Note: Descriptions are shown in the official language in which they were submitted.
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
DEVICES, SYSTEMS AND METHODS RELATING TO THERMOMETER
HOUSINGS FOR ATTACHMENT TO HAND-HELD THERMOMETERS FOR IN
SITU DIFFERENTIATION BETWEEN VIRAL AND NON-VIRAL INFECTIONS
BACKGROUND
[0001] Detection and determination of and between biological infections such
as
bacterial and viral infections have always been difficult and uncertain
processes. The
importance of accurate detection and determination has increased with the
advent of
antibiotic-resistant strains of bacteria such as Methicillin-resistant
Staphylococcus
aureus (MRSA), which some people have attributed to the over-prescription of
antibiotics for virtually all forms of infections including patients with sore
throats even
if those infections are viral and thus not improved by antibiotics.
[0002] Accordingly, there has gone unmet a need to improve the ability of a
doctor,
nurse, dentist or other person or user to detect and diagnose infections as
viral or non-
viral, typically bacterial.
[0003] The present systems and methods, etc., provide improved abilities to
detect
and diagnose infections as viral or non-viral, typically bacterial, using heat
and light
sensing technologies implemented via a hand-held body thermometer, and/or
other
advantages.
SUMMARY
[0004] The present systems, devices and methods, etc., relate to in situ
photonic and
thermic detection systems using heat and light sensing technologies to detect
and
diagnose infections as viral or non-viral, typically bacterial, the systems
sized and
configured to be attached to hand-held body thermometers such as a cell phone.
Methods and systems related to such detection and diagnosis or identification
are
discussed and shown in U.S. Patent Application Ser. No. 15/350,626, filed
November
14, 2016 and entitled Devices, Systems And Methods Relating To In Situ
Differentiation
Between Viral And Bacterial Infections; a copy of such application is appended
to the
end of this provisional application.
[0005] One aspect of the current application provides a thermometer housing
sized
and configured for attachment to a hand-held body thermometer such that the
1
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
thermometer housing and hand-held body thermometer provide a hand-held
fluorescence and temperature detector sized and configured to detect
temperature and
fluorescence emanating from a suspected infection at a target site, wherein,
the thermometer housing can comprise a detection system can comprise a) an
excitation portion can comprise an excitation light source configured to emit
excitation light adequate to elicit selectively detectable fluorescent light
from
the suspected infection at the target site, and b) a detection portion can
comprise
a camera configured to selectively detect substantially only fluorescent light
emanating from the target site,
and wherein the hand-held fluorescence and temperature detector can be
operably connected to computer-implemented programming configured to a)
accept fluorescent light data associated with the fluorescent light and
thermal
data associated with heat levels above ambient body temperature, and b)
interpret the data to determine a probability whether the target site contains
an
infection.
[0006] In some other or further aspects and embodiments, the thermometer
housing
further can comprise a power source operably connected to power the excitation
light
source, and can comprise a computer containing the computer-implemented
programming, and wherein the power source can be operably connected to power
the
computer.
[0007] The hand-held body thermometer can be an oral thermometer, a vaginal
thermometer, a rectal thermometer, or other suitable body thermometer, sized
and
configured for inspection of an animal body cavity such as a human oral,
vaginal or
rectal cavity, and the excitation light source can comprise a light emitting
diode
configured to emit substantially only the excitation light.
[0008] The excitation light source can comprise a light emitting diode
configured to
emit substantially only the excitation light, and can emit substantially only
a single
wavelength or wavelength band of excitation light and/or can comprise multiple
excitation light emitters each emitting a different wavelength or wavelength
band of
excitation light. The excitation light source can comprise a white light
emitter and at
least one short pass filter configured to selectively transmit substantially
only light
below about 485 nm. The excitation light source can comprise a light port
comprising
2
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
at least one short pass filter configured to selectively transmit
substantially only
wavelengths below about 485 nm emitted from a light source disposed within
hand-held
body thermometer.
[0009] The housing camera or camera port can comprise at least a first long
pass filter
configured to block the excitation light and a notch filter configured to
selectively
transmit substantially only fluorescent light emanating from the target area.
The long
pass filter can comprise an about 475 nm long pass filter, and the notch
filter transmits
light have a wavelength of about 590 nm. The housing camera or camera port can
comprise at least one filter configured to selectively transmit substantially
only two
wavelength bands from about 475-585 nm and at about 595 nm. The housing camera
or
camera port can be configured to selectively accept or transmit, respectively,
at least a)
substantially only fluorescent light emanating from the target area, or b) all
visible light
wavelengths emanating from the target area.
