Note: Descriptions are shown in the official language in which they were submitted.
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
FLUORESCENT OXYGEN SENSING INK
BACKGROUND OF THE INVENTION
[0001] Chronic non-healing wounds (e.g., diabetic foot and bed sores)
impact over 6.5
million Americans per year, costs in excess of $25 billion to treat on an
annual basis, and
are on the rise due to increasing levels of obesity and diabetes compounded by
an aging
population. Current treatments are expensive, labor intensive, and generic,
relying on
regular cleaning, debridement, oxygen therapy, and topical or systemic
administration of
antibiotics. Commercially-available dressings (e.g., alginate, hydrogels,
hydro-colloids,
foams, etc.) have not proven to be significantly effective in reducing the
burden. An ideal
dressing integrates sensors (pH, oxygen, and inflammatory mediators),
drug/cell delivery
(antibiotics, growth factors, stem cells, and oxygen), and electronic
intelligence to
drastically improve wound care by measuring individual responses and enabling
appropriate adjustments to therapy.
[0002] Suboptimal oxygenation of the wound bed is a major healing
inhibitor in chronic
wounds. Unlike acute injuries that receive sufficient oxygen via a functional
blood vessel
network, chronic wounds often suffer from the lack of a proper vascular
network; thus
being incapable of providing sufficient oxygen for tissue growth. While the
lack of
oxygen may trigger vascular regeneration, the severity and depth of wounds can
prevent
adequate regeneration, causing wound ischemia. Modern medical treatment of
hypoxic
chronic wounds typically employs hyperbaric oxygen therapy, which requires
bulky
equipment and often exposes large areas of the body to unnecessarily elevated
oxygen
concentrations that can damage healthy tissue. A more practical approach is
topical
oxygen therapy (TOT) in which the dressing itself can generate the required
oxygen.
SUMMARY OF THE INVENTION
[0003] One aspect of the present invention is an ink that can be utilized
to fabricate
"smart" dressings for chronic wounds. A fluorescent oxygen sensing ink
includes an
organic solvent, a polymer binder such as ethyl cellulose, and a fluorescent
dye that is
dispersed or dissolved in the solution. The ink can be printed on a thin
flexible substrate
such as paper, and the ink forms a moisture resistant flexible film that can
be utilized in
1
CA 03055949 2019-09-09
WO 2019/168529
PCT/US2018/020284
an oxygen sensor. The smart dressing measures the amount of oxygen present in
a
wound and pumps more oxygen as necessary. The smart dressing integrates oxygen
delivery and sensing onto a single low-cost, manufacturable, flexible
dressing. The smart
dressing may be fabricated on a biocompatible substrate (e.g. paper) that
incorporates
patterned catalytic oxygen generating regions and an array of oxygen sensors
connected
to an electronic readout module. The use of a paper substrate provides
structural
stability and flexibility while simultaneously offering printability,
selective gaseous
filtering, and physical/chemical protection. However, it will be understood
that the
smart dressing is not limited to paper substrates, and virtually any
hydrophobic to
partially hydrophilic substrate may be utilized.
[0004] These and other features, advantages, and objects of the present
invention will
be further understood and appreciated by those skilled in the art by reference
to the
following specification, claims, and appended drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0005] FIG. 1 is a schematic cross sectional view of a smart wound
dressing with
integrated oxygen sensing and delivery according to one aspect of the present
invention;
[0006] FIG. 2 is a schematic isometric view of a test setup for measuring
oxygen diffusion
into agarose gel;
[0007] FIG. 3 is a graph showing oxygen generation and diffusion into
agarose gel;
[0008] FIG. 4 is a graph showing 3D distribution of oxygen diffusion rate
inside agarose
gel from a single oxygen generation spot;
[0009] FIG. 5A is a graph showing excitation spectrum (RFU) for PdTFPP
dissolved in
chloroform;
[0010] FIG. 5B is a graph showing excitation spectrum (RFU) for PdTFPP dye
dissolved in
chloroform (dried for 10 minutes);
[0011] FIG. 5C is a graph showing excitation spectrum (RFU) for PdTFPP
dissolved in
heptane;
[0012] FIG. 5D is a graph showing excitation spectrum (RFU) for PdTFPP
dissolved in
heptane (dried for 10 minutes);
[0013] FIG. 6 is a graph showing the excitation spectrum of (RFU) of
Ru(dpp)3Cl2
dissolved in chloroform;
2
CA 03055949 2019-09-09
WO 2019/168529
PCT/US2018/020284
[0014] FIG. 7 is a graph showing the excitation spectrum (RFU) of PdTFPP
with PS on
filter paper;
[0015] FIG. 8 is a graph showing the excitation spectrum of PdTFPP with
PDMS on filter
paper;
[0016] FIG. 9A is a graph showing emission spectrum for PdTFPP dissolved
in chloroform;
[0017] FIG. 9B is a graph showing PdTFPP dye dissolved in chloroform
(dried for 10
minutes);
[0018] FIG. 9C is a graph showing PdTFPP dye dissolved in chloroform
(dried for 30
minutes);
[0019] FIG. 10 is a graph showing the emission spectrum of Ru(dpp)3Cl2
dissolved in
chloroform;
[0020] FIG. 11 is a graph showing emission spectrum of PdTFPP with PS on
filter paper;
[0021] FIG. 12 is a graph showing the emission spectrum of PdTFPP with
PDMS on filter
paper;
[0022] FIG. 13A is a graph showing the emission spectrum of a RedEye
patch in water
with 0% dissolved oxygen concentration: (a) 0%; (b) 20%;
[0023] FIG. 13B is a graph showing the emission spectrum of a RedEye
patch in water
with 20% dissolved oxygen concentration;
[0024] FIG. 14A is a graph showing the emission spectrum of PDMS
encapsulated
Ru(dpp)3Cl2 in water with a dissolved oxygen concentration of 0%; 20%;
[0025] FIG. 14B is a graph showing the emission spectrum of PDMS
encapsulated
Ru(dpp)3Cl2 in water with a dissolved oxygen concentration of 20%;
[0026] FIG. 15 is a schematic diagram of an oxygen sensor and electronic
interfacing
circuitry;
[0027] FIG. 16 is a schematic view of inkjet-printed oxygen sensitive dye
with 7.5 mm
diameter circular spot size;
[0028] FIG. 17A is a schematic diagram showing the design of the
microfluidic network in
an oxygen generation patch or module, including: (a) a larger (85 mm x 65 mm)
model;
[0029] FIG. 17B is a schematic diagram showing the design of the
microfluidic network in
an oxygen generation patch or module, including a smaller (52 mm x 45 mm)
model;
[0030] FIG. 18 is a schematic showing the use of pressure rollers for
improving bonding;
3
CA 03055949 2019-09-09
WO 2019/168529
PCT/US2018/020284
[0031] FIG. 19 is a photographic image showing test patches bonded with
the use of
pressure rollers;
[0032] FIG. 20 is a schematic showing an experimental setup and 02
sensing;
[0033] FIG. 21 shows graphs of optical and electrochemical responses of
(a) a
commercially available Redeye oxygen sensing patch, and (b) printing Ruthenium
ink
according to one aspect of the present invention;
[0034] FIG. 22 is an electrical schematic of an oxygen sensing circuit;
[0035] FIG. 23 shows cytotoxicity of smart dressing components for oxygen
generation;
[0036] FIG. 24 is an image of oxygen sensitive dye printed on unrasted
parchment paper;
[0037] FIG. 25 is an image showing inject printed oxygen sensitive dye
with 7.5 mm
diameter circular spot size;
[0038] FIG. 26 is a schematic showing fabrication of an oxygen delivery
patch;
[0039] FIG. 27 is a photographic image of an oxygen delivery patch;
[0040] FIG. 28 is a photographic image of fluid (oxygen) patch arrays of
size 1x2 and 2x2;
[0041] FIG. 29 is a chart showing Cytotoxicity test results of smart
dressing components,
wherein cells were maintained in complete growth medium (Eagle's Minimum
Essential
Medium) ("EMEM") polydimethylsiloxane ("PDMS"), double-sided tape ("TT"), RU
(Ruthenium dye printed on parchment paper as 1, 2 or 3 layers) ("IRU," "2RU,"
and
"3RU," respectively), and negative control extract (NC) made from low density
polyethylene tubing;
[0042] FIG. 30 is a graph showing positive cytotoxicity control for WST-1
assay;
[0043] FIG. 31 is a chart showing cytotoxicity of smart dressing
components following
sterilization by a Sterrad process or by dipping in 100% ethanol;
[0044] FIG. 32 is a graph showing positive cytotoxicity control for WST-1
assay;
[0045] FIG. 33 is a chart showing cytotoxicity of paper sterilized by a
Sterrad process or
70% isopropanol; filter paper ("FP"); parchment paper ("PP"); laser-treated
parchment
paper ("LTPP"); parchment paper calendered by rollers 1 and 2 ("Ca11-2");
parchment
paper calendared by rollers 2 and 3 ("Ca12-3"); positive cytotoxicity control
(PC");
negative cytotoxicity control ("NK"); and
[0046] FIG. 34 is a graph showing positive and negative WST-1
cytotoxicity controls.
4
CA 03055949 2019-09-09
WO 2019/168529
PCT/US2018/020284
DETAILED DESCRIPTION
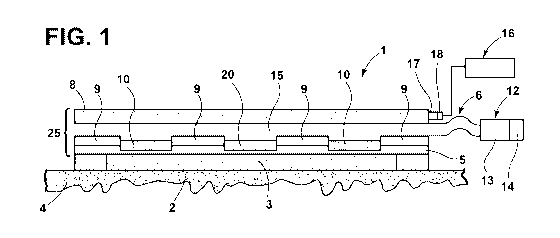
[0047] With reference to FIG. 1, a smart dressing 1 according to one
aspect of the
present invention includes a substrate layer 5 forming a back-bone onto which
one or
more oxygen generating modules 10 and oxygen sensing modules 20 are printed. A
fluid
conduit 6 is fluidly connected to a pump/reservoir unit 12 including a
reservoir 13 and
pump 14. A network of low-profile and flexible microfluidic channels 15 are
formed by
PDMS layers 8 and 9 that are bonded to the substrate layer 5. Fluid channels
15 guide
and delivers hydrogen peroxide from fluid conduit 6 to the catalyst-printed
regions 10. A
wound-facing side 2 of smart dressing 1 includes a collagen-glycosaminoglycan
biodegradable matrix (INTEGRA ) 3 which provides a scaffold for cellular
invasion and
capillary growth while permitting oxygen exchange between the
sensors/generators 20,
and a wound bed 4. The 3 matrix is retained in the wound 4 after initial
application.
The sensors/generators 20, 10 together with layer 5 and PDMS 8, 9 form a
module 25
that can be delaminated from the matrix 3 and replaced periodically.
