Note: Descriptions are shown in the official language in which they were submitted.
85760071
METHODS AND SYSTEMS FOR INDUSTRIAL PROCESSES
FIELD
This disclosure relates generally to information and communication technology
(ICT)
for the cannabis industry. In particular, in some embodiments, the disclosure
relates
to systems and methods for tracing cannabinoid-containing substances through
complex industrial cultivation, extraction, manufacturing and distribution
chains.
BACKGROUND
While the legal market for cannabis-based consumer products is gaining
momentum,
historically, the clandestine nature of the cannabis industry has largely
suppressed
innovation, and led to a market characterised by unsophisticated and small-
scale
production processes, as well as underdeveloped consumer product safety and
characterisation standards.
Even in jurisdictions in which medical cannabis has been legal for some time,
most
governments impose strict controls on cannabinoid-containing substances, for
at
least the reasons of undermining the financial success of organized crime
(e.g. by
reducing the amount of cannabis-based products in the black/grey market) and
ensuring public safety (e.g. by restricting access to psychotropic
substances). As a
result, cannabis producers and processors have been subject to stringent
record
keeping requirements, particularly in regard to tracking the provenance and
chain of
custody of cannabinoid-containing substances.
While these requirements are quite onerous, compliance has not historically
posed a
significant technical problem because the size of the medical cannabis market
has
kept the demand for cannabis-based products relatively limited. As a result,
cannabis
producers and processors have had no need to implement industrial-scale
processes.
CA 3062485 2019-11-21
85760071
Moreover, the limited diversity of legally-available cannabis-based consumer
products (e.g. restricted to cannabis flower, seeds and oils, in Canada) has
also
helped cannabis producers and processors comply with the record keeping
requirements imposed by public health organizations, in that the limited
consumer
product diversity effectively limits record-keeping requirements. Accordingly,
many
cannabis producers and processors have relied on manual and/or labour-
intensive
systems and methods of record keeping.
More recently however, rapidly evolving changes in cannabis legislation in
many
jurisdictions around the world are contributing to a quantum leap in both the
demand
for, and the variety of (e.g. edibles, concentrates, etc.), cannabis-based
consumer
products. This entirely new market for complex and sophisticated cannabis-
based
products will need to be supported by equally complex and sophisticated
industrial
cultivation, extraction, manufacturing and distribution processes.
As such, attempts at scaling known manual and/or labour-intensive systems and
methods of tracking cannabinoid-containing substances to meet this demand can
lead to dilatory, ineffective and unsafe solutions. Applying these known
solutions to
this new and unique industrial environment provides significant financial and
technical
drawbacks. Moreover, scaled versions of known solutions are clearly also
susceptible
to human error and data-security risks, which in turn put the public's safety
at risk and
leave legal production, manufacturing and distributions chains open to
misappropriation by organized crime.
For these and other reasons, there is a clear need for technical improvements
in
data-communication network and computer-based systems and methods for tracking
cannabinoid-containing substances through complex industrial cultivation,
extraction,
manufacturing and distribution chains.
SUMMARY
In accordance with various aspects of this disclosure, the provenance and
chain of
custody of cannabinoid-containing substances are tracked and/or traced through
2
CA 3062485 2019-11-21
85760071
industrial cultivation, extraction, manufacturing and distribution processes
by way of
information and communication technology methods and systems.
In accordance with an aspect, this disclosure relates to a method that
comprises the
step of providing a database in which is stored information associated with a
plurality
of cannabis plants and a plurality of cannabis products. The method also
comprises
the steps of assigning a batch identifier to a batch of the plurality of
cannabis plants
and processing plant material from a portion of the cannabis plants in the
batch using
a first process to produce a plurality of units of a first cannabis product.
The method
also comprises the step of processing plant material from another portion of
the
cannabis plants in the batch using a second process to produce a plurality of
units of
a second cannabis product. The method also comprises the steps of assigning a
first
lot identifier to a lot of the plurality of units of the first cannabis
product and a second
lot identifier to a lot of the plurality of units of the second cannabis
product and
modifying the database to include information conveying the batch identifier,
the first
lot identifier and the second lot identifier, wherein the first lot identifier
and the second
lot identifier are each associated with the batch identifier.
In accordance with another aspect, this disclosure relates to a processor-
readable
storage medium, having processor-executable instructions stored thereon,
which,
when executed by a processor, cause a computing device comprising the
processor
to implement a system configured to implement a database configured to store
information associated with a plurality of cannabis plants and a plurality of
cannabis
products. The system is further configured to assign a batch identifier to a
batch of
the plurality of cannabis plants and receive processing information relating
to the
processing of plant material from a portion of the cannabis plants in the
batch using a
first process to produce a plurality of units of a first cannabis product. The
system is
further configured to receive processing information relating to the
processing of plant
material from another portion of the cannabis plants in the batch using a
second
process to produce a plurality of units of a second cannabis product. The
system is
further configured to assign, using the processing information, a first lot
identifier to a
lot of the plurality of units of the first cannabis product and a second lot
identifier to a
3
CA 3062485 2019-11-21
85760071
lot of the plurality of units of the second cannabis product. The system is
further
configured to modify the database to include information relating to the batch
identifier, the first lot identifier and the second lot identifier, wherein
the first lot
identifier and the second lot identifier are each associated with the batch
identifier.
In accordance with yet another aspect, this disclosure relates to a method
that
comprises the steps of providing a database in which is stored information
associated
with a plurality of cannabis plants and a plurality of cannabis products and
assigning
a batch identifier to a batch of the plurality of cannabis plants. The method
further
comprises the steps of extracting cannabinoids from the plant material of a
portion of
the cannabis plants in the batch using an extraction method to produce a
cannabis
extract and assigning an extract identifier to the cannabis extract. The
method further
comprises processing an amount of the cannabis extract to produce a plurality
of
units of a cannabis product and assigning a lot identifier to a lot of the
plurality of
units of the cannabis product. The method further comprises the step of
modifying the
database to include information relating to the batch identifier, the extract
identifier
and the lot identifier, wherein the lot identifier is associated with the
extract identifier
and the extract identifier is associated with the batch identifier.
In accordance with yet another aspect, this disclosure relates to a processor-
readable
storage medium, having processor-executable instructions stored thereon,
which,
when executed by a processor, cause a computing device comprising the
processor
to implement a system configured to implement a database configured to store
information associated with a plurality of cannabis plants and a plurality of
cannabis
products. The system being further configured to assign a batch identifier to
a batch
of the plurality of cannabis plants. The system being further configured to
receive
extraction information relating to the extraction of cannabinoids from the
plant
material of a portion of the cannabis plants in the batch using an extraction
method to
produce cannabis extract and assign an extract identifier to the cannabis
extract. The
system being further configured to receive processing information related to
the
processing of an amount of the cannabis extract to produce a plurality of
units of a
cannabis product and assign a lot identifier to a lot of the plurality of
units of the
4
CA 3062485 2019-11-21
85760071
cannabis product. The system being further configured to modify the database
to
include information relating to the batch identifier, the extract identifier
and the lot
identifier, wherein the lot identifier is associated with the extract
identifier and the
extract identifier is associated with the batch identifier.
In accordance with yet another aspect, this disclosure relates to a method of
labelling cannabis products in an automated manufacturing process. The method
comprises processing a portion of a first amount of cannabinoid-containing
substance
to sequentially produce a first plurality of units of a cannabis product, the
first amount
of cannabinoid-containing substance being associated with a first cannabinoid-
containing substance identifier. The method further comprises determining a
last unit
of cannabis product produced in the first plurality of units and processing a
portion of
a second amount of cannabinoid-containing substance to sequentially produce a
second plurality of units of a cannabis product, the second amount of
cannabinoid-
containing substance being associated with a second cannabinoid-containing
substance identifier. The method further comprises labelling the first and
second
pluralities of units of cannabis product by controlling an automated labelling
system to
label units of cannabis product with label information conveying a first lot
identifier
associated with the first cannabinoid-containing substance identifier until
the last unit
of cannabis product has been labelled, and to label units of cannabis product
with
label information conveying a second lot identifier associated with the second
cannabinoid-containing substance identifier thereafter.
In accordance with yet another aspect, this disclosure relates to a method for
applying an indicia to containers filled with cannabis-infused beverage. The
method
comprises providing a marking station to mark with an indicia containers
filled with
cannabis-infused beverage, the indicia being indicative of a particular amount
of a
cannabinoid-containing substance derived from cannabis plant material and
containing one or more cannabinoids, from which the cannabis-infused beverage
is
prepared, the marking station configured to receive a succession of containers
filled
with cannabis-infused beverage, the succession of containers being arranged in
successive sets, where each set of containers is filled with cannabis-infused
5
CA 3062485 2019-11-21
85760071
beverage made from a respective amount of the cannabinoid-containing
substance.
The method also comprises applying at each container from a first set a first
indicia
associated with a first amount of cannabinoid-containing substance from which
the
cannabis-infused beverage dispensed in the first set of containers is made.
The
method also comprises detecting in the succession of containers a transition
from a
first set of containers to a second set of containers, wherein the first set
of containers
is filled with cannabis-infused beverage prepared from a first amount of
cannabinoid-
containing substance and the second set of containers is filled with cannabis-
infused
beverage prepared from a second amount of cannabinoid-containing substance.
The
method also comprises controlling the marking station to apply a first indicia
to the
last container of the set in the succession, wherein the first indicia is
associated with
the first amount, and a second indicia to the next container in the succession
of
containers which is the first container of the second set, the second indicia
being
associated with the second amount.
In accordance with yet another aspect, this disclosure relates to a method of
identifying a lot of cannabis products for recall. The method comprises
providing a
database in which is stored information associated with a plurality of batches
of
cannabis plants, each batch being associated with a batch identifier, and a
plurality of
lots of cannabis products, each lot being associated with a lot identifier,
wherein each
batch identifier in the database is associated with at least one lot
identifier. The
method also comprises determining, using a lot identifier associated with a
defective
cannabis product, at least one suspect batch identifier associated with the
lot
identifier. The method also comprises determining, for each archived cannabis
material sample associated with the at least one suspect batch identifier,
whether the
archived cannabis material sample is defective and determining all lot
identifiers in
the database associated with each archived cannabis material sample that is
found to
be defective.
In accordance with yet another aspect, this disclosure relates to a method of
identifying a lot of cannabis products for recall. The method comprises
providing a
database in which is stored information associated with a plurality of batches
of
6
CA 3062485 2019-11-21
85760071
cannabis plants, each batch being associated with a batch identifier, and a
plurality of
lots of cannabis products, each lot being associated with a lot identifier,
wherein each
batch identifier in the database is associated with at least one lot
identifier. The
method also comprises providing a graphical user interface implemented on a
computer system to enable a user to input a suspect lot identifier associated
with a
defective cannabis product. The method also comprises providing a database
search
module implemented on the computer system, the database search module being
configured to determine, in response to a user inputting a suspect lot
identifier, at
least one suspect batch identifier associated with the suspect lot identifier
in the
database and all lot identifiers associated with the at least one suspect
batch
identifier in the database and inputting a suspect lot identifier into the
graphical user
interface.
In accordance with yet another aspect, this disclosure relates to a system for
identifying a lot of cannabis products for recall. The system comprises a
database in
which is stored information associated with a plurality of batches of cannabis
plants,
each batch being associated with a batch identifier, and a plurality of lots
of cannabis
products, each lot being associated with a lot identifier, wherein each batch
identifier
in the database is associated with at least one lot identifier. The system
also
comprises a graphical user interface implemented on a computer system to
enable a
user to input a suspect lot identifier associated with a defective cannabis
product. The
system also comprises a database search module implemented on the computer
system, the database search module being configured to determine, in response
to a
user inputting a suspect lot identifier through the graphical user interface,
at least one
suspect batch identifier associated with the suspect lot identifier in the
database and
recall lot identifiers associated with the at least one suspect batch
identifier in the
database.
In accordance with yet another aspect, this disclosure relates to a method for
dynamically generating a hierarchal dataset having a tree structure,
representative of
a process flow to transform a batch of cannabis plants into a range of
cannabis
products. The method comprises recording on a computer readable storage medium
7
CA 3062485 2019-11-21
85760071
a batch identifier associated with the batch of cannabis plants, the batch
identifier
distinguishing the batch of cannabis plants among a plurality of batches of
cannabis
plants, wherein the batch identifier is a root level of the hierarchal
dataset. The
method also comprises processing a first portion of the batch of cannabis
plants
using a first process to produce a plurality of units of first cannabis
products and
recording on the computer readable storage medium a first lot number
associated
with the first cannabis products. The method also comprises processing a
second
portion of the batch of cannabis plants using a second process, to produce a
plurality
of units of a second cannabis product and recording on the computer readable
storage medium a second lot number associated with the second cannabis
products.
The method also comprises linking the first and second lot numbers to the
batch
identifier in the hierarchal dataset, whereby the first lot number forms a
first branch of
the hierarchal data set ascending from the root node and the second lot number
forms a second branch of the hierarchal data structure ascending from the root
node.
In accordance with yet another aspect, this disclosure relates to a method for
bottling
a cannabis-infused beverage. The method comprises providing a filling line
including
a filling station, a container marking station and a control device, the
control device
configured to control an operation of the container marking station. The
method also
comprises filling containers at the filling station with cannabis-infused
beverage
supplied from a master batch of cannabis-infused beverage, the master batch
being
prepared from an amount of cannabis-containing substance derived from cannabis
plant material, the cannabis-containing substance containing one or more
cannabinoids, the master batch including a quantity of cannabis-infused
beverage to
fill a plurality of containers, the filling station being configured to
perform a supply
switch from a first master batch to a second master batch of cannabis-infused
beverage, whereby a first set of containers is filled with cannabis-infused
beverage
drawn from the first master batch and a second set of containers is filled
with
cannabis infused beverage drawn from the second master batch. The method also
comprises applying an indicia on each container at the marking station, the
indicia
being indicative of the master batch of the cannabis-infused beverage
supplying the
filling station when the container is filled by the filling station. The
method also
8
CA 3062485 2019-11-21
85760071
comprises controlling with the control device the operation of the marking
station
such that when a supply switch is performed from the first master batch to the
second
master batch a marking switchover from a first indicia to a second indicia is
performed by the marking station such that containers filled with cannabis-
infused
beverage drawn from the first master batch are marked with a first indicia
associated
with the first master batch, and containers with cannabis-infused beverage
drawn
from the second master batch are marked with a second indicia associated with
the
second master batch.
In accordance with yet another aspect, this disclosure relates to a method for
manufacturing and packaging a cannabis-infused consumable product made from a
cannabis-containing substance. The method comprises providing multiple amounts
of
cannabis-containing substance, each amount derived from cannabis plant
material,
the cannabis-containing substance containing one or more cannabinoids, each
amount of cannabis-containing substance being associated with an identifier
allowing
distinguishing one amount from another amount. The method further comprises
providing a control device having a machine-readable storage and storing in
the
machine-readable storage identifiers associated with respective ones of the
amounts
of cannabis-containing substance. The method further comprises diluting each
amount of cannabis-containing substance with a diluting agent to produce a
master
batch of consumable product and dispensing the master batch into a set of
packages,
each package holding a portion of the master batch. The method further
comprises
applying an indicia on individual packages. The step of applying an indicia
further
comprises feeding a stream of individual packages to a marking unit and
distinguishing in the stream between individual packages holding a consumable
product made from different amounts of cannabis-containing substance and
controlling the marking unit with the control device to apply to each
individual
package an indicia derived from the identifier of the respective amount from
which the
consumable product in the package was made.
In accordance with yet another aspect, this disclosure relates to a method for
manufacturing and packaging a cannabis-infused consumable product made from a
9
CA 3062485 2019-11-21
85760071
cannabis-containing substance. The method comprises providing multiple amounts
of
a cannabis-containing substance, each amount derived from cannabis plant
material,
the cannabis-containing substance containing one or more cannabinoids. The
method comprises providing a control device having a machine-readable storage
and
storing in the machine-readable storage identifiers associated with respective
ones of
the amounts of cannabis-containing substance, the identifiers allowing
distinguishing
one amount from another amount. The method further comprises diluting each
amount of cannabis-containing substance with a diluting agent to produce
respective
master batches of consumable product and dispensing the master batches into
respective sets of individual packages, each package of a given set holding a
portion
of the respective master batch. The method further comprises feeding a stream
of
individual packages to a marking unit, the stream being arranged in an order
determined by which master batch is the source of the consumable product held
in
each individual package. The method further comprises, under control of the
control
device, synchronizing the operation of the marking unit with the order in
which the
stream of individual packages is arranged such that each individual package
receives
an indicia associated with the particular amount from which the consumable
product
in the package is made.
In accordance with yet another aspect, this disclosure relates to a method for
manufacturing and packaging a cannabis-infused consumable product made from a
cannabis-containing substance. The method comprises providing multiple amounts
of
cannabis-containing substance, each amount derived from cannabis plant
material,
the cannabis-containing substance containing one or more cannabinoids and
providing a control device having a machine-readable storage. The method
further
comprises diluting each amount of cannabis-containing substance with a
diluting
agent to produce respective master batches of consumable product. The method
further comprises, for each master batch, dispensing the master batch into a
set of
individual packages, each package holding a portion of the master batch and
withholding from dispensing into an individual package a residual volume of
consumable product from the master batch that is less than the volume of
consumable product required to fill the individual package to capacity. The
method
CA 3062485 2019-11-21
85760071
further comprises, for one or more master batches, determining the number of
individual packages filled to capacity from the master batch in the respective
set of
individual packages, storing the number in the machine-readable storage,
feeding a
stream of individual packages to a marking unit and controlling with the
control device
the marking unit including deriving from the machine readable storage the
number
and operating the marking unit a corresponding number of times to apply to
each
individual package in the set an indicia linked to the particular amount of
cannabis-
containing substance from which the consumable product in the package is made.
In accordance with yet another aspect, this disclosure relates to a method of
creating
video content. The method comprises receiving video images of a cannabis
operations area in which cannabis material is being processed and receiving
processing information associated with the processing being carried out in in
the
cannabis operations area. The method further comprises generating metadata
using
at least some of the processing information and generating a video record by
combining the video images and the nietadata.
These and other aspects of this disclosure will now become apparent to those
of
ordinary skill in the art upon review of a description of embodiments in
conjunction
with accompanying drawings.
BRIEF DESCRIPTION OF THE DRAWINGS
For a more complete understanding of the present disclosure, reference is now
made
to the following description taken in conjunction with the accompanying
drawings, in
which:
Fig. 1 is a flow diagram illustrating an example process for producing
cannabis
products;
Fig. 2 is a block diagram illustrating an example system for producing
cannabis
products;
Fig. 3 illustrates example formats of machine-readable code for a label;
11
CA 3062485 2019-11-21
85760071
Figs. 4A-4N are block diagrams illustrating an example system implementing an
inventory control system (ICS);
Fig. 5 is a block diagram illustrating an example implementation of a barcode
scanner
in communication with an ICS server;
Fig. 6 is a flow chart illustrating an example method according to one
embodiment;
Fig. 7 illustrates operation of a machine for generating labels, according to
one
embodiment;
Fig. 8 is a flow diagram illustrating an example method of labelling cannabis
products
in an automated manufacturing process.
Fig. 9 is a flow diagram illustrating an example method for drying and/or
curing a
cannabis material;
Fig. 10 is a flow diagram illustrating an example method for milling cannabis
material;
Fig. 11 is a flow diagram illustrating an example method for producing pre-
rolled
cannabis cigarettes with a cone filling machine;
Fig. 12 is a flow diagram illustrating an example process for producing
cannabis
extracts and other cannabis products;
Fig. 13 is a flow diagram illustrating an example method for decarboxylation
of a
cannabis product;
Fig. 14 is a flow diagram illustrating an example method for supercritical
fluid
extraction with CO2;
Fig. 15 is a flow diagram illustrating an example method for resin packaging;
Fig. 16 is a flow diagram illustrating an example process for oil formulation;
Fig. 17 is a flow diagram illustrating an example method for oil packaging;
12
CA 3062485 2019-11-21
85760071
Fig. 18 is a flow diagram illustrating an example method according to another
embodiment.
Fig. 19 is a flow diagram illustrating an example method for cannabis product
irradiation;
Fig. 20 is a flow diagram illustrating an example method for final packaging;
Fig. 21 illustrates an example of a lot record;
Fig. 22 illustrates an example of an extract record;
Fig. 23 illustrates an example of an extraction process record;
Fig. 24 is a block diagram of a cannabis producer and a cannabis processor,
according to one embodiment;
Fig. 25 is a schematic illustrating an example of traceability from a cannabis-
infused
consumer product back to a batch of cannabis plants;
Figs. 26-28 are block diagrams of a system for producing cannabis-infused
beverages, according to one embodiment;
Fig. 29 is a flow diagram illustrating an example method of producing cannabis-
infused beverages, according to one embodiment;
Fig. 30 is a flow diagram illustrating an example method for applying an
indicia to
containers filled with cannabis-infused beverage, according to another
embodiment;
Fig. 31 is a flow diagram illustrating an example method of producing a
cannabis-
infused consumer product, according to one embodiment.
Fig. 32 illustrates a system for identifying a lot of cannabis products for
recall,
according to one embodiment;
Fig. 33 is a flow diagram illustrating an example method of identifying a lot
of
cannabis products for recall;
13
CA 3062485 2019-11-21
85760071
Fig. 34 is a flow diagram illustrating another example of a method of
identifying a lot
of cannabis products for recall; and
Fig. 35 is a flow diagram illustrating an example method of creating video
content.
DETAILED DESCRIPTION
For illustrative purposes, specific example embodiments will be explained in
greater
detail below in conjunction with the figures. It should be appreciated,
however, that
the present disclosure provides many applicable concepts that can be embodied
in
any of a wide variety of specific contexts. The specific embodiments discussed
are
merely illustrative and do not limit the scope of the present disclosure. For
example,
embodiments could include additional, different, or fewer features than shown
in the
drawings. In the flow diagrams illustrated in the accompanying figures, a
rectangle
generally annotates a step, apparatus, device, location or operation, and a
pentagon
generally annotates an input, product or output.
The present disclosure relates, in part, to the production and traceability of
cannabis
products. Cannabis products could be any goods that are produced from cannabis
or
hemp, which include plants, plant material, oils, resins, drinks, food
additives, edibles,
creams, aerosol sprays and vaporization substances, for example. These
cannabis
products could be used for medical and/or recreational purposes. Cannabis
products
could include active substances such as cannabinoids. However, the cannabis
products described herein might not always include an active substance. As
used
herein, the term "cannabinoid" is generally understood to include any chemical
compound that acts upon a cannabinoid receptor. Cannabinoids could include
endocannabinoids (produced naturally by humans and animals), phytocannabinoids
(found in cannabis and some other plants), and synthetic cannabinoids
(manufactured artificially). For the purpose of this specification, the
expression
"cannabinoid" means a compound such as cannabigerolic acid (CBGA),
cannabigerol
(CBG), cannabigerol monomethylether (CBGM), cannabigerovarin (CBGV),
cannabichromene (CBC), cannabichromevarin (CBCV), cannabidiol (CBD),
cannabidiol monomethylether (CBDM), cannabidiol-C4 (CBD-C4), cannabidivarin
14
CA 3062485 2019-11-21
85760071
(CBDV), cannabidiorcol (CBD-C1), delta-9-tetrahydrocannabinol (A9-THC), delta-
9-
tetrahydrocannabinolic acid A (THCA-A), delta-9-tetrahydrocannabionolic acid B
(THCA-B), delta-9-tetrahydrocannabinolic acid-C4 (THCA-C4), delta-9-
tetrahydrocannabinol-C4, delta-9-tetrahydrocannabivarin (THCV),
delta-9-
tetrahydrocannabiorcol (THC-C1), delta-7-cis-iso tetrahydrocannabivarin, delta-
8-
tetrahydrocannabinol (A8-THC), cannabicyclol (CBL), cannabicyclovarin (CBLV),
cannabielsoin (CBE), cannabinol (CBN), cannabinol methylether (CBNM),
cannabinol-C4 (CBN-C4), cannabivarin (CBV), cannabinol-C2 (CBN-C2),
cannabiorcol (CBN-C1), cannabinodiol (CBND), cannabinodivarin (CBVD),
can nabitriol (CBT),
10-ethoxy-9hydroxy-delta-6a-tetrahydrocannabinol, 8,9-
d ihyd roxy-delta-6a-tetrahyd rocan nabinol, cannabitriolvarin
(CBTV), ethoxy-
cannabitriolvarin (CBTVE), dehydrocannabifuran (DCBF), cannabifuran (CBF),
cannabichromanon (CBCN), cannabicitran (CBT),
10-oxo-delta-6a-
tetrahydrocannabionol (OTHC), delta-9-cis-tetrahydrocannabinol (cis-THC),
3,4,5,6-
tetrahydro-7-hydroxy-alpha-alpha-2-trimethy1-9-n-propy1-2, 6-
methano-2H-1-
benzoxocin-5-methanol (OH-iso-HHCV), cannabiripsol (CBR), trihydroxy-delta-9-
tetrahydrocannabinol (tri0H-THC), cannabinol propyl variant (CBNV), and
derivatives
thereof.
For the purpose of this specification, the expressions "cannabidiol" or "CBD"
are
generally understood to refer to one or more of the following compounds, and,
unless
a particular other stereoisorner or stereoisonners are specified, includes the
compound "A2-cannabidiol." These compounds are: (1) A5-cannabidiol (2-(6-
isopropeny1-3-methy1-5-cyclohexen-l-y1)-5-pentyl-1,3-benzenediol); (2) A4-
cannabidiol
(2-(6-isopropeny1-3-methyl-4-cyclohexen-l-y1)-5-pentyl-1,3-benzenediol);
(3) A3-
can nabid lot (2-(6-isopropeny1-3-methyl-3-cyclohexen-l-y1)-5-pentyl-1,3-
benzenediol);
(4) A3,7-cannabidiol
(2-(6-isopropeny1-3-methylenecyclohex-1-y1)-5-penty1-1,3-
benzenediol); (5) A2-cannabidiol (2-(6-isopropeny1-3-methy1-2-cyclohexen-l-y1)-
5-
pentyl-1,3-benzenediol); (6) A1-cannabidiol (2-(6-isopropeny1-3-methyl-l-
cyclohexen-l-
y1)-5-pentyl-1,3-benzenediol); and (7) A6-cannabidiol (2-(6-isopropeny1-3-
methy1-6-
cyclohexen-l-y1)-5-penty1-1,3-benzenediol).
CA 3062485 2019-11-21
85760071
A cannabis product could comprise a cannabinoid in its pure or isolated form
or a
source material comprising the cannabinoid. Examples of source materials
comprising cannabinoids include, but are not limited to, cannabis or hemp
plant
material (for example, flowers, seeds, trichomes, and kief), milled cannabis
or hemp
plant material, extracts obtained from cannabis or hemp plant material (for
example,
resins, waxes and concentrates), and distilled extracts. In some embodiments,
pure
or isolated cannabinoids and/or source materials comprising cannabinoids could
be
combined with water, lipids, hydrocarbons (for example, butane), ethanol,
acetone,
isopropanol, or mixtures thereof.
Examples of phytocannabinoids include, but are not limited to, cannabigerolic
acid
(CBGA), cannabigerol (CBG), cannabigerol monomethylether (CBGM),
cannabigerovarin (CBGV), cannabichromene (CBC), cannabichromevarin (CBCV),
cannabidiol (CBD), cannabidiol monomethylether (CBDM), cannabidiol-C4 (CBD-
C4),
cannabidivarin (CBDV), cannabidiorcol (CBD-C1), delta-9-tetrahydrocannabinol
(A9-
THC), delta-9-tetrahydrocannabinolic acid A (TH CA-A),
delta-9-
tetrahydrocannabionolic acid B (THCA-B), delta-9-tetrahydrocannabinolic acid-
C4
(THCA-C4), delta-9-tetrahydrocannabinol-C4,
delta-9-tetrahydrocannabivarin
(TH CV), delta-9-tetrahydrocannabiorcol (THC-C1),
delta-7-cis-iso
tetrahydrocannabivarin, delta-8-tetrahydrocannabinol (A8-THC), cannabicyclol
(CBL),
cannabicyclovarin (CBLV), cannabielsoin (CBE), cannabinol (CBN), cannabinol
methylether (CBNM), cannabinol-C4 (CBN-C4), cannabivarin (CBV), cannabinol-C2
(CBN-C2), cannabiorcol (CBN-C1), cannabinodiol (CBND), cannabinodivarin
(CBVD), can nabitriol (CBT), 10-ethoxy-9hydroxy-delta-6a-tetrahydrocannabinol,
8,9-
d ihyd roxy-delta-6a-tetrahyd rocan nabinol, cannabitriolvarin
(CBTV), ethoxy-
cannabitriolvarin (CBTVE), dehydrocannabifuran (DCBF), cannabifuran (CBF),
cannabichromanon (CBCN), cannabicitran (CBT),
10-oxo-delta-6a-
tetrahydrocannabionol (OTHC), delta-9-cis-tetrahydrocannabinol (cis-THC),
3,4,5,6-
tetrahydro-7-hyd roxy-alpha-alpha-2-trimethy1-9-n-propy1-2,
6-methano-2H-1-
benzoxocin-5-methanol (OH-iso-HHCV), can nabiripsol (C BR), trihydroxy-delta-9-
tetrahydrocannabinol (tri0H-THC), cannabinol propyl variant (CBNV), and
derivatives
thereof.
16
CA 3062485 2019-11-21
85760071
Examples of synthetic cannabinoids include, but are not limited to,
naphthoylindoles,
naphthylmethylindoles, nap hthoylpyrroles,
naphthylniethylindenes,
phenylacetylindoles, cyclohexylphenols,
tetramethylcyclopropylindoles,
adamantoylindoles, indazole carboxamides, and quinolinyl esters.
A cannabinoid may be in an acid form or a non-acid form, the latter also being
referred to as the decarboxylated form since the non-acid form can be
generated by
decarboxylating the acid form. Within the context of the present disclosure,
where
reference is made to a particular cannabinoid, the cannabinoid can be in its
acid or
non-acid form, or be a mixture of both acid and non-acid forms.
In some embodiments, the cannabinoid is tetrahydrocannabinol (THC). THC is
only
psychoactive in its decarboxylated state. The carboxylic acid form (THCA) is
non-
psychoactive. Delta-9-tetrahydrocannabinol (A9-THC) and
delta-8-
tetrahydrocannabinol (A8-THC) produce the effects associated with cannabis by
binding to the CBI cannabinoid receptors in the brain.
In some embodiments, the cannabinoid is cannabidiol (CBD). The terms
"cannabidiol" or "CBD" are generally understood to refer to one or more of the
following compounds, and, unless a particular other stereoisomer or
stereoisomers
are specified, includes the compound "A2-cannabidiol." These compounds are:
(1)
A5- can nabid iol
(2-(6-isopropeny1-3-methyl-5-cyclohexen-l-y1)-5-pentyl-1, 3-
benzenediol); (2) e_ can nabid iol (2-(6-isopropeny1-3-methy1-4-cyclohexen-l-
y1)-5-
pentyl-1,3-benzenediol); (3) A3- can
(2-(6-isopropeny1-3-methy1-3-cyclohexen-
l-y1)-5-pentyl-1,3-benzenediol); (4) A3'7- cannabidiol
(2-(6-isopropeny1-3-
methylenecyclohex-1-y1)-5-penty1-1,3-benzenediol); (5) A2-
can nabid iol (2-(6-
isopropeny1-3-methy1-2-cyclohexen-l-y1)-5-pentyl-1,3-benzenediol); (6) Al-
cannabidiol
(2-(6-isopropeny1-3-methyl-1-cyclohexen-l-y1)-5-pentyl-1,3-benzenediol); and
(7)
can nabidiol (2-(6-isopropeny1-3-methyl-6-cyclohexen-l-y1)-5-pentyl-1,3-
benzenediol).
In some embodiments, a cannabis product is produced by a cannabis producer. A
cannabis producer refers to any entity (e.g. individual or organization) that
cultivates
17
CA 3062485 2019-11-21
85760071
and/or processes cannabis to produce a cannabis product. A cannabis producer
may
sometimes be called a licensed producer.
Process Overview
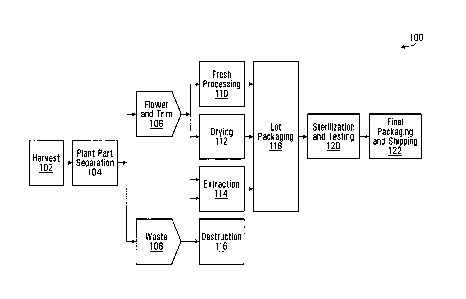
Fig. 1 is a flow diagram illustrating an example process 100 for producing
cannabis
products. Process 100 provides an overview of cannabis product production.
Illustrative examples of various processes for cannabis product production are
also
described in detail elsewhere herein.
Process 100 includes an operation 102 of harvesting at least one batch of
cannabis
plants. A "batch" refers to a group or set of cannabis plants. Each batch of
cannabis
plants could be assigned a unique identifier (ID), which is referred to herein
as a
batch number. In general, a batch number could include alphanumeric characters
and/or other symbols. By way of example, three different batches could be
identified
by the batch numbers "Batch-51", "Batch-52" and "Batch-53". In some
embodiments,
individual cannabis plants are also assigned unique IDs, which are referred to
herein
as plant numbers. Although referred to herein by way of example as numbers,
IDs
associated with plants, and/or other IDs herein, could include alphanumeric
characters and/or other symbols.
A batch of cannabis plants could be cultivated in a particular grow area. In
some
embodiments, a grow area is defined as an area that cultivates similar
cannabis
plants. Grow areas could be provided in greenhouses or other structures that
support
the cultivation of cannabis plants. Grow areas could be contiguous areas, but
this
need not be the case in all embodiments. For example, grow areas that include
multiple non-adjacent areas are also possible. In some embodiments, a grow
area
could be controlled to provide particular and/or consistent growth conditions.
Cannabis plants that are cultivated in a grow area could be from the same
types of
seeds, or be from the same mother plant. A mother plant is a plant grown for
the
purpose of taking cuttings or offsets in order to grow more of the same plant.
A grow
area could instead cultivate plants from multiple different seed types and/or
multiple
different mother plants.
18
CA 3062485 2019-11-21
85760071
Several different batches of cannabis plants could be cultivated and/or
harvested in
parallel. For example, a greenhouse could be divided into several different
grow
areas, each grow area being used to cultivate a respective batch of cannabis
plants.
These batches of cannabis plants could be used for producing different
cannabis
products. Multiple cannabis products could be produced from a single batch of
cannabis plants. Different batches of cannabis plants could also or instead be
combined to produce a single cannabis product.
The cannabis plants that are harvested in operation 102 are sent to operation
104 for
plant part separation, which divides the plants into flower and trim 106, and
waste
108. Cannabis flower could also be referred to as bud, and is typically
harvested from
mature cannabis plants. Trim includes the leaves of the cannabis plant that
are
separated from the flower and stems. Trim could be harvested before the
flower,
while the plants mature. Waste from plant part separation could include
stalks, stems
and leaves that were not separated into trim, for example. In some
embodiments,
plant part separation includes manually cutting or otherwise removing the
leaves
and/or buds from the cannabis plants. However, an automated plant part
separation
process could also or instead be used.
At least a portion of the flower and trim 106 could be sent for fresh
processing at
operation 110, drying at operation 112, and/or extraction at operation 114. At
least a
portion of the waste 108 could also or instead be sent for extraction at
operation 114.
The remainder of the waste 108 could be sent for destruction at operation 116.
Destruction of cannabis waste could include, for example, burning the waste.
Fresh processing at operation 110 could be used to produce fresh cannabis
products.
Fresh cannabis could include flower or trim that has not been dried or cured.
In some
embodiments, fresh processing could include sealing the cannabis after plant
part
separation to help prevent or reduce drying in the ambient atmosphere.
Harvested
flower or trim could also or instead be rapidly transported to a packaging
process, at
operation 118 for example, to help prevent or reduce drying before packaging.
19
CA 3062485 2019-11-21
85760071
Operation 112 is labelled in the drawing as drying, but could include drying
and/or
curing at least a selection of the cannabis flower and trim 106. In some
embodiments,
drying cannabis material could include the use of a commercial dehydrator
system.
Drying could also or instead be performed in the ambient environment. Curing
includes a prolonged process of removing moisture from cannabis plant products
under controlled conditions. Curing could act to preserve cannabis plant
products
and/or increase the concentration of some cannabinoids in the cannabis plant
products.
The extraction process at operation 114 could be used to generate cannabis
products
such as resins. Operation 114 could also include other processes such as
drying,
curing, decarboxylation, winterization, distillation and/or product
formulation
processes. Product formulation processes, such as oil formulation processes
and
liquid formulation processes, could be used to produce cannabis products such
as
cannabis oils, vaporization substances, emulsions, food additives, edibles,
drinks and
oral sprays, for example. Examples of extraction, drying, curing,
decarboxylation,
winterization, distillation and product formulation processes are discussed in
greater
detail elsewhere herein.
The cannabis products produced in operations 110, 112, 114 are sent for lot
packaging at operation 118. "Lots" refer to groups or sets of cannabis
products.
Cannabis products in the same lot have similar properties in some embodiments.
For
example, a lot could be a single type of cannabis product that is produced
from the
same batch of cannabis plants and/or by the same process or processes. Each
lot is
assigned a respective ID, for example, "Lot-5368", "Lot-5369" and "Lot-5370".
A lot ID
could also be referred to as a lot number. In general, a lot number could
include
alphanumeric characters and/or other symbols. In some embodiments, one batch
of
cannabis plants could produce one lot of cannabis products. In other
embodiments,
one batch of cannabis plants could produce multiple lots of cannabis products,
which
could be the same type of cannabis product or include multiple different types
of
cannabis products. For example, one batch of cannabis plants could be
processed to
produce lots of dry cannabis, fresh cannabis, and/or cannabis oil.
CA 3062485 2019-11-21
85760071
In some embodiments, lots are assigned as follows: a particular cannabis
product
originating from one batch of cannabis plants is assigned one lot number; each
different cannabis product originating from that same batch is assigned a
respective
different lot number; and any cannabis products originating from different
batches are
assigned respective different lot numbers. The lot number assigned to each
cannabis
product is used for all units of that cannabis product.
Other methods of lot assignment are also possible. For example, two or more
batches of cannabis plants could be mixed together. In some embodiments, lot
numbers could be assigned to such mixed-batch cannabis products as follows: a
particular cannabis product originating from one mixture of two or more
batches of
cannabis plants is assigned one lot number; each different cannabis product
originating from that same mixture is assigned a respective different lot
number; and
any cannabis products originating from a different mixture of two or more
batches are
assigned respective different lot numbers. The lot number assigned to each
cannabis
product is used for all units of that cannabis product.
Packaging at operation 118 could include transferring lots of cannabis
products into
holding containers. The phrase "holding container", as used herein, refers to
any
container in which cannabis products are or could be contained. Holding
containers
include containers that are used for storing products before, during and after
processing, as well as containers that store products for sale. In some
embodiments,
holding containers are used to seal cannabis products from their environment.
In
some embodiments, holding containers could provide a form of child-resistance,
tamper proofing and/or tamper detection. Examples of holding containers
include
jars, bins, vessels, bags, packets, boxes, bottles and cartridges (for
vaporization
devices, for example), any of which could be made out of wood, paper,
cardboard,
plastic, glass and/or metal, for example. Some holding containers, such as
jars and
bottles, could be sealed with caps or lids. In some embodiments, caps include
tamper-proof induction seals. Holding containers could also or instead be
sealed with
one or more of: foil seals, heat seals, induction seals and shrink wrap, for
example. A
single holding container of cannabis product could be referred to as a unit.
21
CA 3062485 2019-11-21
85760071
During operation 118, labels could be applied to the holding containers. A
label could
associate a holding container with a specific cannabis product. Labels could
be
applied before, while and/or after filling the holding containers with
cannabis
products. Although labels could include material that is glued or otherwise
attached to
a holding container, this might not always be the case. For example, a label
could be
formed on or into a holding container, or be printed directly onto the surface
of a
holding container. In general, markings could be applied to any of various
types of
containers and/or packages. In some embodiments, marking involves printing or
otherwise producing labels and affixing labels to containers and/or packages.
In other
embodiments, marking could also or instead involve printing or otherwise
forming
markings directly on containers and/or packages. As such, features disclosed
herein
in the context of labels or labelling could be applied more generally to other
types of
marking.
A label could include a written description of the holding container and/or
the product
in the holding container. A label could also or instead include a unique
identifier that
distinguishes a holding container from other holding containers. Examples of
unique
identifiers include letters, numbers, symbols, machine-readable code, and
combinations thereof. The unique identifier could encode a description of a
holding
container and/or a product in the holding container. The following is a non-
exhaustive
list of information types, any one or more of which could be included in a
description
of a holding container and/or a product in a holding container:
plant number;
batch number;
lot number;
cannabis producer name, telephone number and/or email address;
cannabis producer number, which is a unique ID assigned to a specific cannabis
producer;
22
CA 3062485 2019-11-21
85760071
customer name, telephone number and/or email address;
shipping information;
Global Trade Item Number (GTIN);
product name;
cannabinoid concentration;
product type;
product composition;
unit or case number;
processing date(s);
packaging date(s);
safety information;
regulatory information;
expiration or "best before" date;
product/container weight;
product/container volume; and
unit size.
In some embodiments, a machine for making labels, such as a label maker, could
be
used in operation 118 to generate labels for and/or apply labels to holding
containers.
During a first time period, the label maker could generate labels for a
particular
cannabis product originating from a particular batch of cannabis plants.
Later, during
a second time period when a cannabis product originating from a new batch of
cannabis plants is being packaged, the label maker could update the labels
being
23
CA 3062485 2019-11-21
85760071
generated such that they map back to the new batch of cannabis plants. In
other
embodiments, the label maker could be replaced with a machine that generates
cannabis holding containers that already include labels.
In process 100, packaged lots of cannabis products are sent for sterilization
and
testing at operation 120. Sterilization could be performed to remove and/or
kill
undesirable biological agents, such as bacteria and fungi. Irradiation is one
example
of a sterilization process, which is discussed in greater detail elsewhere
herein.
Testing could be performed to determine or confirm the composition of the
cannabis
products. For example, testing could determine the uniformity of a product,
the safety
of a product, and/or the amount(s) and type(s) of cannabinoid(s) in a product.
For
example, the concentration of tetrahydrocannabinol (THC) and/or cannabidiol
(CBD)
could be determined through testing. Testing cannabis products could also
include
sampling cannabis products. As discussed in more detail below, in some
embodiments, only certain holding containers for a lot of products could be
sampled,
and in other embodiments, each holding container in a lot could be sampled.
Although lot packaging at operation 118 is illustrated before sterilization
and testing at
operation 120, this might not always be the case. For example, cannabis
products
might not be released for lot packaging until the products have been tested
and the
results are deemed satisfactory.
Final packaging and shipping occurs at operation 122. Operation 122 could also
be
referred to as picking, packaging and shipping (PPS). In some embodiments,
final
packaging includes packing multiple holding containers into larger packages
for
transportation. In general, cannabis products from multiple lots could be
packaged
together in operation 122. Final packaging could also or instead include
removing
cannabis products from one holding container and adding them to another
holding
container. Operation 122 could further include updating and/or adding labels
on
holding containers and/or packages. Final packaging could prepare cannabis
products for shipping, such as by protecting and insulating the cannabis
products.
After final packaging, cannabis products could be released for sale, which
could
24
CA 3062485 2019-11-21
85760071
include shipping the products to customers and/or storing the products in a
particular
area until they are shipped. In some embodiments, shipping could be performed
using a courier service. The term "customer", as used herein, includes any
individual
or organization that receives a cannabis product from a cannabis producer or
processor. Examples of customers include end users of a cannabis product,
distributors of cannabis products, and producers of other cannabis products.
Each
customer could be assigned a unique customer ID.
Fig. 2 is a block diagram illustrating an example system 200 for producing
cannabis
products. In some embodiments, the system 200 could be used to implement any
or
all of the operations 102, 104, 110, 112, 114, 116, 118, 120, 122 of Fig. 1.
The
system 200 includes a cultivation and harvest system 202, a plant part
separation
system 204, a waste destruction system 206, a fresh processing system 208, a
drying system 210, a milling system 212, a decarboxylation system 214, an
extraction
system 216, an oil formulation system 218, a packaging system 220, a
sterilization
system 222, a testing system 224, and a shipping system 226. Various functions
that
could be performed by the systems 202, 204, 206, 208, 210, 212, 214, 216, 218,
220,
222, 224, 226, as well as various components and devices that could be
included in
these systems, are described elsewhere herein.
Fig. 2 illustrates various connections between the systems 202, 204, 206, 208,
210,
212, 214, 216, 218, 220, 222, 224, 226. In general, each of these connections
indicates a transfer of cannabis products between two different systems,
and/or a
means for transferring cannabis products between two different systems.
Transfers of
cannabis product could include physical transfers and/or logical transfers. An
example of a physical transfer includes moving a holding container of cannabis
product from one facility to a different facility. Vehicles, carts and/or
trollies could be
used to physically transfer the holding containers. An example of a logical
transfer
includes processing a cannabis product using one system and then processing
the
same cannabis product using another system, even if the cannabis product does
not
actually move location. These transfers, whether physical or logical, could
include
manual and/or automated transfers.
CA 3062485 2019-11-21
85760071
Fig. 2 includes a connection between the cultivation and harvest system 202
and the
plant part separation system 204, which could enable the transfer of harvested
cannabis plants from the cultivation and harvest system to the plant part
separation
system in holding containers, for example.
The plant part separation system 204 is connected to the waste destruction
system
206, which could enable the transfer of waste material, in holding containers
for
example, from the plant part separation system to the waste destruction
system. The
plant part separation system 204 is further connected to the fresh processing
system
208, the drying system 210, the milling system 212 and the decarboxylation
system
214. These connections could enable transfers of cannabis flower and/or trim,
in
holding containers for example, from the plant part separation system 204 to
the
fresh processing system 208, the drying system 210, the milling system 212
and/or
the decarboxylation system 214.
Plant material could also or instead be serially processed through some or all
of the
drying system 210, the milling system 212, and the decarboxylation system 214.
The
drying system 210 is connected to the milling system 212 for transferring dry
cannabis to the milling system, in holding containers for example. The milling
system
212 is connected to the decarboxylation system 214, which could enable
transfers of
milled cannabis to the decarboxylation system, in holding containers for
example.
The decarboxylation system 214 is connected to the extraction system 216 for
transferring decarboxylated cannabis to the extraction system, in holding
containers
for example. In some embodiments, plant material from the plant part
separation
system 204 is not processed through the drying system 210, the milling system
212,
the decarboxylation system 214, and is instead transferred to the extraction
system
216.
In the illustrated embodiment, the extraction system 216 is connected to the
oil
formulation system 218, which could enable transfer of cannabis extract to the
oil
formulation system, in holding containers for example.
26
CA 3062485 2019-11-21
85760071
The fresh processing system 208, the drying system 210, the milling system
212, the
decarbwrylation system 214, the extraction system 216 and the oil formulation
system 218 are connected to the packaging system 220. Any one or more of
different
types of cannabis products produced by these systems could be transferred from
these systems to the packaging system 220, in the same or one or more
different
types of holding containers, for example.
The packaging system 220 is connected to the sterilization system 222, the
testing
system 224 and the shipping system 226. Packaged cannabis products could be
,
transferred, in packages and/or holding containers for example, from the
packaging
system 220 to any of the sterilization system 222, the testing system 224
and/or the
shipping system 226.
In some embodiments, packaged cannabis products are sterilized and then
tested,
and transfer of sterilized cannabis products from the sterilization system 222
to the
testing system 224 is illustrated in Fig. 2. Sterilized cannabis products
could instead
be shipped without testing, and transfer of sterilized cannabis products from
the
sterilization system 222 to the shipping system 226 is also illustrated. These
transfers
could involve transferring cannabis products in packages and/or holding
containers.
The testing system 224 is also connected to the shipping system 226, which
could
enable the transfer of tested cannabis products to the shipping system, in
holding
containers for example.
The systems and connections illustrated in Fig. 2 represent an example
embodiment.
Other embodiments could include more, fewer and/or different systems, with
similar
and/or different interconnections.
Inventory Control System
An inventory control system (ICS) could be used to record, log, track and/or
monitor
cannabis products throughout cultivation, harvesting, processing, sales,
shipping,
and/or other operations. By way of example, an ICS could record cannabis
products
throughout operations 102, 104, 110, 112, 114, 116, 118, 120, 122 of Fig. 1.
An ICS
27
CA 3062485 2019-11-21
85760071
could also or instead be connected to or otherwise have access to any or all
of the
systems 202, 204, 206, 208, 210, 212, 214, 216, 218, 220, 222, 224, 226 of
Fig. 2, to
provide information to and/or record information from these systems. An ICS
could
also or instead record any or all transfers of cannabis product within and/or
between
the systems 202, 204, 206, 208, 210, 212, 214, 216, 218, 220, 222, 224, 226.
In
general, an ICS could enable traceability of any or all cannabis-containing
substance
through at least part of a production process, including traceability to lot
level, batch
level, or even plant level. This could include plants in cultivation, cannabis
products in
processing, cannabis products in storage, and/or cannabis products that have
been
released for sale or sold. By way of example, in the event of a recall, an ICS
could be
used to determine the status and/or location of all cannabis products that
fall within
the scope of the recall.
For example, transfers of cannabis-containing substances between original
holding
containers and new holding containers could be recorded in the ICS, using a
transfer
action for example. A transfer action could begin by recording information
from labels
on original holding containers in the ICS, for example, and designating them
as labels
for "source" containers. If the new holding containers have pre-existing
labels, then
these labels could be recorded in the ICS and designated as labels for
"target"
holding containers for a transfer. Alternatively, if the new holding
containers do not
have labels, then labels could be created by the ICS, designated as labels for
target
holding containers, and applied to the new holding containers. Once the
transfer is
complete, the ICS could record that the new holding containers now contain the
transferred cannabis-containing substances. For example, a record associated
with a
transferred substance in the ICS could be updated to indicate that the new
holding
containers contain the substance.
In the case of holding containers being moved, information related to the
holding
containers in an ICS could be updated to include such information as any one
or
more of: date of transfer, time of transfer, originating location, and/or
destination.
