Note: Descriptions are shown in the official language in which they were submitted.
PRODUCING HYDROCARBONS FROM SUBTERRANEAN
RESERVOIR WITH SOLVENT INJECTION AT CONTROLLED
SOLVENT DENSITY
FIELD
[0001] This disclosure relates generally to in situ hydrocarbon production
with
solvent injection, and particularly to methods for in situ hydrocarbon
production with
injection of a supercritical solvent or a gas phase solvent.
BACKGROUND
[0002] Recovery of viscous hydrocarbons from subterranean reservoirs can be
facilitated by injection of a suitable solvent into the reservoir, such as
propane, butane
or the like. The solvent can function as a diluent for viscous hydrocarbons.
When the
injected solvent is heated, it may also transfer heat to the hydrocarbons or
the
reservoir. Both effects can reduce the viscosity of viscous hydrocarbons and
increase
their mobility, thus facilitating or improving production of hydrocarbons from
the
reservoir.
[0003] In a solvent-based recovery process, the solvent is injected without
steam
during a production stage of the recovery process. In a solvent-driven
recovery
process, a solvent is co-injected with steam during the production stage where
the
amount of injected steam is less than the amount of the injected solvent.
[0004] In known solvent-based recovery processes implemented on a
commercial
scale, solvents were typically injected in a gas phase. It is generally
considered
desirable to inject the solvent at higher pressures, as the oil production
rate is typically
higher at a higher injection pressure. It is expected that a higher injection
pressure
would drive the reservoir fluid to flow faster in the reservoir. It is also
expected that a
1
CA 3060497 2019-10-29
higher injection pressure would allow the solvent to be injected at a higher
rate and
allow the solvent to condense at a higher temperature, both of which would
increase
the rate of mobilizing the viscous hydrocarbons in the reservoir.
[0005] It has also been previously proposed that injecting a solvent in the
gas
phase at a temperature above, but close to, the boiling point of the solvent
at the
reservoir conditions would be optimal or desirable as it would reduce energy
consumption and achieve efficient production performance, as compared to
injecting
the solvent in the liquid phase or injecting the solvent in the gas phase but
at much
higher temperatures.
[0006] lmanbayev et al., in "Supercritical solvent extraction of oil sand
bitumen",
AIP Conference Proceedings 1879, 0500003 (2017), also disclosed experiments
performed in an autoclave reactor for studying supercritical solvent
extraction of
bitumen from oil sand. Isopropanol and hexane were used as the solvents. For
hexane, the test temperature was 255 C and the test pressure was 29.6 atm (-
3.0
MPa); for isopropanol, the test temperature was 297 C and the test pressure
was
54.8 atm (-5.55 MPa).
[0007] Kharutdinov et al., in "Supercritical Fluid Propane-Butane
Extraction
Treatment of Oil-Bearing Sands", in Theoretical Foundations of Chemical
Engineering, May 2017, Vol. 51, No.3, pp. 288-294, disclosed results of an
experimental study using liquid and supercritical fluid extraction to isolate
hydrocarbons from oil-bearing sands. A mixture of 75 wt% propane and 25 wt%
butane was used as the extracting agent, in extraction processes carried out
at
temperatures of 80 C to 140 C and pressures of 5 to 10 MPa in an
experimental unit.
The critical point temperature of the mixture was 386 K (-113 C) and the
critical point
pressure of the mixture was 4.31 MPa.
[0008] CA 2,767,874 to Meyer disclosed a proposed process for extracting
and
upgrading heavy hydrocarbon mixture by injecting supercritical or near-
supercritical
CO2 at a temperature of around the critical temperature and a pressure of
around the
critical pressure. The temperature in the deposit may be between 25 C to 120
C, and
2
CA 3060497 2019-10-29
the pressure may be 7.4 to 30 MPa. Test results were obtained using a core
flooding
apparatus or in a closed stainless steel cell.
[0009] It remains desirable to improve overall production efficiency in
solvent-based
recovery processes and other recovery processes involving solvents, and
challenges
remain in providing such recovery processes for efficient and effective
commercial
applications.
SUMMARY
[0010] In an aspect of the present disclosure, there is provided a method
of
producing hydrocarbons from a subterranean reservoir, comprising injecting a
solvent
at an injection pressure and an injection temperature into the reservoir to
mobilize
viscous hydrocarbons in the reservoir, wherein the injection pressure and
injection
temperature are selected and matched such that, the solvent has a reduced
density of
less than 0.5 and a second derivative of the reduced density with respect to
temperature is less than 1, at the injection pressure and the injection
temperature; and
producing hydrocarbons mobilized by the solvent from the reservoir.
[0011] In embodiments of the above method, a second derivative of the
reduced
density with respect to pressure may be less than 0.1. In an embodiment, the
solvent
is a supercritical solvent at the injection temperature and the injection
pressure. The
injection pressure may be higher than the critical point pressure of the
solvent. The
solvent may be propane. The injection pressure may be above 2 MPa, the
injection
temperature may be less than 200 C, and the density of the solvent at the
injection
pressure and the injection temperature may be less than 100 kg/m3. In some
embodiments, the density of the solvent may be less than 50 kg/m3. The
injection
pressure may be above 4.3 MPa. The injection pressure may be less than 7 MPa.
The
injection temperature may be less than a coking temperature of the
hydrocarbons. The
injection temperature may be less than 350 C. The injection pressure may be
about 2
MPa and the injection temperature may be higher than 150 C. The injection
pressure
may be about 3 MPa, and the injection temperature may be higher than 200 C.
The
injection pressure may be about 4.5 MPa, and the injection temperature may be
higher
3
CA 3060497 2019-10-29
than 250 C. The injection pressure may be about 5 MPa, and the injection
temperature may be higher than 300 C. In some embodiments, the solvent may
comprise butane, in which case the injection pressure may be above 3.8 MPa and
the
injection temperature may be above 200 C. The solvent may comprise a natural
gas
liquid.
[0012] In another aspect of the disclosure, there is disclosed a method of
delivering
a solvent to an interface region in a reservoir of hydrocarbons through a
solvent
chamber, comprising injecting the solvent into the solvent chamber in the
reservoir at
an injection pressure and an injection temperature selected and matched such
that, at
the injection pressure and the injection temperature the solvent has a reduced
density
of less than 0.5 in the solvent chamber before the solvent reaches the
interface region,
and a second derivative of the reduced density with respect to temperature is
less than
1.
[0013] In a further aspect, there is provided a method of reducing solvent
holdup in
a solvent chamber in a reservoir of hydrocarbons, wherein a solvent is
injected into the
solvent chamber to assist production of hydrocarbons from the reservoir, the
method
comprising injecting the solvent into the solvent chamber in the reservoir at
an
injection pressure and an injection temperature selected and matched such
that, at the
injection pressure and the injection temperature the solvent has a reduced
density of
less than 0.5 in the solvent chamber before the solvent reaches an interface
region
between the solvent chamber and the reservoir, and a second derivative of the
reduced density with respect to temperature is less than 1.
[0014] Other aspects, features, and embodiments of the present disclosure
will
become apparent to those of ordinary skill in the art upon review of the
following
description of specific embodiments in conjunction with the accompanying
figures.
BRIEF DESCRIPTION OF THE DRAWINGS
[0015] In the figures, which illustrate, by way of example only,
embodiments of the
present disclosure:
4
CA 3060497 2019-10-29
[0016] FIG. 1 is a schematic side view of a hydrocarbon reservoir and a
pair of
wells penetrating the reservoir for recovery of hydrocarbons.
[0017] FIG. 2 is a schematic partial end view of the reservoir and wells of
FIG. 1.
[0018] FIGS. 3 and 4 are line graphs illustrating density-pressure phase
diagrams
for propane at various temperatures.
[0019] FIGS. 5-7 are line graphs illustrating propane density dependence on
temperature at various pressures.
[0020] FIG. 8 is a line graph illustrating density-pressure phase diagrams
for
propane at various temperatures.
[0021] FIGS. 9-12 are line graphs illustrating the propane density
dependence on
pressure at various temperatures.
[0022] FIGS. 13 to 15 are line graphs illustrating density-pressure phase
diagrams
for propane at various temperatures.
[0023] FIG. 16 is a graph illustrating simulation results of propane
density
distribution in a solvent chamber.
[0024] FIG. 17 is a line graph showing representative measured solvent
density
variation during controlled solvent injection.
[0025] FIG. 18 is a data graph illustrating the dependence of the first
derivative of
the reduced solvent density with respect to the reduced temperature for the
data
shown in FIG. 17.
CA 3060497 2019-10-29
DETAILED DESCRIPTION
[0026] In brief overview, the present inventors have recognized that
solvent-based,
or solvent-driven, hydrocarbon recovery from a subterranean reservoir can be
improved by injecting a supercritical solvent at matched injection
temperatures and
pressures, which are selected and controlled to limit the density of the
injected solvent
below a selected threshold such that the injected supercritical solvent is
more gas-like
than liquid-like in the reservoir formation, and to limit the temperature and
pressure
dependence of the solvent density at the injection conditions so that slight
deviation
from the selected injection temperature and pressure will cause little or
limited
changes in the in situ solvent density.
[0027] It has been recognized by the present inventors that injecting
solvents with
such controlled density behavior can improve production efficiency by reducing
the
rate or amount of solvent injection, or the solvent-to-oil ratio, as will be
further
discussed below.
[0028] An illustrative embodiment of the present disclosure is described
next with
reference to the figures.
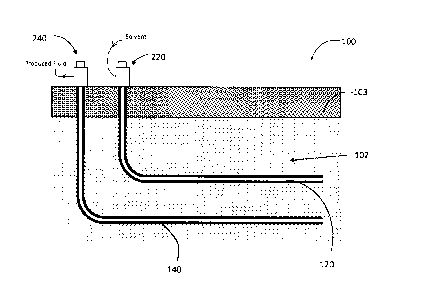
[0029] FIG. 1 schematically depicts a reservoir 100 having a pay zone 102
under a
cap layer 103. An injection well 120 and a production well 140 are provided,
which
penetrate the pay zone 102 of the reservoir 100. Injection wells and
productions wells
are commonly referred to in the art as injectors and producers respectively.
[0030] The reservoir 100 is a subterranean or underground reservoir
containing
recoverable viscous hydrocarbons. At least some of the viscous hydrocarbons
are
immobile under native reservoir conditions (i.e. before the reservoir 100 is
subjected to
heating or before a treatment material has been injected into the reservoir to
mobilize
the hydrocarbons). Immobile materials include materials that are not mobile or
not
mobile enough to drain under gravity without further treatment. In the
reservoir 100,
fluids such as gases and water may also have limited mobility due to a
relatively high
degree of viscous hydrocarbon saturation. In some typical bitumen reservoirs
found in
6
CA 3060497 2019-10-29
Alberta, Canada, the native temperature in the reservoir may be between about
7 C
and about 12 C, and the native pressure in the reservoir may be between about
1
MPa and about 5 MPa. In different reservoirs, the original temperature and
pressure
may be different.