[0048] As discussed in more detail below, other aspects of the present
invention include
reliable processes for inkjet printing the oxygen sensing/generating modules
10, 20 as
well as suitable lamination and bonding techniques (e.g., plasma, adhesives)
for
integrating the various layers of the smart dressing 1. An electronic readout
and control
module 16 is connected to an edge 17 of the smart dressing 1 via an edge-
mounted
connector 18 and a reservoir/pump module 12. The smart dressing 1 is connected
to the
reservoir/pump module 12 via fluid conduit 6 to supply H202through channels
15. The
reservoir/pump module may be fabricated via soft micro-molding techniques or
other
suitable processes.
[0049] Substrate layer 5 may comprise laser-treated parchment paper. In
particular,
laser-treated parchment paper possesses high mechanical strength (> 70MPa) to
withstand human motion, high elastic modulus when dry (>300kPa) for easy
handling
during fabrication, low elastic modulus (<50kPa) when wet for interfacing with
similarly
soft tissue, permeability to gas and not water at low pressures, and permeable
to oxygen
diffusion. When laser-rastered, the surface energy of the paper increases.
[0050] One aspect of the present invention is a fluorescent oxygen sensing
ink and
process for printing the ink. The ink generally includes a solvent, a dye, and
a polymer
5
CA 03055949 2019-09-09
WO 2019/168529
PCT/US2018/020284
binder. The solvent may comprise aqueous buffers or an organic solvent such as
ethanol,
dimethyl sulfoxide (DMSO), dimethyl formamide, isopropyl alcohol, acetone,
toluene,
and mixtures thereof. The fluorescent dye may comprise complexes of ruthenium,
osmium tetroxide, rhodium acetate, chromium, palladium or other dye that
fluoresces
when exposed to UV/visible light in the presence of oxygen. The ink may
comprise any
polymer based material that provides uniform dispersion or is completely
soluble in the
ink system for different additive print manufacturing processes such as
screen, gravure,
flexography, inkjet, aerosol jet. The particle size of the polymers dispersed
in the ink
solution are dependent on the nozzle size of the inkjet heads nozzles if
inkjet printing
processes are utilized. For example, if the nozzle diameter is 21 p.m, then
the particle
size should be less than 0.2 p.m to avoid agglomeration and clogging of print
head
nozzles. For printing the surface tension of the ink is preferably less than
the surface
energy of the substrate to adhere well. The surface energy of the substrate
(e.g. paper)
can be modified by employing UV, corona, plasma, sintering, or laser engraving
processes
to increase a surface energy of the substrate. The surface characteristics of
the substrate
can be modified as desired without adversely affecting or damaging the other
characteristics/properties of the substrate. The fluorescent ink according to
the present
invention can be printed on any hydrophobic to partially hydrophilic
substrates.
However, the ink typically cannot be printed on a substrate that is completely
hydrophilic. An advantage of the ink according to the present invention is
that it does
not require any transparent or translucent substrate or any additional
protective coating
materials.
[0051] As noted above, an ink according to the present invention
includes one or more
polymer binders that include alkyl substituted cellulosic materials. These
alkyl
substituted cellulose materials may be represented by Formula (I):
H oRi
OR2
0 R30
0
\ R30 0
OR2
H ORi (I).
6
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
In Formula (I), R1, R2, and R3 may each independently be hydrogen or an alkyl
group
having 1-8 carbons including, for example, methyl, ethyl, propyl, isopropyl, n-
butyl, sec-
butyl, pentyl, or combinations thereof. In some aspects, the polymer binder is
ethyl
cellulose. Ethyl cellulose has the following chemical structure where R1 is
ethyl, R2 is
ethyl, and R3 is hydrogen as represented by Formula (II):
cA
H #1 H
,
0 k) CH?
_______________________ Y's
$ #1 H H
ft(t tÃP,I4 04;,
4 4 0
(II).
[0052] Ethyl cellulose does not contain any sulphonic or phosphonic
groups or
naphthylene groups. Cellulose containings repeating anhydroglucose rings
having
hydroxyl groups at the 2', 3', and 6' positions that can treated with an
alkaline solution
resulting in an alkali cellulose which in turn is reacted with ethyl chloride
to yield ethyl
cellulose. In this reaction some hydroxyl (-OH) groups are replaced by ethoxyl
(C2H5)
groups. In some aspects, the degree of substitution of the 2', 3', and 6'
hydroxyl groups
may be from about 1.0 to about 3.0, from about 1.2 to about 2.6, from about
2.3 to
about 3.0, or from about 1.8 to about 2.2. In other aspects, the degree of
substitution
may be greater than 10%, greater than 20%, greater than 30%, greater than 40%,
greater
than 50%, greater than 60%, greater than 70%, greater than 80%, greater than
90%,
greater than 95%, greater than 98%, greater than 99% where the percentage is
relative
to the substitution of the 2', 3', and 6' cellulosic hydroxyl groups, or the
hydroxyl groups
may be quantitatively substituted with ethyl or other alkyl group. Not to be
bound by
theory but the increasing reactivity of the 2', 3', and 6' hydroxyl groups,
respectively, will
affect the substitution positioned as would be appreciated by one skilled in
the art.
[0053] Ethyl cellulose is an excellent water barrier film and provides
moisture resistance.
In contrast, other polymers such as nitrocellulose dissolve in water, and have
poor
moisture resistance. In other words, ethyl cellulose has hydrophobicity. Also,
ethyl
cellulose provides excellent film formation, adhesion, high mechanical
flexibility, and also
7
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
allows greater film coverage compared to other cellulose derivatives. For
example,
nitrocellulose requires additional materials including synthetic resins (such
as alkylated
resins, maleic resins, ketone resins, urea resins, polyurethane resins,
polyacrylates,
polyester and polyacrylate resins containing hydroxyl groups) and plasticizers
(diisobutyl
phthalate (DIBP), dicyclohexyl phthalate (DCHP), epoxidized soya oil (ESO),
triphenyl
phosphate) to the ink system in order to provide uniform film formation,
adhesion,
flexibility to printed layer of dye.
[0054] Although polydimethylsiloxane (PDMS) or polystyrene may be utilized
in some ink
formulations according to one aspect of the present invention, PDMS or
polystyrene
disperses in the ink solution and binds to the ruthenium ink. In contrast,
ethyl cellulose
completely dissolves in the ink solution, rather than be dispersed, and binds
with the
ruthenium dye to form a moisture resistant, flexible, continuous, and uniform
film. In
general, binders such as ethyl cellulose that dissolve completely or nearly
completely in
the ink solution provide better film formation, adhesion, and flexibility than
binders that
disperse in the ink system. An ink solution according to the present invention
requires
minimal materials and simple fabrication steps, and forms a continuous uniform
film with
superior adhesion and flexibility.
[0055] An ink formula according to the present invention may include 98
weight percent
of an organic solvent, a 1 weight percentage of dye, and a 1 percentage of a
polymer that
is preferably completely dissolved in the ink solution. The ink compositions
can be varied
as per the requirements of the additive printing processes.
[0056] To evaluate the ability to increase the oxygen concentration in a
wound bed,
oxygen diffusion was investigated on a surrogate wound bed (FIG. 2) comprising
a sample
of 0.3% agarose gel. An acrylic chamber 32 with open top 34 was assembled to
hold the
agarose gel sample 30. The chamber 32 includes an array of 2 mm holes 36
through a
side wall 32 to allow insertion of an oxygen probe 40. Prior to testing, 0.3 %
agarose gel
is prepared and stored in a hypoxic environment until ready for use. During
testing, the
agarose gel 30 is placed in the chamber 32. An oxygenation platform 45 was
constructed
by bonding laser-machined parchment paper 46 to PDMS 47 patterned with a
chamber
(3x3x2mm3) and a channel (18x1x2mm3). The laser- treated region within the
chamber
was a 3x3 mrn2 catalyst spot (deposited as described above). The chamber 34
was filled
8
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
with 30% H202 through the guide channel using a syringe pump to begin oxygen
generation.
[0057] The oxygenation platform 45 was placed on top (in contact with) of
the gel 30.
The test chamber 34 was then sealed with a Parafilm barrier to prevent
significant
oxygenation form the atmosphere. The same oxygen probe 40 is then inserted
into a
hole 36 of the test chamber 34, penetrating the gel 30 until the tip is
positioned 3mm
directly below the catalyst spot of the parchment paper 46. For this test the
oxygen
probe 40 was covered with a protector needle (not shown) to prevent mechanical
damage to the probe 40 during insertion. The remaining holes 36 in the chamber
34
were are sealed with adhesive tape to prevent oxygen diffusion from the
atmosphere.
The oxygen concentration in the gel 30 was monitored over time.
[0058] In clinical applications, the oxygenation platform may have an
interfacial material
between the parchment paper 5 and the wound to create intimate contact with
the
wound bed. To simulate this, the above experiment was repeated with a
commercial
dermal regeneration matrix (Integra , available from Integra Life Sciences
Corp.) as the
interface. The dermal regeneration matrix is 900 p.m thick and is composed of
cross-
linked bovine tendon collagen and glycosaminoglycan that is indicated for the
treatment
of acute and chronic wounds, including diabetic skin ulcers. A 1 cm x 1 cm
sample of the
dermal regeneration matrix was cut with a razor blade and sandwiched between
the
oxygenation platform 45 and the agarose gel 30. The rest of the experiment
proceeded
as above. As a control experiment, this test was repeated with empty
microfluidics (i.e.,
no H202)=
[0059] To investigate the range spatial effect of an oxygenation spot on a
gel substrate,
the oxygenation experiments were repeated for multiple locations, and the rate
of
oxygenation was plotted as a function of both vertical and horizontal distance
from the
generation spot.
[0060] The results from the diffusion experiments into agarose gel 3 mm
deep are
presented in FIG. 3. For the case without a dermal regeneration matrix, the
solid bold
line curve shows a monotonically-increasing oxygen level (from a partially
hypoxic level
of 15 % to 40 % 3 hours later) in the agarose gel 3 mm below an oxygenation
spot. The
curves show saturation in the oxygen level since for these experiments, a
fixed amount of
H202 was used (rather than a continuous flow). Although the level shown is not
100 %
9
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
saturation, the results do show that the platform is able to successfully
raise the oxygen
concentration 3mm within the gel 30 to levels which are far from hypoxic.
Therefore, if
the gel 30 were a wound, it would be reasonable to expect improved healing as
deep as
3mm (or more) as a result of the oxygenation platform.
[0061] The two remaining curves represent the tests with dermal
regeneration matrix
and show a different trend. In particular, the solid thin line curve,
corresponding to the
setup with dermal regeneration matrix and peroxide-filled microfluidics
contains an
initial shallow slope; this lag in the increase of oxygenation can be
attributed to the extra
time required for oxygen to diffuse through the dermal regeneration matrix
(integra)
layer. After 2.5 hours, however, the solid thin line curve exhibits its
highest rate of
change in oxygen concentration (slope of 18.9% per hour); this rate is similar
to the
largest rate of the sample without dermal regeneration matrix (17.1 % per
hour),
suggesting that although the dermal regeneration matrix causes an initial lag
in oxygen
diffusion, the eventual diffusion rate of oxygen approaches that of the oxygen
generation
platform 45. For comparison, the oxygen level does not increase during this
time for the
sample (dashed line curve) that does not contain peroxide in the
microfluidics.
