Note: Descriptions are shown in the official language in which they were submitted.
METHOD FOR DIAGNOSIS OF ALZHEIMER'S DISEASE USING MICRORNA
REFERENCE TO SEQUENCE LISTING SUBMITTED ELECTRONICALLY VIA
EFS-WEB
The content of the electronically submitted sequence listing in ASCII text
file (Name:
4366 0130000_SeqListing_ST25.txt; Size: 2,474 bytes; and Date of Creation:
June 13, 2019)
filed with the application is herein incorporated by reference in its
entirety.
[Technical Field]
The present disclosure relates to a method for diagnosing Alzheimer's disease
or a
brain disease using a microRNA. More particularly, it relates to a method for
diagnosing
Alzheimer's disease or a brain disease, which includes a step of measuring the
expression
level of the microRNA miR-485-3p in a sample. In addition, it relates to a
composition and
a diagnostic kit for diagnosing Alzheimer's disease or a brain disease using
miR-485-3p or
amyloid beta 42.
[Background of the Disclosure]
The treatment of' Alzheimer's disease has recently focused on the observation
that
Alzheimer's disease may be caused by impaired cholinergic signaling and
transmission in the
cerebral cortex and hippocampus (Bartus et al., Science. 217(4558): 408-
14(1982) and Coyle
et al., Science. 219(4589): 1184-90(1983)).
Because these regions of the brain are associated with memory and
intelligence,
functional deficit in these regions may cause loss of memory and intelligence.
Although the
process of impairment in neuronal signaling is still controversial, senile
plaques and
neurofibrillary tangles (NFT) are considered as main pathological causes.
EDC_LAV111204724111
CA 3046766 2019-06-17
2
In particular, development of senile plaques due to the accumulation of
amyloid beta
(Af3) is a notable feature of this disease, and Alzheimer's disease can be
confirmed by post-
mortem examination (Khachaturian, Arch, Arettrol 42(1 ): 1097-105(1985)).
As a way of treating Alzheimer's disease, a method of increasing the amount of
acetylcholine to inhibit the impairment of cholinergic signaling or causing
acetylcholine to
act more effectively on transmission of neuronal cells has been proposed.
Thus, patients
with Alzheimer's disease use a variety of compounds for increasing the
activity of
acetylchol Inc.
Currently, the most effective way is to rapidly decompose acetylcholine in
synapses,
thus inhibiting the activity of acetylcholinesterase that prevents neuronal
signaling. These
inhibitors (e.g., tacrine, donepezil, galantamine and rivastigmine) are
approved by the United
States Food and Drug Adminisitration (FDA) and are currently available on the
market as
Alzheimer's disease medications.
Despite their effectiveness in preventing further
destructive progress of this disease, they are not used to cure the disease.
Some compounds are aimed to improve the neuronal state and maintain aged cells
in
good fuction. For example, some drugs such as NGF or estrogen act as
neuroprotecting
agents to delay neurodegeneration, and other drugs such as antioxidants
decrease cell damage
caused by oxidation of cells resulting from normal aging.
Alzheimer's disease becomes serious as amyloid beta peptide is accumulated in
the
neuritic space. It is thought that the progress of Alzheimer's disease can be
delayed by
reducing the accumulation of amyloid beta. In addition, amyloid precursor
protein (APP) is
considered to play a role in cells in combination with proteinases such as a-,
f3- and y-
secretases. However, because the process of amyloid beta formation has not
been fully
elucidated scientifically, it is not possible to control the formation of
amyloid beta.
EDC_LAW\ 204724111
CA 3046766 2019-06-17
3
It is not certain how the accumulation of amyloid beta acts on neuronal
signaling.
Abnormally cleaved APP induces amyloid beta generation, and plaque formation
is induced
by the accumulation of amyloid beta in neuritic space. Thus, various factors
involved in this
cleavage reaction (e.g., Inflammation reaction, etc.) increase the
phosphorylation of tau
S protein, and also increase the accumulation of paired helical filaments
(PHF) in combination
with NFT, resulting in damage to the nerve. All these factors induce
dysfunction of the
nerve and, ultimately, accelerate the progress of Alzheimer's disease to
dementia.
Although the development of therapeutic methods to reduce the effect of
Alzheimer's
disease is carried out actively, temporary improvement of symptoms is the
current strategy.
. 10 In conclusion, the current treatment of Alzheimer's disease is just
focused on improvement of
symptoms instead of slowing or reversing the progress of the disease. Despite
the biological
knowledge about the disease, clinical application is still not successful.
In this regard, US Patent No. No. 5,532,219 discloses a composition for
treating
Alzheimer's disease containing 4,4'-diaminodiphenylsulfone, etc., US Patent
No. 5,506,097
15 discloses a composition for treating Alzheimer's disease containing para-
atnidinophenylmethanesulfonyl fluoride or ebelactone A, and US Patent No.
6,136,861
discloses a composition for treating Alzheimer's disease containing
bicyclo[2.2.11heptane.
ELAVL2, or ELAVL-like neuron-specific RNA binding protein 2, is a type of
nELAVL2. nELAVL2 is an RNA-binding protein expressed specifically in the brain
and is
20 known to be associated with neurodegenerative diseases. As a result of
conducting high-
throughput RNA sequencing using brain tissue after post-mortem of patients
with
Alzheimer's disease, it was found out that ELAV L2 was expressed with low
levels.
Alzheimer's disease is the most common form of dementia. 75% of patients with
dementia have Alzheimer's disease. In most cases, Alzheimer's disease begins
in people
25 over 65 years of age, although it can occur earlier in rare cases. In
the United States, about
CA 3046766 2019-06-17
4
3% of the population aged 65-74 years, about 19% of the population aged 75-84
years, and
50% of the population aged over 85 years suffer from this disease. In Korea,
according to a
recently reported study on a rural region, about 21% of the population aged
over 60 years in
the rural region showed dementia, and 63% of them had Alzheimer's dementia. In
2006,
266,000 people around the world had the disease. It is expected that the
disease will occur
in one out of every 85 people in 2050.
There is no cure for Alzheimer's dementia and the only method of management is
diagnosis followed by prescription of relievers. In addition, no early
diagnostic system is
available. Although a variety of diagnostic methods exist, they have many
disadvantages.
The questionnaire may have errors due to difference in perception, the
structural brain
imaging is expensive, and the nuclear medical brain imaging has the
disadvantage of using
radioactive isotopes and being expensive. The tau protein measurement through
the
analysis of the cerebrospinal fluid is an invasive method and has a risk
associated with the
extraction of the cerebrospinal fluid,
Thus, the inventors of the present disclosure have made efforts to select a
marker that
can diagnose brain diseases such as Alzheimer's disease and develop a more
accurate
diagnostic method. As a result, they have confirmed the increased expression
level of miR-
485-3p extracted from blood and have completed the present disclosure.
The information described in the Background section is only to enhance the
understanding of the background of the present disclosure, and the information
forming the
prior art already known to those having ordinary skill in the art to Nvhich
the present
disclosure belongs may not be included.
[Summary of the Disclosure]
EDC_LAWI 2047241\1
CA 3046766 2019-06-17
S
The present disclosure is directed to providing a method for diagnosing
Alzheimer's
disease or a brain disease and a method for providing information for the
early diagnosis
thereof by measuring the expression level of miR-485-3p.
The present disclosure is also directed to providing a composition and a
diagnostic
kit for diagnosing Alzheimer's disease or a brain disease.
The present disclosure is also directed to providing a use of a composition
for
measuring the expression level of miR-485-3p or the concentration of amyloid
beta 42 in the
diagnosis of Alzheimer's disease or a brain disease.
In order to achieve the above-described objects, the present disclosure
provides a
method for diagnosing Alzheimer's disease or a brain disease and a method for
providing
information for the diagnosis thereof, which include a step of measuring the
expression level
of miR-485-3p in a sample.
The present disclosure also provides a composition or a diagnostic kit for
diagnosing
a brain disease such as Alzheimer's disease, etc., wherein the kit is used to
measure the
expression level of miR-485-3p or the concentration of amyloid beta 42.
The present disclosure also provides a use of a composition for measuring the
expression level of miR-485-3p or the concentration of amyloid beta 42 in the
diagnosis of
Alzheimer's disease or a brain disease.
[Brief Description of Drawings]
FIGs. IA-I B show a miR.NA expression pattern analysis result (volcano plot)
for a
patient group as compared to a normal group (FIG. A), and a graph comparing
the
expression of miR-485-3p in a patient group compared to a normal group (FIG,
1B).
EDG_LAW1204724111
CA 3046766 2019-06-17
6
FIG. 2 shows the seed sequence of 3'-untranslated region (UTR) mRNA and miR-
485-3p of 5xFAD.
FIGs. 3A-3C show a graph comparing the expression of miR-485-3p in the
hippocampus and cortex (FIG. 3A) and a result of comparing the expression of
ELAVL2 and
related proteins in the hippocampus and cerebral cortex of 5xFAD (FIGs. 3B and
3C).
FIGs. 4A-4B show a graph showing a comparative quantitative analysis result of
Af3
42 in the cerebral cortex of 5xFAD (FIG. 4A), and a comparative quantitative
analysis result
of AP 42 in the hippocampus (FIG. 4B).
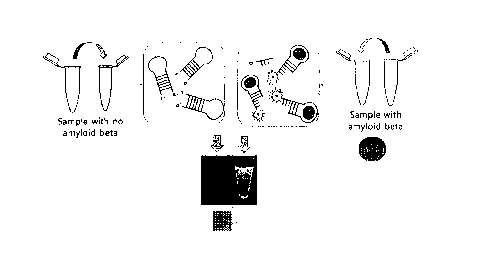
FIG. 5 is a schematic diagram of a magnetic particle collection device. A
process
wherein an antigen, an antibody, a magnetic particle and a fluorescent
material form a
complex is shown.
FIGs. 6A-6B show results of quantifying saliva-derived Ap using a magnetic
particle
collection device.
FIGs. 7A-7I3 show that a dementia patient can be distinguished by measuring
the
.. concentration of saliva-derived AP using a magnetic particle collection
device.
FIG. 8 is a schematic diagram of a process of measuring the level of a nucleic
acid
expressing amyloid beta (Am 42.
