Note: Descriptions are shown in the official language in which they were submitted.
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
IMPROVED DETECTION OF FLUID CHANGES
100011 This application claims priority to U.S. Patent Application Serial No.
15/349,260, titled "Improved Detection of Fluid Changes," filed November 11,
2016
(the only application being claimed as priority), the disclosure of which is
hereby
incorporated by reference in its entirety.
CROSS-REFERENCE TO RELATED APPLICATIONS
[0002] Also, the priority application (15/349,260) is a continuation-in-part
of U.S.
Patent Application Serial No. 14/844,681, titled "Detection and Analysis of
Spatially
Varying Fluid Levels Using Magnetic Signals," filed September 3, 2015, which
is a
continuation-in-part of U.S. Patent Application Ser. No. 14/690,985, titled
"Method for
Detecting and Treating Variations in Fluid," filed Apr. 20, 2015, which is a
continuation of U.S. Patent Application Ser. No. 14/275,549, titled "Method
for
Detecting and Treating Variations of Fluid," filed May 12, 2014, which is a
continuation of U.S. Patent Application Ser. No. 13/745,710, titled
"Diagnostic Method
for Detection of Fluid Changes Using Shielded Transmission Lines as
Transmitters or
Receivers," filed Jan. 18, 2013, and issued as U.S. Pat. No. 8,731,636 on May
20, 2014.
The disclosures of all of the above-referenced patents and patent applications
are
hereby incorporated by reference herein in their entireties.
TECHNICAL FIELD
[0003] This application is related to noninvasive, diagnostic, medical
devices,
systems and methods. More specifically, some embodiments of this disclosure
relate to
devices, systems and methods that use volumetric integral phase-shift
spectroscopy
("VIPS") to monitor changes in fluids in the brain or other parts of the body.
(VIPS
may alternatively be referred to by other acronyms, such as magnetic induction
phase-
shift spectroscopy ("MIPS")).
BACKGROUND
[0004] In many different medical settings, it would be advantageous to be able
to
detect changes in bodily fluid composition and distribution as they occur, in
a
noninvasive manner. For example, it is often critical to monitor changes in
intracranial
fluid content or distribution in an intensive care unit patient. Standard of
care for these
1
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
patients includes invasive monitors that require drilling a hole in the
cranium and
inserting a probe, such as an intracranial pressure (ICP) monitor, or
microdialysis or
"licox" probes, for measuring chemical changes to the fluids in the brain. No
continuous, noninvasive measurement techniques are currently commercially
available
for detecting cerebral fluid changes, such as those that occur with bleeding
or edema.
Furthermore, many brain injuries are not severe enough to warrant drilling a
hole in the
cranium for invasive monitoring. Thus, for many patients with brain injury,
there is no
continuous monitoring technology available to alert clinical staff when there
is a
potentially harmful increase in edema or bleeding. Instead, these patients are
typically
observed by nursing staff, employing a clinical neurological examination, and
it is not
until changes in the fluid composition or distribution in the brain cause
observable
brain function impairment that the physicians or nurses can react. In other
words, there
is no way currently available for monitoring intracranial fluid changes
themselves, and
thus the ability to compensate for such changes is limited.
[0005] VIPS has been previously proposed for diagnosis of brain fluid
abnormalities.
Patents have been awarded for proposed devices, and promising scientific
studies of
prototype devices are described in the literature. For example, Rubinsky et
at.
described the use of VIPS for this purpose, in U.S. Patent Numbers 7,638,341,
7,910,374 and 8,101,421, the disclosures of which are hereby incorporated in
their
entirety herein (referred to herein as the "Rubinsky Patents"). Wyeth et at.
described
additional details of the use and design of VIPS devices in U.S. Patent
8,731,636,
which is hereby incorporated in its entirety herein. However, no practical,
mass-
produced medical device based on VIPS technology has yet emerged, to provide
clinicians specializing in brain treatment or other areas of medicine the
promised
benefits of such a device.
[0006] Ideally, a medical device solution would provide a VIPS system with
improved performance, usability and manufacturability, such that it could be
used for
noninvasive fluid change detection in the brain and/or other areas of the
body. The
embodiments described herein endeavor to address at least some of these
objectives.
BRIEF SUMMARY
[0007] In one aspect of the present disclosure, a method for measuring a fluid
volume
in a patient's brain to help determine the presence of an occlusion of a blood
vessel
2
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
supplying the brain may involve: securing a volumetric integral phase-shift
spectroscopy (VIPS) device to the patient's head; measuring a fluid volume
with the
VIPS device; and determining, using the measured fluid volume, whether an
occlusion
of a blood vessel supplying the brain is present. In some embodiments, the
method
may further involve determining in which hemisphere of the brain the occlusion
is
located. In some embodiments, measuring the fluid volume may involve comparing
a
first fluid volume in one hemisphere of the brain with a second fluid volume
in the
other hemisphere of the brain, and determining whether the occlusion is
present may
involve comparing the first fluid volume with the second fluid volume. Some
embodiments may also involve determining in which hemisphere of the brain the
occlusion is located, based on the comparison of the two fluid volumes.
[0008] In some embodiments, measuring the fluid volume may involve monitoring
the fluid volume over time to detect changes in the fluid volume. Optionally,
the
method may also involve measuring the fluid volume after a procedure is
performed to
remove the occlusion. One example of a type of occlusion that may be detected
using
this method is a large vessel occlusion (LVO).
[0009] In another aspect of the present disclosure, a method for monitoring
fluid
volume changes in a patient's brain after treatment of an occlusion of a blood
vessel
supplying the brain may involve: securing a volumetric integral phase-shift
spectroscopy (VIPS) device to the patient's head; measuring a fluid volume
with the
VIPS device; and providing data regarding the measured fluid volume to a user
to help
the user determine if the treatment was successful. In some embodiments,
measuring
the fluid volume may involve measuring a first fluid volume before the
treatment is
performed and measuring at least a second fluid volume after the treatment is
performed. Optionally, such an embodiment may also involve comparing the first
fluid
volume with the second fluid volume to detect a change between the first and
second
fluid volumes.
[0010] In an alternative embodiment, measuring the fluid volume may involve
comparing a first fluid volume in one hemisphere of the brain with a second
fluid
volume in the other hemisphere of the brain. Such an embodiment may optionally
also
involve comparing the first fluid volume with the second fluid volume. The
method
may also optionally involve determining in which hemisphere of the brain the
occlusion
is located, based on the comparison of the two fluid volumes. In some
embodiments,
3
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
measuring the fluid volume involves monitoring the fluid volume over time to
detect
changes in the fluid volume.
[0011] In another aspect of the present disclosure, a volumetric integral
phase-shift
spectroscopy (VIPS) device for measuring a fluid volume in a patient's brain
to help
determine the presence an occlusion of a blood vessel supplying the brain may
include:
a frame comprising a housing; circuitry housed within the housing; at least
one receiver
housed within the housing; two wrap-around ends, configured to wrap around the
back
of a patient's head and over the ears; two transmitters, wherein one
transmitter is
housed in one of the two wrap-around ends, and the other transmitter is housed
in the
other of the two wrap-around ends; two holding arms extending from the frame
to
contact the patient's ears and help support the device on the patient's head;
and a
nosepiece configured to rest on the patient's nose to help support the device
on the
patient's head. In some embodiments, the device may be configured to detect at
least
two different fluid volumes. One of the fluid volumes may be a first fluid
volume in a
right hemisphere of the brain, and another of the fluid volumes may be a
second fluid
volume in a left hemisphere of the brain.
[0012] In one embodiment, the present disclosure includes a device for
detecting
spatial differences in fluid volume changes in a tissue of a patient. The
device includes
a support structure for securing the device to a body part of a patient, a
processing
element operably connected to the support structure, a wireless networking
interface
operably connected to the support structure and in communication with the
processing
element and an external computing device via a wireless network. The device
also
includes first, second, and third transmission modules, each of the modules
are
connected to the support structure and are in communication with the
processing
element. The second and third transmission modules are spatially separated
from one
another relative to the tissue of the patient, and the first transmission
module is opposed
to the second and third transmission modules, so as to transmit signals form
the
modules as they are transmitted through the tissue. When activated, the first
transmission module transmits a first time varying magnetic field through the
tissue of
the patient, and the second and third transmission modules receive first and
second
versions, respectively, of the first magnetic field and transmit a first
received magnetic
field data corresponding to the first and second versions to the processing
element. The
processing element provides transmission data corresponding to the first
received
4
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
magnetic field data to the wireless networking interface, which in turn
transmits the
transmission data wirelessly to the external computing device. In another
implementation, the first transmission module may be configured to receive
first and
second time varying fields transmitted by the second and third transmission
modules,
respectively.
[0013] In yet another embodiment, the present disclosure includes a method for
detecting symmetry in fluid volumes in a tissue of a patient. The method
includes
securing a device, including a receiver, a first transmitter, and a second
transmitter on
the patient's head, such that the first transmitter and the second transmitter
are spatially
separated from one another and the receiver is positioned to be in
communication with
the first transmitter and the second transmitter via a transmission pathway
through the
tissue. The method further includes transmitting a first time varying magnetic
field
from the first transmitter, transmitting a second time varying magnetic field
from the
second transmitter, receiving a first received field and a second received
with the
receiver, analyzing at least one transmission characteristic with a processing
element,
determining with the processing element that the first received field
corresponding to
the first time varying magnetic field and the second received field
corresponds to the
second time varying magnetic field, determining with the processing element a
first
phase shift between the first time varying magnetic field and the first
received field,
determining with the processing element a second phase shift between the
second time
varying magnetic field and the second received field and determining with the
processing element a change in fluid in the tissue over a period of time based
on the
determined first and second phase shifts.
[0014] In yet another embodiment, the present disclosure includes a method for
detecting variations in fluid volumes in a patient. The method includes
attaching a
headset to the patient, the headset including a support band for securing the
headset to a
head of the patient, a processing element coupled to the support band and
configured to
transfer data wirelessly to an external computer, and multiple transmitter
receiver
components operably connected to the support band at discrete locations. The
method
further includes activating the headset to take one or more fluid volume
readings within
the head of the patient, wirelessly transmitting the fluid data corresponding
to the one
or more fluid volume readings from the processing element to the external
computer,
and analyzing the fluid data with the external computer.
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
[0015] In another aspect of the present disclosure, a method for detecting
evidence of
a stroke in a patient may involve: defining a stroke-specific volumetric
integral phase-
shift spectroscopy (VIPS) signature, comprising a change in phase and/or a
change in
amplitude; securing a VIPS device to the patient's head; measuring multiple
phase
shifts and/or multiple amplitudes in a fluid and/or a tissue in the patient's
head, using
the VIPS device; determining, using a processor in or coupled with the VIPS
device,
that the measured phase shifts and/or amplitudes match the stroke-specific
VIPS
signature; and detecting the evidence of the stroke, based on the determining
step.
[0016] In some embodiments, the method may further involve determining in
which
hemisphere of the patient's brain the evidence of stroke is located. In some
embodiments, the measuring step may involve comparing a first fluid and/or
tissue
volume in one hemisphere of the brain with a second fluid and/or tissue volume
in the
other hemisphere of the brain, and determining in which hemisphere of the
patient's
brain the stroke is located may involve comparing the first volume with the
second
volume. In some embodiments, the multiple phase shifts and/or multiple
amplitudes are
measured over time, to detect changes in at least one of the phase shifts or
the
amplitudes. Some embodiments may further involve measuring multiple phase
shifts
and/or multiple amplitudes, using the VIPS device, after a procedure is
performed to
remove an occlusion in a blood vessel providing blood to the patient's brain.
In some
embodiments, such an occlusion may be a large vessel occlusion.
[0017] In another aspect of the present disclosure, a VIPS device for
detecting
evidence of a stroke in a patient may include: a frame including a housing; at
least one
VIPS receiver in the housing; circuitry in the housing, coupled with the at
least one
VIPS receiver; two wrap-around ends, configured to wrap around the back of the
patient's head and over the patient's ears; a first VIPS transmitter in one of
the two
wrap-around ends; a second VIPS transmitter in the other of the two wrap-
around ends,
and a processor. The first and second VIPS transmitters and the at least one
VIPS
receiver may measure multiple phase shifts and/or multiple amplitudes in a
fluid and/or
a tissue in the patient's head. The processor may determine that the multiple
phase
shifts and/or multiple amplitudes match a predefined stroke-specific VIPS
signature
and detect evidence of the stroke, based on the determination.
[0018] In some embodiments, the device may include two holding arms extending
from the frame to contact the patient's ears and help support the device on
the patient's
6
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
head. Some embodiments may further include a nosepiece configured to rest on
the
patient's nose to help support the device on the patient's head. The nosepiece
may
optionally be detachable, interchangeable and/or adjustable. In some
embodiments, the
device may be configured to detect at least two different fluid and/or tissue
volumes.
One of the two different fluid/tissue volumes may be a first fluid/tissue
volume in a
right hemisphere of the brain, and the other fluid/tissue volume may be a
second
fluid/tissue volume in a left hemisphere of the brain.
[0019] In some embodiments, the processor is disposed within the housing,
while in
alternative embodiments, the processor is located apart from the housing and
is coupled
wirelessly or via a wired connection with the circuitry. In some embodiments,
the first
and second VIPS transmitters and the VIPS receiver(s) are configured to
measure
bioimpedance. In some embodiments, the first and second VIPS transmitters and
the
VIPS receiver(s) are configured to measure multiple phase shifts and/or
multiple
amplitudes continuously over a period of time.
[0020] In another aspect of the present disclosure, a VIPS device for
detecting a
change or an abnormality in a head of a patient may include: a frame
configured to
wrap around at least a portion of the patient's head, the frame including a
housing; at
least one VIPS receiver in the housing; circuitry in the housing, coupled with
the at
least one VIPS receiver; at least two VIPS transmitters coupled with the
frame; and a
processor. The first and second VIPS transmitters and the VIPS receiver(s) are
configured to measure multiple phase shifts and/or multiple amplitudes in a
fluid and/or
a tissue in the patient's head. The processor includes memory containing
instructions
for performing a method, which involves determining that the multiple phase
shifts
and/or multiple amplitudes match a predefined VIPS signature indicative of the
change
or abnormality in the patient's head and detecting the change or abnormality
in the
patient's head, based on the determination.
[0021] In some embodiments, the change or abnormality that is detected is a
stroke.
In other embodiments, the change or abnormality may be a movement of the
patient's
brain toward one side of the patient's cranium. In yet other embodiments, the
change or
abnormality may be a change in a volume of blood and/or tissue on one
hemispheric
side of the patient's cranium compared to the other hemispheric side. In still
other
embodiments, the change or abnormality may be a change in a volume of blood
and/or
tissue within the patient's cranium over time.
7
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
[0022] In some embodiments, the frame of the device may further include two
wraparound ends, configured to fit over the patient' ears, and a nosepiece
positioned
below the housing and configured to fit on the patient's nose. Optionally, the
device
may also include two holding arms extending from the frame to contact the
patient's
ears and help support the device on the patient's head. In various
embodiments, the
processor may be disposed within the housing or may be located apart from the
housing
and coupled wirelessly or via a wired connection with the circuitry.
[0023] These and other aspects and embodiments will be described in more
detail
below, in reference to the attached drawing figures.
BRIEF DESCRIPTION OF THE DRAWINGS
[0024] Fig. 1 is a block diagram of a system for monitoring fluid changes in
the body,
according to one embodiment;
[0025] Fig. 1A is a perspective view of a patient headpiece for use in the
system of
Fig. 1, according to one embodiment;
[0026] Fig. 1B is a perspective exploded view of a patient headpiece for use
in the
system of Fig. 1, according to an alternative embodiment;
[0027] Figs. 2A through 2F illustrate various embodiments of transmitter
transducers
and receiver sensors for use in the system of Fig. 1;
[0028] Fig. 3 is a circuit diagram of a phase shift detection apparatus,
according to
one embodiment;
[0029] Fig. 4 is a simplified logic diagram for a waveform averager processor
for use
in the system of Fig. 1, according to one embodiment;
[0030] Fig. 5 is a simplified logic diagram of a phase shift measurement
processor for
use in the system of Fig. 1, according to one embodiment;
[0031] Fig. 6 is a flow diagram for the operation of the system of Fig. 1,
according to
one embodiment;
[0032] Fig. 7 is a block diagram of a system for monitoring fluid changes in a
body
corresponding to a cardiac signal, according to one embodiment;
[0033] Fig. 8 is an isometric view of a system for monitoring fluid changes
including
a temporary stabilizer, according to one embodiment;
[0034] Fig. 9 is a system diagram for a system for monitoring fluid changes in
a body,
according to an alternative embodiment;
8
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
[0035] Fig. 10A is a left isometric view of a patient wearing the headpiece of
the
system of Fig. 9;
[0036] Fig. 10B is a front elevation view of the patient wearing the headpiece
of Fig.
10A;
[0037] Fig. 10C is a right isometric view of a patient wearing the headpiece
of Fig.
10A;
[0038] Fig. 11 is a front isometric view of a system for monitoring fluid
changes in a
body, according to another embodiment;
[0039] Fig. 12 is a graph illustrating calibrated phase shift measurements as
a
function of time, according to one embodiment;
[0040] Fig. 13 is a graph illustrating changes in phase shift reading as a
function of
time during application of the Valsalva procedure, according to one
embodiment;
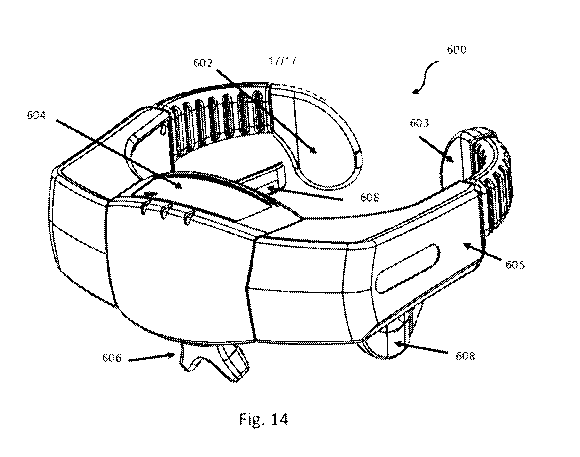
[0041] Fig. 14 is a perspective view of a headpiece for monitoring fluid
changes in a
body, according to an alternative embodiment; and
[0042] Fig. 15 is a chart illustrating measurements of a patient's cerebral
fluid
volumes over time, after a treatment of a cerebrovascular occlusion was
performed.