[0062] One feature of the curves that should be clarified is the initial
drop in oxygen for
the two dermal regeneration matrix samples. For both of these cases, the data
shows an
initially normoxic oxygen level. This corresponds to the reading of the oxygen
probe 40
in atmosphere, prior to insertion into the gel 30 (at time 0). Following
insertion, the
oxygen concentration drops steadily. Although a quick drop in oxygen
concentration (to
hypoxic levels in the gel) might be expected, the curves show a 20-30 minute
steady
decay which may be attributed to atmospheric oxygen trapped in the probe
protector
needle (described in the experimental setup above) which needs time to diffuse
into the
gel 30. After 30 minutes, however, the curves reach their minimum values (the
oxygen
level in the hypoxic gel, 15 %02).
[0063] FIG. 4 shows the 3D spatial oxygen concentration by diffusing
through a 0.9mm
thick dermal regeneration matrix into the hypoxic gel. The maximum oxygen
diffusion
rate is 0.09 %/min (percentage per minute) at the surface of the gel just
below the
catalyst spot (Omm depth and Omm horizontal distance); while the minimum
oxygen
diffusion rate is 0.004 %/min at the position of 2.2mm depth and 15mm
horizontal
distance inside the gel. The oxygen diffusion rate show a normal distribution
in both the
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
depth and horizontal direction. Within the 80% area under the normal curve,
the critical
oxygen diffusion rate is calculated by 0.09/e=0.03 %/min. The oxygen generated
from a
3x3mm2 catalyst spot can therefore cover a range with the radius of 10mm
following the
surface and the depth of 2.2mm directly beneath it. The oxygen concentration
distribution through a single oxygen generation source provides an
experimental baseline
for designing an oxygen generation platform with multiple sources to achieve
an efficient
(optimal) oxygen delivery rate for a large scale chronic wound.
[0064] Another aspect of the present invention is a mass-reproducible
technique for
creating PDMS micro channels and bonding them to parchment paper patterned
with
selective catalyst and oxygen sensitive dye deposited. A repeatable bonding
procedure
between PDMS and parchment paper provides for high-volume production. Several
mass production technologies, such as screen printing, inkjet printing,
lamination, etc.,
may be utilized to produce smart dressings according to the present invention.
[0065] PdTFPP (5,10,15,20-Tetrakis(pentafluorophenyI)-21H,23H-porphine
palladium(II))
and Ru(dpp)3Cl2 (Tris (4,7-dipheny1-1,10-phenanthroline) ruthenium II
dichloride) are
suitable candidates for oxygen sensing materials due to their wide application
as oxygen
indicator. When the fluorescent dyes are exposed to UV/visible light (for
example 455
nm blue light) in the presence of oxygen, the oxygen atoms srike the
fluorescing complex
and cause a change in energy which quenches its fluorescence. A higher
oxygenated
environment creates a higher possibility for such collisions to happen between
oxygen
atoms and the fluorescent material, resulting in a lower fluorescence level.
The
fluorescent properties of two materials being capsulated in both poly-styrene
and PDMS
(polydimethylsiloxane) were then measured to determine the suitability of the
materials
in an oxygen sensing system.
[0066] PdTFPP was purchased from Sigma-Aldrich and Ru(dpp)3 was purchased
from
Cayman Chemical. A solution of the material was made by dissolving 1mg of
PdTFPP or
Ru(dpp)3Cl2 powder into 1mL of chloroform. For PdTFPP, 1mg of powder was also
dissolved into 1mL of heptane for testing. Poly-styrene (PS) sensing patches
were
prepared by mixing PS and dissolved fluorescent solution at a ratio of 1:10 by
weight. 20
pi of mixed solution was then cast onto filter paper with a diameter of 8mm.
The patch
was left in the nitrogen chamber for drying for 24-hours before testing. The
PDMS
encapsulated sensing patches were fabricated by firstly depositing fluorescent
solution
11
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
onto the filter paper. PDMS was then added to the filter paper after the
removal of the
solvent. The same amount of dye was used for all samples. The fluorescence
spectrum
was measured using a UV spectrophotometer. A zero oxygen solution was prepared
by
nitrogen bubbling a 0.15mol/L Na2S03 solution for 30 minutes.
[0067] Excitation spectrums of different oxygen sensing materials were
measured first,
as shown in FIG. 5. The x-axis is the wavelength of the excitation light, and
the y- axis
represents the RFU (relative fluorescence unit). An excitation peak at 407 nm
can be
detected. For comparison, PdTFPP was dissolved into two different solvents at
same
concentration. While comparing the chloroform solution, FIG. 5 (a-b), and
heptane
solution, FIG. 1(c-d), a smoother plot was obtained with chloroform solution.
With
reference to FIG. 5 (b)(d), a higher RFU can also be observed after 10 minutes
of
evaporation of the solvent. These results demonstrate that, the existence of a
solvent
affects the photo-property of the sensing material. Thus, to increase the
sensitive and
stability of the sensing film, any solvent that may be present should be
removed
completely before further fabrication.
[0068] With further reference to FIG. 6, the excitation spectrum of
Ru(dpp)3Cl2 was also
tested. Within the visible light range (390 nm-700 nm), an excitation peak at
460 nm was
detected. As compared to PdTFPP, a blue light source can be used for
Ru(dpp)3Cl2 while
UV light is required for PdTFPP. Ru(dpp)3Cl2 is therefore preferred for oxygen
sensors
that are embedded (integrated) with a wound dressing system.
[0069] The excitation spectrum was also measured for PdTFPP deposited on
filter paper
with PS (poly-styrene) and PDMS, as shown in FIGS. 7 and 8, respectively. A
higher peak
RFU can be observed with PS. When the sensing material being mixed with PDMS,
more
photo noise was introduced and fluorescent intensity (RFU) at the peak
decreased. As a
result, PS can provide a better photo-stability protection to PdTFPP without
compromising the photo characteristic.
[0070] Emission characterization was then conducted. When dissolved with
chloroform,
PdTFPP has three emission peaks. With reference to FIG. 9, a peak at 675 nm
remains
while solvent is being evaporated. Thus, the effect of solvent on the
fluorescent material
can be again confirmed from the emission profile. With reference to FIG. 10,
the photo
property of dissolved Ru(dpp)3Cl2 in chloroform was not affected by the
solvent. A clear
peak is illustrated at 625 nm. FIGS. 11 and 12 compare the emission profile
while PdTFPP
12
CA 03055949 2019-09-09
WO 2019/168529
PCT/US2018/020284
being deposited with PS and PDMS. Same trend was observed as the sample with
PDMS
had three emission peaks which is not expected for oxygen sensing.
[0071] For the validation of the concept, emission intensity under
different oxygen levels
was also tested using the same experiment setup. For comparison, RedEye was
purchased which has been widely used as non-invasive oxygen indicator. PDMS
encapsulated Ru(dpp)3Cl2 and PS encapsulated PdTFPP were also tested. The
results are
shown in FIGS. 13-15. A fluorescent intensity difference of 3.38 times was
obtained from
the commercial patch. For Ru(dpp)3Cl2 and PdTFPP, a difference of 1.4 times
and 2.79
times, respectively, were measured under various oxygenated environments. The
emission intensity decreases with an increase of the oxygen concentration in
water from
0% to 20%.
[0072] In order to protect the functionality of the sensing materials
during sterilization,
the photo properties of different retrieved samples were measured after a
sterilization
process (H202 vapor treatment), as shown in Table 2. Ru(dpp)3Cl2 was tested to
be more
vulnerable to the H202 vapor treatment and PS is needed for protection. PdTFPP
is more
stable as compared to Ru(dpp)3C12; however, a decrease of the photo-reaction
intensity
can be observed after sterilization when no polymer was added.
'rt Beote Met, kstwtioa Atte,: s.telkmtkm
Mz?,+;= F s4'
Rkz(dk:3-30.,- 4-PMS NiM= dO* pa%i
*qge0 tetak't
+PS ph.:1 dae.e.te.; ____ P8A
PdIFPP fat;:r W.4h,a( =trkvb:N4
wmvie
P:ITF:PP +PMI's =i>tn*det.,-;,:*d PeA a4te.:Uxi:
u.,mt.sv=
PaPPP 4S Pa}; ftmave Pe.A Wyge, .mitW
srmtk Emta iw peat mommtnotg Pe.,õu*skAw
13
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
[0073] Printing techniques for the manufacturing of electronics on
flexible substrates
have been developed. Printing methods that have been investigated for the
direct
printing of electronics include, but are not limited to, inkjet, flexographic,
screen and
rotogravure printing.
[0074] An oxygen detection sensor according to one aspect of the present
invention may
be fabricated using inkjet printing process. In inkjet printing, precise
control of ink
interactions at the substrate surface may depend on the ink formulation and
substrate
morphology. Typically, in inject printing, the viscosity of the ink should be
below about
centipose (cP) to properly jet the ink from the nozzles of cartridge. In
printed
electronics, various applications may require substrates with different
surface properties.
[0075] Four different substrate materials were tested for oxygen
generation. These
materials included: unrasted parchment paper, laser rastered parchment paper,
unrasted
Tyvek paper and laser rasted Tyvek paper. For the preparation of ink,
Tris(4,7-
dipheny1-1,10-phenanthroline)ruthenium(II) dichloride dye, ethanol (ACS
spectrophotometric grade) solvent and polymers (binders) such as polystyrene
(38%
emulsified in H20) and polydimethylsiloxane (PDMS) was used.
[0076] Substrate characteristics such as roughness and thickness were
measured using a
Bruker ContourGT-K interferometer. The average thickness of the unrastered
parchment, rastered parchment, unrastered Tyvek and rastered Tyvek
substrates were
measured to be 73.8 2.2 p.m, 73.3 1.1 p.m, 206.2 4.2 p.m, and 208.8
6.3 p.m,
respectively. The root mean square (RMS) roughness of the unrastered
parchment,
rastered parchment, unrastered Tyvek and rastered Tyvek substrates were
measured
to be 7.0 0.5 p.m, 6.5 0.4 p.m, 5.1 0.1 p.m and 4.6 0.4 p.m,
respectively. From the
measured values, it is understood that thickness of the substrates were not
impacted by
laser rastering. The roughness of the rastered Tyvek paper substrate was
decreased by
10.9% when compared to the unrastered Tyvek paper. Similarly, the roughness
of the
rastered parchment paper substrate was decreased by 7.8 % when compared to the
unrastered parchment paper.
[0077] Two test ink solutions were prepared for testing, including:
1. Dye + Ethanol + Polystyrene (1:100:1, by mass) ¨ 0.3 g of dye and
0.3 g of
polystyrene were mixed with 30 g (40 ml) of ethanol solvent.
14
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
2. Dye + Ethanol + PDMS (1:100:1, by mass) ¨0.3 g of dye and 0.3 g of
PDMS were
mixed with 30 g (40 ml) of ethanol solvent.
Both test ink solutions were mixed on a hotplate with magnetic stirrer at 525
rpm;
overnight (12 hours) under a fume hood to obtain homogenous ink solutions.
