Note: Descriptions are shown in the official language in which they were submitted.
1
WATER SOLUBLE CANNABINOID FORMULATIONS COMPRISING AN ENZYME
Field of the Invention
[0001] The present invention generally relates to water soluble
formulations, and more
particularly relates to water soluble formulations comprising a pharmaceutical
agent that is water
insoluble or poorly soluble, such as a cannabinoid, and methods of preparing
such formulations.
Background of the Invention
[00021 Cannabis compounds have a long history of use in humans as an
anticonvulsant,
sedative, hypnotic, anti-depressant, analgesic, anti-inflammatory, anti-
emetic, anti-spasmodic, and
appetite-stimulator. Cannabis contains a broad spectrum of chemical compounds
including:
phytocannabinoids, terpenoicls (essential oils), flavonoids, enzymes, and
steroids, While delta-9-
tetrahydrocannabinol (delta-9-THC) is believed to be the principle
psychoactive component of
hemp, other phytocannabinoids (such as cannabidiol, cannabinol, and
cannabichromene) are
thought to possess numerous medicinal properties without the psychoactive
effects of delta-9-
THC.
10003J However, the oral bioavailability of such phytocannabinoids is
limited. For
example, the oral bioavailability of cannabinoids was found to be about 6% or
less. The limited
bioavailability of phytocannabinoids is believed to be due to the fact that
cannabinoids are
naturally hydrophobic, fat-soluble compounds which limits their absorption,
thereby substantially
decreasing their bioavailability.
[0004] Due to the many desirable properties of phytocannabinoids, it
would be
advantageous to provide phytocannabinoid formulations with enhanced
bioavailability for human
consumption in various convenient dosage forms.
[0005] U.S. Pat. No. 6,045,826 discloses water-soluble compositions
containing a
lipophilic compound (including CoQ10) and a single solubilizing agent having
both hydrophobic
and hydrophilic moieties. Other formulations useful for the delivery of
coenzyme Q (CoQ10) are
described in U.S. Pat. No. 7,438,903 which teaches that soft gels are the most
popular method of
EDC_LAVNA 213454511
CA 3044735 2019-11-12
2
CoQ10 delivery, and CoQ10 is available commercially in the form of tablets,
hard capsules and
softgel capsules, either alone or in combination with vitamins and/or
supplements. U.S. Pat, No.
4,483,873 discloses aqueous solutions of CoQ10 that contain hydrogenated
lecithin to increase
CoQ10 bioavailability. U.S. Pat. Nos. 6,056,971, 6,441,050 and 7,094,804
disclose methods for
solubilizing water-insoluble dietary supplements in liquid form, such as CoQ10
in a softgel, by
mixing CoQ10 with, among other things, an edible polyhydric alcohol solvent.
U.S. Pat. No.
6,300,377 teaches the formulation of a CoQ10 composition that omits polyhydric
alcohol, but
includes other agents to help improve solubility, including a glyceryl ester
molecule having one to
three C2-C7 acyl groups. WO/2005/111224 discloses CoQiO complexes with beta-
cyclodextrin to
increase CoQ10 solubility in water.
100061 WO 00/38655A-1 describes formulations comprising porous calcium
hydrogen
phosphate particulates, sold commercially under the trademark, Fujicalin ,
within which a liquid
formulation of an active agent is absorbed, so that the liquid-absorbing
particulates can be
processed using conventional pharmaceutical equipment. These formulations arc
said to provide
high concentrations of active drug dosage without loss of active
pharmaceutical agent during the
manufacturing process, and to permit delivery of active pharmaceutical agent,
along with suitable
solubilization-enhancers to an absorption site, Other forms of the porous
particulates are also
disclosed including microcrystalline cellulose, silicon dioxide, or magnesium
aluminosilicate, or
blends thereof.
10007) US Patent No. 10/046018 describes methods and formulations for
increasing the
water solubility and/or bioavailability of a phytocannabinoid compound. In one
example, the
water-soluble phytocannabinoid formulation comprises a .phytocannabinoid and a
non-ionic
surfactant in a weight ratio of 1:10,000 to 1:5 phytocannabinoid to non-ionic
surfactant. However,
hydrogenation of cannabinoids is required to render them more water soluble,
and the
hydrogenation method results in decreased absorption and bioavailability of
the cannabinoids. The
formulation also undesirably includes an alcoholic solvent, such as methanol,
ethanol, propanol or
butanol, to dissolve the cannabinoids.
EDC_LAW12016088\1
CA 3044735 2019-05-30
3
[0008] In view of the foregoing, it would be desirable to provide a
stable, water soluble
pharmaceutical formulation.
