Note: Descriptions are shown in the official language in which they were submitted.
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
ELECTROCHEMICAL CELLS AND BATTERIES
RELATED APPLICATIONS
[0001] This application claims priority to, and benefit of, U.S. Provisional
Application
No. 62/470,772, filed on March 13, 2017; U.S. Provisional Application No.
62/479,548,
filed on March 31, 2017; U.S. Provisional Application No. 62/506,422, filed on
May 15,
2017; U.S. Provisional Application No. 62/518,523, filed on June 12, 2017;
U.S.
Provisional Application No. 62/530,687, filed on July 10, 2017; U.S.
Provisional
Application No. 62/531,274, filed on July 11, 2017; and U.S. Provisional
Application No.
62/595,171, filed on December 6, 2017.
BACKGROUND
[0002] The U.S. Energy Information Agency projects a 48% increase in world
energy
consumption by 2041 and global industries are rapidly adapting to these new
opportunities. At the center of their efforts is the demand for a new battery
that is more
efficient, more environmentally friendly, more affordable and more convenient.
The
Department of Defense has stated a goal of generating 25% of its energy from
renewable
sources by the year 2025. Additionally, the military must have readily
available sources
of hydrogen when fielding stealthy fuel-cell powered vehicles.
[0003] Liquid batteries, whether flow or no-flow, are known in the art and
work on the
same principles as solid batteries, except the electrolyte is liquid. Such
batteries are
comprised of electrochemical cells which are based on reduction-oxidation
chemistry.
Oxidation occurs on the anode side of the cell and reduction on the cathode
side. The
solvents used in electrochemical cells are varied. In many circumstances,
aqueous
solutions are used on both sides of an electrochemical cell with each side
(cathode side
and anode side) in contact with an electrode (i.e., the cathode and anode
respectively).
- 1 -
RECTIFIED SHEET (RULE 91)
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
The electrodes of the two-half cells are placed in electrical contact to allow
for current to
flow. To maintain charge balance, an electrochemical cell must also allow for
the passage
of ions. In some batteries, this is done with a salt bridge separating the
cathode solution
from the anode solution. The bridge prevents mixing of the two solutions. The
prior art
teaches that if the solutions were to mix, the half-cells could be destroyed
by direct
chemical reaction.
[0004] Some electrochemical cells deploy membranes or salt bridges to prevent
shorting
and to separate multiple electrolyte solutions. Such membranes are costly and
readily
degrade over time. W02017/106215 reports electrochemical cells which may be
used in
the absence of membranes and which use two or more immiscible electrolytes.
[0005] The present disclosure includes improved designs for electrochemical
cells and
batteries (including flow cells and flow batteries) that, in certain
embodiments, include a
single electrolyte solution, an anode, a current collector, and a porous, non-
conductive
spacer between the current collector and anode. In contrast to typical flow
batteries, the
anode and current collector in such embodiments need not be immersed in an
electrolyte.
Additional embodiments include improved designs for membraneless
electrochemical
cells and batteries that comprise multiple electrolytes.
SUMMARY
[0006] Embodiments of the disclosure include electrochemical cells and
batteries that can
operate with a single electrolyte solution. In one such embodiment, an
electrochemical
cell comprising an anode, a current collector and a porous, non-conductive
spacer between
the anode and the current collector is provided. Additional designs for
electrochemical
cells and batteries that can operate with a single electrolyte solution are
also provided.
- 2 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[0007] In a further embodiment of the disclosure, an electrochemical battery
comprising
one or more of these electrochemical cells and further comprising a load is
provided.
[0008] In yet a further embodiment of the disclosure, methods of delivering
electricity to
applications with these electrochemical cells or electrochemical batteries of
the disclosure
or both are provided.
[0009] In additional embodiments of the disclosure, methods of delivering
hydrogen to
applications with these electrochemical cells or electrochemical batteries of
the disclosure
or both are provided.
[0010] Further embodiments include certain membraneless electrochemical cells
that
contain separate first and second electrolyte solutions at the cathode and
anode,
respectively; batteries comprising these electrochemical cells; methods of
delivering
hydrogen, electricity or both with the cells and batteries to applications;
capacitors; and
various methods related to these embodiments.
[0011] More embodiments and features are included in the detailed description
that
follows, and will be readily apparent to those skilled in the art from the
description or
recognized by practicing the embodiments as described in the description,
including in the
figures and claims.
BREIF DESCRIPTION OF THE DRAWINGS
[0012] The accompanying figures constitute a part of this disclosure. The
figures serve to
provide a further understanding of certain exemplary embodiments. The
disclosure and
claims are not limited to embodiments illustrated in the figures.
[0013] FIG. 1 is a schematic of one embodiment of electrochemical cells of the
disclosure.
[0014] FIG. 2 is a schematic of one embodiment of an electrochemical battery
of the
disclosure.
- 3 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[0015] FIG. 3 is a schematic block diagram of an embodiment of an
electrochemical cell
according to the present disclosure.
[0016] FIG. 4A is a schematic block diagram of an embodiment of an
electrochemical cell
according to the present disclosure.
[0017] FIG. 4B is a schematic block diagram of an embodiment of an
electrochemical cell
according to the present disclosure.
[0018] FIG. 5 is a schematic of an embodiment of an electrochemical cell of
the
disclosure.
[0019] FIG. 6 is a schematic of an embodiment of an electrochemical cell of
the
disclosure.
[0020] FIG. 7 is a schematic of an embodiment of an electrochemical cell of
the
disclosure.
[0021] FIG. 8 is a schematic of an embodiment of an electrochemical cell of
the
disclosure in flow mode.
[0022] FIG. 9 is a schematic of an embodiment of an electrochemical cell of
the
disclosure.
DETAILED DESCRIPTION
[0023] Various embodiments will now be explained in greater detail. It is to
be
understood that both the foregoing general description and the following
detailed
description are exemplary and explanatory only, and are not restrictive of
this disclosure
or of the claims. Any discussion of certain embodiments or features, including
those
depicted in the figures, serve to illustrate certain exemplary aspects of the
disclosure. The
disclosure and claims are not limited to the embodiments specifically
discussed herein or
illustrated in the figures.
- 4 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[0024] Embodiments of the disclosure include electrochemical cells and
batteries that can
operate with a single electrolyte solution. Such cells and batteries do not
require multiple
electrolyte solutions separated by a membrane, by a salt bridge or by other
techniques. In
one such embodiment, an electrochemical cell comprising an anode, a current
collector
and a porous, non-conductive spacer between the anode and the current
collector is
provided.
[0025] The electrochemical cells of the disclosure, including this embodiment
and other
embodiments of electrochemical cells described herein, may be used to form
batteries to
supply electricity, hydrogen, or both to applications. Electrical applications
include, for
example, grid applications such as cell phone tower backup power or backup
power for
wind farms or solar farms. The electricity could also be used to power
vehicles,
household appliances, consumer goods or toys. When configured to run to
produce
hydrogen, the hydrogen may be delivered to an application such as a fuel cell
for
electricity production or a vehicle or to, for example, an engine or furnace
for burning.
The electrochemical cells may be configured such that electricity is primarily
delivered,
hydrogen is primarily delivered, or both are delivered in various ratios. In
some
embodiments, the cells and batteries of the disclosure run in flow mode in
that electrolyte
is added to the cell or battery and then removed after use, thereby
replenishing the supply
of electrolyte to the cell or battery.
[0026] In an example electrochemical cell of the disclosure, the anode is in
physical
contact with the porous, non-conductive spacer, such as non-conductive screen,
which in
turn is in physical contact with the current collector such as carbon foam. In
other
embodiments, the spacer is not in physical contact with the anode, the current
collector, or
both, but is instead positioned at a distance from the anode, current
collector, or both.
- 5 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[0027] The purpose of the porous, non-conductive spacer is to prevent physical
contact
of the current collector with the anode. The term "non-conductive" with
reference to the
porous spacer means that the spacer is not electrically conductive. The spacer
is porous to
electrolyte, and is also porous in a non-selective manner to anions and
cations in the
electrolyte solution. Being electrically non-conductive, the porous spacer
does not permit
the passage of electrons through it. These features of the spacer
differentiate it from salt
bridges and membranes in conventional batteries, which are often used to
maintain
separation of multiple electrolyte solutions. Many different types of non-
conductive
porous materials can be used for the spacer, such as an organic polymer,
surgical tape,
fiberglass film, glass wool, wood, paper, cloth, cardboard, and nylon. One
such organic
polymer is vinyl coated polyester. The thickness of the spacer is often
between about 0.1
mm and about 0.8 mm.
[0028] A microporous material, such as surgical tape, may be used to wrap the
electrochemical cell so as to help maintain its physical integrity. The
wrapping need not
completely encase the electrochemical cell.
[0029] Current collectors and anodes in electrochemical cells in this
disclosure, including
in this embodiment and other embodiments of electrochemical cells described
herein, may
be selected from suitable materials. Examples of suitable current collector
materials
include steel, carbon such as in the graphite allotrope of carbon, carbon
impregnated with
a metal, and carbon foam. Conducting carbon cloth (which is also referred to
as carbon
foam), for example, is a suitable current collector for many embodiments and
is a
conducting material. Suitable anodes for embodiments having a single
electrolyte solution
include metals in Column 13 of the Periodic Table and their alloys. These
metals and
alloys include, for example, aluminum, gallium, indium and thallium, as well
as alloys
- 6 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
comprising at least one of these. One example alloy has the name of Galinstan,
which is
an alloy of gallium, indium and tin.
[0030] In many embodiments, the electrochemical cell further comprises a
single
electrolyte solution. The electrochemical cell may be saturated with respect
to the
electrolyte. In some embodiments, the cell is not immersed in an electrolyte
bath. By
saturating via drip, spray or atomization of the electrolyte, for example, one
can activate
the cells to produce electricity or hydrogen or both. One example of a current
collector is
carbon foam. The electrolyte may be sprayed onto the cell until or before the
carbon foam
is saturated in such an example. As electricity or hydrogen or both is
produced, additional
electrolyte (or other materials such as salts, oxidants, or bases) may be
sprayed or
otherwise delivered to the cell to maintain its saturation and replace salts
and oxides.
Indeed, a recirculator could be used to continuously recycle and deliver
electrolyte or
other materials to the cell. Thus, it is not necessary to keep a large supply
of electrolyte
present, which saves on both cost and weight compared with flow cells of the
prior art.
[0031] In these and other embodiments, the anode of the electrochemical cell
is made
from aluminum, for example. In many embodiments the aluminum is in the form of
a
sheet. In many other embodiments it is in the form of a screen or other thin
porous
structure. The thickness of the aluminum screen may be, for example between
about 0.05
mm and about 0.5 mm, or between about 0.1 mm and about 0.3 mm. In some
embodiments, the current collector can be carbon foam and the porous, non-
conductive
spacer can be a non-conductive screen such as an organic polymer or surgical
tape. One
such polymer is vinyl coated polyester.
[0032] Additional embodiments include 1) an electrochemical cell comprising an
anode, a
current collector, a porous, non-conductive spacer between the anode and the
current
- 7 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
collector, and a single electrolyte solution; 2) an electrochemical cell
consisting essentially
of an anode, a current collector, a porous, non-conductive spacer between the
anode and
the current collector, and a single electrolyte solution; and 3) an
electrochemical cell
consisting of an anode, a current collector, a porous, non-conductive spacer
between the
anode and the current collector, and a single electrolyte solution.
[0033] Electrochemical cells are often connected to one another by metal
conductors to
form electrochemical batteries. An example of a metal conductor is copper such
as copper
wire or wires. Thus, for example, an aluminum anode on one electrochemical
cell is in
contact with a porous, non-conductive spacer on the cell and the spacer is in
turn in
contact with a carbon foam current collector. The aluminum anode is also in
contact with
a metal conductor to the current collector on an adjacent electrochemical
cell, which in
turn is in contact with its own porous, non-conductive spacer and
corresponding aluminum
anode. In this manner, a series of electrochemical cells may be connected. At
the
termination of the series, the terminal electrochemical cells may be connected
to a load,
such as an application or to additional electrochemical cell series so that
the overall
electrochemical battery has both a series and parallel arrangement of
electrochemical cells.
[0034] In some embodiments, the electrochemical cells are also configured to
operate as
flow cells. The cells may be configured as flow cells so as to support a flow
battery, for
example. In such a battery, electrolyte can be provided to cells during the
operation of the
battery continuously during operation so that electrical charging is not
required.
[0035] In many embodiments of the disclosure, the electrolyte comprises water
and one or
more salts. Examples of solvents for use in the electrolyte, including water
and others,
may be found in Table 1.
- 8 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
Table 1 ¨ Polar Solvents
Solvent Dielectric constant
Water 80
Sulfuric acid 101
Ammonia 26.7
Ethanol 24.3
Acetonitrile 36.2
Pyridine 12.3
Methanol 30
Glycerol 47
Ethylene glycol 37
Hydrofluoric acid 134
Furfural 42
Hydrazine 52
Formamide 84
Hydrogen peroxide 128
Hydrocyanic acid 158
[0036] In many embodiments, there are two salts that are added to the
electrolyte.
Examples of such salts include salts of peroxydisulfate (such as sodium
peroxydisulfate),
hypochlorite, and sulfate (such as sodium sulfate). The electrolyte may
additionally
comprise a base, such as sodium hydroxide. One of the salts is an oxidant.
Thus, in many
embodiments, the electrolyte contains an oxidant and also contains an
additional salt, such
as a metal salt found in Table 3, wherein the anion portion of the metal salt
and the
oxidant differ.
[0037] The non-limiting list of compounds in Table 2, or their corresponding
salts and
acids as the case may be, may be delivered and dissociate to form oxidants. As
used
herein, the term "oxidant" refers to the compound added to perform oxidation
as well as
the resulting anion that results from dissociation of that compound. Thus,
peroxydisulfuric acid (H25208), sodium peroxydisulfate (Na25208) and the
peroxydisulfate
anion (52082) are all oxidants as used herein. When the acid or salt form of
the
peroxydisulfate oxidant, for example, is added to an electrolyte of the
disclosure, there
- 9 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
will be dissociation into the anion form. The anion form is the form which
acts to oxidize
another species and which in turn is reduced.
Table ¨ 2 ¨ Oxidants
Hydrogen peroxide
Nitric acid
Sulfuric acid
Peroxydi sulfuric acid
Sodium peroxydi sulfate
Peroxymonosulfuric acid
Chlorite, chlorate, perchlorate, and other analogous halogen compounds
Hypochlorite and other hypohalite compounds, including NaC10
Hexavalent chromium compounds such as chromic and dichromic acids and chromium
trioxide, pyridinium chlorochromate (PCC), and chromate/dichromate compounds
Permanganate compounds such as potassium permanganate
Sodium perborate
Nitrous oxide
Potassium nitrate
Sodium bismuthate
Oxygen
Ozone
Halogens
[0038] The other salt may be, for example, any one of the compounds found in
Table 3.