[0078] The Z-number is a dimensionless constant, and a measure of
density, surface
tension and viscosity. For proper jetting of ink, the Z-number should be in
the range of
about 2 to about 10. The formula for Z is:
tie 1,,
Z ' 0/101'1
Where:
d is the nozzle diameter (21.5 p.m),
p is the liquid density,
y is the surface tension and
n is the ink viscosity.
[0079] Inks with viscosity less than 10 cP are typically preferred for
inkjet printing. For
the dye + ethanol + polystyrene based test ink solution (solution #1), the
surface tension
was measured using the FTA200 and is 21.95 0.1 dynes/cm. The measured
density of
test ink solution #1 was 0.766 g/ml.
[0080] To determine the viscous behavior of ink solution #1 under a broad
range of
temperatures from 20 C to 60 C, the rheometer was used. The shear rate was
maintained at 1000 (1/s) and the viscosity was decreased from 3.77 cP to 2.11
cP for the
temperature range of 20 C to 60 C. After substituting the measured values, Z-
numbers
ranging from 5 to 9 were calculated as the temperature increased from 20 C to
60 C.
The test results show that ink solution #1 is suitable for inkjet printing at
room
temperature.
[0081] For the dye + ethanol + PDMS based ink solution (solution #2), the
measured
surface tension was 21.81 0.08 dynes/cm. The measured density of ink
solution #2 was
0.7669 g/ml. For the shear rate of 1000 (1/s), viscosity decreased from 3.76
cP to 2.10 cP
for the temperature range of 20 C to 60 C. The calculated Z-number for ink
solution #2
increased from 5 to 9 as the temperature increased from 20 C to 60 C. Based
on these
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
test results, it is evident that ink solution #2 is also suitable for inkjet
printing at room
temperature.
[0082] The Z-number and other characteristics such as viscosity, density
and surface
tension for both ink solutions are similar. This may be because the effect of
very small
quantities of polymers, polystyrene and PDMS in the ink solutions is
negligible.
[0083] The contact angle was measured for ink solution #1 on the four
substrate
materials (unrastered parchment paper, laser rasted parchment paper, unrasted
Tyvek
paper and laser rastered Tyvek paper) using an FTA 200 instrument. The
measured
contact values of the ink drops on the unrastered parchment paper, rastered
parchment
paper, unrastered Tyvek paper and rastered Tyvek paper substrates were 20.7
0.1
degrees, 35.3 0.5, 13.2 0.7 degrees and 12.4 0.1 degrees, respectively.
While
measuring contact angles with the FTA200 instrument it was observed that the
ink drops
were spreading rapidly on both the unrastered and rastered Tyvek substrates.
Even
though it is evident from the contact values that all the substrates possess
good wetting
characteristics, the tested Tyvek substrates may not be suitable for printing
due to rapid
spreading of ink on the surface. Thus, surface modifications of Tyvek
substrates may be
required. For example, plasma or UV treatment may be utilized to alter the
surface
properties (to increase contact angle) of Tyvek materials so that spreading
of ink on the
surface can be controlled prior to curing of the ink.
[0084] An oxygen sensing and electronic interfacing system (bandage)
according to the
present invention can be used to measure and maintain a suitable amount of
oxygen at
the interface of the wound and the bandage. In short, the smart dressing 1 of
the
present invention measures the amount of oxygen present, and pumps more oxygen
as
necessary.
[0085] Smart Dressing 1 includes an optical oxygen sensor (module) 20 with
a signal-
processing circuit to monitor the amount of oxygen present. A ruthenium-based
dye may
be utilized as the oxygen-dependent (sensing) compound. Similar to other dyes,
when
the ruthenium dye is excited by a blue LED, it produces an orange
fluorescence. The
fluorescence signal is dependent on the amount of oxygen present. In contrast
to
hypoxic conditions, it is known that when oxygen is present, fluorescence is
less intense
and decays more quickly. By characterizing the fluorescence, the amount of
oxygen
present can be quantified (measured). One method to quantify oxygen is to
excite the
16
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
dye until it reaches a steady state, then directly measure the peak intensity
as well as the
time it takes to decay. However, this method is sensitive to the precise
positioning of the
LED source, the amount of background light present and the inevitable
photobleaching of
the dye over time. To avoid these issues, a system according to one aspect of
the
present invention modulates the excitation blue LED at a frequency between 20
kHz and
75 kHz. The phase difference between blue excitation and the resulting orange
fluorescence signal can be measured. This phase difference changes with the
amount of
oxygen present.
[0086] A DC bias to an excitation source (blue LED) may be provided to
turn it on. An AC
signal may then be superimposed to modulate the intensity of the blue LED. The
resulting fluorescence signal has a phase shift that increases with the amount
of oxygen
present. The fluorescence signal may be amplified and processed so that its
phase can
be compared with the excitation signal. This phase shift is an indicator
(measurement) of
the amount of oxygen present.
[0087] Phase detection may be accomplished with digital logic by using a
single exclusive
or (XOR) gate. When the XOR gate receives two in-phase signals, its output is
low (0 volts
DC). When the XOR gate receives two completely-out-of-phase signals, its
output is high
(e.g. 4.5 V DC). When the signals are slightly out-of-phase, the output of the
XOR gate is
high for a short time, and then low for the rest of the cycle. The assertion
time (pulse
width) for one cycle of the XOR gate's output increases as the input signals
move more
out-of-phase. The output of the XOR gate may be low-pass-filtered to produce a
DC
output that corresponds to the phase of its inputs.
[0088] Signal generation, measurement and decision making may be mediated
by a
microcontroller 50. The microcontroller 50 generates a square wave at a
specified
frequency, which is converted to a DC-biased sine wave by a filter 52. The DC-
biased sine
wave from filter 52 drives a blue LED 52, so that blue light from the LED 54
excites the
ruthenium dye 56. The excited ruthenium dye 56 fluoresces, and the sinusoidal
fluorescence (orange) is measured with a highly-sensitive photodiode 58. The
photodiode 58 includes a light filter so that it picks up only the orange
fluorescence of
the dye 56, and not the blue LED's emission. A processed fluorescence signal
is sent from
the photodiode 58 to a phase comparator (detector) 60. Because the phase
detector 60
requires square wave inputs, the sinusoidal fluorescence signal is converted
to a square
17
CA 03055949 2019-09-09
WO 2019/168529
PCT/US2018/020284
wave by a comparator circuit. After the fluorescence signal is converted, its
output is
sent to the phase comparator 60.
[0089] Finally, the microcontroller 50 receives a DC input from the phase
comparator 60
that represents the current oxygen present. The microcontroller 50 then
decides
whether or not to pump hydrogen peroxide to generate more oxygen.
[0090] As discussed above, untreated Tyvek is generally not suitable for
printing.
Therefore, the surface properties of the Tyvek substrate may be altered by
treating its
surface with a fusion UV system. Typically, UV treatment raises the surface
energy of a
substrate through oxidation which in turn increases the polar energy,
potentially
providing improved wetting. For test purposes, unrastered and rastered Tyvek
substrates were UV treated 1 to 4 times. The contact angle of the ink drops on
the
substrates were then measured (Table 3).
[0091] The contact angle of the unrastered and rastered Tyvek substrate
before UV
treatment was measured as 13.2 0.7 degrees and 12.4 0.1 degrees,
respectively. The
contact angles of the unrastered Tyvek substrates that were UV treated for 1
time, 2
times, 3 times and 4 times were measured as 14.8 0.1 degrees, 13.4 1.1
degrees, 12.4
1.1 degrees and 12.7 1.6 degrees, respectively. Similarly, the contact
angles of the
rastered Tyvek substrates that were UV treated for 1 time, 2 times, 3 times
and 4 times
were 10.5 1.1 degrees, 13.8 1.0 degrees, 14 1.4 degrees and 12.1 0.6
degrees,
respectively.
18
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
Table 3: Contact angles of the UV treated Tyvek substrates.
Contact ange
Tyvek before UV No, of times Contact angle after
Substrate treatment substrate treated UV treatment
(degrees)
(degrees)
14.6
13,4 1.1
Unrested 13.2 O:7
3 12.4 1.1
121 1.6
1 10.5 1..1
2 13.8 1.0
Rested 12.4 0.1
3 14.0 lA
4 12.1
[0092]
From the measured contact angles and through the live video option (spreading
and absorbing behavior of the drops on the substrate can be seen) in the FTA
200
software, it was concluded that the impact of the UV treatment on the surface
of the
Tyvek substrates is minimal. Therefore, further characterizations on the
Tyvek
substrate were not performed.
[0093] It was observed from measured values that the roughness of
parchment paper
samples was not consistent. In order to obtain a similar smoothness over the
surface of
parchment paper, a calendering process was employed. A calendering machine was
used
to calender both sides of the parchment paper, with an applied pressure of 35
Psi (241
kPa). It was observed that, due to calendering, the roughness of the parchment
paper
was reduced from 8.7 1.7 p.m to 5.5 0.4 p.m (Figure 1.3 (a) and (b)).
19
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
[0094] During testing, multi-layer samples (5 layer, 3 layer and 1 layer)
of ruthenium dye
based ink, with ethanol as solvent and PDMS as binder, was inkjet printed on
to both
unrastered and rastered parchment paper in an array of circular spots with a
diameter of
mm with 20 p.m drop spacing, using a DIMATIX inkjet printer (DMP 2831). The
ruthenium ink with polysterene as binder could not be inkjet printed because
of its
comparatively large particle size (<500 nm). The ruthenium ink with PDMS was
loaded
into a DIMATIX DMC-11610 cartridge (10 pl) through a 25 mm disposable Whatman
syringe filter, with a poly vinylidene difluoride filter (PVDF) filter
membrane of 0.2 p.m
pore size, to filter any large particles that may have agglomerated in order
to achieve
smooth printing. Each layer of the printed ink was cured on the stage of the
inkjet
printer for 5 minutes at 60 C. A 27 V actuation voltage was applied at 5 kHz
firing
frequency. The corresponding waveform and cleaning cycles employed for inkjet
printing
are shown in FIGS. 16 and 17, respectively.
[0095] Roughness and thickness measurements were performed to
characterize the print
quality of the printed samples (Table 4). The root mean square (RMS) roughness
of the 5
layer, 3 layer and 1 layer printed sample was measured to be 6.0 0.03 p.m,
6.4 0.48
p.m and 6.7 0.44 p.m, respectively for the unrastered parchment paper.
Similarly, a
roughness of 6.0 0.42 p.m, 6.2 0.31 p.m and 6.8 0.29 p.m was measured
for the 5
layer, 3 layer and 1 layer printed samples, respectively for the rastered
parchment paper.
Before printing, the roughness of the unrastered and rastered parchment paper
substrates were measured to be 7.0 0.5 p.m and 6.5 0.40 p.m, respectively.
From the
measured roughness values, it is thus understood that the thickness of the
substrates
could not be measured because the printed ink did not cover the entire
roughness of the
substrates (Inkjet printing provides a layer that is about 0.5 p.m thick).
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
Table 4 Roughness measurement of the inkjet printed ruthenium dye based ink.