FIGs. 9A-9B are images of a gel electrophoresis (FIG. 9A) and a bar graph
(FIG. 9B)
showing results of comparing the expression of ELAVL2 in hippocampal primary
cells
transfected with AM485-3p.
FIGs. I0A-10C show comparative quantitative analysis results of ELAVL2 and
related proteins and Ap 42 in 5xFAD intranasally treated with AM485-3p.
FIGs. 11A-11B show graphs comparing the cognitive function of 5xFAD
intranasally treated with AM485-3p.
EDC JAVV12047241
CA 3046766 2019-06-17
7
Unless defined otherwise, all technical and scientific terms used herein have
the
same meaning as commonly understood by the skilled experts in the art to which
the present
disclosure belongs. In general, the nomenclature used herein is known well and
commonly
used in the art.
In a specific example of the present disclosure, the expression of miR-485-3p
was
found to increase significantly in the blood obtained from a patient diagnosed
with
Alzheimer's dementia by a physician.
Thus, in an aspect, the present disclosure relates to a method for providing
information for the diagnosis of Alzheimer's disease or a brain disease, which
includes a step
of measuring the expression level of miR-485-3p from a sample.
In another aspect, the present disclosure relates to a method for diagnosing
Alzheimer's disease or a brain disease, which includes a step of measuring the
expression
level of miR-485-3p in a sample.
The present disclosure may include a step of extracting the microRNA mi R-485-
3p
from the sample. The sample may be blood or plasma, although not being limited
thereto.
In the example of the present disclosure, about 3 m1_, of blood was taken from
a
patient diagnosed with Alzheimer's dementia by a physician into a blood tube
(Becton
Dickinson, Germany) to which sodium citrate (3.2% w/v) was added. The blood
was
.==
centrifuged at 3,500 rpm for 10 minutes to separate plasma, and then stored at
-80 C until
RNA extraction. miRNA was extracted using the miRNeasy Serum/Plasma kit
(Qiagen,
USA) according to the manufacturer's recommendations. The miRNA extraction is
performed in the following order. After lysing the blood plasma or serum
sample with the
QIAzol lysis reagent and then adding chloroform, the lysate is separated into
an aqueous
phase and organic phase by centrifugation. RNA partitions to the upper aqueous
phase, while
CA 3046766 2019-06-17
8
DNA partitions to the intermediate layer and proteins to the intermediate
layer or the lower
organic phase. The upper aqueous phase is extracted and ethanol is added to
provide a
condition appropriate for binding with RNA molecules of at least about 18
nucleotides. The
sample is applied to the RNeasy MinElute spin column such that all RNA is
bound to a
membrane and phenol and other contaminants are washed off efficiently. High-
quality
RNA is eluted in a small amount of water without RNase. Because serum and
plasma
mainly contain small RNAs, it is not necessary to additionally purify small
and large RNA
fractions.
The present disclosure includes a step of measuring the expression level of
the
microRNA miR-485-3p.
In the present disclosure, the expression level of miR-485-3p may be measured
by a
method selected from a group consisting of real-time PCR, quantitative PCR,
primer
extension, nucleic acid chip analysis, sequencing, aptamer-based assay and gel
electrophoresis, although not being limited thereto.
In an example of' the present disclosure, the concentration and purity of the
extracted
RNA were analyzed using Bioanalyzer2100 (Agilent, USA). The extracted RNA was
screened using the miRNA array containing 84 different miRNAs known to be
associated
with human neurological development and the progress of neurological disease.
The quantitative PCR assay method can be summarized as follows. A mature
miRNA is generally a 22-nucleotide, non-coding RNA and is responsible for post-
transcriptional regulation. Polyadenylation of mature miRNA was induced by
poly(A)
polymerase, and cDNA was synthesized using oligo-dT primers. The oligo-dT
primer
enables the amplification of the mature miRNA during the real-time PCR process
because it
has a 3' degenerate anchor and a universal tag sequence at the 5' end. The
mature miRNA
EDC_LAW12047241 11
CA 3046766 2019-06-17
9
was quantified during the real-time PCR process using the miScript SYBR Green
PCR kit
(Qiagen).
In the present disclosure, Alzheimer's disease or a brain disease may be
diagnosed if
the expression level of miR-485-3p is 5 times or larger, more specifically 9
times or larger, as
compared to a normal group. Those who were aged between 59 and 64 years and
had no
history of Alzheimer's disease or a brain disease were selected as the control
group.
In the present disclosure, the brain disease may be any one selected from a
group
consisting of autism spectrum disorder, mental retardation, amyotrophic
lateral sclerosis,
seizure, stroke, Parkinson's disease and spinal cord injury, although not
being limited thereto.
In the method for providing information and the diagnostic method according to
the
present disclosure, when diagnosing Alzheimer's disease and/or a brain disease
of a subject or
determining the prognosis thereof using the expression level of the marker,
non-marker
clinical information of the subject showing seizure can be further used. The
non-marker
clinical information of the test subject includes the age, sex, body weight,
diet, body mass,
underlying diseases, brain waves, type of seizure, brain MRI, brain CT,
cerebral spinal fluid
test result, blood test result and saliva test result of the subject, although
not being limited
thereto.
In the present disclosure, the method for providing information for diagnosis
of
Alzheimer's disease or a brain disease may further include a step of measuring
the
.. concentration of amyloid beta 42 (A1342) from a sample.
The sample may be saliva or blood, but is not limited thereto. The use of
saliva as
the sample minimizes the risk for the patient and enables fast and accurate
diagnosis.
The concentration of amyloid beta 42 may be measured using an antigen-antibody
reaction or a nucleic acid aptamer, although not being limited thereto. The
antigen-antibody
reaction may be one well-known in the art, including ELISA (enzyme-linked
immunosorbent
CA 3046766 2019-06-17
10
assay). Specifically, when the concentration of amyloid beta 42 is measured
from saliva,
the measurement may be performed by referring to Korean Patent Publication No.
10-2014-
0025978 or Korean Patent Publication No. 10-2013-0140528, although not being
limited
thereto.
In the present disclosure, the term 'aptamer' refers to a nucleic acid having
a specific
binding affinity for a target molecule. In the present disclosure, the target
molecule may be
specifically amyloid beta 42.
The specific binding affinity of the aptamer to the target means that the
aptamer
binds to the target with a higher degree of affinity than to other components
in the sample.
The aptamer refers to one or more set of such molecules. Different aptamers
may have the
same or different number of nucleotides. The aptamer can be DNA, RNA or a
chemically
modified nucleic acid. It may contain a single-stranded or double-stranded
region and may
include a highly ordered structure. The aptamer may also be photoaptamer as
long as a
photoreactive or chemically reactive functional group is included in the
aptamer to be
covalently bonded to its corresponding target. In the method described in the
present
disclosure, two or more aptamers that bind specifically to the same target
molecule may bc
used.
The aptamer can be identified using any known method, including SELEX. Once
confirmed, the aptamer can be prepared or synthesized according to any known
method,
including chemical synthesis methods and enzymatic synthesis methods.
The terms "SELEX" and "SELEX process" are used interchangeably herein to refer
generally to a combination of (I) the selection of an aptamer that interacts
with a target
molecule in a desirable manner, for example, by binding with high affinity to
a protein, and
(2) the amplification of the selected nucleic acid. The SELEX process can be
used to
.. identify an aptamer having high affinity for a specific target or
biomarker. SELEX
CA 3046766 2019-06-17
11
generally includes preparing a candidate mixture of nucleic acids, binding of
the candidate
mixture to a desired target molecule to form an affinity complex, separating
the affinity
complex from the unbound candidate nucleic acids, separating and isolating the
nucleic acids
from affinity complex, purifying the nucleic acids, and amplifying a specific
aptamer
sequence. The process may include multiple rounds to further refine the
affinity of the
selected aptamer. The process can include an amplification step at one or more
points in the
process (see, "e.g., US Patent No. 5,475,096: Nucleic acid ligands). The SELEX
process can
be used to generate an aptamer that binds covalently to its target as well as
an aptamer that
binds non-covalently to its target (see, e.g., US Patent No, 5,705,337:
Systematic evolution of
nucleic acid ligands by exponential enrichment: Chemi-SELEX). The SELEX
process can
be used to identify a high-affinity aptamer containing a modified nucleotide
that confers
improved characteristics on the aptamer, such as, for example, improved in
vivo stability or =
=
improved delivery characteristics. Examples of such modification include
chemical
substitution at the ribose and/or phosphate and/or base position. The aptamer
identified in
the SELEX process is described in US Patent No. 5,660,985 ("High affinity
nucleic acid
ligands containing modified nucleotides"), which describes an oligonucleotide
containing a
nucleotide derivative chemically modified at the 5'- and 21-positions of
pyrimidine. US
Patent No. 5,580,737 describes a highly specific aptamer containing one or
more nucleotide
modified with 2'-amino (2'-NH2), 2'-fluoro (2'-F) and/or 2'-0-methyl (21-
0114e). See also US
.. Patent Application Publication No. 2009/0098549 (SELEX and PHOTOSELEX),
which
describes nucleic acid libraries having expanded physical and chemical
properties and their in
SELEX and photo-SELEX. SELEX can also be used to identify aptamers that have
desirable off-rate characteristics. See US Patent Application No. 2009/0004667
(Method
for generating aptamers with improved off-rates), which describes an improved
SELEX
method for generating aptamer capable of binding to a target molecule.
CA 3046766 2019-06-17
12
The aptamer is immobilized on a solid support before being contacted with the
sample. However, under certain circumstances, the immobilization of the
aptamer prior to
contact with the sample may not provide an optimal assay. For
example, pre-
immobilization of the aptamer possibly can lead to inefficient mixing of the
target molecule
on the solid support surface and the aptamer due to the long reaction time.
Thus, a long
incubation period is required for efficient binding of the aptamer to its
target molecule, In
addition, if a photoaptamer is used in the assay and if it depends on the
material used as the
solid support, the solid support may tend to scatter or absorb the light used
to form a covalent
bond between the photoaptamer and its target molecule. Furthermore, depending
on the
method used, the surface of the solid support may be exposed to a labeling
reagent and can be
affected by it. Therefore, the detection of the target molecule binding to its
aptamer is
susceptible to imprecision. Finally, the immobilization of the aptamer on the
solid support
generally includes an aptamer preparation (i.e., immobilization) step prior to
exposure of the
aptamer to the sample, and this preparation step can influence the activity or
functionality or
the aptamer.