DETAILED DESCRIPTION
[0043] Certain details are set forth below to provide a sufficient
understanding of
certain embodiments of the present disclosure. However, some embodiments of
the
disclosure may be practiced without these particular details. Moreover, the
particular
embodiments of the present disclosure are provided by way of example and
should not
be used to limit the scope of this disclosure to those particular embodiments.
In some
instances, well-known circuits, control signals, timing protocols, and
software
operations have not been shown in detail, in order to avoid unnecessarily
complicating
the description.
Overall system architecture
[0044] Fig. 1 is a block diagram of one embodiment of a system 100 that may be
used
to detect fluid changes in a human brain. Although this description often
focuses on
use of the system 100 for detecting fluid changes in the brain, this
embodiment of the
system 100 or alternative embodiments may be used for detecting/monitoring
fluid
changes in any other part of the body. Therefore, the exemplary description
provided
9
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
herein that is directed toward the brain should not be interpreted as limiting
the scope
of the invention as it is set forth in the claims.
[0045] The system 100 may include a laptop computer 102 or other computing
device, a processing unit 104, and a patient headpiece 106 in some examples.
The
system 100 may be controlled, for example, by a windows-based labview language
program running on the laptop computer 102. The program generates a graphical
user
interface (GUI) that is displayed on the screen of the laptop 102. The
clinician who
operates the system 100 may initiate monitoring by mouse control after placing
the
headpiece 106 on the patient, which may be similar to an elastic headband or
bandage.
After initiation of monitoring, the program may run unattended as it logs the
phase shift
data on the laptop 102 and applies the appropriate methods to generate alarms
and
suggested corrective actions to a clinician.
[0046] The laptop 10, which may be an external computer relative to the
patient
headpiece 106, may have a USB serial link to the processing unit 104. This USB
link
may be electrically isolated to conform to applicable medical device
requirements. The
processing unit 104 may derive power from a standard universal AC line power
connection consistent with international standards. There may be a medical
grade low-
voltage DC power supply to power all of the processing unit's 104 internal
electronics
that meets applicable standards for patient isolation, line to neutral,
chassis, and patient
leakage as well as earth to ground continuity, EMI susceptibility and
emissions, and
other standard medical device requirements.
[0047] The laptop 102 may initiate phase shift data collection and log the
data in files
on the laptop's 102 hard drive along with other pertinent data and status
information.
[0048] The GUI on the laptop 102 may control the operation of the system 100,
and
may include controls and status indications that guide the clinician through
installation
of the patient headpiece 106 and a preliminary self-test of the entire system
100. If the
self-test passes, the clinician is instructed to initiate monitoring. During
monitoring,
the phase shift angle versus frequency data is collected from the USB
interface and
appropriate status and alert methods are applied to the data. The clinician
may be
informed if additional actions or emergency responses are indicated. The phase
shift
versus frequency data and additional status information is logged in the
laptop 102 for
later reference. A "sanity check" of the data and other built-in-test features
may run
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
continuously in the background, and if a fault is encountered, various levels
of severity
will generate warnings or interrupt operation of the system 100.
[0049] The architecture of hardware and firmware in the processing unit 104
and the
patient headpiece 106 may be optimized to achieve the desired phase
measurement
accuracy and stability while using a minimum number of custom electronics
components in some examples and as illustrated in Fig. 1. For example, in one
embodiment, and with reference to Fig. 1, the system 100 may comprise several
highly
integrated miniaturized off-the-shelf components. The system 100 may include
three
field programmable gate arrays (FPGAs) 110, 112, 114 in the processing unit
104, the
three FPGAs being programmed with appropriate firmware. One FPGA 110 may
synthesize a time-varying signal to be provided to a transmitter (transmitter
may be
alternatively be referred to by emitter) 120 to generate a magnetic field, the
second
FPGA 112 may collect and average digital samples of transmitted and received
magnetic fields, and the third FPGA 114 may measure the phase shift between
the
transmitted and received signals representative of the transmitted and
received
magnetic fields.
[0050] A microcontroller 118 may also be included in the processing unit 104,
and
may supervise the actions of the three FPGAs 110, 112, 114 and communicate
with the
laptop 102 (e.g., by transferring phase data results). The microcontroller 118
may
provide an interface between the external laptop computer 102 (via an
electrically
isolated USB interface) and the FPGAs 110, 112, 114 used for real time signal
processing of the data from the headpiece 106. The microcontroller 118 may
also
perform other miscellaneous functions such as the interface to basic user
controls
including power-on, initiation of data collection, setup of the frequency
synthesizer
110, internal temperature monitoring, power supply monitoring, and other
system status
monitoring and fault detection tasks.
[0051] The processing unit 104 may in some examples be fabricated from larger,
integrated components. In one embodiment, the processing unit 104 may include
an
off-the-shelf electronic signal generator, such as a Techtronix Arbitrary
Waveform
Generator model 3252, and a digital oscilloscope such as LeCroy Model 44xi.
Conversely, processing unit 104 could be integrated into a single ARM
processor.
[0052] The architecture of the system 100 illustrated in Fig. 1 may be
relatively
flexible, allowing improvements in all phases of the data collection, data
processing,
11
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
and data interpretation (e.g., clinical alerts) to be made through relatively
simple
software or firmware modifications. The FPGAs 110, 112, 114 may effectively
function as parallel processors to make data collection and processing proceed
in near
real time. The quantity of phase data transmitted via the microcontroller 118
to the
laptop 102 and archived for later reference may thus be reduced, thereby
requiring less
computation time on the laptop 102 for processing the data. This may in turn
free up
the laptop 102 for checking data consistency and applying methods required for
alerting
clinicians to the need for corrective actions.
[0053] Although the processing unit 104 in Fig. 1 has been illustrated and
described
as a relatively flexible embodiment, in other examples the diagnostic system
100 may
be an embedded system with custom electronics components specially designed
for use
in the diagnostic system 100. For example, one or more analog to digital (A to
D)
converters may be located in the processing unit 104, which may be physically
distinct
and separate from the headpiece 106, or which may be integral with the
headpiece 106
(e.g., the headpiece 106 may, in a custom system 100, include all of the
electronics and
processing equipment needed to capture and process phase shift information).
Also, the
functions executed by the three FPGAs may be combined into one FPGA. In
general,
any suitable architecture may be used.
[0054] Referring again back to Fig. 1, the system 100 may also include a
headpiece
106 with transmission modules, such as one or more transmitters 120 and one or
more
receivers 124, the details of which are explained in more detail below. In one
example,
the headpiece 106 includes a single transmitter 120 and a single receiver 124,
whereas
in other examples, the headpiece 106 includes several transmitters 120 and/or
several
receivers 124. For example, the headpiece 106 may include one transmitter 120
and
two receivers 124. If multiple receivers 124 are placed at different positions
over a
patient's head, they may allow a clinician to triangulate the location of a
fluid change
(e.g., intracerebral bleeding from a blood vessel or tumor) and/or image the
biological
impedance of a patient's brain. In other examples, the headpiece 106 may
include
multiple transmitters 120, which may produce magnetic fields at different or
similar
frequencies. If different frequencies are used, a single or multiple receivers
124 may be
able to distinguish among the several transmitted frequencies in order to, for
example,
further distinguish the type of fluid change. As discussed in more detail
below, other
types of transmission characteristics, such as variations in time
transmission, wave
12
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
shape, frequency, attenuation, amplitude, and/or additional waves may be used
to
identify a particular transmitter for a particular signal.
[0055] In some examples, in addition to the receiver 124 positioned elsewhere
on the
patient's head, an additional receiver may be positioned on the same side of
the
patient's head as the transmitter 120 (e.g., the receiver may be concentric
within or may
circumscribe the transmitter 120, or may be positioned in a separate plane
from the
transmitter 120) in order to obtain a measurement of the transmitted magnetic
field
from the transmitter (not shown in Fig. 1). In other examples, the emitted
magnetic
field may be sampled from the transmitter 120 in another fashion, such as by
measuring
the current and/or voltage present on the transmitter 120. In some examples,
and with
reference to Fig. 1, the patient headpiece 106 includes A to D converters 122,
126 for
one or more of the transmitter(s) 120 and/or receiver(s) 124 proximate the
respective
transmitter(s) 120 and/or receiver(s) 124 themselves ¨ for example, A to D
converters
may be positioned on the same printed circuit board as the respective
transmitters 120
or receivers 124 in some examples.
[0056] In other examples, however, the analog signals are not converted into
digital
signals until after being passed through one or more coaxial cables (or other
transmission lines) connected to a separate processing unit (e.g., the
processing unit
104 shown in Fig. 1). In these examples, various techniques may be employed to
reduce cross-coupling between, for example, a coaxial cable carrying a signal
indicative of the transmitted magnetic field from the transmitter 120 and a
coaxial cable
carrying a signal indicative of the measured magnetic field from the receiver
124. For
example, a relatively flexible RF-316 double shielded cable may be used to
increase the
isolation between the two cables, or, in other examples, triple shielded
cables may be
used. As another option, highly flexible PVC or silicone tubing may be
provided
around the coaxial cable from the receiver 124 and/or transmitter 120.
[0057] Referring again to the headpiece 106 illustrated in Fig. 1, for
repeatable
readings, it may be important for the transmitter 120 and receiver 124 to not
move
during operation of the system 100 because such movement may introduce an
error in
the phase shift measurement. In order to overcome such errors, the transmitter
120 and
receiver 124 may be mounted in a rigid manner in some examples, for example in
an
apparatus that resembles a helmet 140, one example of which is illustrated as
Fig. 1A.
The helmet 140 may provide the necessary support and rigidity to ensure that
the
13
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
transmitter 120 and receiver 124 remain fixed relative to each other and
relative to the
patient's head. However, such a helmet 140 may be uncomfortable or impractical
to
use on a patient while they are lying down. Also, it may not be practical for
a patient to
wear the helmet 140 for several days as may be desirable in some clinical
situations.
[0058] Accordingly, in an alternate embodiment, and with reference to Fig. 1B,
the
transmitter 120 and receiver 124 are held against the head of the patient
using a headset
129, such as an elastic band 129. The transmitter 120 and receiver 124 may be
mounted on the headset 129, for example, by securing them inside a pocket of
the
headset 129, or using stitches, rivets or other fasteners. The transmitter 120
and
receiver 124 may be spaced at a fixed distance from the surface of the skin by
incorporating a non-conductive spacer material 127, such as plastic or fabric.
The
spacers 127 can serve the purpose of maintaining a fixed distance between the
transmitter 120 and receiver 124 from the skin in order to, for example,
reduce
variability of the capacitance between the transmitter 120/receiver 124 and
the skin.
The spacers 127 may be, for example plastic acrylic disks in some embodiments.
Rubber, medical adhesive, or other material may also or alternatively be used
for the
spacers 127, and may be placed at the skin interface surface of the
transmitter 120 and
receiver 124 to aid in keeping them from moving during use.
[0059] The headset 129 may be placed on the patient's head across the forehead
and
around the back of the head in some embodiments; or a different band or other
device
can be placed in other configurations, including around a patient's chest, arm
or leg. In
other words, any suitable positioning device may be used to appropriately
position the
transmitter 120 and receiver 124 proximate the area of the patient's body
under
investigation, of which the headsets 106, 129 and headbands 129 described
herein are
merely examples. Additional features such as a chin strap or a connection over
the top
of the head can be added to the headset 129 to provide additional stability
and to
provide features on which to mount additional transmitters 120 or receivers
124. Since
the patient will often be lying on a pillow, a convenient location for
electrical
components and for cable terminations might be the top of the head. For
example, a
bridge from a point near each ear may be created so that the electronics can
be mounted
at the top of the head, away from the surfaces that the patient may lie on.
Low-profile
components that are lightweight may be used so as to maximize comfort and
minimize
the tendency of the headset to move on the patient's head once in place.
14
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
[0060] In the headset 129 design, a headband 129 may be made of elastic,
rubber,
acrylic, latex or other flexible material, and may be elastic or inelastic.
The headset
129 may be fabricated from inexpensive materials so the headset can be a
disposable
component of the system. Alternatively, the headset 129 may be reusable. If it
is
reusable, the band 129 may be washable so that it can be cleaned between
patients, or
cleaned periodically for the same patient. Washable materials may include
plastic,
rubber, silicone, fabric, or other materials. The headpiece 106 may also
include
mounting means for securing the electronic components and to route the cables
to keep
them from getting in the way of the patient or the clinical staff.
[0061] In some embodiments, including those where a headband 129 is used, in
order
to reduce the relative motion between the transmitter 120/receiver 124 and the
patient,
one or more stabilizers 128 may be used. Stabilizers 128 may be custom-molded
to the
patient's body to hold the transmitter 120 and/or receiver 124 in place. As
one example
of a stabilizer 128, trained clinicians may install the transmitter
120/receiver 124 using
low-melting-point plastic that is similar to orthopedic casts made from the
same
material. Other custom-shapeable materials and methods may be used, such as
materials which polymerize over time, or with activation by heat or chemical
reaction
such as materials used for making orthopedic casts or splints.
[0062] With reference now to the exploded view of Fig. 1B, the operation of
one
embodiment of using a headset 129 will be described, although it will be
understood
that similar bands 129 may be used to monitor fluid change in other parts of
the body,
such as a bandage wrapped around a leg or an arm. Each transmitter
120/receiver 124
may first be coupled to a respective spacer 127 by, for example, a screw or
other
fastener such as glue. The transmitter 120 and respective spacer 127 may then
be
positioned on a patient's head, and the stabilizer 128 may be positioned
around the
transmitter 120/spacer 127 in order to stabilize the transmitter and help
prevent
movement. The stabilizer 128 may need to be soaked in water or otherwise
prepared
for application prior to positioning it around the transmitter 120/spacer 127.
Once the
stabilizer 128 secures the transmitter 120/spacer 127, another stabilizer 128
may
similarly be used to stabilize the receiver 124 and spacer 127 in a similar
manner. The
stabilizers 128 may solidify or dry out to perform the stabilizing function.
Then, a
headset such as a headband 129 may be wrapped around the stabilizers 128 and
transmitter 120/spacer 127 and the receiver 124/spacer 127. In some
embodiments,
CA 03042629 2019-05-02
WO 2018/089035
PCT/US2016/069209
however, no stabilizers may be used, and the headband 129 may instead be used
to
directly position the receiver 124/spacer 127 and the transmitter 120/spacer
128 on the
patient's head. In still other embodiments, and as mentioned above, the
headband 129
may include pockets for the transmitter 120 and receiver 124, with the
headband 129
material itself acting as a spacer. Also, in some embodiments, the headband
129 may
have non-slip material applied to an interior side of the headband 129 to help
prevent
slippage of the headband 129 on the patient's head.
[0063] Other examples of the headset 129 may be used as well. Fig. 8
illustrates an
isometric view of an example of the headset 129. In this embodiment, the
headset 129
may be substantially similar to the headset 129 shown in Fig. 1B. However, in
this
example, a stabilizer 800 may be included with the headset 129. Additionally,
the
headset 129 may include a flexible circuit 802 or other wiring mechanism that
may
extend between the processing unit 104 and the transmitters and receivers 120,
124.
The headset 129 may also include a securing element 804 such as a headband,
elastic,
or the like, which may be flexed and/or stretched to secure the headset 129
around a
patient's head.
[0064] The stabilizer 800 temporarily secures the headset 129 on a user's head
(or
other desired location), but may allow the headset 129 to be removed when
monitoring
is no longer desired. The stabilizer 800 may generally be a skin compatible
adhesive.
The stabilizer 800 may be two-sided adhesive where one side may be secured to
the
headset 129 (such as to the flexible circuit 802 or securing element 804) and
the other
side may be secured to the patient's head. As another example, stabilizer 800
may be
adhesive such as glue or another similar fluid or gel with adhesive
properties. As a
specific example, the stabilizer 800 may be hydrogel.
[0065] In embodiments including the stabilizer 800, the stabilizer 800
stabilizes and
locks the various components of the headset 129 onto a specific position on
the
patient's body. This helps to ensure accurate readings, as the electronics
(e.g.,
transmitters and receivers) and circuit 802 may remain in substantially the
same
orientations and positions, even if the patient moves. Further, the stabilizer
800 may
further help to prevent distortion of the electronics, as the flexible
extensions of the
transmitter and receiver (e.g., the flex circuit 802) can be shaped so as to
curve or wrap
around one dimension of the patient's head (or other monitored area), but do
not
substantially flex or stretch in the other dimension. As one example, the
lateral
16
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
positions of the transmitter and receiver 120, 124 (i.e., front to back) and
the flexible
circuit 802 may remain stable when pressed against the surface of a patient's
head.
[0066] Various embodiments include mechanical mechanisms for determining
correct
placement, alignment, and attachment to a specific position on the patient's
body. For
example, the helmet 140 in Fig. 1A, the headband 129 in Fig. 1B, the headset
906 of
Fig. 9, and the headset 950 of Fig. 11. These mechanisms help ensure accuracy
and
repeatability of the placements, which in turn helps to ensure the accuracy
and
precision of the readings. Further improvements for mechanical stability and
repeatability could be enhanced with sensors to detect and monitor a point of
contact or
series of contacts to the patient's body. For example, sensors could be placed
on arms
962 of headset 950 of Fig. 11 such that they detect when the arms 962 are in
contact
with a location where the scalp meets the ear of the patient. Additionally or
alternatively, a sensor could be located to detect when the backside of the
lenses 960 or
top internal edge of the frame is at the right location to the forehead.
Furthermore, the
sensor or sensors could monitor the continued optimal placement of the headset
during
a measurement sequence. If at any time the headset moves away from the desired
position, a sensor or sensors would send a signal to processing unit 104,
which could in
turn inform the user to correct the headset placement and or identify the
measured data
as non-ideal due to placement. A non-exhaustive list of the types of sensors
that could
be used in these embodiments include impedance, capacitive, conductive,
optical,
thermal, and distance.