- 10 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
Table 3 ¨ Metal Salts
KI NaI LiI BaI2 CO2 MgI2 ZnI2 A113
KBr NaBr LiBr BaBr2 CaBr2 MgBr2 ZnBr2 AlBr3
KC1 NaCl LiC1 BaC12 CaCl2 MgCl2 ZnC12 AlC13
K2SO4 Na2SO4 Li2SO4 BaSO4 CaSO4 MgSO4 ZnSO4 Al2(SO4)3
KNO3 NaNO3 LiNO3 Ba(NO3)2 Ca(NO3)2 Mg(NO3)2 Zn(NO3)2 Al(NO3)3
KF NaF LiF B aF2 CaF2 MgF2 ZnF2 A1F3
K3PO4 Na3PO4 Li3PO4 Ba3(PO4)2 Ca3(PO4)3 Mg3(PO4)2 Zn3(PO4)2 A1PO4
K2S03 Na2S03 Li2S03 BaS03 CaS03 MgS03 ZnS03 Al2(S03)3
K2CO3 Na2CO3 Li2CO3 BaCO3 CaCO3 MgCO3 ZnCO3 Al2(CO3)3
K2S Na2S Li2S BaS CaS MgS ZnS Al2S3
K2SiO3 Na2SiO3 Li2SiO3 BaSiO3 CaSiO3 MgSiO3 ZnSiO3 Al2(SiO3)3
KOH NaOH Li OH B a(OH)2 Ca(OH)2 Mg(OH)2 Zn(OH)2 Al (OH)3
[0039] The second salt should be a compound that dissociates so as to produce
a metal
ion. An example of such a metal salt is sodium sulfate.
[0040] In many embodiments, the electrolyte further contains a base such as a
strong base.
Examples of strong bases include Li0H, RbOH, Cs0H, Sr(OH)2, Ba(OH)2, NaOH,
KOH,
Ca(OH)2, or a combination thereof One particular example is NaOH.
[0041] In further embodiments, the electrolyte comprises one of water or an
alcohol. The
electrolyte can be a catholyte, for example. When an oxidant and a metal salt
are present
in this electrolyte, the two can have different anion components. An example
oxidant
includes sodium peroxydisulfate and an example metal salt is sodium sulfate.
This
electrolyte may further comprise a base, such as sodium hydroxide.
[0042] A further embodiment includes an electrochemical cell comprising:
a. an anode;
b. a current collector; and
c. a porous, non-conductive spacer between the current collector and anode;
wherein the electrochemical cell does not comprise an electrolyte.
-11-
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[0043] The anode, current collector, and spacer may have one or more
characteristics or
features described above for those components. Such an embodiment may, for
example,
be constructed in one location then transported to another location where it
is contacted
with electrolyte (such as a single electrolyte solution) for use.
[0044] The disclosure includes additional embodiments of electrochemical cells
that can
operate with a single electrolyte solution. For example, an electrochemical
cell
comprising a single aqueous electrolyte solution in contact with a non-
metallic current
collector, an oxidant, and a metal solid wherein current travels from the
metal solid to the
current collector via a load, is provided.
[0045] In such a cell, the electrolyte solution can be basic and the oxidant
can be 52082-,
for example. The electrolyte solution may further comprise, for instance,
sodium
hydroxide. Suitable metal solids include those in Column 13 of the Periodic
Table and
their alloys. These metals and alloys include, for example, aluminum, gallium,
indium
and thallium, as well as alloys comprising at least one of these. One example
metal solid
is aluminum, such as aluminum in the form of a foil. The cell may further
comprise a
porous stabilizer. In one example, the cell may comprise a metal sulfate (such
as Na2SO4)
where the current collector is carbon foam and the porous stabilizer is glass
wool or a
borosilicate or both. The pH of the cell may, for example, be greater than 12,
greater than
13 or greater than 14.
[0046] Cells such as this, which can use a single electrolyte solution, may
produce, for
example, between about 10 Watt-hours/(kg of electrolyte + anode metal) and
about 680
Watt-hours/(kg of electrolyte + anode metal). When measured per kg of
electrolyte, the
cell may produce, for example, between about 10 Watt-hours/kg of electrolyte
and about
100 Watt-hours/kg of electrolyte, between about 40 Watt-hours/kg of
electrolyte and about
- 12 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
80 Watt-hours/kg of electrolyte, or between about 10 Watt-hours/kg of
electrolyte and
about 60 Watt-hours/kg of electrolyte. The power produced per square
centimeter of
metal solid can be, for example, between about 600 mW and about 1000 mW. As
with
other electrochemical cells in this disclosure, the cell may be configured to
operate in flow
mode. The cell may include an inflow stream that comprises, for example, an
aqueous
electrolyte solution that may further comprise an oxidant such as sodium
peroxydisulfate
or a solution comprising peroxydisulfate anion or both. Oxidant (such as solid
Na2S208 or
Na2S208 in solution) can be provided to the cell, for example, Na2S208 in an
aqueous
basic solution, where the base may be, for example, sodium hydroxide. The cell
may
further comprise an outflow stream. The outflow stream may comprise an aqueous
solution and may also include, for example, metal sulfate.
[0047] A further embodiment includes a method of creating a capacitor
comprising the
step of disconnecting the load from one side of an electrochemical cell of the
disclosure
that can operate using a single electrolyte solution. The method further
comprises the step
of reconnecting the load. A further embodiment includes a capacitor, prepared
by the
process of alternatively disconnecting and reconnecting the load from at least
one of the
current collector or anode in the electrochemical cell.
[0048] An additional embodiment that can operate with a single electrolyte
solution is an
electrochemical cell comprising a single aqueous electrolyte solution in
contact with a
non-metallic current collector, an oxidant, and a metal solid wherein current
travels from
the metal solid to the current collector via a load, and wherein the pH is
greater or equal to
12. As an example, the non-metallic current collector can be carbon foam, the
oxidant can
be a peroxydisulfate salt, and the metal solid can be aluminum.
- 13 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[0049] An additional embodiment that can operate with a single electrolyte
solution is an
electrochemical cell comprising a single aqueous electrolyte solution in
contact with a
non-metallic current collector, an oxidant, and one or more anodes wherein
current travels
from the one or more anodes to the current collector via a load, and wherein
the pH is
greater or equal to 10.
[0050] Suitable anodes include metals in Column 13 of the Periodic Table and
their
alloys. These metals and alloys include, for example, aluminum, gallium,
indium and
thallium, as well as alloys comprising at least one of these. One example
metal is
aluminum, such as aluminum in the form of foil. The anodes can also be
separated by an
insulator. In some embodiments, the pH is greater or equal to 12.
[0051] An example of an electrochemical cell that can operate with a single
electrolyte
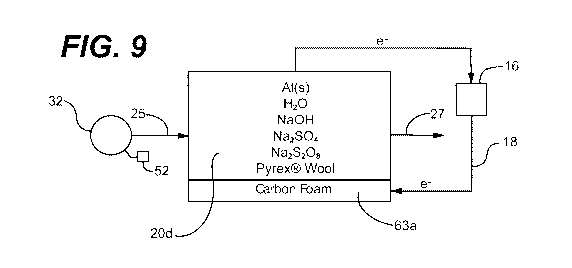
solution is provided in Example 7 and is illustrated in FIG. 9.
[0052] An additional embodiment of the disclosure includes a method of making
an
electrochemical cell of the disclosure. The
method comprises 1) providing an
electrochemical cell comprising an anode, a current collector and a porous,
non-
conductive spacer between the anode and the current collector; and 2)
contacting the cell
with a single electrolyte solution. The cell can be contacted with electrolyte
solution, for
example, by spraying the electrolyte solution onto the cell. The electrolyte
could also be
applied in bulk such as by immersion. The electrolyte solution could also be
applied to
the cell as droplets via drip or as an atomized mist. The electrolyte can also
be supplied in
bulk compartments, or in any combinations of the techniques provided herein.
[0053] A further embodiment includes a method, such as a method of operating
an
electrochemical cell in flow mode, that comprises 1) providing an
electrochemical cell
according to any of the embodiments above and that comprises a single
electrolyte
- 14 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
solution, 2) operating the cell to produce electricity, hydrogen, or both
electricity and
hydrogen (such as when the cell is connected to a load), and 3) providing
additional
electrolyte solution (or one or more components thereof) to the cell during
its operation.
Such an embodiment can further comprise 4) withdrawing spent electrolyte
solution (or
one or more components thereof) from the cell during its operation, for
example,
simultaneously with providing the additional electrolyte solution (or one or
more
components thereof) to the cell. The electrolyte solution in these embodiments
may have
any composition appropriate for the cells as described herein. Components of
the
electrolyte solution can include, for example, an oxidant, a metal salt, and a
base. A
further embodiment includes the method performed on a battery that comprises
the cell.
[0054] In other aspects of the disclosure, electrochemical batteries
comprising
electrochemical cells of the disclosure are provided. An electrochemical
battery contains
one or more cells and is electrically connected to a load. The load could be
the resistance
in a wire or it could be to an application, or both. The electrochemical
batteries of the
disclosure may be used to generate electricity through the load, hydrogen, or
both. The
battery can be configured, for example, to favor electricity or hydrogen.
Hydrogen
production may be controlled by adjusting pH and the specific surface area of
the anode.
Starting with an alkaline solution, as the pH increases and the larger
specific surface of the
anode used, the more hydrogen produced. For example the surface area of the
aluminum
may be increase by folding over an aluminum screen multiple times. Electricity
may be
favored when oxidant concentration is increased and the solution is made
increasingly
alkaline.
[0055] Embodiments of the disclosure further include methods of delivering
electricity,
hydrogen, or both electricity and hydrogen, produced by the cells or batteries
disclosed
- 15 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
herein to an application. Such applications can include, for example, cell
phone towers,
vehicles and fuel cells.
[0056] Without being bound by theory, the following explanation is believed to
explain
how some embodiments of the electrochemical cells and batteries of the
disclosure work.
For example, in many embodiments the anode is aluminum, the cathode is a
catholyte in
contact with a carbon foam current collector, and the catholyte is an
electrolyte comprising
water, sodium sulfate, sodium hydroxide, and sodium peroxydisulfate where the
carbon
current collector and anode are stacked and separated by a porous, non-
conductive spacer
and each stack is electrically connected by a conductor such as copper wire.
Such a
configuration can generally be seen in FIG. 1 and FIG. 2.
[0057] Without being bound by theory, in such embodiments, the aluminum is
oxidized
per equation (A) at the anode and persulfate reduction occurs at the surface
of the current
collector via equation (B)
2A1(s) 2A13+ + 6e- (A)
2Na2S208(aq)+ 4Na0H(aq) + 4e- ¨> 4Na2SO4(aq) + 4(OH)- (B)
[0058] There are, however, two extra electrons available from the oxidation of
aluminum.
In many embodiments, it is observed that protons are reduced to form hydrogen
gas at the
aluminum anode. Thus, it is further believed that water dissociates to form
El+ and OH-
and then the two extra electrons are available to reduce H+ into hydrogen gas
and such
hydrogen gas evolution is observed at the aluminum anode and not the current
collector
where oxidant is reduced. It is also possible that the hydroxide ion itself
may be
dissociating to form H+ because the same hydrogen evolution is observed when
the
electrolyte is ethanol. Accordingly, and as disclosed previously, the
electrolyte could also
be an alcohol and water or an alcohol with one example being ethanol.
- 16 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[0059] In view of the above, the current collector could be characterized as a
"cathode
current collector," because it distributes electrons that reduce oxidant
within the
electrolyte solution at the surface of the current collector (according to
equation B above),
wherein the electrolyte solution can be characterized as a catholyte. The
cathode current
collector can be, for example, embedded in or otherwise suitably contacting
the catholyte,
wherein the catholyte is the source of oxidant for reduction at the cathode
current
collector.
[0060] In FIG. 1, a series of three electrochemical cells are shown. Each cell
contains an
anode 100, such as aluminum in the form of a sheet or screen. The anode 100 is
in
physical contact with a porous, non-conductive spacer 110. The spacer is a
screen that
prevents physical contact between the anode and the current collector 120. The
current
collector is often a carbon foam and the spacer may be surgical tape, for
example, or vinyl
coated polyester. The stack 160 of anode 100, current collector 120 and spacer
110 may
be optionally wrapped in surgical tape for physical integrity. Each cell is in
electrical
contact with an adjacent cell wherein the anode 100 and the current collector
120 of
adjacent cells are in electrical contact via a conductor, such as copper wire
130.
[0061] Electrolyte is deposited onto the cell, but the cell of FIG. 1 is not
immersed in the
electrolyte. The electrolyte may be sprayed on or delivered via atomization or
compartmentalized. Often this is done so when carbon foam is the current
collector, the
carbon foam is saturated. In other embodiments, the cell is immersed in
electrolyte
solution. In a wet condition, the electrolyte acts as a catholyte such that
reduction occurs
in the solution when a circuit is made.
[0062] FIG. 2 represents a battery wherein the cell components of FIG. 1 in
series are
further connected to a Load 150 via a conductor, such as copper 140. In this
state, when
- 17 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
electrolyte is deposited onto the battery, current and/or hydrogen is
produced, with
hydrogen reduction occurring at the anode. Depending on the pH, the surface
area of the
anode and the amount of oxidant used, one can determine whether the battery
produces
primarily electricity, hydrogen, or a combination of the two. The Load 150
could be an
application such as cell tower or other grid application. Alternatively, when
hydrogen is
produced, the hydrogen can be collected at each anode for delivery to, for
example a fuel
cell or to a furnace or engine for burning hydrogen.
[0063] Additional embodiments described below include certain membraneless
electrochemical cells that contain separate first and second electrolyte
solutions at the
cathode and anode, respectively; batteries comprising electrochemical cells of
the
disclosure; methods of delivering hydrogen, electricity or both with the cells
and batteries
to applications; methods for boosting current in electrochemical cells of the
disclosure;
and various methods related to these embodiments.
[0064] For example, one embodiment of the disclosure includes an
electrochemical cell
comprising:
a. a cathode;
b. an anode adjacent to the cathode at a distance;
c. a first polar electrolyte solution in contact with the cathode and disposed
within the
distance comprising an oxidant;
d. a second polar electrolyte solution in contact with the anode and disposed
within
the distance comprising a suitable metal ion; and
e. a separation agent;
- 18 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
wherein the first and second electrolyte solutions are in contact with each
other and are
immiscible, and wherein there is no membrane in between the first and second
electrolyte
solutions.
[0065] In this embodiment, each polar electrolyte solution may comprise a
porous
stabilizer such as borosilicate. The first polar electrolyte solution may be
aqueous and
may also comprise a base such as KOH, NaOH, Ca(OH)2, Li0H, RbOH, Cs0H,
Sr(OH)2,
or Ba(OH)2. The pH of the first polar electrolyte solution may be, for
example, between
about 8 and about 14 or between about 11 and about 14. The oxidant may be, for
example, a Vanadium ion, 52082- or C10. An example separating agent in this
embodiment includes a salt, such as a calcium chloride or sodium sulfate.
[0066] The electrochemical cell may be configured to operate in flow mode. It
may
comprise, for example, an inflow solution comprising a base (such as sodium
hydroxide),
an oxidant (such as S2082) and a separation agent (such as sodium sulfate).
Outflow from
the cell may include a base (including an aqueous solution of a base) and
sodium sulfate,
where the base may be sodium hydroxide.
[0067] A current collector may be placed within the first electrolyte
solution, the second
electrolyte solution, or in both. Example current collectors include metals as
well as non-
metals such as carbon foam. The electrochemical cell may also comprise glass
wool
placed in between the first and second polar electrolyte solutions.
[0068] In some embodiments, the second polar electrolyte solution comprises an
alcohol.
