivers so both of these can take turns sending and receiving according
to
known communication protocols. In certain embodiments, the means of
communication between the two or more individual devices is the mono polar
system, e.g., as described above. In these embodiments, each of these senders
may be configured to take turns sending a high frequency signal into the body
using a monopole pulling charge into and out of the body which is a large
capacitor and a conductor. The receiver, a monopole receiver is detecting at
that
frequency the charge going into and .out of the. body and decoding an
encrypted
signal such as an amplitude modulated signal or frequency modulated signal.
This embodiment of the present invention has broad uses. For example, multiple
sensors can be placed and implanted on various parts of the body that measure
position or acceleration. Without having Wires connecting to a central hub,
they
can communicate that information through a communication medium.
CA 3041518 2019-04-26
70
METI IODS
- in
the methods of the subject invention, an effective amount of a
composition of the invention is administered to a subject in need of the
active
agent present in the composition, where "effective amount" means a dosage
sufficient to produce the desired result, e.g. an improvement in a disease
condition or the symptoms associated therewith, the accomplishment of a
desired
physiological change, etc. The amount that is administered may also be viewed
as a therapeutically effective amount: A "therapeutically effective amount"
means
the amount that, when administered to an subject for treating a disease, is
sufficient to effect treatment for that disease.
The composition may be administered to the subject using any convenient
means capable of producing the desired result, where the administration route
depends, at least in part, on the particular format of the composition, e.g.,
as
reviewed above. As reviewed above, the compositions can be formatted into a
variety of formulations for therapeutic administration, including but not
limited to
solid, semi--solid or liquid, such as tablets, capsules, powders, granules,
ointments, solutions, suppositories and injections. As such, administration of
the
compositions can be achieved in various ways, including, but not limited to:
oral,
buccal, rectal, parenteral, intraperitoneal, intradermal, transdermal,
intracheal,
etc., administration. in pharmaceutical dosage forms, a given composition may
be administered alone or in combination with other pharmaceutically active
compounds, e.g., which may also be compositions having signal generation
elements stably associated therewith.
The subject methods find use in the treatment of a variety of different
conditions, including disease conditions. The specific disease conditions
treatable
by with the subject compositions are as varied as the types of active agents
that
can be present in the subject compositions. Thus, disease conditions include,
but
are not limited to: cardiovascular diseases, cellular proliferative diseases,
such as
neoplastic diseases, autoimmune diseases, hormonal abnormality diseases,
infectious diseases, pain management, and the like.
By treatment is meant at least an amelioration of the symptoms associated
with the disease condition afflicting the subject, where amelioration is used
in a
broad sense to refer to at least a reduction in the magnitude of a parameter,
e.g.
CA 3041518 2019-04-26
71
symptom, associated with the pathological condition being treated. As such,
treatment also includes situations where the pathological condition, or at
least
symptoms associated therewith, are completely inhibited, e.g. prevented from
happening, or stopped, e.g. terminated, such that the subject no longer
suffers
.. from the pathological condition, or at least the symptoms that characterize
the
pathological condition. Accordingly, "treating" or "treatment". of a disease
includes ,
preventing the disease from occurring in an animal that may be predisposed to
the disease but does not yet experience or exhibit symptoms of the disease
(prophylactic treatment), inhibiting the disease (slowing or arresting its
development), providing relief from the symptoms or side-effects of the
disease
(including palliative treatment), and relieving the disease (causing
regression of
the disease). For the purposes of this inventionra "disease" includes pain.
A variety of subjects are treatable according to the present methods.
Generally such subjects are "mammals" or "mammalian," where these terms are
used broadly to describe organisms which are within the class mammalia,
including the orders carnivore (e.g., dogs and cats), rodentia (e.g., mice,
guinea
pigs, and rats), and primates (e.g., humans, chimpanzees, and monkeys). In
representative embodiments, the subjects will be humans.
In certain embodiments, the subject methods, as described above, are
methods of managing a disease condition, e.g., over an extended period of
time,
such as 1 week or longer, 1 month or longer, 6 months or longer, 1 year or
longer, 2 years or longer, 5 years or longer, etc. The subject methods may be
employed in conjunction with one or more additional disease management
protocols, e.g., electrostimulation based protocols in cardiovascular disease
management, such as pacing protocols, cardiac resynchronization protocols,
etc;
lifestyle, such a diet and/or exercise regimens for a variety of different
disease
conditions; etc.
In certain embodiments, the methods include modulating a therapeutic
regimen based data obtained from the compositions. For example, data may be
obtained which includes information about patient compliance with a prescribed
therapeutic regimen. This data, with or without additional physiological data,
e.g.,
obtained using one or more sensors, such as the sensor devices described
above, may be employed, e.g., with appropriate decision tools as desired, to
make determinations of whether a given treatment regimen should be maintained
CA 3041518 2019-04-26
72
or modified in some way, e.g., by modification of a medication regimen and/or
implant activity regimen. As such, methods of invention include methods in
which
a therapeutic regimen is modified based on signals obtained from the
composition (s).