28
CA 3062485 2019-11-21
85760071
Cannabis products and the processes that are used to produce cannabis products
could be recorded and tracked in an ICS in the form of "records". A record in
an ICS
generally relates to a particular combination of variables, functions, and/or
data
structures. Records could help provide a convenient and logical organization
of
information within an ICS. Multiple types of records could be available in an
ICS,
where each record corresponds to a specific type of cannabis product or
process, for
example. Record types could include, for example, extraction process records,
extract records, oil container records, lab sample records and/or oil jar
records.
Records are discussed by way of example in further detail elsewhere herein.
An ICS could create, store and/or update records as cannabis products are
produced, processed, transported and sold. For example, a record could be
created
or updated in an ICS to record a batch of plants, a lot of product, or a
certain instance
of a process. Any measurements and/or data that are related to the batch, lot
or
process could be added to the corresponding record in an ICS. Special
circumstances for a cannabis product or process, such as deviation from a
standard
operating procedure, could also be recorded in a record in an ICS. As such,
records
could provide a consolidated source of information for a product. Records
could be
created and/or updated by entering information into forms, logs and/or tables
associated with an ICS. When a record is created, it could be assigned a
unique ID to
distinguish it from other records stored in an ICS, which could be referred to
as a
record ID. Multiple different records could also be interrelated. For example,
a record
for a process could be associated with a record for a product that is produced
by that
process.
Records and/or other information that is stored in an ICS could be recorded or
updated using "actions". For example, if cannabis product is transferred from
one or
more holding containers to one or more new holding containers, or a holding
container is transferred from one location to a different location, then this
transfer
could be recorded in an ICS using a "transfer" action. Another example of an
action in
an ICS is a "request", which could include a request to receive and/or view
information that is stored on the ICS for example.
29
CA 3062485 2019-11-21
85760071
An ICS could use labels that are applied to holding containers and/or other
packaging
to help record and track cannabis products. In some embodiments, holding
containers with pre-existing labels could be used. For example, a label with a
unique
identifier could be applied to a holding container without any knowledge of
the
cannabis product that will be later held in that holding container. Once the
cannabis
product that has been or will be transferred to the holding container is
known, the
label could be recorded in an ICS along with information relating to that
cannabis
product. Such information could include a cannabis producer number, lot
number,
batch number and/or plant number, for example. Other information could also or
instead be recorded in the ICS, such as the date, time and location of the
transfer.
The recorded information could be stored in the ICS in the form of a record,
for
example. Recording information in the ICS could occur before, while, and/or
after the
cannabis product is transferred to the holding container.
In some embodiments, unique identifiers for holding container labels could be
generated by an ICS. For example, after the cannabis product that will be
transferred
to a holding container is determined, a unique identifier could be generated
by the
ICS for that holding container. Labels and/or unique identifiers could be
generated by
the ICS using a "create new label" action. Generating a unique identifier
could include
generating a lot number for the product that was or will be transferred to the
holding
container. The unique identifier could be recorded in the ICS by adding the
unique
identifier to a record associated with the cannabis product. The unique
identifier could
be printed directly onto a label on the holding container, or printed onto a
label that is
later applied to the holding container. The unique identifier could indicate
the
cannabis producer number, lot number, batch number and/or plant number of the
product, for example. In some embodiments, holding containers include both pre-
existing and ICS generated labels. An ICS itself might not generate or affix
labels to
containers or packages, but could provide label information to a labelling
machine or
equipment, for example.
Unique identifiers on labels could include machine-readable code. Fig. 3
illustrates
example formats of machine-readable code for a label. In Example A, a machine-
CA 3062485 2019-11-21
85760071
readable code 300 is a linear barcode that encodes a number in a pattern that
is
readable by a machine. Specifically, in this example the machine readable-code
300
encodes a cannabis producer number 310, a lot number 312, a batch number 314
and a plant number 316. In some embodiments, the lot number 312 could be part
of
the GTIN or be provided in addition to the GTIN. For example, all units of the
same
cannabis product from the same producer could each include a barcode encoding
the
same GTIN corresponding to the cannabis product, but different lot numbers are
assigned and also included as part of the barcode for different lots of the
cannabis
product.
Not all of the information shown in Example A of Fig. 3 need necessarily be
encoded
by the machine-readable code 300. For example, in some embodiments the
particular plants from which the cannabis product that is in a container might
not be
known, and the plant number 316 might not be encoded.
In Example B, the lot number 312 and the producer number 310 are encoded by a
machine-readable code 302. Additional information could also be included as
part of
the number that is encoded by the machine-readable code 302. For example,
digits
could be reserved for future tracking use, for internal use by regulatory
authorities,
and/or for internal use by cannabis producers. There could also or instead be
digits
that convey other types of information, such as manufacture date of the
cannabis
product and the expiry or 'best before' date of the product, for example.
The machine-readable codes 302, 304 are illustrated in Fig. 3 as linear or one-
dimensional bar codes, but this is only an example. Alternatively, a barcode
could be
a matrix barcode or two-dimensional barcode, such as a quick response (QR)
code.
In Example C, a machine-readable code 304 is a QR code that could convey the
same information as the machines-readable codes 300, 302, and/or different
information. In some embodiments, a QR code could be used to navigate to a
website hosted by an ICS, and the website could provide information related to
the
cannabis product.
31
CA 3062485 2019-11-21
85760071
Machine-readable codes could also or instead be carried by "smart" labels such
as
radio frequency identification (RFID) chips or tags. An RFID chip could be
integrated
into a holding container or label, for example, and encode a unique identifier
and/or
information related to the holding container and/or its contents.
Machine-readable codes that encode information represent one illustrative
example
of how information could be conveyed in markings on a label, container, or
package.
The information itself could be included in markings, for example. These
examples,
and/or other types of encoding or marking, could be used to convey any of
various
types of information.
Figs. 4A-4M are block diagrams illustrating an example system 400 implementing
an
ICS. The system 400 includes, as shown in Figs. 4A-4M, respectively, a
cultivation
and harvest system 420a, a plant part separation system 420b, a waste
destruction
system 420c, a fresh processing system 420d, a drying system 420e, a milling
system 420f, a decarboxylation system 420g, an extraction system 420h, an oil
formulation system 420i, a packaging system 420j, a sterilization system 420k,
a
testing system 4201, and a shipping system 420m. The systems 420a- 420m
provide
illustrative examples of the systems 202, 204, 206, 208, 210, 212, 214, 216,
218,
220, 222, 224, 226 of Fig. 2.
The system 400 also includes a server 402, which could implement, at least in
part,
an ICS. The server 402 includes a memory 404 storing a database 414, a
processor
406, a network interface 408, a display 410, and one or more input/output
(I/O)
devices 412. In some embodiments, these server components are interconnected
to
each other by an internal bus and/or other type(s) of connection(s).
The memory 404 could be or include one or more memory devices, such as one or
more solid state memory devices, and/or one or more memory devices that use
movable or even removable storage media. The database 414 could be formatted
or
otherwise provided in the memory 404 to store any or all information that is
recorded
by the ICS. For example, the database 414 could store records, parameters,
measurements and/or other information for recording and/or tracking by the
ICS.
32
CA 3062485 2019-11-21
85760071
The processor 406 could be implemented by one or more processors that execute
instructions stored in the memory 404. The processor 406 could be implemented,
in
whole or in part, using dedicated circuitry, such as an application specific
integrated
circuit (ASIC), a graphics processing unit (GPU), and/or a programmed field
programmable gate array (FPGA) for performing any of various operations of the
processor, for example.
The network interface 408 is an example of an input-output device, and enables
communications between the server 402 and other devices or systems over a
network 416. The particular structure of the network interface 408 is
implementation-
dependent, and may vary between embodiments that support different types of
connections and/or communication protocols, for example. The network interface
could enable communications over wired and/or wireless connections. In
general, a
network interface include a physical interface such as a port, connector, or
other
component to interface with a communication medium, and a receiver and/or
transmitter to process received signals and/or transmit signals for
transmission. A
transceiver is an example of a component that includes both a receiver and a
transmitter, and could be implemented in the network interface 408.
The display 410 is another example of an input-output device, to allow users
such as
system operators to view any or all information stored on the ICS and/or to
otherwise
interact with the ICS and possibly other components of the system 400. For
example,
the display 410 could show a record for a cannabis product and/or process. The
display 410 could also or instead allow a user to view the current status of
any or all
systems within the system 400, including information regarding which systems
or
devices are currently in use, the processes these systems or devices are
performing,
and/or the operator(s) using the systems or devices, for example. Any of
various
types of displays could be implemented at 410, including touchscreen displays
that
also enable user input.
Other I/O devices 412 could also or instead be provided. For example, one or
more
user input devices that allow a user to manually input information, actions
and/or
33
CA 3062485 2019-11-21
85760071
requests could be provided. Examples of user input devices include keyboards,
computer mice, touchscreens, buttons, dials and switches. The I/O devices 412
could
also or instead include one or more output devices, such as output ports for
exporting
data stored in the database 414. Other types of I/O devices are also
contemplated.
An access card scanner, for example, could provide security and access control
for
the server 402.
In some embodiments, the server 402 itself does not include user I/O devices
such
as a display 410 or user input devices for receiving inputs from a user. User
interaction with the server 402 could be through one or more separate
components
such as one or more workstations that communicate with the server 402 through
local
connections with the server and/or network connections through the network
416.
Such workstations could be identical to or similar in structure to the server
402, but
might not locally store the database 414, for example.
The network 416 could be or include any of various types of network equipment
implementing any of various type(s) of network(s). In some embodiments, the
network 416 includes a corporate network of a cannabis producer. The network
416
could also or instead include the internet. The particular type(s) of
networks(s) in a
system such as 400 could be implementation-dependent. The server 402 could be
located at a corporate office, and at least some of the systems 420a-420m are
located remotely from the server 402. At least the remotely-located systems
could
connect or otherwise communicate with the server 402 through the internet,
whereas
co-located systems that are at the same location as the server 402 could
connect or
otherwise communicate with the server through a local area network (LAN) or
other
type(s) of local network(s).
The network 416 is connected to or otherwise in communication with multiple
servers
418a, 418b, 418c, 418d, 418e, 418f, 418g, 418h, 418i, 418j, 418k, 4181, 418m,
418n
shown in Figs. 4A-4M, respectively. Network/server communications could be
provided, for example, using physical connections such as cables and/or wires,
34
CA 3062485 2019-11-21
85760071
and/or using wireless connections or channels, such as WIFITM connections,
BluetoothTM connection, and/or longer-range wireless communications.
The servers 418a-418n could be generally similar in structure to the sever
402, but
there could be at least operational, and/or possibly structural differences
between
servers. For example, the servers 418a-418n could be involved in maintaining
an ICS
at the server 402 by sending system-related information through the network
416 to
the server 402, but the servers 418a-418n might not locally store a complete
copy of
the database 414.
In some embodiments, the servers 418a-418n could relay information from other
devices to the server 402. Information could also or instead be stored on the
servers
418a-418n. Although the servers 418a-418n could be distributed throughout the
system 400 as shown, this might not always be the case. Two or more of the
servers
418a-418n, for example, could be co-located. Although illustrated separately
in Figs.
4A-4M, in some embodiments two or more of the servers 418a- 418n could be
implemented using a single server. At least some of the systems 420a-420n
could be
connected to or otherwise in communication with the network 416 without an
intervening server 418a-418n. One or more components of a system 420a-420n
could communicate with the network 416 without necessarily traversing a server
in
connecting to the network. A system component could also or instead
communicate
with the network 416 through some other type of communication equipment or
device
that does not necessarily implement a server.
The cultivation and harvest system 420a includes an operator check-in device
422a,
one or more computers 424a, one or more controllers 426a, one or more sensors
428a, one or more scales 430a, one or more label makers 432a and one or more
scanners 434a. These components are each connected to the server 418a in the
example shown. Connections between these components and the server 418 could
include wired and/or wireless connections, through any of various types of
interfaces.
Each component that is connected to or otherwise in communication with the
server
418a includes an interface compatible with an interface that is provided at
the server.
CA 3062485 2019-11-21
85760071
The particular type(s) of interface(s) provided at the system components and
the
server 418a would be dependent upon the type(s) of connection(s) and/or
communication protocol(s) to be supported.
In some embodiments, one or more operator check-in devices such as 422a could
be
implemented in a cannabis production system to record and track one or more
operators involved in a process or using a device. The term operator, as used
herein
at least with reference to Figs. 4A-4M, refers to any person involved in
operating or
using a cannabis production system. Each operator could be assigned a unique
ID
that is recorded by an operator check-in device when the operator wishes to
access
secure premises and/or use system equipment or devices. Examples of operator
check-in devices include punch card readers to read operator information from
an
operator's punch card, magnetic card readers to read operator information from
a
magnetic strip on an operator's identification or access card, and RFID
readers to
read an RFID tag or chip on an operator's identification or access card. An
operator
check-in device could also or instead include a computer or controller into
which an
employee must enter login information, including at least operator information
such
as the operator's unique ID or username and a password.
Operator information that is read by or otherwise obtained by an operator
check-in
device 422a could be stored locally at the cultivation and harvest system
420a,
and/or transmitted to the server 402 for storage in the database 414. Local
storage of
operator information could be by the operator check-in device 442a itself,
and/or one
or more other components of the cultivation and harvest system 420a, such as a
computer 424a and/or the server 418a.
Other information could also or instead be recorded. The operator check-in
device
could record the date and time that an operator enters an area, leaves an
area, starts
a process or equipment and/or stops a process or equipment, for example.
Any or all information that is obtained or generated by an operator check-in
device,
such as operator information and/or other information disclosed by way of
example
above, could be recorded in the ICS. For example, the operator check-in device
422a
36
CA 3062485 2019-11-21
85760071
could transmit information regarding operators and/or their activities in the
cultivation
and harvest system 420a to the server 418a, which could store this information
and/or forward it to the server 402.
In some embodiments, one or more computers such as 424a could be implemented
in a cannabis production system, for such purposes as enabling operators to
manually enter ICS data, otherwise interact with an ICS system, and/or control
system devices. For example, the computer 424a could store entered data and/or
transmit entered data to the server 418a, which could store and/or forward the
data to
the server 402. The computer 424a could also or instead enable an operator to
access ICS data, in the database 414 for example, and output an indication of
that
data on a display screen or other output device. Examples of computers include
desktop computers, laptop computers, tablet computers and other electronic
devices.
In general, the computer 424a could be similar in structure to the server 402,
but
need not necessarily store the database 414. Depending on implementation, the
computer 424a might or might not include a network interface. In a server-
based
implementation as shown in Fig. 4A, for example, the computer 424a could
include
an interface that might or might not be a network interface but is compatible
with an
interface provided at the server 418a.
In some embodiments, one or more controllers such as 426a could be implemented
in a cannabis production system, to control any or all of various types of
devices or
equipment. A controller could be integrated within a controlled device or
equipment,
or be separate from the controlled device or equipment as shown in Fig. 4A.
Controllers could be implemented, for example, using hardware, firmware, one
or
more components that execute software stored in one or more non-transitory
memory
devices. Microprocessors, ASICs, FPGAs, and Programmable Logic Devices (PLDs)
are examples of processing devices that could be used to execute software.
A controller 426a could store, receive, and/or otherwise obtain control
settings, and
control one or more devices or equipment to run according to those settings.
For
example, a controller 426a could be programmable by operators, through a
computer
37
CA 3062485 2019-11-21
85760071
424a and/or through a user interface of the controller for example, and/or by
the ICS.
An ICS-programmable controller 426a could access, download, or otherwise
determine, or be programmed with, control settings from the database 414. In
some
embodiments, a controller 426a could record control settings and/or other
information
in the ICS. Information that is used by and/or obtained by a controller 426a
could be
locally stored, by the controller and/or another component of the cultivation
and
harvest system 420a for example, and/or transmitted to the server 418a for
local
storage and/or transmission to the server 402.
In some embodiments, sensors such as 428a could be implemented in a cannabis
production system to measure or otherwise determine any or a variety of
parameters
involved in production. These parameters, and possibly other information such
as the
time at which measurements were taken, could be recorded in the ICS. Examples
of
sensors, any one or more of which could be implemented in a cannabis
production
system, include the following:
carbon dioxide sensor;
nitrogen oxide sensor;
oxygen sensor;
ozone monitor;
p1-1 sensor;
potentiometric sensor;
redox electrode;
smoke detector;
electrical current sensor;
metal detector;
38
CA 3062485 2019-11-21
85760071
voltage detector;
air pollution sensor;
humidity sensor;
rain sensor;
snow gauge;
soil moisture sensor;
air flow meter;
water meter;
barometer;
pressure sensor;
pressure gauge;
flame detector;
light sensor;
heat flux sensor; and
thermometer.
Sensor readings or measurements could be locally stored, by a sensor 430a
and/or
another component of the cultivation and harvest system 420a for example,
and/or
transmitted to the server 418a for local storage and/or transmission to the
server 402.
In some embodiments, one or more scales such as 430a could be implemented in a
cannabis production system to weigh products, waste material, packages and/or
holding containers, for example. Scales could include, for example, electronic
scales
that are in communication with or otherwise able to access the ICS. When an
39
CA 3062485 2019-11-21
85760071
electronic scale measures the weight of a cannabis product and/or a holding
container, for example, the scale could automatically transmit this weight to
the ICS,
where it could be recorded. Non-electronic scales could also or instead be
used in a
cannabis production system, and the weights measured by these scales could be
manually entered into the ICS using a computer 424a, for example.
A description of a weight measured by a scale 430a could also be recorded in
the
ICS. The description could include information regarding the current stage of
production of a cannabis product and/or holding container when the cannabis
product
and/or holding container was weighed. An operator could manually enter this
description into an electronic scale 430a or a computer 424, for example,
which could
then transmit the description to the ICS. A description of a measured weight
could
also or instead be inferred by the ICS. For example, an electronic scale 430a
could
be associated with a specific step in a cannabis production process or a
specific
device or equipment in a cannabis production system, and a description of the
weights measured by that scale could therefore be predefined in the ICS. In
some
embodiments, a certain scale might only be used to measure the weight of
holding
containers containing extract collected from an extraction process, and the
ICS could
automatically associate any or all weights measured by the scale with that
stage of
production.
A record ID and/or other identifier for the cannabis product and/or holding
container
weighed by a scale 430a could be recorded in the ICS. For example, an
electronic
scale 430a could transmit a record ID and/or other identifier for a weighed
cannabis
product and/or holding container to the ICS, along with the measured weight of
that
cannabis product and/or holding container, allowing the ICS to identify which
record
the weight should be recorded in. To determine the record ID and/or other
identifier,
an operator could manually read a label on the holding container and enter
information from the label into the ICS using the electronic scale or another
device.
Also or alternatively, a label on the holding container could be read and
recorded in
the ICS using a scanner. The scanner could be linked to the scale to
automatically
associate the label of the holding container with the measured weight.
CA 3062485 2019-11-21
85760071
A scale 430 could also receive control information and/or other information. A
scale
could be controlled, for example, to record a weight only when a cannabis
product or
holding container is in proper position for weighing. In some embodiments, a
controller sends a control signal to a scale 430a to trigger a measurement.
Such a
controller could be integrated with a scale 430a, or be separate from the
scale.
Measurement could also or instead be manually initiated or triggered by an
operator,
through a user interface of the scale or another component that is connected
to or
otherwise in communication with the scale.
Weight measurements, and possibly other information that is determined or
otherwise
obtained by or from a scale 430a could be locally stored, by the scale and/or
another
component of the cultivation and harvest system 420a for example, and/or
transmitted to the server 418a for local storage and/or transmission to the
server 402.
In some embodiments, one or more label makers 432a could be implemented in a
cannabis production system to generate labels that are applied to holding
containers,
for example. A label maker 432a could be in communication with or otherwise
have
access to the ICS. In some embodiments, the ICS could control a label maker
432a,
and thereby control the particular labels that are applied to holding
containers. For
example, the ICS could transmit information and/or machine-readable code for a
label to a label maker 432a, and the label maker could generate a label based
on the
information and/or a machine-readable code that encodes the information. The
ICS
could also or instead send an image of a label to a label maker 432a, which
could
print the image onto an adhesive label and/or directly onto a holding
container. In
some embodiments, a label maker 432a could generate information and/or machine-
readable code for a label, produce a label based on the information and/or
machine-
readable code, and apply the label to one or more holding containers.
A label maker 432a could record each label in the ICS. Label information could
be
locally stored, by a label maker 432a and/or another component of the
cultivation and
harvest system 420a for example, and/or transmitted to the server 418a for
local
storage and/or transmission to the server 402.
41
CA 3062485 2019-11-21
85760071
A label maker 432a is an example of a labelling or marking system or station,
which
could include a printing or marking device to print or mark on a label and/or
directly
on a holding container or package. In some embodiments, a single printing or
marking device is suitable for printing or marking, with ink for example, on
multiple
substrates such as labels and holding containers, labels and packages, or
labels,
holding containers, and packages. In embodiments that involve printing or
marking
labels, a labelling system or marking station could also include a label
applicator to
affix labels to holding containers and/or packages. A controller for a
labelling system
or marking station could be integrated with the labelling system or marking
station, or
be a separate component.
In some embodiments, one or more scanners 434a could be implemented in a
cannabis production system to read, record, and/or decode markings, which
could be
directly printed on holding containers and/or packages, and/or on labels that
are
affixed to the holding containers and/or packages. For example, a scanner
could be
used to read, record and/or decode machine-readable code(s) on a label.
Examples of scanners 434a include barcode scanners, image scanners and RFID
readers. A scanner 434a could be provided in the form of a handheld scanner, a
mobile electronic device, a scanner mounted to a structure (a table or
counter, for
example), a scanner embedded in a structure, a scanner integrated into
equipment in
a cannabis production system and/or a wearable scanner. Scanners could be
wired
or wireless. Multiple scanners, of the same type or different types, could be
implemented.
When a marking, on a label affixed to a holding container for example, is
scanned by
a scanner 434a, any of a variety of information could be recorded in the ICS.
For
example, a scanner 434a could be specific to a certain location, device,
equipment,
and/or process in a cannabis production system. Scanning a label using that
scanner
indicates that the holding container or package associated with the label is
at that
specific location, device, equipment, and/or process.
42
CA 3062485 2019-11-21
85760071
A scanner could also receive information. In some embodiments, a scanner could
receive a search or control parameter and generate an alert or other output
when the
search or control parameter is found or satisfied. A search parameter could be
a lot
identifier, for example, and a scanner 434a could generate an alert when a
marking
consistent with the lot identifier is scanned. A count is an example of a
control
parameter, and a scanner 434a could generate an alert or other output when a
certain number of markings have been scanned and/or provide an output
indicating a
count of scanned markings. A scanner 434a could also or instead confirm a
change
in lot and/or batch number at a correct time during a production run.
The example cultivation and harvest system 420a in Fig. 4A also includes one
or
more watering systems 450a, one or more lighting systems 452a, and one or more
ventilation systems 454a. The watering system(s) 450a, the lighting system(s)
452a
and the ventilation system(s) 454a are connected to or otherwise in
communication
with, and are controlled by, one or more of the controllers 426a. Control of a
watering
system 450a could involve controlling one or more valves, for example, to
control
water flow to an irrigation system and/or particular components such as
sprinkler
heads. A lighting system 452a could be controlled by controlling power to
lights
and/or shades, for example. In some embodiments, control of the ventilation
system(s) 454a could involve controlling one or more air inlets, one or more
air
outlets, one or more heaters, one or more coolers, and/or one or more airflow
components such as fans.
Control settings for any or all of the watering system(s) 450a, the lighting
system(s)
452a, and the ventilation system(s) 454a could be provided to, determined by,
or
otherwise obtained by the controller(s) 426a. Watering, lighting, ventilation,
and/or
temperature programs or schedules could be downloaded to one or more
controllers
426a and used to control any or all of the watering system(s) 450a, the
lighting
system(s) 452a, and the ventilation system(s) 454a. In some embodiments, one
or
more controller(s) 426a dynamically control any or all of the watering
system(s) 450a,
the lighting system(s) 452a, and the ventilation system(s) 454a, based on
sensor
readings, for example. Combinations of predetermined and dynamic control are
also
43
CA 3062485 2019-11-21
85760071
contemplated. For example, any or all of the watering system(s) 450a, the
lighting
system(s) 452a, and the ventilation system(s) 454a could be controlled
according to a
predetermined program or schedule as long as one or more monitored parameters
are maintained within target ranges, and dynamic control of one or more of the
systems 450a, 452a, 454a could be initiated in response to an out-of-range
parameter.
The cultivation and harvest system 420a also includes one or more grow areas
456a,
used to cultivate cannabis plants. The watering system(s) 450a, the lighting
system(s) 452a and the ventilation system(s) 454a interact with, and in that
sense
could be considered to be associated with, the grow area(s) 456a. The
interactions
between the watering system(s) 450a, the lighting system(s) 452a, the
ventilation
system(s) 454a, and the grow areas 456a are illustrated using dashed lines in
Fig.
4A.
In Figs. 4A-4M, solid lines are intended to represent wired or wireless
connections for
communications between components. Dashed lines are intended to indicate that
components interact or are related or associated in some way, but are not
necessarily in communication with or coupled to each other. By way of example,
the
watering system(s) 450a could provide water to the grow area(s) 456a, but this
does
not necessarily mean that the watering system(s) would be in communication
with the
grow area(s), or that the watering system(s) would necessarily be in any way
physically coupled to the grow area(s). Similarly, the lighting system(s) 452a
and the
ventilation system(s) 454a provide light and air flow to the grow area(s)
456a, but are
not necessarily coupled to the grow area(s).
Fig. 4A similarly shows the grow area(s) 456a as being associated with the
sensors
428a, which could measure, record and/or track any of a variety of parameters
and/or
growing conditions in the grow area(s). In some embodiments, the sensor(s)
428a
could provide measurements or readings to one or more controller(s) 426a, and
the
controller(s) could control one or more of the watering system(s) 450a, the
lighting
44
CA 3062485 2019-11-21
85760071
system(s) 452a, and the ventilation system(s) 454a based on the measurements
or
readings from the sensor(s).
During and/or after a harvest, cannabis plants from the grow area(s) 456a
could be
transferred to one or more plant holding containers 458a. The plant holding
container(s) 458a could be weighed by the scale(s) 430a, labeled by the label
maker(s) 432a and/or scanned by the scanner(s) 434a, and therefore Fig. 4A
includes dashed lines to represent interactions or associations between these
components.
In some embodiments, a production system includes other systems with at least
some components that may be identical or similar to those in the example
cultivation
and harvest system 420a. With reference to Fig. 4B, for example, a plant part
separation system 420b could include one or more operator check-in devices
422b,
one or more computers 424b, one or more controllers 426b, one or more scales
at
430b-1 and/or 430b-2, one or more label makers 432b and one or more scanners
at
434b-1 and/or 434b-2. These components are connected to or otherwise in
communication with the server 418b. Implementation options for all of these
components are described herein, at least above with reference to Fig. 4A.
Although
two sets of scale(s) and scanner(s) are shown at 430b-1, 430b-2 and 434b-1,
434b-2,
in some embodiments a plant part separation system could include only one set
of
either or both of these components. Different holding containers could be
transferred
to the same weighing station with one set of scales, for example. In some
embodiments, different holding containers could be scanned with the same set
of one
or more portable scanners instead of or in addition to equipment-mounted or
equipment-specific scanners. Separate sets of scale(s) and scanner(s) are
shown at
430b-1, 430b-2 and 434b-1, 434b-2 solely to simplify the illustration of
connecting
lines in Fig. 4B.
The example plant part separation system 420b further includes one or more
plant
holding containers 450b, one or more manual plant part separators 452b, one or
more automated plant part separators 454b, one or more flower holding
containers
CA 3062485 2019-11-21
85760071
456b, one or more trim holding containers 458b, and one or more waste holding
containers 460b. The holding containers 450b, 456b 458b, 460b could be any of
various types of containers, and different types of containers could be used
to hold
harvested and separated plants. In some embodiments, the plant holding
container(s)
450b are the same holding container(s) as shown at 458a in Fig. 4A. In some
embodiments, a manual plant part separator 452b includes one or more sorting
trays
or tables at which an operator sorts harvested plant material. An automated
plant part
separator 454b could include a machine vision system or other means to
distinguish
flower, trim, and waste from each other, and a sorting station to separate
plant
material that has been identified as flower, trim, and waste from each other.
Some
embodiments could include both manual and automated plant part separators.
The plant holding container(s) 450b could be weighed and/or scanned using the
scale(s) 430b-1 and/or the scanner(s) 434b-1, to quantify and/or identify
inputs into
plant part separation. The cannabis plants from the plant holding container(s)
450b
could be transferred to the manual plant part separator(s) 452b and/or the
automated
plant part separator(s) 454b for plant part separation. At least the automated
plant
part separator(s) 454b could be connected to or otherwise in communication
with,
and controlled by, a controller 426b. The flower, trim and waste produced by
the
manual plant part separator(s) 452b and/or the automated plant part
separator(s)
454b could be transferred to the flower holding container(s) 456b, the trim
holding
container(s) 458b, and the waste holding container(s) 460b, respectively. The
flower
holding container(s) 456b, the trim holding container(s) 458b, and the waste
holding
container(s) 460b could be weighed by the scale(s) 430b-2 and/or labeled by
the
label maker(s) 432b, and markings on the holding container(s) or label(s)
could be
scanned by the scanner(s) 434b-2. Weights as measured by the scale(s) 430b-2
could be used to reconcile input plant material with output plant material, to
maintain
desired and/or required records of plant material during processing.
The example waste destruction system 420c in Fig. 4C includes one or more
operator check-in devices 422c, one or more computers 424c, one or more
controllers 426c, one or more scales 430c, one or more sensors 428c, and one
or
46
CA 3062485 2019-11-21
85760071
more scanners 434c. These components are connected to or otherwise in
communication with the server 418c. Implementation options for all of these
components are described herein, at least above with reference to Fig. 4A.
The example waste destruction system 420c further includes one or more waste
holding containers 450c and one or more incinerators 452c. The waste holding
container(s) 452c could include any of various types of containers, and in
some
embodiments the waste holding container(s) 450c are those shown at 460b in
Fig.
4B. The waste holding container(s) 450c could be weighed and/or scanned using
the
scale(s) 430c and/or the scanner(s) 434c, to quantify and/or identify inputs
into waste
destruction. The waste from the waste holding container(s) 450c could be
transferred
to the incinerator(s) 452c for incineration. The incinerator(s) 452c are
connected to or
otherwise in communication with the sensor(s) 428c to measure operating
parameters and/or monitor the process of incineration. The incinerator(s) 452
are
also connected to or otherwise in communication with the controller(s) 426c to
control
the process of incineration.
Referring now to Fig. 4D, an example fresh processing system 420d includes one
or
more operator check-in devices 422d, one or more computers 424d, one or more
scales at 430d-1 and/or 430d-2, one or more label makers 432d and one more
scanners at 434d-1 and/or 434d-2. These components are connected to or
otherwise
in communication with the server 418d. Implementation options for all of these
components are described herein, at least above with reference to Fig. 4A.
Although
two sets of scale(s) and scanner(s) are shown at 430d-1, 430d-2 and 434d-1,
434d-2,
in some embodiments a fresh processing system could include only one set of
either
or both of these components. Separate sets of scale(s) and scanner(s) are
shown at
430d-1, 430d-2 and 434d-1, 434d-2 solely to simplify the illustration of
connecting
lines in Fig. 4D.
The example fresh processing system 420d further includes one or more source
product holding containers 450d and one or more fresh product holding
containers
452d. The containers 450d, 452d could include any of various types of
container, and
47
CA 3062485 2019-11-21
85760071
different container types could be used for source product and fresh product.
The
source product holding container(s) 450d could contain cannabis flower and/or
trim
from plant part separation, for example. In some embodiments, the source
product
holding container(s) 450d are the same holding container(s) as shown at 456b
and/or
458b in Fig. 4B.
The source product holding container(s) 450d could be weighed and/or scanned
using the scale(s) 430d-1 and/or the scanner(s) 434d-1, to quantify and/or
identify
inputs to the fresh processing system 420d. The source product(s) in the
source
product holding container(s) 450d could then be transferred to the fresh
product
holding container(s) 452d and sealed. The fresh product holding container(s)
452d
could be weighed by the scale(s) 430d-2 and/or labeled by the label maker(s)
432d.
Markings on the holding container(s) on the fresh product holding container(s)
452d
or label(s) could be scanned by the scanner(s) 434d-2. Weights as measured by
the
scale(s) 430d-2 could be used to reconcile input source product with total
output
fresh product, to maintain desired and/or required records of source product
during
processing.
An example drying system 420e as shown in Fig. 4E includes one or more
operator
check-in devices 422e, one or more computers 424e, one or more controllers
426e,
one or more sensors 428e, one or more scales at 430e-1 and/or 430e-2, one or
more
label makers 432e and one or more scanners 434e-1 and/or 434e-2. These
components are connected to or otherwise in communication with the server
418e.
Implementation options for all of these components are described herein, at
least
above with reference to Fig. 4A. Although two sets of scale(s) and scanner(s)
are
shown in at 430e-1, 430e-2 and 434e-1, 434e-2, in some embodiments a drying
system could include only one set of either or both of these components.
Separate
sets of scale(s) and scanner(s) are shown at 430e-1, 430e-2 and 434e-1, 434e-2
solely to simplify the illustration of connecting lines in Fig. 4E.
The drying system 420e further includes one or more source product holding
containers 450e, one or more dryers 452e, and one or more dried product
holding
48
CA 3062485 2019-11-21
85760071
containers 454e. The containers 450e, 454e could include any of various types
of
container, and different container types could be used for source product and
dried
product. The source product holding container(s) 450e could contain cannabis
flower
and/or trim from plant part separation, for example, and could include any of
various
types of containers. In some embodiments, the source product holding
container(s)
450e are the same holding container(s) as shown at 456b and/or 458b in Fig.
4B.
The dryer(s) 452e could be or include any of various types of dryers, such as
one or
more commercial dehydrator systems. Although Fig. 4E illustrates only dryer(s)
452e,
a drying system could also or instead provide curing. Curing could be
provided, for
example, using a curing vessel in which a curing solution is applied to source
product
to extract moisture from the source product. One or more of the controller(s)
426e
could control a curing or parameters such as supply of curing solution(s) from
one or
more solution holding container(s) to the curing vessel by controlling one or
more
valves, curing temperature by controlling one or more heaters or coolers to
heat or
cool the vessel and/or curing solution(s), and/or curing pressure by
controlling a
vacuum system or compression system to pressurize or depressurize the curing
vessel, for example.
The source product holding container(s) 450e could be weighed and/or scanned
using the scale(s) 430e-1 and/or the scanner(s) 434e-1, to quantify and/or
identify
inputs to the drying system. The source product(s) in the source product
holding
container(s) 450e could then be transferred to the dryer(s) 452e, to dry the
source
product(s). The controller(s) 426e could be connected to or otherwise in
communication with the dryer(s) 452e, to control the dryer(s). The sensors
428e
could similarly be connected to or otherwise in communication with the
dryer(s) 452e,
to measure one or more parameters and/or otherwise monitor one or more
properties
of a drying process or equipment. Dried product could then be transferred to
the dried
product holding container(s) 454e. The dried product holding container(s) 454e
could
be weighed by the scale(s) 430e-2 and/or labeled by the label maker(s) 432e.
Markings on the dried product holding container(s) 454e or label(s) could be
scanned
by the scanner(s) 434e-2. Weights as measured by the scale(s) 430e-2 could be
49
CA 3062485 2019-11-21
85760071
used to reconcile input source product with total output dried product, to
maintain
desired and/or required records of source product during processing.
Fig. 4F illustrates an example milling system 420f, which includes one or more
operator check-in devices 422f, one or more computers 424f, one or more
controllers
426f, one or more sensors 428f, one or more scales at 430f-1 and/or 430f-2,
one or
more label makers 432f and one or more scanners at 434f-1 and/or 434f-2. These
components are connected to or otherwise in communication with the server
418f.
Implementation options for all of these components are described herein, at
least
above with reference to Fig. 4F. Although two sets of scale(s) and scanner(s)
are
shown at 430f-1, 430f-2 and 434f-1, 434f-2, in some embodiments a milling
system
could include only one set of either or both of these components. Separate
sets of
scale(s) and scanner(s) are shown at 430f-1, 430f-2 and 434f-1, 434f-2 solely
to
simplify the illustration of connecting lines in Fig. 4F.
The milling system 420f further includes one or more source product holding
containers 450f, one or more milling machines 452f, one or more sifters 454f
and one
or more dried product holding containers 456f. The containers 450f, 456f could
include any of various types of container, and different container types could
be used
for source product and dried product. The source product holding container(s)
450f
could contain cannabis flower and/or trim from plant part separation, and/or
dried
cannabis product from a drying process, for example. In some embodiments, the
source product holding container(s) 450f are the same holding container(s) as
shown
at 456b, 458b, and/or 454e in Figs. 4B and 4E.
The source product holding container(s) 450f could be weighed and/or scanned
using
the scale(s) 430f-1 and/or the scanner(s) 434f-1, to quantify and/or identify
inputs to
the milling system 420f. The source product(s) in the source product holding
container(s) 450f could then be transferred to the milling machine(s) 452f,
which
could be implemented as milling equipment to mill the source product(s) and/or
one
or more grinders to grind the source product. One or more of the controller(s)
426f
could be connected to or otherwise in communication with the milling
machine(s) 452f
CA 3062485 2019-11-21
85760071
to control the milling machine(s). The sensor(s) 428f could similarly be
connected to
or otherwise in communication with the milling machine(s) 452f, to measure one
or
more parameters and/or otherwise monitor one or more properties of a milling
process or equipment.
Milled product could then be transferred to one or more sifters 454f and/or to
the
dried product holding container(s) 456f. The sifter(s) 454f could include one
or more
filters or screens to sift the milled cannabis product and separate it based
on particle
size. The outputs from the sifter(s) 454f could also or instead be transferred
to the
milled product holding container(s) 456f. The milled product holding
container(s) 456f
could be weighed by the scale(s) 430f-2 and/or labeled by the label maker(s)
432f.
Markings on the milled product holding container(s) or label(s) could be
scanned by
the scanner(s) 434f-2. Weights as measured by the scale(s) 430f-2 could be
used to
reconcile input source product with total output milled product, to maintain
desired
and/or required records of source product during processing.
Referring now to Fig. 4G, an example decarboxylation system 420g includes one
or
more operator check-in devices 422g, one or more computers 424g, one or more
controllers 426g, one or more sensors 428g, one or more scales at 430g-1
and/or
430g-2, one or more label makers 432g and one or more scanners at 434g-1
and/or
434g-2. These components are connected to or otherwise in communication with
the
server 418g. Implementation options for all of these components are described
herein, at least above with reference to Fig. 4A. Although two sets of
scale(s) and
scanner(s) are shown in at 430g-1, 430g-2 and 434g-1, 434g-2, in some
embodiments a decarboxylation system could include only one set of either or
both of
these components. Separate sets of scale(s) and scanner(s) are shown at 430g-
1,
430g-2 and 434g-1, 434g-2 solely to simplify the illustration of connecting
lines in Fig.
4G.
The decarboxylation system 420g further includes one or more source product
holding containers 450g, one or more decarboxylation ovens 452g and one or
more
decarboxylated product holding containers 454g. The containers 450g, 454g
could
51
CA 3062485 2019-11-21
85760071
include any of various types of container, and different container types could
be used
for source product and decarboxylated product. The source product holding
container(s) 450g could contain cannabis flower and/or trim from plant part
separation, dried cannabis product from a drying process, and/or milled
cannabis
from a milling process, for example. In some embodiments, the source product
holding container(s) 450g are the same holding container(s) as shown at 456b,
458b,
454e, and/or 450f in Figs. 4B, 4E, and 4F.
The source product holding container(s) 450g could be weighed and/or scanned
using the scale(s) 430g-1 and the scanner(s) 434g-1, to quantify and/or
identify
inputs to the decarboxylation system 420g. The source product(s) in the source
product holding container(s) 450g could then be transferred to the
decarboxylation
oven(s) 452g, to heat the source product(s) as described elsewhere herein. One
or
more of the controller(s) 426g could be connected to or otherwise in
communication
with the decarboxylation oven(s) 452g, to control the decarboxylation oven(s).
The
sensor(s) 428g could similarly be connected to or otherwise in communication
with
the decarboxylation oven(s) 452g, to measure one or more parameters and/or
otherwise monitor one or more properties of a decarboxylation process or
equipment.
Decarboxylated product could then be transferred to the decarboxylated product
holding container(s) 454g. The decarboxylated product holding container(s)
454g
could be weighed by the scale(s) 430g-2 and/or labeled by the label maker(s)
432g.
Markings on the milled product holding container(s) 454g or label(s) could be
scanned by the scanner(s) 434g-2. Weights as measured by the scale(s) 430g-2
could be used to reconcile input source product with total output extracted
product, to
maintain desired and/or required records of source product during processing.
An example extraction system 420h is shown in Fig. 4H, and includes one or
more
operator check-in device 422h, one or more computers 424h, one or more
controllers
426h, one or more sensors 428h, one or more scales at 430h-1 and/or 430h-2,
one
or more label makers 432h and one or more scanners at 434h-1 and/or 434h-2.
These components are connected to or otherwise in communication with the
server
52
CA 3062485 2019-11-21
85760071
418g. Implementation options for all of these components are described herein,
at
least above with reference to Fig. 4A. Although two sets of scale(s) and
scanner(s)
are shown in at 430h-1, 430h-2 and 434h-1, 434h-2, in some embodiments an
extraction system could include only one set of either or both of these
components.
Separate sets of scale(s) and scanner(s) are shown at 430h-1, 430h-2 and 434h-
1,
434h-2 solely to simplify the illustration of connecting lines in Fig. 4H.
The extraction system 420h further includes one or more source product holding
containers 450h, one or more extractors 452h, one or more winterization
chillers
454h, one or more distillers 456h and one or more extracted product holding
containers 458h. The containers 450h, 458h could include any of various types
of
container, and different container types could be used for source product and
extracted product. The source product holding container(s) 450h could contain
decarboxylated cannabis products, for example. In some embodiments, the source
product holding container(s) 450h are the same holding container(s) as shown
at
454g in Fig. 4G.
The source product holding container(s) 450h could be weighed and/or scanned
using the scale(s) 430h-1 and the scanner(s) 434h-1, to quantify and/or
identify
inputs to the extraction system 420h. The source product(s) in the source
product
holding container(s) 450h could then be transferred to the extractor(s) 452h,
which
could implement any of various extraction processes to produce one or more
extracts
from the source product(s). Examples of extraction processes and extracts are
disclosed elsewhere herein.
The produced extract(s) could be transferred to the winterization chiller(s)
454h, the
distiller(s) 456h and/or the extract product holding container(s) 458h. The
winterization chillers 454h could include a refrigerator, for example. In some
embodiments, the winterization chiller(s) 454h are provided to cool a mixture
of
extract and polar solvent(s) to a temperature at which waxes and/or lipids
separate
from the extract. One or more outputs of the winterization chiller(s) 454h
could also or
53
CA 3062485 2019-11-21
85760071
instead be transferred to the distiller(s) 456h and/or the extract product
holding
container(s) 458h.
The distiller(s) 456h could include a distillation column, for example, to
separate one
or more cannabinoids and/or terpenes from extract(s). One or more outputs of
the
distiller(s) 456h could also or instead be transferred to the extract product
holding
container(s) 458h.
One or more of the controller(s) 426h could be connected to or otherwise in
communication with the extractor(s) 452h, the winterization chiller(s) 454h
and/or the
distiller(s) 456h, to control these components. The sensor(s) 428h could
similarly be
connected to or otherwise in communication with the extractor(s) 452h, the
winterization chiller(s) 454h and/or the distiller(s) 456h, to measure one or
more
parameters and/or otherwise monitor one or more properties of an extraction
process
or equipment.
The extracted product holding container(s) 458h could be weighed by the
scale(s)
430h-2. The label maker(s) 432h could generate and/or apply labels to the
extracted
product holding container(s) 458h. Markings on the extracted product holding
container(s) 458h could be scanned by the scanner(s) 434h-2. Weights as
measured
by the scale(s) 430h-2 could be used to reconcile input source product with
total
output decarboxylated product, to maintain desired and/or required records of
source
product during processing. In some embodiments, two or more extracted product
holding container(s) 458h could be mixed and marked accordingly.
Fig. 41 illustrates an example oil formulation system 420i, which includes one
or more
operator check-in devices 4221, one or more computers 4241, one or more
controllers
426i, one or more sensors 4281, one or more scales at 4301-1 and/or 430i-2,
one or
more label makers 432i and one or more scanners at 434i-1 and/or 4341-2. These
components are connected to or otherwise in communication with the server
418i.
Implementation options for all of these components are described herein, at
least
above with reference to Fig. 4A. Although two sets of scale(s) and scanner(s)
are
shown in at 430i-1, 430i-2 and 434i-1, 434i-2, in some embodiments an oil
54
CA 3062485 2019-11-21
85760071
formulation system could include only one set of either or both of these
components.
Separate sets of scale(s) and scanner(s) are shown at 430i-1, 430i-2 and 4341-
1,
434i-2 solely to simplify the illustration of connecting lines in Fig. 41.
The oil formulation system 420i further includes one or more source product
holding
containers 450i, one or more carrier oil holding containers 452i, one or more
mixing
devices 454i, one or more dilution devices 456i and one or more cannabis oil
holding
containers 458i. The containers 450i, 452i, 458i could include any of various
types of
container, and different container types could be used for source product,
carrier oil,
and cannabis oil. The source product holding container(s) 450i could contain
cannabis extract-based products, for example, and in some embodiments could
include one or more containers as shown at 458h in Fig. 4H. The carrier oil
holding
container(s) 452i could include carrier oils that, when mixed with a cannabis
extract,
produce a cannabis oil and/or concentrate. Carrier oils are discussed in
greater detail
elsewhere herein.
The source product holding container(s) 450i and/or the carrier oil holding
container(s) 452i could be weighed and/or scanned using the scale(s) 430i-1
and/or
the scanner(s) 434i-1, to quantify and/or identify inputs to the oil
formulation system
420i. Moreover, in some embodiments, the carrier oil and/or the source product
can
be the subject of testing prior to the mixing stage. In some embodiments, such
testing
is part of a Preventable Control Plan (PCP). In some embodiments, such testing
can
include allergen testing, label validation testing, microbiological testing,
mycotoxins
testing, nutritional analysis, organoleptic testing, testing for heavy metals,
foreign
materials, toxins and/or other contaminants. The results of such tests can be
recorded by the ICS in, for example, the database 414 on server 402.
The source product(s) in the source product holding container(s) 450i and the
carrier
oil(s) in the carrier oil holding container(s) 452i could then be transferred
to the mixing
device(s) 454i to be mixed. The carrier oil(s) could also be transferred to
the dilution
devices 456i. The mixing device(s) 454i could dissolve the source product(s)
in the
carrier oil(s) to produce a homogeneous mixture. The dilution device(s) 456i
could
CA 3062485 2019-11-21
85760071
add additional carrier oil(s) to the mixture to decrease concentration of
cannabinoids
in the mixture, for example. A diluted mixture could be further mixed at 456i,
returned
to the mixer(s) 454i for further mixing. Examples of mixing devices and/or
dilution
devices that could be implemented at 454i, 456i include containers, vessels
and/or
tools for mixing, heated water baths, ultrasonic water baths, heated stir
plates and
heat guns.
One or more of the controller(s) 426i could be connected to or otherwise in
communication with the mixing device(s) 454i and/or the dilution device(s)
456i to
control these components. The sensor(s) 428i could similarly be connected to
or
otherwise in communication with the mixing device(s) 454i and/or the dilution
device(s) 456i, to measure one or more parameters and/or otherwise monitor one
or
more properties of an oil formulation process or equipment.
The produced cannabis oil(s) could be transferred from the mixing device(s)
454i
and/or dilution device(s) 456i to the cannabis oil holding container(s) 458i.
The
cannabis oil holding container(s) 458i could be weighed by the scale(s) 430i-
2. The
label maker(s) 432i could generate and/or apply labels to the cannabis oil
holding
container(s) 458i, and/or the scanner(s) 434i-2 could scan markings on the
container(s) or the label(s). Weights as measured by the scale(s) 430i-2 could
be
used to reconcile input source product with total output cannabis oil, to
maintain
desired and/or required records of source product during processing.
In some embodiments, further testing can be carried out after mixing by mixing
device(s) 454i and dilution by dilution device(s) 456i. Such testing can be
carried out
in the holding container(s) 458i, or once the product has been packaged or
partially
packaged. In some embodiments, such further testing is part of a Preventable
Control
Plan (PCP). In some embodiments, such further testing can include allergen
testing,
label validation testing, microbiological testing, mycotoxins testing,
nutritional
analysis, organoleptic testing, testing for heavy metals, foreign materials,
toxins
and/or other contaminants. The results of such further tests can be recorded
by the
ICS in, for example, the database 414 on server 402.
56
CA 3062485 2019-11-21
85760071
Fig. 4N illustrates an example edibles formulation system 420n. Cannabis-
infused
edibles include, but are not limited to, cakes, brownies, other baked goods,
chocolates, gelatin-based chewable sweets (such as gummy or jelly candies) and
other confectionaries, butters, cooking oils, tinctures, dairy-based liquid
edibles (such
as bhang lassi or bhang thandai), capsules containing one or more
cannabinoids, etc.
The edibles formulation system 420n includes one or more operator check-in
devices
422n, one or more computers 424n, one or more controllers 426n, one or more
sensors 428n, one or more scales at 430n-1 and/or 430n-2, one or more label
makers 432n and one or more scanners at 434n-1 and/or 434n-2. These components
are connected to or otherwise in communication with the server 418n.
Implementation options for all of these components are described herein, at
least
above with reference to Fig. 4A. Although two sets of scale(s) and scanner(s)
are
shown in at 430n-1, 430n-2 and 434n-1, 434n-2, in some embodiments an edibles
formulation system could include only one set of either or both of these
components.
Separate sets of scale(s) and scanner(s) are shown at 430n-1, 430n-2 and 434n-
1,
434n-2 solely to simplify the illustration of connecting lines in Fig. 4N.