[0067] Another example of a system for detecting fluid volumes in a body will
now
be discussed. Fig. 9 is a diagram of a system 900 for detecting fluid volumes
in a body.
Figs. 10A-10C illustrate various views of a patient wearing a headset 906 of
the system
900. With reference to Figs. 9-10C, the system 900 may include a headset 906
or
support structure, a processing unit 104 having a network/communication
interface for
communicating with one or more external devices, one or more
transmitters/receivers
124, 124, and a computing device 902. The computing device 902 may be in
communication with the headset 906 and/or the processing unit 904 via a
network 920.
The network 920 may be, for example, WiFi, Bluetooth, wireless, or the like,
and in
many embodiments may be wireless to allow data to be transmitted from the
processing
unit 904 and headset 906 to the computing device 902 without cables, or the
like. In
these embodiments, the computing device 902 may be external from the headset
906, in
17
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
that the computing device may be a standalone device that is in communication
with
the headset 906 via a wireless communication pathway. In other embodiments,
the
networking interface may be in communication via one or more wired pathways to
the
external computer and/or network.
[0068] The computing device 902 may be substantially similar to the computer
102 of
Fig. 1. In some embodiments, the computing device 902 may be portable, to
allow a
treating physician to more easily transport the computing device 902 between
different
patients. However, in embodiments where portability may not be needed, the
computing device 902 may be substantially any other type of computer, such as,
but not
limited to, a server, desktop computer, work station, or the like. It should
be noted that
the computing device 902, the processing unit 904, and/or headset 906 may
include a
networking interface component that provides a communication pathway to the
network 920 from each respective device.
[0069] With reference to Figs. 10A-10C, the headset 906 will now be discussed
in
more detail. The headset 906 in this example includes the processing unit 904
and the
transmitters/receivers 120, 124. The integration of the processing unit 904
and
transmitters/receivers 120, 124 onto a signal device allows the sensing unit
to be more
portable, easier to position on a patient, and enhances the mobility of the
patient while
the patient is wearing the device. Additionally, as discussed in more detail
above, in
embodiments where the processing unit 904 may do a substantial portion of the
processing of the data close to the transmitters/receivers 120, 124, the risk
of errors is
reduced and the signal to noise ratio may also be reduced.
[0070] In one embodiment, the headset 906 includes a front support structure
or
frame 910 that defines the front of the sensing device. The front support
structure 910
may support the processing unit 904 and define a frame for two lenses, e.g.,
for the left
and right eyes of the patient. In embodiments where lenses are not required,
such as
when the patient does not need to wear glasses or have other eye protection,
the lenses
may be omitted to provide clarity for a user. The front support structure 910
may be
varied as desired based on the size and structure of the processing unit 904.
[0071] With continued reference to Figs. 10A-10C, the headset 906 may also
include
two arms 912 that extend from each end of the front support structure 910. The
arms
912 are configured to wrap around a patient's head 930 and be supported above
and/or
on the patient's ears 912. The arms 912 may include contoured portions that
better fit a
18
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
patient's head 930 and/or ears 912 and that may further assist in retaining
the device in
position on the patient's head 930. The headset 906 may be adjustable and in
some
embodiments may include a securing strap 922 connected to the ends of each arm
912.
The securing strap 922 is configured to tighten around the head 930 of the
patient and
secure the headset 906 in position. For example, a fastener or other device
may
selectively adjust the length of the securing strap 922 and assist in securing
it around
the head 930.
[0072] As discussed above, in this example the headset 906 is configured to be
portable and the transmission modules, e.g., the transmitters/receivers 120,
124, are
connected to the headset 906. In one example, such as the one shown in Figs.
10A-
10C, the transmitters/receivers 120, 124 may be connected to the arms 912 of
the frame
so that when the headset 906 is positioned on the patient's head 930, the
transmitters
and receivers 120, 124 will be positioned opposed to one another and oriented
to
receive and transmit signals through the user's head 930. The transmitters and
receivers are configured to be in communication with one another and
positioned so as
to transmit or receive, respectively, signals to the corresponding device.
[0073] The transmitters/receivers 120, 124 or transmission modules may be in
communication with and receive power from the processing unit 904. For
example, a
plurality of connection wires 934 may extend from the processing unit 904 and
electrically connect the transmitters/receivers 120, 124 to the processing
unit 904. The
connection wires 934 may transmit power from a power source, such as a battery
received within the battery slot 936 on the processing unit 904, along with
data and/or
signals from the processing unit 904. Additionally, the transmitters and
receivers 120,
124 may transmit data to the processing unit 904, which may then transmit the
data to
the computing device 902. For example, the receivers 124 may transmit the
received
signals to the processing unit 904, which may then process the signals and
transmit the
data to the computing device 902 via the network 920.
[0074] It should be understood that the arrangement and configuration of the
headset
906 and processing unit 904 may be varied as desired. For example, in another
example, the communication wires 934 may be omitted or incorporated into the
frame
or support structure of the headset 906. Fig. ibis an isometric view of
another example
of the headset 906. With reference to Fig. 11, in this example, the headset
950 may be
substantially similar to the headset 906 illustrated in Figs. 10A-10C, but the
19
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
communication wires 934 may be incorporated into the material and/or structure
of the
frame 910. Additionally, in this example, the headset 920 may include lenses
960 in
the front support structure that may be modified based on the needs of the
patient. The
arms 962 of the headset 950 may extend from each end of the frame 910 and be
configured to support the transmitters/receivers 120, 124 thereon.
Additionally, in this
example a third transmitter/receiver 120,124 could be configured on the
backside of
904, adjacent to the forehead. As can be appreciated, the processing unit 904
may be
smaller and centered on the frame 910, which provides better mobility for the
patient
while wearing the headset 906. Also, as the processing unit 904 is
significantly
smaller, it may be better able to remain in position and more accurately
transmit data to
and from the computing device 902 and/or transmitters/receivers 120, 124.
[0075] In some embodiments, the processing element 904 or unit is configured
to
provide transmission data corresponding to one or more of the received
magnetic field
data as received by the transmitters/receivers to the networking interface,
which in turn
transmits the transmission data to the external computing device 902. In these
embodiments, the processing element 904 may convert the analog data as
received
from the transmitters and receivers into digital data before sending the data
to the
external computing device 902. This allows the speed of the data transmission
between
the headset and the computing device 902 to be increased and more reliable.
[0076] The apparatuses and methods described herein may be used, in various
embodiments, for fluid measurement (often fluid change measurement) in all
parts of
the body and for multiple medical diagnostic applications. The configuration
of the
emitter and detector (detector may alternatively be referred to by receiver)
coils may be
modified, in various embodiments, to be appropriate to the area of the body
and/or the
diagnostic application involved. For example, for an application involving a
limb, such
as the arm, or where it may be more important to measure liquid content at a
shallow
depth in the tissue, the emitter coil and detector coil may be placed on the
same side of
the subject tissue. A co-planar arrangement may be appropriate. Since the
coils may
be separated by a much shorter distance, the received signal strength may be
much
greater, and the size of the coils may be reduced. In various alternative
embodiments,
the coils may be in a side-by-side co-planar arrangement or in a concentric co-
planar
arrangement using coils with different diameters. In some embodiments, it may
be
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
more appropriate to place the plane of the coils at a slight angle to conform
to the shape
of the body part under study.
[0077] With the various examples of the systems described, a method of
operating the
system will now be described in more detail. With reference now to Fig. 6, one
example of the operation of the system 100 will now be briefly described, it
being
understood that various operations illustrated in Fig. 6 will be described in
more detail
below, and various alternative methods and modes of operation will also be
described
below. Beginning at operation 501, the system 100 is powered on and a self-
test is
performed. If the system 100 fails the test, a stop or fail indicator is
displayed on the
laptop 102 in operation 502. If the system 100 passes the power-on self-test,
operation
moves to operation 504. Also, throughout operation of the system 100, a
continuous
status monitor may run in operation 503, and, should the status monitor
determine that
system 100 is failing, the system may display a stop or fail indicator in
operation 502.
[0078] Once the system 100 passes the power-on self-test and operation has
moved to
operation 504, the frequency synthesis FPGA 110 may be initialized and begin
to
provide the transmitter 120 with the transmit signal in operation 504. The
waveform
averager FPGA 112 may begin to collect and average waveforms (e.g., fluid
data) from
the transmitter 120 and the receiver 124 in operation 505. The averaged
waveforms
may be provided to the phase shift measurement FPGA 114, which may determine
the
phase shift between the transmitter 120 and receiver 124 waveforms beginning
in
operation 506, with the ultimate phase calculation of interest being
calculated in
operation 507. The phase calculation may be provided to the laptop 102 in
operation
508. At any point after operation 505, the frequency synthesizer FPGA 110 may
provide another frequency to the transmitter 120, and the process may repeat
for the
next frequency. Multiple frequencies may thus be emitted from the transmitter
120 and
subsequent phase shifts calculated. For example, the frequency synthesis FPGA
110
may provide the next frequency in repeated operation 504 while the phase shift
measurement FPGA 114 measures the phase shift between the waveforms from the
previous frequency, or the frequency synthesis FPGA may not provide the second
frequency until the phase calculation has been provided to the laptop in
operation 508.
In an alternate embodiment, the emitter can emit a single frequency
simultaneously
with harmonic frequencies, or through the use of multiple frequency
generators, for
later separation using techniques such as Fast Fourier Transform (FFT).
Simultaneous
21
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
emission of multiple frequencies can be advantageous for noise cancellation,
motion
rejection and other purposes.
The Transmitter(s) and Receiver(s)
[0079] One range of electromagnetic frequencies appropriate for an inductive
phase
shift measurement based system 100 for brain fluid diagnostics is in the radio
frequency (RF) range from about 20 MHz to 300 MHz, although other frequencies
may
also be used, such as between 1 MHz and 500 MHz, between 3 MHz and 300 MHz,
and so forth. The frequencies chosen may provide relatively low absorption
rates in
human tissues, good signal relative to noise factors, such as capacitive
coupling and
signal line cross-talk, and ease of making accurate phase measurements.
[0080] Previously, certain examples of transmitters (and corresponding
receivers) that
emit (and sense) magnetic fields in these frequency ranges were constructed of
thin
inductive coils of a few circular turns placed such that the plane of the coil
is parallel to
the circumference of the head. The coils of these previous transmitters and
receivers
had diameters of 10 cm or more and 5 or more turns. These relatively large
transmitter
and receiver coils, however, were cumbersome and furthermore had resonances
within
the range of the frequencies of interest for VIPS detection of fluid in a
human brain.
When transmitter or receiver coils are operating in a frequency near one of
their natural
resonant frequencies, a measured phase shift may be largely a function of the
magnitude of the coil's own parasitic capacitances, and very small changes due
to
motion of either of the coils and/or environmental effects can cause large
changes in the
phase shift, creating unacceptable noise in the measurement of phase shift.
[0081] Accordingly, in some embodiments of the present disclosure, the lowest
natural resonant frequency of the transmitter 120 and/or receiver 124 may be
higher
than the intended frequencies of the magnetic fields to be transmitted. In
some
examples, the transmitter 120 may include a coil as a magnetic field generator
or
transducer. From symmetry considerations, this same or a similar coil may act
as a
magnetic field sensor in a receiver 124. In either case, as the diameter of
the coil and
number of turns (i.e., loops) is reduced the first self-resonant frequency
generally
increases. The limit, therefore, is for a coil with a single loop, the loop
having a very
small diameter. As the loop diameter decreases, however, the amount of
magnetic flux
intercepted by the loop is reduced by a factor equal to the ratio of diameters
squared.
Likewise, the induced voltage in the loop is reduced, resulting in a smaller
signal from
22
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
a loop acting as a magnetic field sensor in a receiver 124. Thus, there are
practical
limits on the diameter reduction. In some embodiments, however, an additional
increase in the self-resonant frequency can be achieved by using transmission
line
techniques in the construction of the transmitter 120/receiver 124.
[0082] An alternative to using coils designed for a relatively constant phase
shift over
a wide bandwidth is to add external reactive components in a series-parallel
network to
tune out the phase shift at a single frequency or at a small number of
discrete
frequencies. This concept works best if the approximate value of the
individual
frequencies is known prior to designing the overall system and the number of
discrete
frequencies is small. By using switched or motor driven tunable components,
the phase
shift tuning can be automated and software controlled. An advantage of tuning
to a
constant phase shift is that it provides more freedom in the choice of the
size and shape
of the coils. Using larger coils can increase the detected signal strength and
provide a
field shape that is optimally matched to the portion of the brain or other
body part that
is being sampled.
[0083] In one embodiment, with reference to Fig. 2A, a single loop 250 with a
high
self-resonant frequency and associated stable phase response below the self-
resonant
frequency may be constructed using a shielded transmission line, such as
coaxial cable,
buried strip-line on a printed circuit board, a twisted shielded pair of
wires, a twinaxial
cable, or a triaxial cable. The loop 250 may be used as either a magnetic
field
generator in the transmitter 120 or as a magnetic field sensor in the receiver
124. The
shielded transmission line may include a first conductor as a shield 251 that
at least
partially encloses a second conductor. The first conductor or shield 251 may
be
grounded and may form a faraday cage around the second conductor. The second
conductor may provide an output signal responsive to the changing magnetic
field, and,
due to the faraday cage, the second conductor may be shielded from external
electrostatic effects and from capacitive coupling. For example, in one
embodiment, a
single loop 250 of buried strip line may be sandwiched between two grounded
planes in
a printed circuit board. A plurality of vias may extend between the two
grounded
planes, with the spacing of the vias determined by the wavelengths of the
electromagnetic field being transmitted and/or received, and the vias together
with the
two grounded planes forming an effective electrostatic or faraday cage around
the
buried strip line loop 250. In other embodiments, other types of transmission
lines with
23
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
an outer shield (such as coaxial cable) may be used in order to form a faraday
cage and
thus reduce external electrostatic effects on the loop 250.
[0084] In single loop 250 embodiments of a transmitter 120 or receiver 124,
the
voltage of the loop 250 may not be in phase with the current of the loop 250
due to the
inductive nature of the single loop 250. This phase error may be detected and
accounted for during initialization of the diagnostic system 100, as described
below. In
some embodiments of the single transmitter loop 250, however, and with
reference to
Fig. 2B, a balun transformer 254 may be added, in order to obviate the need to
correct
for this phase error. In still other embodiments, and with reference to Fig.
2C, a
second, independent, smaller, concentric loop 260 is used to sense the
transmitted
magnetic field and provide a current representative of the same to the A to D
converter.
The second, concentric transmitter loop 260 may in some examples be the same
size as
the corresponding receiver loop (e.g., in receiver 124) in order to have
proportional
signals and good uniformity between them, whereas in other examples the
receiver loop
may be larger than the second, concentric transmitter loop 260 in order to be
more
sensitive to the received magnetic field. In those transmitters 120 with the
second,
concentric transmitter loop 260, and with reference to Fig. 2D, a balun
transformer 264
may likewise be used on this second, concentric loop 260 in order to balance
the sensed
voltage and current. Furthermore, for a single-turn receiver loop 250, a balun
254 may
likewise be added in order to also balance its performance, similar to that
shown for the
transmitter cable in Fig. 2B.
[0085] Referring now to Fig. 2E, in another embodiment, the transmission line
concept may be extended from building a single-loop, single-ended device to
building a
dual-loop 270, which may be double-ended or "balanced," for use as a receiver
124 (or,
symmetrically, for use as a balanced transmitter 120). In Fig. 2E, four
conductive (e.g.,
copper) layers 271, 272, 273, 274 may be formed on a printed circuit board as
shown,
with three layers of dielectric material (not shown in Fig. 2E) coupled
between the four
conductive layers 271, 272, 273, 274 when stacked vertically. The top and
bottom
layers 271, 274 may be grounded and thus form an electric shield. Furthermore,
small
linear breaks 271A, 274a may be present in both of the top and bottom layers
271, 274
so that the ground planes 271, 274 don't act like additional shorted turns. In
between
the top and bottom ground layers 271, 274, the +loop 273 and the ¨loop 272 may
be
positioned, with the leads from the two loops 272, 273 being coupled to a
balanced
24
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
amplifier (not shown in Fig. 2E). The +loop 273 and the ¨loop 272 may be
center
tapped in some examples. The inner diameter of the two loops 272, 273 may be
approximately 1 inch, and may be slightly greater than the inner diameter of
the
circular void in the two grounded planes 271, 274. In some embodiments, the
thickness
and permittivity of the dielectric material, the width and thickness of the
conductive
material forming the loops 272, 273, the spacing of the ground planes 271,
274, and so
forth, may be chosen such that the double loop 270 has approximately a 50 ohm
impedance in order to match the transmission line to which it will be coupled.
In this
manner, the self-resonant frequency of the dual loop structure 270 may be
above 200
MHz in some examples.
[0086] Still with reference to Fig. 2E, for a dual loop 270 used as a magnetic
field
sensor in a receiver 124, external noise that is coupled into the system 100
from
environmental changes in the magnetic field due to environmental EMI sources
or
motion of nearby conductors or magnetic materials may be reduced due to the
common-mode rejection of the differential amplifier to which the two loops
272, 273
are coupled. Having the differential amplifier coupled to the loops 272, 273
when used
as a receiver 124 thus may allow the loops' 272, 273 diameters to be reduced
while
keeping the output signal level at a suitable level for transmission to a
remote
processing unit 104 (e.g., for those systems where one or more A to D
converters are
not located directly in the headpiece 106). The amplifier power gain may be
approximately 40db in some embodiments. Low-cost wide-bandwidth amplifiers
offering gains of 40db for the power levels of interest are readily available
in
miniaturized packages from multiple suppliers with negligible phase shift
variation
over a 20 MHz to 200 MHz frequency range.