[00010] The system is suitable for detecting and differentiating between viral
and non-
viral/bacterial infections in an animal body, such as in the throat, on the
skin, or in the
mouth, gut, vagina, lungs or other location capable of hosting such
infections. In one
aspect, the system contains an appropriate sensor (CCD, CMOS, thermopiles,
etc.)
configured to capture at least two groups of data, one corresponding to
emitted
fluorescence wavelengths, typically autofluorescence, from a suspected viral
or non-
viral infection, for example such as bacteria, and one for capturing a heat
signature
caused by such non-viral agent - or not present in the case of a viral
infection.
Exemplary excitation wavelengths include about 340nm and 380nm-500nm, and
detection wavelengths include 500nm to 700nm for fluorescence signatures and
700nm+ for heat signatures (thermal data) when heat is being detected using IR
(infrared). The thermal infrared region for room temperature objects is
generally
considered to be about 1000-1500nm depending on which technology is being used
to
measure it. Suitable thermopiles for use herein can look at window of about
800-
1400nm. Other methods of heat/thermal data detection or measurement can also
be
employed such as measurement of heat conduction or convection, which can in
some
instances be measured using a contact measurement device such as a contact
thermometer. Exemplary temperature levels include any substantial increase
over
3
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
ambient body temperature for the patient/organism commensurate with heat
generated
by bacteria, for example increases of about 0.5 C, 1 C, 2 C, or 3 C.
[00011] The fluorescence can come from fluorophores contained in or caused by
the
target bacteria such as porphyrins or can be introduced into to the target
area if desired,
for example as fluorophores that have been immuno-tagged to be species-
specific or
that are egested by specific species. Further, in the event of a viral
infection, the
autofluorescent signature of the native, ambient tissue is reduced or
eliminated, and thus
the loss of native autofluorescence is an indicator of a viral infection. If
desired, the
system can also detect other wavelengths or wavelengths bands of light such as
white
light, all visible light, or selectively blue light or red light, or
selectively IR (infrared)
etc. Such systems can also provide photographs or video, including real-time
or live
photographs or video.
[00012] The systems can also comprise light sources suitable to provide
interrogative
light for the examination of the target area. Such light sources can include,
for
example, a broad spectrum light source with appropriate selective light
filters to pass
only desired wavelengths such as blue wavelengths suitable for exciting
autofluorescence, infrared wavelengths suitable for heating the target area,
as well as
visible-light imaging wavelengths such as red-green-blue (rgb) or cyan-yellow-
magenta
(cym) wavelengths. The light source can also comprise a plurality of different
light
sources each tasked with providing a desired set(s) of wavelengths or a
wavelength
range(s); such sources can also be used in combination if desired. Examples of
such
sources include LED, metal halide, and xenon light sources.
[00013] The detected fluorescence and heat-based radiation provide a set(s) of
captured data. The captured data can be viewed in real-time by a user and/or
can be
sent to a desired location. For example, the data can be sent as a file or set
of files
preferably with an image representing the target site, to a computer such as
desktop
computer, laptop computer, an iPad or PDA, where the data is processed and/or
can be
viewed by human interrogators. The processed data can be interpreted by the
user
and/or a computer to identify the type of target organism (e.g., whether it is
a virus or
bacterium). Such information can be useful for determining appropriate
treatment
options - or non-treatment options such as choosing not to use antibiotics
against a viral
infection.
4
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
[00014] In some embodiments, the processed data/image can provide a score of
the
combined data points based on infrared hypothermic and/or hyperthermic values
and
can also incorporate or provide a spatial organization of aggregated amounts
of
abnormal thermal and fluorescent conditions within the target area. Generally
speaking, a lack of thermic activity above ambient body temperature indicates
that an
infection is viral, whereas presence of substantial thermic activity above
ambient body
temperature indicates the infection is bacterial. Such spatial organization
can be
provided to the practitioner to improve the ability to visualize the affected
area, and can
also be incorporated in the diagnosis aspect of the systems herein as spatial
organization, such as presence, color and shape of bacterial colonies, can be
indicative
of different types of infections.
[00015] In other words, in some embodiments the devices, etc., herein can
distinguish
between bacterial and viral infections and if desired can also help determine
the
location of the infection(s) within a target area. For the example of a
patient arriving at
a clinic (or other provider) with a sore throat, the processed information can
indicate to
the caregiver a probability, such as more than about 50%, 80%, 90%, 95%, 98%,
99%
or 100%, that the sore throat is an infection and if so, whether it is a
bacterial infection
or viral infection, as well as, if desired, location(s) in the throat of the
infections.
