Note: Descriptions are shown in the official language in which they were submitted.
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
CATHETER INSERTION TRAY WITH INTEGRATED INSTRUCTIONS
PRIORITY
[0001] This application claims the benefit of U.S. Patent Application No.
15/487,297,
filed April 13, 2017, which is a continuation-in-part of U.S. Patent
Application No. 15/029,613,
filed April 14, 2016, which is a U.S. national stage of International Patent
Application No.
PCT/U52014/060963, filed October 16, 2014, which claims the benefit of U.S.
Provisional
Patent Application No. 61/891,496, filed October 16, 2013, and U.S.
Provisional Patent
Application No. 62/015,206, filed June 20, 2014, each of which is hereby
incorporated herein
by reference in its entirety.
BACKGROUND
[0002] Urinary drainage systems are conventionally used in hospitals and
health care
facilities when it is necessary to facilitate, control, monitor urination of
patients, and when it is
necessary to collect urine from a patient. These urinary drainage systems
permit the patient to
remain in bed, without having to use a bedpan or be moved to use a bathroom.
Urinary drainage
systems may include a catheter (e.g., a urinary catheter, such as an
intermittent catheter or a
Foley catheter), a collection container/bag (e.g., a bag made of a polymeric
material or PVC
film), a urine meter, tubing connecting the Foley catheter to the collection
container/bag or
urine meter, and/or other equipment. In operation, the patient is first
catheterized, and the
catheter is connected to the drainage container/bag and/or urine meter through
a length of
tubing (e.g., drainage tubing). The urine drains through the catheter, the
tubing, and then finally
into the collection container/bag and/or urine meter. The urine may be moved
from the catheter
into the collection bag solely due to gravitational forces. On average, a
patient produces about
80-90 mL of urine in 1 hour.
[0003] Accurate monitoring of urine helps the clinician detect
irregularities in urine
flow rate or volume that can signal to the clinician that the patient is
suffering certain problems.
However, urine output cannot be accurately measured if the drainage system
attached to the
Foley catheter is not reliable, or if the Foley catheter and drainage system
are not properly used.
Further, hospitals are using increasingly lower-profile beds in order to
reduce the number of
injuries sustained from falls. With the adoption of lower profile beds, the
amount of height
available to allow the tubing to drain is decreased. Drainage tubing used in
hospitals and
associated venting systems have also undergone changes/revisions. Changes in
venting
-1-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
combined with increasingly lower profile hospital beds have created suboptimal
drainage
performance. For example, urine has been observed pooling in the tubing. This
prevents
accurate urine output and flow rate measurements, which are critical for many
patients.
[0004] Currently, the second-most common form of Hospital Acquired
Infection (HAT)
is catheter-associated urinary tract infection (CAUTI). Hospitals are
interested in ways to cut
their CAUTI rates by conforming to a strict aseptic technique as a standard of
care. However,
there are many factors that influence a hospital's ability to meet the
standard of care. These
factors include: health care practitioner/nurse experience and training,
patient factors (e.g.,
general health, weight, and anatomy), environmental factors, and tray layout
as well as contents
and instructions/indicators. A catheterization package and/or catheterization
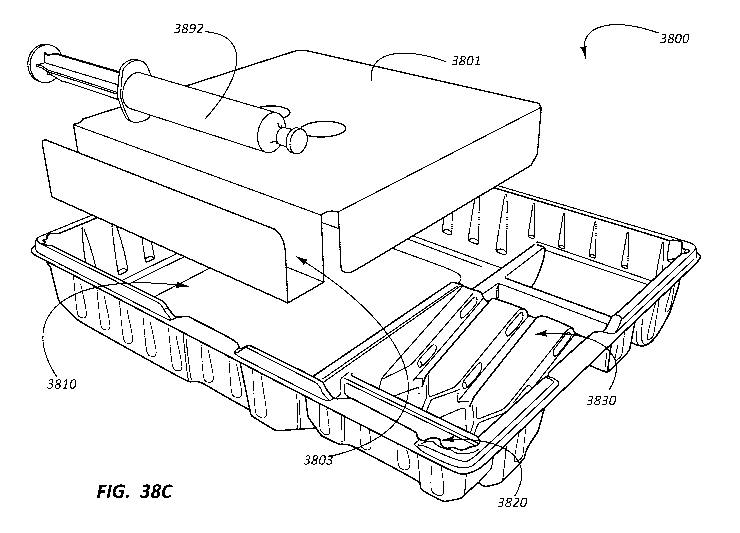
tray with
components optimized to the procedure and an intuitive layout can increase
compliance to
aseptic technique, potentially reducing CAUTI rates.
[0005] There is a need in the healthcare field for a more reliable, safe,
and easy method
for inserting a catheter, such as a urinary catheter, for example an
indwelling or intermittent
catheter, into a patient. More particularly, there is a need to provide a
catheterization package
and/or a catheterization tray (e.g., a Foley catheter tray) that improves and
standardizes the
process for inserting a urinary catheter, such as an indwelling Foley
catheter, into a patient.
[0006] The present disclosure provides a catheterization package,
catheter tray, and
drainage system configured to better meet patients' needs, improve reliability
and ease of use,
reduce incidents of CAUTI, improve safety, and address other issues described
above and
elsewhere herein.
SUMMARY
[0007] Embodiments of, and enhancements for, packages, systems, trays,
assemblies,
devices, methods, etc. used for medical treatment generally and
catheterization, in particular,
are described herein.
[0008] The objectives described herein can be met by providing an
improved medical
procedure package and/or tray (e.g., an improved catheterization package
and/or an improved
catheterization tray). The improved medical procedure package may include the
improved
medical procedure tray therein, and the medical procedure tray may be
intuitively arranged and
may include instructions or procedural indicators to improve the medical
procedure
-2-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
implementation and results. The medical procedure package and/or medical
procedure tray
may include various implements and components necessary and/or helpful to the
medical
procedure. For example, an improved catheterization package may include an
improved
catheterization tray, povidone iodine swabs or swabsticks that allow for
greater coverage and
saturation, hand sanitizer with improved efficacy and that enables single-
handed usage, tubing
that breaks up the surface tension of the fluid in order to improve drainage,
and/or other
components described herein.
[0009] According to various embodiments, the present disclosure provides
a
catheterization package and catheterization tray having a layout and/or
arrangement of
components that may help reduce CAUTI rates by facilitating ease of use and
aiding in proper
aseptic technique during insertion. The present disclosure also provides
methods of
catheterization and use of a catheterization package and/or catheterization
tray that may
facilitate easier and more sterile catheterization to help reduce CAUTI rates.
Further, the
present disclosure provides a system that may improve drainage performance,
which in turn:
(1) helps to eliminate fluid in the drain tubing, and (2) increases the
accuracy of urine
measurements (e.g., measurements of output and flow).
[0010] In some embodiments, a catheterization package is provided
including a
catheterization tray and a sterile wrap around the catheterization tray. The
catheterization tray
can include catheterization instructions imprinted directly on the
catheterization tray. The
catheterization tray can also include a first section, a second section, and a
third section,
wherein in one or more partitions can separate at least the third section from
the first and second
sections. The first section can hold a drainage system, the second section can
hold a container
containing lubricant, and the third section can hold a swab in an inclined
position. The
absorbent head of the swab can angle downwardly into a well of the third
section and an
elongate member of the swab can angle upwardly for gripping and removal of the
swab.
[0011] In such embodiments, the catheterization package can further
include a pen-
care kit, a belly band, and a packaging label. The pen-care kit can be
packaged with the
catheterization tray but outside the sterile wrap. One example of a suitable
peri-care kit is
disclosed in PCT Application Publication No. WO 2016/126555, the disclosure of
which is
incorporated herein by reference in its entirety. The belly band can be
outside the sterile wrap
holding the sterile wrap in a folded configuration around the catheterization
tray. The
packaging label can be outside the sterile wrap for identifying the
catheterization package.
-3-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0012] In such embodiments, the third section of the catheterization
package can
include a swab compartment, an overflow compartment, and a corner storage
compartment
holding a specimen container. The swab compartment can include the well and a
channel
configured to hold the swab. The channel can include at least two snap-in tabs
to hold the swab
in the channel under the snap-in tabs, wherein each longitudinal side of the
channel includes at
least one of the at least two snap-in tabs. The channel can be further
configured to convey a
fluid to the well when the swab is absent from the channel and the fluid is
poured in the channel.
The overflow compartment can be fluidly connected to the well through the
channel.
[0013] In such embodiments, the one or more partitions of the
catheterization package
separating the third section from the first and second sections can further
separate the second
section from the first section. Second section-separating partitions of the
one or more partitions
can have a maximum height equal to a height of the catheterization tray,
wherein a bottom of
the second section can be elevated above a bottom of the catheterization tray.
Alternatively,
the second section-separating partitions of the one or more partitions can
have a maximum
height less than the height of the catheterization tray, wherein the bottom of
the second section
and the bottom of the catheterization tray are substantially co-planar.
[0014] In some embodiments, a medical-procedure package is provided
including a
tray with medical-procedure instructions imprinted directly on the tray, at
least some of which
instructions are revealed in step with steps of the medical procedure. The
tray can include a
first section, a second section, and a third section, wherein one or more
partitions separate at
least the third section from the first and second sections. The tray can
further include a drainage
system, a container containing a lubricant, and one or more swabs. The first
section can include
at least the drainage system, the second section can include at least the
container containing the
lubricant, and the third section includes at least the one or more swabs.
[0015] In such embodiments, the medical-procedure package can further
include a
specimen container. The third section can include a swab compartment, an
overflow
compartment, and a corner storage compartment including the specimen
container. The swab
compartment can include a well and one or more channels configured to
respectively hold the
one or more swabs with snap-in tabs in the one or more channels. The one or
more channels
can also be angled with respect to a bottom of the tray such that a handle end
of each swab of
the one or more swabs held under the snap-in tabs is elevated with respect to
an absorbent-head
end when the absorbent-head end is in the well. The overflow compartment can
be fluidly
-4-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
connected to the well through the one or more channels, wherein the one or
more channels can
be further configured to convey a fluid poured therein to the well when the
one or more swabs
are removed from the one or more channels.
[0016] In such embodiments, the medical-procedure package can further
include a third
section-separating partition of the one or more partitions at a well-end of
the swab compartment
directly separating the well from the second section. Alternatively, a well-
end of the swab
compartment can share with the tray an inner wall of the tray.
[0017] In such embodiments, the one or more partitions of the medical-
procedure
package separating the third section from the first and second sections can
further separate the
second section from the first section. Second section-separating partitions of
the one or more
partitions can have a maximum height equal to a height of the tray, wherein a
bottom of the
second section can be elevated above a bottom of the tray. Alternatively, the
second section-
separating partitions of the one or more partitions can have a maximum height
less than the
height of the tray, wherein the bottom of the second section and the bottom of
the tray are
substantially co-planar.
[0018] In some embodiments, a catheterization package is provided
including a tray
with instructions for a catheterization procedure imprinted directly on the
tray, at least some of
which instructions are revealed in step with steps of the catheterization
procedure. The tray
can include a first section, a second section, and a third section, wherein
one or more partitions
separate at least the third section from the first and second sections. The
tray can further include
a drainage system, a container containing a lubricant, a syringe of sterile
liquid, such as water
or, in some instances, saline, and a skin-preparation kit. The first section
can include at least
the drainage system, which drainage system can include a Foley catheter,
drainage tubing, and
a drainage bag. The second section can include at least the container
containing the lubricant.
The first section can include the syringe of the sterile liquid, such as
sterile water, which syringe
can be configured for inflating a balloon of the Foley catheter with the
sterile water. According
to certain embodiments, the water syringe can be connected to the inflation
port of the Foley
catheter during manufacture of the assembly. Providing a pre-connected syringe
to the Foley
catheter can help minimize the number of steps required to catheterize a
patient. The third
section can include at least the skin-preparation kit, which kit can include a
package of an
antiseptic and one or more swabs.
-5-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0019] In such embodiments, the catheterization package can further
include a
specimen container and a label for the specimen container. The third section
can include a
swab compartment, an overflow compartment, and a corner storage compartment
including the
specimen container. The swab compartment can include a well and one or more
channels
configured to respectively hold the one or more swabs with snap-in tabs in the
one or more
channels. The overflow compartment can be fluidly connected to the well
through the one or
more channels, wherein the one or more channels can be further configured to
convey the
antiseptic poured therein to the well when the one or more swabs are removed
from the one or
more channels. The package of the antiseptic can be placed over the swab
compartment, the
overflow compartment, the corner storage compartment, or a combination
thereof.
[0020] In such embodiments, the catheterization package can further
include a third
section-separating partition of the one or more partitions at a well-end of
the swab compartment
directly separating the well from the second section. Alternatively, a well-
end of the swab
compartment can share with the tray an inner wall of the tray.
[0021] In such embodiments, the one or more partitions of the
catheterization package
separating the third section from the first and second sections can further
separate the second
section from the first section. Second section-separating partitions of the
one or more partitions
can have a maximum height equal to a height of the tray, wherein a bottom of
the second
section can be elevated above a bottom of the tray. Alternatively, the second
section-separating
partitions of the one or more partitions can have a maximum height less than
the height of the
tray, wherein the bottom of the second section and the bottom of the tray are
substantially co-
planar.
[0022] In such embodiments, the catheterization package can further
include a sterile
wrap, a pen-care kit, a belly band, and a packaging label. The sterile wrap
can be in a folded
configuration around the tray. The pen-care kit can be packaged with the
catheterization tray
but outside the sterile wrap. The belly band can be outside the sterile wrap
holding the sterile
wrap in the folded configuration around the catheterization tray. The belly
band can also
include instructions regarding how to orient the tray prior to opening the
sterile wrap. The
packaging label can be outside the sterile wrap for identifying the
catheterization package. In
addition, the packaging label can include information emphasizing key features
of the
catheterization package in a way that is easy to read quickly.
-6-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0023] In some embodiments, a catheterization package comprises a
catheterization
tray including a first compartment holding a drainage system; a second
compartment holding
a syringe; a third compartment holding a swab in an inclined position such
that an absorbent
head of the swab is biased downwardly into a well of the third compartment and
an elongate
member of the swab angles upwardly for easier gripping and removal; and
catheterization
instructions or procedural indicators imprinted directly on the
catheterization tray. The
catheterization package may also include a sterile wrap (e.g., a CSR wrap)
wrapped around the
catheterization tray. The absorbent head of the swab may be formed of
absorbent foam, and
the elongate member of the swab may have a generally rectangular cross section
with rounded
edges. The catheterization package may also include a pen-care kit packaged
with the
catheterization tray at a location outside the sterile wrap. The
catheterization package may also
include a belly band outside the sterile wrap, the belly band including an
instruction or
procedural indicator regarding how to orient the catheterization tray prior to
opening the sterile
wrap. Additionally, the catheterization package may include a packaging label
outside the
sterile wrap, wherein the packaging label includes at least three sides folded
into a different
plane from the top portion of the label that are visible when viewing the
catheterization package
from one or more sides of the catheterization package, and/or wherein the
packaging label
includes information features (e.g., information squares) that each emphasize
a key feature of
the catheterization package in a way that is easy to read quickly. The
catheterization package
may include an outer sealed container or bag (e.g., a plastic, transparent
bag) around the other
components to maintain sterility during shipping and storage.
[0024] In some embodiments, a medical procedure package comprises three
or more
compartments holding implements useful for performance of the medical
procedure and
instructions/procedural indicators imprinted or included directly on the
medical procedure tray
directing a user how to carry out steps of the medical procedure, wherein the
three or more
compartments of the medical procedure tray, the instructions/procedural
indicators, and
implements are arranged in an arrangement such that the medical procedure
proceeds
intuitively from step to step based on the arrangement. The arrangement may
include stacking
various components or implements (e.g., 2 to 20 implements/components or 4 to
10
implements/components) on top of each other in the order that they are to be
used (e.g., with
components or implements to be used before other components or implements
being positioned
on top of the other components or implements to be used later).
-7-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0025] In some embodiments, a method of treating a patient comprises
providing a
medical procedure tray including three or more compartments including
implements useful for
performance of the medical procedure and instructions/procedural indicators
imprinted directly
on the medical procedure tray directing a user how to carry out steps of the
medical procedure.
The method may also include performing the medical procedure on a patient
while following
the instructions/procedural indicators imprinted on the medical procedure
tray. Three or more
compartments of the medical procedure tray, the instructions/procedural
indicators, and/or
implements are arranged such that the medical procedure proceeds intuitively
from step to step
based on how they are arranged on/in the medical procedure tray.
[0026] In some embodiments, a method of catheterizing a patient comprises
providing
a catheterization package having a catheterization tray including a first
compartment holding a
drainage system including a catheter; a second compartment holding a swab in
an inclined
position such that and absorbent head of the swab is biased downwardly into a
well of the
second compartment and an elongate member of the swab angles upwardly; pouring
an
antiseptic solution into the well such that the absorbent head is in contact
with the antiseptic
solution, using the swab to cleanse the patient in a region to be
catheterized, and inserting a
portion of a catheter into a urethra of the patient. A sealed container or bag
may be disposed
around the catheterization tray, and the method may include unsealing the
sealed container or
bag. A sterile wrap may be wrapped around the catheterization tray, and the
method may
include unwrapping the sterile wrap prior to pouring an antiseptic solution
into the well. The
catheterization package may also include a pen-care kit located outside of the
sterile wrap, and
the method may include using the peri-care kit to cleanse a portion of the
patient's perineum
prior to unwrapping the sterile wrap. A belly band may be positioned outside
the sterile wrap,
the belly band may include an instruction/procedural indicator or
instructions/procedural
indicators regarding how to properly orient the catheterization tray prior to
opening the sterile
wrap, and the method may include orienting the tray according to the
instruction(s)/procedural
indicator(s) prior to unwrapping the sterile wrap.
[0027] In some embodiments, a method of manufacturing a catheterization
package
comprises providing a catheterization tray including a first compartment, a
second
compartment, a third compartment having an inclined portion of the bottom of
the compartment
with channels for holding a swab, and catheterization instructions/procedural
indicators
imprinted directly on the catheterization tray; wherein the first compartment,
the second
-8-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
compartment, and the third compartment are each at least partially separated
from each other
by partitions. The method may also include positioning a drainage system in
the first
compartment, a syringe in the second compartment, and a swab in the third
compartment such
that an absorbent head of the swab is biased downwardly into a well of the
third compartment
and an elongate member of the swab angles upwardly for easier gripping and
removal. The
method may further include wrapping the catheterization tray in a sterile wrap
and positioning
a belly band around a portion of the sterile wrap, the belly band including an
instruction/procedural indicator or instructions/procedural indicators
regarding how to properly
orient the catheterization tray prior to opening the sterile wrap. In
addition, the method may
include adding a pen-care kit to the catheterization tray outside the belly
band and sterile wrap,
the pen-care kit including a baggy with a zipper holding cleansing towelettes,
hand sanitizer,
and instructions/procedural indicators for cleansing the patient. The method
may include
adding a document of detailed catheterization instructions and/or procedural
indicators to the
catheterization package. Also, the method may include placing a packaging
label on top of the
catheterization package, the packaging label including at least three side
portions folded from
the top portion, and/or placing a packaging label on top of the
catheterization package, the
packaging label including an information feature or information features that
each emphasize
one or more key features of the catheterization package in a way that is easy
to read quickly.
The information feature or information features may be an information square
or multiple
information squares. The method may also include sealing (e.g., by heat
sealing) a transparent
plastic bag around the catheterization package.
[0028] In some embodiments, a catheterization system includes a container
for
securing components for a catheterization procedure. The container has an
outer shell defining
a general shape of the container and a plurality of compartments within the
outer shell. Each
of the plurality of compartments is separated from one or more adjacent
compartments of the
plurality of compartments by one or more partitions. The plurality of
compartments include a
first compartment sized and configured to contain a catheter assembly and one
or more
secondary compartments separated from the first compartment by the one or more
partitions.
At least one of the one or more secondary compartments includes at least one
partial partition
(e.g., a partition that includes a reduced height portion) of the one or more
partitions.
[0029] In some embodiments, a catheterization system for performing a
catheterization
procedure is provided. The catheterization system includes a catheterization
tray that has an
-9-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
outer shell defining a general shape of the container and a plurality of
compartments within the
outer shell. Each of the plurality of compartments is separated from one or
more adjacent
compartments of the plurality of compartments by one or more partitions. The
plurality of
compartments may include a first compartment containing a catheter assembly
and one or more
secondary compartments separated from the first compartment by the one or more
partitions.
At least one of the one or more secondary compartments may include at least
one partial
partition (e.g., a partition that includes a reduced height portion) of the
one or more partitions.
Additionally, the catheterization system includes one or more swabs located in
a second
compartment of the one or more secondary compartments.
DRAWINGS
[0030] The disclosed embodiments of, and enhancements for, packages,
trays, devices,
systems and methods can be better understood with reference to the following
drawings.
Portions of the material in this patent document are subject to copyright
protection under the
copyright laws of the United States and of other countries. The owner of the
copyright rights
has no objection to the facsimile reproduction by anyone of the patent
document or the patent
disclosure, as it appears in the United States Patent and Trademark Office
publicly available
file or records, but otherwise reserves all copyright rights whatsoever.
[0031] FIG. 1 shows a top view of a medical procedure tray including
integrated
instructions/procedural indicators in the form of a catheterization tray.
[0032] FIG. 2 shows a top, front (or proximal), right perspective view of
the medical
procedure tray of FIG. 1.
[0033] FIG. 3 shows a right side elevation view of the medical procedure
tray of FIG.
1.
[0034] FIG. 4 shows a back (or distal) side elevation view of the medical
procedure
tray of FIG. 1.
[0035] FIG. 5 shows a top view of another medical procedure tray
including integrated
instructions/procedural indicators in the form of a catheterization tray that
is larger than the
tray in FIG. 1.
-10-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0036] FIG. 6A shows a top view of a swab or swabstick that may be
included in a
catheterization package and/or tray.
[0037] FIG. 6B shows a side perspective view of the swab or swabstick in
FIG. 6A.
[0038] FIG. 6C shows an end view of the swab or swabstick in FIG. 6A to
show the
cross-sectional shape of the elongate member or stick.
[0039] FIG. 7A shows a top view of a swab or swabstick that may be
included in a
catheterization package and/or tray.
[0040] FIG. 7B shows a side perspective view of the swab or swabstick in
FIG. 7A.
[0041] FIG. 7C shows an end view of the swab or swabstick in FIG. 7A to
show the
cross-sectional shape of the elongate member or stick, the cross-sectional
shape being different
from that shown in FIG. 6C.
[0042] FIG. 8 shows a top view of a swab compartment and small storage or
overflow
compartment of a catheterization tray including swabs or swabsticks similar to
the swab or
swabstick shown in FIGS. 7A-7C, wherein one swab or swabstick is rotated
approximately 90
to release it from the securement features of the swab compartment.