Brief Description of the Invention
[0009] A formulation that provides enhanced oral bioavailability of
poorly water-soluble
pharmaceutical agents is herein provided. The formulation comprises a
pharmaceutical agent
which is poorly water-soluble, a detergent, a lipase, a plasticizing agent and
an emulsifying agent
in an aqueous solvent.
[00101 Thus, in one aspect of the invention, a formulation is provided
comprising a water-
insoluble or poorly insoluble pharmaceutical agent, a detergent, a lipase, a
plasticizing agent and
an emulsifying agent in an aqueous solvent.
[0011] In another aspect of the invention a water-soluble formulation is
provided
comprising a caimabinoid, a detergent, a lipase, a plasticizing agent and an
emulsifying agent.
[0012] These and other aspects of the invention are described by
reference to the following
Figure.
Brief Description of the Figure
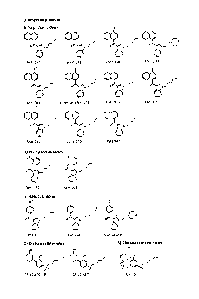
[0013] Figure 1 is a table illustrating the chemical structures of
various cannabinoids.
Detailed Description of the Invention
. [0014] A formulation is provided comprising a water-insoluble or
poorly insoluble
pharmaceutical agent, a detergent, a lipase, a plasticizing agent and an
emulsifying agent.
[00151 The present formulation comprises a water-insoluble or poorly
insoluble
pharmaceutical agent. The phrase "water-insoluble" as used herein is meant to
encompass any
pharmaceutical agent, i.e. an agent that possesses a therapeutic utility, that
does not fully dissolve
in a given aqueous solvent, including agents that are slightly soluble,
sparingly soluble and
completely insoluble, for example, an agent that requires greater than 30 mass
parts of solvent to
dissolve 1 mass part of solute (pharmaceutical agent), e.g. an agent that
requires greater than 50,
EDC_LAW12016088\1
CA 3044735 2019-05-30
4
100, 1000 or more, mass parts of solvent to dissolve 1 mass part of solute.
Examples of water-
insoluble pharmaceutical agents include, but are not limited to:
a. antimicrobial agents, such as trielosan, cetyl pyridium chloride, domiphen
bromide,
quaternary ammonium salts, zinc compounds, sanguinarine, fluorides, alexidine,
octonidine,
EDTA, and the like;
b. non-steroidal anti-inflammatory drugs, such as aspirin, acetaminophen,
ibuprofen,
ketoprofen, diflunisal, fenoprofen calcium, naproxen, tolmetin sodium,
indomethaein, and the like;
c. anti-tussives, such as benzonatate, caramiphen edisylate, menthol,
dextromethorphan
hydrobromide, chlophedianol hydrochloride, and the like;
d. decongestants, such as pseudoephedrine hydrochloride, phenylepherine,
phenylpropanolamine, pseudoephedrine sulfate, and the like;
c. anti-histamines, such as brompheniramine malcate, chlorpheniramine maleate,
carbinoxamine maleate, clemastine fumarate, dexchlorphcniramine maleate,
diphenhydramine
hydrochloride, diphenylpyraline hydrochloride, azatadine meleate,
diphenhydramine citrate,
doxylarnine succinate, promethazine hydrochloride, pyrilamine maleate,
tripelennamine citrate,
triprolidinc hydrochloride, acrivastine, loratadine, brompheniraminc,
dexbrompheniramine, and
the like;
f. expectorants, such as guaifenesin, ipecac, potassium iodide, terpin;
g. anti-diarrheals, such a loperamide, and the like;
h. Hz-antagonists, such as famotidine, ranitidinc, and the like;
i. proton pump inhibitors, such as omeprazole, lansoprazole;
j. general nonselective CNS depressants, such as aliphatic alcohols,
barbiturates and the
like;
k. general nonselective CNS stimulants such as caffeine, nicotine, strychnine,
picrotoxin,
pentylenetetrazol and the like;
I. drugs that selectively modify CNS function such as phenyhydantoin,
phenobarbital,
primidone, carbamazepine, ethosuximide, methsuximide, phensuximide, trim
ethadione,
EDC_LAVV120160880
CA 3044735 2019-05-30
5
diazepam, benzodiazepines, phenacemide, phencturide, acetazolamide, sulthiame,
bromide, and
the like;
m, antiparkinsonism drugs such as levodopa, amantadine and the like;
n. opioid analgesics such as alfentanil, allylprodine, alphaprodine,
anileridine,
benzylmorphine, bczitramide, buprenoorphine, butorphanol, clonitazene,
codeine, cyclazocine,