The aptamer may be constructed to facilitate separation of the assay
components
from an aptamer-biomarker complex (or a photoaptamer-biomarker covalent
complex) and
permit isolation of the aptamer for detection and/or quantification. In one
embodiment, the
construct may contain a cleavable or releasable component within the aptamer
sequence. In
another embodiment, additional functionality such as a labeled or detectable
component, a
spacer component or a specific binding tag or immobilization component can be
introduced
into the aptamer. For example, the aptamer can include a tag connected to the
aptamer via a
cleavable moiety, a label, a spacer component separating the label, and the
cleavable moiety.
The nucleic acid aptamer may be any one selected from a group consisting of
DNA,
RNA, an antagomir (anti-miR), an antisense molecule, a small interfering RNA
(siRNA)
CA 3046766 2019-06-17
13
molecule, a small hairpin RNA (shRNA) molecule, a 2'-0-modified
oligonucleotide,
phosphorothioate-backbone deoxyribonucleotide, a
phosphorothioate-backbone
ribonucleotide, a decoy oligonucleotide, a PNA (peptide nucleic acid)
oligonucleotide, or an
LNA (locked nucleic acid) oligonucleotide, although not being limited thereto.
In an example of the present disclosure, a multi-nucleic acid with the
structural
features that, upon binding to amyloid beta 42, fluorescence is emitted as a
quencher is
detached at the same time was synthesized by using a sequence binding
specifically to
amyloid beta 42 and a sequence self-coupled within the nucleic acid. Because
the size of
the multi-nucleic acid is smaller than an antibody, its specificity and
selectivity are excellent.
Also, because sampling and large-scale analysis are possible within one hour,
it can provide a
diagnostic method with high diagnostic ease.
In the present disclosure, normality may be diagnosed if the concentration of
amyloid
beta 42 is equal to or higher than 0 and below 500 pg/mL, mild cognitive
impairment (MCI)
may be diagnosed if is equal to or higher than 500 pg/mL and below I ng/mL,
and severe
cognitive impairment may be diagnosed if is equal to or higher than I ng/mL.
In an example of the present disclosure, when the amyloid beta 42 level of
over 100
dementia patients was normalized through a previous study, mild cognitive
impairment and
moderate dementia could be distinguished successfully (90% or higher match
with diagnosis
by clinicians).
In an example of the present disclosure, it was confirmed that the increase
pattern of
miR-485-3p significantly affects the expression level of APP, which is known
as an amyloid
beta precursor. -
In another aspect, the present disclosure relates to a composition for
diagnosing
Alzheimer's disease or a brain disease, which contains a primer capable of
amplifying miR-
485-3p or a probe capable or hybridizing with miR-485-3p.
CA 3046766 2019-06-17
14
In the present disclosure, the primer is constructed so as to have
complementarity
roughly to each strand of miR-485-3p to be amplified, and contains a suitable
G or C
nucleotide. This means that the primer has sufficient complementarity to the
corresponding
nucleic acid strand to be hybridized under the conditions for the
polymerization reaction. In
the present disclosure, the primer is used for an amplification process. The
amplification
process is a continuous enzymatic reaction ,in which the number of a target
locus increases
exponentially over the course of the reaction, such as PCR. Typically, one
primer (antisense
primer) has homology to the negative strand of a locus (-), and the other
primer (sense
primer) has homology to the positive (+) strand. When the primer is annealed
to a
denatured nucleic acid, the chain is extended by enzymes and reaction
products, such as DNA
polymerase I (Klenow) and nucleotides. As a result, + and - strands containing
the target
locus sequence are newly synthesized. The newly synthesized target locus is
also used as a
template, such that the synthesis of the target locus sequence proceeds
exponentially as the
cycle of denaturation, primer annealing and chain extension is repeated. The
products of the
continuous reaction are individual double-stranded nucleic acids having
terminals
corresponding to the terminals of the specific primers used in the reaction.
Specifically, the amplification reaction may be PCR, which is commonly used in
the
art. However, alternative methods such as real-time PCR or linear
amplification using an
isothermal enzyme may also be used. The multiplex amplification reaction may
also be
used.
In the present disclosure, the probe may be labeled to be detectable. For
example, it
may be labeled with a radioisotope, a fluorescent compound, a bioluminescent
compound, a
chemiluminescent compound, a metal chelator or an enzyme. The appropriate
labeling of
the probe is a well-known technique in the art and can be carried out by a
method commonly
used in the art. The probe may be a probe specifically binding to a known
nucleic acid
CA 3046766 2019-06-17
15
biomarker for diagnose of diseases. Through binding to the probe, the presence
of a
particular biomarker or a fragment thereof can be detected and a disease may
be diagnosed
based thereon.
In the present disclosure, the sequence of miR-485-3p may be derived from a
mammal, for example, human, mouse or rat.
In an exemplary embodiment of the present disclosure, the sequence of miR-485-
3p
is derived from human, and includes not only a mature sequence (5
'GUCAUACACGGCUCUCCUCUCU 3' (SEQ ID NO I)) but also a precursor sequence (5'-
ACUUGGAGAGAGGCUGGCCG UGAUGAAUUCGA U UCAUCAAAGCGAGUCA UAC
ACGCCUCUCCUCUCUMUAGU- 3' (SEQ ID NO 2)).
Therefore, in the present disclosure, the primer or the probe may contain a
sequence
complementary to the base sequence of SEQ ID NO I or SEQ ID NO 2 or a part
thereof,
although not being limited thereto.
In the present disclosure, the primer may be one represented by SEQ ID NOS 10-
11,
although not being limited thereto.
Forward 5'-NNVNgteatacacggct-3' (SEQ ID NO 10).
Reverse 5'-ccagatatatttattagagagga-3' (SEQ ID NO I I).
In the present disclosure, the SEQ ID NO 10 may contain the 1st through 19th
base
sequences of SEQ ID NO I from the 5' end, and N may be g or c, and. V may be a
or t. The
1st through 19th base sequences and NNVN may be adjusted according to the
length of the
whole primer.
The SEQ ID NO 11 may contain base sequences complementary to the 14th through
22nd base sequences of SEQ ID NO 1 from the 5' end, may contain 15 t's in a
row, and may
be attached with universal gacctgg. The sequences complementary to the 14th
through 22nd
EDC_LAW1204724111
CA 3046766 2019-06-17
16
complementary sequences and the gacctgg may be adjusted according to the
length of the
whole primer.
In the present disclosure, the composition for diagnosing Alzheimer's disease
or a
brain disease may further contain an antibody or a nucleic acid aptamer or
specifically
binding to amyloid beta 42. The antibody or nucleic acid aptamer is for
detecting amyloid
beta 42, and may be used in any form for the detection of amyloid beta 42 from
the sample.
In another aspect, the present disclosure relates to a use of a composition
containing
a primer capable of amplifying miR-485-3p or a probe capable of hybridizing
with miR-485-
3p in diagnosis of Alzheimer's disease or a brain disease.
In the present disclosure, the composition may further contain an antibody or
a
nucleic acid aptamer which specifically binds to amyloid beta 42.
In another aspect, the present disclosure relates to a kit for diagnosing
Alzheimer's
disease or a brain disease, which contains the composition for diagnosing
Alzheimer's disease
or a brain disease.
The diagnostic kit of the present disclosure may be used in the diagnosis of
Alzheimer's disease and/or several brain diseases. Most specifically, it may
be used in the
diagnosis of Alzheimer's disease.
The kit of the present disclosure may further contain other components in
addition to
the aforementioned components. For example, if the kit of the present
disclosure is applied
to a PCR amplification process, it may contain, optionally, a reagent required
for PCR
amplification such as a buffer, a DNA polymerase, a DNA polymerase cofactor
and dNTP,
although not being limited thereto. The kit of the present disclosure may be
prepared into a
number of separate packages or compartments containing the above reagent
component.
In addition, the kit according to the present disclosure may be a microarray,
specifically a gene amplification kit. If the kit is a microarray, a probe is
immobilized on
CA 3046766 2019-06-17
17
the solid surface of the microarray. If
the kit of the present disclosure is a gene
amplification kit, it contains a primer. The probe or primer specifically
recognizes the m iR-
485-3p according to the present disclosure and has a sequence complementary to
its sequence.
The term 'complementary' used herein refers to having complementarity enough
for selective
hybridization to the nucleotide sequence under certain hybridization or
annealing conditions.
The probe or primer of the present disclosure may be fully complementary, or
may have one
or more mismatch base sequence if selective hybridization to the nucleotide
sequence is
possible. The nucleotide sequence of the miRNA of the present disclosure to be
consulted
when constructing the primer or probe may be searched from miRBase, and the
primer or
probe may be designed based on the sequence.
The composition according to the present disclosure, which contains a
substance
capable of inhibiting the activity miR-485-3p and a pharmaceutically
acceptable carrier, may
be provided as a pharmaceutical composition for preventing or treating
Alzheimer's disease
or a brain disease.
The present disclosure provides a pharmaceutical composition for treating or
= preventing Alzheimer's disease, which contains a substance capable of
inhibiting the activity
miR-485-3p and a pharmaceutically acceptable carrier, based on the finding
that the
decreased expression of ELAVL2 by miR-485-3p is associated with Alzheimer's
disease,
The ''miR" mainly refers to a non-coding RNA consisting of 21-23
deoxyribonucleotides, which is known to be involved in post-transcriptional
regulation of
gene expression by suppressing the translation of target RNA or promoting
degradation
thereof.
In addition, the mature sequence of the miRNA can be obtained from the miRNA
database (http://www.mirbase.org). In the miRNA database as of August 13, 2012
(19th
edition, miRBase), 25,141 mature miRNAs derived from 193 species are listed.
CA 3046766 2019-06-17
18
In the present disclosure, the miR-485-3p is expressed in the brain,
particularly in the
hippocampus and the cortex, and it binds to the 3'-untranslated region of
ELAVL2 mRNA
encoding the ELAVL2 protein, thereby inhibiting its expression and decreasing
the
concentration of the ELAVL2 protein in the brain.