[0043] FIG. 9 shows a top view of a catheterization package sealed in a
sealed bag and
having a packaging label with information squares on a top and two sides
thereof, the sides
providing for easy viewing even if the catheterization package were in a stack
of other
catheterization packages.
[0044] FIG. 10 shows a top, front perspective view of another
catheterization package
(different from that shown in FIG. 9) sealed in a sealed bag and having a
packaging label with
information squares on a top and two sides thereof (one side of which is
visible), the sides
providing for easy viewing even if the catheterization package were in a stack
of other
catheterization packages.
[0045] FIG. 11 shows a top view of the catheterization package of FIG. 9
outside of
the sealed bag, but still having the same packaging label thereon.
[0046] FIG. 12 shows atop, flattened view of the packaging label of the
catheterization
package of FIG. 10 including lines added to show where the sides may be folded
when placed
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
over other components of a catheterization package prior to sealing the
catheterization package
in a sealed bag.
[0047] FIG. 13 shows a top view of a catheterization package (e.g.,
either the
catheterization package of FIG. 9 or FIG. 10) after the sealed bag and
packaging label have
been removed, and showing detailed instructions/procedural indicators document
or directions
for use (DFU) document thereon.
[0048] FIG. 14A shows a page of a document of detailed catheterization
instructions/procedural indicators or directions for use (DFU) document that
may be included
in a catheterization package.
[0049] FIG. 14B shows another page of the document of detailed
catheterization
instructions/procedural indicators or directions for use (DFU) document of
FIG. 14A.
[0050] FIG. 14C shows another page of the document of detailed
catheterization
instructions/procedural indicators or directions for use (DFU) document of
FIG. 14A.
[0051] FIG. 14D shows another page of the document of detailed
catheterization
instructions/procedural indicators or directions for use (DFU) document of
FIG. 14A.
[0052] FIG. 15A shows a front side of a patient education information
sheet/pamphlet
that may be included in a catheterization package.
[0053] FIG. 15B shows a back side of the patient education information
sheet/pamphlet
of FIG. 15A.
[0054] FIG. 16 shows a top view of the catheterization package of FIG. 13
without the
detailed instructions/procedural indicators document or directions for use
(DFU) document,
and showing a label or insert sheet thereon.
[0055] FIG. 17 shows a top view of a catheterization package (e.g.,
similar to the
catheterization package of FIG. 16) without the label or insert sheet, and
showing a perineal
care or peri-care kit/packet thereon.
[0056] FIG. 18 shows a top view of a catheterization package (e.g.,
similar to the
catheterization package of FIG. 16) without the label or insert sheet, and
showing another,
different perineal care or peri-care kit/packet thereon.
-12-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0057] FIG. 19 shows a separated view of the components of the perineal
care (or pen-
care) kit or packet of FIG. 18.
[0058] FIG. 20 shows a top view of a catheterization package (e.g.,
similar to the
catheterization package of FIG. 17 or FIG. 18) without the perineal care (or
pen-care) kit or
packet, and showing a belly band including instructions/procedural indicators
thereon.
[0059] FIG. 21 shows a top view of a catheterization package (e.g.,
similar to the
catheterization package of FIG. 20 or FIG. 21) without the belly band, and
showing the sterile
wrap only partially folded/wrapped around a catheterization tray.
[0060] FIG. 22 shows a top view of the catheterization package of FIG. 22
with the
sterile wrap completely unfolded but remaining underneath the catheterization
tray, and
showing the contents of the catheterization tray before any components have
been removed or,
during manufacture/packaging, after all the components to be within the
sterile wrap have been
placed therein.
[0061] FIG. 23 shows a top view of the catheterization package of FIG. 23
with a
package of sterile gloves partially unfolded and sitting on top of the
catheterization tray.
[0062] FIG. 24 shows a top view of the catheterization package of FIG. 24
without the
package of sterile gloves, and revealing a pad/drape (representative of a
waterproof underpad
and/or a fenestrated drape) sitting on top of the catheterization tray.
[0063] FIG. 25 shows a top view of the catheterization package of FIG. 25
with the
pad/drape moved off the swab compartment, and revealing a packet of povidone-
iodine
solution sitting in the swab compartment and extending over the small storage
or overflow
compartment of the catheterization tray.
[0064] FIG. 26 shows a top view of the catheterization package of FIG. 26
without the
povidone-iodine solution, and revealing the swabs or swabsticks in the swab
compartment with
the proximal ends of the sticks extending over the small storage or overflow
compartment of
the catheterization tray.
[0065] FIG. 27 shows a top view of the catheterization package of FIG. 27
with the
syringe of sterile liquid attached to the inflation port of the catheter.
-13-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0066] FIG. 28 shows a top view of the catheterization package of FIG. 28
with a distal
portion of the catheter positioned in the syringe or catheter compartment and
the syringe of
lubricating jelly adjacent to syringe or catheter compartment ready to
dispense the lubricating
jelly on top of the distal region or distal end of the catheter.
[0067] FIG. 29 shows a top view of the catheterization package of FIG. 29
after the
catheter has been removed from the tray and inserted in a patient, with the
drainage tubing
extending in the direction of the patient.
[0068] FIG. 30A is a top view of a catheterization system;
[0069] FIG. 30B is a top view of a catheterization tray of the
catheterization system of
FIG. 30A, which is wrapped in a wrap;
[0070] FIG. 31A is an isometric view of a catheterization tray with
contents thereof;
[0071] FIG. 31B is an isometric view of the catheterization tray of FIG.
31A with some
of the contents thereof removed therefrom;
[0072] FIG. 31C is a back isometric view of the catheterization tray of
FIG. 31A;
[0073] FIG. 32 is a top view of the catheterization tray of FIG. 31A with
all of the
contents thereof removed therefrom;
[0074] FIG. 33 is a top view of a catheterization system;
[0075] FIG. 34 is a top view of a fenestrated drape;
[0076] FIG. 35 is an isometric view of a swab;
[0077] FIG. 36A is an isometric view of a catheterization system;
[0078] FIG. 36B is an isometric view of a catheterization tray of the
catheterization
system of FIG. 36A;
[0079] FIG. 37A is an isometric view of a catheterization system;
[0080] FIG. 37B is an isometric view of a partially unfolded
catheterization tray of the
catheterization system of FIG. 37A; and
-14-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0081] FIG. 37C is a partial isometric view of the unfolded
catheterization tray of the
catheterization system of FIG. 37A.
[0082] FIG. 38A shows a view of another medical-procedure tray in
accordance with
some embodiments.
[0083] FIG. 38B shows another view of the medical-procedure tray of FIG.
38A.
[0084] FIG. 38C shows another view of the medical-procedure tray of FIG.
38A
together with a cover.
[0085] FIG. 38D shows another view of the medical-procedure tray of FIG.
38A
together with a cover.
[0086] FIG. 39A shows a view of another medical-procedure tray in
accordance with
some embodiments.
[0087] FIG. 39B shows another view of the medical-procedure tray of FIG.
39A.
[0088] FIG. 39C shows a view of a medical-procedure tray including a
number of
retainers in a first configuration in accordance with some embodiments.
[0089] FIG. 39D shows a view of a medical-procedure tray including a
number of
retainers in a second configuration in accordance with some embodiments.
[0090] FIG. 40A shows a view of another medical-procedure tray in
accordance with
some embodiments.
[0091] FIG. 40B shows another view of the medical-procedure tray of FIG.
40A.
[0092] FIG. 41A shows a view of another medical-procedure tray in
accordance with
some embodiments.
[0093] FIG. 41B shows another view of the medical-procedure tray of FIG.
41A.
[0094] FIG. 41C shows another view of the medical-procedure tray of FIG.
41A.
[0095] FIG. 42A shows a view of another medical-procedure tray in
accordance with
some embodiments.
-15-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0096] FIGS. 42B and 42C show other views of the medical-procedure tray
of FIG.
42A.
[0097] FIG. 43 shows a view of another medical-procedure tray in
accordance with
some embodiments.
[0098] FIG. 44A shows a view of a removable retainer on a partition or a
side of a
medical-procedure tray in accordance with some embodiments.
[0099] FIG. 44B shows another view of a removable retainer on a partition
or a side of
a medical-procedure tray with a concealed tab of instructions in accordance
with some
embodiments.
[0100] FIG. 44C shows another view of a removable retainer on a partition
or a side of
a medical-procedure tray with a revealed tab of instructions in accordance
with some
embodiments.
[0101] FIG. 45 shows a view of an appendage-type retainer on a side of a
medical-
procedure tray in accordance with some embodiments.
[0102] While the invention is susceptible to various modifications and
alternative
forms, specific embodiments thereof have been shown by way of example in the
drawings and
are herein described in detail. It should be understood, however, that the
description herein of
specific embodiments is not intended to limit the invention to the particular
forms disclosed,
but rather the intention is to cover all modifications, equivalents, and
alternatives falling within
the spirit and scope of the invention as defined by the appended claims.
DESCRIPTION
[0103] The following description and accompanying figures, which describe
and show
certain embodiments, are made to demonstrate, in a non-limiting manner,
several possible
configurations of medical procedure packages, medical procedure trays,
catheterization
packages, catheterization trays, and associated components, assemblies, and
systems, etc. and
various methods of manufacturing and methods of using these according to
various aspects and
features of the present disclosure.
[0104] Various packages, kits, trays, systems, assemblies, devices, and
methods are
described herein, including those used in various medical procedures
(including, for example,
-16-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
catheterization procedures). While specific embodiments are described herein
by way of
example, the embodiments and examples described are not intended to be
limiting.
Accordingly, while aspects of the invention may be described, for example, in
terms of
catheterization packages, catheterization trays, catheterization procedures,
etc. disclosure is not
limited to catheterization-related packages, trays, systems/assemblies,
procedures, etc. Rather,
the inventive principles associated with the embodiments described herein, may
be applied to
other embodiments and other types of packages, trays, systems/assemblies,
devices, methods,
etc.
[0105] According to various embodiments, the objectives described above
and
elsewhere herein may be accomplished by providing an improved medical
procedure package
or kit including a medical procedure tray, for example, with a more intuitive
or better organized
layout, instructions/procedural indicators included on the tray, etc. For
example, an improved
catheterization package or kit may be provided to improve ease of use, improve
adherence to
proper techniques, reduce the likelihood of infection, etc.
[0106] In accordance with various embodiments, the medical procedure
package may
include a medical procedure tray therein. The medical procedure package may
also include
any other components necessary or helpful to the medical procedure. The
medical procedure
tray(s) contemplated herein may be a single level tray or have multiple
levels. The medical
procedure tray(s) may have a variety of shapes and sizes. For example, a
medical procedure
tray may have a generally or approximately rectangular, square, circular,
oval, triangle,
hexagonal, polygon, or other shape. As a non-limiting example, a generally
rectangular
medical procedure tray may have a length in the range from 7 inches to 20
inches, a width in
the range from 4 inches to 12 inches, and a height in the range from 1 inch to
4 inches. In one
embodiment, a generally rectangular medical procedure tray (e.g., a
catheterization tray similar
to that shown in FIGS. 1-4) may have a length of approximately 11 inches, a
width of
approximately 8.5 inches, and a height of approximately 2 inches. In one
embodiment, a
generally rectangular medical procedure tray (e.g., similar to the tray shown
in FIGS. 5, 31A-
31C, & 32) may have a length of approximately 14 inches, a width of
approximately 8.5 inches,
and a height of approximately 2.5 inches.
[0107] The medical procedure tray(s) may also include multiple sections
having a
variety of shapes and sizes, any of which sections can optionally form a
compartment. A
section of the medical procedure tray can be designated for one or more
particular uses (e.g.,
-17-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
well for iodine, section for storage, etc.), whereas a compartment can be a
section at least
partially and up to wholly separated from one or more other sections,
compartments, or a
combination thereof by a physical feature such as one or more partitions. The
partitions can
be formed from the medical procedure tray itself (e.g., formed during an
injection molding
process), and opposing faces of the partitions can be unequal in height if
separating
compartments from other sections or compartments having bottoms of higher or
lower
elevations. Partitions can be separately formed from a same material as the
medical procedure
tray or a different material than the medical procedure tray. The partitions
can also be
transiently formed from any one or more of the other components necessary or
helpful to the
medical procedure ¨ until the any one or more of the other components are
removed from the
medical procedure tray. For example, with respect to components necessary or
helpful to
catheterization, the one or more partitions can be transiently formed from a
specimen or sample
container, instructions/procedural indicators, or other components necessary
or helpful to
catheterization described herein. Like the sections, the compartments can have
a variety of
shapes and sizes (e.g., the compartments may be of any of the shapes described
for the medical
procedure tray itself or of other shapes).
[0108] In accordance with various embodiments, the medical procedure
package may
be a catheterization package that includes one or more catheterization trays
therein. For
example, a catheterization package may include an improved catheterization
tray with a more
intuitive or better organized layout and/or instructions/procedural indicators
included on the
tray. While various features are described below in terms of a catheterization
package and/or
catheterization tray, the features described may also be included in or
applied to medical
procedure packages and/or trays used for procedures other than
catheterization.
[0109] The catheterization package may include any components necessary
or helpful
to catheterization. Some components helpful to catheterization that may be
included in the
catheterization package include a drainage system, a drainage/collection bag,
drainage tubing,
a catheter (e.g., a Foley catheter), a drainage outlet, a stabilization device
(e.g., C. R. Bard,
Inc.'s StatLock Foley stabilization device), a urine meter, swabs or
swabsticks, prepping balls
(e.g., absorbent cotton balls), forceps, a specimen or sample container, a
label that can be filled
out with details regarding the sample and adhered to the specimen or sample
container, a packet
or container of an antiseptic skin cleanser (e.g., a packet or container of
povidone-iodine
solution), a packet or container of lubricant (e.g., a syringe of lubricating
jelly), a syringe of
-18-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
sterile liquid (e.g., a lOcc syringe of sterile water for inflating the
retention balloon of the Foley
catheter), a fenestrated drape to place on patient, an underpad to place under
the buttocks of a
patient (e.g., a waterproof absorbent underpad), gloves (e.g., a package of
rubber gloves, latex
gloves, latex-free gloves), a sterile wrap (e.g., a CSR wrap), a belly band
(e.g., to hold the
sterile wrap in a folded configuration), a perineal care packet, hand
sanitizer (e.g., antiseptic
gel hand rinse), moist towelettes (e.g., a package of castile soap
towelettes),
instructions/procedural indicators (e.g., instruction sheet for health care
provider and/or
instruction pamphlet for patient), a checklist of safety considerations/steps,
a patient
information chart, an insert sheet, a packaging label, an outer container
(e.g., a sealed bag),
and/or other components. The components included in the catheterization
package may be
included in one or more compartments of the catheterization tray, in a
separate package or bag
(e.g., a package outside the tray), or in a second catheterization tray.
[0110] The
catheterization tray(s) may be labeled with step-wise
instructions/procedural indicators arranged in logical locations on the
tray(s). The
catheterization tray(s) may have a layout and design that makes the
catheterization procedure
more intuitive. A single-level catheterization tray having a generally
rectangular shape,
including multiple compartments of varying shapes, including step-wise
instructions/procedural indicators, and having an improved layout is shown,
for example, in
FIGS. 1-4 and described below. Other similar single-level catheterization
trays are shown in
FIGS. 5, 31A-31C, & 32 as well.
[0111]
According to various embodiments and as shown, for example, in FIGS. 1-5,
the catheterization tray may include: a main compartment 1; a syringe or
catheter compartment
2; a swab compartment 3; a small storage or overflow compartment 4; and a
corner storage
compartment 5. The tray also includes stiffening ribs (e.g., stiffening ribs
20 shown in FIG. 2;
see also stiffening ribs 205 in FIGS. 31A-31C), which help to strengthen the
tray and keep the
tray from bending when held, e.g., when held by one hand.
[0112]
With reference to FIGS. 1, 2, and 5, the main compartment 1 is the largest
compartment in the tray and may contain any number of items for the
catheterization procedure,
for example, a drainage system, a collection bag, a drape, an underpad, an
inflation syringe,
and/or gloves. A drainage system that may be included in the catheterization
package and/or
in the main compartment 1 may include, for example, a drainage/collection bag,
drainage
tubing, a catheter (e.g., a Foley catheter), a drainage outlet, a urine meter,
and/or other drainage
-19-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
system components. The drainage system components may be pre-connected and
stored in the
catheterization package in a pre-connected state, or the drainage system
components may be
stored as separate components to be assembled/connected later.
[0113] The drainage system and tubing included in the catheterization
package may be
configured and arranged in a way that helps improve drainage of urine through
the system and
tubing. For example, the tubing or other components of the drainage system may
be designed
to break up the surface tension of the fluid in order to improve drainage. The
drainage tubing
may be short to accommodate lower bed profiles or may have an adjustable
length for various
size beds. The tubing or other drainage system components may have a coating
(e.g., a
lubricious coating) thereon to facilitate drainage therethrough. Optionally,
the tubing or other
drainage system components may include a superhydrophobic pattern thereon to
facilitate
drainage.
[0114] Additionally, there are a range of suitable durometers of the
tubing or other
drainage system components. However, for components that may be coiled in the
tray (e.g.,
drainage tubing and/or the catheter), it is desirable to have a tubing
durometer that does not
tend to hold a set shape at room temperature. For example, one can experiment
with different
durometers to ensure that the durometer ultimately used for coiled components
does not tend
to leave the component in a coiled shape when removed from the tray and put to
use. It is
desirable, for example, to give the drainage tubing a durometer such that,
despite being
packaged in the main compartment in a coiled state, when connected to a
patient, the drainage
tubing does not remain coiled, but tends to straighten out to facilitate
drainage. The drainage
tubing can be made of polyvinyl chloride (PVC), silicone, latex, Teflon, or
another polymer
material.
[0115] As shown in FIG. 1, sides 8 and 9 of the tray form two orthogonal
inner walls
of the main compartment 1 and form a corner of the main compartment 1 where
the sides 8 and
9 intersect each other. Two additional, interior partitions 10 and 12 separate
the main
compartment 1 from other compartments. The two interior partitions 10 and 12
include
reduced height portions 14 and 16 (with reduced heights compared with the
tray's full-height
partitions, inner walls, or sides).
-20-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0116]
Also, the main compartment 1 includes interior fillets 18 at the corners
formed
between the floor of the main compartment 1 and its inner walls. Such fillets
18 may reinforce
the corners of the main compartment 1.
[0117] As
shown, for example, in FIGS. 1, 2, and 5, the main compartment of the tray
may include instructions/procedural indicators and/or other information
integrated thereon.
For example, the main compartment may include a logo or trademark name written
thereon,
e.g., "SureStepTm Foley Tray System" and "BARD." The main compartment may
also, or
alternatively, include instructions/procedural indicators related to the
catheterization printed or
otherwise included thereon. For
example, the main compartment may include
instructions/procedural indicators for how to catheterize a patient or
instructions/procedural
indicators for steps to take after catheterization. Optionally, the main
compartment may
include instructions/procedural indicators for proper Foley care and
maintenance. As shown,
for example, in FIGS. 1 and 5, the instructions/procedural indicators
integrated on the main
compartment may include instructions/procedural indicators stating, "(1)
Secure Foley with
StatLock ", "(2) position bag below bladder", "secure tubing to sheets with
clip", "(3)
document insertion date", "(4) maintain red seal per hospital policy", "(5)
assess need for
catheter routinely." The instructions/procedural indicators or other
information may be in
upper case letters, in bold, or otherwise called out for greater visibility to
ensure the clinician
reads the instructions/procedural indicators or other information.
Optionally,
instructions/procedural indicators may be printed or otherwise included on the
main
compartment to remind the health care provider/clinician to instruct the
patient regarding how
to properly care for and maintain the catheter after catheterization. The
instructions/procedural
indicators may be written to help the health care provider/clinician remember
to cover
important instructions that should be given to the patient.
[0118] The
syringe or catheter compartment 2 has an elongated shape and spans
approximately an entire length of the tray. Partitions and the inner walls of
the tray contribute
to form the syringe or catheter compartment 2. Partition 12 spans between
opposing inner
walls of the tray and separates the syringe or catheter compartment 2 from
other compartments
¨ namely, from the main compartment 1 and from the swab compartment 3. The
syringe or
catheter compartment 2 is sized and shaped to contain one or more syringes
(e.g., a syringe of
lubricating jelly and a syringe of sterile liquid to inflate the retention
balloon) and/or a catheter,
such as a Foley catheter. For example, a syringe of lubricating jelly and a
syringe of 1 Occ
-21-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
sterile water may be packaged in the syringe or catheter compartment 2, but
the end user (e.g.,
a clinician) may remove the syringes at the time of catheterization and place
the catheter in the
syringe or catheter compartment 2 prior to insertion into a patient (e.g., to
facilitate lubrication
of the catheter).
[0119] A portion of the partition 12 separates the catheter compartment 2
from the main
compartment 1. Some of the partition 12 has a full height (e.g., extends as
high as the sides of
the tray), but the partition 12 also includes reduced height portions 16 and
22 (which have a
reduced height relative to the full height of the partition 12, inner walls,
or sides of the tray).
Reduced height portion 16 of the partition 12 may act as a break or opening in
the partition,
which may place the syringe or catheter compartment 2 in fluid communication
with the main
compartment 1 and vice versa. Along reduced height portion 16, the partition
12 may form a
small step between the syringe or catheter compartment 2 and the main
compartment 1, e.g.,
as shown in FIGS. 2 and 6 (see also FIGS. 31A-31C). The step may also be
formed by the
main compartment 1 having a lower/deeper floor than the floor of the syringe
or catheter
compartment 2, e.g., as shown in FIGS. 2 and 6 (see also FIGS. 31A-31C).
[0120] In FIGS. 1 and 2, another reduced height portion of the partition,
i.e., reduced
height portion 22, separates the syringe or catheter compartment 2 from the
swab compartment
3. Reduced height portion 22 of the partition 12 has a partial or reduced
height relative to the
full height of the inner walls or the sides of the tray. FIG. 5 shows an
alternate arrangement of
reduced height portions 16, wherein both reduced height portions 16 are
located along the
partition between the syringe or catheter compartment and the main compartment
(i.e., there is
no reduced height portion 22 between the syringe or catheter compartment and
the swab
compartment). Other arrangements and configurations are also possible. If
syringes are
packaged or included in syringe or catheter compartment 2, the reduced height
portions (e.g.,
reduced height portions 16 and 22) help facilitate easy removal of the
syringes from the syringe
or catheter compartment 2 by providing more open space for the end user to
reach into the tray
and grasp onto the syringes. This makes removal of the syringes easier than if
the partition 12
did not have any reduced height portions and the clinician had to try to grasp
the syringes within
a narrower region.