[0031] Broadly, viscous hydrocarbons in the reservoir 100 may have a
viscosity
higher than about 1,000 centipoise (cP), 10,000 cP, 100,000 cP, or 1,000,000
cP. The
viscous hydrocarbons in the reservoir 100 may be a mixture of various
materials. A
variety of hydrocarbons in the reservoir 100 may exist, as viscous liquids, or
in semi-
solid or solid forms at native reservoir conditions. For example, the viscous
hydrocarbons in reservoir 100 may exist in the form of bitumen, heavy oil,
extra heavy
oil, bituminous sands (also referred to as oil sands), or combinations
thereof. In
bituminous sands, at least some viscous or immobile hydrocarbons are disposed
between, or attached to, sands. In the reservoir 100, hydrocarbons may exist
in
mixtures of varying compositions comprising hydrocarbons in the gaseous,
liquid or
solid states, which may also be in combination with other fluids (liquids and
gases) that
are not hydrocarbons. Bitumen is generally immobile under typical native
reservoir
conditions.
[0032] Each of the wells 120 and 140 has a horizontal section with a
perforated
section. The horizontal sections of the wells 120 and 140 are substantially
parallel to
one another and are vertically spaced by a distance, which may be about 5 to
about 8
m, with the production well 140 positioned below the injection well 120. The
horizontal
sections of the wells 120 and 140 may be about 800 m in length. The distance
between the wells and the well lengths may vary in different embodiments. The
injection well 120 is connected to an injection surface facility 220 (not
shown in detail),
and the production well 140 is connected to a production surface facility 240
(not
shown in detail). Further details of the wells 120 and 140 are provided below
with
reference to FIG. 2.
7
CA 3060497 2019-10-29
[0033] The injection surface facility 220 is configured to supply an
injection fluid,
which includes a solvent, to the injection well 120 for injection into the pay
zone 102 of
the reservoir 100. The injection surface facility 220 may have a supply line
(not shown)
connected to an injection fluid source (not shown) for supplying the injection
fluid.
[0034] The production surface facility 240 and the production well 140 are
configured to produce a fluid from the reservoir 100 to surface through
production well
140. The produced fluid may include a liquid mixture of the injected solvent
and
mobilized hydrocarbons. The production surface facility 240 may include a
fluid
transport pipeline (not shown) for conveying the produced fluid to a
downstream facility
(not shown) for processing or treatment.
[0035] The injection surface facility 220 includes equipment for supplying
the
injection fluid to the injection well 120, and the production surface facility
240 includes
equipment for producing the produced fluid from the production well 140, as
can be
understood by those skilled in the art.
[0036] The wells 120 and 140 may be configured and completed in a similar
manner as the horizontal wells used in conventional steam-assisted gravity
drainage
(SAGD) processes, or vapor extraction (VAPEX) processes, with suitable
modifications to inject a supercritical solvent instead of steam, and
optionally to heat
the production zone as will be further explained below. Wells and well
configurations
as disclosed in United States Provisional Patent Application Nos. 62/565,816
and
62/609,433 may also be used in an embodiment of the present disclosure. The
entire
contents of each one of United States Provisional Patent Application Nos.
62/565,816
and 62/609,433 are incorporated herein by reference.
[0037] For example, in selected embodiments, an injection well may be
provided
with a coiled tubing for injecting the solvent (and other possible injected
fluids or
materials), a casing, a liner assembly, and a liner hanger (all not shown).
The liner
assembly may be slotted to allow injected fluids to pass through. The coiled
tubing
may be connected to a control system (not shown) at the surface for
controlling the
injection operation, as can be understood by those skilled in the art. One or
more
8
CA 3060497 2019-10-29
downhole heaters (not shown) may be provided in the injection well, which may
include a wire or rod coiled around the coiled tubing along a length of the
horizontal
section of the injection well. The heater may be an electric heater, which may
be
operated in the direct-current (DC) mode or in an alternating-current (AC)
mode, and
may be operated at an operating frequency in the range of 1 Hz to 30 kHz. A
temperature sensor (not shown) may be provided in or on the coiled tubing. The
temperature sensor may include a distributed temperature sensing (DTS) device,
and
may include thermocouples (TC). Temperatures at multiple points along the
production
well, such as 4 to 6 points or more, may be monitored during operation.
Electrical
signal and power lines (not separately shown) for the temperature sensors and
the
heater may be connected to the surface control system to provide temperature
signals
from the sensors to the control system and to control operation of the heater.
The
power and signal lines may be attached to the coiled tubing or a tubing string
(not
shown). Additional necessary or optional components, tools, or equipment may
be
installed in the injection well 120. Other sensors and devices (not shown) for
measuring downhole temperature (T) and pressure (P) may also be provided in
the
injection well 120, such as at a heel portion of the injection well 120. The
detailed
constructions of the injection well 120 are not illustrated herein as they are
within the
knowledge of the skilled person in the art and are not particularly relevant
for the
purpose of the present disclosure.
[0038] The production well 140 may be similarly constructed as injection
well 120,
with some modifications or variations for producing a reservoir fluid as can
be
understood by those skilled in the art. In particular, the production well 140
may also
include a coiled tubing, a casing, a slotted liner assembly, a liner hanger, a
heater, and
a temperature sensor (all not shown), which may be similarly constructed and
configured as their counterparts in the injection well 120. The production
well 140 also
additionally includes a pump and a production tubing (not shown) for producing
fluids
entering the production well 140 through the slotted liner assembly to the
surface. As
in the injection well 120, signal and power lines (not shown) for the heater
and
temperature sensor in the production well 140 may be provided and connected to
the
surface control system. As in the injection well 120, additional necessary or
optional
9
CA 3060497 2019-10-29
components, tools, or equipment may be installed in the production well 140.
However, for example, a pressure sensor may not be necessary in the production
well
140 in some embodiments. The production well 140 may also be provided with a
dual-
heater string, four or more TCs, and a DTS fibre in the coiled tubing (all not
shown).
The production tubing may be landed at the heel of the well.
[0039] In operation, a selected solvent is injected through the injection
well 120 into
the pay zone 102 of the reservoir 100 at selected injection temperature and
injection
pressure, and hydrocarbons mobilized by the injected solvent are produced from
the
reservoir 100 through the production well 140. The injected solvent is
selected to
facilitate mobilization and production of viscous hydrocarbons in the
reservoir 100, and
the solvent injection temperature and pressure are selected and matched to
improve
hydrocarbon production efficiency and effectiveness as discussed herein.
[0040] In a particular embodiment, the solvent is injected at supercritical
conditions
so that when the solvent is injected into the reservoir 100 at the selected
injection
conditions the injected solvent will be a supercritical solvent after entering
into the
reservoir formation.
[0041] The injection conditions include the injection temperature and the
injection
pressure. The "injection temperature" as used herein refers to the temperature
of the
solvent when the solvent is in the injection well 120 just prior to entering
the reservoir
100, unless otherwise specified in a particular context. The "injection
pressure" as
used herein refers to the pressure in the injection well 120, which is also
often referred
to as the bottom-hole pressure (BHP) or downhole pressure of the well in the
art.
[0042] In comparison and for clarity, the reservoir conditions refer to the
temperatures and pressures in the reservoir formation. As can be appreciated,
the
reservoir conditions are affected and may be controlled by the injection
conditions and
the injection and production rates, and the local temperatures and pressures
within the
reservoir can vary as will be further discussed below.
CA 3060497 2019-10-29
[0043] Prior to oil production, fluid communication between the injection
well 120
and the production well 140 may be established with any suitable start-up
technique
used for a solvent-based or solvent-driven recovery process. This stage of the
recovery process is commonly referred to as the start-up stage.
[0044] As illustrated in FIG. 2, in the start-up stage, a heated fluid such
as steam or
heaters (not shown) or other heating techniques, or a combination of different
heating
techniques, may be used to heat an inter-well zone 104 between the wells 120,
140 to
soften the viscous hydrocarbons in the inter-well zone 104. The inter-well
zone 104
may be heated for a period of sufficient time to prepare the reservoir
formation, such
as for about 1 month to about 7 months at a heating power/well length of up to
10,000
W/m, such as from about 500 W/m to about 5,000 W/m. As can be appreciated by
those skilled in the art, heating the materials in the reservoir 100,
particularly in the
inter-well zone 104, can soften, or increase the mobility of, viscous
hydrocarbons
within the inter-well zone 104, which can facilitate distribution and
dispersion of the
injected solvent in the inter-well zone 104. After a period of heating, the
temperature in
the inter-well zone 104 may be increased as compared to the native or initial
temperature of the reservoir before heating, so that the viscous hydrocarbons
in the
inter-well zone 104 are at least partially softened and mobilized. For
example, the
average temperature in the inter-well zone 104 may be about 95 C after such
heating.
In different embodiments, the average temperature at this point may vary from
about
80 C to about 290 C if, for example, propane is to be used at the solvent.
[0045] After the inter-well zone 104 is heated to the desired temperature,
an
injection fluid including the selected solvent may be injected into the inter-
well zone
104 from both the injection well 120 and the production well 140 at a selected
pressure, such as about 3 MPa, to establish fluid communication between the
injection
well 120 and the production well 140. The injection pressure and injection
temperature
at the start-up stage may be selected for efficient and effective
establishment of fluid
communication, and may be different from the injection temperature and
pressure
used during the production stage as will be discussed below. In a particular
embodiment, the selected solvent is propane, and the injection temperature for
the
11
CA 3060497 2019-10-29
start-up stage may be higher than about 80 C, and the injection pressure may
be
about 3 MPa to about 3.5 MPa. The solvent such as propane can be injected in a
vapor phase at these temperatures and pressures in the start-up stage.
Alternatively,
the solvent may be injected at supercritical conditions.
[0046] The injected solvent vapor or supercritical solvent will disperse
into the pay
zone 102, particularly the inter-well zone 104, and will cool and eventually
condense in
the cooler regions as the solvent travels away from the wells 120 and 140. The
latent
heat transferred from the solvent to the pay zone 102 further mobilizes the
hydrocarbons therein. The condensed solvent liquid can also dilute the
hydrocarbons it
contacts, thus further softening or mobilizing the hydrocarbons in the inter-
well zone
104. At this point, if heaters (not separately shown) are provided in the
wells 120, 140
they may be activated to heat the inter-well zone 104 to assist heating of the
hydrocarbons in the inter-well zone 104.