Roughness
Roughness Alter
Substrate Before Printing No, of Layers
Printing (pm)
(Prni
Unrested
Parchment 7 0,5 m 3 6A 048
Paper
5
Rasted
Parchment 6,5 0,40 pm 3 6.2 0,31
Paper
1
[0096] After initial tests for oxygen sensing, the circular spot size was
increased to 7.5
mm to increase the concentration of dye. With reference to FIG. 16, an array
of circular
spots was inkjet printed (10 layer, 7 layer and 5 layer) on the unrastered
parchment
paper with 10 p.m drop spacing and cured at 45 C.
[0097] From the printed samples, it was observed that thermally cured
ruthenium
particles were falling off from the parchment paper. This was due to poor
adhesion
between the ink and the parchment paper substrate. If the surface energy of
the
substrate is higher than the surface tension of the ink at least by 10
dynes/cm, then the
ink binds/adheres well to the substrate. The surface energy of the calendered
parchment paper was measured with the FTA 200 using Owens-Wendt method and was
calculated as 21.99 dynes/cm. The surface tension of the ink is 21.81 0.08
dynes/cm.
The poor adhesion of the ink was caused, at least in part, by the small
difference (less
than 10 dynes/cm) between the surface energy of the substrate and surface
tension of
the ink. To improve adhesion, the surface energy of the substrate may be
improved
(raised) either by UV or corona treatment. During testing, the surface of
parchment
21
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
paper was UV treated 4 times using the Fusion UV System 1300MB. The surface
energy of
the UV treated parchment paper was measured to be 22.09 dynes/cm. Thus, the
impact
of the UV treatment on the parchment paper is minimal. Similarly, no impact
was
observed with the corona treatment on the surface of parchment paper.
[0098] As discussed above, smart dressing 1 (FIG. 1) includes fluid
channels 15. FIGS. 17A
and 17B show microfluidic networks 65A and 65B, which represent examples of
the
channels 15 of FIG. 1. White regions or lines 67 comprise fluid channels, and
black
hexagonal areas 69 represent locations (cells) of the oxygen sensing dye.
FIGS. 17A and
17B shows two designs, a smaller one 65A, and a larger one 65B, to allow the
patch to be
adapted for wound of various sizes. The designs 65A and 65B exhibit a
honeycomb
pattern due to the spatial and radial uniformity of the designs. The size of
the hexagonal
unit cells 69 was determined based on the test results discussed above.
Specifically,
testing showed that a 1 mm oxygen-generating spot could influence oxygenation
within a
5-10 mm radius. Thus, the unit cells 69 have a radius of 7.5 mm. Testing of
large-area
bonding of PDMS to parchment paper via the use of partially-cured PDMS (as an
interface between the two layers) was also performed. Testing revealed that
this
technique is not sufficiently strong for assembling larger devices because it
often results
in delamination and/or leakages.
[0099] To remedy the leakage and reliability issue, a bonding process
utilizing lamination
rollers was developed. Such rollers are typically found on hot lamination
machines.
During testing, a commercially available hot laminator machine 68 (Apache) was
incorporated into the fabrication process as shown in FIG. 18. Specifically,
paper/PDMS
bilayers 72 were passed through rollers 70 before curing was complete,
allowing the
rollers 70 to apply pressure and squeeze out trapped gasses from the interface
between
the paper and PDMS. This technique allowed the successful creation of patches
with
stronger bonding and no leakages. Examples of these are shown in FIG. 19. The
left-
hand subfigure (image) shows a patch with Mn02 catalyst deposited on only the
junctions of the microchannels (a few of which are identified with red
circles), whereas
the right-hand subfigure (image) shows a patch with channels that have been
lined with
Mn02 (an alternative, pattern-less technique). The resulting patches exhibit
functionality
and robustness while remaining thin for conforming to human skin.
22
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
[00100] As discussed above, smart dressing 1 senses (measures) oxygen
levels, and
provides controlled flow of oxygen to a wound based, at least in part, on
measured
oxygen levels. Oxygen delivery is initiated with injected H202 over the
printed Mn02 on a
parchment paper. The volume and duration of oxygen delivered to the wound is
precisely controlled. The volume duration of oxygen is also observed
(measured) while
the smart wound dressing is worn by a patient. The concentration of 02 is
monitored
(measured) in real time to control the 02 delivery based on a user's demand,
or the
condition of the patient.
[00101] A Ruthenium complex may be used to measure the concentration of
oxygen at
the wound. During testing, the performance of printed Ru(dpp)3Cl2 (Ruthenium
dye)
with different combinations of materials was characterized. First, a
commercial optical
oxygen sensor (Redeye patch from Ocean Optics) was characterized and
observed.
Then, printed Ruthenium dye was characterized with different compositions of
PDMS,
polystyrene, and ethanol/chloroform.
[00102] The main mechanism of this sensor is the attraction of oxygen atoms
by the
Ruthenium-complex. Ruthenium dye excites when exposed to light have a
wavelength of
about 455 nm (Blue), and emits fluorescence of 610 nm (red). The Ruthenium dye
fluorescence quenching is observed when oxygen atoms collide to fluorescence
Ruthenium-complex transferring its energy. Thus, the quenching fluorophore
results in
lower fluorescent intensity in an environment having a high concentration of
oxygen
environment. This printed oxygen sensor can work in range of 0 to 100 % of
oxygen
environment. It is used mostly for in situ and real-time monitoring of oxygen
generation
in water. For the accuracy in measurement and observations, both emitted
wavelength
of the fluorophore and dissolved oxygen concentration in water were
characterized by
optical and electrochemical sensors.
[00103] For testing purposes, Ruthenium dye 84 (FIG. 20) was printed
(diameter of 7.5
mm) on a parchment paper, then attached to transparent double-sided tape 74.
The
tape 74 was then placed on a wall 76 of a water container 78 facing outside
for optical
measurement as shown in FIG. 20. Water 80 was deoxygenated by pumping N2 gas
over
30 minutes. After deoxygenating, initial dissolved oxygen concentration of the
water was
measured with an electrochemical probe 82. The measured oxygen concentration
was
about 0.2 ppm at room temperature. It will be understood that "normal"
(untreated)
23
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
water contains oxygen concentration of 8 to 9 ppm (1 ppm = 1 mg/L) in air. An
optical
probe 86 was placed about 2 mm away from the wall 76 of the water container 78
and
positioned perpendicular to the dye 84. The electrochemical probe 82 was
dipped into
the water 80 deep enough so that a thermal sensor embedded in the probe 82 was
completely submerged under the water 80. Two probes were calibrated before the
measurement using two-point calibration (0 % and 100 % saturated water). A
stirring
magnet 88 was placed under the water 80 and stirred at 150 rpm. This ensured
that to
make 02 concentration inside the container was at equilibrium at all time.
[00104] Characterization was first conducted with a Redeye patch, and
compared to a
first printed Ruthenium sensor. To optimize the performance of the printed
dye,
multiple layers of sensors with different compositions were tested. These
tests were
conducted with one test sensor unit left in air up to 21 % of oxygen
concentration (9
ppm) and another pumped with 02 gas close to 100 % of oxygen dissolved in the
water.
[00105] Dissolved oxygen measurement and corresponding fluorescent
lifetime of a
Redeye patch (commercially available oxygen sensor, Ocean optics) was first
characterized by leaving deoxygenated water 80 in air above open top 77 of
container
78, thereby slowly dissolving (absorbing) oxygen. An initial reading of the
Redeye patch
in the deoxygenated water had maximum fluorescent lifetime of 20.342 sec at
0.62
ppm of dissolved oxygen. Data was collected every 15 minutes until the oxygen
concentration reached equilibrium to air (9 ppm). The graph (a) of FIG. 21
shows that
fluorescent lifetime of the Redeye patch exponentially decreases over time
until it is in
equilibrium to air. After water reaches the equilibrium with air around 9 ppm,
the
fluorescent lifetime was saturated around 9 sec as shown from the plot.
Resulting
fluorescent lifetime at 9 ppm was 9.247 sec.
[00106] A first batch of printed Ruthenium test sensors according to one
aspect of the
present invention was characterized using the same setup (FIG. 20) as the
Redeye
patch. The samples of Ruthenium dye were printed in multiples layers of 3, 5,
and 10-
layers with compositions of Ruthenium dye, polystyrene, and chloroform in
1:1:100 ratio.
Sensors with 3 multiple layers were printed on two different conditioned
substrates
(parchment paper) that were laser treated and non-laser treated. The laser
treatment
was performed to increase the adhesion of the Ruthenium dye to the paper and
for
better absorbance of 02. Each sample was submerged and placed on the wall 76
of the
24
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
container 78 under deoxygenated water 80, then measured using both optical
probe 86
and electrochemical probe 82, leaving water to dissolve (absorb) oxygen from
air
overtime. Compared to the Redeye patch, the printed Ruthenium dye showed 0.2
times
smaller fluorescence lifetime at the initial reading of deoxygenated water.
The
fluorescent decay of the Redeye patch was 10 times faster than the printed
sensor as
shown by the lower graph (b) of FIG. 21.
[00107] The lower graph (b) of FIG. 21 shows that, the emitted wavelength
from the
excited Ruthenium dye was successfully detected using the optical probe 86.
The
fluorescent lifetime of the printed sensors also had exponential decay over
time like the
Redeye patch. However, multiple layered samples did not show a significant
variation
except 10-layered samples. Printed sensors with 10-layers had the lowest
performance
among the group due to the poor adhesion between the dye and the parchment
paper.
Some fragments of particles of the Ruthenium dye fell off from the parchment
paper.
Other test samples such as the 3 and 5 layer samples also were not uniformly.
However,
the sensing of these non-uniform 3 and 5 layer samples was not significantly
different as
was the case for the 10 layer samples. Laser treatment of parchment paper did
not show
a significant change in sensing as the plot shows between laser treated and
non-treated
samples.
[00108] During testing, a second batch of sensors were printed. These test
samples
included single layer samples and, 2 and 3 layer samples. The samples were
printed on
unrastered parchment paper. Materials used to print this batch were Ruthenium
dye,
ethanol, ethyl cellulose mixed in 1:1:100 ratio by weight. The second batch
samples
showed better uniformity compared to the first printed sensors. These samples
were
primarily characterized in a deoxygenated water container 78 having an opening
77
exposed to air/oxygen such that the water continuously dissolved oxygen from
the air
until the oxygen concentration in the water reached equilibrium.
[00109] Another aspect of the present invention is a portable circuit
which uses the
fluorescence quenching method to monitor oxygen concentration.
[00110] By exciting the ruthenium dye periodically with blue light,
measuring the periodic
fluorescence of the dye and calculating the delay between excitation and
emission, it is
possible to extrapolate oxygen concentration using the Stern-Volmer formula.
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
[00111] As discussed above, in connection with FIG. 15, a microcontroller-
generated
square wave may be fed into a series of low-pass filters, which act as a
square-to-sine
converter. The sine wave is then used to drive a blue LED 54 and excite the
ruthenium
dye 56. A transimpedance amplifier is used to capture the sinusoidal
fluorescence of the
ruthenium dye 56, and this fluorescence signal is then compared to the
original excitation
signal so that the microcontroller 50 can calculate the amount of oxygen
present and
control the hydrogen peroxide pump accordingly.