[0121] As shown, for example, in FIGS. 1 and 5, the syringe or catheter
compartment
of the tray may also include instructions/procedural indicators and/or other
information
integrated thereon. For example, the syringe or catheter compartment may
optionally include
-22-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
instructions/procedural indicators related to the catheterization written
thereon. As shown in
FIGS. 1 and 5, the instructions/procedural indicators integrated on the
syringe or catheter
compartment may include instructions/procedural indicators stating "Dispense &
Lube Foley
here" (alternatively, this instruction may simply say "Lube Foley," "Dispense
Lube," or
something similar), "retract genitalia (non-dominant hand)", "prep patient
with swabs
(dominant hand)", "insert catheter & inflate balloon (dominant hand)." As
shown in FIGS. 1
and 5 and as stated above, the instructions/procedural indicators may inform
the health care
provider which hand to use for a given step, e.g., dominant or non-dominant
hand. The
instructions/procedural indicators or other information may be in upper case
letters, in bold, or
otherwise called out for greater visibility to ensure the clinician reads the
instructions/procedural indicators or other information.
[0122] Note that the sides of the catheterization tray may also include
instructions/procedural indicators or other information thereon. For example,
as shown in
FIGS. 1, 2, and 5, side 6 may include an instruction/procedural indicator 47
to "open iodine,"
while side 7 may include an instruction/procedural indicator 49 to "attach
water syringe." The
instructions/procedural indicators or other information may be in upper case
letters, in bold, or
otherwise called out for greater visibility to ensure the clinician reads the
instructions/procedural indicators or other information.
[0123] As shown in FIGS. 3 and 4, the base 55 of the syringe or catheter
compartment
2 may be offset from the bases of all other compartments. In other words, the
base 55 of the
syringe or catheter compartment 2 may be not as deep or separated from the top
of the tray as,
for example, the base 57 of the main compartment 1. As the base 55 is offset
from the bases
of the other compartments, the bottom/floor of the syringe or catheter
compartment is offset
from bottoms/floors of the other compartments, including being offset from the
bottom/floor
of the main compartment 1.
[0124] The swab compartment 3 spans along a portion of the tray's inner
wall along
side 6 of the tray; that portion of the inner wall forms a side of the swab
compartment 3. The
swab compartment 3 may contain various items, for example, swabs or swabsticks
and one or
more antiseptics such as iodine (e.g., an iodophor such as povidone-iodine,
tincture of iodine,
aqueous iodine, etc.). Portions of partition 10 and partition 12 extend along
two other sides of
the swab compartment 3 forming two other sides of the swab compartment 3, and
separate the
swab compartment 3 from the main compartment 1 and the syringe or catheter
compartment 2,
-23-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
respectively. Also, the small storage or overflow compartment 4 extends along
a fourth side
of the swab compartment 3. In one embodiment as shown, for example, in FIGS. 1
and 2, the
partition 10 has a full height where it separates the swab compartment 3 from
the main
compartment 1, while the partition 12 has a reduced height (i.e., at reduced
height portion 22)
that separates the swab compartment 3 from the syringe or catheter compartment
2. In one
embodiment as shown, for example, in FIG. 5, the partition 10 and the
partition 12 both have
a full height whether they separate the swab compartment 3 from the main
compartment 1 and
the syringe or catheter compartment 2.
[0125] The swab compartment 3 has an angled bottom/floor that spans
between the
small storage or overflow compartment 4 and the syringe or catheter
compartment 2 and has a
downward slope therebetween (i.e., the bottom/floor of the swab compartment 3
near the
catheter compartment 2 is lower than near the storage compartment 4). As such,
fluid poured
into the swab compartment may flow in a direction toward the catheter
compartment 2 and
pool in the lowest/deepest portion or well 24 of the swab compartment near the
partition 12
(e.g., adjacent reduced height portion 22 in FIGS. 1 and 2) where it separates
the swab
compartment 3 from the catheter compartment 2. Fillets may be included in the
corners of the
swab compartment 3 similar to fillets 18 in the main compartment 1, e.g., as
shown in FIGS. 1
and 2.
[0126] FIG. 8 shows a larger view of the swab compartment 3 and the
adjacent small
storage or overflow compartment 4 with swabs or swabsticks contained in the
swab
compartment 3. Near the small storage or overflow compartment 4, the
bottom/floor of the
swab compartment 3 may be supported by another partition 30 (shown on FIG. 2)
that separates
the small storage or overflow compartment 4 from the swab compartment 3. Also,
the partition
30 may extend upward (from the bottom/floor of the small storage or overflow
compartment
4) only to the bottom/floor of the swab compartment 3, leaving the
bottom/floor of the swab
compartment 3 unobstructed near the small storage or overflow compartment 4
(as such, swabs
or swabsticks with a longer stick/stem may extend over a portion of the small
storage or
overflow compartment 4 from the bottom/floor of swab compartment 3 as shown,
for example,
in FIG. 8). This design or arrangement also allows small storage or overflow
compartment 4
to act as an overflow well in case too much fluid/iodine is poured into swab
compartment 3.
[0127] The swab compartment 3 includes one or more channels 28 extending
at least
partially along the region between the small storage or overflow compartment 4
and well 24
-24-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
located at a bottom/floor of the swab compartment 3. On a side of the swab
compartment 3
near the catheter compartment 2, the channels 28 extend to and are in fluid
communication
with the lowest/deepest portion of the well 24 adjacent the partition 12, such
that liquid poured
into the channel(s) 28 can flow downward through the channel(s) 28 along the
downward slope
and pool in the lowest/deepest portion of the well 24. The channel(s) 28 may
be designed to
hold an elongated device (e.g., swabs or swabsticks) and each channel 28 may
be separated by
one or more barriers 26 to help space the elongate devices (e.g., swabs or
swabsticks) apart.
Channel(s) 28 may also include undercutting or snap-in features 32 (e.g., snap-
in tabs) that may
help secure elongated devices, such as swabs, swabbing sticks, or swabsticks
in the channel(s)
28.
[0128] As shown, for example, in FIGS. 1, 5, and 8, the swab compartment
of the tray
may also include instructions/procedural indicators and/or other information
integrated
thereon. For example, the swab compartment (e.g., on one of barriers 26) may
optionally
include instructions/procedural indicators related to the catheterization
printed or otherwise
included thereon. As shown in FIGS. 1, 5, and 8, the instructions/procedural
indicators
integrated on the swab compartment may include an instruction/procedural
indicator stating
"pour iodine here." The instructions/procedural indicators or other
information may be in upper
case letters, in bold, or otherwise called out for greater visibility to
ensure the clinician reads
the instructions/procedural indicators or other information.
[0129] One or more swabs or swabsticks (e.g., swab or swabstick 34 shown
in FIGS.
6A-8) may be packaged in the swab compartment 3 and held in channels 28 (e.g.,
as shown in
FIG. 8). The swabs or swabsticks may be specially designed to fit in swab
compartment 3 and
to be held in channels 28. Swab compartment 3 may also include iodine or
povidone-iodine
solution. The iodine or povidone-iodine solution may be packaged in its own
container, packet,
or syringe in the swab compartment 3, e.g., povidone-iodine packet 52 shown in
FIG. 26.
Alternatively, the iodine or povidone-iodine solution may be stored and/or
sealed in swab
compartment 3 in direct contact with the swab(s) or swabstick(s) 34 (e.g.,
packaged/sealed such
that it is in contact with a portion of the bottom/floor of swab compartment
3).
[0130] FIGS. 6A-6C show one embodiment of swab or swabstick 34 with an
elongate
member or stick/stem 38 having more rounded sides (e.g., in cross-section)
than the swab or
swabstick 34 shown in FIGS. 7A-7C. FIGS. 7A-7C show one embodiment of swab or
swabstick 34 with an elongate member or stick/stem 38 having less rounded or
more angular
-25-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
sides than the swab or swabstick 34 shown in FIGS. 6A-6C. Swab or swabstick
34, as shown
in FIGS. 6A-7C, is representative of other possible swabs or swabsticks that
may be included.
Swab or swabstick 34 includes an absorbent head 36 and an elongate member or
stick/stem 38.
Absorbent head 36 is designed to allow for greater saturation in cleansing
solution (e.g., in
iodine or povidone-iodine solution) and for greater contact with the surface
area of the region
to be cleansed. Absorbent head 36 may be formed from various materials,
including foam,
rayon (e.g., puffs of rayon or a spiral of rayon), cotton, other materials,
and/or a combination
of one or more of these materials. To improve saturation of the absorbent
head, the absorbent
head 36 is desirably made of a material that readily absorbs and distributes
the cleansing
solution. Any material that absorbs a significant percentage of its weight in
cleansing solution
is desirable for use in the absorbent head, especially if it also releases a
large percentage of
absorbed cleansing solution, i.e., it is desirable to use a material that
absorbs a large amount of
cleaning solution and also releases a large amount of cleaning solution when
used on the patient
for cleaning. It has been found that forming the absorbent head 36 of foam (as
opposed to
rayon or other materials) provides the absorbent head 36 with improved
absorption of and
saturation with cleansing solution. Further, a foam absorbent head readily
releases a large
amount of the cleansing solution when put in contact with the region of the
patient to be
cleansed and when compressed slightly against the region being cleansed. This
ready release
of cleansing solution makes cleansing the region of the patient easier and
allows the end user
to be more gentle when cleansing the patient. If absorbent head 36 is made
entirely or partially
of foam, the foam may be open-cell foam, closed-cell foam, polyurethane foam,
high density
foam, latex foam, honey gold foam, or other types of foam. The absorbent head
may be heated
such that it bonds to the elongate member or stick (e.g., the foam may be
heated to bond to the
stick), or other means of attachment may be used, e.g., using an adhesive.
[0131] Preferably the foam or other material(s) selected for the
absorbent head do not
degrade or at least resist degrading when in contact with the cleansing
solution. In one
embodiment, the swabs or swabsticks may be stored in contact with iodine or a
povidone-
iodine solution, so they are already saturated when they are first accessed in
the catheterization
package. For example, a seal may be placed in or over swab compartment 3 that
holds a
cleansing solution in contact with the absorbent head(s) of the swab(s) or
swabstick(s) while
preventing leaks until the seal is removed. When packaged and stored in
contact with the
cleansing solution, it is vital that the absorbent head not degrade over time.
Honey gold foam,
-26-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
for example, is sufficiently resistant to degradation when in contact with an
iodine or povidone-
iodine solution.
[0132] Absorbent head 36 may be of a variety of sizes and shapes.
Preferably, the foam
is shaped in such a way as to provide for greater contact with the surface
area of the region to
be cleansed. For example, the absorbent head 36 may be shaped such that the
distal end or
distal region (e.g., regions that come into contact with the patient), are
substantially larger than
regions that are less likely to come into contact with the patient (e.g., the
proximal end or where
the absorbent head 36 attaches to the elongate member or stick 38). This
provides more surface
area in the patient-contacting end of the absorbent head and makes cleansing
the patient quicker
and easier. Further, with the distal region of the absorbent head being
larger, the majority of
the iodine is absorbed into the area that contacts the patient (in contrast,
when the largest region
of the absorbent head is not at or near the distal end, much of the iodine is
wastefully absorbed
into a location that does not contact or cleanse the patient). The absorbent
head 36 may be
shaped as shown in FIGS. 6A-7B and may be wider than it is thick. The
absorbent head 36
could also be shaped to be generally or approximately circular, rectangular,
or trapezoidal.
Optionally, the shape of absorbent head 36 may be generally or approximately
conical,
pyramidal, tear drop, ovoid, or triangular in shape (with the larger base end
positioned at the
distal tip of the swab or swabstick), or may be another shape. If generally
pyramidal in shape,
the base of the pyramidal shape may be generally triangular, square,
pentagonal, hexagonal,
etc. Even if otherwise generally conical, pyramidal, or another shape, the
edges of the
absorbent head at the distal end may be curved or tapered (e.g., as the distal
edges are curved
in FIGS.6A-7B). Further, the distal end of the elongate member or stick 38 may
also be curved
(e.g., the distal end may be semi-circular rather than flat across the top or
distal-most edge), as
curves are less likely to harm patient when cleansing with a swab or
swabstick.
[0133] In one embodiment, the length of the absorbent head may be between
0.5 and 2
inches (in one embodiment, the length may be approximately 1 inch), the width
of the absorbent
head at its widest may be between 0.5 and 2 inches (in one embodiment, the
width at its widest
may be approximately 1 inch) and the width of the absorbent head at its
narrowest may be
between 0.1 and 1 inches (in one embodiment, the width at its narrowest may be
approximately
1/2 of an inch). Further, if the absorbent head is not generally conical or
pyramidal in shape
(i.e., such that its width and thickness are the same all around), it may have
a thickness less
-27-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
than its width, e.g., a thickness between 0.1 and 1 inches (in one embodiment,
the thickness
may be approximately 3/8 of an inch).
[0134] In one embodiment, the elongate member or stick 38 may have a
length between
2 and 7 inches (e.g., the elongate member or stick 38 may have a length
between 3.5 and 4.5
inches, or a length of approximately 4.25 inches). The length of penetration
of the stick 38 into
the absorbent head 36 (i.e., the length the stick 38 that extends distally
beyond the proximal
end of the absorbent head) may be between 0.25 inches and 1 inch (e.g., the
length of
penetration may be approximately 0.5 inches). It is preferable to ensure that
the length of
penetration is within a good range relative to the overall length of the
absorbent head. If the
stick 38 extends too far into the absorbent head 36, the patient's skin may be
too easily damaged
by the stick 38 (e.g., it may be more likely to scrape against the patient
during cleansing, and
may not be padded by enough foam). Whereas, if the stick 38 does not extend
far enough into
the absorbent head 36, the absorbent head 36 will lack structure and flop
around (especially if
saturated with liquid), which makes it harder for the end user to guide the
absorbent head of
the swab or swabstick and effectively cleanse the patient. Ideally, the length
of penetration of
the stick 38 will extend between 35 and 70% of the length of the absorbent
head 36 (e.g., the
length of penetration may be about 50% of the length of the absorbent head).
The elongate
member or stick 38 may be formed from various materials, including a plastic
or polymer
material.
[0135] The elongate member or stick 38 may have a variety of different
cross-sectional
sizes and shapes. For example, the swab or swab stick may be biased to be
relatively flat or
have a generally rectangular cross-section (or a rectangular-like cross-
section but with rounded
edges as shown, for example in FIG. 6C, or with cut off edges as shown, for
example, in FIG.
7C), so it is easier to hold. The elongate member or stick 38 shown in FIGS.
6A-6C has
rounded edges, but has a roughly rectangular shape otherwise. This is a shape
that is easier to
hold and manipulate while cleaning a patient than a stick with a circular
cross-section. Further,
this and similar cross-sectional shapes for the elongate member or stick 38
make it possible for
the swabs or swabsticks to snap into the tray or be held under features 32 to
prevent movement
of the swabs or swabsticks during shipping or before use. To remove a swab or
swabstick of
this cross-section, one can simply twist the elongate member or stick 38 in a
clockwise or
counter clockwise direction and the features 32 easily release the swab or
swabstick for use.
For example, FIG. 8 shows two swabs or swabsticks that are snapped or held in
place under
-28-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
the features 32, and also shows one swab or swabstick that has been twisted
such that the
features 32 no longer hold the swabstick or prevent its removal. This makes
the swabs or
swabsticks very secure in the swab compartment 3 during shipping and before
use, but also
makes removal of the swabs or swabsticks at the time of catheterization very
simple and easy.
[0136] In one embodiment, if the elongate member or stick is of a
generally flattened
(e.g., generally rectangular) shape, the width or diameter of the elongate
member or stick 38
may be between approximately 1/8 of an inch and 3/4 of an inch (e.g., the
width may be about
1/4 of an inch), whereas the thickness may be between 1/16 of an inch and 1/2
of an inch (e.g.,
the thickness may be approximately 1/8 of an inch). If the elongate member or
stick is of a
generally circular cross-section or is generally cylindrical in shape, the
diameter of the elongate
member or stick 38 may be between 1/8 of an inch and 1/2 of an inch (e.g., the
diameter may
be about 1/4 of an inch).
[0137] The swabs or swabsticks are loaded into the tray and held in such
a way that the
absorbent head 36 is in the lowest/deepest portion or well 24 of the bottom of
the swab
compartment 3 such that they are exposed to the iodine that pools in the
lowest/deepest portion
or well 24 of the bottom of the swab compartment 3 and easily saturate with
the iodine or
povidone-iodine solution. However, the elongate member or stick 38 may be
loaded into the
tray and held in such a way that the elongate member or stick 38 is not itself
exposed to the
iodine or povidone-iodine solution.
[0138] The small storage or overflow compartment 4 spans along a portion
of the tray's
side 6, which forms an inner wall of the tray and a side of the small storage
or overflow
compartment 4. Three other sides of the small storage or overflow compartment
4 are formed
by partial-height partitions that separate the small storage or overflow
compartment 4 from the
main compartment 1, swab compartment 3, and corner compartment 5. These
compartments
may surround the small storage or overflow compartment 4 on three sides
thereof The small
storage or overflow compartment 4 may act as an overflow well to collect any
fluid that might
overflow from the swab compartment. Optionally, the small storage or overflow
compartment
4 may store or hold one or more items useful for catheterization.
[0139] As shown, for example, in FIGS. 1 and 5, the small storage or
overflow
compartment of the tray may also include instructions/procedural indicators
and/or other
information integrated thereon. For example, the small storage or overflow
compartment may
-29-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
optionally include instructions/procedural indicators related to the
catheterization printed or
otherwise included thereon. As shown in FIGS. 1 and 5, the information
integrated on the
small storage or overflow compartment may include a reminder that the health
care
provider/clinician should "refer to directions for use for complete
instructions." The
instructions/procedural indicators or other information may be in upper case
letters, in bold, or
otherwise called out for greater visibility to ensure the clinician reads the
instructions/procedural indicators or other information.
[0140] The corner storage compartment 5 spans along portions of the
tray's side 6 and
side 9, which form sides and a corner of the corner storage compartment 5. Two
other sides of
the corner storage compartment 5 may be formed by partial-height partitions
that separate the
corner storage compartment 5 from the main compartment 1 (e.g., reduced height
portion 14)
and small storage or overflow compartment 4. These compartments surround the
corner
storage compartment 5 on two sides thereof
[0141] The bottom/floor of the corner storage compartment 5 may be non-
planar;
namely, the bottom/floor may have a non-planar, rounded, or partially
cylindrical shape. This
shape may be particularly useful for holding a specimen or sample container.
Corner storage
compartment may store or hold various implements useful for catheterization.
For example,
corner storage compartment may hold a label that can be filled out by a
clinician on a first side
and adhered to a specimen or sample container on a second side. The second
side may already
include an adhesive thereon; the adhesive may be covered by a removable cover
sheet that may
be removed at the time of adhering. The corner storage compartment may also
include a
specimen or sample container 54.
[0142] In one embodiment, at least some of the compartments may be sealed
in a
manner that prevents contamination thereof. In one embodiment, at least one of
the
compartments may be unsealed independently of at least one other compartment.
For example,
a user may first unseal the main compartment, leaving other compartments
sealed. After using
items contained in the main compartment, the user may unseal another
compartment and use
items contained in that compartment.
[0143] In one embodiment, a user may unseal the swab compartment 3 prior
to
unsealing the syringe or catheter compartment 2 or the main compartment 1. The
user may
pour iodine or another antiseptic solution into the swab compartment and may
use the swab
-30-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
compartment for applying the antiseptic solution onto swabs, and then use the
iodine soaked
swabs to cleanse the patient. A user may then unseal the syringe or catheter
compartment 2
and dispense lubricant therein prior to unsealing the main compartment 1. A
user may then
unseal the main compartment 1 to access the catheter. The shape and size of
the catheter
compartment facilitate lubrication of the catheter (e.g., a tip of the
catheter can rotate within
the elongated shape of the catheter compartment and collect lubricant on its
surface during the
rotation). The catheter may be lubricated in the syringe or catheter
compartment 2 and then
inserted into the patient's urethra.
[0144] According to various embodiments, and as shown in FIGS. 1, 2 and
5, and as
discussed above, the tray may have ordered, stepwise instructions/procedural
indicators
thereon for a suitable or preferred catheter insertion technique.
[0145] Other aspects or features of the catheterization package(s) and
catheterization
tray(s) are also described below in the context of non-limiting procedures or
methods of using
the catheterization package and catheterization tray, and in the context of
methods of
manufacturing or packaging a catheterization package. The aspects or features
described below
may be incorporated into the various embodiments already discussed above. The
arrangement
of components and how they are accessed is part of the overall intuitive
design of the
catheterization package, i.e., components and features are arranged in a
logical manner that
flows from step to step to make the catheterization procedure more intuitive
and make
completing the procedure easier and/or quicker. Step-by-step ordering of the
components in
the catheterization package helps a user/clinician to logically know what step
comes next. For
example, a first item may be revealed in the package, then once the first item
is used, a second
item that was underneath the first item is revealed, the second item being
next logical item for
use in the catheterization procedure. Once the second item is used, a third
item that was
underneath the second item may be revealed, the third item being the next
logical item for use
in the catheterization procedure, and so on.
[0146] The catheterization package(s) and catheterization tray(s)
described herein may
be used in many different ways. A non-limiting method of use is described
below. However,
clinicians may vary the steps or procedures described herein, may reorder the
steps, may
perform additional steps beyond those described, and/or may omit certain steps
as
circumstances and a patient's unique needs may require. Further, the
description below can be
-31-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
considered a description of an overall procedure with many steps or can be
considered a series
of individual methods or procedures each including only a subset of the steps
described.
[0147] First, a catheterization package as shown, for example, in FIGS. 9
and 10 may
be provided (e.g., a health care provider/clinician may obtain or select the
catheterization
package from shelf or through a purchase, or may place the catheterization
package in the
hospital room where the patient is waiting for catheterization.) As can be
seen in FIGS. 9 and
10, the catheterization package may be sealed, e.g., by an outer container 60
(e.g., a sealed bag)
surrounding the other contents of the catheterization package, such that the
contents remain
sterile and properly contained. Just inside the sealed bag (or other external
container), a paper
or cardboard packaging label 62 may be included in the catheterization
package. The external
container 60 (or sealed bag) may be transparent, or at least partially
transparent, such that a
clinician may see the label 62 or portions of the label. The label 62 may
include sides 64 that
are foldable such that they extend down the sides of the catheterization
package. The label
may include one, two, three, or four sides. For example, FIGS. 11 and 12 each
show a label
62 that a large top portion 66, and 3 smaller sides or side portions 64
foldable to a different
plane from the plane in which the large top portion 66 resides. Lines on FIG.
12 show where
the folds may be made to form the sides or side portions. In one embodiment,
the label has
four sides or side portions 64 instead of three.