[00016] The devices can rely on auto-generated radiation such as
autofluorescence
generated autonomously within the infecting organism or a heat signature (or
lack
thereof in the case of viruses), or the devices can emit fluorescence-inducing
light
and/or heat-inducing light if desired.
[00017] In some aspects, the current application is directed to detection
systems
configured to scan and interpret a suspected infection at in vivo biological
target site,
the detection system comprising a housing comprising at least one light
emitter
configured to emit excitation light selected to elicit fluorescent light from
the suspected
infection at the target site, a light sensor configured to detect the
fluorescent light, and a
heat sensor configured to detect and identify thermal data indicating heat
above ambient
body temperature emanating from the suspected infection at the target site,
the detection
system further operably connected computer-implemented programming configured
to
a) accept fluorescent light data associated with the fluorescent light and
thermal data
5
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
associated with the heat levels above ambient body temperature, and b)
interpret the
data to determine a probability whether the target site contains an infection.
[00018] The system can be further configured to determine whether the
suspected
infection can be a viral infection or a non-viral infection, can further
comprise an
imaging system aimed and configured to provide an image of the target site.
The image
of the target site can identify a spatial organization of the suspected
infection and the
system can utilizes such spatial organization when determining the probability
whether
the infection can be a viral infection or a non-viral infection and/or when
determining
an identity of an infectious agent in the suspected infection. When the
suspected
infection is a non-viral infection, the computer implemented programming can
further
identify whether the infection may be bacterial.
[00019] The at least one light emitter, the light sensor and the heat sensor
can be all
located at a distal end of the housing and can be all forward-facing and aimed
to
substantially cover a same area of the target site. The housing can be
configured to be
held in a single hand of a user and can be configured to fit within a human
oral cavity
and to scan at least a rear surface of such oral cavity or a throat behind
such oral cavity.
[00020] The system further can comprise a separable distal element sized and
configured to removably attach to the distal end of the housing, wherein the
separable
distal element comprises at least one of light-blocking sides and/or a forward-
facing
window configured to selectively transmit at least the excitation light, the
fluorescent
light and the heat levels without substantial alteration. If desired, at least
two sides of
the separable distal element comprise recesses configured to keep the sides
out of a
view of the heat sensor. The distal end of the housing and the separable
distal element
can be cooperatively configured such that the separable distal element can be
snapped
on and off the distal end of the housing, for example via cooperative
projections and
detents configured such that the separable distal element can be snapped on
and off the
distal end of the housing.
[00021] The distal end of the housing can be configured to be mounted onto a
single
circuit board when the housing can be not being used for scanning, and can
further
comprise a display screen on a dorsal side of the housing.
[00022] The system can be configured to account for heat level distortions due
to
ambient conditions at the target site, for example using specific anti-
distortion
6
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
structures and/or by using at least one algorithm configured to account for
the heat level
distortions.
[00023] In further aspects, the current application is directed methods of
scanning in
vivo biological target site for a suspected infection, the methods comprising:
- emitting excitation light selected to elicit fluorescent light from a
suspected
infection at the target site
- sensing fluorescent light emanating from the target site elicited by such
excitation light;
- sensing thermal data indicating heat above ambient body temperature
emanating from the target site
- based at least in part on the sensed fluorescent light and the heat
levels,
determining a probability whether the target site comprises an infection.
[00024] Such methods can comprise, utilize or implement the structures and
devices
discussed herein. Such methods can also comprise making such structures and
devices
discussed herein
[00025] These and other aspects, features and embodiments are set forth within
this
application, including the following Detailed Description and included
drawings.
Unless expressly stated otherwise, all embodiments, aspects, features, etc.,
can be
mixed and matched, combined and permuted in any desired manner. In addition,
various references are set forth herein, including but not limited to the
Cross-Reference
To Related Applications, that discuss certain systems, apparatus, methods and
other
information; all such references are incorporated herein by reference in their
entirety
and for all their teachings and disclosures, regardless of where the
references may
appear in this application.
BRIEF DESCRIPTION OF THE DRAWINGS
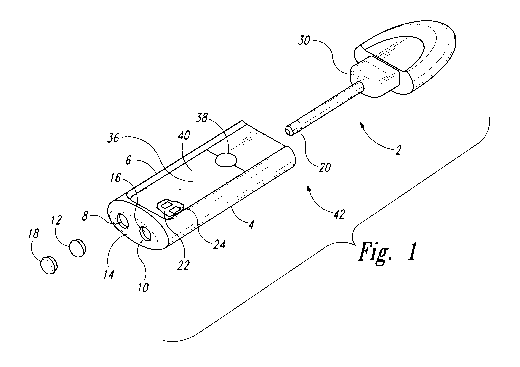
[00026] Figure 1 depicts a perspective view of an exemplary, stylized
depiction of a
thermometer housing sized and configured for attachment to a hand-held body
thermometer to provide a fluorescence-detection thermometer as discussed
herein.