In certain embodiments, also provided are methods of determining the
history of a composition of the invention, where the composition includes an
active agent, an identifier element and a pharmaceutically acceptable carrier.
In
certain embodiments where the identifier emits a signal in response to an
interrogation, the identifier is interrogate, e.g., by a wand or other
suitable
interrogation device, to obtain a signal. The obtained signal is then employed
to
determine historical information about the composition, e.g., source, = chain
of
custody, etc.
In yet other embodiments where the identifier is one that survives
digestion, the methods generally include obtaining the signal generation
element
of the composition, e.g, by retrieving it from a subject that has ingested the
composition, and then determining the history of the composition from obtained
signal generation element. For example, where the signal generation element
includes an engraved identifier, e.g., barcode or other type of identifier,
the
engraved identifier may be retrieved from a subject that has ingested the
composition and then read to identify at least some aspect of the history of
the
composition, such as last known purchaser, additional purchasers in the chain
of
custody of the composition, manufacturer, handling history, etc. In certain
embodiments, this determining step may include accessing a database or
analogous compilation of stored history for the composition.
UTILITY
The present invention provides the clinician an important new tool in their
therapeutic armarnentarium: automatic detection and identification of=
pharmaceutical agents actually delivered into the body. The applications of
this
new information device and system are multi-fold. Applications include, but
are
not limited to: (1) monitoring patient compliance with prescribed therapeutic
regimens; (2) tailoring therapeutic regimens based on patient compliance; (3)
monitoring patient compliance in clinical trials; (4) monitoring usage of
controlled
CA 3041518 2019-04-26
=
73
substances; and the like. Each of these different illUstrative applications is
now
reviewed in greater detail below,
Monitor1nPaent Compliance with Prescribed Therapeutic Regimens
As summarized above, one type of application in which the subject
compositions and systems find use is in monitoring patient compliance with
prescribed therapeutic regimens. By monitoring patient compliance is meant
tracking whether a patient is actually taking medication in the manner
prescribed
to the patient. As such, the present invention provides accurate data of when
a
pill has been taken and which pill has been taken. This allows the precise
determination of which pill was taken at a specific point in time. Such
monitoring
capability assures patients are taking the prescribed medication correctly.
This
information avoids the potential for over prescription of medications that are
not
actually being taken.. By example, if pain killers are intended to be
administered
to a patient, it is possible to verify with the present invention that the
patient did in
fact take those pain killers in a certain period of time. This knowledge is an
important tool in limiting the illicit sale of unconsumed drugs to an
unintended
party. In the case of cardio vascular pills, the clinician or care giver is
able to
= verify that the amount of the drug was taken has been taken at
approximately the
right point and time. Thus, the true efficacy of the drug can be accurately
evaluated. Proper administration and patient compliance is especially critical
in
Alzheimer's, psychiatric, and alcohol aversion drugs, and in the treatment of
rest
home residents. In the case of accidental and other overdoses situations, the
intervening clinician will be able to discern how far the ingestion has
proceeded,
and how many pills are involved.
In more complex embodiments of the present invention, correct, timely
ingestion of the drugs will automatically trigger a prescription refill signal
which is
forwarded to a pharmacy data system, and in some cases the refill will be
automatically delivered directly to the patient's home, or released by a
device in
the patient's home some period of time later. This feature is particularly
valuable
in patients with compromised mental capacity and/or limited physical mobility.
The invention is particularly useful in complex administration regimens,
such as when multiple pharmaceuticals are being taken, and confusion is more
likely to occur. The inventive pills can have multiple external layers, with
only
= CA 3041518 2019-04-26
_
74
correct dosage allowing dissolution and absorption of the pharmaceutical
component. Specific indicators, such as electrical conduction velocity in the
heart
or electrolytic levels in the blood in response to pharmaceutical can also be
titrated.
in certain embodiments, a patient can be alerted when the patient is in
some way non-compliant with a given treatment regimen. For example, by a
sound, visual, or computer reminder, if the pharmacological regimen is not
being
accurately adhered to, a reminder is provided. If that reminder is not
accurately
responded to, the system can provide an alert to family members, caregivers,
or
clinicians in order to remedy the gap in treatment or overdose. The device may
also automatically modify the dosage and timing of the regimen to compensate
for prior non-standard dosing.
Tailoring Therapeutic Regimens Based on Patient Compliance
As summarized above, one type of application in which the subject
compositions and systems find use is in tailoring therapeutic regimens based
on
patient compliance. In such applications, data obtained about whether a
patient
has or has not taken a particular dosage is employed to determine future
dosages and/or timing of such dosages. In certain embodiments, data concerning
patient compliance is combined with additional data, e.g., sensed
physiological
data, to make customized changes or modifications to a given therapeutic
regimen. By example, when data about dosage compliance obtained according to
the invention is used in concert with other medical sensing devices,
correlation
between drug delivery, batch and dosage can be correlated to a physiological
response. in this manner, optimal pharma-therapeutic regimens may be
formulated by the clinician. By example, cardiac stimulating drugs can be
titrated
to the most appropriate dosages, minimizing side effects such as cardiac
muscle
exhaustion and rebound effects .among others, and optimizing both dosage and
timing for each individual patient.