[0148] The packaging label 62 may include instructions and or other
information
thereon. For example, the label 62 may include initial instructions for
catheterization. The
label 62 may include a logo, trademark, or product name printed or otherwise
included thereon,
e.g., "SureStepTm Foley Tray System", "Lubri-sil I.C. Complete Care , and/or
"BARD." The
label 62 may also, or alternatively, include instructions related to the
catheterization written
thereon. For example, the label may include instruction(s) 40 to verify
whether the patient
meets the CDC guidelines for indwelling urethral catheter use, as shown in the
bottom left
quadrant of the top portion 66 of the labels 62 in FIGS. 9-12. The label may
include a checklist
41 to verify the different factors that qualify a patient for indwelling
urethral catheter use under
the CDC guidelines, e.g., as also shown in the bottom left quadrant of the top
portion 66 of the
labels 62 shown in FIGS. 9-12. The label 62 may also include a list of
components 42 of the
catheterization package as shown on the right side the top portion 66 of the
labels 62 in FIGS.
9-12. The instructions or other information may be in upper case letters, in
bold, or otherwise
-32-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
called out for greater visibility to ensure the clinician reads the
instructions or other
information.
[0149] The packaging label 62 may also include key information or
variables called
out in a simplified and easy-to-read manner. For example, visual identifiers,
squares, other
shapes, and/or other features may be included that each have an indicator of
one key piece of
information or variable called out and easy to read. In FIGS. 9-12, the labels
62 are shown as
including information squares 44 that each includes an indicator of one key
piece of
information about the catheterization package. Information squares 44
essentially isolate and
feature particular information about the catheterization tray from a range of
possibilities or
variables. Various features or variables may be called out, including: (1)
whether the tray
includes a drainage bag or a urine meter, (2) the French size of the catheter,
(3) the type of
catheter included, (4) the substrate type of materials used in the catheter or
other components,
e.g., latex or latex-free, (5) special components or features, e.g., inclusion
of a StatLock
securement device, inclusion of special infection control coating, or other
features. This calling
out of particular variables, separated from everything else, allows the health
care provider to
see what is in the catheterization package or tray at a glance. Accordingly,
the health care
provider can quickly determine what the key distinguishing elements of the
catheterization
package or tray are. For example, if the catheterization package shown in FIG.
9 and the
catheterization package in FIG. 10 were both stored on the same shelf, a
health care provider
could quickly tell the major differences between the catheterization packages
just by looking
at the information squares 44. One can readily tell from the information
squares 44 in FIG. 9
that the catheterization package in FIG. 9 includes an 18 French sized
catheter, a drainage bag,
Bard's StatLock , and is a non-latex catheter. One can readily tell from the
information squares
44 in FIG. 10 that the catheterization package in FIG. 10 includes a 16 French
sized catheter,
a drainage bag, and Bard's StatLock .
[0150] Further, the packaging label 62 may be designed to be visible in
multiple planes.
The label may be designed to fold at the edges of the catheterization package
such that the label
(or a portion of the label) forms sides or side portions that are visible when
the catheterization
package is viewed from the sides, in addition to being visible from the top.
In FIGS. 9-12, the
labels 62 are designed such that information squares 44 are visible from the
top of the
catheterization package, and at least two sides of the tray. Optionally, the
label may also be
designed such that information squares 44 appear on all the sides of the
catheterization package
-33-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
(e.g., on three sides or four sides). Accordingly, anyway you stock the
catheterization package,
you can see the label. Further, multiple catheterization packages may be
stacked on top of each
other, but because of the information squares on one or more sides of the
catheterization
package, a clinician can still quickly tell from the sides of the packages in
the stack which
catheterization package from the stack is the one desired for a particular
patient.
[0151] The catheterization package may be selected based on the
information squares
44, e.g., after reviewing the information squares 44. After
providing/obtaining/selecting the
catheterization package, the user may open the container (e.g., the sealed
bag) and remove it
from around the remainder of the catheterization package. After the container
(or sealed bag)
has been removed the remainder of the catheterization package looks, for
example, as shown
in FIG. 11.
[0152] The user may then remove the label 62 and set it aside to reveal
other
components that were underneath the label 62. For example, when the label is
removed from
the catheterization packages shown in FIGS. 9-11, detailed instructions for
catheterization or
the directions for use document 68 of the catheterization package or tray may
be revealed as
shown, for example, in FIG. 13. Detailed instructions and/or information on
safety
considerations are thereby one of the first items accessed in the
catheterization package.
Providing detailed instructions up front allows the user/clinician/health care
provider to review
the entire procedure in advance of any other steps and before breaking the
sterile field formed
by the CSR wrap around the catheterization tray. This helps the procedure to
run more
efficiently and safely. Pages of detailed instructions may be seen on various
pages of document
68 in FIGS. 14A-14D. Any one or more of the steps contained in FIGS. 14A-14D
may be
performed as a catheterization method or procedure.
[0153] Additionally, there may also be associated patient education
information
whether attached to the detailed instructions or separately placed in the
catheterization package.
The patient education information may be used by a health care provider to
instruct the patient
on care of the indwelling catheter and may, ultimately, be given to the
patient for reference.
FIGS. 15A and 15B show an example of patient education information that may be
included
on a patient sheet/pamphlet 70 in the catheterization tray. FIG. 15A shows a
first side of the
patient education information sheet 70 in English, whereas FIG. 15B shows a
second side of
the patient education information sheet 70 in Spanish.
-34-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0154] Underneath the detailed instructions or directions for use
document, the
catheterization package may include a checklist of safety
considerations/steps, a patient
information chart, or an insert sheet including safety consideration/steps and
a patient
information chart combined on the same insert sheet. FIG. 16 shows an insert
sheet 72 on
which patient information can be recorded or charted and including safety
information. This
insert sheet 72 may be accessed after the detailed instructions are removed
from the package.
It may be beneficial to have an insert sheet 72 in this location to do one
last safety check prior
to treating the patient and to have important patient information at hand. The
insert sheet 72
may be configured as a sticker label, e.g., the insert sheet 72 may include a
backing that protects
a sticker adhesive, and when the backing is removed, the insert sheet 72 may
be stuck to a file
or chart associated with the patient.
[0155] After removing the detailed instructions 68 and the insert sheet
72 from the
catheterization package, a perineal care (or pen-care) packet or kit 74 is
revealed, as shown in
FIGS. 17 and 18, and may be accessed. A pen-care kit is helpful to provide an
initial cleaning
of the area where the catheterization takes place prior to using an iodine
solution to further
cleanse and sanitize the area. This is especially true for patients who are
very dirty and must
have an initial cleaning before the iodine solution will be most effective.
The pen-care kit may
include any items helpful for an initial cleaning of the patient's perineum or
for perineum care
in general. For example, as shown in FIGS. 18 and 19, the pen-care kit may
include hand
sanitizer 76 (e.g., antiseptic gel hand rinse) for the health care provider to
sanitize his/her hands,
moist towelettes 78 (e.g., a package of castile soap towelettes),
instructions/procedural
indicators 80 (e.g., instructions/procedural indicators for health care
provider and/or
instructions for patient). The hand sanitizer may be designed to have improved
efficacy and/or
to enable single-handed usage.
[0156] As shown in FIGS. 17-19, the items of the peri-care kit may be
included in a
bag 82 (e.g., a zip lock baggy) or other package to keep the items/components
together. In one
embodiment, the bag type used is a baggy including a zipper 84. It has been
found such a bag
type is easier to open with gloves on rather than, for example, a bag without
a zipper or a bag
that must be opened by tearing. However, a bag with perforations on the bag
instead of a zipper
or zip-lock feature is also contemplated, as such may be cheaper to
manufacture.
[0157] Pen-care instructions/procedural indicators 80 are shown in FIGS.
17-19. The
instructions 80 may inform a health care provider to: "1. Wash hands and don
gloves," "2.
-35-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
Explain procedure to patient and open Pen-Care kit," "3. Use the provided
packet of towelettes
to cleanse the patient's pen-urethral area," and "Remove gloves and perform
hand hygiene with
provided alcohol hand sanitizer gel." Other instructions/procedural indicators
are also possible.
The method may include performing some or all of the four steps recited above
as steps on the
instructions.
[0158] The method may proceed in three main stages, i.e., (A) the initial
pen-care
stage, (B) the catheterization stage, and (C) the catheter care and
maintenance stage. Each of
these stages is denoted on the instructions/procedural indicators and/or other
materials with a
large "A" "B" or "C" to signify the different main stages. As can be seen on
the pen-care
instructions 80, there is a large "A" at the top of the
instructions/procedural indicators to
indicate the first stage. As discussed elsewhere herein, the belly band 46
includes a large "B"
to indicate the second stage, and the main compartment 1 of the
catheterization tray includes a
large "C" to indicate the third stage.
[0159] After removing and using the peri-care kit, a belly band/indicator
wrapper 46
may be revealed as shown in FIG. 20 (see also indicator wrapper 140 in FIG.
30B). The belly
band 46 may circumvent the tray in at least one direction. The belly band 46
may help to keep
the CSR wrap intact and maintain the sterile barrier. The belly band 46 may
also help to keep
all the contents inside the tray, so the items do not move around.
[0160] The belly band/indicator wrapper 46 may include
instructions/procedural
indicators and/or other information thereon. For example, as shown in FIG. 20
(see also FIG.
30B), the belly band may include an instruction/procedural indicator telling
the health care
provider how to orient the tray relative to the patient. For example, the
belly band may say,
"orient toward insertion site" and have an arrow pointing to the end of the
catheterization tray
that should be positioned closest to the patient's perineum and urinary tract.
This ensures that
the tray is properly positioned for most logical and intuitive use when the
CSR wrap is opened.
[0161] Further, as shown in FIG. 20 (see also indicator wrapper 140 in
FIG. 30B), the
belly band 46 may include instructions/procedural indicators to "utilize
proper aseptic
technique," "(1) Open CSR Wrap," "(2) Don Sterile Gloves," "(3) Place
Underpad," "(4) Place
Fenestrated Drape," and (5) "Follow Tray Instructions. These
instructions/procedural
indicators include a large "B" at the top to signify that these
instructions/procedural indicators
are part of the second stage or catheterization stage. The
instructions/procedural indicators
-36-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
inform the health care provider to open the CSR wrap and perform other steps
once the CSR
wrap is opened. Providing this information before breaking the sterile barrier
gives the health
care provider a preview of the procedure, and ensures he/she is prepared when
the sterile barrier
is broken by opening the CSR wrap. Optionally, a label or sticker may be used
instead of a
belly-band, which may provide the same information as the belly band described
herein.
[0162] Once the tray is oriented and the health care provider has read
the belly band
instructions/procedural indicators, the next step is to open and/or remove the
belly band. After
removing the belly band, the sterile wrap 56 (e.g., CSR wrap) may be opened.
FIG. 21 shows
the catheterization tray with three corners of the sterile wrap 56 unfolded to
represent unfolding
the sterile wrap 56. Note that until the belly band and sterile wrap are
opened, the sterile barrier
of the catheterization tray and its components is not broken. This maintains
the sterile barrier
while the initial peri-care stage is completed and while the tray is properly
positioned and
oriented for catheterization. By leaving the sterile barrier intact during the
initial steps of the
method, there are fewer opportunities for contamination and the resulting
patient infection.
[0163] If the catheterization tray is oriented as directed by the belly
band 46, when the
sterile wrap 56 (e.g., CSR wrap) is opened, the tray is properly oriented for
logical and
convenient use of the catheterization tray and its components. The tray is
arranged and ordered
for logical step-by-step use as described herein. The initial view of the tray
after opening the
sterile wrap 56 entirely is shown in FIG. 22. (A breakaway line is used to
indicate that the
actual edges of the sterile wrap 56 extend beyond the breakaway edges shown in
FIGS. 22-29.)
Note that the sterile wrap 56 may remain underneath the catheterization tray
to form a sterile
field for catheterization. The tray shown in FIG. 22 and the other figures are
right-hand biased
to make use easier for a right-handed health care provider when standing near
the lower edge
of the catheterization tray with the syringe or catheter compartment
positioned furthest from
the health care provider (i.e., if the band points toward the genitalia
between the legs, the right-
handed user stands on the side closest to the patient's right leg).
Alternatively, a catheterization
tray may be left-hand biased (e.g., essentially a mirror image of the tray
shown in FIGS. 22-29
with the mirror placed along the tray's side 7 or along the syringe or
catheter compartment).
[0164] As instructed on the belly band 46, after first opening the CSR
wrap, the health
care provider next dons the sterile gloves provided in the tray. As shown in
FIG. 22, the top
item in the tray after the CSR wrap is opened is a package 58 of sterile
gloves (note that the
cuff end of the gloves may be indicated on the package 58). The sterile gloves
may be made
-37-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
of any material known to be suitable, e.g., latex, rubber, or latex-free
materials). This
placement of the gloves on top of the tray under the sterile wrap helps the
health care provider
to intuitively know he/she should don the gloves before proceeding further
(because it is
intuitive, it may be unnecessary to refer back to the instructions/procedural
indicators on the
belly band 46). The package of gloves 58 may be opened by folding the cuff
down to be closest
to the health care provider and then opening the package like a book, as shown
in FIG. 23. If
opened in this intuitive, logical way, the left hand glove is positioned on
the health care
provider's left side and the right hand glove is positioned on the health care
provider's right side
for easy donning of the gloves. A symbol or other indicator may be included on
the package
58 to identify the left and right hand gloves as shown, for example, in FIG.
23.
[0165] As instructed on the belly band 46, after donning the gloves, the
health care
provider places an underpad under the patient's buttocks. As shown in FIG. 24,
once the
package of gloves is removed from the tray, the underpad and fenestrated drape
are revealed.
The folded pad/drape 86 shown in FIG. 24 is representative of: (1) a
waterproof underpad, and
(2) a separate fenestrated drape. The underpad may have a waterproof side and
a liquid
absorbent side. If so, the absorbent side is placed up under the patient, and
the waterproof side
(or plastic side) is placed down. As instructed on the belly band 46, after
placing the underpad,
the health care provider places the fenestrated drape on the patient such that
the genitalia are
visible through the central opening in the fenestrated drape. Although the
underpad and
fenestrated drape are not shown in FIG. 24 as distinct pieces, they are both
included in the area
of the pad/drape 86, and are arranged such that the underpad is on top of the
fenestrated drape
(i.e., because the underpad is placed first, which reveals the fenestrated
drape for placement
thereafter). The fenestrated drape is logically located and folded such that
it is intuitive to open
the drape away from clinician, which is preferable for how the drape is best
used.
[0166] As instructed on the belly band 46, after placing the fenestrated
drape, the health
care provider follows the instructions/procedural indicators and/or takes the
steps written on
the catheterization tray itself. As shown in FIG. 25, removal of the underpad
and fenestrated
drape reveals a package of antiseptic solution 52 (e.g., povidone-iodine
solution). (Note that
FIG. 25 shows the fenestrated drape moved from over the swab compartment 3
into the main
compartment 1. Moving the drape in this way or placing the fenestrated drape
on the patient
will reveal the antiseptic solution in the swab compartment.) Further, the
first
instruction/procedural indicator 47 on the tray (i.e., "(1) Open Iodine";
although, variations on
-38-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
this instruction or other instructions are possible) is visible on the left
side of the tray (which is
also the side closest to the patient and the region to be catheterized, if
oriented as instructed by
the belly band 46). Placement of the first instruction 47 on the left is
logical because people
read English from left to right, and beginning instructions on the left is
intuitive (although in
countries that do not read left to right, this might be adjusted). Also the
instruction/procedural
indicator is located close to the antiseptic or iodine solution 52 in the
tray, which makes
following the instruction/procedural indicator easier and more intuitive.
[0167] Assuming the clinician is right-handed, and has oriented the tray
as instructed
by the belly band 46, the antiseptic or iodine solution 52 is automatically
positioned closest to
patient. (However, as discussed above, another arrangement is possible where
the tray is left-
handed biased (e.g., essentially a mirror image of the tray in the figures)
such that a left-handed
user positioning the tray as instructed by the belly band would also
automatically position the
antiseptic or iodine solution closest to the patient (e.g., with the left-
handed clinician standing
on an opposite side of the tray from where a right-handed clinician would
stand or on the side
closest to the patient's left leg).) Positioning the antiseptic or iodine
solution (and the swab
compartment) closest to the patient is preferable for the use of the tray.
Having the swab
compartment 3 and well of iodine solution closest to the patient is
beneficial, because it
prevents dragging or moving swabs saturated with the antiseptic or iodine
solution across the
tray (which might drip on other components of the tray, if moved over them).
[0168] FIG. 26 shows the catheterization tray after removal of the
povidone-iodine
solution. Note that removal of the packet of antiseptic or iodine solution 52
reveals the swabs
or swabsticks 34 and the second instruction/procedural indicator 48 on the
tray. This intuitively
leads the health care provider to logically follow the second instruction 48
after picking up the
packet of antiseptic or iodine solution. The second instruction 48 on the tray
is in the swab
compartment 3 and says "(2) Pour Iodine Here -->" and points to the
lowest/deepest point or
well of the swab compartment 3 (although, variations of this instruction or
other instructions
are possible). If antiseptic or iodine solution is poured on the barriers or
channels of the swab
compartment 3, the incline ensures the solution will flow to the
lowest/deepest point or well.
[0169] Once the health care provider has poured the antiseptic or iodine
solution into
the well of the swab compartment 3 such that the absorbent heads of the swabs
begin absorbing
the antiseptic or iodine solution, the health care provider may then attach
the syringe of sterile
liquid 88 to the inflation port of the catheter 92 (e.g., a Foley catheter) as
is instructed by the
-39-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
third instruction/procedural indicator 49. The third instruction 49 is
logically located on side
7 of the tray not far from the well of the swab compartment where the health
care provider has
just poured the antiseptic or iodine solution, and near the syringe of sterile
liquid to be used in
the third instruction. The third instruction 49 states "(3) Attach Water
Syringe" (although,
variations on this instruction or other instructions/procedural indicators are
possible). FIG. 27
shows the syringe of sterile liquid or water 88 after it has been attached to
the inflation port of
the catheter 92.
[0170] Once the syringe of sterile liquid 88 is removed from the syringe
or catheter
compartment, the fourth instruction/procedural indicator 50 is revealed (which
was previously
obscured by the syringe of sterile liquid). The fourth instruction 50 may
inform the health care
provider to "(4) Lube Catheter" or "(4) Dispense & Lube Foley Here" or
something similar
(again, variations on this instruction or other instructions/procedural
indicators are possible).
Indeed, lubricating the catheter is the next step taken by the health care
provider. To do so, the
health care provider removes the syringe of lubricating jelly 89, which
removal reveals the
fifth, sixth, and seventh instructions/procedural indicators that were
previously obscured by the
syringe of lubricating jelly 89. In accordance with the present disclosure,
the lubricating jelly
can be provided in other suitable containers well-known to those of ordinary
skill in the art,
with non-limiting examples including a bellows, an ampoule, or the like. The
health care
provider can then lubricate the catheter in any way desired. In one
embodiment, the health care
provider can remove the plastic wrap 90 (shown in FIGS. 27 and 28) from over
the catheter 92
(which can be a Foley catheter) and move a portion of the catheter 92 into the
syringe or
catheter compartment 2, e.g., as shown in FIG. 28. Placing the distal end 94
of the Foley
catheter 92 in the syringe or catheter compartment 2 helps to line the
catheter 92 up for
lubrication and catheterization. For example, as shown in FIG. 28, with the
distal end 94 of
the catheter 92 positioned near the left-most side of the syringe or catheter
compartment 2, the
distal end 94 of the catheter 92 is positioned very near the patient and the
region to be
catheterized (assuming the tray has been oriented as directed by the belly
band 46). Further,
the distal tip of the catheter 92 is positioned such that it essentially
points at the region to be
catheterized such that the health care provider may simply lift the catheter
92 and move it a
short distance in the direction it is pointing to begin insertion of the
catheter 92 into the patient.
Once the catheter 92 is positioned in the syringe or catheter compartment 2,
the user may
dispense the lubricating jelly from the syringe 89 into the syringe or
catheter compartment 2
onto a distal portion of the catheter 92 (e.g., the syringe may be dispensed
on the left side of
-40-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
the syringe or catheter compartment near the patient and the region to be
catheterized), and
twist the catheter 92 in the lubricating jelly to ensure it is properly
lubricated.
[0171] While the above lubrication method is beneficial for the reasons
discussed
above, other methods of lubricating the catheter 92 are also possible. In one
embodiment, the
health care provider may dispense the lubricating jelly onto a portion of the
sterile/CSR wrap
around the tray and put the end of the catheter in the lubricating jelly to
lubricate. In one
embodiment, the user may dispense the lubricating jelly directly into the
syringe or catheter
compartment 2 before moving the catheter 92 from main compartment 1, and then
move the
distal portion of the catheter 92 into the lubricating jelly.
[0172] After lubricating the catheter 92, the health care provider may
leave the catheter
92 temporarily in the syringe and catheter compartment 2 while he/she uses
his/her non-
dominant hand to retract the genitalia, and uses his/her dominant hand to
prepare and sanitize
the patient with the swabs or swabsticks saturated with antiseptic or iodine
solution. The swabs
or swabsticks (e.g., swabs or swabsticks 34 with absorbent head 36 and stick
38 discussed
above) may be twisted either clockwise or counterclockwise to easily release
them from the
channels of the swab compartment as discussed above. Preferably each swab or
swabstick will
be used for one swipe on the patient only. For female patients, one may
cleanse the patient by:
(1) using a downward stroke with one swab or swabstick to cleanse the right
labia minora and
discard the swab or swab stick, (2) using a downward stroke with another swab
or swabstick to
cleanse the left labia minora and discard the swab or swabstick, and (3) using
a third swab or
swabstick to cleanse the middle area between the labia minora. For male
patients, one may
cleanse the patient with a swab or swabstick using a circular motion starting
at the urethral
meatus and working outward. After prepping and sanitizing the patient in this
way, the health
care provider uses his/her dominant hand to insert the catheter into the
patient's bladder (urine
becomes visible in the tubing when the catheter enters the bladder, but two
additional inches
beyond this point should be inserted to allow the retention balloon to enter
the bladder), and
inflate the retention balloon in the bladder by injecting the sterile liquid
from the syringe of
sterile liquid 88 into the catheter 92 through the inflation port. Indeed,
these are the steps
instructed on the tray in the fifth step, sixth step, and seventh step, which
state, respectively,
"(5) Retract Genitalia (Non-Dominant Hand)", "(6) Prep Patient with Swabs
(Dominant
Hand)", and "(7) Insert Catheter & Inflate Balloon (Dominant Hand)."
Variations on these
instructions/procedural indicators or other instructions/procedural indicators
are possible.
-41-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
Once inflated, the health care provider may gently pull the catheter until the
inflated balloon is
snug against the bladder neck. These steps complete the catheterization stage
or stage "B" of
the procedure.
