Note: Descriptions are shown in the official language in which they were submitted.
OXYGENATE SEPARATION FOLLOWING DEHYDROGENATION OF A
LOWER ALKANE
FIELD OF THE INVENTION
The present disclosure relates generally to oxidative dehydrogenation (ODH) of
a
lower alkane into an alkene. In some examples, the present disclosure relates
to separation of
an ODH product from a process stream.
BACKGROUND OF THE INVENTION
Olefins like ethylene, propylene, and butylene, can be basic building blocks
for a
variety of commercially valuable polymers. Since naturally occurring sources
of olefins may
not exist in commercial quantities, polymer producers may rely on methods for
converting
the more abundant lower alkanes into olefins. Typically, a polymer producer
can utilize
steam cracking to produce alkenes from the lower alkanes. Steam cracking is a
highly
endothermic process where steam-diluted lower alkanes are subjected very
briefly to a high
temperature of at least 800 C which requires a high energy demand.
Additionally, steam
cracking can cause coke formation in the reactor which can lead to increased
maintenance
costs.
Oxidative dehydrogenation (ODH) is an alternative to steam cracking that can
be
exothermic, can have a low energy demand, and can produce little or no coke.
In ODH, a
lower alkane is mixed with oxygen in the presence of a catalyst and optionally
an inert
diluent at low temperatures such as, for example 300 C, to produce the
corresponding
alkene. In some examples, various other by-products such as, for example,
carbon monoxide,
carbon dioxide, and an oxygenate may also be produced in the ODH process. The
by-
products may be subject to further processing prior to being a marketable
product or may be
disposed of. The additional processing can increase the complexity of a
chemical complex
and can include a high energy demand.
SUMMARY OF THE INVENTION
In one aspect, a method is provided to convert a lower alkane to an alkene.
More
specifically, an input stream comprising oxygen and the lower alkane is
provided to an
oxidative dehydrogenation (ODH) reactor. At least a portion of the lower
alkane is converted
to the alkene in the ODH reactor and an ODH outlet stream comprising the
alkene, an
oxygenate, and a carbon-based oxide is produced. The ODH outlet stream is
cooled and at
1
CA 3058075 2019-10-09
least a portion of the oxygenate is condensed. The alkene is separated from
the oxygenate to
produce an alkene outlet stream and an oxygenate outlet stream. The alkene
outlet stream
comprises at least a substantial portion of the alkene and at least a
substantial portion of the
carbon-based oxide. The oxygenate outlet stream comprises at least a
substantial portion of
the condensed oxygenate.
In another aspect, an apparatus is provided for oxidative dehydrogenation
(ODH) of a
lower alkane to an alkene. More specifically, the apparatus comprises an ODH
reactor, a
means for cooling, and a flash drum. The ODH reactor comprises an ODH inlet
and an ODH
outlet. The ODH inlet is suitable for transporting an ODH inlet stream
comprising the lower
alkane into the ODH reactor. The ODH outlet is suitable for transporting an
ODH outlet
stream comprising the alkene, an oxygenate, water in the form of steam, and a
carbon-based
oxide. The means for cooling is suitable for cooling the ODH outlet stream and
condensing
at least a portion of the oxygenate. The flash drum comprises a drum inlet, an
oxygenate
outlet, and an alkene outlet. The drum inlet is in fluid communication with
the ODH outlet,
to receive the cooled ODH outlet stream. The flash drum is suitable for
separating the
condensed oxygenate from gaseous alkene and gaseous carbon-based oxide. The
alkene
outlet is suitable for transporting an alkene outlet stream comprising at
least a substantial
portion of the alkene and at least a substantial portion of the carbon-based
oxide. The
oxygenate outlet is suitable for transporting an oxygenate outlet stream
comprising at least a
substantial portion of the condensed oxygenate.
In another aspect, a system is provided for oxidative dehydrogenation (ODH) of
a
lower alkane. More specifically, the system comprises an ODH reactor, a means
for cooling,
and a flash drum. The ODH reactor is configured to receive an input stream
comprising
oxygen and the lower alkane. The ODH reactor is configured to produce an ODH
outlet
.. stream comprising an alkene, an oxygenate, water in the form of steam, and
a carbon-based
oxide. The means for cooling is configured to cool the ODH outlet stream to
produce a
cooled ODH outlet stream. The flash drum is configured to separate the alkene
from the
oxygenate to produce an alkene outlet stream and an oxygenate outlet stream.
The alkene
outlet stream comprises at least a substantial portion of the alkene and at
least a substantial
portion of the carbon-based oxide. The oxygenate outlet stream comprises at
least a
substantial portion of the condensed oxygenate.
2
CA 3058075 2019-10-09
It is understood that the inventions described in this specification are not
limited to the
examples summarized in this Summary. Various other aspects are described and
exemplified
herein.
BRIEF DESCRIPTION OF THE DRAWINGS
The features and advantages of the examples, and the manner of attaining them,
will
become more apparent and the examples will be better understood by reference
to the
following description of examples taken in conjunction with the accompanying
drawings,
wherein:
FIG. 1 is a flow diagram illustrating a non-limiting example of a system to
convert an
alkane to an alkene;
FIG. 2 is a flow diagram illustrating a non-limiting example of a system
comprising a
separation tower;
FIG. 3 is a flow diagram illustrating a non-limiting example of a system
comprising
an oxygen remover;
FIG. 4 is a flow diagram illustrating a non-limiting example of a system
comprising
an amine tower; and
FIG. 5 is a flow diagram illustrating a non-limiting example of a system
comprising a
polymerization reactor.
DETAILED DESCRIPTION OF THE PREFERRED EMBODIMENTS
The exemplifications set out herein illustrate certain examples, in one form,
and such
exemplifications are not to be construed as limiting the scope of the examples
in any manner.
Certain exemplary aspects of the present disclosure will now be described to
provide
an overall understanding of the principles of the structure, function,
manufacture, and use of
the systems, apparatus, and methods disclosed herein. One or more examples of
these
aspects are illustrated in the accompanying drawings. Those of ordinary skill
in the art will
understand that the systems and methods specifically described herein and
illustrated in the
accompanying drawings are non-limiting exemplary aspects and that the scope of
the various
examples of the present invention is defined solely by the claims. The
features illustrated or
described in connection with one exemplary aspect may be combined with the
features of
other aspects. Such modifications and variations are intended to be included
within the scope
of the present invention.
3
CA 3058075 2019-10-09
Reference throughout the specification to "various examples," "some examples,"
"one
example," or "an example", or the like, means that a particular feature,
structure, or
characteristic described in connection with the example is included in at
least one example.
Thus, appearances of the phrases "in various examples," "in some examples,"
"in one
example", or "in an example", or the like, in places throughout the
specification are not
necessarily all referring to the same example. Furthermore, the particular
features, structures,
or characteristics may be combined in any suitable manner in one or more
examples. Thus,
the particular features, structures, or characteristics illustrated or
described in connection with
one example may be combined, in whole or in part, with the features
structures, or
characteristics of one or more other examples without limitation. Such
modifications and
variations are intended to be included within the scope of the present
examples.
Other than in the operating examples or where otherwise indicated, all numbers
or
expressions referring to quantities of ingredients, reaction conditions, etc.
used in the
specification and claims are to be understood as modified in all instances by
the term "about".
Accordingly, unless indicated to the contrary, the numerical parameters set
forth in the
following specification and attached claims are approximations that can vary
depending upon
the desired properties, which the present disclosure desires to obtain. At the
very least, and
not as an attempt to limit the application of the doctrine of equivalents to
the scope of the
claims, each numerical parameter should at least be construed in light of the
number of
reported significant digits and by applying ordinary rounding techniques.
Notwithstanding that the numerical ranges and parameters setting forth the
broad
scope of the disclosure are approximations, the numerical values set forth in
the specific
examples are reported as precisely as possible. Any numerical values, however,
inherently
contain certain errors necessarily resulting from the standard deviation found
in their
respective testing measurements.
Also, it should be understood that any numerical range recited herein is
intended to
include all sub-ranges subsumed therein. For example, a range of "1 to 10" is
intended to
include all sub-ranges between and including the recited minimum value of 1
and the recited
maximum value of 10; that is, having a minimum value equal to or greater than
1 and a
maximum value of equal to or less than 10. Because the disclosed numerical
ranges are
continuous, they include every value between the minimum and maximum values.
Unless
expressly indicated otherwise, the various numerical ranges specified in this
application are
approximations.