[00027] Figure 2 depicts a further a perspective view of an exemplary,
stylized
depiction of a thermometer housing sized and configured for attachment to a
hand-held
body thermometer to provide a fluorescence-detection thermometer as discussed
herein.
7
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
[00028] Figure 3 a perspective view of an exemplary, stylized depiction of a
fluorescence-detection thermometer as discussed herein with the housing closed
with
the body of the thermometer.
[00029] Figure 4 depicts side and top plan views of an exemplary, stylized
depiction of
a fluorescence-detection thermometer as discussed herein in use.
[00030] Figure 5 depicts a flow chart of an exemplary system software
lifecycle.
[00031] Figure 6 depicts an exemplary embedded software architecture and its
composition of individual software components.
[00032] Figure 7 depicts a flow chart of an exemplary application executive
state
diagram.
DETAILED DESCRIPTION
[00033] Turning to the Figures, Figures 1 to 3 depict an exemplary, stylized
depiction
of a hand-held body thermometer 2 comprising a thermometer (heat sensor) 20
and a
on/off button 38 as discussed herein. In Figures 1 and 2, the fluorescence-
detection
thermometer 42 comprises separated hand-held body thermometer 2 and
thermometer
housing 4; in Figure 3 such components are attached to each other. The
fluorescence-
detection thermometer 42 comprising hand-held body thermometer 2 provides a
hand-
held fluorescence and temperature detector sized and configured to detect
temperature
and fluorescence emanating from a suspected infection at a target site. In the
embodiment shown, the thermometer housing 4 includes an excitation light
source 16,
in this instance an LED disposed on the thermometer housing 4, configured to
emit
excitation light selected to elicit fluorescent light from the suspected
infection at the
target site. The excitation light source 16 is configured to emit excitation
light selected
to elicit fluorescent light from the suspected infection at the target site,
for example by
passing white light through a long pass filter 10. The thermometer housing 4
also
includes a camera 8, i.e., a light sensor, preferably able to capture images
and in some
embodiments comprising both a dichroic filter 18 and a notch filter 12. The
camera
port 14 of the camera system 6 is sized and configured to selectively transmit
or admit
substantially only fluorescent light emanating from the target site to an
interior camera
8 disposed within the mobile communication device. The thermometer housing 4
further includes a heat sensor configured to detect and identify thermal data
indicating
8
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
heat above ambient body temperature emanating from the suspected infection at
the
target site.
[00034] The thermometer housing 4 further comprises a power source operably
connected to power the excitation light source 16, as well as a computer 36
containing
the computer-implemented programming, and the computer 36 also operably
connected
to the power source such as battery 24. The thermometer housing 4 further
contains a
wireless communications unit such as Bluetooth communications unit 22 to
transmit
data and diagnoses and other information to/from the thermometer housing 4 and
the
hand-held body thermometer 2, and to other operably connected devices such as
printers, additional computers, viewing screens, etc., if desired.
[00035] Figure 4 depicts an exemplary, stylized depiction of the thermometer
housings
and/or hand-held body thermometers discussed herein in use, with excitation
light 32
shining from the hand-held body thermometer 2 and/or thermometer housing 4
onto a
target site 34. An image 26 and diagnostic information 28 is shown on the
first screen
30 and/or second screen 40 of the hand-held body thermometer.
[00036] Turning to a general discussion of exemplary detection and diagnostic
aspects
and embodiments of the systems herein, such discussions are augmented by, and
hereby
include, the discussions set forth in the appended copy of U.S. Patent
Application Ser.
No. 15/350,626. The illumination and detection aspects of the systems herein
emit the
selected interrogation wavelengths (for example via distally carried LED light
emitters
or via proximally located light sources where such light is conducted through
appropriate conductors such as optic fibers to the target site) and then to
carry the
elicited photonic data (fluorescence data) and heat data/thermal data
(photonic or
otherwise) gathered from the interrogation site to the user such as a doctor
or other
health care provider. The scope can if desired include elements to conduct an
optical
image directly from the target site to the viewer/user. The system can also
include
computers and the like, for example located proximally via hardwire or
wireless links or
within the interrogative device, to process the data and if desired provide
estimates of
the presence or absence of bacteria at the interrogation/target site, and
estimates of
whether the suspected infection, if present, is or is not viral.
[00037] The device can be sized and configured to be held by a human hand,
i.e., is a
"hand-held", for certain embodiments and can be a device shaped to be
maintained
9
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
outside the body as shown, for example, in US patent application no.