[0173] FIG. 29 shows how the tray may look immediately after the catheter
has been
properly placed in the patient. Note that the drainage tubing 95 easily
follows the catheter 92
toward the patient and out of the tray. A folded urine collection bag 96 is
positioned in the
main compartment 1 in FIG. 29 and may be packaged pre-connected to the tubing
95, catheter
92, and/or other components of the catheter assembly. Urine collection bag 96
may be unfolded
and hung below the patient to collect urine as it drains from the patient.
[0174] After catheterization of the patient (i.e., after the catheter has
been inserted and
the retention balloon inflated in the patient's bladder), additional steps may
be taken. For
example, in stage "C" or the Care and Maintenance stage, the health care
provider may: (1)
secure the Foley catheter to the patient (e.g., using a StatLock device), (2)
position the urine
bag and/or urine meter below the patient's bladder (e.g., hang the bag on a
hanger on the bed
rail at the foot of the bed) and secure the tubing to the bed sheets (e.g.,
with a clip) in such a
way that the tubing is not kinked, (3) document the insertion date by
indicating time and date
of catheter insertion on a label and/or chart (e.g., the label/sheet 72 shown
in FIG. 16) and
document the procedure according to hospital protocol, (4) maintain the
catheter (e.g., if it has
a sterile red seal, then maintain the red seal), (5) re-assess the need for
the indwelling catheter
routinely evaluate whether the catheter becomes unnecessary, (6) and/or other
care or
maintenance steps.
[0175] Stage "C" and associated instructions/procedural indicators are
identified in the
main compartment of the catheterization tray as shown in FIGS. 1, 2, and 5.
For example, the
stage "C" instructions/procedural indicators may include: "(1) Secure Foley
with StatLock ,
"(2) position bag below bladder", "secure tubing to sheets with clip", "(3)
document insertion
date", "(4) maintain red seal per hospital policy", "(5) assess need for
catheter routinely."
Variations on these instructions/procedural indicators or other
instructions/procedural
indicators are possible. Urine samples may be taken as instructed in FIG. 14D.
Ultimately,
the catheter may be removed as instructed in FIG. 14C.
[0176] The catheterization package(s) and catheterization tray(s)
described herein
made be manufactured/packaged in different ways. For example, a manufacturer
or vendor of
-42-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
catheterization packages may vary the steps or procedures described herein,
may reorder the
steps, may perform additional steps beyond those described, and/or may omit
certain steps as
circumstances and unique needs may require.
Further, any features, components,
arrangements, designs, ordering of components, etc. described above (e.g.,
features or
arrangements that make using the catheterization package more intuitive or
logical) may be
added to or included in the catheterization package during manufacturing,
e.g., in a method of
manufacturing/packaging. However, non-limiting, methods of
manufacture/packaging are
described below. The steps described herein may be done in order as described
or out of order.
[0177] In
one embodiment, a catheterization package is manufactured/packaged by
providing a catheterization tray (e.g., a catheterization tray similar to or
including features of
those shown in FIGS. 1-5 and described above). The tray may be purchased or
formed by the
manufacturer of the catheterization package. If manufactured, the tray may be
formed using
an injection molding process or other suitable processes.
Instructions/procedural indicators
may be integrated on, printed, or otherwise included on the tray as described
above and as
shown, for example, in FIGS. 1, 2, and 5.
[0178]
Various components (e.g., any of the components discussed above or elsewhere
herein) may be added to the tray. In one embodiment, a pre-connected drainage
system may
be added to/included in the tray, e.g., in the main compartment 1 as shown in
FIG. 26. The
drainage system may include a drainage/collection bag 96, drainage tubing 95,
a catheter 92
(e.g., a Foley catheter), a drainage outlet, a urine meter, or other drainage
components. Swabs
or swabsticks 34 may be added to/included in the tray, e.g., in the swab
compartment 3 as
shown in FIG. 8 or FIG. 26. A specimen or sample container 54 and a label that
can be filled
out with details regarding the sample and adhered to the specimen or sample
container may be
added to/included in the tray, e.g., in the corner storage compartment 5 as
shown in FIG. 26.
A packet or container of an antiseptic skin cleanser 52 (e.g., a packet or
container of povidone-
iodine solution) may be added to/included in the tray, e.g., in the swab
compartment 3 on top
of the swabsticks 34 as shown in FIG. 25. A packet or container of lubricant
89 (e.g., a syringe
of lubricating jelly) may be added to/included in the tray, e.g., in the
syringe or catheter
compartment 2 as shown in FIG. 26. A syringe of sterile liquid 88 (e.g., a 1
Occ syringe of
sterile water for inflating the retention balloon of the Foley catheter) may
be added to/included
in the tray, e.g., in the syringe or catheter compartment 2 on top of or
partially on top of the
syringe of lubricating jelly 89 as shown in FIG. 26. A fenestrated drape to
place on patient
-43-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
may be added to/included in the tray, e.g., on top of other components, and
may span portions
of more than one compartment as shown in FIG 24. An underpad to place under
the buttocks
of a patient (e.g., a waterproof absorbent underpad) may be added to/included
in the tray, e.g.,
on top of the fenestrated drape or other components, and may span portions of
more than one
compartment. Both the underpad and fenestrated drape are represented in the
figures by
pad/drape 86. Gloves (e.g., a package 58 of rubber gloves, latex gloves, latex-
free gloves) may
be added to/included in the tray, e.g., on top of the underpad or other
components and may
span portions of more than one compartment as shown in FIG. 22.
[0179] The tray and components may be sterilized, and a sterile wrap 56
(e.g., a CSR
wrap) may be folded or wrapped around the catheterization tray as shown in
FIGS. 20-21. A
belly band 46 (e.g., to hold the sterile wrap in a folded configuration and
help to keep all the
contents inside the tray, so the items do not move around) may be included
around the sterile
wrap 56 as shown in FIG. 20. A perineal care (or pen-care) packet or kit 74
(e.g., as shown in
FIGS. 17-19) may be added to/included in the catheterization package, e.g.,
placed over the
belly band 46 and/or sterile wrap 56 as shown in FIGS. 17 and 18. The perineal
care packet or
kit 74 may include hand sanitizer 76 (e.g., antiseptic gel hand rinse), moist
towelettes 78 (e.g.,
a package of castile soap towelettes), instructions 80 (e.g., instruction for
health care provider
and/or instructions for patient), and/or other components (see e.g., FIG. 19).
An insert sheet or
label 72 with instructions or other information may be added to/included in
the catheterization
package, e.g., an insert sheet or label 72 with a checklist of safety
considerations/steps and/or
a patient information chart may be included on top of the perineal care packet
or kit as shown
in FIG. 16. A detailed instructions document (e.g., as shown in FIGS. 14A-14D)
and/or patient
educational information (e.g., as shown in FIGS. 15A and 15B) may be added
to/included in
the catheterization package, e.g., a directions for use (DFU) document 68
and/or a patient
educational pamphlet 70 may be included on top of the insert sheet as shown in
FIG. 13. A
packaging label 62 (e.g., a packaging label as described above and shown in
FIGS. 9-12) may
be added to/included in the catheterization package, e.g., on top of all the
other components
and with edges folding down to cover the sides of the catheterization tray.
The catheterization
package may be sterilized and sealed, e.g., in sealed container or bag 60 as
shown in FIGS. 9
and 10. Other components useful to catheterization or care may also be
included.
[0180] In one embodiment, a catheterization system may include a ureteral
catheter
assembly and/or materials or supplies (e.g., catheterization elements or
components, such as
-44-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
sanitizer liquid, lubricant, inflation liquid, gloves, etc.) suitable or
necessary for a ureteral
catheterization procedure. For
instance, the catheterization system may include a
catheterization tray that has multiple compartments for housing and/or
securing a ureteral
catheter assembly (e.g., an assembly including a Foley catheter) as well as
other sterile and/or
non-sterile catheterization elements, including any of those discussed above.
Sterile
catheterization elements may be housed in the catheterization tray in a manner
that the sterile
elements remain uncontaminated by non-sterile catheterization elements.
[0181] The
catheterization tray may include one or more visual, procedural indicators
or instructions that may aid a user during the catheterization procedure. For
instance, the
procedural indicators may aid the user in orienting the catheterization tray
(and catheterization
components or elements thereof) relative to a patient, such that the
catheterization components
and/or elements may be positioned at predictable and suitable or desirable
locations and/or
orientations relative to the user and/or to the patient. Additionally or
alternatively, the
procedural indicators may aid the user in sequencing use of the
catheterization components
and/or removal thereof from the catheterization tray.
[0182] As
mentioned above, the catheterization tray and system including such tray
may facilitate aseptic techniques during a catheterization procedure. In one
embodiment, as
shown in FIG. 30A, catheterization system 100 includes container 110 (e.g., a
sealed bag),
which may hermetically seal or contain the catheterization tray and
catheterization
components. For instance, the container 110 may facilitate maintaining
sterility of sterile
catheterization components. For example, the container 110 may be a plastic
bag (e.g., a
polyethylene bag). Moreover, the container 110 may include a peelable side
111, which may
be detached from a non-peelable side 112 to form an opening in the container
110 (container
60 above may have similar features and properties); the contents located in
the container 110
may be removed through the opening.
[0183] In
one embodiment, the catheterization tray and at least some of the contents
thereof (i.e., catheterization components) may be at least partially
surrounded or enveloped by
an identification cover 120. In one embodiment, the packaging label or
identification cover
120 (e.g., similar to packaging label 62 discussed above) may include one or
more visual
identifiers including key information or variables, such as visual identifiers
121, 122, 123 (e.g.,
these can be similar to the information squares discussed above), which may
provide indication
related to the type and/or contents of the catheterization system 100.
-45-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0184] For example, the packaging label or identification cover 120 may
be fabricated
from a sheet-like material, such as paper, cardboard, plastic (e.g., plastic
film), etc. The visual
identifiers 121, 122, 123 may be configured to convey sufficient information
for the user to
identify the specific type of the catheterization system 100 and/or contents
thereof. For
instance, the visual identifier 121 may identify the size of the catheter
(e.g., 16 French, etc.).
In one example, the visual identifier 122 may indicate that the
catheterization system 100
includes a urine meter (which may be attached to the catheter), and/or the
visual identifier 123
may indicate that the catheterization system 100 includes a catheter
stabilization device (e.g.,
STATLOCK).
[0185] The visual identifiers 121, 122, 123 may be visually separated or
isolated one
from another. For example, the visual identifiers 121, 122, 123 may include a
generally square
or rectangular perimeter outline that may delimit or define the outer contours
thereof In other
words, the identifying information of the visual identifiers 121, 122, 123 may
be contained
within the generally rectangular outlines of the visual identifiers 121, 122,
123. Hence, for
instance, the user may easily and/or quickly locate the pertinent information
of the
identification cover 120, to identify and/or select the particular
catheterization system 100
suitable for the procedure.
[0186] One, some, or all of the visual identifiers 121, 122, 123 may
include any visible
indications or information that may inform the user about specifics of the
catheterization
system 100. For example, the visual identifiers 121, 122, 123 may include
text, images,
symbols, etc. Moreover, the visual identifiers 121, 122, 123 and/or portions
thereof may have
any suitable color or multiple colors, which may aid the user in identifying
and/or
distinguishing among the visual identifiers 121, 122, 123. In one embodiment,
at least one of
the visual identifiers 121, 122, 123 may have a different color than others.
[0187] It should be appreciated that the particular contour shape, size,
thickness, or
combinations thereof, which surrounds or contains the identifying information
of the visual
identifiers 121, 122, 123 may vary from one embodiment to the next. As such,
the visual
identifiers 121, 122, 123 may have generally circular, triangular, or
polygonal shapes.
Furthermore, the identification cover 120 may include any number of suitable
materials that
may carry the visual identifiers 121, 122, 123 may vary from one embodiment to
another. For
instance, the visual identifiers 121, 122, 123 may be printed, embossed, or
otherwise
transferred onto the identification cover 120.
-46-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0188] The identification cover 120 may include any number of visual
identifiers. In
other words, the visual identifiers 121, 122, 123 may be located at any number
of suitable
locations on the identification cover 120 and/or may be duplicated. For
example, the
identification cover 120 may include visual identifiers 121, 122, 123 on a
major face thereof
and on a minor face thereof In one embodiment, the visual identifiers 121,
122, 123 may be
located on one, some, or all visible sides of the catheterization system 100
(e.g., one, some, or
all of the visual identifiers 121, 122, 123 may be visible from any side of
the catheterization
system 100 during storage thereof).
[0189] The identification cover 120 may be removable from the
catheterization tray.
For instance, the identification cover 120 may be generally detached from the
catheterization
tray (e.g., the identification cover 120 may lie on the catheterization tray
and may be secured
relative to the catheterization tray by the container 110). Furthermore, as
shown in FIG. 30B,
removing the identification cover may expose the catheterization tray, which
may be wrapped
by or into a central supply room (CSR) sterile wrap 130. Accordingly, the CSR
sterile wrap
130 may at least temporarily secure components in the catheterization tray.
[0190] The CSR sterile wrap 130 may be unwrapped and removed from the
catheterization tray and may be used during the catheterization procedure. For
example, the
CSR sterile wrap 130 may be placed near and/or under the patient to provide at
least a partially
sterile environment near the site of the catheterization procedure, thereby
reducing risk of
infections associated with the catheterization procedure. The CSR sterile wrap
130 may be
substantially opaque, such that the contents of the catheterization tray
and/or locations
catheterization components are obscured or not be visible to the user.
Optionally, the CSR
sterile wrap 130 may be translucent or transparent, such that the user may
view and/or visually
identify catheterization components contained in the catheterization tray.
[0191] The catheterization system 100 may include a belly band or
directional indicator
wrapper 140, which may provide an indication of relative positioning and/or
orientation of the
components in the catheterization tray, which may not be visible to the user.
For example, the
belly band or indicator wrapper 140 may include a direction arrow 141 that may
be aligned by
the user to point toward the catheter insertion site, which may orient the
catheterization tray
and catheterization components therein relative to the patient. For instance,
orienting the
catheterization tray in a manner that aligns the direction arrow 141 in the
direction of the
insertion site may position the catheterization components and/or elements at
least partially
-47-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
sequentially, such that the catheterization elements and/or components to be
used earlier in the
procedure are located closer to the patient than the catheterization
components intended to be
used later in the procedure.
[0192] In addition, the belly band or indicator wrapper 140 may include a
warning
indicator 142 to alert the user to proper aseptic technique for the
catheterization procedure. For
example, the warning indicator 142 may include one or more specific elements
identifying the
particular steps suitable or necessary for proper aseptic procedure. In one
embodiment, the
warning indicator 142 identifies one or more of the following steps: (1) open
CSR sterile wrap
130; (2) don sterile gloves; (3) place underpad; (4) place fenestrated drape;
or (5) follow steps
identified by procedure indicators located inside the catheterization tray.
[0193] While the indicator wrapper 140 may wrap around the CSR sterile
wrap 130,
this disclosure is not so limited. For example, a sticker or an adhesive label
may be attached
to the CSR sterile wrap and may include the same or similar indicators as the
indicator wrapper
140. Alternatively or additionally, the indicators may be placed directly onto
the CSR sterile
wrap 130 (e.g., may be printed, embossed, or otherwise transferred thereto).
[0194] After the CSR sterile wrap 130 is removed or unwrapped from the
catheterization tray, the contents of the catheterization tray may be exposed.
FIG. 31A
illustrates a catheterization tray 200 of the catheterization system 100. The
catheterization tray
200 may include multiple compartments that may secure or house various
catheterization
components. The shape and size of the catheterization tray 200 may be defined
by the sides
thereof For instance, sides 201, 202, 203, and 204 may define an approximately
rectangular
outer shell or exterior perimeter of the catheterization tray 200.
[0195] The catheterization tray 200 may be molded (e.g., injection
molded,
thermoformed) or stamped (e.g., from a plastic material). Hence, the sides
201, 202, 203, and
204 may be formed from a sheet-like material. One or more of the sides 201,
202, 203, or 204
may include stiffening ribs 205, which may provide rigidity and/or stiffness
to the sides 201,
202, 203, 204. The stiffening ribs 205 may have an approximately tapered shape
and/or may
protrude outward from the respective sides 201, 202, 203, or 204. More
specifically, for
example, lower portions of the stiffening ribs 205 (portions closer to the
bottom/floor of the
catheterization tray 200) may protrude out of the respective sides 201, 202,
203, or 204 more
than the upper portions of the stiffening ribs 205 (portions closer to the
opening of the
-48-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
catheterization tray 200). At least a portion of one, some, or all of the
stiffening ribs 205 may
have a partially conical shape.
[0196] The catheterization tray 200 may include a swab compartment 210,
which may
include one or more swabs 150 therein. Additionally or alternatively, the
compartment 210
may include a cleansing/sanitizing solution or sanitizer packet 160, which may
contain one or
more sanitizing agents or cleansing/sanitizing solutions (e.g., containing
povidone-iodine
solution). The compartment 210 may be positioned near and at least partially
defined by the
side 201 of the catheterization tray 200. In some instances, the exterior of
the sanitizer packet
160 may be non-sterile. Hence, the cleansing/sanitizing solution or sanitizer
packet 160 may
be placed inside another packaging or a bag, which may have a sterile exterior
that may prevent
or reduce contamination of other catheterization components in the
catheterization tray 200
from the unsterilized packet 160 of cleansing solution or sanitizer. The
plastic bag may include
a zipper or similar mechanism that may be operated by the user to open and
access the packet
160 and/or other components therein.
[0197] Catheterization instructions may include images and description of
various
steps or acts taken during the catheterization procedure. In some instances,
instructions also
may identify specific step or act numbers, thereby suggesting a particular
sequence of steps or
acts for the catheterization procedure. Catheterization instructions, which
may be non-sterile,
may be placed into a sterile container, such as a plastic bag, thereby
isolating or separating the
non-sterile instructions from sterile catheterization components in the
catheterization tray 200.
Also, in some examples, the catheterization system may include multiple sets
of catheterization
instructions, which may improve compliance with aseptic catheterization
procedures. For
example, catheterization instructions may could be attached or adhered to
(e.g., as a sticker)
inside the sterile plastic bag and/or to another catheterization element or
component (e.g., to
the sterile container with the sanitizer packet 160), to an exterior sleeve or
portion of the
catheterization system, etc.
[0198] The compartment 210 may be configured to contained sanitizing
substance
(e.g., liquid), which may be dispensed in the compartment 210 from the
sanitizer packet 160.
For example, as described below in more detail, the compartment 210 may have a
slanted
bottom/floor, such that the cleansing or antiseptic solution (e.g., povidone-
iodine solution) or
sanitizer from the packet 160 may pool near and/or at a lowermost corner
between a
bottom/floor and a side (e.g., partition 211) of the compartment 210.
Furthermore, tips of the
-49-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
swabs 150 may be located at or near the lowermost corner between the
bottom/floor and the
side of the compartment 210, such that the tips of the swabs 150 are
positioned in the dispensed
sanitizing liquid.
[0199] The compartment 210 may be at least partially defined by a portion
of the side
201 or inner wall thereof, the partition 211, by a partition 212, and by the
slanted bottom/floor.
For instance, the lowermost portion of the compartment 210 may be located at
the corner edge
formed by and between the partition 211 and the slanted bottom/floor. Hence,
positioning the
tips/absorbent heads of the swabs 150 near the slanted bottom/floor and/or
near the partition
211 of the compartment 210 may locate the tips/absorbent heads of the swabs
150 at or near
the lowermost portion of the compartment 210.
[0200] As described above, the catheterization tray 200 may be oriented
relative to the
patient in a manner that places the side 201 and the compartment 210 closer to
the insertion
site than the side 203 of the catheterization tray 200. In other words,
orienting the
catheterization tray 200 based on the direction arrow of the directional
indicator wrapper (while
the contents and compartments of the catheterization tray 200 are concealed or
covered by the
CSR sterile wrap 130 (FIG. 30B) may place the side 201 and the compartment 210
near the
insertion site. In some instances, the insertion site may be first sterilized
with the swabs 150
that may be saturated with the sanitizing liquid from the sanitizer packet
160. As such,
positioning the compartment 210 together with the swabs 150 and sanitizer
packet 160 near the
insertion site may reduce spillage of the sanitizer liquid (e.g., onto one or
more catheterization
components in the catheterization tray 200) and/or contamination of the swabs
150, which may
lead to contamination of the insertion site.
[0201] As described below in more detail, the compartment 210 may have a
slanted
bottom/floor. For instance, an upper edge (or surface), such as an upper edge
206 of the
catheterization tray 200 may lie in an imaginary plane that may be
approximately parallel to a
support surface that may support the catheterization tray 200 (e.g., surface
of the patient's bed).
The slanted bottom/floor of the compartment 210 may have a non-parallel
orientation relative
to the imaginary plane of the upper edge 206 and/or to the support surface.
[0202] Furthermore, the bottom/floor of the compartment 210 may be non-
parallel
relative to bottoms/floors of one or more other compartments in the
catheterization tray 200.
For example, the catheterization tray 200 may be set on the support surface,
such that
-50-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
bottoms/floors of one or more compartments support and/or orient the
catheterization tray 200
on the support surface. Hence, as described above, the bottom/floor of the
compartment 210
may have a non-parallel orientation relative to the support surface.
[0203] The catheterization tray 200 may include a side compartment 220.
For example,
the compartment 220 may extend along substantially an entire side or length of
the
catheterization tray 200 (e.g., the compartment 220 may extend between the
sides 201 and 203
and/or may be partially defined thereby). Hence, in some instances, one side
of the
compartment 220 may be defined by a side such as by the side 204.
[0204] The compartment 220 may contain any number of suitable elements or
components of the catheterization kit. For example, the compartment 220 may
contain a
syringe 170 with liquid that may be used for inflating a balloon of the
catheter (described
below) and/or a syringe 180 that may contain lubricant. As such, the width of
the compartment
220 may be sufficient to accommodate the syringes 170, 180. In some instances,
the syringes
170, 180 may be oriented lengthwise along the compartment 220, and may lie in
a single row,
one in front of the other therein.
[0205] The catheterization tray 200 may include one or more cutouts or
recesses in a
partition 221, which may separate the compartment 220 from an adjacent
compartment(s) (e.g.,
from a catheter compartment 230). For example, one or more recesses in the
partition 221 may
have top edges thereof located below the upper edge 206 of the catheterization
tray 200. More
specifically, in some instances, the recesses may provide access to one or
more of the syringes
170, 180 from the compartment 230. Hence, a user of the catheterization kit
may remove the
syringe 170 and/or syringe 180 by reaching into the compartment 230, in lieu
of or in addition
to reaching into the compartment 220.
[0206] In one embodiment, the syringes 170 and 180 may be differently
sized. Hence,
in one embodiment, at least a portion of the compartment 220 may be narrower
than another
portion thereof, to accommodate syringes 170, 180 of different sizes. For
example,
compartment 210 may include a narrowing protrusion 222, which may extend into
the space
of the compartment 220, thereby narrowing a portion of the compartment 220.