The edibles formulation system 420n further includes one or more source
product
holding containers 450n, one or more base foodstuff holding containers 452n,
one or
more mixing devices 454n, one or more dilution devices 456n and one or more
cannabis edible holding containers 458n. The containers 450n, 452n, 458n could
include any of various types of container, and different container types could
be used
for source product, base foodstuff, and cannabis edibles. The source product
holding
container(s) 450n could contain cannabis extract-based products (such as a
distillate
or an emulsified cannabinoid mixture), for example, and in some embodiments
could
include one or more containers as shown at 458n in Fig. 4N. The base foodstuff
holding container(s) 452n could include foodstuffs that, when mixed with a
cannabis
extract, produce a cannabis edible. Suitable foodstuffs include, but are not
limited to,
chocolate, gelatin-based chewable sweets, and any other foodstuff suitable for
being
infused with cannabis or a cannabis-based emulsion.
57
CA 3062485 2019-11-21
85760071
The source product holding container(s) 450n and/or the base foodstuff holding
container(s) 452n could be weighed and/or scanned using the scale(s) 430n-1
and/or
the scanner(s) 434n-1, to quantify and/or identify inputs to the edibles
formulation
system 420n. Moreover, in some embodiments, the base foodstuffs and/or the
source
product can be the subject of testing prior to the mixing stage. In some
embodiments,
such testing is part of a Preventable Control Plan (PCP). In some embodiments,
such
testing can include allergen testing, label validation testing,
microbiological testing,
mycotoxins testing, nutritional analysis, organoleptic testing, testing for
heavy metals,
foreign materials, toxins and/or other contaminants. The results of such tests
can be
recorded by the ICS in, for example, the database 414 on server 402.
The source product(s) in the source product holding container(s) 450n and the
food
stuff(s) in the foodstuff holding container(s) 452n could then be transferred
to the
mixing device(s) 454n to be mixed. The foodstuff(s) could also be transferred
to the
dilution devices 456n. The mixing device(s) 454n could dissolve the source
product(s) in the foodstuff(s) to produce a homogeneous mixture. The dilution
device(s) 456n could add additional foodstuff(s) to the mixture to decrease
concentration of cannabinoids in the mixture, for example. A diluted mixture
could be
further mixed at 456n, returned to the mixer(s) 454n for further mixing.
Examples of
mixing devices and/or dilution devices that could be implemented at 454n, 456n
include containers, vessels and/or tools for mixing, such as industrial food
mixers,
industrial blenders, industrial powder mixers, industrial drum/powder mixers,
industrial ring-layer mixers, industrial pelletizers, industrial granulators,
heated water
baths, ultrasonic water baths, heated stir plates and heat guns.
One or more of the controller(s) 426n could be connected to or otherwise in
communication with the mixing device(s) 454n and/or the dilution device(s)
456n to
control these components. The sensor(s) 428n could similarly be connected to
or
otherwise in communication with the mixing device(s) 454n and/or the dilution
device(s) 456n, to measure one or more parameters and/or otherwise monitor one
or
more properties of an edibles formulation process or equipment.
58
CA 3062485 2019-11-21
85760071
The produced cannabis edible(s) could be transferred from the mixing device(s)
454n
and/or dilution device(s) 456n to the cannabis edibles holding container(s)
458n.
The cannabis edibles holding container(s) 458n could be weighed by the
scale(s)
430n-2. The label maker(s) 432n could generate and/or apply labels to the
cannabis
edibles holding container(s) 458n, and/or the scanner(s) 434n-2 could scan
markings
on the container(s) or the label(s). Weights as measured by the scale(s) 430n-
2 could
be used to reconcile input source product with total output cannabis edible,
to
maintain desired and/or required records of source product during processing.
In some embodiments, further testing can be carried out after mixing by mixing
device(s) 454n and dilution by dilution device(s) 456n. Such testing can be
carried
out in the holding container(s) 458n, or once the product has been packaged or
partially packaged. In some embodiments, such further testing is part of a
Preventable Control Plan (PCP). In some embodiments, such further testing can
include allergen testing, label validation testing, microbiological testing,
mycotoxins
testing, nutritional analysis, organoleptic testing, testing for heavy metals,
foreign
materials, toxins and/or other contaminants. The results of such further tests
can be
recorded by the ICS in, for example, the database 414 on server 402.
Referring now to Fig. 4J, an embodiment of a packaging system 420j includes
one or
more operator check-in devices 422j, one or more computers 424j, one or more
controllers 426j, one or more sensors 428j, one or more scales at 430j-1
and/or 430j-
2, one or more label makers 432j and one or more scanners at 434j-1 and/or
434j-2.
These components are connected to or otherwise in communication with the
server
418j. Implementation options for all of these components are described herein,
at
least above with reference to Fig. 4A. Although two sets of scale(s) and
scanner(s)
are shown in at 430j-1, 430j-2 and 434j-1, 434j-2, in some embodiments a
packaging
system could include only one set of either or both of these components.
Separate
sets of scale(s) and scanner(s) are shown at 430j-1, 430j-2 and 434j-1, 434j-2
solely
to simplify the illustration of connecting lines in Fig. 4J.
59
CA 3062485 2019-11-21
85760071
The packaging system 420j further includes one or more source product holding
containers 450j, one or more cone filling machines 452j, one or more bottle
filling
and/or capping machines 454j and one or more target holding containers 456j.
The
containers 450j, 456j could include any of various types of container, and
different
container types could be used as these containers. The source product holding
container(s) 450j could contain cannabis flower and/or trim, dried cannabis,
milled
cannabis, decarboxylated cannabis, cannabis extracts and/or cannabis oils, for
example. In some embodiments, the source product holding container(s) 450j
could
include containers that hold outputs from any one or more of the examples
systems
420a, 420b, 420d, 420e, 420f, 420g, 420h and/or 420i.
The source product holding container(s) 450j could be weighed and/or scanned
using
the scale(s) 430j-1 and/or the scanner(s) 434j-1, to quantify and/or identify
inputs to
the packaging system 420j. Milled cannabis source product(s) could be
transferred to
the cone filling machine(s) 452j, which is provided to fill paper cones with
cannabis
product to produce cannabis cigarettes. Cannabis oil source product(s) could
be
transferred into bottles using the bottle filling and/or capping machine(s)
454j.
Examples of cone filling machines, bottling filling machines and capping
machines
are discussed in further detail elsewhere herein.
One or more of the controller(s) 426j could be connected to or otherwise in
communication with the cone filling machine(s) 452j and/or the bottle filling
and/or
capping machine(s) 454j, to control the cone filling machine(s) and/or the
bottle filling
and/or capping machine(s). The sensor(s) 428j could similarly be connected to
or
otherwise in communication with the cone filling machine(s) 452j and/or the
bottle
filling and/or capping machine(s) 454j, to measure one or more parameters
and/or
otherwise monitor one or more properties of a packaging process or equipment
such
as the cone filling machine(s) and/or the bottle filling and/or capping
machine(s).
Some types of source product could also or instead be transferred from the
source
product holding container(s) 450j to the target holding container(s) 456j,
which could
include transferring cannabis product into holding containers that are
intended for
CA 3062485 2019-11-21
85760071
sale to customers. For example, target holding containers 456j could contain
smaller
quantities of cannabis product than source product holding container(s) 450j.
Target
holding containers 456j could also include packages that store multiple
holding
containers of cannabis product. Cannabis cigarettes produced by the cone
filling
machine(s) 452j and bottles produced by the bottle filling and/or capping
machine(s)
454j could also or instead be transferred to target holding container(s) 456j,
but this
might not always be the case. For example, bottles of cannabis oil produced by
the
bottle filling and/or capping machine(s) 454j could be considered to be a
holding
container that is intended for sale to customers.
Cannabis cigarettes, bottles of cannabis oil, and/or target holding
container(s) 456j
could be weighed by the scale(s) 430j-2. The label maker(s) 432j could
generate
and/or apply labels to the cannabis cigarettes, bottles of cannabis oil and/or
target
holding container(s) 456j, and/or the scanner(s) 434j-2 could scan markings on
these
cannabis products, container(s), or label(s). Weights as measured by the
scale(s)
430j-2 could be used to reconcile input source product with total output
product, to
maintain desired and/or required records of source product during processing.
The example sterilization system 420k in Fig. 4K includes one or more operator
check-in devices 422k, one or more computers 424k, one or more scales and 430k-
1
and/or 430k-2, one or more label makers 432k and one or more scanners at 434k-
1
and/or 434k-2. These components are connected to or otherwise in communication
with the server 418k. Implementation options for all of these components are
described herein, at least above with reference to Fig. 4A. Although two sets
of
scale(s) and scanner(s) are shown in at 430k-1, 430k-2 and 434k-1, 434k-2, in
some
embodiments a sterilization system could include only one set of either or
both of
these components. Separate sets of scale(s) and scanner(s) are shown at 430k-
1,
430k-2 and 434k-1, 434k-2 solely to simplify the illustration of connecting
lines in Fig.
4K.
The sterilization system 420k further includes one or more source product
holding
containers 450k, an irradiation facility 452k and one or more sterilized
product holding
61
CA 3062485 2019-11-21
85760071
containers 454k. The containers 450k, 454k could include any of various types
of
container, and different container types could be used as these containers. In
some
embodiments, the source product holding container(s) 450k could include
containers
that hold outputs from any one or more of the examples systems 420a, 420b,
420d,
420e, 420f, 420g, 420h, 4201 and/or 420j.
The source product holding container(s) 450k could be weighed and/or scanned
using the scale(s) 430k-1 and the scanner(s) 434k-1, to quantify and/or
identify inputs
to the sterilization system 420k. Source product(s) could be transferred from
the
source product holding container(s) 450k, to irradiation facility 452k, and
then to the
sterilized product holding container(s) 454k. The irradiation facility 452k
could include
equipment, with one or more internal and/or or external controllers (not
shown), to
sterilize the source product(s) by irradiation. Other examples of
sterilization
processes that could also or instead be implemented by equipment in a
sterilization
system are also disclosed elsewhere herein.
One or more internal or external sensor(s) (not shown) could also or instead
be
incorporated into, connected to, or otherwise in communication with the
irradiation
facility 452k and/or other sterilization equipment, to measure one or more
parameters
of sterilization equipment and/or otherwise monitor one or more properties of
a
sterilization process or equipment.
The sterilized product holding container(s) 454k could be weighed using the
scale(s)
430k-2. The label maker(s) 432k could label using the sterilized product
holding
container(s) 454k. Markings on the sterilized product holding container(s)
454k or
label(s) could be scanned using the scanner(s) 434k-2. Weights as measured by
the
scale(s) 430k-2 could be used to reconcile input source product with total
output
product, to maintain desired and/or required records of source product during
processing.
An example of a testing system 4201 is shown in Fig. 4L, and includes one or
more
operator check-in devices 4221, one or more computers 4241, one or more
controllers
4261, one or more scales 4301, one or more label makers 4321 and one or more
62
CA 3062485 2019-11-21
85760071
scanners 4341. These components are connected to or otherwise in communication
with the server 4181. Implementation options for all of these components are
described herein, at least above with reference to Fig. 4A.
The testing system 4201 further includes one or more source product holding
containers 4501, one or more sampling containers 4521 and one or more testing
devices 4541. The containers 4501, 4521 could include any of various types of
container, and different container types could be used as these containers. In
some
embodiments, the source product holding container(s) 4501 could include
containers
that hold outputs from any one or more of the examples systems 420a, 420b,
420d,
420e, 420f, 420g, 420h, 420i, 420j and/or 420k.
At least a portion of a source product in each source product holding
container(s)
4501 could be transferred to a sampling container 4521. Each sampling
container 4521
could store one or more samples for testing.
The source product holding container(s) 4501 and/or the sampling container(s)
4521
could be weighed and/or scanned using the scale(s) 4301 and/or the scanner(s)
4341,
to quantify and/or identify inputs to the testing system 4201. Labels could be
applied
to the sampling container(s) 4521 using the label maker(s) 4321. Markings on
either or
both of source product holding container(s) 4501 and the sampling container(s)
4521,
or label(s) thereon, could be scanned by the scanner(s) 4341 to track the
particular
source product(s) being sampled and tested.
The sample(s) in the sampling container(s) 4521 could be tested by the testing
device(s) 4541. Examples of testing devices 4541 include, but are not limited
two,
devices configured to test for mold and/or the presence of pesticides or other
chemicals. The testing device(s) 4541 are connected to or otherwise in
communication with the server 4181, and could transmit test results to the ICS
through the server. Also or alternatively, test results could be recorded
manually
using the computer(s) 4241, for example.
63
CA 3062485 2019-11-21
85760071
One or more of the controller(s) 4261 could be connected to or otherwise in
communication with the testing device(s) 4541, to control the testing
device(s).
One or more internal or external sensor(s) (not shown) could also or instead
be
incorporated into, connected to, or otherwise in communication with the
testing
device(s) 4541, to measure one or more parameters of sterilization equipment
and/or
otherwise monitor one or more properties of a testing process or equipment.
Although not explicitly shown in Fig. 4L, the source product holding
container(s) 4501
could be weighed using the scale(s) 4301 after any samples have been taken, to
reconcile source product input, remaining source product after testing, and
source
product samples. In some embodiments, source product(s) samples are taken and
stored to maintain archived source product samples. Archived source product
samples could be taken in addition to source product samples that are tested
by the
testing device(s) 4541. In some embodiments, archived samples could be weighed
using the scale(s) 4301, possibly labelled using the label maker(s), and have
markings
scanned by the scanner(s) 4341 to enable archived sample recording and
tracking.
In the example shown in Fig. 4M, a shipping system 420m includes one or more
operator check-in devices 422m, one or more computers 424m, one or more scales
430m, one or more label makers 432m and one or more scanners 434m. These
components are connected to or otherwise in communication with the server
418m.
Implementation options for all of these components are described herein, at
least
above with reference to Fig. 4A.
The shipping system 420m further includes one or more customer order databases
450m stored in one or more memory devices, one or more selected holding
containers 452m, one or more packages 454m and one or more shipping services
456m. The customer order database(s) 450m store customer orders for cannabis
products. The memory device(s) in which the customer order database(s) 450m
are
stored are connected to or otherwise in communication with the server 418m,
and in
communication with the ICS in some embodiments. Although Fig. 4M illustrates
the
customer order database(s) 450m as a separate component, in some embodiments
64
CA 3062485 2019-11-21
85760071
the customer order database(s) 450m could be stored within the ICS and/or a
computer 424m.
The selected holding container(s) 452m are intended to represent one or more
holding containers of cannabis product(s) that have been selected to meet one
or
more customer orders stored in the customer order database(s) 450m, and could
include containers that hold outputs from any one or more of the examples
systems
420a, 420b, 420d, 420e, 420f, 420g, 420h, 420i, 420j, 420k and/or 420m. The
selected holding container(s) 452m, or contents such as individual units
therein, are
transferred to one or more packages 454m. Each package could include all of
the
holding containers or units that are selected to meet one customer order.
Selection of
holding container(s) and/or packaging into package(s) could include manual
selection
and packing and/or automated selection and packaging by "picking" machines.
The package(s) 454m could then be transferred to the shipping service(s) 456m,
which ship packages to customers. Shipping service(s) 456m could include, for
example, courier services.
The selected holding container(s) 452m and/or the package(s) 452m could be
weighed and/or scanned using the scale(s) 430m and the scanner(s) 434m. The
selected holding container(s) 452m could be weighed before and after order
fulfilment
if some but not all contents of a holding container are used to fill an order.
Labels could also or instead be applied to the package(s) 454m using the label
maker(s) 432m. The shipping service(s) 456m are connected to or otherwise in
communication with the server 418m. A shipping service 456m could be a
separate
entity from a producer of cannabis products, and connect to server 418m
through a
different type of connection than other shipping system components, using the
internet for example. Tracking numbers provided by the shipping service(s)
456m
could then be stored transmitted to the server 418m and stored by the server
and/or
transmitted to the ICS for storage. Shipping system tracking numbers could be
useful,
for example, to track order fulfilment, to confirm order shipping, to monitor
the
CA 3062485 2019-11-21
85760071
location of the package(s) 454m after shipping, and/or to confirm order
delivery, for
example.
The example system 400 shown in Figs. 4A-4M and described in detail above
represents one illustrative embodiment. Other embodiments are also
contemplated.
For example, although various components are shown separately in these
drawings,
multiple components could be implemented in a single component. In some
embodiments, any two or more of operator check-in device(s), computer(s),
controller(s), sensor(s), scale(s), label maker(s), and scanner(s) could be
implemented using a single device. In one example, plant cultivation and
harvest and
plant part separation are within one facility, and operator check-in devices
422a, 422b
are implemented using a single operator check-in device. In another example, a
label
maker 432a and a scanner 434a are implemented using a single device. Other
combinations are also contemplated.
Various implementations, as well as applications of information, equipment,
and
functions, in an ICS are possible. Illustrative examples are disclosed herein,
and
others may be or become apparent to those skilled in the art.
For example, an ICS could use machine-readable code to identify and record
cannabis products. Fig. 5 is a block diagram illustrating an example
implementation
of a barcode scanner 502 in communication with an ICS through a server 500.
Fig. 3
includes a barcode scanner 502, which is connected to or otherwise in
communication with a server 500. The scanner 502 is illustrated as a portable
barcode scanner, which could be wired or wireless, but this is only an
example. The
server 500 could be the server 418a-m in any of the example systems shown in
Figs.
4A-4M. Various scanner and server implementation examples are disclosed
elsewhere herein, at least with reference to Figs. 4A-4M.
Fig. 5 also includes a package 506, which has a label 508 that includes a
barcode
encoding a number "00536801234". In some embodiments, the number could be a
unique identifier for the package 508. The package 508 stores multiple holding
containers 510, 512, 514, 516. Each of the holding container 510, 512, 514,
516
66
CA 3062485 2019-11-21
85760071
could contain a respective cannabis product. These cannabis products could be
the
same type of product, be from the same batch of cannabis plants, and/or be
from the
same lot of cannabis products. Alternatively, the cannabis products could have
no
relation to each other. The holding containers 510, 512, 514, 516 each include
a
respective label 518, 520, 522, 524 with a barcode. In the case that the
holding
containers 510, 512, 514, 516 contain the same lot of cannabis product, the
labels
518, 520, 522, 524 could be identical, or could at least include some common
information that is identical across multiple labels.
In some embodiments, the scanner 502 could read the label 508 and determine
the
number "00536801234". For example, the scanner 502 could decode the barcode in
the label 508. The scanner 502 could then transmit the number to the server
500 for
local storage and/or transmission to a central ICS database. Alternatively or
additionally, the scanner 502 could send a picture of the label 508 to the
server 500,
and the label could be decoded at the server or another server that hosts the
central
ICS database. The scanner 502 could also or instead transmit an action or
request to
the server 500 with the number. In one example, a user might want to determine
the
contents of the package 506. In that case, the scanner 502 could send a "look-
up"
request to the server 500 with the number "00536801234". Upon receipt of the
request, the server 500 could search a local database or request a search of a
central ICS database for a record or records associated with the number
"00536801234". Any relevant records or information from those records could be
sent
to the server 500 and/or to the scanner 502. For example, any or all
information in an
ICS related to the holding containers 510, 512, 514, 516 could be sent to the
server
500 and/or to the scanner 502. The device 502 could display such information
to an
operator on a screen 504. This could be useful for example, when a customer is
unpacking an order and package contents are to be verified.
In another example, the package 506 could have been received at a new
location,
such as a storage facility. In this case, the scanner 502 could send the
number
"00536801234" and a "received" action to the server 500. The server 500 could
locally store the number and/or update one or more local records to indicate
that the
67
CA 3062485 2019-11-21
85760071
package 506 has been received at the new location. The server 500 could also
or
instead send information to a central ICS database, to enable the central ICS
database to be similarly updated with current status of the package 506. In
some
embodiments, information such as a confirmation or acknowledgement could be
sent
to the scanner 502 to confirm that package status has been successfully
updated.
Other actions and/or requests could also or instead be sent from the scanner
502 to
the server 500, from the server 500 to the scanner 502, and/or from the server
500 to
other components such as a server that hosts a central ICS database. For
example,
the scanner 502 could also or instead communicate with the server 500, and the
server 500 could communicate with other components when the holding container
labels 518, 520, 522, 524 are scanned, to enable traceability of holding
containers
that were actually placed into and unpacked from the package 506, and/or to
detect
potential tampering if packed and unpacked holding container information does
not
match.
The scanner 502 is an illustrative example of an electronic device that could
be in
communication with a server or other component implementing an ICS. Other
electronic devices, such as computers, scales, controllers, and/or sensors,
for
example, could also or instead be in communication with an ICS component such
as
a server. These electronic devices could be portable or stationary, and wired
or
wireless. Examples of these devices are described in further detail elsewhere
herein.
More generally, the implementation with a barcode scanner as illustrated in
Fig. 5 is
provided by way of example. Other ICS implementations could also or instead be
used. For example, an ICS could be implemented using physical files, in
addition to
or instead of electronic files stored in computer memory. In some
implementations,
recording of products and processes in the ICS could be manual, automated, or
a
combination of both. In further implementations, authorization levels could be
implemented within the ICS, such that the type of actions and/or requests that
an
operator or device can perform in the ICS is limited by their authorization
level.
68
CA 3062485 2019-11-21
85760071
An ICS could be applied or used in any of various ways according to
embodiments of
the present disclosure. Fig. 6, for example, is a flow chart illustrating an
example
method according to one embodiment. The example method 530 involves providing,
at 532, a database such as the database 414 in Fig. 4A to store information
associated with cannabis plants and cannabis products. Such a database could
be
stored in one or more memory devices, which could include memory devices of
different types. Examples of memory devices in which a database could be
stored are
disclosed elsewhere herein. The database could be populated with any of
various
types of information. Plant identifiers, such as plant numbers disclosed
elsewhere
herein, represent an example of information that is associated with cannabis
plants
and could be stored in a database. Plant information could also or instead
include
information that conveys such parameters or characteristics as grow area, any
of
various growing conditions, and/or harvest details, for example. Other
examples of
plant information are disclosed elsewhere herein. Similarly, any of various
types of
information associated with cannabis products could be stored in the database,
and
examples of such information are disclosed elsewhere herein. The example
method
530 is not limited to any particular cannabis products. The cannabis product
information stored in the database could include different fields and/or
different types
of information for different types of cannabis products.
A batch identifier is assigned to a batch of the cannabis plants, at 534. A
batch
identifier could be, for example, a batch number as disclosed elsewhere
herein. In
some embodiments, batch identifiers are sequential, and a most recently used
batch
identifier is incremented by a value of one to generate or otherwise determine
a next
sequential batch identifier when a new batch identifier is to be assigned.
Batch
identifiers need not be sequential in other embodiments. In general, any batch
identifier generation or determination approach that enables different batches
to be
distinguished from each other could be applied.
Batch identifiers could be generated or determined on an as-needed basis as
noted
above, but could also or instead be generated in advance and stored to memory,
for
access or retrieval when a new batch identifier is to be assigned. A central
ICS server
69
CA 3062485 2019-11-21
85760071
such as the server 402 in Fig. 4A, the cultivation and harvest system server
418a, the
label maker(s) 432a, and/or another component of the cultivation and harvest
system
420a could generate or determine batch identifiers.
The actual assignment of a batch identifier at 534 could be accomplished in
some
embodiments by marking or otherwise recording the batch identifier as
assigned,
allocated, or reserved in a memory, to indicate that the batch identifier has
been
assigned to a batch of cannabis plants and therefore should not be assigned to
another batch. In some embodiments, batch identifiers are assigned and then
incremented or otherwise changed so that a new batch identifier is assigned to
a next
batch of cannabis plants. Other batch identifier management approaches are
also
possible.
Plant material from a portion of the cannabis plants in the batch is processed
at 536,
using a first process, to produce units of a first cannabis product. Plant
material from
another portion of the cannabis plants in the batch is processed at 538, using
a
second process, to produce units of a second cannabis product. The first and
second
processes could, but need not necessarily, be performed concurrently. Examples
of
processes that could be used to produce different cannabis products are
disclosed
elsewhere herein, and any of those processes could be used to process plant
material at 536, 538.
For example, the processing at 536, 538 could include any one or more of:
separating the plant material; drying the plant material; curing the plant
material; and
extracting one or more cannabinoids from the plant material. An extraction
process
for extracting one or more cannabinoids from the plant material could involve
performing supercritical CO2 extraction of cannabinoids from the plant
material. In
some embodiments, extracting one or more cannabinoids from the plant material
further involves producing a cannabis extract and distilling the cannabis
extract.
At 540, a first lot identifier is assigned to a lot of the units of the first
cannabis product,
and a second lot identifier is assigned to a lot of the units of the second
cannabis
product at 542. The first and second lot identifiers could, but need not
necessarily, be
CA 3062485 2019-11-21
85760071
assigned concurrently. Examples of lots and lot identifiers are disclosed
elsewhere
herein, and any of those examples could be applied in delineating lots and
assigning
lot identifiers at 540, 542. The units of the first and second cannabis
products could
be delineated into lots in similar or different ways. Lot identifiers for the
lots of units of
the first and second cannabis products could be of the same type or different
types.
In some embodiments, lot identifiers could be generated, determined, and/or
assigned in a similar manner as described above for batch numbers. For
example, lot
identifiers could be sequential and generated on an as-needed basis.
Lot identifiers could be managed and/or assigned independently for different
cannabis products. For example, lot identifiers could be unique within each
type of
cannabis product lines to allow lots of each cannabis product to be uniquely
identified, but need not necessarily be "globally" unique across all product
lines. The
same lot identifier could be assigned to lots of units of different cannabis
products,
because those different cannabis products could be distinguished from each
other
based on product type, even though the lot numbers are the same. In other
embodiments, lot identifiers are unique across all product lines, and a
particular lot
identifier is assigned to only one lot.
The example method 530 also involves modifying the database, at 544, to
include
information conveying or indicating the batch identifier, the first lot
identifier and the
second lot identifier, with the first lot identifier and the second lot
identifier each being
associated with the batch identifier. In some embodiments, such associations
between multiple lot identifiers and a batch identifier are inherent in an
arrangement
or organization of information in the database. For example, a database record
could
include multiple fields or entries that are populated with information that
conveys
associated identifiers.
In some embodiments, modifying the database at 544 involves creating a lot
record
for each lot of units of a cannabis product, with the lot record including
information
conveying the lot identifier associated with the lot and information conveying
the
batch identifier associated with the lot identifier. In this example,
information
71
CA 3062485 2019-11-21
85760071
conveying the lot identifier and information conveying the batch identifier
are in the
same lot record, and the lot identifier ¨ batch identifier association is
inherent in the
arrangement of information in the lot record. Respective lot records could be
created
for different lots, such as a first lot record for the lot of units of the
first cannabis
product and a second lot record for the lot of units of the second cannabis
product in
an example described above.
A lot record could also include other information. In some embodiment, a lot
record
includes information indicative of the process or processes used in processing
plant
material to produce units of a cannabis product that is associated with the
lot.
Examples of processes that could be conveyed or indicated in information in a
lot
record are disclosed elsewhere herein. Information conveying or indicating any
of
various parameters or characteristics of such processes could also or instead
be
included in a lot record.
A lot record could also or instead include information indicative of the
number of units
of a cannabis product contained in the lot. This information could be useful,
for
example, to track production output and/or concentration of active
substance(s) in
cannabis products.
Another type of information that could be included in a lot record in some
embodiments is information indicative of the time, date, and/or other details
of the
processing that was used to produce units of a cannabis product contained in
the lot.
The method 530 is an illustrative example of a method according to one
embodiment.
Other embodiments could involve performing operations in a different order
than
shown, and/or performing different operations instead of or in addition to
those shown
in Fig. 6. For example, units of a cannabis product could be packaged, for
storage
and/or shipment.
Considering the first cannabis product in Fig. 6, each of the units of that
cannabis
product could be packaged to produce first product packages, and each product
package could be marked with product information indicative of the first lot
identifier.
72
CA 3062485 2019-11-21
85760071
A method could also or instead involve packaging each of the units of the
second
cannabis product to produce second packages, and marking each second package
with product information indicative of the second lot identifier. Changes of
identifiers
between different lots are also described in further detail elsewhere herein.
In some embodiments, the product information that is marked on packages is
generated, at least in part, from information retrieved from the database.
Product
information could be stored in the database, retrieved, and used to marked
packages,
or information retrieved from the database could be coded or otherwise
processed to
generate the product information with which packages are marked.
The product information for package marking could include any one or more of
the
following:
information conveying an identity or contact information of a licensed
producer of
the cannabis plants;
information conveying an identity or contact information of a licensed
processor of
the cannabis product;
information conveying a brand name of a cannabis product; information
conveying
recommended storage conditions of a cannabis product; and
information conveying a packaging date of a cannabis product.
Package marking could involve printing the product information on a package.
In
some embodiments, marking each product package involves printing a label
including
the product information, and affixing the label to the package. Information
could be
retrieved from the database, and the label could then be generated using the
information retrieved from the database.
The example method 530 illustrates processing of plant material from cannabis
plants
in one batch to produce first and second cannabis products. Other plant
material
could also be processed. For example, the processing at 536 could also involve
processing plant material from a portion of the cannabis plants in a second
batch of
73
CA 3062485 2019-11-21
85760071
cannabis plants using the first process to produce units of the first cannabis
product.
The modifying at 544 could then involve modifying the database to include
information conveying a second batch identifier assigned to the second batch,
and to
associate the first lot identifier with the second batch identifier. In this
example, lot of
units of the first cannabis product are produced from plant material from
cannabis
plants in multiple batches, and the first lot identifier is associated with
multiple batch
identifiers. Using the database, the first lot identifier in this example
could be traced to
two batch identifiers, and the lot of units of the first cannabis product
could thus be
traced to two batches of cannabis plants from which the units of the first
cannabis
product originated.
In some embodiments, as initially described above with reference to Fig. 6,
the units
in one lot of a cannabis product are produced from one batch of cannabis
plants, and
a lot identifier that is assigned to the product lot is associated with only
one batch
identifier. Lot identifiers assigned to different lots could be associated
with the same
batch identifier if cannabis plants from one batch are used to produce the
different
lots. In other embodiments, cannabis plants from multiple batches are used to
produce one lot of units, and a lot identifier for such a lot is associated
with multiple
batch identifiers.
Other variations of the example method 530 may be or become apparent to those
skilled in the art.
A method could be implemented using a processor-readable storage medium,
examples of which are disclosed elsewhere herein. Such a storage medium could
have processor-executable instructions stored thereon, which, when executed by
a
processor, cause the processor to perform a method. Execution of the
instructions
could cause a computing device that includes the processor to implement a
system
configured to perform various operations. In some embodiments, the
instructions,
when executed, cause the computing device to implement a system configured to:
implement a database configured to store information associated with cannabis
plants and cannabis products; assign a batch identifier to a batch of the
cannabis
74
CA 3062485 2019-11-21
85760071
plants; receive processing information relating to the processing of plant
material
from a portion of the cannabis plants in the batch using a first process to
produce
units of a first cannabis product; receive processing information relating to
the
processing of plant material from another portion of the cannabis plants in
the batch
using a second process to produce units of a second cannabis product; assign,
using
the processing information, a first lot identifier to a lot of the units of
the first cannabis
product and a second lot identifier to a lot of the units of the second
cannabis
product; and modify the database to include information relating to the batch
identifier, the first lot identifier and the second lot identifier, with the
first lot identifier
and the second lot identifier each being associated with the batch identifier.
Examples of many of these features are described above with reference to Fig.
530.
A system implemented by a computing device could be configured to implement a
database in one or more memory devices, for example, to store plant and
product
information, examples of which are described above and elsewhere herein. Such
a
system could also be configured to assign a batch identifier and lot
identifiers, and to
modify the database as described above and elsewhere herein.
Fig. 6 and the description thereof refer to processing plant material to
produce units
of cannabis products. A production system could include processing equipment
to
process plant material using processes to produce units of cannabis products.
Different processing equipment could apply different processes to plant
material, for
example. A system that is implemented by a computing device might not itself
include
such processing equipment, but could be part of a production system, or at
least
communicate with processing equipment in a production system. A system that is
implemented by a computing device could receive processing information from
processing equipment, for example. In an embodiment, such a system is
configured
to receive processing information relating to the processing of plant material
from a
portion of the cannabis plants in the batch using a first process to produce
units of a
first cannabis product, and to receive processing information relating to the
processing of plant material from another portion of the cannabis plants in
the batch
using a second process to produce units of a second cannabis product. Any of
CA 3062485 2019-11-21
85760071
various types of processing information could be received, and examples of
information relating to processing of plant material are disclosed elsewhere
herein.
Different types of processing could have different types of related
information.
The server 402 in Fig. 4A, and other servers and computers in Figs. 4A-4E, for
example, could be configured to receive such information in some embodiments.
Processing information could be used to assign a first lot identifier to a lot
of units of
the first cannabis product and a second lot identifier to a lot of units of
the second
cannabis product. For example, each lot identifier could be assigned based on
a type
of the process, conveyed or indicated in the processing information, that was
used to
produce the units in that lot.
A system implemented by a computing device could be configured to provide
other
features disclosed herein.
The example method 530 and the example system described above provide and
modify a database that includes various types of information. In some
embodiments,
a hierarchal dataset could have a tree structure, representative of a process
flow to
transform a batch of cannabis plants into a range of cannabis products. A
method for
dynamically generating such a hierarchal dataset could involve recording, on a
computer readable storage medium, a batch identifier associated with the batch
of
cannabis plants. With reference to Fig. 6, for example, the batch identifier
could be
recorded on the computer readable storage medium by modifying a database as
shown at 544. Although Fig. 6 represents modifying the database at the end of
the
example method 530, in other embodiments information such as the batch
identifier
could be recorded earlier, such as when it is assigned.
The batch identifier distinguishes the batch of cannabis plants among a
plurality of
batches of cannabis plants. Examples of batch identifiers are disclosed
elsewhere
herein. In some embodiments, the batch identifier is a root level of the
hierarchal
dataset.
76
CA 3062485 2019-11-21
85760071
A first portion of the batch of cannabis plants is processed using a first
process to
produce units of first cannabis products, and such processing is disclosed by
way of
example with reference to 536 in Fig. 6. A first lot number associated with
the first
cannabis products is recorded on the computer readable storage medium, and
this is
also represented by way of example at 544 in Fig. 6.
A second portion of the batch of cannabis plants is processed using a second
process, to produce units of a second cannabis product, and a second lot
number
associated with the second cannabis products is recorded on the computer
readable
storage medium. These operations are consistent with 538, 544 in some
embodiments.
Generating a hierarchical dataset could also involve linking the first and
second lot
numbers to the batch identifier in the hierarchal dataset. In some
embodiments, the
first lot number forms a first branch of the hierarchal dataset ascending, or
descending, from the root node and the second lot number forms a second branch
of
the hierarchal dataset ascending, or descending, from the root node.
The example method 1720, like other methods disclosed herein, could include
fewer,
additional, and/or different operations, performed in a similar or different
order.
For example, the hierarchal dataset could include additional information, such
as any
one or more of the following:
information indicative of the process or processes used in the steps of
processing
the first and second portions of the batch of cannabis plants;
information indicative of the number of units produced of the first and second
cannabis products;
information indicative of the time and/or date of the processing used to
produce
the units of the first and second cannabis products.
77
CA 3062485 2019-11-21
85760071
Processing of the first portion of the batch of cannabis plants and the
processing of
the second portion of the batch of cannabis plants could include, for example,
any
one or more of the following, which are also described elsewhere herein:
separating the plant material;
drying the plant material;
curing the plant material; and
extracting one or more cannabinoids from the plant material.
In some embodiments, extracting one or more cannabinoids from the plant
material
involves performing supercritical CO2 extraction of cannabinoids from the
plant
material.
Extracting cannabinoids from the plant material could also or instead involve
such
operations as producing a cannabis extract; and distilling the cannabis
extract.
In some embodiments, each of the units of the first cannabis product is
packaged to
produce first product packages, and each first product package is marked with
.. product information indicative of the first lot number. Similarly, each of
the units of the
second cannabis product could be packaged to produce second product packages
and each second product package could be marked with product information
indicative of the second lot number.
As in other embodiments disclosed herein, marking could involve marking
product
packages directly and/or printing a label including the product information
and affixing
the label to a package.
Product information need not be limited only to information indicative of lot
number.
Product information could also or instead include at least one of: information
conveying an identity or contact information of a licensed producer of the
cannabis
plants; information conveying an identity or contact information of a licensed
processor of the cannabis product; information conveying a brand name of the
78
CA 3062485 2019-11-21
85760071
cannabis product; information conveying recommended storage conditions of the
cannabis product; and information conveying a packaging date of the cannabis
product.
Two portions of a batch of cannabis plants are referenced above. Some
embodiments involve processing plant material from a portion of the cannabis
plants
in a further batch of cannabis plants associated with a further batch
identifier, using
the first process, to produce the units of the first cannabis product; and
linking the first
lot number to the further batch identifier in the hierarchal dataset. Such
linking or
associations between could be accomplished in any of various ways, examples of
which are disclosed elsewhere herein.
Embodiments described above with reference to Fig. 6 involve associating
various
identifiers with each other, and could involve aspects of labelling. For
example, units
of first and second cannabis products could be marked with product information
that
is indicative of different lot numbers. At least these features could impact
product
labelling.
Fig. 7 illustrates operation of a machine, in particular a label maker 552 by
way of
example, for generating labels, according to one embodiment. In the example
shown,
the label maker 554 is connected to or otherwise in communication with a
server 550.
In some embodiments, the server 550 could be the central ICS server 402 in
Fig. 4A
or any one of the other servers in Figs. 4A-4M. Similarly, the label maker 552
could
be a label maker as shown in any of Figs. 4A-4M. Examples of a server and a
label
maker are provided elsewhere herein.
In Fig. 7, during time period A, the label maker 552 generates labels, for
units of a
particular cannabis product originating from a particular batch of cannabis
plants for
example. Each label 554 in this example includes a machine-readable code that
encodes a number specific to the cannabis product and a number that maps back
to
an identity of the batch. In the illustrated example, the number specific to
the
cannabis product is a GTIN and the number that maps back to the identity of
the
batch is the lot number. For example, in Fig. 7 the label 554 is generated for
the
79
CA 3062485 2019-11-21
85760071
cannabis product "Bedtime" dried buds, having a linear barcode 556 that
encodes
GTIN 406972 and the lot number A232. All labels for "Bedtime" dried buds
belonging
to the same lot A232 have the same linear barcode 556, or at least include the
same
GTIN and lot number. Later, during time period B, when units of the cannabis
product
from a different batch are being labelled for example, then the label maker
552
updates the machine-readable code to at least update the number that maps back
to
the identity of the batch. For example, in Fig. 7 the label 560 is generated
for the
cannabis product "Bedtime" dried buds, having a linear barcode 562 that still
encodes
GTIN 406972, but the lot number is changed to A244. All labels for "Bedtime"
dried
buds belonging to the same lot A244 have the same linear barcode 562, or at
least
include the same GTIN and lot number.
The switch in lot numbers in this example is controlled by the server 550, but
in other
embodiments the label maker could count labelled units of cannabis product and
switch lot numbers based on the number of units in a lot. Other control
mechanisms
are also contemplated.
In another embodiment, the label maker 552 is instead replaced with a machine
that
generates cannabis product packaging having the machine-readable code. In
another embodiment, the label maker 552 is instead replaced with a machine
that
generates anything in association with cannabis products that is to have the
machine-
readable code included thereon.
In view of the above, in some embodiments there is provided a cannabis product
including packaging (e.g. a container in which the cannabis product is
contained) and
a machine-readable code included on or as part of the packaging (e.g. on a
label
affixed to the packaging). In some embodiments, the machine-readable code
specifically conveys information that links the cannabis product back to a
particular
batch of cannabis plants from which cannabis in the cannabis product
originates (e.g.
the information may be a lot number and/or the batch number).
Fig. 8 is a flow diagram illustrating an example method of labelling cannabis
products
in an automated manufacturing process, and involves controlling a labelling
system to
CA 3062485 2019-11-21
85760071
label cannabis products with information related to different lot identifiers.
The
example method 570 involves, at 572, processing a portion of a first amount of
cannabinoid-containing substance to sequentially produce first units of a
cannabis
product. The first amount of cannabinoid-containing substance is associated
with a
first cannabinoid-containing substance identifier. Examples of cannabinoid-
containing
substances and identifiers are disclosed elsewhere herein.
The example method 570 also involves, at 574, determining a last unit of
cannabis
product produced in the first units, or in other words determining the last
one of the
first units. The last unit could be determined, for example, based on an
amount of the
cannabinoid-containing substance that is used in producing each unit, and how
many
units can be produced from the first amount of the cannabinoid-containing
substance.
The units are produced sequentially at 572, and in some embodiments are
counted to
identify the last unit of cannabis product that is produced from the first
amount of the
cannabinoid-containing substance.
A portion of a second amount of the same cannabinoid-containing substance or a
different cannabinoid-containing substance could then be processed at 576 to
sequentially produce second units of the same cannabis product, or possibly a
different cannabis product. The second amount of cannabinoid-containing
substance
is associated with a second cannabinoid-containing substance identifier.
Again, it is
noted that examples of cannabinoid-containing substances and identifiers are
disclosed elsewhere herein.
The first and second units of cannabis product are labelled at 578, by
controlling an
automated labelling system to label units of cannabis product with label
information
conveying a first lot identifier associated with the first cannabinoid-
containing
substance identifier until the last unit of cannabis product has been
labelled, and to
then label units of cannabis product with label information conveying a second
lot
identifier associated with the second cannabinoid-containing substance
identifier
thereafter. This is consistent with the type of labelling illustrated in time
period A and
time period B in Fig. 7, for example.
81
CA 3062485 2019-11-21
85760071
The method 570, like other embodiments, represents an illustrative example.
Other
embodiments could involve performing operations in a different order than
shown,
and/or performing different operations instead of or in addition to those
shown in Fig.
8.
For example, although shown in sequence in Fig. 8, the illustrated operations
need
not necessarily be completed in the order shown. A last unit of cannabis
product
could be determined at 574 before processing of the first amount of
cannabinoid-
containing substance at 572 is complete. Processing of the second amount of
cannabinoid-containing substance at 576 could also or instead begin before the
last
unit has been determined at 574. In some embodiments, labelling at 578 could
begin
before other operations have been completed.
As an example of an additional operation that could be performed in some
embodiments, the units of cannabis product could be packaged into product
packages, and the labelling at 578 could then involve affixing labels to the
product
packages. Either or both of product units and product packages could be
labelled at
578.
Regarding the operation at 574, in a sequential production process in which
units are
produced sequentially, determining a last unit of cannabis product produced in
the
first units is equivalent to, and could involve, determining the first unit of
cannabis
product produced in the second units.
Processing a portion of a first amount of cannabinoid-containing substance at
572,
and/or processing a portion of a second amount of cannabinoid-containing
substance
at 576, could involve one or more of the following, and examples of these
types of
processing are disclosed elsewhere herein:
metering out amounts of cannabinoid-containing substance;
diluting cannabinoid-containing substance;
82
CA 3062485 2019-11-21
85760071
emulsifying cannabinoid-containing substance to produce a concentrated
cannabinoid emulsion;
distilling cannabinoid-containing substance to produce distillate;
metering out amounts of distillate;
diluting distillate; and
emulsifying distillate to produce a concentrated cannabinoid emulsion.
Label information with which the first units and/or the second units are
labelled could
include, in addition to information conveying the first or second lot
identifier, at least
one of: information conveying an identity or contact information of a licensed
producer of the cannabinoid-containing substance; information conveying an
identity
or contact information of a licensed processor of the cannabis product;
information
conveying a brand name of a cannabis product; information conveying
recommended
storage conditions of a cannabis product; and information conveying a
packaging
date of a cannabis product.
A processor-readable storage medium could be used in implementing a method
that
is consistent with Fig. 8, with processor-executable instructions being stored
on such
a medium. The instructions, when executed by a processor, cause the processor
to
perform a method. Execution of the instructions could cause a computing device
that
includes the processor to implement a system configured to, in some
embodiments:
receive processing information related to processing of cannabinoid-containing
substance as shown at 572 and described above, determine a last unit as shown
at
574 and described above, receive processing information related to processing
of
cannabinoid-containing substance as shown at 576 and described above, and
control
an automated labelling system receive processing information related to
processing
of cannabinoid-containing substance as shown at 578 and described above.
An automated production system could include such a computing device, as well
as
processing equipment and an automated labelling system. The processing shown
at
83
CA 3062485 2019-11-21
85760071
572 and 574 could be performed in one installation of processing equipment, or
in
separate processing equipment. The first amount of cannabinoid-containing
substance could be supplied to processing equipment until either it runs out
or the
last unit has been produced from the first amount, and then the cannabinoid-
containing substance supply could be switched to the second amount to continue
production.
Various embodiments of an ICS and example systems, methods, and processor-
readable storage media are discussed above. Particular parts of a production
system
or process, and potential implications from an ICS point of view, are
discussed in
further detail below.
Harvesting and Plant Part Separation Processes
For the harvest of cannabis plants, any of a variety of information could be
recorded
in an ICS. For example, information related to a batch of cannabis plants that
is
harvested in operation 102 of Fig. 1 could be recorded in the ICS. Batch
information
could be recorded when the plants are harvested, for example. Batch
information
could also or instead be recorded during cultivation, and then updated when
the
plants are harvested.
In some embodiments, batch information could be recorded in the ICS in the
form of
a batch record that includes or is otherwise associated with a batch
identifier. Harvest
information related to a harvest process could also or instead be recorded in
the ICS,
as part of a batch record and/or in a separate harvest record. A harvest
record could
include or otherwise be associated with a harvest identifier, which could be
similar in
form to other identifiers disclosed herein. A batch record could include a
harvest
identifier or otherwise be associated with a harvest record for a harvest, or
multiple
harvest records if the batch was harvested over multiple days or in different
ways for
example. Similarly, a harvest record could include a batch identifier or
otherwise be
associated with a batch record, or multiple batch records if multiple batches
are
harvested in one harvest.
84
CA 3062485 2019-11-21
85760071
The following is a non-exhaustive list of information that could be recorded
in the ICS
for a batch of cannabis plants. A batch record and/or a harvest record could
include
any one or more of the following:
origin of seeds and/or cuttings used to grow a batch;
storage location of the seeds and/or cuttings;
quantity of plants in the batch, possibly recorded at different points in time
(for
example, number of plants at planting versus number of plants at harvesting);
cannabis plant strain in the batch;
cultivation period prior to harvesting (for example, harvesting performed
after 8
weeks of cultivation);
quantity or percentage of plants that perished during the growing and/or
harvesting process, possibly along with a record of when the plant perished
and/or why/how it perished;
type, quantity, and/or composition of a growing medium used during
cultivation;
type, quantity, composition, and/or application schedule of nutrients (for
example,
fertilizer) during cultivation;
type, quantity, and/or schedule of lighting during cultivation;
schedule of temperature and/or humidity during cultivation;
type, quantity, and/or schedule of air ventilation during cultivation;
quantity of watering and/or watering cycle during cultivation;
quantity and/or percentage of plants that required special attention, possibly
along
with the details of the attention needed;
treatments performed on the plants during cultivation;
CA 3062485 2019-11-21
85760071
root pH levels during cultivation; and
plant nutrients levels during cultivation.
However, not every item listed above is necessarily applicable to every batch,
and
not all items would necessarily be recorded even if applicable to a particular
batch.
Information could be recorded and/or updated in the ICS during plant part
separation.
For example, during operation 104 of Fig. 1, the weight and/or volume of
flower and
trim 106 and waste 108 could be recorded in the ICS for each plant and/or
batch. The
time, date and/or location of plant part separation could also or instead be
recorded in
the ICS. Other information related to plant part separation process could also
or
instead be recorded.
In some embodiments, plant part separation information could be recorded in
the ICS
in the form of a plant part separation record, which includes or is otherwise
associated with a plant part separation record identifier. A plant part
separation
record identifier could be similar in form to other identifiers disclosed
herein.
Plant part separation information related to a plant part separation process
could also
or instead be recorded in the ICS as part of another record such as a batch
record
associated with a batch of plants undergoing plant part separation. A batch
record
could include a plant part separation identifier or otherwise be associated
with a plant
part separation record, or multiple plant part separation record if the batch
was
processed through multiple plant part separation processes or equipment or in
different ways for example. Similarly, a plant part separation record could
include a
batch identifier or otherwise be associated with a batch record, or multiple
batch
records if multiple batches are processed through plant part separation.
In the example cultivation and harvest system 420a in Fig. 4A, any one or more
components such as the operator check-in device(s) 422a, the computer(s) 424a,
the
controller(s) 426a, the sensor(s) 428a, the scale(s) 430a, the label maker(s)
432a,
and the scanner(s) 434a could be involved in populating and/or updating the
ICS. For
example, any one or more of these components could be configured to generate,
86
CA 3062485 2019-11-21
85760071
collect, and/or otherwise obtain batch and/or harvest information and transmit
that
information to the server 402, through the server 418a in some embodiments,
for
populating and/or updating the database 414 or particular records therein.
In some embodiments, one or more components of the example plant part
separation
system 420b in Fig. 4B could be involved in populating and/or updating the
ICS. For
example, any one or more of the operator check-in device(s) 422b, the
computer(s)
424b, the controller(s) 426b, the sensor(s) 428b, the scale(s) 430b-1 and/or
430b-2,
the label maker(s) 432b, and the scanner(s) 434b-1 and/or 434b-2 could be
configured to generate, collect, and/or otherwise obtain plant part separation
information and transmit that information to the server 402 (Fig. 4A), through
the
server 418b in some embodiments, for populating and/or updating the database
414
or particular records therein. In some embodiments, the difference between the
weight of plant material input into the process (e.g. measured by scale(s)
430b-1) and
the weight of plant material output from the process (e.g. measured by
scale(s) 430b-
2) is compared against the amount of waste output from the waste holding
container(s) 460b in order to assess lost and/or theft of material. This
information can
then be recorded by the ICS in, for example, the database 414 on server 402.
Fresh Cannabis Processing
Information relating to fresh cannabis products could be recorded in an ICS.
In some
embodiments, lot numbers are assigned to fresh cannabis products when fresh
cannabis plant material is sent for packaging. For example, a "new lot" action
could
be automatically or manually initiated in the ICS to assign a lot number to
each
different fresh cannabis product originating from a batch of cannabis plants.
Lot
number generation and/or assignment could occur before, during or after
packaging
the fresh cannabis material into holding containers. All of the holding
containers that
contain the fresh cannabis plant material from a single batch could be
associated with
and/or identified by the same lot number.