[0047] At some point a pressure differential between the injection well 120
and the
production well 140 may be established to drive fluid flow from the injection
well 120
towards the production well 140. For example, injection of solvent into the
production
well 140 may be terminated at a selected time, and a pump (not shown) in the
production zone may be operated to produce fluids in the production well 140
to the
surface, while injection of the solvent into the injection well 120 is
maintained. As can
be appreciated, a higher injection pressure or higher pressure differential
between the
wells can drive the solvent into the reservoir 100, or the fluid flow in the
reservoir 100,
more quickly. Eventually, a fluid path between the wells 120 and 140 will be
formed
and fluid communication between the wells 120, 140 will be established. In
some
applications, it may take about 3 months or more to establish fluid
communication
between the wells in a typical well pair. Solvent injection may continue after
initial fluid
communication between the wells 120, 140 to provide improved communication
between the wells. For example, it may be desirable to have generally uniform
communication along the length of the horizontal sections of the wells 120 and
140,
which may take more time to establish.
12
CA 3060497 2019-10-29
[0048] After fluid communication between the wells 120 and 140 is
established,
hydrocarbon production may commence, and the process enters into the
production
stage. At the beginning of the production stage, there may be a ramp-up phase,
in
which the oil production rate is gradually increased, or increased in steps.
[0049] After the start-up stage and the ramp-up phase, an initial "solvent
chamber"
106 will have been developed as some of the hydrocarbons in the reservoir 100
have
been mobilized and drained downward due to gravity, and the pores in the
volume of
the reservoir 100 originally filled by these hydrocarbons are now filled with
the solvent.
The solvent chamber is typically more porous than the initial reservoir
formation and
allows fluids to travel through the solvent chamber.
[0050] In the production stage, a selected solvent 160, which may be the
same or
different from the solvent used in the start-up stage, is injected into the
reservoir 100
through the injection well 120, and a reservoir fluid 170 is produced from the
production well 140. The reservoir fluid 170 includes mobilized hydrocarbons
and
condensed solvent, as well as other possible components as can be appreciated
by
those skilled in the art.
[0051] The injected solvent may be propane or butane, or another suitable
solvent
as will be discussed further below.
[0052] In embodiments disclosed herein, during at least a period of
hydrocarbon
production, the injection temperature may be selected to be higher than the
critical
point temperature of the solvent and the injection pressure may be selected to
be
higher than the critical point pressure of the solvent, so that the injected
solvent is
supercritical when leaving the injection well 120 and entering the reservoir
100. As will
be further discussed below, in selected embodiments, at least one of the
temperature
and pressure is selected to be significantly different from the critical point
value such
that the injection condition is not the same or close to the critical point
conditions of the
solvent, and the reduced density of the injected solvent immediately after
entering into
the reservoir is less than about 0.5 (e.g. less than about 100 kg/m3 for
propane) and
the temperature and pressure dependence of the solvent density at the selected
13
CA 3060497 2019-10-29
injection conditions is relatively weak (see further discussions below). The
"reduced
density" of the solvent refers to the relative solvent density at a given
condition which
is normalized to the density of the solvent at the critical point, and can be
calculated
using Equation (1) discussed below.
[0053] For example, to inject propane as a supercritical solvent, the
injection
temperature should be higher than the critical point temperature of propane,
which is
about 96 C, and the injection pressure should be higher than the critical
point
pressure of propane, which is about 4.23 MPa.
[0054] In another example, when butane is used as the solvent, the
injection
temperature should be higher than the critical point temperature of butane,
which is
about 152 C, and the injection pressure should be higher than the critical
point
pressure of butane, which is about 3.79 MPa.
[0055] In some embodiments, the injection pressure may be below the
critical point
pressure, provided the matching injection temperature is high enough so that
the
solvent is injected in the gas phase with a reduced density of less than 0.5.
For
example, when propane is used as the solvent, the injection pressure may vary
from
about 2 MPa to about 7MPa, where the matching injection temperature is higher
than
125 C.
[0056] For a given injection pressure selected, the matching injection
temperature
has a corresponding lower limit to ensure the reduced density of the solvent
is
controlled as disclosed herein. For example, for reasons to be discussed
later, in an
embodiment when the injection pressure is about 2 MPa, the matching injection
temperature is higher than 150 C; for injection pressure of about 3 MPa, the
matching
injection temperature is higher than 200 C; for injection pressure of about
4.5 MPa,
the matching injection temperature is higher than 250 C; and for injection
pressure of
about 5 MPa, the matching injection temperature is higher than 300 C.
14
CA 3060497 2019-10-29
[0057] As can be appreciated by those skilled in the art, the injection
temperature
should also be controlled so as not to coke the solvent or the hydrocarbons to
be
produced in the reservoir 100. For example, in some bitumen reservoirs the
coking
temperature of the bitumen may be about 350 C. For such reservoirs, the
injection
temperature should be limited to below 350 C.
[0058] The injection pressure should also be safe and may need to be
limited to
comply with local regulatory requirements. For example, in some bitumen
reservoirs, it
is generally safe to inject a fluid into the reservoir at a pressure below
about 7 MPa.
[0059] In accordance with an embodiment of the present disclosure, the
injection
temperature and pressure should also be selected to limit the density of the
supercritical solvent so that the supercritical solvent behaves more gas-like
than liquid-
like in the solvent chamber. In some embodiments, the injection temperature
and
pressure are selected such that the injected supercritical solvent has a
reduced
density of less than about 0.5 at the selected injection temperature and
injection
pressure. For example, when propane is used as the solvent, the density of the
injected propane at the injection conditions may be less than 100 kg/m3 (the
critical
density of propane is about 220 kg/m3 and 0.5 reduced density of propane is
about
110 kg/m3). Suitable or optimal injection temperatures and pressures may be
selected
or determined based computer simulation or laboratory or field test results.
[0060] For ease of illustration, it is assumed that in a specific example
embodiment,
the solvent is propane, and the injection temperature during production may be
from
about 150 C to about 350 C, and the injection pressure may be from about 2
MPa to
about 7 MPa, and the particular pair of injection pressure and temperature are
matched to control the density of the injected solvent as discussed herein.
Detailed
procedures for selecting the matching temperature and pressure pairs will be
further
discussed later below.
CA 3060497 2019-10-29
[0061] When the injected solvent 160 enters the reservoir 100 and comes
into
contact with the hydrocarbons and other materials in the reservoir which are
at a lower
temperature, the solvent 160 can dilute and soften the viscous hydrocarbons to
mobilize the hydrocarbons. Some of the softened or mobilized hydrocarbons
continue
to drain downward due to gravity, leaving behind an increasingly larger porous
volume
in the pay zone 102. The solvent chamber 106 is in a sense analogous to the
"steam
chamber" in a conventional SAGD process. The concept of a "steam chamber" is
well
known and understood by those skilled in the art.
[0062] A solvent can travel more easily and quickly in the solvent chamber
106 as
compared to the original pay zone 102 which has a much lower transmissibility
under
the original conditions before the solvent chamber is formed. In the ramp-up
phase,
the solvent chamber may grow and develop upwards above the injection well 120,
as
the injected solvent is gas-like and tends to rise in the solvent chamber 106.
The
temperature in the central region of the solvent chamber 106 near the
injection well
120 is higher than the temperature at the edges of the solvent chamber 106,
which are
referred to as the "interface region" 150 (sometimes also referred to as the
"chamber
front"). For example, the temperature at the central region of the solvent
chamber 106
may be close to the injection temperature, and may be from about 150 C to
about 350
C in the specific example noted above. The temperature at the interface region
150
may vary, such as from about 70 C to about 20 C, assuming the reservoir
temperature in regions outside the pay zone 102 is about 15 C.
[0063] During the production stage, the solvent is injected into the pay
zone 102 of
the reservoir 100 through the injection well 120 only. The solvent, propane in
the
specific example, enters the reservoir 100 mainly in the supercritical phase.
16
CA 3060497 2019-10-29
[0064] The solvent may be heated and pressurized at surface and supplied to
the
injection well 120 in the supercritical phase, or provided as a liquid to the
injection well
120 and further heated in the injection well 120 to above the supercritical
temperature
before entering the pay zone 102. Alternatively, the solvent may be supplied
to the
injection well 120 as a liquid-vapor mixture, and then heated and pressured to
above
the critical point.
[0065] The solvent may be injected by injecting into the reservoir a fluid
consisting
essentially of the solvent. The fluid may contain impurities or small amounts
of other
substances such as water, steam, methane, other solvents, or the like, but the
total
weight or molar concentration of such impurities and other substances are
relatively
small, such as below about 1 wt% to about 2 wt%.
[0066] In some embodiments, the injection fluid may include a mixture of
two or
more selected solvents, in which case the selected solvents are not considered
impurities. The injection fluid may also include steam, such as for heating
the
solvent(s), and improving mobility of the mobilized hydrocarbons in the
reservoir as
steam can condense in the reservoir and the added water content in the
reservoir fluid
can improve its flow rate towards the production well.
[0067] A heater in the injection well 120 may be used to control the
injection
temperature of the solvent.
[0068] The injection pressure or downhole pressure in the injection well
120 may
be detected and controlled using any suitable technique including known
downhole
pressure monitoring techniques such as a bubble tube downhole pressure
monitoring
system. Using a bubble tube system, the downhole pressure may be automatically
and
continuously measured.
17
CA 3060497 2019-10-29
[0069] The injected solvent will initially travel generally upwards in the
solvent
chamber 106, as indicated by arrows 160 in FIG. 2. The solvent will cool down
and
condense at the interface zone 150 or even earlier due to the cooler
temperature in
the reservoir 100 particularly in interface zone 150. The solvent liquid will
dilute the
hydrocarbons and mix with the mobilized hydrocarbons to form a liquid mixture,
namely, reservoir fluid 170, which drain generally downward.
[0070] Eventually, the reservoir fluid 170 drains into the production zone
108
around the production well 140, and is produced to the surface through the
production
well 140.
[0071] It should be understood that a liquid mixture may contain some
limited
gaseous contents. For example, in the reservoir 100, the solvent may be
partially in
the liquid phase and partially in the vapor phase. A liquid in the liquid
mixture, such as
a liquid solvent, may also be vaporized in the production well 140 when being
produced to surface. Some other gases such as methane, CO2, H25, or a
combination
thereof may also be produced with the liquid mixture.