It may be an alcoholic solution that further comprises a base such as KOH or
NaOH. The
pH of the second polar electrolyte solution may be, for example, between about
8 and
about 14 or between about 11 and about 14. Suitable metal ions in the second
polar
electrolyte include Zn2+ and Al3+.
- 19 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[0069] In some embodiments, the separation agent is a salt (such as CaCl2 or
sodium
sulfate) and the alcohol in the second polar electrolyte solution is ethanol,
methanol, or
both. In some embodiments, the second polar electrolyte comprises an alcohol
and is
configured to operate in flow mode with an inflow stream comprising a polar
solution
comprising an alcohol (such as ethanol, methanol, or both), a base (such as
sodium
hydroxide), a separation agent, and a metal capable of dissociating into a
suitable metal
ion (such as Al3+). An outflow stream for the cell may comprise an alcohol
(such as
ethanol), a base (such as sodium hydroxide) and a separation salt (such as
sodium sulfate).
[0070] The electrochemical cell described here may, for example, generate
hydrogen gas
in the second electrolyte solution and may direct the gas to a hydrogen
compressor. A
further embodiment of the disclosure therefore includes a battery system
comprising one
or more of the electrochemical cells and a hydrogen compressor. Such a system
may be
used, for example, to power a process application such as a fuel cell.
[0071] Another embodiment of the disclosure includes an electrochemical cell
comprising:
a. a cathode;
b. an anode adjacent the cathode at a distance;
c. a first polar aqueous electrolyte solution in contact with the cathode and
disposed
within the distance comprising 52082-;
d. a second polar electrolyte alcoholic solution in contact with the anode and
disposed
within the distance comprising Al3+; and
e. borosilicate within both the first and second electrolyte solutions;
- 20 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
wherein the first and second electrolyte solutions are in contact with each
other and are
immiscible, and wherein there is no membrane in between the first and second
electrolyte
solutions.
[0072] The first polar electrolyte solution and second polar electrolyte
solution may be of
different densities, where the first electrolyte solution may comprise a
halide salt (such as
CaCl2) and the second electrolyte solution may comprise a metal sulfate salt
(such as
Na2SO4). The second polar electrolyte alcoholic solution may comprise, for
example,
ethanol or methanol.
[0073] In some embodiments, the pH of the first electrolyte and second
electrolyte
solutions can be adjusted, for example, to between about 11 to about 13 each.
Each
solution may comprise a base, such as sodium, calcium, or potassium hydroxide.
In some
embodiments, the cathode is copper (such as a copper brush), carbon, or both
and the
anode is aluminum. The borosilicate may be, for example, Pyrex wool. In some
embodiments, the borosilicate has a pore size of about 8 microns.
[0074] This electrochemical cell may be configured to run in flow mode. An
additional
embodiment includes an electrochemical battery comprising one or more of the
cells.
When the battery comprises more than one of the electrochemical cells, the
cells may be
aligned, for example, in parallel geometry and arranged in a voltaic pile. The
electrochemical cell or battery could deliver electricity to a process
application, including
solar farms, wind farms, household appliances, consumer goods, and toys.
[0075] Another embodiment of the disclosure includes an electrochemical cell
comprising:
a. a non-metallic cathode;
b. a non-metallic anode adjacent the cathode at a distance;
-21 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
c. a first polar aqueous electrolyte solution in contact with the cathode and
disposed
within the distance comprising S2082-; and
d. a second polar electrolyte alcoholic solution in contact with the anode and
disposed
within the distance comprising a metal solid;
wherein the first and second electrolyte solutions are in contact with each
other and are
immiscible, and wherein there is no membrane in between the first and second
electrolyte
solutions.
[0076] In some embodiments, the metal solid is dispersed through the second
electrolyte
solution in powder form while borosilicate is placed within both the first and
second
electrolyte solutions. Example metals include zinc and aluminum. Metal in
powder form
may have, for example, an average particle size less than about 5 microns, or
an average
particle size between about 5 and about 30 microns. Non-metallic cathodes and
non-
metallic anodes can be made of carbon foam, for instance. A further embodiment
includes
a method of boosting current in an electrochemical cell comprising the steps
of adding
oxidant to the second electrolyte solution of this electrochemical cell.
[0077] Another embodiment of the disclosure includes an electrochemical cell
comprising:
a. a cathode;
b. an anode adjacent to the cathode at a distance;
c. a first aqueous electrolyte solution in contact with the cathode and
disposed within
the distance comprising an oxidant;
d. a second polar electrolyte solution in contact with the anode and disposed
within
the distance comprising a metal and an oxidant; and
e. a separation agent;
- 22 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
wherein the first and second electrolyte solutions are in contact with each
other and are
immiscible, and wherein there is no membrane in between the first and second
solutions.
[0078] In some embodiments, the second polar electrolyte solution is an
alcoholic solution
such as ethanol or methanol. Also in some embodiments, the oxidant is 82082-
or sodium
peroxydisulfate, or both, the metal is aluminum, the separation agent is
sodium sulfate,
and the cathode and anode are carbon foam. A porous stabilizer (such as glass
wool,
borosilicate, or both) may optionally be provided in the first and second
electrolyte
solutions.
[0079] The electrochemical cell may be configured to operate in flow mode. The
cell may
comprise an inflow stream that comprises an aqueous electrolyte solution and
optionally
also an oxidant (such as sodium peroxydisulfate or a solution comprising
peroxydisulfate
anion or both). Thus, an embodiment of the disclosure is a method which
comprises
providing additional oxidant to the electrochemical cell. The oxidant (such as
Na2S208)
may be provided in an aqueous basic solution, for example, where the base may
be NaOH.
The oxidant may also be provided as a solid. The cell may further comprise an
aqueous
solution that outflows from the cell and that may comprise, for example, metal
sulfate.
[0080] In some embodiments, the cell may produce between about 10 Watt-
hours/(kg of
electrolyte + anode metal) and about 680 Watt-hours/(kg of electrolyte + anode
metal).
When measured per kg of electrolyte, the cell may produce, for example,
between about
Watt-hours/kg of electrolyte and about 100 Watt-hours/kg of electrolyte, and
such as
between about 40 Watt-hours/kg of electrolyte and about 80 Watt-hours/kg of
electrolyte.
[0081] The following disclosure provides further description of the
embodiments detailed
above and other embodiments of this disclosure.
- 23 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[0082] Various embodiments of the disclosure, such as ones mentioned above,
include an
electrochemical cell that contains a single electrolyte solution. In some
embodiments, a
single electrolyte solution contains a metal solid, water, base, an oxidant,
and a current
collector. The solution is basic in some embodiments. Electrical contact is
made between
the current collector, such as carbon foam, and the metal solid via an
external load. A
common metal example is aluminum, such as aluminum foil, a base, such as
sodium
hydroxide, and the oxidant added may be sodium peroxydisulfate, which in turn
dissociates. When operating, the peroxydisulfate reduces to sulfate.
Additional sulfate,
such as by the addition of a metal sulfate such as sodium sulfate, may be
added. A porous
stabilizer, such as glass wool or a borosilicate may be used. In such
embodiments, there is
only a single electrolyte solution and it may be operated in a flow mode
whereby oxidant
is refilled into the cell. The added oxidant may be as an aqueous solution
such as with a
base like sodium hydroxide or added as solid such as with granular sodium
peroxydisulfate. As sodium sulfate forms in the cell, it may optionally be
removed by an
apparatus for desalinization or by other mechanisms.
[0083] Supercharging may also be achieved with two electrolyte solutions. In
these
embodiments, oxidant, such as sodium peroxydisulfate is added to the second
electrolyte
solution. In these embodiments, the metal in the second electrolyte solution
can be
aluminum and the solution is an alcoholic solution such as methanol, ethanol,
or both. A
porous stabilizer such as glass wool or a borosilicate may also be used. A
base such as
sodium hydroxide is present as is a separating agent such as sodium sulfate.
The first
electrolyte solution in such embodiments can be aqueous and is kept basic such
as with
NaOH. An oxidant such as sodium peroxydisulfate is used and a metal sulfate
such as
sodium sulfate is optionally added. A porous stabilizer such as glass wool or
a
- 24 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
borosilicate may also be used. In such embodiments, peroxydisulfate, or
another suitable
oxidant, is added to the second electrolyte solution and this addition causes
a boost in
current production. Such current boosts may be on the order of 50%.
[0084] The power produced by the single-solution embodiments may be, for
example,
between about 10 Watt-hours/(kg of electrolyte + anode metal) and about 680
Watt-
hours/(kg of electrolyte + anode metal). When measured per kg of electrolyte,
the cell
may produce, for example, between 10 and 100 Watt-hours/kg of electrolyte,
including
between about 40 and about 80 Watt-hours/kg electrolyte and further including
between
about 10 and about 60 Watt-hours/kg electrolyte. When measured against the
surface area
of the metal solid used, such as with aluminum, power densities of between
about 600 and
about 1000 mW/cm2 are observed.
[0085] One would typically not expect an electrochemical cell to operate with
a single
electrolyte due to the possibility of creating an electrical short. However,
in the system
presented, it has been possible to demonstrate substantial boosts to current
for sustained
periods of time without shorting. When the electrochemical cell's circuit is
opened and
subsequently closed, such as by disconnecting a lead from one end of the cell
and then
reconnecting it, a spike in current is observed up to about 800 mA.
[0086] Without being bound by theory, it is believed that upon opening the
circuit in a
single electrolyte system, the anode becomes negatively charged and is
surrounded by an
ionic double layer and, for example, when considering a capacitor based on the
electrochemical cell of FIG. 9, the sodium and sulfate ions make up the ionic
double layer.
The sodium concentrates next to the negatively charged surface (the stern
layer) and both
the sodium and sulfate ions form a diffuse layer out from the surface. Such a
capacitor is
in fact a self-charging capacitor. With respect to the cell of FIG. 9, for
example, when the
- 25 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
battery circuit is opened, the S2082- anion or 2H+ cations continue to
interact with the solid
aluminum atoms while trying to place them into the unstable +2 oxidation
state. To
compensate for this instability, the oxidized aluminum atoms shed a third
electron to the
solid aluminum, but because the circuit is open, the solid aluminum cannot
transfer the
electron to the current collector. This effect negatively charges the solid
aluminum, and to
maintain charge neutrality, a classical double layer distribution of ions is
formed, with the
cations packed near the solid aluminum surface. When the circuit is closed
these stored
electrons are shipped to the current collector, but again because of the need
to maintain
charge neutrality, they are not all shipped at once, but rather released with
a peak initially
that gradually declines over time owing to the gradual redistribution of ions
in the double
layer which relaxes on an electro-chemical diffusion gradient. By staggering
the opening
and closing of circuits, multiple anodes can be employed to maximize the
current (while
one anode circuit is open it charges and another can be closed and
discharging).
[0087] Without being bound by theory, aluminum can exist in the non-negative
oxidation
states +3, +2, +1 and 0, but only the +3 and 0 states are energetically stable
under standard
battery operating conditions, with the 0 state being the solid aluminum phase.
When a
strong oxidant such as S2082- or 2H+ reacts with an aluminum atom in the 0
state, it strips
2 electrons from the solid aluminum atom (reducing itself to 2S042-) while
trying to put
the aluminum atom into the energetically unstable +2 oxidation state. Because
this state is
energetically unfavorable, the aluminum atom sheds a third electron to put it
in the stable
+3 state. The third electron is conducted through the solid aluminum to the
load and
subsequently to the current collector, wherein the catholyte reduces another
S2082- anion.
[0088] By comparison, Zn can exist in the non-negative oxidation states +2, +1
and 0, but
only the 0 and +2 states are energetically favored. Thus when the S2082- anion
reacts with
- 26 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
a zinc atom in the 0 oxidation state it strips two electrons from the zinc
atom putting it in
the stable +2 state, and no current is generated. However, if there is an
oxidant in the -1
state which is capable of striping a single electron from the zinc, then as in
the aluminum
case, a current can be generated.
[0089] In such single-electrolyte systems, pairs of oxidants and reductants
may be selected
such that the oxidant removes sufficient electrons to create an unstable
oxidation state in
the reductant which may spontaneously transition to a stable oxidation state
with the
release of one or more additional electrons. In such pairs, the unstable
oxidation state is a
lower oxidation state than the stable oxidation state. The stable oxidation
state may have
an oxidation state that is +1 or +2 or more compared with the unstable
oxidation state. In
aluminum, for example, the stable oxidation state, +3 is one more (+1) than
the unstable
oxidation state.
[0090] In many embodiments of such single-electrolyte electrochemical systems,
multiple
anodes may be used with a single current collector. For example, when the
anode is
aluminum, aluminum foil packets may be separated from each other such as with
an
insulator to create multiple aluminum anodes. If particulate aluminum is used,
such
individual particles may act as multiple anodes.
[0091] The pH can also be used to control current. When the pH is neutral, the
S2082- is
unable to oxidize the solid aluminum because an A1203 film forms on the solid
surface.
When the system is very alkaline, such as greater than pH of 12 or even
higher, the OFF
anion destroys the aluminum oxide film and allows the S2082- anion to thereby
create a
current. By adjusting the pH, either with the addition of OH- ions, such as
from NaOH, or
by the addition of H+ ions, such as with the addition of sulfuric acid, the
availability of
- 27 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
OH- and thus control the current can be regulated. Lower pHs may also work,
such as at
around 10, provided the surface is sufficiently activated.
[0092] In other embodiments, electrochemical cells and batteries of the
disclosure operate
without the need for membranes or other devices to separate a first
electrolyte solution (at
the cathode) from a second electrolyte solution (at the anode). When the terms
"membraneless" or "without a membrane" or "wherein there is no membrane" or
words to
that effect are used, what is meant is that there is no membrane or other kind
of separator
between the first and second electrolyte solutions (and third electrolyte
solutions in those
embodiments).
[0093] Electrochemical batteries of one or more cells, including greater than
one cell, may
be prepared by combining electrochemical cells of the disclosure in parallel
or in series.
Examples include a voltaic pile of cells. Such cells and batteries may be used
to deliver
electricity to process applications such as solar farms and wind farms,
hydrogen
compressors, vehicles, such as electric vehicles, electrical grids, household
appliances,
consumer products, and toys.
[0094] Cathodes and anodes in electrochemical cells in this disclosure,
including in this
embodiment and other embodiments of electrochemical cells described herein,
may be
selected from suitable materials. Examples of suitable cathodes include steel,
carbon such
as in the graphite allotrope of carbon, carbon impregnated with a metal, and
carbon foam.
Conducting carbon cloth (which is also referred to as carbon foam), for
example, is a
suitable cathode for many embodiments and is a conducting material. Suitable
anodes for
embodiments of the membraneless cells with multiple electrolytes include
platinum, zinc,
lithium, nickel, calcium, magnesium or aluminum as well as non-metallic
materials such
as carbon, including carbon foam.
- 28 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[0095] First and second electrolyte solutions included in embodiments of the
disclosure
may be polar and of different densities. In many examples, one or more of the
first polar
electrolyte solution contains water and a separation agent. In these and other
embodiments
of the disclosure, the two polar electrolyte solutions are immiscible. When
the first
electrolyte solution comprises H20 and the second electrolyte solution
comprises ethanol,
methanol, or a combination thereof, the solutions would normally be miscible.