For instance,
the syringe 170 may have a smaller diameter or peripheral size than the
syringe 180. Hence,
the narrowing protrusion 222 may narrow the width of the (s) of the
compartment 220 may be
-51-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
sufficiently wide to accommodate the syringe 180 (e.g., the narrowing
protrusion 222 may
prevent or limit lateral movement of the syringe 170 in the compartment 220).
[0207] As mentioned above, the catheterization tray 200 may include the
main or
catheter compartment 230. The compartment 230 may have an approximately
rectangular
shape. For example, the compartment 230 and the shape thereof may be at least
partially
defined by the side 203, at least a portion of the side 202, and by partitions
212, 221. While in
one embodiment the compartment 230 may have a generally rectangular shape, it
should be
appreciated that the shape of the compartment 230 may vary from one embodiment
to the next.
[0208] In any event, the compartment 230 may be sized (e.g., perimeter
dimensions
and depth), shaped, or otherwise configured to contain or house a catheter
assembly 190. For
instance, the catheter assembly 190 may include a catheter 191 that may be
connected to a urine
collection bag and/or urine meter 192. In some instances, the compartment 230
also may
contain or house a STATLOCK 193, which may secure at least a portion of the
catheter
assembly 190 during the catheterization procedure.
[0209] The compartment 230 may have an approximately flat or planar
bottom/floor,
as described below in more detail. For example, the bottom/floor of the
compartment 230 may
support the catheterization tray 200 on the support surface. Hence, in one
embodiment, the
bottom/floor of the compartment 210 may have a non-parallel orientation
relative to the
bottom/floor of the compartment 230.
[0210] As mentioned above, the compartment 230 may have an approximately
rectangular shape. Furthermore, the compartment 230 may have rounded or
chamfered interior
corners 231 between the inner walls that define the perimeter of the
compartment 230.
Additionally or alternatively, the upper portions of the inner walls and/or
partitions may have
one or more chamfers or fillets 232, which may extend at least partially along
their respective
lengths.
[0211] The catheterization tray 200 also may include an auxiliary
compartment 240
that may contain or house an auxiliary container 195 (e.g., a specimen or
sample container).
For instance, the auxiliary container 195 may be approximately cylindrical and
may include a
cap. Furthermore, the compartment 240 may be shaped, sized, and configured to
secure the
auxiliary container 195 therein. In one embodiment, the length of the
compartment 240 may
be similar to the length of the auxiliary container 195 (e.g., the compartment
240 may include
-52-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
a small clearance or may have an interference fit with the auxiliary container
195). In one
embodiment, the bottom/floor of the compartment 240 may be semi-cylindrical or
generally
arcuate and may approximately follow the general cylindrical shape of the
auxiliary container
195. Hence, for instance, the bottom/floor of the compartment 240 may at least
partially wrap
around the auxiliary container 195.
[0212] For example, as shown in FIG. 31B, the compartment 240 may have a
partially
rounded bottom/floor 241. The compartment 240 may be separated from the
compartment 210
in the catheterization tray 200. For instance, the catheterization tray 200
may include an
overflow cavity 250 located between the compartment 240 and the compartment
210. In one
embodiment, the overflow cavity 250 may form a recess or depression between
the
compartment 210 and the compartment 240. For example, the overflow cavity 250
may
prevent or limit overflow of the sanitizing liquid dispensed into the
compartment 210.
Moreover, the user may tap excess sanitizing liquid from the tips of the swabs
150 into the
overflow cavity 250, before applying the sanitizing liquid at or near the
insertion site.
[0213] The slanted bottom/floor 213 of the compartment 210 may be
elevated or
stepped above the overflow cavity 250. For example, the slanted bottom/floor
213 may
terminate at a fillet or radius 214 that may extend from the slanted
bottom/floor 213 downward
toward the overflow cavity 250 or an edge thereof The radius 214 may provide a
transition
between the slanted bottom/floor 213 and at least a portion of the overflow
cavity 250 located
below the slanted bottom/floor 213. In any event, the slanted bottom/floor 213
may slant
downward and away from the overflow cavity 250.
[0214] As mentioned above, sanitizing liquid may be dispensed at the
lowermost
portion 215 of the compartment 210 (e.g., at or near the lowermost portion of
the slanted
bottom/floor 213). Furthermore, sanitizing liquid dispensed in the compartment
210 may
generally flow toward and pool at the lowermost portion 215 of the compartment
210. In
addition, the tips 151 of the swabs 150 may be set or positioned in the
sanitizing liquid. As
such, the user may remove the swabs 150 from the compartment 210 and apply
sanitizing liquid
at the insertion site without further acts.
[0215] The swabs 150 may be secured in the compartment 210 in a manner
that
positions the tip(s) or absorbent head(s) 151 in the sanitizing liquid
dispense in the
compartment 210. For example, one, some, or each of the swabs 150 may include
an elongated
-53-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
stem/stick 152 and a tip/absorbent head 151 attached to the stem/stick 152.
Moreover, the
compartment 210 may include channels 216 that may house and secure the
elongated stem/stick
152 of the swabs 150. Hence, the stems/sticks 152 of the swabs 150 may be
secured in the
channels 216 of the compartment 210.
[0216] The channels 216 may include one or more retention features (e.g.,
detents,
overhangs), which may secure the stems/sticks 152 therein. For example, the
stems 152 may
have approximately rectangular or generally rectangular cross-sectional
shapes. When the
stems/sticks 152 are oriented along the longer side of the rectangular cross-
sectional shapes
thereof, the retention features may secure the elongated stem 152 in the
channels 216. Also,
rotating or twisting the swabs 150 in the channels 216 (e.g., such that the
shorter side of the
rectangular cross-sectional shapes of the stems 152 may be oriented to lie
within the channels
216) may disengage the stems 152 from the channels 216.
[0217] In other words, spacing between the retention features of the
channels 216 may
be less than the width of the longer side of the rectangular cross-sectional
shape of the elongated
stem 152, such that the retention features may restrain the elongated
stem/stick 152 in the
channels 216. Moreover, the spacing between the retention features may be
greater than the
length of the shorter side of the rectangular cross-sectional shape of the
elongated stem/stick
152. Accordingly, twisting or rotating the swabs 150 within the channels 216
may allow the
elongated stem/stick 152 to pass between the retention features of the
channels 216 in order to
remove the elongated stem/stick 152 therefrom.
[0218] The catheterization tray 200 (as well as the catheterization trays
shown in FIGS.
1-5) may be fabricated in any number of suitable ways and with any number of
suitable
manufacturing techniques. As mentioned above, the catheterization tray(s) may
be injection
molded, thermoformed, or otherwise mass-produced. For instance, the
catheterization tray(s)
may be fabricated from a sheet of plastic material that may be processed by
compressing and
heating the sheet into the suitable configuration, as described herein. As
such, one or more of
the sides of the catheterization tray(s) may comprise a relatively thin
plastic material (e.g.,
0.005 inch, 0.010 inch, 0.020 inch, 0.050 inch, 0.catheterization system 100
inch, etc.). As
described above, the catheterization tray(s) may include stiffening ribs on
one or more sides
thereof, which may provide rigidity to such sides.
-54-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0219] The catheterization tray(s) may include stiffening ribs as
described herein on
one or more partitions that divide the interior space of the catheterization
tray(s) into the
compartments described herein. Also, as described below in more detail, the
partitions (e.g.,
the partition 221) may include folded or dual layers of sheet material. The
stiffening ribs may
protrude away from the compartments (e.g., away from the compartment 210,
compartment
220, compartment 230) and into the space between the layers of the sheet
material.
[0220] As described above, in the compartments, the inside corners and/or
edges
formed between the bottom/floor and inner walls, sides, and/or partitions may
include fillets or
chamfers. For example, such fillets or chamfers may reduce stress at the
inside corners or
edges of the compartments. Accordingly, the fillets and/or chamfers formed at
the inside
corners and/or edges of the compartments may reduce or prevent breakage or
failure of the
sheet material that forms the catheterization tray 200.
[0221] The bottom/floor of the compartment 230 may be substantially flat.
Additionally or alternatively, the bottom/floor of the compartment 230 may
have a protruding
landing 232 and a recessed portion 233. For example, the recessed portion 233
may be lower
or farther away from the upper edge 206 of the catheterization tray 200 than
the landing 232.
Furthermore, the recessed portion 233 may at least partially or completely
wrap around the
landing 232. As such, for instance, the recessed portion 233 may accommodate
at least a
portion of the catheter, which may wrap around the landing 232.
[0222] As described above, the catheterization tray 200 may be placed on
a support
surface, such as a surface of the patient's bed. For example, as shown in FIG.
31C the recessed
portion 233 of the compartment 230 may form a protrusion on the exterior of
the catheterization
tray 200, while the landing 232 may form a recesses on the exterior of the
catheterization tray
200. As such, the exterior surface of the recessed portion 233 may provide or
form a surface
that may orient and/or support the catheterization tray 200 on the support
surface. Moreover,
in one embodiment, the exterior surface of the recessed portion 233 may be
approximately
parallel to the imaginary plane of the upper edge 206.
[0223] Also, as mentioned above, the channels 216 of the compartment 210
may be
generally parallel to the slanted bottom/floor 213 and slanted or non-parallel
relative to the
support surface. The channels 216 of the compartment 210 may have a non-
parallel orientation
relative to the exterior surface of the recessed portion 233. For instance, on
the exterior of the
-55-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
catheterization tray 200, the channels 216 may protrude outward away from the
slanted
bottom/floor 213.
[0224] A compartment of the catheterization tray (e.g., compartment 210)
or a portion
thereof may carry the sanitizing or cleansing liquid (e.g., povidone-iodine
solution) therein.
For example, at least a portion of the compartment may carry the sanitizing
liquid and may be
sealed by a peelable cover. As such, during the catheterization procedure, the
user may avoid
pouring a sanitizing liquid out of a packet (e.g., into the catheterization
tray), which may
prevent or reduce spillage of the sanitizing liquid. The swabs may be at least
partially
submerged in the sanitizing liquid and/or at least partially sealed together
therewith by the
peelable cover. Alternatively or additionally, the swabs may be located in
another
compartment of the catheterization tray.
[0225] As described above, partitions of the catheterization tray 200 may
be formed by
two layers of a sheet material. At least some of the partitions may have space
between the
layers of the sheet material. For example, the partition 221 may include a
first layer 221a and
a second layer 221b spaced apart from each other (e.g., the first layer 221a
may define or form
the perimeter of the compartment 230, and the second layer 221b may define or
form the
perimeter of the compartment 220.
[0226] The stiffening ribs 205 on the first layer 221a of the partition
221 may protrude
outward, away from the compartment 230 and into the space between the first
and second
layers 221a, 221b. The stiffening ribs 205 on the second layer 221b may also
protrude outward,
away from the compartment 220 and toward the outer perimeter or periphery of
the
catheterization tray 200. In any event, as described above, the compartments
of the
catheterization tray 200 may have indents that correspond to the protrusions
formed on the
exterior portions of the catheterization tray 200 by the stiffening ribs 205.
[0227] The catheterization tray 200 may include one or more instructions
or procedural
indicators that may assist a user during a catheterization procedure. For
instance, as illustrated
in FIG. 32, the catheterization tray 200 may include indicators 301-307, which
may be printed
on the catheterization tray 200. For example, a first indicator 301 may
indicate to the user to
open the package containing sanitizing liquid, and a second indicator 302 may
indicate to the
user to pour the sanitizing liquid into the compartment 210, during the
catheterization
procedure.
-56-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0228] A third indicator 303 may indicate to the user to attach to the
catheter the syringe
containing liquid for inflating the catheter balloon. In one embodiment, a
fourth indicator 304
may indicate to the user to dispense lubricant and/or may identify or suggest
a location in the
compartment 220 for dispensing lubricant. Also, indicators 305-307 may
identify additional
or alternative steps to take during the catheterization procedure (e.g.,
retract genitalia (with
non-dominant hand), prepare patient with swabs (with dominant hand), insert
catheter and
inflate balloon (with dominant hand)).
[0229] The catheterization tray 200 may include an enlarged or bolded
print of the
indicators 301-307 for improved visibility. Further, other enlarged
instructions/indicators may
be printed on the bottom/floor of the compartment 230, as shown in FIG. 32.
The presence of
the indicators 301-307 on the catheterization tray 200 may obviate or reduce
the necessity for
printed handouts or similar instructions.
[0230] In one embodiment, as shown in FIG. 33, a catheterization system
100a may
include one or more procedural indicators 260a on a CSR sterile wrap 130a.
Except as
otherwise described herein, the catheterization system 100a and its elements
and components
may be similar to or the same as catheterization system 100 (FIGS. 30A-32) and
its respective
elements and components. For example the catheterization system 100a may
include the
catheterization tray 200 and catheterization elements and/or components
described above in
connection with the catheterization tray 200 above.
[0231] In one embodiment, the CSR sterile wrap 130a may include one or
more
procedural indicators 260a that may assist a user during the catheterization
procedure. For
example, one or more of the procedural indicators 260a may be individually
isolated and/or
delimited, or otherwise visually enclosed by a perimeter outline. In
particular, isolating each
of the procedural indicators 260a may draw or focus user's attention thereto
and may facilitate
identification thereof The procedural indicators 260a may contain similar or
the same
information as indicators 301-307 (FIG. 32).
[0232] In one embodiment, the catheterization tray may include a
fenestrated drape,
which may partially cover the patient and may facilitate insertion of the
catheter. FIG. 34
illustrates a fenestrated drape 270. The fenestrated drape 270 may have a
generally rectangular
shape. Moreover, the fenestrated drape 270 may include perforations 271. In
particular, the
perforations 271 may generally form a shape of a cutout in the fenestrated
drape 270, which
-57-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
may be formed therein by tearing a portion of the fenestrated drape 270
delimited by the
perforations 271. In one embodiment, the central portion of the fenestrated
drape 270 is already
opened, i.e., the central material inside the area circumscribed by
perforations 271 is removed
prior to packaging the fenestrated drape 270.
[0233] The shape defined by the perforations 271 or the shape of the
central opening
may be suitable for accessing the genitalia of the patient for performing the
catheterization
procedure after covering the patient with the fenestrated drape 270. Also, in
some instances,
the fenestrated drape 270 may include side cutouts 272. For instance, the side
cutouts 272 may
be generally V-shaped. In one embodiment, the side cutouts 272 may facilitate
placement of
the fenestrated drape 270 about the legs of the patient.
[0234] As mentioned above, the catheterization system may include various
components that may facilitate the catheterization procedure, such as swabs
that may facilitate
sanitizing area near or at the site of the catheterization. FIG. 35
illustrates a swab 150. For
example, the swabs 150 may include the elongated member or stem/stick 152 and
the
tip/absorbent head 151 attached thereto.
[0235] The tip/absorbent head 151 may have a generally rounded outward or
distal
portion and a tapered proximal portion (e.g., tapering toward the proximal end
of the
tip/absorbent head 151 and toward the elongated stem/stick 152). For example,
the
tip/absorbent head 151 may be narrower at the proximal end of than at the
distal portion (i.e.,
the distal portion may be generally flared and front edge thereof may be
generally rounded).
[0236] The tip/absorbent head 151 may include any suitable material,
which may vary
from one embodiment to the next. The tip/absorbent head 151 may include or
comprise liquid
absorbing material, which may absorb sanitizing liquid and subsequently
dispense the
sanitizing liquid onto and/or near the site of catheterization. For instance,
the tip/absorbent
head 151 may comprise a suitable foam, sponge, cotton, etc.
[0237] The tip/absorbent head 151 may be attached to the elongated
stem/stick 152
with any number of suitable mechanisms, which may vary from one embodiment to
the next.
For example, the tip/absorbent head 151 may be attached to the elongated
stem/stick 152 with
an adhesive, may be ultrasonically welded to the stem/stick 152, etc. The
elongated stem 152
may enter into the tip/absorbent head 151 to a predetermined depth, which may
facilitate
suitably securing together the tip/absorbent head 151 and the elongated
stem/stick 152, while
-58-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
leaving a sufficient portion of the tip/absorbent head 151 beyond the
elongated stem/stick 152
(e.g., in a manner that avoids contacting and/or injuring or hurting the
patient with the elongated
stem/stick 152 during application of the sanitizing liquid at the insertion
site. For instance,
after being secured to the elongated stem/stick 152, the tip/absorbent head
151 may have
sufficient flexibility and sufficient resilience and/or stiffness, such that
the tip may substantially
remain unbent after absorption of the sanitizing liquid and may resiliently
bend during
application of the sanitizing liquid at the insertion site.
[0238] As described above, in some examples, the catheter assembly in the
catheterization tray may be oriented generally parallel to the support surface
that supports the
catheterization tray. The catheter assembly in the catheterization tray may be
generally
oriented vertically or perpendicularly to the support surface. FIGS. 36A-36B
illustrate a
catheterization system 100a that includes a catheterization tray 200a, which
secures a catheter
assembly and/or one or more components or elements for catheterization
procedures. Except
as described herein, the catheterization system 100a and its elements and
components may be
similar to or the same catheterization system 100 (FIGS. 30A) and its elements
or components.
For instance, except as described herein, the catheterization tray 200a and
catheterization
elements and components contained in the catheterization tray 200a may be
similar to the
catheterization tray 200 (FIGS. 31A-32) and catheterization elements or
components contained
therein.
[0239] For example, as shown in FIG. 36A, the catheterization tray 200a
may have a
generally rectangular shape, which may be defined by sides 201a-204a. The
sides 201a-204a
may be approximately planar, hence, may define a box-like structure or
periphery of the
catheterization tray 200a. The catheterization tray 200a also may include a
top compartment
210a that may contain instructions and/or one or more catheterization
components. In one
embodiment, the top compartment 210a may be defined by partitions 211a, 212a
and a bottom
213a. In one embodiment, the partitions 211a, 212a, and/or the bottom 213a may
be removable
from the catheterization tray 200a.
[0240] In one embodiment, removal of the partitions 211a, 212a, and/or
the bottom
213a may provide access to additional catheterization components contained in
the
catheterization tray 200a. For example, as shown in FIG. 36B, after removing
the partitions
211a, 212a, and the bottom 213a (FIG. 36A), a center compartment 220a of the
catheterization
tray 200a may be exposed and/or accessible to the user. For instance, the
compartment 220a
-59-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
may house or contain catheter assembly 190. In one embodiment, the compartment
210a may
be separated from adjacent compartments by one or more partitions (e.g., by
partitions 221a,
222a). As mentioned above, the catheter assembly 190 in the compartment 210a
may be
oriented approximately vertically relative to the support surface (e.g., when
the catheterization
tray 200a is placed on the support surface, such as a surface of the patient's
bed).
[0241] In one embodiment, the partitions 221a, 222a may separate the
center
compartment 220a from adjacent compartments 230a, 240a. Compartments 230a
and/or 240a
may house or contain various catheterization elements or components for
procedure, which
may be similar to or the same as catheterization components described above in
connection
with catheterization tray 200 (FIGS. 31A-32). In some instances, the
partitions 221 and/or
222a may be removable from the catheterization tray 200a, which may facilitate
removal of
catheterization elements or components from the catheterization tray 200a.
[0242] The catheterization tray 200a may be constructed of any suitable
material. For
example, the catheterization tray 200a may be constructed of thick sheet-like
material or panels
(e.g., plastic material). For instance, thickness of the panels may be in one
or more of the
following ranges: about 0.1 inches to about 0.2 inches; about 0.15 inches to
about 0.3 inches;
about 0.25 inches to about 0.5 inches. It should be appreciated, however,
panel thickness may
be less than 0.1 inches or greater than 0.5 inches. Moreover, the
catheterization tray 200a may
be reused after the catheterization procedure (e.g., the catheterization tray
200a may be
sanitized and/or repacked for reuse).
[0243] In one embodiment, the catheterization tray may be at least
partially
disassembled (e.g., in addition to or in lieu of removing partitions). FIG.
37A illustrates a
catheterization system 100b that may include a catheterization tray 200b,
which may be
unfolded to access contents therein. As mentioned above, the catheterization
system 100b may
include a catheterization tray 200b that may be wrapped in a CSR sterile wrap
130b, which
may be unwrapped to access the catheterization tray 200b. For instance,
initially a flap or
portion 131b of the CSR sterile wrap 130b may be unfolded toward the patient
(e.g., thereby
covering the support surface with the sterile portion 13 lb of the CSR sterile
wrap 130b.
[0244] In one embodiment, the CSR sterile wrap 130b may include a pocket
132b,
which may be exposed when the CSR sterile wrap 130b is unwrapped to expose the
catheterization tray 200b. In one embodiment, during and/or after the
catheterization
-60-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
procedure, used items or trash may be placed in the pocket 132b. Subsequently,
the trash may
be disposed together with the CSR sterile wrap 130b.
[0245] A drape (e.g., the fenestrated drape 270) may be attached to the
CSR sterile
wrap 130b, such that after the unwrapping the CSR sterile wrap 130b, the drape
is exposed and
may be removed from the CSR sterile wrap 130b and used in the catheterization
procedure. In
one embodiment, the fenestrated drape 270 may be attached to the CSR sterile
wrap 130b in
any suitable manner, which may vary from one embodiment to the next. In one
example, the
fenestrated drape 270 may be attached to the CSR sterile wrap 130b with light
adhesive, tape,
tabs, etc.
[0246] As mentioned above, the catheterization tray 200b may be exposed
after
unwrapping the CSR sterile wrap 130b. The catheterization tray 200b may
include one or more
identifiers 260b, which may identify or suggest an order of step and/or use of
catheterization
components in the catheterization procedure (e.g., the identifiers may be on
one or more outer
surfaces of the catheterization tray 200b). For example, the identifiers 260b
may be located on
the top surface of the catheterization tray 200b and may indicate further
steps in the
catheterization procedure. According to one embodiment, a top panel 201b of
the
catheterization tray 200b may be opened to unfold the catheterization tray
200b and access the
catheterization components in the catheterization tray 200b.
[0247] As shown in FIG. 37B, after opening the catheterization tray 200b,
the user may
access the compartment 210b, which may contain the catheter assembly 190 for
the
catheterization procedure. For instance, the top panel 201b together with a
side panel 202b
(connected thereto) may be unfolded away from panels 203b, 204b, 205b. More
specifically,
the side panels 203b, 204b, stiffening ribs 205b may partially define the
periphery of the
compartment 210b. Unfolding the side panel 202b and the top panel 201b from
the side panels
203b, 204b, 205b may provide access to the compartment 210b through a side
(from which the
side panel 202b was unfolded) and through the top (where the top panel 201b
was removed).
In one embodiment, the side panel 202b and the top panel 201b may be unfolded
in a general
direction that is opposite to or away from the insertion site (e.g., in a
manner that would not
interfere with the catheterization procedure).