[0072] During production, a heater (not shown) may be provided in the
production
well 140 to heat the production zone 108. The heating may be controlled by a
surface
control system (not shown) based on the temperature signal detected by a
temperature sensor (not shown) provided in the production well 140, to
maintain the
temperature in the production zone 108 to be within a selected temperature
range.
The factors considered for selecting this range have been discussed elsewhere
and
will not be repeated herein.
[0073] Hydrocarbon production may continue until the amount of the
hydrocarbons
in the pay zone 102 has been reduced to a level that is no longer economical.
[0074] After the production stage, the process may enter a blowdown stage, as
can
be understood by those skilled in the art. During the blowdown stage,
injection of the
solvent may be terminated or substantially reduced. The residual hydrocarbons
and
solvent may still be produced for a period of time. A non-condensable gas
(NCG) such
18
CA 3060497 2019-10-29
as methane may be injected instead into the solvent chamber 106 to assist
recovery of
the residual solvent and the remaining hydrocarbons. The injected NCG may keep
the
pressure in the solvent chamber at a relatively high level. During the
blowdown stage,
the production zone 108 may be heated with a heater but the injection well 120
may
not need to be heated any further.
[0075] In different embodiments solvents other than propane may be selected
and
used, and the operating conditions may also vary depending on the selected
solvent
and the native reservoir conditions. To improve the efficiency of hydrocarbon
production, the solvent and the injection and heating conditions may be
selected or
determined based on a number of factors including those disclosed herein.
[0076] Selecting and matching injection pressure and temperature
[0077] It has been recognized by the present inventors that a problem in
some
conventional solvent extraction processes is that the injected solvent is not
efficiently
utilized for extracting hydrocarbons because a large portion of the injected
solvent is
in, or quickly condenses to, the liquid phase before the solvent reaches the
chamber
front or the interface region 150 between the solvent chamber 106 and the pay
zone
102. A liquid solvent generally travels slower than a gaseous solvent in the
solvent
chamber 106 or in the reservoir 100. As a result, a significant amount of
liquid solvent
is held in the reservoir 100, which does not contribute significantly to the
production
process, and cannot be efficiently recovered through the production well 140.
It has
also been discovered that if the density of the injected solvent is lowered
and
controlled as discussed herein, solvent retention or holdup in the reservoir
can be
reduced and recovery efficiency can be improved. In particular, it has been
recognized
that when the injected solvent is either in the gas phase or in the
supercritical phase
but with a gas-like density, where the reduced density of the injected solvent
is less
than about 0.5 (see further discussion about reduced density below), and the
solvent
density dependence on both temperature and pressure is "weak" (see discussion
below) at the injection conditions, the injected solvent is less likely to
condense
prematurely before reaching the chamber front, and solvent "holdup" may be
reduced.
19
CA 3060497 2019-10-29
As a result, more efficient and more economical hydrocarbon recovery can be
achieved, as compared to injecting a liquid solvent, or injecting
supercritical solvent
with liquid-like densities, or injecting a supercritical solvent at conditions
close to the
critical point conditions.
[0078] One of the possible reasons for the improved efficiency is that when
the
solvent is injected at controlled and matched temperatures and pressures as
disclosed
herein, the injected supercritical solvent can move or travel more quickly
through the
solvent chamber 106. As a result, it is expected that less solvent will be
"held up" in
the central portion of the solvent chamber 106. The solvent is thus more
efficiently
delivered from the injection point to the interface region 150 in the
reservoir 100
through the solvent chamber 106. An embodiment described herein can thus
reduce
solvent holdup in the solvent chamber 106 in a hydrocarbon recover process.
[0079] In comparison, liquid solvents or supercritical solvents having
liquid-like
densities are more likely "held-up" in the solvent chamber 106. When a large
amount
of condensed or liquid solvent were present in the central portion of the
solvent
chamber 106 and its residence time in the solvent chamber 106 were long (i.e.,
being
"held-up"), the utilization of the injected solvent for hydrocarbon recovery
is less
efficient and less effective. In some conventional techniques, the solvent
injection
temperature and pressure are optimized to maximize oil yield rate and reduce
energy
consumption, which result in relatively high solvent density in the solvent
chamber
106. As a result, these techniques would require usage of a large amount of
solvent to
the extent that it would not be economical to implement such techniques on a
commercial scale. The usage of solvent in such techniques is also inefficient
and
ineffective.
[0080] To overcome such inefficiency, in an embodiment of the present
disclosure,
the solvent injection temperature and pressure are selected and matched to
reduce or
minimize the solvent residence time in the solvent chamber 106. For example,
limiting
the solvent density and its dependency on temperature and pressure at the
injection
conditions can promote faster passage of the solvent through the solvent
chamber
CA 3060497 2019-10-29
106, thus reducing its residence time in the solvent chamber 106, even when
the
temperature and pressure in the solvent chamber 106 may fluctuate or vary.
[0081] Injecting a supercritical solvent with gas-like density can be more
beneficial
in some embodiments as the solvent can travel through a longer distance in the
reservoir 100 without a phase-change. In comparison, an injected solvent gas
may
quickly condense into the liquid phase in the solvent chamber due to
temperature
drops as the solvent moves away from the injection well.
[0082] Therefore, one of the factors to be considered when selecting and
matching
the injection temperature and pressure is to keep the density of the injected
solvent
below a certain threshold. The threshold may be expressed in terms of the
reduced
density of the solvent or the absolute density of the solvent. It is expected
that it can
be beneficial to inject the solvent at a reduced density of less than about
0.5. Using
propane as an example, a reduced density of 0.5 for propane is equivalent to
about
111 kg/m3 absolute density, as the critical density of propane is 222 kg/m3.
For
butane, the reduced density of 0.5 is equivalent to about 116 kg/m3 absolute
density,
as the critical density of butane is 232.5 kg/m3.
[0083] It has been recognized by the present inventors that a possible
approach to
improve solvent efficiency is to modify the operating conditions in the
reservoir 100 or
in the solvent chamber 106 to reduce or limit solvent "holdup" or solvent
residency
time in the solvent chamber 106 in the reservoir 100. Without being limited to
any
specific theory, it is expected that when the density of the solvent is
relative low, so the
solvent behaves like a gas, the solvent "holdup" can be reduced. Further, to
maintain
the gas-like behaviour along the way to the interface region, the injection
pressure and
temperature should be matched so that the density of the solvent is relatively
insensitive to pressure and temperature changes within the expected conditions
in the
centre portion of the solvent chamber. The derivatives of density with respect
to
temperature and pressure respectively in these zones provide useful and
instructive
information for selecting the suitable densities and corresponding pressure
and
temperature of the solvent.
21
CA 3060497 2019-10-29
[0084] Simulation tests have shown that by operating at example injection
conditions disclosed herein, it is possible to reduce solvent usage and
solvent
residency time in the solvent chamber 106, while maintain relatively high oil
production
rates and low energy intensity, resulting in improved process economics.
[0085] To help explain the selection process, reference is first made to
FIG. 3,
which shows a density-pressure phase diagram for propane, at various selected
temperatures.
[0086] In FIG. 3, the line labelled as "x=1" represents the gas-to-liquid
phase
transition boundary and the line labelled as "x=0" represents the liquid-to-
gas phase
transition boundary. The point at which the liquid phase line and the gas
phase line
meet represents the critical point of propane. At pressures and temperatures
above
the critical point, the solvent is supercritical and the gas and liquid phases
are
indistinguishable. It can also been seen from FIG. 3 that at pressures below
the critical
pressure, the solvent can transition from the gas phase to the liquid phase
with a very
small pressure or temperature change and the density change is quite quick and
substantial at the transition point when the temperature is relatively low so
that the
density-pressure line at that temperature is on the x=1 line or relatively
close to it.
Further, the density of the solvent can change substantially quickly near the
critical
point (see the slope of the line indicated as representing T= 75 C or 100
C).
[0087] To avoid such drastic change in density at or near the injection
conditions,
the injection temperature and pressure should be selected to be at a point
some
distance away from the gas phase line and the critical point.
[0088] The injection conditions should also be selected so that the solvent
density
is relative low and the rate of density change with respect temperature or
pressure at
the injection conditions is low.
[0089] The selection criteria are illustrated herein with reference to
propane as an
example but it should be appreciated that similar approaches can be applied in
embodiments using other solvents.
22
CA 3060497 2019-10-29
[0090] The first selection criterion is that, as already mentioned, the
solvent density
is relatively low at the injection conditions. In particular, the reduced
density of the
solvent should be less than 0.5. In some embodiments, the reduced density may
be
less than 0.25. The reduced density may be 0.05, 0.1, 0.11, 0.12, 0.13, 0.14,
0.15,
0.16, 0.17, 0.18, 0.19, 0.2, 0.21, 0.22, 0.23, 0.24, or 0.25. The reduced
density may
also be 0.3 or 0.4 in some embodiments.
[0091] The reduced density (pr) is the ratio of actual solvent density (p)
to the
solvent critical density (density at the critical point, pc), as defined in
Equation (1):
Pr = (1)
PC
Similarly, the reduced temperature (Tr) and reduced pressure (Pr) may be
expressed
as in Equations (2) and (3) respectively:
T
= ¨ , (2)
Pr =¨ (3)
Pc.
where T is the actual solvent temperature, Tc is the critical temperature, P
is the actual
pressure, and Pc is the critical pressure.
[0092] To provide an economical solvent process, the first criterion is
that the
reduced density is less than about 0.5, or satisfies Equation (4):
Pr~ 5 . (4)
[0093] In FIG. 4, the shaded area indicates the region that satisfies this
criterion (4)
for propane.
[0094] The second criterion is that a relative small change in temperature
or
pressure would not lead to substantial change in the solvent density (i.e. the
dependence of the solvent (reduced) density on temperature and pressure is
"weak").
Quantitatively, it has been found that such a criterion can be expressed as
requiring
the second partial derivative of the solvent density with respect to pressure
or
23
CA 3060497 2019-10-29
temperature to be relatively small. Ideally, the second partial derivatives
should be
a2 a2
zero (i.e., 1- r 2. = o, and 1-'2r =0 , but such ideal solvents may
not exist and in any
aT,.
event are not necessary. For example, the temperature dependence of propane
density at various pressures is illustrated in FIG. 5, and the temperature
dependence
of the first and second derivatives of propane reduced density are
respectively
illustrated in FIG. 6 and FIG. 7.
[0095] It is desirable that the first partial derivatives of density with
respect to
temperature or pressure have weak dependence on the temperature or pressure,
although in ideal situations the first partial derivatives should be
independent of
temperature and pressure.