Examples of fresh product information that could be recorded in an ICS include
the
following, any one or more of which could be included in a lot record, for
example:
87
CA 3062485 2019-11-21
85760071
plant number;
batch number;
category (for example, flower or trim);
brand name;
source (for example, in-house production or external production);
weight of fresh cannabis product;
volume of fresh cannabis product.
The weight of a fresh cannabis product could be recorded, for example, using a
scale
that is connected to or otherwise able to communicate with the ICS. The scale
could
weigh an empty holding container and record this weight in the ICS. An
operator or
equipment in a production system could then add a fresh cannabis product to
the
holding container and weigh the full container, using the same scale or
another scale
that is able to communicate with the ICS. The scale could record the new
weight in
the ICS, and the ICS could compare this weight to the weight of the empty
container
to determine and/or confirm the weight of fresh cannabis product that is
stored in the
holding container.
In some embodiments, fresh cannabis product information could be recorded in
the
ICS in a lot record that includes or is otherwise associated with a lot
identifier. A
batch record could include the lot identifier or otherwise be associated with
the lot
record for a lot of fresh cannabis product, or multiple lot records if the
batch was used
to produce multiple lots of fresh cannabis product. Similarly, a lot record
could include
a batch identifier or otherwise be associated with a batch record, or multiple
batch
records if multiple batches were used to produce fresh cannabis product in one
lot.
In the example fresh processing system 420d in Fig. 4D, any one or more
components such as the operator check-in device(s) 422d, the computer(s) 424d,
the
scale(s) 430d-1 and/or 430d-2, the label maker(s) 432d, and the scanner(s)
434d-1
88
CA 3062485 2019-11-21
85760071
and/or 434d-2 could be involved in populating and/or updating the ICS. For
example,
any one or more of these components could be configured to generate, collect,
and/or otherwise obtain source product and/or fresh product information and
transmit
that information to the server 402, through the server 418d in some
embodiments, for
populating and/or updating the database 414 or particular records therein.
In some embodiments, the difference between the weight of source input into
the
process (e.g. measured by scale(s) 430f-1) and the weight of milling product
output
from the process (e.g. measured by scale(s) 430f-2) is compared in order to
assess
lost and/or theft of material. This information can then be recorded by the
ICS in, for
example, the database 414 on server 402.
Dried Cannabis Manufacturing
Any of various information relating to a drying process and/or dried cannabis
products
could be recorded in an ICS, in the form of a drying record in some
embodiments. A
drying record could include or otherwise be associated with a drying record
identifier,
which could be similar in form to other identifiers disclosed herein. Drying
information
related to a drying process and/or dried cannabis products could also or
instead be
recorded in another type of record such as a lot record.
A lot record could include a drying record identifier or otherwise be
associated with a
drying record for a drying process that was used to produce a lot of dried
cannabis
product, or multiple drying records if the lot was dried in different
equipment, using
different drying processes, and/or over multiple days for example. Similarly,
a drying
record could include a lot identifier or otherwise be associated with a lot
record, or
multiple lot records if a drying process or equipment produced multiple lots
of dried
cannabis product.
The following is a non-exhaustive list of information that could be recorded
in an ICS
for a drying process, and/or curing process if a curing process is also or
instead used
in producing dried cannabis product:
drying and/or curing process(es) used;
89
CA 3062485 2019-11-21
85760071
drying and/or curing time;
type, quantity, and/or schedule of lighting during drying and/or curing;
temperature(s) during drying and/or curing;
humidity during drying and/or curing;
type, quantity, and/or schedule of air ventilation during drying and/or
curing;
type and/or quantity of any other ingredient(s) added during drying and/or
curing.
Fig. 9 is a flow diagram illustrating an example method 580 for drying and/or
curing a
cannabis material, such as the drying performed at operation 112 of Fig. 1.
At step 582, cannabis plant material is selected for the drying process. In
some
embodiments, the cannabis plant material is selected from harvested cannabis
plant
material such as flower and trim. The selection could be performed manually
based
on the weight and/or size of the cannabis plant material, for example. The
selection
could also or instead be performed automatically, using one or more sorting
machines, for example.
Step 584 includes weighing the cannabis plant material that is selected at
step 582.
Alternatively, a holding container containing the cannabis plant material
could be
weighed. Weighing the cannabis plant material at step 584 could be performed
using,
for example, a laboratory scale that is connected to or otherwise able to
communicate
with the ICS, such as the scale(s) 430e-1 in Fig. 4E. The measured weight
could be
recorded in the ICS for the drying process, along with the batch number, lot
number,
and/or any other information associated with the cannabis plant material in
some
embodiments.
At step 586, the selected cannabis material is transferred to one or more
dryer(s),
such as the dryer(s) 452e in Fig. 4E. In some embodiments, a dryer could be or
include a commercial dehydrator. In general, a dryer could include such
components
as a lamp and/or other form of a heater, a fan, and a controller, for example.
The
CA 3062485 2019-11-21
85760071
controller could control the heater and/or the fan according to settings of
the dryer.
Step 586 could include transferring the cannabis material onto carriers such
as racks
or trays and loading the carriers onto shelves inside of the dryer(s). In some
embodiments, step 586 could include adding other ingredients or materials to
the
dryer. For example, ingredients could be added to adjust the flavour,
fragrance, look,
and/or texture of the dried cannabis product.
At step 588, the cannabis material is dried in the dryer(s). Dryer settings
could be
controlled manually, be predefined in a dryer controller, and/or received or
otherwise
obtained or determined by the controller. Examples of dryer settings include
temperature, fan speed, and drying time. In some embodiments, the drying
temperature is 60 C and the drying time is at least 1.5 hours. Other dryer
settings are
possible. The cannabis material could also or instead be actively monitored by
an
operator, and/or one or more sensors such as the sensor(s) 428e in Fig. 4E, to
determine or adjust dryer settings. A controller and/or one or more sensors
could be
connected to or have access to the ICS to record the dryer settings and/or one
or
more properties of the cannabis material during the drying process.
Alternatively,
information could be manually recorded in the ICS for a drying process, using
a
computer such as 424e in Fig. 4E, for example.
At step 590, the dried cannabis material is removed from the dryer(s). Step
590 could
be performed when a predetermined drying time has been reached, or when an
operator or sensor determines that the drying is complete.
Step 592 includes weighing the dried cannabis material, by a scale at 430e-2
in Fig.
4E for example. The measured weight could be recorded in the ICS for the
drying
process.
At step 594, the dried cannabis material is transferred to one or more holding
container(s), shown by way of example at 454e in Fig. 4E. A label could be
applied to
a holding container using the ICS, or a pre-existing label on a holding
container could
be recorded in the ICS to indicate that the holding container now contains the
dried
cannabis product. The label maker(s) 432e and the scanner(s) 434e-2 in Fig. 4E
are
91
CA 3062485 2019-11-21
85760071
examples of system components that could be configured for marking holding
containers and scanning markings on holding containers, respectively. Either
or both
of these components could transmit label information to other components for
storage
in or updating of an ICS, in the database 414 in Fig. 4A for example.
Steps 592 and 594 could be reversed in order in some embodiments, such that
the
dried cannabis material is weighed after being transfer to the holding
container(s).
At step 596 the holding container(s) containing the dried cannabis material is
transferred to one or more storage areas. Any such transfer could be recorded
in an
ICS to help track the location of a holding container. A storage area could be
an area
where holding containers await further processing, such as irradiation,
testing and/or
final packaging. A storage area could also or instead be an area where holding
containers are stored until they are released for sale. In some embodiments, a
storage area is a vault and access is restricted to selected users.
Step 598 includes cleaning the workspace, dryer(s) and/or carrier(s) for the
drying
process. Other components or equipment such as any source product holding
container(s) 450e in Fig. 4E could also or instead be cleaned.
Any of various components of a drying system, such as the example drying
system
420e in Fig. 4E, could be configured to generate, collect, and/or otherwise
obtain
drying information and transmit that information to the server 402 in Fig. 4A,
through
the server 418e in some embodiments, for populating and/or updating the
database
414 or particular records therein. This includes the components which are
referenced
by way of example above in the description of Fig. 9, and/or possibly other
components.
The foregoing description of Fig. 9 refers primarily to drying, but could also
or instead
be applied to curing. Instead of or in addition to one or more dryers, a
curing process
could involve curing equipment to cure selected cannabis plant material.
For some cannabis products that use or include dried cannabis, smaller
particle size
and/or finer granularity of dried cannabis might be desired. For example,
dried
92
CA 3062485 2019-11-21
85760071
cannabis with a fine granularity could be desired for rolling cannabis
cigarettes.
Milling could be used to grind or shred cannabis material, such as the dried
cannabis
produced by method 580, to produce a finer granularity.
Any of various information relating to a milling process and/or milled
cannabis
products could be recorded in an ICS, in the form of a milling record in some
embodiments. A milling record could include or otherwise be associated with a
milling
record identifier, which could be similar in form to other identifiers
disclosed herein.
Milling information related to a milling process and/or milled cannabis
products could
also or instead be recorded in another type of record such as a lot record.
A lot record could include a milling record identifier or otherwise be
associated with a
milling record for a milling process that was used to produce a lot of milled
cannabis
product, or multiple milling records if the lot was milled in different
equipment, using
different milling processes, and/or over multiple days for example. Similarly,
a milling
record could include a lot identifier or otherwise be associated with a lot
record, or
multiple lot records if a milling process or equipment produced multiple lots
of milled
cannabis product.
Fig. 10 is a flow diagram illustrating an example method 600 for milling
cannabis
plant material.
Step 602 includes weighing one or more holding container(s) that contain the
cannabis plant material that is to be milled. By way of example, Fig. 4F
illustrates
source product holding container(s) 450f that could contain source material in
the
form of cannabis plant material, and weight could be measured by the scale(s)
at
430f-1. Alternatively, the cannabis plant material could be removed from the
holding
container(s) and weighed. Measured weight, batch number, lot number, and/or
any
other information associated with the cannabis plant material could be
recorded in the
ICS for the milling process. The weight of the cannabis plant material could
be
recorded as "pre-mill" weight in the ICS, for example.
93
CA 3062485 2019-11-21
85760071
At step 604, the cannabis plant material is transferred to one or more milling
machines, such as the milling machine(s) 452f in Fig. 4F. In some embodiments,
a
milling machine could include a rotating blade driven by a motor. An external
or
integrated controller could be used to control the milling machine. Fig. 4F
illustrates
an external controller embodiment as an example, in which the controller(s)
426f are
connected to or otherwise in communication with the milling machine(s) 452f to
control the milling machine(s).
At step 606, the cannabis plant material is milled using the milling
machine(s). Milling
settings for the milling machine(s) could be controlled manually, be
predefined in a
milling controller, and/or received or otherwise obtained or determined by the
controller. Examples of milling settings include milling time and motor speed.
The
cannabis plant material could also or instead be actively monitored by an
operator,
and/or one or more sensors such as the sensor(s) 428f in Fig. 4F, to determine
or
adjust milling settings. A controller and/or one or more sensors could be
connected to
or have access to the ICS to record the milling settings and/or one or more
properties
of the cannabis plant material during the milling process. Alternatively,
information
could be manually recorded in the ICS for a milling process, using a computer
such
as 424f in Fig. 4F, for example.
At step 608, the milled cannabis plant material is transferred to one or more
holding
container(s), shown by way of example at 456f in Fig. 4F. The holding
container(s)
could include the same holding container(s) that contained un-milled cannabis
plant
material, and/or one or more different holding containers. In some
embodiments, the
milled cannabis material could be sifted, using a sifter or sieve for example,
before
being transferred to a holding container. Sifting could separate the milled
cannabis
material into different size categories. For example, the milled cannabis
product could
be separated into fine particles, "ideal mill" particles, and coarse
particles. Each size
category of milled cannabis material could then be transferred to a respective
holding
container. The example milling system 420f in Fig. 4F includes one or more
sifters
454f.
94
CA 3062485 2019-11-21
85760071
Any transfer of milled cannabis material to a holding container could be
recorded in
the ICS. If the material in a holding container was sifted, then this could
also or
instead be recorded in the ICS.
A label could be applied to a holding container using the ICS, or a pre-
existing label
on a holding container could be recorded in the ICS to indicate that the
holding
container now contains milled cannabis material. The label maker(s) 432f and
the
scanner(s) 434f-2 in Fig. 4F are examples of system components that could be
configured for marking holding containers and scanning markings on holding
containers, respectively. Either or both of these components could transmit
label
information to other components for storage in or updating of an ICS, in the
database
414 in Fig. 4A for example.
Step 610 includes weighing the holding container(s) containing the milled
cannabis
material, using the scale(s) 430f-2 in Fig. 4F for example. The measured
weight could
be recorded in the ICS as a "post-mill" weight. If sifting was also performed
to
separate the milled cannabis material, then the weight of a holding container
could be
recorded as a "post-mill/sift" weight.
The holding container(s) could then be moved to one or more storage areas. Any
such transfer could be recorded in an ICS to help track the location of a
holding
container. Examples of storage areas are provided elsewhere herein.
At step 612, the workspace is cleaned, and this could involve cleaning any
milling
machines and/or sifting machines that were used. Waste that is produced in a
milling
process could be weighed and/or otherwise recorded in the ICS before being
destroyed.
Any of various components of a milling system, such as the example milling
system
420f in Fig. 4F, could be configured to generate, collect, and/or otherwise
obtain
milling information and transmit that information to the server 402 in Fig.
4A, through
the server 418f in some embodiments, for populating and/or updating the
database
414 or particular records therein. This includes the components which are
referenced
CA 3062485 2019-11-21
85760071
by way of example above in the description of Fig. 10, and/or possibly other
cornponents.
Milled and/or dried cannabis could be used to produce "pre-rolled" cannabis
cigarettes, for example. Pre-rolled cigarettes could be rolled by a producer
during lot
packaging, as opposed to being rolled by a user. Pre-rolled cannabis
cigarettes could
be produced manually, or produced with the aid of a cone filling machine.
Any of various information relating to a pre-rolling process and/or pre-rolled
cannabis
products could be recorded in an ICS, in the form of a pre-rolling record in
some
embodiments. A pre-rolling record could include or otherwise be associated
with a
pre-rolling record identifier, which could be similar in form to other
identifiers
disclosed herein. Pre-rolling information related to a pre-rolling process
and/or pre-
rolled cannabis products could also or instead be recorded in another type of
record
such as a lot record.
A lot record could include a pre-rolling record identifier or otherwise be
associated
with a pre-rolling record for a pre-rolling process that was used to produce a
lot of
pre-rolled cannabis product, or multiple pre-rolling records if the lot was
pre-rolling in
different equipment, using different pre-rolling processes, and/or over
multiple days
for example. Similarly, a pre-rolling record could include a lot identifier or
otherwise
be associated with a lot record, or multiple lot records if a pre-rolling
process or
equipment produced multiple lots of pre-rolled cannabis product.
Fig. 11 is a flow diagram illustrating an example method 620 for producing pre-
rolled
cannabis cigarettes with a cone filling machine. In some embodiments, the
method
620 could be performed during the operation 118 of Fig. 1.
Step 602 includes weighing one or more holding containers containing a
cannabis
product. This cannabis product could include dried and/or milled cannabis
plant
material, for example. By way of example, Fig. 4J illustrates source product
holding
container(s) 450j that could contain pre-rolling cannabis product, and weight
could be
measured by the scale(s) at 430j-1. Alternatively, the source product could be
96
CA 3062485 2019-11-21
85760071
removed from the holding container(s) and weighed. Measured weight, batch
number, lot number, and/or any other information associated with the source
product
could be recorded in the ICS for the pre-rolling process. Measured weight
could be
recorded in the ICS as a "pre pre-roll" weight, for example.
At step 624, the cannabis product is transferred from the holding container(s)
to one
or more cone filling machine(s), shown by way of example at 452j in Fig. 4J.
The
cannabis product could be loaded onto trays and/or other supply carriers for
loading
the cone filling machine(s), for example.
Step 626 involves loading the cone filling machine(s) with empty paper cones.
In
some embodiments, empty paper cones are placed onto or into trays or other
carriers, which are loaded into the cone filling machine(s). Paper cones could
be
available in multiple sizes, and cone size determines the cannabis product
capacity
per pre-rolled cigarette and the size of the pre-rolled cigarettes that are
produced.
The cone filling machine(s) could be loaded with cones of one size at a time,
but this
need not be the case in all embodiments. There could also or instead be
multiple pre-
rolling mechanisms in a machine to handle cones of respective different sizes,
and/or
multiple machines to handle cones of respective different sizes. In some
embodiments, a cone-filling machine is not size-specific, and is configured to
handle
cones of multiple sizes. A multi-size cone filling machine could dynamically
detect
cone size and handle multiple different cone sizes at a time, or be
configurable to
handle different cone sizes but only one cone size at a time.
Step 628 involves running or operating the cone filling machine(s), to fill
the paper
cones with the cannabis product. One or more settings for the cone filling
machine(s)
could be adjusted before or during each run. For example, the weight and/or
volume
of cannabis to be added to each cone could be adjusted manually, or
automatically
by a controller in a cone filling machine based on the size and/or type of
cones
currently loaded. In general, settings for a cone filling machine could be
controlled
manually, be predefined in a controller, and/or received or otherwise obtained
or
determined by the controller. Filling weight and/or volume, noted above, are
97
CA 3062485 2019-11-21
85760071
examples of such settings. A cone filling machine could also or instead be
actively
monitored by an operator, and/or one or more sensors such as the sensor(s)
428j in
Fig. 4J, to determine or adjust settings.
A controller and/or one or more sensors could be connected to or have access
to the
ICS to record settings and/or one or more properties of the cone filling
machine(s),
cannabis product, empty paper cones, and/or machine output(s) during the pre-
rolling
process. Alternatively, information could be manually recorded in the ICS for
a pre-
rolling process, using a computer such as 424j in Fig. 4J, for example.
Step 630 includes removing the filled paper cones from the cone filling
machine(s).
Step 630 could be performed manually by an operator, or be automated by one or
more machines. In some embodiments, filled paper cones are ejected from the
machine(s), and could drop or otherwise be transferred to one or more holding
container(s).
Open ends of filled cones, through which the cones were filled, could be
closed by
the cone filling machine(s), or could be closed by folding or twisting after
the cones
are removed from the cone filling machine(s) at 630. Closing the ends of the
filled
cones could reduce or prevent the cannabis product from falling out of the
filled
cones, and forms pre-rolled cannabis cigarettes.
Some of the filled cones that are removed might be damaged or otherwise
unsuitable
for sale. Any cannabis product in damaged cones could be recycled back into
the
cone filling machine(s) or the original holding container(s). The cone filling
machine(s)
could be run multiple times by loading the machine with additional empty cones
and/or with additional cannabis product. For example, steps 624, 626, 628,
630,
could be repeated multiple times, as indicated using dashed lines in Fig. 11.
Producing pre-rolled cigarettes could stop when, for example, a pre-defined
number
of cigarettes have been produced, or the amount of cannabis product remaining
is
less than the amount needed for a run of the cone filling machine(s).
98
CA 3062485 2019-11-21
85760071
At step 632, cannabis product that remains after pre-rolling has finished is
removed
from the cone filling machine(s) and returned to one or more holding
container(s),
which could be the original holding container(s) from which the cone filling
machine(s)
were loaded.
Step 634 involves weighing the holding container(s). If remaining cannabis
product is
transferred back to the original holding container(s) at 632, then the
original holding
container(s) could be weighed again at 634. Otherwise, the holding
container(s) in
which the remaining cannabis product is transferred at 632 are also or instead
weighed at 634. Both the original and remaining cannabis product holding
containers
could be weighed unless the original holding container was completely emptied,
for
example, so that a total remaining "post pre-roll" weight can be measured or
otherwise determined, and could be recorded in the ICS. The difference between
pre
pre-roll and post pre-roll weights should indicate the weight of cannabis in
the pre-
rolled cannabis cigarettes, provided any remaining cannabis product, including
contents of any damaged filled cones, has been returned to one or more holding
containers before measurement of post pre-roll weight(s).
At step 636, the pre-rolled cannabis cigarettes that were removed from the
cone
filling machine at step 630 are transferred to one or more new holding
containers,
shown by way of example as the target holding container(s) 4456j in Fig. 4J .
Step
636 could also include weighing each pre-rolled cannabis cigarette to confirm
that it
does exceed a maximum weight. In one example, if a pre-rolled cigarette weighs
over
1.0g, then it could be destroyed or recycled. In another example, if the
weight and/or
volume of a pre-rolled cigarette deviates from a target weight/volume by more
than a
pre-defined tolerance, then the cigarette could be destroyed or recycled. The
pre-
defined tolerance could be 5% or 10%, for example.
Step 638 includes weighing the new holding container(s) containing the pre-
rolled
cannabis cigarettes, using the scale(s) 430j-2 in Fig. 4J. Holding container
weight in
the case of pre-rolled cigarettes includes the weight of the paper cones and
the
99
CA 3062485 2019-11-21
85760071
cannabis product in the pre-rolled cigarettes. This weight could be entered,
manually
or automatically, into the ICS.
A label could be applied to a holding container using the ICS, or a pre-
existing label
on a holding container could be recorded in the ICS to indicate that the
holding
container now contains pre-rolled cannabis cigarettes. The label maker(s) 432j
and
the scanner(s) 434j-2 in Fig. 4J are examples of system components that could
be
configured for marking holding containers and scanning markings on holding
containers, respectively. Either or both of these components could transmit
label
information to other components for storage in or updating of an ICS, in the
database
414 in Fig. 4A for example.
The holding container(s) could then be moved to one or more storage areas. Any
such transfer could be recorded in an ICS to help track the location of a
holding
container. Examples of storage areas are provided elsewhere herein.
At step 640, the workspace is cleaned, and this could involve cleaning the
cone filling
machine(s) are cleaned. Waste that is produced in a milling process could be
weighed and/or otherwise recorded in the ICS before being destroyed.
Any of various components of a packaging system, such as the example packaging
system 420j in Fig. 4J, could be configured to generate, collect, and/or
otherwise
obtain pre-rolling information and transmit that information to the server 402
in Fig.
4A, through the server 418j in some embodiments, for populating and/or
updating the
database 414 or particular records therein. This includes the components which
are
referenced by way of example above in the description of Fig. 11, and/or
possibly
other components.
Cannabis Extract Manufacturing
Another example of a cannabis product is a cannabis extract, which could be or
include oils, and non-oils such as resins. Cannabis extracts could be further
processed to produce other cannabis products.
100
CA 3062485 2019-11-21
85760071
Fig. 12 is a flow diagram illustrating an example process 700 for producing
cannabis
extracts and other cannabis products. The process 700 includes an operation
702 of
milling, an operation 704 of decarboxylation, an operation 706 of extraction,
an
operation 708 of resin packaging, an operation 710 of oil formulation, and an
operation 712 of oil packaging. These operations are discussed in greater
detail
below, in some instances with additional reference to other drawings. Any or
all of the
operations 702, 704, 706, 708, 710, 712 could be similar to one or more of the
processes performed in operations 114, 118 of Fig. 1. For example, operations
702,
704, 706 of Fig. 12 could be similar to the extraction performed in operation
114 of
Fig. 1. Operations 708, 710, 712 could also or instead be similar to the lot
packaging
performed in operation 118 of Fig. 1.
Harvest material 714 could be a source of cannabis plant material for process
700,
and could include plant material output from a plant part separation process,
such as
the plant part separation process performed in operation 104 of Fig. 1 and/or
by a
plant part separation system such as the example system 420b in Fig. 4B.
Harvest
material 714 could include a single batch or lot of cannabis plant material.
Alternatively, harvest material 714 could include multiple batches or lots of
cannabis
plant material.
Toll processing material 716 could also or instead be a source of cannabis
material
for process 700. Toll processing refers to a situation in which a company or
entity
processes cannabis material or products for another company or entity, and
returns
the resultant product(s) the other company or entity for a fee. For example, a
company performing process 700 could receive cannabis material from an
external
company and process this plant material to produce extracts that are returned
to the
external company. Important considerations for handling toll processing
material 716
could include reducing cross-contamination, preventing the addition of any
extraneous substance, preserving product integrity, and keeping accurate
records of
all products to enable identification and traceability.
101
CA 3062485 2019-11-21
85760071
When toll processing material 716 is received, information such as the name of
the
individual or organization from which it was received, the address of the site
at which
it was received, the date on which is was received, the quantity of material
received,
the intended use of the material received, and/or the brand name of the
material
received could be recorded in an ICS. Each holding container and/or package of
toll
processing material 716 could be weighed, and this weight could be recorded in
the
ICS. The measured weight could be compared to a weight listed in an order
request,
to confirm that the received weight matches what was ordered and/or what was
shipped to the receiver. The received product could then be placed in new
holding
containers, which could be labelled and recorded in the ICS. Alternatively,
the original
packages or holding containers of the toll processing material 716 could be
labelled
and/or recorded in the ICS. The holding containers could then be stored before
they
are processed, tested and/or allocated a lot number.
In some embodiments, toll processing material 716 could be handled in the same
or
substantially the same manner as source material or products in example
methods
disclosed herein. Toll processing material 716 might originate from a
different source
than the harvest material 714, but need not necessarily be handled in a
substantially
different way or by substantially different systems or components because of
its
different origin.
The example process 700 begins with cannabis milling at operation 702, to
grind or
mill cannabis material for extraction. Examples of milling processes, and
potential
implications for an ICS, are disclosed elsewhere herein. The milling at 702
could
reduce the cannabis plant particle size, which could increase the efficiency
of other
processing such as extraction. Harvest material 714 could be sent for milling
at
operation 702. In the case of toll processing material 716 that includes un-
milled
flower, trim or waste, for example, the toll processing material could also or
instead
be sent for milling at operation 702. In some embodiments, only harvest
material 714
or only toll processing material 716 are processed in any operation at one
time.
102
CA 3062485 2019-11-21
85760071
Milled cannabis plant material that is produced at operation 702 could be sent
to
operation 704 for decarboxylation in some embodiments. Decarboxylation is a
process in which acid forms of cannabinoids are converted to their neutral
forms.
More specifically, decarboxylation involves a chemical reaction that removes a
carboxyl group from cannabinoids and releases CO2. It should be noted that
decarboxylation is shown solely for illustrative purposes in Fig. 12, and need
not be
performed in all embodiments.
By way of background in relation to decarboxylation, the term "Cannabis
plant(s)"
encompasses wild type Cannabis and also variants thereof, including cannabis
chemovars which naturally contain different amounts of the individual
cannabinoids.
For example, some Cannabis strains have been bred to produce minimal levels of
THC, the principal psychoactive constituent responsible for the high
associated with it
and other strains have been selectively bred to produce high levels of THC and
other
psychoactive cannabinoids.
Cannabis plants produce a unique family of terpeno-phenolic compounds called
cannabinoids, which produce the cannabis-effect one experiences from consuming
marijuana. There are 483 identifiable chemical constituents known to exist in
the
cannabis plant, and at least 85 different cannabinoids have been isolated from
the
plant. The two cannabinoids usually produced in greatest abundance are
cannabidiol
(CBD) and/or 49-tetrahydrocannabinol (THC), but only THC is psychoactive.
Cannabis plants are categorized by their chemical phenotype or "chemotype,"
based
on the overall amount of THC produced, and on the ratio of THC to CBD.
Although
overall cannabinoid production is influenced by environmental factors, the
THC/CBD
ratio is genetically determined and remains fixed throughout the life of a
plant. Non-
drug plants produce relatively low levels of THC and high levels of CBD, while
drug
plants produce high levels of THC and low levels of CBD.
The best studied cannabinoids include tetrahydrocannabinol (THC), cannabidiol
(CBD) and cannabinol (CBN). Other cannabinoids include for example,
cannabichromene (CBC), cannabigerol (CBG) cannabinidiol (CBND), Cannabicyclol
103
CA 3062485 2019-11-21
85760071
(CBL), Cannabivarin (CBV), Tetrahydrocannabivarin (THCV), Cannabidivarin
(CBDV), Cannabichromevarin (CBCV) Cannabigerovarin (CBGV), Cannabigerol
Monomethyl Ether (CBGM).
Cannabinoids are derived from their respective 2-carboxylic acids (2-COOH) by
decarboxylation (catalyzed by heat, light, or alkaline conditions). As a
general rule,
the carboxylic acids form of the cannabinoid have the function of a
biosynthetic
precursor.
As used herein THC, CBD, CBN, CBC, CBG, CBND, CBL, CBV, THCV, CBDV,
CBCV, CBGV and CBGM refer to the decarboxylated form of the cannabinoid.
Whereas, THCa, CBDa, CBNa, CBCa, CBGa, CBNDa, CBLa, CBVa, THCVa,
CBDVa, CBCVa and CBGVa refer to the acid form of the cannabinoid.
Tetrahydrocannabinol (THC) is the primary psychoactive component of the
Cannabis
plant. THC is only psychoactive in is decarboxylated state. The carboxylic
acid form
(THCa) is non-psychoactive.
Delta-9-tetrahydrocannabinol (A9-THC, THC) and delta-8-tetrahydrocannabinol
(AM-
THC), mimic the action of anandamide, a neurotransmitter produced naturally in
the
body. These two THCs produce the effects associated with cannabis by binding
to
the CBI cannabinoid receptors in the brain. THC appears to ease moderate pain
(analgesic) and to be neuroprotective, while also offering the potential to
reduce
neuroinflammation and to stimulate neurogenesis.
The term "Cannabis plant" encompasses wild type Cannabis sativa, Cannabis
indica,
Cannabis afghanica, and other variants thereof, including cannabis species
which
naturally contain different amounts of the individual cannabinoids. Also
included are
Cannabis subspecies and plants which are the result of genetic crosses, self-
crosses
or hybrids thereof. Also included are hemp plants. The term "Cannabis extract"
is to
be interpreted accordingly as encompassing material extracted from one or more
cannabis plants.
104
CA 3062485 2019-11-21
85760071
THC and CBD are the main medicinally active constituents in Cannabis. However,
these constituents are present as the biologically inactive carboxylic acids
in
Cannabis plants. When extracting THC or CBD from cannabis plants, it has been
the
practice to convert the storage precursor compounds of THCA and CBDA into
their
more readily extractable and pharmacologically active forms. THC and CBD acids
slowly decarboxylate over time, and applying heat increases the rate of
decarboxylation.
Decarboxylation of cannabinoid acids is a function of time and temperature,
thus, at
higher temperatures a shorter period of time will be taken for complete
decarboxylation of a given amount of cannabinoid acid. In selecting
appropriate
conditions for decarboxylation consideration must, however, be given to
minimising
thermal degradation of the desirable, pharmacological cannabinoids into
undesirable
degradation products, particularly thermal degradation of THC to cannabinol
(CBN).
Any of various information relating to a decarboxylation process and/or
decarboxylated cannabis products could be recorded in an ICS, in the form of a
decarboxylation record in some embodiments. A decarboxylation record could
include or otherwise be associated with a decarboxylation record identifier,
which
could be similar in form to other identifiers disclosed herein.
Decarboxylation
information related to a decarboxylation process and/or decarboxylated
cannabis
products could also or instead be recorded in another type of record such as a
lot
record.
A lot record could include a decarboxylation record identifier or otherwise be
associated with a decarboxylation record for a decarboxylation process that
was used
to produce a lot of decarboxylated cannabis product, or multiple
decarboxylation
records if the lot was decarboxylated in different equipment, using different
decarboxylation processes, and/or over multiple days for example. Similarly, a
decarboxylation record could include a lot identifier or otherwise be
associated with a
lot record, or multiple lot records if a decarboxylation process or equipment
produced
multiple lots of decarboxylated cannabis product.
105
CA 3062485 2019-11-21
85760071
Fig. 13 is a flow diagram illustrating an example method 800 for
decarboxylation of a
cannabis product, such as the decarboxylation performed at operation 704 of
Fig. 12
and/or in a decarboxylation system such as the example system 420g in Fig. 4G.
Step 802 involves weighing one or more holding containers containing a pre-
decarboxylation cannabis product. One or more scales and one or more holding
containers are shown by way of example at 430g-1 and 450g, respectively, in
Fig.
4G. The cannabis product could include plant material that was milled at
operation
702 of Fig. 12, for example. The measured weight could be recorded in an ICS,
along
with the batch number, lot number, or any other information associated with
the
cannabis product and/or the holding container(s) in some embodiments. This
weight
could be recorded in the ICS as a "pre-decarboxylation" weight, for example.
At step 804, cannabis product is transferred from the holding container(s) to
one or
more carriers, such as trays. In some embodiments, a carrier is an aluminum
tray.
Before transferring the cannabis product to a carrier, the carrier could be
cleaned
using food grade ethanol, for example.
Step 806 involves placing the carrier(s) into one or more ovens, such as the
decarboxylation oven(s) 452g in Fig. 4G. Removable carriers such as trays
might not
necessarily be used in all embodiments. For example, cannabis product could
instead be loaded into one or more ovens without necessarily using a carrier.
An oven could be preheated to a particular temperature before cannabis product
is
added. In some embodiments, an oven could be set to a temperature of 150 C,
and
cannabis product might not be transferred to the oven until it has reached a
minimum
temperature of 120 C. A temperature probe or thermometer could be inserted
into
the cannabis product to monitor the temperature of the cannabis product during
decarboxylation. This temperature probe could be connected to or have access
to the
ICS to record and track the temperature of the cannabis product. A temperature
probe is an example of a sensor shown at 428g in Fig. 4G. An oven could also
or
instead have access to the ICS, to record its actual and/or set point
temperatures.
106
CA 3062485 2019-11-21
85760071
Oven settings could be controlled manually, be predefined in an oven
controller,
and/or received or otherwise obtained or determined by the controller.
Examples of
oven settings include temperature and decarboxylation time. Other settings are
possible. The cannabis material could also or instead be actively monitored by
an
operator, and/or one or more sensors such as the sensor(s) 428g in Fig. 4G, to
determine or adjust oven settings. A controller and/or one or more sensors
could be
connected to or have access to the ICS to record the oven settings and/or one
or
more properties of the cannabis material during the decarboxylation process.
Alternatively, information could be manually recorded in the ICS for a
decarboxylation
process, using a computer such as 424g in Fig. 4G, for example.
At step 808, the cannabis product is heated. Heating could continue until the
cannabis product reaches a predefined temperature. This temperature could be
the
temperature at which the decarboxylation process occurs. In some embodiments,
the
predefined temperature could be 120 C. It could be desirable to maintain the
temperature of the cannabis product within a certain range of the predefined
temperature. For example, a cannabis product could be maintained within 4 C of
120 C. Heating the cannabis product to temperatures that exceed this range of
the
predefined temperature might be undesirable. Such temperatures could induce
other
reactions, such as vaporization of cannabinoids and terpenes, which might
affect the
properties of the final cannabis product. In some embodiments, if the cannabis
product reaches temperatures greater than 125 C, the set point temperature of
the
oven could be decreased. A damper and/or oven door could also or instead be
opened to decrease the temperature of the oven.
At step 810, the cannabis product is be taken out of the oven(s) and
transferred to
one or more holding container(s). The cannabis product might be taken out of
the
oven(s) once a particular temperature has been maintained or exceeded for a
particular amount of time. This temperature and amount of time could depend,
for
example, on the temperature-dependent rate of the decarboxylation process for
that
particular cannabis product. In some embodiments, a cannabis product might be
removed from an oven if its temperature exceeds 90 C for at least 100 minutes.
The
107
CA 3062485 2019-11-21
85760071
actual temperature of the cannabis product and/or the time the cannabis
product is at
a temperature above a particular temperature could be recorded in the ICS.
Once out
of the oven(s), a carrier containing the cannabis product could be allowed to
cool in
the ambient atmosphere. The cannabis product could then be transferred to the
original holding container(s). Decarboxylated cannabis product could also or
instead
be transferred to one or more different holding container(s), shown by way of
example at 454g in Fig. 4G.
A label could be applied to a holding container using the ICS, or a pre-
existing label
on a holding container could be recorded in the ICS to indicate that the
holding
container now contains the dried cannabis product. The label maker(s) 432g and
the
scanner(s) 434g-2 in Fig. 4G are examples of system components that could be
configured for marking holding containers and scanning markings on holding
containers, respectively. Either or both of these components could transmit
label
information to other components for storage in or updating of an ICS, in the
database
414 in Fig. 4A for example.
Step 812 includes weighing the holding container(s) containing the
decarboxylated
(post-decarboxylation) cannabis products material, by a scale at 430g-2 in
Fig. 4G for
example. The measured weight could be recorded as a "post- decarboxylation"
weight in the ICS, for example.
The holding container(s) could then be moved to one or more storage areas. Any
such transfer could be recorded in an ICS to help track the location of a
holding
container. Examples of storage areas are provided elsewhere herein.
At step 814, the workspace is cleaned, and this could involve cleaning the
oven(s)
and/or any carrier(s) used for decarboxylation. Waste that is produced in a
decarboxylation process could be weighed and/or otherwise recorded in the ICS
before being destroyed.
The decarboxylation method 800 illustrated in Fig. 13 could be used to produce
cannabis material for extraction processes. It should be noted, however, that
108
CA 3062485 2019-11-21
85760071
cannabis products other than decarboxylated cannabis products could be
provided as
inputs to an extraction process. A cannabis product need not necessarily
undergo
decarboxylation before extraction.
Referring again to Fig. 12, operation 706 includes an extraction process to
produce
one or more cannabis extracts. The post-decarboxylation cannabis product from
operation 704, or another cannabis material or product, could be used as a
source
material for the extraction process at operation 706. Harvest material 714
and/or toll
processing material 716 could also or instead be used as source material for
the
extraction process at operation 706. For example, the toll processing material
716
could have undergone milling and/or decarboxylation before being received, or
decarboxylation might not be performed before extraction.
Extraction supplies 718 are provided to support the extraction at operation
706.
Extraction supplies 718 could include extraction solvents and extract
collection
vessels, for example. An extraction solvent is used in solvent extraction
processes,
which separate compounds from a source material based on their relative
solubility in
the extraction solvent. An extract collection vessel is a container for
holding an
extract produced by extraction. In some embodiments, an extract collection
vessel
could be a collection flask or other form of receptacle. However, other
extract
collection vessels could also or instead be used.
In some embodiments, operation 706 includes supercritical fluid extraction
with CO2.
Supercritical fluid extraction with CO2 is the process of separating an
extract from a
matrix using supercritical CO2 as the extraction solvent. When cannabis
material is
used as the matrix, supercritical fluid extraction with CO2 could separate
cannabinoids and terpenes from the cannabis material. These cannabinoids and
terpenes could be captured in the form of a cannabis extract. The remaining
cannabis
material could be considered to be waste.
Any of various information relating to an extraction process and/or cannabis
extracts
could be recorded in an ICS, in the form of an extraction record in some
embodiments. An extraction record could include or otherwise be associated
with an
109
CA 3062485 2019-11-21
85760071
extraction record identifier, which could be similar in form to other
identifiers disclosed
herein. Extraction information related to an extraction process and/or
cannabis
extracts could also or instead be recorded in another type of record such as a
lot
record.
A lot record could include an extraction record identifier or otherwise be
associated
with an extraction record for an extraction process that was used to produce a
lot of
cannabis extract, or multiple extraction records if the lot was produced in
different
extraction equipment, using different extraction processes, and/or over
multiple days
for example. Similarly, an extraction record could include a lot identifier or
otherwise
be associated with a lot record, or multiple lot records if an extraction
process or
equipment produced multiple lots of cannabis extract.
Fig. 14 is a flow diagram illustrating an example method 900 for supercritical
fluid
extraction with CO2. The example method 900 represents one possible option for
an
extraction process at 706 in Fig. 12, and/or could be performed by the
extractor(s)
452h in Fig. 4H.
Step 902 includes preparing one or more supercritical fluid extractors. In
some
embodiments, a supercritical fluid extractor includes a source of compressed
CO2, an
extraction chamber, one or more heaters to heat the extraction chamber, one or
more
collection chambers connected to the extraction chamber to collect extract, a
CO2
monitor, an inlet regulating valve to control the flow of CO2 into the
extraction
chamber, an outlet regulating valve to control the flow of CO2 out of the
extraction
chamber, a venting valve such as a needle valve to controllably vent the
extraction
chamber, and a controller. Preparing a supercritical fluid extractor could
include
venting and opening the extraction chamber, for example. To vent an extraction
chamber, inlet and outlet regulating valves could be closed and a venting
valve could
then be opened to release any CO2 in the extraction chamber. Once the
extraction
chamber is vented, the extraction chamber could be opened. This could include
dismantling a portion of the extraction chamber, such as the top of the
extraction
chamber.
110
CA 3062485 2019-11-21
85760071
At step 904, a cannabis product is prepared for extraction. Step 904 could
include
transferring the cannabis product into an extraction bag. In some embodiments,
a
charge of approximately 5 grams of cannabis product is transferred to the
extraction
bag. Transferring cannabis product into an extraction bag could include
placing and
securing the extraction bag inside of the extraction chamber, adding cannabis
product into the bag using a funnel or other guide if needed, and tying the
top of the
bag with the cannabis product inside. The extraction chamber could then be
closed
and sealed. For example, the top of the extraction chamber could be
reassembled. A
pressure check could be performed to test for any leaks and ensure that all of
the
seals and fittings in the extraction chamber are operating correctly.
Parameters or
information such as the source material used for the extraction process could
be
recorded in the ICS. To record the source material, a batch number, lot
number,
and/or label on the holding container(s) of the source material could be
recorded in
the ICS. The ICS could also or instead record the weight of the source
material
transferred to the extraction bag.
Step 906 involves running the extractor(s). Once an extraction chamber is
closed
without any leaks, its inlet and outlet regulating valves could be adjusted to
allow the
extraction chamber to fill up with CO2. The CO2 monitor, which is an example
of a
sensor 428h in Fig. 4H, could be used to monitor the amount of CO2 in the
extraction
chamber. After the extraction chamber is filled with CO2 and has reached a
stable
pressure, the chamber heater could be started. The chamber could be left for a
predefined time, such as 30 minutes, to allow the chamber to reach a stable
temperature. Chamber temperature and/or pressure could be measured by other
sensor(s) 428h.
With stable temperature and pressure, and extractor could then be run to
produce
extract from the source material. Running an extractor could include adjusting
heat
and/or pressure in the extractor to convert gaseous CO2 into a super critical
fluid. In
some embodiments, running the extractor is an automated process. For example,
an
operating program for the extractor could define parameters for the extraction
run,
including one or more of time duration, CO2 flow rate, temperatures and
pressures.
111
CA 3062485 2019-11-21
85760071
The operation program could be stored on a controller of the extractor. The
controller
could control one or more of the valves, heater, and/or other components of
the
extractor during a run. The parameters of the extraction run could be recorded
in the
ICS. For example, the controller could be in communication with or have access
to
the ICS to record extraction parameters for the extraction process record.
Extraction
parameters could also or instead be recorded in the ICS manually, using a
computer
424h in Fig. 4H for example. Extraction information could also or instead be
collected
and/or provided by other components such as one or more sensor(s) 428h.
The ICS could allow a user to view and monitor the status of an extraction run
via a
computer or other electronic device through which the ICS is accessible.
In some embodiments, steps 902, 904, 906 could be repeated multiple times to
produce larger quantities of extract. This repetition is indicated using a
dashed line in
Fig. 12. In some cases, 8 to 12 extractions could be performed before a
collection.
Each extraction run at step 906 could be recorded using the ICS, using the
same
extraction record or different extraction records for example.
At step 908, the extract produced at step 906 is collected, using a collection
vessel in
some embodiments. Step 908 could include connecting a collection chamber on
the
extractor to a collection vessel. Purging the collection chamber with CO2
could help to
push the extract from the collection chamber into the collection vessel. The
extract
could be collected in the form of a resin. In some embodiments, an "extract"
record
could be created in the ICS to record and track the extract that is collected.
Alternatively, the collected extract could be added to an existing extract
record in the
ICS. Extract records could be identified as, for example, "EXTR-1", "EXTR-2"
and
"EXTR-3". An extract record could be associated with an extraction record in
the ICS.
Before collection of the extract at step 908, an empty extract collection
vessel could
be weighed and recorded in the ICS. A label could be generated by the ICS and
applied to the collection vessel, or a pre-existing label on the collection
vessel could
be recorded in the ICS to indicate that the holding container now contains the
cannabis extract. After collection of the extract in the collection vessel,
the weight
112
CA 3062485 2019-11-21
85760071
and/or volume of the extract in the collection vessel could be recorded in the
ICS.
The weight of the extract could be determined, for example, by comparing the
weight
of the collection vessel before and after it is filled. Either or both of
these weights
could be measured by one or more scales, such as the scale(s) 430h-2 in Fig.
4H.
The volume of the extract could be determined using volume markings on the
collection vessel. At least a portion of the extract that is collected at step
908 could be
sampled and sent for testing to determine, for example, the cannabinoid
concentration in the extract.
A collection vessel is an example of an extracted product holding container
458h in
Fig. 4H. The label maker(s) 432h and the scanner(s) 434h-2 in Fig. 4H are
examples
of system components that could be configured for marking holding containers
and
scanning markings on holding containers, respectively. Either or both of these
components could transmit label information to other components for storage in
or
updating of an ICS, in the database 414 in Fig. 4A for example.
The holding container(s) could then be moved to one or more storage areas. Any
such transfer could be recorded in an ICS to help track the location of a
holding
container. Examples of storage areas are provided elsewhere herein.
At step 910, the workspace is cleaned, and this could involve cleaning the
extractor(s). Waste material and/or residual extract could be removed from the
extractor(s). Waste material could include any cannabis product that remains
in the
extraction bag after the extraction process at step 906. The weight of the
waste
material produced by the extraction run could be recorded in the ICS for the
extraction process record. Comparing the weight of the source material to the
weight
of the waste material could determine the amount of material used in the
extraction
run. Water and/or disinfectant could be sprayed inside of the extractor(s) to
remove
residual extract. Cleaning the extractor could be particularly important if
different
batches or lots of cannabis products are used for subsequent extraction runs
in the
same extractor, as residual extract in the extractor could lead to cross-
contamination
of these subsequent extraction runs.
113
CA 3062485 2019-11-21
85760071
In some embodiments, other processing such as winterization and/or
distillation could
be applied to extracts. Fig. 4H illustrates winterization chiller(s) 454h and
distiller(s)
456h that could be used to perform these processes.
Any of various information relating to a winterization process and/or
winterized
cannabis products could be recorded in an ICS, in the form of a winterization
record
in some embodiments. A winterization record could include or otherwise be
associated with a winterization record identifier, which could be similar in
form to
other identifiers disclosed herein. Winterization information related to a
winterization
process and/or winterized cannabis products could also or instead be recorded
in
another type of record such as a lot record.
A lot record could include a winterization record identifier or otherwise be
associated
with a winterization record for a winterization process that was used to
produce a lot
of winterized cannabis product, or multiple winterization records if the lot
was
winterized in different equipment, using different winterization processes,
and/or over
multiple days for example. Similarly, a winterization record could include a
lot
identifier or otherwise be associated with a lot record, or multiple lot
records if a
winterization process or equipment produced multiple lots of winterized
cannabis
product.
Similarly, any of various information relating to a distillation process
and/or distilled
cannabis products could be recorded in an ICS, in the form of a distillation
record in
some embodiments. A distillation record could include or otherwise be
associated
with a distillation record identifier, which could be similar in form to other
identifiers
disclosed herein. Distillation information related to a distillation process
and/or
distilled cannabis products could also or instead be recorded in another type
of
record such as a lot record.
A lot record could include a distillation record identifier or otherwise be
associated
with a distillation record for a distillation process that was used to produce
a lot of
distilled cannabis product, or multiple distillation records if the lot was
distilled in
different equipment, using different distillation processes, and/or over
multiple days
114
CA 3062485 2019-11-21
85760071
for example. Similarly, a distillation record could include a lot identifier
or otherwise
be associated with a lot record, or multiple lot records if a distillation
process or
equipment produced multiple lots of distilled cannabis product.
A winterization or distillation method could be substantially similar to the
example
method 900 in Fig. 14. For example, winterization or distillation equipment
(such as
the winterization chiller(s) 454h or distiller(s) 456h in Fig. 4H) could be
prepared for
operation, source cannabis product could be prepared for winterization or
distillation,
the winterization or distillation equipment could then be operated for one or
more
runs, and resultant output winterized or distilled extract could then be
collected. A
workspace and/or equipment could then be cleaned. Winterization or
distillation
methods could include any of various information collection, recording and/or
reporting features as well. Any of such parameters as weights of holding
containers
that contain source cannabis products and/or output cannabis products,
winterization
or distillation settings and/or conditions, and/or label information could be
measured
or otherwise collected, recorded, and/or transmitted to populate or update an
ICS.
Other features could also or instead be provided in conjunction with
winterization or
distillation. Examples of winterization and distillation processes are also
provided
below.
Further, typically, supercritical CO2 extraction of cannabinoids involves a
step of
winterization after the CO2 extraction so as to retain the more polar
cannabinoid
molecules while ridding the crude extract of most other waxes, which is often
referred
to as waxy ballast. The secondary extraction or "winterization" is an
ethanolic-
precipitation for removing waxy ballast and purifying the crude Cannabis
extract of
wax esters, glycerides, and unsaturated fatty acids, which hinder the extract
from a
refined liquid state. "Winterization" releases any trapped solvents from the
initial
extraction from the extremely viscous crude extracts.
The process of removing waxy ballast from crude cannabis extract using
"winterization", involves chilling the crude Cannabis extract to a temperature
less than
or equal to about 0 C, alternatively less than or equal to below about -10
C,
115
CA 3062485 2019-11-21
85760071
alternatively less than or equal to below about 20 C. for a time period. The
time
period may be at least 1 hour, alternatively at least about 24 hours,
alternatively at
least about 48 hours, alternatively at least about 50 hours, alternatively at
least about
72 hours. After the chilling freezing period, the crude Cannabis extract can
be cold-
filtered to remove waxy ballast. For example, a Whatman #1 lab filter with
vacuum
assist is initially used to remove the material that is insoluble, and
secondly the crude
extract is run through syringe filters (for example, 0.45 or 0.2 micron
filters), which
takes out any remaining plant material, as well as any bacteria present.
Optionally, the method for obtaining the cannabis concentrate may further
include
purification steps such as a distillation step in order to further purify,
isolate or
crystallize one or more cannabinoids. A cannabis concentrate obtained by
distillation
may be further cut with one or more terpenes (i.e., chemicals made and stored
in the
trichomes of the cannabis plant, with the cannabinoids. Terpenes give cannabis
its
distinctive smell. Alternatively, terpenes can be extracted and obtained from
other
plants).