However,
the separation agent may be used to make such fluids immiscible. The
separation agent
may be added to a mixture of an alcohol and water and at sufficient
concentration it will
separate the two solutions and maintain their immiscibility. The separation
agent is often a
salt. In some embodiments, the solution is saturated with respect to the salt.
Examples of
salts include metal halides or ammonium salts such as sodium chloride,
magnesium
chloride, calcium chloride, lithium chloride and ammonium chloride. Other such
salts
include sodium sulfate, calcium sulfate, potassium sulfate, and ammonium
sulfate among
others. The same or different salts may be present in the first or second
electrolyte
solutions. For example, sodium sulfate may be present in both. In other
embodiments, a
salt such as sodium sulfate or sodium chloride may be present in the first
electrolyte
solution and ammonium chloride in the second electrolyte solution. In still
other
embodiments calcium chloride may be present in the first electrolyte solution
and sodium
sulfate in the second. The salts are often saturated in the respective
solution at or near their
solubility limit.
[0096] In many cells of the disclosure, the first electrolyte solution is an
aqueous solution
and the second electrolyte solution is an alcoholic solution. The solution at
each electrode
must contain the necessary components so that oxidation-reduction will occur,
thus
- 29 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
generating electricity. Suitable alcohols for use in the second electrolyte
solution include
methanol and ethanol.
[0097] Examples of polar solvents for use in first or second electrolyte
solutions may be
found in Table 1 above. The solvent for the particular system selected should
be of
sufficient dipole moment so as to dissociate the corresponding salts placed in
the solvent.
In some embodiments, a strong acid, such as sulfuric acid (e.g., 1M) may be
used to make
the first polar electrolyte solution acidic while the second polar electrolyte
solution is
neutral.
[0098] In other embodiments, both the first and second polar solutions are
basic. These
solutions may be made basic such as by the addition of a base such as Li0H,
RbOH,
Cs0H, Sr(OH)2, Ba(OH)2, NaOH, KOH, Ca(OH)2, or a combination thereof To
complete
an electrochemical circuit, an oxidant is added to the first polar electrolyte
solution and a
suitable metal ion is added to the second polar electrolyte solution. A common
polar
solvent used for the first polar electrolyte solution is water. Common
solvents used for the
second electrolyte solution include ethanol, methanol, acetonitrile, or
combinations
thereof.
[0099] Examples of oxidants can be found in Table 2 above and examples of
compounds
which may dissociate into suitable metal ions in solution can be found in
Table 3 above.
Any metal ion oxidant pair may be chosen provided the metal oxidation by the
oxidant is
thermodynamically spontaneous. Metal ions which are commonly used in the
disclosure
include Al, Zn, Sn, and V.
[00100] FIG. 3 illustrates an embodiment of an electrochemical cell that
comprises
first and second electrolytes. The electrochemical cell 10 includes a cathode
12 and an
anode 14 separated by a first electrolyte solution 20 and a second electrolyte
solution 22
- 30 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
such that the first electrolyte solution 20 is in contact with the cathode 12
and such that the
second electrolyte solution 22 is in contact with the anode 14. The first
electrolyte solution
20 and the second electrolyte solution 22 are immiscible and in contact with
each other
and thus can enable ion and electron exchange (e.g., H+ and e-) between the
anode 14 and
the cathode 12. Each cell 10 may be electrically connected to a load 16 by a
circuit 18 to
enable a current flow via the circuit. Note that the vertical lines connecting
the electrolyte
solutions and the cathode and anode electrodes in the schematic are not
conduits but are
merely to aid in the viewing of the schematic.
[00101] In certain embodiments, the first electrolyte solution 20 may be a
positive
electrolyte or catholyte, and the second electrolyte solution 22 may be a
negative
electrolyte or analyte (and immiscible). In many embodiments, the densities of
the first
electrolyte solution and the second electrolyte solution are different with
the first
electrolyte solution 20 being denser than the second electrolyte solution 22
such that when
the cell 10 is oriented vertically with cathode 12 at the bottom, the buoyancy
effect causes
the second electrolyte solution 22 to layer above the first electrolyte
solution 20.
[00102] In many embodiments, the cell 10 may optionally be configured to
run in
flow mode so as to support a flow battery for example. In such a battery,
electrolyte
solutions are provided to the cell during the operation of the battery
continuously during
operation. For example, the first electrolyte solution 20 and the second
electrolyte
solution 22 may flow into the cell 10 and between the cathode 12 and the anode
14 from a
first source, such as a tank, 30, or other suitable storage device, and a
second source, such
as a tank, 32, or other suitable storage device, respectively, as shown in
FIG. 3 via
conduits 21 and 25 respectively. The first electrolyte solution 20 and second
electrolyte
solution 22 may further flow out of the cell 10 via conduits 23 and 27
respectively. They
-31-
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
may be directed to waste or to other tanks. In some embodiments, flow could be
reversed
from said other tanks to recharge cell 10. The flows may be generated by pumps
50, 52 or
by capillarity, reverse osmosis, a ratchet, swelling pressure, or gravity. The
flows of the
first electrolyte solution 20 and the second electrolyte solution 22 may be
maintained
within a laminar flow regime. In alternative embodiments, the first
electrolyte solution 20
and the second electrolyte solution 22 may not flow through the cell 10 but
may be
replaceable.
[00103] When an electrolyte solution is prepared, typically an
electrolyte, often a
solid, is disposed within a solvent which then becomes an electrolyte
solution. For
example, when an electrolyte is disposed within a solvent where it can
dissolve, the
dissolution of the electrolyte solid will create ions and, if they dissociate
sufficiently, the
solvent becomes an electrolyte solution. In addition, other components are
added to the
solvent so that oxidation will occur at the anode and reduction at the
cathode. Examples of
such a component is zinc metal. When added at the anode of an operating
electrochemical
cell, zinc will oxidize to Zn2+. On the cathode side, one such component
example is
NH4V03 which dissolves and dissociates to produce V5+, which will be reduced
to V4+ in
an operating electrochemical cell. In many such embodiments of the disclosure,
the first
electrolyte solution comprises a component which dissociates into an ion
selected from
C10-, Fe3+, V5+, Br2, and S2082-, which ions are reduced at the cathode. In
these and other
embodiments, the second electrolyte solution comprises a component which
oxidizes into
an ion selected from Lit, ca2+, Ar+, mg2+, v2+, zn2+, siu-32+
, [Zn(CN)4]2-, and [Zn(OH)4]2,
which ions result from oxidation at the anode.
[00104] In some embodiments, the cathode, vanadium undergoes reduction
from
V5+ to V4+. In that embodiment, at the anode, zinc is oxidized from Zn(s) to
Zn2+. To
- 32 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
enable the flow of positively charged ions, a methanol solvent with zinc solid
on the anode
side is further charged with ammonium chloride. The ammonium chloride
dissolves and
dissociates sufficiently to provide NH4 + in solution as a positively charged
ion and Cl- as a
negatively charged ion. On the cathode side, positively charged ions are
provided by
adding both sulfuric acid (H2SO4) and sodium sulfate (Na2SO4) to an aqueous
V5+
solution. The dissolution and dissociation into H+ and Na + provides
positively charged
ions and S042- as a negatively charged ion on the cathode side of the
electrochemical cell.
In addition, the sodium sulfate prevents the mixing of the first and second
electrolyte
solutions and maintains their immiscibility. Further, since water is denser
than methanol,
buoyancy forces cause the methanol solution to layer on top of the denser
aqueous
solution. This layering of immiscible fluids (salt water is immiscible with
methanol or
ethanol) effectively and advantageously eliminates the need for a membrane for
separation. Such embodiments may be configured for flow or for no-flow
operation as
described further herein. Further, in such embodiments the zinc may be in
contact with a
conducting material such as conducting carbon and the cathode solution may
also be in
contact with such a conducting material.
[00105] In some embodiments, the anode is aluminum and the cathode is
carbon or
steel, the first electrolyte solution contains water and C10-, and the second
electrolyte
solution contains ethanol or methanol. In such embodiments, for example, each
electrolyte
contains a base such as NaOH, and a salt, LiC1 which results in immiscible
electrolyte
solutions. The voltage supplied by such an electrochemical cell is between 1.5
and 2.1
volts. Such an electrochemical cell may create amperages of between about 0.1
and about
0.4 amps including about 0.2 and about 0.3 amps. Examples of components
providing
- 33 -
CA 03054957 2019-08-28
WO 2018/169855
PCT/US2018/021981
C10- include Na(C10) and Ca(C10)2. In such a cell, C10- will be reduced at the
cathode
according to equation 1:
C10- + H20 + 2e- ¨> C1-(aq) + 20H- (aq) EQ. 1
[00106] The
second electrolyte may contain a component that is a metal that
oxidizes, such as aluminum oxidizing to Al3+ as per equation 2:
Al(s) ¨> Al3+(aq) + 3e- EQ.2
[00107] Another
anode choice may be magnesium which oxidizes per equation 3:
Mg(s) ¨> Mg2+(aq) + 2e- EQ. 3
or Vanadium which oxidizes per equation 4:
V(s) ¨> V2 + (aq) + 2e- EQ. 4
[00108] In
some embodiments, the anode is lithium solid and the solvent is
propylene carbonate and dimethoxyethane. The cathode may be a suitable metal
such as
copper, with sodium sulfate also added. In these embodiments, both the anode
and cathode
electrolyte solutions contain a salt. An example salt is a metal halide salt
such as MgCl2.
When MgCl2 is used in the electrolyte solutions, a voltage of 3.15 V and a
current of 0.1
A/cm2 may be achieved at 1 ohm resistance. When amperages are recorded as
A/cm2
what is meant is amperage per areal area as opposed to specific area. For
example, a 2
square centimeter piece of carbon foam has a much higher specific area than
its 2 square
centimeter areal area.
[00109] In
other embodiments, the first polar aqueous electrolyte solution contains
S2082-, NaOH, a salt, such as a metal sulfate or metal halide salt such as
CaCl2, and a
metal cathode such as a copper, such as a copper brush, carbon foam, or a
combination
thereof. An example of a metal sulfate salt is Na2SO4. The S2082- ion
originates from a
salt which dissociates into that ion in, for example, the first electrolyte
solution. The salt
- 34 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
may be, for example, Na2S208. The copper may be in wire form such as a brush,
for
example. The second polar alcoholic electrolyte solution, for example of a
different
density that the first electrolyte solution, contains an alcohol such as
methanol, ethanol, or
both, NaOH, a salt such as a sulfate, including metal sulfates such as Na2SO4.
An example
anode is a metal anode such as aluminum. Alternatively, the anode may be a non-
metallic
material such as carbon foam and a metal solid, such as in a powder form, may
be
dispersed within the anode solution. Examples of such metal powders, include
aluminum
and zinc. The metal powder in the solution is oxidized by the action of the
electrochemical cell in a similar manner as it would oxidize if the anode
itself were the
metal.
[00110] In some embodiments, the first polar solution is aqueous and
contains a
separation agent such as a salt like CaCl2 or Na2SO4. The cathode in contact
with the first
electrolyte solution in such embodiments may be a metal like copper or a non-
metal like
carbon foam. A stabilizer such as glass wool or a borosilicate such as Pyrex
may be
used. The oxidant in such solutions is often 52082- which may be sourced from
Na2S208,
for example. In such embodiments, the second polar solution is often an
alcohol such as
ethanol or methanol. The anode in contact with the second electrolyte
solutions in such
embodiments is often aluminum or carbon foam. Separation agents, such as
Na2SO4 are
often deployed at or near saturation concentrations in the first polar
electrolyte solution,
the second polar electrolyte solution, or both. Suitable metal ions are often
Aluminum
such as Al3+ which may be sourced, for example from Aluminum foil or Aluminum
powder. Zn2+ may also be used, for example, in such embodiments. Both the
first and
second polar electrolyte solutions can be rendered basic by a base such as
NaOH where
the pH is greater than and often between 11 and 14. In many such embodiments,
hydrogen
- 35 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
gas is generated in the second polar electrolyte solution. Such gas may be
vented and
delivered to a process application like a fuel cell. The hydrogen can be fed
to a hydrogen
compressor prior to delivery, for example. Electricity generated from one or
more cells
may be used to power the hydrogen compressor. The electrochemical cell may be
run in a
flow mode in such operations where electrolyte solution is replenished and
spent solution
removed.
[00111] When being replenished, the inflow solution may be an aqueous
solution
comprising a base, an oxidizing agent, and a separation agent for the first
polar electrolyte
solution and comprising an alcohol, a metal capable of dissociating into a
suitable metal
ion, a base and a separation agent. The outflow solutions comprise an aqueous
solution of
base and separation agent for the first electrolyte solution and alcohol,
base, and
separation agent for the second polar electrolyte solution. When the suitable
metal ion is
Al, the outflow may also contain A1203.
[00112] In certain aspects of these embodiments, the pH of each solution
is made
basic by the use of NaOH, KOH, Ca(OH)2, or another base. With such a base, the
pH may
be adjusted to between about 8 and about 14, between about 9 and about 14,
between
about 10 and about 14, between about 11 and about 14, between about 12 and
about 14,
between about 11 and about 13 and all values between about 8 and about 14
including
about 8,9, 10, 11, 12, 13, and 14.
[00113] The electrochemical cells may further contain a porous stabilizer.
The
porous stabilizer allows for the passage of fluid and also can be placed
across the first and
second electrolyte solutions and help maintain the separation between the
solutions
including during turbulent movement. A porous stabilizer may be any porous
media,
Examples of porous stabilizers are glass wool and borosilicates including
Pyrex . The
- 36 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
porous stabilization may be used in each of the first and second electrolyte
solutions and it
enables the electrochemical cell containing the two electrolyte solutions to
spin, bounce or
rotate rapidly with little fluid displacement. Examples of borosilicate
include such
borosilicate with a pore size of about 8 .m. Pyrex wool is one such
borosilicate. Such
electrochemical cells may be run in a flow or non-flow mode. Such
electrochemical cells
can have superior current density and voltages than cells of the prior art.
For example,
electrochemical cells of these embodiments have been measured for 18 hours at
1 ohm
resistance with starting voltages and amperages of about 2.07 V and 0.16 A/cm2
and
concluding at about 1.55 V and 0.088 A/cm2.
[00114] The use of porous stabilizers affects some or all of the following
characteristics of batteries and electrochemical cells of the disclosure:
wettability
boundary conditions; no slip and slip boundary conditions; conductivity,
including
resistivity and friction; dispersivity or mixing between adjacent fluids;
porosity (e.g.,
relative volume for flow); tortuosity (e.g., length and complexity of
trajectories);
connectivity (e.g., species and electrical); particle size distribution (e.g.,
packing); relative
conductivity (e.g., multiphase resistivity); multi scale (e.g., discrete scale
separation);
surface absorptivity (e.g., double layer capacitance); surface reactivity
(e.g., pseudo
capacitance); diagenesis (e.g., dissolution or deposition); and swelling
(e.g., interfacial
forces). The porous media may include nanostructures or nanoparticles. Such
porous
media may be used, for example, at the cathode or anode. Further examples of
such
porous media include micro-porous or nano-porous graphite.
[00115] Separators or interconnects may be used to separate adjacent cells
to
prevent short circuiting such batteries but still provide for electrical
communication.
- 37 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
Stabilizers may also be used in between the first and second electrolyte
solutions of an
electrochemical cell.