[0087] With reference to Fig. 2F, as suggested, the dual loop 270 used for a
balanced
receiver 124 has an analogous application as a magnetic field generating
transmitter
120. The balanced approach for constructing a transmitter 120 may result in a
common-mode cancellation of noise in the transmitted magnetic field due to the
opposite winding directions of the dual loops, thus reducing noise in the
transmitted
magnetic field that may otherwise result from electrostatic or magnetic pickup
from
environmental factors.
[0088] Referring still to Figs. 2E and 2F, in some embodiments, the two loops
272,
273 may be formed in different planes, or, in other embodiments, the two loops
may be
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
fabricated in the same plane with concentric circular strip-line traces (thus
reducing the
number of layers required in fabricating the pc board). This concentric design
may be
used for the transmitter 120, and/or the receiver 124.
[0089] Also, with reference to any of Figs. 2A through 2F, in examples where
the
analog to digital conversion is not done proximate the transmitter 120 or
receiver 124, a
resistive attenuator may be added to the pc board with surface-mount resistors
in order
to help reduce cross-coupling of the transmitter signal to the receiver signal
in the cable
through which the analog signals are transmitted, which may help increase
phase
measurement accuracy and stability. The on-board attenuator may result in a
substantial size and cost reduction compared with a bulky separate modular
attenuator.
Also, still continuing with examples where the analog to digital conversion is
not done
proximate the transmitter 120 or receiver 124, with reference still to any of
Figs. 2A
through 2F, one or more amplifiers may be provided to amplify the signals from
the
transmitter 120 and/or the receiver 124 in order to reduce attenuation of the
signals
through the cable to the external analog to digital converter 122, 126. Still
continuing
with examples where the analog to digital conversion is not done proximate the
transmitter 120 or receiver 124, the voltage on the transmitters and receivers
may be in
phase with current on the respective transmitters and receivers because the
"balanced"
transmitter and receivers illustrated in Figs. 2E and 2F are terminated in the
50 ohm
characteristic impedance of coaxial line.
[0090] Referring now to Fig. 3, an alternative design may include an amplifier
256 on
the same printed circuit board as the loop 250. Including an amplifier 256 on
the same
printed circuit board as the loop 250 (that is used, for example, as a
receiver 124) may
help increase the signal to noise ratio, which may be particularly useful for
embodiments where analog to digital conversion is done remotely from the
headpiece
106. An amplifier 256 may also be used in embodiments where analog to digital
conversion of a signal is done near the loop 250. As mentioned above, a balun
transformer may be also included on the printed circuit board between loop 250
and
the amplifier 256, which may help cause the coil to operate in a "balanced"
mode. In
the balanced mode, capacitively coupled electromagnetic interference pickup or
motion
induced fluctuations in the signal level may be reduced or canceled, since
they typically
equally couple into both the negative and positive leads of the balanced
differential
signal.
26
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
Initialization: Air-Scan to Remove Fixed-Phase Errors
[0091] As suggested above, the diagnostic system 100 may be initialized in
some
examples in order to calibrate the transmitter 120 individually, the receiver
124
individually, the transmitter 120 and the receiver 124 with one another and
with the
other associated electronics, and so forth. For example, variations in lead
lengths and
amplifier time delays in signal paths from the transmitter 120 and receiver
124 may be
detected during initialization and removed from the signals during signal
processing in
order to prevent fixed offset errors in the data. Also, any phase shift
between
(measured) voltages and currents in a single-turn loop 250 may be detected.
[0092] The initialization may in one embodiment be an "air-scan" where the
transmitter(s) 120 and receiver(s) 124 are positioned with only air between
them, the
transmitter(s) 120 and receiver(s) 124 positioned approximately as far apart
as they
would be if they were positioned on the head of an average patient. Once thus
spaced,
phase shift data is collected for a range of different frequencies (because
the errors may
be constant across or varying among different frequencies), and the collected
air-scan
values may be subsequently used during signal processing to correct any phase
shift
errors of the system 100 (e.g., by subtracting them from the values obtained
during
operation of the system 100). The initialization may be done when the A to D
converters 122, 126 are in the headpiece 106 proximate to the transmitter 120
and
receiver 124, when the A to D converters 122, 126 are external to the
headpiece 106,
and so forth.
Generation of the Driving and Sampling Signals
[0093] As mentioned above, the diagnostic system 100 collects phase shift data
for
transmitted time-varying magnetic fields at multiple frequencies because the
phase
shifts contributed by various tissue types and body fluids may vary with
frequency.
The diagnostic system 100 illustrated in Fig. 1 provides a flexible frequency
synthesizer 100 within the processing unit 104, although in other embodiments,
a
frequency synthesizer 110 may be provided in, for example, the headpiece 106.
This
frequency synthesizer 110 may have a minimum of 1 MHz resolution over the
range of
about 20 MHz to 200 MHz in some examples (or alternatively about 20 MHz to 300
MHz or about 10 MHz to 300 MHz or any of a number of other suitable ranges).
Standard digital phase-lock loop techniques may be used to derive the
selectable
27
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
frequencies from a single stable crystal-controlled clock oscillator. As
described
above, the digital portions of the synthesizer 110 may be implemented in one
of the
FPGAs 110 in the processing unit 104. The synthesizer 110 may produce both a
basic
square wave clock signal for generating the magnetic field in the transmitter
120 as
well a sampling signal. The sampling signal may be at a slight offset (e.g. 10
KHz) in
frequency from the magnetic field generating signal in some embodiments. The
square
wave signal for generating the magnetic field may, in some embodiments, be
amplified
to correct its level and may also be filtered to eliminate higher order
harmonics and
achieve a low distortion sine wave at one or more fundamental frequencies.
[0094] In other cases, where frequency domain techniques such as FFT
processing of
the time domain data are used to calculate phase, it may be advantageous to
accentuate
the harmonics of the fundamental frequency. For these embodiments, additional
circuits may be added after the basic frequency synthesizer to make the rise-
time or
fall-time of a square-wave or pulse wave-shape much faster, thereby increasing
the
relative amplitude and number of higher order harmonics. As mentioned
previously,
this embodiment allows generation of a "comb" of frequencies with a single
burst of
RF and the processing of the captured time domain data from the emitter and
detector
using Fourier techniques yields a simultaneous time correlated phase
difference data set
for each frequency in the "comb". This simultaneous capture of phase data from
multiple frequencies may yield significant advantages for separating the
desired
information about the patient's brain fluids from motion artifacts or other
effects that
would affect an individual scan of the frequency where the phase data for each
frequency is measured at different times. Sampling each frequency at different
times in
this case introduces noise that may be difficult to detect or remove.
[0095] As the signal used to generate the magnetic field is typically
periodic, it may
not be necessary to use a sampling frequency that is many times greater than
the
frequency of that signal to capture the phase information from a single cycle
of the
waveform, and instead an under-sampling technique may be employed in some
examples. Under-sampling is similar to heterodyning techniques used in modern
radios
where a large portion of amplifier gain and the audio or video signal
demodulation is
performed in much lower intermediate frequency stages of the electronics (IF).
Under-
sampling, in effect, allows a system to collect the same or a similar number
of sample
28
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
points over a longer period of time, while not disturbing the phase
information of the
signal.
[0096] Using under-sampling may eliminate the need for high-speed A to D
converters (which are expensive and may involve many different wired
connections)
that may otherwise be required to capture enough phase samples from a single
cycle of
the waveform to accurately measure phase angle. If a lower speed A to D
converter
may be used, it may be commercially and physically practicable to position the
A to D
converter 122, 126 proximate the transmitter 120 and receiver 124 loops 250,
270, as
described above.
[0097] Therefore, in some embodiments, one or both of the transmitted and
received
magnetic field signals may be under-sampled (e.g., with one sample or less for
each
cycle) and an average record of the waveform may thus be captured using
samples
taken over a much longer interval of time compared to one cycle. In order to
accomplish the under-sampling, both the transmit signal and the sampling
signal may
be derived from a common clock signal, with the sampling signal being
accurately
offset from the transmit signal frequency (or a sub-harmonic frequency) by a
small
amount. If the offset is, for example, 10 KHz from the first harmonic
frequency of the
transmit signal, the result after a period of 100 microseconds will be an
effective
picture of one cycle of the repetitive transmit waveform with f/10000
individual
samples. For a transmit signal frequency of 100 MHz and sample frequency
100.010
MHz, the 10,000 under-sampled individual samples of a single cycle of the
transmit
waveform are spaced at a resolution of 360/10000 or .036 degrees. As one
alternative
to under-sampling, frequency conversion using standard non-linear mixing
technology
before an A to D converter 122, 126 may also be employed.
[0098] In other examples, the frequency of the magnetic field generator signal
and the
frequency of the sampling signal may be otherwise related, one example of
which is
described below when referring to frequency domain signal processing
techniques. In
still other examples, the sampling frequency may be relatively constant (e.g.,
210 MHz,
while the generating frequency may vary over a wide range).
Conversion of the Transmitted and Received Analog Signals to Digital Data
[0099] In some embodiments, electronic phase shift measurements between the
transmit and receive signals may be performed using analog signal processing
29
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
techniques, whereas in other examples the phase shift measurements may be
performed
after converting the analog data to digital data through one or more A to D
converters
122, 126, as described above. The digital waveforms may then be processed to
obtain
the relevant phase shift information. Processing digital data rather than
analog data
may facilitate sampling and averaging many cycles of the waveforms in order
to, for
example, reduce the effects of random noise and, with proper techniques, even
reduce
non-random periodic noise such as AC line pickup at frequencies near 60 Hz.
Also,
after reducing the noise in the waveform data there are many methods, such as
correlation, that may be employed to obtain accurate phase measurement using
digital
signal processing.
[00100] In some examples of the diagnostic system 100 described herein, the A
to D
conversion of both the transmitted and the received signals is performed as
close as
feasible to the point of generation and/or detection of the magnetic fields.
For example,
the A to D conversion may performed in the headpiece 106 by miniaturized
monolithic
single chip A to D converters 122, 126 located integral to the printed
circuits that,
respectively, contain the transmitter 120 and receiver 124. The A to D
converter 122
for the transmitter 120, for example, may differentially sample the voltage
across the
balanced outputs of the transmitter 120 in one example. The A to D converter
126 for
the receiver 124, for example, may be positioned at the output of a wide
bandwidth
signal amplifier coupled to the receiver 124. By locating the A to D
converters 122,
126 on the headpiece 106 rather than in a remote processing unit 104 (which
may,
however, be done in other embodiments described herein) it may be possible to
reduce
or eliminate the effects of phase shifts associated with motion, bending, or
environmental changes on the cables carrying the analog signals to the A to D
converters 122, 126. Other sources of error that may be reduced or eliminated
include
cable length related standing-wave resonances due to small impedance
mismatches at
the terminations and cross-coupling between the transmit and receive signals
on the
interconnecting cables that generate phase errors due to waveform distortion.
To
realize similar advantages in an embodiment where the A to D converters 122,
126 are
not located proximate the transmitter 120 and receiver 124, a single cable may
be used
to bring the sampling signal to the transmitter and receiver A to D converters
122, 126
in the processing unit 104, and/or a high quality semi-rigid cable may be used
between
the two A to D converters 122, 126 in some embodiments.
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
Overall Operation and Pipelining
[00101] Referring again to Fig. 1, the waveform data (which may be under-
sampled in
some embodiments) may be captured for both the transmitted and received
magnetic
fields, and the captured waveforms may be at least partially processed in real-
time (or
substantially real-time). As described herein, one FPGA 112 may average the
data for
each of the two waveforms over many cycles for noise reduction. Another FPGA
114
may then use a correlation technique to perform a phase shift measurement
using the
averaged waveform data. A pipelining technique may be used in some embodiments
to
speed up the data throughput for collection of phase data over multiple
frequency
samples. The transmitter 120 may generate a time-varying magnetic field at a
first
desired frequency, and the requisite number of waveform averages may be
performed
by the waveform averager FPGA 112 at this first frequency.
[00102] After the averager FPGA 112 collects and averages all of the sample
data
points from the transmitter 120 and receiver 124, it may transfer the same to
the phase
shift measurement FPGA 114. In some embodiments, only a single transmit
frequency
is used in detecting a fluid change in a patient, but in other embodiments, a
plurality of
different transmit frequencies within a desired spectral range may be
generated and the
corresponding data collected. In those embodiments with multiple transmit
frequencies, phase determination for a first transmit frequency may proceed in
the
phase shift measurement FPGA 114 (using the data acquired during the first
transmit
frequency) while the frequency synthesizer FPGA 110 causes the transmitter 120
to
generate a magnetic field having a second desired frequency of the spectral
scan and
the waveform data from the second transmit frequency is averaged by the
waveform
averager FPGA 112 (hence the pipelining). In other embodiments, the waveform
averaging for one transmit frequency may occur substantially simultaneously
with
recording a plurality of samples for a second frequency. In general, many
different
types of pipelining (e.g., performing two or more parts of the signal
generation,
acquisition, and data processing at substantially the same time) may be used.
In other
embodiments, however, there may not be any pipelining, and the diagnostic
system 100
may transmit, collect, average, and process all of the data relating to a
single transmit
frequency before moving to a second transmit frequency.
[00103] Regardless of whether pipelining is used, the process of using
different
transmit frequencies may be repeated for any number of transmit frequencies
with a
31
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
desired spectral frequency scan, and may also be repeated for one or more
frequencies
within the spectral scan. The calculated phase shifts for each frequency may
be
transferred to the laptop 102 directly from the phase shift measurement FPGA
114 in
some examples.
Signal Processing ¨ Averaging
[00104] Because of the relatively small size of the transmitter 120 and the
receiver
124, as well as the relatively low power of the transmitted magnetic field (it
is low
power because of, among other things, the need to protect a patient from
overexposure
to RF radiation and the need to minimize electromagnetic field emissions from
the
system 100), the measured magnetic field at the transmitter 120 and/or at the
receiver
124 may have relatively large amounts of noise compared to its relatively
small
amplitude. The noise may include input thermal noise of an amplifier,
background
noise from EMI pickup, and so forth. In some embodiments, the noise may
contribute
a significant fraction to the phase shift measurements relative to the actual
phase shift.
For example, 1 ml of fluid change may correspond with a .3 degree phase shift,
and
thus if the noise in the transmit and receive signals is a substantial portion
of, or even
exceeds, the expected phase shift, the noise may render the data unacceptable.
[00105] In order to reduce the noise, the diagnostic system 100 described
herein may,
in some embodiments, sample many cycles of the transmitted and received
magnetic
fields (e.g., many multiples of 10,000 samples, such as 32,000 samples) and
may
average the individual samples in order to substantially reduce random noise
or filter
specific frequencies. In some examples, the total sampling time interval may
be
extended to be an approximate integer multiple of one 60 Hz AC power period in
order
to reduce the effect of 60 Hz related electromagnetic interference pickup. As
explained
below, these waveforms may be averaged by any appropriate averaging technique,
including multiplying them by one another in the time domain, as well as other
frequency domain averaging techniques.
[00106] Referring now to Fig. 4, one embodiment 300 of a simplified logic
diagram of
the waveform averager FPGA 112 is shown. Of course, in other embodiments,
custom
circuitry may be employed to average data, which custom circuitry may be
located in
headpiece 106, in processing unit 104, in laptop 102, or in another suitable
location.
Fig. 4, however, illustrates one example of logic that may be implemented in
the
32
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
waveform averager FPGA 112 for averaging the transmitted waveform samples
after
they have been digitized by an A to D converter. Similar logic 300 may be used
to
average the received waveform samples after they have been digitized. The
input to the
waveform averager FPGA 112 may be a low voltage differential signaling (LVDS)
type
of format from the A to D converter, in order to reduce the wiring needed
between an A
to D converter and the waveform averager FPGA 112. In the LVDS format, each
word
of digital data representing a single waveform data-point may first be
converted from
serial data to parallel data by the deserialization logic described below.
[00107] The logic illustrated in Fig. 4 includes a synchronous serial-in,
parallel-out
shift register 301 that is clocked by the data transfer clock from the A to D
converter.
The parallel data words are then transferred into a memory buffer 302 with
sufficient
capacity to handle the maximum number of individual waveform samples required
to
construct one complete cycle of the transmitted waveform. An adder 303 may be
used
to accumulate the sum of all of the waveform samples in the memory buffer 302
as the
data words exit the register 301 or after the memory buffer 302 is fully
populated.
Each waveform sum memory location may have a word size in bits that can
accommodate the largest number expected for the sum without overflow. For
example,
a 12 bit resolution A to D converter and 4096 waveform sum requires a 24-bit
memory
word size. After accumulating the sum of the intended number of waveforms in
the
waveform memory for the transmitted signal samples (and, separately, the
receiver
signal samples are similarly summed in a waveform averager), the memory
contents for
both waveforms are serially transferred to the phase shift measurement FPGA
114. It
may not be necessary to divide by the number of waveforms being averaged in
some
examples because, in the next step of the processing, only the relative
magnitudes of
the data-points in the averaged waveforms may be relevant. Because of this, an
appropriate number of least significant bits may also be deleted from each of
the
averaged waveform data points without significant impact to the accuracy of
the overall
phase shift determination.
Signal Processing ¨ Determining Phase Shift
[00108] Referring now to Fig. 5, the phase shift measurement FPGA 114 may also
contain two revolving shift registers 401, 402, a multiplier 403, and an adder
404. It
may also include logic configured to calculate the sum of the product of the
individual
33
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
transmit and receive averaged waveform data points with an adjustable phase
shift
between the two waveforms. The FPGA may be used to find the phase shift where
the
sum of products is closest to zero and the slope of the sum of products versus
phase
shift is also negative.
[00109] Consider the following trigonometric identity for the product of two
sine
waves with frequency f and phase shift (1):
Sin u sin v = 1/2 [cos(u-v)-cos(u+v)] where u = 2aft+d) and v = 2aft (Eq. 1)
= 1/2[cos(c1:1) ¨ cos(27c(2F)t+M] (Eq. 2)
[00110] The first term of the product is a DC term dependent only on the phase
shift.
The second term is another sine wave at twice the frequency which averages to
zero
over one complete cycle of the original frequency. Note that the first term (a
cosine
wave) is also zero when the phase angle (14:1) is either +90 or -90 .
Furthermore the
slope of the product with respect to phase angle changed (sin u sin v)/4 is
negative for
(1)=+90 and positive at =-90 .