[00112] Circuit operation, corresponding to the schematic:
1. T1 MSP430G2553 Microcontroller provides a square wave at a pre-
programmed frequency
2. A series of low-pass filters remove the high frequency content of the
square wave, acting as a square-to-sine converter.
3. The sine wave is fed into a filtering operational amplifier, the output
of
which drives the excitation LED
4. The transimpedance amplifier (R11 = photodiode) picks up the
fluorescence signal
5. The sinusoidal fluorescence is converted into a square wave for
processing
6. An XOR gate compares the original square wave to the fluorescent square
wave. As the phase between the two signals increases, the outputted pulse
width
increases.
7. The output pulse of the XOR gate is low-pass filtered to a DC voltage.
Higher duty cycle of XOR gate corresponds with higher DC voltage.
[00113] The cytotoxicity of the materials used for the fabrication of the
smart wound
dressing 1 described above was investigated following standard ISO 10993-05
(Cytotoxicity) and ISO 10993-12 (Sample preparation and reference materials).
[00114] All samples were 0.5 mm thick and prepared as 8 mm-diameter discs
(surface
area of 0.50 cm2). Samples were sterilized by the STERRAD process (low
temperature
hydrogen peroxide gas plasma) and then extracted for 24 h/37 C in complete
growth
medium (Eagle's Minimum Essential Medium + 10% horse serum + 100 IU/m1
penicillin +
100 ug/mIstreptomycin) using an extraction ratio of 6 cm2/ml. At the time of
the
extraction, L-929 mouse fibroblast cells (NCTC clone 929: CCL 1, American Type
Culture
26
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
Collection, Manassas, VA, USA) in passage 3 were lifted from the culture flask
using
trypsin/EDTA. An aliquot was counted using trypan blue, and then cells were re-
suspended in complete growth medium at a density of 1 x 105 cells/ml. Cells
were
dispensed into wells of 96-well culture plates (1 x 104 cells/well) and
cultured at 37 C in a
humidified atmosphere of 5% CO2/95% air. After 24 h, the culture medium was
removed
and replaced with 100 p.I of extractant. Some wells received sodium dodecyl
sulfate (0
to 400 p.M in EMEM; positive controls), low-density polyethylene extract (1.25
cm2
LDPE/ml EMEM; negative control) or complete growth medium alone. Cells were
then
cultured for an additional 24 h. Images (mag. of 100 and 200x) of cell
cultures were
recorded by photo microscopy using a Olympus CK40 inverted microscope and
Insight2
SPOT camera (Diagnostic Imaging) and the number of attached and dead cells
were
manually counted at a later time using ImageJ (NIH). In addition, images were
graded for
morphological evidence of cytotoxicity using the ISO 10993-5 standard, where
the 0 to 4
scale represents no, slight, mild, moderate or severe cytotoxicity,
respectively.
Subsequently, cells in culture plates were washed once with Hank's Balanced
Salt
Solution and metabolic activity was measured by incubating cells with 100 p.I
of WST-1
cell proliferation reagent (Roche Diagnostics) for up to 4 h at 37 C. To
determine
cytotoxicity, absorbance of the medium in wells was measured at 450 nm after 2
and 4 h
using a microplate reader (PHERAstar) and was corrected using absorbance
measurements at 630 nm and using blanks. To check for mycoplasma contamination
of
the cultures, medium was saved and tested using the luminescent MycoAlert Plus
mycoplasma detection kit (Lonza).
[00115] Absorbance is proportional to the amount of formazan product
generated by the
metabolic activity of cells. Thus, lower absorbance values correlate with
increased
cytotoxicity. Mean absorbance values for cells treated with extracts of
palladium,
palladium + polystyrene or palladium + PDMS on paper substrates (0.734, 0.816
or 0.811,
resp.) are similar to values for cells incubated in EMEM alone (0.827) or the
LDPE extract
(negative control; 0.753). However, cells treated for 24 h with the extracts
of ruthenium
or ruthenium + polystyrene show considerable cytotoxicity (corrected
absorbance
readings of 0.318 or 0.089, resp.), with only 38.4% or 10.7%, respectively, of
the
metabolic activity of cells that were cultured in EMEM alone. The extract
created from
ruthenium + PDMS on paper was borderline non-cytotoxic, having a corrected
mean
27
CA 03055949 2019-09-09
WO 2019/168529
PCT/US2018/020284
absorbance reading of 0.625 or 75.6% (readings below 70% would be considered
cytotoxic according to ISO 10993-05).
[00116] The results are confirmed qualitatively via microscope images.
Micrographs of
cells treated with EMEM, palladium, palladium-polystyrene, palladium-PDMS and
ruthenium-PDMS extracted media had cytotoxicity scores of 0-1, while
micrographs of
cells treated with ruthenium and ruthenium-polystyrene extracted media had
scores of
2-3. According to the standards, scores > 2 are considered to be cytotoxic.
[00117] The results of the cytotoxicity assay on the various combinations
of oxygen-
generation materials are summarized in FIG. 23. Mean absorbance values for
cells
treated for 24 h in EMEM or extracts of Tyvek were 0.768 or 0.822,
respectively.
However, extracts made from Mn02 on Tyvek or Mn02 on parchment paper were
cytotoxic, producing mean absorbance values of 0.005 or 0.399, respectively.
This
represents metabolic activity of only 0.7% or 52%, respectively, of healthy
cells.
Morphological grading confirmed the findings. Cells treated with EMEM or
extracts of
Tyvek , Mn02 on Tyvek and Mn02 on parchment paper produced scores of 0, 1, 4,
and
3, respectively.
[00118] Cells in additional wells were cultured in 0-400 u.M sodium
dodecyl sulfate in
EMEM for 24 h to serve as positive cytotoxicity controls. The test results
confirmed that
increasing concentrations of SDS produced a graded and increasing cytotoxic
response as
expected. Mean absorption readings fell from about 0.7 to 0 between 0 and 300
u.M
SDS. Morphology scores ranged from 0 to 2 between 0 and 150 u.M SDS.
Concentrations
of SDS above 200 u.M were cytotoxic.
[00119] To test cell cultures for mycoplasma contamination, a MycoAlert
Plus kit was
used. Luminescence of the test solution is measured in the presence of reagent
alone or
reagent plus substrate and ratios are calculated and compared to positive and
negative
controls that are purchased with the test kit. Ratios < 0.9 are negative and
>1.2 are
positive for mycoplasma. Borderline values between 0.9 and 1.2 are retested
after 24 h.
The cell cultures used for the cytotoxicity study produced a ratio of 0.31
(negative), while
positive and negative controls produced ratios of 22.38 and 0.32,
respectively.
[00120] Using appropriate positive and negative controls, extracts of
palladium,
palladium + polystyrene and palladium + PDMS were non-cytotoxic. Ruthenium +
PDMS
was marginally non-cytotoxic. Extracts of ruthenium alone on paper and
ruthenium +
28
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
polystyrene were cytotoxic. Extracts of Mn02 on parchment paper or on Tyvek
were
cytotoxic, but Tyvek alone was non-cytotoxic. Cultures tested negative for
mycoplasma.
[00121] Based on the print quality of the ruthenium dye (Ru + Ethanol +
PDMS) ink
discussed above, it was concluded that the film formation of Ru dye and its
adhesion with
parchment paper is poor. This may be due to the insolubility of PDMS with the
ink
system. To improve both the film formation and adhesion, among various binders
such
as ethyl cellulose (polymer) ethyl cellulose was chosen because of its
solubility in ethanol
and better film formation properties. Ruthenium dye (powder form) is mixed
with
ethanol and ethyl cellulose in a 1:100:1 weight ratio on a hotplate with
magnetic stirrer
at 700 rpm for 20 hours at room temperature.
[00122] As discussed above, for ink jet printing the Z-number should be in
the range of
about 2 to about 10. Also, inks having a viscosity that is less than about 10
cP are
typically preferred for inkjet printing.
[00123] For the ruthenium dye + ethanol + ethyl cellulose based ink
solution, the
measured surface tension is 21.48 0.12 dynes/cm. The measured density of the
ink
solution is 0.78 g/ml. To determine the viscous behavior of the ink solution
under a broad
range of temperatures from 20 C to 60 C, a rheometer was used. The shear
rate was
maintained at 1000 (1/s) and the viscosity was decreased from 5.6 cP to 3.4 cP
for the
temperature range of 20 C to 60 C. After substituting the measured values, Z-
numbers
ranging from 3.4 to 5.5 were calculated as the temperature increased from 20
C to 60
C. It is therefore evident that the ink is suitable for inkjet printing at
room temperature.
[00124] During further testing, multi-layer samples (3 layer, 2 layer and
1 layer) of the
ruthenium dye based ink, with ethanol as solvent and ethyl cellulose as
binder, were
inkjet printed on to unrastered parchment paper in an array of circular spots
with a
diameter of 7.5 mm, with 10 p.m drop spacing and resolution of 2540 dpi, using
a
DIMATIX inkjet printer (DMP 2831). The ruthenium ink solution was loaded into
a
DIMATIX DMC-11610 cartridge (10 pl) through a 25 mm disposable Whatman syringe
filter, with a poly vinylidene difluoride filter (PVDF) filter membrane of
0.45 p.m pore size,
to filter any large particles that may have agglomerated in order to achieve
smooth
printing. Each layer of the printed ink was cured on the stage of the inkjet
printer at 55
C. A 40 V actuation voltage, applied at 5 kHz firing frequency, was employed
for inkjet
29
CA 03055949 2019-09-09
WO 2019/168529
PCT/US2018/020284
printing the ruthenium ink. The printed samples on the unrasted parchment
paper are
shown in FIG. 24.
[00125] From the printed samples, it was observed that the film formation
and coverage
of ruthenium dye with ethyl cellulose binder is good when compared to the
ruthenium
dye with PDMS binder. However, the adhesion between the parchment paper and
multiple layers of ruthenium dye (with ethyl cellulose binder) was potentially
insufficient.
[00126] As discussed above, the surface energy of calendered parchment
paper was
measured with the FTA 200 using Owens-Wendt method and was calculated as 21.99
dynes/cm. The surface tension of the ruthenium dye + ethanol + ethyl cellulose
based
ink solution is 21.48 0.12 dynes/cm. As also discussed above, the difference
between
the surface energy of the substrate and surface tension of the ink should be
greater than
dynes/cm to achieve good adhesion between the substrate and ink. Various
surface
treatments such as UV (Fusion UV Systems1300MB), corona (Electro-technic BD-
20v
corona treater) and sintering (Novacentrix pulseforge 1200) have been employed
to
improve/modify the surface energy of calendered unrastered parchment paper.
However, it is observed that these treatments have minimal or no impact on the
surface
of parchment paper.
[00127] However, testing revealed that laser surface treatments
significantly alter the
surface energy of parchment paper. Specifically, when the surface of
calendered
parchment paper is subjected to a laser ablation/rastering process using a PLM
6MW
laser machine (available from Universal Laser Systems), the surface energy
increased to
64 dynes/cm. The surface energy values shows that the laser rastering process
has a
strong impact on the surface of parchment paper. Also, during testing, the
contact angle
of ruthenium ink with ethyl cellulose binder with parchment paper was measured
as
30.37 1.35 degrees. This implies good wetting properties.