[0096] In FIG. 6, it may be seen that the density change is relatively less
when the
propane is injected at relatively higher temperatures for a given pressure. At
lower
pressures (such as 1 MPa to 2.6 MPa) the first derivative is relatively flat
at
temperatures from 125 C to 300 C. However, at a higher pressure, such as 4.2
MPa,
the density is only relatively flat at temperatures higher than about 150 C.
At even
higher pressures, the flat region starts at even higher temperatures.
[0097] FIG. 7 shows the second derivative of the reduced density with
respect to
temperature. To avoid positive, non-linear changes in the solvent density as
the
solvent temperature decreases, such as when the solvent travels from the
injection
well to the interface region through the solvent chamber, should be relatively
small.
[0098] As an example, FIG. 7 shows that for operating pressures of 2.6 MPa,
the
minimum injection temperature may be selected as 200 C. At pressures lower
than
1.8 MPa, the injection temperature may be as low as 150 C. In FIG. 7, points
A, B, C,
D and E represent the points where the second derivative of the propane
density
becomes lower than 1. The same points are also indicated on the phase diagram
in
FIG. 8.
24
CA 3060497 2019-10-29
[0099] In practice, it is expected to be sufficient if the second partial
derivative of
the solvent density with respect to temperature satisfies the following
criterion shown
in Equation (5):
a2pr
<1 (5)
aT2
r
where the Tin; is the injection temperature and Pinj is the injection
pressure.
[00100] From Equation (5), it follows that at different injection
pressures, the
suitable injection temperature ranges may be different and the injection
temperature at
any given injection pressure should be selected to match the given pressure.
[00101] The pressure dependence of the density at the injection conditions
should also be considered. For example, Figs. 9 to 12 show the pressure
dependence
of propane density, its first derivative with respect to pressure, and the
second
derivative of the reduced density with respect to reduced pressure
respectively, at
various temperatures.
[00102] Generally, in a solvent-based recovery process, a higher injection
pressure may be desirable due to increased solubility of the solvent in
hydrocarbons at
higher pressures. However, within the context of the present disclosure, the
injection
pressure should also be selected with consideration to limit the solvent
density and
variation of the solvent density in the central regions of the solvent
chamber.
[00103] For example, as can be seen in FIG. 10, at temperatures below
about
200 C, operating at injection pressures above 2 MPa would involve significant
density
variations with pressure changes.
[00104] Figs. 11 and 12 (enlarged portion of FIG. 11) show the pressure
dependence of the second partial derivative of reduced density of propane with
respect to reduced pressure. It is expected that the density variation will be
within an
acceptable range when the second derivative of the reduced density with
respect to
reduced pressure is less than about 0.1 at the selected injection pressure and
temperature. As can be appreciated, such a low value of the second derivative
of the
reduced density with respect to reduced pressure indicates that the dependence
of the
CA 3060497 2019-10-29
solvent density on the pressure is weak. The points F, G, H at which the
second
derivative crosses the threshold 0.1 are better shown in FIG. 12. The points
of F, G, H
are also shown on the phase diagram illustrated in FIG. 13.
[00105] From these results, it can be expected that for injection
pressures
between 2 MPa and 5 MPa, the minimum injection temperature of propane should
be
higher than 200 *C. Judging from FIGS. 11 and 12, and for propane, at
pressures
above about 6 MPa, the injection temperatures may be significantly lower, such
as as
low as about 175 C, due to the fact that the phase diagram lines are far away
from the
critical point. At pressures below about 2 MPa, the injection temperatures can
also be
as low as 175 C.
[00106] At pressures below about 1 MPa, propane is not expected to be a
suitable solvent in some applications, as the injection temperature needs to
be relative
low at this pressure, and injection of the solvent at such low temperatures
would limit
the heat energy transferable to the reservoir by the injected solvent.
[00107] From FIGS. 3, 8 and 13, it is possible to find a region on the
phase
diagrams where injection of pure propane is expected to be more economical
than in
the other zones. This region is denoted as the "pure solvent economic zone" or
"PSEZ" in FIG. 14.
[00108] However, in the PSEZ region where the pressure is below the
critical
pressure, the solvent is in the vapour phase. If the solvent vapour is at a
temperature
and pressure very close to the dew point conditions, significant solvent
vapour
condensation may occur in the reservoir in the central regions of the solvent
chamber.
Thus, such lower pressure regions may need to be avoided in some embodiments
depending on the injection temperature. For example, in some embodiments, the
injection pressure is selected to be higher than about 2 MPa when such high
pressures are permissible and practical in the particular application.
[00109] FIG. 15 shows the resulting PSEZ truncated at 2 MPa.
26
CA 3060497 2019-10-29
[00110] For reservoirs with maximum operating pressures (MOP) that allow
for
higher pressures, it is expected that injecting the solvent at least initially
at a higher or
the highest possible pressure within the PSEZ would provide improved and
balanced
production efficiency and performance. In particular, the PSEZ at higher
pressures
may overlap with a lower triangle zone within the supercritical region of the
solvent. At
later stages of hydrocarbon production, the injection pressure may be
gradually
dropped to the initial reservoir pressure, such as to avoid cap rock or thief
zone issues.
The corresponding injecting temperatures may be chosen to also be within the
PSEZ
as indicated on the phase diagram as shown in FIG. 15.
[00111] As an example, Table I lists the maximum density and minimum
temperature conditions for the PSEZ at different injection pressures for
propane in
example embodiments.
TABLE I. Example Injection Conditions in the PSEZ for Propane Solvent
Injection Minimum Injection Maximum
Pressure Temperature Solvent Density
(MPa) ( C) (kg/m3)
2.0 150 30
2.0 200 30
3.4 220 35
4.2 245 40
5.0 270 45
5.8 295 50
[00112] Generally, when selecting the solvent and the injection
conditions, the
following conditions or factors should be considered.
27
CA 3060497 2019-10-29
[00113] First, the solvent is soluble in the hydrocarbons to be recovered
at the
operating temperatures and pressures. This condition may be expressed as the
solubility (S*) of the solvent in the hydrocarbons is greater than zero at the
injection
temperature and the reservoir pressure, as in Equation (6):
7.% > 0. (6)
[00114] The solubility is assessed at the injection temperature and the
reservoir
pressure because the solvent solubility decreases with increasing temperature
and
decreasing pressure. Equation (6) ensures the solubility is larger than zero
at all
possible conditions within the reservoir.
[00115] Secondly, it may be desirable if the viscosity (p) of the
hydrocarbons
diluted and heated by the injected solvent in a solvent based process is lower
than the
viscosity of the hydrocarbons if were heated by steam injection in a steam
process at
similar operating temperatures or pressures, or both. One of the operating
temperatures and pressures may be different for this comparison. This
condition may
be expressed, as follows in Equation (7):
< (7)
[00116] In some applications, it may be desirable that the resulting
hydrocarbon
viscosity is an order of magnitude less in a solvent process than in a
comparable
steam process.
[00117] Thirdly, as noted above, the reduced density of the solvent is
less than
0.5 and the second derivative of the reduced density with respect to reduced
temperature is less than 1, as indicated in Equation (5) above. In other
words, the
change in the solvent density with respect to temperature at the injection
conditions
should be at least on the same order of magnitude as the change in the
injection
temperature.
28
CA 3060497 2019-10-29
[00118] As now can be appreciated, according an embodiment of the present
disclosure, a higher injection pressure is not always desirable for increasing
the
hydrocarbon production rate. On the one hand, a higher injection pressure
would
provide a higher driving pressure to increase the fluid flow towards the
production well.
A higher pressure also allows the solvent to be injected at a higher rate and
to
condense at a higher temperature, both of which would increase the rate of
mobilizing
the viscous hydrocarbons. As can be appreciated, a hotter solvent liquid is
more
efficient for mobilizing hydrocarbons. Simulation tests have confirmed that
the
production rate increases as the injection pressure increases at the tested
conditions.
However, in practical applications, the injection pressure is typically
limited by
technical, safety, environmental, or other concerns and may be regulated by
local
authorities. Within the practical limitations, the injection pressure may be
selected to
be as high as is permitted.
[00119] Given the possible injection pressure range, a suitable solvent
may be
selected so that the solvent can be injected as a vapor at the given injection
pressure
and at the possible temperature range and can condense at the expected
temperature
at the interface region of the solvent chamber. The selected solvent should
also be
effective for mobilizing the viscous hydrocarbons solvent at the reservoir
conditions.
Among the possible solvents, the solvents that would provide a similar
recovery rate at
relatively lower temperatures may be selected as heating a solvent and the pay
zone
to a lower temperature requires less energy and less cost. Other factors such
as
chemical compatibility, availability, pre- and post-injection treatment
requirements,
costs, or the like may also be considered when selecting the solvent. As can
be
appreciated, a solvent may be injected as a vapor at temperatures above the
critical
point of the solvent. In this regard, the critical point data are:
- Propane: 96 C, 4.26 MPa
- Butane: 152 C, 3.8 MPa
- Pentane: 197 C, 3.4 MPa
- Hexane: 235 C, 3.02 MPa
29
CA 3060497 2019-10-29
[00120] In this regard, known data including simulation data may be
utilized for
selecting the solvent. For example, it is known from experimental and
simulation
results the bitumen viscosities generally decrease as the temperature
increases. For
practical production, the viscosity of the softened bitumen should be lower
than about
50 cP to about 100 cP, such as from about 1 cP to about 20 cP, although
bitumen with
even lower viscosity is generally easier to produce.
[00121] The injection temperature may be controlled by heating the
solvent
above surface prior to injection. Alternatively or additionally, the solvent
may be heated
in the injection well.
[00122] Known analysis tools and methods including computer-aided methods
may be used to aid the selection of the solvent and operating conditions.
[00123] In some embodiments, propane may be selected as a suitable
candidate
solvent for a number of reasons relating to thermo-physical characteristics of
propane
and propane-bitumen mixtures under the particular reservoir conditions. First,
propane
has a moderate dew point temperature (and the corresponding bubble point
temperature in a propane-bitumen mixture is also moderate), and thus it can be
readily
vaporized at a moderate temperature for injection through the injection well
120 and
the propane vapor can be readily condensed at the interface region 150 of the
solvent
chamber 106. Second, the viscosity of the propane-bitumen mixture decreases
with
decreased temperature at the temperature range of 50 C to 70 C, which is
just below
the propane bubble point in the mixture at the given pressure of about 3 MPa.
This
may be helpful after the solvent propane has condensed to liquid phase and is
recovered with mobilized hydrocarbons through the production well, as the
temperature in the production well or the production zone around the
production well is
substantially lower than the injection temperature and may be lower than the
bubble
point of the solvent at the reservoir pressure.