In the present disclosure, the inhibition of the activity of miR-485-3p means
the
inhibition of or interference with the action or function of miR-485-3p in
cells. Typically, it
includes direct inhibition of binding of miR-485-3p to a target, e.g., a mRNA
molecule
encoding the ELAVL2 protein, direct inhibition of the function of miR-485-3p
using a small
molecule inhibitor, an antibody or an antibody fragment, or indirect
regulation using an
1
inhibitor or a small interfering RNA (siRNA) molecule.
Furthermore, the interference with or inhibition of the activity of miR-485-3p
includes inhibiting the activity of the precursor sequence (SEQ ID NO 2) or
the mature
sequence (SEQ ID NO 1) directly or indirectly. In addition, the inhibition of
the activity of
miR-485-3p includes lowering the concentration thereof in cells by inhibiting
the
transcription of miR-485-3p.
The substance capable of inhibiting the activity of miR-485-3p includes any
substance capable of inhibiting its expression and/or activity. For example,
such substance
,may include a compound (a small molecule or a polymer), an antagomir, an
antisense
molecule, a small hairpin RNA (shRNA) molecule, a small interfering RNA
(siRNA)
molecule, a seed-targeting LNA (locked nucleic acid) oligonucleotide, a decoy
oligonucleotide, an aptamer, a ribozyme, or an antibody that recognizes a DNA:
RNA hybrid.
The antisense oligonucleotide includes a nucleic acid-based molecule having a
sequence complementary to the seed sequence of the miRNA completely or
partially and thus
capable of forming a duplex with miRNA. Thus, the antisense oligonucleotide
may be
referred to as a complementary nucleic acid-based inhibitor.
CA 3046766 2019-06-17
19
In addition, the antisense oligonucleotide includes a variety of molecules,
for
example, a ribonucleic acid (RNA), a deoxyribonucleic acid (DNA), an
antagomir, a 21-0-
modified oligonucleotide, a phosphorothioate-backbone deoxyribonucleotide, a
phosphorothioate-backbone ribonucleotide, a PNA (peptide nucleic acid)
oligonucleotide or
an LNA (locked nucleic acid) oligonucleotide. Specifically, it may be a
ribonucleic acid.
The ribonucleic acid includes a double-stranded small hairpin RNA (shRNA)
molecule, a small interfering RNA (siRNA) molecule and a ribozyme.
The LNA is has a locked conformation due to further modification between the 2
'
and 4' carbon of the ribose moiety of the oligonucleotide and, thus, ensures
thermal stability.
The PNA (peptide nucleic acid) contains a peptide-based backbone instead of a
sugar-phosphate backbone, The 2'-0-modified oligonucleotide is specifically a
21-0-alkyl
oligonucleotide, more specifically a 21-0-C1_3 alkyl oligonucleotide, and most
specifically a
2`-0-methyl oligonucleotide.
The antisense oligonucleotide includes an antisense oligonucleotide in a
narrow
sense, an antagomir and an inhibitory RNA molecule,
The antagomir is a chemically modified single-stranded oligonucleotide and is
used
to silence an endogenous microRNA. The antagomir contains a sequence that is
not
complementary at the Argonaute 2 (Ago2) cleavage site, or inhibits cleavage of
Ago2 such
that the base is modified with, for example, a 2-1methoxy group, a 31-
cholesterol group or a
phosphorothioate. There is a complementary sequence to the target sequence.
In the present disclosure, the antagomir has a sequence which is at least
partially or
completely complementary to miR-485-3p. In an exemplary embodiment of the
present
disclosure, the antagomir includes one or more modification (e.g,, 2'-0-methyl-
sugar
modification or 3'-cholesterol modification). In
another embodiment, the antagomir
EDC_LAVV12047241 \ 1
CA 3046766 2019-06-17
20
contains one or more phosphorothioate linkage and has a phosphorothioate
backbone at least
in part.
In the present disclosure, the appropriate length of the antagomir for
inhibiting the
expression of miR-485-3p is 7-50 nucleotides, particularly 10-40 nucleotides,
more
particularly 15-30 nucleotides, particularly 15-25 nucleotides, more
particularly 16-19
nucleotides, although not being limited thereto.
The term 'complementary' as used the present disclosure means that the
antisense
oligonucleotide is sufficiently complementary to a target of miR-485-3p under
predetermined
hybridization conditions or annealing conditions, specifically under
physiological conditions,
such that it can selectively hybridize to the target, and encompasses both
partially or
substantially complementary and completely (perfectly) complementary.
Specifically, it
means being completely complementary.
Substantially complementary means that,
although not completely complementary, it has complementarity sufficient to
bind to the
target sequence and exert an effect according to the present disclosure, i.e.,
interference with
.. the activity of miR-485-3p.
The 'nucleic acid' includes an oligonucleotide, DNA, RNA, a polynucleotide,
and
analogs derivatives thereof. For example, a peptide nucleic acid (PNA) or a
mixture thereof
is included. In addition, the nucleic acid may be single- or double-stranded
and can encode
molecules including mRNA, microRNA, siRNA, polypeptides, etc.
In an exemplary embodiment of the present disclosure, the substance capable of
inhibiting the activity of miR-485-3p is an antisense oligonucleotide which is
capable of
complementarily binding to all or a portion of the progenitor and/or mature
sequence of miR-
485-3p, particularly the seed sequence, thereby inhibiting its activity. The
inhibition of the
activity is to inhibit the transcription of miR-485-3p and/or binding to the
target mRNA of
miR-485-3p. The antisense oligonucleotide according to the present disclosure
may or may
CA 3046766 2019-06-17
21
not include one or more modification in nucleotides constituting the antisense
oligonucleotide
or the backbone connecting the nucleotides. That is to say, at least one
nucleotide
constituting the antisense oligonucleotide may contain LNA, the sugar of at
least one
nucleotide constituting the same may be 2'-0-methylated or methoxylated, or
one or more
phosphothioate may be contained in the backbone,
In an exemplary embodiment of the present disclosure, the antisense
oligonucleotide
or the nucleic acid molecule contains a sequence complementary to all or a
portion of the
seed sequence of miR-485-3p. The seed sequence conserved in a variety of
species is a
sequence which is very important in recognition of the target molecule of
miRNA (Krenz, M.
et al., .1 Am. Coll. Cordiol, 44: 2390-2397(2004); 1-I. Kiriazis, et al.,
Annu. Rev. Physlol. 62:
321(2000)). Because miRNA binds to its target via the sequence seed, the
translation, etc.
of' the target mRNA may be inhibited effectively by inhibiting the interaction
between the
seed sequence and the target.
In an exemplary embodiment of the present disclosure, the antisense
oligonucleotide
of the present disclosure may contain a sequence which is complementary
completely or
partially to the 1st or 2nd through the 7th or 8th nucleotide sequence of the
nucleotide
sequence of SEQ ID NO I. For example, the antisense oligonucleotide of the
present
disclosure may be 5'-GUGUAUGAC-3 (SEQ ID NO 3), 5'-UGUAUGAC-3' (SEQ ID NO 4),
51-GUGUAUGA-3' (SEQ ID NO 5), 5'-UGUAUGA-3' (SEQ ID NO 6) or 5'-
AGAGAGGAGAGCCGUGUAUGAC-3' (SEQ ID NO 7), and one or more of each
nucleotide constituting the oligonucleotide may be 2'-0-methylated or
methoxylated, ethyl,
one or more of each nucleotide may be LNA, one or more chemical bond
constituting the
backbone may be phosphothiate, or there may be no modification.
EDC_LAIM 204724111
CA 3046766 2019-06-17
22
The present disclosure is based on the finding that the excessive inhibition
of
ELAVL2 expression by miR-485-3p is involved in the development of Alzheimer's
disease
and various brain diseases.
It is known that the decreased expression level of ELAVL2 is associated with
the
onset of Alzheimer's disease, autism spectrum disorder, mental retardation and
amyotrophic
lateral sclerosis. Especially, it is known that the level of the ELAVL2
protein is decreased
by substances inducing excitotoxicity such as kainic acid, NMDA, quisulate,
AMPA,
glutamate, etc., resulting in neuronal cell 'death and disturbance of brain
function, causing a
number of brain diseases such as seizure, stroke, Parkinson's disease, spinal
cord injury, etc.
(Kaminska, B. et al., Acta Biochint Pat 44: 78 1-789).
Therefore, the recovery of the ELAVL2 protein through the inhibition of the
activity
of miR-485-3p according to the present disclosure can be used in the treatment
of various
brain diseases such as Alzheimer's disease, autism spectrum disorder, mental
retardation,
amyotrophic lateral sclerosis, seizure, stroke, Parkinson's disease, spinal
cord injury, etc.
In an exemplary embodiment of the present disclosure, the pharmaceutical
composition containing a substance capable of inhibiting the activity of miR-
485-3p may be
used in the treatment of a brain disease. The brain disease includes
Alzheimer's disease,
autism spectrum disorder, mental retardation, amyotrophic lateral sclerosis,
seizure, stroke,
Parkinson's disease and spinal cord injury, but is not limited thereto.
The term 'improvement', 'treatment', 'alleviation' or 'improvement' as used in
the
present disclosure means any action to change favorably or improve the
symptoms of related
diseases by administering the composition. Those of ordinary skill in the art
to which the
present disclosure belongs will know the exact criteria of diseases by
referring to the data
presented, for example, by the Korean Academy of Medical Sciences, and will be
able to
judge the degree of improvement, progress and treatment.
CA 3046766 2019-06-17
23
The term "prevention" as used in the present disclosure means any action to
inhibit or
delay the onset of related diseases. It will be apparent to those skilled in
the art that the
related diseases can be prevented if the pharmaceutical composition according
to the present
disclosure is administered when or before early symptoms appear.
In the present disclosure, the pharmaceutical composition may further contain,
in
addition to the substance capable of inhibiting the activity of miR-485-3p,
one or more active
ingredient exhibiting the same, similar or synergistic function for the
treatment of related
diseases or a compound which maintains/increases the solubility and/or
absorbency of The
substance capable of inhibiting the activity of miR-485-3p or the active
ingredient. And,
optionally, it may further contain an immunomodulator and/or a
chemotherapeutic agent.