Assessment of a range of alternate medications is made possible by the
present invention without resort to awaiting overt clinical sequel of
treatment,
many of which can be seriously adverse. By example, positive effects would be
quickly ascertainable without being obscured by more random factors. Negative
CA 3041518 2019-04-26
75
responses, such as changes in blood pressure, would become clearly evident as
drug related or independent above background physiologic variation.
In one clinical arena, the present invention allows, in concert with other
sensing devices developed by some of the present inventors, the measurement
and assessment of the cardiac response to those medications. These co-
employed sensing devices can be thoso enumerated below, among others.
Other sensing technology, e.g., as mentioned above, developed by some of the
present inventors allows measurement of heart health and cardiac efficiency.
Using these tools in concert with the present inventive device, the clinician
will be
able to compare the response of the heart and body to the administered
pharmaceutical. The data provided by the present invention can optionally be
recorded over time. The recording system records synchrony or conduction
velocity of a signal going through cardiac tissue and how that is mediated by
the
presence of a certain medication. This unique data is made possible by the
present invention since it can determine electronically exactly when the pill
or
other medication was being absorbed into the body.
In more standard clinical environments, this unique data allows careful
selection and titration of drug administration without resort to more overt
physical
symptoms to ascertain contraindications, efficacy, and optimal dosage levels.
The present invention provides a record for emergency, room technicians or
doctors when a patient is admitted to a hospital so that the patient's status
can be
accurately ascertained. Dosage events within the last hour or day prior to
admission, and the identity of the last medication, will be immediately
available.
As such, future therapeutic regimens can be made based on accurate records of
patient drug medication history.
In certain embodiments, the clinician obtains this information through
simple interrogation of the implanted or portable device. This device would
tell
them without any uncertainty what pills have been taken. As the inventive
technology becomes more wide spread, this data will become more regularly
available. The present inventive microchips are sufficiently inexpensive such
that
when they are put into standard production, most or all pharmaceuticals will
be
fitted with them as a matter of course.
The patient monitoring capacity of the external reporting apparatus is an
importation function which the inventive device can provide. When coordinated
CA 3041518 2019-04-26
76
with internal or external physiologic sensing data, the device can read out
the
physiological response of the patient to the ingestion of medication, and then
transmit this information back to the clinician. The clinician can then modify
therapy to optimal effectiveness, as indicated by the new data in response to
the
modified therapy, and so forth.
In more sophisticated embodiments of the present invention, the dosage
adjustment function, within certain parameters, can be performed by an
intelligence circuit in the apparatus. By
example, for a blood pressure
medication, the patient takes their blood pressure pill. 20 minutes later, the
internal monitoring circuitry in the implantable device registers a drop in
blood
pressure. The circuitry quantifies this drop, and transmits it to this bedside
apparatus. The apparatus then can adjust the dosage of the pill to optimally
treat
the patient. Similarly, when the patient is connected to an IV, the dosage can
be
dispensed directly into the IV fluid. In certain embodiments, the closed-loop
system is provided as a fully implantable device.
Current clinical practice for drug treatment optimization is considerably
more limited than that which is available by use of the present inventive
device.
Currently, blood pressure medication treatment is set at so many pills per
day.
Such a blunt dosage regime takes a long time to optimize appropriately because
the feedback loop is very slow. By contrast, with the present invention, the
feedback loop of physiologic response to pharmaceutical dosage is very rapid
and very efficient. Ultimately, the present invention allows tailoring the
drug
- dosages day to day, or even more finely, to account for change in activity,
change in physiological conditions in the patient, and other dosage parameter.
In more sophisticated embodiments of the present invention, physiological
reactions to specific dosages and time intervals would also be continually
monitored. In some embodiments, the level of drug in the blood stream is
monitored, allowing for individual and time of day variations in drug
metabolism.
This aspect of the present invention effectively minimizes underdosing or
overdosing the controlled substances, in some cases addressing these changes
before they produce external symptoms apparent to the patient or clinician.
The
drug dosage can be automatically titrated so that, by example, the smallest
appropriate level to quell anxiety due to pain, other physiologic reactions to
pain,
or provide steady or gradually diminishing blood levels of the drug would be
CA 3041518 2019-04-26
77
dispensed. This feature of the present invention provides an automatic,
appropriately gradual, weaning off of the drug, lessening the chance of
serious
addiction or severe, adverse withdrawal reactions.