[00128] The ruthenium ink solution with ethyl cellulose binder was inkjet
printed on to
laser rasted parchment paper using the same settings discussed above.
Photographs of
the printed samples with multiple layers of ruthenium dye on the laser
rastered
parchment paper are shown in FIG. 25. It was observed that the adhesion
between the
ruthenium dye and the laser rastered paper is very good (confirmed by
placing/sticking
and removing a scotch tape on the printed dye). Also, digital microscope
images (not
shown) confirmed that the film formation and coverage of the ruthenium dye was
good.
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
[00129] However, some burnt fibers (black spots) were evident in the
rasted area due to
the application of high power intensity during the laser rasting process. In
order to
reduce or eliminate burning of fibers, a profile of power intensity and laser
speed effects
on the surface energies of the parchment paper may be utilized to identify a
suitable
laser rasting process that provides a surface energy value above 32 dynes/cm
without
burning of paper fibers.
[00130] A suitable binder (ethyl cellulose) was identified and used in the
ruthenium ink
system in place of PDMS and z-number has been calculated. The ethyl cellulose
binder
provided acceptable printed ruthenium film formation and coverage. Proper
adhesion
may be provided by laser rastered calendered parchment paper for inkjet
printing.
[00131] As discussed above, an oxygen generation patch may be fabricated
using partial
cured PDMS to bond parchment paper with laser-rastered spots to PDMS with
molded
microchannels. This method is capable of creating a flexible and conformable
wound
dressing patch. However, this process is time consuming, which may interfere
with large
scale production. Thus, processes that are suitable for large scale (high
speed)
production have been developed. Testing showed that the improved processes
improved the mechanical properties of the oxygen delivery system/platform and
reduced
fabrication cost.
[00132] With reference to FIG. 26, a method 90 may be utilized to
fabricate an oxygen
delivery patch. First, at step 91, double sided transparent tape 95 (e.g. 3M
300LSE) is
bonded to a layer of PDMS 96 utilizing an oxygen plasma process. At step 92,
the tape 95
is then laser-rastered (ablated) to form fluid channels 97 in a predefined
honeycomb
pattern 99 (see also FIG. 27). The tape 95 (with channels 97) and PDMS 96 form
a first
subassembly 98. The PDMS layer 96 may also be laser-rastered to a certain
depth,
provided the thickness of the rastered regions of the PDMS layer are not
reduced to a
level affecting the robustness of the patch 100. At step 93, a layer of
parchment paper
102 is laser-rastered (ablated) at selected surface regions 103, and oxygen
catalyst 104 is
inkjet printed on to the rastered spots 102 to form a second subassembly 105.
Oxygen
catalyst 104 may be printed utilizing ruthenium dye/ink (Ru + Ethanol + PDMS)
as
discussed above. It will be understood that forming the first subassembly 98
(steps 91
and 92) and forming the second subassembly 105 (step 93) may occur at the same
time
or at different times. The oxygen catalyst 104 forms a honeycomb pattern that
aligns
31
CA 03055949 2019-09-09
WO 2019/168529
PCT/US2018/020284
with the channels 97 of the tape 95 and PDMS layer 96. At step 94, the first
and second
subassemblies 98 and 105, respectively, are bonded together with catalyst 104
forming a
sidewall that closes off channels 97 to form fluid conduits 106 having a
honeycomb
pattern 99 (FIG. 27). During step 94, the parchment paper 102 is oxygen plasma
bonded
to the tape 95.
[00133] Peel strength testing of a patch 100 fabricated according to
process 90 (FIG. 26)
showed that the interface bond between PDMS layer 96 and parchment paper 102
is
about 7N per 2cm width. This is about twice the peel strength obtained using
partially
cured PDMS as the bonding glue.
[00134] Bonding strength testing was also conducted on a patch fabricated
according to
process 90 (FIG. 26). This testing was conducted to determine if the patch 100
can
withstand the pressure resulting from pumping hydrogen peroxide with a certain
flow
rate through the microchannels 106 during use of patch 100. In one test, the
outlet was
open and fluid was pumped at an escalated flow rate. In a second test, the
outlet was
sealed, and fluid was pumped with a fixed flow rate. Testing showed that a
patch 100
can withstand up to 30 PSI with a flow rate up to 7m1/min in the open outlet
case. Patch
100 can withstand up to about 3 PSI with a fixed flow rate at 30 ul/min in the
closed
outlet case.
[00135] The required flow rate for a wound dressing is about 10 ul/min.
Thus, the test
results show that a patch 100 fabricated according to process 90 (FIG. 26)
meets the
requirement of a sustained H202 pumping with a flow rate of about 10 ul/min
for several
hours.
[00136] Robustness testing to determine the effect of bending/curving of
patch 100 was
also conducted. Patch 100 is designed to conform to a shape/curvature of a
patient's
skin around a wound. The curvature may vary for different patients and wounds.
In
general, the patch 100 must not leak during continuous pumping of H202. During
the
robustness test, the patch 100 was folded into six different configurations
ranging from
about 90 degrees to about 180 degrees (fully folded). Thus, a patch 100 was
first tested
at a bend/fold (curvature) of about 90 degrees, followed by testing at a
greater
bond/fold of about 108 degrees, followed by a bend/fold of about 126 degrees,
etc. until
the maximum bend/fold of 180 degrees (6th curvature) was reached. H202 was
then
32
CA 03055949 2019-09-09
WO 2019/168529
PCT/US2018/020284
continuously pumped through the fluid microchannels/conduits 106 at a constant
flow
rate of about 0.1 ml/min for 6 hours.
[00137] The fluid pressure inside the microchannels 106 was also measured
continuously
for all six curvatures. The test demonstrated that the patch 100 provided a
constant
pressure range from about 0.4 to about 0.5 PSI. This indicates that the patch
100 can
sustain up to at least about 6 hours of continuous operation under a maximum
180
degree folding state (zero pressure would be detected if leakage had
occurred).
[00138] The process 90 (FIG. 26) is scalable to provide increased
production efficiency.
Specifically, with reference to FIG. 28, patch arrays (e.g. 1x2 and 2x2) may
be fabricated
using the procedure 90 (FIG. 26) utilized for a single patch. An array (e.g.
2x2 array) does
not require additional fabrication time compared to fabrication of a single
patch 100.
Thus, the process 90 and patch 100 provide improved mechanical properties and
also
increase fabrication efficiency in a scalable production process.
[00139] Additional characterization (testing) of the Ruthenium oxygen
sensors and
substrate (parchment paper) was conducted by measuring dissolved oxygen in
deoxygenated water. This additional testing was conducted using substantially
the same
test set up described above in connection with FIG. 20. First, multiple layers
of the Ru
dye (ink) (Ruthenium based ink with ethyl cellulose binder) were tested to
optimize its
performance. This formula produced a more uniform printing of the Ru dye (ink)
on the
parchment paper. Also, laser treated parchment paper was tested to determine
if laser
treating increased the adhesion of the printed Ru dye.
[00140] A test oxygen sensor was fabricated by printing Ruthenium (Ru) dye
on a piece of
parchment paper (Diameter = 7.5 mm), and the parchment paper was bonded to
double-
sided tape. Referring again to FIG. 20 the Ru printed side of the test sensor
was taped to
the wall 70 of the water container 78 facing outside for optical measurement
in
substantially the same manner discussed above in connection with FIG. 20.
Deoxygenated water was prepared before the experiment, and oxygen
concentration
was measured with both electrochemical and optical oxygen probes 82, 86
respectively.
During the experiment, oxygen gas was injected into the water 80 through
external
tubing (not shown). The stirring magnet 88 was utilized to ensure uniform 02
concentration. The experiment was conducted with three different sensors,
namely,
sensors having single, double, and triple layers of Ru dye.
33
CA 03055949 2019-09-09
WO 2019/168529
PCT/US2018/020284
[00141] The objective of this experiment was to test the fluorescence
lifetime decay of
the single and multi-layered Ru dye samples. Larger fluorescence lifetime
decay from
multi layered Ru dye was expected. From the previous experiment of printing Ru
dye,
highly concentrated Ru particles showed difficulties in printing due to the
viscosity of the
ink and mixing with solvent. Therefore, a method of multi-layer printing was
selected to
increase its range of quenching decay time of the fluorescence with more
oxygen
absorbance at the sensor. For this test, oxygen gas was injected to the
deoxygenated
water then measured with optical (usec) and electrochemical probes (1 mg/L = 1
ppm).
Oxygen gas injection was stopped when the measurement was taken. The gas
injection
continued until the oxygen concentration reached 27 mg/L, which was the limit
of
electrochemical probe 82. Double and triple layered Ru dye samples were
prepared, and
the fluorescent lifetime decay performance was measured (with oxygen gas
injected).
Fluorescence lifetime decays exponentially over saturation of oxygen gas in
the liquid.
The fluorescence lifetime decay was measured up to about 25 to about 27 mg/L
due to
limit of the measuring device. Nevertheless, measurement was compared at 9
mg/L,
since it is 21 % of oxygen concentration in room temperature. Lifetime decay
were ¨
0.101 and ¨0.109 sec for double and triple layer samples, respectively.
Triple layered
samples showed higher changes in quenching decay time of fluorescence. However
this
different is not significant compared to the results for the double layer
samples. Also,
the single layer samples showed better quenching fluorescence at around 0
percent
dissolved oxygen, resulting in larger changes of fluorescence lifetime at 9
mg/L. Oxygen
absorbed from the parchment paper through the multi-layered Ru dye may not be
effectively diffused through each layer. Also, the gradients of multi-layer
printed Ru dye
samples were more significant compared to the gradients of single layer
samples. Thus,
multiple layers of printed Ru dye do not appear to be effective with respect
to increasing
the performance of quenching fluorescence decay.
[00142] Additional testing was also conducted to compare the performance
of printed
oxygen sensors on rastered and unrastered parchment paper to determine if
rastering
provides increased adhesion. As discussed above, printed Ru dye on unrastered
parchment paper tended to adhere poorly, and particles from the printed sensor
fell off
the unrastered parchment paper.
34
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
[00143] During testing, parchment paper was rastered with a laser
engraving machine.
Test samples were fabricated by printing Ru dye in single, double, and triple
layers on
laser engraved (rastered) parchment paper. Three experiments were repeated for
each
group of test samples. Both unrastered and rastered parchment paper showed
exponential fluorescence lifetime decay. At an atmospheric oxygen level of
21%, the
single layer test samples resulted in a faster fluorescent lifetime drop
compared to the
multiple layer test samples.
[00144] The decay rate up to 9 mg/L was similar in both unrastered and
rastered single
layered test samples. As a result, the multi-layered Ru dye test samples had a
smaller
change of lifetime decay compared to the single layer test samples. Consistent
with prior
observations, the printed Ru dye on unrastered test samples tended to separate
from the
parchment paper, and edge portions of the printed Ru dye fell apart during
most of the
experiments. Test samples having a single layer of Ru dye printed on rastered
parchment
paper had significantly better adhesion. Based on these results, a suitable
oxygen sensor
can be fabricated by printing a single layer of Ru dye onto a rastered
parchment paper
surface.