The pharmaceutical composition may further contain one or more
pharmaceutically
acceptable diluent, carrier and/or adjuvant in addition to the above-mentioned
active
ingredient. As the pharmaceutically acceptable carrier, saline, sterile water,
Ringer's
solution, buffered saline, dextrose solution, maltodextrin solution, glycerol,
ethanol, I iposome,
and a mixture of one or more of these components may be used. If necessary,
other
common additives such as an antioxidant, a buffer, a bacteriostatic agent,
etc. may be added.
In addition, it can be formulated into an injectable formulation such as an
aqueous
solution, a suspension, an emulsion, etc., a pill, a capsule, a granule or a
tablet by additionally
adding a diluent, a dispersant, a surfactant, a binder and/or a lubricant, and
it can be used by
binding a target organ-specific antibody or other ligand with the carrier.
Furthermore, it can be suitably formulated depending on the particular disease
or
ingredient by using appropriate methods in the art or using the methods
disclosed in the
Remington's literature (Remington's Pharmaceutical Science (newest edition),
Mack
Publishing Company, Easton PA). For example, it can be formulated into one of
a
EDC_LAVIA 204724111
CA 3046766 2019-06-17
24
suspension, a liposomal formulation, an emulsion, a tablet, a capsule, a gel,
a syrup or a
suppository.
The administration method of the pharmaceutical composition according to the
present disclosure is not particularly limited and any known administration
method of
inhibitors may be applied. Depending on purposes, parenteral administration
(e.g.,
intranasal, intravenous, subcutaneous, intraperitoncal or topical
administration) or oral
administration may be employed. Specifically, administration by intranasal
injection may
be selected to achieve a quick therapeutic effect.
Also, the pharmaceutical composition according to the present disclosure is
administered in a pharmaceutically effective amount. The 'pharmaceutically or
therapeutically effective amount' means an amount sufficient to treat a
disease at a
reasonable benefit/risk ratio applicable to medical treatment, and an
effective dose level will
depend on factors including the type and severity of the disease, the activity
of a drug,
sensitivity to the drug, the time of administration, the route of
administration, the rate of
excretion, the duration of the treatment, and drugs used together, and other
factors well
known in the medical field.
The pharmaceutical composition may be administered as an individual
therapeutic
agent or in combination with other therapeutic agents, sequentially or
concurrently with
conventional therapeutic agents, and may be administered singly or multiply.
It is important .
that the pharmaceutical composition is administered in such an amount that the
maximum
effect can be obtained with a minimum amount without side effects considering
all of the
above-mentioned factors, which can be easily determined by those skilled in
the art.
The dosage may vary depending on the patient's body weight, age, sex, health
condition and diet, administration time, administration method, excretion
rate, the severity of
the disease, etc., and a proper dosage may also variy depending on the amount
of the drug
CA 3046766 2019-06-17
25
accumulated in the patient's body and/or the specific efficacy of the
polynucicotide used. In
general, it can be calculated on the basis of EC50 measured as effective from
an in-vivo
animal model and in vitro. For example, it may be from 0.01 i.tg to 1 g per 1
kg of body
weight, and may be administered once to several times per unit period in a
daily, weekly,
monthly, or annual unit period. Also, it can be administered continuously for
a long period
of time using an infusion pump. The number of repeated administrations is
determined in
consideration of the time during which the drug remains in the body, the drug
concentration
in the body, and the like. Even after treatment according to the course of
disease treatment,
the pharmaceutical composition can be continuously administered to prevent the
recurrence
of the disease.
In an exemplary embodiment, the present disclosure provides a method for
screening
a substance for treating or preventing Alzheimer's disease or a brain disease,
which includes a
step of contacting miR-485-3p with a test substance and a step of determining
the activity of
miR-485-3p in contact with the test substance, wherein the test substance is
selected as a
candidate substance if the activity of miR-485-3p in contact with the test
substance is
decreased as compared to the activity of miR-485-3p of a control group, not in
contact with
the test substance.
The miR-485-3p is provided in the form of a cell expressing the same, and the
activity is analyzed as the expression level of miR-485-3p. For example, after
contacting a
cell expressing the miR-485-3p according to the present i d ..sc.osure with
test substances, the
change in the expression level of miR-485-3p is compared with that before the
contacting or
with a control group cell not in contact with the test substances and the
substance which
shows change, particularly decrease, in the expression level is selected as a
candidate
substance. The expression level of miR-485-3p may be measured by performing a
known
method such as northern blot, RI-PCR, a hybridization method using a
microarray, etc.
CA 3046766 2019-06-17
26
The miR-485-3p is provided in the form of a cell expressing the same, and the
activity is determined by analyzing the interaction of the miR-485-3p with the
3LUTR of its
typical ELAVL2 protein (ELAV like neuron-specific RNA binding protein 2). For
example,
after contacting a cell expressing the miR-485-3p according to the present
disclosure with test
substances, the degree of interaction between the Y-UTR of the ELAVL2 protein
and miR-
485-3p is compared with that before the contacting or with a control group
cell not in contact
with the test substances and the substance which shows change, particularly
decrease, in the
interaction is selected as a candidate substance. The RNA-RNA interaction used
in the
method according to the present disclosure may be detected by a method known
in the art, for
example, RNA walk (Lusting et al., Nucleic Acids Re,v. 2010; 38 (1): e5) or
yeast two-hybrid
system (Piganeau et at, RNA 2006; 12: 177-184, and RNA: A Laboratory Manual
(Cold
Spring Harbor Laboratory Press 20 I I)).
The type of cell and the amount and kind of the test substances used in the
screening
method will vary depending on the particular test method and test substances
used, and those
skilled in the art will be able to select the suitable type, amount and/or
condition of the cell.
Based on the test result, the substance which leads to decreased activity of
miR-485-3p in the
presence of the test substance as compared to the control group not in contact
with the test
substance is selected as the candidate substance. The decrease means decrease
by about
99% or less, decrease by about 95% or less, decrease by about 90% or less,
decrease by about
85% or less, decrease by about 80% or less, decrease by about 75% or less,
decrease by about
70% or less, decrease by about 65% or less, decrease by about 60% or less,
decrease by about
55% or less, decrease by about 50% or less, decrease by about 45% or less,
decrease by about
40% or less, decrease by about 30% or less, or decrease by about 20% or less,
as compared to
the control group, although not being limited thereto.
EDC_LAVVl 2047241\1
CA 3046766 2019-06-17
27
The 'test substance' means a substance which is expected to inhibit the
activity of
miR-485-3p as described above, and includes a low-molecular-weight compound, a
high-
molecular-weight compound, a mixture of compounds (e.g., a natural extract or
a cell or
tissue culture), a biomedicine (e.g., a protein, an antibody, a peptide, DNA,
RNA, an
antisense oligonucleotide, RNAi, an aptamer, RNAzyme and DNAzyme), a sugar and
a lipid,
although not being limited thereto. The test substance can be a polypeptide
having two or
more amino acid residues, for example, 6, 10, 12, 20 or fewer, or more than
20, e.g., 50,
amino acid residues. The test substance may be obtained from a library of
synthetic or
natural compounds, and a method for obtaining a library of such compounds is
known in the
art. The libraries of synthetic compounds are commercially available from
Maybridge
Chemical Co. (UK), Comgenex (USA), Brandon Associates (USA), Microsource (USA)
and
Sigma-Aldrich (USA), and the libraries of natural compounds are commercially
available
from Pan Laboratories (USA) and MycoSearch (USA). The test substance may be
obtained
by a variety of combinatorial library methods known in the art, for example, a
biological
library, a spatially addressable parallel solid-phase or solution-phase
library, a synthetic
library requiring deconvolution, a "one-bead/one-compound" library, and a
synthetic library
using affinity chromatography selection. Method for the synthesis of molecular
libraries are
disclosed in DeWitt et al., Proc. Nall Acad. Sci. U.S.A. 90, 6909, 1993; Erb
et al. Proc. Natl,
Acrid Sci. U.S.A. 91, 11422, 1994; Zuckertnann et al.õ/ Med. Chew. 37, 2678,
1994; Cho et
al., Science 261, 1303, 1993; Carell et al., Angew. Chew. Mt. Ed. Engl. 33,
2059, 1994; Carell
et al., Angew. Chew. Int. Ed Engl. 33, 2061; Gallop et al., .1. Med. Chew, 37,
1233, 1994, or
the like.
For the screening purpose of a drug which treats Alzheimer's disease and/or
various
brain diseases according to the present disclosure, a low-molecular-weight
exhibiting a
therapeutic effect may be used. For example, a compound with a molecular
weight or about
CA 3046766 2019-06-17
28
1000 Da, e.g., 400 Da, 600 Da or 800 Da, may be used. Depending on purposes,
these
compounds can form a part of a compound library, and the number of compounds
that make
up the library can also vary from dozens to millions. The compound library may
contain
peptides, peptoids, other cyclic or linear oligomeric compounds, template-
based low-
molecular-weight compounds, e.g., benzodiazepines, hydantoins, biaryls,
carbocycles and
polycyclic compounds (e.g., naphthalene, phenothiazine, acridine, steroid,
etc.),
carbohydrates, amino acid derivatives, dihydropyridines, benzhydryls and
heterocycles (e.g.
triazine, indole, thiazolidine, etc.), although not being limited thereto.
In addition, a cell or a biomolecule may be used in the screening. The
biomolecule
refers to a protein, a nucleic acid, a carbohydrate, a lipid or a material
produced in vivo or in
vitro using a cellular system. The biomolecule may be provided either alone or
in
combination with other biomolecules or cells. For example, the biomolecule
includes
polynucleotides, peptides, antibodies or other proteins or biological organic
materials found
in the plasma.
In an exemplary embodiment, the present disclosure provides a method for
detecting
a miR-485-3p marker to provide information required for diagnosis or prognosis
of various
brain disease Alzheimer's disease and/or various brain diseases, which
includes a step of
detecting the expression of a miR-485-3p marker from a sample; and a step of
associating the
expression level of the detected marker with the diagnosis or prognosis of
Alzheimer's
disease and/or various brain diseases.