Clinical Tri'al Applications
An important application of the invention is to provide immediate feedback
of physiological data response to administration of a pharmaceutical agent in
clinical drug trails. A current challenge is that the experimental drug is
administered broadly to a population without a comprehensive foreknowledge of
which sub-groups within this population are most likely to benefit from the
treatment. Another challenge is monitoring patient compliance with the
treatment
regimen, by determining if the tests subjects are taking the medicine as
indicated.
The later challenge is addressed in the sections above. Both patient non-
compliance levels and actual response to drug ingestion can thus be
determined.
As such, compliance intervention can then be addressed early in the study.
In certain embodiments of the present invention, clinical researchers are
provided with immediate access to physiological data. The clinical researchers
are. able to identify the subset for whom the drug is most likely effective
from
within the original test population of possible participants in the trial. The
example
above of a patient receiving blood pressure medication and getting feedback
immediately demonstrates how effectiveness of a novel medicine can be quickly
determined.
Opon administration of the first doses of medication to initial test subjects,
the clinical researchers are likely to find that some subjects in the
population
respond to the medication and others do not. This immediate feedback allows
the administrator of the trail to exclude those patients who do not respond to
the
medication and target only that subgroup for whom there is clear efficacy.
This
culling process allows the overall results of the trail to get a much higher
effective
percentage, because one is able to target the drug to the group for whom it is
effective. It also avoids side effect challenges for subjects who would not
have a
benefit balance to such risks.
As such, from this innovative data, the present invention provides the
clinician an accurate dose response curve showing the response to that
medication and the timing of the digestion of the pill. Such innovative data
has
CA 3041518 2019-04-26
78
many applications. For instance, the clinician now has the ability to
determine
which patients have no response to the medicine in the pill. In a study
situation,
such patients can be removed from a study or a test of the clinical utility of
a
certain medication. This ability provides that only people who have a
beneficial
response to a certain medication are retained in the trail. This feature will
improve the efficacy of medications and to reduce the amount of medications
that
people take that are not being useful. It may also be used in trials to
determine
which patients actually consumed the medicine, and which did not.
The present invention allows identification of physiological proxies for the
efficacy of a drug. By example, for a drug which has a long term
administration
prior to the development of overt clinical changes, there are typically
certain short
term physiological factors which appear immediately after ingestion of the
drug.
By example, cancer medication which requires many months to show an effect,
can have shorter term indicia of its efficacy in one or a constellation of
physiologic
factors. Changes, both local or throughout the whole body, in blood pressure,
body temperature, internal chemical enzymes or other factors will serve as
proxies for the longer term desired effects. A precise correlation of these
factors
with the time of the pills ingestion enhances the ability to find meaningful
indicia.
With the very closely timed correlated response to the ingestion of the pill
provided for the first time by the present innovation, demonstrating that a
physiologic response is a result of the drug ingestion rather than any of the
other
possibility confounding factors, is much more likely. This capacity of the
present
invention can serve as a partial or complete proxy for clinical trials.
The invention provides a way to determine very quickly whether a patient
should be taking the medication or not, whether it will be effective or not,
and
allow its appropriate titration. Synergies between medications, both helpful
and
adverse, will also become more readily apparent.
Monitoring Usage of Controlled Substances
As reviewed above, in other embodiments of the inventive microchips, the
identifiers can be fitted with coils, susceptible of interrogation without
being
dissolved in the body. This is accomplished by transmitting RF energy into the
coil in such a way that the inquirer will be apprised of the presence and
identity of
a pill before it is ingested.
CA 3041518 2019-04-26
, .
79
In an additional embodiment of the present invention, a "smart box" is
provided that can interrogate each pill and ascertain its address. The box can
write a distinctive product number or product code so that .every single pill
ever
made is provided with a unique identifier, Fuses, for example, may be
selectively
destroyed so the addresses may be detected electrically or optically.
Particularly
in the case of controlled substances, such as a narcotic, this will be
important in
limiting the illegal used of previously legitimate medicines. The present
invention
makes it possible to identify precisely who bought such a pill from the
authorized
pharmacist. This use of the present invention will rein in the number of
illicit uses
of controlled substances on the market place.
An important application for the external apparatus aspect of the present
invention is in monitoring and regulating the use of controlled pharmaceutical
substances. A serious risk when patients are prescribed heavy narcotics for
pain
control is the possibility of addiction. In its simplest analysis, addiction
occurs
from the ingestion of too much of the controlled medication by inadvertent
overdosing, purposeful misuse, or through inexact dosage prescription.
Additionally, as described above, individual serial number are provided on
such
pharmaceuticals to track the legitimate distribution of the drug before the
illicit
distribution of such drugs.
In one application of the present invention, a means for locking and
regulating the dosage of a potential addictive drug is provided. An example of
this capacity of the present invention is when a patient takes their narcotic
pill, in
which the ingestion of the medication is registered by the internal device.
This
information is then automatically transmitted to the external apparatus.