[0248] Moreover, on the interior surface of the panel 201b, the
catheterization tray
200b may include additional or alternative catheterization components, such as
the syringe 170
-61-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
and swabs 150. Such catheterization components may be attached or secured to
the top panel
201b and/or to the side panel 202b. For example, the syringe 170 may be
secured to the top
panel 201b with one or more straps 220b. Furthermore, in some instances, the
swabs 150 may
be secured to the top panel 201b within one or more pockets 230b. It should be
appreciated
that, the catheterization components (such as the syringe 170, swabs 150,
etc.) may be attached
or secured to the top panel 201b in any number of suitable ways and with any
number of
suitable mechanisms (e.g., adhesive, tabs, etc.). In any event, the
catheterization components
secured on the interior of the top panel 201b and/or side panel 202b may be
accessible and
removable therefrom after unfolding thereof away from the side panels 203b,
204b, 205b.
[0249]
Moreover, the side panel 202b and/or the top panel 201b may include one or
more procedural indicators 260b near one or more corresponding catheterization
components.
For example, procedural indicators 260b may identify the catheterization
components with a
number that may correspond with a suggested number of a step in a suggested
sequence of
steps for the catheterization procedure.
Also, the catheterization components and
corresponding procedural indicators 260b may be arranged in a sequential
manner, such that
the indicators with higher numbers and corresponding catheterization
components are
generally positioned increasingly farther from the insertion site. In other
words, the
catheterization components that are used earlier in the catheterization
procedure may be closer
to the insertion site, while the catheterization components that are used
later in the
catheterization procedure are located farther away from the insertion site
(based on the
orientation of the catheterization tray 200b).
[0250] In
one embodiment, the catheterization tray 200b may include a horizontal panel
207b, which may be connected to and extend from the side panel 204b (e.g., the
horizontal
panel 207b may be folded into the compartment 210b and/or may be approximately
parallel to
the support surface supporting the catheterization tray 200b). In some
instances, the horizontal
panel 207b may support and/or secure one or more catheterization components.
For example,
the horizontal panel 207b may secure a pouch 180b with lubricant that may be
removed
therefrom and used in the catheterization procedure.
[0251] In
one embodiment, the side panel 204b and horizontal panel 207b may be
unfolded away from the side panels 203b and 205b, as shown in FIG. 37C. For
instance,
unfolding the side panel 204b may provide access into the compartment 210b.
Furthermore,
one or more catheterization components may be attached to the side panel 204b
and/or to the
-62-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
interior side of the horizontal panel 207b, on the interior surface thereof
For example the
sanitizer packet 160 may be attached to the horizontal panel 207b and/or to
the side panel 204b
(e.g., with light adhesive). Also, the catheterization tray 200b may include
indicator 260b
identifying the step or particular order or place in the sequence of the
catheterization procedure
where the sanitizer packet 160 may be used.
[0252] As described above, the catheterization system may include a
catheterization
tray wrapped into a sterile wrap, which may be used during the catheterization
procedure. The
catheterization system may include one or more catheterization components
packaged by
and/or secured in a sterile wrap (e.g., directly in the sterile wrap without
the catheterization
tray). Such catheterization system may reduce waste or materials that are
thrown away after
the catheterization procedure. For instance, after completing the
catheterization material, the
sterile wrap may be disposed and/or composted to reduce long term waste from
the
catheterization system.
[0253] Following on the foregoing, FIGS. 38A, 39A, 40A, 41A, 42A, and 43A
provide
medical-procedure trays for medical-procedure packages (e.g., catheterization
trays for
catheterization packages) in accordance with some alternative embodiments to
previously
described medical-procedure trays. For expository expediency, features of
alternative
embodiments shared with medical-procedure trays previously described in detail
above will
not be described in similar detail below. It should be understood that
description for each of
the shared features is incorporated by reference, as appropriate, by the
shared feature's name
when describing alternative embodiments. For additional expository expediency,
not every
feature of alternative embodiments shared with previously described medical-
procedure trays
will be further described. Instead, it should be understood that shared visual
elements between
the alternative embodiments and the previously described medical-procedure
trays represent
shared features between the alternative embodiments and the previously
described medical-
procedure trays. Thus, the shared visual elements provide a basis for
incorporating by
reference, as appropriate, description for each of the shared features for the
alternative
embodiments. For example, FIGS. 2 and 38A provide medical-procedure trays
including
stiffening ribs. (See stiffening ribs 20 of FIG. 2 and the description
therefor.) While the
stiffening ribs of the alternative embodiment of FIG. 38A are not expressly
described, the
description for the stiffening ribs is incorporated by reference in view of
the shared visual
elements representing the stiffening ribs.
-63-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0254] FIG. 38A shows a view of another medical-procedure tray 3800 in
accordance
with some embodiments. FIG. 38B shows another view of the medical-procedure
tray 3800 of
FIG. 38A. FIG. 38C shows another view of the medical-procedure tray 3800 of
FIG. 38A
together with a cover 3801. FIG. 38D shows another view of the medical-
procedure tray 3801
of FIG. 38A together with the cover 3801.
[0255] The medical-procedure tray 3800 of FIGS. 38A, 38B, 38C, and 38D
can include
a first section 3810, a second section 3820, and a third section 3830. As
described herein, a
section of a medical-procedure tray can be designated for one or more
particular uses (e.g.,
well for iodine, section for storage, etc.), and a section can be at least
partially and up to wholly
separated from one or more other sections by one or more physical features
(e.g., one or more
partitions), thereby forming a compartment for one or more particular uses. As
shown, the
medical-procedure tray 3800 includes a first partition 3842 formed from the
medical-procedure
tray 3800 itself The first partition 3842 or at least a first section-facing
face of the first partition
3842 (see FIG. 38A) has a first height h1 equal to a height of the medical-
procedure tray 3800
at at least an end of the medical-procedure tray 3800 including the second
section 3820. The
first partition 3842 or at least the first section-facing face of the first
partition 3842 (see FIG.
38A) has a second height h2 less than the first height at at least an end of
the medical-procedure
tray 3800 opposite the second section 3820. In view of the first partition
3842, the first section
3810 can be considered a first compartment 3810 at least partially separated
from the third
section 3830 and wholly separated from the second section 3820. As further
shown, the
medical-procedure tray 3800 includes a second partition 3844 formed from the
medical-
procedure tray 3800 itself The second partition 3844 or at least a third
section-facing face of
the second partition 3844 (see FIG. 38A or 38B) has a first height equal to
the height of the
medical-procedure tray 3800. In view of the first partition 3842 and the
second partition 3844,
the second section 3820 can be considered a second compartment 3820 wholly
separated from
the first section 3810 and the third section 3830. Further in view of the
first partition 3842 and
the second partition 3844, the third section 3830 can be considered a third
compartment 3830
at least partially separated from the first section 3810 and wholly separated
from the second
section 3820.
[0256] As with previously described medical-procedure trays, the medical-
procedure
tray 3800 can include medical-procedure instructions imprinted directly on the
medical-
procedure tray 3800, at least some of which instructions can be revealed in
step with steps of
-64-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
the medical procedure. When the medical-procedure tray 3800 is incorporated
into a medical-
procedure package with contents for the medical procedure (e.g., a drainage
system including
a catheter, a container of lubricant, a syringe of water, a skin-preparation
kit, etc. for a
catheterization procedure), step-wise removal of the contents can reveal the
step-wise
instructions for the medical procedure imprinted on the medical-procedure tray
3800.
[0257] With respect to the first section or compartment 3810, the first
section 3810 can
be configured to accommodate a first set of contents for a medical procedure.
In some
embodiments, the medical procedure is a catheterization procedure and the
first set of contents
can include, but is not limited to, a drainage system including a catheter
(e.g., a Foley catheter),
drainage tubing, and a drainage bag. The first set of contents can further
include a syringe of
sterile water for inflating a balloon such as a balloon of a Foley catheter.
When the medical-
procedure tray 3800 is incorporated into a medical-procedure package, the
first section 3810
can include the first set of contents. For a visual reference, the medical-
procedure tray 3800 of
FIGS. 38A, 38B, 38C, and 38D is shown with a syringe of sterile water 3892. As
shown in
FIGS. 38A and 38B, the syringe of sterile water 3892 can be simply placed
alongside an inner
wall of the medical-procedure tray 3800 or fit into position alongside the
inner wall with a
loose-fit interference fit at both end of the syringe of sterile water 3892.
As shown in FIGS.
38C and 38D, the syringe of sterile water 3892 can still be placed alongside
the inner wall of
the medical-procedure tray 3800; however, the syringe of sterile water 3892
can be further
placed within a folded portion or retainer 3803 of the cover 3801.
[0258] Referring to FIG. 38C and 38D, the medical-procedure tray 3800 is
shown
together with the cover 3801 such as the packaging label 62 described in
reference to FIGS. 11
and 12, the identification cover 120 described in reference to FIG. 30A, or a
combination
thereof As further shown, the cover 3801 can be folded to form the retainer
3803 for retaining
certain contents of the medical-procedure package in the first section 3810.
For example, the
retainer 3803 can be configured for keeping the syringe of sterile water 3892
in the first section
3810 apart from certain other contents of the medical-procedure package in the
first section
3810. Other portions of the cover 3801 can be folded to form other retainers
for retaining
contents of the medical-procedure package in the appropriate sections. As
shown, the cover
3801 can be configured to fit within the first section 3810 of the medical-
procedure tray 3800;
however, the cover 3801 can be alternatively configured to fit within the
first section 3810 and
additional sections (e.g., the second section 3820, the third section 3830,
etc.) of the medical-
-65-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
procedure tray 3800 or portions thereof The cover 3801 can be alternatively
configured to fit
over the medical-procedure tray 4100 in any of the foregoing configurations up
to the whole
medical-procedure tray 4100 excepting folded or otherwise shaped portions of
the cover 3801
forming retainers for retaining contents of the medical-procedure package in
the appropriate
sections.
[0259] With respect to the second section or compartment 3820, the second
section
3820 can be configured to accommodate a second set of contents for the medical
procedure.
In some embodiments, the medical procedure is a catheterization procedure and
the second set
of contents can include, but is not limited to, a container containing
lubricant (e.g., lubricant
for a Foley catheter). When the medical-procedure tray 3800 is incorporated
into a medical-
procedure package, the second section 3820 can include the second set of
contents. For a visual
reference, the medical-procedure tray 3800 of FIGS. 38A and 38B is shown with
a container
of lubricant 3894.
[0260] Insofar as configuration of the second section or compartment 3820
for the
second set of contents, the second section 3820 can be configured as a
retainer to retain or
otherwise secure the second set of contents (e.g., the container of lubricant
3894) while
simultaneously providing open access for easy removal of the second set of
contents. The
second partition 3844 formed from the medical-procedure tray 3800 can have the
previously
described first height (e.g., equal to the height of the medical-procedure
tray 3800) at the third
section-facing face of the second partition 3844 (see FIG. 38A or 38B);
however, the second
partition 3844 can further have a second height at a second section-facing
face of the second
partition 3844. The second height at the second section-facing face of the
second partition
3844 can be less than the first height at the third section-facing face of the
second partition
3844, which, in turn, serves to elevate a bottom 3822 of the second section
3820 above a bottom
3812 of the first section 3810 or the tray 3800 (see FIG. 38B). Again, for a
visual reference,
the medical-procedure tray 3800 of FIGS. 38A and 38B is shown with the
container of lubricant
3894. The container of lubricant 3894 can be fit into position alongside an
inner wall (or a
protrusion thereof) of the medical-procedure tray 3800 shared with the second
section 3820
and the second section-facing face of the second partition 3844 with a loose-
fit interference fit
on both sides of the container of lubricant 3894, at both ends of the
container of lubricant 3894,
or a combination thereof. The bottom 3822 of the second section 3820 elevated
above the
-66-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
bottom 3812 of the first section 3810 or the tray 3800 provides for the open
access for easy
removal of the second set of contents.
[0261] With respect to the third section or compartment 3830, the third
section 3830
can be configured to accommodate a third set of contents for the medical
procedure. In some
embodiments, the medical procedure is a catheterization procedure and the
third set of contents
can include, but is not limited to, a specimen container, optionally, a label
for the specimen
container, and a skin-preparation kit including a packet of antiseptic (e.g.,
an iodophor such as
povidone-iodine, tincture of iodine, aqueous iodine, etc.) and one or more
swabs. When the
medical-procedure tray 3800 is incorporated into a medical-procedure package,
the third
section 3830 can include the third set of contents. The medical-procedure tray
3800 of FIGS.
38A and 38B is shown without the specimen container or the skin preparation
kit; however,
FIGS. 8, 26, and 27 provide visual references for inclusion of the one or more
swabs 34 in the
medical-procedure tray 3800, FIGS. 25-29 provide visual references for
inclusion of the
specimen container 54 in the medical-procedure tray 3800, and FIG. 25 provides
a visual
reference for inclusion of the packet of antiseptic 52 in the medical-
procedure tray 3800.
[0262] Insofar as configuration of the third section or compartment 3830
for the third
set of contents, the third section 3830 can be configured with one or more
additional sub-
sections or sub-compartments. As shown in FIGS. 38A and 38B, the one or more
additional
sub-sections or sub-compartments can include a swab compartment 3832, an
overflow
compartment 3834, and a corner storage compartment 3836. The swab compartment
3832, in
turn, can include a well 3831 and one or more channels, one channel of which
is indicated as
channel 3833. The well 3831 can be positioned at a base of the second
partition 3844, which
separates the second section 3820 from the third section 3830 as shown in
FIGS. 38A and 38B.
The well 3831 can be further positioned at a base of the one or more channels,
thereby adjoining
each channel of the one or more channels at a well-end thereof. The well 3831
can be
configured to contain a fluid such as the antiseptic of the medical-procedure
package when
poured therein.
[0263] The one or more channels can be configured to respectively hold
the one or
more swabs of the skin-preparation kit for subsequent use with the antiseptic
of the packet of
antiseptic. Each channel of the one or more channels can be configured to hold
a respective
swab of the one or more swabs optionally under at least one snap-in feature or
snap-in tab
positioned along a length or a longitudinal side of the channel. Any two or
more snap-in tabs,
-67-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
such as any three or more snap-in tabs, and, for example, any four or more
snap-in tabs
positioned along a length or longitudinal sides of a channel can be staggered
with respect to
each other. As shown between FIGS. 38A and 38B, each channel of the one or
more channels
can include three staggered snap-in tabs along the longitudinal sides thereof.
(See also FIG. 8
and the description therefor.) Each channel of the one or more channels can be
further
configured with an incline or an angle with respect to the bottom 3812 of the
tray 3800.
Furthermore, each channel of the one or more channels can be configured with a
delta or delta
shape at the well-end thereof adjoining the well 3831. With such a
configuration, a handle end
of a swab held under the snap-in tabs of a channel can be elevated with
respect to an absorbent-
head end of the swab when the absorbent-head end is positioned in an
accommodating delta
therefor. As such, an absorbent head of a swab can angle downwardly into the
well 3831 of
the swab compartment 3832 of the third section 3830 and an elongate member of
the swab can
angle upwardly for gripping and removal at an appropriate step of unboxing the
medical-
procedure package containing the tray 3800 and the swab.
[0264] The overflow compartment 3834 can be positioned at an end of the
one or more
channels opposite the well 3831. The overflow compartment 3834 can be fluidly
connected to
the well 3831 through the one or more channels, each channel of which can be
further
configured to convey a fluid (e.g., the antiseptic of the skin-preparation
kit) along its length
when the fluid is poured therein or thereon. Should a medical procedure
require additional
antiseptic for skin preparation or the like, the additional antiseptic can be
safely added to the
well 3831 with assurance that any volume of antiseptic exceeding a total
volume of the swab
compartment 3832 (e.g., a combined volume of the well 3831 and the one or more
channels)
can spill into the overflow compartment 3834 via the one or more channels.
[0265] With respect to the corner storage compartment 3836, the corner
storage can be
positioned between the overflow compartment 3834 and an inner wall of the tray
3800, and,
thereby share the inner wall of the tray 3800. The corner storage compartment
3836 can be
configured to accommodate the specimen container of the medical-procedure
package.
Furthermore, while the medical-procedure package can include the packet of
antiseptic in or
over the swab compartment 3832 and the overflow compartment 3834 as shown in
FIG. 25,
the corner storage compartment 3836 can be further configured to accommodate
at least a
portion of the packet of antiseptic. In such a configuration, the packet of
antiseptic can be
placed in or over the swab compartment 3832, the overflow compartment 3834,
the corner
-68-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
storage compartment 3836, or a combination thereof in a medical-procedure
package
containing the tray 3800.
[0266] FIG. 39A shows a view of another medical-procedure tray 3900 in
accordance
with some embodiments. FIG. 39B shows another view of the medical-procedure
tray 3900 of
FIG. 39A.
[0267] The medical-procedure tray 3900A of FIGS. 39A and 39B can have a
configuration similar to the medical-procedure tray 3800 described in
reference to FIGS. 38A,
38B, 38C, and 38D. As such, the tray 3900A can likewise include a first
section or first
compartment 3910, a second section or second compartment 3920, and a third
section of third
compartment 3930, each of which can be configured to accommodate and hold
contents for a
medical procedure. However, as shown, the first section 3910 of the tray 3900A
can have a
different configuration than the first section 3810 of the tray 3800. For
example, the first
section 3810 of the tray 3800 of FIGS. 38A, 38B, 38C, and 38D has more of a
square-shaped
footprint, whereas the first section 3910 of the tray 3900A of FIGS. 39A and
39B has more of
a rectangle-shaped footprint. This results from sides of the tray 3900A about
the first section
3910 (e.g., a side of the tray 3900A adjacent a syringe of sterile water 3892
and an opposite
side thereof) being longer than similar sides of the tray 3800 about the first
section 3810.
Configuring the first section 3910 in this way allows for a comparatively
larger capacity for
comparatively more contents of the medical-procedure package including the
tray 3900A.
[0268] Not only can the first section 3910 of the tray 3900A have a
different
configuration than the first section 3810 of the tray 3800, but one or more
partitions of the tray
3900A can have a different configuration than the one or more partitions of
the tray 3800.
While not shown in FIGS. 39A and 39B, the different configuration can include
a
disproportionally or proportionally shorter height for the one or more
partitions of the tray
3900A compared to the one or more partitions of the tray 3800, which one or
more partitions
can have a height (e.g., a maximum height) equal to a height of the tray. For
an example of a
disproportionally shorter height, a first partition 3942 of the tray 3900A can
have a
disproportionally shorter height than the first partition 3842 of the tray
3800, wherein an
analogous first height h1 of the first partition 3942 can be shorter than the
first height h1 of the
first partition 3842, and wherein an analogous second height h2 of the first
partition 3942 can
be substantially the same as the second height h2 of the first partition 3842.
FIG. 41B provides
a visual reference for such a disproportionally shorter height for the first
partition 3942
-69-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
compared to the first partition 3842 with the understanding that the first
partition 3942 of the
tray 3900A continues to the inner wall of the tray 3900A as shown in FIGS 39A
and 39B. For
an example of a proportionally shorter height, a second partition 3944 of the
tray 3900A can
have a proportionally shorter height than the second partition 3844 of the
tray 3800. FIG. 41B
also provides a visual reference for such a proportionally shorter height for
the second partition
3944 compared to the second partition 3844. Configuring the one or more
partitions of the tray
3900A to be disproportionally or proportionally shorter in height than the one
or more
partitions of the tray 3800 allows for a bottom of the second section 3920 to
be lower than the
bottom 3822 of the second section 3820 of the tray 3800 while still providing
open access for
easy removal of the container of lubricant 3894. For example, the bottom of
the second section
3920 can be substantially co-planar with a bottom of the first section 3910 or
a bottom of the
tray 3900A. FIG. 41B again provides a visual reference for such a bottom of
the second section
3920 of the tray 3900A with the understanding that the first partition 3942 of
the tray 3900A
continues to the inner wall of the tray 3900A as shown in FIGS 39A and 39B.
The embodiment
of the co-planar bottom of the second section 3920 of the tray 3900A and that
of the elevated
bottom 3822 of the tray 3800 provide open access for easy removal of the
container of lubricant
3894 in that both embodiments do not have the container of lubricant 3894
ensconced too
deeply in a second-section well.
[0269] While embodiments are disclosed for an elevated second-section
bottom of a
tray (e.g., the elevated bottom 3822 of the second section 3820 of the tray
3800) and a second-
section bottom co-planar with a bottom of the tray (e.g., the co-planar bottom
of the second
section 3920 of the tray 3900A), it should be understood that a bottom of any
compartment of
any medical-procedure tray disclosed herein can vary in elevation from a
bottom of the tray in
accordance with that described for the trays 3800 and 3900A.
[0270] FIG. 39C shows a view of a medical-procedure tray 3900B including
a number
of retainers in a first configuration in accordance with some embodiments.
[0271] The medical-procedure tray 3900B of FIG. 39C can have a
configuration similar
to the medical-procedure tray 3900A described in reference to FIGS. 39A and
39B. As such,
the tray 3900B can likewise include a first section 3910, a second section
3920, and a third
section 3930, each of which can be configured to accommodate and hold contents
for a medical
procedure. However, as shown, the first section 3910 of the tray 3900B can
have a different
configuration than the first section 3910 of the tray 3900A. For example, the
first section 3910
-70-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
of the tray 3900B of FIG. 39C can have a number of retainers, one retainer of
which is indicated
as retainer 3912. In contrast, the first section 3910 of the tray 3900A of
FIGS. 39A and 39B is
shown without the number of retainers; however, it should be understood that
the tray 3900A,
as well as any tray provided herein, can optionally include one or more
retainers of any type
provided herein. The number of retainers in the first section 3910 of the tray
3900B can be
configured for retaining certain contents of the medical-procedure package in
the first section
3910. For example, the number of retainers can be configured for keeping the
syringe of sterile
water 3892 in the first section 3910 apart from certain other contents of the
medical-procedure
package in the first section 3910. Any of a number of different types of
retainers (e.g., column
or tapered column-type retainers, rack-type retainers, tab-type retainers,
removable retainers,
etc.) can be formed, and any of a number of retainers (e.g., 1, 2, 3, 4, or 5
or more retainers)
can be formed ¨ some of which retainers can be formed from the tray 3900B
itself. As shown,
in FIG. 39C, the first section 3910 of the tray 3900B can include at least 3
tapered column-type
retainers equidistant from the side of the tray 3900B adjacent the syringe of
sterile water 3892.
[0272] FIG. 39D shows a view of a medical-procedure tray 3900C including
a number
of retainers in a second configuration in accordance with some embodiments.
[0273] The medical-procedure tray 3900C of FIG. 39D can have a
configuration
similar to the medical-procedure tray 3900B described in reference to FIG.