At least a portion of resin that is collected in the example method 900 could
be sent
for packaging, which could include transferring the resin from an extract
collection
vessel to one or more other holding containers, for example. In some
embodiments,
the holding container(s) could be recorded in the ICS and assigned a lot
number. The
packaged resin could then be released for sale to consumers. Packaged resin
could
also or instead be transferred to other cannabis producers. For example, a
cannabis
producer could purchase resin in bulk from another producer, and use this
resin to
create their own brand of cannabis oil. Operation 708 of Fig. 12 illustrates
an
example of resin packaging. Operation 708 receives an extract from the
extraction at
operation 706. Operation 708 also receives resin containers 726, which are
examples
of holding containers for non-oil extracts. Resin containers 726 could include
stainless steel containers, for example.
Fig. 15 is a flow diagram illustrating an example method 1000 for resin
packaging.
The example method 1000 could be performed in a vertical laminar flow hood,
for
116
CA 3062485 2019-11-21
85760071
example, to help isolate an operator from any fumes produced by the resin.
More
generally, any or all processes that involve cannabis extracts could be
performed in a
laminar flow hood or other protective structure. Protective equipment, such as
facemasks, could also or instead be used.
Step 1002 includes weighing an empty holding container. The measured weight
could
be recorded in the ICS. At step 1004, the holding container is filled with
resin. Step
1004 could include transferring the resin from an extract collection vessel
into the
holding container, for example. Step 1004 could be performed manually and/or
be
automated by one or more devices.
After the holding container is filled, the holding container is weighed again
at step
1006. This weight could be recorded in the ICS, and could be compared to the
weight
of the empty holding container to determine the weight of resin in the holding
container. The weight of the resin in one or more holding containers could be
compared to the weight of the resin in the collection vessel to help ensure
consistency. A label could be generated for the holding container by the ICS,
or a
pre-existing label on the holding container could be recorded by the ICS. In
some
embodiments, the holding container could be associated with an extract record
in the
ICS, and the label on the holding container could include the extract record's
identification number. Steps 1004, 1006 could be repeated multiple times,
which is
indicated using a dashed line in Fig. 15. For example, step 1004 could be
performed
twice, where in each instance a resin from a different extraction process is
transferred
to the same holding container. At step 1006, the holding container could be
weighed
after each transfer of resin to determine the weight of the respective resin
that was
added.
The holding container of resin is transferred to a storage area at step 1008.
This
transfer could be recorded in the ICS to help track the location of the
holding
container. In some embodiments, the storage area could be a cool, dry and/or
dark
area, such as a refrigerator, to help preserve the resin in the holding
container.
117
CA 3062485 2019-11-21
85760071
The example packaging system 420j in Fig. 4J could be used in performing the
example method 1000. The scale(s) 430j-1 and/or 430j-2 could be used to weight
empty and full holding containers 456j, which could be filled and closed by
the bottle
filling/capping machine(s) 454j. Labelling and/or scanning could be performed
by the
label maker(s) 432j and/or scanners at 434j-1 and/or 434j-2. Information
related to
resin packaging could be transmitted from the packaging system 420j or
components
therein to the server 400 in Fig. 4A, through the server 418j in some
embodiments, to
populate or otherwise update the database 414 or particular records therein.
At least a portion of the resin that is collected during the extraction in
method 900 of
Fig. 14 could be used oil formulation. Oil formulation could be performed in
addition
to or instead of resin packaging. Oil formulation is the process of producing
cannabis
oils from cannabis extracts. In some embodiments, cannabis oils are produced
by
adding carrier oils to cannabis resin. The cannabinoid(s) in the resin could
be infused
into the carrier oil, which becomes a carrier for the cannabinoid(s).
Referring again to
Fig. 12, oil formulation is performed at operation 710. Operation 710 receives
cannabis extracts from the extraction process at operation 706. Operation 710
also
receives oil formulation supplies 720 and carrier oil supplies 722. Oil
formulation
supplies 720 could include, for example, holding containers, flasks,
protective
equipment, cleaning solutions and mixers. Carrier oil supplies 722 could
include any
of a variety of food grade oils, such as peppermint oil, fractionated coconut
oil (also
known as MCT oil), palm oil, olive oil, sunflower oil, canola oil, avocado
oil, hemp
seed oil and grape seed oil, for example. Carrier oil supplies 722 could
include a
mixture of two or more different carrier oils.
Any of various information relating to an oil formulation process and/or oil
formulation
cannabis products could be recorded in an ICS, in the form of an oil
formulation
record in some embodiments. An oil formulation record could include or
otherwise be
associated with an oil formulation record identifier, which could be similar
in form to
other identifiers disclosed herein. Oil formulation information related to an
oil
formulation process and/or oil formulation cannabis products could also or
instead be
recorded in another type of record such as a lot record.
118
CA 3062485 2019-11-21
85760071
A lot record could include an oil formulation record identifier or otherwise
be
associated with an oil formulation record for an oil formulation process that
was used
to produce a lot of oil formulation cannabis product, or multiple oil
formulation records
if the lot was dried in different equipment, using different oil formulation
processes,
and/or over multiple days for example. Similarly, an oil formulation record
could
include a lot identifier or otherwise be associated with a lot record, or
multiple lot
records if an oil formulation process or equipment produced multiple lots of
oil
formulation cannabis product.
Fig. 16 is a flow diagram illustrating an example process 1100 for oil
formulation. An
extract 1102 and a carrier oil 1104 are inputs to the example process 1100. In
some
embodiments, extract 1102 is a cannabis resin produced by an extraction
process.
The resin could be received in a holding container or in an extract collection
vessel,
for example. Carrier oil 1104 could be provided by a supplier, and could
include a
single type of carrier oil or a mixture of multiple types of carrier oils,
examples of
which are provided elsewhere herein.
In some embodiments, at operation 1106, the carrier oil 1104 is sterilized.
Operation
1106 could include transferring at least a portion of the carrier oil 1104
into a clean
flask and measuring the volume and/or weight of the carrier oil. The mouth of
the
flask could then be covered, with aluminum foil for example. The filled flask
could be
transferred to a sterilization device or system, such as a dry heat
sterilization (DHS)
oven. In some embodiments, the oven is operated at 180 C for 2.5 hours for
sterilization. The flask could then be removed from the oven and allowed to
cool.
Sterilization indicator tape could be affixed to the flask before the flask
enters the
oven. At least a portion of the indictor tape could change colors if a
particular
temperature has been reached by the flask, which could indicate that
sterilization was
successful. If sterilization was successful, then the flask could be sealed,
with a cap
for example. Information relating to the sterilization and/or the flask could
be labelled
on the flask and/or recorded in the ICS. For example, any one or more of the
time of
sterilization, the date of sterilization, one or more parameters of the
sterilization
process, the carrier oil volume, and the carrier oil weight could be recorded
on a label
119
CA 3062485 2019-11-21
85760071
and/or in the ICS. Following sterilization, the flask could be stored, in a
cool and dark
location in some embodiments.
By way of example, Fig. 4K illustrates a sterilization system 420k. Such a
system
could be used in sterilization of carrier oil and not only for sterilization
of cannabis
products. In Fig. 4K, sterilization is through irradiation in the irradiation
facility 452k.
Carrier oil sterilization could also or instead involve heating using an oven
instead of
or in addition to the irradiation facility 452k. Recording of sterilization
information
could involve one or more scales such as the scale(s) 430k-1 and/or 431k-2,
and/or
one or more scanners such as the scanner(s) 434k-1 and/or 434k-2. Labelling of
the
source product holding container(s) 450k holding carrier oil and/or the target
holding
container(s) holding sterilized carrier oil, which could be the same
containers in the
case of carrier oil sterilization, could involve one or more label makers such
as the
label maker(s) 432k.
Sterilization of carrier oils might not be performed in all oil formulation
processes. For
example, the carrier oil 1104 could be sent directly to operation 1108 without
first
being sterilized.
One or more other initial treatments of the carrier oil 1104 could be
performed,
instead of or in addition to sterilization, before the carrier oil is used in
operation
1108. For example, operation 1106 could include testing the carrier oil 1104
before
and/or after sterilization, or testing could be performed independently of
sterilization.
A holding container containing untested carrier oil could be marked as
"untested" or
"quality hold" on a label and/or in the ICS, to indicate that the carrier oil
has not yet
been tested and approved for use. To perform testing, a sample of the carrier
oil
1104 could be drawn from the holding container, using a dip tube, dipper or
pipette
for example, and transferred to a sample container such as a glass jar. The
holding
container could then be marked as "sampled" on the label and/or in the ICS. In
some
embodiments, the sample is tested for a United States Pharmacopeia (USP)
monograph that is specific to a type of carrier oil. USP monographs provide
standards for identity, quality, purity and/or strength for certain
substances. USP
120
CA 3062485 2019-11-21
85760071
monographs could confirm that the type of carrier oil being tested matches
what is
indicated on the label and/or what was ordered. By way of example, for testing
olive
oil, the USP monograph USP29-NF24 could be used. The carrier oil sample could
also or instead be screened for heavy metal contaminants.
If the test of the sample returns satisfactory results, then the holding
container that
was sampled could be marked as "cleared for use" on the label and/or in the
ICS. If
the sample failed one or more tests, then a second sample could be drawn from
the
holding container and tested. If the second sample also fails the test, then
the holding
container could be marked as "not for use" on the label and/or in the ICS, and
returned to the supplier of the carrier oil.
By way of example, Fig. 4L illustrates a testing system 4201. Such a system
could be
used in testing carrier oil and not only for testing cannabis products. Source
product
holding container(s) 4501, sampling container(s) 4521, and testing device(s)
4541 are
all shown in Fig. 41, and could be used to hold and test carrier oil.
Recording of
testing information could involve one or more scales such as the scale(s) 4301
and/or
one or more scanners such as the scanner(s) 4341. Labelling of the source
product
holding container(s) 4501 holding carrier oil, sampled carrier oil, and/or
tested carrier
oil, and/or labelling of the sampling container(s) 4521, could involve one or
more label
makers such as the label maker(s) 4321.
For each of the manufacturing examples described above, a lot release process
can
be implemented. In some embodiments, a lot of cannabis product can be tested
in
order to ensure that the batch of cannabis product is within a certain
cannabinoid
concentration range (e.g. milligrams of THC per milliliter of cannabis
product, or
milligrams of THC per gram of cannabis product). In some embodiments, such
testing
can include Quality Assurance (QA) testing, and may be part of a Preventable
Control Plan (PCP). In some embodiments, such QA testing can include allergen
testing, label validation testing, microbiological testing, mycotoxins
testing, nutritional
analysis, organoleptic testing, testing for heavy metals, foreign materials
toxins
121
CA 3062485 2019-11-21
85760071
and/or other contaminants. The results of such concentration and QA tests can
be
recorded by the ICS in, for example, the database 414 on server 402.
In some embodiments, the aforementioned testing can be performed prior to
packaging or bottling. In some embodiments, the aforementioned testing can be
performed once the cannabis product has been bottled or packaged. In such an
embodiment, the testing can be performed on a representative sample of the
bottles
or packages. Once the lot release testing is complete and a lot has passed any
concentration and QA tests, the lot is released.
Any of various information relating to carrier oil testing could be recorded
in an ICS, in
the form of a carrier oil testing record in some embodiments. A carrier oil
testing
record could include or otherwise be associated with a carrier oil testing
record
identifier, which could be similar in form to other identifiers disclosed
herein. Carrier
oil testing information related to testing of a carrier oil could also or
instead be
recorded in another type of record such as a sterilization record or a carrier
oil lot
record.
A sterilization record or a lot record could include a carrier oil testing
record identifier
or otherwise be associated with a carrier oil testing record, or multiple
carrier oil
testing records if carrier oil the lot was tested in different equipment,
using different
testing processes, and/or over multiple days for example. Similarly, a carrier
oil
testing record could include a sterilization identifier and/or a lot
identifier or otherwise
be associated with a sterilization record and/or a lot record, or multiple
sterilization
and/or lot records if multiple carrier oil samples were tested at the same
time and
under the same testing conditions, for example.
With reference again to Fig. 16, at operation 1108 carrier oil 1104, which may
have
been sterilized, tested, and/or otherwise processed, is mixed with extract
1102. This
mixing could include dissolving and/or suspending the extract 1102 in the
carrier oil.
In some embodiments, operation 1108 is intended to prepare a super saturated
solution of cannabis resin and carrier oil, which could also be referred to as
a
cannabinoid concentrate. Operation 1108 could include weighing a holding
container
122
CA 3062485 2019-11-21
85760071
containing cannabis resin, and recording this weight in the ICS. Carrier oil
could be
transferred to a beaker or other vessel, and the weight and/or volume of the
carrier oil
in the beaker could also be recorded in the ICS. The carrier oil could then be
transferred from the beaker to the holding container containing the cannabis
resin.
In some embodiments, the carrier oil is transferred incrementally. For
example, if the
resin in the holding container weighs 50-100g, then the carrier oil could be
added in
10mL increments until all of the resin is dissolved in the oil. If the resin
weighs 100-
300g then the carrier oil could be added in 50mL increments, and if the resign
weighs
over 300g then the carrier oil could be added in 100mL increments.
Alternatively, a
weight ratio of 3:2 for carrier oil to resin could be used. Other extract and
carrier oil
ranges, increments, and/or ratios are also possible, and such parameters could
be
determined and/or controlled dynamically in some embodiments.
Resin could be dissolved and/or suspended by a carrier oil without any
additional
actions to encourage mixing, however this might not always be the case. For
example, a resin with a high wax content might not be dissolved by a carrier
oil
without performing additional actions. The following is a non-limiting list of
actions
that could be used to encourage dissolution of resin in carrier oil, and any
one or
more of these actions could be performed at 1108:
submerging at least a portion of the holding container containing the resin
and the
carrier oil in a heated water bath (for example, the temperature of the water
bath
could be 40 C);
submerging at least a portion of the holding container containing the resin
and the
carrier oil in an ultrasonic water bath (for example, the holding container
could be
sonicated in 5 minute intervals);
placing the holding container on a heated stir plate (for example, the
temperature
of the stir plate could be set to 65 C);
directing a heat gun at the holding container to dissolve resin that is
adhered to
the walls of the holding container;
123
CA 3062485 2019-11-21
85760071
son ication .
Adding carrier oil to the holding container and/or performing any additional
actions to
dissolve the resin could be repeated multiple times until the resin is
substantially
dissolved by the carrier oil. Two or more actions could be performed, possibly
at the
same time, to dissolve the resin in the carrier oil. For example, sonication
and heating
in a water bath could be performed simultaneously. Carrier oil could also or
instead
be added to a holding container while performing additional actions to
dissolve the
resin. Any or all of the actions taken to dissolve the resin could be recorded
in the
ICS. When the resin is substantially dissolved by the carrier oil, the
solution could
appear homogenous. After mixing, the weight of the beaker of carrier oil
and/or the
weight of the holding container containing the produced cannabis oil could be
measured and recorded in the ICS. Comparing one or more of these weights to
their
initial weights could determine the weight of carrier oil that was added to
the holding
container. Using the weight/volume of the resin in the holding container, the
weight/volume of the carrier oil that was added to the holding container, and
the
cannabinoid concentration in the resin (determined by prior testing, for
example), the
cannabinoid concentration in the produced cannabis oil could be determined. In
some embodiments, multiple different resins and/or multiple different carrier
oils could
be mixed in operation 1108. Multiple different cannabis oils could also or
instead be
mixed together in operation 1108.
The operation 1108 could be recorded in the ICS using a "suspend" action, for
example. For example, a suspension process could be recorded in the ICS the
form
of a suspension record, which could be assigned a suspension record ID. The
suspend action could modify an extract record that is associated with extract
1102 to
indicate that at least a portion of this extract is now suspended in a carrier
oil. Using
the suspend action, the volume of the cannabis oil produced in operation 1108
could
be recorded in the ICS, and the extract record in the ICS could be updated to
have
that volume. The suspend action could record the holding container storing the
produced cannabis oil as an "oil container". The oil container record could be
assigned an ID such as "OC-1", "OC-2" or "OC-3".
124
CA 3062485 2019-11-21
85760071
In some embodiments, operation 1108 could further include diluting cannabis
oils
with additional sterilized carrier oil. Dilution could be performed to achieve
a particular
cannabinoid concentration in the final cannabis oil. For example, a cannabis
oil could
be diluted such that the concentration of THC does not exceed 30mg/mL. The
volume and/or weight of carrier oil that should be added to the cannabis oil
to reach a
particular cannabinoid concentration could be calculated beforehand. Prior
testing of
the cannabinoid concentrations in the extract used to produce a cannabis oil,
and/or
in the cannabis oil itself, could help determine the amount of additional
carrier oil that
should be added during dilution. In the case that a specific weight of
additional carrier
oil is calculated, a flask of un-diluted cannabis oil could be placed on a
scale and the
weight of the flask could be monitored as carrier oil is added until the
calculated
weight is reached. Heated water baths, ultrasonic water baths and/or heated
stir
plates could be used to help homogenize the diluted solutions. In some
embodiments, carrier oil could be added to the cannabis oil at a pre-defined
dilution
rate.
Dilution of a cannabis oil could be recorded in the ICS using a "dilute"
action. Using
the dilute action, an extract record and/or an oil container record could be
selected for
dilution. The weight and/or volume of cannabis oil that is being diluted could
be
measured and recorded in the ICS. The weight, volume and/or type of carrier
oil
added during dilution, as well as the final weight and/or volume of the
diluted
cannabis oil, could also be measured and recorded in the ICS. If the diluted
cannabis
oil is transferred to a different holding container, a new oil container
record could be
created by the dilute action.
Fig. 41 illustrates an example oil formulation system 420i. Holding containers
for
carrier oil, source cannabis product, and output cannabis oil(s) are shown at
452i,
450i, 4581, respectively. Recording of information related to an oil
formulation process
could involve such components as the scale(s) 4301-1 and/or 430i-2, the
sensor(s)
4281, and/or the scanner(s) 4341-1 and/or 4341-2. Other components could also
or
instead be used to collect and/or enter oil formulation process information
for
125
CA 3062485 2019-11-21
85760071
recording in an ICS. The label maker(s) 432a could be used in labelling of any
or all
of the holding containers 452i, 450i, 458i.
As referred to herein, cannabis vaping oils have a viscosity and a flash point
which
are suitable for use in a vaping device where the vaping device is configured
for
using a vaping oil having a viscosity at room temperature below a threshold,
and
where the vaping device is further configured to heat the vaping oil at a
vaporization
temperature at which one or more cannabinoids in the vaping oil vaporize.
Generally speaking, several options exist to obtain cannabis vaping oil having
the
herein described desired viscosity and flash point for use in a vaping device.
A first option is to dilute a cannabis concentrate having a viscosity at room
temperature which is above the threshold to the point of obtaining the desired
viscosity with an additive having a flash point equal to or above the
vaporization
temperature. The dilution creates a mixture that has a sufficiently lower
viscosity than
the cannabis concentrate without the additive, while maintaining a flash point
equal to
or above the vaporization temperature for safely vaporizing one or more
cannabinoids contained in the cannabis concentrate. Furthermore, when the
mixture
is loaded into a cartridge component of a vaping device with a pipette at room
temperature, the mixture flows in and out of the pipette into the cartridge
without
much difficulty. In other words, the mixture behaves like a liquid.
A second option is to dilute a cannabis concentrate having a viscosity at room
temperature which is above the threshold to the point of obtaining the desired
viscosity with an additive having a flash point below the vaporization
temperature. In
this option, the cannabis concentrate has a flash point equal to or above the
vaporization temperature such that the dilution creates a mixture that has a
flash
point equal to or above the vaporization temperature for safely vaporizing one
or
more cannabinoids contained in the cannabis concentrate. In this option, the
proportions of cannabis concentrate and additive are selected such that the
mixture
has a viscosity below the threshold while maintaining a flash point equal to
or above
the vaporization temperature.
126
CA 3062485 2019-11-21
85760071
In a practical implementation, the additive includes a compound which operates
to
lower the viscosity of the cannabis concentrate. The additive can be a single
material
or a blend of different materials. Optionally, the rate of addition of the
additive to the
cannabis concentrate can be adjusted according to expected storage or the
vaping
device's operational parameters.
In one embodiment, the additive used in the present disclosure does not
significantly
alter the organoleptic properties of the cannabis concentrate; in other words,
the
taste, smell and touch of the cannabis concentrate is not significantly
altered by the
addition of the additive.
In an advantageous non-limiting embodiment, a single additive is added to the
cannabis concentrate. This simplifies the manufacturing of the cannabis vaping
oil
and may increase regulatory approval likelihood by local regulatory bodies.
However,
it is also conceivable for two or more different additives to be added to the
cannabis
concentrate, especially when particular further advantageous properties are to
be
obtained. For example, a first additive having a flash point equal to or above
the
vaporization temperature may be used together with a second additive having a
flash
point below the vaporization temperature. In such situation, the overall
proportion of
cannabis concentrate required to obtain a suitable flash point for the whole
mixture
may not be as high compared to the situation where the additive(s) has (have)
a flash
point below the vaporization temperature. Accordingly, less cannabis
concentrate
may be required to have a cannabis oil with suitable flash point, although the
person
of skill may still wish to include higher proportion of cannabis concentrate
in other to
increase potency of the cannabis oil, i.e., increase the concentration of
cannabinoid(s) in the cannabis vaping oil.
In one non-limiting embodiment, the cannabis vaping oil of the present
disclosure
includes a mixture of the cannabis concentrate and the additive, where the
cannabis
concentrate is present in a proportion of 40 wt.% relative to the weight of
the
additive. Preferably, the proportion of cannabis concentrate is 5 70 wt.%
relative to
127
CA 3062485 2019-11-21
85760071
the weight of the additive, such that the cannabis vaping oil retains
sufficient free-
flowing liquid properties to afford ease of use with the vaping device.
Examples of additives that typically have a flash point above the vaporization
temperature include Vegetable Glycerin (VG), Polyethylene Glycol (PEG), and
Propylene Glycol (PG). Objectively, those compounds are less desirable than
other
examples provided in this disclosure because they are known to potentially
produce
toxic and carcinogenic impurities as a result of the thermal decomposition of
VG,
PEG and PG.
In one non-limiting embodiment, the additive includes one or more carrier
oil(s). In
one non-limiting embodiment, the one or more carrier oil(s) is (are) of plant
origin. For
example, but without being limited to, terpenes, essential oils, and the like,
such as
for example, d-limonene, Orange sweet (Citrus sinensis), b-myrcene, Pine
(Pinus
sylvestris), Fir (Abies siberica or Abies balsamea), Juniper Berry (Juniperus
communis), lemon Lime Flavor, peppermint oil, and the like.
In one non-limiting embodiment, the additive includes a medium chain
triglyceride
(MCT) or a mixture of MCT and another additive. For example, the additive can
include a mixture of peppermint oil and MCT in proportions such that the
typical taste
of peppermint oil is tamed down with the MCT.
The cannabis concentrate of the present disclosure may be obtained with any
known
method in the art. For example, the cannabis concentrate may be obtained by a
process including an extraction step from plant materials using heat
decarboxylation
to convert cannabinoids in their acid forms to neutral forms followed by or
after CO2
extraction (under sub-critical or super-critical conditions), and then,
optionally,
followed by ethanol winterization to remove waxes. Optionally, the method for
obtaining the cannabis concentrate may further include purification steps such
as a
distillation step in order to further purify, isolate or crystallize one or
more
cannabinoids. A cannabis concentrate obtained by distillation may be further
cut with
one or more terpenes.
128
CA 3062485 2019-11-21
85760071
The cannabis concentrate includes one or more cannabinoid(s). Examples of
cannabinoids include, but are not limited to, cannabigerolic acid (CBGA),
cannabigerol (CBG), cannabigerol monomethylether (CBGM), cannabigerovarin
(CBGV), cannabichromene (CBC), cannabichromevarin (CBCV), cannabidiol (CBD),
cannabidiol monomethylether (CBDM), cannabidiol-C4 (CBD-C4), cannabidivarin
(CBDV), cannabidiorcol (CBD-C1), delta-9-tetrahydrocannabinol (A9-THC), delta-
9-
tetrahydrocannabinolic acid A (THCA-A), delta-9-tetrahydrocannabionolic acid B
(THCA-B), delta-9-tetrahydrocannabinolic acid-C4 (THCA-C4), delta-9-
tetrahydrocannabinol-C4, delta-9-tetrahydrocannabivarin (THCV),
delta-9-
tetrahydrocannabiorcol (THC-C1), delta-7-cis-iso tetrahydrocannabivarin, delta-
8-
tetrahydrocannabinol (A8-THC), cannabicyclol (CBL), cannabicyclovarin (CBLV),
cannabielsoin (CBE), cannabinol (CBN), cannabinol methylether (CBNM),
cannabinol-C4 (CBN-C4), cannabivarin (CBV), cannabinol-C2 (CBN-C2),
cannabiorcol (CBN-C1), cannabinodiol (CBND), cannabinodivarin (CBVD),
cannabitriol (CBT), 10-ethoxy-9hydroxy-delta-6a-tetrahydrocannabinol, 8,9-
d ihyd roxy-delta-6a-tetrahyd rocannabinol, can nabitriolvarin
(CBTV), ethoxy-
cannabitriolvarin (CBTVE), dehydrocannabifuran (DCBF), cannabifuran (CBF),
cannabichromanon (CBCN), cannabicitran (CBT),
10-oxo-delta-6a-
tetrahydrocannabionol (OTHC), delta-9-cis-tetrahydrocannabinol (cis-THC),
3,4,5,6-
tetrahydro-7-hydroxy-alpha-alpha-2-trimethy1-9-n-propy1-2, 6-
methano-2H-1-
benzoxocin-5-methanol (OH-iso-HHCV), can nabiripsol (CBR), trihydroxy-delta-9-
tetrahydrocannabinol (tri0H-THC), cannabinol propyl variant (CBNV), and
derivatives
thereof.
In some embodiments, the cannabinoid is tetrahydrocannabinol (THC). THC is
only
psychoactive in its decarboxylated state. The carboxylic acid form (THCA) is
non-
psychoactive. Delta-9-tetrahydrocannabinol (A9-THC) and
delta-8-
tetrahydrocannabinol (A8-THC) produce the effects associated with cannabis by
binding to the CBI cannabinoid receptors in the brain.
In some embodiments, the cannabinoid is cannabidiol (CBD).
The terms
"cannabidiol" or "CBD" are generally understood to refer to one or more of the
129
CA 3062485 2019-11-21
85760071
following compounds, and, unless a particular other stereoisomer or
stereoisomers
are specified, includes the compound "A2-cannabidiol." These compounds are:
(1)
A5-cannabidiol
(2-(6-isopropeny1-3-methy1-5-cyclohexen-l-y1)-5-penty1-1,3-
benzened101); (2) A4-cannabidiol (2-(6-isopropeny1-3-methy1-4-cyclohexen-l-y1)-
5-
penty1-1,3-benzenediol); (3) A3-cannabidiol (2-(6-isopropeny1-3-methyl-3-
cyclohexen-
l-y1)-5-penty1-1,3-benzenediol); (4) A3,7-cannabidiol
(2-(6-isopropeny1-3-
methylenecyclohex-1-y1)-5-penty1-1,3-benzenediol); (5) A2-cannabidiol
(2-(6-
isopropeny1-3-methy1-2-cyclohexen-l-y1)-5-pentyl-1,3-benzenediol); (6) A1-
cannabidiol
(2-(6-isopropeny1-3-methyl-l-cyclohexen-l-y1)-5-pentyl-1,3-benzenediol); and
(7) A6-
cannabidiol (2-(6-isopropeny1-3-methyl-6-cyclohexen-l-y1)-5-penty1-1,3-
benzenediol).
In one embodiment, the cannabis oil of the present disclosure includes 2 300
mg/ml
of CBD, for example, 2 650 mg/ml, 2 550 mg/ml, 2 550 mg/ml, 2 460 mg/ml, 2 450
mg/ml, 2. 400 mg/ml, and the like.
In one embodiment, the cannabis oil of the present disclosure includes 2 300
mg/ml
of THC, for example, .2 650 mg/ml, 2 550 mg/ml, 2 550 mg/ml, 2. 460 mg/ml, 2
450
mg/ml, 2 400 mg/ml, and the like.
In one embodiment, the cannabis oil of the present disclosure includes 2. 300
mg/ml
of CBD and 5 30 mg/ml THC, for example, 30 mg/ml, 5_ 25 mg/ml, 5. 20 mg/ml,
and
the like.
The cannabis oil of the present disclosure can be used in any suitable
cartridge
component of a vaping device.
Packaging of cannabis oils is illustrated at operation 1110 in Fig. 16.
Operation 1110
includes packaging diluted and/or un-diluted cannabis oils in holding
containers such
as bottles. Packaging could include assigning a lot number to an oil container
record
using a new lot action, for example. All of the holding containers that
contain
cannabis oil from that oil container record could be labelled with or
otherwise
identified by that lot number.
130
CA 3062485 2019-11-21
85760071
In some embodiments, cannabis oils are packaged into bottles using an
automated
bottle filling machine, such as the bottle filling / capping machine(s) 454J
in Fig. 4J.
Fig. 17 is a flow diagram illustrating an example method 1200 for oil
packaging using
a bottle filling machine.
At step 1202, an empty oil vessel from which cannabis oil is supplied to the
bottle
filling machine during a production run is weighed. In some embodiments, this
oil
vessel is a pressure vessel. Weighing the oil vessel could include removing
and/or
decoupling the oil vessel from the bottle filling machine and placing the oil
vessel on a
scale. Alternatively, the oil vessel could be weighed while coupled to the
bottle filling
machine. In this case, the oil vessel and/or bottle filling machine could have
an
integrated scale to measure the weight of the oil vessel.
At step 1204, cannabis oil is added to the oil vessel. The cannabis oil,
and/or any
holding container(s) in which the cannabis oil was stored, could be recorded
in the
ICS. By way of example, cannabis oil could be stored in one or more of the
source
product holding container(s) 450j in Fig. 4J.
At step 1206, the oil vessel is weighed again, to determine the weight of
cannabis oil
that was added to the oil vessel. Any or all of the weights measured in steps
1202,
1206 could be recorded in the ICS, and/or these weights could be used to
determine
cannabis oil weight for recording in the ICS. Cannabis oil could be otherwise
measured, by volume, for example. In some embodiments, volume of oil added to
the
oil vessel from a holding container could be metered as it is added.
If appropriate, the oil vessel could then be re-coupled to the bottle filling
machine. For
example, an input hose of the bottle filling machine could be placed into or
otherwise
fluidly connected to the contents of the oil vessel.
Step 1208 includes loading empty bottles into the bottle filling machine. In
some
embodiments, the bottles could be added to a tray that is loaded into the
bottle filling
machine. These bottles could be cleaned, dried and/or sterilized to prevent
contamination of the cannabis oil. In some embodiments, the bottle filling
machine is
131
CA 3062485 2019-11-21
85760071
part of a production line, and is supplied with bottles as they are needed, by
a
conveyor system for example. Bottles used in a bottle filling machine
represent an
example of the target holding container(s) 456j in Fig. 4J.
The bottle filling machine is then run at step 1210. Before the run, any
pneumatic
connections, electrical connections, valves and/or tubing on the bottle
filling machine
could be cleaned, inspected, aligned and/or tested to confirm that they are
installed
and/or operating correctly. During a run, the bottle filling machine draws
cannabis oil
from the oil vessel and adds a pre-defined volume of cannabis oil to each of
the
empty bottles. The bottle filling machine could include a controller to adjust
the
volume of cannabis oil that is added to each bottle, a rate at which the
cannabis oil is
added and/or a number of bottles filled.
Some settings of the bottling filling machine could be adjusted based on
properties of
the cannabis oil. For example, a pressure that is applied to the cannabis oil
during a
run could be adjusted based on viscosity of the oil. The controller could be
connected
or otherwise have access to the ICS to receive and/or record any or all
settings of the
bottle filling machine.
Settings for a bottle filling machine could be controlled manually, be
predefined in a
controller, and/or received or otherwise obtained or determined by the
controller.
Bottle filling machine operation could also or instead be actively monitored
by an
operator, and/or one or more sensors such as the sensor(s) 428j in Fig. 4J, to
determine or adjust settings. A controller and/or one or more sensors could be
connected to or have access to the ICS to record the bottle filling machine
settings
and/or one or more parameters of the production run. Alternatively,
information could
be manually recorded in the ICS for a drying process, using a computer such as
424j
in Fig. 4J, for example.
In some embodiments, the bottle filling machine could be run multiple times
before
the oil vessel is emptied. For example, a full oil vessel could contain enough
cannabis
oil to supply two or more runs of the bottling filling machine. In these
embodiments,
132
CA 3062485 2019-11-21
85760071
steps 1208, 1210 could be performed multiple times, as indicated using a
dashed line
in Fig. 17.
At step 1212, the oil vessel is weighed again. This weight could be recorded
in the
ICS and/or used to determine and/or confirm the weight of cannabis oil that
was
added to the bottles in step 1210. Any unused cannabis oil in the oil vessel
could be
returned to the original holding container, or transferred to a new holding
container.
This transfer could be recorded in the ICS.
At step 1214, the filled bottles are sealed. This could include removing the
bottles
from the bottle filling machine and loading them into a capping machine to
apply caps
to the bottles, for example. Capping could be performed, entirely or in part,
manually
by one or more operators. In some embodiments, capping could be performed in
the
same equipment as bottling, as in the example packaging system 420j in Fig.
4J.
Each bottle could be weighed, by one or more scales such as the scale(s) 430j-
2 in
Fig. 4J, to determine whether the correct amount of cannabis oil was added. If
the
weight of a bottle differs from a target weight by more than a threshold, such
as 5%
for example, then the bottle could be rejected, and its contents could be
recycled into
another production run or destroyed.
At step 1216, labels are applied to the bottles. These labels could be
generated by
the ICS, and could include any of various types of information regarding the
cannabis
oil that they contain. The label could also or instead include the volume of
cannabis
oil in the bottle, the date on which the bottle was packaged, and/or any other
information regarding the bottle contents. The label maker(s) 432j and the
scanner(s)
434j-2 in Fig. 4J are examples of system components that could be configured
for
marking holding containers and scanning markings on holding containers,
respectively. Either or both of these components could transmit label
information to
other components for storage in or updating of an ICS, in the database 414 in
Fig. 4A
for example.
133
CA 3062485 2019-11-21
85760071
At step 1218, the bottles are transferred to a storage area, examples of which
are
provided elsewhere herein. Any such transfer could be recorded in an ICS to
help
track the location of a holding container.
At step 1220, the workspace, bottle filling machine(s) and/or oil vessel(s)
are cleaned.
Step 1220 could include washing or flushing certain components of the bottle
filling
machine with solvents (for example, water and/or ethanol) and/or compressed
air.
Any of various components of a packaging system, such as the example packaging
system 420j in Fig. 4J, could be configured to generate, collect, and/or
otherwise
obtain information and transmit that information to the server 402 in Fig. 4A,
through
the server 418j in some embodiments, for populating and/or updating the
database
414 or particular records therein. This includes the components which are
referenced
by way of example above in the description of Fig. 17, and/or possibly other
components.
In some embodiments, a holding container that contains cannabis oil could be
recorded in the ICS as an "oil jar record". By way of example, each bottle
that was
filled with cannabis oil in the example method 1200 could be recorded as an
oil jar
record. Oil jar records could be identified as "JAR-1", "JAR-2" and "JAR-3".
These
records could be created using a "new oil jar" action, for example. The new
oil jar
action could record, for example, any one or more of the following
information:
the label(s) on the holding container;
the volume of cannabis oil in the holding container;
the weight of the holding container; and
the location of the holding container.
Multiple holding containers of cannabis oil could also or instead be recorded
in the
ICS as an oil jar record. In this case, a "new bulk" action could be used to
create the
oil jar record, for example. The new bulk action could record, for example,
any one or
more of the following information:
134
CA 3062485 2019-11-21
85760071
an oil container record associated with the holding containers;
the number of holding containers;
the volume of cannabis oil in each holding container;
the weight of cannabis oil in each holding container; and
the weight of each empty holding container.
Referring again to Fig. 16, at least a portion the cannabis oil produced at
operation
1108, and/or a portion of the cannabis oil packaged at operation 1110, could
be sent
for sampling at operation 1112. Sampling could be performed to collect
cannabis oil
for testing and/or archiving for future testing.
A holding container of cannabis oil that has been selected for sampling could
be
weighed, using a scale 4301 in Fig. 4L for example, and this weight could be
recorded
in the ICS as a "pre-sample weight". A sample container such as a glass vial,
shown
by way of example in Fig. 4L at 4521, could be used to hold the sample of
cannabis
oil. The weight of the empty sample container could be measured, using a scale
4301
in Fig. 4L for example, and recorded in the ICS. Cannabis oil could then be
transferred from the holding container into the sample container, using a
pipette for
example. The amount of cannabis oil that is transferred could be pre-
determined. For
example, a desired sample volume could be 50mL. The sample container could
then
be closed, using a cap and/or an induction seal for example. The filled sample
container and/or the holding container that contained the cannabis oil could
be
reweighed, using a scale 4301 in Fig. 4L for example. Any or all measured
weights
could be recorded in the ICS. The weight of the holding container after
sampling
could be recorded as a "post-sample weight", which might be used to determine
or
confirm the weight of cannabis oil that was transferred to the sample
container. The
sample container could then be labelled, using a label maker(s) 4321 in Fig.
4L for
example, and/or stored in a storage area to await testing. In some
embodiments,
samples are tested when they are taken.
135
CA 3062485 2019-11-21
85760071
A sampling process could be recorded in the ICS using a "create sample"
action, for
example. This action could create a "lab sample" record in the ICS to record
any of
various information regarding a given sample of cannabis oil. Lab sample
records
could be assigned specific identifiers, such "LS-1", "LS-2" and "LS-3", for
example.
When a lab sample is sent for testing, the associated lab sample record could
be
labelled as "sent to lab" in the ICS.
Although the sampling process described above primarily relates to cannabis
oils, a
similar process could be performed to collect samples of other cannabis
products,
such as resin and/or plant material. Similar processes could also or instead
be
applied to sampling cannabis plants during cultivation, harvest and/or plant
part
separation. In some embodiments, samples of cannabis products could be
archived
and possibly tested at a later date.
Fig. 18 is a flow diagram illustrating an example method according to another
embodiment relating to cannabis extracts. The example method 1230 involves
providing, at 1232, a database to store information associated with cannabis
plants
and cannabis products, and assigning, at 1234, a batch identifier to a batch
of the
cannabis plants. These operations are described by way of example above, with
reference to Fig. 6, for example. Fig. 6 and the description thereof refer to
processing
plant material using first and second processes. The example method 1230
relates to
a process, at 1236, of extracting one or more cannabinoids from the plant
material of
a portion of the cannabis plants in the batch using an extraction method to
produce a
cannabis extract. In some embodiments, extracting cannabinoids from the plant
material at 1236 involves performing supercritical CO2 extraction of
cannabinoids.
Extracting cannabinoids from the plant material at 1236 could also or instead
involve
distilling the cannabis extract, and/or other operations as disclosed
elsewhere herein.
An extract identifier is assigned to the cannabis extract at 1238. An extract
identifier
could include alphanumeric characters and/or other symbols, and could be
managed
and/or assigned in the same way as lot identifiers or batch identifiers, for
example.
136
CA 3062485 2019-11-21
85760071
An amount of the cannabis extract is processed at 1240 to produce units of a
cannabis product. The processing at 1240 could involve, for example, any one
or
more of:
metering out amounts of the cannabis extract, illustratively by weight and/or
by
volume;
diluting the cannabis extract with one or more diluents such as a carrier oil;
emulsifying the cannabis extract to create a cannabinoid emulsion;
distilling the cannabis extract to produce a distillate;
metering out amounts of the distillate, illustratively by weight and/or by
volume;
diluting the distillate with one or more diluents such as water and/or oil;
emulsifying the distillate to create a cannabinoid emulsion.
Examples of these processes are disclosed elsewhere herein.
At 1242, a lot identifier is assigned to a lot of the units of the cannabis
product, and
the database is modified at 1244 to include information relating to the batch
identifier,
the extract identifier and the lot identifier, with the lot identifier being
associated with
the extract identifier and the extract identifier being associated with the
batch
identifier. Lot delineation, lot identifiers, modifying a database, and
identifier
associations are all disclosed by way of example elsewhere herein.
For instance, similar to an example described above, modifying the database at
1244
could involve creating a lot record for the lot of units of a cannabis
product. The lot
record could include information conveying or indicating the lot identifier
associated
with the lot and information conveying or indicating at least one of the batch
identifier
and the extract identifier associated with the lot identifier.
A lot record could include other information, such as information indicative
of the
process or processes used at 1240 to produce the units of cannabis product.
137
CA 3062485 2019-11-21
85760071
In some embodiments, the lot record further includes information indicative of
the
number of units of a cannabis product contained in the lot. This type of
information
could be useful in confirming that the cannabis extract was used to produce an
expected number of units of a cannabis product, for example.
Other examples of information that could be part of a lot record include
information
indicative of at least one of:
the time of the extracting that was used to produce the cannabis extract;
the date of the extracting that was used to produce the cannabis extract;
the time of the processing that was used to produce the units of cannabis
product
contained in the lot;
the date of the processing that was used to produce the units of cannabis
product
contained in the lot.
The method 1230, like other methods disclosed herein, is an illustrative
example.
Other embodiments could involve performing operations in a different order
than
shown, and/or performing different operations instead of or in addition to
those shown
in Fig. 18. For example, units of a cannabis product could be packaged, for
storage
and/or shipment, and a method could involve packaging each of the units of the
cannabis product to produce product packages. Each product package could be
marked with product information indicative of the lot identifier. A product
package
could be marked with other information in some embodiments.
Product information could be generated, at least in part, from information
retrieved
from the database, and examples of product information generation are
disclosed
elsewhere herein.
Marking each product package could involve marking the product package
directly,
and/or printing a label including the product information and affixing the
label to the
package. In some embodiments, a method involves retrieving information from
the
database, and generating the label using information retrieved from the
database.
138
CA 3062485 2019-11-21
85760071
The product information with which a product package is marked could include
at
least one of the following:
information conveying or indicating an identity or contact information of a
licensed
producer of the cannabis product;
information conveying or indicating an identity or contact information of a
licensed
processor of the cannabis product;
information conveying a brand name of a cannabis product;
information conveying recommended storage conditions of a cannabis product;
information conveying a packaging date of a cannabis product.
The example method 1230 has been described above in the context of extracting
one
or more cannabinoids from plant material of a portion of cannabis plants in
one batch.
It should be appreciated, however, that extracting cannabinoid(s) at 1236
could also
include extracting cannabinoids from plant material of a portion of cannabis
plants in
a second batch of cannabis plants using an extraction method to produce the
same
or a different cannabis extract, with the second batch of cannabis plants
having a
second batch identifier. The modifying at 1244 could then involve modifying
the
database to include information conveying or indicating the second batch
identifier,
and to associate the extract identifier with the second batch identifier.
Other variations of the example method 1230 may be or become apparent to those
skilled in the art.
A processor-readable storage medium could be used in implementing a method,
with
processor-executable instructions being stored on such a medium. The
instructions,
when executed by a processor, cause the processor to perform a method.
Execution
of the instructions could cause a computing device that includes the processor
to
implement a system configured to, in some embodiments: implement a database
configured to store information associated with cannabis plants and cannabis
products; assign a batch identifier to a batch of the cannabis plants; receive
139
CA 3062485 2019-11-21
85760071
extraction information relating to the extraction of one or more cannabinoids
from the
plant material of a portion of the cannabis plants in the batch using an
extraction
method to produce cannabis extract; assign an extract identifier to the
cannabis
extract; receive processing information related to the processing of an amount
of the
cannabis extract to produce units of a cannabis product; assign a lot
identifier to a lot
of the units of the cannabis product; and modify the database to include
information
relating to the batch identifier, the extract identifier and the lot
identifier, with the lot
identifier being associated with the extract identifier and the extract
identifier is
associated with the batch identifier.
Examples of many of these features are described above with reference to Fig.
18. A
system implemented by a computing device could be configured to implement a
database in one or more memory devices, for example, to store plant and
product
information, examples of which are described above and elsewhere herein. Such
a
system could also be configured to assign a batch identifier, an extract
identifier, and
a lot identifier, and to modify the database as described above and elsewhere
herein.
Fig. 18 and the description thereof refer to extracting one or more
cannabinoids from
plant material and processing cannabis extract to produce units of a cannabis
product. A production system could include equipment such as extraction
equipment
to extract cannabinoids from plant material and processing equipment to
process
cannabis extract. A system that is implemented by a computing device might not
itself
include such equipment, but could be part of a production system, or at least
communicate with equipment in a production system. A system that is
implemented
by a computing device could receive information from production system
equipment,
for example. In an embodiment, such a system is configured to receive
extraction
information relating to the extraction of one or more cannabinoids from the
plant
material using an extraction method to produce cannabis extract, and to
receive
processing information related to the processing of an amount of the cannabis
extract
to produce units of a cannabis product. Any of various types of processing
information could be received, and examples of information relating to
extraction and
140
CA 3062485 2019-11-21
85760071
processing are disclosed elsewhere herein. Different types of operations, such
as
extraction and processing, could have different types of related information.
Extraction information could be used to assign an extract identifier, and
processing
information could be used to assign a lot identifier to cannabis product units
in a lot.
For example, an extract identifier could be assigned based on a type of
extraction as
conveyed or indicated in the extraction information, and/or a lot identifier
could be
assigned based on a type of process, conveyed or indicated in the processing
information, that was used to produce the units in a lot.
A system implemented by a computing device could be configured to provide
other
features disclosed herein.
Other implementations are also contemplated. For example, the features
described
above with reference to Fig. 18 could involve various components of the
example
system 400 illustrated in Figs. 4A-4M.
Sterilization is shown at 1106 in Fig. 16 for carrier oil, but could also or
instead be
performed on cannabis material and/or cannabis products at any of various
stages of
production. For example, sterilization could be performed during or following
harvest,
plant part separation, drying, milling, decarboxylation, pre-rolling,
extraction and/or
packaging. Sterilization could be performed before and/or after cannabis
products are
assigned lot numbers. A sterilization process could be recorded in the ICS, in
the
form of a sterilization record for example, which could be assigned a
sterilization
record ID.
Irradiation is one method for sterilizing cannabis products, as in the example
sterilization system 420k in Fig. 4K. During an irradiation process, ionizing
radiation
could be directed towards a cannabis product to kill bacteria and/or other
organic
material that is present on and/or in the cannabis product. Examples of
ionizing
radiation include gamma rays, X-rays and electron beams. In some embodiments,
ionizing radiation could penetrate the walls of the holding containers that
contain a
cannabis product, such as the source product holding container(s) 450k in Fig.
4K,
141
CA 3062485 2019-11-21
85760071
and therefore a cannabis product might not be removed from a holding container
during irradiation. Irradiation processes could be performed by a cannabis
producer,
but this might not always be the case. For example, cannabis products could be
sent
to another company for irradiation.
Fig. 19 is a flow diagram illustrating an example method 1300 for cannabis
product
irradiation. In the example method 1300, step 1302 includes weighing an empty
shipping box that is used to ship cannabis products to an external facility
for
irradiation. This irradiation facility could be owned and/or managed by the
producer of
the cannabis product, or it could be owned and/or managed by another company.
The weight of the empty shipping box could be recorded in the ICS. Although
the
example shipping system 420m in Fig. 4M is described above primarily in the
context
of customer order fulfillment, a similar system could be used to ship cannabis
product
for irradiation. For example, empty shipping boxes could be weighed by one or
more
scales such as the scale(s) 430m.
At step 1304, the holding containers that contain the cannabis product to be
irradiated are weighed, by one or more scales such as the scale(s) 430m in
Fig. 4M.
These weights could be recorded in the ICS as "pre-irradiation" weights. The
holding
containers are then transferred to the shipping box at step 1306, and the full
shipping
box is weighed at step 1308, by one or more scales such as the scale(s) 430m
in Fig.
4M.. The weight of the full shipping box could be recorded in the ICS, and
compared
to the combined weights of the holding containers and the empty shipping box
to
confirm that the full shipping box weight is consistent with the total weights
of the
empty shipping box and the holding containers. A label for the shipping box
could be
generated by the ICS and/or recorded by the ICS. Labelling and/or recording
could
involve components such as one or more label makers and/or one or more
scanners,
examples of which are shown at 432m and 434m in Fig. 4M.
One or more tamper evidence seals could be provided on the shipping box. Such
seals could be incorporated into labels or separate from labels.
142
CA 3062485 2019-11-21
85760071
At step 1310 the shipping box is shipped to the irradiation facility, and at
step the
1312 the shipping box is received from the irradiation facility sometime
later.
Receiving the shipping box could include recording the label on the shipping
box in
the ICS. The ICS could then be updated to indicate that the shipping box has
been
received. The shipping box could also be weighed and compared to the weight
that
was recorded at step 1308 to confirm that no cannabis products were lost or
added.
A received shipping box and the holding containers inside the shipping box
represent
examples of the source product holding container(s) 450k in Fig. 4K. Weight
and/or
label recording could involve one or more scales 430k-1 and/or one or more
scanners
434k-1, for example.
At 1314, the holding containers are irradiated, in the irradiation facility
452k in Fig. 4K
for example. The holding containers could be removed from the shipping box
before
irradiation, or irradiated without being removed from the shipping box.
Step 1316 includes weighing the holding containers, individually and/or in the
received shipping box, and recording these weights as "post-irradiation"
weights in
the ICS. The weights measured at step 1314, using the scale(s) 430k-2 for
example,
could be compared to the weights that were measured at step 1304 and/or step
1308, to confirm or re-confirm that no cannabis products were lost or added.
Step
1316 could include inspecting the irradiated holding containers. For example,
tamper
detection devices on the holding containers could be inspected to detect any
evidence of tampering. Step 1316 could further include sampling one or more of
the
irradiated holding containers to test the effectiveness of the irradiation
process, for
example.
At step 1318, the holding containers are transferred to one or more storage
areas, to
await further processing and/or shipping for example. Any such transfer could
be
recorded in the ICS.