desomorphine, dextromoramide, dezocine, diampromide, dihydrocodeine,
dihydromorphine,
dimenoxadol, dhnepheptanol, dimethylthiatnbutene, dioxaphetyl butyrate,
dipipanone,
eptazocine, ethoheptazine, ethylmethylthiambutene, ethylmorphine, etonitazine,
fentanyl, heroin,
hydrocodone, hydromorphone, hydroxypethidine, isomethadone, ketobemidone,
levallorphan,
levorphanol, levophenacylmorphan, lofentanil, meperidine, meptazinol,
metazocine, methadone,
metopon, morphine, myrophine, nalbuphine, nareeine, nicomorphine,
norlevorphanol,
normethadone, nalorphine, normorphine, norpipanone, opium, oxycodone,
oxymorphone,
papaveretum, pentazocine, phenadoxone, phenomorphan, phenazocine,
phenoperidine,
piminodine, piritramide, propheptazine, promedol, properidine, propiram,
propoxyphene,
sufentanil, tramadol, tilidine, salts thereof, mixtures of any of the
foregoing, mixed mu-
agonists/antagonists, mu-antagonist combinations, free base forms,
pharmaceutically acceptable
salts, or in the form of a pharmaceutically acceptable complex;
o. analgesic-antipyretics such as salycilates, phenylbutazone, indomethacin,
phenacetin
and the like;
p. psychopharmacological drugs such as chlorpromazine, methotrimeprazine,
haloperidol,
clozapine, reserpine, imipramine, tranyleypromine, phenelzine, lithium and the
like;
q. hypnotics, sedatives, antiepilepties, awakening agents;
r. vitamins and minerals, caffeine, nicotine;
s. amino acids and peptides;
t. compounds such as sildenafil citrate (Viagra etc);
u. proteins, hormones and peptides e.g., insulin, erythropoietin, etc.;
EDC_LAVV1201608811
CA 3044735 2019-05-30
6
v. antidiabetic drugs, e.g., metformin, glyburide and insulin secretart agent,
insulin
stimulators, fat metabolizers, carbohydrates metabolizers, insulin,
cholesterol lowering
agents like statins, etc.;
w. a cannabinoid, terpene and analogues thereof;
x. pharmaceutically acceptable salts of any of the foregoing,
[0016] As used herein, the terms "cannabinoid" or "cannabinoid compound"
refer to
naturally occurring cannabinoids or terpenes from a Cannabis plant, typically
from the Cannabis
sativa or hemp plant, as well as analogues thereof, including synthetically
prepared earmabinoids
and analogues thereof. Exemplary eannabinoids include cannabidiol (CBD),
cannabinol (CBN),
cannabichromene (CBC), cannabiehromenic acid (CBCA), cannabidiolic acid
(CBDA),
cannabidivarin (CBDV), catmabigerol (CRC), cannabigcrolic acid (CBGA),
cannabigerivarin
(CBG V), cannabidivarin acid (CBDVA), cannabinovarin (CBN V), cannabinodiol
(CBDI.),
cannabicyclol (CBL), cannabielsoita (CBE), cannabitriol (CBT), cannabivarin
(CBV),
cannabichromevarin (CBCV), cannabigerol monocthyl ether (CBGM),
tetrahydrocannabinols
(THC), tetrahydrocannabivarin (THCV), naphthoylindoles such as JWH-018, JWI-1-
073õTWH-
398, JWH-200, JWI-1-081, 4-methyl-JWH-073, JWH-015, JWH-122, JW1-1-220, JWH-
019, JWH-
007; phenylacetylindoles such as JWH-250 and JW1-1-203; benzoylindoles such as
RCS-4, AM-
694 and WIN 48,098; cyclohcxylphenoles such as CP 47,497-C8 and CP 47,497; H11-
210 and
pharmaceutically acceptable salts thereof.
[0017] The term "pharmaceutically acceptable salts" or "salts" is meant to
include a salt
of a pharmaceutical agent prepared with nontoxic or relatively non-toxic acids
or bases, depending
on the particular substituent moieties found on the compounds described
herein, Examples of acid
addition salts include those derived from nontoxic inorganic acids, such as
hydrochloric, nitric,
phosphoric, sulfuric, hydrobromic, hydroiodic, phosphorous and the like, as
well as from nontoxic
organic acids such as aliphatic mono- and dicarboxylic acids, phenyl-
substituted alkanoic acids,
hydroxy alkanoic acids, aromatic acids, aliphatic and aromatic sulfonic acids
and the like. Base
EDC_LAW12016088N1
CA 3044735 2019-05-30
7
addition salts include those derived from alkaline earth metals, such as
sodium, potassium,
magnesium, calcium and the like, as well as from nontoxic organic amines, such
as N,NI-
dibenzylethylenediamine, N-methylglucamine, chloroprocaine, choline,
diethanolamine,
ethylenediamine, procaine and the like.