4
CA 3058075 2019-10-09
The grammatical articles "a", "an", and "the", as used herein, are intended to
include
"at least one" or "one or more", unless otherwise indicated, even if "at least
one" or "one or
more" is expressly used in certain instances. Thus, the foregoing grammatical
articles are
used herein to refer to one or more than one (i.e., to "at least one") of the
particular identified
elements. Further, the use of a singular noun includes the plural, and the use
of a plural noun
includes the singular, unless the context of the usage requires otherwise.
As used herein, the term "substantial portion" means at least 50 percent by
weight. A
substantial portion can be 50 % to 100 % by weight such as, for example, at
least 60 % by
weight, at least 70 % by weight, at least 80 % by weight, at least 90 % by
weight, or at least
95 % by weight.
As used herein, the term "alkane" refers to an acyclic saturated hydrocarbon.
In
various examples, an alkane consists of hydrogen and carbon atoms arranged in
a linear
structure in which all of the carbon-carbon bonds are single bonds. An alkane
has the general
chemical formula C,1-12n+2 and in various examples, for a lower alkane, 'n' is
in a range of 2
to 4. In various examples, an alkane refers to one or more of ethane, propane,
butane,
pentane, hexane, octane, decane and dodecane. In various examples, a lower
alkane refers to
one or more of ethane, propane, and butane.
As used herein, the term "alkene" refers to an unsaturated hydrocarbon that
contains
at least one carbon¨carbon double bond. In various examples, alkene refers to
alpha olefins.
For example, alkene can refer to one or more of ethylene, propylene, 1-butene,
butadiene,
pentene, pentadiaene hexene, octene, decene, and dodecene.
As used herein, the terms "alpha olefin" or "a-olefin" refer to a family of
organic
compounds which are an alkene (also known as olefin) with a chemical formula
CJI2x,
distinguished by having a double bond at the primary or alpha (a) position. In
various
examples, alpha olefin refers to one or more of ethylene, propylene, 1-butene,
1-pentene, 1-
hexene, 1-octene, 1-decene, and 1-dodecene.
As used herein, the term "fixed bed reactor" refers to one or more reactors,
in series or
parallel, often including a cylindrical tube filled with catalyst pellets with
reactants flowing
through the bed and being converted into products. The catalyst in the reactor
may have
multiple configurations including, for example, one large bed, several
horizontal beds,
several parallel packed tubes, multiple beds in their own shells, and/or
combinations thereof.
As used herein, the term "fluidized bed reactor" refers to one or more
reactors, in
series or parallel, often including a fluid (e.g., gas or liquid) which can be
passed through a
5
CA 3058075 2019-10-09
solid granular catalyst, which can be shaped as tiny spheres, at a velocity
high enough to
suspend the solid granular catalyst and cause the solid granular catalyst to
behave like a fluid.
As used herein, the term "HDPE" refers to high density polyethylene, which
generally
has a density of greater or equal to 0.941 g/cm3. HDPE has a low degree of
branching.
HDPE can be often produced using chromium/silica catalysts, Ziegler-Natta
catalysts or
metallocene catalysts.
As used herein, the term "LDPE" refers to low density polyethylene, which can
be a
polyethylene with a high degree of branching with long chains. Often, the
density of a
LDPE will range from 0.910 - 0.940 g/cm3. LDPE can be created by free radical
polymerization.
As used herein, the term "LLDPE" refers to linear low density polyethylene,
which
can be a polyethylene that can have significant numbers of short branches
resulting from
copolymerization of ethylene with at least one a-olefin comonomer. In some
examples,
LLDPE has a density in the range of 0.915 - 0.925 g/cm3. In some examples, the
LLDPE can
be an ethylene hexene copolymer, ethylene octene copolymer, or ethylene butene
copolymer.
The amount of comonomer incorporated can be from 0.5 mole % to 12 mole %
relative to
ethylene, in some examples from 1.5 mole % to 10 mole %, and in other examples
from 2
mole % to 8 mole %.
As used herein, the term "MDPE" refers to medium density polyethylene, which
can
be a polyethylene with some short and/or long chain branching and a density in
the range of
0.926 - 0.940 g/cm3. MDPE can be produced using chromium/silica catalysts,
Ziegler-Natta
catalysts or metallocene catalysts.
As used herein, the term "VLDPE" refers to very low density polyethylene,
which can
be a polyethylene with high levels of short chain branching with a typical
density in the range
of 0.880 - 0.915 g/cc. In some examples, VLDPE can be a substantially linear
polymer.
VLDPE can be typically produced by copolymerization of ethylene with a-
olefins. VLDPE
can be produced using metallocene catalysts.
As used herein, the term "gas phase polyethylene process" refers to a process
where a
mixture of ethylene, optional alpha olefin comonomers, and hydrogen can be
passed over a
catalyst in a fixed or fluidized bed reactor. The ethylene and optional alpha
olefins
polymerize to form grains of polyethylene, suspended in the flowing gas, which
can pass out
of the reactor. In various examples, two or more of the individual reactors
are placed in
parallel or in series, each of which are under slightly different conditions,
so that the
6
CA 3058075 2019-10-09
properties of different polyethylenes from the reactors are present in the
resulting
polyethylene blend. In some examples, the catalyst system includes, for
example, chromium
catalysts, Ziegler-Natta catalysts, zirconocene catalysts, and metallocene
catalysts and
combinations thereof.
As used herein, the term "high pressure polyethylene process" refers to
converting
ethylene gas into a white solid by heating it at very high pressures in the
presence of minute
quantities of oxygen (less than 10 ppm oxygen) at 1000 bar - 3000 bar and at
80 C - 300 C.
In some examples, the high pressure polyethylene process produces LDPE.
As used herein, the term "low pressure polyethylene process" refers to
polymerizing
ethylene using a catalyst that in some examples includes aluminum at generally
lower
pressures than the high pressure polyethylene process. In some examples, the
low pressure
polyethylene process can be carried out at 10 bar - 80 bar and at 70 C - 300
C. In various
examples, the low pressure polyethylene process provides HDPE. In various
examples, an a-
olefin comonomer can be included in the low pressure polyethylene process to
provide
LLDPE.
As used herein, the term "solution polyethylene process" refers to processes
that
polymerize ethylene and one or more optional a-olefins in a mixture of lower
alkane
hydrocarbons in the presence of one or more catalysts. In various examples,
two or more of
the individual reactors can be placed in parallel or in series, each of which
can be under
slightly different conditions, so that the properties of different
polyethylenes from the
reactors are present in the resulting polyethylene blend. In some examples the
catalysts
include, but are not limited to, chromium catalysts, Ziegler-Natta catalysts,
zirconocene
catalysts, hafnocene catalysts, phosphinimine catalysts, metallocene
catalysts, and
combinations thereof.
As used herein, the term "slurry polyethylene process" refers to single-tube
loop
reactors, double-tube loop reactors or autoclaves (stirred-tank reactors) used
to polymerize
ethylene and optional a-olefins in the presence of a catalyst system and a
diluent. Non-
limiting examples of diluents include isobutane, n-hexane, or n-heptane. In
some examples,
two or more of the individual reactors are placed in parallel or in series,
each of which can be
under slightly different conditions, so that the properties of different
polyethylenes from the
reactors are present in the resulting polyethylene blend. In some examples,
the catalyst
system includes, for example, chromium catalysts, Ziegler-Natta catalysts,
zirconocene
7
CA 3058075 2019-10-09
catalysts, hafnocene catalysts, phosphinimine catalysts, metallocene
catalysts, and
combinations thereof.
As used herein, the term "long chain branching" refers to a situation where
during a-
olefin polymerization, a vinyl terminated polymer chain can be incorporated
into a growing
polymer chain. Long branches often have a length that can be longer than the
average critical
entanglement distance of a linear (e.g., no long chain branching) polymer
chain. In some
examples, long chain branching effects melt rheological behavior.
As used herein, the term "short chain branching" refers to a copolymer of
ethylene
with an a-olefin or with branches of less than 40 carbon atoms. In some
examples, the a-
olefin or branches are present at less than 20 % by weight of the
polyethylene, in some
examples less than 15 % by weight. In some examples, the presence of short
chain branches
can interfere with the formation of the polyethylene crystal structure and can
be observed as a
lower density compared with a linear (no short chain branching) polyethylene
of the same
molecular weight.
As used herein, the term "monomer" refers to small molecules containing at
least one
double bond that can react in the presence of a free radical polymerization
initiator to become
chemically bonded to other monomers to form a polymer.
As used herein, the term, "olefinic monomer" includes, without limitation, a-
olefins,
and in some examples, ethylene, propylene, 1-butene, 1-hexene, 1-octene, and
combinations
thereof.
As used herein, the term "polyolefin" refers to a material, which is prepared
by
polymerizing a monomer composition containing at least one olefinic monomer.