[00111] By iteration, the FPGA may determine the value of noffset where the
transmitted wave and received wave are closest to a +90 phase shift. For an
offset of
noffset samples, and nt samples for one complete 360 waveform, the phase
shift is then
calculated using the following equation:
Phase shift = 90 + (noffsetint) * 360 (eq. 3)
[00112] The resolution of the determination may be limited to the number of
samples
(resolution= 360 /nt). If this resolution is insufficient for the needed
precision of the
measurement, then interpolation may be used to find the fractional value of
noffset where
the sum of product terms exactly passes through zero.
Frequency Domain Signal Processing Methods for Phase Shift Measurement
[00113] As explained above (see e.g., sections on averaging and multiplying
waveforms together to obtain phase shift data), the signal processing of the
measured
and digitized magnetic field traces from both the transmitter 120 and the
receiver 124
may proceed in the time domain. In other embodiments, however, the signals may
be
processed in the frequency domain using, for example, Fast Fourier Transforms
(FFTs)
[00114] In one embodiment of Fourier domain analysis, the signals from the
transmitter 120 and receiver 124 are digitized at, for example, about a 200
MHz
34
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
sampling rate with a relatively high resolution (e.g., 14 bits). The A to D
converter and
the data capture electronics may be included in a relatively small printed
circuit
assembly packaging. The captured data may be transferred via a high-speed USB
serial
link to the laptop computer 102. Time domain processing can then be replaced
by
frequency-domain processing on the laptop 102 to calculate the phase shift
between the
waveforms.
[00115] Once the data is on the laptop 102, the FFT for each of the
transmitter and
receiver time domain waveforms can be calculated (in other embodiments,
however,
the FFT may be calculated by an FPGA or other processor proximate the A to D
converters). The resulting real and imaginary solutions which represent the
resistive
and reactive frequency domain data can then be converted from cartesian to
polar
coordinates, thus yielding frequency domain plots of the magnitude and phase
of the
waveforms. The phase of each waveform can be obtained from the frequency
domain
plots of phase for the frequency of interest. If the fundamental frequency is
off-scale,
then a difference frequency between the sampling frequency and the transmitted
wavefield frequency can be used. For example, a sample frequency of 210 MHz
yields
an FFT with a frequency range of 0 to 105 MHz, and the fundamental frequency
is used
for phase shift measurement when the transmitted wavefield frequency lies in
this
range. The difference frequency is used if the transmitted wavefield frequency
is in the
higher end of the range, for example, 105 MHz to 315 MHz.
[00116] After the FFT for both of the transmitted and received wavefield
signals is
calculated, the phase shift for a particular frequency of interest can then be
calculated
from the difference of the phase values obtained from the transformed
transmitter and
receiver waveforms. Note that some sign reversals for the phase information in
various
frequency regions may be needed when calculating the shift.
[00117] In order to allow FFTs to be computed for samples from the transmitter
120
and the receiver 124, the frequencies used for the sampling and the
transmitted
waveform may be determined so as to allow coherent sampling so that both the
transmitted and received waveforms contain an integer number of complete time
periods of the repeated waveform, and the number of samples collected for the
waveforms is an even power of two. One method for implementing coherent
sampling
is to choose transmitter and receiver sampling frequencies such that
primei/ftransmit =
prime2/freceive= The prime numbers prime' and prime2, as well as the number of
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
samples, can be very large in some embodiments, thereby reducing the spacing
between
the allowable values for the signal frequencies (e.g., the tuning resolution
may be
approximately 1 Hz). This may be accomplished by using digital frequency
synthesis
techniques, such as by combining a stable frequency source and the appropriate
combinations of integer frequency multipliers, integer frequency dividers, and
phase
lock loops.
[00118] With coherent sampling, the theoretical accuracy of the phase
calculation may
only be limited by the number of samples of the time domain waveform and the
digital
resolution of the A to D converter. Dc noise and low frequency noise sources
such as
1/f noise may be inherently rejected by the frequency domain processing
technique.
The use of coherent sampling also reduces the probability that harmonic and
intermodulation product frequency components will lie on top of the
frequencies of
interest for calculating phase. Furthermore, using an FFT frequency domain
solution to
determining phase may provide information regarding the magnitude or amplitude
of
the measured transmitted and received magnetic fields. The ratio of the
magnitude
values can be used to determine the attenuation of the transmitted magnetic
field, which
may be expressed in logarithmic dB power ratio units.
Alternative Signal Processing in the Time Domain
[00119] As one additional alternative signal processing technique in the time
domain,
the phase shift measurement may be done via one or more relatively low-cost
analog
phase detectors or by measuring time delays between zero crossings of the
transmitted
and received wavefield signals. For example, an integrated phase detector
circuit may
include an amplifier that converts sine waves of transmitted and received
wavefields to
square waves by clipping the sine waves (e.g., with an extra high gain), and
then
compares the clipped/square wave from the transmitter with that from the
receiver
using an analog exclusive OR (XOR) gate, with the pulse width provided by the
XOR
gate being indicative of the phase shift between the transmitted and received
magnetic
fields.
Reduction of Phase Measurement Errors Due to Motion
[00120] Among all of the factors that contribute to phase measurement error,
many are
related to motion ¨ motion of the patient, movement of the transmitter 120,
movement
36
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
of the receiver 124, bending of the connection or transmission cables, etc.
For
example, relative motion between the patient and the transmitter 120/receiver
124
results in path length and location variations for the magnetic field lines as
they pass
through the patient's head. Conductive or magnetic objects moving near the
transmitter
120 and/or near the receiver 124 can also change the shape of the magnetic
field lines
as they pass from the transmitter 120 to the receiver 124.
[00121] In some embodiments, methods may be deployed to reduce artifacts
attributable to patient movement. These algorithms may, for example, detect
statistical
variations in the differential phase shift data across the frequency spectrum
of interest
(e.g., from about 30 MHz to 300 MHz or about 20 MHz to 200 MHz) that could not
possibly be the result of biological changes, as determined by their rates of
change or
other characteristics. This thresholding-type of method may thus be used to
eliminate
data corrupted by means other than true biological changes.
[00122] As another example, the attenuation data that is obtained from the
magnitude
portion of the FFT processing can be utilized in algorithms by examining the
way it
varies across the frequency spectrum to aid in the detection and correction of
motion
artifacts in the phase shift data.
[00123] As still another example, electronic accelerometers can additionally
or
alternatively be used to detect motion of one or more of the transmitter 120,
the
receiver 124, the patient, or the transmission cables. In some examples,
accelerometers
may be coupled to the same printed circuit board as the transmitter or
receiver (e.g.,
using a MEMS type accelerometer).
[00124] In addition to detecting any motion above a threshold level, a
relationship
between the transmitter/receiver accelerometer data and patient accelerometer
data may
be examined for relative differences. For example, small amplitude changes
sensed in
both the patient and the transmitter/receiver may be of little consequence.
Some patient
motion is almost always present (because, e.g., even comatose patients
breathe).
Larger or non-correlated accelerometer readings, however, may be used to
trigger data
rejection or correction. Because the separate motion of totally independent
objects near
the patient can also present motion artifacts in the data then some types of
motion
detection and correction based on statistical analyses of the phase data may
still be
required.
37
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
Medical Diagnostic Methods for Alerting Clinicians
[00125] The system 100 described herein may be used to, among other things,
measure
the change in phase shift induced by changes in fluid content within, for
example, a
patient's head ("intracranial fluid"). Methods can be employed to analyze the
phase
data and make a determination as to whether the fluid change represents a
tissue change
that is troubling to the clinician user. For example, a baseline reading of
the phase shift
between a magnetic field transmitted from a transmitter 120 positioned on one
side of a
patient's head and a magnetic field received at a receiver 124 positioned on
the other
side of the patient's head at one or more frequencies may be recorded when the
patient
first arrives at the hospital. Then, any significant changes in the measured
phase shift
that occurs during subsequent scans can be tracked and trended by clinicians
to aid in
understanding the patient's clinical condition, and certain thresholds,
patterns or trends
may trigger an alarm. Many methods may be employed and optimized to provide
the
clinicians with the most useful fluid change information. For example, if the
phase
shifts by more than a certain number of degrees, the system may sound an alarm
to alert
the clinician that the patient may have clinically significant bleeding or
edema. For
some conditions, it may be useful to alert the clinician if the rate of change
of the phase
shift exceeds a threshold.
[00126] The phase shifts at different frequencies may vary with different
fluid changes,
as described, for example, U.S. Patent No. 7,638,341, which is hereby
incorporated by
reference in its entirety for all purposes. Certain patterns of phase shift
may be
correlated with certain clinical conditions. For example, a condition such as
bleeding
or edema may be evidenced by an increase in phase angle at one frequency, with
a
concurrent decrease at a different frequency. Using ratios of phase shifts at
different
frequencies can provide additional information about the types of fluids and
how they
are changing. For example, the ratio of phase shift at a first frequency to
the phase shift
at a second frequency may be a good parameter to assess blood content or to
separate
edema from bleeding or other fluid change. For example, the phase shift
frequency
response of saline may be different from the phase shift frequency response of
blood,
thus allowing a clinician to separately identify changes in blood and saline
content in a
patient's brain cavity. Changes in amounts of water may have relatively little
effect on
phase shift in some instances, although the concentration of electrolytes in
an ionic
solution may have a more pronounced effect.
38
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
[00127] The phase shift patterns may also be time dependent. A hypothetical
clinical
condition may be characterized by an increase in phase shift for some period
of time,
then stabilizing, and then returning to baseline after some other time period.
Noise
factors such as patient activities like getting up out of bed, eating, getting
blood drawn
or speaking with visitors may cause changes to the phase shift readings from
baseline.
Clinically meaningful fluid changes may be differentiated from noise by
examining the
patterns associated with different activities.
[00128] Using combinations of phase shift and /or attenuation data at various
frequencies, ratios or other functions of those phase shifts and/ or
attenuations, and/or
time-based methods may all be combined and optimized in various embodiments to
provide a range of useful information about tissue and/or fluid changes to
clinicians.
The clinicians can then respond to the tissue changes by using more specific
diagnostic
techniques such as medical imaging to diagnose a clinical problem.
[00129] In some cases, therapies may be changed in response to fluid and/or
tissue
change information. For example, the diagnostic system described herein may
monitor
fluid changes in a patient who is on blood thinners to dissolve a clot in a
cerebral artery.
If the system detects an intracerebral bleed, the blood thinners may be
reduced or
stopped to help manage the bleeding, or other interventions such as vascular
surgery
may be performed to stop the bleeding. As another example, a patient who
begins to
experience cerebral edema may undergo medical interventions to control or
reduce the
edema, or can undergo surgical procedures to drain fluid or even have a
hemicraniectomy to reduce intracerebral pressure due to the edema.
[00130] Clinicians may, in some cases, use fluid change information to manage
medication dosage by examining what is effectively feedback from the
diagnostic
system. For example, if mannitol is used to reduce intracerebral pressure by
drawing
water out of the brain, a treating clinician may use the diagnostic system
described
herein in order to receive feedback regarding how the patient's brain water is
changing
in response to the medication.
[00131] Similarly, drugs for blood pressure management, electrolyte
concentration and
other parameters may be more effectively administered when dosage amounts are
controlled responsive to feedback from the diagnostic system described herein.
For
example, cerebral sodium concentrations may be controlled using intravenous
hypertonic or hypotonic saline solutions. Changes to the ion concentrations
can be
39
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
detected as a shift in phase angle or some function of shift in phase angle at
one or
more frequencies. Such information can be used as feedback to the physician to
better
manage the patient.
Additional Embodiments
[00132] One embodiment of a VIPS system for monitoring intracranial/brain
fluid(s)
houses all of the electronics in the headpiece 129. The headpiece 129 could be
constructed like a helmet or hardhat. The radiofrequency oscillators can be
placed near
the emitter or multiple emitters 120/124, potentially on the same printed
circuit board.
One oscillator can generate the transmitter signal, and another oscillator can
be used to
generate the sampling signal. As will be discussed later, multiple
transmitters or
receivers may be used, and it may be desirable to have different oscillators
for different
transmitters. Therefore, multiple oscillators may be used. In another
embodiment, the
headpiece 129 could be constructed like a pair of spectacles. One advantage of
such an
embodiment is that the position may be better controlled because the device
would be
mechanically registered to the nose and two ears, making it possible to remove
and
replace the device with good repeatability of antenna location. The antennas
can be
placed on the temples of the glasses, just above and in front of the ears,
providing a
location approximately at the center of the brain. The antenna placement near
the ears
has the feature of being close to the mechanical reference points, and
therefore
providing for good position repeatability.
[00133] In some embodiments, multiple transmitters may be used, transmitting
frequencies that are offset from each other. For example, three transmitter
antennae
may be used, and each antenna may transmit a frequency that is several KHz
different
from the others. The frequency of all three oscillators should be derived from
the same
stable reference oscillator, using digital phase locked loop synthesis
techniques to
reduce phase errors due to the differences in thermal frequency drift and
phase noise of
separate oscillators. One advantage of having slightly different frequencies
for each
transmitter is that the system could then identify and separate out the
signals produced
from each transmitter, for example, using a Fast Fourier Transform (FFT).
Using this
technique, all transmitters could briefly be powered on simultaneously, and
all of the
received phase information for each transmitter/receiver combination could
simultaneously be determined using FFTs of the transmitted and received
waveforms
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
for the same extremely small time interval. This information can allow the
system to
resolve the location of a fluid change within the tissue and also
differentiate from phase
changes caused by motion of the patient, tissue fluid flow, or motion of the
antennae or
field motion generated from moving objects in the environment. For example,
such a
system may be used to specifically identify the location of a hematoma or
volume of
ischemia inside the brain of a patient.
[00134] For medical applications, it may be desirable to transmit signals
within the
industrial, scientific and medical radio band (referred to herein as the "ism
band").
However, it may be desirable to design the system to transmit outside this
band so as to
reduce exposure to more ambient radiofrequency noise coming from other devices
operating in the ism band.
[00135] In order to improve the system's robustness against ambient
radiofrequency
noise, the system can detect the ambient radiofrequency noise during time
periods
when the oscillators are not transmitting any signals. If the noise at certain
frequencies
is too high, then the system can shift to generating signals at a different
frequency, thus
improving the signal-to-noise ratio. In some applications, it may be desirable
to use
spread spectrum techniques for measuring the phase, in order to spread the
electromagnetic interference frequencies over a wider range of frequencies to
improve
the signal-to-noise ratio. To facilitate changing frequencies, multiple
crystals could be
installed in the device, and the system could select between the crystals to
allow for
selecting the most appropriate frequency given the noise environment.
Alternately, the
digital RF frequency synthesizer could have sufficient bandwidth and
resolution to
facilitate rapid frequency synthesis for the new frequencies from a single
reference
crystal oscillator. If necessary, the reference crystal oscillators could be
oven stabilized
to further reduce phase errors from temperate drift.
[00136] When generating the signals, a variety of wave shapes may be employed.
A
square wave will provide more power at harmonics of the fundamental frequency.
Sine
waves and distorted square waves can be used to push more of the
radiofrequency
power into the higher frequencies, or to provide power at various harmonic
frequencies.
Alternatively, a base frequency and higher frequency can be summed together
for
additional power at the different frequencies. A separate RF frequency may
also be
required for the sample signal for analog to digital conversion. For adequate
resolution
in the phase measurement, the required resolution on the sampling frequency
may also
41
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
be very high to allow coherent sampling. Digital frequency synthesizers could
utilize
various combinations of phase lock loop stabilized frequency multipliers and
frequency
dividers to achieve the high resolution needed for coherent sampling, while
also
generating the slightly offset frequencies for multiple transmitters. A
receiver amplifier
with high gain and good phase stability is needed. In one embodiment,
amplification of
about 40 dB of gain is used. In some embodiments, the receiver amplifier may
employ
two or more gain stages, for example, 20 dB on the antenna and an additional
20 dB on
the analog-to-digital conversion board.
[00137] Analog-to-digital converters can also be included on the same printed
circuit
boards with the emitter and receiver antennas, along with any amplifiers that
are
appropriate to amplify the signals to an optimum level.
[00138] Data can be transferred from the helmet to the console with various
high-speed
cable connections and protocols. Using metal cables can induce a source of
error by
changing the shape of the magnetic field. To avoid this problem, fiber-optic
cables can
be used as an alternative to metal cables.
[00139] Data can be transmitted wirelessly from the patient headset or helmet
to a
console with a wireless protocol such as Bluetooth, WiFi, wireless, or other
suitable
protocol. The data transmitted can be time domain data, or an FFT can be
performed by
a processor in the headpiece, and the resulting digital data may then be sent
wirelessly
to the console. The primary advantage to sending the data in the frequency
domain with
an FFT is a reduction in the amount of data, resulting in a lower required
data
transmission rate. The FFT may be performed by a processing element, such as a
field-
programmable gate array (FPGA) hardwired to perform FFT inside the helmet.
Alternatively, other types of microprocessors, including general-purpose
microprocessors, could be used to perform the FFT. Because all of these
electronics are
mounted inside the helmet or other headpiece 129, in some embodiments, it may
be
advantageous to minimize the size and power consumption of the components. To
further reduce the need for an electrical cable connection to the console, a
portable
rechargeable battery based power system may be included in the helmet.
[00140] In one embodiment, the system is designed to take multiple samples per
second, either continuously or in short bursts, so that the data may be
analyzed to
measure a patient's heart rate, or provide other useful information. This
technique may
help to differentiate arterial from venous blood volume measurements, much
like the
42
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
technique used in pulse oximetry. In another embodiment, the system may be
configured to synchronize to an EKG, pulse oximetry, or other cardiac signal.
This may
provide a very accurate timing trigger for measuring the arterial and venous
blood
volume simultaneously with a particular portion of the cardiac cycle.
Synchronizing
VIPS readings to an external cardiac signal allows under-sampling relative to
cardiac
rhythm, with VIPS readings which can be spaced seconds apart. By comparing
VIPS
readings at different portions of the cardiac cycle, a series of VIPS readings
can be
processed to reconstruct fluid composition changes associated with the cardiac
rhythm,
revealing a measure of the global perfusion within the brain.