[00124] For clarity, it is noted that an embodiment of a solvent-based
recovery
process may include injection of steam at different stages (such as the start-
up stage)
other than the oil production stage, where a solvent is injected in the
production stage
CA 3060497 2019-10-29
without steam. Embodiments of the present disclosure also include recovery
processes in which a solvent is injected in an oil production stage to drive
oil
production, but steam is not co-injected with the solvent as a primary heating
source to
maintain or control the temperature in the production zone of the reservoir
during the
production stage.
[00125] Conveniently, an embodiment of the solvent-based recovery process
as
described herein may provide effective and efficient hydrocarbon production at
reduced energy and solvent consumption and lower costs.
[00126] In some embodiments, it may be less efficient to heat the solvent
in the
production zone to a temperature above the bubble point of the solvent in the
fluid
mixture to be produced, as compared to subcool heating where the heating
temperature is maintained below the bubble point temperature.
[00127] In an embodiment, the recovery process may be a solvent-driven
recovery process, and a relatively small amount of steam may be co-injected
with the
solvent in the production stage.
[00128] In a different embodiment, butane may be selected as the solvent,
and
the operation parameters and conditions may be selected based on the factors
and
considerations described herein.
[00129] In some embodiments, a natural gas liquid (NGL) may be used as the
solvent. Natural gas liquids may include ethane, propane, butane (n-butane, or
isobutane), pentanes, or heavier hydrocarbons.
[00130] In view of the foregoing description of example embodiments, a
skilled
person will appreciate the working principles of the present disclosure, which
is in no
way bound to the example embodiments set out above or below. The foregoing
description will now be supplemented to elucidate other aspects and
embodiments of
the present disclosure.
31
CA 3060497 2019-10-29
[00131] For instance, in different embodiments, different solvents may be
used
as a solvent in one or more selected stages of the recovery process. Example
candidates for suitable solvents may include, for example, the following
materials, and
may be selected based on factors including the factors discussed below.
[00132] Some factors to be considered for selecting the solvent include
the
reservoir pressure, maximum operating pressure (may be dictated by local
regulatory
requirement), solvent solubility, solvent cost and availability, solvent-rock
interaction
properties, capital expenditure (capex) constraints, possible solvent losses,
and other
factors.
[00133] Generally, an operator may not be able to change the reservoir
pressure
and the maximum permissible operating pressure, and may need to work within
these
constraints. For example, in a shallow reservoir with a regulatory constraint
that the
operating pressure should not be significantly above the initial reservoir
pressure,
lighter hydrocarbon solvents such as propane may be used.
[00134] Among solvents which can work within the same operating conditions
(pressure and temperature), the solvent that provides the highest oil mobility
within the
reservoir operating ranges may be selected and may be expected to provide
better
production performance than other solvents in the group. Alternatively, the
solvent
associated with the lowest operating temperature may be selected, such as when
it is
desirable to reduce energy consumption or to lower green-house gas (GHG)
emissions. For example, at an operating pressure of 3 MPa, in some cases using
butane as the solvent may provide better oil production rates than using
propane,
while using propane may reduce energy requirements and GHG emissions as
compared to butane.
[00135] A person of skill in the art may also appreciate that objective
functions
(used in optimization) may be formulated by combining maximizing oil
production rates
and minimizing energy requirements and GHG emissions, with a selected weight
for
each objective.
32
CA 3060497 2019-10-29
[00136] Solvent cost and availability are economic factors that can change
and
are mainly driven by demand and supply in the market. However, such economic
factors should also be considered along with other factors including technical
factors.
Economic considerations may be balanced against technical advantages or
disadvantages of selecting a particular solvent.
[00137] Hydrocarbon solvents, such as organic solvents, do not generally
interact with the mineral rocks present in the reservoir, and may be used.
However,
non-hydrocarbon solvents may also be used. When selecting a non-hydrocarbon
solvent for use in a recovery process as described herein, one should consider
the
possible interaction between the particular solvent and the rock matrix in the
reservoir.
If the particular solvent would interact deleteriously with the rock matrix,
it should not
be used. For example, carbon dioxide (CO2) may not be a good solvent for
carbonate
reservoirs because CO2 can interact with the rock matrix to form calcium
carbonate
(CaCO3), which can precipitate and potentially block reservoir pores, thus
limiting or
preventing fluid flow in the reservoir and negatively affecting oil
production.
[00138] The costs of obtaining and handling solvent should also be
considered.
On a balanced approach considering both economic and technical factors, in
some
cases a technically less optimal solvent may be selected over the technically
optimal
solvent.
[00139] As another example, to reduce solvent residue (trapped solvent) in
the
reservoir formation (particularly before the blowdown phase or stage), heavier
solvents
may be selected as they are less likely to be trapped. However, heavier
solvents tend
to be more expensive. Thus, a detailed analysis may be required to determine
the
actual overall costs for selecting a heavier solvent over another lighter
solvent.
[00140] In some embodiments, a mixture of solvents, such as propane and
butane, may be injected, which may provide some advantages over using a single
solvent. A mixture of NLGs may also be used. For example, the solvent mixture
may
be selected to optimize a combined objective function of oil production rate
and heater
energy intensity. An example of such a combined objective function is the net
present
33
CA 3060497 2019-10-29
value (NPV) for a proposed process, which may take into account the amount of
oil
produced, the capital and operating costs required for the production, and
carbon tax
savings from possible GHG emission reductions. The operating costs include the
costs
of the injected solvent, so a lower cost solvent may be a criterion to be
considered
when selecting the solvent.
[00141] As a skilled person in the art will appreciate, in a liquid
mixture containing
multiple solvents, the bubble point condition of the liquid mixture is
different from the
bubble point condition of a mixture containing only one of the solvents.
[00142] The candidate solvent should be suitable for dissolving at least
one of
the viscous hydrocarbons in the reservoir 100, such that it can function as a
diluent for
the hydrocarbons. Possible solvents may include non-polar solvents such as C3-
C15
hydrocarbons, for example, a C3, C4, C5, C6 or C7 alkane. In some embodiments,
the
solvent may be propane, iso-butane, n-butane, pentane, hexane, heptane, octane
or a
combination thereof. Cyclohexane, 2,2-dimethylpentane, 2,2,4-trimethylpentane,
or
combinations thereof may also be suitable solvents alone or in combination
with other
non-polar solvents. Other possible solvents may include polar solvents. Polar
solvents
may include one or more of the following functional groups: an ether group, an
epoxide
group, a carboxylic acid group, an aldehyde group, a ketone group, an
anhydride
group, an ester group, an alcohol group, an amine group, and the like as
disclosed in
CA 1,887,405, which is incorporated by reference herein. Other possible
solvents may
be multi-component solvents such as gas condensates, naphtha, diesel, other
diluents, or combinations thereof.
[00143] Not all solvents will work under all conditions, as would be
understood by
the skilled person. The solvent thus should be carefully selected for given
reservoir
conditions and for given overall production objectives. Some properties of the
solvent
may be readily recognized by a person skilled in the art. For example, the
skilled
person may be able to select a solvent that is vaporizable under given
injection
conditions (temperature and pressure) such that it can be injected into the
reservoir
100 in the gas (vapor) phase and so that it can substantially remain in the
vapor phase
34
CA 3060497 2019-10-29
until it reaches the interface region in the solvent chamber 106. In this
regard, heavier
solvents, such as C8-C15 hydrocarbons, may not be suitable under some
reservoir
conditions. If heavier solvents are desirable under such conditions, they may
be
combined with another lighter solvent to form a solvent mixture. The skilled
person
may also be able to recognize solvents that are condensable under given
temperature
and pressure conditions. In this regard, non-condensable solvent gases (under
reservoir conditions), such as methane and ethane, are not suitable solvents
for
embodiments disclosed herein.
[00144] In selecting a suitable solvent for use, the skilled person may be
guided,
by initially determining the pressure and temperature conditions of the
particular
reservoir. Typically, injection pressures and temperatures are also subject to
limitations set by regulatory bodies. The skilled person may select an
injection
pressure/temperature at a point which is at or near the upper
pressure/temperature
limit for the particular conditions in order to obtain maximum solvent
diffusivity and to
broaden the choice of solvents for use. Once the initial temperature and
pressure
conditions are set, the choice of potential solvents may be determined based
on the
guidance provided in this disclosure, and may be additionally based on routine
calculation, routine experimentation or routine simulation and analysis of
solvent
behaviour and properties in a given reservoir composition.
[00145] In selecting a suitable solvent, the skilled person may also be
guided by
the solvent-crude hydrocarbon miscibility profiles for the solvents that meet
the
pressure/temperature requirements set out above. Solvent-crude hydrocarbon
miscibility profiles for a wide array of solvents are known, as discussed in
H.
Nouroozieh, M Kariznovi and J. Abedi, "Experimental and modeling studies of
phase
behavior for propane/Athabasca bitumen mixtures," Journal of Fluid Phase
Equilibria,
397 (2015) 37-43, the entire contents of which are incorporated by reference
herein. In
general, the skilled person may select a solvent which has a suitable solvent-
crude
hydrocarbon mixing coefficient, such that it will serve to mobilize
hydrocarbons within
the reservoir 100 during the development and expansion of the solvent chamber
106.
For this reason, highly polar solvents may not be appropriate under some
reservoir
CA 3060497 2019-10-29
conditions. Likewise, the skilled person may select a solvent which has a
suitable
solvent-asphaltene miscibility (or precipitation) coefficient. In order to
select an
appropriate solvent for a particular set of reservoir conditions, the skilled
person may
also rely on the teachings in this disclosure, in combination with routine
calculation,
routine experimentation, or routine simulation related to solvent-crude
hydrocarbon
miscibility profiles, or solvent-asphaltene miscibility profiles.
[00146] In selecting a suitable solvent, the skilled person may be further
guided
by the solvent bubble point in the fluid mixture in the production zone under
the
reservoir operating conditions. As noted, to avoid excess heating, which is
non-
productive or less efficient, substantial solvent re-vaporization within the
production
zone 108 may be prevented. Further, solvent re-vaporization may increase the
viscosity of the liquid mixture in which the solvent acts as a diluent.
Solvents which
substantially evaporate or remain substantially in the vapor phase at a very
low
temperature, such as below about 50 C to about 60 C, may not be suitable,
because
if such solvents were used, the production zone would need to be maintained at
even
lower temperatures, and the oil mobility at these lower temperatures would be
too low
to allow efficient production. At such low temperatures, other potential
problems may
arise which may negatively affect the production process, such as hydrate
formation or
the like.