[00116] When
the anode is non-metallic, such as carbon foam, and a metal, such as
aluminum or zinc, is added as a solid to the anode solution, it is often in
the form of a
powder and a common particle size is one where the average size is less than 5
microns. In
other embodiments, the average size is between about 5 microns and 30 microns.
In many
embodiments, the powder is dispersed throughout the solution so that the
solution appears
cloudy. Such a dispersion is useful for the performance of the cell.
Generally, the smaller
the particle, the longer it takes for the particles to settle out from the
dispersion in
accordance with Stokes' law. Thus, from a suspension stability stand point,
smaller
particles are favored.
[00117] At the cathode, the half-cell reaction for some such embodiments
is:
S2082- + 2e- ¨> 2S042- EQ. 5
whereas the half-cell reaction at the anode for some such embodiments is:
Al(s) ¨> Al3+ + 3e- EQ. 6
when aluminum is the anode or is suspended in the anode solution as a solid
and
Zn(s) ¨> Zn2+ + 2e- EQ. 7
is the half-cell reaction at the anode the anode itself is zinc, or is
suspended in the anode
solution as a solid.
[00118] In
these and other embodiments, hydrogen gas may be liberated by the
chemical reactions within the electrochemical cell under basic conditions.
Advantageously, and unlike with hydrogen production from petroleum products,
hydrogen
may be created without the liberation of CO2 or CO. The resulting hydrogen may
be
collected for storage or redistribution or routed to a second electrochemical
cell with a
- 38 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
platinum membrane for additional energy generation. For example, when the
suitable
metal ion is Al3+ and the second polar electrolyte solution contains a polar
solvent such as
a methanol, ethanol or both, hydrogen gas may be liberated as a result of
subsequent
reactions in such electrochemical cells. A suitable oxidant herein is 52082-.
[00119] Thus, there are three separate reactions that are occurring. In
one, protons
are being reduced to form hydrogen gas at the anode. In another, aluminum is
being
oxidized and forms NaAl(OH)4. Lastly, at the cathode, oxidant (such as S2082-)
is reduced
to 5042-.
[00120] The rate at which hydrogen gas is generated, for fixed
concentrations of
reactants, is a function of the distance between the copper/carbon and metal,
such as
aluminum, in the second polar electrolyte solution, for example, aluminum. The
greater
the distance, the lower the rate hydrogen gas is released and the lower the
current. In
addition surface area affects the rate in that the greater the surface area,
generally the
greater the rate of hydrogen produced. Hydrogen may be produced in a flow or
non-flow
mode.
[00121] In order for the electrochemical cell to produce electricity
spontaneously,
the metal anode is oxidizable by the oxidant and the oxidant is reduced at the
cathode.
When the electrochemical cell of the disclosure is further used to generate
hydrogen gas,
then a suitable metal ion is present in the anode side along with a base such
as NaOH in a
polar solution comprising for example, methanol or ethanol. Suitable metals
are those
which produce hydrogen when placed in contact with a base in the second polar
electrolyte solution. Such metals have a metal-metal ion redox potential that
is negative.
Examples of such fluids include alcohols such as methanol, ethanol, propyl
alcohol, or
isopropyl alcohol. Water is another such fluid such as in a mixture with an
alcohol,
- 39 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
however, in the presence of a separating salt, water tends to collect on the
cathode side of
an electrochemical cell of the disclosure. The amount of hydrogen that is
created from a
battery may be up to about 100kg of hydrogen per day including between about
10 kg and
100 kg and all values in between. Hydrogen gas may be collected for use in
such
electrochemical cells. A single electrochemical cell with 3cm2 Al metal placed
in the
second polar electrolyte solution was measured in an electrochemical cell set
up generally
in accordance with Fig. 8 to produce about 0.5 kg of the H2 per hour. A larger
surface
area metal placed in the second polar electrolyte solution will produce more
hydrogen.
Metals placed in the second polar electrolyte solution to produce suitable
metal ions may
be provided as sheets such as Aluminum sheets, folded over or serpentine in
shape may
also be provided. Other forms of Aluminum solid include powders such as in a
slurry, or
nano structures. The hydrogen may be stored for future use, such as under
compressed
conditions. Examples of uses of hydrogen includes fuel cells. Such fuel cells
may be used
to power vehicles, for instance.
[00122] In some embodiments of the disclosure, the current may be boosted
or
"super charged" by the addition of oxidant to the second polar electrolyte
solution. For
example, in electrochemical cells where aluminum is disposed with an alcohol
or aqueous
solution, sodium peroxydisulfate may be added to the alcohol solution to boost
the current
observed from the electrochemical cell. In still other embodiments, a second
polar
electrolyte solution is not needed to create such boosted current. In
electrochemical
batteries of the disclosure, it may be useful to stack more than one
electrochemical cell.
The stacking of cells may be enabled, for example, by the use of three or more
immiscible
fluids having three or more different densities. In such embodiments, a second
cathode
opposite the first cathode at a second distance from the anode is provided and
a third
- 40 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
electrolyte solution in contact with the second cathode and the second
electrolyte solution
is further provided wherein the third and second electrolyte solutions are in
contact with
each other and are immiscible, and wherein there is no membrane in between the
third and
second electrolyte solutions. The third electrolyte solution may be polar and
will be a
greater density than the first two electrolyte solutions. An example of a
third electrolyte
solution that is denser than water is one that contains propylene carbonate as
a solvent.
The third electrolyte solution may contain a salt and may be saturated with
respect to that
salt. Batteries with such cells may be configured in flow or no flow mode.
[00123] In at least one embodiment according to the present disclosure, as
shown in
FIG. 4A, a battery may include a cell 11 including a first electrolyte
solution 20, a second
electrolyte solution 22, and a third electrolyte solution 24. In such an
embodiment, the
cell 11 includes one cathode 12 operating with two anodes 14 to generate
electricity
supplied to the load 16 via circuit 18. In such an embodiment, the third
electrolyte
solution 24 is denser than the first electrolyte solution 20 and the second
electrolyte
solution 22. The third electrolyte solution 24 is immiscible relative to the
first electrolyte
solution 20 and/or the second electrolyte solution 22. Accordingly, the second
electrolyte
solution 20 is disposed in a layer above the first electrolyte solution 22,
and the first
electrolyte solution 22 is disposed in a separate layer above the third
electrolyte solution
24. Optional tanks 30 and 32, acting as sources for electrolyte solutions, and
pumps 50
and 52 (or by capillarity, reverse osmosis, a ratchet, swelling pressure, or
gravity), may be
used to deliver electrolyte solutions to the cell in flow mode, for example
via conduits 21
and 25 respectively. The first electrolyte solution 20 and second electrolyte
solution 22
may further flow out of the cell 11 via conduits 23 and 27 respectively. They
may be
directed to waste or to other tanks. In some embodiments, flow could be
reversed from
-41 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
said other tanks to recharge cell 11. In other embodiments, the cell can be
arranged with a
cathode on top and bottom and an anode in the middle.
[00124] As shown in FIG. 4A, the third electrolyte solution 24 may be
supplied to
the cell 11 from a third source 34 via conduit 31 with pump 54 (or by
capillarity, reverse
osmosis, a ratchet, swelling pressure, or gravity) which may be used in flow
mode. The
third electrolyte solution 24 may further flow out of cell 11 via conduit 33.
This may be
directed to waste or to another tank. In some embodiments, flow could be
reversed from
said another tank to recharge cell 11. In embodiments in which the third
electrolyte
solution 24 flows through the cell 11, the third electrolyte solution 24 may
be directed to
waste or to other tanks. In some embodiments, flow could be reversed and from
the other
tanks to recharge the cells. Alternatively, the third electrolyte solution 24
may not flow
through the cell 11 but may be replaceable. Note that the vertical lines
connecting the
electrolyte solutions and the cathode and anode electrodes in the schematic
are not
conduits but are merely to aid in the viewing of the schematic.
[00125] In FIG. 4B, electrochemical cell 1 la is a three-layer system
presented with
two cathodes 12 and one anode 14. First electrolyte solution 20 is in contact
with cathode
12 and second electrolyte solution 22 which in turn is in contact with anode
14 and third
electrolyte solution 24. Cathode 12 is in contact with the third electrolyte
solution 24 and
load 16 via circuit 18. An optional reductant (such as H2 gas) 26 may supply
the H2 to
anode 14 via conduit 31a. Flow tanks, conduits, and pumps (or by capillarity,
reverse
osmosis, a ratchet, swelling pressure, or gravity) may be used to run
electrochemical cell
11 a in flow mode and thus allow for recharging. Note that the vertical lines
connecting the
electrolyte solutions and the cathode and anode electrodes in the schematic
are not
conduits but are merely to aid in the viewing of the schematic.
- 42 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00126] In some embodiments, a first and second electrolyte solution are
of
different densities and immiscible due to the presence of a salt in the first
electrolyte
solution and are in contact without a membrane. Further, the cell is
configured to run in a
no flow mode. Batteries may be made of such cells such as in parallel or
series geometry
and/or a voltaic pile. The electricity from such batteries may be delivered to
a process
application such as solar farms, wind farms, household appliances, consumer
goods, or
toys.
Example 1 ¨ Creating an electrochemical cell
[00127] Al Anode: A strip of aluminum screen which is 200 mm by 25 mm is
cut
and the screen is folded in half lengthwise three times, resulting in a 25 mm
by 25 mm
square 8 layers thick.
[00128] Carbon foam current collector: A strip of carbon felt which is 100
mm by
25 mm is cut. The carbon felt is folded in half lengthwise two times,
resulting in a 25 mm
by 25 mm square 4 layers thick.
[00129] Copper wire: A length of bare copper wire is cut which is about100
mm
long.
[00130] Assembly of copper wire and Al Anode: The piece of copper wire is
slid
through the middle of the aluminum screen, so there will be four layers on top
of the
copper and four layers below the copper. 10 mm on the end of the wire is bent
so it
partially wraps around resulting in copper wire, 4 layers aluminum, copper
wire, and 4
layers aluminum. Once the wire is bent, the assembly is stapled together to
avoid the wire
falling out and to ensure good contact.
[00131] Assembly of copper wire and Carbon Foam Collector: The piece of
copper
wire is slid through the middle of the carbon foam, resulting in two layers on
top of the
- 43 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
copper and two layers below the copper. 10 mm on the end of the wire is bent
so it
partially wraps around the copper wire, 2 layers carbon foam, copper wire, and
2 layers of
carbon foam. Once the wire is bent, the assembly is stapled together to avoid
the wire
falling out and to ensure good contact.
[00132] The overall assembly of aluminum, copper wire, and carbon foam is
referred herein as a "unit".
Example 2 ¨ Assembling units together
[00133] Cutting the porous, non-conductive spacer: A 35 mm by 35 mm piece
of
vinyl coated polyester screen is cut. This screen will act as a spacer to
ensure that the
aluminum anode of one unit will not touch the carbon foam collector of a
separate unit,
preventing a short in the battery.
[00134] Assembling two units together: A sandwich of the aluminum from a
first
unit, the spacer, and the carbon foam from a second unit is made. This
sandwich is then
wrapped in one layer of 3M surgical tape, which ensures that there is light
compression of
the sandwich construct so that the gap between the aluminum and carbon is the
thickness
of the spacer. The surgical tape also works as a sponge to absorb the
electrolyte when so
exposed to ensure adequate saturation of the battery. This sandwich may be
referred to as
a stack.
[00135] The assembly can be repeated for as many units as desired to
create
electrochemical cells in series. The ends of the cells can be connected via a
common load
to create an electrochemical battery. Upon exposure to electrolytes of the
disclosure,
electricity or hydrogen or both may be generated and may be delivered to an
application.
Example 3 ¨ Al/peroxydi sulfate electrochemical cell/battery with immiscible
electrolyte
solutions without a membrane
- 44 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00136] A no flow electrochemical cell/battery configured in accordance
with the
schematic of FIG. 5 (other than the flow portion of the schematic) was
prepared. The
figure represents both an electrochemical cell and a battery with a battery
being defined as
containing one or more electrochemical cells. The cell/battery was made in a
glass beaker.
Aluminum solid was used as anode 62 and electrically connected via circuit 18
and Load
16 to cathode 63, conducting carbon cloth or copper depending on the specific
experiment.
The anode was placed in an electrolyte solution 22c containing ethanol or
methanol
(multiple were tested), which was loaded with sodium sulfate and sodium
hydroxide. The
cathode was placed in contact with an electrolyte solution 20c and loaded with
sodium
hydroxide, calcium chloride, and Na2S208. Although each solution is polar, the
different
electrolyte solutions are immiscible. Further, they have different densities
with the
peroxydisulfate solution being denser, and thus on the bottom and the less
dense neutral
alcohol (ethanol or methanol) solution on top. The anode electrode is
aluminum. Pyrex
wool is loaded into the beaker in between the cathode and anode. The Pyrex
wool forms
a porous medium in which fluids are imbedded. Other porous media materials,
such as
other borosilicates, may be used.
[00137] The concentrations of sodium and calcium salts were such that they
were
near or at their solubility limit for the system. The cell/battery of FIG. 5
could be run in
flow mode by attaching optional tanks 30 and 32 and pumps 50 and 52 (or by
capillarity,
reverse osmosis, a ratchet, swelling pressure, or gravity) via conduits 21 and
25
respectively. The outflow from the cell/battery 23 and 27 could be routed to
waste or to
an external tank for recharge purposes via reversing polarity. In flow
operation, the rate of
addition of a fluid to increase or decrease the thickness of a layer can be
used to change
the pressure in the layer. The anode oxidation reaction rate, such as Al(s)
¨>A13+, can be
- 45 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
controlled by adjusting the two pressures in electrolyte solutions (by
adjusting the rate
which controls the thickness of the two solutions) which in turn changes the
proximity of
the aluminum to the ions in the cathode solution such as, for example, the
82082- ion).
Example 4 ¨ Zn/peroxydi sulfate electrochemical cell/battery with immiscible
electrolyte
solutions without a membrane
[00138] A no flow electrochemical cell/battery configured in accordance
with the
schematic of FIG. 6 (other than the flow portion of the schematic) was
prepared. The
figure represents both an electrochemical cell and a battery with a battery
being defined as
containing one or more electrochemical cells. The cell/battery was made in a
glass beaker.
Carbon foam was used as an anode-electrode 62a and electrically connected via
circuit 18
and Load 16 to cathode 63a, which was also carbon foam. The anode was placed
in an
electrolyte solution 22d containing ethanol or methanol depending on the
experiment
(multiple runs were performed), which was loaded with zinc powder, sodium
sulfate and
sodium hydroxide. The cathode was placed in contact with an electrolyte
solution 20c in
water and loaded with sodium hydroxide, calcium chloride, and Na2S208.
Although each
solution is polar, the different electrolyte solutions are immiscible.
Further, they have
different densities with the peroxydisulfate solution being denser, and thus
on the bottom
and the less dense neutral alcohol (ethanol or methanol) solution on top.
Pyrex wool was
loaded into the beaker in between the cathode and anode. The Pyrex wool forms
a
porous medium in which fluids are imbedded. Other porous media materials, such
as other
borosilicates, may be used.