[0018] The present formulation will generally comprise the pharmaceutical
agent in an
amount in the range of about 1-50% by wt,
10019] The present formulation comprises at least one biological
detergent. Biological
detergents contain one or more enzymes that break down lipids (including
triglycerides, fats,
oils), e.g, a lipase, one or more enzymes that break down proteins, e.g. a
protease, and/or one or
more enzymes that break down starches. The detergent may be an ionic, non-
ionic or
zwitterionic detergent. Detergents are amphipathic molecules, containing a
polar hydrophilic
head group attached to a long-chain hydrophobic carbon tail. The polar head
group of ionic
detergents contain either a positive (cationic) or negative (anionic) charge.
10020] Anionic detergents typically have negatively-charged sulfate or ail
foliate groups
as the hydrophilic head; whereas cationic detergents contain a positively-
charged ammonium
group. Bile acids, such as cholic acid, deoxycholic acid, glycoeholic acid,
chenodeoxycholic
acid, taurocholie acid, glycodeoxycholic acid, taurodeoxycholic acid, or a
salts thereof, and
aliphatic sulphate esters (e.g., sodium dodecyl sulphate or sodium lauryl
sulfate) .are examples of
anionic detergents, and quaternary ammonium salts of acetates, chlorides, or
bromides are
examples of cationic detergents.
[0021] Non-ionic detergents have a neutral, polar head group. Non-ionic
detergents are
typically based on polyoxycthylcne or a glycoside. Polyoxyethylene detergents
have a tail
composed of hydrophobic oxyethylene or ethylene glycoether chains. Examples of
polyoxyethylene-based detergents include ethoxylates, PEGylates and
metabolites thereof',
including Tweens such as polysorbate 20 (polyoxyethylene (20) sorbitan
monolaurate),
EDC_UMAIN 201608811
CA 3044735 2019-05-30
8
polysorbate 40 (polyoxyethylene (20) sorbitan menopalmitate), polysorbate 60
(polyoxyethylenc
(60) sorbitan monostearate), polysorbate 80 (polyoxyethylene (80) sorbitan
monooleate),
alkylphenol ethoxylates such as nonoxynols and Tritonim, and the BrijIm
compounds, e.g. Brij
20 (polyoxyethylene (20) cetyl ether) or Brij 35 (polyoxyethylene (23) lauryl
ether). Glycosidic-
based detergents have a sugar, such as glucose or maltose, as their uncharged
hydrophilic
headgroup, and may have an alkyl polymer tail. Examples include octyl
thioglucoside and
maltosides. Fatty acid esters of sorbitol, such as sorbitan monolaurate,
sorbitan monostearate
and sorbitan tristearate, fatty acid esters of glycerol, such as glycerol
monostearate and glycerol
monolaurate and fatty acid esters of sucrose are also non-ionic detergents.
[0022] The polar head groups of zwitterionic detergents contain both
negatively and
positively charged atomic groups, therefore the overall charge is neutral,
e.g.
(dimethylmyristylammonio)-propanesulfonate and (tart-Buty1-1-pyridi nio)-1-
propanesulfonate .
Other examples include 3-[(3-cholamidopropyl)dimethylammonio]-1-
propanesulfonate (CHAPS)
and 3-[(3-eholamidopropyI)-dimethylammonio]-2-hydroxy-1-propanesulfonate
(CHAPS 0).
[0023] As one of skill in the art will appreciate, the appropriate
detergent for inclusion in
the present formulation will depend on factors such as the pharmaceutical
agent in the formulation,
pH, ionic charges, the desired denaturing effect and the desired end result,
including structure and
charge of the final product.
[0024] Examples of enzymes that may be used in conjunction with the
detergent to provide
a biological detergent include, but are not limited to, lipases such as
pancreatic lipase (PL),
pancreatic lipase-related protein 1 or 2 (PLRP1/PLI{P2), hepatic lipase,
endothelial lipase,
lipoprotein lipase, lysosomal lipase, gastric lipase and lingual lipase. Other
examples include
termamyl (amylase), lipolase (lipase), celluzyme (cellulase), mannanase and
pectinase. The
enzymes may be naturally occurring enzymes or recombinant enzymes. Individual
enzymes or
combinations of enzymes may be used.