[00141] An illustrative system for synchronizing VIPS readings with the
cardiac signal
will now be discussed. As should be understood, the embodiment of Fig. 7 may
be
modified with substantially any type of physiologic sensor for detecting
variations in a
patient's body and should not be limited to the cardiac signal specifically
discussed.
Fig. 7 is a block diagram of a system 700 for detecting and monitoring bodily
fluid
volumes due to or occurring with a cardiac cycle of the patient. The system
700 of Fig.
7 may be substantially similar to the system 100 of Fig. 1. However, in the
embodiment of Fig. 7, the system 700 includes a cardiac module 701, which may
include a cardiac cycle sensor 702 and a trigger 704. The cardiac cycle sensor
702 may
be substantially any type of sensor or combination of sensors that detect the
electrical
activity of a patient's heart. For example, the cardiac cycle sensor 702 may
be
configured to detect the polarization and depolarization of cardiac tissue.
The cardiac
cycle sensor 702 may further be in communication with the processing unit 104,
microcontroller 118, or other processing element that may transform the
various signals
into a cardiac waveform or other desired form. In a specific example, the
cardiac sensor
702 may be a pressure sensor that detects changes in pressure within a
patient's body to
detect characteristics of the cardiac cycle. In another example, the cardiac
sensor 702
may be an acoustic sensor that senses changes in sound to detect
characteristics of the
cardiac system. The cardiac cycle sensor 702 may be formed integrally with the
headpiece 106 or may be a separate component therefrom.
[00142] The trigger 704 may be substantially any type of device that may
receive
and/or transmit signals. The trigger 704 may be in electrical communication
with the
cardiac sensor(s) 702 and may be configured to transmit a signal, such as an
infrared
43
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
pulse (open air or optical fiber), a radio frequency pulse, and/or radio
frequency digital
communication based timing pulse, to the processing unit 104 and/or headset
106.
[00143] Using the system 700 of Fig. 7, a VIPS measurement may be triggered
wirelessly or wired by the trigger 704. For example, based on detection of a
particular
cardiac event (e.g., pulse oximetry) or other cardiac signal, the trigger 704
may indicate
to the processing unit 104 to activate a VIPS reading, so that data may be
detected and
collected at specific portions of the cardiac cycle. In this example, the VIPS
detection
may be based on a cardiac event. However, in other embodiments, the detection
antenna or wiring on the cardiac sensor 702 may be sensitive to VIPS radio
transmission frequencies and may be configured to be activated by the VIPS in
order to
capture the instant of each VIPS data acquisition pulse within the EKG record
(an
augmentation and/or alternative means of assuring very accurate correlation of
VIPS
data to cardiac cycle data)
[00144] With each heartbeat, the volume of arterial blood, venous blood, and
cerebrospinal fluid in the brain fluctuate, and these changes, as detected by
VIPS
monitoring, may yield valuable diagnostic information. In one embodiment, the
system
is designed to take multiple samples per second, either continuously or in
short bursts,
so that the data may be analyzed to measure a patient's heart rate. In another
embodiment, the system may be configured to be triggered by and synchronized
to an
EKG, pulse oximetry, or other cardiac signal. This may provide a very accurate
timing
trigger for measuring fluid conditions, including arterial blood volume,
venous blood
volume, and cerebrospinal fluid volume at one or more particular portions of
the
cardiac cycle. This technique may help to differentiate arterial from venous
blood
volume measurements, much like the technique used in pulse oximetry.
[00145] In yet another embodiment, the VIPS measurements are not triggered to
synchronize to an EKG or other external cardiac signal, but are time tagged
with
sufficient precision to assign each VIPS measurement to the portion of the
cardiac
cycle under which it was collected. By comparing VIPS readings at different
portions
of the cardiac cycle, either by synchronous acquisition or by subsequent
analysis, a
series of VIPS readings can be processed to reconstruct fluid composition
changes
associated with the cardiac cycle. Such analysis of VIPS measurements may
reveal a
measure of the global perfusion within the brain, as well as valuable
information for the
diagnosis of conditions, such as shunt failure (detailed later in the
specification). These
44
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
methods (synchronizing VIPS readings to an external cardiac signal or time-
based
correlation with an external cardiac signal) allow under-sampling relative to
cardiac
rhythm, so that individual VIPS readings may be spaced even many seconds
apart,
while still providing valuable information relating to fluid fluctuations
associated with
the cardiac cycle. Other examples include synchronizing (or isolating
irregularities) to
a ventilation signal, such as a capnography signal.
[00146] A variety of signal processing analysis techniques, including
frequency
domain approaches, such as discrete Fourier transforms (DFT) and Fast Fourier
Transforms (FFT) analysis, may be applied to the VIPS measurements to reveal
the
frequency distribution of the oscillations in cerebral fluids, which derive
from the
patient's heart rate. These techniques may be applied to the measured VIPS
phases
and/or magnitude data for multiple radio frequencies, either alone or in
combination.
Useful combinations for analysis include theoretically and empirically derived
formulae that use weighted combinations of VIPS phases and amplitude data to
create
indicators that correlate with blood volume, cerebrospinal fluids, edema, or
other
relevant fluid characteristics. When an external cardiac signal is available
for
correlation, the period and frequency of the cardiac cycle is provided and may
be used
with processing approaches, such as applying averages, medians, or other
statistics to
VIPS measurements at each of the measured portions of the cardiac cycle, then
calculating the differences between bins to determine the magnitudes of the
fluid
changes associated with the cardiac cycle.
[00147] In another embodiment, the system is designed to take multiple samples
per
second and is configured to generate a signal that corresponds to the
magnitude of the
change in intracranial blood volume that results from each arterial pulse. It
is well
known in the art of intracranial pressure (ICP) measurement that ICP increases
during
the diastole phase of the cardiac cycle, and decreases during systole, because
of the
induced changes in intracranial blood volume. Using an ICP monitor, therefore,
a
plethysmogram can be generated, which approximately plots the intracranial
blood
volume over time as it fluctuates through repeated cardiac cycles.
[00148] The amplitude of ICP changes due to cardiac pulsation is significantly
damped
in patients who have cranial vents, for example, an intraventricular catheter.
This is
because the pressure pulses are relieved as fluid moves back and forth through
the
catheter. The same dampening of the ICP plethysmogram occurs in patients with
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
intraventricular shunts, as are commonly used in patients with chronic
hydrocephalus.
When the shunt is working normally, cerebrospinal fluid will move back and
forth in
the shunt catheter, dampening the ICP excursions during cardiac cycles.
However,
when the shunt is clogged or otherwise malfunctions, the fluid is unable to
move during
cardiac cycles, and the amplitude of the ICP variation increases. The current
invention
can be configured to monitor the changes in blood and cerebrospinal fluid
volumes that
result during cardiac cycles and detect shunt clogs or malfunctions.
[00149] Once a plethysmogram is generated, there are a variety of ways one can
use
the information to help to diagnose the condition of a patient. For example,
after the
peak of the cardiac pressure/volume pulse, the following portion of the
waveform
represents the recovery period during which the fluid volume returns to
baseline. The
time it takes from the peak to another subsequent point in the cardiac cycle
can provide
information about intracranial compliance or intracranial pressure. It can
help identify
specific characteristics of intraventricular shunt performance or failure.
Ratios,
differences and other mathematical relationships of amplitudes of the
plethysmogram at
various time points along the cardiac cycle can be developed to indicate a
variety of
clinical conditions and physiologic parameters.
[00150] There is a need during administration of cardiopulmonary resuscitation
(CPR)
for providing feedback on the effectiveness of cardiac compressions.
Currently, there
are devices which can measure displacement distance, which is correlated to
cardiac
compression and induced blood volume changes. However, these devices do not
directly measure the effectiveness of the compressions at inducing blood flow
to the
brain, which is the primary goal of CPR. The present invention can be applied
to the
head of a patient undergoing CPR, and direct readings can be made to detect
the
amplitude of the change in blood volume in the brain during CPR. In this
embodiment
of the present invention, the effectiveness of CPR can be monitored and
improved by
providing direct feedback to the CPR administrator as to the actual change in
blood
volume with each cardiac compression.
[00151] In addition to using the VIPS technology to produce a plethysmogram of
intracranial fluid changes, the present invention also can be implemented
using other
technologies. For example, a plethysmogram can be generated using near-
infrared
spectroscopy (nirs), or by measuring the absorption of light at a variety of
wavelengths.
By way of example, pulse oximetry devices typically use two wavelengths of
light and
46
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
rapidly sample the absorption of those wavelengths during cardiac pulsations,
creating
a plethysmogram. This can also be accomplished with one wavelength. This type
of
light absorption technology can be applied to the brain, yielding a
plethysmogram that
can be used to evaluate shunt malfunction. One skilled in the art of
plethysmography
will recognize that a plethysmogram of the intracranial fluid can be created
by a variety
of technologies, and the present invention is not limited to any particular
technical
means of producing the plethysmogram.
[00152] In the art of ICP monitoring, skilled neurologists and other experts
can
examine the shape of the ICP plots and identify important clinical conditions.
With a
high sample rate, the plethysmogram produced by the present invention can
produce a
similar curve and can provide clinical practitioners with similar diagnostic
information
without the need for an invasive ICP probe. Information about arterial and
venous
blood flow and volume, intracranial compliance, edema, CSF volume and
pulsation,
can all be derived from a high resolution plethysmogram. In some cases, it may
be
useful to combine the VIPS plethysmogram with ICP monitors to better
understand the
patient's clinical condition, especially when information about multiple
distinct fluids
is needed. This technique can also be used to inform the clinician about
intracranial
compliance.
[00153] In another embodiment, detection of intracranial compliance can be
accomplished by examining the changes in the volume of one or more
intracranial
fluids over time, or in response to an external stimulus, such as a valsalva
maneuver,
jugular vein compression, cerebrospinal fluid injection or withdrawal (as with
a spinal
tap), hyperventilation, hypoventilation, or change in patient position. The
recovery after
the initial stimulus can also be an indication of intracranial compliance and
autoregulation. The present invention can be used in combination with an ICP
monitor
to establish the relationship between pressure and volume, and therefore
provide
information about intracranial fluid compliance and autoregulation. The
present device
could be combined with other monitoring technologies, such as, but not limited
to,
ECG, EEG, pulse oximetry, ultrasound, transcranial Doppler, and/or infrared
SPEectroscopy, to spectroscopy to correlate intracranial fluid volume to other
physiologic parameters that may be useful in detecting, managing or treating
disease.
[00154] In another embodiment, the current device can be used to detect CSF
leaks.
For example, a patient who is at risk for a CSF leak, such as a patient
undergoing a
47
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
procedure with epidural anesthesia, could be monitored with the current
device, and the
device could alert the treating physician when there is a change in the volume
of CSF.
Since there is currently no way to directly detect CSF leaks during or
following spinal
or epidural anesthesia, the anesthesiologist will typically leave before the
symptoms of
the leaks become manifest, hours or days later. Because most patients are
still in a
recumbent position during immediate post-operative recovery, they generally
will not
experience any neurological symptoms until well after the surgery, when they
stand up.
Because of the depletion of CSF inside the skull, the brain will sag due to
gravity and
the absence of the normal buoyant force supplied by an adequate amount of CSF.
It is
commonly hypothesized that this sagging induces stress on some of the vessels
supplying the brain, resulting in a severe headache, commonly known as a
"spinal
headache". One common treatment for this type of CSF leak, which is the result
of an
inadvertent dural puncture, is to inject the patient's autologous blood into
the epidural
space, near the puncture. This is called a blood patch. Other treatments
involve
injection of saline or other fluids into the space, or surgical repair of the
dural tear.
With the appropriate application of the current device, a novel method for
treating
patients can be formulated, comprising the following steps: applying an
intracranial
fluid monitor to a patient undergoing a procedure which may result in a CSF
leak,
detecting the CSF leak, and repairing the leak during the same operative
session.
Variations on this method could include detecting a CSF leak in a patient
using an
intracranial fluid monitor, and repairing the leak as a result of the leak
detection. Or, a
measurement of the intracranial CSF volume of a patient can be made prior to a
procedure that may cause a CSF leak, and a second measurement of the
intracranial
CSF volume can be made during or after the procedure, and if a significant
reduction
has been detected, the repair can be made before the conclusion of the
procedure.
Alternatively, the second measurement can be made at any time after the
procedure,
and a repair can be made after the detection of the leak.
[00155] In another embodiment of the current invention, plethysmography is
used to
detect respiratory rate and volume, heart rate, or penile erectile function.
For instance,
sensors could be designed so that they would adhere to the torso in such a way
as to
detect the extent of thoracic excursion due to the breath cycle. Sensors could
also be
integrated into an arm band, ear phones, or a watch bracelet to monitor
changes in
blood volume of the underlying tissue that would then be related via
mathematical
48
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
transformations to the cardiac and respiratory cycles. Sensors adherent to the
base of
the penis could measure volumetric changes associated with erectile response.
[00156] The console of the VIPS system, according to one embodiment, may
include a
custom electronic device with a display. A laptop computer or tablet, such as
an iPad,
could alternatively be used. Using one of these off-the-shelf computers has
the
advantage of having already integrated wireless communications capability,
including
Bluetooth or WiFi. But custom consoles comprised of off-the-shelf or custom
components can also be used.
[00157] In order to detect an asymmetry (or other symmetrical or non-
symmetrical
characteristics) of the fluids in the brain, multiple transmitters and
receivers can be
strategically located. The transmitters and receivers may be located such that
the
transmitters transmit through different portions of the bulk tissue of the
patient and the
receivers are located generally opposite to the transmitters so as to receive
the signals
through the tissue. For instance, a single transmitter (or receiver) could be
located on or
near the forehead of the patient, and two receivers (or transmitters) are
spatially
separated from one another and could be located on either side of the head,
preferably
toward the back, such that the time varying magnetic field propagates through
each
hemisphere, or in the case of two transmitters each of the time varying
magnetic fields
propagates uniquely biased to different sides of the brain. In this example,
the
magnetic fields received by the receivers (or in instances where two
transmitters are
used, the two magnetic fields received by the single transmitter) will be
transmitted
substantially through different portions (e.g., a first portion and a second
portion) of the
overall tissue sample. Depending on the orientation of the
transmitter/receiver, there
may be some overlap in the tissue portions, but generally the transmitters are
arranged
to be transmitted through discrete sections of the overall bulk tissue.
[00158] Continuing with this example, uneven signals between the two receivers
and
one transmitter, or one receiver and two transmitters, could be an indication
that a
stroke or hemorrhage was present on one side. This is useful, because most
brain
lesions are not directly in the center of the brain. So, detecting an
asymmetry would be
an indication of a lesion. To identify the signals sent from each of the
transmitters, the
signals may include a transmission characteristic as an identifier, such as a
synchronization pulse, amplitude or frequency modulation, and or each
transmitter
could transmit at different fundamental frequencies or a different series of
frequencies.
49
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
For example, the signal sent from the first transmitter may have a different
frequency
from the signal sent from the second transmitter. As another example, the
signal sent
from the first transmitter may be shifted in time as compared to the signal
sent from the
second transmitter. As yet another example, each or one of the signals may
include a
bit of data (e.g., an amplitude value, or the like) that corresponds to the
particular
transmitter from which it was transmitted.
[00159] It is possible to allow a single antenna or coil to act as either a
transmitter or
receiver at different times, thus creating a transceiver. A switch could be
implemented,
to switch the antenna from being a receiver to being a transmitter and vice
versa. For
example, the use of a gallium arsenide FET or PIN diode switch could be used.
Alternatively, two concentric loop antennas could be located on the same
printed circuit
board or other substrate.
[00160] In measuring phase shift, some of the electronic components can be
sensitive
to temperature changes. To minimize the effect of temperature-induced
variation, it
may be desirable to design the cable from the transmitter to the analog-to-
digital
converter to be the same length as the cable from the receiver. The addition
of
compensating electrical resistance or reactance in the form of series/parallel
networks
of resistors, capacitors, and inductors can also minimize the effect of
temperature.
Furthermore, heaters or thermo-electro-coolers and thermal insulation may be
used to
temperature stabilize amplifiers or other components that are inherently
temperature
sensitive.
[00161] To reduce the effect of the mismatch of the transmit antennae to the
cable that
delivers the RF transmit signal, a directional coupler may be used to remove
cable
reflections and provide a pure sample of the transmit signal that may be
utilized for
analog-to-digital conversion.
[00162] To reduce the sensitivity of the system to movement of people or other
objects
near the antennae or in the magnetic field, shielding of the antennae to
direct the
magnetic field may be useful. Various field shaping passive devices formed
from
ferrites, other magnetic materials, or electrical conductors may be
incorporated with the
antennae to best match the field profile to the human brain cavity.
Algorithms
[00163] As has been described, the VIPS device may capture electrical property
data at
a multitude of frequencies. This data may include measurements of the phase
shift and
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
attenuation of voltage or current signals between the emitter and detector. In
some
embodiments, there will be measurements of phase shift or attenuation between
multiple emitters and detectors.
[00164] Different biological tissues have varying electrical properties and
thus induce
different phase shifts and attenuations. By examining the frequency response
of the
electrical property changes¨e.g., phase shift¨it is possible to examine volume
changes of each of the types of fluid separately. Because the skull is a rigid
and closed
volume, changes to the volumes of different fluids, such as blood,
intracellular fluid,
extracellular fluid, and cerebrospinal fluid, affect each other, since the
total fluid
volume must remain essentially constant. The fundamental relationship between
intracranial pressure and intracranial fluid volume was first published over
two
centuries ago by Professors Monro and Kellie. Monro and Kellie established the
doctrine that, because the skull is essentially a rigid, closed volume, venous
flow of
blood out of the cranium is necessary to allow arterial blood flow into the
cranium.
This phenomenon also applies to other intracranial fluids.