[00139] The concentrations of sodium and calcium salts were such that they
were
near or at their solubility limit for the system. The cell/battery of FIG. 6
could be run in
flow mode by attaching optional tanks 30 and 32 and pumps 50 and 52 (or by
capillarity,
- 46 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
reverse osmosis, a ratchet, swelling pressure, or gravity) via conduits 21 and
25
respectively. The outflow from the cell/battery 23 and 27 could be routed to
waste or to
an external tank for recharge purposes via reversing polarity. After running
for three
hours, voltages of between 1.75V and 1.89 V were recorded and a current of .12
A/cm2
were recorded at one ohm in ethanol and about 1.52V at 0.12 A/cm2 in methanol.
[00140] In flow operation, the rate of addition of a fluid to increase or
decrease the
thickness of a layer can be used to change the pressure in the layer. The
anode oxidation
reaction rate, such as Zn(s) ¨>Zn2+, can be controlled by adjusting the two
pressures in
electrolyte solutions (by adjusting the rate which controls the thickness of
the two
solutions) which in turn changes the proximity of the zinc to the ions in the
cathode
solution such as, for example, the S2082- ion. The reaction may also be
reversible so that
the cell or a battery with multiple cells may be recharged.
Example 5 ¨ Additional embodiment of Al/peroxydi sulfate electrochemical
cell/battery
with immiscible electrolyte solutions without a membrane
[00141] A no flow electrochemical cell/battery configured in accordance
with the
schematic of FIG. 7 (other than the flow portion of the schematic) was
prepared. The
figure represents both an electrochemical cell and a battery with a battery
being defined as
containing one or more electrochemical cells. The cell/battery was made in a
glass beaker.
Carbon foam was used as an anode-electrode 62a and electrically connected via
circuit 18
and Load 16 to cathode 63a, which was also carbon foam. The anode was placed
in an
electrolyte solution 22e containing ethanol or methanol depending on the
experiment
(multiple runs were performed), which was loaded with aluminum powder, sodium
sulfate
and sodium hydroxide. The cathode was placed in contact with an electrolyte
water
solution 20c and loaded with sodium hydroxide, calcium chloride, and Na2S208.
- 47 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
Although each solution is polar, the different electrolyte solutions are
immiscible. Further,
they have different densities with the peroxydisulfate solution being denser,
and thus on
the bottom and the less dense neutral alcohol (ethanol or methanol) solution
on top.
Pyrex wool is loaded into the beaker in between the cathode and anode. The
Pyrex
wool forms a porous medium in which fluids are imbedded. Other porous media
materials,
such as other borosilicates, may be used.
[00142] The concentrations of sodium and calcium salts were such that they
were
near or at their solubility limit for the system. The cell/battery of FIG. 7
could be run in
flow mode by attaching optional tanks 30 and 32 and pumps 50 and 52 (or by
capillarity,
reverse osmosis, a ratchet, swelling pressure, or gravity) via conduits 21 and
25
respectively. The outflow from the cell/battery 23 and 27 could be routed to
waste or to
an external tank for recharge purposes via reversing polarity. After running
for three
hours, a voltage of 1.9 V was recorded and a current of 0.14 A/cm2.
[00143] In flow operation, the rate of addition of a fluid to increase or
decrease the
thickness of a layer can be used to change the pressure in the layer. The
anode oxidation
reaction rate, such as Al(s) ¨>A13+, can be controlled by adjusting the two
pressures in
electrolyte solutions (by adjusting the rate which controls the thickness of
the two
solutions) which in turn changes the proximity of the aluminum to the ions in
the cathode
solution such as, for example, the S2082- ion).
Example 6 ¨ Al/Peroxydisulfate flow battery and hydrogen production
[00144] FIG. 8 is a schematic of an electrochemical cell in flow mode.
Flow
batteries have the advantage that they are instantly rechargeable via
continual replacement
of the electrolytes. In many ways flow batteries are like fuel cells, but
rather than using
hydrogen and oxygen, they employ liquid electrolytes. The metal that is often
on the
- 48 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
anode side of redox flow-batteries is in the form of a very fine powder that
is premixed
with the analyte to form a colloidal suspension and pumped through a porous
carbon
current collector where oxidation takes place. On the cathode side a strong
oxidant is
dissolved in the catholyte for the reduction step. Example single cell
amperages range
from 0.25A/cm2 to 0.1A/cm2 with voltages ranging from 1.5V to 3.25V. A first
electrolyte
solution is made by loading water with NaOH, Na2SO4 and Na2S208. Inflow and
outflow
ports are provided so that electrolyte solution can be replenished and removed
for flow
purposes. The first electrolyte solution uses a carbon foam current collector
and is
stabilized with glass wool. Inflowing solution contains water, NaOH, Na2SO4,
and
Na2S208 whereas outflow is water, NaOH, and Na2SO4. A material, often made of
plastic,
with holes may be used to separate the first electrolyte solution from the
second and may
contain glass wool.
[00145] A second electrolyte is made by adding NaOH, Al powder and Na2SO4
to
ethanol. The Na2SO4 is added at a sufficiently high concentration to keep
separate the
aqueous first electrolyte solution from the ethanolic second electrolyte
solution. Inflows
and outflows are provided for the second electrolyte solution with an inflow
being an
ethanolic solution of NaOH and Na2SO4 with added Al in powder form. The
outflow
contains ethanol, sodium hydroxide, A1203,Na2SO4, and NaAl(OH)4. The current
collector
is carbon foam which is in electrical contact via a load with the current
collector of the
first electrolyte solution. Glass wool stabilizes the second electrolyte
solution. During the
electrochemical process hydrogen gas is formed in the second electrolyte
solution and may
be removed as set forth in FIG. 8 to, for example, a hydrogen compressor, for
hydrogen
delivery to a process application such as a fuel cell. The electricity
generated by the
electrochemical cell may be used to power the hydrogen compressor. For
example, a 900
- 49 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
CM2 Aluminum sheet placed in the second electrolyte solution should provide
current of at
least 0.1A/cm2 resulting in 90A of current, at 1 ohm and 2 volts, this
provides 180 watts of
power. Thus, 6 electrochemical cells in series would be more than sufficient
to run a
typical hydrogen compressor.
[00146] Thus, on the anode side (ethanol), solid aluminum produces
electrons and
on the cathode side a strong oxidant (like 52082- or C10-) accepts electrons.
The
Al(s)¨>A13++3e- and the S2082-+2e-->2S042- half reactions yield a substantial
current
(1.4A/cm2-anode) which can be used to drive a compressor. Without being bound
by
theory, if these reactions are carried out in a basic environment, then there
are three other
very important reactions on the anode side that take place which also lead to
the oxidation
of aluminum and the resulting production of hydrogen gas.
Example 7 ¨ Electrochemical Cell with Single Solution which may be used as a
capacitor
[00147] An electrochemical cell was prepared in accordance with FIG. 9 in
a no-
flow mode. The cell could be run in flow mode, however, and FIG. 9 illustrates
how that
could be done.
[00148] An electrolyte solution was made from 10 grams of sodium
peroxydisulfate, 10 grams of sodium hydroxide, 10 grams of sodium sulfate and
200 mls
of water. Within an electrochemical cell, 1.5 grams of Al foil was compressed
into a
lx2x1 cm block and connected via copper wire to a meter in the cell and 20 mls
of the
electrolyte solution was added to the cell. A carbon foam current collector
was also
connected to the meter in the cell. Electrochemical cell 20d, therefore, is
similar to 20c,
except for the addition of sodium sulfate. Over the course of about 45
minutes,
concentrated (2M) sodium peroxydisulfate and NaOH (pH15) was added dropwise to
the
cell near the Al foil. Drops were added so that the current provided by the
cell ranged
- 50 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
between about 400 and 600 milliwatts until a total of about 20 ml were added.
Subsequently, a small amount of solid sodium peroxydisulfate was added near
the anode
and it was observed that the power spiked to 750 milliwatts and then reached a
steady state
of 600 milliwatts for about 10 minutes. The addition of solid peroxydisulfate
also caused
the release of heat and substantial hydrogen gas.
[00149] Spikes in power may be obtained by adding oxidant, such as with
the
addition of sodium peroxydisulfate and sodium hydroxide followed by opening
the
electrochemical circuit such as by disconnecting the lead. The disconnection
may be used
to build a capacitor. The capacitor charge may then be discharged by
reconnecting the
lead and closing the circuit. It is believed that reduction-oxidation
capacitance is charged
chemically by continuous oxidation of the aluminum while the circuit is open
creating a
net negative charge on the aluminum, which in turn induces a double ionic
layer (sodium
and sulfate) to form next to the aluminum surface with cations favored toward
the surface.
Subsequent closing the circuit causes the anode to rapidly discharge through
the load, with
the coincident breakdown of the double layer, creating what is commonly known
as a
capacitor. Rapidly cycling an open and closed circuit can create a large and
sustainable
current. This capacitor is self-charging and can be controlled by the amount
of oxidant
deployed and pH.
[00150] This cycle, of opening and closing the circuit may be repeated at
the same
time that bulk electrolyte and solid aluminum is passed through the system.
Alternatively,
the resulting sodium sulfate, aluminum oxide and sodium aluminate may be
removed.
[00151] In the various descriptions above, electrochemical cells are
stacked
vertically. In alternative embodiments, adjacent electrochemical cells, for
example, may
be disposed in other orientations to make batteries.
-51 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00152] A variety of embodiments according to the present disclosure are
contemplated. Such embodiments may be employed in a variety of methods,
processes,
procedures, steps, and operations as a means of providing electrochemical
cells and
batteries. While the disclosure has been illustrated and described in detail
in the drawings
and foregoing description, the same is to be considered as illustrative and
not restrictive in
character, it being understood that only certain exemplary embodiments have
been shown
and described. Those skilled in the art will appreciate that many
modifications are
possible in the example embodiments without materially departing from this
disclosure.
Accordingly, all such modifications are intended to be included within the
scope of this
disclosure as defined in the following claims. Indeed, this disclosure is not
intended to be
exhaustive or to limit the scope of the disclosure.
[00153] An additional list of non-limiting embodiments of this disclosure
is
provided below in the form of clauses.
[00154] Clause 1. An electrochemical cell comprising:
a. an anode;
b. a current collector; and
c. a porous, non-conductive spacer between the current collector and anode.
[00155] Clause 2. The electrochemical cell of Clause 1, further comprising
a single
electrolyte solution.
[00156] Clause 3. The electrochemical cell of Clause 2, wherein the cell
is
saturated with electrolyte.
[00157] Clause 4. The electrochemical cell of Clause 3, wherein the cell
is not
immersed in an electrolyte bath.
- 52 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00158] Clause 5. The electrochemical cell of Clause 1, wherein the cell
is wrapped
in a microporous material.
[00159] Clause 6. The electrochemical cell of Clause 1, wherein the anode
is
selected from aluminum, gallium, indium, thallium and alloys comprising at
least one of
these.
[00160] Clause 7. The electrochemical cell of Clause 6, wherein the anode
is
aluminum.
[00161] Clause 8. The electrochemical cell of Clause 1, wherein the
current
collector is selected from steel, the graphite allotrope of carbon, carbon
impregnated with
a metal and carbon foam.
[00162] Clause 9. The electrochemical cell of Clause 8, wherein the
current
collector is carbon foam.
[00163] Clause 10. The electrochemical cell of Clause 1, wherein the
porous, non-
conductive spacer is selected from an organic polymer, surgical tape,
fiberglass film, glass
wool, wood, paper, cloth, cardboard and nylon.
[00164] Clause 11. The electrochemical cell of Clause 10, wherein the
spacer is
vinyl coated polyester.
[00165] Clause 12. The electrochemical cell of Clause 2, wherein the
electrolyte
comprises water and one or more salts.
[00166] Clause 13. The electrochemical cell of Clause 12, wherein at least
one salt
is an oxidant.
[00167] Clause 14. The electrochemical cell of Clause 12, wherein the
electrolyte
comprises two salts.
- 53 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00168] Clause 15. The electrochemical cell of Clause 14, wherein the
electrolyte
comprises a salt of peroxydisulfate and a salt of sulfate and further
comprises a base.
[00169] Clause 16. The electrochemical cell of Clause 15, wherein the
peroxydisulfate salt is sodium peroxydisulfate and the sulfate salt is sodium
sulfate and the
base is sodium hydroxide.
[00170] Clause 17. The electrochemical cell of Clause 2, wherein the
electrolyte
comprises one of water or an alcohol.
[00171] Clause 18. The electrochemical cell of Clause 17, wherein the
electrolyte
is a catholyte.
[00172] Clause 19. The electrochemical cell of Clause 17, further
comprising an
oxidant.
[00173] Clause 20. The electrochemical cell of Clause 19, further
comprising a
metal salt.
[00174] Clause 21. The electrochemical cell of Clause 20, wherein the
oxidant and
metal salt have different anion components.
[00175] Clause 22. The electrochemical cell of Clause 21, wherein the
oxidant is
sodium peroxydisulfate and the metal salt is sodium sulfate.
[00176] Clause 23. The electrochemical cell of Clause 17, further
comprising a
base.
[00177] Clause 24. The electrochemical cell of Clause 23, wherein the base
is
NaOH.
[00178] Clause 25. An electrochemical cell of any one of Clauses 1-24,
wherein
the cell is electrically connected to a load.
- 54 -
CA 03054957 2019-08-28
WO 2018/169855
PCT/US2018/021981
[00179] Clause 26. An electrochemical battery comprising one or more
electrochemical cells of any one of Clauses 1-24, wherein the battery is
electrically
connected to a load.
[00180] Clause 27. A method which comprises producing electricity or
hydrogen
with an electrochemical cell of Clause 25 and delivering the electricity to an
application.
[00181] Clause 28. A method which comprises producing electricity or
hydrogen
with an electrochemical battery of Clause 26 and delivering the electricity or
hydrogen to
an application.
[00182] Clause 29. A method of making an electrochemical cell, which
comprises:
1) providing an electrochemical cell of any one of Clauses 1 and 5-11, wherein
the
electrochemical cell does not comprise an electrolyte; and
2) contacting the cell with a single electrolyte solution.
[00183] Clause 30. The method of Clause 29, which comprises contacting the
cell
with the single electrolyte solution by spraying the electrolyte solution onto
the cell.
[00184] Clause 31. The method of Clause 29, which comprises contacting the
cell
with droplets of the single electrolyte solution via drip.
[00185] Clause 32. The method of Clause 29, which comprises contacting the
cell
with an atomized mist of the single electrolyte solution.
[00186] Clause 33. A method of operating an electrochemical cell, which
comprises:
1) providing an electrochemical cell of Clause 1, wherein the electrochemical
cell
further comprises a single electrolyte solution;
- 55 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
2) electrically connecting the electrochemical cell to a load such that the
electrochemical cell operates to produce electricity, hydrogen, or both
electricity and
hydrogen; and
3) providing additional electrolyte solution, or one or more components
thereof, to
the cell during its operation.
[00187] Clause 34. The method of Clause 33, which further comprises:
4) withdrawing spent electrolyte solution, or one or more components thereof,
from the electrochemical cell during its operation.
[00188] Clause 35. The method of Clause 34, which comprises 4) withdrawing
spent electrolyte solution, or one or more components thereof, simultaneously
with 3)
providing additional electrolyte solution, or one or more components thereof.
[00189] Clause 36. The method of any one of Clauses 33-35, wherein the
single
electrolyte solution comprises one or more of the following components:
solvent, oxidant,
metal salt, and base.
[00190] Clause 37. A method of creating a capacitor, which comprises
disconnecting a load from the current collector or anode side of the
electrochemical cell of
any one of Clauses 1-25.