EDC_LAVV1 201608811
CA 3044735 2019-05-30
9
[0025] The amount of detergent in the present formulation is in the range
of about 0.01 to
% by wt of the composition, The amount of enzyme in the formulation is in the
range of about
0.01 to 10 % by wt of the composition.
[0026] The present formulation includes one or more plasticizing agents
to attain
desired flexibility and mold-releasing properties. Suitable plasticizing
agents include, for example,
triacetin, monoacetin, diacctin and glycerin. Plasticizing agent may be added
to the formulation
in an amount ranging from about 0.01 to about 20 wt 1)/0, preferably an amount
of about 0.1 to
about 2 wt % of the formulation.
[0027] The present formulation also includes an emulsifying agent such as
triethanolam Inc
stearate, quaternary ammonium compounds, acacia, gelatin, lecithin, bentonite,
veegum, and the
like, in amounts ranging from about 0,01 to about 5 wt %, and preferably about
0.01 to about 0,7 1
wt % of the formulation.
[00281 The present formulation may include a stabilizing agent such as
xanthan gum,
locust bean gum, guar gum and carrageenan, in amounts ranging from about 0.01
to about 10 wt
%, preferably about 0.1 to about 2 wt % of the formulation.
[00291 The present formulation may also include one or more saliva
stimulating agents
such as a food acid, e.g. citric, lactic, malic, succinic, ascorbic, adipic,
fumaric or tartaric acid, or
mixtures thereof Preferred food acids are citric, malic and ascorbic acids.
The amount of saliva
stimulating agent suitable for inclusion in the present formulation may range
from about 0.01 to
about 12 wt %, preferably about 1 wt % to about 10 wt %.
[0030] The present formulation may additonally include a thickening agent
such as
methylcellulose, carboxyl methylcellulose, and the like, in amounts ranging
from about 0.01 to
about 20 wt %, and preferably about 0.01 to about 5 wt %,
EDC_LAVVI 2016088 \ 1
CA 3044735 2019-05-30
10
[0031] The present formulation may fluffier include one or more
pharmaceutically
acceptable adjuvants or carriers. The expression "pharmaceutically acceptable"
means acceptable
for use in the pharmaceutical arts, i.e. not being unacceptably toxic, or
otherwise unsuitable for
administration to a mammal. Examples of pharmaceutically acceptable adjuvants
include, but are
not limited to, diluents, excipients and the like. Reference may be made to
"Remington's: The
Science and Practice of Pharmacy", 21st Ed., Lippincott Williams & Wilkins,
2005, for guidance
on drug formulations generally. The selection of adjuvant depends on the
intended mode of
administration of the composition. In one embodiment of the invention, the
compounds are
formulated for oral administration via tablet, capsule, lozenge, solution or
suspension in an
aqueous or non-aqueous liquid, an oil-in-water or water-in-oil liquid
emulsion, an elixir or syrup
are prepared using adjuvants including sugars, such as lactose, glucose and
sucrose; starches such
as corn starch and potato starch; cellulose and derivatives thereof, including
sodium
carboxymethyleellulose, ethylcellulose and cellulose acetates; powdered
tragancanth; malt;
gelatin; talc; stearic acids; magnesium stearate; calcium sulfate; vegetable
oils, such as peanut oils,
cotton seed oil, sesame oil, olive oil and corn oil; polyols such as propylene
glycol, glycerine,
sorbital, mannitol and polyethylene glycol; agar; alginic acids; water;
isotonic saline and phosphate
buffer solutions, wetting agents, lubricants, stabilizer's, anti-oxidants and
preservatives.
[0032] To render the formulation more desirable for oral administration,
natural and/or
artificial sweeteners, flavouring agents and colouring agents may be included
in the formulation.