[00165] A variety of algorithms can be generated to reliably detect changes to
the
intracranial fluids. Formulas may be derived from the phase shift, attenuation
or other
electrical parameters at certain frequencies for certain fluids. One formula,
B(p(f1),
a(f2)), may be empirically derived which is strongly correlated with
intracranial blood
volume. In the present example, the formula B, is a function of phase shift
(p) at a
particular frequency (fl) and attenuation (a) at the same or another frequency
f(2). In
live patients or animals, as blood volume increases, we would expect the
volume of
cerebrospinal fluid to decrease. Therefore, if we derive a formula for
cerebrospinal
fluid and call it C, then, a rise in the ratio of B/C may be a good indicator
of venous
blood pooling, or an intracerebral hemorrhage. As another example, it is well
known
that as cerebral edema develops, the increased intracellular and extracellular
fluid
volume pushes some of the intracranial blood out of the skull. Therefore, if
we derive a
formula for cellular fluid, and call it CF, then the ratio CF/B can be used as
a metric to
quantify edema. Using ratio formulas can be particularly helpful to divide out
noise
factors that may affect both the numerator and denominator.
[00166] Going further with this general method, one of ordinary skill in the
art may
develop many such algorithms which take advantage of formulas which correlate
strongly with one or more particular intracranial fluids and or location of
the fluids in
51
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
the brain's hemispheres. Relationship between two or more fluids can be
expressed in
mathematical formulas which may include ratios, products, sums, differences,
or a
variety of other mathematical relationships.
[00167] The present invention can be used to diagnose conditions, such as
cerebral
bleeding or edema. But it can also be used to help control the administration
of
treatments for some of these conditions. For example, the device could be used
for
measuring cellular fluid in brain tissue. In a case of dangerous edema,
physicians will
often administer intravenous drugs like mannitol and hypertonic saline
solution to draw
water out of the brain. If not administered properly and in the right dose,
these drugs
can be dangerous. For the treating physician, it would be useful to know how
much
fluid was removed from the brain tissue. Therefore the use of a device such as
the one
described here would have utility as a means for providing feedback for
treatments to
reduce intracranial fluid volume. Another example would be to use such a
device to
provide a measure of intracranial blood volume as feedback for administering
drugs
that alter blood pressure and flow rate, that are sometimes used to treat
patients with
brain injury. Other examples where intracranial fluid measurements could be
used as
feedback include: hydration during intense exercise such as running marathons;
sodium
concentration during intense exercise; or in treating patients with improper
levels of
sodium.
[00168] Although the examples used here are focused on intracranial fluids,
algorithms
and treatment methods using a device that can distinguish different types of
fluids can
be used in other fields of medicine as well. Algorithms and feedback
techniques such as
are described above can be used to reliably measure ratios of different types
of fluids in
other parts of the body. For instance examining the fluid that builds up
inside the lung
tissue in patients with congestive heart failure can be read as a change in
the ratio of
lung fluid to blood in the same region. Lymphedema that commonly occurs in the
arms
of patients after breast cancer surgery can be measured as a ratio of
extracellular fluid
to blood or muscle tissue volume. Treatments for patients that affect tissue
fluid
volume, such as compression garments for lymphedema, or diuretics for
congestive
heart failure patients, can be dosed using feedback as has been described
above.
52
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
Clinical Applications
[00169] During hemodialysis, blood is withdrawn from a patient's vein, and
substances
including sodium and urea are filtered out. The blood brain barrier prevents
these
larger molecules, called osmoles, from leaving the brain quickly. This sets up
a
concentration gradient that provides osmotic pressure to draw water across the
blood
brain barrier into the brain, resulting in cerebral edema. In extreme cases,
this cerebral
edema causes a condition called dialysis disequilibrium syndrome and can be
severe
enough to cause degradation of brain function, or even permanent brain injury.
Partly
for this reason, dialysis is performed over a prolonged period of time,
typically about 4
hours. It is believed that many patients could undergo a more rapid dialysis
protocol,
but it is difficult to ascertain which patients could tolerate the faster
rate. A new dialysis
protocol could be enabled by the VIPS system described herein, by monitoring
the
intracranial fluids during dialysis. The steps of this method would involve
placing a
fluid monitor on the patient prior to initiation of dialysis, initiating
dialysis at a
relatively fast rate, and checking for signs of cerebral edema. As edema
progresses, the
dialysis can be slowed in response to the fluid readings, thereby customizing
the
dialysis rate for each patient based on their ability to tolerate the process.
[00170] For patients with sodium imbalances, the VIPS system described herein
may
be used to detect changes to the sodium level that may result in conditions
such as
hypernatremia and hyponatremia. In patients suspected of such conditions, the
system
may be deployed to detect and diagnose the condition, or to aid the clinician
in the
treatment of the patient to correct their sodium balance by providing real-
time feedback
during administration of fluid or drug therapies.
[00171] During heart surgery, there is a risk that not enough blood is getting
to the
brain. This can be the result of an embolism or of lack of circulation or low
blood
pressure to the brain. One article that discusses this problem is "Silent
Brain Injury
After Cardiac Surgery: A Review" by Sun et at, journal of the American College
of
Cardiology, 2012. A fluid monitor could detect a reduction in the amount of
blood in
the brain, and it could detect ischemia in the brain tissue. Thus, a new
monitoring
technique could involve placing a fluid monitor, such as the system described
herein,
on a patient at the beginning of a cardiac surgery and monitoring the patient
during the
surgery. In the event that the device detects brain ischemia or a reduction in
the blood
53
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
volume in the brain, the physician may be alerted and may attempt to correct
the
problem through a variety of clinical means.
[00172] A VIPS device can be configured to monitor intracranial pressure
noninvasively. It is well known in the field of neurology that intracranial
pressure and
volume are approximately linearly related when the intracranial fluids are
properly
regulated by the body's own intracranial fluid control systems. It has been
established
in clinical studies that a VIPS device can detect fluid shifts that are
proportional to
pressure changes.
[00173] There is a need for detecting ischemia in the G.I. tract, especially
in neonates.
The VIPS system described herein may be used to detect ischemia, either with
continuous monitoring or with instantaneous measurements.
[00174] Prevention and detection of head injuries in automobile accident
victims,
football players, in the military, and other types of head injuries is a
critical need.
Accelerometers have been added to football helmets to monitor accelerations
due to
impact, and companies like Nike, Inc. have acceleration detectors integrated
into caps.
But accelerometers are, at best, an indirect way to help determine likelihood
of head
injury. It is the movement of the brain within the skull in response to the
external
acceleration forces that lead to concussion or brain injury. VIPS could also
be added to
helmets, caps, headbands, or applied directly to the head, and could detect
the
movement of the brain within the skull during the impact. This could be used
instead
of accelerometers, but would be most effective if used in conjunction with
accelerometers. Monitoring brain movement within skull with VIPS would provide
a
better measure of potential for brain injury than accelerometers alone.
Football is one
application. Crash testing is another. Research in vehicle safety could
benefit greatly
from a better understanding of brain movement during impact (e.g., crash
testing with
cadavers monitored with VIPS).
[00175] Detection of concussion is important, especially in sports injuries.
If a person
has a concussion, a second concussion before the first has resolved can result
in a very
severe injury called second impact syndrome. ("second impact syndrome", Bey &
Ostick, West J Emerg Med. 2009 February; 10(1): 6-10.) Although the science of
concussion and its effect on intracranial fluids is still evolving, VIPS could
be used to
detect early stages of intracranial swelling, hyperemia, venous pooling,
hemorrhage,
ischemia, blood flow rate changes or other biologic changes affecting the
tissue's
54
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
bioimpedance. With a VIPS device, readings may be taken prior to a game or at
some
other baseline time, and readings after a potential injury event may be
compared to the
baseline to establish the presence or degree of injury.
[00176] A variety of other medical conditions may be monitored with the VIPS
system
described herein. Peripheral edema can be caused by a variety of medical
conditions.
Swelling in the feet and legs is common among patients with congestive heart
failure.
Swelling in the arms is common after breast cancer surgery when patients
develop
lymphedema. Swelling is common in limbs or other parts of the body after
surgery. In
some types of surgery, there is a flap of tissue that is at risk for ischemia,
edema, or
venous pooling. Compartment syndrome can result after an injury when there is
insufficient blood flow to muscles and nerves due to increased pressure within
the
compartment such as an arm, leg, or any enclosed space within the body.
Current
devices measure compartment syndrome pressure using a minimally invasive
device
involving a needle to penetrate the tissue and take a reading of the pressure.
("accuracy
in the measurement of compartment pressures: a comparison of three commonly
used
devices", Boody & Wongworawat, J Bone Joint Surg Am. 2005 Nov;87(11):2415-22.)
Patients with congestive heart failure or other conditions can have a buildup
of fluid in
their lungs or chest cavity. The VIPS device described herein may be used to
monitor
changes related to swelling, blood flow, perfusion, and/or other fluid
characteristics of
limbs and other parts of the body due to any of these or other conditions. A
baseline
reading may be taken, and subsequent measurements may be compared to that
baseline
to monitor and detect changes, for example, to swelling or perfusion of the
tissue.
Continuous monitoring of swelling may provide feedback for medical therapies
to
control edema, blood flow, or other clinical parameters.
[00177] Dehydration can be a life-threatening medical condition and can occur
during
athletic activities, such as marathon running, and in patients with a variety
of medical
conditions. The VIPS device described herein may be used for quantifying the
hydration level of a patient for purposes of an initial diagnosis, for
monitoring
effectiveness of treatment, and/or as an alarm to a worsening condition of a
patient.
[00178] Fighter pilots and other people undergoing extreme accelerations can
sometimes lose consciousness, as a result of sudden fluid shifts within their
brain.
Similar conditions can occur in deep sea divers, astronauts, skydivers and
mountain
climbers who are exposed to extreme conditions that may affect their
intracranial
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
fluids. The VIPS device described herein may be installed inside a helmet or
otherwise
affixed to a person's head during activities that put them at risk for changes
to their
intracranial fluids could be monitored in real time. If a dangerous change in
fluids were
to occur, the individual or a third party could be alerted to provide
intervention.
[00179] Migraine headaches are well known to be caused by expansion of blood
vessels in and around the brain. Regular or continuous monitoring of
intracranial blood
volume may be used to diagnose or better understand the physiology of
migraine. An
individual migraine patient may quantify the effect of various migraine
treatments
during administration, and may use that information as feedback to titrate
medication or
otherwise adjust therapy. Regular periodic monitoring by migraine patients,
for
example brief VIPS spot check readings nightly and upon waking in the morning,
would allow individuals to detect characteristic intracranial fluid changes
that precede
migraine headache symptoms, thus facilitating earlier interventions that more
effectively reduce symptoms.
[00180] Penile plethysmography is commonly used in urologic surgery to
evaluate
erectile function before and after prostate resection. Currently, this is
typically
accomplished via circumferential strain-gauge transducers. A VIPS sensor could
be
utilized to provide direct volumetric measurements of penile filling. Such a
device
could also be used in the ambulatory setting to evaluate the etiology of
erectile
dysfunction, i.e. whether physiologic or psychogenic, or monitoring night-time
arousal.
[00181] As described above, various methods using the systems 100, 700 to
detect
bodily fluids (either directly or indirectly) may be used. For example, in one
method,
asynchronous EKG and VIPS readings may be time-stamped and the VIPS readings
may be binned as a function of position in cardiac cycle for subsequent
analysis.
Exemplary analysis includes, as some examples, statistics such as median or
mean
values in each bin, then differences between mean values for bins associated
with
diastolic and systolic portions could indicate the extent of fluid exchange.
[00182] As another example of a method, a signal processing algorithms, e.g.
FFT,
DFT, may be applied by the processing unit 104 and/or computing device (e.g.,
laptop,
desktop, server) to the measured phases, amplitudes, and/or weighted
combinations
such as the computed indicators that correlate with blood, CSF, etc. In order
to
determine heart rate (a frequency) and/or amplitudes of fluid changes
associated
cardiac cycle.
56
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
[00183] Physiologic monitoring is commonly utilized in a variety of medical
settings,
to include such parameters as heart rate and respiratory rate. While a variety
of
modalities currently exist to derive these values ¨ electrical, optical, and
others ¨ VIPS
could also be used to provide data on these vital signs, thereby obviating the
need for
additional monitors when a VIPS device is already being utilized for cranial
fluid
surveillance, or as an additional source of the same information. That is, a
physiologic
sensor can be used to detect, either directly or indirectly, one or more
characteristics of
fluid flow or other conditions within the patient's body and then these
conditions may
be used to calibrate or filter the data from the VIPS system.
[00184] Autoregulation of intracranial fluids is a complex biological process,
involving
vasodilation, vasoconstriction, movement of cerebrospinal fluid (CSF) between
various
compartments of the brain and the spinal column, and production of CSF.
Patients with
a variety of neurological disorders can have poor autoregulation, which can
lead to
elevated or reduced intracranial pressure. The VIPS device described herein
may be
used to evaluate the autoregulation and intracranial compliance of a
particular patient.
Tests may be developed to measure the fluid changes that occur as a result of
a
procedure or posture change. For example, a patient may lie flat on his or her
back, and
a clinician may take a fluid volume reading, raise the patient's legs into an
elevated
position, and measure the fluid changes that occur. Other tests may include
intravenous
infusion of bulk fluids, administration of medications, and/or moving the
patient from a
flat to vertical position, all of which will induce a change to the blood, CSF
and other
fluids in the brain. The results from a particular patient test may be
compared against a
baseline measurement of the same patient performed at a different time, or
against a
database of known normal and pathologic responses, helping the clinician to
better
understand the patient's autoregulation and intracranial compliance status.
With a
better understanding of a patient's intracranial fluid function, the clinician
may be
better able to select a course of treatment that is most beneficial to the
patient.
[00185] Studies comparing the return to normal cerebrovascular reactivity
(CVR) in
subjects after voluntary manipulations of the blood flow to the brain show a
difference
between those with a concussion and healthy subjects. Unlike healthy subjects,
those
with concussions failed to return to normal CVR after hyperventilation tests.
This
condition lasted for several days after the concussion. In contrast, in
healthy subjects,
the CVR returned to normal conditions in a much shorter time. Our experiment
shows
57
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
that bulk measurements of the electromagnetic properties of the brain have
measurable
changes during tests that affect the CVR, such as the valsalva maneuver and
jugular
vein compression. The results show that return to both temporal and magnitude
normal
can be detected precisely with the devices and methods described in this
patent
application. This illustrates that the devices and methods can be used to
detect a variety
of diseases, such as concussion, by evaluating the temporal and magnitude
patterns of
the excursion from a normal signature, due to maneuvers that produce well-
controlled
voluntary changes in blood flow.
Experimental Example
[00186] This experiment was based on the idea that substantial insight can be
found in
the electromagnetic signature response to a voluntary change in tissue
condition. This
could lead to a much more controlled diagnostic method, based on
electromagnetic
measurements of biological tissue condition. In our experiment, a voluntary
change was
produced in the interrogated organ or tissue, and the diagnostics were
performed by
evaluating the changes of electromagnetic properties that occurred in those
organs or
tissue in response to the voluntary produced change and correlating these
changes to
the voluntary action.
[00187] One example of the method relates to brain concussion, an important
medical
problem in sports medicine. Sports induced concussion or mild traumatic brain
injury
(mTBI) is of increasing concern in sports medicine. Neuropsychological
examination is
the main diagnostic tool for detecting mTBI. However, mTBI also produces
physiological effects that include changes in heart rate and decreases in
baroreflex
sensitivity, cellular metabolism and cerebral blood flow. Cerebrovascular
reactivity (or
"cerebrovascular response," CVR), which is a measure of cerebrovascular flow,
is
impaired by brain trauma. Various methods are used to assess CVR. They include
hyperventilation, breath holding, CO2 inhalation, and administration of
acetazolamide.
It has been shown that Doppler ultrasound measurements on the carotid artery
can be
used to monitor changes in CVR, which can then be correlated with mTBI and
used for
diagnosis of the condition. The methods and devices described herein provide
an
alternative means for measuring changes in CVR, with a practical application
in
diagnosis of mTBI.
[00188] This experiment demonstrates that the various methods used to assess
CVR
through voluntary actions on the body produce changes in the electromagnetic
58
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
properties of the brain. These properties produce a distinct signature in
magnitude and
time and can therefore be used with our device for brain diagnostics.
Experimental System: Inductive Spectrometer
[00189] An experimental multi-frequency inductive spectrometer was designed
and
constructed. The system consisted of four modules: function generator,
transceiver,
dual-channel demodulator and analog-digital converter. A personal computer was
used
to control the system and process the data. The function generator module used
two
identical programmable synthesizers (NI 5401 synthesizers, National
Instruments, Inc.,
Austin, TX) as oscillators. The first oscillator supplied an excitation signal
Icos(wet) of
approximately 20mA, in the range of 1 to 10 MHz, at pre-programmed steps. A
modulation signal Icos(wint) was generated by the second oscillator. The
difference 0e
w= w0=100(27c) was maintained constant in the whole bandwidth, in order to
produce
a narrow band measured voltage signal on a constant low intermediate frequency
for
processing and demodulation.
[00190] The excitation and modulation signals were connected to the
transceiver and
the dual-channel demodulator modules, respectively. The transceiver consisted
of an
excitation coil and a sensing coil, coaxially centered at a distance d=18cm
and two
differential receiver amplifiers AD8130. Both coils were built with magnet
wire
AWG32 rolled on a cylindrical plastic former with radius r=2 cm, five turns.
The coil
inductance, as calculated from Faraday's law, was approximately 40 mH. The
excitation coil generated a primary oscillating magnetic field. The sensing
coil detected
the primary magnetic field and its perturbation through a proximal conductive
sample.
To avoid inductive pickup, the leads of the coils were twisted. The amplifiers
were
connected as conventional operational amplifiers and collected the reference
voltage
(Võf) and the induced voltage (Vmd) in the excitation and sensing coils,
respectively.
The gain of the amplifiers was adjusted in order to obtain a dynamic range of
5V
throughout the whole bandwidth.
[00191] The dual-channel demodulator module used a mixer and a narrow band
pass
filter to transfer the information of any excitation and sensing frequency to
the same
low frequency (wo). This module used two similar channels for demodulation of
the
reference and induced signals. To avoid additional inductance and stray
capacitance in
the circuit, the amplifiers and dual channel-demodulator circuits were
shielded by a
59
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
metallic box and connected to the coils with short coaxial cables (length less
than
0.8m). The current passed through the shield to minimize any inductance mutual
between the circuit and the coils.