[00191] Clause 38. The method of Clause 37, further comprising the step of
reconnecting the load.
[00192] Clause 39. A capacitor prepared by the process of alternately
disconnecting and reconnecting a load from at least one of the current
collector or anode
in an electrochemical cell of any one of Clauses 1-25.
[00193] Clause 40. The electrochemical cell of any one of Clauses 1-4,
wherein the
cell is wrapped in a microporous material.
- 56 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00194] Clause 41. The electrochemical cell of any one of Clauses 1-4 and
40,
wherein the anode is selected from aluminum, gallium, indium, thallium and
alloys
comprising at least one of these.
[00195] Clause 42. The electrochemical cell of Clause 41, wherein the
anode is
aluminum.
[00196] Clause 43. The electrochemical cell of Clause 42, wherein the
aluminum is
in the form of a screen.
[00197] Clause 44. The electrochemical cell of Clause 43, wherein the
aluminum
thickness in the screen is between about 0.1 mm and about 0.3 mm.
[00198] Clause 45. The electrochemical cell of any one of Clauses 1-4 and
40-44,
wherein the current collector is carbon foam.
[00199] Clause 46. The electrochemical cell of any one of Clauses 1-4 and
40-45,
wherein the porous, non-conductive spacer is selected from an organic polymer,
surgical
tape, fiberglass film, glass wool, wood, paper, cloth, cardboard and nylon.
[00200] Clause 47. The electrochemical cell of Clause 46, wherein the
spacer is
vinyl coated polyester.
[00201] Clause 48. The electrochemical cell of Clause 46, wherein the
spacer is a
screen.
[00202] Clause 49. The electrochemical cell of Clause 48, wherein the
spacer
thickness in the screen is between about 0.1 mm and about 0.8 mm and the
spacer is vinyl
coated polyester.
[00203] Clause 50. The electrochemical cell of Clause 1, further
comprising a
metal conductor between the anode and an adjacent current collector from a
second
- 57 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
electrochemical cell and wherein the electrochemical cell of Clause 1 is
configured to
operate as a flow cell.
[00204] Clause 51. The electrochemical cell of Clause 50, wherein the
metal
conductor is copper wire.
[00205] Clause 52. The electrochemical cell of any one of Clauses 40-51,
wherein
the microporous material is surgical tape.
[00206] Clause 53. The electrochemical cell of any one of Clauses 2-4 and
40-52,
wherein the electrolyte comprises water and one or more salts, wherein at
least one of the
salts is an oxidant.
[00207] Clause 54. The electrochemical cell of Clause 53, wherein the
electrolyte
comprises two salts.
[00208] Clause 55. The electrochemical cell of Clause 54, wherein the
electrolyte
comprises a salt of peroxydisulfate and a salt of sulfate and further
comprises a base.
[00209] Clause 56. The electrochemical cell of Clause 55, wherein the
peroxydisulfate salt is sodium peroxydisulfate and the sulfate salt is sodium
sulfate and the
base is sodium hydroxide.
[00210] Clause 57. An electrochemical battery comprising one or more
electrochemical cells of any one of Clauses 1-4 and 40-56, wherein the battery
is
electrically connected to a load.
[00211] Clause 58. The electrochemical battery of Clause 57, comprising
two or
more electrochemical cells arranged in series.
[00212] Clause 59. The electrochemical battery of Clause 57, comprising
two or
more electrochemical cells arranged in parallel.
- 58 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00213] Clause 60. The electrochemical battery of Clause 57, comprising
electrochemical cells arranged in series and in parallel.
[00214] Clause 61. The electrochemical battery of any one of Clauses 57-
60,
further comprising an electrolyte wherein the electrolyte comprises water and
one or more
salts, wherein at least one of the salts is an oxidant.
[00215] Clause 62. The electrochemical battery of Clause 61, wherein the
electrolyte comprises two salts.
[00216] Clause 63. The electrochemical battery of Clause 62, wherein the
electrolyte comprises a salt of peroxydisulfate and a salt of sulfate and
further comprises a
base and wherein the electrochemical battery is configured as a flow battery.
[00217] Clause 64. The electrochemical battery of Clause 63, wherein the
peroxydisulfate salt is sodium peroxydisulfate and the sulfate salt is sodium
sulfate and the
base is sodium hydroxide.
[00218] Clause 65. The electrochemical battery of any one of Clauses 57-
64, which
produces electricity.
[00219] Clause 66. The electrochemical battery of any one of Clauses 57-
64, which
produces hydrogen.
[00220] Clause 67. The electrochemical battery of any one of Clauses 57-
64, which
produces electricity and hydrogen.
[00221] Clause 68. A method which comprises delivering electricity
produced by
the electrochemical battery of Clause 65 to an application.
[00222] Clause 69. A method which comprises delivering hydrogen produced
by
the electrochemical battery of Clause 66 to an application.
- 59 -
CA 03054957 2019-08-28
WO 2018/169855
PCT/US2018/021981
[00223] Clause 70. The method of Clause 68, wherein the application is a
cell
phone tower or a vehicle.
[00224] Clause 71. The method of Clause 69, wherein the application is a
fuel cell
or a vehicle.
[00225] Clause 72. The electrochemical cell of any one of Clauses 2-4 and
40-56,
wherein the electrolyte comprises one of water or an alcohol.
[00226] Clause 73. The electrochemical cell of Clause 72, wherein the
electrolyte
is a catholyte.
[00227] Clause 74. The electrochemical cell of Clause 72 or 73, further
comprising
an oxidant.
[00228] Clause 75. The electrochemical cell of Clause 74, further
comprising a
metal salt.
[00229] Clause 76. The electrochemical cell of Clause 75, wherein the
oxidant and
metal salt have different anion components.
[00230] Clause 77. The electrochemical cell of Clause 76, wherein the
oxidant is
sodium peroxydisulfate and the metal salt is sodium sulfate.
[00231] Clause 78. The electrochemical cell of any one of Clauses 72-77,
further
comprising a base.
[00232] Clause 79. The electrochemical cell of Clause 78, wherein the base
is
NaOH.
[00233] Clause 80. The electrochemical battery of any one of Clauses 57-
60,
further comprising an electrolyte wherein the electrolyte comprises one of
water or an
alcohol.
- 60 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00234] Clause 81. The electrochemical battery of Clause 80, wherein the
electrolyte is a catholyte.
[00235] Clause 82. The electrochemical battery of Clause 80 or 81, further
comprising an oxidant.
[00236] Clause 83. The electrochemical battery of Clause 82, further
comprising a
metal salt.
[00237] Clause 84. The electrochemical battery of Clause 83, wherein the
oxidant
and metal salt have different anion components.
[00238] Clause 85. The electrochemical battery of Clause 84, wherein the
oxidant
is sodium peroxydisulfate and the metal salt is sodium sulfate.
[00239] Clause 86. The electrochemical battery of any one of Clauses 80-85
further
comprising a base.
[00240] Clause 87. The electrochemical battery of Clause 86, wherein the
base is
NaOH.
[00241] Clause 88. An electrochemical cell comprising:
a) an anode;
b) a current collector; and
c) a porous, non-conductive spacer between the current collector and anode;
wherein the electrochemical cell does not comprise an electrolyte.
[00242] Clause 89. The electrochemical cell of Clause 88, wherein the cell
is
wrapped in a microporous material.
[00243] Clause 90. The electrochemical cell of Clause 88 or Clause 89,
wherein the
anode is selected from aluminum, gallium, indium, thallium and alloys
comprising at least
one of these.
- 61 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00244] Clause 91. The electrochemical cell of Clause 90, wherein the
anode is
aluminum.
[00245] Clause 92. The electrochemical cell of Clause 90 or Clause 91,
wherein the
anode is in the form of a screen.
[00246] Clause 93. The electrochemical cell of Clause 92, wherein the
anode
thickness in the screen is between about 0.1 mm and about 0.3 mm.
[00247] Clause 94. The electrochemical cell of any one of Clauses 88-93,
wherein
the current collector is selected from steel, the graphite allotrope of
carbon, carbon
impregnated with a metal and carbon foam.
[00248] Clause 95. The electrochemical cell of Clause 94, wherein the
current
collector is carbon foam.
[00249] Clause 96. The electrochemical cell of any one of Clauses 88-95,
wherein
the porous, non-conductive spacer is selected from an organic polymer,
surgical tape,
fiberglass film, glass wool, wood, paper, cloth, cardboard and nylon.
[00250] Clause 97. The electrochemical cell of Clause 96, wherein the
spacer is
vinyl coated polyester.
[00251] Clause 98. The electrochemical cell of any one of Clauses 88-97,
wherein
the spacer is a screen.
[00252] Clause 99. The electrochemical cell of Clause 98, wherein the
spacer
thickness in the screen is between about 0.1 mm and about 0.8 mm and the
spacer is vinyl
coated polyester.
[00253] Clause 100. The electrochemical cell of Clause 1, wherein the
electrochemical cell comprises a single electrolyte solution and is
electrically connected to
- 62 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
a load, and wherein the cell produces between about 10 Watt-hours/(kg of
electrolyte +
anode metal) and about 680 Watt-hours/(kg of electrolyte + anode metal).
[00254] Clause 101. The electrochemical cell of Clause 1, wherein the
electrochemical cell comprises a single electrolyte solution and is
electrically connected to
a load, and wherein the cell produces between about 10 Watt-hours/kg of
electrolyte and
about 100 Watt-hours/kg of electrolyte.
[00255] Clause 102. An electrochemical cell comprising a single aqueous
electrolyte solution in contact with a non-metallic current collector, an
oxidant, and a
metal solid wherein current travels from the metal solid to the current
collector via a load.
[00256] Clause 103. The electrochemical cell of Clause 102, wherein the
aqueous
electrolyte solution is basic and the oxidant is S2082-.
[00257] Clause 104. The electrochemical cell of Clause 102, wherein the
aqueous
electrolyte solution further comprises sodium hydroxide.
[00258] Clause 105. The electrochemical cell of any one of Clauses 102-
104,
wherein the metal solid is selected from aluminum, gallium, indium, thallium
and alloys
comprising at least one of these.
[00259] Clause 106. The electrochemical cell of Clause 105, wherein the
method
solid is aluminum in foil form.
[00260] Clause 107. The electrochemical cell of any one of Clauses 102-
106,
further comprising a porous stabilizer.
[00261] Clause 108. The electrochemical cell of any one of Clauses 102-
107,
further comprising a metal sulfate and wherein the current collector is carbon
foam and the
porous stabilizer is glass wool or a borosilicate or both.
- 63 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00262] Clause 109. The electrochemical cell of Clause 108, wherein the
metal
sulfate is Na2 S 04.
[00263] Clause 110. The electrochemical cell of any one of Clauses 102-109
wherein the pH is greater than 12.
[00264] Clause 111. The electrochemical cell of Clause 110, wherein the pH
is
greater than 13.
[00265] Clause 112. The electrochemical cell of Clause 111, wherein the pH
is
greater than 14.
[00266] Clause 113. The electrochemical cell of any one of Clauses 102-
112,
wherein between about 10 Watt-hours/kg of electrolyte and about 100 Watt-
hours/kg of
electrolyte is produced.
[00267] Clause 114. The electrochemical cell of Clause 113, wherein the
power
produced per square centimeter of metal solid is between about 600 mW and
about 1000
mW.
[00268] Clause 115. The electrochemical cell of any one of Clauses 102-114
configured to operate in a flow mode.
[00269] Clause 116. A method which comprises providing additional oxidant
to the
electrochemical cell of any one of Clauses 102-115.
[00270] Clause 117. The electrochemical cell of Clause 115, further
comprising an
inflow stream comprising an aqueous electrolyte solution.
[00271] Clause 118. The electrochemical cell of Clause 117, wherein the
inflow
stream further comprises an oxidant.
[00272] Clause 119. The electrochemical cell of Clause 118, wherein the
oxidant is
sodium peroxydisulfate or a solution comprising peroxydisulfate anion or both.
- 64 -
CA 03054957 2019-08-28
WO 2018/169855
PCT/US2018/021981
[00273] Clause 120. The electrochemical cell of any one of Clauses 115-
119,
wherein an aqueous solution outflows from the cell.
[00274] Clause 121. The electrochemical cell of Clause 120, wherein the
aqueous
solution outflowing from the cell comprises a metal sulfate.
[00275] Clause 122. The electrochemical cell of Clause 118 or 119, wherein
the
oxidant is in an aqueous basic solution.
[00276] Clause 123. The electrochemical cell of Clause 122, wherein the
base is
NaOH.
[00277] Clause 124. The electrochemical cell of Clause 118 or 119 wherein
the
oxidant is solid Na2S208.
[00278] Clause 125. The electrochemical cell of any one of Clauses 115-
124,
wherein between about 10 Watt-hours/kg of electrolyte and about 100 Watt-
hours/kg of
electrolyte is produced.
[00279] Clause 126. The electrochemical cell of Clause 125, wherein
between
about 40 Watt-hours/kg of electrolyte and 80 Watt-hours/kg of electrolyte is
produced.
[00280] Clause 127. A method of creating a capacitor comprising the steps
of
disconnecting the load from one side of the electrochemical cell of any one of
Clauses
115-126.
[00281] Clause 128. The method of Clause 127, further comprising the step
of
reconnecting the load.
[00282] Clause 129. A capacitor, prepared by the process of alternatively
disconnecting and reconnecting the load from at least one of the current
collector or anode
in the cell of any one of Clauses 102-126.
- 65 -
CA 03054957 2019-08-28
WO 2018/169855
PCT/US2018/021981
[00283] Clause 130. The electrochemical cell of Clause 125, wherein
between
about 10 Watt-hours/kg of electrolyte and about 60 Watt-hours/kg of
electrolyte is
produced.
[00284] Clause 131. An electrochemical cell comprising a single aqueous
electrolyte solution in contact with a non-metallic current collector, an
oxidant, and a
metal solid wherein current travels from the metal solid to the current
collector via a load,
and wherein the pH is greater or equal to 12.
[00285] Clause 132. The electrochemical cell of Clause 131, wherein the
non-
metallic current collector is carbon foam, the oxidant is a peroxydisulfate
salt, and the
metal solid is aluminum.
[00286] Clause 133. An electrochemical cell comprising a single aqueous
electrolyte solution in contact with a non-metallic current collector, an
oxidant, and one or
more anodes wherein current travels from the one or more anodes to the current
collector
via a load, and wherein the pH is greater or equal to 10.
[00287] Clause 134. The electrochemical cell of Clause 133, wherein the
one or
more anodes are a metal.
[00288] Clause 135. The electrochemical cell of Clause 134, wherein the
metal is
aluminum, gallium, indium, thallium, or an alloy comprising at least one of
these.
[00289] Clause 136. The electrochemical cell of any one of Clause 133-135,
wherein the anodes are separated by an insulator.
[00290] Clause 137. The electrochemical cell of Clause 135, wherein the
anode is
aluminum and is in a foil form.
[00291] Clause 138. The electrochemical cell of any one of Clauses 133-
137,
wherein the pH is 12 or greater.
- 66 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00292] Clause 139. An electrochemical cell comprising:
a. a cathode;
b. an anode adjacent to the cathode at a distance;
c. a first polar electrolyte solution in contact with the cathode and disposed
within the
distance comprising an oxidant;
d. a second polar electrolyte solution in contact with the anode and disposed
within
the distance comprising a suitable metal ion; and
e. a separation agent;
wherein the first and second electrolyte solutions are in contact with each
other and are
immiscible, and wherein there is no membrane in between the first and second
electrolyte
solutions.