The inventive apparatus is so configured that only after the patient has
taken the pill and at the appropriate time has elapsed does this accessory
apparatus dispense a further pill. In this manner, the addiction rate for the
drug is
dramatically lowered by limiting legal drug availability by dispensing exactly
the
prescribed dosage at precisely the appropriate time interval.
The external apparatus can also be effectively employed in mandatory
medication forensic applications. For example, in the case of a convicted
criminal, the criminal can be required to take court ordered medication as a
condition of release from jail. Using the present invention, the court or
probation
officer has access to a real-time record of the administration of this drug as
this
CA 3041518 2019-04-26
80
information is fed back through the accessory apparatus to the appropriate
official. There is a current trend towards court mandated psycholropio or
chemical sterilization drug maintenance for sex offenders which would be
addressed by this aspect of the present invention. This use of the present
invention is analogous to house arrests where physical position monitoring
bands
are worn on the ankle of the offender.
KITS
Also provided are kits for practicing the subject methods. Kits may include
one or more compositions of the invention, as described above. The dosage
amount of the one or more pharmacological agents provided in a kit may be
sufficient for a single application or for multiple applications. Accordingly,
in
certain embodiments of the subject kits a single dosage amount of a
pharmacological agent is present and in certain other embodiments multiple
dosage amounts of a pharmacological agent may be present in a kit. In those
embodiments having multiple dosage amounts of pharmacological agent, such
may be packaged in a single container, e.g., a single tube, bottle, vial, and
the
like, or one or more dosage amounts may be individually packaged such that
certain kits may have more than one container of a pharmacological agent.
Suitable means for delivering one or more pharmacological agents to a
subject may also be provided in a subject kit. The particular delivery means
provided in a kit is dictated by the particular pharmacological agent
employed, as
describe above, e.g., the particular form of the agent such as whether the
pharmacological agent is formulated into preparations in solid, semi solid,
liquid
or gaseous forms, such as tablets, capsules, powders, granules, ointments,
solutions, suppositories, injections, inhalants and aerosols, and the like,
and the
particular mode of administration of the agent, e.g., whether oral, buccal,
rectal,
parenteral, intraperitoneal, intradermal, transdermal, intracheal, etc.
Accordingly,
certain systems may include a suppository applicator, syringe, 1.V. bag and
tubing, electrode, etc.
In certain embodiments the kits may also include a signal receiving
element, as reviewed above. In certain embodiments, the kits may also include
an external monitor device, e.g., as described above, which may provide for
CA 3041518 2019-04-26
81
communication with a remote location, e.g., a doctor's office, a central
facility etc.,
which obtains and .processes data obtained about the usage of the composition.
The subject kits may also include instructions for how to practice the
subject methods using the components of the kit The instructions may be
recorded on a suitable recording medium or substrate. For example, the
instructions may be printed on a substrate, such as paper or plastic, etc. As
such,
the instructions may be present in the kits as a package insert, in the
labeling of
the container of the kit or components thereof (i.e., associated with the
packaging
or sub-packaging) etc. In other embodiments, the instructions are present as
an
electronic storage data file present on a suitable computer readable storage
medium, e.g. CD-ROM, diskette, etc. In yet other embodiments, the actual
instructions are not present in the kit, but means for obtaining the
instructions
from a remote source, e.g. via the internet, are provided. An example of this
embodiment is a kit that includes a web address where the instructions can be
is viewed and/or from which the instructions can be downloaded. As with the
instructions, this means for obtaining the instructions is recorded on a
suitable
substrate.
Some or all Components of the subject kits may be packaged in suitable
packaging to maintain sterility. In many embodiments of the subject kits, the
components of the kit are packaged in a kit containment element to make a
single, easily handled unit, where the kit containment element, e.g.', box or
analogous structure, may or may not be an airtight container, e.g., to further
preserve the sterility of some or all of the components of the kit.
The following example is offered by way of illustration and not by way of
limitation.
EXPERIMENTAL
Example 1.
In the following experiment, a transmitter (Tx) and receiver (Rx), each
powered by batteries and encased in a water-tight RubbermaidTM container, are
employed. The Tx and Rx float in a bath of saline, and an LED glows on the Rx
CA 3041518 2019-04-26
S2
when the Tx is placed in the bath. Both Tx and Rx are completely isolated from
the outside world.
The Tx, representing the compositions of the present application, e.g., a
pill composition having an active agent and signal generation element, is an
S oscillator circuit based on a CMOS timer chip. It produces a square wave
at
about 80 kHz of 3V amplitude from a Lithium battery. A tightly twisted pair of
wires extends from the circuit, out of the container, and into the bath. At
the end
of the twisted pair, the wires are striped of insulation by about 1 mm and
separated to form a dipole antenna. The signal amplitude was found to scale
linearly with the separation distance characterizing this dipole. The signal
was
easily detectable with this setup when the dipole was 5mm in extent.