Any of various components of a shipping system and/or a sterilization system,
such
as the example systems 420m, 420k in Figs. 4M and 4J, could be configured to
generate, collect, and/or otherwise obtain information and transmit that
information to
143
CA 3062485 2019-11-21
85760071
the server 402 in Fig. 4A, through the servers 418m, 418j in some embodiments,
for
populating and/or updating the database 414 or particular records therein.
This
includes the components which are referenced by way of example above in the
description of Fig. 19, and/or possibly other components.
Testing of cannabis products is disclosed herein by way of example, and could
be
performed on cannabis material and/or cannabis products at any or all stages
of
production. For example, testing could be performed before and/or after
sterilization.
In some embodiments, sampling might first be performed to collect a
representative
sample of a cannabis material and/or cannabis product for testing. For
example, the
number n of holding containers to be selected for testing, from a lot of
cannabis
product, could be defined as n = 1 + VW, where N is the total number of
holding
containers in the lot. Other selection criteria could also or instead be used.
Some the
samples could be tested when taken, and/or some samples could be stored as
archived samples for future testing.
Testing could be performed by a cannabis producer, and/or by another entity. A
test
could be recorded in the ICS, in the form of a test record for example, which
could be
assigned a test record ID. In some embodiments, a lot number is not assigned
to a
cannabis product until after at least one sample of the cannabis product has
passed
one or more quality assurance tests. The result(s) of the test(s) could be
recorded in
the ICS and/or on a label of the cannabis product. For example, a cannabinoid
concentration that is determined for a cannabis product through testing could
be
recorded in the ICS using a new lot action, and/or added to a label.
Testing could also or instead be used to determine safety and/or effectiveness
of the
holding containers that contain a cannabis product. For example, a sample of
holding
containers for cannabis oil could be tested for leakage before and/or after
the holding
containers are filled with cannabis oil.
Testing for leaks could be performed in any of a variety of ways. In some
embodiments, one or more holding containers could be filled with a test liquid
and
positioned over a clean piece of blotting paper or any other material that
stains or
144
CA 3062485 2019-11-21
85760071
otherwise changes appearance upon contact with a liquid. In one example of
leak
testing, the holding containers could be positioned at an inverted angle of 45
below
horizontal with a container closure in the lowest position and free of any
obstruction.
After a particular amount of time, such as one hour, the blotting paper could
be
examined for any evidence of leakage. If a visual examination of the blotting
paper
discloses any trace of the test liquid, then the holding container has failed
the leakage
test and a holding container of the same type might not be used for packaging
cannabis products. If no trace of the test liquid is found on the paper, the
sample has
passed the leakage test and a holding container of the same type could be used
for
cannabis oil. The result(s) of the leak test(s) could be recorded in the ICS.
Other tests
of holding containers could include testing tamper detection seals and/or
child-
resistant features, for example.
A testing system 4201 is shown by way of example in Fig. 4L. Such a testing
system
could be used to test cannabis material and/or cannabis products, and holding
container testing could be implemented in the same or a similar manner.
In each of the aforementioned systems, the difference between the weight of
material
input into a process (or series of processes) and the weight of material
output from
the process (or series of processes) can be compared against any amount of
waste
output from the process (or series of processes) in order to assess lost
and/or theft of
material. This information can then be recorded by the ICS in, for example,
the
database 414 on server 402.
Packaging and Shipping
Final packaging and shipping could be managed by an ICS. The ICS could include
a
database to store information related to final packaging and shipping, for
example.
Fig. 20 is a flow diagram illustrating an example method 1400 for final
packaging.
Method 1400 could be similar to the final packaging performed at operation 122
in
Fig. 1.
145
CA 3062485 2019-11-21
85760071
At step 1402, one or more holding containers containing the same or different
cannabis products are selected for final packaging. By way of example, Fig. 4M
illustrates one or more selected holding containers 452m.
The number of holding containers selected at step 1402, and/or the type(s) of
cannabis product(s) stored in these holding containers, could be based on a
customer order. The selection could be performed automatically by the ICS,
and/or
manually by an operator. Labels on the holding containers could be used to
help
identify the desired holding container(s) for selection. Step 1402 could
include
selecting and removing the holding containers from a storage area, for
example. Step
1402 could also or instead include selecting and transferring holding
containers
directly from a production system or process. In some embodiments, cannabis
products from one or more holding containers could be transferred to one or
more
different holding containers at step 1402. For example, a lot of cannabis
product
could be stored in a large holding container, and then transferred to multiple
smaller
holding containers during final packaging.
The holding containers selected at step 1402 could be transferred to a
packaging
and/or shipping area or equipment, and the weight of and/or other information
related
to each selected holding container could be recorded in the ICS. One or more
scales
such as the scale(s) 430m in Fig. 4M could measure weight(s) of selected
holding
container(s) 452m. The scanner(s) 434m in Fig. 4M are illustrative of devices
that
could collect other information related to each selected holding container
452m.
Step 1404 includes transferring the selected holding container(s) to one or
more
primary boxes. In some embodiments, a primary box could store all of the
holding
containers that have been selected to meet a customer order. The selected
holding
container(s) could be compared to a customer order as they are transferred to
the
primary box to confirm that the order is being met. Protective materials, such
as
bubble wrap and/or Styrofoam for example, could be added to the primary
box(es) to
help protect the holding container(s) during shipping. Insulation could also
or instead
146
CA 3062485 2019-11-21
85760071
be added to protect the holding container(s) from hot or cold environments
during
shipping.
Step 1404 could include adding or updating labels on the holding container(s)
before
they are added to the primary box(es). Labels could also or instead be added
to
and/or updated on the primary box(es). In some embodiments, a label associated
with the customer could be added to the primary box(es) and/or the holding
container(s), using one or more label makers such as the label maker(s) 432m
in Fig.
4M.
The ICS could record all of the holding container(s) that are transferred to
the primary
box(es) by scanning their labels, for example. The ICS could also record any
or all
labels on the primary box(es). One or more scanners such as the scanner(s)
434m in
Fig. 4M could be used for container, primary box and/or label scanning.
In some embodiments, as shown at step 1406, the primary box(es) are
transferred
into one or more shipping boxes. The shipping box(es) could include protective
and/or insulating materials to help protect the cannabis products. In some
embodiments, these shipping boxes could be specific to a courier service that
is used
to ship cannabis products. Similar to the primary box(es), the shipping
box(es) could
be labelled and/or recorded in an ICS, using the label maker(s) 432m and/or
scanner(s) 434m in Fig. 4M for example. The weight of the shipping box(es),
and/or
the primary box(es), could also or instead be recorded in the ICS, using the
scale(s)
430m in Fig. 4M for example. In some embodiments, orders are placed directly
into
shipping boxes, and no separate primary boxes are used.
Labels on shipping boxes could include shipping information, such as the name
and
address of the customer. Step 1406 could include transferring the shipping
boxes to a
pick-up location for a courier service, and/or actually shipping the shipping
box(es).
Any such transfer and/or shipping could be recorded in the ICS. Other
information
such as the date, time and/or location of final packaging and/or shipping
could also or
instead be recorded in the ICS.
147
CA 3062485 2019-11-21
85760071
Step 1408 includes transferring any unused holding containers to one or more
storage areas. Any such transfer could be recorded in the ICS. For example, a
number of containers could be retrieved from storage, but only some of those
containers might be selected for order fulfillment. The remaining containers
could
then be returned to storage.
The following example describes a specific example implementation of the
method
1400 for final packaging of dried cannabis according to a customer order. At
step
1402, a holding container of dried cannabis is selected by the ICS to meet the
customer order. The ICS indicates that the holding container is stored in a
particular
storage area. Using a barcode scanner, an operator could locate the holding
container and scan the label on the holding container to confirm that it is
the holding
container selected by the ICS. The holding container could then be transferred
to a
final packaging area. Step 1402 also includes transferring the dried cannabis
from the
holding container into multiple bags, which are recorded in the ICS. The
number of
bags and the weight of dried cannabis in each bag could be specified by the
customer order and/or the ICS. These bags are then transferred to a primary
box at
step 1404, which is also recorded in the ICS. The primary box is then
transferred to a
courier shipping box at step 1406, and the shipping box is placed in a courier
pick-up
location. At step 1408, the dried cannabis that remains the original holding
container
could be sealed, and the holding container could be transferred back to the
storage
area.
Shipping could be performed by a courier service and/or postal service,
however
other means for shipping are also possible. Cannabis products could be shipped
to a
store or a private residence. Cannabis products could also or instead be
shipped to
another cannabis producer, in a bulk transaction for example. Further,
cannabis
products could be shipped to another producer for further processing, as
discussed
elsewhere herein.
The ICS could record and track any or all aspects of final packaging and
shipping.
For example, the weight, volume and/or type of any or all products that
undergo final
148
CA 3062485 2019-11-21
85760071
packaging and shipping could be recorded in the ICS. Information related to
shipping
destination(s) could also or instead be recorded in the ICS. A tracking number
that is
assigned to a shipped package could be recorded in the ICS. The ICS could have
access to a package tracking system provided by a courier/postal service to
actively
track the location of a shipped package. Proof of delivery could also or
instead be
recorded in the ICS. In some embodiments, the amount of cannabis products
shipped
to each customer could be recorded to ensure that any allowance and/or
shipping
limits are not exceeded. The ICS could convert the shipped cannabis products
into
an equivalent amount of cannabis plants for the purposes of the recording.
Any of various components of a shipping system such as the example shipping
system 420m in Fig. 4M, could be configured to generate, collect, and/or
otherwise
obtain information and transmit that information to the server 402 in Fig. 4A,
through
the server 418m in some embodiments, for populating and/or updating the
database
414 or particular records therein. This includes the components which are
referenced
by way of example above in the description of Fig. 20, and/or possibly other
components.
Various types of records that could be stored in an ICS are referenced herein.
Several detailed examples are shown in Figs. 21-23. Fig. 21 illustrates an
example of
a lot record, Fig. 22 illustrates an example of an extract record, and Fig. 23
illustrates
an example of an extraction process record.
The example lot record in Fig. 21 includes a Record ID and a Record Type. Date
of
creation and creator of the record are also included, in Record Created On and
Record Created By fields in this example.
The Batch Number(s) field in this example illustrates one way in which
batch(es) and
lot(s), and/or identifiers thereof, could be associated with each other. The
batch
numbers in the Batch Number(s) field are explicitly associated with the lot to
which
the lot record corresponds, by including the batch numbers in the lot record.
Plant
numbers, if specified in the Plant Number(s) field, similarly associate plants
and/or
149
CA 3062485 2019-11-21
85760071
plant numbers with a lot and/or lot number, and possibly with one or more
batches
and/or batch numbers as well.
In Fig. 21, the lot record also includes a Lot Number(s) field, which could be
useful if
a lot record has a different Record ID that does not match the lot number. For
a lot
record, it may be preferred to use the lot number as the Record ID, but this
might not
always be the case.
Other information regarding the lot is also included in the example lot
record, in the
following fields: GTIN, Cannabis Producer ID(s), Product Type, Product Volume,
Product Weight, Number of Holding Containers in Lot, and THC Concentration(s)
(by
weight).
Other explicit associations are included in the example lot record as well, in
the fields
Harvest Record ID(s), Plant Part Separation Record ID(s), Drying Record ID(s),
Milling Record ID(s), Decarboxylation Record ID(s), Extraction Process Record
ID(s),
Extract ID(s), Suspension (i.e. Mixing / Dilution) Process Record ID(s), Oil
Container
Record ID(s), Sterilization Record ID(s), Holding Container ID(s), Sample
Record
ID(s) and Test Record IDs.
Lot information, whether stored in a lot record or otherwise, could be
searchable.
Searchable lot information could be especially useful in facilitating
traceability. A
search for a batch number in an ICS database using a computer, for example,
could
identify any associated lots much more quickly and reliably than a manual
search of
lot information by an operator. Speed and reliability could be crucial in such
applications as identifying lots for product recalls for example.
Explicit associations as shown in the example lot record in Fig. 21 could also
impact
search speed and reliability.
The example lot record in Fig. 21 represents one embodiment. All fields are
populated in Fig. 21, but this might not be the case for every lot. Also, in
other
embodiments, a lot record could include further, fewer, and/or different
fields,
150
CA 3062485 2019-11-21
85760071
arranged in a similar or different order. Lot records might not even be used
in other
embodiments in which information related to lots is instead stored in some
other way.
With reference now to the example extract record in Fig. 22, this example
record, like
the example lot record in Fig. 21, also includes a Record ID field, a Record
Type field,
a Record Created On field, and a Record Created By field. In Fig. 22, the
example
record includes a Record ID without a separate extract identifier, and this
illustrates
an embodiment in which a cannabis product identifier, in this case an extract
identifier, is used as a Record ID and need not be separately specified in a
record.
Inclusion of the Batch Number(s) field in an extract record is one way in
which
batch(es) and extract(s), and/or identifiers thereof, could be associated with
each
other. The batch numbers in the Batch Number(s) field are explicitly
associated with
the extract and/or extract identifier to which the extract record corresponds.
Plant
numbers, if specified in the Plant Number(s) field, similarly associate plants
and/or
plant numbers with an extract and/or extract number, and possibly with one or
more
batches and/or batch numbers as well.
In some embodiments, an extract record could include a Lot Number(s) field in
addition to or instead of the Batch Number(s) field. A Lot Number(s) field,
without a
Batch Number(s) field, could provide for "indirect" associations between
extracts and
batches. For example, one or more lot numbers could be specified in a Lot
Number(s) field, to associate the lot number(s) with an extract number, and
extract-
batch association could then be determined from a lot record that includes one
or
more associated batch numbers in a Batch Number(s) field. In the examples
shown
in Figs. 21 and 22, batch numbers are explicitly associated with a lot number
(Fig. 21)
and an extract number (Fig. 22), and from these associations a lot-extract
association
could be determined. There is also an explicit association between lot and
extract as
well, in that the lot record in Fig. 21 includes the Record ID of the extract
record in
Fig. 22, in the Extract ID(s) field of the lot record.
Other information regarding the extract is included in the example extract
record, in
the following fields: Cannabis Producer ID(s), Product Type, Product Volume,
151
CA 3062485 2019-11-21
85760071
Product Weight, and THC Concentration(s) (by weight). The Collection Vessel
(full)
and Collection Vessel (empty) fields in the example extract record represent
an
example of fields that could be used to determine or verify values in other
fields. As
shown in Fig. 22, the Collection Vessel (full) weight minus the Collection
Vessel
(empty) weight is consistent with the Product Weight entry. Product Weight
entry
could be calculated from the Collection Vessel (full) weight and the
Collection Vessel
(empty) weight, or could be measured and verified using the Collection Vessel
(full)
weight and the Collection Vessel (empty) weight.
Other explicit associations are also included in the example extract record as
well, in
the fields Harvest Record ID(s), Plant Part Separation Record ID(s), Drying
Record
ID(s), Milling Record ID(s), Decarboxylation Record ID(s), Extraction Process
Record
ID(s), Collection Vessel ID(s), Suspension (i.e. Mixing / Dilution) Process
Record
ID(s), Oil Container Record ID(s), Sample Record ID(s), and Test Record ID(s).
Extract information, whether stored in an extract record or otherwise, could
be
searchable. Searchable extract information could be especially useful in
facilitating
traceability. A search for a batch number in an ICS database using a computer,
for
example, could identify any associated extracts much more quickly and reliably
than
a manual search of extract information by an operator. Speed and reliability
could be
crucial in such applications as identifying lots for product recalls for
example.
Explicit associations as shown in the example extract record in Fig. 22 could
also
impact search speed and reliability.
The example extract record in Fig. 22, like the example lot record in Fig. 21,
represents one embodiment. All fields are populated in Fig. 22, but this might
not be
the case for every extract. Also, in other embodiments, an extract record
could
include further, fewer, and/or different fields, arranged in a similar or
different order.
Extract records might not even be used in other embodiments in which
information
related to extracts is instead stored in some other way.
152
CA 3062485 2019-11-21
85760071
Turning to Fig. 23, the example extraction process record, like the example
records in
Figs. 21 and 22, also includes a Record ID field, a Record Type field, a
Record
Created On field, and a Record Created By field. In Fig. 23, as in Fig. 22,
the
example record includes a Record ID without a separate extraction process
identifier.
This illustrates another embodiment in which a process identifier, in this
case an
extraction process identifier, is used as a Record ID and need not be
separately
specified in a record.
Other extraction process details are specified in the following fields:
Cannabis
Producer ID(s), Date of Extraction, Start Time of Extraction, End Time of
Extraction,
Extraction Performed By, Number of Extraction Runs, Weight of Source Material
Before Run, Weight of Source Material After Run, Extractor Operating Program
ID(s),
Extractor Temperature(s), Extractor Pressure(s), Extraction Run Time(s), CO2
Flow
Rate(s), Winterization Process(es), and Distillation Process(es).
Several associations are also explicitly specified in the example extraction
record, in
the Extract Record ID(s) field, the Source Material Batch Number(s) field, and
the
Source Material Plant Number(s) field. The entry in the Extract Record ID(s)
field
references the extract record in Fig. 22, which cross-references the
extraction
process record in its Extraction Process Record ID(s) field. The entries in
the Source
Material Batch Number(s) field and the Source Material Plant Number(s) field
in Fig.
23 include the same batch and plant numbers as the entries in the Batch
Number(s)
fields and Plant Number(s) fields in the example lot record in Fig. 21 and the
example
extract record in Fig. 22. The example lot record in Fig. 21 also cross-
references the
extraction process record in its Extraction Process Record ID(s) field.
Such associations and cross-references could enable a much higher level of
accessibility to various types of information in an ICS database, relative to
manually
maintained records and/or even electronic records that are not as highly
organized or
cross-referenced as in the examples shown. Common information that appears in
multiple records and/or explicit associations between related records could
vastly
improve search speed and reliability. Automated collection of information,
creation of
153
CA 3062485 2019-11-21
85760071
records, and/or population of fields in records, could be especially preferred
to
maintain record integrity and accuracy.
Extraction process information, whether stored in an extraction process record
or
otherwise, could be searchable. Searchable extraction process information
could be
especially useful in facilitating traceability. A search for a batch number in
an ICS
database using a computer, for example, could identify any associated
extraction
processes much more quickly and reliably than a manual search of extraction
process information by an operator. As noted at least above for the example
records
in Figs. 21 and 22, speed and reliability could be crucial in such
applications as
identifying lots for product recalls for example, and explicit associations as
shown in
the example extract record in Fig. 23 could also impact search speed and
reliability.
The example extraction process record in Fig. 23, like the example lot record
in Fig.
21 and the example extract record in Fig. 23, represents one embodiment. All
fields
are populated in Fig. 23, but this might not be the case for every extraction
process.
Also, in other embodiments, an extraction process record could include
further, fewer,
and/or different fields, arranged in a similar or different order. Extraction
process
records might not even be used in other embodiments in which information
related to
extraction processes is instead stored in some other way.
Figs. 21-23 provide illustrative examples of records that could be used to
store
information relating to lots, extracts, and extraction processes,
respectively, in an
ICS. Similar or different records could be used to store other types of
information.
Examples of information that could be stored for cannabis plant material,
other
cannabis products, and/or other processes are disclosed by way of example
elsewhere herein.
Some embodiments disclosed herein relate to a hierarchical dataset in which a
batch
identifier is a root node and lot numbers form branches of the hierarchical
dataset
from the root node. Consider, for example, two lot records of the form shown
in Fig.
21. Two lots of cannabis product could be produced from the same batch of
plants,
and each lot could have a corresponding lot record including the same batch
number.
154
CA 3062485 2019-11-21
85760071
In this sense, the lot numbers could be considered branches from the same
batch
number in a hierarchical dataset. Other branches in such a dataset are also
possible.
For example, units of a cannabis product with a certain lot number could be
further
processed to produce multiple different cannabis products, such as beverages
and
edibles, to which further identifiers could be assigned. Those further
identifiers form
branches from their originating lot number, which in turn branches from a
batch
number or possibly multiple batch numbers as in the example shown in Fig. 21.
A hierarchical dataset is not restricted to only two levels (batch level and
lot level), or
even to levels associated with batches and/or lots.
Cannabis-Infused Consumer Product Manufacturing
In some embodiments, a cannabis producer processes cannabis plants from a
batch
of cannabis plants in order to produce one or more units of a cannabinoid-
containing
substance. A cannabinoid-containing substance is any substance that contains
cannabinoids. A cannabinoid-containing substance is itself a cannabis product.
However, a subsequent product produced using a cannabinoid-containing
substance
may also be called a cannabis product. Also, a cannabinoid-containing
substance
may sometimes instead be called a cannabis-containing substance.
In some embodiments, the tracking and traceability system disclosed herein is
also
used to track other materials which can be used in the manufacture of cannabis
products and/or materials which can form part of cannabis products. For
example, in
some embodiments, edible ingredients such as chocolate, gelling agents,
emulsifiers,
etc., can be tracked and/or traced by the traceability system disclosed
herein. As
described in more detail, above, in some embodiments, the process of tracking
such
edible ingredients form part of a Preventative Control Plan (PCP), or other
such
protocol that demonstrates how risks to food and food animals are identified
and
controlled.
In some embodiments, some or all of a unit of a cannabinoid-containing
substance is
used as an ingredient in a process to produce one or more cannabis-infused
155
CA 3062485 2019-11-21
85760071
consumer products. The following is a non-exhaustive list of examples of
cannabinoid-containing substances that may be used as an ingredient in a
process to
produce one or more cannabis-infused consumer products:
an extract (e.g. the substance output from an extraction process or machine,
e.g.
a resin resulting from a CO2 extraction process);
a distillate (e.g. the substance output from a distillation or fractionation
process or
machine, e.g., a distilled extract containing almost pure cannabinoid or
mixture of
cannabinoids, such as at least 90 wt.% pure cannabinoid);
a distillate/extract in an emulsification system (e.g. a substance in which a
distillate or an extract has been mixed with one or more emulsifiers, e.g.,
hydrophobic cannabinoid molecules that have been covered/coated with, or
incorporated into, an emulsifier);
a cannabinoid emulsion (e.g. distillate/extract in an emulsification system +
aqueous liquid);
a concentrated cannabinoid emulsion (e.g. a cannabinoid emulsion that has a
high concentration of cannabinoids, e.g. having at least 3 wt.% of a
cannabinoid,
taking into account both the acid form of the cannabinoid, such as THC-a, and
the
decarboxylated form of the cannabinoid, such as THC).
The following is a non-exhaustive list of examples of cannabis-infused
consumer
products that may be produced using a cannabinoid-containing substance as an
ingredient:
Cannabis-infused beverages (beverages incorporating cannabinoid-containing
substance(s) and which are intended to be consumed in the same manner as
beverage drinks);
Cannabis-infused edibles (products incorporating cannabinoid-containing
substance(s) and which are intended to be consumed in the same manner as
food);
156
CA 3062485 2019-11-21
85760071
Cannabis-infused topicals (products that incorporate cannabinoid-containing
substance(s) and which are intended to be used on external body surfaces, such
as skin, hair, and/or nails);
Cannabis-infused mucoadhesive delivery systems (products that incorporate
cannabinoid-containing substance(s) and which are intended to be used on
mucosa body surfaces, such as mouth, anal, nasal and vaginal cavities);
Cannabis-infused vaping oil (oil products incorporating cannabinoid-containing
substance(s) and which are intended to be consumed in a vaping device, such as
an electronic cigarette);
A cartridge containing cannabis-infused vaping oil.
An entity that uses some or all of a unit or amount of a cannabinoid-
containing
substance as an ingredient to produce one or more cannabis-infused consumer
products will be referred to as a cannabis processor. A cannabis processor may
sometimes be called a licensed processor. In some embodiments, the cannabis
producer and the cannabis processor may be the same entity. However, more
generally, the cannabis processor is a separate entity from the cannabis
producer,
and the cannabis processor receives one or more units of a cannabinoid-
containing
substance from a cannabis producer, and then uses some or all of the one or
more
units of the cannabinoid-containing substance to produce one or more units of
a
cannabis-infused consumer product. A consumer product is sometimes referred to
instead as a consumable product.
Fig. 24 is a block diagram of a cannabis producer 1502 and a cannabis
processor
1504, according to one embodiment. The cannabis producer 1502 utilizes an ICS
1506, e.g. the ICS described earlier in relation to Figs. 4A-4M. The cannabis
processor 1504 also utilizes an ICS 1508. The ICS 1506 and the ICS 1508 may be
the same ICS, e.g. if the cannabis producer 1502 and the cannabis processor
1504
are the same entity or related entities. In the description of Fig. 24 below,
it will be
assumed that the cannabis producer 1502 and the cannabis processor 1504 are
different entities, and that the ICS 1506 and ICS 1508 are different ICS's.
157
CA 3062485 2019-11-21
85760071
An example of ICS 1506 is illustrated in stippled bubble 1522. The ICS 1506
includes
a server 1524 having a memory 1526, processor 1528, and network interface
1530.
The processor 1528 controls the operations of the ICS 1506. The processor 1528
may be implemented by one or more processors that execute instructions stored
in
the memory 1526. Alternatively, some or all of the processor 1528 may be
implemented using dedicated circuitry, such as an ASIC, GPU, or FPGA for
performing the operations of the processor 1528. Several input/output (I/O)
devices
1534 are connected to the server 1524 via a network 1532. Examples of I/O
devices
1534 include computers, displays, scanners, scales, label makers, etc. which
are
used as part of the cannabis production process by the cannabis producer 1502.
For
example, server 1524 may be server 402 in Fig. 4A, and I/O devices 1534 may
include items such as:
computer(s) 424a, sensors(s) 428a, scale(s) 430a, label maker(s) 432a, and/or
scanner(s) 434a in the cultivation and harvest system 420a; and/or
scale(s) 430b-1 and 430b-2, scanner(s) 434b-1 and 434b-2, computer(s) 424b,
and/or label maker(s) 432b in the plant part separation system 420b; and/or
scales(s) 430c, scanner(s) 434c, computer(s) 424c, and/or sensor(s) 428c in
the
waste destruction system 420c; and/or
scale(s) 430d-1 and 430d-2, scanner(s) 434d-1 and 434d-2, computer(s) 424d,
and/or label maker(s) 432d in the fresh processing system 420d; and/or
scale(s) 430e-1 and 430e-2, scanner(s) 434e-1 and 434e-2, computer(s) 424e,
sensor(s) 428e and/or label maker(s) 432e in the drying system 420e; and/or
scale(s) 430f-1 and 430f-2, scanner(s) 434f-1 and 434f-2, computer(s) 424f,
sensor(s) 428f, and/or label maker(s) 432f in the milling system 420f; and/or
scale(s) 430g-1 and 430g-2, scanner(s) 434g-1 and 434g-2, computer(s) 424g,
sensor(s) 428g, and/or label maker(s) 432g in the decarboxylation system 420g;
and/or
158
CA 3062485 2019-11-21
85760071
scale(s) 430h-1 and 430h-2, scanner(s) 434h-1 and 434h-2, computer(s) 424h,
sensor(s) 428h, and/or label maker(s) 432h in the extraction system 420h;
and/or
scale(s) 430i-1 and 4301-2, scanner(s) 434i-1 and 434i-2, computer(s) 424i,
sensor(s) 428i, and/or label maker(s) 432i in the oil formulation system 420i;
and/or
scale(s) 430j-1 and 430j-2, scanner(s) 434j-1 and 434j-2, computer(s) 424j,
sensor(s) 428j, and/or label maker(s) 432j in the packaging system 420j;
and/or
scale(s) 430k-1 and 430k-2, scanner(s) 434k-1 and 434k-2, computer(s) 424k,
and/or label maker(s) 432k in the sterilization system 420k; and/or
scale(s) 4301, scanner(s) 4341, label maker(s) 4321, and/or computer(s) 4241
in the
testing system 4201; and/or
scale(s) 430m, scanner(s) 434m, label maker(s) 432m, and/or computer(s) 424m
in the shipping system 420m.
An example of ICS 1508 is illustrated in stippled bubble 1542. The ICS 1508
includes
a server 1544 having a memory 1546, processor 1548, and network interface
1550.
The processor 1548 controls the operations of the ICS 1508. The processor 1548
may be implemented by one or more processors that execute instructions stored
in
the memory 1546. Alternatively, some or all of the processor 1548 may be
implemented using dedicated circuitry, such as an ASIC, GPU, or FPGA for
performing the operations of the processor 1548. Several I/O devices 1554 are
connected to the server 1544 via a network 1552. Examples of I/O devices 1554
include computers, displays, scanners, scales, label makers, etc. which are
used as
part of the processing by the cannabis processor 1504.
In some embodiments, ICS 1506 and ICS 1508 may share information, as shown by
stippled line 1510. The shared information may be transferred over a network
in
some embodiments, and the information may relate to an association between
records and/or lots, e.g. linking a lot number on a cannabis-infused consumer
product
159
CA 3062485 2019-11-21
85760071
produced by the cannabis processor 1504 back to the lot number of a unit of a
cannabinoid-containing substance received from the cannabis producer 1502 and
used to make that cannabis-infused consumer product.
In operation, the cannabis producer 1502 produces one or more units of a
cannabinoid-containing substance, e.g. a cannabinoid emulsion, which is used
as a
raw material/ingredient by the cannabis processor 1504 to produce one or more
units
of a cannabis-infused consumer product, e.g. a cannabis-infused edible,
beverage,
and/or topical product. The cannabis producer 1502 uses its ICS 1506 to
record, log,
track and/or monitor production of the cannabinoid-containing substance
throughout
cultivation, harvesting, processing, sales, shipping, and/or other operations,
e.g. as
described in detail earlier. The cannabis processor 1504 similarly uses its
ICS 1508
to record, log, track and/or monitor its cannabis-infused consumer products,
e.g. from
receipt of the cannabinoid-containing substance from the cannabis producer
1502
through to production of the cannabis-infused consumer product and shipping
and/or
sale of the cannabis-infused consumer product.
For example, the ICS 1508 may be used by the cannabis processor 1504 to record
information relating to the production of each lot of cannabis-infused
consumer
product. The ICS 1508 may record any or all transfers of the cannabinoid-
containing
substance or a product or intermediary product incorporating some of all of
the
cannabinoid-containing substance within and/or between the systems used by the
cannabis processor 1504. The ICS 1508 may enable traceability of any or all
cannabis through at least part of a production process, including traceability
to lot
level and/or batch level. This may include enabling traceability through and
back to: a
master batch of the cannabis-infused consumer product that was produced using
a
particular lot of a cannabinoid-containing substance; and/or the cannabinoid-
containing substance as received from the cannabis producer 1502; and/or a
diluted
form of the cannabinoid-containing substance (e.g. if the cannabinoid-
containing
substance received from the cannabis producer 1502 is diluted or added to a
larger
volume of other liquid); and/or units of consumer product produced using the
160
CA 3062485 2019-11-21
85760071
cannabinoid-containing substance; and/or units of consumer product in storage;
and/or units of consumer product that have been released for sale or sold.
By way of example, in the event of a recall, the ICS 1508 may be used to
determine
the status and/or location of all cannabis-infused consumer products that fall
within
the scope of the recall. As another example, the ICS 1508 may be used to trace
a
particular cannabis-infused consumer product (e.g. an edible, beverage, or
topical) to
a particular lot of cannabinoid-containing substance received from the
cannabis
producer 1502. The ICS 1508 may therefore facilitate traceability back through
to
cannabis producer 1502, e.g. the lot number associated with a particular unit
of
cannabis-infused consumer product may be traced back to the lot number of a
unit of
cannabinoid-containing substance received from the cannabis producer 1502.
This
may allow for both: (1) the cannabis processor 1504 to determine which other
cannabis-infused consumer products may be subject to the recall; and (2) the
cannabis producer 1502 to use their ICS 1506 to determine which batch of
cannabis
plants, and hence which other cannabinoid-containing substances produced by
the
cannabis producer 1502, may be subject to the recall.
Fig. 25 is a schematic illustrating an example of traceability from a cannabis-
infused
consumer product back to a batch of cannabis plants. A cannabis producer 1502
cultivates and harvests different batches of cannabis plants, two of which are
illustrated and respectively assigned batch numbers B376 and B377. The batches
may be cultivated or harvested in parallel or serially. The batch numbers B376
and
B377 are stored in the ICS 1506. In this illustrated example, at least some of
the
plants from batch B376 undergo extraction processing to create an extract,
which is
optionally subjected to additional processing (e.g. distillation, adding an
emulsifier,
etc.). The result is a plurality of units 1572 of a cannabinoid-containing
substance,
each of the units 1572 being cannabis in concentrated form in this particular
embodiment. The extraction process is assigned an extraction process number
E231,
and the additional processing (if performed) is assigned a process number
P402. The
numbers E231 and P402 are stored in the ICS 1506 in association with/linked to
the
batch number B376. The plurality of units 1572 of cannabinoid-containing
substance
161
CA 3062485 2019-11-21
85760071
are each assigned the same lot number Al2, which is marked on the holding
container of each one of the plurality of units 1572, e.g. via a label. The
lot number
Al2 is stored in the ICS 1506 in association with/linked to the processing and
batch
numbers E231, P402, and B376.
Similarly, in this illustrated example, at least some of the plants from batch
B377
undergo extraction processing to create an extract, which is optionally
subjected to
additional processing (e.g. distillation, adding an emulsifier, etc.) to
produce a
plurality of units 1574 of a cannabinoid-containing substance, each of the
units 1574
being cannabis in concentrated form in this particular embodiment. The
extraction
process is assigned an extraction process number E232, and the additional
processing (if performed) is assigned a process number P403. The numbers E232
and P403 are stored in the ICS 1506 in association with/linked to the batch
number
B377. The plurality of units 1574 of cannabinoid-containing substance are each
assigned the same lot number A13, which is marked on the holding container of
each
one of the plurality of units 1574, e.g. via a label. The lot number Al 3 is
stored in the
ICS 1506 in association with/linked to the processing and batch numbers E232,
P403, and B377.
Some or all of the batch, process, and lot numbers (e.g. numbers B376, B377,
E231,
E232, P402, P403, Al2, and A13) may be generated by the ICS 1506, or instead
generated manually or by local equipment and stored in the ICS 1506.
In the embodiment illustrated in Fig. 25, the lot number Al2 assigned to each
unit
1572 of cannabinoid-containing substance originating from batch B376 and
output
from extraction process E231 (and optional additional processing P402) is
different
from the lot number A13 assigned to each unit 1574 of cannabinoid-containing
substance originating from batch B377 and output from extraction process E232
(and
optional additional processing P403). The lot number therefore allows, through
the
ICS 1506, traceability from a unit of cannabinoid-containing substance all the
way
back to the particular batch of cannabis plants used to produce that unit of
cannabinoid-containing substance.
162
CA 3062485 2019-11-21
85760071
The cannabis processor 1504 receives a unit 1572 of cannabinoid-containing
substance having lot number Al2. The lot number Al2 is stored in the ICS 1508.
Some or all of the unit 1572 of cannabinoid-containing substance is used in a
consumer product production process, in combination with other ingredients, to
produce a plurality of units 1582 of cannabis-infused consumer products for
sale, e.g.
a plurality of cannabis-infused beverages. The plurality of units 1582 of
cannabis-
infused consumer product are each assigned the same lot number 3Y3, which is
marked on each one of the plurality of units 1582, e.g. via a label. The lot
number
3Y3 is stored in the ICS 1508 in association with/linked to the cannabinoid-
containing
substance lot number Al2. Other processing numbers may be assigned during the
consumer product production and linked to the lot numbers 3Y3 and Al2, e.g. a
master consumer product batch number, a processing number, a holding tank
number, etc., depending upon the implementation.
Similarly, in this illustrated example, the cannabis processor 1504 receives a
unit
1574 of cannabinoid-containing substance having lot number A13. The lot number
Al 3 is stored in the ICS 1508. Some or all of the unit 1574 of cannabinoid-
containing
substance is used in a consumer product production process, in combination
with
other ingredients, to produce a plurality of units 1584 of cannabis-infused
consumer
products for sale, e.g. a plurality of cannabis-infused beverages. The
plurality of units
1584 of cannabis-infused consumer product are each assigned the same lot
number
3Y4, which is marked on each one of the plurality of units 1584, e.g. via a
label. The
lot number 3Y4 is stored in the ICS 1508 in association with/linked to the
cannabinoid-containing substance lot number A13. Other processing numbers may
be assigned during the consumer product production and linked to the lot
numbers
3Y4 and A13, e.g. a master consumer product batch number, a processing number,
a
holding tank number, etc., depending upon the implementation.
In this example, the lot number 3Y4 assigned to each consumer product unit
1584 is
the same because units 1584 originate from the same amount (lot) A13 of
cannabinoid-containing substance. Similarly, the lot number 3Y3 assigned to
each
consumer product unit 1582 is the same because units 1582 originate from the
same
163
CA 3062485 2019-11-21
85760071
amount (lot) Al2 of cannabinoid-containing substance. However, the lot number
3Y4
is different from the lot number 3Y3 because consumer product units 1582
originate
from a different amount (lot) of cannabinoid-containing substance than
consumer
product units 1584.
In the embodiment illustrated in Fig. 25, indicia marked on a unit of cannabis-
infused
consumer product (e.g. lot number 3Y3) is indicative of (e.g. mapped back to)
a
particular amount (e.g. particular lot) of a cannabinoid-containing substance.
That
particular amount of cannabinoid-containing substance was derived from
particular
cannabis plant material and contains one or more cannabinoids. That particular
amount of cannabinoid-containing substance may be a particular lot of
cannabinoid-
containing substance, which is assigned a particular lot number, and which
differs
from another particular amount (lot) of cannabinoid-containing substance. For
example, in Fig. 25 indicia in the form of lot number 3Y3 is mapped back to
(indicative of) lot number Al2, and lot number Al2 was derived from and
ultimately
maps back to a particular batch of cannabis plant material from which the
cannabis
originated (e.g. lot number 3Y3 is mapped back to lot number Al2, which is
mapped
back to cannabis plants batch number B376). In some embodiments, the lot
number
on a unit of the cannabis-infused consumer product (e.g. lot number 3Y3) may
also
be used to identify particular processing or other steps in the process of
creating that
unit of cannabis-infused consumer product from a batch of cannabis plants
(e.g. lot
number 3Y3 links back to extraction process E231). The ICS 1506 and 1508
facilitate
the traceability by storing the processing records and numbers in association
with
each other.
The exact processing performed by cannabis processor 1504 is implementation
specific and depends upon the cannabis-infused consumer product being
produced.
Some examples will now be described in the context of producing cannabis-
infused
beverages.
Fig. 26 is a block diagram of a system 1650 for producing cannabis-infused
beverages, according to one embodiment. The system 1650 includes: a filling
line
164
CA 3062485 2019-11-21
85760071
1652; a filling station 1654 having a plurality of nozzles 1656a-n; a
container marking
station 1658 for applying indicia to containers or packaging for the units of
cannabis-
infused beverages, which in this embodiment is a labeling station that applies
a label
on each container; a control device 1660 including a processor 1662 and
computer
readable storage in the form of memory 1664; a plurality of holding tanks
1666a-k,
each having a respective liquid level sensor 1668a-k; and a supply selection
valve
1670 interposed between and in fluid communication with the holding tanks
1666a-k
and the filling station 1654. The processor 1662 may be implemented by one or
more
processors that execute instructions stored in the memory 1664. Alternatively,
some
or all of the processor 1662 may be implemented using dedicated circuitry,
such as
an ASIC, GPU, or FPGA. The processor 1662 implements the operations of the
control device 1660. A control device may alternatively be called a
controller.
The filling line 1652 comprises a conveyor of containers. In the illustrated
embodiment the containers are bottles 1675. In general, any type of container
may
be used, e.g. a glass, plastic, or aluminum container. Although the bottles
1675 are
illustrated in a conveyor line, a conveyor line is only an example. A
different
configuration may be used instead, e.g. pallets or disks holding bottles that
are
presented to the filling station 1654 and filled in batches. The filling
station 1654 uses
nozzles 1656a-n to fill the bottles 1675 with cannabis-infused beverage from
holding
tanks 1666a-k. Several bottles are filled in parallel, with each bottle being
filled by a
respective one of the nozzles 1656a-n. The container marking station 1658
prints and
applies a label on each bottle. Instead of generating labels, the marking
station 1658
may apply indicia to each bottle in another manner instead, e.g. by stamping
each
bottle or providing indentations in each bottle, etc. The supply selection
valve 1670 is
capable of selectively acquiring a plurality of supply positions, each supply
position
associating a respective holding tank with the filling station 1654 to supply
the filling
station 1654 from the respective holding tank.
Operation of the system 1650 will be explained in the context of the example
introduced in relation to Fig. 25. A unit of cannabinoid-containing substance
having
lot number Al2 is received by the cannabis processor 1504 from the cannabis
165
CA 3062485 2019-11-21
85760071
producer 1502. A first master batch of cannabis-infused beverage is prepared
by the
cannabis processor 1504 using that unit of cannabinoid-containing substance,
and
the first master batch of cannabis-infused beverage is stored in holding tank
1666a.
Preparing the first master batch may include processing steps such as adding
additional ingredients (e.g. water, flavourants), and possibly first diluting
or otherwise
modifying the cannabinoid-containing substance to put it in a form suitable
for adding
as an ingredient (e.g. forming an emulsion from the cannabinoid-containing
substance if the lot of cannabinoid-containing substance as received is not an
emulsion). In some embodiments, the mater batch may be tested for cannabinoid
concentration levels. In some embodiments, the cannabinoid concentration
levels
determined as a result of testing can be recorded by way of the ICS 1508. In
some
embodiments, the cannabinoid concentration levels can be recorded in a master
batch record.
The control device 1660 is in communication with the ICS 1508 (not
illustrated), and
the control device 1660 associates product lot number 3Y3 with each bottle to
be
filled with cannabis-infused beverage from the first master batch. The control
device
1660 controls marking station 1658 to affix a label having lot number 3Y3 to
each
bottle that is to be filled with cannabis-infused beverage from the first
master batch.
The label is from a supply of labels used by the marking station 1658.
In some embodiments, the ICS 1508 may also store a holding tank number and/or
master batch number and/or other processing number(s) in association with /
linked
to the product lot number 3Y3 to allow for traceability throughout the process
performed by the cannabis processor 1504. As an example, product lot number
3Y3
may also be associated with: (1) master batch number MB35, where "MB35" is a
number associated with a particular unit of master batch that was produced
using
cannabinoid-containing substance lot number Al2 and that is held in a
particular
holding tank 1666a; and (2) holding tank number HT1, where "HT1" is a number
assigned to the holding tank 1666a that holds a unit of master batch. This may
allow
for traceability within the cannabis processor's operations, e.g. if there was
a problem
with a particular bottle of cannabis-infused beverage, then the lot number 3Y3
on the
166
CA 3062485 2019-11-21
85760071
label of the bottle may be used by the ICS 1508 to identify that the cannabis-
infused
beverage for that particular bottle was stored in holding tank HT1 and from
master
batch MB35, and that master batch MB35 is a batch of cannabis-infused beverage
made using a unit of cannabinoid-containing substance from lot number Al2. The
traceability could extend back through the cannabis producer 1502 also: the
lot
number Al2 of cannabinoid-containing substance was generated using an extract
from extraction process E231, and the input to that extraction process was
cannabis
from batch B376 of cultivated/harvested cannabis plants. In this way, in some
embodiments it is possible to use to lot number 3Y3 on a bottle 1675 to trace
some or
all steps of the processes involving the cannabis ingredient, possibly all the
way back
to the batch of plants cultivated and harvested to produce the cannabis
ingredient.
In Fig. 26, a second master batch of the cannabis-infused beverage is prepared
using
a unit of cannabinoid-containing substance from another lot A13, and that
second
master batch of cannabis-infused beverage is stored in holding tanks 1666b and
1666k. The second master batch of cannabis-infused beverage is held in the
holding
tanks until the first master batch of cannabis-infused beverage has depleted.
For
example, the control device 1660 controls the valve 1670 to open the flowline
from
holding tank 1666a, as shown at 1680, and to close the flowline from holding
tanks
1666a and 1666k, as shown at 1682.
Turning to Fig. 27, in some embodiments a signal 1684 from liquid level sensor
1668a in holding tank 1666a indicates to control device 1660 that the cannabis-
infused beverage in holding tank 1666a is becoming depleted, such that the
control
device 1660 knows the point at which bottles will need to start being filled
from
holding tank 1668b instead. Alternatively, the number of bottles that can be
filled with
cannabis-infused beverage in a holding tank may be fixed or known in advance
by
the control device 1660, such that the control device 1660 may simply count
the
number of bottles and switch over to the next holding tank when the maximum
number of bottles for a holding tank have been filled, in which case the
liquid level
sensors 1668a-k may not be utilized or present. In some embodiments, the
control
device 1660 may count and store the counted number, e.g. so that the number of
167
CA 3062485 2019-11-21
85760071
bottles that can filled is known for future master batches and/or known for
future lots
(amounts) of can nabinoid-containing substance.
The control device 1660 stores information from the ICS 1508 indicating that
holding
tank 1668b holds cannabis-infused beverage from a second master batch, which
is
associated with a different product lot number 3Y4. Therefore, the control
device
1660 sends a signal 1686 to marking station 1658 to modify the label being
affixed to
reflect lot number 3Y4, and to begin applying that label to each bottle,
starting at the
first bottle that is to receive the cannabis-infused beverage from the second
master
batch.
Turning to Fig. 28, at the appropriate switching point, the control device
1660
transmits a signal 1688 to valve 1670 to close the filling line from holding
tank 1666a,
as shown at 1690, and to open the filling line from holding tank 1666b, as
shown at
1692. Although not illustrated, a similar switch occurs to go from holding
tank 1666b
to holding tank 1666k when holding tank 1666b is depleted of beverage.
However, in
this embodiment the lot number 3Y4 would not be changed because both holding
tanks 1666b and 1666k include beverage from the same second master batch that
originates from lot number A13 of the cannabinoid-containing substance. In an
alternative embodiment, the lot number 3Y4 may be changed when switching from
holding tank 1666b to holding tank 1666k in order to uniquely associate a lot
number
with a particular holding tank.
In some embodiments, a lot number on a unit of cannabis-infused consumer
product
(e.g. 3Y3 and 3Y4) and/or a lot number on a unit of cannabinoid-containing
substance (e.g. Al2 and A13) may be encoded in a machine-readable code, e.g. a
barcode. The barcode may be decoded by a computer to obtain the lot number. In
some embodiments, the computer may be connected to (or in network
communication with) ICS 1506 and/or ICS 1508. In some embodiments, any of the
numbers discussed herein, e.g. B376, B377, E231, E232, P402, P403, 3Y3, and
3Y4
may be encoded in a machine-readable code. Also, each number is an identifier,
which in general may include alphanumeric characters and/or other symbols.
168
CA 3062485 2019-11-21
85760071
The bottles 1675 marked with label 3Y3 form one set of containers, and the
bottles
1675 marked with label 3Y4 form another set of containers, such that the
conveyor
provides to the marking station 1658 successive sets of containers, each set
having a
label unique to that set in that the indicia applied to containers in one set
would differ
from the indicia applied to containers in another set. For example, each set
would at
least have its own lot number, and possibly other information listed also
(e.g. date of
bottling or creation of master batch, cannabis concentration if it differs
amongst lots,
etc.).
In Figs. 26-28, the container marking station 1658 is located upstream of
(i.e. before)
the filling station 1654. However, in other embodiments, the container marking
station
1658 may be located downstream of (i.e. after) the filling station 1654 and
apply the
labels after the bottles are filled, which may be useful in situations in
which the
controller 1660 cannot determine in advance of filling the exact switching
point from
one master batch (e.g. one master batch or lot number) to another master batch
(e.g.
another master batch or lot number). For example, the controller 1660 may
receive a
signal from sensor 1668a indicating that the liquid in tank 1666a is empty,
near-
empty, or depleted, perhaps such that there is not enough liquid left for even
filling
another bottle, at which point the controller 1660 may perform a supply switch
to the
second master batch in tank 1666b. The controller 1660 would then control the
marking station 1658 downstream of the filling station 1654 to change the
indicia
applied to each bottle to update the lot number 3Y3 to 3Y4 at the point at
which the
cannabis-infused beverage supply switched from tank 1666a to tank 1666b. In
any
case, the control device 1660 synchronizes the operation of the marking
station 1658
with the order in which the stream of individual containers is arranged such
that each
individual container receives an indicia (e.g. lot number) associated with the
particular
lot of cannabinoid-containing substance from which the consumer product in the
container is made.
It will be appreciated that the general method and approach described above in
relation to Figs. 26-28 can also apply to non-beverage cannabis-infused
consumer
products in a similar way, e.g. to cannabis-infused edibles, topicals,
mucoadhesive
169
CA 3062485 2019-11-21
85760071
delivery systems, vape oils, vape oil cartridges, etc. For example, a set of
units of
cannabis-infused consumer product originating from the same lot of cannabinoid-
containing substance may have the same marking (e.g. label) on the packaging
of
each unit, where the marking conveys the same lot number, and that lot number
is
different from the lot number used for a set of units of consumer product
originating
from a different lot of cannabinoid-containing substance. The control device
1660
would synchronizes the operation of the marking station 1658 with the order in
which
the stream of individual packages are arranged such that each individual
package
receives an indicia (e.g. lot number) associated with the particular lot of
cannabinoid-
containing substance from which the consumer product in the package is made.
Fig. 29 is a method of producing cannabis-infused beverages, according to one
embodiment.
In step 1702, a unit of cannabinoid-containing substance is received from a
cannabis
producer 1502. The unit of cannabinoid-containing substance has a particular
dose
and particular lot number. The unit of cannabinoid-containing substance is of
or from
a particular amount, e.g. of or from a particular lot comprising an amount of
cannabinoid-containing substance derived from cannabis plant material to
produce
the lot, which has a particular lot number. The unit of cannabinoid-containing
substance may be associated with a particular extraction process record,
and/or an
extract record, and/or an oil container record, and/or a lab sample record,
and/or an
oil jar record, and/or a lot record, which is/are stored in the ICS 1506 of
the cannabis
producer 1502. However, all of this record information is not necessarily
transferred
to the ICS 1508 of the cannabis processor 1504. In some embodiments, perhaps
only
the lot number and other required information (e.g. dose of the cannabinoid-
containing substance, name or ID of the cannabis producer 1502, etc.) is
provided to
the cannabis processor 1504. The lot number and possibly other information
(e.g. the
dose of the cannabinoid-containing substance and name/ID of cannabis producer
1502) is stored in the ICS 1508, e.g. in a record created and stored in the
ICS 1508 in
association with the received unit of cannabinoid-containing substance.