[00293] Clause 140. The electrochemical cell of Clause 139, wherein each
polar
electrolyte solution further comprising a porous stabilizer.
[00294] Clause 141. The electrochemical cell of any one of Clauses 139-
140,
configured for flow-mode.
[00295] Clause 142. The electrochemical cell of any one of Clauses 139-
141,
wherein the first polar electrolyte solution is aqueous.
[00296] Clause 143. The electrochemical cell of any one of Clauses 139-
142,
wherein the oxidant is a Vanadium ion.
[00297] Clause 144. The electrochemical cell of any one of Clauses 139-
142,
wherein the oxidant is S2082-.
[00298] Clause 145. The electrochemical cell of any one of Clauses 139-
142,
wherein the oxidant is C10-.
- 67 -
CA 03054957 2019-08-28
WO 2018/169855
PCT/US2018/021981
[00299] Clause 146. The electrochemical cell of any one of Clauses 139-
145,
wherein the first polar electrolyte solution further comprises a base.
[00300] Clause 147. The electrochemical cell of Clause 146, wherein the
base is
selected from KOH, NaOH, Ca(OH)2, Li0H, RbOH, Cs0H, Sr(OH)2, and Ba(OH)2.
[00301] Clause 148. The electrochemical cell of Clause 147, wherein the
base is
NaOH.
[00302] Clause 149. The electrochemical cell of any one of Clauses 146-
148,
wherein the pH of the first polar electrolyte solution is between about 8 and
about 14.
[00303] Clause 150. The electrochemical cell of Clause 149, wherein the pH
of the
first polar electrolyte solution is between about 11 and about 14.
[00304] Clause 151. The electrochemical cell of any one of Clauses 140-
150,
wherein the porous stabilizer is borosilicate.
[00305] Clause 152. The electrochemical cell of any one of Clauses 139-
151,
wherein the separation agent is a salt.
[00306] Clause 153. The electrochemical cell of Clause 152, wherein the
salt is
calcium chloride.
[00307] Clause 154. The electrochemical cell of Clause 152, wherein the
salt is
sodium sulfate.
[00308] Clause 155. The electrochemical cell of any one of Clauses 139-
154,
wherein the cell is configured to operate in a flow mode.
[00309] Clause 156. The electrochemical cell of Clause 155, further
comprising an
inflow solution comprising an aqueous solution comprising a base, an oxidant,
and a
separation agent.
- 68 -
CA 03054957 2019-08-28
WO 2018/169855
PCT/US2018/021981
[00310] Clause 157. The electrochemical cell of Clause 156, wherein the
base is
sodium hydroxide, the separate agent is sodium sulfate, and the oxidant 82082-
.
[00311] Clause 158. The electrochemical cell of any one of Clauses 155-
157,
wherein an aqueous solution comprising base and sodium sulfate outflows from
the cell.
[00312] Clause 159. The electrochemical cell of any one of Clauses 155-
157,
further comprising an outflow solution comprising an aqueous solution of a
base.
[00313] Clause 160. The electrochemical cell of Clause 159, wherein the
base is
sodium hydroxide.
[00314] Clause 161. The electrochemical cell of any one of Clauses 139-
160,
wherein a current collector is placed within the first electrolyte solution.
[00315] Clause 162. The electrochemical cell of Clause 161, wherein the
current
collector is a metal.
[00316] Clause 163. The electrochemical cell of Clause 161, wherein the
current
collector is a non-metal.
[00317] Clause 164. The electrochemical cell of Clause 163, wherein the
current
collector is carbon foam.
[00318] Clause 165. The electrochemical cell of any one of Clauses 139-
164,
further comprising glass wool placed in between the first and second polar
electrolyte
solutions.
[00319] Clause 166. The electrochemical cell of any one of Clauses 139-
165,
wherein the second polar electrolyte solution comprises an alcohol.
[00320] Clause 167. The electrochemical cell of any one of Clauses 139-
166,
wherein the suitable metal ion is Zn2+.
- 69 -
CA 03054957 2019-08-28
WO 2018/169855
PCT/US2018/021981
[00321] Clause 168. The electrochemical cell of any one of Clauses 139-
166,
wherein the suitable metal ion is Al3+.
[00322] Clause 169. The electrochemical cell of any one of Clauses 139-
168,
wherein the second polar electrolyte solution is an alcoholic solution and
further
comprises a base.
[00323] Clause 170. The electrochemical cell of Clause 169, wherein the
base is
KOH.
[00324] Clause 171. The electrochemical cell of Clause 169, wherein the
base is
NaOH.
[00325] Clause 172. The electrochemical cell of any one of Clauses 169-
171,
wherein the pH of the second polar electrolyte solution is between about 8 and
about 14.
[00326] Clause 173. The electrochemical cell of Clause 172, wherein the pH
of the
second polar electrolyte solution is between about 11 and about 14.
[00327] Clause 174. The electrochemical cell of any one of Clauses 166-
173,
wherein the separation agent is a salt and the alcohol is ethanol, methanol,
or both.
[00328] Clause 175. The electrochemical cell of Clause 174, wherein the
salt is
CaCl2.
[00329] Clause 176. The electrochemical cell of Clause 174, wherein the
salt is
sodium sulfate.
[00330] Clause 177. The electrochemical cell of any one of Clauses 166-
176,
wherein the alcohol is ethanol.
[00331] Clause 178. The electrochemical cell of any one of Clauses 139-
177,
wherein a current collector is placed within the second electrolyte solution.
- 70 -
CA 03054957 2019-08-28
WO 2018/169855
PCT/US2018/021981
[00332] Clause 179. The electrochemical cell of Clause 178, wherein the
current
collector is a metal.
[00333] Clause 180. The electrochemical cell of Clause 178, wherein the
current
collector is a non-metal.
[00334] Clause 181. The electrochemical cell of Clause 180, wherein the
current
collector is carbon foam.
[00335] Clause 182. The electrochemical cell of any one of Clauses 166-
181,
wherein the cell is configured to operate in a flow mode.
[00336] Clause 183. The electrochemical cell of Clause 182, further
comprising an
inflow stream comprising a polar solution comprising an alcohol, a base, a
separation
agent, and a metal capable of dissociating into a suitable metal ion.
[00337] Clause 184. The electrochemical cell of Clause 183, wherein the
alcohol is
ethanol or methanol or both, the base is sodium hydroxide, and the suitable
metal ion is
Al3+.
[00338] Clause 185. The electrochemical cell of any one of Clauses 166-
184,
further comprising an outflow stream comprising an alcohol, a base, and a
separation salt.
[00339] Clause 186. The electrochemical cell of Clause 185, wherein the
separation salt is sodium sulfate, and the base is sodium hydroxide.
[00340] Clause 187. The electrochemical cell of Clauses 185 or 186,
wherein the
alcohol is ethanol.
[00341] Clause 188. The electrochemical cell of any one of Clauses 139-
187,
wherein hydrogen gas is generated in the second electrolyte solution.
[00342] Clause 189. The electrochemical cell of Clause 188, wherein the
hydrogen
gas is directed to a hydrogen compressor.
- 71 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00343] Clause 190. A battery system comprising one or more
electrochemical
cells of any one of Clauses 139-189 and a hydrogen compressor.
[00344] Clause 191. The battery system of Clause 190 wherein the hydrogen
is
used to power a process application.
[00345] Clause 192. The battery system of Clause 191, wherein the process
application is a fuel cell.
[00346] Clause 193. An electrochemical cell comprising:
a. a cathode;
b. an anode adjacent the cathode at a distance;
c. a first polar aqueous electrolyte solution in contact with the cathode and
disposed
within the distance comprising S2082-;
d. a second polar electrolyte alcoholic solution in contact with the anode and
disposed
within the distance comprising Al3+; and
e. borosilicate within both the first and second electrolyte solutions;
wherein the first and second electrolyte solutions are in contact with each
other and are
immiscible, and wherein there is no membrane in between the first and second
electrolyte
solutions.
[00347] Clause 194. The electrochemical cell of Clause 193, wherein the
first polar
electrolyte solution and second polar electrolyte solution are of different
densities and
wherein the first electrolyte solution further comprises a halide salt and the
second
electrolyte solution further comprises a metal sulfate salt.
[00348] Clause 195. The electrochemical cell of Clause 194, wherein the
alcohol is
methanol or ethanol, the halide salt is CaCl2 and the metal sulfate salt is
Na2SO4.
- 72 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00349] Clause 196. The electrochemical cell of Clause 195, wherein the pH
of the
first electrolyte and second electrolyte solutions are adjusted to between
about 11 to about
13 each.
[00350] Clause 197. The electrochemical solution of Clause 193, wherein
the first
and second electrolyte solutions further comprise a base.
[00351] Clause 198. The electrochemical solution of Clause 197, wherein
the base
is sodium, calcium or potassium hydroxide.
[00352] Clause 199. The electrochemical cell of any one of Clauses 193-
198,
wherein the cathode is copper, carbon, or both and the anode is aluminum.
[00353] Clause 200. The electrochemical cell of Clause 199, wherein the
cathode is
a copper brush.
[00354] Clause 201. The electrochemical cell of any one of Clauses 193-
200,
wherein the borosilicate is Pyrex wool.
[00355] Clause 202. The electrochemical cell of any one of Clauses 193-201
wherein the cell is configured to run in a flow mode.
[00356] Clause 203. An electrochemical battery comprising one or more
electrochemical cells of any one of Clauses 193-202.
[00357] Clause 204. The electrochemical battery of Clause 203, wherein the
number of electrochemical cells is greater than one and the electrochemical
cells are
arranged in a parallel geometry.
[00358] Clause 205. The electrochemical battery of Clause 204, wherein the
cells
are arranged in a voltaic pile.
[00359] Clause 206. The electrochemical battery of any one of Clauses 203-
205,
wherein the battery delivers electricity to a process application.
- 73 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
[00360] Clause 207. The electrochemical battery of Clause 206, wherein the
process application is selected from solar farms, wind farms, household
appliances,
consumer goods, and toys.
[00361] Clause 208. A method of delivering electricity from an
electrochemical
cell of any one of Clauses 193-202 to a process application.
[00362] Clause 209. The method of Clause 208, wherein the process
application is
selected from solar farms, wind farms, household appliances, consumer
products, and
toys.
[00363] Clause 210. The electrochemical cell of Clause 201, wherein the
borosilicate has a pore size of about 8 microns.
[00364] Clause 211. An electrochemical cell comprising:
a. a non-metallic cathode;
b. a non-metallic anode adjacent the cathode at a distance;
c. a first polar aqueous electrolyte solution in contact with the cathode and
disposed
within the distance comprising S2082-; and
d. a second polar electrolyte alcoholic solution in contact with the anode and
disposed
within the distance comprising a metal solid;
wherein the first and second electrolyte solutions are in contact with each
other and are
immiscible, and wherein there is no membrane in between the first and second
electrolyte
solutions.
[00365] Clause 212. The electrochemical cell of Clause 211, wherein the
metal
solid is dispersed through the solution in powder form and wherein
borosilicate is placed
within both the first and second electrolyte solutions.
- 74 -
CA 03054957 2019-08-28
WO 2018/169855
PCT/US2018/021981
[00366] Clause 213. The electrochemical cell of Clause 212, wherein the
metal is
zinc.
[00367] Clause 214. The electrochemical cell of Clause 212, wherein the
metal is
aluminum.
[00368] Clause 215. The electrochemical cell of any one of Clauses 211-
214,
wherein the non-metallic cathode is carbon foam.
[00369] Clause 216. The electrochemical cell of any one of Clauses 211-
215,
wherein the non-metallic anode is carbon foam.
[00370] Clause 217. The electrochemical cell of any one of Clauses 212-216
wherein the average particle size of the powder is less than about 5 microns.
[00371] Clause 218. The electrochemical cell of any one of Clauses 212-
216,
wherein the average particle size of the powder is between about 5 and about
30 microns.
[00372] Clause 219. A method of boosting current in an electrochemical
cell
comprising the steps of adding oxidant to the second electrolyte solution of
any one of
Clauses 211-218.
[00373] Clause 220. An electrochemical cell comprising:
a. a cathode;
b. an anode adjacent to the cathode at a distance;
c. a first aqueous electrolyte solution in contact with the cathode and
disposed within
the distance comprising an oxidant;
d. a second polar electrolyte solution in contact with the anode and disposed
within
the distance comprising a metal and an oxidant; and
e. a separation agent;
- 75 -
CA 03054957 2019-08-28
WO 2018/169855 PCT/US2018/021981
wherein the first and second electrolyte solutions are in contact with each
other and are
immiscible, and wherein there is no membrane in between the first and second
solutions.
[00374] Clause 221. The electrochemical cell of Clause 220, wherein the
second
polar electrolyte solution is an alcoholic solution.
[00375] Clause 222. The electrochemical cell of Clause 221, wherein the
alcohol is
ethanol or methanol.
[00376] Clause 223. The electrochemical cell of any one of Clauses 220-
222,
wherein the oxidant is S2082- or sodium peroxydisulfate, or both, the metal is
aluminum,
the separation agent is sodium sulfate, and the cathode and anode are carbon
foam.
[00377] Clause 224. The electrochemical cell of Clause 223, wherein a
porous
stabilizer is in the first and second electrolyte solutions.
[00378] Clause 225. The electrochemical cell of Clause 224, wherein the
porous
stabilizer is glass wool, a borosilicate, or both.
[00379] Clause 226. The electrochemical cell of any one of Clauses 220-
225,
configured to operate in a flow mode.
[00380] Clause 227. A method which comprises providing additional oxidant
to the
electrochemical cell of any one of Clauses 220-226.
[00381] Clause 228. The electrochemical cell of Clause 226 or 227, further
comprising an inflow stream comprising an aqueous electrolyte solution.
[00382] Clause 229. The electrochemical cell of Clause 228, wherein the
inflow
stream further comprises an oxidant.
[00383] Clause 230. The electrochemical cell of Clause 229, wherein the
oxidant is
sodium peroxydisulfate or a solution comprising peroxydisulfate anion or both.
- 76 -
CA 03054957 2019-08-28
WO 2018/169855
PCT/US2018/021981
[00384] Clause 231. The electrochemical cell of any one of Clauses 226 and
228-
230, wherein an aqueous solution outflows from the cell.
[00385] Clause 232. A method which comprises removing metal sulfate from
the
electrochemical cell of any one of Clauses 226 and 228-230.
[00386] Clause 233. The electrochemical cell of Clause 229, wherein the
oxidant is
in an aqueous basic solution.
[00387] Clause 234. The electrochemical cell of Clause 233, wherein the
base is
NaOH.
[00388] Clause 235. The electrochemical cell of Clause 229 or 230 wherein
the
oxidant is solid Na2S208.
[00389] Clause 236. The electrochemical cell of any one of Clauses 220-
235,
which produces between about 10 Watt-hours/kg of electrolyte and about 100
Watt-
hours/kg of electrolyte.
[00390] Clause 237. The electrochemical cell of Clause 208, wherein
between
about 40 Watt-hours/kg of electrolyte and about 80 Watt-hours/kg of
electrolyte is
produced.
- 77 -