The Rx is a filtered amplifier circuit with outputs to detect the transmitted
signal. A square of copper, 10cm on a side, was attached to the bottom of the
outside of the container and attached to the negative differential input of
the
circuit; this represents the pacing can. A bipolar pacing lead, about 40cm
long,
was attached to the positive differential input of the circuit; the ring
electrode
was selected for the input. The differential signal was coupled into the
inputs of a
gain 100 instrumentation amplifier through 0.1uF series capacitors. The output
of
the instrumentation amplifier was fed into a 4-pole high-pass filter, with
gain of
100 and cutoff frequency 5 kHz. This output was fed into a 2-pole low-pass
filter
with gain 20 and cutoff frequency 100 kHz. Thus, the overall gain of the
circuit is
200,000. This output signal is applied across an LED and resistor in series,
which
glows when the output signal exceeds a few volts.
When not in the bath, the Rx LED was on all the time as it picked up
interference and power line noise from the environment. When the pacing lead
was shorted to the mock can the LED turned off.
When placed in solution, the LED turned off. When the Tx was also
placed in solution, the Rx LED turned on and the dependence on position and
orientation was investigated. The intensity of the LED was found to depend on
the cosine of the angle between the Rx and Tx dipole, with a null for
perpendicular orientation and sign inversion as the sense of the dipole was
reversed, as observed with an external oscilloscope. The intensity of the LED
was found to vary directly with position, With a bright, saturated glow
observed for
spacing less than 5cm and a dim, diffuse glow observed for the maximum
CA 3041518 2019-04-26
,
83
spacing allowed bythe bath, about 60cm.
The key to making the detection robust is differentiating the desired
signal from spurious interference. Such was accomplished in this experiment by
restricting the frequency band of sensitivity to between 5 and 100 kHz. To the
extent this band can be narrowed, the more robust the system will be. The
challenge here is to match the frequency of the Tx and Rx circuits, in light
of the
fact that the Tx frequency may vary by 30% due to manufacturing variation. The
Rx circuit can be very narrow through the use of a narrow bandpass or by using
demodulation techniques from the radio. The Rx circuit can be swept across a
.. tuning frequency range to detect the presence of the pill. The presence of
the pill
can be confirmed by encoding an unlikely bit sequence in the digital
information
transmitted by the pill.
Two problems with this approach are that it consumes power from the
Rx circuit while it scans frequencies, and that synchronization with multiple
pills,
which may burst the transmission of their codes, is difficUlt. If the
frequency of
the Tx is known ab intio, as is possible with circuit trimming or advanced
manufacturing processes, an elegant solution to both these problems is
presented. At the input to the Rx circuit, a tuned LC oscillator matched to
the Tx
frequency will "ring up" when the desired signal is present. This power can be
detected by a simple diode circuit, which serves as a trigger to turn the
detection
circuit on, greatly reducing the time it must draw current. This tuned input
also
serves to narrow the bandwidth and reject spurious signals.
This above experiment demonstrates the ability to transmit and detect
signals through a synthetic biological medium. The Tx may be readily powered
off a chemical battery, such as a Pt/Mg system. Furthermore, digital
information
is readily encoded in the signal using a variety of encoding techniques to
eliminate errors and improve the overall reliability of the system.
Example 2.
A transmitter according to the subject invention was set up as follows. The
circuit was powered off a 9V battery and floated on a bath of saline. The
circuit
was an oscillator based on the TL.C551 chip, a CMOS version of the popular 555
timer. The oscillator was run at ¨7 kHz, with a duty cycle of perhaps 15%. The
outputs of the oscillator were each capacitively coupled through 7 uF to a
twisted
CA 3041518 2019-04-26
84
pair, which was terminated in a small "Y" shaped dipole, with the arms
separated
by -1mm, and -2mm of bare wire exposed to the saline bath.
The signal was received through two Cu electrodes, each with -1
cm2 exposed to the bath. This was routed to the input of a Stanford pre-amp
.. operated off batteries, set to a gain of 1000 with a pass band between 3
kHz and
30 kHz. The output of the pre-amp was observed on a battery powered
oscilloscope.
A maximum signal of -200 uV referenced to the amplifier input was
observed for an Rx electrode separation of -20 cm. A dipolar coupling strength
was observed, displaying a sinusoidal angular dependence, with a null in
received signal for perpendicular orientation; phase inversion was seen
between
parallel and anti- parallel orientations. The received signal strength was
seen to
scale linearly with separation of the Rx electrodes.
The above demonstrates that the signal is clearly detectable with proper
amplification and filtering. Furthermore, a capacitor on the input of the Rx
.
amplifier is not necessary; as the same results were obtained using DC
coupling
on the input with a high-pass filter later in the signal chain.
The above results also verified that the Tx can run off an Mg/Pt potato
battery.
.. Example 3.