[0033] Suitable sweeteners include, water-soluble sweetening agents such
as
monosaccharides, disaccharides and polysaccharides such as xylose, ribose,
glucose (dextrose),
mannose, galactose, fructose (levulose), sucrose (sugar), maltose, invert
sugar (a mixture of
fructose and glucose derived from sucrose), partially hydrolyzed starch, corn
syrup solids,
dihydrochalcones, monellin, steviosides, and glycyrrhizim water-soluble
artificial sweeteners such
as the soluble saccharin salts, i.e., sodium or calcium saccharin salts,
cyclamate salts, the sodium,
ammonium or calcium salt of 3,4-dihydro-6-methyl-1,2,3-oxathiazine-4-one-2,2-
dioxide, the
EDC_LAW\ 201608811
CA 3044735 2019-05-30
11
potassium salt of 3,4-dihydro-6-methyl-1,2,3-oxathiazine-4-one-2,2-dioxide
(acesulfame-K), the
free acid form of saccharin, and the like; dipeptide based sweeteners, such as
Laspartic acid
derived sweeteners, such as L-aspartyl-L-phenylalanine methyl ester
(aspartame) and materials
described in U.S. Pat. No. 3,492,131, L-alpha-aspartyl-N-(2,2,4,4-tetramethy1-
3-thietany1)-D-
alaninamide hydrate, methyl esters of L-aspartyl-L-phenylglycerin and L-
aspartyl-L-
2,5,dihydrophenyl-glyeine, L-
asparty1-2,5-dihydro- L-phenylalanine, L-aspartyl-L-( -
cyclohexyen)-alanine, and the like; derivatives of water-soluble sweeteners
such as a chlorinated
derivative of sucrose, i.e. sucralose; and protein based sweeteners such as
thaumatoccons danielli
(Thaumatin 1 and 11). In general, an effective amount of an auxiliary
sweetener is utilized to
provide the desired level of sweetness. The amount of sweetener will vary with
the selected
sweetener. Generally, an amount of sweetener in the range of 0.0 I% to about
10% by weight of
the formulation is appropriate.
[00341
Flavorings that may be used in the formulation include both natural
anclartificial
flavors. These flavorings may be chosen from synthetic flavor oils and
flavoring aromatics and/or
oils, oleo resins and extracts derived from plants, leaves, flowers, fruits
and so forth, and
combinations thereof. Representative flavor oils include: spearmint oil,
cinnamon oil, peppermint
oil, clove oil, bay oil, thyme oil, cedar leaf oil, oil of nutmeg, oil of
sage, and oil of bitter almonds.
Also useful are artificial, natural or synthetic fruit flavors such as lemon,
orange, grape, lime,
grapefruit, apple, pear, peach, strawberry, raspberry, cherry, plum,
pineapple, apricot and so forth.
These flavorings may be used individually or in admixture. Other flavours
include vanilla,
chocolate and coffee. Aldehydes and esters may be used as flavourants as well,
e.g. cinnamyl
acetate, cinnamaldehyde, citral, diethylacetal, dihydrocarvyl acetate, eugenyl
formate, p-
methylanisole, acetaldehyde (apple); benzaldehyde (cherry, almond); cinnamic
aldehyde
(cinnamon); eitral, (lemon, lime); decanal (orange, lemon); ethyl vanillin
(vanilla, cream);
heliotropine, i.e., piperonal cream);
vanillin (vanilla, cream); butyraldehyde (butter,
cheese); valeraldehyde (butter, cheese); citronellal (modifies, many types);
aldehyde C-8 (citrus
fruits); aldehyde C-9 (citrus fruits); aldehyde C-12 (citrus fruits); 2-ethyl
butyraldehyde (berry
EDC JAVV12016088 \1
CA 3044735 2019-05-30
12
fruits); hexenal, i.e. trans-2 (berry fruits); tolyl aldehyde (cherry,
almond); veratraldehyde
(vanilla); 2,6-dimethy1-5-heptenal, i.e. melonal (melon); 2-6-dimethyloctanal
(green fruit); and 2-
dodecenal (citrus, mandarin); cherry; grape; mixtures thereof; and the like.
The amount of
flavoring employed may be in the range of about 0.1 to about 30 wt. %.
[0035] The formulation may also contain coloring agents or colorants. The
coloring agents
may include natural food colors and dyes suitable for consumption such as FD&C
dyes and lakes,
such as FD&C Blue No. 2, which is the disodium salt of 5,5-indigotindisulfonic
acid and FD&C
Green No. 3 which is the monosodium salt of 444-N-ethyl-p-sullobenzylamino)
diphenyl-
methyleneHl-N-ethyl-N-p-sulfonium benzy1)-2,5-cyclo-hexadieniminej. FD&C and
D&C dyes
and their corresponding chemical structures are described in the Kirk-Oihmer
Encyclopedia of
Chemical Technology, Volume 5, pages 857-884. Pigments such as titanium
dioxide may be used.
Colorants may be incorporated in amounts of up to about 5 wt %, and preferably
less than about
%.
100361 Cooling agents may be added to the formulation to increase the
boiling point
thereof. Examples of cooling agents that may be added to the formulation are
monomenthyl
succinate, WS3, WS23 and Ultracool II in an amount ranging from about 0.001 to
about 2.0 wt %,
preferably about 0.2 to about 0.4 wt % of the formulation.