As used herein, the term "polyethylene" can include, for example, a
homopolymer of
ethylene, a copolymer of ethylene, and an a-olefin.
As used herein, the term "polypropylene" can include a homopolymer of
propylene
such as, for example, isotactic polypropylene and syndiotactic polypropylene,
a copolymer of
propylene, and an a-olefin.
As used herein, the term "polymer" refers to macromolecules composed of
repeating
structural units connected by covalent chemical bonds and can include, for
example, a
homopolymer, a random copolymer, a block copolymer, and a graft copolymer.
As used herein, the term "thermoplastic" refers to a class of polymers that
can soften
or become liquid when heated and can harden when cooled. In some examples, a
thermoplastic can be a high-molecular-weight polymer that can be repeatedly
heated and
8
CA 3058075 2019-10-09
remolded. In various examples, a thermoplastic resin can include a polyolefin
and an
elastomer that has thermoplastic properties.
As used herein, the terms "thermoplastic elastomers" and "TPE" refer to a
class of
copolymers or a blend of polymers (in some examples a blend of a thermoplastic
and a
rubber) which includes materials having both thermoplastic and elastomeric
properties.
As used herein, the terms "thermoplastic olefin" or "TPO" refer to
polymer/filler
blends that contain some fraction of polyethylene, polypropylene, block
copolymers of
polypropylene, rubber, and a reinforcing filler. The fillers can include, for
example, talc,
fiberglass, carbon fiber, wollastonite, metal oxy sulfate, and combinations
thereof. The
rubber can include, for example, ethylene-propylene rubber, EPDM (ethylene-
propylene-
diene rubber), ethylene-butadiene copolymer, styrene-ethylene-butadiene-
styrene block
copolymers, styrene-butadiene copolymers, ethylene-vinyl acetate copolymers,
ethylene-
alkyl (meth)acrylate copolymers, and VLDPE such as those available under the
Flexomere
resin trade name from the Dow Chemical Co., Midland, MI, styrene-ethylene-
ethylene-
propylene-styrene (SEEPS). These can also be used as the materials to be
modified by the
interpolymer to tailor their rheological properties.
Unless otherwise specified, all molecular weight values are determined using
gel
permeation chromatography (GPC). Molecular weights are expressed as
polyethylene
equivalents with a relative standard deviation of 2.9 % for the number average
molecular
weight ("Mn") and 5.0 % for the weight average molecular weight ("Mw"). Unless
otherwise
indicated, the molecular weight values indicated herein are weight average
molecular weights
(Mw).
Unless otherwise specified, all pressure values are absolute pressure values.
Composition of gaseous streams can be described in many ways, as known to a
person skilled in the art. For oxidative dehydrogenation, numerous streams are
described and
can be partially describe in terms of the chemical composition. Unless
otherwise specified,
the chemical composition of a chemical species within streams is measured in
terms of mole
percent (mol%) which is calculated by determining the moles of the species and
dividing by
the total number of moles of all species in the stream and multiplying by 100.
Converting to
mass fraction or mass percent is within the knowledge of the person skilled in
the art and can
be performed given sufficient information about the stream with respect to
flow rates,
temperature, and pressure.
9
CA 3058075 2019-10-09
Oxidative dehydration (ODH) can couple the endothermic dehydration of an
alkane
with the strongly exothermic oxidation of hydrogen. For example, ODH of an
alkane can
comprise contacting an alkane and oxygen in an ODH reactor with an ODH
catalyst under
reaction conditions (e.g., temperature, pressure, flow rate, etc.) that can
promote oxidation of
the alkane into the corresponding alkene. The corresponding alkene includes
hydrocarbons
with the same number of carbons as the alkane used in the ODH reactor, but
with the addition
of one carbon to carbon double bond. For example, utilizing ODH, ethane can be
converted
to ethylene, propane can be converted to propylene, and butane can be
converted to butylene.
Any ODH catalyst known in the art can be suitable for use with the present
disclosure.
For example, an ODH catalyst containing a mixed metal oxide can be used.
Additionally,
reaction conditions can be controlled to adjust the selectively and yield of
the ODH reactor
products. As known in the art, conditions will vary and can be optimized for a
particular
alkane, for a specific catalyst, a select product, and/or a particular inert
diluent.
A product of an ODH reaction can be an oxygenate such as, for example, acetic
acid,
acrylic acid, maleic acid, and maleic anhydride. The oxygenate can require
purification
and/or further processing in order to generate a marketable product. For
example, water may
have to be removed from the oxygenate. Separation of the oxygenate from water
can
increase the complexity of a quench tower and/or a separation tower due to the
small thermal
(e.g., boiling point) separation between the oxygenate and the water. In
various examples, a
mixture of oxygenate and water can be azeotropic. The separation tower may
employ a large
column, a high quantity of stages, a high reflux ratio, and a high energy
demand to separate
an azeotropic mixture of oxygenate and water.
Thus, a method, a system, and an apparatus are provided which can enhance the
purification of the oxygenate and reduce energy requirements for the
purification. More
.. specifically, a method, a system, and an apparatus are provided for
converting a lower alkane
to an alkene. An input stream comprising oxygen and the lower alkane can be
provided to an
ODH reactor. At least a portion of the lower alkane can be converted to the
alkene in the
ODH reactor and an ODH outlet stream comprising the alkene, an oxygenate,
water in the
form of steam, and a carbon-based oxide can be produced. The ODH outlet stream
can then
be cooled to promote condensation of at least a substantial portion of the
oxygenate and a
portion of the steam. The ODH outlet stream can then be subjected to a means
for liquid-gas
separation to produce a first oxygenate outlet stream comprising at least a
substantial portion
of the condensed oxygenate and water and an alkene outlet stream comprising at
least a
CA 3058075 2019-10-09
substantial portion of the alkane, at least a substantial portion of the
carbon-based oxide, and
any remaining oxygenate. Using condensation and liquid-gas separation for
removing a
substantial portion of the oxygenate from the ODH outlet stream is non-
dilutive as no
additional components are added to the ODH outlet stream.
The alkene outlet stream can be provided to a quench tower and remaining
oxygenate
can be removed from the alkene outlet stream. The quench tower, as known to a
person
skilled in the art, includes addition of a quench agent, usually water, and is
therefore dilutive
as an additional component is added to the stream. A first quench outlet
stream comprising at
least a substantial portion of the alkene and at least a substantial portion
of the carbon-based
oxide can be produced in the quench tower. Additionally, a second quench
outlet stream
comprising at least a substantial portion of the remaining oxygenate can be
produced in the
quench tower. The first quench outlet stream can be provided to a caustic wash
tower. The
first quench outlet stream can be contacted with a hydroxide in the caustic
wash tower to
form a caustic outlet stream comprising a carbonate. The caustic outlet stream
can be
provided to the quench tower. The alkene outlet stream can be contacted with
the caustic
outlet stream to form an acetate. The second quench outlet stream can comprise
a substantial
portion of the acetate.
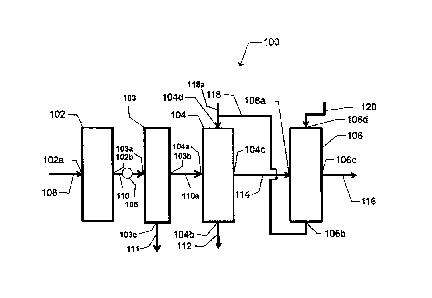
Referring to FIG. 1, illustrated is a flow diagram of a non-limiting example
of a
system 100 to convert an alkane to an alkene. As illustrated, an ODH reactor
102, a flash
tower 103, a quench tower 104, and a caustic wash tower 106 can be in
operative
communication. For example, an ODH outlet 102b of the ODH reactor 102 can be
in fluid
communication with a flash tower inlet 103a of flash tower 103 via an ODH
outlet line 110.
Additionally, a flash tower outlet 103b of flash tower 103 can be in fluid
communication with
a quench inlet 104a of the quench tower 104 via alkene outlet line 110a.
Additionally, a
quench outlet 104c of the quench tower 104 can be in fluid communication with
a wash inlet
106a of the caustic wash tower 106 via a quench outlet line 114. Accordingly,
the ODH
reactor 102 can be in fluid communication with the caustic wash tower 106 via
the flash
tower 103 and the quench tower 104.