A prototype smart pill microchip, which broadcasts a fixed code using
frequency shift keying, was first powered by a 1.5V AA battery. The conductive
signal was applied to a physiological saline bath with a twisted pair T-shaped
dipole, approximately 1 cm across with 1 mm of conductor exposed on each arm
of the T. The signal was detected by two copper electrodes, spaced
approximately 10 cm apart, which feed into a battery powered, isolated
differential pre-amp. The signal was observed on an oscilloscope. An
oscillatory
signal, clearly representative of the transmitted data, was observed with a
frequency of about 300 kHz and an input-referenced amplitude of about 10 mV.
Furthermore, a dependence of the received signal strength on the cosine of the
angle between the transmit and receive conductors, as is characteristic of a
dipolar interaction, was observed.
A Mg-Cul water-activated battery, with each electrode having an exposed
surface area of -1 mm2 was constructed. The Mg electrode was formed by
CA 3041518 2019-04-26
., =
simply potting commercial grade Mg ribbon in epoxy and polishing the end flat
with sandpaper. The Cul electrode was produced by first polishing the end of
Cu
wire potted in epoxy.
Approximately 100 p,rn of Cu was then electroplated on the end of the Cu
5 wire using standard techniques, with the parameters chosen to give a
large
roughness coefficient, increasing the effective area of the electrode. The
surface
of this plated Cu was then transformed electrochemically to Cul by applying a
potential corresponding to the potential of the Cu+ ion in a solution of I-
ions. In
approximately 15 min 40 mC of Cul was produced. The battery was
10 demonstrated to have an open cell voltage of ¨1.05V in a pH 2 solution,
corresponding to the acidity of a typical stomach.
The Cul-Mg battery was connected to the power terminals of the chip, and
the output terminals were connected to the dipole conductor described above in
a
physiological saline bath. The battery was activated by dropping the
electrodes
15 in the bath, and a signal of amplitude .-2 mV at a frequency of 20 kHz
was
observed for at least a minute.
Finally, the output terminals of the chip were shorted together, effectively
configuring the chip for the 2-terminal operation described above. An output
signal was observed, but its amplitude was much weaker, probably becaOse of
20 the decreased effective transmitter dipole length in this configuration.
That is, in
the 4-terminal configuration, the 'effective transmit conductor size is
determined
by the spacing between the battery and dipolar T, which was several
centimeters;
in the 2-terminal mode, the effective dipole length is reduced to the
separation
between the Mg and Cul electrodes, which was less than 1 cm. The observed
25 signal was perhaps a few hundred 1.1,V, and could be quantified using
averaging to
overcome an interfering signal amplified by the broadband receiver. More
sophisticated detection schemes will have little problem detecting such a
signal
reliably.
Example 4. -
30 A pill composition as described above prior to ingestion may be composed
of two main components, an address generating logic circuit and a signal
transmission circuit. The address generation circuit is powered with low
current
adequate to the required tasks. However, if the voltage supplied to the
address
CA 3041518 2019-04-26
86
generation circuit changes, the frequency of the oscillator therein will also
change. This may produce changes in signal transmission, introduce noise into
the transmission, and cause other undesired effects.
For design purposes, it is simpler to power the address generation circuit
with a constant voltage. However, in certain embodiments a more complex
configuration may be desired. By example, when the transmission starts, the
transmitter consumes considerable energy. As a result, the voltage will drop
because as more energy is consumed, the voltage of the power source drops.
The change in voltage will result in a change of frequency in the oscillator
within
the address generation circuit.
An example of this challenge in a different area of engineering is when a
remote control device is made from a receiver and servos. By contrast, the
receiver works permanently, and consumes low current in proportion to the
servo
which consumes a very large current. The servos work only when a signal is
transmitted to the remote site. In that case, the whole system consumes a
relatively large amount of power when the servos start to work. When the servo
starts to work, the voltage drops, and produces some noise. As a result, the
stability of signal transmission is compromised.
In order to avoid this problem, embodiments of the system are powered
with two voltage sources. The receivers are powered with one battery, and the
servos are powered with another battery. With this configuration, whatever
occurs
in the servo does not affect the receiver. As such, a more stable remote
control
results, theerby improving the performance of the complete system.
In one embodiment, a common cathode is provided. There are also two
positive electrodes, Al and A2. In this case, with the battery divided into
two
parts, one part of the battery will power the address generation circuit, and
the
other will power the transmission circuit. This configuration provides a
stable
voltage to the address generation circuit. When the transmitter section of the
device is turned on, only the voltage on the transmitter will change, but no
change of voltage will occur in the address generation section of the device.
Hence, the changes will typically cause a change in signal amplitude, but not
in
the frequency. As a result, the transition will be more stable, and the
frequency
of RF transmission will be unaffected, or minimally effected.
CA 3041518 2019-04-26
87
The above phenomena are not of concern if there is a big area of battery
electrode, because the voltage of the system as a whole will not change.