[00147] In selecting a suitable solvent, the skilled person may be
additionally
guided by additional factors such as solvent cost, solvent recoverability,
solvent
toxicity, and solvent recyclability. A skilled person can weigh these
exemplary
additional factors when selecting an appropriate solvent without requiring
undue
experimentation and without requiring inventive ingenuity.
[00148] As can be appreciated, the temperatures under native conditions in
different reservoirs may vary. For example, the native temperature may be from
about
7 C to about 22 C, from about 9 C to about 15 C, or from about 10 C to about
13 C,
depending on the location of the reservoirs and the time. The native pressures
may
also vary in different reservoirs. For example, the native pressure in a
reservoir may
36
CA 3060497 2019-10-29
be from about 0.1 MPa to about 4 MPa, from about 0.5 MPa to about 3.5 MPa, or
from
about 1 MPa to about 3 MPa. The pressure and temperature profiles in a
reservoir
may also vary depending on the location and other characteristics of the
reservoir.
[00149] The types of viscous hydrocarbons within different reservoirs may
also
vary. Depending on the in situ density and viscosity of the viscous
hydrocarbons, the
viscous hydrocarbons may comprise, for example, a combination of heavy oil,
extra
heavy oil and bitumen. Heavy oil, for example, may be defined as any liquid
petroleum
hydrocarbon having an American Petroleum Institute (API) Gravity of less than
about
20 and a viscosity greater than 1,000 mPa-s. Extra heavy oil, for example,
may be
defined as having a viscosity of over 10,000 mPa-s and about 100 API Gravity.
The
API Gravity of bitumen ranges from about 12 to about 7 and the viscosity is
greater
than about 100,000 mPa-s. For example, the bitumen in a reservoir may have an
API
of 10 and a viscosity of about 110,000 mPa-s. API Gravity is also referred to
as API for
brevity.
[00150] The recovery processes described herein are not limited to any
particular
type of reservoirs or hydrocarbon compositions in the reservoir.
[00151] As noted earlier, in selected embodiments, the injection well 120
may be
completed with, for example, a perforated or slotted liner along the
horizontal section
of the well. The production well 140 may also be completed with a slotted
liner along
the horizontal section of the well. In other embodiments, the wells may be
completed
differently as described above. For example, the injection or production well
may
include perforations, slotted liners, screens, oufflow control devices
(injection well),
inflow control devices (production well), or a combination thereof as known to
one
skilled in the art.
[00152] In selected embodiments, one or both of the wells 120 and 140 may
be
provided with standard completion devices and equipment used in a typical
solvent
aided process, or used in wells that are suitable for use in a SAGD process
with
suitable modifications for solvent injection. Such devices and equipment may
include
flow control devices (FCDs), temperature measuring devices such as distributed
37
CA 3060497 2019-10-29
temperature sensing (DTS) devices or fibre optic measurement or control
components,
or the like.
[00153] In selected embodiments, the injection well 120 may be vertically
spaced
from the production well 140 by a distance within a range of from 3 m to 10 m,
or from
4 m to 6 m. These distances are exemplary and may be varied to optimize the
operation performance. A skilled person could select the well spacing by
considering
relevant processing parameters such as the temperature and pressure of the
reservoir
100 and the mobility of the viscous hydrocarbons present therein. In selected
embodiments, the length of the horizontal sections of the wells 120 and 140
may vary.
For example, in some embodiments, the horizontal sections of the wells 120 and
140
may have a length from 200 m to 1400 m, or from 600 m to 1000 m. The injection
well
120 and the production well 140 may be configured and completed in any
suitable
manner so long as the wells are suitable for injection of the selected solvent
and
production of a fluid from the reservoir as described herein. In some
embodiments, the
terminal sections of the wells 120 and 140 may be substantially parallel to
one
another. A person of skill in the art will appreciate that while there may be
some
variation in the vertical or lateral trajectory of the wells 120 and 140
(causing increased
or decreased separation there between), such wells for the purpose of this
application
will still be considered substantially horizontal and substantially parallel
to one another.
[00154] In selected embodiments, the surface facility 220 may have a
supply line
(not shown) connected to an injection fluid source for supplying the solvent.
In
selected embodiments, one or more additional supply lines may be provided for
supplying other fluids, additives or the like (not shown) for co-injection
with the solvent.
Each supply line may be connected to an appropriate source of supply, which
may
include, for example, a truck, a fluid tank, or the like. In some embodiments,
co-
injected fluids or materials may be pre-mixed before injection. In other
embodiments,
co-injected fluids may be separately supplied into the injection well 120.
38
CA 3060497 2019-10-29
[00155] In selected embodiments, the surface facility 240 may include a
fluid
transport pipeline (not shown) for conveying the produced fluids to a
downstream
facility (not shown) for processing or treatment. The surface facility 240 may
also
include additional optional equipment for producing a fluid from the
production well
140, as can be understood by one skilled in the art.
[00156] In selected embodiments, other necessary or optional surface
facilities
(not shown) may also be provided, as can be understood by one skilled in the
art. For
example, the surface facilities 220 and 240 may include one or more of a pre-
injection
treatment facility for treating a material to be injected into the formation,
a post-
production treatment facility for treating a produced material, a solvent
recycling
facility, and a control or data processing system for controlling production /
operation
or for processing collected operational data.
[00157] Downhole heaters (not shown), such as those disclosed in CA
2,304,938, may be used in selected embodiments as described herein. The
heaters
may include an electric heater. A suitable heating system may also be used.
[00158] In addition to solvents, other suitable injection fluids such as
steam,
diesel, natural gas liquids, gas condensate, C3-C15 hydrocarbons, non-
condensable
gases (NCGs), or a combination thereof may be injected during a start-up
stage. While
not all of these fluids or solvents will work under all conditions, a suitable
fluid for use
in a start-up stage may be selected by a person skilled in the art having
regard to the
particular reservoir conditions (e.g. temperature, pressure, composition), in
view of the
guidance provided in this disclosure. NCGs include, but are not limited to
air, nitrogen,
carbon dioxide, methane, natural gas, other light hydrocarbons, or a
combination
thereof. The NCG may facilitate maintaining at least a portion of the solvent
in the
vapor phase due to a partial pressure effect, allowing the solvent to travel
further
before completely condensing.
39
CA 3060497 2019-10-29
[00159] The injection temperature and injection pressure for any given
injection
fluid in the start-up stages may also vary. Possible injection temperatures
may be, for
example, from the ambient temperature to about 250 C or about 290 C.
Possible
injection pressures may be from about 2 MPa to about 7 MPa.
[00160] In selected embodiments, the time period of the production stage
may
vary. For example, it may last for a period of about 1 year to about 10 years.
Likewise,
the injection temperature and injection pressure during the production stage
may vary
over time and may vary in different applications. The injection temperatures
may be,
for example, from about 175 C to about 350 C, depending on the solvent
selected
and the reservoir conditions. The injection pressures may be from about 2 MPa
to
about 7 MPa.
[00161] The wells 120 and 140 may be positioned towards the bottom of the
pay
zone 102, which may be more efficient as the heated solvent vapor may tend to
rise
up in the solvent chamber 106.
[00162] In selected embodiments, the time period of the blowdown stage may
vary. For example, the blowdown stage may last for a period of about 1 month
to
about 12 months. In selected embodiments, the injected fluid, injection
temperature
and pressure used during the blown-down stage may vary. Possible fluids for
blowdown may include methane, ethane, propane, N2, CO2 or the like. Possible
blowdown pressures may range from 2 MPa to 7 MPa, and possible blowdown
temperatures may range from ambient temperature to about 250 C or about 350
C.
[00163] As discussed earlier with respect to FIG. 2, the temperatures of
the
various regions of the reservoir 100 generally decrease as the distance from
the
injection well 120 and the production well 140 becomes longer, towards the
interface
region 150 of the solvent chamber 106. In the interface region 150, the
temperature
may decrease quickly, and the temperature just outside the solvent chamber may
be
close to or at the reservoir native temperature. Thus, the temperature of the
injected
solvent may be the highest at the injection well 120, and may drop modestly as
the
solvent travels through the central region of the solvent chamber 106.
CA 3060497 2019-10-29
[00164] In select embodiments, the injection temperature may be between
125
C and 350 C, and the injected solvent may cool down to a temperature between
about 45 C and to about 145 C as it passes through the solvent chamber 106.
As the
solvent vapor contacts materials within the cooler interface region 150 its
temperature
may decrease more quickly, and the solvent may condense and mix with
hydrocarbons in the interface region to form a liquid mixture containing the
solvent and
mobilized hydrocarbons. The liquid mixture may also contain asphaltenes. In
select
embodiments the temperature at the interface region may be between 25 C and
125 C.
[00165] In different embodiments, the process parameters may be selected
to
improve overall process efficiency, with an aim to recover the maximum amount
of oil
from the reservoir. The process may also be designed to reduce the amount of
the
solvent used, or to recapture injected solvent quickly. Convenient recycling
and re-use
of the solvent may be a factor, but reducing or avoiding solvent recycling may
be
beneficial in some embodiments; recycling a solvent may not be as efficient as
for
recycling steam because the gravity is not as efficient for driving solvent
drainage as
compared to driving steam drainage.
[00166] Another factor to consider is overall reduced energy usage. Such a
factor
may be assessed using a net energy intensity (El). The El for a given process
may be
assessed by a person skilled in the art based on known methods and tools.
[00167] For example, some analysis has shown that substantial energy
savings
can be obtained for a given recovery factor (RF) (such as at 70% RF) with an
embodiment of the present disclosure in a homogenous reservoir, as compared to
other processes. In particular, using the El of a typical SAGD process as the
base line,
using propane according to the present disclosure may reduce the El by as much
as
75%, and using butane may reduce the El by as much as 45%, at 3 MPa. In other
words, propane may reduce the El to about 1/14 of the SAGD value, butane may
reduce the El to about 1/8 of the SAGD value. Pentane may reduce the El to
about 1/4
of the SAGD value. The reduced effect of butane as compared to propane is
expected
41
CA 3060497 2019-10-29
to be largely due to the higher heating temperature permitted in the butane
process.
[00168] The process parameters may be selected to reduce or minimize the
amount or volume of injected solvent without sacrificing the production rate,
production
efficiency, or recovery factor.
[00169] It is noted that it has been recognized that when a solvent is
injected in
the vapor phase into the reservoir at lower temperatures, such as when the
injection
temperature is close to the bubble point of the solvent at the given pressure,
the
solvent can condense quickly in the reservoir after entering into the
reservoir and
losing some of its heat energy to the surrounding materials. Further, the
temperature
in the production zone 108 around the production well 140 may be controlled to
be
lower than the bubble point temperature of the solvent to ensure that the
solvent will
be substantially in the liquid phase in the production zone 108.