20050234526, or
can be a catheter or endoscope or other configuration (e.g., colposcope,
laparascope,
etc.) shaped to be inserted into or otherwise introduced into or aimed toward
the body
of a patient.
[00038] The scope, for example where the scope provides an image to an ocular,
can
comprise a hollow casing with desired optics that returns light from the
target tissue to
the detector and/or an ocular eye piece. The hollow casing if desired can also
transmit
light from an external (typically proximally-located) light source to the
target tissue.
Suitable ocular eye pieces include an eye cup or frosted glass, and can be
monocular or
binocular as desired. If desired, the scope can alternatively, or
additionally, be
configured to contain one or more internal light sources, distally located
light sources
(such as LEDs), and/or proximally located light sources, and one or more fiber
optic
light guides, fiber optic cables or other such light transmission guides, in
addition to, or
instead of, the light guide formed by the hollow casing discussed above.
[00039] Typically, the scope comprises a power source suitable to power the
light
sources and/or sensors, data transmitters, and other electronics associated
with the
device. The power source can be an external power source such as a battery
pack
connected by a wire, a battery pack maintained in the handle or otherwise
within the
scope itself, or a cord and plug or other appropriate structure linking the
device to a
wall outlet or other power source. In some embodiments, the housing of the
light
source includes a retaining structure configured to hold the scope to a
desired location
when not in use.
[00040] As noted previously, the scope comprises one or more sensors such as
CCDs,
CIDs, CMOSs, thermopiles, etc., and/or is operably connected to one or more
display
devices, which can be located on the scope and/or in an operably connected
computer.
Such sensors, either in combination or as wide-sensing singular sensors, can
detect at
least any desired fluorescence, such as autofluorescence in the 400nm-600nm
range and
700nm+ range. Suitable sensors including infrared (IR) and detectors are well
known.
[00041] Exemplary display devices include CRTs, flat panel displays, computer
screens, etc. The diagnostic systems include one or more computers that
control,
process, and/or interpret the data sets and if desired various other functions
of the scope,
including, for example, diagnostic, investigative and/or therapeutic
functions.
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
Typically, a computer comprises a central processing unit (CPU) or other logic-
implementation device, for example a stand-alone computer such as a desk top
or laptop
computer, a computer with peripherals, a handheld, a local or internet
network, etc.
Computers are well known and selection of a desirable computer for a
particular aspect
or feature is within the scope of a skilled person in view of the present
disclosure.
[00042] As noted above, suitable heat detectors include well known infrared
(IR) and
including for example thermopiles and microbolometer arrays, provided that
when such
devices are included within the scopes/housings herein, such are suitably
sized to fit
within or on the scope without making the overall device too large for its
purpose.
Where the detection light gathered from the target sight is transported, such
as by fiber
optics, outside the scope and body, size concerns for the heat detector
elements (and
other detection elements) are reduced. Such
sensors can also comprise heat-
neutralization structures configured to reduce or eliminate improper ambient
heat
readings due to outside influences, such as a patient's breath when
interrogating the
back of the mouth or throat. Heat-neutralization structures can include, for
example, an
anti-fog element such as a hydrophobic material, a spray or coating that does
not skew
the signal determined by the sensor, or a dichroic mirror that transmits the
signal to a
proximate sensor removed from the impeding outside influence.
EXAMPLES
Example 1: Exemplary Software Desi2n
[00043] An exemplary system comprises embedded system software and host client
software. The embedded system software will run on a Raspberry PI (RPI)
Compute
Module. This software will comprise device drivers, kernel services, the Linux
kernel
and bootloader, and application level software. The host software is a client
Graphical
User Interface (GUI) that will run on a PC. The client GUI aids users in
interacting
with the system.
[00044] Table 1 in Figure 5 shows an exemplary system level software lifecycle
for
system during a typical use case scenario. Aspects of the system functionality
can be
encapsulated within the "Application Executive" sub-process.
[00045] In Figure 5, the exemplary software lifecycle comprises power on 500
followed by bootloader 502, which in turn leads to splash screen 504. The
splash
11
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
screen 504 is followed by kernel boot startup scripts 506, which then invokes
the
application executive 508. At the end of the cycle, power-off 510 takes place.
EMBEDDED SYSTEM SOFTWARE
[00046] Turning to Fig. 6, the embedded hardware platform 602 can comprise a
RPI
Compute Module with a number of hardware peripherals 604 that make use of the
Compute Module's Input/Output (I/O) 606. The compute module utilizes a
Broadcom
BCM2835 processor with on-board 512MB of RAM and 4GB of eMMC flash.