The ODH reactor 102 can comprise an ODH inlet 102a which can be configured to
receive an ODH inlet stream from an ODH inlet line 108 and can be suitable to
transport the
ODH inlet stream into the ODH reactor 102. The ODH inlet stream can comprise a
gaseous
mixture of a lower alkane and oxygen. In various examples, the ODH inlet
stream
additionally can include at least one of a carbon-based oxide, steam, and an
inert diluent. The
11
CA 3058075 2019-10-09
inert diluent can comprise, for example, nitrogen, carbon dioxide, steam, and
methane. In
various examples, the carbon-based oxide can comprise at least one of carbon
dioxide and
carbon monoxide. The concentration of the oxygen and the lower alkane within
the mixture
in the ODH inlet stream and the temperature and pressure of the ODH inlet
stream can be
adjusted such that the mixture can be outside of the flammability limits of
the mixture.
In various examples, there may be multiple ODH inlet lines configured to
provide the
ODH inlet stream to the ODH reactor 102. For example, each component (e.g.,
lower alkane,
oxygen, steam, carbon-based oxide, and inert diluent) may be added directly to
the ODH
reactor 102, each in separate inlet lines (not shown). Alternatively, one or
more components
may be pre-mixed and added in more than one inlet line. In various example,
components
may be mixed together prior to the ODH reactor 102 and subsequently introduced
into the
ODH reactor in a common ODH inlet. In various examples, steam may be added
indirectly
as water mixed with an additional reactant and the resulting mixture can be
preheated before
entering the ODH reactor 102. When adding steam indirectly as water, the
preheating
process can increase the temperature of the mixture so that the water can be
substantially
converted to steam before entering the ODH reactor 102.
The ODH reactor 102 can include a catalyst capable of catalyzing the ODH of
the
reactants within the ODH inlet stream to products such as, for example, an
alkene, a carbon-
based oxide, water, and an oxygenate. The catalyst may be, for example, a
mixed metal
oxide catalyst.
The catalyst composition, temperature and pressure of the ODH reactor 102, and
the
composition of the ODH inlet stream can be adjusted in order to vary the
composition of
products as known by one of ordinary skill in the art. For example, the ratio
of the lower
alkane to oxygen can be outside of the upper flammability limit of the
mixture. In various
examples, the oxygen concentration in the ODH inlet stream can be in a range
of 0.1 % to
% by weight of the ODH inlet stream, and in some examples range from 0.1% to
less than
30 % by weight, less than 25 % by weight, or less than 20 % by weight. In
various
examples, the lower alkane concentration in the ODH inlet stream can range
from 0.1% to
50% by weight of the ODH inlet stream, and in some examples range from 0.1% to
less than
30 50 % by weight or less than 40 % by weight.
In various examples increasing the steam concentration in the ODH inlet stream
can
increase the amount of oxygenate produced relative to the alkene produced in
the ODH
reactor 102. In various examples, reducing the steam concentration in the ODH
inlet stream
12
CA 3058075 2019-10-09
can decrease the amount of oxygenate produced relative to the alkene produced
in the ODH
reactor 102. The concentration of steam in the ODH inlet stream can be in a
range of 0.1 %
to 40 % by weight of the total ODH inlet stream 108, and in some examples
range from 0.1%
to less than 40 % by weight, or less than 25 % by weight. In various examples,
the
.. concentration of the stream in the ODH inlet stream can be at least 1 % by
weight. In various
examples, the ODH inlet stream can comprise 20 % oxygen by weight, 40 % lower
alkane by
weight, and the balance being steam, carbon dioxide, and/or an inert diluent.
In various examples, the ODH process has a selectivity for the corresponding
alkene
(e.g., ethylene in the case of ethane ODH) of greater than 95% such as, for
example, greater
than 98%. The gas hourly space velocity (GHSV) within the ODH reactor 102 can
be from
500 to 30000 WI and in some examples the GHSV within the ODH reactor 102 can
be greater
than 1000 WI. In various examples, the linear velocity within the ODH reactor
102 can be
from 10 cm/s to 500 cm/s. In various examples, the weight hourly space
velocity (WHSV)
within the ODH reactor can be from 2.1 to 25 WI. In various examples, the
space-time yield
of corresponding alkene (e.g., productivity) in grams(g)/hour per kilogram
(kg) of the catalyst
can be at least 900 such as, for example, greater than 1500, greater than
3000, or greater than
3500, at an ODH reactor temperature of, for example, 350 C to 400 C. In
various
examples, the productivity of the catalyst can increase with increasing
temperature in the
ODH reactor 102 until the selectivity of the alkene decreases.
Use of an ODH reactor for performing an ODH reaction consistent with the
disclosure
falls within the knowledge of the person skilled in the art. In various
examples, the reaction
can be conducted at temperatures in a range of 300 C to 450 C such as, for
example, 300 C
to 425 C, or 330 C to 400 C. In various examples, the reaction can be
conducted at
pressures in a range of 0.5 pounds per square inch (psi) to 100 psi (3.447 to
689.47 kPa) such
as, for example, 15 psi to 50 psi (103.4 to 344.73 kPa). In various examples,
the lower alkane
can have a residence time in the ODH reactor 102 in a range of 0.002 seconds
(s) to 30 s, or
from 1 s to 10 s.
The products of the ODH reaction can leave the ODH reactor 102 through the ODH
outlet 102b in an ODH outlet stream. The ODH outlet 102b can be configured to
receive the
ODH outlet stream and can be suitable to transport the ODH outlet stream 110
out of the
ODH reactor 102 and into the ODH outlet line 110. In various examples, in
addition to the
products, the ODH outlet stream can include unreacted components from the ODH
inlet
stream such as, for example, lower alkane, carbon-based oxide, oxygen, steam,
inert diluent,
13
CA 3058075 2019-10-09
and combinations thereof. In various examples, the temperature of the ODH
outlet stream
can be in a range of 100 C to 450 C, such as for example, 300 C to 425 C,
and in certain
examples 330 C to 400 C.
Any of the known reactor types applicable for the ODH of an alkane may be used
with the present disclosure. For example, a fixed bed reactor, a fluidized bed
reactor, or
combinations thereof can be used for the ODH reactor 102. In a typical fixed
bed reactor,
reactants are introduced into the reactor at an inlet and flow past an
immobilized catalyst.
Products are formed and leave through the outlet of the reactor. A person
skilled in the art
would understand which features are required with respect to shape and
dimensions of the
reactor, inputs for reactants, outputs for products, temperature and pressure
control, and
means for immobilizing the catalyst.
In a typical fluidized bed reactor, the catalyst bed can be supported by a
porous
structure or a distributor plate and located near a lower end of the reactor.
Reactants flow
through the fluidized bed reactor at a velocity sufficient to fluidize the bed
(e.g., the catalyst
rises and begins to swirl around in a fluidized manner). The reactants can be
converted to
products upon contact with the fluidized catalyst and the reactants are
subsequently removed
from an upper end of the reactor. A person of ordinary skill in the art would
understand
which features are required with respect to shape and dimensions of the
reactor, the shape and
size of the distributor plate, the input temperature, the output temperature,
the reactor
temperature and pressure, inputs for reactors, outputs for reactants, and
velocities to achieve
fluidization.
In various examples, there may be multiple ODH reactors connected in series or
in
parallel. Each ODH reactor may be the same or different. For example, each ODH
reactor
can contain the same or different ODH catalyst. In various examples, the
multiple ODH
reactors can each be a fixed bed reactor, can each be a fluidized bed reactor,
or the multiple
ODH reactors can be combinations of fixed bed reactors and fluidized bed
reactors.
Regardless of the configuration of the ODH reactor 102, the ODH outlet 102b
can be
in fluid communication with the flash tower inlet 103a of the flash tower 103
via ODH outlet
line 110 to direct the ODH outlet stream to the flash tower 103. The ODH
outlet stream is
subjected to a cooling means 105 prior to reaching flash tower inlet 103a or
within flash
tower 103. The flash tower outlet 103b can be in fluid communication with the
quench inlet
104a of the quench tower 104 via the alkene outlet line 110a to direct the
alkene outlet stream
to the quench tower 104. The quench inlet 104a can be configured to receive
the alkene
14
CA 3058075 2019-10-09
outlet stream from the alkene outlet line 110a and can be suitable to
transport the alkene
outlet stream into the quench tower 104.
The cooling means 105 can be any means that cools the ODH outlet stream after
it
leaves the ODH reactor. This can include using a sufficiently long ODH outlet
line 110 that
allows the ODH outlet stream to cool to a temperature where the oxygenate
begins to
condense before reaching flash tower 103. In some embodiments, the most
preferable
cooling means 105 comprise a heat exchanger, use of which is well known within
the art. In
some embodiments, the cooling means 105 are an integral part of the flash
tower 103. In
some examples, the flash tower may be surrounded by a cooling jacket that
cools the ODH
outlet stream as it enters flash tower 103. In another example, cooling tubes
are arranged
within the space inside flash tower 103. In some embodiments, a heat exchanger
in
combination with integrated cooling means are used to cool to ODH outlet
stream.