[00170] When the solvent injection temperature is relatively high, viscous
hydrocarbons in the reservoir may be mobilized mainly by viscosity reduction
due to
heat transfer, which will be higher at elevated temperatures. In this case,
solvent
dissolution can still have an effect on mobilizing the hydrocarbons but such
effect may
be secondary depending on the injection temperature and other factors such as
solvent type and injection pressure. At a higher injection temperature, the
solvent will
have a lower density at a given pressure and thus the same amount of solvent
can
occupy more space within the reservoir. Consequently, a lower amount of the
solvent
may be required to achieve similar hydrocarbon recovery at a higher solvent
injection
temperature. However, to heat the solvent to these higher injection
temperatures, the
process may be more energy intensive.
[00171] In some embodiments, it may be beneficial to vary the solvent
injection
temperature during hydrocarbon production based on reservoir conditions and
production progress, so as to balance and optimize energy efficiency, solvent
usage,
and hydrocarbon recovery performance. Varying the injection temperature may
also
provide other benefits in different embodiments.
42
CA 3060497 2019-10-29
[00172] ALTERNATIVES AND VARIATIONS
[00173] In some embodiments, injection pressure may be controlled using
known
techniques that have been used in other processes such as SAGD processes and
other processes involving injection of a solvent. Alternatively, different or
additional
techniques may be sued.
[00174] As discussed above, the solvent may be delivered relatively hot to
the
reservoir formation. However, it is possible that the solvent may be fed into
the
injection well with or without pre-heating at the surface.
[00175] In some embodiments, the solvent condensed in the reservoir will
be
recovered (produced) in the oleic phase. Additionally or alternatively, vapor
solvent
could remain in the reservoir formation, and may also be recovered with a
reservoir
fluid in the gaseous phase.
[00176] In some embodiments, an additive or chemical such as toluene may
be
injected during solvent injection or during a post-production phase. Injection
of toluene
may help to reduce asphaltene precipitation. About 5 wt% of toluene may be co-
injected with a solvent.
[00177] In some embodiments, fluids recovered at the surface may be
separated
from produced solvent to undergo recycling.
[00178] In some embodiments, the injected solvent may be recovered and
recycled.
[00179] In some embodiments, particularly in a process with higher
injection
temperatures, a barrier or insulation layer may be formed at the overburden,
which
may assist in reducing heat loss through the overburden once the solvent
chamber
has substantially reached full vertical growth. For example, a barrier layer
may be
formed after this condition is reached. Alternatively, a barrier may be formed
at an
earlier or later point in time. In another example, a barrier layer may be
formed at or
about the time that the peak process threshold has been reached and detected.
The
43
CA 3060497 2019-10-29
barrier layer may be formed of an insulation composition such as described in
US
2015/0159476 to Warren et al., the entire contents of which are incorporated
herein by
reference. The barrier layer may also be formed from an artificial layer such
as those
disclosed in US 2011/0186295 or CA 2,729,430 to Kaminski et al., the entire
contents
of which are incorporated herein by reference.
[00180] In some embodiments, non-condensable gases (NCGs) may be
generated in the reservoir such as due to heating. Additionally or
alternatively, an NCG
may be injected as an additive in some embodiments. Conveniently, the presence
of
NCGs in the formation can enhance lateral dispersion of the solvent vapor to
spread
the solvent laterally into the reservoir formation. Increased lateral
dispersion of the
solvent is expected to assist lateral growth of the solvent chamber, and hence
enhance oil production.
[00181] While in some of the above discussed embodiments a pair of wells
is
employed for injection and production respectively, it can be appreciated that
some
processes as disclosed herein may be implemented with a single well or
unpaired
wells. The single well, or an unpaired well, may be used alternately for
injection or
production. The single well may have a substantially horizontal or vertical
section in
fluid communication with the reservoir. The single well may be a well that is
configured
and completed for use in a cyclic steam stimulation (CSS) recovery process.
[00182] As alluded to earlier, the injected solvent may be an alkane or
another
hydrocarbon solvent, or may be a mixture of different solvents. The solvent
mixture
may also include a relatively small portion of another material. For example,
the
mixture may contain 90 wt% or more of a hydrocarbon solvent and 10 wt% or less
of
the other material. The other material may be light weight filler included to
reduce the
overall density of the injected mixture. The other material may also be a
material for
selective zone shutoff, or to reduce interfacial tension between various
materials in the
formation, or perform other functions. The other material may include one or
more of
Di-methyl ether (DME), methanol, ethanol, methane, CO2, surfactant, polymer,
gel,
nanoparticles, or the like.
44
CA 3060497 2019-10-29
[00183] The following examples further illustrate embodiments of the
present
disclosure, or demonstrate functionalities or results that could be achieved
in various
aspects, configurations, or combinations of the features described herein.
[00184] Examples
[00185] Example I
[00186] Computer simulations have been conducted to predict solvent
density
distribution in the solvent chamber of a simulated reservoir. A representative
simulation result is shown in FIG. 16.
[00187] Figure 16 shows the average in-situ density profile of a pure
solvent
process, with propane as the solvent. As can be seen, the solvent density
varies in the
formation, increasing from the injection well towards the interface region.
The solvent
density is about 30 kg/m3 in the region closer to the injection zone, but
about 100
kg/m3 in the region closer to the chamber/bitumen interface. The density
variation is
expected to be due to heat loss from the solvent to the bitumen material in
the
chamber after the solvent enters the formation from the injection well. For
the model
simulation, the solvent injection temperature was modelled to be 225 C near
the
injection well, and the native reservoir temperature was modeled to be 15 C.
The
reservoir pressure was modelled to be about 3MPa.
[00188] Figure 16 also shows that the solvent at the chamber/bitumen
interface
behaves more like a liquid than a gas, and hence the solvent is expected to
dissolve
more into the bitumen phase, which helps oil production. Such a solvent
density profile
is desirable for more efficient oil recovery, as in this case, the solvent in
the majority of
the solvent chamber is more gas-like and of lower density.
[00189] Example ll
[00190] FIG. 17 shows representative experimental data obtained from field
tests.
CA 3060497 2019-10-29
[00191] For these tests, steam was initially injected and then both steam
and
propane solvent were co-injected. As can be seen, during the illustrated
period, after
propane injection was initiated, the propane injection rate was maintained at
about 40
ton/day. The steam injection rate was maintained relatively constant at about
10
ton/day before and after solvent injection.
[00192] With relatively constant heat energy input from the steam
injection, it was
expected that the reservoir temperature would gradually drop with time and
continued
solvent injection, as illustrated in FIG. 17, where the reservoir temperature
dropped
from about 200 C at the left-hand side of the graph (start of propane
injection) to
about 125 C at the right-hand side of the graph, within a time period of
about 13
months.
[00193] Further decrease in the reservoir temperature below about 125 C
had a
negative effect on the oil production rate. To increase or maintain the oil
production
rate, the propane injection rate needed to be increased, or the steam
injection rate
would need to be increased, which would increase the reservoir temperature.
[00194] It is noted that the oil production rate at the right-hand end of
the graph
was about 25% of the oil production rate at the left-hand end of the graph.
[00195] The injection pressure during solvent injection was as high as 5
MPa for
injecting supercritical propane, and was gradually reduced to eventually about
3.2
MPa as temperature decreased.
[00196] FIG. 17 also shows the reduced density of the injected propane in
the
solvent chamber in the formation, which varied from about 0.2 to about 0.3,
and was
less than 0.5.
[00197] FIG. 17 further shows the first partial derivative of the reduced
density of
the solvent with respect to temperature (denoted as P.D.R.D_T), which varied
substantially (increasing significantly) as the reservoir temperature dropped
to below
150 C, which indicated that the first derivative is a strong function of
temperature at
temperatures below 150 C.
46
CA 3060497 2019-10-29
[00198] The median value of the second derivative of the reduced density
with
respect to reduced temperature at a high temperature region was about 0.43,
indicating a weak dependence of the first derivate of the reduced density on
temperature. In comparison, the median value of the corresponding second
derivative
at lower temperatures was found to be about 1.5, indicating relatively strong
temperature dependence.
[00199] FIG. 18 shows an illustrative representation of the temperature
dependence of the reduced solvent density and its first derivative as a
function of
reduced temperature (Tr) for representative field data. As can be seen, there
are two
(2) distinguishable regions or clusters of data: a first region in which the
First
P.D.R.D_T depends linearly with Tr (weak dependency region, represented by
diamond shaped data points), and a second region in which the First P.D.R.D_T
depends non-linearly, up to the 3rd order, with Tr (strong dependence region,
represented by triangular shaped data points). The degree of correlation (R2)
among
the data points is 0.9699 in the first region, and 0.265 in the second region.
A line fit of
the data points in the first region can be represented by a linear equation, y
(First
P.D.R.D_T) = -0.8153 x (Tr) + 1.2387. In comparison, a line fit of the data
points in the
second region can be represented by a third order equation, y = - 273.41 x3 +
922.7 x2
+ 1038.1 x + 389.68.
[00200] The First P.D.R.D_T may be considered to have a weak dependence on
temperature if the condition in Equation (5) is satisfied. The First P.D.R.D_T
may be
considered to not have a weak dependence on temperature if the second partial
derivative of the reduced solvent density with respect to temperature is
higher than 1,
i.e., the condition in Equation (5) is not satisfied. In other words, if the
First P.D.R.D_T
scales generally linearly (first order) with temperature, or with a less order
of
dependence, it is considered to have weak temperature dependence. If the First
P.D.R.D_T scales on a higher order (>1) with temperature, it is considered to
have
strong temperature dependence.
47
CA 3060497 2019-10-29
[00201] CONCLUDING REMARKS
[00202] It will be understood that any range of values herein is intended
to
specifically include any intermediate value or sub-range within the given
range, and all
such intermediate values and sub-ranges are individually and specifically
disclosed.
[00203] It will also be understood that the word "a" or "an" is intended
to mean
"one or more" or "at least one", and any singular form is intended to include
plurals
herein.
[00204] It will be further understood that the term "comprise", including
any
variation thereof, is intended to be open-ended and means "include, but not
limited to,"
unless otherwise specifically indicated to the contrary.
[00205] When a list of items is given herein with an "or" before the last
item, any
one of the listed items or any suitable combination of two or more of the
listed items
may be selected and used.
[00206] Of course, the above described embodiments of the present
disclosure
are intended to be illustrative only and in no way limiting. The described
embodiments
are susceptible to many modifications of form, arrangement of parts, details
and order
of operation. The invention, rather, is intended to encompass all such
modification
within its scope, as defined by the claims.
48
CA 3060497 2019-10-29