In the present disclosure, in the step of the association, the determined
expression
level of the marker is compared with the detection result for each of the
marker determined
for a control group. The miR-485-3p marker shows increased expression level as
compared
to the control group.
EDC_LAWN 204724111
CA 3046766 2019-06-17
29
During the diagnostic or prognostic determination of Alzheimer's disease
and/or
various brain diseases, non-marker clinical information of' the test subject
may be used in
addition to the expression level of the marker. The non-marker clinical
information of the
test subject includes the age, sex, body weight, diet, body mass, underlying
diseases, brain
waves, type of seizure, brain MRI, brain CT, cerebral spinal fluid test
result, blood test result
and saliva test result of the subject, although not being limited thereto.
The present disclosure is based on the finding that the excessive inhibition
of the
expression of the ELAVL2 protein by miR-485-3p is involved in the development
of
Alzheimer's disease and/or various brain diseases. In another exemplary
embodiment, the
present disclosure provides a method for treating or preventing Alzheimer's
disease and/or a
brain disease by inhibiting the activity of miR-485-3p in the cells or
tissues, particularly in
the brain cells, brain tissues or brain, of a subject.
In an exemplary embodiment, the present disclosure provides a method for
treating
or preventing Alzheimer's disease and/or a brain disease, which includes a
step of
administering a therapeutically or prophylactically effective amount of a miR-
485-3p activity
inhibitor to a subject in need of treatment or prevention of Alzheimer's
disease and/or a brain
disease.
Further, in an exemplary embodiment, the present disclosure provides a
substance
capable of inhibiting the activity of miR-485-3p for the purpose of treating
or preventing a
brain disease diseases. In particular, the substance capable of inhibiting the
activity of miR-
485-3p is delivered to the brain.
Reference can be made to the foregoing descriptions about the miR-485-3p
activity
inhibitor that can be used, the regulation or inhibition of the activity of
miR-485-3p, the
method of administration, the type of diseases that can be treated, etc.
CA 3046766 2019-06-17
30
Hereinafter, the present disclosure will be described in detail through
examples.
. However, the following examples are for illustrative purposes only and it
will be apparent to
those of ordinary skill in the art that the scope of the present disclosure is
not limited by the
examples.
Example I: Analysis of miRNA expression pattern in plasma of Alzheimer's
patients
using miRNA ciPCR array
(1) Patients and sample preparation
Table 1 shows the characteristics of the patients used in the study. About 3
mL of
blood was collected in blood tubes (Becton Dickinson, Germany) containing
sodium citrate
(3.2% w/v) from 4 patients diagnosed with Alzheimer's dementia by physicians.
Four
healthy adults of corresponding ages ( 4 years) were included as a control
group.
[Table I]
Sex and age of normal group and patient group
Group Sample No. Sex Age
Normal group N1 Female 78
Normal group N2 Male 72
Normal group N3 Female 74
Normal group N4 Male 79
Patient Group S I Female 72
Patient Group S2 Female 82
Patient Group S3 Female 84
Patient Group S4 Male 75
EOC_LAV \ A 2047241 \ 1
CA 3046766 2019-06-17
31
The blood was centrifuged for 10 minutes at 3,500 rpm to separate plasma and
then
stored at -80 C until RNA extraction. miRNA was extracted using the miRNAeasy
Serum/Plasma kit (Qiagen, USA) according to the manufacturer's
recommendations. Thc
concentration and purity of the extracted RNA were analyzed using Bioanalyzer
2100
(Agilent, USA). Eight groups including a normal group satisfied the quality
criteria and
were used in the study.
(2) miRNA qPCR array
EDC_LAWN 2047241\1
CA 3046766 2019-06-17
32
1
The genes used in miRNA qPCR array assay are listed in Table 2. The mature
sequence of
each miRNA is available from the miRNA database (http://www.mirbase.org). The
extracted RNA was screened using the miRNA array containing 84 different
miRNAs known
to be associated with human neurological development and the progress of
neurological
disease.
[Table 2]
List of genes used in miRNA qPCR array assay
No. Mature miRNA list No. Mature miRNA list
1 hsa-let-7b-5p 43 hsa-miR-298
2 hsa-let-7c-5p 44 hsa-miR-29a-3p
3 hsa-let-7d-5p 45 hsa-miR-2911-3p
4 hsa-let-7e-5p 46 hsa-miR-29c-3p
_________________________________________________ ._........____.
5 hsa-let-7i-5p 47 hsa-miR-302a-5p
6 hsa-miR-101-3p 48 hsa-miR-3025-5p
7 hsa-miR-105-5p 49 hsa-iniR-30t1-5p
8 hsa-miR-106b-5p 50 hsa-miR-320a
9 hsa-miR-107 51 hsa-miR-328-3p
hsa-miR-124-3p 52 hsa-miR-337-3p
11 hsa-miR-125b-5p 53 hsa-miR-338-3p
12 hsa-raiR-126-5p 54 hsa-miR-339-50
13 ' hsa-miR-128-3p 55 hsa-miR-342-3p
14 hsa-m1R-130a-3p 56 hsa-miR-346
hsa-miR-132-3p 57 hsa-miR-34a-5p
16 hsa-miR-133b 58 ha-miR-3761)-3p
17 hsa-miR-134-5p 59 hsa-miR-381-3p
18 hsa-miR-135b-5p 60 hsa-miR'409-3p
19 hsa-miR-138-5p 61 hsa-miR-431-5p
hsa-miR-139-5p ' 62 hsa-miR-432-5p
21 hsa-miR-140-5p 63 hsa-miR-433-3p
22 hsa-miR-146a-5p 64 hsa-miR-455-5p
EDCJAM204724111
i
!
1
CA 3046766 2019-06-17
33
23 hi-miR-1465-5p 65 1isa-miR-484
24 ! hsa-miR-148b-3p 66 hsa-miR-485-3p
25 : hsa-miR-151a-3p 67 hsa-miR-485-5p
26 = hsa-miR-152-3p 68 ha-miR-487a-3p
27 hsa-mi6-15a-5p 69 ! h5a-miR-488-3p
28 , hsa-miR-155-5p 70 ! hsa-miR-489-3p
29 hsa-miR-181a-5p 71 hsa-miR-499a-5p
4-
30 hsa-miR-181d-5p 72 hsa-mi8-509-3p
31 hsa-miR-191-5p 73 1isa-miR-511-50
32 hsa-miR-193b-3p 74 : hsa-miR-512-3p
33 : hsa-miR-195-5p 75 ha-miR-518b
34 hsa-miR-19b-3p 76 hsa-miR-539-5p
35 hsa-miR-203a-3p 77 - hsa-miR-652-3p
36 hsa-miR-20a-5p 78 hsa-miR-7-5p
37 hsa-wiR-212-3p 79 hsa-miR-9-5p
38 hsa-miR-22-3p 80 hsa-miR-9-3p
39 hsa-miR-24-3p 81 hsa-miR-92a-3p
40 = hsa-nuR-26b-5p 82 hsa-miR-93-5p
41 hsa-miR-27a-3p 83 . hsa-miR-95-3p
42 hsa-miR-28-5p 84 ' hsa-miR-98-5p
The Quantitative PCR assay method can be summarized as follows. A mature
miRNA is generally a 22-nucleotide, non-coding RNA and is responsible for post-
transcriptional regulation. Polyadenylation of mature miRNA was induced by
poly(A)
polymerase, and cDNA was synthesized using oligo-dT primers. The oligo-dT
primer
enables the amplification of the mature miRNA during the real-time PCR process
because it
has a 3' degenerate anchor and a universal tag sequence at the 5' end. The
mature miRNA
was quantified during the real-time PCR process using the miScript SYBR Green
PCR kit
(Qiagen).
EDC_LAVVI 204724111
CA 3046766 2019-06-17
34
(3) Analysis of miRNA expression pattern through volcano plot
FIG. IA shows a miRNA expression pattern analysis result (volcano plot) for
the
patient group as compared to the normal group. The expression pattern of 84
miRNAs was
analyzed as compared to the normal group.
The x axis represents fold-change and the y axis represents -log10 of the p
value.
The horizontal black line shows where the p value is 0.05 or smaller. As a
result of the
volcano plot analysis, it was confirmed that the expression of hsa-miR-105-5p,
hsa-miR-98-
5p, hsa-miR-15a-5p, hsa-miR- I34-5p, hsa-miR-409-3p, hsa-miR-19b-3p, hsa-miR-
92a-3p,
hsa-miR-28-5p, hsa-miR-30d-5p, Itsa-miR-212-3p, hsa-miR-93-5p, hsa-miR-342-3p,
hsa-
miR-38 I -3p, hsa-miR-431-5p, hsa-miR- 130a-3p, hsa-m iR-146b-5p, hsa-rniR-29a-
3p, hsa-
miR-132-3p, hsa-miR-376b-3p, hsa-miR-22-3p, hsa-miR-509-3p, hsa-miR- I 39-5p,
lisa-milt-
499a-5p, hsa-miR-203a-3p, hsa-miR- 95-3p, hsa-miR-128-3p, hsa-miR-487a-3p, hsa-
miR-
485-3p, hsa-miR- I95-5p, hsa-rniR-433-3p, hsa-miR-133b, hsa-miR-191-5p, hsa-
miR-489-3p,
hsa-miR-432-5p, hsa-miR-29c-3p, hsa-miR-485-5p, hsa-miR-652-3p. hsa- miR-126-
5p. hsa-
in R-328-3p, hsa-let-7b-5p, hsa-m iR-539-5p, hsa-miR-106b-5p, hsa-miR- l01-3p,
hsa-in iR-
302a-5p, hsa-miR-484, hsa-miR-518b, hsa-miR-148b-3p, hsa-miR-181d-5p, hsa-miR-
7-5p,
hsa-miR-512-3p, hsa-miR-151a-3p, hsa-miR-15b-5p, hsa-let-7e-5p, hsa-miR-135b-
5p, hsa-
miR-18 la-5p, hsa-tniR-138-5p, hsa-miR- 34a-5p, hsa-miR-346, hsa-miR-511-5p,
hsa-miR-
485-3p, hsa-miR-485-5p, hsa-rniR-487a-3p, hsa-miR-489-3p, hsa-miR-499a-5p, hsa-
miR-
509-3p, hsa-miR-511-5p, hsa-miR-512-3p, hsa-miR -518b, hsa-miR-539-5p, hsa-miR-
652-3p,
hsa-miR-7-5p, hsa-miR-92a-3p, hsa-rniR-93-5p hsa-miR-95-3p and hsa-miR-98-5p
was
increased in the patient group. However, the regulation of miRNA was not
statistically
significant except for hsa-miR-485-3p. The expression of hsa-485-3p was
significantly
increased in the Alzheimer's patients as compared to the normal group, with a
p value of
0.00439. The expression level was different by about 9-fold for severe
dementia as compared
CA 3046766 2019-06-17
35
to the normal group with I-fold. The intermediacy between 1-fold and 9-fold
can be
distinguished as mild cognitive impairment (FIG, 1B). Therefore, hsa-miR-485-
3p can be
used as a marker for diagnosis of Alzheimer's disease,
Example 2: Prediction of human miRNA target gene, conservation of same miRNA
target gene in mouse and synthesis of anti-mmu-miR-485-3p
In order to analyze the base sequence and target location of hsa-m iR-485-3p,
it was
confirmed using a target prediction software (miRDB) that the 3'-untranslated
region (UTR)
of human-derived ELAVL2 is the target of hsa-miR-485-3p. It was confirmed that
the
identified seed sequence was conserved also in mmu-miR-485-3p and the 3'-
untranslated
region of mouse-derived ELAVL2.