The cooling means can cool the ODH outlet stream to a temperature of less than
200
C such as, for example, less than 100 C, less than 50 C, less than 40 C,
and in some
examples, the cooling means 105 can cool the ODH outlet stream to a
temperature of 20 C
to 80 C. In various examples, the cooling means 105 can cool the ODH outlet
stream to a
temperature which induces condensation of the oxygenate such as, for example,
to a
temperature less than or equal to the boiling point of the oxygenate and/or a
temperature that
reduces the vapor pressure of the oxygenate. The lower the temperature,
without going
below a temperature that results in freezing of the water or oxygenate, the
greater the degree
of condensation, which would be understood by a person skilled in the art.
Flash tower 103 can comprise a flash tower, or any other means that provides
for gas-
liquid separation. Use of flash towers is well known. At least a substantial
portion of the
oxygenate and water, in the form of steam, within the ODH outlet stream may be
in a liquid
state after being subjected to cooling means 105 and may exit flash tower 103
through a first
oxygenate outlet 103c, as a first oxygenate outlet stream, and into the first
oxygenate outlet
line 111. In various examples, the first oxygenate outlet stream can comprise
at least 0.5
mol% oxygenate, such as, for example, at least 2.0 mol% oxygenate, at least 5
mol%, or 0.5
mol% to 15 mol% oxygenate. The first oxygenate outlet stream can additionally
comprise
water of from 80 mol% to 99.5 mol%.
A portion of the oxygenate and water within the ODH outlet stream, including
liquid
and gaseous forms, may leave the flash tower 103 as part of the alkane outlet
stream. These
portions are referred to as remaining oxygenate and remaining water,
respectively.
CA 3058075 2019-10-09
In various examples, the alkene outlet stream may be subject to a second
cooling
means prior to or as an integral part of quench tower 104. The second cooling
means can be
configured to adjust the temperature of the alkene outlet stream, for example,
by cooling to a
temperature of less than 200 C such as, for example, less than 100 C, less
than 50 C, less
.. than 40 C, and in some examples, the second cooling means can cool the
alkene outlet
stream to a temperature of 20 C to 80 C. In various examples, the second
cooling means
can cool the alkene outlet stream to a temperature which induces condensation
of the
remaining oxygenate such as, for example, a temperature less than or equal to
the boiling
point of the remaining oxygenate and/or a temperature that reduces the vapor
pressure of the
.. remaining oxygenate. The second cooling means can use any means known in
the art. For
example, the second cooling means can be a standalone heat exchanger separate
from a
quench tower. In various examples, the second cooling means can be an
integrated heat
exchanger that is part of a quench tower. In further examples, the second
cooling means may
include a combination of standalone heat exchanger and an integrated heat
exchanger.
The quench tower 104 can comprise a quench tower, an oxygenate scrubber, the
like,
or combinations thereof. The quench tower 104 can be configured to quench the
components
in the alkene outlet stream and remove at least a substantial portion of the
alkene from the
alkene outlet stream. In various examples, the quench tower 104 can facilitate
the removal of
remaining oxygenate and water from the alkene outlet stream. The quench tower
104 can
produce a first quench outlet stream comprising at least a substantial portion
of the alkene
and at least a substantial portion of the carbon-based oxide from the alkene
outlet stream. In
various examples, the first quench outlet stream can comprise additional
components from
the alkene outlet stream such as, for example, a portion of the oxygen, a
portion of the
oxygenate, a portion of the inert diluent, a portion of the steam, and a
portion of the unreacted
alkane. The first quench outlet stream exits the quench tower 104 through the
quench outlet
104c. The quench outlet 104c can be configured to receive the first quench
outlet stream and
can be suitable to transport the first quench outlet stream out of the quench
tower 104 into the
quench outlet line 114.
The quench tower 104 can produce a second quench outlet stream comprising at
least
a substantial portion of any remaining oxygenate present in the alkene outlet
stream and in
some examples, an acetate as discussed herein. In various examples, the second
quench
outlet stream can comprise additional components from the alkene outlet stream
such as, for
example, a substantial portion of the remaining water (e.g., steam), as well
as lower alkane,
16
CA 3058075 2019-10-09
alkene, oxygen, and carbon-based oxide. The second quench outlet stream can
exit the
quench tower 104 through second quench outlet 104b of the quench tower 104.
The second
quench outlet 104b can be configured to receive the second quench outlet
stream and can be
suitable to transport the second quench outlet stream out of the quench tower
104 into the
second quench outlet line 112.
The quench tower 104 also comprises a carbonate inlet 104d for providing a
quenching agent such as water via quench agent line 118a, or a carbonate
solution via return
line 118. Use of quench towers is well known. A person skilled in the art
would understand
that quenching condenses and dilutes the oxygenate. The result is that second
quench outlet
stream comprises a lower mol% of the oxygenate compared to the first oxygenate
outlet
stream. When using a carbonate solution as the quench agent the effect is more
pronounced
as a portion of the oxygenate is converted to an acetate, as will be
described.
In some examples, the quench agent is provided to the quench tower at a
temperature
of less than 200 C such as, for example, less than 100 C, less than 50 C,
less than 40 C,
and in some examples, the quench agent is provided to the quench tower at a
temperature of
C to 80 C. In various examples, the quench agent can be provided to the
quench to a
temperature which induces condensation of the remaining oxygenate such as, for
example, a
temperature less than or equal to the boiling point of the remaining oxygenate
and/or a
temperature that reduces the vapor pressure of the remaining oxygenate.
20 The quench outlet 104c can be in fluid communication with the wash inlet
106a of the
caustic wash tower, 106 via the quench outlet line 114 to direct the first
quench outlet stream
to the caustic wash tower 106. The wash inlet 106a can be configured to
receive the first
quench outlet stream from the quench outlet line 114 and can be suitable to
transport the first
quench outlet stream into the caustic wash tower 106.
The caustic wash tower 106 can comprise the wash inlet 106a, a wash outlet
106c, a
caustic inlet 106d, and a caustic outlet 106b. The caustic inlet 106d can be
configured to
receive a hydroxide stream comprising a hydroxide from a hydroxide line 120
and can be
suitable to transport the hydroxide stream into the caustic wash tower 106.
The hydroxide
may be, for example, an aqueous solution of at least one of sodium hydroxide,
potassium
hydroxide, and ammonia hydroxide. In various examples, the aqueous solution
comprises at
least 0.5 mol% hydroxide, such as, for example, at least 1.0 mol% hydroxide,
at least 1.25
mol%, or 0.5 mol% to 1.75 mol% hydroxide.
17
CA 3058075 2019-10-09
The caustic wash tower 106 can be configured to contact the hydroxide stream
with
the first quench outlet stream. In various examples, where the carbon-based
oxide comprises
carbon dioxide, the hydroxide can react with carbon dioxide in the first
quench outlet stream
to form a carbonate. The reaction can remove at least a substantial portion of
the carbon-
based oxide (e.g., carbon dioxide) from the first quench outlet stream and
produce a wash
outlet stream and a caustic outlet stream. The carbonate may be, for example,
at least one of
sodium bicarbonate, potassium carbonate, and ammonium bicarbonate. For
example, the
reaction of sodium hydroxide and carbon dioxide is shown in Scheme 1.
Scheme 1
CO2 + NaOH 4-0 NaHCO3
The wash outlet stream can comprise unreacted components from the first quench
outlet stream. The wash outlet 106c can be configured to receive the wash
outlet stream and
can be suitable to transport the wash outlet stream out of the caustic wash
tower 106 into the
wash outlet line 116.
The caustic outlet stream can comprise a substantial portion of the carbonate
and in
some examples, at least one of water, hydroxide, and oxygenate. The caustic
outlet 106b can
be configured to receive the caustic outlet stream and can be suitable to
transport the caustic
outlet stream into the return line 118. The return line 118 can be configured
to receive the
caustic outlet stream and output the caustic outlet stream into a carbonate
inlet 104d of the
quench tower 104.
In various examples, the caustic outlet stream can comprise at least 0.5 mol%
carbonate, such as, for example, at least 2.0 mol% carbonate, at least 5 mol%,
or 0.5 mol% to
15 mol% carbonate. The caustic outlet stream can additionally comprise water
of from 80
mol% to 99.5 mol%.