Additionally, the Compute Module pulls out all of the I/O pins of the
processor for
developer use. The Compute Module has a rich embedded Linux ecosystem making
it
ideally suited for rapid prototyping and deployment of embedded Linux. The
embedded
software implementation provides a custom streamlined Linux Kernel, the
necessary
kernel-mode drivers, and user-mode application functions suitable to implement
the
unit. Table 2 in Figure 6 shows the embedded software architecture and its
composition
of individual software components. Exemplary embedded system software is also
shown in Figure 6 and/or discussed in the following sections in Table 2.
APPLICATION EXECUTIVE
[00047] The Application Executive is a Linux User-mode Process that is
launched at
boot that runs until the unit is powered off The purpose of the Application
Executive is
to serve as a high level state machine that coordinates the various underlying
functional
components of the system based on user interaction with the unit.
[00048] Table 3 in Figure 7 shows a high level state diagram of the
Application
Executive 700 which is comprised of a loop 724 and a number of functional
components and sub-processes that handles user-events and the various
interactions
with the hardware components of the system.
[00049] The application executive 700 can launch automatically at system boot.
[00050] The application executive 700 can start within a desired number of
seconds
after power-on.
[00051] The application executive 700 can run continuously until power-off
[00052] In Figure 7, application executive 700 is entered, which causes the
display to
update 702, the a check of the GPIO/Button driver 704. Illumination button
706, take
12
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
picture button 710, take temperature button 714, and BLE event button 719 are
checked
for pressings. If a pressing is detected, then, respectively, the following
happens: the
LED state is toggled 708, the image sequence is initiated 712, the thermopile
(or other
temperature sensor) sampling algorithm is implemented, and/or the handle BLE
event
processes are implemented. After such button pressing check 704 is performed
(as
many iterations as desired), power mode 22 is invoked, which can also lead via
loop
724 to update display 702 or other desired location in the loop.
IMAGE STORAGE
[00053] The unit is capable of storing images within its flash file system.
Image
storage will persist through power cycles. The user of the unit will have the
ability to
associate a unique patient identifier to a grouping of one or more images. The
file
system will reside on the same flash part that contains the Linux Kernel and
application
software; a region of 40MB is reserved for system software binary storage.
[00054] A 40MB partition of flash can be reserved for Linux Kernel and
application
software storage.
[00055] There can be a Memory Technology Device (MTD) driver suitable to
control
the eMMC flash interface for use with a Flash File System (FFS)
[00056] There can be a FFS implemented.
[00057] Image storage can persist through power-cycle.
[00058] There can be a unique patient identifier associated with each image.
[00059] There can be a method to erase files from the FFS.
[00060] Images can be stored using a desired compression algorithm.
IMAGE CAPTURE
[00061] The unit is capable of using its camera to capture images for
analysis.
[00062] There can be a Camera Serial Interface (CSI) driver for image upload
from the
camera.
[00063] There can be an I2C driver for Camera Control Interface (CCI)
functionality.
[00064] Image data can automatically be written to flash.
[00065] Image acquisition sequence can occur automatically when prompted by
the
user.
13
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
DISPLAY AND MENU
[00066] The unit will have a Serial Peripheral Interface (SPI) 128x64
graphical/character. The display will show information pertaining to the
current state or
function of the unit, as well as host communication status. The display will
also be
capable of displaying Unique Identifier (UID) information pertaining to the
specific
unit as well as the current patient. Note: on-device display can be capable or
incapable
of presenting camera images as desired.
[00067] There can be a SPI driver for communications with the display.
[00068] The display can be capable of showing current state information.
[00069] The display can show a splash screen during system boot.
[00070] The display can show the Bluetooth UID of the unit.
[00071] The display can show the temperature measurements when prompted by
user.
[00072] The display can show the current UID of the patient under test.
TEMPERATURE ACQUISITION
[00073] The unit is capable of reading a thermal sensor for patient
temperature
acquisition.
[00074] There can be an I2C driver for communication with a thermopile sensor
[00075] There can be an algorithm for temperature acquisition.
[00076] The unit can acquire temperature when prompted by the user.
[00077] There can be a method to associate and store temperature data with the
patient
UID.
[00078] Button Controls
[00079] The unit will have three buttons for user interaction. The first
button controls
the illumination LED (white). The second button initiates the image
acquisition
procedure. The third button initiates the temperature acquisition procedure.
Other
buttons can also be provided
[00080] There can be a GPIO driver for controlling three button inputs.
[00081] There can be a button de-bounce algorithm implemented to filter button
noise.
[00082] Button-1 can control the state of the illumination LED.
[00083] Button-2 can initiate the image acquisition procedure.
14
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
[00084] Button-3 can initiate the temperature acquisition procedure.