[0037] The balance of the formulation is an aqueous solvent such as water
or other water-
containing solvent, e.g. saline, etc.
(0038] The present formulation may be in the form ()la beverage, juice,
soil drink, bottled .
water or a liquid concentrate.
[00391 The present formulation may be prepared as follows. The selected
pharmaceutical
agent is added to a volume of the selected detergent or mixture of detergents
and heated to a
temperature in the range of about 35-65 C. The heated combination is mixed to
form a clear
emulsion in which the pharmaceutical agent is dissolved, e.g. generally with
high speed mixing.
CDC_LAW1 2016088 \ 1
CA 3044735 2019-05-30
13
Hot water may additionally be added to the combination to achieve dissolution,
e.g. a crystal clear
solution. Other non-aqueous components may then be added with heat and
stirring. An aqueous
solution comprising water-soluble components (e.g. sweetener, flavor, colour)
is then added to the
emulsion and mixed to form a clear solution. Enzyme, plasticizer, saliva
stimulating agent,
stabilizing agent and any additional emulsifying agent are added once the
solution or suspension
is made. The mixture is further stirred to form a clear or ahnost clear
solution, and then allowed
to cool for storage.
[0040] The present invention advantageously provides an oral formulation
in which water
insoluble pharmaceutical agents are solubilized without using alcohols, i.e.
an alcohol-free
formulation. In addition, the formulation is prepared using hydrogenation
methods to form a clear
aqueous solution that exhibits improved bioavailability. As used herein, the
term "clear is
intended to relate to a solution or aqueous solution that is free, or
essentially free, of visible
particles ofundissolved compound. A clear solution or clear aqueous solution
includes, thus, both
solutions as well as very fine dispersions that remain clear upon sitting
undisturbed for one hour
or more, Essentially in a clear solution no visible (to the naked eye)
particles or micelles are
present.
[0041] Embodiments of the invention are described in the following
specific examples
which are not to be construed as limiting.
Example 1
[0042] A formulation comprising the water insoluble pharmaceutical agent,
eannabidiol
(CBD), was prepared with the following ingredients:
0.01-50% CBD oil
5% vitamin 2 (d,l-a-locopheryl acetate) (emulsifier)
1-15% omega-3 fatty acid ethyl ester (lncromegaTM 3322) (emulsifier)
15% mono-, di-glycerides of caprylic acid (detergent)
20% polyoxyl 35 (CremophorTM EL) (emulsifier)
3% Na lauryl sulfate (SLS) (ionic detergent)
0. [-25% glycerin (plasticizing agent)
2% Brij 80 detergent (Tween)
E DC_LAW \ 2016088\1
CA 3044735 2019-05-30
14
5% triethanolamine stearate (emulsifier)
3% pancreatic lipase related protein 2 and I (lingual lipase)
O. I% sodium citrate (saliva stimulating agent)
27% distilled water
[0043] The method of making the present formulation was as follows. A
water soluble
formulation comprising cannabidiol and THC was prepared by admixing the
cannabidiol oil with
the detergents, Na lauryl sulfate + Brij 80 (polyoxyl ether 80). The
cannabidiol oil .contained 80
wt % cannabidiol (CBD) and 20% oil, The mixture was heated with stirring to a
temperature of
about 60 C and mixed at 1000-1500rpm until a clear viscous emulsion phase,
with dissolved C131)
oil was formed (cannabidiol emulsion). Water was boiled at 212 F. The heated
water was then
slowly added to the cannabidiol emulsion until a crystal clear solution was
formed, in a separate
container, Vitamin E oil, Omega-3 oil fatty acid ethyl ester, mono/di-
glyceride of caprylic acid
detergent, Cremophor, glycerin and Tween were combined and mixed to form an
emulsion. This
emulsion was then added slowly to the oil-water mixture at 60 C slowly while
stirring
continuously at 1000 rpm. An aqueous solution comprising water-soluble
components, if any (e.g.
sweetener, flavor, colour), are then added to the emulsion and mixed to form a
clear solution.
Enzyme, saliva stimulating agent and emulsifying agent (triethanolamine
stearate) were then
added to the solution. The mixture thus prepared was stirred additionally for
30-45 minutes to
form an essentially clear solution. The solution was then cooled down slowly
to room temperature
and stored in a brown glass bottle.