The quench tower 104 can be configured to contact the caustic outlet stream
with the
alkene outlet stream. In various examples, the quench tower 104 can be
configured to react
the caustic outlet stream with the alkene outlet stream to form an acetate. In
various
examples, the quench tower 104 can react the carbonate with the oxygenate, and
in some
examples, with water and hydroxide, to form the acetate. The acetate can
comprise at least
one of sodium acetate, potassium acetate, and ammonium acetate. As an example,
the
reaction of sodium bicarbonate and the oxygenate to form sodium acetate is
illustrated by the
reaction in Scheme 2.
Scheme 2
18
CA 3058075 2019-10-09
NaHCO3 CH3COOH 4-, CO2 + H20 + NaC2H302
In various examples, the mole ratio of the carbonate in the caustic outlet
stream to
oxygenate in the alkene outlet stream can be in a range of 0.8:1 to 1.2:1 such
as for example,
1:1. In various examples, the mole ratio of the carbonate in the caustic
outlet stream to
oxygenate in the alkene outlet stream can be greater than 1:1 such as, for
example, 2:1.
In various examples, the quench tower 104 can be configured to maintain a pH
in a
range of 2 to 12 such as, for example, 4 to 7. In various examples, the quench
tower 104 can
be configured to maintain a pH in a range of a pKa of the oxygenate to a pKa
of the carbonate
in order to facilitate the formation of the acetate. In various example, the
oxygenate
comprises acetic acid having a pKa of 4.7 and sodium bicarbonate having a pKa
of 6.4.
The first quench outlet stream can comprise a substantial portion of the
carbon-based
oxide produced in the quench tower 104 and from the alkene outlet stream. The
second
quench outlet stream can comprise the oxygenate, the acetate, and water.
Adding the caustic
outlet stream to the quench tower can decrease the amount of oxygenate and
increase the
amount of acetate in the first quench outlet stream. The decrease in oxygenate
in the first
quench outlet stream can be a result of the conversion of the oxygenate to the
acetate. The
conversion of the oxygenate to the acetate can facilitate the removal of the
oxygenate from
the alkene outlet stream and limit the oxygenate from exiting the quench tower
104 in the
first quench outlet stream.
In various examples, the second quench outlet stream can comprise at least 0.1
mol%
oxygenate, such as, for example, at least 0.5 mol% oxygenate, at least 1 mol%,
or 0.1 mol%
to 5 mol% oxygenate. The second quench outlet stream can additionally comprise
at least
0.25 mol% acetate, such as, for example, at least 1.75 mol% oxygenate, at
least 5 mol%, or
0.25 mol% to 15 mol% acetate water of from 80 mol% to 99.5 mol%.
In various examples, the oxygenate in the first oxygenate outlet stream and
the second
quench outlet stream may be subject to further processing. For example,
referring to FIG. 2,
the oxygenate can be separated from the acetate in a separation tower 326.
FIG. 2 is a flow
diagram of a non-limiting example of a system 300 comprising the separation
tower 326. As
illustrated, the separation tower 326 has a separation inlet 326a, a first
separation outlet 326b,
and a second separation outlet 326c. The separation inlet 326a can be
configured to receive
the first oxygenate outlet stream from first oxygenate outlet line 111 and the
second quench
outlet stream from the second quench outline line 112 and may be suitable to
transport at
19
CA 3058075 2019-10-09
least one of the first oxygenate outlet stream and the second quench outlet
stream into the
separation tower 326.
The separation tower 326 can separate the oxygenate from the acetate and, in
various
examples, the separation tower 326 can separate the oxygenate from water. The
presence of
.. the acetate in the separation tower 326 can enhance the separation of
oxygenate from the
water. For example, the acetate and oxygenate may disassociate and/or react
with water to
form an acetate ion (e.g., CH3C00-) and an acid (e.g., H30+, Na+) . Since the
acetate and
oxygenate can form a common ion, an increase in the concentration of one of
the acetate and
oxygenate can affect the other. For example, the reactions of sodium acetate
(C2H3Na02),
acetic acid (CH3COOH), bicarbonate ion (HC0i), carbon dioxide (CO2), and water
(H20) is
illustrated in Scheme 3.
Scheme 3
CH3COOH + H20 4-> CH3C00- + H30'
1120 + HCO3- <-> C01- + H30"
2H20+ CO2 HCO3- + H30"
C2H3Na02 <-4 ( or -4) CH3C00- + Na"
As illustrated in Scheme 3, sodium acetate can form an acetate ion which can
affect
the equilibrium reaction of acetic acid and water. For example, the sodium
acetate can cause
the equilibrium reaction of acetic acid and water to have a higher preference
for the separate
species of acetic acid and water than an acetate ion and an acid relative to
without the
presence of acetate.
The separation tower 326 can comprise various equipment known to those of
ordinary
skill in the art. For example, the separation tower 326 can comprise an
extraction tower, a
packed column, a sieve-tray column, a spray column, a KARR column, a rotating
disc
contactor, a stirred cell extractor, a rectification tower, a stripper, and
combinations thereof
In various examples, the separation tower 326 can comprise a liquid-liquid
extractor.
Accordingly, the acetate in the oxygenate inlet stream can increase the
efficiency of the
separation tower 326 and can facilitate efficient separation of the oxygenate
from water.
The separation tower 326 can produce a second separation outlet stream
comprising a
substantial portion of the oxygenate from at least one of the first oxygenate
outlet stream and
the second quench outlet stream. In various examples, the second separation
outlet stream
can comprise additional components from at least one of the first oxygenate
outlet stream and
the second quench outlet stream, such as, for example, water. In various
examples, the
CA 3058075 2019-10-09
second separation outlet stream can comprise at least 80 mol% oxygenate such
as, for
example, at least 90 mol% oxygenate, at least 95 mol% oxygenate, or 80 mol% to
100 mol%
oxygenate by weight. The second separation outlet stream can exit the
separation tower 326
through the second separation outlet 326c of the separation tower 326. The
second separation
outlet 326c can be configured to receive the second separation outlet stream
and can be
suitable to transport the second separation outlet stream out of the
separation tower 326 into
the second separation outlet line 328.
The separation tower 326 can produce a first separation outlet stream
comprising a
substantial portion of the acetate from the second quench outlet stream and in
various
examples, a substantial portion of the water from the second quench outlet
stream. In various
examples, the first separation outlet stream can comprise at least 10 %
acetate by weight such
as, for example, at least 30 % acetate by weight, at least 50 % acetate by
weight, or 30 % to
70 % acetate by weight. In various examples, the first separation outlet
stream can comprise
at least 5 % water by weight such as, for example, at least 10 % water by
weight, at least 25
% water by weight, or 15 % to 50 % water by weight. The first separation
outlet stream can
exit the separation tower 326 through the first separation outlet 326b of the
separation tower
326. The first separation outlet 326b can be configured to receive the first
separation outlet
stream and can be suitable to transport the first separation outlet stream out
of the separation
tower 326 into the first separation outlet line 330.
The separation tower 326 can be configured with a recycle line 332 in fluid
communication with the first separation outlet line 330 and/or first
separation outlet 326b.
The recycle line 332 can be configured to recycle a portion of the acetate
from the first
separation outlet stream to the separation tower 326 via the recycle inlet
326d. The recycle
line 332 can be configured to receive a portion of the first separation outlet
stream and can be
suitable to transport a recycle stream to a recycle inlet 326d of the
separation tower 326. The
recycle inlet 326d can be configured to receive the recycle stream and can be
suitable to
transport the recycle stream into the separation tower 326. For example, the
recycle stream
can comprise a portion of the acetate from the first separation outlet stream,
and in various
examples, a portion of the water from the first separation outlet stream.
The recycle line 332 can be configured to recycle the acetate from the first
separation
tower outlet stream until a select concentration of acetate is achieved in the
separation tower
326. In various examples and referring to FIGs. 1 and 3, the return line 118
can enable
additional generation of acetate in the quench tower 104 which would flow to
the separation
21
CA 3058075 2019-10-09
tower 326 through the second quench outlet line 112 to increase the
concentration of acetate
in the separation tower 316.
In various examples, a supplemental acetate stream can be added to the
separation
tower 326. In various examples, the supplemental acetate can comprise ethyl
acetate.
In various examples, an oxygen remover 444 can be disposed at any point
intermediate the ODH reactor 102 and the caustic wash tower 106. FIG. 3 is a
flow diagram
of a non-limiting embodiment of a system 400 comprising an oxygen remover 444
when
situated intermediate the flash tower 103 and the quench tower 104. As
illustrated, the
oxygen remover 444, comprising a remover inlet 444a and a remover outlet 444b,
can be
.. provided in fluid communication with the flash tower 103 (FIG. 1) via
alkene outlet line 110a
and the quench tower 104 via remover outlet line 446. The remover inlet 444a
can be
configured to receive the alkene outlet stream and can be suitable to
transport the alkene
outlet stream into the oxygen remover 444. The oxygen remover 444 can remove a
substantial portion of the oxygen in the alkene outlet stream and produce a
remover outlet
stream comprising the alkene outlet stream with the substantial portion of the
oxygen
removed. The oxygen remover 444 can be of various designs as known in the art.