LED CONTROLS
[00085] The unit will have three LEDs comprising a white illumination LED, and
a red
and blue LED used in the image acquisition.
[00086] There can be a GPIO driver for controlling three LED outputs.
[00087] The white illumination LED output can go active or inactive when
prompted
by the user.
[00088] The red and blue LEDs can be controlled automatically as part of the
image
acquisition sequence.
[00089] Host Communications
[00090] Communications with the host PC is achieved through the incorporation
of an
integrated USB-Bluetooth dongle implementing Bluetooth Low Energy (BLE).
Device
pairing is performed on the host PC.
[00091] There can be a USB-Bluetooth driver and firmware to control the USB-
Bluetooth dongle.
[00092] After Bluetooth driver registration is complete, the Bluetooth unique
identifier
can be read and displayed.
[00093] The Kernel can include the BlueZ Bluetooth stack.
[00094] The unit can present itself as a Basic Imaging Profile (BIP) Bluetooth
device if
desired.
[00095] The unit can transfer images to the host at any desired rate.
DEBUG CONSOLE (TERMINAL)
[00096] The unit will have a serial port used for displaying the Linux
Terminal for
development and debug.
[00097] There can be a UART for serial I/O debug console.
[00098] The embedded Linux distribution can include a Terminal console such as
bash.
HOST CLIENT GUI SOFTWARE
GRAPHICAL USER INTERFACE
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
[00099] The host client software can comprise a GUI with minimal functions to
utilize
the unit. The GUI will have the ability to execute Bluetooth device pairing,
file upload
and browsing, patient ID display, image display, device wiping, and possibly
other
functions as desired.
[000100] The GUI can be designed to run on the Windows7 or 10 Operating
Systems.
[000101] The GUI can provide an interface for Bluetooth device pairing with
one or
more units based on the unique Bluetooth device ID.
[000102] The GUI can provide an interface to browse the filesystem on the
paired unit.
[000103] The GUI can provide an interface to upload files from the paired unit
to the
host PC filesystem.
[000104] The GUI can provide the ability to erase files from the paired unit.
[000105] The GUI can provide a method of displaying the association of patient
unique
identifier with patient images and temperature if desired.
[000106] The GUI can provide a method of opening and displaying image files.
[000107] Turning to some other embodiments and other general discussion, in
some
embodiments the light path can comprise an illumination light path extending
from the
scope to the target and the scope can comprise in order a collimator, a 430+/-
30 nm
notch filter (filter 1), a dichroic filter (filter 2), an unwanted-light
absorber, then a glass
or other transmissive/transparent window. Such a window can both enhance
cleaning
and reduce cross-contamination of the device and/or between patients. The
illumination
light contacts the mucosal tissue or other target tissue then returns through
a dichroic
filter (filter 2 (the light can pass back past the same dichroic filter), a
475 long pass
filter (filter 3), a 590 nm notch filter (filter 4), a filter configured to
receive IR and/or
NIR light, and then be passed to the detectors and if desired an eyepiece
ocular. The
filters can be either separate (discrete) or combined (e.g., reflective
coatings).
[000108] The systems can if desired comprise binocular eyepieces such as
loops/filtered
glasses or sunglasses/goggles with/without magnification. Some other features
that can
be included are a light wand, a treatment light, a mirror and/or fiber optic,
typically
collimated, or an LED on the wand which can have a sleeve with a filter at the
end to
provide particularly desired light and thus function as the light wand, and
thus as the
light source or as an additional light source for fluorescence or other
desired response.
16
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
[000109] The scopes' designs can have multi-wavelength light processing within
and
outside the detector or camera. The light can be piped through the system or a
light
source can be incorporated or there can be a separate sleeve (or other
suitable light
emitter) with its own light. The
sleeve could have appropriate wavelength
emission/excitation filters. Filter and other optical element position can
vary within the
pathway provided the desired functions are achieved.
[000110] The illumination light and viewing pathways can be combined or
separate as
in a light source with loupes/eyewear. The pathways can enhance user ability
to use the
device to have a standard method of viewing and illumination. The size of the
spot of
interrogation in some embodiments is sized to compare a full lesion to
surrounding
normal tissue, which enhances viewing and identifying anatomical landmarks for
location.
[00011111n some embodiments, intensity is optimized to bathe the tissue with
excitation light for detection and diagnosis, to excite the necessary
fluorophores, to
induce or avoid heat-based responses, etc. The wavelengths/fluorescence
enhance the
ability to recognize a shift in the fluorescent emission spectra to permit
differentiation
between normal and abnormal for cancerous tissue. For example, dual monitoring
of
two wavelength bands from about 475-585 and from about 595 and up enhances
monitoring of cellular activity for the metabolic co-factors NAD and FAD. NAD
and
FAD produce fluorescence with peak levels at such wavelengths.