Example 2
(0044] A water soluble formulation comprising cannabidiol (CBD) was
prepared by
admixing cannabidiol oil with the ionic detergents, Na lauryl sulfate + Brij
80 (polyoxyl ether 80).
The cannabidiol oil contained 80 wt % cannabidiol (CBD). The ionic detergents
were heated and
stirred to a temperature of about 120 F. Then the cannabidiol oil was added
slowly and mixed at
1000-1500rpm until a clear viscous emulsion phase with dissolved CBD oil was
formed
(cannabidiol emulsion). Water was boiled at 212 F. The heated water was then
slowly added to
EDC_LAW\ 2016088\1
CA 3044735 2019-05-30
15
the cannabidiol emulsion until a crystal clear solution was formed. The
remaining ingredients
were then added.
[0045) The weight percentage of each component in the water soluble
composition is
presented in Table 1.
Formulation wt%
CBD 5.000
Avicel 0.250
Thymol NF 0.400
Menthol NF 0.550
Methyl Salicylate 0.500
Mint flavor 8.500
Citric Acid 0.750 (saliva stimulating agent)
Copper gluconate 1.250
Purified water, USP 68.500
Sodium lauryl sulfate 1.500 (surfactant, detergent)
Aspartame 6.500 (sweetener)
Cooling' agent 0.075 =
Sorbitol (crystalline) 1.000 (sweetener)
Glycetin 5.000 (plasticizer)
Polysorbate 80 NF 0.550 (emulsifier)
Atmos 300 0.550 (emulsifier)
FD&C Green #3 = 0.009
Macro golglycerol 13.116
D&C Yellow #10 0.002
Lipase 0.003
Example 3
[0046] A water soluble formulation comprising cannabidiol was prepared as
follows.
Polyoxyl 40 castor oil (Brij 80) was heated and stirred to a temperature of
about 100 F. Then
grapefruit seed oil (a natural preservative) and a cannabidiol oil containing
80 wt % cannabidiol
(CBD) were added slowly and mixed until a clear viscous solution was formed.
The clear emulsion
was then slowly added to warm water (120 F-180 F) until a crystal clear
solution was formed
and then remaining ingredients were added.
EDC_LAiM 213454511
CA 3044735 2019-11-12
16
[0047] The weight percentage of each component in the formulation was as
follows:
Ingredient Wt %
Grapefruit seed Extract Oil 0.5
Cannabidiol Oil (80% CBD) 2.0
Water 62.45
Sodium Lauryl Sulfate + Brij 80 10.0 (surfactant/detergent ¨ 5 wt% each)
Glycerin 10.0 (plasticizer)
Cooling agent 0.0750
Sorbitol (crystalline) 5.0000
Aspartum 10.000
Green color 0.05
Citric Acid 0.2 (saliva stimulating agent)
Polysorbate 80 NF 0.550 (emulsifier)
Atmos 300 0.550 (emulsifier)
Lipase 0.0030
Example 4
[0048] A pharmacokinetic, bioavailability (blood absorption) study was
conducted with
the water soluble cannabidiol formulation from Example 2 above to compare the
bioavailability
of cannabinoids in the formulation as compared to cannabinoid oil.
[0049] Male Sprague-Dawley rats (mean wt 0.298 kg) were administered 50
nig doses by
oral gavage. Blood plasma samples were collected at various intervals from 0-
24 hours, post dose,
and the plasma concentration of cannabidiol was determined by liquid
chromatography/tandem
mass spectrometry (LC-MS/MS). The animal data collected is shown below and
displays the mean
blood plasma level over a 24 hour period.
PLASMA CONC'N OF CBD (ng/ml)
Time Formulation Cannabis Oil
0 hour (pre-dose)
0.25 hour 103 14.6
0.5 hour 342 19.2
1.0 hour 681 91.7
2.0 hours 3470 265
4.0 hours 2370 192
8.0 hours 1570 127
24 hours 551 30.6
E DC_LAW1 201 608811
CA 3044735 2019-05-30
= 17
[0050] Consumption of the present formulation resulted in a much greater
plasma
concentration of CBD than consumption of CBD oil itself. The Cnia of the
present formulation
2310 ng/ml as compared to 192 for CBD oil itself. Thus, bioavailability of CBD
was
significantly improved using the present formulation, e.g. 76.9% uptake of CBD
when
administered the present formulation vs. 6.3% CBD uptake for CBD oil.
[00511 The total absorption level AUCco (hr = kg - ng/intimg) was
determined to be 89
for CBD oil as compared to 296 for the present water-soluble CBD formulation.
=
EDC_LAW\ 2016088\1
CA 3044735 2019-05-30