The
remover outlet 444b can be configured to receive the remover outlet stream and
can be
suitable to transport the remover outlet stream out of the oxygen remover 444
into the
remover outlet line 446. The quench inlet 104a of the quench tower 104 can be
configured to
receive the remover outlet stream.
In another embodiment, the oxygen remover 444 can be situated downstream the
ODH reactor 102 and upstream the flash tower 103. In another embodiment, the
oxygen
remover 444 can be situated down stream the quench tower 104 and upstream the
caustic
tower 106. The oxygen remover precedes the caustic wash tower 106, or an amine
tower
(described below), as residual oxygen within the first quench outlet stream
may effect
operability of the caustic wash tower, or amine tower. Minimizing the level of
oxygen within
the first alkene outlet stream may also be achieved by altering ODH reaction
conditions, as
would be apparent to a person skilled in the art.
Referring to FIG. 4, in various examples, an amine tower 548 can be disposed
intermediate the quench tower 104 and the caustic wash tower 106. FIG. 4 is a
flow diagram
of a non-limiting example of a system 500 comprising an amine tower 548. As
illustrated,
the amine tower 548, comprising an amine tower inlet 548a and an amine tower
outlet 548b,
can be provided in fluid communication with the quench tower 104 (FIG. 1) via
quench outlet
22
CA 3058075 2019-10-09
line 114 and the caustic wash tower 106 via amine tower outlet line 550. The
amine tower
inlet 548a can be configured to receive the first quench outlet stream and can
be suitable to
transport the first quench outlet stream into the amine tower 548. The amine
tower 548 can
remove a substantial portion of carbon dioxide in the quench outlet stream and
produce an
amine tower outlet stream comprising the first quench outlet stream with the
substantial
portion of the carbon dioxide removed. The amine tower 548 can be of various
designs as
known in the art.
The amine tower outlet 548b can be configured to receive the amine tower
outlet
stream and can be suitable to transport the amine tower outlet stream out of
the amine tower
548 into the amine tower outlet line 550. The wash inlet 106a of the caustic
wash tower 106
can be configured to receive the amine tower outlet stream from the amine
tower outlet line
550.
Having a high efficiency oxygenate removal prior to the amine tower 548 can
limit,
and in some examples prevent, amine degradation to presence of the oxygenate
in the amine
tower 548. For example, the oxygenate can form heat stable salts with amine in
the amine
tower 548 which can degrade the efficiency and shorten the operational life of
the amine
tower 548.
Referring to FIG. 5, in various examples, a polymerization reactor 652 can be
in fluid
communication with the caustic wash tower 106 via the wash outlet line 116.
FIG. 5 is a
flow diagram of a non-limiting example of a system 600 comprising a
polymerization reactor
652. As illustrated, the polymerization reactor 652, comprising a
polymerization inlet 652a
and a polymerization outlet 652b, can be provided in fluid communication with
the caustic
wash tower 106 via the wash outlet line 116. The polymerization inlet 652a can
be
configured to receive the ODH outlet stream and can be suitable to transport
the ODH outlet
stream into the polymerization reactor 652. The polymerization reactor 652 can
produce a
polymer from the alkene and produce a polymerization outlet stream comprising
the polymer.
In various examples, the polymer comprises at least one of polyethylene,
polypropylene, and
polybutlyene. The polymerization reactor 652 can be of various designs as
known in the art.
The polymerization outlet 652b can be configured to receive the polymerization
outlet stream
and can be suitable to transport the polymerization outlet stream out of the
polymerization
reactor 652 into the polymerization outlet line 654.
Concentrations of the components within the system can be measured any at
point in
the process using any means known in the art. For example, a detector such as
a gas
23
CA 3058075 2019-10-09
chromatograph, an infrared spectrometer, and a Raman spectrometer can be
disposed
downstream or upstream of ODH reactor 102, quench tower 104, caustic wash
tower 106,
separator 238, separation tower 326, oxygen remover 444, amine tower 548, and
polymerization reactor 652.
In various examples, the ODH inlet stream 108 can comprise mixtures that fall
within
the flammability limits of the components. For example, the mixture may exist
in conditions
that prevent propagation of an explosive event. In these examples, the
flammable mixture
can be created within a medium where ignition can be immediately quenched. In
various
examples, oxygen and the lower alkanes can be mixed at a point where they are
surrounded
by a flame arresting material. Thus, any ignition can be quenched by the
surrounding
material. Flame arresting material includes, for example, metallic or ceramic
components,
such as stainless steel walls or ceramic supports. In various examples, oxygen
and lower
alkanes can be mixed at a low temperature, where an ignition event may not
lead to an
explosion, then the mixture can be introduced into the ODH reactor before
increasing the
temperature. Therefore, the flammable conditions may not exist until the
mixture can be
surrounded by the flame arresting material inside of the reactor.
In various examples, the olefins produced using an ODH reactor, or any of the
processes or complexes described herein, can be used to make various olefin
derivatives
utilizing a polymerization reactor. Olefin derivatives include, but are not
limited to,
polyethylene, polypropylene, ethylene oxide, propylene oxide, polyethylene
oxide,
polypropylene oxide, vinyl acetate, vinyl chloride, acrylic esters (e.g.,
methyl methacrylate),
thermoplastic elastomers, thermoplastic olefins, blends thereof, and
combinations thereof.
In various examples, ethylene and optionally a-olefins can be produced in an
ODH
reactor, or any of the processes or complexes described herein, and are used
to make
polyethylene utilizing a polymerization reactor. The polyethylene made from
the ethylene
and optional a-olefins described herein can include homopolymers of ethylene,
copolymers
of ethylene and a-olefins, resulting in HDPE, MDPE, LDPE, LLDPE and VLDPE.
The polyethylene produced using the ethylene and optional a-olefins described
herein
can be produced using any suitable polymerization process and equipment.
Suitable ethylene
polymerization processes include, but are not limited to gas phase
polyethylene processes,
high pressure polyethylene processes, low pressure polyethylene processes,
solution
polyethylene processes, slurry polyethylene processes and suitable
combinations of the above
arranged either in parallel or in series.
24
CA 3058075 2019-10-09
A process for converting a lower alkane to an alkene according to the present
disclosure can include providing an input stream comprising oxygen and the
lower alkane to
an ODH reactor 102. In various examples, providing the input stream to an ODH
includes
providing oxygen and the lower alkane via separate streams. At least a portion
of the lower
alkane can be converted to the alkene in the ODH reactor 102. In various
examples, the
alkane can comprise ethane and the alkene comprises ethylene. In various
examples, the
alkane can comprise propane and the alkene comprises propylene. In various
examples, the
alkane comprises butane and the alkene can comprise butylene. An ODH outlet
stream
comprising the alkene, water in the form of steam, an oxygenate, and a carbon-
based oxide
may be produced. In various examples, the ODH outlet stream can comprise at
least one of
an unreacted alkane and oxygen.
The ODH outlet stream is cooled to allow condensation of a portion of the
oxygenate
and a portion of the steam. The cooled ODH outlet stream is provided to a
flash tower 103,
or other means for gas-liquid separation, and at least a substantial portion
of the condensed
oxygenate and at least a substantial portion of the condensed steam are
removed from the
ODH outlet stream to produce an alkene outlet stream comprising at least a
substantial
portion of the alkene and at least a substantial portion of the carbon-based
oxide and a first
oxygenate outlet stream comprising at least a substantial portion of the
condensed oxygenate
and at least a substantial portion of the condensed steam. In various
examples, the alkene
outlet stream can comprise at least one of oxygenate, steam, unreacted alkane,
and oxygen.
Oxygenate and steam present in the alkene outlet stream, referred to as
remaining oxygenate
and remaining steam, comprise the portions of the oxygenate and steam within
the ODH
outlet stream that fail to condense during cooling, or are carried by the
gaseous alkene and
gaseous carbon-based oxide as the alkene outlet stream exits the flash tower
103.
The alkene outlet stream can be provided to a quench tower 104 and the
remaining
oxygenate and the remaining steam can be removed from the alkene outlet stream
in the
quench tower 104 to produce a first quench outlet stream comprising at least a
substantial
portion of the alkene and at least a substantial portion of the carbon-based
oxide.