However, in the case of a small battery electrode, the transmitter can
potentially
lower the voltage of the battery, and there will be a change in voltage over
the
entire circuitry. If the battery is divided into two parts, the voltage of one
battery
can be changed while the other will continue to power the address generation
circuit with a constant voltage.
A consideration in the design development is how one battery will affect the
other. Experiments conducted by some of the present inventors show that a
change of load on one battery does not affect the other; i.e., they worked
independently. In this experiment, as is shown in FIG. 23, two copper iodine
anode electrodes were provided with a magnesium electrode as a common
cathode. These were connected to a zero-resistance ammeter, and performance
= was measured. One copper iodine electrode was connected through a 2.5 KO
resistor, and the other through a 200 KO resistor. All the electrodes are
submerged
in a pH2 HC1 solution at about 37 C. The data derived from this experiment is
shown in FIG. 24. The two _copper iodine electrodes work independently of each
other.
An ordinary skilled artesian will easily identify different materials and
configurations for the above device. The chemistry of this copper iodine and
various manners of preparation will be understood or quickly developed.
The surface preparation before the copper iodine is forming is of interest.
One approach is to use copper wire embedded in epoxy. This can be plated with
electrolytic copper. After the copper is polarized in solution of potassium
iodide,
copper iodine is formed on the tip of the electrode. Copper iodine can also be
formed by chemically deposition. Other means are also available.
10 jam is a typical range for thickness of copper iodide to produce an
adequate amount of electricity to accomplish the activity of the device for a
15
minute period. If less thickness is employed, the transmission will last a
shorter
time. Thus, the thickness of copper iodide is determined by the time required
to
produce electricity to provide the results needed for a particular
application. For
several seconds of transmission, less than 1 um of copper iodide would be
adequate. For one microsecond of transmission, a few nanometers of copper
CA 3041518 2019-04-26
88
iodine thickness, such as in the range of about 10-100 nanometers, more
specifically, about 20-50 nanorneters is sufficient.
It is to be understood that this invention is not limited to particular
embodiments described, as such may vary. It is also to be understood that the
terminology used herein is for the purpose of describing particular
embodiments
only, and is not intended to be limiting, since the scope of the present
invention
will be limited only by the appended claims.
Where a range of values is provided, it is understood that each intervening
value, to the tenth of the unit of the lower limit unless the context clearly
dictates
otherwise, between the upper and lower limit of that range and any other
stated
or intervening value in that stated range, is encompassed within the
invention.
The upper and lower limits of these smaller ranges may independently be
included in the smaller ranges and are also encompassed within the invention,
subject to any specifically excluded limit in the stated range. Where the
stated
range includes one or both of the limits, ranges excluding either or both of
those
included limits are also included in the invention.
Unless defined otherwise, all technical and scientific terms used herein
have the same meaning as commonly understood by one of ordinary skill in the
art to which this invention belongs. Although any methods and materials
similar
or equivalent to those described herein can also be used in the practice or
testing
of the present invention, representative illustrative methods and materials
are
now described.
The citation of any
publication is for its disclosure prior to the filing date and should not be
construed
as an admission that the present invention is not entitled to antedate such
publication by virtue of prior invention. Further, the dates of publication
provided
may be different from the actual publication dates which may need to be
independently confirmed.
CA 3041518 2019-04-26
89
It is further noted that the claims may be drafted to exclude
any optional element. As such, this statement is intended to serve as
antecedent
basis for use of such exclusive terminology as "solely," "only" and the like
in
connection with the recitation of claim elements, or use of a "negative"
limitation.
As will be apparent to those of skill in the art upon reading this disclosure,
each of the individual embodiments described and illustrated herein has
discrete
components and features which may be readily separated from or combined with
the features of any of the other several embodiments.
Any recited method can be carried out in
the order of events recited or in any other order which is logically possible.
Although the foregoing invention has been described in some detail by
way of illustration and example for purposes of clarity of understanding, it
is
1,5 .. readily apparent to those of ordinary skill in the art in light of the
teachings of this
invention that certain changes and modifications may be made thereto
Accordingly, the preceding merely illustrates the principles of the invention.
It will be appreciated that those skilled in the art will be able: to devise
Various
arrangements which, although not explicitly described or shown herein, embody
the principles of .the invention.
Furthermore, all examples and conditional language recited herein are
principally
intended to aid the reader in understanding the principles of the invention
and the
concepts contributed by the inventors to furthering the art, and are to be
construed as being without limitation to such specifically recited examples
and
:conditions. Moreover, all statements herein reciting principles, aspects, and
embodiments of the invention as well as specific examples thereof, are
intended
to encompass both structural and functional equivalents thereof. Additionally,
it is
intended that such equivalents include both currently known equivalents and
equivalents developed in the future, Le., any elements developed that perform
the same function, regardless of structure. The scope of the present
invention,
therefore, is not intended to be limited to the exemplary embodiments shown
and
described herein.
CA 3041518 2019-04-26