[00192] The analog-digital conversion module digitized the reference and
induced
voltage signals on the constant low frequency. A data acquisition card (NI
6071E,
National Instruments, Inc., Austin, TX), with a sample rate of
1.25MSamples/seg and a
resolution of 12 bits, was used as an analog-digital converter.
[00193] The phase of the reference and induced voltages are calculated in
software
over approximately five cycles by an extract single tone function available in
LAB VIEW V6.1 (National Instruments Inc, Austin, TX). The phase shift between
the
reference and induced voltage was estimated as AO = 0(Võf) - 0(V,,d). The
ratio signal to
noise (SNR) for phase shift measurement was improved by averaging over twenty
spectra (39 dB at 1MHz).
Experimental protocol:
External jugular vein compression
[00194] The two external jugular veins, found on both lateral sides of the
neck, are one
of the main routes for cerebral venous drainage. By applying light pressure to
both
sides of the neck, a person can inhibit drainage. In doing so, intracranial
fluid volumes
increase 20-30cc. The purpose of this experiment was to evaluate the ability
of the
phase shift intracranial fluid monitoring device, as described in this patent
application,
to detect these changes in blood volume.
[00195] The experiment showed that, following release of the jugular vein
after
compression, there was an exponential decay in reading. It also showed that,
following
a second compression and release, the reading did not return to the original
value. This
is typical of CVR when the metabolism is exhausted due to partial ischemia. It
suggests that this method can provide another technique for evaluating CVR and
thereby assess concussion.
[00196] With reference to Fig. 12, the results of the experience are presented
in the
graph. As shown in Fig. 2, calibrated phase shift measurements are plotted as
a
function of time and the increase in phase shift is caused by the vein
compression and
the decrease during the release. Further, following the blood vessel release
there is an
exponential decay in reading that does not return to the original value. This
is typical
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
of CVR when the metabolism is exhausted due to partial ischemia and indicates
that the
method can provide another technique for evaluating CVR and assessing
concussion.
Valsalva maneuver
[00197] The Valsalva maneuver is performed by moderately forceful attempted
exhalation against a closed airway, usually done by closing ones mouth and
pinching
ones nose while pressing out, as if blowing up a balloon. The Valsalva
maneuver tests
the body's ability to compensate for changes in the amount of blood that
returns to the
heart (preload) and affects the blood flow into and from the head. The dynamic
response of the circulation system through the maneuver is indicative of
several
physiological functions, including the CVR. There are other conditions that
can be
evaluated with this procedure. For instance, patients with autonomic
dysfunction will
have changes in heart rate and/or blood pressure that differ from those
expected in
healthy patients.
[00198] A temporal response to the Valsalva maneuver was measured, using a
device
as described herein. The measurement had several typical temporal aspects that
may be
used for diagnostic purposes. These include the time constant of the increase
in the
reading, the peak value, the time constant of the decays, and the final short
term and
long term values.
[00199] Fig. 13 illustrates a graph shown the changes in shift reading as a
function of
time during the Valsalva procedure. As shown in Fig. 13, the reading has
several
typical temporal aspects that can be used for diagnostics and these include
the time
constant of the increase in reading, the peak value, the time constant of the
decay, as
well as the final short term and long term value.
Detecting concussions
[00200] Return to normal CVR in subjects after voluntary manipulations of the
blood
flow to the brain is different for those with a concussion than it is for
healthy subjects.
Subjects with concussions failed to return to normal CVR after
hyperventilation tests
for several days after the concussion. In healthy subjects, on the other hand,
the CVR
returned to normal conditions in a much shorter timeframe. Our experiment
shows that
our bulk measurements of the electromagnetic properties of the brain show
measurable
changes during tests that affect the CVR, such as the Valsalva maneuver and
jugular
61
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
vein compression. The results show that return to normal can be detected
precisely with
our measurements. This proves that our device can be used to detect a variety
of
diseases, such as concussion, by evaluating the temporal and magnitude
patterns of the
excursion from a normal signature due to maneuvers that produce well-
controlled
voluntary changes in blood flow.
Detecting Large Vessel Occlusions
[00201] Almost 50% of ischemic strokes are clinically referred to as either
cerebral
thrombosis or cerebral infarction. These strokes fall into two categories:
small vessel
occlusions (or "thrombosis") and large vessel occlusions (LVO). Large vessel
occlusion occurs when there is a blockage in one of the brain's larger blood-
supplying
arteries, such as the carotid, middle cerebral, or basilar arteries. Small
vessel occlusion
involves one of the brain's smaller and deeper arteries. The effect of an
occlusion of
one or more cerebral blood vessels is reduction or elimination of arterial,
oxygen rich
blood flowing beyond the occlusion, resulting in hypoxia in (i.e.,
insufficient oxygen
delivery to) "downstream" brain tissue. If undetected and therefore untreated,
an LVO
will result in brain cell death, causing lasting brain damage and in some
cases death. If
detected, arterial recanalization may be performed, to allow blood to flow
again to the
parts of the brain that were blocked from blood flow by the occlusion. Two
methods
for recanalization are intravenous (IV) thrombosis with tPa (tissue
plasminogen
activator) and mechanical recanalization. One of the main challenges in
treating an
LVO is detecting the LVO early enough to be able to provide effective
treatment.
[00202] Earlier detection of LVO would result in earlier clinical intervention
and
therefore minimize brain cell damage. The ability to continually, non-
invasively
monitor for evidence of LVO may eliminate the need to keep the patient awake,
may
reduce or eliminate the need for hospital staff to perform continual visual
monitoring
and interaction with the patient, and may limit the patient's exposure to
radiation from
multiple CT (computed tomography) scans. Additionally, in some cases LVO is
actually a secondary brain injury, which may occur hours or days after the
primary
brain injury. The ability to monitor brain injury patients for secondary LVO,
especially
while they are asleep, is critical to improving outcomes.
[00203] Any of the embodiments of noninvasive, diagnostic, VIPS systems and
methods described above may be used to monitor changes in fluids in the brain
or other
parts of the body to detect LVO. In any given case of LVO, there may be a
detectible
62
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
change in the fluid in the region of the brain affected by the LVO after the
occlusion
has occurred. Thus, one way to detect LVO is by monitoring a patient's
cerebral
fluid(s) over time and watching for changes that would indicate a possible
LVO. Such a
detection method may involve either multiple "snapshots" of fluids over time
or
continuous monitoring, according to various embodiments. As LVO persists,
brain
cells begin to die, due lack of perfusion, which results in edema and
swelling. This
edema and swelling may be detected using the VIPS systems and methods
described in
this application.
[00204] Another way to detect an LVO is to take just one "snapshot" of the
brain,
using a VIPS system as described herein, and compare blood volume in the right
hemisphere of the brain with blood volume in the left hemisphere of the brain.
In the
event of an LVO on the right side of the brain, for example, there will be
less blood on
the right side than on the left side, thus indicating a likely LVO on the
right. This
method of detection can be performed instantly, and one advantage of this
method is
that it does not require a baseline fluid measurement for comparison.
Therefore, this
"single snapshot" method may be performed in a number of different settings,
such as
an ambulance or emergency department, to quickly detect an LVO. It may also be
used
in the hospital setting, for example to quickly detect a second stroke in a
patient who
earlier suffered a first stroke. Of course, for some patients the two methods
of LVO
detection may be used together¨i.e., the monitoring of fluids over time and
the single
snapshot method. The VIPS systems and methods described herein allow for any
combination of such methods to be applied to any given patient.
[00205] Although these techniques are being described here for use with LVO
detection, they may have other applications for stroke detection as well. For
example,
in some embodiments, the techniques may be used to detect small vessel
occlusions or
hemorrhagic strokes, such as those caused by ruptured aneurysms.
[00206] Referring now to Fig. 15, detection of occlusion removal may also be
important in patient management. After a successful recanalization (e.g.,
mechanical or
intravenous tPa), cerebrovascular reactivity will occur, which will impart a
fluid change
as the blood rushes into the depleted arterial vascular system. Over time, the
fluids in
the brain (blood, edema, parenchymal fluid, etc.) will reach homeostasis, thus
providing
additional clinical feedback on effectiveness. Again, any of the VIPS systems
and
methods described herein may be used for detecting fluid changes associated
with
63
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
removal of a cerebrovascular occlusion. As detailed in this application, VIPS
systems
may also be used to identify one or more types of fluid in the brain, such as
blood, CSF,
edema, etc. Fig. 15 illustrates one clinical example of using a VIPS system as
described
herein to monitor a patient undergoing occlusion removal. Each of the dots on
the chart
represents one snapshot measurement of fluid volumes in the brain, using a
VIPS
system as described herein. The first dot, to the far left and at the bottom
of the chart,
represents a baseline measurement. The second dot (i.e., the next dot to the
right from
the baseline) represents the change in measured fluid volumes immediately
after the
occlusion removal procedure was performed. Subsequent dots, moving to the
right on
the chart, represent follow-up VIPS fluid measurements, showing a slower rise
and then
a tapering off of fluid volumes. This is but one example of a way in which a
VIPS
system as described herein may be used to measure fluid changes after an
occlusion
removal procedure.
[00207] In addition to detecting the presence of an LVO, the VIPS systems and
methods described herein may be used to help determine where an LVO is located
within the cerebrovasculature. Determining where the LVO is located, such as
which
hemisphere of the brain it is located in, may provide important clinical
diagnostic
feedback for preferential treatment. For example, a VIPS device with two
transmitters
and one receiver spatially separated to discriminate fluid changes in a
particular region
of the brain may be used to detect the hemisphere in which there is a fluid
change. As
mentioned above, this method may be used to detect the presence of the LVO,
and it
may also be used to help locate the LVO in the brain. The ability to determine
whether
an occlusion or other pathology is located in the right hemisphere or the left
hemisphere
is important beyond LVO conditions. Additionally, it may be possible in other
embodiments to determine a location of an occlusion or lesion in other
anatomical areas
of the brain, vasculature or the like.
Bilateral Detection
[00208] Whether for use in locating LVO or for other applications, the ability
to
spatially detect fluid changes in the right versus left hemisphere can be
critical in the
diagnosis and care of a patient. For example, determining what type of stroke
has
occurred¨ischemic versus hemorrhagic¨and which hemisphere it occurred in,
provides crucial information to a clinician for administering an appropriate
therapy.
The ability to detect the plethysmograph (measure changes in volume in an
organ) of
64
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
the brain is useful beyond determining the patient's heart rate. For example,
it may be
used in detecting carotid artery stenosis, where the blood flow on one side of
the brain
is restricted versus the other. This could result in different plethysmograph
amplitudes,
thus providing clinically relevant information. It may also be used in
detecting acute
ischemic stroke (stenosis, thrombosis, infarction), which may lead to
detection of a
difference in plethysmograph amplitudes. In some cases, a hemorrhagic event in
one
hemisphere may attenuate the plethysmograph amplitude on one hemisphere only,
thus
providing additional clinically relevant data. The ability to detect "non-
symmetry" in
the plethysmograph information can provide critical clinically significant
feedback for
appropriate intervention.
[00209] Fig. 14 illustrates one embodiment of a headpiece 600 for use in a
fluid
monitoring system that may provide bilateral detection as described above. The
headpiece 600 includes a frame 605, extending around the front of the head and
out to
two wrap-around ends 602, 603. Each of the wrap-around ends 602, 603 contains
a
transmitter, and each transmitter preferentially transmits through one
hemisphere of the
brain to one or more receiving antennae in the headpiece 600. The wrap-around
ends
602, 603 are also designed to wrap around the back of a patient's head, to
help hold the
headpiece 600 snugly onto the head.
[00210] The frame 605 also includes a housing 604, which houses controlling
circuitry
for the headpiece 600 and at least one receiving antenna. Two holding arms 608
may
also be coupled with the frame 605, to hold onto the patient's ears and thus
to help hold
the headpiece 600 onto the patient's head. A nosepiece 606 may also be coupled
to the
frame 605, to provide a surface for the headpiece 600 to rest on the patient's
nose and
thus provide additional stability, as well as a consistent
alignment/registration reference
for subsequent placements of the headset 600 on the patient.
[00211] In alternative embodiments, other devices illustrated and described
farther
above may be used (or modified for use) in bilateral detection. The headset
600
described here is provided for exemplary purposes only.
[00212] Although specific embodiments of the disclosure have been described
herein
for purposes of illustration, various modifications may be made without
deviating from
the spirit and scope of the disclosure. For example, although the present
application
includes several examples of monitoring fluid changes in the human brain as
one
potential application for the systems and methods described herein, the
present
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
disclosure finds broad application in a host of other applications, including
monitoring
fluid changes in other areas of the human body (e.g., arms, legs, lungs,
etc.), in
monitoring fluid changes in other animals (e.g., sheep, pigs, cows, etc.), and
in other
medical diagnostic settings. Fluid changes in an arm, for example, may be
detected by
having an arm wrapped in a bandage that includes a transmitter and a receiver.
[00213] A few examples of the other medical diagnostic settings in which the
systems
and methods described herein may be used include determining an absolute
proportion
of a particular fluid, tissue (e.g., muscle, fat, parenchymal organs, etc.),
or other solid
matter (e.g., a tumor) in a given area of a human body, determining relative
permittivity
and/or relative permeability of an object, and so forth. Further clinical
applications
include a wide variety of monitoring and diagnostic uses, including internal
bleeding
detection, distinction between different types of fluid (e.g. blood,
extracellular fluid,
intracellular fluid, etc.), assessing edema including cerebral edema as well
as
lymphedema, and assessing lung fluid build-up resulting from such conditions
as
congestive heart failure. All of these applications and many more may be
addressed by
various embodiments described herein. Accordingly, the scope of the claims is
not
limited to the specific examples given herein.
[00214] VIPS technology has been previously discussed and disclosed as,
"volumetric
integral phase-shift spectroscopy". This technology is based on the principles
of
spectroscopy, in that it generates and directs a spectrum (a range) of
frequencies toward
a part of the body (for examples, chest or brain) and measures/detects the
effect (for
example, absorption and/or propagation phase delay) of the electromagnetic
radiation
due to the matter within the body part (for example, fluids or electrolytes).
However,
within this application the concept of the generation and detection of a
single
frequency, and not a spectra or spectrum, is disclosed. Furthermore, the
acronym VIPS
is used as "VIPS technology", "VIPS system", and the "VIPS device", as
examples,
and can represent a single frequency or spectra/spectrum/range of frequencies.
[00215] As described throughout this application, in many embodiments,
multiple
frequencies, phases and/or magnitudes may be used to measure fluid changes in
a
bodily organ or portion of the body, such as the brain, using VIPS technology
(volumetric integral phase-shift spectroscopy). The focus of the above
description, in
fact, is on the use of multiple transmitters and/or receivers in a system,
often used to
distinguish between different types of fluid in a given space. In some
embodiments,
66
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
however, the systems described herein may use only one frequency, phase or
magnitude to make any of a number of types of measurements. Plethysmography,
as
mentioned above, is the measurement of changes in volume in an organ or a
whole
body, usually resulting from changes in blood, motion (for example, if the
brain moves
due to a pressure increase caused by a ventilator) or air volume. In some
embodiments,
the systems described herein may use one frequency to measure changes in blood
volume in the brain or cranium to determine whether, for example, one
hemisphere of
the brain is receiving less blood flow than the other hemisphere. Similarly,
one
frequency may be used to measure overall changes in blood flow to the brain.
This is
but one example, which is provided to illustrate that although the description
in this
application focuses on the use of multiple frequencies, phases and magnitudes,
some
embodiments may employ only one frequency, phase and/or magnitude.
[00216] Although many embodiments of this disclosure describe VIPS technology
for
use in the measurement, monitoring, and/or detecting of fluid volume change,
these
same embodiments and/or alternative embodiments may use VIPS technology for
the
detection of non-fluid volume or change, such as a volume and/or change in a
tissue in
the patient's head. Furthermore, the association of fluid and/or volume change
may or
may not be a direct correlation to a pathology. Additional embodiments, for
example,
may not take into consideration any specific fluid, fluid volume, tissue or
organ
(example brain), but instead may assess the phase(s) and/or amplitude(s)
change, and/or
a combination of change(s). Such changes, volumes and the like may be defined
and
referred to as a "VIPS signature", which may correlate or be abstracted to a
particular
condition or pathology. For example, the effect of an ischemic stroke may not
present
as a volume/fluid change, but the VIPS technology may detect a frequency or
frequencies and/or amplitude(s) signature indicative of an ischemic stroke.
Furthermore, in some embodiments, the VIPS technology may detect a shift (or
translation) of an organ of the body, for example a shift in the brain itself,
which would
present as a VIPS signature. There may be a number of different VIPS
signatures, each
relating to a different condition, abnormality, anatomy, physiology or the
like. VIPS
signatures may be defined as changes, thresholds, specific measurements and/or
other
parameters related to measured phase(s) and/or amplitude(s). In various
embodiments,
a VIPS device may include any number of predefined, stored VIPS signatures,
and the
device may compare phase and/or amplitude measurements to the VIPS signatures
to
67
CA 03042629 2019-05-02
WO 2018/089035 PCT/US2016/069209
determine whether a particular condition, abnormality, anatomy or physiology
is
present.
[00217] Other changes might be changes in properties of tissues/liquids, such
as
electrolytes, enzymes, glial fibers, phagocyte cells, and/or iron. For
example, iron and
protein content in tissue sometimes change after an injury (stroke, impact,
bruise, etc.).
In another example, cell membrane permeability may change, thus altering or
breaking
the sodium-potassium pump. Bio-electric potentials may change, osmotic
pressure may
change, etc. There are different phases to a stroke, for example, and with
each there is
continual "remodeling" of the injury site, which may or may not be solely
dependent on
fluid volume changes.
[00218] Again, the above examples and embodiments are not intended to limit
the
scope of the invention, which is set forth in the following claims. The above
description
is meant to be exemplary in nature and not limiting.
68