39C. As such, the
tray 3900C can likewise include a first section 3910, a second section 3920,
and a third section
3930, each of which can be configured to accommodate and hold contents for a
medical
procedure. However, as shown, the first section 3910 of the tray 3900C can
have a different
configuration than the first section 3910 of the tray 3900B. For example, the
first section 3910
of the tray 3900C of FIG. 39D can similarly have at least 3 tapered column-
type retainers, one
retainer of which is indicated as retainer 3912, and another retainer of which
is indicated as
retainer 3913. However, in contrast to the at least 3 tapered column-type
retainers equidistantly
spaced from the side of the tray 3900B, at least one retainer (e.g., the
retainer 3913) of the at
least 3 tapered column-type retainers of the tray 3900C can be offset from the
others as shown
by the dotted line. Such a different configuration can further restrict
degrees of freedom of
certain contents (e.g., the syringe of sterile water 3892) of the medical-
procedure package in
the first section 3910. As such, such a different configuration can further
keep such certain
contents in the first section 3910 apart from certain other contents of the
medical-procedure
package in the first section 3910.
-71-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0274] FIG. 40A shows a view of another medical-procedure tray 4000 in
accordance
with some embodiments. FIG. 40B shows another view of the medical-procedure
tray 4000 of
FIG. 40A.
[0275] The medical-procedure tray 4000 of FIGS. 40A and 40B can have a
configuration similar to the medical-procedure tray 3900A described in
reference to FIGS. 39A
and 39B. As such, the tray 4000 can likewise include a first section or first
compartment 4010,
a second section or second compartment 4020, and a third section or third
compartment 4030,
each of which can be configured to accommodate and hold contents for a medical
procedure.
However, as shown, the second section 4020 of the tray 4000 can have a
different configuration
than the second section 3920 of the tray 3900A. For example, the second
section 4020 of the
tray 4000 can be an extended section extending into the first section 4010,
whereas the second
section 3920 of the tray 3900A terminates at a common partition to both the
second section
3920 and the third section 3930 of the tray 3900A, namely at the first
partition 3942. The
extended second section 4020 of the tray 4000 can be formed from an extended
second partition
4044 extending into the first section 4010 and terminating at a third
partition 4046 in the first
section 4010 of the tray 4000. In addition, a first partition 4042 separating
the third section
4030 from the first section 4010 can terminate at a medial portion of the
extended second
partition 4044 instead of at an inner wall of the tray 4000. With the second
section 4020
configured as an extended section, the extended second section 4020 can
accommodate more
contents, larger contents, or both as needed for a medical procedure. As
shown, the extended
second section 4020 includes a larger container of lubricant 4094 compared to
the container of
lubricant 3894 shown in FIGS. 39A and 39B.
[0276] FIG. 41A shows a view of another medical-procedure tray 4100 in
accordance
with some embodiments. FIG. 41B shows another view of the medical-procedure
tray 4100 of
FIG. 41A. FIG. 41C shows a third view of the medical-procedure tray 4100 of
FIG. 41A.
[0277] The medical-procedure tray 4100 of FIGS. 41A, 41B, and 41C can
include a
first section 4110, a second section 4120, and a third section 4130, each of
which can be
configured to accommodate and hold contents for a medical procedure. As
described herein,
a section of a medical-procedure tray can be designated for one or more
particular uses, and a
section can be at least partially (e.g., the second section 4120) and up to
wholly (e.g., the third
section 4130) separated from one or more other sections by one or more
physical features (e.g.,
one or more partitions), thereby forming a compartment for one or more
particular uses. As
-72-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
shown, the tray 4100 includes a first partition 4142 and a second partition
4144 wholly
separating the third section 4130 from the first section 4110 and the second
section 4120. In
addition, the second partition 4144 partially separates the second section
4120 from the first
section 4110. Like the medical-procedure tray 3800 of FIGS. 38A and 38B, the
first partition
4142 of the tray 4100 ¨ or at least a first section-facing face of the first
partition 4142 ¨ can
have a first height hi and a second height h2 (see FIG. 41B), and the second
partition 4144 ¨ or
at least the third section-facing face of the second partition 4144 ¨ can have
a first height (not
shown). Unlike the tray 3800 of FIGS. 38A and 38B, the first height of the
first partition 4142
and the first height of the second partition 4144 are, as shown, less than a
height of the medical-
procedure tray 4100. In addition, a second section-facing face of the second
partition 4144 can
have a height equal to the height of the third section-facing face of the
second partition 4144
making a bottom of the second section 4120 co-planar with a bottom of the
first section 4110
or the tray 4100. (See FIG. 41B.)
[0278] With respect to the first section 4110 of the tray 4100, the first
section 4110 can
be configured to accommodate a first set of contents for a medical procedure
such as a
catheterization procedure. As previously described, the first set of contents
can include, but is
not limited to, the drainage system including the catheter (e.g., the Foley
catheter), the drainage
tubing, the drainage bag, and the syringe of sterile water 3892 for inflating
the balloon of the
Foley catheter. For a visual reference, the syringe of sterile water 3892 is
shown mounted in
or on a retainer (e.g., a rack-type retainer) of the first section 4110 of a
different type than the
retainers (e.g., tapered column-type retainers) described in reference to
trays 3900B and 3900C
respectively of FIGS. 39C and 39D. Like the number of retainers of trays 3900B
and 3900C,
the retainer for the syringe of sterile water 3892 can be formed of the tray
4100 itself and
configured to keep the syringe of sterile water 3892 in the first section 4110
apart from other
contents of the medical-procedure package in the first section 4110. The
syringe of sterile
water 3892 can simply lay in the retainer or rack, or the syringe of sterile
water 3892 can be fit
into the retainer or rack with a loose-fit interference fit about the syringe
of sterile water 3892.
[0279] With respect to the second section 4120 of the tray 4100, the
second section
4120 can be configured to accommodate a second set of contents for the medical
procedure
such as the catheterization procedure. As previously described, the second set
of contents can
include, but is not limited to, the container containing lubricant (e.g.,
lubricant for the Foley
catheter) such as a syringe of lubricant 4194. For a visual reference, the
syringe of lubricant
-73-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
4194 is shown mounted in or on a retainer 4114 (e.g., a rack-type retainer)
like that described
in reference to the retainer in the first section 4110 for the syringe of
sterile water 3892. The
syringe of lubricant 4194 can simply lay in the retainer or rack 4194, or the
syringe of lubricant
4194 can be fit into the retainer or rack 4194 with a loose-fit interference
fit about the syringe
of lubricant 4194.
[0280] The retainer 4194 and the retainer for the syringe of sterile
water 3892 can have
the same or different dimensions as required by the size of the syringe of
lubricant 4194 and
the size of the syringe of sterile water 3892 required by the medical
procedure. When the size
of the syringe of lubricant 4194 and the size of the syringe of sterile water
3892 are the same,
the retainers or racks therefor can have the same dimensions, and the syringe
of lubricant 4194
and the syringe of sterile water 3892 can be swapped with respect to their
placement in the
medical-procedure package, for example, to better orchestrate steps of the
medical procedure.
Therefore, the second section 4120 is not limited to accommodating and holding
the syringe of
lubricant 4194, and the first section 4110 is not limited to accommodating and
holding the
syringe of sterile water 3892. The second section 4120 can be configured to
accommodate and
hold the syringe of sterile water 3892, and the first section 4110 can be
configured to
accommodate the syringe of lubricant 4194 ¨ even if the size of the syringe of
lubricant 4194
and the size of the syringe of sterile water 3892 are different.
[0281] With respect to the third section 4130, the third section 4130
includes a swab
compartment 4132, an overflow compartment 4134, and a corner storage
compartment 4136
configured as described herein to accommodate a third set of contents for the
medical procedure
such as the catheterization procedure. As previously described, the third set
of contents can
include, but is not limited to, the specimen container, optionally, the label
for the specimen
container, and the skin-preparation kit including the packet of antiseptic
(e.g., an iodophor such
as povidone-iodine, tincture of iodine, aqueous iodine, etc.) and the one or
more swabs. The
medical-procedure tray 4100 of FIGS. 41A, 41B, and 41C is shown without the
specimen
container or the skin preparation kit for which the third section 4130 is
configured; however,
FIGS. 8, 26, and 27 provide visual references for inclusion of the one or more
swabs 34 in the
swab compartment 4132, FIGS. 25-29 provide visual references for inclusion of
the specimen
container 54 in the corner storage compartment 4136, and FIG. 25 provides a
visual reference
for inclusion of the packet of antiseptic 52 in the medical-procedure tray
4100 over the swab
compartment 4132 and the overflow compartment 4134.
-74-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
[0282] Adverting to FIG. 41C, the medical-procedure tray 4100 is shown
together with
a cover 4101 such as the packaging label 62 described in reference to FIGS. 11
and 12, the
identification cover 120 described in reference to FIG. 30A, or a combination
thereof. As
shown, the cover 4101 can be configured to fit over the whole medical-
procedure tray 4100;
however, the cover 4101 can be alternatively configured to fit over a portion
of the medical-
procedure tray 4100, the portion including any one or more sections selected
from the first
section 4110, the second section 4120, and the third section 4130. The cover
4101 can be
alternatively configured to fit within the medical-procedure tray 4100 in any
of the foregoing
configurations such as within the whole medical-procedure tray 4100 or a
portion of the
medical-procedure tray 4100. (See, for example, cover 3801 of FIGS. 38C and
38D.) As
further shown, the tray 4100 can be configured with an optional support 4102
in the first section
4110 of the tray 4100. The support 4102 can prevent caving of the cover 4101
and damage to
the contents of the tray 4100 thereunder when one or more medical-procedure
packages are
stacked on the medical-procedure package including the medical-procedure tray
4100 and the
cover 4101. The support can be configured with a height substantially equal to
a height of the
tray 4100 and a width sufficient to withstand the weight of a stack of one or
more medical-
procedure packages stacked thereon.
[0283] FIG. 42A shows a view of another medical-procedure tray 4200 in
accordance
with some embodiments. FIG. 42B shows another view of the medical-procedure
tray 4200 of
FIG. 42A.
[0284] The medical-procedure tray 4200 of FIGS. 42A and 42B can have a
configuration similar to the medical-procedure tray 4100 described in
reference to FIGS. 41A
and 41B. As such, the tray 4200 can likewise include a first section 4210, a
second section
4220, and a third section 4230, each of which can be configured to accommodate
and hold
contents for a medical procedure. The first section 4210, for example, can be
configured to
accommodate the previously described first set of contents for the
catheterization procedure
including the drainage system including the catheter (e.g., the Foley
catheter), the drainage
tubing, the drainage bag, and the syringe of sterile water 3892 for inflating
the balloon of the
Foley catheter. For a visual reference, the syringe of sterile water 3892 is
shown mounted in
or on a rack-type retainer of the first section 4210. In addition, the first
section can be
configured with an optional support 4202A to prevent caving of a cover placed
thereon or
thereover as described herein. The second section 4220, for example, can be
configured to
-75-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
accommodate the previously described second set of contents for the
catheterization procedure
including the container containing lubricant (e.g., lubricant for the Foley
catheter) such as a
syringe of lubricant 4194. For a visual reference, the syringe of lubricant
4194 is shown
mounted in or on a rack-type retainer 4214 in the second section 4220. The
third section 4230,
for example, can be configured with a swab compartment 4232, an overflow
compartment
4234, and a corner storage compartment 4236 configured as described herein to
accommodate
the previously described third set of contents for the catheterization
procedure including the
specimen container, optionally, the label for the specimen container, and the
skin-preparation
kit including the packet of antiseptic (e.g., an iodophor such as povidone-
iodine, tincture of
iodine, aqueous iodine, etc.) and the one or more swabs.
[0285] With respect to the third section 4230, however, the third section
4230 of the
tray 4200 can have a different configuration than, for example, the third
section of any one tray
of the trays 3800, 3900A-C, 4000, and 4100 in that at least the orientation of
the third section
4230 of the tray 4200 is in a reversed orientation with respect to any one
tray of the other
foregoing trays. For example, the orientation of the third section 3830 of the
tray 3800 is such
that the well 3831 of the swab compartment 3832 or a well-end of the third
section 3830 is
adjacent the second section 3820 of the tray 3800. In such an orientation, the
corner storage
compartment 3836 shares with the tray 3800 an inner wall of the tray 3800. In
contrast, the
reversed orientation of the third section 4230 of the tray 4200 is such that
the well (not shown)
of the swab compartment 4232 or a well-end of the third section 4230 shares
with the tray 4200
an inner wall of the tray 4200. In such an orientation, the corner storage
compartment 4236 is
adjacent the second section 4220 of the tray 4200. Any one or more of the
trays provided
herein including any one or more of the trays selected from the trays 3800,
3900A-C, 4000,
and 4100 can be configured with a third section in the reverse orientation.
[0286] Adverting to FIG. 42B, the medical-procedure tray 4200 is shown
together with
a cover 4201 such as the packaging label 62 described in reference to FIGS. 11
and 12, the
identification cover 120 described in reference to FIG. 30A, or a combination
thereof. As
shown, the cover 4201 can be configured to fit over the whole medical-
procedure tray 4200;
however, the cover 4201 can be alternatively configured to fit over a portion
of the medical-
procedure tray 4200, the portion including any one or more sections selected
from the first
section 4210, the second section 4220, and the third section 4230. The cover
4201 can be
alternatively configured to fit within the medical-procedure tray 4200 in any
of the foregoing
-76-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
configurations such as within the whole medical-procedure tray 4200 or a
portion of the
medical-procedure tray 4200. (See, for example, cover 3801 of FIGS. 38C and
38D.) As
further shown, the tray 4200 can be configured with an optional support 4202B
in the first
section 4110 of the tray 4100, which support 4202B is shown in a different
location of the first
section 4110 than the optional support 4202A of FIG. 42A. The optional
supports 4202A and
4202B can vary in location in in accordance with packaging and stacking needs.
Again, a
support such as the support 4202B can prevent caving of the cover 4201 and
damage to the
contents of the tray 4200 thereunder when one or more medical-procedure
packages are stacked
on the medical-procedure package including the medical-procedure tray 4200 and
the cover
4201. Each support of the supports 4202A and 4202B can be configured with a
height
substantially equal to a height of the tray 4200 and a width sufficient to
withstand the weight
of a stack of one or more medical-procedure packages stacked thereon.
[0287] FIG. 43 shows a view of another medical-procedure tray 4300 in
accordance
with some embodiments.
[0288] The medical-procedure tray 4300 of FIG. 43 can have a
configuration similar to
the medical-procedure tray 4200 described in reference to FIGS. 42A and 42B.
As such, the
tray 4300 can likewise include a first section 4310, a second section 4320,
and a third section
4330 in the reverse orientation described in reference to tray 4200. Each of
the first section
4310, the second section 4320, and the third section 4330 can be configured to
accommodate
and hold contents for a medical procedure. The first section 4310, for
example, can be
configured to accommodate the previously described first set of contents for
the catheterization
procedure including the drainage system including the catheter (e.g., the
Foley catheter), the
drainage tubing, the drainage bag, and the syringe of sterile water for
inflating the balloon of
the Foley catheter. While not shown, the first section 4310 can be configured
with a tab-type
retainer to retain or otherwise secure the syringe of sterile water. The
second section 4320, for
example, can be configured to accommodate the previously described second set
of contents
for the catheterization procedure including the container containing lubricant
(e.g., lubricant
for the Foley catheter), which container of lubricant can be retained or
otherwise secured in the
second section 4320 by a tab-type retainer 4314 in the second section 4320.
The reverse-
orientation third section 4330, for example, can be configured with a swab
compartment 4332,
an overflow compartment 4334, and a corner storage compartment 4336 configured
as
described herein to accommodate the previously described third set of contents
for the
-77-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
catheterization procedure including the specimen container, optionally, the
label for the
specimen container, and the skin-preparation kit including the packet of
antiseptic (e.g., an
iodophor such as povidone-iodine, tincture of iodine, aqueous iodine, etc.)
and the one or more
swabs. Like the tray 4200 of FIGS. 42A and 42B, the corner storage compartment
4336 is
adjacent the second section 4320 of the tray 4300; however, the second section
4320 of the tray
4300 extends from an inner wall of the first section 4310 of the tray 4300 all
the way to an
inner wall on an opposite side of the tray 4300 shared by the third section
4330, whereas the
second section 4220 of the tray 4200 does not. That being said, the second
section 4220 of the
tray 4200 can be configured to likewise extend from an inner wall of the first
section 4210 of
the tray 4200 all the way to an inner wall on an opposite side of the tray
4200 shared by the
third section 4230.
[0289] FIG. 44A shows a view of a removable retainer 4412 on a partition
or a side of
a medical-procedure tray in accordance with some embodiments. FIG. 44B shows
another
view of the removable retainer 4412 on the partition or the side of the
medical-procedure tray
with a concealed tab of instructions in accordance with some embodiments. FIG.
44C shows
another view of the removable retainer on the partition or the side of the
medical-procedure
tray with a revealed tab of instructions in accordance with some embodiments.
[0290] Any medical-procedure tray provided herein can optionally include
any number
of retainers of any type selected from at least column or tapered column-type
retainers, rack-
type retainers, and tab-type retainers for retaining certain contents of the
medical-procedure
package in the appropriate sections. For example, the tray 3900A can include
three tapered
column-type retainers providing the tray 3900B in which the three tapered
column-type
retainers retain or otherwise keep the syringe of sterile water 3892 in the
first section 3910
apart from other contents of the medical-procedure package including the other
contents in the
first section 3810. (See FIG. 39C and the retainer 3912 in the first section
3910 retaining the
syringe 3892.) Furthermore, any medical-procedure tray provided herein can
optionally
include one or more appendage-type retainers for retaining certain contents of
the medical-
procedure package in the appropriate sections. For example, as shown in FIG.
45 an
appendage-type retainer 4512 can include a clip section 4593 for clipping the
syringe of sterile
water 3892 (or the container of lubricant 3894) to the appendage-type retainer
4512, and the
appendage-type retainer 4512 can include an appending belt or strap section
4514 for
appending the clip section 4593 to either the tray (e.g., a side of the tray
as shown) or a cover
-78-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
(e.g., an underside of the cover) therefor. Instructions for one or more steps
of a medical
procedure can be printed on or imprinted in a visible surface of the belt or
strap section 4514.
[0291] Whether or not a medical-procedure tray includes any of the
foregoing retainers
(e.g., the column or tapered column-type retainers, the rack-type retainers,
the tab-type
retainers, and the appendage-type retainers), the medical-procedure tray can
include one or
more removable retainers. An example of a removable retainer is the removable
retainer 4412
of FIGS. 44A, 44B, and 44C, which is configured to keep the syringe of sterile
water 3892 and
the container of lubricant 3894 apart from other contents of the medical-
procedure package.
However, it should be understood that removable retainers are not limited to
the removable
retainer 4412. For example, a removable retainer can be configured to retain
only the syringe
of sterile water 3892 or only the container of lubricant 3894. Furthermore, a
removable retainer
can be configured to retain other contents of the medical-procedure package in
the appropriate
sections therefor.
[0292] As shown in FIGS. 44A, 44B, and 44C, a removable retainer such as
the
removable retainer 4412 can include a clip such as clip 4414 for removably
attaching the
removable retainer to a partition or a side of a medical-procedure tray. The
clip can be
configured to clip the removable retainer onto a partition or a side of a
medical-procedure tray
such that the removable retainer is either inside the medical-procedure tray
(e.g., as packaged)
or outside the medical-procedure tray (e.g., when in use during a medical
procedure). The clip
can be configured to clip the removable retainer onto a partition or a side of
a medical-
procedure tray with a clip force sufficient to restrict slideable movement of
the removable
retainer along the side or the partition of a medical-procedure tray.
Alternatively, the clip can
be configured to allow such slideable movement. When the clip is configured to
allow slideable
movement of the removable retainer along a partition or a side of a medical-
procedure tray,
less clip force or another captivating means other than clip force can be used
to captivate or
otherwise hold the removable retainer on the partition or the side of a
medical-procedure tray
until the removable retainer is deliberately removed.
[0293] The slideable movement of the removable retainer can be used to
reveal or
conceal instructions for one or more steps of a medical procedure. As shown in
FIG. 44B, a
removable retainer such as the removable retainer 4412 can be packaged on an
inside of a
medical-procedure tray concealing a folded-down tab of the instructions for
the one or more
steps of the medical procedure. While the folded-down tab of the instructions
is shown in
-79-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
FIGS. 44B and 44C on an outside of the medical-procedure tray, the tray can be
alternatively
configured with the folded-down tab of the instructions on the inside of the
tray. Regardless
of the outside or the inside configuration of the folded-down tab of the
instructions, the
removable retainer can be slid aside as shown in FIG. 44B to reveal the
instructions shown in
FIG. 44C, which instructions can be printed or imprinted on the tab. The
instructions can again
be concealed by pushing the tab of the instructions in toward the inside or
the outside of the
tray and sliding the removable retainer thereover. Concealing the instructions
in the folded-
down tab can reduce an amount of information a health care provider needs to
know to only
the information immediately needed to perform one or more immediate steps of
the medical
procedure. This can, in turn, reduce errors in the medical procedure.
Furthermore, the folded-
down tab of the instructions can further conceal sharp edges of the tab that
might otherwise
compromise sterile packaging if left in an unfolded or extended position.
[0294] Again, the removable retainer 4412 of FIGS. 44A, 44B, and 44C is
an example
of a removable retainer configured to keep the syringe of sterile water 3892
and the container
of lubricant 3894 apart from other contents of the medical-procedure package.
As shown in
FIGS. 44A, 44B, and 44C, the removable retainer 4412 can include another,
wider clip (e.g.,
wider than clip 4414) configured with a first clip section 4493 for clipping
the syringe of sterile
water 3892 and a second clip section 4495 for the container of lubricant 3894.
Depending upon
dimensions of the syringe of sterile water 3892 and the container of lubricant
3894, the first
clip section 4493 and the second clip section 4495 can have the same
dimensions as each other
or different dimensions from each other. Regardless, the syringe of sterile
water 3892 and the
container of lubricant 3894 can be arranged in the removable retainer 4412 in
step with one or
more steps of a medical procedure. For example, instructions for the one or
more steps of the
medical procedure might recommend connecting the syringe of sterile water 3892
to a Foley
catheter in a first step, and the instructions might recommend lubricating the
Foley catheter in
a second step.
[0295] While the invention has been described in terms of particular
variations and
illustrative figures, those of ordinary skill in the art will recognize that
the invention is not
limited to the variations or figures described. In addition, where methods and
steps described
above indicate certain events occurring in certain order, those of ordinary
skill in the art will
recognize that the ordering of certain steps may be modified and that such
modifications are in
accordance with the variations of the invention. Additionally, certain of the
steps may be
-80-
CA 03058887 2019-10-02
WO 2018/190865 PCT/US2017/027628
performed concurrently in a parallel process when possible, as well as
performed sequentially
as described above. Therefore, to the extent there are variations of the
invention, which are
within the spirit of the disclosure or equivalent to the inventions found in
the claims, it is the
intent that this patent will cover those variations as well.
-81-