Fluorescence lifetime decay up to 9 mg/L
Iwair clinraste1e0 (Rastere4
1 -1i47 -1.891
- 0357 -1,066
3 -0.771 -0.579
[00145] The cytotoxicity of the materials used for the fabrication of the
smart wound
dressing 1 was investigated following standard ISO 10993-05 (Cytotoxicity) and
ISO
10993-12 (Sample preparation and reference materials). This subsection
describes the
methods and presents the results of the cytotoxicity experiments.
[00146] Samples were sterilized by the STERRAD process (low temperature
hydrogen
peroxide gas plasma) and then extracted for 24 h/37 C in complete growth
medium
(Eagle's Minimum Essential Medium + 10% horse serum + 100 IU/m1 penicillin +
100
ug/mIstreptomycin) using an extraction ratio of 6 cm2/ml. In some experiments,
additional samples were sterilized by dipping samples into 100% ethanol or 75%
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
isopropanol for 5 minutes and allowing time to air dry before extraction. At
the time of
the extraction, L-929 mouse fibroblast cells (NCTC clone 929: CCL 1, American
Type
Culture Collection, Manassas, VA, USA) in passage 3-10 were lifted from the
culture flask
using trypsin/EDTA. An aliquot was counted using trypan blue, and then cells
were re-
suspended in complete growth medium at a density of 1 x 105 cells/ml. Cells
were
dispensed into wells of 96-well culture plates (1 x 104 cells/well) and
cultured at 37 C in a
humidified atmosphere of 5% CO2/95% air. After 24 h, the culture medium was
removed
and replaced with 100 ul of extractant. Some wells received sodium dodecyl
sulfate
(SDS; 0 to 400 u.M in EMEM; positive controls), low-density polyethylene
extract (1.25
cm2 LDPE/ml EMEM; negative control) or complete growth medium alone. Cells
were
then cultured for an additional 24 h. Subsequently, cells in culture plates
were washed
once with HBSS and metabolic activity was measured by incubating cells with
100 ul of
WST-1 cell proliferation reagent (Roche Diagnostics) for up to 4 h at 37 C.
[00147] To determine cytotoxicity, absorbance of the medium in wells was
measured at
450 nm after 2 and/or 4 h using a microplate reader (PHERAstar) and was
corrected using
absorbance measurements at 630 nm and using blanks. Absorbance levels are
proportional to the metabolic activity of cells and therefore inversely
related to
cytotoxicity. To check for mycoplasma contamination of the cultures, medium
was saved
and tested using the luminescent MycoAlert Plus mycoplasma detection kit
(Lonza).
Statistical significance was determined using analysis of variance and Tukey-
Kramer post-
test.
[00148] The results of the cytotoxicity measurements of the various
materials used in the
smart dressing are shown in FIGS. 31 and 32. The low metabolic activity of
cells treated
with the extracts of parchment paper, PDMS, double-sided tape, and 3-, 2- or 1-
layer
Ruthenium dye printed on parchment paper was significantly less than the
activity of
cells treated with the LDPE extract (negative control) or cells treated with
growth
medium and was comparable to cells treated with 300-400 u.M SDS (positive
controls)
(FIG. 30). We hypothesized that the apparent toxicity of the individual
materials could be
related to residual contaminants from the Sterrad process. To test this,
samples of
parchment paper, double-sided tape, PDMS and the three materials combined were
sterilized by Sterrad. Duplicate samples were sterilized by dipping in 100%
ethanol for 5
minutes and then air-drying before extraction with complete growth medium (37
C/24
36
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
h). FIG. 31 shows that the cytotoxicity of parchment paper ("paper"), alone or
combined
with double sided tape ("tape") and PDMS ("3-Layer"), was independent of the
sterilization method. However, the effect of the double-sided tape extract on
metabolic
activity was not significantly different than the negative control ("NC") or
Eagle's
Minimum Essential Medium ("EMEM") treated samples. This was in contrast to
experiment 1, where the extract of double-sided tape induced significant
cytotoxicity.
This may have been a result of extractingthe tape with the backing paper left
on in
experiment 1 and removing it in experiment 2. Cellulosics are known absorbers
of H202
and can be chemically modified by H202.
[00149] To further examine a possible interaction between paper and the
Sterrad process,
samples of filter paper and parchment paper where treated with the Sterrad
process or
sterilized by immersion in 70% isopropanol. Additional samples of parchment
paper
calendered between specific rollers where sterilized by Sterrad to determine
if the
devices could be the source of the toxic contaminants. FIG. 33 shows that
extracts of
filter paper (FP), parchment paper (PP), laser-treated parchment paper (LTPP)
and
calendered parchment paper (CAL1-2 and CAL 2-3) sterilized by the Sterrad
process were
significantly cytotoxic and comparable to the cytotoxicity of 400 u.M SDS
(FIG. 34). By
contrast, extracts of filter paper and parchment paper dipped in isopropanol
were not
cytotoxic and were comparable to cells maintained in EMEM or extracts of
double-sided
tape without backing. This confirms the previous findings of an interaction
between
paper and the Sterrad process, which renders the paper cytotoxic.
[00150] Sterilized samples of parchment paper appeared to be cytotoxic due
to possible
contaminants resulting from the Sterrad process. The cytotoxicity associated
with the
Sterrad process was reduced by washing parchment paper samples for 5 minutes
in HBSS
followed by equilibration for 5 minutes in complete growth medium.
LIST OF NON-LIMITING EMBODIMENTS
[00151] Embodiment A is a fluorescent oxygen sensing ink. The composition
of
Embodiment A includes an organic solvent, polymer binder in the organic
solvent, and
fluorescent dye particles disposed in the organic solvent wherein the
fluorescent dye
37
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
particles bind to the alkyl cellulose particles after printing to form a
moisture resistant
flexible and comformable film.
[00152] The composition of Embodiment A wherein the polymer binder includes
alkyl
cellulose particles comprising methyl cellulose, ethyl cellulose, propyl
cellulose, isopropyl
cellulose, n-butyl cellulose, sec-butyl cellulose, pentyl cellulose, or
combinations thereof;
silicone based polymers such as polydimethylsiloxane (PDMS), Ecoflex; and
polystyrene.
[00153] The composition of Embodiment A or Embodiment A with any of the
intervening
features wherein the alkyl cellulose polymer have a degree of substitution
from about 1.0
to about 3Ø
[00154] The composition of Embodiment A or Embodiment A with any of the
intervening
features wherein the organic solvent includes at least one substance or a
mixture of
substances chosen from the group consisting of ethanol, dimethyl sulfoxide
(DMSO),
dimethyl-formamide, iso propyl alcohol, acetone, and toluene.
[00155] The composition of Embodiment A or Embodiment A with any of the
intervening
features wherein the fluorescent dye complexes comprise a material selected
from the
group consisting of ruthenium, osmium tetroxide, rhodium acetate, palladium
and
chromium.
[00156] The composition of Embodiment A or Embodiment A with any of the
intervening
features wherein the size of particles in the ink system should be less than
1/100 of the
nozzle diameter to avoid agglomeration and clogging of print nozzles during
inkjet
printing. For example, if the nozzle diameter is 21 p.m, then the particle
size should be
less than 0.2 p.m to avoid agglomeration and clogging of print head nozzles.
[00157] The composition of Embodiment A or Embodiment A with any of the
intervening
features wherein the ink is capable of being printed on hydrophobic to
partially
hydrophilic substrates, but not completely hydrophilic substrates.
[00158] Embodiment B is a method of fabricating an oxygen sensor. The
method
comprising: providing a liquid ink solution including a solvent, fluorescent
ink particles
dispersed in the solvent, and a polymer binder dissolved in the solution,
wherein the
polymer binder particles are bound to the fluorescent ink particles, providing
a thin
flexible substrate having a surface that is hydrophobic to partially
hydrophilic, printing
the liquid ink solution on the surface of the thin flexible substrate.
38
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
[00159] The method of Embodiment B wherein the polymer binder comprises an
alkyl
cellulose, silicone based polymers such as PDMS, Ecoflex; and polystyrene.
[00160] The method of Embodiment B or Embodiment B with any of the
intervening
features wherein the alkyl cellulose comprises methyl cellulose, ethyl
cellulose, propyl
cellulose, isopropyl cellulose, n-butyl cellulose, sec-butyl cellulose, pentyl
cellulose, or
combinations thereof.
[00161] The method of Embodiment B or Embodiment B with any of the
intervening
features wherein the alkyl cellulose has a degree of substitution from about
1.0 to about
3Ø
[00162] The method of Embodiment B or Embodiment B with any of the
intervening
features wherein the size of particles in the ink system should be less than
1/100 of the
nozzle diameter to avoid agglomeration and clogging of print nozzles during
inkjet
printing. For example, if the nozzle diameter is 21 p.m, then the particle
size should be
less than 0.2 p.m to avoid agglomeration and clogging of print head nozzles.
[00163] The method of Embodiment B or Embodiment B with any of the
intervening
features wherein the fluorescent dye complexes comprise a material selected
from the
group consisting of ruthenium, osmium tetroxide, rhodium acetate, palladium
and
chromium.
[00164] The method of Embodiment B or Embodiment B with any of the
intervening
features wherein the substrate comprises any paper/coated papers such as
parchment,
TYVErm wax coated, chromatography; any polyester films such as polyethylene
terephthalate (PET), polyethylene-naphthalate (PEN); any polyimide films such
as
KAPTONTm, UPILEXTM; any polyurethane plastics/thermoplastic elastomers such as
thermoplastic polyurethane; any silicon based organic polymers such as
polydimethylsiloxane (PDMS) and ECOFLEXTM.
[00165] The method of Embodiment B or Embodiment B with any of the
intervening
features wherein treating a surface of the substrate, to alter its surface
energy, by
utilizing a process selected from the group consisting of UV treatment, corona
treatment,
plasma treatment, sintering, and laser engraving.
[00166] The method of Embodiment B or Embodiment B with any of the
intervening
features wherein the organic solvent includes at least one substance chosen
from the
39
CA 03055949 2019-09-09
WO 2019/168529 PCT/US2018/020284
group consisting of ethanol, DMSO, dimethyl formamide, iso propyl alcohol,
acetone, and
toluene.
[00167] The method of Embodiment B or Embodiment B with any of the
intervening
features wherein the ink can be deposited on the substrate using additive
print
manufacturing processes such as screen, inkjet, flexography, aerosol jet or
gravure.
[00168] The method of Embodiment B or Embodiment B with any of the
intervening
features wherein the organic solvent includes at least one substance or a
mixture of
substances chosen from the group consisting of ethanol, DMSO, dimethyl
formamide, iso
propyl alcohol, acetone, and toluene.
[00169] The method of Embodiment B or Embodiment B with any of the
intervening
features wherein the liquid ink solution includes about 75% to about 99%
solvent, from
about .1% to about 5% fluorescent ink particles, and from about .1% to about
20%
polymer binder particles.