170
CA 3062485 2019-11-21
85760071
Optionally, in step 1704, processing is performed on the cannabinoid-
containing
substance to put it in a form that is ready for use by the cannabis processor
1504.
Whether step 1704 is performed, and if so, the extent of the processing in
step 1704,
will depend upon the form of the unit of cannabinoid-containing substance as
received from the cannabis producer 1502. For example, if the unit of
cannabinoid-
containing substance is received as a "ready-to-mix" concentrated cannabinoid
emulsion, then step 1704 may not need to be performed at all, whereas if the
unit of
cannabinoid-containing substance is received as a distillate, then step 1704
may be
performed and include mixing the distillate with one or more emulsifiers.
In step 1706, the cannabinoid-containing substance is added to liquid in one
or more
vats. For example, the liquid may be or primarily consist of water. The ICS
1508 may
generate a record documenting the transfer of cannabinoid-containing
substance,
e.g. date and/or time of transfer, amount transferred to each vat, vats
receiving the
cannabinoid-containing substance, etc.
In step 1708, any other required ingredients are added to the cannabis-infused
liquid
in the one or more vats, and/or any required processing is performed, in order
to
produce a master batch of the cannabis-infused beverage. The master batch may
sometimes instead be referred to simply as a "batch". The ICS 1508 may
generate a
record for the master batch, e.g. assigning a master batch number that is
associated
with / linked to the record documenting the transfer of the cannabinoid-
containing
substance and the lot number of the cannabinoid-containing substance.
In step 1710, the master batch is transferred to one or more holding tanks.
The ICS
1508 may generate a record documenting the transfer. The record may include
assigned holding tank number(s) to which the master batch was transferred.
In step 1712, the master batch of cannabis-infused beverage, which is held in
the one
or more holding containers, is transferred into a set of containers, e.g. into
bottles or
cans.
171
CA 3062485 2019-11-21
85760071
In step 1714A, optionally, the workspace, bottle filling machine(s) and lines
and/or
holding tanks are cleaned. Step 1714A could include washing or flushing
certain
components of the bottle filling machine with solvents (for example, water
and/or
ethanol) and/or compressed air. Cleaning/flushing the equipment helps prevent
cross-contamination of toxins/contaminants between lots/batches, and also
isolates
cannabinoids between lots/batches, which further improves traceability of
cannabinoids.
In step 1714B, indicia (e.g. labels having a lot number) are provided, a
respective one
on each container in the set of containers. In some embodiments, the indicia
may be
provided prior to filling the set of containers, e.g. as in Fig. 26, which
shows a
marking station upstream of a filling station. The indicia are markings, and
each
indicia includes an identification (e.g. text, number, and/or machine-readable
code)
that is unique to the set of containers filled with the cannabis-infused
beverage
originating from that particular unit of cannabinoid-containing substance
received
from the cannabis producer 1502. For example, the indicia may include a
consumer
product lot number (e.g. lot number 3Y3 in the examples above). In some
embodiments, each container in the set of containers has the same indicia
(e.g. same
lot number and/or same other information) applied because each container in
the set
of containers has cannabis-infused beverage originating from the same lot of
cannabinoid-containing substance. In some embodiments, the indicia includes
the
dose of cannabis in the beverage, which is labelled as the same for each
container in
the set, e.g. "Cannabis content: 2.5 milligrams THC per bottle".
The ICS 1508 may assign the indicia, and/or may store the indicia, and/or may
control a marking station, e.g. a label maker, to print the indicia on the set
of
containers. In some embodiments, the indicia is the same for all containers in
the set
and is indicative of: the master batch of cannabis-infused beverage used to
fill the
container, and/or the lot of cannabinoid-containing substance used to produce
the
cannabis-infused beverage, and/or the particular dose of cannabis, etc. In
some
embodiments, the indicia links each container in the set back to the same
particular
172
CA 3062485 2019-11-21
85760071
cannabinoid-containing substance (e.g. lot number) received from the cannabis
producer.
In some embodiments, steps 1702 to 1714B are repeated for each subsequent
different lot number of cannabinoid-containing substance received from the
cannabis
producer 1502. A different record and/or indicia is created in the ICS 1508
for each
received lot of cannabinoid-containing substance used to produce a respective
batch
of cannabis-infused beverage containers. In this way, the indicia on a
particular
container of cannabis-infused beverage may be used to trace back to the
particular
cannabinoid-containing substance (e.g. lot number of cannabinoid-containing
substance) used by the cannabis processor 1504 to produce that particular
container
of cannabis-infused beverage, and may also indicate the particular dose, e.g.
particular concentration of the cannabinoid(s) in that particular container,
which in
some embodiments could vary between different lots of cannabinoid-containing
substance. The same indicia may be used for containers holding beverage from
the
same batch (e.g. bottles having cannabis originating from the same lot).
Figs. 26-28 and the descriptions thereof relate primarily to cannabis-infused
beverages. Fig. 30 is a flow diagram illustrating an example method for
applying an
indicia to containers filled with cannabis-infused beverage, according to
another
embodiment. The containers could be glass bottles, plastic bottles, or cans,
for
example. The example method 1720 involves, at 1722, providing a marking
station to
mark with an indicia containers that are filled with cannabis-infused
beverage.
Providing a marking station is not intended to imply that a marking station is
manufactured as part of the example method 1720. A marking station could be
purchased or otherwise acquired, for example.
In some embodiments, the indicia is indicative of a particular amount of a
cannabinoid-containing substance derived from cannabis plant material and
containing one or more cannabinoids, from which the cannabis-infused beverage
is
prepared, and the marking station is configured to receive a succession of
containers
filled with cannabis-infused beverage. The succession of containers is
arranged in
173
CA 3062485 2019-11-21
85760071
successive sets, where each set of containers is filled with cannabis-infused
beverage made from a respective amount of the cannabinoid-containing
substance.
At 1724, a first indicia is applied at each container from a first set. The
first indicia is
associated with a first amount of cannabinoid-containing substance from which
the
cannabis-infused beverage dispensed in the first set of containers is made.
For
completeness, it is noted that the cannabis-infused beverage could be
dispensed into
containers by any of various types of dispensing or container filling
equipment, and
be conveyed or otherwise provided to the marking station for marking. Applying
the
indicia at 1724 could involve, for example, printing the indicia onto the
containers,
otherwise marking the indicia on the containers, or generating labels that
include the
indicia and affixing the labels to the containers.
A transition from a first set of containers to a second set of containers in
the
succession of containers is detected at 1726. The first set of containers is
filled with
cannabis-infused beverage prepared from a first amount of cannabinoid-
containing
substance and the second set of containers is filled with cannabis-infused
beverage
prepared from a second amount of cannabinoid-containing substance. This
detection
could be made based on a count of a predetermined number of containers, a
dynamically determined number of containers that can be filled from an
available
supply of cannabinoid-containing substance, and/or other production,
processing, or
control parameters.
The example method 1720 also involves controlling the marking station at 1728,
to
apply the first indicia to the last container of the first set in the
succession and to
apply a second indicia to the next container in the succession of containers,
which is
the first container of the second set. The first indicia is associated with
the first
amount as noted above, and the second indicia is associated with the second
amount.
A beverage production run could include cannabis-infused beverages that are
prepared from different amounts, which could be different lots for example, of
cannabinoid-containing substance. The description of Fig. 30 above refers to
first and
174
CA 3062485 2019-11-21
85760071
second amounts, but there could be additional amounts as well. Subsequent
transitions between sets of containers that are filled with cannabis-infused
beverage
prepared from different amounts of cannabinoid-containing substance could be
detected, to initiate marking system control and indicia changes for
additional sets of
containers. Containers that are filled with cannabis-infused beverage prepared
from
different amounts of cannabinoid-containing substance can therefore be
labelled with
respective different indicia, to allow each container to be traced to the
amount of
cannabinoid-containing substance from which the cannabis-infused beverage that
it
contains was prepared.
A processor-readable storage medium could be used in implementing at least the
operations at 1724, 1726, 1728, with processor-executable instructions being
stored
on such a medium. The instructions, when executed by a processor, cause the
processor to perform a method. Execution of the instructions could cause a
computing device that includes the processor to implement a system configured
to, in
some embodiments: control a marking station to apply indicia as shown at 1724
and
described above, detect one or more transitions as shown at 1726 and described
above, and control a marking system to change indicia between sets of
containers as
shown at 1728 and described above.
An automated marking system could include such a computing device, as well as
a
marking station such as an automated labelling system. These and/or other
possible
implementation options in respect of a system that could be configured or used
to
perform a method consistent with Fig. 30 could be or become apparent. Figs. 26-
28,
for example, illustrate one possible embodiment of a system in which
components
could be configured to perform such a method.
In an embodiment, a variation of the example method 1720 relates to a method
for
bottling a cannabis-infused beverage. In the context of such a method, or a
system
that implements or performs such a method, the term "bottling" is intended to
be
generally indicative of filling containers, which could include bottles such
as glass
bottles and/or plastic bottles, and could also or instead include other types
of
175
CA 3062485 2019-11-21
85760071
containers such as cans, for example. In general, containers could be or
include one
or more of: glass containers, plastic containers, and/or other containers such
as
aluminum containers.
Fig. 30 illustrates, at 1722, providing a marking station. In some
embodiments, a
filling line including a filling station, a container marking station and a
control device is
provided. Providing a filling line is not intended to imply that a filling
line is
manufactured as part of a bottling method. A filling line or filling line
equipment could
be purchased or otherwise acquired, and thereby be provided for use in a
bottling
method, as noted above.
A filling station is operative to fill containers, and various examples of
fillings stations
will be apparent to those familiar with bottling or filling lines. Although
the particular
structure of a filling station may vary depending on the type(s) of containers
to be
filled, a filling station includes a supply or input stage or substation to
prepare or
receive the beverage(s) to be with which containers are to be filled, a
dispensing
station or substation including a dispenser or set of dispensers such as
nozzles to
dispense the beverage(s) into one or more containers at a time, and an output
stage
through which filled containers are output for further handling. Closing of
containers,
for example, could be performed by a closing stage or substation of a filling
station or
by separate equipment on a filling line.
Examples of marking stations are provided elsewhere herein.
The control device of a filling line is configured to control an operation of
the container
marking station, and could also control other filling line components. A
control device
could be implemented, for example, as part of a production control system.
Examples
of control devices, such as controllers, are provided elsewhere herein.
Although not shown in Fig. 30, some embodiments could involve filling
containers, at
the filling station, with cannabis-infused beverage supplied from a master
batch of
cannabis-infused beverage. The master batch could be prepared from an amount
of
cannabis-containing substance derived from cannabis plant material. The
cannabis-
176
CA 3062485 2019-11-21
85760071
containing substance contains one or more cannabinoids. The master batch
includes
a quantity of cannabis-infused beverage to fill a plurality of containers, and
the filling
station is configured to perform a supply switch from a first master batch to
a second
master batch of cannabis-infused beverage. The supply switch could switch from
one
supply source to another, and involve controlling one or more valves for
example. A
first set of containers is filled with cannabis-infused beverage drawn from
the first
master batch and, after the supply switch, a second set of containers is
filled with
cannabis infused beverage drawn from the second master batch.
An indicia could be applied on each container at the marking station, as
discussed
above with reference to operation 1724 for example. In the present embodiment
involving multiple master batches, the indicia is indicative of the master
batch of the
cannabis-infused beverage supplying the filling station when the container is
filled by
the filling station. In some embodiments, the indicia is, includes, conveys,
or is
indicative of a lot number.
The example method 1720 includes controlling the marking station at 1728, and
a
bottling method could similarly involve controlling, with the control device
of the filling
line, the operation of the marking station such that when a supply switch is
performed
from the first master batch to the second master batch, a marking switchover
from a
first indicia to a second indicia is performed by the marking station. Marking
station
operation is controlled to perform the marking switchover such that containers
filled
with cannabis-infused beverage drawn from the first master batch are marked
with a
first indicia associated with the first master batch, and containers filled
with cannabis-
infused beverage drawn from the second master batch are marked with a second
indicia associated with the second master batch. Examples of indicia and how
indicia
could be applied to containers, are disclosed elsewhere herein.
A bottling method could also involve other operations, such as preparing a
master
batch from an amount of cannabis-containing substance. Preparing the master
batch
could include diluting the cannabis-containing substance with a diluent. In an
embodiment, the diluent includes water.
177
CA 3062485 2019-11-21
85760071
In some embodiments, a bottling method includes adjusting an amount of diluent
added to the cannabis-containing substance to achieve a target concentration,
which
could be a predetermined concentration or a dynamically determined
concentration,
of the cannabinoid in the master batch. A method could involve holding the
prepared
master batch, and/or a master batch that was provided in a prepared and
filling-ready
form, in or into a holding tank. The filling station could then be supplied
with
cannabis-infused beverage from the holding tank.
As part of a bottling method, multiple holding tanks, each configured to hold
a
respective master batch of cannabis-infused beverage, could be provided. As
noted
elsewhere herein for other components such as a marking station and a filling
line,
providing holding tanks could involve purchasing or otherwise acquiring
holding tanks
and not necessarily manufacturing holding tanks.
Some embodiments involve providing, by manufacturing or otherwise, a supply
selection valve in fluid communication with the holding tanks and with the
filling
station. The supply selection valve is capable of selectively acquiring any of
a number
of supply positions, with each supply position associating a respective
holding tank
with the filling station to supply the filling station from the respective
holding tank.
Such a supply selection valve could be manually operable. In an automated
bottling
system, however, the control device of the filling line could control the
supply
selection valve and direct the supply selection valve to acquire a selected
supply
position among its supply positions.
For example, in an embodiment, the control device is configured to command the
supply selection valve to switch a supply position to perform a supply switch
from a
first holding tank holding the first master batch to a second holding tank
holding the
second master batch. The control device could command the supply selection
valve
to switch the supply position to perform the supply switch from the first
holding tank to
the second holding tank when sensing that the first holding tank is empty, or
is at or
below a minimum threshold volume of the first master batch.
178
CA 3062485 2019-11-21
85760071
In some embodiments, the first holding tank and the second holding tank
include
respective level sensors generating outputs indicative of the level of
cannabis-infused
beverage in the respective holding tanks, and the control device receives the
outputs
of the respective level sensors. The control device is thereby able to
determine fill
level of a current supply holding tank from which containers are currently
being filled,
and switch supply to another holding tank when the current supply holding tank
runs
low or empty. A supply switch could be made when the beverage volume remaining
in the current supply holding tank is at or below a volume required to fill a
certain
number of containers, for example. The number of containers from which a
minimum
volume threshold is determined could be one, to minimize production loss or
waste,
or more than one, to potentially reduce the likelihood of a holding tank
running dry
and interrupting production. Different minimum volumes could be used for
different
holding tanks and/or different master batches.
Supply switching could alternate between holding tanks, and not switch only
from one
holding tank to another. For example, a filling line that is operable with
either of two
holding tanks could switch supply from a first holding tank to a second
holding tank
when a volume of beverage in the first holding tank is at or below a minimum
volume.
The first holding tank could then be refilled with a further master batch, a
supply
switch back to the first holding tank could be performed when a volume of
beverage
in the second holding tank is at or below a minimum volume, and then the
second
holding tank could be refilled with another master batch. Filling lines that
work in
conjunction with more than two holding tanks or supply sources are also
contemplated.
In some embodiments, the filling station receives a succession of empty
containers
and fills the empty containers with cannabis-infused beverage. Containers
could be
filled in succession, one at a time. In other embodiments, the filling station
includes
nozzles, or other types of dispensers, so that a set of multiple empty
containers can
be filled simultaneously.
179
CA 3062485 2019-11-21
85760071
The marking station could be or include, for example, a labeling station for
applying a
label on each container, in which case the label bears the indicia that is to
be applied
to a container. In such an implementation, the marking station could include a
supply
of labels and be configured to apply to each container a label from the supply
of
labels.
The marking station could be configured to apply the indicia to a label from
the supply
of labels and apply the label to the container. In some embodiments, the
labels in the
supply of labels are pre-printed with indicia. A label supply could include
sets of
labels that are pre-printed with respective different indicia, and the marking
station
could then select one of the sets of labels for a container based on the
indicia with
which the container is to be marked.
Label-based marking is one illustrative embodiment. The marking station could
also
or instead print the indicia on a container.
Whether label-based marking or another type of marking is applied by the
marking
station, the marking station could apply the indicia to each container before
the
container is filled with cannabis-infused beverage. The marking station could
apply
the indicia to each container after the container is filled with cannabis-
infused
beverage in some embodiments.
A control device could include, or at least access, a computer readable
storage, and
be configured to determine a number of containers filled with cannabis-infused
beverage from a particular master batch and store in the computer readable
storage
the determined number. This could be useful, for example, in tracking
productivity
and/or inventory control.
The control device could include an input to receive an identifier associated
with the
amount of cannabis-containing substance, and be further operative to link in
the
computer readable storage the determined number of containers filled with
cannabis-
infused beverage made from the amount of cannabis-containing substance and the
identifier. The control device could also or instead be operative to link, in
the
180
CA 3062485 2019-11-21
85760071
computer readable storage, the indicia applied on the containers filled with
cannabis-
infused beverage made from the amount of cannabis-containing substance and the
identifier associated with the predetermined amount of cannabis-containing
substance.
A processor-readable storage medium could be used in implementing at least
some
of the operations in these variations of the example method 1720, with
processor-
executable instructions being stored on such a medium. The instructions, when
executed by a processor, cause the processor to perform a method. Execution of
the
instructions could cause a computing device that includes the processor to
implement
a system configured to, in some embodiments: fill containers, apply indicia,
and
control a marking station as discussed above and/or elsewhere herein.
An automated marking system could include such a computing device, as well as
a
filling station and a marking station such as an automated labelling system.
These
and/or other possible implementation options in respect of a system that could
be
configured or used to perform a method consistent with these variations in the
example method illustrated in Fig. 30 could be or become apparent. Figs. 26-
28, for
example, illustrate one possible embodiment of a system in which components
could
be configured to perform such a method.
Fig. 31 is a method of producing a cannabis-infused consumer product,
according to
one embodiment. The cannabis-infused consumer product may be any one of the
examples described above, e.g. an edible, beverage, topical, mucoadhesive
delivery
system, vape oil, vape oil cartridge, etc.
In step 1732, a unit of cannabinoid-containing substance is received from a
cannabis
producer 1502. The unit of cannabinoid-containing substance has a particular
dose
and particular lot number. The unit of can nabinoid-containing substance is of
or from
a particular amount, e.g. of or from a particular lot comprising an amount of
cannabinoid-containing substance derived from cannabis plant material to
produce
the lot, which has a particular lot number. The unit of cannabinoid-containing
substance may be associated with a particular extraction process record,
and/or an
181
CA 3062485 2019-11-21
85760071
extract record, and/or an oil container record, and/or a lab sample record,
and/or an
oil jar record, and/or a lot record, which is/are stored in the ICS 1506 of
the cannabis
producer 1502. However, all of this record information is not necessarily
transferred
to the ICS 1508 of the cannabis processor 1504. In some embodiments, perhaps
only
the lot number and other required information (e.g. dose of the cannabinoid-
containing substance, name or ID of the cannabis producer 1502, etc.) is
provided to
the cannabis processor 1504. The lot number and possibly other information
(e.g. the
dose of the cannabinoid-containing substance and name/ID of cannabis producer
1502) is stored in the ICS 1508, e.g. in a record created and stored in the
ICS 1508 in
association with the received unit of cannabinoid-containing substance.
Optionally, in step 1734, processing is performed on the cannabinoid-
containing
substance to put it in a form that is ready for use by the cannabis processor
1504.
Whether step 1704 is performed, and if so, the extent of the processing in
step 1704,
will depend upon the form of the unit of cannabinoid-containing substance as
received from the cannabis producer 1502.
Optionally, in step 1736, the cannabinoid-containing substance is diluted with
a
diluting agent. In some embodiments, the diluting agent may be water and/or
oil.
In step 1738, the cannabinoid-containing substance is combined with other
ingredients to produce a master batch of a consumer product. The ICS 1508 may
generate a record for the master batch, e.g. assigning a master batch number
that is
associated with / linked to the record documenting the transfer of the
cannabinoid-
containing substance and the lot number of the cannabinoid-containing
substance.
An identifier, e.g. a lot number, may be stored in the ICS 1508 and/or in
memory in a
control device (e.g. control device 1660) in association with the master batch
and/or
in association with units of consumer product produced from the master batch.
In step 1740, the master batch is dispensed into one or more packages. In some
embodiments, the packages are containers or bottles depending upon the
consumer
product. Each package holds a portion of the master batch.
182
CA 3062485 2019-11-21
85760071
In step 1742, indicia (e.g. a lot number) is applied on individual packages by
feeding
a stream of individual packages to a marking station. A marking station is
sometimes
called a marking unit.
In step 1744A, optionally, the workspace, bottle filling machine(s) and lines
and/or
holding tanks are cleaned. Step 1744A could include washing or flushing
certain
components of the bottle filling machine with solvents (for example, water
and/or
ethanol) and/or compressed air. Cleaning/flushing the equipment helps prevent
cross-contamination of toxins/contaminants between lots/batches, and also
isolates
cannabinoids between lots/batches, which further improves traceability of
cannabinoids.
In step 1744B, steps 1732-1742 are repeated for each unit of cannabinoid-
containing
substance, and in step 1742 a control device (e.g. control device 1660)
distinguishes
between individual packages holding a unit of consumer product made from
different
lots of cannabinoid-containing substance. The marking station is controlled by
the
control device to apply to each individual package an indicia (e.g. lot
number) derived
from the identifier (e.g. lot number) of the respective lot of cannabinoid-
containing
substance from which the consumer product in the package was made.
In some embodiments, when a master batch of a consumer product is being
dispensed into individual packages, there may result in a residual volume of
consumer product from the master batch that is less than the volume of
consumer
product required to fill the individual package to capacity. A control device,
e.g.
control device 1660 described earlier, may obtain the number of individual
packages
that are or can be filled to capacity from that master batch. The number may
be
stored in machine-readable storage (e.g. memory 1664) accessible by the
control
device. In some embodiments, the control device controls the marking station
to
operate the marking station a corresponding number times to apply to each
individual
package from the master batch an indicia associated with/linked back to the
lot of
cannabinoid-containing substance from which the master batch (and each
consumer
product in each package originating from the master batch) was made. In this
way,
183
CA 3062485 2019-11-21
85760071
the control device controls the marking station to apply the correct indicia
(e.g. lot
number) to each package across different master batches originating from
different
lots of cannabinoid-containing substance.
In some embodiments, the dispensing of the master batch is performed such that
the
consumer product in each package of a set of packages originates from a same
single amount (e.g. same lot) of cannabinoid-containing substance. In some
embodiments, the number of packages may be counted by a control device and
stored.
In another embodiment, a method for manufacturing and packaging a cannabis-
infused consumable product made from a cannabis-containing substance could
include at least some operations similar to those in Fig. 30. For example, a
manufacturing method could include providing one or more manufacturing inputs,
such as multiple amounts of cannabis-containing substance that contains one or
more cannabinoids. Each amount of cannabis-containing substance could be
derived
from cannabis plant material, and be associated with an identifier allowing
distinguishing of one amount from another amount. Extract identifiers and lot
identifiers as disclosed elsewhere herein are examples of identifiers with
which each
amount could be associated to enable amounts to be distinguished from each
another.
One or more manufacturing line or system components could also be provided.
For
example, a manufacturing and packaging method could involve providing a
control
device that has, or at least has access to, a machine-readable storage. A
method
could then include storing, in the machine-readable storage, identifiers
associated
with respective ones of the amounts of cannabis-containing substance.
In manufacturing a consumable product, a method could involve, for example
diluting
each amount of cannabis-containing substance with a diluent or diluting agent,
such
as water or oil, to produce a master batch of consumable product. The master
batch
could then be dispensed into a set of packages, with each package holding a
portion
of the master batch. A marking unit or station could be provided, as shown at
1722
184
CA 3062485 2019-11-21
85760071
for example, and a method could include applying an indicia on individual
packages,
as shown by way of example at 1744B. Applying an indicia could include feeding
a
stream of individual packages to a marking unit or station, and
distinguishing, in the
stream, between individual packages holding a consumable product made from
different amounts of cannabis-containing substance and controlling the marking
unit
or station, as shown at 1728 for example, with the control device to apply to
each
individual package an indicia derived from the identifier of the respective
amount from
which the consumable product in the package was made. The indicia could be,
include, convey, or indicate the identifier of the amount.
In some embodiments, the cannabis-infused consumable product is vaping oil. In
such embodiments, each package could be a vaping cartridge containing vaping
oil.
Another example of a consumable product is a cannabis-infused beverage.
The consumable product is an emulsion in some embodiments.
Dispensing of the master batch could be performed such that the consumable
product in each package originates from a single amount of cannabis-containing
substance. This could involve the control device controlling filling or
dispensing
equipment to fill the packages from a single source of diluted concentrate,
for
example. A different source could subsequently be used to fill another set of
packages.
A method could include determining, by counting for example, the number of
packages produced from a particular amount of cannabis-containing substance
and
storing the counted number in the machine-readable storage. The number of
packages could be useful for production monitoring and/or inventory control,
for
example.
Another embodiment of a method for manufacturing and packaging a cannabis-
infused consumable product made from a cannabis-containing substance also
involves providing multiple amounts of cannabis-containing substance
containing one
or more cannabinoids, with each dose being derived from cannabis plant
material;
185
CA 3062485 2019-11-21
85760071
providing a control device having a machine-readable storage; storing in the
machine-readable storage identifiers associated with respective ones of the
amounts
of cannabis-containing substance to allow distinguishing one amount from
another
amount; diluting each amount of cannabis-containing substance with a diluting
agent
to produce respective master batches of consumable product; and dispensing the
master batches into respective sets of individual packages, with each package
of a
given set holding a portion of the respective master batch, as described in an
example above. In the present embodiment, a stream of individual packages is
fed to
a marking unit, and the stream is arranged in an order determined by which
master
batch is the source of the consumable product held in each individual package.
Packages for which one master batch is the source could be fed to the marking
unit
first, followed by packages for which a different master batch is the source,
for
example. Other arrangements are also possible.
Under control of the control device, the operation of the marking unit is
synchronized
with the order in which the stream of individual packages is arranged, such
that each
individual package receives an indicia associated with the particular dose
from which
the consumable product in the package is made. Such synchronization could be
based on a number of packages for which each amount is the source. For
example, if
one amount was the source for "x" packages, then the marking unit, the control
device, or another component could count packages until "x" packages have
received
an indicia associated with that amount, and then the control device could
control the
marking unit to change the indicia to a different indicia associated with a
different
amount of cannabis-containing substance that is the source for subsequent
packages
in the package stream.
In another embodiment, a method for manufacturing and packaging a cannabis-
infused consumable product made from a cannabis-containing substance involves
operations of providing multiple amounts of cannabis-containing substance,
providing
a control device, and diluting each amount of cannabis-containing substance
with a
diluent or diluting agent as described above. In an embodiment, each amount is
diluted to produce respective master batches of consumable product. For each
186
CA 3062485 2019-11-21
85760071
master batch, the master batch is dispensed into a set of individual packages,
with
each package holding a portion of the master batch, and a residual volume of
consumable product from the master batch that is less than the volume of
consumable product required to fill the individual package to capacity is
withheld from
dispensing into an individual package.
For one or more master batches, the number of individual packages filled to
capacity
from the master batch in the respective set of individual packages could be
determined, and the number could then be stored in the machine-readable
storage.
A stream of individual packages could be fed to a marking unit, which is
controlled
with the control device. Controlling the marking unit could include deriving
from the
machine readable storage the number of filled packages and operating the
marking
unit a corresponding number of times to apply to each individual package in
the set
an indicia linked to the particular amount of cannabis-containing substance
from
which the consumable product in the package is made. In this manner, sets of
packages including consumable product produced from respective different
amounts
of cannabis-containing substance have an indicia, applied by that marking
unit, which
is linked to the respective amount.
In some embodiments, residual amounts from multiple cannabis-containing
substance amounts could be combined in order to collect sufficient volume to
fill one
or more packages. An indicia, or multiple indicia, linked to each of the
multiple
amounts of cannabis-containing substance, could then be applied to such
packages
by the marking unit under control of the control device.
Residual amounts could instead be designated as waste and collected for
destruction. The residual amounts could be measured and recorded, and used in
production monitoring and/or inventory control, for example.
Features disclosed elsewhere herein could be implemented in conjunction with
such
a manufacturing and packaging method. For example, such a method could be
employed in manufacturing and packaging a cannabis-infused consumable product
in
187
CA 3062485 2019-11-21
85760071
the form of vaping oil. In some embodiments, each package for vaping oil could
be a
vaping cartridge containing vaping oil. Oil is one example of a diluting agent
that
could be used in manufacturing vaping oil.
A cannabis-infused beverage is another example of a consumable product. In
some
embodiments, the diluting agent for manufacturing a cannabis-infused beverage
is
water.
Yet another example of a consumable product is an emulsion.
In a manufacturing method, the step of dispensing the master batch could be
performed such that the consumable product in each package originates from a
single amount of cannabis-containing substance.
Some embodiments could include counting the number of packages produced from a
particular amount of cannabis-containing substance and storing the counted
number
in machine-readable storage.
The cannabis-containing substance could be a cannabis extract in some
embodiments.
As noted at least above in respect of other embodiments, a processor-readable
storage medium could be used in implementing at least some of the operations
in
these example methods relating to cannabis-infused consumer products, with
processor-executable instructions being stored on such a medium. The
instructions,
when executed by a processor, cause the processor to perform a method.
Execution
of the instructions could cause a computing device that includes the processor
to
implement a system configured to, in some embodiments, perform at least some
of
the method operations discussed above and/or elsewhere herein.
A production system could include such a computing device, as well as other
components involved in producing a cannabis-infused consumer product. These
and/or other possible implementation options in respect of a system that could
be
configured or used to perform a method consistent with these example methods
188
CA 3062485 2019-11-21
85760071
disclosed herein could be or become apparent. Figs. 26-28, for example,
illustrate
one possible embodiment of a system in which components could be configured to
perform such methods.
In some embodiments, the cannabis-containing substance is a food additive. A
food
additive provided herein comprising an emulsion or nanoemulsion
microencapsulation system may be formed using any of the techniques available
to
fabricate emulsions and nanoemulsions. The techniques available are commonly
classified as either high or low energy approaches.
High energy approaches use mechanical devices known as "homogenizers" that
generate intense disruptive forces that mix the oil and water phases together,
as well
as break larger droplets into smaller ones. 0/W emulsions are usually prepared
by
homogenizing an oil phase and a watery phase together in the presence of a
water-
soluble hydrophilic emulsifier. A variety of specialized homogenization
equipment is
available for fabricating emulsions and nanoennulsions that include, but are
not
limited to, high shear mixers, high pressure valve homogenizers,
microfluidizers,
colloid mills, ultrasonic homogenizers, and membrane and microchannel
homogenizers.
High shear mixers are a type of rotor-stator device that homogenizes oil,
water, and
other ingredients in a batch process. Typically, the droplets produced by a
high shear
mixer range between about 1 and about 10 pm in diameter. A suitable vessel may
contain as a few cm3 or as large as several m3. The rapid rotation of the
mixing head
generates a combination of longitudinal, rotational, and radial velocity
gradients in the
fluids, which disrupts the interfaces between the oil and water phases,
causing the
liquids to become intermingled, and breaks the larger droplets into smaller
ones.
Efficient homogenization is achieved when the horizontal and vertical flow
profiles
distribute the liquids evenly throughout the vessel, which can be facilitated
by having
baffles fixed to the inside walls of the vessel. The design of the mixing head
determines the efficiency of the homogenization process, and a number of
different
189
CA 3062485 2019-11-21
85760071
types are available for different situations, for example, blades, propellers,
and
turbines.
High-pressure valve homogenizers are used to produce fine emulsions from pre-
existing emulsions ("coarse emulsion"), with emulsion droplets as small as 0.1
pm.
The homogenizer has a pump that pulls the coarse emulsion into a chamber on
its
backstroke and then forces it through a narrow valve at the end of the chamber
and
on its forwards stroke it experiences a combination of intense disruptive
forces that
cause the larger droplets to be broken down to smaller ones. The flow regime
that is
responsible for disrupting the droplets in a particular high pressure valve
homogenizer depends on the characteristics of the material being homogenized,
the
size of the homogenizer, and the design of the homogenization nozzle.
Microfluidization creates emulsions with very fine droplets whose diameter can
be
less than 0.1 pm. This type of homogenizer typically consists of a fluid inlet
(single or
double), some kind of pumping device, and an interaction chamber containing
two
channels. Fluids are introduced into the homogenizer, accelerated to a high
velocity
and then made to simultaneously impinge with each other on a solid surface,
which
causes the fluids to intermingle and disrupt larger droplets.
Colloid mills are used to homogenize medium and high viscosity liquids. A
colloid mill
typically contains two disks: a rotor (a rotating disk) and a stator (a static
disk). The
liquids and other ingredients to be homogenized are usually fed into the
center of the
colloid mill in the form of a pre-existing emulsion. The intensity of the
shear stresses
(and therefore the droplet disruption forces) can be altered by varying the
rotation
speed, gap thickness, rotor/stator type, and throughput to reduce droplet
sizes.
Typically, colloid mills can be used to produce emulsions with droplet
diameters in the
range between about 1 and about 5 pm.
Ultrasonic homogenizers use high-intensity ultrasonic waves that generate
intense
shear and pressure gradients within a material that disrupt droplets mainly
through
cavitation and turbulent effects. The present invention can use any of the
available
190
CA 3062485 2019-11-21
85760071
methods that are available for generating high-intensity ultrasonic waves
including,
but not limited to, piezoelectric transducers and liquid jet generators.
Membrane homogenizers can be used in two main ways to process emulsions,
direct
homogenization and premix homogenization. Direct homogenization involves
forming
an emulsion directly from the separate oil and water phases in the presence of
a
suitable emulsifier. Premix homogenization involves reducing the size of the
droplets
present within an existing coarse emulsion. The droplet size attained depends
on the
membrane pore size, the oil-water interfacial tension, the applied pressure,
the flow
profile of the continuous phase, and the type and amount of emulsifier used.
Low energy approaches to produce emulsions and nanoemulsions rely on the
spontaneous formation of oil droplets in surfactant-oil-water mixtures which
either
their composition or environment is altered in a controlled way. Examples of
low
energy methods include, but are not limited to, spontaneous emulsification
methods,
emulsion inversion point methods, and phase inversion temperature methods.
Spontaneous emulsification involves titrating a mixture of oil and water-
soluble
surfactant into a water phase with continuous stirring. Small oil droplets are
spontaneously formed at the oil-water boundary as the surfactant molecules
move
from the oil phase to the water phase. The spontaneous emulsification method
has
been used widely within the pharmaceutical industry to encapsulate and deliver
lipophilic drugs. Such systems are known as either self-emulsifying drug
delivery
systems (SEDDS) or self-nanoemulsifying drug delivery systems (SNEDDS)
depending on the droplet size produced. Self-emulsifying formulations are
readily
dispersed in the gastrointestinal tract, where the motility of the stomach and
small
intestine provides the agitation necessary for emulsification.
Emulsion inversion point methods involve titrating water into a mixture of oil
and
water-soluble surfactant with continuous stirring. As increasing amounts of
water are
added, a W/O emulsion is initially formed, then an 0/W/0 emulsion, and then an
0/W
emulsion.
191
CA 3062485 2019-11-21
85760071
Phase inversion temperature (PIT) methods rely on heating a surfactant-oil-
water
mixture around or slightly above its PIT and the quench cooling with
continuous
stirring. When the emulsion passes through the PIT, the optimum curvature
tends
towards unity, thereby leading to an ultralow interfacial tension and a highly
dynamic
interface. For a general overview of emulsification technology, see, e.g.,
McClements, David J., Food Emulsions: Principles, Practices, and Techniques,
3rd ed
(Boca Raton, FL: CRC Press, 2016).
In some embodiments, the herein described emulsion of cannabinoids may
include,
for example, per total volume of emulsion up to 1g/ml, up to 750 mg/ml, up to
700
mg/ml, up to 650 mg/ml, up to 600 mg/ml, up to 550 mg/ml, up to 500 mg/ml, up
to
450 mg/ml, up to 400 mg/ml, up to 350 mg/ml, up to 300 mg/ml, up to 250 mg/ml,
up
to 200 mg/ml, up to 150 mg/ml, up to 100 mg/ml, up to 50 mg/ml, up to 40
mg/ml, up
to 35 mg/ml, up to 30 mg/ml, up to 25 mg/ml, up to 20 mg/ml, or up to 15 mg/ml
of a
specific cannabis extract such as THC, CBD, terpene (e.g., D-limonene) or any
mixtures thereof, and the like.
In some embodiments, once a suitable emulsion of the cannabinoid has been
produced, the emulsion is dehydrated to form a powder, typically using spray
drying.
For example, the emulsion may be dried to obtain a water activity (aw) of less
than
0.6, for example 0.04 s. aõ, .s. 0.3. Water activity may be measured using an
Aqualab
Water Activity Meter 4TE (Decagon Devices, Inc., U.S.A.). For extra
protection, the
resulting powder can be atomized and coated with a secondary layer, typically
a high
melting fat or starch.
In some embodiments, the food additive is a beverage additive which includes
the
herein described emulsion of cannabinoid. Dilution or infusion of the beverage
additive in a cannabinoid-less beverage or blending with a beverage base
results in a
beverage product comprising at least 0.002 mg/ml of cannabinoid in total
volume of
the beverage product. For example, the beverage product may include from 0.002
mg/ml to about 1 mg/ml of cannabinoid in volume of the beverage product.
192
CA 3062485 2019-11-21
85760071
In some embodiments, the food additive is a beverage additive which includes
the
herein described emulsion of cannabinoid. Dilution or infusion of the beverage
additive in a cannabinoid-less beverage or blending with a beverage base
results in a
beverage product comprising at least 0.002 mg/ml of cannabinoid in volume of
the
beverage product, the beverage product having a turbidity of less than 0.05 cm-
1 at
600 nm.
In some embodiments, the food additive is a beverage additive which includes
the
herein described emulsion of cannabinoid. Dilution or infusion of the beverage
additive in a cannabinoid-less beverage or blending with a beverage base
results in a
beverage product comprising at least 0.002 mg/ml of cannabinoid in volume of
the
beverage product, the beverage product having a viscosity selected in the
range of
from 50 mPas (for juice-like beverages) to 1500 mPas (for more honey-like
beverages, such as fruit juice concentrates) measured at room temperature. In
some
embodiments, the beverage product may have a viscosity which is substantially
the
same as that one of the cannabinoid-less beverage.
In some embodiments, the food additive is a beverage additive which includes
the
herein described emulsion of cannabinoid. Dilution or infusion of the beverage
additive in a cannabinoid-less beverage or blending with a beverage base
results in a
beverage product comprising at least 0.002 mg/ml of cannabinoid in volume of
the
beverage product, the beverage product having an odor index which is
substantially
the same as that one of the cannabinoid-less beverage. Odor index can be
determined based on odor intensity index measuring method known in the art
with
which practical odor intensity can be objectively and easily measured, for
example
but without being limited thereto, as described in Somchai Rice and Jacek
Koziel,
PLOS ONE 10(12): e0144160.
In some embodiments, the food additive is a beverage additive which includes
the
herein described emulsion of cannabinoid. Dilution or infusion of the beverage
additive in a cannabinoid-less liquid beverage results in a beverage
comprising at
least 0.002 mg/ml of cannabinoid in total volume of the liquid beverage and
having a
193
CA 3062485 2019-11-21
85760071
taste index which is substantially the same as that one of the cannabinoid-
less
beverage. Testing methods for assessing taste index are known in the art, and
some
of which are described in McDaniel, ACS Symposium Series, Vol. 289, chapter 1,
p.
1-10.
In one embodiment, the expression "substantially the same" as used herein when
referring to a tested parameter of a cannabinoid-containing beverage when
compared
to the same parameter tested in the cannabinoid-less beverage generally refers
to
the value resulting from the test being more or less 20%, identical, or more
or less
15% identical, or more or less 10% identical. Typically, such will occur when
a
sensory evaluation (by a subject, e.g., tasting, smelling, looking, touching)
will not
detect any significant variations and yet, depending on the instrumentation
used, may
result in slight measured variations, e.g., more or less 20%, identical, or
more or less
15% identical, or more or less 10% identical. However, because it is the
sensory
evaluation which likely has a more significant effect on the user experience
and/or
derived commercial benefit, even such slight variations will be deemed to be
"substantially the same" for the purposes of the user's perspective, i.e., the
consumer.
In some embodiments, a cannabinoid may be microencapsulated in micelles.
Micelles consist of small clusters of surfactant molecules that self-assemble
into a
structure where the hydrophobic tails are located in the interior and the
hydrophilic
heads are located at the exterior. Micelles are thermodynamically stable
systems
under a particular range of compositional and environmental conditions, and
should
therefore form spontaneously. Nevertheless, some form of energy often has to
be
applied during their formation (such as simple mixing) to overcome kinetic
energy
barriers to the self-assembly of the surfactant molecules. Micelles are one of
the
smallest colloidal particles that are widely used as delivery systems, with
diameters
typically in the range from about 5 to 20 nm. Nonpolar active agents can be
solubilized within the hydrophobic interior of micelles, whereas amphiphilic
active
agents can be incorporated at their exterior, with the loading capacity
depending on
the molecular dimensions of the active agents and the optimum curvature of the
194
CA 3062485 2019-11-21
85760071
surfactant monolayer. Larger thermodynamically stable micelles (e.g.,
diameters up
to 100 nm) may also contain an oil phase and possibly a co-surfactant. Termed
"microemulsions" by IUPAC, larger thermodynamically stable micelles can
solubilize
higher levels of nonpolar active agents. They are usually fabricated from one
or more
small-molecule surfactants, but amphiphilic block copolymers can also be used.
In some embodiments, a cannabinoid may be microencapsulated in solid lipid
nanoparticles or nanostructured lipid carriers. Solid lipid nanoparticles
(SLNs) have
similar structures to nanoemulsions (or emulsions), but the oil phase is
crystallized
rather than liquid. SLNs are typically fabricated by preparing an oil-in-water
nanoemulsion at a temperature above the melting point (Tm) of the oil phase,
and
then cooling the system well below Tm to promote droplet crystallization. In
principle,
the crystallization of the lipid phase slows down molecular diffusion
processes inside
the particles, which may help to protect an encapsulated active agent from
chemical
degradation. SLNs have proven to be useful delivery systems for many
applications
in the pharmaceutical industry, where they are mainly used to encapsulate
hydrophobic drugs. However, if the lipid phase is not carefully selected there
can be
appreciable challenges to their utilization for this purpose. Lipids that form
highly
regular crystalline structures (such as pure triacylglycerols) have a tendency
to expel
other nonpolar substances when they undergo a liquid-to-solid transition.
Moreover,
there may be an appreciable change in the morphology of the lipid
nanoparticles,
from spherical to irregular, when the lipid phase crystallizes or undergoes a
polymorphic transition. As a result of the increase in particle surface area,
there may
be insufficient emulsifier to coat the particles, which leads to extensive
aggregation.
These problems can be overcome by using nanostructured lipid carriers (NLCs).
In
this case, a lipid phase is selected that forms more irregular crystals when
it solidifies,
which leads to less expulsion of encapsulated active agents and less particle
aggregation.
In some embodiments, a cannabinoid may be microencapsulated in liposomes,
nanoliposomes, or niosomes. Liposomes (diameter > 100 nm) and nanoliposomes
(diameter < 100 nm) are colloidal systems that are composed of particles made
up of
195
CA 3062485 2019-11-21
85760071
concentric layers of phospholipid bilayers. Niosomes are formed when non-ionic
surfactants assemble into similar structures. The bilayers form due to the
hydrophobic effect, that is, the tendency for the system to reduce the contact
area
between the nonpolar phospholipid or surfactant tails and water. These systems
may
contain one (unilamellar) or numerous (multilamellar) phospholipid bilayers
depending on the preparation method and ingredients used. Hydrophilic
functional
ingredients can be trapped inside the aqueous interior of liposomes and
nanoliposomes, whereas amphiphilic and lipophilic active agents can be trapped
in
the bilayer region. Liposomes and nanoliposomes can be fabricated from natural
components, such as phospholipids. Cholesterol is often added to the
formulation as
it increases rigidity strength of the membrane and confers steric stability.
Egg yolk-
and soy-derived phosphatidylcholines are commonly used to form liposomes,
whereas Tweene 80, Span 80 and sucrose laurate have been used to form
niosomes.
In some embodiments, a cannabinoid may be microencapsulated in polymer or
hydrogel particles. Polymer microparticles (diameter > 100 nm) and
nanoparticles
(diameter < 100 nm) are fabricated from either synthetic or natural polymers,
such as
proteins and polysaccharides. Commonly, they are produced from antisolvent
precipitation methods where a polymer dissolved in a good solvent is injected
into a
poor solvent, which promotes spontaneous particle formation. Hydrogel
particles
(sometimes called nanogels or microgels) may also be fabricated from synthetic
or
natural polymers, but they contain higher levels of water (typically >80% to
90%). A
wide variety of different methods are available for producing hydrogel
particles
including injection, templating, emulsion, and phase separation methods. The
composition and porosity of hydrogel particles must be carefully controlled to
ensure
appropriate loading, retention, and release properties.
In some embodiments, a food additive provided herein may further comprise a
terpene or terpenoid. The term "terpene" is generally understood to include
any
organic compound derived biosynthetically from units of isoprene, and the term
"terpenoid" generally refers to a chemically modified terpene (e.g., by
oxidation). As
196
CA 3062485 2019-11-21
85760071
used herein, terpenes include terpenoids. Terpenes may be classified in
various
ways, such as by their sizes. For example, suitable terpenes may include
monoterpenes, sesquiterpenes, or triterpenes. At least some terpenes are
expected
to interact with, and potentiate the activity of, cannabinoids.
Examples of terpenes known to be extractable from cannabis include
aromadendrene, bergamottin, bergamotol, bisabolene, borneol, 4-3-carene,
caryophyllene, cineole/eucalyptol, p-cymene, dihydroj asmone, elemene,
farnesene,
fenchol, geranylacetate, guaiol, humulene, isopulegol, limonene, linalool,
menthone,
menthol, menthofuran, myrcene, nerylacetate, neomenthylacetate, ocimene,
perillylalcohol, phellandrene, pinene, pulegone, sabinene, terpinene,
terpineol, 4-
terpineol, terpinolene, and derivatives thereof.
Additional examples of terpenes include nerolidol, phytol, geraniol, alpha-
bisabolol,
thymol, genipin, astragaloside, asiaticoside, camphene, beta-amyrin, thujone,
citronellol, 1,8-cineole, cycloartenol, and derivatives thereof. Further
examples of
terpenes are discussed in US Patent Application Pub. No. US2016/0250270.
In some embodiments, an edible product provided herein may further comprise
other
additives. Examples of suitable other additives include, but are not limited
to,
carbonation, pH control agents, vitamins, minerals, chelating agents,
antioxidants,
antimicrobial agents, flavors, sweeteners, colorants, weighting agents, fat
replacers,
and mixtures thereof.
In some embodiments, a food additive provided herein may be made using a
method
comprising the steps of: (1) adding cannabis oil, powder, distillate, or
isolate to water;
(2) adding an emulsifier; (3) subjecting the mixture to a high shear mixer as
described
in the Internet site of Proscientific which can be found on
https://proscientific.com/cannabis on July 31, 2018.
In some embodiments, a food additive provided herein may be made using a
method
comprising the steps of: (1) gently warming a cannabis extract by water bath;
(2)
addition of a starch-based powder, such as maltodextrin, to the warm cannabis
197
CA 3062485 2019-11-21
85760071
extract; (3) mix the cannabis extract and starch-based powder together to
create a
uniform concentrated cannabis extract powder, and (4) addition of powder to
hot
water to dissolve the powder and emulsify the extract, as disclosed in U.S.
Patent No.
9,629,886 B2. The preferred temperature of the water bath is between 80 and
100
degrees Fahrenheit and more preferably between 84 and 90 degrees Fahrenheit.
The ratio of the starch-based powder to the cannabis extract may be at least
24:1
w/w. The mixing step may be performed using an industrial blender to ensure
even
absorption of the powder by the extract. Other types of powders fit for human
consumption may be used in place of the starch-based powder, including but not
limited to, whey protein isolate (both dairy-based and plant-based), xanthan
gum,
guar gum (guaran), mono-and diglycerides, and carboxymethylcellulose
(cellulose
gum) so long as they absorb the oil when blended together, dissolve when added
to
a liquid, remain dissolved in that liquid and have no post-mixing separation
of the
powder and the oil.
In some embodiments, a food additive provided herein may be made using a
method
comprising the steps of: (1) heating an oil; (2) addition of a cannabis
extract to the
heated mixture; (3) addition of water or an aqueous solution to the heated
mixture; (4)
addition of at least one emulsifying agent to the heated mixture; and (5)
mixing the
heated mixture and added ingredients, as disclosed in WO 2017/180948A1. The
oil is
preferably in the range of 0.1% to 40% of the liquid formulation. The
preferred oil
temperature is between 120 to 220 degrees Fahrenheit. The amount of cannabis
extract will be in the range of about 5 mg to 30 mg per 2 ounces of liquid
formulation.
The water or aqueous solution will be present in the range of 60% to 99.9% of
the
liquid formulation. The emulsifying agent(s) will be added in the amount of
0.15% and
2% of the total volume of the edible product and may be selected from the
group
consisting of xanthan gum, guar gum, cyclodextrin, lecithin, carrageen,
monoglycerides, natural emulsifiers and organic emulsifiers that are safe for
ingestion
by humans. The mixing step may be performed using a high speed blender (or
similar
machine). The blender is run at high speed for between 30 seconds and 2
minutes. In
some embodiments, caffeine (or anhydrous caffeine) may be added after the
mixing
step in the amounts ranging from 10-300 mg per 2 ounces of the emulsification.
198
CA 3062485 2019-11-21
85760071
Alternatively, the caffeine can be added prior to adding the emulsifying agent
or at the
same time.