FIG. 2 shows a list of the 31-untranslated region (UTR) mRNAs of ELAVL2 known
as a target known of hsa-miR485-3p, and shows the 31-untranslated region (UTR)
mRNAs of
target ELAVL2 of miR485-3p. The 5' seed sequence of miR-485-3p (ELAVL2) is
shown
in red color. Table 3 shows the base sequence of has-miR-485-3p. A functional
study was
conducted to elucidate the physiological functions of miR-485-3p using an
Alzheimer's
disease model by synthesizing the sequence.
[Table 3]
Base sequence of hsa-miR485-3p
Gene Sequence (5'-6') SEQ ID NO
hsa-miR485-3p GUCAUACACGGCUCUCCUCUCU
Table 4 shows the results of analyzing the base sequence and target location
of mmu-
miR485-3p. It was confirmed using target prediction softwares (TargetScan,
PicTar, and
microT) that the 31-untranslated region (UTR) of mouse ELAVL2 mouse and the
target
EDC_LAW1204724111
CA 3046766 2019-06-17
36
sequence of mmu-miR-485-3p were conserved. It was confirmed that the 31-
untranslated
region (UTR) of mouse ELAVE2 is the target of mmu-miR-485-3p,
[Table 4]
Analysis of base Sequence and target location of mmu-miR485-3p
Gene Sequence( ->3' ) SEQ ID NO
R-485-3p AGIICAUACAMGCUCUCCUCUC 8
Target Total Representative
Gene name 3P-sec tags + 5
gene sites m i RNA
ELAY1,2 ELM I i kc neuron-specific 78 3 umni-rni R-485-3p
= RNA binding protein 2
-
For the mmu-miR-485-3p overexpressed in the Alzheimer's disease mouse model,
an
antisense oligonucleotide modified with a target gene associated with
Alzheimer's and a brain
disease, a specific sequence antagomir, 2'-0-methytation and phosphorothioatc
was
synthesized.
= Example 3: Analysis of miR'-485-3p expression in hippocampus and cerebral
cortex
of 5xFAD mouse (RT-qPCR)
(1) Research methods
5xFAD transgenic mouse is an animal model of Alzheimer disease obtained by
overexpressing mutant forms of APP and PSEN I, which exhibits severe
accumulation of
intraneuronal Af342 from about 6 weeks.
Given the result of Example 1, RT-qPCR was performed to confirm the expression
of
miR-485-3p in the dementia animal model. 10-month-old 5xFAD transgenic mouse
and
wild-type (WT) mouse were deeply anesthetized and sacrificed by decapitation.
After
excising the brain immediately, the hippocampus and cerebral cortex were
dissected from the
remaining brain structure. Total miRNA was isolated from the hippocampus using
the
EDC_LAVV1204724111
CA 3046766 2019-06-17
37
PAXgene Tissue miRNA kit (Qiagen, USA) according to the manufacturer's
instructions.
cDNA was synthesized using the miScript II RT kit (Qiagen, USA), and qPCR was
performed using the mmu_miR-485-3p miScript Primer Assay kit and the miScript
SYBR
Green PCR kit. The miRNA level was quantified by normalizing to snoRNA202
(control
mouse).
(2) Research results
FIG. 3A compares the expression of miR-485-3p in the hippocampus and the
cortex.
RT-PCR was conducted to investigate the expression pattern of miR-485-3p in
the
hippocampus and the cerebral cortex of 5xFAD. The result showed that the
expression of
miR-485-3p was increased in the hippocampus of' 5xFAD as compared to wild-type
(WT).
This, together with the results of Example I, shows that the expression of miR-
485-3p is
increased in Alzheimer's dementia. Therefore, the neuronal target mRNA or
protein that
may be affected by miR-485-3p was investigated.
Example 4: Confirmation of expression of related proteins including ELAVI.,2
and
amyloid beta 42 protein in hippocampus and cerebral cortex of 5xFAD mouse
(1) Research methods
Given the results of Example 3, the expression of related proteins including
ELAVL2
and the amyloid beta42 protein in the hippocampus and the cerebral cortex of'
5xFAD was
investigated. After sacrificing an anesthetized mouse (9-month-old) by
decapitation, the
brain was extracted immediately. After preparing a homogenate of the brain
(hippocampus
and cerebral cortex), western blot was conducted using an anti-ELAVL2 antibody
(Abeam,
USA), a cFOS antibody (Cell Signaling, USA), an APP antibody (Cell Signaling,
USA) and
an amyloid beta antibody (Cell Signaling, USA). The immunoreactive protein was
CA 3046766 2019-06-17
38
visualized with a chemiluminescence reagent (GE Healthcare, UK) and was
measured and
quantified using a chemiluminescence analyzer (Fusion SL). The amyloid beta 42
protein in
the hippocampus and the cerebral cortex was quantified by using the mouse/rat
amyloid beta
(1-42) ELISA kit (1BL) according to the manufacturer's instructions.
(2) Research results
I) Confirmation of ELAVL2 expression in hippocampus and cerebral cortex
FIG. 3B and FIG. 3C show the result of comparing the expression of ELAVL2 and
related proteins in the hippocampus and the cerebral cortex of 5xFAD. ELAVL2,
an
ELAV-like RNA-binding to protein, is known as a protein that regulates neural
functions
such as neuronal excitation or synaptic transmission, which are directly
associated with
cognitive and behavioral functions. Also, ELAVL2 is responsible for post-
transcriptional
gene regulation as a neural-specific RNA-binding protein by recognizing the
GAAA motif of
RNA. As its target, the transcription factor cFOS which affects the expression
level of the
protein is known. Also, cFOS is known to influence the expression of the APP
protein,
which is known as an amyloid beta precursor in brain cells. It was confirmed
that the
increase pattern of miR-485-3p significantly affects the expression level of
APP.
2) Comparison of AP42 expression in cerebral cortex and hippocampus
FIGs. 4A-4B show results of quantitatively comparing the expression of Af342
in the
cerebral cortex and the hippocampus of 5xFAD. It was confirmed that Af342 was
significantly increased as compared to WT both in the cerebral cortex (FIG.
4A) and in the
hippocampus (FIG. 4B) of 5xFAD. Based on this result, it can be seen that the
regulation of
miR-485-3p can be a way to reduce amyloid beta, which known as the causative
agent of
Alzheimer's disease.
CA 3046766 2019-06-17
39
Example 5: Measurement of amyloid beta (Af3) 42 isolated from saliva
(1) Research methods
The measurement of amyloid beta in saliva is advantageous in that the accuracy
is
better than for blood because or the proximity to the brain and early
diagnosis is possible
because even a small amount of amyloid beta can be quantified. In addition, a
higher
accuracy (> 99%) can be ensured by doubly checking blood, miRNA, etc.
A multi-nucleic acid having the structural features that, upon binding to
amyloid beta
42, fluorescence is emitted as a quencher is detached at the same time was
synthesized by
using a sequence binding specifically to amyloid beta 42 and a sequence self-
coupled within
the nucleic acid. Because the size of the multi-nucleic acid is smaller than
an antibody, its
specificity and selectivity are excellent. Also, because sampling and large-
scale analysis are
possible within one hour, it can provide a diagnostic method with high
diagnostic ease. The
amyloid beta 42 in saliva was quantified by using the platform technology
(magnetic
is immunoassay system) which can assemble a very small amount of a
biomarker as a
monolayer (FIG. 5). The assemblage of nanoparticles on a specified area could
be achieved.
The aggregate of the complex of the saliva-derived amyloid beta and the
magnetic particle
was 300 tm or smaller in diameter, and the number of the magnetic particles in
the aggregate
was ¨1.0x 104 (FIG. 6A). The region of interest (R01) in photomultiplier tube
(PMT)
analysis was 100x100 un2. The fluorescent substances was measured 3 times
using the
PMT, and then the average value was calculated for each sample (FIG. 6B).
A normal group of 100 people and a patient group of 100 patients from mild
cognitive impairment to severe dementia were recruited. Their ages ranged from
early 30s
to late 80s. The patients with other diseases that can affect dementia were
excluded.
CA 3046766 2019-06-17
40
(2) Research results
A trace amount of amyloid beta in saliva, which was not detectable by ELISA,
could
be measured. As a result of comparing with commercially available ELISA,
amyloid beta
was not detected for the normal subjects in their 30s.
The magnetic immunoassay system used in this example can easily detect the
amyloid beta peptide in saliva at a slightly high concentration of ¨20 pg/mL
as compared to
the commercially available ELISA system. According to the protocol of the
ELISA system
used, the lowest measurable concentration of the standard amyloid beta peptide
is 7.4 pg/mL,
which can be a lirtle ambiguous because the curve fitting for measurement
cannot meet the
exact linearity in very low concentration ranges.
As a result of measuring saliva-derived amyloid beta 42 for the dementia
patients, it
was possible to distinguish normal eases, mild cognitive impairment (MCI) and
severe cases.