Additionally, the quench tower 104 can produce a second quench outlet stream
comprising at
least a substantial portion of the remaining oxygenate and at least a portion
of the remaining
steam. Quench towers typically involve the quenching of a gaseous stream with
water, or
other quench agent, to promote condensation of components within the gaseous
stream. The
condensed components along with the quench agent fall to the bottom of the
tower where
CA 3058075 2019-10-09
they can be removed. The gaseous components rise and can be removed from a
location near
the top end of the quench tower. The temperature of the quench agent is
ideally below that of
the condensation point of the component that is targeted for removal. For
oxygenates, such
as acetic acid, the temperature of the quench agent is, for example, between
20 C and 100 C.
In various examples, at least one of the ODH outlet stream and the alkene
outlet
stream can be provided to an oxygen remover 444 prior to the quench tower 104.
Oxygen
can be removed from at least one of the ODH outlet stream and the alkene
outlet stream in
the oxygen remover 444 and to reduce the levels of oxygen within at least one
of the ODH
outlet stream and the alkene outlet stream to from 0 to 5 parts per million
(ppm)
The first quench outlet stream can be provided to a caustic wash tower 106.
The
quench outlet stream can be contacted with a hydroxide to form a caustic
outlet stream
comprising a carbonate. In various examples, the first quench outlet stream is
contacted with
the hydroxide in the caustic wash tower 106.
In various examples, the first quench outlet stream can be provided to an
amine wash
.. tower 548 prior to the caustic waste tower 106. A substantial portion of
the carbon-based
oxide can be removed from the first quench outlet stream. The first quench
outlet stream
with the substantial portion of the carbon-based oxide removed can be provided
to the caustic
waste tower 106. Use of amines such as diethanolamine, monoethanolamine,
methyldiethanolamine, is well known for treating gases to remove carbon-based
oxides.
The caustic outlet stream can be provided the quench tower 104 where it acts
as a
quench agent, and the alkene outlet stream can be contacted with the caustic
outlet stream to
form an acetate. In various examples, the alkene outlet stream is contacted
with the caustic
outlet stream in the quench tower 104. The second quench outlet stream outlet
stream can
comprise a substantial portion of the acetate. In various examples, the pH of
the quench
tower 104 can be maintained in a range of 2 to 12 such as, for example 4 to 7.
In various
examples, the pH of the quench tower 104 can be maintained in a range of a pKa
of the
oxygenate to a pKa of the carbonate.
In various examples, the second quench outlet stream can be provided to a
separation
tower 326. The oxygenate can be separated from the acetate within the second
quench outlet
stream. A second oxygenate outlet stream comprising a substantial portion of
the oxygenate
from the second quench outlet stream can be produced. An extraction outlet
stream
comprising a substantial portion of the acetate from the oxygenate stream can
be produced.
In various examples, a portion of the acetate from the extraction outlet
stream can be recycled
26
CA 3058075 2019-10-09
to the separation tower 326. In various examples, a supplemental acetate can
be provided to
the separation tower 326 such as, for example, ethyl acetate. In various
examples, the first
oxygenate outlet stream can be provided to the separation tower 326.
In various examples, olefin derivatives can be produced from the alkene.
The present disclosure can provide an alternative use for the caustic waste
stream
which limits, and in some examples, can eliminate a need to disposed of the
caustic waste
stream. Additionally, the reuse of the caustic waste stream can provide a
useful product of
acetate which can aid in oxygenate separation from the quench outlet stream
and purification
of oxygenate in the separation tower. The efficient removal of the oxygenate
from the
quench outlet stream can length the operational light of downstream equipment
such as
protecting the amine tower against fouling and amine solution degradation.
Moreover, the
acetate can be sold. Furthermore, the efficient purification of the oxygenate
can create a
marketable product such as, for example, glacial acetic acid.
EXAMPLES
Computational modeling of a liquid-liquid separation vessel using equations
from
Scheme 3 using ASPEN Plus version 8.6 chemical process simulation software,
commercially available from Aspen Technology, Inc. Bedford, Massachusetts, was
used to
demonstrate the effect of altering the composition of the feed, as mass
fraction, to the
separation tower, the feed representing the second quench outlet stream, on
the composition
of the second oxygenate outlet stream and the composition of the extraction
outlet stream.
The feed components chosen are typical for an ODH process of ethane, and
include water and
acetic acid, with trace amounts (not shown) of ethane, ethylene, carbon
dioxide. Use of
acetate in the quench tower results in sodium acetate contributing to the feed
composition.
Example 1
Example 1 represents a feed composition that corresponds to a second quench
outlet
stream where the ODH outlet stream was not cooled or subjected to non-dilutive
separation
prior to quenching in the presence of sodium acetate. The feed was modeled at
a total mass
flow rate of 6980 kg/hr and at a pressure of 185.7 kPa gauge.
Example 2
Example 2 represents a feed composition that corresponds to a second quench
outlet
stream where the ODH outlet stream was not cooled or subjected to non-dilutive
separation
27
CA 3058075 2019-10-09
prior to quenching in the presence of sodium acetate. The feed was modeled at
a total mass
flow rate of 55891 kg/hr and at a pressure of 465 kPa gauge.
Example 3
Example 3 represents a feed composition that corresponds to a second quench
outlet
stream where the ODH outlet stream was cooled and subjected to non-dilutive
separation
prior to quenching in the presence of sodium acetate. The feed was modeled at
a total mass
flow rate of 11259 kg/hr and at a pressure of 450 kPa gauge.
Example 4
Example 4 represents a feed composition that corresponds to a second quench
outlet
stream where the ODH outlet stream was cooled and subjected to non-dilutive
separation
prior to quenching in the presence of sodium acetate. The feed was modeled at
a total mass
flow rate of 9259 kg/hr and at a pressure of 450 kPa gauge.
Table 1:
Second oxygenate outlet
Feed Extraction Outlet Stream
stream
Example 1 2 3 4 1 2 3 4 1 2 3 4
CH300H 0.219 0.024 0.144 0.175 0.013 0.000 0.070 0.016 0.942 0.954 0.855 0.923
.c) ___________________________________________________________________
Na+ 0.153
0.228 0.145 0.176 0.196 0.240 0.160 0.213 0.000 0.000 0.000 0.000
l' ____________________________________________________________________
CH3C00- 0.392 0.586 0.372 0.453 0.504 0.616 0.411 0.548 0.000 0.000 0.001
0.000
A _____________________________________________________________________
H20 0.235
0.162 0.338 0.196 0.235 0.144 0.359 0.222 0.052 0.046 0.144 0.366
As shown in Table 1, the separation tower, as aided by the sodium acetate,
produced
the second oxygenate outlet stream comprising at least 85 % by weight of
acetic acid. The
separation tower produced an extraction outlet stream comprising at least 99%
of the sodium
acetate. The sodium acetate can be recycled into the separation tower and/or
can be a
commercially marketable product. Additionally, the acetic acid in the second
separation
outlet can be a commercially marketable product.
The feed streams in the examples take into account the effect of cooling prior
to the
quench tower. One skilled in the art would recognize that when the ODH outlet
stream is not
cooled prior to quenching that a larger quantity of water would be required to
quench the
steam and acetic acid present in the ODH outlet stream. This explains why in
example 2 a
feed composition lower in acetic acid than examples 3 and 4 was chosen,
despite the fact that
28
CA 3058075 2019-10-09
in examples 3 and 4 a substantial portion of the acetic acid was removed prior
to the quench
tower. A smaller quantity of water was required for quenching in examples 3
and 4 resulting
in a higher mass fraction for those samples.
The examples demonstrate that acetic acid produced in an ODH process can be
.. isolated in a more concentrated from using non-dilutive separation, and
that the remainder
can be captured as a more dilute solution. The dilute solution can be treated
to increase the
concentration to a marketable level, and this treatment is simplified by
adding spent caustic in
the form of a carbonate to the quenching step.
One skilled in the art will recognize that the herein described components,
devices,
operations/actions, and objects, and the discussion accompanying them are used
as examples
for the sake of conceptual clarity and that various configuration
modifications are
contemplated. Consequently, as used herein, the specific examples/embodiments
set forth
and the accompanying discussion are intended to be representative of their
more general
classes. In general, use of any specific exemplar is intended to be
representative of its class,
.. and the non-inclusion of specific components, devices, operations/actions,
and objects should
not be taken limiting.
While the present disclosure provides descriptions of various specific aspects
for the
purpose of illustrating various aspects of the present disclosure and/or its
potential
applications, it is understood that variations and modifications will occur to
those skilled in
.. the art. Accordingly, the invention or inventions described herein should
be understood to be
at least as broad as they are claimed, and not as more narrowly defined by
particular
illustrative aspects provided herein.
29
CA 3058075 2019-10-09