In some embodiments, a food additive provided herein may be made using a
method
comprising the steps of: (1) adding water; (2) adding one or more surfactant;
(3)
mixing water and one or more emulsifier using a magnetic stirring plate or
stick; (4)
adding cannabis oil to the mixture; (5) subjecting the mixture to a low shear
mixer; (6)
subjecting the mixture to a high shear mixer as described in the Internet site
of
Analytic company in the UK which can be found at https://analytik.co.uk/wp-
content/uploads/2017/03/application-note-use-of-microfluidizer-technolocy-for-
cannabis-products.pdf on July 31, 2018. The low-shear mixer may be a rotor-
stator
mixer. The high shear mixer may be a microfluidizer. The mixture may be passed
through each mixer one or more times. Pressure, number of passes, and
temperature
of the process may be adjusted.
In some embodiments, a food additive provided herein may be made using a
method
comprising the steps of: (1) mixing cannabis oil and a first emulsifier; (2)
adding
baicalein; (3) adding ethanol; (4) heating the mixture to 50 C until all
ingredients melt
to form the oil phase mixture; (5) mixing a second emulsifier with water to
form an
aqueous phase mixture; (6) mixing the aqueous and oil phase mixtures; (7)
subjecting the mixture to a high shear mixer for 5 minutes; (8) subjecting the
mixture
to a microfluidizer as described in Juntao Yin et al, "Biocompatible
nanoemulsions
based on hemp oil and less surfactants for oral delivery of baicalein with
enhanced
bioavailability" (2017) Int J Nanomedicine, 12, 2923. A particle size of 90.6
nm can be
achieved using a formulation comprising 40 mg of baicalein, 1,000 mg of hemp
oil, 50
mg of poly(ethylene glycol) monooleate as the first emulsifier, and 50 mg of
sodium
oleate as the second emulsifier mixed with 20 mL of water. The ratio of the
first
emulsifier to the second emulsifier may be about 1:1.
In some embodiments, a food additive provided herein may be made using a
method
comprising the steps of: (1) dilution of the cannabis extract with an oil; (2)
addition of
an emulsifier; (3) sonication to produce an oil-cannabis mixture; and (4)
emulsification
199
CA 3062485 2019-11-21
85760071
of the oil-cannabis mixture with water, as described at the Internet site of
the
hielscher company at https://www.hielscher.com/ultrasonic-cannabis-oil-
emulsion.htm
on July 29, 2018. The oil may be vegetable oil such as olive oil or coconut
oil. The
ratio of the extract to the oil may be about 1:40 v/v. The emulsifier may be
lecithin,
arabic gum, or a starch-based emulsifier. The ratio of the extract to the
emulsifier
may be between 1:10 and 1:15 w/v. The sonication step may be performed using
an
ultrasonic homogenizer. The ratio of cannabis-oil mixture to water may be
about 2:5
v/v. The emulsification step may be performed using an ultrasonic homogenizer.
In some embodiments, a food additive provided herein may be made using a
method
comprising the steps of: (1) adding cannabis oil, distillate, or isolate; (2)
adding a
carrier oil; (3) adding a mixture of emulsifiers; (4) adding of distilled
water; (5)
subjecting the mixture to sonication to produce a nanoemulsion with droplet
sizes of
about 20 to 40 nm, as described in the Internet site of Sonomechanics which
can be
retrieved at http://blog.sonomechanics.com/blog/stabilizer-package-for-
producing-
water-soluble-cannabis-extracts on July 31, 2018. The sonication may be
performed
using an ultrasonic homogenizer.
In some embodiments, a food additive provided herein may be made using a
method
comprising the steps of: (1) adding cannabis oil, distillate, or isolate; (2)
adding a
carrier oil; (3) adding a first emulsifier; (4) heating the mixture up to 110
C; (5) cooling
the mixture for 24 hours; (6) mixing water and a second emulsifier and heating
the
mixture up to 45 C, then allowing the mixture to cool for 24 hours; (7) mixing
the two
mixtures using a magnetic stirrer at room temperature; (8) subjecting the
mixture to
sonication as described in
https://leherbe.com/knowledge-
center/experiment/emulsification on July 31, 2018. The first emulsifier may be
Span 80. The second emulsifier may be Tween 80. Preferably, the oil volume
fraction
is at To = 0.10 and the total emulsifier volume fraction is at cps = 0.08.
Preferably, the
sonication time is between 5 and 7.5 minutes.
In some embodiments, a food additive provided herein may be made using a
method
comprising the steps of: (1) adding cannabis oil; (2) adding a suitable pair
of
200
CA 3062485 2019-11-21
85760071
emulsifiers 5 or 10 wt % with a hydrophilic-lipophilic balance (HLB) ranging
from 6 to
10; (3) adding distilled water; (4); heating the mixture to 70 C; (5)
subjecting the
mixture immediately to sonication for 15 minutes as described in Mikulcova et
al.,
"Formulation, Characterization and Properties of Hemp Seed Oil and Its
Emulsions",
Molecules (2017) 22, 700. The use of Tween 85 and Span 85 at 10 wt % as
emulsifiers produced particles ranging in diameter from 84nm to 122 nm.
In some embodiments, a food additive provided herein may be made using a
method
comprising the steps of: (1) spreading cannabis oil on a thin film of
parchment paper
or PTFE sheets; (2) subjecting oil to a 100 hour purge in a vacuum oven until
cannabis shatter forms; (3) flipping the extracted solution during the process
on a 12-
hour schedule or twice daily; (4) allowing the cannabis shatter to cool; (5)
heating the
chatter to 50-60 C to a semi-smooth texture; (5) adding 190/200 proof ethanol;
(6)
heating the mixture and reducing it to nearly the starting weight; (7) cooling
the
mixture in an ice bath; (8) subjecting the mixture to slow clockwise
sonication,
pausing the sonicator on one minute intervals for two minutes and stirring in
between;
(9) subjecting the mixture to sonication at a 30000J output for five to eight
minutes;
(10) subjecting the mixture to magnetic stirring hotplate [at a temperature of
60C,
300-320 rpm, and 72 hours of continuous mixing] as described in
https://cdn.shopify.com/s/files/1/1726/3473/files/A Methodology for the
Preparation
of Liquid Textured Cannabinoids.pdf?14822043847272496341 on July 31, 2018.
A preferred catalyst for enthalpy of vaporization may be added to step (6). A
ratio of
the oil to the catalyst may be 1:1.
In some embodiments, a food additive provided herein may be made using a
method
comprising the steps of: (1) adding a water-soluble surfactant to distilled
water to
form the aqueous phase; (2) heating the aqueous phase mixture to 70 C; (3)
mixing
an oil-soluble surfactant and cannabis oil to form the oil phase; (4) heating
the oil
phase mixture to 70 C; (5) adding the aqueous phase drop-by-drop to the oil
phase;
(6) stirring the mixture at a constant rate for 30 minutes; (7) maintaining
the
temperature of the process at 70 C as described in Mikulcova et al.,
"Formulation,
Characterization and Properties of Hemp Seed Oil and Its Emulsions", Molecules
201
CA 3062485 2019-11-21
85760071
(2017) 22, 700. The water-soluble surfactant may be a Tween surfactant. The
oil-
soluble surfactant may be a Span surfactant. The use of Tween 80 and Span 80
at
wt % as emulsifiers produced particles ranging in diameter from 502 nm to 1050
nm.
5 In some embodiments, a food additive provided herein may be made using a
method comprising the steps of: (1) preparing a mixture comprised of
triglyceride,
polyoxyl 40-hydroxy castor oil, Tween 20, and Span 80; (2) preparing a
separate
mixture comprised of amphiphilic co-solvent with soy phospholipid and heating
the
mixture to 40 C until complete dissolution; (3) mixing the mixtures in steps
(1) and
(2); (4) stirring gently; (5) heating the mixture to 40 C until homogenous pre-
concentrate solution is formed; (6) adding a cannabinoid to the pre-
concentrate; (7)
stirring the mixture gently, where upon gentle agitation of the cannabinoid in
the
aqueous phase, the pre-concentrate spontaneously forms drug encapsulated 0/W
nano-dispersion; (8) heating the mixture to 40 C until homogenous solution is
formed, as described in W02013/108254 Al.
The ratio of triglyceride to polyoxyl 40-hydroxy castor oil to Tween 20 to
Span 80 may
be about 1:1:1:1. The amphiphilic co-solvent may be ethyl lactate. The ratio
of
amphiphilic co-solvent to lechitin may be about 4:1. The mixture of
emulsifiers in step
(1) may be comprised of polysorbate 20 at 14.1% w/w, sorbitan monoleate at
14.1% w/w, lechitin at 8.3% w/w, tricaprine at 14.1% w/w, polyoxyl 40-hydroxy
castor
oil at 14.1% w/w, and ethyl lactate at 35.4 % w/w. The mixture comprised of an
amphiphilic co-solvent with soy phospholipid may be heated in a scintillation
tube. In
some embodiments, the cannabinoid may be tetrahydrocannabinol or cannabidiol.
The cannabinoid may be added at 3% w/w.
In some embodiments, a food additive provided herein may be made using a
method
comprising the steps of: (1) preparing a water and a lipid source mixture in a
flask; (2)
heating the water-lipid source mixture to boiling; (3) removing the boiling
water-lipid
source from heat; (3) immediately adding cannabis material enclosed in a tea
bag (or
similar porous enclosure) to the boiling water-lipid source; and (4) steeping
the
202
CA 3062485 2019-11-21
85760071
cannabis mixture. The lipid source may include, but is not limited to, milk
such as
10% milk, or butter, or combinations thereof. The ratio of the water to the
lipid source
may be about 4:1. The cannabis material may be the bud or the trim. The
cannabis
material may be processed using a hand miller, such as a handheld food
processor,
or an industrial miller. The heating step may be performed using an electric
water
heater or a microwave (e.g., set to a length of time of 2 minutes). The
steeping step
may last from about 3 minutes to about 10 minutes.
In some embodiments, an edible product provided herein further comprises an
antidote to the cannabinoid. The person of skill will readily understand that
in one
embodiment, the antidote may be included in the food additive comprising the
cannabinoid. In an alternate embodiment, the person of skill will readily
understand
that the antidote may be included in the edible product, separate from the
food
additive containing the cannabinoid.
As used herein, the term "antidote" means any compound capable of reducing or
neutralizing the effects of a cannabinoid.
In some embodiments, the cannabinoid is psychoactive. In the context of the
present
disclosure, a cannabinoid is psychoactive if it affects mood, perception,
consciousness, cognition or behaviour of a subject when consumed, as a result
of
changes in the functioning of the nervous system. Psychoactive effects of a
cannabinoid may include euphoria, enhanced well-being, easy laughter,
relaxation,
fatigue, sleepiness, dysphoria, anxiety, panic, paranoia, depersonalisation,
increased
sensory perception, feeling of the body floating or sinking, heightened sexual
experience, hallucinations, alteration of time perception, aggravation of
psychotic
states, fragmented thinking, enhanced creativity, disturbed memory, difficulty
in
concentration, headache, unsteady gait, ataxia, slurred speech, weakness,
deterioration or amelioration of motor coordination, impaired learning,
analgesia,
muscle relaxation, improved taste responsiveness, appetite stimulation,
cravings for
cannabis, nausea, vomiting, and antiemetic effects. An antidote to a
psychoactive
203
CA 3062485 2019-11-21
85760071
cannabinoid is a compound capable of reducing or neutralizing the psychoactive
effects of a cannabinoid.
In some embodiments, the psychoactive cannabinoid provided herein is THC, and
the antidote is CBD; acorus calamus or extracts thereof; black pepper or
extracts
thereof; citrus or extracts thereof; pine nuts or extracts thereof; pistachio
nuts or
extracts thereof; fruits of Pistacia terebinthus or extracts thereof;
piperine; or
terpenes, such as 6-caryophyllene, limonene, myrcene, or a-pinene. The
antidote
may be encapsulated in a microencapsulation system that is different from the
microencapsulation system of THC.
Complaint, Recall, Return and Feedback Handling
The ICS discussed herein (e.g. the ICS implemented via system 400 and/or ICS
1506
and/or ICS 1508) may be used to manage and record complaints, recalls, returns
and/or feedback for cannabis products. Complaints could be recorded in the ICS
using a "create complaint" action. Complaints could originate from a customer
due to
an adverse reaction to a cannabis product and/or a dislike for a cannabis
product, for
example. However, complaints might not always relate directly to cannabis
products.
For example, issues with the holding containers that contain cannabis products
could
also or instead result in a complaint. Complaints could be received in the
form of
phone calls, emails or written letters, for example. Any or all of the
information
regarding a complaint could be recorded in the ICS. A non-limiting list of
complaint
information includes:
type and/or brand of product that initiated the complaint;
any or all identification numbers for the product (for example, lot number(s),
batch
number(s) and/or plant number(s));
quantity of the product used by the customer;
quantity of the product remaining in the customer's possession;
time and date the complaint was received;
204
CA 3062485 2019-11-21
85760071
name and contact information of the customer providing the complaint; and
the customer's explanation for the complaint.
A recall could be initiated in the ICS using a "new recall" action. For
example, a recall
could be initiated upon receipt of a complaint from a customer. A test of a
product
that returned an undesirable result could also or instead initiate a recall of
a product.
For example, a customer complaint could lead to an archived sample of a
cannabis
product being tested or re-tested, which might produce a failed result leading
to a
recall of that cannabis product. The new recall action could record the
complaint, test
results and/or other reason for initiating the recall. The product to be
recalled could
be identified in the ICS by a batch number, plant number, lot number, or any
other
form of product identifier. When the recall is created, the ICS could be
automatically
updated to reflect the recall. The products that are affected by the recall
and still held
by the cannabis producer could be frozen in the ICS such that they are not
sold or
shipped. In some embodiments, these products could also or instead be labelled
to
indicate that they have been recalled, transferred to a quarantine area,
and/or
destroyed.
In the event of a recall, the ICS could generate a list of customers affected
by the
recall. Customers affected by the recall could include distributors who have
received
and/or sold the recalled product, cannabis processors, other producers who
have
used the recalled product to produce other products, and end users who have
received the recalled product or a product that includes or incorporates the
recalled
product. This list could be organized into different regions that the recalled
product
was distributed to. Any or all customers on the list could be notified of the
recall and
provided with instructions to return the affected products. For example, a
distributor
could be instructed to stop the sale of the recalled products, provide an
inventory of
the recalled products, and/or contact customers who bought the recalled
products.
Return kits could also or instead be sent to customers to help them safely
return the
recalled products. The return kits could include labelled packaging for
sending a
recalled product back to a cannabis producer. The return kits could be
packaged,
205
CA 3062485 2019-11-21
85760071
shipped and/or recorded in the ICS using any of the methods described herein.
After
the recalled products have been returned to the cannabis producer, these
products
could be weighed and/or recorded in the ICS. Labels could also or instead be
added
and/or updated on the returned products. At some point, the returned products
could
be transferred to a quarantine area and/or destroyed. Replacement products
could be
sent to affected customers at any time during and/or after a recall. In some
embodiments, at least some of communication with the customers during a recall
could be automated using the ICS.
Products could also be returned without a recall being issued. For example, a
customer could file a complaint that does not warrant a recall of a product,
and that
customer could be provided with instructions to return the relevant products,
a return
kit, and/or a replacement product. These returns could be recorded in the ICS
using a
"create return" action. The create return action could record any or all
information
regarding the complaint, information regarding the product that was returned,
and/or
information regarding the replacement product that was shipped.
Fig. 32 illustrates a system 1802 for identifying a lot of cannabis products
for recall,
according to one embodiment. The system 1802 includes memory, e.g. a database
1804, and processing modules 1806. The processing modules 1806 may be
implemented by one or more processors that execute instructions stored in the
memory 1804. Alternatively, some or all of the processing modules 1806 may be
implemented using dedicated circuitry, such as an ASIC, GPU, or FPGA. In Fig.
32,
the processing modules 1806 include a database search module 1806a and a
filter
module 1806b, which operate in the manner described below.
In the example illustrated in Fig. 32, the database 1804 and processing
modules
.. 1806 are part of an ICS. However, this is only an example. In other
embodiments, the
database 1804 and/or processing modules 1806 may be separate from and/or
independent of an ICS.
Stored in the database 1804 is information associated with a plurality of
batches of
cannabis plants. Each batch is associated with a batch identifier, which will
be called
206
CA 3062485 2019-11-21
85760071
a batch number. Also stored in the database 1804 is information associated
with a
plurality of lots of cannabis products. Each lot is associated with a lot
identifier, which
will be called a lot number. Each batch number is linked to / associated with
the lot
number for each lot of cannabis product originating from that batch. An
example is
illustrated in Fig. 32 in which some plants from batch B803 are processed to
produce
lot A22 of dried buds, other plants from batch B803 are processed to produce
lot A23
of dried buds, and other plants from batch B803 are processed to produce lot
A24 of
a distillate product. The batch number B803 is therefore associated with lot
numbers
A22, A23, and A24. Also illustrated in the example of Fig. 32 is a single lot
A25
produced from batch B804, and two lots A26 and A27 produced from batch B805.
Although not illustrated in the example, it could be the case that one or more
lots
originate from more than one batch (e.g. another lot A28 ¨ not illustrated ¨
may be
produced using plants from batch B803 and B805).
A user interface, e.g. a graphical user interface (GUI) 1808 in the form of a
display, is
coupled to the processing modules 1806 and database 1804. In the example of
Fig.
32, this is implemented by the GUI 1808 being communicatively coupled to the
ICS
via a network 1810. The GUI 1808 allows for a user to input information
relating to a
defective unit of cannabis product, e.g. to input a lot number for the unit of
cannabis
product, as shown at 1812. In an alternative embodiment, the user interface
may not
be a GUI, and/or it may include other components. For example, the user
interface
may be or include a barcode scanner that reads the lot number encoded in a
machine-readable code on a unit of cannabis product.
The particular GUI 1808 illustrated in Fig. 32 also allows for the user to
enter defect
information indicative of the nature of the defect resulting in the defective
unit of
cannabis product, as shown at 1814. However, other embodiments may not support
this functionality.
The lot number of a defective unit of cannabis product, which is provided by
the user,
e.g. via GUI 1808, will be referred to as a "suspect lot number". It is a lot
number of a
lot suspected to be defective. In some embodiments, one or more of the
processing
207
CA 3062485 2019-11-21
85760071
modules 1806, e.g. the database search module 1806a, queries the database 1804
to identify any batch number associated with the suspect lot number. An
associated
batch number will be referred to as a "suspect batch number". The database
search
module 1806a may then query the database 1804 to determine all of the lot
numbers
associated with each suspect batch number. For example, if the suspect lot
number
is A22, then there is one suspect batch number (B803), and the associated lot
numbers are A22, A23, and A24. Each lot number associated with a suspect batch
number will be referred to as a "recall lot number" (or recall lot identifier)
because it is
a lot number that is possibly subject to a recall. For example, if the suspect
batch
number is B803, then the recall lot numbers are A22, A23, and A24.
In some embodiments, there may be one or more units of archived cannabis
material
associated with each batch and/or lot, and information used to identify the
archived
material may be stored in database 1804. For example, in Fig. 32, an archived
sample exists for each lot, and is identified by a respective number, which is
stored in
database 1804. For example, lot A22 is associated with the archived sample
identified as X637, lot A23 is associated with archived sample X638, and lot
A24 is
associated with archived sample X639, such that batch number B803 is
associated
with three archived samples X637, X638, and X639. In some embodiments, any
archived cannabis material sample that is associated with a suspect batch
identifier is
examined or tested to determine whether it is defective. If a tested archived
cannabis
material sample is found to be defective, then the associated lot number(s) in
the
database 1804 are identified, and a recall of the affected lot(s) may be
triggered.
In some embodiments, each lot number may have process information stored in
the
database 1804 and associated with the lot. The process information may be
associated with a manufacturing process used to manufacture the lot of
cannabis
product. An example is illustrated in Fig. 32 in which process information is
included
in database 1804. For example, the process information for lot A22 identifies
that the
product of lot A22 is dried buds, which was produced using drying and curing
process
D12, and was packaged into containers using packaging process P135, etc.
Examples of manufacturing processes may include processes such as: separating
208
CA 3062485 2019-11-21
85760071
the plant material; and/or drying the plant material; and/or curing the plant
material;
and/or extracting cannabinoids from the plant material to produce a cannabis
extract;
and/or distilling cannabis extract to produce a distillate.
In some embodiments, the GUI 1808 enables a user to input defect information
indicative of the nature of the defect resulting in the defective cannabis
product. The
filter module 1806b then obtains the defect information from the GUI 1808. The
filter
module 1806b also obtains, from the database 1804, the process information
associated with the recall lot identifiers that are associated with the at
least one
suspect batch identifier. The recall lot identifiers may then be filtered by
the filter
module 1806b using the process information and the defect information, e.g. to
identify which lots may need to be recalled (and which lots would perhaps be
exempt
from the recall) based on the defect information and the process information.
As an example: The defect information entered on the GUI 1808 is that a unit
of lot
A22 of cannabis product contains mold, as shown in GUI 1808 as illustrated.
The
suspect batch number is therefore B803, and so the recall lot identifiers are
A22,
A23, and A24. The filter module 1806b retrieves process information for each
of the
recall lot identifiers. The process information associated with recall lot
number A24
indicates that the manufacturing process used in the production of lot A24
included
extracting cannabinoids from the plant material to produce a cannabis extract
and
distilling a cannabis extract to produce a distillate. The act of distillation
is known to
eliminate the possibility of mold, i.e. distillation remedies the defect of
mold, and so
lot A24 should not need to be recalled. The filter module 1806b therefore
exempts lot
A24 from recall by filtering out recall lot number A24. Only products from lot
numbers
A22 and A23 are identified as being subject to recall.
Recalls represent an example of an application of various assigned and
recorded
identifiers disclosed herein. These identifiers could be used to trace
cannabis
products through at least part of a processing or production chain, and
potentially to
plant batch or even individual plant, depending on the depth or granularity of
identifiers.
209
CA 3062485 2019-11-21
85760071
Fig. 33 is a flow diagram illustrating an example method of identifying a lot
of
cannabis products for recall. The example method 1900 involves, at 1902,
providing
a database in which information associated with batches of cannabis plants is
stored.
Each batch is associated with a batch identifier. Information associated with
lots of
cannabis products is also stored in the database. Each lot is associated with
a lot
identifier. Each batch identifier in the database is also associated with at
least one lot
identifier.
Provision of a database at 1902 does not necessarily involve populating the
database. The database could have been previously populated with information
during harvest of cannabis plants, processing of those plants into any of
various
cannabis products, and/or packaging of those products, for example. Therefore,
providing a database at 1902 could, but need not necessarily, involve
populating or
otherwise generating the database. For the purposes of identifying a lot of
cannabis
products for recall, and/or possibly other embodiments that involve using
information
in a database, providing a database could entail providing access to an
existing
database.
The example method 1900 also involves, at 1904, determining, using a lot
identifier
associated with a defective cannabis product, at least one suspect batch
identifier
associated with the lot identifier. As described above, each batch identifier
in the
database is also associated with at least one lot identifier, and accordingly
the lot
identifier associated with the defective cannabis product can be used to
determine a
batch identifier associated with the lot identifier, or each associated batch
identifier if
there is more than one batch identifier associated with the lot identifier.
Multiple batch
identifiers could be associated with the same lot identifier if plant material
from
multiple batches of cannabis plants is used in producing the defective
cannabis
product. A determined batch identifier could be considered a "suspect" batch
identifier
in the sense that it has an association with the lot identifier of a defective
cannabis
product.
210
CA 3062485 2019-11-21
85760071
The lot identifier associated with a defective cannabis product could be
received or
entered into a recall process in any of various ways. Markings on a product
container,
a product package, a product container label, and/or a product package label,
for
example, could be scanned and at least information conveying or indicating the
lot
identifier could be transmitted to or otherwise entered into a recall process.
Manual
entry of a lot identifier or other information that enables determination of
the lot
identifier is also contemplated.
In some embodiments, one or more cannabis material samples are archived for
each
batch of cannabis plants. The sample analysis at 1906 represents determining,
for
each archived cannabis material sample associated with the at least one
suspect
batch identifier, whether the archived cannabis material sample is defective.
Sample
analysis could involve any of various analysis processes. In some embodiments,
sample analysis could involve consulting records of testing that was
previously
conducted during processing or production, at 120 in Fig. 1, for example.
Sample
analysis could also or instead involve repeating previous testing and/or
conducting
different testing or analysis on one or more archived samples. The type(s) of
testing
or analysis performed at 1906 could be predetermined and/or selected based on
one
or more factors such as the type of cannabis product with which the lot
identifier is
associated, the manner in which the cannabis product is defective, parameters
or
characteristics associated with the batch(es) associated with the at least one
suspect
batch identifier, and/or other factors.
The sample analysis at 1906 could find that one or more archived materials
samples
are defective. A method could include, as shown at 1908, determining all lot
identifiers in the database associated with each archived cannabis material
sample
that is found to be defective. A batch of cannabis plants could have been
processed
to produce multiple lots of one or more cannabis products, in which case
multiple lot
identifiers could be associated with the same batch identifier. The
determining at
1906 could involve determining such lot identifiers, possibly including
further lot
identifiers in addition to the lot identifier associated with the defective
cannabis
product, using a batch identifier with which each defective archived cannabis
material
211
CA 3062485 2019-11-21
85760071
sample is associated. For example, lot records could be searched for each
batch
identifier that is associated with a defective archived cannabis material
sample, and
the lot identifier associated with each lot record that includes any searched
batch
identifier can then be determined.
The example method 1900 could therefore involve "bi-directional" searching or
tracing in the database. At 1904, searching or tracing is from lot to batch,
and then at
1908 the searching or tracing is in the opposite direction, from batch to lot.
Fig. 33 helps demonstrate not only the potential importance of traceability
for the
purpose of recalls, but also how depth or granularity of identifiers could
impact
functions or tasks for which it is necessary or desirable to determine batch
or plant
origin of cannabis products. Larger plant batches and/or smaller lot sizes,
for
example, could result in a larger number of product lots being associated with
a
batch. This could in turn lead to more extensive recalls if any lot from a
batch is
determined to be defective. Smaller plant batches and/or larger lot sizes
might result
in fewer associated lots for each batch, but it may be necessary to use plant
material
from multiple batches to produce enough product for a lot, in which case a
recall for a
defective lot could extend to multiple batches and potentially all lots
associated with
any one of those multiple batches. Any of these and/or other factors could be
taken
into account in determining manageable batch and/or lot sizes.
A recall process could also include other features as well. One or more of any
lot
identifiers that are determined at 1908 could be included in a product recall,
for
example. Not all determined lot identifiers might necessarily be included in a
recall.
For example, a defect could be related to a particular substance that is used
only in
certain production processes and not in others. A defect could be associated
with a
processing or treatment residue that only affects particular types of
products. Only
lots of those particular types of products could be recalled, even if other
products
were also produced from the same batch(es). Other defects could affect the
same
and/or other product types, or all products.
212
CA 3062485 2019-11-21
85760071
Another example of a method of identifying a lot of cannabis products for
recall is
illustrated in the flow diagram in Fig. 34. The example method 1910 involves,
at 1912,
providing a database in which information associated with a plurality of
batches of
cannabis plants and a plurality of lots of cannabis products is stored. Each
batch is
associated with a batch identifier, each lot is associated with a lot
identifier, and each
batch identifier in the database is associated with at least one lot
identifier. Providing
a database is discussed elsewhere herein, such as above with reference to Fig.
33.
A GUI implemented on a computer system is provided at 1914, to enable a user
to
input a suspect lot identifier associated with a defective cannabis product. A
database
search module implemented on the computer system is also provided, at 1916.
The
database search module is configured to determine, in response to a user
inputting a
suspect lot identifier, at least one suspect batch identifier associated with
the suspect
lot identifier in the database and all lot identifiers associated with the at
least one
suspect batch identifier in the database. Providing the GUI and the database
search
module could involve, for example, accessing the computer system in which the
GUI
and the database search module are implemented. In some embodiments, these
features are implemented at least in part using software and one or more
components of the computer system, such as a processor, that execute the
software.
Software could also or instead configure the database search module to
determine
the at least one suspect batch identifier and all lot identifiers associated
with the at
least one suspect batch identifier in the database. Such lot-batch-lot
searching or
tracing is disclosed elsewhere herein, such as above with reference to Fig.
33.
The example method 1910 also involves inputting a suspect lot identifier into
the
graphical user interface, at 1918. In response to this input of a suspect lot
identifier,
at least one suspect batch identifier associated with the suspect lot
identifier in the
database and all lot identifiers associated with the at least one suspect
batch
identifier in the database are determined by the database search module. The
database search module could provide an output indicative of any one or more
of the
suspect lot identifier, the at least one suspect batch identifier, and the lot
identifiers
associated with the at least one suspect batch identifier in the database. The
output
213
CA 3062485 2019-11-21
85760071
could be provided to a user and/or to other components of a system, and could
be
used to generate recall notices or orders for one or more lots, for example.
A processor-readable storage medium could be used in implementing at least
some
of the operations in the example recall-related methods 1900 and/or 1910, with
processor-executable instructions being stored on such a medium. The
instructions,
when executed by a processor, cause the processor to perform a method.
Execution
of the instructions could cause a computing device that includes the processor
to
implement a system configured to perform such a method.
A system for identifying a lot of cannabis products for recall, whether
implemented
using a processor-readable storage medium and a processor or in some other
way,
could include in some embodiments a database, a graphical user interface, and
a
database search module. In the database, information associated with a
plurality of
batches of cannabis plants and a plurality of lots of cannabis products is
stored. As in
other embodiments disclosed herein, such as with reference to Figs. 29 and 30,
each
batch is associated with a batch identifier, each lot is associated with a lot
identifier,
and each batch identifier in the database is associated with at least one lot
identifier.
The graphical user interface is implemented on a computer system, to enable a
user
to input a suspect lot identifier associated with a defective cannabis
product, and the
database search module is also implemented on the computer system. The
database
search module is configured to determine, in response to a user inputting a
suspect
lot identifier through the graphical user interface, at least one suspect
batch identifier
associated with the suspect lot identifier in the database and recall lot
identifiers
associated with the at least one suspect batch identifier in the database.
These
operations are discussed elsewhere herein, for example above with reference to
Fig.
34.
In some embodiments, the database further includes, for each lot identifier,
process
information associated with the manufacturing process(es) used to manufacture
the
associated lot of cannabis products from plant material of one or more batches
of
cannabis plants. The graphical user interface could be further configured to
enable a
214
CA 3062485 2019-11-21
85760071
user to input defect information indicative of the nature of the defect
resulting in the
defective cannabis product.
A filter module could also be implemented on the computer system, using
software
and a component of the computer system such as a processor to execute the
software. The filter module could be configured to: receive the defect
information that
is input by the user; receive the process information associated with the
recall lot
identifiers associated with the at least one suspect batch identifier in the
database;
and filter the recall lot identifiers using the process information and the
defect
information. Such filtering represents an example of how only lot identifiers
associated with product lots that are potentially affected by a defect could
be
distinguished from unaffected product lots, for at least certain defects that
do not
necessarily affect all products or all product types that originated from an
affected
batch of plants.
Examples of processing or manufacturing processes are provided elsewhere
herein.
The manufacturing processes used to manufacture the lot of cannabis product
from
plant material of one or more batches of cannabis plants could include one or
more
of: separating the plant material; drying the plant material; curing the plant
material;
extracting cannabinoids from the plant material to produce a cannabis extract;
distilling cannabis extract to produce a distillate, and/or others disclosed
herein.
In some embodiments, the filter module is configured to filter out the recall
lot
identifiers associated with manufacturing processes that are known to result
in a
remediation of the defect resulting in the defective cannabis product. A
defect could
relate to plant bacteria that would be killed by certain types of
manufacturing
processes such as extraction, for example. Recall lot identifiers associated
with
extraction could be filtered out by the filter module in this example.
As another example, if the defect information conveys that the nature of the
defect
resulting in the defective cannabis product relates to the presence of mold,
then the
filter module could be configured to filter out the recall lot identifiers
associated with
process information that conveys that the manufacturing processes used in the
215
CA 3062485 2019-11-21
85760071
production of the lot included one of extracting cannabinoids from the plant
material
to produce a cannabis extract and distilling a cannabis extract to produce a
distillate.
The recall-related methods and systems described above are intended solely for
illustrative purposes. Other embodiments could include fewer, further, and/or
different
features, performed or arranged in a similar or different order than
described. For
example, features described in the context of a method could be provided in a
system
embodiment, and features described in the context of a system could be
provided in
a method embodiment.
Furthermore, recall-related features need not necessarily be specific only to
recalls.
The same or similar features could also or instead be used in other
applications in
which it may be necessary or useful to determine batch or plant origin of
cannabis
products. For example, it might be desirable to enable a cannabis product that
has
high customer ratings to be traced back through a production stream. This
could
enable growth, harvest, and/or processing parameters or conditions to be
determined, and potentially replicated in an effort to reproduce highly rated
cannabis
products that are expected to be well-received by customers.
As noted at least above in respect of other embodiments, a processor-readable
storage medium could be used in implementing at least some of the operations
in
these example methods relating to recalls, with processor-executable
instructions
being stored on such a medium. The instructions, when executed by a processor,
cause the processor to perform a method. Execution of the instructions could
cause a
computing device that includes the processor to implement a system configured
to, in
some embodiments, perform at least some of the method operations discussed
above and/or elsewhere herein.
A system could include such a computing device, as well as other components
involved in producing a cannabis-infused consumer product. These and/or other
possible implementation options in respect of a system that could be
configured or
used to perform a method consistent with these example methods disclosed
herein
could be or become apparent. Fig. 32, for example, illustrate one possible
216
CA 3062485 2019-11-21
85760071
embodiment of a system in which components could be configured to perform such
methods.
Manufacturing Area Surveillance
In some embodiments, video cameras are installed on-site to record activities
relating
to the handling and/or processing of cannabis, e.g. for security and/or
regulatory
purposes. For example, a video camera may record images of a cannabis
operations
area in which cannabis material is being processed.
In addition to the video recordings, processing information associated with
the
processing of the cannabis material may also be recorded and stored, e.g. in
the ICS.
The processing information may include information such as:
a batch identifier/number identifying a batch of cannabis plants associated
with
the cannabis material being processed in the operations area; and/or
a lot identifier/number identifying a lot of cannabis products associated with
the
cannabis material being processed in the cannabis operations area; and/or
the identity of the person or people carrying out the processing in the
cannabis
operations area; and/or
the date and/or time at which the processing is being performed; and/or
the date and/or time at which the video images are recorded; and/or
the location of the cannabis operations area.
As an example, it may be recorded in the ICS that harvested plants from batch
B378
are placed into holding container H212 at 2pm on April 15, 2019. As another
example, it may be recorded in the ICS that fifty containers of dried buds are
packaged on May 1, 2019 at 4pm to produce lot number A75. During this time,
the
video camera(s) is/are recording video images of all such activities.
217
CA 3062485 2019-11-21
85760071
In some embodiments, the processing information is used as metadata that is
tagged
to the video record by combining the video images with the metadata. The
metadata
may then be used, e.g. by the ICS, to automatically retrieve and present to a
user
interface (e.g. a GUI) the relevant video footage when it is necessary to
review video
footage to investigate a problem.
As one example, fifty containers of dried buds are packaged on May 1, 2019 at
4pm
to produce lot number A75. The ICS stores in memory (e.g. in a record) that
lot
number A75 has been created. Meanwhile, a digital video recording of the event
is
also stored in a database. The ICS then associates: (1) the packaging of
containers
to produce lot number A75, and (2) the video recording of the event. For
example, the
start and end times of the packaging may be input by a person or machine into
the
ICS, or the ICS may select a predefined window of time around the time
indicated by
the person or machine, e.g. if the packaging happened at 4pm, then a window of
3:45pm-4:15pm may be selected. The video footage of that time and at that
location
may then be indexed with this processing information. Then, for example, if it
is later
determined that there is a problem with a container of dried buds from lot
number
A75, the ICS may automatically retrieve the indexed video footage that
recorded the
packaging of lot number A75, and present that to a user interface. The user
therefore
does not have to sort through vast amounts of video footage manually. Instead,
the
relevant video footage is presented to the user for viewing.
In some embodiments, the processing information metadata may be overlaid onto
the
video footage. For example, in the scenario described above, when the video
footage
of lot packaging around 4pm on May 1, 2019 is presented to the user, the
information
"packaging lot number A75" may be overlaid on top of the video images,
possibly
along with other metadata (e.g. the date/time, the person performing the
packaging,
etc., as recorded in the ICS).
In some embodiments, the ICS uses the link between batch, processing, and lot
identifiers to associate together all video footage relevant to the creation
of a
particular lot of cannabis product. For example, if a problem is identified
with a unit of
218
CA 3062485 2019-11-21
85760071
cannabis product belonging to lot number A75, then the ICS may retrieve and
present
(for user selection) all video footage relating to the creation of lot A75,
e.g. from the
harvesting of the batch of plants from which the cannabis in lot A75
originated, to the
recording of movement/transfer of that cannabis, to the recording of any
processing
performed on that cannabis, all the way through to the packaging of the
containers of
lot A75. The ICS is able to automatically retrieve this video because of: (1)
the
association of records in the ICS relating to harvesting of a particular batch
of
cannabis plants from which the cannabis in the lot originated, through all
steps in the
process up to and including creation of the lot; and (2) the association of
video
images with each step of the processing.
As an example: video footage 'A' is associated with the harvesting of batch
B378;
video footage 'B' is associated with transferring the harvested plants from
batch B378
into holding container H212; video footage 'C' is associated with extraction
process
E567 performed on the plants in holding container H212; video footage D' is
associated with lot packaging of the output of extraction process E567 to
create lot
A93. The association between batch B378, holding container H212, extraction
process E567, and lot A93 is stored in the ICS to link lot A93 to all previous
processing operations and have traceability all the way back to the batch
B378.
Subsequently, if there is a problem with a unit of cannabis product from lot
A93, the
ICS may retrieve, for presentation to the user interface, any or all of video
footage 'A'
to 'D', depending upon the user's request.
In this way, in some embodiments the ICS associates a unit of cannabis product
from
a particular lot with a plurality of digital video segments, each digital
video segment
corresponding to a respective different part of a multi-step process for
producing that
unit of cannabis product from a particular batch of cannabis plants.
In some embodiments, video images may be tagged with metadata associated with
a
detected security event. An example of a security event is attempted or actual
unauthorized access to the cannabis operations area or illicit conduct within
the
cannabis operations area. For example, if an alert signal is triggered by a
security
219
CA 3062485 2019-11-21
85760071
system, the relevant video images (e.g. the video recording of the location at
which
the alert was triggered around the time at which the alert was triggered) may
be
stored in the ICS in association with the alert signal. Metadata indicative of
the alert
signal may be generated and possibly overlaid on top of the video images.
Method embodiments related to video content are also contemplated. Fig. 35 is
a
flow diagram illustrating an example method of creating video content,
according to
one embodiment. The example method 1920 involves receiving, at 1922, video
images of a cannabis operations area in which cannabis material is being
processed.
The video images are captured by one or more video cameras installed to record
activities at one or more operations areas, and could be transmitted to a
central ICS
server that hosts and ICS database, and/or to one or more other components.
For example, referring to Fig. 4A, one or more video cameras could be provided
to
record activities during cultivation, in the grow area(s) 456a for example,
and/or
during harvest. A video camera could be connected to or otherwise in
communication
with the server 418a, and/or to other components of the example cultivation
and
harvest system 420a such as a computer 424a and/or a controller at 426a, to
transmit video images to the server 418a and/or other component(s). Video
images
could be locally stored by the video camera(s) and/or other component(s) to
which
video images are transmitted by the video camera(s), and/or further
transmitted, to
the server 402 for example.
In some embodiments, a video camera is connected to or otherwise in
communication with one or more controllers 426a, to control operation of the
video
camera. A video camera could be configured or controlled to record
continuously, or
according to a program or schedule. Dynamic video camera control and/or
recording
are also contemplated. For example, a video camera could be turned on when any
operator first checks in, using an operator check-in device 422a, to an empty
facility
or area in which no other operators are currently checked in, and could record
video
images until all operators have checked out. A video camera could also or
instead be
responsive to intrusion detection by a security system. Such dynamic control
could be
220
CA 3062485 2019-11-21
85760071
implemented in combination with programmed or scheduled recording. A video
camera operate in accordance with a program or schedule unless or until a
trigger
event such as an operator check-in or intrusion detection occurs. After a
trigger event
is no longer valid or active, such as after all operators check out of a
monitored
facility or area or an intrusion detector is reset, a video camera could
return to
programmed or scheduled recording.
Other video camera settings or parameters could also or instead be
predetermined
and/or controlled. Examples of such settings or parameters include
illumination
settings for a camera light or controller, video speed such as frames per
second,
and/or focus settings.
Camera orientation could also or instead be controlled in some embodiments.
This
could involve controlling a camera and/or a movable platform or mount on which
the
camera is mounted, for example.
One or more video cameras could be provided to record activities in any
cannabis
operations area. Video monitoring of cultivation and harvest, and provision of
one or
more video cameras in the example cultivation and harvest system 420a, are
intended as illustrative example embodiments. One or more video cameras could
also or instead be provided to monitor other cannabis operations areas.
At 1914 in Fig. 35, processing information associated with the processing
being
carried out in in the cannabis operations area is received. Examples of such
processing information and how such information could be used with video
images
are provided elsewhere herein, at least above in the context of creating video
content. The processing information could be received from other components,
such
as one or more of the computer(s) 424a, controller(s) 426a, sensor(s) 428a,
scale(s)
430a, label maker(s) 432a, and scanner(s) 434a in Fig. 4A. As noted above, the
example cultivation and harvest system 420a is an illustrative example
application of
video monitoring, which could also or instead be provided for other cannabis
operations areas. For other cannabis operations areas, processing information
could
be received from similar and/or different components.
221
CA 3062485 2019-11-21
85760071
The example method 1920 in Fig. 35 also includes, at 1916, generating metadata
using at least some of the processing information. Metadata could include
excerpts
from the processing information itself. In some embodiments, a one-way
transformation such as a hashing function is applied at least some of the
processing
information to generate metadata. A resulting transformed value such as a hash
value could subsequently be used to verify the part(s) of the processing
information
to which the transformation was applied. Another example of metadata is a code
generated based on at least some of the processing information. Such a code
could
encode at least some of the processing information and be used to recover the
coded
part(s) of the processing information. In some embodiments, the code is a
machine-
readable code. Other types of metadata based on at least some of the
processing
information are also contemplated.
Referring again to the example cultivation and harvest system 420a in Fig. 4A
as an
illustrative example, metadata could be generated by any one or more of a
computer
424a, another component that generates or collects the processing information,
the
server 418a, and the server 402. For example, if metadata is generated based
only in
processing information from a particular sensor or particular equipment, then
that
sensor or that equipment or its controller could generate the metadata and
store
and/or transmit the metadata with the processing information.
At 1918, a video record is generating by combining the video images and the
metadata. The video images and the metadata could be combined in any of
various
ways to generate a video record. The metadata could be added, as file metadata
for
example, to a video file that includes the video images. The metadata and
video
images could also or instead be stored in the video record. Generating a video
record
could involve overlaying at least part of the metadata onto the video images.
File
metadata, storage of the metadata and video images in the video record, and
overlaying a part of the metadata onto the video images represent illustrative
examples of how metadata and video images could be combined to generate a
video
record.
222
CA 3062485 2019-11-21
85760071
A video record could be stored in a database, such as the database 414 in Fig.
4A for
example. A video record stored in such a database could be indexed using the
processing information. For example, the processing information could include
a
batch identifier/number and/or a lot identifier/number. As disclosed elsewhere
herein,
other types of records in an ICS could include such identifiers/numbers, and a
video
record could similarly include and/or otherwise be indexed by batch and/or lot
identifier/number. Examples of records are provided herein at least with
reference to
Figs. 21-23, and a video record could include at least some similar record
fields in
some embodiments.
The method 1920, like other methods disclosed herein, is an illustrative
embodiments. Variations of the method are also contemplated. For example, a
method could involve receiving an alert signal. An alert signal could be
indicative of
an attempted or actual unauthorized access to the cannabis operations area or
illicit
conduct within the cannabis operations area, for example. Metadata indicative
of the
alert signal could be generated, and combined with the video data in
generating a
video record. Examples of metadata generation are provided herein, at least
above
with reference to step 1918 in Fig. 35. In some embodiments, both metadata
indicative of an alert signal and metadata that is based on at least some
processing
information is combined with video data in generating a video record.
Video images as discussed herein represent one example of visual content.
Images
need not necessarily be continuous in time. For example, a series of images
spaced
apart by more than a visually perceivable time gap could be sufficient for the
purposes of monitoring a cannabis operations area. Such series of images could
be
considered a form of video images, despite the time gaps. In general, any
features
disclosed herein in the context of video content or video images could also or
instead
be applied to still images.
As noted at least above in respect of other method embodiments, a processor-
readable storage medium could be used in implementing at least some of the
operations in methods relating to video content, with processor-executable
223
CA 3062485 2019-11-21
85760071
instructions being stored on such a medium. The instructions, when executed by
a
processor, cause the processor to perform a method. Execution of the
instructions
could cause a computing device that includes the processor to implement a
system
configured to, in some embodiments, receive video images, receive processing
information, generate metadata, and generate a video record.
A system could include such a computing device, and possibly other components.
Embodiments that involve video content could be implemented in any of various
ways
in the example system 400 in Figs. 4A-4M, for example.
Reporting
In some embodiments, the ICS system described herein can use any of the
information collected and/or stored in order to generate regular or ad hoc
reports
relating to any aspect of cultivation, extraction, processing, manufacturing,
testing,
packaging, shipping, or any other activity, task or operation described
herein. Such
reports can be used to feed into integrated systems for managing business
processes (e.g. Enterprise Resource Planning (ERP) platforms). The ICS system
described herein can also generate compliance, operational and Business
Intelligence (BI) reports. Examples of such reports include, but are not
limited to:
= Regulatory reports, such as monthly reports, annual reports,
notifications to
regulatory bodies, onsite inspection reporting, including:
o Amount of cannabis reported lost or theft,
o Lists of cannabis products made available for sale,
o Lists of amounts of cannabis produced and types of cannabis classes,
o Number and nature of deviations and corrective actions taken,
o Number of shipments and associated geographic locations,
o List of adverse reactions per batch/lot,
224
CA 3062485 2019-11-21
85760071
o List of complains per batch/lot,
o Amount of cannabis product recalled (and batch/lot identification),
o Amount of cannabis product produced for research and development,
o Inventory reporting, including:
= lists of items
held in inventory (oils, extracts, distillates, terpenes,
etc.)
= Physical locations in inventory, current weight / volume;
o Reports relating to whether or not processes are executed in
accordance with predetermined Standard Operating Procedures
(SOPs), which can include the reviewing of camera footage associated
with particular tasks and/or time periods and/or batches/lots; and
o Adverse reaction reports relating to customers having adverse reactions
to particular batches/lots;
= Financial reports, such as government agency reports relating to taxation
and
statistics;
= Business Intelligence reports relating to the cost for each product line
/ task,
Cost of Manufacturing (COM) reports, evaluation cost; and
= Quality Assurance (QA) reports including test results of cannabinoid
concentration levels, and the presence of heavy metals, microbiological
contaminants and/or pesticides in finished products.
Conclusion
An ICS as disclosed herein could be leveraged in any of various ways, to
track,
monitor, verify, and/or control any of a multitude of logistical or
operational aspects of
cannabis production, from cultivation to final sale of cannabis products, and
225
CA 3062485 2019-11-21
85760071
anywhere in between. An ICS could include at least an inventory of assets,
including
asset locations, status, and/or other information relating to assets. Any or
all transfers
of cannabis-containing substances between different holding containers and/or
different locations could also or instead be recorded. In general, any time a
cannabis-
containing substance is produced, combined, separated, and/or transferred, an
ICS
could be updated with any of various types of information.
Whenever "number" is used herein, it encompasses any arrangement of characters
or symbols, e.g. it encompasses alphanumeric numbers, characters, and/or
symbols
also. The word "number" may be used interchangeably with "identifier" or
"indicia".
Although the foregoing has been described with reference to certain specific
embodiments, various modifications thereof will be apparent to those skilled
in the art
without departing from the scope of the invention as defined by the claims.
For example, embodiments disclosed in the context of a cannabis producer or a
cannabis processor are not necessarily exclusive to cannabis producer
applications
or cannabis processor applications. Embodiments could potentially be applied
to
cannabis producers and/or cannabis processors.
Embodiments are disclosed primarily in terms of collecting and recording
information
and controlling labelling for the purpose of enabling traceability. Other
features could
also or instead be provided. Inventory stored in an ICS could be routinely
audited to
verify that the ICS is accurate. An inventory audit could include taking
account of
assets, for example by counting and/or weighing all cannabis seeds and plants,
counting and/or weighing all cannabis being dried, counting and/or weighing
all
holding containers for dried and fresh cannabis, counting and/or weighing all
holding
containers for cannabis oil and resin, and counting and/or weighing all
cannabis
waste. Alternatively, a random selection of cannabis products could be
counted,
weighed, and/or otherwise accounted. Results of an inventory audit could be
checked
against the ICS to determine whether the ICS is consistent with the inventory
audit. In
the event of a discrepancy between the ICS and the inventory audit is
discovered, an
investigation could be launched to determine the cause of the discrepancy.
226
CA 3062485 2019-11-21
85760071
Any module, component, or device exemplified herein that executes instructions
may
include or otherwise have access to a non-transitory computer/processor
readable
storage medium or media for storage of information, such as computer/processor
readable instructions, data structures, program modules, and/or other data. A
non-
exhaustive list of examples of non-transitory computer/processor readable
storage
media includes magnetic cassettes, magnetic tape, magnetic disk storage or
other
magnetic storage devices, optical disks such as compact disc read-only memory
(CD-ROM), digital video discs or digital versatile disc (DVDs), Blu-ray
DiscTM, or other
optical storage, volatile and non-volatile, removable and non-removable media
implemented in any method or technology, random-access memory (RAM), read-only
memory (ROM), electrically erasable programmable read-only memory (EEPROM),
flash memory or other memory technology. Any such non-transitory
computer/processor storage media may be part of a device or accessible or
connectable thereto. Any application or module herein described may be
implemented using computer/processor readable/executable instructions that may
be
stored or otherwise held by such non-transitory computer/processor readable
storage
media.
227
CA 3062485 2019-11-21