The concentration of beta amyloid was 0-500 pg/mL for normal eases, 500 pg to
I ng/ml, for
mild cognitive impairment, and I ng/mL or higher for severe cases (FIGs. 7A-
7D). In
addition, the amyloid beta level of over 100 dementia patients was normalized,
and mild
cognitive impairment and moderate dementia could be distinguished successfully
(90% or
higher match with diagnosis by clinicians).
Example 6: Preparation and in-vitro transfection of hippocampal primary cell
line
(I) Research methods
' Primary cells derived from the tissues of the hippocampus and the cerebral
cortex
excised from the embryo of 5x.FAD were cultured. The methods for cell
preparation and
culture followed the previous research (Seibenhener, M.L & Woonten M.W,
Isolation and
culture of Hippocattipal Neurons from Prenatal Mice, Jove, 2012). 50 nM of miR-
485-3p
duplex (or scrambled miRNA duplex; Bioneer, Daejon, South Korea) and 50 nM of
CA 3046766 2019-06-17
41
antagomir (AM) 485-3p were transfected into primary cells in vitro using
Lipofectamine
2000. A cell homogenate was obtained 48 hours after the transfeetion, which
was subjected
to western blot using ELAVL2 antibody (Abeam, UK). The immunoreactive protein
was
visualized with a chemiluminescence reagent (GE Healthcare, UK) and was
measured and
quantified using a chemiluminescence analyzer (Fusion SL). The amyloid beta 42
protein
was measured by using the mouse/rat amyloid beta (1-42) ELISA kit (IBL)
according to the
manufacturer's instructions,
(2) Research results
The expression of ELAVL2 in the hippocampal primary cells depending on the
transfection with AM485-3p (2'-0-methylated)-5'-gagaggagagccguguaugacu-31(SEQ
ID NO
9)) was compared (FIGs. 9A-9B). It was confirmed that ELAVL2 was expressed in
the
hippocampal primary cells of 5xFAD, and the expression of ELAVL2 expression
was
increased in the cells transfeeted with AM485-3p as compared to the control.
This means
that miR-485-3p inhibits the expression of E1AVL2 in the cells treated with
AM485-3p.
Because ELAVL2 is an important factor affecting cognitive function by being
involved in
excitation of neurons, the development of a drug or a composition that
increases ELAVL,2,
such as a miR-485-3p inhibitor, can be a key strategy in preventing/treating
of Alzheimer's
disease.
Example 7: Comparative quantification of related proteins including ELAVL2
amyloid beta 42 protein in 5xFAD intranasally treated with AM485-3p
(I) Research methods
The inhibition of miR-485-3p was induced by intranasally administering a
sequence-
specific antagomir or a scrambled sequence antagomir. The intranasal
administration of the
CA 3046766 2019-06-17
42
antagomir carried out according to a method targeting the brain without
anesthetizing in the
mouse (Leah R.T., et al. (2013) Intranasal Administration of CNS Therapeutics
to Awake
Mice. I Vis Exp. 2013; (74): 4440). After immobilizing the accustomed mouse
for
intranasal inhalation (intranasal grip) and positioning so that the abdomen
faces upward, a
pipette was positioned in front of one nasal cavity. 6 i_LL, was inhaled
dropwise twice using
the pipette (1 drop = 3 }IL). After maintaining the position for 15 seconds,
intranasal
inhalation into the right nasal cavity was conducted in the same manner. The
same
procedure was repeated 2 minutes later. A total of 24 111., was inhaled (AM485
(21-0-
methylated)-5'-gagaggagagceguguaugacu-3' (SEQ ID NO 9); 5 nmol in 24 0_, of
distilled
water treated with 0.1% v/v diethylpyrocarbonate; Bioneer, Korea). A vehicle
of the same
volume was administered to a control mouse. 12 weeks after the nasal
administration (once a
week), the anesthetized mouse was sacrificed by decapitation and the brain was
excised
immediately. After preparing a homogenate of the brain (hippocampus and
cerebral cortex),
western blot was conducted using anti-ELAVL2 antibody (Abeam, USA), cFOS
antibody
(Cell Signaling, USA), APP antibody (Cell Signaling, USA) and amyloid beta
antibody (Cell
Signaling, USA). The immunoreactive protein was visualized with a
chemiluminescence
reagent (GE Healthcare, UK) and was measured and quantified using a
chemiluminescence
analyzer (Fusion SL). The amyloid beta 42 protein was measured by using the
mouse/rat
amyloid beta (1-42) EL1SA kit (IBL) according to the manufacturer's
instructions.
(2) Research results
The related proteins including ELAVL2 and the amyloid beta 42 protein were
quantitatively compared for the 5xFAD intranasally treated with AM485-3p ((2r-
0-
methy1ated)-5'-gagaggagagccguguaugacu-3' (SEQ ID NO 9); FIGs. I0A-10C).
Because it
was confirmed in Example 6 that the treatment of a mouse primary cell line
with AM485-3p
CA 3046766 2019-06-17
43
induces change in ELAVL2, the effect of AM485-3p in vivo was investigated
intranasally
treating 5xFAD with AM485-3p. The ELAVL2 group showed increased expression of
AM485-3p as compared to the control group. With the recovery of ELAVL2, the
expression of cF0S, which is a transcription factor involved in APP
expression, was
normalized and this led to the decreased expression of APP, resulting in
decrease in the
amyloid beta 42 protein (FIGs. I OA and I OB). This suggests that the
development of a drug
such as a miR-485-3p inhibitor or a composition thereof is a key strategy in
preventing/treating Alzheimer's disease. In addition, since it was confirmed
in the animal
model that the treatment with AM485-3p affects the inhibition of A1342
production (FIG.
10C), it seems that treatment with the related inhibitor or drug can relieve
the pathological
symptoms of Alzheimer's dementia.
Example 8: Comparison of cognitive function of 5xFAD mouse intranasally
treated
with AM485-3p
(1) Research methods
Y-maze and passive avoidance tests were carried out to investigate whether the
intranasal treatment of AM485-3p (2'-0-methylatcd)-5'-gagaggagagccguguaugacu-3
(SEC?
ID NO 9)) improved the cognitive function of 5xFAD.
I) Y-maze test
A Y-maze test apparatus is composed of Y-shaped maze prepared with black
acrylic
plates (10 cm wide, 41 cm long, 25 cm high). The maze is arranged with an
angle of 120 .
After dividing each maze into A, B and C zones, the experimental animals were
placed
carefully in each zone and allowed to move freely for 8 minutes. Spontaneous
alternation
(%) was evaluated by measuring the number and sequence of entries to each
maze. The
CA 3046766 2019-06-17
44
entrance into the three different zones in sequence was given one point
(actual alternation,
e.g., A-B-C, B-C-A, C-A-B, etc.). No point was given to discontinuous
entrance. The
spontaneous alternation (%) was calculated by the following formula.
% Spontaneous alteration = total number of alternation / (total number of
entries - 2)
x
2) Passive avoidance test
The passive avoidance test passive is a widely used method for measuring the
working memory ability of rodents. A passive avoidance test apparatus is a
shuttle box
divided into two chambers, one equipped with a light bulb to create a bright
environment that
the test animals dislike, and the other with light blocked to create an
environment which is
comfortable for the animals. After two hours of stress application, the
passive avoidance
response was tested (training test). Aluminum grids were placed on the floor
of the dark
chamber at regular intervals so as to apply electric shocks to the sole of the
animals. The
.. experimental animals tend to enter the dark chamber. After keeping the
animal in the bright
chamber and then allowing to enter the dark chamber, electric shock (5 V, 0.5
mA, 10 see)
was applied so that it could remember it. 24 hours later, the time (latency
time) lapsed until
the entry into the dark chamber was measured up to 90 seconds without applying
electric
shock (retention tests I, 2 and 3).
(2) Research results
The cognitive function of the 5xFAD intranasally treated with ANI485-3p (21-0-
methylated)-5'-gagaggagagceguguaugacu-3 (SEQ ID NO 9)) was compared (FIGs. I
IA-11B.
As a result, both the spontaneous alteration (FIG. I IA) and the latency time
(FIG. 11B) were
decreased in 5xFAD as compared to WT. Because the typical symptoms of
Alzheimer's
CA 3046766 2019-06-17
45
dementia are behavior disorder and memory decline, the behavior disorder of
5xFAD seems
to be due to the excessive accumulation and pathology of amyloid beta.
However, the group
intranasally treated with AM485-3p showed significant increase in both the
spontaneous
alteration and the latency time as compared to 5xFAD. It means that the
treatment with
.. AM485-3p can improve the main symptoms of Alzheimer's by relieving the
pathological
symptoms such as behavioral disorder and memory decline caused by the
production of the
amyloid.beta42 protein facilitated by miR-485-3p. Therefore, the preparation
of a drug that
regulates miR-485-3p manufacturer or a composition thereof can be a new
strategy to
improve the main symptoms of Alzheimer's dementia, i.e., behavioral disorder
and cognitive
function.
Example 9: Statistical analysis
Two groups were compared by the Student's t-test, and three or more groups
were
compared by the Krushall-Wallis test. When the P value obtained from the
Krushall-Wallis
test was < 0.05, two groups were tested post-hoc by the Mann-Whitney U test. P
value of
0.05 or smaller for the two-tailed test was considered statistically
significant.
According to the present disclosure, objective data analysis for diagnosis of
Alzheimer's disease or a brain disease is possible by measuring the expression
level of miR-
.. 485-3p in blood, the risk of a patient can be minimized by measuring the
concentration of
atnyloid beta 42 in saliva, and fast and accurate diagnosis is possible.
Therefore, the present
disclosure is very useful in preventing Alzheimer's disease or a brain disease
by diagnosing
the Alzheimer's disease or the brain disease early.
EDC_LAIAA 204724111
CA 3046766 2019-06-17
46
While the specific embodiments of the present disclosure have been described
in
detail above, those skilled of ordinary skill in the art will appreciate that
the specific
embodiments are merely specific exemplary embodiments and the scope of the
present
disclosure is not limited by them. It is to be understood that the substantial
scope of the
disclosure is defined by the appended claims and their equivalents.
EDC_LAIM 204724111
CA 3046766 2019-06-17