100011211n certain embodiments, it is desirable to get as much power as
possible
without smearing emission signal too much, to keep the output spectrum narrow
to
prevent Stokes shift, and to exclude UV light and to avoid
illuminating/exciting with
light in the emission band (overlapping fluorescence).
[000113] In certain embodiments, the systems can further comprise a diffuser
to make
spot-size more regular, remove hot spots, etc. Also sometimes desirable is a
collimator
to straighten light out at the filter, and to limit the divergence of the beam
with
increases in power density, or to use a liquid light guide and not fibers so
as to get more
efficiency by reducing wasted space between fibers, and achieving better
transmission
per cost and higher numerical aperture (which contributes to better light
collection). In
still other embodiments, the systems can further comprise metal halide light
sources to
enhance power in certain emission ranges, dichroic filters or similar optical
elements to
17
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
enhance overlapping viewing and illumination light paths (can simultaneously
direct
illumination light away from the source and emanation light from the tissue).
A glass
or other transparent window at the front of the scope can keep out the dust,
bodily
fluids, infectious organisms, etc. The scopes can be black internally to
absorb stray
reflected illumination and released fluorescent (unwanted fluorescent
feedback) light.
[000114] The shape of the scope can be preferably set to be ergonomically
comfortable,
optimize the excitation and emission pathways. The proximal eyepiece can be
set at a
length, such that tilting the proximal filter (e.g., a 590 nm notch filter)
creates a
geometry such that incoming ambient light (if any is relevant) from behind the
practitioner can be reduced and what passes can be reflected into the
absorbing internal
tube surface. This reduces reflection and prevents the user from seeing
themselves. For
example, the proximal filter can be tilted with its top closer to the
clinician and bottom
closer to the dichroic mirror so as to make a reflecting surface that would
direct
incoming light into the bottom of the optical pathway tube.
[000115] As noted elsewhere, sometimes multiple light sources can be provided
with a
single scope. For white light viewing if desired, there could be provision for
a greater
bandwidth in the output. The larger bandwidth could be obtained by having an
extra
light (LED, halide, etc.) or by using different filters at the output of a
single light
source. The systems can also provide illumination with multiple peaks. For
example,
pharmacology/physiology testing of biological markers may sometimes use this
for
when fluorescence emitted (by the tissue, markers, or chemical signals)
changes in the
presence of various ions/molecules/pH. This can
also be used to provide a
normalization as the power of fluorescence produced by each wavelength can be
being
compared, normalized against each other.
[0001161AI] terms used herein, are used in accordance with their ordinary
meanings
unless the context or definition clearly indicates otherwise. Also unless
expressly
indicated otherwise, the use of "or" includes "and" and vice-versa. Non-
limiting terms
are not to be construed as limiting unless expressly stated, or the context
clearly
indicates, otherwise (for example, "including," "having," and "comprising"
typically
indicate "including without limitation"). Singular forms, including in the
claims, such
18
CA 03062900 2019-11-08
WO 2018/205030
PCT/CA2018/050556
as "a," "an," and "the" include the plural reference unless expressly stated,
or the
context clearly indicates, otherwise.
[000117] The scope of the present systems and methods, etc., includes both
means plus
function and step plus function concepts. However, the terms set forth in this
application are not to be interpreted in the claims as indicating a "means
plus function"
relationship unless the word "means" is specifically recited in a claim, and
are to be
interpreted in the claims as indicating a "means plus function" relationship
where the
word "means" is specifically recited in a claim. Similarly, the terms set
forth in this
application are not to be interpreted in method or process claims as
indicating a "step
plus function" relationship unless the word "step" is specifically recited in
the claims,
and are to be interpreted in the claims as indicating a "step plus function"
relationship
where the word "step" is specifically recited in a claim.
[000118] The innovations herein include not just the devices, systems, etc.,
discussed
herein but all associated methods including methods of making the systems,
making
elements of the systems such as particular devices of the scopes, as well as
methods of
using the devices and systems, such as to interrogate a tissue (or otherwise
using the
scope to diagnose, treat, etc., a tissue).
[000119] From the foregoing, it will be appreciated that, although specific
embodiments
have been discussed herein for purposes of illustration, various modifications
may be
made without deviating from the spirit and scope of the discussion herein.
Accordingly, the systems and methods, etc., include such modifications as well
as all
permutations and combinations of the subject matter set forth herein and are
not limited
except as by the appended claims or other claim having adequate support in the
discussion and figures herein.
19